User login
Think outside the box─and outside your hospital─when planning your next hire
Hospitalists aren’t urban planners, but it doesn’t take a zoning expert to realize that when a community sees hundreds of new homes built, some of the residents of those homes will end up in the hospital. The same logic applies when a company moves thousands of jobs to an office building a few blocks away from a hospital.
HM group leaders might not normally think about such things when analyzing whether they need to add staff, but at least one practice consultant says they should.
Hospitals often have community data available, Hertz says, but group leaders don’t always think to access it. He suggests they view the information as a routine part of their strategic planning.
Of course, Hertz adds, it’s not the only information that goes into the expansion equation, but administrators often respect group leaders who come armed with data from inside and outside the hospital about why it is necessary to make a new hire.
“It’s about open, honest discussion,” he says. “It’s about looking at information both inside the four walls and outside in the community. It’s not easy, but it can be done. But you’ve got to plan.”
Hertz says HM group leaders should plan at least 12 to 18 months out for a hire, “which I know is hard these days,” he says. But, he adds, short-term forecasting makes it “very difficult” to know when and how best to grow your group. TH
Richard Quinn is a freelance writer in New Jersey.
Hospitalists aren’t urban planners, but it doesn’t take a zoning expert to realize that when a community sees hundreds of new homes built, some of the residents of those homes will end up in the hospital. The same logic applies when a company moves thousands of jobs to an office building a few blocks away from a hospital.
HM group leaders might not normally think about such things when analyzing whether they need to add staff, but at least one practice consultant says they should.
Hospitals often have community data available, Hertz says, but group leaders don’t always think to access it. He suggests they view the information as a routine part of their strategic planning.
Of course, Hertz adds, it’s not the only information that goes into the expansion equation, but administrators often respect group leaders who come armed with data from inside and outside the hospital about why it is necessary to make a new hire.
“It’s about open, honest discussion,” he says. “It’s about looking at information both inside the four walls and outside in the community. It’s not easy, but it can be done. But you’ve got to plan.”
Hertz says HM group leaders should plan at least 12 to 18 months out for a hire, “which I know is hard these days,” he says. But, he adds, short-term forecasting makes it “very difficult” to know when and how best to grow your group. TH
Richard Quinn is a freelance writer in New Jersey.
Hospitalists aren’t urban planners, but it doesn’t take a zoning expert to realize that when a community sees hundreds of new homes built, some of the residents of those homes will end up in the hospital. The same logic applies when a company moves thousands of jobs to an office building a few blocks away from a hospital.
HM group leaders might not normally think about such things when analyzing whether they need to add staff, but at least one practice consultant says they should.
Hospitals often have community data available, Hertz says, but group leaders don’t always think to access it. He suggests they view the information as a routine part of their strategic planning.
Of course, Hertz adds, it’s not the only information that goes into the expansion equation, but administrators often respect group leaders who come armed with data from inside and outside the hospital about why it is necessary to make a new hire.
“It’s about open, honest discussion,” he says. “It’s about looking at information both inside the four walls and outside in the community. It’s not easy, but it can be done. But you’ve got to plan.”
Hertz says HM group leaders should plan at least 12 to 18 months out for a hire, “which I know is hard these days,” he says. But, he adds, short-term forecasting makes it “very difficult” to know when and how best to grow your group. TH
Richard Quinn is a freelance writer in New Jersey.
Tighter rules for ad hoc PCI
The increased frequency in recent years of what has been termed "ad hoc" percutaneous coronary intervention is of concern to both interventional cardiologists and third-party payers.
The definition of ad hoc PCI that accompanied recent guidelines on that subject in a statement by the Society of Cardiovascular Angiography and Interventions (SCAI) is a "diagnostic catheterization followed in the same session or same sitting by PCI." Much of this increase has occurred in patients without symptoms and with minimal if any evidence of ischemia. Convenience and economics also play a role. As a result, cardiologists presume that they can do no harm without asking the question of whether they are doing any good.
A recent report on 144,737 nonacute PCIs using the National Cardiovascular Data Registry indicated that almost 30,000 PCIs (24.4%) were performed in patients without symptoms or class I angina and 30% were at low risk by noninvasive testing. In these nonacute patients, 67% were considered either inappropriate or uncertain (JAMA 2011;306:53-61). The rate of performing inappropriate PCI in hospitals varied between 6% and 16%. A number of hospitals had inappropriate rates exceeding 25%, and some had rates as high as 48%. The registry does not provide the number of ad hoc procedures performed, but one might presume that many of these patients would have fit the criteria for entry into the COURAGE trial (N. Engl. J. Med. 2007;356:1503-16), in which patients with stable coronary disease, 43% of whom had either no angina or class I angina, did as well with medical treatment as with PCI.
Angiographers have admitted having difficulty assessing the severity of stenosis, and therefore often proceeding to ad hoc PCI. The recent FAME study suggests that the measure of fractional flow reserve (FFR) is able to define coronary lesions that are clinically significant (N. Engl. J. Med. 2009;360;213-24). However, the conclusions of FAME have been challenged in regard to the clinical importance of FFR measurement.
Included in the recent SCAI guidelines is the requirement that before ad hoc PCI is performed, patients should be given information about the appropriateness, relative risk, and benefit of the procedure as well as therapeutic alternatives to PCI. For patients with ongoing symptoms and positive diagnostic tests for ischemia, this is easily obtained prior to intervention. But patients without symptoms and without evidence by stress testing may not be given the real story before the procedure. For these patients, SCAI advises that "time-out" be called and that they be given time to consider the alternatives for treatment of their disease (Catheter. Cardiovasc. Interv. 2012 Nov. 29 [doi: 10.1002/ccd.24701]).
Unfortunately for all of us, the federal government is also concerned about the issue of appropriateness. A recent whistleblower lawsuit in Ohio was resolved with a payment of fines of $3 million by the hospital and more than $500,000 by the physician group involved in the lawsuit. According to press reports, the physicians defended their "high rates as a result of their aggressive style of medicine." The physicians defended the medical care that they provided although they "might not have met the government’s guidelines of reimbursement" (New York Times, Jan. 5, 2013, sec. B1).
Unless we adhere to good practice guidelines, the federal government will force our adherence, whether we like it or not.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies. This column, "Heart of the Matter," appears regularly in Cardiology News.
The increased frequency in recent years of what has been termed "ad hoc" percutaneous coronary intervention is of concern to both interventional cardiologists and third-party payers.
The definition of ad hoc PCI that accompanied recent guidelines on that subject in a statement by the Society of Cardiovascular Angiography and Interventions (SCAI) is a "diagnostic catheterization followed in the same session or same sitting by PCI." Much of this increase has occurred in patients without symptoms and with minimal if any evidence of ischemia. Convenience and economics also play a role. As a result, cardiologists presume that they can do no harm without asking the question of whether they are doing any good.
A recent report on 144,737 nonacute PCIs using the National Cardiovascular Data Registry indicated that almost 30,000 PCIs (24.4%) were performed in patients without symptoms or class I angina and 30% were at low risk by noninvasive testing. In these nonacute patients, 67% were considered either inappropriate or uncertain (JAMA 2011;306:53-61). The rate of performing inappropriate PCI in hospitals varied between 6% and 16%. A number of hospitals had inappropriate rates exceeding 25%, and some had rates as high as 48%. The registry does not provide the number of ad hoc procedures performed, but one might presume that many of these patients would have fit the criteria for entry into the COURAGE trial (N. Engl. J. Med. 2007;356:1503-16), in which patients with stable coronary disease, 43% of whom had either no angina or class I angina, did as well with medical treatment as with PCI.
Angiographers have admitted having difficulty assessing the severity of stenosis, and therefore often proceeding to ad hoc PCI. The recent FAME study suggests that the measure of fractional flow reserve (FFR) is able to define coronary lesions that are clinically significant (N. Engl. J. Med. 2009;360;213-24). However, the conclusions of FAME have been challenged in regard to the clinical importance of FFR measurement.
Included in the recent SCAI guidelines is the requirement that before ad hoc PCI is performed, patients should be given information about the appropriateness, relative risk, and benefit of the procedure as well as therapeutic alternatives to PCI. For patients with ongoing symptoms and positive diagnostic tests for ischemia, this is easily obtained prior to intervention. But patients without symptoms and without evidence by stress testing may not be given the real story before the procedure. For these patients, SCAI advises that "time-out" be called and that they be given time to consider the alternatives for treatment of their disease (Catheter. Cardiovasc. Interv. 2012 Nov. 29 [doi: 10.1002/ccd.24701]).
Unfortunately for all of us, the federal government is also concerned about the issue of appropriateness. A recent whistleblower lawsuit in Ohio was resolved with a payment of fines of $3 million by the hospital and more than $500,000 by the physician group involved in the lawsuit. According to press reports, the physicians defended their "high rates as a result of their aggressive style of medicine." The physicians defended the medical care that they provided although they "might not have met the government’s guidelines of reimbursement" (New York Times, Jan. 5, 2013, sec. B1).
Unless we adhere to good practice guidelines, the federal government will force our adherence, whether we like it or not.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies. This column, "Heart of the Matter," appears regularly in Cardiology News.
The increased frequency in recent years of what has been termed "ad hoc" percutaneous coronary intervention is of concern to both interventional cardiologists and third-party payers.
The definition of ad hoc PCI that accompanied recent guidelines on that subject in a statement by the Society of Cardiovascular Angiography and Interventions (SCAI) is a "diagnostic catheterization followed in the same session or same sitting by PCI." Much of this increase has occurred in patients without symptoms and with minimal if any evidence of ischemia. Convenience and economics also play a role. As a result, cardiologists presume that they can do no harm without asking the question of whether they are doing any good.
A recent report on 144,737 nonacute PCIs using the National Cardiovascular Data Registry indicated that almost 30,000 PCIs (24.4%) were performed in patients without symptoms or class I angina and 30% were at low risk by noninvasive testing. In these nonacute patients, 67% were considered either inappropriate or uncertain (JAMA 2011;306:53-61). The rate of performing inappropriate PCI in hospitals varied between 6% and 16%. A number of hospitals had inappropriate rates exceeding 25%, and some had rates as high as 48%. The registry does not provide the number of ad hoc procedures performed, but one might presume that many of these patients would have fit the criteria for entry into the COURAGE trial (N. Engl. J. Med. 2007;356:1503-16), in which patients with stable coronary disease, 43% of whom had either no angina or class I angina, did as well with medical treatment as with PCI.
Angiographers have admitted having difficulty assessing the severity of stenosis, and therefore often proceeding to ad hoc PCI. The recent FAME study suggests that the measure of fractional flow reserve (FFR) is able to define coronary lesions that are clinically significant (N. Engl. J. Med. 2009;360;213-24). However, the conclusions of FAME have been challenged in regard to the clinical importance of FFR measurement.
Included in the recent SCAI guidelines is the requirement that before ad hoc PCI is performed, patients should be given information about the appropriateness, relative risk, and benefit of the procedure as well as therapeutic alternatives to PCI. For patients with ongoing symptoms and positive diagnostic tests for ischemia, this is easily obtained prior to intervention. But patients without symptoms and without evidence by stress testing may not be given the real story before the procedure. For these patients, SCAI advises that "time-out" be called and that they be given time to consider the alternatives for treatment of their disease (Catheter. Cardiovasc. Interv. 2012 Nov. 29 [doi: 10.1002/ccd.24701]).
Unfortunately for all of us, the federal government is also concerned about the issue of appropriateness. A recent whistleblower lawsuit in Ohio was resolved with a payment of fines of $3 million by the hospital and more than $500,000 by the physician group involved in the lawsuit. According to press reports, the physicians defended their "high rates as a result of their aggressive style of medicine." The physicians defended the medical care that they provided although they "might not have met the government’s guidelines of reimbursement" (New York Times, Jan. 5, 2013, sec. B1).
Unless we adhere to good practice guidelines, the federal government will force our adherence, whether we like it or not.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies. This column, "Heart of the Matter," appears regularly in Cardiology News.
Patient empowerment: A coming of age story
The February 2013 issue of Health Affairs explores a surprisingly underutilized concept in health care that, until recently, has essentially been ignored – patient empowerment.
For some, this term may conjure up unpleasant memories of annoying encounters in which demanding patients (and family members) tried to dictate their own hospital course. Yet others may recall how some well-informed patients have helped them significantly expedite, as well as optimize, the care they provided.
In an article titled "What the Evidence Shows about Patient Activation: Better Health Outcomes and Care Experiences; Fewer Data on Costs," the authors define patient activation as "the skills and confidence that equip patients to become actively engaged in their health care." The authors note that patients who are less "activated" are three times as likely to have their medical needs go unmet and twice as likely to delay medical care, when compared to patients who are more engaged. On the other hand, highly activated patients were found to be at least twice as likely to prepare questions for their doctors and seek out health information, including the quality of health care providers.
In another article in the same issue, "Rx for the ‘Blockbuster Drug’ of Patient Engagement," Susan Denzter noted that evidence is emerging that patients who are actively involved in their medical care have better outcomes and lower medical bills compared with those who are not.
The medical community is finally embracing this crucial issue. We have always known that well-informed patients can bolster their own health care – and make our lives much easier as well. But it seems that in our historically paternalistic health care system, doctors tightly held onto the reins and patients, patients blindly complied (or so we thought).
