User login
Getting past bad drug outcomes
In my first year of fellowship, I met a delightful old man who had temporal arteritis. We naturally treated him with steroids, but he consequently suffered a vertebral fracture. He passed away soon after that from pneumonia that was probably aggravated by his inability to breathe deeply and cough appropriately.
An elderly patient with rheumatoid arthritis was diagnosed with lymphoma. For want of something to blame, his children blamed it on the methotrexate.
A woman with lupus nephritis got pregnant while on mycophenolate despite being on contraception. Her baby was born with malformed ears and eyes, and by all accounts will probably be deaf and blind.
We have been gifted with this mind-blowing ability to make our patients’ lives much better. That sense of accomplishment can be intoxicating. After all, how many of your polymyalgia rheumatica patients worship you because you made the diagnosis and made them 100% better by putting them on prednisone? Yet we forget that although bad things rarely happen, that does not mean that they won’t happen.
In a beautiful book called "Where’d You Go, Bernadette?" the husband of the title character says that the brain is a discounting mechanism: "Let’s say you get a crack in your windshield and you’re really upset. Oh no, my windshield, it’s ruined, I can hardly see out of it, this is a tragedy! But you don’t have enough money to fix it, so you drive with it. In a month, someone asks you what happened to your windshield, and you say, What do you mean? Because your brain has discounted it. ... It’s for survival. You need to be prepared for novel experiences because often they signal danger."
The book is about an artist who we are led to believe has completed her downward spiral, going from genius to wacko. In the above passage, the artist’s husband is explaining to their daughter why they loved their family home so much, despite its state of extreme disrepair. They loved the house so much that they couldn’t see that it was a safety hazard.
As a fresh graduate I insisted on weaning everyone off prednisone, terrified of the potential side effects. Five years later and with the benefit of the collected wisdom of hundreds of rheumatologists before me, I have accepted that some people need a low dose of steroid to keep their disease quiet. I have used this and other, more toxic drugs to such great effects – taking for granted their ability to make people better – that I forget sometimes that they can cause serious problems.
Bad outcomes can and do happen in spite of our best intentions. In my case, my default is to blame myself. In my more melodramatic moments, I wonder if I deserve to be a doctor. But when I am done feeling angry or sad, or, frankly, feeling sorry for myself, then I need that discounting mechanism to kick in, to remind myself that this is one bad outcome out of many good outcomes. There are things beyond my control, and I cannot let a bad outcome keep me from doing the good work that I am still able to do.
There is a scene from the TV series "The West Wing" where the president asks one of his staffers if he thought the president was being kept from doing a great job because his demons were "shouting down the better angels" in his brain. Thankfully, my brain’s discounting mechanism helps keep the demons at bay.
Dr. Chan practices rheumatology in Pawtucket, R.I.
In my first year of fellowship, I met a delightful old man who had temporal arteritis. We naturally treated him with steroids, but he consequently suffered a vertebral fracture. He passed away soon after that from pneumonia that was probably aggravated by his inability to breathe deeply and cough appropriately.
An elderly patient with rheumatoid arthritis was diagnosed with lymphoma. For want of something to blame, his children blamed it on the methotrexate.
A woman with lupus nephritis got pregnant while on mycophenolate despite being on contraception. Her baby was born with malformed ears and eyes, and by all accounts will probably be deaf and blind.
We have been gifted with this mind-blowing ability to make our patients’ lives much better. That sense of accomplishment can be intoxicating. After all, how many of your polymyalgia rheumatica patients worship you because you made the diagnosis and made them 100% better by putting them on prednisone? Yet we forget that although bad things rarely happen, that does not mean that they won’t happen.
In a beautiful book called "Where’d You Go, Bernadette?" the husband of the title character says that the brain is a discounting mechanism: "Let’s say you get a crack in your windshield and you’re really upset. Oh no, my windshield, it’s ruined, I can hardly see out of it, this is a tragedy! But you don’t have enough money to fix it, so you drive with it. In a month, someone asks you what happened to your windshield, and you say, What do you mean? Because your brain has discounted it. ... It’s for survival. You need to be prepared for novel experiences because often they signal danger."
The book is about an artist who we are led to believe has completed her downward spiral, going from genius to wacko. In the above passage, the artist’s husband is explaining to their daughter why they loved their family home so much, despite its state of extreme disrepair. They loved the house so much that they couldn’t see that it was a safety hazard.
As a fresh graduate I insisted on weaning everyone off prednisone, terrified of the potential side effects. Five years later and with the benefit of the collected wisdom of hundreds of rheumatologists before me, I have accepted that some people need a low dose of steroid to keep their disease quiet. I have used this and other, more toxic drugs to such great effects – taking for granted their ability to make people better – that I forget sometimes that they can cause serious problems.
Bad outcomes can and do happen in spite of our best intentions. In my case, my default is to blame myself. In my more melodramatic moments, I wonder if I deserve to be a doctor. But when I am done feeling angry or sad, or, frankly, feeling sorry for myself, then I need that discounting mechanism to kick in, to remind myself that this is one bad outcome out of many good outcomes. There are things beyond my control, and I cannot let a bad outcome keep me from doing the good work that I am still able to do.
There is a scene from the TV series "The West Wing" where the president asks one of his staffers if he thought the president was being kept from doing a great job because his demons were "shouting down the better angels" in his brain. Thankfully, my brain’s discounting mechanism helps keep the demons at bay.
Dr. Chan practices rheumatology in Pawtucket, R.I.
In my first year of fellowship, I met a delightful old man who had temporal arteritis. We naturally treated him with steroids, but he consequently suffered a vertebral fracture. He passed away soon after that from pneumonia that was probably aggravated by his inability to breathe deeply and cough appropriately.
An elderly patient with rheumatoid arthritis was diagnosed with lymphoma. For want of something to blame, his children blamed it on the methotrexate.
A woman with lupus nephritis got pregnant while on mycophenolate despite being on contraception. Her baby was born with malformed ears and eyes, and by all accounts will probably be deaf and blind.
We have been gifted with this mind-blowing ability to make our patients’ lives much better. That sense of accomplishment can be intoxicating. After all, how many of your polymyalgia rheumatica patients worship you because you made the diagnosis and made them 100% better by putting them on prednisone? Yet we forget that although bad things rarely happen, that does not mean that they won’t happen.
In a beautiful book called "Where’d You Go, Bernadette?" the husband of the title character says that the brain is a discounting mechanism: "Let’s say you get a crack in your windshield and you’re really upset. Oh no, my windshield, it’s ruined, I can hardly see out of it, this is a tragedy! But you don’t have enough money to fix it, so you drive with it. In a month, someone asks you what happened to your windshield, and you say, What do you mean? Because your brain has discounted it. ... It’s for survival. You need to be prepared for novel experiences because often they signal danger."
The book is about an artist who we are led to believe has completed her downward spiral, going from genius to wacko. In the above passage, the artist’s husband is explaining to their daughter why they loved their family home so much, despite its state of extreme disrepair. They loved the house so much that they couldn’t see that it was a safety hazard.
As a fresh graduate I insisted on weaning everyone off prednisone, terrified of the potential side effects. Five years later and with the benefit of the collected wisdom of hundreds of rheumatologists before me, I have accepted that some people need a low dose of steroid to keep their disease quiet. I have used this and other, more toxic drugs to such great effects – taking for granted their ability to make people better – that I forget sometimes that they can cause serious problems.
Bad outcomes can and do happen in spite of our best intentions. In my case, my default is to blame myself. In my more melodramatic moments, I wonder if I deserve to be a doctor. But when I am done feeling angry or sad, or, frankly, feeling sorry for myself, then I need that discounting mechanism to kick in, to remind myself that this is one bad outcome out of many good outcomes. There are things beyond my control, and I cannot let a bad outcome keep me from doing the good work that I am still able to do.
There is a scene from the TV series "The West Wing" where the president asks one of his staffers if he thought the president was being kept from doing a great job because his demons were "shouting down the better angels" in his brain. Thankfully, my brain’s discounting mechanism helps keep the demons at bay.
Dr. Chan practices rheumatology in Pawtucket, R.I.
New Breast Cancer Research Group Aims To Improve Veteran Survival Rates
Over 200,000 new cases of breast cancer are diagnosed in the U.S. annually, according to the National Cancer Institute. Dr. Anita Aggarwal, an oncologist at the Washington, DC VAMC, recently completed an extensive study to compare male and female breast cancer in VA patients. The study found that males with breast cancer had higher stage and grade at presentation and higher mortality compared with females. But, when adjusted for age, stage, and grade, males had better survival rates.
Federal Practitioner talked with Dr. Aggarwal about the new breast cancer research group she is currently developing at the Washington, DC VAMC. Dr. Aggarwal’s hope is to help doctors in the federal health system to identify etiology, biology, and improve treatment of both male and female patients with breast cancer.
Federal Practitioner: What is a breast cancer research group, and why do you think one needs to be created at the VA?
Anita Aggarwal, MD: I would like to build a breast cancer research group with the help of all oncologists and health professionals who take care of patients with breast cancer at all VAMCs nationwide. From my retrospective comparison study, breast cancer in our veterans seems to be different than in the general population. The goal of this research group will be to build a data bank with all the pertinent information as well as tissue gene profiling. This will help us to diagnose them early and treat accordingly in a timely fashion.
FP: As more women join the military, do you think breast cancer treatment at the VA will change?
AA: As the number of female veterans increases, I suspect we will see an increase in the number of female patients with breast cancer. As reported by the 2012 Women’s Task Force, women are now the fastest growing cohort within the veteran community. In 2011, there were about 1.8 million women veterans, which is about 8% of the 22.2 million vets in the VA system. That is expected to increase to 2 million in 2020, at which time women will make up to 10.7% of the total vet population. To accommodate these changing needs, the VHA made women’s health programs a priority in 2007, including a recommendation to improve access to screening, mammograms, and related breast care services. The treatment of breast cancer is becoming more personalized with the advent of new, targeted therapies. The treatment will change if we can identify different biological targets in veterans with breast cancer.
FP: Do you think all veterans, male and female, are more susceptible to breast cancer than is the general population?
AA: In general, incidence of breast cancer is decreasing but, as per the Walter Reed General Hospital and USA Today, breast cancer is one of the most common cancers in our veterans. Not only is the number of women with breast cancer increasing, but so too is the number of male veterans with breast cancer. In general, breast cancer in males is rare, < 1% of all breast cancer cases. Our retrospective data from 1995 to 2012 had more than 6,000 patients with breast cancer; out of that, 1,100 were males with breast cancer.
FP: What do you think needs to be changed about how breast cancer is approached in veterans?
AA: I don’t have an answer to that, but if we can build a breast cancer research group, we may be able to answer some of these questions. Collection of the data prospectively on all of breast cancer at all VA facilities will help us to understand etiology, risk factors, and biology by molecular profiling. In turn, this will help health professionals to give personalized treatment to veterans.
Email: [email protected]
Read more about Dr. Aggarwal’s breast cancer initiative: http://www.research.va.gov/currents/spring2014/spring2014-45.cfm
Over 200,000 new cases of breast cancer are diagnosed in the U.S. annually, according to the National Cancer Institute. Dr. Anita Aggarwal, an oncologist at the Washington, DC VAMC, recently completed an extensive study to compare male and female breast cancer in VA patients. The study found that males with breast cancer had higher stage and grade at presentation and higher mortality compared with females. But, when adjusted for age, stage, and grade, males had better survival rates.
Federal Practitioner talked with Dr. Aggarwal about the new breast cancer research group she is currently developing at the Washington, DC VAMC. Dr. Aggarwal’s hope is to help doctors in the federal health system to identify etiology, biology, and improve treatment of both male and female patients with breast cancer.
Federal Practitioner: What is a breast cancer research group, and why do you think one needs to be created at the VA?
Anita Aggarwal, MD: I would like to build a breast cancer research group with the help of all oncologists and health professionals who take care of patients with breast cancer at all VAMCs nationwide. From my retrospective comparison study, breast cancer in our veterans seems to be different than in the general population. The goal of this research group will be to build a data bank with all the pertinent information as well as tissue gene profiling. This will help us to diagnose them early and treat accordingly in a timely fashion.
FP: As more women join the military, do you think breast cancer treatment at the VA will change?
AA: As the number of female veterans increases, I suspect we will see an increase in the number of female patients with breast cancer. As reported by the 2012 Women’s Task Force, women are now the fastest growing cohort within the veteran community. In 2011, there were about 1.8 million women veterans, which is about 8% of the 22.2 million vets in the VA system. That is expected to increase to 2 million in 2020, at which time women will make up to 10.7% of the total vet population. To accommodate these changing needs, the VHA made women’s health programs a priority in 2007, including a recommendation to improve access to screening, mammograms, and related breast care services. The treatment of breast cancer is becoming more personalized with the advent of new, targeted therapies. The treatment will change if we can identify different biological targets in veterans with breast cancer.
FP: Do you think all veterans, male and female, are more susceptible to breast cancer than is the general population?
AA: In general, incidence of breast cancer is decreasing but, as per the Walter Reed General Hospital and USA Today, breast cancer is one of the most common cancers in our veterans. Not only is the number of women with breast cancer increasing, but so too is the number of male veterans with breast cancer. In general, breast cancer in males is rare, < 1% of all breast cancer cases. Our retrospective data from 1995 to 2012 had more than 6,000 patients with breast cancer; out of that, 1,100 were males with breast cancer.
FP: What do you think needs to be changed about how breast cancer is approached in veterans?
AA: I don’t have an answer to that, but if we can build a breast cancer research group, we may be able to answer some of these questions. Collection of the data prospectively on all of breast cancer at all VA facilities will help us to understand etiology, risk factors, and biology by molecular profiling. In turn, this will help health professionals to give personalized treatment to veterans.
Email: [email protected]
Read more about Dr. Aggarwal’s breast cancer initiative: http://www.research.va.gov/currents/spring2014/spring2014-45.cfm
Over 200,000 new cases of breast cancer are diagnosed in the U.S. annually, according to the National Cancer Institute. Dr. Anita Aggarwal, an oncologist at the Washington, DC VAMC, recently completed an extensive study to compare male and female breast cancer in VA patients. The study found that males with breast cancer had higher stage and grade at presentation and higher mortality compared with females. But, when adjusted for age, stage, and grade, males had better survival rates.
Federal Practitioner talked with Dr. Aggarwal about the new breast cancer research group she is currently developing at the Washington, DC VAMC. Dr. Aggarwal’s hope is to help doctors in the federal health system to identify etiology, biology, and improve treatment of both male and female patients with breast cancer.
Federal Practitioner: What is a breast cancer research group, and why do you think one needs to be created at the VA?
Anita Aggarwal, MD: I would like to build a breast cancer research group with the help of all oncologists and health professionals who take care of patients with breast cancer at all VAMCs nationwide. From my retrospective comparison study, breast cancer in our veterans seems to be different than in the general population. The goal of this research group will be to build a data bank with all the pertinent information as well as tissue gene profiling. This will help us to diagnose them early and treat accordingly in a timely fashion.
FP: As more women join the military, do you think breast cancer treatment at the VA will change?
AA: As the number of female veterans increases, I suspect we will see an increase in the number of female patients with breast cancer. As reported by the 2012 Women’s Task Force, women are now the fastest growing cohort within the veteran community. In 2011, there were about 1.8 million women veterans, which is about 8% of the 22.2 million vets in the VA system. That is expected to increase to 2 million in 2020, at which time women will make up to 10.7% of the total vet population. To accommodate these changing needs, the VHA made women’s health programs a priority in 2007, including a recommendation to improve access to screening, mammograms, and related breast care services. The treatment of breast cancer is becoming more personalized with the advent of new, targeted therapies. The treatment will change if we can identify different biological targets in veterans with breast cancer.
FP: Do you think all veterans, male and female, are more susceptible to breast cancer than is the general population?
AA: In general, incidence of breast cancer is decreasing but, as per the Walter Reed General Hospital and USA Today, breast cancer is one of the most common cancers in our veterans. Not only is the number of women with breast cancer increasing, but so too is the number of male veterans with breast cancer. In general, breast cancer in males is rare, < 1% of all breast cancer cases. Our retrospective data from 1995 to 2012 had more than 6,000 patients with breast cancer; out of that, 1,100 were males with breast cancer.
FP: What do you think needs to be changed about how breast cancer is approached in veterans?
AA: I don’t have an answer to that, but if we can build a breast cancer research group, we may be able to answer some of these questions. Collection of the data prospectively on all of breast cancer at all VA facilities will help us to understand etiology, risk factors, and biology by molecular profiling. In turn, this will help health professionals to give personalized treatment to veterans.
Email: [email protected]
Read more about Dr. Aggarwal’s breast cancer initiative: http://www.research.va.gov/currents/spring2014/spring2014-45.cfm
Circadian disruption negatively affects RBCs

