User login
Adipose-derived SCs can resist methotrexate

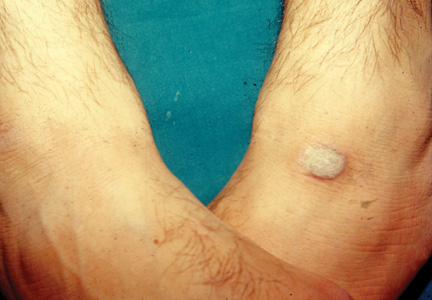
A new study indicates that adipose-derived stem cells (ASCs) are highly resistant to the chemotherapy drug methotrexate (MTX).
Cultured ASCs and tissue samples that included ASCs were able to withstand exposure to MTX quite well. The drug had little or no effect on ASC viability, division, senescence, or differentiation.
The researchers believe these findings could prove significant for cancer patients, particularly children with acute lymphoblastic leukemia.
“Kids undergo chemotherapy at such an important time, when they should be growing, but, instead, they are introduced to this very harsh environment where bone cells are damaged with these drugs,” said study author Olivia Beane, a graduate student at Brown University in Providence, Rhode Island.
“That leads to major long-term side effects, including osteoporosis and bone defects. If we found a stem cell that was resistant to the chemotherapeutic agent and could promote bone growth by becoming bone itself, then maybe they wouldn’t have these issues.”
Beane’s work, which appears in Experimental Cell Research, grew out of more basic research. She was originally looking for chemicals that could help purify ASCs from mixed cell cultures to encourage their proliferation.
Among other things, she tried chemotherapy drugs, speculating that ASCs might withstand a drug that other cells could not. The idea that this could help cancer patients did not come until later.
To explore potential cancer applications, Beane and her colleagues exposed pure human ASC cultures, stromal vascular fraction (SVF) tissue samples (which include ASCs and several other cell types), and cultures of human fibroblast cells to medically relevant concentrations of chemotherapy drugs for 24 hours.
The researchers then measured how those cell populations fared over the next 10 days. They also measured the ability of MTX-exposed ASCs, both alone and in SVF, to proliferate and differentiate.
In contrast to the fibroblast controls, the ASCs withstood a variety of MTX doses. The drug had little or no effect on ASC viability, cell division, senescence, or differentiation. ASCs also resisted vincristine to an extent, but they could not withstand exposure to cytarabine or etoposide.
The SVF tissue samples withstood MTX doses well too. That is significant, according to the researchers, because the tissue would be clinically useful if an ASC-based therapy were ever developed for cancer patients. Hypothetically, fresh SVF could be harvested from the fat of a donor and injected into bone tissue, delivering ASCs to the site.
To understand why ASCs resist MTX, the researchers conducted further tests. MTX shuts down DNA biosynthesis by binding the protein dihydrofolate reductase so it is unavailable to assist in that essential task.
The testing showed that ASCs ramped up dihydrofolate reductase levels upon exposure to the drug. They produced enough to overcome a clinically relevant dose of MTX.
Now, the researchers are eager to see if they can translate these findings to deliver a medical benefit for cancer patients. To that end, the team is planning several more experiments.
One is to test ASC survival and performance after 48- and 72-hour exposures to MTX. Another is to begin examining how the cells fare in mouse models of chemotherapy. The researchers plan to directly compare ASCs and bone marrow-derived stem cells exposed to various chemotherapies. ![]()

A new study indicates that adipose-derived stem cells (ASCs) are highly resistant to the chemotherapy drug methotrexate (MTX).
Cultured ASCs and tissue samples that included ASCs were able to withstand exposure to MTX quite well. The drug had little or no effect on ASC viability, division, senescence, or differentiation.
The researchers believe these findings could prove significant for cancer patients, particularly children with acute lymphoblastic leukemia.
“Kids undergo chemotherapy at such an important time, when they should be growing, but, instead, they are introduced to this very harsh environment where bone cells are damaged with these drugs,” said study author Olivia Beane, a graduate student at Brown University in Providence, Rhode Island.
“That leads to major long-term side effects, including osteoporosis and bone defects. If we found a stem cell that was resistant to the chemotherapeutic agent and could promote bone growth by becoming bone itself, then maybe they wouldn’t have these issues.”
Beane’s work, which appears in Experimental Cell Research, grew out of more basic research. She was originally looking for chemicals that could help purify ASCs from mixed cell cultures to encourage their proliferation.
Among other things, she tried chemotherapy drugs, speculating that ASCs might withstand a drug that other cells could not. The idea that this could help cancer patients did not come until later.
To explore potential cancer applications, Beane and her colleagues exposed pure human ASC cultures, stromal vascular fraction (SVF) tissue samples (which include ASCs and several other cell types), and cultures of human fibroblast cells to medically relevant concentrations of chemotherapy drugs for 24 hours.
The researchers then measured how those cell populations fared over the next 10 days. They also measured the ability of MTX-exposed ASCs, both alone and in SVF, to proliferate and differentiate.
In contrast to the fibroblast controls, the ASCs withstood a variety of MTX doses. The drug had little or no effect on ASC viability, cell division, senescence, or differentiation. ASCs also resisted vincristine to an extent, but they could not withstand exposure to cytarabine or etoposide.
The SVF tissue samples withstood MTX doses well too. That is significant, according to the researchers, because the tissue would be clinically useful if an ASC-based therapy were ever developed for cancer patients. Hypothetically, fresh SVF could be harvested from the fat of a donor and injected into bone tissue, delivering ASCs to the site.
To understand why ASCs resist MTX, the researchers conducted further tests. MTX shuts down DNA biosynthesis by binding the protein dihydrofolate reductase so it is unavailable to assist in that essential task.
The testing showed that ASCs ramped up dihydrofolate reductase levels upon exposure to the drug. They produced enough to overcome a clinically relevant dose of MTX.
Now, the researchers are eager to see if they can translate these findings to deliver a medical benefit for cancer patients. To that end, the team is planning several more experiments.
One is to test ASC survival and performance after 48- and 72-hour exposures to MTX. Another is to begin examining how the cells fare in mouse models of chemotherapy. The researchers plan to directly compare ASCs and bone marrow-derived stem cells exposed to various chemotherapies. ![]()

A new study indicates that adipose-derived stem cells (ASCs) are highly resistant to the chemotherapy drug methotrexate (MTX).
Cultured ASCs and tissue samples that included ASCs were able to withstand exposure to MTX quite well. The drug had little or no effect on ASC viability, division, senescence, or differentiation.
The researchers believe these findings could prove significant for cancer patients, particularly children with acute lymphoblastic leukemia.
“Kids undergo chemotherapy at such an important time, when they should be growing, but, instead, they are introduced to this very harsh environment where bone cells are damaged with these drugs,” said study author Olivia Beane, a graduate student at Brown University in Providence, Rhode Island.
“That leads to major long-term side effects, including osteoporosis and bone defects. If we found a stem cell that was resistant to the chemotherapeutic agent and could promote bone growth by becoming bone itself, then maybe they wouldn’t have these issues.”
Beane’s work, which appears in Experimental Cell Research, grew out of more basic research. She was originally looking for chemicals that could help purify ASCs from mixed cell cultures to encourage their proliferation.
Among other things, she tried chemotherapy drugs, speculating that ASCs might withstand a drug that other cells could not. The idea that this could help cancer patients did not come until later.
To explore potential cancer applications, Beane and her colleagues exposed pure human ASC cultures, stromal vascular fraction (SVF) tissue samples (which include ASCs and several other cell types), and cultures of human fibroblast cells to medically relevant concentrations of chemotherapy drugs for 24 hours.
The researchers then measured how those cell populations fared over the next 10 days. They also measured the ability of MTX-exposed ASCs, both alone and in SVF, to proliferate and differentiate.
In contrast to the fibroblast controls, the ASCs withstood a variety of MTX doses. The drug had little or no effect on ASC viability, cell division, senescence, or differentiation. ASCs also resisted vincristine to an extent, but they could not withstand exposure to cytarabine or etoposide.
The SVF tissue samples withstood MTX doses well too. That is significant, according to the researchers, because the tissue would be clinically useful if an ASC-based therapy were ever developed for cancer patients. Hypothetically, fresh SVF could be harvested from the fat of a donor and injected into bone tissue, delivering ASCs to the site.
To understand why ASCs resist MTX, the researchers conducted further tests. MTX shuts down DNA biosynthesis by binding the protein dihydrofolate reductase so it is unavailable to assist in that essential task.
The testing showed that ASCs ramped up dihydrofolate reductase levels upon exposure to the drug. They produced enough to overcome a clinically relevant dose of MTX.
Now, the researchers are eager to see if they can translate these findings to deliver a medical benefit for cancer patients. To that end, the team is planning several more experiments.
One is to test ASC survival and performance after 48- and 72-hour exposures to MTX. Another is to begin examining how the cells fare in mouse models of chemotherapy. The researchers plan to directly compare ASCs and bone marrow-derived stem cells exposed to various chemotherapies. ![]()
Factor affects thrombus size and content

Credit: Kevin MacKenzie
The activity of transglutaminase factor XIII is critical for retaining red blood cells in a thrombus and therefore affects the clot’s size, investigators have reported in the Journal of Clinical Investigation.
The team showed that mice deficient in factor XIII had smaller thrombi containing fewer red blood cells than wild-type mice. And the same phenomenon occurred in humans.
According to the researchers, this discovery could aid the development of a safer alternative to anticoagulants.
“If we can develop a treatment that exploits this discovery to reduce the size of blood clots, it would represent a whole new approach to treating thrombosis that’s different from anything else on the market,” said study author Alisa Wolberg, PhD, of the University of North Carolina at Chapel Hill.
“We think reducing factor XIII activity could be helpful to a large number of people, perhaps including some who cannot take existing blood-thinning medications.”
Dr Wolberg and her colleagues examined the role of factor XIII in clot formation, first in mice and then in human samples. To their surprise, the team found that mice incapable of producing factor XIII formed thrombi that were half the size of the clots produced by wild-type mice.
“That difference in itself was extremely striking,” said study author Maria Aleman, PhD, also of the University of North Carolina at Chapel Hill.
“Then, the second surprise was discovering that the size difference was actually due to a reduced number of red blood cells in the clot. Since no previous studies had suggested that it was possible to manipulate the number of red blood cells, we knew we had found something new.”
Factor XIII appears to play a crucial role in helping the fibrin matrix keep its integrity during thrombus formation. Normally, the fibrin matrix forms a strong mesh in and around the clot, trapping red blood cells within. Without factor XIII, some red blood cells are squeezed out, resulting in a much smaller clot.
Unlike existing drugs that reduce the formation of fibrin, a drug that reduces factor XIII could potentially cut the body’s ability to produce large, dangerous thrombi without sacrificing the ability to produce small, beneficial clots.
“What’s needed is a drug that reduces the risk of forming large clots but still allows you to form a clot when you need one to stanch bleeding,” Dr Wolberg said. “The biological pathway we’ve discovered may make it possible to strike that balance.” ![]()

Credit: Kevin MacKenzie
The activity of transglutaminase factor XIII is critical for retaining red blood cells in a thrombus and therefore affects the clot’s size, investigators have reported in the Journal of Clinical Investigation.
The team showed that mice deficient in factor XIII had smaller thrombi containing fewer red blood cells than wild-type mice. And the same phenomenon occurred in humans.
According to the researchers, this discovery could aid the development of a safer alternative to anticoagulants.
“If we can develop a treatment that exploits this discovery to reduce the size of blood clots, it would represent a whole new approach to treating thrombosis that’s different from anything else on the market,” said study author Alisa Wolberg, PhD, of the University of North Carolina at Chapel Hill.
“We think reducing factor XIII activity could be helpful to a large number of people, perhaps including some who cannot take existing blood-thinning medications.”
Dr Wolberg and her colleagues examined the role of factor XIII in clot formation, first in mice and then in human samples. To their surprise, the team found that mice incapable of producing factor XIII formed thrombi that were half the size of the clots produced by wild-type mice.
“That difference in itself was extremely striking,” said study author Maria Aleman, PhD, also of the University of North Carolina at Chapel Hill.
“Then, the second surprise was discovering that the size difference was actually due to a reduced number of red blood cells in the clot. Since no previous studies had suggested that it was possible to manipulate the number of red blood cells, we knew we had found something new.”
Factor XIII appears to play a crucial role in helping the fibrin matrix keep its integrity during thrombus formation. Normally, the fibrin matrix forms a strong mesh in and around the clot, trapping red blood cells within. Without factor XIII, some red blood cells are squeezed out, resulting in a much smaller clot.
Unlike existing drugs that reduce the formation of fibrin, a drug that reduces factor XIII could potentially cut the body’s ability to produce large, dangerous thrombi without sacrificing the ability to produce small, beneficial clots.
“What’s needed is a drug that reduces the risk of forming large clots but still allows you to form a clot when you need one to stanch bleeding,” Dr Wolberg said. “The biological pathway we’ve discovered may make it possible to strike that balance.” ![]()

Credit: Kevin MacKenzie
The activity of transglutaminase factor XIII is critical for retaining red blood cells in a thrombus and therefore affects the clot’s size, investigators have reported in the Journal of Clinical Investigation.
The team showed that mice deficient in factor XIII had smaller thrombi containing fewer red blood cells than wild-type mice. And the same phenomenon occurred in humans.
According to the researchers, this discovery could aid the development of a safer alternative to anticoagulants.
“If we can develop a treatment that exploits this discovery to reduce the size of blood clots, it would represent a whole new approach to treating thrombosis that’s different from anything else on the market,” said study author Alisa Wolberg, PhD, of the University of North Carolina at Chapel Hill.
“We think reducing factor XIII activity could be helpful to a large number of people, perhaps including some who cannot take existing blood-thinning medications.”
Dr Wolberg and her colleagues examined the role of factor XIII in clot formation, first in mice and then in human samples. To their surprise, the team found that mice incapable of producing factor XIII formed thrombi that were half the size of the clots produced by wild-type mice.
“That difference in itself was extremely striking,” said study author Maria Aleman, PhD, also of the University of North Carolina at Chapel Hill.
“Then, the second surprise was discovering that the size difference was actually due to a reduced number of red blood cells in the clot. Since no previous studies had suggested that it was possible to manipulate the number of red blood cells, we knew we had found something new.”
Factor XIII appears to play a crucial role in helping the fibrin matrix keep its integrity during thrombus formation. Normally, the fibrin matrix forms a strong mesh in and around the clot, trapping red blood cells within. Without factor XIII, some red blood cells are squeezed out, resulting in a much smaller clot.
Unlike existing drugs that reduce the formation of fibrin, a drug that reduces factor XIII could potentially cut the body’s ability to produce large, dangerous thrombi without sacrificing the ability to produce small, beneficial clots.
“What’s needed is a drug that reduces the risk of forming large clots but still allows you to form a clot when you need one to stanch bleeding,” Dr Wolberg said. “The biological pathway we’ve discovered may make it possible to strike that balance.” ![]()
EC expands indication for ofatumumab

Credit: Linda Bartlett
The European Commission (EC) has expanded the indication for the anti-CD20 monoclonal antibody ofatumumab (Arzerra).
The EC granted conditional approval for ofatumumab in combination with chlorambucil or bendamustine to treat patients with chronic lymphocytic leukemia (CLL) who have not received prior therapy and are not eligible for fludarabine-based therapy.
In 2010, the EC granted ofatumumab conditional approval to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses. Ofatumumab will not receive full approval until the drug’s developers, GlaxoSmithKline and GenMab, submit results of additional research to the EC.
Trial results
The EC’s new approval of ofatumumab, for previously untreated CLL patients, is based on results from 2 trials, COMPLEMENT 1 and OMB115991.
The phase 3 COMPLEMENT 1 trial was a comparison of ofatumumab plus chlorambucil (n=221) with chlorambucil alone (n=226) in CLL patients for whom fludarabine-based treatment was considered inappropriate.
In this study, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
In the phase 2 trial known as OMB115991, researchers evaluated ofatumumab in combination with bendamustine in 44 patients with previously untreated CLL for whom fludarabine-based treatment was considered inappropriate.
The combination elicited an overall response rate of 95% and a complete response rate of 43%.
The overall safety profile of ofatumumab in CLL (previously untreated and relapsed/refractory) is based on data from 511 patients in clinical trials.
This includes 250 patients with relapsed/refractory CLL who were treated with ofatumumab alone and 261 patients with previously untreated CLL who were treated in combination with an alkylating agent.
The most common adverse effects associated with ofatumumab were infusion reactions, neutropenia, anemia, febrile neutropenia, thrombocytopenia, leukopenia, lower respiratory tract infection (including pneumonia), upper respiratory tract infection, sepsis (including neutropenic sepsis and septic shock), herpes virus infection, and urinary tract infection. ![]()

Credit: Linda Bartlett
The European Commission (EC) has expanded the indication for the anti-CD20 monoclonal antibody ofatumumab (Arzerra).
The EC granted conditional approval for ofatumumab in combination with chlorambucil or bendamustine to treat patients with chronic lymphocytic leukemia (CLL) who have not received prior therapy and are not eligible for fludarabine-based therapy.
In 2010, the EC granted ofatumumab conditional approval to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses. Ofatumumab will not receive full approval until the drug’s developers, GlaxoSmithKline and GenMab, submit results of additional research to the EC.
Trial results
The EC’s new approval of ofatumumab, for previously untreated CLL patients, is based on results from 2 trials, COMPLEMENT 1 and OMB115991.
The phase 3 COMPLEMENT 1 trial was a comparison of ofatumumab plus chlorambucil (n=221) with chlorambucil alone (n=226) in CLL patients for whom fludarabine-based treatment was considered inappropriate.
In this study, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
In the phase 2 trial known as OMB115991, researchers evaluated ofatumumab in combination with bendamustine in 44 patients with previously untreated CLL for whom fludarabine-based treatment was considered inappropriate.
The combination elicited an overall response rate of 95% and a complete response rate of 43%.
The overall safety profile of ofatumumab in CLL (previously untreated and relapsed/refractory) is based on data from 511 patients in clinical trials.
This includes 250 patients with relapsed/refractory CLL who were treated with ofatumumab alone and 261 patients with previously untreated CLL who were treated in combination with an alkylating agent.
The most common adverse effects associated with ofatumumab were infusion reactions, neutropenia, anemia, febrile neutropenia, thrombocytopenia, leukopenia, lower respiratory tract infection (including pneumonia), upper respiratory tract infection, sepsis (including neutropenic sepsis and septic shock), herpes virus infection, and urinary tract infection. ![]()

