User login
‘Nanodaisies’ deliver drug cocktail to leukemia cells

Credit: PNAS
Biomedical engineers have reported that daisy-shaped, nanoscale structures can deliver a cocktail of drugs directly to cancer cells.
The “nanodaisies” effectively delivered a 2-drug combination in a range of cell lines, including the leukemia cell line HL-60.
The drug-delivery vehicles also proved effective in a mouse model of lung cancer.
Zhen Gu, PhD, of North Carolina State University and the University of North Carolina at Chapel Hill, and his colleagues detailed these results in Biomaterials.
“We found that this technique was much better than conventional drug-delivery techniques at inhibiting the growth of lung cancer tumors in mice,” Dr Gu said.
“And based on in vitro tests in 9 different cell lines, the technique is also promising for use against leukemia, breast, prostate, liver, ovarian, and brain cancers.”
To make the “nanodaisies,” the researchers begin with a solution that contains a polymer called polyethylene glycol (PEG). The PEG forms long strands that have much shorter strands branching off to either side.
The researchers directly link the anticancer drug camptothecin (CPT) onto the shorter strands and introduce the anticancer drug doxorubicin (Dox) into the solution.
PEG is hydrophilic, but CPT and Dox are hydrophobic. As a result, the CPT and Dox cluster together in the solution, wrapping the PEG around themselves. This results in a daisy-shaped drug cocktail, only 50 nanometers in diameter, that can (in theory) be injected into a cancer patient.
Once injected, the nanodaisies float through the bloodstream until they are absorbed by cancer cells. In fact, one of the reasons the researchers chose to use PEG is because it has chemical properties that prolong the life of the drugs in the bloodstream. Once in a cancer cell, the drugs are released.
“Both drugs attack the cell’s nucleus but via different mechanisms,” said study author Wanyi Tai, PhD, who was previously a researcher in Dr Gu’s lab but is now at the University of Washington in Seattle.
“Combined, the drugs are more effective than either drug is by itself,” Dr Gu added. “We are very optimistic about this technique and are hoping to begin preclinical testing in the near future.” ![]()

Credit: PNAS
Biomedical engineers have reported that daisy-shaped, nanoscale structures can deliver a cocktail of drugs directly to cancer cells.
The “nanodaisies” effectively delivered a 2-drug combination in a range of cell lines, including the leukemia cell line HL-60.
The drug-delivery vehicles also proved effective in a mouse model of lung cancer.
Zhen Gu, PhD, of North Carolina State University and the University of North Carolina at Chapel Hill, and his colleagues detailed these results in Biomaterials.
“We found that this technique was much better than conventional drug-delivery techniques at inhibiting the growth of lung cancer tumors in mice,” Dr Gu said.
“And based on in vitro tests in 9 different cell lines, the technique is also promising for use against leukemia, breast, prostate, liver, ovarian, and brain cancers.”
To make the “nanodaisies,” the researchers begin with a solution that contains a polymer called polyethylene glycol (PEG). The PEG forms long strands that have much shorter strands branching off to either side.
The researchers directly link the anticancer drug camptothecin (CPT) onto the shorter strands and introduce the anticancer drug doxorubicin (Dox) into the solution.
PEG is hydrophilic, but CPT and Dox are hydrophobic. As a result, the CPT and Dox cluster together in the solution, wrapping the PEG around themselves. This results in a daisy-shaped drug cocktail, only 50 nanometers in diameter, that can (in theory) be injected into a cancer patient.
Once injected, the nanodaisies float through the bloodstream until they are absorbed by cancer cells. In fact, one of the reasons the researchers chose to use PEG is because it has chemical properties that prolong the life of the drugs in the bloodstream. Once in a cancer cell, the drugs are released.
“Both drugs attack the cell’s nucleus but via different mechanisms,” said study author Wanyi Tai, PhD, who was previously a researcher in Dr Gu’s lab but is now at the University of Washington in Seattle.
“Combined, the drugs are more effective than either drug is by itself,” Dr Gu added. “We are very optimistic about this technique and are hoping to begin preclinical testing in the near future.” ![]()

Credit: PNAS
Biomedical engineers have reported that daisy-shaped, nanoscale structures can deliver a cocktail of drugs directly to cancer cells.
The “nanodaisies” effectively delivered a 2-drug combination in a range of cell lines, including the leukemia cell line HL-60.
The drug-delivery vehicles also proved effective in a mouse model of lung cancer.
Zhen Gu, PhD, of North Carolina State University and the University of North Carolina at Chapel Hill, and his colleagues detailed these results in Biomaterials.
“We found that this technique was much better than conventional drug-delivery techniques at inhibiting the growth of lung cancer tumors in mice,” Dr Gu said.
“And based on in vitro tests in 9 different cell lines, the technique is also promising for use against leukemia, breast, prostate, liver, ovarian, and brain cancers.”
To make the “nanodaisies,” the researchers begin with a solution that contains a polymer called polyethylene glycol (PEG). The PEG forms long strands that have much shorter strands branching off to either side.
The researchers directly link the anticancer drug camptothecin (CPT) onto the shorter strands and introduce the anticancer drug doxorubicin (Dox) into the solution.
PEG is hydrophilic, but CPT and Dox are hydrophobic. As a result, the CPT and Dox cluster together in the solution, wrapping the PEG around themselves. This results in a daisy-shaped drug cocktail, only 50 nanometers in diameter, that can (in theory) be injected into a cancer patient.
Once injected, the nanodaisies float through the bloodstream until they are absorbed by cancer cells. In fact, one of the reasons the researchers chose to use PEG is because it has chemical properties that prolong the life of the drugs in the bloodstream. Once in a cancer cell, the drugs are released.
“Both drugs attack the cell’s nucleus but via different mechanisms,” said study author Wanyi Tai, PhD, who was previously a researcher in Dr Gu’s lab but is now at the University of Washington in Seattle.
“Combined, the drugs are more effective than either drug is by itself,” Dr Gu added. “We are very optimistic about this technique and are hoping to begin preclinical testing in the near future.” ![]()
PICC Placement and Related Complications
Peripherally inserted central venous catheters (PICCs) are used for a variety of indications, including administration of long‐term intravenous (IV) antibiotics, home IV medications, chemotherapy, and parenteral nutrition.[1, 2, 3] Additionally, PICCs have also been recognized as an alternative to large‐bore central venous catheters such as subclavian or internal jugular central venous catheters. PICCs have been associated with fewer bloodstream infections in patients with cancer than tunneled catheters.[4] Compared to central venous catheters, they demonstrate reduced complication rates,[5] decreased cost,[6] and increased safety for longer durations of use.[1, 2, 3, 7, 8, 9]
Despite the numerous benefits of PICCs, Prandoni et al. estimate an all‐cause complication rate of 12% to 17% with the use of PICCs.[10] Associated complications include infection,[11] pain, bleeding, and mechanical dysfunction, all of which contribute to patient discomfort and additional healthcare costs.[12] Bloodstream infections, for example, had previously been thought to occur at a substantially lower rate in PICCs than central venous catheters.[13] However, a recent systematic review suggests the rate of PICC‐associated bloodstream infections in the inpatient setting is actually comparable to that of central venous catheters.[14] Perhaps the most serious PICC‐associated complication is catheter‐related venous thrombosis. A recent systematic review and meta‐analysis found evidence to suggest the rate of catheter‐related venous thrombosis was highest in patients with cancer or critical illness15; additionally, rates of thrombosis associated with PICCs were higher than those associated with subclavian or internal jugular central venous catheters.[15, 16] Fletcher et al. showed an 8.1% incidence of symptomatic PICC‐related upper extremity deep vein thrombosis (DVT) in the neurosurgical intensive care unit, with 15% of patients subsequently developing a pulmonary embolism.[17] A recent prospective, randomized controlled trial by Itkin et al. similarly demonstrated symptomatic DVT rates of approximately 4%.[18] However, in this study, when PICCs were routinely screened for thrombosis (with or without associated symptoms), approximately 72% demonstrated thrombosis,[18] suggesting that many PICC‐associated thromboses may be clinically undetected. This may have far‐reaching clinical significance, as pulmonary embolism complicates upper extremity DVT in 9% of cases and can result in a mortality rate as high as 25%.[10, 19]
Some strategies to reduce the rate of catheter‐related complications include identification of characteristics that put patients at risk. Many potential risk factors have been investigated, including catheter size,[12, 20, 21, 22, 23, 24] choice of vein,[24] location of catheter tip,[25] and history of malignancy or prior DVT.[12] However, to date, no definitive consensus has been reached. Special attention has been paid to the investigation of underlying risk factors and treatment for catheter‐related DVT, given its significant morbidity and mortality. Results have been equivocal, though, and in some instances, complicated by a diagnosis of underlying malignancy.[26, 27, 28]
As PICCs become more widely utilized, assessments of factors that place patients at greater risk of PICC‐related complications are needed.[21] The purpose of this study was to establish the incidence of complications associated with PICCs placed in the inpatient setting and examine risk factors predisposing patients to these complications.
MATERIALS AND METHODS
Study Design
A case control analysis of adult inpatients who underwent PICC placement between January 2009 and January 2010 was conducted at Scott & White Healthcare (now Baylor Scott & White Healthcare) to determine the incidence and risk factors for PICC‐associated complications.
Study Site
The study took place at Scott & White Memorial Hospital in Temple, Texas, a 636‐bed multispecialty teaching hospital and level 1 trauma center. It is part of a healthcare system that includes 12 hospitals and more than 60 regional clinics, all of which share an electronic medical record to enable full integration.
Human Subjects Approval
This study received approval from the institutional review board at Scott & White Healthcare.
PICC Placement Technique
Inpatient PICC placement was performed by the PICC consult service. The consult service was comprised of 3 separate provider teams: (1) internal medicine, including select hospitalists and internal medicine residents; (2) radiology, including interventional radiologists and radiology residents; and (3) nursing, including registered nurses with advanced training in PICC placement. Following placement of a consult, the PICC consult service assessed the patient, obtained consent, and subsequently placed the catheter. Members of the PICC consult service followed a system‐wide protocol wherein target veins were identified by ultrasound prior to attempting catheter placement, and actual placement of the PICC was ultrasound guided. Images obtained during the procedure were permanently documented in the medical record. At the time of this study, no formal protocol existed wherein target veins were mapped for caliber. Operators relied on their professional judgment to determine if vein caliber appeared sufficient to accommodate catheter placement.
All PICCs were placed using industry standard sterile precautions. A universally accepted modified Seldinger technique was used to obtain venous access.[29] A guidewire was then positioned in the desired vessel to facilitate proper venous placement of the catheter. During the course of the study period, catheters used were either single‐ (4 Fr) or double lumen (5 Fr).
Catheters were placed at the bedside by hospitalists or registered nurse teams; the location of the catheter tip at the cavoatrial junction was confirmed by chest radiography. Catheter insertions by radiologists were performed in the interventional radiology suite, and confirmation of location of the catheter tip was obtained with fluoroscopy.
PICC Maintenance
Following placement, nurses managed the PICC site according to nursing policy. Per policy, the site was assessed each shift. Documentation of assessment was recorded in nursing notes. Routine dressing changes were performed every 7 days, and as needed, to maintain a sterile site. Date and time of dressing changes were documented in nursing notes and on the PICC dressing. Catheter hubs and injection ports were disinfected with an antiseptic preparation for 15 seconds and allowed to air dry for 30 seconds prior to accessing the catheter. Catheters were flushed with 10 mL of normal saline before and after use. Any abnormality noted during PICC assessment was relayed to the primary provider. If the catheter did not flush readily or demonstrate appropriate blood return, nursing staff obtained an order for alteplase to be administered in an effort to salvage the line. PICCs were discontinued at the discretion of the healthcare provider.
Participants
Records of all patients 18 years of age and older who underwent PICC placement between January 2009 and January 2010 were reviewed (N=1444) for study inclusion. There were no exclusion criteria.
Data Collection
Patients who experienced complications were identified by electronic medical record review. One‐to‐one matching was performed for age and gender‐matched controls randomly selected from inpatients who underwent PICC placement during the same time period without complications. A total of 170 cases with PICC‐related complications were identified. One hundred seventy exact age‐ and gender‐matched controls, who based upon documentation available in the electronic medical record did not experience complications, were then randomly selected. Prior to data collection, the research team reviewed and discussed the data collection form and agreed upon a standardized protocol for data collection. Data collection was completed by authors J.M. and J.H. on the standardized data collection form. Although a formal analysis of inter‐rater agreement was not performed, J.M. and J.H. discussed any items where questions arose and arrived at a consensus decision regarding completion of the data point.
End points of the chart review were completion of medical therapy for which the PICC was indicated (eg, IV antibiotics or total parenteral nutrition [TPN]) or documentation of a complication that led to 1 of the following: discontinuation of the PICC or adjustment of either catheter placement or medical therapy. All complications were identified via International Classification of Diseases, 9th Revision codes and systematic chart review.
Complications resulting in discontinuation of the PICC, adjustment of catheter placement, or change in medical therapy were identified by review of nursing or physician documentation, and were categorized as follows: mechanical complications (defined as loss of the ability of the catheter to flush or draw properly, inadvertent catheter dislodgement, or retained portion of the catheter following catheter removal), catheter‐associated bloodstream infection (development of a positive blood culture attributable to the central catheter with no other clearly identifiable source of bacteremia present), cellulitis (defined as cellulitis in the extremity where the catheter was placed), bleeding from the site of catheter, fever (for which no other cause could be identified), and catheter‐associated thrombosis (identified by Doppler ultrasonography in patients exhibiting symptoms such as pain, swelling, redness, or warmth in the extremity in which the PICC was placed).[30]
Demographic data were collected, including insurance status, age, ethnicity, and gender. Clinical data included body mass index (BMI), presence of malnutrition (defined by a serum albumin of less than 3 g/dL),[31] previous or active cancer, previous DVT, use of anticoagulants (eg, warfarin, heparin, or low‐molecular‐weight heparin) or antiplatelet agent (eg, aspirin or clopidogrel) at the time of placement, and indication for PICC placement. A patient's history of previous or active cancer and previous DVT were identified by clinical documentation. Indications for PICC placement included: treating infectious processes (ie, infusion of antimicrobials), providing TPN, chemotherapy administration, and IV access. Catheter‐specific data were also collected and included venous access obtained (cephalic, basilic, brachial), catheter size (single lumen [4 Fr] or double lumen [5 Fr]), type of complication, and time to complication. The procedure note accompanying PICC placement was reviewed for data regarding time of day inserted (with after hours defined as documentation of placement occurring after 5 pm), and procedure operator to identify type of team (internal medicine, radiology, nursing) responsible for placement.
Data Analysis
Demographic characteristics and potential risk factors for patients in both the case and control groups of the study were summarized using descriptive statistics: mean ( standard deviation [SD]) for continuous variables and frequency (percent) for categorical variables. Univariate and multivariable conditional logistic regression analyses of variables that were potential risk factors of PICC‐related complications were utilized. A stepwise selection method was used for multivariable conditional logistic regression models. Alpha=0.2 was used for the significance to enter the model, and =0.05 was used for significance level to remain in the model. Attribution of PICC‐related complications was evaluated in terms of odds ratios (OR) and 95% confidence interval (CI). A P value of <0.05 indicated statistical significance. No prospective power analysis was performed. However, for a retrospective power analysis for 1:1 matching with 170 cases and 170 matched controls, assuming 20% of controls were affected and an of 0.05, one would achieve 80% power to detect an odds ratio of 2. SAS 9.2 (SAS Institute Inc., Cary, NC) was used for data analysis.
RESULTS
In 2009, 1444 PICCs were placed, and 170 cases in which patients experienced complications associated with PICC placement were identified, resulting in a complication rate of 11.77% (95% CI: 10.11%‐13.44%). The most common complications experienced by our patient population included catheter‐associated thrombosis (3%, n = 46), mechanical complications (4%, n=67), inadvertent catheter dislodgement (2%, n=36), mechanical dysfunction (2%, n=30), retained portion of the catheter following catheter removal (<1%, n=1), catheter‐associated bloodstream infections (2%, n=24), and cellulitis at the catheter insertion site (1%, n=15). Other documented complications included unexplained fever and bleeding (Table 1).
| Complication | N (%) |
|---|---|
| |
| Thrombosis | 46 (3) |
| Infection | 24 (2) |
| Cellulitis | 15 (1) |
| Mechanical complications* | 67 (4) |
| Unexplained fever | 15 (1) |
| Bleeding | 3 (0) |
| No complication | 1,274 (88) |
The mean age of the total cohort (N=340), comprised of case (N=170) and control (N=170) groups, was 58 years (SD 17), and 55% (n=94) were females. There were no significant differences in complications between groups based on ethnicity (P=0.66). In the case group, 46% (n=78) of PICCs were placed by the radiology team, 41% (n=69) were placed by the internal medicine team, and 14% (n=23) were placed by nursing. In the control group, 44% (n=74) of PICCs were placed by radiology, 36% (n=62) by internal medicine, and 20% (n=34) by nursing. Based on univariate conditional analysis, provider team was not significantly associated with complications (P=0.29).
Predictors of All‐Cause Complications
Based upon univariate conditional logistic regression analyses of complications related to PICC placement (N=340), the following variables demonstrated a statistically significant increased risk for complications: malnutrition (OR: 1.88 [95% CI: 1.023.44], P=0.04) and after‐hours placement (OR: 8.67 [95% CI: 2.62‐28.63], P=0.0004) (Table 2). Anticoagulation was associated with a decreased risk of complications (OR: 0.27 [95% CI: 0.16‐0.45], P=0.04). Based upon multivariable logistic regression analysis, after‐hours placement (OR: 9.52 [95% CI: 2.68‐33.78], P=0.0005) and BMI >30 (OR: 1.98 [95% CI: 1.09‐3.61], P=0.02) were significantly associated with an increased risk of PICC‐associated complications. Conversely, anticoagulation/antiplatelet use was associated with a decreased risk of complications (OR: 0.24 [95% CI: 0.14‐0.43], P<0.0001).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5817 | 5817 | ||||
| BMI, meanSD | 29.29.5 | 27.97.9 | 1.02 (0.991.05) | 0.12 | ||
| 30 | 108 (64) | 116 (68%) | 1.00 | 1.00 | ||
| >30 | 62 (36) | 54 (32%) | 1.29 (0.792.11) | 0.32 | 1.98 (1.093.61) | 0.02 |
| Length of stay, d, meanSD | 1822 | 1416 | 1.01 (1.001.03) | 0.06 | ||
| Length of stay group, d | 0.11a | |||||
| <7 | 41 (24) | 52 (31) | 1.00 | |||
| 729 | 101 (59) | 103 (61) | 1.19 (0.721.98) | 0.49 | ||
| 30 | 28 (16) | 15 (9) | 2.21 (1.074.58) | 0.03 | ||
| Gender | ||||||
| Female | 94 (55) | 94 (55) | ||||
| Male | 76 (45) | 76 (45) | ||||
| Ethnicity | 0.66a | |||||
| Caucasian | 131 (77) | 125 (74) | 1.00 | |||
| African American | 26 (15) | 28 (16) | 0.88 (0.481.60) | 0.67 | ||
| Hispanic/Asian | 13 (8) | 17 (10) | 0.70 (0.311.58) | 0.38 | ||
| Provider team | 0.29a | |||||
| Radiology | 78 (46) | 74 (44) | 1.00 | |||
| Internal medicine | 69 (41) | 62 (36) | 1.05 (0.681.64) | 0.82 | ||
| Nursing | 23 (14) | 34 (20) | 0.65 (0.351.19) | 0.16 | ||
| Insuranceb | 0.22a | |||||
| Private insurance | 46 (27) | 42 (25) | 1.00 | |||
| Uninsured | 17 (10) | 24 (14) | 0.73 (0.351.55) | 0.41 | ||
| Medicare | 57 (34) | 62 (37) | 0.73 (0.381.40) | 0.34 | ||
| Medicaid | 39 (23) | 25 (15) | 1.51 (0.743.06) | 0.26 | ||
| Tricare/Veterans Administration | 11 (6) | 16 (9) | 0.59 (0.241.45) | 0.25 | ||
| History of DVT | 27 (16) | 26 (15) | 1.05 (0.581.91) | 0.88 | ||
| Malnutritionb | 149 (88) | 134 (79) | 1.88 (1.023.44) | 0.04 | ||
| Cancer | 25 (15) | 36 (21) | 0.58 (0.311.09) | 0.09 | ||
| Fluoroscopy | 129 (76) | 139 (82) | 0.71 (0.421.19) | 0.19 | ||
| Anticoagulation use | 50 (29) | 100 (59) | 0.27 (0.160.45) | <0.0001 | 0.24 (0.140.43) | <0.0001 |
| Multilumenc | 99 (58) | 111 (66) | 0.70 (0.441.11) | 0.13 | ||
| Veinb | 0.39a | |||||
| Basilic | 98 (58) | 86 (51) | 1.00 | |||
| Cephalic | 11 (6) | 8 (5) | 1.37 (0.483.89) | 0.55 | ||
| Brachial | 61 (36) | 74 (44) | 0.70 (0.451.09) | 0.12 | ||
| Internal mammary | 0 (0) | 1 (1) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 144 (85) | 166 (98) | 1.00 | 1.00 | ||
| After hours | 26 (15) | 3 (2) | 8.67 (2.6228.63) | 0.0004 | 9.52 (2.6833.78) | 0.0005 |
| Indication for PICC | 0.02a | |||||
| Infection | 88 (52) | 71 (42) | 1.00 | |||
| Pneumonia | 21 (12) | 14 (8) | 1.07 (0.502.29) | 0.87 | ||
| Chemotherapy | 5 (3) | 2 (1) | 1.84 (0.349.93) | 0.48 | ||
| IV access | 36 (21) | 66 (39) | 0.44 (0.250.75) | 0.003 | ||
| Total parenteral nutrition | 20 (12) | 17 (10) | 0.96 (0.442.14) | 0.93 | ||
Predictors of Nonmechanical Complications
To study risk factors related to nonmechanical complications, a secondary analysis (N=206) was performed in which all patients who experienced mechanical complications (N=67) and matched controls (N=67) were excluded. Based upon multivariable logistic regression analysis, after‐hours placement (OR: 6.93 [95% CI: 1.35‐35.56], P=0.02) and malnutrition (OR: 2.83 [95% CI: 1.037.81], P=0.04) were significantly associated with increased risk of nonmechanical complications. The use of anticoagulation/antiplatelet agents was associated with decreased risk of nonmechanical complications (OR: 0.17 [95% CI: 0.07‐0.40], P<0.0001). Variables not significantly associated with nonmechanical complications included BMI>30, previous history of DVT, history of cancer, catheter size, and venous access choice (Table 3).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5816 | 5816 | ||||
| BMI, meanSD | 29.79.8 | 28.57.9 | 1.03 (0.991.07) | 0.22 | ||
| 30 | 64 (62) | 68 (66) | 1.00 | |||
| >30 | 39 (38) | 35 (34) | 1.27 (0.642.49) | 0.49 | ||
| Length of stay, d, meanSD | 2026 | 1418 | 1.02 (1.001.03) | 0.08 | ||
| Length of stay group, d | 0.03a | |||||
| <7 | 22 (21) | 28 (27) | 1.00 | |||
| 729 | 60 (58) | 68 (66) | 0.95 (0.491.82) | 0.87 | ||
| 30 | 21 (20) | 7 (7) | 3.24 (1.238.54) | 0.02 | ||
| Gender | ||||||
| Female | 63 (61) | 63 (61) | ||||
| Male | 40 (39) | 40 (39) | ||||
| Ethnicity | 0.95a | |||||
| Caucasian | 75 (73) | 75 (73) | 1.00 | |||
| African American | 19 (18) | 18 (17) | 1.06 (0.512.21) | 0.87 | ||
| Hispanic/Asian | 9 (9) | 10 (10) | 0.88 (0.322.44) | 0.81 | ||
| Provider team | 0.81a | |||||
| Radiology | 43 (42) | 44 (43) | 1.00 | |||
| Internal medicine | 45 (44) | 41 (40) | 1.11 (0.621.96) | 0.73 | ||
| Nursing | 15 (15) | 18 (17) | 0.86 (0.391.90) | 0.71 | ||
| Insuranceb | 0.22a | |||||
| Private insurance | 29 (28) | 27 (26) | 1.00 | |||
| Uninsured | 13 (13) | 12 (12) | 1.18 (0.433.26) | 0.74 | ||
| Medicare | 32 (31) | 40 (39) | 0.52 (0.211.29) | 0.16 | ||
| Medicaid | 21 (20) | 12 (12) | 1.81 (0.694.74) | 0.23 | ||
| Tricare/Veterans Administration | 8 (8) | 11 (11) | 0.58 (0.191.79) | 0.34 | ||
| History of DVT | 15 (15) | 15 (15) | 1.00 (0.462.16) | 1.00 | ||
| Malnutritionb | 93 (90) | 79 (77) | 2.86 (1.216.76) | 0.02 | 2.83 (1.037.81) | 0.04 |
| Cancer | 17 (17) | 22 (21) | 0.67 (0.301.48) | 0.32 | ||
| Fluoroscopy | 78 (76) | 85 (83) | 0.65 (0.321.31) | 0.23 | ||
| Anticoagulation use | 29 (28) | 60 (58) | 0.21 (0.100.44) | <0.0001 | 0.17 (0.070.40) | <0.0001 |
| Multilumenc | 64 (62) | 67 (66) | 0.83 (0.461.51) | 0.55 | ||
| Veinb | 0.32a | |||||
| Basilic | 54 (52) | 49 (48) | 1.00 | |||
| Cephalic | 8 (8) | 3 (3) | 2.45 (0.649.32) | 0.19 | ||
| Brachial | 41 (40) | 49 (48) | 0.72 (0.421.24) | 0.24 | ||
| Internal mammary | 0 (0) | 1 (1) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 87 (84) | 100 (98) | 1.00 | 1.00 | ||
| After hours | 16 (16) | 2 (2) | 8.00 (1.8434.79) | 0.006 | 6.93 (1.3535.56) | 0.02 |
| Indication for PICC | 0.13 | |||||
| Infectiond | 52 (50) | 45 (44) | 1.00 | |||
| Pneumonia | 14 (14) | 7 (7) | 1.46 (0.514.18) | 0.48 | ||
| Chemotherapy | 5 (5) | 0 (0) | >999 (<0.001>999) | 0.99 | ||
| IV access | 22 (21) | 43 (42) | 0.48 (0.240.96) | 0.04 | ||
| Total parenteral nutrition | 10 (10) | 8 (8) | 1.08 (0.323.62) | 0.90 | ||
Predictors of Thrombotic Complications
Of 1444 patients who underwent PICC placement, 3% (n=46) were subsequently diagnosed with a catheter‐associated thrombosis, representing 27% of all observed complications. In an attempt to better identify factors predisposing patients to thrombotic complications, an additional subgroup analysis (N=92) was performed on those patients who experienced catheter‐associated thrombosis (N=46) and matched controls (N=46). Variables examined in the analysis included BMI, length of stay (LOS), history of DVT, history of cancer, utilization of anticoagulation/antiplatelet agents, malnutrition, and catheter size.
Based on conditional univariate analyses, the following variables were significantly associated with increased risk of catheter‐associated thrombosis: LOS (as a continuous variable) (OR: 1.04 [95% CI: 1.001.09], P=0.05), malnutrition (OR: 4 [95% CI: 1.1314.18], P=0.03), and after‐hours placement (OR: 8.00 [95% CI: 1.0063.96], P=0.05) (Table 4). Use of anticoagulation/antiplatelet agents (OR: 0.29 [95% CI: 0.11‐0.80], P=0.02) was associated with decreased risk of thrombosis. History of previous DVT and history of cancer were nonsignificant. In the multivariable logistic regression model, malnutrition (OR: 10.16 [95% CI: 1.76‐58.71], P=0.01) remained associated with increased risk of catheter‐associated thrombosis, whereas use of anticoagulation/antiplatelet agents (OR: 0.11 [95% CI: 0.02‐0.51], P=0.005) was associated with decreased risk of catheter‐associated thrombosis (Table 4).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5818 | 5818 | ||||
| BMI, meanSD | 27.77.1 | 27.77.8 | 1.00 (0.931.08) | 0.98 | ||
| 30 | 34 (74) | 33 (72) | ||||
| >30 | 12 (26) | 13 (28) | 0.83 (0.252.73) | 0.76 | ||
| Length of stay, d, meanSD | 1712 | 119 | 1.04 (1.001.09) | 0.05 | ||
| Length of stay group, d | 0.15 | |||||
| <7 | 8 (17) | 14 (30) | 1.00 | |||
| 729 | 29 (63) | 30 (65) | 1.13 (0.413.07) | 0.82 | ||
| 30 | 9 (20) | 2 (4) | 4.65 (0.9822.13) | 0.05 | ||
| Gender | ||||||
| Female | 26 (57) | 26 (57) | ||||
| Male | 20 (43) | 20 (43) | ||||
| Ethnicity | 0.44a | |||||
| Caucasian | 31 (67) | 36 (78) | 1.00 | |||
| African American | 11 (24) | 6 (13) | 2.02 (0.695.93) | 0.20 | ||
| Hispanic/Asian | 4 (9) | 4 (9) | 1.12 (0.225.68) | 0.89 | ||
| Provider team | 0.26a | |||||
| Radiology | 23 (50) | 19 (41) | 1.00 | |||
| Internal medicine | 20 (43) | 18 (39) | 1.00 (0.432.31) | 1.00 | ||
| Nursing | 3(7) | 9 (20) | 0.33 (0.091.27) | 0.11 | ||
| Insuranceb | 0.38a | |||||
| Private insurance | 13 (28) | 11 (24) | 1.00 | |||
| Uninsured | 8 (17) | 4 (9) | 2.01 (0.3810.58) | 0.41 | ||
| Medicare | 14 (30) | 21 (47) | 0.39 (0.101.47) | 0.16 | ||
| Medicaid | 8 (17) | 7 (16) | 1.23 (0.285.36) | 0.78 | ||
| Tricare/Veterans Administration | 3 (7) | 2 (4) | 1.01 (0.128.27) | 1.00 | ||
| History of DVT | 7 (15) | 8 (17) | 0.88 (0.322.41) | 0.80 | ||
| Malnutritionb | 43 (93) | 33 (73) | 4.00 (1.1314.18) | 0.03 | 10.16 (1.7658.71) | 0.01 |
| Cancer | 10 (22) | 13 (28) | 0.67 (0.241.87) | 0.44 | ||
| Fluoroscopy | 33 (72) | 39 (85) | 0.46 (0.161.31) | 0.14 | ||
| Anticoagulation use | 16 (35) | 28 (61) | 0.29 (0.110.80) | 0.02 | 0.11 (0.020.51) | 0.005 |
| Multilumenc | 22 (48) | 28 (62) | 0.53 (0.231.26) | 0.15 | ||
| Veinb | 0.93a | |||||
| Basilic | 24 (52) | 21 (47) | 1.00 | |||
| Cephalic | 1 (2) | 1 (2) | 0.86 (0.0514.39) | 0.92 | ||
| Brachial | 21 (46) | 22 (49) | 0.75 (0.311.79) | 0.51 | ||
| Internal mammary | 0 (0) | 1 (2) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 38 (83) | 44 (98) | 1.00 | |||
| After hours | 8 (17) | 1 (2) | 8.00 (1.0063.96) | 0.05 | ||
| Indication for PICC | 0.80a | |||||
| Infectiond | 20 (43) | 17 (37) | 1.00 | |||
| Pneumonia | 5 (11) | 6 (13) | 0.60 (0.142.56) | 0.49 | ||
| Chemotherapy | 3 (7) | 0 (0) | >999 (<0.001>999) | 0.99 | ||
| IV access | 14 (30) | 20 (43) | 0.58 (0.231.44) | 0.24 | ||
| Total parenteral nutrition | 4 (9) | 3 (7) | 1.22 (0.197.70) | 0.83 | ||
DISCUSSION
The goal of this study was to identify factors related to PICC placement that place the general population of patients at risk. The type and rate of complications associated with PICCs in this study were similar to those previously reported in the literature including catheter‐related infection and thrombosis.[10, 32] Two unique risk factors, not well recognized previously,[10, 27, 28, 33] were observed in this study: malnutrition and after‐hours placement. Malnutrition, defined as serum albumin <3 g/dL was associated with an increase in PICC‐related complications (such as catheter‐associated bloodstream infections and cellulitis) and catheter‐related thrombosis. Malnutrition itself has long been associated with a decreased resistance to infection[34]; in addition, low serum albumin may also be a marker of the presence of other severe comorbidities, which may contribute to increased risk of thrombosis. It has been noted in previous studies that critical illness increases risk of thrombosis.[15] Despite an exhaustive search of the literature, we have been unable to find additional studies examining the extent to which malnutrition may impact PICC‐associated complications.
