User login
Pretibial Myxedema
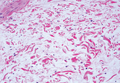
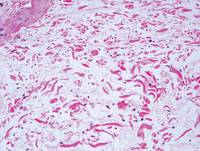
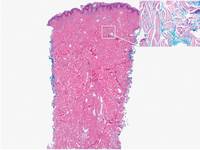
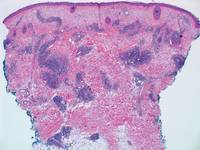
Pretibial myxedema (PM) is a cutaneous mucinosis associated with thyroid dysfunction. It most commonly presents in the setting of Graves disease and is seen less often in patients with hypothyroidism and euthyroidism.1 The anterior tibia is most frequently affected, but lesions also may present on the feet, thighs, and upper extremities. Physical examination generally demonstrates skin thickening, hyperkeratosis, hyperpigmentation, yellow-red discoloration, and hyperhidrosis. Classically, the term peau d’orange has been used to characterize these clinical features.1 Histologic examination of PM typically reveals marked deposition of mucin throughout the reticular dermis with sparing of the papillary dermis (Figure 1) and may be accompanied by overlying hyperkeratosis. Collagen fibers are splayed and appear decreased in density (Figure 2). Alcian blue, periodic acid–Schiff, colloidal iron, and toluidine blue staining can be used to highlight dermal mucin.

| 
|
Figure 1. Prominent mucin deposition throughout the reticular dermis in pretibial myxedema (H&E, original magnification ×40). | Figure 2. Increased mucin deposition and collagen fiber splaying in pretibial myxedema (H&E, original magnification ×200). |
| 
|
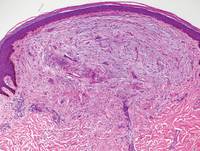
Figure 3. Scleredema with increased dermal thickness (H&E, original magnification ×20) and interstitial mucin on colloidal iron–stained sections (inset in upper right corner, original magnification ×400). | Figure 4. Scleromyxedema with mucin deposition primarily in the superficial dermis as well as increased cellularity and fibrosis (H&E, original magnification ×200). |
| 
|
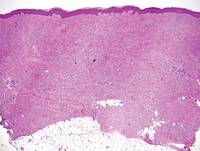
Figure 5. Nephrogenic systemic fibrosis with prominent mucinous fibrosis (H&E, original magnification ×40). | Figure 6. Tumid lupus erythematosus with a superficial and perivascular lymphoid infiltrate as well as increased dermal mucin (H&E, original magnification ×40). |
Similar to PM, histologic examination of scleredema and scleromyxedema usually demonstrate prominent mucin deposition. In scleredema, mucin primarily is visualized in the deep dermis between thick collagen bundles and typically is localized to the back (Figure 3). Scleromyxedema is distinguished by mucin deposition in the superficial dermis with associated fibroblast proliferation and fibrosis (Figure 4).
Nephrogenic systemic fibrosis is characterized by proliferation of CD34+ dermal spindle cells, fibroblasts, interstitial mucin, altered elastic fibers, and thickened collagen bundles that involve the dermis and subcutaneous septa (Figure 5).2 Tumid lupus erythematosus classically demonstrates perivascular and periadnexal superficial and deep lymphocytic inflammation (Figure 6). Similar to scleredema and PM, mucin deposition in tumid lupus erythematosus is interspersed between collagen bundles in the reticular dermis.3
1. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
2. Cowper SE, Lyndon DS, Bhawan J, et al. Nephro-genic fibrosing dermopathy. Am J Dermatopathol.2001;23:383-393.
3. Kuhn A, Dagmar RH, Oslislo C, et al. Lupus erythematosus tumidus: a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
Pretibial myxedema (PM) is a cutaneous mucinosis associated with thyroid dysfunction. It most commonly presents in the setting of Graves disease and is seen less often in patients with hypothyroidism and euthyroidism.1 The anterior tibia is most frequently affected, but lesions also may present on the feet, thighs, and upper extremities. Physical examination generally demonstrates skin thickening, hyperkeratosis, hyperpigmentation, yellow-red discoloration, and hyperhidrosis. Classically, the term peau d’orange has been used to characterize these clinical features.1 Histologic examination of PM typically reveals marked deposition of mucin throughout the reticular dermis with sparing of the papillary dermis (Figure 1) and may be accompanied by overlying hyperkeratosis. Collagen fibers are splayed and appear decreased in density (Figure 2). Alcian blue, periodic acid–Schiff, colloidal iron, and toluidine blue staining can be used to highlight dermal mucin.

| 
|
Figure 1. Prominent mucin deposition throughout the reticular dermis in pretibial myxedema (H&E, original magnification ×40). | Figure 2. Increased mucin deposition and collagen fiber splaying in pretibial myxedema (H&E, original magnification ×200). |

| 
|
Figure 3. Scleredema with increased dermal thickness (H&E, original magnification ×20) and interstitial mucin on colloidal iron–stained sections (inset in upper right corner, original magnification ×400). | Figure 4. Scleromyxedema with mucin deposition primarily in the superficial dermis as well as increased cellularity and fibrosis (H&E, original magnification ×200). |

| 
|
Figure 5. Nephrogenic systemic fibrosis with prominent mucinous fibrosis (H&E, original magnification ×40). | Figure 6. Tumid lupus erythematosus with a superficial and perivascular lymphoid infiltrate as well as increased dermal mucin (H&E, original magnification ×40). |
Similar to PM, histologic examination of scleredema and scleromyxedema usually demonstrate prominent mucin deposition. In scleredema, mucin primarily is visualized in the deep dermis between thick collagen bundles and typically is localized to the back (Figure 3). Scleromyxedema is distinguished by mucin deposition in the superficial dermis with associated fibroblast proliferation and fibrosis (Figure 4).
Nephrogenic systemic fibrosis is characterized by proliferation of CD34+ dermal spindle cells, fibroblasts, interstitial mucin, altered elastic fibers, and thickened collagen bundles that involve the dermis and subcutaneous septa (Figure 5).2 Tumid lupus erythematosus classically demonstrates perivascular and periadnexal superficial and deep lymphocytic inflammation (Figure 6). Similar to scleredema and PM, mucin deposition in tumid lupus erythematosus is interspersed between collagen bundles in the reticular dermis.3
Pretibial myxedema (PM) is a cutaneous mucinosis associated with thyroid dysfunction. It most commonly presents in the setting of Graves disease and is seen less often in patients with hypothyroidism and euthyroidism.1 The anterior tibia is most frequently affected, but lesions also may present on the feet, thighs, and upper extremities. Physical examination generally demonstrates skin thickening, hyperkeratosis, hyperpigmentation, yellow-red discoloration, and hyperhidrosis. Classically, the term peau d’orange has been used to characterize these clinical features.1 Histologic examination of PM typically reveals marked deposition of mucin throughout the reticular dermis with sparing of the papillary dermis (Figure 1) and may be accompanied by overlying hyperkeratosis. Collagen fibers are splayed and appear decreased in density (Figure 2). Alcian blue, periodic acid–Schiff, colloidal iron, and toluidine blue staining can be used to highlight dermal mucin.

| 
|
Figure 1. Prominent mucin deposition throughout the reticular dermis in pretibial myxedema (H&E, original magnification ×40). | Figure 2. Increased mucin deposition and collagen fiber splaying in pretibial myxedema (H&E, original magnification ×200). |

| 
|
Figure 3. Scleredema with increased dermal thickness (H&E, original magnification ×20) and interstitial mucin on colloidal iron–stained sections (inset in upper right corner, original magnification ×400). | Figure 4. Scleromyxedema with mucin deposition primarily in the superficial dermis as well as increased cellularity and fibrosis (H&E, original magnification ×200). |

