User login
Getting Hip to Vitamin D
Hip fracture is a common clinical problem, with an incidence of 957 cases/100,000 adults in the United States.[1] Studies have found a high prevalence of vitamin D deficiency among elderly patients with fragility fractures, though many of these studies were performed in high latitude regions.[2, 3, 4, 5, 6, 7, 8, 9, 10] Endocrine Society clinical practice guidelines recommend screening patients with fragility fractures for vitamin D deficiency.[11]
Our hospitalist group practices in an academic tertiary care facility in the southeastern United States. Beginning in June 2010, all patients with acute hip fracture were admitted to our service with consultative comanagement from orthopedics. Our group did not have a standardized approach for the assessment or treatment of vitamin D deficiency in this population. Preliminary analysis of a subgroup of our patients with acute hip fracture revealed that only 29% had been screened for vitamin D deficiency. Of these patients, 68% were deficient or insufficient, yet less than half had been discharged on an appropriate dose of vitamin D. We concluded that our group practice was both varied and substandard.
In this report we describe the creation and implementation of a process for improving the assessment and treatment of vitamin D deficiency in elderly patients with fragility hip fracture. We evaluated the effect of our process on the percentages of patients screened and treated appropriately for vitamin D deficiency.
METHODS
Creation of Intervention
We assembled a task force, consisting of 4 hospitalist physicians. The task force reviewed available literature on the prevalence of vitamin D deficiency in elderly patients with fragility fracture and major practice guidelines related to vitamin D. We utilized Endocrine Society clinical practice guidelines to define vitamin D deficiency, insufficiency, and recommended treatment dosing for each condition[11] (Table 1).
| Vitamin D Level (25‐OH) | Vitamin D Status | Treatment Dose Recommendation |
|---|---|---|
| 019 ng/mL | Deficient | 50,000 IU/week for 68 weeks |
| 2029 ng/mL | Insufficient | 1,000 to 2,000 IU/day or 50,000 IU/month |
We developed 2 processes for improving group practice. First, we presented a review of evidence and preliminary data from our group practice at a meeting of hospitalist staff. Second, we revised the computerized physician order entry (CPOE) set for patients with hip fractures to include 2 new orders: (1) an automatic order for 25‐OH vitamin D level to be drawn the morning after admission and (2) an order for initiation of 1000 IU daily of vitamin D at admission.
The reasons for starting empiric vitamin D supplementation were 2fold. First was to prompt dosing of vitamin D at the time of discharge by already having it on the patient's medication list. Second was to conform to US Preventive Services Task Force guidelines for fall prevention.[12] The dose of 1000 IU was selected due to its being adequate treatment for insufficient (though not deficient) patients, and yet a low enough dose to minimize risk of toxicity.
Providers
Our hospitalist group includes 21 physicians and 3 physician extenders. Two nocturnist positions were added to our group in July 2013, part way through our intervention. There were no other additions or subtractions to the staff during the study period.
Patients
Patients were identified by search of University of North Carolina (UNC) Hospitals' database using International Classification of Diseases, 9th Revision codes for femoral neck fracture (821.x) and femur fracture NOS (820.x), linked to hospital services covered by our group. Exclusion criteria included age 50 years, fracture due to high‐speed trauma, fracture due to malignancy, end‐stage renal disease, and death or transition to comfort care during the index hospitalization.
Outcome Measures
Primary outcome measures were the percentage of patients with acute hip fracture with vitamin D level checked during hospitalization and the percentage of deficient patients discharged on the recommended dose of vitamin D. Outcomes were measured for the 28 months before intervention (when our group assumed direct care for hip fracture patients) and were compared with the 12 months after intervention. We also report the prevalence of vitamin D deficiency in our population.
Laboratory Methodology
25‐OH vitamin D assays were performed by UNC Hospitals' core laboratories. Assays were performed using liquid chromatography tandem mass spectroscopy technique. Methodology remained constant through the study period.
During implementation of the project, we identified slow turnaround time in reporting of the vitamin D assays as an issue. We subsequently plotted the percentage of assays returned within 48 hours for each month of the study period on a run chart.
Analysis
Primary outcome measures and demographic data were tested for statistical significance with the 2 test. As a separate means of analysis, we plotted a control chart for the percentage of patients with vitamin D level checked and a run chart for the percentage of deficient or insufficient patients discharged on the recommended dose of vitamin D. To ensure a constant sample size, consecutive samples of patients were plotted in chronologic order. Results were interpreted with standard Shewhart rules.[13] 2 testing and plotting of control and run charts were performed using Microsoft Excel (Microsoft Corp., Redmond, WA) and QI Charts (Process Improvement Products, Austin, TX).
Implementation
In October 2012, we presented the review of evidence and preliminary data to the hospitalist group and made the new CPOE hip fracture order set available. Implementation was monitored by solicitation of qualitative feedback from group physicians and analysis of outcome data every 6 months. Issues that arose during implementation are described in a project timeline (Figure 1) and discussed in detail in manuscript discussion. We received institutional review board approval to study the project's implementation.

RESULTS
Patients
There were 220 patients identified in the 28 months before implementation. Twenty‐four were excluded by criteria, leaving 196 for analysis. One hundred thirteen patients were identified after implementation. Six patients were excluded by criteria, leaving 107 for analysis.
The mean patient age was 80 years, and the median age was 83 years. Seventy‐five percent were female. Race categories were 85% Caucasian, 8% African American, 3% Asian, 1% Native American, 1% Hispanic, and 3% other.
The preintervention group had mean and median ages of 80 and 82 years, respectively, compared with 81 and 84 years, respectively, in the postintervention group. Seventy‐five percent of the preintervention group was female, compared with 74% postintervention. The only statistically significant difference was in the percentage of Caucasian patients81% of preintervention group compared with 91% of the postintervention group (P = 0.028).
Primary Outcomes
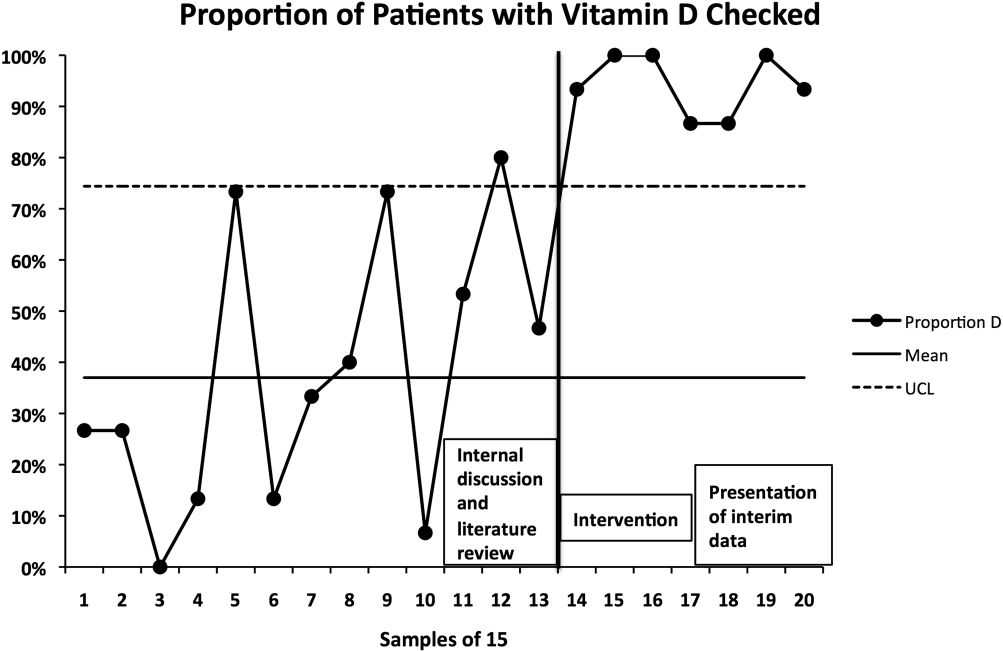
The percentage of patients with acute hip fracture with vitamin D level checked before project implementation was 37.2% (n = 196). After implementation, the percentage improved to 93.5% (n = 107, P < 0.001).
The proportion chart plot of the same data (Figure 2) shows evidence of a fundamental change after intervention. Data points showing the proportion of consecutive samples of 15 patients were plotted chronologically. All points after implementation were above the upper control limit, meeting Shewhart control chart rules for special cause variation.[13]
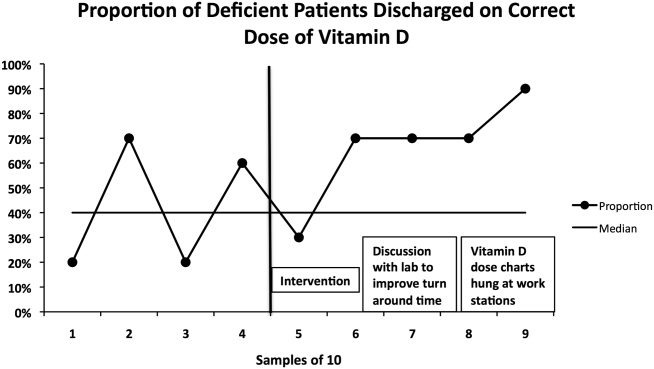
The percentage of vitamin D deficient/emnsufficient patients discharged on the recommended dose of vitamin D also improved, rising from 40.9% (n = 44) before to 68.0% (n = 50) after implementation (P = 0.008). Because there were fewer candidates for this outcome, we plotted samples of 10 patients consecutively on a run chart (Figure 3). Although there were insufficient data to establish a trend by run chart rules, the last 4 consecutive data points showed sequential improvement.
Prevalence of Vitamin D Insufficiency and Deficiency
Before implementation, 44 of the 73 patients (60.3%) with vitamin D levels checked were deficient or insufficient (25‐OH vitamin D <30 ng/mL); of those 44 patients, 21 (28.8% of total checked) had 25‐OH vitamin D levels <20 ng/mL. After implementation, 50 of 100 patients with levels checked were identified as deficient or insufficient (50%); of those 50 patients, 23 (23% of total) had 25‐OH vitamin D levels <20 ng/mL.
DISCUSSION
Our interventions correlated with significant improvements in the assessment and treatment of vitamin D deficiency in elderly patients with fragility hip fractures. Our study demonstrates a systematic method groups may use to adopt and reliably implement practice guidelines. Moreover, we report several steps to implementation that enhanced our ability to standardize clinical care.
The prevalence of vitamin D deficiency and insufficiency we identified50.0% after change implementationis within the range reported in prior studies, though our result is notable for being in a southern region of the United States. The prevalence we found before implementation (60.3%) may have been subject to selection bias in screening, so 50.0% is likely the more correct prevalence. Other US studies of vitamin D deficiency prevalence in hip fracture patients report rates from 50% to 65.8%.[2, 8, 10]
The percentage of hip fracture patients screened for vitamin D deficiency showed significant improvement after our interventions, rising to 93.5%. As a comparison with our results, a 2008 study after implementation of a hip fracture pathway reported only screening 37% of patients for vitamin D deficiency.[14] The main barrier we identified was occasional failure to use the electronic order set. This was in large part due to moonlighting physicians, who occasionally cover hospitalist shifts. They accounted for 5 of the 7 missed patients. The other 2 misses were due to group physicians not using the order set. These findings were first identified after 6 months of data were analyzed. These data were presented to the hospitalist group, with reminders to reinforce order set use with moonlighters and to manually order levels after admission if the order set was not utilized.
We found more difficulty with discharging deficient patients on the recommended dose of vitamin D. Our low level at the time of implementation40.9%was actually higher than a recent Swiss study, which found that only 27% of patients with acute hip fracture were discharged on any vitamin D, despite 91% of patients having 25‐OH vitamin D levels <30 ng/mL.[15] However, our proportion of deficient patients discharged on the recommended vitamin D dose only improved to 68.0% during our interventions. This is similar to Glowacki et al., who reported discharging 76% of hip fracture patients on vitamin D and/or calcium through utilization of a discharge pathway, though they did not differentiate vitamin D from calcium in results or attempt to identify patient‐specific vitamin D dosing based on serum levels.[14]
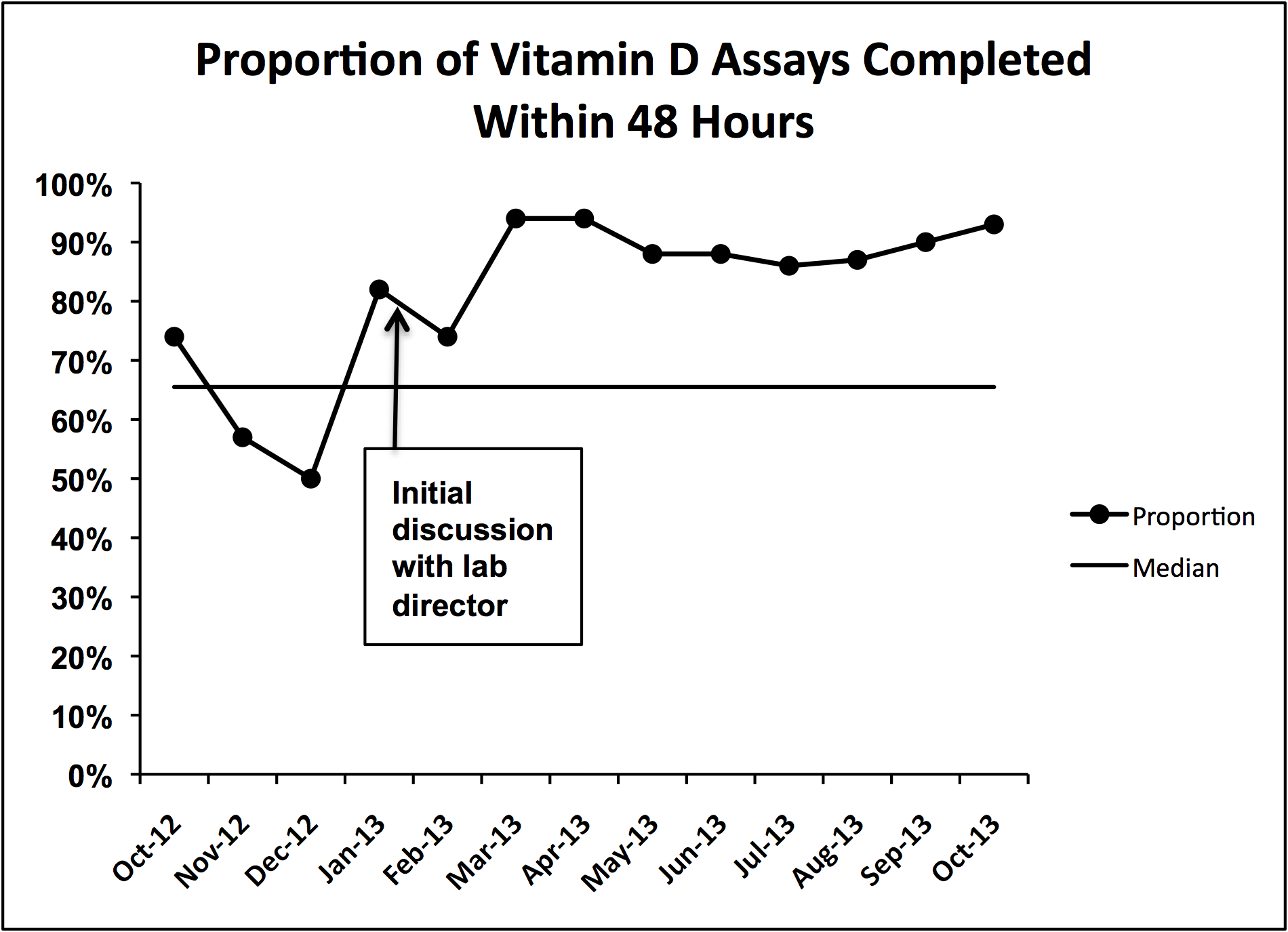
We did identify and address several barriers to discharging patients on the recommended dose. First, we experienced slow turnaround time in measurement of 25‐OH vitamin D. Early into the project, we received several reports of patients being discharged before vitamin D levels had returned. We communicated with the director of UNC Hospitals' core laboratories. A major issue was that the special chemistry section of the core laboratory did not report results directly into the hospital's main electronic reporting system, so that the results had to be hand entered. Over several months, the laboratory worked to improve turnaround times. A run chart plot of the percentage of assays reported within 48 hours for each month showed significant improvement with these efforts (see Supporting Information, Figure 1, in the online version of this article). All 9 data points after our initial discussion with the laboratory director were above the mean established during the prior 4 months, meeting run chart rules for a fundamental change in the system.[13]
The second issue identified was that the ranges for deficiency and insufficiency recommended by Endocrine Society guidelines did not match the reference ranges provided by UNC Hospitals. UNC Hospitals reported levels of 25‐OH vitamin D as normal if above 24, whereas the Endocrine Society defined normal as above 29. When analyzing data after 6 months, we found several patients who had been screened appropriately with results available and noted by the discharging physician, but with results in the normal range per our laboratory. Several of these patients, though low in vitamin D by Endocrine Society standards, were not treated. The laboratory director was again contacted, who noted that the UNC reference ranges had been formed before the Endocrine Society guidelines had been published. We elected to continue with the more conservative ranges recommended by the Endocrine Society. We presented results to the group after 6 months of data had been collected and emphasized our recommended reference ranges and vitamin D dosing (Table 1). We also created reference charts with this information and hung them by all computer workstations in the hospitalist office. With this continued assessment of data and provider education, we did note further improvement through the implementation period, with 90.0% of the last sample of deficient/emnsufficient patients discharged on the recommended dose of vitamin D (Figure 3).