After‐hours placement was also associated with increased nonmechanical complications, as well as catheter‐related thrombosis. In an effort to improve both patient and consulting provider satisfaction and provide more expedient service, PICCs were often placed after hours (between 5 pm and 8 am) by both interventional radiology (n=14) and internal medicine (n=15) teams.
LOS has been associated with PICC placement complications in other studies.[12] In both primary and secondary analyses, hospital stays >30 days were associated with a higher risk of complications than hospitalizations <7 days. In light of the clinical significance of catheter‐related thrombosis, a subgroup analysis of patients with an LOS >30 days was conducted. The conditional univariate regression analysis showed an increased risk with greater LOS, malnutrition, and after‐hours placement. Use of anticoagulant or antiplatelet agents were associated with decreased risk of thrombosis (Table 4). The association between LOS and PICC‐related thrombosis is consistent with findings from Evans et al. involving 1728 patients in a similar center.[12] In these circumstances, increased LOS may be a surrogate marker for increased severity of illness, in that those patients who are more ill require lengthier hospitalizations. In a systematic review and meta‐analysis, Chopra et al. observed that increased severity of illness correlated with higher rates of catheter‐associated thrombosis, which is supportive of these findings.[15]
In the multivariate logistic regression analysis, BMI >30 was associated with a statistically significant increased risk for PICC‐associated complications after adjusting for anticoagulation and time of placement (Table 2). In the secondary analysis, where patients with mechanical complications were removed, BMI >30 was no longer associated with an increased risk for PICC‐associated complications (Table 3). This suggests that patients with a BMI >30 had an increased risk of mechanical complications, but were not necessarily at increased risk of developing other complications, such as catheter‐related thrombosis, infection, or bleeding. This finding is congruent with studies by Evans et al.,[12] who found no association between BMI and catheter‐associated thrombosis. Our association between BMI and complications is unique; to date, there are few additional studies that examine the extent to which BMI impacts the rate and type of complications associated with PICCs. At this time, the mechanism of the association between mechanical complications (such as inadvertent catheter removal or mechanical malfunction) and BMI is uncertain and warrants further investigation.
Use of Anticoagulant Agents
Anticoagulant (ie, any agent used for DVT prophylaxis or therapeutic anticoagulation) or antiplatelet agent use at the time of PICC placement and during the patient's hospitalization was associated with a decreased risk of thrombosis in our analysis. However, it should be noted that no specific anticoagulant agent was studied, and that antiplatelet agents were included in this analysis, unlike that of Evans et al.[12] Although current literature in oncologic populations, as well as the evidence‐based clinical practice guidelines, recommend against routine use of venous thromboprophylaxis in patients with central venous catheters,[33, 35, 36, 37] we believe this deserves further study, particularly in light of conflicting data in this area.[38, 39] Evans et al.[12] noted that although use of anticoagulants initially appeared to be associated with greater incidence of upper extremity venous thrombosis, when previous diagnosis of DVT was removed from the analysis the association was no longer significant.
In our analyses, no associations between catheter size, choice of venous access, history of previous deep venous thrombosis, or history of malignancy and risk for complications were found. Our findings differed from previous studies, where a relationship between increasing catheter bore size and site of access have been associated with increased PICC‐related thrombosis or other complications.[12, 20, 40, 41] There were also no significant differences in risk for complications between provider teams (eg, internal medicine, radiology, nursing) for PICCs placed during the morning or afternoon, which is consistent with findings by Funk et al.[1] Yet, after‐hours placement of PICCs was associated with greater complications than daytime placement. Although the exploration of factors associated with after‐hours placement was beyond the scope of this study, the findings from this study caused the authors, primarily comprised of members of the internal medicine inpatient medicine division, to reexamine the division's protocol on PICC placement. A consensus decision was made to discontinue after‐hours placement of PICCs by internal medicine teams in an effort to promote patient safety until further data could be collected. As a result, internal medicine teams no longer place PICCs after regular working hours at our institution.
Limitations
Limitations include the categorization of antiplatelet and anticoagulant agents together. We did not distinguish between high‐ and low‐dose aspirin, nor did we distinguish between therapeutic dosing of heparin and low‐molecular‐weight heparin versus DVT prophylaxis dosing. Additionally, for patients who were on warfarin or heparin drip, we did not evaluate for therapeutic range of international normalized ratio or partial thromboplastin time, as this was beyond the present scope of this study. In addition, malnutrition defined by albumin alone may have been somewhat narrow, as conditions aside from malnutrition can impact albumin levels. In future evaluations, this relationship may be clarified by including other determinants of clinical malnutrition including BMI <18 or the measurement of prealbumin. For determination of after‐hours placement of PICCs, we relied upon time of procedure dictation, assuming that all dictations immediately followed catheter placement. If there was a lapse in time between catheter placement and dictation, the category may have been recorded in error. Another limitation of after‐hours categorization was that we were unable to determine whether the PICC was placed on a weekend or holiday.
CONCLUSIONS AND FUTURE DIRECTIONS
Our results suggest that more stringent screening of patients undergoing PICC placement may reduce the risk of complications, with special attention to characteristics such as BMI >30, increased LOS, and protein‐calorie malnutrition (albumin <3). Furthermore, placement of PICC lines in emergent or after‐hours settings should be carefully considered and weighed against relative risks of central venous catheter placement. Further examination of the role anticoagulant and antiplatelet agents may have in the prevention of catheter‐related thrombosis should be undertaken. We hope that the identification of these risk factors will decrease the rate of complications and ultimately enhance patient safety and satisfaction.
Acknowledgments
The authors sincerely thank Glen Cryer, Publications Manager, Baylor Scott & White Health, for his assistance with this article.
Disclosures: Nothing to report.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22:377–379.
- , , . Peripherally inserted central catheters in the intensive care unit. J Intensive Care Med. 1996;11:49–54.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154:1833–1837.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78:26–30.
- , . Peripherally inserted central catheters: a report of 2506 catheter days. JPEN J Parenter Enteral Nutr. 1995;19:133–136.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72:225–233.
- , , . Bedside placement of peripherally inserted central catheters: a cost‐effectiveness analysis. Radiology. 1998;206:423–428.
- , , , . Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271:223–225.
- , . Experience with PICC at a university medical center. J Intraven Nurs. 1997;20:141–147.
- , , , et al. Upper‐extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57–62.
- , , , . Previous PICC placement may be associated with catheter‐related infections in hemodialysis patients. Cardiovasc Intervent Radiol. 2011;34:120–123.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138:803–810.
- . Be picky about PICCs. ACP Hospitalist, American College of Physicians website. Available at: http://www.acphospitalist.org/archives/2013/09/coverstory.htm. Accessed January 4, 2014.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34:908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382:311–325.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based practice guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15:454–460.
- , , , , , . Peripherally inserted central catheter thrombosis‐reverse tapered versus nontapered catheters: a randomized controlled study. J Vasc Interv Radiol. 2014;25:85–91.
- , , , et al. Upper extremity venous thrombosis diagnosed by duplex scanning. Am J Surg. 1990;160:202–206.
- , , , et al. Reduction of peripherally inserted central catheter‐associated DVT. Chest. 2013;143:627–633.
- . Preventing DVT in peripherally inserted central catheters. Chest. 2013;143:589–590.
- , , , et al. Incidence of upper limb venous thrombosis associated with peripherally inserted central catheters (PICC). Br J Radiol. 2005;78:596–600.
- , , , , , . Computer identification of symptomatic deep venous thrombosis associated with peripherally inserted central catheters. AMIA Annu Symp Proc. 2007:226–230.
- , . Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11:837–884.
- , , , , . To clot or not to clot? That is the question in central venous catheters. Clin Radiol. 2004;59:349–355.
- , , , et al. The Incidence of PICC line–associated thrombosis with and without the use of prophylactic anticoagulants. JPEN J Parenter Enteral Nutr. 2008;32:443–447.
- , , , , . Efficacy of multifaceted interventions in reducing complications of peripherally inserted central catheter in adult oncology patients. Support Care Cancer. 2010;18:1293–1298.
- , , , , . Peripherally inserted central venous catheter‐associated thrombosis: retrospective analysis of clinical risk factors in adult patients. South Med J. 2006;99:1073–1077.
- . The Seldinger method for PICC insertion. J Intraven Nurs. 1989;12:238–243.
- , , , et al. Venous thrombosis associated with the placement of peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11:1309–1314.
- . Protein‐energy undernutrition. Merk Manual of Diagnosis and Therapy. 18th ed. Available at: http://www.merckmanuals.com/professional/nutritional_disorders/undernutrition/protein‐energy_undernutrition.html. Accessed May 26, 2013.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4:417–422.
- , , , . Risk factors for upper extremity venous thrombosis associated with peripherally inserted central venous catheters. J Vasc Access. 2012;13:231–238.
- . Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr. 2003;133:316S–321S.
- , , , et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20:1459–1471.
- , , , , . Thromboprophylaxis in cancer patients with central venous catheters. A systematic review and meta‐analysis. Thromb Haemost. 2008;99:38–43.
- , , , . Thromboprophylaxis for catheter‐related thrombosis in patients with cancer: a systematic review of the randomized, controlled trials. J Thromb Haemost. 2007;5:2552–2554.
- , , , et al. Prevention of central venous catheter associated thrombosis using minidose warfarin in patients with haematological malignancies. Br J Haematol. 1998;10:483–486.
- , , , et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112:423–428.
- , , , , , . The peripherally inserted central catheter (PICC): a prospective study of its natural history after cubital fossa insertion. Anesth Intensive Care. 2002;30:21–24.
- . Thrombotic complications in intravenous access. J Intraven Nurs. 1998;21:96–100.
Peripherally inserted central venous catheters (PICCs) are used for a variety of indications, including administration of long‐term intravenous (IV) antibiotics, home IV medications, chemotherapy, and parenteral nutrition.[1, 2, 3] Additionally, PICCs have also been recognized as an alternative to large‐bore central venous catheters such as subclavian or internal jugular central venous catheters. PICCs have been associated with fewer bloodstream infections in patients with cancer than tunneled catheters.[4] Compared to central venous catheters, they demonstrate reduced complication rates,[5] decreased cost,[6] and increased safety for longer durations of use.[1, 2, 3, 7, 8, 9]
Despite the numerous benefits of PICCs, Prandoni et al. estimate an all‐cause complication rate of 12% to 17% with the use of PICCs.[10] Associated complications include infection,[11] pain, bleeding, and mechanical dysfunction, all of which contribute to patient discomfort and additional healthcare costs.[12] Bloodstream infections, for example, had previously been thought to occur at a substantially lower rate in PICCs than central venous catheters.[13] However, a recent systematic review suggests the rate of PICC‐associated bloodstream infections in the inpatient setting is actually comparable to that of central venous catheters.[14] Perhaps the most serious PICC‐associated complication is catheter‐related venous thrombosis. A recent systematic review and meta‐analysis found evidence to suggest the rate of catheter‐related venous thrombosis was highest in patients with cancer or critical illness15; additionally, rates of thrombosis associated with PICCs were higher than those associated with subclavian or internal jugular central venous catheters.[15, 16] Fletcher et al. showed an 8.1% incidence of symptomatic PICC‐related upper extremity deep vein thrombosis (DVT) in the neurosurgical intensive care unit, with 15% of patients subsequently developing a pulmonary embolism.[17] A recent prospective, randomized controlled trial by Itkin et al. similarly demonstrated symptomatic DVT rates of approximately 4%.[18] However, in this study, when PICCs were routinely screened for thrombosis (with or without associated symptoms), approximately 72% demonstrated thrombosis,[18] suggesting that many PICC‐associated thromboses may be clinically undetected. This may have far‐reaching clinical significance, as pulmonary embolism complicates upper extremity DVT in 9% of cases and can result in a mortality rate as high as 25%.[10, 19]
Some strategies to reduce the rate of catheter‐related complications include identification of characteristics that put patients at risk. Many potential risk factors have been investigated, including catheter size,[12, 20, 21, 22, 23, 24] choice of vein,[24] location of catheter tip,[25] and history of malignancy or prior DVT.[12] However, to date, no definitive consensus has been reached. Special attention has been paid to the investigation of underlying risk factors and treatment for catheter‐related DVT, given its significant morbidity and mortality. Results have been equivocal, though, and in some instances, complicated by a diagnosis of underlying malignancy.[26, 27, 28]
As PICCs become more widely utilized, assessments of factors that place patients at greater risk of PICC‐related complications are needed.[21] The purpose of this study was to establish the incidence of complications associated with PICCs placed in the inpatient setting and examine risk factors predisposing patients to these complications.
MATERIALS AND METHODS
Study Design
A case control analysis of adult inpatients who underwent PICC placement between January 2009 and January 2010 was conducted at Scott & White Healthcare (now Baylor Scott & White Healthcare) to determine the incidence and risk factors for PICC‐associated complications.
Study Site
The study took place at Scott & White Memorial Hospital in Temple, Texas, a 636‐bed multispecialty teaching hospital and level 1 trauma center. It is part of a healthcare system that includes 12 hospitals and more than 60 regional clinics, all of which share an electronic medical record to enable full integration.
Human Subjects Approval
This study received approval from the institutional review board at Scott & White Healthcare.
PICC Placement Technique
Inpatient PICC placement was performed by the PICC consult service. The consult service was comprised of 3 separate provider teams: (1) internal medicine, including select hospitalists and internal medicine residents; (2) radiology, including interventional radiologists and radiology residents; and (3) nursing, including registered nurses with advanced training in PICC placement. Following placement of a consult, the PICC consult service assessed the patient, obtained consent, and subsequently placed the catheter. Members of the PICC consult service followed a system‐wide protocol wherein target veins were identified by ultrasound prior to attempting catheter placement, and actual placement of the PICC was ultrasound guided. Images obtained during the procedure were permanently documented in the medical record. At the time of this study, no formal protocol existed wherein target veins were mapped for caliber. Operators relied on their professional judgment to determine if vein caliber appeared sufficient to accommodate catheter placement.
All PICCs were placed using industry standard sterile precautions. A universally accepted modified Seldinger technique was used to obtain venous access.[29] A guidewire was then positioned in the desired vessel to facilitate proper venous placement of the catheter. During the course of the study period, catheters used were either single‐ (4 Fr) or double lumen (5 Fr).
Catheters were placed at the bedside by hospitalists or registered nurse teams; the location of the catheter tip at the cavoatrial junction was confirmed by chest radiography. Catheter insertions by radiologists were performed in the interventional radiology suite, and confirmation of location of the catheter tip was obtained with fluoroscopy.
PICC Maintenance
Following placement, nurses managed the PICC site according to nursing policy. Per policy, the site was assessed each shift. Documentation of assessment was recorded in nursing notes. Routine dressing changes were performed every 7 days, and as needed, to maintain a sterile site. Date and time of dressing changes were documented in nursing notes and on the PICC dressing. Catheter hubs and injection ports were disinfected with an antiseptic preparation for 15 seconds and allowed to air dry for 30 seconds prior to accessing the catheter. Catheters were flushed with 10 mL of normal saline before and after use. Any abnormality noted during PICC assessment was relayed to the primary provider. If the catheter did not flush readily or demonstrate appropriate blood return, nursing staff obtained an order for alteplase to be administered in an effort to salvage the line. PICCs were discontinued at the discretion of the healthcare provider.
Participants
Records of all patients 18 years of age and older who underwent PICC placement between January 2009 and January 2010 were reviewed (N=1444) for study inclusion. There were no exclusion criteria.
Data Collection
Patients who experienced complications were identified by electronic medical record review. One‐to‐one matching was performed for age and gender‐matched controls randomly selected from inpatients who underwent PICC placement during the same time period without complications. A total of 170 cases with PICC‐related complications were identified. One hundred seventy exact age‐ and gender‐matched controls, who based upon documentation available in the electronic medical record did not experience complications, were then randomly selected. Prior to data collection, the research team reviewed and discussed the data collection form and agreed upon a standardized protocol for data collection. Data collection was completed by authors J.M. and J.H. on the standardized data collection form. Although a formal analysis of inter‐rater agreement was not performed, J.M. and J.H. discussed any items where questions arose and arrived at a consensus decision regarding completion of the data point.
End points of the chart review were completion of medical therapy for which the PICC was indicated (eg, IV antibiotics or total parenteral nutrition [TPN]) or documentation of a complication that led to 1 of the following: discontinuation of the PICC or adjustment of either catheter placement or medical therapy. All complications were identified via International Classification of Diseases, 9th Revision codes and systematic chart review.
Complications resulting in discontinuation of the PICC, adjustment of catheter placement, or change in medical therapy were identified by review of nursing or physician documentation, and were categorized as follows: mechanical complications (defined as loss of the ability of the catheter to flush or draw properly, inadvertent catheter dislodgement, or retained portion of the catheter following catheter removal), catheter‐associated bloodstream infection (development of a positive blood culture attributable to the central catheter with no other clearly identifiable source of bacteremia present), cellulitis (defined as cellulitis in the extremity where the catheter was placed), bleeding from the site of catheter, fever (for which no other cause could be identified), and catheter‐associated thrombosis (identified by Doppler ultrasonography in patients exhibiting symptoms such as pain, swelling, redness, or warmth in the extremity in which the PICC was placed).[30]
Demographic data were collected, including insurance status, age, ethnicity, and gender. Clinical data included body mass index (BMI), presence of malnutrition (defined by a serum albumin of less than 3 g/dL),[31] previous or active cancer, previous DVT, use of anticoagulants (eg, warfarin, heparin, or low‐molecular‐weight heparin) or antiplatelet agent (eg, aspirin or clopidogrel) at the time of placement, and indication for PICC placement. A patient's history of previous or active cancer and previous DVT were identified by clinical documentation. Indications for PICC placement included: treating infectious processes (ie, infusion of antimicrobials), providing TPN, chemotherapy administration, and IV access. Catheter‐specific data were also collected and included venous access obtained (cephalic, basilic, brachial), catheter size (single lumen [4 Fr] or double lumen [5 Fr]), type of complication, and time to complication. The procedure note accompanying PICC placement was reviewed for data regarding time of day inserted (with after hours defined as documentation of placement occurring after 5 pm), and procedure operator to identify type of team (internal medicine, radiology, nursing) responsible for placement.
Data Analysis
Demographic characteristics and potential risk factors for patients in both the case and control groups of the study were summarized using descriptive statistics: mean ( standard deviation [SD]) for continuous variables and frequency (percent) for categorical variables. Univariate and multivariable conditional logistic regression analyses of variables that were potential risk factors of PICC‐related complications were utilized. A stepwise selection method was used for multivariable conditional logistic regression models. Alpha=0.2 was used for the significance to enter the model, and =0.05 was used for significance level to remain in the model. Attribution of PICC‐related complications was evaluated in terms of odds ratios (OR) and 95% confidence interval (CI). A P value of <0.05 indicated statistical significance. No prospective power analysis was performed. However, for a retrospective power analysis for 1:1 matching with 170 cases and 170 matched controls, assuming 20% of controls were affected and an of 0.05, one would achieve 80% power to detect an odds ratio of 2. SAS 9.2 (SAS Institute Inc., Cary, NC) was used for data analysis.
RESULTS
In 2009, 1444 PICCs were placed, and 170 cases in which patients experienced complications associated with PICC placement were identified, resulting in a complication rate of 11.77% (95% CI: 10.11%‐13.44%). The most common complications experienced by our patient population included catheter‐associated thrombosis (3%, n = 46), mechanical complications (4%, n=67), inadvertent catheter dislodgement (2%, n=36), mechanical dysfunction (2%, n=30), retained portion of the catheter following catheter removal (<1%, n=1), catheter‐associated bloodstream infections (2%, n=24), and cellulitis at the catheter insertion site (1%, n=15). Other documented complications included unexplained fever and bleeding (Table 1).
| Complication | N (%) |
|---|---|
| |
| Thrombosis | 46 (3) |
| Infection | 24 (2) |
| Cellulitis | 15 (1) |
| Mechanical complications* | 67 (4) |
| Unexplained fever | 15 (1) |
| Bleeding | 3 (0) |
| No complication | 1,274 (88) |
The mean age of the total cohort (N=340), comprised of case (N=170) and control (N=170) groups, was 58 years (SD 17), and 55% (n=94) were females. There were no significant differences in complications between groups based on ethnicity (P=0.66). In the case group, 46% (n=78) of PICCs were placed by the radiology team, 41% (n=69) were placed by the internal medicine team, and 14% (n=23) were placed by nursing. In the control group, 44% (n=74) of PICCs were placed by radiology, 36% (n=62) by internal medicine, and 20% (n=34) by nursing. Based on univariate conditional analysis, provider team was not significantly associated with complications (P=0.29).
Predictors of All‐Cause Complications
Based upon univariate conditional logistic regression analyses of complications related to PICC placement (N=340), the following variables demonstrated a statistically significant increased risk for complications: malnutrition (OR: 1.88 [95% CI: 1.023.44], P=0.04) and after‐hours placement (OR: 8.67 [95% CI: 2.62‐28.63], P=0.0004) (Table 2). Anticoagulation was associated with a decreased risk of complications (OR: 0.27 [95% CI: 0.16‐0.45], P=0.04). Based upon multivariable logistic regression analysis, after‐hours placement (OR: 9.52 [95% CI: 2.68‐33.78], P=0.0005) and BMI >30 (OR: 1.98 [95% CI: 1.09‐3.61], P=0.02) were significantly associated with an increased risk of PICC‐associated complications. Conversely, anticoagulation/antiplatelet use was associated with a decreased risk of complications (OR: 0.24 [95% CI: 0.14‐0.43], P<0.0001).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5817 | 5817 | ||||
| BMI, meanSD | 29.29.5 | 27.97.9 | 1.02 (0.991.05) | 0.12 | ||
| 30 | 108 (64) | 116 (68%) | 1.00 | 1.00 | ||
| >30 | 62 (36) | 54 (32%) | 1.29 (0.792.11) | 0.32 | 1.98 (1.093.61) | 0.02 |
| Length of stay, d, meanSD | 1822 | 1416 | 1.01 (1.001.03) | 0.06 | ||
| Length of stay group, d | 0.11a | |||||
| <7 | 41 (24) | 52 (31) | 1.00 | |||
| 729 | 101 (59) | 103 (61) | 1.19 (0.721.98) | 0.49 | ||
| 30 | 28 (16) | 15 (9) | 2.21 (1.074.58) | 0.03 | ||
| Gender | ||||||
| Female | 94 (55) | 94 (55) | ||||
| Male | 76 (45) | 76 (45) | ||||
| Ethnicity | 0.66a | |||||
| Caucasian | 131 (77) | 125 (74) | 1.00 | |||
| African American | 26 (15) | 28 (16) | 0.88 (0.481.60) | 0.67 | ||
| Hispanic/Asian | 13 (8) | 17 (10) | 0.70 (0.311.58) | 0.38 | ||
| Provider team | 0.29a | |||||
| Radiology | 78 (46) | 74 (44) | 1.00 | |||
| Internal medicine | 69 (41) | 62 (36) | 1.05 (0.681.64) | 0.82 | ||
| Nursing | 23 (14) | 34 (20) | 0.65 (0.351.19) | 0.16 | ||
| Insuranceb | 0.22a | |||||
| Private insurance | 46 (27) | 42 (25) | 1.00 | |||
| Uninsured | 17 (10) | 24 (14) | 0.73 (0.351.55) | 0.41 | ||
| Medicare | 57 (34) | 62 (37) | 0.73 (0.381.40) | 0.34 | ||
| Medicaid | 39 (23) | 25 (15) | 1.51 (0.743.06) | 0.26 | ||
| Tricare/Veterans Administration | 11 (6) | 16 (9) | 0.59 (0.241.45) | 0.25 | ||
| History of DVT | 27 (16) | 26 (15) | 1.05 (0.581.91) | 0.88 | ||
| Malnutritionb | 149 (88) | 134 (79) | 1.88 (1.023.44) | 0.04 | ||
| Cancer | 25 (15) | 36 (21) | 0.58 (0.311.09) | 0.09 | ||
| Fluoroscopy | 129 (76) | 139 (82) | 0.71 (0.421.19) | 0.19 | ||
| Anticoagulation use | 50 (29) | 100 (59) | 0.27 (0.160.45) | <0.0001 | 0.24 (0.140.43) | <0.0001 |
| Multilumenc | 99 (58) | 111 (66) | 0.70 (0.441.11) | 0.13 | ||
| Veinb | 0.39a | |||||
| Basilic | 98 (58) | 86 (51) | 1.00 | |||
| Cephalic | 11 (6) | 8 (5) | 1.37 (0.483.89) | 0.55 | ||
| Brachial | 61 (36) | 74 (44) | 0.70 (0.451.09) | 0.12 | ||
| Internal mammary | 0 (0) | 1 (1) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 144 (85) | 166 (98) | 1.00 | 1.00 | ||
| After hours | 26 (15) | 3 (2) | 8.67 (2.6228.63) | 0.0004 | 9.52 (2.6833.78) | 0.0005 |
| Indication for PICC | 0.02a | |||||
| Infection | 88 (52) | 71 (42) | 1.00 | |||
| Pneumonia | 21 (12) | 14 (8) | 1.07 (0.502.29) | 0.87 | ||
| Chemotherapy | 5 (3) | 2 (1) | 1.84 (0.349.93) | 0.48 | ||
| IV access | 36 (21) | 66 (39) | 0.44 (0.250.75) | 0.003 | ||
| Total parenteral nutrition | 20 (12) | 17 (10) | 0.96 (0.442.14) | 0.93 | ||
Predictors of Nonmechanical Complications
To study risk factors related to nonmechanical complications, a secondary analysis (N=206) was performed in which all patients who experienced mechanical complications (N=67) and matched controls (N=67) were excluded. Based upon multivariable logistic regression analysis, after‐hours placement (OR: 6.93 [95% CI: 1.35‐35.56], P=0.02) and malnutrition (OR: 2.83 [95% CI: 1.037.81], P=0.04) were significantly associated with increased risk of nonmechanical complications. The use of anticoagulation/antiplatelet agents was associated with decreased risk of nonmechanical complications (OR: 0.17 [95% CI: 0.07‐0.40], P<0.0001). Variables not significantly associated with nonmechanical complications included BMI>30, previous history of DVT, history of cancer, catheter size, and venous access choice (Table 3).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5816 | 5816 | ||||
| BMI, meanSD | 29.79.8 | 28.57.9 | 1.03 (0.991.07) | 0.22 | ||
| 30 | 64 (62) | 68 (66) | 1.00 | |||
| >30 | 39 (38) | 35 (34) | 1.27 (0.642.49) | 0.49 | ||
| Length of stay, d, meanSD | 2026 | 1418 | 1.02 (1.001.03) | 0.08 | ||
| Length of stay group, d | 0.03a | |||||
| <7 | 22 (21) | 28 (27) | 1.00 | |||
| 729 | 60 (58) | 68 (66) | 0.95 (0.491.82) | 0.87 | ||
| 30 | 21 (20) | 7 (7) | 3.24 (1.238.54) | 0.02 | ||
| Gender | ||||||
| Female | 63 (61) | 63 (61) | ||||
| Male | 40 (39) | 40 (39) | ||||
| Ethnicity | 0.95a | |||||
| Caucasian | 75 (73) | 75 (73) | 1.00 | |||
| African American | 19 (18) | 18 (17) | 1.06 (0.512.21) | 0.87 | ||
| Hispanic/Asian | 9 (9) | 10 (10) | 0.88 (0.322.44) | 0.81 | ||
| Provider team | 0.81a | |||||
| Radiology | 43 (42) | 44 (43) | 1.00 | |||
| Internal medicine | 45 (44) | 41 (40) | 1.11 (0.621.96) | 0.73 | ||
| Nursing | 15 (15) | 18 (17) | 0.86 (0.391.90) | 0.71 | ||
| Insuranceb | 0.22a | |||||
| Private insurance | 29 (28) | 27 (26) | 1.00 | |||
| Uninsured | 13 (13) | 12 (12) | 1.18 (0.433.26) | 0.74 | ||
| Medicare | 32 (31) | 40 (39) | 0.52 (0.211.29) | 0.16 | ||
| Medicaid | 21 (20) | 12 (12) | 1.81 (0.694.74) | 0.23 | ||
| Tricare/Veterans Administration | 8 (8) | 11 (11) | 0.58 (0.191.79) | 0.34 | ||
| History of DVT | 15 (15) | 15 (15) | 1.00 (0.462.16) | 1.00 | ||
| Malnutritionb | 93 (90) | 79 (77) | 2.86 (1.216.76) | 0.02 | 2.83 (1.037.81) | 0.04 |
| Cancer | 17 (17) | 22 (21) | 0.67 (0.301.48) | 0.32 | ||
| Fluoroscopy | 78 (76) | 85 (83) | 0.65 (0.321.31) | 0.23 | ||
| Anticoagulation use | 29 (28) | 60 (58) | 0.21 (0.100.44) | <0.0001 | 0.17 (0.070.40) | <0.0001 |
| Multilumenc | 64 (62) | 67 (66) | 0.83 (0.461.51) | 0.55 | ||
| Veinb | 0.32a | |||||
| Basilic | 54 (52) | 49 (48) | 1.00 | |||
| Cephalic | 8 (8) | 3 (3) | 2.45 (0.649.32) | 0.19 | ||
| Brachial | 41 (40) | 49 (48) | 0.72 (0.421.24) | 0.24 | ||
| Internal mammary | 0 (0) | 1 (1) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 87 (84) | 100 (98) | 1.00 | 1.00 | ||
| After hours | 16 (16) | 2 (2) | 8.00 (1.8434.79) | 0.006 | 6.93 (1.3535.56) | 0.02 |
| Indication for PICC | 0.13 | |||||
| Infectiond | 52 (50) | 45 (44) | 1.00 | |||
| Pneumonia | 14 (14) | 7 (7) | 1.46 (0.514.18) | 0.48 | ||
| Chemotherapy | 5 (5) | 0 (0) | >999 (<0.001>999) | 0.99 | ||
| IV access | 22 (21) | 43 (42) | 0.48 (0.240.96) | 0.04 | ||
| Total parenteral nutrition | 10 (10) | 8 (8) | 1.08 (0.323.62) | 0.90 | ||
Predictors of Thrombotic Complications
Of 1444 patients who underwent PICC placement, 3% (n=46) were subsequently diagnosed with a catheter‐associated thrombosis, representing 27% of all observed complications. In an attempt to better identify factors predisposing patients to thrombotic complications, an additional subgroup analysis (N=92) was performed on those patients who experienced catheter‐associated thrombosis (N=46) and matched controls (N=46). Variables examined in the analysis included BMI, length of stay (LOS), history of DVT, history of cancer, utilization of anticoagulation/antiplatelet agents, malnutrition, and catheter size.
