User login
Method could speed up cancer diagnosis

Credit: NIGMS
A new technique could enable faster diagnosis of cancer and various prenatal conditions, according to a paper published in Proceedings of the National Academy of Sciences.
The method, known as convex lens-induced confinement (CLIC), allows researchers to load long strands of DNA into a tunable, nanoscale imaging chamber in ways that maintain their structural identity and under conditions that are similar to those in the human body.
CLIC lets researchers map large genomes rapidly and identify specific gene sequences from single cells with single-molecule resolution, a process that is critical to diagnosing diseases like cancer.
“Current practices of genomic analysis typically require tens of thousands of cells worth of genomic material to obtain the information we need, but this new approach works with single cells,” said study author Rob Sladek, MD, of McGill University in Montreal, Canada.
“CLIC will allow researchers to avoid having to spend time stitching together maps of entire genomes, as we do under current techniques, and promises to make genomic analysis a much simpler and more efficient process.”
The CLIC imaging chamber can sit on top of a standard inverted fluorescence microscope, and strands of DNA can be loaded into the chamber from above, which allows the strands to maintain their integrity.
Existing tools used for genomic analysis rely on side-loading DNA under pressure into nanochannels in the imaging chamber. This breaks the DNA molecules into small pieces, making it a challenge to reconstruct the genome.
CLIC, on the other hand, is “like squeezing many soft spaghetti noodles into long, narrow tubes without breaking them,” according to study author Sabrina Leslie, PhD, also of McGill University.
“Once these long strands of DNA are gently squeezed down into nanochannels from a nanoscale bath above, they become effectively rigid, which means that we can map positions along uniformly stretched strands of DNA, while holding them still,” she said.
“This means diagnostics can be performed quickly, one cell at a time, which is critical for diagnosing many prenatal conditions and the onset of cancer.” ![]()

Credit: NIGMS
A new technique could enable faster diagnosis of cancer and various prenatal conditions, according to a paper published in Proceedings of the National Academy of Sciences.
The method, known as convex lens-induced confinement (CLIC), allows researchers to load long strands of DNA into a tunable, nanoscale imaging chamber in ways that maintain their structural identity and under conditions that are similar to those in the human body.
CLIC lets researchers map large genomes rapidly and identify specific gene sequences from single cells with single-molecule resolution, a process that is critical to diagnosing diseases like cancer.
“Current practices of genomic analysis typically require tens of thousands of cells worth of genomic material to obtain the information we need, but this new approach works with single cells,” said study author Rob Sladek, MD, of McGill University in Montreal, Canada.
“CLIC will allow researchers to avoid having to spend time stitching together maps of entire genomes, as we do under current techniques, and promises to make genomic analysis a much simpler and more efficient process.”
The CLIC imaging chamber can sit on top of a standard inverted fluorescence microscope, and strands of DNA can be loaded into the chamber from above, which allows the strands to maintain their integrity.
Existing tools used for genomic analysis rely on side-loading DNA under pressure into nanochannels in the imaging chamber. This breaks the DNA molecules into small pieces, making it a challenge to reconstruct the genome.
CLIC, on the other hand, is “like squeezing many soft spaghetti noodles into long, narrow tubes without breaking them,” according to study author Sabrina Leslie, PhD, also of McGill University.
“Once these long strands of DNA are gently squeezed down into nanochannels from a nanoscale bath above, they become effectively rigid, which means that we can map positions along uniformly stretched strands of DNA, while holding them still,” she said.
“This means diagnostics can be performed quickly, one cell at a time, which is critical for diagnosing many prenatal conditions and the onset of cancer.” ![]()

Credit: NIGMS
A new technique could enable faster diagnosis of cancer and various prenatal conditions, according to a paper published in Proceedings of the National Academy of Sciences.
The method, known as convex lens-induced confinement (CLIC), allows researchers to load long strands of DNA into a tunable, nanoscale imaging chamber in ways that maintain their structural identity and under conditions that are similar to those in the human body.
CLIC lets researchers map large genomes rapidly and identify specific gene sequences from single cells with single-molecule resolution, a process that is critical to diagnosing diseases like cancer.
“Current practices of genomic analysis typically require tens of thousands of cells worth of genomic material to obtain the information we need, but this new approach works with single cells,” said study author Rob Sladek, MD, of McGill University in Montreal, Canada.
“CLIC will allow researchers to avoid having to spend time stitching together maps of entire genomes, as we do under current techniques, and promises to make genomic analysis a much simpler and more efficient process.”
The CLIC imaging chamber can sit on top of a standard inverted fluorescence microscope, and strands of DNA can be loaded into the chamber from above, which allows the strands to maintain their integrity.
Existing tools used for genomic analysis rely on side-loading DNA under pressure into nanochannels in the imaging chamber. This breaks the DNA molecules into small pieces, making it a challenge to reconstruct the genome.
CLIC, on the other hand, is “like squeezing many soft spaghetti noodles into long, narrow tubes without breaking them,” according to study author Sabrina Leslie, PhD, also of McGill University.
“Once these long strands of DNA are gently squeezed down into nanochannels from a nanoscale bath above, they become effectively rigid, which means that we can map positions along uniformly stretched strands of DNA, while holding them still,” she said.
“This means diagnostics can be performed quickly, one cell at a time, which is critical for diagnosing many prenatal conditions and the onset of cancer.” ![]()
Drug gets fast track designation for MF

Credit: Peter Anderson
The US Food and Drug Administration (FDA) is expediting its review of pacritinib, a tyrosine kinase inhibitor with activity against JAK2 and FLT3, by granting the drug fast track designation.
Pacritinib is under review as a treatment for patients with intermediate- and high-risk myelofibrosis (MF), including those with disease-related or treatment-induced thrombocytopenia and those who cannot tolerate or do not respond well to other JAK2 therapy.
The FDA’s fast track process is designed to expedite the review of drugs to treat serious conditions and fill an unmet medical need.
The program enables a company—in this case, CTI BioPharma—to submit sections of a new drug application on a rolling basis as data becomes available.
That way, the FDA can review sections of the application as they are received, rather than waiting until every section of the application is completed before the entire application can be reviewed. This often leads to faster approval.
Pacritinib is currently under investigation in two phase 3 clinical trials, known as the PERSIST program, for patients with MF.
One of these trials, known as PERSIST-1, includes a broad set of patients without limitations on platelet counts. The other, PERSIST-2, includes patients with low platelet counts.
PERSIST-1
In July 2014, CTI Biopharma completed enrollment in the PERSIST-1 trial, which was designed to enroll approximately 320 patients.
This randomized trial was designed to compared the efficacy and safety of pacritinib with that of best available therapy, other than JAK inhibitors, in patients with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF, without exclusion for low platelet counts.
The primary endpoint is the percentage of patients achieving at least a 35% reduction in spleen volume, measured by MRI or CT from baseline to 24 weeks of treatment.
PERSIST-2
In March 2014, CTI announced the initiation of the PERSIST-2 trial, a comparison of pacritinib and best available therapy, including approved JAK2 inhibitors that are dosed according to product label, in patients with MF whose platelet counts are 100,000/uL or lower.
The trial is designed to enroll up to 300 patients in North America, Europe, Australia, and New Zealand. In October 2013, CTI reached agreement with the FDA on a special protocol assessment for the trial, a written agreement between CTI and the FDA regarding the planned design, endpoints, and statistical analysis approach of the trial to be used in support of a potential new drug application.
Under the special protocol assessment, the trial will have two primary endpoints. The first is the percentage of patients achieving a 35% or greater reduction in spleen volume, measured by MRI or CT scan from baseline to 24 weeks of treatment.
The second primary endpoint is the percentage of patients achieving a total symptom score reduction of 50% or greater using 6 key symptoms, as measured by the modified Myeloproliferative Neoplasm Symptom Assessment (MPN-SAF TSS 2.0) diary from baseline to 24 weeks.
More details on the PERSIST-1 and PERSIST-2 trials can be found at www.clinicaltrials.gov. ![]()

Credit: Peter Anderson
The US Food and Drug Administration (FDA) is expediting its review of pacritinib, a tyrosine kinase inhibitor with activity against JAK2 and FLT3, by granting the drug fast track designation.
Pacritinib is under review as a treatment for patients with intermediate- and high-risk myelofibrosis (MF), including those with disease-related or treatment-induced thrombocytopenia and those who cannot tolerate or do not respond well to other JAK2 therapy.
The FDA’s fast track process is designed to expedite the review of drugs to treat serious conditions and fill an unmet medical need.
The program enables a company—in this case, CTI BioPharma—to submit sections of a new drug application on a rolling basis as data becomes available.
That way, the FDA can review sections of the application as they are received, rather than waiting until every section of the application is completed before the entire application can be reviewed. This often leads to faster approval.
Pacritinib is currently under investigation in two phase 3 clinical trials, known as the PERSIST program, for patients with MF.
One of these trials, known as PERSIST-1, includes a broad set of patients without limitations on platelet counts. The other, PERSIST-2, includes patients with low platelet counts.
PERSIST-1
In July 2014, CTI Biopharma completed enrollment in the PERSIST-1 trial, which was designed to enroll approximately 320 patients.
This randomized trial was designed to compared the efficacy and safety of pacritinib with that of best available therapy, other than JAK inhibitors, in patients with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF, without exclusion for low platelet counts.
The primary endpoint is the percentage of patients achieving at least a 35% reduction in spleen volume, measured by MRI or CT from baseline to 24 weeks of treatment.
PERSIST-2
In March 2014, CTI announced the initiation of the PERSIST-2 trial, a comparison of pacritinib and best available therapy, including approved JAK2 inhibitors that are dosed according to product label, in patients with MF whose platelet counts are 100,000/uL or lower.
The trial is designed to enroll up to 300 patients in North America, Europe, Australia, and New Zealand. In October 2013, CTI reached agreement with the FDA on a special protocol assessment for the trial, a written agreement between CTI and the FDA regarding the planned design, endpoints, and statistical analysis approach of the trial to be used in support of a potential new drug application.
Under the special protocol assessment, the trial will have two primary endpoints. The first is the percentage of patients achieving a 35% or greater reduction in spleen volume, measured by MRI or CT scan from baseline to 24 weeks of treatment.
The second primary endpoint is the percentage of patients achieving a total symptom score reduction of 50% or greater using 6 key symptoms, as measured by the modified Myeloproliferative Neoplasm Symptom Assessment (MPN-SAF TSS 2.0) diary from baseline to 24 weeks.
More details on the PERSIST-1 and PERSIST-2 trials can be found at www.clinicaltrials.gov. ![]()

Credit: Peter Anderson
The US Food and Drug Administration (FDA) is expediting its review of pacritinib, a tyrosine kinase inhibitor with activity against JAK2 and FLT3, by granting the drug fast track designation.
Pacritinib is under review as a treatment for patients with intermediate- and high-risk myelofibrosis (MF), including those with disease-related or treatment-induced thrombocytopenia and those who cannot tolerate or do not respond well to other JAK2 therapy.
The FDA’s fast track process is designed to expedite the review of drugs to treat serious conditions and fill an unmet medical need.
The program enables a company—in this case, CTI BioPharma—to submit sections of a new drug application on a rolling basis as data becomes available.
That way, the FDA can review sections of the application as they are received, rather than waiting until every section of the application is completed before the entire application can be reviewed. This often leads to faster approval.
Pacritinib is currently under investigation in two phase 3 clinical trials, known as the PERSIST program, for patients with MF.
One of these trials, known as PERSIST-1, includes a broad set of patients without limitations on platelet counts. The other, PERSIST-2, includes patients with low platelet counts.
PERSIST-1
In July 2014, CTI Biopharma completed enrollment in the PERSIST-1 trial, which was designed to enroll approximately 320 patients.
This randomized trial was designed to compared the efficacy and safety of pacritinib with that of best available therapy, other than JAK inhibitors, in patients with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF, without exclusion for low platelet counts.
The primary endpoint is the percentage of patients achieving at least a 35% reduction in spleen volume, measured by MRI or CT from baseline to 24 weeks of treatment.
PERSIST-2
In March 2014, CTI announced the initiation of the PERSIST-2 trial, a comparison of pacritinib and best available therapy, including approved JAK2 inhibitors that are dosed according to product label, in patients with MF whose platelet counts are 100,000/uL or lower.
The trial is designed to enroll up to 300 patients in North America, Europe, Australia, and New Zealand. In October 2013, CTI reached agreement with the FDA on a special protocol assessment for the trial, a written agreement between CTI and the FDA regarding the planned design, endpoints, and statistical analysis approach of the trial to be used in support of a potential new drug application.
Under the special protocol assessment, the trial will have two primary endpoints. The first is the percentage of patients achieving a 35% or greater reduction in spleen volume, measured by MRI or CT scan from baseline to 24 weeks of treatment.
The second primary endpoint is the percentage of patients achieving a total symptom score reduction of 50% or greater using 6 key symptoms, as measured by the modified Myeloproliferative Neoplasm Symptom Assessment (MPN-SAF TSS 2.0) diary from baseline to 24 weeks.
More details on the PERSIST-1 and PERSIST-2 trials can be found at www.clinicaltrials.gov. ![]()
ACIP and 2014 flu vaccine
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
Study challenges traditional cancer classification

a tumor sample in a test tube
Credit: Rhoda Baer
Defining cancers by molecular criteria rather than their tissue of origin can provide patients with more accurate diagnoses, researchers have reported in Cell.
The group analyzed the molecular characteristics of more than 3500 samples of 12 different cancers and reclassified them according to the new information.
For 5 of the cancer types, including acute myeloid leukemia (AML), the molecular classification largely matched the tissue-of-origin classification.
For the remaining malignancies, that was not the case.
“This genomic study not only challenges our existing system of classifying cancers based on tissue type, but also provides a massive new data resource for further exploration, as well as a comprehensive list of the molecular features distinguishing each of the newly described cancer classes,” said study author Christopher Benz, MD, of the University of California, San Francisco.