We debated whether to include calcium supplementation as part of our intervention, but given known potential harms from calcium supplementation, including nephrolithiasis and possible increased cardiovascular risk,[16] we elected to focus exclusively on vitamin D. Although studies of primary and secondary fragility fracture prevention with vitamin D have not demonstrated consistently positive results, the studies were not specifically targeted to vitamin D‐deficient patients.[17, 18] Even in the absence of definitively proven secondary fracture prevention, given the multiple health issues associated with vitamin D deficiency, we believe that screening high prevalence populations and treating appropriately is best practice. With minimal patient costs (our institution charges $93 per assay) and a high prevalence (50% in our population), we believe universal screening of elderly patients with hip fracture for vitamin D deficiency is also cost‐effective.
Our project was specifically designed to address the issue of vitamin D deficiency in elderly hip fracture patients, but most of these patients also have osteoporosis. Although vitamin D deficiency contributes to osteoporosis, it is certainly not the only factor. It is also recognized that a minority of patients with fragility fractures receives subsequent evaluation and treatment for osteoporosis, <20% in a recent large population‐based study.[19] The American Orthopedic Association has recently launched a website and campaign entitled Own the Bone to improve the quality of care for patients after osteoporotic fracture.[20] A number of measures have been studied to improve the deficit in care, often termed the osteoporosis treatment gap. Edwards and colleagues recently described an intervention based on their institutional electronic medical record.[21] The intervention included order sets for diagnosing osteoporosis and educational materials for patients and providers, but did not demonstrate any change in percentage of patients evaluated for osteoporosis after fragility fracture. Successful randomized controlled trials have been reported using mail notification of physicians and patients after osteoporotic fracture[22]; multifaceted telephone, education and mail notification interventions after wrist fracture[23]; and through the use of a central osteoporosis coordinator to coordinate osteoporosis treatment after a fragility fracture.[24] These successful trials were broad in scope and yet reported modest (10%20%) gains in improvement.
Although bisphophonate therapy is of proven benefit in secondary fracture prevention, there are a number of barriers to initiating it in the acute setting after fragility fracture, as the difficulty in getting large improvement during the above trials suggests. These include recommendations from some experts for bone density testing before starting treatment and theoretic concerns of impairing fracture healing in the initial weeks after acute fracture. Both of these concerns make a hospitalist‐based intervention for osteoporosis evaluation and treatment challenging and beyond the scope of our project's quality improvement efforts.
Our study has some limitations. It was conducted in a single institution and electronic order entry system, which could limit the ability to generalize the results. We did not assess vitamin D compliance or follow‐up after hospitalization, so we are unable to determine if patients successfully completed treatment after it was prescribed. We also found slight differences in race between the pre‐ and postintervention groups. Although we did not perform multivariable regression to account for these differences, we feel such analyses would be unlikely to alter our results. Last, it should be noted that there may be unintended consequences from preselected orders, such as the ones we utilized for vitamin D assays and empiric supplementation. For example, patients with a recently checked vitamin D assay would have duplication of that lab. Similarly, patients who were already taking vitamin D could theoretically be placed on double therapy at admission. With safeguards in the electronic system to flag duplicate medications, low toxicity of standard doses of vitamin D, and minimal economic harm with duplicate laboratory therapy in the context of a hospitalization for hip fracture, we believe the risks are outweighed by the benefits of screening.
In summary, with review of evidence, modification of a computerized physician order set, provider education and feedback, and collaboration with our clinical laboratory, we were able to standardize and improve group practice for the assessment and treatment of vitamin D deficiency in elderly patients with hip fracture. We believe that our model could be applied to other institutions to further improve patient care. Given the extremely high incidence of hip fracture and consistently high prevalence of vitamin D deficiency in this population across studies, these findings have important implications for the care of this commonly encountered and vulnerable group of patients.
Disclosures: Data from this project were presented in abstract form at the Society of Hospital Medicine Annual Meetings in 2013 and 2014 and as an abstract at the Society of General Internal Medicine Annual Meeting in 2014. Dr. Catherine Hammett‐Stabler, Director of UNC Hospitals McLendon Core Laboratories, provided data on vitamin D assay turnaround times. The authors report no conflicts of interest.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , , , . Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281(16):1505–1511.
- , , , , , . Prevalence of vitamin D inadequacy in Scottish adults with non‐vertebral fragility fractures. Curr Med Res Opin. 2005;21(9):1355–1361.
- , , . Prevalence of vitamin D inadequacy in osteoporotic hip fracture patients in London. Curr Med Res Opin. 2005;21(12):1891–1894.
- , , , et al. Half of the patients with an acute hip fracture suffer from hypovitaminosis D: a prospective study in southeastern Finland. Osteoporos Int. 2005;16(12):2018–2024.
- , , , et al. Prevalence of vitamin D inadequacy in Belfast following fragility fracture. Curr Med Res Opin. 2006;22(1):101–105.
- , , , , , . High prevalence of hypovitaminosis D and K in patients with hip fracture. Asia Pac J Clin Nutr. 2011;20(1):56–61.
- , , , . Vitamin D insufficiency in patients with acute hip fractures of all ages and both sexes in a sunny climate. J Orthop Trauma. 2013;27(12):e275–e280.
- , , , et al. Vitamin D and intact PTH status in patients with hip fracture. Osteoporos Int. 2006;17(11):1608–1614.
- , , , et al. Distribution and correlates of serum 25‐hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335–340.
- , , , et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.
- , . Prevention of falls in community‐dwelling older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(3):197–204.
- , . The Health Care Data Guide: Learning From Data for Improvement. 1st ed. San Francisco, CA: Jossey‐Bass; 2011.
- , , , , . Importance of vitamin D in hospital‐based fracture care pathways. J Nutr Health Aging. 2008;12(5):291–293.
- , , , et al. Before and after hip fracture, vitamin D deficiency may not be treated sufficiently. Osteoporos Int. 2013;24(11):2765–2773.
- , , , et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta‐analysis. BMJ. 2010;341:c3691.
- , , , et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–49.
- , , , et al. Oral vitamin D3 and calcium for secondary prevention of low‐trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo‐controlled trial. Lancet. 2005;365(9471):1621–1628.
- , , , et al. A population‐based analysis of the post‐fracture care gap 1996–2008: the situation is not improving. Osteoporos Int. 2012;23(5):1623–1629.
- American Orthopedic Association. Own the Bone website. 2011. Available at: http://www.ownthebone.org. Accessed August 1, 2014.
- , , , et al. Development of an electronic medical record based intervention to improve medical care of osteoporosis. Osteoporos Int. 2012;23(10):2489–2498.
- , , , , . Closing the gap in postfracture care at the population level: a randomized controlled trial. CMAJ. 2012;184(3):290–296.
- , , , et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ. 2008;178(5):569–575.
- , , , et al. Impact of a centralized osteoporosis coordinator on post‐fracture osteoporosis management: a cluster randomized trial. Osteoporos Int. 2012;23(1):87–95.
Hip fracture is a common clinical problem, with an incidence of 957 cases/100,000 adults in the United States.[1] Studies have found a high prevalence of vitamin D deficiency among elderly patients with fragility fractures, though many of these studies were performed in high latitude regions.[2, 3, 4, 5, 6, 7, 8, 9, 10] Endocrine Society clinical practice guidelines recommend screening patients with fragility fractures for vitamin D deficiency.[11]
Our hospitalist group practices in an academic tertiary care facility in the southeastern United States. Beginning in June 2010, all patients with acute hip fracture were admitted to our service with consultative comanagement from orthopedics. Our group did not have a standardized approach for the assessment or treatment of vitamin D deficiency in this population. Preliminary analysis of a subgroup of our patients with acute hip fracture revealed that only 29% had been screened for vitamin D deficiency. Of these patients, 68% were deficient or insufficient, yet less than half had been discharged on an appropriate dose of vitamin D. We concluded that our group practice was both varied and substandard.
In this report we describe the creation and implementation of a process for improving the assessment and treatment of vitamin D deficiency in elderly patients with fragility hip fracture. We evaluated the effect of our process on the percentages of patients screened and treated appropriately for vitamin D deficiency.
METHODS
Creation of Intervention
We assembled a task force, consisting of 4 hospitalist physicians. The task force reviewed available literature on the prevalence of vitamin D deficiency in elderly patients with fragility fracture and major practice guidelines related to vitamin D. We utilized Endocrine Society clinical practice guidelines to define vitamin D deficiency, insufficiency, and recommended treatment dosing for each condition[11] (Table 1).
| Vitamin D Level (25‐OH) | Vitamin D Status | Treatment Dose Recommendation |
|---|---|---|
| 019 ng/mL | Deficient | 50,000 IU/week for 68 weeks |
| 2029 ng/mL | Insufficient | 1,000 to 2,000 IU/day or 50,000 IU/month |
We developed 2 processes for improving group practice. First, we presented a review of evidence and preliminary data from our group practice at a meeting of hospitalist staff. Second, we revised the computerized physician order entry (CPOE) set for patients with hip fractures to include 2 new orders: (1) an automatic order for 25‐OH vitamin D level to be drawn the morning after admission and (2) an order for initiation of 1000 IU daily of vitamin D at admission.
The reasons for starting empiric vitamin D supplementation were 2fold. First was to prompt dosing of vitamin D at the time of discharge by already having it on the patient's medication list. Second was to conform to US Preventive Services Task Force guidelines for fall prevention.[12] The dose of 1000 IU was selected due to its being adequate treatment for insufficient (though not deficient) patients, and yet a low enough dose to minimize risk of toxicity.
Providers
Our hospitalist group includes 21 physicians and 3 physician extenders. Two nocturnist positions were added to our group in July 2013, part way through our intervention. There were no other additions or subtractions to the staff during the study period.
Patients
Patients were identified by search of University of North Carolina (UNC) Hospitals' database using International Classification of Diseases, 9th Revision codes for femoral neck fracture (821.x) and femur fracture NOS (820.x), linked to hospital services covered by our group. Exclusion criteria included age 50 years, fracture due to high‐speed trauma, fracture due to malignancy, end‐stage renal disease, and death or transition to comfort care during the index hospitalization.
Outcome Measures
Primary outcome measures were the percentage of patients with acute hip fracture with vitamin D level checked during hospitalization and the percentage of deficient patients discharged on the recommended dose of vitamin D. Outcomes were measured for the 28 months before intervention (when our group assumed direct care for hip fracture patients) and were compared with the 12 months after intervention. We also report the prevalence of vitamin D deficiency in our population.
Laboratory Methodology
25‐OH vitamin D assays were performed by UNC Hospitals' core laboratories. Assays were performed using liquid chromatography tandem mass spectroscopy technique. Methodology remained constant through the study period.
During implementation of the project, we identified slow turnaround time in reporting of the vitamin D assays as an issue. We subsequently plotted the percentage of assays returned within 48 hours for each month of the study period on a run chart.
Analysis
Primary outcome measures and demographic data were tested for statistical significance with the 2 test. As a separate means of analysis, we plotted a control chart for the percentage of patients with vitamin D level checked and a run chart for the percentage of deficient or insufficient patients discharged on the recommended dose of vitamin D. To ensure a constant sample size, consecutive samples of patients were plotted in chronologic order. Results were interpreted with standard Shewhart rules.[13] 2 testing and plotting of control and run charts were performed using Microsoft Excel (Microsoft Corp., Redmond, WA) and QI Charts (Process Improvement Products, Austin, TX).
Implementation
In October 2012, we presented the review of evidence and preliminary data to the hospitalist group and made the new CPOE hip fracture order set available. Implementation was monitored by solicitation of qualitative feedback from group physicians and analysis of outcome data every 6 months. Issues that arose during implementation are described in a project timeline (Figure 1) and discussed in detail in manuscript discussion. We received institutional review board approval to study the project's implementation.

RESULTS
Patients
There were 220 patients identified in the 28 months before implementation. Twenty‐four were excluded by criteria, leaving 196 for analysis. One hundred thirteen patients were identified after implementation. Six patients were excluded by criteria, leaving 107 for analysis.
The mean patient age was 80 years, and the median age was 83 years. Seventy‐five percent were female. Race categories were 85% Caucasian, 8% African American, 3% Asian, 1% Native American, 1% Hispanic, and 3% other.
The preintervention group had mean and median ages of 80 and 82 years, respectively, compared with 81 and 84 years, respectively, in the postintervention group. Seventy‐five percent of the preintervention group was female, compared with 74% postintervention. The only statistically significant difference was in the percentage of Caucasian patients81% of preintervention group compared with 91% of the postintervention group (P = 0.028).
Primary Outcomes
The percentage of patients with acute hip fracture with vitamin D level checked before project implementation was 37.2% (n = 196). After implementation, the percentage improved to 93.5% (n = 107, P < 0.001).
The proportion chart plot of the same data (Figure 2) shows evidence of a fundamental change after intervention. Data points showing the proportion of consecutive samples of 15 patients were plotted chronologically. All points after implementation were above the upper control limit, meeting Shewhart control chart rules for special cause variation.[13]

The percentage of vitamin D deficient/emnsufficient patients discharged on the recommended dose of vitamin D also improved, rising from 40.9% (n = 44) before to 68.0% (n = 50) after implementation (P = 0.008). Because there were fewer candidates for this outcome, we plotted samples of 10 patients consecutively on a run chart (Figure 3). Although there were insufficient data to establish a trend by run chart rules, the last 4 consecutive data points showed sequential improvement.

