User login
Antibody may treat cancer cachexia

Preclinical research raises the prospect of more effective treatments for cachexia, a profound wasting of fat and muscle that can increase the risk of death in cancer patients.
In mouse models, an antibody effectively improved or prevented symptoms of cachexia.
The antibody inhibited the effects of parathyroid hormone-related protein (PTHrP), which is released from many types of cancer cells.
The researchers said their findings, published in Nature, are the first to explain in detail how PTHrP from tumors switches on a thermogenic process in fatty tissues, resulting in unhealthy weight loss.
The team carried out 2 experiments using mice that developed lung tumors and cachexia. In the first, a polyclonal antibody that specifically neutralizes PTHrP prevented cachexia almost completely, while untreated animals became mildly cachexic.
Anti-PTHrP treatment prevented the shrinkage of fat droplets. It blocked thermogenic gene expression in epididymal white adipose tissue, interscapular brown adipose tissue, and inguinal white adipose tissue, which suggests thermogenesis has a causal role in fat wasting.
Treatment with the anti-PTHrP antibody also lowered oxygen consumption in the mice, increased their physical activity, and reduced their heat production.
In the second experiment, the researchers treated mice with the anti-PTHrP antibody until they observed severe cachexia in control animals. The antibody significantly preserved muscle mass, which was evident by improved grip strength and in situ muscle contraction.
“You would have expected, based on our first experiments in cell culture, that blocking PTHrP in the mice would reduce browning of the fat,” said study author Bruce Spiegelman, PhD, of the Dana-Farber Cancer Institute in Boston.
“But we were surprised that it also affected the loss of muscle mass and improved health.”
Additional experiments, in which the researchers injected PTHrP into healthy and tumor-bearing mice, suggested that PTHrP alone doesn’t directly cause muscle wasting. But blocking the protein’s activity still prevents cachexia.
Thus, the role of PTHrP “is definitely not the whole answer” to the riddle of cachexia, Dr Spiegelman noted. Furthermore, it may turn out that the PTHrP mechanism is responsible for cachexia in only a subset of cancer patients.
The researchers analyzed blood samples from 47 cachexic patients with lung or colon cancer. And they found increased levels of PTHrP in 17 of the patients. Those patients had significantly lower lean body mass and were producing more heat energy at rest than the other patients in the group.
Dr Spiegelman noted that, before they test the anti-PTHrP antibody in clinical trials, clinicians would likely want to determine if the protein is elevated in certain cancers and determine which patients would be good candidates for the treatment. ![]()

Preclinical research raises the prospect of more effective treatments for cachexia, a profound wasting of fat and muscle that can increase the risk of death in cancer patients.
In mouse models, an antibody effectively improved or prevented symptoms of cachexia.
The antibody inhibited the effects of parathyroid hormone-related protein (PTHrP), which is released from many types of cancer cells.
The researchers said their findings, published in Nature, are the first to explain in detail how PTHrP from tumors switches on a thermogenic process in fatty tissues, resulting in unhealthy weight loss.
The team carried out 2 experiments using mice that developed lung tumors and cachexia. In the first, a polyclonal antibody that specifically neutralizes PTHrP prevented cachexia almost completely, while untreated animals became mildly cachexic.
Anti-PTHrP treatment prevented the shrinkage of fat droplets. It blocked thermogenic gene expression in epididymal white adipose tissue, interscapular brown adipose tissue, and inguinal white adipose tissue, which suggests thermogenesis has a causal role in fat wasting.
Treatment with the anti-PTHrP antibody also lowered oxygen consumption in the mice, increased their physical activity, and reduced their heat production.
In the second experiment, the researchers treated mice with the anti-PTHrP antibody until they observed severe cachexia in control animals. The antibody significantly preserved muscle mass, which was evident by improved grip strength and in situ muscle contraction.
“You would have expected, based on our first experiments in cell culture, that blocking PTHrP in the mice would reduce browning of the fat,” said study author Bruce Spiegelman, PhD, of the Dana-Farber Cancer Institute in Boston.
“But we were surprised that it also affected the loss of muscle mass and improved health.”
Additional experiments, in which the researchers injected PTHrP into healthy and tumor-bearing mice, suggested that PTHrP alone doesn’t directly cause muscle wasting. But blocking the protein’s activity still prevents cachexia.
Thus, the role of PTHrP “is definitely not the whole answer” to the riddle of cachexia, Dr Spiegelman noted. Furthermore, it may turn out that the PTHrP mechanism is responsible for cachexia in only a subset of cancer patients.
The researchers analyzed blood samples from 47 cachexic patients with lung or colon cancer. And they found increased levels of PTHrP in 17 of the patients. Those patients had significantly lower lean body mass and were producing more heat energy at rest than the other patients in the group.
Dr Spiegelman noted that, before they test the anti-PTHrP antibody in clinical trials, clinicians would likely want to determine if the protein is elevated in certain cancers and determine which patients would be good candidates for the treatment. ![]()

Preclinical research raises the prospect of more effective treatments for cachexia, a profound wasting of fat and muscle that can increase the risk of death in cancer patients.
In mouse models, an antibody effectively improved or prevented symptoms of cachexia.
The antibody inhibited the effects of parathyroid hormone-related protein (PTHrP), which is released from many types of cancer cells.
The researchers said their findings, published in Nature, are the first to explain in detail how PTHrP from tumors switches on a thermogenic process in fatty tissues, resulting in unhealthy weight loss.
The team carried out 2 experiments using mice that developed lung tumors and cachexia. In the first, a polyclonal antibody that specifically neutralizes PTHrP prevented cachexia almost completely, while untreated animals became mildly cachexic.
Anti-PTHrP treatment prevented the shrinkage of fat droplets. It blocked thermogenic gene expression in epididymal white adipose tissue, interscapular brown adipose tissue, and inguinal white adipose tissue, which suggests thermogenesis has a causal role in fat wasting.
Treatment with the anti-PTHrP antibody also lowered oxygen consumption in the mice, increased their physical activity, and reduced their heat production.
In the second experiment, the researchers treated mice with the anti-PTHrP antibody until they observed severe cachexia in control animals. The antibody significantly preserved muscle mass, which was evident by improved grip strength and in situ muscle contraction.
“You would have expected, based on our first experiments in cell culture, that blocking PTHrP in the mice would reduce browning of the fat,” said study author Bruce Spiegelman, PhD, of the Dana-Farber Cancer Institute in Boston.
“But we were surprised that it also affected the loss of muscle mass and improved health.”
Additional experiments, in which the researchers injected PTHrP into healthy and tumor-bearing mice, suggested that PTHrP alone doesn’t directly cause muscle wasting. But blocking the protein’s activity still prevents cachexia.
Thus, the role of PTHrP “is definitely not the whole answer” to the riddle of cachexia, Dr Spiegelman noted. Furthermore, it may turn out that the PTHrP mechanism is responsible for cachexia in only a subset of cancer patients.
The researchers analyzed blood samples from 47 cachexic patients with lung or colon cancer. And they found increased levels of PTHrP in 17 of the patients. Those patients had significantly lower lean body mass and were producing more heat energy at rest than the other patients in the group.
Dr Spiegelman noted that, before they test the anti-PTHrP antibody in clinical trials, clinicians would likely want to determine if the protein is elevated in certain cancers and determine which patients would be good candidates for the treatment. ![]()
HSC engraftment across the species barrier

Scientists say they’ve generated a mouse model that supports the transplantation of human hematopoietic stem cells (HSCs), despite the species barrier and without the need for irradiation.
The group used a mutation of the Kit receptor in the mouse stem cells to facilitate the engraftment of human cells.
In this model, human HSCs can expand and differentiate into all blood cell types without any additional treatment.
Even cells of the innate immune system that are not typically found in “humanized” mice were efficiently generated in this mouse.
Furthermore, the stem cells can be maintained in the mouse over a longer period of time.
The researchers reported these results in Cell Stem Cell.
“Our goal was to develop an optimal model for the transplantation and study of human blood stem cells,” said study author Claudia Waskow, PhD, of Technische Universität Dresden in Germany.
To achieve optimal stem cell engraftment, she and her colleagues introduced a naturally occurring mutation of the Kit receptor into mice lacking a functional immune system.
In this way, the team circumvented the 2 major obstacles of HSC transplantation: the rejection by the recipient’s immune system and the absence of free niche space for the incoming donor stem cells in the recipient’s bone marrow.
The Kit mutation in the new mouse model impairs the recipient’s stem cell compartment in such a way that the endogenous HSCs can be easily replaced by human donor stem cells with a functional Kit receptor.
The researchers said this replacement works so efficiently that irradiation can be completely omitted, allowing the study of human blood development in a physiological setting. The model can now be used to study diseases of the human blood and immune system or to test new treatment options.
The results of this research also show that the Kit receptor is important for the function of human HSCs, notably in a transplant setting. The researchers said future studies will focus on using this knowledge to improve conditioning therapy for patients undergoing HSC transplant. ![]()

Scientists say they’ve generated a mouse model that supports the transplantation of human hematopoietic stem cells (HSCs), despite the species barrier and without the need for irradiation.
The group used a mutation of the Kit receptor in the mouse stem cells to facilitate the engraftment of human cells.
In this model, human HSCs can expand and differentiate into all blood cell types without any additional treatment.
Even cells of the innate immune system that are not typically found in “humanized” mice were efficiently generated in this mouse.
Furthermore, the stem cells can be maintained in the mouse over a longer period of time.
The researchers reported these results in Cell Stem Cell.
“Our goal was to develop an optimal model for the transplantation and study of human blood stem cells,” said study author Claudia Waskow, PhD, of Technische Universität Dresden in Germany.
To achieve optimal stem cell engraftment, she and her colleagues introduced a naturally occurring mutation of the Kit receptor into mice lacking a functional immune system.
In this way, the team circumvented the 2 major obstacles of HSC transplantation: the rejection by the recipient’s immune system and the absence of free niche space for the incoming donor stem cells in the recipient’s bone marrow.
The Kit mutation in the new mouse model impairs the recipient’s stem cell compartment in such a way that the endogenous HSCs can be easily replaced by human donor stem cells with a functional Kit receptor.
The researchers said this replacement works so efficiently that irradiation can be completely omitted, allowing the study of human blood development in a physiological setting. The model can now be used to study diseases of the human blood and immune system or to test new treatment options.
The results of this research also show that the Kit receptor is important for the function of human HSCs, notably in a transplant setting. The researchers said future studies will focus on using this knowledge to improve conditioning therapy for patients undergoing HSC transplant. ![]()

