User login
Klaus Suehler, MD, FHM, Is Rooted in Hospital Medicine
Klaus Suehler, MD, FHM, grew up in Germany and studied in Munich but dreamed of one day becoming the next Marcus Welby, MD. So he trained in Minnesota and has now worked for the same physician group for nearly two decades.
“Like many of us, I had some romantic ideas of being a doctor, running around with my black bag, figuring out a patient’s diagnosis that everyone else had missed,” says Dr. Suehler, one of the newest members of Team Hospitalist, the volunteer editorial advisory group for The Hospitalist. “As I was more seriously contemplating medicine, hospital medicine [presented] an intellectual challenge, the opportunity of applying science outside of a lab, and to be of service and to develop relationships with one’s patients.”
Dr. Suehler says internal medicine appealed to him because it was, and still is, “somewhat of an art” and affords him professional freedom. In 1994, straight out of residency, he joined Midwest Internal Medicine in Coon Rapids, Minn., an internal medicine group that, at the time, was breaking ground by dedicating physicians to hospital-based positions.
“The time was right. … Being at the hospital was kind of a continuation of residency anyhow. Some of my friends at my teaching hospital were starting a hospitalist service as well, and the whole concept appealed to me,” he says. “After juggling outpatient clinic and my weeks at the hospital for about five to six years, I finally became a full-time hospitalist.”
Since 2000, he has worked only as a hospitalist with Midwest Internal Medicine, serving as the hospitalist group’s leader for eight of those years.
Question: What do you like most about working as a hospitalist?
Answer: I like the challenging cases and the opportunity to collaborate with other specialists and the staff on the wards. We always learn from each other within our hospitalist group, as we are taking care of each other’s patients. There is ample opportunity to informally discuss complex patients and their treatment.
Q: What do you dislike most?
A: Overly busy call nights. I must say, though, at least at our hospital we have the opportunity to close our service for admissions, if we feel the workload would no longer be safe.
Q: What’s the best advice you ever received?
A: There was no single advice that I can recall related to my HM practice. What really helped very much was the ongoing input and advice that I received through formal leadership training, which I received during the time as the leader of our group. It helped me to be more grounded, resilient, and effective, both as a professional and a person.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The field has essentially become its own specialty. There is an increased focus on the operational part of taking care of patients in the hospital, as well as the transitions, which goes beyond the medical aspects of patient care for, let’s say, patients presenting with congestive heart failure.
Q: Why is it important for group leaders to continue seeing patients?
A: It adds credibility to their leadership. There is nothing like shouldering new responsibilities or high workloads together with your partners.
Q: Outside of patient care, what about your job interests you the most?
A: Quality and patient safety are of great interest for me. Our hospitalist group consists of about 25 hospitalists, but there are about 200 hospitalists within our health system. I participate in a steering committee within the larger system to improve medication reconciliation.
I also continue to be interested in leadership. Especially in the years to come, with all the upcoming changes in the delivery of care, performance measures, and modes of reimbursement, there will be an increased need for physician leaders.
Q: What is your biggest professional challenge?
A: It is a good sign that I have to think for a while to come up with an answer. It probably is taking care of patients with narcotic addictions or difficult personalities. Every given week there are probably one or two such patients on the service. It is challenging to do these patients justice and to provide the appropriate medical care.
Q: What is your biggest professional reward?
A: The respect of my physician colleagues, the nursing staff, and the gratitude of my patients and their families. One of my most moving experiences was when a former patient of mine walked over to my table in a Chinese restaurant and said: “Thank you for saving my life!”
Q: You were one of the first hospitalists designated Fellow in Hospital Medicine. What does that mean to you?
A: It gave me some formal recognition for the years of service I have put into HM, both in patient care and leadership, as well as for my level of experience.
Q: When you aren’t working, what is important to you?
A: The relationships with the people around me (marriage, family, friendship). In terms of what I do, it is traveling, exercising, reading, and being appreciative of the moment.
Q: Where do you see yourself in 10 years?
A: I want to continue to work as a hospitalist. I am looking into leadership opportunities. I can see shifting my focus more toward leadership or consulting roles again within the next 10 years.
Q: If you weren’t a doctor, what would you be doing right now?
A: I think I would be a lawyer. I know there is a perceived friction between these professions, but there are some common themes, such as providing expert advice or professional help in times of need.
Q: What’s the best book you’ve read recently?
A: The Angel’s Game by Carlos Ruiz Zafon. It is an incredibly captivating story, with a marvelous imagery of old Barcelona before the Spanish Civil War.