Prevalence of Vitamin D Insufficiency and Deficiency
Before implementation, 44 of the 73 patients (60.3%) with vitamin D levels checked were deficient or insufficient (25‐OH vitamin D <30 ng/mL); of those 44 patients, 21 (28.8% of total checked) had 25‐OH vitamin D levels <20 ng/mL. After implementation, 50 of 100 patients with levels checked were identified as deficient or insufficient (50%); of those 50 patients, 23 (23% of total) had 25‐OH vitamin D levels <20 ng/mL.
DISCUSSION
Our interventions correlated with significant improvements in the assessment and treatment of vitamin D deficiency in elderly patients with fragility hip fractures. Our study demonstrates a systematic method groups may use to adopt and reliably implement practice guidelines. Moreover, we report several steps to implementation that enhanced our ability to standardize clinical care.
The prevalence of vitamin D deficiency and insufficiency we identified50.0% after change implementationis within the range reported in prior studies, though our result is notable for being in a southern region of the United States. The prevalence we found before implementation (60.3%) may have been subject to selection bias in screening, so 50.0% is likely the more correct prevalence. Other US studies of vitamin D deficiency prevalence in hip fracture patients report rates from 50% to 65.8%.[2, 8, 10]
The percentage of hip fracture patients screened for vitamin D deficiency showed significant improvement after our interventions, rising to 93.5%. As a comparison with our results, a 2008 study after implementation of a hip fracture pathway reported only screening 37% of patients for vitamin D deficiency.[14] The main barrier we identified was occasional failure to use the electronic order set. This was in large part due to moonlighting physicians, who occasionally cover hospitalist shifts. They accounted for 5 of the 7 missed patients. The other 2 misses were due to group physicians not using the order set. These findings were first identified after 6 months of data were analyzed. These data were presented to the hospitalist group, with reminders to reinforce order set use with moonlighters and to manually order levels after admission if the order set was not utilized.
We found more difficulty with discharging deficient patients on the recommended dose of vitamin D. Our low level at the time of implementation40.9%was actually higher than a recent Swiss study, which found that only 27% of patients with acute hip fracture were discharged on any vitamin D, despite 91% of patients having 25‐OH vitamin D levels <30 ng/mL.[15] However, our proportion of deficient patients discharged on the recommended vitamin D dose only improved to 68.0% during our interventions. This is similar to Glowacki et al., who reported discharging 76% of hip fracture patients on vitamin D and/or calcium through utilization of a discharge pathway, though they did not differentiate vitamin D from calcium in results or attempt to identify patient‐specific vitamin D dosing based on serum levels.[14]
We did identify and address several barriers to discharging patients on the recommended dose. First, we experienced slow turnaround time in measurement of 25‐OH vitamin D. Early into the project, we received several reports of patients being discharged before vitamin D levels had returned. We communicated with the director of UNC Hospitals' core laboratories. A major issue was that the special chemistry section of the core laboratory did not report results directly into the hospital's main electronic reporting system, so that the results had to be hand entered. Over several months, the laboratory worked to improve turnaround times. A run chart plot of the percentage of assays reported within 48 hours for each month showed significant improvement with these efforts (see Supporting Information, Figure 1, in the online version of this article). All 9 data points after our initial discussion with the laboratory director were above the mean established during the prior 4 months, meeting run chart rules for a fundamental change in the system.[13]
The second issue identified was that the ranges for deficiency and insufficiency recommended by Endocrine Society guidelines did not match the reference ranges provided by UNC Hospitals. UNC Hospitals reported levels of 25‐OH vitamin D as normal if above 24, whereas the Endocrine Society defined normal as above 29. When analyzing data after 6 months, we found several patients who had been screened appropriately with results available and noted by the discharging physician, but with results in the normal range per our laboratory. Several of these patients, though low in vitamin D by Endocrine Society standards, were not treated. The laboratory director was again contacted, who noted that the UNC reference ranges had been formed before the Endocrine Society guidelines had been published. We elected to continue with the more conservative ranges recommended by the Endocrine Society. We presented results to the group after 6 months of data had been collected and emphasized our recommended reference ranges and vitamin D dosing (Table 1). We also created reference charts with this information and hung them by all computer workstations in the hospitalist office. With this continued assessment of data and provider education, we did note further improvement through the implementation period, with 90.0% of the last sample of deficient/emnsufficient patients discharged on the recommended dose of vitamin D (Figure 3).
We debated whether to include calcium supplementation as part of our intervention, but given known potential harms from calcium supplementation, including nephrolithiasis and possible increased cardiovascular risk,[16] we elected to focus exclusively on vitamin D. Although studies of primary and secondary fragility fracture prevention with vitamin D have not demonstrated consistently positive results, the studies were not specifically targeted to vitamin D‐deficient patients.[17, 18] Even in the absence of definitively proven secondary fracture prevention, given the multiple health issues associated with vitamin D deficiency, we believe that screening high prevalence populations and treating appropriately is best practice. With minimal patient costs (our institution charges $93 per assay) and a high prevalence (50% in our population), we believe universal screening of elderly patients with hip fracture for vitamin D deficiency is also cost‐effective.
Our project was specifically designed to address the issue of vitamin D deficiency in elderly hip fracture patients, but most of these patients also have osteoporosis. Although vitamin D deficiency contributes to osteoporosis, it is certainly not the only factor. It is also recognized that a minority of patients with fragility fractures receives subsequent evaluation and treatment for osteoporosis, <20% in a recent large population‐based study.[19] The American Orthopedic Association has recently launched a website and campaign entitled Own the Bone to improve the quality of care for patients after osteoporotic fracture.[20] A number of measures have been studied to improve the deficit in care, often termed the osteoporosis treatment gap. Edwards and colleagues recently described an intervention based on their institutional electronic medical record.[21] The intervention included order sets for diagnosing osteoporosis and educational materials for patients and providers, but did not demonstrate any change in percentage of patients evaluated for osteoporosis after fragility fracture. Successful randomized controlled trials have been reported using mail notification of physicians and patients after osteoporotic fracture[22]; multifaceted telephone, education and mail notification interventions after wrist fracture[23]; and through the use of a central osteoporosis coordinator to coordinate osteoporosis treatment after a fragility fracture.[24] These successful trials were broad in scope and yet reported modest (10%20%) gains in improvement.
Although bisphophonate therapy is of proven benefit in secondary fracture prevention, there are a number of barriers to initiating it in the acute setting after fragility fracture, as the difficulty in getting large improvement during the above trials suggests. These include recommendations from some experts for bone density testing before starting treatment and theoretic concerns of impairing fracture healing in the initial weeks after acute fracture. Both of these concerns make a hospitalist‐based intervention for osteoporosis evaluation and treatment challenging and beyond the scope of our project's quality improvement efforts.
Our study has some limitations. It was conducted in a single institution and electronic order entry system, which could limit the ability to generalize the results. We did not assess vitamin D compliance or follow‐up after hospitalization, so we are unable to determine if patients successfully completed treatment after it was prescribed. We also found slight differences in race between the pre‐ and postintervention groups. Although we did not perform multivariable regression to account for these differences, we feel such analyses would be unlikely to alter our results. Last, it should be noted that there may be unintended consequences from preselected orders, such as the ones we utilized for vitamin D assays and empiric supplementation. For example, patients with a recently checked vitamin D assay would have duplication of that lab. Similarly, patients who were already taking vitamin D could theoretically be placed on double therapy at admission. With safeguards in the electronic system to flag duplicate medications, low toxicity of standard doses of vitamin D, and minimal economic harm with duplicate laboratory therapy in the context of a hospitalization for hip fracture, we believe the risks are outweighed by the benefits of screening.
In summary, with review of evidence, modification of a computerized physician order set, provider education and feedback, and collaboration with our clinical laboratory, we were able to standardize and improve group practice for the assessment and treatment of vitamin D deficiency in elderly patients with hip fracture. We believe that our model could be applied to other institutions to further improve patient care. Given the extremely high incidence of hip fracture and consistently high prevalence of vitamin D deficiency in this population across studies, these findings have important implications for the care of this commonly encountered and vulnerable group of patients.
Disclosures: Data from this project were presented in abstract form at the Society of Hospital Medicine Annual Meetings in 2013 and 2014 and as an abstract at the Society of General Internal Medicine Annual Meeting in 2014. Dr. Catherine Hammett‐Stabler, Director of UNC Hospitals McLendon Core Laboratories, provided data on vitamin D assay turnaround times. The authors report no conflicts of interest.
Hip fracture is a common clinical problem, with an incidence of 957 cases/100,000 adults in the United States.[1] Studies have found a high prevalence of vitamin D deficiency among elderly patients with fragility fractures, though many of these studies were performed in high latitude regions.[2, 3, 4, 5, 6, 7, 8, 9, 10] Endocrine Society clinical practice guidelines recommend screening patients with fragility fractures for vitamin D deficiency.[11]
Our hospitalist group practices in an academic tertiary care facility in the southeastern United States. Beginning in June 2010, all patients with acute hip fracture were admitted to our service with consultative comanagement from orthopedics. Our group did not have a standardized approach for the assessment or treatment of vitamin D deficiency in this population. Preliminary analysis of a subgroup of our patients with acute hip fracture revealed that only 29% had been screened for vitamin D deficiency. Of these patients, 68% were deficient or insufficient, yet less than half had been discharged on an appropriate dose of vitamin D. We concluded that our group practice was both varied and substandard.
In this report we describe the creation and implementation of a process for improving the assessment and treatment of vitamin D deficiency in elderly patients with fragility hip fracture. We evaluated the effect of our process on the percentages of patients screened and treated appropriately for vitamin D deficiency.
METHODS
Creation of Intervention
We assembled a task force, consisting of 4 hospitalist physicians. The task force reviewed available literature on the prevalence of vitamin D deficiency in elderly patients with fragility fracture and major practice guidelines related to vitamin D. We utilized Endocrine Society clinical practice guidelines to define vitamin D deficiency, insufficiency, and recommended treatment dosing for each condition[11] (Table 1).
| Vitamin D Level (25‐OH) | Vitamin D Status | Treatment Dose Recommendation |
|---|---|---|
| 019 ng/mL | Deficient | 50,000 IU/week for 68 weeks |
| 2029 ng/mL | Insufficient | 1,000 to 2,000 IU/day or 50,000 IU/month |
We developed 2 processes for improving group practice. First, we presented a review of evidence and preliminary data from our group practice at a meeting of hospitalist staff. Second, we revised the computerized physician order entry (CPOE) set for patients with hip fractures to include 2 new orders: (1) an automatic order for 25‐OH vitamin D level to be drawn the morning after admission and (2) an order for initiation of 1000 IU daily of vitamin D at admission.
The reasons for starting empiric vitamin D supplementation were 2fold. First was to prompt dosing of vitamin D at the time of discharge by already having it on the patient's medication list. Second was to conform to US Preventive Services Task Force guidelines for fall prevention.[12] The dose of 1000 IU was selected due to its being adequate treatment for insufficient (though not deficient) patients, and yet a low enough dose to minimize risk of toxicity.
Providers
Our hospitalist group includes 21 physicians and 3 physician extenders. Two nocturnist positions were added to our group in July 2013, part way through our intervention. There were no other additions or subtractions to the staff during the study period.
Patients
Patients were identified by search of University of North Carolina (UNC) Hospitals' database using International Classification of Diseases, 9th Revision codes for femoral neck fracture (821.x) and femur fracture NOS (820.x), linked to hospital services covered by our group. Exclusion criteria included age 50 years, fracture due to high‐speed trauma, fracture due to malignancy, end‐stage renal disease, and death or transition to comfort care during the index hospitalization.
Outcome Measures
Primary outcome measures were the percentage of patients with acute hip fracture with vitamin D level checked during hospitalization and the percentage of deficient patients discharged on the recommended dose of vitamin D. Outcomes were measured for the 28 months before intervention (when our group assumed direct care for hip fracture patients) and were compared with the 12 months after intervention. We also report the prevalence of vitamin D deficiency in our population.
Laboratory Methodology
25‐OH vitamin D assays were performed by UNC Hospitals' core laboratories. Assays were performed using liquid chromatography tandem mass spectroscopy technique. Methodology remained constant through the study period.
During implementation of the project, we identified slow turnaround time in reporting of the vitamin D assays as an issue. We subsequently plotted the percentage of assays returned within 48 hours for each month of the study period on a run chart.
Analysis
Primary outcome measures and demographic data were tested for statistical significance with the 2 test. As a separate means of analysis, we plotted a control chart for the percentage of patients with vitamin D level checked and a run chart for the percentage of deficient or insufficient patients discharged on the recommended dose of vitamin D. To ensure a constant sample size, consecutive samples of patients were plotted in chronologic order. Results were interpreted with standard Shewhart rules.[13] 2 testing and plotting of control and run charts were performed using Microsoft Excel (Microsoft Corp., Redmond, WA) and QI Charts (Process Improvement Products, Austin, TX).
Implementation
In October 2012, we presented the review of evidence and preliminary data to the hospitalist group and made the new CPOE hip fracture order set available. Implementation was monitored by solicitation of qualitative feedback from group physicians and analysis of outcome data every 6 months. Issues that arose during implementation are described in a project timeline (Figure 1) and discussed in detail in manuscript discussion. We received institutional review board approval to study the project's implementation.

RESULTS
Patients
There were 220 patients identified in the 28 months before implementation. Twenty‐four were excluded by criteria, leaving 196 for analysis. One hundred thirteen patients were identified after implementation. Six patients were excluded by criteria, leaving 107 for analysis.
The mean patient age was 80 years, and the median age was 83 years. Seventy‐five percent were female. Race categories were 85% Caucasian, 8% African American, 3% Asian, 1% Native American, 1% Hispanic, and 3% other.
The preintervention group had mean and median ages of 80 and 82 years, respectively, compared with 81 and 84 years, respectively, in the postintervention group. Seventy‐five percent of the preintervention group was female, compared with 74% postintervention. The only statistically significant difference was in the percentage of Caucasian patients81% of preintervention group compared with 91% of the postintervention group (P = 0.028).
Primary Outcomes
The percentage of patients with acute hip fracture with vitamin D level checked before project implementation was 37.2% (n = 196). After implementation, the percentage improved to 93.5% (n = 107, P < 0.001).
The proportion chart plot of the same data (Figure 2) shows evidence of a fundamental change after intervention. Data points showing the proportion of consecutive samples of 15 patients were plotted chronologically. All points after implementation were above the upper control limit, meeting Shewhart control chart rules for special cause variation.[13]

The percentage of vitamin D deficient/emnsufficient patients discharged on the recommended dose of vitamin D also improved, rising from 40.9% (n = 44) before to 68.0% (n = 50) after implementation (P = 0.008). Because there were fewer candidates for this outcome, we plotted samples of 10 patients consecutively on a run chart (Figure 3). Although there were insufficient data to establish a trend by run chart rules, the last 4 consecutive data points showed sequential improvement.

Prevalence of Vitamin D Insufficiency and Deficiency
Before implementation, 44 of the 73 patients (60.3%) with vitamin D levels checked were deficient or insufficient (25‐OH vitamin D <30 ng/mL); of those 44 patients, 21 (28.8% of total checked) had 25‐OH vitamin D levels <20 ng/mL. After implementation, 50 of 100 patients with levels checked were identified as deficient or insufficient (50%); of those 50 patients, 23 (23% of total) had 25‐OH vitamin D levels <20 ng/mL.