Scientists say they’ve generated a mouse model that supports the transplantation of human hematopoietic stem cells (HSCs), despite the species barrier and without the need for irradiation.
The group used a mutation of the Kit receptor in the mouse stem cells to facilitate the engraftment of human cells.
In this model, human HSCs can expand and differentiate into all blood cell types without any additional treatment.
Even cells of the innate immune system that are not typically found in “humanized” mice were efficiently generated in this mouse.
Furthermore, the stem cells can be maintained in the mouse over a longer period of time.
The researchers reported these results in Cell Stem Cell.
“Our goal was to develop an optimal model for the transplantation and study of human blood stem cells,” said study author Claudia Waskow, PhD, of Technische Universität Dresden in Germany.
To achieve optimal stem cell engraftment, she and her colleagues introduced a naturally occurring mutation of the Kit receptor into mice lacking a functional immune system.
In this way, the team circumvented the 2 major obstacles of HSC transplantation: the rejection by the recipient’s immune system and the absence of free niche space for the incoming donor stem cells in the recipient’s bone marrow.
The Kit mutation in the new mouse model impairs the recipient’s stem cell compartment in such a way that the endogenous HSCs can be easily replaced by human donor stem cells with a functional Kit receptor.
The researchers said this replacement works so efficiently that irradiation can be completely omitted, allowing the study of human blood development in a physiological setting. The model can now be used to study diseases of the human blood and immune system or to test new treatment options.
The results of this research also show that the Kit receptor is important for the function of human HSCs, notably in a transplant setting. The researchers said future studies will focus on using this knowledge to improve conditioning therapy for patients undergoing HSC transplant. ![]()
RBC Transfusion Reduction
Historically, red blood cell (RBC) transfusions have been viewed as safe and effective means of treating anemia and improving oxygen delivery to tissues. Beginning in the early 1980s, primarily driven by concerns related to the risks of transfusion‐related infection, transfusion practice began to come under scrutiny.
Numerous studies over the past 2 decades have failed to demonstrate a benefit of RBC transfusion in many of the clinical situations in which RBC transfusions are routinely given, and many of these studies have in fact shown that RBC transfusion may lead to worse clinical outcomes in some patients.[1, 2] The few available large, randomized clinical trials and prospective observational studies that have assessed the effectiveness of allogeneic RBC transfusion have demonstrated that a more restrictive approach to RBC transfusion results in at least equivalent patient outcomes as compared to a liberal approach, and may in fact reduce morbidity and mortality rates.[1, 2]
Over the last decade, RBC transfusion best‐practice guidelines have been developed by a number of professional societies,[3] addressing RBC transfusion practice in specific patient populations including critical care as well as more general hospitalized populations. These guidelines are generally consistent, strongly recommending a restrictive RBC transfusion approach in most clinical populations. However, despite the general consistency of the guidelines and the lack of evidence for the efficacy of RBC transfusion, there still remains significant variability in clinical RBC transfusion practice.[4, 5]
The difficulty in getting physicians to follow clinical guidelines in general has been well described.[6] Over the last 2 decades there have been reports of a variety of interventions directed toward improving RBC transfusion practice either in specific care units (eg, intensive care units [ICUs]) or institution wide.[7, 8, 9, 10, 11, 12, 13, 14] These initiatives have had varying degrees of success and have employed strategies that have included clinical guidelines, education, audit/feedback, and most recently computer order entry and decision support. We report on the effectiveness of an institution‐wide intervention to align RBC transfusion practice with best‐practice clinical guidelines. Our approach included institutional endorsement of a RBC transfusion guideline coupled with an ongoing education program and RBC transfusion order set.
METHODS
Study Setting
The University of Arkansas for Medical Sciences (UAMS) is a tertiary care university teaching hospital with a total of 437 patient beds. UAMS is a level 1 trauma center and has 52 ICU beds. The study took place between July 2012 and December 2013. At the time of study initiation, there was no institutional RBC transfusion protocol or guideline.
Study Design
In June 2012, a program was initiated to align RBC transfusion practice at UAMS with best‐practice RBC transfusion guidelines. This initiative consisted of several components: a series of educational programs, followed by hospital medical board approval of an intuitional RBC transfusion guideline, and initiation of an RBC transfusion order set of approved RBC transfusion guideline recommendations (Table 1).
| RBC Transfusion Guideline | |
|---|---|
| |
| PURPOSE: Unnecessary blood transfusions increase healthcare costs and expose patients to potential infectious and noninfectious risks. The purpose of this clinical practice guideline is to establish an evidence‐based approach to the transfusion of RBCs in hospitalized patients at UAMS. | |
| GUIDELINE: In order to avoid the potential risks and increased costs associated with unnecessary blood transfusions, the medical staff of UAMS will adhere to a restrictive transfusion strategy in which: | |
| (I) RBC transfusion should be considered unnecessary for hospitalized, hemodynamically stable patients unless the hemoglobin concentration is <78 g/dL. | |
| (II) RBC transfusion is appropriate for patients who have evidence of acute hemorrhage or hemorrhagic shock. | |
| (III) RBC transfusion is appropriate for patients with acute MI or unstable myocardial ischemia if the hemoglobin concentration is 8 g/dL. | |
| (IV) The use of the hemoglobin concentration alone as a trigger for RBC transfusion should be avoided. The decision to order an RBC transfusion should also consider a patient's intravascular volume status, evidence of shock, duration and extent of anemia, and cardiopulmonary physiologic parameters as well as other symptomatology. | |
| (V) In the absence of acute hemorrhage, an RBC transfusion should be ordered and administered as single units. | |
| (VI) It is the physician's responsibility to weigh the risks and benefits of an RBC transfusion for a particular patient based on their medical condition. As such, it is recognized that there will be situations in which an RBC transfusion is appropriate outside of the guidelines put forth in this document. In these instances, the physician should document in the medical record his/her rationale for the RBC transfusion. | |
| RBC Transfusion Order Form | |
| The following are RBC transfusion indications consistent with UAMS‐approved guidelines (check 1): | |
| Acute hemorrhage or hemorrhagic shock | Yes |
| Hgb <78 g/dL | Yes |
| Acute MI, Hgb 8 g/dL | Yes |
| Acute coronary syndrome Hgb 8 g/dL | Yes |
| If the RBC transfusion is for an indication other than those listed above, please note the indication and attending physician in the space provided. | |
| Other indications/attending physician | Free text of other indications. |
| In the absence of acute hemorrhage or a hemoglobin concentration <6.5 g/dL, it is recommended that RBCs be ordered as single units. | |
The educational program included grand rounds presentations for all major clinical departments (internal medicine, surgery, obstetrics and gynecology, geriatrics, anesthesiology), presentations to high‐volume transfusing services (hematology, vascular surgery, cardiac surgery), presentations to hightransfusion‐volume nursing units (eg, medical and surgical ICUs, intermediate care unit, hematology), and scheduled and ad hoc resident educational programs. Educational sessions were repeated over the 18 months of the study and were presented by a clinical content expert.
A UAMS‐specific transfusion guideline was developed based on published best‐practice guidelines.[15, 16] The UAMS medical board approved this guideline in November 2012 (Table 1). The guidelines were disseminated to the entire medical staff in December 2012 via email communication from the hospital's chief medical officer. Membership of the medical board included clinical leadership of the medical center (ie, department chairs) as well as ad hoc members from the hospital administrative leadership.
An RBC transfusion order form that included the guideline recommendations was implemented in the electronic medical record (Sunrise Enterprise 5.5; Eclipsys Corp., Atlanta, GA) in March 2013. There was no hard stop for an RBC transfusion order that was outside of the guideline recommendations; however, for documentation, the ordering physician was required to note the indication and the supervising attending physician for these out‐of‐guideline RBC transfusions. RBC transfusion orders are entered in an electronic medical record. There was no alert triggered by an RBC transfusion order outside of the RBC transfusion guideline.
Outcomes
The number of RBC units transfused during the baseline period of January 2011 through June 2012 was compared with RBC units transfused July 2012 through December 2013. The latter period was further divided into the time period July 2012 through December 2012, during which the education program was initiated (education) as well as the time period January 2013 through December 2013 following the transfusion guideline approval and the initiation of the transfusion order set (decision support). All adult inpatient RBC units transfused, excluding RBC units transfused in the operating room and emergency room, were included in the analysis. RBC transfusions per month were normalized to RBC transfusions per 28 days. RBC transfusions were also calculated as RBC units per adult hospital admission and RBC units per 100 patient‐days.
Hospital mortality is presented as mortality index (observed/predicted mortality). The mean weighted diagnosis‐related group (DRG) was calculated using the monthly average of the Centers for Medicare and Medicaid Services (CMS)‐derived relative weighted DRGs.
Statistical Analysis
Data are presented as meanstandard deviation. Comparisons were by Student t test or analysis of variance as appropriate. GraphPad InStat (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis, and Minitab (Minitab Inc., State College, PA) was used for control graphs.
RESULTS
There were 28,393 adult admissions (excluding psychiatry) during the baseline period (January 2011June 2012) and 35,743 (12,353 education, 23,390 decision support) adult admissions during the study period (July 2012December 2013). The patient demographics for the 3 time periods were comparable (Table 2).
| Baseline | Education | Decision Support | |
|---|---|---|---|
| |||
| Total patients | 28,393 | 12,353 | 23,390 |
| Age, mean, y* | 48.20.6 | 480.1 | 480.5 |
| Gender, % female | 56 | 57 | 58 |
| Race, % non‐Caucasian | 63 | 61 | 61 |
| Weighted DRG | 1.60 | 1.59 | 1.59 |
| MDC, % | |||
| Nervous system | 13 | 13 | 12 |
| Circulatory system | 11 | 12 | 11 |
| Digestive system | 10 | 10 | 10 |
| Respiratory system | 9 | 8 | 9 |
| Musculoskeletal system | 8 | 8 | 8 |
| Kidney and urinary tract | 8 | 8 | 8 |
| Hepatobiliary system | 5 | 5 | 5 |
| Infectious and parasitic | 5 | 5 | 6 |
| Endocrine, metabolic | 3 | 4 | 3 |
| Blood, immunologic | 3 | 2 | 2 |
| Myeloproliferative | 4 | 4 | 3 |
| Multiple significant trauma | 1 | 1 | 1 |
| Other | 20 | 20 | 22 |
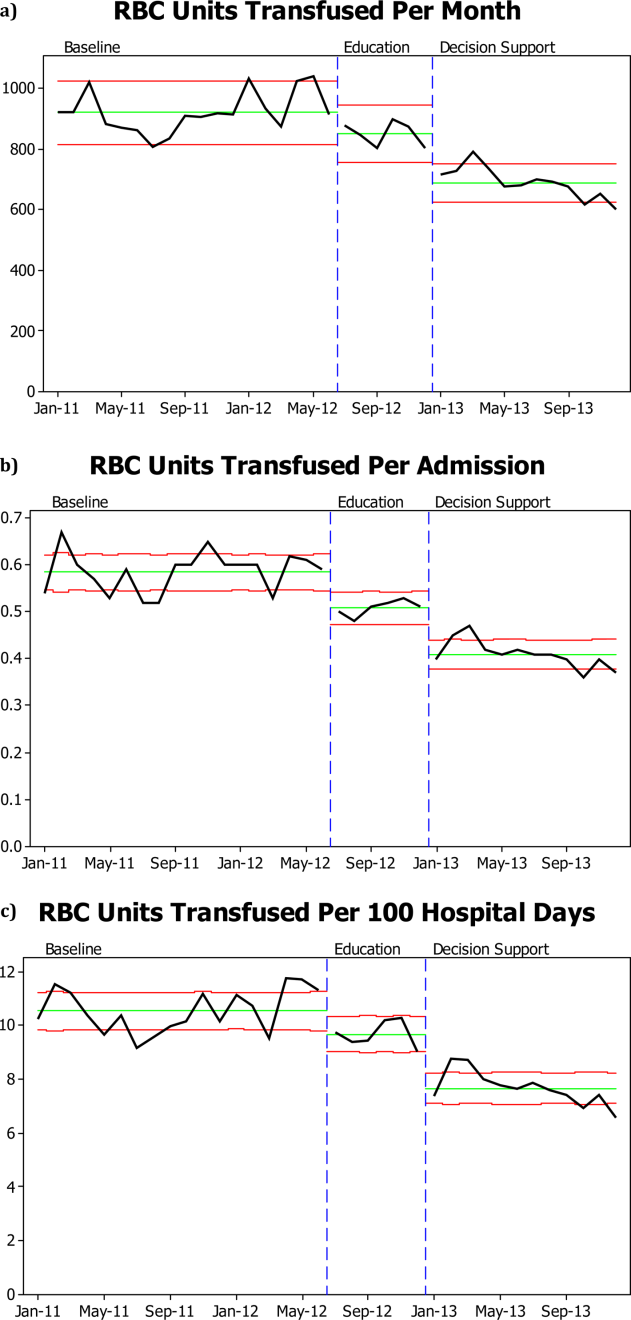
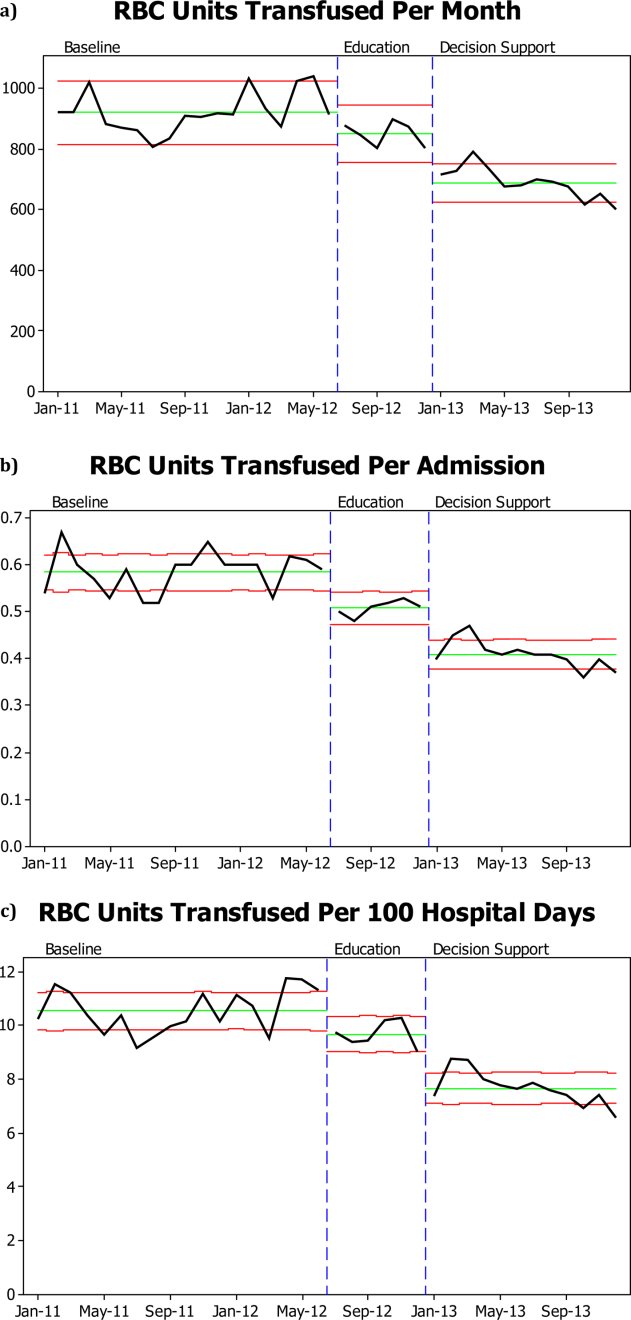
There was a significant decrease in the mean number of RBC units transfused as a result of the RBC transfusion program (Figure 1A). As compared to the baseline period, the mean number of RBC units transfused fell immediately during the 6 months following the initiation of the education program (92368 to 85240, P=0.02), and further still during the subsequent 12 months following the approval of the RBC transfusion guideline by the UAMS medical board and initiation of the RBC transfusion order set (to 69052, P<0.0001). These results do not reflect a change in the number of hospital admissions or length of stay; results are comparable if calculated based on RBC units transfused per patient admission or RBC per 100 patient‐days (Figure 1B,C). Overall, there was a 29% reduction in mean RBC units transfused per hospital admission (0.580.040.410.03, P=0.0001) and a 27% reduction in mean RBC units transfused per 100 hospital‐days (10.560.87.680.63, P=0.0001).
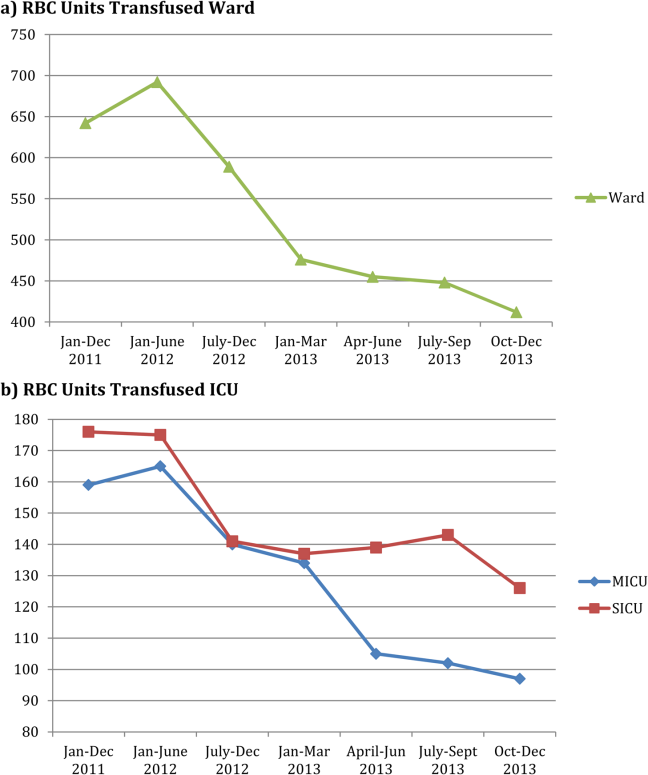
RBC transfusion reduction was observed in both the medical and surgical ICUs (Figure 2B) as well as the general patient wards (Figure 2A). The trends noted above were similar in the medical ICU and general patient wards; however, in the surgical ICU, the RBC transfusion rate fell on initiation of the education program and remained stable at this lower rate for the subsequent 18 months, with no further decrease following RBC transfusion guideline approval and initiation of the RBC order set.
There was no significant difference in hospital mortality observed pre‐ versus post‐RBC transfusion program (mortality index 0.890.05 vs 0.840.04, P=0.13).
DISCUSSION
We were able to demonstrate a 25% reduction in total RBC units transfused with an ongoing education program coupled with an institutional adoption of an RBC transfusion guideline that was incorporated into an RBC transfusion order set. Our program was novel in that the RBC transfusion guideline was approved by the hospital medical board as an institutional practice guideline. Importantly, the RBC transfusion reduction has been maintained over a 18‐month period. The program was instituted in stages: educational program, followed by guideline approval by the hospital medical board, and the initiation of an RBC transfusion order set. At each stage we observed an additive increase in RBC transfusion reduction, with the largest reduction following guideline approval and initiation of the order set.
The pattern of RBC transfusion reduction was observed in all areas of the hospital with the exception of the surgical ICU, where transfusion practice remained stable after the initial decrease in RBC transfusions following initiation of the education program. That RBC transfusion practice on the general surgical wards mirrored practice in other areas of the hospital suggests that the difference seen in the surgical ICU reflects factors unique to that specific area rather than the general approach of surgeons to RBC transfusion.
Despite the substantial data now available regarding RBC transfusion risks and the proliferation of RBC transfusion practice guidelines, wide variation in clinical practice still exists.[4, 5] The delay for evidence from clinical studies to be incorporated into clinical practice can be considerable. Balas and Boren[17] have estimated that it may take over 15 years from publication of a landmark study for the results to reach a 50% utilization rate in clinical practice. The barriers to guideline adherence have been described, including lack of familiarity, lack of agreement, and external factors.[6] Overcoming these barriers involve approaches toward knowledge, attitudes, and behavior.
There have been a number of approaches to changing RBC transfusion practice over the last 2 decades.[7, 8, 9, 10, 11, 12, 13, 14] These interventions have all achieved varying degrees of success. Most have involved some combination of education, practice guideline, and audit/feedback. More recently, technology has allowed computer‐assisted order entry and feedback. Goodnough et al.,[7] employing real‐time clinical decision support and best‐practice alerts, were able to achieve sustained adherence to clinical guidelines and a 24% reduction in RBC units transfused. Other recent reports have shown improvement in RBC transfusion practices comparable to what we observed with programs including audit/feedback and educational efforts.[13, 14]
Our approach to RBC transfusion practice was relatively simple, involving education followed by institutional adoption of a best‐practice guideline and simple RBC transfusion order form. We were able to begin to change RBC transfusion practice with the initiation of an education program; however, there was a more marked and persistent decrease in RBC transfusions following the adoption of the institution's RBC transfusion guideline and RBC transfusion order set. Although education alone is often ineffective in causing sustained change in behavior, a key aspect of our program was the approval of the RBC transfusion guideline by the hospital medical board. The approval by the hospital medical board, made up in part by the clinical leadership, was instrumental in changing the transfusion culture, or beliefs, in the institution. The consistency of practice seen within the time periods both before and after our intervention suggest a given set of beliefs driving RBC transfusion in each time period. Further supporting this view is the consistency of RBC transfusion practice change throughout the institution, and the fact that patient volumes and severity of illness were comparable pre‐ and postintervention. It is difficult to know which elements of the program were most important. It is likely that optimal transfusion practices promoted by the education program were reinforced by the guideline, which were further reinforced by the order set.
Given the known risks of RBC transfusion and the data supporting a restrictive approach to RBC transfusion practice, improved patient safety by aligning RBC transfusion with best‐practice guidelines was the primary goal of our RBC transfusion program.[1, 2] Although we were not able to look at specific complications such as infection rate, there was no change in overall hospital mortality. The total RBC units transfused at our institution fell by almost 30%. We estimate that in the 18 months following initiation of our program we saved approximately 3200 RBC units as compared with the number of RBC units that would have been transfused based on the transfusion rate prior to the initiation of our educational program. This preserves a scarce resource, RBCs, as a well as reduces cost. The cost of an RBC transfusion involves both the direct cost of the RBC unit as well as the cost of activities surrounding an RBC transfusion. Shander et al.,[18] using an activities‐based costing model, have estimated the direct and indirect cost of an RBC transfusion as between $522 and $1183 (mean $761). Over the last 18 months we have achieved a direct savings of $704,000 for purchase of RBC units and, using the low estimate based on the activities‐based costing model, a total savings of at least $1.7 million.
This study is limited by the fact that it reflects a single‐institution experience. Although we cannot exclude other factors contributing to the decrease in RBC transfusion, the pattern of response suggests that the RBC transfusion program was largely responsible for the results observed. Further, patient volumes at our institution have remained constant, as have surgical volumes. RBC transfusions are reduced comparably whether analyzed as total units transfused, units transfused per admission, or units transfused per 100 patient‐days. The complexity of care also limits our ability to draw any conclusions regarding the impact of RBC transfusion reduction on patient outcome. We also do not know how consistent RBC transfusion practice prior to our program was with our guideline; however, the significant decline in RBC units transfused following our intervention suggests that there was a discrepancy in RBC transfusion practice preintervention.
In conclusion, an education program coupled with institutional adoption of a best‐practice RBC transfusion guideline and a RBC transfusion order set resulted in consistent reduction in RBC units transfused. The improvement in RBC transfusion practice was additive with implementation of each intervention. RBC transfusion practice was changed in all areas of the hospital and resulted in less exposure of patients to RBC transfusion risks, preserved a scarce resource, and was a direct cost savings.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042.
- , . Efficacy of RBC transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674.
- , , , , . A new perspective on best transfusion practice. Blood Transfus. 2013;11:193–202.
- , , , et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–1575.
- , , , et al. RBC transfusion practices among critically ill patients: has evidence changed practice? Crit Care Med. 2013;41:2344–2353.
- , , , et al. Why don't physicians follow clinical practice guidelines? JAMA. 1999;282:1458–1465.
- , , , , , . Improved blood utilization using real‐time clinical decision support. Transfusion. 2014;54:1358–1365.
- , , , , , . Computerized physician order entry with decision support decreases blood transfusion in children. Pediatrics. 2011;127:e1112–e1119.
- , , , et al. Reducing the amount of blood transfused. Arch Intern Med. 2005;165:845–852.
- , , , et al. Assessment of education and computerized decision support interventions for improving transfusion practice. Transfusion. 2007;47:228–239.
- , , , , Transfusion insurgency: practice change through education and evidence‐based recommendations. Am J Surg. 2009;197:279–283.
- , , , et al. Evidence‐based red cell transfusion in the critically ill: quality improvement using computerized physician order entry. Crit Care Med. 2006;34:1892–1897.
- , , . The addition of decision support into computerize physician order entry reduces red blood cell transfusion resource utilization in the intensive care unit. Am J Hematol. 2007;82:631–633.
- , , , et al. How we closed the gap between red blood cell utilization and whole blood collections in our institution. Transfusion. 2012;52:1857–1867.
- , , , et al. American College of Critical Care and Eastern Association of Trauma. Clinical practice guideline: red blood cell transfusion practice in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157.
- , , , et al. Red blood cell transfusion: a clinical practice guideline of the AABB. Ann Intern Med. 2012;157:49–58.
- , . Managing clinical knowledge for health care improvement. In: Bemmel J, McCray AT, eds. Yearbook of Medical Informatics 2000: Patient‐Centered Systems. Stuttgart, Germany: Schattauer Verlagsgesellschaft; 2000:65–70.
- , , , et al. Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–764.
Historically, red blood cell (RBC) transfusions have been viewed as safe and effective means of treating anemia and improving oxygen delivery to tissues. Beginning in the early 1980s, primarily driven by concerns related to the risks of transfusion‐related infection, transfusion practice began to come under scrutiny.
Numerous studies over the past 2 decades have failed to demonstrate a benefit of RBC transfusion in many of the clinical situations in which RBC transfusions are routinely given, and many of these studies have in fact shown that RBC transfusion may lead to worse clinical outcomes in some patients.[1, 2] The few available large, randomized clinical trials and prospective observational studies that have assessed the effectiveness of allogeneic RBC transfusion have demonstrated that a more restrictive approach to RBC transfusion results in at least equivalent patient outcomes as compared to a liberal approach, and may in fact reduce morbidity and mortality rates.[1, 2]
Over the last decade, RBC transfusion best‐practice guidelines have been developed by a number of professional societies,[3] addressing RBC transfusion practice in specific patient populations including critical care as well as more general hospitalized populations. These guidelines are generally consistent, strongly recommending a restrictive RBC transfusion approach in most clinical populations. However, despite the general consistency of the guidelines and the lack of evidence for the efficacy of RBC transfusion, there still remains significant variability in clinical RBC transfusion practice.[4, 5]
The difficulty in getting physicians to follow clinical guidelines in general has been well described.[6] Over the last 2 decades there have been reports of a variety of interventions directed toward improving RBC transfusion practice either in specific care units (eg, intensive care units [ICUs]) or institution wide.[7, 8, 9, 10, 11, 12, 13, 14] These initiatives have had varying degrees of success and have employed strategies that have included clinical guidelines, education, audit/feedback, and most recently computer order entry and decision support. We report on the effectiveness of an institution‐wide intervention to align RBC transfusion practice with best‐practice clinical guidelines. Our approach included institutional endorsement of a RBC transfusion guideline coupled with an ongoing education program and RBC transfusion order set.
METHODS
Study Setting
The University of Arkansas for Medical Sciences (UAMS) is a tertiary care university teaching hospital with a total of 437 patient beds. UAMS is a level 1 trauma center and has 52 ICU beds. The study took place between July 2012 and December 2013. At the time of study initiation, there was no institutional RBC transfusion protocol or guideline.
Study Design
In June 2012, a program was initiated to align RBC transfusion practice at UAMS with best‐practice RBC transfusion guidelines. This initiative consisted of several components: a series of educational programs, followed by hospital medical board approval of an intuitional RBC transfusion guideline, and initiation of an RBC transfusion order set of approved RBC transfusion guideline recommendations (Table 1).
| RBC Transfusion Guideline | |
|---|---|
| |
| PURPOSE: Unnecessary blood transfusions increase healthcare costs and expose patients to potential infectious and noninfectious risks. The purpose of this clinical practice guideline is to establish an evidence‐based approach to the transfusion of RBCs in hospitalized patients at UAMS. | |
| GUIDELINE: In order to avoid the potential risks and increased costs associated with unnecessary blood transfusions, the medical staff of UAMS will adhere to a restrictive transfusion strategy in which: | |
| (I) RBC transfusion should be considered unnecessary for hospitalized, hemodynamically stable patients unless the hemoglobin concentration is <78 g/dL. | |
| (II) RBC transfusion is appropriate for patients who have evidence of acute hemorrhage or hemorrhagic shock. | |
| (III) RBC transfusion is appropriate for patients with acute MI or unstable myocardial ischemia if the hemoglobin concentration is 8 g/dL. | |
| (IV) The use of the hemoglobin concentration alone as a trigger for RBC transfusion should be avoided. The decision to order an RBC transfusion should also consider a patient's intravascular volume status, evidence of shock, duration and extent of anemia, and cardiopulmonary physiologic parameters as well as other symptomatology. | |
| (V) In the absence of acute hemorrhage, an RBC transfusion should be ordered and administered as single units. | |
| (VI) It is the physician's responsibility to weigh the risks and benefits of an RBC transfusion for a particular patient based on their medical condition. As such, it is recognized that there will be situations in which an RBC transfusion is appropriate outside of the guidelines put forth in this document. In these instances, the physician should document in the medical record his/her rationale for the RBC transfusion. | |
| RBC Transfusion Order Form | |
| The following are RBC transfusion indications consistent with UAMS‐approved guidelines (check 1): | |
| Acute hemorrhage or hemorrhagic shock | Yes |
| Hgb <78 g/dL | Yes |
| Acute MI, Hgb 8 g/dL | Yes |
| Acute coronary syndrome Hgb 8 g/dL | Yes |
| If the RBC transfusion is for an indication other than those listed above, please note the indication and attending physician in the space provided. | |
| Other indications/attending physician | Free text of other indications. |
| In the absence of acute hemorrhage or a hemoglobin concentration <6.5 g/dL, it is recommended that RBCs be ordered as single units. | |
The educational program included grand rounds presentations for all major clinical departments (internal medicine, surgery, obstetrics and gynecology, geriatrics, anesthesiology), presentations to high‐volume transfusing services (hematology, vascular surgery, cardiac surgery), presentations to hightransfusion‐volume nursing units (eg, medical and surgical ICUs, intermediate care unit, hematology), and scheduled and ad hoc resident educational programs. Educational sessions were repeated over the 18 months of the study and were presented by a clinical content expert.
A UAMS‐specific transfusion guideline was developed based on published best‐practice guidelines.[15, 16] The UAMS medical board approved this guideline in November 2012 (Table 1). The guidelines were disseminated to the entire medical staff in December 2012 via email communication from the hospital's chief medical officer. Membership of the medical board included clinical leadership of the medical center (ie, department chairs) as well as ad hoc members from the hospital administrative leadership.
An RBC transfusion order form that included the guideline recommendations was implemented in the electronic medical record (Sunrise Enterprise 5.5; Eclipsys Corp., Atlanta, GA) in March 2013. There was no hard stop for an RBC transfusion order that was outside of the guideline recommendations; however, for documentation, the ordering physician was required to note the indication and the supervising attending physician for these out‐of‐guideline RBC transfusions. RBC transfusion orders are entered in an electronic medical record. There was no alert triggered by an RBC transfusion order outside of the RBC transfusion guideline.
Outcomes
The number of RBC units transfused during the baseline period of January 2011 through June 2012 was compared with RBC units transfused July 2012 through December 2013. The latter period was further divided into the time period July 2012 through December 2012, during which the education program was initiated (education) as well as the time period January 2013 through December 2013 following the transfusion guideline approval and the initiation of the transfusion order set (decision support). All adult inpatient RBC units transfused, excluding RBC units transfused in the operating room and emergency room, were included in the analysis. RBC transfusions per month were normalized to RBC transfusions per 28 days. RBC transfusions were also calculated as RBC units per adult hospital admission and RBC units per 100 patient‐days.
Hospital mortality is presented as mortality index (observed/predicted mortality). The mean weighted diagnosis‐related group (DRG) was calculated using the monthly average of the Centers for Medicare and Medicaid Services (CMS)‐derived relative weighted DRGs.
Statistical Analysis
Data are presented as meanstandard deviation. Comparisons were by Student t test or analysis of variance as appropriate. GraphPad InStat (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis, and Minitab (Minitab Inc., State College, PA) was used for control graphs.
RESULTS
There were 28,393 adult admissions (excluding psychiatry) during the baseline period (January 2011June 2012) and 35,743 (12,353 education, 23,390 decision support) adult admissions during the study period (July 2012December 2013). The patient demographics for the 3 time periods were comparable (Table 2).
| Baseline | Education | Decision Support | |
|---|---|---|---|
| |||
| Total patients | 28,393 | 12,353 | 23,390 |
| Age, mean, y* | 48.20.6 | 480.1 | 480.5 |
| Gender, % female | 56 | 57 | 58 |
| Race, % non‐Caucasian | 63 | 61 | 61 |
| Weighted DRG | 1.60 | 1.59 | 1.59 |
| MDC, % | |||
| Nervous system | 13 | 13 | 12 |
| Circulatory system | 11 | 12 | 11 |
| Digestive system | 10 | 10 | 10 |
| Respiratory system | 9 | 8 | 9 |
| Musculoskeletal system | 8 | 8 | 8 |
| Kidney and urinary tract | 8 | 8 | 8 |
| Hepatobiliary system | 5 | 5 | 5 |
| Infectious and parasitic | 5 | 5 | 6 |
| Endocrine, metabolic | 3 | 4 | 3 |
| Blood, immunologic | 3 | 2 | 2 |
| Myeloproliferative | 4 | 4 | 3 |
| Multiple significant trauma | 1 | 1 | 1 |
| Other | 20 | 20 | 22 |
There was a significant decrease in the mean number of RBC units transfused as a result of the RBC transfusion program (Figure 1A). As compared to the baseline period, the mean number of RBC units transfused fell immediately during the 6 months following the initiation of the education program (92368 to 85240, P=0.02), and further still during the subsequent 12 months following the approval of the RBC transfusion guideline by the UAMS medical board and initiation of the RBC transfusion order set (to 69052, P<0.0001). These results do not reflect a change in the number of hospital admissions or length of stay; results are comparable if calculated based on RBC units transfused per patient admission or RBC per 100 patient‐days (Figure 1B,C). Overall, there was a 29% reduction in mean RBC units transfused per hospital admission (0.580.040.410.03, P=0.0001) and a 27% reduction in mean RBC units transfused per 100 hospital‐days (10.560.87.680.63, P=0.0001).

