User login
Can private practice survive?
I ran into Peter the other day. We weren’t close back in high school 50 years ago – Peter was a jock and I wasn’t – but both of us ended up in medicine.
"How’s the radiology business?" I asked him.
"Two more years," he said. "I should be able to hang on."
"That bad?"
"We were taken over by the academic department of a big teaching hospital," Peter said.
"What’s the problem? They want you to publish papers?" I asked.
"They’re not crazy," he said. "They know what kind of papers we’d write. They just want to measure things."
"Measure what?"
"Anything they can," he said. "Productivity, quality. They micromanage everything. We’re not a private practice anymore. We don’t call our own shots."
You hear a lot of talk these days about whether private practice can survive. Judging by anecdotal chats with colleagues in other specialties, I’d say prospects are iffy. A lot of the future is already here.
"I’m going crazy," a local community rheumatologist told me. "There was just one other guy in town. The local hospital knocked on his door and said, ‘Either sell out and work for us on our terms, or you’ll never get another referral.’ So he just closed up and moved out of state."
"How about you?" I asked him. "Have they made you an offer you can’t refuse?"
"Not yet," he said.
Then I met an oncologist from my wife’s family. He is a couple of years younger than I am. "I never thought I’d be working harder than I ever did at my age," he said. "And earning less.
"But we just sold our practice. No choice, really. Reimbursements don’t cover the cost of chemo drugs, so we lose money on every patient we give them to. The hospital that’s buying us can bill for drugs at a higher rate. So I’ll work for them for 5 years, then I’m out."
Then I heard about a neurologist, whose practice his hospital covets. He orders scans and other diagnostic procedures that generate hundreds of thousands of dollars annually, but that of course he can’t bill for. The hospital can. I don’t get how that works, but obviously the hospital does.
The trend toward consolidation proceeds. Doctors become salaried employees of large organizations. Maybe the main reason we dermatologists aren’t being swallowed yet by the big fish is that we’re just not tasty enough. We don’t generate admissions or enough costly procedures to make gulping us down worthwhile.
Switching from being an independent practitioner to a salaried employee means more than a change of location or even lower income; it signals a loss of status. I got a glimpse of how that plays out when I met another friend, an internist who used to be out on his own, but who now works for a big health care organization. "Have you got a quota of patients you have to meet?" I asked him.
"Yes," he said. "They just set that up recently."
"How did you find out?" I asked him.
"They sent out a memo," he said.
In other words, management notified doctors of productivity guidelines the same way that they would tell clerks or auxiliary personnel by which quality metrics their performance and salary would be judged.
"What if you don’t meet your quota because patients cancel or don’t keep their appointments?" I asked him. "You have no control over how patients are booked or reminded to come?"
"They blame the doctors anyway," he said. "Anyway, I’m not full time anymore. I’ll be retiring soon."
To say that hanging around colleagues who talk this way is dispiriting would be an understatement.
Peter the radiologist spoke in similar downbeat tones. "I’m adapting to the new reality fairly well," he said, "because I see the end in sight. But my younger colleagues are having a harder time. They thought they were joining a private practice, and none of them would have signed on for what they’re getting now: harder work, longer hours, no autonomy. They show up every morning cranky, muttering under their breath."
"I can see why," I said. "Whom do they take it out on?"
"Not the administrators," said Peter. "They don’t care. And certainly not the patients. They take it out on their families, I guess."
We agreed that seemed the best strategy.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Skin & Allergy News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
I ran into Peter the other day. We weren’t close back in high school 50 years ago – Peter was a jock and I wasn’t – but both of us ended up in medicine.
"How’s the radiology business?" I asked him.
"Two more years," he said. "I should be able to hang on."
"That bad?"
"We were taken over by the academic department of a big teaching hospital," Peter said.
"What’s the problem? They want you to publish papers?" I asked.
"They’re not crazy," he said. "They know what kind of papers we’d write. They just want to measure things."
"Measure what?"
"Anything they can," he said. "Productivity, quality. They micromanage everything. We’re not a private practice anymore. We don’t call our own shots."
You hear a lot of talk these days about whether private practice can survive. Judging by anecdotal chats with colleagues in other specialties, I’d say prospects are iffy. A lot of the future is already here.
"I’m going crazy," a local community rheumatologist told me. "There was just one other guy in town. The local hospital knocked on his door and said, ‘Either sell out and work for us on our terms, or you’ll never get another referral.’ So he just closed up and moved out of state."
"How about you?" I asked him. "Have they made you an offer you can’t refuse?"
"Not yet," he said.
Then I met an oncologist from my wife’s family. He is a couple of years younger than I am. "I never thought I’d be working harder than I ever did at my age," he said. "And earning less.
"But we just sold our practice. No choice, really. Reimbursements don’t cover the cost of chemo drugs, so we lose money on every patient we give them to. The hospital that’s buying us can bill for drugs at a higher rate. So I’ll work for them for 5 years, then I’m out."
Then I heard about a neurologist, whose practice his hospital covets. He orders scans and other diagnostic procedures that generate hundreds of thousands of dollars annually, but that of course he can’t bill for. The hospital can. I don’t get how that works, but obviously the hospital does.
The trend toward consolidation proceeds. Doctors become salaried employees of large organizations. Maybe the main reason we dermatologists aren’t being swallowed yet by the big fish is that we’re just not tasty enough. We don’t generate admissions or enough costly procedures to make gulping us down worthwhile.
Switching from being an independent practitioner to a salaried employee means more than a change of location or even lower income; it signals a loss of status. I got a glimpse of how that plays out when I met another friend, an internist who used to be out on his own, but who now works for a big health care organization. "Have you got a quota of patients you have to meet?" I asked him.
"Yes," he said. "They just set that up recently."
"How did you find out?" I asked him.
"They sent out a memo," he said.
In other words, management notified doctors of productivity guidelines the same way that they would tell clerks or auxiliary personnel by which quality metrics their performance and salary would be judged.
"What if you don’t meet your quota because patients cancel or don’t keep their appointments?" I asked him. "You have no control over how patients are booked or reminded to come?"
"They blame the doctors anyway," he said. "Anyway, I’m not full time anymore. I’ll be retiring soon."
To say that hanging around colleagues who talk this way is dispiriting would be an understatement.
Peter the radiologist spoke in similar downbeat tones. "I’m adapting to the new reality fairly well," he said, "because I see the end in sight. But my younger colleagues are having a harder time. They thought they were joining a private practice, and none of them would have signed on for what they’re getting now: harder work, longer hours, no autonomy. They show up every morning cranky, muttering under their breath."
"I can see why," I said. "Whom do they take it out on?"
"Not the administrators," said Peter. "They don’t care. And certainly not the patients. They take it out on their families, I guess."
We agreed that seemed the best strategy.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Skin & Allergy News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
I ran into Peter the other day. We weren’t close back in high school 50 years ago – Peter was a jock and I wasn’t – but both of us ended up in medicine.
"How’s the radiology business?" I asked him.
"Two more years," he said. "I should be able to hang on."
"That bad?"
"We were taken over by the academic department of a big teaching hospital," Peter said.
"What’s the problem? They want you to publish papers?" I asked.
"They’re not crazy," he said. "They know what kind of papers we’d write. They just want to measure things."
"Measure what?"
"Anything they can," he said. "Productivity, quality. They micromanage everything. We’re not a private practice anymore. We don’t call our own shots."
You hear a lot of talk these days about whether private practice can survive. Judging by anecdotal chats with colleagues in other specialties, I’d say prospects are iffy. A lot of the future is already here.
"I’m going crazy," a local community rheumatologist told me. "There was just one other guy in town. The local hospital knocked on his door and said, ‘Either sell out and work for us on our terms, or you’ll never get another referral.’ So he just closed up and moved out of state."
"How about you?" I asked him. "Have they made you an offer you can’t refuse?"
"Not yet," he said.
Then I met an oncologist from my wife’s family. He is a couple of years younger than I am. "I never thought I’d be working harder than I ever did at my age," he said. "And earning less.
"But we just sold our practice. No choice, really. Reimbursements don’t cover the cost of chemo drugs, so we lose money on every patient we give them to. The hospital that’s buying us can bill for drugs at a higher rate. So I’ll work for them for 5 years, then I’m out."
Then I heard about a neurologist, whose practice his hospital covets. He orders scans and other diagnostic procedures that generate hundreds of thousands of dollars annually, but that of course he can’t bill for. The hospital can. I don’t get how that works, but obviously the hospital does.
The trend toward consolidation proceeds. Doctors become salaried employees of large organizations. Maybe the main reason we dermatologists aren’t being swallowed yet by the big fish is that we’re just not tasty enough. We don’t generate admissions or enough costly procedures to make gulping us down worthwhile.
Switching from being an independent practitioner to a salaried employee means more than a change of location or even lower income; it signals a loss of status. I got a glimpse of how that plays out when I met another friend, an internist who used to be out on his own, but who now works for a big health care organization. "Have you got a quota of patients you have to meet?" I asked him.
"Yes," he said. "They just set that up recently."
"How did you find out?" I asked him.
"They sent out a memo," he said.
In other words, management notified doctors of productivity guidelines the same way that they would tell clerks or auxiliary personnel by which quality metrics their performance and salary would be judged.
"What if you don’t meet your quota because patients cancel or don’t keep their appointments?" I asked him. "You have no control over how patients are booked or reminded to come?"
"They blame the doctors anyway," he said. "Anyway, I’m not full time anymore. I’ll be retiring soon."
To say that hanging around colleagues who talk this way is dispiriting would be an understatement.
Peter the radiologist spoke in similar downbeat tones. "I’m adapting to the new reality fairly well," he said, "because I see the end in sight. But my younger colleagues are having a harder time. They thought they were joining a private practice, and none of them would have signed on for what they’re getting now: harder work, longer hours, no autonomy. They show up every morning cranky, muttering under their breath."
"I can see why," I said. "Whom do they take it out on?"
"Not the administrators," said Peter. "They don’t care. And certainly not the patients. They take it out on their families, I guess."
We agreed that seemed the best strategy.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Skin & Allergy News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
Laparoscopic power morcellation coverage will end as of September 1 for Blue Cross Blue Shield affiliate
About 5.2 million people insured by Highmark Inc, a Blue Cross and Blue Shield (BCBS)–affiliated company in Pennsylvania, Delaware, and West Virginia will be denied coverage for laparoscopic power morcellation used in gynecologic surgery. Highmark is the fourth-largest BCBS affiliate.1
On August 2, spokesman Aaron Billger announced by email that, in the best interest of their members, Highmark is halting coverage as of September 1, 2014.2 This decision comes in response to the US Food and Drug Administration (FDA) Safety Communication of April 17, 2014, that discouraged the use of power morcellation during hysterectomy and myomectomy because of the increased risk of dispersion of benign myoma tissue and occult malignant tissue through the abdominal cavity.2,3
University of Pittsburgh Medical Center (UPMC), the largest hospital group in western Pennsylvania, publicized that it will stop performing laparoscopic power morcellation as of September 1. UPMC spokesperson Gloria Kreps said the decision was an “appropriate and prudent course of action,” and that the hospital was “looking to the FDA for further guidance.”4 Other medical centers and hospitals have banned the use of power morcellation, beginning with Brigham and Women’s and Massachusetts General in April.5
In April 2014, Ethicon, a unit of Johnson & Johnson, suspended sales of all its morcellation devices. In an urgent Medical Device Market Withdrawal dated July 30, 2014, Ethicon initiated a “worldwide voluntary market withdrawal of all Ethicon Morcellation Devices that currently remain on the market.”6 Ethicon will issue prorated credit for morcellation devices returned by December 30, 2014, with full credit issued for unopened, unexpired disposable products.6
The FDA Obstetrics and Gynecology Devices Panel Advisory Committee held a 2-day hearing in July 2014 to weigh the risks and benefits of power morcellation. The panel will send recommendations to the FDA, and a final decision on the use of laparoscopic power morcellation will be forthcoming.7
1.
Reuters. U.S. insurer to stop coverage of gynecological procedure. http://in.reuters.com/article/2014/08/02/highmark-procedure-idINL2N0Q80IY20140802. Published August 3, 2014. Accessed August 6, 2014.
2. Kreiter M. Highmark Inc. says it won’t cover laparoscopic power morcellation because of cancer risk. International Business Times. http://www.ibtimes.com/highmark-inc-says-it-wont-cover-laparoscopic-power-morcellation-because-cancer-risk-1647160. Published August 2, 2014. Accessed August 6, 2014.
3. Yates J. FDA, hospitals caution against laparoscopic power morcellation during hysterectomy and myomectomy. OBG Manag. 2014;26(5):68, 67. http://www.obgmanagement.com/topic-collections/morcellation/article/fda-hospitals-caution-against-laparoscopic-power-morcellation-during-hysterectomy-and-myomectomy.html.
4. Mamula KB. UPMC drops controversial surgical procedure. Pittsburgh Business Times. http://www.bizjournals.com/pittsburgh/news/2014/08/01/upmc-drops-controversial-surgical-procedure.html. Published August 1, 2014. Accessed August 6, 2014.
5. Yates J. Open power morcellation of uterine tumors during hysterectomy banned at two Boston hospitals. OBG Manag. 2014;26(4). http://www.obgmanagement.com/topic-collections/morcellation/article/open-power-morcellation-of-uterine-tumors-during-hysterectomy-banned-at-two-boston-hospitals.html
6. Ethicon. Urgent: Medical device market withdrawal. http://www.ethicon.com/sites/default/files/managed-documents/Ethicon%20Morcellation%20Devices%20Customer%20Letter%20Final.pdf. Published July 30, 2014. Accessed August 6, 2014.
7. Iglesia C, Yates J. Why FDA hearing on morcellation safety could drive innovation [audiocast]. OBG Manag. 2014;26(7). http://www.obgmanagement.com/home/article/why-fda-hearing-on-morcellation-safety-could-drive-innovation/d1071c5e8326e8a2de76a30f0446b1ab.html. Published July 17, 2014. Accessed August 6, 2014.
About 5.2 million people insured by Highmark Inc, a Blue Cross and Blue Shield (BCBS)–affiliated company in Pennsylvania, Delaware, and West Virginia will be denied coverage for laparoscopic power morcellation used in gynecologic surgery. Highmark is the fourth-largest BCBS affiliate.1
On August 2, spokesman Aaron Billger announced by email that, in the best interest of their members, Highmark is halting coverage as of September 1, 2014.2 This decision comes in response to the US Food and Drug Administration (FDA) Safety Communication of April 17, 2014, that discouraged the use of power morcellation during hysterectomy and myomectomy because of the increased risk of dispersion of benign myoma tissue and occult malignant tissue through the abdominal cavity.2,3
University of Pittsburgh Medical Center (UPMC), the largest hospital group in western Pennsylvania, publicized that it will stop performing laparoscopic power morcellation as of September 1. UPMC spokesperson Gloria Kreps said the decision was an “appropriate and prudent course of action,” and that the hospital was “looking to the FDA for further guidance.”4 Other medical centers and hospitals have banned the use of power morcellation, beginning with Brigham and Women’s and Massachusetts General in April.5
In April 2014, Ethicon, a unit of Johnson & Johnson, suspended sales of all its morcellation devices. In an urgent Medical Device Market Withdrawal dated July 30, 2014, Ethicon initiated a “worldwide voluntary market withdrawal of all Ethicon Morcellation Devices that currently remain on the market.”6 Ethicon will issue prorated credit for morcellation devices returned by December 30, 2014, with full credit issued for unopened, unexpired disposable products.6
The FDA Obstetrics and Gynecology Devices Panel Advisory Committee held a 2-day hearing in July 2014 to weigh the risks and benefits of power morcellation. The panel will send recommendations to the FDA, and a final decision on the use of laparoscopic power morcellation will be forthcoming.7
About 5.2 million people insured by Highmark Inc, a Blue Cross and Blue Shield (BCBS)–affiliated company in Pennsylvania, Delaware, and West Virginia will be denied coverage for laparoscopic power morcellation used in gynecologic surgery. Highmark is the fourth-largest BCBS affiliate.1
On August 2, spokesman Aaron Billger announced by email that, in the best interest of their members, Highmark is halting coverage as of September 1, 2014.2 This decision comes in response to the US Food and Drug Administration (FDA) Safety Communication of April 17, 2014, that discouraged the use of power morcellation during hysterectomy and myomectomy because of the increased risk of dispersion of benign myoma tissue and occult malignant tissue through the abdominal cavity.2,3
University of Pittsburgh Medical Center (UPMC), the largest hospital group in western Pennsylvania, publicized that it will stop performing laparoscopic power morcellation as of September 1. UPMC spokesperson Gloria Kreps said the decision was an “appropriate and prudent course of action,” and that the hospital was “looking to the FDA for further guidance.”4 Other medical centers and hospitals have banned the use of power morcellation, beginning with Brigham and Women’s and Massachusetts General in April.5
In April 2014, Ethicon, a unit of Johnson & Johnson, suspended sales of all its morcellation devices. In an urgent Medical Device Market Withdrawal dated July 30, 2014, Ethicon initiated a “worldwide voluntary market withdrawal of all Ethicon Morcellation Devices that currently remain on the market.”6 Ethicon will issue prorated credit for morcellation devices returned by December 30, 2014, with full credit issued for unopened, unexpired disposable products.6
The FDA Obstetrics and Gynecology Devices Panel Advisory Committee held a 2-day hearing in July 2014 to weigh the risks and benefits of power morcellation. The panel will send recommendations to the FDA, and a final decision on the use of laparoscopic power morcellation will be forthcoming.7
1.
Reuters. U.S. insurer to stop coverage of gynecological procedure. http://in.reuters.com/article/2014/08/02/highmark-procedure-idINL2N0Q80IY20140802. Published August 3, 2014. Accessed August 6, 2014.
2. Kreiter M. Highmark Inc. says it won’t cover laparoscopic power morcellation because of cancer risk. International Business Times. http://www.ibtimes.com/highmark-inc-says-it-wont-cover-laparoscopic-power-morcellation-because-cancer-risk-1647160. Published August 2, 2014. Accessed August 6, 2014.
3. Yates J. FDA, hospitals caution against laparoscopic power morcellation during hysterectomy and myomectomy. OBG Manag. 2014;26(5):68, 67. http://www.obgmanagement.com/topic-collections/morcellation/article/fda-hospitals-caution-against-laparoscopic-power-morcellation-during-hysterectomy-and-myomectomy.html.
4. Mamula KB. UPMC drops controversial surgical procedure. Pittsburgh Business Times. http://www.bizjournals.com/pittsburgh/news/2014/08/01/upmc-drops-controversial-surgical-procedure.html. Published August 1, 2014. Accessed August 6, 2014.
5. Yates J. Open power morcellation of uterine tumors during hysterectomy banned at two Boston hospitals. OBG Manag. 2014;26(4). http://www.obgmanagement.com/topic-collections/morcellation/article/open-power-morcellation-of-uterine-tumors-during-hysterectomy-banned-at-two-boston-hospitals.html
6. Ethicon. Urgent: Medical device market withdrawal. http://www.ethicon.com/sites/default/files/managed-documents/Ethicon%20Morcellation%20Devices%20Customer%20Letter%20Final.pdf. Published July 30, 2014. Accessed August 6, 2014.
7. Iglesia C, Yates J. Why FDA hearing on morcellation safety could drive innovation [audiocast]. OBG Manag. 2014;26(7). http://www.obgmanagement.com/home/article/why-fda-hearing-on-morcellation-safety-could-drive-innovation/d1071c5e8326e8a2de76a30f0446b1ab.html. Published July 17, 2014. Accessed August 6, 2014.
1.
Reuters. U.S. insurer to stop coverage of gynecological procedure. http://in.reuters.com/article/2014/08/02/highmark-procedure-idINL2N0Q80IY20140802. Published August 3, 2014. Accessed August 6, 2014.
2. Kreiter M. Highmark Inc. says it won’t cover laparoscopic power morcellation because of cancer risk. International Business Times. http://www.ibtimes.com/highmark-inc-says-it-wont-cover-laparoscopic-power-morcellation-because-cancer-risk-1647160. Published August 2, 2014. Accessed August 6, 2014.
3. Yates J. FDA, hospitals caution against laparoscopic power morcellation during hysterectomy and myomectomy. OBG Manag. 2014;26(5):68, 67. http://www.obgmanagement.com/topic-collections/morcellation/article/fda-hospitals-caution-against-laparoscopic-power-morcellation-during-hysterectomy-and-myomectomy.html.