| 
|
Figure 5. Nephrogenic systemic fibrosis with prominent mucinous fibrosis (H&E, original magnification ×40). | Figure 6. Tumid lupus erythematosus with a superficial and perivascular lymphoid infiltrate as well as increased dermal mucin (H&E, original magnification ×40). |
Similar to PM, histologic examination of scleredema and scleromyxedema usually demonstrate prominent mucin deposition. In scleredema, mucin primarily is visualized in the deep dermis between thick collagen bundles and typically is localized to the back (Figure 3). Scleromyxedema is distinguished by mucin deposition in the superficial dermis with associated fibroblast proliferation and fibrosis (Figure 4).
Nephrogenic systemic fibrosis is characterized by proliferation of CD34+ dermal spindle cells, fibroblasts, interstitial mucin, altered elastic fibers, and thickened collagen bundles that involve the dermis and subcutaneous septa (Figure 5).2 Tumid lupus erythematosus classically demonstrates perivascular and periadnexal superficial and deep lymphocytic inflammation (Figure 6). Similar to scleredema and PM, mucin deposition in tumid lupus erythematosus is interspersed between collagen bundles in the reticular dermis.3
1. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
2. Cowper SE, Lyndon DS, Bhawan J, et al. Nephro-genic fibrosing dermopathy. Am J Dermatopathol.2001;23:383-393.
3. Kuhn A, Dagmar RH, Oslislo C, et al. Lupus erythematosus tumidus: a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
1. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
2. Cowper SE, Lyndon DS, Bhawan J, et al. Nephro-genic fibrosing dermopathy. Am J Dermatopathol.2001;23:383-393.
3. Kuhn A, Dagmar RH, Oslislo C, et al. Lupus erythematosus tumidus: a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
Findings support endovascular-first approach for ruptured VAAs
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a the 2014 Vascular Annual Meeting.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures
Visceral Artery Aneurysms (VAA) can be challenging to treat from an open approach especially when the aneurysm is adherent to the surrounding pancreatic, visceral or retroperitoneal tissue. In most areas of vascular disease endovascular therapy is easier. But, endovascular therapy for VAA can be as challenging as the open surgical repair because of vessel tortuosity and access, imaging challenges, and the few options available for durable aneurysm treatment. Dr. Shukla and colleagues reviewed their outcomes in treating VAA over a 10-year period and report high rates of technical success, low morbidity and mortality using endovascular means for both intact and ruptured VAA. The results for endovascular therapy for ruptured VAA were particularly promising. These results indicate that coils and other ablative maneuvers may suffice in the setting of hemorrhage and be preferable to open surgical repair or ligation. This study provides important information on a rare problem and reassures us that the mid-term durability of ablative techniques for VAA is acceptable.
Dr. Vikram Kashyap is professor of surgery, Case Western Reserve University and chief, Division of Vascular Surgery and Endovascular Therapy, and co-director, Harrington Heart & Vascular Institute, University Hospitals Case Medical Center,Cleveland.
Visceral Artery Aneurysms (VAA) can be challenging to treat from an open approach especially when the aneurysm is adherent to the surrounding pancreatic, visceral or retroperitoneal tissue. In most areas of vascular disease endovascular therapy is easier. But, endovascular therapy for VAA can be as challenging as the open surgical repair because of vessel tortuosity and access, imaging challenges, and the few options available for durable aneurysm treatment. Dr. Shukla and colleagues reviewed their outcomes in treating VAA over a 10-year period and report high rates of technical success, low morbidity and mortality using endovascular means for both intact and ruptured VAA. The results for endovascular therapy for ruptured VAA were particularly promising. These results indicate that coils and other ablative maneuvers may suffice in the setting of hemorrhage and be preferable to open surgical repair or ligation. This study provides important information on a rare problem and reassures us that the mid-term durability of ablative techniques for VAA is acceptable.
Dr. Vikram Kashyap is professor of surgery, Case Western Reserve University and chief, Division of Vascular Surgery and Endovascular Therapy, and co-director, Harrington Heart & Vascular Institute, University Hospitals Case Medical Center,Cleveland.
Visceral Artery Aneurysms (VAA) can be challenging to treat from an open approach especially when the aneurysm is adherent to the surrounding pancreatic, visceral or retroperitoneal tissue. In most areas of vascular disease endovascular therapy is easier. But, endovascular therapy for VAA can be as challenging as the open surgical repair because of vessel tortuosity and access, imaging challenges, and the few options available for durable aneurysm treatment. Dr. Shukla and colleagues reviewed their outcomes in treating VAA over a 10-year period and report high rates of technical success, low morbidity and mortality using endovascular means for both intact and ruptured VAA. The results for endovascular therapy for ruptured VAA were particularly promising. These results indicate that coils and other ablative maneuvers may suffice in the setting of hemorrhage and be preferable to open surgical repair or ligation. This study provides important information on a rare problem and reassures us that the mid-term durability of ablative techniques for VAA is acceptable.
Dr. Vikram Kashyap is professor of surgery, Case Western Reserve University and chief, Division of Vascular Surgery and Endovascular Therapy, and co-director, Harrington Heart & Vascular Institute, University Hospitals Case Medical Center,Cleveland.
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a the 2014 Vascular Annual Meeting.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures
BOSTON – Endovascular interventions for ruptured visceral artery aneurysms are associated with reduced morbidity and mortality, compared with open interventions, according to findings from a retrospective chart review.
Both endovascular and open repairs are safe and durable for intact visceral artery aneurysms, Dr. Ankur J. Shukla reported at a the 2014 Vascular Annual Meeting.
Of 261 patients who presented with visceral artery aneurysms (VAAs), 174 underwent repair: 74 who presented with ruptured VAA and 100 who presented with intact VAA. The majority – 73% of ruptured VAA and 62% of intact VAA – were repaired with an endovascular approach.
Among those with ruptured VAA, 30-day mortality was 7.4% following endovascular repair, compared with 28.6% following open repair, a significant difference, said Dr. Shukla of the University of Pittsburgh Medical Center.
Survival at 3 years of ruptured VAA was about 70% vs. 46.4% in the endovascular and open repair groups, respectively, he said.
About 65% of patients with ruptured VAA presented with pain, and about 30% presented in hemodynamic shock. The most commonly identified etiology was "inflammatory/pancreatitis inflammatory," and 80% of the aneurysms were pseudoaneurysmal in nature.
A large proportion of the aneurysms were in the splenic and arterial beds, but 26% were located in the pancreaticoduodenal arcade, and those had a mean size of 12.7 mm. Most (95%) were pseudoaneurysms.
The outcomes with ruptured VAA were quite good, Dr. Shukla said, noting that the technical success rate was 98.7%.
Although the 30-day reintervention rate with endovascular repair was higher, the difference between the groups was not statistically significant, and there was a trend toward a lower rate of major complications with endovascular repair.
Factors found to be predictors of mortality risk were older age and steroid use, while endovascular repair was found to be protective.
As for the patients with intact VAA, most presented without symptoms, and the most common etiology was atherosclerosis.
"When we looked at the distribution, this was very consistent with what has been reported in the literature, with the splenic and arterial beds really making up the lion’s share of this group. Notable is the fact that 6.7% of our patients had intact aneurysms in the pancreaticoduodenal arcade," he said.
Outcomes in those with intact aneurysms were good. A slightly higher 30-day reintervention rate in those who underwent endovascular repair did not reach statistical significance, and both the endovascular and open repair groups had low rates of major complications.
Survival at 3 years for intact aneurysms did not differ in the endovascular and open repair groups. This was partly due to a 0% 30-day mortality, and – despite the fact that the overall mortality in those with intact aneurysms was 10% – there was zero overall aneurysm-related mortality, he said.
Patients in the study were treated at a single institution between 2003 and 2013. Most were in their mid to late 50s, and there were more men and more individuals on immunosuppressive therapy in the ruptured VAA group. However, comorbidities were similar in the ruptured and intact VAA groups.
Visceral artery aneurysms occur only rarely, affecting 0.1% to 2% of the general population, but because of the increasing use of noninvasive imaging, more of these aneurysms are being detected incidentally.
When they are not found incidentally, they often go undetected and present when they rupture, Dr. Shukla said.
"Because of the increasing utilization and improvement of endovascular technology, we now have a lot of options to fix these aneurysms. But the outcomes are not well defined. Even less well defined is the outcome of ruptured visceral artery aneurysms," he said, noting that most studies have a small sample size or look only at endovascular or open repairs.
Overall, the current study showed that there is "an acute and sharp drop-off in survival with open repair," related, most likely, to operative mortality, he said.
"Based on the findings, we recommend aggressive treatment of pseudoaneurysms and true aneurysms in the pancreaticoduodenal arcade, and advocate for an endovascular-first approach to treating ruptured visceral artery aneurysms, acknowledging that success in this is really predicated on good planning based on advanced imaging and endovascular set-up," he concluded.
Dr. Shukla reported having no disclosures
AT THE 2014 VASCULAR ANNUAL MEETING
Key clinical point: The researchers recommend aggressive treatment of visceral artery pseudoaneurysms and true aneurysms, with an endovascular-first approach to treating ruptured aneurysms.
Major finding: Thirty-day mortality was 7.4% vs. 26% with endovascular vs. open repair of ruptured VAAs.
Data source: A retrospective chart review involving 174 cases.
Disclosures: Dr. Shukla reported having no disclosures.
Self-care for the weary
"Try and be nice to people, avoid eating fat, read a good book every now and then get some walking in, and try and live together in peace and harmony with people of all creeds and nations."
–Monty Python, "The Meaning of Life"
1. Set boundaries. This is arguably the most important aspect of the physician-patient relationship. A relationship like this is inherently fertile ground for such Freudian defenses as transference and projection. It’s important to recognize, too, that the process is not necessarily a one-way street. Freud was, after all, flawed like his subjects.
2. Don’t sweat the small stuff. This is self-evident, yet surprisingly difficult to remember.
3. Keep a journal. You’ll want to remember the good times, and keeping track of the bad times just shows you how far you’ve come.
4. Nourish your spirit – church, yoga, meditation. Take your pick. The best life advice I’ve ever received is that the mind needs structured space for quiet because otherwise you are never alone; there is always that voice in your head, constantly narrating your life and probably judging you for it.
5. There is a whole world outside of medicine. How easy is it to let our lives be consumed by our profession? How much happier would it make us to be reminded that music and literature and art exist? For my birthday this year, I asked my sister to give me a book that she thinks I should own.
6. Related to #5: Learn something outside of medicine. There are brilliant podcasts on science, the economy, politics. There are audio courses on great books or philosophy. Pick up a new instrument or a new language.
7. Indulge in the experiences that make you happy, whether that’s going to the beach, or eating out, throwing parties, or traveling. In the arguably dubious field of happiness research, studies show that spending money on experiences leads to happiness much more so than spending money on objects. (Of course, there may be a selection bias problem, in that it is entirely possible that people who are likely to buy experiences are happier at baseline than people who are likely to buy objects. But you get my meaning.)
8. Exercise. Run, take bike rides, walk your dog. Go for hikes. Take rowing lessons.
9. Sleep is crucial. We are not teenagers any longer, and it is a losing proposition to think that you can still get away with barely sleeping. If I get 8 hours of sleep, I am almost guaranteed to not be as grumpy at work. I feel refreshed, my mind is clearer, and I feel better equipped to deal with the challenges of the workday.
10. Forgive yourself – for the grumpiness, for mistakes, for bad outcomes that you could not possibly have done anything about.
11. Bonus: Mental health therapy, if done right, is a fantastic investment in yourself.
Dr. Chan practices rheumatology in Pawtucket, R.I.
"Try and be nice to people, avoid eating fat, read a good book every now and then get some walking in, and try and live together in peace and harmony with people of all creeds and nations."
–Monty Python, "The Meaning of Life"
1. Set boundaries. This is arguably the most important aspect of the physician-patient relationship. A relationship like this is inherently fertile ground for such Freudian defenses as transference and projection. It’s important to recognize, too, that the process is not necessarily a one-way street. Freud was, after all, flawed like his subjects.
2. Don’t sweat the small stuff. This is self-evident, yet surprisingly difficult to remember.
3. Keep a journal. You’ll want to remember the good times, and keeping track of the bad times just shows you how far you’ve come.
4. Nourish your spirit – church, yoga, meditation. Take your pick. The best life advice I’ve ever received is that the mind needs structured space for quiet because otherwise you are never alone; there is always that voice in your head, constantly narrating your life and probably judging you for it.
5. There is a whole world outside of medicine. How easy is it to let our lives be consumed by our profession? How much happier would it make us to be reminded that music and literature and art exist? For my birthday this year, I asked my sister to give me a book that she thinks I should own.
6. Related to #5: Learn something outside of medicine. There are brilliant podcasts on science, the economy, politics. There are audio courses on great books or philosophy. Pick up a new instrument or a new language.
7. Indulge in the experiences that make you happy, whether that’s going to the beach, or eating out, throwing parties, or traveling. In the arguably dubious field of happiness research, studies show that spending money on experiences leads to happiness much more so than spending money on objects. (Of course, there may be a selection bias problem, in that it is entirely possible that people who are likely to buy experiences are happier at baseline than people who are likely to buy objects. But you get my meaning.)
8. Exercise. Run, take bike rides, walk your dog. Go for hikes. Take rowing lessons.
9. Sleep is crucial. We are not teenagers any longer, and it is a losing proposition to think that you can still get away with barely sleeping. If I get 8 hours of sleep, I am almost guaranteed to not be as grumpy at work. I feel refreshed, my mind is clearer, and I feel better equipped to deal with the challenges of the workday.
10. Forgive yourself – for the grumpiness, for mistakes, for bad outcomes that you could not possibly have done anything about.
11. Bonus: Mental health therapy, if done right, is a fantastic investment in yourself.
Dr. Chan practices rheumatology in Pawtucket, R.I.
"Try and be nice to people, avoid eating fat, read a good book every now and then get some walking in, and try and live together in peace and harmony with people of all creeds and nations."
–Monty Python, "The Meaning of Life"
1. Set boundaries. This is arguably the most important aspect of the physician-patient relationship. A relationship like this is inherently fertile ground for such Freudian defenses as transference and projection. It’s important to recognize, too, that the process is not necessarily a one-way street. Freud was, after all, flawed like his subjects.
2. Don’t sweat the small stuff. This is self-evident, yet surprisingly difficult to remember.
3. Keep a journal. You’ll want to remember the good times, and keeping track of the bad times just shows you how far you’ve come.
4. Nourish your spirit – church, yoga, meditation. Take your pick. The best life advice I’ve ever received is that the mind needs structured space for quiet because otherwise you are never alone; there is always that voice in your head, constantly narrating your life and probably judging you for it.
5. There is a whole world outside of medicine. How easy is it to let our lives be consumed by our profession? How much happier would it make us to be reminded that music and literature and art exist? For my birthday this year, I asked my sister to give me a book that she thinks I should own.
6. Related to #5: Learn something outside of medicine. There are brilliant podcasts on science, the economy, politics. There are audio courses on great books or philosophy. Pick up a new instrument or a new language.
7. Indulge in the experiences that make you happy, whether that’s going to the beach, or eating out, throwing parties, or traveling. In the arguably dubious field of happiness research, studies show that spending money on experiences leads to happiness much more so than spending money on objects. (Of course, there may be a selection bias problem, in that it is entirely possible that people who are likely to buy experiences are happier at baseline than people who are likely to buy objects. But you get my meaning.)
8. Exercise. Run, take bike rides, walk your dog. Go for hikes. Take rowing lessons.
9. Sleep is crucial. We are not teenagers any longer, and it is a losing proposition to think that you can still get away with barely sleeping. If I get 8 hours of sleep, I am almost guaranteed to not be as grumpy at work. I feel refreshed, my mind is clearer, and I feel better equipped to deal with the challenges of the workday.
10. Forgive yourself – for the grumpiness, for mistakes, for bad outcomes that you could not possibly have done anything about.
11. Bonus: Mental health therapy, if done right, is a fantastic investment in yourself.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Drugs can increase risk of MDS and AML
A class of immunosuppressive agents appear to increase the risk of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) in patients with inflammatory bowel disease (IBD).
In an observational study of more than 19,000 IBD patients, past exposure to the agents—thiopurines—increased the risk of developing AML or MDS nearly 7-fold, when compared to the general population.
However, the absolute risk to an individual patient was about 1 in 10,000.
The researchers reported these results in Clinical Gastroenterology and Hepatology.
Thiopurines are an established treatment for IBD patients, but the drugs are also used to prevent rejection after a kidney transplant, to treat rheumatoid arthritis, as maintenance therapy for acute lymphocytic leukemia, and to induce remission in patients with AML.
Previous research showed that long-term use of thiopurines can increase a person’s risk of developing lymphoma.
“In order to make appropriate, informed decisions about thiopurines, patients and providers need to be well-educated about the risks and benefits of this treatment,” said study author Laurent Peyrin-Biroulet, MD, PhD, of the University Hospital of Nancy-Brabois in France.
“According to our research, the risk of myeloid disorders was not increased among the overall IBD population, compared with the general population. However, it was increased amongst those taking thiopurines. We hope these findings encourage other researchers to investigate more about the drug and its potentially harmful effects.”
The researchers analyzed 19,486 patients who were enrolled in the Cancers Et Surrisque Associé aux Maladies inflammatoires intestinales En France study from May 2004 through June 2005.
At study entry, 10,810 patients had never received thiopurines, 2810 patients had discontinued such drugs, and 5866 patients were still receiving them.
After 3 years of follow up, 5 patients were diagnosed with incident myeloid disorders—2 with AML and 3 with MDS. Four of these patients had been exposed to thiopurines—1 with ongoing treatment and 3 with past exposure.
The risk of myeloid disorders was not increased among the overall IBD population, compared with the general population. The standardized incidence ratio (SIR) was 1.80.
Similarly, the risk of myeloid disorders was not increased among IBD patients still receiving thiopurine treatment. The SIR was 1.54.
However, patients with prior exposure to thiopurines did have a significantly increased risk of myeloid disorders, with an SIR of 6.98.
The researchers noted that, although these findings provide evidence of a connection between thiopurines and myeloid disorders in IBD patients, the absolute risk to an individual patient was low.
So it seems the link between thiopurines and myeloid disorders remains complex. And physicians must balance the risk against the known benefits of thiopurines in the management of IBD.
The American Gastroenterological Association has developed a guideline-based clinical decision support tool to help providers determine when to use thiopurines in these patients. ![]()

