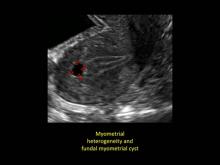
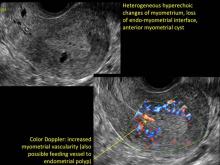
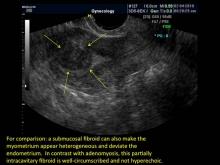
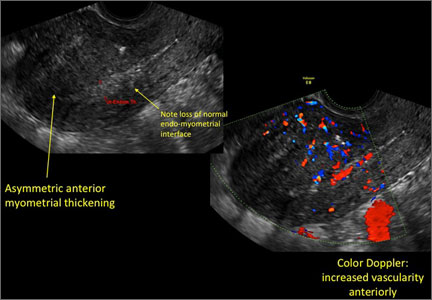
User login
Novel agent shows promising activity in heavily pretreated NHL

©ASCO/Rodney White
CHICAGO—The novel, oral selective inhibitor of nuclear transport known as selinexor (KPT-330) can safely be given as monotherapy to patients with heavily pretreated non-Hodgkin lymphoma (NHL), according to a presentation at the 2014 ASCO Annual Meeting.
“Selinexor has favorable pharmacokinetic and pharmacodynamic characteristics,” said presenter Martin Gutierrez, MD, of the John Theurer Cancer Center at Hackensack University Medical Center in New Jersey.
Selinexor is a slowly reversible, selective inhibitor of nuclear transport that inhibits XPO1, which is elevated in NHL, chronic lymphocytic leukemia (CLL), and other malignances.
“It shows single-agent anti-tumor activity across all NHL types, with durable cancer control of more than 9 months [and] marked activity across germ cell B (GCB), non-germ cell B, and double-hit diffuse large B-cell lymphoma (DLBCL),” Dr Gutierrez said.
He provided an update of the ongoing phase 1 dose-escalation study at the meeting as abstract 8518.
The study now includes 51 patients, half of whom have NHL. Their median age is 60 years.
The patients received selinexor across 8 dose levels, ranging from 3 mg/m2 to 60 mg/m2. Dosing at 60 mg/m2 twice weekly is ongoing, and the maximum-tolerated dose has not been reached.
Among the 43 evaluable patients, the disease control rate was 74%, the overall response rate was 28%, and the complete response rate was 5%.
“All patients who had their disease controlled had a reduction in lymph nodes and some degree of activity across all dose levels,” Dr Gutierrez said. “GCB and non-GCB patients responded similarly.”
The length of response was up to 632 days in the DLBCL group.
Most adverse events were gastrointestinal in nature, and most of them were grade 1 or 2. The most common adverse events were nausea, anorexia, and fatigue.
The investigators found a higher incidence of side effects in the first treatment cycle. The dosing schedule was interrupted and reduced to maintain steady state levels.
“The results suggest that an intermittent dosing schedule optimally induces a steady state with maximal induction of XPO1 mRNA,” Dr Gutierrez said.
There were 3 dose-limiting toxicities, including 1 multiple myeloma patient with grade 4 thrombocytopenia, 1 follicular lymphoma patient with grade 4 thrombocytopenia, and 1 CLL patient with grade 2 fatigue.
ASCO discussant Owen O’Connor, MD, from Columbia University Medical Center in New York, commented, “These clinical data are interesting with a provocative target. I applaud the investigators for doing a trial across a diversity of B- and T-cell lymphomas . . . . The results suggest a potential effect in a rare subset of lymphoma patients that have little treatment options.”
Frontline trials of selinexor are planned, including patients with Richter transformation and follicular lymphoma.
Selinexor recently received orphan drug status from the US Food and Drug Administration for the treatment of DLBCL. ![]()

©ASCO/Rodney White
CHICAGO—The novel, oral selective inhibitor of nuclear transport known as selinexor (KPT-330) can safely be given as monotherapy to patients with heavily pretreated non-Hodgkin lymphoma (NHL), according to a presentation at the 2014 ASCO Annual Meeting.
“Selinexor has favorable pharmacokinetic and pharmacodynamic characteristics,” said presenter Martin Gutierrez, MD, of the John Theurer Cancer Center at Hackensack University Medical Center in New Jersey.
Selinexor is a slowly reversible, selective inhibitor of nuclear transport that inhibits XPO1, which is elevated in NHL, chronic lymphocytic leukemia (CLL), and other malignances.
“It shows single-agent anti-tumor activity across all NHL types, with durable cancer control of more than 9 months [and] marked activity across germ cell B (GCB), non-germ cell B, and double-hit diffuse large B-cell lymphoma (DLBCL),” Dr Gutierrez said.
He provided an update of the ongoing phase 1 dose-escalation study at the meeting as abstract 8518.
The study now includes 51 patients, half of whom have NHL. Their median age is 60 years.
The patients received selinexor across 8 dose levels, ranging from 3 mg/m2 to 60 mg/m2. Dosing at 60 mg/m2 twice weekly is ongoing, and the maximum-tolerated dose has not been reached.
Among the 43 evaluable patients, the disease control rate was 74%, the overall response rate was 28%, and the complete response rate was 5%.
“All patients who had their disease controlled had a reduction in lymph nodes and some degree of activity across all dose levels,” Dr Gutierrez said. “GCB and non-GCB patients responded similarly.”
The length of response was up to 632 days in the DLBCL group.
Most adverse events were gastrointestinal in nature, and most of them were grade 1 or 2. The most common adverse events were nausea, anorexia, and fatigue.
The investigators found a higher incidence of side effects in the first treatment cycle. The dosing schedule was interrupted and reduced to maintain steady state levels.
“The results suggest that an intermittent dosing schedule optimally induces a steady state with maximal induction of XPO1 mRNA,” Dr Gutierrez said.
There were 3 dose-limiting toxicities, including 1 multiple myeloma patient with grade 4 thrombocytopenia, 1 follicular lymphoma patient with grade 4 thrombocytopenia, and 1 CLL patient with grade 2 fatigue.
ASCO discussant Owen O’Connor, MD, from Columbia University Medical Center in New York, commented, “These clinical data are interesting with a provocative target. I applaud the investigators for doing a trial across a diversity of B- and T-cell lymphomas . . . . The results suggest a potential effect in a rare subset of lymphoma patients that have little treatment options.”
Frontline trials of selinexor are planned, including patients with Richter transformation and follicular lymphoma.
Selinexor recently received orphan drug status from the US Food and Drug Administration for the treatment of DLBCL. ![]()

©ASCO/Rodney White
CHICAGO—The novel, oral selective inhibitor of nuclear transport known as selinexor (KPT-330) can safely be given as monotherapy to patients with heavily pretreated non-Hodgkin lymphoma (NHL), according to a presentation at the 2014 ASCO Annual Meeting.
“Selinexor has favorable pharmacokinetic and pharmacodynamic characteristics,” said presenter Martin Gutierrez, MD, of the John Theurer Cancer Center at Hackensack University Medical Center in New Jersey.
Selinexor is a slowly reversible, selective inhibitor of nuclear transport that inhibits XPO1, which is elevated in NHL, chronic lymphocytic leukemia (CLL), and other malignances.
“It shows single-agent anti-tumor activity across all NHL types, with durable cancer control of more than 9 months [and] marked activity across germ cell B (GCB), non-germ cell B, and double-hit diffuse large B-cell lymphoma (DLBCL),” Dr Gutierrez said.
He provided an update of the ongoing phase 1 dose-escalation study at the meeting as abstract 8518.
The study now includes 51 patients, half of whom have NHL. Their median age is 60 years.
The patients received selinexor across 8 dose levels, ranging from 3 mg/m2 to 60 mg/m2. Dosing at 60 mg/m2 twice weekly is ongoing, and the maximum-tolerated dose has not been reached.
Among the 43 evaluable patients, the disease control rate was 74%, the overall response rate was 28%, and the complete response rate was 5%.
“All patients who had their disease controlled had a reduction in lymph nodes and some degree of activity across all dose levels,” Dr Gutierrez said. “GCB and non-GCB patients responded similarly.”
The length of response was up to 632 days in the DLBCL group.
Most adverse events were gastrointestinal in nature, and most of them were grade 1 or 2. The most common adverse events were nausea, anorexia, and fatigue.
The investigators found a higher incidence of side effects in the first treatment cycle. The dosing schedule was interrupted and reduced to maintain steady state levels.
“The results suggest that an intermittent dosing schedule optimally induces a steady state with maximal induction of XPO1 mRNA,” Dr Gutierrez said.
There were 3 dose-limiting toxicities, including 1 multiple myeloma patient with grade 4 thrombocytopenia, 1 follicular lymphoma patient with grade 4 thrombocytopenia, and 1 CLL patient with grade 2 fatigue.
ASCO discussant Owen O’Connor, MD, from Columbia University Medical Center in New York, commented, “These clinical data are interesting with a provocative target. I applaud the investigators for doing a trial across a diversity of B- and T-cell lymphomas . . . . The results suggest a potential effect in a rare subset of lymphoma patients that have little treatment options.”
Frontline trials of selinexor are planned, including patients with Richter transformation and follicular lymphoma.
Selinexor recently received orphan drug status from the US Food and Drug Administration for the treatment of DLBCL. ![]()
Method allows for high-resolution cell imaging

filaments (red), microtubules
(green), and nuclei (blue)
National Institutes of Health
A new technique allows scientists to view the cell cytoskeleton with “unprecedented resolution,” according to a paper published in Nature Methods.
A group of researchers exploited the properties of a fluorescent molecule they developed to generate 2 probes for imaging the cytoskeleton.
The team said these probes can easily enter live cells, are non-toxic, have long-lasting signals, and offer better image resolution than current imaging techniques.
This research began when Kai Johnsson, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland, and his colleagues developed a fluorescent molecule called silicon-rhodamine (SiR).
The molecule switches “on” only when it binds to the charged surface of a protein like those found on the cytoskeleton. When SiR switches on, it emits light at far-red wavelengths.
The challenge was getting SiR to bind specifically to the cytoskeleton’s proteins, actin and tubulin. To achieve this, the researchers fused SiR molecules with compounds that bind tubulin or actin.
The resulting hybrid molecules consist of a SiR molecule, which provides the fluorescent signal, and a molecule of a natural compound that can bind the target protein.
One such compound was docetaxel, an anticancer drug that binds tubulin, and the other was jasplakinolide, which specifically binds the cytoskeletal form of actin. Both compounds, which are used here in very low, non-toxic concentrations, can easily pass through the cell’s membrane and into the cell itself.
The probes, called SiR-tubulin and SiR-actin, were used to visualize the dynamics of the cytoskeleton in human skin cells. Because the light signal of the probes is emitted in the far-red spectrum, it is easy to isolate from background noise, which generates images of unprecedented resolution when used with super-resolution microscopy.
An additional advantage of the probes, according to the researchers, is their practicality.
“You just add them directly into your cell culture, and they are taken up by the cells,” Dr Johnsson said.
The probes don’t require any washing or preparation of the cells before administration or any subsequent washing steps, which helps in maintaining the stability of their environment and their natural biological functions.
“Up to now, no probes were available that would allow you to get high-quality images of microtubules and microfilaments in living cells without some kind of genetic modification,” Dr Johnsson said.
“With this work, we provide the biological community with 2 high-performing, high-contrast fluorogenic probes that emit in the non-phototoxic part of the light spectrum, and can be even used in tissues like whole-blood samples.” ![]()

filaments (red), microtubules
(green), and nuclei (blue)
National Institutes of Health
A new technique allows scientists to view the cell cytoskeleton with “unprecedented resolution,” according to a paper published in Nature Methods.
A group of researchers exploited the properties of a fluorescent molecule they developed to generate 2 probes for imaging the cytoskeleton.
The team said these probes can easily enter live cells, are non-toxic, have long-lasting signals, and offer better image resolution than current imaging techniques.
This research began when Kai Johnsson, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland, and his colleagues developed a fluorescent molecule called silicon-rhodamine (SiR).
The molecule switches “on” only when it binds to the charged surface of a protein like those found on the cytoskeleton. When SiR switches on, it emits light at far-red wavelengths.
The challenge was getting SiR to bind specifically to the cytoskeleton’s proteins, actin and tubulin. To achieve this, the researchers fused SiR molecules with compounds that bind tubulin or actin.
The resulting hybrid molecules consist of a SiR molecule, which provides the fluorescent signal, and a molecule of a natural compound that can bind the target protein.
One such compound was docetaxel, an anticancer drug that binds tubulin, and the other was jasplakinolide, which specifically binds the cytoskeletal form of actin. Both compounds, which are used here in very low, non-toxic concentrations, can easily pass through the cell’s membrane and into the cell itself.
The probes, called SiR-tubulin and SiR-actin, were used to visualize the dynamics of the cytoskeleton in human skin cells. Because the light signal of the probes is emitted in the far-red spectrum, it is easy to isolate from background noise, which generates images of unprecedented resolution when used with super-resolution microscopy.
An additional advantage of the probes, according to the researchers, is their practicality.
“You just add them directly into your cell culture, and they are taken up by the cells,” Dr Johnsson said.
The probes don’t require any washing or preparation of the cells before administration or any subsequent washing steps, which helps in maintaining the stability of their environment and their natural biological functions.
“Up to now, no probes were available that would allow you to get high-quality images of microtubules and microfilaments in living cells without some kind of genetic modification,” Dr Johnsson said.
“With this work, we provide the biological community with 2 high-performing, high-contrast fluorogenic probes that emit in the non-phototoxic part of the light spectrum, and can be even used in tissues like whole-blood samples.” ![]()

filaments (red), microtubules
(green), and nuclei (blue)
National Institutes of Health
A new technique allows scientists to view the cell cytoskeleton with “unprecedented resolution,” according to a paper published in Nature Methods.
A group of researchers exploited the properties of a fluorescent molecule they developed to generate 2 probes for imaging the cytoskeleton.
The team said these probes can easily enter live cells, are non-toxic, have long-lasting signals, and offer better image resolution than current imaging techniques.
This research began when Kai Johnsson, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland, and his colleagues developed a fluorescent molecule called silicon-rhodamine (SiR).
The molecule switches “on” only when it binds to the charged surface of a protein like those found on the cytoskeleton. When SiR switches on, it emits light at far-red wavelengths.
The challenge was getting SiR to bind specifically to the cytoskeleton’s proteins, actin and tubulin. To achieve this, the researchers fused SiR molecules with compounds that bind tubulin or actin.
The resulting hybrid molecules consist of a SiR molecule, which provides the fluorescent signal, and a molecule of a natural compound that can bind the target protein.
One such compound was docetaxel, an anticancer drug that binds tubulin, and the other was jasplakinolide, which specifically binds the cytoskeletal form of actin. Both compounds, which are used here in very low, non-toxic concentrations, can easily pass through the cell’s membrane and into the cell itself.
The probes, called SiR-tubulin and SiR-actin, were used to visualize the dynamics of the cytoskeleton in human skin cells. Because the light signal of the probes is emitted in the far-red spectrum, it is easy to isolate from background noise, which generates images of unprecedented resolution when used with super-resolution microscopy.
An additional advantage of the probes, according to the researchers, is their practicality.
“You just add them directly into your cell culture, and they are taken up by the cells,” Dr Johnsson said.
The probes don’t require any washing or preparation of the cells before administration or any subsequent washing steps, which helps in maintaining the stability of their environment and their natural biological functions.
“Up to now, no probes were available that would allow you to get high-quality images of microtubules and microfilaments in living cells without some kind of genetic modification,” Dr Johnsson said.
“With this work, we provide the biological community with 2 high-performing, high-contrast fluorogenic probes that emit in the non-phototoxic part of the light spectrum, and can be even used in tissues like whole-blood samples.” ![]()
A3 to Improve STAT
STAT is an abbreviation of the Latin word statim, meaning immediately,[1] and has been a part of healthcare's lexicon for almost as long as there have been hospitals. STAT conveys a sense of urgency, compelling those who hear STAT to act quickly. Unfortunately, given the lack of a consistent understanding of STAT, the term in reality often has an alternate use: to hurry up or to complete sooner than routine, and is sometimes used to circumvent a system that is perceived to be too slow to accomplish a routine task in a timely manner.
As part of a larger systems redesign effort to improve patient safety and quality of care, an institutional review board (IRB)‐approved qualitative study was conducted on 2 medical‐surgical units in a US Department of Veterans Affairs (VA) hospital to explore communication patterns between physicians and nurses.[2] The study revealed wide variation in understanding between physicians and nurses on the ordering and administration of STAT medication. Physicians were unaware that when they placed a STAT order into the computerized patient record system (CPRS), nurses were not automatically alerted about the order. At this facility, nurses did not carry pagers. Although each unit had a supply of wireless telephones, they were often unreliable and therefore not used consistently. Nurses were required by policy to check the CPRS for new orders every 2 hours. This was an inefficient and possibly dangerous process,[3] because if a nurse was not expecting a STAT order, 2 hours could elapse before she or he saw the order in the CPRS and began to look for the medication. A follow‐up survey completed by physicians, nurses, pharmacists, and pharmacy technicians demonstrated stark differences on the definition of STAT and overlap with similar terms such as NOW and ASAP. Interviews with ordering providers indicated that 36% of the time a STAT was ordered it was not clinically urgent, but instead ordered STAT to speed up the process.
The STAT medication process was clearly in need of improvement, but previous quality improvement projects in our organization had varying degrees of success. For example, we used Lean methodology in an attempt to improve our discharge process. We conducted a modified rapid process discharge improvement workshop[4] structured in phases over 4 weeks. During the workshops, a strong emphasis remained on the solutions to the problem, and we were unable to help the team move from a mindset of fix it to create it. This limited the buy‐in of team members, the creativity of their ideas for improvement, and ultimately the momentum to improve the process.