Q: How many Apple products do you interface with in a given week?
A: Two: iPhone and iPad.
Richard Quinn is a freelance writer in New Jersey.
Klaus Suehler, MD, FHM, grew up in Germany and studied in Munich but dreamed of one day becoming the next Marcus Welby, MD. So he trained in Minnesota and has now worked for the same physician group for nearly two decades.
“Like many of us, I had some romantic ideas of being a doctor, running around with my black bag, figuring out a patient’s diagnosis that everyone else had missed,” says Dr. Suehler, one of the newest members of Team Hospitalist, the volunteer editorial advisory group for The Hospitalist. “As I was more seriously contemplating medicine, hospital medicine [presented] an intellectual challenge, the opportunity of applying science outside of a lab, and to be of service and to develop relationships with one’s patients.”
Dr. Suehler says internal medicine appealed to him because it was, and still is, “somewhat of an art” and affords him professional freedom. In 1994, straight out of residency, he joined Midwest Internal Medicine in Coon Rapids, Minn., an internal medicine group that, at the time, was breaking ground by dedicating physicians to hospital-based positions.
“The time was right. … Being at the hospital was kind of a continuation of residency anyhow. Some of my friends at my teaching hospital were starting a hospitalist service as well, and the whole concept appealed to me,” he says. “After juggling outpatient clinic and my weeks at the hospital for about five to six years, I finally became a full-time hospitalist.”
Since 2000, he has worked only as a hospitalist with Midwest Internal Medicine, serving as the hospitalist group’s leader for eight of those years.
Question: What do you like most about working as a hospitalist?
Answer: I like the challenging cases and the opportunity to collaborate with other specialists and the staff on the wards. We always learn from each other within our hospitalist group, as we are taking care of each other’s patients. There is ample opportunity to informally discuss complex patients and their treatment.
Q: What do you dislike most?
A: Overly busy call nights. I must say, though, at least at our hospital we have the opportunity to close our service for admissions, if we feel the workload would no longer be safe.
Q: What’s the best advice you ever received?
A: There was no single advice that I can recall related to my HM practice. What really helped very much was the ongoing input and advice that I received through formal leadership training, which I received during the time as the leader of our group. It helped me to be more grounded, resilient, and effective, both as a professional and a person.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The field has essentially become its own specialty. There is an increased focus on the operational part of taking care of patients in the hospital, as well as the transitions, which goes beyond the medical aspects of patient care for, let’s say, patients presenting with congestive heart failure.
Q: Why is it important for group leaders to continue seeing patients?
A: It adds credibility to their leadership. There is nothing like shouldering new responsibilities or high workloads together with your partners.
Q: Outside of patient care, what about your job interests you the most?
A: Quality and patient safety are of great interest for me. Our hospitalist group consists of about 25 hospitalists, but there are about 200 hospitalists within our health system. I participate in a steering committee within the larger system to improve medication reconciliation.
I also continue to be interested in leadership. Especially in the years to come, with all the upcoming changes in the delivery of care, performance measures, and modes of reimbursement, there will be an increased need for physician leaders.
Q: What is your biggest professional challenge?
A: It is a good sign that I have to think for a while to come up with an answer. It probably is taking care of patients with narcotic addictions or difficult personalities. Every given week there are probably one or two such patients on the service. It is challenging to do these patients justice and to provide the appropriate medical care.
Q: What is your biggest professional reward?
A: The respect of my physician colleagues, the nursing staff, and the gratitude of my patients and their families. One of my most moving experiences was when a former patient of mine walked over to my table in a Chinese restaurant and said: “Thank you for saving my life!”
Q: You were one of the first hospitalists designated Fellow in Hospital Medicine. What does that mean to you?
A: It gave me some formal recognition for the years of service I have put into HM, both in patient care and leadership, as well as for my level of experience.
Q: When you aren’t working, what is important to you?
A: The relationships with the people around me (marriage, family, friendship). In terms of what I do, it is traveling, exercising, reading, and being appreciative of the moment.
Q: Where do you see yourself in 10 years?
A: I want to continue to work as a hospitalist. I am looking into leadership opportunities. I can see shifting my focus more toward leadership or consulting roles again within the next 10 years.
Q: If you weren’t a doctor, what would you be doing right now?
A: I think I would be a lawyer. I know there is a perceived friction between these professions, but there are some common themes, such as providing expert advice or professional help in times of need.
Q: What’s the best book you’ve read recently?