Credit: NHLBI
MANCHESTER—Preclinical research indicates that circadian disruption has severe adverse effects on red blood cells (RBCs), a finding that might possibly explain the high incidence of heart disease observed in shift workers.
The study also showed the negative effects could be reduced under hypoxic conditions. Hypoxia in combination with circadian disruption produced fresh RBCs.
And this, according to researchers, suggests blood donations might help decrease the risk of cardiovascular disease in shift workers.
This research was presented at the 2014 Annual Main Meeting of the Society for Experimental Biology (SEB). It was also published in Chronobiology International.
The researchers, led by Margit Egg, PhD, of the University of Innsbruck in Austria, set out to investigate the impact of circadian disruption on hypoxic signaling and the cardiovascular system.
The team used zebrafish, a model organism that, like humans, is active during the day. To disrupt circadian rhythms, the researchers subjected the fish to alternate short days (7 hours) and long days (21 hours), resembling shift patterns common in industry.
Results showed that circadian disruption increased the number of aged RBCs that accumulated in the blood vessels.
“Normally, there is a balance between newly produced red blood cells and old ones which are removed from the blood,” Dr Egg noted.
Old cells are less flexible and become stuck in the spleen and liver, where they are engulfed by white blood cells. Circadian disruption appears to inhibit this removal process, but the researchers are unsure why this is the case.
They do know that having large aggregates of old RBCs in the vessels increases the chance of a clot that could lead to a heart attack. This may explain why shift workers have a 30% higher risk of cardiovascular disease. In addition, the decreased functionality of the aged cells reduces the oxygen-carrying capacity of the blood.
However, the researchers also found that zebrafish were less affected by circadian disruption if they were simultaneously exposed to hypoxic conditions. This is because hypoxia stimulates the production of fresh RBCs.
The team noted that the cell signaling pathways that regulate circadian rhythms and the hypoxic response are intrinsically linked. This is based on the observation that genes activated by hypoxia, such as erythropoietin, normally show a daily rhythm of activity that becomes disturbed under hypoxic conditions.
“In zebrafish, hypoxia in combination with jetlag led to the production of fresh red blood cells, counteracting the harmful consequences of jetlag and reducing mortality by 10%,” Dr Egg noted.
“Blood donations in humans also stimulate the generation of new fresh erythrocytes. Therefore, blood donations on a regular basis might be a very simple measure to help decrease the cardiovascular risk in human shift workers.”
Dr Egg and her colleagues are currently investigating whether circadian disruption affects any other physiological processes, apart from the cardiovascular system. ![]()

Credit: NHLBI
MANCHESTER—Preclinical research indicates that circadian disruption has severe adverse effects on red blood cells (RBCs), a finding that might possibly explain the high incidence of heart disease observed in shift workers.
The study also showed the negative effects could be reduced under hypoxic conditions. Hypoxia in combination with circadian disruption produced fresh RBCs.
And this, according to researchers, suggests blood donations might help decrease the risk of cardiovascular disease in shift workers.
This research was presented at the 2014 Annual Main Meeting of the Society for Experimental Biology (SEB). It was also published in Chronobiology International.
The researchers, led by Margit Egg, PhD, of the University of Innsbruck in Austria, set out to investigate the impact of circadian disruption on hypoxic signaling and the cardiovascular system.
The team used zebrafish, a model organism that, like humans, is active during the day. To disrupt circadian rhythms, the researchers subjected the fish to alternate short days (7 hours) and long days (21 hours), resembling shift patterns common in industry.
Results showed that circadian disruption increased the number of aged RBCs that accumulated in the blood vessels.
“Normally, there is a balance between newly produced red blood cells and old ones which are removed from the blood,” Dr Egg noted.
Old cells are less flexible and become stuck in the spleen and liver, where they are engulfed by white blood cells. Circadian disruption appears to inhibit this removal process, but the researchers are unsure why this is the case.
They do know that having large aggregates of old RBCs in the vessels increases the chance of a clot that could lead to a heart attack. This may explain why shift workers have a 30% higher risk of cardiovascular disease. In addition, the decreased functionality of the aged cells reduces the oxygen-carrying capacity of the blood.
However, the researchers also found that zebrafish were less affected by circadian disruption if they were simultaneously exposed to hypoxic conditions. This is because hypoxia stimulates the production of fresh RBCs.
The team noted that the cell signaling pathways that regulate circadian rhythms and the hypoxic response are intrinsically linked. This is based on the observation that genes activated by hypoxia, such as erythropoietin, normally show a daily rhythm of activity that becomes disturbed under hypoxic conditions.
“In zebrafish, hypoxia in combination with jetlag led to the production of fresh red blood cells, counteracting the harmful consequences of jetlag and reducing mortality by 10%,” Dr Egg noted.
“Blood donations in humans also stimulate the generation of new fresh erythrocytes. Therefore, blood donations on a regular basis might be a very simple measure to help decrease the cardiovascular risk in human shift workers.”
Dr Egg and her colleagues are currently investigating whether circadian disruption affects any other physiological processes, apart from the cardiovascular system. ![]()