In this article we describe our adaptation of A3 Thinking,[5, 6] a structure for guiding quality improvement based in Lean methodology, to improve the STAT medication process. We chose A3 Thinking for several reasons. A3 Thinking focuses on process improvement and thus aligned well with our interest in improving the STAT medication process. A3 Thinking also reveals otherwise hidden nonvalue‐added activities that should be eliminated.[7] Finally A3 Thinking reinforces a deeper understanding of the way the work is currently being done, providing critical information needed before making a change. This provides a tremendous opportunity to look at work differently and see opportunities for improvement.[8] Given these strengths as well as the lack of congruence between what the STAT process should consist of and how the STAT process was actually being used in our organization, A3 Thinking offered the best fit between an improvement process and the problem to be solved.
METHODS
A search of healthcare literature yielded very few studies on the STAT process.[9, 10] Only 1 intervention to improve the process was found, and this focused on a specific procedure.[10] An informal survey of local VA and non‐VA hospitals regarding their experiences with the STAT medication process revealed insufficient information to aid our efforts. We next searched the business and manufacturing literature and found examples of how the Lean methodology was successfully applied to other problems in healthcare, including improving pediatric surgery workflow and decreasing ventilator‐associated pneumonia.[11, 12]
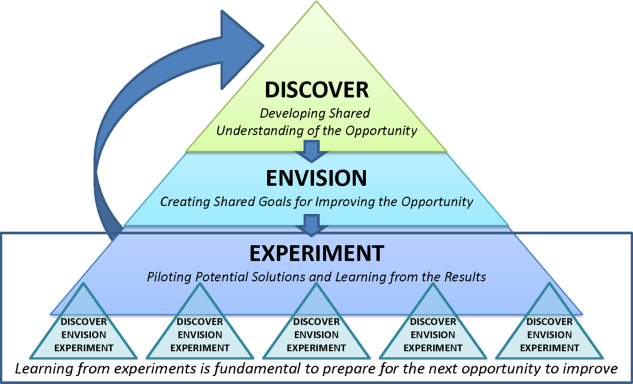
Therefore, the STAT project was structured to adapt a problem‐solving process commonly used in Lean organizationsA3 Thinkingwhich challenges team members to work through a discovery phase to develop a shared understanding of the process, an envisioning phase to conceptualize an ideal process experience, and finally an experimentation phase to identify and trial possible solutions through prioritization, iterative testing, structured reflection, and adjustment on resulting changes. Our application of the term experimentation in this context is distinct from that of controlled experimentation in clinical research; the term is intended to convey iterative learning as changes are tested, evaluated, and modified during this quality improvement project. Figure 1 displays a conceptual model of our adaptation of A3 Thinking. As this was a quality‐improvement project, it was exempt from IRB review.
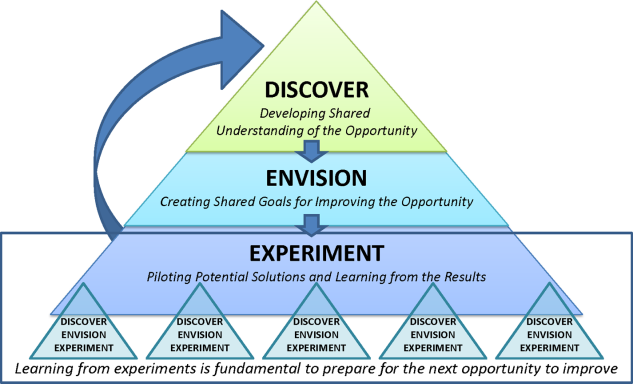
DISCOVERY
To begin the discovery phase, a workgroup consisting of representatives of all groups that had a role in the STAT process (ie, physician, pharmacist, nurse, pharmacy technician, clerk) gathered to identify the opportunity we are looking to address and learn from each other's individual experiences with the STAT medication process. The group was facilitated by an industrial engineer familiar with the A3 Thinking process. The team completed a mapping exercise to lay out, step‐by‐step, the current STAT medication process. This activity allowed the team to build shared empathy with others' experiences and to appreciate the challenges experienced by others through their individual responsibilities in the process. The current process was found to consist of 4 overarching components: a provider entered the STAT order into the CPRS; the order was verified by a pharmacist; a pharmacy technician delivered the medication to the unit (or a nurse retrieved the medication from the Omnicell (Omnicell Inc., Mountain View, CA), a proprietary automated medication dispensing system); and finally the nurse administered the medication to a patient.
A large, color‐coded flow map of the STAT medication process was constructed over several meetings to capture all perspectives and allow team members to gather feedback from their peers. To further our understanding of the current process, the team participated in a modified Go to the Gemba (ie, go to where the work is done)[13] on a real‐time STAT order. Once all workgroup members were satisfied that the flow map represented the current state of the STAT medication process, we came to a consensus on the goals needed to meet our main objective.
We agreed that our main objective was that STAT medication orders should be recognized, verified, and administered to patients in a timely and appropriate manner to ensure quality care. We identified 3 goals to meet this objective: (1) STAT should be consistently defined and understood by everyone; (2) an easy, intuitive STAT process should be available for all stakeholders; and (3) the STAT process should be transparent and ideally visual so that everyone involved can understand at which point in the process a specific STAT order is currently situated. We also identified additional information we would need to reach the goals.
Shortly after the process‐mapping sessions, 2 workgroup members conducted real‐time STAT order time studies to track medications from order to administration. Three time periods in the STAT process were identified for observation and measurement: the time from physician order entry in the CPRS to the time a pharmacist verified the medication, the time from verification to when the medication arrived on the nursing unit, and the time from arrival on the nursing unit to when that medication was administered. Using a data‐collection template, each time period was recorded, and 28 time studies were collected over 1 month. To monitor the progress of our initiatives, the time study was repeated 3 months into the project.
ENVISIONING
Following the discovery phase, the team was better equipped to identify the specific changes needed to achieve an improved process. The envisioning phase allowed the team freedom to imagine an ideal process barring any preconceived notion of constraints within the current process.
In 2 meetings we brainstormed as many improvement ideas as possible. To prioritize and focus our ideas, we developed a matrix (see Supporting Information, Appendix A, in the online version of this article), placing our ideas in 1 of 4 quadrants based on the anticipated effort to implement the change (x‐axis) and impact of making the change (y‐axis). The matrix helped us see that some ideas would be relatively simple to implement (eg, color‐coded bags for STAT medication delivery), whereas others would require more sophisticated efforts and involvement of other people (eg, monthly education sessions to resident physicians).
EXPERIMENTING
Experiments were conducted to meet each of the 3 goals identified above. The team used the outcomes of the prioritization exercise to identify initial experiments to test. To build momentum by showing progress and improvement with a few quick wins, the team began with low‐effort/high‐impact opportunities. Each experiment followed a standard Plan‐Do‐Study‐Act (PDSA) cycle to encourage reflection, learning, adaptation, and adjustment as a result of the experiential learning process.[5]
Goal 1: STAT Should Be Consistently Defined and Understood by Everyone
To address the first goal, a subgroup collected policies and procedures related to the STAT medication administration process. The policy defined a STAT medication as a medication that has the potential to significantly and negatively impact a patient's clinical condition if not given within 30 minutes. The group found that the policy requiring a 30‐minute time to administration was clinically appropriate, reinforcing our goals to create a practice congruent with the policy.
A subgroup led by the pharmacy department collected data related to STAT medications on the 3 medical‐surgical units. Within 1 month, 550 STAT medications were ordered, consisting of medications ranging from furosemide to nicotine lozenges, the latter being a medication clearly outside of the policy definition of STAT. The workgroup reviewed the information and realized education would be required to align practice with policy. According to our matrix, education was a high‐impact/high‐effort activity, so efforts were focused on the high‐impact/low‐effort activities initially. We addressed educational opportunities in later PDSA cycles.
Goal 2: An Easy, Intuitive STAT Process for All Stakeholders
The CPRS contains prefabricated templates that conform to regulatory requirements and ensure completeness. However, the CPRS does not intuitively enable ordering providers to choose the time for the first dose of a new routine medication. This often creates a situation where a provider orders the medication STAT, so that the medication can be given earlier than the CPRS would otherwise allow. Although there is a check box, Give additional dose now, it was not being used because it was visually obscure in the interface. The CPRS restricted our ability to change the template for ordering medications to include a specific time for first‐dose administration before defaulting to the routine order; thus, complementary countermeasures were trialed first. These are outlined in Table 1.
| Countermeasure | Intended Outcome |
|---|---|
| Remove duplicate dosing frequencies from medication order template | Reduce list of dosing frequencies to sort through to find desired selection |
| Develop 1‐page job aid for ordering providers to utilize | Assist in the correct methods of ordering STAT, NOW, and routine medications |
| Added STAT ONCE as a dosing frequency selection | Clarify the medication, if ordered STAT, will only be a 1‐time administration to avoid the recurrence of a STAT order should the orders be transferred to a new unit with the patient |
| Modify existing policies to add STAT ONCE option | Ensure documentation is congruent with new expectations |
| Educate interns and residents with the job aid and a hands‐on how to ordering exercise | Inform ordering physicians on the available references for ordering and educate according to desired practice |
| Provide interns and residents with a visual job aid at their workstation and a hands‐on how to ordering exercise | In addition to providing information and educating according to desired practice, provide a just‐in‐time reference resource |
Goal 3: The STAT Process Should Be Transparent and Ideally Visual
During the time studies, the time period from when the medication arrived on the unit to the time it was administered to the patient averaged 34 minutes. Of 28 STAT orders followed through the entire process, 5 pharmacy technicians (26%) were not informed of 19 STAT medication orders requiring delivery, and 12 nurses (63%) were not notified of the delivery of those 19 medications. The remaining 9 STAT medications were stocked in the Omnicell. Informal interviews with nurses and pharmacy technicians, as well as input from the nurses and pharmacy technicians in our workgroup, revealed several explanations for these findings.
First, the delivering technicians could not always find the patient's nurse, and because the delivery procedure was not standardized, there was no consistency between technicians in where medications were delivered. Second, each unit had a different medication inventory stored in the Omnicell, and the inventory was frequently changed (eg, due to unit‐specific needs, backorders), which made it difficult for nurses to keep track of what was available in Omnicell at any given time. Finally, the STAT medication was not consistently labeled with a visual STAT notation, so even if a nurse saw that new medications had been delivered, he or she would not be able to easily identify which was STAT. The team made several low‐tech process changes to improve the visibility of a STAT medication and ensure reliable communication upon delivery. A subgroup of pharmacists, technicians, and nurses developed and implemented the countermeasures described in Table 2.
| Countermeasure | Intended Outcome |
|---|---|
| Designate delivery preferences with the patient's nurse as the first preference and a set location in the med room as the only alternative preference | Attempt to deliver medications directly to the patient's nurse as frequently as possible to eliminate any unnecessary delays and avoid miscommunication |
| Identify a location in each unit's med room to place a red bin to deliver the STAT medications that are unable to be delivered to the patient's nurse directly | Provide 1 alternate location to retrieve STAT medications if the technician is unable to locate the patient's nurse to deliver the medication directly |
| Utilize a plastic bag with a red STAT indication for transportation of STAT medications to the units | Provide a visual to assist in pharmacy technicians prioritizing their deliveries to the inpatient units |
| Utilize red STAT magnets on the patient's door frame to signal nurses a medication had been delivered to the med room | Provide a visual to assist in timely recognition of a STAT medication delivery given the technician was unable to find the nurse to hand it off directly |
RESULTS
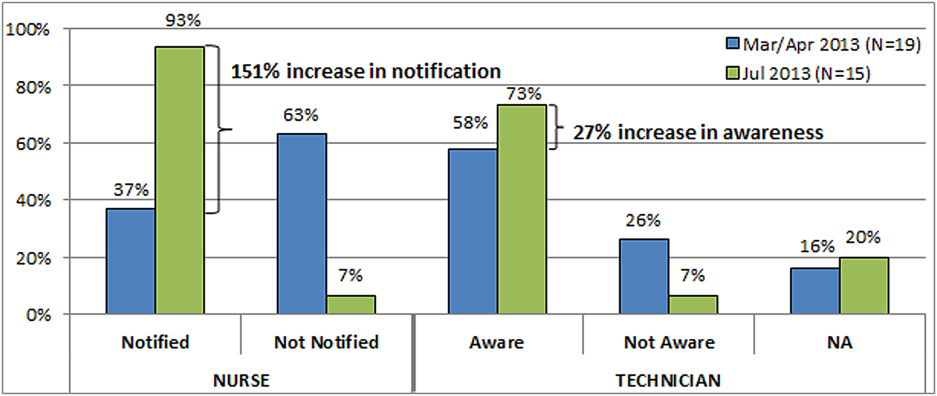
At the start of our project, the average time from STAT order to medication administration was 1 hour and 7 minutes (range, 6 minutes 2 hours and 22 minutes). As a result of the 2 sets of countermeasures outlined in Tables 1 and 2, the average total time from STAT order entry to administration decreased by 21% to an average of 53 minutes. The total time from medication delivery to administration decreased by 26% from 34 minutes to 25 minutes postimplementation. On average, 391 STAT medications were ordered per month during the project period, which represents a decrease of 9.5% from the 432 orders per month for the same time period the previous year. After implementing the countermeasures in Table 2, we followed another 26 STAT medications through the process to evaluate our efforts. Of 15 STAT medications requiring delivery, only 1 nurse (7%) was not notified of the delivery of a STAT medication, and 1 pharmacy technician (7%) was not informed the medication was STAT. The 151% increase in notification of nurses to delivery of a STAT medication suggests that use of the STAT bags, STAT magnets on patient doors, and whenever possible direct delivery of STAT medications to the nurse has improved communication between the technicians and nurses. Similarly, the 27% increase in technician awareness of a STAT designation suggests STAT is being better communicated to them. The improvement in awareness and notification of a STAT medication is summarized in Figure 2.
Due to time and financial constraints, the following limitations may have affected our findings. First, resident physicians were not directly represented in our discussions. Attending medicine hospitalists provided the physician perspective, which provides a biased view given their intimate knowledge of the CPRS and additional years of experience. Similarly, nurse perspectives were limited to staff and clinical nurse leaders. Last, our low‐cost approach was mandated by limited resources; a more resource‐rich environment may have devised alternative approaches.
CONCLUSIONS
Adapting A3 Thinking for process improvement was a low‐cost/low‐tech option for a VA facility. Having buy‐in from all levels was crucial to the success of the project. The size and diversity of the group was also very important, as different opinions and aspects of the process were represented. Cross‐discipline relationships and respect were formed, which will be valuable for collaboration in future projects. Although we focused on the STAT medication process, other quality‐improvement projects could also benefit from A3 Thinking. Moreover, there were enough people to serve as ambassadors, taking the project back to their work areas to share with their peers, gather consensus, and elicit additional feedback. The collaboration led to comprehensive understanding of the process, the nature of the problems within the process, and the complexity of solving the problem. For example, although the number of STAT orders did not decrease dramatically, we have learned from these experiments that we may need to change how we approach structuring additional experiments. Future work will focus on increasing communication between physicians and nurses when placing STAT medication orders, enhancing resident education to ensure appropriate use of the STAT designation, and continuing our efforts to improve the delivery process of STAT medications.
Other quality‐improvement methodologies we could have used include: total quality management (TQM), continuous quality improvement (CQI), business process redesign, Lean, Six Sigma, and others.[14] Differences between these can be broadly classified as putting an emphasis on people (eg, inclusion of front line staff in CQI or leadership in TQM) or on process (eg, understanding process function to reduce waste in Lean or statistical process control in Six Sigma).[14] Using A3 Thinking methodology was more useful than these others for the STAT medication process for some very important reasons. The A3 process not only led to a better understanding of the meaning of STAT across disciplines, increasing the intuitive nature, transparency and visual aspects of the whole process, but also promoted a collaborative, multidisciplinary, integrative culture, in which other hospital‐wide problems may be addressed in the future.
Acknowledgements
This work could not have been done without the contribution of all members of the STAT Improvement Workgroup, including Charles Alday; Allison Brenner, PharmD; Paula Carroll; Garry Davis; Michele Delaney, RN, MSN, CWCN; Mary East, MD; Stacy Frick, MSN, RN, CNL; Corry Gessner, CPhT; Kenya Harbin, MSN, RN, CNL; Crystal Heath, MS, RN‐BC; Tom Kerr, MPH; Diane Klemer, RPh; Diane Kohmescher, PharmD, BCPS; Sara Oberdick; Antanita Pickett; Ana Preda, CPhT; Joseph Pugh, RPh, MS; Gloria Salazar, CPhT; Samar Sheth, MD; Andrea Starnes, RN; Christine Wagner, PharmD; Leo Wallace; Roderick Williams; and Marilyn Woodruff.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability Grant and the Veterans in Partnership (VISN11) Healthcare Network. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs. The authors have no other disclosures or conflicts to report.
- The American Heritage Medical Dictionary of the English Language website. 2011. Available at: http://ahdictionary.com/word/search.html?q=STAT. Accessed December 22, 2013.
- , , , , , . The use of multiple qualitative methods to characterize communication events between physicians and nurses [published online ahead of print January 31, 2014]. Health Commun. doi: 10.1080/10410236.2013.835894.
- , , . Fifteen best practice recommendations for bar‐code medication administration in the Veterans Health Administration. Jt Comm J Qual Saf. 2004;30(7):355–365.
- , , , , . Going lean in health care. Cambridge, MA: Institute for Healthcare Improvement; 2005. Available at: http://www.ihi.org. Accessed March 19, 2014.
- , . Understanding A3 Thinking: A Critical Component of Toyota's PDCA Management System. New York, NY: Productivity Press, Taylor 2008.
- . Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor and Lead. Cambridge, MA: Lean Enterprise Institute; 2008.
- , , . Basics of quality improvement in health care. Mayo Clin Proc. 2007;82(6):735–739.
- , . A3 problem solving: unique features of the A3 problem solving method. Available at: http://leanhealthcarewest.com/Page/A3‐Problem‐Solving. Accessed March 27, 2014.
- , , . Evaluation of stat orders in a teaching hospital: a chart review. Clin Drug Investig. 2011;31(4):231–235.
- . Using STAT properly. Radiol Manage. 2006;28(1):26–30; quiz 31–33.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , , . Lean health care: what can hospitals learn from a world‐class automaker? J Hosp Med. 2006;1(3):191–199.
- . Gemba Kaizen: A Commonsense Approach to a Continuous Improvement Strategy. 2nd ed. New York, NY: McGraw‐Hill; 2012.