Credit: Linda Bartlett
The European Commission (EC) has expanded the indication for the anti-CD20 monoclonal antibody ofatumumab (Arzerra).
The EC granted conditional approval for ofatumumab in combination with chlorambucil or bendamustine to treat patients with chronic lymphocytic leukemia (CLL) who have not received prior therapy and are not eligible for fludarabine-based therapy.
In 2010, the EC granted ofatumumab conditional approval to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses. Ofatumumab will not receive full approval until the drug’s developers, GlaxoSmithKline and GenMab, submit results of additional research to the EC.
Trial results
The EC’s new approval of ofatumumab, for previously untreated CLL patients, is based on results from 2 trials, COMPLEMENT 1 and OMB115991.
The phase 3 COMPLEMENT 1 trial was a comparison of ofatumumab plus chlorambucil (n=221) with chlorambucil alone (n=226) in CLL patients for whom fludarabine-based treatment was considered inappropriate.
In this study, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
In the phase 2 trial known as OMB115991, researchers evaluated ofatumumab in combination with bendamustine in 44 patients with previously untreated CLL for whom fludarabine-based treatment was considered inappropriate.
The combination elicited an overall response rate of 95% and a complete response rate of 43%.
The overall safety profile of ofatumumab in CLL (previously untreated and relapsed/refractory) is based on data from 511 patients in clinical trials.
This includes 250 patients with relapsed/refractory CLL who were treated with ofatumumab alone and 261 patients with previously untreated CLL who were treated in combination with an alkylating agent.
The most common adverse effects associated with ofatumumab were infusion reactions, neutropenia, anemia, febrile neutropenia, thrombocytopenia, leukopenia, lower respiratory tract infection (including pneumonia), upper respiratory tract infection, sepsis (including neutropenic sepsis and septic shock), herpes virus infection, and urinary tract infection. ![]()
USPSTF: Don’t screen general population for carotid stenosis
Asymptomatic adults in the general population who have no history of stroke, transient ischemic attack, or neurologic signs or symptoms should not be screened for carotid artery stenosis, according to a U.S. Preventive Services Task Force recommendation published online July 7 in Annals of Internal Medicine.
All screening strategies, even a noninvasive one that has minimal harmful effects such as ultrasonography, are insufficiently sensitive for detecting the condition. And all of them can lead to unnecessary treatment or can themselves induce serious harms including death, stroke, and myocardial infarction. Therefore, at this time, "the harms of screening for asymptomatic carotid artery stenosis outweigh the benefits," said Dr. Michael L. LeFevre of the University of Missouri, Columbia, and his associates with the USPSTF.
The recommendation is an update of the previous one issued in 2007, which also concluded that screening the general population for carotid stenosis was unwarranted. For this update, the USPSTF performed an exhaustive review and meta-analysis of the data that have accrued since that time, which addressed advances in screening tests, risk stratification tools, both screening and treatment using carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAAS), and optimal medical therapies.
Dr. LeFevre, a cochair of USPSTF, and his colleagues reviewed recent randomized controlled trials, meta-analyses, and cohort studies of these topics. They found that the prevalence of carotid artery stenosis is only 0.5%-1% in the general population of adults. The most feasible screen for the condition is duplex ultrasonography; but in real-world practice, even this screen yields many false-positive results in such patients, and so exposes them to harm.
There also is no evidence that another noninvasive screen for carotid artery stenosis – auscultation of the neck to detect carotid bruits – is accurate or provides any benefit. Only four studies examined this strategy; none of them used angiography as a gold standard for diagnosis, and only two involved patients from the general population.
Moreover, even when screening of asymptomatic patients leads to detection and early intervention, "the magnitude of benefit is small to none." In particular, adding medications to current optimal medical management does not appear to convey any benefit, Dr. LeFevre and his associates said.
On the other side of the benefit-to-harm scale, carotid endarterectomy is associated with a 30-day rate of stroke or mortality of approximately 2.4% overall. However, the rates are as high as 5% in low-volume medical centers and 6% in certain states. The 30-day rate of stroke or mortality associated with CAAS is 3.1%-3.8%. Those risks are far too high to counterbalance the small benefit of screening, the USPSTF reviewers noted.
Other important harms after CEA or CAAS include myocardial infarction, surgical complications, cranial nerve injury, lung embolism, pneumonia, and local hematoma requiring further surgery.
The review and meta-analysis were hampered by a dearth of high-quality data. Specifically, much more data are needed comparing patient outcomes after CEA or CAAS with those after optimal medical therapy. The planned CREST-2 (Carotid Revascularization Endarterectomy vs Stenting Trial 2) will include a comparator group on medical management alone, and should provide important findings in this regard, Dr. LeFevre and his associates said.
They added that the USPSTF recommendation against screening the general population for carotid stenosis agrees with recommendations from the American Heart Association, American Stroke Association, American College of Cardiology, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Society for Vascular Surgery, Society for Vascular Medicine, and the American Academy of Family Physicians.
More information on this recommendation – as well as recommendations for the related issues of hypertension, dyslipidemia, CHD, and diet – is available at the USPSTF website.
The USPSTF is an independent group that makes recommendations about the effectiveness of specific preventive care services and is funded by the Agency for Healthcare Research and Quality.
The available data clearly support the USPSTF’s reaffirmation of its previous recommendation against screening for asymptomatic carotid artery stenosis in the general population, yet "such screenings are offered throughout the country in health fairs and other settings," said Dr. Larry B. Goldstein.
Patients should be aware that such tests are unlikely to prevent them from having a stroke or to otherwise improve their health. The population-attributable risk for stroke related to asymptomatic CAS is only 0.7% – a figure that is dwarfed by such factors as hypertension (population-attributable risk greater than 95%), atrial fibrillation (population-attributable risk as high as 24%, depending on patient age and other factors), cigarette smoking (population-attributable risk of up to 14%), and hyperlipidemia (population-attributable risk of 9%), he noted.
Dr. Goldstein is at Duke University’s Stroke Center and Durham Veterans Affairs Medical Center in Durham, N.C. These remarks were taken from his editorial accompanying Dr. LeFevre’s report.
The available data clearly support the USPSTF’s reaffirmation of its previous recommendation against screening for asymptomatic carotid artery stenosis in the general population, yet "such screenings are offered throughout the country in health fairs and other settings," said Dr. Larry B. Goldstein.
Patients should be aware that such tests are unlikely to prevent them from having a stroke or to otherwise improve their health. The population-attributable risk for stroke related to asymptomatic CAS is only 0.7% – a figure that is dwarfed by such factors as hypertension (population-attributable risk greater than 95%), atrial fibrillation (population-attributable risk as high as 24%, depending on patient age and other factors), cigarette smoking (population-attributable risk of up to 14%), and hyperlipidemia (population-attributable risk of 9%), he noted.
Dr. Goldstein is at Duke University’s Stroke Center and Durham Veterans Affairs Medical Center in Durham, N.C. These remarks were taken from his editorial accompanying Dr. LeFevre’s report.
The available data clearly support the USPSTF’s reaffirmation of its previous recommendation against screening for asymptomatic carotid artery stenosis in the general population, yet "such screenings are offered throughout the country in health fairs and other settings," said Dr. Larry B. Goldstein.
Patients should be aware that such tests are unlikely to prevent them from having a stroke or to otherwise improve their health. The population-attributable risk for stroke related to asymptomatic CAS is only 0.7% – a figure that is dwarfed by such factors as hypertension (population-attributable risk greater than 95%), atrial fibrillation (population-attributable risk as high as 24%, depending on patient age and other factors), cigarette smoking (population-attributable risk of up to 14%), and hyperlipidemia (population-attributable risk of 9%), he noted.
Dr. Goldstein is at Duke University’s Stroke Center and Durham Veterans Affairs Medical Center in Durham, N.C. These remarks were taken from his editorial accompanying Dr. LeFevre’s report.
Asymptomatic adults in the general population who have no history of stroke, transient ischemic attack, or neurologic signs or symptoms should not be screened for carotid artery stenosis, according to a U.S. Preventive Services Task Force recommendation published online July 7 in Annals of Internal Medicine.
All screening strategies, even a noninvasive one that has minimal harmful effects such as ultrasonography, are insufficiently sensitive for detecting the condition. And all of them can lead to unnecessary treatment or can themselves induce serious harms including death, stroke, and myocardial infarction. Therefore, at this time, "the harms of screening for asymptomatic carotid artery stenosis outweigh the benefits," said Dr. Michael L. LeFevre of the University of Missouri, Columbia, and his associates with the USPSTF.
The recommendation is an update of the previous one issued in 2007, which also concluded that screening the general population for carotid stenosis was unwarranted. For this update, the USPSTF performed an exhaustive review and meta-analysis of the data that have accrued since that time, which addressed advances in screening tests, risk stratification tools, both screening and treatment using carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAAS), and optimal medical therapies.
Dr. LeFevre, a cochair of USPSTF, and his colleagues reviewed recent randomized controlled trials, meta-analyses, and cohort studies of these topics. They found that the prevalence of carotid artery stenosis is only 0.5%-1% in the general population of adults. The most feasible screen for the condition is duplex ultrasonography; but in real-world practice, even this screen yields many false-positive results in such patients, and so exposes them to harm.
There also is no evidence that another noninvasive screen for carotid artery stenosis – auscultation of the neck to detect carotid bruits – is accurate or provides any benefit. Only four studies examined this strategy; none of them used angiography as a gold standard for diagnosis, and only two involved patients from the general population.
Moreover, even when screening of asymptomatic patients leads to detection and early intervention, "the magnitude of benefit is small to none." In particular, adding medications to current optimal medical management does not appear to convey any benefit, Dr. LeFevre and his associates said.
On the other side of the benefit-to-harm scale, carotid endarterectomy is associated with a 30-day rate of stroke or mortality of approximately 2.4% overall. However, the rates are as high as 5% in low-volume medical centers and 6% in certain states. The 30-day rate of stroke or mortality associated with CAAS is 3.1%-3.8%. Those risks are far too high to counterbalance the small benefit of screening, the USPSTF reviewers noted.
Other important harms after CEA or CAAS include myocardial infarction, surgical complications, cranial nerve injury, lung embolism, pneumonia, and local hematoma requiring further surgery.
The review and meta-analysis were hampered by a dearth of high-quality data. Specifically, much more data are needed comparing patient outcomes after CEA or CAAS with those after optimal medical therapy. The planned CREST-2 (Carotid Revascularization Endarterectomy vs Stenting Trial 2) will include a comparator group on medical management alone, and should provide important findings in this regard, Dr. LeFevre and his associates said.
They added that the USPSTF recommendation against screening the general population for carotid stenosis agrees with recommendations from the American Heart Association, American Stroke Association, American College of Cardiology, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Society for Vascular Surgery, Society for Vascular Medicine, and the American Academy of Family Physicians.
More information on this recommendation – as well as recommendations for the related issues of hypertension, dyslipidemia, CHD, and diet – is available at the USPSTF website.
The USPSTF is an independent group that makes recommendations about the effectiveness of specific preventive care services and is funded by the Agency for Healthcare Research and Quality.
Asymptomatic adults in the general population who have no history of stroke, transient ischemic attack, or neurologic signs or symptoms should not be screened for carotid artery stenosis, according to a U.S. Preventive Services Task Force recommendation published online July 7 in Annals of Internal Medicine.
All screening strategies, even a noninvasive one that has minimal harmful effects such as ultrasonography, are insufficiently sensitive for detecting the condition. And all of them can lead to unnecessary treatment or can themselves induce serious harms including death, stroke, and myocardial infarction. Therefore, at this time, "the harms of screening for asymptomatic carotid artery stenosis outweigh the benefits," said Dr. Michael L. LeFevre of the University of Missouri, Columbia, and his associates with the USPSTF.
The recommendation is an update of the previous one issued in 2007, which also concluded that screening the general population for carotid stenosis was unwarranted. For this update, the USPSTF performed an exhaustive review and meta-analysis of the data that have accrued since that time, which addressed advances in screening tests, risk stratification tools, both screening and treatment using carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAAS), and optimal medical therapies.
Dr. LeFevre, a cochair of USPSTF, and his colleagues reviewed recent randomized controlled trials, meta-analyses, and cohort studies of these topics. They found that the prevalence of carotid artery stenosis is only 0.5%-1% in the general population of adults. The most feasible screen for the condition is duplex ultrasonography; but in real-world practice, even this screen yields many false-positive results in such patients, and so exposes them to harm.
There also is no evidence that another noninvasive screen for carotid artery stenosis – auscultation of the neck to detect carotid bruits – is accurate or provides any benefit. Only four studies examined this strategy; none of them used angiography as a gold standard for diagnosis, and only two involved patients from the general population.
Moreover, even when screening of asymptomatic patients leads to detection and early intervention, "the magnitude of benefit is small to none." In particular, adding medications to current optimal medical management does not appear to convey any benefit, Dr. LeFevre and his associates said.
On the other side of the benefit-to-harm scale, carotid endarterectomy is associated with a 30-day rate of stroke or mortality of approximately 2.4% overall. However, the rates are as high as 5% in low-volume medical centers and 6% in certain states. The 30-day rate of stroke or mortality associated with CAAS is 3.1%-3.8%. Those risks are far too high to counterbalance the small benefit of screening, the USPSTF reviewers noted.
Other important harms after CEA or CAAS include myocardial infarction, surgical complications, cranial nerve injury, lung embolism, pneumonia, and local hematoma requiring further surgery.
The review and meta-analysis were hampered by a dearth of high-quality data. Specifically, much more data are needed comparing patient outcomes after CEA or CAAS with those after optimal medical therapy. The planned CREST-2 (Carotid Revascularization Endarterectomy vs Stenting Trial 2) will include a comparator group on medical management alone, and should provide important findings in this regard, Dr. LeFevre and his associates said.
They added that the USPSTF recommendation against screening the general population for carotid stenosis agrees with recommendations from the American Heart Association, American Stroke Association, American College of Cardiology, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Society for Vascular Surgery, Society for Vascular Medicine, and the American Academy of Family Physicians.
More information on this recommendation – as well as recommendations for the related issues of hypertension, dyslipidemia, CHD, and diet – is available at the USPSTF website.
The USPSTF is an independent group that makes recommendations about the effectiveness of specific preventive care services and is funded by the Agency for Healthcare Research and Quality.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical finding: Don’t screen asymptomatic adults for carotid artery stenosis.
Major finding: The harms of screening asymptomatic adults in the general population for carotid artery stenosis outweigh the benefits, because all currently available screens "have imperfect sensitivity" and can lead to unnecessary treatment that induces serious harms, including death, stroke, myocardial infarction, cranial nerve injury, and embolism.
Data source: A comprehensive review and meta-analysis of data from randomized controlled trials, meta-analyses, and cohort studies performed since 2007 regarding CAS screening tests, risk stratification tools, for both screening and treatment using CEA and CAAS, and optimal medical therapies.
Disclosures: The USPSTF is an independent group that makes recommendations about the effectiveness of specific preventive care services and is funded by the Agency for Healthcare Research and Quality.
Delayed revisions led to worse congenital outcomes
In previous studies on patients under 6 months of age undergoing a wide range of congenital cardiac operations, Dr. Meena Nathan and her colleagues at Boston Children’s Hospital found that immediate revisions of procedures intraoperatively that resulted in adequate anatomic correction of residual defects did not affect outcomes, but that delayed revisions of residual lesions resulted in worse patient outcomes.
Dr. Nathan presented the results of a larger prospective cohort of patients that she and her colleagues studied who were followed from index surgery to discharge from January 2011 to September 2013.
Patients were divided into four groups: a) intraoperative revisions of residual lesion, b) delayed postoperative revision of residual lesions during the same hospital stay, c) both intraoperative and delayed (BOTH) revision of residual lesions, d) and no revisions (neither intraoperative nor postoperative revision), Dr. Nathan said at the annual meeting of the American Association for Thoracic Surgery.
They used linear and logistic regression to compare the outcomes mortality, complications (excluding unplanned postoperative reinterventions) and postoperative hospital length of stay across the four groups (using patients who had intraoperative revisions only as reference group).
"We adjusted for baseline patient risk including age, prematurity, presence of extracardiac anomalies, and RACHS-1 risk category, each of which could contribute to the difference in outcomes," according to Dr. Nathan.
"In addition, to allow inclusion of all patients in the risk-adjusted analyses, we added two additional categories to the RACHS-1 categories: all patients less than 18 years of age with non–RACHS-1 categorizable procedures, and adults greater than 18 years who are not eligible for RACHS-1 risk adjustment," Dr. Nathan added.
A total of 2,427 patients were discharged after congenital cardiac operations during the time period studied.
As might be expected, on multivariable modeling, adjusting for other significant patient factors, the no-revisions group fared better than the other three groups. The intraoperative revision group had significantly lower postoperative length of stay and complication rates when compared to the delayed postoperative revision and the BOTH group, but they showed no significant differences in mortality compared to these two groups.
On subgroup analysis of the intraoperative revision group, 86% left the hospital with an optimal or adequate repair on discharge echocardiogram, Dr. Nathan added.
"We found that the intraoperative correction of residual lesions results in a shorter length of stay and lower complications when compared to those patients who underwent delayed postoperative revision of residual lesion," she concluded.
Dr. Nathan reported that she had no relevant disclosures.
In previous studies on patients under 6 months of age undergoing a wide range of congenital cardiac operations, Dr. Meena Nathan and her colleagues at Boston Children’s Hospital found that immediate revisions of procedures intraoperatively that resulted in adequate anatomic correction of residual defects did not affect outcomes, but that delayed revisions of residual lesions resulted in worse patient outcomes.
Dr. Nathan presented the results of a larger prospective cohort of patients that she and her colleagues studied who were followed from index surgery to discharge from January 2011 to September 2013.
Patients were divided into four groups: a) intraoperative revisions of residual lesion, b) delayed postoperative revision of residual lesions during the same hospital stay, c) both intraoperative and delayed (BOTH) revision of residual lesions, d) and no revisions (neither intraoperative nor postoperative revision), Dr. Nathan said at the annual meeting of the American Association for Thoracic Surgery.
They used linear and logistic regression to compare the outcomes mortality, complications (excluding unplanned postoperative reinterventions) and postoperative hospital length of stay across the four groups (using patients who had intraoperative revisions only as reference group).
"We adjusted for baseline patient risk including age, prematurity, presence of extracardiac anomalies, and RACHS-1 risk category, each of which could contribute to the difference in outcomes," according to Dr. Nathan.
"In addition, to allow inclusion of all patients in the risk-adjusted analyses, we added two additional categories to the RACHS-1 categories: all patients less than 18 years of age with non–RACHS-1 categorizable procedures, and adults greater than 18 years who are not eligible for RACHS-1 risk adjustment," Dr. Nathan added.
A total of 2,427 patients were discharged after congenital cardiac operations during the time period studied.
As might be expected, on multivariable modeling, adjusting for other significant patient factors, the no-revisions group fared better than the other three groups. The intraoperative revision group had significantly lower postoperative length of stay and complication rates when compared to the delayed postoperative revision and the BOTH group, but they showed no significant differences in mortality compared to these two groups.
On subgroup analysis of the intraoperative revision group, 86% left the hospital with an optimal or adequate repair on discharge echocardiogram, Dr. Nathan added.
"We found that the intraoperative correction of residual lesions results in a shorter length of stay and lower complications when compared to those patients who underwent delayed postoperative revision of residual lesion," she concluded.
Dr. Nathan reported that she had no relevant disclosures.
In previous studies on patients under 6 months of age undergoing a wide range of congenital cardiac operations, Dr. Meena Nathan and her colleagues at Boston Children’s Hospital found that immediate revisions of procedures intraoperatively that resulted in adequate anatomic correction of residual defects did not affect outcomes, but that delayed revisions of residual lesions resulted in worse patient outcomes.
Dr. Nathan presented the results of a larger prospective cohort of patients that she and her colleagues studied who were followed from index surgery to discharge from January 2011 to September 2013.
Patients were divided into four groups: a) intraoperative revisions of residual lesion, b) delayed postoperative revision of residual lesions during the same hospital stay, c) both intraoperative and delayed (BOTH) revision of residual lesions, d) and no revisions (neither intraoperative nor postoperative revision), Dr. Nathan said at the annual meeting of the American Association for Thoracic Surgery.
They used linear and logistic regression to compare the outcomes mortality, complications (excluding unplanned postoperative reinterventions) and postoperative hospital length of stay across the four groups (using patients who had intraoperative revisions only as reference group).
"We adjusted for baseline patient risk including age, prematurity, presence of extracardiac anomalies, and RACHS-1 risk category, each of which could contribute to the difference in outcomes," according to Dr. Nathan.
"In addition, to allow inclusion of all patients in the risk-adjusted analyses, we added two additional categories to the RACHS-1 categories: all patients less than 18 years of age with non–RACHS-1 categorizable procedures, and adults greater than 18 years who are not eligible for RACHS-1 risk adjustment," Dr. Nathan added.
A total of 2,427 patients were discharged after congenital cardiac operations during the time period studied.
As might be expected, on multivariable modeling, adjusting for other significant patient factors, the no-revisions group fared better than the other three groups. The intraoperative revision group had significantly lower postoperative length of stay and complication rates when compared to the delayed postoperative revision and the BOTH group, but they showed no significant differences in mortality compared to these two groups.
On subgroup analysis of the intraoperative revision group, 86% left the hospital with an optimal or adequate repair on discharge echocardiogram, Dr. Nathan added.
"We found that the intraoperative correction of residual lesions results in a shorter length of stay and lower complications when compared to those patients who underwent delayed postoperative revision of residual lesion," she concluded.
Dr. Nathan reported that she had no relevant disclosures.
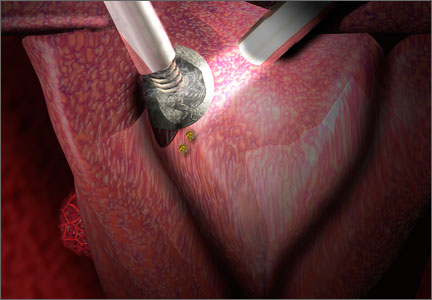
Will open power morcellation of uterine tissue remain an option during hysterectomy and myomectomy?
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In February 2014, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Not surprisingly, the numerous statements and warnings since then have led to some confusion in the specialty about the safest course of action for tissue extraction during hysterectomy and myomectomy in women with a large uterus.
To explore the options more deeply and address the future of minimally invasive surgery (MIS) in women’s health, OBG Management invited two experts to comment: Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches to patients
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, though neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be utilized whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?2 How does it compare with the ACOG and FDA statements on the use of power morcellation?
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. I’ve been practicing for almost 40 years in academic and private settings, and I found this group to be the brightest, most caring and compassionate group with whom I’ve ever worked. Our review is the most complete report to date, more comprehensive than the reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL task force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. One of the problems we encountered was that there are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction in individual patients undergoing uterine surgery.
How to manage tissue extraction going forward
OBG Management: The FDA will convene another meeting on power morcellation July 10 and 11. Regardless of its final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: Yes, AAGL will be at the FDA’s July hearing because we are the experts. MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the current data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. Risk factors include advanced age, history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Education, at the hospital and national level, is in the works
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, Dr. Reich and I, along with Albert Steren, MD, a minimally invasive surgeon from Rockville, Maryland, are hosting an educational dinner meeting on tissue extraction on July 24 in northern Virginia. I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November, since this is such a pressing issue.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs (NSAIDs) by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, since you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today the majority of gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.3 However, the AAGL task force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views of the professional societies:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopically and microscopically at the subsequent laparotomy? We on the AAGL task force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL task force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
What should the FDA’s next move be?
OBG Management: Care to make any predictions about the FDA’s final decision?
Dr. Wertheim: This has become a highly emotional and controversial issue with little good existing data. During the preoperative visit, this issue should be discussed with the patient using clear, lay-friendly language. Having said that, I also do not believe we should hide behind informed consent. The FDA has a responsibility to keep the public safe. If open power morcellation is allowed to continue, there will be another morcellated sarcoma or complications from retained benign tissue fragments. I doubt the FDA can live with this. I believe the risk factors identified by the AAGL task force should be ruled out, the appropriate workup done and then, if power morcellation is performed, it should be done inside a bag. In addition, I think the FDA should require that complications be reported and recorded in a registry.
Dr. Reich: I disagree. The FDA has to back off. It’s important to note that this is an American problem, as the rest of the world cannot afford power morcellators. The data are not in yet. The decision about what kind of hysterectomy is performed will be made by the “informed” patient, who undoubtedly will be very afraid to have MIS because of the surrounding negative publicity. We must do a better job of promoting the advantages of a minimally invasive approach.
OBG Management: Thank you both for your time and expertise.
Dr. Wertheim: Thank you for giving us the opportunity to express our opinions regarding this highly emotional and controversial issue.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
1. Barbieri RL. Benefits and pitfalls of open power morcellation of uterine fibroids. OBG Manag. 2014;26(2):10–15.
2. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation During Uterine Tissue Extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 014.
3. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In February 2014, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Not surprisingly, the numerous statements and warnings since then have led to some confusion in the specialty about the safest course of action for tissue extraction during hysterectomy and myomectomy in women with a large uterus.
To explore the options more deeply and address the future of minimally invasive surgery (MIS) in women’s health, OBG Management invited two experts to comment: Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches to patients
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, though neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be utilized whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?2 How does it compare with the ACOG and FDA statements on the use of power morcellation?
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. I’ve been practicing for almost 40 years in academic and private settings, and I found this group to be the brightest, most caring and compassionate group with whom I’ve ever worked. Our review is the most complete report to date, more comprehensive than the reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL task force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. One of the problems we encountered was that there are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction in individual patients undergoing uterine surgery.
How to manage tissue extraction going forward
OBG Management: The FDA will convene another meeting on power morcellation July 10 and 11. Regardless of its final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: Yes, AAGL will be at the FDA’s July hearing because we are the experts. MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the current data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. Risk factors include advanced age, history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Education, at the hospital and national level, is in the works
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, Dr. Reich and I, along with Albert Steren, MD, a minimally invasive surgeon from Rockville, Maryland, are hosting an educational dinner meeting on tissue extraction on July 24 in northern Virginia. I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November, since this is such a pressing issue.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs (NSAIDs) by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, since you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today the majority of gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.3 However, the AAGL task force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views of the professional societies:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopically and microscopically at the subsequent laparotomy? We on the AAGL task force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL task force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
What should the FDA’s next move be?
OBG Management: Care to make any predictions about the FDA’s final decision?
Dr. Wertheim: This has become a highly emotional and controversial issue with little good existing data. During the preoperative visit, this issue should be discussed with the patient using clear, lay-friendly language. Having said that, I also do not believe we should hide behind informed consent. The FDA has a responsibility to keep the public safe. If open power morcellation is allowed to continue, there will be another morcellated sarcoma or complications from retained benign tissue fragments. I doubt the FDA can live with this. I believe the risk factors identified by the AAGL task force should be ruled out, the appropriate workup done and then, if power morcellation is performed, it should be done inside a bag. In addition, I think the FDA should require that complications be reported and recorded in a registry.
Dr. Reich: I disagree. The FDA has to back off. It’s important to note that this is an American problem, as the rest of the world cannot afford power morcellators. The data are not in yet. The decision about what kind of hysterectomy is performed will be made by the “informed” patient, who undoubtedly will be very afraid to have MIS because of the surrounding negative publicity. We must do a better job of promoting the advantages of a minimally invasive approach.
OBG Management: Thank you both for your time and expertise.
Dr. Wertheim: Thank you for giving us the opportunity to express our opinions regarding this highly emotional and controversial issue.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In February 2014, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Not surprisingly, the numerous statements and warnings since then have led to some confusion in the specialty about the safest course of action for tissue extraction during hysterectomy and myomectomy in women with a large uterus.
To explore the options more deeply and address the future of minimally invasive surgery (MIS) in women’s health, OBG Management invited two experts to comment: Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches to patients
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, though neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be utilized whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?2 How does it compare with the ACOG and FDA statements on the use of power morcellation?
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. I’ve been practicing for almost 40 years in academic and private settings, and I found this group to be the brightest, most caring and compassionate group with whom I’ve ever worked. Our review is the most complete report to date, more comprehensive than the reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL task force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. One of the problems we encountered was that there are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction in individual patients undergoing uterine surgery.
How to manage tissue extraction going forward
OBG Management: The FDA will convene another meeting on power morcellation July 10 and 11. Regardless of its final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: Yes, AAGL will be at the FDA’s July hearing because we are the experts. MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the current data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. Risk factors include advanced age, history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Education, at the hospital and national level, is in the works
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, Dr. Reich and I, along with Albert Steren, MD, a minimally invasive surgeon from Rockville, Maryland, are hosting an educational dinner meeting on tissue extraction on July 24 in northern Virginia. I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November, since this is such a pressing issue.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs (NSAIDs) by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, since you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today the majority of gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.3 However, the AAGL task force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views of the professional societies:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopically and microscopically at the subsequent laparotomy? We on the AAGL task force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL task force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
What should the FDA’s next move be?
OBG Management: Care to make any predictions about the FDA’s final decision?
Dr. Wertheim: This has become a highly emotional and controversial issue with little good existing data. During the preoperative visit, this issue should be discussed with the patient using clear, lay-friendly language. Having said that, I also do not believe we should hide behind informed consent. The FDA has a responsibility to keep the public safe. If open power morcellation is allowed to continue, there will be another morcellated sarcoma or complications from retained benign tissue fragments. I doubt the FDA can live with this. I believe the risk factors identified by the AAGL task force should be ruled out, the appropriate workup done and then, if power morcellation is performed, it should be done inside a bag. In addition, I think the FDA should require that complications be reported and recorded in a registry.
Dr. Reich: I disagree. The FDA has to back off. It’s important to note that this is an American problem, as the rest of the world cannot afford power morcellators. The data are not in yet. The decision about what kind of hysterectomy is performed will be made by the “informed” patient, who undoubtedly will be very afraid to have MIS because of the surrounding negative publicity. We must do a better job of promoting the advantages of a minimally invasive approach.
OBG Management: Thank you both for your time and expertise.
Dr. Wertheim: Thank you for giving us the opportunity to express our opinions regarding this highly emotional and controversial issue.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
1. Barbieri RL. Benefits and pitfalls of open power morcellation of uterine fibroids. OBG Manag. 2014;26(2):10–15.
2. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation During Uterine Tissue Extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 014.
3. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
1. Barbieri RL. Benefits and pitfalls of open power morcellation of uterine fibroids. OBG Manag. 2014;26(2):10–15.
2. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation During Uterine Tissue Extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 014.
3. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
Visit the Morcellation Topic Collection Page for additional articles, videos, and audiocasts.
Bariatric Surgery Leads to 3-Year Resolution of Diabetes in 24% to 38% of Patients
Study Overview
Objective. To examine the 3-year efficacy of bariatric surgery on resolution of diabetes.
Design. Randomized controlled trial.
Setting and participants. Patients were participants in the STAMPEDE trial, a single-center study with enrollment from March 2007 to January 2011. 150 patients aged 20 to 60 years with a hemoglobin A1cof > 7% and a BMI of 27 to 43 kg/m2 were studied. Patients were excluded for a history of bariatric surgery or complex abdominal surgery and poorly controlled medical or psychiatric conditions [1]. Patients were randomized to intensive medical therapy, Roux-en-Y gastric bypass, or sleeve gastrectomy. All participants received intensive medical therapy, including lifestyle education, diabetes medical management, and cardiovascular risk reduction administered by a diabetes specialist every 3 months for 2 years and every 6 months thereafter. All surgeries were performed by a single surgeon, using equipment by Ethicon (a sponsor of the study, along with the National Institutes of Health, LifeScan, and the Cleveland Clinic).
Main outcome measure. HbA1c of ≤ 6% at 3 years.
Main results. At baseline, 68% were women and 74% were white. Participants had a mean age of 48 years (SD 8), mean A1c of 9.3% (1.5%), and mean BMI of 36 (3.5). 43% required insulin at baseline. Follow-up at 3 years was 91% (9 participants dropped out after enrollment, 4 lost to follow-up), and at this time, A1c levels were ≤ 6% for 5% of intensive medical therapy participants, 38% who had gastric bypass (P < 0.001 compared with medical therapy), and 24% who had sleeve gastrectomy (P = 0.01 compared with medical therapy); the difference between bypass and sleeve gastrectomy arms was not significant (P = 0.17). Nearly all of the participants reaching the primary outcome in the bariatric surgery arms achieved this goal A1c without using diabetic medications (35% and 20%). For the secondary outcome of A1c ≤ 7% without using diabetic medications, 0%, 58%, and 33% reached this endpoint in the medical therapy, bypass, and sleeve gastrectomy arms, respectively (P < 0.001 for both surgery arms compared to medical therapy; P = 0.01 comparing gastric bypass to sleeve gastrectomy). At 3 years, 2%, 69%, and 43% of participants were not taking any diabetic medications; 55% of medical therapy participants were taking insulin compared with 6% and 8% in the surgery arms. Weight loss was significantly greater in the gastric bypass and sleeve gastrectomy arms (24.5% and 21.1% of baseline body weight compared with the medical therapy arm with 4.2%). HDL cholesterol was higher and triglycerides were lower in both surgery arms, compared with medical therapy, but LDL cholesterol and blood pressure were not significantly different. Surgery participants also were taking fewer cardiovascular medications at 3 years. Quality of life was improved in 5 of 8 domains for the bypass arm compared with medical therapy and in 3 of 8 domains for the sleeve gastrectomy arm.
Conclusion. Gastric bypass and sleeve gastrectomy surgery leads to substantial resolution of diabetes compared to medical therapy.
Commentary
Over the last several decades, bariatric surgery has emerged as important treatment for obesity. Observational studies have demonstrated sustained weight loss persisting up to 15 years, as well as reductions in cardiovascular risk, diabetes, and even mortality [2–5]. In the Swedish Obesity Study, a nonrandomized study of 2010 participants undergoing bariatric surgery and 2037 matched controls, gastric bypass led to a 32% reduction from baseline body weight at 1–2 years after surgery with sustained weight loss of 27% at 15 years [2,3]. Patients undergoing gastric banding lost a bit less weight, with 20% weight loss at 1–2 years and 13% at 15 years. Control subjects lost very little.
Among diabetic Swedish Obesity Study participants, bariatric surgery led to a much higher rate of remission from diabetes over 10 years compared with control patients (36% after surgery, 13% among controls) [2] and lower rates of microvascular and macrovascular complications [6]. Among participants who were not diabetic at baseline, the incidence of diabetes was just 7% in the surgery arm and 24% in the control arm [2]; this difference in incidence persisted for 15 years of follow-up [4].
Among randomized controlled trials, several studies have found short-term resolution of diabetes after surgery. A study of 60 patients (age 30 to 60 years, BMI ≥ 35, A1c ≥ 7%) found that 75% of patients undergoing gastric bypass and 95% of patients undergoing biliopancreatic diversion had fasting glucose of < 100 mg/dL and A1c < 6.5% at 2 years; none of the control subjects met these thresholds for diabetes resolution [7]. Another 1-year trial of 120 US and Taiwanese patients (age 30 to 67 years, BMI 30 to 39.9, A1c ≥ 8%) found that 48% randomized to gastric bypass met a combination endpoint of A1c < 7%, LDL cholesterol < 100 mg/dL, and systolic blood pressure of < 130 mm Hg after 1 year compared with 19% assigned to intensive medical therapy [8]. In the gastric bypass arm, 75% reached an A1c of < 7% compared with 32% receiving medical therapy.
What does the study by Schauer and colleagues contribute? First, the study extended data on diabetes resolution to 3 years, longer than prior studies, and found substantial diabetes resolution in more than 1/3 of gastric bypass patients and 1/4 of sleeve gastrectomy patients (5% receiving medical therapy); over 2/3 and 1/3, respectively, were no longer taking any diabetes medications compared with 2% receiving medical therapy. In an earlier published study reporting on 1-year outcomes of this study, Schauer found diabetes resolution in 42% of those undergoing gastric bypass, 37% with sleeve gastrectomy, and 12% with medical therapy, demonstrating some regression over time [1]. Second, the study compared patients undergoing gastric bypass and sleeve gastrectomy. Sleeve gastrectomy is a newer procedure with less long-term outcome data; for example, none of the Swedish Obesity Study participants had sleeve gastrectomy. Schauer et al demonstrated that both procedures provide similar results for the primary outcome, but use of glucose-lowering medications was less and weight loss was more in the gastric bypass arm. These results provide some evidence that bypass surgery might be superior. Third, the study provided important data on cardiovascular risk factors, showing improvement in triglycerides and HDL cholesterol and quality of life. Quality of life was better after surgery than with medical therapy.
In this study, only 4 patients required reoperations, and no deaths or life-threatening complications were reported. However, mortality and morbidity remain a concern in bariatric surgery. In the earlier published study of this trial, authors noted that 22% of gastric bypass required hospitalization in the year after surgery compared with 8% in the sleeve gastrectomy and 9% in the medical therapy arms [1]. Observational data has shown higher rates of complications. In a study of patients at 10 clinical sites across the US from 2005 to 2007, 30-day mortality was 2.1% for open Roux-en Y gastric bypass and 0.2% for laparoscopic bypass [9]. That study also found substantial morbidity, with nearly 8% of patients after open bypass surgery reaching a composite end-point of death, deep venous thromboembolism, a repeat operation, or persistent hospitalization for 30 days after surgery; 4.8% reached this composite outcome after laparoscopic bypass. In another study of Medicare patients, 30-day mortality was 4.8% after open gastric bypass surgery compared with 1.7% for younger patients [10].
This trial by Schauer and colleagues demonstrates important benefits of gastric bypass and sleeve gastrectomy. While bariatric surgery still has some risk, it increasingly appears to be a viable treatment for patients with obesity, especially if they also have diabetes. Ideal future studies would be large enough to provide more data on predictors of diabetes resolution and long-term successful weight loss. Such information would allow clinicians and patients to better predict how patients might respond to surgery over the long term.
Applications for Clinical Practice
Bariatric surgery leads to a substantial reduction in diabetes over 3 years. While reduction was similar after gastric bypass and sleeve gastrectomy, secondary endpoints demonstrate some superiority of gastric bypass surgery. Clinicians should feel increasingly confident recommending bariatric surgery for their patients with diabetes and obesity.
—Jason P. Block, MD, MPH
1. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
2. Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93.
3. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52.
4. Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704.
5. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–61.
6. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
7. Mingrone G, Panunzi S, DeGaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
8. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
9. The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445–54.
10. Flum DR, Salem L, Elrod JA, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA 2005;294:1903–8.
Study Overview
Objective. To examine the 3-year efficacy of bariatric surgery on resolution of diabetes.
Design. Randomized controlled trial.
Setting and participants. Patients were participants in the STAMPEDE trial, a single-center study with enrollment from March 2007 to January 2011. 150 patients aged 20 to 60 years with a hemoglobin A1cof > 7% and a BMI of 27 to 43 kg/m2 were studied. Patients were excluded for a history of bariatric surgery or complex abdominal surgery and poorly controlled medical or psychiatric conditions [1]. Patients were randomized to intensive medical therapy, Roux-en-Y gastric bypass, or sleeve gastrectomy. All participants received intensive medical therapy, including lifestyle education, diabetes medical management, and cardiovascular risk reduction administered by a diabetes specialist every 3 months for 2 years and every 6 months thereafter. All surgeries were performed by a single surgeon, using equipment by Ethicon (a sponsor of the study, along with the National Institutes of Health, LifeScan, and the Cleveland Clinic).
Main outcome measure. HbA1c of ≤ 6% at 3 years.
Main results. At baseline, 68% were women and 74% were white. Participants had a mean age of 48 years (SD 8), mean A1c of 9.3% (1.5%), and mean BMI of 36 (3.5). 43% required insulin at baseline. Follow-up at 3 years was 91% (9 participants dropped out after enrollment, 4 lost to follow-up), and at this time, A1c levels were ≤ 6% for 5% of intensive medical therapy participants, 38% who had gastric bypass (P < 0.001 compared with medical therapy), and 24% who had sleeve gastrectomy (P = 0.01 compared with medical therapy); the difference between bypass and sleeve gastrectomy arms was not significant (P = 0.17). Nearly all of the participants reaching the primary outcome in the bariatric surgery arms achieved this goal A1c without using diabetic medications (35% and 20%). For the secondary outcome of A1c ≤ 7% without using diabetic medications, 0%, 58%, and 33% reached this endpoint in the medical therapy, bypass, and sleeve gastrectomy arms, respectively (P < 0.001 for both surgery arms compared to medical therapy; P = 0.01 comparing gastric bypass to sleeve gastrectomy). At 3 years, 2%, 69%, and 43% of participants were not taking any diabetic medications; 55% of medical therapy participants were taking insulin compared with 6% and 8% in the surgery arms. Weight loss was significantly greater in the gastric bypass and sleeve gastrectomy arms (24.5% and 21.1% of baseline body weight compared with the medical therapy arm with 4.2%). HDL cholesterol was higher and triglycerides were lower in both surgery arms, compared with medical therapy, but LDL cholesterol and blood pressure were not significantly different. Surgery participants also were taking fewer cardiovascular medications at 3 years. Quality of life was improved in 5 of 8 domains for the bypass arm compared with medical therapy and in 3 of 8 domains for the sleeve gastrectomy arm.
Conclusion. Gastric bypass and sleeve gastrectomy surgery leads to substantial resolution of diabetes compared to medical therapy.
Commentary
Over the last several decades, bariatric surgery has emerged as important treatment for obesity. Observational studies have demonstrated sustained weight loss persisting up to 15 years, as well as reductions in cardiovascular risk, diabetes, and even mortality [2–5]. In the Swedish Obesity Study, a nonrandomized study of 2010 participants undergoing bariatric surgery and 2037 matched controls, gastric bypass led to a 32% reduction from baseline body weight at 1–2 years after surgery with sustained weight loss of 27% at 15 years [2,3]. Patients undergoing gastric banding lost a bit less weight, with 20% weight loss at 1–2 years and 13% at 15 years. Control subjects lost very little.
Among diabetic Swedish Obesity Study participants, bariatric surgery led to a much higher rate of remission from diabetes over 10 years compared with control patients (36% after surgery, 13% among controls) [2] and lower rates of microvascular and macrovascular complications [6]. Among participants who were not diabetic at baseline, the incidence of diabetes was just 7% in the surgery arm and 24% in the control arm [2]; this difference in incidence persisted for 15 years of follow-up [4].
Among randomized controlled trials, several studies have found short-term resolution of diabetes after surgery. A study of 60 patients (age 30 to 60 years, BMI ≥ 35, A1c ≥ 7%) found that 75% of patients undergoing gastric bypass and 95% of patients undergoing biliopancreatic diversion had fasting glucose of < 100 mg/dL and A1c < 6.5% at 2 years; none of the control subjects met these thresholds for diabetes resolution [7]. Another 1-year trial of 120 US and Taiwanese patients (age 30 to 67 years, BMI 30 to 39.9, A1c ≥ 8%) found that 48% randomized to gastric bypass met a combination endpoint of A1c < 7%, LDL cholesterol < 100 mg/dL, and systolic blood pressure of < 130 mm Hg after 1 year compared with 19% assigned to intensive medical therapy [8]. In the gastric bypass arm, 75% reached an A1c of < 7% compared with 32% receiving medical therapy.
What does the study by Schauer and colleagues contribute? First, the study extended data on diabetes resolution to 3 years, longer than prior studies, and found substantial diabetes resolution in more than 1/3 of gastric bypass patients and 1/4 of sleeve gastrectomy patients (5% receiving medical therapy); over 2/3 and 1/3, respectively, were no longer taking any diabetes medications compared with 2% receiving medical therapy. In an earlier published study reporting on 1-year outcomes of this study, Schauer found diabetes resolution in 42% of those undergoing gastric bypass, 37% with sleeve gastrectomy, and 12% with medical therapy, demonstrating some regression over time [1]. Second, the study compared patients undergoing gastric bypass and sleeve gastrectomy. Sleeve gastrectomy is a newer procedure with less long-term outcome data; for example, none of the Swedish Obesity Study participants had sleeve gastrectomy. Schauer et al demonstrated that both procedures provide similar results for the primary outcome, but use of glucose-lowering medications was less and weight loss was more in the gastric bypass arm. These results provide some evidence that bypass surgery might be superior. Third, the study provided important data on cardiovascular risk factors, showing improvement in triglycerides and HDL cholesterol and quality of life. Quality of life was better after surgery than with medical therapy.
In this study, only 4 patients required reoperations, and no deaths or life-threatening complications were reported. However, mortality and morbidity remain a concern in bariatric surgery. In the earlier published study of this trial, authors noted that 22% of gastric bypass required hospitalization in the year after surgery compared with 8% in the sleeve gastrectomy and 9% in the medical therapy arms [1]. Observational data has shown higher rates of complications. In a study of patients at 10 clinical sites across the US from 2005 to 2007, 30-day mortality was 2.1% for open Roux-en Y gastric bypass and 0.2% for laparoscopic bypass [9]. That study also found substantial morbidity, with nearly 8% of patients after open bypass surgery reaching a composite end-point of death, deep venous thromboembolism, a repeat operation, or persistent hospitalization for 30 days after surgery; 4.8% reached this composite outcome after laparoscopic bypass. In another study of Medicare patients, 30-day mortality was 4.8% after open gastric bypass surgery compared with 1.7% for younger patients [10].
This trial by Schauer and colleagues demonstrates important benefits of gastric bypass and sleeve gastrectomy. While bariatric surgery still has some risk, it increasingly appears to be a viable treatment for patients with obesity, especially if they also have diabetes. Ideal future studies would be large enough to provide more data on predictors of diabetes resolution and long-term successful weight loss. Such information would allow clinicians and patients to better predict how patients might respond to surgery over the long term.
Applications for Clinical Practice
Bariatric surgery leads to a substantial reduction in diabetes over 3 years. While reduction was similar after gastric bypass and sleeve gastrectomy, secondary endpoints demonstrate some superiority of gastric bypass surgery. Clinicians should feel increasingly confident recommending bariatric surgery for their patients with diabetes and obesity.
—Jason P. Block, MD, MPH
Study Overview
Objective. To examine the 3-year efficacy of bariatric surgery on resolution of diabetes.
Design. Randomized controlled trial.
Setting and participants. Patients were participants in the STAMPEDE trial, a single-center study with enrollment from March 2007 to January 2011. 150 patients aged 20 to 60 years with a hemoglobin A1cof > 7% and a BMI of 27 to 43 kg/m2 were studied. Patients were excluded for a history of bariatric surgery or complex abdominal surgery and poorly controlled medical or psychiatric conditions [1]. Patients were randomized to intensive medical therapy, Roux-en-Y gastric bypass, or sleeve gastrectomy. All participants received intensive medical therapy, including lifestyle education, diabetes medical management, and cardiovascular risk reduction administered by a diabetes specialist every 3 months for 2 years and every 6 months thereafter. All surgeries were performed by a single surgeon, using equipment by Ethicon (a sponsor of the study, along with the National Institutes of Health, LifeScan, and the Cleveland Clinic).
Main outcome measure. HbA1c of ≤ 6% at 3 years.
Main results. At baseline, 68% were women and 74% were white. Participants had a mean age of 48 years (SD 8), mean A1c of 9.3% (1.5%), and mean BMI of 36 (3.5). 43% required insulin at baseline. Follow-up at 3 years was 91% (9 participants dropped out after enrollment, 4 lost to follow-up), and at this time, A1c levels were ≤ 6% for 5% of intensive medical therapy participants, 38% who had gastric bypass (P < 0.001 compared with medical therapy), and 24% who had sleeve gastrectomy (P = 0.01 compared with medical therapy); the difference between bypass and sleeve gastrectomy arms was not significant (P = 0.17). Nearly all of the participants reaching the primary outcome in the bariatric surgery arms achieved this goal A1c without using diabetic medications (35% and 20%). For the secondary outcome of A1c ≤ 7% without using diabetic medications, 0%, 58%, and 33% reached this endpoint in the medical therapy, bypass, and sleeve gastrectomy arms, respectively (P < 0.001 for both surgery arms compared to medical therapy; P = 0.01 comparing gastric bypass to sleeve gastrectomy). At 3 years, 2%, 69%, and 43% of participants were not taking any diabetic medications; 55% of medical therapy participants were taking insulin compared with 6% and 8% in the surgery arms. Weight loss was significantly greater in the gastric bypass and sleeve gastrectomy arms (24.5% and 21.1% of baseline body weight compared with the medical therapy arm with 4.2%). HDL cholesterol was higher and triglycerides were lower in both surgery arms, compared with medical therapy, but LDL cholesterol and blood pressure were not significantly different. Surgery participants also were taking fewer cardiovascular medications at 3 years. Quality of life was improved in 5 of 8 domains for the bypass arm compared with medical therapy and in 3 of 8 domains for the sleeve gastrectomy arm.
Conclusion. Gastric bypass and sleeve gastrectomy surgery leads to substantial resolution of diabetes compared to medical therapy.
Commentary
Over the last several decades, bariatric surgery has emerged as important treatment for obesity. Observational studies have demonstrated sustained weight loss persisting up to 15 years, as well as reductions in cardiovascular risk, diabetes, and even mortality [2–5]. In the Swedish Obesity Study, a nonrandomized study of 2010 participants undergoing bariatric surgery and 2037 matched controls, gastric bypass led to a 32% reduction from baseline body weight at 1–2 years after surgery with sustained weight loss of 27% at 15 years [2,3]. Patients undergoing gastric banding lost a bit less weight, with 20% weight loss at 1–2 years and 13% at 15 years. Control subjects lost very little.
Among diabetic Swedish Obesity Study participants, bariatric surgery led to a much higher rate of remission from diabetes over 10 years compared with control patients (36% after surgery, 13% among controls) [2] and lower rates of microvascular and macrovascular complications [6]. Among participants who were not diabetic at baseline, the incidence of diabetes was just 7% in the surgery arm and 24% in the control arm [2]; this difference in incidence persisted for 15 years of follow-up [4].
Among randomized controlled trials, several studies have found short-term resolution of diabetes after surgery. A study of 60 patients (age 30 to 60 years, BMI ≥ 35, A1c ≥ 7%) found that 75% of patients undergoing gastric bypass and 95% of patients undergoing biliopancreatic diversion had fasting glucose of < 100 mg/dL and A1c < 6.5% at 2 years; none of the control subjects met these thresholds for diabetes resolution [7]. Another 1-year trial of 120 US and Taiwanese patients (age 30 to 67 years, BMI 30 to 39.9, A1c ≥ 8%) found that 48% randomized to gastric bypass met a combination endpoint of A1c < 7%, LDL cholesterol < 100 mg/dL, and systolic blood pressure of < 130 mm Hg after 1 year compared with 19% assigned to intensive medical therapy [8]. In the gastric bypass arm, 75% reached an A1c of < 7% compared with 32% receiving medical therapy.
What does the study by Schauer and colleagues contribute? First, the study extended data on diabetes resolution to 3 years, longer than prior studies, and found substantial diabetes resolution in more than 1/3 of gastric bypass patients and 1/4 of sleeve gastrectomy patients (5% receiving medical therapy); over 2/3 and 1/3, respectively, were no longer taking any diabetes medications compared with 2% receiving medical therapy. In an earlier published study reporting on 1-year outcomes of this study, Schauer found diabetes resolution in 42% of those undergoing gastric bypass, 37% with sleeve gastrectomy, and 12% with medical therapy, demonstrating some regression over time [1]. Second, the study compared patients undergoing gastric bypass and sleeve gastrectomy. Sleeve gastrectomy is a newer procedure with less long-term outcome data; for example, none of the Swedish Obesity Study participants had sleeve gastrectomy. Schauer et al demonstrated that both procedures provide similar results for the primary outcome, but use of glucose-lowering medications was less and weight loss was more in the gastric bypass arm. These results provide some evidence that bypass surgery might be superior. Third, the study provided important data on cardiovascular risk factors, showing improvement in triglycerides and HDL cholesterol and quality of life. Quality of life was better after surgery than with medical therapy.
In this study, only 4 patients required reoperations, and no deaths or life-threatening complications were reported. However, mortality and morbidity remain a concern in bariatric surgery. In the earlier published study of this trial, authors noted that 22% of gastric bypass required hospitalization in the year after surgery compared with 8% in the sleeve gastrectomy and 9% in the medical therapy arms [1]. Observational data has shown higher rates of complications. In a study of patients at 10 clinical sites across the US from 2005 to 2007, 30-day mortality was 2.1% for open Roux-en Y gastric bypass and 0.2% for laparoscopic bypass [9]. That study also found substantial morbidity, with nearly 8% of patients after open bypass surgery reaching a composite end-point of death, deep venous thromboembolism, a repeat operation, or persistent hospitalization for 30 days after surgery; 4.8% reached this composite outcome after laparoscopic bypass. In another study of Medicare patients, 30-day mortality was 4.8% after open gastric bypass surgery compared with 1.7% for younger patients [10].
This trial by Schauer and colleagues demonstrates important benefits of gastric bypass and sleeve gastrectomy. While bariatric surgery still has some risk, it increasingly appears to be a viable treatment for patients with obesity, especially if they also have diabetes. Ideal future studies would be large enough to provide more data on predictors of diabetes resolution and long-term successful weight loss. Such information would allow clinicians and patients to better predict how patients might respond to surgery over the long term.
Applications for Clinical Practice
Bariatric surgery leads to a substantial reduction in diabetes over 3 years. While reduction was similar after gastric bypass and sleeve gastrectomy, secondary endpoints demonstrate some superiority of gastric bypass surgery. Clinicians should feel increasingly confident recommending bariatric surgery for their patients with diabetes and obesity.
—Jason P. Block, MD, MPH
1. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
2. Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93.
3. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52.
4. Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704.
5. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–61.
6. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
7. Mingrone G, Panunzi S, DeGaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
8. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
9. The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445–54.
10. Flum DR, Salem L, Elrod JA, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA 2005;294:1903–8.
1. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–76.
2. Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93.
3. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52.
4. Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704.
5. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–61.
6. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304.
7. Mingrone G, Panunzi S, DeGaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–85.
8. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–9.
9. The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445–54.
10. Flum DR, Salem L, Elrod JA, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA 2005;294:1903–8.
Experiencing Age-Related Vision and Hearing Impairment: The Psychosocial Dimension
From the University of Education (Dr. Heyl) and Heidelberg University (Dr. Wahl), Heidelberg, Germany.
Abstract
- Objective: To summarize the current state of research regarding the experience of age-related vision and hearing impairment.
- Methods:Review of the literature.
- Results: Negative consequences of age-related vision and hearing impairment manifest in the domains of health and longevity, everyday competence, cognitive functioning, social functioning, and subjective well-being. However, while vision impairment strongly impacts everyday competence, the burden of hearing impairment can mainly be found in the social domain. Psychosocially framed intervention research has shown promising findings, but many studies rely on small samples or do not include a control condition.
- Conclusions: Although more research is needed, it is clear that traditional rehabilitation programs targeting age-related vision and hearing impairments need a strong psychosocial component.
Vision and hearing are essential for person–environment interaction and both are subject to pronounced age-related changes. Ongoing demographic changes and increasing life expectancy is contributing to a significant increase in the number of very old individuals [1]. It is projected that by 2030 about 50% of older Americans may have some significant eye disease, ie, cataract, glaucoma, or age-related macular degeneration [2]. Presbycusis as the major cause of age-related hearing impairment is present in 40% of the American senior citizens [3]. In this narrative review, we review the epidemiological data on age-related vision and hearing impairment, research on its psychosocial impact, and intervention research aimed to improve coping processes and rehabilitative outcomes. We close with future recommendations directed both toward research and clinical practice.
Epidemiology
Vision and hearing impairment is highly prevalent in old age, yet prevalence rates reported in the literature are quite different, depending on the definition of vision and hearing impairment used. A widely used criterion for low vision is the one used by the World Health Organization, ie, visual acuity less than 20/60 and equal to or better than 20/400 in the better eye with best correction. A best corrected visual acuity of less than 20/400 in the better eye is used to define blindness [4]. A disabling hearing impairment is defined by an average hearing loss in decibel (dB HL) of at least 41 dB HL at 500, 1000, 2000, and 4000 Hz. [5]. Translated to everyday life, such hearing impairment mainly manifests in severe difficulties in understanding normal conversation. Besides differing definitions, different methods to assess vision and hearing impairment and heterogenous study populations make comparisons of prevalence rates difficult [6]. In particular, relying solely on self-report data to assess vision and hearing loss seems generally problematic. In addition, the strong focus in vision impairment assessment on visual acuity measures has limitations, as other indicators, such as contrast sensitivity or useful field of vision, may be more important for out-of-home mobility or driving [7].
A recent study on the prevalence of visual impairment (defined as best corrected visual acuity < 20⁄40) in 6 European countries found quite similar prevalence rates as reported for the US: Prevalence of visual impairment was 3% in those aged 65 to 74 years, 13% in those over 75 years and 33% in those over 85 years [8]. At first glance, vision loss seems to be more prevalent among older women than among older men, but this relationship is not sustained in multivariate analyses considering age, health, and social support variables [9].
Regarding hearing loss, Gopinath et al [10] found prevalence rates of 29% among men and 17% among women aged 60 to 69 years. Moreover, for every 10 years of age, the prevalence of hearing loss doubled. In their review of epidemiologic data on prevalence of age-related hearing impairment in Europe, Roth and coauthors [11] report that at the age of 70 years, about 30% of men and 20% of women were found to have a hearing loss of at least 30 dB HL, while at the age of 80 years about 55% of men and 45% of women were affected. Lin et al found that 63% of those 70 years and older had a hearing loss of more than 25 dB in the better ear [12].
According to a recent review by Schneider et al [6], prevalence of impairment in both vision and hearing in older age (dual sensory impairment) varies between 1.6% and 22.5% due to different sample characteristics (eg, size, age) and different definitions and assessments of vision and hearing impairment (see also [13]). However, there is good evidence that dual sensory impairment increases with age, and that it is more common among frailer subpopulations such as older individuals consulting care services [6].
Quality of Life Impact of Vision and Hearing Impairment
Health and Longevity
There is inconsistent evidence that both age-related vision and hearing impairment are accompanied by heightened multimorbidity and an increased mortality rate. For example, while some older as well as more recent studies have found that visual and hearing declines over time predict death in very old age [14–16], other studies have detected no significant relationship after adjusting for confounders such as age, gender, and education [17,18]. Among the hearing impaired, only men seem to have a significant increase in mortality risk [15,19]. Dual sensory impairment appears to be more consistently and more strongly related to increased mortality than vision or hearing impairment alone [19–21].
Everyday Competence
The term everyday competence includes both basic (eg, self-care behaviors) and instrumental (eg, using public transport) activities of daily living (ADL/IADL [22]). Age-related vision impairment has been found to be robustly associated with significantly lower everyday competence, because visual capacity is a critical prerequisite for such behaviors [23,24]. Indeed, lowered everyday competence appeared as the best of a range of variables (including cognitive function and well-being–related measures) used to differentiate between visually impaired and visually unimpaired older adults [18]. Furthermore, vision impairment impacts cross-sectionally as well as longitudinally—more strongly on IADL as compared to ADL—because the execution of IADL is more complex and depends more strongly on environmental enhancing or hindering factors [25–27]. Hence, shrinkage in IADL competence reflects a kind of early behavioral marker of severe vision impairment, whereas significant ADL decrease only happens later in the process of chronic vision loss.
In contrast to vision impairment, age-related hearing loss has been found not to have a major impact in particular on ADL/IADL [28]. However, as has been found elsewhere [29], hearing loss is associated with increased reliance on community and informal supports, suggesting that while IADL function may not deteriorate with hearing loss, the way it is conducted may change (ie, need for support to maintain participation).
It should also be mentioned in this context that assessment strategies have been developed to better consider the specific life conditions of those with vision and hearing impairment. The best-known and frequently applied instruments in this context are the National Eye Institute Visual Function Questionnaire [30] and the Hearing Handicap Inventory for the Elderly [31].
Cognitive Functioning
Previous research largely supports the notion that reduced vision and hearing function is accompanied by a decrease in cognitive performance in older adults. The work of Lindenberger and Baltes, based on the Berlin Aging Study (BASE)—but also including additional studies with a wider age-range—is central in supporting a strong connection among vision, hearing, balance, and cognitive functioning in later life. Lindenberger and Baltes [32] found that general intelligence correlated just as strongly with visual as with auditory ability. In a model conjoining age, sensory function, as well as intelligence, visual and auditory function predicted a large portion of interindividual differences in intelligence and indeed fully mediated the negative correlation between age and intelligence. This finding has meanwhile been replicated by a number of other research groups and may be regarded as rather robust [33,34]. In addition, Baltes and Lindenberger [35] observed that sensory measures were better predictors of intelligence than socio-structural variables such as education or social class. They also showed that the connection among sensory functioning and intelligence was much closer in older adults as compared to adults in early and middle adulthood [34].
No clear difference between the sensory modalities of vision and hearing has been identified regarding their relationship with cognitive performance. On the one hand, there is research supporting the view that both vision and hearing impairment are connected with cognitive decline [12,36,37], while some evidence also supports that the linkage may be stronger with vision [14]. On the other hand, there are also data not supporting a close connection between vision and hearing impairment and cognitive function [38]. Explanations for such inconsistencies may refer to a number of reasons, such as pronounced positive selectivity of samples (which may lead to underestimation of connections among sensory and cognitive function), the application of established cognitive tests not appropriate for sensory impaired older adults (which may lead to overestimation of connections among sensory and cognitive function), and the application of different cut-off scores for significant vision and hearing impairment (possibly, higher cut-offs may lead to higher, lower to lower connections). Longitudinal data using the latest in causal modeling data analysis support the view that the causal dynamics involved in sensory and intelligence change are complex and that each of these variables can drive change in the other across longer periods of later life [39].
Vision status also plays a role when it comes to the connection between cognitive function and everyday competence—a linkage that is generally challenged as people age and that may lead to endpoints such as dependence on others and transition to long-term care. Heyl et al [40] observed that the link between vision status and out-of-home leisure activities is mediated by cognitive status. In a more recent study, able to add to the understanding of such a mediation process, Heyl and Wahl [38,41] showed that the connection between cognitive function and everyday function is much closer in visually and hearing impaired older adults as compared with visually unimpaired older adults, which possibly means that both visually and hearing impaired elders rely more intensely on their cognitive resources. Causality dynamics may however also work in the opposite direction. As Rovner and colleagues [42,43] observed in a study with age-related macular degeneration patients over 64 years of age covering a 3-year observation period and 2 measurement occasions, activity loss over time due to the visual loss led to cognitive decline happening between T1 and T2. This finding fits well with the more general finding in the cognitive aging literature that the exertion of social and leisure activities is important for maintaining cognitive functioning [44].
Social Functioning