A: The Angel’s Game by Carlos Ruiz Zafon. It is an incredibly captivating story, with a marvelous imagery of old Barcelona before the Spanish Civil War.
Q: How many Apple products do you interface with in a given week?
A: Two: iPhone and iPad.
Richard Quinn is a freelance writer in New Jersey.
Klaus Suehler, MD, FHM, grew up in Germany and studied in Munich but dreamed of one day becoming the next Marcus Welby, MD. So he trained in Minnesota and has now worked for the same physician group for nearly two decades.
“Like many of us, I had some romantic ideas of being a doctor, running around with my black bag, figuring out a patient’s diagnosis that everyone else had missed,” says Dr. Suehler, one of the newest members of Team Hospitalist, the volunteer editorial advisory group for The Hospitalist. “As I was more seriously contemplating medicine, hospital medicine [presented] an intellectual challenge, the opportunity of applying science outside of a lab, and to be of service and to develop relationships with one’s patients.”
Dr. Suehler says internal medicine appealed to him because it was, and still is, “somewhat of an art” and affords him professional freedom. In 1994, straight out of residency, he joined Midwest Internal Medicine in Coon Rapids, Minn., an internal medicine group that, at the time, was breaking ground by dedicating physicians to hospital-based positions.
“The time was right. … Being at the hospital was kind of a continuation of residency anyhow. Some of my friends at my teaching hospital were starting a hospitalist service as well, and the whole concept appealed to me,” he says. “After juggling outpatient clinic and my weeks at the hospital for about five to six years, I finally became a full-time hospitalist.”
Since 2000, he has worked only as a hospitalist with Midwest Internal Medicine, serving as the hospitalist group’s leader for eight of those years.
Question: What do you like most about working as a hospitalist?
Answer: I like the challenging cases and the opportunity to collaborate with other specialists and the staff on the wards. We always learn from each other within our hospitalist group, as we are taking care of each other’s patients. There is ample opportunity to informally discuss complex patients and their treatment.
Q: What do you dislike most?
A: Overly busy call nights. I must say, though, at least at our hospital we have the opportunity to close our service for admissions, if we feel the workload would no longer be safe.
Q: What’s the best advice you ever received?
A: There was no single advice that I can recall related to my HM practice. What really helped very much was the ongoing input and advice that I received through formal leadership training, which I received during the time as the leader of our group. It helped me to be more grounded, resilient, and effective, both as a professional and a person.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The field has essentially become its own specialty. There is an increased focus on the operational part of taking care of patients in the hospital, as well as the transitions, which goes beyond the medical aspects of patient care for, let’s say, patients presenting with congestive heart failure.
Q: Why is it important for group leaders to continue seeing patients?
A: It adds credibility to their leadership. There is nothing like shouldering new responsibilities or high workloads together with your partners.
Q: Outside of patient care, what about your job interests you the most?
A: Quality and patient safety are of great interest for me. Our hospitalist group consists of about 25 hospitalists, but there are about 200 hospitalists within our health system. I participate in a steering committee within the larger system to improve medication reconciliation.
I also continue to be interested in leadership. Especially in the years to come, with all the upcoming changes in the delivery of care, performance measures, and modes of reimbursement, there will be an increased need for physician leaders.
Q: What is your biggest professional challenge?
A: It is a good sign that I have to think for a while to come up with an answer. It probably is taking care of patients with narcotic addictions or difficult personalities. Every given week there are probably one or two such patients on the service. It is challenging to do these patients justice and to provide the appropriate medical care.
Q: What is your biggest professional reward?
A: The respect of my physician colleagues, the nursing staff, and the gratitude of my patients and their families. One of my most moving experiences was when a former patient of mine walked over to my table in a Chinese restaurant and said: “Thank you for saving my life!”
Q: You were one of the first hospitalists designated Fellow in Hospital Medicine. What does that mean to you?
A: It gave me some formal recognition for the years of service I have put into HM, both in patient care and leadership, as well as for my level of experience.
Q: When you aren’t working, what is important to you?
A: The relationships with the people around me (marriage, family, friendship). In terms of what I do, it is traveling, exercising, reading, and being appreciative of the moment.
Q: Where do you see yourself in 10 years?
A: I want to continue to work as a hospitalist. I am looking into leadership opportunities. I can see shifting my focus more toward leadership or consulting roles again within the next 10 years.
Q: If you weren’t a doctor, what would you be doing right now?
A: I think I would be a lawyer. I know there is a perceived friction between these professions, but there are some common themes, such as providing expert advice or professional help in times of need.