A class of immunosuppressive agents appear to increase the risk of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) in patients with inflammatory bowel disease (IBD).
In an observational study of more than 19,000 IBD patients, past exposure to the agents—thiopurines—increased the risk of developing AML or MDS nearly 7-fold, when compared to the general population.
However, the absolute risk to an individual patient was about 1 in 10,000.
The researchers reported these results in Clinical Gastroenterology and Hepatology.
Thiopurines are an established treatment for IBD patients, but the drugs are also used to prevent rejection after a kidney transplant, to treat rheumatoid arthritis, as maintenance therapy for acute lymphocytic leukemia, and to induce remission in patients with AML.
Previous research showed that long-term use of thiopurines can increase a person’s risk of developing lymphoma.
“In order to make appropriate, informed decisions about thiopurines, patients and providers need to be well-educated about the risks and benefits of this treatment,” said study author Laurent Peyrin-Biroulet, MD, PhD, of the University Hospital of Nancy-Brabois in France.
“According to our research, the risk of myeloid disorders was not increased among the overall IBD population, compared with the general population. However, it was increased amongst those taking thiopurines. We hope these findings encourage other researchers to investigate more about the drug and its potentially harmful effects.”
The researchers analyzed 19,486 patients who were enrolled in the Cancers Et Surrisque Associé aux Maladies inflammatoires intestinales En France study from May 2004 through June 2005.
At study entry, 10,810 patients had never received thiopurines, 2810 patients had discontinued such drugs, and 5866 patients were still receiving them.
After 3 years of follow up, 5 patients were diagnosed with incident myeloid disorders—2 with AML and 3 with MDS. Four of these patients had been exposed to thiopurines—1 with ongoing treatment and 3 with past exposure.
The risk of myeloid disorders was not increased among the overall IBD population, compared with the general population. The standardized incidence ratio (SIR) was 1.80.
Similarly, the risk of myeloid disorders was not increased among IBD patients still receiving thiopurine treatment. The SIR was 1.54.
However, patients with prior exposure to thiopurines did have a significantly increased risk of myeloid disorders, with an SIR of 6.98.
The researchers noted that, although these findings provide evidence of a connection between thiopurines and myeloid disorders in IBD patients, the absolute risk to an individual patient was low.
So it seems the link between thiopurines and myeloid disorders remains complex. And physicians must balance the risk against the known benefits of thiopurines in the management of IBD.
The American Gastroenterological Association has developed a guideline-based clinical decision support tool to help providers determine when to use thiopurines in these patients. ![]()

A class of immunosuppressive agents appear to increase the risk of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) in patients with inflammatory bowel disease (IBD).
In an observational study of more than 19,000 IBD patients, past exposure to the agents—thiopurines—increased the risk of developing AML or MDS nearly 7-fold, when compared to the general population.
However, the absolute risk to an individual patient was about 1 in 10,000.
The researchers reported these results in Clinical Gastroenterology and Hepatology.
Thiopurines are an established treatment for IBD patients, but the drugs are also used to prevent rejection after a kidney transplant, to treat rheumatoid arthritis, as maintenance therapy for acute lymphocytic leukemia, and to induce remission in patients with AML.
Previous research showed that long-term use of thiopurines can increase a person’s risk of developing lymphoma.
“In order to make appropriate, informed decisions about thiopurines, patients and providers need to be well-educated about the risks and benefits of this treatment,” said study author Laurent Peyrin-Biroulet, MD, PhD, of the University Hospital of Nancy-Brabois in France.
“According to our research, the risk of myeloid disorders was not increased among the overall IBD population, compared with the general population. However, it was increased amongst those taking thiopurines. We hope these findings encourage other researchers to investigate more about the drug and its potentially harmful effects.”
The researchers analyzed 19,486 patients who were enrolled in the Cancers Et Surrisque Associé aux Maladies inflammatoires intestinales En France study from May 2004 through June 2005.
At study entry, 10,810 patients had never received thiopurines, 2810 patients had discontinued such drugs, and 5866 patients were still receiving them.
After 3 years of follow up, 5 patients were diagnosed with incident myeloid disorders—2 with AML and 3 with MDS. Four of these patients had been exposed to thiopurines—1 with ongoing treatment and 3 with past exposure.
The risk of myeloid disorders was not increased among the overall IBD population, compared with the general population. The standardized incidence ratio (SIR) was 1.80.
Similarly, the risk of myeloid disorders was not increased among IBD patients still receiving thiopurine treatment. The SIR was 1.54.
However, patients with prior exposure to thiopurines did have a significantly increased risk of myeloid disorders, with an SIR of 6.98.
The researchers noted that, although these findings provide evidence of a connection between thiopurines and myeloid disorders in IBD patients, the absolute risk to an individual patient was low.
So it seems the link between thiopurines and myeloid disorders remains complex. And physicians must balance the risk against the known benefits of thiopurines in the management of IBD.
The American Gastroenterological Association has developed a guideline-based clinical decision support tool to help providers determine when to use thiopurines in these patients. ![]()
Ofatumumab prompts fatal reaction in CLL patient

Credit: Bill Branson
Health Canada and GlaxoSmithKline (GSK) have reported a fatal infusion reaction in a patient receiving the monoclonal antibody ofatumumab (Arzerra) to treat
chronic lymphocytic leukemia (CLL).
The patient had no known history of cardiac disease.
Ofatumumab’s product monograph is being updated to include a warning about the potential for fatal infusion reactions.
In Canada, ofatumumab is approved to treat patients with CLL that is refractory to fludarabine and alemtuzumab.
The drug received this marketing authorization with conditions, pending the results of trials to verify its clinical benefit.
In light of the fatal infusion reaction, GSK and Health Canada are reminding healthcare professionals that ofatumumab should be administered under the supervision of a physician experienced in the use of cancer therapy. The drug should be given in an environment where facilities to adequately monitor and treat infusion reactions are available.
Prior to infusion, patients should always receive the appropriate premedication, as outlined in the product label. However, serious infusion reactions may occur despite premedication.
If you suspect a severe infusion reaction, stop the infusion immediately and provide symptomatic treatment. Signs and symptoms of an infusion reaction may include swelling of the face or mouth, fever, chills, difficulty breathing, tightness of the chest and/or throat, light headedness, nausea, diarrhea, and rash.
These symptoms can occur during or shortly after the infusion, predominantly with the first 2 infusions. So ensure patients are closely monitored, especially those with heart conditions. And inform patients about the risk of fatal infusion reactions associated with ofatumumab.
GSK has sent a letter to healthcare professionals detailing the risk of fatal infusion reactions. The information is also available on the Canadian website of GSK and the Health Canada website.
Any case of serious hypersensitivity, infusion reactions, or other serious or unexpected side effects in patients receiving ofatumumab should be reported to GSK or Health Canada.
Ofatumumab is also known to pose a risk of hepatitis B virus reactivation, progressive multifocal leukoencephalopathy, serious and/or fatal cardiovascular events, and serious and/or fatal infections (bacterial, fungal, and viral).
Ofatumumab recently received approval in the European Union to be used in combination with chlorambucil or bendamustine for untreated CLL patients who are not eligible for fludarabine-based therapy. The drug previously received conditional approval in Europe as monotherapy to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab is approved for both of these indications in the US as well. ![]()

Credit: Bill Branson
Health Canada and GlaxoSmithKline (GSK) have reported a fatal infusion reaction in a patient receiving the monoclonal antibody ofatumumab (Arzerra) to treat
chronic lymphocytic leukemia (CLL).
The patient had no known history of cardiac disease.
Ofatumumab’s product monograph is being updated to include a warning about the potential for fatal infusion reactions.
In Canada, ofatumumab is approved to treat patients with CLL that is refractory to fludarabine and alemtuzumab.
The drug received this marketing authorization with conditions, pending the results of trials to verify its clinical benefit.
In light of the fatal infusion reaction, GSK and Health Canada are reminding healthcare professionals that ofatumumab should be administered under the supervision of a physician experienced in the use of cancer therapy. The drug should be given in an environment where facilities to adequately monitor and treat infusion reactions are available.
Prior to infusion, patients should always receive the appropriate premedication, as outlined in the product label. However, serious infusion reactions may occur despite premedication.
If you suspect a severe infusion reaction, stop the infusion immediately and provide symptomatic treatment. Signs and symptoms of an infusion reaction may include swelling of the face or mouth, fever, chills, difficulty breathing, tightness of the chest and/or throat, light headedness, nausea, diarrhea, and rash.
These symptoms can occur during or shortly after the infusion, predominantly with the first 2 infusions. So ensure patients are closely monitored, especially those with heart conditions. And inform patients about the risk of fatal infusion reactions associated with ofatumumab.
GSK has sent a letter to healthcare professionals detailing the risk of fatal infusion reactions. The information is also available on the Canadian website of GSK and the Health Canada website.
Any case of serious hypersensitivity, infusion reactions, or other serious or unexpected side effects in patients receiving ofatumumab should be reported to GSK or Health Canada.
Ofatumumab is also known to pose a risk of hepatitis B virus reactivation, progressive multifocal leukoencephalopathy, serious and/or fatal cardiovascular events, and serious and/or fatal infections (bacterial, fungal, and viral).
Ofatumumab recently received approval in the European Union to be used in combination with chlorambucil or bendamustine for untreated CLL patients who are not eligible for fludarabine-based therapy. The drug previously received conditional approval in Europe as monotherapy to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab is approved for both of these indications in the US as well. ![]()