Social relations as well as social support have generally been found to be of key importance for older adults [45]. Reinhardt [46] found that visually impaired older adults nominated on average 5.4 persons of intimate relation within their family network, and 3.5 persons within their friendship network, which is similar to sensory-unimpaired older adults, such as those assessed in the BASE [47]. In addition, in Wahl et al’s study [18], visually and hearing impaired older adults nominated practically the same number of persons as being in the most intimate circle of their social network (4.70 versus 4.71); the respective number in a comparison group of visually unimpaired older adults amounted to 5.2 persons, which was not significantly different from both sensory impaired group means.
Neither vision nor hearing impairment seem to affect the experience of loneliness dramatically [18,23,48], although some research did report an increased risk of loneliness in older adults with vision impairment [49]. It is clear however that hearing impairment more strongly than vision impairment negatively impacts social communication and carries a strong stigma for those affected [48,50]. The stigma particularly implies that hearing deficits and concomitant communication disturbances (eg, giving an answer that does not match the question) elicits the view of a cognitively impaired, if not demented older person. In some contrast, vision loss seems to raise rather strong helping impulses and feelings of compassion. The dark side of this tendency is that it seems a challenge to provide visually impaired older adults with the instrumental support needed while at the same time fostering remaining capabilities [51]. Overprotection may put constraints on the visually impaired older adults’ “true” functional capacity and thereby contribute to loss in competence over the longer run due to disuse [52].
Subjective Well Being–related Outcomes and Depression
Visually impaired older adults have shown evidence of diminished well-being as compared with sensory unimpaired older adults [53], although effect sizes were rather small in a respective meta-analysis [54]. Differences in well-being between hearing impaired and unimpaired older adults seem small or nonexistent in some studies [18,55], but considerable in others [56]. The latter study covering a 16-year observational period as well as other longitudinal work (eg, [57]) also support the notion that remaining ADLs and social engagement mediate the linkage between sensory loss and well-being and depression. The “well-being paradox” in old age, pointing to pronounced adaptive resources to maintain well-being in spite of adverse conditions [58], may also apply to sensory impaired older adults [57,59].
At the same time, it is critical to acknowledge that visually impaired older adults represent an at-risk population, in which the positive impact of human adaptation and the drawback of reaching the limits of psychological resilience go hand in hand. Affect balance (ratio of positive and negative affect) has been found to be more toward the negative pole in visually impaired older adults [60] and depression has consistently been found to be significantly increased in visually impaired older adults [61–63]. Rates roughly vary between 15% and 30% and are particularly high in age-related macular degeneration patients [61]. This is also important, because depressive symptoms may accelerate both cognitive decline and decline in everyday competence in age-related macular degeneration patients [42]. Perceived overprotection may also lead to negative consequences in terms of heightened depression and anxiety over time [64].
Regarding the impact of hearing loss on depression, findings are quite inconsistent. Some studies found evidence for a significant relationship between hearing impairment and depressive symptoms among older adults [65, 66], while others did not [67, 68]. Gopinath and co-workers [66] observed that hearing impaired individuals, particularly women, younger than 70 years of age and those who were infrequently using a hearing aid (less than one hour per day) were more likely to suffer from depressive symptoms. According to a population-based study among older Italians, hearing impairment might be more closely related to anxiety symptoms than to depression [69].
Dual Sensory Impairment
Previous research supports the notion that the overall psychosocial situation of those with dual sensory impairment is even worse as compared to those with sole vision or hearing impairment. In particular, higher rates of ADL/IADL impairment, depression and lowered well-being have been found in older adults affected by dual sensory loss [6,13,18,56]. Also, dual sensory impairment has been found to be linked with cognitive decline cross-sectionally [37] as well as longitudinally [36].
Improving Quality of Life in Sensory Impaired Older Adults
The research summary provided in the previous section underscores that the experience of age-related visual and hearing impairment comes with pronounced challenges that deserve evidence-based professional support. In the following, we give an overview and evaluation of major work in the area of psychosocially framed intervention research targeting older adults with vision and hearing loss. By psychosocially framed interventions we mean studies containing programs that focused on psychosocial processes (eg, consultation on how to better cope with sensory impairment, educative components, problem solving strategies, coping with negative affect) and assessed psychosocial outcomes (eg, everyday functioning, depression, emotional stress experiences). Such interventions may have been integrated into regular rehabilitation programs or offered as a separate strategy in addition to classic rehabilitation. We also consider physical activity–related and overall “way of living” interventions such as tai chi and yoga. In doing so, our aim is to highlight the bandwidth of psychosocial interventions and respective outcomes, not comprehensiveness.
Age-related Vision Impairment
Most psychosocially framed interventions could be characterized as self-management– and disease management–like efforts and are promising for visually impaired older adults. Major elements of such programs include stress-reducing strategies (eg, muscle-relaxation exercises), goal-directed problem-solving, strategies to evoke positive affect, activating available resources, and information and consultation. Typically, such programs are conducted in a group format in an eye clinic, bringing together 6 to 8 visually impaired older adults for weekly sessions of 2 to 3 hours over 6 to 8 weeks.
More recent work provides additional support for the usefulness of self-management programs for visually impaired older adults [72,73]. In addition, emerging evidence supports the notion that psychosocially framed interventions may contribute to saving health costs (eg, via reduced psychopharmacy) and may also enhance commitment
It is also obvious that such programs should find a strong liaison with classic high-caliber rehabilitation programs for visually impaired older adults, including effective reading training [75].
Furthermore, physical training programs, which have proven efficiency with old and very old individuals—including those who are cognitively vulnerable—also seem to be of significant advantage for visually impaired older adults. As has been found, such programs not only increase posture, gait, and general physical fitness, they also prevent falls and enhance well-being, self-efficacy, and cognitive function, especially executive control [76]. Postural control has been improved by multimodal balance and strength exercises among older individuals with visual impairments as well [77]. Participation in physical activity and being in better physical condition buffered the relationship between dual sensory impairment and depression, pointing to the importance of physical training programs for the mental health of older persons with dual sensory impairment [78]. According to a randomized control study as well as to some case studies, tai chi seems to be an effective tool to improve balance control in visually impaired older adults, and thus to reduce an important risk factor for falls [79–81]. Visually impaired adults might also benefit from yoga in terms of balance improvement as well as psychosocial improvements [82]. To teach tai chi efficiently, it is necessary to adapt instructions to the needs of the visually impaired seniors by relying on verbal cuing and manual body placement [80]. The need to adapt instructions and to provide an accessible environment (including transportation arrangements) to motivate older individuals with visual impairments to perform regular physical exercises is also highlighted by Surakka and Kivela [83].
Furthermore, there is evidence that state of the art low vision rehabilitation as such also has beneficial effects on psychosocial outcomes, such as general and vision-related quality of life and emotional well-being.
Age-related Hearing Impairment
Interventions concerning older adults with hearing impairment center on amplification and aural rehabilitation, including auditory training [84]. It has been shown that using hearing aids improves the quality of life, in particular hearing-related quality of life, of hearing impaired adults [85]. Yet many older adults who would benefit from hearing aids do not wear them [86]. From the reasons identified in the review by McCormack and Fortnum [86], perceived hearing aid value, in particular poor benefit in noisy situations, fit and comfort, as well as care and maintenance of the hearing aid emerged as most important. Improvements in these areas are necessary to enhance hearing aid usage among older adults with hearing impairment. Meyer and Hickson [87] identified 5 factors increasing the likelihood to seek help for hearing impairment and/or adopt hearing aids: (1) moderate to severe hearing impairment and perceived hearing-related everyday limitations; (2) older age; (3) poor subjective hearing; (4) perceiving more benefits than barriers to amplification; and (5) perceiving significant others as supportive of hearing rehabilitation. Thus, the involvement of family members in the rehabilitation process appears necessary and promising.
Beyond amplification, aural rehabilitation seeks to improve the situation of hearing impaired older adults by providing listening and communication techniques to enhance communication effectiveness. We have summarized major work in this area in Table 2.
In sum, it seems clear for both vision impairment [95] and hearing impairment [96] that classic rehabilitation strategies, such as fitting a reading device or hearing aid, need significant enrichment by psychosocial training components in order to achieve the best outcomes possible. Furthermore, given the findings on the role of cognitive resources in visually impaired older adults (see respective section above), cognitive training may be an important addition to psychosocial intervention and rehabilitation [38,97]. It must be noted, however, that many of the available studies reveal a number of methodological limitations, such as small sample sizes, missing control condition, and no follow-up assessments to estimate the maintenance of effects.
A significant future need is intervention research addressing older adults with dual sensory impairment. Although we found study protocols related to important trials underway [98,99] and a physical training study with visually impaired older adults that included also some dual sensory impaired individuals [83], it seems that there is not much research in terms of completed interventions and respective findings. Furthermore, it may be important to better involve significant others such as family members and friends in psychosocially framed programs and emerging research with low vision adults has revealed advantages and disadvantages of such an approach [100].
Practical Implications
The older patient is on the way to become the “standard” patient for eye care and hearing specialists and thus a challenge for public health at large. Based on the evidence compiled above, we argue that best practice in medical treatment and traditional rehabilitation of vision and hearing impairment should better consider the psychosocial dimension of age-related vision and hearing impairment. We see different levels at which a stronger psychosocially framed input is needed. First, at the diagnostic level, having a better understanding of everyday competence, the role of cognitive functioning, social resources, and well-being–related dynamics in visually impaired and hearing impaired older adults may significantly enrich the professional background knowledge about the patient. Such knowledge may become important for diagnostic evaluation, treatment decisions, and predictions of long-term outcomes. It seems also critical to have an understanding of the more fundamental mechanisms and systemic inter-relations in older patients (eg, among visual, hearing, mobility, and cognitive impairment), because such evidence helps to evaluate overall vulnerability and likely future trajectories of respective patients.
Second, at the intervention level against the background of the available empirical effectiveness evidence, self-management–oriented and psycho-educative programs should become a regular component of low vision rehabilitation. Similarly, psychosocial programs educating older adults in hearing tactics and hearing loss–oriented coping strategies should become a regular part of hearing rehabilitation. In addition, we argue that cognitive training and physical activity–oriented interventions should have their place in rehabilitation programs designed for older visually and hearing impaired adults. The major reason is that respective programs generally have been found to positively impact quality of life in old age. This impact may be particularly valuable for more vulnerable populations, such as sensory impaired older adults. We therefore recommend implementing psychosocially framed programs as a regular service in eye clinics as well as in ear, nose, and throat clinics, because this seems to be the setting best suited to approach visually and hearing impaired older adults as well as to offer the logistic opportunities to conduct such programs. It would also be critical to extend such programs to in-home services as well as services covering long-term care settings. Older adults with dual sensory impairment bring specific challenges to such interventions, such as the optimal cooperation and combination of rehabilitation and psychosocial expertise related to each domain and traditionally offered side by side. It is also good news that new trials are underway to learn more about psychosocial interventions aimed to address older adults with dual sensory loss [98,99].
In conclusion, we argue for a better implementation of both age-related psycho-ophthalmology as well as psycho-audiology. Although we regard psychologists with a clinical training background as a key profession to be involved in psychosocially framed interventions with older adults with vision and hearing impairment, other professions (eg, occupational therapists, sport scientists) should also play an important role. A multiprofessional enrichment of classic sensory rehabilitation based on the training principles as described above is a major future need.
Corresponding author: Vera Heyl, PhD, Zeppelinstr, 1, D-69121, Heidelberg, Germany, [email protected].
Financial disclosures: None.
1. Oeppen J, Vaupel JW. Broken limits to life expectancy. Science 2002;296:1029–31.
2. Eichenbaum JW. Geriatric vision loss due to cataracts, macular degeneration, and glaucoma. Mt Sinai J Med 2012;79:276–94.
3. Ciorba A, Bianchini C, Pelucchi S, et al. The impact of hearing loss on the quality of life of elderly adults. Clin Interven Aging 2012;7:159–63.
4. WHO - World Health Organization and International Agency for the Prevention of Blindness. Developing an action plan to prevent blindness at national, provincial and district levels. Vision 2020 The right to sight. Geneva: World Health Organization; 2004.
5. WHO - World Health Organizaton. Grades of hearing impairment. Geneva: World Health Organization; 2013.
6. Schneider JM, Gopinath B, McMahon CM, et al. Dual sensory impairment in older age. J Aging Health 2011;23:1309–24.
7. Clay OJ, Wadley VG, Edwards JD, et al. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: current and future implications. Optom Vis Sci 2005;82:724–31.
8. Seland JH, Vingerling JR, Augood CA, et al. Visual Impairment and quality of life in the Older European Population, the EUREYE study. Acta Ophthal 2011;89:608–13.
9. Horowitz A, Brennan M, Reinhardt JP. Prevalence and risk factors for self-reported visual impairment among middle-aged and older adults. Research Aging 2005;27:307–26.
10. Gopinath B, Rochtchina E, Wang JJ, et al. Prevalence of age-related hearing loss in older adults: Blue mountains study. Arch Intern Med 2009;169:415–6.
11. Roth TN, Hanebuth D, Probst R. Prevalence of age-related hearing loss in Europe: a review. Eur Arch Otorhinolaryngol 2011;268:1101–7.
12. Lin FR, Thorpe R, Gordon-Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol Series A: Biolog Sci Med Sci 2011;66A:582–90.
13. Brennan M, Horowitz A, Su Y-p. Dual sensory loss and its impact on everyday competence. Gerontologist 2005;45:337–46.
14. Anstey KJ, Luszcz MA, Giles LC, et al. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging 2001;16:3–11.
15. Appollonio I, Carabellese C, Magni E, et al. Sensory impairments and mortality in an elderly community population: A six-year follow-up study. Age Ageing 1995;24:30–6.
16. McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol 2001;85:322–6.
17. Ostbye T, Steenhuis R, Wolfson C, et al. Predictors of five-year mortality in older Canadians: the Canadian Study of Health and Aging. J Am Geriatr Soc 1999 47:1249–54.
18. Wahl H-W, Heyl V, Drapaniotis PM, et al. Severe vision and hearing impairment and successful aging: A multidimensional view. Gerontologist 2013;53:950–62.
19. Fisher D, Li C-M, Chiu MS, et al. Impairments in hearing and vision impact on mortality in older people: the AGES-Reykjavik Study. Age Ageing 2013.
20. Bamini G, Schneider J, McMahon CM, et al. Dual sensory impairment in older adults inceases the risk of mortality: A population-based study. PLoS ONE 2013;8:1–6.
21. Lam BL, Lee DJ, Gómez-Marín O, et al. Concurrent visual and hearing impairment and risk of mortality: The National Health Interview Survey. Arch Ophthalmol 2006;124:95–101.
22. Baltes MM, Maas I, Wilms H-U, et al. Everyday competence in old and very old age: Theoretical considerations and empirical findings. In: Baltes PB, Mayer K-U, editors. The Berlin Aging Study Cambridge, UK: Cambridge University Press; 1999: 384–402.
23. Burmedi D, Becker S, Heyl V, et al. Behavioral consequences of age-related low vision: A narrative review. Vis Impair Res 2002;4:15–45.
24. Wahl H-W, Heyl V, Schilling O. Robustness of personality and affect relations under chronic conditions: the case of age-related vision and hearing impairment. J Gerontol B Psycholog Sci Soc Sci 2012;67:687–96.
25. Heyl V, Wahl H-W. On the long-term psychosocial adaptation to vision loss in the later years. In: Wahl H-W, Schulze H-E, editors. On the special needs of blind and low vision seniors: Research and practice concepts. Amsterdam: IOS-Press; 2001: 77–83.
26. Wahl H-W, Schilling O, Oswald F, et al. Psychosocial consequences of age-related visual impairment: Comparison with mobility-impaired older adults and long-term outcome. J Gerontol Psych Sci Soc Sci 1999;54B:P304–P16.
27. Wahl H-W, Becker S, Burmedi D, et al. The role of primary and secondary control in adaptation to age-related vision loss: A study of older adults with macular degeneration Psychol Aging 2004;19:235–9.
28. Rudberg MA, Furner SE, Dunn JE, et al. The relationship of visual and hearing impairments to disability: An analysis using the Longitudinal Study of Aging. J Gerontol Med Sci 1993;48:M261–M5.
29. Schneider J, Gopinath B, Karpa MJ, et al. Hearing loss impacts on the use of community and informal supports. Age Ageing 2010;39:458–64.
30. Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute visual function questionnaire. Arch Ophthalmol 2001;119:1050–8.
31. Ventry I, Weinstein B. The hearing handicap inventory for the elderly: A new tool. Ear Hearing 1982;3:128–34.
32. Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol Aging 1994;9:339–55.
33. Clay OJ, Edwards JD, Ross LA, et al. Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. J Aging Health 2009;21:547–66.
34. Salthouse TA, Hancock HE, Meinz EJ, et al. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol Psychol Sci 1996;51B:P317–P30.
35. Baltes PB, Lindenberger U. Emergence of powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging 1997;12:12–21.
36. Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 2004;52:1996–2002.
37. Tay T, Wang JJ, Kifley A, et al. Sensory and cognitive association in older persons: Findings from an older Australian population. Gerontology 2006;52:386–94.
38. Heyl V, Wahl H-W. Managing daily life with age-related sensory loss: Cogni-tive resources gain in importance. Psychol Aging 2012;27:510–21.
39. Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychol Aging 2009;24:1-16.
40. Heyl V, Wahl H-W, Mollenkopf H. Visual capacity, out-of-home activities and emotional well-being in old age: Basic relations and contextual variation. Soc Indicat Res 2005;74:159–89.
41. Heyl V, Wahl H-W. Cognitive ability as a resource for everyday functioning among older adults who are visually impaired. J Vis Impair Blind 2010;104:391–403.
42. Rovner BW, Casten RJ, Leiby BE. Variability in depressive symptoms predicts cognitive decline in age-related macular degeneration. Am J Geriatr Psych 2009;17:574–81
43. Rovner BW, Casten RJ, Leiby BE, et al. Activity loss is associated with cognitive decline in age-related macular degeneration. Thomas Jefferson University, Department of Psychiatry and Human Behavior, Faculty Papers. Paper 5. 2009.
44. Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psych Aging 2005;20:423–34.
45. Antonucci TC. Social relations. An examination of social networks, social support, and sense of control. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5th ed. San Diego: Academic Press; 2001: 427–53.
46. Reinhardt JP. The importance of friendship and family support in adaptation to chronic vision impairment. J Gerontol Series B Psychol Sci Soc Sci 1996;51B:P268–P78.
47. Wagner M, Schütze Y, Lang FR. Social relationships in old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study Aging from 70 to 100. Cambridge, UK: Cambridge University Press; 1999: 282–301.
48. Wahl H-W, Tesch-Römer C. Aging, sensory loss, and social functioning. In: Charness N, Park D, Sabel B, editors. Aging and communication: Opportunities and challenges of technology. New York: Springer; 2001: 108-26.
49. Nachtegaal J, Smit JH, Smits C, et al. The association between hearing status and psychosocial health before the age of 70 years: Results from an internet-based national survey on hearing. Ear Hearing 2009;30:302-12
50. Wallhagen MI. The stigma of hearing loss. Gerontologist 2010;50:66–75.
51. Cimarolli VR, Boerner K. Social support and well-being in adults who are visually impaired. J Vis Impair Blind 2005 99:521–34.
52. Cicirelli VG. Fear of death in mid-old age. J Gerontol Series B Psychol Sci Soc Sci 2006;61:P75–P81.
53. Horowitz A, Reinhardt JP. Depression among low vision elders. In: Stuen. C, Arditi A, Horowitz A, et al, editors. Vision rehabilitation assessment, intervention and outcomes. Lisse: Swets & Zeitlinger Publishers; 2000: 655–8.
54. Pinquart M, Pfeiffer JP. Psychological well-being in visually impaired and unimpaired individuals: A meta-analysis. Br J Vis Impair 2011;29:27–45.
55. Tesch-Römer C. Schwerhörigkeit im Alter. Belastung, Bewältigung, Rehabilitation. Heidelberg: Median-Verlag; 2001.
56. Kiely KM, Anstey KJ, Luszcz MA. Dual sensory loss and depressive symptoms: the importance of hearing, daily functioning, and activity engagement. Front Hum Neurosci 2013;7:1–13.
57. Schilling OK, Wahl H-W, Horowitz A, et al. The adaptation dynamics of chronic functional impairment: What we can learn from older adults with vision loss. Psychol Aging 2011;26:203–13.
58. Kunzmann U, Little T, Smith J. Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study. Psychol Aging 2000;15:511-26.
59. Schilling O, Wahl H-W. Modeling late life adaptation in affective well-being under a severe chronic health condition: The case of age-related macular degeneration. Psychol Aging. 2006;21:703–14.
60. Wahl H-W, Schilling O, Becker S, et al. A German research program on the psychosocial adaptation to age-related vision impairment: Recent findings based on a control theory approach. Europ Psychol 2003;8:168-77.
61. Casten R, Rovner B. Depression in age-related macular degeneration. J Visual Impair Blind 2008;102:591-9.
62. Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Pub Health 2004;94:823–9.
63. Horowitz A, Reinhardt JP, Boerner K. The effect of rehabilitation on depression among visually disabled older adults. Aging Mental Health 2005;9:563–70.
64. Cimarolli VR. Perceived overprotection and distress in adults with visual impairment. Rehabil Psychol 2006;51:338-45.
65. Ishine M, Okumiya K, Matsubayashi K. A close association between hearing impairment and activities of daily living, depression, and quality of life in community-dwelling older people in Japan. J Am Geriatr Soc 2007;55:316-7.
66. Gopinath B, Wang JJ, Schneider J, et al. Depressive symptoms in older adults with hearing impairments: The Blue Mountains Study. J Am Geriatr Soc 2009;57:1306-8.
67. Chou K-L, Chi I. Combined effect of vision and hearing impairment on depression in elderly Chinese. Int J Geriatr Psych 2004;19:825-32.
68. Tambs K. Moderate effects of hearing loss on mental health and subjective well-being: Results from the Nord-Trøndelag Hearing Loss Study. Psychosom Med 2004;66:776-82.
69. Bernabei V, Morini V, Moretti F, et al. Vision and hearing impairments are associated with depressive--anxiety syndrome in Italian elderly. Aging Ment Health 2011 15:467-74.
70. Wahl H-W, Heyl V, Langer N. Lebensqualität bei Seheinschränkung im Alter: Das Beispiel altersabhängige Makuladegeneration [Quality of life by limited vision in old age: the example of age-related macula degeneration]. Der Ophthalmologe 2008;105:735-43.
71. Wahl H-W, Kämmerer A, Holz F, et al. Psychosocial intervention for age-related macular degeneration: A pilot project. J Vis Impair Blind 2006;100:533-44.
72. Rees G, Keeffe JE, Hassell J, et al. A self-management program for low vision: Program overview and pilot evaluation. Disabil Rehab 2010;32:808-15.
73. Rovner BW, Casten RJ. Preventing late-life depression in age-related macular degeneration. Am J Geriatr Psych 2008;16:454-9
74. Eklund K, Sonn U, Nystedt P, et al. A cost-effectiveness analysis of a health education programme for elderly persons with age-related macular degeneration: A longitudinal study. Disabil Rehab 2005;27:1203-12.
75. Pijnacker J, Verstraten P, van Damme W, et al. Rehabilitation of reading in older individuals with macular degeneration: A review of effective training programs. Aging Neuropsych Cognition. 2011;18:708-32.
76. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci 2003;14:125-30.
77. Kovács É, Tóth K, Dénes L, et al. Effects of exercise programs on balance in older women with age-related visual problems: A pilot study. Arch Gerontol Geriatr 2012;55:446-52.
78. McDonnall MC. Physical status as a moderator of depressive symptoms among older adults with dual sensory loss. Rehab Psychol 2011;56:67-76.
79. Chen EW, Fu ASN, Chan KM, et al. The effects of Tai Chi on the balance control of elderly persons with visual impairment: a randomised clinical trial. Age Ageing 2012;41:254-9.
80. Miszko TA, Ramsey VK, Blasch BB. Tai Chi for people with visual impairments: a pilot study. J Vis Impair Blind 2004;98:5-13.
81. Ray C, Horvat M, Keen K, et al. Using Tai Chi as an exercise intervention for improving balance in adults with visual impairments: Two case studies. RE:view 2005;37:17-24.
82. Jeter PE, Dagnelie G, Khalsa SBS, et al. Yoga for persons with severe visual impairment: a feasibility study. Altern Med Stud 2012;2:18-25.
83. Surakka A, Kivela T. Motivating visually impaired and deaf-blind people to perform regular physical exercises. Br J Visual Impair 2008;26:255-68.
84. Li-Korotky H-S. Age-related hearing loss: Quality of care for quality of life. Gerontologist 2012;52:265-71.
85. Chisolm TH, Johnson CE, Danhauer JL, et al. A systematic review of health-related quality of life and hearing aids: Final report of the American Academy of Audiology Task Force on the Health-Related Quality of Life Benefits of Amplification in Adults. J Am Acad Audiol 2007;18:151-83.
86. McCormack A, Fortnum H. Why do people fitted with hearing aids not wear them? Int J Audiol 2013 52:360-8.
87. Meyer C, Hickson L. What factors influence help-seeking for hearing impairment and hearing aid adoption in older adults? Int J Audiol 2012;51:66-74.
88. Andersson G, Green M, Melin L. Behavioural hearing tactics: a controlled trial of a short treatment programme. Behav Res Ther 1997;35:523-30.
89. Andersson G, Melin L, Scott B, et al. Behavioural counselling for subjects with acquired hearing loss. A new approach to hearing tactics. Scandinav Audiol 1994;23:249-56.
90. Burk MH, Humes LE. Effects of long-term training on aided speech-recognition performance in noise in older adults. J Speech Lang Hear Res 2008;51:759-71.
91. Henderson Sabes J, Sweetow RW. Variables predicting outcomes on listening and communication enhancement (LACETM) training. Int J Audiol 2007;46:374-83.
92. Kramer SE, Allessie GH, Dondorp AW, et al. A home education program for older adults with hearing impairment and their significant others: a randomized trial evaluating short- and long-term effects. Int J Audiol 2005;44:255-64.
93. Hickson L, Worrall L, Scarinci N. Measuring outcomes of a communication program for older people with hearing impairment using the International Outcome Inventory. Int J Audiol 2006;45:238-46.
94. Barcroft J, Sommers MS, Tye-Murray N, et al. Tailoring auditory training to patient needs with single and multiple talkers: Transfer-appropriate gains on a four-choice discrimination test. Int J Audiol 2011;50:802-8.
95. Wahl H-W. The psychological challenge of late-life vision impairment: Concepts, Findings, and practical implications. J Ophthalmol 2013.
96. Lin FR. Hearing loss in older adults. Who’s listening? JAMA 2012;307:1147-8.
97. Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296:2805-14.
98. Roets-Merken LM, Graff MJL, Zuidema SU, et al. Effectiveness of a self-management program for dual sensory impaired seniors in aged care settings: study protocol for a cluster randomized controlled trial. Trials 2013;14.
99. Vreeken HL, van Rens GHMB, Kramer SE, et al. Dual sensory loss: development of a dual sensory loss protocol and design of a randomized controlled trial. BMC Geriatr 2013;13.
100. Rees G, Saw C, Larizza M, et al. Should family and friends be involved in group-based rehabilitation programs for adults with low vision? Br J Visual Impair 2007;25:155-68.
101. Birk T, Hickl S, Wahl H-W, et al. Development and pilot evaluation of a psychosocial intervention program for patients with age-related macular degeneration. Gerontologist 2004;44:836-43.
102. Bradley P, Mitchell J, Bradley C, editors. Peer support for people newly diagnosed with macular degeneration: a pilot study. International Congress Vision 2005 September. London, UK.
103. Brody B, Williams R, Thomas R, et al. Age-related macular degeneration: A randomized clinical trial of a self-management intervention. Ann Behavi Med 1999;21:322-9.
104. Brody BL, Roch-Levecq A-C, Gamst AC, et al. Self-management of age-related macular degeneration and quality of life: a randomized controlled trial. Arch Ophthalmol 2002;120:1477-83.
105. Brody BL, Roch-Levecq A-C, Thomas RG, et al. Self-management of age-related macular degeneration at the 6-month follow-up. a randomized controlled trial. Arch Ophthalmol 2005;123:46-53.
106. Brody BL, Roch-Levecq A-C, Kaplan RM, et al. Age-related macular degeneration: self-management and reduction of depressive symptoms in a randomized, controlled study. J Am Geriatr Soc 2006;54:1557-62.
107. Dahlin-Ivanoff S, Sonn U, Svensson E. A health education program for elderly persons with visual impairments and perceived security in the performance of daily occupations: a randomized study. Am J Occup Thera 2002;56:322-30.
108. Eklund K, Sonn U, Dahlin-Ivanoff S. Long-term evaluation of a health education programme for elderly persons with visual impairment. A randomized study. Disabil Rehab 2004;26:401-9.
109. Eklund K, Dahlin-Ivanoff S. Health education for people with macular degeneration: Learning experiences and the effect on daily occupations. Can J Occup Ther 2006;73:272-80.
110. Eklund K, Sjöstrand J, Dahlin-Ivanoff S. A randomized controlled trial of a health-promotion programme and its effect on ADL dependence and self-reported health problems for the elderly visually impaired. Scand J Occupat Ther 2008;15:68-74.
111. Kämmerer A, Wahl H-W, Becker S, et al. Psychosoziale Unterstützung von älteren Menschen mit einer chronischen Sehbeeinträchtigung: Anwendung und Überprüfung einer problemlöse- und einer emotionsfokussierten Kurzintervention. Zeitschrift für Gesundheitspsychologie 2006;14:95-105.
112. Rovner BW, Casten RJ, Hegel MT, et al. Preventing depression in age-related macular degeneration. Arch Gen Psychiatr 2007;64:886-92.
113. Rovner BW, Casten RJ, Hegel MT, et al. Improving function in age-related macular degeneration: A randomized clinical trial. Ophthalmology 2013;120:1649-55.
114. Barcroft J, Mauzé E, Schroy C, et al. Improving the quality of auditory training by making tasks meaningful. Persp Audiol 2011;7:15-28.
From the University of Education (Dr. Heyl) and Heidelberg University (Dr. Wahl), Heidelberg, Germany.
Abstract
- Objective: To summarize the current state of research regarding the experience of age-related vision and hearing impairment.
- Methods:Review of the literature.
- Results: Negative consequences of age-related vision and hearing impairment manifest in the domains of health and longevity, everyday competence, cognitive functioning, social functioning, and subjective well-being. However, while vision impairment strongly impacts everyday competence, the burden of hearing impairment can mainly be found in the social domain. Psychosocially framed intervention research has shown promising findings, but many studies rely on small samples or do not include a control condition.
- Conclusions: Although more research is needed, it is clear that traditional rehabilitation programs targeting age-related vision and hearing impairments need a strong psychosocial component.
Vision and hearing are essential for person–environment interaction and both are subject to pronounced age-related changes. Ongoing demographic changes and increasing life expectancy is contributing to a significant increase in the number of very old individuals [1]. It is projected that by 2030 about 50% of older Americans may have some significant eye disease, ie, cataract, glaucoma, or age-related macular degeneration [2]. Presbycusis as the major cause of age-related hearing impairment is present in 40% of the American senior citizens [3]. In this narrative review, we review the epidemiological data on age-related vision and hearing impairment, research on its psychosocial impact, and intervention research aimed to improve coping processes and rehabilitative outcomes. We close with future recommendations directed both toward research and clinical practice.
Epidemiology
Vision and hearing impairment is highly prevalent in old age, yet prevalence rates reported in the literature are quite different, depending on the definition of vision and hearing impairment used. A widely used criterion for low vision is the one used by the World Health Organization, ie, visual acuity less than 20/60 and equal to or better than 20/400 in the better eye with best correction. A best corrected visual acuity of less than 20/400 in the better eye is used to define blindness [4]. A disabling hearing impairment is defined by an average hearing loss in decibel (dB HL) of at least 41 dB HL at 500, 1000, 2000, and 4000 Hz. [5]. Translated to everyday life, such hearing impairment mainly manifests in severe difficulties in understanding normal conversation. Besides differing definitions, different methods to assess vision and hearing impairment and heterogenous study populations make comparisons of prevalence rates difficult [6]. In particular, relying solely on self-report data to assess vision and hearing loss seems generally problematic. In addition, the strong focus in vision impairment assessment on visual acuity measures has limitations, as other indicators, such as contrast sensitivity or useful field of vision, may be more important for out-of-home mobility or driving [7].
A recent study on the prevalence of visual impairment (defined as best corrected visual acuity < 20⁄40) in 6 European countries found quite similar prevalence rates as reported for the US: Prevalence of visual impairment was 3% in those aged 65 to 74 years, 13% in those over 75 years and 33% in those over 85 years [8]. At first glance, vision loss seems to be more prevalent among older women than among older men, but this relationship is not sustained in multivariate analyses considering age, health, and social support variables [9].
Regarding hearing loss, Gopinath et al [10] found prevalence rates of 29% among men and 17% among women aged 60 to 69 years. Moreover, for every 10 years of age, the prevalence of hearing loss doubled. In their review of epidemiologic data on prevalence of age-related hearing impairment in Europe, Roth and coauthors [11] report that at the age of 70 years, about 30% of men and 20% of women were found to have a hearing loss of at least 30 dB HL, while at the age of 80 years about 55% of men and 45% of women were affected. Lin et al found that 63% of those 70 years and older had a hearing loss of more than 25 dB in the better ear [12].
According to a recent review by Schneider et al [6], prevalence of impairment in both vision and hearing in older age (dual sensory impairment) varies between 1.6% and 22.5% due to different sample characteristics (eg, size, age) and different definitions and assessments of vision and hearing impairment (see also [13]). However, there is good evidence that dual sensory impairment increases with age, and that it is more common among frailer subpopulations such as older individuals consulting care services [6].
Quality of Life Impact of Vision and Hearing Impairment
Health and Longevity
There is inconsistent evidence that both age-related vision and hearing impairment are accompanied by heightened multimorbidity and an increased mortality rate. For example, while some older as well as more recent studies have found that visual and hearing declines over time predict death in very old age [14–16], other studies have detected no significant relationship after adjusting for confounders such as age, gender, and education [17,18]. Among the hearing impaired, only men seem to have a significant increase in mortality risk [15,19]. Dual sensory impairment appears to be more consistently and more strongly related to increased mortality than vision or hearing impairment alone [19–21].
Everyday Competence
The term everyday competence includes both basic (eg, self-care behaviors) and instrumental (eg, using public transport) activities of daily living (ADL/IADL [22]). Age-related vision impairment has been found to be robustly associated with significantly lower everyday competence, because visual capacity is a critical prerequisite for such behaviors [23,24]. Indeed, lowered everyday competence appeared as the best of a range of variables (including cognitive function and well-being–related measures) used to differentiate between visually impaired and visually unimpaired older adults [18]. Furthermore, vision impairment impacts cross-sectionally as well as longitudinally—more strongly on IADL as compared to ADL—because the execution of IADL is more complex and depends more strongly on environmental enhancing or hindering factors [25–27]. Hence, shrinkage in IADL competence reflects a kind of early behavioral marker of severe vision impairment, whereas significant ADL decrease only happens later in the process of chronic vision loss.
In contrast to vision impairment, age-related hearing loss has been found not to have a major impact in particular on ADL/IADL [28]. However, as has been found elsewhere [29], hearing loss is associated with increased reliance on community and informal supports, suggesting that while IADL function may not deteriorate with hearing loss, the way it is conducted may change (ie, need for support to maintain participation).
It should also be mentioned in this context that assessment strategies have been developed to better consider the specific life conditions of those with vision and hearing impairment. The best-known and frequently applied instruments in this context are the National Eye Institute Visual Function Questionnaire [30] and the Hearing Handicap Inventory for the Elderly [31].
Cognitive Functioning
Previous research largely supports the notion that reduced vision and hearing function is accompanied by a decrease in cognitive performance in older adults. The work of Lindenberger and Baltes, based on the Berlin Aging Study (BASE)—but also including additional studies with a wider age-range—is central in supporting a strong connection among vision, hearing, balance, and cognitive functioning in later life. Lindenberger and Baltes [32] found that general intelligence correlated just as strongly with visual as with auditory ability. In a model conjoining age, sensory function, as well as intelligence, visual and auditory function predicted a large portion of interindividual differences in intelligence and indeed fully mediated the negative correlation between age and intelligence. This finding has meanwhile been replicated by a number of other research groups and may be regarded as rather robust [33,34]. In addition, Baltes and Lindenberger [35] observed that sensory measures were better predictors of intelligence than socio-structural variables such as education or social class. They also showed that the connection among sensory functioning and intelligence was much closer in older adults as compared to adults in early and middle adulthood [34].
No clear difference between the sensory modalities of vision and hearing has been identified regarding their relationship with cognitive performance. On the one hand, there is research supporting the view that both vision and hearing impairment are connected with cognitive decline [12,36,37], while some evidence also supports that the linkage may be stronger with vision [14]. On the other hand, there are also data not supporting a close connection between vision and hearing impairment and cognitive function [38]. Explanations for such inconsistencies may refer to a number of reasons, such as pronounced positive selectivity of samples (which may lead to underestimation of connections among sensory and cognitive function), the application of established cognitive tests not appropriate for sensory impaired older adults (which may lead to overestimation of connections among sensory and cognitive function), and the application of different cut-off scores for significant vision and hearing impairment (possibly, higher cut-offs may lead to higher, lower to lower connections). Longitudinal data using the latest in causal modeling data analysis support the view that the causal dynamics involved in sensory and intelligence change are complex and that each of these variables can drive change in the other across longer periods of later life [39].
Vision status also plays a role when it comes to the connection between cognitive function and everyday competence—a linkage that is generally challenged as people age and that may lead to endpoints such as dependence on others and transition to long-term care. Heyl et al [40] observed that the link between vision status and out-of-home leisure activities is mediated by cognitive status. In a more recent study, able to add to the understanding of such a mediation process, Heyl and Wahl [38,41] showed that the connection between cognitive function and everyday function is much closer in visually and hearing impaired older adults as compared with visually unimpaired older adults, which possibly means that both visually and hearing impaired elders rely more intensely on their cognitive resources. Causality dynamics may however also work in the opposite direction. As Rovner and colleagues [42,43] observed in a study with age-related macular degeneration patients over 64 years of age covering a 3-year observation period and 2 measurement occasions, activity loss over time due to the visual loss led to cognitive decline happening between T1 and T2. This finding fits well with the more general finding in the cognitive aging literature that the exertion of social and leisure activities is important for maintaining cognitive functioning [44].
Social Functioning
Social relations as well as social support have generally been found to be of key importance for older adults [45]. Reinhardt [46] found that visually impaired older adults nominated on average 5.4 persons of intimate relation within their family network, and 3.5 persons within their friendship network, which is similar to sensory-unimpaired older adults, such as those assessed in the BASE [47]. In addition, in Wahl et al’s study [18], visually and hearing impaired older adults nominated practically the same number of persons as being in the most intimate circle of their social network (4.70 versus 4.71); the respective number in a comparison group of visually unimpaired older adults amounted to 5.2 persons, which was not significantly different from both sensory impaired group means.
Neither vision nor hearing impairment seem to affect the experience of loneliness dramatically [18,23,48], although some research did report an increased risk of loneliness in older adults with vision impairment [49]. It is clear however that hearing impairment more strongly than vision impairment negatively impacts social communication and carries a strong stigma for those affected [48,50]. The stigma particularly implies that hearing deficits and concomitant communication disturbances (eg, giving an answer that does not match the question) elicits the view of a cognitively impaired, if not demented older person. In some contrast, vision loss seems to raise rather strong helping impulses and feelings of compassion. The dark side of this tendency is that it seems a challenge to provide visually impaired older adults with the instrumental support needed while at the same time fostering remaining capabilities [51]. Overprotection may put constraints on the visually impaired older adults’ “true” functional capacity and thereby contribute to loss in competence over the longer run due to disuse [52].
Subjective Well Being–related Outcomes and Depression
Visually impaired older adults have shown evidence of diminished well-being as compared with sensory unimpaired older adults [53], although effect sizes were rather small in a respective meta-analysis [54]. Differences in well-being between hearing impaired and unimpaired older adults seem small or nonexistent in some studies [18,55], but considerable in others [56]. The latter study covering a 16-year observational period as well as other longitudinal work (eg, [57]) also support the notion that remaining ADLs and social engagement mediate the linkage between sensory loss and well-being and depression. The “well-being paradox” in old age, pointing to pronounced adaptive resources to maintain well-being in spite of adverse conditions [58], may also apply to sensory impaired older adults [57,59].
At the same time, it is critical to acknowledge that visually impaired older adults represent an at-risk population, in which the positive impact of human adaptation and the drawback of reaching the limits of psychological resilience go hand in hand. Affect balance (ratio of positive and negative affect) has been found to be more toward the negative pole in visually impaired older adults [60] and depression has consistently been found to be significantly increased in visually impaired older adults [61–63]. Rates roughly vary between 15% and 30% and are particularly high in age-related macular degeneration patients [61]. This is also important, because depressive symptoms may accelerate both cognitive decline and decline in everyday competence in age-related macular degeneration patients [42]. Perceived overprotection may also lead to negative consequences in terms of heightened depression and anxiety over time [64].
Regarding the impact of hearing loss on depression, findings are quite inconsistent. Some studies found evidence for a significant relationship between hearing impairment and depressive symptoms among older adults [65, 66], while others did not [67, 68]. Gopinath and co-workers [66] observed that hearing impaired individuals, particularly women, younger than 70 years of age and those who were infrequently using a hearing aid (less than one hour per day) were more likely to suffer from depressive symptoms. According to a population-based study among older Italians, hearing impairment might be more closely related to anxiety symptoms than to depression [69].
Dual Sensory Impairment
Previous research supports the notion that the overall psychosocial situation of those with dual sensory impairment is even worse as compared to those with sole vision or hearing impairment. In particular, higher rates of ADL/IADL impairment, depression and lowered well-being have been found in older adults affected by dual sensory loss [6,13,18,56]. Also, dual sensory impairment has been found to be linked with cognitive decline cross-sectionally [37] as well as longitudinally [36].
Improving Quality of Life in Sensory Impaired Older Adults
The research summary provided in the previous section underscores that the experience of age-related visual and hearing impairment comes with pronounced challenges that deserve evidence-based professional support. In the following, we give an overview and evaluation of major work in the area of psychosocially framed intervention research targeting older adults with vision and hearing loss. By psychosocially framed interventions we mean studies containing programs that focused on psychosocial processes (eg, consultation on how to better cope with sensory impairment, educative components, problem solving strategies, coping with negative affect) and assessed psychosocial outcomes (eg, everyday functioning, depression, emotional stress experiences). Such interventions may have been integrated into regular rehabilitation programs or offered as a separate strategy in addition to classic rehabilitation. We also consider physical activity–related and overall “way of living” interventions such as tai chi and yoga. In doing so, our aim is to highlight the bandwidth of psychosocial interventions and respective outcomes, not comprehensiveness.
Age-related Vision Impairment
Most psychosocially framed interventions could be characterized as self-management– and disease management–like efforts and are promising for visually impaired older adults. Major elements of such programs include stress-reducing strategies (eg, muscle-relaxation exercises), goal-directed problem-solving, strategies to evoke positive affect, activating available resources, and information and consultation. Typically, such programs are conducted in a group format in an eye clinic, bringing together 6 to 8 visually impaired older adults for weekly sessions of 2 to 3 hours over 6 to 8 weeks.
More recent work provides additional support for the usefulness of self-management programs for visually impaired older adults [72,73]. In addition, emerging evidence supports the notion that psychosocially framed interventions may contribute to saving health costs (eg, via reduced psychopharmacy) and may also enhance commitment
It is also obvious that such programs should find a strong liaison with classic high-caliber rehabilitation programs for visually impaired older adults, including effective reading training [75].
Furthermore, physical training programs, which have proven efficiency with old and very old individuals—including those who are cognitively vulnerable—also seem to be of significant advantage for visually impaired older adults. As has been found, such programs not only increase posture, gait, and general physical fitness, they also prevent falls and enhance well-being, self-efficacy, and cognitive function, especially executive control [76]. Postural control has been improved by multimodal balance and strength exercises among older individuals with visual impairments as well [77]. Participation in physical activity and being in better physical condition buffered the relationship between dual sensory impairment and depression, pointing to the importance of physical training programs for the mental health of older persons with dual sensory impairment [78]. According to a randomized control study as well as to some case studies, tai chi seems to be an effective tool to improve balance control in visually impaired older adults, and thus to reduce an important risk factor for falls [79–81]. Visually impaired adults might also benefit from yoga in terms of balance improvement as well as psychosocial improvements [82]. To teach tai chi efficiently, it is necessary to adapt instructions to the needs of the visually impaired seniors by relying on verbal cuing and manual body placement [80]. The need to adapt instructions and to provide an accessible environment (including transportation arrangements) to motivate older individuals with visual impairments to perform regular physical exercises is also highlighted by Surakka and Kivela [83].
Furthermore, there is evidence that state of the art low vision rehabilitation as such also has beneficial effects on psychosocial outcomes, such as general and vision-related quality of life and emotional well-being.
Age-related Hearing Impairment
Interventions concerning older adults with hearing impairment center on amplification and aural rehabilitation, including auditory training [84]. It has been shown that using hearing aids improves the quality of life, in particular hearing-related quality of life, of hearing impaired adults [85]. Yet many older adults who would benefit from hearing aids do not wear them [86]. From the reasons identified in the review by McCormack and Fortnum [86], perceived hearing aid value, in particular poor benefit in noisy situations, fit and comfort, as well as care and maintenance of the hearing aid emerged as most important. Improvements in these areas are necessary to enhance hearing aid usage among older adults with hearing impairment. Meyer and Hickson [87] identified 5 factors increasing the likelihood to seek help for hearing impairment and/or adopt hearing aids: (1) moderate to severe hearing impairment and perceived hearing-related everyday limitations; (2) older age; (3) poor subjective hearing; (4) perceiving more benefits than barriers to amplification; and (5) perceiving significant others as supportive of hearing rehabilitation. Thus, the involvement of family members in the rehabilitation process appears necessary and promising.
Beyond amplification, aural rehabilitation seeks to improve the situation of hearing impaired older adults by providing listening and communication techniques to enhance communication effectiveness. We have summarized major work in this area in Table 2.
In sum, it seems clear for both vision impairment [95] and hearing impairment [96] that classic rehabilitation strategies, such as fitting a reading device or hearing aid, need significant enrichment by psychosocial training components in order to achieve the best outcomes possible. Furthermore, given the findings on the role of cognitive resources in visually impaired older adults (see respective section above), cognitive training may be an important addition to psychosocial intervention and rehabilitation [38,97]. It must be noted, however, that many of the available studies reveal a number of methodological limitations, such as small sample sizes, missing control condition, and no follow-up assessments to estimate the maintenance of effects.
A significant future need is intervention research addressing older adults with dual sensory impairment. Although we found study protocols related to important trials underway [98,99] and a physical training study with visually impaired older adults that included also some dual sensory impaired individuals [83], it seems that there is not much research in terms of completed interventions and respective findings. Furthermore, it may be important to better involve significant others such as family members and friends in psychosocially framed programs and emerging research with low vision adults has revealed advantages and disadvantages of such an approach [100].
Practical Implications
The older patient is on the way to become the “standard” patient for eye care and hearing specialists and thus a challenge for public health at large. Based on the evidence compiled above, we argue that best practice in medical treatment and traditional rehabilitation of vision and hearing impairment should better consider the psychosocial dimension of age-related vision and hearing impairment. We see different levels at which a stronger psychosocially framed input is needed. First, at the diagnostic level, having a better understanding of everyday competence, the role of cognitive functioning, social resources, and well-being–related dynamics in visually impaired and hearing impaired older adults may significantly enrich the professional background knowledge about the patient. Such knowledge may become important for diagnostic evaluation, treatment decisions, and predictions of long-term outcomes. It seems also critical to have an understanding of the more fundamental mechanisms and systemic inter-relations in older patients (eg, among visual, hearing, mobility, and cognitive impairment), because such evidence helps to evaluate overall vulnerability and likely future trajectories of respective patients.
Second, at the intervention level against the background of the available empirical effectiveness evidence, self-management–oriented and psycho-educative programs should become a regular component of low vision rehabilitation. Similarly, psychosocial programs educating older adults in hearing tactics and hearing loss–oriented coping strategies should become a regular part of hearing rehabilitation. In addition, we argue that cognitive training and physical activity–oriented interventions should have their place in rehabilitation programs designed for older visually and hearing impaired adults. The major reason is that respective programs generally have been found to positively impact quality of life in old age. This impact may be particularly valuable for more vulnerable populations, such as sensory impaired older adults. We therefore recommend implementing psychosocially framed programs as a regular service in eye clinics as well as in ear, nose, and throat clinics, because this seems to be the setting best suited to approach visually and hearing impaired older adults as well as to offer the logistic opportunities to conduct such programs. It would also be critical to extend such programs to in-home services as well as services covering long-term care settings. Older adults with dual sensory impairment bring specific challenges to such interventions, such as the optimal cooperation and combination of rehabilitation and psychosocial expertise related to each domain and traditionally offered side by side. It is also good news that new trials are underway to learn more about psychosocial interventions aimed to address older adults with dual sensory loss [98,99].
In conclusion, we argue for a better implementation of both age-related psycho-ophthalmology as well as psycho-audiology. Although we regard psychologists with a clinical training background as a key profession to be involved in psychosocially framed interventions with older adults with vision and hearing impairment, other professions (eg, occupational therapists, sport scientists) should also play an important role. A multiprofessional enrichment of classic sensory rehabilitation based on the training principles as described above is a major future need.
Corresponding author: Vera Heyl, PhD, Zeppelinstr, 1, D-69121, Heidelberg, Germany, [email protected].
Financial disclosures: None.
From the University of Education (Dr. Heyl) and Heidelberg University (Dr. Wahl), Heidelberg, Germany.
Abstract
- Objective: To summarize the current state of research regarding the experience of age-related vision and hearing impairment.
- Methods:Review of the literature.
- Results: Negative consequences of age-related vision and hearing impairment manifest in the domains of health and longevity, everyday competence, cognitive functioning, social functioning, and subjective well-being. However, while vision impairment strongly impacts everyday competence, the burden of hearing impairment can mainly be found in the social domain. Psychosocially framed intervention research has shown promising findings, but many studies rely on small samples or do not include a control condition.
- Conclusions: Although more research is needed, it is clear that traditional rehabilitation programs targeting age-related vision and hearing impairments need a strong psychosocial component.
Vision and hearing are essential for person–environment interaction and both are subject to pronounced age-related changes. Ongoing demographic changes and increasing life expectancy is contributing to a significant increase in the number of very old individuals [1]. It is projected that by 2030 about 50% of older Americans may have some significant eye disease, ie, cataract, glaucoma, or age-related macular degeneration [2]. Presbycusis as the major cause of age-related hearing impairment is present in 40% of the American senior citizens [3]. In this narrative review, we review the epidemiological data on age-related vision and hearing impairment, research on its psychosocial impact, and intervention research aimed to improve coping processes and rehabilitative outcomes. We close with future recommendations directed both toward research and clinical practice.
Epidemiology
Vision and hearing impairment is highly prevalent in old age, yet prevalence rates reported in the literature are quite different, depending on the definition of vision and hearing impairment used. A widely used criterion for low vision is the one used by the World Health Organization, ie, visual acuity less than 20/60 and equal to or better than 20/400 in the better eye with best correction. A best corrected visual acuity of less than 20/400 in the better eye is used to define blindness [4]. A disabling hearing impairment is defined by an average hearing loss in decibel (dB HL) of at least 41 dB HL at 500, 1000, 2000, and 4000 Hz. [5]. Translated to everyday life, such hearing impairment mainly manifests in severe difficulties in understanding normal conversation. Besides differing definitions, different methods to assess vision and hearing impairment and heterogenous study populations make comparisons of prevalence rates difficult [6]. In particular, relying solely on self-report data to assess vision and hearing loss seems generally problematic. In addition, the strong focus in vision impairment assessment on visual acuity measures has limitations, as other indicators, such as contrast sensitivity or useful field of vision, may be more important for out-of-home mobility or driving [7].
A recent study on the prevalence of visual impairment (defined as best corrected visual acuity < 20⁄40) in 6 European countries found quite similar prevalence rates as reported for the US: Prevalence of visual impairment was 3% in those aged 65 to 74 years, 13% in those over 75 years and 33% in those over 85 years [8]. At first glance, vision loss seems to be more prevalent among older women than among older men, but this relationship is not sustained in multivariate analyses considering age, health, and social support variables [9].
Regarding hearing loss, Gopinath et al [10] found prevalence rates of 29% among men and 17% among women aged 60 to 69 years. Moreover, for every 10 years of age, the prevalence of hearing loss doubled. In their review of epidemiologic data on prevalence of age-related hearing impairment in Europe, Roth and coauthors [11] report that at the age of 70 years, about 30% of men and 20% of women were found to have a hearing loss of at least 30 dB HL, while at the age of 80 years about 55% of men and 45% of women were affected. Lin et al found that 63% of those 70 years and older had a hearing loss of more than 25 dB in the better ear [12].
According to a recent review by Schneider et al [6], prevalence of impairment in both vision and hearing in older age (dual sensory impairment) varies between 1.6% and 22.5% due to different sample characteristics (eg, size, age) and different definitions and assessments of vision and hearing impairment (see also [13]). However, there is good evidence that dual sensory impairment increases with age, and that it is more common among frailer subpopulations such as older individuals consulting care services [6].
Quality of Life Impact of Vision and Hearing Impairment
Health and Longevity
There is inconsistent evidence that both age-related vision and hearing impairment are accompanied by heightened multimorbidity and an increased mortality rate. For example, while some older as well as more recent studies have found that visual and hearing declines over time predict death in very old age [14–16], other studies have detected no significant relationship after adjusting for confounders such as age, gender, and education [17,18]. Among the hearing impaired, only men seem to have a significant increase in mortality risk [15,19]. Dual sensory impairment appears to be more consistently and more strongly related to increased mortality than vision or hearing impairment alone [19–21].
Everyday Competence
The term everyday competence includes both basic (eg, self-care behaviors) and instrumental (eg, using public transport) activities of daily living (ADL/IADL [22]). Age-related vision impairment has been found to be robustly associated with significantly lower everyday competence, because visual capacity is a critical prerequisite for such behaviors [23,24]. Indeed, lowered everyday competence appeared as the best of a range of variables (including cognitive function and well-being–related measures) used to differentiate between visually impaired and visually unimpaired older adults [18]. Furthermore, vision impairment impacts cross-sectionally as well as longitudinally—more strongly on IADL as compared to ADL—because the execution of IADL is more complex and depends more strongly on environmental enhancing or hindering factors [25–27]. Hence, shrinkage in IADL competence reflects a kind of early behavioral marker of severe vision impairment, whereas significant ADL decrease only happens later in the process of chronic vision loss.
In contrast to vision impairment, age-related hearing loss has been found not to have a major impact in particular on ADL/IADL [28]. However, as has been found elsewhere [29], hearing loss is associated with increased reliance on community and informal supports, suggesting that while IADL function may not deteriorate with hearing loss, the way it is conducted may change (ie, need for support to maintain participation).
It should also be mentioned in this context that assessment strategies have been developed to better consider the specific life conditions of those with vision and hearing impairment. The best-known and frequently applied instruments in this context are the National Eye Institute Visual Function Questionnaire [30] and the Hearing Handicap Inventory for the Elderly [31].
Cognitive Functioning
Previous research largely supports the notion that reduced vision and hearing function is accompanied by a decrease in cognitive performance in older adults. The work of Lindenberger and Baltes, based on the Berlin Aging Study (BASE)—but also including additional studies with a wider age-range—is central in supporting a strong connection among vision, hearing, balance, and cognitive functioning in later life. Lindenberger and Baltes [32] found that general intelligence correlated just as strongly with visual as with auditory ability. In a model conjoining age, sensory function, as well as intelligence, visual and auditory function predicted a large portion of interindividual differences in intelligence and indeed fully mediated the negative correlation between age and intelligence. This finding has meanwhile been replicated by a number of other research groups and may be regarded as rather robust [33,34]. In addition, Baltes and Lindenberger [35] observed that sensory measures were better predictors of intelligence than socio-structural variables such as education or social class. They also showed that the connection among sensory functioning and intelligence was much closer in older adults as compared to adults in early and middle adulthood [34].
No clear difference between the sensory modalities of vision and hearing has been identified regarding their relationship with cognitive performance. On the one hand, there is research supporting the view that both vision and hearing impairment are connected with cognitive decline [12,36,37], while some evidence also supports that the linkage may be stronger with vision [14]. On the other hand, there are also data not supporting a close connection between vision and hearing impairment and cognitive function [38]. Explanations for such inconsistencies may refer to a number of reasons, such as pronounced positive selectivity of samples (which may lead to underestimation of connections among sensory and cognitive function), the application of established cognitive tests not appropriate for sensory impaired older adults (which may lead to overestimation of connections among sensory and cognitive function), and the application of different cut-off scores for significant vision and hearing impairment (possibly, higher cut-offs may lead to higher, lower to lower connections). Longitudinal data using the latest in causal modeling data analysis support the view that the causal dynamics involved in sensory and intelligence change are complex and that each of these variables can drive change in the other across longer periods of later life [39].
Vision status also plays a role when it comes to the connection between cognitive function and everyday competence—a linkage that is generally challenged as people age and that may lead to endpoints such as dependence on others and transition to long-term care. Heyl et al [40] observed that the link between vision status and out-of-home leisure activities is mediated by cognitive status. In a more recent study, able to add to the understanding of such a mediation process, Heyl and Wahl [38,41] showed that the connection between cognitive function and everyday function is much closer in visually and hearing impaired older adults as compared with visually unimpaired older adults, which possibly means that both visually and hearing impaired elders rely more intensely on their cognitive resources. Causality dynamics may however also work in the opposite direction. As Rovner and colleagues [42,43] observed in a study with age-related macular degeneration patients over 64 years of age covering a 3-year observation period and 2 measurement occasions, activity loss over time due to the visual loss led to cognitive decline happening between T1 and T2. This finding fits well with the more general finding in the cognitive aging literature that the exertion of social and leisure activities is important for maintaining cognitive functioning [44].
Social Functioning
Social relations as well as social support have generally been found to be of key importance for older adults [45]. Reinhardt [46] found that visually impaired older adults nominated on average 5.4 persons of intimate relation within their family network, and 3.5 persons within their friendship network, which is similar to sensory-unimpaired older adults, such as those assessed in the BASE [47]. In addition, in Wahl et al’s study [18], visually and hearing impaired older adults nominated practically the same number of persons as being in the most intimate circle of their social network (4.70 versus 4.71); the respective number in a comparison group of visually unimpaired older adults amounted to 5.2 persons, which was not significantly different from both sensory impaired group means.
Neither vision nor hearing impairment seem to affect the experience of loneliness dramatically [18,23,48], although some research did report an increased risk of loneliness in older adults with vision impairment [49]. It is clear however that hearing impairment more strongly than vision impairment negatively impacts social communication and carries a strong stigma for those affected [48,50]. The stigma particularly implies that hearing deficits and concomitant communication disturbances (eg, giving an answer that does not match the question) elicits the view of a cognitively impaired, if not demented older person. In some contrast, vision loss seems to raise rather strong helping impulses and feelings of compassion. The dark side of this tendency is that it seems a challenge to provide visually impaired older adults with the instrumental support needed while at the same time fostering remaining capabilities [51]. Overprotection may put constraints on the visually impaired older adults’ “true” functional capacity and thereby contribute to loss in competence over the longer run due to disuse [52].
Subjective Well Being–related Outcomes and Depression
Visually impaired older adults have shown evidence of diminished well-being as compared with sensory unimpaired older adults [53], although effect sizes were rather small in a respective meta-analysis [54]. Differences in well-being between hearing impaired and unimpaired older adults seem small or nonexistent in some studies [18,55], but considerable in others [56]. The latter study covering a 16-year observational period as well as other longitudinal work (eg, [57]) also support the notion that remaining ADLs and social engagement mediate the linkage between sensory loss and well-being and depression. The “well-being paradox” in old age, pointing to pronounced adaptive resources to maintain well-being in spite of adverse conditions [58], may also apply to sensory impaired older adults [57,59].
At the same time, it is critical to acknowledge that visually impaired older adults represent an at-risk population, in which the positive impact of human adaptation and the drawback of reaching the limits of psychological resilience go hand in hand. Affect balance (ratio of positive and negative affect) has been found to be more toward the negative pole in visually impaired older adults [60] and depression has consistently been found to be significantly increased in visually impaired older adults [61–63]. Rates roughly vary between 15% and 30% and are particularly high in age-related macular degeneration patients [61]. This is also important, because depressive symptoms may accelerate both cognitive decline and decline in everyday competence in age-related macular degeneration patients [42]. Perceived overprotection may also lead to negative consequences in terms of heightened depression and anxiety over time [64].
Regarding the impact of hearing loss on depression, findings are quite inconsistent. Some studies found evidence for a significant relationship between hearing impairment and depressive symptoms among older adults [65, 66], while others did not [67, 68]. Gopinath and co-workers [66] observed that hearing impaired individuals, particularly women, younger than 70 years of age and those who were infrequently using a hearing aid (less than one hour per day) were more likely to suffer from depressive symptoms. According to a population-based study among older Italians, hearing impairment might be more closely related to anxiety symptoms than to depression [69].
Dual Sensory Impairment
Previous research supports the notion that the overall psychosocial situation of those with dual sensory impairment is even worse as compared to those with sole vision or hearing impairment. In particular, higher rates of ADL/IADL impairment, depression and lowered well-being have been found in older adults affected by dual sensory loss [6,13,18,56]. Also, dual sensory impairment has been found to be linked with cognitive decline cross-sectionally [37] as well as longitudinally [36].
Improving Quality of Life in Sensory Impaired Older Adults
The research summary provided in the previous section underscores that the experience of age-related visual and hearing impairment comes with pronounced challenges that deserve evidence-based professional support. In the following, we give an overview and evaluation of major work in the area of psychosocially framed intervention research targeting older adults with vision and hearing loss. By psychosocially framed interventions we mean studies containing programs that focused on psychosocial processes (eg, consultation on how to better cope with sensory impairment, educative components, problem solving strategies, coping with negative affect) and assessed psychosocial outcomes (eg, everyday functioning, depression, emotional stress experiences). Such interventions may have been integrated into regular rehabilitation programs or offered as a separate strategy in addition to classic rehabilitation. We also consider physical activity–related and overall “way of living” interventions such as tai chi and yoga. In doing so, our aim is to highlight the bandwidth of psychosocial interventions and respective outcomes, not comprehensiveness.
Age-related Vision Impairment
Most psychosocially framed interventions could be characterized as self-management– and disease management–like efforts and are promising for visually impaired older adults. Major elements of such programs include stress-reducing strategies (eg, muscle-relaxation exercises), goal-directed problem-solving, strategies to evoke positive affect, activating available resources, and information and consultation. Typically, such programs are conducted in a group format in an eye clinic, bringing together 6 to 8 visually impaired older adults for weekly sessions of 2 to 3 hours over 6 to 8 weeks.
More recent work provides additional support for the usefulness of self-management programs for visually impaired older adults [72,73]. In addition, emerging evidence supports the notion that psychosocially framed interventions may contribute to saving health costs (eg, via reduced psychopharmacy) and may also enhance commitment
It is also obvious that such programs should find a strong liaison with classic high-caliber rehabilitation programs for visually impaired older adults, including effective reading training [75].
Furthermore, physical training programs, which have proven efficiency with old and very old individuals—including those who are cognitively vulnerable—also seem to be of significant advantage for visually impaired older adults. As has been found, such programs not only increase posture, gait, and general physical fitness, they also prevent falls and enhance well-being, self-efficacy, and cognitive function, especially executive control [76]. Postural control has been improved by multimodal balance and strength exercises among older individuals with visual impairments as well [77]. Participation in physical activity and being in better physical condition buffered the relationship between dual sensory impairment and depression, pointing to the importance of physical training programs for the mental health of older persons with dual sensory impairment [78]. According to a randomized control study as well as to some case studies, tai chi seems to be an effective tool to improve balance control in visually impaired older adults, and thus to reduce an important risk factor for falls [79–81]. Visually impaired adults might also benefit from yoga in terms of balance improvement as well as psychosocial improvements [82]. To teach tai chi efficiently, it is necessary to adapt instructions to the needs of the visually impaired seniors by relying on verbal cuing and manual body placement [80]. The need to adapt instructions and to provide an accessible environment (including transportation arrangements) to motivate older individuals with visual impairments to perform regular physical exercises is also highlighted by Surakka and Kivela [83].
Furthermore, there is evidence that state of the art low vision rehabilitation as such also has beneficial effects on psychosocial outcomes, such as general and vision-related quality of life and emotional well-being.
Age-related Hearing Impairment
Interventions concerning older adults with hearing impairment center on amplification and aural rehabilitation, including auditory training [84]. It has been shown that using hearing aids improves the quality of life, in particular hearing-related quality of life, of hearing impaired adults [85]. Yet many older adults who would benefit from hearing aids do not wear them [86]. From the reasons identified in the review by McCormack and Fortnum [86], perceived hearing aid value, in particular poor benefit in noisy situations, fit and comfort, as well as care and maintenance of the hearing aid emerged as most important. Improvements in these areas are necessary to enhance hearing aid usage among older adults with hearing impairment. Meyer and Hickson [87] identified 5 factors increasing the likelihood to seek help for hearing impairment and/or adopt hearing aids: (1) moderate to severe hearing impairment and perceived hearing-related everyday limitations; (2) older age; (3) poor subjective hearing; (4) perceiving more benefits than barriers to amplification; and (5) perceiving significant others as supportive of hearing rehabilitation. Thus, the involvement of family members in the rehabilitation process appears necessary and promising.