Q: What’s the best book you’ve read recently?
A: The Angel’s Game by Carlos Ruiz Zafon. It is an incredibly captivating story, with a marvelous imagery of old Barcelona before the Spanish Civil War.
Q: How many Apple products do you interface with in a given week?
A: Two: iPhone and iPad.
Richard Quinn is a freelance writer in New Jersey.
When Should You Suspect Kawasaki Disease as the Cause of Fever in an Infant?
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
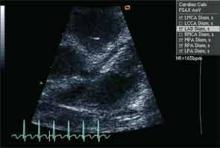
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Movers and Shakers in Hospital Medicine
Michael Campbell, MD, has been named one of the 2013 Physicians of the Year at Lake Health community health system in Lake County, Ohio. Dr. Campbell is a hospitalist who is board certified in family practice. He has been with Lake Health since 2011.
Nitish Kosaraju, MD, and Jocelyn Hendricks, DO, both received the 2013 Hospitalist of the Year award for an acute care practice from IPC The Hospitalist Company, based in North Hollywood, Calif. Dr. Kosaraju is a practice group leader for IPC in Houston and has been an IPC hospitalist since 2010. Dr. Hendricks is a practice group leader in Tucson, Ariz., and has been part of IPC since 2004.
Pedram Taher, MD, received IPC’s 2013 Hospitalist of the Year award for a post-acute care practice. Dr. Taher has worked for IPC since 2009 and is a practice group leader in the San Francisco Bay area.
Tammy Hilliard, FNP-C, earned IPC’s 2013 Hospitalist of the Year award for a non-physician provider. Hilliard has been with IPC since 2009 and now serves as a nurse practitioner and practice group representative in Phoenix, Ariz.
Jeffrey Harris, MD, received IPC’s 2013 Newcomer Clinician of the Year award. Dr. Harris is a neuro-hospitalist in San Antonio, Texas, and joined IPC in January 2013.
Corbi Milligan, MD, was featured in Murfreesboro Magazine for her exemplary leadership as the EmCare hospitalist site medical director for TriStar StoneCrest Medical Center in Smyrna, Tenn. Dr. Milligan oversees 10 hospitalists in her role and has been with TriStar StoneCrest since 2004.
Business Moves
St. Anthony’s Memorial Hospital in Effingham, Ill., has announced a brand new hospitalist program. The 146-bed acute care center will staff two full-time hospitalists.
Laurens County Memorial Hospital (LCMH) in Clinton, S.C., has partnered with the hospitalist program at Greenville Health System (GHS) in Greenville, S.C., to provide 24-hour hospitalist services. GHS’s lead hospitalist, Kevin Gilroy, MD, will oversee the new program at LCMH. GHS is a public, nonprofit healthcare system comprising seven regional medical centers, including LCMH, as well as numerous post-acute care facilities and offices.
The hospitalist program at Erlanger Health System in Chattanooga, Tenn., will now be managed by MDP Management, a Chattanooga-based physician management company. Erlanger’s hospitalist program has 18 full-time physicians at the nonprofit, level-one trauma center.
IPC The Hospitalist Company has acquired the post-acute hospitalist practice CAP Medical Group, PLLC, in New Hartford, N.Y. CAP Medical Group has served the Oneida County area of upstate New York since 2007. IPC oversees hospitalist services in over 400 hospitals and 1,100 post-acute care practices across the country.
Michael Campbell, MD, has been named one of the 2013 Physicians of the Year at Lake Health community health system in Lake County, Ohio. Dr. Campbell is a hospitalist who is board certified in family practice. He has been with Lake Health since 2011.
Nitish Kosaraju, MD, and Jocelyn Hendricks, DO, both received the 2013 Hospitalist of the Year award for an acute care practice from IPC The Hospitalist Company, based in North Hollywood, Calif. Dr. Kosaraju is a practice group leader for IPC in Houston and has been an IPC hospitalist since 2010. Dr. Hendricks is a practice group leader in Tucson, Ariz., and has been part of IPC since 2004.
Pedram Taher, MD, received IPC’s 2013 Hospitalist of the Year award for a post-acute care practice. Dr. Taher has worked for IPC since 2009 and is a practice group leader in the San Francisco Bay area.
Tammy Hilliard, FNP-C, earned IPC’s 2013 Hospitalist of the Year award for a non-physician provider. Hilliard has been with IPC since 2009 and now serves as a nurse practitioner and practice group representative in Phoenix, Ariz.