RBC transfusion reduction was observed in both the medical and surgical ICUs (Figure 2B) as well as the general patient wards (Figure 2A). The trends noted above were similar in the medical ICU and general patient wards; however, in the surgical ICU, the RBC transfusion rate fell on initiation of the education program and remained stable at this lower rate for the subsequent 18 months, with no further decrease following RBC transfusion guideline approval and initiation of the RBC order set.

There was no significant difference in hospital mortality observed pre‐ versus post‐RBC transfusion program (mortality index 0.890.05 vs 0.840.04, P=0.13).
DISCUSSION
We were able to demonstrate a 25% reduction in total RBC units transfused with an ongoing education program coupled with an institutional adoption of an RBC transfusion guideline that was incorporated into an RBC transfusion order set. Our program was novel in that the RBC transfusion guideline was approved by the hospital medical board as an institutional practice guideline. Importantly, the RBC transfusion reduction has been maintained over a 18‐month period. The program was instituted in stages: educational program, followed by guideline approval by the hospital medical board, and the initiation of an RBC transfusion order set. At each stage we observed an additive increase in RBC transfusion reduction, with the largest reduction following guideline approval and initiation of the order set.
The pattern of RBC transfusion reduction was observed in all areas of the hospital with the exception of the surgical ICU, where transfusion practice remained stable after the initial decrease in RBC transfusions following initiation of the education program. That RBC transfusion practice on the general surgical wards mirrored practice in other areas of the hospital suggests that the difference seen in the surgical ICU reflects factors unique to that specific area rather than the general approach of surgeons to RBC transfusion.
Despite the substantial data now available regarding RBC transfusion risks and the proliferation of RBC transfusion practice guidelines, wide variation in clinical practice still exists.[4, 5] The delay for evidence from clinical studies to be incorporated into clinical practice can be considerable. Balas and Boren[17] have estimated that it may take over 15 years from publication of a landmark study for the results to reach a 50% utilization rate in clinical practice. The barriers to guideline adherence have been described, including lack of familiarity, lack of agreement, and external factors.[6] Overcoming these barriers involve approaches toward knowledge, attitudes, and behavior.
There have been a number of approaches to changing RBC transfusion practice over the last 2 decades.[7, 8, 9, 10, 11, 12, 13, 14] These interventions have all achieved varying degrees of success. Most have involved some combination of education, practice guideline, and audit/feedback. More recently, technology has allowed computer‐assisted order entry and feedback. Goodnough et al.,[7] employing real‐time clinical decision support and best‐practice alerts, were able to achieve sustained adherence to clinical guidelines and a 24% reduction in RBC units transfused. Other recent reports have shown improvement in RBC transfusion practices comparable to what we observed with programs including audit/feedback and educational efforts.[13, 14]
Our approach to RBC transfusion practice was relatively simple, involving education followed by institutional adoption of a best‐practice guideline and simple RBC transfusion order form. We were able to begin to change RBC transfusion practice with the initiation of an education program; however, there was a more marked and persistent decrease in RBC transfusions following the adoption of the institution's RBC transfusion guideline and RBC transfusion order set. Although education alone is often ineffective in causing sustained change in behavior, a key aspect of our program was the approval of the RBC transfusion guideline by the hospital medical board. The approval by the hospital medical board, made up in part by the clinical leadership, was instrumental in changing the transfusion culture, or beliefs, in the institution. The consistency of practice seen within the time periods both before and after our intervention suggest a given set of beliefs driving RBC transfusion in each time period. Further supporting this view is the consistency of RBC transfusion practice change throughout the institution, and the fact that patient volumes and severity of illness were comparable pre‐ and postintervention. It is difficult to know which elements of the program were most important. It is likely that optimal transfusion practices promoted by the education program were reinforced by the guideline, which were further reinforced by the order set.
Given the known risks of RBC transfusion and the data supporting a restrictive approach to RBC transfusion practice, improved patient safety by aligning RBC transfusion with best‐practice guidelines was the primary goal of our RBC transfusion program.[1, 2] Although we were not able to look at specific complications such as infection rate, there was no change in overall hospital mortality. The total RBC units transfused at our institution fell by almost 30%. We estimate that in the 18 months following initiation of our program we saved approximately 3200 RBC units as compared with the number of RBC units that would have been transfused based on the transfusion rate prior to the initiation of our educational program. This preserves a scarce resource, RBCs, as a well as reduces cost. The cost of an RBC transfusion involves both the direct cost of the RBC unit as well as the cost of activities surrounding an RBC transfusion. Shander et al.,[18] using an activities‐based costing model, have estimated the direct and indirect cost of an RBC transfusion as between $522 and $1183 (mean $761). Over the last 18 months we have achieved a direct savings of $704,000 for purchase of RBC units and, using the low estimate based on the activities‐based costing model, a total savings of at least $1.7 million.
This study is limited by the fact that it reflects a single‐institution experience. Although we cannot exclude other factors contributing to the decrease in RBC transfusion, the pattern of response suggests that the RBC transfusion program was largely responsible for the results observed. Further, patient volumes at our institution have remained constant, as have surgical volumes. RBC transfusions are reduced comparably whether analyzed as total units transfused, units transfused per admission, or units transfused per 100 patient‐days. The complexity of care also limits our ability to draw any conclusions regarding the impact of RBC transfusion reduction on patient outcome. We also do not know how consistent RBC transfusion practice prior to our program was with our guideline; however, the significant decline in RBC units transfused following our intervention suggests that there was a discrepancy in RBC transfusion practice preintervention.
In conclusion, an education program coupled with institutional adoption of a best‐practice RBC transfusion guideline and a RBC transfusion order set resulted in consistent reduction in RBC units transfused. The improvement in RBC transfusion practice was additive with implementation of each intervention. RBC transfusion practice was changed in all areas of the hospital and resulted in less exposure of patients to RBC transfusion risks, preserved a scarce resource, and was a direct cost savings.
Historically, red blood cell (RBC) transfusions have been viewed as safe and effective means of treating anemia and improving oxygen delivery to tissues. Beginning in the early 1980s, primarily driven by concerns related to the risks of transfusion‐related infection, transfusion practice began to come under scrutiny.
Numerous studies over the past 2 decades have failed to demonstrate a benefit of RBC transfusion in many of the clinical situations in which RBC transfusions are routinely given, and many of these studies have in fact shown that RBC transfusion may lead to worse clinical outcomes in some patients.[1, 2] The few available large, randomized clinical trials and prospective observational studies that have assessed the effectiveness of allogeneic RBC transfusion have demonstrated that a more restrictive approach to RBC transfusion results in at least equivalent patient outcomes as compared to a liberal approach, and may in fact reduce morbidity and mortality rates.[1, 2]
Over the last decade, RBC transfusion best‐practice guidelines have been developed by a number of professional societies,[3] addressing RBC transfusion practice in specific patient populations including critical care as well as more general hospitalized populations. These guidelines are generally consistent, strongly recommending a restrictive RBC transfusion approach in most clinical populations. However, despite the general consistency of the guidelines and the lack of evidence for the efficacy of RBC transfusion, there still remains significant variability in clinical RBC transfusion practice.[4, 5]
The difficulty in getting physicians to follow clinical guidelines in general has been well described.[6] Over the last 2 decades there have been reports of a variety of interventions directed toward improving RBC transfusion practice either in specific care units (eg, intensive care units [ICUs]) or institution wide.[7, 8, 9, 10, 11, 12, 13, 14] These initiatives have had varying degrees of success and have employed strategies that have included clinical guidelines, education, audit/feedback, and most recently computer order entry and decision support. We report on the effectiveness of an institution‐wide intervention to align RBC transfusion practice with best‐practice clinical guidelines. Our approach included institutional endorsement of a RBC transfusion guideline coupled with an ongoing education program and RBC transfusion order set.
METHODS
Study Setting
The University of Arkansas for Medical Sciences (UAMS) is a tertiary care university teaching hospital with a total of 437 patient beds. UAMS is a level 1 trauma center and has 52 ICU beds. The study took place between July 2012 and December 2013. At the time of study initiation, there was no institutional RBC transfusion protocol or guideline.
Study Design
In June 2012, a program was initiated to align RBC transfusion practice at UAMS with best‐practice RBC transfusion guidelines. This initiative consisted of several components: a series of educational programs, followed by hospital medical board approval of an intuitional RBC transfusion guideline, and initiation of an RBC transfusion order set of approved RBC transfusion guideline recommendations (Table 1).
| RBC Transfusion Guideline | |
|---|---|
| |
| PURPOSE: Unnecessary blood transfusions increase healthcare costs and expose patients to potential infectious and noninfectious risks. The purpose of this clinical practice guideline is to establish an evidence‐based approach to the transfusion of RBCs in hospitalized patients at UAMS. | |
| GUIDELINE: In order to avoid the potential risks and increased costs associated with unnecessary blood transfusions, the medical staff of UAMS will adhere to a restrictive transfusion strategy in which: | |
| (I) RBC transfusion should be considered unnecessary for hospitalized, hemodynamically stable patients unless the hemoglobin concentration is <78 g/dL. | |
| (II) RBC transfusion is appropriate for patients who have evidence of acute hemorrhage or hemorrhagic shock. | |
| (III) RBC transfusion is appropriate for patients with acute MI or unstable myocardial ischemia if the hemoglobin concentration is 8 g/dL. | |
| (IV) The use of the hemoglobin concentration alone as a trigger for RBC transfusion should be avoided. The decision to order an RBC transfusion should also consider a patient's intravascular volume status, evidence of shock, duration and extent of anemia, and cardiopulmonary physiologic parameters as well as other symptomatology. | |
| (V) In the absence of acute hemorrhage, an RBC transfusion should be ordered and administered as single units. | |
| (VI) It is the physician's responsibility to weigh the risks and benefits of an RBC transfusion for a particular patient based on their medical condition. As such, it is recognized that there will be situations in which an RBC transfusion is appropriate outside of the guidelines put forth in this document. In these instances, the physician should document in the medical record his/her rationale for the RBC transfusion. | |
| RBC Transfusion Order Form | |
| The following are RBC transfusion indications consistent with UAMS‐approved guidelines (check 1): | |
| Acute hemorrhage or hemorrhagic shock | Yes |
| Hgb <78 g/dL | Yes |
| Acute MI, Hgb 8 g/dL | Yes |
| Acute coronary syndrome Hgb 8 g/dL | Yes |
| If the RBC transfusion is for an indication other than those listed above, please note the indication and attending physician in the space provided. | |
| Other indications/attending physician | Free text of other indications. |
| In the absence of acute hemorrhage or a hemoglobin concentration <6.5 g/dL, it is recommended that RBCs be ordered as single units. | |
The educational program included grand rounds presentations for all major clinical departments (internal medicine, surgery, obstetrics and gynecology, geriatrics, anesthesiology), presentations to high‐volume transfusing services (hematology, vascular surgery, cardiac surgery), presentations to hightransfusion‐volume nursing units (eg, medical and surgical ICUs, intermediate care unit, hematology), and scheduled and ad hoc resident educational programs. Educational sessions were repeated over the 18 months of the study and were presented by a clinical content expert.
A UAMS‐specific transfusion guideline was developed based on published best‐practice guidelines.[15, 16] The UAMS medical board approved this guideline in November 2012 (Table 1). The guidelines were disseminated to the entire medical staff in December 2012 via email communication from the hospital's chief medical officer. Membership of the medical board included clinical leadership of the medical center (ie, department chairs) as well as ad hoc members from the hospital administrative leadership.
An RBC transfusion order form that included the guideline recommendations was implemented in the electronic medical record (Sunrise Enterprise 5.5; Eclipsys Corp., Atlanta, GA) in March 2013. There was no hard stop for an RBC transfusion order that was outside of the guideline recommendations; however, for documentation, the ordering physician was required to note the indication and the supervising attending physician for these out‐of‐guideline RBC transfusions. RBC transfusion orders are entered in an electronic medical record. There was no alert triggered by an RBC transfusion order outside of the RBC transfusion guideline.
Outcomes
The number of RBC units transfused during the baseline period of January 2011 through June 2012 was compared with RBC units transfused July 2012 through December 2013. The latter period was further divided into the time period July 2012 through December 2012, during which the education program was initiated (education) as well as the time period January 2013 through December 2013 following the transfusion guideline approval and the initiation of the transfusion order set (decision support). All adult inpatient RBC units transfused, excluding RBC units transfused in the operating room and emergency room, were included in the analysis. RBC transfusions per month were normalized to RBC transfusions per 28 days. RBC transfusions were also calculated as RBC units per adult hospital admission and RBC units per 100 patient‐days.
Hospital mortality is presented as mortality index (observed/predicted mortality). The mean weighted diagnosis‐related group (DRG) was calculated using the monthly average of the Centers for Medicare and Medicaid Services (CMS)‐derived relative weighted DRGs.
Statistical Analysis
Data are presented as meanstandard deviation. Comparisons were by Student t test or analysis of variance as appropriate. GraphPad InStat (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis, and Minitab (Minitab Inc., State College, PA) was used for control graphs.
RESULTS
There were 28,393 adult admissions (excluding psychiatry) during the baseline period (January 2011June 2012) and 35,743 (12,353 education, 23,390 decision support) adult admissions during the study period (July 2012December 2013). The patient demographics for the 3 time periods were comparable (Table 2).
| Baseline | Education | Decision Support | |
|---|---|---|---|
| |||
| Total patients | 28,393 | 12,353 | 23,390 |
| Age, mean, y* | 48.20.6 | 480.1 | 480.5 |
| Gender, % female | 56 | 57 | 58 |
| Race, % non‐Caucasian | 63 | 61 | 61 |
| Weighted DRG | 1.60 | 1.59 | 1.59 |
| MDC, % | |||
| Nervous system | 13 | 13 | 12 |
| Circulatory system | 11 | 12 | 11 |
| Digestive system | 10 | 10 | 10 |
| Respiratory system | 9 | 8 | 9 |
| Musculoskeletal system | 8 | 8 | 8 |
| Kidney and urinary tract | 8 | 8 | 8 |
| Hepatobiliary system | 5 | 5 | 5 |
| Infectious and parasitic | 5 | 5 | 6 |
| Endocrine, metabolic | 3 | 4 | 3 |
| Blood, immunologic | 3 | 2 | 2 |
| Myeloproliferative | 4 | 4 | 3 |
| Multiple significant trauma | 1 | 1 | 1 |
| Other | 20 | 20 | 22 |
There was a significant decrease in the mean number of RBC units transfused as a result of the RBC transfusion program (Figure 1A). As compared to the baseline period, the mean number of RBC units transfused fell immediately during the 6 months following the initiation of the education program (92368 to 85240, P=0.02), and further still during the subsequent 12 months following the approval of the RBC transfusion guideline by the UAMS medical board and initiation of the RBC transfusion order set (to 69052, P<0.0001). These results do not reflect a change in the number of hospital admissions or length of stay; results are comparable if calculated based on RBC units transfused per patient admission or RBC per 100 patient‐days (Figure 1B,C). Overall, there was a 29% reduction in mean RBC units transfused per hospital admission (0.580.040.410.03, P=0.0001) and a 27% reduction in mean RBC units transfused per 100 hospital‐days (10.560.87.680.63, P=0.0001).

RBC transfusion reduction was observed in both the medical and surgical ICUs (Figure 2B) as well as the general patient wards (Figure 2A). The trends noted above were similar in the medical ICU and general patient wards; however, in the surgical ICU, the RBC transfusion rate fell on initiation of the education program and remained stable at this lower rate for the subsequent 18 months, with no further decrease following RBC transfusion guideline approval and initiation of the RBC order set.