Beyond amplification, aural rehabilitation seeks to improve the situation of hearing impaired older adults by providing listening and communication techniques to enhance communication effectiveness. We have summarized major work in this area in Table 2.
In sum, it seems clear for both vision impairment [95] and hearing impairment [96] that classic rehabilitation strategies, such as fitting a reading device or hearing aid, need significant enrichment by psychosocial training components in order to achieve the best outcomes possible. Furthermore, given the findings on the role of cognitive resources in visually impaired older adults (see respective section above), cognitive training may be an important addition to psychosocial intervention and rehabilitation [38,97]. It must be noted, however, that many of the available studies reveal a number of methodological limitations, such as small sample sizes, missing control condition, and no follow-up assessments to estimate the maintenance of effects.
A significant future need is intervention research addressing older adults with dual sensory impairment. Although we found study protocols related to important trials underway [98,99] and a physical training study with visually impaired older adults that included also some dual sensory impaired individuals [83], it seems that there is not much research in terms of completed interventions and respective findings. Furthermore, it may be important to better involve significant others such as family members and friends in psychosocially framed programs and emerging research with low vision adults has revealed advantages and disadvantages of such an approach [100].
Practical Implications
The older patient is on the way to become the “standard” patient for eye care and hearing specialists and thus a challenge for public health at large. Based on the evidence compiled above, we argue that best practice in medical treatment and traditional rehabilitation of vision and hearing impairment should better consider the psychosocial dimension of age-related vision and hearing impairment. We see different levels at which a stronger psychosocially framed input is needed. First, at the diagnostic level, having a better understanding of everyday competence, the role of cognitive functioning, social resources, and well-being–related dynamics in visually impaired and hearing impaired older adults may significantly enrich the professional background knowledge about the patient. Such knowledge may become important for diagnostic evaluation, treatment decisions, and predictions of long-term outcomes. It seems also critical to have an understanding of the more fundamental mechanisms and systemic inter-relations in older patients (eg, among visual, hearing, mobility, and cognitive impairment), because such evidence helps to evaluate overall vulnerability and likely future trajectories of respective patients.
Second, at the intervention level against the background of the available empirical effectiveness evidence, self-management–oriented and psycho-educative programs should become a regular component of low vision rehabilitation. Similarly, psychosocial programs educating older adults in hearing tactics and hearing loss–oriented coping strategies should become a regular part of hearing rehabilitation. In addition, we argue that cognitive training and physical activity–oriented interventions should have their place in rehabilitation programs designed for older visually and hearing impaired adults. The major reason is that respective programs generally have been found to positively impact quality of life in old age. This impact may be particularly valuable for more vulnerable populations, such as sensory impaired older adults. We therefore recommend implementing psychosocially framed programs as a regular service in eye clinics as well as in ear, nose, and throat clinics, because this seems to be the setting best suited to approach visually and hearing impaired older adults as well as to offer the logistic opportunities to conduct such programs. It would also be critical to extend such programs to in-home services as well as services covering long-term care settings. Older adults with dual sensory impairment bring specific challenges to such interventions, such as the optimal cooperation and combination of rehabilitation and psychosocial expertise related to each domain and traditionally offered side by side. It is also good news that new trials are underway to learn more about psychosocial interventions aimed to address older adults with dual sensory loss [98,99].
In conclusion, we argue for a better implementation of both age-related psycho-ophthalmology as well as psycho-audiology. Although we regard psychologists with a clinical training background as a key profession to be involved in psychosocially framed interventions with older adults with vision and hearing impairment, other professions (eg, occupational therapists, sport scientists) should also play an important role. A multiprofessional enrichment of classic sensory rehabilitation based on the training principles as described above is a major future need.
Corresponding author: Vera Heyl, PhD, Zeppelinstr, 1, D-69121, Heidelberg, Germany, [email protected].
Financial disclosures: None.
1. Oeppen J, Vaupel JW. Broken limits to life expectancy. Science 2002;296:1029–31.
2. Eichenbaum JW. Geriatric vision loss due to cataracts, macular degeneration, and glaucoma. Mt Sinai J Med 2012;79:276–94.
3. Ciorba A, Bianchini C, Pelucchi S, et al. The impact of hearing loss on the quality of life of elderly adults. Clin Interven Aging 2012;7:159–63.
4. WHO - World Health Organization and International Agency for the Prevention of Blindness. Developing an action plan to prevent blindness at national, provincial and district levels. Vision 2020 The right to sight. Geneva: World Health Organization; 2004.
5. WHO - World Health Organizaton. Grades of hearing impairment. Geneva: World Health Organization; 2013.
6. Schneider JM, Gopinath B, McMahon CM, et al. Dual sensory impairment in older age. J Aging Health 2011;23:1309–24.
7. Clay OJ, Wadley VG, Edwards JD, et al. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: current and future implications. Optom Vis Sci 2005;82:724–31.
8. Seland JH, Vingerling JR, Augood CA, et al. Visual Impairment and quality of life in the Older European Population, the EUREYE study. Acta Ophthal 2011;89:608–13.
9. Horowitz A, Brennan M, Reinhardt JP. Prevalence and risk factors for self-reported visual impairment among middle-aged and older adults. Research Aging 2005;27:307–26.
10. Gopinath B, Rochtchina E, Wang JJ, et al. Prevalence of age-related hearing loss in older adults: Blue mountains study. Arch Intern Med 2009;169:415–6.
11. Roth TN, Hanebuth D, Probst R. Prevalence of age-related hearing loss in Europe: a review. Eur Arch Otorhinolaryngol 2011;268:1101–7.
12. Lin FR, Thorpe R, Gordon-Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol Series A: Biolog Sci Med Sci 2011;66A:582–90.
13. Brennan M, Horowitz A, Su Y-p. Dual sensory loss and its impact on everyday competence. Gerontologist 2005;45:337–46.
14. Anstey KJ, Luszcz MA, Giles LC, et al. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging 2001;16:3–11.
15. Appollonio I, Carabellese C, Magni E, et al. Sensory impairments and mortality in an elderly community population: A six-year follow-up study. Age Ageing 1995;24:30–6.
16. McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol 2001;85:322–6.
17. Ostbye T, Steenhuis R, Wolfson C, et al. Predictors of five-year mortality in older Canadians: the Canadian Study of Health and Aging. J Am Geriatr Soc 1999 47:1249–54.
18. Wahl H-W, Heyl V, Drapaniotis PM, et al. Severe vision and hearing impairment and successful aging: A multidimensional view. Gerontologist 2013;53:950–62.
19. Fisher D, Li C-M, Chiu MS, et al. Impairments in hearing and vision impact on mortality in older people: the AGES-Reykjavik Study. Age Ageing 2013.
20. Bamini G, Schneider J, McMahon CM, et al. Dual sensory impairment in older adults inceases the risk of mortality: A population-based study. PLoS ONE 2013;8:1–6.
21. Lam BL, Lee DJ, Gómez-Marín O, et al. Concurrent visual and hearing impairment and risk of mortality: The National Health Interview Survey. Arch Ophthalmol 2006;124:95–101.
22. Baltes MM, Maas I, Wilms H-U, et al. Everyday competence in old and very old age: Theoretical considerations and empirical findings. In: Baltes PB, Mayer K-U, editors. The Berlin Aging Study Cambridge, UK: Cambridge University Press; 1999: 384–402.
23. Burmedi D, Becker S, Heyl V, et al. Behavioral consequences of age-related low vision: A narrative review. Vis Impair Res 2002;4:15–45.
24. Wahl H-W, Heyl V, Schilling O. Robustness of personality and affect relations under chronic conditions: the case of age-related vision and hearing impairment. J Gerontol B Psycholog Sci Soc Sci 2012;67:687–96.
25. Heyl V, Wahl H-W. On the long-term psychosocial adaptation to vision loss in the later years. In: Wahl H-W, Schulze H-E, editors. On the special needs of blind and low vision seniors: Research and practice concepts. Amsterdam: IOS-Press; 2001: 77–83.
26. Wahl H-W, Schilling O, Oswald F, et al. Psychosocial consequences of age-related visual impairment: Comparison with mobility-impaired older adults and long-term outcome. J Gerontol Psych Sci Soc Sci 1999;54B:P304–P16.
27. Wahl H-W, Becker S, Burmedi D, et al. The role of primary and secondary control in adaptation to age-related vision loss: A study of older adults with macular degeneration Psychol Aging 2004;19:235–9.
28. Rudberg MA, Furner SE, Dunn JE, et al. The relationship of visual and hearing impairments to disability: An analysis using the Longitudinal Study of Aging. J Gerontol Med Sci 1993;48:M261–M5.
29. Schneider J, Gopinath B, Karpa MJ, et al. Hearing loss impacts on the use of community and informal supports. Age Ageing 2010;39:458–64.
30. Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute visual function questionnaire. Arch Ophthalmol 2001;119:1050–8.
31. Ventry I, Weinstein B. The hearing handicap inventory for the elderly: A new tool. Ear Hearing 1982;3:128–34.
32. Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol Aging 1994;9:339–55.
33. Clay OJ, Edwards JD, Ross LA, et al. Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. J Aging Health 2009;21:547–66.
34. Salthouse TA, Hancock HE, Meinz EJ, et al. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol Psychol Sci 1996;51B:P317–P30.
35. Baltes PB, Lindenberger U. Emergence of powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging 1997;12:12–21.
36. Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 2004;52:1996–2002.
37. Tay T, Wang JJ, Kifley A, et al. Sensory and cognitive association in older persons: Findings from an older Australian population. Gerontology 2006;52:386–94.
38. Heyl V, Wahl H-W. Managing daily life with age-related sensory loss: Cogni-tive resources gain in importance. Psychol Aging 2012;27:510–21.
39. Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychol Aging 2009;24:1-16.
40. Heyl V, Wahl H-W, Mollenkopf H. Visual capacity, out-of-home activities and emotional well-being in old age: Basic relations and contextual variation. Soc Indicat Res 2005;74:159–89.
41. Heyl V, Wahl H-W. Cognitive ability as a resource for everyday functioning among older adults who are visually impaired. J Vis Impair Blind 2010;104:391–403.
42. Rovner BW, Casten RJ, Leiby BE. Variability in depressive symptoms predicts cognitive decline in age-related macular degeneration. Am J Geriatr Psych 2009;17:574–81
43. Rovner BW, Casten RJ, Leiby BE, et al. Activity loss is associated with cognitive decline in age-related macular degeneration. Thomas Jefferson University, Department of Psychiatry and Human Behavior, Faculty Papers. Paper 5. 2009.
44. Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psych Aging 2005;20:423–34.
45. Antonucci TC. Social relations. An examination of social networks, social support, and sense of control. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5th ed. San Diego: Academic Press; 2001: 427–53.
46. Reinhardt JP. The importance of friendship and family support in adaptation to chronic vision impairment. J Gerontol Series B Psychol Sci Soc Sci 1996;51B:P268–P78.
47. Wagner M, Schütze Y, Lang FR. Social relationships in old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study Aging from 70 to 100. Cambridge, UK: Cambridge University Press; 1999: 282–301.
48. Wahl H-W, Tesch-Römer C. Aging, sensory loss, and social functioning. In: Charness N, Park D, Sabel B, editors. Aging and communication: Opportunities and challenges of technology. New York: Springer; 2001: 108-26.
49. Nachtegaal J, Smit JH, Smits C, et al. The association between hearing status and psychosocial health before the age of 70 years: Results from an internet-based national survey on hearing. Ear Hearing 2009;30:302-12
50. Wallhagen MI. The stigma of hearing loss. Gerontologist 2010;50:66–75.
51. Cimarolli VR, Boerner K. Social support and well-being in adults who are visually impaired. J Vis Impair Blind 2005 99:521–34.
52. Cicirelli VG. Fear of death in mid-old age. J Gerontol Series B Psychol Sci Soc Sci 2006;61:P75–P81.
53. Horowitz A, Reinhardt JP. Depression among low vision elders. In: Stuen. C, Arditi A, Horowitz A, et al, editors. Vision rehabilitation assessment, intervention and outcomes. Lisse: Swets & Zeitlinger Publishers; 2000: 655–8.
54. Pinquart M, Pfeiffer JP. Psychological well-being in visually impaired and unimpaired individuals: A meta-analysis. Br J Vis Impair 2011;29:27–45.
55. Tesch-Römer C. Schwerhörigkeit im Alter. Belastung, Bewältigung, Rehabilitation. Heidelberg: Median-Verlag; 2001.
56. Kiely KM, Anstey KJ, Luszcz MA. Dual sensory loss and depressive symptoms: the importance of hearing, daily functioning, and activity engagement. Front Hum Neurosci 2013;7:1–13.
57. Schilling OK, Wahl H-W, Horowitz A, et al. The adaptation dynamics of chronic functional impairment: What we can learn from older adults with vision loss. Psychol Aging 2011;26:203–13.
58. Kunzmann U, Little T, Smith J. Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study. Psychol Aging 2000;15:511-26.
59. Schilling O, Wahl H-W. Modeling late life adaptation in affective well-being under a severe chronic health condition: The case of age-related macular degeneration. Psychol Aging. 2006;21:703–14.
60. Wahl H-W, Schilling O, Becker S, et al. A German research program on the psychosocial adaptation to age-related vision impairment: Recent findings based on a control theory approach. Europ Psychol 2003;8:168-77.
61. Casten R, Rovner B. Depression in age-related macular degeneration. J Visual Impair Blind 2008;102:591-9.
62. Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Pub Health 2004;94:823–9.
63. Horowitz A, Reinhardt JP, Boerner K. The effect of rehabilitation on depression among visually disabled older adults. Aging Mental Health 2005;9:563–70.
64. Cimarolli VR. Perceived overprotection and distress in adults with visual impairment. Rehabil Psychol 2006;51:338-45.
65. Ishine M, Okumiya K, Matsubayashi K. A close association between hearing impairment and activities of daily living, depression, and quality of life in community-dwelling older people in Japan. J Am Geriatr Soc 2007;55:316-7.
66. Gopinath B, Wang JJ, Schneider J, et al. Depressive symptoms in older adults with hearing impairments: The Blue Mountains Study. J Am Geriatr Soc 2009;57:1306-8.
67. Chou K-L, Chi I. Combined effect of vision and hearing impairment on depression in elderly Chinese. Int J Geriatr Psych 2004;19:825-32.
68. Tambs K. Moderate effects of hearing loss on mental health and subjective well-being: Results from the Nord-Trøndelag Hearing Loss Study. Psychosom Med 2004;66:776-82.
69. Bernabei V, Morini V, Moretti F, et al. Vision and hearing impairments are associated with depressive--anxiety syndrome in Italian elderly. Aging Ment Health 2011 15:467-74.
70. Wahl H-W, Heyl V, Langer N. Lebensqualität bei Seheinschränkung im Alter: Das Beispiel altersabhängige Makuladegeneration [Quality of life by limited vision in old age: the example of age-related macula degeneration]. Der Ophthalmologe 2008;105:735-43.
71. Wahl H-W, Kämmerer A, Holz F, et al. Psychosocial intervention for age-related macular degeneration: A pilot project. J Vis Impair Blind 2006;100:533-44.
72. Rees G, Keeffe JE, Hassell J, et al. A self-management program for low vision: Program overview and pilot evaluation. Disabil Rehab 2010;32:808-15.
73. Rovner BW, Casten RJ. Preventing late-life depression in age-related macular degeneration. Am J Geriatr Psych 2008;16:454-9
74. Eklund K, Sonn U, Nystedt P, et al. A cost-effectiveness analysis of a health education programme for elderly persons with age-related macular degeneration: A longitudinal study. Disabil Rehab 2005;27:1203-12.
75. Pijnacker J, Verstraten P, van Damme W, et al. Rehabilitation of reading in older individuals with macular degeneration: A review of effective training programs. Aging Neuropsych Cognition. 2011;18:708-32.
76. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci 2003;14:125-30.
77. Kovács É, Tóth K, Dénes L, et al. Effects of exercise programs on balance in older women with age-related visual problems: A pilot study. Arch Gerontol Geriatr 2012;55:446-52.
78. McDonnall MC. Physical status as a moderator of depressive symptoms among older adults with dual sensory loss. Rehab Psychol 2011;56:67-76.
79. Chen EW, Fu ASN, Chan KM, et al. The effects of Tai Chi on the balance control of elderly persons with visual impairment: a randomised clinical trial. Age Ageing 2012;41:254-9.
80. Miszko TA, Ramsey VK, Blasch BB. Tai Chi for people with visual impairments: a pilot study. J Vis Impair Blind 2004;98:5-13.
81. Ray C, Horvat M, Keen K, et al. Using Tai Chi as an exercise intervention for improving balance in adults with visual impairments: Two case studies. RE:view 2005;37:17-24.
82. Jeter PE, Dagnelie G, Khalsa SBS, et al. Yoga for persons with severe visual impairment: a feasibility study. Altern Med Stud 2012;2:18-25.
83. Surakka A, Kivela T. Motivating visually impaired and deaf-blind people to perform regular physical exercises. Br J Visual Impair 2008;26:255-68.
84. Li-Korotky H-S. Age-related hearing loss: Quality of care for quality of life. Gerontologist 2012;52:265-71.
85. Chisolm TH, Johnson CE, Danhauer JL, et al. A systematic review of health-related quality of life and hearing aids: Final report of the American Academy of Audiology Task Force on the Health-Related Quality of Life Benefits of Amplification in Adults. J Am Acad Audiol 2007;18:151-83.
86. McCormack A, Fortnum H. Why do people fitted with hearing aids not wear them? Int J Audiol 2013 52:360-8.
87. Meyer C, Hickson L. What factors influence help-seeking for hearing impairment and hearing aid adoption in older adults? Int J Audiol 2012;51:66-74.
88. Andersson G, Green M, Melin L. Behavioural hearing tactics: a controlled trial of a short treatment programme. Behav Res Ther 1997;35:523-30.
89. Andersson G, Melin L, Scott B, et al. Behavioural counselling for subjects with acquired hearing loss. A new approach to hearing tactics. Scandinav Audiol 1994;23:249-56.
90. Burk MH, Humes LE. Effects of long-term training on aided speech-recognition performance in noise in older adults. J Speech Lang Hear Res 2008;51:759-71.
91. Henderson Sabes J, Sweetow RW. Variables predicting outcomes on listening and communication enhancement (LACETM) training. Int J Audiol 2007;46:374-83.
92. Kramer SE, Allessie GH, Dondorp AW, et al. A home education program for older adults with hearing impairment and their significant others: a randomized trial evaluating short- and long-term effects. Int J Audiol 2005;44:255-64.
93. Hickson L, Worrall L, Scarinci N. Measuring outcomes of a communication program for older people with hearing impairment using the International Outcome Inventory. Int J Audiol 2006;45:238-46.
94. Barcroft J, Sommers MS, Tye-Murray N, et al. Tailoring auditory training to patient needs with single and multiple talkers: Transfer-appropriate gains on a four-choice discrimination test. Int J Audiol 2011;50:802-8.
95. Wahl H-W. The psychological challenge of late-life vision impairment: Concepts, Findings, and practical implications. J Ophthalmol 2013.
96. Lin FR. Hearing loss in older adults. Who’s listening? JAMA 2012;307:1147-8.
97. Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296:2805-14.
98. Roets-Merken LM, Graff MJL, Zuidema SU, et al. Effectiveness of a self-management program for dual sensory impaired seniors in aged care settings: study protocol for a cluster randomized controlled trial. Trials 2013;14.
99. Vreeken HL, van Rens GHMB, Kramer SE, et al. Dual sensory loss: development of a dual sensory loss protocol and design of a randomized controlled trial. BMC Geriatr 2013;13.
100. Rees G, Saw C, Larizza M, et al. Should family and friends be involved in group-based rehabilitation programs for adults with low vision? Br J Visual Impair 2007;25:155-68.
101. Birk T, Hickl S, Wahl H-W, et al. Development and pilot evaluation of a psychosocial intervention program for patients with age-related macular degeneration. Gerontologist 2004;44:836-43.
102. Bradley P, Mitchell J, Bradley C, editors. Peer support for people newly diagnosed with macular degeneration: a pilot study. International Congress Vision 2005 September. London, UK.
103. Brody B, Williams R, Thomas R, et al. Age-related macular degeneration: A randomized clinical trial of a self-management intervention. Ann Behavi Med 1999;21:322-9.
104. Brody BL, Roch-Levecq A-C, Gamst AC, et al. Self-management of age-related macular degeneration and quality of life: a randomized controlled trial. Arch Ophthalmol 2002;120:1477-83.
105. Brody BL, Roch-Levecq A-C, Thomas RG, et al. Self-management of age-related macular degeneration at the 6-month follow-up. a randomized controlled trial. Arch Ophthalmol 2005;123:46-53.
106. Brody BL, Roch-Levecq A-C, Kaplan RM, et al. Age-related macular degeneration: self-management and reduction of depressive symptoms in a randomized, controlled study. J Am Geriatr Soc 2006;54:1557-62.
107. Dahlin-Ivanoff S, Sonn U, Svensson E. A health education program for elderly persons with visual impairments and perceived security in the performance of daily occupations: a randomized study. Am J Occup Thera 2002;56:322-30.
108. Eklund K, Sonn U, Dahlin-Ivanoff S. Long-term evaluation of a health education programme for elderly persons with visual impairment. A randomized study. Disabil Rehab 2004;26:401-9.
109. Eklund K, Dahlin-Ivanoff S. Health education for people with macular degeneration: Learning experiences and the effect on daily occupations. Can J Occup Ther 2006;73:272-80.
110. Eklund K, Sjöstrand J, Dahlin-Ivanoff S. A randomized controlled trial of a health-promotion programme and its effect on ADL dependence and self-reported health problems for the elderly visually impaired. Scand J Occupat Ther 2008;15:68-74.
111. Kämmerer A, Wahl H-W, Becker S, et al. Psychosoziale Unterstützung von älteren Menschen mit einer chronischen Sehbeeinträchtigung: Anwendung und Überprüfung einer problemlöse- und einer emotionsfokussierten Kurzintervention. Zeitschrift für Gesundheitspsychologie 2006;14:95-105.
112. Rovner BW, Casten RJ, Hegel MT, et al. Preventing depression in age-related macular degeneration. Arch Gen Psychiatr 2007;64:886-92.
113. Rovner BW, Casten RJ, Hegel MT, et al. Improving function in age-related macular degeneration: A randomized clinical trial. Ophthalmology 2013;120:1649-55.
114. Barcroft J, Mauzé E, Schroy C, et al. Improving the quality of auditory training by making tasks meaningful. Persp Audiol 2011;7:15-28.
1. Oeppen J, Vaupel JW. Broken limits to life expectancy. Science 2002;296:1029–31.
2. Eichenbaum JW. Geriatric vision loss due to cataracts, macular degeneration, and glaucoma. Mt Sinai J Med 2012;79:276–94.
3. Ciorba A, Bianchini C, Pelucchi S, et al. The impact of hearing loss on the quality of life of elderly adults. Clin Interven Aging 2012;7:159–63.
4. WHO - World Health Organization and International Agency for the Prevention of Blindness. Developing an action plan to prevent blindness at national, provincial and district levels. Vision 2020 The right to sight. Geneva: World Health Organization; 2004.
5. WHO - World Health Organizaton. Grades of hearing impairment. Geneva: World Health Organization; 2013.
6. Schneider JM, Gopinath B, McMahon CM, et al. Dual sensory impairment in older age. J Aging Health 2011;23:1309–24.
7. Clay OJ, Wadley VG, Edwards JD, et al. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: current and future implications. Optom Vis Sci 2005;82:724–31.
8. Seland JH, Vingerling JR, Augood CA, et al. Visual Impairment and quality of life in the Older European Population, the EUREYE study. Acta Ophthal 2011;89:608–13.
9. Horowitz A, Brennan M, Reinhardt JP. Prevalence and risk factors for self-reported visual impairment among middle-aged and older adults. Research Aging 2005;27:307–26.
10. Gopinath B, Rochtchina E, Wang JJ, et al. Prevalence of age-related hearing loss in older adults: Blue mountains study. Arch Intern Med 2009;169:415–6.
11. Roth TN, Hanebuth D, Probst R. Prevalence of age-related hearing loss in Europe: a review. Eur Arch Otorhinolaryngol 2011;268:1101–7.
12. Lin FR, Thorpe R, Gordon-Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol Series A: Biolog Sci Med Sci 2011;66A:582–90.
13. Brennan M, Horowitz A, Su Y-p. Dual sensory loss and its impact on everyday competence. Gerontologist 2005;45:337–46.
14. Anstey KJ, Luszcz MA, Giles LC, et al. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging 2001;16:3–11.
15. Appollonio I, Carabellese C, Magni E, et al. Sensory impairments and mortality in an elderly community population: A six-year follow-up study. Age Ageing 1995;24:30–6.
16. McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol 2001;85:322–6.
17. Ostbye T, Steenhuis R, Wolfson C, et al. Predictors of five-year mortality in older Canadians: the Canadian Study of Health and Aging. J Am Geriatr Soc 1999 47:1249–54.
18. Wahl H-W, Heyl V, Drapaniotis PM, et al. Severe vision and hearing impairment and successful aging: A multidimensional view. Gerontologist 2013;53:950–62.
19. Fisher D, Li C-M, Chiu MS, et al. Impairments in hearing and vision impact on mortality in older people: the AGES-Reykjavik Study. Age Ageing 2013.
20. Bamini G, Schneider J, McMahon CM, et al. Dual sensory impairment in older adults inceases the risk of mortality: A population-based study. PLoS ONE 2013;8:1–6.
21. Lam BL, Lee DJ, Gómez-Marín O, et al. Concurrent visual and hearing impairment and risk of mortality: The National Health Interview Survey. Arch Ophthalmol 2006;124:95–101.
22. Baltes MM, Maas I, Wilms H-U, et al. Everyday competence in old and very old age: Theoretical considerations and empirical findings. In: Baltes PB, Mayer K-U, editors. The Berlin Aging Study Cambridge, UK: Cambridge University Press; 1999: 384–402.
23. Burmedi D, Becker S, Heyl V, et al. Behavioral consequences of age-related low vision: A narrative review. Vis Impair Res 2002;4:15–45.
24. Wahl H-W, Heyl V, Schilling O. Robustness of personality and affect relations under chronic conditions: the case of age-related vision and hearing impairment. J Gerontol B Psycholog Sci Soc Sci 2012;67:687–96.
25. Heyl V, Wahl H-W. On the long-term psychosocial adaptation to vision loss in the later years. In: Wahl H-W, Schulze H-E, editors. On the special needs of blind and low vision seniors: Research and practice concepts. Amsterdam: IOS-Press; 2001: 77–83.
26. Wahl H-W, Schilling O, Oswald F, et al. Psychosocial consequences of age-related visual impairment: Comparison with mobility-impaired older adults and long-term outcome. J Gerontol Psych Sci Soc Sci 1999;54B:P304–P16.
27. Wahl H-W, Becker S, Burmedi D, et al. The role of primary and secondary control in adaptation to age-related vision loss: A study of older adults with macular degeneration Psychol Aging 2004;19:235–9.
28. Rudberg MA, Furner SE, Dunn JE, et al. The relationship of visual and hearing impairments to disability: An analysis using the Longitudinal Study of Aging. J Gerontol Med Sci 1993;48:M261–M5.
29. Schneider J, Gopinath B, Karpa MJ, et al. Hearing loss impacts on the use of community and informal supports. Age Ageing 2010;39:458–64.
30. Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute visual function questionnaire. Arch Ophthalmol 2001;119:1050–8.
31. Ventry I, Weinstein B. The hearing handicap inventory for the elderly: A new tool. Ear Hearing 1982;3:128–34.
32. Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol Aging 1994;9:339–55.
33. Clay OJ, Edwards JD, Ross LA, et al. Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. J Aging Health 2009;21:547–66.
34. Salthouse TA, Hancock HE, Meinz EJ, et al. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol Psychol Sci 1996;51B:P317–P30.
35. Baltes PB, Lindenberger U. Emergence of powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging 1997;12:12–21.
36. Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 2004;52:1996–2002.
37. Tay T, Wang JJ, Kifley A, et al. Sensory and cognitive association in older persons: Findings from an older Australian population. Gerontology 2006;52:386–94.
38. Heyl V, Wahl H-W. Managing daily life with age-related sensory loss: Cogni-tive resources gain in importance. Psychol Aging 2012;27:510–21.
39. Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychol Aging 2009;24:1-16.
40. Heyl V, Wahl H-W, Mollenkopf H. Visual capacity, out-of-home activities and emotional well-being in old age: Basic relations and contextual variation. Soc Indicat Res 2005;74:159–89.
41. Heyl V, Wahl H-W. Cognitive ability as a resource for everyday functioning among older adults who are visually impaired. J Vis Impair Blind 2010;104:391–403.
42. Rovner BW, Casten RJ, Leiby BE. Variability in depressive symptoms predicts cognitive decline in age-related macular degeneration. Am J Geriatr Psych 2009;17:574–81
43. Rovner BW, Casten RJ, Leiby BE, et al. Activity loss is associated with cognitive decline in age-related macular degeneration. Thomas Jefferson University, Department of Psychiatry and Human Behavior, Faculty Papers. Paper 5. 2009.
44. Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psych Aging 2005;20:423–34.
45. Antonucci TC. Social relations. An examination of social networks, social support, and sense of control. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5th ed. San Diego: Academic Press; 2001: 427–53.
46. Reinhardt JP. The importance of friendship and family support in adaptation to chronic vision impairment. J Gerontol Series B Psychol Sci Soc Sci 1996;51B:P268–P78.
47. Wagner M, Schütze Y, Lang FR. Social relationships in old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study Aging from 70 to 100. Cambridge, UK: Cambridge University Press; 1999: 282–301.
48. Wahl H-W, Tesch-Römer C. Aging, sensory loss, and social functioning. In: Charness N, Park D, Sabel B, editors. Aging and communication: Opportunities and challenges of technology. New York: Springer; 2001: 108-26.
49. Nachtegaal J, Smit JH, Smits C, et al. The association between hearing status and psychosocial health before the age of 70 years: Results from an internet-based national survey on hearing. Ear Hearing 2009;30:302-12
50. Wallhagen MI. The stigma of hearing loss. Gerontologist 2010;50:66–75.
51. Cimarolli VR, Boerner K. Social support and well-being in adults who are visually impaired. J Vis Impair Blind 2005 99:521–34.
52. Cicirelli VG. Fear of death in mid-old age. J Gerontol Series B Psychol Sci Soc Sci 2006;61:P75–P81.
53. Horowitz A, Reinhardt JP. Depression among low vision elders. In: Stuen. C, Arditi A, Horowitz A, et al, editors. Vision rehabilitation assessment, intervention and outcomes. Lisse: Swets & Zeitlinger Publishers; 2000: 655–8.
54. Pinquart M, Pfeiffer JP. Psychological well-being in visually impaired and unimpaired individuals: A meta-analysis. Br J Vis Impair 2011;29:27–45.
55. Tesch-Römer C. Schwerhörigkeit im Alter. Belastung, Bewältigung, Rehabilitation. Heidelberg: Median-Verlag; 2001.
56. Kiely KM, Anstey KJ, Luszcz MA. Dual sensory loss and depressive symptoms: the importance of hearing, daily functioning, and activity engagement. Front Hum Neurosci 2013;7:1–13.
57. Schilling OK, Wahl H-W, Horowitz A, et al. The adaptation dynamics of chronic functional impairment: What we can learn from older adults with vision loss. Psychol Aging 2011;26:203–13.
58. Kunzmann U, Little T, Smith J. Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study. Psychol Aging 2000;15:511-26.
59. Schilling O, Wahl H-W. Modeling late life adaptation in affective well-being under a severe chronic health condition: The case of age-related macular degeneration. Psychol Aging. 2006;21:703–14.
60. Wahl H-W, Schilling O, Becker S, et al. A German research program on the psychosocial adaptation to age-related vision impairment: Recent findings based on a control theory approach. Europ Psychol 2003;8:168-77.
61. Casten R, Rovner B. Depression in age-related macular degeneration. J Visual Impair Blind 2008;102:591-9.
62. Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Pub Health 2004;94:823–9.
63. Horowitz A, Reinhardt JP, Boerner K. The effect of rehabilitation on depression among visually disabled older adults. Aging Mental Health 2005;9:563–70.
64. Cimarolli VR. Perceived overprotection and distress in adults with visual impairment. Rehabil Psychol 2006;51:338-45.
65. Ishine M, Okumiya K, Matsubayashi K. A close association between hearing impairment and activities of daily living, depression, and quality of life in community-dwelling older people in Japan. J Am Geriatr Soc 2007;55:316-7.
66. Gopinath B, Wang JJ, Schneider J, et al. Depressive symptoms in older adults with hearing impairments: The Blue Mountains Study. J Am Geriatr Soc 2009;57:1306-8.
67. Chou K-L, Chi I. Combined effect of vision and hearing impairment on depression in elderly Chinese. Int J Geriatr Psych 2004;19:825-32.
68. Tambs K. Moderate effects of hearing loss on mental health and subjective well-being: Results from the Nord-Trøndelag Hearing Loss Study. Psychosom Med 2004;66:776-82.
69. Bernabei V, Morini V, Moretti F, et al. Vision and hearing impairments are associated with depressive--anxiety syndrome in Italian elderly. Aging Ment Health 2011 15:467-74.
70. Wahl H-W, Heyl V, Langer N. Lebensqualität bei Seheinschränkung im Alter: Das Beispiel altersabhängige Makuladegeneration [Quality of life by limited vision in old age: the example of age-related macula degeneration]. Der Ophthalmologe 2008;105:735-43.
71. Wahl H-W, Kämmerer A, Holz F, et al. Psychosocial intervention for age-related macular degeneration: A pilot project. J Vis Impair Blind 2006;100:533-44.
72. Rees G, Keeffe JE, Hassell J, et al. A self-management program for low vision: Program overview and pilot evaluation. Disabil Rehab 2010;32:808-15.
73. Rovner BW, Casten RJ. Preventing late-life depression in age-related macular degeneration. Am J Geriatr Psych 2008;16:454-9
74. Eklund K, Sonn U, Nystedt P, et al. A cost-effectiveness analysis of a health education programme for elderly persons with age-related macular degeneration: A longitudinal study. Disabil Rehab 2005;27:1203-12.
75. Pijnacker J, Verstraten P, van Damme W, et al. Rehabilitation of reading in older individuals with macular degeneration: A review of effective training programs. Aging Neuropsych Cognition. 2011;18:708-32.
76. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci 2003;14:125-30.
77. Kovács É, Tóth K, Dénes L, et al. Effects of exercise programs on balance in older women with age-related visual problems: A pilot study. Arch Gerontol Geriatr 2012;55:446-52.
78. McDonnall MC. Physical status as a moderator of depressive symptoms among older adults with dual sensory loss. Rehab Psychol 2011;56:67-76.
79. Chen EW, Fu ASN, Chan KM, et al. The effects of Tai Chi on the balance control of elderly persons with visual impairment: a randomised clinical trial. Age Ageing 2012;41:254-9.
80. Miszko TA, Ramsey VK, Blasch BB. Tai Chi for people with visual impairments: a pilot study. J Vis Impair Blind 2004;98:5-13.
81. Ray C, Horvat M, Keen K, et al. Using Tai Chi as an exercise intervention for improving balance in adults with visual impairments: Two case studies. RE:view 2005;37:17-24.
82. Jeter PE, Dagnelie G, Khalsa SBS, et al. Yoga for persons with severe visual impairment: a feasibility study. Altern Med Stud 2012;2:18-25.
83. Surakka A, Kivela T. Motivating visually impaired and deaf-blind people to perform regular physical exercises. Br J Visual Impair 2008;26:255-68.
84. Li-Korotky H-S. Age-related hearing loss: Quality of care for quality of life. Gerontologist 2012;52:265-71.
85. Chisolm TH, Johnson CE, Danhauer JL, et al. A systematic review of health-related quality of life and hearing aids: Final report of the American Academy of Audiology Task Force on the Health-Related Quality of Life Benefits of Amplification in Adults. J Am Acad Audiol 2007;18:151-83.
86. McCormack A, Fortnum H. Why do people fitted with hearing aids not wear them? Int J Audiol 2013 52:360-8.
87. Meyer C, Hickson L. What factors influence help-seeking for hearing impairment and hearing aid adoption in older adults? Int J Audiol 2012;51:66-74.
88. Andersson G, Green M, Melin L. Behavioural hearing tactics: a controlled trial of a short treatment programme. Behav Res Ther 1997;35:523-30.
89. Andersson G, Melin L, Scott B, et al. Behavioural counselling for subjects with acquired hearing loss. A new approach to hearing tactics. Scandinav Audiol 1994;23:249-56.
90. Burk MH, Humes LE. Effects of long-term training on aided speech-recognition performance in noise in older adults. J Speech Lang Hear Res 2008;51:759-71.
91. Henderson Sabes J, Sweetow RW. Variables predicting outcomes on listening and communication enhancement (LACETM) training. Int J Audiol 2007;46:374-83.
92. Kramer SE, Allessie GH, Dondorp AW, et al. A home education program for older adults with hearing impairment and their significant others: a randomized trial evaluating short- and long-term effects. Int J Audiol 2005;44:255-64.
93. Hickson L, Worrall L, Scarinci N. Measuring outcomes of a communication program for older people with hearing impairment using the International Outcome Inventory. Int J Audiol 2006;45:238-46.
94. Barcroft J, Sommers MS, Tye-Murray N, et al. Tailoring auditory training to patient needs with single and multiple talkers: Transfer-appropriate gains on a four-choice discrimination test. Int J Audiol 2011;50:802-8.
95. Wahl H-W. The psychological challenge of late-life vision impairment: Concepts, Findings, and practical implications. J Ophthalmol 2013.
96. Lin FR. Hearing loss in older adults. Who’s listening? JAMA 2012;307:1147-8.
97. Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296:2805-14.
98. Roets-Merken LM, Graff MJL, Zuidema SU, et al. Effectiveness of a self-management program for dual sensory impaired seniors in aged care settings: study protocol for a cluster randomized controlled trial. Trials 2013;14.
99. Vreeken HL, van Rens GHMB, Kramer SE, et al. Dual sensory loss: development of a dual sensory loss protocol and design of a randomized controlled trial. BMC Geriatr 2013;13.
100. Rees G, Saw C, Larizza M, et al. Should family and friends be involved in group-based rehabilitation programs for adults with low vision? Br J Visual Impair 2007;25:155-68.
101. Birk T, Hickl S, Wahl H-W, et al. Development and pilot evaluation of a psychosocial intervention program for patients with age-related macular degeneration. Gerontologist 2004;44:836-43.
102. Bradley P, Mitchell J, Bradley C, editors. Peer support for people newly diagnosed with macular degeneration: a pilot study. International Congress Vision 2005 September. London, UK.
103. Brody B, Williams R, Thomas R, et al. Age-related macular degeneration: A randomized clinical trial of a self-management intervention. Ann Behavi Med 1999;21:322-9.
104. Brody BL, Roch-Levecq A-C, Gamst AC, et al. Self-management of age-related macular degeneration and quality of life: a randomized controlled trial. Arch Ophthalmol 2002;120:1477-83.
105. Brody BL, Roch-Levecq A-C, Thomas RG, et al. Self-management of age-related macular degeneration at the 6-month follow-up. a randomized controlled trial. Arch Ophthalmol 2005;123:46-53.
106. Brody BL, Roch-Levecq A-C, Kaplan RM, et al. Age-related macular degeneration: self-management and reduction of depressive symptoms in a randomized, controlled study. J Am Geriatr Soc 2006;54:1557-62.
107. Dahlin-Ivanoff S, Sonn U, Svensson E. A health education program for elderly persons with visual impairments and perceived security in the performance of daily occupations: a randomized study. Am J Occup Thera 2002;56:322-30.
108. Eklund K, Sonn U, Dahlin-Ivanoff S. Long-term evaluation of a health education programme for elderly persons with visual impairment. A randomized study. Disabil Rehab 2004;26:401-9.
109. Eklund K, Dahlin-Ivanoff S. Health education for people with macular degeneration: Learning experiences and the effect on daily occupations. Can J Occup Ther 2006;73:272-80.
110. Eklund K, Sjöstrand J, Dahlin-Ivanoff S. A randomized controlled trial of a health-promotion programme and its effect on ADL dependence and self-reported health problems for the elderly visually impaired. Scand J Occupat Ther 2008;15:68-74.
111. Kämmerer A, Wahl H-W, Becker S, et al. Psychosoziale Unterstützung von älteren Menschen mit einer chronischen Sehbeeinträchtigung: Anwendung und Überprüfung einer problemlöse- und einer emotionsfokussierten Kurzintervention. Zeitschrift für Gesundheitspsychologie 2006;14:95-105.
112. Rovner BW, Casten RJ, Hegel MT, et al. Preventing depression in age-related macular degeneration. Arch Gen Psychiatr 2007;64:886-92.
113. Rovner BW, Casten RJ, Hegel MT, et al. Improving function in age-related macular degeneration: A randomized clinical trial. Ophthalmology 2013;120:1649-55.
114. Barcroft J, Mauzé E, Schroy C, et al. Improving the quality of auditory training by making tasks meaningful. Persp Audiol 2011;7:15-28.
Using Patient Navigators to Help Adults with Sickle Cell Disease Obtain a Primary Care Home
From the Colorado Sickle Cell Network, University of Colorado School of Medicine, Aurora, CO.
This article is the fifth in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the development and implementation of a patient navigation program to help individuals with sickle cell disease (SCD) overcome barriers to finding adult primary care.
- Methods: Six patient navigators were recruited and received training. A workgroup was formed to clarify goals and objectives and develop standard procedures. Navigators were instrumental in establishing a network of primary care offices that were willing to accept new patients with SCD. Navigators assisted patients in making calls to primary care offices and in some cases would attend appointments with them.
- Results: About two-thirds of patients who were referred to the navigator program for primary care follow-up attended an initial appointment with a new primary care provider.
- Conclusion: Patient navigation is a feasible and useful strategy to help individuals with SCD overcome barriers to receiving comprehensive care.
With advances in the management of sickle cell disease (SCD), adults with SCD are living longer [1,2]. Adequate care for individuals with SCD requires that they receive both specialized services and comprehensive primary care. A lack of comprehensive outpatient care can translate into suboptimal outcomes and increased reliance on the emergency room [3].
In the metropolitan area of Denver, specialty care for individuals with SCD is centralized and easily accessible at a tertiary academic medical center. However, we found that many adult patients treated in our specialty setting had not established care with an adult primary care provider (PCP) or had not been seen regularly by their PCP for ongoing preventive primary care services. Thus, they were not getting their comprehensive care needs met. Although support was available from community-based organizations to help them access certain resources (eg, directions to the food bank), patients reported difficulties in accessing the adult care health system, for example, securing appointments with PCPs and securing/maintaining insurance. No services existed to specifically help them navigate through the complexities of obtaining needed care.
Patient navigation is a strategy commonly used in cancer care settings [4–7] to to help patients overcome barriers in accessing the health care system. Patient navigators can not only facilitate improved health care access and quality for underserved populations through advocacy and care coordination, but they can also address the information needs of patients and assist in overcoming language and cultural barriers. Navigation has been proposed as a strategy to help reduce health disparities [8].
We developed a patient navigation program to address unmet needs of children and adults with SCD receving care in our clinics. In this paper we describe our program.
Patient Navigator Program
Setting
The SCD Treatment Demonstration Program was created in 2004 by the federal government to improve care and outcomes for persons with SCD [9]. As a grantee of this program, we developed the Colorado Sickle Cell Care Network (CSCCN) to care for scd patients in the Denver metropolitan and surrounding area. The CSCCN is a collaboration between the Colorado Sickle Cell Treatment and Research Center and the Division of General Internal Medicine and Department of Hematology at the University of Colorado Denver Anschutz Medical Campus, the Center for Cancer and Blood Disorders at Children’s Hospital Colorado, and 2 community-based organizations. With other grantees, we are participating in the Hemoglobinopathy Learning Collaborative, a collaborative of teams utilizing iterative cycles of testing to learn what changes can be made to improve care processes [10].
Navigators
We developed the patient navigator program to help patients overcome barriers to receiving comprehensive care in a primary care medical home. We hired 4 patient navigators to serve individuals living in or seeking resources in the Denver metropolitan area. Persons interested in being navigators were required to first qualify to be official hospital volunteers at the University of Colorado Hospital and Children’s Hospital Colorado, a process that involved attending an orientation and obtaining official facility badging. They received patient navigation training at the Harold P. Freeman Patient Navigation Institute in New York [11] as well as completed the Colorado Patient Navigator Training Program [12]. Navigators in training learned about the history of patient navigation, health promotion and communication models, motivational interviewing techniques, and systemic and individual barriers to care. All patient navigators received training in HIPAA and went through a volunteer credentialing process at the hospital; however they do not have access to patient’s medical records or the electronic health records.
The navigators are from various backgrounds, and 2 of our patient navigators are bilingual Spanish-English speakers, enhancing our ability to outreach to individuals whose preferred language is Spanish and who may otherwise not be able to access available resources. Some of our patient navigators are family members of individuals being treated for SCD and are able to provide a unique perspective that aids program development.
Once training for the initial group of navigators was completed, a patient navigation workgroup was created to clarify goals and objectives, develop standard procedures, and define navigator responsibilities. This workgroup included the CSCCN program staff, adult and pediatric hematology trained SCD specialists, and the pediatric coordinator. We used the Hemoglobinopathy Learning Collaborative process [10] to develop and refine a process map for referrals made for primary care. Process mapping was an iterative process with regular input from the navigators and other members of the patient navigation workgroup, as well as input from case managers and social workers when needed.
Process
Upon receiving a referral, navigators made contact with the patient within 1 business day and obtained preferred contact information. The navigator completed a patient intake form and needs assessment. Each patient referral was logged into a secure database. Most referrals were generated by our specialty health care providers, but as awareness of the program grew referrals also came from the community-based organizations as well as self-referrals from patients or caregivers. Most of the referrals (68%) were specifically for assistance in finding a primary care medical home. Other reasons patients were referred to the navigator program included needing assistance with housing, financial assistance, or insurance application questions.
Barriers Addressed
Patient navigators spent much of their time proactively seeking local sources of adult primary care for clients and in doing so established a network of primary care offices in the community that would be able and willing to accept new patients with SCD. This was accomplished through personal outreach and communication with stakeholders at the academic medical center and in the community, utilizing skills learned during patient navigation training and by sorting through vast informational resources available to the public. When feasible the navigators, accompanied by the project director and adult hematology specialist, personally visited sites of primary care in the community to help provide information about the CSCCN and establish a working relationship.
Our navigators would make calls together with the patient to make a PCP appointment and would remind them of upcoming PCP appointments. In some cases, navigators would attend appointments with the patient; in addition, they would help advocate for them when they had to go to the emergency department. If a patient missed a PCP appointment, the navigator followed up to find out why and how to secure another appointment. Navigators would follow-up every 10 days if a patient had not yet seen a PCP. As many of our patients did not understand why they needed to have a PCP when they had a specialist, the navigators educated patients on the importance of PCP care.
In additon, navigators helped our patients deal with insurance coverage problems, such as frequent insurance changes/insurance getting dropped. Some patients had low literacy or had difficulties in filling out disability or insurance forms properly. Navigators received training from state coordinators for Medicaid and SSI disability on filling out the respective forms, which can be confusing.
Our navigators were trained in motivational interviewing and used it to identify other barriers patients were facing. In trying to obtain proper care, our patients struggled with competing priorities (eg, food, shelter, child care). Because of the numerous challenges that our patients and families face, an important role for the patients navigators was creating a bridge to accessing social and community resources.
Outcomes
The help and support provided by the navigators is helpful in mitigating the mistrust our patients have about the health care system, in part due to unfavorable initial experiences as young adults or being stereotyped for pain issues. Our patients have said that navigators make them feel like they have an advocate—someone who is on their side.
Subsequent to forming the initial navigator group, we expanded our program by adding 2 patient navigators in Colorado Springs, a smaller metropolitan area about an hour and a half south of Denver. In Colorado Springs, 16 referrals for services have been made but none of these referrals were specifically for a PCP, since access to a PCP was not a barrier to care in that locale. Patients were more likely to need help with things such as transportation to get to their location of specialty care.
Conclusion
Coordination of care is essential for individuals with chronic diseases. While the focus to date has been on coordination of care for common chronic illness such as diabetes, it is essential to use best practices for individuals with less common, but often more complex, chronic diseases such as SCD. To facilitate coordination of care for this population who receive specialty care in an academic medical center, we developed a patient navigation program for adults with SCD as a quality improvement project. Our navigation program assisted 62% of referred adults in the Denver metropolitan area in identifying a PCP and attending an initial appointment.
The program is continuing for the duration of the grant funding and at this time we will likely need to seek mechanisms for additional support to continue the work that has been started, as well as to consider measurement of outcomes that are meaningful to the institutions in which the program is housed in order to become more sustainable internally.
Patient navigation is an acceptable and feasible way to enhance the care for adults with SCD and can help to bridge systems of clinical care, support and resources. Our program can serve as a model for similar patient populations with orphan chronic diseases that have both primary care and subspecialty needs that have both distinct and overlapping roles. Sustainability is a crucial issue to address and the use of outcome measures that can accurately reflect both successes and challenges of program implementation will be important to share with institutional leaders.
Corresponding author: Linda S. Overholser, MD, MPH, 12631 E 17th Ave., Mail Stop B180, Academic Office 1, Aurora, CO 80045, [email protected].
1. Prabhakar H, Haywood C Jr, Molokie R. Sickle cell disease in the United States: looking back and forward at 100 years of progress in management and survival. Am J Hematol 2010;85:346–53.
2. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
3. Hemker BG, Brousseau DC, Yan K, et al. When children with sickle-cell disease become adults: lack of outpatient care leads to increased use of the emergency department. Am J Hematol 2011;86:863–5.
4. Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract 1995 3:19–30.
5. Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer 2011;117(15 Suppl):3539–42.
6. Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer 2011;117(15 Suppl):3543–52.
7. Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer 2005;104:848–55.
8. Chin MH, Clarke AR, Nocon RS, et al. A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med 2012;27:992–1000.
9. Grosse SD, Schechter MS, Kulkarni R, et al. Models of comprehensive multidisciplinary care for individuals in the United States with genetic disorders. Pediatrics 2009;123:407–12.
10. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34–48.
11. Harold P. Freeman Patient Navigation Institute. Available at www.hpfreemanpni.org.
12. Patient Navigator Training Collaborative. Available at www.patientnavigatortraining.org.
From the Colorado Sickle Cell Network, University of Colorado School of Medicine, Aurora, CO.
This article is the fifth in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the development and implementation of a patient navigation program to help individuals with sickle cell disease (SCD) overcome barriers to finding adult primary care.
- Methods: Six patient navigators were recruited and received training. A workgroup was formed to clarify goals and objectives and develop standard procedures. Navigators were instrumental in establishing a network of primary care offices that were willing to accept new patients with SCD. Navigators assisted patients in making calls to primary care offices and in some cases would attend appointments with them.
- Results: About two-thirds of patients who were referred to the navigator program for primary care follow-up attended an initial appointment with a new primary care provider.
- Conclusion: Patient navigation is a feasible and useful strategy to help individuals with SCD overcome barriers to receiving comprehensive care.
With advances in the management of sickle cell disease (SCD), adults with SCD are living longer [1,2]. Adequate care for individuals with SCD requires that they receive both specialized services and comprehensive primary care. A lack of comprehensive outpatient care can translate into suboptimal outcomes and increased reliance on the emergency room [3].
In the metropolitan area of Denver, specialty care for individuals with SCD is centralized and easily accessible at a tertiary academic medical center. However, we found that many adult patients treated in our specialty setting had not established care with an adult primary care provider (PCP) or had not been seen regularly by their PCP for ongoing preventive primary care services. Thus, they were not getting their comprehensive care needs met. Although support was available from community-based organizations to help them access certain resources (eg, directions to the food bank), patients reported difficulties in accessing the adult care health system, for example, securing appointments with PCPs and securing/maintaining insurance. No services existed to specifically help them navigate through the complexities of obtaining needed care.
Patient navigation is a strategy commonly used in cancer care settings [4–7] to to help patients overcome barriers in accessing the health care system. Patient navigators can not only facilitate improved health care access and quality for underserved populations through advocacy and care coordination, but they can also address the information needs of patients and assist in overcoming language and cultural barriers. Navigation has been proposed as a strategy to help reduce health disparities [8].
We developed a patient navigation program to address unmet needs of children and adults with SCD receving care in our clinics. In this paper we describe our program.
Patient Navigator Program
Setting
The SCD Treatment Demonstration Program was created in 2004 by the federal government to improve care and outcomes for persons with SCD [9]. As a grantee of this program, we developed the Colorado Sickle Cell Care Network (CSCCN) to care for scd patients in the Denver metropolitan and surrounding area. The CSCCN is a collaboration between the Colorado Sickle Cell Treatment and Research Center and the Division of General Internal Medicine and Department of Hematology at the University of Colorado Denver Anschutz Medical Campus, the Center for Cancer and Blood Disorders at Children’s Hospital Colorado, and 2 community-based organizations. With other grantees, we are participating in the Hemoglobinopathy Learning Collaborative, a collaborative of teams utilizing iterative cycles of testing to learn what changes can be made to improve care processes [10].
Navigators
We developed the patient navigator program to help patients overcome barriers to receiving comprehensive care in a primary care medical home. We hired 4 patient navigators to serve individuals living in or seeking resources in the Denver metropolitan area. Persons interested in being navigators were required to first qualify to be official hospital volunteers at the University of Colorado Hospital and Children’s Hospital Colorado, a process that involved attending an orientation and obtaining official facility badging. They received patient navigation training at the Harold P. Freeman Patient Navigation Institute in New York [11] as well as completed the Colorado Patient Navigator Training Program [12]. Navigators in training learned about the history of patient navigation, health promotion and communication models, motivational interviewing techniques, and systemic and individual barriers to care. All patient navigators received training in HIPAA and went through a volunteer credentialing process at the hospital; however they do not have access to patient’s medical records or the electronic health records.
The navigators are from various backgrounds, and 2 of our patient navigators are bilingual Spanish-English speakers, enhancing our ability to outreach to individuals whose preferred language is Spanish and who may otherwise not be able to access available resources. Some of our patient navigators are family members of individuals being treated for SCD and are able to provide a unique perspective that aids program development.
Once training for the initial group of navigators was completed, a patient navigation workgroup was created to clarify goals and objectives, develop standard procedures, and define navigator responsibilities. This workgroup included the CSCCN program staff, adult and pediatric hematology trained SCD specialists, and the pediatric coordinator. We used the Hemoglobinopathy Learning Collaborative process [10] to develop and refine a process map for referrals made for primary care. Process mapping was an iterative process with regular input from the navigators and other members of the patient navigation workgroup, as well as input from case managers and social workers when needed.
Process
Upon receiving a referral, navigators made contact with the patient within 1 business day and obtained preferred contact information. The navigator completed a patient intake form and needs assessment. Each patient referral was logged into a secure database. Most referrals were generated by our specialty health care providers, but as awareness of the program grew referrals also came from the community-based organizations as well as self-referrals from patients or caregivers. Most of the referrals (68%) were specifically for assistance in finding a primary care medical home. Other reasons patients were referred to the navigator program included needing assistance with housing, financial assistance, or insurance application questions.
Barriers Addressed
Patient navigators spent much of their time proactively seeking local sources of adult primary care for clients and in doing so established a network of primary care offices in the community that would be able and willing to accept new patients with SCD. This was accomplished through personal outreach and communication with stakeholders at the academic medical center and in the community, utilizing skills learned during patient navigation training and by sorting through vast informational resources available to the public. When feasible the navigators, accompanied by the project director and adult hematology specialist, personally visited sites of primary care in the community to help provide information about the CSCCN and establish a working relationship.
Our navigators would make calls together with the patient to make a PCP appointment and would remind them of upcoming PCP appointments. In some cases, navigators would attend appointments with the patient; in addition, they would help advocate for them when they had to go to the emergency department. If a patient missed a PCP appointment, the navigator followed up to find out why and how to secure another appointment. Navigators would follow-up every 10 days if a patient had not yet seen a PCP. As many of our patients did not understand why they needed to have a PCP when they had a specialist, the navigators educated patients on the importance of PCP care.
In additon, navigators helped our patients deal with insurance coverage problems, such as frequent insurance changes/insurance getting dropped. Some patients had low literacy or had difficulties in filling out disability or insurance forms properly. Navigators received training from state coordinators for Medicaid and SSI disability on filling out the respective forms, which can be confusing.
Our navigators were trained in motivational interviewing and used it to identify other barriers patients were facing. In trying to obtain proper care, our patients struggled with competing priorities (eg, food, shelter, child care). Because of the numerous challenges that our patients and families face, an important role for the patients navigators was creating a bridge to accessing social and community resources.
Outcomes
The help and support provided by the navigators is helpful in mitigating the mistrust our patients have about the health care system, in part due to unfavorable initial experiences as young adults or being stereotyped for pain issues. Our patients have said that navigators make them feel like they have an advocate—someone who is on their side.
Subsequent to forming the initial navigator group, we expanded our program by adding 2 patient navigators in Colorado Springs, a smaller metropolitan area about an hour and a half south of Denver. In Colorado Springs, 16 referrals for services have been made but none of these referrals were specifically for a PCP, since access to a PCP was not a barrier to care in that locale. Patients were more likely to need help with things such as transportation to get to their location of specialty care.
Conclusion
Coordination of care is essential for individuals with chronic diseases. While the focus to date has been on coordination of care for common chronic illness such as diabetes, it is essential to use best practices for individuals with less common, but often more complex, chronic diseases such as SCD. To facilitate coordination of care for this population who receive specialty care in an academic medical center, we developed a patient navigation program for adults with SCD as a quality improvement project. Our navigation program assisted 62% of referred adults in the Denver metropolitan area in identifying a PCP and attending an initial appointment.
The program is continuing for the duration of the grant funding and at this time we will likely need to seek mechanisms for additional support to continue the work that has been started, as well as to consider measurement of outcomes that are meaningful to the institutions in which the program is housed in order to become more sustainable internally.
Patient navigation is an acceptable and feasible way to enhance the care for adults with SCD and can help to bridge systems of clinical care, support and resources. Our program can serve as a model for similar patient populations with orphan chronic diseases that have both primary care and subspecialty needs that have both distinct and overlapping roles. Sustainability is a crucial issue to address and the use of outcome measures that can accurately reflect both successes and challenges of program implementation will be important to share with institutional leaders.
Corresponding author: Linda S. Overholser, MD, MPH, 12631 E 17th Ave., Mail Stop B180, Academic Office 1, Aurora, CO 80045, [email protected].
From the Colorado Sickle Cell Network, University of Colorado School of Medicine, Aurora, CO.
This article is the fifth in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the development and implementation of a patient navigation program to help individuals with sickle cell disease (SCD) overcome barriers to finding adult primary care.
- Methods: Six patient navigators were recruited and received training. A workgroup was formed to clarify goals and objectives and develop standard procedures. Navigators were instrumental in establishing a network of primary care offices that were willing to accept new patients with SCD. Navigators assisted patients in making calls to primary care offices and in some cases would attend appointments with them.
- Results: About two-thirds of patients who were referred to the navigator program for primary care follow-up attended an initial appointment with a new primary care provider.
- Conclusion: Patient navigation is a feasible and useful strategy to help individuals with SCD overcome barriers to receiving comprehensive care.
With advances in the management of sickle cell disease (SCD), adults with SCD are living longer [1,2]. Adequate care for individuals with SCD requires that they receive both specialized services and comprehensive primary care. A lack of comprehensive outpatient care can translate into suboptimal outcomes and increased reliance on the emergency room [3].
In the metropolitan area of Denver, specialty care for individuals with SCD is centralized and easily accessible at a tertiary academic medical center. However, we found that many adult patients treated in our specialty setting had not established care with an adult primary care provider (PCP) or had not been seen regularly by their PCP for ongoing preventive primary care services. Thus, they were not getting their comprehensive care needs met. Although support was available from community-based organizations to help them access certain resources (eg, directions to the food bank), patients reported difficulties in accessing the adult care health system, for example, securing appointments with PCPs and securing/maintaining insurance. No services existed to specifically help them navigate through the complexities of obtaining needed care.
Patient navigation is a strategy commonly used in cancer care settings [4–7] to to help patients overcome barriers in accessing the health care system. Patient navigators can not only facilitate improved health care access and quality for underserved populations through advocacy and care coordination, but they can also address the information needs of patients and assist in overcoming language and cultural barriers. Navigation has been proposed as a strategy to help reduce health disparities [8].
We developed a patient navigation program to address unmet needs of children and adults with SCD receving care in our clinics. In this paper we describe our program.
Patient Navigator Program
Setting
The SCD Treatment Demonstration Program was created in 2004 by the federal government to improve care and outcomes for persons with SCD [9]. As a grantee of this program, we developed the Colorado Sickle Cell Care Network (CSCCN) to care for scd patients in the Denver metropolitan and surrounding area. The CSCCN is a collaboration between the Colorado Sickle Cell Treatment and Research Center and the Division of General Internal Medicine and Department of Hematology at the University of Colorado Denver Anschutz Medical Campus, the Center for Cancer and Blood Disorders at Children’s Hospital Colorado, and 2 community-based organizations. With other grantees, we are participating in the Hemoglobinopathy Learning Collaborative, a collaborative of teams utilizing iterative cycles of testing to learn what changes can be made to improve care processes [10].
Navigators
We developed the patient navigator program to help patients overcome barriers to receiving comprehensive care in a primary care medical home. We hired 4 patient navigators to serve individuals living in or seeking resources in the Denver metropolitan area. Persons interested in being navigators were required to first qualify to be official hospital volunteers at the University of Colorado Hospital and Children’s Hospital Colorado, a process that involved attending an orientation and obtaining official facility badging. They received patient navigation training at the Harold P. Freeman Patient Navigation Institute in New York [11] as well as completed the Colorado Patient Navigator Training Program [12]. Navigators in training learned about the history of patient navigation, health promotion and communication models, motivational interviewing techniques, and systemic and individual barriers to care. All patient navigators received training in HIPAA and went through a volunteer credentialing process at the hospital; however they do not have access to patient’s medical records or the electronic health records.
The navigators are from various backgrounds, and 2 of our patient navigators are bilingual Spanish-English speakers, enhancing our ability to outreach to individuals whose preferred language is Spanish and who may otherwise not be able to access available resources. Some of our patient navigators are family members of individuals being treated for SCD and are able to provide a unique perspective that aids program development.
Once training for the initial group of navigators was completed, a patient navigation workgroup was created to clarify goals and objectives, develop standard procedures, and define navigator responsibilities. This workgroup included the CSCCN program staff, adult and pediatric hematology trained SCD specialists, and the pediatric coordinator. We used the Hemoglobinopathy Learning Collaborative process [10] to develop and refine a process map for referrals made for primary care. Process mapping was an iterative process with regular input from the navigators and other members of the patient navigation workgroup, as well as input from case managers and social workers when needed.
Process
Upon receiving a referral, navigators made contact with the patient within 1 business day and obtained preferred contact information. The navigator completed a patient intake form and needs assessment. Each patient referral was logged into a secure database. Most referrals were generated by our specialty health care providers, but as awareness of the program grew referrals also came from the community-based organizations as well as self-referrals from patients or caregivers. Most of the referrals (68%) were specifically for assistance in finding a primary care medical home. Other reasons patients were referred to the navigator program included needing assistance with housing, financial assistance, or insurance application questions.
Barriers Addressed
Patient navigators spent much of their time proactively seeking local sources of adult primary care for clients and in doing so established a network of primary care offices in the community that would be able and willing to accept new patients with SCD. This was accomplished through personal outreach and communication with stakeholders at the academic medical center and in the community, utilizing skills learned during patient navigation training and by sorting through vast informational resources available to the public. When feasible the navigators, accompanied by the project director and adult hematology specialist, personally visited sites of primary care in the community to help provide information about the CSCCN and establish a working relationship.
Our navigators would make calls together with the patient to make a PCP appointment and would remind them of upcoming PCP appointments. In some cases, navigators would attend appointments with the patient; in addition, they would help advocate for them when they had to go to the emergency department. If a patient missed a PCP appointment, the navigator followed up to find out why and how to secure another appointment. Navigators would follow-up every 10 days if a patient had not yet seen a PCP. As many of our patients did not understand why they needed to have a PCP when they had a specialist, the navigators educated patients on the importance of PCP care.
In additon, navigators helped our patients deal with insurance coverage problems, such as frequent insurance changes/insurance getting dropped. Some patients had low literacy or had difficulties in filling out disability or insurance forms properly. Navigators received training from state coordinators for Medicaid and SSI disability on filling out the respective forms, which can be confusing.
Our navigators were trained in motivational interviewing and used it to identify other barriers patients were facing. In trying to obtain proper care, our patients struggled with competing priorities (eg, food, shelter, child care). Because of the numerous challenges that our patients and families face, an important role for the patients navigators was creating a bridge to accessing social and community resources.
Outcomes
The help and support provided by the navigators is helpful in mitigating the mistrust our patients have about the health care system, in part due to unfavorable initial experiences as young adults or being stereotyped for pain issues. Our patients have said that navigators make them feel like they have an advocate—someone who is on their side.
Subsequent to forming the initial navigator group, we expanded our program by adding 2 patient navigators in Colorado Springs, a smaller metropolitan area about an hour and a half south of Denver. In Colorado Springs, 16 referrals for services have been made but none of these referrals were specifically for a PCP, since access to a PCP was not a barrier to care in that locale. Patients were more likely to need help with things such as transportation to get to their location of specialty care.
Conclusion
Coordination of care is essential for individuals with chronic diseases. While the focus to date has been on coordination of care for common chronic illness such as diabetes, it is essential to use best practices for individuals with less common, but often more complex, chronic diseases such as SCD. To facilitate coordination of care for this population who receive specialty care in an academic medical center, we developed a patient navigation program for adults with SCD as a quality improvement project. Our navigation program assisted 62% of referred adults in the Denver metropolitan area in identifying a PCP and attending an initial appointment.
The program is continuing for the duration of the grant funding and at this time we will likely need to seek mechanisms for additional support to continue the work that has been started, as well as to consider measurement of outcomes that are meaningful to the institutions in which the program is housed in order to become more sustainable internally.
Patient navigation is an acceptable and feasible way to enhance the care for adults with SCD and can help to bridge systems of clinical care, support and resources. Our program can serve as a model for similar patient populations with orphan chronic diseases that have both primary care and subspecialty needs that have both distinct and overlapping roles. Sustainability is a crucial issue to address and the use of outcome measures that can accurately reflect both successes and challenges of program implementation will be important to share with institutional leaders.
Corresponding author: Linda S. Overholser, MD, MPH, 12631 E 17th Ave., Mail Stop B180, Academic Office 1, Aurora, CO 80045, [email protected].
1. Prabhakar H, Haywood C Jr, Molokie R. Sickle cell disease in the United States: looking back and forward at 100 years of progress in management and survival. Am J Hematol 2010;85:346–53.
2. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
3. Hemker BG, Brousseau DC, Yan K, et al. When children with sickle-cell disease become adults: lack of outpatient care leads to increased use of the emergency department. Am J Hematol 2011;86:863–5.
4. Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract 1995 3:19–30.
5. Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer 2011;117(15 Suppl):3539–42.
6. Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer 2011;117(15 Suppl):3543–52.
7. Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer 2005;104:848–55.
8. Chin MH, Clarke AR, Nocon RS, et al. A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med 2012;27:992–1000.
9. Grosse SD, Schechter MS, Kulkarni R, et al. Models of comprehensive multidisciplinary care for individuals in the United States with genetic disorders. Pediatrics 2009;123:407–12.
10. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34–48.
11. Harold P. Freeman Patient Navigation Institute. Available at www.hpfreemanpni.org.
12. Patient Navigator Training Collaborative. Available at www.patientnavigatortraining.org.
1. Prabhakar H, Haywood C Jr, Molokie R. Sickle cell disease in the United States: looking back and forward at 100 years of progress in management and survival. Am J Hematol 2010;85:346–53.
2. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
3. Hemker BG, Brousseau DC, Yan K, et al. When children with sickle-cell disease become adults: lack of outpatient care leads to increased use of the emergency department. Am J Hematol 2011;86:863–5.
4. Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract 1995 3:19–30.
5. Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer 2011;117(15 Suppl):3539–42.
6. Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer 2011;117(15 Suppl):3543–52.
7. Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer 2005;104:848–55.
8. Chin MH, Clarke AR, Nocon RS, et al. A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med 2012;27:992–1000.
9. Grosse SD, Schechter MS, Kulkarni R, et al. Models of comprehensive multidisciplinary care for individuals in the United States with genetic disorders. Pediatrics 2009;123:407–12.
10. Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved 2012;23(3 Suppl):34–48.
11. Harold P. Freeman Patient Navigation Institute. Available at www.hpfreemanpni.org.
12. Patient Navigator Training Collaborative. Available at www.patientnavigatortraining.org.
Cutaneous Signs of Piety
Religious practices can lead to cutaneous changes, and awareness of these changes is of paramount importance in establishing the cause. We review the cutaneous changes related to religious practices, including the Semitic religions, Hinduism, and Sikhism (Table). The most widely followed Semitic religions are Christianity, Islam, and Judaism. Christianity and Islam collectively account for more than half of the world’s population.1