The researchers said each molecular subtype they identified may reflect tumors arising from distinct cell types. For example, the data showed a marked difference between cancers of epithelial and non-epithelial origins.
“We think the subtypes reflect, primarily, the cell of origin,” said study author Joshua Stuart, PhD, of the University of California, Santa Cruz.
“Another factor is the nature of the genomic lesion, and third is the microenvironment of the cell and how surrounding cells influence it. We are disentangling the signals from these different factors so we can gauge each one for its prognostic power.”
Identifying molecular subtypes
The researchers performed an integrative analysis using 5 genome-wide platforms and 1 proteomic platform on 3527 specimens from 12 cancer types.
This included AML, glioblastoma multiforme, serous ovarian carcinoma, colon and rectal adenocarcinomas, lung squamous cell carcinoma, breast cancer, endometrial cancer, renal cell carcinoma, bladder urothelial adenocarcinoma, lung adenocarcinoma, and head and neck squamous cell carcinoma.
The group’s analyses allowed them to classify these cancer types into 11 major cellular/molecular subtypes. Two of the initial 13 subtypes (numbers 11 and 12) were eliminated from further analysis because they included fewer than 10 samples.
Five of the classification types—C5-renal cell carcinoma, C6-endometrial cancer, C9-serous ovarian carcinoma, C10-glioblastoma multiforme, and C13-AML—showed near 1-to-1 relationships with the tissue site of origin. However, there were a few cases of reclassification here and there, such as a case of breast cancer that fell in the AML subtype.
Another subtype stayed pretty true to its tissues of origin. C7-colon adenocarcinoma/rectal adenocarcinoma was composed mainly of colon and rectal adenocarcinomas but also included a case of endometrial cancer.
The C1-lung adenocarcinoma-enriched subtype was predominantly composed of non-small cell lung adenocarcinoma samples. But it also included cases of bladder cancer, breast cancer, colon adenocarcinoma, glioblastoma multiforme, head and neck squamous cell carcinoma, renal cell carcinoma, lung squamous cell carcinoma , serous ovarian carcinoma, and endometrial cancer.
The C2-squamous-like subtype consisted largely of head and neck squamous cell carcinoma and lung squamous cell carcinoma but also included bladder urothelial adenocarcinoma and breast cancer.
Breast cancers were further divided into the C3-breast cancer/luminal subtype and the C4-breast cancer/basal subtype. The C4 subtype also included lung adenocarcinoma and lung squamous cell carcinoma.
The researchers noted that breast cancers were present in 7 of the subtype classifications. And while this study confirmed known differences between the subtypes of breast cancer, the team was surprised to discover that basal-like breast cancers actually constitute their own cancer class.
“Even though these basal-like cancers arise in the breast, on the molecular level, they have more in common with ovarian cancers and cancers of squamous-cell origin than with other subtypes of breast cancer,” said study author Christina Yau, PhD, of the University of California, San Francisco.
Like breast cancers, bladder cancers were present in 7 of the subtype classifications. There were 1 or 2 cases in C5, C10, C11, and C12. But most bladder cancer samples fell into 1 of 3 categories: C1-lung adenocarcinoma-enriched, C2-squamous-like, and C8-bladder urothelial adenocarcinoma.
Although the C8-bladder urothelial adenocarcinoma subtype consisted largely of bladder cancer, it also included breast cancer, head and neck squamous cell carcinoma, lung adenocarcinoma, and lung squamous cell carcinoma.
These findings may help explain why patients with bladder cancer “often respond very differently when treated with the same systemic therapy for their seemingly identical cancer type,” Dr Benz said.
In fact, the researchers found the bladder cancers that clustered with other tumor types had a worse prognosis.
Next steps
The researchers noted that follow-up studies are needed to validate these findings, but this analysis lays the groundwork for classifying tumors into molecularly defined subtypes. The new classification scheme could be used to enroll patients in clinical trials and could lead to different treatment options based on molecular subtypes.
“We can now say what the telltale signatures of the subtypes are, so you can classify a patient’s tumor just based on the gene expression data, or just based on mutation data, if that’s what you have,” Dr Stuart said. “Having a molecular map like this could help get a patient into the right clinical trial.”
The researchers believe the percentage of tumors that should be reclassified based on molecular signatures is likely to grow as more samples and tumor types are analyzed. This study suggested that 1 in 10 cancers could be reclassified in clinically meaningful ways, but the researchers said their next analysis will include 21 tumor types instead of 12.
“We’re just appreciating the tip of the iceberg when considering the potential of this multiplatform type of genomic analysis,” Dr Benz said. “It could be that as many as 30% or 50% of cancers need to be reclassified.”
The data sets and results from this study have been made available to other researchers through the Synapse website. ![]()

a tumor sample in a test tube
Credit: Rhoda Baer
Defining cancers by molecular criteria rather than their tissue of origin can provide patients with more accurate diagnoses, researchers have reported in Cell.
The group analyzed the molecular characteristics of more than 3500 samples of 12 different cancers and reclassified them according to the new information.
For 5 of the cancer types, including acute myeloid leukemia (AML), the molecular classification largely matched the tissue-of-origin classification.
For the remaining malignancies, that was not the case.
“This genomic study not only challenges our existing system of classifying cancers based on tissue type, but also provides a massive new data resource for further exploration, as well as a comprehensive list of the molecular features distinguishing each of the newly described cancer classes,” said study author Christopher Benz, MD, of the University of California, San Francisco.
The researchers said each molecular subtype they identified may reflect tumors arising from distinct cell types. For example, the data showed a marked difference between cancers of epithelial and non-epithelial origins.
“We think the subtypes reflect, primarily, the cell of origin,” said study author Joshua Stuart, PhD, of the University of California, Santa Cruz.
“Another factor is the nature of the genomic lesion, and third is the microenvironment of the cell and how surrounding cells influence it. We are disentangling the signals from these different factors so we can gauge each one for its prognostic power.”
Identifying molecular subtypes
The researchers performed an integrative analysis using 5 genome-wide platforms and 1 proteomic platform on 3527 specimens from 12 cancer types.
This included AML, glioblastoma multiforme, serous ovarian carcinoma, colon and rectal adenocarcinomas, lung squamous cell carcinoma, breast cancer, endometrial cancer, renal cell carcinoma, bladder urothelial adenocarcinoma, lung adenocarcinoma, and head and neck squamous cell carcinoma.
The group’s analyses allowed them to classify these cancer types into 11 major cellular/molecular subtypes. Two of the initial 13 subtypes (numbers 11 and 12) were eliminated from further analysis because they included fewer than 10 samples.
Five of the classification types—C5-renal cell carcinoma, C6-endometrial cancer, C9-serous ovarian carcinoma, C10-glioblastoma multiforme, and C13-AML—showed near 1-to-1 relationships with the tissue site of origin. However, there were a few cases of reclassification here and there, such as a case of breast cancer that fell in the AML subtype.
Another subtype stayed pretty true to its tissues of origin. C7-colon adenocarcinoma/rectal adenocarcinoma was composed mainly of colon and rectal adenocarcinomas but also included a case of endometrial cancer.
The C1-lung adenocarcinoma-enriched subtype was predominantly composed of non-small cell lung adenocarcinoma samples. But it also included cases of bladder cancer, breast cancer, colon adenocarcinoma, glioblastoma multiforme, head and neck squamous cell carcinoma, renal cell carcinoma, lung squamous cell carcinoma , serous ovarian carcinoma, and endometrial cancer.
The C2-squamous-like subtype consisted largely of head and neck squamous cell carcinoma and lung squamous cell carcinoma but also included bladder urothelial adenocarcinoma and breast cancer.
Breast cancers were further divided into the C3-breast cancer/luminal subtype and the C4-breast cancer/basal subtype. The C4 subtype also included lung adenocarcinoma and lung squamous cell carcinoma.
The researchers noted that breast cancers were present in 7 of the subtype classifications. And while this study confirmed known differences between the subtypes of breast cancer, the team was surprised to discover that basal-like breast cancers actually constitute their own cancer class.
“Even though these basal-like cancers arise in the breast, on the molecular level, they have more in common with ovarian cancers and cancers of squamous-cell origin than with other subtypes of breast cancer,” said study author Christina Yau, PhD, of the University of California, San Francisco.
Like breast cancers, bladder cancers were present in 7 of the subtype classifications. There were 1 or 2 cases in C5, C10, C11, and C12. But most bladder cancer samples fell into 1 of 3 categories: C1-lung adenocarcinoma-enriched, C2-squamous-like, and C8-bladder urothelial adenocarcinoma.
Although the C8-bladder urothelial adenocarcinoma subtype consisted largely of bladder cancer, it also included breast cancer, head and neck squamous cell carcinoma, lung adenocarcinoma, and lung squamous cell carcinoma.
These findings may help explain why patients with bladder cancer “often respond very differently when treated with the same systemic therapy for their seemingly identical cancer type,” Dr Benz said.
In fact, the researchers found the bladder cancers that clustered with other tumor types had a worse prognosis.
Next steps
The researchers noted that follow-up studies are needed to validate these findings, but this analysis lays the groundwork for classifying tumors into molecularly defined subtypes. The new classification scheme could be used to enroll patients in clinical trials and could lead to different treatment options based on molecular subtypes.
“We can now say what the telltale signatures of the subtypes are, so you can classify a patient’s tumor just based on the gene expression data, or just based on mutation data, if that’s what you have,” Dr Stuart said. “Having a molecular map like this could help get a patient into the right clinical trial.”
The researchers believe the percentage of tumors that should be reclassified based on molecular signatures is likely to grow as more samples and tumor types are analyzed. This study suggested that 1 in 10 cancers could be reclassified in clinically meaningful ways, but the researchers said their next analysis will include 21 tumor types instead of 12.
“We’re just appreciating the tip of the iceberg when considering the potential of this multiplatform type of genomic analysis,” Dr Benz said. “It could be that as many as 30% or 50% of cancers need to be reclassified.”
The data sets and results from this study have been made available to other researchers through the Synapse website. ![]()

a tumor sample in a test tube
Credit: Rhoda Baer
Defining cancers by molecular criteria rather than their tissue of origin can provide patients with more accurate diagnoses, researchers have reported in Cell.
The group analyzed the molecular characteristics of more than 3500 samples of 12 different cancers and reclassified them according to the new information.
For 5 of the cancer types, including acute myeloid leukemia (AML), the molecular classification largely matched the tissue-of-origin classification.
For the remaining malignancies, that was not the case.
“This genomic study not only challenges our existing system of classifying cancers based on tissue type, but also provides a massive new data resource for further exploration, as well as a comprehensive list of the molecular features distinguishing each of the newly described cancer classes,” said study author Christopher Benz, MD, of the University of California, San Francisco.
The researchers said each molecular subtype they identified may reflect tumors arising from distinct cell types. For example, the data showed a marked difference between cancers of epithelial and non-epithelial origins.
“We think the subtypes reflect, primarily, the cell of origin,” said study author Joshua Stuart, PhD, of the University of California, Santa Cruz.
“Another factor is the nature of the genomic lesion, and third is the microenvironment of the cell and how surrounding cells influence it. We are disentangling the signals from these different factors so we can gauge each one for its prognostic power.”
Identifying molecular subtypes
The researchers performed an integrative analysis using 5 genome-wide platforms and 1 proteomic platform on 3527 specimens from 12 cancer types.
This included AML, glioblastoma multiforme, serous ovarian carcinoma, colon and rectal adenocarcinomas, lung squamous cell carcinoma, breast cancer, endometrial cancer, renal cell carcinoma, bladder urothelial adenocarcinoma, lung adenocarcinoma, and head and neck squamous cell carcinoma.
The group’s analyses allowed them to classify these cancer types into 11 major cellular/molecular subtypes. Two of the initial 13 subtypes (numbers 11 and 12) were eliminated from further analysis because they included fewer than 10 samples.
Five of the classification types—C5-renal cell carcinoma, C6-endometrial cancer, C9-serous ovarian carcinoma, C10-glioblastoma multiforme, and C13-AML—showed near 1-to-1 relationships with the tissue site of origin. However, there were a few cases of reclassification here and there, such as a case of breast cancer that fell in the AML subtype.
Another subtype stayed pretty true to its tissues of origin. C7-colon adenocarcinoma/rectal adenocarcinoma was composed mainly of colon and rectal adenocarcinomas but also included a case of endometrial cancer.
The C1-lung adenocarcinoma-enriched subtype was predominantly composed of non-small cell lung adenocarcinoma samples. But it also included cases of bladder cancer, breast cancer, colon adenocarcinoma, glioblastoma multiforme, head and neck squamous cell carcinoma, renal cell carcinoma, lung squamous cell carcinoma , serous ovarian carcinoma, and endometrial cancer.
The C2-squamous-like subtype consisted largely of head and neck squamous cell carcinoma and lung squamous cell carcinoma but also included bladder urothelial adenocarcinoma and breast cancer.
Breast cancers were further divided into the C3-breast cancer/luminal subtype and the C4-breast cancer/basal subtype. The C4 subtype also included lung adenocarcinoma and lung squamous cell carcinoma.
The researchers noted that breast cancers were present in 7 of the subtype classifications. And while this study confirmed known differences between the subtypes of breast cancer, the team was surprised to discover that basal-like breast cancers actually constitute their own cancer class.
“Even though these basal-like cancers arise in the breast, on the molecular level, they have more in common with ovarian cancers and cancers of squamous-cell origin than with other subtypes of breast cancer,” said study author Christina Yau, PhD, of the University of California, San Francisco.
Like breast cancers, bladder cancers were present in 7 of the subtype classifications. There were 1 or 2 cases in C5, C10, C11, and C12. But most bladder cancer samples fell into 1 of 3 categories: C1-lung adenocarcinoma-enriched, C2-squamous-like, and C8-bladder urothelial adenocarcinoma.
Although the C8-bladder urothelial adenocarcinoma subtype consisted largely of bladder cancer, it also included breast cancer, head and neck squamous cell carcinoma, lung adenocarcinoma, and lung squamous cell carcinoma.