- . Pseudoinnovation: the development and spread of healthcare quality improvement methodologies. Int J Qual Health Care. 2009;21(3):153–159.
STAT is an abbreviation of the Latin word statim, meaning immediately,[1] and has been a part of healthcare's lexicon for almost as long as there have been hospitals. STAT conveys a sense of urgency, compelling those who hear STAT to act quickly. Unfortunately, given the lack of a consistent understanding of STAT, the term in reality often has an alternate use: to hurry up or to complete sooner than routine, and is sometimes used to circumvent a system that is perceived to be too slow to accomplish a routine task in a timely manner.
As part of a larger systems redesign effort to improve patient safety and quality of care, an institutional review board (IRB)‐approved qualitative study was conducted on 2 medical‐surgical units in a US Department of Veterans Affairs (VA) hospital to explore communication patterns between physicians and nurses.[2] The study revealed wide variation in understanding between physicians and nurses on the ordering and administration of STAT medication. Physicians were unaware that when they placed a STAT order into the computerized patient record system (CPRS), nurses were not automatically alerted about the order. At this facility, nurses did not carry pagers. Although each unit had a supply of wireless telephones, they were often unreliable and therefore not used consistently. Nurses were required by policy to check the CPRS for new orders every 2 hours. This was an inefficient and possibly dangerous process,[3] because if a nurse was not expecting a STAT order, 2 hours could elapse before she or he saw the order in the CPRS and began to look for the medication. A follow‐up survey completed by physicians, nurses, pharmacists, and pharmacy technicians demonstrated stark differences on the definition of STAT and overlap with similar terms such as NOW and ASAP. Interviews with ordering providers indicated that 36% of the time a STAT was ordered it was not clinically urgent, but instead ordered STAT to speed up the process.
The STAT medication process was clearly in need of improvement, but previous quality improvement projects in our organization had varying degrees of success. For example, we used Lean methodology in an attempt to improve our discharge process. We conducted a modified rapid process discharge improvement workshop[4] structured in phases over 4 weeks. During the workshops, a strong emphasis remained on the solutions to the problem, and we were unable to help the team move from a mindset of fix it to create it. This limited the buy‐in of team members, the creativity of their ideas for improvement, and ultimately the momentum to improve the process.
In this article we describe our adaptation of A3 Thinking,[5, 6] a structure for guiding quality improvement based in Lean methodology, to improve the STAT medication process. We chose A3 Thinking for several reasons. A3 Thinking focuses on process improvement and thus aligned well with our interest in improving the STAT medication process. A3 Thinking also reveals otherwise hidden nonvalue‐added activities that should be eliminated.[7] Finally A3 Thinking reinforces a deeper understanding of the way the work is currently being done, providing critical information needed before making a change. This provides a tremendous opportunity to look at work differently and see opportunities for improvement.[8] Given these strengths as well as the lack of congruence between what the STAT process should consist of and how the STAT process was actually being used in our organization, A3 Thinking offered the best fit between an improvement process and the problem to be solved.
METHODS
A search of healthcare literature yielded very few studies on the STAT process.[9, 10] Only 1 intervention to improve the process was found, and this focused on a specific procedure.[10] An informal survey of local VA and non‐VA hospitals regarding their experiences with the STAT medication process revealed insufficient information to aid our efforts. We next searched the business and manufacturing literature and found examples of how the Lean methodology was successfully applied to other problems in healthcare, including improving pediatric surgery workflow and decreasing ventilator‐associated pneumonia.[11, 12]
Therefore, the STAT project was structured to adapt a problem‐solving process commonly used in Lean organizationsA3 Thinkingwhich challenges team members to work through a discovery phase to develop a shared understanding of the process, an envisioning phase to conceptualize an ideal process experience, and finally an experimentation phase to identify and trial possible solutions through prioritization, iterative testing, structured reflection, and adjustment on resulting changes. Our application of the term experimentation in this context is distinct from that of controlled experimentation in clinical research; the term is intended to convey iterative learning as changes are tested, evaluated, and modified during this quality improvement project. Figure 1 displays a conceptual model of our adaptation of A3 Thinking. As this was a quality‐improvement project, it was exempt from IRB review.

DISCOVERY
To begin the discovery phase, a workgroup consisting of representatives of all groups that had a role in the STAT process (ie, physician, pharmacist, nurse, pharmacy technician, clerk) gathered to identify the opportunity we are looking to address and learn from each other's individual experiences with the STAT medication process. The group was facilitated by an industrial engineer familiar with the A3 Thinking process. The team completed a mapping exercise to lay out, step‐by‐step, the current STAT medication process. This activity allowed the team to build shared empathy with others' experiences and to appreciate the challenges experienced by others through their individual responsibilities in the process. The current process was found to consist of 4 overarching components: a provider entered the STAT order into the CPRS; the order was verified by a pharmacist; a pharmacy technician delivered the medication to the unit (or a nurse retrieved the medication from the Omnicell (Omnicell Inc., Mountain View, CA), a proprietary automated medication dispensing system); and finally the nurse administered the medication to a patient.
A large, color‐coded flow map of the STAT medication process was constructed over several meetings to capture all perspectives and allow team members to gather feedback from their peers. To further our understanding of the current process, the team participated in a modified Go to the Gemba (ie, go to where the work is done)[13] on a real‐time STAT order. Once all workgroup members were satisfied that the flow map represented the current state of the STAT medication process, we came to a consensus on the goals needed to meet our main objective.
We agreed that our main objective was that STAT medication orders should be recognized, verified, and administered to patients in a timely and appropriate manner to ensure quality care. We identified 3 goals to meet this objective: (1) STAT should be consistently defined and understood by everyone; (2) an easy, intuitive STAT process should be available for all stakeholders; and (3) the STAT process should be transparent and ideally visual so that everyone involved can understand at which point in the process a specific STAT order is currently situated. We also identified additional information we would need to reach the goals.
Shortly after the process‐mapping sessions, 2 workgroup members conducted real‐time STAT order time studies to track medications from order to administration. Three time periods in the STAT process were identified for observation and measurement: the time from physician order entry in the CPRS to the time a pharmacist verified the medication, the time from verification to when the medication arrived on the nursing unit, and the time from arrival on the nursing unit to when that medication was administered. Using a data‐collection template, each time period was recorded, and 28 time studies were collected over 1 month. To monitor the progress of our initiatives, the time study was repeated 3 months into the project.
ENVISIONING
Following the discovery phase, the team was better equipped to identify the specific changes needed to achieve an improved process. The envisioning phase allowed the team freedom to imagine an ideal process barring any preconceived notion of constraints within the current process.
In 2 meetings we brainstormed as many improvement ideas as possible. To prioritize and focus our ideas, we developed a matrix (see Supporting Information, Appendix A, in the online version of this article), placing our ideas in 1 of 4 quadrants based on the anticipated effort to implement the change (x‐axis) and impact of making the change (y‐axis). The matrix helped us see that some ideas would be relatively simple to implement (eg, color‐coded bags for STAT medication delivery), whereas others would require more sophisticated efforts and involvement of other people (eg, monthly education sessions to resident physicians).
EXPERIMENTING
Experiments were conducted to meet each of the 3 goals identified above. The team used the outcomes of the prioritization exercise to identify initial experiments to test. To build momentum by showing progress and improvement with a few quick wins, the team began with low‐effort/high‐impact opportunities. Each experiment followed a standard Plan‐Do‐Study‐Act (PDSA) cycle to encourage reflection, learning, adaptation, and adjustment as a result of the experiential learning process.[5]
Goal 1: STAT Should Be Consistently Defined and Understood by Everyone
To address the first goal, a subgroup collected policies and procedures related to the STAT medication administration process. The policy defined a STAT medication as a medication that has the potential to significantly and negatively impact a patient's clinical condition if not given within 30 minutes. The group found that the policy requiring a 30‐minute time to administration was clinically appropriate, reinforcing our goals to create a practice congruent with the policy.
A subgroup led by the pharmacy department collected data related to STAT medications on the 3 medical‐surgical units. Within 1 month, 550 STAT medications were ordered, consisting of medications ranging from furosemide to nicotine lozenges, the latter being a medication clearly outside of the policy definition of STAT. The workgroup reviewed the information and realized education would be required to align practice with policy. According to our matrix, education was a high‐impact/high‐effort activity, so efforts were focused on the high‐impact/low‐effort activities initially. We addressed educational opportunities in later PDSA cycles.
Goal 2: An Easy, Intuitive STAT Process for All Stakeholders
The CPRS contains prefabricated templates that conform to regulatory requirements and ensure completeness. However, the CPRS does not intuitively enable ordering providers to choose the time for the first dose of a new routine medication. This often creates a situation where a provider orders the medication STAT, so that the medication can be given earlier than the CPRS would otherwise allow. Although there is a check box, Give additional dose now, it was not being used because it was visually obscure in the interface. The CPRS restricted our ability to change the template for ordering medications to include a specific time for first‐dose administration before defaulting to the routine order; thus, complementary countermeasures were trialed first. These are outlined in Table 1.
| Countermeasure | Intended Outcome |
|---|---|
| Remove duplicate dosing frequencies from medication order template | Reduce list of dosing frequencies to sort through to find desired selection |
| Develop 1‐page job aid for ordering providers to utilize | Assist in the correct methods of ordering STAT, NOW, and routine medications |
| Added STAT ONCE as a dosing frequency selection | Clarify the medication, if ordered STAT, will only be a 1‐time administration to avoid the recurrence of a STAT order should the orders be transferred to a new unit with the patient |
| Modify existing policies to add STAT ONCE option | Ensure documentation is congruent with new expectations |
| Educate interns and residents with the job aid and a hands‐on how to ordering exercise | Inform ordering physicians on the available references for ordering and educate according to desired practice |
| Provide interns and residents with a visual job aid at their workstation and a hands‐on how to ordering exercise | In addition to providing information and educating according to desired practice, provide a just‐in‐time reference resource |
Goal 3: The STAT Process Should Be Transparent and Ideally Visual
During the time studies, the time period from when the medication arrived on the unit to the time it was administered to the patient averaged 34 minutes. Of 28 STAT orders followed through the entire process, 5 pharmacy technicians (26%) were not informed of 19 STAT medication orders requiring delivery, and 12 nurses (63%) were not notified of the delivery of those 19 medications. The remaining 9 STAT medications were stocked in the Omnicell. Informal interviews with nurses and pharmacy technicians, as well as input from the nurses and pharmacy technicians in our workgroup, revealed several explanations for these findings.
First, the delivering technicians could not always find the patient's nurse, and because the delivery procedure was not standardized, there was no consistency between technicians in where medications were delivered. Second, each unit had a different medication inventory stored in the Omnicell, and the inventory was frequently changed (eg, due to unit‐specific needs, backorders), which made it difficult for nurses to keep track of what was available in Omnicell at any given time. Finally, the STAT medication was not consistently labeled with a visual STAT notation, so even if a nurse saw that new medications had been delivered, he or she would not be able to easily identify which was STAT. The team made several low‐tech process changes to improve the visibility of a STAT medication and ensure reliable communication upon delivery. A subgroup of pharmacists, technicians, and nurses developed and implemented the countermeasures described in Table 2.
| Countermeasure | Intended Outcome |
|---|---|
| Designate delivery preferences with the patient's nurse as the first preference and a set location in the med room as the only alternative preference | Attempt to deliver medications directly to the patient's nurse as frequently as possible to eliminate any unnecessary delays and avoid miscommunication |
| Identify a location in each unit's med room to place a red bin to deliver the STAT medications that are unable to be delivered to the patient's nurse directly | Provide 1 alternate location to retrieve STAT medications if the technician is unable to locate the patient's nurse to deliver the medication directly |
| Utilize a plastic bag with a red STAT indication for transportation of STAT medications to the units | Provide a visual to assist in pharmacy technicians prioritizing their deliveries to the inpatient units |
| Utilize red STAT magnets on the patient's door frame to signal nurses a medication had been delivered to the med room | Provide a visual to assist in timely recognition of a STAT medication delivery given the technician was unable to find the nurse to hand it off directly |
RESULTS
At the start of our project, the average time from STAT order to medication administration was 1 hour and 7 minutes (range, 6 minutes 2 hours and 22 minutes). As a result of the 2 sets of countermeasures outlined in Tables 1 and 2, the average total time from STAT order entry to administration decreased by 21% to an average of 53 minutes. The total time from medication delivery to administration decreased by 26% from 34 minutes to 25 minutes postimplementation. On average, 391 STAT medications were ordered per month during the project period, which represents a decrease of 9.5% from the 432 orders per month for the same time period the previous year. After implementing the countermeasures in Table 2, we followed another 26 STAT medications through the process to evaluate our efforts. Of 15 STAT medications requiring delivery, only 1 nurse (7%) was not notified of the delivery of a STAT medication, and 1 pharmacy technician (7%) was not informed the medication was STAT. The 151% increase in notification of nurses to delivery of a STAT medication suggests that use of the STAT bags, STAT magnets on patient doors, and whenever possible direct delivery of STAT medications to the nurse has improved communication between the technicians and nurses. Similarly, the 27% increase in technician awareness of a STAT designation suggests STAT is being better communicated to them. The improvement in awareness and notification of a STAT medication is summarized in Figure 2.

Due to time and financial constraints, the following limitations may have affected our findings. First, resident physicians were not directly represented in our discussions. Attending medicine hospitalists provided the physician perspective, which provides a biased view given their intimate knowledge of the CPRS and additional years of experience. Similarly, nurse perspectives were limited to staff and clinical nurse leaders. Last, our low‐cost approach was mandated by limited resources; a more resource‐rich environment may have devised alternative approaches.
CONCLUSIONS
Adapting A3 Thinking for process improvement was a low‐cost/low‐tech option for a VA facility. Having buy‐in from all levels was crucial to the success of the project. The size and diversity of the group was also very important, as different opinions and aspects of the process were represented. Cross‐discipline relationships and respect were formed, which will be valuable for collaboration in future projects. Although we focused on the STAT medication process, other quality‐improvement projects could also benefit from A3 Thinking. Moreover, there were enough people to serve as ambassadors, taking the project back to their work areas to share with their peers, gather consensus, and elicit additional feedback. The collaboration led to comprehensive understanding of the process, the nature of the problems within the process, and the complexity of solving the problem. For example, although the number of STAT orders did not decrease dramatically, we have learned from these experiments that we may need to change how we approach structuring additional experiments. Future work will focus on increasing communication between physicians and nurses when placing STAT medication orders, enhancing resident education to ensure appropriate use of the STAT designation, and continuing our efforts to improve the delivery process of STAT medications.
Other quality‐improvement methodologies we could have used include: total quality management (TQM), continuous quality improvement (CQI), business process redesign, Lean, Six Sigma, and others.[14] Differences between these can be broadly classified as putting an emphasis on people (eg, inclusion of front line staff in CQI or leadership in TQM) or on process (eg, understanding process function to reduce waste in Lean or statistical process control in Six Sigma).[14] Using A3 Thinking methodology was more useful than these others for the STAT medication process for some very important reasons. The A3 process not only led to a better understanding of the meaning of STAT across disciplines, increasing the intuitive nature, transparency and visual aspects of the whole process, but also promoted a collaborative, multidisciplinary, integrative culture, in which other hospital‐wide problems may be addressed in the future.
Acknowledgements
This work could not have been done without the contribution of all members of the STAT Improvement Workgroup, including Charles Alday; Allison Brenner, PharmD; Paula Carroll; Garry Davis; Michele Delaney, RN, MSN, CWCN; Mary East, MD; Stacy Frick, MSN, RN, CNL; Corry Gessner, CPhT; Kenya Harbin, MSN, RN, CNL; Crystal Heath, MS, RN‐BC; Tom Kerr, MPH; Diane Klemer, RPh; Diane Kohmescher, PharmD, BCPS; Sara Oberdick; Antanita Pickett; Ana Preda, CPhT; Joseph Pugh, RPh, MS; Gloria Salazar, CPhT; Samar Sheth, MD; Andrea Starnes, RN; Christine Wagner, PharmD; Leo Wallace; Roderick Williams; and Marilyn Woodruff.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability Grant and the Veterans in Partnership (VISN11) Healthcare Network. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs. The authors have no other disclosures or conflicts to report.
STAT is an abbreviation of the Latin word statim, meaning immediately,[1] and has been a part of healthcare's lexicon for almost as long as there have been hospitals. STAT conveys a sense of urgency, compelling those who hear STAT to act quickly. Unfortunately, given the lack of a consistent understanding of STAT, the term in reality often has an alternate use: to hurry up or to complete sooner than routine, and is sometimes used to circumvent a system that is perceived to be too slow to accomplish a routine task in a timely manner.
As part of a larger systems redesign effort to improve patient safety and quality of care, an institutional review board (IRB)‐approved qualitative study was conducted on 2 medical‐surgical units in a US Department of Veterans Affairs (VA) hospital to explore communication patterns between physicians and nurses.[2] The study revealed wide variation in understanding between physicians and nurses on the ordering and administration of STAT medication. Physicians were unaware that when they placed a STAT order into the computerized patient record system (CPRS), nurses were not automatically alerted about the order. At this facility, nurses did not carry pagers. Although each unit had a supply of wireless telephones, they were often unreliable and therefore not used consistently. Nurses were required by policy to check the CPRS for new orders every 2 hours. This was an inefficient and possibly dangerous process,[3] because if a nurse was not expecting a STAT order, 2 hours could elapse before she or he saw the order in the CPRS and began to look for the medication. A follow‐up survey completed by physicians, nurses, pharmacists, and pharmacy technicians demonstrated stark differences on the definition of STAT and overlap with similar terms such as NOW and ASAP. Interviews with ordering providers indicated that 36% of the time a STAT was ordered it was not clinically urgent, but instead ordered STAT to speed up the process.
The STAT medication process was clearly in need of improvement, but previous quality improvement projects in our organization had varying degrees of success. For example, we used Lean methodology in an attempt to improve our discharge process. We conducted a modified rapid process discharge improvement workshop[4] structured in phases over 4 weeks. During the workshops, a strong emphasis remained on the solutions to the problem, and we were unable to help the team move from a mindset of fix it to create it. This limited the buy‐in of team members, the creativity of their ideas for improvement, and ultimately the momentum to improve the process.