In 2000, I published "Your Family Medical Record: An Interactive Guide to Getting the Best Care," a book designed to address the tremendous void between how patients think and how we, their doctors, think. At that time, Americans had not yet grasped the importance of patient engagement, and my book is no longer in print. I was a doctor desperately trying to introduce the concept of patient engagement to the American public. At the time, I had high hopes of bridging important gaps by teaching patients easy-to-understand concepts about keeping and understanding their own health records and expediting their own care through applying basic "patient skills," such as how to prepare for visits in advance and how to think through their symptoms in a methodical, concise manner. Thirteen years later, I am thrilled to see others succeeding where I did not, for this concept is far too important to sweep under the carpet.
In the burgeoning age of the Affordable Care Act, physicians are challenged to seek innovative cost-effective new means by which we can optimize the medical care we provide. If we teach our patients a patient skill or two when time allows, we can play an important role in this important paradigm shift in the American health care system that over time will, undoubtedly, help lower health care costs and improve patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. This blog, "Teachable Moments," appears regularly in Hospitalist News.
The February 2013 issue of Health Affairs explores a surprisingly underutilized concept in health care that, until recently, has essentially been ignored – patient empowerment.
For some, this term may conjure up unpleasant memories of annoying encounters in which demanding patients (and family members) tried to dictate their own hospital course. Yet others may recall how some well-informed patients have helped them significantly expedite, as well as optimize, the care they provided.
In an article titled "What the Evidence Shows about Patient Activation: Better Health Outcomes and Care Experiences; Fewer Data on Costs," the authors define patient activation as "the skills and confidence that equip patients to become actively engaged in their health care." The authors note that patients who are less "activated" are three times as likely to have their medical needs go unmet and twice as likely to delay medical care, when compared to patients who are more engaged. On the other hand, highly activated patients were found to be at least twice as likely to prepare questions for their doctors and seek out health information, including the quality of health care providers.
In another article in the same issue, "Rx for the ‘Blockbuster Drug’ of Patient Engagement," Susan Denzter noted that evidence is emerging that patients who are actively involved in their medical care have better outcomes and lower medical bills compared with those who are not.
The medical community is finally embracing this crucial issue. We have always known that well-informed patients can bolster their own health care – and make our lives much easier as well. But it seems that in our historically paternalistic health care system, doctors tightly held onto the reins and patients, patients blindly complied (or so we thought).
In 2000, I published "Your Family Medical Record: An Interactive Guide to Getting the Best Care," a book designed to address the tremendous void between how patients think and how we, their doctors, think. At that time, Americans had not yet grasped the importance of patient engagement, and my book is no longer in print. I was a doctor desperately trying to introduce the concept of patient engagement to the American public. At the time, I had high hopes of bridging important gaps by teaching patients easy-to-understand concepts about keeping and understanding their own health records and expediting their own care through applying basic "patient skills," such as how to prepare for visits in advance and how to think through their symptoms in a methodical, concise manner. Thirteen years later, I am thrilled to see others succeeding where I did not, for this concept is far too important to sweep under the carpet.
In the burgeoning age of the Affordable Care Act, physicians are challenged to seek innovative cost-effective new means by which we can optimize the medical care we provide. If we teach our patients a patient skill or two when time allows, we can play an important role in this important paradigm shift in the American health care system that over time will, undoubtedly, help lower health care costs and improve patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. This blog, "Teachable Moments," appears regularly in Hospitalist News.
The February 2013 issue of Health Affairs explores a surprisingly underutilized concept in health care that, until recently, has essentially been ignored – patient empowerment.
For some, this term may conjure up unpleasant memories of annoying encounters in which demanding patients (and family members) tried to dictate their own hospital course. Yet others may recall how some well-informed patients have helped them significantly expedite, as well as optimize, the care they provided.
In an article titled "What the Evidence Shows about Patient Activation: Better Health Outcomes and Care Experiences; Fewer Data on Costs," the authors define patient activation as "the skills and confidence that equip patients to become actively engaged in their health care." The authors note that patients who are less "activated" are three times as likely to have their medical needs go unmet and twice as likely to delay medical care, when compared to patients who are more engaged. On the other hand, highly activated patients were found to be at least twice as likely to prepare questions for their doctors and seek out health information, including the quality of health care providers.
In another article in the same issue, "Rx for the ‘Blockbuster Drug’ of Patient Engagement," Susan Denzter noted that evidence is emerging that patients who are actively involved in their medical care have better outcomes and lower medical bills compared with those who are not.
The medical community is finally embracing this crucial issue. We have always known that well-informed patients can bolster their own health care – and make our lives much easier as well. But it seems that in our historically paternalistic health care system, doctors tightly held onto the reins and patients, patients blindly complied (or so we thought).
In 2000, I published "Your Family Medical Record: An Interactive Guide to Getting the Best Care," a book designed to address the tremendous void between how patients think and how we, their doctors, think. At that time, Americans had not yet grasped the importance of patient engagement, and my book is no longer in print. I was a doctor desperately trying to introduce the concept of patient engagement to the American public. At the time, I had high hopes of bridging important gaps by teaching patients easy-to-understand concepts about keeping and understanding their own health records and expediting their own care through applying basic "patient skills," such as how to prepare for visits in advance and how to think through their symptoms in a methodical, concise manner. Thirteen years later, I am thrilled to see others succeeding where I did not, for this concept is far too important to sweep under the carpet.
In the burgeoning age of the Affordable Care Act, physicians are challenged to seek innovative cost-effective new means by which we can optimize the medical care we provide. If we teach our patients a patient skill or two when time allows, we can play an important role in this important paradigm shift in the American health care system that over time will, undoubtedly, help lower health care costs and improve patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center, Glen Burnie, Md., who has a passion for empowering patients to partner in their health care. This blog, "Teachable Moments," appears regularly in Hospitalist News.
Obesity, diabetes fuel liver disease epidemic
Many physicians do not consider liver disease and liver cancer classic complications of obesity, type 2 diabetes, or metabolic syndrome, but they should.
Research findings over the past decade offer substantial evidence for links between obesity, diabetes, or metabolic syndrome and the earliest hepatic manifestation of these derangements: nonalcoholic fatty liver disease (NAFLD). Equally compelling links tie obesity, diabetes, and metabolic syndrome to more advanced liver pathology: nonalcoholic steatohepatitis (NASH), cirrhosis, and liver cancer, especially hepatocellular carcinoma (HCC).
Although the link between obesity, diabetes, or metabolic syndrome and NASH or liver cancer is not yet strong enough to justify major changes in disease surveillance or management, the link between these metabolic disorders and NAFLD is powerful and common enough to warrant routinely considering these patients as having NAFLD, say experts. And if NAFLD is found, the next step is deciding if a patient is the right candidate for NASH or cirrhosis assessment; and if those disorders develop, cancer screening follows.
A new dimension of obesity and diabetes morbidity
"For decades, attention to the patient with type 2 diabetes focused on the control of hyperglycemia and of risk factors associated with macrovascular disease. The epidemic of obesity now presents endocrinologists with new challenges. Among them is the need to identify early complications related to obesity in the setting of type 2 diabetes. NAFLD is a common complication of patients with type 2 diabetes that ... does not fit into the traditional realm of diabetic complications," Dr. Romina Lomonaco and Dr. Kenneth Cusi wrote in a recently published book chapter ("Evidence-based Management of Diabetes," chapter 21; TFM Publishing, 2012).
Not until recently has NAFLD been recognized as another common complication of patients with type 2 diabetes that requires special attention. NAFLD’s low profile as a complication of obesity and diabetes contrasts with its ubiquity. About 70% of patients with type 2 diabetes have NAFLD (compared with about 20% of all American adults), and perhaps up to 90% of morbidly obese patients have NAFLD. The prevalence of impaired fasting glucose and of newly diagnosed type 2 diabetes is about threefold higher in patients with NAFLD than in those without liver disease.
"Insulin resistance and obesity are probably the biggest factors" causing NAFLD, said Dr. Cusi, professor of medicine and chief of adult endocrinology, diabetes, and metabolism at the University of Florida in Gainesville. Moreover, "diabetes will worsen NAFLD, producing more fibrosis and an increased rate of cirrhosis," he said in an interview.
That’s significant because it is NAFLD progression that poses the biggest risk. NAFLD severity can range from mild, early-stage disease in an asymptomatic patient with normal liver enzyme levels to the development of inflammation –NASH – which can cause liver injury and fibrosis, lead to cirrhosis, and set up progression to organ failure or development of HCC or other liver cancer.
Overall, about 40% of patients with NAFLD progress to NASH, but both obesity and diabetes ratchet up NAFLD progression, and so roughly half of all patients with diabetes have NASH. Patients with type 2 diabetes also have a two- to fourfold increased risk of developing advanced liver disease, cirrhosis, and HCC compared with people without diabetes. "About 15% of NASH patients develop cirrhosis, and a significant percent also develop cancer," Dr. Cusi said.
"NASH represents the hepatic manifestation of the metabolic syndrome, a constellation of abdominal obesity, hypertension, diabetes, and dyslipidemia. It is projected that 25 million Americans will develop NASH by 2025, with 20% progressing to cirrhosis, hepatocellular carcinoma, or both, that may require liver transplantation," wrote Dr. Vatche G. Agopian and his associates from the Dumont-University of California, Los Angeles (UCLA), Transplant and Liver Cancer Center in a recent report (Ann. Surg. 2012;256:624-33).
From 2001 to 2009, the nationwide frequency of NASH as the primary indication for liver transplantation rose from 1% to 10%, with NASH becoming the third most common U.S. indication for liver transplantation (Gastroenterology 2011;141:1249-53). The UCLA surgeons reviewed their experience with 1,294 patients who underwent primary liver transplantation at their center between January 2002 and August 2011, and found 136 patients (11%) who met NASH criteria. But during the 10-year period studied, NASH as the trigger for liver transplant soared from 3% of transplants in 2002 to 19% in 2011, a jump that by 2011 made NASH the second most common cause for liver transplant at UCLA, trailing only hepatitis C virus. In fact, NASH "is poised to surpass hepatitis C as the leading indication in the next 10-20 years," wrote Dr. Agopian, a liver surgeon, and Dr. Busuttil, professor and chief of liver and pancreatic transplantation at UCLA, and their associates (Ann. Surg. 2012;256:624-33).
In their report, Dr. Agopian and Dr. Busuttil called the current surge in liver transplants for patients with NASH "the new epidemic."
"The future of [liver] transplantation is here with these patients who have nonalcoholic steatohepatitis and subsequent cirrhosis," commented Dr. John P. Roberts, professor and chief of transplant surgery at the University of California, San Francisco. "Currently, there are about 6,000 [liver] transplants [per year] in the United States. Half of those are done for hepatitis C. In the overall population of the United States, 1.3% have hepatitis C, and that provides about half of liver transplant patients. Twelve percent of the U.S. population have nonalcoholic steatohepatitis, a 10-fold increase over the percentage of the population with hepatitis C. Due to the kinetics of the hepatitis C epidemic, we are going to see a falloff in the number of patients with hepatitis C eligible for transplantation in the next 10 years. [Patients with NASH] are going to replace them, potentially by 10-fold," said Dr. Roberts, who commented on the report by Dr. Agopian and Dr. Busuttil on the UCLA experience during the 2012 annual meeting of the American Surgical Association in San Francisco.
The NAFLD, NASH, and HCC connections
"The link between obesity, NASH, cirrhosis, and HCC is very strong" said Dr. Stephen H. Caldwell, professor of medicine and director of hepatology at the University of Virginia in Charlottesville.
"What remains unknown is whether NASH and hepatic scar formation are essential to cause cancer, or can carcinomas arise in a noncirrhotic, non-NASH fatty liver? Scar formation itself is a carcinogenic process, especially when it progresses to stage 3 – bridging fibrosis – or to stage 4," when cirrhosis occurs.
"It’s difficult to justify screening all patients with a fatty liver; that would be a huge undertaking," Dr. Caldwell said in an interview. "The more important clinical message is to consider whether a patient has NASH, but that is hard to diagnose without a liver biopsy."
So far, no markers have been unquestionably accurate for diagnosing NASH. Any patient who is obese or has metabolic syndrome should be considered for NASH, said Dr. Caldwell. Signs of more advanced liver injury include cirrhosis or portal hypertension. Other, more subtle signs include spider angiomas, reddening of the palms, declining platelet counts, or a family history of liver disease. Any of these could be a reason to look for NASH, he said.
Last year, guidelines issued by the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology, and the American Gastroenterological Association recommended against routinely testing for NAFLD, even among patients in diabetes or obesity clinics. Evidence was lacking for routine screening, even of high-risk patients, the guidelines said, with no data on cost effectiveness and uncertainties about diagnostic tests and treatment options (Hepatology 2012;55:2005-32).
But the guidelines do call for targeted assessment of NAFLD, and targeting NASH workups for selected NAFLD patients. The guidelines recommend ruling out all other possible etiologies and establishing NAFLD by histology or imaging. When a patient is diagnosed with NAFLD, the guidelines say that "as the metabolic syndrome predicts the presence of steatohepatitis in patients with NAFLD, its presence can be used to target patients for liver biopsy." The 2012 guidelines also highlighted the NAFLD Fibrosis Score (Hepatology 2007;45:846-54) as another useful tool to identify NAFLD patients at increased risk for NASH or cirrhosis. The guidelines called the plasma biomarker cytokeretin-18 "promising," but cautioned that it was "premature to recommend in routine clinical practice."
Major issues for patients who develop NASH are their risk for cirrhosis and liver failure, as well as that for liver cancer. Although the case already exists for obesity, diabetes, and metabolic syndrome as factors leading to NAFLD and NASH, evidence also links each of these three conditions to an increased rate of HCC and other liver cancer, such as cholangiocarcinoma.