Christianity
Christian individuals are prone to blisters that develop below the knees due to repeated kneeling in prayer.2 A case of allergic contact dermatitis to a wooden cross made from Dalbergia nigra has been reported.3 Localized swelling with hypertrichosis due to muscular hypertrophy in the lower neck above the interscapular region has been described in well-built men who lift weights to bear pasos (floats with wooden sculptures) during Holy Week in Seville, Spain.4
Islam
Cutaneous signs of piety have been well documented in Muslim individuals. The most common presentation is hyperpigmentation of the forehead, usually noted as a secondary finding in patients seeking treatment of unrelated symptoms.5 Cutaneous changes in this region correspond with the area of the forehead that rests on the carpet during prayer. Macules typically present on the upper central aspect of the forehead close to the hairline and/or in pairs above the medial ends of the eyebrows; sometimes 3 or 4 lesions may be present in this area with involvement of the nasion (Figure 1).6
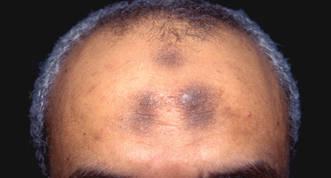
In Saudi Arabia where Sunni Islam predominates, Muslim individuals observe prayer 5 times per day. Calluses have been observed in areas of the body that are frequently subject to friction during this practice.7 For instance, calluses are more prominent on the right knee (Figure 2) and the left ankle, which bear the individual’s weight during prayer, and typically become nodular over time (Figure 3). In Arabic, these calluses are referred to as zabiba.8