DISCUSSION
Our interventions correlated with significant improvements in the assessment and treatment of vitamin D deficiency in elderly patients with fragility hip fractures. Our study demonstrates a systematic method groups may use to adopt and reliably implement practice guidelines. Moreover, we report several steps to implementation that enhanced our ability to standardize clinical care.
The prevalence of vitamin D deficiency and insufficiency we identified50.0% after change implementationis within the range reported in prior studies, though our result is notable for being in a southern region of the United States. The prevalence we found before implementation (60.3%) may have been subject to selection bias in screening, so 50.0% is likely the more correct prevalence. Other US studies of vitamin D deficiency prevalence in hip fracture patients report rates from 50% to 65.8%.[2, 8, 10]
The percentage of hip fracture patients screened for vitamin D deficiency showed significant improvement after our interventions, rising to 93.5%. As a comparison with our results, a 2008 study after implementation of a hip fracture pathway reported only screening 37% of patients for vitamin D deficiency.[14] The main barrier we identified was occasional failure to use the electronic order set. This was in large part due to moonlighting physicians, who occasionally cover hospitalist shifts. They accounted for 5 of the 7 missed patients. The other 2 misses were due to group physicians not using the order set. These findings were first identified after 6 months of data were analyzed. These data were presented to the hospitalist group, with reminders to reinforce order set use with moonlighters and to manually order levels after admission if the order set was not utilized.
We found more difficulty with discharging deficient patients on the recommended dose of vitamin D. Our low level at the time of implementation40.9%was actually higher than a recent Swiss study, which found that only 27% of patients with acute hip fracture were discharged on any vitamin D, despite 91% of patients having 25‐OH vitamin D levels <30 ng/mL.[15] However, our proportion of deficient patients discharged on the recommended vitamin D dose only improved to 68.0% during our interventions. This is similar to Glowacki et al., who reported discharging 76% of hip fracture patients on vitamin D and/or calcium through utilization of a discharge pathway, though they did not differentiate vitamin D from calcium in results or attempt to identify patient‐specific vitamin D dosing based on serum levels.[14]
We did identify and address several barriers to discharging patients on the recommended dose. First, we experienced slow turnaround time in measurement of 25‐OH vitamin D. Early into the project, we received several reports of patients being discharged before vitamin D levels had returned. We communicated with the director of UNC Hospitals' core laboratories. A major issue was that the special chemistry section of the core laboratory did not report results directly into the hospital's main electronic reporting system, so that the results had to be hand entered. Over several months, the laboratory worked to improve turnaround times. A run chart plot of the percentage of assays reported within 48 hours for each month showed significant improvement with these efforts (see Supporting Information, Figure 1, in the online version of this article). All 9 data points after our initial discussion with the laboratory director were above the mean established during the prior 4 months, meeting run chart rules for a fundamental change in the system.[13]
The second issue identified was that the ranges for deficiency and insufficiency recommended by Endocrine Society guidelines did not match the reference ranges provided by UNC Hospitals. UNC Hospitals reported levels of 25‐OH vitamin D as normal if above 24, whereas the Endocrine Society defined normal as above 29. When analyzing data after 6 months, we found several patients who had been screened appropriately with results available and noted by the discharging physician, but with results in the normal range per our laboratory. Several of these patients, though low in vitamin D by Endocrine Society standards, were not treated. The laboratory director was again contacted, who noted that the UNC reference ranges had been formed before the Endocrine Society guidelines had been published. We elected to continue with the more conservative ranges recommended by the Endocrine Society. We presented results to the group after 6 months of data had been collected and emphasized our recommended reference ranges and vitamin D dosing (Table 1). We also created reference charts with this information and hung them by all computer workstations in the hospitalist office. With this continued assessment of data and provider education, we did note further improvement through the implementation period, with 90.0% of the last sample of deficient/emnsufficient patients discharged on the recommended dose of vitamin D (Figure 3).
We debated whether to include calcium supplementation as part of our intervention, but given known potential harms from calcium supplementation, including nephrolithiasis and possible increased cardiovascular risk,[16] we elected to focus exclusively on vitamin D. Although studies of primary and secondary fragility fracture prevention with vitamin D have not demonstrated consistently positive results, the studies were not specifically targeted to vitamin D‐deficient patients.[17, 18] Even in the absence of definitively proven secondary fracture prevention, given the multiple health issues associated with vitamin D deficiency, we believe that screening high prevalence populations and treating appropriately is best practice. With minimal patient costs (our institution charges $93 per assay) and a high prevalence (50% in our population), we believe universal screening of elderly patients with hip fracture for vitamin D deficiency is also cost‐effective.
Our project was specifically designed to address the issue of vitamin D deficiency in elderly hip fracture patients, but most of these patients also have osteoporosis. Although vitamin D deficiency contributes to osteoporosis, it is certainly not the only factor. It is also recognized that a minority of patients with fragility fractures receives subsequent evaluation and treatment for osteoporosis, <20% in a recent large population‐based study.[19] The American Orthopedic Association has recently launched a website and campaign entitled Own the Bone to improve the quality of care for patients after osteoporotic fracture.[20] A number of measures have been studied to improve the deficit in care, often termed the osteoporosis treatment gap. Edwards and colleagues recently described an intervention based on their institutional electronic medical record.[21] The intervention included order sets for diagnosing osteoporosis and educational materials for patients and providers, but did not demonstrate any change in percentage of patients evaluated for osteoporosis after fragility fracture. Successful randomized controlled trials have been reported using mail notification of physicians and patients after osteoporotic fracture[22]; multifaceted telephone, education and mail notification interventions after wrist fracture[23]; and through the use of a central osteoporosis coordinator to coordinate osteoporosis treatment after a fragility fracture.[24] These successful trials were broad in scope and yet reported modest (10%20%) gains in improvement.
Although bisphophonate therapy is of proven benefit in secondary fracture prevention, there are a number of barriers to initiating it in the acute setting after fragility fracture, as the difficulty in getting large improvement during the above trials suggests. These include recommendations from some experts for bone density testing before starting treatment and theoretic concerns of impairing fracture healing in the initial weeks after acute fracture. Both of these concerns make a hospitalist‐based intervention for osteoporosis evaluation and treatment challenging and beyond the scope of our project's quality improvement efforts.
Our study has some limitations. It was conducted in a single institution and electronic order entry system, which could limit the ability to generalize the results. We did not assess vitamin D compliance or follow‐up after hospitalization, so we are unable to determine if patients successfully completed treatment after it was prescribed. We also found slight differences in race between the pre‐ and postintervention groups. Although we did not perform multivariable regression to account for these differences, we feel such analyses would be unlikely to alter our results. Last, it should be noted that there may be unintended consequences from preselected orders, such as the ones we utilized for vitamin D assays and empiric supplementation. For example, patients with a recently checked vitamin D assay would have duplication of that lab. Similarly, patients who were already taking vitamin D could theoretically be placed on double therapy at admission. With safeguards in the electronic system to flag duplicate medications, low toxicity of standard doses of vitamin D, and minimal economic harm with duplicate laboratory therapy in the context of a hospitalization for hip fracture, we believe the risks are outweighed by the benefits of screening.
In summary, with review of evidence, modification of a computerized physician order set, provider education and feedback, and collaboration with our clinical laboratory, we were able to standardize and improve group practice for the assessment and treatment of vitamin D deficiency in elderly patients with hip fracture. We believe that our model could be applied to other institutions to further improve patient care. Given the extremely high incidence of hip fracture and consistently high prevalence of vitamin D deficiency in this population across studies, these findings have important implications for the care of this commonly encountered and vulnerable group of patients.
Disclosures: Data from this project were presented in abstract form at the Society of Hospital Medicine Annual Meetings in 2013 and 2014 and as an abstract at the Society of General Internal Medicine Annual Meeting in 2014. Dr. Catherine Hammett‐Stabler, Director of UNC Hospitals McLendon Core Laboratories, provided data on vitamin D assay turnaround times. The authors report no conflicts of interest.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , , , . Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281(16):1505–1511.
- , , , , , . Prevalence of vitamin D inadequacy in Scottish adults with non‐vertebral fragility fractures. Curr Med Res Opin. 2005;21(9):1355–1361.
- , , . Prevalence of vitamin D inadequacy in osteoporotic hip fracture patients in London. Curr Med Res Opin. 2005;21(12):1891–1894.
- , , , et al. Half of the patients with an acute hip fracture suffer from hypovitaminosis D: a prospective study in southeastern Finland. Osteoporos Int. 2005;16(12):2018–2024.
- , , , et al. Prevalence of vitamin D inadequacy in Belfast following fragility fracture. Curr Med Res Opin. 2006;22(1):101–105.
- , , , , , . High prevalence of hypovitaminosis D and K in patients with hip fracture. Asia Pac J Clin Nutr. 2011;20(1):56–61.
- , , , . Vitamin D insufficiency in patients with acute hip fractures of all ages and both sexes in a sunny climate. J Orthop Trauma. 2013;27(12):e275–e280.
- , , , et al. Vitamin D and intact PTH status in patients with hip fracture. Osteoporos Int. 2006;17(11):1608–1614.
- , , , et al. Distribution and correlates of serum 25‐hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335–340.
- , , , et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.
- , . Prevention of falls in community‐dwelling older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(3):197–204.
- , . The Health Care Data Guide: Learning From Data for Improvement. 1st ed. San Francisco, CA: Jossey‐Bass; 2011.
- , , , , . Importance of vitamin D in hospital‐based fracture care pathways. J Nutr Health Aging. 2008;12(5):291–293.
- , , , et al. Before and after hip fracture, vitamin D deficiency may not be treated sufficiently. Osteoporos Int. 2013;24(11):2765–2773.
- , , , et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta‐analysis. BMJ. 2010;341:c3691.
- , , , et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–49.
- , , , et al. Oral vitamin D3 and calcium for secondary prevention of low‐trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo‐controlled trial. Lancet. 2005;365(9471):1621–1628.
- , , , et al. A population‐based analysis of the post‐fracture care gap 1996–2008: the situation is not improving. Osteoporos Int. 2012;23(5):1623–1629.
- American Orthopedic Association. Own the Bone website. 2011. Available at: http://www.ownthebone.org. Accessed August 1, 2014.
- , , , et al. Development of an electronic medical record based intervention to improve medical care of osteoporosis. Osteoporos Int. 2012;23(10):2489–2498.
- , , , , . Closing the gap in postfracture care at the population level: a randomized controlled trial. CMAJ. 2012;184(3):290–296.
- , , , et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ. 2008;178(5):569–575.
- , , , et al. Impact of a centralized osteoporosis coordinator on post‐fracture osteoporosis management: a cluster randomized trial. Osteoporos Int. 2012;23(1):87–95.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , , , . Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281(16):1505–1511.
- , , , , , . Prevalence of vitamin D inadequacy in Scottish adults with non‐vertebral fragility fractures. Curr Med Res Opin. 2005;21(9):1355–1361.
- , , . Prevalence of vitamin D inadequacy in osteoporotic hip fracture patients in London. Curr Med Res Opin. 2005;21(12):1891–1894.
- , , , et al. Half of the patients with an acute hip fracture suffer from hypovitaminosis D: a prospective study in southeastern Finland. Osteoporos Int. 2005;16(12):2018–2024.
- , , , et al. Prevalence of vitamin D inadequacy in Belfast following fragility fracture. Curr Med Res Opin. 2006;22(1):101–105.
- , , , , , . High prevalence of hypovitaminosis D and K in patients with hip fracture. Asia Pac J Clin Nutr. 2011;20(1):56–61.
- , , , . Vitamin D insufficiency in patients with acute hip fractures of all ages and both sexes in a sunny climate. J Orthop Trauma. 2013;27(12):e275–e280.
- , , , et al. Vitamin D and intact PTH status in patients with hip fracture. Osteoporos Int. 2006;17(11):1608–1614.
- , , , et al. Distribution and correlates of serum 25‐hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5(4):335–340.
- , , , et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.
- , . Prevention of falls in community‐dwelling older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(3):197–204.
- , . The Health Care Data Guide: Learning From Data for Improvement. 1st ed. San Francisco, CA: Jossey‐Bass; 2011.
- , , , , . Importance of vitamin D in hospital‐based fracture care pathways. J Nutr Health Aging. 2008;12(5):291–293.
- , , , et al. Before and after hip fracture, vitamin D deficiency may not be treated sufficiently. Osteoporos Int. 2013;24(11):2765–2773.
- , , , et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta‐analysis. BMJ. 2010;341:c3691.
- , , , et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–49.
- , , , et al. Oral vitamin D3 and calcium for secondary prevention of low‐trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo‐controlled trial. Lancet. 2005;365(9471):1621–1628.
- , , , et al. A population‐based analysis of the post‐fracture care gap 1996–2008: the situation is not improving. Osteoporos Int. 2012;23(5):1623–1629.
- American Orthopedic Association. Own the Bone website. 2011. Available at: http://www.ownthebone.org. Accessed August 1, 2014.
- , , , et al. Development of an electronic medical record based intervention to improve medical care of osteoporosis. Osteoporos Int. 2012;23(10):2489–2498.
- , , , , . Closing the gap in postfracture care at the population level: a randomized controlled trial. CMAJ. 2012;184(3):290–296.
- , , , et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ. 2008;178(5):569–575.
- , , , et al. Impact of a centralized osteoporosis coordinator on post‐fracture osteoporosis management: a cluster randomized trial. Osteoporos Int. 2012;23(1):87–95.
© 2014 Society of Hospital Medicine
Systems Automation for Cancer Surveillance: A Useful Tool for Tracking the Care of Head and Neck Cancer Patients in the Ear, Nose, and Throat Clinic
Purpose: About 400,000 new cases of Head and Neck Cancer (HNC) are diagnosed and reported each year. HNC patients require frequent follow-up care and additional interventions due to the potential for disease recurrence and second primaries. A robust and automated HNC identification and surveillance program can aid in case identification and can track appointments and required care, using the guidelines of the National Comprehensive Cancer Network (NCCN). An automated tool would provide for optimal treatment interventions while preventing any patients from being inadvertently lost to follow-up, enhancing veteran centered care.
Methods: The ear, nose, and throat (ENT) Cancer Tracking System (CTS) queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with a HNC. All patients residing in the Albuquerque, New Mexico, and Big Spring, Texas, catchment areas are included in the capture pool. Cases are identified by examining outpatient visits and inpatient discharge diagnosis International Classification of Diseases (ICD) codes, surgical pathology Systematized Nomenclature of Medicine—Clinical Terms (SNOMED codes), and VistA problem list diagnoses. Patients identified as having cancer are presented, using an interactive report hosted on a secure SharePoint site. Newly identified patients are automatically assigned to “active” management status, minimizing the risk of missing a new patient. The coordinator can toggle a patient’s status between “inactive” and “active” at any time, but can never delete a patient from the CTS. Inactive patients with a new cancer diagnosis are automatically toggled to active status. The CTS report tracks and presents a number of pertinent medical indicators, including patient identifiers and residence location, most recent diagnosis date, days since last diagnosis, diagnosis ICD code, date captured on the CTS, most recent ear, nose, and throat (ENT) visit, most recent ENT appointment, days since last visit, date of last thyroid-stimulating hormone (TSH) test, and date of last PET scan. Cancellations, no-shows, and patients overdue for TSH testing are highlighted.
