User login
No benefit to ultrafiltration for treatment of acute cardiorenal syndrome (CARRESS-HF)
Clinical question
Does ultrafiltration therapy result in improved diuresis in hospitalized patients with decompensated heart failure and worsening renal function?
Bottom line
For hospitalized patients with acute decompensated heart failure and worsening renal function, intravenous diuretic therapy is superior to ultrafiltration for preserving renal function while providing similar weight reduction. Moreover, ultrafiltration is a costly and invasive therapy that is associated with more adverse events. LOE = 1b
Reference
Study Design
Randomized controlled trial (nonblinded)
Funding Source
Government
Allocation
Concealed
Setting
Inpatient (any location)
Synopsis
In hospitalized patients with acute cardiorenal syndrome (acute heart failure exacerbation and worsening renal function), ultrafiltration is an alternative strategy for fluid removal. These investigators used concealed allocation to randomize 188 of these patients to receive either ultrafiltration therapy or pharmacologic therapy with intravenous diuretics for fluid removal. Patients with severe renal impairment were excluded (creatinine >3.5 mg/dL [> 309.4 umol/L]). In the pharmacologic therapy group, diuretic doses were adjusted as needed to achieve a urine output of 3 liters to 5 liters per day. In the ultrafiltration group, fluid removal was performed at a rate of 200 mL per hour. Both therapies were continued until symptoms and signs of congestion were optimally reduced. Participants had a median age of 68 years and a median ejection fraction of 33%. More than 75% had been hospitalized for heart failure within the previous year. Analysis was by intention to treat. The primary endpoint was change in weight and change in serum creatinine level at 96 hours postrandomization. Although there was no significant difference in weight loss between the 2 groups at 96 hours, there was a significant increase in serum creatinine level in the ultrafiltration group (an increase of 0.23 mg/dL [20.3 umol/L] in ultrafiltration group vs a decrease in creatinine of 0.04 mg/dL [3.5 umol/L] in diuretic group; P = .003). Despite the worsened renal function in the short-term, there were no differences in long-term outcomes between the 2 groups, including mortality and rehospitalization within 60 days. Finally, ultrafiltration patients were more likely to experience serious adverse events (72% vs 57%; P = .03) during the 60-day follow-up period, mainly due to kidney failure and intravenous catheter-related complications. Although the outcomes assessed were objective, the nonmasked methodology of this study may have introduced a bias on the part of the investigators as to how aggressively they pursued the 2 therapies.
Clinical question
Does ultrafiltration therapy result in improved diuresis in hospitalized patients with decompensated heart failure and worsening renal function?
Bottom line
For hospitalized patients with acute decompensated heart failure and worsening renal function, intravenous diuretic therapy is superior to ultrafiltration for preserving renal function while providing similar weight reduction. Moreover, ultrafiltration is a costly and invasive therapy that is associated with more adverse events. LOE = 1b
Reference
Study Design
Randomized controlled trial (nonblinded)
Funding Source
Government
Allocation
Concealed
Setting
Inpatient (any location)
Synopsis
In hospitalized patients with acute cardiorenal syndrome (acute heart failure exacerbation and worsening renal function), ultrafiltration is an alternative strategy for fluid removal. These investigators used concealed allocation to randomize 188 of these patients to receive either ultrafiltration therapy or pharmacologic therapy with intravenous diuretics for fluid removal. Patients with severe renal impairment were excluded (creatinine >3.5 mg/dL [> 309.4 umol/L]). In the pharmacologic therapy group, diuretic doses were adjusted as needed to achieve a urine output of 3 liters to 5 liters per day. In the ultrafiltration group, fluid removal was performed at a rate of 200 mL per hour. Both therapies were continued until symptoms and signs of congestion were optimally reduced. Participants had a median age of 68 years and a median ejection fraction of 33%. More than 75% had been hospitalized for heart failure within the previous year. Analysis was by intention to treat. The primary endpoint was change in weight and change in serum creatinine level at 96 hours postrandomization. Although there was no significant difference in weight loss between the 2 groups at 96 hours, there was a significant increase in serum creatinine level in the ultrafiltration group (an increase of 0.23 mg/dL [20.3 umol/L] in ultrafiltration group vs a decrease in creatinine of 0.04 mg/dL [3.5 umol/L] in diuretic group; P = .003). Despite the worsened renal function in the short-term, there were no differences in long-term outcomes between the 2 groups, including mortality and rehospitalization within 60 days. Finally, ultrafiltration patients were more likely to experience serious adverse events (72% vs 57%; P = .03) during the 60-day follow-up period, mainly due to kidney failure and intravenous catheter-related complications. Although the outcomes assessed were objective, the nonmasked methodology of this study may have introduced a bias on the part of the investigators as to how aggressively they pursued the 2 therapies.
Clinical question
Does ultrafiltration therapy result in improved diuresis in hospitalized patients with decompensated heart failure and worsening renal function?
Bottom line
For hospitalized patients with acute decompensated heart failure and worsening renal function, intravenous diuretic therapy is superior to ultrafiltration for preserving renal function while providing similar weight reduction. Moreover, ultrafiltration is a costly and invasive therapy that is associated with more adverse events. LOE = 1b
Reference
Study Design
Randomized controlled trial (nonblinded)
Funding Source
Government
Allocation
Concealed
Setting
Inpatient (any location)
Synopsis
In hospitalized patients with acute cardiorenal syndrome (acute heart failure exacerbation and worsening renal function), ultrafiltration is an alternative strategy for fluid removal. These investigators used concealed allocation to randomize 188 of these patients to receive either ultrafiltration therapy or pharmacologic therapy with intravenous diuretics for fluid removal. Patients with severe renal impairment were excluded (creatinine >3.5 mg/dL [> 309.4 umol/L]). In the pharmacologic therapy group, diuretic doses were adjusted as needed to achieve a urine output of 3 liters to 5 liters per day. In the ultrafiltration group, fluid removal was performed at a rate of 200 mL per hour. Both therapies were continued until symptoms and signs of congestion were optimally reduced. Participants had a median age of 68 years and a median ejection fraction of 33%. More than 75% had been hospitalized for heart failure within the previous year. Analysis was by intention to treat. The primary endpoint was change in weight and change in serum creatinine level at 96 hours postrandomization. Although there was no significant difference in weight loss between the 2 groups at 96 hours, there was a significant increase in serum creatinine level in the ultrafiltration group (an increase of 0.23 mg/dL [20.3 umol/L] in ultrafiltration group vs a decrease in creatinine of 0.04 mg/dL [3.5 umol/L] in diuretic group; P = .003). Despite the worsened renal function in the short-term, there were no differences in long-term outcomes between the 2 groups, including mortality and rehospitalization within 60 days. Finally, ultrafiltration patients were more likely to experience serious adverse events (72% vs 57%; P = .03) during the 60-day follow-up period, mainly due to kidney failure and intravenous catheter-related complications. Although the outcomes assessed were objective, the nonmasked methodology of this study may have introduced a bias on the part of the investigators as to how aggressively they pursued the 2 therapies.
Beware of subgroup analyses in trial results
Studies often include subgroup analyses outlining how a specific treatment is more or less effective in one group of patients compared with another. But clinicians, beware: Subgroup analyses too often are not clinically meaningful and should be interpreted cautiously, Dr. Sarah R. Barton and her associates reported in a poster presentation at the American Society for Clinical Oncology’s Gastrointestinal Cancers Symposium.
The investigators reviewed 145 randomized, controlled phase III trials published in peer-reviewed journals from January 2003 to January 2012 that tested an investigational therapy in GI cancer and that involved at least 150 patients. Subgroup analyses appeared in 100 studies (69%), more often in larger ones.
Here’s the shocking part: Only 25% of trials that claimed the treatment worked in a subgroup of patients had the statistical measures to back that up, reported Dr. Barton of Royal Marsden Hospital, Sutton, England. That proportion was the same for industry-sponsored and nonindustry trials.
The study, which won a Merit Award at the meeting, conducted some interesting subgroup analyses of its own. Trials sponsored by for-profit companies included a significantly higher number of subgroup analyses compared with nonindustry trials – a median of six versus two, respectively.
Trials of targeted therapies were more than three times as likely to report subgroup analyses compared with studies of cytotoxic therapies and included significantly more subgroup analyses (a median of six vs. two, respectively). Studies that reported a positive effect in the primary outcome also included a significantly higher median number of subgroup analyses compared with negative trials (again, six versus two).
Industry-sponsored trials that reported a positive effect in the primary outcome of the study were the most likely to report subgroup analyses (23 of 25 studies, or 95%) and to include the highest median number of subgroup analyses (eight) compared with industry-funded trials with a negative primary outcome or nonindustry trials, positive or negative.
Dr. Barton gave some clues that, in general, should cause physicians to look closely at efficacy claims. These include subgroup analyses conducted post hoc, when multiple tests are applied, when multiple endpoints are used, and if there’s no statistically significant test of interaction.
This is not just a problem in oncology. A previous study of 469 randomized, controlled trials published in 118 journals reported that industry-funded trials were less likely to define subgroups before starting the trial, less likely to use the interaction test for analyses of subgroup effects, and more likely to report on subgroups if the primary outcome in the study did not show a positive result (BMJ 2011;342:d1569)
The New England Journal of Medicine provides similar cautions in its guidelines for investigators reporting on subgroup analyses (N. Engl. J. Med. 2007;357:2189-2194).
Dr. Barton reported having no financial disclosures.
– Sherry Boschert
On Twitter @sherryboschert
Studies often include subgroup analyses outlining how a specific treatment is more or less effective in one group of patients compared with another. But clinicians, beware: Subgroup analyses too often are not clinically meaningful and should be interpreted cautiously, Dr. Sarah R. Barton and her associates reported in a poster presentation at the American Society for Clinical Oncology’s Gastrointestinal Cancers Symposium.
The investigators reviewed 145 randomized, controlled phase III trials published in peer-reviewed journals from January 2003 to January 2012 that tested an investigational therapy in GI cancer and that involved at least 150 patients. Subgroup analyses appeared in 100 studies (69%), more often in larger ones.
Here’s the shocking part: Only 25% of trials that claimed the treatment worked in a subgroup of patients had the statistical measures to back that up, reported Dr. Barton of Royal Marsden Hospital, Sutton, England. That proportion was the same for industry-sponsored and nonindustry trials.
The study, which won a Merit Award at the meeting, conducted some interesting subgroup analyses of its own. Trials sponsored by for-profit companies included a significantly higher number of subgroup analyses compared with nonindustry trials – a median of six versus two, respectively.
Trials of targeted therapies were more than three times as likely to report subgroup analyses compared with studies of cytotoxic therapies and included significantly more subgroup analyses (a median of six vs. two, respectively). Studies that reported a positive effect in the primary outcome also included a significantly higher median number of subgroup analyses compared with negative trials (again, six versus two).
Industry-sponsored trials that reported a positive effect in the primary outcome of the study were the most likely to report subgroup analyses (23 of 25 studies, or 95%) and to include the highest median number of subgroup analyses (eight) compared with industry-funded trials with a negative primary outcome or nonindustry trials, positive or negative.
Dr. Barton gave some clues that, in general, should cause physicians to look closely at efficacy claims. These include subgroup analyses conducted post hoc, when multiple tests are applied, when multiple endpoints are used, and if there’s no statistically significant test of interaction.
This is not just a problem in oncology. A previous study of 469 randomized, controlled trials published in 118 journals reported that industry-funded trials were less likely to define subgroups before starting the trial, less likely to use the interaction test for analyses of subgroup effects, and more likely to report on subgroups if the primary outcome in the study did not show a positive result (BMJ 2011;342:d1569)
The New England Journal of Medicine provides similar cautions in its guidelines for investigators reporting on subgroup analyses (N. Engl. J. Med. 2007;357:2189-2194).
Dr. Barton reported having no financial disclosures.
– Sherry Boschert
On Twitter @sherryboschert
Studies often include subgroup analyses outlining how a specific treatment is more or less effective in one group of patients compared with another. But clinicians, beware: Subgroup analyses too often are not clinically meaningful and should be interpreted cautiously, Dr. Sarah R. Barton and her associates reported in a poster presentation at the American Society for Clinical Oncology’s Gastrointestinal Cancers Symposium.
The investigators reviewed 145 randomized, controlled phase III trials published in peer-reviewed journals from January 2003 to January 2012 that tested an investigational therapy in GI cancer and that involved at least 150 patients. Subgroup analyses appeared in 100 studies (69%), more often in larger ones.
Here’s the shocking part: Only 25% of trials that claimed the treatment worked in a subgroup of patients had the statistical measures to back that up, reported Dr. Barton of Royal Marsden Hospital, Sutton, England. That proportion was the same for industry-sponsored and nonindustry trials.
The study, which won a Merit Award at the meeting, conducted some interesting subgroup analyses of its own. Trials sponsored by for-profit companies included a significantly higher number of subgroup analyses compared with nonindustry trials – a median of six versus two, respectively.
Trials of targeted therapies were more than three times as likely to report subgroup analyses compared with studies of cytotoxic therapies and included significantly more subgroup analyses (a median of six vs. two, respectively). Studies that reported a positive effect in the primary outcome also included a significantly higher median number of subgroup analyses compared with negative trials (again, six versus two).
Industry-sponsored trials that reported a positive effect in the primary outcome of the study were the most likely to report subgroup analyses (23 of 25 studies, or 95%) and to include the highest median number of subgroup analyses (eight) compared with industry-funded trials with a negative primary outcome or nonindustry trials, positive or negative.
Dr. Barton gave some clues that, in general, should cause physicians to look closely at efficacy claims. These include subgroup analyses conducted post hoc, when multiple tests are applied, when multiple endpoints are used, and if there’s no statistically significant test of interaction.
This is not just a problem in oncology. A previous study of 469 randomized, controlled trials published in 118 journals reported that industry-funded trials were less likely to define subgroups before starting the trial, less likely to use the interaction test for analyses of subgroup effects, and more likely to report on subgroups if the primary outcome in the study did not show a positive result (BMJ 2011;342:d1569)
The New England Journal of Medicine provides similar cautions in its guidelines for investigators reporting on subgroup analyses (N. Engl. J. Med. 2007;357:2189-2194).
Dr. Barton reported having no financial disclosures.
– Sherry Boschert
On Twitter @sherryboschert
Heavy Workloads Burden Hospitalists, Raise Concerns about Patient Safety
A recent study in which 36% of hospitalists reported that their workload exceeds safe patient census levels at least once a week could spur serious discussions on productivity and quality of care, according to one of its authors.
Daniel Brotman, MD, FACP, FHM, director of the hospitalist program at Johns Hopkins Hospital in Baltimore and one of the study's authors, says the results highlight the delicate balance between pushing hospitalists to generate revenue and maintaining patient safety.
"It's certainly not in the best interest of our patients or our healthcare system to fix financial stress by expecting more clinical productivity of doctors year over year,” he says. "At some point, and it's self-evident—at least in my mind—quality starts to suffer when workload gets excessive."
The report, "Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists," details findings of the first study to assess perception of unsafe workloads by directly questioning physicians, according to its authors. They electronically queried 506 hospitalists enrolled in the physicians' online network and information site QuantiaMD.com.
As many as 40% of physicians reported their typical inpatient census exceeded safe levels at least once monthly, the report noted, and physicians pegged 15 as the optimal number of patients to see on a shift dedicated to clinical work.
John Nelson, MD, MHM, a principal in Nelson Flores Hospital Medicine Consultants in La Quinta, Calif., says staffing shortages are likely the most common cause of heavy workloads, and that the high number of physicians reporting overloaded censuses is evidence that hospitalists are concerned their job performance is adversely affected.
"I suspect that as belt-tightening continues to occur," Dr. Brotman adds, "we're going to see the importance of [research] like this increasing, because we're going to see more and more stressed-out, overextended doctors who are having trouble delivering the care that they know they can deliver if they had more time."
Visit our website for more information on hospital medicine workloads.
A recent study in which 36% of hospitalists reported that their workload exceeds safe patient census levels at least once a week could spur serious discussions on productivity and quality of care, according to one of its authors.
Daniel Brotman, MD, FACP, FHM, director of the hospitalist program at Johns Hopkins Hospital in Baltimore and one of the study's authors, says the results highlight the delicate balance between pushing hospitalists to generate revenue and maintaining patient safety.
"It's certainly not in the best interest of our patients or our healthcare system to fix financial stress by expecting more clinical productivity of doctors year over year,” he says. "At some point, and it's self-evident—at least in my mind—quality starts to suffer when workload gets excessive."
The report, "Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists," details findings of the first study to assess perception of unsafe workloads by directly questioning physicians, according to its authors. They electronically queried 506 hospitalists enrolled in the physicians' online network and information site QuantiaMD.com.
As many as 40% of physicians reported their typical inpatient census exceeded safe levels at least once monthly, the report noted, and physicians pegged 15 as the optimal number of patients to see on a shift dedicated to clinical work.
John Nelson, MD, MHM, a principal in Nelson Flores Hospital Medicine Consultants in La Quinta, Calif., says staffing shortages are likely the most common cause of heavy workloads, and that the high number of physicians reporting overloaded censuses is evidence that hospitalists are concerned their job performance is adversely affected.
"I suspect that as belt-tightening continues to occur," Dr. Brotman adds, "we're going to see the importance of [research] like this increasing, because we're going to see more and more stressed-out, overextended doctors who are having trouble delivering the care that they know they can deliver if they had more time."
Visit our website for more information on hospital medicine workloads.
A recent study in which 36% of hospitalists reported that their workload exceeds safe patient census levels at least once a week could spur serious discussions on productivity and quality of care, according to one of its authors.
Daniel Brotman, MD, FACP, FHM, director of the hospitalist program at Johns Hopkins Hospital in Baltimore and one of the study's authors, says the results highlight the delicate balance between pushing hospitalists to generate revenue and maintaining patient safety.
"It's certainly not in the best interest of our patients or our healthcare system to fix financial stress by expecting more clinical productivity of doctors year over year,” he says. "At some point, and it's self-evident—at least in my mind—quality starts to suffer when workload gets excessive."
The report, "Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists," details findings of the first study to assess perception of unsafe workloads by directly questioning physicians, according to its authors. They electronically queried 506 hospitalists enrolled in the physicians' online network and information site QuantiaMD.com.
As many as 40% of physicians reported their typical inpatient census exceeded safe levels at least once monthly, the report noted, and physicians pegged 15 as the optimal number of patients to see on a shift dedicated to clinical work.
John Nelson, MD, MHM, a principal in Nelson Flores Hospital Medicine Consultants in La Quinta, Calif., says staffing shortages are likely the most common cause of heavy workloads, and that the high number of physicians reporting overloaded censuses is evidence that hospitalists are concerned their job performance is adversely affected.
"I suspect that as belt-tightening continues to occur," Dr. Brotman adds, "we're going to see the importance of [research] like this increasing, because we're going to see more and more stressed-out, overextended doctors who are having trouble delivering the care that they know they can deliver if they had more time."
Visit our website for more information on hospital medicine workloads.
Former SHM President Lands South Carolina Hospital’s Top Post
Patrick Cawley, MD, MBA, MHM, a past president of SHM and a recipient of its prestigious Master of Hospital Medicine award, has been named vice president for clinical operations and executive director of the Medical University Hospital Authority at the Medical University of South Carolina (MUSC) in Charleston.
"What distinguished Dr. Cawley from the rest of the field is his intimate knowledge of MUSC, his medical expertise combined with graduate education in management, and his track record of improving our performance in quality and patient safety," says Raymond S. Greenberg, MD, PhD, and president of MUSC. "Given his familiarity with the issues here, Dr. Cawley can step in quickly to assume his new responsibilities. He's already demonstrating steady and thoughtful leadership."
Dr. Cawley took his first leadership course 15 years ago. That led to other courses on such topics as marketing and finance, and that led to his earning a master’s degree in business administration from the University of Massachusetts at Amherst. "Just like medicine, you never stop learning in business or trying to do things better," he says.
Dr. Cawley says hospitalists have a leg up on other physicians when it comes to moving into hospital administration. "Being a hospitalist allowed me to get into every nook and cranny of this hospital," he says. He advises other hospitalists interested in this path to seek out progressive leadership roles. “Show what you can do,” he says, “and get that management degree.”
As Dr. Cawley's administrative responsibilities continue to expand, the time he spends in clinical practice continues to decrease. Although he plans to give up clinical work for the crucial first six months in his new position, he says he hopes to return to hospitalist practice at least 10% of the time after that.
"Any CEO worth his or her salt goes out to the front lines to see what is happening," Dr. Cawley notes. "As a physician, it is easier to get to those front lines. Once there, you get a feel for how the hospital really runs."
Visit our website for more information about executive leadership positions in hospitals.
Patrick Cawley, MD, MBA, MHM, a past president of SHM and a recipient of its prestigious Master of Hospital Medicine award, has been named vice president for clinical operations and executive director of the Medical University Hospital Authority at the Medical University of South Carolina (MUSC) in Charleston.
"What distinguished Dr. Cawley from the rest of the field is his intimate knowledge of MUSC, his medical expertise combined with graduate education in management, and his track record of improving our performance in quality and patient safety," says Raymond S. Greenberg, MD, PhD, and president of MUSC. "Given his familiarity with the issues here, Dr. Cawley can step in quickly to assume his new responsibilities. He's already demonstrating steady and thoughtful leadership."
Dr. Cawley took his first leadership course 15 years ago. That led to other courses on such topics as marketing and finance, and that led to his earning a master’s degree in business administration from the University of Massachusetts at Amherst. "Just like medicine, you never stop learning in business or trying to do things better," he says.
Dr. Cawley says hospitalists have a leg up on other physicians when it comes to moving into hospital administration. "Being a hospitalist allowed me to get into every nook and cranny of this hospital," he says. He advises other hospitalists interested in this path to seek out progressive leadership roles. “Show what you can do,” he says, “and get that management degree.”
As Dr. Cawley's administrative responsibilities continue to expand, the time he spends in clinical practice continues to decrease. Although he plans to give up clinical work for the crucial first six months in his new position, he says he hopes to return to hospitalist practice at least 10% of the time after that.
"Any CEO worth his or her salt goes out to the front lines to see what is happening," Dr. Cawley notes. "As a physician, it is easier to get to those front lines. Once there, you get a feel for how the hospital really runs."
Visit our website for more information about executive leadership positions in hospitals.