These findings may help explain why patients with bladder cancer “often respond very differently when treated with the same systemic therapy for their seemingly identical cancer type,” Dr Benz said.
In fact, the researchers found the bladder cancers that clustered with other tumor types had a worse prognosis.
Next steps
The researchers noted that follow-up studies are needed to validate these findings, but this analysis lays the groundwork for classifying tumors into molecularly defined subtypes. The new classification scheme could be used to enroll patients in clinical trials and could lead to different treatment options based on molecular subtypes.
“We can now say what the telltale signatures of the subtypes are, so you can classify a patient’s tumor just based on the gene expression data, or just based on mutation data, if that’s what you have,” Dr Stuart said. “Having a molecular map like this could help get a patient into the right clinical trial.”
The researchers believe the percentage of tumors that should be reclassified based on molecular signatures is likely to grow as more samples and tumor types are analyzed. This study suggested that 1 in 10 cancers could be reclassified in clinically meaningful ways, but the researchers said their next analysis will include 21 tumor types instead of 12.
“We’re just appreciating the tip of the iceberg when considering the potential of this multiplatform type of genomic analysis,” Dr Benz said. “It could be that as many as 30% or 50% of cancers need to be reclassified.”
The data sets and results from this study have been made available to other researchers through the Synapse website. ![]()
Two new tests can detect CJD

Credit: Elise Amendola
Two groups of scientists have developed new tests to diagnose Creutzfeldt-Jakob disease (CJD).
One test uses samples collected from nasal passages to detect sporadic CJD, and the other uses urine samples to identify variant CJD.
The researchers said these tests provide simple methods for differentiating CJD from other diseases and could help prevent the transmission of CJD via blood
transfusions, transplants, or contaminated surgical instruments.
Both tests are described in The New England Journal of Medicine.
Nasal test for sporadic CJD
In one NEJM article, Byron Caughey, PhD, of the National Institute of Allergy and Infectious Diseases in Rockville, Maryland, and his colleagues detailed their results with the nasal test.
The researchers collected 31 nasal samples from patients with sporadic CJD and 43 samples from patients who had other neurologic diseases or no neurologic disease. The team brushed the inside of a subject’s nose to collect olfactory neurons connected to the brain.
Testing these samples allowed the researchers to correctly identify 30 of the 31 sporadic CJD patients (97% sensitivity). The tests also correctly showed negative results for all 43 of the non-CJD patients (100% specificity).
By comparison, tests using cerebral spinal fluid, which is currently used to detect sporadic CJD, were 77% sensitive and 100% specific. And these results took twice as long to obtain.
While continuing to validate the new testing method in CJD patients, the scientists are looking to expand their research to diagnose forms of prion diseases in sheep, cattle, and wildlife. The team also hopes to replace the nasal brush with an even simpler swabbing approach.
Urine test for variant CJD
In another NEJM article, Fabio Moda, PhD, of the University of Texas Medical School at Houston, and his colleagues described results observed with their urine test.
The team noted that the infectious agent in transmissible spongiform encephalopathies appears to be composed exclusively of the misfolded form of the prion protein, PrPSc. So they set out to determine if they could detect PrPSc in the urine of patients with CJD.
The researchers analyzed urine samples from healthy individuals (n=52) and patients with variant CJD (n=68), sporadic CJD (n=14), genetic forms of prion disease (n=4), other neurodegenerative disorders (n=50), and nondegenerative neurologic disorders (n=50).
The group found they could only detect PrPSc in samples from patients with variant CJD. They found “minute quantities” of PrPSc in 13 of the 14 urine samples from variant CJD patients, but PrPSc was not present in any of the samples from the other patients or the healthy individuals.
This suggests the test has a sensitivity of 92.9% and a specificity of 100%. ![]()

Credit: Elise Amendola
Two groups of scientists have developed new tests to diagnose Creutzfeldt-Jakob disease (CJD).
One test uses samples collected from nasal passages to detect sporadic CJD, and the other uses urine samples to identify variant CJD.
The researchers said these tests provide simple methods for differentiating CJD from other diseases and could help prevent the transmission of CJD via blood
transfusions, transplants, or contaminated surgical instruments.
Both tests are described in The New England Journal of Medicine.
Nasal test for sporadic CJD
In one NEJM article, Byron Caughey, PhD, of the National Institute of Allergy and Infectious Diseases in Rockville, Maryland, and his colleagues detailed their results with the nasal test.
The researchers collected 31 nasal samples from patients with sporadic CJD and 43 samples from patients who had other neurologic diseases or no neurologic disease. The team brushed the inside of a subject’s nose to collect olfactory neurons connected to the brain.
Testing these samples allowed the researchers to correctly identify 30 of the 31 sporadic CJD patients (97% sensitivity). The tests also correctly showed negative results for all 43 of the non-CJD patients (100% specificity).
By comparison, tests using cerebral spinal fluid, which is currently used to detect sporadic CJD, were 77% sensitive and 100% specific. And these results took twice as long to obtain.
While continuing to validate the new testing method in CJD patients, the scientists are looking to expand their research to diagnose forms of prion diseases in sheep, cattle, and wildlife. The team also hopes to replace the nasal brush with an even simpler swabbing approach.
Urine test for variant CJD
In another NEJM article, Fabio Moda, PhD, of the University of Texas Medical School at Houston, and his colleagues described results observed with their urine test.
The team noted that the infectious agent in transmissible spongiform encephalopathies appears to be composed exclusively of the misfolded form of the prion protein, PrPSc. So they set out to determine if they could detect PrPSc in the urine of patients with CJD.
The researchers analyzed urine samples from healthy individuals (n=52) and patients with variant CJD (n=68), sporadic CJD (n=14), genetic forms of prion disease (n=4), other neurodegenerative disorders (n=50), and nondegenerative neurologic disorders (n=50).
The group found they could only detect PrPSc in samples from patients with variant CJD. They found “minute quantities” of PrPSc in 13 of the 14 urine samples from variant CJD patients, but PrPSc was not present in any of the samples from the other patients or the healthy individuals.
This suggests the test has a sensitivity of 92.9% and a specificity of 100%. ![]()

Credit: Elise Amendola
Two groups of scientists have developed new tests to diagnose Creutzfeldt-Jakob disease (CJD).
One test uses samples collected from nasal passages to detect sporadic CJD, and the other uses urine samples to identify variant CJD.
The researchers said these tests provide simple methods for differentiating CJD from other diseases and could help prevent the transmission of CJD via blood
transfusions, transplants, or contaminated surgical instruments.
Both tests are described in The New England Journal of Medicine.
Nasal test for sporadic CJD
In one NEJM article, Byron Caughey, PhD, of the National Institute of Allergy and Infectious Diseases in Rockville, Maryland, and his colleagues detailed their results with the nasal test.
The researchers collected 31 nasal samples from patients with sporadic CJD and 43 samples from patients who had other neurologic diseases or no neurologic disease. The team brushed the inside of a subject’s nose to collect olfactory neurons connected to the brain.
Testing these samples allowed the researchers to correctly identify 30 of the 31 sporadic CJD patients (97% sensitivity). The tests also correctly showed negative results for all 43 of the non-CJD patients (100% specificity).
By comparison, tests using cerebral spinal fluid, which is currently used to detect sporadic CJD, were 77% sensitive and 100% specific. And these results took twice as long to obtain.
While continuing to validate the new testing method in CJD patients, the scientists are looking to expand their research to diagnose forms of prion diseases in sheep, cattle, and wildlife. The team also hopes to replace the nasal brush with an even simpler swabbing approach.
Urine test for variant CJD
In another NEJM article, Fabio Moda, PhD, of the University of Texas Medical School at Houston, and his colleagues described results observed with their urine test.
The team noted that the infectious agent in transmissible spongiform encephalopathies appears to be composed exclusively of the misfolded form of the prion protein, PrPSc. So they set out to determine if they could detect PrPSc in the urine of patients with CJD.
The researchers analyzed urine samples from healthy individuals (n=52) and patients with variant CJD (n=68), sporadic CJD (n=14), genetic forms of prion disease (n=4), other neurodegenerative disorders (n=50), and nondegenerative neurologic disorders (n=50).
The group found they could only detect PrPSc in samples from patients with variant CJD. They found “minute quantities” of PrPSc in 13 of the 14 urine samples from variant CJD patients, but PrPSc was not present in any of the samples from the other patients or the healthy individuals.
This suggests the test has a sensitivity of 92.9% and a specificity of 100%. ![]()
Gene plays crucial role in cancer development, team says
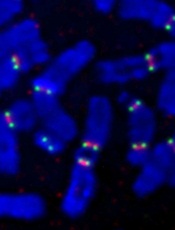
Credit: Beth A. Sullivan
New research suggests DNA ligase 3 is crucial for the evolutionary processes that drive cancer.
“We have identified a gene that, as cells age, seems to regulate whether the cells become cancerous or not,” said Eric A. Hendrickson, PhD, of the University of Minnesota in Minneapolis.
“This gene has never been identified before in this role, so this makes it a potentially very important therapeutic target.”
Dr Hendrickson and his colleagues recounted this discovery in Cell Reports.
The researchers noted that short, dysfunctional telomeres can fuse, thereby generating dicentric chromosomes and initiating breakage-fusion-bridge cycles. The cells that manage to escape the subsequent crisis have genomic rearrangements that drive clonal evolution and malignant progression.
The team wanted to determine exactly what allows these malignant cells to escape telomere-driven crisis and avoid death.
To find out, the group disabled certain genes in human cells and then studied the impact this had on telomere fusion.
They found that cells escaped death when ligase 3 was active but not when its action, which appears to promote fusion within like chromosomes rather than between different chromosomes, was inhibited.
“Telomere dysfunction has been identified in many human cancers,” said study author Duncan Baird, PhD, of Cardiff University in the UK.
“And, as we have shown previously, short telomeres can predict the outcome of patients with [chronic lymphocytic leukemia] and probably many other tumor types. Thus, the discovery that ligase 3 is required for this process is fundamentally important.”
This research was made possible by a chance meeting between Dr Baird and Dr Hendrickson at an international conference. The pair discovered they were both looking at the role of ligase 3 in cancer and decided to collaborate.
“The collaboration paid off, as we were able to uncover something that neither one of us could have done on our own,” Dr Hendrickson said.
Additional studies are already underway. The researchers are investigating the discovery that the reliance on ligase 3 appears to be dependent upon the activity of another key DNA repair gene, p53.
“Since p53 is the most commonly mutated gene in human cancer, it now behooves us to discover how these two genes are interacting and to see if we can’t use that information to develop synergistic treatment modalities,” Dr Hendrickson concluded. ![]()

Credit: Beth A. Sullivan
New research suggests DNA ligase 3 is crucial for the evolutionary processes that drive cancer.
“We have identified a gene that, as cells age, seems to regulate whether the cells become cancerous or not,” said Eric A. Hendrickson, PhD, of the University of Minnesota in Minneapolis.
“This gene has never been identified before in this role, so this makes it a potentially very important therapeutic target.”
Dr Hendrickson and his colleagues recounted this discovery in Cell Reports.
The researchers noted that short, dysfunctional telomeres can fuse, thereby generating dicentric chromosomes and initiating breakage-fusion-bridge cycles. The cells that manage to escape the subsequent crisis have genomic rearrangements that drive clonal evolution and malignant progression.
The team wanted to determine exactly what allows these malignant cells to escape telomere-driven crisis and avoid death.
To find out, the group disabled certain genes in human cells and then studied the impact this had on telomere fusion.
They found that cells escaped death when ligase 3 was active but not when its action, which appears to promote fusion within like chromosomes rather than between different chromosomes, was inhibited.
“Telomere dysfunction has been identified in many human cancers,” said study author Duncan Baird, PhD, of Cardiff University in the UK.
“And, as we have shown previously, short telomeres can predict the outcome of patients with [chronic lymphocytic leukemia] and probably many other tumor types. Thus, the discovery that ligase 3 is required for this process is fundamentally important.”
This research was made possible by a chance meeting between Dr Baird and Dr Hendrickson at an international conference. The pair discovered they were both looking at the role of ligase 3 in cancer and decided to collaborate.
“The collaboration paid off, as we were able to uncover something that neither one of us could have done on our own,” Dr Hendrickson said.
Additional studies are already underway. The researchers are investigating the discovery that the reliance on ligase 3 appears to be dependent upon the activity of another key DNA repair gene, p53.
“Since p53 is the most commonly mutated gene in human cancer, it now behooves us to discover how these two genes are interacting and to see if we can’t use that information to develop synergistic treatment modalities,” Dr Hendrickson concluded. ![]()

Credit: Beth A. Sullivan
New research suggests DNA ligase 3 is crucial for the evolutionary processes that drive cancer.
“We have identified a gene that, as cells age, seems to regulate whether the cells become cancerous or not,” said Eric A. Hendrickson, PhD, of the University of Minnesota in Minneapolis.
“This gene has never been identified before in this role, so this makes it a potentially very important therapeutic target.”
Dr Hendrickson and his colleagues recounted this discovery in Cell Reports.
The researchers noted that short, dysfunctional telomeres can fuse, thereby generating dicentric chromosomes and initiating breakage-fusion-bridge cycles. The cells that manage to escape the subsequent crisis have genomic rearrangements that drive clonal evolution and malignant progression.
The team wanted to determine exactly what allows these malignant cells to escape telomere-driven crisis and avoid death.
To find out, the group disabled certain genes in human cells and then studied the impact this had on telomere fusion.
They found that cells escaped death when ligase 3 was active but not when its action, which appears to promote fusion within like chromosomes rather than between different chromosomes, was inhibited.
“Telomere dysfunction has been identified in many human cancers,” said study author Duncan Baird, PhD, of Cardiff University in the UK.
“And, as we have shown previously, short telomeres can predict the outcome of patients with [chronic lymphocytic leukemia] and probably many other tumor types. Thus, the discovery that ligase 3 is required for this process is fundamentally important.”