Credit: Bill Branson
Health Canada and GlaxoSmithKline (GSK) have reported a fatal infusion reaction in a patient receiving the monoclonal antibody ofatumumab (Arzerra) to treat
chronic lymphocytic leukemia (CLL).
The patient had no known history of cardiac disease.
Ofatumumab’s product monograph is being updated to include a warning about the potential for fatal infusion reactions.
In Canada, ofatumumab is approved to treat patients with CLL that is refractory to fludarabine and alemtuzumab.
The drug received this marketing authorization with conditions, pending the results of trials to verify its clinical benefit.
In light of the fatal infusion reaction, GSK and Health Canada are reminding healthcare professionals that ofatumumab should be administered under the supervision of a physician experienced in the use of cancer therapy. The drug should be given in an environment where facilities to adequately monitor and treat infusion reactions are available.
Prior to infusion, patients should always receive the appropriate premedication, as outlined in the product label. However, serious infusion reactions may occur despite premedication.
If you suspect a severe infusion reaction, stop the infusion immediately and provide symptomatic treatment. Signs and symptoms of an infusion reaction may include swelling of the face or mouth, fever, chills, difficulty breathing, tightness of the chest and/or throat, light headedness, nausea, diarrhea, and rash.
These symptoms can occur during or shortly after the infusion, predominantly with the first 2 infusions. So ensure patients are closely monitored, especially those with heart conditions. And inform patients about the risk of fatal infusion reactions associated with ofatumumab.
GSK has sent a letter to healthcare professionals detailing the risk of fatal infusion reactions. The information is also available on the Canadian website of GSK and the Health Canada website.
Any case of serious hypersensitivity, infusion reactions, or other serious or unexpected side effects in patients receiving ofatumumab should be reported to GSK or Health Canada.
Ofatumumab is also known to pose a risk of hepatitis B virus reactivation, progressive multifocal leukoencephalopathy, serious and/or fatal cardiovascular events, and serious and/or fatal infections (bacterial, fungal, and viral).
Ofatumumab recently received approval in the European Union to be used in combination with chlorambucil or bendamustine for untreated CLL patients who are not eligible for fludarabine-based therapy. The drug previously received conditional approval in Europe as monotherapy to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab is approved for both of these indications in the US as well. ![]()
CHMP recommends antifungal agent
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for intravenous (IV) posaconazole (Noxafil), an antifungal agent.
If the European Commission affirms the CHMP opinion, IV posaconazole will be authorized for use in the European Union, Iceland, Liechtenstein, and Norway.
The commission previously granted marketing authorization for posaconazole delayed-release tablets and oral suspension.
Posaconazole is used to prevent invasive fungal infections in severely immunocompromised patients, such as hematopoietic stem cell transplant recipients with graft-vs-host disease or patients with hematologic malignancies and prolonged neutropenia from chemotherapy.
The drug is also used to treat fungal diseases—invasive aspergillosis, fusariosis, chromoblastomycosis, mycetoma, and coccidioidomycosis—when other antifungal agents—amphotericin B, itraconazole, or fluconazole—cannot be tolerated or have failed.
And posaconazole oral suspension is used as a first-line treatment for thrush, a fungal infection of the mouth and throat due to Candida.
Posaconazole injection is administered with a loading dose of 300 mg twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by IV infusion over approximately 90 minutes.
Once combined with a mixture of IV solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2°-8° C (36°-46° F).
The safety and effectiveness of IV posaconazole in patients younger than 18 years has not been established. IV posaconazole should not be used in pediatric patients because of non-clinical safety concerns.
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Patients with known hypersensitivity to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
Hepatic reactions have been reported as well. This includes mild to moderate elevations in ALT, AST, alkaline phosphatase, total bilirubin, and/or clinical hepatitis. Monitoring or discontinuation may be necessary in patients with hepatic reactions to posaconazole.
IV posaconazole should be avoided in patients with moderate or severe renal impairment (estimated glomerular filtration rate <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of posaconazole.
In clinical trials, the adverse events associated with IV posaconazole were generally similar to those in trials of posaconazole oral suspension. The most frequently reported events were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
IV posaconazole is under development by MSD (known as Merck in the US and Canada). ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for intravenous (IV) posaconazole (Noxafil), an antifungal agent.
If the European Commission affirms the CHMP opinion, IV posaconazole will be authorized for use in the European Union, Iceland, Liechtenstein, and Norway.
The commission previously granted marketing authorization for posaconazole delayed-release tablets and oral suspension.
Posaconazole is used to prevent invasive fungal infections in severely immunocompromised patients, such as hematopoietic stem cell transplant recipients with graft-vs-host disease or patients with hematologic malignancies and prolonged neutropenia from chemotherapy.
The drug is also used to treat fungal diseases—invasive aspergillosis, fusariosis, chromoblastomycosis, mycetoma, and coccidioidomycosis—when other antifungal agents—amphotericin B, itraconazole, or fluconazole—cannot be tolerated or have failed.
And posaconazole oral suspension is used as a first-line treatment for thrush, a fungal infection of the mouth and throat due to Candida.
Posaconazole injection is administered with a loading dose of 300 mg twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by IV infusion over approximately 90 minutes.
Once combined with a mixture of IV solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2°-8° C (36°-46° F).
The safety and effectiveness of IV posaconazole in patients younger than 18 years has not been established. IV posaconazole should not be used in pediatric patients because of non-clinical safety concerns.
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Patients with known hypersensitivity to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
Hepatic reactions have been reported as well. This includes mild to moderate elevations in ALT, AST, alkaline phosphatase, total bilirubin, and/or clinical hepatitis. Monitoring or discontinuation may be necessary in patients with hepatic reactions to posaconazole.
IV posaconazole should be avoided in patients with moderate or severe renal impairment (estimated glomerular filtration rate <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of posaconazole.
In clinical trials, the adverse events associated with IV posaconazole were generally similar to those in trials of posaconazole oral suspension. The most frequently reported events were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
IV posaconazole is under development by MSD (known as Merck in the US and Canada). ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for intravenous (IV) posaconazole (Noxafil), an antifungal agent.
If the European Commission affirms the CHMP opinion, IV posaconazole will be authorized for use in the European Union, Iceland, Liechtenstein, and Norway.
The commission previously granted marketing authorization for posaconazole delayed-release tablets and oral suspension.
Posaconazole is used to prevent invasive fungal infections in severely immunocompromised patients, such as hematopoietic stem cell transplant recipients with graft-vs-host disease or patients with hematologic malignancies and prolonged neutropenia from chemotherapy.
The drug is also used to treat fungal diseases—invasive aspergillosis, fusariosis, chromoblastomycosis, mycetoma, and coccidioidomycosis—when other antifungal agents—amphotericin B, itraconazole, or fluconazole—cannot be tolerated or have failed.
And posaconazole oral suspension is used as a first-line treatment for thrush, a fungal infection of the mouth and throat due to Candida.
Posaconazole injection is administered with a loading dose of 300 mg twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by IV infusion over approximately 90 minutes.
Once combined with a mixture of IV solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2°-8° C (36°-46° F).
The safety and effectiveness of IV posaconazole in patients younger than 18 years has not been established. IV posaconazole should not be used in pediatric patients because of non-clinical safety concerns.
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Patients with known hypersensitivity to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
Hepatic reactions have been reported as well. This includes mild to moderate elevations in ALT, AST, alkaline phosphatase, total bilirubin, and/or clinical hepatitis. Monitoring or discontinuation may be necessary in patients with hepatic reactions to posaconazole.
IV posaconazole should be avoided in patients with moderate or severe renal impairment (estimated glomerular filtration rate <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of posaconazole.
In clinical trials, the adverse events associated with IV posaconazole were generally similar to those in trials of posaconazole oral suspension. The most frequently reported events were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
IV posaconazole is under development by MSD (known as Merck in the US and Canada). ![]()
Malaria treatment in pregnancy often not up to snuff

Credit: Nina Matthews
Pregnant women are at risk of being undertreated or inappropriately treated for malaria, a new study suggests.
The research showed that some pregnant women with symptoms of malaria did not seek care from their formal healthcare system.
Those who did seek care sometimes received inappropriate treatment because healthcare providers did not to adhere to standard diagnostic and treatment guidelines recommended by the World Health Organization (WHO).
Jenny Hill, of the Liverpool School of Tropical Medicine in the UK, and her colleagues reported these discoveries in PLOS Medicine.
The team reviewed 37 studies investigating pregnant women’s access to malaria treatment and healthcare provider practices for managing malaria during pregnancy. The studies were conducted in Africa (30), Asia (4), Brazil (2), and Yemen (1).
Twenty-five percent to 75% of the women studied reported malaria episodes during pregnancy. More than 85% of women who reported a malaria episode during pregnancy sought some form of treatment, though this included self-treatment, herbal remedies, and other options not recommended by WHO.
Barriers to WHO-approved treatment included poor knowledge of drug safety, prohibitive costs, and self-treatment practices. Factors that determined whether a woman sought professional treatment included education, previous miscarriage, and prior use of antenatal care.
Other barriers to appropriate malaria treatment included healthcare providers’ reliance on a clinical diagnosis of malaria and poor adherence to treatment policy.
Seventy-two percent of healthcare providers followed standard treatment guidelines for malaria during a patient’s second or third trimester, but only 28% of providers followed the guidelines for patients in their first trimester (P=0.02).
Healthcare providers’ prescribing practices were driven by concerns about drug safety, patient preference, drug availability, and cost. Other factors that determined provider practices included access to training and the type of healthcare facility (public vs private).
Hill and her colleagues noted that this research is limited by the sparseness of data and the inconsistencies in study methodologies. Nevertheless, these findings highlight the need to develop interventions to improve access to and delivery of appropriate malaria treatment in pregnant women. ![]()

Credit: Nina Matthews
Pregnant women are at risk of being undertreated or inappropriately treated for malaria, a new study suggests.
The research showed that some pregnant women with symptoms of malaria did not seek care from their formal healthcare system.
Those who did seek care sometimes received inappropriate treatment because healthcare providers did not to adhere to standard diagnostic and treatment guidelines recommended by the World Health Organization (WHO).
Jenny Hill, of the Liverpool School of Tropical Medicine in the UK, and her colleagues reported these discoveries in PLOS Medicine.
The team reviewed 37 studies investigating pregnant women’s access to malaria treatment and healthcare provider practices for managing malaria during pregnancy. The studies were conducted in Africa (30), Asia (4), Brazil (2), and Yemen (1).
Twenty-five percent to 75% of the women studied reported malaria episodes during pregnancy. More than 85% of women who reported a malaria episode during pregnancy sought some form of treatment, though this included self-treatment, herbal remedies, and other options not recommended by WHO.
Barriers to WHO-approved treatment included poor knowledge of drug safety, prohibitive costs, and self-treatment practices. Factors that determined whether a woman sought professional treatment included education, previous miscarriage, and prior use of antenatal care.
Other barriers to appropriate malaria treatment included healthcare providers’ reliance on a clinical diagnosis of malaria and poor adherence to treatment policy.
Seventy-two percent of healthcare providers followed standard treatment guidelines for malaria during a patient’s second or third trimester, but only 28% of providers followed the guidelines for patients in their first trimester (P=0.02).
Healthcare providers’ prescribing practices were driven by concerns about drug safety, patient preference, drug availability, and cost. Other factors that determined provider practices included access to training and the type of healthcare facility (public vs private).
Hill and her colleagues noted that this research is limited by the sparseness of data and the inconsistencies in study methodologies. Nevertheless, these findings highlight the need to develop interventions to improve access to and delivery of appropriate malaria treatment in pregnant women. ![]()