4. Mamula KB. UPMC drops controversial surgical procedure. Pittsburgh Business Times. http://www.bizjournals.com/pittsburgh/news/2014/08/01/upmc-drops-controversial-surgical-procedure.html. Published August 1, 2014. Accessed August 6, 2014.
5. Yates J. Open power morcellation of uterine tumors during hysterectomy banned at two Boston hospitals. OBG Manag. 2014;26(4). http://www.obgmanagement.com/topic-collections/morcellation/article/open-power-morcellation-of-uterine-tumors-during-hysterectomy-banned-at-two-boston-hospitals.html
6. Ethicon. Urgent: Medical device market withdrawal. http://www.ethicon.com/sites/default/files/managed-documents/Ethicon%20Morcellation%20Devices%20Customer%20Letter%20Final.pdf. Published July 30, 2014. Accessed August 6, 2014.
7. Iglesia C, Yates J. Why FDA hearing on morcellation safety could drive innovation [audiocast]. OBG Manag. 2014;26(7). http://www.obgmanagement.com/home/article/why-fda-hearing-on-morcellation-safety-could-drive-innovation/d1071c5e8326e8a2de76a30f0446b1ab.html. Published July 17, 2014. Accessed August 6, 2014.
Ebola, fear, and us
Recently, two American missionary workers in Liberia made headlines when they became infected with the Ebola virus, which causes a highly fatal hemorrhagic fever. They both received an experimental "secret serum" called ZMapp while in Africa and have since been flown back to the United States to be cared for at Emory University Hospital in Atlanta.
The response to their return has been mixed. While some hail their incredible selflessness and the mission they set out to accomplish, others are lukewarm, indifferent, or even very opposed to their return – so opposed that security was heightened in response to threats. I, of course, am among the former.
I once went on a 5-day missionary trip to Nicaragua. Optimistic about the potential to help the poor and hurting, my biggest challenges were the long plane ride and the hotel accommodations, which were not quite to my liking. The thought of contracting a potentially fatal disease and never returning home to my family never, ever crossed my mind.
Dr. Kent Brantly and Nancy Writebol, both missionary workers, were of a completely different mindset; they had different goals, they faced different challenges. They willingly boarded a plane to fly thousands of miles away from the comfort of their homes, the love of their families, the security of their close friends. They risked everything to care for complete strangers, strangers who posed a real threat to their lives. They never expected to receive any earthly thing in return. Interestingly, after contracting Ebola, Dr. Brantley did receive a unit of blood from a 14-year-old who had survived his fight with the Ebola virus while under his care.
Personally, I can only hope that one day I will have even half of the compassion and servant’s heart that these brave, incredibly selfless individuals possess.
I have to believe that those who object to their presence on American soil are afraid. They do not know what to expect and fear the epidemic spreading to the United States. I remember when I first started treating HIV/AIDS patients in the early ’90s. It was new and it was very, very scary. In those days, we donned protective garments above and beyond what we now know is necessary. Still, at times I, along with countless other health care workers, was overwhelmed by fear.
Like the human immunodeficiency virus, the Ebola virus is spread through direct contact with the blood or other body fluids of an infected person or through exposure to infected objects such as needles. It is not airborne, nor is it spread by contaminated food and water. Unlike with HIV, however, Ebola can be fatal within days. Another difference is that it is believed to be transmissible only if the individual is symptomatic, while countless cases of HIV were the result of exposure to seemingly healthy individuals.
While some in the American public still decry that Dr. Brantley and Mrs. Writebol were brought to a hospital in the United States, I have to believe that the great majority of Americans keep these two incredible individuals in their thoughts and prayers.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
Recently, two American missionary workers in Liberia made headlines when they became infected with the Ebola virus, which causes a highly fatal hemorrhagic fever. They both received an experimental "secret serum" called ZMapp while in Africa and have since been flown back to the United States to be cared for at Emory University Hospital in Atlanta.
The response to their return has been mixed. While some hail their incredible selflessness and the mission they set out to accomplish, others are lukewarm, indifferent, or even very opposed to their return – so opposed that security was heightened in response to threats. I, of course, am among the former.
I once went on a 5-day missionary trip to Nicaragua. Optimistic about the potential to help the poor and hurting, my biggest challenges were the long plane ride and the hotel accommodations, which were not quite to my liking. The thought of contracting a potentially fatal disease and never returning home to my family never, ever crossed my mind.
Dr. Kent Brantly and Nancy Writebol, both missionary workers, were of a completely different mindset; they had different goals, they faced different challenges. They willingly boarded a plane to fly thousands of miles away from the comfort of their homes, the love of their families, the security of their close friends. They risked everything to care for complete strangers, strangers who posed a real threat to their lives. They never expected to receive any earthly thing in return. Interestingly, after contracting Ebola, Dr. Brantley did receive a unit of blood from a 14-year-old who had survived his fight with the Ebola virus while under his care.
Personally, I can only hope that one day I will have even half of the compassion and servant’s heart that these brave, incredibly selfless individuals possess.
I have to believe that those who object to their presence on American soil are afraid. They do not know what to expect and fear the epidemic spreading to the United States. I remember when I first started treating HIV/AIDS patients in the early ’90s. It was new and it was very, very scary. In those days, we donned protective garments above and beyond what we now know is necessary. Still, at times I, along with countless other health care workers, was overwhelmed by fear.
Like the human immunodeficiency virus, the Ebola virus is spread through direct contact with the blood or other body fluids of an infected person or through exposure to infected objects such as needles. It is not airborne, nor is it spread by contaminated food and water. Unlike with HIV, however, Ebola can be fatal within days. Another difference is that it is believed to be transmissible only if the individual is symptomatic, while countless cases of HIV were the result of exposure to seemingly healthy individuals.
While some in the American public still decry that Dr. Brantley and Mrs. Writebol were brought to a hospital in the United States, I have to believe that the great majority of Americans keep these two incredible individuals in their thoughts and prayers.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
Recently, two American missionary workers in Liberia made headlines when they became infected with the Ebola virus, which causes a highly fatal hemorrhagic fever. They both received an experimental "secret serum" called ZMapp while in Africa and have since been flown back to the United States to be cared for at Emory University Hospital in Atlanta.
The response to their return has been mixed. While some hail their incredible selflessness and the mission they set out to accomplish, others are lukewarm, indifferent, or even very opposed to their return – so opposed that security was heightened in response to threats. I, of course, am among the former.
I once went on a 5-day missionary trip to Nicaragua. Optimistic about the potential to help the poor and hurting, my biggest challenges were the long plane ride and the hotel accommodations, which were not quite to my liking. The thought of contracting a potentially fatal disease and never returning home to my family never, ever crossed my mind.
Dr. Kent Brantly and Nancy Writebol, both missionary workers, were of a completely different mindset; they had different goals, they faced different challenges. They willingly boarded a plane to fly thousands of miles away from the comfort of their homes, the love of their families, the security of their close friends. They risked everything to care for complete strangers, strangers who posed a real threat to their lives. They never expected to receive any earthly thing in return. Interestingly, after contracting Ebola, Dr. Brantley did receive a unit of blood from a 14-year-old who had survived his fight with the Ebola virus while under his care.
Personally, I can only hope that one day I will have even half of the compassion and servant’s heart that these brave, incredibly selfless individuals possess.
I have to believe that those who object to their presence on American soil are afraid. They do not know what to expect and fear the epidemic spreading to the United States. I remember when I first started treating HIV/AIDS patients in the early ’90s. It was new and it was very, very scary. In those days, we donned protective garments above and beyond what we now know is necessary. Still, at times I, along with countless other health care workers, was overwhelmed by fear.
Like the human immunodeficiency virus, the Ebola virus is spread through direct contact with the blood or other body fluids of an infected person or through exposure to infected objects such as needles. It is not airborne, nor is it spread by contaminated food and water. Unlike with HIV, however, Ebola can be fatal within days. Another difference is that it is believed to be transmissible only if the individual is symptomatic, while countless cases of HIV were the result of exposure to seemingly healthy individuals.
While some in the American public still decry that Dr. Brantley and Mrs. Writebol were brought to a hospital in the United States, I have to believe that the great majority of Americans keep these two incredible individuals in their thoughts and prayers.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
When ‘normal’ just isn’t normal
As pediatricians, we are constantly evaluating children of all ages. We make determinations of normal and abnormal all the time. But, sometimes determining normal can be a challenge because children come in all shapes, sizes, and complexions, so "normal" can appear in a variety of ways.
When it comes to the adolescent, this is an even greater challenge because the onset of puberty is so varied that children of the same age can look vastly different, and pubertal changes only widen the variety. Obesity also has impacted the appearance of normal because it makes children look older, pubertal changes more advanced, and a thorough exam more difficult. So when is it okay to say, "They will just grow out of it?" Well, the best answer is when all the serious illnesses have been considered and ruled out.
Gynecomastia is a common finding in the adolescent wellness exam; 50%-60% of adolescent males experience some degree of breast enlargement starting at the age of 10 years. This peaks at ages 13-14, then regresses over a period of 18 months (N. Engl. J. Med. 2007;357:1229-37). For approximately 25% of children, the breast tissue persists, which leads to significant anxiety and insecurities among adolescent males. Even when asked if they have concerns, few will admit to it because the thought of the evaluation is more than they can handle.
Gynecomastia is caused from the increased ratio of estrogen to androgen. Antiandrogens, drugs, and weight gain have all been implicated. But in the evaluation of increased breast tissue, normal as well as abnormal causes have to be considered.
Exogenous causes include herbal products, such as tea tree oil, or medications. The most common drugs are cimetidine, ranitidine, and omeprazole, as well spirolactone and ketoconazole. With the exception of spirolactone, these are all drugs that are used commonly for minor illness in children, but have been identified as a cause for gynecomastia. Discontinuation of these products usually resolves the issue within a few months (Pharmacotherapy 1993;13:37-45).
Obesity can cause a pseudogynecomastia as well as a true gynecomastia because aromatase enzyme increases with the increase in fat tissue, which converts testosterone to estradiol. Clinically, pseudogynecomastia can be distinguished from true gynecomastia by doing a breast exam. True gynecomastia is a concentric, rubbery firm mass greater than 0.5cm, and directly below the areola, where pseudogynecomastia has diffuse enlargement and no discernable glandular tissue.
Abnormal causes of gynecomastia are much less common, but do occur. A careful physical examination and a detailed review of systems can be very helpful in ruling in or out serious causes.
An imbalance of estrogen and testosterone can result from estrogen or testosterone going up or down. These changes can be caused by other hormonal stimulation. Human chorionic gonadotropin (HGC) is increased with germ cell tumors, which can be found in abdominal or testicular masses, resulting in secondary hypogonadism. Elevated estradiol is found with testicular tumors and adrenal tumors.
Hyperthyroidism can cause gynecomastia. Additional symptoms include palpitations, weight loss, and anxiety. Physical findings include a goiter, exophthalmoses, and tremors.
Klinefelter’s syndrome, a condition that occurs in men who have an extra X chromosome, includes gynecomastia and hypogonadism. There is a 20%-60% increased risk of breast cancer in these patients, who tend to have less facial and body hair, reduced muscle tone, and narrower shoulders and wider hips (N. Engl. J. Med. 2007;357:1229-37). Suspicion of breast cancer should increase if the mass is unilateral, nontender, and eccentric to the areola.
Although the vast majority of patients with gynecomastia will resolve spontaneously, careful evaluation and consideration of abnormal causes can lead to early diagnosis and treatment.
Experienced pediatricians know it’s never "nothing" unless all the possible "somethings" have been ruled out!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].
As pediatricians, we are constantly evaluating children of all ages. We make determinations of normal and abnormal all the time. But, sometimes determining normal can be a challenge because children come in all shapes, sizes, and complexions, so "normal" can appear in a variety of ways.
When it comes to the adolescent, this is an even greater challenge because the onset of puberty is so varied that children of the same age can look vastly different, and pubertal changes only widen the variety. Obesity also has impacted the appearance of normal because it makes children look older, pubertal changes more advanced, and a thorough exam more difficult. So when is it okay to say, "They will just grow out of it?" Well, the best answer is when all the serious illnesses have been considered and ruled out.
Gynecomastia is a common finding in the adolescent wellness exam; 50%-60% of adolescent males experience some degree of breast enlargement starting at the age of 10 years. This peaks at ages 13-14, then regresses over a period of 18 months (N. Engl. J. Med. 2007;357:1229-37). For approximately 25% of children, the breast tissue persists, which leads to significant anxiety and insecurities among adolescent males. Even when asked if they have concerns, few will admit to it because the thought of the evaluation is more than they can handle.
Gynecomastia is caused from the increased ratio of estrogen to androgen. Antiandrogens, drugs, and weight gain have all been implicated. But in the evaluation of increased breast tissue, normal as well as abnormal causes have to be considered.
Exogenous causes include herbal products, such as tea tree oil, or medications. The most common drugs are cimetidine, ranitidine, and omeprazole, as well spirolactone and ketoconazole. With the exception of spirolactone, these are all drugs that are used commonly for minor illness in children, but have been identified as a cause for gynecomastia. Discontinuation of these products usually resolves the issue within a few months (Pharmacotherapy 1993;13:37-45).
Obesity can cause a pseudogynecomastia as well as a true gynecomastia because aromatase enzyme increases with the increase in fat tissue, which converts testosterone to estradiol. Clinically, pseudogynecomastia can be distinguished from true gynecomastia by doing a breast exam. True gynecomastia is a concentric, rubbery firm mass greater than 0.5cm, and directly below the areola, where pseudogynecomastia has diffuse enlargement and no discernable glandular tissue.
Abnormal causes of gynecomastia are much less common, but do occur. A careful physical examination and a detailed review of systems can be very helpful in ruling in or out serious causes.
An imbalance of estrogen and testosterone can result from estrogen or testosterone going up or down. These changes can be caused by other hormonal stimulation. Human chorionic gonadotropin (HGC) is increased with germ cell tumors, which can be found in abdominal or testicular masses, resulting in secondary hypogonadism. Elevated estradiol is found with testicular tumors and adrenal tumors.
Hyperthyroidism can cause gynecomastia. Additional symptoms include palpitations, weight loss, and anxiety. Physical findings include a goiter, exophthalmoses, and tremors.
Klinefelter’s syndrome, a condition that occurs in men who have an extra X chromosome, includes gynecomastia and hypogonadism. There is a 20%-60% increased risk of breast cancer in these patients, who tend to have less facial and body hair, reduced muscle tone, and narrower shoulders and wider hips (N. Engl. J. Med. 2007;357:1229-37). Suspicion of breast cancer should increase if the mass is unilateral, nontender, and eccentric to the areola.
Although the vast majority of patients with gynecomastia will resolve spontaneously, careful evaluation and consideration of abnormal causes can lead to early diagnosis and treatment.
Experienced pediatricians know it’s never "nothing" unless all the possible "somethings" have been ruled out!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].
As pediatricians, we are constantly evaluating children of all ages. We make determinations of normal and abnormal all the time. But, sometimes determining normal can be a challenge because children come in all shapes, sizes, and complexions, so "normal" can appear in a variety of ways.
When it comes to the adolescent, this is an even greater challenge because the onset of puberty is so varied that children of the same age can look vastly different, and pubertal changes only widen the variety. Obesity also has impacted the appearance of normal because it makes children look older, pubertal changes more advanced, and a thorough exam more difficult. So when is it okay to say, "They will just grow out of it?" Well, the best answer is when all the serious illnesses have been considered and ruled out.
Gynecomastia is a common finding in the adolescent wellness exam; 50%-60% of adolescent males experience some degree of breast enlargement starting at the age of 10 years. This peaks at ages 13-14, then regresses over a period of 18 months (N. Engl. J. Med. 2007;357:1229-37). For approximately 25% of children, the breast tissue persists, which leads to significant anxiety and insecurities among adolescent males. Even when asked if they have concerns, few will admit to it because the thought of the evaluation is more than they can handle.
Gynecomastia is caused from the increased ratio of estrogen to androgen. Antiandrogens, drugs, and weight gain have all been implicated. But in the evaluation of increased breast tissue, normal as well as abnormal causes have to be considered.
Exogenous causes include herbal products, such as tea tree oil, or medications. The most common drugs are cimetidine, ranitidine, and omeprazole, as well spirolactone and ketoconazole. With the exception of spirolactone, these are all drugs that are used commonly for minor illness in children, but have been identified as a cause for gynecomastia. Discontinuation of these products usually resolves the issue within a few months (Pharmacotherapy 1993;13:37-45).
Obesity can cause a pseudogynecomastia as well as a true gynecomastia because aromatase enzyme increases with the increase in fat tissue, which converts testosterone to estradiol. Clinically, pseudogynecomastia can be distinguished from true gynecomastia by doing a breast exam. True gynecomastia is a concentric, rubbery firm mass greater than 0.5cm, and directly below the areola, where pseudogynecomastia has diffuse enlargement and no discernable glandular tissue.
Abnormal causes of gynecomastia are much less common, but do occur. A careful physical examination and a detailed review of systems can be very helpful in ruling in or out serious causes.
An imbalance of estrogen and testosterone can result from estrogen or testosterone going up or down. These changes can be caused by other hormonal stimulation. Human chorionic gonadotropin (HGC) is increased with germ cell tumors, which can be found in abdominal or testicular masses, resulting in secondary hypogonadism. Elevated estradiol is found with testicular tumors and adrenal tumors.
Hyperthyroidism can cause gynecomastia. Additional symptoms include palpitations, weight loss, and anxiety. Physical findings include a goiter, exophthalmoses, and tremors.
Klinefelter’s syndrome, a condition that occurs in men who have an extra X chromosome, includes gynecomastia and hypogonadism. There is a 20%-60% increased risk of breast cancer in these patients, who tend to have less facial and body hair, reduced muscle tone, and narrower shoulders and wider hips (N. Engl. J. Med. 2007;357:1229-37). Suspicion of breast cancer should increase if the mass is unilateral, nontender, and eccentric to the areola.
Although the vast majority of patients with gynecomastia will resolve spontaneously, careful evaluation and consideration of abnormal causes can lead to early diagnosis and treatment.
Experienced pediatricians know it’s never "nothing" unless all the possible "somethings" have been ruled out!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected].
Method corrects β-thalassemia mutations

Credit: Salk Institute
Genome editing technology allows for seamless correction of disease-causing mutations in cells from patients with β-thalassemia, investigators have reported in Genome Research.
The team noted that β-thalassemia results from inherited mutations in the hemoglobin beta (HBB) gene, which prompt reduced HBB expression in red blood cells, as well as anemia.
The only established curative treatment is hematopoietic stem cell transplant, but this requires a matched donor.
Gene therapy could eliminate this need.
To correct HBB mutations directly in a patient’s genome, Yuet Wai Kan, MD, of the University of California, San Francisco, and his colleagues first generated induced pluripotent stem cells (iPSCs) from patients’ skin cells.
The team then used CRISPR/Cas9 technology to precisely engineer a double-strand DNA break at the HBB locus in the iPSCs, allowing a donor plasmid with the corrected sites to be efficiently integrated, thus replacing the mutated sites.
The donor plasmid also contained selectable markers to identify cells with corrected copies of the gene. These selectable markers were subsequently removed with transposase and a second round of selection, generating a seamless, corrected version of HBB in the patient’s genome.
The investigators found the corrected iPSCs could differentiate into mature blood cells, and these blood cells showed restored expression of hemoglobin.
However, the team said a lot more work is needed before these cells could be transplanted to treat a patient with β-thalassemia.
“Although we and others are able to differentiate iPSCs into blood cell progenitors as well as mature blood cells, the transplantation of the progenitors into mouse models to test them has, so far, proven very difficult,” Dr Kan said. “I believe it will take quite a few more years before we can apply it in a clinical setting.” ![]()

Credit: Salk Institute
Genome editing technology allows for seamless correction of disease-causing mutations in cells from patients with β-thalassemia, investigators have reported in Genome Research.
The team noted that β-thalassemia results from inherited mutations in the hemoglobin beta (HBB) gene, which prompt reduced HBB expression in red blood cells, as well as anemia.
The only established curative treatment is hematopoietic stem cell transplant, but this requires a matched donor.
Gene therapy could eliminate this need.
To correct HBB mutations directly in a patient’s genome, Yuet Wai Kan, MD, of the University of California, San Francisco, and his colleagues first generated induced pluripotent stem cells (iPSCs) from patients’ skin cells.