Credit: NHLBI
MANCHESTER—Preclinical research indicates that circadian disruption has severe adverse effects on red blood cells (RBCs), a finding that might possibly explain the high incidence of heart disease observed in shift workers.
The study also showed the negative effects could be reduced under hypoxic conditions. Hypoxia in combination with circadian disruption produced fresh RBCs.
And this, according to researchers, suggests blood donations might help decrease the risk of cardiovascular disease in shift workers.
This research was presented at the 2014 Annual Main Meeting of the Society for Experimental Biology (SEB). It was also published in Chronobiology International.
The researchers, led by Margit Egg, PhD, of the University of Innsbruck in Austria, set out to investigate the impact of circadian disruption on hypoxic signaling and the cardiovascular system.
The team used zebrafish, a model organism that, like humans, is active during the day. To disrupt circadian rhythms, the researchers subjected the fish to alternate short days (7 hours) and long days (21 hours), resembling shift patterns common in industry.
Results showed that circadian disruption increased the number of aged RBCs that accumulated in the blood vessels.
“Normally, there is a balance between newly produced red blood cells and old ones which are removed from the blood,” Dr Egg noted.
Old cells are less flexible and become stuck in the spleen and liver, where they are engulfed by white blood cells. Circadian disruption appears to inhibit this removal process, but the researchers are unsure why this is the case.
They do know that having large aggregates of old RBCs in the vessels increases the chance of a clot that could lead to a heart attack. This may explain why shift workers have a 30% higher risk of cardiovascular disease. In addition, the decreased functionality of the aged cells reduces the oxygen-carrying capacity of the blood.
However, the researchers also found that zebrafish were less affected by circadian disruption if they were simultaneously exposed to hypoxic conditions. This is because hypoxia stimulates the production of fresh RBCs.
The team noted that the cell signaling pathways that regulate circadian rhythms and the hypoxic response are intrinsically linked. This is based on the observation that genes activated by hypoxia, such as erythropoietin, normally show a daily rhythm of activity that becomes disturbed under hypoxic conditions.
“In zebrafish, hypoxia in combination with jetlag led to the production of fresh red blood cells, counteracting the harmful consequences of jetlag and reducing mortality by 10%,” Dr Egg noted.
“Blood donations in humans also stimulate the generation of new fresh erythrocytes. Therefore, blood donations on a regular basis might be a very simple measure to help decrease the cardiovascular risk in human shift workers.”
Dr Egg and her colleagues are currently investigating whether circadian disruption affects any other physiological processes, apart from the cardiovascular system. ![]()
Why Transfusion BPAs Are Overridden
Transfusion of blood products has multiple clinical applications, yet when used outside of recommended guidelines it is associated with increased cost, waste, morbidity, and mortality.[1] Studies reviewing restrictive versus liberal blood transfusion strategies have demonstrated no benefit to liberal strategies over restrictive strategies[2, 3, 4, 5, 6, 7, 8, 9, 10] and possibly even harm.[11] Given the risks and real costs of blood transfusions occurring outside of recommended guidelines, professional societies in hematology, anesthesiology, and hospital medicine each include excessive blood transfusions among their top 5 questionable clinical practices in the American Board of Internal Medicine Foundation's Choosing Wisely initiative.[12] To modify behaviors around blood transfusion practices, hospitals and blood banks may need to provide clinical decision support (CDS) for physicians.
The conventional approach to CDS is direct education and training campaigns, but there is a unique opportunity for intelligent decision support at the point of care through electronic medical record (EMR) systems directly integrated with computerized physician order entry (CPOE).[13, 14, 15] Prior work at Stanford toward reducing unnecessary blood transfusions started with hospital‐wide education campaigns, which brought down the percentage of transfusions ordered for patients with hemoglobin (Hgb) >8 g/dL from 57% to 52%. Further reduction to <30% was achieved after the introduction of an interruptive best practice alert (BPA) integrated into the CPOE transfusion ordering process.[16, 17] Specifically, providers attempting to order a blood transfusion for patients with an EMR‐determined Hgb >7 are presented with a BPA popup reminding them of best practice guidelines and a prompt to either abort the transfusion or provide a reason to override the BPA and proceed.
It remains uncertain why up to 30% of transfusions continue to occur outside of recommended guidelines in spite of interruptive prompts. This study demonstrates a general approach to secondary use of clinical data from the EMR toward understanding provider behavior, specifically by analyzing free‐text comments linked to transfusion override behavior and identifying the type of providers interacting with the BPAs.
MATERIALS AND METHODS
At Stanford University Hospital, a 447‐bed academic tertiary care center servicing adult patients, clinicians order blood transfusions through an EMR+CPOE system. When an order for red blood cell transfusion is attempted, the EMR evaluates the patient chart for specific criteria based on previously published guidelines.[18] Specifically, the BPA will review the last recorded Hgb value and trigger if the Hgb is >8, or if the Hgb is >7 and there is no concurrent EMR problem‐list entry related to acute coronary syndrome or acute hemorrhage. Once the blood transfusion BPA triggers, the ordering provider is presented with an interruptive prompt reminding them of best practice guidelines and the 3 most recent Hgb values for the patient.[16] From there, the provider may either abort the transfusion or override the BPA and proceed. Overrides require the provider to select a reason from a predefined list of institutionally accepted transfusion indications including acute bleeding, acute coronary syndrome and Hgb <8, and postoperative cardiothoracic surgery and Hgb <8. If none of the predefined override reasons are selected, the provider simply selects other, with the option of a free‐text comment to elaborate their rationale.
Data from provider interactions with the BPA were collected from the EMR across all inpatient wards for 8 months after the implementation period. Data collected from each interaction included the patient identifier, alert description, action taken, ordering provider identifier, ordering provider type (job title), optional comments for overriding the BPA, and date and time.
The free‐text override comments were independently reviewed by 2 licensed physicians, tagging them into a set of general categories by iterative inductive analysis of the comment content. Individual comments were allowed multiple possible category tags (eg, coronary artery disease and symptomatic). The initial tagging process was expedited by isolating common keywords in the override comments and assigning likely category tags to each, as in Table 2. The 2 physician reviewers then manually inspected all comments with the option to revise the initial category tagging to ensure validity. Many category tags occurred sporadically and were aggregated into more general categories, such as hematologic disorder (includes myelodysplastic syndrome, myelofibrosis, multiple myeloma, sickle cell, thalassemia, Waldenstrom's), symptomatic (includes fatigue, lightheaded, short of breath), and per other medical doctor [MD] (includes any other physician direction, primarily specialty consultation services).
To assess the inter‐rater agreement of this category tagging between the 2 reviewers, a confusion matrix similar to the example in Table 1 was setup for each tag used. Several agreement statistics are calculated based on the confusion matrix, including the positive agreement rate (Pa+) and Cohen's kappa statistic (). Kappa statistic values range from 1 to +1, with values <0 indicating no agreement and values >0.8 indicating near perfect agreement.[19] To reject the null hypothesis that the 2 reviewers could have independently arrived at their similar tagging assignments by chance, a 2 test was applied for each confusion matrix, with Yates' correction to avoid overestimating statistical significance given the low rates of inter‐rater disagreement.[20]
| Reviewer 1 Tags "Surgery" | ||||
|---|---|---|---|---|
| Yes | No | Total | ||
| ||||
| Reviewer 2 Tags "Surgery" | Yes | 143 | 11 | 154 |
| No | 5 | 820 | 825 | |
| Total | 148 | 831 | 979 | |
| Category Tag | Keyword | Keyword Count |
|---|---|---|
| ||
| Hgb 78 | 7.1, 7.2, 7.8, 7.9, 8, <8 | 360 |
| BMT | BMT | 359 |
| Symptomatic | Symptomatic | 187 |
| Surgery | Postop, post‐op, surgery, surgical | 176 |
| Dropping Hgb | Down, drop, dropping | 117 |
| Chemotherapy | Chemo, chemotherapy | 88 |
| Per other MD | Per | 87 |
| Transplant | Transplant | 70 |
| Cardiac | Cardiac | 66 |
| Bleeding | Bleeding | 65 |
| Procedure | Procedure | 65 |
| Hgb <7 | 7 | 58 |
| Hypotension | Hypotension | 51 |
| Protocol | Protocol | 51 |
| Cirrhosis | Liver | 50 |
| Imminent discharge | Discharge | 49 |
| Leukemia, acute | AML | 44 |
| Cancer | Cancer | 37 |
| Sepsis | Sepsis | 32 |
| Tachycardia | Tachycardia | 28 |
RESULTS
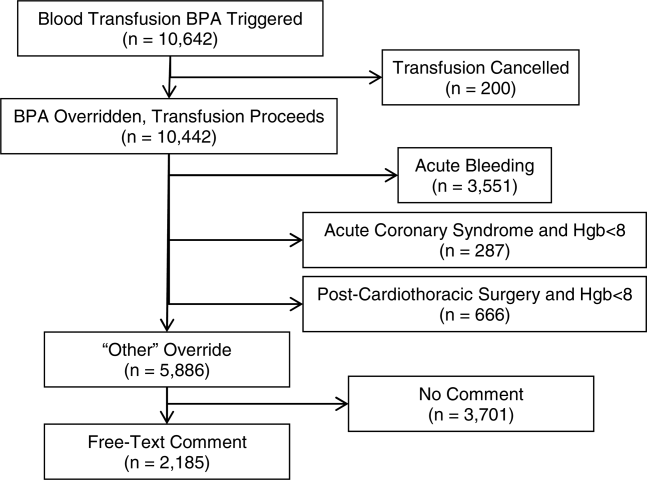
During the data collection period from January 1, 2011 to August 31, 2012, the blood transfusion BPA fired in 11,791 instances, of which 10,642 recorded sufficient data for analysis (Figure 1). The ordering provider proceeded to override the BPA and continued with transfusion in 98% of cases (10,442/10,642). Acute bleeding" was the most common structured response (34%). The majority of BPA overrides used the general purpose other structured response option accounting for 56% (5886/10,442) of override responses, of which 37% (2185/5886) entered a free‐text comment elaborating the override reason. With 3701 nonresponders (other overrides with no free‐text comment), the overall response rate was 65% (6941/10,642).
With a handful of free‐text comments included with structured override responses (eg, 28 acute bleeding overrides included additional comment from the provider), a total of 2216 override comments were available for analysis. Using an initial selection of keyword‐tag associations, as in Table 2, 95% (2104/2216) of the override comments had a preliminary category tagging assigned. After manual review and revision by the first physician reviewer, 74% (1633/2216) of the comments retained their automated tags, whereas 26% (583/2216) were updated based on the reviewer's assessment of validity. This included 112 comments lacking automated tags the reviewer manually added, as well as 471 comments with automated tags revised by the reviewer.
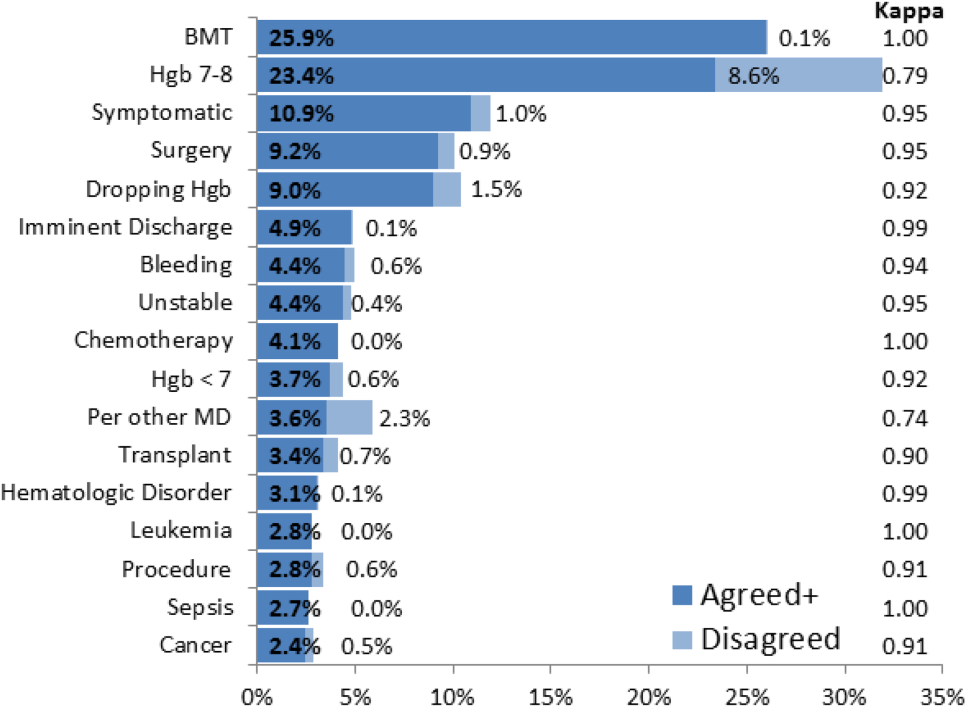
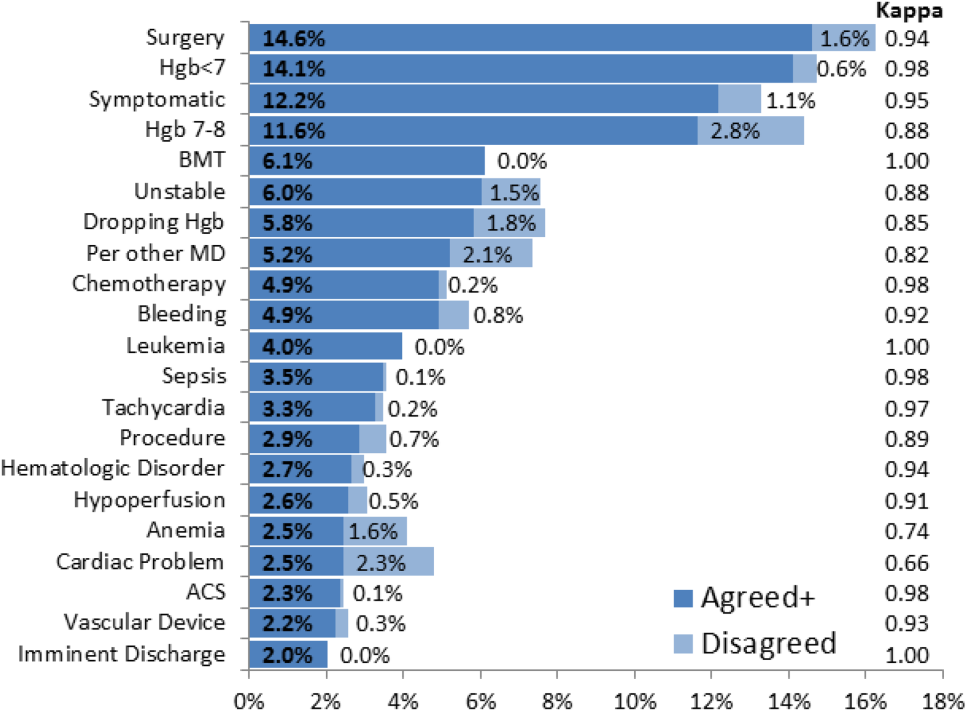
The most common override category tags are presented in Figure 2 and Figure 3 for BPAs triggered in response to blood transfusions ordered for patients with Hgb 78 and Hgb >8, respectively. The agreed+ percentages correspond to the number of comments where the 2 physician reviewers both assigned the respective category tag (Pa+), whereas the disagreed percentages correspond to comments where the reviewers differed (Pd=1‐Pa). By Yates' corrected 2 analysis, P was <1010 for independence between reviewer tag assignments for all tags assessed. Kappa statistics are reported in the figures to describe inter‐rater agreement.
Resident physicians were the primary ordering provider group, accounting for approximately 55% (5863/10,642) of BPA interactions, followed by registered nurses, fellows, and attending physicians.
DISCUSSION
Prior work has established that an interruptive BPA to provide decision support against inappropriate blood transfusions can drive a significant and sustained reduction in unnecessary transfusions,[16] especially when satisfying the primary features of effective decision support.[14] The quantity of transfusions directly aborted by the BPA (only 2% in this case) does not nearly account for the total observed reductions in transfusions, suggesting that the BPA yields an indirect teaching effect over time beyond direct interruption. In other words, once a user has been interrupted by the BPA several times, they will be discouraged from even attempting to order inappropriate blood transfusions in the future.
Despite the improvements above, there remains a substantial fraction (up to 30%) of blood transfusions occurring outside of recommended guidelines where providers specifically override the BPA prompt.[21] This work is the first structured analysis of such BPA override behavior by generalizable methods of EMR data analysis to guide further improvements.
Analysis of the override behavior focused primarily on the free‐text comments explaining provider rationales for overriding the BPA. These comments were categorized by 2 physician reviewers, with P<1010 for all category tags, rejecting the null hypothesis that the 2 reviewers could have independently arrived at their similar category tags by chance. Further assessment of inter‐rater agreement was generally excellent, with >0.8 in the majority of cases. The remaining cases (ie, Hgb 78, per other MD, anemia, cardiac) still had substantial agreement, with >0.6. These disagreements almost universally occurred when a reviewer assigned a subset of the category tags assigned by the other reviewer. For example, 1 reviewer may tag a comment bone marrow transplant (BMT) patient with Hgb 7.2 with BMT and Hgb 78, whereas the other would only cite BMT.
Figure 2 and Figure 3 highlight the varied categories of responses, with most categories comprising <4% of the available responses. Among the most common override reasons are structured protocols for specialty services, as in the stem cell transplant service, whose protocol recommends transfusing blood when Hgb <8 rather than Hgb <7. In these cases, the BPA is unlikely to change protocolized behavior, thus overall workflow would likely be improved by adding a structured BPA override option for these protocols.
Analysis of the override comments did expose some relatively common and questionable transfusion practices, including perioperative and periprocedural transfusions, as well as anticipation of imminent discharge. Prior studies indicate that transfusions in anticipation of surgical or procedural intervention provide no benefit compared to responding to blood requirements intraoperatively as needed,[3, 22, 23] and imminent discharge of a patient is not a well‐recognized reason to transfuse outside of guidelines. The identification of these questionable and relatively common practices identifies opportunities for targeted education and training campaigns.
Symptomatic anemia was 1 of the more commonly cited BPA override reasons with a fraction providing a specific symptom such as fatigue, lightheadedness, or shortness of breath. Although the pervasiveness of this rationale may speak to adding it as a structured BPA override option, the nonspecific, subjective, and nonevidence‐based nature of symptom‐driven blood transfusions suggests that ongoing interruptive BPA prompts can still be useful to remind providers of the risks and guideline‐based approaches to such cases.
Limitations of the analysis are revealed as a fraction of BPA events did not fully record all relevant data, preventing proper analysis. Override comments suggest the BPA was triggering inappropriately for patients appropriately below the recommended transfusion threshold of Hgb <7, assuming provider free‐textentered values were accurate. Review of these cases showed some variability, such as when providers based their transfusion decision on a hematocrit of 20 rather than a Hgb of 7. Many comments also stated nonthreshold Hgb values, such as Hgb 7.2, seeming to imply that the value was close enough to the recommended threshold to justify overriding the transfusion prompt.
The most significant limitation of this study is the substantial fraction of nonresponder BPA transfusion overrides with a nonspecific other reason and no text commentary, comprising 34% (3670/10,642) of all BPA interactions and 62% (3670/5886) of all other overrides. Although the BPA is easily overridden by design to avoid workflow disruption that could compromise the priority of patient care, the nonresponses raise concern for skewed interpretation of the override data. General studies in survey responses provide reassurance that lower response rates do not necessarily indicate response bias,[24] with response rates as low as 25% yielding results statistically indistinguishable from more rigorous methods achieving >50% response rates.[25] In this specific case, response bias is better characterized by comparing ordering provider characteristics for the other overrides with and without free‐text comments. Specifically, Figure 4 shows the distribution of other overrides by provider type (job title) and provider home department (where available from physician department registries), separated by whether a free‐text comment was left. For each sub‐group, a 2 analysis compared the observed versus expected proportion of providers leaving comments based on the null hypothesis that leaving a comment was independent of membership in the subgroup. Similar proportions with nonsignificant P values suggest against significant response bias for most subgroups, but the data do indicate that this work likely under‐represents the opinion of fellows, physician assistants, and neurosurgeons, while slightly over‐representing the opinion of medicine, general surgery, and obstetrics/gynecology practitioners. Given that the under‐represented groups overall constitute a small minority of the total BPA interactions, this work should still be generalizable to the majority of transfusion behavior.
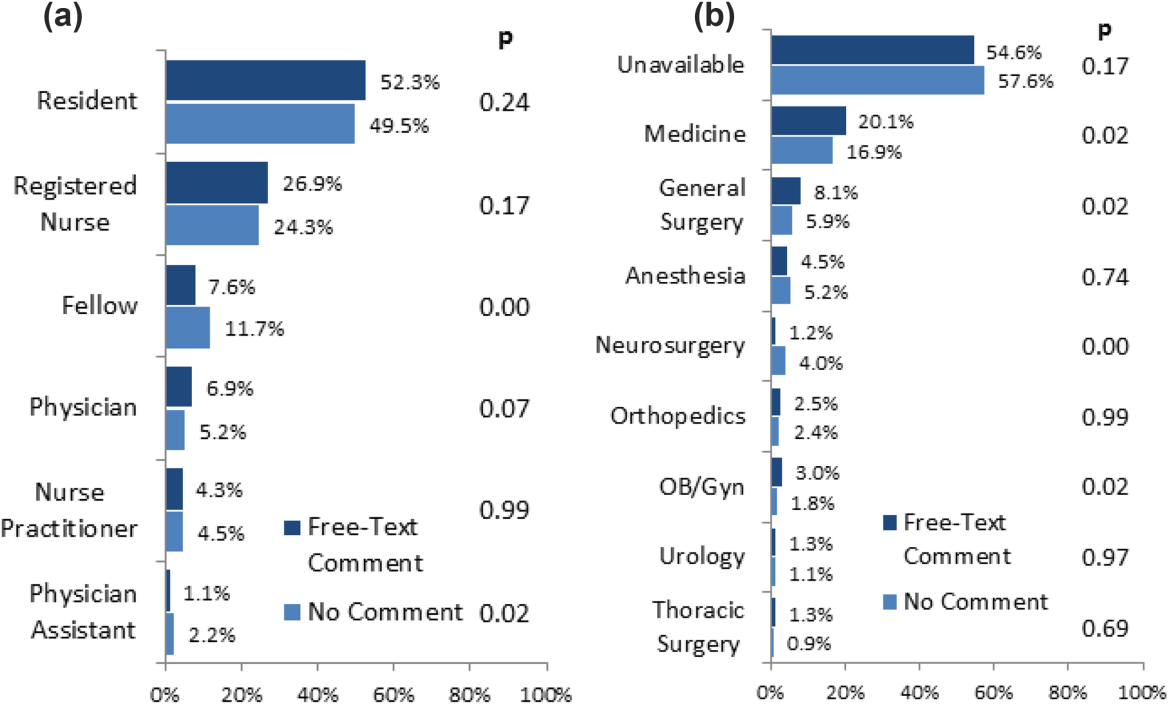
Further review of the ordering provider type (job title) distribution indicates that the vast majority of providers who actually interact with BPAs in this academic hospital are resident physicians. Similarly notable is that 25% of BPAs trigger for registered nurses. Upon review of hospital practices, we confirmed that nurses can enter transfusion orders on behalf of MD cosigners, generally in the context of a verbal order or routine treatment protocol, most notably on the stem cell transplant service. The prevalence of resident, nurse, nurse practitioner, and physician assistant order entry overall indicates that the providers encountering the BPA often do not have the negotiating power to accept or override the prompts, specifically defying the meaningful use goal of decision‐making providers directly interacting with CPOE and decision support prompts.[26]
The theoretical intelligence of the BPA not to trigger in well‐accepted cases of transfusion, where acute bleeding is occurring based on the presence of bleeding related items in the patient's problem list, is demonstrated to be limited. Acute bleeding represented the most common structured override response. This is likely multifactorial, but is largely the result of inconsistent usage of the patient problem lists that the BPA would depend upon. Another commonly cited override explanation was for a dropping Hgb without a specific bleeding source. This could point toward the BPA triggering an algorithm incorporating the last several Hgb values to assess the trajectory. Even then, however, guidelines would advocate holding transfusion and proceeding with serial monitoring of Hgb levels until an accepted transfusion threshold was actually breached.
This analysis demonstrates a general approach to secondary analysis of EMR data, in this case to provide insight into the specific question of why providers continue to order costly, unnecessary, and potentially harmful blood transfusions in spite of an interruptive BPA CDS tool. Limitations of the BPA are now better understood, including technical aspects of accounting for all decision‐making data, the frequency of nonresponse for override rationale, and that the providers interacting with the BPAs often do not have the negotiating power to change ordering behavior. The analysis recognizes protocolized behaviors that should be integrated into the CDS algorithms, and specification of symptomatic anemia as a common albeit subjective indication for blood transfusion. A wide swath of individually uncommon rationales drive transfusion override behavior, motivating further EMR‐based analysis to more efficiently recognize common scenarios (eg, perioperative/procedural, imminent discharge) for directed education and training interventions than can be achieved by conventional chart review.
Acknowledgements
The authors thank Sylvia Bereknyei for consultation on qualitative analysis methods, C.J. Garst for providing the dataset, David Iberri for contributing to early manuscript drafts, Paul Maggio for designing the original BPA, and Debra Green and Brett Toensing for providing staff department registries.
Disclosure: Nothing to report.
Transfusion of blood products has multiple clinical applications, yet when used outside of recommended guidelines it is associated with increased cost, waste, morbidity, and mortality.[1] Studies reviewing restrictive versus liberal blood transfusion strategies have demonstrated no benefit to liberal strategies over restrictive strategies[2, 3, 4, 5, 6, 7, 8, 9, 10] and possibly even harm.[11] Given the risks and real costs of blood transfusions occurring outside of recommended guidelines, professional societies in hematology, anesthesiology, and hospital medicine each include excessive blood transfusions among their top 5 questionable clinical practices in the American Board of Internal Medicine Foundation's Choosing Wisely initiative.[12] To modify behaviors around blood transfusion practices, hospitals and blood banks may need to provide clinical decision support (CDS) for physicians.
The conventional approach to CDS is direct education and training campaigns, but there is a unique opportunity for intelligent decision support at the point of care through electronic medical record (EMR) systems directly integrated with computerized physician order entry (CPOE).[13, 14, 15] Prior work at Stanford toward reducing unnecessary blood transfusions started with hospital‐wide education campaigns, which brought down the percentage of transfusions ordered for patients with hemoglobin (Hgb) >8 g/dL from 57% to 52%. Further reduction to <30% was achieved after the introduction of an interruptive best practice alert (BPA) integrated into the CPOE transfusion ordering process.[16, 17] Specifically, providers attempting to order a blood transfusion for patients with an EMR‐determined Hgb >7 are presented with a BPA popup reminding them of best practice guidelines and a prompt to either abort the transfusion or provide a reason to override the BPA and proceed.
It remains uncertain why up to 30% of transfusions continue to occur outside of recommended guidelines in spite of interruptive prompts. This study demonstrates a general approach to secondary use of clinical data from the EMR toward understanding provider behavior, specifically by analyzing free‐text comments linked to transfusion override behavior and identifying the type of providers interacting with the BPAs.
MATERIALS AND METHODS
At Stanford University Hospital, a 447‐bed academic tertiary care center servicing adult patients, clinicians order blood transfusions through an EMR+CPOE system. When an order for red blood cell transfusion is attempted, the EMR evaluates the patient chart for specific criteria based on previously published guidelines.[18] Specifically, the BPA will review the last recorded Hgb value and trigger if the Hgb is >8, or if the Hgb is >7 and there is no concurrent EMR problem‐list entry related to acute coronary syndrome or acute hemorrhage. Once the blood transfusion BPA triggers, the ordering provider is presented with an interruptive prompt reminding them of best practice guidelines and the 3 most recent Hgb values for the patient.[16] From there, the provider may either abort the transfusion or override the BPA and proceed. Overrides require the provider to select a reason from a predefined list of institutionally accepted transfusion indications including acute bleeding, acute coronary syndrome and Hgb <8, and postoperative cardiothoracic surgery and Hgb <8. If none of the predefined override reasons are selected, the provider simply selects other, with the option of a free‐text comment to elaborate their rationale.
Data from provider interactions with the BPA were collected from the EMR across all inpatient wards for 8 months after the implementation period. Data collected from each interaction included the patient identifier, alert description, action taken, ordering provider identifier, ordering provider type (job title), optional comments for overriding the BPA, and date and time.
The free‐text override comments were independently reviewed by 2 licensed physicians, tagging them into a set of general categories by iterative inductive analysis of the comment content. Individual comments were allowed multiple possible category tags (eg, coronary artery disease and symptomatic). The initial tagging process was expedited by isolating common keywords in the override comments and assigning likely category tags to each, as in Table 2. The 2 physician reviewers then manually inspected all comments with the option to revise the initial category tagging to ensure validity. Many category tags occurred sporadically and were aggregated into more general categories, such as hematologic disorder (includes myelodysplastic syndrome, myelofibrosis, multiple myeloma, sickle cell, thalassemia, Waldenstrom's), symptomatic (includes fatigue, lightheaded, short of breath), and per other medical doctor [MD] (includes any other physician direction, primarily specialty consultation services).
To assess the inter‐rater agreement of this category tagging between the 2 reviewers, a confusion matrix similar to the example in Table 1 was setup for each tag used. Several agreement statistics are calculated based on the confusion matrix, including the positive agreement rate (Pa+) and Cohen's kappa statistic (). Kappa statistic values range from 1 to +1, with values <0 indicating no agreement and values >0.8 indicating near perfect agreement.[19] To reject the null hypothesis that the 2 reviewers could have independently arrived at their similar tagging assignments by chance, a 2 test was applied for each confusion matrix, with Yates' correction to avoid overestimating statistical significance given the low rates of inter‐rater disagreement.[20]
| Reviewer 1 Tags "Surgery" | ||||
|---|---|---|---|---|
| Yes | No | Total | ||
| ||||
| Reviewer 2 Tags "Surgery" | Yes | 143 | 11 | 154 |
| No | 5 | 820 | 825 | |
| Total | 148 | 831 | 979 | |
| Category Tag | Keyword | Keyword Count |
|---|---|---|
| ||
| Hgb 78 | 7.1, 7.2, 7.8, 7.9, 8, <8 | 360 |
| BMT | BMT | 359 |
| Symptomatic | Symptomatic | 187 |
| Surgery | Postop, post‐op, surgery, surgical | 176 |
| Dropping Hgb | Down, drop, dropping | 117 |
| Chemotherapy | Chemo, chemotherapy | 88 |
| Per other MD | Per | 87 |
| Transplant | Transplant | 70 |
| Cardiac | Cardiac | 66 |
| Bleeding | Bleeding | 65 |
| Procedure | Procedure | 65 |
| Hgb <7 | 7 | 58 |
| Hypotension | Hypotension | 51 |
| Protocol | Protocol | 51 |
| Cirrhosis | Liver | 50 |
| Imminent discharge | Discharge | 49 |
| Leukemia, acute | AML | 44 |
| Cancer | Cancer | 37 |
| Sepsis | Sepsis | 32 |
| Tachycardia | Tachycardia | 28 |
RESULTS
During the data collection period from January 1, 2011 to August 31, 2012, the blood transfusion BPA fired in 11,791 instances, of which 10,642 recorded sufficient data for analysis (Figure 1). The ordering provider proceeded to override the BPA and continued with transfusion in 98% of cases (10,442/10,642). Acute bleeding" was the most common structured response (34%). The majority of BPA overrides used the general purpose other structured response option accounting for 56% (5886/10,442) of override responses, of which 37% (2185/5886) entered a free‐text comment elaborating the override reason. With 3701 nonresponders (other overrides with no free‐text comment), the overall response rate was 65% (6941/10,642).