Credit: Nina Matthews
Pregnant women are at risk of being undertreated or inappropriately treated for malaria, a new study suggests.
The research showed that some pregnant women with symptoms of malaria did not seek care from their formal healthcare system.
Those who did seek care sometimes received inappropriate treatment because healthcare providers did not to adhere to standard diagnostic and treatment guidelines recommended by the World Health Organization (WHO).
Jenny Hill, of the Liverpool School of Tropical Medicine in the UK, and her colleagues reported these discoveries in PLOS Medicine.
The team reviewed 37 studies investigating pregnant women’s access to malaria treatment and healthcare provider practices for managing malaria during pregnancy. The studies were conducted in Africa (30), Asia (4), Brazil (2), and Yemen (1).
Twenty-five percent to 75% of the women studied reported malaria episodes during pregnancy. More than 85% of women who reported a malaria episode during pregnancy sought some form of treatment, though this included self-treatment, herbal remedies, and other options not recommended by WHO.
Barriers to WHO-approved treatment included poor knowledge of drug safety, prohibitive costs, and self-treatment practices. Factors that determined whether a woman sought professional treatment included education, previous miscarriage, and prior use of antenatal care.
Other barriers to appropriate malaria treatment included healthcare providers’ reliance on a clinical diagnosis of malaria and poor adherence to treatment policy.
Seventy-two percent of healthcare providers followed standard treatment guidelines for malaria during a patient’s second or third trimester, but only 28% of providers followed the guidelines for patients in their first trimester (P=0.02).
Healthcare providers’ prescribing practices were driven by concerns about drug safety, patient preference, drug availability, and cost. Other factors that determined provider practices included access to training and the type of healthcare facility (public vs private).
Hill and her colleagues noted that this research is limited by the sparseness of data and the inconsistencies in study methodologies. Nevertheless, these findings highlight the need to develop interventions to improve access to and delivery of appropriate malaria treatment in pregnant women. ![]()
Interventions for the Treatment of Stretch Marks: A Systematic Review
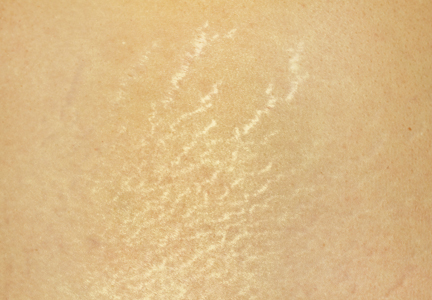
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
Stretch marks (striae cutis distensae) are a common disfiguring skin condition characterized by linear bands of atrophic-appearing skin.1 The prevalence of stretch marks associated with pregnancy ranges from 50% to 90%.2 Although stretch marks do not pose a health risk, they often cause burning, itching, and emotional distress, and they can have a deep psychological impact on patients, particularly in young healthy women who are commonly affected by this condition.3
The cause of stretch marks currently is unknown, but they are known to develop in a variety of physiological and pathological states (eg, pregnancy, adolescent growth spurts, obesity, large weight gain, Cushing syndrome, Marfan syndrome, diabetes mellitus, long-term systemic or topical steroid use).2-5 Clinically, newly formed stretch marks present as pink or purple linear lesions without substantial depression of the skin (striae rubra). Over time, the lesions lose their pigmentation, becoming depressed, atrophic, and white (striae alba).2,3,6 The most commonly affected sites are the breasts, upper arms, abdomen, buttocks, and thighs.3,4
Regardless of the etiology, the same histologic changes can be noted in the epidermis of all stretch marks, such as atrophy and loss of rete ridges, with features that are similar to scarring.3 Additionally, reorganization and diminution of the elastic fiber network of skin can be observed.7
A variety of treatment strategies are available for stretch marks, including topical preparations (eg, tretinoin, glycolic acid) and lasers.4 With current methods, no consistently effective therapies have been established yet. In this article, we present the results of a systematic review of the literature to address the effectiveness and safety of the available treatment options for stretch marks.
Methods
A literature search for randomized controlled trials (RCTs) related to the treatment of stretch marks was conducted on March 13, 2013, using the Cochrane Central Register of Controlled Trials, PubMed (from 1966), Embase (from 1974), Chinese Biomedical Literature Database (from 1978), China National Knowledge Infrastructure (from 1994), Chinese Science Journals Database (from 1989), and Wanfang Data (from 1995). Search terms included stretch marks, stretch mark, striae atrophicae, striae distensae, striae gravidarum, striae rubra, striae alba, lineae albicantes, striae, kikkisa, and random*.
We attempted to contact the original investigators of the 25 articles assessed for eligibility by e-mail to identify the randomization and answer other methodology questions to ensure that the studies included in the analysis were RCTs. Each of the 8 RCTs selected for inclusion was assessed independently by 2 investigators (L.L. and H.M.), and data extraction also was performed independently. Any differences in opinion were resolved by discussion. The risk of biases were assessed and 5 domains—random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data—were judged for each study included in the analysis using the Cochrane Collaboration’s domain-based evaluation tool as described in the Cochrane Handbook for Systematic Reviews of Interventions.8 Publication bias was not assessed due to insufficient data.
Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias or low risk of bias for all key domains; (2) unclear risk of bias or unclear risk of bias for 1 or more key domains; and (3) high risk of bias or high risk of 1 or more key domains.
Results
Search Results
Figure 1 presents the literature search results. Of 300 total search results, 8 RCTs were selected for assessment,9-16 which included a total of 240 patients (Table). The investigators of all 8 reports were contacted, but only 2 responses were received.11,14 The full text of one article could not be obtained; therefore, we could not confirm that it was a true RCT and excluded it.17
Risk of Bias
The risk of bias in methodology was evaluated for all 8 RCTs and the judgments were given for each domain (Figure 2). All the included studies claimed to be RCTs, but only 37.5% (3/8) of them used adequate randomizations, which were from a including computer-generated code,10 a table of randomized numbers,13 or the Microsoft Excel RND function (from the author by e-mail).14 The randomization methods in the other 5 studies were unclear. Allocation concealment was adequate in 1 trial13 but was unclear in the others. Three trials were double-blinded with the participants and outcome assessors blinded10,13,16; in 2 of these studies investigators also were blinded.10,13 There were 5 single-blinded trials; in 3 of these trials the outcome assessors were blinded12,14,15 and 1 was investigator-blinded.9 The other study was stated to be single-blinded but with no further detail.11 Due to the nature of the experimental design in 2 of the trials12,15 (ie, effects of laser therapy compared to topical treatment or no therapy), participants could not be blinded to treatment types; however, participants were blinded in 1 trial that compared different types of lasers.16 Investigators from all studies reported participants who did not complete the trial or were lost to follow-up, ranging from 0% to 65.6%. Two trials reported no loss of follow-up.11,12 Most trials had losses less than 20% except Pribanich et al13 who reported a loss of 65.6% of participants. One trial included a full analysis set,9 and none of the studies included an intention-to-treat analysis.
The overall risk of bias was assessed for each study and none could be categorized as low risk. Six studies had 1 or more domains assessed as high risk of bias and were classified as high risk of bias.9,11-15 The remaining 2 studies without high-risk domains had one or more domains assessed as unclear10,16 and were therefore considered to be at unclear risk of bias overall.
Effects of Treatments
Among the 8 studies we assessed, there were different treatments, methods of comparison, product concentrations, and times of application. The methods for assessing outcomes (eg, the size and severity of stretch marks) also were varied. Therefore, it is difficult to perform a meta-analysis of the data, and all the evidence was from individual studies. A summary of the results is presented in the Table.
All of the studies we evaluated assessed clinical improvement. Three studies reported the effects of topical tretinoin on stretch marks.10,12,13 A small parallel study with unclear risk of bias indicated that white participants with erythematous stretch marks seemed to have a better response to treatment with tretinoin cream 0.1% for 24 weeks versus placebo.10 However, there was no significant difference between tretinoin cream 0.025% and placebo for patients with abdominal striae in another trial.13 The latter trial was performed with low risk of bias in methodology, but the dropout rate was high (65.6%), with only 11 of 32 participants completing the trial. It is likely that the small number of patients makes the power too low to detect significant differences between tretinoin cream 0.025% and placebo if such a difference indeed existed.13 Because the outcomes in these 2 trials were assessed in different ways, it is difficult to perform a meta-analysis on the data. More adverse effects, mainly erythema and scaling associated with itching or burning sensations, were reported with the higher concentration (0.05%) of tretinoin.10 Another study at a high risk of bias found that the combined use of tretinoin cream 0.05% and glycolic acid 10% was not as effective as fractional CO2 laser therapy in improving the appearance of striae alba.12 There also were 3 studies comparing the effects of laser therapy with another treatment or no treatment.12,15,16 Two within-participant comparison studies with unclear or high risk of bias compared CO2 fractional laser therapy with other active treatment methods in female participants with striae alba.12,16 No difference between the fractional CO2 laser and the 1550-nm nonablative fractional erbium:glass laser was reported,16 but the fractional CO2 laser may be more effective than the topical therapy.12 A small study (11 participants) at high risk of bias reported negative results for the 1450-nm mid-infrared diode laser compared to no treatment.15 Data on the adverse effects of laser therapy were available from these studies. Postinflammatory hyperpigmentation was found in all the 3 studies12,15,16 and posttreatment erythema was mentioned in 2 studies.15,16 Based on the individual studies, treatment with a cosmetic oil formulation was more effective than a moisturizer in improving clinical presentation of stretch marks in white patients.14 Women with striae rubra showed better response to treatment with onion extract cream versus no treatment.9 Limited data from 1 study showed that combined use of Active B (sodium ascorbyl phosphate, 3-aminopropyl-L-ascorbyl phosphate, carboxybetaglucan, hyaluronic acid) and Active A (hyaluronic acid, sodium salt 2 mg, sodium carboxymethyl betaglucan 0.1 mg, ascorbic acid 0.5 mg, arginine 1 mg, sodium chloride 9 mg, sterile water) might be more effective than the use of Active B or placebo.11 These 3 studies are at high risk of bias and no obvious adverse effects were reported.9,11,14
Comment
In the 8 trials included in our assessment, 5 used a within-participant design in which 2 different treatments were randomly administered to the left and right sides of the body, respectively.9,12,14-16 Because the comparison of treatments was made based on results in the same patient versus 2 different treatment groups, the results may be more accurate. In the studies we reviewed, only 3 were placebo-controlled, which may only provide limited evidence on the comparative efficacy of the treatments used in these studies.10,11,13 Most treatments were evaluated in single studies, and most studies had a small number of participants (range, 6–66 participants). A considerable number of the total participants withdrew from their respective studies or were lost during follow-up. In some cases, no reason was given,13 but in the others, it was because of an obvious side effect16 or noncompliance.14 Overall, the methodology quality was low, especially the methods of randomization and allocation concealment. Unsuccessful attempts to contact the original investigators made it difficult to make accurate assessments of the risk of bias in most of the studies included in our assessment. No study met all the risk of bias criteria, and none were classified as having a low risk of bias.
The impact of industry sponsorship on the direction and completeness of the results of the studies we reviewed is unclear. One study was funded by a grant from the manufacturer of the study product,14 and the medication used in another study was supplied by the manufacturer.13 Another study was supported in part by a company that had no part in the conduct, analysis, or reporting of the study.10 In one instance, the authors were employees of the manufacturer of the study product.9 The remaining studies made no declaration.11,12,15,16
Thus the evidence from this review was insufficient to provide clear guidelines for practice. Because the results were based on a small number of patients and were of high or unclear risk of bias, caution must be taken when comparing the efficacy of the treatments administered in these studies; however, given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could reasonably be considered for the treatment of stretch marks. Laser therapies such as the fractional CO2 laser or the 1550-nm fractional erbium:glass laser may be another effective choice.
Conclusion
In future investigations of stretch mark treatments, more high-quality, placebo-controlled trials are needed. One important issue is the varied outcome assessment among different studies, which makes the evaluation and pooling of different studies difficult. Therefore, future RCTs should measure clinical features with a uniform score system such as the visual analog scale or the patient and observer scar assessment scale and try to avoid individual made-up system to assess the outcome. Furthermore, quality-of-life assessment was not included in any of the reports we evaluated; rather all 8 studies focused on changes in the appearance of stretch marks only. Given the deep psychological impact that stretch marks can have on patients, measures for quality-of-life assessment, such as the dermatology life quality index, should be incorporated into future study designs to improve the relevance of the trial and allow comparisons among studies using different interventions.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
1. Viennet C, Bride J, Cohen-Letessier A, et al. Mechanical behavior of fibroblasts included in collagen lattices. J Soc Biol. 2001;195:427-430.
2. Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004;51:881-885.
3. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24:97-100.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
5. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71:370-372.
6. Jiménez GP, Flores F, Berman B, et al. Treatment of striae rubra and striae alba with the 585-nm pulsed-dye laser. Dermatol Surg. 2003;29:362-365.
7. Watson RE, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-937.
8. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, United Kingdom: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 8, 2014.
9. Draelos ZD, Gold MH, Kaur M, et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed. 2010;8:80-86.
10. Kang S, Kim KJ, Griffiths CE, et al. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996;132:519-526.
11. Morganti P, Palombo P, Fabrizi G, et al. Biweekly in-office injectable treatment of striae distensae vs a long-term daily use of topical vitamin C. J Appl Cosmetol. 2001;19:107-112.
12. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci. 2012;17:928-933.
13. Pribanich S, Simpson FG, Held B, et al. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994;54:121-124.
14. Summers B, Lategan M. The effect of a topically-applied cosmetic oil formulation on striae distensae. SA Fam Pract. 2009;51:332-336.
15. Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006;38:196-199.
16. Yang YJ, Lee GY. Treatment of striae distensae with nonablative fractional laser versus ablative CO2 fractional laser: a randomized controlled trial. Ann Dermatol. 2011;23:481-489.
17. Joshi J, Donga SB, Pandya MA. A comparative study of Savarnakara Ghrita and Savarnakara Cream in the management of Kikkisa w.s.r. to Striae Gravidarum. Ayu. 2008;29:260-265.
Practice Points
- Given the negligible reported side effects, tretinoin cream 0.1%, a cosmetic oil formulation, onion extract cream, or the combined use of Active A and Active B could be considered for the treatment of stretch marks, though the evidence is insufficient.
- High-quality, randomized, placebo-controlled trials are needed in the future.
Weathering the ‘Perfect Storm?’