Jeffrey Harris, MD, received IPC’s 2013 Newcomer Clinician of the Year award. Dr. Harris is a neuro-hospitalist in San Antonio, Texas, and joined IPC in January 2013.
Corbi Milligan, MD, was featured in Murfreesboro Magazine for her exemplary leadership as the EmCare hospitalist site medical director for TriStar StoneCrest Medical Center in Smyrna, Tenn. Dr. Milligan oversees 10 hospitalists in her role and has been with TriStar StoneCrest since 2004.
Business Moves
St. Anthony’s Memorial Hospital in Effingham, Ill., has announced a brand new hospitalist program. The 146-bed acute care center will staff two full-time hospitalists.
Laurens County Memorial Hospital (LCMH) in Clinton, S.C., has partnered with the hospitalist program at Greenville Health System (GHS) in Greenville, S.C., to provide 24-hour hospitalist services. GHS’s lead hospitalist, Kevin Gilroy, MD, will oversee the new program at LCMH. GHS is a public, nonprofit healthcare system comprising seven regional medical centers, including LCMH, as well as numerous post-acute care facilities and offices.
The hospitalist program at Erlanger Health System in Chattanooga, Tenn., will now be managed by MDP Management, a Chattanooga-based physician management company. Erlanger’s hospitalist program has 18 full-time physicians at the nonprofit, level-one trauma center.
IPC The Hospitalist Company has acquired the post-acute hospitalist practice CAP Medical Group, PLLC, in New Hartford, N.Y. CAP Medical Group has served the Oneida County area of upstate New York since 2007. IPC oversees hospitalist services in over 400 hospitals and 1,100 post-acute care practices across the country.
Michael Campbell, MD, has been named one of the 2013 Physicians of the Year at Lake Health community health system in Lake County, Ohio. Dr. Campbell is a hospitalist who is board certified in family practice. He has been with Lake Health since 2011.
Nitish Kosaraju, MD, and Jocelyn Hendricks, DO, both received the 2013 Hospitalist of the Year award for an acute care practice from IPC The Hospitalist Company, based in North Hollywood, Calif. Dr. Kosaraju is a practice group leader for IPC in Houston and has been an IPC hospitalist since 2010. Dr. Hendricks is a practice group leader in Tucson, Ariz., and has been part of IPC since 2004.
Pedram Taher, MD, received IPC’s 2013 Hospitalist of the Year award for a post-acute care practice. Dr. Taher has worked for IPC since 2009 and is a practice group leader in the San Francisco Bay area.
Tammy Hilliard, FNP-C, earned IPC’s 2013 Hospitalist of the Year award for a non-physician provider. Hilliard has been with IPC since 2009 and now serves as a nurse practitioner and practice group representative in Phoenix, Ariz.
Jeffrey Harris, MD, received IPC’s 2013 Newcomer Clinician of the Year award. Dr. Harris is a neuro-hospitalist in San Antonio, Texas, and joined IPC in January 2013.
Corbi Milligan, MD, was featured in Murfreesboro Magazine for her exemplary leadership as the EmCare hospitalist site medical director for TriStar StoneCrest Medical Center in Smyrna, Tenn. Dr. Milligan oversees 10 hospitalists in her role and has been with TriStar StoneCrest since 2004.
Business Moves
St. Anthony’s Memorial Hospital in Effingham, Ill., has announced a brand new hospitalist program. The 146-bed acute care center will staff two full-time hospitalists.
Laurens County Memorial Hospital (LCMH) in Clinton, S.C., has partnered with the hospitalist program at Greenville Health System (GHS) in Greenville, S.C., to provide 24-hour hospitalist services. GHS’s lead hospitalist, Kevin Gilroy, MD, will oversee the new program at LCMH. GHS is a public, nonprofit healthcare system comprising seven regional medical centers, including LCMH, as well as numerous post-acute care facilities and offices.
The hospitalist program at Erlanger Health System in Chattanooga, Tenn., will now be managed by MDP Management, a Chattanooga-based physician management company. Erlanger’s hospitalist program has 18 full-time physicians at the nonprofit, level-one trauma center.
IPC The Hospitalist Company has acquired the post-acute hospitalist practice CAP Medical Group, PLLC, in New Hartford, N.Y. CAP Medical Group has served the Oneida County area of upstate New York since 2007. IPC oversees hospitalist services in over 400 hospitals and 1,100 post-acute care practices across the country.