Results: Baseline data obtained in 2012 prior to the activation of the CTS revealed that about 31.1% of diagnosed HNC patients in the ENT clinic experienced delays in care or were lost to follow-up care through cancellations, no shows, and nonrescheduled appointments. A dedicated cancer care coordinator (CCC) was assigned to the ENT clinic to record, monitor, and track HNC patients manually using an Excel spreadsheet. Although cancer surveillance reports proved that launching a cancer surveillance program prevented patients with cancer from being lost to follow-up care, the manual tracking system was time consuming and labor intensive. The automated CTS has optimized cancer surveillance by providing the CCC with immediate identification of new HNC diagnoses, appointment tracking, alerts for HNC patients that have not been scheduled, alerts of overdue required lab tests, tracking of completed PET CT imaging, and improved timeliness in obtaining quality improvement data; all were accomplished without the CCC manually tracking anything.
Conclusions: During the first 4 months of operation (February to May 2014), 14 new HNC patients were identified automatically—patients that manual tracking might have missed or incurred delays in care. The CTS has proved to be a vital tool to the CCC and will continue to assist in the identification of new HNC patients, provide access to patient information on follow-up care, and improve access to recommended diagnostic procedures from NCCN guidelines. Other benefits of an electronic tracking system are optimized time, improved workflows, and improvements to patient safety by providing timely access to care and treatment.
Purpose: About 400,000 new cases of Head and Neck Cancer (HNC) are diagnosed and reported each year. HNC patients require frequent follow-up care and additional interventions due to the potential for disease recurrence and second primaries. A robust and automated HNC identification and surveillance program can aid in case identification and can track appointments and required care, using the guidelines of the National Comprehensive Cancer Network (NCCN). An automated tool would provide for optimal treatment interventions while preventing any patients from being inadvertently lost to follow-up, enhancing veteran centered care.
Methods: The ear, nose, and throat (ENT) Cancer Tracking System (CTS) queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with a HNC. All patients residing in the Albuquerque, New Mexico, and Big Spring, Texas, catchment areas are included in the capture pool. Cases are identified by examining outpatient visits and inpatient discharge diagnosis International Classification of Diseases (ICD) codes, surgical pathology Systematized Nomenclature of Medicine—Clinical Terms (SNOMED codes), and VistA problem list diagnoses. Patients identified as having cancer are presented, using an interactive report hosted on a secure SharePoint site. Newly identified patients are automatically assigned to “active” management status, minimizing the risk of missing a new patient. The coordinator can toggle a patient’s status between “inactive” and “active” at any time, but can never delete a patient from the CTS. Inactive patients with a new cancer diagnosis are automatically toggled to active status. The CTS report tracks and presents a number of pertinent medical indicators, including patient identifiers and residence location, most recent diagnosis date, days since last diagnosis, diagnosis ICD code, date captured on the CTS, most recent ear, nose, and throat (ENT) visit, most recent ENT appointment, days since last visit, date of last thyroid-stimulating hormone (TSH) test, and date of last PET scan. Cancellations, no-shows, and patients overdue for TSH testing are highlighted.
Results: Baseline data obtained in 2012 prior to the activation of the CTS revealed that about 31.1% of diagnosed HNC patients in the ENT clinic experienced delays in care or were lost to follow-up care through cancellations, no shows, and nonrescheduled appointments. A dedicated cancer care coordinator (CCC) was assigned to the ENT clinic to record, monitor, and track HNC patients manually using an Excel spreadsheet. Although cancer surveillance reports proved that launching a cancer surveillance program prevented patients with cancer from being lost to follow-up care, the manual tracking system was time consuming and labor intensive. The automated CTS has optimized cancer surveillance by providing the CCC with immediate identification of new HNC diagnoses, appointment tracking, alerts for HNC patients that have not been scheduled, alerts of overdue required lab tests, tracking of completed PET CT imaging, and improved timeliness in obtaining quality improvement data; all were accomplished without the CCC manually tracking anything.
Conclusions: During the first 4 months of operation (February to May 2014), 14 new HNC patients were identified automatically—patients that manual tracking might have missed or incurred delays in care. The CTS has proved to be a vital tool to the CCC and will continue to assist in the identification of new HNC patients, provide access to patient information on follow-up care, and improve access to recommended diagnostic procedures from NCCN guidelines. Other benefits of an electronic tracking system are optimized time, improved workflows, and improvements to patient safety by providing timely access to care and treatment.
Purpose: About 400,000 new cases of Head and Neck Cancer (HNC) are diagnosed and reported each year. HNC patients require frequent follow-up care and additional interventions due to the potential for disease recurrence and second primaries. A robust and automated HNC identification and surveillance program can aid in case identification and can track appointments and required care, using the guidelines of the National Comprehensive Cancer Network (NCCN). An automated tool would provide for optimal treatment interventions while preventing any patients from being inadvertently lost to follow-up, enhancing veteran centered care.
Methods: The ear, nose, and throat (ENT) Cancer Tracking System (CTS) queries the VA Corporate Data Warehouse each night to identify all patients recently diagnosed with a HNC. All patients residing in the Albuquerque, New Mexico, and Big Spring, Texas, catchment areas are included in the capture pool. Cases are identified by examining outpatient visits and inpatient discharge diagnosis International Classification of Diseases (ICD) codes, surgical pathology Systematized Nomenclature of Medicine—Clinical Terms (SNOMED codes), and VistA problem list diagnoses. Patients identified as having cancer are presented, using an interactive report hosted on a secure SharePoint site. Newly identified patients are automatically assigned to “active” management status, minimizing the risk of missing a new patient. The coordinator can toggle a patient’s status between “inactive” and “active” at any time, but can never delete a patient from the CTS. Inactive patients with a new cancer diagnosis are automatically toggled to active status. The CTS report tracks and presents a number of pertinent medical indicators, including patient identifiers and residence location, most recent diagnosis date, days since last diagnosis, diagnosis ICD code, date captured on the CTS, most recent ear, nose, and throat (ENT) visit, most recent ENT appointment, days since last visit, date of last thyroid-stimulating hormone (TSH) test, and date of last PET scan. Cancellations, no-shows, and patients overdue for TSH testing are highlighted.
Results: Baseline data obtained in 2012 prior to the activation of the CTS revealed that about 31.1% of diagnosed HNC patients in the ENT clinic experienced delays in care or were lost to follow-up care through cancellations, no shows, and nonrescheduled appointments. A dedicated cancer care coordinator (CCC) was assigned to the ENT clinic to record, monitor, and track HNC patients manually using an Excel spreadsheet. Although cancer surveillance reports proved that launching a cancer surveillance program prevented patients with cancer from being lost to follow-up care, the manual tracking system was time consuming and labor intensive. The automated CTS has optimized cancer surveillance by providing the CCC with immediate identification of new HNC diagnoses, appointment tracking, alerts for HNC patients that have not been scheduled, alerts of overdue required lab tests, tracking of completed PET CT imaging, and improved timeliness in obtaining quality improvement data; all were accomplished without the CCC manually tracking anything.
Conclusions: During the first 4 months of operation (February to May 2014), 14 new HNC patients were identified automatically—patients that manual tracking might have missed or incurred delays in care. The CTS has proved to be a vital tool to the CCC and will continue to assist in the identification of new HNC patients, provide access to patient information on follow-up care, and improve access to recommended diagnostic procedures from NCCN guidelines. Other benefits of an electronic tracking system are optimized time, improved workflows, and improvements to patient safety by providing timely access to care and treatment.
Preventing Skeletal-Related Events in Veterans on Bisphosphonates for Bone Metastases
Purpose: Multiple myeloma and solid tumor metastases can cause bone disease leading to skeletal-related events (SREs) such as bone pain, fractures, and spinal cord compression. Intravenous bisphosphonate therapy—which is indicated in such cases—can lead to osteonecrosis of the jaw and hypocalcemia further putting patients at risk for SREs. These risks can be avoided by dental evaluation before bisphosphonate therapy and calcium and vitamin D supplementation throughout treatment. Our study of veterans treated with bisphosphonates for bone metastases or multiple myeloma aimed to (1) assess screening dental evaluation prior to treatment; and (2) measure effectiveness of calcium and vitamin D supplementation.
Methods: We performed a retrospective chart review at the James J. Peters VAMC of 117 veterans with multiple myeloma or bone metastases who received intravenous bisphosphonate therapy between January 2008 and November 2013. Those receiving bisphosphonates for other morbidities such as osteoporosis or hypercalcemia were excluded. Those getting dental clearance before intravenous bisphosphonate therapy and supplementation of vitamin D and calcium were assessed. Charts were further reviewed to gather outcomes data on incidence of osteonecrosis of the jaw and SREs such as bone pain, pathologic and traumatic fractures, orthopedic surgery, spine or nerve root compression. These data were analyzed using descriptive statistics to calculate frequencies, mean/median, and proportions. Odds ratios were calculated to assess differences in SRE outcomes for those who received supplementation as compared to those who did not get supplementation with calcium and vitamin D.
Results: Of the 117 patients included in the study, 97% were males aged from 58 to 92 years. Of these, 55 (47%) had prostate cancer, 21 (17%) had multiple myeloma, and 16 (14%) had lung cancer. All patients receiving bisphosphonates for bone metastases had undergone a dental evaluation prior to starting therapy; none were reported to have osteonecrosis of the jaw. However, only 78% had vitamin D levels checked before therapy; 69% of these were vitamin D deficient and received vitamin D supplementation. Overall, rates of calcium and vitamin D supplementation were very low (34% and 41%, respectively). Fifty-four percent of the patients reported an SRE; 49% with bone pain, 13% with pathological fractures, 7% with traumatic fractures, and 8% with nerve root compression. Vitamin D supplementation significantly reduced the odds of an SRE for our patients (OR 0.37, 95% CI = 0.19- 0.74, P < .05).
Conclusions: Onset of SREs can be reduced or delayed with bisphosphonates; however, patients need prior screening for osteonecrosis of the jaw and optimized calcium and vitamin D levels. Our study showed that although screening for osteonecrosis of the jaw was at optimum levels, supplementation with calcium and vitamin D was lacking in patients on bisphosphonates. In our study, vitamin D supplementation reduced the risk of an SRE by 63%. Hence, adequate prevention with vitamin D supplementation can improve bone health among veterans with multiple myeloma or bone metastases. Data-based policies and practices need to be incorporated to provide care to ensure adequate bone health.
Purpose: Multiple myeloma and solid tumor metastases can cause bone disease leading to skeletal-related events (SREs) such as bone pain, fractures, and spinal cord compression. Intravenous bisphosphonate therapy—which is indicated in such cases—can lead to osteonecrosis of the jaw and hypocalcemia further putting patients at risk for SREs. These risks can be avoided by dental evaluation before bisphosphonate therapy and calcium and vitamin D supplementation throughout treatment. Our study of veterans treated with bisphosphonates for bone metastases or multiple myeloma aimed to (1) assess screening dental evaluation prior to treatment; and (2) measure effectiveness of calcium and vitamin D supplementation.
Methods: We performed a retrospective chart review at the James J. Peters VAMC of 117 veterans with multiple myeloma or bone metastases who received intravenous bisphosphonate therapy between January 2008 and November 2013. Those receiving bisphosphonates for other morbidities such as osteoporosis or hypercalcemia were excluded. Those getting dental clearance before intravenous bisphosphonate therapy and supplementation of vitamin D and calcium were assessed. Charts were further reviewed to gather outcomes data on incidence of osteonecrosis of the jaw and SREs such as bone pain, pathologic and traumatic fractures, orthopedic surgery, spine or nerve root compression. These data were analyzed using descriptive statistics to calculate frequencies, mean/median, and proportions. Odds ratios were calculated to assess differences in SRE outcomes for those who received supplementation as compared to those who did not get supplementation with calcium and vitamin D.
Results: Of the 117 patients included in the study, 97% were males aged from 58 to 92 years. Of these, 55 (47%) had prostate cancer, 21 (17%) had multiple myeloma, and 16 (14%) had lung cancer. All patients receiving bisphosphonates for bone metastases had undergone a dental evaluation prior to starting therapy; none were reported to have osteonecrosis of the jaw. However, only 78% had vitamin D levels checked before therapy; 69% of these were vitamin D deficient and received vitamin D supplementation. Overall, rates of calcium and vitamin D supplementation were very low (34% and 41%, respectively). Fifty-four percent of the patients reported an SRE; 49% with bone pain, 13% with pathological fractures, 7% with traumatic fractures, and 8% with nerve root compression. Vitamin D supplementation significantly reduced the odds of an SRE for our patients (OR 0.37, 95% CI = 0.19- 0.74, P < .05).
Conclusions: Onset of SREs can be reduced or delayed with bisphosphonates; however, patients need prior screening for osteonecrosis of the jaw and optimized calcium and vitamin D levels. Our study showed that although screening for osteonecrosis of the jaw was at optimum levels, supplementation with calcium and vitamin D was lacking in patients on bisphosphonates. In our study, vitamin D supplementation reduced the risk of an SRE by 63%. Hence, adequate prevention with vitamin D supplementation can improve bone health among veterans with multiple myeloma or bone metastases. Data-based policies and practices need to be incorporated to provide care to ensure adequate bone health.
Purpose: Multiple myeloma and solid tumor metastases can cause bone disease leading to skeletal-related events (SREs) such as bone pain, fractures, and spinal cord compression. Intravenous bisphosphonate therapy—which is indicated in such cases—can lead to osteonecrosis of the jaw and hypocalcemia further putting patients at risk for SREs. These risks can be avoided by dental evaluation before bisphosphonate therapy and calcium and vitamin D supplementation throughout treatment. Our study of veterans treated with bisphosphonates for bone metastases or multiple myeloma aimed to (1) assess screening dental evaluation prior to treatment; and (2) measure effectiveness of calcium and vitamin D supplementation.
Methods: We performed a retrospective chart review at the James J. Peters VAMC of 117 veterans with multiple myeloma or bone metastases who received intravenous bisphosphonate therapy between January 2008 and November 2013. Those receiving bisphosphonates for other morbidities such as osteoporosis or hypercalcemia were excluded. Those getting dental clearance before intravenous bisphosphonate therapy and supplementation of vitamin D and calcium were assessed. Charts were further reviewed to gather outcomes data on incidence of osteonecrosis of the jaw and SREs such as bone pain, pathologic and traumatic fractures, orthopedic surgery, spine or nerve root compression. These data were analyzed using descriptive statistics to calculate frequencies, mean/median, and proportions. Odds ratios were calculated to assess differences in SRE outcomes for those who received supplementation as compared to those who did not get supplementation with calcium and vitamin D.
Results: Of the 117 patients included in the study, 97% were males aged from 58 to 92 years. Of these, 55 (47%) had prostate cancer, 21 (17%) had multiple myeloma, and 16 (14%) had lung cancer. All patients receiving bisphosphonates for bone metastases had undergone a dental evaluation prior to starting therapy; none were reported to have osteonecrosis of the jaw. However, only 78% had vitamin D levels checked before therapy; 69% of these were vitamin D deficient and received vitamin D supplementation. Overall, rates of calcium and vitamin D supplementation were very low (34% and 41%, respectively). Fifty-four percent of the patients reported an SRE; 49% with bone pain, 13% with pathological fractures, 7% with traumatic fractures, and 8% with nerve root compression. Vitamin D supplementation significantly reduced the odds of an SRE for our patients (OR 0.37, 95% CI = 0.19- 0.74, P < .05).
Conclusions: Onset of SREs can be reduced or delayed with bisphosphonates; however, patients need prior screening for osteonecrosis of the jaw and optimized calcium and vitamin D levels. Our study showed that although screening for osteonecrosis of the jaw was at optimum levels, supplementation with calcium and vitamin D was lacking in patients on bisphosphonates. In our study, vitamin D supplementation reduced the risk of an SRE by 63%. Hence, adequate prevention with vitamin D supplementation can improve bone health among veterans with multiple myeloma or bone metastases. Data-based policies and practices need to be incorporated to provide care to ensure adequate bone health.