There was no significant difference in hospital mortality observed pre‐ versus post‐RBC transfusion program (mortality index 0.890.05 vs 0.840.04, P=0.13).
DISCUSSION
We were able to demonstrate a 25% reduction in total RBC units transfused with an ongoing education program coupled with an institutional adoption of an RBC transfusion guideline that was incorporated into an RBC transfusion order set. Our program was novel in that the RBC transfusion guideline was approved by the hospital medical board as an institutional practice guideline. Importantly, the RBC transfusion reduction has been maintained over a 18‐month period. The program was instituted in stages: educational program, followed by guideline approval by the hospital medical board, and the initiation of an RBC transfusion order set. At each stage we observed an additive increase in RBC transfusion reduction, with the largest reduction following guideline approval and initiation of the order set.
The pattern of RBC transfusion reduction was observed in all areas of the hospital with the exception of the surgical ICU, where transfusion practice remained stable after the initial decrease in RBC transfusions following initiation of the education program. That RBC transfusion practice on the general surgical wards mirrored practice in other areas of the hospital suggests that the difference seen in the surgical ICU reflects factors unique to that specific area rather than the general approach of surgeons to RBC transfusion.
Despite the substantial data now available regarding RBC transfusion risks and the proliferation of RBC transfusion practice guidelines, wide variation in clinical practice still exists.[4, 5] The delay for evidence from clinical studies to be incorporated into clinical practice can be considerable. Balas and Boren[17] have estimated that it may take over 15 years from publication of a landmark study for the results to reach a 50% utilization rate in clinical practice. The barriers to guideline adherence have been described, including lack of familiarity, lack of agreement, and external factors.[6] Overcoming these barriers involve approaches toward knowledge, attitudes, and behavior.
There have been a number of approaches to changing RBC transfusion practice over the last 2 decades.[7, 8, 9, 10, 11, 12, 13, 14] These interventions have all achieved varying degrees of success. Most have involved some combination of education, practice guideline, and audit/feedback. More recently, technology has allowed computer‐assisted order entry and feedback. Goodnough et al.,[7] employing real‐time clinical decision support and best‐practice alerts, were able to achieve sustained adherence to clinical guidelines and a 24% reduction in RBC units transfused. Other recent reports have shown improvement in RBC transfusion practices comparable to what we observed with programs including audit/feedback and educational efforts.[13, 14]
Our approach to RBC transfusion practice was relatively simple, involving education followed by institutional adoption of a best‐practice guideline and simple RBC transfusion order form. We were able to begin to change RBC transfusion practice with the initiation of an education program; however, there was a more marked and persistent decrease in RBC transfusions following the adoption of the institution's RBC transfusion guideline and RBC transfusion order set. Although education alone is often ineffective in causing sustained change in behavior, a key aspect of our program was the approval of the RBC transfusion guideline by the hospital medical board. The approval by the hospital medical board, made up in part by the clinical leadership, was instrumental in changing the transfusion culture, or beliefs, in the institution. The consistency of practice seen within the time periods both before and after our intervention suggest a given set of beliefs driving RBC transfusion in each time period. Further supporting this view is the consistency of RBC transfusion practice change throughout the institution, and the fact that patient volumes and severity of illness were comparable pre‐ and postintervention. It is difficult to know which elements of the program were most important. It is likely that optimal transfusion practices promoted by the education program were reinforced by the guideline, which were further reinforced by the order set.
Given the known risks of RBC transfusion and the data supporting a restrictive approach to RBC transfusion practice, improved patient safety by aligning RBC transfusion with best‐practice guidelines was the primary goal of our RBC transfusion program.[1, 2] Although we were not able to look at specific complications such as infection rate, there was no change in overall hospital mortality. The total RBC units transfused at our institution fell by almost 30%. We estimate that in the 18 months following initiation of our program we saved approximately 3200 RBC units as compared with the number of RBC units that would have been transfused based on the transfusion rate prior to the initiation of our educational program. This preserves a scarce resource, RBCs, as a well as reduces cost. The cost of an RBC transfusion involves both the direct cost of the RBC unit as well as the cost of activities surrounding an RBC transfusion. Shander et al.,[18] using an activities‐based costing model, have estimated the direct and indirect cost of an RBC transfusion as between $522 and $1183 (mean $761). Over the last 18 months we have achieved a direct savings of $704,000 for purchase of RBC units and, using the low estimate based on the activities‐based costing model, a total savings of at least $1.7 million.
This study is limited by the fact that it reflects a single‐institution experience. Although we cannot exclude other factors contributing to the decrease in RBC transfusion, the pattern of response suggests that the RBC transfusion program was largely responsible for the results observed. Further, patient volumes at our institution have remained constant, as have surgical volumes. RBC transfusions are reduced comparably whether analyzed as total units transfused, units transfused per admission, or units transfused per 100 patient‐days. The complexity of care also limits our ability to draw any conclusions regarding the impact of RBC transfusion reduction on patient outcome. We also do not know how consistent RBC transfusion practice prior to our program was with our guideline; however, the significant decline in RBC units transfused following our intervention suggests that there was a discrepancy in RBC transfusion practice preintervention.
In conclusion, an education program coupled with institutional adoption of a best‐practice RBC transfusion guideline and a RBC transfusion order set resulted in consistent reduction in RBC units transfused. The improvement in RBC transfusion practice was additive with implementation of each intervention. RBC transfusion practice was changed in all areas of the hospital and resulted in less exposure of patients to RBC transfusion risks, preserved a scarce resource, and was a direct cost savings.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042.
- , . Efficacy of RBC transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674.
- , , , , . A new perspective on best transfusion practice. Blood Transfus. 2013;11:193–202.
- , , , et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–1575.
- , , , et al. RBC transfusion practices among critically ill patients: has evidence changed practice? Crit Care Med. 2013;41:2344–2353.
- , , , et al. Why don't physicians follow clinical practice guidelines? JAMA. 1999;282:1458–1465.
- , , , , , . Improved blood utilization using real‐time clinical decision support. Transfusion. 2014;54:1358–1365.
- , , , , , . Computerized physician order entry with decision support decreases blood transfusion in children. Pediatrics. 2011;127:e1112–e1119.
- , , , et al. Reducing the amount of blood transfused. Arch Intern Med. 2005;165:845–852.
- , , , et al. Assessment of education and computerized decision support interventions for improving transfusion practice. Transfusion. 2007;47:228–239.
- , , , , Transfusion insurgency: practice change through education and evidence‐based recommendations. Am J Surg. 2009;197:279–283.
- , , , et al. Evidence‐based red cell transfusion in the critically ill: quality improvement using computerized physician order entry. Crit Care Med. 2006;34:1892–1897.
- , , . The addition of decision support into computerize physician order entry reduces red blood cell transfusion resource utilization in the intensive care unit. Am J Hematol. 2007;82:631–633.
- , , , et al. How we closed the gap between red blood cell utilization and whole blood collections in our institution. Transfusion. 2012;52:1857–1867.
- , , , et al. American College of Critical Care and Eastern Association of Trauma. Clinical practice guideline: red blood cell transfusion practice in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157.
- , , , et al. Red blood cell transfusion: a clinical practice guideline of the AABB. Ann Intern Med. 2012;157:49–58.
- , . Managing clinical knowledge for health care improvement. In: Bemmel J, McCray AT, eds. Yearbook of Medical Informatics 2000: Patient‐Centered Systems. Stuttgart, Germany: Schattauer Verlagsgesellschaft; 2000:65–70.
- , , , et al. Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–764.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042.
- , . Efficacy of RBC transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674.
- , , , , . A new perspective on best transfusion practice. Blood Transfus. 2013;11:193–202.
- , , , et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–1575.
- , , , et al. RBC transfusion practices among critically ill patients: has evidence changed practice? Crit Care Med. 2013;41:2344–2353.
- , , , et al. Why don't physicians follow clinical practice guidelines? JAMA. 1999;282:1458–1465.
- , , , , , . Improved blood utilization using real‐time clinical decision support. Transfusion. 2014;54:1358–1365.
- , , , , , . Computerized physician order entry with decision support decreases blood transfusion in children. Pediatrics. 2011;127:e1112–e1119.
- , , , et al. Reducing the amount of blood transfused. Arch Intern Med. 2005;165:845–852.
- , , , et al. Assessment of education and computerized decision support interventions for improving transfusion practice. Transfusion. 2007;47:228–239.
- , , , , Transfusion insurgency: practice change through education and evidence‐based recommendations. Am J Surg. 2009;197:279–283.
- , , , et al. Evidence‐based red cell transfusion in the critically ill: quality improvement using computerized physician order entry. Crit Care Med. 2006;34:1892–1897.
- , , . The addition of decision support into computerize physician order entry reduces red blood cell transfusion resource utilization in the intensive care unit. Am J Hematol. 2007;82:631–633.
- , , , et al. How we closed the gap between red blood cell utilization and whole blood collections in our institution. Transfusion. 2012;52:1857–1867.
- , , , et al. American College of Critical Care and Eastern Association of Trauma. Clinical practice guideline: red blood cell transfusion practice in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157.
- , , , et al. Red blood cell transfusion: a clinical practice guideline of the AABB. Ann Intern Med. 2012;157:49–58.
- , . Managing clinical knowledge for health care improvement. In: Bemmel J, McCray AT, eds. Yearbook of Medical Informatics 2000: Patient‐Centered Systems. Stuttgart, Germany: Schattauer Verlagsgesellschaft; 2000:65–70.
- , , , et al. Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–764.
© 2014 Society of Hospital Medicine
Hospitalist Care by Geriatricians
Care for hospitalized seniors in acute geriatric units including acute care for the elderly (ACE) units have been shown to reduce function impairment and nursing home admission and possibly mortality, length of stay (LOS), and readmission.[1, 2, 3, 4, 5, 6] These units are run by specialized multidisciplinary teams with direct responsibility for the care of seniors with acute medical illnesses and are often led by geriatricians.[1] However, it is unclear whether these benefits are also achieved by hospitalist care by geriatricians working alongside other internists in general internal medicine units[7] and hospitalist care models.[8] Questions on effectiveness are relevant given the shortage of geriatricians in most healthcare systems and the escalating numbers of seniors requiring acute care. Many of these seniors have cognitive impairment, delirium, and functional decline, and longer hospital stays.[9] Beyond care settings, it is likely that specific subgroups of seniors benefit more from care delivered by geriatricians and their multidisciplinary teams. Patient characteristics defining these subgroups constitute potential targeting criteria, and these include advanced age, functional impairment, and geriatric syndromes.[10] However, to date, supporting evidence that these subgroups accrue greater benefit from care by geriatricians is lacking.[1]
Over this backdrop, our primary study aim was to determine whether hospitalist care by geriatricians for seniors aged 80 years and older in general internal medicine units improves short‐term outcomes compared with care by other internists in the setting of a busy acute‐care hospital. The secondary aim was to determine whether subgroups with premorbid functional impairment and with acute geriatric syndromes receive greater benefit from this care. Our hypotheses were that hospitalist care by geriatricians reduces hospital mortality, 30‐day mortality or readmission, and hospital LOS compared with care by other internists, and that these improvements are greater for the 2 subgroups.
METHODS
Design
This is a retrospective cohort study employing secondary analysis of merged data from clinical records, hospital administrative information, and the national death registry. The local institutional review board approved waiver of consent and other study procedures.
Setting and Patients
Hospital episodes of seniors aged 80 years and over admitted to the 350‐bed general internal medicine department of an acute‐care hospital in Singapore across calendar years 2005 to 2008 comprised the sampling frame. The choice of the study period was influenced by 2 factors. First, geriatricians consistently provided hospitalist care in the general internal medicine department at the study hospital up to 2008 but not after that. Second, administrative data were judged to be less reliable prior to 2005. Those with human immunodeficiency virus disease or acquired immune deficiency syndrome were excluded. Equal numbers of hospital episodes with attending physicians as geriatricians and other internists, and from each calendar year, were randomly sampled for analysis.
Intervention
Hospitalist care by geriatricians was compared with care by other internists who comprised a mix of generalists (with advanced internal medicine training) and subspecialists (including gastroenterologists, endocrinologists, and rheumatologists). Geriatricians and other internists were first certified in internal medicine in a 3‐year training program, before proceeding to either their respective subspecialty training for 3 years or additional training in advanced internal medicine for 2 years. At the general internal medicine department of the study hospital, 10 to 12 internists provided hospitalist care at any time. Of them, 1 to 2 would be geriatricians. All were hospital‐based physicians.
All attending physicians provided hospitalist care for adult patients at general internal medicine wards and led teams of medical residents drawn from a common departmental pool. Nurses, including those with added certification in gerontology, and allied health professionals were generally similar across these wards. In addition, nurse specialists in dementia and continence were accessible for specific consultation. Geriatricians and other internists were rotated to these wards in accordance with monthly rosters that did not have any systematic assignment criteria. They and their team of 2 to 3 residents would typically care for 20 to 30 patients at any time.
In both intervention and control groups, interdisciplinary rounds were not carried out. Rather, ad hoc discussions between physicians and other attending healthcare professionals including physiotherapists, occupational therapists, speech therapists, dieticians, pharmacists, social workers, and case managers took place. Different patients would have varying permutations of these professionals involved in their care at different times during the course of their hospital episode.
Variables
Outcome variables measured were hospital mortality, 30‐day mortality or readmission, and LOS. The latter 2 outcomes were only for hospital admissions of patients who survived and were discharged. Besides attending physicians' specialty, other explanatory variables included demography, living arrangement, hospitalization in the prior 30 days, Elixhauser comorbidity conditions,[11] modified Severity of Illness Index (SII),[12] premorbid functional impairment measured by basic activities of daily living (BADL), acute geriatric syndromes (delirium, falls, impaired mobility), and calendar year. The modified SII is based on 4 clinical parameters items (systolic blood pressure, body temperature, heart rate, and respiratory rate) at admission and was extracted from the clinical charts. It was scaled 0 to 4, with higher scores indicating more severe acute illness. Information on premorbid functional status was extracted from the section of the clinical charts that was mandatory for attending doctors to complete. In a previous study of older hospitalized patients in the general internal medicine department of the study hospital, agreement between data on premorbid functional status from chart review and interview was good.[13] Finally, the presence of acute geriatric syndromes at admission was determined by their documentation in the clinical charts.
Statistical Analysis
Sample size calculation indicated that 1812 patients (906 in each of intervention and control groups) were sufficient to detect a difference of 5% in hospital mortality between the intervention and control groups (15% vs 20%) with 80% power and alpha of 0.05. With anticipated loss of 8% due to unavailability of clinical charts for review, 2000 hospital episodes were sampled (1000 for each group, of which 250 were from each calendar year).
The 3 unadjusted outcome measures for the intervention and control groups constituted the main results. To adjust for any observed differences between the intervention and control groups, logistic regression was performed for hospital mortality and 30‐day mortality or readmission as binary outcomes. Generalized linear models with gamma family and log link were used for the continuous variable of LOS because of its expected right‐skewed distribution. Through these regression analyses, outcome measures were adjusted for age, gender, nursing home residence, hospitalization in the prior 30 days, premorbid functional status, comorbidity, severity of illness, and acute geriatric syndromes. In addition, clustering of hospital episodes within calendar years was addressed using fixed effects with dummy variables. These analyses were repeated for the 2 subgroups of those with premorbid functional impairment (defined as assisted or dependent BADL) and with acute geriatric syndromes (delirium, falls, impaired mobility, incontinence, and impaired self‐care). Listwise deletion was used to address missing values for explanatory variables where they occurred in <5% of hospital episodes analyzed. Clustering due to physicians was not addressed, as only information on whether the attending physician was a geriatrician or another internist was available in the study dataset rather than individual physician identifiers.
As additional analyses to adjust for difference in Diagnosis‐Related Groups (DRG) between intervention and control groups, we identified DRG codes that accounted more than 20 hospital episodes. Remaining DRG codes were aggregated into a single category designated as others. We then included these DRG codes as additional dummy variables in the regression models to observe the extent to which odds ratios for the treatment effect of geriatricians providing hospitalist care were changed.
Statistical analyses were performed using Stata version 13.1 (StateCorp, College Station, TX) with significance taken at the 5% level.
RESULTS
Among 1944 hospital episodes with data available for analysis, 968 received care by geriatricians and 976 by other internists. Death and readmission information at 30 days postdischarge was available for all. Seniors were predominantly female. About one‐quarter of seniors were nursing home residents. Only one‐third had premorbid functional independence. They had a mean number of 3 out of 30 Elixhauser comorbid conditions. Circulatory, respiratory, and genitourinary disorders accounted for more than half of primary diagnoses. The most common acute geriatric syndrome at presentation was delirium, which occurred in 3 out of every 10 seniors. More importantly, intervention and control groups only had minor differences on baseline characteristics, including nursing home residence, which was slightly more common in the intervention group (Table 1). Missing values occurred only for the explanatory variables, living arrangement, and premorbid basic activities of daily living in 0.4% to 2.7% of included hospital episodes.
| Care Led by Geriatricians (n=968) | Care Led by Other Internists (n=976) | P Value | |
|---|---|---|---|
| |||
| Age, mean (SD), y | 86.0 (5.1) | 85.8 (5.1) | 0.52 |
| Male, n (%) | 377 (39.0) | 361 (37.0) | 0.37 |
| Living arrangement, n (%) | 0.09 | ||
| Alone | 19 (2.0) | 21 (2.2) | |
| With other people | 690 (71.3) | 730 (74.8) | |
| Nursing home | 255 (26.3) | 214 (21.9) | |
| Missing | 4 (0.4) | 11 (1.1) | |
| Admission source, n (%) | 0.91 | ||
| Emergency department | 943 (97.4) | 950 (97.3) | |
| Others | 25 (2.6) | 26 (2.7) | |
| Hospital admissions in the prior 30 days, n (%) | 214 (22.1) | 210 (21.5) | 0.75 |
| Year, n (%) | 1.00 | ||
| 2005 | 244 (25.2) | 242 (24.8) | |
| 2006 | 237 (24.5) | 243 (24.9) | |
| 2007 | 241 (24.9) | 244 (25.0) | |
| 2008 | 246 (25.4) | 247 (25.3) | |
| Premorbid basic activities of daily living, n (%) | 0.28 | ||
| Independent | 317 (32.7) | 345 (35.3) | |
| Assisted or dependent | 625 (64.6) | 613 (62.9) | |
| Missing | 26 (2.7) | 18 (1.8) | |
| Elixhauser comorbidity count, mean (SD) | 3.2 (1.6) | 3.2 (1.7) | 0.58 |
| Modified Severity of Illness Index, n (%) | 0.30 | ||
| 1 or 2 | 541 (55.9) | 568 (58.2) | |
| 3 or 4 | 427 (44.1) | 408 (41.8) | |
| Diagnosis‐Related Group category, n (%) | 0.88 | ||
| Circulatory | 110 (11.4) | 110 (11.3) | |
| Digestive | 55 (5.7) | 60 (6.1) | |
| Endocrine, nutritional and metabolic diseases, and immunological | 60 (6.2) | 54 (5.5) | |
| Genitourinary | 146 (15.1) | 172 (17.6) | |
| Mental and nervous | 16 (1.7) | 16 (1.6) | |
| Musculoskeletal | 9 (0.9) | 10 (1.0) | |
| Respiratory | 364 (37.6) | 356 (36.5) | |
| Others | 208 (21.5) | 198 (20.3) | |
| Acute geriatric syndromes, n (%) | |||
| Mobility impairment | 75 (7.7) | 79 (8.1) | 0.78 |
| Falls | 82 (8.5) | 99 (10.1) | 0.21 |
| Delirium | 290 (30.0) | 279 (28.6) | 0.51 |
There were no significant differences in hospital mortality, 30‐day mortality or readmission, and LOS between hospital episodes with care by geriatricians and other internists for the whole group and the 2 subgroups (Table 2). However, nonsignificant reduction in hospital mortality was observed for the whole group (15.5% vs 16.9%, P=0.40), with greater magnitude for the subgroup with acute geriatric syndromes (20.2% vs 23.1%, P=0.31).
| All | Those With Premorbid Functional Impairment | Those With Acute Geriatric Syndromes | ||||
|---|---|---|---|---|---|---|
| Care Led by Geriatricians | Care Led by Other Internists | Care Led by Geriatricians | Care Led by Other Internists | Care Led by Geriatricians | Care Led by Other Internists | |
| ||||||
| Hospital mortality, n (%) | 150/968 (15.5) | 165/976 (16.9), P=0.40 | 125/625 (20.0) | 137/613 (22.4), P=0.31 | 79/392 (20.2) | 92/398 (23.1), P=0.31 |
| 30‐day mortality or readmission, n (%) | 206/818 (25.2) | 200/811 (24.7), P=0.81 | 147/500, (29.4) | 144/476, (30.3), P=0.77 | 88/313, (28.1) | 83/306, (27.1), P=0.78 |
| Mean length of stay, days (SD) | 9.7 (10.2), n=818 | 9.7 (10.9), n=811, P=0.87 | 11.1 (10.7), n=500 | 11.1 (12.3), n=476, P=0.93 | 11.4 (12.5), n=321 | 10.8 (13.0), n=312, P=0.57 |
When adjusted for age, gender, premorbid functional status, comorbidity, severity of illness, acute geriatric syndromes, hospitalization in the prior 30 days, and calendar year, care by geriatricians was associated with nonsignificant trends toward lower hospital mortality, with odds ratios between 0.80 and 0.89. However, 30‐day mortality or readmission and LOS for the intervention and control groups were generally equivalent (Table 3). There are only minor differences between the odds ratios and their 95% confidence intervals for regression analyses without and with additional adjustment for DRG codes (results not shown). Thus, they do not change the study results in any significant way.
| Care by Geriatricians (Ref: Care by Other Internists) | All | Those With Premorbid Functional Impairment | Those With Acute Geriatric Syndromes |
|---|---|---|---|
| |||
| Hospital mortality: odds ratio (95% confidence interval) | 0.89 (0.69 to 1.16), n=1,886, P=0.40 | 0.85 (0.64 to 1.13), n=1,233, P=0.27 | 0.80 (0.55 to 1.16), n=764, P=0.24 |
| 30‐day mortality or readmission: odds ratio (95% confidence interval) | 1.05 (0.82 to 1.33), n=1,580, P=0.71 | 0.94 (0.71 to 1.25), n=973, P=0.69 | 1.03 (0.70 to 1.50), n=600, P=0.89 |
| Length of stay: log days (95% confidence interval) | 0.03 (0.14 to 0.07), n=1,580, P=0.52 | 0.03 (0.16 to 0.10), n=973, P=0.68 | 0.00 (0.18 to 0.18), n=600, P=1.00 |
DISCUSSION
Geriatricians provide direct acute hospital care for seniors either in dedicated acute geriatric units including ACE units[14] or alongside generalists or subspecialty physicians in general internal medicine units. Through an unique opportunity to study the latter arrangement, we found that hospitalist care by geriatricians for seniors aged 80 years and older in general internal medicine units did not improve their short‐term outcomes vis‐‐vis care by other internists. These findings are in contrast to those of studies on acute geriatric units. This is the first report on the effectiveness of hospitalist care for seniors provided by geriatricians. Although not a randomized controlled trial, our study is in essence a natural experiment which does not impose any major inclusion restrictions other than age of 80 years and above. Internal validity was enhanced by intervention and control groups being similar on individual‐level characteristics, whereas external validity was boosted by an all‐comers approach to enrollment.
It is pertinent to ask why hospitalist care by geriatricians in a general internal medicine department did not benefit seniors with advanced age, many of whom have functional impairment and multimorbidity. After all, improved care and outcomes seem plausible for these seniors who appear to be more vulnerable. We propose 4 possible explanations. First, unmeasured differences between intervention and control groups could have led to unobserved confounding. However, this is less likely given the nonsystematic assignment of attending physicians to different wards and similarity of intervention and control groups on a broad range of baseline characteristics. Second, care processes in wards allocated to geriatricians may not differ very much from those in other wards. Irrespective of ward, care delivered by medical residents and other healthcare professionals were also expected to be similar. Unlike acute geriatric units where comprehensive geriatric assessment (CGA) by a multidisciplinary team is thought to be responsible for the improved outcomes,[12] the influence of geriatricians outside of these units may not necessarily achieve the same level of geriatric care.[15, 16] This is precisely the challenge encountered by geriatricians in their care of acutely ill older patients in settings other than acute geriatric units, Third, diffusion of geriatric care practices across general internal medicine wards over the past decade at our hospital may have resulted in narrowed differences in the care processes particularly relevant to seniors, such as those related to functional retraining, swallowing assessment, and discharge planning, although we do not have any specific data to confirm this. These differences may in turn not be wide enough for hospitalist care by geriatricians to influence these short‐term outcomes positively. Last, our study was not designed to measure patient‐reported outcomes such as functional status, mood, quality of life, and satisfaction, which may arguably be more responsive to geriatric intervention.
It might be noted that the average LOS for hospital episodes in this study was almost 10 days, which is longer than that typically seen in North America. There are 2 possible reasons for this. First, these are hospital episodes of very old patients, and longer LOS among survivors is expected. Second, post‐acute care was not as well developed in Singapore during the study period. Since then, the system of community hospitals has expanded, thereby allowing earlier transfer to these facilities for post‐acute care and shorter LOS at acute‐care hospitals.
There are a number of limitations of our study. First, this is an observational study where treatment assignment is not allocated. Although a randomized controlled trial may be the ideal design to evaluate treatment effects, operational and ethical considerations at a busy acute‐care hospital render this very challenging to conduct. As mentioned, nonsystematic assignment of attending physicians to different wards and lack of important baseline differences between intervention and control groups support the notion that important unmeasured differences are less likely. Second, and as alluded to, we did not measure relevant patient‐reported outcomes. Nonetheless, we argue that survival is still important to many seniors, particularly those without advanced illness, whereas readmission avoidance and shorter hospital stay matter almost universally. Third, clinical charts were unavailable for data extraction in almost 3% of hospital episodes. In addition, there were missing values in 2 explanatory variables in <3% of available clinical charts. These missing values were handled by listwise deletion in the regression analyses. Doing so carries with it the risk of introducing bias in the estimation of the treatment effect of care by geriatricians. However, given the relatively small proportions of missing charts and values, it is less likely that any bias would have changed the study conclusions. Fourth, we did not account for clustering at the physician level, which would have widened the confidence intervals for the odds ratios. However, because all treatment effects on the 3 outcomes were clearly not statistically significant, widening of confidence intervals would not have changed the results and study conclusions. Finally, this is a single institution study in a single health system. Thus, caution is necessary when attempting to extrapolate its results. On the other hand, the major strength of this study is its real‐world setting, which allows the results to be more generalizable to other hospital systems with similar organization and practice of general internal medicine.
Our findings need to be placed in the context of emerging innovative models of care for hospitalized seniors, which directly or indirectly involve geriatricians. Besides traditional ACE units, which have fixed geographical locations within a hospital, a mobile acute care of the elderly service achieved shorter LOS and reduced cost than the established ACE unit with similar mortality and readmission rates.[17] Others include a proactive geriatrics consultation model in collaboration with hospitalists.[18] Another variant of the ACE unit is the hospitalist‐run acute care for the elderly (hospitalist‐ACE) service, which improved care processes without improving clinical outcomes or increasing cost.[19] Clearly, there needs to be better collaboration between hospitalists and geriatricians to improve care of acutely ill seniors.[20] Ultimately, any form of direct geriatrician care for seniors needs to be complimented by indirect care through hospital‐wide systems such the Hospital Elder Life Program. This model of care aims to prevent cognitive and functional decline in hospitalized seniors by combining CGA with protocol‐driven interventions ranging from orientation, visitation, feeding assistance, early mobilization, and visual and hearing adaptations.[21, 22]
In conclusion, hospitalist care for seniors aged 80 years and above by geriatricians based in general internal medicine units is not more effective than care by other internists, at least where reducing short‐term mortality and readmission and LOS are concerned. This is particularly applicable to hospital systems where geriatric care elements have already been widely adopted beyond the confines of acute geriatric units. However, these findings do not by any means indicate that hospitalist care provided by geriatricians is altogether not more beneficial for these seniors than care by other internists in general internal medicine units. Rather, further research on patient‐reported outcomes can clarify more fully the geriatrician's true role in this setting.
Disclosures
This study was wholly funded by the National Healthcare Group Small Innovative Grants. The funders did not play any other role in this study. The authors report no conflicts of interest.
- , , , , . Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta‐analysis. BMJ. 2009;338:b50.
- , , , , . Comprehensive geriatric assessment: a meta‐analysis of controlled trials. Lancet. 1993;342(8878):1032–1036.
- , , , et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346(12):905–912.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , , . Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. J Am Geriatr Soc. 2002;50(5):792–798.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60:2237–2245.
- , . Age‐related geriatric medicine or integrated medical care? Age Ageing. 1999;28:245–247.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- . Care of hospitalized older patients: opportunities for hospital‐based physicians. J Hosp Med. 2006;1:42–47.
- , , , . Which patients benefit the most from a geriatrician's care? Consensus among directors of geriatrics academic programs. J Am Geriatr Soc. 2008;56(10):1796–1801.
- , , , et al. Comorbidity measure for use with administrative data. Med Care. 1998;36:8–27.
- , , , , . Resource consumption in hospitalised, frail older patients. Ann Acad Med Singapore. 2010;39:830–836.
- , , , , . Impact of data source and time reference of functional status on hospital mortality prediction. BMC Health Serv Res. 2012;12:115.
- , , , , . A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–1344.
- , , . Acute hospital care for frail older people. Age Ageing. 2006;35:551–552.
- , . Comprehensive geriatric assessment for older hospital patients. Br Med Bull. 2005;71:45–59.
- , , , et al. Operational and quality outcomes of a mobile acute care for the elderly service. J Hosp Med. 2011;6:358–363.
- , , , . Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57:2139–2145.
- , , , et al. Evaluation of a hospitalist‐run acute care for the elderly service. J Hosp Med. 2011;6:313–321.
- , , . Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs. J Hosp Med. 2006;1:29–35.
- , , , et al. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in hospitalized older patients. J Am Geriatr Soc. 2000;48:1697–1706.
- . Organizational interventions to improve health outcomes of older persons. Med Care. 2002;40:416–428.
Care for hospitalized seniors in acute geriatric units including acute care for the elderly (ACE) units have been shown to reduce function impairment and nursing home admission and possibly mortality, length of stay (LOS), and readmission.[1, 2, 3, 4, 5, 6] These units are run by specialized multidisciplinary teams with direct responsibility for the care of seniors with acute medical illnesses and are often led by geriatricians.[1] However, it is unclear whether these benefits are also achieved by hospitalist care by geriatricians working alongside other internists in general internal medicine units[7] and hospitalist care models.[8] Questions on effectiveness are relevant given the shortage of geriatricians in most healthcare systems and the escalating numbers of seniors requiring acute care. Many of these seniors have cognitive impairment, delirium, and functional decline, and longer hospital stays.[9] Beyond care settings, it is likely that specific subgroups of seniors benefit more from care delivered by geriatricians and their multidisciplinary teams. Patient characteristics defining these subgroups constitute potential targeting criteria, and these include advanced age, functional impairment, and geriatric syndromes.[10] However, to date, supporting evidence that these subgroups accrue greater benefit from care by geriatricians is lacking.[1]
Over this backdrop, our primary study aim was to determine whether hospitalist care by geriatricians for seniors aged 80 years and older in general internal medicine units improves short‐term outcomes compared with care by other internists in the setting of a busy acute‐care hospital. The secondary aim was to determine whether subgroups with premorbid functional impairment and with acute geriatric syndromes receive greater benefit from this care. Our hypotheses were that hospitalist care by geriatricians reduces hospital mortality, 30‐day mortality or readmission, and hospital LOS compared with care by other internists, and that these improvements are greater for the 2 subgroups.
METHODS
Design
This is a retrospective cohort study employing secondary analysis of merged data from clinical records, hospital administrative information, and the national death registry. The local institutional review board approved waiver of consent and other study procedures.
Setting and Patients
Hospital episodes of seniors aged 80 years and over admitted to the 350‐bed general internal medicine department of an acute‐care hospital in Singapore across calendar years 2005 to 2008 comprised the sampling frame. The choice of the study period was influenced by 2 factors. First, geriatricians consistently provided hospitalist care in the general internal medicine department at the study hospital up to 2008 but not after that. Second, administrative data were judged to be less reliable prior to 2005. Those with human immunodeficiency virus disease or acquired immune deficiency syndrome were excluded. Equal numbers of hospital episodes with attending physicians as geriatricians and other internists, and from each calendar year, were randomly sampled for analysis.
Intervention
Hospitalist care by geriatricians was compared with care by other internists who comprised a mix of generalists (with advanced internal medicine training) and subspecialists (including gastroenterologists, endocrinologists, and rheumatologists). Geriatricians and other internists were first certified in internal medicine in a 3‐year training program, before proceeding to either their respective subspecialty training for 3 years or additional training in advanced internal medicine for 2 years. At the general internal medicine department of the study hospital, 10 to 12 internists provided hospitalist care at any time. Of them, 1 to 2 would be geriatricians. All were hospital‐based physicians.
All attending physicians provided hospitalist care for adult patients at general internal medicine wards and led teams of medical residents drawn from a common departmental pool. Nurses, including those with added certification in gerontology, and allied health professionals were generally similar across these wards. In addition, nurse specialists in dementia and continence were accessible for specific consultation. Geriatricians and other internists were rotated to these wards in accordance with monthly rosters that did not have any systematic assignment criteria. They and their team of 2 to 3 residents would typically care for 20 to 30 patients at any time.
In both intervention and control groups, interdisciplinary rounds were not carried out. Rather, ad hoc discussions between physicians and other attending healthcare professionals including physiotherapists, occupational therapists, speech therapists, dieticians, pharmacists, social workers, and case managers took place. Different patients would have varying permutations of these professionals involved in their care at different times during the course of their hospital episode.
Variables
Outcome variables measured were hospital mortality, 30‐day mortality or readmission, and LOS. The latter 2 outcomes were only for hospital admissions of patients who survived and were discharged. Besides attending physicians' specialty, other explanatory variables included demography, living arrangement, hospitalization in the prior 30 days, Elixhauser comorbidity conditions,[11] modified Severity of Illness Index (SII),[12] premorbid functional impairment measured by basic activities of daily living (BADL), acute geriatric syndromes (delirium, falls, impaired mobility), and calendar year. The modified SII is based on 4 clinical parameters items (systolic blood pressure, body temperature, heart rate, and respiratory rate) at admission and was extracted from the clinical charts. It was scaled 0 to 4, with higher scores indicating more severe acute illness. Information on premorbid functional status was extracted from the section of the clinical charts that was mandatory for attending doctors to complete. In a previous study of older hospitalized patients in the general internal medicine department of the study hospital, agreement between data on premorbid functional status from chart review and interview was good.[13] Finally, the presence of acute geriatric syndromes at admission was determined by their documentation in the clinical charts.
Statistical Analysis
Sample size calculation indicated that 1812 patients (906 in each of intervention and control groups) were sufficient to detect a difference of 5% in hospital mortality between the intervention and control groups (15% vs 20%) with 80% power and alpha of 0.05. With anticipated loss of 8% due to unavailability of clinical charts for review, 2000 hospital episodes were sampled (1000 for each group, of which 250 were from each calendar year).
The 3 unadjusted outcome measures for the intervention and control groups constituted the main results. To adjust for any observed differences between the intervention and control groups, logistic regression was performed for hospital mortality and 30‐day mortality or readmission as binary outcomes. Generalized linear models with gamma family and log link were used for the continuous variable of LOS because of its expected right‐skewed distribution. Through these regression analyses, outcome measures were adjusted for age, gender, nursing home residence, hospitalization in the prior 30 days, premorbid functional status, comorbidity, severity of illness, and acute geriatric syndromes. In addition, clustering of hospital episodes within calendar years was addressed using fixed effects with dummy variables. These analyses were repeated for the 2 subgroups of those with premorbid functional impairment (defined as assisted or dependent BADL) and with acute geriatric syndromes (delirium, falls, impaired mobility, incontinence, and impaired self‐care). Listwise deletion was used to address missing values for explanatory variables where they occurred in <5% of hospital episodes analyzed. Clustering due to physicians was not addressed, as only information on whether the attending physician was a geriatrician or another internist was available in the study dataset rather than individual physician identifiers.
As additional analyses to adjust for difference in Diagnosis‐Related Groups (DRG) between intervention and control groups, we identified DRG codes that accounted more than 20 hospital episodes. Remaining DRG codes were aggregated into a single category designated as others. We then included these DRG codes as additional dummy variables in the regression models to observe the extent to which odds ratios for the treatment effect of geriatricians providing hospitalist care were changed.
Statistical analyses were performed using Stata version 13.1 (StateCorp, College Station, TX) with significance taken at the 5% level.
RESULTS
Among 1944 hospital episodes with data available for analysis, 968 received care by geriatricians and 976 by other internists. Death and readmission information at 30 days postdischarge was available for all. Seniors were predominantly female. About one‐quarter of seniors were nursing home residents. Only one‐third had premorbid functional independence. They had a mean number of 3 out of 30 Elixhauser comorbid conditions. Circulatory, respiratory, and genitourinary disorders accounted for more than half of primary diagnoses. The most common acute geriatric syndrome at presentation was delirium, which occurred in 3 out of every 10 seniors. More importantly, intervention and control groups only had minor differences on baseline characteristics, including nursing home residence, which was slightly more common in the intervention group (Table 1). Missing values occurred only for the explanatory variables, living arrangement, and premorbid basic activities of daily living in 0.4% to 2.7% of included hospital episodes.
| Care Led by Geriatricians (n=968) | Care Led by Other Internists (n=976) | P Value | |
|---|---|---|---|
| |||
| Age, mean (SD), y | 86.0 (5.1) | 85.8 (5.1) | 0.52 |
| Male, n (%) | 377 (39.0) | 361 (37.0) | 0.37 |
| Living arrangement, n (%) | 0.09 | ||
| Alone | 19 (2.0) | 21 (2.2) | |
| With other people | 690 (71.3) | 730 (74.8) | |
| Nursing home | 255 (26.3) | 214 (21.9) | |
| Missing | 4 (0.4) | 11 (1.1) | |
| Admission source, n (%) | 0.91 | ||
| Emergency department | 943 (97.4) | 950 (97.3) | |
| Others | 25 (2.6) | 26 (2.7) | |
| Hospital admissions in the prior 30 days, n (%) | 214 (22.1) | 210 (21.5) | 0.75 |
| Year, n (%) | 1.00 | ||
| 2005 | 244 (25.2) | 242 (24.8) | |
| 2006 | 237 (24.5) | 243 (24.9) | |
| 2007 | 241 (24.9) | 244 (25.0) | |
| 2008 | 246 (25.4) | 247 (25.3) | |
| Premorbid basic activities of daily living, n (%) | 0.28 | ||
| Independent | 317 (32.7) | 345 (35.3) | |
| Assisted or dependent | 625 (64.6) | 613 (62.9) | |
| Missing | 26 (2.7) | 18 (1.8) | |
| Elixhauser comorbidity count, mean (SD) | 3.2 (1.6) | 3.2 (1.7) | 0.58 |
| Modified Severity of Illness Index, n (%) | 0.30 | ||
| 1 or 2 | 541 (55.9) | 568 (58.2) | |
| 3 or 4 | 427 (44.1) | 408 (41.8) | |
| Diagnosis‐Related Group category, n (%) | 0.88 | ||
| Circulatory | 110 (11.4) | 110 (11.3) | |
| Digestive | 55 (5.7) | 60 (6.1) | |
| Endocrine, nutritional and metabolic diseases, and immunological | 60 (6.2) | 54 (5.5) | |
| Genitourinary | 146 (15.1) | 172 (17.6) | |
| Mental and nervous | 16 (1.7) | 16 (1.6) | |
| Musculoskeletal | 9 (0.9) | 10 (1.0) | |
| Respiratory | 364 (37.6) | 356 (36.5) | |
| Others | 208 (21.5) | 198 (20.3) | |
| Acute geriatric syndromes, n (%) | |||
| Mobility impairment | 75 (7.7) | 79 (8.1) | 0.78 |
| Falls | 82 (8.5) | 99 (10.1) | 0.21 |
| Delirium | 290 (30.0) | 279 (28.6) | 0.51 |
There were no significant differences in hospital mortality, 30‐day mortality or readmission, and LOS between hospital episodes with care by geriatricians and other internists for the whole group and the 2 subgroups (Table 2). However, nonsignificant reduction in hospital mortality was observed for the whole group (15.5% vs 16.9%, P=0.40), with greater magnitude for the subgroup with acute geriatric syndromes (20.2% vs 23.1%, P=0.31).
| All | Those With Premorbid Functional Impairment | Those With Acute Geriatric Syndromes | ||||
|---|---|---|---|---|---|---|
| Care Led by Geriatricians | Care Led by Other Internists | Care Led by Geriatricians | Care Led by Other Internists | Care Led by Geriatricians | Care Led by Other Internists | |
| ||||||
| Hospital mortality, n (%) | 150/968 (15.5) | 165/976 (16.9), P=0.40 | 125/625 (20.0) | 137/613 (22.4), P=0.31 | 79/392 (20.2) | 92/398 (23.1), P=0.31 |
| 30‐day mortality or readmission, n (%) | 206/818 (25.2) | 200/811 (24.7), P=0.81 | 147/500, (29.4) | 144/476, (30.3), P=0.77 | 88/313, (28.1) | 83/306, (27.1), P=0.78 |
| Mean length of stay, days (SD) | 9.7 (10.2), n=818 | 9.7 (10.9), n=811, P=0.87 | 11.1 (10.7), n=500 | 11.1 (12.3), n=476, P=0.93 | 11.4 (12.5), n=321 | 10.8 (13.0), n=312, P=0.57 |
When adjusted for age, gender, premorbid functional status, comorbidity, severity of illness, acute geriatric syndromes, hospitalization in the prior 30 days, and calendar year, care by geriatricians was associated with nonsignificant trends toward lower hospital mortality, with odds ratios between 0.80 and 0.89. However, 30‐day mortality or readmission and LOS for the intervention and control groups were generally equivalent (Table 3). There are only minor differences between the odds ratios and their 95% confidence intervals for regression analyses without and with additional adjustment for DRG codes (results not shown). Thus, they do not change the study results in any significant way.
| Care by Geriatricians (Ref: Care by Other Internists) | All | Those With Premorbid Functional Impairment | Those With Acute Geriatric Syndromes |
|---|---|---|---|
| |||
| Hospital mortality: odds ratio (95% confidence interval) | 0.89 (0.69 to 1.16), n=1,886, P=0.40 | 0.85 (0.64 to 1.13), n=1,233, P=0.27 | 0.80 (0.55 to 1.16), n=764, P=0.24 |
| 30‐day mortality or readmission: odds ratio (95% confidence interval) | 1.05 (0.82 to 1.33), n=1,580, P=0.71 | 0.94 (0.71 to 1.25), n=973, P=0.69 | 1.03 (0.70 to 1.50), n=600, P=0.89 |
| Length of stay: log days (95% confidence interval) | 0.03 (0.14 to 0.07), n=1,580, P=0.52 | 0.03 (0.16 to 0.10), n=973, P=0.68 | 0.00 (0.18 to 0.18), n=600, P=1.00 |
DISCUSSION
Geriatricians provide direct acute hospital care for seniors either in dedicated acute geriatric units including ACE units[14] or alongside generalists or subspecialty physicians in general internal medicine units. Through an unique opportunity to study the latter arrangement, we found that hospitalist care by geriatricians for seniors aged 80 years and older in general internal medicine units did not improve their short‐term outcomes vis‐‐vis care by other internists. These findings are in contrast to those of studies on acute geriatric units. This is the first report on the effectiveness of hospitalist care for seniors provided by geriatricians. Although not a randomized controlled trial, our study is in essence a natural experiment which does not impose any major inclusion restrictions other than age of 80 years and above. Internal validity was enhanced by intervention and control groups being similar on individual‐level characteristics, whereas external validity was boosted by an all‐comers approach to enrollment.
It is pertinent to ask why hospitalist care by geriatricians in a general internal medicine department did not benefit seniors with advanced age, many of whom have functional impairment and multimorbidity. After all, improved care and outcomes seem plausible for these seniors who appear to be more vulnerable. We propose 4 possible explanations. First, unmeasured differences between intervention and control groups could have led to unobserved confounding. However, this is less likely given the nonsystematic assignment of attending physicians to different wards and similarity of intervention and control groups on a broad range of baseline characteristics. Second, care processes in wards allocated to geriatricians may not differ very much from those in other wards. Irrespective of ward, care delivered by medical residents and other healthcare professionals were also expected to be similar. Unlike acute geriatric units where comprehensive geriatric assessment (CGA) by a multidisciplinary team is thought to be responsible for the improved outcomes,[12] the influence of geriatricians outside of these units may not necessarily achieve the same level of geriatric care.[15, 16] This is precisely the challenge encountered by geriatricians in their care of acutely ill older patients in settings other than acute geriatric units, Third, diffusion of geriatric care practices across general internal medicine wards over the past decade at our hospital may have resulted in narrowed differences in the care processes particularly relevant to seniors, such as those related to functional retraining, swallowing assessment, and discharge planning, although we do not have any specific data to confirm this. These differences may in turn not be wide enough for hospitalist care by geriatricians to influence these short‐term outcomes positively. Last, our study was not designed to measure patient‐reported outcomes such as functional status, mood, quality of life, and satisfaction, which may arguably be more responsive to geriatric intervention.
It might be noted that the average LOS for hospital episodes in this study was almost 10 days, which is longer than that typically seen in North America. There are 2 possible reasons for this. First, these are hospital episodes of very old patients, and longer LOS among survivors is expected. Second, post‐acute care was not as well developed in Singapore during the study period. Since then, the system of community hospitals has expanded, thereby allowing earlier transfer to these facilities for post‐acute care and shorter LOS at acute‐care hospitals.
There are a number of limitations of our study. First, this is an observational study where treatment assignment is not allocated. Although a randomized controlled trial may be the ideal design to evaluate treatment effects, operational and ethical considerations at a busy acute‐care hospital render this very challenging to conduct. As mentioned, nonsystematic assignment of attending physicians to different wards and lack of important baseline differences between intervention and control groups support the notion that important unmeasured differences are less likely. Second, and as alluded to, we did not measure relevant patient‐reported outcomes. Nonetheless, we argue that survival is still important to many seniors, particularly those without advanced illness, whereas readmission avoidance and shorter hospital stay matter almost universally. Third, clinical charts were unavailable for data extraction in almost 3% of hospital episodes. In addition, there were missing values in 2 explanatory variables in <3% of available clinical charts. These missing values were handled by listwise deletion in the regression analyses. Doing so carries with it the risk of introducing bias in the estimation of the treatment effect of care by geriatricians. However, given the relatively small proportions of missing charts and values, it is less likely that any bias would have changed the study conclusions. Fourth, we did not account for clustering at the physician level, which would have widened the confidence intervals for the odds ratios. However, because all treatment effects on the 3 outcomes were clearly not statistically significant, widening of confidence intervals would not have changed the results and study conclusions. Finally, this is a single institution study in a single health system. Thus, caution is necessary when attempting to extrapolate its results. On the other hand, the major strength of this study is its real‐world setting, which allows the results to be more generalizable to other hospital systems with similar organization and practice of general internal medicine.
Our findings need to be placed in the context of emerging innovative models of care for hospitalized seniors, which directly or indirectly involve geriatricians. Besides traditional ACE units, which have fixed geographical locations within a hospital, a mobile acute care of the elderly service achieved shorter LOS and reduced cost than the established ACE unit with similar mortality and readmission rates.[17] Others include a proactive geriatrics consultation model in collaboration with hospitalists.[18] Another variant of the ACE unit is the hospitalist‐run acute care for the elderly (hospitalist‐ACE) service, which improved care processes without improving clinical outcomes or increasing cost.[19] Clearly, there needs to be better collaboration between hospitalists and geriatricians to improve care of acutely ill seniors.[20] Ultimately, any form of direct geriatrician care for seniors needs to be complimented by indirect care through hospital‐wide systems such the Hospital Elder Life Program. This model of care aims to prevent cognitive and functional decline in hospitalized seniors by combining CGA with protocol‐driven interventions ranging from orientation, visitation, feeding assistance, early mobilization, and visual and hearing adaptations.[21, 22]
In conclusion, hospitalist care for seniors aged 80 years and above by geriatricians based in general internal medicine units is not more effective than care by other internists, at least where reducing short‐term mortality and readmission and LOS are concerned. This is particularly applicable to hospital systems where geriatric care elements have already been widely adopted beyond the confines of acute geriatric units. However, these findings do not by any means indicate that hospitalist care provided by geriatricians is altogether not more beneficial for these seniors than care by other internists in general internal medicine units. Rather, further research on patient‐reported outcomes can clarify more fully the geriatrician's true role in this setting.
Disclosures
This study was wholly funded by the National Healthcare Group Small Innovative Grants. The funders did not play any other role in this study. The authors report no conflicts of interest.
Care for hospitalized seniors in acute geriatric units including acute care for the elderly (ACE) units have been shown to reduce function impairment and nursing home admission and possibly mortality, length of stay (LOS), and readmission.[1, 2, 3, 4, 5, 6] These units are run by specialized multidisciplinary teams with direct responsibility for the care of seniors with acute medical illnesses and are often led by geriatricians.[1] However, it is unclear whether these benefits are also achieved by hospitalist care by geriatricians working alongside other internists in general internal medicine units[7] and hospitalist care models.[8] Questions on effectiveness are relevant given the shortage of geriatricians in most healthcare systems and the escalating numbers of seniors requiring acute care. Many of these seniors have cognitive impairment, delirium, and functional decline, and longer hospital stays.[9] Beyond care settings, it is likely that specific subgroups of seniors benefit more from care delivered by geriatricians and their multidisciplinary teams. Patient characteristics defining these subgroups constitute potential targeting criteria, and these include advanced age, functional impairment, and geriatric syndromes.[10] However, to date, supporting evidence that these subgroups accrue greater benefit from care by geriatricians is lacking.[1]
Over this backdrop, our primary study aim was to determine whether hospitalist care by geriatricians for seniors aged 80 years and older in general internal medicine units improves short‐term outcomes compared with care by other internists in the setting of a busy acute‐care hospital. The secondary aim was to determine whether subgroups with premorbid functional impairment and with acute geriatric syndromes receive greater benefit from this care. Our hypotheses were that hospitalist care by geriatricians reduces hospital mortality, 30‐day mortality or readmission, and hospital LOS compared with care by other internists, and that these improvements are greater for the 2 subgroups.
METHODS
Design
This is a retrospective cohort study employing secondary analysis of merged data from clinical records, hospital administrative information, and the national death registry. The local institutional review board approved waiver of consent and other study procedures.
Setting and Patients
Hospital episodes of seniors aged 80 years and over admitted to the 350‐bed general internal medicine department of an acute‐care hospital in Singapore across calendar years 2005 to 2008 comprised the sampling frame. The choice of the study period was influenced by 2 factors. First, geriatricians consistently provided hospitalist care in the general internal medicine department at the study hospital up to 2008 but not after that. Second, administrative data were judged to be less reliable prior to 2005. Those with human immunodeficiency virus disease or acquired immune deficiency syndrome were excluded. Equal numbers of hospital episodes with attending physicians as geriatricians and other internists, and from each calendar year, were randomly sampled for analysis.
Intervention
Hospitalist care by geriatricians was compared with care by other internists who comprised a mix of generalists (with advanced internal medicine training) and subspecialists (including gastroenterologists, endocrinologists, and rheumatologists). Geriatricians and other internists were first certified in internal medicine in a 3‐year training program, before proceeding to either their respective subspecialty training for 3 years or additional training in advanced internal medicine for 2 years. At the general internal medicine department of the study hospital, 10 to 12 internists provided hospitalist care at any time. Of them, 1 to 2 would be geriatricians. All were hospital‐based physicians.
All attending physicians provided hospitalist care for adult patients at general internal medicine wards and led teams of medical residents drawn from a common departmental pool. Nurses, including those with added certification in gerontology, and allied health professionals were generally similar across these wards. In addition, nurse specialists in dementia and continence were accessible for specific consultation. Geriatricians and other internists were rotated to these wards in accordance with monthly rosters that did not have any systematic assignment criteria. They and their team of 2 to 3 residents would typically care for 20 to 30 patients at any time.
In both intervention and control groups, interdisciplinary rounds were not carried out. Rather, ad hoc discussions between physicians and other attending healthcare professionals including physiotherapists, occupational therapists, speech therapists, dieticians, pharmacists, social workers, and case managers took place. Different patients would have varying permutations of these professionals involved in their care at different times during the course of their hospital episode.
Variables
Outcome variables measured were hospital mortality, 30‐day mortality or readmission, and LOS. The latter 2 outcomes were only for hospital admissions of patients who survived and were discharged. Besides attending physicians' specialty, other explanatory variables included demography, living arrangement, hospitalization in the prior 30 days, Elixhauser comorbidity conditions,[11] modified Severity of Illness Index (SII),[12] premorbid functional impairment measured by basic activities of daily living (BADL), acute geriatric syndromes (delirium, falls, impaired mobility), and calendar year. The modified SII is based on 4 clinical parameters items (systolic blood pressure, body temperature, heart rate, and respiratory rate) at admission and was extracted from the clinical charts. It was scaled 0 to 4, with higher scores indicating more severe acute illness. Information on premorbid functional status was extracted from the section of the clinical charts that was mandatory for attending doctors to complete. In a previous study of older hospitalized patients in the general internal medicine department of the study hospital, agreement between data on premorbid functional status from chart review and interview was good.[13] Finally, the presence of acute geriatric syndromes at admission was determined by their documentation in the clinical charts.
Statistical Analysis
Sample size calculation indicated that 1812 patients (906 in each of intervention and control groups) were sufficient to detect a difference of 5% in hospital mortality between the intervention and control groups (15% vs 20%) with 80% power and alpha of 0.05. With anticipated loss of 8% due to unavailability of clinical charts for review, 2000 hospital episodes were sampled (1000 for each group, of which 250 were from each calendar year).
The 3 unadjusted outcome measures for the intervention and control groups constituted the main results. To adjust for any observed differences between the intervention and control groups, logistic regression was performed for hospital mortality and 30‐day mortality or readmission as binary outcomes. Generalized linear models with gamma family and log link were used for the continuous variable of LOS because of its expected right‐skewed distribution. Through these regression analyses, outcome measures were adjusted for age, gender, nursing home residence, hospitalization in the prior 30 days, premorbid functional status, comorbidity, severity of illness, and acute geriatric syndromes. In addition, clustering of hospital episodes within calendar years was addressed using fixed effects with dummy variables. These analyses were repeated for the 2 subgroups of those with premorbid functional impairment (defined as assisted or dependent BADL) and with acute geriatric syndromes (delirium, falls, impaired mobility, incontinence, and impaired self‐care). Listwise deletion was used to address missing values for explanatory variables where they occurred in <5% of hospital episodes analyzed. Clustering due to physicians was not addressed, as only information on whether the attending physician was a geriatrician or another internist was available in the study dataset rather than individual physician identifiers.
As additional analyses to adjust for difference in Diagnosis‐Related Groups (DRG) between intervention and control groups, we identified DRG codes that accounted more than 20 hospital episodes. Remaining DRG codes were aggregated into a single category designated as others. We then included these DRG codes as additional dummy variables in the regression models to observe the extent to which odds ratios for the treatment effect of geriatricians providing hospitalist care were changed.
Statistical analyses were performed using Stata version 13.1 (StateCorp, College Station, TX) with significance taken at the 5% level.
RESULTS
Among 1944 hospital episodes with data available for analysis, 968 received care by geriatricians and 976 by other internists. Death and readmission information at 30 days postdischarge was available for all. Seniors were predominantly female. About one‐quarter of seniors were nursing home residents. Only one‐third had premorbid functional independence. They had a mean number of 3 out of 30 Elixhauser comorbid conditions. Circulatory, respiratory, and genitourinary disorders accounted for more than half of primary diagnoses. The most common acute geriatric syndrome at presentation was delirium, which occurred in 3 out of every 10 seniors. More importantly, intervention and control groups only had minor differences on baseline characteristics, including nursing home residence, which was slightly more common in the intervention group (Table 1). Missing values occurred only for the explanatory variables, living arrangement, and premorbid basic activities of daily living in 0.4% to 2.7% of included hospital episodes.
| Care Led by Geriatricians (n=968) | Care Led by Other Internists (n=976) | P Value | |
|---|---|---|---|
| |||
| Age, mean (SD), y | 86.0 (5.1) | 85.8 (5.1) | 0.52 |
| Male, n (%) | 377 (39.0) | 361 (37.0) | 0.37 |
| Living arrangement, n (%) | 0.09 | ||
| Alone | 19 (2.0) | 21 (2.2) | |
| With other people | 690 (71.3) | 730 (74.8) | |
| Nursing home | 255 (26.3) | 214 (21.9) | |
| Missing | 4 (0.4) | 11 (1.1) | |
| Admission source, n (%) | 0.91 | ||
| Emergency department | 943 (97.4) | 950 (97.3) | |
| Others | 25 (2.6) | 26 (2.7) | |
| Hospital admissions in the prior 30 days, n (%) | 214 (22.1) | 210 (21.5) | 0.75 |
| Year, n (%) | 1.00 | ||
| 2005 | 244 (25.2) | 242 (24.8) | |
| 2006 | 237 (24.5) | 243 (24.9) | |
| 2007 | 241 (24.9) | 244 (25.0) | |
| 2008 | 246 (25.4) | 247 (25.3) | |
| Premorbid basic activities of daily living, n (%) | 0.28 | ||
| Independent | 317 (32.7) | 345 (35.3) | |
| Assisted or dependent | 625 (64.6) | 613 (62.9) | |
| Missing | 26 (2.7) | 18 (1.8) | |
| Elixhauser comorbidity count, mean (SD) | 3.2 (1.6) | 3.2 (1.7) | 0.58 |
| Modified Severity of Illness Index, n (%) | 0.30 | ||
| 1 or 2 | 541 (55.9) | 568 (58.2) | |
| 3 or 4 | 427 (44.1) | 408 (41.8) | |
| Diagnosis‐Related Group category, n (%) | 0.88 | ||
| Circulatory | 110 (11.4) | 110 (11.3) | |
| Digestive | 55 (5.7) | 60 (6.1) | |
| Endocrine, nutritional and metabolic diseases, and immunological | 60 (6.2) | 54 (5.5) | |
| Genitourinary | 146 (15.1) | 172 (17.6) | |
| Mental and nervous | 16 (1.7) | 16 (1.6) | |
| Musculoskeletal | 9 (0.9) | 10 (1.0) | |
| Respiratory | 364 (37.6) | 356 (36.5) | |
| Others | 208 (21.5) | 198 (20.3) | |
| Acute geriatric syndromes, n (%) | |||
| Mobility impairment | 75 (7.7) | 79 (8.1) | 0.78 |
| Falls | 82 (8.5) | 99 (10.1) | 0.21 |
| Delirium | 290 (30.0) | 279 (28.6) | 0.51 |
There were no significant differences in hospital mortality, 30‐day mortality or readmission, and LOS between hospital episodes with care by geriatricians and other internists for the whole group and the 2 subgroups (Table 2). However, nonsignificant reduction in hospital mortality was observed for the whole group (15.5% vs 16.9%, P=0.40), with greater magnitude for the subgroup with acute geriatric syndromes (20.2% vs 23.1%, P=0.31).
| All | Those With Premorbid Functional Impairment | Those With Acute Geriatric Syndromes | ||||
|---|---|---|---|---|---|---|
| Care Led by Geriatricians | Care Led by Other Internists | Care Led by Geriatricians | Care Led by Other Internists | Care Led by Geriatricians | Care Led by Other Internists | |
| ||||||
| Hospital mortality, n (%) | 150/968 (15.5) | 165/976 (16.9), P=0.40 | 125/625 (20.0) | 137/613 (22.4), P=0.31 | 79/392 (20.2) | 92/398 (23.1), P=0.31 |
| 30‐day mortality or readmission, n (%) | 206/818 (25.2) | 200/811 (24.7), P=0.81 | 147/500, (29.4) | 144/476, (30.3), P=0.77 | 88/313, (28.1) | 83/306, (27.1), P=0.78 |
| Mean length of stay, days (SD) | 9.7 (10.2), n=818 | 9.7 (10.9), n=811, P=0.87 | 11.1 (10.7), n=500 | 11.1 (12.3), n=476, P=0.93 | 11.4 (12.5), n=321 | 10.8 (13.0), n=312, P=0.57 |
When adjusted for age, gender, premorbid functional status, comorbidity, severity of illness, acute geriatric syndromes, hospitalization in the prior 30 days, and calendar year, care by geriatricians was associated with nonsignificant trends toward lower hospital mortality, with odds ratios between 0.80 and 0.89. However, 30‐day mortality or readmission and LOS for the intervention and control groups were generally equivalent (Table 3). There are only minor differences between the odds ratios and their 95% confidence intervals for regression analyses without and with additional adjustment for DRG codes (results not shown). Thus, they do not change the study results in any significant way.
| Care by Geriatricians (Ref: Care by Other Internists) | All | Those With Premorbid Functional Impairment | Those With Acute Geriatric Syndromes |
|---|---|---|---|
| |||
| Hospital mortality: odds ratio (95% confidence interval) | 0.89 (0.69 to 1.16), n=1,886, P=0.40 | 0.85 (0.64 to 1.13), n=1,233, P=0.27 | 0.80 (0.55 to 1.16), n=764, P=0.24 |
| 30‐day mortality or readmission: odds ratio (95% confidence interval) | 1.05 (0.82 to 1.33), n=1,580, P=0.71 | 0.94 (0.71 to 1.25), n=973, P=0.69 | 1.03 (0.70 to 1.50), n=600, P=0.89 |
| Length of stay: log days (95% confidence interval) | 0.03 (0.14 to 0.07), n=1,580, P=0.52 | 0.03 (0.16 to 0.10), n=973, P=0.68 | 0.00 (0.18 to 0.18), n=600, P=1.00 |
DISCUSSION
Geriatricians provide direct acute hospital care for seniors either in dedicated acute geriatric units including ACE units[14] or alongside generalists or subspecialty physicians in general internal medicine units. Through an unique opportunity to study the latter arrangement, we found that hospitalist care by geriatricians for seniors aged 80 years and older in general internal medicine units did not improve their short‐term outcomes vis‐‐vis care by other internists. These findings are in contrast to those of studies on acute geriatric units. This is the first report on the effectiveness of hospitalist care for seniors provided by geriatricians. Although not a randomized controlled trial, our study is in essence a natural experiment which does not impose any major inclusion restrictions other than age of 80 years and above. Internal validity was enhanced by intervention and control groups being similar on individual‐level characteristics, whereas external validity was boosted by an all‐comers approach to enrollment.
It is pertinent to ask why hospitalist care by geriatricians in a general internal medicine department did not benefit seniors with advanced age, many of whom have functional impairment and multimorbidity. After all, improved care and outcomes seem plausible for these seniors who appear to be more vulnerable. We propose 4 possible explanations. First, unmeasured differences between intervention and control groups could have led to unobserved confounding. However, this is less likely given the nonsystematic assignment of attending physicians to different wards and similarity of intervention and control groups on a broad range of baseline characteristics. Second, care processes in wards allocated to geriatricians may not differ very much from those in other wards. Irrespective of ward, care delivered by medical residents and other healthcare professionals were also expected to be similar. Unlike acute geriatric units where comprehensive geriatric assessment (CGA) by a multidisciplinary team is thought to be responsible for the improved outcomes,[12] the influence of geriatricians outside of these units may not necessarily achieve the same level of geriatric care.[15, 16] This is precisely the challenge encountered by geriatricians in their care of acutely ill older patients in settings other than acute geriatric units, Third, diffusion of geriatric care practices across general internal medicine wards over the past decade at our hospital may have resulted in narrowed differences in the care processes particularly relevant to seniors, such as those related to functional retraining, swallowing assessment, and discharge planning, although we do not have any specific data to confirm this. These differences may in turn not be wide enough for hospitalist care by geriatricians to influence these short‐term outcomes positively. Last, our study was not designed to measure patient‐reported outcomes such as functional status, mood, quality of life, and satisfaction, which may arguably be more responsive to geriatric intervention.
It might be noted that the average LOS for hospital episodes in this study was almost 10 days, which is longer than that typically seen in North America. There are 2 possible reasons for this. First, these are hospital episodes of very old patients, and longer LOS among survivors is expected. Second, post‐acute care was not as well developed in Singapore during the study period. Since then, the system of community hospitals has expanded, thereby allowing earlier transfer to these facilities for post‐acute care and shorter LOS at acute‐care hospitals.
There are a number of limitations of our study. First, this is an observational study where treatment assignment is not allocated. Although a randomized controlled trial may be the ideal design to evaluate treatment effects, operational and ethical considerations at a busy acute‐care hospital render this very challenging to conduct. As mentioned, nonsystematic assignment of attending physicians to different wards and lack of important baseline differences between intervention and control groups support the notion that important unmeasured differences are less likely. Second, and as alluded to, we did not measure relevant patient‐reported outcomes. Nonetheless, we argue that survival is still important to many seniors, particularly those without advanced illness, whereas readmission avoidance and shorter hospital stay matter almost universally. Third, clinical charts were unavailable for data extraction in almost 3% of hospital episodes. In addition, there were missing values in 2 explanatory variables in <3% of available clinical charts. These missing values were handled by listwise deletion in the regression analyses. Doing so carries with it the risk of introducing bias in the estimation of the treatment effect of care by geriatricians. However, given the relatively small proportions of missing charts and values, it is less likely that any bias would have changed the study conclusions. Fourth, we did not account for clustering at the physician level, which would have widened the confidence intervals for the odds ratios. However, because all treatment effects on the 3 outcomes were clearly not statistically significant, widening of confidence intervals would not have changed the results and study conclusions. Finally, this is a single institution study in a single health system. Thus, caution is necessary when attempting to extrapolate its results. On the other hand, the major strength of this study is its real‐world setting, which allows the results to be more generalizable to other hospital systems with similar organization and practice of general internal medicine.
Our findings need to be placed in the context of emerging innovative models of care for hospitalized seniors, which directly or indirectly involve geriatricians. Besides traditional ACE units, which have fixed geographical locations within a hospital, a mobile acute care of the elderly service achieved shorter LOS and reduced cost than the established ACE unit with similar mortality and readmission rates.[17] Others include a proactive geriatrics consultation model in collaboration with hospitalists.[18] Another variant of the ACE unit is the hospitalist‐run acute care for the elderly (hospitalist‐ACE) service, which improved care processes without improving clinical outcomes or increasing cost.[19] Clearly, there needs to be better collaboration between hospitalists and geriatricians to improve care of acutely ill seniors.[20] Ultimately, any form of direct geriatrician care for seniors needs to be complimented by indirect care through hospital‐wide systems such the Hospital Elder Life Program. This model of care aims to prevent cognitive and functional decline in hospitalized seniors by combining CGA with protocol‐driven interventions ranging from orientation, visitation, feeding assistance, early mobilization, and visual and hearing adaptations.[21, 22]
In conclusion, hospitalist care for seniors aged 80 years and above by geriatricians based in general internal medicine units is not more effective than care by other internists, at least where reducing short‐term mortality and readmission and LOS are concerned. This is particularly applicable to hospital systems where geriatric care elements have already been widely adopted beyond the confines of acute geriatric units. However, these findings do not by any means indicate that hospitalist care provided by geriatricians is altogether not more beneficial for these seniors than care by other internists in general internal medicine units. Rather, further research on patient‐reported outcomes can clarify more fully the geriatrician's true role in this setting.
Disclosures
This study was wholly funded by the National Healthcare Group Small Innovative Grants. The funders did not play any other role in this study. The authors report no conflicts of interest.
- , , , , . Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta‐analysis. BMJ. 2009;338:b50.
- , , , , . Comprehensive geriatric assessment: a meta‐analysis of controlled trials. Lancet. 1993;342(8878):1032–1036.
- , , , et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346(12):905–912.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , , . Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. J Am Geriatr Soc. 2002;50(5):792–798.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60:2237–2245.
- , . Age‐related geriatric medicine or integrated medical care? Age Ageing. 1999;28:245–247.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- . Care of hospitalized older patients: opportunities for hospital‐based physicians. J Hosp Med. 2006;1:42–47.
- , , , . Which patients benefit the most from a geriatrician's care? Consensus among directors of geriatrics academic programs. J Am Geriatr Soc. 2008;56(10):1796–1801.
- , , , et al. Comorbidity measure for use with administrative data. Med Care. 1998;36:8–27.
- , , , , . Resource consumption in hospitalised, frail older patients. Ann Acad Med Singapore. 2010;39:830–836.
- , , , , . Impact of data source and time reference of functional status on hospital mortality prediction. BMC Health Serv Res. 2012;12:115.
- , , , , . A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–1344.
- , , . Acute hospital care for frail older people. Age Ageing. 2006;35:551–552.
- , . Comprehensive geriatric assessment for older hospital patients. Br Med Bull. 2005;71:45–59.
- , , , et al. Operational and quality outcomes of a mobile acute care for the elderly service. J Hosp Med. 2011;6:358–363.
- , , , . Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57:2139–2145.
- , , , et al. Evaluation of a hospitalist‐run acute care for the elderly service. J Hosp Med. 2011;6:313–321.
- , , . Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs. J Hosp Med. 2006;1:29–35.
- , , , et al. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in hospitalized older patients. J Am Geriatr Soc. 2000;48:1697–1706.
- . Organizational interventions to improve health outcomes of older persons. Med Care. 2002;40:416–428.
- , , , , . Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta‐analysis. BMJ. 2009;338:b50.
- , , , , . Comprehensive geriatric assessment: a meta‐analysis of controlled trials. Lancet. 1993;342(8878):1032–1036.
- , , , et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346(12):905–912.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , , . Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. J Am Geriatr Soc. 2002;50(5):792–798.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60:2237–2245.
- , . Age‐related geriatric medicine or integrated medical care? Age Ageing. 1999;28:245–247.
- . An introduction to the hospitalist model. Ann Intern Med. 1999;130(4 pt 2):338–342.
- . Care of hospitalized older patients: opportunities for hospital‐based physicians. J Hosp Med. 2006;1:42–47.
- , , , . Which patients benefit the most from a geriatrician's care? Consensus among directors of geriatrics academic programs. J Am Geriatr Soc. 2008;56(10):1796–1801.
- , , , et al. Comorbidity measure for use with administrative data. Med Care. 1998;36:8–27.
- , , , , . Resource consumption in hospitalised, frail older patients. Ann Acad Med Singapore. 2010;39:830–836.
- , , , , . Impact of data source and time reference of functional status on hospital mortality prediction. BMC Health Serv Res. 2012;12:115.
- , , , , . A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–1344.
- , , . Acute hospital care for frail older people. Age Ageing. 2006;35:551–552.
- , . Comprehensive geriatric assessment for older hospital patients. Br Med Bull. 2005;71:45–59.
- , , , et al. Operational and quality outcomes of a mobile acute care for the elderly service. J Hosp Med. 2011;6:358–363.
- , , , . Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57:2139–2145.
- , , , et al. Evaluation of a hospitalist‐run acute care for the elderly service. J Hosp Med. 2011;6:313–321.
- , , . Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs. J Hosp Med. 2006;1:29–35.
- , , , et al. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in hospitalized older patients. J Am Geriatr Soc. 2000;48:1697–1706.
- . Organizational interventions to improve health outcomes of older persons. Med Care. 2002;40:416–428.
© 2014 Society of Hospital Medicine
Cerebral microbleeds’ depth may reveal dementia type
COPENHAGEN – The location of cerebral microbleeds appears to be strongly associated with dementia subtypes, perhaps reflecting the disorders’ underlying pathologies.
"This regional association with dementia subtypes is consistent with the hypothesis that lobar cerebral microbleeds reflect cerebral amyloid angiopathy, which is consistent with Alzheimer’s disease, while those in deep areas reflect hypertensive arteriopathy, which is important in vascular dementia," Lenore Launer, Ph.D., said at the Alzheimer’s Association International Conference 2014.
Dr. Launer, chief of the neuroepidemiology section in the laboratory of epidemiology and population science at the National Institute on Aging, and her colleagues examined the relationship between incident microbleeds and dementia in the AGES (Age, Gene/Environment Susceptibility) Reykjavik Study.
The population-based study is a project of the Icelandic Heart Association. It was established in 1967 and has followed more than 9,000 people, all of whom were born between 1907 and 1935. In 2000, the group partnered with the National Institute on Aging to further study diseases of old age, with an emphasis on imaging.
Since then, participants have undergone extensive phenotyping and repeat brain imaging to look specifically at cerebral microbleeds, infarcts, white-matter hyperintensities, and whole-brain volume.
Dr. Launer’s study comprised 2,482 people who were without stroke or dementia at baseline and who had complete brain MRI data during two 5-year periods: 2002-2006 and 2007-2011.
At baseline, patients’ mean age was 75 years. About 25% were carriers of the apolipoprotein E epsilon 4 allele. Hypertension was common (77% of patients). Almost a third of patients (29%) had brain infarct–like lesions, 11% had white-matter hyperintensities, and 16% had cerebral microbleeds.
Over the study period, 458 (18%) of the cohort’s patients developed new microbleeds, with 30% of those developing multiple bleeds. Of those new microbleeds, 64% were strictly lobar, with 1-15 bleeds per person. The remainder were deep lesions, numbering 1-19 per person.
There were 111 new dementia cases; of those, 83 were diagnosed as Alzheimer’s and 17 as vascular dementia. The rest were designated as "other dementia."
Two multivariate regression analyses examined the relationship between microbleed location and dementia subtype. Both controlled for a number of clinical and demographic factors. The first analysis included age, gender, and baseline cerebral microbleeds. The second analysis included all of those factors, plus education, depression, baseline vascular risk factors (hypertension, smoking, diabetes, body mass index, and total cholesterol), and baseline MRI markers (infarcts, total brain volume, and hyperintense lesions).
In the fully adjusted model, microbleed location showed a significant relationship with dementia subtypes. Lobar microbleeds were associated with a doubling in the risk of Alzheimer’s disease, while deep bleeds increased the risk of vascular dementia sixfold.
"It’s difficult to disentangle the temporal relationship here," Dr. Launer said. "But incident cerebral microbleeds may indicate more severe small-vessel disease, and be the thing that pushes a person off the cliff into the clinical presentation of dementia."
The Icelandic Heart Association and the National Institute on Aging sponsored the study. As a government employee, Dr. Launer has no financial disclosures.
On Twitter @alz_gal
COPENHAGEN – The location of cerebral microbleeds appears to be strongly associated with dementia subtypes, perhaps reflecting the disorders’ underlying pathologies.
"This regional association with dementia subtypes is consistent with the hypothesis that lobar cerebral microbleeds reflect cerebral amyloid angiopathy, which is consistent with Alzheimer’s disease, while those in deep areas reflect hypertensive arteriopathy, which is important in vascular dementia," Lenore Launer, Ph.D., said at the Alzheimer’s Association International Conference 2014.
Dr. Launer, chief of the neuroepidemiology section in the laboratory of epidemiology and population science at the National Institute on Aging, and her colleagues examined the relationship between incident microbleeds and dementia in the AGES (Age, Gene/Environment Susceptibility) Reykjavik Study.
The population-based study is a project of the Icelandic Heart Association. It was established in 1967 and has followed more than 9,000 people, all of whom were born between 1907 and 1935. In 2000, the group partnered with the National Institute on Aging to further study diseases of old age, with an emphasis on imaging.
Since then, participants have undergone extensive phenotyping and repeat brain imaging to look specifically at cerebral microbleeds, infarcts, white-matter hyperintensities, and whole-brain volume.
Dr. Launer’s study comprised 2,482 people who were without stroke or dementia at baseline and who had complete brain MRI data during two 5-year periods: 2002-2006 and 2007-2011.
At baseline, patients’ mean age was 75 years. About 25% were carriers of the apolipoprotein E epsilon 4 allele. Hypertension was common (77% of patients). Almost a third of patients (29%) had brain infarct–like lesions, 11% had white-matter hyperintensities, and 16% had cerebral microbleeds.
Over the study period, 458 (18%) of the cohort’s patients developed new microbleeds, with 30% of those developing multiple bleeds. Of those new microbleeds, 64% were strictly lobar, with 1-15 bleeds per person. The remainder were deep lesions, numbering 1-19 per person.
There were 111 new dementia cases; of those, 83 were diagnosed as Alzheimer’s and 17 as vascular dementia. The rest were designated as "other dementia."
Two multivariate regression analyses examined the relationship between microbleed location and dementia subtype. Both controlled for a number of clinical and demographic factors. The first analysis included age, gender, and baseline cerebral microbleeds. The second analysis included all of those factors, plus education, depression, baseline vascular risk factors (hypertension, smoking, diabetes, body mass index, and total cholesterol), and baseline MRI markers (infarcts, total brain volume, and hyperintense lesions).
In the fully adjusted model, microbleed location showed a significant relationship with dementia subtypes. Lobar microbleeds were associated with a doubling in the risk of Alzheimer’s disease, while deep bleeds increased the risk of vascular dementia sixfold.
"It’s difficult to disentangle the temporal relationship here," Dr. Launer said. "But incident cerebral microbleeds may indicate more severe small-vessel disease, and be the thing that pushes a person off the cliff into the clinical presentation of dementia."
The Icelandic Heart Association and the National Institute on Aging sponsored the study. As a government employee, Dr. Launer has no financial disclosures.
On Twitter @alz_gal
COPENHAGEN – The location of cerebral microbleeds appears to be strongly associated with dementia subtypes, perhaps reflecting the disorders’ underlying pathologies.
"This regional association with dementia subtypes is consistent with the hypothesis that lobar cerebral microbleeds reflect cerebral amyloid angiopathy, which is consistent with Alzheimer’s disease, while those in deep areas reflect hypertensive arteriopathy, which is important in vascular dementia," Lenore Launer, Ph.D., said at the Alzheimer’s Association International Conference 2014.
Dr. Launer, chief of the neuroepidemiology section in the laboratory of epidemiology and population science at the National Institute on Aging, and her colleagues examined the relationship between incident microbleeds and dementia in the AGES (Age, Gene/Environment Susceptibility) Reykjavik Study.
The population-based study is a project of the Icelandic Heart Association. It was established in 1967 and has followed more than 9,000 people, all of whom were born between 1907 and 1935. In 2000, the group partnered with the National Institute on Aging to further study diseases of old age, with an emphasis on imaging.
Since then, participants have undergone extensive phenotyping and repeat brain imaging to look specifically at cerebral microbleeds, infarcts, white-matter hyperintensities, and whole-brain volume.
Dr. Launer’s study comprised 2,482 people who were without stroke or dementia at baseline and who had complete brain MRI data during two 5-year periods: 2002-2006 and 2007-2011.
At baseline, patients’ mean age was 75 years. About 25% were carriers of the apolipoprotein E epsilon 4 allele. Hypertension was common (77% of patients). Almost a third of patients (29%) had brain infarct–like lesions, 11% had white-matter hyperintensities, and 16% had cerebral microbleeds.
Over the study period, 458 (18%) of the cohort’s patients developed new microbleeds, with 30% of those developing multiple bleeds. Of those new microbleeds, 64% were strictly lobar, with 1-15 bleeds per person. The remainder were deep lesions, numbering 1-19 per person.
There were 111 new dementia cases; of those, 83 were diagnosed as Alzheimer’s and 17 as vascular dementia. The rest were designated as "other dementia."
Two multivariate regression analyses examined the relationship between microbleed location and dementia subtype. Both controlled for a number of clinical and demographic factors. The first analysis included age, gender, and baseline cerebral microbleeds. The second analysis included all of those factors, plus education, depression, baseline vascular risk factors (hypertension, smoking, diabetes, body mass index, and total cholesterol), and baseline MRI markers (infarcts, total brain volume, and hyperintense lesions).
In the fully adjusted model, microbleed location showed a significant relationship with dementia subtypes. Lobar microbleeds were associated with a doubling in the risk of Alzheimer’s disease, while deep bleeds increased the risk of vascular dementia sixfold.
"It’s difficult to disentangle the temporal relationship here," Dr. Launer said. "But incident cerebral microbleeds may indicate more severe small-vessel disease, and be the thing that pushes a person off the cliff into the clinical presentation of dementia."
The Icelandic Heart Association and the National Institute on Aging sponsored the study. As a government employee, Dr. Launer has no financial disclosures.
On Twitter @alz_gal
AT AAIC 2014
Key clinical point: The location of cerebral microbleeds is strongly associated with the type of dementia that may develop.
Major finding: Lobar microbleeds doubled the risk of Alzheimer’s disease, while deep bleeds increased the risk of vascular dementia sixfold.
Data source: The population-based AGES Reykjavik Study has followed more than 9,000 patients since 1967.
Disclosures: The Icelandic Heart Association and the National Institute on Aging sponsored the study. As a government employee, Dr. Launer has no financial disclosures.
High dietary omega-3 fatty acids are associated with lower ALS risk
Adults who consumed high levels of omega-3 polyunsaturated fatty acids showed a markedly reduced risk of developing amyotrophic lateral sclerosis in a pooled analysis of five large prospective cohort studies that assessed diet.
Diet-derived omega-3 polyunsaturated fatty acids (PUFAs) are known to have neuroprotective effects, and those present in neural plasma membranes can modulate oxidative stress, excitotoxicity, and inflammation. But no prospective studies have explored a possible relationship between omega-3 PUFA intake and amyotrophic lateral sclerosis (ALS) risk, according to Kathryn C. Fitzgerald of the department of nutrition, Harvard School of Public Health, Boston, and her associates.
In a study published July 14 in JAMA Neurology, Ms. Fitzgerald and her colleagues pooled data from the Health Professionals Follow-up Study, the Nurses’ Health Study, the Cancer Prevention Study II Nutrition Cohort, the Multiethnic Cohort Study, and the National Institutes of Health-AARP Diet and Health Study. A total of 995 ALS patients were identified among 1,002,082 participants in these studies. The five studies included detailed dietary information and tracked the occurrence of ALS in the study participants through the National Death Index.
Omega-3 PUFA intake in the highest quintile of consumption at a median of 2.11 g/day was associated with a 34% reduced risk of developing ALS, compared with the lowest quintile of consumption at a median of 0.94 g/day. This finding was consistent across all five studies. This means that adding 0.5% of energy from omega-3 PUFAs and maintaining a constant intake of omega-6 fatty acids while reducing the intake of other types of fat would reduce ALS risk by 34%. Consumption of alpha-linolenic acid, another PUFA, also was associated with significantly reduced risk of developing ALS. In contrast, consumption of omega-6 PUFAs, consumption of linolenic acid, total energy intake, and percentage of energy from other types of fat showed no association with ALS risk, the investigators said (JAMA Neurol. 2014 July 14 [doi:10.1001/jamaneurol.2014.1214]).
Foods that are rich in omega-3 PUFAs include fatty fish (salmon, sardines, tuna, herring) and fish oils; vegetable oils (corn, safflower, canola, soy, and flaxseed oils); and nuts and seeds (walnuts, chia seeds, butternuts, and sunflower seeds). Further studies are needed to confirm this protective effect in ALS and to determine whether patients who already have the disease would benefit from the addition of omega-3 PUFAs to their diets, Ms. Fitzgerald and her associates added.
The findings from Ms. Fitzgerald and her associates are persuasive and consistent with earlier suggestions that PUFAs may play a role in other neurodegenerative conditions, Dr. Michael Swash said in a related editorial (JAMA Neurol. 2014 July 14 [doi:10.1001/jamaneurol.2014.1894]).
"Ideas on long-term risk-susceptibility factors are very much welcomed in trying to unravel the mystery that is ALS. Now, in addition to genetic factors, there are the following five risk factors to work on: male sex, smoking status, BMI, physical exercise, and dietary intake of PUFAs," said Dr. Swash of the Royal London Hospital, Queen Mary University of London, and the Institute of Neuroscience at the University of Lisbon.
This study was supported by the National Institute of Neurological Diseases and Stroke, the National Cancer Institute, and the ALS Therapy Alliance Foundation. The study authors and Dr. Swash had no financial disclosures.
Adults who consumed high levels of omega-3 polyunsaturated fatty acids showed a markedly reduced risk of developing amyotrophic lateral sclerosis in a pooled analysis of five large prospective cohort studies that assessed diet.
Diet-derived omega-3 polyunsaturated fatty acids (PUFAs) are known to have neuroprotective effects, and those present in neural plasma membranes can modulate oxidative stress, excitotoxicity, and inflammation. But no prospective studies have explored a possible relationship between omega-3 PUFA intake and amyotrophic lateral sclerosis (ALS) risk, according to Kathryn C. Fitzgerald of the department of nutrition, Harvard School of Public Health, Boston, and her associates.
In a study published July 14 in JAMA Neurology, Ms. Fitzgerald and her colleagues pooled data from the Health Professionals Follow-up Study, the Nurses’ Health Study, the Cancer Prevention Study II Nutrition Cohort, the Multiethnic Cohort Study, and the National Institutes of Health-AARP Diet and Health Study. A total of 995 ALS patients were identified among 1,002,082 participants in these studies. The five studies included detailed dietary information and tracked the occurrence of ALS in the study participants through the National Death Index.
Omega-3 PUFA intake in the highest quintile of consumption at a median of 2.11 g/day was associated with a 34% reduced risk of developing ALS, compared with the lowest quintile of consumption at a median of 0.94 g/day. This finding was consistent across all five studies. This means that adding 0.5% of energy from omega-3 PUFAs and maintaining a constant intake of omega-6 fatty acids while reducing the intake of other types of fat would reduce ALS risk by 34%. Consumption of alpha-linolenic acid, another PUFA, also was associated with significantly reduced risk of developing ALS. In contrast, consumption of omega-6 PUFAs, consumption of linolenic acid, total energy intake, and percentage of energy from other types of fat showed no association with ALS risk, the investigators said (JAMA Neurol. 2014 July 14 [doi:10.1001/jamaneurol.2014.1214]).
Foods that are rich in omega-3 PUFAs include fatty fish (salmon, sardines, tuna, herring) and fish oils; vegetable oils (corn, safflower, canola, soy, and flaxseed oils); and nuts and seeds (walnuts, chia seeds, butternuts, and sunflower seeds). Further studies are needed to confirm this protective effect in ALS and to determine whether patients who already have the disease would benefit from the addition of omega-3 PUFAs to their diets, Ms. Fitzgerald and her associates added.
The findings from Ms. Fitzgerald and her associates are persuasive and consistent with earlier suggestions that PUFAs may play a role in other neurodegenerative conditions, Dr. Michael Swash said in a related editorial (JAMA Neurol. 2014 July 14 [doi:10.1001/jamaneurol.2014.1894]).
"Ideas on long-term risk-susceptibility factors are very much welcomed in trying to unravel the mystery that is ALS. Now, in addition to genetic factors, there are the following five risk factors to work on: male sex, smoking status, BMI, physical exercise, and dietary intake of PUFAs," said Dr. Swash of the Royal London Hospital, Queen Mary University of London, and the Institute of Neuroscience at the University of Lisbon.
This study was supported by the National Institute of Neurological Diseases and Stroke, the National Cancer Institute, and the ALS Therapy Alliance Foundation. The study authors and Dr. Swash had no financial disclosures.
Adults who consumed high levels of omega-3 polyunsaturated fatty acids showed a markedly reduced risk of developing amyotrophic lateral sclerosis in a pooled analysis of five large prospective cohort studies that assessed diet.
Diet-derived omega-3 polyunsaturated fatty acids (PUFAs) are known to have neuroprotective effects, and those present in neural plasma membranes can modulate oxidative stress, excitotoxicity, and inflammation. But no prospective studies have explored a possible relationship between omega-3 PUFA intake and amyotrophic lateral sclerosis (ALS) risk, according to Kathryn C. Fitzgerald of the department of nutrition, Harvard School of Public Health, Boston, and her associates.
In a study published July 14 in JAMA Neurology, Ms. Fitzgerald and her colleagues pooled data from the Health Professionals Follow-up Study, the Nurses’ Health Study, the Cancer Prevention Study II Nutrition Cohort, the Multiethnic Cohort Study, and the National Institutes of Health-AARP Diet and Health Study. A total of 995 ALS patients were identified among 1,002,082 participants in these studies. The five studies included detailed dietary information and tracked the occurrence of ALS in the study participants through the National Death Index.
Omega-3 PUFA intake in the highest quintile of consumption at a median of 2.11 g/day was associated with a 34% reduced risk of developing ALS, compared with the lowest quintile of consumption at a median of 0.94 g/day. This finding was consistent across all five studies. This means that adding 0.5% of energy from omega-3 PUFAs and maintaining a constant intake of omega-6 fatty acids while reducing the intake of other types of fat would reduce ALS risk by 34%. Consumption of alpha-linolenic acid, another PUFA, also was associated with significantly reduced risk of developing ALS. In contrast, consumption of omega-6 PUFAs, consumption of linolenic acid, total energy intake, and percentage of energy from other types of fat showed no association with ALS risk, the investigators said (JAMA Neurol. 2014 July 14 [doi:10.1001/jamaneurol.2014.1214]).
Foods that are rich in omega-3 PUFAs include fatty fish (salmon, sardines, tuna, herring) and fish oils; vegetable oils (corn, safflower, canola, soy, and flaxseed oils); and nuts and seeds (walnuts, chia seeds, butternuts, and sunflower seeds). Further studies are needed to confirm this protective effect in ALS and to determine whether patients who already have the disease would benefit from the addition of omega-3 PUFAs to their diets, Ms. Fitzgerald and her associates added.
The findings from Ms. Fitzgerald and her associates are persuasive and consistent with earlier suggestions that PUFAs may play a role in other neurodegenerative conditions, Dr. Michael Swash said in a related editorial (JAMA Neurol. 2014 July 14 [doi:10.1001/jamaneurol.2014.1894]).
"Ideas on long-term risk-susceptibility factors are very much welcomed in trying to unravel the mystery that is ALS. Now, in addition to genetic factors, there are the following five risk factors to work on: male sex, smoking status, BMI, physical exercise, and dietary intake of PUFAs," said Dr. Swash of the Royal London Hospital, Queen Mary University of London, and the Institute of Neuroscience at the University of Lisbon.
This study was supported by the National Institute of Neurological Diseases and Stroke, the National Cancer Institute, and the ALS Therapy Alliance Foundation. The study authors and Dr. Swash had no financial disclosures.
FROM JAMA NEUROLOGY
Key clinical point: Eating more omega-3 PUFAs, maintaining a constant intake of omega-6 fatty acids, and reducing the intake of other types of fat is associated with a reduced risk of ALS.
Major finding: High omega-3 PUFA intake was associated with a 34% reduction in the relative risk of developing ALS.
Data source: A pooled analysis of five large prospective cohorts totaling 1,002,082 participants to explore any association between dietary intake of various fatty acids and ALS risk.
Disclosures: This study was supported by the National Institute of Neurological Diseases and Stroke, the National Cancer Institute, and the ALS Therapy Alliance Foundation. The study authors and Dr. Swash had no financial disclosures.
Survival differences in blood cancers across Europe