"The evidence supports both an independent role for obesity, insulin resistance, and diabetes, as well as boosting the risk from other major risk factors such as hepatitis. The missing evidence is it has not been shown that treatment of diabetes or weight loss can reduce the risk of liver cancer," said Dr. Hashem B. El-Serag, professor and chief of gastroenterology and hepatology at the Baylor College of Medicine in Houston. "Screening for fatty liver by liver enzymes and ultrasound is probably a prudent first step" for obese or insulin-resistant patients, noted Dr. El-Serag. But surveillance for HCC by twice-annual ultrasound exams is only for patients with demonstrated advanced fibrosis or cirrhosis, he said in an interview.
"We currently recommend that anyone with NAFLD cirrhosis or cirrhosis of unknown etiology who is also obese or had diabetes should receive routine HCC surveillance," said Dr. György Baffy, chief of gastroenterology at the VA Boston Healthcare System. He predicts that "we may soon reach a general conclusion that people with morbid obesity (a body mass index of greater that 40 kg/m2) and poorly controlled diabetes should be considered for liver cancer surveillance even without clear evidence for cirrhosis," he said in an interview. But in general, "HCC occurrence in noncirrhotic liver is so low that surveillance would be rather inefficient."
Despite that, Dr. Baffy admits that the connection between diabetes and HCC may go beyond cirrhosis. "Up to half of all HCC may develop in noncirrhotic livers," he wrote in a recent editorial (Am. J. Gastroenterol. 2012;107:53-5). "It is more difficult to determine the need for HCC surveillance in diabetic patients with noncirrhotic liver or with no established liver disease."
To avoid missing a diagnosis of HCC, Dr. Baffy suggested awareness of the risk factors for advanced background liver disease and for HCC in patients with diabetes: male sex, older age, morbid obesity, poorly controlled and long-standing disease, and coexisting hepatitis C.
"For now, cirrhosis remains the primary indication for implementing HCC surveillance," but the new findings on liver cancer developing in liver disease associated with obesity and diabetes so far provide insufficient evidence to warrant any firm screening recommendations for these patients, Dr. Baffy wrote in another recent article along with Dr. Caldwell and Dr. Elizabeth M. Brunt (J. Hepatology 2012;56:1384-91). "The greater dilemma comes from new evidence that HCC may complicate NAFLD when fibrosis is mild or absent. Observations that diabetes may increase the risk of HCC regardless of the presence of cirrhosis remain a major concern for the 26 million Americans estimated to have diabetes or prediabetes," they wrote. "We may need to contemplate a paradigm shift in liver cancer surveillance, but for now at least, HCC appears to be a rare complication of NAFLD in the complete absence of fibrosis."
In addition, the value of regular cancer surveillance, even in patients with cirrhosis, remains uncertain, just as surveillance for breast cancer and prostate cancer has come under similar criticism. "It gets a little shaky when you look for evidence that [HCC] surveillance led to prolonged survival," said Dr. Caldwell. "You have all the same controversy as breast cancer, but surveillance is even less established for HCC."
Diabetes also linked to HCC spread
Once hepatocellular carcinoma forms in a patient with diabetes, the cancer may act more aggressively, according to studies from the University of Rochester (N.Y.).
A review of 265 consecutive patients treated for hepatocellular carcinoma (HCC) at Rochester’s Wilmot Cancer Center identified 91 (34%) with diabetes at the time of HCC diagnosis. Forty-seven of the 265 patients (18%) had distant metastases at the time of diagnosis. A multivariate analysis that controlled for age and etiologic risk factors showed that patients with diabetes were 10 times more likely to have distant metastases at the time of HCC diagnosis, compared with patients without diabetes, Dr. Aram F. Hezel and his associates reported last year (Cancer Investigation 2012;30:698-702). The analysis showed no statistically significant impact of diabetes on survival rate.
In a second analysis they found that patients with newly diagnosed HCC and diabetes were also significantly more likely to have macrovascular invasion by the HCC.
"We don’t treat patients with HCC differently if they have diabetes or obesity, but our findings show an association between diabetes and greater spread of HCC, more invasive cancer," said Dr. Hezel, an oncologist and director of hepatic and pancreatic cancer research at the Wilmot Cancer Center of the University of Rochester (N.Y.). "We don’t know whether we can treat the diabetes and change the behavior of the cancer by having patients under better control. Are cancers different in patients with diabetes or obesity? Do some metabolic states help push a cancer to more invasive behavior?" he asked in an interview.
"We use liver transplant to treat patients with liver cancer. In early stages of liver cancer the tumor is less likely to spread, so liver transplant can be curative. But if there are patients with a greater propensity for cancer spread at an earlier stage" then the efficacy of transplantation needs reassessment, Dr. Hezel said.
Few proven treatments for NAFLD, NASH, and to prevent HCC
Although diagnosing NAFLD is an important step in identifying patients at risk for NASH, cirrhosis, and liver cancer, interventions with proven benefits for NAFLD are limited. No approved drug treatments exist for NAFLD; lifestyle modification is the standard treatment to reduce steatosis and plasma levels of liver aminotransferases. Reductions in liver fat correlate closely with weight loss, Dr. Cusi, Dr. Lomonaco, and their associates said in a recently published analysis of NAFLD (Drugs 2013; Jan. 11 [Epub ahead of print]). A weight loss of 7%-10% has been linked with a roughly 50% drop in liver fat levels in NAFLD patients, they said. But long-term controlled studies are needed to better assess the impact of lifestyle changes on NAFLD and fatty livers.
Pioglitazone received endorsement from the AASLD panel for treating NASH in their 2012 NAFLD management recommendations. The recommendations cautioned that most NASH patients who received pioglitazone treatment in trials did not have diabetes, and that long-term safety and efficacy of pioglitazone in NASH patients are not established.
The AASLD guidelines also call for using vitamin E at a daily dosage of 800 IU, but only for patients with biopsy-proven NASH and no diabetes; the guidelines call it "first line" in this setting. But the guidelines also specifically caution against using vitamin E in patients with NASH and diabetes, patients with NAFLD who have not undergone a liver biopsy, patients with NASH and cirrhosis, and those with cryptogenic cirrhosis. The guidelines also caution against using metformin to treat NASH. No other drug intervention gets guideline endorsement for treating NASH.
"You can say diet and exercise minimize the risk of fatty liver, but beyond that drug therapy is unclear," said Dr. Caldwell. "I think as we see treatment evolve, we’ll see more interest [in treating NAFLD and NASH] by endocrinologists," he predicted.
The intervention picture changes when the goal is preventing liver cancer. "Effective treatment of insulin resistance and hyperinsulinemia may be critical to prevent hepatocarcinogenesis," wrote Dr. Baffy, Dr. Brunt, and Dr. Caldwell in their recent review (J. Hepatology 2012;56:1384-91). "Insulin sensitizing agents in diabetes may reduce the risk of HCC." They especially cited the epidemiologic evidence supporting a role for thiazolidinediones, which were linked to a 70% reduction in HCC incidence among patients with diabetes compared with patients treated with insulin or a sulfonylurea in a case-control study (Cancer 2010;116:1938-46). The same study also showed a similar, 70% reduction in HCC among patients treated with a biguanide like metformin.
"While current guidelines for the management of HCC have no specific recommendations for cases associated with NAFLD, obesity, and diabetes, the use of insulin-sensitizing drugs and avoidance of treatments that contribute to hyperinsulinemia are likely to enhance prevention and improve disease outcomes of HCC," said Dr. Baffy, Dr. Brunt, and Dr. Caldwell.
Similar evidence recently came from other epidemiologic studies that suggest damping down of HCC development in patients treated with a thiazolidinedione or metformin. A report last year that analyzed health records of about 98,000 Taiwan residents found that treatment with a thiazolidinedione or with metformin reduced the rate of HCC in patients with diabetes by about 50% compared with other treatments (Am. J. Gastroenterol. 2012;107:46-52). More evidence supporting protection from metformin against formation of both HCC and a second, less common type of liver cancer, intrahepatic cholangiocarcinoma, came in two studies reported last May at the annual Digestive Disease Week in San Diego.
"Metformin has not proved useful in the therapy of NAFLD, but it is helpful in decreasing the risk of HCC in patients with obesity- or diabetes-associated liver disease. Metformin should be part of antidiabetic management whenever possible," Dr. Baffy said in an interview.
But other experts regard the evidence accumulated so far as too preliminary to guide management. "It is premature to recommend using [metformin or a thiazolidinedione] for the primary reason of HCC prevention," said Dr. El-Serag.
"I don’t think the evidence is convincing at this point" regarding preventing HCC, said Dr. Caldwell. "The thiazolidinediones seem to retard progression of NASH fibrosis, but they also have adverse effects and their popularity has decreased."
Early days for a complex pathology
It seems as if the links between obesity, diabetes, and metabolic syndrome and NAFLD, NASH, and liver cancer are so tangled that it will take more time to fully resolve the etiologic relationships and the implications for diagnosis and management. The bottom line today is that a growing segment of American adults face risks for significant liver disease because of obesity, type 2 diabetes, and other elements of the metabolic syndrome.
"We see more and more patients over the last decade with liver cancer who didn’t have hepatitis or alcohol use but have diabetes and obesity. It’s a changing demographic," said Dr. Hezel. "We increasingly see liver cancer in patients without one of the classic risk factors. There are two possible mechanisms. Fibrosis and inflammation" caused by NAFLD and NASH trigger cancer formation and growth, "or it could be a more direct effect of high insulin levels or other hormonal effects. This is an emerging area; it follows on the epidemic of obesity and diabetes."
Dr. Cusi, Dr. Caldwell, Dr. Baffy, Dr. El-Serag, Dr. Busuttil, and Dr. Hezel all said that they had no relevant disclosures.
On Twitter @mitchelzoler
Many physicians do not consider liver disease and liver cancer classic complications of obesity, type 2 diabetes, or metabolic syndrome, but they should.
Research findings over the past decade offer substantial evidence for links between obesity, diabetes, or metabolic syndrome and the earliest hepatic manifestation of these derangements: nonalcoholic fatty liver disease (NAFLD). Equally compelling links tie obesity, diabetes, and metabolic syndrome to more advanced liver pathology: nonalcoholic steatohepatitis (NASH), cirrhosis, and liver cancer, especially hepatocellular carcinoma (HCC).
Although the link between obesity, diabetes, or metabolic syndrome and NASH or liver cancer is not yet strong enough to justify major changes in disease surveillance or management, the link between these metabolic disorders and NAFLD is powerful and common enough to warrant routinely considering these patients as having NAFLD, say experts. And if NAFLD is found, the next step is deciding if a patient is the right candidate for NASH or cirrhosis assessment; and if those disorders develop, cancer screening follows.
A new dimension of obesity and diabetes morbidity
"For decades, attention to the patient with type 2 diabetes focused on the control of hyperglycemia and of risk factors associated with macrovascular disease. The epidemic of obesity now presents endocrinologists with new challenges. Among them is the need to identify early complications related to obesity in the setting of type 2 diabetes. NAFLD is a common complication of patients with type 2 diabetes that ... does not fit into the traditional realm of diabetic complications," Dr. Romina Lomonaco and Dr. Kenneth Cusi wrote in a recently published book chapter ("Evidence-based Management of Diabetes," chapter 21; TFM Publishing, 2012).
Not until recently has NAFLD been recognized as another common complication of patients with type 2 diabetes that requires special attention. NAFLD’s low profile as a complication of obesity and diabetes contrasts with its ubiquity. About 70% of patients with type 2 diabetes have NAFLD (compared with about 20% of all American adults), and perhaps up to 90% of morbidly obese patients have NAFLD. The prevalence of impaired fasting glucose and of newly diagnosed type 2 diabetes is about threefold higher in patients with NAFLD than in those without liver disease.
"Insulin resistance and obesity are probably the biggest factors" causing NAFLD, said Dr. Cusi, professor of medicine and chief of adult endocrinology, diabetes, and metabolism at the University of Florida in Gainesville. Moreover, "diabetes will worsen NAFLD, producing more fibrosis and an increased rate of cirrhosis," he said in an interview.
That’s significant because it is NAFLD progression that poses the biggest risk. NAFLD severity can range from mild, early-stage disease in an asymptomatic patient with normal liver enzyme levels to the development of inflammation –NASH – which can cause liver injury and fibrosis, lead to cirrhosis, and set up progression to organ failure or development of HCC or other liver cancer.
Overall, about 40% of patients with NAFLD progress to NASH, but both obesity and diabetes ratchet up NAFLD progression, and so roughly half of all patients with diabetes have NASH. Patients with type 2 diabetes also have a two- to fourfold increased risk of developing advanced liver disease, cirrhosis, and HCC compared with people without diabetes. "About 15% of NASH patients develop cirrhosis, and a significant percent also develop cancer," Dr. Cusi said.
"NASH represents the hepatic manifestation of the metabolic syndrome, a constellation of abdominal obesity, hypertension, diabetes, and dyslipidemia. It is projected that 25 million Americans will develop NASH by 2025, with 20% progressing to cirrhosis, hepatocellular carcinoma, or both, that may require liver transplantation," wrote Dr. Vatche G. Agopian and his associates from the Dumont-University of California, Los Angeles (UCLA), Transplant and Liver Cancer Center in a recent report (Ann. Surg. 2012;256:624-33).