The era of the Affordable Care Act is upon us, and short of an unlikely repeal following midterm elections, this will remain the law of the land. As surgical residents, most of us have neither the time nor mental stamina to become significantly entrenched in politics. As a result, many of us know less about the impact that the Affordable Care Act will have on our future livelihood than many of the Senators did when they passed the bill on December 24, 2009. While most of the public focus has been on the individual mandate, pre-existing conditions, and insurance exchanges, further hidden from the public eye are the methods by which our fundamental model for health care reimbursement will change.
Much of the mystery was dispelled for me this May, when I heard a lecture entitled "The Perfect Storm: The Affordable Care Act and the Repeal of the SGR" by Dr. Jeffrey Rich. Dr. Rich’s presentation was the 2014 Norman E. Shumway, MD, Visiting Professorship Lecture at Stanford (Calif.) University, a webcast of which is available at http://ctsurgery.stanford.edu/media/.
Dr. Rich has a unique perspective on the issue of health care reimbursement, as he has served both as president of STS from 2012-2013 and director of the Center for Medicare Management, part of the Centers for Medicare & Medicaid Services, in 2008, in the political tumult leading up to the passage of the Affordable Care Act. On top of this, he has remained a practicing cardiothoracic surgeon in the Sentara Health System and sits as director-at-large for the Virginia Cardiac Surgery Quality Initiative. It is impossible to overstate the impact that he and his staff have had on the future of health care reimbursement. His recent lecture highlighted the ideological change in payment models that the Affordable Care Act embodies, along with the carrots and the sticks that the government will be wielding over the next 5 years to change physician and hospital behavior. This, along with the untenable continuation of the Sustainable Growth Rate (SGR) with all of its problems, portends huge swings both positive and negative for the reimbursement of all doctors, and cardiothoracic surgeons in particular. Early adopters may find themselves with a much-needed windfall, while those who do not anticipate the changes may find themselves in dire financial straits.
First, let us examine where we stand. The United States spends 17.6% of its gross domestic product (GDP) on health care. The nearest rival sits at 12%. State and federal government together spent $1.5 trillion on health care in 2013. Add private insurance into the mix and the figure is $2.8 trillion. Our life expectancy has not followed the money, and the rate of increase in health care spending is far outstripping inflation and the growth in our GDP. Our spending has increased exponentially since the passage of the Social Security Amendments of 1965 and shows no sign of slowing down. These statistics are well publicized, and should no longer be a surprise to anyone.
Keeping in mind that hindsight is 20/20, it seems obvious how we got here. The private health insurance industry took off during WWII, when competitive wage controls were put in place to keep skilled laborers in jobs supporting the war effort. To compete for laborers, private sector employers began offering health insurance policies. Shortly thereafter, public pressure to provide a health care safety net culminated in the creation of Medicare and Medicaid in 1965, and our complex public/private health insurance environment was born. For the first decade or so, physician reimbursement was based on "reasonable charge," meaning that doctors sent a bill to Medicare and, if it was considered reasonable, the doctor was paid. This fee-for-service model can be seen as a blank check of sorts, in that it contained few stipulations to withhold repayment for redundant or unnecessary tests and procedures. Expenses associated with complications also were reimbursable. The incentive to "do more" was set. It is worth noting that the Social Security Amendments of 1965 are federal law, and the law stipulates that reimbursement is tied to the amount of work that a physician performs, which also forbids associating reimbursement with the quality of work that the physician produces. It takes an act of Congress to change such law.
The Affordable Care Act is that act. Dr. Rich’s work at CMS paved the way for the inclusion of "Title III: Improving the quality and efficiency of health care," which allows Medicare and Medicaid reimbursements to be altered based on efficiency and outcomes, moving away (although not disintegrating) the fee-for-service model. It incentivizes the development of Accountable Care Organizations and Clinically Integrated Networks to encourage cross-specialty collaboration within the fee-for-service model and lays the groundwork for physician and hospital reimbursement to be based on high-quality, efficient, and appropriate care.
This is the most comprehensive change to the status quo, but it is by no means the first. By 1975, the federal government could see that open fee-for-service was leading to skyrocketing health care costs. It began experimenting with ways to curb physician charges. It pegged reimbursements to the Medicare Economic Index (still used to update hospital reimbursements by 3.2%-3.6% per year) and then tried basing reimbursement on relative value units. Costs continued to rise. Thirty years after the 1965 law, as health care spending continued to spiral out of control, the Sustainable Growth Rate was applied to physician repayment as an attempt to reel it in. The basic premise was that increased costs from increased patient and procedure volume would be curbed by decreasing the reimbursement per procedure.
The sustainable growth model essentially placed a spending target that would grow in step with GDP using the total expenditures beginning in 1996 as a benchmark. If, during a given year, spending outstripped the target, the following year a compensatory decrease in physician reimbursement would be enacted. If spending were less than the target, then physician payments would increase. Expenditures have exceeded the target every year since 2002, and each year our spending gets further and further from the benchmark, compounding the penalty. If the SGR penalties were allowed, it is estimated that physician repayment would drop by 25%-35% in the next few years. Each time the penalty is about to be applied, a fix is passed by Congress, saving our livelihoods at the last minute. Although we should be thankful not to take a 35% pay cut, the SGR and its fixes increased physician repayment by a mere 5.1% between 1992 and 2012. For comparison, Social Security benefits, adjusted annually to compensate increasing cost of living, have risen 52.9% in the same period. Meanwhile, as I mentioned earlier, hospital reimbursement continues to be tied to the Medicare Economic Index, which yields a fairly predictable payment increase of 3.2%-3.6% each year. The Affordable Care Act operates as a law separate from the SGR law, though the two are closely intertwined.
Title III of the Affordable Care Act provides a number of new incentives and penalties that will help make efficiency and quality goals that affect profit at least as much as procedural volume. It will impact hospitals and physicians in a number of new and potentially positive ways. Most immediately concerning to hospitals and medical groups are the incentives for quality. With value-based purchasing, Medicare will withhold 2% of diagnosis-related group (DRG) reimbursements to hospitals at the beginning of a year, giving them the chance to earn it back at the end of the year if they meet quality and efficiency performance goals. The top performers will receive a bonus from the funds collected from those who do not meet goals, making this a budget-neutral operation. Cardiothoracic surgeons will feel this scrutiny early, as the first five DRGs subject to the law are acute myocardial infarction, heart failure, pneumonia, surgeries, and health care–associated infections. As time passes, more diagnoses will be added.
Payments will be based on bundled care, meaning that a hospital will be paid one sum to cover the peri-admission period, starting from 3 days prior to 30 days after admission. Complications, readmissions, and repeat tests will not generate additional funds for the hospital. You can expect that daily chest x-rays and multiple echocardiograms will generate a lot of e-mails to attending physicians. Other preventable hospital-acquired conditions, such as catheter-associated urinary tract infections and pressure ulcers, if present in rates beyond the norm for the country, could cut reimbursements an additional 1%. Patient satisfaction scores will influence hospital reimbursement. Readmission rates beyond the specified cutoff for each admission will result in a 3% hospital pay cut, again, starting with the same set of diagnoses. When meaningful use of electronic health records incentives are factored in, hospitals are looking at a 7% swing on reimbursements for the DRGs listed above by 2017. Hospitals typically operate on a profit margin around 3.5%.
On the individual physician level, there are a number of changes. Already in place was a bonus for participating in physician quality reporting systems (PQRS), such as the STS database. By 2016 the bonus for participating will become a 2% pay cut for not participating. Thankfully, our specialty has been forward thinking in this regard, and the majority of cardiothoracic practices already participate in the STS database. Similar to value-based purchasing, the physician value modifier will apply a 2% bonus or penalty to reimbursements, based on a broad spectrum of quality measures, including patient safety, population and community health, total cost per patient by condition, and patient experience. Again, this will be budget neutral. When all of the items are tallied, the lowest-performing providers could see a 6% decrease in their personal reimbursement.
The SGR has not been fixed with the Affordable Care Act. Dr. Rich, in his role as STS president, provided testimony to Congress leading up to the most recent attempt to reform the law. Part of the main thrust of his testimony was that each specialty needs to set its own outcomes standards through database-driven research. Incentives for improved outcomes need to be in place for all members of the heart team, not just the physicians. All three of the proposed bills that followed his testimony included such incentives, but they also included even more dismal updates to physician payments than we have seen in the past 20 years. For better or for worse, none of the bills passed, and we can look forward to more anxiety as we await the next SGR patch. Whatever durable solution passes will likely focus on these new models of payment but without a significant boost in hospital income.
Currently, the alternative payment model (APM) pilot programs are still being developed. CMS has a $10 billion budget to fund the pilot programs, and consulting groups that advised the agency chose cardiothoracic surgery as a top priority for APM development. Current discussions indicate that participants in APMs could get a 5% bonus and would not be subject to the physician value modifier.
So how does this apply to us residents? Despite our ground-level perspective, we must recognize that we are straddling two drastically different eras in the practice of medicine. It will be the duty of all of us, not just our attendings, to reduce our costs and provide better patient care. This may mean using our stethoscope more effectively or making those extra phone calls to avoid unnecessary or repeated tests. We need to rebel against the ideology of physician shift work by owning our patients, but still work effectively in that system. When it comes time to seek our first jobs, we should focus not just on the department that we will work on, but its context within the local hospital system. The most vibrant department within an unresponsive hospital system will drown in the future penalties, and likewise for an unenthusiastic department within a forward-thinking system. In short, we need to start training ourselves to be keener, sharper, and more agile physicians, and to position ourselves within like-minded environments. Perhaps more important than any of these, we need to reclaim the right to shape our own profession. In recent history there has not been a better opportunity for cardiothoracic surgeons as a group to assert themselves as adept physicians and leaders. Whether we become head of CMS, participate in STS fly-ins to Capitol Hill, write our congressman about the issues we face, or engage our hospitals to anticipate the coming changes, it is up to us to ensure that we have a future.
Dr. Zeigler is one of the outgoing resident medical editors for Thoracic Surgery News.
The era of the Affordable Care Act is upon us, and short of an unlikely repeal following midterm elections, this will remain the law of the land. As surgical residents, most of us have neither the time nor mental stamina to become significantly entrenched in politics. As a result, many of us know less about the impact that the Affordable Care Act will have on our future livelihood than many of the Senators did when they passed the bill on December 24, 2009. While most of the public focus has been on the individual mandate, pre-existing conditions, and insurance exchanges, further hidden from the public eye are the methods by which our fundamental model for health care reimbursement will change.
Much of the mystery was dispelled for me this May, when I heard a lecture entitled "The Perfect Storm: The Affordable Care Act and the Repeal of the SGR" by Dr. Jeffrey Rich. Dr. Rich’s presentation was the 2014 Norman E. Shumway, MD, Visiting Professorship Lecture at Stanford (Calif.) University, a webcast of which is available at http://ctsurgery.stanford.edu/media/.
Dr. Rich has a unique perspective on the issue of health care reimbursement, as he has served both as president of STS from 2012-2013 and director of the Center for Medicare Management, part of the Centers for Medicare & Medicaid Services, in 2008, in the political tumult leading up to the passage of the Affordable Care Act. On top of this, he has remained a practicing cardiothoracic surgeon in the Sentara Health System and sits as director-at-large for the Virginia Cardiac Surgery Quality Initiative. It is impossible to overstate the impact that he and his staff have had on the future of health care reimbursement. His recent lecture highlighted the ideological change in payment models that the Affordable Care Act embodies, along with the carrots and the sticks that the government will be wielding over the next 5 years to change physician and hospital behavior. This, along with the untenable continuation of the Sustainable Growth Rate (SGR) with all of its problems, portends huge swings both positive and negative for the reimbursement of all doctors, and cardiothoracic surgeons in particular. Early adopters may find themselves with a much-needed windfall, while those who do not anticipate the changes may find themselves in dire financial straits.
First, let us examine where we stand. The United States spends 17.6% of its gross domestic product (GDP) on health care. The nearest rival sits at 12%. State and federal government together spent $1.5 trillion on health care in 2013. Add private insurance into the mix and the figure is $2.8 trillion. Our life expectancy has not followed the money, and the rate of increase in health care spending is far outstripping inflation and the growth in our GDP. Our spending has increased exponentially since the passage of the Social Security Amendments of 1965 and shows no sign of slowing down. These statistics are well publicized, and should no longer be a surprise to anyone.
Keeping in mind that hindsight is 20/20, it seems obvious how we got here. The private health insurance industry took off during WWII, when competitive wage controls were put in place to keep skilled laborers in jobs supporting the war effort. To compete for laborers, private sector employers began offering health insurance policies. Shortly thereafter, public pressure to provide a health care safety net culminated in the creation of Medicare and Medicaid in 1965, and our complex public/private health insurance environment was born. For the first decade or so, physician reimbursement was based on "reasonable charge," meaning that doctors sent a bill to Medicare and, if it was considered reasonable, the doctor was paid. This fee-for-service model can be seen as a blank check of sorts, in that it contained few stipulations to withhold repayment for redundant or unnecessary tests and procedures. Expenses associated with complications also were reimbursable. The incentive to "do more" was set. It is worth noting that the Social Security Amendments of 1965 are federal law, and the law stipulates that reimbursement is tied to the amount of work that a physician performs, which also forbids associating reimbursement with the quality of work that the physician produces. It takes an act of Congress to change such law.
The Affordable Care Act is that act. Dr. Rich’s work at CMS paved the way for the inclusion of "Title III: Improving the quality and efficiency of health care," which allows Medicare and Medicaid reimbursements to be altered based on efficiency and outcomes, moving away (although not disintegrating) the fee-for-service model. It incentivizes the development of Accountable Care Organizations and Clinically Integrated Networks to encourage cross-specialty collaboration within the fee-for-service model and lays the groundwork for physician and hospital reimbursement to be based on high-quality, efficient, and appropriate care.