In this article we describe our adaptation of A3 Thinking,[5, 6] a structure for guiding quality improvement based in Lean methodology, to improve the STAT medication process. We chose A3 Thinking for several reasons. A3 Thinking focuses on process improvement and thus aligned well with our interest in improving the STAT medication process. A3 Thinking also reveals otherwise hidden nonvalue‐added activities that should be eliminated.[7] Finally A3 Thinking reinforces a deeper understanding of the way the work is currently being done, providing critical information needed before making a change. This provides a tremendous opportunity to look at work differently and see opportunities for improvement.[8] Given these strengths as well as the lack of congruence between what the STAT process should consist of and how the STAT process was actually being used in our organization, A3 Thinking offered the best fit between an improvement process and the problem to be solved.
METHODS
A search of healthcare literature yielded very few studies on the STAT process.[9, 10] Only 1 intervention to improve the process was found, and this focused on a specific procedure.[10] An informal survey of local VA and non‐VA hospitals regarding their experiences with the STAT medication process revealed insufficient information to aid our efforts. We next searched the business and manufacturing literature and found examples of how the Lean methodology was successfully applied to other problems in healthcare, including improving pediatric surgery workflow and decreasing ventilator‐associated pneumonia.[11, 12]
Therefore, the STAT project was structured to adapt a problem‐solving process commonly used in Lean organizationsA3 Thinkingwhich challenges team members to work through a discovery phase to develop a shared understanding of the process, an envisioning phase to conceptualize an ideal process experience, and finally an experimentation phase to identify and trial possible solutions through prioritization, iterative testing, structured reflection, and adjustment on resulting changes. Our application of the term experimentation in this context is distinct from that of controlled experimentation in clinical research; the term is intended to convey iterative learning as changes are tested, evaluated, and modified during this quality improvement project. Figure 1 displays a conceptual model of our adaptation of A3 Thinking. As this was a quality‐improvement project, it was exempt from IRB review.

DISCOVERY
To begin the discovery phase, a workgroup consisting of representatives of all groups that had a role in the STAT process (ie, physician, pharmacist, nurse, pharmacy technician, clerk) gathered to identify the opportunity we are looking to address and learn from each other's individual experiences with the STAT medication process. The group was facilitated by an industrial engineer familiar with the A3 Thinking process. The team completed a mapping exercise to lay out, step‐by‐step, the current STAT medication process. This activity allowed the team to build shared empathy with others' experiences and to appreciate the challenges experienced by others through their individual responsibilities in the process. The current process was found to consist of 4 overarching components: a provider entered the STAT order into the CPRS; the order was verified by a pharmacist; a pharmacy technician delivered the medication to the unit (or a nurse retrieved the medication from the Omnicell (Omnicell Inc., Mountain View, CA), a proprietary automated medication dispensing system); and finally the nurse administered the medication to a patient.
A large, color‐coded flow map of the STAT medication process was constructed over several meetings to capture all perspectives and allow team members to gather feedback from their peers. To further our understanding of the current process, the team participated in a modified Go to the Gemba (ie, go to where the work is done)[13] on a real‐time STAT order. Once all workgroup members were satisfied that the flow map represented the current state of the STAT medication process, we came to a consensus on the goals needed to meet our main objective.
We agreed that our main objective was that STAT medication orders should be recognized, verified, and administered to patients in a timely and appropriate manner to ensure quality care. We identified 3 goals to meet this objective: (1) STAT should be consistently defined and understood by everyone; (2) an easy, intuitive STAT process should be available for all stakeholders; and (3) the STAT process should be transparent and ideally visual so that everyone involved can understand at which point in the process a specific STAT order is currently situated. We also identified additional information we would need to reach the goals.
Shortly after the process‐mapping sessions, 2 workgroup members conducted real‐time STAT order time studies to track medications from order to administration. Three time periods in the STAT process were identified for observation and measurement: the time from physician order entry in the CPRS to the time a pharmacist verified the medication, the time from verification to when the medication arrived on the nursing unit, and the time from arrival on the nursing unit to when that medication was administered. Using a data‐collection template, each time period was recorded, and 28 time studies were collected over 1 month. To monitor the progress of our initiatives, the time study was repeated 3 months into the project.
ENVISIONING
Following the discovery phase, the team was better equipped to identify the specific changes needed to achieve an improved process. The envisioning phase allowed the team freedom to imagine an ideal process barring any preconceived notion of constraints within the current process.
In 2 meetings we brainstormed as many improvement ideas as possible. To prioritize and focus our ideas, we developed a matrix (see Supporting Information, Appendix A, in the online version of this article), placing our ideas in 1 of 4 quadrants based on the anticipated effort to implement the change (x‐axis) and impact of making the change (y‐axis). The matrix helped us see that some ideas would be relatively simple to implement (eg, color‐coded bags for STAT medication delivery), whereas others would require more sophisticated efforts and involvement of other people (eg, monthly education sessions to resident physicians).
EXPERIMENTING
Experiments were conducted to meet each of the 3 goals identified above. The team used the outcomes of the prioritization exercise to identify initial experiments to test. To build momentum by showing progress and improvement with a few quick wins, the team began with low‐effort/high‐impact opportunities. Each experiment followed a standard Plan‐Do‐Study‐Act (PDSA) cycle to encourage reflection, learning, adaptation, and adjustment as a result of the experiential learning process.[5]
Goal 1: STAT Should Be Consistently Defined and Understood by Everyone
To address the first goal, a subgroup collected policies and procedures related to the STAT medication administration process. The policy defined a STAT medication as a medication that has the potential to significantly and negatively impact a patient's clinical condition if not given within 30 minutes. The group found that the policy requiring a 30‐minute time to administration was clinically appropriate, reinforcing our goals to create a practice congruent with the policy.
A subgroup led by the pharmacy department collected data related to STAT medications on the 3 medical‐surgical units. Within 1 month, 550 STAT medications were ordered, consisting of medications ranging from furosemide to nicotine lozenges, the latter being a medication clearly outside of the policy definition of STAT. The workgroup reviewed the information and realized education would be required to align practice with policy. According to our matrix, education was a high‐impact/high‐effort activity, so efforts were focused on the high‐impact/low‐effort activities initially. We addressed educational opportunities in later PDSA cycles.
Goal 2: An Easy, Intuitive STAT Process for All Stakeholders
The CPRS contains prefabricated templates that conform to regulatory requirements and ensure completeness. However, the CPRS does not intuitively enable ordering providers to choose the time for the first dose of a new routine medication. This often creates a situation where a provider orders the medication STAT, so that the medication can be given earlier than the CPRS would otherwise allow. Although there is a check box, Give additional dose now, it was not being used because it was visually obscure in the interface. The CPRS restricted our ability to change the template for ordering medications to include a specific time for first‐dose administration before defaulting to the routine order; thus, complementary countermeasures were trialed first. These are outlined in Table 1.
| Countermeasure | Intended Outcome |
|---|---|
| Remove duplicate dosing frequencies from medication order template | Reduce list of dosing frequencies to sort through to find desired selection |
| Develop 1‐page job aid for ordering providers to utilize | Assist in the correct methods of ordering STAT, NOW, and routine medications |
| Added STAT ONCE as a dosing frequency selection | Clarify the medication, if ordered STAT, will only be a 1‐time administration to avoid the recurrence of a STAT order should the orders be transferred to a new unit with the patient |
| Modify existing policies to add STAT ONCE option | Ensure documentation is congruent with new expectations |
| Educate interns and residents with the job aid and a hands‐on how to ordering exercise | Inform ordering physicians on the available references for ordering and educate according to desired practice |
| Provide interns and residents with a visual job aid at their workstation and a hands‐on how to ordering exercise | In addition to providing information and educating according to desired practice, provide a just‐in‐time reference resource |
Goal 3: The STAT Process Should Be Transparent and Ideally Visual
During the time studies, the time period from when the medication arrived on the unit to the time it was administered to the patient averaged 34 minutes. Of 28 STAT orders followed through the entire process, 5 pharmacy technicians (26%) were not informed of 19 STAT medication orders requiring delivery, and 12 nurses (63%) were not notified of the delivery of those 19 medications. The remaining 9 STAT medications were stocked in the Omnicell. Informal interviews with nurses and pharmacy technicians, as well as input from the nurses and pharmacy technicians in our workgroup, revealed several explanations for these findings.
First, the delivering technicians could not always find the patient's nurse, and because the delivery procedure was not standardized, there was no consistency between technicians in where medications were delivered. Second, each unit had a different medication inventory stored in the Omnicell, and the inventory was frequently changed (eg, due to unit‐specific needs, backorders), which made it difficult for nurses to keep track of what was available in Omnicell at any given time. Finally, the STAT medication was not consistently labeled with a visual STAT notation, so even if a nurse saw that new medications had been delivered, he or she would not be able to easily identify which was STAT. The team made several low‐tech process changes to improve the visibility of a STAT medication and ensure reliable communication upon delivery. A subgroup of pharmacists, technicians, and nurses developed and implemented the countermeasures described in Table 2.
| Countermeasure | Intended Outcome |
|---|---|
| Designate delivery preferences with the patient's nurse as the first preference and a set location in the med room as the only alternative preference | Attempt to deliver medications directly to the patient's nurse as frequently as possible to eliminate any unnecessary delays and avoid miscommunication |
| Identify a location in each unit's med room to place a red bin to deliver the STAT medications that are unable to be delivered to the patient's nurse directly | Provide 1 alternate location to retrieve STAT medications if the technician is unable to locate the patient's nurse to deliver the medication directly |
| Utilize a plastic bag with a red STAT indication for transportation of STAT medications to the units | Provide a visual to assist in pharmacy technicians prioritizing their deliveries to the inpatient units |
| Utilize red STAT magnets on the patient's door frame to signal nurses a medication had been delivered to the med room | Provide a visual to assist in timely recognition of a STAT medication delivery given the technician was unable to find the nurse to hand it off directly |
RESULTS
At the start of our project, the average time from STAT order to medication administration was 1 hour and 7 minutes (range, 6 minutes 2 hours and 22 minutes). As a result of the 2 sets of countermeasures outlined in Tables 1 and 2, the average total time from STAT order entry to administration decreased by 21% to an average of 53 minutes. The total time from medication delivery to administration decreased by 26% from 34 minutes to 25 minutes postimplementation. On average, 391 STAT medications were ordered per month during the project period, which represents a decrease of 9.5% from the 432 orders per month for the same time period the previous year. After implementing the countermeasures in Table 2, we followed another 26 STAT medications through the process to evaluate our efforts. Of 15 STAT medications requiring delivery, only 1 nurse (7%) was not notified of the delivery of a STAT medication, and 1 pharmacy technician (7%) was not informed the medication was STAT. The 151% increase in notification of nurses to delivery of a STAT medication suggests that use of the STAT bags, STAT magnets on patient doors, and whenever possible direct delivery of STAT medications to the nurse has improved communication between the technicians and nurses. Similarly, the 27% increase in technician awareness of a STAT designation suggests STAT is being better communicated to them. The improvement in awareness and notification of a STAT medication is summarized in Figure 2.

Due to time and financial constraints, the following limitations may have affected our findings. First, resident physicians were not directly represented in our discussions. Attending medicine hospitalists provided the physician perspective, which provides a biased view given their intimate knowledge of the CPRS and additional years of experience. Similarly, nurse perspectives were limited to staff and clinical nurse leaders. Last, our low‐cost approach was mandated by limited resources; a more resource‐rich environment may have devised alternative approaches.
CONCLUSIONS
Adapting A3 Thinking for process improvement was a low‐cost/low‐tech option for a VA facility. Having buy‐in from all levels was crucial to the success of the project. The size and diversity of the group was also very important, as different opinions and aspects of the process were represented. Cross‐discipline relationships and respect were formed, which will be valuable for collaboration in future projects. Although we focused on the STAT medication process, other quality‐improvement projects could also benefit from A3 Thinking. Moreover, there were enough people to serve as ambassadors, taking the project back to their work areas to share with their peers, gather consensus, and elicit additional feedback. The collaboration led to comprehensive understanding of the process, the nature of the problems within the process, and the complexity of solving the problem. For example, although the number of STAT orders did not decrease dramatically, we have learned from these experiments that we may need to change how we approach structuring additional experiments. Future work will focus on increasing communication between physicians and nurses when placing STAT medication orders, enhancing resident education to ensure appropriate use of the STAT designation, and continuing our efforts to improve the delivery process of STAT medications.
Other quality‐improvement methodologies we could have used include: total quality management (TQM), continuous quality improvement (CQI), business process redesign, Lean, Six Sigma, and others.[14] Differences between these can be broadly classified as putting an emphasis on people (eg, inclusion of front line staff in CQI or leadership in TQM) or on process (eg, understanding process function to reduce waste in Lean or statistical process control in Six Sigma).[14] Using A3 Thinking methodology was more useful than these others for the STAT medication process for some very important reasons. The A3 process not only led to a better understanding of the meaning of STAT across disciplines, increasing the intuitive nature, transparency and visual aspects of the whole process, but also promoted a collaborative, multidisciplinary, integrative culture, in which other hospital‐wide problems may be addressed in the future.
Acknowledgements
This work could not have been done without the contribution of all members of the STAT Improvement Workgroup, including Charles Alday; Allison Brenner, PharmD; Paula Carroll; Garry Davis; Michele Delaney, RN, MSN, CWCN; Mary East, MD; Stacy Frick, MSN, RN, CNL; Corry Gessner, CPhT; Kenya Harbin, MSN, RN, CNL; Crystal Heath, MS, RN‐BC; Tom Kerr, MPH; Diane Klemer, RPh; Diane Kohmescher, PharmD, BCPS; Sara Oberdick; Antanita Pickett; Ana Preda, CPhT; Joseph Pugh, RPh, MS; Gloria Salazar, CPhT; Samar Sheth, MD; Andrea Starnes, RN; Christine Wagner, PharmD; Leo Wallace; Roderick Williams; and Marilyn Woodruff.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability Grant and the Veterans in Partnership (VISN11) Healthcare Network. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs. The authors have no other disclosures or conflicts to report.
- The American Heritage Medical Dictionary of the English Language website. 2011. Available at: http://ahdictionary.com/word/search.html?q=STAT. Accessed December 22, 2013.
- , , , , , . The use of multiple qualitative methods to characterize communication events between physicians and nurses [published online ahead of print January 31, 2014]. Health Commun. doi: 10.1080/10410236.2013.835894.
- , , . Fifteen best practice recommendations for bar‐code medication administration in the Veterans Health Administration. Jt Comm J Qual Saf. 2004;30(7):355–365.
- , , , , . Going lean in health care. Cambridge, MA: Institute for Healthcare Improvement; 2005. Available at: http://www.ihi.org. Accessed March 19, 2014.
- , . Understanding A3 Thinking: A Critical Component of Toyota's PDCA Management System. New York, NY: Productivity Press, Taylor 2008.
- . Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor and Lead. Cambridge, MA: Lean Enterprise Institute; 2008.
- , , . Basics of quality improvement in health care. Mayo Clin Proc. 2007;82(6):735–739.
- , . A3 problem solving: unique features of the A3 problem solving method. Available at: http://leanhealthcarewest.com/Page/A3‐Problem‐Solving. Accessed March 27, 2014.
- , , . Evaluation of stat orders in a teaching hospital: a chart review. Clin Drug Investig. 2011;31(4):231–235.
- . Using STAT properly. Radiol Manage. 2006;28(1):26–30; quiz 31–33.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , , . Lean health care: what can hospitals learn from a world‐class automaker? J Hosp Med. 2006;1(3):191–199.
- . Gemba Kaizen: A Commonsense Approach to a Continuous Improvement Strategy. 2nd ed. New York, NY: McGraw‐Hill; 2012.
- . Pseudoinnovation: the development and spread of healthcare quality improvement methodologies. Int J Qual Health Care. 2009;21(3):153–159.
- The American Heritage Medical Dictionary of the English Language website. 2011. Available at: http://ahdictionary.com/word/search.html?q=STAT. Accessed December 22, 2013.
- , , , , , . The use of multiple qualitative methods to characterize communication events between physicians and nurses [published online ahead of print January 31, 2014]. Health Commun. doi: 10.1080/10410236.2013.835894.
- , , . Fifteen best practice recommendations for bar‐code medication administration in the Veterans Health Administration. Jt Comm J Qual Saf. 2004;30(7):355–365.
- , , , , . Going lean in health care. Cambridge, MA: Institute for Healthcare Improvement; 2005. Available at: http://www.ihi.org. Accessed March 19, 2014.
- , . Understanding A3 Thinking: A Critical Component of Toyota's PDCA Management System. New York, NY: Productivity Press, Taylor 2008.
- . Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor and Lead. Cambridge, MA: Lean Enterprise Institute; 2008.
- , , . Basics of quality improvement in health care. Mayo Clin Proc. 2007;82(6):735–739.
- , . A3 problem solving: unique features of the A3 problem solving method. Available at: http://leanhealthcarewest.com/Page/A3‐Problem‐Solving. Accessed March 27, 2014.
- , , . Evaluation of stat orders in a teaching hospital: a chart review. Clin Drug Investig. 2011;31(4):231–235.
- . Using STAT properly. Radiol Manage. 2006;28(1):26–30; quiz 31–33.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , , . Lean health care: what can hospitals learn from a world‐class automaker? J Hosp Med. 2006;1(3):191–199.
- . Gemba Kaizen: A Commonsense Approach to a Continuous Improvement Strategy. 2nd ed. New York, NY: McGraw‐Hill; 2012.
- . Pseudoinnovation: the development and spread of healthcare quality improvement methodologies. Int J Qual Health Care. 2009;21(3):153–159.