The team then used CRISPR/Cas9 technology to precisely engineer a double-strand DNA break at the HBB locus in the iPSCs, allowing a donor plasmid with the corrected sites to be efficiently integrated, thus replacing the mutated sites.
The donor plasmid also contained selectable markers to identify cells with corrected copies of the gene. These selectable markers were subsequently removed with transposase and a second round of selection, generating a seamless, corrected version of HBB in the patient’s genome.
The investigators found the corrected iPSCs could differentiate into mature blood cells, and these blood cells showed restored expression of hemoglobin.
However, the team said a lot more work is needed before these cells could be transplanted to treat a patient with β-thalassemia.
“Although we and others are able to differentiate iPSCs into blood cell progenitors as well as mature blood cells, the transplantation of the progenitors into mouse models to test them has, so far, proven very difficult,” Dr Kan said. “I believe it will take quite a few more years before we can apply it in a clinical setting.” ![]()

Credit: Salk Institute
Genome editing technology allows for seamless correction of disease-causing mutations in cells from patients with β-thalassemia, investigators have reported in Genome Research.
The team noted that β-thalassemia results from inherited mutations in the hemoglobin beta (HBB) gene, which prompt reduced HBB expression in red blood cells, as well as anemia.
The only established curative treatment is hematopoietic stem cell transplant, but this requires a matched donor.
Gene therapy could eliminate this need.
To correct HBB mutations directly in a patient’s genome, Yuet Wai Kan, MD, of the University of California, San Francisco, and his colleagues first generated induced pluripotent stem cells (iPSCs) from patients’ skin cells.
The team then used CRISPR/Cas9 technology to precisely engineer a double-strand DNA break at the HBB locus in the iPSCs, allowing a donor plasmid with the corrected sites to be efficiently integrated, thus replacing the mutated sites.
The donor plasmid also contained selectable markers to identify cells with corrected copies of the gene. These selectable markers were subsequently removed with transposase and a second round of selection, generating a seamless, corrected version of HBB in the patient’s genome.
The investigators found the corrected iPSCs could differentiate into mature blood cells, and these blood cells showed restored expression of hemoglobin.
However, the team said a lot more work is needed before these cells could be transplanted to treat a patient with β-thalassemia.
“Although we and others are able to differentiate iPSCs into blood cell progenitors as well as mature blood cells, the transplantation of the progenitors into mouse models to test them has, so far, proven very difficult,” Dr Kan said. “I believe it will take quite a few more years before we can apply it in a clinical setting.” ![]()
Battling Multidrug Resistant UTIs With Methenamine Hippurate
Recently, Federal Practitioner talked with Rebecca McAllister, MS, FNP-BC, about her role in treating complicated urinary tract infections (UTIs) in elderly patients at the Community Living Center of the Bay Pines VA Healthcare System in Florida. The original July 2014 Case in Point, “Recurrent Multidrug Resistant Urinary Tract Infections in Geriatric Patients,” discussed 4 case studies, which suggested the safety and efficacy of treatment with methenamine hippurate.
Federal Practitioner: Much of the focus in your article was on the use of methenamine in Norway and Sweden and a lack thereof in the U.S. How are multidrug resistant UTIs generally treated in the U.S., and how could this be handled differently?
Rebecca McAllister, MS, FNP-BC: Because of the increased rates of bacterial resistance, treating recurrent UTIs prophylactically with low-dose antibiotics is no longer the standard of care. Currently, multidrug resistant UTIs are treated with broad-spectrum antibiotics, that organisms are susceptible to. The promise of methenamine relies on the bacteria not developing resistance to it; in turn, long-term use in patients does not contribute to developing resistance.
FP: Is methenamine hippurate readily available within the VA, and what are the guidelines surrounding its use?
RM: Methenamine is on the VA formulary available in 1-gm doses. Standard guidelines per Micromedex are for prophylaxis of recurrent UTIs, as mentioned in the article, and contraindicates use in patients with impaired renal function, although specific parameters are not identified, because testing was never done with geriatric patients.
FP: Of the 4 patients discussed in the article, 3 were aged > 89 years and the fourth was aged exactly 89 years. Was this a coincidence, or does the success of methenamine in this oldest-old cohort highlight UTI recurrence rate late in life, the failed efficacy of other drugs over time, or both?
RM: The success of methenamine highlights both UTI recurrence rate late in life and the failed efficacy of other drugs over time. The primary patient group in these case studies was composed of homebound veterans.
FP: At the beginning of the discussion portion of your article, following 4 case studies, you mention, “Patients with similar profiles to those discussed in this report were treated with less dramatic results.” How do you, as a family nurse practitioner, consider treatment a success, and how might this differ from expectations set by a medical facility?
RM: As is always the challenge with preventive interventions, it is difficult to measure what does not happen. Successful treatment for recurrent UTIs is lack of recurrence, also asymptomatic colonization vs a symptomatic UTI, which can be measured by a urinalysis is a success. In the oldest of the old, delayed hospitalization for urosepsis, reduced risk of falls, and increased mortality are also successes. Cost savings to the health care system by administering an inexpensive preventive medication vs very expensive IV antibiotic therapy, another success. The observed changes in bacterial resistance in patients treated with methenamine offers great hope in the battle against bacteria.
Ms. McAllister coauthored the July 2014 article, “Recurrent Multidrug Resistant Urinary Tract Infections in Geriatric Patients,” with Janice Allwood, MS, ARNP, CUNP.
Ms. McAllister is a Community Living Center family nurse practitioner and Ms. Allwood is an advanced registered nurse practitioner in Urology Surgery, both at the Bay Pines VA Healthcare System in Florida.
Recently, Federal Practitioner talked with Rebecca McAllister, MS, FNP-BC, about her role in treating complicated urinary tract infections (UTIs) in elderly patients at the Community Living Center of the Bay Pines VA Healthcare System in Florida. The original July 2014 Case in Point, “Recurrent Multidrug Resistant Urinary Tract Infections in Geriatric Patients,” discussed 4 case studies, which suggested the safety and efficacy of treatment with methenamine hippurate.
Federal Practitioner: Much of the focus in your article was on the use of methenamine in Norway and Sweden and a lack thereof in the U.S. How are multidrug resistant UTIs generally treated in the U.S., and how could this be handled differently?
Rebecca McAllister, MS, FNP-BC: Because of the increased rates of bacterial resistance, treating recurrent UTIs prophylactically with low-dose antibiotics is no longer the standard of care. Currently, multidrug resistant UTIs are treated with broad-spectrum antibiotics, that organisms are susceptible to. The promise of methenamine relies on the bacteria not developing resistance to it; in turn, long-term use in patients does not contribute to developing resistance.
FP: Is methenamine hippurate readily available within the VA, and what are the guidelines surrounding its use?
RM: Methenamine is on the VA formulary available in 1-gm doses. Standard guidelines per Micromedex are for prophylaxis of recurrent UTIs, as mentioned in the article, and contraindicates use in patients with impaired renal function, although specific parameters are not identified, because testing was never done with geriatric patients.
FP: Of the 4 patients discussed in the article, 3 were aged > 89 years and the fourth was aged exactly 89 years. Was this a coincidence, or does the success of methenamine in this oldest-old cohort highlight UTI recurrence rate late in life, the failed efficacy of other drugs over time, or both?
RM: The success of methenamine highlights both UTI recurrence rate late in life and the failed efficacy of other drugs over time. The primary patient group in these case studies was composed of homebound veterans.
FP: At the beginning of the discussion portion of your article, following 4 case studies, you mention, “Patients with similar profiles to those discussed in this report were treated with less dramatic results.” How do you, as a family nurse practitioner, consider treatment a success, and how might this differ from expectations set by a medical facility?
RM: As is always the challenge with preventive interventions, it is difficult to measure what does not happen. Successful treatment for recurrent UTIs is lack of recurrence, also asymptomatic colonization vs a symptomatic UTI, which can be measured by a urinalysis is a success. In the oldest of the old, delayed hospitalization for urosepsis, reduced risk of falls, and increased mortality are also successes. Cost savings to the health care system by administering an inexpensive preventive medication vs very expensive IV antibiotic therapy, another success. The observed changes in bacterial resistance in patients treated with methenamine offers great hope in the battle against bacteria.
Ms. McAllister coauthored the July 2014 article, “Recurrent Multidrug Resistant Urinary Tract Infections in Geriatric Patients,” with Janice Allwood, MS, ARNP, CUNP.
Ms. McAllister is a Community Living Center family nurse practitioner and Ms. Allwood is an advanced registered nurse practitioner in Urology Surgery, both at the Bay Pines VA Healthcare System in Florida.
Recently, Federal Practitioner talked with Rebecca McAllister, MS, FNP-BC, about her role in treating complicated urinary tract infections (UTIs) in elderly patients at the Community Living Center of the Bay Pines VA Healthcare System in Florida. The original July 2014 Case in Point, “Recurrent Multidrug Resistant Urinary Tract Infections in Geriatric Patients,” discussed 4 case studies, which suggested the safety and efficacy of treatment with methenamine hippurate.
Federal Practitioner: Much of the focus in your article was on the use of methenamine in Norway and Sweden and a lack thereof in the U.S. How are multidrug resistant UTIs generally treated in the U.S., and how could this be handled differently?
Rebecca McAllister, MS, FNP-BC: Because of the increased rates of bacterial resistance, treating recurrent UTIs prophylactically with low-dose antibiotics is no longer the standard of care. Currently, multidrug resistant UTIs are treated with broad-spectrum antibiotics, that organisms are susceptible to. The promise of methenamine relies on the bacteria not developing resistance to it; in turn, long-term use in patients does not contribute to developing resistance.
FP: Is methenamine hippurate readily available within the VA, and what are the guidelines surrounding its use?
RM: Methenamine is on the VA formulary available in 1-gm doses. Standard guidelines per Micromedex are for prophylaxis of recurrent UTIs, as mentioned in the article, and contraindicates use in patients with impaired renal function, although specific parameters are not identified, because testing was never done with geriatric patients.
FP: Of the 4 patients discussed in the article, 3 were aged > 89 years and the fourth was aged exactly 89 years. Was this a coincidence, or does the success of methenamine in this oldest-old cohort highlight UTI recurrence rate late in life, the failed efficacy of other drugs over time, or both?
RM: The success of methenamine highlights both UTI recurrence rate late in life and the failed efficacy of other drugs over time. The primary patient group in these case studies was composed of homebound veterans.
FP: At the beginning of the discussion portion of your article, following 4 case studies, you mention, “Patients with similar profiles to those discussed in this report were treated with less dramatic results.” How do you, as a family nurse practitioner, consider treatment a success, and how might this differ from expectations set by a medical facility?
RM: As is always the challenge with preventive interventions, it is difficult to measure what does not happen. Successful treatment for recurrent UTIs is lack of recurrence, also asymptomatic colonization vs a symptomatic UTI, which can be measured by a urinalysis is a success. In the oldest of the old, delayed hospitalization for urosepsis, reduced risk of falls, and increased mortality are also successes. Cost savings to the health care system by administering an inexpensive preventive medication vs very expensive IV antibiotic therapy, another success. The observed changes in bacterial resistance in patients treated with methenamine offers great hope in the battle against bacteria.
Ms. McAllister coauthored the July 2014 article, “Recurrent Multidrug Resistant Urinary Tract Infections in Geriatric Patients,” with Janice Allwood, MS, ARNP, CUNP.
Ms. McAllister is a Community Living Center family nurse practitioner and Ms. Allwood is an advanced registered nurse practitioner in Urology Surgery, both at the Bay Pines VA Healthcare System in Florida.
Malaria prophylaxis appears safe, effective in kids

Credit: Malayaka house
Year-round prophylaxis with a newer antimalaria treatment can reduce the risk of malaria in young children without posing a risk of serious adverse events, according to a study published in PLOS Medicine.
The researchers found that dihydroartemisinin-piperaquine (DP), an artemisinin-based combination therapy, was the most effective of 3 treatments at reducing malaria risk in children aged 6 months to 24 months.
The other 2 treatments, the antifolates sulfadoxine-pyrimethamine (SP) and trimethoprim-sulfamethoxazole (TS), have been in use longer than DP. And, in many locations, the malaria parasite has developed a resistance to them.
The researchers conducted this study to determine if the benefits of malaria prophylaxis outweighed the potential risk of anemia and other side effects from the drugs.
And they found the benefits did outweigh the risks. There was no significant increase in grade 3/4 adverse events with any of the treatments when compared to a control group.
The researchers also wanted to look specifically at the effects of year-round treatment. They noted that most previous studies of malaria prophylaxis have been limited to areas where there is only a seasonal risk of the disease. But this study took place in Uganda, where the risk persists throughout the year.
“Our study showed that preventive drug treatment can greatly reduce malaria in young children in areas where there are year-round high rates of transmission,” said study author Grant Dorsey, MD, of the University of California, San Francisco.
To make this discovery, the researchers studied 393 children from Tororo, Uganda. Beginning at 6 months of age, the children were randomized to 1 of 4 groups: monthly DP, monthly SP, daily TS, or a group that didn’t receive any prophylaxis, which is the standard medical practice in the area.
Treatments were given at home without supervision. Piperaquine levels were used as a measure of compliance in the DP arm.
All of the families involved in the study received insecticide-treated bed nets to put over the children when they slept. By 24 months of age, 352 children were still taking part in the study.
There were 3.02 malaria episodes per person-year in the DP group, 5.21 in the TS group, 6.73 in the SP group, and 6.95 in the control group. Protective efficacy measured 58% for the DP group, 28% for the TS group, and 7% for the SP group.
Piperaquine levels were below the detection limit 52% of the time when malaria was diagnosed in the DP group, which suggests non-adherence to treatment.
Between the groups, there was no significant difference in the rate of grade 3/4 adverse events related to treatment. There were 8 such events in the SP group, 8 in the TS group, and 3 in the DP group. Events included elevated temperature, anemia, neutropenia, thrombocytopenia, and elevated ALT/AST.
Considering all grade 3/4 adverse events regardless of their relationship to treatment, the researchers found the overall incidence was significantly lower in the DP group, but not the SP or TS groups, compared to the control group. The same was true for the incidence of elevated temperature, anemia, and thrombocytopenia.
After discontinuing the children’s treatment at 24 months of age, the researchers followed the children until age 3 and found no difference in malaria rates between the groups.
The team said these results suggest monthly administration of DP is a safe and effective option for reducing malaria among infants in regions with year-round transmission and high resistance to antifolates.
The findings also help to allay any concerns that continuous treatment might interfere with the children’s ability to develop an immune response against malaria, thereby making them more likely to contract the disease after treatment stops.
The researchers noted, however, that additional research is needed to evaluate the preventive efficacy of DP in other areas, maintain surveillance for potential selection of drug-resistant parasites, and evaluate the role of preventative treatment in the context of other malaria control interventions. ![]()

Credit: Malayaka house
Year-round prophylaxis with a newer antimalaria treatment can reduce the risk of malaria in young children without posing a risk of serious adverse events, according to a study published in PLOS Medicine.
The researchers found that dihydroartemisinin-piperaquine (DP), an artemisinin-based combination therapy, was the most effective of 3 treatments at reducing malaria risk in children aged 6 months to 24 months.
The other 2 treatments, the antifolates sulfadoxine-pyrimethamine (SP) and trimethoprim-sulfamethoxazole (TS), have been in use longer than DP. And, in many locations, the malaria parasite has developed a resistance to them.
The researchers conducted this study to determine if the benefits of malaria prophylaxis outweighed the potential risk of anemia and other side effects from the drugs.
And they found the benefits did outweigh the risks. There was no significant increase in grade 3/4 adverse events with any of the treatments when compared to a control group.
The researchers also wanted to look specifically at the effects of year-round treatment. They noted that most previous studies of malaria prophylaxis have been limited to areas where there is only a seasonal risk of the disease. But this study took place in Uganda, where the risk persists throughout the year.
“Our study showed that preventive drug treatment can greatly reduce malaria in young children in areas where there are year-round high rates of transmission,” said study author Grant Dorsey, MD, of the University of California, San Francisco.
To make this discovery, the researchers studied 393 children from Tororo, Uganda. Beginning at 6 months of age, the children were randomized to 1 of 4 groups: monthly DP, monthly SP, daily TS, or a group that didn’t receive any prophylaxis, which is the standard medical practice in the area.
Treatments were given at home without supervision. Piperaquine levels were used as a measure of compliance in the DP arm.
All of the families involved in the study received insecticide-treated bed nets to put over the children when they slept. By 24 months of age, 352 children were still taking part in the study.
There were 3.02 malaria episodes per person-year in the DP group, 5.21 in the TS group, 6.73 in the SP group, and 6.95 in the control group. Protective efficacy measured 58% for the DP group, 28% for the TS group, and 7% for the SP group.
Piperaquine levels were below the detection limit 52% of the time when malaria was diagnosed in the DP group, which suggests non-adherence to treatment.
Between the groups, there was no significant difference in the rate of grade 3/4 adverse events related to treatment. There were 8 such events in the SP group, 8 in the TS group, and 3 in the DP group. Events included elevated temperature, anemia, neutropenia, thrombocytopenia, and elevated ALT/AST.
Considering all grade 3/4 adverse events regardless of their relationship to treatment, the researchers found the overall incidence was significantly lower in the DP group, but not the SP or TS groups, compared to the control group. The same was true for the incidence of elevated temperature, anemia, and thrombocytopenia.
After discontinuing the children’s treatment at 24 months of age, the researchers followed the children until age 3 and found no difference in malaria rates between the groups.
The team said these results suggest monthly administration of DP is a safe and effective option for reducing malaria among infants in regions with year-round transmission and high resistance to antifolates.
The findings also help to allay any concerns that continuous treatment might interfere with the children’s ability to develop an immune response against malaria, thereby making them more likely to contract the disease after treatment stops.
The researchers noted, however, that additional research is needed to evaluate the preventive efficacy of DP in other areas, maintain surveillance for potential selection of drug-resistant parasites, and evaluate the role of preventative treatment in the context of other malaria control interventions. ![]()

Credit: Malayaka house
Year-round prophylaxis with a newer antimalaria treatment can reduce the risk of malaria in young children without posing a risk of serious adverse events, according to a study published in PLOS Medicine.
The researchers found that dihydroartemisinin-piperaquine (DP), an artemisinin-based combination therapy, was the most effective of 3 treatments at reducing malaria risk in children aged 6 months to 24 months.
The other 2 treatments, the antifolates sulfadoxine-pyrimethamine (SP) and trimethoprim-sulfamethoxazole (TS), have been in use longer than DP. And, in many locations, the malaria parasite has developed a resistance to them.
The researchers conducted this study to determine if the benefits of malaria prophylaxis outweighed the potential risk of anemia and other side effects from the drugs.
And they found the benefits did outweigh the risks. There was no significant increase in grade 3/4 adverse events with any of the treatments when compared to a control group.
The researchers also wanted to look specifically at the effects of year-round treatment. They noted that most previous studies of malaria prophylaxis have been limited to areas where there is only a seasonal risk of the disease. But this study took place in Uganda, where the risk persists throughout the year.
“Our study showed that preventive drug treatment can greatly reduce malaria in young children in areas where there are year-round high rates of transmission,” said study author Grant Dorsey, MD, of the University of California, San Francisco.
To make this discovery, the researchers studied 393 children from Tororo, Uganda. Beginning at 6 months of age, the children were randomized to 1 of 4 groups: monthly DP, monthly SP, daily TS, or a group that didn’t receive any prophylaxis, which is the standard medical practice in the area.
Treatments were given at home without supervision. Piperaquine levels were used as a measure of compliance in the DP arm.
All of the families involved in the study received insecticide-treated bed nets to put over the children when they slept. By 24 months of age, 352 children were still taking part in the study.
There were 3.02 malaria episodes per person-year in the DP group, 5.21 in the TS group, 6.73 in the SP group, and 6.95 in the control group. Protective efficacy measured 58% for the DP group, 28% for the TS group, and 7% for the SP group.
Piperaquine levels were below the detection limit 52% of the time when malaria was diagnosed in the DP group, which suggests non-adherence to treatment.
Between the groups, there was no significant difference in the rate of grade 3/4 adverse events related to treatment. There were 8 such events in the SP group, 8 in the TS group, and 3 in the DP group. Events included elevated temperature, anemia, neutropenia, thrombocytopenia, and elevated ALT/AST.
Considering all grade 3/4 adverse events regardless of their relationship to treatment, the researchers found the overall incidence was significantly lower in the DP group, but not the SP or TS groups, compared to the control group. The same was true for the incidence of elevated temperature, anemia, and thrombocytopenia.