Society of Hospital Medicine Improves Online Career Center
Looking to recruit new hospitalists? Or are you a hospitalist looking for a change? Visit the new SHM Career Center (www.shmcareercenter.org) to recruit and look for new jobs. The new site enables visitors to highlight their favorite opportunities, get alerts via e-mail, and search through hospitalist jobs across the country. Recruiters will find easy-to-use instructions for posting jobs and searching through resumes.
Looking to recruit new hospitalists? Or are you a hospitalist looking for a change? Visit the new SHM Career Center (www.shmcareercenter.org) to recruit and look for new jobs. The new site enables visitors to highlight their favorite opportunities, get alerts via e-mail, and search through hospitalist jobs across the country. Recruiters will find easy-to-use instructions for posting jobs and searching through resumes.
Looking to recruit new hospitalists? Or are you a hospitalist looking for a change? Visit the new SHM Career Center (www.shmcareercenter.org) to recruit and look for new jobs. The new site enables visitors to highlight their favorite opportunities, get alerts via e-mail, and search through hospitalist jobs across the country. Recruiters will find easy-to-use instructions for posting jobs and searching through resumes.
Hospitalists Share Q&As on SHM's Hospital Medicine Exchange
Thousands of hospitalists have logged into HMX (www.hmxchange.org) to ask questions and share answers to some of the most pressing questions for hospitalists everywhere. Recent conversations include:
- How residents can get involved with SHM;
- How hospitalists can simplify the process of transferring a patient from one facility to another;
- How hospitalists can staff long-term acute care hospitals; and
- Pediatric hospital medicine subspecialty certification.
Thousands of hospitalists have logged into HMX (www.hmxchange.org) to ask questions and share answers to some of the most pressing questions for hospitalists everywhere. Recent conversations include:
- How residents can get involved with SHM;
- How hospitalists can simplify the process of transferring a patient from one facility to another;
- How hospitalists can staff long-term acute care hospitals; and
- Pediatric hospital medicine subspecialty certification.
Thousands of hospitalists have logged into HMX (www.hmxchange.org) to ask questions and share answers to some of the most pressing questions for hospitalists everywhere. Recent conversations include:
- How residents can get involved with SHM;
- How hospitalists can simplify the process of transferring a patient from one facility to another;
- How hospitalists can staff long-term acute care hospitals; and
- Pediatric hospital medicine subspecialty certification.
The Hospital Leader Blog Showcases Latest Ideas, Fresh Perspectives from Hospitalists
SHM’s blog, “The Hospital Leader,” showcases some of the most cutting-edge ideas and fresh perspectives in the hospitalist movement. For all posts, visit www.hospitalleader.org. Here’s a sampling of our most popular recent posts:
- The Next Dose: Hospitalist Brett Hendel-Paterson juxtaposes his experience as a cancer patient with his experience as a caregiver, especially when it comes to prescribing treatments for his patients.
- 15 Patients a Day: Starling Curve or Sweet Spot?: SHM President Burke Kealey asks whether a 15-patient census is really the most important factor in a hospitalist’s day.
- Broken RAC System Continues to Hurt Patients, Providers: Hospitalist Bart Caponi dissects the Recovery Audit Contractor (RAC) system in a post that was featured on the Forbes.com Pharma & Healthcare section.
SHM’s blog, “The Hospital Leader,” showcases some of the most cutting-edge ideas and fresh perspectives in the hospitalist movement. For all posts, visit www.hospitalleader.org. Here’s a sampling of our most popular recent posts:
- The Next Dose: Hospitalist Brett Hendel-Paterson juxtaposes his experience as a cancer patient with his experience as a caregiver, especially when it comes to prescribing treatments for his patients.
- 15 Patients a Day: Starling Curve or Sweet Spot?: SHM President Burke Kealey asks whether a 15-patient census is really the most important factor in a hospitalist’s day.
- Broken RAC System Continues to Hurt Patients, Providers: Hospitalist Bart Caponi dissects the Recovery Audit Contractor (RAC) system in a post that was featured on the Forbes.com Pharma & Healthcare section.
SHM’s blog, “The Hospital Leader,” showcases some of the most cutting-edge ideas and fresh perspectives in the hospitalist movement. For all posts, visit www.hospitalleader.org. Here’s a sampling of our most popular recent posts:
- The Next Dose: Hospitalist Brett Hendel-Paterson juxtaposes his experience as a cancer patient with his experience as a caregiver, especially when it comes to prescribing treatments for his patients.
- 15 Patients a Day: Starling Curve or Sweet Spot?: SHM President Burke Kealey asks whether a 15-patient census is really the most important factor in a hospitalist’s day.