Patrick Cawley, MD, MBA, MHM, a past president of SHM and a recipient of its prestigious Master of Hospital Medicine award, has been named vice president for clinical operations and executive director of the Medical University Hospital Authority at the Medical University of South Carolina (MUSC) in Charleston.
"What distinguished Dr. Cawley from the rest of the field is his intimate knowledge of MUSC, his medical expertise combined with graduate education in management, and his track record of improving our performance in quality and patient safety," says Raymond S. Greenberg, MD, PhD, and president of MUSC. "Given his familiarity with the issues here, Dr. Cawley can step in quickly to assume his new responsibilities. He's already demonstrating steady and thoughtful leadership."
Dr. Cawley took his first leadership course 15 years ago. That led to other courses on such topics as marketing and finance, and that led to his earning a master’s degree in business administration from the University of Massachusetts at Amherst. "Just like medicine, you never stop learning in business or trying to do things better," he says.
Dr. Cawley says hospitalists have a leg up on other physicians when it comes to moving into hospital administration. "Being a hospitalist allowed me to get into every nook and cranny of this hospital," he says. He advises other hospitalists interested in this path to seek out progressive leadership roles. “Show what you can do,” he says, “and get that management degree.”
As Dr. Cawley's administrative responsibilities continue to expand, the time he spends in clinical practice continues to decrease. Although he plans to give up clinical work for the crucial first six months in his new position, he says he hopes to return to hospitalist practice at least 10% of the time after that.
"Any CEO worth his or her salt goes out to the front lines to see what is happening," Dr. Cawley notes. "As a physician, it is easier to get to those front lines. Once there, you get a feel for how the hospital really runs."
Visit our website for more information about executive leadership positions in hospitals.
FDA approves pomalidomide for MM

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has granted accelerated approval for the immunomodulatory agent pomalidomide (Pomalyst) to treat patients with advanced multiple myeloma (MM).
Continued FDA approval for the drug may be contingent upon verification and description of clinical benefit in confirmatory trials.
Pomalidomide is intended for use in combination with dexamethasone to treat MM patients who have received at least 2 prior
therapies (including lenalidomide and a proteasome inhibitor) and who experienced progression within 60 days of their last treatment.
Pomalidomide has demonstrated some efficacy in this patient population in a number of studies.
In a study published in Blood last year (PG Richardson et al.), pomalidomide elicited responses in MM patients who were refractory to lenalidomide, bortezomib, or both drugs.
In a study presented at ASH 2011 (abstract 634), pomalidomide did not fare as well when given alone to patients with refractory MM. However, combining the drug with low-dose dexamethasone significantly improved responses.
A study presented at ASH 2012 (LBA-6) built upon those findings, showing that pomalidomide plus low-dose dexamethasone was superior to high-dose dexamethasone in MM patients who were refractory to lenalidomide and bortezomib.
Common side effects observed with pomalidomide include neutropenia, anemia, thrombocytopenia, fatigue, weakness, constipation, diarrhea, upper respiratory tract infections, back pain, and fever.
In addition, pomalidomide has been shown to cause venous thromboembolism, as well as severe, life-threatening birth defects in pregnant women. The drug carries a boxed warning alerting patients and healthcare professionals to both of these risks.
Because of the embryo-fetal risk, pomalidomide is available only through the Pomalyst Risk Evaluation and Mitigation Strategy (REMS) Program. Prescribers must be certified with the program by enrolling and complying with the REMS requirements.
Patients must sign a patient-physician agreement form and comply with the REMS requirements. In particular, female patients who are not pregnant but can become pregnant must comply with the pregnancy testing and contraception requirements, and males must comply with contraception requirements.
Pharmacies must be certified with the Pomalyst REMS Program, must only dispense the drug to patients who are authorized to receive it, and must comply with REMS requirements. Both lenalidomide and thalidomide have similar REMS.
Pomalidomide is marketed by Celgene, which is based in Summit, New Jersey. ![]()

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has granted accelerated approval for the immunomodulatory agent pomalidomide (Pomalyst) to treat patients with advanced multiple myeloma (MM).
Continued FDA approval for the drug may be contingent upon verification and description of clinical benefit in confirmatory trials.
Pomalidomide is intended for use in combination with dexamethasone to treat MM patients who have received at least 2 prior
therapies (including lenalidomide and a proteasome inhibitor) and who experienced progression within 60 days of their last treatment.
Pomalidomide has demonstrated some efficacy in this patient population in a number of studies.
In a study published in Blood last year (PG Richardson et al.), pomalidomide elicited responses in MM patients who were refractory to lenalidomide, bortezomib, or both drugs.
In a study presented at ASH 2011 (abstract 634), pomalidomide did not fare as well when given alone to patients with refractory MM. However, combining the drug with low-dose dexamethasone significantly improved responses.
A study presented at ASH 2012 (LBA-6) built upon those findings, showing that pomalidomide plus low-dose dexamethasone was superior to high-dose dexamethasone in MM patients who were refractory to lenalidomide and bortezomib.
Common side effects observed with pomalidomide include neutropenia, anemia, thrombocytopenia, fatigue, weakness, constipation, diarrhea, upper respiratory tract infections, back pain, and fever.
In addition, pomalidomide has been shown to cause venous thromboembolism, as well as severe, life-threatening birth defects in pregnant women. The drug carries a boxed warning alerting patients and healthcare professionals to both of these risks.
Because of the embryo-fetal risk, pomalidomide is available only through the Pomalyst Risk Evaluation and Mitigation Strategy (REMS) Program. Prescribers must be certified with the program by enrolling and complying with the REMS requirements.
Patients must sign a patient-physician agreement form and comply with the REMS requirements. In particular, female patients who are not pregnant but can become pregnant must comply with the pregnancy testing and contraception requirements, and males must comply with contraception requirements.
Pharmacies must be certified with the Pomalyst REMS Program, must only dispense the drug to patients who are authorized to receive it, and must comply with REMS requirements. Both lenalidomide and thalidomide have similar REMS.
Pomalidomide is marketed by Celgene, which is based in Summit, New Jersey. ![]()

Credit: Steven Harbour
The US Food and Drug Administration (FDA) has granted accelerated approval for the immunomodulatory agent pomalidomide (Pomalyst) to treat patients with advanced multiple myeloma (MM).
Continued FDA approval for the drug may be contingent upon verification and description of clinical benefit in confirmatory trials.
Pomalidomide is intended for use in combination with dexamethasone to treat MM patients who have received at least 2 prior
therapies (including lenalidomide and a proteasome inhibitor) and who experienced progression within 60 days of their last treatment.
Pomalidomide has demonstrated some efficacy in this patient population in a number of studies.
In a study published in Blood last year (PG Richardson et al.), pomalidomide elicited responses in MM patients who were refractory to lenalidomide, bortezomib, or both drugs.
In a study presented at ASH 2011 (abstract 634), pomalidomide did not fare as well when given alone to patients with refractory MM. However, combining the drug with low-dose dexamethasone significantly improved responses.
A study presented at ASH 2012 (LBA-6) built upon those findings, showing that pomalidomide plus low-dose dexamethasone was superior to high-dose dexamethasone in MM patients who were refractory to lenalidomide and bortezomib.
Common side effects observed with pomalidomide include neutropenia, anemia, thrombocytopenia, fatigue, weakness, constipation, diarrhea, upper respiratory tract infections, back pain, and fever.
In addition, pomalidomide has been shown to cause venous thromboembolism, as well as severe, life-threatening birth defects in pregnant women. The drug carries a boxed warning alerting patients and healthcare professionals to both of these risks.
Because of the embryo-fetal risk, pomalidomide is available only through the Pomalyst Risk Evaluation and Mitigation Strategy (REMS) Program. Prescribers must be certified with the program by enrolling and complying with the REMS requirements.
Patients must sign a patient-physician agreement form and comply with the REMS requirements. In particular, female patients who are not pregnant but can become pregnant must comply with the pregnancy testing and contraception requirements, and males must comply with contraception requirements.
Pharmacies must be certified with the Pomalyst REMS Program, must only dispense the drug to patients who are authorized to receive it, and must comply with REMS requirements. Both lenalidomide and thalidomide have similar REMS.
Pomalidomide is marketed by Celgene, which is based in Summit, New Jersey. ![]()
Report: Hospitals Show Improvement on Infection Rates, but Progress Slows on CAUTIs
U.S. hospitals in 2011 showed improvements in their rates of central line-associated bloodstream infections (CLABSI) and in some surgical-site infections, compared with 2010, but the rate essentially hit a plateau for catheter-associated urinary tract infections (CAUTI), according to a new CDC report.
“Reductions in some of the deadliest healthcare-associated infections are encouraging, especially when you consider the costs to both patients and the health care system,” CDC director Thomas R. Frieden, MD, MPH, says. “However, the slower progress in reducing catheter-associated urinary tract infections is a call to action for hospitals to redouble their efforts to track these infections and implement control strategies we know that work.”
The report showed a 41% reduction in 2011 central-line infections compared with 2008, the baseline year for the report. In 2010, the reduction was 32% over the 2008 baseline. The improvement was seen across ICUs, general wards, and neonatal ICUs.

—Scott Flanders, MD, SFHM, professor of medicine, director of hospital medicine, University of Michigan Health System, Ann Arbor, former SHM president
The CDC also reported a 17% drop in surgical-site infections since 2008, better than the 7% reduction in 2010. The biggest reductions were seen in coronary artery bypass graft surgery and cardiac surgery; little improvement was seen in infections from hip arthroplasty and vaginal hysterectomy procedures.
The rate of infections from CAUTIs was 7%, nearly the same as the 6% rate in 2010 data. The infection rate in ICUs actually went up—a 1% drop in 2011 compared with a 3% drop from baseline in 2010.
SHM is a partner in two initiatives that aim to reduce CAUTI infections: the University HealthSystems Consortium’s Partnership for Patients project and On the CUSP: STOP CAUTI, an American Hospital Association HRET effort that’s funded by the Agency for Healthcare Research and Quality-funded project.
Gregory Maynard, MD, SFHM, director of hospital medicine at the University of San Diego Medical Center and senior vice president of SHM’s Center for Healthcare Improvement and Innovation is encouraged by the CLABSI and SSI figures. The report highlights the need for more effort on CAUTI.
“I think all the tools and information are available for improvement teams,” he says. “The CDC, the HRET On the CUSP group, and others all have great toolkits.”
He also says it was telling that the CAUTI numbers were worse in the ICU than in general wards.
“The more complex the environment, the easier it is for those things to get lost,” he says. “It just will probably take more attention to it and making it more of a priority.
“The more complex the environment, the easier it is for those things to get lost. It just will probably take more attention to it and making it more of a priority…. We’re supposed to reduce these adverse events by a very significant amount and obviously we’re not getting there based on this report. We have to do a better job. Reducing CAUTI by 40% is one of goals for the $500 million Partnerships for Patients effort. With that much money involved, it should increase the pressure to get this done.”
Click here to hear more of Dr. Maynard's interview with The Hospitalist
Scott Flanders, MD, SFHM, a former SHM president and SHM’s physician leader for STOP CAUTI, says the report shows that CAUTIs may be more difficult to prevent. In part, that is because catheters are used more broadly throughout a hospital than, say, central lines, which are most common in ICUs.
It takes a multi-disciplinary team implementing a variety of tools: critieria for putting catheters in, managing them appropriately once they are in, and developing protocols for removing them as quickly as possible, he adds.
“Having all those elements in place are critical to preventing CAUTI and I think many hospitals around the country have not implemented all of those strategies to reduce CAUTI,” says Dr. Flanders, professor of medicine and director of hospital medicine at the University of Michigan Health System in Ann Arbor. “No single strategy used in isolation is going to be effective.”
Efforts to reduce CAUTIs have been launched more recently than efforts to reduce other infection types, he says.
“There’s been less of a drive for CAUTI,” he says. “It’s a tougher problem to tackle than some of these other issues, which is a contributing factor in the lower rate of improvement.” TH
Tom Collins is a freelance writer in South Florida.
U.S. hospitals in 2011 showed improvements in their rates of central line-associated bloodstream infections (CLABSI) and in some surgical-site infections, compared with 2010, but the rate essentially hit a plateau for catheter-associated urinary tract infections (CAUTI), according to a new CDC report.
“Reductions in some of the deadliest healthcare-associated infections are encouraging, especially when you consider the costs to both patients and the health care system,” CDC director Thomas R. Frieden, MD, MPH, says. “However, the slower progress in reducing catheter-associated urinary tract infections is a call to action for hospitals to redouble their efforts to track these infections and implement control strategies we know that work.”
The report showed a 41% reduction in 2011 central-line infections compared with 2008, the baseline year for the report. In 2010, the reduction was 32% over the 2008 baseline. The improvement was seen across ICUs, general wards, and neonatal ICUs.

—Scott Flanders, MD, SFHM, professor of medicine, director of hospital medicine, University of Michigan Health System, Ann Arbor, former SHM president
The CDC also reported a 17% drop in surgical-site infections since 2008, better than the 7% reduction in 2010. The biggest reductions were seen in coronary artery bypass graft surgery and cardiac surgery; little improvement was seen in infections from hip arthroplasty and vaginal hysterectomy procedures.
The rate of infections from CAUTIs was 7%, nearly the same as the 6% rate in 2010 data. The infection rate in ICUs actually went up—a 1% drop in 2011 compared with a 3% drop from baseline in 2010.
SHM is a partner in two initiatives that aim to reduce CAUTI infections: the University HealthSystems Consortium’s Partnership for Patients project and On the CUSP: STOP CAUTI, an American Hospital Association HRET effort that’s funded by the Agency for Healthcare Research and Quality-funded project.
Gregory Maynard, MD, SFHM, director of hospital medicine at the University of San Diego Medical Center and senior vice president of SHM’s Center for Healthcare Improvement and Innovation is encouraged by the CLABSI and SSI figures. The report highlights the need for more effort on CAUTI.
“I think all the tools and information are available for improvement teams,” he says. “The CDC, the HRET On the CUSP group, and others all have great toolkits.”
He also says it was telling that the CAUTI numbers were worse in the ICU than in general wards.
“The more complex the environment, the easier it is for those things to get lost,” he says. “It just will probably take more attention to it and making it more of a priority.
“The more complex the environment, the easier it is for those things to get lost. It just will probably take more attention to it and making it more of a priority…. We’re supposed to reduce these adverse events by a very significant amount and obviously we’re not getting there based on this report. We have to do a better job. Reducing CAUTI by 40% is one of goals for the $500 million Partnerships for Patients effort. With that much money involved, it should increase the pressure to get this done.”
Click here to hear more of Dr. Maynard's interview with The Hospitalist
Scott Flanders, MD, SFHM, a former SHM president and SHM’s physician leader for STOP CAUTI, says the report shows that CAUTIs may be more difficult to prevent. In part, that is because catheters are used more broadly throughout a hospital than, say, central lines, which are most common in ICUs.
It takes a multi-disciplinary team implementing a variety of tools: critieria for putting catheters in, managing them appropriately once they are in, and developing protocols for removing them as quickly as possible, he adds.
“Having all those elements in place are critical to preventing CAUTI and I think many hospitals around the country have not implemented all of those strategies to reduce CAUTI,” says Dr. Flanders, professor of medicine and director of hospital medicine at the University of Michigan Health System in Ann Arbor. “No single strategy used in isolation is going to be effective.”
Efforts to reduce CAUTIs have been launched more recently than efforts to reduce other infection types, he says.
“There’s been less of a drive for CAUTI,” he says. “It’s a tougher problem to tackle than some of these other issues, which is a contributing factor in the lower rate of improvement.” TH
Tom Collins is a freelance writer in South Florida.
U.S. hospitals in 2011 showed improvements in their rates of central line-associated bloodstream infections (CLABSI) and in some surgical-site infections, compared with 2010, but the rate essentially hit a plateau for catheter-associated urinary tract infections (CAUTI), according to a new CDC report.
“Reductions in some of the deadliest healthcare-associated infections are encouraging, especially when you consider the costs to both patients and the health care system,” CDC director Thomas R. Frieden, MD, MPH, says. “However, the slower progress in reducing catheter-associated urinary tract infections is a call to action for hospitals to redouble their efforts to track these infections and implement control strategies we know that work.”
The report showed a 41% reduction in 2011 central-line infections compared with 2008, the baseline year for the report. In 2010, the reduction was 32% over the 2008 baseline. The improvement was seen across ICUs, general wards, and neonatal ICUs.

—Scott Flanders, MD, SFHM, professor of medicine, director of hospital medicine, University of Michigan Health System, Ann Arbor, former SHM president
The CDC also reported a 17% drop in surgical-site infections since 2008, better than the 7% reduction in 2010. The biggest reductions were seen in coronary artery bypass graft surgery and cardiac surgery; little improvement was seen in infections from hip arthroplasty and vaginal hysterectomy procedures.
The rate of infections from CAUTIs was 7%, nearly the same as the 6% rate in 2010 data. The infection rate in ICUs actually went up—a 1% drop in 2011 compared with a 3% drop from baseline in 2010.
SHM is a partner in two initiatives that aim to reduce CAUTI infections: the University HealthSystems Consortium’s Partnership for Patients project and On the CUSP: STOP CAUTI, an American Hospital Association HRET effort that’s funded by the Agency for Healthcare Research and Quality-funded project.
Gregory Maynard, MD, SFHM, director of hospital medicine at the University of San Diego Medical Center and senior vice president of SHM’s Center for Healthcare Improvement and Innovation is encouraged by the CLABSI and SSI figures. The report highlights the need for more effort on CAUTI.
“I think all the tools and information are available for improvement teams,” he says. “The CDC, the HRET On the CUSP group, and others all have great toolkits.”
He also says it was telling that the CAUTI numbers were worse in the ICU than in general wards.
“The more complex the environment, the easier it is for those things to get lost,” he says. “It just will probably take more attention to it and making it more of a priority.
“The more complex the environment, the easier it is for those things to get lost. It just will probably take more attention to it and making it more of a priority…. We’re supposed to reduce these adverse events by a very significant amount and obviously we’re not getting there based on this report. We have to do a better job. Reducing CAUTI by 40% is one of goals for the $500 million Partnerships for Patients effort. With that much money involved, it should increase the pressure to get this done.”
Click here to hear more of Dr. Maynard's interview with The Hospitalist
Scott Flanders, MD, SFHM, a former SHM president and SHM’s physician leader for STOP CAUTI, says the report shows that CAUTIs may be more difficult to prevent. In part, that is because catheters are used more broadly throughout a hospital than, say, central lines, which are most common in ICUs.
It takes a multi-disciplinary team implementing a variety of tools: critieria for putting catheters in, managing them appropriately once they are in, and developing protocols for removing them as quickly as possible, he adds.
“Having all those elements in place are critical to preventing CAUTI and I think many hospitals around the country have not implemented all of those strategies to reduce CAUTI,” says Dr. Flanders, professor of medicine and director of hospital medicine at the University of Michigan Health System in Ann Arbor. “No single strategy used in isolation is going to be effective.”
Efforts to reduce CAUTIs have been launched more recently than efforts to reduce other infection types, he says.
“There’s been less of a drive for CAUTI,” he says. “It’s a tougher problem to tackle than some of these other issues, which is a contributing factor in the lower rate of improvement.” TH
Tom Collins is a freelance writer in South Florida.
If you’ve ever heard someone say, "I’ve been pinning for an hour. I’m addicted!" and had no idea what this person was talking about, I have one word for you: Pinterest.
Pinterest is a fabulously popular social media site that allows users to find, share, and organize images called "pins" that are displayed or "pinned" on electronic "boards." A "board" is like a digital folder that helps you organize your pins. For example, you might have boards for Healthy Recipes, Exercise, and Places I’ve Traveled. Images are uploaded from the web or from your own computer or smartphone. Since it’s social, users can "like" other people’s pins, comment on them, and "repin" or share them. They can also add friends and become part of a "pin group board," where you and selected others upload pins to the shared boards.
Why is this important for you and your medical practice? Pinterest is one of the fastest-growing social media sites in history. It launched in March 2010, and by October 2012 it had reached more than 25,000,000 active monthly users and debuted on the list of top 50 most-visited web sites in the United States.
According to the Pew Research Center, 72% of adults who are online are searching for health, and Pinterest is another social media channel you can use to reach them. If you’re thinking, "But I already do Twitter and Facebook," consider this: Approximately 80% of Pinterest users are female and, according to the U.S. Department of Labor, women make 80% of health care decisions for their families. See the connection? It’s not farfetched to posit that Pinterest may turn out to be one of the most effective social media sites for the health care industry.
Because many people are visual learners, Pinterest can be an effective tool for patient education. Several renowned institutions, including St. Jude Children’s Hospital and the Mayo Clinic, use Pinterest effectively to educate the public, share patient stories, and discuss newsworthy topics.
As physicians, you can use Pinterest similarly to build your brand and help market your practice more creatively.
You’ll find that Pinterest is very easy to learn and use. And because it’s a visual site with little to no text, it requires minimal effort on your part, or your staff’s part. A few minutes per day or every few days are sufficient to establish a presence and make connections.
There are many ways you can use Pinterest to build brand awareness and reach patients. Here are a few:
• Explain how medical or cosmetic procedures work, such as fillers and sclerotherapy.
• Explain how medical devices work, such as lasers and dermatoscopes.
• Generate awareness of medical conditions, such as psoriasis, eczema, and skin cancers. Infographics are especially effective.
• Provide inspiration. Many skin conditions are psychologically challenging. Pinning inspirational images can give patients hope.
• Share your product recommendations.
• Share uplifting patient stories and testimonials.
• Introduce and update the public to you, your staff, your office, and your services.
As for creating pin boards, the categories are endless, but here are some ideas to get you started: Patient Stories, Healthy Skin Habits, Sun Safety, Before and After, Acne Tips, Cosmetic Services, Parenting Tips, Words of Inspiration, and Meet Our Staff.
If you haven’t been on Pinterest yet, take a visit there and explore what it has to offer. And don’t be surprised if you become addicted.
Dr. Benabio is physician director at Kaiser Permanente in San Diego. Visit his consumer health blog at thedermblog.com; connect with him on Twitter @Dermdoc, and on Facebook (DermDoc).
If you’ve ever heard someone say, "I’ve been pinning for an hour. I’m addicted!" and had no idea what this person was talking about, I have one word for you: Pinterest.