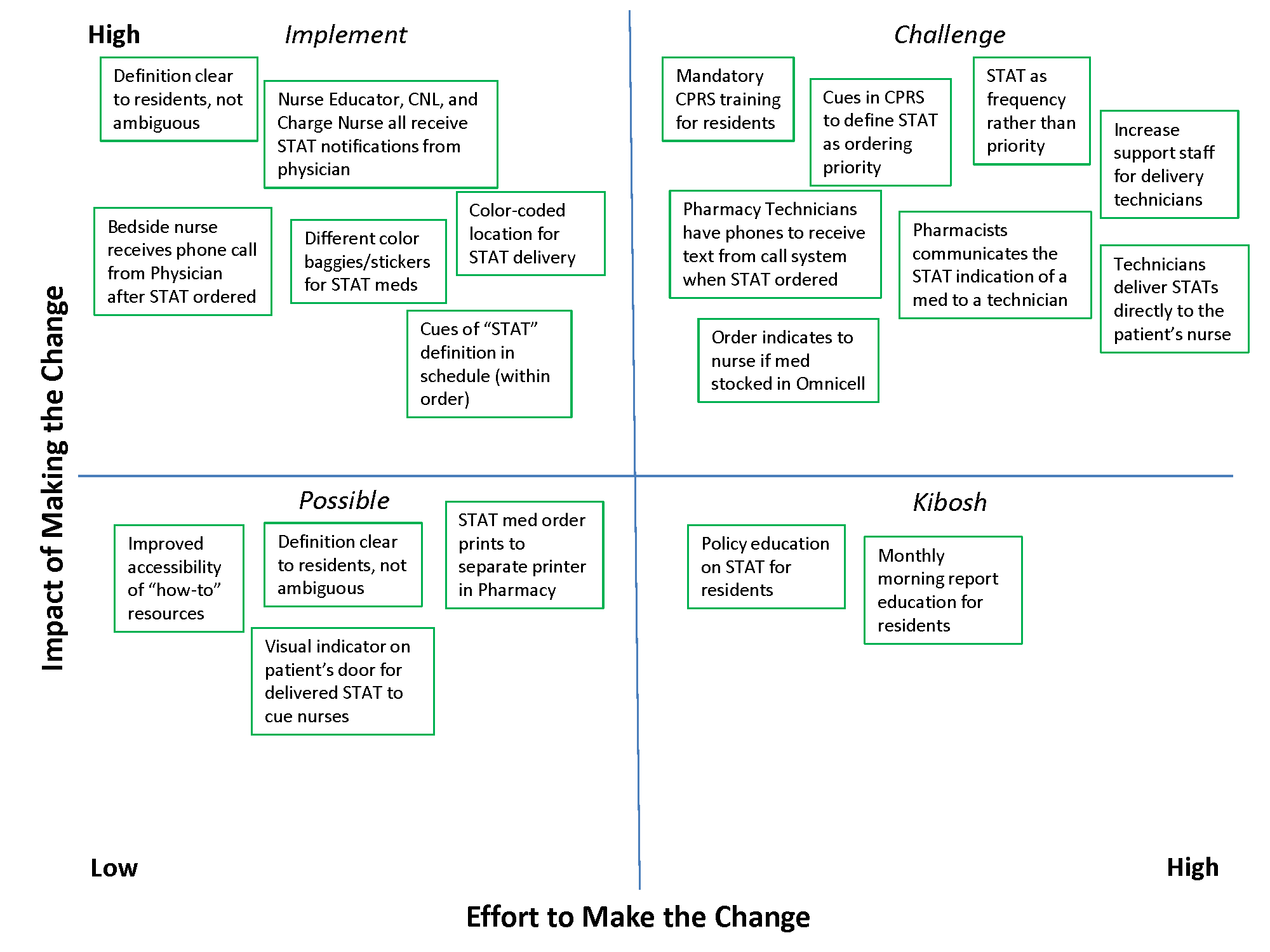
VIDEO EXCLUSIVE: Baystate Medical Center's Unit-Based, Multidisciplinary Rounding Enhances Inpatient Care
Click on the 5-minute video below...
)Click on the 5-minute video below...
)Click on the 5-minute video below...
)Uterine adenomyosis: Noninvasive diagnosis
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s installment of Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of describing what adenomyosis will look like on transvaginal ultrasound
In my first book, entitled Endovaginal Ultrasound,1 I coined the phrase “sonomicoscopy.” I maintain that we are seeing things with transvaginal ultrasound that you could not see with your naked eye if you could hold the structure at arms length and squint at it.
Adenomyosis is defined as endometrial glands and stroma embedded within the myometrium. Literature has shown that if you do three sections on a routine hysterectomy specimen the incidence of adenomyosis is 31%; with six sections the incidence is 61%! In other words, it is a very prevalent occurrence.
There is no question that adenomyosis CAN be a source of uterine enlargement, pain, and bleeding. But it is such a prevalent finding that the real question is: What percent of women, especially parous women, will have sonographic evidence of adenomyosis but be totally asymptomatic? Such women represent the denominator while the symptomatic ones represent the numerator. I worry about labeling asymptomatic patients with this entity—when they become perimenopausal and oligo-ovulatory, and may have irregular bleeding—their symptoms can be judged to be FROM adenomyosis and surgical correction is offered.
An important part of successful ultrasound use is being sure that we redefine what is “normal” as we examine patients with this “low power microscope.” So, while transvaginal ultrasound CAN identify glands and stroma within the myometrium, we must be careful not to automatically label this finding as a “disease.”
Reference
1. Goldstein SR. Endovaginal Ultrasound. 2nd ed. John Wiley & Sons, Inc: Hoboken, NJ; January 1991.
Uterine adenomyosis: Noninvasive diagnosis
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine adenomyosis is a pathologic condition in which endometrial glands and stroma are present in the uterine myometrium. Uterine adenomyosis is common, and may coexist with leiomyomata or endometriosis. When present, it may cause dysmenorrhea and heavy menses.
Until recently, the best way to establish a diagnosis of uterine adenomyosis was through histologic examination of a hysterectomy specimen. However, transvaginal ultrasound and pelvic magnetic resonance imaging have been shown to be accurate for noninvasive diagnosis.
Signs on imaging include:
- Globular/bulky uterus
- Asymmetric thickening of myometrium
- Loss of clarity of endo-myometrial interface
- Diffuse heterogenous myometrial echogenicity
- Myometrial cysts
| Click to enlarge image |
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.
1. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstetricia et Gynecologica. 2010;89(11):1374-1384.
2. Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and meta-analysis. Am J Obstet Gynecol. 2009;201(1):107.e1-e6.
3. Munro MG. Update: abnormal uterine bleeding. OBG Manag. 2014;26(3):27-32.
Tests pinpoint primary sources of neuroendocrine bowel, pancreatic metastases
BOSTON – With a little chemical or genetic snooping, or both, clinicians may be able to pinpoint the source of nearly all metastatic neuroendocrine tumors of the small bowel or pancreas.
By looking at expression patterns of four genes, investigators were able to determine that a surgically obtained metastatic neuroendocrine tumor (NET) originated in the small bowel with more than 96% accuracy, and, with the use of an immunohistochemistry algorithm, they identified the pancreas as the primary source of metastases in 10 of 10 cases.
"All the NETs that were misclassified by one method were correctly identified by the other method," said Dr. Jessica Maxwell of the department of surgery at the University of Iowa Hospitals and Clinics in Iowa City.
In about 15%-20% of cases of metastatic NETs, the primary tumor site is unknown, but is most likely to be in the small bowel or pancreas. Failure to identify the primary tumor site, despite optimal work-up, could delay referral for surgery or complicate choice of systemic medical therapies, she said at the annual meeting of the American Association of Endocrine Surgeons.
The authors tested the mettle of immunohistochemistry and gene expression classification (GEC) methods on 136 metastatic NETs collected intraoperatively from 97 patients with small-bowel NETs (38 with metastases to liver and 59 with metastases to lymph nodes) and 39 with pancreatic NETs (17 liver and 22 lymph node metastases).
The GEC uses quantitative or "real-time" polymerase chain reaction (qPCR) to evaluate expression of four key genes, encoding for the secretin receptor (SCTR), oxytocin receptor (OXTR), bombesin-like receptor-3 (BRS3), and opioid receptor kappa-1 (OPRK1).
The differential patterns of gene expression mark the metastases as originating either in the pancreas or small bowel.
They also tested a two-tiered immunohistochemistry algorithm using the markers CDX2, PAX6, and ISLET1 for tier 1, and PrAP, PRm NESP55, and PDX1 in tier 2. They tested the algorithm on six primary tumors and validated their findings on 37 metastases.
The immunohistochemistry method can identify a primary tumor site with as few as three markers, but if the findings are indeterminate, the addition of the four tier 2 markers can help to nail down the tumor site, Dr. Maxwell said.
They found that the GEC accurately identified 94 of 97 small bowel NETs (96.9%), and 34 of 39 pancreatic NETS (87.2%).
In contrast, the immunohistochemistry algorithm correctly identified the primary site in 23 of 27 small bowel metastases (85.2%), and in 10 of 10 (100%) pancreatic metastases.
When the methods were compared head to head in 27 metastases, GEC had a 96.2% overall accuracy and immunohistochemistry an 85.2% accuracy.
As noted before, the methods were complementary, with all NETs misclassified by one method called accurately by the other.
The investigators suggest that because the methods are highly accurate and complementary, they may best be used sequentially, starting with immunohistochemistry which is both inexpensive and widely available, and if immunohistochemistry fails, moving on to GEC.
"Sequential use allows for identification of nearly all metastatic neuroendocrine tumors from small bowel or pancreatic sites," Dr. Maxwell said.
In the discussion, Dr. Eren Berber of the Center for Endocrine Surgery at the Cleveland Clinic, who was not involved in the study, questioned whether knowing the primary site had any practical implications for surgeons.
Dr. Maxwell noted that some pancreatic NETs are not detected by preoperative studies and that given the risks of pancreatectomy or pancreaticoduodenectomy, accurately identifying the source of an NET may be helpful for patient counseling and preoperative planning.
The study was supported by a grant from the National Institutes of Health. Dr. Maxwell and Dr. Berber reported having no financial disclosures.
BOSTON – With a little chemical or genetic snooping, or both, clinicians may be able to pinpoint the source of nearly all metastatic neuroendocrine tumors of the small bowel or pancreas.
By looking at expression patterns of four genes, investigators were able to determine that a surgically obtained metastatic neuroendocrine tumor (NET) originated in the small bowel with more than 96% accuracy, and, with the use of an immunohistochemistry algorithm, they identified the pancreas as the primary source of metastases in 10 of 10 cases.
"All the NETs that were misclassified by one method were correctly identified by the other method," said Dr. Jessica Maxwell of the department of surgery at the University of Iowa Hospitals and Clinics in Iowa City.
In about 15%-20% of cases of metastatic NETs, the primary tumor site is unknown, but is most likely to be in the small bowel or pancreas. Failure to identify the primary tumor site, despite optimal work-up, could delay referral for surgery or complicate choice of systemic medical therapies, she said at the annual meeting of the American Association of Endocrine Surgeons.
The authors tested the mettle of immunohistochemistry and gene expression classification (GEC) methods on 136 metastatic NETs collected intraoperatively from 97 patients with small-bowel NETs (38 with metastases to liver and 59 with metastases to lymph nodes) and 39 with pancreatic NETs (17 liver and 22 lymph node metastases).
The GEC uses quantitative or "real-time" polymerase chain reaction (qPCR) to evaluate expression of four key genes, encoding for the secretin receptor (SCTR), oxytocin receptor (OXTR), bombesin-like receptor-3 (BRS3), and opioid receptor kappa-1 (OPRK1).
The differential patterns of gene expression mark the metastases as originating either in the pancreas or small bowel.
They also tested a two-tiered immunohistochemistry algorithm using the markers CDX2, PAX6, and ISLET1 for tier 1, and PrAP, PRm NESP55, and PDX1 in tier 2. They tested the algorithm on six primary tumors and validated their findings on 37 metastases.
The immunohistochemistry method can identify a primary tumor site with as few as three markers, but if the findings are indeterminate, the addition of the four tier 2 markers can help to nail down the tumor site, Dr. Maxwell said.
They found that the GEC accurately identified 94 of 97 small bowel NETs (96.9%), and 34 of 39 pancreatic NETS (87.2%).
In contrast, the immunohistochemistry algorithm correctly identified the primary site in 23 of 27 small bowel metastases (85.2%), and in 10 of 10 (100%) pancreatic metastases.
When the methods were compared head to head in 27 metastases, GEC had a 96.2% overall accuracy and immunohistochemistry an 85.2% accuracy.
As noted before, the methods were complementary, with all NETs misclassified by one method called accurately by the other.
The investigators suggest that because the methods are highly accurate and complementary, they may best be used sequentially, starting with immunohistochemistry which is both inexpensive and widely available, and if immunohistochemistry fails, moving on to GEC.
"Sequential use allows for identification of nearly all metastatic neuroendocrine tumors from small bowel or pancreatic sites," Dr. Maxwell said.
In the discussion, Dr. Eren Berber of the Center for Endocrine Surgery at the Cleveland Clinic, who was not involved in the study, questioned whether knowing the primary site had any practical implications for surgeons.
Dr. Maxwell noted that some pancreatic NETs are not detected by preoperative studies and that given the risks of pancreatectomy or pancreaticoduodenectomy, accurately identifying the source of an NET may be helpful for patient counseling and preoperative planning.
The study was supported by a grant from the National Institutes of Health. Dr. Maxwell and Dr. Berber reported having no financial disclosures.
BOSTON – With a little chemical or genetic snooping, or both, clinicians may be able to pinpoint the source of nearly all metastatic neuroendocrine tumors of the small bowel or pancreas.
By looking at expression patterns of four genes, investigators were able to determine that a surgically obtained metastatic neuroendocrine tumor (NET) originated in the small bowel with more than 96% accuracy, and, with the use of an immunohistochemistry algorithm, they identified the pancreas as the primary source of metastases in 10 of 10 cases.
"All the NETs that were misclassified by one method were correctly identified by the other method," said Dr. Jessica Maxwell of the department of surgery at the University of Iowa Hospitals and Clinics in Iowa City.
In about 15%-20% of cases of metastatic NETs, the primary tumor site is unknown, but is most likely to be in the small bowel or pancreas. Failure to identify the primary tumor site, despite optimal work-up, could delay referral for surgery or complicate choice of systemic medical therapies, she said at the annual meeting of the American Association of Endocrine Surgeons.
The authors tested the mettle of immunohistochemistry and gene expression classification (GEC) methods on 136 metastatic NETs collected intraoperatively from 97 patients with small-bowel NETs (38 with metastases to liver and 59 with metastases to lymph nodes) and 39 with pancreatic NETs (17 liver and 22 lymph node metastases).
The GEC uses quantitative or "real-time" polymerase chain reaction (qPCR) to evaluate expression of four key genes, encoding for the secretin receptor (SCTR), oxytocin receptor (OXTR), bombesin-like receptor-3 (BRS3), and opioid receptor kappa-1 (OPRK1).
The differential patterns of gene expression mark the metastases as originating either in the pancreas or small bowel.
They also tested a two-tiered immunohistochemistry algorithm using the markers CDX2, PAX6, and ISLET1 for tier 1, and PrAP, PRm NESP55, and PDX1 in tier 2. They tested the algorithm on six primary tumors and validated their findings on 37 metastases.
The immunohistochemistry method can identify a primary tumor site with as few as three markers, but if the findings are indeterminate, the addition of the four tier 2 markers can help to nail down the tumor site, Dr. Maxwell said.
They found that the GEC accurately identified 94 of 97 small bowel NETs (96.9%), and 34 of 39 pancreatic NETS (87.2%).
In contrast, the immunohistochemistry algorithm correctly identified the primary site in 23 of 27 small bowel metastases (85.2%), and in 10 of 10 (100%) pancreatic metastases.
When the methods were compared head to head in 27 metastases, GEC had a 96.2% overall accuracy and immunohistochemistry an 85.2% accuracy.
As noted before, the methods were complementary, with all NETs misclassified by one method called accurately by the other.
The investigators suggest that because the methods are highly accurate and complementary, they may best be used sequentially, starting with immunohistochemistry which is both inexpensive and widely available, and if immunohistochemistry fails, moving on to GEC.
"Sequential use allows for identification of nearly all metastatic neuroendocrine tumors from small bowel or pancreatic sites," Dr. Maxwell said.
In the discussion, Dr. Eren Berber of the Center for Endocrine Surgery at the Cleveland Clinic, who was not involved in the study, questioned whether knowing the primary site had any practical implications for surgeons.
Dr. Maxwell noted that some pancreatic NETs are not detected by preoperative studies and that given the risks of pancreatectomy or pancreaticoduodenectomy, accurately identifying the source of an NET may be helpful for patient counseling and preoperative planning.
The study was supported by a grant from the National Institutes of Health. Dr. Maxwell and Dr. Berber reported having no financial disclosures.
AT AAES 2014
Key clinical point: Gene expression can be used to identify the primary source of neuroendocrine small bowel and pancreatic metastases.
Major finding: Gene expression classification accurately identified the primary source of 94 of 97 small bowel neuroendocrine tumor metastases, (96.9%), and 34 of 39 pancreatic metastases (87.2%).
Data source: Retrospective single institution study of metastases from 136 patients with neuroendocrine tumors.
Disclosures: The study was supported by a grant from the National Institutes of Health. Dr. Maxwell and Dr. Berber reported having no financial disclosures.
Swapping bortezomib for vincristine improves PFS in mantle cell lymphoma
CHICAGO – Tweaking the R-CHOP recipe to substitute bortezomib for vincristine may bring clinical benefit to patients with newly diagnosed mantle cell lymphoma who are ineligible for bone marrow transplant.
In a randomized phase III trial in patients with MCL who could not undergo bone marrow transplant due to age or comorbidities, those patients who received a combination of rituximab, doxorubicin, bortezomib (Velcade), cyclophosphamide, and prednisone (the VR-CAP regimen) had significantly better progression-free survival than did patients treated with R-CHOP, the same regimen but with vincristine instead of bortezomib.
The VR-CAP regimen "could be considered a new standard of care for newly diagnosed mantle cell lymphoma patients not considered for intensive treatment and bone marrow transplant," Dr. Franco Cavalli reported at the annual meeting of the American Society of Clinical Oncology.