Credit: Rhoda Baer
Differences in treatment access and quality may explain why survival rates vary widely for European patients with hematologic malignancies, researchers have reported in The Lancet Oncology.
“The good news is that 5-year survival for most cancers of the blood has increased over the past 11 years, most likely reflecting the approval of new targeted drugs in the early 2000s . . . ,” said Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy.
“But there continue to be persistent differences between regions. For example, the uptake and use of new technologies and effective treatments has been far slower in eastern Europe than other regions. This might have contributed to the large differences in the management and outcomes of patients.”
Dr Sant and her colleagues uncovered these differences by analyzing data from 30 cancer registries covering all patients diagnosed in 20 European countries.*
The researchers compared changes in 5-year survival for 560,444 adults (aged 15 years and older) who were diagnosed with 11 lymphoid and myeloid cancers between 1997 and 2008, and followed up to the end of 2008.
Some cancers have shown particularly large increases in survival between 1997-1999 and 2006-2008, such as follicular lymphoma (59% to 74%), diffuse large B-cell lymphoma (42% to 55%), chronic myeloid leukemia (32% to 54%), and acute promyelocytic leukemia (50% to 62%).
The greatest improvements in survival have been in northern, central, and eastern Europe, even though adults in eastern Europe (where survival in 1997 was the lowest) continue to have lower survival for most hematologic malignancies than elsewhere.
Survival gains have been lower in southern Europe and the UK. For example, improvements in 5-year chronic myeloid leukemia survival in northern Europe (29% to 60%) and central Europe (34% to 65%) have been persistently higher than in the UK (35% to 56%) and southern Europe (37% to 55%).
Overall, the risk of death within 5 years from diagnosis fell significantly for all malignancies except myelodysplastic syndromes. But not all regions have seen such improvements.
For example, compared with the UK, the excess risk of death was significantly higher in eastern Europe than in other regions for most of the cancers investigated, but significantly lower in northern Europe.
The researchers said the most likely reasons for continuing geographical differences in survival are inequalities in the provision of care and in the availability and use of new treatments.
“We know that rituximab, imatinib, thalidomide, and bortezomib were first made available for general use in Europe in 1997, 2001, 1998, and 2003, respectively,” the researchers wrote.
“The years following general release of these drugs coincided with large increases in survival for chronic myeloid leukemia, diffuse large B-cell lymphoma, and follicular lymphoma, with a smaller but still significant survival increase for multiple myeloma plasmacytoma.”
However, they pointed out that the uptake and use of these drugs has not been uniform across Europe. For example, market uptake of rituximab, imatinib, and bortezomib was lower in eastern Europe than elsewhere and might explain the consistently lower survival in this region.
Writing in a linked comment article, Alastair Munro, MD, of the University of Dundee Medical School in Scotland, questioned whether improvements in survival can be attributed to drugs alone.
He said that better understanding of the conclusions from this study (called EUROCARE-5) requires additional information about changes affecting survival according to disease categories, the distribution of histological subtypes and their relation with the age distribution of the population, the distribution of stages at diagnosis, and the timing of active intervention for indolent tumors. ![]()
*The areas included in the study were northern Europe (Denmark, Iceland, and Norway), the UK (England, Northern Ireland, Scotland, and Wales), central Europe (Austria, France, Germany, Switzerland, and The Netherlands), eastern Europe (Bulgaria, Estonia, Lithuania, Poland, and Slovakia), and southern Europe (Italy, Malta, and Slovenia).