This is the most comprehensive change to the status quo, but it is by no means the first. By 1975, the federal government could see that open fee-for-service was leading to skyrocketing health care costs. It began experimenting with ways to curb physician charges. It pegged reimbursements to the Medicare Economic Index (still used to update hospital reimbursements by 3.2%-3.6% per year) and then tried basing reimbursement on relative value units. Costs continued to rise. Thirty years after the 1965 law, as health care spending continued to spiral out of control, the Sustainable Growth Rate was applied to physician repayment as an attempt to reel it in. The basic premise was that increased costs from increased patient and procedure volume would be curbed by decreasing the reimbursement per procedure.
The sustainable growth model essentially placed a spending target that would grow in step with GDP using the total expenditures beginning in 1996 as a benchmark. If, during a given year, spending outstripped the target, the following year a compensatory decrease in physician reimbursement would be enacted. If spending were less than the target, then physician payments would increase. Expenditures have exceeded the target every year since 2002, and each year our spending gets further and further from the benchmark, compounding the penalty. If the SGR penalties were allowed, it is estimated that physician repayment would drop by 25%-35% in the next few years. Each time the penalty is about to be applied, a fix is passed by Congress, saving our livelihoods at the last minute. Although we should be thankful not to take a 35% pay cut, the SGR and its fixes increased physician repayment by a mere 5.1% between 1992 and 2012. For comparison, Social Security benefits, adjusted annually to compensate increasing cost of living, have risen 52.9% in the same period. Meanwhile, as I mentioned earlier, hospital reimbursement continues to be tied to the Medicare Economic Index, which yields a fairly predictable payment increase of 3.2%-3.6% each year. The Affordable Care Act operates as a law separate from the SGR law, though the two are closely intertwined.
Title III of the Affordable Care Act provides a number of new incentives and penalties that will help make efficiency and quality goals that affect profit at least as much as procedural volume. It will impact hospitals and physicians in a number of new and potentially positive ways. Most immediately concerning to hospitals and medical groups are the incentives for quality. With value-based purchasing, Medicare will withhold 2% of diagnosis-related group (DRG) reimbursements to hospitals at the beginning of a year, giving them the chance to earn it back at the end of the year if they meet quality and efficiency performance goals. The top performers will receive a bonus from the funds collected from those who do not meet goals, making this a budget-neutral operation. Cardiothoracic surgeons will feel this scrutiny early, as the first five DRGs subject to the law are acute myocardial infarction, heart failure, pneumonia, surgeries, and health care–associated infections. As time passes, more diagnoses will be added.
Payments will be based on bundled care, meaning that a hospital will be paid one sum to cover the peri-admission period, starting from 3 days prior to 30 days after admission. Complications, readmissions, and repeat tests will not generate additional funds for the hospital. You can expect that daily chest x-rays and multiple echocardiograms will generate a lot of e-mails to attending physicians. Other preventable hospital-acquired conditions, such as catheter-associated urinary tract infections and pressure ulcers, if present in rates beyond the norm for the country, could cut reimbursements an additional 1%. Patient satisfaction scores will influence hospital reimbursement. Readmission rates beyond the specified cutoff for each admission will result in a 3% hospital pay cut, again, starting with the same set of diagnoses. When meaningful use of electronic health records incentives are factored in, hospitals are looking at a 7% swing on reimbursements for the DRGs listed above by 2017. Hospitals typically operate on a profit margin around 3.5%.
On the individual physician level, there are a number of changes. Already in place was a bonus for participating in physician quality reporting systems (PQRS), such as the STS database. By 2016 the bonus for participating will become a 2% pay cut for not participating. Thankfully, our specialty has been forward thinking in this regard, and the majority of cardiothoracic practices already participate in the STS database. Similar to value-based purchasing, the physician value modifier will apply a 2% bonus or penalty to reimbursements, based on a broad spectrum of quality measures, including patient safety, population and community health, total cost per patient by condition, and patient experience. Again, this will be budget neutral. When all of the items are tallied, the lowest-performing providers could see a 6% decrease in their personal reimbursement.
The SGR has not been fixed with the Affordable Care Act. Dr. Rich, in his role as STS president, provided testimony to Congress leading up to the most recent attempt to reform the law. Part of the main thrust of his testimony was that each specialty needs to set its own outcomes standards through database-driven research. Incentives for improved outcomes need to be in place for all members of the heart team, not just the physicians. All three of the proposed bills that followed his testimony included such incentives, but they also included even more dismal updates to physician payments than we have seen in the past 20 years. For better or for worse, none of the bills passed, and we can look forward to more anxiety as we await the next SGR patch. Whatever durable solution passes will likely focus on these new models of payment but without a significant boost in hospital income.
Currently, the alternative payment model (APM) pilot programs are still being developed. CMS has a $10 billion budget to fund the pilot programs, and consulting groups that advised the agency chose cardiothoracic surgery as a top priority for APM development. Current discussions indicate that participants in APMs could get a 5% bonus and would not be subject to the physician value modifier.
So how does this apply to us residents? Despite our ground-level perspective, we must recognize that we are straddling two drastically different eras in the practice of medicine. It will be the duty of all of us, not just our attendings, to reduce our costs and provide better patient care. This may mean using our stethoscope more effectively or making those extra phone calls to avoid unnecessary or repeated tests. We need to rebel against the ideology of physician shift work by owning our patients, but still work effectively in that system. When it comes time to seek our first jobs, we should focus not just on the department that we will work on, but its context within the local hospital system. The most vibrant department within an unresponsive hospital system will drown in the future penalties, and likewise for an unenthusiastic department within a forward-thinking system. In short, we need to start training ourselves to be keener, sharper, and more agile physicians, and to position ourselves within like-minded environments. Perhaps more important than any of these, we need to reclaim the right to shape our own profession. In recent history there has not been a better opportunity for cardiothoracic surgeons as a group to assert themselves as adept physicians and leaders. Whether we become head of CMS, participate in STS fly-ins to Capitol Hill, write our congressman about the issues we face, or engage our hospitals to anticipate the coming changes, it is up to us to ensure that we have a future.
Dr. Zeigler is one of the outgoing resident medical editors for Thoracic Surgery News.
The era of the Affordable Care Act is upon us, and short of an unlikely repeal following midterm elections, this will remain the law of the land. As surgical residents, most of us have neither the time nor mental stamina to become significantly entrenched in politics. As a result, many of us know less about the impact that the Affordable Care Act will have on our future livelihood than many of the Senators did when they passed the bill on December 24, 2009. While most of the public focus has been on the individual mandate, pre-existing conditions, and insurance exchanges, further hidden from the public eye are the methods by which our fundamental model for health care reimbursement will change.
Much of the mystery was dispelled for me this May, when I heard a lecture entitled "The Perfect Storm: The Affordable Care Act and the Repeal of the SGR" by Dr. Jeffrey Rich. Dr. Rich’s presentation was the 2014 Norman E. Shumway, MD, Visiting Professorship Lecture at Stanford (Calif.) University, a webcast of which is available at http://ctsurgery.stanford.edu/media/.
Dr. Rich has a unique perspective on the issue of health care reimbursement, as he has served both as president of STS from 2012-2013 and director of the Center for Medicare Management, part of the Centers for Medicare & Medicaid Services, in 2008, in the political tumult leading up to the passage of the Affordable Care Act. On top of this, he has remained a practicing cardiothoracic surgeon in the Sentara Health System and sits as director-at-large for the Virginia Cardiac Surgery Quality Initiative. It is impossible to overstate the impact that he and his staff have had on the future of health care reimbursement. His recent lecture highlighted the ideological change in payment models that the Affordable Care Act embodies, along with the carrots and the sticks that the government will be wielding over the next 5 years to change physician and hospital behavior. This, along with the untenable continuation of the Sustainable Growth Rate (SGR) with all of its problems, portends huge swings both positive and negative for the reimbursement of all doctors, and cardiothoracic surgeons in particular. Early adopters may find themselves with a much-needed windfall, while those who do not anticipate the changes may find themselves in dire financial straits.
First, let us examine where we stand. The United States spends 17.6% of its gross domestic product (GDP) on health care. The nearest rival sits at 12%. State and federal government together spent $1.5 trillion on health care in 2013. Add private insurance into the mix and the figure is $2.8 trillion. Our life expectancy has not followed the money, and the rate of increase in health care spending is far outstripping inflation and the growth in our GDP. Our spending has increased exponentially since the passage of the Social Security Amendments of 1965 and shows no sign of slowing down. These statistics are well publicized, and should no longer be a surprise to anyone.
Keeping in mind that hindsight is 20/20, it seems obvious how we got here. The private health insurance industry took off during WWII, when competitive wage controls were put in place to keep skilled laborers in jobs supporting the war effort. To compete for laborers, private sector employers began offering health insurance policies. Shortly thereafter, public pressure to provide a health care safety net culminated in the creation of Medicare and Medicaid in 1965, and our complex public/private health insurance environment was born. For the first decade or so, physician reimbursement was based on "reasonable charge," meaning that doctors sent a bill to Medicare and, if it was considered reasonable, the doctor was paid. This fee-for-service model can be seen as a blank check of sorts, in that it contained few stipulations to withhold repayment for redundant or unnecessary tests and procedures. Expenses associated with complications also were reimbursable. The incentive to "do more" was set. It is worth noting that the Social Security Amendments of 1965 are federal law, and the law stipulates that reimbursement is tied to the amount of work that a physician performs, which also forbids associating reimbursement with the quality of work that the physician produces. It takes an act of Congress to change such law.
The Affordable Care Act is that act. Dr. Rich’s work at CMS paved the way for the inclusion of "Title III: Improving the quality and efficiency of health care," which allows Medicare and Medicaid reimbursements to be altered based on efficiency and outcomes, moving away (although not disintegrating) the fee-for-service model. It incentivizes the development of Accountable Care Organizations and Clinically Integrated Networks to encourage cross-specialty collaboration within the fee-for-service model and lays the groundwork for physician and hospital reimbursement to be based on high-quality, efficient, and appropriate care.
This is the most comprehensive change to the status quo, but it is by no means the first. By 1975, the federal government could see that open fee-for-service was leading to skyrocketing health care costs. It began experimenting with ways to curb physician charges. It pegged reimbursements to the Medicare Economic Index (still used to update hospital reimbursements by 3.2%-3.6% per year) and then tried basing reimbursement on relative value units. Costs continued to rise. Thirty years after the 1965 law, as health care spending continued to spiral out of control, the Sustainable Growth Rate was applied to physician repayment as an attempt to reel it in. The basic premise was that increased costs from increased patient and procedure volume would be curbed by decreasing the reimbursement per procedure.
The sustainable growth model essentially placed a spending target that would grow in step with GDP using the total expenditures beginning in 1996 as a benchmark. If, during a given year, spending outstripped the target, the following year a compensatory decrease in physician reimbursement would be enacted. If spending were less than the target, then physician payments would increase. Expenditures have exceeded the target every year since 2002, and each year our spending gets further and further from the benchmark, compounding the penalty. If the SGR penalties were allowed, it is estimated that physician repayment would drop by 25%-35% in the next few years. Each time the penalty is about to be applied, a fix is passed by Congress, saving our livelihoods at the last minute. Although we should be thankful not to take a 35% pay cut, the SGR and its fixes increased physician repayment by a mere 5.1% between 1992 and 2012. For comparison, Social Security benefits, adjusted annually to compensate increasing cost of living, have risen 52.9% in the same period. Meanwhile, as I mentioned earlier, hospital reimbursement continues to be tied to the Medicare Economic Index, which yields a fairly predictable payment increase of 3.2%-3.6% each year. The Affordable Care Act operates as a law separate from the SGR law, though the two are closely intertwined.
Title III of the Affordable Care Act provides a number of new incentives and penalties that will help make efficiency and quality goals that affect profit at least as much as procedural volume. It will impact hospitals and physicians in a number of new and potentially positive ways. Most immediately concerning to hospitals and medical groups are the incentives for quality. With value-based purchasing, Medicare will withhold 2% of diagnosis-related group (DRG) reimbursements to hospitals at the beginning of a year, giving them the chance to earn it back at the end of the year if they meet quality and efficiency performance goals. The top performers will receive a bonus from the funds collected from those who do not meet goals, making this a budget-neutral operation. Cardiothoracic surgeons will feel this scrutiny early, as the first five DRGs subject to the law are acute myocardial infarction, heart failure, pneumonia, surgeries, and health care–associated infections. As time passes, more diagnoses will be added.
Payments will be based on bundled care, meaning that a hospital will be paid one sum to cover the peri-admission period, starting from 3 days prior to 30 days after admission. Complications, readmissions, and repeat tests will not generate additional funds for the hospital. You can expect that daily chest x-rays and multiple echocardiograms will generate a lot of e-mails to attending physicians. Other preventable hospital-acquired conditions, such as catheter-associated urinary tract infections and pressure ulcers, if present in rates beyond the norm for the country, could cut reimbursements an additional 1%. Patient satisfaction scores will influence hospital reimbursement. Readmission rates beyond the specified cutoff for each admission will result in a 3% hospital pay cut, again, starting with the same set of diagnoses. When meaningful use of electronic health records incentives are factored in, hospitals are looking at a 7% swing on reimbursements for the DRGs listed above by 2017. Hospitals typically operate on a profit margin around 3.5%.