From 2001 to 2009, the nationwide frequency of NASH as the primary indication for liver transplantation rose from 1% to 10%, with NASH becoming the third most common U.S. indication for liver transplantation (Gastroenterology 2011;141:1249-53). The UCLA surgeons reviewed their experience with 1,294 patients who underwent primary liver transplantation at their center between January 2002 and August 2011, and found 136 patients (11%) who met NASH criteria. But during the 10-year period studied, NASH as the trigger for liver transplant soared from 3% of transplants in 2002 to 19% in 2011, a jump that by 2011 made NASH the second most common cause for liver transplant at UCLA, trailing only hepatitis C virus. In fact, NASH "is poised to surpass hepatitis C as the leading indication in the next 10-20 years," wrote Dr. Agopian, a liver surgeon, and Dr. Busuttil, professor and chief of liver and pancreatic transplantation at UCLA, and their associates (Ann. Surg. 2012;256:624-33).
In their report, Dr. Agopian and Dr. Busuttil called the current surge in liver transplants for patients with NASH "the new epidemic."
"The future of [liver] transplantation is here with these patients who have nonalcoholic steatohepatitis and subsequent cirrhosis," commented Dr. John P. Roberts, professor and chief of transplant surgery at the University of California, San Francisco. "Currently, there are about 6,000 [liver] transplants [per year] in the United States. Half of those are done for hepatitis C. In the overall population of the United States, 1.3% have hepatitis C, and that provides about half of liver transplant patients. Twelve percent of the U.S. population have nonalcoholic steatohepatitis, a 10-fold increase over the percentage of the population with hepatitis C. Due to the kinetics of the hepatitis C epidemic, we are going to see a falloff in the number of patients with hepatitis C eligible for transplantation in the next 10 years. [Patients with NASH] are going to replace them, potentially by 10-fold," said Dr. Roberts, who commented on the report by Dr. Agopian and Dr. Busuttil on the UCLA experience during the 2012 annual meeting of the American Surgical Association in San Francisco.
The NAFLD, NASH, and HCC connections
"The link between obesity, NASH, cirrhosis, and HCC is very strong" said Dr. Stephen H. Caldwell, professor of medicine and director of hepatology at the University of Virginia in Charlottesville.
"What remains unknown is whether NASH and hepatic scar formation are essential to cause cancer, or can carcinomas arise in a noncirrhotic, non-NASH fatty liver? Scar formation itself is a carcinogenic process, especially when it progresses to stage 3 – bridging fibrosis – or to stage 4," when cirrhosis occurs.
"It’s difficult to justify screening all patients with a fatty liver; that would be a huge undertaking," Dr. Caldwell said in an interview. "The more important clinical message is to consider whether a patient has NASH, but that is hard to diagnose without a liver biopsy."
So far, no markers have been unquestionably accurate for diagnosing NASH. Any patient who is obese or has metabolic syndrome should be considered for NASH, said Dr. Caldwell. Signs of more advanced liver injury include cirrhosis or portal hypertension. Other, more subtle signs include spider angiomas, reddening of the palms, declining platelet counts, or a family history of liver disease. Any of these could be a reason to look for NASH, he said.
Last year, guidelines issued by the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology, and the American Gastroenterological Association recommended against routinely testing for NAFLD, even among patients in diabetes or obesity clinics. Evidence was lacking for routine screening, even of high-risk patients, the guidelines said, with no data on cost effectiveness and uncertainties about diagnostic tests and treatment options (Hepatology 2012;55:2005-32).
But the guidelines do call for targeted assessment of NAFLD, and targeting NASH workups for selected NAFLD patients. The guidelines recommend ruling out all other possible etiologies and establishing NAFLD by histology or imaging. When a patient is diagnosed with NAFLD, the guidelines say that "as the metabolic syndrome predicts the presence of steatohepatitis in patients with NAFLD, its presence can be used to target patients for liver biopsy." The 2012 guidelines also highlighted the NAFLD Fibrosis Score (Hepatology 2007;45:846-54) as another useful tool to identify NAFLD patients at increased risk for NASH or cirrhosis. The guidelines called the plasma biomarker cytokeretin-18 "promising," but cautioned that it was "premature to recommend in routine clinical practice."
Major issues for patients who develop NASH are their risk for cirrhosis and liver failure, as well as that for liver cancer. Although the case already exists for obesity, diabetes, and metabolic syndrome as factors leading to NAFLD and NASH, evidence also links each of these three conditions to an increased rate of HCC and other liver cancer, such as cholangiocarcinoma.
"The evidence supports both an independent role for obesity, insulin resistance, and diabetes, as well as boosting the risk from other major risk factors such as hepatitis. The missing evidence is it has not been shown that treatment of diabetes or weight loss can reduce the risk of liver cancer," said Dr. Hashem B. El-Serag, professor and chief of gastroenterology and hepatology at the Baylor College of Medicine in Houston. "Screening for fatty liver by liver enzymes and ultrasound is probably a prudent first step" for obese or insulin-resistant patients, noted Dr. El-Serag. But surveillance for HCC by twice-annual ultrasound exams is only for patients with demonstrated advanced fibrosis or cirrhosis, he said in an interview.
"We currently recommend that anyone with NAFLD cirrhosis or cirrhosis of unknown etiology who is also obese or had diabetes should receive routine HCC surveillance," said Dr. György Baffy, chief of gastroenterology at the VA Boston Healthcare System. He predicts that "we may soon reach a general conclusion that people with morbid obesity (a body mass index of greater that 40 kg/m2) and poorly controlled diabetes should be considered for liver cancer surveillance even without clear evidence for cirrhosis," he said in an interview. But in general, "HCC occurrence in noncirrhotic liver is so low that surveillance would be rather inefficient."
Despite that, Dr. Baffy admits that the connection between diabetes and HCC may go beyond cirrhosis. "Up to half of all HCC may develop in noncirrhotic livers," he wrote in a recent editorial (Am. J. Gastroenterol. 2012;107:53-5). "It is more difficult to determine the need for HCC surveillance in diabetic patients with noncirrhotic liver or with no established liver disease."
To avoid missing a diagnosis of HCC, Dr. Baffy suggested awareness of the risk factors for advanced background liver disease and for HCC in patients with diabetes: male sex, older age, morbid obesity, poorly controlled and long-standing disease, and coexisting hepatitis C.
"For now, cirrhosis remains the primary indication for implementing HCC surveillance," but the new findings on liver cancer developing in liver disease associated with obesity and diabetes so far provide insufficient evidence to warrant any firm screening recommendations for these patients, Dr. Baffy wrote in another recent article along with Dr. Caldwell and Dr. Elizabeth M. Brunt (J. Hepatology 2012;56:1384-91). "The greater dilemma comes from new evidence that HCC may complicate NAFLD when fibrosis is mild or absent. Observations that diabetes may increase the risk of HCC regardless of the presence of cirrhosis remain a major concern for the 26 million Americans estimated to have diabetes or prediabetes," they wrote. "We may need to contemplate a paradigm shift in liver cancer surveillance, but for now at least, HCC appears to be a rare complication of NAFLD in the complete absence of fibrosis."
In addition, the value of regular cancer surveillance, even in patients with cirrhosis, remains uncertain, just as surveillance for breast cancer and prostate cancer has come under similar criticism. "It gets a little shaky when you look for evidence that [HCC] surveillance led to prolonged survival," said Dr. Caldwell. "You have all the same controversy as breast cancer, but surveillance is even less established for HCC."
Diabetes also linked to HCC spread
Once hepatocellular carcinoma forms in a patient with diabetes, the cancer may act more aggressively, according to studies from the University of Rochester (N.Y.).
A review of 265 consecutive patients treated for hepatocellular carcinoma (HCC) at Rochester’s Wilmot Cancer Center identified 91 (34%) with diabetes at the time of HCC diagnosis. Forty-seven of the 265 patients (18%) had distant metastases at the time of diagnosis. A multivariate analysis that controlled for age and etiologic risk factors showed that patients with diabetes were 10 times more likely to have distant metastases at the time of HCC diagnosis, compared with patients without diabetes, Dr. Aram F. Hezel and his associates reported last year (Cancer Investigation 2012;30:698-702). The analysis showed no statistically significant impact of diabetes on survival rate.
In a second analysis they found that patients with newly diagnosed HCC and diabetes were also significantly more likely to have macrovascular invasion by the HCC.
"We don’t treat patients with HCC differently if they have diabetes or obesity, but our findings show an association between diabetes and greater spread of HCC, more invasive cancer," said Dr. Hezel, an oncologist and director of hepatic and pancreatic cancer research at the Wilmot Cancer Center of the University of Rochester (N.Y.). "We don’t know whether we can treat the diabetes and change the behavior of the cancer by having patients under better control. Are cancers different in patients with diabetes or obesity? Do some metabolic states help push a cancer to more invasive behavior?" he asked in an interview.
"We use liver transplant to treat patients with liver cancer. In early stages of liver cancer the tumor is less likely to spread, so liver transplant can be curative. But if there are patients with a greater propensity for cancer spread at an earlier stage" then the efficacy of transplantation needs reassessment, Dr. Hezel said.
Few proven treatments for NAFLD, NASH, and to prevent HCC
Although diagnosing NAFLD is an important step in identifying patients at risk for NASH, cirrhosis, and liver cancer, interventions with proven benefits for NAFLD are limited. No approved drug treatments exist for NAFLD; lifestyle modification is the standard treatment to reduce steatosis and plasma levels of liver aminotransferases. Reductions in liver fat correlate closely with weight loss, Dr. Cusi, Dr. Lomonaco, and their associates said in a recently published analysis of NAFLD (Drugs 2013; Jan. 11 [Epub ahead of print]). A weight loss of 7%-10% has been linked with a roughly 50% drop in liver fat levels in NAFLD patients, they said. But long-term controlled studies are needed to better assess the impact of lifestyle changes on NAFLD and fatty livers.
Pioglitazone received endorsement from the AASLD panel for treating NASH in their 2012 NAFLD management recommendations. The recommendations cautioned that most NASH patients who received pioglitazone treatment in trials did not have diabetes, and that long-term safety and efficacy of pioglitazone in NASH patients are not established.
The AASLD guidelines also call for using vitamin E at a daily dosage of 800 IU, but only for patients with biopsy-proven NASH and no diabetes; the guidelines call it "first line" in this setting. But the guidelines also specifically caution against using vitamin E in patients with NASH and diabetes, patients with NAFLD who have not undergone a liver biopsy, patients with NASH and cirrhosis, and those with cryptogenic cirrhosis. The guidelines also caution against using metformin to treat NASH. No other drug intervention gets guideline endorsement for treating NASH.
"You can say diet and exercise minimize the risk of fatty liver, but beyond that drug therapy is unclear," said Dr. Caldwell. "I think as we see treatment evolve, we’ll see more interest [in treating NAFLD and NASH] by endocrinologists," he predicted.
The intervention picture changes when the goal is preventing liver cancer. "Effective treatment of insulin resistance and hyperinsulinemia may be critical to prevent hepatocarcinogenesis," wrote Dr. Baffy, Dr. Brunt, and Dr. Caldwell in their recent review (J. Hepatology 2012;56:1384-91). "Insulin sensitizing agents in diabetes may reduce the risk of HCC." They especially cited the epidemiologic evidence supporting a role for thiazolidinediones, which were linked to a 70% reduction in HCC incidence among patients with diabetes compared with patients treated with insulin or a sulfonylurea in a case-control study (Cancer 2010;116:1938-46). The same study also showed a similar, 70% reduction in HCC among patients treated with a biguanide like metformin.
"While current guidelines for the management of HCC have no specific recommendations for cases associated with NAFLD, obesity, and diabetes, the use of insulin-sensitizing drugs and avoidance of treatments that contribute to hyperinsulinemia are likely to enhance prevention and improve disease outcomes of HCC," said Dr. Baffy, Dr. Brunt, and Dr. Caldwell.
Similar evidence recently came from other epidemiologic studies that suggest damping down of HCC development in patients treated with a thiazolidinedione or metformin. A report last year that analyzed health records of about 98,000 Taiwan residents found that treatment with a thiazolidinedione or with metformin reduced the rate of HCC in patients with diabetes by about 50% compared with other treatments (Am. J. Gastroenterol. 2012;107:46-52). More evidence supporting protection from metformin against formation of both HCC and a second, less common type of liver cancer, intrahepatic cholangiocarcinoma, came in two studies reported last May at the annual Digestive Disease Week in San Diego.
"Metformin has not proved useful in the therapy of NAFLD, but it is helpful in decreasing the risk of HCC in patients with obesity- or diabetes-associated liver disease. Metformin should be part of antidiabetic management whenever possible," Dr. Baffy said in an interview.
But other experts regard the evidence accumulated so far as too preliminary to guide management. "It is premature to recommend using [metformin or a thiazolidinedione] for the primary reason of HCC prevention," said Dr. El-Serag.
"I don’t think the evidence is convincing at this point" regarding preventing HCC, said Dr. Caldwell. "The thiazolidinediones seem to retard progression of NASH fibrosis, but they also have adverse effects and their popularity has decreased."
Early days for a complex pathology
It seems as if the links between obesity, diabetes, and metabolic syndrome and NAFLD, NASH, and liver cancer are so tangled that it will take more time to fully resolve the etiologic relationships and the implications for diagnosis and management. The bottom line today is that a growing segment of American adults face risks for significant liver disease because of obesity, type 2 diabetes, and other elements of the metabolic syndrome.
"We see more and more patients over the last decade with liver cancer who didn’t have hepatitis or alcohol use but have diabetes and obesity. It’s a changing demographic," said Dr. Hezel. "We increasingly see liver cancer in patients without one of the classic risk factors. There are two possible mechanisms. Fibrosis and inflammation" caused by NAFLD and NASH trigger cancer formation and growth, "or it could be a more direct effect of high insulin levels or other hormonal effects. This is an emerging area; it follows on the epidemic of obesity and diabetes."