The bortezomib-containing regimen was associated with more grade 3 or 4 toxicities than standard R-CHOP, but adverse events were manageable, and most patients in each study arm were able to stay on chemotherapy for all prescribed cycles, said Dr. Cavalli of the Oncology Institute of Southern Switzerland, Bellinzona.
R-CHOP is a standard frontline therapy for patients with MCL who are deemed to be ineligible for intensive therapy and/or bone marrow transplant. But the regimen offers only limited progression-free survival (PFS) in this population, Dr. Cavalli said.
Because bortezomib is approved for the treatment of relapsed MCL in the United States and 53 other nations, the authors investigated whether it could improve outcomes when given to patients with newly diagnosed disease.
The LYM-3002 trial was a phase III study conducted at 128 centers in 28 countries in Europe, Asia, the Americas, and Africa. Patients with newly diagnosed MCL stage II-IV, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and who were ineligible or not considered for bone marrow transplant, were randomized to receive either R-CHOP or VR-CAP. In R-CHOP, vincristine 1.4 mg/m2 was delivered to a maximum of 2 mg intravenously on day 1 of each cycle. In VR-CAP, bortezomib 1.3 mg/m2 was delivered via intravenous infusion on days 1, 4, 8, and 11 of each cycle. Patients were assigned to receive at least six cycles of therapy, with an additional two cycles possible if investigator-assessed responses were first documented at the end of cycle 6.
A total of 487 patients (244 assigned to R-CHOP and 243 to VR-CAP) were included in the intention-to-treat analysis.
At a median follow-up of 40 months, median PFS, the primary endpoint, was 24.7 months in the VR-CAP arm, compared with 14.4 months for R-CHOP (hazard ratio, 0.63; P less than .001), as judged by an independent review committee. Investigator-rated PFS was 30.7 months vs. 16.1 months, respectively (HR, 0.51; P less than .001).
Clinical responses according to International Working Group revised response criteria for malignant lymphoma included overall response rates (complete response, complete unconfirmed response, and partial response) of 90% in the R-CHOP–treated patients and 92% in those who received VR-CAP.
However, there was a higher proportion of combined complete response and complete unconfirmed response in the VR-CAP group: 42% for R-CHOP vs. 53% for VR-CAP (odds ratio, 1.69; P = .007).
Median time to response was also shorter with VR-CAP (1.6 vs. 1.4 months; HR, 1.54; P less than .001).
Independent reviewer-rated median time-to-progression was 16.1 months for R-CHOP vs. 30.5 months for VR-CAP (HR, 0.58; P less than .001). Median time to next therapy was 24.8 vs. 44.5 months, respectively (HR, 0.50; P less than .001), and median treatment-free interval was 20.5 vs. 40.6 months (HR, 0.50; P less than .001).
Median overall survival was 56.3 months among R-CHOP–treated patients, vs. not reached among VR-CAP–treated patients.
Grade 3 or higher drug-related adverse events occurred in 85% and 93% of patients, respectively. The events were considered serious in 21% of R-CHOP–treated patients and in 33% of VR-CAP–treated patients. In all, 7% of patients on R-CHOP and 9% of those on VR-CAP discontinued therapy because of adverse events.
Grade 3 adverse events were more frequent with VR-CAP and included neutropenia, leukopenia, lymphopenia, and thrombocytopenia, the last of which occurred in 6% of patients on R-CHOP, compared with 57% for VR-CAP. Despite this difference, however, rates of grade 3 or higher bleeding were similar between the groups, occurring in 1.2% and 1.7%, respectively.
The invited discussant, Dr. Michael E. Williams, chief of hematology/oncology at the University of Virginia Cancer Center, Charlottesville, commented that the study provides proof of principle "that if you add an active single agent and substitute bortezomib for vincristine, which would appear to be a less active agent, that you can certainly improve PFS significantly."
Dr. Williams said that it remains to be seen, however, whether, as Dr. Cavalli suggested, certain treatment strategies could be used to lower the incidence of drug-related adverse events and improve PFS rates further, such as the use of subcutaneous rather than intravenous bortezomib, different dosing schedules, or rituximab in the maintenance phase.
The study was supported by Janssen Global Services and Millennium. Dr. Cavalli disclosed receiving travel support for attending the ASCO annual meeting, but reported having no other conflicts of interest. Dr. Williams disclosed consulting/advising for Millennium and receiving research funding from Janssen and Millennium.
CHICAGO – Tweaking the R-CHOP recipe to substitute bortezomib for vincristine may bring clinical benefit to patients with newly diagnosed mantle cell lymphoma who are ineligible for bone marrow transplant.
In a randomized phase III trial in patients with MCL who could not undergo bone marrow transplant due to age or comorbidities, those patients who received a combination of rituximab, doxorubicin, bortezomib (Velcade), cyclophosphamide, and prednisone (the VR-CAP regimen) had significantly better progression-free survival than did patients treated with R-CHOP, the same regimen but with vincristine instead of bortezomib.
The VR-CAP regimen "could be considered a new standard of care for newly diagnosed mantle cell lymphoma patients not considered for intensive treatment and bone marrow transplant," Dr. Franco Cavalli reported at the annual meeting of the American Society of Clinical Oncology.
The bortezomib-containing regimen was associated with more grade 3 or 4 toxicities than standard R-CHOP, but adverse events were manageable, and most patients in each study arm were able to stay on chemotherapy for all prescribed cycles, said Dr. Cavalli of the Oncology Institute of Southern Switzerland, Bellinzona.
R-CHOP is a standard frontline therapy for patients with MCL who are deemed to be ineligible for intensive therapy and/or bone marrow transplant. But the regimen offers only limited progression-free survival (PFS) in this population, Dr. Cavalli said.
Because bortezomib is approved for the treatment of relapsed MCL in the United States and 53 other nations, the authors investigated whether it could improve outcomes when given to patients with newly diagnosed disease.
The LYM-3002 trial was a phase III study conducted at 128 centers in 28 countries in Europe, Asia, the Americas, and Africa. Patients with newly diagnosed MCL stage II-IV, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and who were ineligible or not considered for bone marrow transplant, were randomized to receive either R-CHOP or VR-CAP. In R-CHOP, vincristine 1.4 mg/m2 was delivered to a maximum of 2 mg intravenously on day 1 of each cycle. In VR-CAP, bortezomib 1.3 mg/m2 was delivered via intravenous infusion on days 1, 4, 8, and 11 of each cycle. Patients were assigned to receive at least six cycles of therapy, with an additional two cycles possible if investigator-assessed responses were first documented at the end of cycle 6.
A total of 487 patients (244 assigned to R-CHOP and 243 to VR-CAP) were included in the intention-to-treat analysis.
At a median follow-up of 40 months, median PFS, the primary endpoint, was 24.7 months in the VR-CAP arm, compared with 14.4 months for R-CHOP (hazard ratio, 0.63; P less than .001), as judged by an independent review committee. Investigator-rated PFS was 30.7 months vs. 16.1 months, respectively (HR, 0.51; P less than .001).
Clinical responses according to International Working Group revised response criteria for malignant lymphoma included overall response rates (complete response, complete unconfirmed response, and partial response) of 90% in the R-CHOP–treated patients and 92% in those who received VR-CAP.
However, there was a higher proportion of combined complete response and complete unconfirmed response in the VR-CAP group: 42% for R-CHOP vs. 53% for VR-CAP (odds ratio, 1.69; P = .007).
Median time to response was also shorter with VR-CAP (1.6 vs. 1.4 months; HR, 1.54; P less than .001).
Independent reviewer-rated median time-to-progression was 16.1 months for R-CHOP vs. 30.5 months for VR-CAP (HR, 0.58; P less than .001). Median time to next therapy was 24.8 vs. 44.5 months, respectively (HR, 0.50; P less than .001), and median treatment-free interval was 20.5 vs. 40.6 months (HR, 0.50; P less than .001).
Median overall survival was 56.3 months among R-CHOP–treated patients, vs. not reached among VR-CAP–treated patients.
Grade 3 or higher drug-related adverse events occurred in 85% and 93% of patients, respectively. The events were considered serious in 21% of R-CHOP–treated patients and in 33% of VR-CAP–treated patients. In all, 7% of patients on R-CHOP and 9% of those on VR-CAP discontinued therapy because of adverse events.
Grade 3 adverse events were more frequent with VR-CAP and included neutropenia, leukopenia, lymphopenia, and thrombocytopenia, the last of which occurred in 6% of patients on R-CHOP, compared with 57% for VR-CAP. Despite this difference, however, rates of grade 3 or higher bleeding were similar between the groups, occurring in 1.2% and 1.7%, respectively.
The invited discussant, Dr. Michael E. Williams, chief of hematology/oncology at the University of Virginia Cancer Center, Charlottesville, commented that the study provides proof of principle "that if you add an active single agent and substitute bortezomib for vincristine, which would appear to be a less active agent, that you can certainly improve PFS significantly."
Dr. Williams said that it remains to be seen, however, whether, as Dr. Cavalli suggested, certain treatment strategies could be used to lower the incidence of drug-related adverse events and improve PFS rates further, such as the use of subcutaneous rather than intravenous bortezomib, different dosing schedules, or rituximab in the maintenance phase.
The study was supported by Janssen Global Services and Millennium. Dr. Cavalli disclosed receiving travel support for attending the ASCO annual meeting, but reported having no other conflicts of interest. Dr. Williams disclosed consulting/advising for Millennium and receiving research funding from Janssen and Millennium.
CHICAGO – Tweaking the R-CHOP recipe to substitute bortezomib for vincristine may bring clinical benefit to patients with newly diagnosed mantle cell lymphoma who are ineligible for bone marrow transplant.
In a randomized phase III trial in patients with MCL who could not undergo bone marrow transplant due to age or comorbidities, those patients who received a combination of rituximab, doxorubicin, bortezomib (Velcade), cyclophosphamide, and prednisone (the VR-CAP regimen) had significantly better progression-free survival than did patients treated with R-CHOP, the same regimen but with vincristine instead of bortezomib.
The VR-CAP regimen "could be considered a new standard of care for newly diagnosed mantle cell lymphoma patients not considered for intensive treatment and bone marrow transplant," Dr. Franco Cavalli reported at the annual meeting of the American Society of Clinical Oncology.
The bortezomib-containing regimen was associated with more grade 3 or 4 toxicities than standard R-CHOP, but adverse events were manageable, and most patients in each study arm were able to stay on chemotherapy for all prescribed cycles, said Dr. Cavalli of the Oncology Institute of Southern Switzerland, Bellinzona.
R-CHOP is a standard frontline therapy for patients with MCL who are deemed to be ineligible for intensive therapy and/or bone marrow transplant. But the regimen offers only limited progression-free survival (PFS) in this population, Dr. Cavalli said.
Because bortezomib is approved for the treatment of relapsed MCL in the United States and 53 other nations, the authors investigated whether it could improve outcomes when given to patients with newly diagnosed disease.
The LYM-3002 trial was a phase III study conducted at 128 centers in 28 countries in Europe, Asia, the Americas, and Africa. Patients with newly diagnosed MCL stage II-IV, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and who were ineligible or not considered for bone marrow transplant, were randomized to receive either R-CHOP or VR-CAP. In R-CHOP, vincristine 1.4 mg/m2 was delivered to a maximum of 2 mg intravenously on day 1 of each cycle. In VR-CAP, bortezomib 1.3 mg/m2 was delivered via intravenous infusion on days 1, 4, 8, and 11 of each cycle. Patients were assigned to receive at least six cycles of therapy, with an additional two cycles possible if investigator-assessed responses were first documented at the end of cycle 6.
A total of 487 patients (244 assigned to R-CHOP and 243 to VR-CAP) were included in the intention-to-treat analysis.
At a median follow-up of 40 months, median PFS, the primary endpoint, was 24.7 months in the VR-CAP arm, compared with 14.4 months for R-CHOP (hazard ratio, 0.63; P less than .001), as judged by an independent review committee. Investigator-rated PFS was 30.7 months vs. 16.1 months, respectively (HR, 0.51; P less than .001).
Clinical responses according to International Working Group revised response criteria for malignant lymphoma included overall response rates (complete response, complete unconfirmed response, and partial response) of 90% in the R-CHOP–treated patients and 92% in those who received VR-CAP.
However, there was a higher proportion of combined complete response and complete unconfirmed response in the VR-CAP group: 42% for R-CHOP vs. 53% for VR-CAP (odds ratio, 1.69; P = .007).
Median time to response was also shorter with VR-CAP (1.6 vs. 1.4 months; HR, 1.54; P less than .001).
Independent reviewer-rated median time-to-progression was 16.1 months for R-CHOP vs. 30.5 months for VR-CAP (HR, 0.58; P less than .001). Median time to next therapy was 24.8 vs. 44.5 months, respectively (HR, 0.50; P less than .001), and median treatment-free interval was 20.5 vs. 40.6 months (HR, 0.50; P less than .001).
Median overall survival was 56.3 months among R-CHOP–treated patients, vs. not reached among VR-CAP–treated patients.
Grade 3 or higher drug-related adverse events occurred in 85% and 93% of patients, respectively. The events were considered serious in 21% of R-CHOP–treated patients and in 33% of VR-CAP–treated patients. In all, 7% of patients on R-CHOP and 9% of those on VR-CAP discontinued therapy because of adverse events.
Grade 3 adverse events were more frequent with VR-CAP and included neutropenia, leukopenia, lymphopenia, and thrombocytopenia, the last of which occurred in 6% of patients on R-CHOP, compared with 57% for VR-CAP. Despite this difference, however, rates of grade 3 or higher bleeding were similar between the groups, occurring in 1.2% and 1.7%, respectively.
The invited discussant, Dr. Michael E. Williams, chief of hematology/oncology at the University of Virginia Cancer Center, Charlottesville, commented that the study provides proof of principle "that if you add an active single agent and substitute bortezomib for vincristine, which would appear to be a less active agent, that you can certainly improve PFS significantly."
Dr. Williams said that it remains to be seen, however, whether, as Dr. Cavalli suggested, certain treatment strategies could be used to lower the incidence of drug-related adverse events and improve PFS rates further, such as the use of subcutaneous rather than intravenous bortezomib, different dosing schedules, or rituximab in the maintenance phase.
The study was supported by Janssen Global Services and Millennium. Dr. Cavalli disclosed receiving travel support for attending the ASCO annual meeting, but reported having no other conflicts of interest. Dr. Williams disclosed consulting/advising for Millennium and receiving research funding from Janssen and Millennium.
AT THE ASCO ANNUAL MEETING 2014
Key clinical point: Substituting bortezomib for vincristine may bring clinical benefit to patients with newly diagnosed mantle cell lymphoma who are ineligible for bone marrow transplant.
Major finding: At a median follow-up of 40 months, median progression-free survival was 24.7 months in the bortezomib-containing VR-CAP arm, compared with 14.4 months for R-CHOP, a significant difference.
Data source: Randomized, open-label phase III study in 487 patients with newly diagnosed mantle cell lymphoma.
Disclosures: The study was supported by Janssen Global Services and Millennium. Dr. Cavalli disclosed receiving travel support for attending the ASCO annual meeting, but reported having no other conflicts of interest. Dr. Williams disclosed consulting/advising for Millennium and receiving research funding from Janssen and Millennium.
Bellis perennis
Known also as the common daisy or English daisy, Bellis perennis is a perennial plant belonging to the Asteraceae or Compositae (aster, daisy, or sunflower) family. Native to Europe and North Africa, it has been used in traditional medicine in Europe since the Middle Ages to treat bruises, broken bones, muscle pain, cutaneous wounds, and rheumatism. Other skin applications include eczema, boils, inflammation, and purulent skin disease. In addition, B. perennis has been used in folk medicine to treat upper respiratory tract infections, gastritis, stomachache, diarrhea, bleeding, rheumatism, common colds, and headache (Pharm. Biol. 2014 March 12 [Epub ahead of print]; Pharm. Biol. 2012;50:1031-7; Chem. Pharm. Bull. [Tokyo] 2008;56:559-68; Chem. Pharm. Bull. [Tokyo] 2011;59:889-95; Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Chemistry
B. perennis roots and flowers have been shown to contain several important bioactive constituents, including triterpene saponins, anthocyanins, flavonoids, polysaccharides, and polyacetylenes (Chem. Pharm. Bull. [Tokyo] 2008;56:559-68; SOFW J. 2005;131:40-9). In 2008, Morikawa et al. identified newly isolated triterpene saponins in B. perennis. These compounds, labeled as perennisosides I-VII, exhibited inhibitory activity on serum triglyceride elevation in olive oil–treated mice (J. Nat. Prod. 2008;71:828-35). That same year, Yoshikawa et al. isolated six new acylated oleanane-type triterpene oligoglycosides (perennisaponins A-F) from the flowers of B. perennis in addition to 14 saponins, 9 flavonoids, and 2 glycosides (Chem. Pharm. Bull. [Tokyo] 2008;56:559-68).
In 2011, Morikawa et al. isolated five new triterpene saponins (perennisosides VIII-XII) from the methanolic extract of B. perennis flowers. The extract was shown to suppress gastric emptying in olive oil–laded mice (Chem. Pharm. Bull. [Tokyo] 2011;59:889-95).
Early in 2014, Pehlivan et al. used bioassay-guided fractionation and isolation procedures to isolate an oleanane-type saponin from B. perennis that exhibited antitumor activity, the first such finding associated with B. perennis flowers. Tumor inhibition of 99% was achieved by the most active fraction at 3,000 mg/L (Pharm. Biol. 2014 March 12 [Epub ahead of print]).
Wound-healing capacity
In 2012, Karakas et al. studied the wound-healing activity displayed by the dried flowers of B. perennis in 12 male adult Wistar albino rats over a 30-day period. Six wounds were introduced onto each animal, with two treated topically once a day with a hydrophilic ointment containing an n-butanol fraction of B. perennis, two treated daily with the ointment minus the B. perennis fraction, and two untreated wounds used as control. Statistically significant differences were noted with 100% wound closure in the B. perennis group, 87% in the control group, and 85% in the other treatment group. The investigators concluded that their findings represented the first scientific confirmation supporting the traditional usage of B. perennis for wound healing. They noted that the topical administration of an ointment formulated with an n-butanol fraction of B. perennis flowers exhibits wound healing activity without inducing scars in a circular excision wound model in rats (Pharm. Biol. 2012;50:1031-7).