- Broken RAC System Continues to Hurt Patients, Providers: Hospitalist Bart Caponi dissects the Recovery Audit Contractor (RAC) system in a post that was featured on the Forbes.com Pharma & Healthcare section.
Three Ways to Improve Quality of Patient Care in Your Hospital
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
Improving the quality of care in your hospital isn’t just good for your hospital medicine group or your hospital; it’s good for the community. Each year, SHM leads some of the best quality improvement programs in healthcare, and you can get involved.
SHM is now accepting applications for the Glycemic Control Mentored Implementation Program. An informational webinar about the program will be available on Aug. 14. For details, visit www.hospitalmedicine.org/gcmi.
There is still time to apply for the Project BOOST fall cohort. For details, visit www.hospitalmedicine.org/boost.
Are you implementing Choosing Wisely in your hospital? You could win SHM’s Choosing Wisely competition and share your expertise with thousands of other hospitalists.
Visit www.hospitalmedicine.org/choosingwisely to learn more.
Pre-Order Your State of Hospital Medicine Report Now
The State of Hospital Medicine Report is the authoritative source for hospitalists to compare and contrast their staffing, productivity, and compensation with other HM groups across the country. SHM publishes the State of Hospital Medicine every two years. SHM is accepting pre-orders now. For more information, visit www.hospitalmedicine.org/sohm.
The State of Hospital Medicine Report is the authoritative source for hospitalists to compare and contrast their staffing, productivity, and compensation with other HM groups across the country. SHM publishes the State of Hospital Medicine every two years. SHM is accepting pre-orders now. For more information, visit www.hospitalmedicine.org/sohm.
The State of Hospital Medicine Report is the authoritative source for hospitalists to compare and contrast their staffing, productivity, and compensation with other HM groups across the country. SHM publishes the State of Hospital Medicine every two years. SHM is accepting pre-orders now. For more information, visit www.hospitalmedicine.org/sohm.
Code-H Interactive Tool Helps Hospital Medicine Groups with Coding
Looking for ways to make your HM group run better? SHM is introducing new tools and information to keep you ahead of the curve.
CODE-H Interactive is an industry first: an interactive tool to help hospitalist groups code effectively and efficiently. CODE-H Interactive allows users to validate documentation against coding criteria and provides a guided tour through clinical documentation, allowing users to ensure they are choosing the correct billing code while providing a conceptual framework that enables the user to easily “connect the dots” between clinical documentation and the applicable CPT coding.
CODE-H Interactive includes two modules: one that reviews three admission notes and a second that reviews three daily notes. It also enables users to assess other E/M codes, such as consultations and ED visits. To get started, visit www.hospitalmedicine.org/CODEHI.
Looking for ways to make your HM group run better? SHM is introducing new tools and information to keep you ahead of the curve.
CODE-H Interactive is an industry first: an interactive tool to help hospitalist groups code effectively and efficiently. CODE-H Interactive allows users to validate documentation against coding criteria and provides a guided tour through clinical documentation, allowing users to ensure they are choosing the correct billing code while providing a conceptual framework that enables the user to easily “connect the dots” between clinical documentation and the applicable CPT coding.
CODE-H Interactive includes two modules: one that reviews three admission notes and a second that reviews three daily notes. It also enables users to assess other E/M codes, such as consultations and ED visits. To get started, visit www.hospitalmedicine.org/CODEHI.
Looking for ways to make your HM group run better? SHM is introducing new tools and information to keep you ahead of the curve.
CODE-H Interactive is an industry first: an interactive tool to help hospitalist groups code effectively and efficiently. CODE-H Interactive allows users to validate documentation against coding criteria and provides a guided tour through clinical documentation, allowing users to ensure they are choosing the correct billing code while providing a conceptual framework that enables the user to easily “connect the dots” between clinical documentation and the applicable CPT coding.
CODE-H Interactive includes two modules: one that reviews three admission notes and a second that reviews three daily notes. It also enables users to assess other E/M codes, such as consultations and ED visits. To get started, visit www.hospitalmedicine.org/CODEHI.
Society of Hospital Medicine Leadership Academy Provides Practical Tips, Hands-On Collaboration, Networking for Hospitalists
In April, The Hospitalist explored the many paths that hospitalists have taken to leadership positions within their hospitals. Among the many skill sets required to grow as a leader in the hospital, hospital CEOs, presidents, and others noted that hospitalists have a unique grasp on the ability to deal with complexity and solve problems within the hospital.