After discontinuing the children’s treatment at 24 months of age, the researchers followed the children until age 3 and found no difference in malaria rates between the groups.
The team said these results suggest monthly administration of DP is a safe and effective option for reducing malaria among infants in regions with year-round transmission and high resistance to antifolates.
The findings also help to allay any concerns that continuous treatment might interfere with the children’s ability to develop an immune response against malaria, thereby making them more likely to contract the disease after treatment stops.
The researchers noted, however, that additional research is needed to evaluate the preventive efficacy of DP in other areas, maintain surveillance for potential selection of drug-resistant parasites, and evaluate the role of preventative treatment in the context of other malaria control interventions. ![]()
Protein-targeting drug could treat cancers, other diseases

surrounding fused cells
Credit: IRB Barcelona
Experiments in mice suggest the mitochondrial chaperone TRAP-1 is involved in the development of cancers and age-related diseases.
Previous research showed that TRAP-1 is overexpressed in leukemia, lymphoma, and many other cancers.
The new research, published in Cell Reports, clarifies TRAP-1’s role in cancers and age-related conditions. It also suggests gamitrinib, a novel agent targeting TRAP-1, could prove useful in treating these diseases.
TRAP-1 is a member of the heat shock protein 90 (HSP90) family, chaperone proteins that guide the physical formation of other proteins and serve a regulatory function within mitochondria. Tumors use HSP90 proteins like TRAP-1 to help survive therapeutic attack.
To further investigate the effects of TRAP-1, researchers bred TRAP-1 knockout mice. The team found the mice compensate for losing the protein by switching to alternative cellular mechanisms for making energy.
“We see this astounding change in TRAP-1 knockout mice, where they show fewer signs of aging and are less likely to develop cancers,” said Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania.
“Our findings provide an unexpected explanation for how TRAP-1 and related proteins regulate metabolism within our cells. We usually link the reprogramming of metabolic pathways with human diseases, such as cancer. What we didn’t expect to see were healthier mice with fewer tumors.”
Dr Altieri and his colleagues created the TRAP-1 knockout mice as part of their ongoing investigation into their novel drug, gamitrinib, which targets TRAP-1 in the mitochondria of tumor cells.
“In tumors, the loss of TRAP-1 is devastating, triggering a host of catastrophic defects, including metabolic problems that ultimately result in the death of the tumor cells,” Dr Altieri said. ”Mice that lack TRAP-1 from the start, however, have 3 weeks in the womb to compensate for the loss of the protein.”
The researchers found that, in the knockout mice, the loss of TRAP-1 causes mitochondrial proteins to misfold, which triggers a compensatory response that causes cells to consume more oxygen and metabolize more sugar. This prompts the mitochondria to produce deregulated levels of ATP.
This increased mitochondrial activity actually creates a moderate boost in oxidative stress (free radical damage) and the associated DNA damage. While DNA damage may seem counterproductive to longevity and good health, the low level of DNA damage actually reduces cell proliferation, slowing growth to allow the cell’s natural repair mechanisms to take effect.
According to Dr Altieri, his group’s observations provide a mechanistic foundation for the role of chaperone molecules like HSP90 in the regulation of bioenergetics in mitochondria—how cells produce and use the chemical energy they need to survive and grow.
Their results explain some contradictory findings in the scientific literature regarding the regulation of bioenergetics and show how compensatory mechanisms can arise when these chaperone molecules are taken out of the equation.
“Our findings strengthen the case for targeting HSP90 in tumor cells, but they also open up a fascinating array of questions that may have implications for metabolism and longevity,” Dr Altieri said. “I predict that the TRAP-1 knockout mouse will be a valuable tool for answering these questions.” ![]()

surrounding fused cells
Credit: IRB Barcelona
Experiments in mice suggest the mitochondrial chaperone TRAP-1 is involved in the development of cancers and age-related diseases.
Previous research showed that TRAP-1 is overexpressed in leukemia, lymphoma, and many other cancers.
The new research, published in Cell Reports, clarifies TRAP-1’s role in cancers and age-related conditions. It also suggests gamitrinib, a novel agent targeting TRAP-1, could prove useful in treating these diseases.
TRAP-1 is a member of the heat shock protein 90 (HSP90) family, chaperone proteins that guide the physical formation of other proteins and serve a regulatory function within mitochondria. Tumors use HSP90 proteins like TRAP-1 to help survive therapeutic attack.
To further investigate the effects of TRAP-1, researchers bred TRAP-1 knockout mice. The team found the mice compensate for losing the protein by switching to alternative cellular mechanisms for making energy.
“We see this astounding change in TRAP-1 knockout mice, where they show fewer signs of aging and are less likely to develop cancers,” said Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania.
“Our findings provide an unexpected explanation for how TRAP-1 and related proteins regulate metabolism within our cells. We usually link the reprogramming of metabolic pathways with human diseases, such as cancer. What we didn’t expect to see were healthier mice with fewer tumors.”
Dr Altieri and his colleagues created the TRAP-1 knockout mice as part of their ongoing investigation into their novel drug, gamitrinib, which targets TRAP-1 in the mitochondria of tumor cells.
“In tumors, the loss of TRAP-1 is devastating, triggering a host of catastrophic defects, including metabolic problems that ultimately result in the death of the tumor cells,” Dr Altieri said. ”Mice that lack TRAP-1 from the start, however, have 3 weeks in the womb to compensate for the loss of the protein.”
The researchers found that, in the knockout mice, the loss of TRAP-1 causes mitochondrial proteins to misfold, which triggers a compensatory response that causes cells to consume more oxygen and metabolize more sugar. This prompts the mitochondria to produce deregulated levels of ATP.
This increased mitochondrial activity actually creates a moderate boost in oxidative stress (free radical damage) and the associated DNA damage. While DNA damage may seem counterproductive to longevity and good health, the low level of DNA damage actually reduces cell proliferation, slowing growth to allow the cell’s natural repair mechanisms to take effect.
According to Dr Altieri, his group’s observations provide a mechanistic foundation for the role of chaperone molecules like HSP90 in the regulation of bioenergetics in mitochondria—how cells produce and use the chemical energy they need to survive and grow.
Their results explain some contradictory findings in the scientific literature regarding the regulation of bioenergetics and show how compensatory mechanisms can arise when these chaperone molecules are taken out of the equation.
“Our findings strengthen the case for targeting HSP90 in tumor cells, but they also open up a fascinating array of questions that may have implications for metabolism and longevity,” Dr Altieri said. “I predict that the TRAP-1 knockout mouse will be a valuable tool for answering these questions.” ![]()

surrounding fused cells
Credit: IRB Barcelona
Experiments in mice suggest the mitochondrial chaperone TRAP-1 is involved in the development of cancers and age-related diseases.
Previous research showed that TRAP-1 is overexpressed in leukemia, lymphoma, and many other cancers.
The new research, published in Cell Reports, clarifies TRAP-1’s role in cancers and age-related conditions. It also suggests gamitrinib, a novel agent targeting TRAP-1, could prove useful in treating these diseases.
TRAP-1 is a member of the heat shock protein 90 (HSP90) family, chaperone proteins that guide the physical formation of other proteins and serve a regulatory function within mitochondria. Tumors use HSP90 proteins like TRAP-1 to help survive therapeutic attack.
To further investigate the effects of TRAP-1, researchers bred TRAP-1 knockout mice. The team found the mice compensate for losing the protein by switching to alternative cellular mechanisms for making energy.
“We see this astounding change in TRAP-1 knockout mice, where they show fewer signs of aging and are less likely to develop cancers,” said Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania.
“Our findings provide an unexpected explanation for how TRAP-1 and related proteins regulate metabolism within our cells. We usually link the reprogramming of metabolic pathways with human diseases, such as cancer. What we didn’t expect to see were healthier mice with fewer tumors.”
Dr Altieri and his colleagues created the TRAP-1 knockout mice as part of their ongoing investigation into their novel drug, gamitrinib, which targets TRAP-1 in the mitochondria of tumor cells.
“In tumors, the loss of TRAP-1 is devastating, triggering a host of catastrophic defects, including metabolic problems that ultimately result in the death of the tumor cells,” Dr Altieri said. ”Mice that lack TRAP-1 from the start, however, have 3 weeks in the womb to compensate for the loss of the protein.”
The researchers found that, in the knockout mice, the loss of TRAP-1 causes mitochondrial proteins to misfold, which triggers a compensatory response that causes cells to consume more oxygen and metabolize more sugar. This prompts the mitochondria to produce deregulated levels of ATP.
This increased mitochondrial activity actually creates a moderate boost in oxidative stress (free radical damage) and the associated DNA damage. While DNA damage may seem counterproductive to longevity and good health, the low level of DNA damage actually reduces cell proliferation, slowing growth to allow the cell’s natural repair mechanisms to take effect.
According to Dr Altieri, his group’s observations provide a mechanistic foundation for the role of chaperone molecules like HSP90 in the regulation of bioenergetics in mitochondria—how cells produce and use the chemical energy they need to survive and grow.
Their results explain some contradictory findings in the scientific literature regarding the regulation of bioenergetics and show how compensatory mechanisms can arise when these chaperone molecules are taken out of the equation.
“Our findings strengthen the case for targeting HSP90 in tumor cells, but they also open up a fascinating array of questions that may have implications for metabolism and longevity,” Dr Altieri said. “I predict that the TRAP-1 knockout mouse will be a valuable tool for answering these questions.” ![]()
STAP cell researcher commits suicide

Credit: NIH
An author of the retracted Nature papers on STAP cells (stimulus-triggered acquisition of pluripotency cells) has committed suicide at the age of 52.
Yoshiki Sasai, MD, PhD, was found dead at the RIKEN Center for Developmental Biology in Kobe, Japan, where he was deputy director.
Dr Sasai reportedly hanged himself and left several suicide notes.
Members of the scientific community have expressed shock and sadness upon learning of Dr Sasai’s death.
RIKEN President Ryoji Noyori, PhD, said he was “overcome with grief” when he heard the unfortunate news.
“The scientific world has lost a talented and dedicated researcher, who earned our deep respect for the advanced research he carried out over many years,” Dr Noyori said.
Nature’s editor-in-chief, Phil Campbell, PhD, echoed that sentiment, saying, “Yoshiki Sasai was an exceptional scientist, and he has left an extraordinary legacy of pioneering work across many fields within stem cell and developmental biology.”
Dr Sasai was a respected expert on embryonic stem cells, but the STAP cell scandal damaged his reputation and reportedly took a toll on his health. According to a spokesperson at RIKEN, Dr Sasai was hospitalized for stress and required counseling in the wake of the scandal.
Dr Sasai had worked closely with the lead author of the STAP cell papers, Haruko Obokata, PhD, although he said his main duty was editing the papers.
The papers, an article and a letter, were published in Nature in January. They recounted the creation of STAP cells—inducing pluripotency in somatic cells by exposing them to a low-pH environment.
Not long after the papers were published, members of the scientific community began to question the validity of the research. They voiced concerns about published images, possible plagiarism, and an inability to replicate the experiments described.
So RIKEN launched an investigation. In April, the investigative committee concluded that Dr Obokata was guilty of research misconduct, while Dr Sasai and another author from RIKEN, Teruhiko Wakayama, PhD, were guilty of negligence.
RIKEN also said the researchers would be disciplined, although details were not released.
At a subsequent news conference, Dr Sasai said the Nature papers should be retracted because of the errors and inconsistencies, but the data do indicate the STAP cell phenomenon is real.
Likewise, Dr Obokata insisted the phenomenon is real and appealed the findings of RIKEN’s investigation. But RIKEN said another investigation was not warranted and called for a retraction of the papers. In July, Nature published retractions.
A RIKEN group is still attempting to recreate the STAP cell phenomenon, with Dr Obokata’s help. RIKEN plans to release an interim report on this attempt soon.
Other researchers said they have tried and failed to replicate the STAP cell experiments. One group reported their failed attempt in F1000Research. ![]()

Credit: NIH
An author of the retracted Nature papers on STAP cells (stimulus-triggered acquisition of pluripotency cells) has committed suicide at the age of 52.
Yoshiki Sasai, MD, PhD, was found dead at the RIKEN Center for Developmental Biology in Kobe, Japan, where he was deputy director.
Dr Sasai reportedly hanged himself and left several suicide notes.
Members of the scientific community have expressed shock and sadness upon learning of Dr Sasai’s death.
RIKEN President Ryoji Noyori, PhD, said he was “overcome with grief” when he heard the unfortunate news.
“The scientific world has lost a talented and dedicated researcher, who earned our deep respect for the advanced research he carried out over many years,” Dr Noyori said.
Nature’s editor-in-chief, Phil Campbell, PhD, echoed that sentiment, saying, “Yoshiki Sasai was an exceptional scientist, and he has left an extraordinary legacy of pioneering work across many fields within stem cell and developmental biology.”
Dr Sasai was a respected expert on embryonic stem cells, but the STAP cell scandal damaged his reputation and reportedly took a toll on his health. According to a spokesperson at RIKEN, Dr Sasai was hospitalized for stress and required counseling in the wake of the scandal.
Dr Sasai had worked closely with the lead author of the STAP cell papers, Haruko Obokata, PhD, although he said his main duty was editing the papers.
The papers, an article and a letter, were published in Nature in January. They recounted the creation of STAP cells—inducing pluripotency in somatic cells by exposing them to a low-pH environment.
Not long after the papers were published, members of the scientific community began to question the validity of the research. They voiced concerns about published images, possible plagiarism, and an inability to replicate the experiments described.
So RIKEN launched an investigation. In April, the investigative committee concluded that Dr Obokata was guilty of research misconduct, while Dr Sasai and another author from RIKEN, Teruhiko Wakayama, PhD, were guilty of negligence.
RIKEN also said the researchers would be disciplined, although details were not released.
At a subsequent news conference, Dr Sasai said the Nature papers should be retracted because of the errors and inconsistencies, but the data do indicate the STAP cell phenomenon is real.
Likewise, Dr Obokata insisted the phenomenon is real and appealed the findings of RIKEN’s investigation. But RIKEN said another investigation was not warranted and called for a retraction of the papers. In July, Nature published retractions.
A RIKEN group is still attempting to recreate the STAP cell phenomenon, with Dr Obokata’s help. RIKEN plans to release an interim report on this attempt soon.
Other researchers said they have tried and failed to replicate the STAP cell experiments. One group reported their failed attempt in F1000Research. ![]()

Credit: NIH
An author of the retracted Nature papers on STAP cells (stimulus-triggered acquisition of pluripotency cells) has committed suicide at the age of 52.
Yoshiki Sasai, MD, PhD, was found dead at the RIKEN Center for Developmental Biology in Kobe, Japan, where he was deputy director.
Dr Sasai reportedly hanged himself and left several suicide notes.
Members of the scientific community have expressed shock and sadness upon learning of Dr Sasai’s death.
RIKEN President Ryoji Noyori, PhD, said he was “overcome with grief” when he heard the unfortunate news.
“The scientific world has lost a talented and dedicated researcher, who earned our deep respect for the advanced research he carried out over many years,” Dr Noyori said.
Nature’s editor-in-chief, Phil Campbell, PhD, echoed that sentiment, saying, “Yoshiki Sasai was an exceptional scientist, and he has left an extraordinary legacy of pioneering work across many fields within stem cell and developmental biology.”
Dr Sasai was a respected expert on embryonic stem cells, but the STAP cell scandal damaged his reputation and reportedly took a toll on his health. According to a spokesperson at RIKEN, Dr Sasai was hospitalized for stress and required counseling in the wake of the scandal.
Dr Sasai had worked closely with the lead author of the STAP cell papers, Haruko Obokata, PhD, although he said his main duty was editing the papers.
The papers, an article and a letter, were published in Nature in January. They recounted the creation of STAP cells—inducing pluripotency in somatic cells by exposing them to a low-pH environment.
Not long after the papers were published, members of the scientific community began to question the validity of the research. They voiced concerns about published images, possible plagiarism, and an inability to replicate the experiments described.
So RIKEN launched an investigation. In April, the investigative committee concluded that Dr Obokata was guilty of research misconduct, while Dr Sasai and another author from RIKEN, Teruhiko Wakayama, PhD, were guilty of negligence.
RIKEN also said the researchers would be disciplined, although details were not released.
At a subsequent news conference, Dr Sasai said the Nature papers should be retracted because of the errors and inconsistencies, but the data do indicate the STAP cell phenomenon is real.
Likewise, Dr Obokata insisted the phenomenon is real and appealed the findings of RIKEN’s investigation. But RIKEN said another investigation was not warranted and called for a retraction of the papers. In July, Nature published retractions.
A RIKEN group is still attempting to recreate the STAP cell phenomenon, with Dr Obokata’s help. RIKEN plans to release an interim report on this attempt soon.
Other researchers said they have tried and failed to replicate the STAP cell experiments. One group reported their failed attempt in F1000Research. ![]()
Readmissions in Primary Care Clinics
Reducing hospital readmissions is a national healthcare priority. In October 2012, the Centers for Medicare and Medicaid Services (CMS) enacted financial penalties on hospitals with higher than average risk‐adjusted readmissions, offering an incentive pool of $850 million in the first year.[1] As a result, a wide range of activities to understand and reduce readmissions among patients with congestive heart failure (CHF), acute myocardial infarction (AMI), and pneumonia have emerged.[2, 3, 4, 5] Some have proposed that to effectively reduce hospital readmissions, a community of inpatient and outpatient providers and local support organizations must coordinate efforts.[6, 7] In fact, CMS has recognized the important role of community support groups and outpatient providers in safe discharge transitions in 2 ways. First, in 2011 CMS launched the Community‐based Care Transitions Program to fund community‐based organizations to assist Medicare patients with care transitions.[8] Second, CMS introduced 2 new reimbursement codes for primary care providers (PCPs) to perform care coordination immediately after hospital discharge.[9] Both of these new payment programs represent an evolving perspective that reducing hospital readmissions requires active participation among outpatient partners.
As leaders of the outpatient care team, PCPs play a significant role in reducing hospital readmissions. One way in which PCPs can begin to understand the magnitude of the issue within their clinic is to evaluate the clinic's 30‐day readmission rates. Currently, CMS calculates readmission rates at the hospital level. However, understanding these rates at the clinic level is critical for developing strategies for improvement across the care continuum. Our current understanding of effective outpatient interventions to reduce hospital readmission is limited.[3] As clinics introduce and refine strategies to reduce readmissions, tracking the impact of these strategies on readmission rates will be critical for identifying effective outpatient interventions. Clinics with similar patient case‐mix can also benchmark readmission rates, sharing best practices from clinics with lower‐than‐expected rates.
There are no available studies or proposed methodologies to guide primary care clinics in calculating their 30‐day readmission rates. A particularly difficult challenge is obtaining the admission information when patients may be admitted to 1 of several area hospitals. For large integrated delivery networks where primary care patients are relatively loyal to the network of physicians and hospitals, an opportunity exists to explore the data. Furthermore, variations in readmission rates across primary care specialties (such as internal medicine and family practice) are not well understood. In this study, we set out to develop a methodology for calculating all‐cause 30‐day hospital readmission rates at the level of individual primary care practices and to identify factors associated with variations in these rates.
METHODS
Study Design
We conducted a retrospective observational study of adult primary care patients at the University of California, San Francisco (UCSF) who were hospitalized at UCSF Medical Center between July 1, 2009 and June 30, 2012. UCSF Medical Center is comprised of Moffitt‐Long Hospital (a 600‐bed facility) and UCSF‐Mount Zion Hospital (a 90‐bed facility) located in San Francisco, CA. The patient population was limited to adults ages 18 and over with a PCP at UCSF. UCSF has 7 adult primary care clinics: General Internal Medicine (IM), Family Practice (FP), Women's Health, Geriatrics, a combined IM/FP clinic, Human Immunodeficiency Virus (HIV) Primary Care, and a Concierge Internal Medicine clinic staffed by IM physicians. Between 2009 and 2011, all clinics completed the process of enpanelment, or defining the population of patients for which each PCP and the clinic is responsible. We obtained the list of patient and PCP assignments at each of the 7 clinics across the time period of study. We then obtained UCSF Medical Center hospital claims data for this group, including dates of admission and discharge, patient age, sex, race/ethnicity, language, insurance, admitting service, diagnosis codes, information on intensive care unit stay, and discharge disposition. Hospital claims data are housed in Transition Systems International (Boston, MA) administrative databases, a cost‐accounting system that collects data abstracted from patient charts upon discharge from UCSF Medical Center.