Dr. Cusi, Dr. Caldwell, Dr. Baffy, Dr. El-Serag, Dr. Busuttil, and Dr. Hezel all said that they had no relevant disclosures.
On Twitter @mitchelzoler
Many physicians do not consider liver disease and liver cancer classic complications of obesity, type 2 diabetes, or metabolic syndrome, but they should.
Research findings over the past decade offer substantial evidence for links between obesity, diabetes, or metabolic syndrome and the earliest hepatic manifestation of these derangements: nonalcoholic fatty liver disease (NAFLD). Equally compelling links tie obesity, diabetes, and metabolic syndrome to more advanced liver pathology: nonalcoholic steatohepatitis (NASH), cirrhosis, and liver cancer, especially hepatocellular carcinoma (HCC).
Although the link between obesity, diabetes, or metabolic syndrome and NASH or liver cancer is not yet strong enough to justify major changes in disease surveillance or management, the link between these metabolic disorders and NAFLD is powerful and common enough to warrant routinely considering these patients as having NAFLD, say experts. And if NAFLD is found, the next step is deciding if a patient is the right candidate for NASH or cirrhosis assessment; and if those disorders develop, cancer screening follows.
A new dimension of obesity and diabetes morbidity
"For decades, attention to the patient with type 2 diabetes focused on the control of hyperglycemia and of risk factors associated with macrovascular disease. The epidemic of obesity now presents endocrinologists with new challenges. Among them is the need to identify early complications related to obesity in the setting of type 2 diabetes. NAFLD is a common complication of patients with type 2 diabetes that ... does not fit into the traditional realm of diabetic complications," Dr. Romina Lomonaco and Dr. Kenneth Cusi wrote in a recently published book chapter ("Evidence-based Management of Diabetes," chapter 21; TFM Publishing, 2012).
Not until recently has NAFLD been recognized as another common complication of patients with type 2 diabetes that requires special attention. NAFLD’s low profile as a complication of obesity and diabetes contrasts with its ubiquity. About 70% of patients with type 2 diabetes have NAFLD (compared with about 20% of all American adults), and perhaps up to 90% of morbidly obese patients have NAFLD. The prevalence of impaired fasting glucose and of newly diagnosed type 2 diabetes is about threefold higher in patients with NAFLD than in those without liver disease.
"Insulin resistance and obesity are probably the biggest factors" causing NAFLD, said Dr. Cusi, professor of medicine and chief of adult endocrinology, diabetes, and metabolism at the University of Florida in Gainesville. Moreover, "diabetes will worsen NAFLD, producing more fibrosis and an increased rate of cirrhosis," he said in an interview.
That’s significant because it is NAFLD progression that poses the biggest risk. NAFLD severity can range from mild, early-stage disease in an asymptomatic patient with normal liver enzyme levels to the development of inflammation –NASH – which can cause liver injury and fibrosis, lead to cirrhosis, and set up progression to organ failure or development of HCC or other liver cancer.
Overall, about 40% of patients with NAFLD progress to NASH, but both obesity and diabetes ratchet up NAFLD progression, and so roughly half of all patients with diabetes have NASH. Patients with type 2 diabetes also have a two- to fourfold increased risk of developing advanced liver disease, cirrhosis, and HCC compared with people without diabetes. "About 15% of NASH patients develop cirrhosis, and a significant percent also develop cancer," Dr. Cusi said.
"NASH represents the hepatic manifestation of the metabolic syndrome, a constellation of abdominal obesity, hypertension, diabetes, and dyslipidemia. It is projected that 25 million Americans will develop NASH by 2025, with 20% progressing to cirrhosis, hepatocellular carcinoma, or both, that may require liver transplantation," wrote Dr. Vatche G. Agopian and his associates from the Dumont-University of California, Los Angeles (UCLA), Transplant and Liver Cancer Center in a recent report (Ann. Surg. 2012;256:624-33).
From 2001 to 2009, the nationwide frequency of NASH as the primary indication for liver transplantation rose from 1% to 10%, with NASH becoming the third most common U.S. indication for liver transplantation (Gastroenterology 2011;141:1249-53). The UCLA surgeons reviewed their experience with 1,294 patients who underwent primary liver transplantation at their center between January 2002 and August 2011, and found 136 patients (11%) who met NASH criteria. But during the 10-year period studied, NASH as the trigger for liver transplant soared from 3% of transplants in 2002 to 19% in 2011, a jump that by 2011 made NASH the second most common cause for liver transplant at UCLA, trailing only hepatitis C virus. In fact, NASH "is poised to surpass hepatitis C as the leading indication in the next 10-20 years," wrote Dr. Agopian, a liver surgeon, and Dr. Busuttil, professor and chief of liver and pancreatic transplantation at UCLA, and their associates (Ann. Surg. 2012;256:624-33).
In their report, Dr. Agopian and Dr. Busuttil called the current surge in liver transplants for patients with NASH "the new epidemic."
"The future of [liver] transplantation is here with these patients who have nonalcoholic steatohepatitis and subsequent cirrhosis," commented Dr. John P. Roberts, professor and chief of transplant surgery at the University of California, San Francisco. "Currently, there are about 6,000 [liver] transplants [per year] in the United States. Half of those are done for hepatitis C. In the overall population of the United States, 1.3% have hepatitis C, and that provides about half of liver transplant patients. Twelve percent of the U.S. population have nonalcoholic steatohepatitis, a 10-fold increase over the percentage of the population with hepatitis C. Due to the kinetics of the hepatitis C epidemic, we are going to see a falloff in the number of patients with hepatitis C eligible for transplantation in the next 10 years. [Patients with NASH] are going to replace them, potentially by 10-fold," said Dr. Roberts, who commented on the report by Dr. Agopian and Dr. Busuttil on the UCLA experience during the 2012 annual meeting of the American Surgical Association in San Francisco.
The NAFLD, NASH, and HCC connections
"The link between obesity, NASH, cirrhosis, and HCC is very strong" said Dr. Stephen H. Caldwell, professor of medicine and director of hepatology at the University of Virginia in Charlottesville.
"What remains unknown is whether NASH and hepatic scar formation are essential to cause cancer, or can carcinomas arise in a noncirrhotic, non-NASH fatty liver? Scar formation itself is a carcinogenic process, especially when it progresses to stage 3 – bridging fibrosis – or to stage 4," when cirrhosis occurs.
"It’s difficult to justify screening all patients with a fatty liver; that would be a huge undertaking," Dr. Caldwell said in an interview. "The more important clinical message is to consider whether a patient has NASH, but that is hard to diagnose without a liver biopsy."
So far, no markers have been unquestionably accurate for diagnosing NASH. Any patient who is obese or has metabolic syndrome should be considered for NASH, said Dr. Caldwell. Signs of more advanced liver injury include cirrhosis or portal hypertension. Other, more subtle signs include spider angiomas, reddening of the palms, declining platelet counts, or a family history of liver disease. Any of these could be a reason to look for NASH, he said.
Last year, guidelines issued by the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology, and the American Gastroenterological Association recommended against routinely testing for NAFLD, even among patients in diabetes or obesity clinics. Evidence was lacking for routine screening, even of high-risk patients, the guidelines said, with no data on cost effectiveness and uncertainties about diagnostic tests and treatment options (Hepatology 2012;55:2005-32).
But the guidelines do call for targeted assessment of NAFLD, and targeting NASH workups for selected NAFLD patients. The guidelines recommend ruling out all other possible etiologies and establishing NAFLD by histology or imaging. When a patient is diagnosed with NAFLD, the guidelines say that "as the metabolic syndrome predicts the presence of steatohepatitis in patients with NAFLD, its presence can be used to target patients for liver biopsy." The 2012 guidelines also highlighted the NAFLD Fibrosis Score (Hepatology 2007;45:846-54) as another useful tool to identify NAFLD patients at increased risk for NASH or cirrhosis. The guidelines called the plasma biomarker cytokeretin-18 "promising," but cautioned that it was "premature to recommend in routine clinical practice."
Major issues for patients who develop NASH are their risk for cirrhosis and liver failure, as well as that for liver cancer. Although the case already exists for obesity, diabetes, and metabolic syndrome as factors leading to NAFLD and NASH, evidence also links each of these three conditions to an increased rate of HCC and other liver cancer, such as cholangiocarcinoma.
"The evidence supports both an independent role for obesity, insulin resistance, and diabetes, as well as boosting the risk from other major risk factors such as hepatitis. The missing evidence is it has not been shown that treatment of diabetes or weight loss can reduce the risk of liver cancer," said Dr. Hashem B. El-Serag, professor and chief of gastroenterology and hepatology at the Baylor College of Medicine in Houston. "Screening for fatty liver by liver enzymes and ultrasound is probably a prudent first step" for obese or insulin-resistant patients, noted Dr. El-Serag. But surveillance for HCC by twice-annual ultrasound exams is only for patients with demonstrated advanced fibrosis or cirrhosis, he said in an interview.
"We currently recommend that anyone with NAFLD cirrhosis or cirrhosis of unknown etiology who is also obese or had diabetes should receive routine HCC surveillance," said Dr. György Baffy, chief of gastroenterology at the VA Boston Healthcare System. He predicts that "we may soon reach a general conclusion that people with morbid obesity (a body mass index of greater that 40 kg/m2) and poorly controlled diabetes should be considered for liver cancer surveillance even without clear evidence for cirrhosis," he said in an interview. But in general, "HCC occurrence in noncirrhotic liver is so low that surveillance would be rather inefficient."
Despite that, Dr. Baffy admits that the connection between diabetes and HCC may go beyond cirrhosis. "Up to half of all HCC may develop in noncirrhotic livers," he wrote in a recent editorial (Am. J. Gastroenterol. 2012;107:53-5). "It is more difficult to determine the need for HCC surveillance in diabetic patients with noncirrhotic liver or with no established liver disease."
To avoid missing a diagnosis of HCC, Dr. Baffy suggested awareness of the risk factors for advanced background liver disease and for HCC in patients with diabetes: male sex, older age, morbid obesity, poorly controlled and long-standing disease, and coexisting hepatitis C.
"For now, cirrhosis remains the primary indication for implementing HCC surveillance," but the new findings on liver cancer developing in liver disease associated with obesity and diabetes so far provide insufficient evidence to warrant any firm screening recommendations for these patients, Dr. Baffy wrote in another recent article along with Dr. Caldwell and Dr. Elizabeth M. Brunt (J. Hepatology 2012;56:1384-91). "The greater dilemma comes from new evidence that HCC may complicate NAFLD when fibrosis is mild or absent. Observations that diabetes may increase the risk of HCC regardless of the presence of cirrhosis remain a major concern for the 26 million Americans estimated to have diabetes or prediabetes," they wrote. "We may need to contemplate a paradigm shift in liver cancer surveillance, but for now at least, HCC appears to be a rare complication of NAFLD in the complete absence of fibrosis."
In addition, the value of regular cancer surveillance, even in patients with cirrhosis, remains uncertain, just as surveillance for breast cancer and prostate cancer has come under similar criticism. "It gets a little shaky when you look for evidence that [HCC] surveillance led to prolonged survival," said Dr. Caldwell. "You have all the same controversy as breast cancer, but surveillance is even less established for HCC."
Diabetes also linked to HCC spread
Once hepatocellular carcinoma forms in a patient with diabetes, the cancer may act more aggressively, according to studies from the University of Rochester (N.Y.).
A review of 265 consecutive patients treated for hepatocellular carcinoma (HCC) at Rochester’s Wilmot Cancer Center identified 91 (34%) with diabetes at the time of HCC diagnosis. Forty-seven of the 265 patients (18%) had distant metastases at the time of diagnosis. A multivariate analysis that controlled for age and etiologic risk factors showed that patients with diabetes were 10 times more likely to have distant metastases at the time of HCC diagnosis, compared with patients without diabetes, Dr. Aram F. Hezel and his associates reported last year (Cancer Investigation 2012;30:698-702). The analysis showed no statistically significant impact of diabetes on survival rate.
In a second analysis they found that patients with newly diagnosed HCC and diabetes were also significantly more likely to have macrovascular invasion by the HCC.
"We don’t treat patients with HCC differently if they have diabetes or obesity, but our findings show an association between diabetes and greater spread of HCC, more invasive cancer," said Dr. Hezel, an oncologist and director of hepatic and pancreatic cancer research at the Wilmot Cancer Center of the University of Rochester (N.Y.). "We don’t know whether we can treat the diabetes and change the behavior of the cancer by having patients under better control. Are cancers different in patients with diabetes or obesity? Do some metabolic states help push a cancer to more invasive behavior?" he asked in an interview.
"We use liver transplant to treat patients with liver cancer. In early stages of liver cancer the tumor is less likely to spread, so liver transplant can be curative. But if there are patients with a greater propensity for cancer spread at an earlier stage" then the efficacy of transplantation needs reassessment, Dr. Hezel said.
Few proven treatments for NAFLD, NASH, and to prevent HCC
Although diagnosing NAFLD is an important step in identifying patients at risk for NASH, cirrhosis, and liver cancer, interventions with proven benefits for NAFLD are limited. No approved drug treatments exist for NAFLD; lifestyle modification is the standard treatment to reduce steatosis and plasma levels of liver aminotransferases. Reductions in liver fat correlate closely with weight loss, Dr. Cusi, Dr. Lomonaco, and their associates said in a recently published analysis of NAFLD (Drugs 2013; Jan. 11 [Epub ahead of print]). A weight loss of 7%-10% has been linked with a roughly 50% drop in liver fat levels in NAFLD patients, they said. But long-term controlled studies are needed to better assess the impact of lifestyle changes on NAFLD and fatty livers.