With a handful of free‐text comments included with structured override responses (eg, 28 acute bleeding overrides included additional comment from the provider), a total of 2216 override comments were available for analysis. Using an initial selection of keyword‐tag associations, as in Table 2, 95% (2104/2216) of the override comments had a preliminary category tagging assigned. After manual review and revision by the first physician reviewer, 74% (1633/2216) of the comments retained their automated tags, whereas 26% (583/2216) were updated based on the reviewer's assessment of validity. This included 112 comments lacking automated tags the reviewer manually added, as well as 471 comments with automated tags revised by the reviewer.
The most common override category tags are presented in Figure 2 and Figure 3 for BPAs triggered in response to blood transfusions ordered for patients with Hgb 78 and Hgb >8, respectively. The agreed+ percentages correspond to the number of comments where the 2 physician reviewers both assigned the respective category tag (Pa+), whereas the disagreed percentages correspond to comments where the reviewers differed (Pd=1‐Pa). By Yates' corrected 2 analysis, P was <1010 for independence between reviewer tag assignments for all tags assessed. Kappa statistics are reported in the figures to describe inter‐rater agreement.


Resident physicians were the primary ordering provider group, accounting for approximately 55% (5863/10,642) of BPA interactions, followed by registered nurses, fellows, and attending physicians.
DISCUSSION
Prior work has established that an interruptive BPA to provide decision support against inappropriate blood transfusions can drive a significant and sustained reduction in unnecessary transfusions,[16] especially when satisfying the primary features of effective decision support.[14] The quantity of transfusions directly aborted by the BPA (only 2% in this case) does not nearly account for the total observed reductions in transfusions, suggesting that the BPA yields an indirect teaching effect over time beyond direct interruption. In other words, once a user has been interrupted by the BPA several times, they will be discouraged from even attempting to order inappropriate blood transfusions in the future.
Despite the improvements above, there remains a substantial fraction (up to 30%) of blood transfusions occurring outside of recommended guidelines where providers specifically override the BPA prompt.[21] This work is the first structured analysis of such BPA override behavior by generalizable methods of EMR data analysis to guide further improvements.
Analysis of the override behavior focused primarily on the free‐text comments explaining provider rationales for overriding the BPA. These comments were categorized by 2 physician reviewers, with P<1010 for all category tags, rejecting the null hypothesis that the 2 reviewers could have independently arrived at their similar category tags by chance. Further assessment of inter‐rater agreement was generally excellent, with >0.8 in the majority of cases. The remaining cases (ie, Hgb 78, per other MD, anemia, cardiac) still had substantial agreement, with >0.6. These disagreements almost universally occurred when a reviewer assigned a subset of the category tags assigned by the other reviewer. For example, 1 reviewer may tag a comment bone marrow transplant (BMT) patient with Hgb 7.2 with BMT and Hgb 78, whereas the other would only cite BMT.
Figure 2 and Figure 3 highlight the varied categories of responses, with most categories comprising <4% of the available responses. Among the most common override reasons are structured protocols for specialty services, as in the stem cell transplant service, whose protocol recommends transfusing blood when Hgb <8 rather than Hgb <7. In these cases, the BPA is unlikely to change protocolized behavior, thus overall workflow would likely be improved by adding a structured BPA override option for these protocols.
Analysis of the override comments did expose some relatively common and questionable transfusion practices, including perioperative and periprocedural transfusions, as well as anticipation of imminent discharge. Prior studies indicate that transfusions in anticipation of surgical or procedural intervention provide no benefit compared to responding to blood requirements intraoperatively as needed,[3, 22, 23] and imminent discharge of a patient is not a well‐recognized reason to transfuse outside of guidelines. The identification of these questionable and relatively common practices identifies opportunities for targeted education and training campaigns.
Symptomatic anemia was 1 of the more commonly cited BPA override reasons with a fraction providing a specific symptom such as fatigue, lightheadedness, or shortness of breath. Although the pervasiveness of this rationale may speak to adding it as a structured BPA override option, the nonspecific, subjective, and nonevidence‐based nature of symptom‐driven blood transfusions suggests that ongoing interruptive BPA prompts can still be useful to remind providers of the risks and guideline‐based approaches to such cases.
Limitations of the analysis are revealed as a fraction of BPA events did not fully record all relevant data, preventing proper analysis. Override comments suggest the BPA was triggering inappropriately for patients appropriately below the recommended transfusion threshold of Hgb <7, assuming provider free‐textentered values were accurate. Review of these cases showed some variability, such as when providers based their transfusion decision on a hematocrit of 20 rather than a Hgb of 7. Many comments also stated nonthreshold Hgb values, such as Hgb 7.2, seeming to imply that the value was close enough to the recommended threshold to justify overriding the transfusion prompt.
The most significant limitation of this study is the substantial fraction of nonresponder BPA transfusion overrides with a nonspecific other reason and no text commentary, comprising 34% (3670/10,642) of all BPA interactions and 62% (3670/5886) of all other overrides. Although the BPA is easily overridden by design to avoid workflow disruption that could compromise the priority of patient care, the nonresponses raise concern for skewed interpretation of the override data. General studies in survey responses provide reassurance that lower response rates do not necessarily indicate response bias,[24] with response rates as low as 25% yielding results statistically indistinguishable from more rigorous methods achieving >50% response rates.[25] In this specific case, response bias is better characterized by comparing ordering provider characteristics for the other overrides with and without free‐text comments. Specifically, Figure 4 shows the distribution of other overrides by provider type (job title) and provider home department (where available from physician department registries), separated by whether a free‐text comment was left. For each sub‐group, a 2 analysis compared the observed versus expected proportion of providers leaving comments based on the null hypothesis that leaving a comment was independent of membership in the subgroup. Similar proportions with nonsignificant P values suggest against significant response bias for most subgroups, but the data do indicate that this work likely under‐represents the opinion of fellows, physician assistants, and neurosurgeons, while slightly over‐representing the opinion of medicine, general surgery, and obstetrics/gynecology practitioners. Given that the under‐represented groups overall constitute a small minority of the total BPA interactions, this work should still be generalizable to the majority of transfusion behavior.

Further review of the ordering provider type (job title) distribution indicates that the vast majority of providers who actually interact with BPAs in this academic hospital are resident physicians. Similarly notable is that 25% of BPAs trigger for registered nurses. Upon review of hospital practices, we confirmed that nurses can enter transfusion orders on behalf of MD cosigners, generally in the context of a verbal order or routine treatment protocol, most notably on the stem cell transplant service. The prevalence of resident, nurse, nurse practitioner, and physician assistant order entry overall indicates that the providers encountering the BPA often do not have the negotiating power to accept or override the prompts, specifically defying the meaningful use goal of decision‐making providers directly interacting with CPOE and decision support prompts.[26]
The theoretical intelligence of the BPA not to trigger in well‐accepted cases of transfusion, where acute bleeding is occurring based on the presence of bleeding related items in the patient's problem list, is demonstrated to be limited. Acute bleeding represented the most common structured override response. This is likely multifactorial, but is largely the result of inconsistent usage of the patient problem lists that the BPA would depend upon. Another commonly cited override explanation was for a dropping Hgb without a specific bleeding source. This could point toward the BPA triggering an algorithm incorporating the last several Hgb values to assess the trajectory. Even then, however, guidelines would advocate holding transfusion and proceeding with serial monitoring of Hgb levels until an accepted transfusion threshold was actually breached.
This analysis demonstrates a general approach to secondary analysis of EMR data, in this case to provide insight into the specific question of why providers continue to order costly, unnecessary, and potentially harmful blood transfusions in spite of an interruptive BPA CDS tool. Limitations of the BPA are now better understood, including technical aspects of accounting for all decision‐making data, the frequency of nonresponse for override rationale, and that the providers interacting with the BPAs often do not have the negotiating power to change ordering behavior. The analysis recognizes protocolized behaviors that should be integrated into the CDS algorithms, and specification of symptomatic anemia as a common albeit subjective indication for blood transfusion. A wide swath of individually uncommon rationales drive transfusion override behavior, motivating further EMR‐based analysis to more efficiently recognize common scenarios (eg, perioperative/procedural, imminent discharge) for directed education and training interventions than can be achieved by conventional chart review.
Acknowledgements
The authors thank Sylvia Bereknyei for consultation on qualitative analysis methods, C.J. Garst for providing the dataset, David Iberri for contributing to early manuscript drafts, Paul Maggio for designing the original BPA, and Debra Green and Brett Toensing for providing staff department registries.
Disclosure: Nothing to report.
Transfusion of blood products has multiple clinical applications, yet when used outside of recommended guidelines it is associated with increased cost, waste, morbidity, and mortality.[1] Studies reviewing restrictive versus liberal blood transfusion strategies have demonstrated no benefit to liberal strategies over restrictive strategies[2, 3, 4, 5, 6, 7, 8, 9, 10] and possibly even harm.[11] Given the risks and real costs of blood transfusions occurring outside of recommended guidelines, professional societies in hematology, anesthesiology, and hospital medicine each include excessive blood transfusions among their top 5 questionable clinical practices in the American Board of Internal Medicine Foundation's Choosing Wisely initiative.[12] To modify behaviors around blood transfusion practices, hospitals and blood banks may need to provide clinical decision support (CDS) for physicians.
The conventional approach to CDS is direct education and training campaigns, but there is a unique opportunity for intelligent decision support at the point of care through electronic medical record (EMR) systems directly integrated with computerized physician order entry (CPOE).[13, 14, 15] Prior work at Stanford toward reducing unnecessary blood transfusions started with hospital‐wide education campaigns, which brought down the percentage of transfusions ordered for patients with hemoglobin (Hgb) >8 g/dL from 57% to 52%. Further reduction to <30% was achieved after the introduction of an interruptive best practice alert (BPA) integrated into the CPOE transfusion ordering process.[16, 17] Specifically, providers attempting to order a blood transfusion for patients with an EMR‐determined Hgb >7 are presented with a BPA popup reminding them of best practice guidelines and a prompt to either abort the transfusion or provide a reason to override the BPA and proceed.
It remains uncertain why up to 30% of transfusions continue to occur outside of recommended guidelines in spite of interruptive prompts. This study demonstrates a general approach to secondary use of clinical data from the EMR toward understanding provider behavior, specifically by analyzing free‐text comments linked to transfusion override behavior and identifying the type of providers interacting with the BPAs.
MATERIALS AND METHODS
At Stanford University Hospital, a 447‐bed academic tertiary care center servicing adult patients, clinicians order blood transfusions through an EMR+CPOE system. When an order for red blood cell transfusion is attempted, the EMR evaluates the patient chart for specific criteria based on previously published guidelines.[18] Specifically, the BPA will review the last recorded Hgb value and trigger if the Hgb is >8, or if the Hgb is >7 and there is no concurrent EMR problem‐list entry related to acute coronary syndrome or acute hemorrhage. Once the blood transfusion BPA triggers, the ordering provider is presented with an interruptive prompt reminding them of best practice guidelines and the 3 most recent Hgb values for the patient.[16] From there, the provider may either abort the transfusion or override the BPA and proceed. Overrides require the provider to select a reason from a predefined list of institutionally accepted transfusion indications including acute bleeding, acute coronary syndrome and Hgb <8, and postoperative cardiothoracic surgery and Hgb <8. If none of the predefined override reasons are selected, the provider simply selects other, with the option of a free‐text comment to elaborate their rationale.
Data from provider interactions with the BPA were collected from the EMR across all inpatient wards for 8 months after the implementation period. Data collected from each interaction included the patient identifier, alert description, action taken, ordering provider identifier, ordering provider type (job title), optional comments for overriding the BPA, and date and time.
The free‐text override comments were independently reviewed by 2 licensed physicians, tagging them into a set of general categories by iterative inductive analysis of the comment content. Individual comments were allowed multiple possible category tags (eg, coronary artery disease and symptomatic). The initial tagging process was expedited by isolating common keywords in the override comments and assigning likely category tags to each, as in Table 2. The 2 physician reviewers then manually inspected all comments with the option to revise the initial category tagging to ensure validity. Many category tags occurred sporadically and were aggregated into more general categories, such as hematologic disorder (includes myelodysplastic syndrome, myelofibrosis, multiple myeloma, sickle cell, thalassemia, Waldenstrom's), symptomatic (includes fatigue, lightheaded, short of breath), and per other medical doctor [MD] (includes any other physician direction, primarily specialty consultation services).
To assess the inter‐rater agreement of this category tagging between the 2 reviewers, a confusion matrix similar to the example in Table 1 was setup for each tag used. Several agreement statistics are calculated based on the confusion matrix, including the positive agreement rate (Pa+) and Cohen's kappa statistic (). Kappa statistic values range from 1 to +1, with values <0 indicating no agreement and values >0.8 indicating near perfect agreement.[19] To reject the null hypothesis that the 2 reviewers could have independently arrived at their similar tagging assignments by chance, a 2 test was applied for each confusion matrix, with Yates' correction to avoid overestimating statistical significance given the low rates of inter‐rater disagreement.[20]
| Reviewer 1 Tags "Surgery" | ||||
|---|---|---|---|---|
| Yes | No | Total | ||
| ||||
| Reviewer 2 Tags "Surgery" | Yes | 143 | 11 | 154 |
| No | 5 | 820 | 825 | |
| Total | 148 | 831 | 979 | |
| Category Tag | Keyword | Keyword Count |
|---|---|---|
| ||
| Hgb 78 | 7.1, 7.2, 7.8, 7.9, 8, <8 | 360 |
| BMT | BMT | 359 |
| Symptomatic | Symptomatic | 187 |
| Surgery | Postop, post‐op, surgery, surgical | 176 |
| Dropping Hgb | Down, drop, dropping | 117 |
| Chemotherapy | Chemo, chemotherapy | 88 |
| Per other MD | Per | 87 |
| Transplant | Transplant | 70 |
| Cardiac | Cardiac | 66 |
| Bleeding | Bleeding | 65 |
| Procedure | Procedure | 65 |
| Hgb <7 | 7 | 58 |
| Hypotension | Hypotension | 51 |
| Protocol | Protocol | 51 |
| Cirrhosis | Liver | 50 |
| Imminent discharge | Discharge | 49 |
| Leukemia, acute | AML | 44 |
| Cancer | Cancer | 37 |
| Sepsis | Sepsis | 32 |
| Tachycardia | Tachycardia | 28 |
RESULTS
During the data collection period from January 1, 2011 to August 31, 2012, the blood transfusion BPA fired in 11,791 instances, of which 10,642 recorded sufficient data for analysis (Figure 1). The ordering provider proceeded to override the BPA and continued with transfusion in 98% of cases (10,442/10,642). Acute bleeding" was the most common structured response (34%). The majority of BPA overrides used the general purpose other structured response option accounting for 56% (5886/10,442) of override responses, of which 37% (2185/5886) entered a free‐text comment elaborating the override reason. With 3701 nonresponders (other overrides with no free‐text comment), the overall response rate was 65% (6941/10,642).