A notable finding in followers of Shia Islam, which predominates in Iran, is the development of small nodules on the forehead, possibly caused by rubbing the forehead on a flat disclike prayer stone called a mohr during daily prayer,9 which is said to enhance public esteem.10 The nodules generally are asymptomatic, but some individuals experience minimal pain on pressure.8 Ulceration of the nodules has rarely been observed.7
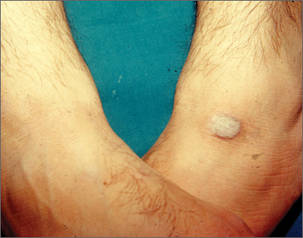
Limited access to thick and soft carpets and rarely bony exostoses or obesity are factors associated with prayer that can lead to skin changes (known as prayer signs), as they render the skin sensitive to pressure. Localized alopecia may occur on the forehead in individuals with low or pointed hairlines. An unexplained finding noted by one of the authors (K.A.) in some elderly Muslim individuals is that hair located on the forehead at the point of pressure during prayer remains pigmented, while the rest of the hair on the scalp turns white. Hyperpigmentation of the knuckles may be seen in individuals who use closed fists to rise from the ground following prayer. Except for mild hyperpigmentation of the knees,7 Muslim women rarely develop these changes, as they either do not pray,10 particularly during menstruation or puerperium, or they have more subcutaneous fat for protection.7 Some Muslim individuals who pray regularly at home may be conscious of these skin changes and therefore use a soft pillow to rest the forehead during prayer.
The histopathologic findings of prayer signs depend on the extent of lichenification and typically show compact hyperkeratosis or orthokeratosis, hypergranulosis, acanthosis, and mild dermal inflammation.8 Increased dermal vascularization and papillary fibrosis unlike that seen in lichen simplex chronicus have been described from skin changes in the lower limbs due to prayer practices.7 Additional findings in forehead biopsies include multiple comedones and epidermoid cysts in elderly patients showing a foreign body granulomatous reaction to hair fragments.10 Deposition of mucinous material in the dermal collagen in a prayer nodule on the forehead has been described in a Shiite individual, possibly due to repetitive microtrauma from the use of a prayer stone.9 Infections developed from sharing communal facilities or performing ritual sacrifices (eg, tinea,11 orf12) are prevalent during the yearly Hajj pilgrimage at Makkah, Saudi Arabia, in addition to other infectious and noninfectious dermatoses.13 Muslim women wearing headscarves secured at the neck with a safety pin have developed vitiligo at that site due to friction.14 Occasionally, Muslim individuals may apply perfumes before prayers, which may cause allergic contact dermatitis.
Judaism
Hyperpigmentation has been described in Jewish men at Talmudic seminaries due to the practice of reciting scriptures, which involves a rocking motion known as daven that leads to friction on the back.15 Lesions associated with this practice typically appear as isolated macules or a continuous linear patch over the skin of the bony protuberances of the inferior thoracic and lumbar vertebrae. Allergic contact dermatitis has been reported in Jewish individuals due to exposure to a variety of agents during religious practices, such as potassium dichromate, which is present in the leather used to make phylacteries or tefillin (boxes containing scripture that are secured to the forehead with straps that are then tied to the left arm during prayer). This finding has been noted in some or all areas of contact including the forehead, scalp, neck, left wrist, and waist.16
It is customary for both Orthodox Jewish and Muslim women to be concealed by clothing, which predisposes them to vitamin D deficiency17,18 but also protects them from developing malignant melanoma.19 Neonates have developed genital herpetic infections following circumcision due to the ancient practice of having the mohel (the person who performs the Jewish circumcision) suck on the wound until the bleeding stops.20
Hinduism
Hinduism espouses an eclectic philosophy of life subsuming numerous beliefs involving guardian deities, invoked by sacred marks, symbols, and rituals. Marks generally are placed on the forehead or other specified sites on the body. Sandalwood paste as well as vibhuti and kumkum powders most commonly are used, which can cause allergic contact dermatitis. Vibhuti is holy ash prepared by burning balls of dried cow dung in a fire pit with rice husk and clarified butter. Kumkum is prepared by alkalinizing turmeric powder, which turns red in color. A case of contact allergic dermatitis was reported in a Hindu priest who regularly used sandalwood paste on the forehead and as a balm for an ailment of the hands and feet.21 In our experience, vibhuti also has caused dermatitis on the forehead as well as on the neck and arms. The main difference between the 2 eruptions is that sandalwood dermatitis generally is localized to the center of the forehead as a circular or vertical mark or often in the center of the left palm, which is used to mix sandalwood powder with water to make a paste (Figure 4), while vibhuti contact dermatitis typically presents as a broad horizontal patch on the forehead because the powder is smeared with the middle 3 fingers (Figure 5). Perfumes used by some Muslim individuals before prayer that are applied on the clothes can mimic this type of contact dermatitis, but eruptions typically are confined to the fingers and palms.22 Contact dermatitis caused by necklaces made with beads of the stem of the Ocimum sanctum (holy basil) plant and seeds of the evergreen tree Elaeocarpus ganitrus have been reported.23 Calluses are sometimes seen in individuals who meditate for long hours while sitting in a cross-legged position and usually occur on or uncommonly below the lateral malleolus of the right foot, similar to practitioners of yoga.24
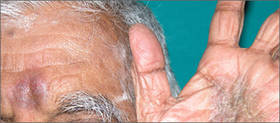
Hemorrhaging and crusting below the lateral malleolus of the right foot have been reported in Buddhist monks due to sitting in a cross-legged position for prolonged periods of meditation.25 Hyperpigmentation of the knees, ankles, and interphalangeal joints of the feet has been seen after sitting in the traditional Japanese meditative position.26 Tattoos of Hindu gods are common, while tattoos are forbidden in Islam and Judaism. Attributes of prominent deities branded on the body may be seen. Discrete sarcoidlike nodules along the axillae and chest wall have been attributed to a Hindu ritual (kavadi) that is performed annually as a form of self-inflicted punishment for their sins in which devotees pierce the chest wall with spokes to form a base over a heavy cage in which offerings are carried, and skewers passed through the cheeks have resulted in similar nodules in the oral cavity.27,28 Consumption of cow’s urine during rituals may induce acute urticaria.29 Lichen planus of the trunk30 and leukoderma of the waist31 may be induced by köbnerization or contact allergy from wearing sacred threads, respectively.
Sikhism
Sikhism, a religion founded in the 15th century, epitomizes the high-water mark of the syncretism between Hinduism and Islam. Men must abstain from cutting their hair; pulling and knotting the hair to maintain a coiffure can cause traction alopecia in the submandibular region and the frontal and parietal areas of the scalp as well as ridging and furrowing of the scalp resembling cutis verticis gyrata. Fixer, a product used to keep the beard intact, can cause contact dermatitis. The tight broad band of cloth (known as a ribbon) that is worn around the head to keep hair intact beneath a turban may cause forehead lesions. Discoid lupus erythematosus–like lesions or painful chondrodermatitis of the pinnae due to pressure from wearing a starched turban have been observed, also called “turban ear” from prominence of both anthelices.32,33 A case of a Sikh man who developed oral sarcoidal lesions from body piercing has been reported.28
Conclusion
Knowledge of the religious practices of patients would help in recognizing puzzling and peculiar dermatoses. It may not be possible to eliminate the causes of these conditions, but methods to reduce their effects on the skin can be discussed with patients.
Acknowledgments—We are grateful to the valuable help rendered by Joginder Kumar, MD, New Delhi, India, and C. Indira, MD, Hyderabad, India.
- The Pew Forum on Religion & Public Life. The Global Religious Landscape: A Report on the Size and Distribution of the World’s Major Religious Groups as of 2010. Washington, DC: The Pew Forum on Religion & Public Life, The Pew Research Center; 2012.
- Goodheart HP. “Devotional dermatoses”: a new nosologic entity? J Am Acad Dermatol. 2001;44:543.
- Fisher AA, Bikowski J. Allergic dermatitis due to a wooden cross made of Dalbergia nigra. Contact Dermatitis. 1981;7:45-46.
- Camacho F. Acquired circumscribed hypertrichosis in the ‘costaleros’ who bear the ‘pasos’ during Holy Week in Seville, Spain. Arch Dermatol. 1995;131:361-363.
- Mishriki YY. Skin commotion from repetitive devotion. prayer callus. Postgrad Med. 1999;105:153-154.
- Barankin B. Prayer marks. Int J Dermatol. 2004;43:985-986.
- Abanmi AA, Al Zouman AY, Al Hussaini H, et al. Prayer marks. Int J Dermatol. 2002;41:411-414.
- Kahana M, Cohen M, Ronnen M, et al. Prayer nodules in Moslem men. Cutis. 1986;38:281-282.
- O’Goshi KI, Aoyama H, Tagami H. Mucin deposition in a prayer nodule on the forehead. Dermatology. 1998;196:364.
- Vollum DI, Azadeh B. Prayer nodules. Clin Exp Dermatol. 1979;4:39-47.
- Arrese JE, Piérard-Franchimont C, Piérard GE. Scytalidium dimidiatum melanonychia and scaly plantar skin in four patients from the Maghreb: imported disease or outbreak in a Belgian mosque? Dermatology. 2001;202:183-185.
- Malik M, Bharier M, Tahan S, et al. Orf acquired during religious observance. Arch Dermatol. 2009;145:606-608.
- Mimesh SA, Al-Khenaizan S, Memish ZA. Dermatologic challenge of pilgrimage. Clin Dermatol. 2008;26:52-61.
- El-Din Anbar T, Abdel-Rahman AT, El-Khayyat MA, et al. Vitiligo on anterior aspect of neck in Muslim females: case series. Int J Dermatol. 2008;47:178-179.
- Naimer SA, Trattner A, Biton A, et al. Davener’s dermatosis: a variant of friction hypermelanosis. J Am Acad Dermatol. 2000;42:442-445.
- Feit NE, Weinberg JM, DeLeo VA. Cutaneous disease and religious practice: case of allergic contact dermatitis to tefillin and review of the literature. Int J Dermatol. 2004;43:886-888.
- Mukamel MN, Weisman Y, Somech R, et al. Vitamin D deficiency and insufficiency in Orthodox and non-Orthodox Jewish mothers in Israel. Isr Med Assoc J. 2001;3:419-421.
- Hatun S, Islam O, Cizmecioglu F, et al. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005;135:218-222.
- Vardi G, Modan B, Golan R, et al. Orthodox Jews have a lower incidence of malignant melanoma. a note on the potentially protective role of traditional clothing. Int J Cancer. 1993;53:771-773.
- Gesundheit B, Grisaru-Soen G, Greenberg G, et al. Neonatal genital herpes virus type 1 infection after Jewish ritual circumcision: modern medicine and religious tradition. Pediatrics. 2004;114:e259-e263.
- Pasricha JS, Ramam M. Contact dermatitis due to sandalwood (Santalum album Linn). Indian J Dermatol Venereol Leprol. 1986;52:232-233.
- Carmichael AJ, Foulds IS. Sensitization as a result of a religious ritual. Br J Dermatol. 1990;123:846.
- Bajaj AK, Saraswat A. Contact dermatitis. In: Valia RG, Valia AR, eds. Textbook of Dermatology. 3rd ed. Mumbai, India: Bhalani Publishing House; 2008:545-549.
- Verma SB, Wollina U. Callosities of cross-legged sitting: “yoga sign”—an under-recognized cultural cutaneous presentation. Int J Dermatol. 2008;47:1212-1214.
- Rehman H, Asfour NA. Clinical images: prayer nodules [published online ahead of print November 16, 2009]. CMAJ. 2010;182:e19.
- Ruhnke WG, Serizawa Y. Viral pericarditis. BMJ. 2010;340:b5579.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Ng KH, Siar CH, Ganesapillai T. Sarcoid-like foreign body reaction in body piercing: a report of two cases. Oral Surg Oral Med Oral Pathol Radiol Endod. 1997;84:28-31.
- Bhalla M, Thami GP. Acute urticaria following ‘gomutra’ (cow’s urine) gargles. Clin Exp Dermatol. 2005;30:722-723.
- Joshi A, Agarwalla A, Agrawal S, et al. Köbner phenomenon due to sacred thread in lichen planus. J Dermatol. 2000;27:129-130.
- Banerjee K, Banerjee R, Mandal B. Amulet string contact leukoderma and its differentiation from vitiligo. Indian J Dermatol Venereol Leprol. 2004;70:180-181.
- Kanwar AJ, Kaur S. Some dermatoses peculiar to Sikh men. Int J Dermatol. 1990;29:739-740.
- Williams HC. Turban ear. Arch Dermatol. 1994;130:117-119.
Religious practices can lead to cutaneous changes, and awareness of these changes is of paramount importance in establishing the cause. We review the cutaneous changes related to religious practices, including the Semitic religions, Hinduism, and Sikhism (Table). The most widely followed Semitic religions are Christianity, Islam, and Judaism. Christianity and Islam collectively account for more than half of the world’s population.1