On the individual physician level, there are a number of changes. Already in place was a bonus for participating in physician quality reporting systems (PQRS), such as the STS database. By 2016 the bonus for participating will become a 2% pay cut for not participating. Thankfully, our specialty has been forward thinking in this regard, and the majority of cardiothoracic practices already participate in the STS database. Similar to value-based purchasing, the physician value modifier will apply a 2% bonus or penalty to reimbursements, based on a broad spectrum of quality measures, including patient safety, population and community health, total cost per patient by condition, and patient experience. Again, this will be budget neutral. When all of the items are tallied, the lowest-performing providers could see a 6% decrease in their personal reimbursement.
The SGR has not been fixed with the Affordable Care Act. Dr. Rich, in his role as STS president, provided testimony to Congress leading up to the most recent attempt to reform the law. Part of the main thrust of his testimony was that each specialty needs to set its own outcomes standards through database-driven research. Incentives for improved outcomes need to be in place for all members of the heart team, not just the physicians. All three of the proposed bills that followed his testimony included such incentives, but they also included even more dismal updates to physician payments than we have seen in the past 20 years. For better or for worse, none of the bills passed, and we can look forward to more anxiety as we await the next SGR patch. Whatever durable solution passes will likely focus on these new models of payment but without a significant boost in hospital income.
Currently, the alternative payment model (APM) pilot programs are still being developed. CMS has a $10 billion budget to fund the pilot programs, and consulting groups that advised the agency chose cardiothoracic surgery as a top priority for APM development. Current discussions indicate that participants in APMs could get a 5% bonus and would not be subject to the physician value modifier.
So how does this apply to us residents? Despite our ground-level perspective, we must recognize that we are straddling two drastically different eras in the practice of medicine. It will be the duty of all of us, not just our attendings, to reduce our costs and provide better patient care. This may mean using our stethoscope more effectively or making those extra phone calls to avoid unnecessary or repeated tests. We need to rebel against the ideology of physician shift work by owning our patients, but still work effectively in that system. When it comes time to seek our first jobs, we should focus not just on the department that we will work on, but its context within the local hospital system. The most vibrant department within an unresponsive hospital system will drown in the future penalties, and likewise for an unenthusiastic department within a forward-thinking system. In short, we need to start training ourselves to be keener, sharper, and more agile physicians, and to position ourselves within like-minded environments. Perhaps more important than any of these, we need to reclaim the right to shape our own profession. In recent history there has not been a better opportunity for cardiothoracic surgeons as a group to assert themselves as adept physicians and leaders. Whether we become head of CMS, participate in STS fly-ins to Capitol Hill, write our congressman about the issues we face, or engage our hospitals to anticipate the coming changes, it is up to us to ensure that we have a future.
Dr. Zeigler is one of the outgoing resident medical editors for Thoracic Surgery News.
ICD-10 Will Allow Dermatologists to Effectively Communicate With Payors About Patient Visits
It is important that dermatologists do not overlook the changes associated with the transition to International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Some physicians believe that providing this level of specificity is not important because at the end of the day, who is watching? You will see with several of the codes highlighted in this column that specificity may be required before a claim can be submitted to the payor for processing. In addition, some older catchall codes will go away, forcing us to provide additional specificity.1
Scabies coding is a good example of an added specificity requirement. Currently, with the International Classification of Diseases, Ninth Revision (ICD-9), we have a code for scabies but no code to address the postscabetic pruritus that patients often develop. The ICD-9 code 133.0 applies to scabies, and if we would like to code for the itch, we must enter a second code for unspecified pruritic disorder (698.9).2 With ICD-10-CM, we can be more specific and actually code for the cause of the itch. We will be able to mark the initial visit as B86 (scabies), but subsequent visits for the itch related to scabies can be coded as pruritus using the primary code L29.8 (other pruritus) and a new sequelae or late effect code of B94 (sequelae of other and unspecified infectious and parasitic diseases).3 You will still be able to submit the second visit with scabies as the primary diagnosis, but this practice should be avoided to prevent claim rejection on the backend. In addition, there will be codes to allow for evaluation of a family member or close contact for this condition. Although coding these patients as an initial visit for scabies may be easier, for epidemiologic purposes a more complete code to provide would be the ICD-10-CM code of Z11.8 (encounter for screening for other infectious and parasitic diseases), particularly if they are not found to have scabies.3 This code also would be useful when screening for head lice when a close contact is not found to have it. What do we currently code when someone comes for screening of head lice because a classmate or sibling has it? With ICD-10-CM we will have a more accurate way to communicate with the payor.
The increase in the number of codes with ICD-10-CM permits us to give a description on a situation or circumstance when a person who may not be sick comes into the office for a specific reason or when circumstances influence a person’s health status but those circumstances are not an actual illness or injury. It is frustrating that I currently am not able to code for a skin examination appropriately using ICD-9. Let me be clear, I am not saying that this new code will be reimbursable. We will have to wait and see how we are reimbursed for all of these codes, but at least we will have the option of coding for the reason the patient presents to me, which is often a skin examination, and then the secondary code could be benign nevi or seborrheic keratosis. The code Z12.83 (encounter for screening for malignant neoplasm of skin) will now be the best code for these purposes.3 In addition, codes that address postoperative nursing visits have not been available. With ICD-10-CM we will have a new code for encounter for surgical aftercare following surgery on the skin and subcutaneous tissue that does not include a standard suture removal but is a necessary visit (Z48.817). In addition, we will have a specific code to document encounters for allergy testing (Z01.82).3 With ICD-9, rash not otherwise specified or eczema would have to be the primary code (782.1) and you were forced to place a code for the patient’s allergy, regardless of whether or not the allergy was known.
The reimbursement of these codes has not been addressed, so ideally during the testing period there can be a head-to-head comparison. By adhering to some of the new rules, hopefully we will be able to more completely communicate about what is occurring during the patient visit.
1. Lamb A. Dermatology coding changes with ICD-10. Cutis. 2014;93:284-285.
2. ICD-9 code lookup. Centers for Medicare & Medicaid Services Web site. http://www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspx. Accessed July 17, 2014.
3. Centers for Medicare & Medicaid Services. ICD-10-CM Tabular List of Diseases and Injuries. http://www.cms.gov/Medicare/Coding/ICD10/downloads/6_I10tab2010.pdf. Published 2010. Accessed July 17, 2014.
It is important that dermatologists do not overlook the changes associated with the transition to International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Some physicians believe that providing this level of specificity is not important because at the end of the day, who is watching? You will see with several of the codes highlighted in this column that specificity may be required before a claim can be submitted to the payor for processing. In addition, some older catchall codes will go away, forcing us to provide additional specificity.1
Scabies coding is a good example of an added specificity requirement. Currently, with the International Classification of Diseases, Ninth Revision (ICD-9), we have a code for scabies but no code to address the postscabetic pruritus that patients often develop. The ICD-9 code 133.0 applies to scabies, and if we would like to code for the itch, we must enter a second code for unspecified pruritic disorder (698.9).2 With ICD-10-CM, we can be more specific and actually code for the cause of the itch. We will be able to mark the initial visit as B86 (scabies), but subsequent visits for the itch related to scabies can be coded as pruritus using the primary code L29.8 (other pruritus) and a new sequelae or late effect code of B94 (sequelae of other and unspecified infectious and parasitic diseases).3 You will still be able to submit the second visit with scabies as the primary diagnosis, but this practice should be avoided to prevent claim rejection on the backend. In addition, there will be codes to allow for evaluation of a family member or close contact for this condition. Although coding these patients as an initial visit for scabies may be easier, for epidemiologic purposes a more complete code to provide would be the ICD-10-CM code of Z11.8 (encounter for screening for other infectious and parasitic diseases), particularly if they are not found to have scabies.3 This code also would be useful when screening for head lice when a close contact is not found to have it. What do we currently code when someone comes for screening of head lice because a classmate or sibling has it? With ICD-10-CM we will have a more accurate way to communicate with the payor.
The increase in the number of codes with ICD-10-CM permits us to give a description on a situation or circumstance when a person who may not be sick comes into the office for a specific reason or when circumstances influence a person’s health status but those circumstances are not an actual illness or injury. It is frustrating that I currently am not able to code for a skin examination appropriately using ICD-9. Let me be clear, I am not saying that this new code will be reimbursable. We will have to wait and see how we are reimbursed for all of these codes, but at least we will have the option of coding for the reason the patient presents to me, which is often a skin examination, and then the secondary code could be benign nevi or seborrheic keratosis. The code Z12.83 (encounter for screening for malignant neoplasm of skin) will now be the best code for these purposes.3 In addition, codes that address postoperative nursing visits have not been available. With ICD-10-CM we will have a new code for encounter for surgical aftercare following surgery on the skin and subcutaneous tissue that does not include a standard suture removal but is a necessary visit (Z48.817). In addition, we will have a specific code to document encounters for allergy testing (Z01.82).3 With ICD-9, rash not otherwise specified or eczema would have to be the primary code (782.1) and you were forced to place a code for the patient’s allergy, regardless of whether or not the allergy was known.
The reimbursement of these codes has not been addressed, so ideally during the testing period there can be a head-to-head comparison. By adhering to some of the new rules, hopefully we will be able to more completely communicate about what is occurring during the patient visit.
It is important that dermatologists do not overlook the changes associated with the transition to International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Some physicians believe that providing this level of specificity is not important because at the end of the day, who is watching? You will see with several of the codes highlighted in this column that specificity may be required before a claim can be submitted to the payor for processing. In addition, some older catchall codes will go away, forcing us to provide additional specificity.1
Scabies coding is a good example of an added specificity requirement. Currently, with the International Classification of Diseases, Ninth Revision (ICD-9), we have a code for scabies but no code to address the postscabetic pruritus that patients often develop. The ICD-9 code 133.0 applies to scabies, and if we would like to code for the itch, we must enter a second code for unspecified pruritic disorder (698.9).2 With ICD-10-CM, we can be more specific and actually code for the cause of the itch. We will be able to mark the initial visit as B86 (scabies), but subsequent visits for the itch related to scabies can be coded as pruritus using the primary code L29.8 (other pruritus) and a new sequelae or late effect code of B94 (sequelae of other and unspecified infectious and parasitic diseases).3 You will still be able to submit the second visit with scabies as the primary diagnosis, but this practice should be avoided to prevent claim rejection on the backend. In addition, there will be codes to allow for evaluation of a family member or close contact for this condition. Although coding these patients as an initial visit for scabies may be easier, for epidemiologic purposes a more complete code to provide would be the ICD-10-CM code of Z11.8 (encounter for screening for other infectious and parasitic diseases), particularly if they are not found to have scabies.3 This code also would be useful when screening for head lice when a close contact is not found to have it. What do we currently code when someone comes for screening of head lice because a classmate or sibling has it? With ICD-10-CM we will have a more accurate way to communicate with the payor.
The increase in the number of codes with ICD-10-CM permits us to give a description on a situation or circumstance when a person who may not be sick comes into the office for a specific reason or when circumstances influence a person’s health status but those circumstances are not an actual illness or injury. It is frustrating that I currently am not able to code for a skin examination appropriately using ICD-9. Let me be clear, I am not saying that this new code will be reimbursable. We will have to wait and see how we are reimbursed for all of these codes, but at least we will have the option of coding for the reason the patient presents to me, which is often a skin examination, and then the secondary code could be benign nevi or seborrheic keratosis. The code Z12.83 (encounter for screening for malignant neoplasm of skin) will now be the best code for these purposes.3 In addition, codes that address postoperative nursing visits have not been available. With ICD-10-CM we will have a new code for encounter for surgical aftercare following surgery on the skin and subcutaneous tissue that does not include a standard suture removal but is a necessary visit (Z48.817). In addition, we will have a specific code to document encounters for allergy testing (Z01.82).3 With ICD-9, rash not otherwise specified or eczema would have to be the primary code (782.1) and you were forced to place a code for the patient’s allergy, regardless of whether or not the allergy was known.
The reimbursement of these codes has not been addressed, so ideally during the testing period there can be a head-to-head comparison. By adhering to some of the new rules, hopefully we will be able to more completely communicate about what is occurring during the patient visit.
1. Lamb A. Dermatology coding changes with ICD-10. Cutis. 2014;93:284-285.
2. ICD-9 code lookup. Centers for Medicare & Medicaid Services Web site. http://www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspx. Accessed July 17, 2014.
3. Centers for Medicare & Medicaid Services. ICD-10-CM Tabular List of Diseases and Injuries. http://www.cms.gov/Medicare/Coding/ICD10/downloads/6_I10tab2010.pdf. Published 2010. Accessed July 17, 2014.
1. Lamb A. Dermatology coding changes with ICD-10. Cutis. 2014;93:284-285.
2. ICD-9 code lookup. Centers for Medicare & Medicaid Services Web site. http://www.cms.gov/medicare-coverage-database/staticpages/icd-9-code-lookup.aspx. Accessed July 17, 2014.
3. Centers for Medicare & Medicaid Services. ICD-10-CM Tabular List of Diseases and Injuries. http://www.cms.gov/Medicare/Coding/ICD10/downloads/6_I10tab2010.pdf. Published 2010. Accessed July 17, 2014.
Practice Points
- With International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), dermatologists will have a more accurate way to communicate with the payor. For example, physicians will be able to code for scabies as well as the cause of postscabetic pruritus.
- Physicians will have the option of coding for the reason the patient presented. For example, dermatologists may code for a skin examination to screen for a malignant neoplasm.
- The reimbursement of the new codes has not been addressed; a head-to-head comparison will be needed during the testing period.