Pinterest is a fabulously popular social media site that allows users to find, share, and organize images called "pins" that are displayed or "pinned" on electronic "boards." A "board" is like a digital folder that helps you organize your pins. For example, you might have boards for Healthy Recipes, Exercise, and Places I’ve Traveled. Images are uploaded from the web or from your own computer or smartphone. Since it’s social, users can "like" other people’s pins, comment on them, and "repin" or share them. They can also add friends and become part of a "pin group board," where you and selected others upload pins to the shared boards.
Why is this important for you and your medical practice? Pinterest is one of the fastest-growing social media sites in history. It launched in March 2010, and by October 2012 it had reached more than 25,000,000 active monthly users and debuted on the list of top 50 most-visited web sites in the United States.
According to the Pew Research Center, 72% of adults who are online are searching for health, and Pinterest is another social media channel you can use to reach them. If you’re thinking, "But I already do Twitter and Facebook," consider this: Approximately 80% of Pinterest users are female and, according to the U.S. Department of Labor, women make 80% of health care decisions for their families. See the connection? It’s not farfetched to posit that Pinterest may turn out to be one of the most effective social media sites for the health care industry.
Because many people are visual learners, Pinterest can be an effective tool for patient education. Several renowned institutions, including St. Jude Children’s Hospital and the Mayo Clinic, use Pinterest effectively to educate the public, share patient stories, and discuss newsworthy topics.
As physicians, you can use Pinterest similarly to build your brand and help market your practice more creatively.
You’ll find that Pinterest is very easy to learn and use. And because it’s a visual site with little to no text, it requires minimal effort on your part, or your staff’s part. A few minutes per day or every few days are sufficient to establish a presence and make connections.
There are many ways you can use Pinterest to build brand awareness and reach patients. Here are a few:
• Explain how medical or cosmetic procedures work, such as fillers and sclerotherapy.
• Explain how medical devices work, such as lasers and dermatoscopes.
• Generate awareness of medical conditions, such as psoriasis, eczema, and skin cancers. Infographics are especially effective.
• Provide inspiration. Many skin conditions are psychologically challenging. Pinning inspirational images can give patients hope.
• Share your product recommendations.
• Share uplifting patient stories and testimonials.
• Introduce and update the public to you, your staff, your office, and your services.
As for creating pin boards, the categories are endless, but here are some ideas to get you started: Patient Stories, Healthy Skin Habits, Sun Safety, Before and After, Acne Tips, Cosmetic Services, Parenting Tips, Words of Inspiration, and Meet Our Staff.
If you haven’t been on Pinterest yet, take a visit there and explore what it has to offer. And don’t be surprised if you become addicted.
Dr. Benabio is physician director at Kaiser Permanente in San Diego. Visit his consumer health blog at thedermblog.com; connect with him on Twitter @Dermdoc, and on Facebook (DermDoc).
If you’ve ever heard someone say, "I’ve been pinning for an hour. I’m addicted!" and had no idea what this person was talking about, I have one word for you: Pinterest.
Pinterest is a fabulously popular social media site that allows users to find, share, and organize images called "pins" that are displayed or "pinned" on electronic "boards." A "board" is like a digital folder that helps you organize your pins. For example, you might have boards for Healthy Recipes, Exercise, and Places I’ve Traveled. Images are uploaded from the web or from your own computer or smartphone. Since it’s social, users can "like" other people’s pins, comment on them, and "repin" or share them. They can also add friends and become part of a "pin group board," where you and selected others upload pins to the shared boards.
Why is this important for you and your medical practice? Pinterest is one of the fastest-growing social media sites in history. It launched in March 2010, and by October 2012 it had reached more than 25,000,000 active monthly users and debuted on the list of top 50 most-visited web sites in the United States.
According to the Pew Research Center, 72% of adults who are online are searching for health, and Pinterest is another social media channel you can use to reach them. If you’re thinking, "But I already do Twitter and Facebook," consider this: Approximately 80% of Pinterest users are female and, according to the U.S. Department of Labor, women make 80% of health care decisions for their families. See the connection? It’s not farfetched to posit that Pinterest may turn out to be one of the most effective social media sites for the health care industry.
Because many people are visual learners, Pinterest can be an effective tool for patient education. Several renowned institutions, including St. Jude Children’s Hospital and the Mayo Clinic, use Pinterest effectively to educate the public, share patient stories, and discuss newsworthy topics.
As physicians, you can use Pinterest similarly to build your brand and help market your practice more creatively.
You’ll find that Pinterest is very easy to learn and use. And because it’s a visual site with little to no text, it requires minimal effort on your part, or your staff’s part. A few minutes per day or every few days are sufficient to establish a presence and make connections.
There are many ways you can use Pinterest to build brand awareness and reach patients. Here are a few:
• Explain how medical or cosmetic procedures work, such as fillers and sclerotherapy.
• Explain how medical devices work, such as lasers and dermatoscopes.
• Generate awareness of medical conditions, such as psoriasis, eczema, and skin cancers. Infographics are especially effective.
• Provide inspiration. Many skin conditions are psychologically challenging. Pinning inspirational images can give patients hope.
• Share your product recommendations.
• Share uplifting patient stories and testimonials.
• Introduce and update the public to you, your staff, your office, and your services.
As for creating pin boards, the categories are endless, but here are some ideas to get you started: Patient Stories, Healthy Skin Habits, Sun Safety, Before and After, Acne Tips, Cosmetic Services, Parenting Tips, Words of Inspiration, and Meet Our Staff.
If you haven’t been on Pinterest yet, take a visit there and explore what it has to offer. And don’t be surprised if you become addicted.
Dr. Benabio is physician director at Kaiser Permanente in San Diego. Visit his consumer health blog at thedermblog.com; connect with him on Twitter @Dermdoc, and on Facebook (DermDoc).
New antihemophilic factors last longer than standard treatments
WARSAW—Recombinant Fc fusion proteins can provide long-lasting protection from bleeding in patients with hemophilia A or B, according to data presented at the 6th Annual Congress of the European Association for Haemophilia and Allied Disorders.
Data from the phase 3 A-LONG study indicated that patients with hemophilia A could maintain low bleeding rates with once- to twice-weekly prophylactic injections of a recombinant factor VIII Fc fusion protein (rFVIIIFc, efmoroctocog alfa/Elocta, Eloctate).
Similarly, results of the phase 3 B-LONG study showed that patients with hemophilia B had low bleeding rates when they received prophylactic injections of a recombinant factor IX Fc fusion protein (rFIXFc, eftrenonacog alfa/Alprolix) every 1 to 2 weeks.
Both studies were sponsored by the companies developing these factors, Biogen Idec and Swedish Orphan Biovitrum (Sobi).
A-LONG data
In the A-LONG study, researchers evaluated the efficacy, safety, and pharmacokinetics of intravenous rFVIIIFc in 165 male patients aged 12 years and older. The team found that 98% of bleeding episodes were controlled by 1 or 2 injections of rFVIIIFc.
The factor was generally well-tolerated, and no inhibitors were detected. The most common adverse events (with an incidence of 5% or higher) were nasopharyngitis, arthralgia, headache, and upper respiratory tract infection.
The study also showed that rFVIIIFc stays in the body for 50% longer than Advate [antihemophilic factor (recombinant), plasma/albumin-free method], the most frequently used factor VIII therapy. The terminal half-life for rFVIIIFc was 19 hours, compared to 12 hours for Advate.
Additionally, the mean time for maintaining a clotting factor activity level associated with less bleeding (time to 1%) was approximately 5 days for rFVIIIFc, compared to 3.5 days for Advate. And the average rate at which rFVIIIFc was cleared from the body was 2.0 mL/hr/kg, compared with 3.0 mL/hr/kg for Advate.
In the study’s individualized prophylaxis arm, patients received rFVIIIFc at a median dosing interval of 3.5 days and a median weekly dose of 78 IU/kg to prevent bleeding, which compares favorably to the recommended dose for the standard of care. Nearly one-third of patients were able to achieve every-5-days dosing in this arm.
The A-LONG data were presented in the late-breaking oral abstract session and in poster 104, “Phase 3 clinical study of recombinant FC fusion factor FVIII (rFVIIIFc) demonstrated safety, efficacy, and improved pharmacokinetics (A-LONG).”
B-LONG data
In the B-LONG study, researchers evaluated the efficacy, safety, and pharmacokinetics of intravenous rFIXFc in 123 male patients aged 12 years and older. The team found that more than 90% of bleeding episodes were controlled by a single injection of rFIXFc.
rFIXFc was generally well-tolerated, and no inhibitors were detected. The most common adverse events (with an incidence of 5% or more) were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event, obstructive uropathy in the setting of hematuria, may have been related to rFIXFc treatment. However, the patient continued to receive rFIXFc, and the event resolved with medical management.
The study also showed that rFIXFc stays in the body more than twice as long as BeneFIX [Coagulation Factor IX (Recombinant)], the only recombinant factor IX therapy currently approved for prophylactic use. The terminal half-life for rFIXFc was 82 hours, compared to 34 hours for BeneFIX.
In addition, the mean time for maintaining a normal clotting factor activity level (time to 1%) was 11 days for rFIXFc, compared to 5 days for BeneFIX. And the average rate at which rFIXFc was cleared from the body was 3.2 mL/hr/kg, compared with 6.3 mL/hr/kg for BeneFIX.
All patients in the individualized interval prophylaxis arm of the study were able to go at least 1 week between rFIXFc injections, and 50% could go 14 days or longer before needing another dose to prevent bleeding. The median weekly dose was 45 IU/kg, which is comparable to the recommended dose for the current standard of care.
The B-LONG data were presented in poster 115, “Safety, efficacy, and improved pharmacokinetics (PK) demonstrated in a phase 3 clinical trial of extended half-life recombinant FC fusion factor IX (B-LONG).” ![]()
WARSAW—Recombinant Fc fusion proteins can provide long-lasting protection from bleeding in patients with hemophilia A or B, according to data presented at the 6th Annual Congress of the European Association for Haemophilia and Allied Disorders.
Data from the phase 3 A-LONG study indicated that patients with hemophilia A could maintain low bleeding rates with once- to twice-weekly prophylactic injections of a recombinant factor VIII Fc fusion protein (rFVIIIFc, efmoroctocog alfa/Elocta, Eloctate).
Similarly, results of the phase 3 B-LONG study showed that patients with hemophilia B had low bleeding rates when they received prophylactic injections of a recombinant factor IX Fc fusion protein (rFIXFc, eftrenonacog alfa/Alprolix) every 1 to 2 weeks.
Both studies were sponsored by the companies developing these factors, Biogen Idec and Swedish Orphan Biovitrum (Sobi).
A-LONG data
In the A-LONG study, researchers evaluated the efficacy, safety, and pharmacokinetics of intravenous rFVIIIFc in 165 male patients aged 12 years and older. The team found that 98% of bleeding episodes were controlled by 1 or 2 injections of rFVIIIFc.
The factor was generally well-tolerated, and no inhibitors were detected. The most common adverse events (with an incidence of 5% or higher) were nasopharyngitis, arthralgia, headache, and upper respiratory tract infection.
The study also showed that rFVIIIFc stays in the body for 50% longer than Advate [antihemophilic factor (recombinant), plasma/albumin-free method], the most frequently used factor VIII therapy. The terminal half-life for rFVIIIFc was 19 hours, compared to 12 hours for Advate.
Additionally, the mean time for maintaining a clotting factor activity level associated with less bleeding (time to 1%) was approximately 5 days for rFVIIIFc, compared to 3.5 days for Advate. And the average rate at which rFVIIIFc was cleared from the body was 2.0 mL/hr/kg, compared with 3.0 mL/hr/kg for Advate.
In the study’s individualized prophylaxis arm, patients received rFVIIIFc at a median dosing interval of 3.5 days and a median weekly dose of 78 IU/kg to prevent bleeding, which compares favorably to the recommended dose for the standard of care. Nearly one-third of patients were able to achieve every-5-days dosing in this arm.
The A-LONG data were presented in the late-breaking oral abstract session and in poster 104, “Phase 3 clinical study of recombinant FC fusion factor FVIII (rFVIIIFc) demonstrated safety, efficacy, and improved pharmacokinetics (A-LONG).”
B-LONG data
In the B-LONG study, researchers evaluated the efficacy, safety, and pharmacokinetics of intravenous rFIXFc in 123 male patients aged 12 years and older. The team found that more than 90% of bleeding episodes were controlled by a single injection of rFIXFc.
rFIXFc was generally well-tolerated, and no inhibitors were detected. The most common adverse events (with an incidence of 5% or more) were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event, obstructive uropathy in the setting of hematuria, may have been related to rFIXFc treatment. However, the patient continued to receive rFIXFc, and the event resolved with medical management.
The study also showed that rFIXFc stays in the body more than twice as long as BeneFIX [Coagulation Factor IX (Recombinant)], the only recombinant factor IX therapy currently approved for prophylactic use. The terminal half-life for rFIXFc was 82 hours, compared to 34 hours for BeneFIX.
In addition, the mean time for maintaining a normal clotting factor activity level (time to 1%) was 11 days for rFIXFc, compared to 5 days for BeneFIX. And the average rate at which rFIXFc was cleared from the body was 3.2 mL/hr/kg, compared with 6.3 mL/hr/kg for BeneFIX.
All patients in the individualized interval prophylaxis arm of the study were able to go at least 1 week between rFIXFc injections, and 50% could go 14 days or longer before needing another dose to prevent bleeding. The median weekly dose was 45 IU/kg, which is comparable to the recommended dose for the current standard of care.
The B-LONG data were presented in poster 115, “Safety, efficacy, and improved pharmacokinetics (PK) demonstrated in a phase 3 clinical trial of extended half-life recombinant FC fusion factor IX (B-LONG).” ![]()
WARSAW—Recombinant Fc fusion proteins can provide long-lasting protection from bleeding in patients with hemophilia A or B, according to data presented at the 6th Annual Congress of the European Association for Haemophilia and Allied Disorders.
Data from the phase 3 A-LONG study indicated that patients with hemophilia A could maintain low bleeding rates with once- to twice-weekly prophylactic injections of a recombinant factor VIII Fc fusion protein (rFVIIIFc, efmoroctocog alfa/Elocta, Eloctate).
Similarly, results of the phase 3 B-LONG study showed that patients with hemophilia B had low bleeding rates when they received prophylactic injections of a recombinant factor IX Fc fusion protein (rFIXFc, eftrenonacog alfa/Alprolix) every 1 to 2 weeks.
Both studies were sponsored by the companies developing these factors, Biogen Idec and Swedish Orphan Biovitrum (Sobi).
A-LONG data
In the A-LONG study, researchers evaluated the efficacy, safety, and pharmacokinetics of intravenous rFVIIIFc in 165 male patients aged 12 years and older. The team found that 98% of bleeding episodes were controlled by 1 or 2 injections of rFVIIIFc.
The factor was generally well-tolerated, and no inhibitors were detected. The most common adverse events (with an incidence of 5% or higher) were nasopharyngitis, arthralgia, headache, and upper respiratory tract infection.
The study also showed that rFVIIIFc stays in the body for 50% longer than Advate [antihemophilic factor (recombinant), plasma/albumin-free method], the most frequently used factor VIII therapy. The terminal half-life for rFVIIIFc was 19 hours, compared to 12 hours for Advate.
Additionally, the mean time for maintaining a clotting factor activity level associated with less bleeding (time to 1%) was approximately 5 days for rFVIIIFc, compared to 3.5 days for Advate. And the average rate at which rFVIIIFc was cleared from the body was 2.0 mL/hr/kg, compared with 3.0 mL/hr/kg for Advate.
In the study’s individualized prophylaxis arm, patients received rFVIIIFc at a median dosing interval of 3.5 days and a median weekly dose of 78 IU/kg to prevent bleeding, which compares favorably to the recommended dose for the standard of care. Nearly one-third of patients were able to achieve every-5-days dosing in this arm.
The A-LONG data were presented in the late-breaking oral abstract session and in poster 104, “Phase 3 clinical study of recombinant FC fusion factor FVIII (rFVIIIFc) demonstrated safety, efficacy, and improved pharmacokinetics (A-LONG).”
B-LONG data
In the B-LONG study, researchers evaluated the efficacy, safety, and pharmacokinetics of intravenous rFIXFc in 123 male patients aged 12 years and older. The team found that more than 90% of bleeding episodes were controlled by a single injection of rFIXFc.
rFIXFc was generally well-tolerated, and no inhibitors were detected. The most common adverse events (with an incidence of 5% or more) were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event, obstructive uropathy in the setting of hematuria, may have been related to rFIXFc treatment. However, the patient continued to receive rFIXFc, and the event resolved with medical management.
The study also showed that rFIXFc stays in the body more than twice as long as BeneFIX [Coagulation Factor IX (Recombinant)], the only recombinant factor IX therapy currently approved for prophylactic use. The terminal half-life for rFIXFc was 82 hours, compared to 34 hours for BeneFIX.
In addition, the mean time for maintaining a normal clotting factor activity level (time to 1%) was 11 days for rFIXFc, compared to 5 days for BeneFIX. And the average rate at which rFIXFc was cleared from the body was 3.2 mL/hr/kg, compared with 6.3 mL/hr/kg for BeneFIX.
All patients in the individualized interval prophylaxis arm of the study were able to go at least 1 week between rFIXFc injections, and 50% could go 14 days or longer before needing another dose to prevent bleeding. The median weekly dose was 45 IU/kg, which is comparable to the recommended dose for the current standard of care.
The B-LONG data were presented in poster 115, “Safety, efficacy, and improved pharmacokinetics (PK) demonstrated in a phase 3 clinical trial of extended half-life recombinant FC fusion factor IX (B-LONG).” ![]()
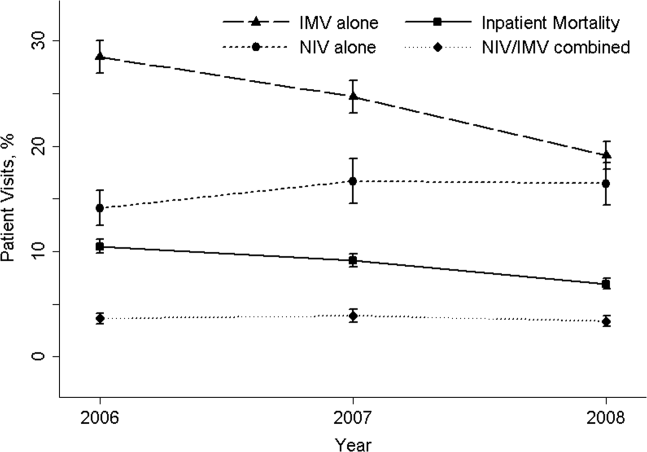
Effectiveness of NIV vs. IMV in AECOPD
Chronic obstructive pulmonary disease (COPD) is now the third leading cause of death in the United States,[1] and its rising mortality trend is unique among the top 5 causes of death.[2] Acute exacerbations of COPD (AECOPD) are important events in the natural history of COPD, accounting for 1.5 million emergency department (ED) visits and 726,000 hospitalizations each year in the United States.[3, 4] Given the significant morbidity and mortality from AECOPD, Healthy People 2020 lists reducing deaths, hospitalizations, and ED visits as the key objectives for COPD.[5]
Over the past 2 decades, noninvasive ventilation (NIV) has emerged as a potentially useful treatment modality in AECOPD patients with acute respiratory failure. Noninvasive ventilation commonly refers to positive‐pressure ventilatory support delivered through a nasal or full‐face mask, such as bilevel positive airway pressure.[6] A number of randomized controlled trials[7, 8, 9] and meta‐analyses[10] have suggested a mortality‐reduction benefit with NIV use compared with standard medical care in AECOPD. To our knowledge, however, very few small randomized controlled trials compared NIV vs invasive mechanical ventilation (IMV) head‐to‐head,[11, 12, 13] and a recent evidence review found only 5 studies (405 subjects) on this topic.[14] Collectively, the limited evidence from randomized trials showed that NIV use resulted in similar intensive care unit (ICU) and in‐hospital mortality, fewer complications (eg, ventilator‐associated pneumonia and sepsis), and shorter hospital length of stays (LOS). Given that these trials have a smaller sample size and tend to exclude older patient (age >75 years) or patients with multiple comorbidities, there is a need to better understand the adoption and effectiveness of NIV treatment for AECOPD in a much larger patient population in the real‐world setting using observational data.
To address these knowledge gaps in the literature, we analyzed data from a large, nationally representative ED and inpatient sample. The objective of the present analysis was 2‐fold: (1) to characterize the use of NIV and IMV in AECOPD patients with acute respiratory failure at a national level; and (2) to compare the effectiveness of NIV vs IMV in the real‐world setting.
METHODS
Study Design and Setting
We conducted a retrospective cohort study using data from the 20062008 Nationwide Emergency Department Sample (NEDS),[15] a component of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality. The NEDS is nationally representative of all community hospitalbased EDs in the United States, defined by the American Hospital Association as all nonfederal, short‐term, general, and other specialty hospitals.[16] Community hospitals include academic medical centers if they are nonfederal short‐term hospitals. The NEDS was constructed using administrative records from the State Emergency Department Databases and the State Inpatient Databases. The former captures information on ED visits that do not result in an admission (ie, treat‐and‐release visits or transfers to another hospital); the latter contains information on patients initially seen in the ED and then admitted to the same hospital. Taken together, the resulting NEDS represents all ED visits regardless of disposition and contains information on short‐term outcomes for patients admitted through the ED. In other words, the NEDS is the largest all‐payer ED and inpatient database in the United States. The NEDS represents an approximately 20% stratified sample of US hospital‐based EDs, containing more than 28 million records of ED visits from approximately 1000 hospitals each year. Additional details of the NEDS can be found elsewhere.[15, 17] We received a waiver for this analysis from our institutional review board.
Study Population
Patient visits were included in this analysis if they carried any COPD‐related diagnostic code (ie, International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code of 491.xx [chronic bronchitis], 492.xx [emphysema], or 496.xx [chronic airway obstruction, not elsewhere classified]) as their primary ED diagnosis and any acute respiratory failure code (ie, 518.81 [acute respiratory failure], 518.82 [pulmonary insufficiency not elsewhere classified, 518.84 [acute and chronic respiratory failure], or 799.1 [respiratory arrest]) as their secondary diagnosis. Patient visits with a primary diagnosis of acute respiratory failure and a secondary diagnosis of COPD were also included. Patients age <40 years were excluded, because they are much less likely to have COPD.[18]
Modes of Mechanical Ventilation
The primary exposure variable was mode of mechanical ventilation. To compare the effectiveness of different ventilatory modes, patients were divided into 3 groups according to the ventilation mode they received: (1) NIV alone, (2) IMV alone, and (3) combined modes of NIV and IMV. The use of NIV was identified by using Current Procedural Terminology (CPT) code of 94660 or ICD‐9 procedure code 93.90, whereas the use of IMV was identified by using CPT code of 31500 or ICD‐9 procedure code 96.04 or 96.7x.