Based on conditional univariate analyses, the following variables were significantly associated with increased risk of catheter‐associated thrombosis: LOS (as a continuous variable) (OR: 1.04 [95% CI: 1.001.09], P=0.05), malnutrition (OR: 4 [95% CI: 1.1314.18], P=0.03), and after‐hours placement (OR: 8.00 [95% CI: 1.0063.96], P=0.05) (Table 4). Use of anticoagulation/antiplatelet agents (OR: 0.29 [95% CI: 0.11‐0.80], P=0.02) was associated with decreased risk of thrombosis. History of previous DVT and history of cancer were nonsignificant. In the multivariable logistic regression model, malnutrition (OR: 10.16 [95% CI: 1.76‐58.71], P=0.01) remained associated with increased risk of catheter‐associated thrombosis, whereas use of anticoagulation/antiplatelet agents (OR: 0.11 [95% CI: 0.02‐0.51], P=0.005) was associated with decreased risk of catheter‐associated thrombosis (Table 4).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5818 | 5818 | ||||
| BMI, meanSD | 27.77.1 | 27.77.8 | 1.00 (0.931.08) | 0.98 | ||
| 30 | 34 (74) | 33 (72) | ||||
| >30 | 12 (26) | 13 (28) | 0.83 (0.252.73) | 0.76 | ||
| Length of stay, d, meanSD | 1712 | 119 | 1.04 (1.001.09) | 0.05 | ||
| Length of stay group, d | 0.15 | |||||
| <7 | 8 (17) | 14 (30) | 1.00 | |||
| 729 | 29 (63) | 30 (65) | 1.13 (0.413.07) | 0.82 | ||
| 30 | 9 (20) | 2 (4) | 4.65 (0.9822.13) | 0.05 | ||
| Gender | ||||||
| Female | 26 (57) | 26 (57) | ||||
| Male | 20 (43) | 20 (43) | ||||
| Ethnicity | 0.44a | |||||
| Caucasian | 31 (67) | 36 (78) | 1.00 | |||
| African American | 11 (24) | 6 (13) | 2.02 (0.695.93) | 0.20 | ||
| Hispanic/Asian | 4 (9) | 4 (9) | 1.12 (0.225.68) | 0.89 | ||
| Provider team | 0.26a | |||||
| Radiology | 23 (50) | 19 (41) | 1.00 | |||
| Internal medicine | 20 (43) | 18 (39) | 1.00 (0.432.31) | 1.00 | ||
| Nursing | 3(7) | 9 (20) | 0.33 (0.091.27) | 0.11 | ||
| Insuranceb | 0.38a | |||||
| Private insurance | 13 (28) | 11 (24) | 1.00 | |||
| Uninsured | 8 (17) | 4 (9) | 2.01 (0.3810.58) | 0.41 | ||
| Medicare | 14 (30) | 21 (47) | 0.39 (0.101.47) | 0.16 | ||
| Medicaid | 8 (17) | 7 (16) | 1.23 (0.285.36) | 0.78 | ||
| Tricare/Veterans Administration | 3 (7) | 2 (4) | 1.01 (0.128.27) | 1.00 | ||
| History of DVT | 7 (15) | 8 (17) | 0.88 (0.322.41) | 0.80 | ||
| Malnutritionb | 43 (93) | 33 (73) | 4.00 (1.1314.18) | 0.03 | 10.16 (1.7658.71) | 0.01 |
| Cancer | 10 (22) | 13 (28) | 0.67 (0.241.87) | 0.44 | ||
| Fluoroscopy | 33 (72) | 39 (85) | 0.46 (0.161.31) | 0.14 | ||
| Anticoagulation use | 16 (35) | 28 (61) | 0.29 (0.110.80) | 0.02 | 0.11 (0.020.51) | 0.005 |
| Multilumenc | 22 (48) | 28 (62) | 0.53 (0.231.26) | 0.15 | ||
| Veinb | 0.93a | |||||
| Basilic | 24 (52) | 21 (47) | 1.00 | |||
| Cephalic | 1 (2) | 1 (2) | 0.86 (0.0514.39) | 0.92 | ||
| Brachial | 21 (46) | 22 (49) | 0.75 (0.311.79) | 0.51 | ||
| Internal mammary | 0 (0) | 1 (2) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 38 (83) | 44 (98) | 1.00 | |||
| After hours | 8 (17) | 1 (2) | 8.00 (1.0063.96) | 0.05 | ||
| Indication for PICC | 0.80a | |||||
| Infectiond | 20 (43) | 17 (37) | 1.00 | |||
| Pneumonia | 5 (11) | 6 (13) | 0.60 (0.142.56) | 0.49 | ||
| Chemotherapy | 3 (7) | 0 (0) | >999 (<0.001>999) | 0.99 | ||
| IV access | 14 (30) | 20 (43) | 0.58 (0.231.44) | 0.24 | ||
| Total parenteral nutrition | 4 (9) | 3 (7) | 1.22 (0.197.70) | 0.83 | ||
DISCUSSION
The goal of this study was to identify factors related to PICC placement that place the general population of patients at risk. The type and rate of complications associated with PICCs in this study were similar to those previously reported in the literature including catheter‐related infection and thrombosis.[10, 32] Two unique risk factors, not well recognized previously,[10, 27, 28, 33] were observed in this study: malnutrition and after‐hours placement. Malnutrition, defined as serum albumin <3 g/dL was associated with an increase in PICC‐related complications (such as catheter‐associated bloodstream infections and cellulitis) and catheter‐related thrombosis. Malnutrition itself has long been associated with a decreased resistance to infection[34]; in addition, low serum albumin may also be a marker of the presence of other severe comorbidities, which may contribute to increased risk of thrombosis. It has been noted in previous studies that critical illness increases risk of thrombosis.[15] Despite an exhaustive search of the literature, we have been unable to find additional studies examining the extent to which malnutrition may impact PICC‐associated complications.
After‐hours placement was also associated with increased nonmechanical complications, as well as catheter‐related thrombosis. In an effort to improve both patient and consulting provider satisfaction and provide more expedient service, PICCs were often placed after hours (between 5 pm and 8 am) by both interventional radiology (n=14) and internal medicine (n=15) teams.
LOS has been associated with PICC placement complications in other studies.[12] In both primary and secondary analyses, hospital stays >30 days were associated with a higher risk of complications than hospitalizations <7 days. In light of the clinical significance of catheter‐related thrombosis, a subgroup analysis of patients with an LOS >30 days was conducted. The conditional univariate regression analysis showed an increased risk with greater LOS, malnutrition, and after‐hours placement. Use of anticoagulant or antiplatelet agents were associated with decreased risk of thrombosis (Table 4). The association between LOS and PICC‐related thrombosis is consistent with findings from Evans et al. involving 1728 patients in a similar center.[12] In these circumstances, increased LOS may be a surrogate marker for increased severity of illness, in that those patients who are more ill require lengthier hospitalizations. In a systematic review and meta‐analysis, Chopra et al. observed that increased severity of illness correlated with higher rates of catheter‐associated thrombosis, which is supportive of these findings.[15]
In the multivariate logistic regression analysis, BMI >30 was associated with a statistically significant increased risk for PICC‐associated complications after adjusting for anticoagulation and time of placement (Table 2). In the secondary analysis, where patients with mechanical complications were removed, BMI >30 was no longer associated with an increased risk for PICC‐associated complications (Table 3). This suggests that patients with a BMI >30 had an increased risk of mechanical complications, but were not necessarily at increased risk of developing other complications, such as catheter‐related thrombosis, infection, or bleeding. This finding is congruent with studies by Evans et al.,[12] who found no association between BMI and catheter‐associated thrombosis. Our association between BMI and complications is unique; to date, there are few additional studies that examine the extent to which BMI impacts the rate and type of complications associated with PICCs. At this time, the mechanism of the association between mechanical complications (such as inadvertent catheter removal or mechanical malfunction) and BMI is uncertain and warrants further investigation.
Use of Anticoagulant Agents
Anticoagulant (ie, any agent used for DVT prophylaxis or therapeutic anticoagulation) or antiplatelet agent use at the time of PICC placement and during the patient's hospitalization was associated with a decreased risk of thrombosis in our analysis. However, it should be noted that no specific anticoagulant agent was studied, and that antiplatelet agents were included in this analysis, unlike that of Evans et al.[12] Although current literature in oncologic populations, as well as the evidence‐based clinical practice guidelines, recommend against routine use of venous thromboprophylaxis in patients with central venous catheters,[33, 35, 36, 37] we believe this deserves further study, particularly in light of conflicting data in this area.[38, 39] Evans et al.[12] noted that although use of anticoagulants initially appeared to be associated with greater incidence of upper extremity venous thrombosis, when previous diagnosis of DVT was removed from the analysis the association was no longer significant.
In our analyses, no associations between catheter size, choice of venous access, history of previous deep venous thrombosis, or history of malignancy and risk for complications were found. Our findings differed from previous studies, where a relationship between increasing catheter bore size and site of access have been associated with increased PICC‐related thrombosis or other complications.[12, 20, 40, 41] There were also no significant differences in risk for complications between provider teams (eg, internal medicine, radiology, nursing) for PICCs placed during the morning or afternoon, which is consistent with findings by Funk et al.[1] Yet, after‐hours placement of PICCs was associated with greater complications than daytime placement. Although the exploration of factors associated with after‐hours placement was beyond the scope of this study, the findings from this study caused the authors, primarily comprised of members of the internal medicine inpatient medicine division, to reexamine the division's protocol on PICC placement. A consensus decision was made to discontinue after‐hours placement of PICCs by internal medicine teams in an effort to promote patient safety until further data could be collected. As a result, internal medicine teams no longer place PICCs after regular working hours at our institution.
Limitations
Limitations include the categorization of antiplatelet and anticoagulant agents together. We did not distinguish between high‐ and low‐dose aspirin, nor did we distinguish between therapeutic dosing of heparin and low‐molecular‐weight heparin versus DVT prophylaxis dosing. Additionally, for patients who were on warfarin or heparin drip, we did not evaluate for therapeutic range of international normalized ratio or partial thromboplastin time, as this was beyond the present scope of this study. In addition, malnutrition defined by albumin alone may have been somewhat narrow, as conditions aside from malnutrition can impact albumin levels. In future evaluations, this relationship may be clarified by including other determinants of clinical malnutrition including BMI <18 or the measurement of prealbumin. For determination of after‐hours placement of PICCs, we relied upon time of procedure dictation, assuming that all dictations immediately followed catheter placement. If there was a lapse in time between catheter placement and dictation, the category may have been recorded in error. Another limitation of after‐hours categorization was that we were unable to determine whether the PICC was placed on a weekend or holiday.
CONCLUSIONS AND FUTURE DIRECTIONS
Our results suggest that more stringent screening of patients undergoing PICC placement may reduce the risk of complications, with special attention to characteristics such as BMI >30, increased LOS, and protein‐calorie malnutrition (albumin <3). Furthermore, placement of PICC lines in emergent or after‐hours settings should be carefully considered and weighed against relative risks of central venous catheter placement. Further examination of the role anticoagulant and antiplatelet agents may have in the prevention of catheter‐related thrombosis should be undertaken. We hope that the identification of these risk factors will decrease the rate of complications and ultimately enhance patient safety and satisfaction.
Acknowledgments
The authors sincerely thank Glen Cryer, Publications Manager, Baylor Scott & White Health, for his assistance with this article.
Disclosures: Nothing to report.
Peripherally inserted central venous catheters (PICCs) are used for a variety of indications, including administration of long‐term intravenous (IV) antibiotics, home IV medications, chemotherapy, and parenteral nutrition.[1, 2, 3] Additionally, PICCs have also been recognized as an alternative to large‐bore central venous catheters such as subclavian or internal jugular central venous catheters. PICCs have been associated with fewer bloodstream infections in patients with cancer than tunneled catheters.[4] Compared to central venous catheters, they demonstrate reduced complication rates,[5] decreased cost,[6] and increased safety for longer durations of use.[1, 2, 3, 7, 8, 9]
Despite the numerous benefits of PICCs, Prandoni et al. estimate an all‐cause complication rate of 12% to 17% with the use of PICCs.[10] Associated complications include infection,[11] pain, bleeding, and mechanical dysfunction, all of which contribute to patient discomfort and additional healthcare costs.[12] Bloodstream infections, for example, had previously been thought to occur at a substantially lower rate in PICCs than central venous catheters.[13] However, a recent systematic review suggests the rate of PICC‐associated bloodstream infections in the inpatient setting is actually comparable to that of central venous catheters.[14] Perhaps the most serious PICC‐associated complication is catheter‐related venous thrombosis. A recent systematic review and meta‐analysis found evidence to suggest the rate of catheter‐related venous thrombosis was highest in patients with cancer or critical illness15; additionally, rates of thrombosis associated with PICCs were higher than those associated with subclavian or internal jugular central venous catheters.[15, 16] Fletcher et al. showed an 8.1% incidence of symptomatic PICC‐related upper extremity deep vein thrombosis (DVT) in the neurosurgical intensive care unit, with 15% of patients subsequently developing a pulmonary embolism.[17] A recent prospective, randomized controlled trial by Itkin et al. similarly demonstrated symptomatic DVT rates of approximately 4%.[18] However, in this study, when PICCs were routinely screened for thrombosis (with or without associated symptoms), approximately 72% demonstrated thrombosis,[18] suggesting that many PICC‐associated thromboses may be clinically undetected. This may have far‐reaching clinical significance, as pulmonary embolism complicates upper extremity DVT in 9% of cases and can result in a mortality rate as high as 25%.[10, 19]
Some strategies to reduce the rate of catheter‐related complications include identification of characteristics that put patients at risk. Many potential risk factors have been investigated, including catheter size,[12, 20, 21, 22, 23, 24] choice of vein,[24] location of catheter tip,[25] and history of malignancy or prior DVT.[12] However, to date, no definitive consensus has been reached. Special attention has been paid to the investigation of underlying risk factors and treatment for catheter‐related DVT, given its significant morbidity and mortality. Results have been equivocal, though, and in some instances, complicated by a diagnosis of underlying malignancy.[26, 27, 28]
As PICCs become more widely utilized, assessments of factors that place patients at greater risk of PICC‐related complications are needed.[21] The purpose of this study was to establish the incidence of complications associated with PICCs placed in the inpatient setting and examine risk factors predisposing patients to these complications.
MATERIALS AND METHODS
Study Design
A case control analysis of adult inpatients who underwent PICC placement between January 2009 and January 2010 was conducted at Scott & White Healthcare (now Baylor Scott & White Healthcare) to determine the incidence and risk factors for PICC‐associated complications.
Study Site
The study took place at Scott & White Memorial Hospital in Temple, Texas, a 636‐bed multispecialty teaching hospital and level 1 trauma center. It is part of a healthcare system that includes 12 hospitals and more than 60 regional clinics, all of which share an electronic medical record to enable full integration.
Human Subjects Approval
This study received approval from the institutional review board at Scott & White Healthcare.
PICC Placement Technique
Inpatient PICC placement was performed by the PICC consult service. The consult service was comprised of 3 separate provider teams: (1) internal medicine, including select hospitalists and internal medicine residents; (2) radiology, including interventional radiologists and radiology residents; and (3) nursing, including registered nurses with advanced training in PICC placement. Following placement of a consult, the PICC consult service assessed the patient, obtained consent, and subsequently placed the catheter. Members of the PICC consult service followed a system‐wide protocol wherein target veins were identified by ultrasound prior to attempting catheter placement, and actual placement of the PICC was ultrasound guided. Images obtained during the procedure were permanently documented in the medical record. At the time of this study, no formal protocol existed wherein target veins were mapped for caliber. Operators relied on their professional judgment to determine if vein caliber appeared sufficient to accommodate catheter placement.
All PICCs were placed using industry standard sterile precautions. A universally accepted modified Seldinger technique was used to obtain venous access.[29] A guidewire was then positioned in the desired vessel to facilitate proper venous placement of the catheter. During the course of the study period, catheters used were either single‐ (4 Fr) or double lumen (5 Fr).
Catheters were placed at the bedside by hospitalists or registered nurse teams; the location of the catheter tip at the cavoatrial junction was confirmed by chest radiography. Catheter insertions by radiologists were performed in the interventional radiology suite, and confirmation of location of the catheter tip was obtained with fluoroscopy.
PICC Maintenance
Following placement, nurses managed the PICC site according to nursing policy. Per policy, the site was assessed each shift. Documentation of assessment was recorded in nursing notes. Routine dressing changes were performed every 7 days, and as needed, to maintain a sterile site. Date and time of dressing changes were documented in nursing notes and on the PICC dressing. Catheter hubs and injection ports were disinfected with an antiseptic preparation for 15 seconds and allowed to air dry for 30 seconds prior to accessing the catheter. Catheters were flushed with 10 mL of normal saline before and after use. Any abnormality noted during PICC assessment was relayed to the primary provider. If the catheter did not flush readily or demonstrate appropriate blood return, nursing staff obtained an order for alteplase to be administered in an effort to salvage the line. PICCs were discontinued at the discretion of the healthcare provider.
Participants
Records of all patients 18 years of age and older who underwent PICC placement between January 2009 and January 2010 were reviewed (N=1444) for study inclusion. There were no exclusion criteria.
Data Collection
Patients who experienced complications were identified by electronic medical record review. One‐to‐one matching was performed for age and gender‐matched controls randomly selected from inpatients who underwent PICC placement during the same time period without complications. A total of 170 cases with PICC‐related complications were identified. One hundred seventy exact age‐ and gender‐matched controls, who based upon documentation available in the electronic medical record did not experience complications, were then randomly selected. Prior to data collection, the research team reviewed and discussed the data collection form and agreed upon a standardized protocol for data collection. Data collection was completed by authors J.M. and J.H. on the standardized data collection form. Although a formal analysis of inter‐rater agreement was not performed, J.M. and J.H. discussed any items where questions arose and arrived at a consensus decision regarding completion of the data point.
End points of the chart review were completion of medical therapy for which the PICC was indicated (eg, IV antibiotics or total parenteral nutrition [TPN]) or documentation of a complication that led to 1 of the following: discontinuation of the PICC or adjustment of either catheter placement or medical therapy. All complications were identified via International Classification of Diseases, 9th Revision codes and systematic chart review.
Complications resulting in discontinuation of the PICC, adjustment of catheter placement, or change in medical therapy were identified by review of nursing or physician documentation, and were categorized as follows: mechanical complications (defined as loss of the ability of the catheter to flush or draw properly, inadvertent catheter dislodgement, or retained portion of the catheter following catheter removal), catheter‐associated bloodstream infection (development of a positive blood culture attributable to the central catheter with no other clearly identifiable source of bacteremia present), cellulitis (defined as cellulitis in the extremity where the catheter was placed), bleeding from the site of catheter, fever (for which no other cause could be identified), and catheter‐associated thrombosis (identified by Doppler ultrasonography in patients exhibiting symptoms such as pain, swelling, redness, or warmth in the extremity in which the PICC was placed).[30]
Demographic data were collected, including insurance status, age, ethnicity, and gender. Clinical data included body mass index (BMI), presence of malnutrition (defined by a serum albumin of less than 3 g/dL),[31] previous or active cancer, previous DVT, use of anticoagulants (eg, warfarin, heparin, or low‐molecular‐weight heparin) or antiplatelet agent (eg, aspirin or clopidogrel) at the time of placement, and indication for PICC placement. A patient's history of previous or active cancer and previous DVT were identified by clinical documentation. Indications for PICC placement included: treating infectious processes (ie, infusion of antimicrobials), providing TPN, chemotherapy administration, and IV access. Catheter‐specific data were also collected and included venous access obtained (cephalic, basilic, brachial), catheter size (single lumen [4 Fr] or double lumen [5 Fr]), type of complication, and time to complication. The procedure note accompanying PICC placement was reviewed for data regarding time of day inserted (with after hours defined as documentation of placement occurring after 5 pm), and procedure operator to identify type of team (internal medicine, radiology, nursing) responsible for placement.
Data Analysis
Demographic characteristics and potential risk factors for patients in both the case and control groups of the study were summarized using descriptive statistics: mean ( standard deviation [SD]) for continuous variables and frequency (percent) for categorical variables. Univariate and multivariable conditional logistic regression analyses of variables that were potential risk factors of PICC‐related complications were utilized. A stepwise selection method was used for multivariable conditional logistic regression models. Alpha=0.2 was used for the significance to enter the model, and =0.05 was used for significance level to remain in the model. Attribution of PICC‐related complications was evaluated in terms of odds ratios (OR) and 95% confidence interval (CI). A P value of <0.05 indicated statistical significance. No prospective power analysis was performed. However, for a retrospective power analysis for 1:1 matching with 170 cases and 170 matched controls, assuming 20% of controls were affected and an of 0.05, one would achieve 80% power to detect an odds ratio of 2. SAS 9.2 (SAS Institute Inc., Cary, NC) was used for data analysis.
RESULTS
In 2009, 1444 PICCs were placed, and 170 cases in which patients experienced complications associated with PICC placement were identified, resulting in a complication rate of 11.77% (95% CI: 10.11%‐13.44%). The most common complications experienced by our patient population included catheter‐associated thrombosis (3%, n = 46), mechanical complications (4%, n=67), inadvertent catheter dislodgement (2%, n=36), mechanical dysfunction (2%, n=30), retained portion of the catheter following catheter removal (<1%, n=1), catheter‐associated bloodstream infections (2%, n=24), and cellulitis at the catheter insertion site (1%, n=15). Other documented complications included unexplained fever and bleeding (Table 1).
| Complication | N (%) |
|---|---|
| |
| Thrombosis | 46 (3) |
| Infection | 24 (2) |
| Cellulitis | 15 (1) |
| Mechanical complications* | 67 (4) |
| Unexplained fever | 15 (1) |
| Bleeding | 3 (0) |
| No complication | 1,274 (88) |
The mean age of the total cohort (N=340), comprised of case (N=170) and control (N=170) groups, was 58 years (SD 17), and 55% (n=94) were females. There were no significant differences in complications between groups based on ethnicity (P=0.66). In the case group, 46% (n=78) of PICCs were placed by the radiology team, 41% (n=69) were placed by the internal medicine team, and 14% (n=23) were placed by nursing. In the control group, 44% (n=74) of PICCs were placed by radiology, 36% (n=62) by internal medicine, and 20% (n=34) by nursing. Based on univariate conditional analysis, provider team was not significantly associated with complications (P=0.29).
Predictors of All‐Cause Complications
Based upon univariate conditional logistic regression analyses of complications related to PICC placement (N=340), the following variables demonstrated a statistically significant increased risk for complications: malnutrition (OR: 1.88 [95% CI: 1.023.44], P=0.04) and after‐hours placement (OR: 8.67 [95% CI: 2.62‐28.63], P=0.0004) (Table 2). Anticoagulation was associated with a decreased risk of complications (OR: 0.27 [95% CI: 0.16‐0.45], P=0.04). Based upon multivariable logistic regression analysis, after‐hours placement (OR: 9.52 [95% CI: 2.68‐33.78], P=0.0005) and BMI >30 (OR: 1.98 [95% CI: 1.09‐3.61], P=0.02) were significantly associated with an increased risk of PICC‐associated complications. Conversely, anticoagulation/antiplatelet use was associated with a decreased risk of complications (OR: 0.24 [95% CI: 0.14‐0.43], P<0.0001).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5817 | 5817 | ||||
| BMI, meanSD | 29.29.5 | 27.97.9 | 1.02 (0.991.05) | 0.12 | ||
| 30 | 108 (64) | 116 (68%) | 1.00 | 1.00 | ||
| >30 | 62 (36) | 54 (32%) | 1.29 (0.792.11) | 0.32 | 1.98 (1.093.61) | 0.02 |
| Length of stay, d, meanSD | 1822 | 1416 | 1.01 (1.001.03) | 0.06 | ||
| Length of stay group, d | 0.11a | |||||
| <7 | 41 (24) | 52 (31) | 1.00 | |||
| 729 | 101 (59) | 103 (61) | 1.19 (0.721.98) | 0.49 | ||
| 30 | 28 (16) | 15 (9) | 2.21 (1.074.58) | 0.03 | ||
| Gender | ||||||
| Female | 94 (55) | 94 (55) | ||||
| Male | 76 (45) | 76 (45) | ||||
| Ethnicity | 0.66a | |||||
| Caucasian | 131 (77) | 125 (74) | 1.00 | |||
| African American | 26 (15) | 28 (16) | 0.88 (0.481.60) | 0.67 | ||
| Hispanic/Asian | 13 (8) | 17 (10) | 0.70 (0.311.58) | 0.38 | ||
| Provider team | 0.29a | |||||
| Radiology | 78 (46) | 74 (44) | 1.00 | |||
| Internal medicine | 69 (41) | 62 (36) | 1.05 (0.681.64) | 0.82 | ||
| Nursing | 23 (14) | 34 (20) | 0.65 (0.351.19) | 0.16 | ||
| Insuranceb | 0.22a | |||||
| Private insurance | 46 (27) | 42 (25) | 1.00 | |||
| Uninsured | 17 (10) | 24 (14) | 0.73 (0.351.55) | 0.41 | ||
| Medicare | 57 (34) | 62 (37) | 0.73 (0.381.40) | 0.34 | ||
| Medicaid | 39 (23) | 25 (15) | 1.51 (0.743.06) | 0.26 | ||
| Tricare/Veterans Administration | 11 (6) | 16 (9) | 0.59 (0.241.45) | 0.25 | ||
| History of DVT | 27 (16) | 26 (15) | 1.05 (0.581.91) | 0.88 | ||
| Malnutritionb | 149 (88) | 134 (79) | 1.88 (1.023.44) | 0.04 | ||
| Cancer | 25 (15) | 36 (21) | 0.58 (0.311.09) | 0.09 | ||
| Fluoroscopy | 129 (76) | 139 (82) | 0.71 (0.421.19) | 0.19 | ||
| Anticoagulation use | 50 (29) | 100 (59) | 0.27 (0.160.45) | <0.0001 | 0.24 (0.140.43) | <0.0001 |
| Multilumenc | 99 (58) | 111 (66) | 0.70 (0.441.11) | 0.13 | ||
| Veinb | 0.39a | |||||
| Basilic | 98 (58) | 86 (51) | 1.00 | |||
| Cephalic | 11 (6) | 8 (5) | 1.37 (0.483.89) | 0.55 | ||
| Brachial | 61 (36) | 74 (44) | 0.70 (0.451.09) | 0.12 | ||
| Internal mammary | 0 (0) | 1 (1) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 144 (85) | 166 (98) | 1.00 | 1.00 | ||
| After hours | 26 (15) | 3 (2) | 8.67 (2.6228.63) | 0.0004 | 9.52 (2.6833.78) | 0.0005 |
| Indication for PICC | 0.02a | |||||
| Infection | 88 (52) | 71 (42) | 1.00 | |||
| Pneumonia | 21 (12) | 14 (8) | 1.07 (0.502.29) | 0.87 | ||
| Chemotherapy | 5 (3) | 2 (1) | 1.84 (0.349.93) | 0.48 | ||
| IV access | 36 (21) | 66 (39) | 0.44 (0.250.75) | 0.003 | ||
| Total parenteral nutrition | 20 (12) | 17 (10) | 0.96 (0.442.14) | 0.93 | ||
Predictors of Nonmechanical Complications
To study risk factors related to nonmechanical complications, a secondary analysis (N=206) was performed in which all patients who experienced mechanical complications (N=67) and matched controls (N=67) were excluded. Based upon multivariable logistic regression analysis, after‐hours placement (OR: 6.93 [95% CI: 1.35‐35.56], P=0.02) and malnutrition (OR: 2.83 [95% CI: 1.037.81], P=0.04) were significantly associated with increased risk of nonmechanical complications. The use of anticoagulation/antiplatelet agents was associated with decreased risk of nonmechanical complications (OR: 0.17 [95% CI: 0.07‐0.40], P<0.0001). Variables not significantly associated with nonmechanical complications included BMI>30, previous history of DVT, history of cancer, catheter size, and venous access choice (Table 3).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5816 | 5816 | ||||
| BMI, meanSD | 29.79.8 | 28.57.9 | 1.03 (0.991.07) | 0.22 | ||
| 30 | 64 (62) | 68 (66) | 1.00 | |||
| >30 | 39 (38) | 35 (34) | 1.27 (0.642.49) | 0.49 | ||
| Length of stay, d, meanSD | 2026 | 1418 | 1.02 (1.001.03) | 0.08 | ||
| Length of stay group, d | 0.03a | |||||
| <7 | 22 (21) | 28 (27) | 1.00 | |||
| 729 | 60 (58) | 68 (66) | 0.95 (0.491.82) | 0.87 | ||
| 30 | 21 (20) | 7 (7) | 3.24 (1.238.54) | 0.02 | ||
| Gender | ||||||
| Female | 63 (61) | 63 (61) | ||||
| Male | 40 (39) | 40 (39) | ||||
| Ethnicity | 0.95a | |||||
| Caucasian | 75 (73) | 75 (73) | 1.00 | |||
| African American | 19 (18) | 18 (17) | 1.06 (0.512.21) | 0.87 | ||
| Hispanic/Asian | 9 (9) | 10 (10) | 0.88 (0.322.44) | 0.81 | ||
| Provider team | 0.81a | |||||
| Radiology | 43 (42) | 44 (43) | 1.00 | |||
| Internal medicine | 45 (44) | 41 (40) | 1.11 (0.621.96) | 0.73 | ||
| Nursing | 15 (15) | 18 (17) | 0.86 (0.391.90) | 0.71 | ||
| Insuranceb | 0.22a | |||||
| Private insurance | 29 (28) | 27 (26) | 1.00 | |||
| Uninsured | 13 (13) | 12 (12) | 1.18 (0.433.26) | 0.74 | ||
| Medicare | 32 (31) | 40 (39) | 0.52 (0.211.29) | 0.16 | ||
| Medicaid | 21 (20) | 12 (12) | 1.81 (0.694.74) | 0.23 | ||
| Tricare/Veterans Administration | 8 (8) | 11 (11) | 0.58 (0.191.79) | 0.34 | ||
| History of DVT | 15 (15) | 15 (15) | 1.00 (0.462.16) | 1.00 | ||
| Malnutritionb | 93 (90) | 79 (77) | 2.86 (1.216.76) | 0.02 | 2.83 (1.037.81) | 0.04 |
| Cancer | 17 (17) | 22 (21) | 0.67 (0.301.48) | 0.32 | ||
| Fluoroscopy | 78 (76) | 85 (83) | 0.65 (0.321.31) | 0.23 | ||
| Anticoagulation use | 29 (28) | 60 (58) | 0.21 (0.100.44) | <0.0001 | 0.17 (0.070.40) | <0.0001 |
| Multilumenc | 64 (62) | 67 (66) | 0.83 (0.461.51) | 0.55 | ||
| Veinb | 0.32a | |||||
| Basilic | 54 (52) | 49 (48) | 1.00 | |||
| Cephalic | 8 (8) | 3 (3) | 2.45 (0.649.32) | 0.19 | ||
| Brachial | 41 (40) | 49 (48) | 0.72 (0.421.24) | 0.24 | ||
| Internal mammary | 0 (0) | 1 (1) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 87 (84) | 100 (98) | 1.00 | 1.00 | ||
| After hours | 16 (16) | 2 (2) | 8.00 (1.8434.79) | 0.006 | 6.93 (1.3535.56) | 0.02 |
| Indication for PICC | 0.13 | |||||
| Infectiond | 52 (50) | 45 (44) | 1.00 | |||
| Pneumonia | 14 (14) | 7 (7) | 1.46 (0.514.18) | 0.48 | ||
| Chemotherapy | 5 (5) | 0 (0) | >999 (<0.001>999) | 0.99 | ||
| IV access | 22 (21) | 43 (42) | 0.48 (0.240.96) | 0.04 | ||
| Total parenteral nutrition | 10 (10) | 8 (8) | 1.08 (0.323.62) | 0.90 | ||
Predictors of Thrombotic Complications
Of 1444 patients who underwent PICC placement, 3% (n=46) were subsequently diagnosed with a catheter‐associated thrombosis, representing 27% of all observed complications. In an attempt to better identify factors predisposing patients to thrombotic complications, an additional subgroup analysis (N=92) was performed on those patients who experienced catheter‐associated thrombosis (N=46) and matched controls (N=46). Variables examined in the analysis included BMI, length of stay (LOS), history of DVT, history of cancer, utilization of anticoagulation/antiplatelet agents, malnutrition, and catheter size.