Pioglitazone received endorsement from the AASLD panel for treating NASH in their 2012 NAFLD management recommendations. The recommendations cautioned that most NASH patients who received pioglitazone treatment in trials did not have diabetes, and that long-term safety and efficacy of pioglitazone in NASH patients are not established.
The AASLD guidelines also call for using vitamin E at a daily dosage of 800 IU, but only for patients with biopsy-proven NASH and no diabetes; the guidelines call it "first line" in this setting. But the guidelines also specifically caution against using vitamin E in patients with NASH and diabetes, patients with NAFLD who have not undergone a liver biopsy, patients with NASH and cirrhosis, and those with cryptogenic cirrhosis. The guidelines also caution against using metformin to treat NASH. No other drug intervention gets guideline endorsement for treating NASH.
"You can say diet and exercise minimize the risk of fatty liver, but beyond that drug therapy is unclear," said Dr. Caldwell. "I think as we see treatment evolve, we’ll see more interest [in treating NAFLD and NASH] by endocrinologists," he predicted.
The intervention picture changes when the goal is preventing liver cancer. "Effective treatment of insulin resistance and hyperinsulinemia may be critical to prevent hepatocarcinogenesis," wrote Dr. Baffy, Dr. Brunt, and Dr. Caldwell in their recent review (J. Hepatology 2012;56:1384-91). "Insulin sensitizing agents in diabetes may reduce the risk of HCC." They especially cited the epidemiologic evidence supporting a role for thiazolidinediones, which were linked to a 70% reduction in HCC incidence among patients with diabetes compared with patients treated with insulin or a sulfonylurea in a case-control study (Cancer 2010;116:1938-46). The same study also showed a similar, 70% reduction in HCC among patients treated with a biguanide like metformin.
"While current guidelines for the management of HCC have no specific recommendations for cases associated with NAFLD, obesity, and diabetes, the use of insulin-sensitizing drugs and avoidance of treatments that contribute to hyperinsulinemia are likely to enhance prevention and improve disease outcomes of HCC," said Dr. Baffy, Dr. Brunt, and Dr. Caldwell.
Similar evidence recently came from other epidemiologic studies that suggest damping down of HCC development in patients treated with a thiazolidinedione or metformin. A report last year that analyzed health records of about 98,000 Taiwan residents found that treatment with a thiazolidinedione or with metformin reduced the rate of HCC in patients with diabetes by about 50% compared with other treatments (Am. J. Gastroenterol. 2012;107:46-52). More evidence supporting protection from metformin against formation of both HCC and a second, less common type of liver cancer, intrahepatic cholangiocarcinoma, came in two studies reported last May at the annual Digestive Disease Week in San Diego.
"Metformin has not proved useful in the therapy of NAFLD, but it is helpful in decreasing the risk of HCC in patients with obesity- or diabetes-associated liver disease. Metformin should be part of antidiabetic management whenever possible," Dr. Baffy said in an interview.
But other experts regard the evidence accumulated so far as too preliminary to guide management. "It is premature to recommend using [metformin or a thiazolidinedione] for the primary reason of HCC prevention," said Dr. El-Serag.
"I don’t think the evidence is convincing at this point" regarding preventing HCC, said Dr. Caldwell. "The thiazolidinediones seem to retard progression of NASH fibrosis, but they also have adverse effects and their popularity has decreased."
Early days for a complex pathology
It seems as if the links between obesity, diabetes, and metabolic syndrome and NAFLD, NASH, and liver cancer are so tangled that it will take more time to fully resolve the etiologic relationships and the implications for diagnosis and management. The bottom line today is that a growing segment of American adults face risks for significant liver disease because of obesity, type 2 diabetes, and other elements of the metabolic syndrome.
"We see more and more patients over the last decade with liver cancer who didn’t have hepatitis or alcohol use but have diabetes and obesity. It’s a changing demographic," said Dr. Hezel. "We increasingly see liver cancer in patients without one of the classic risk factors. There are two possible mechanisms. Fibrosis and inflammation" caused by NAFLD and NASH trigger cancer formation and growth, "or it could be a more direct effect of high insulin levels or other hormonal effects. This is an emerging area; it follows on the epidemic of obesity and diabetes."
Dr. Cusi, Dr. Caldwell, Dr. Baffy, Dr. El-Serag, Dr. Busuttil, and Dr. Hezel all said that they had no relevant disclosures.
On Twitter @mitchelzoler
Itching
My patients itch. Do yours?
This time of year, many of them say their backs itch, but the itch is not really their main concern. What worries them more is what the itch means. They know there are spots back there. They can feel them even if they can’t see them very well. Does the itch mean those spots are turning into something?
Sometimes those spots on their backs are moles. Sometimes they are seborrheic keratoses. But basically they’re all just innocent bystanders. Even if there does happen to be a superficial basal cell back there, any itch in the vicinity has nothing to do with any of the spots.
"Itching," I tell my patients, "is a sign that you are alive." After a short pause for mental processing, most of them smile. Being alive is good. Itch is your friend.
If they don’t smile and instead continue to look anguished, I sometimes freeze off some of their keratoses, just so they can feel reassured. You never know about those pesky growths. They’re benign today, but who knows about tomorrow? And they’re itchy, aren’t they? Doesn’t an itch mean something?
As far as I’m concerned, it doesn’t mean much, or at least not much about malignant transformation. Sometimes a cigar is just a cigar, and mostly an itch is just an itch. But to many of my patients, an itch is much more: Itch is change, itch is instability. Something is happening, something is changing, something is going on. Maybe one thing is turning into something else. Maybe it will.
Last week, I saw a thirtyish woman who wanted a skin check. One of her concerns was an itchy spot on her left shoulder. Lately, it had started to "move down" to her upper arm. As she admitted herself, there was absolutely nothing to be seen on the skin. She couldn’t possibly be worried about ...
Yes, she could. "This isn’t skin cancer, is it?" she asked. I assured her it was not. She seemed to believe me. I couldn’t remove anything anyway, because there was nothing to remove.
I don’t know where people get the idea that itch, especially when it applies to a mole or growth, means possible cancer. But wherever they get the idea, many of them certainly have it. They ask about it all the time. "I’m worried about that mole," they say.
"Do you think it’s changed, gotten larger or darker?"
"No, it looks the same. But now it itches."
People worry, not just about the itch, but about what happens when they scratch it. They’ve been warned since childhood not to scratch. Scratching can cause damage or infection. If what they’re scratching is a spot, then scratching can possibly turn the spot into ... don’t say it!
Of course, people complain about itching for a lot of reasons: They have eczema, or dry skin, or winter itch. Older folks have trouble sleeping because of itch. Office workers are embarrassed by itch – they have to leave meetings to keep their colleagues from twitching uncomfortably when they see them scratch. ("Like a monkey," is usually how they put it.) People who work in nursing homes or homeless shelters worry that they picked up a creepy-crawly from one of their clients. I once read that a king of England forbade commoners from scratching their itches, because scratching was so much fun that he wanted to reserve it for royalty. Couples married 7 years may get the itch. Treatises have been written about itching and scratching. I have not read them. Some things are better enjoyed than read about.
When the itch is accompanied by a visible rash – atopic eczema is the parade example – you treat the itch by treating the rash. When the patient has an itch but no rash other than scratch marks, it’s often best not just to treat the symptom, but to eliminate the worry that accompanies and exaggerates the symptom. No, the itch is not bugs. No, the itch is not liver disease. No, scratching will not cause damage, or you-know-what.
No, the itch is not cancer. There, I said it.
You itch. Itch is life. Celebrate!
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at [email protected].
My patients itch. Do yours?
This time of year, many of them say their backs itch, but the itch is not really their main concern. What worries them more is what the itch means. They know there are spots back there. They can feel them even if they can’t see them very well. Does the itch mean those spots are turning into something?
Sometimes those spots on their backs are moles. Sometimes they are seborrheic keratoses. But basically they’re all just innocent bystanders. Even if there does happen to be a superficial basal cell back there, any itch in the vicinity has nothing to do with any of the spots.
"Itching," I tell my patients, "is a sign that you are alive." After a short pause for mental processing, most of them smile. Being alive is good. Itch is your friend.
If they don’t smile and instead continue to look anguished, I sometimes freeze off some of their keratoses, just so they can feel reassured. You never know about those pesky growths. They’re benign today, but who knows about tomorrow? And they’re itchy, aren’t they? Doesn’t an itch mean something?
As far as I’m concerned, it doesn’t mean much, or at least not much about malignant transformation. Sometimes a cigar is just a cigar, and mostly an itch is just an itch. But to many of my patients, an itch is much more: Itch is change, itch is instability. Something is happening, something is changing, something is going on. Maybe one thing is turning into something else. Maybe it will.
Last week, I saw a thirtyish woman who wanted a skin check. One of her concerns was an itchy spot on her left shoulder. Lately, it had started to "move down" to her upper arm. As she admitted herself, there was absolutely nothing to be seen on the skin. She couldn’t possibly be worried about ...
Yes, she could. "This isn’t skin cancer, is it?" she asked. I assured her it was not. She seemed to believe me. I couldn’t remove anything anyway, because there was nothing to remove.
I don’t know where people get the idea that itch, especially when it applies to a mole or growth, means possible cancer. But wherever they get the idea, many of them certainly have it. They ask about it all the time. "I’m worried about that mole," they say.
"Do you think it’s changed, gotten larger or darker?"
"No, it looks the same. But now it itches."
People worry, not just about the itch, but about what happens when they scratch it. They’ve been warned since childhood not to scratch. Scratching can cause damage or infection. If what they’re scratching is a spot, then scratching can possibly turn the spot into ... don’t say it!
Of course, people complain about itching for a lot of reasons: They have eczema, or dry skin, or winter itch. Older folks have trouble sleeping because of itch. Office workers are embarrassed by itch – they have to leave meetings to keep their colleagues from twitching uncomfortably when they see them scratch. ("Like a monkey," is usually how they put it.) People who work in nursing homes or homeless shelters worry that they picked up a creepy-crawly from one of their clients. I once read that a king of England forbade commoners from scratching their itches, because scratching was so much fun that he wanted to reserve it for royalty. Couples married 7 years may get the itch. Treatises have been written about itching and scratching. I have not read them. Some things are better enjoyed than read about.
When the itch is accompanied by a visible rash – atopic eczema is the parade example – you treat the itch by treating the rash. When the patient has an itch but no rash other than scratch marks, it’s often best not just to treat the symptom, but to eliminate the worry that accompanies and exaggerates the symptom. No, the itch is not bugs. No, the itch is not liver disease. No, scratching will not cause damage, or you-know-what.
No, the itch is not cancer. There, I said it.
You itch. Itch is life. Celebrate!
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at [email protected].
My patients itch. Do yours?
This time of year, many of them say their backs itch, but the itch is not really their main concern. What worries them more is what the itch means. They know there are spots back there. They can feel them even if they can’t see them very well. Does the itch mean those spots are turning into something?
Sometimes those spots on their backs are moles. Sometimes they are seborrheic keratoses. But basically they’re all just innocent bystanders. Even if there does happen to be a superficial basal cell back there, any itch in the vicinity has nothing to do with any of the spots.
"Itching," I tell my patients, "is a sign that you are alive." After a short pause for mental processing, most of them smile. Being alive is good. Itch is your friend.
If they don’t smile and instead continue to look anguished, I sometimes freeze off some of their keratoses, just so they can feel reassured. You never know about those pesky growths. They’re benign today, but who knows about tomorrow? And they’re itchy, aren’t they? Doesn’t an itch mean something?
As far as I’m concerned, it doesn’t mean much, or at least not much about malignant transformation. Sometimes a cigar is just a cigar, and mostly an itch is just an itch. But to many of my patients, an itch is much more: Itch is change, itch is instability. Something is happening, something is changing, something is going on. Maybe one thing is turning into something else. Maybe it will.
Last week, I saw a thirtyish woman who wanted a skin check. One of her concerns was an itchy spot on her left shoulder. Lately, it had started to "move down" to her upper arm. As she admitted herself, there was absolutely nothing to be seen on the skin. She couldn’t possibly be worried about ...
Yes, she could. "This isn’t skin cancer, is it?" she asked. I assured her it was not. She seemed to believe me. I couldn’t remove anything anyway, because there was nothing to remove.
I don’t know where people get the idea that itch, especially when it applies to a mole or growth, means possible cancer. But wherever they get the idea, many of them certainly have it. They ask about it all the time. "I’m worried about that mole," they say.
"Do you think it’s changed, gotten larger or darker?"
"No, it looks the same. But now it itches."
People worry, not just about the itch, but about what happens when they scratch it. They’ve been warned since childhood not to scratch. Scratching can cause damage or infection. If what they’re scratching is a spot, then scratching can possibly turn the spot into ... don’t say it!
Of course, people complain about itching for a lot of reasons: They have eczema, or dry skin, or winter itch. Older folks have trouble sleeping because of itch. Office workers are embarrassed by itch – they have to leave meetings to keep their colleagues from twitching uncomfortably when they see them scratch. ("Like a monkey," is usually how they put it.) People who work in nursing homes or homeless shelters worry that they picked up a creepy-crawly from one of their clients. I once read that a king of England forbade commoners from scratching their itches, because scratching was so much fun that he wanted to reserve it for royalty. Couples married 7 years may get the itch. Treatises have been written about itching and scratching. I have not read them. Some things are better enjoyed than read about.