The Use and Beliefs of Complementary and Alternative Medicine Among Veteran Cancer Patients
Purpose: Population studies indicate that > 40% of cancer patients use complementary and alternative medicine (CAM) therapies. Data suggest that most patients are not well informed of their chosen CAM therapy and do not discuss it with their health care providers (HCPs). However, no information exists on the use of CAM among veterans with cancer. We evaluated the type and prevalence of CAM use among VA patients with cancer, the disclosure of its use, and the beliefs veterans hold regarding CAM therapies.
Methods: Cancer patients from the radiation oncology and medical oncology clinic waiting areas of a VA hospital were surveyed by convenience sampling over a 3-month period. A hard copy-validated survey regarding attitudes and beliefs of CAM was used to evaluate patient responses. Relationships of the use and beliefs regarding CAM therapies were examined and analyzed using chi-square test and Spearman correlation.
Results: Two hundred twenty-two questionnaires were distributed to predominantly male veteran outpatient cancer patients, and 196 patients (97% male) returned completed questionnaires (88.3% response rate). Twenty-nine of the 196 (14.8%) patients reported CAM use. Dietary supplements (55.2%) and herbal supplements (41.4%) were the most commonly used forms of CAM; acupuncture (6.9%) and meditation techniques (6.9%) were the least common. Of the patients reporting CAM use, 89.7% of patients discussed their using CAM with their physicians. CAM users compared with nonusers believed that using CAM would improve their physical health (P < .0001), boost their immune system (P < .0001), reduce stress (P < .05), reduce symptoms such as pain or fatigue related to cancer and its treatment (P < .05), help them to live longer (P < .05), help cure their cancer (P < .05), prevent development of future health problems (P < .001), and help them to cope with the experience of having cancer (P < .0001). Neither group felt that CAM would decrease their emotional distress. Users of CAM believed that the use of CAM was encouraged by their family (P < .001) as well as their HCPs (P < .05), and their HCPs were open to their use of CAM (P < .05). Nonusers of CAM more often believed that CAM treatments were not based on scientific research (P < .0001), might interfere with the conventional cancer treatments (P < .001),CAM treatments cost too much money (P < .05), they do not have time to go to CAM treatments (P < .05), and they do not have adequate knowledge about CAM treatments (P < .05).
Conclusions: Whereas the prevalence of CAM use among veteran cancer patients is lower than that of the general population, they are more likely to report its use to their HCPs. Veterans have divergent beliefs regarding the potential benefits of CAM therapies. Because both users and nonusers of CAM express lack of knowledge regarding CAM treatments, veteran patients with cancer may benefit from education regarding the various CAM modalities and their utilities.
Purpose: Population studies indicate that > 40% of cancer patients use complementary and alternative medicine (CAM) therapies. Data suggest that most patients are not well informed of their chosen CAM therapy and do not discuss it with their health care providers (HCPs). However, no information exists on the use of CAM among veterans with cancer. We evaluated the type and prevalence of CAM use among VA patients with cancer, the disclosure of its use, and the beliefs veterans hold regarding CAM therapies.
Methods: Cancer patients from the radiation oncology and medical oncology clinic waiting areas of a VA hospital were surveyed by convenience sampling over a 3-month period. A hard copy-validated survey regarding attitudes and beliefs of CAM was used to evaluate patient responses. Relationships of the use and beliefs regarding CAM therapies were examined and analyzed using chi-square test and Spearman correlation.
Results: Two hundred twenty-two questionnaires were distributed to predominantly male veteran outpatient cancer patients, and 196 patients (97% male) returned completed questionnaires (88.3% response rate). Twenty-nine of the 196 (14.8%) patients reported CAM use. Dietary supplements (55.2%) and herbal supplements (41.4%) were the most commonly used forms of CAM; acupuncture (6.9%) and meditation techniques (6.9%) were the least common. Of the patients reporting CAM use, 89.7% of patients discussed their using CAM with their physicians. CAM users compared with nonusers believed that using CAM would improve their physical health (P < .0001), boost their immune system (P < .0001), reduce stress (P < .05), reduce symptoms such as pain or fatigue related to cancer and its treatment (P < .05), help them to live longer (P < .05), help cure their cancer (P < .05), prevent development of future health problems (P < .001), and help them to cope with the experience of having cancer (P < .0001). Neither group felt that CAM would decrease their emotional distress. Users of CAM believed that the use of CAM was encouraged by their family (P < .001) as well as their HCPs (P < .05), and their HCPs were open to their use of CAM (P < .05). Nonusers of CAM more often believed that CAM treatments were not based on scientific research (P < .0001), might interfere with the conventional cancer treatments (P < .001),CAM treatments cost too much money (P < .05), they do not have time to go to CAM treatments (P < .05), and they do not have adequate knowledge about CAM treatments (P < .05).
Conclusions: Whereas the prevalence of CAM use among veteran cancer patients is lower than that of the general population, they are more likely to report its use to their HCPs. Veterans have divergent beliefs regarding the potential benefits of CAM therapies. Because both users and nonusers of CAM express lack of knowledge regarding CAM treatments, veteran patients with cancer may benefit from education regarding the various CAM modalities and their utilities.
Purpose: Population studies indicate that > 40% of cancer patients use complementary and alternative medicine (CAM) therapies. Data suggest that most patients are not well informed of their chosen CAM therapy and do not discuss it with their health care providers (HCPs). However, no information exists on the use of CAM among veterans with cancer. We evaluated the type and prevalence of CAM use among VA patients with cancer, the disclosure of its use, and the beliefs veterans hold regarding CAM therapies.
Methods: Cancer patients from the radiation oncology and medical oncology clinic waiting areas of a VA hospital were surveyed by convenience sampling over a 3-month period. A hard copy-validated survey regarding attitudes and beliefs of CAM was used to evaluate patient responses. Relationships of the use and beliefs regarding CAM therapies were examined and analyzed using chi-square test and Spearman correlation.
Results: Two hundred twenty-two questionnaires were distributed to predominantly male veteran outpatient cancer patients, and 196 patients (97% male) returned completed questionnaires (88.3% response rate). Twenty-nine of the 196 (14.8%) patients reported CAM use. Dietary supplements (55.2%) and herbal supplements (41.4%) were the most commonly used forms of CAM; acupuncture (6.9%) and meditation techniques (6.9%) were the least common. Of the patients reporting CAM use, 89.7% of patients discussed their using CAM with their physicians. CAM users compared with nonusers believed that using CAM would improve their physical health (P < .0001), boost their immune system (P < .0001), reduce stress (P < .05), reduce symptoms such as pain or fatigue related to cancer and its treatment (P < .05), help them to live longer (P < .05), help cure their cancer (P < .05), prevent development of future health problems (P < .001), and help them to cope with the experience of having cancer (P < .0001). Neither group felt that CAM would decrease their emotional distress. Users of CAM believed that the use of CAM was encouraged by their family (P < .001) as well as their HCPs (P < .05), and their HCPs were open to their use of CAM (P < .05). Nonusers of CAM more often believed that CAM treatments were not based on scientific research (P < .0001), might interfere with the conventional cancer treatments (P < .001),CAM treatments cost too much money (P < .05), they do not have time to go to CAM treatments (P < .05), and they do not have adequate knowledge about CAM treatments (P < .05).
Conclusions: Whereas the prevalence of CAM use among veteran cancer patients is lower than that of the general population, they are more likely to report its use to their HCPs. Veterans have divergent beliefs regarding the potential benefits of CAM therapies. Because both users and nonusers of CAM express lack of knowledge regarding CAM treatments, veteran patients with cancer may benefit from education regarding the various CAM modalities and their utilities.
Breast Cancer Treatment Among Rural and Urban Women at the Veterans Health Administration
Purpose: Women with breast cancer are increasingly being diagnosed and cared for within the VA. Breast cancer specialists are available only at large VA hospitals in urban regions, possibly impacting the outcomes of rural women. The health outcomes of rural women at the VA have not been well described and are currently a research priority. We described the differences between urban and rural women’s demographics and breast cancer characteristics. We then compared urban and rural women with nonmetastatic breast cancer on type of lymph node biopsy, type of breast surgery, adjuvant radiation, adjuvant chemotherapy, and hormone therapy.
Methods: Following IRB approval, 4,025 women with nonmetastatic breast cancer from 1995 to 2012 were identified from the Veterans Affairs Central Cancer Registry (VACCR). This dataset contained diagnosis date, histology, tumor size, tumor grade, lymph node status, and estrogen receptor status. The VACCR also gathered type of lymph node surgery, type of breast surgery, adjuvant radiation, adjuvant chemotherapy, and adjuvant hormone therapy. Patient-specific data included date of birth, ethnicity, and zip code of residence at the time of diagnosis. The Rural Urban Commuting Areas 2.0 (RUCA) was used to define rural status and collated further into 3 categories of urban, large rural, and small rural. STATA data analysis and statistical software was used to organize and analyze data. The associations between the 3 rural/urban categories and diagnosis year, age, ethnicity, histology and tumor grade were assessed by ordinal logistic regression. Tumor size was compared using rank sum test. Lymph node and estrogen receptor status were compared with logistic regression, and lymph node sampling methods with multinomial regression. All other treatments were compared between small rural and urban women using logistic regression, and further analyzed with adjustments for factors that could influence treatment choices, including diagnosis year, age, ethnicity, tumor size and grade, lymph node status, and estrogen receptor status.
Results: Most women (n = 3,192) with nonmetastatic breast cancer resided in urban regions, 423 women in large rural regions, and 410 in small rural regions. The number of women living in urban and rural regions did not shift significantly over time (P = .48). The age distributions of rural and urban women did not differ. Women with breast cancer in rural regions were more likely to be white (P ≤ .001, 69% white urban; 90% white small rural; 24% black urban, and 6% black small rural). Tumor histology, size, grade, and lymph node and estrogen receptor status did not differ significantly between rural and urban. Mastectomy was more common among rural women initially, but after adjustments for patient demographics and breast cancer characteristics, urban and rural women received similar proportions of mastectomies. After adjustments, urban and rural women received equivalent breast cancer surgery, adjuvant radiation and adjuvant hormone therapy. However, after controlling for confounding factors, a disproportionate number of urban women receive no lymph node biopsy (P = .05). Additionally, women from large rural regions were statistically more likely to receive adjuvant chemotherapy (P = .04), although the chemotherapy administration did not differ statistically between women from urban and small rural regions (P = .7).
Conclusions: Most women diagnosed with breast cancer at the VA from 1995 to 2012 resided in urban areas. Rural women were much more likely to be white, but the age at diagnosis did not differ. Breast cancer characteristics were similar between rural and urban women. Women living in large rural regions were more likely to receive adjuvant chemotherapy than were women from urban or small rural regions; however reporting differences should be considered as an explanation. A higher proportion of urban women received no lymph node biopsy, which merits further investigation. Breast conservation therapy was administered consistently among rural and urban women veterans.
Purpose: Women with breast cancer are increasingly being diagnosed and cared for within the VA. Breast cancer specialists are available only at large VA hospitals in urban regions, possibly impacting the outcomes of rural women. The health outcomes of rural women at the VA have not been well described and are currently a research priority. We described the differences between urban and rural women’s demographics and breast cancer characteristics. We then compared urban and rural women with nonmetastatic breast cancer on type of lymph node biopsy, type of breast surgery, adjuvant radiation, adjuvant chemotherapy, and hormone therapy.
Methods: Following IRB approval, 4,025 women with nonmetastatic breast cancer from 1995 to 2012 were identified from the Veterans Affairs Central Cancer Registry (VACCR). This dataset contained diagnosis date, histology, tumor size, tumor grade, lymph node status, and estrogen receptor status. The VACCR also gathered type of lymph node surgery, type of breast surgery, adjuvant radiation, adjuvant chemotherapy, and adjuvant hormone therapy. Patient-specific data included date of birth, ethnicity, and zip code of residence at the time of diagnosis. The Rural Urban Commuting Areas 2.0 (RUCA) was used to define rural status and collated further into 3 categories of urban, large rural, and small rural. STATA data analysis and statistical software was used to organize and analyze data. The associations between the 3 rural/urban categories and diagnosis year, age, ethnicity, histology and tumor grade were assessed by ordinal logistic regression. Tumor size was compared using rank sum test. Lymph node and estrogen receptor status were compared with logistic regression, and lymph node sampling methods with multinomial regression. All other treatments were compared between small rural and urban women using logistic regression, and further analyzed with adjustments for factors that could influence treatment choices, including diagnosis year, age, ethnicity, tumor size and grade, lymph node status, and estrogen receptor status.
Results: Most women (n = 3,192) with nonmetastatic breast cancer resided in urban regions, 423 women in large rural regions, and 410 in small rural regions. The number of women living in urban and rural regions did not shift significantly over time (P = .48). The age distributions of rural and urban women did not differ. Women with breast cancer in rural regions were more likely to be white (P ≤ .001, 69% white urban; 90% white small rural; 24% black urban, and 6% black small rural). Tumor histology, size, grade, and lymph node and estrogen receptor status did not differ significantly between rural and urban. Mastectomy was more common among rural women initially, but after adjustments for patient demographics and breast cancer characteristics, urban and rural women received similar proportions of mastectomies. After adjustments, urban and rural women received equivalent breast cancer surgery, adjuvant radiation and adjuvant hormone therapy. However, after controlling for confounding factors, a disproportionate number of urban women receive no lymph node biopsy (P = .05). Additionally, women from large rural regions were statistically more likely to receive adjuvant chemotherapy (P = .04), although the chemotherapy administration did not differ statistically between women from urban and small rural regions (P = .7).
Conclusions: Most women diagnosed with breast cancer at the VA from 1995 to 2012 resided in urban areas. Rural women were much more likely to be white, but the age at diagnosis did not differ. Breast cancer characteristics were similar between rural and urban women. Women living in large rural regions were more likely to receive adjuvant chemotherapy than were women from urban or small rural regions; however reporting differences should be considered as an explanation. A higher proportion of urban women received no lymph node biopsy, which merits further investigation. Breast conservation therapy was administered consistently among rural and urban women veterans.
Purpose: Women with breast cancer are increasingly being diagnosed and cared for within the VA. Breast cancer specialists are available only at large VA hospitals in urban regions, possibly impacting the outcomes of rural women. The health outcomes of rural women at the VA have not been well described and are currently a research priority. We described the differences between urban and rural women’s demographics and breast cancer characteristics. We then compared urban and rural women with nonmetastatic breast cancer on type of lymph node biopsy, type of breast surgery, adjuvant radiation, adjuvant chemotherapy, and hormone therapy.
Methods: Following IRB approval, 4,025 women with nonmetastatic breast cancer from 1995 to 2012 were identified from the Veterans Affairs Central Cancer Registry (VACCR). This dataset contained diagnosis date, histology, tumor size, tumor grade, lymph node status, and estrogen receptor status. The VACCR also gathered type of lymph node surgery, type of breast surgery, adjuvant radiation, adjuvant chemotherapy, and adjuvant hormone therapy. Patient-specific data included date of birth, ethnicity, and zip code of residence at the time of diagnosis. The Rural Urban Commuting Areas 2.0 (RUCA) was used to define rural status and collated further into 3 categories of urban, large rural, and small rural. STATA data analysis and statistical software was used to organize and analyze data. The associations between the 3 rural/urban categories and diagnosis year, age, ethnicity, histology and tumor grade were assessed by ordinal logistic regression. Tumor size was compared using rank sum test. Lymph node and estrogen receptor status were compared with logistic regression, and lymph node sampling methods with multinomial regression. All other treatments were compared between small rural and urban women using logistic regression, and further analyzed with adjustments for factors that could influence treatment choices, including diagnosis year, age, ethnicity, tumor size and grade, lymph node status, and estrogen receptor status.