This research was made possible by a chance meeting between Dr Baird and Dr Hendrickson at an international conference. The pair discovered they were both looking at the role of ligase 3 in cancer and decided to collaborate.
“The collaboration paid off, as we were able to uncover something that neither one of us could have done on our own,” Dr Hendrickson said.
Additional studies are already underway. The researchers are investigating the discovery that the reliance on ligase 3 appears to be dependent upon the activity of another key DNA repair gene, p53.
“Since p53 is the most commonly mutated gene in human cancer, it now behooves us to discover how these two genes are interacting and to see if we can’t use that information to develop synergistic treatment modalities,” Dr Hendrickson concluded. ![]()
Interim data appear positive for MM drug
Interim results of the phase 3 ASPIRE trial suggest carfilzomib can improve progression-free survival (PFS) in patients with relapsed multiple myeloma (MM).
Patients who received carfilzomib, lenalidomide, and dexamethasone lived 8.7 months longer without progression than patients who received only lenalidomide and dexamethasone.
The companies developing carfilzomib, Amgen and its subsidiary, Onyx Pharmaceuticals, Inc., recently shared these results.
They said additional results will be submitted for presentation at the 56th Annual ASH Meeting in December.
The companies also said data from the ASPIRE trial will form the basis for regulatory submissions for carfilzomib throughout the world.
In the US, the data may support the conversion of accelerated approval to full approval and expand the current indication for carfilzomib.
The ASPIRE trial includes 792 patients with relapsed MM who were randomized to treatment at sites in North America, Europe, and Israel. Prior to study treatment, the patients had received 1 to 3 therapeutic regimens.
The patients were randomized to receive carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1 only, then 27 mg/m2), in addition to a standard dosing schedule of lenalidomide (25 mg per day for 21 days on, 7 days off) and low-dose dexamethasone (40 mg per week in 4-week cycles), or lenalidomide and low-dose dexamethasone alone.
The primary endpoint was PFS, and secondary endpoints included overall survival, overall response rate, duration of response, disease control rate, health-related quality of life, and safety.
At a planned interim analysis, patients in the carfilzomib arm had a significantly longer median PFS than patients in the other arm—26.3 months and 17.6 months, respectively (P<0.0001).
The data for overall survival are not yet mature, but the analysis showed a trend in favor of carfilzomib that did not reach statistical significance, according to Amgen and Onyx.
The companies said the safety profile in this study is consistent with previous studies, including the rate of cardiac events.
Treatment discontinuation due to adverse events and on-study deaths were comparable between the 2 arms, and researchers did not identify any new safety signals. ![]()
Interim results of the phase 3 ASPIRE trial suggest carfilzomib can improve progression-free survival (PFS) in patients with relapsed multiple myeloma (MM).
Patients who received carfilzomib, lenalidomide, and dexamethasone lived 8.7 months longer without progression than patients who received only lenalidomide and dexamethasone.
The companies developing carfilzomib, Amgen and its subsidiary, Onyx Pharmaceuticals, Inc., recently shared these results.
They said additional results will be submitted for presentation at the 56th Annual ASH Meeting in December.
The companies also said data from the ASPIRE trial will form the basis for regulatory submissions for carfilzomib throughout the world.
In the US, the data may support the conversion of accelerated approval to full approval and expand the current indication for carfilzomib.
The ASPIRE trial includes 792 patients with relapsed MM who were randomized to treatment at sites in North America, Europe, and Israel. Prior to study treatment, the patients had received 1 to 3 therapeutic regimens.
The patients were randomized to receive carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1 only, then 27 mg/m2), in addition to a standard dosing schedule of lenalidomide (25 mg per day for 21 days on, 7 days off) and low-dose dexamethasone (40 mg per week in 4-week cycles), or lenalidomide and low-dose dexamethasone alone.
The primary endpoint was PFS, and secondary endpoints included overall survival, overall response rate, duration of response, disease control rate, health-related quality of life, and safety.
At a planned interim analysis, patients in the carfilzomib arm had a significantly longer median PFS than patients in the other arm—26.3 months and 17.6 months, respectively (P<0.0001).
The data for overall survival are not yet mature, but the analysis showed a trend in favor of carfilzomib that did not reach statistical significance, according to Amgen and Onyx.
The companies said the safety profile in this study is consistent with previous studies, including the rate of cardiac events.
Treatment discontinuation due to adverse events and on-study deaths were comparable between the 2 arms, and researchers did not identify any new safety signals. ![]()
Interim results of the phase 3 ASPIRE trial suggest carfilzomib can improve progression-free survival (PFS) in patients with relapsed multiple myeloma (MM).
Patients who received carfilzomib, lenalidomide, and dexamethasone lived 8.7 months longer without progression than patients who received only lenalidomide and dexamethasone.
The companies developing carfilzomib, Amgen and its subsidiary, Onyx Pharmaceuticals, Inc., recently shared these results.
They said additional results will be submitted for presentation at the 56th Annual ASH Meeting in December.
The companies also said data from the ASPIRE trial will form the basis for regulatory submissions for carfilzomib throughout the world.
In the US, the data may support the conversion of accelerated approval to full approval and expand the current indication for carfilzomib.
The ASPIRE trial includes 792 patients with relapsed MM who were randomized to treatment at sites in North America, Europe, and Israel. Prior to study treatment, the patients had received 1 to 3 therapeutic regimens.
The patients were randomized to receive carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1 only, then 27 mg/m2), in addition to a standard dosing schedule of lenalidomide (25 mg per day for 21 days on, 7 days off) and low-dose dexamethasone (40 mg per week in 4-week cycles), or lenalidomide and low-dose dexamethasone alone.
The primary endpoint was PFS, and secondary endpoints included overall survival, overall response rate, duration of response, disease control rate, health-related quality of life, and safety.
At a planned interim analysis, patients in the carfilzomib arm had a significantly longer median PFS than patients in the other arm—26.3 months and 17.6 months, respectively (P<0.0001).
The data for overall survival are not yet mature, but the analysis showed a trend in favor of carfilzomib that did not reach statistical significance, according to Amgen and Onyx.
The companies said the safety profile in this study is consistent with previous studies, including the rate of cardiac events.
Treatment discontinuation due to adverse events and on-study deaths were comparable between the 2 arms, and researchers did not identify any new safety signals. ![]()
Combative Behavior and Delirium
Delirium affects up to 82% of critical‐care patients and 29% to 64% of general medical patients, resulting in medical morbidity, decreased function, and mortality.[1, 2, 3, 4, 5] Delirium is associated with specific, adverse hospital outcomes, including falls, aspiration pneumonia, pressure ulcers, and restraint use.[6, 7] As a consequence of delirium, individuals may be transformed from independent and active at the time of admission to requiring skilled nursing supervision at discharge, in some cases resulting in permanent cognitive disability for the remainder of the person's life.[4, 8]
Combative behavior in hospitalized patients can be a threat to self or others, including other patients and staff. An emergency alert to staff regarding the presence of a combative patient requiring intervention is known as a behavioral code or Code Gray.[9] Guidelines addressing this particular hospital emergency typically refer to de‐escalation methods and implementation of security measures. Ideally, however, at‐risk patients would be identified prior to development into a full behavioral code. Unfortunately, the medical literature on the causes, prevention, and interventions for combative behavior requiring intervention is limited. Interventions published to date focus on patients living with severe and persistent mental illness, such as schizophrenia or bipolar disorder.[9, 10, 11, 12]
We hypothesized that delirium contributes to combative behavior in hospitalized patients, leading to the adverse outcome of behavioral codes. Delirium identification would therefore provide an opportunity for prevention and early identification of patients at risk, thereby improving safety for patients and staff. However, no studies published to date address the impact of delirium on the likelihood of a patient hospitalized in a general medical/surgical setting becoming combative and requiring intervention such as a behavioral code. The purpose of this article is to determine the strength of the association between delirium and combative behavior requiring intervention.
METHODS
This study was conducted as part of a quality improvement project, resulting in a waiver by the institutional review board of the hospital. All data with patient‐specific information were securely handled and deidentified prior to analysis. The setting is a 336‐bed, nonuniversity, teaching hospital serving adults in the Pacific Northwest, with approximately 16,000 admissions per year, and 31 critical‐care beds. Delirium prevention has been identified as an institutional priority, and we have been screening for delirium on admission and with twice‐daily Confusion Assessment Method (CAM) scores since 2010.
The study design was a retrospective case control study of hospital inpatients. Consecutive patients experiencing combative behavior requiring a specific behavioral code intervention (n=125) between January 1, 2011 to December 31, 2011 were identified using security reports and operator code logs. Five patients were excluded because the combative behavior requiring intervention occurred prior to hospital admission, in the emergency department or short‐stay observation unit. Interventions for combative behavior are institution specific. At our institution, a behavioral code is called when a patient or visitor is disruptive and exhibiting behavior that, if not controlled immediately, may result in serious injury to self or others, in line with the approach recommended by the Washington State Hospital Association.[9] When this situation arises, a staff member calls the hospital operator and an alert is paged overhead. Security staff then comes to the area to assist the clinical staff in determining the proper response.
The sample of 120 patients with behavioral codes was compared to a control group of 159 inpatients from the same year, randomly selected from all hospital discharges. For both groups, patients under the age of 18 and patients who were cared for only in the emergency department or short stay observation unit were excluded.
The presence or absence of delirium, the primary exposure of interest, was determined using a combined reference standard. First, by institutional protocol, all inpatients underwent nursing administration of the CAM[13] or the CAM for the Intensive Care Unit[14] on admission and every 12 hours thereafter. Patients with a positive CAM score within the 48 hours preceding the behavioral code event in cases, or anytime during the hospitalization in controls, were considered to have delirium. The CAM was performed as part of routine care at our institution by clinical nurses. However, when used outside of the research setting, untrained nurses using the CAM may substantially underestimate the incidence of delirium.[15, 16] Therefore, as a second reference standard, among patients who did not have delirium by the CAM criteria above, chart review was performed to identify delirium. Though chart review is also imperfect for the determination of both delirium and potential confounders, given the limitations of CAM scores in clinical rather than research settings, the use of this combined reference standard improves detection of delirium. Our chart review method is based on the previously reported work by Lakatos et al. and Inouye et al., identifying delirium from key words in the medical record demonstrating the diagnostic criteria for delirium.[6, 16] The Lakatos et al. study provided the specific key words used in our chart review.[6]
For each case and control subject, 1 of 2 experienced chart reviewers abstracted data from the electronic medical record retrospectively. The abstractors were not informed as to whether or not the patients were cases or controls, but could not be blinded as this information may be clear from the medical record. (The chart abstraction tool, with the key words mapped to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for delirium and the CAM is included in the Supporting Information, Appendix, in the online version of this article). We assessed interobserver agreement for delirium diagnosis through double review of 20% of charts. The presence or absence of potential confounders, including dementia, substance use or other psychiatric illness, and use of drugs associated with delirium specific (opiates, sedatives, anticholinergics, and antihistamines), and demographic information, including time of admission; hospital length of stay; any intensive care unit (ICU) visit; and discharge disposition was also determined from the electronic medical record.
Bivariate statistics were completed comparing patients with a behavioral code to the control group. Categorical variables were compared using 2, and continuous variables were compared using the t test. Logistic regression using stepwise regression, threshold of 0.1, dependent variable of behavioral code, and independent variable of delirium was performed to determine the association of delirium with behavioral codes after adjustment for confounders. In the multivariate model, use of any medication associated with delirium (Table 1) was considered as a single binary variable, regardless of drug type or class. Agreement was assessed through the kappa statistic. All statistics were performed using Stata MP v.12 (StataCorp, College Station, TX).
| Patients With Behavioral Code, N=120 | Patients Without Behavioral Code, N=159 | P Value | |
|---|---|---|---|
| |||
| Narcotic analgesics (%) | 78 (65) | 111 (70) | 0.40 |
| Fentanyl (%) | 47 (39) | 84 (53) | 0.024 |
| Hydromorphone (%) | 51 (43) | 78 (49) | 0.28 |
| Oxycodone (%) | 34 (28) | 46 (29) | 0.91 |
| Sedative hypnotics (%) | 76 (63) | 84 (53) | 0.08 |
| Midazolam (%) | 35 (29) | 70 (44) | 0.011 |
| Lorazepam (%) | 58 (48) | 15 (9) | <0.001 |
| No. of different drugs/person, mean (SD), range 010 | 2.6 (2.2) | 2.3 (1.9) | 0.27 |
RESULTS
Patients experiencing combative behavior requiring intervention through a behavioral code were significantly more likely to be male, admitted overnight, require an ICU stay during their hospitalization, and have a diagnosis of dementia or substance‐use disorder (Table 2). Patients with a behavioral code demonstrated an increased hospital length of stay (9.4 vs 4.5 days) and were significantly more likely to be discharged to a skilled nursing facility (31/120, 26% vs 16/159, 10%), or leave against medical advice (10% 12/120 vs 0%, P<0.001). Of the patients leaving against medical advice, none had evidence of delirium or dementia in the record; 92% (11/12) had International Classification of Disease9th Revision codes reflecting alcohol and/or drug abuse or dependence. Exposure to medications commonly associated with delirium was common in both groups, with differences in usage patterns for different drugs (Table 1).
| Characteristic | Patients With Behavioral Code, N=120 | Patients Without Behavioral Code, N=159 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 64.8 (19.5) | 63.9 (16.7) | 0.66 |
| Female (%) | 39 (33) | 78 (49) | 0.006 |
| Race/ethnicity (%) | 0.68 | ||
| White non‐Hispanic | 95 (79) | 129 (81) | |
| Other or unknown | 25 (21) | 30 (19) | |
| Patient Diagnosis Related Group (%) | 0.017 | ||
| Medical | 69 (57) | 69 (43) | |
| Gynecological or surgical | 51 (43) | 90 (57) | |
| Hospital admit between 6 pm and 6 am (%) | 63 (53) | 54 (34) | 0.002 |
| Any intensive care unit visit (%) | 43 (36) | 26 (16) | <0.001 |
| Any positive Confusion Assessment Method score | 70 (58) | 16 (10) | 0.002 |
| Delirium (%)a | 87 (73) | 26 (16) | <0.001 |
| Mental disorders as any discharge diagnosis code (%) | 110 (92) | 58 (36) | <0.001 |
| Dementia and other persistent (%) | 29 (24) | 9 (6) | <0.001 |
| Alcohol/drugs (%) | 61 (51) | 23 (14) | <0.001 |
Although combative behavior requiring intervention occurred throughout the stay, the majority (84/120, 70%) of behavioral codes occurred during the first 72 hours of hospital admission. Behavioral codes occurred throughout all nursing shifts, with 27% (32/120) between 7:00 am and 3:00 pm, 32% (39/120) between 3:00 pm and 11:00 pm, and 41% (49/120) between 11:00 pm and 7:00 am. Psychiatric consultation occurred in only 17% (20/120) of patients prior to the behavioral code.