Based on conditional univariate analyses, the following variables were significantly associated with increased risk of catheter‐associated thrombosis: LOS (as a continuous variable) (OR: 1.04 [95% CI: 1.001.09], P=0.05), malnutrition (OR: 4 [95% CI: 1.1314.18], P=0.03), and after‐hours placement (OR: 8.00 [95% CI: 1.0063.96], P=0.05) (Table 4). Use of anticoagulation/antiplatelet agents (OR: 0.29 [95% CI: 0.11‐0.80], P=0.02) was associated with decreased risk of thrombosis. History of previous DVT and history of cancer were nonsignificant. In the multivariable logistic regression model, malnutrition (OR: 10.16 [95% CI: 1.76‐58.71], P=0.01) remained associated with increased risk of catheter‐associated thrombosis, whereas use of anticoagulation/antiplatelet agents (OR: 0.11 [95% CI: 0.02‐0.51], P=0.005) was associated with decreased risk of catheter‐associated thrombosis (Table 4).
| Variable | Case, N (%) | Control, N (%) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | AOR (95% CI) | P Value | |||
| ||||||
| Age, y, meanSD | 5818 | 5818 | ||||
| BMI, meanSD | 27.77.1 | 27.77.8 | 1.00 (0.931.08) | 0.98 | ||
| 30 | 34 (74) | 33 (72) | ||||
| >30 | 12 (26) | 13 (28) | 0.83 (0.252.73) | 0.76 | ||
| Length of stay, d, meanSD | 1712 | 119 | 1.04 (1.001.09) | 0.05 | ||
| Length of stay group, d | 0.15 | |||||
| <7 | 8 (17) | 14 (30) | 1.00 | |||
| 729 | 29 (63) | 30 (65) | 1.13 (0.413.07) | 0.82 | ||
| 30 | 9 (20) | 2 (4) | 4.65 (0.9822.13) | 0.05 | ||
| Gender | ||||||
| Female | 26 (57) | 26 (57) | ||||
| Male | 20 (43) | 20 (43) | ||||
| Ethnicity | 0.44a | |||||
| Caucasian | 31 (67) | 36 (78) | 1.00 | |||
| African American | 11 (24) | 6 (13) | 2.02 (0.695.93) | 0.20 | ||
| Hispanic/Asian | 4 (9) | 4 (9) | 1.12 (0.225.68) | 0.89 | ||
| Provider team | 0.26a | |||||
| Radiology | 23 (50) | 19 (41) | 1.00 | |||
| Internal medicine | 20 (43) | 18 (39) | 1.00 (0.432.31) | 1.00 | ||
| Nursing | 3(7) | 9 (20) | 0.33 (0.091.27) | 0.11 | ||
| Insuranceb | 0.38a | |||||
| Private insurance | 13 (28) | 11 (24) | 1.00 | |||
| Uninsured | 8 (17) | 4 (9) | 2.01 (0.3810.58) | 0.41 | ||
| Medicare | 14 (30) | 21 (47) | 0.39 (0.101.47) | 0.16 | ||
| Medicaid | 8 (17) | 7 (16) | 1.23 (0.285.36) | 0.78 | ||
| Tricare/Veterans Administration | 3 (7) | 2 (4) | 1.01 (0.128.27) | 1.00 | ||
| History of DVT | 7 (15) | 8 (17) | 0.88 (0.322.41) | 0.80 | ||
| Malnutritionb | 43 (93) | 33 (73) | 4.00 (1.1314.18) | 0.03 | 10.16 (1.7658.71) | 0.01 |
| Cancer | 10 (22) | 13 (28) | 0.67 (0.241.87) | 0.44 | ||
| Fluoroscopy | 33 (72) | 39 (85) | 0.46 (0.161.31) | 0.14 | ||
| Anticoagulation use | 16 (35) | 28 (61) | 0.29 (0.110.80) | 0.02 | 0.11 (0.020.51) | 0.005 |
| Multilumenc | 22 (48) | 28 (62) | 0.53 (0.231.26) | 0.15 | ||
| Veinb | 0.93a | |||||
| Basilic | 24 (52) | 21 (47) | 1.00 | |||
| Cephalic | 1 (2) | 1 (2) | 0.86 (0.0514.39) | 0.92 | ||
| Brachial | 21 (46) | 22 (49) | 0.75 (0.311.79) | 0.51 | ||
| Internal mammary | 0 (0) | 1 (2) | <0.001 (<0.001>999) | 0.99 | ||
| Time of dayb | ||||||
| Morning/afternoon | 38 (83) | 44 (98) | 1.00 | |||
| After hours | 8 (17) | 1 (2) | 8.00 (1.0063.96) | 0.05 | ||
| Indication for PICC | 0.80a | |||||
| Infectiond | 20 (43) | 17 (37) | 1.00 | |||
| Pneumonia | 5 (11) | 6 (13) | 0.60 (0.142.56) | 0.49 | ||
| Chemotherapy | 3 (7) | 0 (0) | >999 (<0.001>999) | 0.99 | ||
| IV access | 14 (30) | 20 (43) | 0.58 (0.231.44) | 0.24 | ||
| Total parenteral nutrition | 4 (9) | 3 (7) | 1.22 (0.197.70) | 0.83 | ||
DISCUSSION
The goal of this study was to identify factors related to PICC placement that place the general population of patients at risk. The type and rate of complications associated with PICCs in this study were similar to those previously reported in the literature including catheter‐related infection and thrombosis.[10, 32] Two unique risk factors, not well recognized previously,[10, 27, 28, 33] were observed in this study: malnutrition and after‐hours placement. Malnutrition, defined as serum albumin <3 g/dL was associated with an increase in PICC‐related complications (such as catheter‐associated bloodstream infections and cellulitis) and catheter‐related thrombosis. Malnutrition itself has long been associated with a decreased resistance to infection[34]; in addition, low serum albumin may also be a marker of the presence of other severe comorbidities, which may contribute to increased risk of thrombosis. It has been noted in previous studies that critical illness increases risk of thrombosis.[15] Despite an exhaustive search of the literature, we have been unable to find additional studies examining the extent to which malnutrition may impact PICC‐associated complications.
After‐hours placement was also associated with increased nonmechanical complications, as well as catheter‐related thrombosis. In an effort to improve both patient and consulting provider satisfaction and provide more expedient service, PICCs were often placed after hours (between 5 pm and 8 am) by both interventional radiology (n=14) and internal medicine (n=15) teams.
LOS has been associated with PICC placement complications in other studies.[12] In both primary and secondary analyses, hospital stays >30 days were associated with a higher risk of complications than hospitalizations <7 days. In light of the clinical significance of catheter‐related thrombosis, a subgroup analysis of patients with an LOS >30 days was conducted. The conditional univariate regression analysis showed an increased risk with greater LOS, malnutrition, and after‐hours placement. Use of anticoagulant or antiplatelet agents were associated with decreased risk of thrombosis (Table 4). The association between LOS and PICC‐related thrombosis is consistent with findings from Evans et al. involving 1728 patients in a similar center.[12] In these circumstances, increased LOS may be a surrogate marker for increased severity of illness, in that those patients who are more ill require lengthier hospitalizations. In a systematic review and meta‐analysis, Chopra et al. observed that increased severity of illness correlated with higher rates of catheter‐associated thrombosis, which is supportive of these findings.[15]
In the multivariate logistic regression analysis, BMI >30 was associated with a statistically significant increased risk for PICC‐associated complications after adjusting for anticoagulation and time of placement (Table 2). In the secondary analysis, where patients with mechanical complications were removed, BMI >30 was no longer associated with an increased risk for PICC‐associated complications (Table 3). This suggests that patients with a BMI >30 had an increased risk of mechanical complications, but were not necessarily at increased risk of developing other complications, such as catheter‐related thrombosis, infection, or bleeding. This finding is congruent with studies by Evans et al.,[12] who found no association between BMI and catheter‐associated thrombosis. Our association between BMI and complications is unique; to date, there are few additional studies that examine the extent to which BMI impacts the rate and type of complications associated with PICCs. At this time, the mechanism of the association between mechanical complications (such as inadvertent catheter removal or mechanical malfunction) and BMI is uncertain and warrants further investigation.
Use of Anticoagulant Agents
Anticoagulant (ie, any agent used for DVT prophylaxis or therapeutic anticoagulation) or antiplatelet agent use at the time of PICC placement and during the patient's hospitalization was associated with a decreased risk of thrombosis in our analysis. However, it should be noted that no specific anticoagulant agent was studied, and that antiplatelet agents were included in this analysis, unlike that of Evans et al.[12] Although current literature in oncologic populations, as well as the evidence‐based clinical practice guidelines, recommend against routine use of venous thromboprophylaxis in patients with central venous catheters,[33, 35, 36, 37] we believe this deserves further study, particularly in light of conflicting data in this area.[38, 39] Evans et al.[12] noted that although use of anticoagulants initially appeared to be associated with greater incidence of upper extremity venous thrombosis, when previous diagnosis of DVT was removed from the analysis the association was no longer significant.
In our analyses, no associations between catheter size, choice of venous access, history of previous deep venous thrombosis, or history of malignancy and risk for complications were found. Our findings differed from previous studies, where a relationship between increasing catheter bore size and site of access have been associated with increased PICC‐related thrombosis or other complications.[12, 20, 40, 41] There were also no significant differences in risk for complications between provider teams (eg, internal medicine, radiology, nursing) for PICCs placed during the morning or afternoon, which is consistent with findings by Funk et al.[1] Yet, after‐hours placement of PICCs was associated with greater complications than daytime placement. Although the exploration of factors associated with after‐hours placement was beyond the scope of this study, the findings from this study caused the authors, primarily comprised of members of the internal medicine inpatient medicine division, to reexamine the division's protocol on PICC placement. A consensus decision was made to discontinue after‐hours placement of PICCs by internal medicine teams in an effort to promote patient safety until further data could be collected. As a result, internal medicine teams no longer place PICCs after regular working hours at our institution.
Limitations
Limitations include the categorization of antiplatelet and anticoagulant agents together. We did not distinguish between high‐ and low‐dose aspirin, nor did we distinguish between therapeutic dosing of heparin and low‐molecular‐weight heparin versus DVT prophylaxis dosing. Additionally, for patients who were on warfarin or heparin drip, we did not evaluate for therapeutic range of international normalized ratio or partial thromboplastin time, as this was beyond the present scope of this study. In addition, malnutrition defined by albumin alone may have been somewhat narrow, as conditions aside from malnutrition can impact albumin levels. In future evaluations, this relationship may be clarified by including other determinants of clinical malnutrition including BMI <18 or the measurement of prealbumin. For determination of after‐hours placement of PICCs, we relied upon time of procedure dictation, assuming that all dictations immediately followed catheter placement. If there was a lapse in time between catheter placement and dictation, the category may have been recorded in error. Another limitation of after‐hours categorization was that we were unable to determine whether the PICC was placed on a weekend or holiday.
CONCLUSIONS AND FUTURE DIRECTIONS
Our results suggest that more stringent screening of patients undergoing PICC placement may reduce the risk of complications, with special attention to characteristics such as BMI >30, increased LOS, and protein‐calorie malnutrition (albumin <3). Furthermore, placement of PICC lines in emergent or after‐hours settings should be carefully considered and weighed against relative risks of central venous catheter placement. Further examination of the role anticoagulant and antiplatelet agents may have in the prevention of catheter‐related thrombosis should be undertaken. We hope that the identification of these risk factors will decrease the rate of complications and ultimately enhance patient safety and satisfaction.
Acknowledgments
The authors sincerely thank Glen Cryer, Publications Manager, Baylor Scott & White Health, for his assistance with this article.
Disclosures: Nothing to report.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22:377–379.
- , , . Peripherally inserted central catheters in the intensive care unit. J Intensive Care Med. 1996;11:49–54.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154:1833–1837.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78:26–30.
- , . Peripherally inserted central catheters: a report of 2506 catheter days. JPEN J Parenter Enteral Nutr. 1995;19:133–136.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72:225–233.
- , , . Bedside placement of peripherally inserted central catheters: a cost‐effectiveness analysis. Radiology. 1998;206:423–428.
- , , , . Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271:223–225.
- , . Experience with PICC at a university medical center. J Intraven Nurs. 1997;20:141–147.
- , , , et al. Upper‐extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57–62.
- , , , . Previous PICC placement may be associated with catheter‐related infections in hemodialysis patients. Cardiovasc Intervent Radiol. 2011;34:120–123.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138:803–810.
- . Be picky about PICCs. ACP Hospitalist, American College of Physicians website. Available at: http://www.acphospitalist.org/archives/2013/09/coverstory.htm. Accessed January 4, 2014.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34:908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382:311–325.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based practice guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15:454–460.
- , , , , , . Peripherally inserted central catheter thrombosis‐reverse tapered versus nontapered catheters: a randomized controlled study. J Vasc Interv Radiol. 2014;25:85–91.
- , , , et al. Upper extremity venous thrombosis diagnosed by duplex scanning. Am J Surg. 1990;160:202–206.
- , , , et al. Reduction of peripherally inserted central catheter‐associated DVT. Chest. 2013;143:627–633.
- . Preventing DVT in peripherally inserted central catheters. Chest. 2013;143:589–590.
- , , , et al. Incidence of upper limb venous thrombosis associated with peripherally inserted central catheters (PICC). Br J Radiol. 2005;78:596–600.
- , , , , , . Computer identification of symptomatic deep venous thrombosis associated with peripherally inserted central catheters. AMIA Annu Symp Proc. 2007:226–230.
- , . Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11:837–884.
- , , , , . To clot or not to clot? That is the question in central venous catheters. Clin Radiol. 2004;59:349–355.
- , , , et al. The Incidence of PICC line–associated thrombosis with and without the use of prophylactic anticoagulants. JPEN J Parenter Enteral Nutr. 2008;32:443–447.
- , , , , . Efficacy of multifaceted interventions in reducing complications of peripherally inserted central catheter in adult oncology patients. Support Care Cancer. 2010;18:1293–1298.
- , , , , . Peripherally inserted central venous catheter‐associated thrombosis: retrospective analysis of clinical risk factors in adult patients. South Med J. 2006;99:1073–1077.
- . The Seldinger method for PICC insertion. J Intraven Nurs. 1989;12:238–243.
- , , , et al. Venous thrombosis associated with the placement of peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11:1309–1314.
- . Protein‐energy undernutrition. Merk Manual of Diagnosis and Therapy. 18th ed. Available at: http://www.merckmanuals.com/professional/nutritional_disorders/undernutrition/protein‐energy_undernutrition.html. Accessed May 26, 2013.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4:417–422.
- , , , . Risk factors for upper extremity venous thrombosis associated with peripherally inserted central venous catheters. J Vasc Access. 2012;13:231–238.
- . Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr. 2003;133:316S–321S.
- , , , et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20:1459–1471.
- , , , , . Thromboprophylaxis in cancer patients with central venous catheters. A systematic review and meta‐analysis. Thromb Haemost. 2008;99:38–43.
- , , , . Thromboprophylaxis for catheter‐related thrombosis in patients with cancer: a systematic review of the randomized, controlled trials. J Thromb Haemost. 2007;5:2552–2554.
- , , , et al. Prevention of central venous catheter associated thrombosis using minidose warfarin in patients with haematological malignancies. Br J Haematol. 1998;10:483–486.
- , , , et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112:423–428.
- , , , , , . The peripherally inserted central catheter (PICC): a prospective study of its natural history after cubital fossa insertion. Anesth Intensive Care. 2002;30:21–24.
- . Thrombotic complications in intravenous access. J Intraven Nurs. 1998;21:96–100.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22:377–379.
- , , . Peripherally inserted central catheters in the intensive care unit. J Intensive Care Med. 1996;11:49–54.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154:1833–1837.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78:26–30.
- , . Peripherally inserted central catheters: a report of 2506 catheter days. JPEN J Parenter Enteral Nutr. 1995;19:133–136.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72:225–233.
- , , . Bedside placement of peripherally inserted central catheters: a cost‐effectiveness analysis. Radiology. 1998;206:423–428.
- , , , . Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271:223–225.
- , . Experience with PICC at a university medical center. J Intraven Nurs. 1997;20:141–147.
- , , , et al. Upper‐extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57–62.
- , , , . Previous PICC placement may be associated with catheter‐related infections in hemodialysis patients. Cardiovasc Intervent Radiol. 2011;34:120–123.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138:803–810.
- . Be picky about PICCs. ACP Hospitalist, American College of Physicians website. Available at: http://www.acphospitalist.org/archives/2013/09/coverstory.htm. Accessed January 4, 2014.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34:908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382:311–325.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based practice guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15:454–460.
- , , , , , . Peripherally inserted central catheter thrombosis‐reverse tapered versus nontapered catheters: a randomized controlled study. J Vasc Interv Radiol. 2014;25:85–91.
- , , , et al. Upper extremity venous thrombosis diagnosed by duplex scanning. Am J Surg. 1990;160:202–206.
- , , , et al. Reduction of peripherally inserted central catheter‐associated DVT. Chest. 2013;143:627–633.
- . Preventing DVT in peripherally inserted central catheters. Chest. 2013;143:589–590.
- , , , et al. Incidence of upper limb venous thrombosis associated with peripherally inserted central catheters (PICC). Br J Radiol. 2005;78:596–600.
- , , , , , . Computer identification of symptomatic deep venous thrombosis associated with peripherally inserted central catheters. AMIA Annu Symp Proc. 2007:226–230.
- , . Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11:837–884.
- , , , , . To clot or not to clot? That is the question in central venous catheters. Clin Radiol. 2004;59:349–355.
- , , , et al. The Incidence of PICC line–associated thrombosis with and without the use of prophylactic anticoagulants. JPEN J Parenter Enteral Nutr. 2008;32:443–447.
- , , , , . Efficacy of multifaceted interventions in reducing complications of peripherally inserted central catheter in adult oncology patients. Support Care Cancer. 2010;18:1293–1298.
- , , , , . Peripherally inserted central venous catheter‐associated thrombosis: retrospective analysis of clinical risk factors in adult patients. South Med J. 2006;99:1073–1077.
- . The Seldinger method for PICC insertion. J Intraven Nurs. 1989;12:238–243.
- , , , et al. Venous thrombosis associated with the placement of peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11:1309–1314.
- . Protein‐energy undernutrition. Merk Manual of Diagnosis and Therapy. 18th ed. Available at: http://www.merckmanuals.com/professional/nutritional_disorders/undernutrition/protein‐energy_undernutrition.html. Accessed May 26, 2013.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4:417–422.
- , , , . Risk factors for upper extremity venous thrombosis associated with peripherally inserted central venous catheters. J Vasc Access. 2012;13:231–238.
- . Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr. 2003;133:316S–321S.
- , , , et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20:1459–1471.
- , , , , . Thromboprophylaxis in cancer patients with central venous catheters. A systematic review and meta‐analysis. Thromb Haemost. 2008;99:38–43.
- , , , . Thromboprophylaxis for catheter‐related thrombosis in patients with cancer: a systematic review of the randomized, controlled trials. J Thromb Haemost. 2007;5:2552–2554.
- , , , et al. Prevention of central venous catheter associated thrombosis using minidose warfarin in patients with haematological malignancies. Br J Haematol. 1998;10:483–486.
- , , , et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112:423–428.
- , , , , , . The peripherally inserted central catheter (PICC): a prospective study of its natural history after cubital fossa insertion. Anesth Intensive Care. 2002;30:21–24.
- . Thrombotic complications in intravenous access. J Intraven Nurs. 1998;21:96–100.
© 2014 Society of Hospital Medicine
Study elucidates enzyme’s role in cell survival

Credit: Egelberg
Investigators say they have solved the mystery of an enzyme’s role in cell survival, thereby offering clues as to how the immune system fights infection and pointing to possible strategies for treating cancers.
The enzyme, receptor-interacting protein kinase 1 (RIPK1), is known to play a pivotal role in survival after birth.
But the new research, published in Cell, reveals that RIPK1 inhibits the pathways that control apoptosis and necroptosis.
By removing different components of each pathway in different combinations, the investigators demonstrated that, after birth, RIPK1 helps cells maintain a balanced response to signals that promote either pathway.
“We are learning that, in disease, this balancing act can be perturbed to produce damage and cell death,” said study author Douglas Green, PhD, of the St Jude Children’s Research Hospital in Memphis, Tennessee.
The results resolve long-standing questions about RIPK1’s role in cell survival and provide clues about how the immune system might use these pathways to contain infections.
The findings have also prompted the researchers to launch an investigation into whether RIPK1 could be harnessed to kill cancer cells or provide insight into tumor development.
“This study fundamentally changes the way we think about RIPK1, a molecule that we care about because it is required for life,” Dr Green said. “The results helped us identify new pathways involved in regulating programmed cell death and suggest that we might be able to develop cancer therapies that target these pathways or engage them in other ways to advance treatment of a range of diseases.”
The report builds on previous research from Dr Green’s lab regarding regulation of the pathways that control apoptosis and necroptosis. The investigators knew that apoptosis is driven by the enzyme caspase-8, which forms a complex with FADD and other proteins.
And necroptosis involves a pathway orchestrated by the enzyme receptor-interacting protein kinase 3 (RIPK3). Before birth, RIPK1 works through RIPK3 to trigger cell death by necroptosis, but, until now, the enzyme’s primary role after birth was uncertain.
So the investigators bred mice lacking different combinations of genes for RIPK1, RIPK3, caspase-8, FADD and other components of both the apoptotic and necroptotic pathways.
Mice lacking RIPK1 died. Mice missing 2 genes—RIPK1 plus RIPK3 or RIPK1 plus caspase-8 or FADD—also died soon after birth.
However, mice survived and developed normally when the investigators removed 3 genes—RIPK1, RIPK3, and either caspase-8 or FADD.
“The fact that the mice survived was totally unexpected and made us rethink how these pathways work,” Dr Green said.
The results also demonstrated that other pathways must exist in cells to maintain a balanced response to signals pushing for cell death via apoptosis or necroptosis.
Evidence in this study, for example, suggested one possible new pathway that triggered necroptosis using interferon and other elements of the immune response to infections. ![]()

Credit: Egelberg
Investigators say they have solved the mystery of an enzyme’s role in cell survival, thereby offering clues as to how the immune system fights infection and pointing to possible strategies for treating cancers.
The enzyme, receptor-interacting protein kinase 1 (RIPK1), is known to play a pivotal role in survival after birth.
But the new research, published in Cell, reveals that RIPK1 inhibits the pathways that control apoptosis and necroptosis.
By removing different components of each pathway in different combinations, the investigators demonstrated that, after birth, RIPK1 helps cells maintain a balanced response to signals that promote either pathway.
“We are learning that, in disease, this balancing act can be perturbed to produce damage and cell death,” said study author Douglas Green, PhD, of the St Jude Children’s Research Hospital in Memphis, Tennessee.
The results resolve long-standing questions about RIPK1’s role in cell survival and provide clues about how the immune system might use these pathways to contain infections.
The findings have also prompted the researchers to launch an investigation into whether RIPK1 could be harnessed to kill cancer cells or provide insight into tumor development.
“This study fundamentally changes the way we think about RIPK1, a molecule that we care about because it is required for life,” Dr Green said. “The results helped us identify new pathways involved in regulating programmed cell death and suggest that we might be able to develop cancer therapies that target these pathways or engage them in other ways to advance treatment of a range of diseases.”
The report builds on previous research from Dr Green’s lab regarding regulation of the pathways that control apoptosis and necroptosis. The investigators knew that apoptosis is driven by the enzyme caspase-8, which forms a complex with FADD and other proteins.
And necroptosis involves a pathway orchestrated by the enzyme receptor-interacting protein kinase 3 (RIPK3). Before birth, RIPK1 works through RIPK3 to trigger cell death by necroptosis, but, until now, the enzyme’s primary role after birth was uncertain.
So the investigators bred mice lacking different combinations of genes for RIPK1, RIPK3, caspase-8, FADD and other components of both the apoptotic and necroptotic pathways.
Mice lacking RIPK1 died. Mice missing 2 genes—RIPK1 plus RIPK3 or RIPK1 plus caspase-8 or FADD—also died soon after birth.
However, mice survived and developed normally when the investigators removed 3 genes—RIPK1, RIPK3, and either caspase-8 or FADD.
“The fact that the mice survived was totally unexpected and made us rethink how these pathways work,” Dr Green said.
The results also demonstrated that other pathways must exist in cells to maintain a balanced response to signals pushing for cell death via apoptosis or necroptosis.
Evidence in this study, for example, suggested one possible new pathway that triggered necroptosis using interferon and other elements of the immune response to infections. ![]()

Credit: Egelberg
Investigators say they have solved the mystery of an enzyme’s role in cell survival, thereby offering clues as to how the immune system fights infection and pointing to possible strategies for treating cancers.
The enzyme, receptor-interacting protein kinase 1 (RIPK1), is known to play a pivotal role in survival after birth.
But the new research, published in Cell, reveals that RIPK1 inhibits the pathways that control apoptosis and necroptosis.
By removing different components of each pathway in different combinations, the investigators demonstrated that, after birth, RIPK1 helps cells maintain a balanced response to signals that promote either pathway.
“We are learning that, in disease, this balancing act can be perturbed to produce damage and cell death,” said study author Douglas Green, PhD, of the St Jude Children’s Research Hospital in Memphis, Tennessee.
The results resolve long-standing questions about RIPK1’s role in cell survival and provide clues about how the immune system might use these pathways to contain infections.
The findings have also prompted the researchers to launch an investigation into whether RIPK1 could be harnessed to kill cancer cells or provide insight into tumor development.
“This study fundamentally changes the way we think about RIPK1, a molecule that we care about because it is required for life,” Dr Green said. “The results helped us identify new pathways involved in regulating programmed cell death and suggest that we might be able to develop cancer therapies that target these pathways or engage them in other ways to advance treatment of a range of diseases.”
The report builds on previous research from Dr Green’s lab regarding regulation of the pathways that control apoptosis and necroptosis. The investigators knew that apoptosis is driven by the enzyme caspase-8, which forms a complex with FADD and other proteins.