With a handful of free‐text comments included with structured override responses (eg, 28 acute bleeding overrides included additional comment from the provider), a total of 2216 override comments were available for analysis. Using an initial selection of keyword‐tag associations, as in Table 2, 95% (2104/2216) of the override comments had a preliminary category tagging assigned. After manual review and revision by the first physician reviewer, 74% (1633/2216) of the comments retained their automated tags, whereas 26% (583/2216) were updated based on the reviewer's assessment of validity. This included 112 comments lacking automated tags the reviewer manually added, as well as 471 comments with automated tags revised by the reviewer.
The most common override category tags are presented in Figure 2 and Figure 3 for BPAs triggered in response to blood transfusions ordered for patients with Hgb 78 and Hgb >8, respectively. The agreed+ percentages correspond to the number of comments where the 2 physician reviewers both assigned the respective category tag (Pa+), whereas the disagreed percentages correspond to comments where the reviewers differed (Pd=1‐Pa). By Yates' corrected 2 analysis, P was <1010 for independence between reviewer tag assignments for all tags assessed. Kappa statistics are reported in the figures to describe inter‐rater agreement.


Resident physicians were the primary ordering provider group, accounting for approximately 55% (5863/10,642) of BPA interactions, followed by registered nurses, fellows, and attending physicians.
DISCUSSION
Prior work has established that an interruptive BPA to provide decision support against inappropriate blood transfusions can drive a significant and sustained reduction in unnecessary transfusions,[16] especially when satisfying the primary features of effective decision support.[14] The quantity of transfusions directly aborted by the BPA (only 2% in this case) does not nearly account for the total observed reductions in transfusions, suggesting that the BPA yields an indirect teaching effect over time beyond direct interruption. In other words, once a user has been interrupted by the BPA several times, they will be discouraged from even attempting to order inappropriate blood transfusions in the future.
Despite the improvements above, there remains a substantial fraction (up to 30%) of blood transfusions occurring outside of recommended guidelines where providers specifically override the BPA prompt.[21] This work is the first structured analysis of such BPA override behavior by generalizable methods of EMR data analysis to guide further improvements.
Analysis of the override behavior focused primarily on the free‐text comments explaining provider rationales for overriding the BPA. These comments were categorized by 2 physician reviewers, with P<1010 for all category tags, rejecting the null hypothesis that the 2 reviewers could have independently arrived at their similar category tags by chance. Further assessment of inter‐rater agreement was generally excellent, with >0.8 in the majority of cases. The remaining cases (ie, Hgb 78, per other MD, anemia, cardiac) still had substantial agreement, with >0.6. These disagreements almost universally occurred when a reviewer assigned a subset of the category tags assigned by the other reviewer. For example, 1 reviewer may tag a comment bone marrow transplant (BMT) patient with Hgb 7.2 with BMT and Hgb 78, whereas the other would only cite BMT.
Figure 2 and Figure 3 highlight the varied categories of responses, with most categories comprising <4% of the available responses. Among the most common override reasons are structured protocols for specialty services, as in the stem cell transplant service, whose protocol recommends transfusing blood when Hgb <8 rather than Hgb <7. In these cases, the BPA is unlikely to change protocolized behavior, thus overall workflow would likely be improved by adding a structured BPA override option for these protocols.
Analysis of the override comments did expose some relatively common and questionable transfusion practices, including perioperative and periprocedural transfusions, as well as anticipation of imminent discharge. Prior studies indicate that transfusions in anticipation of surgical or procedural intervention provide no benefit compared to responding to blood requirements intraoperatively as needed,[3, 22, 23] and imminent discharge of a patient is not a well‐recognized reason to transfuse outside of guidelines. The identification of these questionable and relatively common practices identifies opportunities for targeted education and training campaigns.
Symptomatic anemia was 1 of the more commonly cited BPA override reasons with a fraction providing a specific symptom such as fatigue, lightheadedness, or shortness of breath. Although the pervasiveness of this rationale may speak to adding it as a structured BPA override option, the nonspecific, subjective, and nonevidence‐based nature of symptom‐driven blood transfusions suggests that ongoing interruptive BPA prompts can still be useful to remind providers of the risks and guideline‐based approaches to such cases.
Limitations of the analysis are revealed as a fraction of BPA events did not fully record all relevant data, preventing proper analysis. Override comments suggest the BPA was triggering inappropriately for patients appropriately below the recommended transfusion threshold of Hgb <7, assuming provider free‐textentered values were accurate. Review of these cases showed some variability, such as when providers based their transfusion decision on a hematocrit of 20 rather than a Hgb of 7. Many comments also stated nonthreshold Hgb values, such as Hgb 7.2, seeming to imply that the value was close enough to the recommended threshold to justify overriding the transfusion prompt.
The most significant limitation of this study is the substantial fraction of nonresponder BPA transfusion overrides with a nonspecific other reason and no text commentary, comprising 34% (3670/10,642) of all BPA interactions and 62% (3670/5886) of all other overrides. Although the BPA is easily overridden by design to avoid workflow disruption that could compromise the priority of patient care, the nonresponses raise concern for skewed interpretation of the override data. General studies in survey responses provide reassurance that lower response rates do not necessarily indicate response bias,[24] with response rates as low as 25% yielding results statistically indistinguishable from more rigorous methods achieving >50% response rates.[25] In this specific case, response bias is better characterized by comparing ordering provider characteristics for the other overrides with and without free‐text comments. Specifically, Figure 4 shows the distribution of other overrides by provider type (job title) and provider home department (where available from physician department registries), separated by whether a free‐text comment was left. For each sub‐group, a 2 analysis compared the observed versus expected proportion of providers leaving comments based on the null hypothesis that leaving a comment was independent of membership in the subgroup. Similar proportions with nonsignificant P values suggest against significant response bias for most subgroups, but the data do indicate that this work likely under‐represents the opinion of fellows, physician assistants, and neurosurgeons, while slightly over‐representing the opinion of medicine, general surgery, and obstetrics/gynecology practitioners. Given that the under‐represented groups overall constitute a small minority of the total BPA interactions, this work should still be generalizable to the majority of transfusion behavior.

Further review of the ordering provider type (job title) distribution indicates that the vast majority of providers who actually interact with BPAs in this academic hospital are resident physicians. Similarly notable is that 25% of BPAs trigger for registered nurses. Upon review of hospital practices, we confirmed that nurses can enter transfusion orders on behalf of MD cosigners, generally in the context of a verbal order or routine treatment protocol, most notably on the stem cell transplant service. The prevalence of resident, nurse, nurse practitioner, and physician assistant order entry overall indicates that the providers encountering the BPA often do not have the negotiating power to accept or override the prompts, specifically defying the meaningful use goal of decision‐making providers directly interacting with CPOE and decision support prompts.[26]
The theoretical intelligence of the BPA not to trigger in well‐accepted cases of transfusion, where acute bleeding is occurring based on the presence of bleeding related items in the patient's problem list, is demonstrated to be limited. Acute bleeding represented the most common structured override response. This is likely multifactorial, but is largely the result of inconsistent usage of the patient problem lists that the BPA would depend upon. Another commonly cited override explanation was for a dropping Hgb without a specific bleeding source. This could point toward the BPA triggering an algorithm incorporating the last several Hgb values to assess the trajectory. Even then, however, guidelines would advocate holding transfusion and proceeding with serial monitoring of Hgb levels until an accepted transfusion threshold was actually breached.
This analysis demonstrates a general approach to secondary analysis of EMR data, in this case to provide insight into the specific question of why providers continue to order costly, unnecessary, and potentially harmful blood transfusions in spite of an interruptive BPA CDS tool. Limitations of the BPA are now better understood, including technical aspects of accounting for all decision‐making data, the frequency of nonresponse for override rationale, and that the providers interacting with the BPAs often do not have the negotiating power to change ordering behavior. The analysis recognizes protocolized behaviors that should be integrated into the CDS algorithms, and specification of symptomatic anemia as a common albeit subjective indication for blood transfusion. A wide swath of individually uncommon rationales drive transfusion override behavior, motivating further EMR‐based analysis to more efficiently recognize common scenarios (eg, perioperative/procedural, imminent discharge) for directed education and training interventions than can be achieved by conventional chart review.
Acknowledgements
The authors thank Sylvia Bereknyei for consultation on qualitative analysis methods, C.J. Garst for providing the dataset, David Iberri for contributing to early manuscript drafts, Paul Maggio for designing the original BPA, and Debra Green and Brett Toensing for providing staff department registries.
Disclosure: Nothing to report.
© 2014 Society of Hospital Medicine
Inhibitor gets accelerated approval for PTCL
The US Food and Drug Administration (FDA) has granted accelerated approval for belinostat (Beleodaq) to treat relapsed or refractory peripheral T-cell lymphoma (PTCL).
Belinostat is a histone deacetylase inhibitor with antineoplastic activity. The drug works by inhibiting tumor cell proliferation, inducing apoptosis, promoting cellular differentiation, and inhibiting angiogenesis.
The FDA’s accelerated approval program allows for approval of a drug based on surrogate or intermediate endpoints reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs.
Drugs receiving accelerated approval are subject to confirmatory trials verifying clinical benefit.
The FDA granted belinostat accelerated approval based on results of a phase 2 trial, which included 129 patients with relapsed or refractory PTCL. All patients received belinostat until disease progression or unacceptable toxicity.
About 26% of patients achieved a complete or partial response. The most common side effects were nausea, fatigue, pyrexia, anemia, and vomiting.
“[Belinostat] is the third drug that has been approved since 2009 for the treatment of peripheral T-cell lymphoma,” said Richard Pazdur, MD, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA granted accelerated approval to pralatrexate (Folotyn) in 2009 for use in patients with relapsed or refractory PTCL and romidepsin (Istodax) in 2011 for PTCL patients who had received at least 1 prior therapy.
Beleodaq and Folotyn are marketed by Spectrum Pharmaceuticals, Inc., based in Henderson, Nevada. Istodax is marketed by Celgene Corporation based in Summit, New Jersey. ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval for belinostat (Beleodaq) to treat relapsed or refractory peripheral T-cell lymphoma (PTCL).
Belinostat is a histone deacetylase inhibitor with antineoplastic activity. The drug works by inhibiting tumor cell proliferation, inducing apoptosis, promoting cellular differentiation, and inhibiting angiogenesis.
The FDA’s accelerated approval program allows for approval of a drug based on surrogate or intermediate endpoints reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs.
Drugs receiving accelerated approval are subject to confirmatory trials verifying clinical benefit.
The FDA granted belinostat accelerated approval based on results of a phase 2 trial, which included 129 patients with relapsed or refractory PTCL. All patients received belinostat until disease progression or unacceptable toxicity.
About 26% of patients achieved a complete or partial response. The most common side effects were nausea, fatigue, pyrexia, anemia, and vomiting.
“[Belinostat] is the third drug that has been approved since 2009 for the treatment of peripheral T-cell lymphoma,” said Richard Pazdur, MD, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA granted accelerated approval to pralatrexate (Folotyn) in 2009 for use in patients with relapsed or refractory PTCL and romidepsin (Istodax) in 2011 for PTCL patients who had received at least 1 prior therapy.
Beleodaq and Folotyn are marketed by Spectrum Pharmaceuticals, Inc., based in Henderson, Nevada. Istodax is marketed by Celgene Corporation based in Summit, New Jersey. ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval for belinostat (Beleodaq) to treat relapsed or refractory peripheral T-cell lymphoma (PTCL).
Belinostat is a histone deacetylase inhibitor with antineoplastic activity. The drug works by inhibiting tumor cell proliferation, inducing apoptosis, promoting cellular differentiation, and inhibiting angiogenesis.
The FDA’s accelerated approval program allows for approval of a drug based on surrogate or intermediate endpoints reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs.
Drugs receiving accelerated approval are subject to confirmatory trials verifying clinical benefit.
The FDA granted belinostat accelerated approval based on results of a phase 2 trial, which included 129 patients with relapsed or refractory PTCL. All patients received belinostat until disease progression or unacceptable toxicity.
About 26% of patients achieved a complete or partial response. The most common side effects were nausea, fatigue, pyrexia, anemia, and vomiting.
“[Belinostat] is the third drug that has been approved since 2009 for the treatment of peripheral T-cell lymphoma,” said Richard Pazdur, MD, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
The FDA granted accelerated approval to pralatrexate (Folotyn) in 2009 for use in patients with relapsed or refractory PTCL and romidepsin (Istodax) in 2011 for PTCL patients who had received at least 1 prior therapy.
Beleodaq and Folotyn are marketed by Spectrum Pharmaceuticals, Inc., based in Henderson, Nevada. Istodax is marketed by Celgene Corporation based in Summit, New Jersey. ![]()
New compound blocks essential enzyme

Credit: Peter H. Seeberger
A novel compound can inhibit an enzyme that is essential for malaria parasite survival, according to research published in PLOS Biology.
Researchers believe that creating this compound, WEHI-916, is the first step toward developing a new class of antimalarial drugs that could cure and prevent malaria infections caused by all species of the parasite, including those resistant to existing drugs.
The group developed WEHI-916 to block the enzyme Plasmepsin V. They previously showed Plasmepsin V is responsible for controlling the transport of proteins in and out of the malaria parasite.
Now, they’ve used WEHI-916 to prove the importance of Plasmepsin V to the survival of both Plasmodium vivax and Plasmodium falciparum.
“Researchers, including us, had been trying, without success, to learn more about Plasmepsin V using standard genetic techniques,” said study author Just Boddey, PhD, of The Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“Our idea was to create a drug-like compound that would block Plasmepsin V so we could investigate its importance. We found that blocking Plasmepsin V kills malaria parasites and delivered a new and effective potential drug at the same time.”
Plasmepsin V was an ideal drug target because its inhibition effectively halted the transport of hundreds of malaria proteins, Dr Boddey noted.
“The Plasmodium parasite needs to produce and deliver over 300 different proteins to the red blood cell to survive in the body and hide from the host’s immune system,” he said. “Instead of targeting individual proteins, we can block Plasmepsin V and prevent all of those proteins from leaving the parasite.”
The researchers believe these findings could aid the development of drugs that are effective in curing malaria caused by all 5 species of Plasmodium parasite.
“Our study has shown that Plasmepsin V is a key enzyme in [P vivax and P falciparum], and WEHI-916 can inhibit Plasmepsin V isolated from both of them,” said study author Brad Sleebs, PhD, also of The Walter and Eliza Hall Institute.
“Not only does this compound enable us to prove Plasmepsin V is an excellent drug target, it is a starting point for a research program that could lead to a new class of antimalarial drugs.”
Now, the researchers have turned their attention to developing WEHI-916 and related compounds for human use.
“We are now examining in our insectary whether Plasmepsin V could be a target during other stages of the malaria lifecycle,” Dr Boddey said. “The enzyme is present in the parasites that first infect humans in the liver, as well as in parasite forms that exit humans and infect mosquitoes.”
“If WEHI-916 kills the parasite during these stages as well, it will mean any drugs that target Plasmepsin V can be used as a preventative as well as a cure.” ![]()

Credit: Peter H. Seeberger
A novel compound can inhibit an enzyme that is essential for malaria parasite survival, according to research published in PLOS Biology.
Researchers believe that creating this compound, WEHI-916, is the first step toward developing a new class of antimalarial drugs that could cure and prevent malaria infections caused by all species of the parasite, including those resistant to existing drugs.
The group developed WEHI-916 to block the enzyme Plasmepsin V. They previously showed Plasmepsin V is responsible for controlling the transport of proteins in and out of the malaria parasite.
Now, they’ve used WEHI-916 to prove the importance of Plasmepsin V to the survival of both Plasmodium vivax and Plasmodium falciparum.
“Researchers, including us, had been trying, without success, to learn more about Plasmepsin V using standard genetic techniques,” said study author Just Boddey, PhD, of The Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“Our idea was to create a drug-like compound that would block Plasmepsin V so we could investigate its importance. We found that blocking Plasmepsin V kills malaria parasites and delivered a new and effective potential drug at the same time.”
Plasmepsin V was an ideal drug target because its inhibition effectively halted the transport of hundreds of malaria proteins, Dr Boddey noted.
“The Plasmodium parasite needs to produce and deliver over 300 different proteins to the red blood cell to survive in the body and hide from the host’s immune system,” he said. “Instead of targeting individual proteins, we can block Plasmepsin V and prevent all of those proteins from leaving the parasite.”
The researchers believe these findings could aid the development of drugs that are effective in curing malaria caused by all 5 species of Plasmodium parasite.
“Our study has shown that Plasmepsin V is a key enzyme in [P vivax and P falciparum], and WEHI-916 can inhibit Plasmepsin V isolated from both of them,” said study author Brad Sleebs, PhD, also of The Walter and Eliza Hall Institute.
“Not only does this compound enable us to prove Plasmepsin V is an excellent drug target, it is a starting point for a research program that could lead to a new class of antimalarial drugs.”
Now, the researchers have turned their attention to developing WEHI-916 and related compounds for human use.
“We are now examining in our insectary whether Plasmepsin V could be a target during other stages of the malaria lifecycle,” Dr Boddey said. “The enzyme is present in the parasites that first infect humans in the liver, as well as in parasite forms that exit humans and infect mosquitoes.”
“If WEHI-916 kills the parasite during these stages as well, it will mean any drugs that target Plasmepsin V can be used as a preventative as well as a cure.” ![]()