Patient‐Level and Emergency DepartmentLevel Variables
The NEDS contains information on patient demographics, national quartiles for median household income based on the patient's ZIP code, payment sources, ICD‐9‐CM diagnoses and procedures, ED disposition, hospital LOS, and hospital disposition. Hospital characteristics include annual visit volume, urban‐rural status, ownership, teaching status, and US region. Geographic regions (Northeast, South, Midwest, and West) were defined according to Census Bureau boundaries.[19] To adjust for confounding by patient mix, Elixhauser comorbidity measures were derived based on the ICD‐9 codes, using the Agency for Healthcare Research and Quality's Comorbidity Software.[20] This risk‐adjustment tool has been derived and validated extensively.[21]
Outcome Measures
The outcome measures were all‐cause inpatient mortality, hospital LOS, hospital charges, and ventilator‐related complications. Three ventilator‐related complications were identified using ICD‐9 procedure codes: ventilator‐associated pneumonia (997.31), facial injury (910.x), and iatrogenic pneumothorax (512.1).
Statistical Analysis
Summary statistics are presented as proportions (with 95% confidence intervals [CI]), means (with standard deviations [SD]), or medians (with interquartile ranges). Bivariate associations were examined using Student t tests, Kruskal‐Wallis tests, and [2] tests, as appropriate. Emergency department and discharge weights were used to obtain national estimates at the ED and visit level. At all other times (eg, the propensity score and instrumental variable analyses), the unweighted cohort was analyzed, because survey weights are generally not advised for propensity score analysis using complex survey data.[22]
Propensity Score Analysis
To adjust for baseline patient and ED characteristics that may have confounded the relationship between ventilation mode and clinical outcomes, we performed propensity score and instrumental variable analyses. To compare the effectiveness of NIV vs IMV, a propensity score or predicted probability of NIV was estimated using a logistic‐regression model with all patient characteristics (age, sex, quartiles for median household income, weekend admission, insurance status, season, calendar year, and comorbid conditions) and ED characteristics (urban/rural and teaching status, US region, annual ED volume, and annual volume of AECOPD with respiratory failure) as the independent variables. We then performed 1:1 propensity score matching based on a nearest‐neighbor algorithm with caliper distance of 0.01. Although propensity score matching may result in a smaller sample, it provides a clinically relevant estimate of treatment effect because subjects in the matched sample are potential candidates for either treatment option.[23, 24] An absolute standardized difference between characteristics of <10% was considered as adequate balance.[25]
Instrumental Variable Analysis
When hospitals always or nearly always use NIV or IMV, this suggests the choice is largely independent of patient characteristics, and it is possible to use the hospital preference as a proxy for the actual treatment choice (ie, an instrument variable). The instrumental variable analysis simulates a natural randomization of patients to 2 hospital groups with high and low NIV use.
The main difference between instrumental variable and propensity score analysis is that the former could potentially adjust for unmeasured confounders.[26] We used Stata procedure IVREG to estimate the outcome differences between NIV‐preferring hospitals (NIV use in 90% of patients) and IMV‐preferring hospitals (NIV use in 10% of patients).
All odds ratios (ORs) and ‐coefficients are presented with 95% CIs. All analyses were performed using Stata 12.0 software (StataCorp, College Station, TX). All P values are 2‐sided, with P<0.05 considered statistically significant.
Sensitivity Analyses
We conducted a sensitivity analysis to determine whether it was plausible that an unmeasured confounder could completely explain the observed results. The risk ratio of a hypothetical unmeasured confounder on study outcome and the exposure‐confounder imbalance were both varied to see at what point the observed association was reduced to 1.0.[27]
RESULTS
Patient and ED Characteristics
The 20062008 NEDS sample contained 67,651 ED visits for AECOPD with acute respiratory failure from 1594 US EDs. After the weighting procedure, there were an estimated 101,000 visits annually for AECOPD with acute respiratory failure from approximately 4700 US EDs. In the weighted analysis, the mean patient age of these visits was 68 years, and 56% were made by women. Ninety‐six percent were admitted to the hospital. Of these, the mortality rate was 9% and the mean hospital LOS was 7 days. Figure 1 shows the secular trends in NIV, IMV, and the combined use over the 3‐year study period. Use of IMV decreased from 28% in 2006 to 19% in 2008 (P<0.001), whereas NIV use increased slightly from 14% in 2006 to 16% in 2008 (P=0.049); the combined use of both ventilation modalities remained stable (4%). Inpatient mortality decreased from 10% in 2006 to 7% in 2008 (P<0.001).
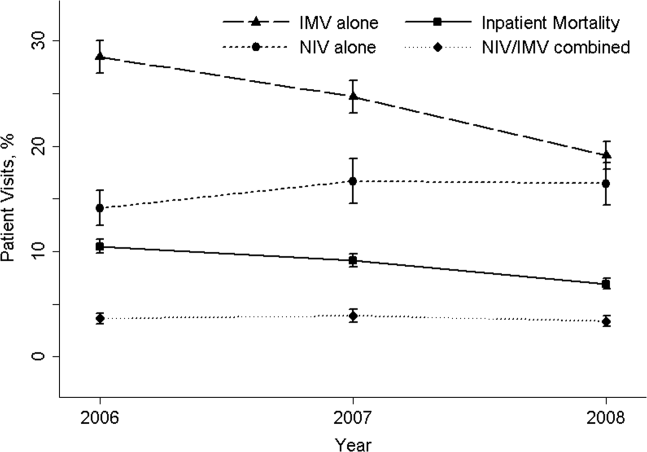
Figure 2 shows that the frequency of NIV use (including combined use of NIV and IMV) varied widely between hospitals, ranging from 0% to 100% with a median of 11%. In the unweighted cohort of AECOPD with acute respiratory failure, 43% received some forms of ventilatory support. Table 1 shows the patient and hospital characteristics of the patients receiving ventilatory support: 36% received NIV, 56% received IMV, and 8% received combined use. In general, patients receiving combined use of NIV and IMV tended to have more comorbidities (eg, congestive heart failure and pneumonia) compared with the NIV‐alone or IMV‐alone groups. With respect to hospital characteristics, NIV was used more often in hospitals with higher volumes of COPD exacerbation and respiratory failure, in nonmetropolitan hospitals, and in hospitals in the Northeast.
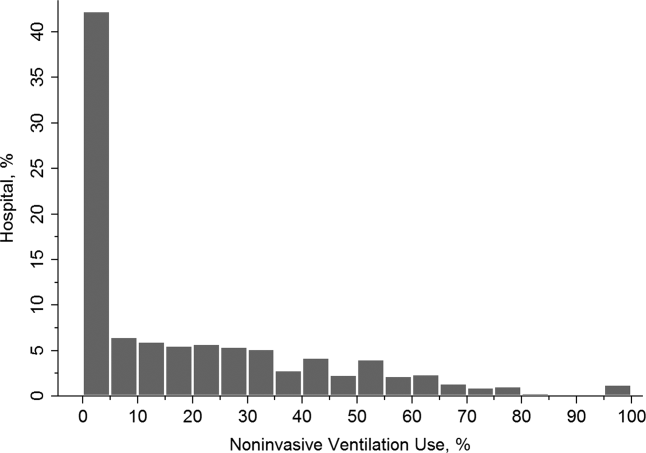
| NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value, A vs B | P Value, B vs C | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age, y, | <0.001 | 0.64 | |||
| 4049 | 5 | 5 | 5 | ||
| 5059 | 17 | 18 | 19 | ||
| 6069 | 31 | 33 | 33 | ||
| 7079 | 30 | 29 | 29 | ||
| 80 | 17 | 15 | 13 | ||
| Female sex, % | 57 | 53 | 54 | <0.001 | 0.87 |
| Quartile for median household income of patient ZIP code, $, % | <0.001 | <0.001 | |||
| 138,999 | 30 | 34 | 29 | ||
| 39,00047,999 | 28 | 28 | 28 | ||
| 48,00062,999 | 24 | 22 | 24 | ||
| 63,000 | 18 | 15 | 19 | ||
| Weekend admission, % | 27 | 28 | 28 | 0.07 | 0.80 |
| Insurance status, % | <0.001 | 0.91 | |||
| Medicare | 74 | 70 | 70 | ||
| Medicaid | 9 | 12 | 12 | ||
| Private | 12 | 13 | 13 | ||
| Self‐pay | 2 | 3 | 2 | ||
| Other | 2 | 2 | 2 | ||
| Season, % | <0.001 | 0.16 | |||
| Winter (January 1March 31) | 29 | 32 | 31 | ||
| Spring (April 1June 30) | 24 | 25 | 26 | ||
| Summer (July 1September 30) | 22 | 20 | 19 | ||
| Fall (October 1December 31) | 25 | 22 | 24 | ||
| No. of comorbidities, median (IQR) | 4 (35) | 4 (35) | 4 (36) | <0.001 | <0.001 |
| Selected comorbidities, % | |||||
| Hypertension | 56 | 55 | 55 | 0.01 | 0.65 |
| CHF | 38 | 40 | 44 | 0.001 | <0.001 |
| Fluid and electrolyte disorders | 37 | 44 | 49 | <0.001 | <0.001 |
| Diabetes, uncomplicated | 27 | 26 | 29 | 0.04 | 0.002 |
| Pneumonia | 19 | 34 | 39 | <0.001 | <0.001 |
| Deficiency anemia | 16 | 19 | 19 | <0.001 | 0.39 |
| Obesity | 18 | 12 | 17 | <0.001 | <0.001 |
| Depression | 15 | 11 | 11 | <0.001 | 0.54 |
| Pulmonary circulatory diseases | 15 | 11 | 14 | <0.001 | <0.001 |
| Hospital characteristics | |||||
| Annual ED visit volume, median (IQR) | 42,704 (29,50562,470) | 44,119 (29,89564,097) | 46,695 (31,29866,235) | 0.02 | 0.0003 |
| Annual ED volume of COPD exacerbation with respiratory failure, median (IQR) | 45 (2672) | 42 (2368) | 38 (2364) | <0.001 | <0.001 |
| Urban/rural and teaching status, % | <0.001 | <0.001 | |||
| Metropolitan nonteaching | 53 | 52 | 47 | ||
| Metropolitan teaching | 31 | 35 | 39 | ||
| Nonmetropolitan | 16 | 13 | 13 | ||
| US region, % | <0.001 | <0.001 | |||
| Northeast | 28 | 16 | 36 | ||
| Midwest | 17 | 22 | 15 | ||
| South | 41 | 45 | 32 | ||
| West | 14 | 17 | 17 | ||
The unadjusted differences in outcomes are shown in Table 2. The combined‐use group had the highest inpatient mortality, longest LOS, and highest charges, followed by the IMV and NIV groups. In general, complications were few across all 3 groups, but the rate of iatrogenic pneumothorax was notably lower in the NIV group. Table 3 details the statistically significant predictors of NIV use in the propensity score model. Similar to the unadjusted analysis, older age, high‐income neighborhoods, Medicare insurance, and some comorbidities were positively associated with NIV use (eg, pulmonary circulatory disorders and liver disease), whereas a few comorbidities were negatively associated with NIV use (eg, pneumonia, and alcohol and drug abuse). With respect to hospital characteristics, higher case volumes of COPD exacerbation/respiratory failure, Northeastern and nonmetropolitan hospitals, and more recent years were associated with NIV use.
| Outcome | NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value. A vs B | P Value, B vs C |
|---|---|---|---|---|---|
| |||||
| Inpatient mortality, n (%) | 825 (8) | 2,454 (16) | 407 (18) | <0.001 | 0.04 |
| Hospital length of stay, median (IQR), d | 5 (48) | 8 (513) | 10 (716) | <0.001 | <0.001 |
| Hospital charge per visit, median (IQR), $ | 26,002 (15,74744,638) | 53,432 (31,99892,664) | 64,585 (39,024110,336) | <0.001 | <0.001 |
| Complications* | |||||
| Ventilator‐associated pneumonia, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.09 | 1.00 |
| Facial injury, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.26 | 1.00 |
| Iatrogenic pneumothorax, n (%) | 10 (0.1) | 90 (0.6) | 14 (0.6) | <0.001 | 0.90 |
| Patient Characteristics | Adjusted OR (95% CI)* | P Value |
|---|---|---|
| ||
| Age, y | ||
| 4049 | 1.00 (Reference) | |
| 5059 | 0.96 (0.84‐1.11) | 0.61 |
| 6069 | 0.96 (0.84‐1.10) | 0.56 |
| 7079 | 1.09 (0.94‐1.25) | 0.25 |
| 80 | 1.30 (1.12‐1.52) | 0.001 |
| Quartile for median household income of patient ZIP code, $ | ||
| 138,999 | 1.00 (Reference) | |
| 39,00047,999 | 1.13 (1.05‐1.21) | 0.001 |
| 48,00062,999 | 1.21 (1.12‐1.30) | <0.001 |
| 63,000 | 1.21 (1.11‐1.32) | <0.001 |
| Insurance status | ||
| Medicare | 1.00 (Reference) | |
| Medicaid | 0.79 (0.72‐0.88) | <0.001 |
| Private | 0.88 (0.81‐0.96) | 0.004 |
| Self‐pay | 0.68 (0.56‐0.82) | <0.001 |
| Other | 0.88 (0.73‐1.07) | 0.22 |
| Season | ||
| Winter (January 1March 31) | 1.00 (Reference) | |
| Spring (April 1June 30) | 1.06 (0.99‐1.14) | 0.11 |
| Summer (July 1September 30) | 1.17 (1.08‐1.26) | <0.001 |
| Fall (October 1December 31) | 1.24 (1.15‐1.33) | <0.001 |
| Comorbidity | ||
| CHF | 0.90 (0.85‐0.95) | <0.001 |
| Pulmonary circulatory disorders | 1.40 (1.29‐1.52) | <0.001 |
| Diabetes, complicated | 1.25 (1.08‐1.44) | 0.002 |
| Liver disease | 1.79 (1.40‐2.28) | <0.001 |
| Coagulopathy | 0.54 (0.46‐0.63) | <0.001 |
| Obesity | 1.52 (1.41‐1.65) | <0.001 |
| Weight loss | 0.50 (0.44‐0.57) | <0.001 |
| Fluid and electrolyte disorders | 0.84 (0.80‐0.89) | <0.001 |
| Deficiency anemia | 0.83 (0.78‐0.90) | <0.001 |
| Alcohol abuse | 0.66 (0.58‐0.76) | <0.001 |
| Drug abuse | 0.74 (0.62‐0.88) | 0.001 |
| Psychoses | 1.22 (1.10‐1.37) | <0.001 |
| Depression | 1.45 (1.34‐1.57) | <0.001 |
| Pneumonia | 0.48 (0.45‐0.51) | <0.001 |
| Valvular heart disease | 0.87 (0.77‐0.97) | 0.01 |
| Neurological disorders | 0.89 (0.80‐0.98) | 0.02 |
| RA/collagen vascular diseases | 1.25 (1.02‐1.53) | 0.04 |
| Blood‐loss anemia | 0.72 (0.53‐0.97) | 0.03 |
| Hospital characteristics | ||
| Annual ED visit volume, per 1000‐visit increase | 0.997 (0.996‐0.998) | <0.001 |
| Annual ED volume of COPD exacerbation with respiratory failure, per 10‐visit increase | 1.03 (1.02‐1.03) | <0.001 |
| Urban/rural and teaching status | ||
| Metropolitan nonteaching | 1.00 (Reference) | |
| Metropolitan teaching | 0.91 (0.85‐0.97) | 0.006 |
| Nonmetropolitan | 1.30 (1.20‐1.42) | <0.001 |
| US region | ||
| Northeast | 1.00 (Reference) | |
| Midwest | 0.44 (0.40‐0.48) | <0.001 |
| South | 0.54 (0.50‐0.58) | <0.001 |
| West | 0.51 (0.46‐0.56) | <0.001 |
| Calendar year | ||
| 2006 | 1.00 (Reference) | |
| 2007 | 1.30 (1.22‐1.39) | <0.001 |
| 2008 | 1.65 (1.54‐1.76) | <0.001 |
In terms of propensity score distributions (see Supporting Information, Figure E1, in the online version of this article), there was sufficient overlap of the NIV and IMV groups. After matching on propensity score for the NIV and IMV groups, the differences in baseline characteristics were all balanced (see Supporting Information, Table E1, in the online version of this article), as indicated by <10% standardized differences in all covariates between the 2 groups. Finally, in the propensity scorematched cohort (see Supporting Information, Table E2, in the online version of this article), NIV use remained associated with significantly lower inpatient mortality (risk ratio: 0.54; 95% CI: 0.50‐0.59, P<0.001), a shorter hospital LOS (mean difference, 3.2 days; 95% CI: 3.4 to 2.9 days, P<0.001), and lower hospital charges (mean difference, P<$35,012; 95% CI: $36,848 to $33,176, P<0.001), compared with IMV use. Use of NIV also was associated with a lower rate of iatrogenic pneumothorax than IMV use (0.05% vs 0.5%, P<0.001).
Using hospital preference for NIV vs IMV as an instrument, the instrumental analysis confirmed the benefits of NIV use, with a 5% reduction in inpatient mortality in the NIV‐preferring hospitals (risk difference, P<5%; 95% CI: P<1.8% to P<8.3%).
In the sensitivity analysis to assess the impact of an unmeasured confounder, the confounder would have had to have a very strong impact on outcome (risk ratio: 5) and a severe exposure‐confounder imbalance (odds ratio of exposure on confounder: 5) to reduce the observed association to 1.0. In other words, an individual unmeasured confounder is unlikely to explain the observed association.
DISCUSSION
In this nationally representative sample of 67,651 ED visits for AECOPD with acute respiratory failure, we found that NIV use was increasing from 2006 to 2008. However, the utilization of NIV remained low (16% in 2008) and varied widely by patient and hospital characteristic. As with all observational studies, causality cannot be inferred definitely; however, our study suggests that, NIV usecompared with IMV usewas associated with potentially important benefits: a reduction of inpatient mortality by 46%, shortened hospital LOS by 3 days, reduced hospital charges by approximately $35,000 per visit, and modestly reduced risk of iatrogenic pneumothorax.
A recent analysis using the US Nationwide Inpatient Sample has shown increasing use of NIV and concomitant decreasing mortality in AECOPD over time.[28] Our analysis confirmed these favorable trends in the United States using a much larger NEDS sample (28 million visits in the NEDS vs 8 million visits in the Nationwide Inpatient Sample per year). Despite these favorable trends, NIV was still underutilized for AECOPD with respiratory failure in the United States (16% in 2008) compared with major European countries (40%).[29] Although our study lacked clinical details to arrive at the optimal rate of NIV use, the low rate of NIV use is concerning and suggests room for improvement in NIV use in appropriate patients as outlined by the current COPD guidelines.[18, 30] Why is NIV not widely adopted, given its demonstrated efficacy? Previous surveys have identified several perceived reasons for low NIV use, including lack of physician knowledge, insufficient respiratory therapist training, inadequate equipment, and time required for setting up NIV.[29, 31, 32] Our study adds to the literature by showing the actual predictors of NIV use in the real world. Our data showed that the early adopters were hospitals with higher case volumes, and hospitals in the Northeast and in nonmetropolitan areas. A higher case volume has been linked with lower mortality in AECOPD (ie, practice makes perfect),[33] and frequent NIV use could explain the lower AECOPD mortality in highcase volume centers. Alternatively, smaller hospitals tend to have moonlighters working in EDs who may not be board certified in emergency medicine. Perhaps the logical next step is to conduct a qualitative study to understand the specifics of best practices and provider characteristics in these Northeastern, highercase volume centers. Another incentive to promote NIV use in clinical practice is the cost‐effectiveness associated with this intervention, as previous studies have shown that, compared with usual care, receiving NIV was associated with a reduction in costs, mainly through reduced use of the ICU.[34, 35]
Some patient factors associated with NIV use may be well justified. For example, older AECOPD patients may have an advance directive describing their treatment wishes (eg, do‐not‐intubate order),[36] and therefore NIV was preferred to IMV. Also, our data suggested AECOPD patients with a suspected pneumonia component were less likely to be placed on NIV, which is consistent with COPD guideline recommendations.[18, 30] As outlined in the current guidelines, the major contraindications to NIV include impending respiratory arrest, excessive respiratory secretions, massive gastrointestinal bleeding, recent facial trauma, or altered mental status.[18, 30] By contrast, some factors associated with NIV use may be targeted for intervention, such as lower rates of NIV use in the uninsured, patients who live in low‐income neighborhoods, and hospitals in US regions other than the Northeast.
Current guidelines recommend using NIV in AECOPD patients with early signs of respiratory failure, such as arterial pH of 7.257.35 or pCO2 45 mm Hg.[18, 30] When NIV is considered as the modality of ventilatory support, it should probably be used as early as possible,[37] because evidence suggests that delayed use of NIV may lead to severe respiratory acidosis and increased mortality.[38] Other than in ICUs, NIV can be used on general wards and in EDs that have adequate staff training and experience, because the success rates of NIV in these settings are similar to those reported in ICU studies.[8, 36, 39] In addition, NIV is more cost‐effective when performed outside the ICU.[35] In fact, studies have found a substantial portion of patients had NIV started in the ED (one‐fourth) and on the general ward (one‐fourth).[31, 40] Given the shortage of intensivists in the United States, hospitalists begin to play an important role in provision of critical care outside the ICU.[41] Once NIV is used, it is important to ensure that it is delivered effectively and monitored closely because NIV failure has been shown to be associated with high mortality.[28, 42]
This study has some potential limitations. First, we used administrative claims that lack clinical details such as data on arterial blood gases and severity scores, and thus potential residual confounding may exist. In our study, the IMV group may be sicker than the NIV group, which could partially explain the increased mortality with IMV. However, the propensity scores overlap to a great extent between the 2 study groups, suggesting that a strong confounding bias is less likely, given the observed covariates. Furthermore, the instrumental variable and sensitivity analyses taking into account unmeasured confounders still suggested the benefits of NIV. Second, the NEDS does not contain data on the location where NIV was initiated (eg, ED, ward, or ICU) or the timing of initiating NIV or IMV. As a result, for the combined‐use group, we could not further distinguish the group switching from NIV to IMV (ie, NIV failure)[42] or from IMV to NIV (ie, NIV as a weaning strategy).[43] Accordingly, we chose to focus on the comparativeness effectiveness of NIV vs IMV. Third, although the NEDS data have undergone quality‐control procedures,[44] some misclassification may exist in identifying patient population and interventions. Finally, the analysis may not reflect the most recent trend in NIV use, as the 2010 NEDS data have just been released. In addition, although the study is the largest to date on this topic, our findings may not be generalizable to EDs that were not part of the NEDS.