And necroptosis involves a pathway orchestrated by the enzyme receptor-interacting protein kinase 3 (RIPK3). Before birth, RIPK1 works through RIPK3 to trigger cell death by necroptosis, but, until now, the enzyme’s primary role after birth was uncertain.
So the investigators bred mice lacking different combinations of genes for RIPK1, RIPK3, caspase-8, FADD and other components of both the apoptotic and necroptotic pathways.
Mice lacking RIPK1 died. Mice missing 2 genes—RIPK1 plus RIPK3 or RIPK1 plus caspase-8 or FADD—also died soon after birth.
However, mice survived and developed normally when the investigators removed 3 genes—RIPK1, RIPK3, and either caspase-8 or FADD.
“The fact that the mice survived was totally unexpected and made us rethink how these pathways work,” Dr Green said.
The results also demonstrated that other pathways must exist in cells to maintain a balanced response to signals pushing for cell death via apoptosis or necroptosis.
Evidence in this study, for example, suggested one possible new pathway that triggered necroptosis using interferon and other elements of the immune response to infections. ![]()
Method reveals new targets of p53

Credit: A.T. Tikhonenko
A novel sequencing technique has allowed researchers to identify direct targets of p53, providing new insight into this gene’s anticancer activity.
The research, published in eLife, revealed nearly 200 genes that were directly regulated by p53, and many of these had never been identified before.
The study’s authors said this work lays the foundation for investigations into which of these genes are necessary for p53’s cancer-killing effects and how cancer cells evade these genes.
The researchers noted that all cancers must deal with p53’s antitumor effects. Generally, there are 2 ways they do this: by mutating p53 directly or by producing the protein MDM2, which inhibits p53 function. With the current study, the team explored the second strategy.
“MDM2 inhibitors, which are through phase 1 human trials, effectively activate p53 but manage to kill only about 1 in 20 tumors,” said study author Joaquín Espinosa, PhD, of the University of Colorado in Boulder.
“The question is why. What else is happening in these cancer cells that allow them to evade p53?”
According to the researchers, the answer is in the downstream effects of p53. The gene sets in motion a cascade of events that lead to cancer cell destruction. But it has been unclear exactly which other genes are directly activated by p53.
To identify genetic targets of p53, Dr Espinosa and his colleagues used a technique called Global Run-On Sequencing (GRO-Seq). Unlike other methods, GRO-Seq measures new RNA being created, not overall RNA levels.
“Many teams around the world have been getting cancer cells, treating them with MDM2 inhibitors, and waiting hours and hours to see what genes turn on, and then, only imprecisely,” Dr Espinosa said. “GRO-Seq lets us do it in minutes, and the discoveries are massive.”
The technique generates a large quantity of data because it requires counting tens of thousands of RNA molecules before and after p53 activation. So this research required designing algorithms to sort through the data, as well as a computational biologist driving a supercomputer.
But the researchers were able to pinpoint new genes directly regulated by p53. And they believe this could aid the future development of cancer-fighting strategies. ![]()

Credit: A.T. Tikhonenko
A novel sequencing technique has allowed researchers to identify direct targets of p53, providing new insight into this gene’s anticancer activity.
The research, published in eLife, revealed nearly 200 genes that were directly regulated by p53, and many of these had never been identified before.
The study’s authors said this work lays the foundation for investigations into which of these genes are necessary for p53’s cancer-killing effects and how cancer cells evade these genes.
The researchers noted that all cancers must deal with p53’s antitumor effects. Generally, there are 2 ways they do this: by mutating p53 directly or by producing the protein MDM2, which inhibits p53 function. With the current study, the team explored the second strategy.
“MDM2 inhibitors, which are through phase 1 human trials, effectively activate p53 but manage to kill only about 1 in 20 tumors,” said study author Joaquín Espinosa, PhD, of the University of Colorado in Boulder.
“The question is why. What else is happening in these cancer cells that allow them to evade p53?”
According to the researchers, the answer is in the downstream effects of p53. The gene sets in motion a cascade of events that lead to cancer cell destruction. But it has been unclear exactly which other genes are directly activated by p53.
To identify genetic targets of p53, Dr Espinosa and his colleagues used a technique called Global Run-On Sequencing (GRO-Seq). Unlike other methods, GRO-Seq measures new RNA being created, not overall RNA levels.
“Many teams around the world have been getting cancer cells, treating them with MDM2 inhibitors, and waiting hours and hours to see what genes turn on, and then, only imprecisely,” Dr Espinosa said. “GRO-Seq lets us do it in minutes, and the discoveries are massive.”
The technique generates a large quantity of data because it requires counting tens of thousands of RNA molecules before and after p53 activation. So this research required designing algorithms to sort through the data, as well as a computational biologist driving a supercomputer.
But the researchers were able to pinpoint new genes directly regulated by p53. And they believe this could aid the future development of cancer-fighting strategies. ![]()

Credit: A.T. Tikhonenko
A novel sequencing technique has allowed researchers to identify direct targets of p53, providing new insight into this gene’s anticancer activity.
The research, published in eLife, revealed nearly 200 genes that were directly regulated by p53, and many of these had never been identified before.
The study’s authors said this work lays the foundation for investigations into which of these genes are necessary for p53’s cancer-killing effects and how cancer cells evade these genes.
The researchers noted that all cancers must deal with p53’s antitumor effects. Generally, there are 2 ways they do this: by mutating p53 directly or by producing the protein MDM2, which inhibits p53 function. With the current study, the team explored the second strategy.
“MDM2 inhibitors, which are through phase 1 human trials, effectively activate p53 but manage to kill only about 1 in 20 tumors,” said study author Joaquín Espinosa, PhD, of the University of Colorado in Boulder.
“The question is why. What else is happening in these cancer cells that allow them to evade p53?”
According to the researchers, the answer is in the downstream effects of p53. The gene sets in motion a cascade of events that lead to cancer cell destruction. But it has been unclear exactly which other genes are directly activated by p53.
To identify genetic targets of p53, Dr Espinosa and his colleagues used a technique called Global Run-On Sequencing (GRO-Seq). Unlike other methods, GRO-Seq measures new RNA being created, not overall RNA levels.
“Many teams around the world have been getting cancer cells, treating them with MDM2 inhibitors, and waiting hours and hours to see what genes turn on, and then, only imprecisely,” Dr Espinosa said. “GRO-Seq lets us do it in minutes, and the discoveries are massive.”
The technique generates a large quantity of data because it requires counting tens of thousands of RNA molecules before and after p53 activation. So this research required designing algorithms to sort through the data, as well as a computational biologist driving a supercomputer.
But the researchers were able to pinpoint new genes directly regulated by p53. And they believe this could aid the future development of cancer-fighting strategies. ![]()
Long-term follow-up shows 22% survival rate for advanced GIST
CHICAGO – More than 10 years on, nearly one-fourth of patients with gastrointestinal stromal tumors treated initially with imatinib are still alive, according to results from a collaborative trial reported at the annual meeting of the American Society of Clinical Oncology.
"A significant fraction of patients can survive for more than 10 years with imatinib [Gleevec] as their initial therapy for advanced GIST; and for almost half as their only systemic therapy for advanced GIST, understanding the pathobiology of these exceptional outcomes will be important to understanding the disease better," said Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology, Dana-Farber Cancer Institute, Boston.
In the 14 years that have ensued since the first patient with GIST received imatinib and had an "extraordinary" response, new drugs in the tyrosine kinase inhibitor (TKI) class have become available for patients whose disease has progressed on the TKI imatinib. The evolution in the treatment of GIST emphasizes the fact that overall survival as a trial endpoint is really "a composite endpoint of on-study and poststudy interventions," Dr. Demetri said at meeting.
In the phase III Southwest Oncology Group (SWOG) Intergroup S0033 trial, initiated in 2000, 746 patients with metastatic or unresectable GIST were randomly assigned to receive daily imatinib at a dose of either 400 mg or 800 mg (400 mg twice daily). Patients in the 400-mg qd group had the option of crossing over to the 800-mg dose at the time of disease progression, and 130 patients chose to do so.
The trial was sparked by the discovery by Japanese investigators in 1998 of gain-of-function mutations in the gene encoding for KIT kinase.
As the SWOG S0033 investigators reported in 2008, median progression-free survival at a median follow-up of 4.5 years was 18 months for patients on the 400-mg dose and 20 months for those receiving 800 mg. The median overall survival was 55 months for patients in the 400-mg arm and 51 months for those in the 800-mg arm.
"A question we ask ourselves is, what accounts for this 33-month survival median difference after objective disease progression? It seems like a long time, which is why we wanted to know what happened to these patients after progression," Dr. Demetri said.
The investigators looked at survival by mutational status. Data on the GIST genotype were available on 395 patients, 282 of whom (71%) had KIT exon 11 mutations, 32 of whom (8%) had KIT exon 9 mutations, and 14 (4%) had other KIT or PDGFRA (platelet-derived growth factor receptor–alpha) mutations. Another 67 patients (17%) had no detectable KIT or PDGFRA mutations.
An analysis of data on these patients at 4.5 months’ median follow-up showed that patients with KIT exon 11 mutations had significantly better overall survival than patients with either wild-type (no mutation) KIT (P = .0011) or exon 9 mutations (P = .049).
Updated data with follow-up out to 10 years shows that patients with the KIT exon 9 mutation had significantly worse overall survival than patients with either exon 11 mutations (P = .0001) or no mutations (P = .047). There was no significant difference between patients with exon 11 mutations and no KIT or PDGFRA mutations.
Other factors significantly associated with overall survival in multivariate analysis included age by decade, male sex, performance status, maximum tumor diameter, and serum albumin (3.5 g/dL or less vs. more than 3.5 g/dL).
An analysis of on-study and postprogression therapies among 137 of 180 long-term survivors (8 years and longer) showed that 67 of the 137 (49%) had taken imatinib continuously as the only long-term therapy.
The remaining 70 patients (51%) had some additional therapy, including systemic therapies such as sunitinib (Sutent; 30% of the 137 patients), sorafenib (Nexavar; 12%), and other agents (31%).
In addition, 41 patients (30%) had metastasectomy or other type of surgery, 10 (7%) had radiofrequency ablation of tumors, and 6 (4%) had radiation therapy.
"We know that the landscape of therapeutic options for GIST has evolved greatly since this early large-scale study. We have new TKI therapies like sunitinib, sorafenib, and regorafenib [Stivarga], which has been approved for patients following progression of first-line imatinib, and we now accept the fact that multidisciplinary management of GIST, with resection of limited sites of oligoclonal resistant disease, is a standard option, with continuation of TKI therapy to control residual, unresectable disease," he said.
"Nonetheless , what the survival curves show us is that new options for management of KIT exon 9 mutant and other resistant genotypes are still needed," he concluded.
Dr. Jon Trent, director of the bone and soft-tissue program at the University of Miami Sylvester Cancer Center, the invited discussant, commented that "molecular subtyping should be required for all GIST patients."
"Most of all, I think we really need a tool other than the current version of RECIST [Response Evaluation Criteria in Solid Tumors], in order to really identify early makers of response and early markers of progression in our GIST patients," he said.
RECIST criteria often fail to provide useful information about early responses to therapy in GIST, he said.
The study was supported by the National Cancer Institute. Dr. Demetri disclosed serving as a consultant or adviser to ARIAD, Bayer, Novartis, and Pfizer; receiving research funding from Bayer, Novartis, and Pfizer; and receiving other remuneration from Novartis. Dr. Trent disclosed consulting/advising for Ariad, Bayer/Onyx, Novartis, and Pfizer, and receiving honoraria from Pfizer.
CHICAGO – More than 10 years on, nearly one-fourth of patients with gastrointestinal stromal tumors treated initially with imatinib are still alive, according to results from a collaborative trial reported at the annual meeting of the American Society of Clinical Oncology.
"A significant fraction of patients can survive for more than 10 years with imatinib [Gleevec] as their initial therapy for advanced GIST; and for almost half as their only systemic therapy for advanced GIST, understanding the pathobiology of these exceptional outcomes will be important to understanding the disease better," said Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology, Dana-Farber Cancer Institute, Boston.
In the 14 years that have ensued since the first patient with GIST received imatinib and had an "extraordinary" response, new drugs in the tyrosine kinase inhibitor (TKI) class have become available for patients whose disease has progressed on the TKI imatinib. The evolution in the treatment of GIST emphasizes the fact that overall survival as a trial endpoint is really "a composite endpoint of on-study and poststudy interventions," Dr. Demetri said at meeting.
In the phase III Southwest Oncology Group (SWOG) Intergroup S0033 trial, initiated in 2000, 746 patients with metastatic or unresectable GIST were randomly assigned to receive daily imatinib at a dose of either 400 mg or 800 mg (400 mg twice daily). Patients in the 400-mg qd group had the option of crossing over to the 800-mg dose at the time of disease progression, and 130 patients chose to do so.
The trial was sparked by the discovery by Japanese investigators in 1998 of gain-of-function mutations in the gene encoding for KIT kinase.
As the SWOG S0033 investigators reported in 2008, median progression-free survival at a median follow-up of 4.5 years was 18 months for patients on the 400-mg dose and 20 months for those receiving 800 mg. The median overall survival was 55 months for patients in the 400-mg arm and 51 months for those in the 800-mg arm.
"A question we ask ourselves is, what accounts for this 33-month survival median difference after objective disease progression? It seems like a long time, which is why we wanted to know what happened to these patients after progression," Dr. Demetri said.
The investigators looked at survival by mutational status. Data on the GIST genotype were available on 395 patients, 282 of whom (71%) had KIT exon 11 mutations, 32 of whom (8%) had KIT exon 9 mutations, and 14 (4%) had other KIT or PDGFRA (platelet-derived growth factor receptor–alpha) mutations. Another 67 patients (17%) had no detectable KIT or PDGFRA mutations.
An analysis of data on these patients at 4.5 months’ median follow-up showed that patients with KIT exon 11 mutations had significantly better overall survival than patients with either wild-type (no mutation) KIT (P = .0011) or exon 9 mutations (P = .049).
Updated data with follow-up out to 10 years shows that patients with the KIT exon 9 mutation had significantly worse overall survival than patients with either exon 11 mutations (P = .0001) or no mutations (P = .047). There was no significant difference between patients with exon 11 mutations and no KIT or PDGFRA mutations.
Other factors significantly associated with overall survival in multivariate analysis included age by decade, male sex, performance status, maximum tumor diameter, and serum albumin (3.5 g/dL or less vs. more than 3.5 g/dL).
An analysis of on-study and postprogression therapies among 137 of 180 long-term survivors (8 years and longer) showed that 67 of the 137 (49%) had taken imatinib continuously as the only long-term therapy.
The remaining 70 patients (51%) had some additional therapy, including systemic therapies such as sunitinib (Sutent; 30% of the 137 patients), sorafenib (Nexavar; 12%), and other agents (31%).
In addition, 41 patients (30%) had metastasectomy or other type of surgery, 10 (7%) had radiofrequency ablation of tumors, and 6 (4%) had radiation therapy.
"We know that the landscape of therapeutic options for GIST has evolved greatly since this early large-scale study. We have new TKI therapies like sunitinib, sorafenib, and regorafenib [Stivarga], which has been approved for patients following progression of first-line imatinib, and we now accept the fact that multidisciplinary management of GIST, with resection of limited sites of oligoclonal resistant disease, is a standard option, with continuation of TKI therapy to control residual, unresectable disease," he said.
"Nonetheless , what the survival curves show us is that new options for management of KIT exon 9 mutant and other resistant genotypes are still needed," he concluded.
Dr. Jon Trent, director of the bone and soft-tissue program at the University of Miami Sylvester Cancer Center, the invited discussant, commented that "molecular subtyping should be required for all GIST patients."
"Most of all, I think we really need a tool other than the current version of RECIST [Response Evaluation Criteria in Solid Tumors], in order to really identify early makers of response and early markers of progression in our GIST patients," he said.
RECIST criteria often fail to provide useful information about early responses to therapy in GIST, he said.
The study was supported by the National Cancer Institute. Dr. Demetri disclosed serving as a consultant or adviser to ARIAD, Bayer, Novartis, and Pfizer; receiving research funding from Bayer, Novartis, and Pfizer; and receiving other remuneration from Novartis. Dr. Trent disclosed consulting/advising for Ariad, Bayer/Onyx, Novartis, and Pfizer, and receiving honoraria from Pfizer.
CHICAGO – More than 10 years on, nearly one-fourth of patients with gastrointestinal stromal tumors treated initially with imatinib are still alive, according to results from a collaborative trial reported at the annual meeting of the American Society of Clinical Oncology.
"A significant fraction of patients can survive for more than 10 years with imatinib [Gleevec] as their initial therapy for advanced GIST; and for almost half as their only systemic therapy for advanced GIST, understanding the pathobiology of these exceptional outcomes will be important to understanding the disease better," said Dr. George Demetri, director of the Center for Sarcoma and Bone Oncology, Dana-Farber Cancer Institute, Boston.
In the 14 years that have ensued since the first patient with GIST received imatinib and had an "extraordinary" response, new drugs in the tyrosine kinase inhibitor (TKI) class have become available for patients whose disease has progressed on the TKI imatinib. The evolution in the treatment of GIST emphasizes the fact that overall survival as a trial endpoint is really "a composite endpoint of on-study and poststudy interventions," Dr. Demetri said at meeting.
In the phase III Southwest Oncology Group (SWOG) Intergroup S0033 trial, initiated in 2000, 746 patients with metastatic or unresectable GIST were randomly assigned to receive daily imatinib at a dose of either 400 mg or 800 mg (400 mg twice daily). Patients in the 400-mg qd group had the option of crossing over to the 800-mg dose at the time of disease progression, and 130 patients chose to do so.
The trial was sparked by the discovery by Japanese investigators in 1998 of gain-of-function mutations in the gene encoding for KIT kinase.
As the SWOG S0033 investigators reported in 2008, median progression-free survival at a median follow-up of 4.5 years was 18 months for patients on the 400-mg dose and 20 months for those receiving 800 mg. The median overall survival was 55 months for patients in the 400-mg arm and 51 months for those in the 800-mg arm.
"A question we ask ourselves is, what accounts for this 33-month survival median difference after objective disease progression? It seems like a long time, which is why we wanted to know what happened to these patients after progression," Dr. Demetri said.
The investigators looked at survival by mutational status. Data on the GIST genotype were available on 395 patients, 282 of whom (71%) had KIT exon 11 mutations, 32 of whom (8%) had KIT exon 9 mutations, and 14 (4%) had other KIT or PDGFRA (platelet-derived growth factor receptor–alpha) mutations. Another 67 patients (17%) had no detectable KIT or PDGFRA mutations.
An analysis of data on these patients at 4.5 months’ median follow-up showed that patients with KIT exon 11 mutations had significantly better overall survival than patients with either wild-type (no mutation) KIT (P = .0011) or exon 9 mutations (P = .049).
Updated data with follow-up out to 10 years shows that patients with the KIT exon 9 mutation had significantly worse overall survival than patients with either exon 11 mutations (P = .0001) or no mutations (P = .047). There was no significant difference between patients with exon 11 mutations and no KIT or PDGFRA mutations.
Other factors significantly associated with overall survival in multivariate analysis included age by decade, male sex, performance status, maximum tumor diameter, and serum albumin (3.5 g/dL or less vs. more than 3.5 g/dL).
An analysis of on-study and postprogression therapies among 137 of 180 long-term survivors (8 years and longer) showed that 67 of the 137 (49%) had taken imatinib continuously as the only long-term therapy.
The remaining 70 patients (51%) had some additional therapy, including systemic therapies such as sunitinib (Sutent; 30% of the 137 patients), sorafenib (Nexavar; 12%), and other agents (31%).
In addition, 41 patients (30%) had metastasectomy or other type of surgery, 10 (7%) had radiofrequency ablation of tumors, and 6 (4%) had radiation therapy.
"We know that the landscape of therapeutic options for GIST has evolved greatly since this early large-scale study. We have new TKI therapies like sunitinib, sorafenib, and regorafenib [Stivarga], which has been approved for patients following progression of first-line imatinib, and we now accept the fact that multidisciplinary management of GIST, with resection of limited sites of oligoclonal resistant disease, is a standard option, with continuation of TKI therapy to control residual, unresectable disease," he said.
"Nonetheless , what the survival curves show us is that new options for management of KIT exon 9 mutant and other resistant genotypes are still needed," he concluded.
Dr. Jon Trent, director of the bone and soft-tissue program at the University of Miami Sylvester Cancer Center, the invited discussant, commented that "molecular subtyping should be required for all GIST patients."
"Most of all, I think we really need a tool other than the current version of RECIST [Response Evaluation Criteria in Solid Tumors], in order to really identify early makers of response and early markers of progression in our GIST patients," he said.
RECIST criteria often fail to provide useful information about early responses to therapy in GIST, he said.
The study was supported by the National Cancer Institute. Dr. Demetri disclosed serving as a consultant or adviser to ARIAD, Bayer, Novartis, and Pfizer; receiving research funding from Bayer, Novartis, and Pfizer; and receiving other remuneration from Novartis. Dr. Trent disclosed consulting/advising for Ariad, Bayer/Onyx, Novartis, and Pfizer, and receiving honoraria from Pfizer.
AT THE ASCO ANNUAL MEETING 2014
Major finding: Ten-year overall survival for patients with metastatic or unresectable gastrointestinal tumors (GIST) treated with imatinib was 22%.
Data source: Long-term follow-up of data on 746 patients enrolled in the randomized phase III SWOG Intergroup S0033 trial.
Disclosures: The study was supported by the National Cancer Institute. Dr. Demetri disclosed serving as a consultant or adviser to ARIAD, Bayer, Novartis, and Pfizer; receiving research funding from Bayer, Novartis, and Pfizer; and receiving other remuneration from Novartis. Dr. Trent disclosed consulting/advising for Ariad, Bayer/Onyx, Novartis, and Pfizer, and receiving honoraria from Pfizer.
My patient got arrested! What do I do?
Nonforensic psychiatrists in private practice rarely expect to be dealing with patients involved in the correctional system, but unexpected things happen even with the most carefully chosen patients. I’m writing this column to offer guidance to clinicians facing this situation for the first time, based upon the most common questions I get asked.
The most common situation I hear about is that a patient has missed an appointment, and the clinician hears from a family member that the patient has been arrested. The conscientious doctor wants to make sure that his seriously mentally ill client doesn’t experience an interruption in treatment, and that an appointment will be ready after release. The first challenge is to locate the patient.
In small communities, or when a family member was present at the time of arrest, it’s relatively easy to figure out which detention center or jail the patient was taken to. If the patient was arrested in a large urban area, or even out of state, this can be more of a challenge. Fortunately, many states and even now some local county or city jurisdictions have inmate locator web pages. The website will provide search capabilities to identify anyone currently in custody, and will generally provide a unique booking or inmate number that should be used in any facility communication, along with a date of birth and the address of the facility. Be aware that a large jail with high turnover may not have real-time data capability, meaning that new arrests may not show up on the website for 24 hours.
For psychiatrists who spend a lot of time tracking down their patients in custody, there is even an iPhone- and Android-compatible app called MobilePatrol, which provides a convenient interface to many inmate locator databases nationwide. MobilePatrol does not provide information about charges or date of birth, so it’s mainly useful if the patient can be identified by age and has a unique name.
The next step is to ensure that the patient has been identified as needing psychiatric care within the facility. Almost all jails and prisons now have routine multilevel screens to identify arrestees with chronic medical or mental health needs, and to assess suicide risk at intake. This is required by any jail or prison accredited by the National Commission on Correctional Health Care. Nevertheless, some patients are reluctant to self-identify out of fear they might be inappropriately or precipitously thrown into a suicide observation cell.
When it comes to transmitting information to a correctional facility, don’t rely on custody staff. They aren’t clinicians, they change with every shift, and they won’t know what questions to ask about the patient. This includes the warden’s office. The best thing to do is call the psychology department to transmit the patient’s name, date of birth, jail or prison number, and any pertinent clinical information. Don’t rely on an administrative assistant or nonmedical therapist to do this for you – I can’t tell you the number of times I’ve gotten a message that "...John Doe is in your jail and he needs to be seen..." with absolutely no information about medication names, dosage, and frequency, or even a diagnosis! An initial phone call will ensure that the patient is found within the facility and scheduled to see the institutional psychiatrist.
Follow the phone call up with a letter. This will ensure that the clinical information is still available on the day the psychiatrist comes in, and for the next institutional physician if the patient is transferred to another facility. The letter should summarize pertinent symptoms, violence or suicide risk factors, and previous medication trials. The past med trial information is particularly important for correctional psychiatrists, given that many jails and prisons require "fail-first" prescribing policies. Outside documentation that supports a current nonformulary medication regimen can be crucial to ensuring a smooth transition of care. But please, resist the temptation to reprimand the correctional psychiatrist in advance for making a medication change – there are many valid clinical reasons for a correctional psychiatrist to alter a treatment regimen upon arrest that have nothing to do with formulary issues.
Finally, encourage the patient’s family members to maintain contact with their incarcerated loved one if that relationship is healthy and supportive. No one knows a patient better than those in his own household, and a family member can be particularly sensitive to early signs of relapse sometimes through nothing more than a patient’s tone of voice during a phone call. Give the primary caregivers contact information for the institutional psychology department and encourage them to call if they observe anything concerning during a visit or court appearance. Court dates are particularly stressful times and may serve as a crisis point for a suicidal inmate. Having an extra pair of eyes on the scene could be lifesaving.
Once the patient has been identified and referred, and treatment started, your job is done until release. For misdemeanor offenders in local detention, this could take place within days or a few weeks, or even the day of arrest if the patient is able to make bail. Following the steps I’ve recommended to ensure continuity of care will help your patient return to you in at least as good a condition as when he came in.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Nonforensic psychiatrists in private practice rarely expect to be dealing with patients involved in the correctional system, but unexpected things happen even with the most carefully chosen patients. I’m writing this column to offer guidance to clinicians facing this situation for the first time, based upon the most common questions I get asked.
The most common situation I hear about is that a patient has missed an appointment, and the clinician hears from a family member that the patient has been arrested. The conscientious doctor wants to make sure that his seriously mentally ill client doesn’t experience an interruption in treatment, and that an appointment will be ready after release. The first challenge is to locate the patient.
In small communities, or when a family member was present at the time of arrest, it’s relatively easy to figure out which detention center or jail the patient was taken to. If the patient was arrested in a large urban area, or even out of state, this can be more of a challenge. Fortunately, many states and even now some local county or city jurisdictions have inmate locator web pages. The website will provide search capabilities to identify anyone currently in custody, and will generally provide a unique booking or inmate number that should be used in any facility communication, along with a date of birth and the address of the facility. Be aware that a large jail with high turnover may not have real-time data capability, meaning that new arrests may not show up on the website for 24 hours.
For psychiatrists who spend a lot of time tracking down their patients in custody, there is even an iPhone- and Android-compatible app called MobilePatrol, which provides a convenient interface to many inmate locator databases nationwide. MobilePatrol does not provide information about charges or date of birth, so it’s mainly useful if the patient can be identified by age and has a unique name.
The next step is to ensure that the patient has been identified as needing psychiatric care within the facility. Almost all jails and prisons now have routine multilevel screens to identify arrestees with chronic medical or mental health needs, and to assess suicide risk at intake. This is required by any jail or prison accredited by the National Commission on Correctional Health Care. Nevertheless, some patients are reluctant to self-identify out of fear they might be inappropriately or precipitously thrown into a suicide observation cell.
When it comes to transmitting information to a correctional facility, don’t rely on custody staff. They aren’t clinicians, they change with every shift, and they won’t know what questions to ask about the patient. This includes the warden’s office. The best thing to do is call the psychology department to transmit the patient’s name, date of birth, jail or prison number, and any pertinent clinical information. Don’t rely on an administrative assistant or nonmedical therapist to do this for you – I can’t tell you the number of times I’ve gotten a message that "...John Doe is in your jail and he needs to be seen..." with absolutely no information about medication names, dosage, and frequency, or even a diagnosis! An initial phone call will ensure that the patient is found within the facility and scheduled to see the institutional psychiatrist.
Follow the phone call up with a letter. This will ensure that the clinical information is still available on the day the psychiatrist comes in, and for the next institutional physician if the patient is transferred to another facility. The letter should summarize pertinent symptoms, violence or suicide risk factors, and previous medication trials. The past med trial information is particularly important for correctional psychiatrists, given that many jails and prisons require "fail-first" prescribing policies. Outside documentation that supports a current nonformulary medication regimen can be crucial to ensuring a smooth transition of care. But please, resist the temptation to reprimand the correctional psychiatrist in advance for making a medication change – there are many valid clinical reasons for a correctional psychiatrist to alter a treatment regimen upon arrest that have nothing to do with formulary issues.
Finally, encourage the patient’s family members to maintain contact with their incarcerated loved one if that relationship is healthy and supportive. No one knows a patient better than those in his own household, and a family member can be particularly sensitive to early signs of relapse sometimes through nothing more than a patient’s tone of voice during a phone call. Give the primary caregivers contact information for the institutional psychology department and encourage them to call if they observe anything concerning during a visit or court appearance. Court dates are particularly stressful times and may serve as a crisis point for a suicidal inmate. Having an extra pair of eyes on the scene could be lifesaving.
Once the patient has been identified and referred, and treatment started, your job is done until release. For misdemeanor offenders in local detention, this could take place within days or a few weeks, or even the day of arrest if the patient is able to make bail. Following the steps I’ve recommended to ensure continuity of care will help your patient return to you in at least as good a condition as when he came in.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Nonforensic psychiatrists in private practice rarely expect to be dealing with patients involved in the correctional system, but unexpected things happen even with the most carefully chosen patients. I’m writing this column to offer guidance to clinicians facing this situation for the first time, based upon the most common questions I get asked.
The most common situation I hear about is that a patient has missed an appointment, and the clinician hears from a family member that the patient has been arrested. The conscientious doctor wants to make sure that his seriously mentally ill client doesn’t experience an interruption in treatment, and that an appointment will be ready after release. The first challenge is to locate the patient.
In small communities, or when a family member was present at the time of arrest, it’s relatively easy to figure out which detention center or jail the patient was taken to. If the patient was arrested in a large urban area, or even out of state, this can be more of a challenge. Fortunately, many states and even now some local county or city jurisdictions have inmate locator web pages. The website will provide search capabilities to identify anyone currently in custody, and will generally provide a unique booking or inmate number that should be used in any facility communication, along with a date of birth and the address of the facility. Be aware that a large jail with high turnover may not have real-time data capability, meaning that new arrests may not show up on the website for 24 hours.