Credit: Peter H. Seeberger
A novel compound can inhibit an enzyme that is essential for malaria parasite survival, according to research published in PLOS Biology.
Researchers believe that creating this compound, WEHI-916, is the first step toward developing a new class of antimalarial drugs that could cure and prevent malaria infections caused by all species of the parasite, including those resistant to existing drugs.
The group developed WEHI-916 to block the enzyme Plasmepsin V. They previously showed Plasmepsin V is responsible for controlling the transport of proteins in and out of the malaria parasite.
Now, they’ve used WEHI-916 to prove the importance of Plasmepsin V to the survival of both Plasmodium vivax and Plasmodium falciparum.
“Researchers, including us, had been trying, without success, to learn more about Plasmepsin V using standard genetic techniques,” said study author Just Boddey, PhD, of The Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“Our idea was to create a drug-like compound that would block Plasmepsin V so we could investigate its importance. We found that blocking Plasmepsin V kills malaria parasites and delivered a new and effective potential drug at the same time.”
Plasmepsin V was an ideal drug target because its inhibition effectively halted the transport of hundreds of malaria proteins, Dr Boddey noted.
“The Plasmodium parasite needs to produce and deliver over 300 different proteins to the red blood cell to survive in the body and hide from the host’s immune system,” he said. “Instead of targeting individual proteins, we can block Plasmepsin V and prevent all of those proteins from leaving the parasite.”
The researchers believe these findings could aid the development of drugs that are effective in curing malaria caused by all 5 species of Plasmodium parasite.
“Our study has shown that Plasmepsin V is a key enzyme in [P vivax and P falciparum], and WEHI-916 can inhibit Plasmepsin V isolated from both of them,” said study author Brad Sleebs, PhD, also of The Walter and Eliza Hall Institute.
“Not only does this compound enable us to prove Plasmepsin V is an excellent drug target, it is a starting point for a research program that could lead to a new class of antimalarial drugs.”
Now, the researchers have turned their attention to developing WEHI-916 and related compounds for human use.
“We are now examining in our insectary whether Plasmepsin V could be a target during other stages of the malaria lifecycle,” Dr Boddey said. “The enzyme is present in the parasites that first infect humans in the liver, as well as in parasite forms that exit humans and infect mosquitoes.”
“If WEHI-916 kills the parasite during these stages as well, it will mean any drugs that target Plasmepsin V can be used as a preventative as well as a cure.” ![]()
Unsuspected aspect of immune regulation revealed

Immunologists may have discovered an additional role for B cells. Their research suggests the cells participate in the development of regulatory T cells (Tregs).
Until now, the only non-thymic cells known to aid Treg production were dendritic cells, which travel to the thymus to deliver antigens.
The new research, published in the Journal of Immunology, suggests B cells can do the same thing.
B cells were previously thought to specialize only in antibody production. With their newly discovered role, the cells become much more interesting and complex characters, according to the researchers.
The findings mean B cells could have useful applications for treating transplant patients and those with autoimmune disorders.
“Regulatory T cells are critical in the outcome of an immune response, so anything that regulates them becomes very interesting to immunologists,” said study author Shane Grey, PhD, of the Garvan Institute of Medical Research in Darlinghurst, New South Wales, Australia.
“Right now, there are clinical trials around the world looking to expand populations of these cells in patients. Researchers are also working on ways to grow regulatory cells in the laboratory—to infuse into patients as therapy. Our finding suggests it should be possible to set up systems that harness B cells to expand regulatory cells.”
Dr Grey and his colleagues worked with mice genetically modified to express high levels of BAFF, which increases B-cell survival. The higher number of B cells overall allowed researchers to track the activity of B cells in the thymus.
“It has been known for years that some B cells travel to the thymus, but no one has understood why,” said study author Stacey Walters, also of the Garvan Institute of Medical Research.
“Our experiments showed clearly that B cells participated in the creation of regulatory T cells. The more B cells that were in the thymus, the higher the number of regulatory cells generated. That direct correlation raises interesting possibilities. One possibility is using BAFF, a non-toxic substance, to ramp up the B-cell count of patients before transplant procedures.”
Research has suggested that Tregs can reduce the risk of graft-vs-host disease, promote enhanced immune reconstitution, and decrease the incidence of infectious complications in stem cell transplant recipients. And several studies have shown that high levels of Tregs can prevent graft rejection after solid organ transplant. ![]()

Immunologists may have discovered an additional role for B cells. Their research suggests the cells participate in the development of regulatory T cells (Tregs).
Until now, the only non-thymic cells known to aid Treg production were dendritic cells, which travel to the thymus to deliver antigens.
The new research, published in the Journal of Immunology, suggests B cells can do the same thing.
B cells were previously thought to specialize only in antibody production. With their newly discovered role, the cells become much more interesting and complex characters, according to the researchers.
The findings mean B cells could have useful applications for treating transplant patients and those with autoimmune disorders.
“Regulatory T cells are critical in the outcome of an immune response, so anything that regulates them becomes very interesting to immunologists,” said study author Shane Grey, PhD, of the Garvan Institute of Medical Research in Darlinghurst, New South Wales, Australia.
“Right now, there are clinical trials around the world looking to expand populations of these cells in patients. Researchers are also working on ways to grow regulatory cells in the laboratory—to infuse into patients as therapy. Our finding suggests it should be possible to set up systems that harness B cells to expand regulatory cells.”
Dr Grey and his colleagues worked with mice genetically modified to express high levels of BAFF, which increases B-cell survival. The higher number of B cells overall allowed researchers to track the activity of B cells in the thymus.
“It has been known for years that some B cells travel to the thymus, but no one has understood why,” said study author Stacey Walters, also of the Garvan Institute of Medical Research.
“Our experiments showed clearly that B cells participated in the creation of regulatory T cells. The more B cells that were in the thymus, the higher the number of regulatory cells generated. That direct correlation raises interesting possibilities. One possibility is using BAFF, a non-toxic substance, to ramp up the B-cell count of patients before transplant procedures.”
Research has suggested that Tregs can reduce the risk of graft-vs-host disease, promote enhanced immune reconstitution, and decrease the incidence of infectious complications in stem cell transplant recipients. And several studies have shown that high levels of Tregs can prevent graft rejection after solid organ transplant. ![]()