Antimicrobial activity against gram-positive and gram-negative bacteria, as well as anticancer activity against human leukemia cells in vitro, has also been associated with B. perennis (Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Skin-lightening activity
Extracts of B. perennis are included in the product Belides that has been combined in a formulation with emblica and licorice for use as a skin-lightening agent. In 2010, Costa et al. conducted a monoblind clinical study to assess the clinical efficacy of the combination of Belides, emblica, and licorice 7%, compared with hydroquinone 2% for the treatment of epidermal or mixed melasma in 56 women aged 18-60 years. Subjects (ranging from Fitzpatrick skin type I to IV) exclusively used an SPF 35 sunscreen for 60 days before being selected for either the herbal combination cream treatment, applied twice daily, or the hydroquinone group, applied nightly.
Depigmentation was observed in 78.3% of the herbal combination group and 88.9% of the hydroquinone group, among the 23 volunteers in the herbal group and 27 in the hydroquinone group who completed the study. No statistically significant differences were found between the treatment regimens in ameliorating melasma, but fewer adverse cutaneous reactions were associated with the herbal treatment. The investigators found the combination of Belides, emblica, and licorice to be a safe and effective option for treating melasma (An. Bras. Dermatol. 2010;85:613-20). Previously, Belides was shown to be nearly twice as active as arbutin and an effective skin-lightening agent in a pilot study with human volunteers (SOFW J. 2005;131:40-9; Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Conclusion
The roots and flowers of B. perennis have been used for many years in traditional medicine to treat various conditions, including skin disorders. While modern scientific interest has been piqued, the current body of evidence is meager. Much more research is necessary to determine the potential role of topical B. perennis in the dermatologic armamentarium. But recent data and the history of traditional use suggest that such research is warranted.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Known also as the common daisy or English daisy, Bellis perennis is a perennial plant belonging to the Asteraceae or Compositae (aster, daisy, or sunflower) family. Native to Europe and North Africa, it has been used in traditional medicine in Europe since the Middle Ages to treat bruises, broken bones, muscle pain, cutaneous wounds, and rheumatism. Other skin applications include eczema, boils, inflammation, and purulent skin disease. In addition, B. perennis has been used in folk medicine to treat upper respiratory tract infections, gastritis, stomachache, diarrhea, bleeding, rheumatism, common colds, and headache (Pharm. Biol. 2014 March 12 [Epub ahead of print]; Pharm. Biol. 2012;50:1031-7; Chem. Pharm. Bull. [Tokyo] 2008;56:559-68; Chem. Pharm. Bull. [Tokyo] 2011;59:889-95; Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Chemistry
B. perennis roots and flowers have been shown to contain several important bioactive constituents, including triterpene saponins, anthocyanins, flavonoids, polysaccharides, and polyacetylenes (Chem. Pharm. Bull. [Tokyo] 2008;56:559-68; SOFW J. 2005;131:40-9). In 2008, Morikawa et al. identified newly isolated triterpene saponins in B. perennis. These compounds, labeled as perennisosides I-VII, exhibited inhibitory activity on serum triglyceride elevation in olive oil–treated mice (J. Nat. Prod. 2008;71:828-35). That same year, Yoshikawa et al. isolated six new acylated oleanane-type triterpene oligoglycosides (perennisaponins A-F) from the flowers of B. perennis in addition to 14 saponins, 9 flavonoids, and 2 glycosides (Chem. Pharm. Bull. [Tokyo] 2008;56:559-68).
In 2011, Morikawa et al. isolated five new triterpene saponins (perennisosides VIII-XII) from the methanolic extract of B. perennis flowers. The extract was shown to suppress gastric emptying in olive oil–laded mice (Chem. Pharm. Bull. [Tokyo] 2011;59:889-95).
Early in 2014, Pehlivan et al. used bioassay-guided fractionation and isolation procedures to isolate an oleanane-type saponin from B. perennis that exhibited antitumor activity, the first such finding associated with B. perennis flowers. Tumor inhibition of 99% was achieved by the most active fraction at 3,000 mg/L (Pharm. Biol. 2014 March 12 [Epub ahead of print]).
Wound-healing capacity
In 2012, Karakas et al. studied the wound-healing activity displayed by the dried flowers of B. perennis in 12 male adult Wistar albino rats over a 30-day period. Six wounds were introduced onto each animal, with two treated topically once a day with a hydrophilic ointment containing an n-butanol fraction of B. perennis, two treated daily with the ointment minus the B. perennis fraction, and two untreated wounds used as control. Statistically significant differences were noted with 100% wound closure in the B. perennis group, 87% in the control group, and 85% in the other treatment group. The investigators concluded that their findings represented the first scientific confirmation supporting the traditional usage of B. perennis for wound healing. They noted that the topical administration of an ointment formulated with an n-butanol fraction of B. perennis flowers exhibits wound healing activity without inducing scars in a circular excision wound model in rats (Pharm. Biol. 2012;50:1031-7).
Antimicrobial activity against gram-positive and gram-negative bacteria, as well as anticancer activity against human leukemia cells in vitro, has also been associated with B. perennis (Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Skin-lightening activity
Extracts of B. perennis are included in the product Belides that has been combined in a formulation with emblica and licorice for use as a skin-lightening agent. In 2010, Costa et al. conducted a monoblind clinical study to assess the clinical efficacy of the combination of Belides, emblica, and licorice 7%, compared with hydroquinone 2% for the treatment of epidermal or mixed melasma in 56 women aged 18-60 years. Subjects (ranging from Fitzpatrick skin type I to IV) exclusively used an SPF 35 sunscreen for 60 days before being selected for either the herbal combination cream treatment, applied twice daily, or the hydroquinone group, applied nightly.
Depigmentation was observed in 78.3% of the herbal combination group and 88.9% of the hydroquinone group, among the 23 volunteers in the herbal group and 27 in the hydroquinone group who completed the study. No statistically significant differences were found between the treatment regimens in ameliorating melasma, but fewer adverse cutaneous reactions were associated with the herbal treatment. The investigators found the combination of Belides, emblica, and licorice to be a safe and effective option for treating melasma (An. Bras. Dermatol. 2010;85:613-20). Previously, Belides was shown to be nearly twice as active as arbutin and an effective skin-lightening agent in a pilot study with human volunteers (SOFW J. 2005;131:40-9; Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Conclusion
The roots and flowers of B. perennis have been used for many years in traditional medicine to treat various conditions, including skin disorders. While modern scientific interest has been piqued, the current body of evidence is meager. Much more research is necessary to determine the potential role of topical B. perennis in the dermatologic armamentarium. But recent data and the history of traditional use suggest that such research is warranted.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Known also as the common daisy or English daisy, Bellis perennis is a perennial plant belonging to the Asteraceae or Compositae (aster, daisy, or sunflower) family. Native to Europe and North Africa, it has been used in traditional medicine in Europe since the Middle Ages to treat bruises, broken bones, muscle pain, cutaneous wounds, and rheumatism. Other skin applications include eczema, boils, inflammation, and purulent skin disease. In addition, B. perennis has been used in folk medicine to treat upper respiratory tract infections, gastritis, stomachache, diarrhea, bleeding, rheumatism, common colds, and headache (Pharm. Biol. 2014 March 12 [Epub ahead of print]; Pharm. Biol. 2012;50:1031-7; Chem. Pharm. Bull. [Tokyo] 2008;56:559-68; Chem. Pharm. Bull. [Tokyo] 2011;59:889-95; Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Chemistry
B. perennis roots and flowers have been shown to contain several important bioactive constituents, including triterpene saponins, anthocyanins, flavonoids, polysaccharides, and polyacetylenes (Chem. Pharm. Bull. [Tokyo] 2008;56:559-68; SOFW J. 2005;131:40-9). In 2008, Morikawa et al. identified newly isolated triterpene saponins in B. perennis. These compounds, labeled as perennisosides I-VII, exhibited inhibitory activity on serum triglyceride elevation in olive oil–treated mice (J. Nat. Prod. 2008;71:828-35). That same year, Yoshikawa et al. isolated six new acylated oleanane-type triterpene oligoglycosides (perennisaponins A-F) from the flowers of B. perennis in addition to 14 saponins, 9 flavonoids, and 2 glycosides (Chem. Pharm. Bull. [Tokyo] 2008;56:559-68).
In 2011, Morikawa et al. isolated five new triterpene saponins (perennisosides VIII-XII) from the methanolic extract of B. perennis flowers. The extract was shown to suppress gastric emptying in olive oil–laded mice (Chem. Pharm. Bull. [Tokyo] 2011;59:889-95).
Early in 2014, Pehlivan et al. used bioassay-guided fractionation and isolation procedures to isolate an oleanane-type saponin from B. perennis that exhibited antitumor activity, the first such finding associated with B. perennis flowers. Tumor inhibition of 99% was achieved by the most active fraction at 3,000 mg/L (Pharm. Biol. 2014 March 12 [Epub ahead of print]).
Wound-healing capacity
In 2012, Karakas et al. studied the wound-healing activity displayed by the dried flowers of B. perennis in 12 male adult Wistar albino rats over a 30-day period. Six wounds were introduced onto each animal, with two treated topically once a day with a hydrophilic ointment containing an n-butanol fraction of B. perennis, two treated daily with the ointment minus the B. perennis fraction, and two untreated wounds used as control. Statistically significant differences were noted with 100% wound closure in the B. perennis group, 87% in the control group, and 85% in the other treatment group. The investigators concluded that their findings represented the first scientific confirmation supporting the traditional usage of B. perennis for wound healing. They noted that the topical administration of an ointment formulated with an n-butanol fraction of B. perennis flowers exhibits wound healing activity without inducing scars in a circular excision wound model in rats (Pharm. Biol. 2012;50:1031-7).
Antimicrobial activity against gram-positive and gram-negative bacteria, as well as anticancer activity against human leukemia cells in vitro, has also been associated with B. perennis (Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Skin-lightening activity
Extracts of B. perennis are included in the product Belides that has been combined in a formulation with emblica and licorice for use as a skin-lightening agent. In 2010, Costa et al. conducted a monoblind clinical study to assess the clinical efficacy of the combination of Belides, emblica, and licorice 7%, compared with hydroquinone 2% for the treatment of epidermal or mixed melasma in 56 women aged 18-60 years. Subjects (ranging from Fitzpatrick skin type I to IV) exclusively used an SPF 35 sunscreen for 60 days before being selected for either the herbal combination cream treatment, applied twice daily, or the hydroquinone group, applied nightly.
Depigmentation was observed in 78.3% of the herbal combination group and 88.9% of the hydroquinone group, among the 23 volunteers in the herbal group and 27 in the hydroquinone group who completed the study. No statistically significant differences were found between the treatment regimens in ameliorating melasma, but fewer adverse cutaneous reactions were associated with the herbal treatment. The investigators found the combination of Belides, emblica, and licorice to be a safe and effective option for treating melasma (An. Bras. Dermatol. 2010;85:613-20). Previously, Belides was shown to be nearly twice as active as arbutin and an effective skin-lightening agent in a pilot study with human volunteers (SOFW J. 2005;131:40-9; Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Springer: Dordrecht, 2014, pp. 204-11).
Conclusion
The roots and flowers of B. perennis have been used for many years in traditional medicine to treat various conditions, including skin disorders. While modern scientific interest has been piqued, the current body of evidence is meager. Much more research is necessary to determine the potential role of topical B. perennis in the dermatologic armamentarium. But recent data and the history of traditional use suggest that such research is warranted.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Approach to newborns exposed to HSV at the time of delivery
Recently the American Academy of Pediatrics issued recommendations that address management of asymptomatic newborns whose mothers have active herpes simplex virus (HSV) lesions noted at the time of delivery. Implementing these recommendations requires proactive coordination between the director of the laboratory and the obstetrical and pediatric providers to ensure success (Pediatrics 2013;131:e635-46).
Approximately 1,500 infants are diagnosed and treated for neonatal HSV infection each year in the United States. Most pediatricians are knowledgeable about the three forms of neonatal HSV infection and the role of prompt diagnosis and utilization of acyclovir. Even so, the outcomes of this disease may be devastating. Skin–eye–mucous membrane disease has the best prognosis (98% neurologically normal), as finding the culprit lesion generally ensures timely diagnosis and treatment. With central nervous system infection or disseminated disease, a skin lesion is not noted in 25%-40% of cases. So the diagnosis is sometimes delayed or missed initially because the initial presentations (seizures in CNS infection; sepsis picture or liver failure in disseminated disease) may sidetrack the provider into considering other working diagnoses, such as bacterial sepsis or metabolic disease. Neurologic sequelae occur in 25% of those with disseminated infection, but in upward of 70% in those with CNS disease.
Ideally, both the obstetrician and the pediatric provider play a role in ensuring appropriate care for the baby whose mother has active HSV lesions at time of delivery. Appropriate care includes preemptive treatment for neonatal HSV infection, which has the potential to improve outcomes and so should be a high priority for all providers.
The new guidance is evidence based and predicated on the availability of HSV typing of the HSV from the maternal lesion and type-specific serology. It allows the provider to define the newborn risk of acquiring HSV infection more explicitly and utilize preemptive evaluation/therapy. Providers should ensure that their hospital laboratory can perform such testing with reasonable turnaround time for results.
Obstetrical role and implications of testing
The obstetric provider should swab the maternal lesion for HSV polymerase chain reaction (PCR) assay/culture and typing (HSV-1 or HSV-2). These data can be utilized with maternal history and serologic results to calculate the neonatal risk for infection.
Calculation of relative neonatal risk
• First episode, primary infection. Defined as the first maternal HSV episode with type-specific serology being negative, this makes the risk of neonatal infection approximately 50%. If maternal history of prior disease is negative AND either the maternal lesion test results or serology results are unavailable, follow the plan of care for first episode primary infection.
• First episode, nonprimary infection. Defined as the first maternal episode but antibody to detected HSV type is not present (e.g., HSV-2 confirmed from lesion, with type 1 but NOT type 2 maternal antibody present; OR HSV-1 confirmed, with type 2 but NOT type 1 maternal antibody present), the risk of neonatal infection is approximately 25%.
• Recurrent. If the mother has a history of genital herpes and the mother’s type-specific antibody is the same as the type detected in the lesions, the risk for neonatal infection is lower and approximately 2%.
Pediatrician’s role and plan of care
The first order of business is to identify neonates who demonstrate signs or symptoms suggestive of HSV infection at birth or in the perinatal period (whether or not any lesions were noted at time of delivery). In this case, all infants should undergo full evaluation for both viral and bacterial causes and should have prompt initiation of preemptive antiviral and antibacterial therapy. The evaluation of an ill-appearing infant at birth should include CBC; liver function studies; blood, urine, and cerebrospinal fluid examination with bacterial cultures of blood, urine, and cerebrospinal fluid, plus blood and cerebrospinal fluid HSV PCR. Also, HSV surface (conjunctivae, nasopharynx, and rectum) and lesion cultures are needed. Infectious disease consultation is recommended if HSV infection is confirmed. Acyclovir should continue for 14 days for skin–eye–mucous membrane disease or 21 days for CNS or disseminated infection. Further evaluation toward the end of therapy can determine if a longer course of therapy should be considered.
The recent guideline addresses care for those infants who are born to mothers with active HSV lesions noted at time of delivery, and should be initiated only if the infant is asymptomatic at birth.
In this situation, for babies whose mothers have primary infection (risk 50% for neonatal infection) or first episode, nonprimary infection (risk 25% for neonatal infection):
• Approximately 24 hours after the infant’s birth, obtain blood HSV DNA PCR and HSV surface cultures of conjunctivae, nasopharynx, and rectum as well as from the scalp electrode site if there was one.
• Cerebrospinal fluid examination with HSV DNA PCR testing should be obtained.
• Acyclovir (20 mg/kg per dose every 8 hours IV) should be initiated. Preemptive therapy (acyclovir 20 mg/kg per dose every 8 hours IV) should be continued for 10 days and until all studies are negative.
For babies whose mothers have recurrent infection:
• Cerebrospinal fluid examination may be deferred.
• But the rest of the workup should be completed and IV acyclovir initiated.
• IV acyclovir can be stopped at the time that studies are negative (usually at 48 hours, assuming negative results of blood PCR and preliminary negative surface cultures), with close follow-up of the infant.
Use of this guideline can improve care of infants only when the laboratory and the obstetrical and pediatric providers have established a good working relationship. This ensures the availability of necessary HSV studies, complete implementation, and proper interpretation of testing to guide the newborn’s care.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled "Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions," but said she had no other conflicts of interest to disclose. E-mail her at [email protected].
Recently the American Academy of Pediatrics issued recommendations that address management of asymptomatic newborns whose mothers have active herpes simplex virus (HSV) lesions noted at the time of delivery. Implementing these recommendations requires proactive coordination between the director of the laboratory and the obstetrical and pediatric providers to ensure success (Pediatrics 2013;131:e635-46).
Approximately 1,500 infants are diagnosed and treated for neonatal HSV infection each year in the United States. Most pediatricians are knowledgeable about the three forms of neonatal HSV infection and the role of prompt diagnosis and utilization of acyclovir. Even so, the outcomes of this disease may be devastating. Skin–eye–mucous membrane disease has the best prognosis (98% neurologically normal), as finding the culprit lesion generally ensures timely diagnosis and treatment. With central nervous system infection or disseminated disease, a skin lesion is not noted in 25%-40% of cases. So the diagnosis is sometimes delayed or missed initially because the initial presentations (seizures in CNS infection; sepsis picture or liver failure in disseminated disease) may sidetrack the provider into considering other working diagnoses, such as bacterial sepsis or metabolic disease. Neurologic sequelae occur in 25% of those with disseminated infection, but in upward of 70% in those with CNS disease.
Ideally, both the obstetrician and the pediatric provider play a role in ensuring appropriate care for the baby whose mother has active HSV lesions at time of delivery. Appropriate care includes preemptive treatment for neonatal HSV infection, which has the potential to improve outcomes and so should be a high priority for all providers.