All‐cause 30‐day hospital readmission rates were calculated for each primary care clinic, using an adaptation of the CMS definition. First, we defined index discharges as the first discharge for an individual patient in any given 30‐day interval. Only 1 index discharge is flagged for each 30‐day interval. The first admission within 30 days after the index discharge was flagged as the readmission. Consistent with CMS methodology, only the first readmission in the 30‐day period was counted. We included all inpatient and observation status admissions and excluded patients who died during the index encounter, left against medical advice, or transferred to another acute care hospital after the index encounter.
We used secondary diagnosis codes in the administrative data to classify comorbidities by the Elixhauser methodology.[10] All but 1 adult primary care clinic at UCSF are faculty‐only clinics, whereby the assigned PCP is an attending physician. In the general internal medicine clinic, an attending or a resident can serve as the PCP, and approximately 20% of IM clinic patients have a resident as their PCP. For the IM clinic, we classified patients' PCP as attending, resident, or departed. The latter category refers to patients whose PCPs were residents who had graduated or faculty who had departed and had not been assigned to a new PCP prior to the index admission or readmission.
Statistical Analysis
We built a model to predict readmissions using the demographic and clinical variables with [2] P<0.20 in an initial bivariate analysis, and then removed, with backward selection, the least significant variables until only those with P0.05 remained. Age, log‐length of stay (LOS), and intensive care unit stay were forced in the model, as studies evaluating factors related to readmissions have often included these as important covariates.[11, 12, 13, 14] Results were expressed as adjusted odds ratio (OR) with 95% confidence interval (CI). All analyses were carried out using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
This study was exempt from review by the institutional review board of UCSF.
RESULTS
During the study period, there were 12,564 discharges from UCSF Medical Center for primary care patients belonging to the 7 clinics. Of these, 8685 were index discharges and 1032 were readmissions within 30 days. Table 1 shows the characteristics of the patients who had at least 1 admission during the study period. In all but 2 clinics (HIV Primary Care and Concierge Internal Medicine), there were more women hospitalized than men. Age and gender differences across clinics are consistent with the patient populations served by these clinics, with Women's Health having more female patients and younger patients hospitalized compared to Geriatrics.
| General Internal Medicine | Family Practice | Women's Health | Geriatrics | Combined IM/FP | HIV Primary Care | Concierge IM | |
|---|---|---|---|---|---|---|---|
| |||||||
| Panel size | 26,521 | 10,495 | 8,526 | 190 | 4,109 | 1,039 | 706 |
| FTE attending MD and NP providers | 18.8 | 7.3 | 4.5 | 1.7 | 5.0 | 2.3 | 2.1 |
| Mean panel size per FTE | 1,311 | 1,448 | 1,895 | 112 | 822 | 452 | 336 |
| Clinic visits in FY 2012 | 50,362 | 20,647 | 11,014 | 4,425 | 8,120 | 4,182 | 1,713 |
| No. of index discharges | 5,388 | 1,204 | 983 | 409 | 339 | 249 | 113 |
| No. of readmissions | 718 | 104 | 76 | 56 | 30 | 37 | 11 |
| No. of patients discharged during study period | 4,063 | 1,003 | 818 | 289* | 290 | 185 | 84 |
| % Male | 40.1% | 33.9% | 4.3% | 35.3% | 33.8% | 69.7% | 52.4% |
| Average age (SD), y | 60.4 (18.6) | 52.0 (19.0) | 47.0 (15.7) | 83.2 (6.9) | 46.3 (16.9) | 49.5 (9.9) | 64.5 (15.6) |
| Age range, y | 19104 | 1896 | 1997 | 6399 | 1890 | 2273 | 2092 |
| Race | |||||||
| % White | 42.2% | 41.3% | 58.8% | 61.9% | 49.3% | 61.0% | 91.7% |
| % Black | 16.3% | 8.5% | 7.5% | 7.3% | 10.0% | 27.6% | 0.0% |
| % Asian | 23.6% | 31.4% | 19.0% | 17.0% | 16.6% | 2.2% | 4.8% |
| % Native America/ Alaskan Native | 0.7% | 1.6% | 0.7% | 0.7% | 0.7% | 0.5% | 0.0% |
| % Other | 16.3% | 15.7% | 13.1% | 12.5% | 18.3% | 8.1% | 2.4% |
| Not available | 1.0% | 1.6% | 1.0% | 0.7% | 5.2% | 0.5% | 1.2% |
| Ethnicity | |||||||
| % Hispanic | 8.8% | 10.6% | 5.0% | 6.2% | 8.3% | 4.9% | 0.0% |
| % Non‐Hispanic | 73.8% | 74.5% | 81.8% | 73.0% | 74.5% | 84.9% | 75.0% |
| Not available | 17.4% | 15.0% | 13.2% | 20.8% | 17.2% | 10.3% | 25.0% |
| Language | |||||||
| % English | 69.7% | 75.6% | 88.8% | 69.6% | 85.9% | 89.7% | 89.3% |
| % Spanish | 3.8% | 1.9% | 0.6% | 4.2% | 1.0% | 0.5% | 0.0% |
| % Chinese (Mandarin or Cantonese) | 8.0% | 6.7% | 1.2% | 4.8% | 2.1% | 0.0% | 0.0% |
| % Russian | 1.7% | 0.6% | 0.1% | 0.4% | 1.0% | 0.0% | 0.0% |
| % Vietnamese | 1.0% | 1.4% | 0.0% | 0.4% | 0.0% | 0.0% | 0.0% |
| % Other | 11.7% | 9.3% | 5.6% | 18.0% | 6.9% | 8.1% | 4.8% |
| Not available | 4.1% | 4.6% | 3.7% | 2.8% | 3.1% | 1.6% | 6.0% |
| Insurance type | |||||||
| % Private | 35.4% | 58.7% | 77.4% | 10.7% | 63.8% | 36.8% | 66.7% |
| % Medicare | 22.0% | 17.2% | 12.0% | 67.8% | 14.8% | 8.7% | 33.3% |
| % Medicaid | 15.5% | 12.5% | 3.1% | 1.4% | 12.8% | 24.3% | 0.0% |
| % Dual eligible | 24.4% | 8.8% | 5.4% | 19.4% | 6.6% | 27.6% | 0.0% |
| % Self‐pay/other | 2.6% | 2.9% | 2.2% | 0.7% | 2.1% | 2.7% | 0.0% |
All‐cause 30‐day readmission rates varied across practices, with HIV Primary Care being the highest at 14.9%, followed by Geriatrics at 13.7%, General Internal Medicine at 13.3%, Concierge Internal Medicine at 9.7%, combined IM/FP at 8.9%, Family Practice at 8.6%, and Women's Health at 7.7% (Figure 1). Despite HIV Primary Care having the highest readmission rate, the number of index discharges during the 3‐year period was relatively low (249) compared to General Internal Medicine (5388). For the index admission, the top 5 admitting services were medicine, obstetrics, cardiology, orthopedic surgery, and general surgery (Table 2). Medicine was the primary admitting service for patients in the following clinics: General Internal Medicine, Geriatrics, HIV Primary Care, and Concierge Internal Medicine. Obstetrics was the primary admitting service for patients in the Family Practice, Women's Health, and combined IM/FP clinics. Vaginal delivery was the top discharge diagnosis‐related group (DRG) for patients in the General Internal Medicine, Family Practice, Women's Health, and combined IM/FP clinics. The top discharge DRG was urinary tract infection for Geriatrics, HIV for the HIV clinic (13.6%), and chemotherapy (8.0%) for Concierge Internal Medicine. Average LOS varied from 4.7 days for patients in the HIV Primary Care clinic to 2.8 days for patients in the Concierge Internal Medicine clinic. Average LOS was 3.4, 3.1, and 3.2 days in the Family Practice, Women's Health, and Geriatrics clinics, respectively, and 3.8 days in the General Internal Medicine and combined IM/FP clinics. For all clinics except Geriatrics, the majority of patients were discharged home without home health. A larger proportion of patients in the Geriatrics clinic were discharged home with home health or discharged to a skilled nursing facility.
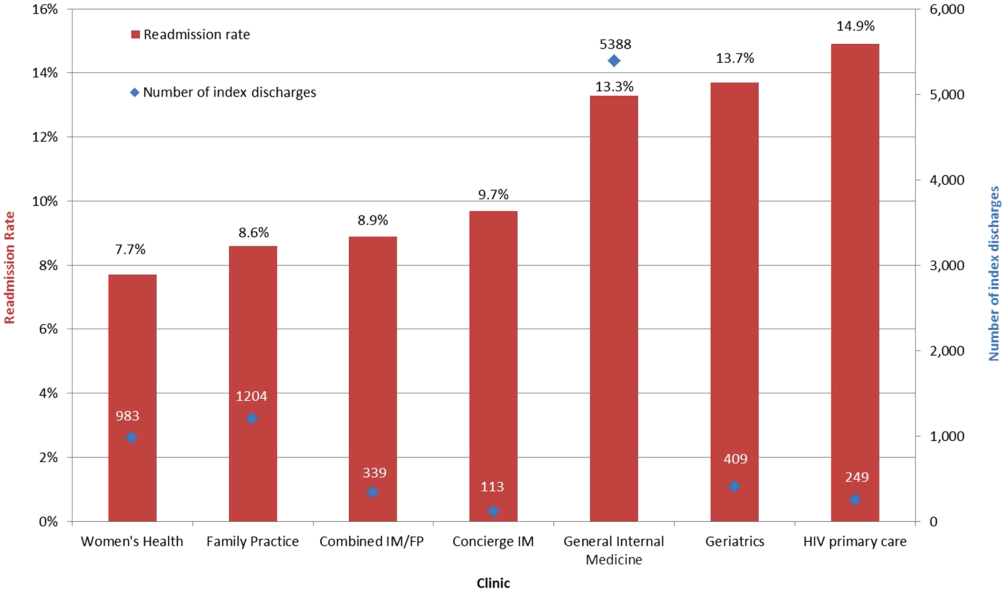
| General Internal Medicine, N=5,388 | Family Practice, N=1,204 | Women's Health, N=983 | Geriatrics, N=409 | Combined IM/FP, N=339 | HIV Primary Care, N=249 | Concierge IM, N=113 | |
|---|---|---|---|---|---|---|---|
| |||||||
| Top 5 admitting services | |||||||
| Medicine | 41.8% | 25.0% | 15.2% | 56.4% | 23.5% | 59.7% | 19.0% |
| Obstetrics | 7.5% | 24.7% | 38.7% | 0.0% | 35.6% | 2.1% | 9.0% |
| Cardiology | 15.1% | 10.9% | 6.2% | 16.5% | 10.3% | 6.5% | 17.0% |
| Orthopedic surgery | 8.3% | 8.3% | 8.2% | 7.3% | 5.3% | 4.8% | 12.0% |
| Adult general surgery | 7.2% | 9.8% | 10.0% | 6.0% | 5.0% | 5.8% | 7.0% |
| Top 5 discharge diagnoses | |||||||
| Vaginal delivery | 3.5% | 13.6% | 20.6% | 0.0% | 17.9% | 0.3% | 3.0% |
| Major joint replacement | 3.5% | 3.4% | 4.8% | 4.3% | 3.8% | 0.7% | 5.0% |
| Vaginal delivery with complications | 1.3% | 5.0% | 6.9% | 0.0% | 4.1% | 1.0% | 2.0% |
| Simple pneumonia and pleurisy | 2.8% | 1.2% | 0.6% | 3.8% | 0.3% | 2.1% | 0.0% |
| Urinary tract infection | 2.0% | 1.5% | 1.4% | 6.2% | 0.9% | 2.4% | 1.0% |
| Discharge disposition | |||||||
| % Home | 69.1% | 74.6% | 76.6% | 47.8% | 69.9% | 78.6% | 68.4% |
| % Home with home health | 19.4% | 16.5% | 17.8% | 24.1% | 21.7% | 11.8% | 18.8% |
| % Skilled nursing facility | 7.2% | 4.9% | 3.0% | 18.9% | 3.3% | 5.0% | 0.8% |
| % Other | 4.3% | 4.0% | 2.6% | 9.2% | 5.1% | 4.6% | 12.0% |
| Average length of stay (SD) | 3.8 (5.6) | 3.4 (7.5) | 3.1 (3.7) | 3.2 (3.7) | 3.8 (5.2) | 4.7 (6.5) | 2.8 (2.8) |
| % Discharges with ICU stay | 11.0% | 10.6% | 5.9% | 8.5% | 9.2% | 11.1% | 15.8% |
Factors associated with variation in readmission rates included: male gender (OR: 1.21, 95% CI: 1.051.40), Medicare (OR: 1.31, 95% CI: 1.051.64; Ref=private) and dual‐eligible Medicare‐Medicaid insurance (OR: 1.26, 95% CI: 1.011.56), unknown primary language (OR: 0.06, 95% CI: 0.020.25; Ref=English), and the following comorbidities: peripheral vascular disease, renal failure, lymphoma, fluid and electrolyte disorders, and anemia. Multivariable logistic regression modeling results are listed in Table 3. Patients having a resident PCP showed no increased odds of readmission (OR: 1.13, 95% CI: 0.931.37; Ref=attending PCP). However, patients with a graduated resident PCP or departed faculty PCP awaiting transfer to a new PCP had an OR of 1.59 (95% CI: 1.162.17) compared with having a current faculty PCP. The C‐statistic for this model was 0.67.
| Adjusted Odds Ratio (95% CI) | |
|---|---|
| |
| Age | |
| <65 years | Ref |
| >65 years | 0.91 (0.751.10) |
| Clinic | |
| General Internal Medicine | 1.24 (0.991.56) |
| Family Practice | Ref |
| Women's Health | 1.13 (0.821.56) |
| Geriatrics | 1.20 (0.841.74) |
| Combined Internal Medicine/Family Practice | 1.13 (0.731.74) |
| HIV Primary Care | 1.24 (0.811.89) |
| Concierge Internal Medicine | 1.06 (0.542.07) |
| Sex | |
| Female | Ref |
| Male | 1.21 (1.051.40) |
| Insurance | |
| Private | Ref |
| Medicaid | 1.09 (0.871.37) |
| Medicare | 1.31 (1.051.64) |
| Dual eligible MedicareMedicaid | 1.26 (1.011.56) |
| Self/other | 1.20 (0.741.94) |
| Language | |
| English | Ref |
| Spanish | 1.02 (0.701.47) |
| Chinese | 1.13 (0.871.47) |
| Other | 0.87 (0.701.08) |
| Unknown | 0.06 (0.020.25) |
| Comorbidities | |
| Pulmonary circulation disease | 1.51 (0.982.32) |
| Peripheral vascular disease | 1.50 (1.141.98) |
| Renal failure | 1.31 (1.091.58) |
| Lymphoma | 2.50 (1.434.36) |
| Fluid and electrolyte disorders | 1.27 (1.071.52) |
| Deficiency anemia | 1.25 (1.041.51) |
| Physician | |
| Attending | Ref |
| Resident | 1.13 (0.931.37) |
| Departed PCP (internal medicine only) | 1.59 (1.162.17) |
| ICU stay | 1.03 (0.831.28) |
| LOS (log) | 1.02 (0.981.06) |
| Admitting service | |
| Medicine | Ref |
| Obstetrics | 0.41 (0.300.56) |
| Cardiology | 1.12 (0.911.37) |
| Orthopedic surgery | 0.36 (0.250.51) |
| Adult general surgery | 0.64 (0.470.87) |
| Other services | 0.76 (0.630.92) |
DISCUSSION
In this study we introduce a complementary way to view hospital readmissions, from the perspective of primary care practices. We found variation in readmission rates across primary care practices. After controlling for admitting service, clinic, provider, and patient factors, the specific characteristics of male gender, patients with Medicare or Medicare with Medicaid, and patients in our General Internal Medicine clinic with a departed PCP were independent risk factors for hospital readmission. Patients with specific comorbidities were also at increased risk for admissions, including those with peripheral vascular disease, renal failure, lymphoma, fluid and electrolyte disorders, and anemia. Because this is the first study viewing readmissions from the perspective of primary care practices, our findings are unique in the literature. However, hospital‐based studies have found similar relationships between readmission rates and these specific comorbidities.[11, 12, 15] Unlike other studies, our study cohort did not show CHF as an independent risk factor. We hypothesize this is because in 2008, UCSF Medical Center introduced an inpatient‐based intervention to reduce readmissions in patients with heart failure. By 2011, the readmission rates of patients with heart failure (primary or secondary diagnoses) had dropped by 30%. The success of this program, focused only on patients with heart failure, likely affected our analysis of comorbidities as risk factors for readmissions.
Models developed to predict hospital readmissions, overall and for specific disease conditions, have inconsistently identified predictive factors, and there is not a specific set of variables that dominate.[16, 17, 18, 19] A recent review of readmission risk prediction models suggested that models that take into account psychosocial factors such as social support, substance abuse, and functional status improved model performance.[16] We hypothesize that the reason why male gender was significant in our model may be related to lack of social support, especially among those who may be single or widowed. Other studies have also showed male gender as a predictor for hospital readmissions.[14, 20]
People with Medicare as the primary payor or Medicare with Medicaid also tended to have higher risk of readmission. We believe that this may be a proxy for the combined effect of age, multiple comorbidities, and psychosocial factors. In a multicenter study of general medicine inpatients, Medicare, but not age, was also found to be a predictive variable.[21] Unlike other studies, our study did not find Medicaid alone as a significant predictive variable for readmissions.[15, 21] One explanation may be that in hospital‐based studies, people with Medicaid who are at high risk for readmission may be high‐risk because they do not have a PCP or good access to outpatient care. In our study, all patients have a PCP in 1 of the UCSF clinics, and access to care is improved with this established relationship. These other studies did not examine Medicare‐Medicaid dual eligibility status. Our results are consistent with a national study on avoidable hospital admissions that showed the dual eligibility population experiencing 60% higher avoidable admission rates compared to the Medicaid‐only population.[22]
An interesting finding was that there were no statistically significant differences in readmissions among patients who report a language other than English as their primary language. Although language barriers and health literacy can affect a patient's ability to understand discharge instructions, the use of translators at UCSF Medical Center may have decreased the risk of readmissions. For a small number of patients whose primary language was not recorded (unknown language in our model), they appeared to have a lower risk of readmissions. Language preferences are recorded by our admitting staff during the process of admission. This step may have been skipped after hours or if the patient was not able to answer the question. However, we do not have a good hypothesis as to why these patients may have a reduced risk of readmissions.
Having a departed PCP in the General Internal Medicine clinic was an independent predictor of readmissions. These patients have access to primary care; they can schedule acute appointments with covering providers for new medical issues or follow‐up of chronic conditions. However, until they are transferred to a new PCP, they do not have a provider who is proactively managing their preventive and chronic disease care, including follow‐up and coordinating care after hospital discharge. Our study is not the first to suggest adverse outcomes for patients who are in transition from 1 primary care provider to the next.[23] Studies conducted in General Internal Medicine clinics have shown missed opportunities for cancer screening and overlooked test results during the transition period,[24] and as many as one‐fifth of patients whom residents identified as high‐risk were lost to follow‐up.[25] However, our study is the first to show the link between PCP transition in a teaching clinic and hospital readmissions. This finding underscores the importance of continuity of care in the optimal management of patients following hospital discharge.
There are several limitations to this study. First, our study only considers hospitalizations to UCSF Medical Center, potentially undercounting readmissions to area hospitals. Because our study population are patients with PCPs at UCSF, these patients tend to seek specialty and acute care at UCSF Medical Center as well. We obtained payor data from our medical group (Hill Physicians), which covers our largest private payors, to understand whether our results can be applied on a global basis. We found that in 87.4% of index admissions with readmissions from January 1, 2010 through May 30, 2012, the readmission occurred at UCSF Medical Center. We conducted a similar analysis with CMS data from October 1, 2008 to June 30, 2011, the latest data we have from CMS. For patients with UCSF PCPs and a diagnosis of AMI or CHF, 100% were readmitted back to UCSF Medical Center. For patients with UCSF PCPs and a diagnosis of pneumonia, 89% were readmitted back to UCSF Medical Center. Given that only a small percentage of patients with PCPs at UCSF present for readmission at other area hospitals, we believe that limiting our analysis to UCSF Medical Center is reasonable.