Immunologists may have discovered an additional role for B cells. Their research suggests the cells participate in the development of regulatory T cells (Tregs).
Until now, the only non-thymic cells known to aid Treg production were dendritic cells, which travel to the thymus to deliver antigens.
The new research, published in the Journal of Immunology, suggests B cells can do the same thing.
B cells were previously thought to specialize only in antibody production. With their newly discovered role, the cells become much more interesting and complex characters, according to the researchers.
The findings mean B cells could have useful applications for treating transplant patients and those with autoimmune disorders.
“Regulatory T cells are critical in the outcome of an immune response, so anything that regulates them becomes very interesting to immunologists,” said study author Shane Grey, PhD, of the Garvan Institute of Medical Research in Darlinghurst, New South Wales, Australia.
“Right now, there are clinical trials around the world looking to expand populations of these cells in patients. Researchers are also working on ways to grow regulatory cells in the laboratory—to infuse into patients as therapy. Our finding suggests it should be possible to set up systems that harness B cells to expand regulatory cells.”
Dr Grey and his colleagues worked with mice genetically modified to express high levels of BAFF, which increases B-cell survival. The higher number of B cells overall allowed researchers to track the activity of B cells in the thymus.
“It has been known for years that some B cells travel to the thymus, but no one has understood why,” said study author Stacey Walters, also of the Garvan Institute of Medical Research.
“Our experiments showed clearly that B cells participated in the creation of regulatory T cells. The more B cells that were in the thymus, the higher the number of regulatory cells generated. That direct correlation raises interesting possibilities. One possibility is using BAFF, a non-toxic substance, to ramp up the B-cell count of patients before transplant procedures.”
Research has suggested that Tregs can reduce the risk of graft-vs-host disease, promote enhanced immune reconstitution, and decrease the incidence of infectious complications in stem cell transplant recipients. And several studies have shown that high levels of Tregs can prevent graft rejection after solid organ transplant. ![]()
Adolescent Obesity and Its Risks: How to Screen and When to Refer
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
From the Department of Pediatrics, University of Wisconsin, Madison, WI.
Abstract
- Objective: To provide information that will assist clinicians in assessing and addressing risk for obesity-related comorbidities in adolescents.
- Methods: Review of the literature.
- Results: Childhood obesity is a major public health concern. Prevention of obesity or early detection of its health consequences are important responsibilities or opportunities for primary care clinicians. While body mass index (BMI) screening is valuable, insulin resistance and other obesity-related comorbidities can develop even when BMI falls below the 95th percentile threshold for obesity. Detailed history and physical examination can help identify comorbidities and guide diagnostic evaluation. Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention.
- Conclusion: For optimal health outcomes, management of adolescent obesity and associated comorbidities is should be adapted based on an individual’s overall risk rather than BMI alone.
Case Study
Initial Presentation
A 14-year-old Hispanic male presents for a well child check.
History and Physical Examination
The patient and his mother have no complaints or concerns. A comprehensive review of systems is positive for fatigue and snoring but is otherwise unremarkable. Past medical history is unremarkable except for mild intermittent asthma. Family history is positive for type 2 diabetes in paternal grandmother and a maternal uncle and cardiovascular disease and hypertension in multiple extended family members. Both maternal and paternal grandparents are from Mexico.
Vital signs are within normal limits. Height is 160 cm (30th percentile for age), weight is 58.4 kg (75th percentile for age), and body mass index (BMI) is 22.8 kg/m2 (85th percentile for age). Blood pressure is 127/81 mm Hg (95th percentile for age and gender). Physical exam is pertinent for acanthosis nigricans on neck and axilla and nonviolaceous striae on abdomen. Waist circumference is 88 cm (90th percentile for age and ethnicity). Otherwise, physical exam is within normal limits.
• Does this child’s physical examination findings pose a cause for concern?
Yes. A key concept is that while obesity is widespread, the adverse health complications of adiposity and overnutrition affect some children much earlier and more profoundly than others. Some children exhibit adiposity-associated comorbidities even prior to meeting obesity criteria defined by BMI. Careful history and examination can help identify those most at risk for developing adiposity-associated comorbidities, prompting earlier intervention and, when appropriate, subspecialty referral.
Obesity is caused by a complex interplay of genetic, environmental, and metabolic programming, especially early in life, and lifestyle habits [1,2]. The vast majority of obesity is due to excess nutrition leading to energy imbalance, while less than 1% is due to endocrine or syndromic causes [3]. Obesity is defined as excessive body fat and is often estimated indirectly by using a surrogate marker, BMI. Diagnostically, a BMI > 95th percentile for age on sex-specific CDC growth charts is defined as obese, while a BMI from the 85th to 94th percentile is defined as overweight [4]. Using these criteria, the prevalence of childhood obesity more than tripled in the past 3 decades [5], leading to its classification as an epidemic and public health crisis [2]. Today, an estimated 12.5 million American children are obese [5]. For adolescents specifically, the prevalence of obesity is 18.4%, with more than one-third overweight [6].
Childhood obesity is associated with both short- and long-term morbidities including insulin resistance and type 2 diabetes, hypertension, dyslipidemia, asthma, obstructive sleep apnea, psychosocial problems, and decreased quality of life [7,8]. Obese children, particularly older children and adolescents, are more likely become obese adults [2,7]. Obesity in adulthood is associated with both significant morbidity and premature death [9]. Individual characteristics such as lifestyle habits, fitness level, and genetic predisposition influence the likelihood of development of both obesity and associated comorbidities [10].
The burden of obesity and its associated comorbidities are not equally distributed among racial/ethnic and socioeconomic groups. Hispanic and non-Hispanic black children are much more likely to be obese and overweight than non-Hispanic white children [6]. Low socioeconomic status is associated with increased rates of obesity in certain subgroups, including adolescents [2]. In addition, certain ethnic/racial minorities are more likely to develop obesity-associated comorbidities, such as insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). With regard to insulin resistance and development of type 2 diabetes, the risk is greatest in Native Americans, but there is also increased risk in Hispanic/Latinos, non-Hispanic blacks, and Asian Americans as compared with non-Hispanic whites [11–13]. Collectively, these findings highlight the need for individualized assessment and the importance of obesity prevention and early intervention to improve long-term health outcomes. Primary care providers play a pivotal role in this process of preventing, identifying and treating childhood obesity and associated comorbidities [14]. In the case history, the child’s ethnicity, family history, and borderline overweight BMI indicate a high risk for future obesity-related morbidity and a critical opportunity for prevention intervention.
• What are the initial steps a practitioner can take to address overweight and obesity?
To encourage the development of healthy lifestyles and prevention of obesity, dietary and exercise counseling should be routinely provided as part of anticipatory guidance to all children and families regardless of weight status. It is critical to recognize individuals at high risk for becoming obese starting early in life. Risk factors for obesity in healthy weight children include rapid crossing of BMI percentiles, obese parent(s), maternal history of gestational diabetes during pregnancy, ethnicity, sedentary lifestyle, and excessive caloric intake [2]. Identification of these high-risk individuals can prompt more intensive counseling and early intervention with the goal of preventing the development of obesity and its complications. The use of automated BMI calculation and electronic medical records can facilitate identification of overweight and obesity status when already present and improve counseling rates [15].
Obesity due to excess nutrition is typically associated with linear growth acceleration that occurs subsequent to and to a lesser degree than the percentile shift in weight gain. A declining height velocity associated with obesity, therefore, is concerning and should prompt investigation for endocrine disease such as hypothyroidism, glucocorticoid excess, and growth hormone deficiency. Additional factors that warrant further investigation and/or referral include growth trajectory significantly below genetic potential, developmental delay, and dysmorphic features. A complete physical examination should be performed to evaluate for signs consistent with these conditions (eg, violaceous striae in glucocorticoid excess, microcephaly, and small hands/feet in Prader-Willi syndrome), and signs of obesity-associated comorbidities (eg, acanthosis nigricans). Accurate height, weight, BMI calculation, and blood pressure assessment using an appropriately sized cuff are essential.
While BMI screening is valuable, as noted above it is important to appreciate that insulin resistance (and other obesity-related comorbidities) can develop even when BMI is below the 95th percentile. Detailed history and physical examination can help identify these comorbidities of excess adiposity and guide diagnostic evaluation. Independent risk factors for insulin resistance and the development of type 2 diabetes include family history of diabetes, minority race/ethnicity, elevated waist circumference, and poor fitness level [18–20].
Further History
The patient reports skipping breakfast on most days, eats lunch at school, and snacks on chips and soda after school. Dinner is variable but usually contains carbohydrates and a protein and rarely includes vegetables. Family eats “take-out” about 3 times per week. Patient reports spending 3 hours a day watching television and playing on computer. He had gym last semester but currently reports very limited to no physical activity on most days.
• What are effective ways to raise the issue of obesity during an office visit?
Despite the strong connection of obesity with adverse health outcomes, discussion of obesity in routine office settings can be difficult and is often limited by many factors such as time, training, availability of support services, perceived lack of patient motivation, and low outcome expectations [21,22]. Perhaps most challenging is tactfully handling the stigma associated with obesity, which can make discussion awkward and difficult for patients, parents, and providers. To do this, efforts to choose words that convey a nonjudgmental message while maintaining focus on obesity as a health concern are helpful. For example, terms such as “fat” and “obese” are often perceived as stigmatizing and blaming while using the term “unhealthy weight” is less pejorative and can be motivating [23]. It can also be important to acknowledge and emphasize that some individuals are more susceptible to weight gain and its consequences than others and as a result can tolerate fewer calories without unwanted weight gain and health problems. These approaches shift the focus of the discussion toward the goal of restoring and preserving health rather than changing physical appearance without placing blame on the individual and/or family. Motivational interviewing techniques which can be performed effectively even in short office visits can help to actively engage families, reveal familial perception of obesity and assess readiness to change [2]. Their use may also improve the efficacy of other interventions [24].
Case Continued
The patient and his mother were asked if they had any concerns today, including concerns about future health. Mother expressed worry about the potential for diabetes given their family history. The clinician used this as an opportunity to discuss pertinent factors associated with insulin resistance and type 2 diabetes, including modifiable factors such as diet, fitness level, and weight.
• Should this non-obese adolescent be assessed for obesity comorbidities?
Yes. While there are multiple guidelines available for pediatric screening, all highlight the importance of obtaining individualized risk assessment to guide the extent of diagnostic workup. An Expert Committee comprised of representatives from 15 professional organizations appointed 3 writing groups to review the literature and recommend approaches to prevention, assessment, and treatment. Because effective strategies remain poorly defined, the writing groups used both available evidence and expert opinion to develop the recommendations [2]. In addition to routine blood pressure monitoring and universal lipid screening, the Expert Committee recommends obtaining additional laboratory assessment for obese children (BMI ≥ 95th percentile) including a fasting glucose and ALT/AST levels every 2 years starting at age 10 years. For overweight children (BMI > 85th percentile), the Expert Committee recommends obtaining these studies if additional risk factors are present [2]. The American Diabetes Association (ADA) recommends obtaining diabetes screening in all children classified as overweight (defined as either a BMI > 85th percentile for age and sex, weight for height > 85th percentile, or weight > 120% of ideal for height) once every 3 years beginning at age 10 or at pubertal onset (whichever is earliest) when 2 additional risk factors for diabetes are also present, including: (1) history of type 2 diabetes in a first- or second-degree relative, (2) race/ethnicity with increased risk for diabetes development (eg, Native American, African American, Latino, Asian American), (3) signs of insulin resistance or conditions associated with insulin resistance (eg, small for gestational age, polycystic ovary syndrome, hypertension) and (4) maternal history of gestational diabetes during pregnancy [25]. The ADA recommendations for diabetes screening test include either fasting plasma glucose, HgA1C, or oral glucose tolerance test [25].
With a BMI at the 85th percentile, on initial assessment our patient might be perceived as being at moderate or even low risk for obesity and its associated comorbidities. However, a more careful review has elicited several additional risk factors suggesting more appropriate classification in the high-risk category. First, family history of type 2 diabetes on both sides of his family suggests a degree of genetic predisposition. Second, Hispanic ethnicity is known to be independently associated with insulin resistance, type 2 diabetes, and NAFLD [26]. Moreover, physical exam findings of an elevated waist circumference (90th percentile for age and ethnicity [27]) and acanthosis nigricans are also supportive of insulin resistance. As a result, despite having a BMI at the 85th percentile, this adolescent is at high risk and further evaluation is warranted based on both Expert Committee and ADA guidelines. Detailed discussion of certain risk factors is outlined below.
Pattern of Adipose Tissue Distribution: Utility of BMI and Waist Circumference
BMI is a clinical tool that serves as a surrogate marker of adiposity, but since it does not directly measure body fat it provides a statistical, rather than inherent, description of risk. While it is a relatively specific marker (~95%) with moderately high sensitivity and positive predictive value (~70–80%) at BMI levels > 95th percentile, sensitivity and positive predictive value decrease substantially at lower BMI percentiles (PPV 18% in a sample of overweight children) [28]. Current CDC BMI percentile charts consider age and gender differences but do not take into account sexual maturation level or race/ethnicity, both of which are independently correlated with BMI [29]. That is, children with similar BMIs of the same age and sex may exhibit varying degrees of adiposity and risk attributable to their pubertal stage and/or ethnicity [30]. For example, many studies have demonstrated that at the same BMI percentile, Asian Americans tend to have more adiposity compared with non-Hispanic whites [31], whereas African Americans tend to have more fat-free mass compared with non-Hispanic whites [32]. As a result of these differences, some advocate for adjusting cut-offs for BMI based on ethnicity and/or utilizing alternative measures of adiposity such as waist circumference or waist to hip ratio. However, in order for these latter methods to be useful, standardized methods of measurement and normative reference values must be developed. In summary, though BMI can be a useful screening tool, it is an indirect measure of adiposity and cannot discern adipose distribution. Therefore, it is important to remember that when used alone, BMI may overlook children with high inherent risk for disease.
Abdominal adiposity is associated with increased metabolic risk, including insulin resistance, type 2 diabetes, hypertension, cardiovascular disease, and mortality [33]. Waist circumference, a marker of abdominal/truncal obesity, has been considered as a potential marker in place of or in combination with BMI to identify children with increased metabolic risk. In adults, it is well established that an elevated waist circumference is associated with increased health risk, even among those within a normal-weight BMI category [34], and it is recommended that waist circumference in addition to BMI be used to assess health risk [35]. Many studies have documented similar associations between increased waist circumference and metabolic risk factors in childhood and adolescence [36–38]. Specifically, waist circumference is an independent predictor of both insulin sensitivity and increased visceral adiposity tissue (VAT) in children and adolescents [39]. Waist circumference can provide valuable information beyond BMI alone and may be beneficial in the clinical setting in identifying adolescents at risk for obesity-associated comorbidities.
The use of waist circumference in routine clinical settings is complicated and limited by many factors. First, there is no universal method for waist circumference measurement. For example, the WHO recommends measurement at the midpoint between the superior iliac crest and inferior most rib, while the NIH and NHANES recommend measurement immediately above the iliac crest [40]. Since nationally representative data published by Fernandez et al [27] uses the latter method for waist circumference measurement, we recommend this method to allow for comparison of waist circumference percentile with available data for age, sex, and ethnicity. Second, while absolute waist circumference values are used as cut-offs in adulthood, in childhood use of waist circumference percentiles would be more appropriate to account for expected increases during childhood and changes related to pubertal stage. Unfortunately, a lack of standardized waist circumference percentile charts makes meaningful interpretation of waist circumference difficult. Moreover, even if standardized waist circumference percentile charts were developed, there are currently no accepted standards defining an abnormally elevated waist circumference percentile.
Many studies have identified increased metabolic risk factors associated with a waist circumference at or above the 90th percentile for age [41–43]. Based on these studies, the International Diabetes Federation uses waist circumference > 90th percentile as part of the criteria for metabolic syndrome in adolescents. While this ensures a high degree of specificity, use of waist circumference at the 75th percentile would allow for increased sensitivity. For example, Lee et al found that for insulin resistance use of waist circumference at the 75th percentile compared with the 90th percentile increased sensitivity from 61.3% to 86.1% while decreasing specificity from 91.4% to 71.5% [44]. Thus, for individuals at low risk based on history and clinical findings, a waist circumference threshold at the 90th percentile might be reasonable, while for individuals with additional risk factors for insulin resistance use of a lower waist circumference threshold (such as the 75th percentile) may be beneficial. Finally, since risk for insulin resistance and type 2 diabetes varies by race/ethnicity, which may correspond with visceral fat deposition, utilizing various threshold cut-offs based on race/ethnicity has been proposed by some. However, current data do not support this practice [44]. In summary, though there are many challenges to using waist circumference measurements in routine settings, if performed correctly determination of elevated waist circumference measurement can provide some additional information on an individual’s overall risk for complications of obesity.
Acanthosis Nigricans as an Indicator of Insulin Resistance
Insulin resistance, independent of adiposity, is associated with increased risk for type 2 diabetes, cardiovascular disease, ovarian hyperandrogenism, and certain forms of cancer [45]. Identification of insulin resistance in the clinical setting can lead to appropriate intervention (both lifestyle and, when warranted, pharmacologic) to reduce insulin resistance and improve health outcomes. Several risk factors for insulin resistance have been discussed above. Acanthosis nigricans, which is characterized by thick, velvety hyperpigmentation of the skin in intertriginous areas such as the neck and axilla, is an additional finding that is associated with insulin resistance. Its pathogenesis is felt to be related to activation of the IGF-1 receptor by high levels of circulating insulin [46]. Acanthosis nigricans is independently associated with fasting insulin levels and impaired glucose tolerance [47,48]. In addition to increased insulin resistance, one study found that 1 in 4 youths with acanthosis nigricans demonstrated abnormalities in glucose homeostasis and identified 2 individuals with diabetes who would not have been diagnosed based on fasting glucose levels alone [48]. The presence of acanthosis nigricans should alert the clinician to the likelihood of insulin resistance and prompt further investigation. Of note, the prevalence of acanthosis nigricans is increased among African American and Hispanic patients [49,50].
• What laboratory evaluation is warranted and practical in the office setting?
Laboratory evaluation is warranted when obesity or risk factors for comorbidities of obesity are present. At minimum, this should include lipid screening, liver enzymes (ALT and AST), and fasting glucose as outlined above. This approach, however, fails to identify all individuals with obesity-associated comorbidities. ALT is only moderately sensitive in detecting NAFLD [51], and fasting glucose levels only become abnormal when compensation for the degree of insulin resistance is inadequate to maintain normal fasting glucose homeostasis. As a result, while abnormal results on screening are suggestive of disease, normal results do not necessarily confer its absence. Thus, for high-risk subjects, additional testing and/or referral should be considered.
The hyperinsulinemic euglycemic clamp is the “gold standard” for measuring insulin sensitivity, but it is labor intensive and impractical in routine clinical settings. Alter-native approaches using surrogate markers have commonly been utilized, including fasting insulin and glucose levels and 2-hour oral glucose tolerance test (OGTT). The utility of these approaches in the clinical setting has been limited by several factors, including lack of a universal insulin assay. However, despite these limitations, obtaining fasting insulin in addition to fasting glucose or performing 2-hour OGTT can be useful in providing crude estimates of insulin resistance in certain high-risk subpopulations [52,53]. Recently, the ADA added HgA1C measurement as diagnostic criteria for pre-diabetes (5.7%–6.4%) and diabetes (> 6.5%) [54]. Benefits of HgA1C measurement include reliable measurements in nonfasting conditions and reflection of glucose over time. Studies in pediatric patients have shown the usefulness of HgA1C as a measure of future glucose intolerance or diabetes [55]. When fasting insulin or HgA1C are elevated and/or OGTT is abnormal, this suggests the presence of insulin resistance and need for intervention.
Proposed guideline criteria for the diagnosis of “metabolic syndrome” in adolescents include the following: (1) glucose intolerance, (2) elevated waist circumference or BMI, (3) hypertriglyceridemia, (4) low HDL, and 5) hypertension. There is no universal definition for metabolic syndrome in childhood and adolescence, and cut-off values in each category vary by study group [41–43,56]. When insulin resistance is present, it should alert the clinician to the increased likelihood for metabolic syndrome and NAFLD, and additional screening should be performed accordingly. NAFLD is present in about 25% of all overweight children and is strongly associated with insulin resistance and the metabolic syndrome [57]. Hispanic patients have an increased prevalence of NAFLD compared with patients of other ethnicities [58,59]. Elevated liver transaminases (AST and ALT) are commonly used to screen for NAFLD. However, since these markers are indicative of hepatocellular damage, they may remain within normal limits and correlate poorly with early steatosis [51]. Alternative approaches have been proposed in high-risk populations to detect early steatosis and improve long-term prognosis [60].
Case Continued
The patient underwent laboratory assessment that included fasting glucose and insulin, fasting lipid panel, and ALT. Results were suggestive of insulin resistance and metabolic syndrome and included the following: fasting glucose 108 mg/dL, fasting insulin 65 uIU/mL (reference range 3–25), HgA1C 5.9% (reference range 4.2–5.8), total cholesterol 178 mg/dL, HDL cholesterol 35 mg/dL, LDL cholesterol 110 mg/dL, triglycerides 157 mg/dL, and ALT 40 u/L. Blood pressure, as noted above, is at the 95th percentile for age and height.
• What is the recommended approach to intervention? When is referral warranted?
Staged Obesity Treatment
The initial stage, termed “Prevention Plus,” is similar to obesity prevention strategies and is focused on institution of healthy dietary and activity lifestyle habits tailored to the individual and family. Frequent follow-up and monitoring can be helpful and should be offered to families. Failure to demonstrate progress after 3 to 6 months warrants advancement to Stage 2, “Structured Weight Management,” which includes a planned diet with structured meals and snacks, reduction of screen time to 1 hour or less, 60 minutes of supervised physical activity, use of logs to document diet and activity levels, monthly follow-ups and positive reinforcement for achieving goals. Consultation with a dietician and health psychologist/counseling can be helpful at this level.
If no progress is noted after 3 to 6 months, progression to Stage 3, “Comprehensive Multidisciplinary Intervention,” is recommended. This stage emphasizes the importance of a multidisciplinary team including behavioral counselor, registered dietician and exercise specialist in addition to a medical provider. Current evidence suggests modest improvement of obesity and related comorbidities in adolescents participating in multidisciplinary weight management programs [62,63]. While these interventions can be implemented in community settings, coordination in this setting can be difficult and implementation more commonly involves weight management programs in tertiary care centers. Access to such programs can be limited by geographic accessibility, insurance coverage and physician awareness of available programs/resources [64]. Utilization of technology such as telemedicine visits is one way to overcome limited access [65]. Finally, Stage 4 “Tertiary Care Intervention”, involving discussion of pharmacologic or intensive/surgical weight loss options, can be considered for those who fail to show progression after successful intervention of previous stages.
Specialty Referral
Referral to multidisciplinary clinics specializing in childhood obesity is warranted when obesity is particularly severe, comorbidities are present at baseline, or no improvement is noted after 6 months of intense lifestyle intervention. Insulin resistance evidenced by impaired glucose tolerance (abnormal fasting or 2-hour glucose levels), HgA1C in the pre-diabetes range or higher (> 5.7%), or persistently elevated fasting insulin levels after 3 to 6 months of intensive lifestyle modification should prompt referral for consideration of metformin initiation. Metformin can reduce insulin resistance in children and may reduce progression from impaired glucose tolerance to diabetes [66]. For dyslipidemia related to metabolic syndrome, lifestyle interventions are most likely to be efficacious. Referral to preventative cardiology for consideration of pharmacologic intervention should be considered when severe hypertriglyceridemia is present (> 400 mg/dL) or LDL remains elevated after implementation of healthy lifestyle interventions. Elevations in ALT are highly specific for NAFLD and should prompt referral to gastroenterology. In addition, given the poor sensitivity of ALT for detection of early hepatic steatosis, referral might be considered when ALT is in the high normal ranges, especially in those with increased risk such as Hispanic patients [67]. Finally, when signs of obstructive sleep apnea are present, a sleep study should be performed. In summary, while specialty referral can aid targeted treatment of obesity-related morbidities, the central role of the primary care clinician in anticipating and preventing or minimizing their occurrence remains paramount.
Case Conclusion
The patient was referred to a multidisciplinary obesity clinic where he and his family met with dietician, exercise physiologist, health psychologist, and endocrinologist. Healthy lifestyle modifications with specific goals were instituted, including elimination of all calorie-containing beverages (except daily recommended intake of fat-free milk) and initiation of physical activity for 30 minutes a day 5 days per week. He was started on metformin due to glucose intolerance and increased risk for diabetes. Follow-up occurred at monthly intervals for the first 3 months. Additional goals and lifestyle interventions were implemented at each follow-up. At 6 months’ follow-up, the patient’s height was 164 cm, weight was stable at 58.4 kg and BMI was 21.7 (79th percentile). Blood pressure was slightly improved at 123/80 mm Hg. Repeat labs showed mild but consistent improvement in all areas. Specifically, fasting glucose 100 mg/dL, fasting insulin 40 uIU/mL, HgA1C 5.6%, total cholesterol 162 mg/dL, HDL cholesterol 40 mg/dL, LDL cholesterol 105 mg/dL, triglycerides 140 mg/dL, and ALT 38 u/L. The patient continues to be monitored closely with goal to improve metabolic health and long-term health outcomes.
Summary
Childhood obesity is a major public health concern. The health impact of obesity on children is broad and profound. Since treatment of obesity is often unsuccessful, prevention of obesity or early detection of its health consequences are crucial responsibilities and opportunities for primary care clinicians. While clinical guidelines can be instructive, application of clinical guidelines must be tailored to individual adolescent patients according to accompanying risk factors. This review aims to help clinicians stratify risk based on susceptibility to development of insulin resistance and other morbidities associated with adolescent obesity. While the enormity of the obesity epidemic can appear overwhelming to primary care providers, they remain in the best position to initiate early intervention strategies. Coordinating care between primary care providers and specialty clinics will continue to be an important partnership for the care of those experiencing health-threatening effects of adolescent obesity.
Corresponding author: Aaron L Carrel, MD, University of Wisconsin, 600 Highland Ave, H4-436, Madison, WI 53792.
Financial disclosures: Drs. Seibert and Carrel have received fellowship grants from Genentech.
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
1. CDC. Obesity task force report. 2010. Available at www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. Accessed 4 Sept 2013.
2. Barlow SE, AAP Expert Committee. AAP Expert Committee Recommendations regarding prevention, assessment and treatment of child obesity. Pediatrics 2007;120:s164–92.
3. Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9.
4. Centers for Disease Control and Prevention (CDC) 2012; Overweight and obesity. Available at www.cdc.gov/obesity/childhood/basics.html. Accessed 3 Sept 2013.
5. Centers for Disease Control and Prevention (CDC). Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2009–2010. Available at www.cdc.gov/nchs/data/hestat/obesity_child_09_10/obesity_child_09_10.pdf.
6. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90.
7. August GP, Caprio S, Fennoy I, et al; Endocrine Society. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab 2008;93:4576–99.
8. Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health 2008;5:294–307.
9. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32.
10. Sharifi M, Marshall G, Marshall R, et al. Accelerating progress in reducing childhood obesity disparities: exploring best practices of positive outliers. J Health Care Poor Underserved 2013;24(2 Suppl):193–9.
11. Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 2004;89:2590–4.
12. Rosenbaum M, Fennoy I, Accacha S, et al. Racial/ethnic differences in clinical and biochemical type 2 diabetes mellitus risk factors in children. Obesity (Silver Spring) 2013;21:2081–90.
13. NIDDK. National diabetes statistics, 2011. Available at http://diabetes.niddk.nih.gov/dm/pubs/statistics/. Accessed 18 Sept 2013.
14. Janz KF, Butner KL, Pate RR. The role of pediatricians in increasing physical activity in youth. JAMA Pediatr 2013:1–2.
15. Coleman KJ, Hsii AC, Koebnick C, et al. Implementation of clinical practice guidelines for pediatric weight management. J Pediatrics 2012;160:918–22.
16. Ratcliff MB, Jenkins TM, Reiter-Purtill J, et al. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics 2011;127:827–34.
17. Goldenring J, Rosen D. Getting into adolescent heads: An essential update. Contemp Pediatr 2004;21:64.
18. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007;39:1251–6.
19. Weiss R, Shaw M, Savoye M, Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Ped Diabetes 2009;10:360–7.
20. Rizzo NS, Ruiz JR, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The European Youth Heart Study. J Pediatr 2007;150:388–94.
21. Story MT, Neumark-Stzainer DR, Sherwood NE, et al. Management of child and adolescent obesity: attitudes, barriers, skills, and training needs among health care professionals. Pediatrics 2002;110(1 Pt 2):210–4.
22. Alexander SC, Ostbye T, Pollak KI, et al. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
23. Puhl RM, Peterson JL, Luedicke J. Weight-based victimization: bullying experiences of weight loss treatment-seeking youth. Pediatrics 2013;131:e1–9.
24. Christie D, Channon S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: a clinical review. Diabetes Obes Metab 2013. Aug 8 [Epub ahead of print].
25. Standards of medical care in diabetes--2010. Diabetes Care 2010;33 Suppl 1:S11–61.
26. Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–51.
27. Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatrics 2004;145:439–44.
28. Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34.
29. Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7.
30. Curtis VA, Carrel AL, Eickhoff JC, Allen DB. Gender and race influence metabolic benefits of fitness in children: a cross-sectional study. Int J Pediatr Endocrinol 2012;2012:4.
31. Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among U.K. Children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care 2013;36:1712–9.
32. Gutin B, Yin Z, Humphries MC, Hoffman WH, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr 2004;145:737–43.
33. Cook S. The metabolic syndrome: Antecedent of adult cardiovascular disease in pediatrics. J Pediatr 2004;145:427–30.
34. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med 2002;162:2074–9.
35. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. NIH Pub No. 98-4083. Available at www.ncbi.nlm.nih.gov/books/NBK2003/pdf/TOC.pdf. Accessed 29 Sept 2013.
36. Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30.
37. Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17.
38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Rel Metab Disorders 2000;24:1453–8.
39. Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatrics 2006;148:188–94.
40. Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutrition 2003;77:379–84.
41. Cook S, Weitzman M, Auinger P, et al. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Ped Adol Med 2003;157:821–7.
42. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
43. Cruz ML, Weigensberg MJ, Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrin Metab 2004;89:108–13.
44. Lee JM, Davis MM, Woolford SJ, Gurney JG. Waist circumference percentile thresholds for identifying adolescents with insulin resistance in clinical practice. Pediatric Diabetes 2009;10:336–42.
45. Li S, Chen W, Srinivasan SR, et al. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012;1:S142–9.
46. Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002;147:1096–101.
47. Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J. Adolesc Health 2001;28:372–6.
48. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatrics 2010;156:87–92.
49. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269–72.
50. Brickman WJ, Binns HJ, Jovanovic BD, et al. Acanthosis nigricans: a common finding in overweight youth. Pediatr Dermatol 2007;24:601–6.
51. Yang HR, Kim HR, Kim MJ, et al. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:1525–30.
52. Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159 Suppl 1:S67–74.
53. Adam TC, Hasson RE, Lane CJ, Goran MI. Fasting indicators of insulin sensitivity: effects of ethnicity and pubertal status. Diabetes Care 2011;34:994–9.
54. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36 Suppl 1:S67–74.
55. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
56. Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74.
57. Martins C, Pizarro A, Aires L, et al. Fitness and metabolic syndrome in obese fatty liver children. Ann Hum Biol 2013;40:99–101.
58. Taveras EM, Gillman MW, Kleinman KP, et al. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013;167:731–8.
59. Wolfgram PM, Connor EL, Rehm JL, et al. Ethnic differences in the effects of hepatic fat deposition on insulin resistance in non-obese middle school girls. Obesity (Silver Spring) 2014;22:243–8.
60. Sowa JP, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS One 2013;8:e62439.
61. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–192.
62. Woolford SJ, Sallinen BJ, Clark SJ, Freed GL. Results from a clinical multidisciplinary weight management program. Clin Pediatrics 2011;50:187–91.
63. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297:2697–704.
64. Woolford SJ, Clark SJ, Gebremariam A, et al. Physicians’ perspectives on referring obese adolescents to pediatric multidisciplinary weight management programs. Clin Pediatrics 2010;49:871–5.
65. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J Ehealth 2013;19:806–8.
66. Park MH, Kinra S, Ward KJ, et al. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–5.
67. Urrutia-Rojas X, McConathy W, Willis B, et al. Abnormal glucose metabolism in Hispanic parents of children with acanthosis nigricans. ISRN Endocrinol 2011(Epub 2011 Dec 25.).
Mixing meds and supplements to dangerous effect