The new guidance is evidence based and predicated on the availability of HSV typing of the HSV from the maternal lesion and type-specific serology. It allows the provider to define the newborn risk of acquiring HSV infection more explicitly and utilize preemptive evaluation/therapy. Providers should ensure that their hospital laboratory can perform such testing with reasonable turnaround time for results.
Obstetrical role and implications of testing
The obstetric provider should swab the maternal lesion for HSV polymerase chain reaction (PCR) assay/culture and typing (HSV-1 or HSV-2). These data can be utilized with maternal history and serologic results to calculate the neonatal risk for infection.
Calculation of relative neonatal risk
• First episode, primary infection. Defined as the first maternal HSV episode with type-specific serology being negative, this makes the risk of neonatal infection approximately 50%. If maternal history of prior disease is negative AND either the maternal lesion test results or serology results are unavailable, follow the plan of care for first episode primary infection.
• First episode, nonprimary infection. Defined as the first maternal episode but antibody to detected HSV type is not present (e.g., HSV-2 confirmed from lesion, with type 1 but NOT type 2 maternal antibody present; OR HSV-1 confirmed, with type 2 but NOT type 1 maternal antibody present), the risk of neonatal infection is approximately 25%.
• Recurrent. If the mother has a history of genital herpes and the mother’s type-specific antibody is the same as the type detected in the lesions, the risk for neonatal infection is lower and approximately 2%.
Pediatrician’s role and plan of care
The first order of business is to identify neonates who demonstrate signs or symptoms suggestive of HSV infection at birth or in the perinatal period (whether or not any lesions were noted at time of delivery). In this case, all infants should undergo full evaluation for both viral and bacterial causes and should have prompt initiation of preemptive antiviral and antibacterial therapy. The evaluation of an ill-appearing infant at birth should include CBC; liver function studies; blood, urine, and cerebrospinal fluid examination with bacterial cultures of blood, urine, and cerebrospinal fluid, plus blood and cerebrospinal fluid HSV PCR. Also, HSV surface (conjunctivae, nasopharynx, and rectum) and lesion cultures are needed. Infectious disease consultation is recommended if HSV infection is confirmed. Acyclovir should continue for 14 days for skin–eye–mucous membrane disease or 21 days for CNS or disseminated infection. Further evaluation toward the end of therapy can determine if a longer course of therapy should be considered.
The recent guideline addresses care for those infants who are born to mothers with active HSV lesions noted at time of delivery, and should be initiated only if the infant is asymptomatic at birth.
In this situation, for babies whose mothers have primary infection (risk 50% for neonatal infection) or first episode, nonprimary infection (risk 25% for neonatal infection):
• Approximately 24 hours after the infant’s birth, obtain blood HSV DNA PCR and HSV surface cultures of conjunctivae, nasopharynx, and rectum as well as from the scalp electrode site if there was one.
• Cerebrospinal fluid examination with HSV DNA PCR testing should be obtained.
• Acyclovir (20 mg/kg per dose every 8 hours IV) should be initiated. Preemptive therapy (acyclovir 20 mg/kg per dose every 8 hours IV) should be continued for 10 days and until all studies are negative.
For babies whose mothers have recurrent infection:
• Cerebrospinal fluid examination may be deferred.
• But the rest of the workup should be completed and IV acyclovir initiated.
• IV acyclovir can be stopped at the time that studies are negative (usually at 48 hours, assuming negative results of blood PCR and preliminary negative surface cultures), with close follow-up of the infant.
Use of this guideline can improve care of infants only when the laboratory and the obstetrical and pediatric providers have established a good working relationship. This ensures the availability of necessary HSV studies, complete implementation, and proper interpretation of testing to guide the newborn’s care.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled "Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions," but said she had no other conflicts of interest to disclose. E-mail her at [email protected].
Recently the American Academy of Pediatrics issued recommendations that address management of asymptomatic newborns whose mothers have active herpes simplex virus (HSV) lesions noted at the time of delivery. Implementing these recommendations requires proactive coordination between the director of the laboratory and the obstetrical and pediatric providers to ensure success (Pediatrics 2013;131:e635-46).
Approximately 1,500 infants are diagnosed and treated for neonatal HSV infection each year in the United States. Most pediatricians are knowledgeable about the three forms of neonatal HSV infection and the role of prompt diagnosis and utilization of acyclovir. Even so, the outcomes of this disease may be devastating. Skin–eye–mucous membrane disease has the best prognosis (98% neurologically normal), as finding the culprit lesion generally ensures timely diagnosis and treatment. With central nervous system infection or disseminated disease, a skin lesion is not noted in 25%-40% of cases. So the diagnosis is sometimes delayed or missed initially because the initial presentations (seizures in CNS infection; sepsis picture or liver failure in disseminated disease) may sidetrack the provider into considering other working diagnoses, such as bacterial sepsis or metabolic disease. Neurologic sequelae occur in 25% of those with disseminated infection, but in upward of 70% in those with CNS disease.
Ideally, both the obstetrician and the pediatric provider play a role in ensuring appropriate care for the baby whose mother has active HSV lesions at time of delivery. Appropriate care includes preemptive treatment for neonatal HSV infection, which has the potential to improve outcomes and so should be a high priority for all providers.
The new guidance is evidence based and predicated on the availability of HSV typing of the HSV from the maternal lesion and type-specific serology. It allows the provider to define the newborn risk of acquiring HSV infection more explicitly and utilize preemptive evaluation/therapy. Providers should ensure that their hospital laboratory can perform such testing with reasonable turnaround time for results.
Obstetrical role and implications of testing
The obstetric provider should swab the maternal lesion for HSV polymerase chain reaction (PCR) assay/culture and typing (HSV-1 or HSV-2). These data can be utilized with maternal history and serologic results to calculate the neonatal risk for infection.
Calculation of relative neonatal risk
• First episode, primary infection. Defined as the first maternal HSV episode with type-specific serology being negative, this makes the risk of neonatal infection approximately 50%. If maternal history of prior disease is negative AND either the maternal lesion test results or serology results are unavailable, follow the plan of care for first episode primary infection.
• First episode, nonprimary infection. Defined as the first maternal episode but antibody to detected HSV type is not present (e.g., HSV-2 confirmed from lesion, with type 1 but NOT type 2 maternal antibody present; OR HSV-1 confirmed, with type 2 but NOT type 1 maternal antibody present), the risk of neonatal infection is approximately 25%.
• Recurrent. If the mother has a history of genital herpes and the mother’s type-specific antibody is the same as the type detected in the lesions, the risk for neonatal infection is lower and approximately 2%.
Pediatrician’s role and plan of care
The first order of business is to identify neonates who demonstrate signs or symptoms suggestive of HSV infection at birth or in the perinatal period (whether or not any lesions were noted at time of delivery). In this case, all infants should undergo full evaluation for both viral and bacterial causes and should have prompt initiation of preemptive antiviral and antibacterial therapy. The evaluation of an ill-appearing infant at birth should include CBC; liver function studies; blood, urine, and cerebrospinal fluid examination with bacterial cultures of blood, urine, and cerebrospinal fluid, plus blood and cerebrospinal fluid HSV PCR. Also, HSV surface (conjunctivae, nasopharynx, and rectum) and lesion cultures are needed. Infectious disease consultation is recommended if HSV infection is confirmed. Acyclovir should continue for 14 days for skin–eye–mucous membrane disease or 21 days for CNS or disseminated infection. Further evaluation toward the end of therapy can determine if a longer course of therapy should be considered.
The recent guideline addresses care for those infants who are born to mothers with active HSV lesions noted at time of delivery, and should be initiated only if the infant is asymptomatic at birth.
In this situation, for babies whose mothers have primary infection (risk 50% for neonatal infection) or first episode, nonprimary infection (risk 25% for neonatal infection):
• Approximately 24 hours after the infant’s birth, obtain blood HSV DNA PCR and HSV surface cultures of conjunctivae, nasopharynx, and rectum as well as from the scalp electrode site if there was one.
• Cerebrospinal fluid examination with HSV DNA PCR testing should be obtained.
• Acyclovir (20 mg/kg per dose every 8 hours IV) should be initiated. Preemptive therapy (acyclovir 20 mg/kg per dose every 8 hours IV) should be continued for 10 days and until all studies are negative.
For babies whose mothers have recurrent infection:
• Cerebrospinal fluid examination may be deferred.
• But the rest of the workup should be completed and IV acyclovir initiated.
• IV acyclovir can be stopped at the time that studies are negative (usually at 48 hours, assuming negative results of blood PCR and preliminary negative surface cultures), with close follow-up of the infant.
Use of this guideline can improve care of infants only when the laboratory and the obstetrical and pediatric providers have established a good working relationship. This ensures the availability of necessary HSV studies, complete implementation, and proper interpretation of testing to guide the newborn’s care.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled "Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions," but said she had no other conflicts of interest to disclose. E-mail her at [email protected].
Novel noninvasive therapy for extraesophageal reflux
CHICAGO – A novel, externally worn device for the treatment of extraesophageal reflux proved effective, safe, and well tolerated in a multicenter clinical trial.
"Given the poor response to PPI [proton-pump inhibitors] therapy in many patients with extraesophageal reflux, this device may be an alternative form of treating this difficult disorder," Dr. Michael F. Vaezi said in a presentation of the study findings at the annual Digestive Disease Week.
Extraesophageal reflux (EER) affects more than 15 million Americans. The cost of care is estimated to be in excess of $50 billion annually, most of which goes for PPIs, which are overused and generally not effective in treating this common disorder, observed Dr. Vaezi, professor of medicine at Vanderbilt University, Nashville, Tenn.
EER is caused by reflux of gastroduodenal contents into the laryngopharynx, resulting in a range of symptoms including chronic cough, postnasal drip, throat clearing, hoarseness, and a sensation of a lump in the throat.
The investigational device, known as the Reza-Band, is an individually fitted upper esophageal sphincter assist device to be worn at bedtime. It is designed to apply slight pressure to the cricoid cartilage area sufficient to increase the intraluminal pressure of the upper esophageal sphincter to 20 mm Hg. In earlier studies, this modest increase in pressure eliminated pharyngeal reflux without affecting normal activities such as swallowing and belching.
Dr. Vaezi reported on 47 patients with EER treated with the upper esophageal assist device at four medical centers. Their mean age was 50 years, with an average body mass index of 26.1 kg/m2. Three-quarters of patients were on PPI therapy, which they felt to be ineffective.
The primary study endpoint was the change in Reflux Symptom Index scores from baseline through week 4 of nighttime use of the assist device. The median score improved from 27 at baseline to 14 at week 2 and 12 at week 4. That translated to a mean 54% reduction. A total of 86% of subjects were deemed to have experienced treatment success as defined by at least a 25% improvement in their symptom score; 30% of participants showed a greater than 75% improvement.
Treatment side effects were few, mild, and brief, consisting chiefly of skin irritation from the device’s Velcro strap. No one withdrew from the study.
The Reza-Band device was developed by gastroenterologists at the Medical College of Wisconsin, Milwaukee. Dr. Vaezi said he and his coinvestigators decided to formally investigate the device because "we were intrigued and impressed by the fact that it has helped so many patients."
"For me, it’s a welcome development," Dr. Vaezi added. "Just anecdotally, many patients in the study wanted to know how they could purchase this device. It’s really welcome in an area [in which] we don’t have many therapeutic options."
Still, he noted, it’s probably a good idea for device users to continue to keep a couple of bricks under the head of the bed, in accord with standard medical advice.
Future plans include a sham-controlled study designed to assess the device’s true benefit over placebo therapy; this will be an important study because EER is known to have a substantial placebo response rate, he continued. Also, plans are afoot for a safety study assessing whether the device has any clinically meaningful impact upon intraocular pressure.
The study was supported by Somna Therapeutics, which is developing the device. Dr. Vaezi reported having no financial relationship with the company.
CHICAGO – A novel, externally worn device for the treatment of extraesophageal reflux proved effective, safe, and well tolerated in a multicenter clinical trial.
"Given the poor response to PPI [proton-pump inhibitors] therapy in many patients with extraesophageal reflux, this device may be an alternative form of treating this difficult disorder," Dr. Michael F. Vaezi said in a presentation of the study findings at the annual Digestive Disease Week.
Extraesophageal reflux (EER) affects more than 15 million Americans. The cost of care is estimated to be in excess of $50 billion annually, most of which goes for PPIs, which are overused and generally not effective in treating this common disorder, observed Dr. Vaezi, professor of medicine at Vanderbilt University, Nashville, Tenn.
EER is caused by reflux of gastroduodenal contents into the laryngopharynx, resulting in a range of symptoms including chronic cough, postnasal drip, throat clearing, hoarseness, and a sensation of a lump in the throat.
The investigational device, known as the Reza-Band, is an individually fitted upper esophageal sphincter assist device to be worn at bedtime. It is designed to apply slight pressure to the cricoid cartilage area sufficient to increase the intraluminal pressure of the upper esophageal sphincter to 20 mm Hg. In earlier studies, this modest increase in pressure eliminated pharyngeal reflux without affecting normal activities such as swallowing and belching.
Dr. Vaezi reported on 47 patients with EER treated with the upper esophageal assist device at four medical centers. Their mean age was 50 years, with an average body mass index of 26.1 kg/m2. Three-quarters of patients were on PPI therapy, which they felt to be ineffective.
The primary study endpoint was the change in Reflux Symptom Index scores from baseline through week 4 of nighttime use of the assist device. The median score improved from 27 at baseline to 14 at week 2 and 12 at week 4. That translated to a mean 54% reduction. A total of 86% of subjects were deemed to have experienced treatment success as defined by at least a 25% improvement in their symptom score; 30% of participants showed a greater than 75% improvement.
Treatment side effects were few, mild, and brief, consisting chiefly of skin irritation from the device’s Velcro strap. No one withdrew from the study.
The Reza-Band device was developed by gastroenterologists at the Medical College of Wisconsin, Milwaukee. Dr. Vaezi said he and his coinvestigators decided to formally investigate the device because "we were intrigued and impressed by the fact that it has helped so many patients."
"For me, it’s a welcome development," Dr. Vaezi added. "Just anecdotally, many patients in the study wanted to know how they could purchase this device. It’s really welcome in an area [in which] we don’t have many therapeutic options."
Still, he noted, it’s probably a good idea for device users to continue to keep a couple of bricks under the head of the bed, in accord with standard medical advice.
Future plans include a sham-controlled study designed to assess the device’s true benefit over placebo therapy; this will be an important study because EER is known to have a substantial placebo response rate, he continued. Also, plans are afoot for a safety study assessing whether the device has any clinically meaningful impact upon intraocular pressure.
The study was supported by Somna Therapeutics, which is developing the device. Dr. Vaezi reported having no financial relationship with the company.
CHICAGO – A novel, externally worn device for the treatment of extraesophageal reflux proved effective, safe, and well tolerated in a multicenter clinical trial.
"Given the poor response to PPI [proton-pump inhibitors] therapy in many patients with extraesophageal reflux, this device may be an alternative form of treating this difficult disorder," Dr. Michael F. Vaezi said in a presentation of the study findings at the annual Digestive Disease Week.
Extraesophageal reflux (EER) affects more than 15 million Americans. The cost of care is estimated to be in excess of $50 billion annually, most of which goes for PPIs, which are overused and generally not effective in treating this common disorder, observed Dr. Vaezi, professor of medicine at Vanderbilt University, Nashville, Tenn.
EER is caused by reflux of gastroduodenal contents into the laryngopharynx, resulting in a range of symptoms including chronic cough, postnasal drip, throat clearing, hoarseness, and a sensation of a lump in the throat.
The investigational device, known as the Reza-Band, is an individually fitted upper esophageal sphincter assist device to be worn at bedtime. It is designed to apply slight pressure to the cricoid cartilage area sufficient to increase the intraluminal pressure of the upper esophageal sphincter to 20 mm Hg. In earlier studies, this modest increase in pressure eliminated pharyngeal reflux without affecting normal activities such as swallowing and belching.
Dr. Vaezi reported on 47 patients with EER treated with the upper esophageal assist device at four medical centers. Their mean age was 50 years, with an average body mass index of 26.1 kg/m2. Three-quarters of patients were on PPI therapy, which they felt to be ineffective.
The primary study endpoint was the change in Reflux Symptom Index scores from baseline through week 4 of nighttime use of the assist device. The median score improved from 27 at baseline to 14 at week 2 and 12 at week 4. That translated to a mean 54% reduction. A total of 86% of subjects were deemed to have experienced treatment success as defined by at least a 25% improvement in their symptom score; 30% of participants showed a greater than 75% improvement.
Treatment side effects were few, mild, and brief, consisting chiefly of skin irritation from the device’s Velcro strap. No one withdrew from the study.
The Reza-Band device was developed by gastroenterologists at the Medical College of Wisconsin, Milwaukee. Dr. Vaezi said he and his coinvestigators decided to formally investigate the device because "we were intrigued and impressed by the fact that it has helped so many patients."
"For me, it’s a welcome development," Dr. Vaezi added. "Just anecdotally, many patients in the study wanted to know how they could purchase this device. It’s really welcome in an area [in which] we don’t have many therapeutic options."
Still, he noted, it’s probably a good idea for device users to continue to keep a couple of bricks under the head of the bed, in accord with standard medical advice.
Future plans include a sham-controlled study designed to assess the device’s true benefit over placebo therapy; this will be an important study because EER is known to have a substantial placebo response rate, he continued. Also, plans are afoot for a safety study assessing whether the device has any clinically meaningful impact upon intraocular pressure.
The study was supported by Somna Therapeutics, which is developing the device. Dr. Vaezi reported having no financial relationship with the company.
AT DDW 2014
Key clinical point: An investigational device that modestly increases intraluminal pressure at the upper esophageal sphincter shows promise as a noninvasive therapy for extraesophageal reflux, a common disorder for which no effective pharmacotherapy exists.
Major finding: Patients with extraesophageal reflux experienced a mean 54% reduction in scores on the Reflux Symptom Index after 4 weeks of nighttime use of the upper esophageal sphincter assist device.
Data source: This was a 4-week multicenter, prospective, uncontrolled study involving 47 patients.
Disclosures: The study was supported by Somna Therapeutics. The presenter reported having no financial relationship with the company.