Delirium was evident in the 48 hours preceding the behavioral code event in 50% (60/120) of cases, and was present overall in 16% of the comparison group. In the cases, delirium prior to behavioral code was identified by positive CAM scores in 23% (28/120) and by chart review in 27% (32/120). In the control subjects, delirium was identified by CAM scores in 10% (16/159) and by chart review in 10% (16/159). The chart review delirium assessment demonstrated high interobserver reliability (kappa=0.71). Among patients with behavioral codes, only 28/60 (46.7%) of delirium cases were identified by the CAM score.
The unadjusted odds ratio (OR) for having a behavioral code in the setting of delirium (within 48 hours prior to the code) was 5.1 (95% confidence interval [CI]: 2.9‐8.9, P<0.001), with the combined reference standard of positive CAM or delirium by chart review. Because each reference standard (chart review or CAM) only identified some of the delirium, the OR with only a single reference standard was lower. The risk of behavioral code in the setting of delirium when only CAM scores are considered for the diagnosis of delirium, the OR for behavioral code was 2.7 (95% CI: 1.4‐ 5.3, P=0.003), and when only chart review was used for delirium diagnosis, the OR was 2.7 (95% CI: 1.5‐5.0, P=0.001).
In the stepwise logistic regression model (using the composite reference standard of positive CAM score or delirium on chart review), the odds of having a behavioral code was 3.8 times greater in the setting of delirium (OR: 3.8, 95% CI: 2.07.3, P<0.001), after adjustment for substance abuse (OR: 5.3, 95% CI: 2.810.2, P<0.001), dementia (OR: 6.5, 95% CI: 2.616.1, P<0.001), other mental health diagnosis (OR: 3.2, 95% CI: 1.7‐6.1, P<0.001), and gender (OR male gender: 2.4, 95% CI: 1.34.5, P=0.006). Other potential confounders (age, use of delirium‐associated medications, ICU stay, time of admission) were not significant and so were not included in the final multivariate model.
DISCUSSION
In this study, we identify a 3.8‐fold increased odds of combative behavior requiring a behavioral code intervention in hospitalized patients with delirium. Although a previous association between delirium and restraint use among mechanically ventilated patients in the ICU has been published,[6] this is the first article to describe the strong, statistically significant association between combative patient behavior and delirium in the general medical/surgical acute‐care setting. This association raises the possibility that prevention, early identification, and treatment of delirium in hospitalized patients can decrease the incidence of such combative behavior, and may lead to shorter length of stay and less institutionalization after discharge.
In the multivariate model, we did identify other predictors of combative behavior requiring intervention, including substance abuse, dementia, and other psychiatric diagnoses. Our results do not support age as predictive of combative behavior after adjustment for other predictors. However, our hospital population is relatively old (mean age in controls, 63.9 years). In populations different from ours, age may still be an important predictor. We also did not identify use of medications potentially associated with delirium as a predictor of combative behavior requiring intervention, after adjustment for other predictors. We did, however, consider all medications together, and are not able to differentiate the potential predictive ability of any single drug or class. Finally, we report relatively high rates of opiate and sedative use in our sample, likely because we included short‐acting agents (ie, midazolam, fentanyl) that are commonly used for procedures and perioperative care.
Our study also highlights the challenges of accurately identifying delirium for quality improvement interventions. The CAM is a validated and widely accepted method of prospective screening for delirium, but in relatively untrained hands outside of research settings (as in our institution) does underestimate the true incidence of delirium.[15, 16, 17] Further, both CAM assessment and chart review may underestimate the incidence of hypoactive delirium. In our study, we note that the CAM scores only identified 46.7% of the behavioral code patients with delirium. Chart review also has limitations, identified by Inouye et al.,[16] particularly in the setting of comorbid dementia, high baseline delirium risk, and other comorbid conditions. Comorbidities as defined by APACHE II (Acute Physiology and Chronic Health Evaluation II) score may contribute to false positives, and poor documentation may result in false negatives. Our use of a combined reference standard of CAM assessment and chart review for delirium is supported by the fact that the incidence of delirium we report in the control group is similar to the published literature.[5]
Combative behavior in the hospital setting may be a threat to patients, staff, and visitors, and multiple state hospital associations have called for standardized responses, including the calling of behavioral codes when such circumstances arise.[9] In general, de‐escalation techniques and security measures are sufficient for patients exhibiting combative behavior. However, in cases of delirium‐associated combative behavior, clinical evaluation of root causes and both pharmacological and nonpharmacological interventions, including proactive psychiatric consultation, may also be beneficial.[18, 19, 20, 21] Many nonpharmacological interventions may need to be multicomponent in nature, as has been described previously.[18, 19, 20, 21]
We acknowledge the limitations of this article. First, we identify a strong association between combative behavior requiring intervention and delirium, but cannot prove causality. Second, though the chart reviewers where not told if reviewing cases or controls, we were not able to blind them to information on combative behavior that might have been present in the medical record. The use of unblinded reviewers could lead to overestimation of the presence of delirium in cases, and overestimation of the association between delirium and combative behavior requiring intervention. Third, chart review methods may underestimate the prevalence of dementia, which can confound the diagnosis of delirium. Finally, we defined delirium in the behavioral code cases as occurring in the 48 hours prior to the code event. However, as no such event occurred in the controls, we considered delirium as present if identified any time during the hospitalization. This could potentially lead to underestimation of the true association of delirium with combative behavior requiring intervention. It is also worth noting that many of the identified predictors for combative behavior are also predictors for delirium and may be identifying a subset of combative behavior related to agitated delirium. Overall, however, the strength of the association we report does strongly support identification and treatment of delirium in the context of combative behavior.
In this article, we identify a strong association between delirium and combative behavior requiring intervention, even after adjustment for other predictors. Understanding this association can help providers consider delirium as a potential cause of combative behavior in a medical/surgical setting, beyond behavioral issues associated with community violence, serious mental illness, progressive dementia, or substance use. Overall, therefore, delirium risk assessment, screening, prevention, and early intervention may potentially decrease combative behavior, and contribute to improving patient and staff safety in hospital settings.
Disclosures: C. Craig Blackmore, MD, reports royalties from the Springer Verlag publishing company for a textbook series on evidence‐based imaging, which is not relevant to this article. None of the authors report any conflicts of interest.
- Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the ICU. JAMA. 2004;291:1753–1762.
- , , . Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106:565–573.
- , , , et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia. JAMA. 2010;304:443–451.
- , , . Delirium in elderly people. Lancet. 2014;383(9920):911–922.
- , , , et al. Falls in the general hospital: association with delirium, advanced age, and specific surgical procedures. Psychosomatics. 2009;50:218–226.
- , , , , . Delirium as detected by the CAM‐ICU predicts restraint use among mechanically ventilated patients. Crit Care Med. 2005;33:226–229.
- , , . Delirium: prevention, treatment and outcome studies. J Geriatr Psychiatry Neurol. 1998;11:126–137.
- Washington State Hospital Association. Standardization of emergency code calls in Washington: implementation toolkit. Available at: http://www.wsha.org/files/82/EmergencyCodesExceutiveSummary.pdf. Accessed October 23, 2013.
- . Words for the wise: defusing combative patient behavior through verbal intervention. J Healthc Prot Manage. 2005;21:81–88.
- , , , . Rapid response team for behavioral emergencies. J Am Psychiatric Nurses Assoc. 2010;16:93–100.
- , . Physical and chemical restraints. Emerg Med Clin N Am. 2009;27:655–667.
- , , , , , . Clarifying confusion: the Confusion Assessment Method. Ann Int Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , , , . A researchh algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
- , , , , , . A chart‐based method for identification of delirium: validation compared with interviewer ratings using the Confusion Assessment Method. J Am Geriatic Soc. 2005;53:312–318.
- , , , , . Nurses' recognition of delirium and its symptoms. Arch Int Med. 2001;161:2467–2473.
- , , , et al. A multi‐component intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676.
- , , . Delirium: screening, prevention and diagnosis—a systematic review of the evidence. Evidence‐based Synthesis Program (ESP). Washington, DC: Department of Veterans Affairs; 2011.
- , , , , , . A longitudinal study of motor subtypes in delirium: frequency and stability during episodes. J Psychsom Res. 2012;72:236–241.
- , , , et al. Pharmacologic management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med. 2009;24:848–853.
Delirium affects up to 82% of critical‐care patients and 29% to 64% of general medical patients, resulting in medical morbidity, decreased function, and mortality.[1, 2, 3, 4, 5] Delirium is associated with specific, adverse hospital outcomes, including falls, aspiration pneumonia, pressure ulcers, and restraint use.[6, 7] As a consequence of delirium, individuals may be transformed from independent and active at the time of admission to requiring skilled nursing supervision at discharge, in some cases resulting in permanent cognitive disability for the remainder of the person's life.[4, 8]
Combative behavior in hospitalized patients can be a threat to self or others, including other patients and staff. An emergency alert to staff regarding the presence of a combative patient requiring intervention is known as a behavioral code or Code Gray.[9] Guidelines addressing this particular hospital emergency typically refer to de‐escalation methods and implementation of security measures. Ideally, however, at‐risk patients would be identified prior to development into a full behavioral code. Unfortunately, the medical literature on the causes, prevention, and interventions for combative behavior requiring intervention is limited. Interventions published to date focus on patients living with severe and persistent mental illness, such as schizophrenia or bipolar disorder.[9, 10, 11, 12]
We hypothesized that delirium contributes to combative behavior in hospitalized patients, leading to the adverse outcome of behavioral codes. Delirium identification would therefore provide an opportunity for prevention and early identification of patients at risk, thereby improving safety for patients and staff. However, no studies published to date address the impact of delirium on the likelihood of a patient hospitalized in a general medical/surgical setting becoming combative and requiring intervention such as a behavioral code. The purpose of this article is to determine the strength of the association between delirium and combative behavior requiring intervention.
METHODS
This study was conducted as part of a quality improvement project, resulting in a waiver by the institutional review board of the hospital. All data with patient‐specific information were securely handled and deidentified prior to analysis. The setting is a 336‐bed, nonuniversity, teaching hospital serving adults in the Pacific Northwest, with approximately 16,000 admissions per year, and 31 critical‐care beds. Delirium prevention has been identified as an institutional priority, and we have been screening for delirium on admission and with twice‐daily Confusion Assessment Method (CAM) scores since 2010.
The study design was a retrospective case control study of hospital inpatients. Consecutive patients experiencing combative behavior requiring a specific behavioral code intervention (n=125) between January 1, 2011 to December 31, 2011 were identified using security reports and operator code logs. Five patients were excluded because the combative behavior requiring intervention occurred prior to hospital admission, in the emergency department or short‐stay observation unit. Interventions for combative behavior are institution specific. At our institution, a behavioral code is called when a patient or visitor is disruptive and exhibiting behavior that, if not controlled immediately, may result in serious injury to self or others, in line with the approach recommended by the Washington State Hospital Association.[9] When this situation arises, a staff member calls the hospital operator and an alert is paged overhead. Security staff then comes to the area to assist the clinical staff in determining the proper response.
The sample of 120 patients with behavioral codes was compared to a control group of 159 inpatients from the same year, randomly selected from all hospital discharges. For both groups, patients under the age of 18 and patients who were cared for only in the emergency department or short stay observation unit were excluded.
The presence or absence of delirium, the primary exposure of interest, was determined using a combined reference standard. First, by institutional protocol, all inpatients underwent nursing administration of the CAM[13] or the CAM for the Intensive Care Unit[14] on admission and every 12 hours thereafter. Patients with a positive CAM score within the 48 hours preceding the behavioral code event in cases, or anytime during the hospitalization in controls, were considered to have delirium. The CAM was performed as part of routine care at our institution by clinical nurses. However, when used outside of the research setting, untrained nurses using the CAM may substantially underestimate the incidence of delirium.[15, 16] Therefore, as a second reference standard, among patients who did not have delirium by the CAM criteria above, chart review was performed to identify delirium. Though chart review is also imperfect for the determination of both delirium and potential confounders, given the limitations of CAM scores in clinical rather than research settings, the use of this combined reference standard improves detection of delirium. Our chart review method is based on the previously reported work by Lakatos et al. and Inouye et al., identifying delirium from key words in the medical record demonstrating the diagnostic criteria for delirium.[6, 16] The Lakatos et al. study provided the specific key words used in our chart review.[6]
For each case and control subject, 1 of 2 experienced chart reviewers abstracted data from the electronic medical record retrospectively. The abstractors were not informed as to whether or not the patients were cases or controls, but could not be blinded as this information may be clear from the medical record. (The chart abstraction tool, with the key words mapped to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for delirium and the CAM is included in the Supporting Information, Appendix, in the online version of this article). We assessed interobserver agreement for delirium diagnosis through double review of 20% of charts. The presence or absence of potential confounders, including dementia, substance use or other psychiatric illness, and use of drugs associated with delirium specific (opiates, sedatives, anticholinergics, and antihistamines), and demographic information, including time of admission; hospital length of stay; any intensive care unit (ICU) visit; and discharge disposition was also determined from the electronic medical record.