In our study, we did not remove vaginal deliveries or Caesarian sections prior to building the model. Primary care physicians and their clinic leadership are accustomed to taking a population‐health perspective. We anticipate they would be interested in designing interventions and addressing readmission for the entire primary care panel. Although readmissions after delivery are not frequent, they can still occur, and interventions should not necessarily exclude this population. We did run a sensitivity analysis by removing vaginal delivery and Caesarian sections from the analysis. As expected, readmission rates for all practices increased except for Geriatrics. However, the independent predictors of readmissions did not change.
Our study is based on a population of patients at 1 urban academic medical center and may not be generalizable across all delivery systems. Our population is racially and ethnically diverse, and many do not speak English as their primary language. The study also spans different types of primary care clinics, capturing a wide range of ages and case mix. As PCP assignments fluctuate over time, there may be errors with PCP attribution in the UCSF Medical Center data systems. We do not believe errors in PCP attribution would differ across primary care practices. Because the primary care practices' performance reports on quality measures are based on PCP assignment, each clinic regularly updates their clinic panels and has specific protocols to address errors with PCP attribution.
Finally, our study includes only the variables that we were able to extract from administrative claims. Other explanatory variables that have been suggested as important for evaluation, such as social support, functional assessment, access to care, hospital discharge process, and posthospitalization follow‐up, were not included. Each of these could be explored in future studies.
This study offers a unique perspective of hospital readmission by introducing a new methodology for primary care clinics to calculate and evaluate their all‐cause 30‐day readmission rates. Although this methodology is not intended to provide real‐time feedback to clinics on readmitted patients, it opens the door for benchmarking based on specific case‐mix indices. Another direction for future research is to design robust evaluations of the impact of interventions, both inpatient and outpatient, on primary care clinic readmission rates. Finally, future research should replicate this analysis across teaching clinics to identify whether provider turnover is a consistent independent predictor of hospital readmissions.
This study also has implications for inpatient interventions. For example, discharging physicians may consider proactively identifying whether the patient has a continuous primary care provider. Patients who are in between PCPs may need closer follow‐up after discharge, until they re‐establish with a new PCP. This can be accomplished through a postdischarge clinic visit, either run by inpatient providers or covering physicians in the primary care clinic. In addition, the discharging physician may work with the case manager to increase the level of care coordination. The case manager could contact the primary care clinic and proactively ask for immediate PCP re‐assignment. Once a new PCP has been identified, the discharging physician could consider a warm hand‐off. With a warm hand‐off, the new PCP may feel more comfortable managing problems that may arise after hospital discharge, and especially before the first outpatient visit with the new PCP. Future research can test whether these interventions could effectively reduce hospital readmissions across a broad primary care population.
CONCLUSION
Primary care providers and their clinics play an important role in managing population health, decreasing healthcare spending, and keeping patients out of the hospital. In this study, we introduce a tool in which primary care clinics can begin to understand their hospital readmission rates. This may be particularly valuable as primary care providers enter global payment arrangements such as accountable care organizations or bundled payments and are responsible for a population of patients across the continuum of care. We found significant variation in readmission rates between different primary care practices, but much of this variation appears to be due to differences between practices in patient demographics, comorbidities, and hospitalization factors. Our study is the first to show the association between provider transitions and higher hospital readmissions. Continuity of care is critical for the optimal management of patients following hospital discharge. More attention will need to be focused on providing good continuity outpatient care for patients at high risk for readmissions.
Acknowledgements
The authors acknowledge Janelle Lee, MBA, MHA, DrPH, for her assistance with extracting the hospital claims data at UCSF Medical Center.
Disclosure: Dr. Tang and Ms. Maselli were supported by a University of California, Center for Health Quality and Innovation grant. The authors report no conflicts of interest.
- , . Medicare's new hospital value‐based purchasing program is likely to have only a small impact on hospital payments. Health Aff. 2012;31:1932–1940.
- , , , et al. Contemporary evidence about hospital strategies for reducing 30‐day readmissions. J Am Coll Cardiol. 2012;60:607–614.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Reducing hospital readmissions: lessons from top‐performing hospitals. The Commonwealth Fund Synthesis Report. Available at: http://www.commonwealthfund.org/publications/case‐studies/2011/apr/reducing‐hospital‐readmissions. Published April 6, 2011. Accessed February 8, 2013.
- , . Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
- , , . Recasting readmissions by placing the hospital role in community context. JAMA. 2013;309:351–352.
- , , , . Hospitalists and care transitions: the divorce of inpatient and outpatient care. Health Affairs. 2008;27:1315–1327.
- Center for Medicare and Medicaid Innovation. Community‐based Care Transitions Program. Available at: http://innovation.cms.gov/initiatives/CCTP/#collapse‐tableDetails. Accessed February 8, 2013.
- , , . Medicare's transitional care payment—a step toward the medical home. N Engl J Med. 2013;368:692–694.
- , , . Volume thresholds and hospital characteristics in the United States. Health Aff (Millwood). 2003;22:167–177.
- , , , , , . Thirty‐day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157:11–18.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157:837–845.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54–60.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;206:1688–1698.
- , , , et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386.
- , , , . Statistical models and patient predictors of readmission for acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes. 2009;2:500–507.
- , , , et al. Predictors of hospital readmission after stroke: a systematic review. Stroke. 2010;41:2525–2533.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21:363–372.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25:211–219.
- , , . Users of Medicaid home and community‐based services are especially vulnerable to costly avoidable hospital admissions. Health Affairs. 2012;31:1167–1175.
- , . Academic year‐end transfers of outpatients from outgoing to incoming residents: an unaddressed patient safety issue. JAMA. 2009;302:1327–1329.
- , , , , , . The revolving door of resident continuity practice: identifying gaps in transitions of care. J Gen Intern Med. 2011;26:995–998.
- , , , et al. Outcomes for resident‐identified high‐risk patients and resident perspectives of year‐end continuity clinic handoffs. J Gen Intern Med. 2012;27:1438–1444.
Reducing hospital readmissions is a national healthcare priority. In October 2012, the Centers for Medicare and Medicaid Services (CMS) enacted financial penalties on hospitals with higher than average risk‐adjusted readmissions, offering an incentive pool of $850 million in the first year.[1] As a result, a wide range of activities to understand and reduce readmissions among patients with congestive heart failure (CHF), acute myocardial infarction (AMI), and pneumonia have emerged.[2, 3, 4, 5] Some have proposed that to effectively reduce hospital readmissions, a community of inpatient and outpatient providers and local support organizations must coordinate efforts.[6, 7] In fact, CMS has recognized the important role of community support groups and outpatient providers in safe discharge transitions in 2 ways. First, in 2011 CMS launched the Community‐based Care Transitions Program to fund community‐based organizations to assist Medicare patients with care transitions.[8] Second, CMS introduced 2 new reimbursement codes for primary care providers (PCPs) to perform care coordination immediately after hospital discharge.[9] Both of these new payment programs represent an evolving perspective that reducing hospital readmissions requires active participation among outpatient partners.
As leaders of the outpatient care team, PCPs play a significant role in reducing hospital readmissions. One way in which PCPs can begin to understand the magnitude of the issue within their clinic is to evaluate the clinic's 30‐day readmission rates. Currently, CMS calculates readmission rates at the hospital level. However, understanding these rates at the clinic level is critical for developing strategies for improvement across the care continuum. Our current understanding of effective outpatient interventions to reduce hospital readmission is limited.[3] As clinics introduce and refine strategies to reduce readmissions, tracking the impact of these strategies on readmission rates will be critical for identifying effective outpatient interventions. Clinics with similar patient case‐mix can also benchmark readmission rates, sharing best practices from clinics with lower‐than‐expected rates.
There are no available studies or proposed methodologies to guide primary care clinics in calculating their 30‐day readmission rates. A particularly difficult challenge is obtaining the admission information when patients may be admitted to 1 of several area hospitals. For large integrated delivery networks where primary care patients are relatively loyal to the network of physicians and hospitals, an opportunity exists to explore the data. Furthermore, variations in readmission rates across primary care specialties (such as internal medicine and family practice) are not well understood. In this study, we set out to develop a methodology for calculating all‐cause 30‐day hospital readmission rates at the level of individual primary care practices and to identify factors associated with variations in these rates.
METHODS
Study Design
We conducted a retrospective observational study of adult primary care patients at the University of California, San Francisco (UCSF) who were hospitalized at UCSF Medical Center between July 1, 2009 and June 30, 2012. UCSF Medical Center is comprised of Moffitt‐Long Hospital (a 600‐bed facility) and UCSF‐Mount Zion Hospital (a 90‐bed facility) located in San Francisco, CA. The patient population was limited to adults ages 18 and over with a PCP at UCSF. UCSF has 7 adult primary care clinics: General Internal Medicine (IM), Family Practice (FP), Women's Health, Geriatrics, a combined IM/FP clinic, Human Immunodeficiency Virus (HIV) Primary Care, and a Concierge Internal Medicine clinic staffed by IM physicians. Between 2009 and 2011, all clinics completed the process of enpanelment, or defining the population of patients for which each PCP and the clinic is responsible. We obtained the list of patient and PCP assignments at each of the 7 clinics across the time period of study. We then obtained UCSF Medical Center hospital claims data for this group, including dates of admission and discharge, patient age, sex, race/ethnicity, language, insurance, admitting service, diagnosis codes, information on intensive care unit stay, and discharge disposition. Hospital claims data are housed in Transition Systems International (Boston, MA) administrative databases, a cost‐accounting system that collects data abstracted from patient charts upon discharge from UCSF Medical Center.
All‐cause 30‐day hospital readmission rates were calculated for each primary care clinic, using an adaptation of the CMS definition. First, we defined index discharges as the first discharge for an individual patient in any given 30‐day interval. Only 1 index discharge is flagged for each 30‐day interval. The first admission within 30 days after the index discharge was flagged as the readmission. Consistent with CMS methodology, only the first readmission in the 30‐day period was counted. We included all inpatient and observation status admissions and excluded patients who died during the index encounter, left against medical advice, or transferred to another acute care hospital after the index encounter.
We used secondary diagnosis codes in the administrative data to classify comorbidities by the Elixhauser methodology.[10] All but 1 adult primary care clinic at UCSF are faculty‐only clinics, whereby the assigned PCP is an attending physician. In the general internal medicine clinic, an attending or a resident can serve as the PCP, and approximately 20% of IM clinic patients have a resident as their PCP. For the IM clinic, we classified patients' PCP as attending, resident, or departed. The latter category refers to patients whose PCPs were residents who had graduated or faculty who had departed and had not been assigned to a new PCP prior to the index admission or readmission.
Statistical Analysis
We built a model to predict readmissions using the demographic and clinical variables with [2] P<0.20 in an initial bivariate analysis, and then removed, with backward selection, the least significant variables until only those with P0.05 remained. Age, log‐length of stay (LOS), and intensive care unit stay were forced in the model, as studies evaluating factors related to readmissions have often included these as important covariates.[11, 12, 13, 14] Results were expressed as adjusted odds ratio (OR) with 95% confidence interval (CI). All analyses were carried out using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
This study was exempt from review by the institutional review board of UCSF.
RESULTS
During the study period, there were 12,564 discharges from UCSF Medical Center for primary care patients belonging to the 7 clinics. Of these, 8685 were index discharges and 1032 were readmissions within 30 days. Table 1 shows the characteristics of the patients who had at least 1 admission during the study period. In all but 2 clinics (HIV Primary Care and Concierge Internal Medicine), there were more women hospitalized than men. Age and gender differences across clinics are consistent with the patient populations served by these clinics, with Women's Health having more female patients and younger patients hospitalized compared to Geriatrics.
| General Internal Medicine | Family Practice | Women's Health | Geriatrics | Combined IM/FP | HIV Primary Care | Concierge IM | |
|---|---|---|---|---|---|---|---|
| |||||||
| Panel size | 26,521 | 10,495 | 8,526 | 190 | 4,109 | 1,039 | 706 |
| FTE attending MD and NP providers | 18.8 | 7.3 | 4.5 | 1.7 | 5.0 | 2.3 | 2.1 |
| Mean panel size per FTE | 1,311 | 1,448 | 1,895 | 112 | 822 | 452 | 336 |
| Clinic visits in FY 2012 | 50,362 | 20,647 | 11,014 | 4,425 | 8,120 | 4,182 | 1,713 |
| No. of index discharges | 5,388 | 1,204 | 983 | 409 | 339 | 249 | 113 |
| No. of readmissions | 718 | 104 | 76 | 56 | 30 | 37 | 11 |
| No. of patients discharged during study period | 4,063 | 1,003 | 818 | 289* | 290 | 185 | 84 |
| % Male | 40.1% | 33.9% | 4.3% | 35.3% | 33.8% | 69.7% | 52.4% |
| Average age (SD), y | 60.4 (18.6) | 52.0 (19.0) | 47.0 (15.7) | 83.2 (6.9) | 46.3 (16.9) | 49.5 (9.9) | 64.5 (15.6) |
| Age range, y | 19104 | 1896 | 1997 | 6399 | 1890 | 2273 | 2092 |
| Race | |||||||
| % White | 42.2% | 41.3% | 58.8% | 61.9% | 49.3% | 61.0% | 91.7% |
| % Black | 16.3% | 8.5% | 7.5% | 7.3% | 10.0% | 27.6% | 0.0% |
| % Asian | 23.6% | 31.4% | 19.0% | 17.0% | 16.6% | 2.2% | 4.8% |
| % Native America/ Alaskan Native | 0.7% | 1.6% | 0.7% | 0.7% | 0.7% | 0.5% | 0.0% |
| % Other | 16.3% | 15.7% | 13.1% | 12.5% | 18.3% | 8.1% | 2.4% |
| Not available | 1.0% | 1.6% | 1.0% | 0.7% | 5.2% | 0.5% | 1.2% |
| Ethnicity | |||||||
| % Hispanic | 8.8% | 10.6% | 5.0% | 6.2% | 8.3% | 4.9% | 0.0% |
| % Non‐Hispanic | 73.8% | 74.5% | 81.8% | 73.0% | 74.5% | 84.9% | 75.0% |
| Not available | 17.4% | 15.0% | 13.2% | 20.8% | 17.2% | 10.3% | 25.0% |
| Language | |||||||
| % English | 69.7% | 75.6% | 88.8% | 69.6% | 85.9% | 89.7% | 89.3% |
| % Spanish | 3.8% | 1.9% | 0.6% | 4.2% | 1.0% | 0.5% | 0.0% |
| % Chinese (Mandarin or Cantonese) | 8.0% | 6.7% | 1.2% | 4.8% | 2.1% | 0.0% | 0.0% |
| % Russian | 1.7% | 0.6% | 0.1% | 0.4% | 1.0% | 0.0% | 0.0% |
| % Vietnamese | 1.0% | 1.4% | 0.0% | 0.4% | 0.0% | 0.0% | 0.0% |
| % Other | 11.7% | 9.3% | 5.6% | 18.0% | 6.9% | 8.1% | 4.8% |
| Not available | 4.1% | 4.6% | 3.7% | 2.8% | 3.1% | 1.6% | 6.0% |
| Insurance type | |||||||
| % Private | 35.4% | 58.7% | 77.4% | 10.7% | 63.8% | 36.8% | 66.7% |
| % Medicare | 22.0% | 17.2% | 12.0% | 67.8% | 14.8% | 8.7% | 33.3% |
| % Medicaid | 15.5% | 12.5% | 3.1% | 1.4% | 12.8% | 24.3% | 0.0% |
| % Dual eligible | 24.4% | 8.8% | 5.4% | 19.4% | 6.6% | 27.6% | 0.0% |
| % Self‐pay/other | 2.6% | 2.9% | 2.2% | 0.7% | 2.1% | 2.7% | 0.0% |
All‐cause 30‐day readmission rates varied across practices, with HIV Primary Care being the highest at 14.9%, followed by Geriatrics at 13.7%, General Internal Medicine at 13.3%, Concierge Internal Medicine at 9.7%, combined IM/FP at 8.9%, Family Practice at 8.6%, and Women's Health at 7.7% (Figure 1). Despite HIV Primary Care having the highest readmission rate, the number of index discharges during the 3‐year period was relatively low (249) compared to General Internal Medicine (5388). For the index admission, the top 5 admitting services were medicine, obstetrics, cardiology, orthopedic surgery, and general surgery (Table 2). Medicine was the primary admitting service for patients in the following clinics: General Internal Medicine, Geriatrics, HIV Primary Care, and Concierge Internal Medicine. Obstetrics was the primary admitting service for patients in the Family Practice, Women's Health, and combined IM/FP clinics. Vaginal delivery was the top discharge diagnosis‐related group (DRG) for patients in the General Internal Medicine, Family Practice, Women's Health, and combined IM/FP clinics. The top discharge DRG was urinary tract infection for Geriatrics, HIV for the HIV clinic (13.6%), and chemotherapy (8.0%) for Concierge Internal Medicine. Average LOS varied from 4.7 days for patients in the HIV Primary Care clinic to 2.8 days for patients in the Concierge Internal Medicine clinic. Average LOS was 3.4, 3.1, and 3.2 days in the Family Practice, Women's Health, and Geriatrics clinics, respectively, and 3.8 days in the General Internal Medicine and combined IM/FP clinics. For all clinics except Geriatrics, the majority of patients were discharged home without home health. A larger proportion of patients in the Geriatrics clinic were discharged home with home health or discharged to a skilled nursing facility.

| General Internal Medicine, N=5,388 | Family Practice, N=1,204 | Women's Health, N=983 | Geriatrics, N=409 | Combined IM/FP, N=339 | HIV Primary Care, N=249 | Concierge IM, N=113 | |
|---|---|---|---|---|---|---|---|
| |||||||
| Top 5 admitting services | |||||||
| Medicine | 41.8% | 25.0% | 15.2% | 56.4% | 23.5% | 59.7% | 19.0% |
| Obstetrics | 7.5% | 24.7% | 38.7% | 0.0% | 35.6% | 2.1% | 9.0% |
| Cardiology | 15.1% | 10.9% | 6.2% | 16.5% | 10.3% | 6.5% | 17.0% |
| Orthopedic surgery | 8.3% | 8.3% | 8.2% | 7.3% | 5.3% | 4.8% | 12.0% |
| Adult general surgery | 7.2% | 9.8% | 10.0% | 6.0% | 5.0% | 5.8% | 7.0% |
| Top 5 discharge diagnoses | |||||||
| Vaginal delivery | 3.5% | 13.6% | 20.6% | 0.0% | 17.9% | 0.3% | 3.0% |
| Major joint replacement | 3.5% | 3.4% | 4.8% | 4.3% | 3.8% | 0.7% | 5.0% |
| Vaginal delivery with complications | 1.3% | 5.0% | 6.9% | 0.0% | 4.1% | 1.0% | 2.0% |
| Simple pneumonia and pleurisy | 2.8% | 1.2% | 0.6% | 3.8% | 0.3% | 2.1% | 0.0% |
| Urinary tract infection | 2.0% | 1.5% | 1.4% | 6.2% | 0.9% | 2.4% | 1.0% |
| Discharge disposition | |||||||
| % Home | 69.1% | 74.6% | 76.6% | 47.8% | 69.9% | 78.6% | 68.4% |
| % Home with home health | 19.4% | 16.5% | 17.8% | 24.1% | 21.7% | 11.8% | 18.8% |
| % Skilled nursing facility | 7.2% | 4.9% | 3.0% | 18.9% | 3.3% | 5.0% | 0.8% |
| % Other | 4.3% | 4.0% | 2.6% | 9.2% | 5.1% | 4.6% | 12.0% |
| Average length of stay (SD) | 3.8 (5.6) | 3.4 (7.5) | 3.1 (3.7) | 3.2 (3.7) | 3.8 (5.2) | 4.7 (6.5) | 2.8 (2.8) |
| % Discharges with ICU stay | 11.0% | 10.6% | 5.9% | 8.5% | 9.2% | 11.1% | 15.8% |
Factors associated with variation in readmission rates included: male gender (OR: 1.21, 95% CI: 1.051.40), Medicare (OR: 1.31, 95% CI: 1.051.64; Ref=private) and dual‐eligible Medicare‐Medicaid insurance (OR: 1.26, 95% CI: 1.011.56), unknown primary language (OR: 0.06, 95% CI: 0.020.25; Ref=English), and the following comorbidities: peripheral vascular disease, renal failure, lymphoma, fluid and electrolyte disorders, and anemia. Multivariable logistic regression modeling results are listed in Table 3. Patients having a resident PCP showed no increased odds of readmission (OR: 1.13, 95% CI: 0.931.37; Ref=attending PCP). However, patients with a graduated resident PCP or departed faculty PCP awaiting transfer to a new PCP had an OR of 1.59 (95% CI: 1.162.17) compared with having a current faculty PCP. The C‐statistic for this model was 0.67.