When the itch is accompanied by a visible rash – atopic eczema is the parade example – you treat the itch by treating the rash. When the patient has an itch but no rash other than scratch marks, it’s often best not just to treat the symptom, but to eliminate the worry that accompanies and exaggerates the symptom. No, the itch is not bugs. No, the itch is not liver disease. No, scratching will not cause damage, or you-know-what.
No, the itch is not cancer. There, I said it.
You itch. Itch is life. Celebrate!
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at [email protected].
Implementing Peer Evaluation of Handoffs
The advent of restricted residency duty hours has thrust the safety risks of handoffs into the spotlight. More recently, the Accreditation Council of Graduate Medical Education (ACGME) has restricted hours even further to a maximum of 16 hours for first‐year residents and up to 28 hours for residents beyond their first year.[1] Although the focus on these mandates has been scheduling and staffing in residency programs, another important area of attention is for handoff education and evaluation. The Common Program Requirements for the ACGME state that all residency programs should ensure that residents are competent in handoff communications and that programs should monitor handoffs to ensure that they are safe.[2] Moreover, recent efforts have defined milestones for handoffs, specifically that by 12 months, residents should be able to effectively communicate with other caregivers to maintain continuity during transitions of care.[3] Although more detailed handoff‐specific milestones have to be flushed out, a need for evaluation instruments to assess milestones is critical. In addition, handoffs continue to represent a vulnerable time for patients in many specialties, such as surgery and pediatrics.[4, 5]
Evaluating handoffs poses specific challenges for internal medicine residency programs because handoffs are often conducted on the fly or wherever convenient, and not always at a dedicated time and place.[6] Even when evaluations could be conducted at a dedicated time and place, program faculty and leadership may not be comfortable evaluating handoffs in real time due to lack of faculty development and recent experience with handoffs. Although supervising faculty may be in the most ideal position due to their intimate knowledge of the patient and their ability to evaluate the clinical judgment of trainees, they may face additional pressures of supervision and direct patient care that prevent their attendance at the time of the handoff. For these reasons, potential people to evaluate the quality of a resident handoff may be the peers to whom they frequently handoff. Because handoffs are also conceptualized as an interactive dialogue between sender and receiver, an ideal handoff performance evaluation would capture both of these roles.[7] For these reasons, peer evaluation may be a viable modality to assist programs in evaluating handoffs. Peer evaluation has been shown to be an effective method of rating performance of medical students,[8] practicing physicians,[9] and residents.[10] Moreover, peer evaluation is now a required feature in assessing internal medicine resident performance.[11] Although enthusiasm for peer evaluation has grown in residency training, the use of it can still be limited by a variety of problems, such as reluctance to rate peers poorly, difficulty obtaining evaluations, and the utility of such evaluations. For these reasons, it is important to understand whether peer evaluation of handoffs is feasible. Therefore, the aim of this study was to assess feasibility of an online peer evaluation survey tool of handoffs in an internal medicine residency and to characterize performance over time as well and associations between workload and performance.
METHODS
From July 2009 to March 2010, all interns on the general medicine inpatient service at 2 hospitals were asked to complete an end‐of‐month anonymous peer evaluation that included 14‐items addressing all core competencies. The evaluation tool was administered electronically using New Innovations (New Innovations, Inc., Uniontown, OH). Interns signed out to each other in a cross‐cover circuit that included 3 other interns on an every fourth night call cycle.[12] Call teams included 1 resident and 1 intern who worked from 7 am on the on‐call day to noon on the postcall day. Therefore, postcall interns were expected to hand off to the next on‐call intern before noon. Although attendings and senior residents were not required to formally supervise the handoff, supervising senior residents were often present during postcall intern sign‐out to facilitate departure of the team. When interns were not postcall, they were expected to sign out before they went to the clinic in the afternoon or when their foreseeable work was complete. The interns were provided with a 45‐minute lecture on handoffs and introduced to the peer evaluation tool in July 2009 at an intern orientation. They were also prompted to complete the tool to the best of their ability after their general medicine rotation. We chose the general medicine rotation because each intern completed approximately 2 months of general medicine in their first year. This would provide ratings over time without overburdening interns to complete 3 additional evaluations after every inpatient rotation.
The peer evaluation was constructed to correspond to specific ACGME core competencies and was also linked to specific handoff behaviors that were known to be effective. The questions were adapted from prior items used in a validated direct‐observation tool previously developed by the authors (the Handoff Clinical Evaluation Exercise), which was based on literature review as well as expert opinion.[13, 14] For example, under the core competency of communication, interns were asked to rate each other on communication skills using the anchors of No questions, no acknowledgement of to do tasks, transfer of information face to face is not a priority for low unsatisfactory (1) and Appropriate use of questions, acknowledgement and read‐back of to‐do and priority tasks, face to face communication a priority for high superior (9). Items that referred to behaviors related to both giving handoff and receiving handoff were used to capture the interactive dialogue between senders and receivers that characterize ideal handoffs. In addition, specific items referring to written sign‐out and verbal sign‐out were developed to capture the specific differences. For instance, for the patient care competency in written sign‐out, low unsatisfactory (1) was defined as Incomplete written content; to do's omitted or requested with no rationale or plan, or with inadequate preparation (ie, request to transfuse but consent not obtained), and high superior (9) was defined as Content is complete with to do's accompanied by clear plan of action and rationale. Pilot testing with trainees was conducted, including residents not involved in the study and clinical students. The tool was also reviewed by the residency program leadership, and in an effort to standardize the reporting of the items with our other evaluation forms, each item was mapped to a core competency that it was most related to. Debriefing of the instrument experience following usage was performed with 3 residents who had an interest in medical education and handoff performance.
The tool was deployed to interns following a brief educational session for interns, in which the tool was previewed and reviewed. Interns were counseled to use the form as a global performance assessment over the course of the month, in contrast to an episodic evaluation. This would also avoid the use of negative event bias by raters, in which the rater allows a single negative event to influence the perception of the person's performance, even long after the event has passed into history.
To analyze the data, descriptive statistics were used to summarize mean performance across domains. To assess whether intern performance improved over time, we split the academic year into 3 time periods of 3 months each, which we have used in earlier studies assessing intern experience.[15] Prior to analysis, postcall interns were identified by using the intern monthly call schedule located in the AMiON software program (Norwich, VT) to label the evaluation of the postcall intern. Then, all names were removed and replaced with a unique identifier for the evaluator and the evaluatee. In addition, each evaluation was also categorized as either having come from the main teaching hospital or the community hospital affiliate.
Multivariate random effects linear regression models, controlling for evaluator, evaluatee, and hospital, were used to assess the association between time (using indicator variables for season) and postcall status on intern performance. In addition, because of the skewness in the ratings, we also undertook additional analysis by transforming our data into dichotomous variables reflecting superior performance. After conducting conditional ordinal logistic regression, the main findings did not change. We also investigated within‐subject and between‐subject variation using intraclass correlation coefficients. Within‐subject intraclass correlation enabled assessment of inter‐rater reliability. Between‐subject intraclass correlation enabled the assessment of evaluator effects. Evaluator effects can encompass a variety of forms of rater bias such as leniency (in which evaluators tended to rate individuals uniformly positively), severity (rater tends to significantly avoid using positive ratings), or the halo effect (the individual being evaluated has 1 significantly positive attribute that overrides that which is being evaluated). All analyses were completed using STATA 10.0 (StataCorp, College Station, TX) with statistical significance defined as P < 0.05. This study was deemed to be exempt from institutional review board review after all data were deidentified prior to analysis.
RESULTS
From July 2009 to March 2010, 31 interns (78%) returned 60% (172/288) of the peer evaluations they received. Almost all (39/40, 98%) interns were evaluated at least once with a median of 4 ratings per intern (range, 19). Thirty‐five percent of ratings occurred when an intern was rotating at the community hospital. Ratings were very high on all domains (mean, 8.38.6). Overall sign‐out performance was rated as 8.4 (95% confidence interval [CI], 8.3‐8.5), with over 55% rating peers as 9 (maximal score). The lowest score given was 5. Individual items ranged from a low of 8.34 (95% CI, 8.21‐8.47) for updating written sign‐outs, to a high of 8.60 (95% CI, 8.50‐8.69) for collegiality (Table 1) The internal consistency of the instrument was calculated using all items and was very high, with a Cronbach = 0.98.
| ACGME Core Competency | Role | Items | Item | Mean | 95% CI | Range | % Receiving 9 as Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Patient care | Sender | Written sign‐out | Q1 | 8.34 | 8.25 to 8.48 | 69 | 53.2 |
| Sender | Updated content | Q2 | 8.35 | 8.22 to 8.47 | 59 | 54.4 | |
| Receiver | Documentation of overnight events | Q6 | 8.41 | 8.30 to 8.52 | 69 | 56.3 | |
| Medical knowledge | Sender | Anticipatory guidance | Q3 | 8.40 | 8.28 to 8.51 | 69 | 56.3 |
| Receiver | Clinical decision making during cross‐cover | Q7 | 8.45 | 8.35 to 8.55 | 69 | 56.0 | |
| Professionalism | Sender | Collegiality | Q4 | 8.60 | 8.51 to 8.68 | 69 | 65.7 |
| Receiver | Acknowledgement of professional responsibility | Q10 | 8.53 | 8.43 to 8.62 | 69 | 62.4 | |
| Receiver | Timeliness/responsiveness | Q11 | 8.50 | 8.39 to 8.60 | 69 | 61.9 | |
| Interpersonal and communication skills | Receiver | Listening behavior when receiving sign‐outs | Q8 | 8.52 | 8.42 to 8.62 | 69 | 63.6 |
| Receiver | Communication when receiving sign‐out | Q9 | 8.52 | 8.43 to 8.62 | 69 | 63.0 | |
| Systems‐based practice | Receiver | Resource use | Q12 | 8.45 | 8.35 to 8.55 | 69 | 55.6 |
| Practice‐based learning and improvement | Sender | Accepting of feedback | Q5 | 8.45 | 8.34 to 8.55 | 69 | 58.7 |
| Overall | Both | Overall sign‐out quality | Q13 | 8.44 | 8.34 to 8.54 | 69 | 55.3 |
Mean ratings for each item increased in season 2 and 3 and were statistically significant using a test for trend across ordered groups. However, in multivariate regression models, improvements remained statistically significant for only 4 items (Figure 1): 1) communication skills, 2) listening behavior, 3) accepting professional responsibility, and 4) accessing the system (Table 2). Specifically, when compared to season 1, improvements in communication skill were seen in season 2 (+0.34 [95% CI, 0.08‐0.60], P = 0.009) and were sustained in season 3 (+0.34 [95% CI, 0.06‐0.61], P = 0.018). A similar pattern was observed for listening behavior, with improvement in ratings that were similar in magnitude with increasing intern experience (season 2, +0.29 [95% CI, 0.04‐0.55], P = 0.025 compared to season 1). Although accessing the system scores showed a similar pattern of improvement with an increase in season 2 compared to season 1, the magnitude of this change was smaller (season 2, +0.21 [95% CI, 0.03‐0.39], P = 0.023). Interestingly, improvements in accepting professional responsibility rose during season 2, but the difference did not reach statistical significance until season 3 (+0.37 [95% CI, 0.08‐0.65], P = 0.012 compared to season 1).
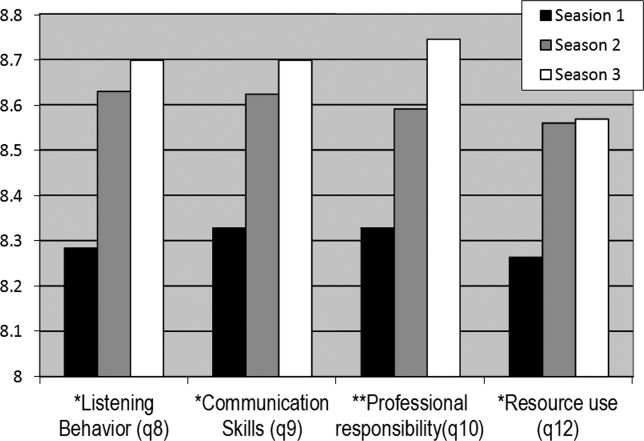
| Outcome | |||||
|---|---|---|---|---|---|
| Coefficient (95% CI) | |||||
| Predictor | Communication Skills | Listening Behavior | Professional Responsibility | Accessing the System | Written Sign‐out Quality |
| |||||
| Season 1 | Ref | Ref | Ref | Ref | Ref |
| Season 2 | 0.29 (0.04 to 0.55)a | 0.34 (0.08 to 0.60)a | 0.24 (0.03 to 0.51) | 0.21 (0.03 to 0.39)a | 0.05 (0.25 to 0.15) |
| Season 3 | 0.29 (0.02 to 0.56)a | 0.34 (0.06 to 0.61)a | 0.37 (0.08 to 0.65)a | 0.18 (0.01 to 0.36)a | 0.08 (0.13 to 0.30) |
| Community hospital | 0.18 (0.00 to 0.37) | 0.23 (0.04 to 0.43)a | 0.06 (0.13 to 0.26) | 0.13 (0.00 to 0.25) | 0.24 (0.08 to 0.39)a |
| Postcall | 0.10 (0.25 to 0.05) | 0.04 (0.21 to 0.13) | 0.02 (0.18 to 0.13) | 0.05 (0.16 to 0.05) | 0.18 (0.31,0.05)a |
| Constant | 7.04 (6.51 to 7.58) | 6.81 (6.23 to 7.38) | 7.04 (6.50 to 7.60) | 7.02 (6.59 to 7.45) | 6.49 (6.04 to 6.94) |
In addition to increasing experience, postcall interns were rated significantly lower than nonpostcall interns in 2 items: 1) written sign‐out quality (8.21 vs 8.39, P = 0.008) and 2) accepting feedback (practice‐based learning and improvement) (8.25 vs 8.42, P = 0.006). Interestingly, when interns were at the community hospital general medicine rotation, where overall census was much lower than at the teaching hospital, peer ratings were significantly higher for overall handoff performance and 7 (written sign‐out, update content, collegiality, accepting feedback, documentation of overnight events, clinical decision making during cross‐cover, and listening behavior) of the remaining 12 specific handoff domains (P < 0.05 for all, data not shown).