Bivariate statistics were completed comparing patients with a behavioral code to the control group. Categorical variables were compared using 2, and continuous variables were compared using the t test. Logistic regression using stepwise regression, threshold of 0.1, dependent variable of behavioral code, and independent variable of delirium was performed to determine the association of delirium with behavioral codes after adjustment for confounders. In the multivariate model, use of any medication associated with delirium (Table 1) was considered as a single binary variable, regardless of drug type or class. Agreement was assessed through the kappa statistic. All statistics were performed using Stata MP v.12 (StataCorp, College Station, TX).
| Patients With Behavioral Code, N=120 | Patients Without Behavioral Code, N=159 | P Value | |
|---|---|---|---|
| |||
| Narcotic analgesics (%) | 78 (65) | 111 (70) | 0.40 |
| Fentanyl (%) | 47 (39) | 84 (53) | 0.024 |
| Hydromorphone (%) | 51 (43) | 78 (49) | 0.28 |
| Oxycodone (%) | 34 (28) | 46 (29) | 0.91 |
| Sedative hypnotics (%) | 76 (63) | 84 (53) | 0.08 |
| Midazolam (%) | 35 (29) | 70 (44) | 0.011 |
| Lorazepam (%) | 58 (48) | 15 (9) | <0.001 |
| No. of different drugs/person, mean (SD), range 010 | 2.6 (2.2) | 2.3 (1.9) | 0.27 |
RESULTS
Patients experiencing combative behavior requiring intervention through a behavioral code were significantly more likely to be male, admitted overnight, require an ICU stay during their hospitalization, and have a diagnosis of dementia or substance‐use disorder (Table 2). Patients with a behavioral code demonstrated an increased hospital length of stay (9.4 vs 4.5 days) and were significantly more likely to be discharged to a skilled nursing facility (31/120, 26% vs 16/159, 10%), or leave against medical advice (10% 12/120 vs 0%, P<0.001). Of the patients leaving against medical advice, none had evidence of delirium or dementia in the record; 92% (11/12) had International Classification of Disease9th Revision codes reflecting alcohol and/or drug abuse or dependence. Exposure to medications commonly associated with delirium was common in both groups, with differences in usage patterns for different drugs (Table 1).
| Characteristic | Patients With Behavioral Code, N=120 | Patients Without Behavioral Code, N=159 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 64.8 (19.5) | 63.9 (16.7) | 0.66 |
| Female (%) | 39 (33) | 78 (49) | 0.006 |
| Race/ethnicity (%) | 0.68 | ||
| White non‐Hispanic | 95 (79) | 129 (81) | |
| Other or unknown | 25 (21) | 30 (19) | |
| Patient Diagnosis Related Group (%) | 0.017 | ||
| Medical | 69 (57) | 69 (43) | |
| Gynecological or surgical | 51 (43) | 90 (57) | |
| Hospital admit between 6 pm and 6 am (%) | 63 (53) | 54 (34) | 0.002 |
| Any intensive care unit visit (%) | 43 (36) | 26 (16) | <0.001 |
| Any positive Confusion Assessment Method score | 70 (58) | 16 (10) | 0.002 |
| Delirium (%)a | 87 (73) | 26 (16) | <0.001 |
| Mental disorders as any discharge diagnosis code (%) | 110 (92) | 58 (36) | <0.001 |
| Dementia and other persistent (%) | 29 (24) | 9 (6) | <0.001 |
| Alcohol/drugs (%) | 61 (51) | 23 (14) | <0.001 |
Although combative behavior requiring intervention occurred throughout the stay, the majority (84/120, 70%) of behavioral codes occurred during the first 72 hours of hospital admission. Behavioral codes occurred throughout all nursing shifts, with 27% (32/120) between 7:00 am and 3:00 pm, 32% (39/120) between 3:00 pm and 11:00 pm, and 41% (49/120) between 11:00 pm and 7:00 am. Psychiatric consultation occurred in only 17% (20/120) of patients prior to the behavioral code.
Delirium was evident in the 48 hours preceding the behavioral code event in 50% (60/120) of cases, and was present overall in 16% of the comparison group. In the cases, delirium prior to behavioral code was identified by positive CAM scores in 23% (28/120) and by chart review in 27% (32/120). In the control subjects, delirium was identified by CAM scores in 10% (16/159) and by chart review in 10% (16/159). The chart review delirium assessment demonstrated high interobserver reliability (kappa=0.71). Among patients with behavioral codes, only 28/60 (46.7%) of delirium cases were identified by the CAM score.
The unadjusted odds ratio (OR) for having a behavioral code in the setting of delirium (within 48 hours prior to the code) was 5.1 (95% confidence interval [CI]: 2.9‐8.9, P<0.001), with the combined reference standard of positive CAM or delirium by chart review. Because each reference standard (chart review or CAM) only identified some of the delirium, the OR with only a single reference standard was lower. The risk of behavioral code in the setting of delirium when only CAM scores are considered for the diagnosis of delirium, the OR for behavioral code was 2.7 (95% CI: 1.4‐ 5.3, P=0.003), and when only chart review was used for delirium diagnosis, the OR was 2.7 (95% CI: 1.5‐5.0, P=0.001).
In the stepwise logistic regression model (using the composite reference standard of positive CAM score or delirium on chart review), the odds of having a behavioral code was 3.8 times greater in the setting of delirium (OR: 3.8, 95% CI: 2.07.3, P<0.001), after adjustment for substance abuse (OR: 5.3, 95% CI: 2.810.2, P<0.001), dementia (OR: 6.5, 95% CI: 2.616.1, P<0.001), other mental health diagnosis (OR: 3.2, 95% CI: 1.7‐6.1, P<0.001), and gender (OR male gender: 2.4, 95% CI: 1.34.5, P=0.006). Other potential confounders (age, use of delirium‐associated medications, ICU stay, time of admission) were not significant and so were not included in the final multivariate model.
DISCUSSION
In this study, we identify a 3.8‐fold increased odds of combative behavior requiring a behavioral code intervention in hospitalized patients with delirium. Although a previous association between delirium and restraint use among mechanically ventilated patients in the ICU has been published,[6] this is the first article to describe the strong, statistically significant association between combative patient behavior and delirium in the general medical/surgical acute‐care setting. This association raises the possibility that prevention, early identification, and treatment of delirium in hospitalized patients can decrease the incidence of such combative behavior, and may lead to shorter length of stay and less institutionalization after discharge.
In the multivariate model, we did identify other predictors of combative behavior requiring intervention, including substance abuse, dementia, and other psychiatric diagnoses. Our results do not support age as predictive of combative behavior after adjustment for other predictors. However, our hospital population is relatively old (mean age in controls, 63.9 years). In populations different from ours, age may still be an important predictor. We also did not identify use of medications potentially associated with delirium as a predictor of combative behavior requiring intervention, after adjustment for other predictors. We did, however, consider all medications together, and are not able to differentiate the potential predictive ability of any single drug or class. Finally, we report relatively high rates of opiate and sedative use in our sample, likely because we included short‐acting agents (ie, midazolam, fentanyl) that are commonly used for procedures and perioperative care.
Our study also highlights the challenges of accurately identifying delirium for quality improvement interventions. The CAM is a validated and widely accepted method of prospective screening for delirium, but in relatively untrained hands outside of research settings (as in our institution) does underestimate the true incidence of delirium.[15, 16, 17] Further, both CAM assessment and chart review may underestimate the incidence of hypoactive delirium. In our study, we note that the CAM scores only identified 46.7% of the behavioral code patients with delirium. Chart review also has limitations, identified by Inouye et al.,[16] particularly in the setting of comorbid dementia, high baseline delirium risk, and other comorbid conditions. Comorbidities as defined by APACHE II (Acute Physiology and Chronic Health Evaluation II) score may contribute to false positives, and poor documentation may result in false negatives. Our use of a combined reference standard of CAM assessment and chart review for delirium is supported by the fact that the incidence of delirium we report in the control group is similar to the published literature.[5]
Combative behavior in the hospital setting may be a threat to patients, staff, and visitors, and multiple state hospital associations have called for standardized responses, including the calling of behavioral codes when such circumstances arise.[9] In general, de‐escalation techniques and security measures are sufficient for patients exhibiting combative behavior. However, in cases of delirium‐associated combative behavior, clinical evaluation of root causes and both pharmacological and nonpharmacological interventions, including proactive psychiatric consultation, may also be beneficial.[18, 19, 20, 21] Many nonpharmacological interventions may need to be multicomponent in nature, as has been described previously.[18, 19, 20, 21]
We acknowledge the limitations of this article. First, we identify a strong association between combative behavior requiring intervention and delirium, but cannot prove causality. Second, though the chart reviewers where not told if reviewing cases or controls, we were not able to blind them to information on combative behavior that might have been present in the medical record. The use of unblinded reviewers could lead to overestimation of the presence of delirium in cases, and overestimation of the association between delirium and combative behavior requiring intervention. Third, chart review methods may underestimate the prevalence of dementia, which can confound the diagnosis of delirium. Finally, we defined delirium in the behavioral code cases as occurring in the 48 hours prior to the code event. However, as no such event occurred in the controls, we considered delirium as present if identified any time during the hospitalization. This could potentially lead to underestimation of the true association of delirium with combative behavior requiring intervention. It is also worth noting that many of the identified predictors for combative behavior are also predictors for delirium and may be identifying a subset of combative behavior related to agitated delirium. Overall, however, the strength of the association we report does strongly support identification and treatment of delirium in the context of combative behavior.
In this article, we identify a strong association between delirium and combative behavior requiring intervention, even after adjustment for other predictors. Understanding this association can help providers consider delirium as a potential cause of combative behavior in a medical/surgical setting, beyond behavioral issues associated with community violence, serious mental illness, progressive dementia, or substance use. Overall, therefore, delirium risk assessment, screening, prevention, and early intervention may potentially decrease combative behavior, and contribute to improving patient and staff safety in hospital settings.
Disclosures: C. Craig Blackmore, MD, reports royalties from the Springer Verlag publishing company for a textbook series on evidence‐based imaging, which is not relevant to this article. None of the authors report any conflicts of interest.
Delirium affects up to 82% of critical‐care patients and 29% to 64% of general medical patients, resulting in medical morbidity, decreased function, and mortality.[1, 2, 3, 4, 5] Delirium is associated with specific, adverse hospital outcomes, including falls, aspiration pneumonia, pressure ulcers, and restraint use.[6, 7] As a consequence of delirium, individuals may be transformed from independent and active at the time of admission to requiring skilled nursing supervision at discharge, in some cases resulting in permanent cognitive disability for the remainder of the person's life.[4, 8]
Combative behavior in hospitalized patients can be a threat to self or others, including other patients and staff. An emergency alert to staff regarding the presence of a combative patient requiring intervention is known as a behavioral code or Code Gray.[9] Guidelines addressing this particular hospital emergency typically refer to de‐escalation methods and implementation of security measures. Ideally, however, at‐risk patients would be identified prior to development into a full behavioral code. Unfortunately, the medical literature on the causes, prevention, and interventions for combative behavior requiring intervention is limited. Interventions published to date focus on patients living with severe and persistent mental illness, such as schizophrenia or bipolar disorder.[9, 10, 11, 12]
We hypothesized that delirium contributes to combative behavior in hospitalized patients, leading to the adverse outcome of behavioral codes. Delirium identification would therefore provide an opportunity for prevention and early identification of patients at risk, thereby improving safety for patients and staff. However, no studies published to date address the impact of delirium on the likelihood of a patient hospitalized in a general medical/surgical setting becoming combative and requiring intervention such as a behavioral code. The purpose of this article is to determine the strength of the association between delirium and combative behavior requiring intervention.
METHODS
This study was conducted as part of a quality improvement project, resulting in a waiver by the institutional review board of the hospital. All data with patient‐specific information were securely handled and deidentified prior to analysis. The setting is a 336‐bed, nonuniversity, teaching hospital serving adults in the Pacific Northwest, with approximately 16,000 admissions per year, and 31 critical‐care beds. Delirium prevention has been identified as an institutional priority, and we have been screening for delirium on admission and with twice‐daily Confusion Assessment Method (CAM) scores since 2010.
The study design was a retrospective case control study of hospital inpatients. Consecutive patients experiencing combative behavior requiring a specific behavioral code intervention (n=125) between January 1, 2011 to December 31, 2011 were identified using security reports and operator code logs. Five patients were excluded because the combative behavior requiring intervention occurred prior to hospital admission, in the emergency department or short‐stay observation unit. Interventions for combative behavior are institution specific. At our institution, a behavioral code is called when a patient or visitor is disruptive and exhibiting behavior that, if not controlled immediately, may result in serious injury to self or others, in line with the approach recommended by the Washington State Hospital Association.[9] When this situation arises, a staff member calls the hospital operator and an alert is paged overhead. Security staff then comes to the area to assist the clinical staff in determining the proper response.
The sample of 120 patients with behavioral codes was compared to a control group of 159 inpatients from the same year, randomly selected from all hospital discharges. For both groups, patients under the age of 18 and patients who were cared for only in the emergency department or short stay observation unit were excluded.
The presence or absence of delirium, the primary exposure of interest, was determined using a combined reference standard. First, by institutional protocol, all inpatients underwent nursing administration of the CAM[13] or the CAM for the Intensive Care Unit[14] on admission and every 12 hours thereafter. Patients with a positive CAM score within the 48 hours preceding the behavioral code event in cases, or anytime during the hospitalization in controls, were considered to have delirium. The CAM was performed as part of routine care at our institution by clinical nurses. However, when used outside of the research setting, untrained nurses using the CAM may substantially underestimate the incidence of delirium.[15, 16] Therefore, as a second reference standard, among patients who did not have delirium by the CAM criteria above, chart review was performed to identify delirium. Though chart review is also imperfect for the determination of both delirium and potential confounders, given the limitations of CAM scores in clinical rather than research settings, the use of this combined reference standard improves detection of delirium. Our chart review method is based on the previously reported work by Lakatos et al. and Inouye et al., identifying delirium from key words in the medical record demonstrating the diagnostic criteria for delirium.[6, 16] The Lakatos et al. study provided the specific key words used in our chart review.[6]
For each case and control subject, 1 of 2 experienced chart reviewers abstracted data from the electronic medical record retrospectively. The abstractors were not informed as to whether or not the patients were cases or controls, but could not be blinded as this information may be clear from the medical record. (The chart abstraction tool, with the key words mapped to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for delirium and the CAM is included in the Supporting Information, Appendix, in the online version of this article). We assessed interobserver agreement for delirium diagnosis through double review of 20% of charts. The presence or absence of potential confounders, including dementia, substance use or other psychiatric illness, and use of drugs associated with delirium specific (opiates, sedatives, anticholinergics, and antihistamines), and demographic information, including time of admission; hospital length of stay; any intensive care unit (ICU) visit; and discharge disposition was also determined from the electronic medical record.