In summary, in this nationally representative ED and inpatient database, NIV use is increasing for AECOPD with acute respiratory failure; however, its adoption remains low and varies widely between US hospitals. Our observational study suggests that NIV appears to be more effective and safer than IMV in the real‐world setting. There is an opportunity to increase the use of NIV as recommended in guidelines and to promote the use NIV in replacement of IMV in patients with severe AECOPD. Given the increasing mortality burden of COPD, such a strategy may help reduce COPD mortality at the population level, thereby fulfilling the objectives of Healthy People 2020.
Disclosure
Partial results from this study were presented at the 2012 Society for Academic Emergency Medicine Annual Meeting, Chicago, Illinois, May 912, 2012. This project was supported by grant number R03HS020722 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to disclose.
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics Reports, 2011. Deaths: final data for 2008. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_10.pdf. Accessed August 15, 2012.
- , , , . Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259.
- , , . National study of emergency department visits for acute exacerbation of chronic obstructive pulmonary disease. Acad Emerg Med. 2008;15:1275–1283.
- , , , , . Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16.
- US Department of Health and Human Services. Healthy People 2020. Objectives for Respiratory Diseases. Available at: http://www.healthypeople. gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=36. Accessed May 3, 2012.
- . Mechanical ventilation: invasive versus noninvasive. Eur Respir J Suppl. 2003;47:31s–37s.
- , , , et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935.
- , , , et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104.
- , , , et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707.
- . Noninvasive vs conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128:3916–3924.
- , , , , , . Mechanical ventilation in chronic obstructive pulmonary disease patients, noninvasive vs. invasive method (randomized prospective study). Coll Antropol. 2009;33:791–797.
- , , , et al. Noninvasive Positive‐Pressure Ventilation (NPPV) for Acute Respiratory Failure. Rockville, MD: Agency for Healthcare Research and Quality; July 2012: Report 12‐EHC089‐EF. Available at: http://effectivehealthcareahrqgov/ehc/products/273/1180/CER68_NPPV_FinalReport_20120706pdf. Accessed December 11, 2012.
- Healthcare Cost and Utilization Project (HCUP). HCUP Nationwide Emergency Department Sample (NEDS). Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/nedsoverview.jsp. Accessed April 15, 2012.
- American Hospital Association. Annual survey database. Available at: http://www.ahadata.com/ahadata/html/AHASurvey.html. Accessed April 15, 2012.
- , , , . Age‐related differences in clinical outcomes for acute asthma in the United States, 2006–2008. J Allergy Clin Immunol. 2012;129:1252e1–1258e1.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). NHLBI/WHO Global Strategy for the Diagnosis, Management and Prevention of COPD. Available at: http://www.goldcopd.org. Accessed April 15, 2012.
- .US Bureau of the Census. Census regions and divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed April 9, 2012.
- .Healthcare Cost and Utilization Project. Comorbidity software. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed April 15, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Healthcare Cost and Utilization Project (HCUP). HCUP Methods Series. Hierarchical Modeling Using HCUP Data. Rockville, MD: Agency for Healthcare Research and Quality; 2012: Report 2007‐01. Available at: http://www.hcup‐us.ahrq.gov/reports/methods/2007_01.pdf. Accessed April 15, 2012.
- , , . Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259.
- , , , , , . Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042.
- . An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–554.
- . Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159.
- , , , , . A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36:362–369.
- , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
- , , , . Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest. 2006;129:1226–1233.
- , , . A survey of the use of noninvasive ventilation in academic emergency departments in the United States. Respir Care. 2009;54:1306–1312.
- , , . Emergency department case volume and patient outcomes in acute exacerbations of chronic obstructive pulmonary disease. Acad Emerg Med. 2012;19:656–663.
- , , , , . Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28:2094–2102.
- , , , . Cost effectiveness of ward based non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ. 2003;326:956.
- , , . Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do‐not‐intubate” patients. Crit Care Med. 2005;33:1976–1982.
- , . Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: “Don't think twice, it's alright!”. Am J Respir Crit Care Med. 2012;185:121–123.
- , , , , . Acidosis, non‐invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66:43–48.
- , , , , . Use of noninvasive positive‐pressure ventilation on the regular hospital ward: experience and correlates of success. Respir Care. 2006;51:1237–1243.
- , , , et al. Bilevel noninvasive positive pressure ventilation for acute respiratory failure: survey of Ontario practice. Crit Care Med. 2005;33:1477–1483.
- . Hospitalists and intensivists: partners in caring for the critically ill—the time has come. J Hosp Med. 2010;5:1–3.
- , , , , , . Incidence and causes of non‐invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–825.
- , , , . Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010:CD004127.
- Healthcare Cost and Utilization Project (HCUP). HCUP Quality Control Procedures. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/db/quality.jsp. Accessed December 15, 2012.
Chronic obstructive pulmonary disease (COPD) is now the third leading cause of death in the United States,[1] and its rising mortality trend is unique among the top 5 causes of death.[2] Acute exacerbations of COPD (AECOPD) are important events in the natural history of COPD, accounting for 1.5 million emergency department (ED) visits and 726,000 hospitalizations each year in the United States.[3, 4] Given the significant morbidity and mortality from AECOPD, Healthy People 2020 lists reducing deaths, hospitalizations, and ED visits as the key objectives for COPD.[5]
Over the past 2 decades, noninvasive ventilation (NIV) has emerged as a potentially useful treatment modality in AECOPD patients with acute respiratory failure. Noninvasive ventilation commonly refers to positive‐pressure ventilatory support delivered through a nasal or full‐face mask, such as bilevel positive airway pressure.[6] A number of randomized controlled trials[7, 8, 9] and meta‐analyses[10] have suggested a mortality‐reduction benefit with NIV use compared with standard medical care in AECOPD. To our knowledge, however, very few small randomized controlled trials compared NIV vs invasive mechanical ventilation (IMV) head‐to‐head,[11, 12, 13] and a recent evidence review found only 5 studies (405 subjects) on this topic.[14] Collectively, the limited evidence from randomized trials showed that NIV use resulted in similar intensive care unit (ICU) and in‐hospital mortality, fewer complications (eg, ventilator‐associated pneumonia and sepsis), and shorter hospital length of stays (LOS). Given that these trials have a smaller sample size and tend to exclude older patient (age >75 years) or patients with multiple comorbidities, there is a need to better understand the adoption and effectiveness of NIV treatment for AECOPD in a much larger patient population in the real‐world setting using observational data.
To address these knowledge gaps in the literature, we analyzed data from a large, nationally representative ED and inpatient sample. The objective of the present analysis was 2‐fold: (1) to characterize the use of NIV and IMV in AECOPD patients with acute respiratory failure at a national level; and (2) to compare the effectiveness of NIV vs IMV in the real‐world setting.
METHODS
Study Design and Setting
We conducted a retrospective cohort study using data from the 20062008 Nationwide Emergency Department Sample (NEDS),[15] a component of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality. The NEDS is nationally representative of all community hospitalbased EDs in the United States, defined by the American Hospital Association as all nonfederal, short‐term, general, and other specialty hospitals.[16] Community hospitals include academic medical centers if they are nonfederal short‐term hospitals. The NEDS was constructed using administrative records from the State Emergency Department Databases and the State Inpatient Databases. The former captures information on ED visits that do not result in an admission (ie, treat‐and‐release visits or transfers to another hospital); the latter contains information on patients initially seen in the ED and then admitted to the same hospital. Taken together, the resulting NEDS represents all ED visits regardless of disposition and contains information on short‐term outcomes for patients admitted through the ED. In other words, the NEDS is the largest all‐payer ED and inpatient database in the United States. The NEDS represents an approximately 20% stratified sample of US hospital‐based EDs, containing more than 28 million records of ED visits from approximately 1000 hospitals each year. Additional details of the NEDS can be found elsewhere.[15, 17] We received a waiver for this analysis from our institutional review board.
Study Population
Patient visits were included in this analysis if they carried any COPD‐related diagnostic code (ie, International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code of 491.xx [chronic bronchitis], 492.xx [emphysema], or 496.xx [chronic airway obstruction, not elsewhere classified]) as their primary ED diagnosis and any acute respiratory failure code (ie, 518.81 [acute respiratory failure], 518.82 [pulmonary insufficiency not elsewhere classified, 518.84 [acute and chronic respiratory failure], or 799.1 [respiratory arrest]) as their secondary diagnosis. Patient visits with a primary diagnosis of acute respiratory failure and a secondary diagnosis of COPD were also included. Patients age <40 years were excluded, because they are much less likely to have COPD.[18]
Modes of Mechanical Ventilation
The primary exposure variable was mode of mechanical ventilation. To compare the effectiveness of different ventilatory modes, patients were divided into 3 groups according to the ventilation mode they received: (1) NIV alone, (2) IMV alone, and (3) combined modes of NIV and IMV. The use of NIV was identified by using Current Procedural Terminology (CPT) code of 94660 or ICD‐9 procedure code 93.90, whereas the use of IMV was identified by using CPT code of 31500 or ICD‐9 procedure code 96.04 or 96.7x.
Patient‐Level and Emergency DepartmentLevel Variables
The NEDS contains information on patient demographics, national quartiles for median household income based on the patient's ZIP code, payment sources, ICD‐9‐CM diagnoses and procedures, ED disposition, hospital LOS, and hospital disposition. Hospital characteristics include annual visit volume, urban‐rural status, ownership, teaching status, and US region. Geographic regions (Northeast, South, Midwest, and West) were defined according to Census Bureau boundaries.[19] To adjust for confounding by patient mix, Elixhauser comorbidity measures were derived based on the ICD‐9 codes, using the Agency for Healthcare Research and Quality's Comorbidity Software.[20] This risk‐adjustment tool has been derived and validated extensively.[21]
Outcome Measures
The outcome measures were all‐cause inpatient mortality, hospital LOS, hospital charges, and ventilator‐related complications. Three ventilator‐related complications were identified using ICD‐9 procedure codes: ventilator‐associated pneumonia (997.31), facial injury (910.x), and iatrogenic pneumothorax (512.1).
Statistical Analysis
Summary statistics are presented as proportions (with 95% confidence intervals [CI]), means (with standard deviations [SD]), or medians (with interquartile ranges). Bivariate associations were examined using Student t tests, Kruskal‐Wallis tests, and [2] tests, as appropriate. Emergency department and discharge weights were used to obtain national estimates at the ED and visit level. At all other times (eg, the propensity score and instrumental variable analyses), the unweighted cohort was analyzed, because survey weights are generally not advised for propensity score analysis using complex survey data.[22]
Propensity Score Analysis
To adjust for baseline patient and ED characteristics that may have confounded the relationship between ventilation mode and clinical outcomes, we performed propensity score and instrumental variable analyses. To compare the effectiveness of NIV vs IMV, a propensity score or predicted probability of NIV was estimated using a logistic‐regression model with all patient characteristics (age, sex, quartiles for median household income, weekend admission, insurance status, season, calendar year, and comorbid conditions) and ED characteristics (urban/rural and teaching status, US region, annual ED volume, and annual volume of AECOPD with respiratory failure) as the independent variables. We then performed 1:1 propensity score matching based on a nearest‐neighbor algorithm with caliper distance of 0.01. Although propensity score matching may result in a smaller sample, it provides a clinically relevant estimate of treatment effect because subjects in the matched sample are potential candidates for either treatment option.[23, 24] An absolute standardized difference between characteristics of <10% was considered as adequate balance.[25]
Instrumental Variable Analysis
When hospitals always or nearly always use NIV or IMV, this suggests the choice is largely independent of patient characteristics, and it is possible to use the hospital preference as a proxy for the actual treatment choice (ie, an instrument variable). The instrumental variable analysis simulates a natural randomization of patients to 2 hospital groups with high and low NIV use.
The main difference between instrumental variable and propensity score analysis is that the former could potentially adjust for unmeasured confounders.[26] We used Stata procedure IVREG to estimate the outcome differences between NIV‐preferring hospitals (NIV use in 90% of patients) and IMV‐preferring hospitals (NIV use in 10% of patients).
All odds ratios (ORs) and ‐coefficients are presented with 95% CIs. All analyses were performed using Stata 12.0 software (StataCorp, College Station, TX). All P values are 2‐sided, with P<0.05 considered statistically significant.
Sensitivity Analyses
We conducted a sensitivity analysis to determine whether it was plausible that an unmeasured confounder could completely explain the observed results. The risk ratio of a hypothetical unmeasured confounder on study outcome and the exposure‐confounder imbalance were both varied to see at what point the observed association was reduced to 1.0.[27]
RESULTS
Patient and ED Characteristics
The 20062008 NEDS sample contained 67,651 ED visits for AECOPD with acute respiratory failure from 1594 US EDs. After the weighting procedure, there were an estimated 101,000 visits annually for AECOPD with acute respiratory failure from approximately 4700 US EDs. In the weighted analysis, the mean patient age of these visits was 68 years, and 56% were made by women. Ninety‐six percent were admitted to the hospital. Of these, the mortality rate was 9% and the mean hospital LOS was 7 days. Figure 1 shows the secular trends in NIV, IMV, and the combined use over the 3‐year study period. Use of IMV decreased from 28% in 2006 to 19% in 2008 (P<0.001), whereas NIV use increased slightly from 14% in 2006 to 16% in 2008 (P=0.049); the combined use of both ventilation modalities remained stable (4%). Inpatient mortality decreased from 10% in 2006 to 7% in 2008 (P<0.001).

Figure 2 shows that the frequency of NIV use (including combined use of NIV and IMV) varied widely between hospitals, ranging from 0% to 100% with a median of 11%. In the unweighted cohort of AECOPD with acute respiratory failure, 43% received some forms of ventilatory support. Table 1 shows the patient and hospital characteristics of the patients receiving ventilatory support: 36% received NIV, 56% received IMV, and 8% received combined use. In general, patients receiving combined use of NIV and IMV tended to have more comorbidities (eg, congestive heart failure and pneumonia) compared with the NIV‐alone or IMV‐alone groups. With respect to hospital characteristics, NIV was used more often in hospitals with higher volumes of COPD exacerbation and respiratory failure, in nonmetropolitan hospitals, and in hospitals in the Northeast.

| NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value, A vs B | P Value, B vs C | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age, y, | <0.001 | 0.64 | |||
| 4049 | 5 | 5 | 5 | ||
| 5059 | 17 | 18 | 19 | ||
| 6069 | 31 | 33 | 33 | ||
| 7079 | 30 | 29 | 29 | ||
| 80 | 17 | 15 | 13 | ||
| Female sex, % | 57 | 53 | 54 | <0.001 | 0.87 |
| Quartile for median household income of patient ZIP code, $, % | <0.001 | <0.001 | |||
| 138,999 | 30 | 34 | 29 | ||
| 39,00047,999 | 28 | 28 | 28 | ||
| 48,00062,999 | 24 | 22 | 24 | ||
| 63,000 | 18 | 15 | 19 | ||
| Weekend admission, % | 27 | 28 | 28 | 0.07 | 0.80 |
| Insurance status, % | <0.001 | 0.91 | |||
| Medicare | 74 | 70 | 70 | ||
| Medicaid | 9 | 12 | 12 | ||
| Private | 12 | 13 | 13 | ||
| Self‐pay | 2 | 3 | 2 | ||
| Other | 2 | 2 | 2 | ||
| Season, % | <0.001 | 0.16 | |||
| Winter (January 1March 31) | 29 | 32 | 31 | ||
| Spring (April 1June 30) | 24 | 25 | 26 | ||
| Summer (July 1September 30) | 22 | 20 | 19 | ||
| Fall (October 1December 31) | 25 | 22 | 24 | ||
| No. of comorbidities, median (IQR) | 4 (35) | 4 (35) | 4 (36) | <0.001 | <0.001 |
| Selected comorbidities, % | |||||
| Hypertension | 56 | 55 | 55 | 0.01 | 0.65 |
| CHF | 38 | 40 | 44 | 0.001 | <0.001 |
| Fluid and electrolyte disorders | 37 | 44 | 49 | <0.001 | <0.001 |
| Diabetes, uncomplicated | 27 | 26 | 29 | 0.04 | 0.002 |
| Pneumonia | 19 | 34 | 39 | <0.001 | <0.001 |
| Deficiency anemia | 16 | 19 | 19 | <0.001 | 0.39 |
| Obesity | 18 | 12 | 17 | <0.001 | <0.001 |
| Depression | 15 | 11 | 11 | <0.001 | 0.54 |
| Pulmonary circulatory diseases | 15 | 11 | 14 | <0.001 | <0.001 |
| Hospital characteristics | |||||
| Annual ED visit volume, median (IQR) | 42,704 (29,50562,470) | 44,119 (29,89564,097) | 46,695 (31,29866,235) | 0.02 | 0.0003 |
| Annual ED volume of COPD exacerbation with respiratory failure, median (IQR) | 45 (2672) | 42 (2368) | 38 (2364) | <0.001 | <0.001 |
| Urban/rural and teaching status, % | <0.001 | <0.001 | |||
| Metropolitan nonteaching | 53 | 52 | 47 | ||
| Metropolitan teaching | 31 | 35 | 39 | ||
| Nonmetropolitan | 16 | 13 | 13 | ||
| US region, % | <0.001 | <0.001 | |||
| Northeast | 28 | 16 | 36 | ||
| Midwest | 17 | 22 | 15 | ||
| South | 41 | 45 | 32 | ||
| West | 14 | 17 | 17 | ||
The unadjusted differences in outcomes are shown in Table 2. The combined‐use group had the highest inpatient mortality, longest LOS, and highest charges, followed by the IMV and NIV groups. In general, complications were few across all 3 groups, but the rate of iatrogenic pneumothorax was notably lower in the NIV group. Table 3 details the statistically significant predictors of NIV use in the propensity score model. Similar to the unadjusted analysis, older age, high‐income neighborhoods, Medicare insurance, and some comorbidities were positively associated with NIV use (eg, pulmonary circulatory disorders and liver disease), whereas a few comorbidities were negatively associated with NIV use (eg, pneumonia, and alcohol and drug abuse). With respect to hospital characteristics, higher case volumes of COPD exacerbation/respiratory failure, Northeastern and nonmetropolitan hospitals, and more recent years were associated with NIV use.
| Outcome | NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value. A vs B | P Value, B vs C |
|---|---|---|---|---|---|
| |||||
| Inpatient mortality, n (%) | 825 (8) | 2,454 (16) | 407 (18) | <0.001 | 0.04 |
| Hospital length of stay, median (IQR), d | 5 (48) | 8 (513) | 10 (716) | <0.001 | <0.001 |
| Hospital charge per visit, median (IQR), $ | 26,002 (15,74744,638) | 53,432 (31,99892,664) | 64,585 (39,024110,336) | <0.001 | <0.001 |
| Complications* | |||||
| Ventilator‐associated pneumonia, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.09 | 1.00 |
| Facial injury, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.26 | 1.00 |
| Iatrogenic pneumothorax, n (%) | 10 (0.1) | 90 (0.6) | 14 (0.6) | <0.001 | 0.90 |
| Patient Characteristics | Adjusted OR (95% CI)* | P Value |
|---|---|---|
| ||
| Age, y | ||
| 4049 | 1.00 (Reference) | |
| 5059 | 0.96 (0.84‐1.11) | 0.61 |
| 6069 | 0.96 (0.84‐1.10) | 0.56 |
| 7079 | 1.09 (0.94‐1.25) | 0.25 |
| 80 | 1.30 (1.12‐1.52) | 0.001 |
| Quartile for median household income of patient ZIP code, $ | ||
| 138,999 | 1.00 (Reference) | |
| 39,00047,999 | 1.13 (1.05‐1.21) | 0.001 |
| 48,00062,999 | 1.21 (1.12‐1.30) | <0.001 |
| 63,000 | 1.21 (1.11‐1.32) | <0.001 |
| Insurance status | ||
| Medicare | 1.00 (Reference) | |
| Medicaid | 0.79 (0.72‐0.88) | <0.001 |
| Private | 0.88 (0.81‐0.96) | 0.004 |
| Self‐pay | 0.68 (0.56‐0.82) | <0.001 |
| Other | 0.88 (0.73‐1.07) | 0.22 |
| Season | ||
| Winter (January 1March 31) | 1.00 (Reference) | |
| Spring (April 1June 30) | 1.06 (0.99‐1.14) | 0.11 |
| Summer (July 1September 30) | 1.17 (1.08‐1.26) | <0.001 |
| Fall (October 1December 31) | 1.24 (1.15‐1.33) | <0.001 |
| Comorbidity | ||
| CHF | 0.90 (0.85‐0.95) | <0.001 |
| Pulmonary circulatory disorders | 1.40 (1.29‐1.52) | <0.001 |
| Diabetes, complicated | 1.25 (1.08‐1.44) | 0.002 |
| Liver disease | 1.79 (1.40‐2.28) | <0.001 |
| Coagulopathy | 0.54 (0.46‐0.63) | <0.001 |
| Obesity | 1.52 (1.41‐1.65) | <0.001 |
| Weight loss | 0.50 (0.44‐0.57) | <0.001 |
| Fluid and electrolyte disorders | 0.84 (0.80‐0.89) | <0.001 |
| Deficiency anemia | 0.83 (0.78‐0.90) | <0.001 |
| Alcohol abuse | 0.66 (0.58‐0.76) | <0.001 |
| Drug abuse | 0.74 (0.62‐0.88) | 0.001 |
| Psychoses | 1.22 (1.10‐1.37) | <0.001 |
| Depression | 1.45 (1.34‐1.57) | <0.001 |
| Pneumonia | 0.48 (0.45‐0.51) | <0.001 |
| Valvular heart disease | 0.87 (0.77‐0.97) | 0.01 |
| Neurological disorders | 0.89 (0.80‐0.98) | 0.02 |
| RA/collagen vascular diseases | 1.25 (1.02‐1.53) | 0.04 |
| Blood‐loss anemia | 0.72 (0.53‐0.97) | 0.03 |
| Hospital characteristics | ||
| Annual ED visit volume, per 1000‐visit increase | 0.997 (0.996‐0.998) | <0.001 |
| Annual ED volume of COPD exacerbation with respiratory failure, per 10‐visit increase | 1.03 (1.02‐1.03) | <0.001 |
| Urban/rural and teaching status | ||
| Metropolitan nonteaching | 1.00 (Reference) | |
| Metropolitan teaching | 0.91 (0.85‐0.97) | 0.006 |
| Nonmetropolitan | 1.30 (1.20‐1.42) | <0.001 |
| US region | ||
| Northeast | 1.00 (Reference) | |
| Midwest | 0.44 (0.40‐0.48) | <0.001 |
| South | 0.54 (0.50‐0.58) | <0.001 |
| West | 0.51 (0.46‐0.56) | <0.001 |
| Calendar year | ||
| 2006 | 1.00 (Reference) | |
| 2007 | 1.30 (1.22‐1.39) | <0.001 |
| 2008 | 1.65 (1.54‐1.76) | <0.001 |
In terms of propensity score distributions (see Supporting Information, Figure E1, in the online version of this article), there was sufficient overlap of the NIV and IMV groups. After matching on propensity score for the NIV and IMV groups, the differences in baseline characteristics were all balanced (see Supporting Information, Table E1, in the online version of this article), as indicated by <10% standardized differences in all covariates between the 2 groups. Finally, in the propensity scorematched cohort (see Supporting Information, Table E2, in the online version of this article), NIV use remained associated with significantly lower inpatient mortality (risk ratio: 0.54; 95% CI: 0.50‐0.59, P<0.001), a shorter hospital LOS (mean difference, 3.2 days; 95% CI: 3.4 to 2.9 days, P<0.001), and lower hospital charges (mean difference, P<$35,012; 95% CI: $36,848 to $33,176, P<0.001), compared with IMV use. Use of NIV also was associated with a lower rate of iatrogenic pneumothorax than IMV use (0.05% vs 0.5%, P<0.001).