For psychiatrists who spend a lot of time tracking down their patients in custody, there is even an iPhone- and Android-compatible app called MobilePatrol, which provides a convenient interface to many inmate locator databases nationwide. MobilePatrol does not provide information about charges or date of birth, so it’s mainly useful if the patient can be identified by age and has a unique name.
The next step is to ensure that the patient has been identified as needing psychiatric care within the facility. Almost all jails and prisons now have routine multilevel screens to identify arrestees with chronic medical or mental health needs, and to assess suicide risk at intake. This is required by any jail or prison accredited by the National Commission on Correctional Health Care. Nevertheless, some patients are reluctant to self-identify out of fear they might be inappropriately or precipitously thrown into a suicide observation cell.
When it comes to transmitting information to a correctional facility, don’t rely on custody staff. They aren’t clinicians, they change with every shift, and they won’t know what questions to ask about the patient. This includes the warden’s office. The best thing to do is call the psychology department to transmit the patient’s name, date of birth, jail or prison number, and any pertinent clinical information. Don’t rely on an administrative assistant or nonmedical therapist to do this for you – I can’t tell you the number of times I’ve gotten a message that "...John Doe is in your jail and he needs to be seen..." with absolutely no information about medication names, dosage, and frequency, or even a diagnosis! An initial phone call will ensure that the patient is found within the facility and scheduled to see the institutional psychiatrist.
Follow the phone call up with a letter. This will ensure that the clinical information is still available on the day the psychiatrist comes in, and for the next institutional physician if the patient is transferred to another facility. The letter should summarize pertinent symptoms, violence or suicide risk factors, and previous medication trials. The past med trial information is particularly important for correctional psychiatrists, given that many jails and prisons require "fail-first" prescribing policies. Outside documentation that supports a current nonformulary medication regimen can be crucial to ensuring a smooth transition of care. But please, resist the temptation to reprimand the correctional psychiatrist in advance for making a medication change – there are many valid clinical reasons for a correctional psychiatrist to alter a treatment regimen upon arrest that have nothing to do with formulary issues.
Finally, encourage the patient’s family members to maintain contact with their incarcerated loved one if that relationship is healthy and supportive. No one knows a patient better than those in his own household, and a family member can be particularly sensitive to early signs of relapse sometimes through nothing more than a patient’s tone of voice during a phone call. Give the primary caregivers contact information for the institutional psychology department and encourage them to call if they observe anything concerning during a visit or court appearance. Court dates are particularly stressful times and may serve as a crisis point for a suicidal inmate. Having an extra pair of eyes on the scene could be lifesaving.
Once the patient has been identified and referred, and treatment started, your job is done until release. For misdemeanor offenders in local detention, this could take place within days or a few weeks, or even the day of arrest if the patient is able to make bail. Following the steps I’ve recommended to ensure continuity of care will help your patient return to you in at least as good a condition as when he came in.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Screening catches breast cancer early in HL survivors

Results of a new study indicate that MRI and mammography can detect invasive breast tumors at very early stages in female survivors of Hodgkin lymphoma (HL).
Researchers said the findings underscore the need for at-risk childhood HL survivors and their physicians to be aware of screening guidelines.
The guidelines recommend survivors undergo breast MRI screening beginning at age 25 or 8 years after they received chest radiation, whichever is later.
“Female survivors of childhood HL who had chest radiation should speak with their family doctor about after-care assessment and breast cancer screening,” said David Hodgson, MD, of Princess Margaret Cancer Centre in Toronto, Canada.
“We estimate that 75% of women who are at high risk because of prior radiotherapy to the chest are not being screened. So my hope is that this new evidence will encourage these survivors to discuss early screening with their doctors.”
Dr Hodgson and his colleagues reported this evidence in Cancer.
The researchers evaluated the results of breast MRI and mammography screening among 96 female survivors of childhood HL who had been treated with chest radiotherapy.
The median patient age at first screening was 30 years, and the median number of MRI screening rounds was 3. Ten breast cancers—half of them invasive tumors—were diagnosed in 9 women during 363 person-years follow up.
The median age at breast cancer diagnosis was 39 years (range, 24 to 43 years), and the median latency period between HL diagnosis and age at breast cancer diagnoses was 21 years (range, 12 to 27 years).
“This illustrates the young age at which these cancers can occur,” Dr Hodgson said. “For some of these women, if they had been screened starting at age 40 or 50, like average-risk women, it would have been too late.”
MRI alone detected tumors with 80% sensitivity and 93.5% specificity. Mammography alone detected tumors with 70% sensitivity and 95% specificity. And both modalities combined detected tumors with 100% sensitivity and 88.6% specificity. All invasive tumors were detected by MRI.
In other words, of the 10 breast tumors, 5 were visible on both MRI and mammogram, 3 were visible only on MRI, and 2 were detected via mammogram alone (but were non-invasive). The median size of invasive tumors size was 8 mm (range, 3-15 mm), and none had spread to the lymph nodes.
The researchers noted that these results are substantially better than prior studies using only mammographic screening in young patients.
Dr Hodgson also pointed out that, because MRI screening is so much more sensitive to small changes in the appearance of the breast tissue than mammography, up to a third of patients may be called back for further testing of suspicious findings. But many of these are benign or not clinically significant and, therefore, require no treatment. ![]()

Results of a new study indicate that MRI and mammography can detect invasive breast tumors at very early stages in female survivors of Hodgkin lymphoma (HL).
Researchers said the findings underscore the need for at-risk childhood HL survivors and their physicians to be aware of screening guidelines.
The guidelines recommend survivors undergo breast MRI screening beginning at age 25 or 8 years after they received chest radiation, whichever is later.
“Female survivors of childhood HL who had chest radiation should speak with their family doctor about after-care assessment and breast cancer screening,” said David Hodgson, MD, of Princess Margaret Cancer Centre in Toronto, Canada.
“We estimate that 75% of women who are at high risk because of prior radiotherapy to the chest are not being screened. So my hope is that this new evidence will encourage these survivors to discuss early screening with their doctors.”
Dr Hodgson and his colleagues reported this evidence in Cancer.
The researchers evaluated the results of breast MRI and mammography screening among 96 female survivors of childhood HL who had been treated with chest radiotherapy.
The median patient age at first screening was 30 years, and the median number of MRI screening rounds was 3. Ten breast cancers—half of them invasive tumors—were diagnosed in 9 women during 363 person-years follow up.
The median age at breast cancer diagnosis was 39 years (range, 24 to 43 years), and the median latency period between HL diagnosis and age at breast cancer diagnoses was 21 years (range, 12 to 27 years).
“This illustrates the young age at which these cancers can occur,” Dr Hodgson said. “For some of these women, if they had been screened starting at age 40 or 50, like average-risk women, it would have been too late.”
MRI alone detected tumors with 80% sensitivity and 93.5% specificity. Mammography alone detected tumors with 70% sensitivity and 95% specificity. And both modalities combined detected tumors with 100% sensitivity and 88.6% specificity. All invasive tumors were detected by MRI.
In other words, of the 10 breast tumors, 5 were visible on both MRI and mammogram, 3 were visible only on MRI, and 2 were detected via mammogram alone (but were non-invasive). The median size of invasive tumors size was 8 mm (range, 3-15 mm), and none had spread to the lymph nodes.
The researchers noted that these results are substantially better than prior studies using only mammographic screening in young patients.
Dr Hodgson also pointed out that, because MRI screening is so much more sensitive to small changes in the appearance of the breast tissue than mammography, up to a third of patients may be called back for further testing of suspicious findings. But many of these are benign or not clinically significant and, therefore, require no treatment. ![]()

Results of a new study indicate that MRI and mammography can detect invasive breast tumors at very early stages in female survivors of Hodgkin lymphoma (HL).
Researchers said the findings underscore the need for at-risk childhood HL survivors and their physicians to be aware of screening guidelines.
The guidelines recommend survivors undergo breast MRI screening beginning at age 25 or 8 years after they received chest radiation, whichever is later.
“Female survivors of childhood HL who had chest radiation should speak with their family doctor about after-care assessment and breast cancer screening,” said David Hodgson, MD, of Princess Margaret Cancer Centre in Toronto, Canada.
“We estimate that 75% of women who are at high risk because of prior radiotherapy to the chest are not being screened. So my hope is that this new evidence will encourage these survivors to discuss early screening with their doctors.”
Dr Hodgson and his colleagues reported this evidence in Cancer.
The researchers evaluated the results of breast MRI and mammography screening among 96 female survivors of childhood HL who had been treated with chest radiotherapy.
The median patient age at first screening was 30 years, and the median number of MRI screening rounds was 3. Ten breast cancers—half of them invasive tumors—were diagnosed in 9 women during 363 person-years follow up.
The median age at breast cancer diagnosis was 39 years (range, 24 to 43 years), and the median latency period between HL diagnosis and age at breast cancer diagnoses was 21 years (range, 12 to 27 years).
“This illustrates the young age at which these cancers can occur,” Dr Hodgson said. “For some of these women, if they had been screened starting at age 40 or 50, like average-risk women, it would have been too late.”
MRI alone detected tumors with 80% sensitivity and 93.5% specificity. Mammography alone detected tumors with 70% sensitivity and 95% specificity. And both modalities combined detected tumors with 100% sensitivity and 88.6% specificity. All invasive tumors were detected by MRI.
In other words, of the 10 breast tumors, 5 were visible on both MRI and mammogram, 3 were visible only on MRI, and 2 were detected via mammogram alone (but were non-invasive). The median size of invasive tumors size was 8 mm (range, 3-15 mm), and none had spread to the lymph nodes.
The researchers noted that these results are substantially better than prior studies using only mammographic screening in young patients.
Dr Hodgson also pointed out that, because MRI screening is so much more sensitive to small changes in the appearance of the breast tissue than mammography, up to a third of patients may be called back for further testing of suspicious findings. But many of these are benign or not clinically significant and, therefore, require no treatment. ![]()
Prevention of Inpatient Hypoglycemia
Insulin therapy in the hospital setting can cause hypoglycemia, which may lead to increased mortality and length of stay (LOS).[1, 2, 3] Hypoglycemia is associated with cardiovascular, cerebrovascular, and patient fall events.[4, 5] The Centers for Medicare and Medicaid Services have designated both severe hypoglycemia (SH) with harm and diabetic ketoacidosis as hospital acquired conditions (HAC) or never events. The Society for Hospital Medicine (SHM) defines SH in the hospital as a blood glucose (BG) <40 mg/dL. Minimizing episodes of SH is important for patient health outcomes, patient safety, and for healthcare facilities' safety metrics.
Many factors contribute to SH including excessive insulin doses, medication errors, inappropriate timing of insulin doses with food intake, changes in nutritional status, impaired renal function, and changes in medications such as steroids.[6] As part of a multiyear project in patient safety, an inpatient hypoglycemia alert algorithm was developed based on a multivariate analysis of individual patient demographic, pharmacy, laboratory, and glucometric data. The algorithm was previously shown to have a 75% sensitivity to predict episodes of SH.[7] In this study, we tested whether a predictive real‐time informatics hypoglycemia alert based on the tested algorithm, along with trained nurses, would result in a decreased frequency of SH events compared to usual care. We hypothesized that this alert would result in a reduction of SH events in those patients at high risk for hypoglycemia.
METHODS
Study Design and Population
This prospective cohort‐intervention study involved inpatients admitted to Barnes‐Jewish Hospital in St. Louis, Missouri, the academic hospital of Washington University School of Medicine (WUSM), from August 2011 through December 2011. Fourteen floors, including 10 internal medicine and 4 cardiology medicine floors, were selected based upon a high frequency of severe hypoglycemic events noted in 2010. Six of the internal medicine floors were designated as intervention floors, and 8 were designated as control floors, including the 4 cardiology units. The study population consisted of patients receiving diabetic medications on study floors who had a BG <90 mg/dL during their hospital stay (Figure 1). The study was approved by the WUSM institutional review board and included a waiver of consent for individual patients.
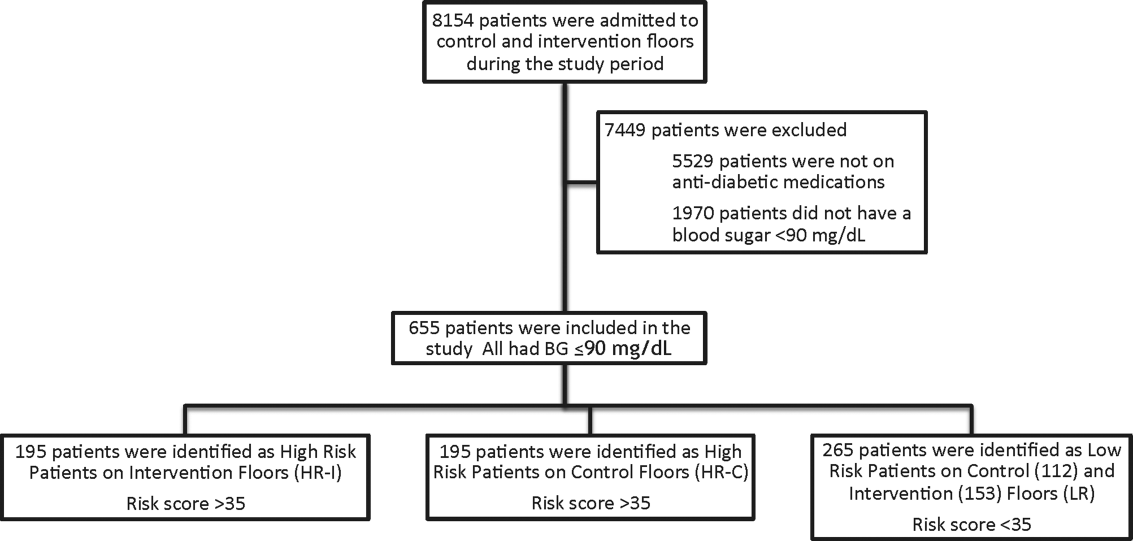
The pharmacy informatics system was programmed with the previously developed hypoglycemia alert to prospectively identify those patients at high risk of hypoglycemia based on real‐time patient information.[7] Patients were identified as high risk on study floors if insulin or an oral antihyperglycemic agent was prescribed and if their hypoglycemia informatics generated risk score was >35 within 24 hours of having a capillary or venous BG<90 mg/dL. The risk score of 35 corresponded to a 50% sensitivity for a subsequent BG <60 mg/dL and a 75% sensitivity for a BG <40 mg/dL. Patients who generated an alert once during their hospital stay were assigned to 1 of 3 categories based on their admission division and risk score: high‐risk intervention (HR‐I), high‐risk control (HR‐C), or low risk (LR). LR patients also had a BG <90 mg/dL during their stay, but a risk score of <35.
The electronic alert for HR‐I patients was sent by pager to division‐specific charge nurses. Fourteen charge nurses on intervention divisions were trained to assess the alert, interview the patient, identify an alternate dosing strategy, and collaborate with the patient's physicians. HR‐C patients were identified on control divisions based on the same criteria as intervention patients, but no alert was generated. Control patients' charts were reviewed and evaluated upon discharge by the research team‐certified diabetes nurse educator to determine whether the treating physician had identified the SH risk and had changed insulin orders.
Nurses and physicians caring for patients on study divisions provided informed consent to participate in the study. Nurses' satisfaction with the alert process and physician interaction was assessed with a collaboration scale that was completed after each alert (see Supporting Information, Appendix A, in the online version of this article).[8]
Alert Development Process
The alert equation algorithm was developed at Barnes‐Jewish Hospital after a retrospective analysis of hospital glucometric data, including capillary and venous BG measurements, and demographic and pharmacy data over a 6‐month time period.[9] The analysis identified factors that were independently associated with hypoglycemia and used these variables in a mathematical model to achieve a 50% sensitivity to predict a subsequent BG of <60 mg/dL and a 75% sensitivity to predict SH.[7] Table 1 outlines the variables in the model and provides the risk‐score equation used to generate an alert.
| Variable | Description of Variable |
|---|---|
| Body weight | Patients at a lower weight were at an increased risk. The variable had a linear response, and 3 levels were used to modify the risk equation: <69 kg, 7079 kg, and >80 kg. |
| Creatinine clearance | Patients with a lower creatinine clearance were at an increased risk. This variable had a linear response, and 2 levels were used to modify the risk equation: <48 mL/min or >48 mL/min. |
| Basal insulin dose | Increased risk was noted at a doses of basal insulin >0.25 U/kg. |
| Basal‐only dosing | Dosing of basal insulin without meal‐time insulin conferred increased risk. |
| Nonstandard insulin therapy | The use of 70/30 insulin was associated with increased risk. |
| Oral diabetic therapy | Use of sulfonylureas was associated with increased risk. |
| Risk score equation | (Value <60)=0.055+1.062 * (Basal <0.25 U/kg)+1.234 * (Basal 0.25 U/kg) & minus;0.294 * (Weight <6069 kg)0.540 * (Weight 7079 kg)0.786 * (Weight 80 kg) & minus;0.389 * (Creatinine Clearance <3847) 0.680 * (Creatinine Clearance 48) 0.239 * (Sliding Yes) 0.556*(Meal Yes)+0.951 * (Sliding and Meal)+0.336 * (Sulfonylurea Yes) Score=100 * (Exp (Value <60)/(1+Exp (Value <60)) |
The alert used a BG cutoff of 90 mg/dL in accordance with the American College of Endocrinology Hospital Guideline. Although current guidelines from the SHM recommend keeping BG values >100 mg/dL for patient safety, our analysis found that the cutoff of 90 mg/dL had better sensitivity and specificity than the <100 mg/dL guideline for the risk algorithm.[10, 11]
Nurse and Physician Training
Charge nurses received 5 hours of hyperglycemia management training in 3 sessions utilizing a structured curriculum. Session 1 included a pretest followed by diabetes management education. Session 2 was devoted to an interactive workshop utilizing case‐based scenarios of diabetes management problems and hypoglycemia prevention. The final session provided instructions on the electronic alert communication process. Nurses were empowered with tools for effective communication practices using the situation‐background‐assessment‐recommendation (SBAR) technique.[12]
Physicians, including hospitalists and medicine residents on intervention and control floors, took a pretest, received a 1‐hour lecture, and completed the same curriculum of case‐based scenarios in an online self‐directed learning module. Physicians did not receive SBAR training. Both nurses and physicians received pocket cards with insulin management guidelines developed by our research team to ensure that all clinicians had common prescribing practices.[13]
Outcomes
The primary outcome was the incidence of SH occurring in HR‐I versus HR‐C patients. Secondary outcomes included: episodes of SH in LR study patients, incidence of BG<60 mg/dL frequency of transfer to a higher level of care, incidence of severe hyperglycemia defined as BG >299 mg/dL, frequency that high‐risk patient's orders were changed to reduce hypoglycemia risk in response to the alert‐intervention process, LOS, mortality, and a nurse‐physician collaboration scale score.[14]
Statistical Analysis
Demographic and clinical metrics were compared between HR‐I and HR‐C patients to evaluate potential sources of bias. These included age, weight, serum creatinine, creatinine clearance (measured by Cockcroft‐Gault), hemoglobin A1c (HbA1c) if available, LOS, gender, admitting diagnosis, type of diabetes, and Charlson Comorbidity Index score. The alert risk‐score was also compared between intervention and control floors. Two‐tailed t tests assessed differences between the study groups on normally distributed variables, whereas Wilcoxon rank sum tests were used for non‐normally distributed variables, and 2 tests were used for categorical variables. Two‐tailed Fisher exact tests compared the prevalence of hypoglycemia thresholds between the study groups. 2 analysis was used to compare the proportion of patients who experienced a BG >299 mg/dL between intervention and controls and the proportion of orders changed in HR‐I versus HR‐C patients. Logistic regression was used to test the association of nurse collaboration score with the likelihood of orders being changed.
Based on previous research, we estimated a 48% rate of hypoglycemia <60 mg/dL in HR‐C patients on control floors.[7] We calculated a sample size of 195 subjects in each high‐risk group as the number needed for the intervention to produce a clinically meaningful reduction in hypoglycemia of 25% on the intervention floor compared to the control floors with 90% power.
RESULTS
Study Cohort and Patient Characteristics
One hundred ninety‐five patients who met criteria for high‐risk status were enrolled on HR‐I floors and HR‐C floors for a total of 390 high‐risk patients. During the same time period, 265 LR patients were identified on intervention (153 patients) and control (112 patients) floors. The HR‐I patients were similar to the HR‐C patients by baseline demographics, as shown in Table 2. HbA1c was not available on all patients, but the mean HbA1c in the HR‐I group was 7.93% versus 7.40% in the HR‐C group (P=0.048). The Charlson Comorbidity Index score was significantly different between the high‐risk groups (HR‐I: 6.48 vs HR‐C: 7.48, P=0.002), indicating that the HR‐C patients had more comorbidities.[15] There were significant differences in 2 of the 3 most common admitting diagnoses between groups, with more HR‐C patients admitted for circulatory system diseases (HR‐C: 22.3% vs HR‐I: 4.4%, P=0.001), and more HR‐I patients admitted for digestive system diseases (HR‐I: 13.7% vs HR‐C: 3.3%, P<0.001). The proportion of patients with preexisting type 2 diabetes did not differ by intervention status (HR‐I: 89.8% vs HR‐C: 92.0%, P=0.462).
| Demographic | HR‐I, Mean SD/Frequency (%), N=195 | HR‐C, Mean, SD/Frequency (%), N=195 | Low Risk, Mean, SD/Frequency (%), N=265 | P Value* |
|---|---|---|---|---|
| ||||
| Age, y | 60.2 (15.1) | 60.3 (16.9) | 61.0 (13.8) | 0.940 |
| Weight, kg | 84.9 (31.9) | 80.8 (26.6) | 93.6 (28.7) | 0.173 |
| Serum creatinine, mg/dL | 2.06 (2.56) | 2.03 (1.87) | 1.89 (2.17) | 0.910 |
| Creatinine clearance, mL/min | 50.6 (29.8) | 45.4 (27.1) | 55.5 (29.3) | 0.077 |
| Hemoglobin A1c, n (%) with data | 7.93 (2.46), n=130 (67%) | 7.40 (1.75), n=115 (59%) | 6.65 (2.05), n=152 (57%) | 0.048 |
| Risk score | 52 (11) | 54 (11) | 26 (6) | 0.111 |
| Length of stay, median, d | 5.83 | 5.88 | 5.79 | 0.664 |
| Male gender | 84 (43.1%) | 98 (50.3%) | 145 (54.7%) | 0.155 |
| Type 2 diabetes | 167 (89.8%) | 172 (92.0%) | 219 (95.6%) | 0.462 |
| Charlson Comorbidity Index score | 6.48 (3.06) | 7.48 (3.28) | 6.66 (3.24) | 0.002 |
| Admit diagnosis endocrine, nutritional, metabolic diseases, and immunity disorders (codes 240279) | 17 (9.3%) | 10 (5.4%) | 11 (4.3%) | 0.153 |
| Admit diagnosis disease of circulatory system (codes 390459) | 8 (4.4%) | 41 (22.3%) | 26 (10.1%) | <0.001 |
| Admit diagnosis disease of digestive system (codes 520579) | 25 (13.7%) | 6 (3.3%) | 27 (10.5%) | <0.001 |
| Admit diagnosis diseases of the genitourinary system (codes 580629) | 6 (3.3%) | 4 (2.2%) | 15 (5.8%) | 0.510 |
| Admit diagnosis reported only as signs, symptoms, or ill‐defined conditions (codes 780799) | 77 (42.3%) | 92 (50.0%) | 121 (46.9%) | 0.140 |
Study Outcomes
The rate of hypoglycemia was compared between 195 HR‐I and 195 HR‐C patients, and it should be noted that each patient could generate only 1 episode of hypoglycemia during an admission. As shown in Table 3, the incidence of a BG <60 mg/dL was significantly lower in the HR‐I patients versus the HR‐C patients (13.3% vs 26.7%, P=0.002) as was the incidence of a BG <40 mg/dL (3.1% HR‐I vs 9.7% HR‐C, P=0.012). This represents a decrease of 50% in moderate hypoglycemia (BG <60 mg/dL) and a decrease of 68% in SH (BG <40 mg/dL) between HR‐I and HR‐C patients. Severe hyperglycemia occurrences were not significantly different between intervention and control floors at 28% each.
| Alerted Patients Glucose Threshold | HR‐I (%), N=195 | HR‐C (%), N=195 | Low Risk (%), N=265 | P Value* |
|---|---|---|---|---|
| ||||
| With BG <40 mg/dL | 6 (3.1%) | 19 (9.7%) | 10 (3.8%) | 0.012 |
| With BG <60 mg/dL | 26 (13.3%) | 51 (26.7%) | 50 (18.9%) | 0.002 |
| With BG >299 mg/dL | 53 (28.0%) | 53 (27.9%) | 29 (11.9%) | 0.974 |
The sensitivity, specificity, and predictive values of the alert for BG thresholds of <40 mg/dL and <60 mg/dL are presented in Table 4. On control floors, the alert exhibited a modest sensitivity and high negative predictive value for BG <40 mg/dL. Sensitivity for a BG <40 mg/dL was 76% and 51.5% for BG <60 mg/dL. The alert was developed with a 50% sensitivity for a BG of <60 mg/dL, and the sensitivities calculated on control floors were consistent with the original modeling. The predictive value of an LR classification was 98.2% for not having a BG <40 mg/dL. The predictive value of a positive alert was 9.7% for BG <40 mg/dL.
| Variable | 40 mg/dL Threshold | 60 mg/dL Threshold |
|---|---|---|
| ||
| Sensitivity: probability of an alert given BG <40 or 60 mg/dL | 76.0% | 51.5% |
| Specificity: probability of no alert given BG >40 or 60 mg/dL | 64.6% | 66.0% |
| Positive predictive value | 9.7% | 26.7% |
| Negative predictive value (nonalerted patients identified as low risk) | 98.2% | 85.0% |
There was no significant difference in mortality (P=0.726), transfer to a higher level of care (P=0.296), or LOS between the 2 groups (HR‐I: 5.83 days vs HR‐C: 5.88 days, P=0.664). However, patients with a BG <40 mg/dL had an LOS of 12.2 days (N=45) versus 8.1 days for those without an SH event (N=610), which was statistically significant (P=0.005). There was no increase in the incidence of BG >299 mg/dL in the HR‐I versus HR‐C groups (P=0.53).
Nurse‐physician satisfaction with the alert process was evaluated using a collaboration scale completed after each alert.[8] Of the 195 hypoglycemia alerts, there were 167 (85.6%) nurse and 25 (12.8%) physician collaboration scales completed. Scores were similar among nurses (average 1.52) and physicians (average 1.72), reflecting positive experiences with collaboration. Orders were changed in 40.7% HR‐I patients in response to the collaboration, but in only 20.5% of HR‐C patients after the initial BG of <90 mg/dL occurred. A change in orders constituted a modification consistent with lowering the risk of hypoglycemia and included discontinuing an oral antidiabetic agent, lowering the dose of insulin, and rarely the addition of dextrose‐containing fluids. The most common change in orders was a reduction in the total dose of insulin. A difference in orders changed was partially explained by the collaboration score; a 1‐unit increase in the score correlated to an odds ratio of 2.10 that the orders would be changed (P=0.002).
DISCUSSION
Hospitals are accountable for safe and effective care of patients with hyperglycemia, which includes prevention of medication‐induced hypoglycemia. We have developed a predictive informatics hypoglycemia risk alert that, when tested in a real‐world situation, significantly reduced the rate of SH in hospitalized patients without increasing severe hyperglycemia. The alert algorithm correctly identified patients who were at high risk for hypoglycemia and allowed caretakers the opportunity to lower that risk. The positive predictive value of the alert was low but acceptable at 9.7%, owing to the overall low rate of hypoglycemia in the patient population.
The alert model tested involved 3 components for success: the automated alert, trained charge nurse responders, and an interaction between the nurse responder and the care provider. HR‐I patients were interviewed and assessed for problems associated with oral intake, dietary habits, medication compliance, and hypoglycemia at home prior to communicating with physicians. The extensive training and proficiency in patient assessment and SBAR communication process required by nurses was paramount in the success of the alert. However, the alert provided a definitive risk assessment that was actionable, versus more global instruction, which has not had the same impact in risk reduction. Based on feedback collected from nurses at the study end, they felt the alert process was within their scope of practice and was not unduly burdensome. They also found that the training in diabetes management and SBAR communication techniques, in addition to the alert system, were useful in protecting patients from medication harm.
Physicians for HR‐C patients missed many opportunities to effectively intervene and thereby reduce the likelihood of an SH event. Our assumption is that the clinicians did not ascertain the risk of SH, which was reflected by the fact that orders were changed in 40.7% of HR‐I patients versus only 20.5% in the HR‐C group. Having alerts go directly to nurses rather than physicians permitted inclusion of additional information, such as caloric intake and testing schedules, so that changes in orders would have greater context, and the importance of mild hypoglycemia would not be overlooked.[16] Glycemic control is challenging for providers in the inpatient setting, as there is little time to test and titrate doses of insulin to achieve control. Tight glycemic control has become the primary focus of diabetes management in the outpatient setting to reduce long‐term risks of microvascular complications.[17, 18] However, establishing glycemic targets in the inpatient setting has been difficult because the risk for hypoglycemia increases with tighter control.[19, 20] Inpatient hypoglycemia has been associated with increased mortality, particularly in critically ill patients.[21, 22] Many factors contribute to hypoglycemia including low creatinine clearance, low body weight, untested insulin doses, errors in insulin administration, unexpected dietary changes, changes in medications affecting BG levels, poor communication during times of patient transfer to different care teams, and poor coordination of BG testing with insulin administration at meal times. A multifaceted approach aimed at improving both clinician and nurse awareness, and providing real‐time risk assessment is clearly required to insure patient safety.[6, 13, 23, 24]
There are significant economic benefits to avoiding SH in the hospital given the adverse outcomes associated with HACs and the extra cost associated with these conditions. In hospitalized patients, hypoglycemia worsens outcomes leading to higher costs due to longer LOS (by 3 days), higher inpatient charges (38.9%), and higher risk of discharge to a skilled nursing facility.[1, 3, 25, 26] Conversely, improved glycemic control can reduce surgical site infections, perioperative morbidity, and hospital LOS.[27] The high prevalence of insulin use among inpatients, many of whom have high‐risk characteristics, creates a milieu for both hyper‐ and hypoglycemia. Other groups have described a drop in hypoglycemia rates related to the use of standardized diabetes order sets and nurse and physician education, but this is the first study that used informatics in a prospective manner to identify patients who are at high risk for developing hypoglycemia and then specifically targeted those patients.[28] The alert process was modeled after a similar alert developed in our institution for identifying medicine patients at risk for sepsis.[29] Given the paucity of data related to inpatient glycemia risk reduction, this study is particularly relevant for improving patient safety.