Christianity
Christian individuals are prone to blisters that develop below the knees due to repeated kneeling in prayer.2 A case of allergic contact dermatitis to a wooden cross made from Dalbergia nigra has been reported.3 Localized swelling with hypertrichosis due to muscular hypertrophy in the lower neck above the interscapular region has been described in well-built men who lift weights to bear pasos (floats with wooden sculptures) during Holy Week in Seville, Spain.4
Islam
Cutaneous signs of piety have been well documented in Muslim individuals. The most common presentation is hyperpigmentation of the forehead, usually noted as a secondary finding in patients seeking treatment of unrelated symptoms.5 Cutaneous changes in this region correspond with the area of the forehead that rests on the carpet during prayer. Macules typically present on the upper central aspect of the forehead close to the hairline and/or in pairs above the medial ends of the eyebrows; sometimes 3 or 4 lesions may be present in this area with involvement of the nasion (Figure 1).6

In Saudi Arabia where Sunni Islam predominates, Muslim individuals observe prayer 5 times per day. Calluses have been observed in areas of the body that are frequently subject to friction during this practice.7 For instance, calluses are more prominent on the right knee (Figure 2) and the left ankle, which bear the individual’s weight during prayer, and typically become nodular over time (Figure 3). In Arabic, these calluses are referred to as zabiba.8