Using hospital preference for NIV vs IMV as an instrument, the instrumental analysis confirmed the benefits of NIV use, with a 5% reduction in inpatient mortality in the NIV‐preferring hospitals (risk difference, P<5%; 95% CI: P<1.8% to P<8.3%).
In the sensitivity analysis to assess the impact of an unmeasured confounder, the confounder would have had to have a very strong impact on outcome (risk ratio: 5) and a severe exposure‐confounder imbalance (odds ratio of exposure on confounder: 5) to reduce the observed association to 1.0. In other words, an individual unmeasured confounder is unlikely to explain the observed association.
DISCUSSION
In this nationally representative sample of 67,651 ED visits for AECOPD with acute respiratory failure, we found that NIV use was increasing from 2006 to 2008. However, the utilization of NIV remained low (16% in 2008) and varied widely by patient and hospital characteristic. As with all observational studies, causality cannot be inferred definitely; however, our study suggests that, NIV usecompared with IMV usewas associated with potentially important benefits: a reduction of inpatient mortality by 46%, shortened hospital LOS by 3 days, reduced hospital charges by approximately $35,000 per visit, and modestly reduced risk of iatrogenic pneumothorax.
A recent analysis using the US Nationwide Inpatient Sample has shown increasing use of NIV and concomitant decreasing mortality in AECOPD over time.[28] Our analysis confirmed these favorable trends in the United States using a much larger NEDS sample (28 million visits in the NEDS vs 8 million visits in the Nationwide Inpatient Sample per year). Despite these favorable trends, NIV was still underutilized for AECOPD with respiratory failure in the United States (16% in 2008) compared with major European countries (40%).[29] Although our study lacked clinical details to arrive at the optimal rate of NIV use, the low rate of NIV use is concerning and suggests room for improvement in NIV use in appropriate patients as outlined by the current COPD guidelines.[18, 30] Why is NIV not widely adopted, given its demonstrated efficacy? Previous surveys have identified several perceived reasons for low NIV use, including lack of physician knowledge, insufficient respiratory therapist training, inadequate equipment, and time required for setting up NIV.[29, 31, 32] Our study adds to the literature by showing the actual predictors of NIV use in the real world. Our data showed that the early adopters were hospitals with higher case volumes, and hospitals in the Northeast and in nonmetropolitan areas. A higher case volume has been linked with lower mortality in AECOPD (ie, practice makes perfect),[33] and frequent NIV use could explain the lower AECOPD mortality in highcase volume centers. Alternatively, smaller hospitals tend to have moonlighters working in EDs who may not be board certified in emergency medicine. Perhaps the logical next step is to conduct a qualitative study to understand the specifics of best practices and provider characteristics in these Northeastern, highercase volume centers. Another incentive to promote NIV use in clinical practice is the cost‐effectiveness associated with this intervention, as previous studies have shown that, compared with usual care, receiving NIV was associated with a reduction in costs, mainly through reduced use of the ICU.[34, 35]
Some patient factors associated with NIV use may be well justified. For example, older AECOPD patients may have an advance directive describing their treatment wishes (eg, do‐not‐intubate order),[36] and therefore NIV was preferred to IMV. Also, our data suggested AECOPD patients with a suspected pneumonia component were less likely to be placed on NIV, which is consistent with COPD guideline recommendations.[18, 30] As outlined in the current guidelines, the major contraindications to NIV include impending respiratory arrest, excessive respiratory secretions, massive gastrointestinal bleeding, recent facial trauma, or altered mental status.[18, 30] By contrast, some factors associated with NIV use may be targeted for intervention, such as lower rates of NIV use in the uninsured, patients who live in low‐income neighborhoods, and hospitals in US regions other than the Northeast.
Current guidelines recommend using NIV in AECOPD patients with early signs of respiratory failure, such as arterial pH of 7.257.35 or pCO2 45 mm Hg.[18, 30] When NIV is considered as the modality of ventilatory support, it should probably be used as early as possible,[37] because evidence suggests that delayed use of NIV may lead to severe respiratory acidosis and increased mortality.[38] Other than in ICUs, NIV can be used on general wards and in EDs that have adequate staff training and experience, because the success rates of NIV in these settings are similar to those reported in ICU studies.[8, 36, 39] In addition, NIV is more cost‐effective when performed outside the ICU.[35] In fact, studies have found a substantial portion of patients had NIV started in the ED (one‐fourth) and on the general ward (one‐fourth).[31, 40] Given the shortage of intensivists in the United States, hospitalists begin to play an important role in provision of critical care outside the ICU.[41] Once NIV is used, it is important to ensure that it is delivered effectively and monitored closely because NIV failure has been shown to be associated with high mortality.[28, 42]
This study has some potential limitations. First, we used administrative claims that lack clinical details such as data on arterial blood gases and severity scores, and thus potential residual confounding may exist. In our study, the IMV group may be sicker than the NIV group, which could partially explain the increased mortality with IMV. However, the propensity scores overlap to a great extent between the 2 study groups, suggesting that a strong confounding bias is less likely, given the observed covariates. Furthermore, the instrumental variable and sensitivity analyses taking into account unmeasured confounders still suggested the benefits of NIV. Second, the NEDS does not contain data on the location where NIV was initiated (eg, ED, ward, or ICU) or the timing of initiating NIV or IMV. As a result, for the combined‐use group, we could not further distinguish the group switching from NIV to IMV (ie, NIV failure)[42] or from IMV to NIV (ie, NIV as a weaning strategy).[43] Accordingly, we chose to focus on the comparativeness effectiveness of NIV vs IMV. Third, although the NEDS data have undergone quality‐control procedures,[44] some misclassification may exist in identifying patient population and interventions. Finally, the analysis may not reflect the most recent trend in NIV use, as the 2010 NEDS data have just been released. In addition, although the study is the largest to date on this topic, our findings may not be generalizable to EDs that were not part of the NEDS.
In summary, in this nationally representative ED and inpatient database, NIV use is increasing for AECOPD with acute respiratory failure; however, its adoption remains low and varies widely between US hospitals. Our observational study suggests that NIV appears to be more effective and safer than IMV in the real‐world setting. There is an opportunity to increase the use of NIV as recommended in guidelines and to promote the use NIV in replacement of IMV in patients with severe AECOPD. Given the increasing mortality burden of COPD, such a strategy may help reduce COPD mortality at the population level, thereby fulfilling the objectives of Healthy People 2020.
Disclosure
Partial results from this study were presented at the 2012 Society for Academic Emergency Medicine Annual Meeting, Chicago, Illinois, May 912, 2012. This project was supported by grant number R03HS020722 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to disclose.
Chronic obstructive pulmonary disease (COPD) is now the third leading cause of death in the United States,[1] and its rising mortality trend is unique among the top 5 causes of death.[2] Acute exacerbations of COPD (AECOPD) are important events in the natural history of COPD, accounting for 1.5 million emergency department (ED) visits and 726,000 hospitalizations each year in the United States.[3, 4] Given the significant morbidity and mortality from AECOPD, Healthy People 2020 lists reducing deaths, hospitalizations, and ED visits as the key objectives for COPD.[5]
Over the past 2 decades, noninvasive ventilation (NIV) has emerged as a potentially useful treatment modality in AECOPD patients with acute respiratory failure. Noninvasive ventilation commonly refers to positive‐pressure ventilatory support delivered through a nasal or full‐face mask, such as bilevel positive airway pressure.[6] A number of randomized controlled trials[7, 8, 9] and meta‐analyses[10] have suggested a mortality‐reduction benefit with NIV use compared with standard medical care in AECOPD. To our knowledge, however, very few small randomized controlled trials compared NIV vs invasive mechanical ventilation (IMV) head‐to‐head,[11, 12, 13] and a recent evidence review found only 5 studies (405 subjects) on this topic.[14] Collectively, the limited evidence from randomized trials showed that NIV use resulted in similar intensive care unit (ICU) and in‐hospital mortality, fewer complications (eg, ventilator‐associated pneumonia and sepsis), and shorter hospital length of stays (LOS). Given that these trials have a smaller sample size and tend to exclude older patient (age >75 years) or patients with multiple comorbidities, there is a need to better understand the adoption and effectiveness of NIV treatment for AECOPD in a much larger patient population in the real‐world setting using observational data.
To address these knowledge gaps in the literature, we analyzed data from a large, nationally representative ED and inpatient sample. The objective of the present analysis was 2‐fold: (1) to characterize the use of NIV and IMV in AECOPD patients with acute respiratory failure at a national level; and (2) to compare the effectiveness of NIV vs IMV in the real‐world setting.
METHODS
Study Design and Setting
We conducted a retrospective cohort study using data from the 20062008 Nationwide Emergency Department Sample (NEDS),[15] a component of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality. The NEDS is nationally representative of all community hospitalbased EDs in the United States, defined by the American Hospital Association as all nonfederal, short‐term, general, and other specialty hospitals.[16] Community hospitals include academic medical centers if they are nonfederal short‐term hospitals. The NEDS was constructed using administrative records from the State Emergency Department Databases and the State Inpatient Databases. The former captures information on ED visits that do not result in an admission (ie, treat‐and‐release visits or transfers to another hospital); the latter contains information on patients initially seen in the ED and then admitted to the same hospital. Taken together, the resulting NEDS represents all ED visits regardless of disposition and contains information on short‐term outcomes for patients admitted through the ED. In other words, the NEDS is the largest all‐payer ED and inpatient database in the United States. The NEDS represents an approximately 20% stratified sample of US hospital‐based EDs, containing more than 28 million records of ED visits from approximately 1000 hospitals each year. Additional details of the NEDS can be found elsewhere.[15, 17] We received a waiver for this analysis from our institutional review board.
Study Population
Patient visits were included in this analysis if they carried any COPD‐related diagnostic code (ie, International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code of 491.xx [chronic bronchitis], 492.xx [emphysema], or 496.xx [chronic airway obstruction, not elsewhere classified]) as their primary ED diagnosis and any acute respiratory failure code (ie, 518.81 [acute respiratory failure], 518.82 [pulmonary insufficiency not elsewhere classified, 518.84 [acute and chronic respiratory failure], or 799.1 [respiratory arrest]) as their secondary diagnosis. Patient visits with a primary diagnosis of acute respiratory failure and a secondary diagnosis of COPD were also included. Patients age <40 years were excluded, because they are much less likely to have COPD.[18]
Modes of Mechanical Ventilation
The primary exposure variable was mode of mechanical ventilation. To compare the effectiveness of different ventilatory modes, patients were divided into 3 groups according to the ventilation mode they received: (1) NIV alone, (2) IMV alone, and (3) combined modes of NIV and IMV. The use of NIV was identified by using Current Procedural Terminology (CPT) code of 94660 or ICD‐9 procedure code 93.90, whereas the use of IMV was identified by using CPT code of 31500 or ICD‐9 procedure code 96.04 or 96.7x.
Patient‐Level and Emergency DepartmentLevel Variables
The NEDS contains information on patient demographics, national quartiles for median household income based on the patient's ZIP code, payment sources, ICD‐9‐CM diagnoses and procedures, ED disposition, hospital LOS, and hospital disposition. Hospital characteristics include annual visit volume, urban‐rural status, ownership, teaching status, and US region. Geographic regions (Northeast, South, Midwest, and West) were defined according to Census Bureau boundaries.[19] To adjust for confounding by patient mix, Elixhauser comorbidity measures were derived based on the ICD‐9 codes, using the Agency for Healthcare Research and Quality's Comorbidity Software.[20] This risk‐adjustment tool has been derived and validated extensively.[21]
Outcome Measures
The outcome measures were all‐cause inpatient mortality, hospital LOS, hospital charges, and ventilator‐related complications. Three ventilator‐related complications were identified using ICD‐9 procedure codes: ventilator‐associated pneumonia (997.31), facial injury (910.x), and iatrogenic pneumothorax (512.1).
Statistical Analysis
Summary statistics are presented as proportions (with 95% confidence intervals [CI]), means (with standard deviations [SD]), or medians (with interquartile ranges). Bivariate associations were examined using Student t tests, Kruskal‐Wallis tests, and [2] tests, as appropriate. Emergency department and discharge weights were used to obtain national estimates at the ED and visit level. At all other times (eg, the propensity score and instrumental variable analyses), the unweighted cohort was analyzed, because survey weights are generally not advised for propensity score analysis using complex survey data.[22]
Propensity Score Analysis
To adjust for baseline patient and ED characteristics that may have confounded the relationship between ventilation mode and clinical outcomes, we performed propensity score and instrumental variable analyses. To compare the effectiveness of NIV vs IMV, a propensity score or predicted probability of NIV was estimated using a logistic‐regression model with all patient characteristics (age, sex, quartiles for median household income, weekend admission, insurance status, season, calendar year, and comorbid conditions) and ED characteristics (urban/rural and teaching status, US region, annual ED volume, and annual volume of AECOPD with respiratory failure) as the independent variables. We then performed 1:1 propensity score matching based on a nearest‐neighbor algorithm with caliper distance of 0.01. Although propensity score matching may result in a smaller sample, it provides a clinically relevant estimate of treatment effect because subjects in the matched sample are potential candidates for either treatment option.[23, 24] An absolute standardized difference between characteristics of <10% was considered as adequate balance.[25]
Instrumental Variable Analysis
When hospitals always or nearly always use NIV or IMV, this suggests the choice is largely independent of patient characteristics, and it is possible to use the hospital preference as a proxy for the actual treatment choice (ie, an instrument variable). The instrumental variable analysis simulates a natural randomization of patients to 2 hospital groups with high and low NIV use.
The main difference between instrumental variable and propensity score analysis is that the former could potentially adjust for unmeasured confounders.[26] We used Stata procedure IVREG to estimate the outcome differences between NIV‐preferring hospitals (NIV use in 90% of patients) and IMV‐preferring hospitals (NIV use in 10% of patients).
All odds ratios (ORs) and ‐coefficients are presented with 95% CIs. All analyses were performed using Stata 12.0 software (StataCorp, College Station, TX). All P values are 2‐sided, with P<0.05 considered statistically significant.
Sensitivity Analyses
We conducted a sensitivity analysis to determine whether it was plausible that an unmeasured confounder could completely explain the observed results. The risk ratio of a hypothetical unmeasured confounder on study outcome and the exposure‐confounder imbalance were both varied to see at what point the observed association was reduced to 1.0.[27]
RESULTS
Patient and ED Characteristics
The 20062008 NEDS sample contained 67,651 ED visits for AECOPD with acute respiratory failure from 1594 US EDs. After the weighting procedure, there were an estimated 101,000 visits annually for AECOPD with acute respiratory failure from approximately 4700 US EDs. In the weighted analysis, the mean patient age of these visits was 68 years, and 56% were made by women. Ninety‐six percent were admitted to the hospital. Of these, the mortality rate was 9% and the mean hospital LOS was 7 days. Figure 1 shows the secular trends in NIV, IMV, and the combined use over the 3‐year study period. Use of IMV decreased from 28% in 2006 to 19% in 2008 (P<0.001), whereas NIV use increased slightly from 14% in 2006 to 16% in 2008 (P=0.049); the combined use of both ventilation modalities remained stable (4%). Inpatient mortality decreased from 10% in 2006 to 7% in 2008 (P<0.001).

Figure 2 shows that the frequency of NIV use (including combined use of NIV and IMV) varied widely between hospitals, ranging from 0% to 100% with a median of 11%. In the unweighted cohort of AECOPD with acute respiratory failure, 43% received some forms of ventilatory support. Table 1 shows the patient and hospital characteristics of the patients receiving ventilatory support: 36% received NIV, 56% received IMV, and 8% received combined use. In general, patients receiving combined use of NIV and IMV tended to have more comorbidities (eg, congestive heart failure and pneumonia) compared with the NIV‐alone or IMV‐alone groups. With respect to hospital characteristics, NIV was used more often in hospitals with higher volumes of COPD exacerbation and respiratory failure, in nonmetropolitan hospitals, and in hospitals in the Northeast.

| NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value, A vs B | P Value, B vs C | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age, y, | <0.001 | 0.64 | |||
| 4049 | 5 | 5 | 5 | ||
| 5059 | 17 | 18 | 19 | ||
| 6069 | 31 | 33 | 33 | ||
| 7079 | 30 | 29 | 29 | ||
| 80 | 17 | 15 | 13 | ||
| Female sex, % | 57 | 53 | 54 | <0.001 | 0.87 |
| Quartile for median household income of patient ZIP code, $, % | <0.001 | <0.001 | |||
| 138,999 | 30 | 34 | 29 | ||
| 39,00047,999 | 28 | 28 | 28 | ||
| 48,00062,999 | 24 | 22 | 24 | ||
| 63,000 | 18 | 15 | 19 | ||
| Weekend admission, % | 27 | 28 | 28 | 0.07 | 0.80 |
| Insurance status, % | <0.001 | 0.91 | |||
| Medicare | 74 | 70 | 70 | ||
| Medicaid | 9 | 12 | 12 | ||
| Private | 12 | 13 | 13 | ||
| Self‐pay | 2 | 3 | 2 | ||
| Other | 2 | 2 | 2 | ||
| Season, % | <0.001 | 0.16 | |||
| Winter (January 1March 31) | 29 | 32 | 31 | ||
| Spring (April 1June 30) | 24 | 25 | 26 | ||
| Summer (July 1September 30) | 22 | 20 | 19 | ||
| Fall (October 1December 31) | 25 | 22 | 24 | ||
| No. of comorbidities, median (IQR) | 4 (35) | 4 (35) | 4 (36) | <0.001 | <0.001 |
| Selected comorbidities, % | |||||
| Hypertension | 56 | 55 | 55 | 0.01 | 0.65 |
| CHF | 38 | 40 | 44 | 0.001 | <0.001 |
| Fluid and electrolyte disorders | 37 | 44 | 49 | <0.001 | <0.001 |
| Diabetes, uncomplicated | 27 | 26 | 29 | 0.04 | 0.002 |
| Pneumonia | 19 | 34 | 39 | <0.001 | <0.001 |
| Deficiency anemia | 16 | 19 | 19 | <0.001 | 0.39 |
| Obesity | 18 | 12 | 17 | <0.001 | <0.001 |
| Depression | 15 | 11 | 11 | <0.001 | 0.54 |
| Pulmonary circulatory diseases | 15 | 11 | 14 | <0.001 | <0.001 |
| Hospital characteristics | |||||
| Annual ED visit volume, median (IQR) | 42,704 (29,50562,470) | 44,119 (29,89564,097) | 46,695 (31,29866,235) | 0.02 | 0.0003 |
| Annual ED volume of COPD exacerbation with respiratory failure, median (IQR) | 45 (2672) | 42 (2368) | 38 (2364) | <0.001 | <0.001 |
| Urban/rural and teaching status, % | <0.001 | <0.001 | |||
| Metropolitan nonteaching | 53 | 52 | 47 | ||
| Metropolitan teaching | 31 | 35 | 39 | ||
| Nonmetropolitan | 16 | 13 | 13 | ||
| US region, % | <0.001 | <0.001 | |||
| Northeast | 28 | 16 | 36 | ||
| Midwest | 17 | 22 | 15 | ||
| South | 41 | 45 | 32 | ||
| West | 14 | 17 | 17 | ||
The unadjusted differences in outcomes are shown in Table 2. The combined‐use group had the highest inpatient mortality, longest LOS, and highest charges, followed by the IMV and NIV groups. In general, complications were few across all 3 groups, but the rate of iatrogenic pneumothorax was notably lower in the NIV group. Table 3 details the statistically significant predictors of NIV use in the propensity score model. Similar to the unadjusted analysis, older age, high‐income neighborhoods, Medicare insurance, and some comorbidities were positively associated with NIV use (eg, pulmonary circulatory disorders and liver disease), whereas a few comorbidities were negatively associated with NIV use (eg, pneumonia, and alcohol and drug abuse). With respect to hospital characteristics, higher case volumes of COPD exacerbation/respiratory failure, Northeastern and nonmetropolitan hospitals, and more recent years were associated with NIV use.