The major limitation of this study is that it was not randomized at the patient level. Patients were assigned to intervention and control groups based on their occupancy on specific hospital floors to avoid treatment bias. Bias was assessed due to this nonrandom assignment by comparing demographic and clinical factors of HR patients between intervention and control floors, and found significant differences in HbA1c and admitting diagnosis. As the control group had lower HbA1c values than the intervention group, and it is known from the Diabetes Control and Complications Trial and Action to Control Cardiovascular Risk in Diabetes trial that lower HbA1c increases the risk of hypoglycemia, our results may be biased by the level of glucose control on admission.[30, 31] Admitting diagnoses differed significantly between intervention and control patients as did the Charlson Comorbidity Index score; however, the hypoglycemia alert system does not include patient diagnoses or comorbidities, and as such provided equipoise with regard to risk reduction regardless of presenting illness. This study included trained nurses, which may be beyond the scope of every institution and thereby limit the effectiveness of the alert in reducing risk. However, as a result of this study, the alert was expanded to other acute care floors at our hospital as well as other hospitals in the Barnes‐Jewish Hospital system.
In summary, this study showed a 68% decrease in episodes of SH in a high‐risk patient cohort on diabetic medications using a hypoglycemia alert system. The results of this study demonstrate the validity of a systems‐based approach to reduce SH in high‐risk inpatients.
Disclosures
This work was funded by the Barnes‐Jewish Hospital Foundation The authors report no conflicts of interest.
- , , , , , . Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29:e445–e448.
- , , , et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med. 2003;163:1825–1829.
- , , , , , . Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32:1153–1157.
- , , , , . Association of hypoglycemia and cardiac ischemia: a study based on continuous glucose monitoring. Diabetes Care. 2003;26:1485–1489.
- , , , et al. Diabetes‐related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396.
- , , , , . Inpatient insulin orders: are patients getting what is prescribed? J Hosp Med. 2011;9:526–529.
- , , , . Prediction and prevention of treatment‐related inpatient hypoglycemia. J Diabetes Sci Technol. 2012;6:302–309.
- , . Collaboration between nurses and physicians. Image J Nurs Sch. 1988;20:145–149.
- , , , et al. Glucometrics—assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2005;8:560–569.
- ACE/ADA Task Force on Inpatient Diabetes. American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Diabetes Care. 2006;29:1955–1962.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353–369.
- . Role‐play using SBAR technique to improve observed communication skills in senior nursing students. J Nurs Educ. 2011;50:79–87.
- SHM Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Society of Hospital Medicine website, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org. Accessed on February 12, 2011.
- , . An internet service supporting quality assessment of inpatient glycemic control. J Diabetes Sci and Technol. 2008;2:402–408.
- , , , et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682.
- , , . Glycemic management in medical and surgical patients in the non‐ICU setting. Curr Diab Rep. 2013;13:96–106.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
- UK Prospective Diabetes Study Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853.
- , , . Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912.
- . Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14:750–756.
- , , , et al.; for the NICE‐SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–1118.
- , , , , , . Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–1901.
- , , , et al. Risk factors for inpatient hypoglycemia during subcutaneous insulin therapy in non‐critically ill patients with type 2 diabetes. J Diabetes Sci Technol. 2012;6:1022–1029.
- , , . Minimizing hypoglycemia in the wake of a tight glycemic control protocol in hospitalized patients. J Nurs Care Qual. 2010;25:255–260.
- , , , . The economic and quality of life impact of hypoglycemia. Eur J Health Econ. 2005;6:197–202.
- , , , , , . Economic and clinical impact of inpatient diabetic hypoglycemia. Endocr Pract. 2009;15:302–312.
- , , , et al. Mild hypoglycemia is strongly associated with increased intensive care unit length of stay. Ann Intensive Care. 2011;49:1–49.
- , , , et al. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Jt Comm J Qual Patient Saf. 2012;38:195–206.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39:469–473.
- The Diabetes Control and Complications Trial Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med. 1991;90:450–459.
- , , , et al. The effects of baseline characteristics, glycemia treatment approach, and glycated hemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:1–12.
Insulin therapy in the hospital setting can cause hypoglycemia, which may lead to increased mortality and length of stay (LOS).[1, 2, 3] Hypoglycemia is associated with cardiovascular, cerebrovascular, and patient fall events.[4, 5] The Centers for Medicare and Medicaid Services have designated both severe hypoglycemia (SH) with harm and diabetic ketoacidosis as hospital acquired conditions (HAC) or never events. The Society for Hospital Medicine (SHM) defines SH in the hospital as a blood glucose (BG) <40 mg/dL. Minimizing episodes of SH is important for patient health outcomes, patient safety, and for healthcare facilities' safety metrics.
Many factors contribute to SH including excessive insulin doses, medication errors, inappropriate timing of insulin doses with food intake, changes in nutritional status, impaired renal function, and changes in medications such as steroids.[6] As part of a multiyear project in patient safety, an inpatient hypoglycemia alert algorithm was developed based on a multivariate analysis of individual patient demographic, pharmacy, laboratory, and glucometric data. The algorithm was previously shown to have a 75% sensitivity to predict episodes of SH.[7] In this study, we tested whether a predictive real‐time informatics hypoglycemia alert based on the tested algorithm, along with trained nurses, would result in a decreased frequency of SH events compared to usual care. We hypothesized that this alert would result in a reduction of SH events in those patients at high risk for hypoglycemia.
METHODS
Study Design and Population
This prospective cohort‐intervention study involved inpatients admitted to Barnes‐Jewish Hospital in St. Louis, Missouri, the academic hospital of Washington University School of Medicine (WUSM), from August 2011 through December 2011. Fourteen floors, including 10 internal medicine and 4 cardiology medicine floors, were selected based upon a high frequency of severe hypoglycemic events noted in 2010. Six of the internal medicine floors were designated as intervention floors, and 8 were designated as control floors, including the 4 cardiology units. The study population consisted of patients receiving diabetic medications on study floors who had a BG <90 mg/dL during their hospital stay (Figure 1). The study was approved by the WUSM institutional review board and included a waiver of consent for individual patients.

The pharmacy informatics system was programmed with the previously developed hypoglycemia alert to prospectively identify those patients at high risk of hypoglycemia based on real‐time patient information.[7] Patients were identified as high risk on study floors if insulin or an oral antihyperglycemic agent was prescribed and if their hypoglycemia informatics generated risk score was >35 within 24 hours of having a capillary or venous BG<90 mg/dL. The risk score of 35 corresponded to a 50% sensitivity for a subsequent BG <60 mg/dL and a 75% sensitivity for a BG <40 mg/dL. Patients who generated an alert once during their hospital stay were assigned to 1 of 3 categories based on their admission division and risk score: high‐risk intervention (HR‐I), high‐risk control (HR‐C), or low risk (LR). LR patients also had a BG <90 mg/dL during their stay, but a risk score of <35.
The electronic alert for HR‐I patients was sent by pager to division‐specific charge nurses. Fourteen charge nurses on intervention divisions were trained to assess the alert, interview the patient, identify an alternate dosing strategy, and collaborate with the patient's physicians. HR‐C patients were identified on control divisions based on the same criteria as intervention patients, but no alert was generated. Control patients' charts were reviewed and evaluated upon discharge by the research team‐certified diabetes nurse educator to determine whether the treating physician had identified the SH risk and had changed insulin orders.
Nurses and physicians caring for patients on study divisions provided informed consent to participate in the study. Nurses' satisfaction with the alert process and physician interaction was assessed with a collaboration scale that was completed after each alert (see Supporting Information, Appendix A, in the online version of this article).[8]
Alert Development Process
The alert equation algorithm was developed at Barnes‐Jewish Hospital after a retrospective analysis of hospital glucometric data, including capillary and venous BG measurements, and demographic and pharmacy data over a 6‐month time period.[9] The analysis identified factors that were independently associated with hypoglycemia and used these variables in a mathematical model to achieve a 50% sensitivity to predict a subsequent BG of <60 mg/dL and a 75% sensitivity to predict SH.[7] Table 1 outlines the variables in the model and provides the risk‐score equation used to generate an alert.
| Variable | Description of Variable |
|---|---|
| Body weight | Patients at a lower weight were at an increased risk. The variable had a linear response, and 3 levels were used to modify the risk equation: <69 kg, 7079 kg, and >80 kg. |
| Creatinine clearance | Patients with a lower creatinine clearance were at an increased risk. This variable had a linear response, and 2 levels were used to modify the risk equation: <48 mL/min or >48 mL/min. |
| Basal insulin dose | Increased risk was noted at a doses of basal insulin >0.25 U/kg. |
| Basal‐only dosing | Dosing of basal insulin without meal‐time insulin conferred increased risk. |
| Nonstandard insulin therapy | The use of 70/30 insulin was associated with increased risk. |
| Oral diabetic therapy | Use of sulfonylureas was associated with increased risk. |
| Risk score equation | (Value <60)=0.055+1.062 * (Basal <0.25 U/kg)+1.234 * (Basal 0.25 U/kg) & minus;0.294 * (Weight <6069 kg)0.540 * (Weight 7079 kg)0.786 * (Weight 80 kg) & minus;0.389 * (Creatinine Clearance <3847) 0.680 * (Creatinine Clearance 48) 0.239 * (Sliding Yes) 0.556*(Meal Yes)+0.951 * (Sliding and Meal)+0.336 * (Sulfonylurea Yes) Score=100 * (Exp (Value <60)/(1+Exp (Value <60)) |
The alert used a BG cutoff of 90 mg/dL in accordance with the American College of Endocrinology Hospital Guideline. Although current guidelines from the SHM recommend keeping BG values >100 mg/dL for patient safety, our analysis found that the cutoff of 90 mg/dL had better sensitivity and specificity than the <100 mg/dL guideline for the risk algorithm.[10, 11]
Nurse and Physician Training
Charge nurses received 5 hours of hyperglycemia management training in 3 sessions utilizing a structured curriculum. Session 1 included a pretest followed by diabetes management education. Session 2 was devoted to an interactive workshop utilizing case‐based scenarios of diabetes management problems and hypoglycemia prevention. The final session provided instructions on the electronic alert communication process. Nurses were empowered with tools for effective communication practices using the situation‐background‐assessment‐recommendation (SBAR) technique.[12]
Physicians, including hospitalists and medicine residents on intervention and control floors, took a pretest, received a 1‐hour lecture, and completed the same curriculum of case‐based scenarios in an online self‐directed learning module. Physicians did not receive SBAR training. Both nurses and physicians received pocket cards with insulin management guidelines developed by our research team to ensure that all clinicians had common prescribing practices.[13]
Outcomes
The primary outcome was the incidence of SH occurring in HR‐I versus HR‐C patients. Secondary outcomes included: episodes of SH in LR study patients, incidence of BG<60 mg/dL frequency of transfer to a higher level of care, incidence of severe hyperglycemia defined as BG >299 mg/dL, frequency that high‐risk patient's orders were changed to reduce hypoglycemia risk in response to the alert‐intervention process, LOS, mortality, and a nurse‐physician collaboration scale score.[14]
Statistical Analysis
Demographic and clinical metrics were compared between HR‐I and HR‐C patients to evaluate potential sources of bias. These included age, weight, serum creatinine, creatinine clearance (measured by Cockcroft‐Gault), hemoglobin A1c (HbA1c) if available, LOS, gender, admitting diagnosis, type of diabetes, and Charlson Comorbidity Index score. The alert risk‐score was also compared between intervention and control floors. Two‐tailed t tests assessed differences between the study groups on normally distributed variables, whereas Wilcoxon rank sum tests were used for non‐normally distributed variables, and 2 tests were used for categorical variables. Two‐tailed Fisher exact tests compared the prevalence of hypoglycemia thresholds between the study groups. 2 analysis was used to compare the proportion of patients who experienced a BG >299 mg/dL between intervention and controls and the proportion of orders changed in HR‐I versus HR‐C patients. Logistic regression was used to test the association of nurse collaboration score with the likelihood of orders being changed.
Based on previous research, we estimated a 48% rate of hypoglycemia <60 mg/dL in HR‐C patients on control floors.[7] We calculated a sample size of 195 subjects in each high‐risk group as the number needed for the intervention to produce a clinically meaningful reduction in hypoglycemia of 25% on the intervention floor compared to the control floors with 90% power.
RESULTS
Study Cohort and Patient Characteristics
One hundred ninety‐five patients who met criteria for high‐risk status were enrolled on HR‐I floors and HR‐C floors for a total of 390 high‐risk patients. During the same time period, 265 LR patients were identified on intervention (153 patients) and control (112 patients) floors. The HR‐I patients were similar to the HR‐C patients by baseline demographics, as shown in Table 2. HbA1c was not available on all patients, but the mean HbA1c in the HR‐I group was 7.93% versus 7.40% in the HR‐C group (P=0.048). The Charlson Comorbidity Index score was significantly different between the high‐risk groups (HR‐I: 6.48 vs HR‐C: 7.48, P=0.002), indicating that the HR‐C patients had more comorbidities.[15] There were significant differences in 2 of the 3 most common admitting diagnoses between groups, with more HR‐C patients admitted for circulatory system diseases (HR‐C: 22.3% vs HR‐I: 4.4%, P=0.001), and more HR‐I patients admitted for digestive system diseases (HR‐I: 13.7% vs HR‐C: 3.3%, P<0.001). The proportion of patients with preexisting type 2 diabetes did not differ by intervention status (HR‐I: 89.8% vs HR‐C: 92.0%, P=0.462).
| Demographic | HR‐I, Mean SD/Frequency (%), N=195 | HR‐C, Mean, SD/Frequency (%), N=195 | Low Risk, Mean, SD/Frequency (%), N=265 | P Value* |
|---|---|---|---|---|
| ||||
| Age, y | 60.2 (15.1) | 60.3 (16.9) | 61.0 (13.8) | 0.940 |
| Weight, kg | 84.9 (31.9) | 80.8 (26.6) | 93.6 (28.7) | 0.173 |
| Serum creatinine, mg/dL | 2.06 (2.56) | 2.03 (1.87) | 1.89 (2.17) | 0.910 |
| Creatinine clearance, mL/min | 50.6 (29.8) | 45.4 (27.1) | 55.5 (29.3) | 0.077 |
| Hemoglobin A1c, n (%) with data | 7.93 (2.46), n=130 (67%) | 7.40 (1.75), n=115 (59%) | 6.65 (2.05), n=152 (57%) | 0.048 |
| Risk score | 52 (11) | 54 (11) | 26 (6) | 0.111 |
| Length of stay, median, d | 5.83 | 5.88 | 5.79 | 0.664 |
| Male gender | 84 (43.1%) | 98 (50.3%) | 145 (54.7%) | 0.155 |
| Type 2 diabetes | 167 (89.8%) | 172 (92.0%) | 219 (95.6%) | 0.462 |
| Charlson Comorbidity Index score | 6.48 (3.06) | 7.48 (3.28) | 6.66 (3.24) | 0.002 |
| Admit diagnosis endocrine, nutritional, metabolic diseases, and immunity disorders (codes 240279) | 17 (9.3%) | 10 (5.4%) | 11 (4.3%) | 0.153 |
| Admit diagnosis disease of circulatory system (codes 390459) | 8 (4.4%) | 41 (22.3%) | 26 (10.1%) | <0.001 |
| Admit diagnosis disease of digestive system (codes 520579) | 25 (13.7%) | 6 (3.3%) | 27 (10.5%) | <0.001 |
| Admit diagnosis diseases of the genitourinary system (codes 580629) | 6 (3.3%) | 4 (2.2%) | 15 (5.8%) | 0.510 |
| Admit diagnosis reported only as signs, symptoms, or ill‐defined conditions (codes 780799) | 77 (42.3%) | 92 (50.0%) | 121 (46.9%) | 0.140 |
Study Outcomes
The rate of hypoglycemia was compared between 195 HR‐I and 195 HR‐C patients, and it should be noted that each patient could generate only 1 episode of hypoglycemia during an admission. As shown in Table 3, the incidence of a BG <60 mg/dL was significantly lower in the HR‐I patients versus the HR‐C patients (13.3% vs 26.7%, P=0.002) as was the incidence of a BG <40 mg/dL (3.1% HR‐I vs 9.7% HR‐C, P=0.012). This represents a decrease of 50% in moderate hypoglycemia (BG <60 mg/dL) and a decrease of 68% in SH (BG <40 mg/dL) between HR‐I and HR‐C patients. Severe hyperglycemia occurrences were not significantly different between intervention and control floors at 28% each.
| Alerted Patients Glucose Threshold | HR‐I (%), N=195 | HR‐C (%), N=195 | Low Risk (%), N=265 | P Value* |
|---|---|---|---|---|
| ||||
| With BG <40 mg/dL | 6 (3.1%) | 19 (9.7%) | 10 (3.8%) | 0.012 |
| With BG <60 mg/dL | 26 (13.3%) | 51 (26.7%) | 50 (18.9%) | 0.002 |
| With BG >299 mg/dL | 53 (28.0%) | 53 (27.9%) | 29 (11.9%) | 0.974 |
The sensitivity, specificity, and predictive values of the alert for BG thresholds of <40 mg/dL and <60 mg/dL are presented in Table 4. On control floors, the alert exhibited a modest sensitivity and high negative predictive value for BG <40 mg/dL. Sensitivity for a BG <40 mg/dL was 76% and 51.5% for BG <60 mg/dL. The alert was developed with a 50% sensitivity for a BG of <60 mg/dL, and the sensitivities calculated on control floors were consistent with the original modeling. The predictive value of an LR classification was 98.2% for not having a BG <40 mg/dL. The predictive value of a positive alert was 9.7% for BG <40 mg/dL.
| Variable | 40 mg/dL Threshold | 60 mg/dL Threshold |
|---|---|---|
| ||
| Sensitivity: probability of an alert given BG <40 or 60 mg/dL | 76.0% | 51.5% |
| Specificity: probability of no alert given BG >40 or 60 mg/dL | 64.6% | 66.0% |
| Positive predictive value | 9.7% | 26.7% |
| Negative predictive value (nonalerted patients identified as low risk) | 98.2% | 85.0% |
There was no significant difference in mortality (P=0.726), transfer to a higher level of care (P=0.296), or LOS between the 2 groups (HR‐I: 5.83 days vs HR‐C: 5.88 days, P=0.664). However, patients with a BG <40 mg/dL had an LOS of 12.2 days (N=45) versus 8.1 days for those without an SH event (N=610), which was statistically significant (P=0.005). There was no increase in the incidence of BG >299 mg/dL in the HR‐I versus HR‐C groups (P=0.53).
Nurse‐physician satisfaction with the alert process was evaluated using a collaboration scale completed after each alert.[8] Of the 195 hypoglycemia alerts, there were 167 (85.6%) nurse and 25 (12.8%) physician collaboration scales completed. Scores were similar among nurses (average 1.52) and physicians (average 1.72), reflecting positive experiences with collaboration. Orders were changed in 40.7% HR‐I patients in response to the collaboration, but in only 20.5% of HR‐C patients after the initial BG of <90 mg/dL occurred. A change in orders constituted a modification consistent with lowering the risk of hypoglycemia and included discontinuing an oral antidiabetic agent, lowering the dose of insulin, and rarely the addition of dextrose‐containing fluids. The most common change in orders was a reduction in the total dose of insulin. A difference in orders changed was partially explained by the collaboration score; a 1‐unit increase in the score correlated to an odds ratio of 2.10 that the orders would be changed (P=0.002).
DISCUSSION
Hospitals are accountable for safe and effective care of patients with hyperglycemia, which includes prevention of medication‐induced hypoglycemia. We have developed a predictive informatics hypoglycemia risk alert that, when tested in a real‐world situation, significantly reduced the rate of SH in hospitalized patients without increasing severe hyperglycemia. The alert algorithm correctly identified patients who were at high risk for hypoglycemia and allowed caretakers the opportunity to lower that risk. The positive predictive value of the alert was low but acceptable at 9.7%, owing to the overall low rate of hypoglycemia in the patient population.
The alert model tested involved 3 components for success: the automated alert, trained charge nurse responders, and an interaction between the nurse responder and the care provider. HR‐I patients were interviewed and assessed for problems associated with oral intake, dietary habits, medication compliance, and hypoglycemia at home prior to communicating with physicians. The extensive training and proficiency in patient assessment and SBAR communication process required by nurses was paramount in the success of the alert. However, the alert provided a definitive risk assessment that was actionable, versus more global instruction, which has not had the same impact in risk reduction. Based on feedback collected from nurses at the study end, they felt the alert process was within their scope of practice and was not unduly burdensome. They also found that the training in diabetes management and SBAR communication techniques, in addition to the alert system, were useful in protecting patients from medication harm.
Physicians for HR‐C patients missed many opportunities to effectively intervene and thereby reduce the likelihood of an SH event. Our assumption is that the clinicians did not ascertain the risk of SH, which was reflected by the fact that orders were changed in 40.7% of HR‐I patients versus only 20.5% in the HR‐C group. Having alerts go directly to nurses rather than physicians permitted inclusion of additional information, such as caloric intake and testing schedules, so that changes in orders would have greater context, and the importance of mild hypoglycemia would not be overlooked.[16] Glycemic control is challenging for providers in the inpatient setting, as there is little time to test and titrate doses of insulin to achieve control. Tight glycemic control has become the primary focus of diabetes management in the outpatient setting to reduce long‐term risks of microvascular complications.[17, 18] However, establishing glycemic targets in the inpatient setting has been difficult because the risk for hypoglycemia increases with tighter control.[19, 20] Inpatient hypoglycemia has been associated with increased mortality, particularly in critically ill patients.[21, 22] Many factors contribute to hypoglycemia including low creatinine clearance, low body weight, untested insulin doses, errors in insulin administration, unexpected dietary changes, changes in medications affecting BG levels, poor communication during times of patient transfer to different care teams, and poor coordination of BG testing with insulin administration at meal times. A multifaceted approach aimed at improving both clinician and nurse awareness, and providing real‐time risk assessment is clearly required to insure patient safety.[6, 13, 23, 24]
There are significant economic benefits to avoiding SH in the hospital given the adverse outcomes associated with HACs and the extra cost associated with these conditions. In hospitalized patients, hypoglycemia worsens outcomes leading to higher costs due to longer LOS (by 3 days), higher inpatient charges (38.9%), and higher risk of discharge to a skilled nursing facility.[1, 3, 25, 26] Conversely, improved glycemic control can reduce surgical site infections, perioperative morbidity, and hospital LOS.[27] The high prevalence of insulin use among inpatients, many of whom have high‐risk characteristics, creates a milieu for both hyper‐ and hypoglycemia. Other groups have described a drop in hypoglycemia rates related to the use of standardized diabetes order sets and nurse and physician education, but this is the first study that used informatics in a prospective manner to identify patients who are at high risk for developing hypoglycemia and then specifically targeted those patients.[28] The alert process was modeled after a similar alert developed in our institution for identifying medicine patients at risk for sepsis.[29] Given the paucity of data related to inpatient glycemia risk reduction, this study is particularly relevant for improving patient safety.
The major limitation of this study is that it was not randomized at the patient level. Patients were assigned to intervention and control groups based on their occupancy on specific hospital floors to avoid treatment bias. Bias was assessed due to this nonrandom assignment by comparing demographic and clinical factors of HR patients between intervention and control floors, and found significant differences in HbA1c and admitting diagnosis. As the control group had lower HbA1c values than the intervention group, and it is known from the Diabetes Control and Complications Trial and Action to Control Cardiovascular Risk in Diabetes trial that lower HbA1c increases the risk of hypoglycemia, our results may be biased by the level of glucose control on admission.[30, 31] Admitting diagnoses differed significantly between intervention and control patients as did the Charlson Comorbidity Index score; however, the hypoglycemia alert system does not include patient diagnoses or comorbidities, and as such provided equipoise with regard to risk reduction regardless of presenting illness. This study included trained nurses, which may be beyond the scope of every institution and thereby limit the effectiveness of the alert in reducing risk. However, as a result of this study, the alert was expanded to other acute care floors at our hospital as well as other hospitals in the Barnes‐Jewish Hospital system.
In summary, this study showed a 68% decrease in episodes of SH in a high‐risk patient cohort on diabetic medications using a hypoglycemia alert system. The results of this study demonstrate the validity of a systems‐based approach to reduce SH in high‐risk inpatients.
Disclosures
This work was funded by the Barnes‐Jewish Hospital Foundation The authors report no conflicts of interest.
Insulin therapy in the hospital setting can cause hypoglycemia, which may lead to increased mortality and length of stay (LOS).[1, 2, 3] Hypoglycemia is associated with cardiovascular, cerebrovascular, and patient fall events.[4, 5] The Centers for Medicare and Medicaid Services have designated both severe hypoglycemia (SH) with harm and diabetic ketoacidosis as hospital acquired conditions (HAC) or never events. The Society for Hospital Medicine (SHM) defines SH in the hospital as a blood glucose (BG) <40 mg/dL. Minimizing episodes of SH is important for patient health outcomes, patient safety, and for healthcare facilities' safety metrics.
Many factors contribute to SH including excessive insulin doses, medication errors, inappropriate timing of insulin doses with food intake, changes in nutritional status, impaired renal function, and changes in medications such as steroids.[6] As part of a multiyear project in patient safety, an inpatient hypoglycemia alert algorithm was developed based on a multivariate analysis of individual patient demographic, pharmacy, laboratory, and glucometric data. The algorithm was previously shown to have a 75% sensitivity to predict episodes of SH.[7] In this study, we tested whether a predictive real‐time informatics hypoglycemia alert based on the tested algorithm, along with trained nurses, would result in a decreased frequency of SH events compared to usual care. We hypothesized that this alert would result in a reduction of SH events in those patients at high risk for hypoglycemia.
METHODS
Study Design and Population
This prospective cohort‐intervention study involved inpatients admitted to Barnes‐Jewish Hospital in St. Louis, Missouri, the academic hospital of Washington University School of Medicine (WUSM), from August 2011 through December 2011. Fourteen floors, including 10 internal medicine and 4 cardiology medicine floors, were selected based upon a high frequency of severe hypoglycemic events noted in 2010. Six of the internal medicine floors were designated as intervention floors, and 8 were designated as control floors, including the 4 cardiology units. The study population consisted of patients receiving diabetic medications on study floors who had a BG <90 mg/dL during their hospital stay (Figure 1). The study was approved by the WUSM institutional review board and included a waiver of consent for individual patients.

The pharmacy informatics system was programmed with the previously developed hypoglycemia alert to prospectively identify those patients at high risk of hypoglycemia based on real‐time patient information.[7] Patients were identified as high risk on study floors if insulin or an oral antihyperglycemic agent was prescribed and if their hypoglycemia informatics generated risk score was >35 within 24 hours of having a capillary or venous BG<90 mg/dL. The risk score of 35 corresponded to a 50% sensitivity for a subsequent BG <60 mg/dL and a 75% sensitivity for a BG <40 mg/dL. Patients who generated an alert once during their hospital stay were assigned to 1 of 3 categories based on their admission division and risk score: high‐risk intervention (HR‐I), high‐risk control (HR‐C), or low risk (LR). LR patients also had a BG <90 mg/dL during their stay, but a risk score of <35.
The electronic alert for HR‐I patients was sent by pager to division‐specific charge nurses. Fourteen charge nurses on intervention divisions were trained to assess the alert, interview the patient, identify an alternate dosing strategy, and collaborate with the patient's physicians. HR‐C patients were identified on control divisions based on the same criteria as intervention patients, but no alert was generated. Control patients' charts were reviewed and evaluated upon discharge by the research team‐certified diabetes nurse educator to determine whether the treating physician had identified the SH risk and had changed insulin orders.
Nurses and physicians caring for patients on study divisions provided informed consent to participate in the study. Nurses' satisfaction with the alert process and physician interaction was assessed with a collaboration scale that was completed after each alert (see Supporting Information, Appendix A, in the online version of this article).[8]
Alert Development Process
The alert equation algorithm was developed at Barnes‐Jewish Hospital after a retrospective analysis of hospital glucometric data, including capillary and venous BG measurements, and demographic and pharmacy data over a 6‐month time period.[9] The analysis identified factors that were independently associated with hypoglycemia and used these variables in a mathematical model to achieve a 50% sensitivity to predict a subsequent BG of <60 mg/dL and a 75% sensitivity to predict SH.[7] Table 1 outlines the variables in the model and provides the risk‐score equation used to generate an alert.
| Variable | Description of Variable |
|---|---|
| Body weight | Patients at a lower weight were at an increased risk. The variable had a linear response, and 3 levels were used to modify the risk equation: <69 kg, 7079 kg, and >80 kg. |
| Creatinine clearance | Patients with a lower creatinine clearance were at an increased risk. This variable had a linear response, and 2 levels were used to modify the risk equation: <48 mL/min or >48 mL/min. |
| Basal insulin dose | Increased risk was noted at a doses of basal insulin >0.25 U/kg. |
| Basal‐only dosing | Dosing of basal insulin without meal‐time insulin conferred increased risk. |
| Nonstandard insulin therapy | The use of 70/30 insulin was associated with increased risk. |
| Oral diabetic therapy | Use of sulfonylureas was associated with increased risk. |
| Risk score equation | (Value <60)=0.055+1.062 * (Basal <0.25 U/kg)+1.234 * (Basal 0.25 U/kg) & minus;0.294 * (Weight <6069 kg)0.540 * (Weight 7079 kg)0.786 * (Weight 80 kg) & minus;0.389 * (Creatinine Clearance <3847) 0.680 * (Creatinine Clearance 48) 0.239 * (Sliding Yes) 0.556*(Meal Yes)+0.951 * (Sliding and Meal)+0.336 * (Sulfonylurea Yes) Score=100 * (Exp (Value <60)/(1+Exp (Value <60)) |
The alert used a BG cutoff of 90 mg/dL in accordance with the American College of Endocrinology Hospital Guideline. Although current guidelines from the SHM recommend keeping BG values >100 mg/dL for patient safety, our analysis found that the cutoff of 90 mg/dL had better sensitivity and specificity than the <100 mg/dL guideline for the risk algorithm.[10, 11]
Nurse and Physician Training
Charge nurses received 5 hours of hyperglycemia management training in 3 sessions utilizing a structured curriculum. Session 1 included a pretest followed by diabetes management education. Session 2 was devoted to an interactive workshop utilizing case‐based scenarios of diabetes management problems and hypoglycemia prevention. The final session provided instructions on the electronic alert communication process. Nurses were empowered with tools for effective communication practices using the situation‐background‐assessment‐recommendation (SBAR) technique.[12]
Physicians, including hospitalists and medicine residents on intervention and control floors, took a pretest, received a 1‐hour lecture, and completed the same curriculum of case‐based scenarios in an online self‐directed learning module. Physicians did not receive SBAR training. Both nurses and physicians received pocket cards with insulin management guidelines developed by our research team to ensure that all clinicians had common prescribing practices.[13]
Outcomes
The primary outcome was the incidence of SH occurring in HR‐I versus HR‐C patients. Secondary outcomes included: episodes of SH in LR study patients, incidence of BG<60 mg/dL frequency of transfer to a higher level of care, incidence of severe hyperglycemia defined as BG >299 mg/dL, frequency that high‐risk patient's orders were changed to reduce hypoglycemia risk in response to the alert‐intervention process, LOS, mortality, and a nurse‐physician collaboration scale score.[14]
Statistical Analysis
Demographic and clinical metrics were compared between HR‐I and HR‐C patients to evaluate potential sources of bias. These included age, weight, serum creatinine, creatinine clearance (measured by Cockcroft‐Gault), hemoglobin A1c (HbA1c) if available, LOS, gender, admitting diagnosis, type of diabetes, and Charlson Comorbidity Index score. The alert risk‐score was also compared between intervention and control floors. Two‐tailed t tests assessed differences between the study groups on normally distributed variables, whereas Wilcoxon rank sum tests were used for non‐normally distributed variables, and 2 tests were used for categorical variables. Two‐tailed Fisher exact tests compared the prevalence of hypoglycemia thresholds between the study groups. 2 analysis was used to compare the proportion of patients who experienced a BG >299 mg/dL between intervention and controls and the proportion of orders changed in HR‐I versus HR‐C patients. Logistic regression was used to test the association of nurse collaboration score with the likelihood of orders being changed.