Credit: Rhoda Baer
Differences in treatment access and quality may explain why survival rates vary widely for European patients with hematologic malignancies, researchers have reported in The Lancet Oncology.
“The good news is that 5-year survival for most cancers of the blood has increased over the past 11 years, most likely reflecting the approval of new targeted drugs in the early 2000s . . . ,” said Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy.
“But there continue to be persistent differences between regions. For example, the uptake and use of new technologies and effective treatments has been far slower in eastern Europe than other regions. This might have contributed to the large differences in the management and outcomes of patients.”
Dr Sant and her colleagues uncovered these differences by analyzing data from 30 cancer registries covering all patients diagnosed in 20 European countries.*
The researchers compared changes in 5-year survival for 560,444 adults (aged 15 years and older) who were diagnosed with 11 lymphoid and myeloid cancers between 1997 and 2008, and followed up to the end of 2008.
Some cancers have shown particularly large increases in survival between 1997-1999 and 2006-2008, such as follicular lymphoma (59% to 74%), diffuse large B-cell lymphoma (42% to 55%), chronic myeloid leukemia (32% to 54%), and acute promyelocytic leukemia (50% to 62%).
The greatest improvements in survival have been in northern, central, and eastern Europe, even though adults in eastern Europe (where survival in 1997 was the lowest) continue to have lower survival for most hematologic malignancies than elsewhere.
Survival gains have been lower in southern Europe and the UK. For example, improvements in 5-year chronic myeloid leukemia survival in northern Europe (29% to 60%) and central Europe (34% to 65%) have been persistently higher than in the UK (35% to 56%) and southern Europe (37% to 55%).
Overall, the risk of death within 5 years from diagnosis fell significantly for all malignancies except myelodysplastic syndromes. But not all regions have seen such improvements.
For example, compared with the UK, the excess risk of death was significantly higher in eastern Europe than in other regions for most of the cancers investigated, but significantly lower in northern Europe.
The researchers said the most likely reasons for continuing geographical differences in survival are inequalities in the provision of care and in the availability and use of new treatments.
“We know that rituximab, imatinib, thalidomide, and bortezomib were first made available for general use in Europe in 1997, 2001, 1998, and 2003, respectively,” the researchers wrote.
“The years following general release of these drugs coincided with large increases in survival for chronic myeloid leukemia, diffuse large B-cell lymphoma, and follicular lymphoma, with a smaller but still significant survival increase for multiple myeloma plasmacytoma.”
However, they pointed out that the uptake and use of these drugs has not been uniform across Europe. For example, market uptake of rituximab, imatinib, and bortezomib was lower in eastern Europe than elsewhere and might explain the consistently lower survival in this region.
Writing in a linked comment article, Alastair Munro, MD, of the University of Dundee Medical School in Scotland, questioned whether improvements in survival can be attributed to drugs alone.
He said that better understanding of the conclusions from this study (called EUROCARE-5) requires additional information about changes affecting survival according to disease categories, the distribution of histological subtypes and their relation with the age distribution of the population, the distribution of stages at diagnosis, and the timing of active intervention for indolent tumors. ![]()
*The areas included in the study were northern Europe (Denmark, Iceland, and Norway), the UK (England, Northern Ireland, Scotland, and Wales), central Europe (Austria, France, Germany, Switzerland, and The Netherlands), eastern Europe (Bulgaria, Estonia, Lithuania, Poland, and Slovakia), and southern Europe (Italy, Malta, and Slovenia).