| Outcome | NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value. A vs B | P Value, B vs C |
|---|---|---|---|---|---|
| |||||
| Inpatient mortality, n (%) | 825 (8) | 2,454 (16) | 407 (18) | <0.001 | 0.04 |
| Hospital length of stay, median (IQR), d | 5 (48) | 8 (513) | 10 (716) | <0.001 | <0.001 |
| Hospital charge per visit, median (IQR), $ | 26,002 (15,74744,638) | 53,432 (31,99892,664) | 64,585 (39,024110,336) | <0.001 | <0.001 |
| Complications* | |||||
| Ventilator‐associated pneumonia, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.09 | 1.00 |
| Facial injury, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.26 | 1.00 |
| Iatrogenic pneumothorax, n (%) | 10 (0.1) | 90 (0.6) | 14 (0.6) | <0.001 | 0.90 |
| Patient Characteristics | Adjusted OR (95% CI)* | P Value |
|---|---|---|
| ||
| Age, y | ||
| 4049 | 1.00 (Reference) | |
| 5059 | 0.96 (0.84‐1.11) | 0.61 |
| 6069 | 0.96 (0.84‐1.10) | 0.56 |
| 7079 | 1.09 (0.94‐1.25) | 0.25 |
| 80 | 1.30 (1.12‐1.52) | 0.001 |
| Quartile for median household income of patient ZIP code, $ | ||
| 138,999 | 1.00 (Reference) | |
| 39,00047,999 | 1.13 (1.05‐1.21) | 0.001 |
| 48,00062,999 | 1.21 (1.12‐1.30) | <0.001 |
| 63,000 | 1.21 (1.11‐1.32) | <0.001 |
| Insurance status | ||
| Medicare | 1.00 (Reference) | |
| Medicaid | 0.79 (0.72‐0.88) | <0.001 |
| Private | 0.88 (0.81‐0.96) | 0.004 |
| Self‐pay | 0.68 (0.56‐0.82) | <0.001 |
| Other | 0.88 (0.73‐1.07) | 0.22 |
| Season | ||
| Winter (January 1March 31) | 1.00 (Reference) | |
| Spring (April 1June 30) | 1.06 (0.99‐1.14) | 0.11 |
| Summer (July 1September 30) | 1.17 (1.08‐1.26) | <0.001 |
| Fall (October 1December 31) | 1.24 (1.15‐1.33) | <0.001 |
| Comorbidity | ||
| CHF | 0.90 (0.85‐0.95) | <0.001 |
| Pulmonary circulatory disorders | 1.40 (1.29‐1.52) | <0.001 |
| Diabetes, complicated | 1.25 (1.08‐1.44) | 0.002 |
| Liver disease | 1.79 (1.40‐2.28) | <0.001 |
| Coagulopathy | 0.54 (0.46‐0.63) | <0.001 |
| Obesity | 1.52 (1.41‐1.65) | <0.001 |
| Weight loss | 0.50 (0.44‐0.57) | <0.001 |
| Fluid and electrolyte disorders | 0.84 (0.80‐0.89) | <0.001 |
| Deficiency anemia | 0.83 (0.78‐0.90) | <0.001 |
| Alcohol abuse | 0.66 (0.58‐0.76) | <0.001 |
| Drug abuse | 0.74 (0.62‐0.88) | 0.001 |
| Psychoses | 1.22 (1.10‐1.37) | <0.001 |
| Depression | 1.45 (1.34‐1.57) | <0.001 |
| Pneumonia | 0.48 (0.45‐0.51) | <0.001 |
| Valvular heart disease | 0.87 (0.77‐0.97) | 0.01 |
| Neurological disorders | 0.89 (0.80‐0.98) | 0.02 |
| RA/collagen vascular diseases | 1.25 (1.02‐1.53) | 0.04 |
| Blood‐loss anemia | 0.72 (0.53‐0.97) | 0.03 |
| Hospital characteristics | ||
| Annual ED visit volume, per 1000‐visit increase | 0.997 (0.996‐0.998) | <0.001 |
| Annual ED volume of COPD exacerbation with respiratory failure, per 10‐visit increase | 1.03 (1.02‐1.03) | <0.001 |
| Urban/rural and teaching status | ||
| Metropolitan nonteaching | 1.00 (Reference) | |
| Metropolitan teaching | 0.91 (0.85‐0.97) | 0.006 |
| Nonmetropolitan | 1.30 (1.20‐1.42) | <0.001 |
| US region | ||
| Northeast | 1.00 (Reference) | |
| Midwest | 0.44 (0.40‐0.48) | <0.001 |
| South | 0.54 (0.50‐0.58) | <0.001 |
| West | 0.51 (0.46‐0.56) | <0.001 |
| Calendar year | ||
| 2006 | 1.00 (Reference) | |
| 2007 | 1.30 (1.22‐1.39) | <0.001 |
| 2008 | 1.65 (1.54‐1.76) | <0.001 |
In terms of propensity score distributions (see Supporting Information, Figure E1, in the online version of this article), there was sufficient overlap of the NIV and IMV groups. After matching on propensity score for the NIV and IMV groups, the differences in baseline characteristics were all balanced (see Supporting Information, Table E1, in the online version of this article), as indicated by <10% standardized differences in all covariates between the 2 groups. Finally, in the propensity scorematched cohort (see Supporting Information, Table E2, in the online version of this article), NIV use remained associated with significantly lower inpatient mortality (risk ratio: 0.54; 95% CI: 0.50‐0.59, P<0.001), a shorter hospital LOS (mean difference, 3.2 days; 95% CI: 3.4 to 2.9 days, P<0.001), and lower hospital charges (mean difference, P<$35,012; 95% CI: $36,848 to $33,176, P<0.001), compared with IMV use. Use of NIV also was associated with a lower rate of iatrogenic pneumothorax than IMV use (0.05% vs 0.5%, P<0.001).
Using hospital preference for NIV vs IMV as an instrument, the instrumental analysis confirmed the benefits of NIV use, with a 5% reduction in inpatient mortality in the NIV‐preferring hospitals (risk difference, P<5%; 95% CI: P<1.8% to P<8.3%).
In the sensitivity analysis to assess the impact of an unmeasured confounder, the confounder would have had to have a very strong impact on outcome (risk ratio: 5) and a severe exposure‐confounder imbalance (odds ratio of exposure on confounder: 5) to reduce the observed association to 1.0. In other words, an individual unmeasured confounder is unlikely to explain the observed association.
DISCUSSION
In this nationally representative sample of 67,651 ED visits for AECOPD with acute respiratory failure, we found that NIV use was increasing from 2006 to 2008. However, the utilization of NIV remained low (16% in 2008) and varied widely by patient and hospital characteristic. As with all observational studies, causality cannot be inferred definitely; however, our study suggests that, NIV usecompared with IMV usewas associated with potentially important benefits: a reduction of inpatient mortality by 46%, shortened hospital LOS by 3 days, reduced hospital charges by approximately $35,000 per visit, and modestly reduced risk of iatrogenic pneumothorax.
A recent analysis using the US Nationwide Inpatient Sample has shown increasing use of NIV and concomitant decreasing mortality in AECOPD over time.[28] Our analysis confirmed these favorable trends in the United States using a much larger NEDS sample (28 million visits in the NEDS vs 8 million visits in the Nationwide Inpatient Sample per year). Despite these favorable trends, NIV was still underutilized for AECOPD with respiratory failure in the United States (16% in 2008) compared with major European countries (40%).[29] Although our study lacked clinical details to arrive at the optimal rate of NIV use, the low rate of NIV use is concerning and suggests room for improvement in NIV use in appropriate patients as outlined by the current COPD guidelines.[18, 30] Why is NIV not widely adopted, given its demonstrated efficacy? Previous surveys have identified several perceived reasons for low NIV use, including lack of physician knowledge, insufficient respiratory therapist training, inadequate equipment, and time required for setting up NIV.[29, 31, 32] Our study adds to the literature by showing the actual predictors of NIV use in the real world. Our data showed that the early adopters were hospitals with higher case volumes, and hospitals in the Northeast and in nonmetropolitan areas. A higher case volume has been linked with lower mortality in AECOPD (ie, practice makes perfect),[33] and frequent NIV use could explain the lower AECOPD mortality in highcase volume centers. Alternatively, smaller hospitals tend to have moonlighters working in EDs who may not be board certified in emergency medicine. Perhaps the logical next step is to conduct a qualitative study to understand the specifics of best practices and provider characteristics in these Northeastern, highercase volume centers. Another incentive to promote NIV use in clinical practice is the cost‐effectiveness associated with this intervention, as previous studies have shown that, compared with usual care, receiving NIV was associated with a reduction in costs, mainly through reduced use of the ICU.[34, 35]
Some patient factors associated with NIV use may be well justified. For example, older AECOPD patients may have an advance directive describing their treatment wishes (eg, do‐not‐intubate order),[36] and therefore NIV was preferred to IMV. Also, our data suggested AECOPD patients with a suspected pneumonia component were less likely to be placed on NIV, which is consistent with COPD guideline recommendations.[18, 30] As outlined in the current guidelines, the major contraindications to NIV include impending respiratory arrest, excessive respiratory secretions, massive gastrointestinal bleeding, recent facial trauma, or altered mental status.[18, 30] By contrast, some factors associated with NIV use may be targeted for intervention, such as lower rates of NIV use in the uninsured, patients who live in low‐income neighborhoods, and hospitals in US regions other than the Northeast.
Current guidelines recommend using NIV in AECOPD patients with early signs of respiratory failure, such as arterial pH of 7.257.35 or pCO2 45 mm Hg.[18, 30] When NIV is considered as the modality of ventilatory support, it should probably be used as early as possible,[37] because evidence suggests that delayed use of NIV may lead to severe respiratory acidosis and increased mortality.[38] Other than in ICUs, NIV can be used on general wards and in EDs that have adequate staff training and experience, because the success rates of NIV in these settings are similar to those reported in ICU studies.[8, 36, 39] In addition, NIV is more cost‐effective when performed outside the ICU.[35] In fact, studies have found a substantial portion of patients had NIV started in the ED (one‐fourth) and on the general ward (one‐fourth).[31, 40] Given the shortage of intensivists in the United States, hospitalists begin to play an important role in provision of critical care outside the ICU.[41] Once NIV is used, it is important to ensure that it is delivered effectively and monitored closely because NIV failure has been shown to be associated with high mortality.[28, 42]
This study has some potential limitations. First, we used administrative claims that lack clinical details such as data on arterial blood gases and severity scores, and thus potential residual confounding may exist. In our study, the IMV group may be sicker than the NIV group, which could partially explain the increased mortality with IMV. However, the propensity scores overlap to a great extent between the 2 study groups, suggesting that a strong confounding bias is less likely, given the observed covariates. Furthermore, the instrumental variable and sensitivity analyses taking into account unmeasured confounders still suggested the benefits of NIV. Second, the NEDS does not contain data on the location where NIV was initiated (eg, ED, ward, or ICU) or the timing of initiating NIV or IMV. As a result, for the combined‐use group, we could not further distinguish the group switching from NIV to IMV (ie, NIV failure)[42] or from IMV to NIV (ie, NIV as a weaning strategy).[43] Accordingly, we chose to focus on the comparativeness effectiveness of NIV vs IMV. Third, although the NEDS data have undergone quality‐control procedures,[44] some misclassification may exist in identifying patient population and interventions. Finally, the analysis may not reflect the most recent trend in NIV use, as the 2010 NEDS data have just been released. In addition, although the study is the largest to date on this topic, our findings may not be generalizable to EDs that were not part of the NEDS.
In summary, in this nationally representative ED and inpatient database, NIV use is increasing for AECOPD with acute respiratory failure; however, its adoption remains low and varies widely between US hospitals. Our observational study suggests that NIV appears to be more effective and safer than IMV in the real‐world setting. There is an opportunity to increase the use of NIV as recommended in guidelines and to promote the use NIV in replacement of IMV in patients with severe AECOPD. Given the increasing mortality burden of COPD, such a strategy may help reduce COPD mortality at the population level, thereby fulfilling the objectives of Healthy People 2020.
Disclosure
Partial results from this study were presented at the 2012 Society for Academic Emergency Medicine Annual Meeting, Chicago, Illinois, May 912, 2012. This project was supported by grant number R03HS020722 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to disclose.
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics Reports, 2011. Deaths: final data for 2008. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_10.pdf. Accessed August 15, 2012.
- , , , . Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259.
- , , . National study of emergency department visits for acute exacerbation of chronic obstructive pulmonary disease. Acad Emerg Med. 2008;15:1275–1283.
- , , , , . Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16.
- US Department of Health and Human Services. Healthy People 2020. Objectives for Respiratory Diseases. Available at: http://www.healthypeople. gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=36. Accessed May 3, 2012.
- . Mechanical ventilation: invasive versus noninvasive. Eur Respir J Suppl. 2003;47:31s–37s.
- , , , et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935.
- , , , et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104.
- , , , et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707.
- . Noninvasive vs conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128:3916–3924.
- , , , , , . Mechanical ventilation in chronic obstructive pulmonary disease patients, noninvasive vs. invasive method (randomized prospective study). Coll Antropol. 2009;33:791–797.
- , , , et al. Noninvasive Positive‐Pressure Ventilation (NPPV) for Acute Respiratory Failure. Rockville, MD: Agency for Healthcare Research and Quality; July 2012: Report 12‐EHC089‐EF. Available at: http://effectivehealthcareahrqgov/ehc/products/273/1180/CER68_NPPV_FinalReport_20120706pdf. Accessed December 11, 2012.
- Healthcare Cost and Utilization Project (HCUP). HCUP Nationwide Emergency Department Sample (NEDS). Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/nedsoverview.jsp. Accessed April 15, 2012.
- American Hospital Association. Annual survey database. Available at: http://www.ahadata.com/ahadata/html/AHASurvey.html. Accessed April 15, 2012.
- , , , . Age‐related differences in clinical outcomes for acute asthma in the United States, 2006–2008. J Allergy Clin Immunol. 2012;129:1252e1–1258e1.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). NHLBI/WHO Global Strategy for the Diagnosis, Management and Prevention of COPD. Available at: http://www.goldcopd.org. Accessed April 15, 2012.
- .US Bureau of the Census. Census regions and divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed April 9, 2012.
- .Healthcare Cost and Utilization Project. Comorbidity software. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed April 15, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Healthcare Cost and Utilization Project (HCUP). HCUP Methods Series. Hierarchical Modeling Using HCUP Data. Rockville, MD: Agency for Healthcare Research and Quality; 2012: Report 2007‐01. Available at: http://www.hcup‐us.ahrq.gov/reports/methods/2007_01.pdf. Accessed April 15, 2012.
- , , . Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259.
- , , , , , . Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042.
- . An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–554.
- . Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159.
- , , , , . A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36:362–369.
- , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
- , , , . Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest. 2006;129:1226–1233.
- , , . A survey of the use of noninvasive ventilation in academic emergency departments in the United States. Respir Care. 2009;54:1306–1312.
- , , . Emergency department case volume and patient outcomes in acute exacerbations of chronic obstructive pulmonary disease. Acad Emerg Med. 2012;19:656–663.
- , , , , . Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28:2094–2102.
- , , , . Cost effectiveness of ward based non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ. 2003;326:956.
- , , . Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do‐not‐intubate” patients. Crit Care Med. 2005;33:1976–1982.
- , . Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: “Don't think twice, it's alright!”. Am J Respir Crit Care Med. 2012;185:121–123.
- , , , , . Acidosis, non‐invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66:43–48.
- , , , , . Use of noninvasive positive‐pressure ventilation on the regular hospital ward: experience and correlates of success. Respir Care. 2006;51:1237–1243.
- , , , et al. Bilevel noninvasive positive pressure ventilation for acute respiratory failure: survey of Ontario practice. Crit Care Med. 2005;33:1477–1483.
- . Hospitalists and intensivists: partners in caring for the critically ill—the time has come. J Hosp Med. 2010;5:1–3.
- , , , , , . Incidence and causes of non‐invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–825.
- , , , . Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010:CD004127.
- Healthcare Cost and Utilization Project (HCUP). HCUP Quality Control Procedures. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/db/quality.jsp. Accessed December 15, 2012.
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics Reports, 2011. Deaths: final data for 2008. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_10.pdf. Accessed August 15, 2012.
- , , , . Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259.
- , , . National study of emergency department visits for acute exacerbation of chronic obstructive pulmonary disease. Acad Emerg Med. 2008;15:1275–1283.
- , , , , . Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16.
- US Department of Health and Human Services. Healthy People 2020. Objectives for Respiratory Diseases. Available at: http://www.healthypeople. gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=36. Accessed May 3, 2012.
- . Mechanical ventilation: invasive versus noninvasive. Eur Respir J Suppl. 2003;47:31s–37s.
- , , , et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935.
- , , , et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104.
- , , , et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707.
- . Noninvasive vs conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128:3916–3924.
- , , , , , . Mechanical ventilation in chronic obstructive pulmonary disease patients, noninvasive vs. invasive method (randomized prospective study). Coll Antropol. 2009;33:791–797.
- , , , et al. Noninvasive Positive‐Pressure Ventilation (NPPV) for Acute Respiratory Failure. Rockville, MD: Agency for Healthcare Research and Quality; July 2012: Report 12‐EHC089‐EF. Available at: http://effectivehealthcareahrqgov/ehc/products/273/1180/CER68_NPPV_FinalReport_20120706pdf. Accessed December 11, 2012.
- Healthcare Cost and Utilization Project (HCUP). HCUP Nationwide Emergency Department Sample (NEDS). Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/nedsoverview.jsp. Accessed April 15, 2012.
- American Hospital Association. Annual survey database. Available at: http://www.ahadata.com/ahadata/html/AHASurvey.html. Accessed April 15, 2012.
- , , , . Age‐related differences in clinical outcomes for acute asthma in the United States, 2006–2008. J Allergy Clin Immunol. 2012;129:1252e1–1258e1.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). NHLBI/WHO Global Strategy for the Diagnosis, Management and Prevention of COPD. Available at: http://www.goldcopd.org. Accessed April 15, 2012.
- .US Bureau of the Census. Census regions and divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed April 9, 2012.
- .Healthcare Cost and Utilization Project. Comorbidity software. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed April 15, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Healthcare Cost and Utilization Project (HCUP). HCUP Methods Series. Hierarchical Modeling Using HCUP Data. Rockville, MD: Agency for Healthcare Research and Quality; 2012: Report 2007‐01. Available at: http://www.hcup‐us.ahrq.gov/reports/methods/2007_01.pdf. Accessed April 15, 2012.
- , , . Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259.
- , , , , , . Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042.
- . An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–554.
- . Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159.
- , , , , . A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36:362–369.
- , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
- , , , . Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest. 2006;129:1226–1233.
- , , . A survey of the use of noninvasive ventilation in academic emergency departments in the United States. Respir Care. 2009;54:1306–1312.
- , , . Emergency department case volume and patient outcomes in acute exacerbations of chronic obstructive pulmonary disease. Acad Emerg Med. 2012;19:656–663.
- , , , , . Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28:2094–2102.
- , , , . Cost effectiveness of ward based non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ. 2003;326:956.
- , , . Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do‐not‐intubate” patients. Crit Care Med. 2005;33:1976–1982.
- , . Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: “Don't think twice, it's alright!”. Am J Respir Crit Care Med. 2012;185:121–123.
- , , , , . Acidosis, non‐invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66:43–48.
- , , , , . Use of noninvasive positive‐pressure ventilation on the regular hospital ward: experience and correlates of success. Respir Care. 2006;51:1237–1243.
- , , , et al. Bilevel noninvasive positive pressure ventilation for acute respiratory failure: survey of Ontario practice. Crit Care Med. 2005;33:1477–1483.
- . Hospitalists and intensivists: partners in caring for the critically ill—the time has come. J Hosp Med. 2010;5:1–3.
- , , , , , . Incidence and causes of non‐invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–825.
- , , , . Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010:CD004127.
- Healthcare Cost and Utilization Project (HCUP). HCUP Quality Control Procedures. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/db/quality.jsp. Accessed December 15, 2012.
Copyright © 2013 Society of Hospital Medicine
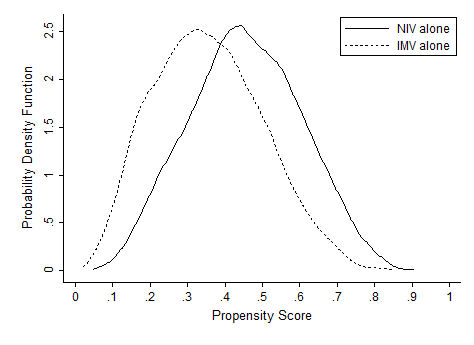
Intellectual Agenda for Hospitalists
The practice of bloodletting, performed using sticks, thorns, bones, or anything sharp, probably began in Egypt about 3,000 years ago.[1] The practice continued in Greece, where Hippocrates recommended bloodletting to balance the body's four humorsblood, phlegm, yellow bile, and black bileand continued during Roman times under the influence of Galen. In the United States, perhaps the most infamous use of bloodletting was when doctors reportedly bled as much as 5 U of blood from George Washington before he died from what was probably either acute epiglottitis or streptococcal pharyngitis.[2, 3] Although many infectious organisms, especially malaria parasites, require iron to proliferateand therefore may be less virulent in iron‐deficient people[4]acute near‐exsanguination undoubtedly did more harm than good in the elderly ex‐president.
But the practice of bloodletting continued and even flourished. In 1833 alone, France reportedly imported more than 40 million leeches to assist in bloodletting,[5] which oftentimes was thought to be sufficiently aggressive only when the patient actually fainted. Enthusiasm for bloodletting declined in the second half of the 19th century, influenced in part by a nonrandomized study that compared mortality rates among patients who were bled early in their illness with those who were bled later.[6] Nevertheless, Sir William Osler still recommended small amounts of bloodletting for pneumonia in his last edition of his famous textbook, The Principles and Practice of Medicine, published in 1920.[7] By 1927, however, the first edition of the Cecil's A Textbook of Medicine thankfully no longer recommended venesection except to treat conditions such as pulmonary edema.[8]
Why would I start this essay with a history of bloodletting? Surely, one might argue, nothing could be less relevant to a modern discussion of the quality of in‐hospital medical care. The substantial literature on quality improvement emphasizes the practical implementation of strategies to increase the appropriate adherence to processes that are known to improve outcomes. A number of common quality measures quickly come to mind: the use of aspirin, ‐blockers, angiotensin‐converting enzyme inhibitors, and statins in post‐myocardial infarction patients without contraindications,[9, 10] the rapid initiation of appropriate antibiotics to patients with community‐acquired pneumonia,[11] and early endoscopy for patients with acute upper gastrointestinal hemorrhage.[12] I could go on and on, listing in‐hospital interventions supported by class 1 evidence from more than one definitive randomized trial. In essentially all of these situations, the creation of quality metrics, often accompanied by measurement and feedback, have improved adherence and undoubtedly saved lives. But although adherence has improved, the explosion in evidence‐based medicine means that even the best hospitals may be in perpetual catch‐up mode as they try to ensure adherence with the next wave of improvement interventions.