Those very skills—and many others—are the focus of SHM’s Leadership Academy, presented Nov. 3-6 in Honolulu, Hawaii. Leadership Academy comprises three different courses, each of which will be available at the Hilton Hawaiian Village Waikiki Beach Resort:
- Leadership Foundations;
- Advanced Leadership: Influential Management; and
- Advanced Leadership: Mastering Teamwork.
Details and registration are now available at www.hospitalmedicine.org/leadership.
Hospitalist Binu Pappachen, MD, who will be attending one of the Advanced Leadership sessions in Hawaii, plans on completing the program and earning SHM’s Certificate of Leadership in Hospital Medicine.
“I consider this as a great opportunity for my road ahead, get to know more people, and networking,” says Dr. Pappachen, who highly recommends the training to fellow hospitalists. “The first academy course was an eye opener to the different aspects of medicine, team-building, problem solving, and business aspects.”
In fact, Dr. Pappachen feels as though Leadership Academy already has made him a better hospitalist. “A better team player, committed and taking ownership of what I do,” he says.
All three courses focus on practical leadership, hands-on collaboration, and networking. In fact, Leadership Academy alumni have begun their own community on SHM’s online collaboration forum, HMX (www.hmxchange.com).
Brendon Shank is SHM’s associate vice president of communications.
In April, The Hospitalist explored the many paths that hospitalists have taken to leadership positions within their hospitals. Among the many skill sets required to grow as a leader in the hospital, hospital CEOs, presidents, and others noted that hospitalists have a unique grasp on the ability to deal with complexity and solve problems within the hospital.
Those very skills—and many others—are the focus of SHM’s Leadership Academy, presented Nov. 3-6 in Honolulu, Hawaii. Leadership Academy comprises three different courses, each of which will be available at the Hilton Hawaiian Village Waikiki Beach Resort:
- Leadership Foundations;
- Advanced Leadership: Influential Management; and
- Advanced Leadership: Mastering Teamwork.
Details and registration are now available at www.hospitalmedicine.org/leadership.
Hospitalist Binu Pappachen, MD, who will be attending one of the Advanced Leadership sessions in Hawaii, plans on completing the program and earning SHM’s Certificate of Leadership in Hospital Medicine.
“I consider this as a great opportunity for my road ahead, get to know more people, and networking,” says Dr. Pappachen, who highly recommends the training to fellow hospitalists. “The first academy course was an eye opener to the different aspects of medicine, team-building, problem solving, and business aspects.”
In fact, Dr. Pappachen feels as though Leadership Academy already has made him a better hospitalist. “A better team player, committed and taking ownership of what I do,” he says.
All three courses focus on practical leadership, hands-on collaboration, and networking. In fact, Leadership Academy alumni have begun their own community on SHM’s online collaboration forum, HMX (www.hmxchange.com).
Brendon Shank is SHM’s associate vice president of communications.
In April, The Hospitalist explored the many paths that hospitalists have taken to leadership positions within their hospitals. Among the many skill sets required to grow as a leader in the hospital, hospital CEOs, presidents, and others noted that hospitalists have a unique grasp on the ability to deal with complexity and solve problems within the hospital.
Those very skills—and many others—are the focus of SHM’s Leadership Academy, presented Nov. 3-6 in Honolulu, Hawaii. Leadership Academy comprises three different courses, each of which will be available at the Hilton Hawaiian Village Waikiki Beach Resort:
- Leadership Foundations;
- Advanced Leadership: Influential Management; and
- Advanced Leadership: Mastering Teamwork.
Details and registration are now available at www.hospitalmedicine.org/leadership.
Hospitalist Binu Pappachen, MD, who will be attending one of the Advanced Leadership sessions in Hawaii, plans on completing the program and earning SHM’s Certificate of Leadership in Hospital Medicine.
“I consider this as a great opportunity for my road ahead, get to know more people, and networking,” says Dr. Pappachen, who highly recommends the training to fellow hospitalists. “The first academy course was an eye opener to the different aspects of medicine, team-building, problem solving, and business aspects.”
In fact, Dr. Pappachen feels as though Leadership Academy already has made him a better hospitalist. “A better team player, committed and taking ownership of what I do,” he says.
All three courses focus on practical leadership, hands-on collaboration, and networking. In fact, Leadership Academy alumni have begun their own community on SHM’s online collaboration forum, HMX (www.hmxchange.com).
Brendon Shank is SHM’s associate vice president of communications.