Credit: Rhoda Baer
Differences in treatment access and quality may explain why survival rates vary widely for European patients with hematologic malignancies, researchers have reported in The Lancet Oncology.
“The good news is that 5-year survival for most cancers of the blood has increased over the past 11 years, most likely reflecting the approval of new targeted drugs in the early 2000s . . . ,” said Milena Sant, MD, of the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy.
“But there continue to be persistent differences between regions. For example, the uptake and use of new technologies and effective treatments has been far slower in eastern Europe than other regions. This might have contributed to the large differences in the management and outcomes of patients.”
Dr Sant and her colleagues uncovered these differences by analyzing data from 30 cancer registries covering all patients diagnosed in 20 European countries.*
The researchers compared changes in 5-year survival for 560,444 adults (aged 15 years and older) who were diagnosed with 11 lymphoid and myeloid cancers between 1997 and 2008, and followed up to the end of 2008.
Some cancers have shown particularly large increases in survival between 1997-1999 and 2006-2008, such as follicular lymphoma (59% to 74%), diffuse large B-cell lymphoma (42% to 55%), chronic myeloid leukemia (32% to 54%), and acute promyelocytic leukemia (50% to 62%).
The greatest improvements in survival have been in northern, central, and eastern Europe, even though adults in eastern Europe (where survival in 1997 was the lowest) continue to have lower survival for most hematologic malignancies than elsewhere.
Survival gains have been lower in southern Europe and the UK. For example, improvements in 5-year chronic myeloid leukemia survival in northern Europe (29% to 60%) and central Europe (34% to 65%) have been persistently higher than in the UK (35% to 56%) and southern Europe (37% to 55%).
Overall, the risk of death within 5 years from diagnosis fell significantly for all malignancies except myelodysplastic syndromes. But not all regions have seen such improvements.
For example, compared with the UK, the excess risk of death was significantly higher in eastern Europe than in other regions for most of the cancers investigated, but significantly lower in northern Europe.
The researchers said the most likely reasons for continuing geographical differences in survival are inequalities in the provision of care and in the availability and use of new treatments.
“We know that rituximab, imatinib, thalidomide, and bortezomib were first made available for general use in Europe in 1997, 2001, 1998, and 2003, respectively,” the researchers wrote.
“The years following general release of these drugs coincided with large increases in survival for chronic myeloid leukemia, diffuse large B-cell lymphoma, and follicular lymphoma, with a smaller but still significant survival increase for multiple myeloma plasmacytoma.”
However, they pointed out that the uptake and use of these drugs has not been uniform across Europe. For example, market uptake of rituximab, imatinib, and bortezomib was lower in eastern Europe than elsewhere and might explain the consistently lower survival in this region.
Writing in a linked comment article, Alastair Munro, MD, of the University of Dundee Medical School in Scotland, questioned whether improvements in survival can be attributed to drugs alone.
He said that better understanding of the conclusions from this study (called EUROCARE-5) requires additional information about changes affecting survival according to disease categories, the distribution of histological subtypes and their relation with the age distribution of the population, the distribution of stages at diagnosis, and the timing of active intervention for indolent tumors. ![]()
*The areas included in the study were northern Europe (Denmark, Iceland, and Norway), the UK (England, Northern Ireland, Scotland, and Wales), central Europe (Austria, France, Germany, Switzerland, and The Netherlands), eastern Europe (Bulgaria, Estonia, Lithuania, Poland, and Slovakia), and southern Europe (Italy, Malta, and Slovenia).
FDA puts clinical hold on REGULATE-PCI trial