Unfortunately, every now and then a lot of attention is paid to meeting a quality metric that turns out to be misguided. Perhaps the best recent in‐hospital example was the metric of prophylactic ‐blocker use before major noncardiac surgery. Although this recommendation initially appeared to be based on reasonable data,[13] the large Perioperative Ischemic Evaluation Study (POISE) trial showed that reductions in rates of myocardial infarction were more than offset by an increased risk of stroke and other complications; therefore, average‐risk patients actually did worse, not better, with the ‐blocker regimen used in the trial. Although some have questioned whether these results were a function of the precise ‐blocker regimen that was used, the results of POISE are actually remarkably consistent with prior data on the risk of myocardial infarction and stroke.[14, 15] What was really different was the relative importance of these and other end points in patients whose risk of cardiac death was lower than those of higher risk patients in prior studies. But more recently, an even more disturbing reality has emerged: a number of key reports on which the guidelines were based came from an investigator whose publications included data that could not be confirmed when his studies were reviewed by his home institution.[16] Regardless of the precise reasons, we no longer routinely recommend an intervention that at one time was a key quality indicator.
The ‐blocker fiasco brings me back to bloodletting. In the early 19th century, a hypothetical visionary physician interested in quality improvement would likely have looked for ways to improve the efficiency and reduce the cost of bloodletting. Perhaps the leeches could be bigger, hungrier, or applied in a more effective fashion? Or perhaps vacuum tubes would have been invented sooner?
Of course, I am overemphasizing to prove a point. I truly believe that more and more of what we recommend is based on solid evidence to document, at least for now, that we are doing the right thing. If we do it more often and in more people, net benefit will be realized.
What does all this mean for the future of hospital medicine and its emerging research endeavors? For me at least, the message is pretty clear. First, we must be careful not to over extrapolate from limited studies in high risk patients, or we will jump to more conclusions like we did with ‐blockers. Second, most advances in medicine require new and better data.
How can new clinical data be generated most quickly and efficiently? One‐off studies at individual institutions are logistically and financially challenging, whereas an enduring research infrastructure is a treasure that can study a series of questions as they arise. The Thrombolysis in Myocardial Infarction Study Group has published scores of papers looking at a series of interventions in patients with acute myocardial infarction and the acute coronary syndrome.[17, 18] The Acute Respiratory Distress Syndrome Network has demonstrated the value of lower tidal volumes and less aggressive fluid strategies in patients with respiratory failure.[19, 20]
The success of these large, multicenter research networks should become the paradigm for the study of common hospital problems, ranging from the conditions that result in admission on the medical service to the problems that have engendered surgical comanagement services. New data can be gleaned by studying the medical care system, by studying routinely gathered administrative and clinical data, or even better yet, by gathering prospective data on patients and their diseases. For hospitalized patients, a variety of unanswered questions remain regarding the epidemiology of common diseases, the value of diagnostic tests, the impact of various therapies and treatment protocols, and the incremental value of new technologies, ranging from self‐monitoring to handheld ultrasound. High‐quality research may address the genetic epidemiology of why one person is admitted with pneumococcal pneumonia, whereas family members seem perfectly healthy; which patients with a particular diagnosis might be managed for different lengths of time in different settings; what physical findings or diagnostic tests best stratify prognosis; what new technologies are truly worth their cost; and especially, what therapies really work.
I do not dispute that hospital medicine researchers should try to improve the current use of interventions that are deemed to be valuable right now. But if that is all the field does, it will be a huge disappointment. Hospitalists should not be relegated to being adherence police who spend their collective research energy finding ways to force themselves to follow recommendations based on data gathered by others.
Hospital medicine researchers are uniquely positioned to discover new information that will change what should be done and help create the quality metrics for the future. Unless both of these two goalsimproving the implementation of today's knowledge and generating new and better knowledgeare part of the research agenda, we run the risk that some of the best minds in internal medicine may, when all is said and done, have spent an inordinate number of IQ hours on what, a century from now, will be reminiscent of improving the quality of bloodletting.
- . Bloodletting over the centuries. NY State J Med. 1980;80:2022–2028.
- . The death of George Washington: an end to the controversy? Am Surg. 2008;74:770–774.
- . The death of George Washington (1732–1799) and the history of cynanche. J Med Biogr. 2005;13:225–231.
- , . Hepcidin and the iron‐infection axis. Science. 2012;338:768–772.
- . Leech therapy: a history. J Hist Dent. 2005;53:25–27.
- . Pierre‐Charles‐Alexandre Louis and the evaluation of bloodletting. J R Soc Med. 2006;99:158–160.
- , . The Principles and Practice of Medicine. New York, NY: Appleton and Co.; 1920.
- Cecil RL, Kennedy F, eds. A Textbook of Medicine by American Authors. 1st ed. Philadelphia, PA: WB Saunders; 1927.
- Department of Health and Human Services. Hospital Compare: hospital process of care measures tables. Available at: http://www.hospitalcompare.hhs.gov/staticpages/for‐consumers/poc/explainations‐of‐measures.aspx. Accessed January 3, 2013.
- , , . Trends in the use of lipid‐lowering medications at discharge in patients with acute myocardial infarction: 1998 to 2006. Am Heart J. 2009;157:185–194.
- , , , et al. Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169:1525–1531.
- , , , et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113.
- , . β‐blockers and reduction of cardiac events in noncardiac surgery. JAMA. 2002;287:1445–1447.
- , , , et al; POISE Study Group. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847.
- , , , et al. Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis. Lancet. 2008;372:1962–1976.
- Report on the 2012 follow‐up investigation of possible breaches of academic integrity. Erasmus MC Follow‐up Investigation Committee, 2012. Available at: http://www.eramusmc.nl. . Accessed January 3, 2013.
- , , , et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19.
- , , , et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation. N Engl J Med. 2005;352:1179–1189.
- Acute Respiratory Distress Syndrome Network: ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network: comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575.
The practice of bloodletting, performed using sticks, thorns, bones, or anything sharp, probably began in Egypt about 3,000 years ago.[1] The practice continued in Greece, where Hippocrates recommended bloodletting to balance the body's four humorsblood, phlegm, yellow bile, and black bileand continued during Roman times under the influence of Galen. In the United States, perhaps the most infamous use of bloodletting was when doctors reportedly bled as much as 5 U of blood from George Washington before he died from what was probably either acute epiglottitis or streptococcal pharyngitis.[2, 3] Although many infectious organisms, especially malaria parasites, require iron to proliferateand therefore may be less virulent in iron‐deficient people[4]acute near‐exsanguination undoubtedly did more harm than good in the elderly ex‐president.
But the practice of bloodletting continued and even flourished. In 1833 alone, France reportedly imported more than 40 million leeches to assist in bloodletting,[5] which oftentimes was thought to be sufficiently aggressive only when the patient actually fainted. Enthusiasm for bloodletting declined in the second half of the 19th century, influenced in part by a nonrandomized study that compared mortality rates among patients who were bled early in their illness with those who were bled later.[6] Nevertheless, Sir William Osler still recommended small amounts of bloodletting for pneumonia in his last edition of his famous textbook, The Principles and Practice of Medicine, published in 1920.[7] By 1927, however, the first edition of the Cecil's A Textbook of Medicine thankfully no longer recommended venesection except to treat conditions such as pulmonary edema.[8]
Why would I start this essay with a history of bloodletting? Surely, one might argue, nothing could be less relevant to a modern discussion of the quality of in‐hospital medical care. The substantial literature on quality improvement emphasizes the practical implementation of strategies to increase the appropriate adherence to processes that are known to improve outcomes. A number of common quality measures quickly come to mind: the use of aspirin, ‐blockers, angiotensin‐converting enzyme inhibitors, and statins in post‐myocardial infarction patients without contraindications,[9, 10] the rapid initiation of appropriate antibiotics to patients with community‐acquired pneumonia,[11] and early endoscopy for patients with acute upper gastrointestinal hemorrhage.[12] I could go on and on, listing in‐hospital interventions supported by class 1 evidence from more than one definitive randomized trial. In essentially all of these situations, the creation of quality metrics, often accompanied by measurement and feedback, have improved adherence and undoubtedly saved lives. But although adherence has improved, the explosion in evidence‐based medicine means that even the best hospitals may be in perpetual catch‐up mode as they try to ensure adherence with the next wave of improvement interventions.
Unfortunately, every now and then a lot of attention is paid to meeting a quality metric that turns out to be misguided. Perhaps the best recent in‐hospital example was the metric of prophylactic ‐blocker use before major noncardiac surgery. Although this recommendation initially appeared to be based on reasonable data,[13] the large Perioperative Ischemic Evaluation Study (POISE) trial showed that reductions in rates of myocardial infarction were more than offset by an increased risk of stroke and other complications; therefore, average‐risk patients actually did worse, not better, with the ‐blocker regimen used in the trial. Although some have questioned whether these results were a function of the precise ‐blocker regimen that was used, the results of POISE are actually remarkably consistent with prior data on the risk of myocardial infarction and stroke.[14, 15] What was really different was the relative importance of these and other end points in patients whose risk of cardiac death was lower than those of higher risk patients in prior studies. But more recently, an even more disturbing reality has emerged: a number of key reports on which the guidelines were based came from an investigator whose publications included data that could not be confirmed when his studies were reviewed by his home institution.[16] Regardless of the precise reasons, we no longer routinely recommend an intervention that at one time was a key quality indicator.
The ‐blocker fiasco brings me back to bloodletting. In the early 19th century, a hypothetical visionary physician interested in quality improvement would likely have looked for ways to improve the efficiency and reduce the cost of bloodletting. Perhaps the leeches could be bigger, hungrier, or applied in a more effective fashion? Or perhaps vacuum tubes would have been invented sooner?
Of course, I am overemphasizing to prove a point. I truly believe that more and more of what we recommend is based on solid evidence to document, at least for now, that we are doing the right thing. If we do it more often and in more people, net benefit will be realized.
What does all this mean for the future of hospital medicine and its emerging research endeavors? For me at least, the message is pretty clear. First, we must be careful not to over extrapolate from limited studies in high risk patients, or we will jump to more conclusions like we did with ‐blockers. Second, most advances in medicine require new and better data.
How can new clinical data be generated most quickly and efficiently? One‐off studies at individual institutions are logistically and financially challenging, whereas an enduring research infrastructure is a treasure that can study a series of questions as they arise. The Thrombolysis in Myocardial Infarction Study Group has published scores of papers looking at a series of interventions in patients with acute myocardial infarction and the acute coronary syndrome.[17, 18] The Acute Respiratory Distress Syndrome Network has demonstrated the value of lower tidal volumes and less aggressive fluid strategies in patients with respiratory failure.[19, 20]
The success of these large, multicenter research networks should become the paradigm for the study of common hospital problems, ranging from the conditions that result in admission on the medical service to the problems that have engendered surgical comanagement services. New data can be gleaned by studying the medical care system, by studying routinely gathered administrative and clinical data, or even better yet, by gathering prospective data on patients and their diseases. For hospitalized patients, a variety of unanswered questions remain regarding the epidemiology of common diseases, the value of diagnostic tests, the impact of various therapies and treatment protocols, and the incremental value of new technologies, ranging from self‐monitoring to handheld ultrasound. High‐quality research may address the genetic epidemiology of why one person is admitted with pneumococcal pneumonia, whereas family members seem perfectly healthy; which patients with a particular diagnosis might be managed for different lengths of time in different settings; what physical findings or diagnostic tests best stratify prognosis; what new technologies are truly worth their cost; and especially, what therapies really work.
I do not dispute that hospital medicine researchers should try to improve the current use of interventions that are deemed to be valuable right now. But if that is all the field does, it will be a huge disappointment. Hospitalists should not be relegated to being adherence police who spend their collective research energy finding ways to force themselves to follow recommendations based on data gathered by others.
Hospital medicine researchers are uniquely positioned to discover new information that will change what should be done and help create the quality metrics for the future. Unless both of these two goalsimproving the implementation of today's knowledge and generating new and better knowledgeare part of the research agenda, we run the risk that some of the best minds in internal medicine may, when all is said and done, have spent an inordinate number of IQ hours on what, a century from now, will be reminiscent of improving the quality of bloodletting.
The practice of bloodletting, performed using sticks, thorns, bones, or anything sharp, probably began in Egypt about 3,000 years ago.[1] The practice continued in Greece, where Hippocrates recommended bloodletting to balance the body's four humorsblood, phlegm, yellow bile, and black bileand continued during Roman times under the influence of Galen. In the United States, perhaps the most infamous use of bloodletting was when doctors reportedly bled as much as 5 U of blood from George Washington before he died from what was probably either acute epiglottitis or streptococcal pharyngitis.[2, 3] Although many infectious organisms, especially malaria parasites, require iron to proliferateand therefore may be less virulent in iron‐deficient people[4]acute near‐exsanguination undoubtedly did more harm than good in the elderly ex‐president.
But the practice of bloodletting continued and even flourished. In 1833 alone, France reportedly imported more than 40 million leeches to assist in bloodletting,[5] which oftentimes was thought to be sufficiently aggressive only when the patient actually fainted. Enthusiasm for bloodletting declined in the second half of the 19th century, influenced in part by a nonrandomized study that compared mortality rates among patients who were bled early in their illness with those who were bled later.[6] Nevertheless, Sir William Osler still recommended small amounts of bloodletting for pneumonia in his last edition of his famous textbook, The Principles and Practice of Medicine, published in 1920.[7] By 1927, however, the first edition of the Cecil's A Textbook of Medicine thankfully no longer recommended venesection except to treat conditions such as pulmonary edema.[8]
Why would I start this essay with a history of bloodletting? Surely, one might argue, nothing could be less relevant to a modern discussion of the quality of in‐hospital medical care. The substantial literature on quality improvement emphasizes the practical implementation of strategies to increase the appropriate adherence to processes that are known to improve outcomes. A number of common quality measures quickly come to mind: the use of aspirin, ‐blockers, angiotensin‐converting enzyme inhibitors, and statins in post‐myocardial infarction patients without contraindications,[9, 10] the rapid initiation of appropriate antibiotics to patients with community‐acquired pneumonia,[11] and early endoscopy for patients with acute upper gastrointestinal hemorrhage.[12] I could go on and on, listing in‐hospital interventions supported by class 1 evidence from more than one definitive randomized trial. In essentially all of these situations, the creation of quality metrics, often accompanied by measurement and feedback, have improved adherence and undoubtedly saved lives. But although adherence has improved, the explosion in evidence‐based medicine means that even the best hospitals may be in perpetual catch‐up mode as they try to ensure adherence with the next wave of improvement interventions.
Unfortunately, every now and then a lot of attention is paid to meeting a quality metric that turns out to be misguided. Perhaps the best recent in‐hospital example was the metric of prophylactic ‐blocker use before major noncardiac surgery. Although this recommendation initially appeared to be based on reasonable data,[13] the large Perioperative Ischemic Evaluation Study (POISE) trial showed that reductions in rates of myocardial infarction were more than offset by an increased risk of stroke and other complications; therefore, average‐risk patients actually did worse, not better, with the ‐blocker regimen used in the trial. Although some have questioned whether these results were a function of the precise ‐blocker regimen that was used, the results of POISE are actually remarkably consistent with prior data on the risk of myocardial infarction and stroke.[14, 15] What was really different was the relative importance of these and other end points in patients whose risk of cardiac death was lower than those of higher risk patients in prior studies. But more recently, an even more disturbing reality has emerged: a number of key reports on which the guidelines were based came from an investigator whose publications included data that could not be confirmed when his studies were reviewed by his home institution.[16] Regardless of the precise reasons, we no longer routinely recommend an intervention that at one time was a key quality indicator.
The ‐blocker fiasco brings me back to bloodletting. In the early 19th century, a hypothetical visionary physician interested in quality improvement would likely have looked for ways to improve the efficiency and reduce the cost of bloodletting. Perhaps the leeches could be bigger, hungrier, or applied in a more effective fashion? Or perhaps vacuum tubes would have been invented sooner?
Of course, I am overemphasizing to prove a point. I truly believe that more and more of what we recommend is based on solid evidence to document, at least for now, that we are doing the right thing. If we do it more often and in more people, net benefit will be realized.
What does all this mean for the future of hospital medicine and its emerging research endeavors? For me at least, the message is pretty clear. First, we must be careful not to over extrapolate from limited studies in high risk patients, or we will jump to more conclusions like we did with ‐blockers. Second, most advances in medicine require new and better data.
How can new clinical data be generated most quickly and efficiently? One‐off studies at individual institutions are logistically and financially challenging, whereas an enduring research infrastructure is a treasure that can study a series of questions as they arise. The Thrombolysis in Myocardial Infarction Study Group has published scores of papers looking at a series of interventions in patients with acute myocardial infarction and the acute coronary syndrome.[17, 18] The Acute Respiratory Distress Syndrome Network has demonstrated the value of lower tidal volumes and less aggressive fluid strategies in patients with respiratory failure.[19, 20]
The success of these large, multicenter research networks should become the paradigm for the study of common hospital problems, ranging from the conditions that result in admission on the medical service to the problems that have engendered surgical comanagement services. New data can be gleaned by studying the medical care system, by studying routinely gathered administrative and clinical data, or even better yet, by gathering prospective data on patients and their diseases. For hospitalized patients, a variety of unanswered questions remain regarding the epidemiology of common diseases, the value of diagnostic tests, the impact of various therapies and treatment protocols, and the incremental value of new technologies, ranging from self‐monitoring to handheld ultrasound. High‐quality research may address the genetic epidemiology of why one person is admitted with pneumococcal pneumonia, whereas family members seem perfectly healthy; which patients with a particular diagnosis might be managed for different lengths of time in different settings; what physical findings or diagnostic tests best stratify prognosis; what new technologies are truly worth their cost; and especially, what therapies really work.
I do not dispute that hospital medicine researchers should try to improve the current use of interventions that are deemed to be valuable right now. But if that is all the field does, it will be a huge disappointment. Hospitalists should not be relegated to being adherence police who spend their collective research energy finding ways to force themselves to follow recommendations based on data gathered by others.
Hospital medicine researchers are uniquely positioned to discover new information that will change what should be done and help create the quality metrics for the future. Unless both of these two goalsimproving the implementation of today's knowledge and generating new and better knowledgeare part of the research agenda, we run the risk that some of the best minds in internal medicine may, when all is said and done, have spent an inordinate number of IQ hours on what, a century from now, will be reminiscent of improving the quality of bloodletting.
- . Bloodletting over the centuries. NY State J Med. 1980;80:2022–2028.
- . The death of George Washington: an end to the controversy? Am Surg. 2008;74:770–774.
- . The death of George Washington (1732–1799) and the history of cynanche. J Med Biogr. 2005;13:225–231.
- , . Hepcidin and the iron‐infection axis. Science. 2012;338:768–772.
- . Leech therapy: a history. J Hist Dent. 2005;53:25–27.
- . Pierre‐Charles‐Alexandre Louis and the evaluation of bloodletting. J R Soc Med. 2006;99:158–160.
- , . The Principles and Practice of Medicine. New York, NY: Appleton and Co.; 1920.
- Cecil RL, Kennedy F, eds. A Textbook of Medicine by American Authors. 1st ed. Philadelphia, PA: WB Saunders; 1927.
- Department of Health and Human Services. Hospital Compare: hospital process of care measures tables. Available at: http://www.hospitalcompare.hhs.gov/staticpages/for‐consumers/poc/explainations‐of‐measures.aspx. Accessed January 3, 2013.
- , , . Trends in the use of lipid‐lowering medications at discharge in patients with acute myocardial infarction: 1998 to 2006. Am Heart J. 2009;157:185–194.
- , , , et al. Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169:1525–1531.
- , , , et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113.
- , . β‐blockers and reduction of cardiac events in noncardiac surgery. JAMA. 2002;287:1445–1447.
- , , , et al; POISE Study Group. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847.
- , , , et al. Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis. Lancet. 2008;372:1962–1976.
- Report on the 2012 follow‐up investigation of possible breaches of academic integrity. Erasmus MC Follow‐up Investigation Committee, 2012. Available at: http://www.eramusmc.nl. . Accessed January 3, 2013.
- , , , et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19.
- , , , et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation. N Engl J Med. 2005;352:1179–1189.
- Acute Respiratory Distress Syndrome Network: ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network: comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575.
- . Bloodletting over the centuries. NY State J Med. 1980;80:2022–2028.
- . The death of George Washington: an end to the controversy? Am Surg. 2008;74:770–774.
- . The death of George Washington (1732–1799) and the history of cynanche. J Med Biogr. 2005;13:225–231.
- , . Hepcidin and the iron‐infection axis. Science. 2012;338:768–772.
- . Leech therapy: a history. J Hist Dent. 2005;53:25–27.
- . Pierre‐Charles‐Alexandre Louis and the evaluation of bloodletting. J R Soc Med. 2006;99:158–160.
- , . The Principles and Practice of Medicine. New York, NY: Appleton and Co.; 1920.
- Cecil RL, Kennedy F, eds. A Textbook of Medicine by American Authors. 1st ed. Philadelphia, PA: WB Saunders; 1927.
- Department of Health and Human Services. Hospital Compare: hospital process of care measures tables. Available at: http://www.hospitalcompare.hhs.gov/staticpages/for‐consumers/poc/explainations‐of‐measures.aspx. Accessed January 3, 2013.
- , , . Trends in the use of lipid‐lowering medications at discharge in patients with acute myocardial infarction: 1998 to 2006. Am Heart J. 2009;157:185–194.
- , , , et al. Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169:1525–1531.
- , , , et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113.
- , . β‐blockers and reduction of cardiac events in noncardiac surgery. JAMA. 2002;287:1445–1447.
- , , , et al; POISE Study Group. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847.
- , , , et al. Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis. Lancet. 2008;372:1962–1976.
- Report on the 2012 follow‐up investigation of possible breaches of academic integrity. Erasmus MC Follow‐up Investigation Committee, 2012. Available at: http://www.eramusmc.nl. . Accessed January 3, 2013.
- , , , et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19.
- , , , et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation. N Engl J Med. 2005;352:1179–1189.
- Acute Respiratory Distress Syndrome Network: ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308.
- The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network: comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575.