A notable finding in followers of Shia Islam, which predominates in Iran, is the development of small nodules on the forehead, possibly caused by rubbing the forehead on a flat disclike prayer stone called a mohr during daily prayer,9 which is said to enhance public esteem.10 The nodules generally are asymptomatic, but some individuals experience minimal pain on pressure.8 Ulceration of the nodules has rarely been observed.7

Limited access to thick and soft carpets and rarely bony exostoses or obesity are factors associated with prayer that can lead to skin changes (known as prayer signs), as they render the skin sensitive to pressure. Localized alopecia may occur on the forehead in individuals with low or pointed hairlines. An unexplained finding noted by one of the authors (K.A.) in some elderly Muslim individuals is that hair located on the forehead at the point of pressure during prayer remains pigmented, while the rest of the hair on the scalp turns white. Hyperpigmentation of the knuckles may be seen in individuals who use closed fists to rise from the ground following prayer. Except for mild hyperpigmentation of the knees,7 Muslim women rarely develop these changes, as they either do not pray,10 particularly during menstruation or puerperium, or they have more subcutaneous fat for protection.7 Some Muslim individuals who pray regularly at home may be conscious of these skin changes and therefore use a soft pillow to rest the forehead during prayer.
The histopathologic findings of prayer signs depend on the extent of lichenification and typically show compact hyperkeratosis or orthokeratosis, hypergranulosis, acanthosis, and mild dermal inflammation.8 Increased dermal vascularization and papillary fibrosis unlike that seen in lichen simplex chronicus have been described from skin changes in the lower limbs due to prayer practices.7 Additional findings in forehead biopsies include multiple comedones and epidermoid cysts in elderly patients showing a foreign body granulomatous reaction to hair fragments.10 Deposition of mucinous material in the dermal collagen in a prayer nodule on the forehead has been described in a Shiite individual, possibly due to repetitive microtrauma from the use of a prayer stone.9 Infections developed from sharing communal facilities or performing ritual sacrifices (eg, tinea,11 orf12) are prevalent during the yearly Hajj pilgrimage at Makkah, Saudi Arabia, in addition to other infectious and noninfectious dermatoses.13 Muslim women wearing headscarves secured at the neck with a safety pin have developed vitiligo at that site due to friction.14 Occasionally, Muslim individuals may apply perfumes before prayers, which may cause allergic contact dermatitis.
Judaism
Hyperpigmentation has been described in Jewish men at Talmudic seminaries due to the practice of reciting scriptures, which involves a rocking motion known as daven that leads to friction on the back.15 Lesions associated with this practice typically appear as isolated macules or a continuous linear patch over the skin of the bony protuberances of the inferior thoracic and lumbar vertebrae. Allergic contact dermatitis has been reported in Jewish individuals due to exposure to a variety of agents during religious practices, such as potassium dichromate, which is present in the leather used to make phylacteries or tefillin (boxes containing scripture that are secured to the forehead with straps that are then tied to the left arm during prayer). This finding has been noted in some or all areas of contact including the forehead, scalp, neck, left wrist, and waist.16
It is customary for both Orthodox Jewish and Muslim women to be concealed by clothing, which predisposes them to vitamin D deficiency17,18 but also protects them from developing malignant melanoma.19 Neonates have developed genital herpetic infections following circumcision due to the ancient practice of having the mohel (the person who performs the Jewish circumcision) suck on the wound until the bleeding stops.20
Hinduism
Hinduism espouses an eclectic philosophy of life subsuming numerous beliefs involving guardian deities, invoked by sacred marks, symbols, and rituals. Marks generally are placed on the forehead or other specified sites on the body. Sandalwood paste as well as vibhuti and kumkum powders most commonly are used, which can cause allergic contact dermatitis. Vibhuti is holy ash prepared by burning balls of dried cow dung in a fire pit with rice husk and clarified butter. Kumkum is prepared by alkalinizing turmeric powder, which turns red in color. A case of contact allergic dermatitis was reported in a Hindu priest who regularly used sandalwood paste on the forehead and as a balm for an ailment of the hands and feet.21 In our experience, vibhuti also has caused dermatitis on the forehead as well as on the neck and arms. The main difference between the 2 eruptions is that sandalwood dermatitis generally is localized to the center of the forehead as a circular or vertical mark or often in the center of the left palm, which is used to mix sandalwood powder with water to make a paste (Figure 4), while vibhuti contact dermatitis typically presents as a broad horizontal patch on the forehead because the powder is smeared with the middle 3 fingers (Figure 5). Perfumes used by some Muslim individuals before prayer that are applied on the clothes can mimic this type of contact dermatitis, but eruptions typically are confined to the fingers and palms.22 Contact dermatitis caused by necklaces made with beads of the stem of the Ocimum sanctum (holy basil) plant and seeds of the evergreen tree Elaeocarpus ganitrus have been reported.23 Calluses are sometimes seen in individuals who meditate for long hours while sitting in a cross-legged position and usually occur on or uncommonly below the lateral malleolus of the right foot, similar to practitioners of yoga.24

Hemorrhaging and crusting below the lateral malleolus of the right foot have been reported in Buddhist monks due to sitting in a cross-legged position for prolonged periods of meditation.25 Hyperpigmentation of the knees, ankles, and interphalangeal joints of the feet has been seen after sitting in the traditional Japanese meditative position.26 Tattoos of Hindu gods are common, while tattoos are forbidden in Islam and Judaism. Attributes of prominent deities branded on the body may be seen. Discrete sarcoidlike nodules along the axillae and chest wall have been attributed to a Hindu ritual (kavadi) that is performed annually as a form of self-inflicted punishment for their sins in which devotees pierce the chest wall with spokes to form a base over a heavy cage in which offerings are carried, and skewers passed through the cheeks have resulted in similar nodules in the oral cavity.27,28 Consumption of cow’s urine during rituals may induce acute urticaria.29 Lichen planus of the trunk30 and leukoderma of the waist31 may be induced by köbnerization or contact allergy from wearing sacred threads, respectively.

Sikhism
Sikhism, a religion founded in the 15th century, epitomizes the high-water mark of the syncretism between Hinduism and Islam. Men must abstain from cutting their hair; pulling and knotting the hair to maintain a coiffure can cause traction alopecia in the submandibular region and the frontal and parietal areas of the scalp as well as ridging and furrowing of the scalp resembling cutis verticis gyrata. Fixer, a product used to keep the beard intact, can cause contact dermatitis. The tight broad band of cloth (known as a ribbon) that is worn around the head to keep hair intact beneath a turban may cause forehead lesions. Discoid lupus erythematosus–like lesions or painful chondrodermatitis of the pinnae due to pressure from wearing a starched turban have been observed, also called “turban ear” from prominence of both anthelices.32,33 A case of a Sikh man who developed oral sarcoidal lesions from body piercing has been reported.28
Conclusion
Knowledge of the religious practices of patients would help in recognizing puzzling and peculiar dermatoses. It may not be possible to eliminate the causes of these conditions, but methods to reduce their effects on the skin can be discussed with patients.
Acknowledgments—We are grateful to the valuable help rendered by Joginder Kumar, MD, New Delhi, India, and C. Indira, MD, Hyderabad, India.
Religious practices can lead to cutaneous changes, and awareness of these changes is of paramount importance in establishing the cause. We review the cutaneous changes related to religious practices, including the Semitic religions, Hinduism, and Sikhism (Table). The most widely followed Semitic religions are Christianity, Islam, and Judaism. Christianity and Islam collectively account for more than half of the world’s population.1

Christianity
Christian individuals are prone to blisters that develop below the knees due to repeated kneeling in prayer.2 A case of allergic contact dermatitis to a wooden cross made from Dalbergia nigra has been reported.3 Localized swelling with hypertrichosis due to muscular hypertrophy in the lower neck above the interscapular region has been described in well-built men who lift weights to bear pasos (floats with wooden sculptures) during Holy Week in Seville, Spain.4
Islam
Cutaneous signs of piety have been well documented in Muslim individuals. The most common presentation is hyperpigmentation of the forehead, usually noted as a secondary finding in patients seeking treatment of unrelated symptoms.5 Cutaneous changes in this region correspond with the area of the forehead that rests on the carpet during prayer. Macules typically present on the upper central aspect of the forehead close to the hairline and/or in pairs above the medial ends of the eyebrows; sometimes 3 or 4 lesions may be present in this area with involvement of the nasion (Figure 1).6

In Saudi Arabia where Sunni Islam predominates, Muslim individuals observe prayer 5 times per day. Calluses have been observed in areas of the body that are frequently subject to friction during this practice.7 For instance, calluses are more prominent on the right knee (Figure 2) and the left ankle, which bear the individual’s weight during prayer, and typically become nodular over time (Figure 3). In Arabic, these calluses are referred to as zabiba.8

A notable finding in followers of Shia Islam, which predominates in Iran, is the development of small nodules on the forehead, possibly caused by rubbing the forehead on a flat disclike prayer stone called a mohr during daily prayer,9 which is said to enhance public esteem.10 The nodules generally are asymptomatic, but some individuals experience minimal pain on pressure.8 Ulceration of the nodules has rarely been observed.7

Limited access to thick and soft carpets and rarely bony exostoses or obesity are factors associated with prayer that can lead to skin changes (known as prayer signs), as they render the skin sensitive to pressure. Localized alopecia may occur on the forehead in individuals with low or pointed hairlines. An unexplained finding noted by one of the authors (K.A.) in some elderly Muslim individuals is that hair located on the forehead at the point of pressure during prayer remains pigmented, while the rest of the hair on the scalp turns white. Hyperpigmentation of the knuckles may be seen in individuals who use closed fists to rise from the ground following prayer. Except for mild hyperpigmentation of the knees,7 Muslim women rarely develop these changes, as they either do not pray,10 particularly during menstruation or puerperium, or they have more subcutaneous fat for protection.7 Some Muslim individuals who pray regularly at home may be conscious of these skin changes and therefore use a soft pillow to rest the forehead during prayer.
The histopathologic findings of prayer signs depend on the extent of lichenification and typically show compact hyperkeratosis or orthokeratosis, hypergranulosis, acanthosis, and mild dermal inflammation.8 Increased dermal vascularization and papillary fibrosis unlike that seen in lichen simplex chronicus have been described from skin changes in the lower limbs due to prayer practices.7 Additional findings in forehead biopsies include multiple comedones and epidermoid cysts in elderly patients showing a foreign body granulomatous reaction to hair fragments.10 Deposition of mucinous material in the dermal collagen in a prayer nodule on the forehead has been described in a Shiite individual, possibly due to repetitive microtrauma from the use of a prayer stone.9 Infections developed from sharing communal facilities or performing ritual sacrifices (eg, tinea,11 orf12) are prevalent during the yearly Hajj pilgrimage at Makkah, Saudi Arabia, in addition to other infectious and noninfectious dermatoses.13 Muslim women wearing headscarves secured at the neck with a safety pin have developed vitiligo at that site due to friction.14 Occasionally, Muslim individuals may apply perfumes before prayers, which may cause allergic contact dermatitis.
Judaism
Hyperpigmentation has been described in Jewish men at Talmudic seminaries due to the practice of reciting scriptures, which involves a rocking motion known as daven that leads to friction on the back.15 Lesions associated with this practice typically appear as isolated macules or a continuous linear patch over the skin of the bony protuberances of the inferior thoracic and lumbar vertebrae. Allergic contact dermatitis has been reported in Jewish individuals due to exposure to a variety of agents during religious practices, such as potassium dichromate, which is present in the leather used to make phylacteries or tefillin (boxes containing scripture that are secured to the forehead with straps that are then tied to the left arm during prayer). This finding has been noted in some or all areas of contact including the forehead, scalp, neck, left wrist, and waist.16
It is customary for both Orthodox Jewish and Muslim women to be concealed by clothing, which predisposes them to vitamin D deficiency17,18 but also protects them from developing malignant melanoma.19 Neonates have developed genital herpetic infections following circumcision due to the ancient practice of having the mohel (the person who performs the Jewish circumcision) suck on the wound until the bleeding stops.20
Hinduism
Hinduism espouses an eclectic philosophy of life subsuming numerous beliefs involving guardian deities, invoked by sacred marks, symbols, and rituals. Marks generally are placed on the forehead or other specified sites on the body. Sandalwood paste as well as vibhuti and kumkum powders most commonly are used, which can cause allergic contact dermatitis. Vibhuti is holy ash prepared by burning balls of dried cow dung in a fire pit with rice husk and clarified butter. Kumkum is prepared by alkalinizing turmeric powder, which turns red in color. A case of contact allergic dermatitis was reported in a Hindu priest who regularly used sandalwood paste on the forehead and as a balm for an ailment of the hands and feet.21 In our experience, vibhuti also has caused dermatitis on the forehead as well as on the neck and arms. The main difference between the 2 eruptions is that sandalwood dermatitis generally is localized to the center of the forehead as a circular or vertical mark or often in the center of the left palm, which is used to mix sandalwood powder with water to make a paste (Figure 4), while vibhuti contact dermatitis typically presents as a broad horizontal patch on the forehead because the powder is smeared with the middle 3 fingers (Figure 5). Perfumes used by some Muslim individuals before prayer that are applied on the clothes can mimic this type of contact dermatitis, but eruptions typically are confined to the fingers and palms.22 Contact dermatitis caused by necklaces made with beads of the stem of the Ocimum sanctum (holy basil) plant and seeds of the evergreen tree Elaeocarpus ganitrus have been reported.23 Calluses are sometimes seen in individuals who meditate for long hours while sitting in a cross-legged position and usually occur on or uncommonly below the lateral malleolus of the right foot, similar to practitioners of yoga.24

Hemorrhaging and crusting below the lateral malleolus of the right foot have been reported in Buddhist monks due to sitting in a cross-legged position for prolonged periods of meditation.25 Hyperpigmentation of the knees, ankles, and interphalangeal joints of the feet has been seen after sitting in the traditional Japanese meditative position.26 Tattoos of Hindu gods are common, while tattoos are forbidden in Islam and Judaism. Attributes of prominent deities branded on the body may be seen. Discrete sarcoidlike nodules along the axillae and chest wall have been attributed to a Hindu ritual (kavadi) that is performed annually as a form of self-inflicted punishment for their sins in which devotees pierce the chest wall with spokes to form a base over a heavy cage in which offerings are carried, and skewers passed through the cheeks have resulted in similar nodules in the oral cavity.27,28 Consumption of cow’s urine during rituals may induce acute urticaria.29 Lichen planus of the trunk30 and leukoderma of the waist31 may be induced by köbnerization or contact allergy from wearing sacred threads, respectively.

Sikhism
Sikhism, a religion founded in the 15th century, epitomizes the high-water mark of the syncretism between Hinduism and Islam. Men must abstain from cutting their hair; pulling and knotting the hair to maintain a coiffure can cause traction alopecia in the submandibular region and the frontal and parietal areas of the scalp as well as ridging and furrowing of the scalp resembling cutis verticis gyrata. Fixer, a product used to keep the beard intact, can cause contact dermatitis. The tight broad band of cloth (known as a ribbon) that is worn around the head to keep hair intact beneath a turban may cause forehead lesions. Discoid lupus erythematosus–like lesions or painful chondrodermatitis of the pinnae due to pressure from wearing a starched turban have been observed, also called “turban ear” from prominence of both anthelices.32,33 A case of a Sikh man who developed oral sarcoidal lesions from body piercing has been reported.28
Conclusion
Knowledge of the religious practices of patients would help in recognizing puzzling and peculiar dermatoses. It may not be possible to eliminate the causes of these conditions, but methods to reduce their effects on the skin can be discussed with patients.
Acknowledgments—We are grateful to the valuable help rendered by Joginder Kumar, MD, New Delhi, India, and C. Indira, MD, Hyderabad, India.
- The Pew Forum on Religion & Public Life. The Global Religious Landscape: A Report on the Size and Distribution of the World’s Major Religious Groups as of 2010. Washington, DC: The Pew Forum on Religion & Public Life, The Pew Research Center; 2012.
- Goodheart HP. “Devotional dermatoses”: a new nosologic entity? J Am Acad Dermatol. 2001;44:543.
- Fisher AA, Bikowski J. Allergic dermatitis due to a wooden cross made of Dalbergia nigra. Contact Dermatitis. 1981;7:45-46.
- Camacho F. Acquired circumscribed hypertrichosis in the ‘costaleros’ who bear the ‘pasos’ during Holy Week in Seville, Spain. Arch Dermatol. 1995;131:361-363.
- Mishriki YY. Skin commotion from repetitive devotion. prayer callus. Postgrad Med. 1999;105:153-154.
- Barankin B. Prayer marks. Int J Dermatol. 2004;43:985-986.
- Abanmi AA, Al Zouman AY, Al Hussaini H, et al. Prayer marks. Int J Dermatol. 2002;41:411-414.
- Kahana M, Cohen M, Ronnen M, et al. Prayer nodules in Moslem men. Cutis. 1986;38:281-282.
- O’Goshi KI, Aoyama H, Tagami H. Mucin deposition in a prayer nodule on the forehead. Dermatology. 1998;196:364.
- Vollum DI, Azadeh B. Prayer nodules. Clin Exp Dermatol. 1979;4:39-47.
- Arrese JE, Piérard-Franchimont C, Piérard GE. Scytalidium dimidiatum melanonychia and scaly plantar skin in four patients from the Maghreb: imported disease or outbreak in a Belgian mosque? Dermatology. 2001;202:183-185.
- Malik M, Bharier M, Tahan S, et al. Orf acquired during religious observance. Arch Dermatol. 2009;145:606-608.
- Mimesh SA, Al-Khenaizan S, Memish ZA. Dermatologic challenge of pilgrimage. Clin Dermatol. 2008;26:52-61.
- El-Din Anbar T, Abdel-Rahman AT, El-Khayyat MA, et al. Vitiligo on anterior aspect of neck in Muslim females: case series. Int J Dermatol. 2008;47:178-179.
- Naimer SA, Trattner A, Biton A, et al. Davener’s dermatosis: a variant of friction hypermelanosis. J Am Acad Dermatol. 2000;42:442-445.
- Feit NE, Weinberg JM, DeLeo VA. Cutaneous disease and religious practice: case of allergic contact dermatitis to tefillin and review of the literature. Int J Dermatol. 2004;43:886-888.
- Mukamel MN, Weisman Y, Somech R, et al. Vitamin D deficiency and insufficiency in Orthodox and non-Orthodox Jewish mothers in Israel. Isr Med Assoc J. 2001;3:419-421.
- Hatun S, Islam O, Cizmecioglu F, et al. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005;135:218-222.
- Vardi G, Modan B, Golan R, et al. Orthodox Jews have a lower incidence of malignant melanoma. a note on the potentially protective role of traditional clothing. Int J Cancer. 1993;53:771-773.
- Gesundheit B, Grisaru-Soen G, Greenberg G, et al. Neonatal genital herpes virus type 1 infection after Jewish ritual circumcision: modern medicine and religious tradition. Pediatrics. 2004;114:e259-e263.
- Pasricha JS, Ramam M. Contact dermatitis due to sandalwood (Santalum album Linn). Indian J Dermatol Venereol Leprol. 1986;52:232-233.
- Carmichael AJ, Foulds IS. Sensitization as a result of a religious ritual. Br J Dermatol. 1990;123:846.
- Bajaj AK, Saraswat A. Contact dermatitis. In: Valia RG, Valia AR, eds. Textbook of Dermatology. 3rd ed. Mumbai, India: Bhalani Publishing House; 2008:545-549.
- Verma SB, Wollina U. Callosities of cross-legged sitting: “yoga sign”—an under-recognized cultural cutaneous presentation. Int J Dermatol. 2008;47:1212-1214.
- Rehman H, Asfour NA. Clinical images: prayer nodules [published online ahead of print November 16, 2009]. CMAJ. 2010;182:e19.
- Ruhnke WG, Serizawa Y. Viral pericarditis. BMJ. 2010;340:b5579.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Ng KH, Siar CH, Ganesapillai T. Sarcoid-like foreign body reaction in body piercing: a report of two cases. Oral Surg Oral Med Oral Pathol Radiol Endod. 1997;84:28-31.
- Bhalla M, Thami GP. Acute urticaria following ‘gomutra’ (cow’s urine) gargles. Clin Exp Dermatol. 2005;30:722-723.
- Joshi A, Agarwalla A, Agrawal S, et al. Köbner phenomenon due to sacred thread in lichen planus. J Dermatol. 2000;27:129-130.
- Banerjee K, Banerjee R, Mandal B. Amulet string contact leukoderma and its differentiation from vitiligo. Indian J Dermatol Venereol Leprol. 2004;70:180-181.
- Kanwar AJ, Kaur S. Some dermatoses peculiar to Sikh men. Int J Dermatol. 1990;29:739-740.
- Williams HC. Turban ear. Arch Dermatol. 1994;130:117-119.
- The Pew Forum on Religion & Public Life. The Global Religious Landscape: A Report on the Size and Distribution of the World’s Major Religious Groups as of 2010. Washington, DC: The Pew Forum on Religion & Public Life, The Pew Research Center; 2012.
- Goodheart HP. “Devotional dermatoses”: a new nosologic entity? J Am Acad Dermatol. 2001;44:543.
- Fisher AA, Bikowski J. Allergic dermatitis due to a wooden cross made of Dalbergia nigra. Contact Dermatitis. 1981;7:45-46.
- Camacho F. Acquired circumscribed hypertrichosis in the ‘costaleros’ who bear the ‘pasos’ during Holy Week in Seville, Spain. Arch Dermatol. 1995;131:361-363.
- Mishriki YY. Skin commotion from repetitive devotion. prayer callus. Postgrad Med. 1999;105:153-154.
- Barankin B. Prayer marks. Int J Dermatol. 2004;43:985-986.
- Abanmi AA, Al Zouman AY, Al Hussaini H, et al. Prayer marks. Int J Dermatol. 2002;41:411-414.
- Kahana M, Cohen M, Ronnen M, et al. Prayer nodules in Moslem men. Cutis. 1986;38:281-282.
- O’Goshi KI, Aoyama H, Tagami H. Mucin deposition in a prayer nodule on the forehead. Dermatology. 1998;196:364.
- Vollum DI, Azadeh B. Prayer nodules. Clin Exp Dermatol. 1979;4:39-47.
- Arrese JE, Piérard-Franchimont C, Piérard GE. Scytalidium dimidiatum melanonychia and scaly plantar skin in four patients from the Maghreb: imported disease or outbreak in a Belgian mosque? Dermatology. 2001;202:183-185.
- Malik M, Bharier M, Tahan S, et al. Orf acquired during religious observance. Arch Dermatol. 2009;145:606-608.
- Mimesh SA, Al-Khenaizan S, Memish ZA. Dermatologic challenge of pilgrimage. Clin Dermatol. 2008;26:52-61.
- El-Din Anbar T, Abdel-Rahman AT, El-Khayyat MA, et al. Vitiligo on anterior aspect of neck in Muslim females: case series. Int J Dermatol. 2008;47:178-179.
- Naimer SA, Trattner A, Biton A, et al. Davener’s dermatosis: a variant of friction hypermelanosis. J Am Acad Dermatol. 2000;42:442-445.
- Feit NE, Weinberg JM, DeLeo VA. Cutaneous disease and religious practice: case of allergic contact dermatitis to tefillin and review of the literature. Int J Dermatol. 2004;43:886-888.
- Mukamel MN, Weisman Y, Somech R, et al. Vitamin D deficiency and insufficiency in Orthodox and non-Orthodox Jewish mothers in Israel. Isr Med Assoc J. 2001;3:419-421.
- Hatun S, Islam O, Cizmecioglu F, et al. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005;135:218-222.
- Vardi G, Modan B, Golan R, et al. Orthodox Jews have a lower incidence of malignant melanoma. a note on the potentially protective role of traditional clothing. Int J Cancer. 1993;53:771-773.
- Gesundheit B, Grisaru-Soen G, Greenberg G, et al. Neonatal genital herpes virus type 1 infection after Jewish ritual circumcision: modern medicine and religious tradition. Pediatrics. 2004;114:e259-e263.
- Pasricha JS, Ramam M. Contact dermatitis due to sandalwood (Santalum album Linn). Indian J Dermatol Venereol Leprol. 1986;52:232-233.
- Carmichael AJ, Foulds IS. Sensitization as a result of a religious ritual. Br J Dermatol. 1990;123:846.
- Bajaj AK, Saraswat A. Contact dermatitis. In: Valia RG, Valia AR, eds. Textbook of Dermatology. 3rd ed. Mumbai, India: Bhalani Publishing House; 2008:545-549.
- Verma SB, Wollina U. Callosities of cross-legged sitting: “yoga sign”—an under-recognized cultural cutaneous presentation. Int J Dermatol. 2008;47:1212-1214.
- Rehman H, Asfour NA. Clinical images: prayer nodules [published online ahead of print November 16, 2009]. CMAJ. 2010;182:e19.
- Ruhnke WG, Serizawa Y. Viral pericarditis. BMJ. 2010;340:b5579.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Ng KH, Siar CH, Ganesapillai T. Sarcoid-like foreign body reaction in body piercing: a report of two cases. Oral Surg Oral Med Oral Pathol Radiol Endod. 1997;84:28-31.
- Bhalla M, Thami GP. Acute urticaria following ‘gomutra’ (cow’s urine) gargles. Clin Exp Dermatol. 2005;30:722-723.
- Joshi A, Agarwalla A, Agrawal S, et al. Köbner phenomenon due to sacred thread in lichen planus. J Dermatol. 2000;27:129-130.
- Banerjee K, Banerjee R, Mandal B. Amulet string contact leukoderma and its differentiation from vitiligo. Indian J Dermatol Venereol Leprol. 2004;70:180-181.
- Kanwar AJ, Kaur S. Some dermatoses peculiar to Sikh men. Int J Dermatol. 1990;29:739-740.
- Williams HC. Turban ear. Arch Dermatol. 1994;130:117-119.
Practice Points
- Cutaneous changes may be seen in specified areas of the skin following regular worship in almost all major religions of the world.
- Cutaneous lesions are most commonly associated with friction from praying, along with contact allergic dermatitis from products and substances commonly used in worshipping and granulomas due to practices such as tattoos and skin piercing.
- Uncommon skin manifestations include urticaria and leukoderma.
- Some religious practices may render individuals prone to infections that manifest on the skin.