Bivariate statistics were completed comparing patients with a behavioral code to the control group. Categorical variables were compared using 2, and continuous variables were compared using the t test. Logistic regression using stepwise regression, threshold of 0.1, dependent variable of behavioral code, and independent variable of delirium was performed to determine the association of delirium with behavioral codes after adjustment for confounders. In the multivariate model, use of any medication associated with delirium (Table 1) was considered as a single binary variable, regardless of drug type or class. Agreement was assessed through the kappa statistic. All statistics were performed using Stata MP v.12 (StataCorp, College Station, TX).
| Patients With Behavioral Code, N=120 | Patients Without Behavioral Code, N=159 | P Value | |
|---|---|---|---|
| |||
| Narcotic analgesics (%) | 78 (65) | 111 (70) | 0.40 |
| Fentanyl (%) | 47 (39) | 84 (53) | 0.024 |
| Hydromorphone (%) | 51 (43) | 78 (49) | 0.28 |
| Oxycodone (%) | 34 (28) | 46 (29) | 0.91 |
| Sedative hypnotics (%) | 76 (63) | 84 (53) | 0.08 |
| Midazolam (%) | 35 (29) | 70 (44) | 0.011 |
| Lorazepam (%) | 58 (48) | 15 (9) | <0.001 |
| No. of different drugs/person, mean (SD), range 010 | 2.6 (2.2) | 2.3 (1.9) | 0.27 |
RESULTS
Patients experiencing combative behavior requiring intervention through a behavioral code were significantly more likely to be male, admitted overnight, require an ICU stay during their hospitalization, and have a diagnosis of dementia or substance‐use disorder (Table 2). Patients with a behavioral code demonstrated an increased hospital length of stay (9.4 vs 4.5 days) and were significantly more likely to be discharged to a skilled nursing facility (31/120, 26% vs 16/159, 10%), or leave against medical advice (10% 12/120 vs 0%, P<0.001). Of the patients leaving against medical advice, none had evidence of delirium or dementia in the record; 92% (11/12) had International Classification of Disease9th Revision codes reflecting alcohol and/or drug abuse or dependence. Exposure to medications commonly associated with delirium was common in both groups, with differences in usage patterns for different drugs (Table 1).
| Characteristic | Patients With Behavioral Code, N=120 | Patients Without Behavioral Code, N=159 | P Value |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 64.8 (19.5) | 63.9 (16.7) | 0.66 |
| Female (%) | 39 (33) | 78 (49) | 0.006 |
| Race/ethnicity (%) | 0.68 | ||
| White non‐Hispanic | 95 (79) | 129 (81) | |
| Other or unknown | 25 (21) | 30 (19) | |
| Patient Diagnosis Related Group (%) | 0.017 | ||
| Medical | 69 (57) | 69 (43) | |
| Gynecological or surgical | 51 (43) | 90 (57) | |
| Hospital admit between 6 pm and 6 am (%) | 63 (53) | 54 (34) | 0.002 |
| Any intensive care unit visit (%) | 43 (36) | 26 (16) | <0.001 |
| Any positive Confusion Assessment Method score | 70 (58) | 16 (10) | 0.002 |
| Delirium (%)a | 87 (73) | 26 (16) | <0.001 |
| Mental disorders as any discharge diagnosis code (%) | 110 (92) | 58 (36) | <0.001 |
| Dementia and other persistent (%) | 29 (24) | 9 (6) | <0.001 |
| Alcohol/drugs (%) | 61 (51) | 23 (14) | <0.001 |
Although combative behavior requiring intervention occurred throughout the stay, the majority (84/120, 70%) of behavioral codes occurred during the first 72 hours of hospital admission. Behavioral codes occurred throughout all nursing shifts, with 27% (32/120) between 7:00 am and 3:00 pm, 32% (39/120) between 3:00 pm and 11:00 pm, and 41% (49/120) between 11:00 pm and 7:00 am. Psychiatric consultation occurred in only 17% (20/120) of patients prior to the behavioral code.
Delirium was evident in the 48 hours preceding the behavioral code event in 50% (60/120) of cases, and was present overall in 16% of the comparison group. In the cases, delirium prior to behavioral code was identified by positive CAM scores in 23% (28/120) and by chart review in 27% (32/120). In the control subjects, delirium was identified by CAM scores in 10% (16/159) and by chart review in 10% (16/159). The chart review delirium assessment demonstrated high interobserver reliability (kappa=0.71). Among patients with behavioral codes, only 28/60 (46.7%) of delirium cases were identified by the CAM score.
The unadjusted odds ratio (OR) for having a behavioral code in the setting of delirium (within 48 hours prior to the code) was 5.1 (95% confidence interval [CI]: 2.9‐8.9, P<0.001), with the combined reference standard of positive CAM or delirium by chart review. Because each reference standard (chart review or CAM) only identified some of the delirium, the OR with only a single reference standard was lower. The risk of behavioral code in the setting of delirium when only CAM scores are considered for the diagnosis of delirium, the OR for behavioral code was 2.7 (95% CI: 1.4‐ 5.3, P=0.003), and when only chart review was used for delirium diagnosis, the OR was 2.7 (95% CI: 1.5‐5.0, P=0.001).
In the stepwise logistic regression model (using the composite reference standard of positive CAM score or delirium on chart review), the odds of having a behavioral code was 3.8 times greater in the setting of delirium (OR: 3.8, 95% CI: 2.07.3, P<0.001), after adjustment for substance abuse (OR: 5.3, 95% CI: 2.810.2, P<0.001), dementia (OR: 6.5, 95% CI: 2.616.1, P<0.001), other mental health diagnosis (OR: 3.2, 95% CI: 1.7‐6.1, P<0.001), and gender (OR male gender: 2.4, 95% CI: 1.34.5, P=0.006). Other potential confounders (age, use of delirium‐associated medications, ICU stay, time of admission) were not significant and so were not included in the final multivariate model.
DISCUSSION
In this study, we identify a 3.8‐fold increased odds of combative behavior requiring a behavioral code intervention in hospitalized patients with delirium. Although a previous association between delirium and restraint use among mechanically ventilated patients in the ICU has been published,[6] this is the first article to describe the strong, statistically significant association between combative patient behavior and delirium in the general medical/surgical acute‐care setting. This association raises the possibility that prevention, early identification, and treatment of delirium in hospitalized patients can decrease the incidence of such combative behavior, and may lead to shorter length of stay and less institutionalization after discharge.
In the multivariate model, we did identify other predictors of combative behavior requiring intervention, including substance abuse, dementia, and other psychiatric diagnoses. Our results do not support age as predictive of combative behavior after adjustment for other predictors. However, our hospital population is relatively old (mean age in controls, 63.9 years). In populations different from ours, age may still be an important predictor. We also did not identify use of medications potentially associated with delirium as a predictor of combative behavior requiring intervention, after adjustment for other predictors. We did, however, consider all medications together, and are not able to differentiate the potential predictive ability of any single drug or class. Finally, we report relatively high rates of opiate and sedative use in our sample, likely because we included short‐acting agents (ie, midazolam, fentanyl) that are commonly used for procedures and perioperative care.
Our study also highlights the challenges of accurately identifying delirium for quality improvement interventions. The CAM is a validated and widely accepted method of prospective screening for delirium, but in relatively untrained hands outside of research settings (as in our institution) does underestimate the true incidence of delirium.[15, 16, 17] Further, both CAM assessment and chart review may underestimate the incidence of hypoactive delirium. In our study, we note that the CAM scores only identified 46.7% of the behavioral code patients with delirium. Chart review also has limitations, identified by Inouye et al.,[16] particularly in the setting of comorbid dementia, high baseline delirium risk, and other comorbid conditions. Comorbidities as defined by APACHE II (Acute Physiology and Chronic Health Evaluation II) score may contribute to false positives, and poor documentation may result in false negatives. Our use of a combined reference standard of CAM assessment and chart review for delirium is supported by the fact that the incidence of delirium we report in the control group is similar to the published literature.[5]
Combative behavior in the hospital setting may be a threat to patients, staff, and visitors, and multiple state hospital associations have called for standardized responses, including the calling of behavioral codes when such circumstances arise.[9] In general, de‐escalation techniques and security measures are sufficient for patients exhibiting combative behavior. However, in cases of delirium‐associated combative behavior, clinical evaluation of root causes and both pharmacological and nonpharmacological interventions, including proactive psychiatric consultation, may also be beneficial.[18, 19, 20, 21] Many nonpharmacological interventions may need to be multicomponent in nature, as has been described previously.[18, 19, 20, 21]
We acknowledge the limitations of this article. First, we identify a strong association between combative behavior requiring intervention and delirium, but cannot prove causality. Second, though the chart reviewers where not told if reviewing cases or controls, we were not able to blind them to information on combative behavior that might have been present in the medical record. The use of unblinded reviewers could lead to overestimation of the presence of delirium in cases, and overestimation of the association between delirium and combative behavior requiring intervention. Third, chart review methods may underestimate the prevalence of dementia, which can confound the diagnosis of delirium. Finally, we defined delirium in the behavioral code cases as occurring in the 48 hours prior to the code event. However, as no such event occurred in the controls, we considered delirium as present if identified any time during the hospitalization. This could potentially lead to underestimation of the true association of delirium with combative behavior requiring intervention. It is also worth noting that many of the identified predictors for combative behavior are also predictors for delirium and may be identifying a subset of combative behavior related to agitated delirium. Overall, however, the strength of the association we report does strongly support identification and treatment of delirium in the context of combative behavior.
In this article, we identify a strong association between delirium and combative behavior requiring intervention, even after adjustment for other predictors. Understanding this association can help providers consider delirium as a potential cause of combative behavior in a medical/surgical setting, beyond behavioral issues associated with community violence, serious mental illness, progressive dementia, or substance use. Overall, therefore, delirium risk assessment, screening, prevention, and early intervention may potentially decrease combative behavior, and contribute to improving patient and staff safety in hospital settings.
Disclosures: C. Craig Blackmore, MD, reports royalties from the Springer Verlag publishing company for a textbook series on evidence‐based imaging, which is not relevant to this article. None of the authors report any conflicts of interest.
- Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the ICU. JAMA. 2004;291:1753–1762.
- , , . Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106:565–573.
- , , , et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia. JAMA. 2010;304:443–451.
- , , . Delirium in elderly people. Lancet. 2014;383(9920):911–922.
- , , , et al. Falls in the general hospital: association with delirium, advanced age, and specific surgical procedures. Psychosomatics. 2009;50:218–226.
- , , , , . Delirium as detected by the CAM‐ICU predicts restraint use among mechanically ventilated patients. Crit Care Med. 2005;33:226–229.
- , , . Delirium: prevention, treatment and outcome studies. J Geriatr Psychiatry Neurol. 1998;11:126–137.
- Washington State Hospital Association. Standardization of emergency code calls in Washington: implementation toolkit. Available at: http://www.wsha.org/files/82/EmergencyCodesExceutiveSummary.pdf. Accessed October 23, 2013.
- . Words for the wise: defusing combative patient behavior through verbal intervention. J Healthc Prot Manage. 2005;21:81–88.
- , , , . Rapid response team for behavioral emergencies. J Am Psychiatric Nurses Assoc. 2010;16:93–100.
- , . Physical and chemical restraints. Emerg Med Clin N Am. 2009;27:655–667.
- , , , , , . Clarifying confusion: the Confusion Assessment Method. Ann Int Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , , , . A researchh algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
- , , , , , . A chart‐based method for identification of delirium: validation compared with interviewer ratings using the Confusion Assessment Method. J Am Geriatic Soc. 2005;53:312–318.
- , , , , . Nurses' recognition of delirium and its symptoms. Arch Int Med. 2001;161:2467–2473.
- , , , et al. A multi‐component intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676.
- , , . Delirium: screening, prevention and diagnosis—a systematic review of the evidence. Evidence‐based Synthesis Program (ESP). Washington, DC: Department of Veterans Affairs; 2011.
- , , , , , . A longitudinal study of motor subtypes in delirium: frequency and stability during episodes. J Psychsom Res. 2012;72:236–241.
- , , , et al. Pharmacologic management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med. 2009;24:848–853.
- Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the ICU. JAMA. 2004;291:1753–1762.
- , , . Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106:565–573.
- , , , et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia. JAMA. 2010;304:443–451.
- , , . Delirium in elderly people. Lancet. 2014;383(9920):911–922.
- , , , et al. Falls in the general hospital: association with delirium, advanced age, and specific surgical procedures. Psychosomatics. 2009;50:218–226.
- , , , , . Delirium as detected by the CAM‐ICU predicts restraint use among mechanically ventilated patients. Crit Care Med. 2005;33:226–229.
- , , . Delirium: prevention, treatment and outcome studies. J Geriatr Psychiatry Neurol. 1998;11:126–137.
- Washington State Hospital Association. Standardization of emergency code calls in Washington: implementation toolkit. Available at: http://www.wsha.org/files/82/EmergencyCodesExceutiveSummary.pdf. Accessed October 23, 2013.
- . Words for the wise: defusing combative patient behavior through verbal intervention. J Healthc Prot Manage. 2005;21:81–88.