| Adjusted Odds Ratio (95% CI) | |
|---|---|
| |
| Age | |
| <65 years | Ref |
| >65 years | 0.91 (0.751.10) |
| Clinic | |
| General Internal Medicine | 1.24 (0.991.56) |
| Family Practice | Ref |
| Women's Health | 1.13 (0.821.56) |
| Geriatrics | 1.20 (0.841.74) |
| Combined Internal Medicine/Family Practice | 1.13 (0.731.74) |
| HIV Primary Care | 1.24 (0.811.89) |
| Concierge Internal Medicine | 1.06 (0.542.07) |
| Sex | |
| Female | Ref |
| Male | 1.21 (1.051.40) |
| Insurance | |
| Private | Ref |
| Medicaid | 1.09 (0.871.37) |
| Medicare | 1.31 (1.051.64) |
| Dual eligible MedicareMedicaid | 1.26 (1.011.56) |
| Self/other | 1.20 (0.741.94) |
| Language | |
| English | Ref |
| Spanish | 1.02 (0.701.47) |
| Chinese | 1.13 (0.871.47) |
| Other | 0.87 (0.701.08) |
| Unknown | 0.06 (0.020.25) |
| Comorbidities | |
| Pulmonary circulation disease | 1.51 (0.982.32) |
| Peripheral vascular disease | 1.50 (1.141.98) |
| Renal failure | 1.31 (1.091.58) |
| Lymphoma | 2.50 (1.434.36) |
| Fluid and electrolyte disorders | 1.27 (1.071.52) |
| Deficiency anemia | 1.25 (1.041.51) |
| Physician | |
| Attending | Ref |
| Resident | 1.13 (0.931.37) |
| Departed PCP (internal medicine only) | 1.59 (1.162.17) |
| ICU stay | 1.03 (0.831.28) |
| LOS (log) | 1.02 (0.981.06) |
| Admitting service | |
| Medicine | Ref |
| Obstetrics | 0.41 (0.300.56) |
| Cardiology | 1.12 (0.911.37) |
| Orthopedic surgery | 0.36 (0.250.51) |
| Adult general surgery | 0.64 (0.470.87) |
| Other services | 0.76 (0.630.92) |
DISCUSSION
In this study we introduce a complementary way to view hospital readmissions, from the perspective of primary care practices. We found variation in readmission rates across primary care practices. After controlling for admitting service, clinic, provider, and patient factors, the specific characteristics of male gender, patients with Medicare or Medicare with Medicaid, and patients in our General Internal Medicine clinic with a departed PCP were independent risk factors for hospital readmission. Patients with specific comorbidities were also at increased risk for admissions, including those with peripheral vascular disease, renal failure, lymphoma, fluid and electrolyte disorders, and anemia. Because this is the first study viewing readmissions from the perspective of primary care practices, our findings are unique in the literature. However, hospital‐based studies have found similar relationships between readmission rates and these specific comorbidities.[11, 12, 15] Unlike other studies, our study cohort did not show CHF as an independent risk factor. We hypothesize this is because in 2008, UCSF Medical Center introduced an inpatient‐based intervention to reduce readmissions in patients with heart failure. By 2011, the readmission rates of patients with heart failure (primary or secondary diagnoses) had dropped by 30%. The success of this program, focused only on patients with heart failure, likely affected our analysis of comorbidities as risk factors for readmissions.
Models developed to predict hospital readmissions, overall and for specific disease conditions, have inconsistently identified predictive factors, and there is not a specific set of variables that dominate.[16, 17, 18, 19] A recent review of readmission risk prediction models suggested that models that take into account psychosocial factors such as social support, substance abuse, and functional status improved model performance.[16] We hypothesize that the reason why male gender was significant in our model may be related to lack of social support, especially among those who may be single or widowed. Other studies have also showed male gender as a predictor for hospital readmissions.[14, 20]
People with Medicare as the primary payor or Medicare with Medicaid also tended to have higher risk of readmission. We believe that this may be a proxy for the combined effect of age, multiple comorbidities, and psychosocial factors. In a multicenter study of general medicine inpatients, Medicare, but not age, was also found to be a predictive variable.[21] Unlike other studies, our study did not find Medicaid alone as a significant predictive variable for readmissions.[15, 21] One explanation may be that in hospital‐based studies, people with Medicaid who are at high risk for readmission may be high‐risk because they do not have a PCP or good access to outpatient care. In our study, all patients have a PCP in 1 of the UCSF clinics, and access to care is improved with this established relationship. These other studies did not examine Medicare‐Medicaid dual eligibility status. Our results are consistent with a national study on avoidable hospital admissions that showed the dual eligibility population experiencing 60% higher avoidable admission rates compared to the Medicaid‐only population.[22]
An interesting finding was that there were no statistically significant differences in readmissions among patients who report a language other than English as their primary language. Although language barriers and health literacy can affect a patient's ability to understand discharge instructions, the use of translators at UCSF Medical Center may have decreased the risk of readmissions. For a small number of patients whose primary language was not recorded (unknown language in our model), they appeared to have a lower risk of readmissions. Language preferences are recorded by our admitting staff during the process of admission. This step may have been skipped after hours or if the patient was not able to answer the question. However, we do not have a good hypothesis as to why these patients may have a reduced risk of readmissions.
Having a departed PCP in the General Internal Medicine clinic was an independent predictor of readmissions. These patients have access to primary care; they can schedule acute appointments with covering providers for new medical issues or follow‐up of chronic conditions. However, until they are transferred to a new PCP, they do not have a provider who is proactively managing their preventive and chronic disease care, including follow‐up and coordinating care after hospital discharge. Our study is not the first to suggest adverse outcomes for patients who are in transition from 1 primary care provider to the next.[23] Studies conducted in General Internal Medicine clinics have shown missed opportunities for cancer screening and overlooked test results during the transition period,[24] and as many as one‐fifth of patients whom residents identified as high‐risk were lost to follow‐up.[25] However, our study is the first to show the link between PCP transition in a teaching clinic and hospital readmissions. This finding underscores the importance of continuity of care in the optimal management of patients following hospital discharge.
There are several limitations to this study. First, our study only considers hospitalizations to UCSF Medical Center, potentially undercounting readmissions to area hospitals. Because our study population are patients with PCPs at UCSF, these patients tend to seek specialty and acute care at UCSF Medical Center as well. We obtained payor data from our medical group (Hill Physicians), which covers our largest private payors, to understand whether our results can be applied on a global basis. We found that in 87.4% of index admissions with readmissions from January 1, 2010 through May 30, 2012, the readmission occurred at UCSF Medical Center. We conducted a similar analysis with CMS data from October 1, 2008 to June 30, 2011, the latest data we have from CMS. For patients with UCSF PCPs and a diagnosis of AMI or CHF, 100% were readmitted back to UCSF Medical Center. For patients with UCSF PCPs and a diagnosis of pneumonia, 89% were readmitted back to UCSF Medical Center. Given that only a small percentage of patients with PCPs at UCSF present for readmission at other area hospitals, we believe that limiting our analysis to UCSF Medical Center is reasonable.
In our study, we did not remove vaginal deliveries or Caesarian sections prior to building the model. Primary care physicians and their clinic leadership are accustomed to taking a population‐health perspective. We anticipate they would be interested in designing interventions and addressing readmission for the entire primary care panel. Although readmissions after delivery are not frequent, they can still occur, and interventions should not necessarily exclude this population. We did run a sensitivity analysis by removing vaginal delivery and Caesarian sections from the analysis. As expected, readmission rates for all practices increased except for Geriatrics. However, the independent predictors of readmissions did not change.
Our study is based on a population of patients at 1 urban academic medical center and may not be generalizable across all delivery systems. Our population is racially and ethnically diverse, and many do not speak English as their primary language. The study also spans different types of primary care clinics, capturing a wide range of ages and case mix. As PCP assignments fluctuate over time, there may be errors with PCP attribution in the UCSF Medical Center data systems. We do not believe errors in PCP attribution would differ across primary care practices. Because the primary care practices' performance reports on quality measures are based on PCP assignment, each clinic regularly updates their clinic panels and has specific protocols to address errors with PCP attribution.
Finally, our study includes only the variables that we were able to extract from administrative claims. Other explanatory variables that have been suggested as important for evaluation, such as social support, functional assessment, access to care, hospital discharge process, and posthospitalization follow‐up, were not included. Each of these could be explored in future studies.
This study offers a unique perspective of hospital readmission by introducing a new methodology for primary care clinics to calculate and evaluate their all‐cause 30‐day readmission rates. Although this methodology is not intended to provide real‐time feedback to clinics on readmitted patients, it opens the door for benchmarking based on specific case‐mix indices. Another direction for future research is to design robust evaluations of the impact of interventions, both inpatient and outpatient, on primary care clinic readmission rates. Finally, future research should replicate this analysis across teaching clinics to identify whether provider turnover is a consistent independent predictor of hospital readmissions.
This study also has implications for inpatient interventions. For example, discharging physicians may consider proactively identifying whether the patient has a continuous primary care provider. Patients who are in between PCPs may need closer follow‐up after discharge, until they re‐establish with a new PCP. This can be accomplished through a postdischarge clinic visit, either run by inpatient providers or covering physicians in the primary care clinic. In addition, the discharging physician may work with the case manager to increase the level of care coordination. The case manager could contact the primary care clinic and proactively ask for immediate PCP re‐assignment. Once a new PCP has been identified, the discharging physician could consider a warm hand‐off. With a warm hand‐off, the new PCP may feel more comfortable managing problems that may arise after hospital discharge, and especially before the first outpatient visit with the new PCP. Future research can test whether these interventions could effectively reduce hospital readmissions across a broad primary care population.
CONCLUSION
Primary care providers and their clinics play an important role in managing population health, decreasing healthcare spending, and keeping patients out of the hospital. In this study, we introduce a tool in which primary care clinics can begin to understand their hospital readmission rates. This may be particularly valuable as primary care providers enter global payment arrangements such as accountable care organizations or bundled payments and are responsible for a population of patients across the continuum of care. We found significant variation in readmission rates between different primary care practices, but much of this variation appears to be due to differences between practices in patient demographics, comorbidities, and hospitalization factors. Our study is the first to show the association between provider transitions and higher hospital readmissions. Continuity of care is critical for the optimal management of patients following hospital discharge. More attention will need to be focused on providing good continuity outpatient care for patients at high risk for readmissions.
Acknowledgements
The authors acknowledge Janelle Lee, MBA, MHA, DrPH, for her assistance with extracting the hospital claims data at UCSF Medical Center.
Disclosure: Dr. Tang and Ms. Maselli were supported by a University of California, Center for Health Quality and Innovation grant. The authors report no conflicts of interest.
Reducing hospital readmissions is a national healthcare priority. In October 2012, the Centers for Medicare and Medicaid Services (CMS) enacted financial penalties on hospitals with higher than average risk‐adjusted readmissions, offering an incentive pool of $850 million in the first year.[1] As a result, a wide range of activities to understand and reduce readmissions among patients with congestive heart failure (CHF), acute myocardial infarction (AMI), and pneumonia have emerged.[2, 3, 4, 5] Some have proposed that to effectively reduce hospital readmissions, a community of inpatient and outpatient providers and local support organizations must coordinate efforts.[6, 7] In fact, CMS has recognized the important role of community support groups and outpatient providers in safe discharge transitions in 2 ways. First, in 2011 CMS launched the Community‐based Care Transitions Program to fund community‐based organizations to assist Medicare patients with care transitions.[8] Second, CMS introduced 2 new reimbursement codes for primary care providers (PCPs) to perform care coordination immediately after hospital discharge.[9] Both of these new payment programs represent an evolving perspective that reducing hospital readmissions requires active participation among outpatient partners.
As leaders of the outpatient care team, PCPs play a significant role in reducing hospital readmissions. One way in which PCPs can begin to understand the magnitude of the issue within their clinic is to evaluate the clinic's 30‐day readmission rates. Currently, CMS calculates readmission rates at the hospital level. However, understanding these rates at the clinic level is critical for developing strategies for improvement across the care continuum. Our current understanding of effective outpatient interventions to reduce hospital readmission is limited.[3] As clinics introduce and refine strategies to reduce readmissions, tracking the impact of these strategies on readmission rates will be critical for identifying effective outpatient interventions. Clinics with similar patient case‐mix can also benchmark readmission rates, sharing best practices from clinics with lower‐than‐expected rates.
There are no available studies or proposed methodologies to guide primary care clinics in calculating their 30‐day readmission rates. A particularly difficult challenge is obtaining the admission information when patients may be admitted to 1 of several area hospitals. For large integrated delivery networks where primary care patients are relatively loyal to the network of physicians and hospitals, an opportunity exists to explore the data. Furthermore, variations in readmission rates across primary care specialties (such as internal medicine and family practice) are not well understood. In this study, we set out to develop a methodology for calculating all‐cause 30‐day hospital readmission rates at the level of individual primary care practices and to identify factors associated with variations in these rates.
METHODS
Study Design
We conducted a retrospective observational study of adult primary care patients at the University of California, San Francisco (UCSF) who were hospitalized at UCSF Medical Center between July 1, 2009 and June 30, 2012. UCSF Medical Center is comprised of Moffitt‐Long Hospital (a 600‐bed facility) and UCSF‐Mount Zion Hospital (a 90‐bed facility) located in San Francisco, CA. The patient population was limited to adults ages 18 and over with a PCP at UCSF. UCSF has 7 adult primary care clinics: General Internal Medicine (IM), Family Practice (FP), Women's Health, Geriatrics, a combined IM/FP clinic, Human Immunodeficiency Virus (HIV) Primary Care, and a Concierge Internal Medicine clinic staffed by IM physicians. Between 2009 and 2011, all clinics completed the process of enpanelment, or defining the population of patients for which each PCP and the clinic is responsible. We obtained the list of patient and PCP assignments at each of the 7 clinics across the time period of study. We then obtained UCSF Medical Center hospital claims data for this group, including dates of admission and discharge, patient age, sex, race/ethnicity, language, insurance, admitting service, diagnosis codes, information on intensive care unit stay, and discharge disposition. Hospital claims data are housed in Transition Systems International (Boston, MA) administrative databases, a cost‐accounting system that collects data abstracted from patient charts upon discharge from UCSF Medical Center.
All‐cause 30‐day hospital readmission rates were calculated for each primary care clinic, using an adaptation of the CMS definition. First, we defined index discharges as the first discharge for an individual patient in any given 30‐day interval. Only 1 index discharge is flagged for each 30‐day interval. The first admission within 30 days after the index discharge was flagged as the readmission. Consistent with CMS methodology, only the first readmission in the 30‐day period was counted. We included all inpatient and observation status admissions and excluded patients who died during the index encounter, left against medical advice, or transferred to another acute care hospital after the index encounter.
We used secondary diagnosis codes in the administrative data to classify comorbidities by the Elixhauser methodology.[10] All but 1 adult primary care clinic at UCSF are faculty‐only clinics, whereby the assigned PCP is an attending physician. In the general internal medicine clinic, an attending or a resident can serve as the PCP, and approximately 20% of IM clinic patients have a resident as their PCP. For the IM clinic, we classified patients' PCP as attending, resident, or departed. The latter category refers to patients whose PCPs were residents who had graduated or faculty who had departed and had not been assigned to a new PCP prior to the index admission or readmission.
Statistical Analysis
We built a model to predict readmissions using the demographic and clinical variables with [2] P<0.20 in an initial bivariate analysis, and then removed, with backward selection, the least significant variables until only those with P0.05 remained. Age, log‐length of stay (LOS), and intensive care unit stay were forced in the model, as studies evaluating factors related to readmissions have often included these as important covariates.[11, 12, 13, 14] Results were expressed as adjusted odds ratio (OR) with 95% confidence interval (CI). All analyses were carried out using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
This study was exempt from review by the institutional review board of UCSF.
RESULTS
During the study period, there were 12,564 discharges from UCSF Medical Center for primary care patients belonging to the 7 clinics. Of these, 8685 were index discharges and 1032 were readmissions within 30 days. Table 1 shows the characteristics of the patients who had at least 1 admission during the study period. In all but 2 clinics (HIV Primary Care and Concierge Internal Medicine), there were more women hospitalized than men. Age and gender differences across clinics are consistent with the patient populations served by these clinics, with Women's Health having more female patients and younger patients hospitalized compared to Geriatrics.
| General Internal Medicine | Family Practice | Women's Health | Geriatrics | Combined IM/FP | HIV Primary Care | Concierge IM | |
|---|---|---|---|---|---|---|---|
| |||||||
| Panel size | 26,521 | 10,495 | 8,526 | 190 | 4,109 | 1,039 | 706 |
| FTE attending MD and NP providers | 18.8 | 7.3 | 4.5 | 1.7 | 5.0 | 2.3 | 2.1 |
| Mean panel size per FTE | 1,311 | 1,448 | 1,895 | 112 | 822 | 452 | 336 |
| Clinic visits in FY 2012 | 50,362 | 20,647 | 11,014 | 4,425 | 8,120 | 4,182 | 1,713 |
| No. of index discharges | 5,388 | 1,204 | 983 | 409 | 339 | 249 | 113 |
| No. of readmissions | 718 | 104 | 76 | 56 | 30 | 37 | 11 |
| No. of patients discharged during study period | 4,063 | 1,003 | 818 | 289* | 290 | 185 | 84 |
| % Male | 40.1% | 33.9% | 4.3% | 35.3% | 33.8% | 69.7% | 52.4% |
| Average age (SD), y | 60.4 (18.6) | 52.0 (19.0) | 47.0 (15.7) | 83.2 (6.9) | 46.3 (16.9) | 49.5 (9.9) | 64.5 (15.6) |
| Age range, y | 19104 | 1896 | 1997 | 6399 | 1890 | 2273 | 2092 |
| Race | |||||||
| % White | 42.2% | 41.3% | 58.8% | 61.9% | 49.3% | 61.0% | 91.7% |
| % Black | 16.3% | 8.5% | 7.5% | 7.3% | 10.0% | 27.6% | 0.0% |
| % Asian | 23.6% | 31.4% | 19.0% | 17.0% | 16.6% | 2.2% | 4.8% |
| % Native America/ Alaskan Native | 0.7% | 1.6% | 0.7% | 0.7% | 0.7% | 0.5% | 0.0% |
| % Other | 16.3% | 15.7% | 13.1% | 12.5% | 18.3% | 8.1% | 2.4% |
| Not available | 1.0% | 1.6% | 1.0% | 0.7% | 5.2% | 0.5% | 1.2% |
| Ethnicity | |||||||
| % Hispanic | 8.8% | 10.6% | 5.0% | 6.2% | 8.3% | 4.9% | 0.0% |
| % Non‐Hispanic | 73.8% | 74.5% | 81.8% | 73.0% | 74.5% | 84.9% | 75.0% |
| Not available | 17.4% | 15.0% | 13.2% | 20.8% | 17.2% | 10.3% | 25.0% |
| Language | |||||||
| % English | 69.7% | 75.6% | 88.8% | 69.6% | 85.9% | 89.7% | 89.3% |
| % Spanish | 3.8% | 1.9% | 0.6% | 4.2% | 1.0% | 0.5% | 0.0% |
| % Chinese (Mandarin or Cantonese) | 8.0% | 6.7% | 1.2% | 4.8% | 2.1% | 0.0% | 0.0% |
| % Russian | 1.7% | 0.6% | 0.1% | 0.4% | 1.0% | 0.0% | 0.0% |
| % Vietnamese | 1.0% | 1.4% | 0.0% | 0.4% | 0.0% | 0.0% | 0.0% |
| % Other | 11.7% | 9.3% | 5.6% | 18.0% | 6.9% | 8.1% | 4.8% |
| Not available | 4.1% | 4.6% | 3.7% | 2.8% | 3.1% | 1.6% | 6.0% |
| Insurance type | |||||||
| % Private | 35.4% | 58.7% | 77.4% | 10.7% | 63.8% | 36.8% | 66.7% |
| % Medicare | 22.0% | 17.2% | 12.0% | 67.8% | 14.8% | 8.7% | 33.3% |
| % Medicaid | 15.5% | 12.5% | 3.1% | 1.4% | 12.8% | 24.3% | 0.0% |
| % Dual eligible | 24.4% | 8.8% | 5.4% | 19.4% | 6.6% | 27.6% | 0.0% |
| % Self‐pay/other | 2.6% | 2.9% | 2.2% | 0.7% | 2.1% | 2.7% | 0.0% |
All‐cause 30‐day readmission rates varied across practices, with HIV Primary Care being the highest at 14.9%, followed by Geriatrics at 13.7%, General Internal Medicine at 13.3%, Concierge Internal Medicine at 9.7%, combined IM/FP at 8.9%, Family Practice at 8.6%, and Women's Health at 7.7% (Figure 1). Despite HIV Primary Care having the highest readmission rate, the number of index discharges during the 3‐year period was relatively low (249) compared to General Internal Medicine (5388). For the index admission, the top 5 admitting services were medicine, obstetrics, cardiology, orthopedic surgery, and general surgery (Table 2). Medicine was the primary admitting service for patients in the following clinics: General Internal Medicine, Geriatrics, HIV Primary Care, and Concierge Internal Medicine. Obstetrics was the primary admitting service for patients in the Family Practice, Women's Health, and combined IM/FP clinics. Vaginal delivery was the top discharge diagnosis‐related group (DRG) for patients in the General Internal Medicine, Family Practice, Women's Health, and combined IM/FP clinics. The top discharge DRG was urinary tract infection for Geriatrics, HIV for the HIV clinic (13.6%), and chemotherapy (8.0%) for Concierge Internal Medicine. Average LOS varied from 4.7 days for patients in the HIV Primary Care clinic to 2.8 days for patients in the Concierge Internal Medicine clinic. Average LOS was 3.4, 3.1, and 3.2 days in the Family Practice, Women's Health, and Geriatrics clinics, respectively, and 3.8 days in the General Internal Medicine and combined IM/FP clinics. For all clinics except Geriatrics, the majority of patients were discharged home without home health. A larger proportion of patients in the Geriatrics clinic were discharged home with home health or discharged to a skilled nursing facility.