Results: Most women (n = 3,192) with nonmetastatic breast cancer resided in urban regions, 423 women in large rural regions, and 410 in small rural regions. The number of women living in urban and rural regions did not shift significantly over time (P = .48). The age distributions of rural and urban women did not differ. Women with breast cancer in rural regions were more likely to be white (P ≤ .001, 69% white urban; 90% white small rural; 24% black urban, and 6% black small rural). Tumor histology, size, grade, and lymph node and estrogen receptor status did not differ significantly between rural and urban. Mastectomy was more common among rural women initially, but after adjustments for patient demographics and breast cancer characteristics, urban and rural women received similar proportions of mastectomies. After adjustments, urban and rural women received equivalent breast cancer surgery, adjuvant radiation and adjuvant hormone therapy. However, after controlling for confounding factors, a disproportionate number of urban women receive no lymph node biopsy (P = .05). Additionally, women from large rural regions were statistically more likely to receive adjuvant chemotherapy (P = .04), although the chemotherapy administration did not differ statistically between women from urban and small rural regions (P = .7).
Conclusions: Most women diagnosed with breast cancer at the VA from 1995 to 2012 resided in urban areas. Rural women were much more likely to be white, but the age at diagnosis did not differ. Breast cancer characteristics were similar between rural and urban women. Women living in large rural regions were more likely to receive adjuvant chemotherapy than were women from urban or small rural regions; however reporting differences should be considered as an explanation. A higher proportion of urban women received no lymph node biopsy, which merits further investigation. Breast conservation therapy was administered consistently among rural and urban women veterans.
Durable Palliation of Lung Tumors Using Stereotactic Body Radiotherapy
Purpose: Stereotactic body radiotherapy (SBRT) is a safe and effective modality for treatment of early stage non-small cell lung cancer. We report our single institution experience in using protracted course of SBRT as a palliative treatment for lung tumors.
Methods: Patients with symptomatic lung lesions treated with palliative intent SBRT were retrospectively reviewed. These patients were not amenable to curative treatment due to previous irradiation, large-sized lesions, or advanced disease. Patients received 50-52 Gy in 10-12 fractions daily for 2 weeks.
Results: Ten patients, 5 males and 5 females, were treated over 3 years. Seven primary lesions, 2 metastatic lesions, and 1 recurrent primary lesion were treated. Patients ranged from aged 41-84 years with a mean age of 72 years. With a median follow-up of 11.5 months, the median overall survival was 18 months. Of 14 symptoms that were treated, 9 (64%) had complete resolution. Two patients (14%) had partial improvement, and 2 patients (14%) had no response. One patient (7%) had worsening symptoms following treatment. The median time to response was 27 days. Symptoms relapsed in 3 (21%) patients with median time of 3.7 months. A majority of patients (70%) remained symptom free until last follow-up. None of the patients experienced grade 3 or higher toxicity.
Conclusions: SBRT is a safe, effective, and durable treatment modality for palliating lung tumors that are not suitable for curative treatment.
Purpose: Stereotactic body radiotherapy (SBRT) is a safe and effective modality for treatment of early stage non-small cell lung cancer. We report our single institution experience in using protracted course of SBRT as a palliative treatment for lung tumors.
Methods: Patients with symptomatic lung lesions treated with palliative intent SBRT were retrospectively reviewed. These patients were not amenable to curative treatment due to previous irradiation, large-sized lesions, or advanced disease. Patients received 50-52 Gy in 10-12 fractions daily for 2 weeks.
Results: Ten patients, 5 males and 5 females, were treated over 3 years. Seven primary lesions, 2 metastatic lesions, and 1 recurrent primary lesion were treated. Patients ranged from aged 41-84 years with a mean age of 72 years. With a median follow-up of 11.5 months, the median overall survival was 18 months. Of 14 symptoms that were treated, 9 (64%) had complete resolution. Two patients (14%) had partial improvement, and 2 patients (14%) had no response. One patient (7%) had worsening symptoms following treatment. The median time to response was 27 days. Symptoms relapsed in 3 (21%) patients with median time of 3.7 months. A majority of patients (70%) remained symptom free until last follow-up. None of the patients experienced grade 3 or higher toxicity.
Conclusions: SBRT is a safe, effective, and durable treatment modality for palliating lung tumors that are not suitable for curative treatment.
Purpose: Stereotactic body radiotherapy (SBRT) is a safe and effective modality for treatment of early stage non-small cell lung cancer. We report our single institution experience in using protracted course of SBRT as a palliative treatment for lung tumors.
Methods: Patients with symptomatic lung lesions treated with palliative intent SBRT were retrospectively reviewed. These patients were not amenable to curative treatment due to previous irradiation, large-sized lesions, or advanced disease. Patients received 50-52 Gy in 10-12 fractions daily for 2 weeks.
Results: Ten patients, 5 males and 5 females, were treated over 3 years. Seven primary lesions, 2 metastatic lesions, and 1 recurrent primary lesion were treated. Patients ranged from aged 41-84 years with a mean age of 72 years. With a median follow-up of 11.5 months, the median overall survival was 18 months. Of 14 symptoms that were treated, 9 (64%) had complete resolution. Two patients (14%) had partial improvement, and 2 patients (14%) had no response. One patient (7%) had worsening symptoms following treatment. The median time to response was 27 days. Symptoms relapsed in 3 (21%) patients with median time of 3.7 months. A majority of patients (70%) remained symptom free until last follow-up. None of the patients experienced grade 3 or higher toxicity.
Conclusions: SBRT is a safe, effective, and durable treatment modality for palliating lung tumors that are not suitable for curative treatment.
Effects of Feeding Tube Placement on Weight and Treatment Breaks in Patients With Locally Advanced Head and Neck Cancer Who Undergo Definitive Radiotherapy
Purpose: To evaluate the effects of feeding tube placement on patient weight and length of treatment breaks during definitive radiotherapy with at least 50 Gy to the bilateral necks of patients with head and neck (H&N) cancer.
Methods: Thirty-five H&N cancer patients underwent definitive radiotherapy at the Radiation Oncology Department at Michael E. DeBakey VA Medical Center from July 23, 2012 to April 25, 2013. Twenty-three patients received doses of ≥ 50 Gy to bilateral necks, and constituted the study group. The remaining 12 patients did not receive ≥ 50 Gy and were excluded from the study. Among the 23 patients, 11 underwent feeding tube placement (group 1). Group 2 consisted of 12 patients without feeding tubes. All patients with feeding tube placement had concurrent chemotherapy. Some patients in group 2 received radiation treatment only.
Results: Twenty-two patients had weight loss, 1 patient gained 6.9 lb during the course of treatment. The median weight loss for group 1 was 17.8 lb (ranging from 4.4 to 34.4 lb), compared with 18.6 lb in patients in group 2. Those in group 2 who only received radiation therapy had the least median weight loss (5.4 lb). The average treatment break was 3.3 days for patients in group 1, 3.7 days for those in group 2 with concurrent chemo-radiotherapy (chemoRT), and 3.2 days for group 2 subjects receiving radiation therapy only.
Conclusions: In H&N cancer patients, feeding tube placement did not minimize weight loss and did not reduce average treatment breaks in those given concurrent chemoRT. An interesting additional finding of the study was that chemotherapy seems to have greater impact on a patient’s ability to tolerate radiation therapy. Our findings in this small, retrospective study, though suggestive, are insufficient to draw any definitive conclusions about the effectiveness of prophylactic feeding tube placement in the target patient population. Published studies on this subject are contradictory. Treatment decisions should be based on physician expertise and individualized to clinical needs of patients.
Purpose: To evaluate the effects of feeding tube placement on patient weight and length of treatment breaks during definitive radiotherapy with at least 50 Gy to the bilateral necks of patients with head and neck (H&N) cancer.
Methods: Thirty-five H&N cancer patients underwent definitive radiotherapy at the Radiation Oncology Department at Michael E. DeBakey VA Medical Center from July 23, 2012 to April 25, 2013. Twenty-three patients received doses of ≥ 50 Gy to bilateral necks, and constituted the study group. The remaining 12 patients did not receive ≥ 50 Gy and were excluded from the study. Among the 23 patients, 11 underwent feeding tube placement (group 1). Group 2 consisted of 12 patients without feeding tubes. All patients with feeding tube placement had concurrent chemotherapy. Some patients in group 2 received radiation treatment only.
Results: Twenty-two patients had weight loss, 1 patient gained 6.9 lb during the course of treatment. The median weight loss for group 1 was 17.8 lb (ranging from 4.4 to 34.4 lb), compared with 18.6 lb in patients in group 2. Those in group 2 who only received radiation therapy had the least median weight loss (5.4 lb). The average treatment break was 3.3 days for patients in group 1, 3.7 days for those in group 2 with concurrent chemo-radiotherapy (chemoRT), and 3.2 days for group 2 subjects receiving radiation therapy only.
Conclusions: In H&N cancer patients, feeding tube placement did not minimize weight loss and did not reduce average treatment breaks in those given concurrent chemoRT. An interesting additional finding of the study was that chemotherapy seems to have greater impact on a patient’s ability to tolerate radiation therapy. Our findings in this small, retrospective study, though suggestive, are insufficient to draw any definitive conclusions about the effectiveness of prophylactic feeding tube placement in the target patient population. Published studies on this subject are contradictory. Treatment decisions should be based on physician expertise and individualized to clinical needs of patients.
Purpose: To evaluate the effects of feeding tube placement on patient weight and length of treatment breaks during definitive radiotherapy with at least 50 Gy to the bilateral necks of patients with head and neck (H&N) cancer.
Methods: Thirty-five H&N cancer patients underwent definitive radiotherapy at the Radiation Oncology Department at Michael E. DeBakey VA Medical Center from July 23, 2012 to April 25, 2013. Twenty-three patients received doses of ≥ 50 Gy to bilateral necks, and constituted the study group. The remaining 12 patients did not receive ≥ 50 Gy and were excluded from the study. Among the 23 patients, 11 underwent feeding tube placement (group 1). Group 2 consisted of 12 patients without feeding tubes. All patients with feeding tube placement had concurrent chemotherapy. Some patients in group 2 received radiation treatment only.
Results: Twenty-two patients had weight loss, 1 patient gained 6.9 lb during the course of treatment. The median weight loss for group 1 was 17.8 lb (ranging from 4.4 to 34.4 lb), compared with 18.6 lb in patients in group 2. Those in group 2 who only received radiation therapy had the least median weight loss (5.4 lb). The average treatment break was 3.3 days for patients in group 1, 3.7 days for those in group 2 with concurrent chemo-radiotherapy (chemoRT), and 3.2 days for group 2 subjects receiving radiation therapy only.
Conclusions: In H&N cancer patients, feeding tube placement did not minimize weight loss and did not reduce average treatment breaks in those given concurrent chemoRT. An interesting additional finding of the study was that chemotherapy seems to have greater impact on a patient’s ability to tolerate radiation therapy. Our findings in this small, retrospective study, though suggestive, are insufficient to draw any definitive conclusions about the effectiveness of prophylactic feeding tube placement in the target patient population. Published studies on this subject are contradictory. Treatment decisions should be based on physician expertise and individualized to clinical needs of patients.
Comparison of Low-Dose Platinum vs High-Dose Platinum vs Cetuximab and Intensity-Modulated Radiation Therapy in Advanced Head and Neck Cancers
Purpose: High-dose cisplatin is standard in head and neck cancers. Recently, weekly low-dose platinum alone or with cetuximab has been used. All patients received 70 Gy standard fraction intensity-modulated radiation therapy. We are comparing the former used at the VAMC with the latter regimens used in the department of Oncology at the University of Mississippi. We will present the toxicity, response, patterns of failure, and survival by the use of the different types of chemotherapy.
Purpose: High-dose cisplatin is standard in head and neck cancers. Recently, weekly low-dose platinum alone or with cetuximab has been used. All patients received 70 Gy standard fraction intensity-modulated radiation therapy. We are comparing the former used at the VAMC with the latter regimens used in the department of Oncology at the University of Mississippi. We will present the toxicity, response, patterns of failure, and survival by the use of the different types of chemotherapy.
Purpose: High-dose cisplatin is standard in head and neck cancers. Recently, weekly low-dose platinum alone or with cetuximab has been used. All patients received 70 Gy standard fraction intensity-modulated radiation therapy. We are comparing the former used at the VAMC with the latter regimens used in the department of Oncology at the University of Mississippi. We will present the toxicity, response, patterns of failure, and survival by the use of the different types of chemotherapy.
Blastic Plasmacytoid Dendritic Cell Neoplasm: A Case Successfully Treated With HyperCVAD Followed by Allogeneic Stem Cell Transplantation
Introduction: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, clinically aggressive tumor derived from the precursors of palsmacytoid dendritic cells with a high frequency of cutaneous and bone marrow involvement and leukaemic dissemination. The prognosis is poor, and an optimal treatment approach has not been defined. We describe a case of BPDCN that has been successfully treated with acute leukaemia-type induction (hyperCVAD) followed by allogeneic stem cell transplantation (ASCT).
Purpose: To evaluate the efficacy of hyperCVAD and ASCT in treatment of BPDCN.
Method: A case report and literature review. Result: An African American male, aged 49 years, presented with firm violaceous nodules on the left calf, back, and shoulders, accompanied by 40 lb weight loss. Patient did not experience night sweats, fevers, and chill. Complete blood cell count and serum lactate dehydrogenase were normal. A skin biopsy revealed dense dermal infiltrates consisting of intermediate-sized cells with high N:C ratio. The tumor cells were strongly positive for CD4, CD56; partially positive for TdT; weakly positive for CD45 and CD43; and negative for CD3, CD20, CD30, MPO, CD34, CD117. Bone marrow biopsy and aspirate smear showed hypercellular marrow with predominant blastic cells with high nuclear-cytoplasmic ratio, finely chromatin, and prominent nucleoli. Flow cytometric analysis demonstrated 85% blasts that were positive for HLA-DR, CD4, CD56, CD38, and TdT (partial); but negative for CD34, CD117, CD33, CD13, CD14, CD15, CD2, CD3, CD5, CD11c, CD7, CD19, CD10, CD20, CD22, CD24, Kappa, Lambda, CD25, CD52, and MPO. Cyotogenetic analysis reported an abnormal complex chromosome abnormality: 46, XY, add (7)(q22), add (8)(p11.2), add (9)(q13), psu dic(13:6)(p12;q16), del(13)(q12q22), -17, +21, +mar[9]. The peripheral blood smear revealed rare blasts. The patient responded well to hyperCVAD chemo followed by ASCT. He has remained disease free for > 5 years.
Conclusions: Aggressive chemotherapy followed by ASCT is a favorable treatment plan for BPDCN.
Introduction: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, clinically aggressive tumor derived from the precursors of palsmacytoid dendritic cells with a high frequency of cutaneous and bone marrow involvement and leukaemic dissemination. The prognosis is poor, and an optimal treatment approach has not been defined. We describe a case of BPDCN that has been successfully treated with acute leukaemia-type induction (hyperCVAD) followed by allogeneic stem cell transplantation (ASCT).
Purpose: To evaluate the efficacy of hyperCVAD and ASCT in treatment of BPDCN.