- , , , . Rapid response team for behavioral emergencies. J Am Psychiatric Nurses Assoc. 2010;16:93–100.
- , . Physical and chemical restraints. Emerg Med Clin N Am. 2009;27:655–667.
- , , , , , . Clarifying confusion: the Confusion Assessment Method. Ann Int Med. 1990;113:941–948.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU). JAMA. 2001;286:2703–2710.
- , , , , , . A researchh algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121.
- , , , , , . A chart‐based method for identification of delirium: validation compared with interviewer ratings using the Confusion Assessment Method. J Am Geriatic Soc. 2005;53:312–318.
- , , , , . Nurses' recognition of delirium and its symptoms. Arch Int Med. 2001;161:2467–2473.
- , , , et al. A multi‐component intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676.
- , , . Delirium: screening, prevention and diagnosis—a systematic review of the evidence. Evidence‐based Synthesis Program (ESP). Washington, DC: Department of Veterans Affairs; 2011.
- , , , , , . A longitudinal study of motor subtypes in delirium: frequency and stability during episodes. J Psychsom Res. 2012;72:236–241.
- , , , et al. Pharmacologic management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med. 2009;24:848–853.
© 2014 Society of Hospital Medicine
BEST PRACTICES IN: Acne Vulgaris
A Best Practices Supplement to Skin & Allergy News®. This supplement was sponsored by Valeant Dermatology, a division of Valeant Pharmaceuticals.
- Introduction
- ACANYA (clindamycin phosphate and benzoyl peroxide) Gel Indication and Mechanisms
- ACANYA Gel Efficacy
- ACANYA Gel Tolerability
- IMPORTANT SAFETY INFORMATION
Faculty/Faculty Disclosures
Hilary Baldwin, MD
SUNY Downstate Medical Center
Brooklyn, NY
Dr. Baldwin discloses that she serves as a member on the advisory board for Valeant Pharmaceuticals.
Copyright © by Frontline Medical Communications Inc.
A Best Practices Supplement to Skin & Allergy News®. This supplement was sponsored by Valeant Dermatology, a division of Valeant Pharmaceuticals.
- Introduction
- ACANYA (clindamycin phosphate and benzoyl peroxide) Gel Indication and Mechanisms
- ACANYA Gel Efficacy
- ACANYA Gel Tolerability
- IMPORTANT SAFETY INFORMATION
Faculty/Faculty Disclosures
Hilary Baldwin, MD
SUNY Downstate Medical Center
Brooklyn, NY
Dr. Baldwin discloses that she serves as a member on the advisory board for Valeant Pharmaceuticals.
Copyright © by Frontline Medical Communications Inc.
A Best Practices Supplement to Skin & Allergy News®. This supplement was sponsored by Valeant Dermatology, a division of Valeant Pharmaceuticals.
- Introduction
- ACANYA (clindamycin phosphate and benzoyl peroxide) Gel Indication and Mechanisms
- ACANYA Gel Efficacy
- ACANYA Gel Tolerability
- IMPORTANT SAFETY INFORMATION
Faculty/Faculty Disclosures
Hilary Baldwin, MD
SUNY Downstate Medical Center
Brooklyn, NY
Dr. Baldwin discloses that she serves as a member on the advisory board for Valeant Pharmaceuticals.
Copyright © by Frontline Medical Communications Inc.
Product News: 08 2014
Acticlate
Aqua Pharmaceuticals, LLC, announces US Food and Drug Administration approval of the new drug application for Acticlate (doxycycline hyclate) tablets. Acticlate is a tetracycline-class antibacterial indicated for the treatment of a number of infections, including adjunctive therapy in severe acne. Acticlate is available in round 75-mg tablets and oval-shaped, dual-scored 150-mg tablets. Utilizing the latest manufacturing technology, the 150-mg tablets are formulated in a substantially reduced tablet size for Acticlate, allowing for dosing flexibility and making it easier for the patient to swallow. For more information, visit www.aquapharm.com.
ATX-101
Kythera Biopharmaceuticals, Inc, announces that its new drug application for ATX-101 (deoxycholic acid) has been accepted for filing by the US Food and Drug Administration. ATX-101 is an injectable treatment for the reduction of submental fat. The treatment contours the area under the chin by destroying fat cells while leaving surrounding tissue largely unaffected. The new drug application review should be completed by May 13, 2015. For more information, visit www.kytherabiopharma.com/pipeline/ATX-101.
EpiCeram Airless Pump
PuraCap Pharmaceutical LLC adds the EpiCeram 225-g airless pump to its product line. This airless pump gives patients more EpiCeram to treat any size area of atopic dermatitis with twice-daily application. The convenient and portable pump dispenser gives patients more control in applying EpiCeram. A 90-g tube is already available. The EpiCeram Controlled Release Skin Barrier Emulsion helps to relieve the burning and itching associated with skin conditions such as atopic dermatitis and eczema. For more information, visit www.epiceram-us.com.
Hair Regrowth System
brandMD Skin Care introduces the Hair Regrowth System, a 3-step daily regimen to prevent and improve signs of hair loss. The regimen includes the Replenish Shampoo, Restorative Conditioning Treatment, and Rapid Growth Serum. By reducing the production of dihydrotestosterone, the system stimulates hair growth and improves hair follicle anchoring. Both men and women can use the Hair Regrowth System. For more information, visit www.brandMDskincare.com.
Rasuvo
Medac Pharma Inc obtains US Food and Drug Administration approval for Rasuvo, a subcutaneous injectable methotrexate therapy delivered in a single-dose autoinjector for rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, and psoriasis. Rasuvo is available in 10 dosage strengths ranging from 7.5 to 30 mg in 2.5-mg increments. Because of the possibility of serious toxic reactions, Rasuvo should be used only in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease that is not adequately responsive to other forms of therapy. For more information, visit www.rasuvo.com.
Restylane Silk
Valeant Pharmaceuticals International, Inc, obtains US Food and Drug Administration marketing clearance for Restylane Silk injectable gel with 0.3% lidocaine, which is indicated for submucosal implantation for lip augmentation and dermal implantation for correction of perioral rhytides in patients older than 21 years. Restylane Silk is composed of hyaluronic acid and is free from animal protein. Allergy pretesting is not necessary. Restylane Silk should not be used in patients with prior hypersensitivity to local anesthetics of the amide type, such as lidocaine. For more information, visit www.valeant.com.
Zenatane Provider Portal
Promius Pharma, LLC, launches a Web-based provider portal to follow a patient taking Zenatane (isotretinoin). It is designed to allow any authorized prescriber using the Promius Promise pharmacy program to obtain instant access to secure patient data. This portal is valuable for dermatologists to track patient adherence to guidelines for using isotretinoin. It will provide patient demographics, dates of prescriptions received and shipped, and information on pending prescription shipments. A messaging tool also allows health care providers to send questions and receive answers within the portal itself. It maintains strict privacy in accordance with the Health Insurance Portability and Accountability Act.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Acticlate
Aqua Pharmaceuticals, LLC, announces US Food and Drug Administration approval of the new drug application for Acticlate (doxycycline hyclate) tablets. Acticlate is a tetracycline-class antibacterial indicated for the treatment of a number of infections, including adjunctive therapy in severe acne. Acticlate is available in round 75-mg tablets and oval-shaped, dual-scored 150-mg tablets. Utilizing the latest manufacturing technology, the 150-mg tablets are formulated in a substantially reduced tablet size for Acticlate, allowing for dosing flexibility and making it easier for the patient to swallow. For more information, visit www.aquapharm.com.
ATX-101
Kythera Biopharmaceuticals, Inc, announces that its new drug application for ATX-101 (deoxycholic acid) has been accepted for filing by the US Food and Drug Administration. ATX-101 is an injectable treatment for the reduction of submental fat. The treatment contours the area under the chin by destroying fat cells while leaving surrounding tissue largely unaffected. The new drug application review should be completed by May 13, 2015. For more information, visit www.kytherabiopharma.com/pipeline/ATX-101.
EpiCeram Airless Pump
PuraCap Pharmaceutical LLC adds the EpiCeram 225-g airless pump to its product line. This airless pump gives patients more EpiCeram to treat any size area of atopic dermatitis with twice-daily application. The convenient and portable pump dispenser gives patients more control in applying EpiCeram. A 90-g tube is already available. The EpiCeram Controlled Release Skin Barrier Emulsion helps to relieve the burning and itching associated with skin conditions such as atopic dermatitis and eczema. For more information, visit www.epiceram-us.com.
Hair Regrowth System
brandMD Skin Care introduces the Hair Regrowth System, a 3-step daily regimen to prevent and improve signs of hair loss. The regimen includes the Replenish Shampoo, Restorative Conditioning Treatment, and Rapid Growth Serum. By reducing the production of dihydrotestosterone, the system stimulates hair growth and improves hair follicle anchoring. Both men and women can use the Hair Regrowth System. For more information, visit www.brandMDskincare.com.
Rasuvo
Medac Pharma Inc obtains US Food and Drug Administration approval for Rasuvo, a subcutaneous injectable methotrexate therapy delivered in a single-dose autoinjector for rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, and psoriasis. Rasuvo is available in 10 dosage strengths ranging from 7.5 to 30 mg in 2.5-mg increments. Because of the possibility of serious toxic reactions, Rasuvo should be used only in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease that is not adequately responsive to other forms of therapy. For more information, visit www.rasuvo.com.
Restylane Silk
Valeant Pharmaceuticals International, Inc, obtains US Food and Drug Administration marketing clearance for Restylane Silk injectable gel with 0.3% lidocaine, which is indicated for submucosal implantation for lip augmentation and dermal implantation for correction of perioral rhytides in patients older than 21 years. Restylane Silk is composed of hyaluronic acid and is free from animal protein. Allergy pretesting is not necessary. Restylane Silk should not be used in patients with prior hypersensitivity to local anesthetics of the amide type, such as lidocaine. For more information, visit www.valeant.com.
Zenatane Provider Portal
Promius Pharma, LLC, launches a Web-based provider portal to follow a patient taking Zenatane (isotretinoin). It is designed to allow any authorized prescriber using the Promius Promise pharmacy program to obtain instant access to secure patient data. This portal is valuable for dermatologists to track patient adherence to guidelines for using isotretinoin. It will provide patient demographics, dates of prescriptions received and shipped, and information on pending prescription shipments. A messaging tool also allows health care providers to send questions and receive answers within the portal itself. It maintains strict privacy in accordance with the Health Insurance Portability and Accountability Act.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Acticlate
Aqua Pharmaceuticals, LLC, announces US Food and Drug Administration approval of the new drug application for Acticlate (doxycycline hyclate) tablets. Acticlate is a tetracycline-class antibacterial indicated for the treatment of a number of infections, including adjunctive therapy in severe acne. Acticlate is available in round 75-mg tablets and oval-shaped, dual-scored 150-mg tablets. Utilizing the latest manufacturing technology, the 150-mg tablets are formulated in a substantially reduced tablet size for Acticlate, allowing for dosing flexibility and making it easier for the patient to swallow. For more information, visit www.aquapharm.com.
ATX-101
Kythera Biopharmaceuticals, Inc, announces that its new drug application for ATX-101 (deoxycholic acid) has been accepted for filing by the US Food and Drug Administration. ATX-101 is an injectable treatment for the reduction of submental fat. The treatment contours the area under the chin by destroying fat cells while leaving surrounding tissue largely unaffected. The new drug application review should be completed by May 13, 2015. For more information, visit www.kytherabiopharma.com/pipeline/ATX-101.
EpiCeram Airless Pump
PuraCap Pharmaceutical LLC adds the EpiCeram 225-g airless pump to its product line. This airless pump gives patients more EpiCeram to treat any size area of atopic dermatitis with twice-daily application. The convenient and portable pump dispenser gives patients more control in applying EpiCeram. A 90-g tube is already available. The EpiCeram Controlled Release Skin Barrier Emulsion helps to relieve the burning and itching associated with skin conditions such as atopic dermatitis and eczema. For more information, visit www.epiceram-us.com.
Hair Regrowth System
brandMD Skin Care introduces the Hair Regrowth System, a 3-step daily regimen to prevent and improve signs of hair loss. The regimen includes the Replenish Shampoo, Restorative Conditioning Treatment, and Rapid Growth Serum. By reducing the production of dihydrotestosterone, the system stimulates hair growth and improves hair follicle anchoring. Both men and women can use the Hair Regrowth System. For more information, visit www.brandMDskincare.com.
Rasuvo
Medac Pharma Inc obtains US Food and Drug Administration approval for Rasuvo, a subcutaneous injectable methotrexate therapy delivered in a single-dose autoinjector for rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, and psoriasis. Rasuvo is available in 10 dosage strengths ranging from 7.5 to 30 mg in 2.5-mg increments. Because of the possibility of serious toxic reactions, Rasuvo should be used only in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease that is not adequately responsive to other forms of therapy. For more information, visit www.rasuvo.com.
Restylane Silk
Valeant Pharmaceuticals International, Inc, obtains US Food and Drug Administration marketing clearance for Restylane Silk injectable gel with 0.3% lidocaine, which is indicated for submucosal implantation for lip augmentation and dermal implantation for correction of perioral rhytides in patients older than 21 years. Restylane Silk is composed of hyaluronic acid and is free from animal protein. Allergy pretesting is not necessary. Restylane Silk should not be used in patients with prior hypersensitivity to local anesthetics of the amide type, such as lidocaine. For more information, visit www.valeant.com.
Zenatane Provider Portal
Promius Pharma, LLC, launches a Web-based provider portal to follow a patient taking Zenatane (isotretinoin). It is designed to allow any authorized prescriber using the Promius Promise pharmacy program to obtain instant access to secure patient data. This portal is valuable for dermatologists to track patient adherence to guidelines for using isotretinoin. It will provide patient demographics, dates of prescriptions received and shipped, and information on pending prescription shipments. A messaging tool also allows health care providers to send questions and receive answers within the portal itself. It maintains strict privacy in accordance with the Health Insurance Portability and Accountability Act.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].