| General Internal Medicine, N=5,388 | Family Practice, N=1,204 | Women's Health, N=983 | Geriatrics, N=409 | Combined IM/FP, N=339 | HIV Primary Care, N=249 | Concierge IM, N=113 | |
|---|---|---|---|---|---|---|---|
| |||||||
| Top 5 admitting services | |||||||
| Medicine | 41.8% | 25.0% | 15.2% | 56.4% | 23.5% | 59.7% | 19.0% |
| Obstetrics | 7.5% | 24.7% | 38.7% | 0.0% | 35.6% | 2.1% | 9.0% |
| Cardiology | 15.1% | 10.9% | 6.2% | 16.5% | 10.3% | 6.5% | 17.0% |
| Orthopedic surgery | 8.3% | 8.3% | 8.2% | 7.3% | 5.3% | 4.8% | 12.0% |
| Adult general surgery | 7.2% | 9.8% | 10.0% | 6.0% | 5.0% | 5.8% | 7.0% |
| Top 5 discharge diagnoses | |||||||
| Vaginal delivery | 3.5% | 13.6% | 20.6% | 0.0% | 17.9% | 0.3% | 3.0% |
| Major joint replacement | 3.5% | 3.4% | 4.8% | 4.3% | 3.8% | 0.7% | 5.0% |
| Vaginal delivery with complications | 1.3% | 5.0% | 6.9% | 0.0% | 4.1% | 1.0% | 2.0% |
| Simple pneumonia and pleurisy | 2.8% | 1.2% | 0.6% | 3.8% | 0.3% | 2.1% | 0.0% |
| Urinary tract infection | 2.0% | 1.5% | 1.4% | 6.2% | 0.9% | 2.4% | 1.0% |
| Discharge disposition | |||||||
| % Home | 69.1% | 74.6% | 76.6% | 47.8% | 69.9% | 78.6% | 68.4% |
| % Home with home health | 19.4% | 16.5% | 17.8% | 24.1% | 21.7% | 11.8% | 18.8% |
| % Skilled nursing facility | 7.2% | 4.9% | 3.0% | 18.9% | 3.3% | 5.0% | 0.8% |
| % Other | 4.3% | 4.0% | 2.6% | 9.2% | 5.1% | 4.6% | 12.0% |
| Average length of stay (SD) | 3.8 (5.6) | 3.4 (7.5) | 3.1 (3.7) | 3.2 (3.7) | 3.8 (5.2) | 4.7 (6.5) | 2.8 (2.8) |
| % Discharges with ICU stay | 11.0% | 10.6% | 5.9% | 8.5% | 9.2% | 11.1% | 15.8% |
Factors associated with variation in readmission rates included: male gender (OR: 1.21, 95% CI: 1.051.40), Medicare (OR: 1.31, 95% CI: 1.051.64; Ref=private) and dual‐eligible Medicare‐Medicaid insurance (OR: 1.26, 95% CI: 1.011.56), unknown primary language (OR: 0.06, 95% CI: 0.020.25; Ref=English), and the following comorbidities: peripheral vascular disease, renal failure, lymphoma, fluid and electrolyte disorders, and anemia. Multivariable logistic regression modeling results are listed in Table 3. Patients having a resident PCP showed no increased odds of readmission (OR: 1.13, 95% CI: 0.931.37; Ref=attending PCP). However, patients with a graduated resident PCP or departed faculty PCP awaiting transfer to a new PCP had an OR of 1.59 (95% CI: 1.162.17) compared with having a current faculty PCP. The C‐statistic for this model was 0.67.
| Adjusted Odds Ratio (95% CI) | |
|---|---|
| |
| Age | |
| <65 years | Ref |
| >65 years | 0.91 (0.751.10) |
| Clinic | |
| General Internal Medicine | 1.24 (0.991.56) |
| Family Practice | Ref |
| Women's Health | 1.13 (0.821.56) |
| Geriatrics | 1.20 (0.841.74) |
| Combined Internal Medicine/Family Practice | 1.13 (0.731.74) |
| HIV Primary Care | 1.24 (0.811.89) |
| Concierge Internal Medicine | 1.06 (0.542.07) |
| Sex | |
| Female | Ref |
| Male | 1.21 (1.051.40) |
| Insurance | |
| Private | Ref |
| Medicaid | 1.09 (0.871.37) |
| Medicare | 1.31 (1.051.64) |
| Dual eligible MedicareMedicaid | 1.26 (1.011.56) |
| Self/other | 1.20 (0.741.94) |
| Language | |
| English | Ref |
| Spanish | 1.02 (0.701.47) |
| Chinese | 1.13 (0.871.47) |
| Other | 0.87 (0.701.08) |
| Unknown | 0.06 (0.020.25) |
| Comorbidities | |
| Pulmonary circulation disease | 1.51 (0.982.32) |
| Peripheral vascular disease | 1.50 (1.141.98) |
| Renal failure | 1.31 (1.091.58) |
| Lymphoma | 2.50 (1.434.36) |
| Fluid and electrolyte disorders | 1.27 (1.071.52) |
| Deficiency anemia | 1.25 (1.041.51) |
| Physician | |
| Attending | Ref |
| Resident | 1.13 (0.931.37) |
| Departed PCP (internal medicine only) | 1.59 (1.162.17) |
| ICU stay | 1.03 (0.831.28) |
| LOS (log) | 1.02 (0.981.06) |
| Admitting service | |
| Medicine | Ref |
| Obstetrics | 0.41 (0.300.56) |
| Cardiology | 1.12 (0.911.37) |
| Orthopedic surgery | 0.36 (0.250.51) |
| Adult general surgery | 0.64 (0.470.87) |
| Other services | 0.76 (0.630.92) |
DISCUSSION
In this study we introduce a complementary way to view hospital readmissions, from the perspective of primary care practices. We found variation in readmission rates across primary care practices. After controlling for admitting service, clinic, provider, and patient factors, the specific characteristics of male gender, patients with Medicare or Medicare with Medicaid, and patients in our General Internal Medicine clinic with a departed PCP were independent risk factors for hospital readmission. Patients with specific comorbidities were also at increased risk for admissions, including those with peripheral vascular disease, renal failure, lymphoma, fluid and electrolyte disorders, and anemia. Because this is the first study viewing readmissions from the perspective of primary care practices, our findings are unique in the literature. However, hospital‐based studies have found similar relationships between readmission rates and these specific comorbidities.[11, 12, 15] Unlike other studies, our study cohort did not show CHF as an independent risk factor. We hypothesize this is because in 2008, UCSF Medical Center introduced an inpatient‐based intervention to reduce readmissions in patients with heart failure. By 2011, the readmission rates of patients with heart failure (primary or secondary diagnoses) had dropped by 30%. The success of this program, focused only on patients with heart failure, likely affected our analysis of comorbidities as risk factors for readmissions.
Models developed to predict hospital readmissions, overall and for specific disease conditions, have inconsistently identified predictive factors, and there is not a specific set of variables that dominate.[16, 17, 18, 19] A recent review of readmission risk prediction models suggested that models that take into account psychosocial factors such as social support, substance abuse, and functional status improved model performance.[16] We hypothesize that the reason why male gender was significant in our model may be related to lack of social support, especially among those who may be single or widowed. Other studies have also showed male gender as a predictor for hospital readmissions.[14, 20]
People with Medicare as the primary payor or Medicare with Medicaid also tended to have higher risk of readmission. We believe that this may be a proxy for the combined effect of age, multiple comorbidities, and psychosocial factors. In a multicenter study of general medicine inpatients, Medicare, but not age, was also found to be a predictive variable.[21] Unlike other studies, our study did not find Medicaid alone as a significant predictive variable for readmissions.[15, 21] One explanation may be that in hospital‐based studies, people with Medicaid who are at high risk for readmission may be high‐risk because they do not have a PCP or good access to outpatient care. In our study, all patients have a PCP in 1 of the UCSF clinics, and access to care is improved with this established relationship. These other studies did not examine Medicare‐Medicaid dual eligibility status. Our results are consistent with a national study on avoidable hospital admissions that showed the dual eligibility population experiencing 60% higher avoidable admission rates compared to the Medicaid‐only population.[22]
An interesting finding was that there were no statistically significant differences in readmissions among patients who report a language other than English as their primary language. Although language barriers and health literacy can affect a patient's ability to understand discharge instructions, the use of translators at UCSF Medical Center may have decreased the risk of readmissions. For a small number of patients whose primary language was not recorded (unknown language in our model), they appeared to have a lower risk of readmissions. Language preferences are recorded by our admitting staff during the process of admission. This step may have been skipped after hours or if the patient was not able to answer the question. However, we do not have a good hypothesis as to why these patients may have a reduced risk of readmissions.
Having a departed PCP in the General Internal Medicine clinic was an independent predictor of readmissions. These patients have access to primary care; they can schedule acute appointments with covering providers for new medical issues or follow‐up of chronic conditions. However, until they are transferred to a new PCP, they do not have a provider who is proactively managing their preventive and chronic disease care, including follow‐up and coordinating care after hospital discharge. Our study is not the first to suggest adverse outcomes for patients who are in transition from 1 primary care provider to the next.[23] Studies conducted in General Internal Medicine clinics have shown missed opportunities for cancer screening and overlooked test results during the transition period,[24] and as many as one‐fifth of patients whom residents identified as high‐risk were lost to follow‐up.[25] However, our study is the first to show the link between PCP transition in a teaching clinic and hospital readmissions. This finding underscores the importance of continuity of care in the optimal management of patients following hospital discharge.
There are several limitations to this study. First, our study only considers hospitalizations to UCSF Medical Center, potentially undercounting readmissions to area hospitals. Because our study population are patients with PCPs at UCSF, these patients tend to seek specialty and acute care at UCSF Medical Center as well. We obtained payor data from our medical group (Hill Physicians), which covers our largest private payors, to understand whether our results can be applied on a global basis. We found that in 87.4% of index admissions with readmissions from January 1, 2010 through May 30, 2012, the readmission occurred at UCSF Medical Center. We conducted a similar analysis with CMS data from October 1, 2008 to June 30, 2011, the latest data we have from CMS. For patients with UCSF PCPs and a diagnosis of AMI or CHF, 100% were readmitted back to UCSF Medical Center. For patients with UCSF PCPs and a diagnosis of pneumonia, 89% were readmitted back to UCSF Medical Center. Given that only a small percentage of patients with PCPs at UCSF present for readmission at other area hospitals, we believe that limiting our analysis to UCSF Medical Center is reasonable.
In our study, we did not remove vaginal deliveries or Caesarian sections prior to building the model. Primary care physicians and their clinic leadership are accustomed to taking a population‐health perspective. We anticipate they would be interested in designing interventions and addressing readmission for the entire primary care panel. Although readmissions after delivery are not frequent, they can still occur, and interventions should not necessarily exclude this population. We did run a sensitivity analysis by removing vaginal delivery and Caesarian sections from the analysis. As expected, readmission rates for all practices increased except for Geriatrics. However, the independent predictors of readmissions did not change.
Our study is based on a population of patients at 1 urban academic medical center and may not be generalizable across all delivery systems. Our population is racially and ethnically diverse, and many do not speak English as their primary language. The study also spans different types of primary care clinics, capturing a wide range of ages and case mix. As PCP assignments fluctuate over time, there may be errors with PCP attribution in the UCSF Medical Center data systems. We do not believe errors in PCP attribution would differ across primary care practices. Because the primary care practices' performance reports on quality measures are based on PCP assignment, each clinic regularly updates their clinic panels and has specific protocols to address errors with PCP attribution.
Finally, our study includes only the variables that we were able to extract from administrative claims. Other explanatory variables that have been suggested as important for evaluation, such as social support, functional assessment, access to care, hospital discharge process, and posthospitalization follow‐up, were not included. Each of these could be explored in future studies.
This study offers a unique perspective of hospital readmission by introducing a new methodology for primary care clinics to calculate and evaluate their all‐cause 30‐day readmission rates. Although this methodology is not intended to provide real‐time feedback to clinics on readmitted patients, it opens the door for benchmarking based on specific case‐mix indices. Another direction for future research is to design robust evaluations of the impact of interventions, both inpatient and outpatient, on primary care clinic readmission rates. Finally, future research should replicate this analysis across teaching clinics to identify whether provider turnover is a consistent independent predictor of hospital readmissions.
This study also has implications for inpatient interventions. For example, discharging physicians may consider proactively identifying whether the patient has a continuous primary care provider. Patients who are in between PCPs may need closer follow‐up after discharge, until they re‐establish with a new PCP. This can be accomplished through a postdischarge clinic visit, either run by inpatient providers or covering physicians in the primary care clinic. In addition, the discharging physician may work with the case manager to increase the level of care coordination. The case manager could contact the primary care clinic and proactively ask for immediate PCP re‐assignment. Once a new PCP has been identified, the discharging physician could consider a warm hand‐off. With a warm hand‐off, the new PCP may feel more comfortable managing problems that may arise after hospital discharge, and especially before the first outpatient visit with the new PCP. Future research can test whether these interventions could effectively reduce hospital readmissions across a broad primary care population.
CONCLUSION
Primary care providers and their clinics play an important role in managing population health, decreasing healthcare spending, and keeping patients out of the hospital. In this study, we introduce a tool in which primary care clinics can begin to understand their hospital readmission rates. This may be particularly valuable as primary care providers enter global payment arrangements such as accountable care organizations or bundled payments and are responsible for a population of patients across the continuum of care. We found significant variation in readmission rates between different primary care practices, but much of this variation appears to be due to differences between practices in patient demographics, comorbidities, and hospitalization factors. Our study is the first to show the association between provider transitions and higher hospital readmissions. Continuity of care is critical for the optimal management of patients following hospital discharge. More attention will need to be focused on providing good continuity outpatient care for patients at high risk for readmissions.
Acknowledgements
The authors acknowledge Janelle Lee, MBA, MHA, DrPH, for her assistance with extracting the hospital claims data at UCSF Medical Center.
Disclosure: Dr. Tang and Ms. Maselli were supported by a University of California, Center for Health Quality and Innovation grant. The authors report no conflicts of interest.
- , . Medicare's new hospital value‐based purchasing program is likely to have only a small impact on hospital payments. Health Aff. 2012;31:1932–1940.
- , , , et al. Contemporary evidence about hospital strategies for reducing 30‐day readmissions. J Am Coll Cardiol. 2012;60:607–614.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Reducing hospital readmissions: lessons from top‐performing hospitals. The Commonwealth Fund Synthesis Report. Available at: http://www.commonwealthfund.org/publications/case‐studies/2011/apr/reducing‐hospital‐readmissions. Published April 6, 2011. Accessed February 8, 2013.
- , . Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
- , , . Recasting readmissions by placing the hospital role in community context. JAMA. 2013;309:351–352.
- , , , . Hospitalists and care transitions: the divorce of inpatient and outpatient care. Health Affairs. 2008;27:1315–1327.
- Center for Medicare and Medicaid Innovation. Community‐based Care Transitions Program. Available at: http://innovation.cms.gov/initiatives/CCTP/#collapse‐tableDetails. Accessed February 8, 2013.
- , , . Medicare's transitional care payment—a step toward the medical home. N Engl J Med. 2013;368:692–694.
- , , . Volume thresholds and hospital characteristics in the United States. Health Aff (Millwood). 2003;22:167–177.
- , , , , , . Thirty‐day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157:11–18.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157:837–845.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54–60.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;206:1688–1698.
- , , , et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386.
- , , , . Statistical models and patient predictors of readmission for acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes. 2009;2:500–507.
- , , , et al. Predictors of hospital readmission after stroke: a systematic review. Stroke. 2010;41:2525–2533.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21:363–372.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25:211–219.
- , , . Users of Medicaid home and community‐based services are especially vulnerable to costly avoidable hospital admissions. Health Affairs. 2012;31:1167–1175.
- , . Academic year‐end transfers of outpatients from outgoing to incoming residents: an unaddressed patient safety issue. JAMA. 2009;302:1327–1329.
- , , , , , . The revolving door of resident continuity practice: identifying gaps in transitions of care. J Gen Intern Med. 2011;26:995–998.
- , , , et al. Outcomes for resident‐identified high‐risk patients and resident perspectives of year‐end continuity clinic handoffs. J Gen Intern Med. 2012;27:1438–1444.
- , . Medicare's new hospital value‐based purchasing program is likely to have only a small impact on hospital payments. Health Aff. 2012;31:1932–1940.
- , , , et al. Contemporary evidence about hospital strategies for reducing 30‐day readmissions. J Am Coll Cardiol. 2012;60:607–614.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Reducing hospital readmissions: lessons from top‐performing hospitals. The Commonwealth Fund Synthesis Report. Available at: http://www.commonwealthfund.org/publications/case‐studies/2011/apr/reducing‐hospital‐readmissions. Published April 6, 2011. Accessed February 8, 2013.
- , . Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
- , , . Recasting readmissions by placing the hospital role in community context. JAMA. 2013;309:351–352.
- , , , . Hospitalists and care transitions: the divorce of inpatient and outpatient care. Health Affairs. 2008;27:1315–1327.
- Center for Medicare and Medicaid Innovation. Community‐based Care Transitions Program. Available at: http://innovation.cms.gov/initiatives/CCTP/#collapse‐tableDetails. Accessed February 8, 2013.
- , , . Medicare's transitional care payment—a step toward the medical home. N Engl J Med. 2013;368:692–694.
- , , . Volume thresholds and hospital characteristics in the United States. Health Aff (Millwood). 2003;22:167–177.
- , , , , , . Thirty‐day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157:11–18.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157:837–845.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104.
- , , , . Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54–60.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;206:1688–1698.
- , , , et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386.
- , , , . Statistical models and patient predictors of readmission for acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes. 2009;2:500–507.
- , , , et al. Predictors of hospital readmission after stroke: a systematic review. Stroke. 2010;41:2525–2533.
- , , , , . Risk factors for 30‐day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent). 2008;21:363–372.
- , , , et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25:211–219.
- , , . Users of Medicaid home and community‐based services are especially vulnerable to costly avoidable hospital admissions. Health Affairs. 2012;31:1167–1175.
- , . Academic year‐end transfers of outpatients from outgoing to incoming residents: an unaddressed patient safety issue. JAMA. 2009;302:1327–1329.
- , , , , , . The revolving door of resident continuity practice: identifying gaps in transitions of care. J Gen Intern Med. 2011;26:995–998.
- , , , et al. Outcomes for resident‐identified high‐risk patients and resident perspectives of year‐end continuity clinic handoffs. J Gen Intern Med. 2012;27:1438–1444.
© 2014 Society of Hospital Medicine