Credit: Kevin MacKenzie
The US Food and Drug Administration (FDA) has placed a hold on patient enrollment and dosing of drugs under investigation in the phase 3 REGULATE-PCI trial.
The trial is a comparison of the reversible thrombin inhibitor bivalirudin and the Revolixys Kit—a 2-component system consisting of pegnivacogin, an anticoagulant aptamer targeting coagulation Factor IXa, and its complementary oligonucleotide active control agent, anivamersen—in patients undergoing percutaneous coronary intervention (PCI).
The company sponsoring the REGULATE-PCI trial, Regado Biosciences, had voluntarily halted enrollment prior to the FDA’s announcement. The FDA’s clinical hold formalizes its involvement in any decision to re-initiate enrollment and dosing in the trial in the future.
Regado stopped enrollment after its Data Safety Monitoring Board (DSMB) started an unplanned review of the study data. The board has said the review is focusing on serious adverse events and allergic reactions.
“[W]e remain blinded to REGULATE-PCI study data and are awaiting the outcome of the full safety and efficacy analysis, including an analysis of benefit/risk ratio, being performed by our DSMB,” said David J Mazzo, PhD, CEO of Regado.
“Any recommendation to re-initiate patient enrollment in REGULATE-PCI will be based on the DSMB’s conclusions and would always be implemented in agreement with FDA.”
The REGULATE-PCI trial is a comparison of the Revolixys Kit (formerly known as REG-1) with bivalirudin (Angiomax) in patients undergoing PCI. The goal is to enroll 13,200 patients, and 3234 have been enrolled to date.
Eligible patients are those receiving PCI electively or for the treatment of unstable angina or non-ST elevated myocardial infarction.
Patients randomized to the Revolixys arm receive pegnivacogin at a dose of 1 mg/kg along with an 80% reversal dose of anivamersen. The timing of the remaining 20% is at the discretion of the treating physician.
The trial is powered to show superiority in efficacy and noninferiority in safety of the Revolixys Kit compared to bivalirudin. The study’s primary endpoint is a composite of death, nonfatal myocardial infarction, nonfatal stroke, and urgent target lesion revascularization through day 3 post-PCI.
For more details, visit clinicaltrials.gov. ![]()

Credit: Kevin MacKenzie
The US Food and Drug Administration (FDA) has placed a hold on patient enrollment and dosing of drugs under investigation in the phase 3 REGULATE-PCI trial.
The trial is a comparison of the reversible thrombin inhibitor bivalirudin and the Revolixys Kit—a 2-component system consisting of pegnivacogin, an anticoagulant aptamer targeting coagulation Factor IXa, and its complementary oligonucleotide active control agent, anivamersen—in patients undergoing percutaneous coronary intervention (PCI).
The company sponsoring the REGULATE-PCI trial, Regado Biosciences, had voluntarily halted enrollment prior to the FDA’s announcement. The FDA’s clinical hold formalizes its involvement in any decision to re-initiate enrollment and dosing in the trial in the future.
Regado stopped enrollment after its Data Safety Monitoring Board (DSMB) started an unplanned review of the study data. The board has said the review is focusing on serious adverse events and allergic reactions.
“[W]e remain blinded to REGULATE-PCI study data and are awaiting the outcome of the full safety and efficacy analysis, including an analysis of benefit/risk ratio, being performed by our DSMB,” said David J Mazzo, PhD, CEO of Regado.
“Any recommendation to re-initiate patient enrollment in REGULATE-PCI will be based on the DSMB’s conclusions and would always be implemented in agreement with FDA.”
The REGULATE-PCI trial is a comparison of the Revolixys Kit (formerly known as REG-1) with bivalirudin (Angiomax) in patients undergoing PCI. The goal is to enroll 13,200 patients, and 3234 have been enrolled to date.
Eligible patients are those receiving PCI electively or for the treatment of unstable angina or non-ST elevated myocardial infarction.
Patients randomized to the Revolixys arm receive pegnivacogin at a dose of 1 mg/kg along with an 80% reversal dose of anivamersen. The timing of the remaining 20% is at the discretion of the treating physician.
The trial is powered to show superiority in efficacy and noninferiority in safety of the Revolixys Kit compared to bivalirudin. The study’s primary endpoint is a composite of death, nonfatal myocardial infarction, nonfatal stroke, and urgent target lesion revascularization through day 3 post-PCI.
For more details, visit clinicaltrials.gov. ![]()

Credit: Kevin MacKenzie
The US Food and Drug Administration (FDA) has placed a hold on patient enrollment and dosing of drugs under investigation in the phase 3 REGULATE-PCI trial.
The trial is a comparison of the reversible thrombin inhibitor bivalirudin and the Revolixys Kit—a 2-component system consisting of pegnivacogin, an anticoagulant aptamer targeting coagulation Factor IXa, and its complementary oligonucleotide active control agent, anivamersen—in patients undergoing percutaneous coronary intervention (PCI).
The company sponsoring the REGULATE-PCI trial, Regado Biosciences, had voluntarily halted enrollment prior to the FDA’s announcement. The FDA’s clinical hold formalizes its involvement in any decision to re-initiate enrollment and dosing in the trial in the future.
Regado stopped enrollment after its Data Safety Monitoring Board (DSMB) started an unplanned review of the study data. The board has said the review is focusing on serious adverse events and allergic reactions.
“[W]e remain blinded to REGULATE-PCI study data and are awaiting the outcome of the full safety and efficacy analysis, including an analysis of benefit/risk ratio, being performed by our DSMB,” said David J Mazzo, PhD, CEO of Regado.
“Any recommendation to re-initiate patient enrollment in REGULATE-PCI will be based on the DSMB’s conclusions and would always be implemented in agreement with FDA.”
The REGULATE-PCI trial is a comparison of the Revolixys Kit (formerly known as REG-1) with bivalirudin (Angiomax) in patients undergoing PCI. The goal is to enroll 13,200 patients, and 3234 have been enrolled to date.
Eligible patients are those receiving PCI electively or for the treatment of unstable angina or non-ST elevated myocardial infarction.
Patients randomized to the Revolixys arm receive pegnivacogin at a dose of 1 mg/kg along with an 80% reversal dose of anivamersen. The timing of the remaining 20% is at the discretion of the treating physician.
The trial is powered to show superiority in efficacy and noninferiority in safety of the Revolixys Kit compared to bivalirudin. The study’s primary endpoint is a composite of death, nonfatal myocardial infarction, nonfatal stroke, and urgent target lesion revascularization through day 3 post-PCI.
For more details, visit clinicaltrials.gov. ![]()
Pair details ‘promise and perils’ of antioxidants

Two researchers have offered an explanation as to why antioxidants are not effective in fighting cancers and suggested a way to change that.
The duo proposed that antioxidants from supplements or dietary sources are proving ineffective because they are not acting where reactive oxygen species (ROS) are produced.
So therapies that directly inhibit the production of mitochondrial- and NADPH oxidase-derived ROS, or that scavenge ROS at these sites, may be more effective.
David Tuveson, MD, PhD, of the Cold Spring Harbor Laboratory in New York, and Navdeep S. Chandel, PhD, of the Feinberg School of Medicine at Northwestern University in Chicago, detailed these theories in a report published in The New England Journal of Medicine.
The pair’s insights are based on recent advances in understanding the cell system that establishes a natural balance between oxidizing and antioxidizing compounds.
Oxidants like hydrogen peroxide are manufactured within cells and are essential in small quantities. But oxidants are toxic in large amounts, and cells naturally generate their own antioxidants to neutralize oxidants.
It has seemed logical, therefore, to boost a person’s intake of antioxidants to counter the effects of hydrogen peroxide and other similarly toxic ROS. All the more because cancer cells are known to generate higher levels of ROS to help feed their abnormal growth.
However, Drs Tuveson and Chandel proposed that taking antioxidant pills or eating foods rich in antioxidants may be failing to show a beneficial effect against cancer because antioxidants do not act where tumor-promoting ROS are produced—at mitochondria.
Rather, supplements and dietary antioxidants tend to accumulate at scattered distant sites in the cell, “leaving tumor-promoting ROS relatively unperturbed.”
Therefore, the authors suggested therapies that directly inhibit the production of mitochondrial- and NADPH oxidase-derived ROS, or that scavenge ROS at these sites, will be more effective than dietary antioxidants.
An alternative approach
Drs Tuveson and Chandel also proposed an alternative approach: disabling antioxidants in cancer cells. They noted that quantities of both ROS and natural antioxidants are higher in cancer cells. The higher levels of antioxidants are a natural defense by cancer cells to keep their higher levels of oxidants in check so that growth can continue.
In fact, therapies that raise the levels of oxidants in cells can be beneficial, whereas those that act as antioxidants may further stimulate the cancer cells.
So the authors suggested that genetic or pharmacologic inhibition of antioxidant proteins—a concept tested successfully in rodent models of lung and pancreatic cancers—may be a useful therapeutic approach in humans.
The key challenge is to identify antioxidant proteins and pathways in cells that are used only by cancer cells and not by healthy cells. Impeding antioxidant production in healthy cells will upset the delicate redox balance upon which normal cellular function depends.
So it seems research is needed to profile antioxidant pathways in tumor and adjacent normal cells, to identify possible therapeutic targets. ![]()

Two researchers have offered an explanation as to why antioxidants are not effective in fighting cancers and suggested a way to change that.
The duo proposed that antioxidants from supplements or dietary sources are proving ineffective because they are not acting where reactive oxygen species (ROS) are produced.
So therapies that directly inhibit the production of mitochondrial- and NADPH oxidase-derived ROS, or that scavenge ROS at these sites, may be more effective.
David Tuveson, MD, PhD, of the Cold Spring Harbor Laboratory in New York, and Navdeep S. Chandel, PhD, of the Feinberg School of Medicine at Northwestern University in Chicago, detailed these theories in a report published in The New England Journal of Medicine.
The pair’s insights are based on recent advances in understanding the cell system that establishes a natural balance between oxidizing and antioxidizing compounds.
Oxidants like hydrogen peroxide are manufactured within cells and are essential in small quantities. But oxidants are toxic in large amounts, and cells naturally generate their own antioxidants to neutralize oxidants.
It has seemed logical, therefore, to boost a person’s intake of antioxidants to counter the effects of hydrogen peroxide and other similarly toxic ROS. All the more because cancer cells are known to generate higher levels of ROS to help feed their abnormal growth.
However, Drs Tuveson and Chandel proposed that taking antioxidant pills or eating foods rich in antioxidants may be failing to show a beneficial effect against cancer because antioxidants do not act where tumor-promoting ROS are produced—at mitochondria.
Rather, supplements and dietary antioxidants tend to accumulate at scattered distant sites in the cell, “leaving tumor-promoting ROS relatively unperturbed.”
Therefore, the authors suggested therapies that directly inhibit the production of mitochondrial- and NADPH oxidase-derived ROS, or that scavenge ROS at these sites, will be more effective than dietary antioxidants.
An alternative approach
Drs Tuveson and Chandel also proposed an alternative approach: disabling antioxidants in cancer cells. They noted that quantities of both ROS and natural antioxidants are higher in cancer cells. The higher levels of antioxidants are a natural defense by cancer cells to keep their higher levels of oxidants in check so that growth can continue.
In fact, therapies that raise the levels of oxidants in cells can be beneficial, whereas those that act as antioxidants may further stimulate the cancer cells.
So the authors suggested that genetic or pharmacologic inhibition of antioxidant proteins—a concept tested successfully in rodent models of lung and pancreatic cancers—may be a useful therapeutic approach in humans.
The key challenge is to identify antioxidant proteins and pathways in cells that are used only by cancer cells and not by healthy cells. Impeding antioxidant production in healthy cells will upset the delicate redox balance upon which normal cellular function depends.
So it seems research is needed to profile antioxidant pathways in tumor and adjacent normal cells, to identify possible therapeutic targets. ![]()

Two researchers have offered an explanation as to why antioxidants are not effective in fighting cancers and suggested a way to change that.
The duo proposed that antioxidants from supplements or dietary sources are proving ineffective because they are not acting where reactive oxygen species (ROS) are produced.
So therapies that directly inhibit the production of mitochondrial- and NADPH oxidase-derived ROS, or that scavenge ROS at these sites, may be more effective.
David Tuveson, MD, PhD, of the Cold Spring Harbor Laboratory in New York, and Navdeep S. Chandel, PhD, of the Feinberg School of Medicine at Northwestern University in Chicago, detailed these theories in a report published in The New England Journal of Medicine.
The pair’s insights are based on recent advances in understanding the cell system that establishes a natural balance between oxidizing and antioxidizing compounds.
Oxidants like hydrogen peroxide are manufactured within cells and are essential in small quantities. But oxidants are toxic in large amounts, and cells naturally generate their own antioxidants to neutralize oxidants.
It has seemed logical, therefore, to boost a person’s intake of antioxidants to counter the effects of hydrogen peroxide and other similarly toxic ROS. All the more because cancer cells are known to generate higher levels of ROS to help feed their abnormal growth.
However, Drs Tuveson and Chandel proposed that taking antioxidant pills or eating foods rich in antioxidants may be failing to show a beneficial effect against cancer because antioxidants do not act where tumor-promoting ROS are produced—at mitochondria.
Rather, supplements and dietary antioxidants tend to accumulate at scattered distant sites in the cell, “leaving tumor-promoting ROS relatively unperturbed.”
Therefore, the authors suggested therapies that directly inhibit the production of mitochondrial- and NADPH oxidase-derived ROS, or that scavenge ROS at these sites, will be more effective than dietary antioxidants.
An alternative approach
Drs Tuveson and Chandel also proposed an alternative approach: disabling antioxidants in cancer cells. They noted that quantities of both ROS and natural antioxidants are higher in cancer cells. The higher levels of antioxidants are a natural defense by cancer cells to keep their higher levels of oxidants in check so that growth can continue.
In fact, therapies that raise the levels of oxidants in cells can be beneficial, whereas those that act as antioxidants may further stimulate the cancer cells.
So the authors suggested that genetic or pharmacologic inhibition of antioxidant proteins—a concept tested successfully in rodent models of lung and pancreatic cancers—may be a useful therapeutic approach in humans.
The key challenge is to identify antioxidant proteins and pathways in cells that are used only by cancer cells and not by healthy cells. Impeding antioxidant production in healthy cells will upset the delicate redox balance upon which normal cellular function depends.
So it seems research is needed to profile antioxidant pathways in tumor and adjacent normal cells, to identify possible therapeutic targets. ![]()
Product recalled due to mold contamination
Image courtesy of Hospira
Hospira, Inc., has announced a US-wide, user-level recall of one lot of Lactated Ringers and 5% Dextrose Injection, USP, 1000 mL, Flexible Container (NDC 0409-7929-09, Lot 35-118-JT).
The company confirmed a report of particulate within the solution of the primary container. It was a filamentous-like structured particulate indicative of mold.
An analysis of the primary container and overwrap revealed a puncture that had caused the container to leak.
Intravenous administration of a non-sterile product can result in infections that may be life-threatening and could result in prolonged hospitalization or organ failure.
However, Hospira has not received reports of any adverse events associated with this issue for this lot and has not identified any quality issues with retention samples for this lot.
Lactated Ringers and 5% Dextrose Injection is indicated for parenteral replacement of extracellular losses of fluid and electrolytes, with or without minimal carbohydrate calories, as required by the clinical condition of the patient.
The product is packaged in 1000mL flexible containers, 1 container per overwrap, and 12 overwrapped containers in each case. The lot number is located in the upper left hand side of the primary container.
This lot (35-118-JT) was distributed nationwide—from December 2013 through February 2014—to hospitals, clinics, wholesalers, and distributors.
Anyone with an existing inventory should stop use and distribution, quarantine the product immediately, and call Stericycle at 1-888-912-8457 (8am to 5pm EST, Monday through Friday) to arrange for the return of the product.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187 (available 24 hours a day, 7 days per week) or [email protected].
To report adverse events or product complaints, contact Hospira Global
Complaint Management at 1-800-441-4100 (8am to 5pm CT, Monday through Friday) or
Adverse events or quality problems can also be reported to the Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()

Image courtesy of Hospira
Hospira, Inc., has announced a US-wide, user-level recall of one lot of Lactated Ringers and 5% Dextrose Injection, USP, 1000 mL, Flexible Container (NDC 0409-7929-09, Lot 35-118-JT).
The company confirmed a report of particulate within the solution of the primary container. It was a filamentous-like structured particulate indicative of mold.
An analysis of the primary container and overwrap revealed a puncture that had caused the container to leak.
Intravenous administration of a non-sterile product can result in infections that may be life-threatening and could result in prolonged hospitalization or organ failure.
However, Hospira has not received reports of any adverse events associated with this issue for this lot and has not identified any quality issues with retention samples for this lot.
Lactated Ringers and 5% Dextrose Injection is indicated for parenteral replacement of extracellular losses of fluid and electrolytes, with or without minimal carbohydrate calories, as required by the clinical condition of the patient.
The product is packaged in 1000mL flexible containers, 1 container per overwrap, and 12 overwrapped containers in each case. The lot number is located in the upper left hand side of the primary container.
This lot (35-118-JT) was distributed nationwide—from December 2013 through February 2014—to hospitals, clinics, wholesalers, and distributors.
Anyone with an existing inventory should stop use and distribution, quarantine the product immediately, and call Stericycle at 1-888-912-8457 (8am to 5pm EST, Monday through Friday) to arrange for the return of the product.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187 (available 24 hours a day, 7 days per week) or [email protected].
To report adverse events or product complaints, contact Hospira Global
Complaint Management at 1-800-441-4100 (8am to 5pm CT, Monday through Friday) or
Adverse events or quality problems can also be reported to the Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()

Image courtesy of Hospira
Hospira, Inc., has announced a US-wide, user-level recall of one lot of Lactated Ringers and 5% Dextrose Injection, USP, 1000 mL, Flexible Container (NDC 0409-7929-09, Lot 35-118-JT).
The company confirmed a report of particulate within the solution of the primary container. It was a filamentous-like structured particulate indicative of mold.
An analysis of the primary container and overwrap revealed a puncture that had caused the container to leak.
Intravenous administration of a non-sterile product can result in infections that may be life-threatening and could result in prolonged hospitalization or organ failure.
However, Hospira has not received reports of any adverse events associated with this issue for this lot and has not identified any quality issues with retention samples for this lot.
Lactated Ringers and 5% Dextrose Injection is indicated for parenteral replacement of extracellular losses of fluid and electrolytes, with or without minimal carbohydrate calories, as required by the clinical condition of the patient.
The product is packaged in 1000mL flexible containers, 1 container per overwrap, and 12 overwrapped containers in each case. The lot number is located in the upper left hand side of the primary container.
This lot (35-118-JT) was distributed nationwide—from December 2013 through February 2014—to hospitals, clinics, wholesalers, and distributors.
Anyone with an existing inventory should stop use and distribution, quarantine the product immediately, and call Stericycle at 1-888-912-8457 (8am to 5pm EST, Monday through Friday) to arrange for the return of the product.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187 (available 24 hours a day, 7 days per week) or [email protected].
To report adverse events or product complaints, contact Hospira Global
Complaint Management at 1-800-441-4100 (8am to 5pm CT, Monday through Friday) or
Adverse events or quality problems can also be reported to the Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()