Credit: CDC
A new study indicates that a fair share of patients may be mixing the herbal supplement St. John’s wort with prescribed medications, which can have dangerous results.
St. John’s wort can reduce the concentration of numerous drugs in the body, including anticoagulants and chemotherapeutic agents. And this can result in impaired effectiveness and treatment failure.
But the supplement can also interact with medications to produce serious adverse events.
“Patients may have a false sense of safety with so-called ‘natural’ treatments like St. John’s wort,” said study author Sarah Taylor, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“And it is crucial for physicians to know the dangers of ‘natural’ treatments and to communicate the risks to patients effectively.”
Dr Taylor and her colleagues investigated the use of St. John’s wort and reported their findings in The Journal of Alternative and Complementary Medicine.
To determine how often the supplement was being prescribed or taken with other medications, the researchers conducted a retrospective analysis of nationally representative data collected by the National Ambulatory Medical Care Survey from 1993 to 2010.
The team found the use of St. John’s wort in potentially harmful combinations in 28% of the cases reviewed. The drugs involved were warfarin, selective serotonin reuptake inhibitors, benzodiazepines, statins, verapamil, digoxin, and oral contraceptives.
Possible drug interactions include serotonin syndrome (a potentially fatal condition that causes high levels of the chemical serotonin to accumulate in the body), heart disease due to impaired efficacy of blood pressure medications, or unplanned pregnancy due to contraceptive failure, Dr Taylor said.
A key limitation of this study is that only medications recorded by the physician were analyzed. And Dr Taylor said the rate of St. John’s wort interactions may actually be underestimated because the database did not include patients who were using St. John’s wort but did not tell their doctor.
“Labeling requirements for helpful supplements such as St. John’s wort need to provide appropriate cautions and risk information,” Dr Taylor said, adding that France has banned the use of St. John’s wort products, and several other countries, including Japan, the UK, and Canada, are in the process of including drug-herb interaction warnings on St. John’s wort products.
“Doctors also need to be trained to always ask if the patient is taking any supplements, vitamins, minerals or herbs, especially before prescribing any of the common drugs that might interact with St. John’s wort.” ![]()

Credit: CDC
A new study indicates that a fair share of patients may be mixing the herbal supplement St. John’s wort with prescribed medications, which can have dangerous results.
St. John’s wort can reduce the concentration of numerous drugs in the body, including anticoagulants and chemotherapeutic agents. And this can result in impaired effectiveness and treatment failure.
But the supplement can also interact with medications to produce serious adverse events.
“Patients may have a false sense of safety with so-called ‘natural’ treatments like St. John’s wort,” said study author Sarah Taylor, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“And it is crucial for physicians to know the dangers of ‘natural’ treatments and to communicate the risks to patients effectively.”
Dr Taylor and her colleagues investigated the use of St. John’s wort and reported their findings in The Journal of Alternative and Complementary Medicine.
To determine how often the supplement was being prescribed or taken with other medications, the researchers conducted a retrospective analysis of nationally representative data collected by the National Ambulatory Medical Care Survey from 1993 to 2010.
The team found the use of St. John’s wort in potentially harmful combinations in 28% of the cases reviewed. The drugs involved were warfarin, selective serotonin reuptake inhibitors, benzodiazepines, statins, verapamil, digoxin, and oral contraceptives.
Possible drug interactions include serotonin syndrome (a potentially fatal condition that causes high levels of the chemical serotonin to accumulate in the body), heart disease due to impaired efficacy of blood pressure medications, or unplanned pregnancy due to contraceptive failure, Dr Taylor said.
A key limitation of this study is that only medications recorded by the physician were analyzed. And Dr Taylor said the rate of St. John’s wort interactions may actually be underestimated because the database did not include patients who were using St. John’s wort but did not tell their doctor.
“Labeling requirements for helpful supplements such as St. John’s wort need to provide appropriate cautions and risk information,” Dr Taylor said, adding that France has banned the use of St. John’s wort products, and several other countries, including Japan, the UK, and Canada, are in the process of including drug-herb interaction warnings on St. John’s wort products.
“Doctors also need to be trained to always ask if the patient is taking any supplements, vitamins, minerals or herbs, especially before prescribing any of the common drugs that might interact with St. John’s wort.” ![]()

Credit: CDC
A new study indicates that a fair share of patients may be mixing the herbal supplement St. John’s wort with prescribed medications, which can have dangerous results.
St. John’s wort can reduce the concentration of numerous drugs in the body, including anticoagulants and chemotherapeutic agents. And this can result in impaired effectiveness and treatment failure.
But the supplement can also interact with medications to produce serious adverse events.
“Patients may have a false sense of safety with so-called ‘natural’ treatments like St. John’s wort,” said study author Sarah Taylor, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
“And it is crucial for physicians to know the dangers of ‘natural’ treatments and to communicate the risks to patients effectively.”
Dr Taylor and her colleagues investigated the use of St. John’s wort and reported their findings in The Journal of Alternative and Complementary Medicine.
To determine how often the supplement was being prescribed or taken with other medications, the researchers conducted a retrospective analysis of nationally representative data collected by the National Ambulatory Medical Care Survey from 1993 to 2010.
The team found the use of St. John’s wort in potentially harmful combinations in 28% of the cases reviewed. The drugs involved were warfarin, selective serotonin reuptake inhibitors, benzodiazepines, statins, verapamil, digoxin, and oral contraceptives.
Possible drug interactions include serotonin syndrome (a potentially fatal condition that causes high levels of the chemical serotonin to accumulate in the body), heart disease due to impaired efficacy of blood pressure medications, or unplanned pregnancy due to contraceptive failure, Dr Taylor said.
A key limitation of this study is that only medications recorded by the physician were analyzed. And Dr Taylor said the rate of St. John’s wort interactions may actually be underestimated because the database did not include patients who were using St. John’s wort but did not tell their doctor.
“Labeling requirements for helpful supplements such as St. John’s wort need to provide appropriate cautions and risk information,” Dr Taylor said, adding that France has banned the use of St. John’s wort products, and several other countries, including Japan, the UK, and Canada, are in the process of including drug-herb interaction warnings on St. John’s wort products.
“Doctors also need to be trained to always ask if the patient is taking any supplements, vitamins, minerals or herbs, especially before prescribing any of the common drugs that might interact with St. John’s wort.” ![]()
FISH may help predict survival in ALCL
Researchers have discovered 3 subgroups of ALK-negative anaplastic large-cell lymphoma (ALCL) that have markedly different survival rates, according to a paper published in Blood.
They found that ALCL patients with TP63 rearrangements had a 17% chance of living 5 years beyond diagnosis, compared to 90% of patients who had DUSP22 rearrangements.
A third group of patients, those with neither rearrangement, had a 42% survival rate.
The researchers noted that these subgroups cannot be differentiated by routine pathology but can be identified via fluorescence in situ hybridization (FISH).
“This is the first study to demonstrate unequivocal genetic and clinical heterogeneity among systemic ALK-negative anaplastic large-cell lymphomas,” said study author Andrew L. Feldman, MD, of the Mayo Clinic in Rochester, Minnesota.
“Most strikingly, patients with DUSP22-rearranged ALCL had excellent overall survival rates, while patients with TP63-rearranged ALCL had dismal outcomes and nearly always failed standard therapy.”
Currently, all ALK-negative ALCLs are treated the same, using chemotherapy and, in some institutions, stem cell transplantation. But these new findings make a case for additional testing and possible changes to the standard of care.
“This is a great example of where individualized medicine can make a difference,” Dr Feldman said. “Patients whose chance of surviving is 1 in 6 are receiving the same therapy as patients whose odds are 9 in 10. Developing tests that identify how tumors are different is a critical step toward being able to tailor therapy to each individual patient.”
Therefore, Dr Feldman and his colleagues recommend performing FISH in all patients with ALK-negative ALCL.
To learn more about testing for DUSP22 and TP63:
- 6p25.3 FISH (DUSP22/IRF4): http://www.mayomedicallaboratories.com/test-catalog/Overview/60506
- 3q28 FISH (TP63): http://www.mayomedicallaboratories.com/test-catalog/Overview/70014.


Researchers have discovered 3 subgroups of ALK-negative anaplastic large-cell lymphoma (ALCL) that have markedly different survival rates, according to a paper published in Blood.
They found that ALCL patients with TP63 rearrangements had a 17% chance of living 5 years beyond diagnosis, compared to 90% of patients who had DUSP22 rearrangements.
A third group of patients, those with neither rearrangement, had a 42% survival rate.
The researchers noted that these subgroups cannot be differentiated by routine pathology but can be identified via fluorescence in situ hybridization (FISH).
“This is the first study to demonstrate unequivocal genetic and clinical heterogeneity among systemic ALK-negative anaplastic large-cell lymphomas,” said study author Andrew L. Feldman, MD, of the Mayo Clinic in Rochester, Minnesota.
“Most strikingly, patients with DUSP22-rearranged ALCL had excellent overall survival rates, while patients with TP63-rearranged ALCL had dismal outcomes and nearly always failed standard therapy.”
Currently, all ALK-negative ALCLs are treated the same, using chemotherapy and, in some institutions, stem cell transplantation. But these new findings make a case for additional testing and possible changes to the standard of care.
“This is a great example of where individualized medicine can make a difference,” Dr Feldman said. “Patients whose chance of surviving is 1 in 6 are receiving the same therapy as patients whose odds are 9 in 10. Developing tests that identify how tumors are different is a critical step toward being able to tailor therapy to each individual patient.”
Therefore, Dr Feldman and his colleagues recommend performing FISH in all patients with ALK-negative ALCL.
To learn more about testing for DUSP22 and TP63:
- 6p25.3 FISH (DUSP22/IRF4): http://www.mayomedicallaboratories.com/test-catalog/Overview/60506
- 3q28 FISH (TP63): http://www.mayomedicallaboratories.com/test-catalog/Overview/70014.


Researchers have discovered 3 subgroups of ALK-negative anaplastic large-cell lymphoma (ALCL) that have markedly different survival rates, according to a paper published in Blood.
They found that ALCL patients with TP63 rearrangements had a 17% chance of living 5 years beyond diagnosis, compared to 90% of patients who had DUSP22 rearrangements.
A third group of patients, those with neither rearrangement, had a 42% survival rate.
The researchers noted that these subgroups cannot be differentiated by routine pathology but can be identified via fluorescence in situ hybridization (FISH).
“This is the first study to demonstrate unequivocal genetic and clinical heterogeneity among systemic ALK-negative anaplastic large-cell lymphomas,” said study author Andrew L. Feldman, MD, of the Mayo Clinic in Rochester, Minnesota.
“Most strikingly, patients with DUSP22-rearranged ALCL had excellent overall survival rates, while patients with TP63-rearranged ALCL had dismal outcomes and nearly always failed standard therapy.”
Currently, all ALK-negative ALCLs are treated the same, using chemotherapy and, in some institutions, stem cell transplantation. But these new findings make a case for additional testing and possible changes to the standard of care.
“This is a great example of where individualized medicine can make a difference,” Dr Feldman said. “Patients whose chance of surviving is 1 in 6 are receiving the same therapy as patients whose odds are 9 in 10. Developing tests that identify how tumors are different is a critical step toward being able to tailor therapy to each individual patient.”
Therefore, Dr Feldman and his colleagues recommend performing FISH in all patients with ALK-negative ALCL.
To learn more about testing for DUSP22 and TP63:
- 6p25.3 FISH (DUSP22/IRF4): http://www.mayomedicallaboratories.com/test-catalog/Overview/60506
- 3q28 FISH (TP63): http://www.mayomedicallaboratories.com/test-catalog/Overview/70014.