Based on previous research, we estimated a 48% rate of hypoglycemia <60 mg/dL in HR‐C patients on control floors.[7] We calculated a sample size of 195 subjects in each high‐risk group as the number needed for the intervention to produce a clinically meaningful reduction in hypoglycemia of 25% on the intervention floor compared to the control floors with 90% power.
RESULTS
Study Cohort and Patient Characteristics
One hundred ninety‐five patients who met criteria for high‐risk status were enrolled on HR‐I floors and HR‐C floors for a total of 390 high‐risk patients. During the same time period, 265 LR patients were identified on intervention (153 patients) and control (112 patients) floors. The HR‐I patients were similar to the HR‐C patients by baseline demographics, as shown in Table 2. HbA1c was not available on all patients, but the mean HbA1c in the HR‐I group was 7.93% versus 7.40% in the HR‐C group (P=0.048). The Charlson Comorbidity Index score was significantly different between the high‐risk groups (HR‐I: 6.48 vs HR‐C: 7.48, P=0.002), indicating that the HR‐C patients had more comorbidities.[15] There were significant differences in 2 of the 3 most common admitting diagnoses between groups, with more HR‐C patients admitted for circulatory system diseases (HR‐C: 22.3% vs HR‐I: 4.4%, P=0.001), and more HR‐I patients admitted for digestive system diseases (HR‐I: 13.7% vs HR‐C: 3.3%, P<0.001). The proportion of patients with preexisting type 2 diabetes did not differ by intervention status (HR‐I: 89.8% vs HR‐C: 92.0%, P=0.462).
| Demographic | HR‐I, Mean SD/Frequency (%), N=195 | HR‐C, Mean, SD/Frequency (%), N=195 | Low Risk, Mean, SD/Frequency (%), N=265 | P Value* |
|---|---|---|---|---|
| ||||
| Age, y | 60.2 (15.1) | 60.3 (16.9) | 61.0 (13.8) | 0.940 |
| Weight, kg | 84.9 (31.9) | 80.8 (26.6) | 93.6 (28.7) | 0.173 |
| Serum creatinine, mg/dL | 2.06 (2.56) | 2.03 (1.87) | 1.89 (2.17) | 0.910 |
| Creatinine clearance, mL/min | 50.6 (29.8) | 45.4 (27.1) | 55.5 (29.3) | 0.077 |
| Hemoglobin A1c, n (%) with data | 7.93 (2.46), n=130 (67%) | 7.40 (1.75), n=115 (59%) | 6.65 (2.05), n=152 (57%) | 0.048 |
| Risk score | 52 (11) | 54 (11) | 26 (6) | 0.111 |
| Length of stay, median, d | 5.83 | 5.88 | 5.79 | 0.664 |
| Male gender | 84 (43.1%) | 98 (50.3%) | 145 (54.7%) | 0.155 |
| Type 2 diabetes | 167 (89.8%) | 172 (92.0%) | 219 (95.6%) | 0.462 |
| Charlson Comorbidity Index score | 6.48 (3.06) | 7.48 (3.28) | 6.66 (3.24) | 0.002 |
| Admit diagnosis endocrine, nutritional, metabolic diseases, and immunity disorders (codes 240279) | 17 (9.3%) | 10 (5.4%) | 11 (4.3%) | 0.153 |
| Admit diagnosis disease of circulatory system (codes 390459) | 8 (4.4%) | 41 (22.3%) | 26 (10.1%) | <0.001 |
| Admit diagnosis disease of digestive system (codes 520579) | 25 (13.7%) | 6 (3.3%) | 27 (10.5%) | <0.001 |
| Admit diagnosis diseases of the genitourinary system (codes 580629) | 6 (3.3%) | 4 (2.2%) | 15 (5.8%) | 0.510 |
| Admit diagnosis reported only as signs, symptoms, or ill‐defined conditions (codes 780799) | 77 (42.3%) | 92 (50.0%) | 121 (46.9%) | 0.140 |
Study Outcomes
The rate of hypoglycemia was compared between 195 HR‐I and 195 HR‐C patients, and it should be noted that each patient could generate only 1 episode of hypoglycemia during an admission. As shown in Table 3, the incidence of a BG <60 mg/dL was significantly lower in the HR‐I patients versus the HR‐C patients (13.3% vs 26.7%, P=0.002) as was the incidence of a BG <40 mg/dL (3.1% HR‐I vs 9.7% HR‐C, P=0.012). This represents a decrease of 50% in moderate hypoglycemia (BG <60 mg/dL) and a decrease of 68% in SH (BG <40 mg/dL) between HR‐I and HR‐C patients. Severe hyperglycemia occurrences were not significantly different between intervention and control floors at 28% each.
| Alerted Patients Glucose Threshold | HR‐I (%), N=195 | HR‐C (%), N=195 | Low Risk (%), N=265 | P Value* |
|---|---|---|---|---|
| ||||
| With BG <40 mg/dL | 6 (3.1%) | 19 (9.7%) | 10 (3.8%) | 0.012 |
| With BG <60 mg/dL | 26 (13.3%) | 51 (26.7%) | 50 (18.9%) | 0.002 |
| With BG >299 mg/dL | 53 (28.0%) | 53 (27.9%) | 29 (11.9%) | 0.974 |
The sensitivity, specificity, and predictive values of the alert for BG thresholds of <40 mg/dL and <60 mg/dL are presented in Table 4. On control floors, the alert exhibited a modest sensitivity and high negative predictive value for BG <40 mg/dL. Sensitivity for a BG <40 mg/dL was 76% and 51.5% for BG <60 mg/dL. The alert was developed with a 50% sensitivity for a BG of <60 mg/dL, and the sensitivities calculated on control floors were consistent with the original modeling. The predictive value of an LR classification was 98.2% for not having a BG <40 mg/dL. The predictive value of a positive alert was 9.7% for BG <40 mg/dL.
| Variable | 40 mg/dL Threshold | 60 mg/dL Threshold |
|---|---|---|
| ||
| Sensitivity: probability of an alert given BG <40 or 60 mg/dL | 76.0% | 51.5% |
| Specificity: probability of no alert given BG >40 or 60 mg/dL | 64.6% | 66.0% |
| Positive predictive value | 9.7% | 26.7% |
| Negative predictive value (nonalerted patients identified as low risk) | 98.2% | 85.0% |
There was no significant difference in mortality (P=0.726), transfer to a higher level of care (P=0.296), or LOS between the 2 groups (HR‐I: 5.83 days vs HR‐C: 5.88 days, P=0.664). However, patients with a BG <40 mg/dL had an LOS of 12.2 days (N=45) versus 8.1 days for those without an SH event (N=610), which was statistically significant (P=0.005). There was no increase in the incidence of BG >299 mg/dL in the HR‐I versus HR‐C groups (P=0.53).
Nurse‐physician satisfaction with the alert process was evaluated using a collaboration scale completed after each alert.[8] Of the 195 hypoglycemia alerts, there were 167 (85.6%) nurse and 25 (12.8%) physician collaboration scales completed. Scores were similar among nurses (average 1.52) and physicians (average 1.72), reflecting positive experiences with collaboration. Orders were changed in 40.7% HR‐I patients in response to the collaboration, but in only 20.5% of HR‐C patients after the initial BG of <90 mg/dL occurred. A change in orders constituted a modification consistent with lowering the risk of hypoglycemia and included discontinuing an oral antidiabetic agent, lowering the dose of insulin, and rarely the addition of dextrose‐containing fluids. The most common change in orders was a reduction in the total dose of insulin. A difference in orders changed was partially explained by the collaboration score; a 1‐unit increase in the score correlated to an odds ratio of 2.10 that the orders would be changed (P=0.002).
DISCUSSION
Hospitals are accountable for safe and effective care of patients with hyperglycemia, which includes prevention of medication‐induced hypoglycemia. We have developed a predictive informatics hypoglycemia risk alert that, when tested in a real‐world situation, significantly reduced the rate of SH in hospitalized patients without increasing severe hyperglycemia. The alert algorithm correctly identified patients who were at high risk for hypoglycemia and allowed caretakers the opportunity to lower that risk. The positive predictive value of the alert was low but acceptable at 9.7%, owing to the overall low rate of hypoglycemia in the patient population.
The alert model tested involved 3 components for success: the automated alert, trained charge nurse responders, and an interaction between the nurse responder and the care provider. HR‐I patients were interviewed and assessed for problems associated with oral intake, dietary habits, medication compliance, and hypoglycemia at home prior to communicating with physicians. The extensive training and proficiency in patient assessment and SBAR communication process required by nurses was paramount in the success of the alert. However, the alert provided a definitive risk assessment that was actionable, versus more global instruction, which has not had the same impact in risk reduction. Based on feedback collected from nurses at the study end, they felt the alert process was within their scope of practice and was not unduly burdensome. They also found that the training in diabetes management and SBAR communication techniques, in addition to the alert system, were useful in protecting patients from medication harm.
Physicians for HR‐C patients missed many opportunities to effectively intervene and thereby reduce the likelihood of an SH event. Our assumption is that the clinicians did not ascertain the risk of SH, which was reflected by the fact that orders were changed in 40.7% of HR‐I patients versus only 20.5% in the HR‐C group. Having alerts go directly to nurses rather than physicians permitted inclusion of additional information, such as caloric intake and testing schedules, so that changes in orders would have greater context, and the importance of mild hypoglycemia would not be overlooked.[16] Glycemic control is challenging for providers in the inpatient setting, as there is little time to test and titrate doses of insulin to achieve control. Tight glycemic control has become the primary focus of diabetes management in the outpatient setting to reduce long‐term risks of microvascular complications.[17, 18] However, establishing glycemic targets in the inpatient setting has been difficult because the risk for hypoglycemia increases with tighter control.[19, 20] Inpatient hypoglycemia has been associated with increased mortality, particularly in critically ill patients.[21, 22] Many factors contribute to hypoglycemia including low creatinine clearance, low body weight, untested insulin doses, errors in insulin administration, unexpected dietary changes, changes in medications affecting BG levels, poor communication during times of patient transfer to different care teams, and poor coordination of BG testing with insulin administration at meal times. A multifaceted approach aimed at improving both clinician and nurse awareness, and providing real‐time risk assessment is clearly required to insure patient safety.[6, 13, 23, 24]
There are significant economic benefits to avoiding SH in the hospital given the adverse outcomes associated with HACs and the extra cost associated with these conditions. In hospitalized patients, hypoglycemia worsens outcomes leading to higher costs due to longer LOS (by 3 days), higher inpatient charges (38.9%), and higher risk of discharge to a skilled nursing facility.[1, 3, 25, 26] Conversely, improved glycemic control can reduce surgical site infections, perioperative morbidity, and hospital LOS.[27] The high prevalence of insulin use among inpatients, many of whom have high‐risk characteristics, creates a milieu for both hyper‐ and hypoglycemia. Other groups have described a drop in hypoglycemia rates related to the use of standardized diabetes order sets and nurse and physician education, but this is the first study that used informatics in a prospective manner to identify patients who are at high risk for developing hypoglycemia and then specifically targeted those patients.[28] The alert process was modeled after a similar alert developed in our institution for identifying medicine patients at risk for sepsis.[29] Given the paucity of data related to inpatient glycemia risk reduction, this study is particularly relevant for improving patient safety.
The major limitation of this study is that it was not randomized at the patient level. Patients were assigned to intervention and control groups based on their occupancy on specific hospital floors to avoid treatment bias. Bias was assessed due to this nonrandom assignment by comparing demographic and clinical factors of HR patients between intervention and control floors, and found significant differences in HbA1c and admitting diagnosis. As the control group had lower HbA1c values than the intervention group, and it is known from the Diabetes Control and Complications Trial and Action to Control Cardiovascular Risk in Diabetes trial that lower HbA1c increases the risk of hypoglycemia, our results may be biased by the level of glucose control on admission.[30, 31] Admitting diagnoses differed significantly between intervention and control patients as did the Charlson Comorbidity Index score; however, the hypoglycemia alert system does not include patient diagnoses or comorbidities, and as such provided equipoise with regard to risk reduction regardless of presenting illness. This study included trained nurses, which may be beyond the scope of every institution and thereby limit the effectiveness of the alert in reducing risk. However, as a result of this study, the alert was expanded to other acute care floors at our hospital as well as other hospitals in the Barnes‐Jewish Hospital system.
In summary, this study showed a 68% decrease in episodes of SH in a high‐risk patient cohort on diabetic medications using a hypoglycemia alert system. The results of this study demonstrate the validity of a systems‐based approach to reduce SH in high‐risk inpatients.
Disclosures
This work was funded by the Barnes‐Jewish Hospital Foundation The authors report no conflicts of interest.
- , , , , , . Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29:e445–e448.
- , , , et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med. 2003;163:1825–1829.
- , , , , , . Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32:1153–1157.
- , , , , . Association of hypoglycemia and cardiac ischemia: a study based on continuous glucose monitoring. Diabetes Care. 2003;26:1485–1489.
- , , , et al. Diabetes‐related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396.
- , , , , . Inpatient insulin orders: are patients getting what is prescribed? J Hosp Med. 2011;9:526–529.
- , , , . Prediction and prevention of treatment‐related inpatient hypoglycemia. J Diabetes Sci Technol. 2012;6:302–309.
- , . Collaboration between nurses and physicians. Image J Nurs Sch. 1988;20:145–149.
- , , , et al. Glucometrics—assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2005;8:560–569.
- ACE/ADA Task Force on Inpatient Diabetes. American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Diabetes Care. 2006;29:1955–1962.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353–369.
- . Role‐play using SBAR technique to improve observed communication skills in senior nursing students. J Nurs Educ. 2011;50:79–87.
- SHM Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Society of Hospital Medicine website, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org. Accessed on February 12, 2011.
- , . An internet service supporting quality assessment of inpatient glycemic control. J Diabetes Sci and Technol. 2008;2:402–408.
- , , , et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682.
- , , . Glycemic management in medical and surgical patients in the non‐ICU setting. Curr Diab Rep. 2013;13:96–106.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
- UK Prospective Diabetes Study Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853.
- , , . Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912.
- . Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14:750–756.
- , , , et al.; for the NICE‐SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–1118.
- , , , , , . Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–1901.
- , , , et al. Risk factors for inpatient hypoglycemia during subcutaneous insulin therapy in non‐critically ill patients with type 2 diabetes. J Diabetes Sci Technol. 2012;6:1022–1029.
- , , . Minimizing hypoglycemia in the wake of a tight glycemic control protocol in hospitalized patients. J Nurs Care Qual. 2010;25:255–260.
- , , , . The economic and quality of life impact of hypoglycemia. Eur J Health Econ. 2005;6:197–202.
- , , , , , . Economic and clinical impact of inpatient diabetic hypoglycemia. Endocr Pract. 2009;15:302–312.
- , , , et al. Mild hypoglycemia is strongly associated with increased intensive care unit length of stay. Ann Intensive Care. 2011;49:1–49.
- , , , et al. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Jt Comm J Qual Patient Saf. 2012;38:195–206.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39:469–473.
- The Diabetes Control and Complications Trial Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med. 1991;90:450–459.
- , , , et al. The effects of baseline characteristics, glycemia treatment approach, and glycated hemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:1–12.
- , , , , , . Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29:e445–e448.
- , , , et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med. 2003;163:1825–1829.
- , , , , , . Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32:1153–1157.
- , , , , . Association of hypoglycemia and cardiac ischemia: a study based on continuous glucose monitoring. Diabetes Care. 2003;26:1485–1489.
- , , , et al. Diabetes‐related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396.
- , , , , . Inpatient insulin orders: are patients getting what is prescribed? J Hosp Med. 2011;9:526–529.
- , , , . Prediction and prevention of treatment‐related inpatient hypoglycemia. J Diabetes Sci Technol. 2012;6:302–309.
- , . Collaboration between nurses and physicians. Image J Nurs Sch. 1988;20:145–149.
- , , , et al. Glucometrics—assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2005;8:560–569.
- ACE/ADA Task Force on Inpatient Diabetes. American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Diabetes Care. 2006;29:1955–1962.
- , , , et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353–369.
- . Role‐play using SBAR technique to improve observed communication skills in senior nursing students. J Nurs Educ. 2011;50:79–87.
- SHM Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Society of Hospital Medicine website, Glycemic Control Quality Improvement Resource Room. Available at: http://www.hospitalmedicine.org. Accessed on February 12, 2011.
- , . An internet service supporting quality assessment of inpatient glycemic control. J Diabetes Sci and Technol. 2008;2:402–408.
- , , , et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682.
- , , . Glycemic management in medical and surgical patients in the non‐ICU setting. Curr Diab Rep. 2013;13:96–106.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
- UK Prospective Diabetes Study Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853.
- , , . Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912.
- . Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14:750–756.
- , , , et al.; for the NICE‐SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–1118.
- , , , , , . Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–1901.
- , , , et al. Risk factors for inpatient hypoglycemia during subcutaneous insulin therapy in non‐critically ill patients with type 2 diabetes. J Diabetes Sci Technol. 2012;6:1022–1029.
- , , . Minimizing hypoglycemia in the wake of a tight glycemic control protocol in hospitalized patients. J Nurs Care Qual. 2010;25:255–260.
- , , , . The economic and quality of life impact of hypoglycemia. Eur J Health Econ. 2005;6:197–202.
- , , , , , . Economic and clinical impact of inpatient diabetic hypoglycemia. Endocr Pract. 2009;15:302–312.
- , , , et al. Mild hypoglycemia is strongly associated with increased intensive care unit length of stay. Ann Intensive Care. 2011;49:1–49.
- , , , et al. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Jt Comm J Qual Patient Saf. 2012;38:195–206.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39:469–473.
- The Diabetes Control and Complications Trial Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med. 1991;90:450–459.
- , , , et al. The effects of baseline characteristics, glycemia treatment approach, and glycated hemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:1–12.
© 2014 Society of Hospital Medicine
Telemedicine on Capitol Hill
Hospitalist Ateev Mehrotra, MD, MPH, garnered an audience in Congress last month with his speech on telemedicine that called on lawmakers to take a deliberate approach to the healthcare strategy.
Dr. Mehrotra, a staff physician at Beth Israel Deaconess Medical Center in Boston and a policy analyst for RAND Corporation in Santa Monica, Calif., testified before a health subcommittee of the Energy & Commerce Committee[PDF]. He urged politicians to understand that telemedicine has immense potential but needs to be implemented deliberately to ensure that it provides quality care, improves access to those who need it most, and is used in the most cost-efficient manner.
He spoke with The Hospitalist after testifying:
Question: What do you hope the committee took away from your speech?
Answer: Go in with [your] eyes wide open. Experience tells us this is going to work in some ways and is not going to work in some ways. I think some people are naive and think telemedicine is perfect.
Q: Overutilization is a fear of yours. Tell me why.
A: For every great and remarkable intervention we have introduced in medicine, there has been this potential concern. I gave the example of cardiac catheterization, [which] has saved tens of thousands of lives probably. I can cite many other examples from MRIs to CTs [computed tomography] to robot-assisted surgery, etc., where that overuse issue is very significant. Economists believe [new technologies] are one of the greatest drivers of increased healthcare spending in the United States. With that as background, one shouldn’t be surprised that telemedicine would face the same issues.
Q: With a national push for telemedicine, is that overall a good thing?
A: Maybe I’m just too much of a doctor, but I think about this very much like I think about a drug. You have positive benefits, and you’ve got side effects. You need to be aware of the side effects … it doesn’t mean in many cases you don’t prescribe the drug because the drug is helping overall. If you take that same framework to telemedicine, I would say I’m overall very enthusiastic about the multitude of benefits … but not all telemedicine is the same. TH
Visit our website for more information on telemedicine and hospitalists.
Hospitalist Ateev Mehrotra, MD, MPH, garnered an audience in Congress last month with his speech on telemedicine that called on lawmakers to take a deliberate approach to the healthcare strategy.
Dr. Mehrotra, a staff physician at Beth Israel Deaconess Medical Center in Boston and a policy analyst for RAND Corporation in Santa Monica, Calif., testified before a health subcommittee of the Energy & Commerce Committee[PDF]. He urged politicians to understand that telemedicine has immense potential but needs to be implemented deliberately to ensure that it provides quality care, improves access to those who need it most, and is used in the most cost-efficient manner.
He spoke with The Hospitalist after testifying:
Question: What do you hope the committee took away from your speech?
Answer: Go in with [your] eyes wide open. Experience tells us this is going to work in some ways and is not going to work in some ways. I think some people are naive and think telemedicine is perfect.
Q: Overutilization is a fear of yours. Tell me why.
A: For every great and remarkable intervention we have introduced in medicine, there has been this potential concern. I gave the example of cardiac catheterization, [which] has saved tens of thousands of lives probably. I can cite many other examples from MRIs to CTs [computed tomography] to robot-assisted surgery, etc., where that overuse issue is very significant. Economists believe [new technologies] are one of the greatest drivers of increased healthcare spending in the United States. With that as background, one shouldn’t be surprised that telemedicine would face the same issues.
Q: With a national push for telemedicine, is that overall a good thing?
A: Maybe I’m just too much of a doctor, but I think about this very much like I think about a drug. You have positive benefits, and you’ve got side effects. You need to be aware of the side effects … it doesn’t mean in many cases you don’t prescribe the drug because the drug is helping overall. If you take that same framework to telemedicine, I would say I’m overall very enthusiastic about the multitude of benefits … but not all telemedicine is the same. TH
Visit our website for more information on telemedicine and hospitalists.
Hospitalist Ateev Mehrotra, MD, MPH, garnered an audience in Congress last month with his speech on telemedicine that called on lawmakers to take a deliberate approach to the healthcare strategy.
Dr. Mehrotra, a staff physician at Beth Israel Deaconess Medical Center in Boston and a policy analyst for RAND Corporation in Santa Monica, Calif., testified before a health subcommittee of the Energy & Commerce Committee[PDF]. He urged politicians to understand that telemedicine has immense potential but needs to be implemented deliberately to ensure that it provides quality care, improves access to those who need it most, and is used in the most cost-efficient manner.
He spoke with The Hospitalist after testifying:
Question: What do you hope the committee took away from your speech?
Answer: Go in with [your] eyes wide open. Experience tells us this is going to work in some ways and is not going to work in some ways. I think some people are naive and think telemedicine is perfect.
Q: Overutilization is a fear of yours. Tell me why.
A: For every great and remarkable intervention we have introduced in medicine, there has been this potential concern. I gave the example of cardiac catheterization, [which] has saved tens of thousands of lives probably. I can cite many other examples from MRIs to CTs [computed tomography] to robot-assisted surgery, etc., where that overuse issue is very significant. Economists believe [new technologies] are one of the greatest drivers of increased healthcare spending in the United States. With that as background, one shouldn’t be surprised that telemedicine would face the same issues.
Q: With a national push for telemedicine, is that overall a good thing?
A: Maybe I’m just too much of a doctor, but I think about this very much like I think about a drug. You have positive benefits, and you’ve got side effects. You need to be aware of the side effects … it doesn’t mean in many cases you don’t prescribe the drug because the drug is helping overall. If you take that same framework to telemedicine, I would say I’m overall very enthusiastic about the multitude of benefits … but not all telemedicine is the same. TH
Visit our website for more information on telemedicine and hospitalists.
Overworked Hospitalists Linked to Higher Costs, Longer Lengths of Stay
As hospitalist workloads increase, so do hospital costs and patients' lengths of stay (LOS), according to findings in a recent study.
Those results, says SHM President Burke T. Kealey, MD, SFHM, provide a good starting point to determine an ideal patient census for hospitalists.
"Pushing hospitalist workloads ever higher to meet the demands of patient-care needs or flawed payment models has costs associated with it," says Dr. Kealey, associate medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn. "The costs may be borne by the system or by patients, but there are costs."
For the study published in JAMA Internal Medicine, researchers analyzed data from 20,241 hospitalizations involving 13,916 patients seen by hospitalists at the Christiana Care Health System in Newark, Del., between February 2008 and January 2011.
For hospital occupancies less than 75%, they found that LOS increased from 5.5 to 7.5 days as workload increased. For occupancies of 75% to 85%, LOS increased to about 8 days with higher workloads. For occupancies greater than 85%, the LOS decreased slightly and then increased significantly with higher workloads, with this change occurring at about 15 patients or more per hospitalist.
Costs were also significantly associated with an increase in workload. As the study notes, benchmark recommendations for an individual hospitalist’s workload range from 10 to 15 patient encounters per day.
Dr. Kealey says the findings seem to support the conventional wisdom that hospitalists should ideally see no more than 15 patients a day. He notes, however, that deciding the optimal number of cases for a given practice depends on several factors, including duration of shift, the availability of physician extenders, and the addition of surgical or cardiology cases.
"We won't be able as a specialty to fully realize our potential until we understand and apply the learnings about workload into our practices to ensure hospitalist career sustainability, system health, and best patient care," Dr. Kealey says. "This paper really gets the discussion going."
For more from Dr. Kealey on hospitalist workloads, read his recent blog post on "The Hospital Leader." TH
Visit our website for more information about hospitalist workloads.
As hospitalist workloads increase, so do hospital costs and patients' lengths of stay (LOS), according to findings in a recent study.
Those results, says SHM President Burke T. Kealey, MD, SFHM, provide a good starting point to determine an ideal patient census for hospitalists.
"Pushing hospitalist workloads ever higher to meet the demands of patient-care needs or flawed payment models has costs associated with it," says Dr. Kealey, associate medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn. "The costs may be borne by the system or by patients, but there are costs."
For the study published in JAMA Internal Medicine, researchers analyzed data from 20,241 hospitalizations involving 13,916 patients seen by hospitalists at the Christiana Care Health System in Newark, Del., between February 2008 and January 2011.
For hospital occupancies less than 75%, they found that LOS increased from 5.5 to 7.5 days as workload increased. For occupancies of 75% to 85%, LOS increased to about 8 days with higher workloads. For occupancies greater than 85%, the LOS decreased slightly and then increased significantly with higher workloads, with this change occurring at about 15 patients or more per hospitalist.
Costs were also significantly associated with an increase in workload. As the study notes, benchmark recommendations for an individual hospitalist’s workload range from 10 to 15 patient encounters per day.
Dr. Kealey says the findings seem to support the conventional wisdom that hospitalists should ideally see no more than 15 patients a day. He notes, however, that deciding the optimal number of cases for a given practice depends on several factors, including duration of shift, the availability of physician extenders, and the addition of surgical or cardiology cases.
"We won't be able as a specialty to fully realize our potential until we understand and apply the learnings about workload into our practices to ensure hospitalist career sustainability, system health, and best patient care," Dr. Kealey says. "This paper really gets the discussion going."
For more from Dr. Kealey on hospitalist workloads, read his recent blog post on "The Hospital Leader." TH
Visit our website for more information about hospitalist workloads.
As hospitalist workloads increase, so do hospital costs and patients' lengths of stay (LOS), according to findings in a recent study.
Those results, says SHM President Burke T. Kealey, MD, SFHM, provide a good starting point to determine an ideal patient census for hospitalists.
"Pushing hospitalist workloads ever higher to meet the demands of patient-care needs or flawed payment models has costs associated with it," says Dr. Kealey, associate medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn. "The costs may be borne by the system or by patients, but there are costs."
For the study published in JAMA Internal Medicine, researchers analyzed data from 20,241 hospitalizations involving 13,916 patients seen by hospitalists at the Christiana Care Health System in Newark, Del., between February 2008 and January 2011.
For hospital occupancies less than 75%, they found that LOS increased from 5.5 to 7.5 days as workload increased. For occupancies of 75% to 85%, LOS increased to about 8 days with higher workloads. For occupancies greater than 85%, the LOS decreased slightly and then increased significantly with higher workloads, with this change occurring at about 15 patients or more per hospitalist.
Costs were also significantly associated with an increase in workload. As the study notes, benchmark recommendations for an individual hospitalist’s workload range from 10 to 15 patient encounters per day.
Dr. Kealey says the findings seem to support the conventional wisdom that hospitalists should ideally see no more than 15 patients a day. He notes, however, that deciding the optimal number of cases for a given practice depends on several factors, including duration of shift, the availability of physician extenders, and the addition of surgical or cardiology cases.
"We won't be able as a specialty to fully realize our potential until we understand and apply the learnings about workload into our practices to ensure hospitalist career sustainability, system health, and best patient care," Dr. Kealey says. "This paper really gets the discussion going."
For more from Dr. Kealey on hospitalist workloads, read his recent blog post on "The Hospital Leader." TH
Visit our website for more information about hospitalist workloads.