Method: A case report and literature review. Result: An African American male, aged 49 years, presented with firm violaceous nodules on the left calf, back, and shoulders, accompanied by 40 lb weight loss. Patient did not experience night sweats, fevers, and chill. Complete blood cell count and serum lactate dehydrogenase were normal. A skin biopsy revealed dense dermal infiltrates consisting of intermediate-sized cells with high N:C ratio. The tumor cells were strongly positive for CD4, CD56; partially positive for TdT; weakly positive for CD45 and CD43; and negative for CD3, CD20, CD30, MPO, CD34, CD117. Bone marrow biopsy and aspirate smear showed hypercellular marrow with predominant blastic cells with high nuclear-cytoplasmic ratio, finely chromatin, and prominent nucleoli. Flow cytometric analysis demonstrated 85% blasts that were positive for HLA-DR, CD4, CD56, CD38, and TdT (partial); but negative for CD34, CD117, CD33, CD13, CD14, CD15, CD2, CD3, CD5, CD11c, CD7, CD19, CD10, CD20, CD22, CD24, Kappa, Lambda, CD25, CD52, and MPO. Cyotogenetic analysis reported an abnormal complex chromosome abnormality: 46, XY, add (7)(q22), add (8)(p11.2), add (9)(q13), psu dic(13:6)(p12;q16), del(13)(q12q22), -17, +21, +mar[9]. The peripheral blood smear revealed rare blasts. The patient responded well to hyperCVAD chemo followed by ASCT. He has remained disease free for > 5 years.
Conclusions: Aggressive chemotherapy followed by ASCT is a favorable treatment plan for BPDCN.
Introduction: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, clinically aggressive tumor derived from the precursors of palsmacytoid dendritic cells with a high frequency of cutaneous and bone marrow involvement and leukaemic dissemination. The prognosis is poor, and an optimal treatment approach has not been defined. We describe a case of BPDCN that has been successfully treated with acute leukaemia-type induction (hyperCVAD) followed by allogeneic stem cell transplantation (ASCT).
Purpose: To evaluate the efficacy of hyperCVAD and ASCT in treatment of BPDCN.
Method: A case report and literature review. Result: An African American male, aged 49 years, presented with firm violaceous nodules on the left calf, back, and shoulders, accompanied by 40 lb weight loss. Patient did not experience night sweats, fevers, and chill. Complete blood cell count and serum lactate dehydrogenase were normal. A skin biopsy revealed dense dermal infiltrates consisting of intermediate-sized cells with high N:C ratio. The tumor cells were strongly positive for CD4, CD56; partially positive for TdT; weakly positive for CD45 and CD43; and negative for CD3, CD20, CD30, MPO, CD34, CD117. Bone marrow biopsy and aspirate smear showed hypercellular marrow with predominant blastic cells with high nuclear-cytoplasmic ratio, finely chromatin, and prominent nucleoli. Flow cytometric analysis demonstrated 85% blasts that were positive for HLA-DR, CD4, CD56, CD38, and TdT (partial); but negative for CD34, CD117, CD33, CD13, CD14, CD15, CD2, CD3, CD5, CD11c, CD7, CD19, CD10, CD20, CD22, CD24, Kappa, Lambda, CD25, CD52, and MPO. Cyotogenetic analysis reported an abnormal complex chromosome abnormality: 46, XY, add (7)(q22), add (8)(p11.2), add (9)(q13), psu dic(13:6)(p12;q16), del(13)(q12q22), -17, +21, +mar[9]. The peripheral blood smear revealed rare blasts. The patient responded well to hyperCVAD chemo followed by ASCT. He has remained disease free for > 5 years.
Conclusions: Aggressive chemotherapy followed by ASCT is a favorable treatment plan for BPDCN.
Liver grafts donated after circulatory death increase early risk of diabetes
SAN FRANCISCO – The type of liver graft used in transplantation plays a large role in early development of new-onset diabetes, according to a retrospective study of 430 patients from the United Kingdom.
A team led by Dr. Hermien Hartog, an honorary clinical fellow in the Liver Unit, Queen Elizabeth Hospital, Birmingham, England, studied patients undergoing primary liver transplant between 2008 and 2012. Patients were excluded from the study if they had preexisting diabetes, had died, or had undergone retransplantation within 90 days.
The investigators assessed both the development of new-onset diabetes after transplant (NODAT), using criteria adapted from a published article (Transplantation 2013;96:58-64), and its resolution, defined as the date of cessation of antihyperglycemic therapy or the last episode of hyperglycemia.
Seventy-nine percent of the patients received grafts donated after brain death (DBD), Dr. Hartog reported at the annual meeting of the 2014 World Transplant Congress. Among the recipients of grafts donated after circulatory death (DCD), the mean warm ischemic time was 21 minutes.
With a median follow-up of 2.5 years, the cumulative 1-year incidence of NODAT was 19% in the entire cohort, with a median time to onset of 30 days. In the 44% of affected patients whose NODAT resolved, the median time to resolution was 150 days post transplantation, Dr. Hartog reported at the congress, which was sponsored by the American Society of Transplant Surgeons.
The cumulative 1-year incidence of NODAT was 23% in DCD graft recipients and 18% in DBD graft recipients, a nonsignificant difference. But when patients were stratified by graft type, "we saw an early occurrence and high peak incidence of NODAT in DCD graft recipients. Also, a larger proportion of these patients resolved their NODAT over time," she commented.
The overall temporal pattern suggested that "the effect that we see of graft type seems to be temporary and [lessens] over time when multifactorial factors come into play," according to Dr. Hartog.
In multivariate analyses, the risk of NODAT within 90 days of transplantation was higher for patients who received a DCD graft (hazard ratio, 1.8). More detailed analysis showed that the elevation of risk was greatest within the first 15 days.
"Our study confirms known associations with NODAT after liver transplantation but identifies DCD graft as a novel risk factor. This causes a temporary effect in the early post-transplant period that is independent from known risk factors," Dr. Hartog commented.
"Based on our observations, we hypothesize that hyperglycemia may be related to liver graft function through ischemia-reperfusion–induced hepatic insulin resistance," she added. "We are currently trying to confirm our data in an independent data set, which will also include postreperfusion glucose levels and correlation with the insulin receptor pathway in time-zero liver biopsies."
"The clinical relevance of our findings is as yet unknown," she acknowledged. However, they may help inform new approaches for graft optimization and selection.
Session cochair Dr. Darius Mirza, also of the University of Birmingham, asked, "Why does the pattern of recovery seem to be different in the DCDs versus the DBDs? Also, why are the cumulative incidence and the time frame so different?"
"Actually, in the literature, I have not seen any reports looking at the early post-transplant period. So most reports look at one time point, normally 1 year," Dr. Hartog replied. "What I think is that there is an early peak caused by DCD grafts that would explain why there is an early peak, but also why those patients recover later on. I think this peak is a bit obscure because there are also other factors that come into play, maybe after a while, that will obscure that first peak. If you would take those other factors out of the equation, I think you would just see a peak in the early period."
Dr. Mirza also wondered about the role of using DCD grafts that are accepted under extended criteria. "So you start off using mainly young, fit DCD livers. Now, the vast majority are extended-criteria DCD livers. Do you think that plays a role, or is it too early to say?"
"Yes, I think so," Dr. Hartog said, while adding that this phenomenon is likely not restricted to DCD grafts. "From earlier literature, there is a clear difference between a living donated graft and deceased donation. And it might also be that the extended grafts or the more steatotic grafts may exhibit this effect more than the better grafts."
Dr. Hartog disclosed no conflicts of interest relevant to the research.
SAN FRANCISCO – The type of liver graft used in transplantation plays a large role in early development of new-onset diabetes, according to a retrospective study of 430 patients from the United Kingdom.
A team led by Dr. Hermien Hartog, an honorary clinical fellow in the Liver Unit, Queen Elizabeth Hospital, Birmingham, England, studied patients undergoing primary liver transplant between 2008 and 2012. Patients were excluded from the study if they had preexisting diabetes, had died, or had undergone retransplantation within 90 days.
The investigators assessed both the development of new-onset diabetes after transplant (NODAT), using criteria adapted from a published article (Transplantation 2013;96:58-64), and its resolution, defined as the date of cessation of antihyperglycemic therapy or the last episode of hyperglycemia.
Seventy-nine percent of the patients received grafts donated after brain death (DBD), Dr. Hartog reported at the annual meeting of the 2014 World Transplant Congress. Among the recipients of grafts donated after circulatory death (DCD), the mean warm ischemic time was 21 minutes.
With a median follow-up of 2.5 years, the cumulative 1-year incidence of NODAT was 19% in the entire cohort, with a median time to onset of 30 days. In the 44% of affected patients whose NODAT resolved, the median time to resolution was 150 days post transplantation, Dr. Hartog reported at the congress, which was sponsored by the American Society of Transplant Surgeons.
The cumulative 1-year incidence of NODAT was 23% in DCD graft recipients and 18% in DBD graft recipients, a nonsignificant difference. But when patients were stratified by graft type, "we saw an early occurrence and high peak incidence of NODAT in DCD graft recipients. Also, a larger proportion of these patients resolved their NODAT over time," she commented.
The overall temporal pattern suggested that "the effect that we see of graft type seems to be temporary and [lessens] over time when multifactorial factors come into play," according to Dr. Hartog.
In multivariate analyses, the risk of NODAT within 90 days of transplantation was higher for patients who received a DCD graft (hazard ratio, 1.8). More detailed analysis showed that the elevation of risk was greatest within the first 15 days.
"Our study confirms known associations with NODAT after liver transplantation but identifies DCD graft as a novel risk factor. This causes a temporary effect in the early post-transplant period that is independent from known risk factors," Dr. Hartog commented.
"Based on our observations, we hypothesize that hyperglycemia may be related to liver graft function through ischemia-reperfusion–induced hepatic insulin resistance," she added. "We are currently trying to confirm our data in an independent data set, which will also include postreperfusion glucose levels and correlation with the insulin receptor pathway in time-zero liver biopsies."
"The clinical relevance of our findings is as yet unknown," she acknowledged. However, they may help inform new approaches for graft optimization and selection.
Session cochair Dr. Darius Mirza, also of the University of Birmingham, asked, "Why does the pattern of recovery seem to be different in the DCDs versus the DBDs? Also, why are the cumulative incidence and the time frame so different?"
"Actually, in the literature, I have not seen any reports looking at the early post-transplant period. So most reports look at one time point, normally 1 year," Dr. Hartog replied. "What I think is that there is an early peak caused by DCD grafts that would explain why there is an early peak, but also why those patients recover later on. I think this peak is a bit obscure because there are also other factors that come into play, maybe after a while, that will obscure that first peak. If you would take those other factors out of the equation, I think you would just see a peak in the early period."
Dr. Mirza also wondered about the role of using DCD grafts that are accepted under extended criteria. "So you start off using mainly young, fit DCD livers. Now, the vast majority are extended-criteria DCD livers. Do you think that plays a role, or is it too early to say?"
"Yes, I think so," Dr. Hartog said, while adding that this phenomenon is likely not restricted to DCD grafts. "From earlier literature, there is a clear difference between a living donated graft and deceased donation. And it might also be that the extended grafts or the more steatotic grafts may exhibit this effect more than the better grafts."
Dr. Hartog disclosed no conflicts of interest relevant to the research.
SAN FRANCISCO – The type of liver graft used in transplantation plays a large role in early development of new-onset diabetes, according to a retrospective study of 430 patients from the United Kingdom.
A team led by Dr. Hermien Hartog, an honorary clinical fellow in the Liver Unit, Queen Elizabeth Hospital, Birmingham, England, studied patients undergoing primary liver transplant between 2008 and 2012. Patients were excluded from the study if they had preexisting diabetes, had died, or had undergone retransplantation within 90 days.
The investigators assessed both the development of new-onset diabetes after transplant (NODAT), using criteria adapted from a published article (Transplantation 2013;96:58-64), and its resolution, defined as the date of cessation of antihyperglycemic therapy or the last episode of hyperglycemia.
Seventy-nine percent of the patients received grafts donated after brain death (DBD), Dr. Hartog reported at the annual meeting of the 2014 World Transplant Congress. Among the recipients of grafts donated after circulatory death (DCD), the mean warm ischemic time was 21 minutes.
With a median follow-up of 2.5 years, the cumulative 1-year incidence of NODAT was 19% in the entire cohort, with a median time to onset of 30 days. In the 44% of affected patients whose NODAT resolved, the median time to resolution was 150 days post transplantation, Dr. Hartog reported at the congress, which was sponsored by the American Society of Transplant Surgeons.
The cumulative 1-year incidence of NODAT was 23% in DCD graft recipients and 18% in DBD graft recipients, a nonsignificant difference. But when patients were stratified by graft type, "we saw an early occurrence and high peak incidence of NODAT in DCD graft recipients. Also, a larger proportion of these patients resolved their NODAT over time," she commented.
The overall temporal pattern suggested that "the effect that we see of graft type seems to be temporary and [lessens] over time when multifactorial factors come into play," according to Dr. Hartog.
In multivariate analyses, the risk of NODAT within 90 days of transplantation was higher for patients who received a DCD graft (hazard ratio, 1.8). More detailed analysis showed that the elevation of risk was greatest within the first 15 days.
"Our study confirms known associations with NODAT after liver transplantation but identifies DCD graft as a novel risk factor. This causes a temporary effect in the early post-transplant period that is independent from known risk factors," Dr. Hartog commented.
"Based on our observations, we hypothesize that hyperglycemia may be related to liver graft function through ischemia-reperfusion–induced hepatic insulin resistance," she added. "We are currently trying to confirm our data in an independent data set, which will also include postreperfusion glucose levels and correlation with the insulin receptor pathway in time-zero liver biopsies."
"The clinical relevance of our findings is as yet unknown," she acknowledged. However, they may help inform new approaches for graft optimization and selection.
Session cochair Dr. Darius Mirza, also of the University of Birmingham, asked, "Why does the pattern of recovery seem to be different in the DCDs versus the DBDs? Also, why are the cumulative incidence and the time frame so different?"
"Actually, in the literature, I have not seen any reports looking at the early post-transplant period. So most reports look at one time point, normally 1 year," Dr. Hartog replied. "What I think is that there is an early peak caused by DCD grafts that would explain why there is an early peak, but also why those patients recover later on. I think this peak is a bit obscure because there are also other factors that come into play, maybe after a while, that will obscure that first peak. If you would take those other factors out of the equation, I think you would just see a peak in the early period."
Dr. Mirza also wondered about the role of using DCD grafts that are accepted under extended criteria. "So you start off using mainly young, fit DCD livers. Now, the vast majority are extended-criteria DCD livers. Do you think that plays a role, or is it too early to say?"
"Yes, I think so," Dr. Hartog said, while adding that this phenomenon is likely not restricted to DCD grafts. "From earlier literature, there is a clear difference between a living donated graft and deceased donation. And it might also be that the extended grafts or the more steatotic grafts may exhibit this effect more than the better grafts."
Dr. Hartog disclosed no conflicts of interest relevant to the research.
AT THE 2014 WORLD TRANSPLANT CONGRESS
Key clinical point: Recipients of liver grafts donated after circulatory death are at a slightly higher risk for post-transplant new-onset diabetes.
Major finding: The risk of new-onset diabetes within 90 days of transplantation was 1.8-fold higher for patients who received a DCD graft than for peers who received a DBD graft.
Data source: A retrospective cohort study of 430 primary liver transplant recipients
Disclosures: Dr. Hartog disclosed no relevant conflicts of interest.