Last, significant evaluator effects were observed, which contributed to the variance in ratings given. For example, using intraclass correlation coefficients (ICC), we found that there was greater within‐intern variation than between‐intern variation, highlighting that evaluator scores tended to be strongly correlated with each other (eg, ICC overall performance = 0.64) and more so than scores of multiple evaluations of the same intern (eg, ICC overall performance = 0.18).
Because ratings of handoff performance were skewed, we also conducted a sensitivity analysis using ordinal logistic regression to ascertain if our findings remained significant. Using ordinal logistic regression models, significant improvements were seen in season 3 for 3 of the above‐listed behaviors, specifically listening behavior, professional responsibility, and accessing the system. Although there was no improvement in communication, there was an improvement observed in collegiality scores that were significant in season 3.
DISCUSSION
Using an end‐of‐rotation online peer assessment of handoff skills, it is feasible to obtain ratings of intern handoff performance from peers. Although there is evidence of rater bias toward leniency and low inter‐rater reliability, peer ratings of intern performance did increase over time. In addition, peer ratings were lower for interns who were handing off their postcall service. Working on a rotation at a community affiliate with a lower census was associated with higher peer ratings of handoffs.
It is worth considering the mechanism of these findings. First, the leniency observed in peer ratings likely reflects peers unwilling to critique each other due to a desire for an esprit de corps among their classmates. The low intraclass correlation coefficient for ratings of the same intern highlight that peers do not easily converge on their ratings of the same intern. Nevertheless, the ratings on the peer evaluation did demonstrate improvements over time. This improvement could easily reflect on‐the‐job learning, as interns become more acquainted with their roles and efficient and competent in their tasks. Together, these data provide a foundation for developing milestone handoffs that reflect the natural progression of intern competence in handoffs. For example, communication appeared to improve at 3 months, whereas transfer of professional responsibility improved at 6 months after beginning internship. However, alternative explanations are also important to consider. Although it is easy and somewhat reassuring to assume that increases over time reflect a learning effect, it is also possible that interns are unwilling to critique their peers as familiarity with them increases.
There are several reasons why postcall interns could have been universally rated lower than nonpostcall interns. First, postcall interns likely had the sickest patients with the most to‐do tasks or work associated with their sign‐out because they were handing off newly admitted patients. Because the postcall sign‐out is associated with the highest workload, it may be that interns perceive that a good handoff is nothing to do, and handoffs associated with more work are not highly rated. It is also important to note that postcall interns, who in this study were at the end of a 30‐hour duty shift, were also most fatigued and overworked, which may have also affected the handoff, especially in the 2 domains of interest. Due to the time pressure to leave coupled with fatigue, they may have had less time to invest in written sign‐out quality and may not have been receptive to feedback on their performance. Likewise, performance on handoffs was rated higher when at the community hospital, which could be due to several reasons. The most plausible explanation is that the workload associated with that sign‐out is less due to lower patient census and lower patient acuity. In the community hospital, fewer residents were also geographically co‐located on a quieter ward and work room area, which may contribute to higher ratings across domains.
This study also has implications for future efforts to improve and evaluate handoff performance in residency trainees. For example, our findings suggest the importance of enhancing supervision and training for handoffs during high workload rotations or certain times of the year. In addition, evaluation systems for handoff performance that rely solely on peer evaluation will not likely yield an accurate picture of handoff performance, difficulty obtaining peer evaluations, the halo effect, and other forms of evaluator bias in ratings. Accurate handoff evaluation may require direct observation of verbal communication and faculty audit of written sign‐outs.[16, 17] Moreover, methods such as appreciative inquiry can help identify the peers with the best practices to emulate.[18] Future efforts to validate peer assessment of handoffs against these other assessment methods, such as direct observation by service attendings, are needed.
There are limitations to this study. First, although we have limited our findings to 1 residency program with 1 type of rotation, we have already expanded to a community residency program that used a float system and have disseminated our tool to several other institutions. In addition, we have a small number of participants, and our 60% return rate on monthly peer evaluations raises concerns of nonresponse bias. For example, a peer who perceived the handoff performance of an intern to be poor may be less likely to return the evaluation. Because our dataset has been deidentified per institutional review board request, we do not have any information to differentiate systematic reasons for not responding to the evaluation. Anecdotally, a critique of the tool is that it is lengthy, especially in light of the fact that 1 intern completes 3 additional handoff evaluations. It is worth understanding why the instrument had such a high internal consistency. Although the items were designed to address different competencies initially, peers may make a global assessment about someone's ability to perform a handoff and then fill out the evaluation accordingly. This speaks to the difficulty in evaluating the subcomponents of various actions related to the handoff. Because of the high internal consistency, we were able to shorten the survey to a 5‐item instrument with a Cronbach of 0.93, which we are currently using in our program and have disseminated to other programs. Although it is currently unclear if the ratings of performance on the longer peer evaluation are valid, we are investigating concurrent validity of the shorter tool by comparing peer evaluations to other measures of handoff quality as part of our current work. Last, we are only able to test associations and not make causal inferences.
CONCLUSION
Peer assessment of handoff skills is feasible via an electronic competency‐based tool. Although there is evidence of score inflation, intern performance does increase over time and is associated with various aspects of workload, such as postcall status or working on a rotation at a community affiliate with a lower census. Together, these data can provide a foundation for developing milestones handoffs that reflect the natural progression of intern competence in handoffs.
Acknowledgments
The authors thank the University of Chicago Medicine residents and chief residents, the members of the Curriculum and Housestaff Evaluation Committee, Tyrece Hunter and Amy Ice‐Gibson, and Meryl Prochaska and Laura Ruth Venable for assistance with manuscript preparation.
Disclosures
This study was funded by the University of Chicago Department of Medicine Clinical Excellence and Medical Education Award and AHRQ R03 5R03HS018278‐02 Development of and Validation of a Tool to Evaluate Hand‐off Quality.
- , , ; the ACGME Duty Hour Task Force. The new recommendations on duty hours from the ACGME Task Force. N Engl J Med. 2010; 363.
- Common program requirements. Available at: http://acgme‐2010standards.org/pdf/Common_Program_Requirements_07012011.pdf. Accessed December 10, 2012.
- , , , et al. Charting the road to competence: developmental milestones for internal medicine residency training. J Grad Med Educ. 2009;1(1):5–20.
- , , , et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204(4):533–540.
- , , , . Patient handoffs: pediatric resident experiences and lessons learned. Clin Pediatr (Phila). 2011;50(1):57–63.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , , . Communication, communication, communication: the art of the handoff. Ann Emerg Med. 2010;55(2):181–183.
- , , , , . Use of peer evaluation in the assessment of medical students. J Med Educ. 1981;56:35–42.
- , , , , , . Use of peer ratings to evaluate physician performance. JAMA. 1993;269:1655–1660.
- , , . A pilot study of peer review in residency training. J Gen Intern Med. 1999;14(9):551–554.
- ACGME Program Requirements for Graduate Medical Education in Internal Medicine Effective July 1, 2009. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/140_internal_medicine_07012009.pdf. Accessed December 10, 2012.
- , , , , , . The effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Hand‐off education and evaluation: piloting the observed simulated hand‐off experience (OSHE). J Gen Intern Med. 2010;25(2):129–134.
- , , , , , . Validation of a handoff assessment tool: the Handoff CEX [published online ahead of print June 7, 2012]. J Clin Nurs. doi: 10.1111/j.1365‐2702.2012.04131.x.
- , , , et al. Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities. JAMA. 2008;300(10):1146–1153.
- , . Using direct observation, formal evaluation, and an interactive curriculum to improve the sign‐out practices of internal medicine interns. Acad Med. 2010;85(7):1182–1188.
- , , , . Faculty member review and feedback using a sign‐out checklist: improving intern written sign‐out. Acad Med. 2012;87(8):1125–1131.
- , , , et al. Use of an appreciative inquiry approach to improve resident sign‐out in an era of multiple shift changes. J Gen Intern Med. 2012;27(3):287–291.
The advent of restricted residency duty hours has thrust the safety risks of handoffs into the spotlight. More recently, the Accreditation Council of Graduate Medical Education (ACGME) has restricted hours even further to a maximum of 16 hours for first‐year residents and up to 28 hours for residents beyond their first year.[1] Although the focus on these mandates has been scheduling and staffing in residency programs, another important area of attention is for handoff education and evaluation. The Common Program Requirements for the ACGME state that all residency programs should ensure that residents are competent in handoff communications and that programs should monitor handoffs to ensure that they are safe.[2] Moreover, recent efforts have defined milestones for handoffs, specifically that by 12 months, residents should be able to effectively communicate with other caregivers to maintain continuity during transitions of care.[3] Although more detailed handoff‐specific milestones have to be flushed out, a need for evaluation instruments to assess milestones is critical. In addition, handoffs continue to represent a vulnerable time for patients in many specialties, such as surgery and pediatrics.[4, 5]
Evaluating handoffs poses specific challenges for internal medicine residency programs because handoffs are often conducted on the fly or wherever convenient, and not always at a dedicated time and place.[6] Even when evaluations could be conducted at a dedicated time and place, program faculty and leadership may not be comfortable evaluating handoffs in real time due to lack of faculty development and recent experience with handoffs. Although supervising faculty may be in the most ideal position due to their intimate knowledge of the patient and their ability to evaluate the clinical judgment of trainees, they may face additional pressures of supervision and direct patient care that prevent their attendance at the time of the handoff. For these reasons, potential people to evaluate the quality of a resident handoff may be the peers to whom they frequently handoff. Because handoffs are also conceptualized as an interactive dialogue between sender and receiver, an ideal handoff performance evaluation would capture both of these roles.[7] For these reasons, peer evaluation may be a viable modality to assist programs in evaluating handoffs. Peer evaluation has been shown to be an effective method of rating performance of medical students,[8] practicing physicians,[9] and residents.[10] Moreover, peer evaluation is now a required feature in assessing internal medicine resident performance.[11] Although enthusiasm for peer evaluation has grown in residency training, the use of it can still be limited by a variety of problems, such as reluctance to rate peers poorly, difficulty obtaining evaluations, and the utility of such evaluations. For these reasons, it is important to understand whether peer evaluation of handoffs is feasible. Therefore, the aim of this study was to assess feasibility of an online peer evaluation survey tool of handoffs in an internal medicine residency and to characterize performance over time as well and associations between workload and performance.
METHODS
From July 2009 to March 2010, all interns on the general medicine inpatient service at 2 hospitals were asked to complete an end‐of‐month anonymous peer evaluation that included 14‐items addressing all core competencies. The evaluation tool was administered electronically using New Innovations (New Innovations, Inc., Uniontown, OH). Interns signed out to each other in a cross‐cover circuit that included 3 other interns on an every fourth night call cycle.[12] Call teams included 1 resident and 1 intern who worked from 7 am on the on‐call day to noon on the postcall day. Therefore, postcall interns were expected to hand off to the next on‐call intern before noon. Although attendings and senior residents were not required to formally supervise the handoff, supervising senior residents were often present during postcall intern sign‐out to facilitate departure of the team. When interns were not postcall, they were expected to sign out before they went to the clinic in the afternoon or when their foreseeable work was complete. The interns were provided with a 45‐minute lecture on handoffs and introduced to the peer evaluation tool in July 2009 at an intern orientation. They were also prompted to complete the tool to the best of their ability after their general medicine rotation. We chose the general medicine rotation because each intern completed approximately 2 months of general medicine in their first year. This would provide ratings over time without overburdening interns to complete 3 additional evaluations after every inpatient rotation.
The peer evaluation was constructed to correspond to specific ACGME core competencies and was also linked to specific handoff behaviors that were known to be effective. The questions were adapted from prior items used in a validated direct‐observation tool previously developed by the authors (the Handoff Clinical Evaluation Exercise), which was based on literature review as well as expert opinion.[13, 14] For example, under the core competency of communication, interns were asked to rate each other on communication skills using the anchors of No questions, no acknowledgement of to do tasks, transfer of information face to face is not a priority for low unsatisfactory (1) and Appropriate use of questions, acknowledgement and read‐back of to‐do and priority tasks, face to face communication a priority for high superior (9). Items that referred to behaviors related to both giving handoff and receiving handoff were used to capture the interactive dialogue between senders and receivers that characterize ideal handoffs. In addition, specific items referring to written sign‐out and verbal sign‐out were developed to capture the specific differences. For instance, for the patient care competency in written sign‐out, low unsatisfactory (1) was defined as Incomplete written content; to do's omitted or requested with no rationale or plan, or with inadequate preparation (ie, request to transfuse but consent not obtained), and high superior (9) was defined as Content is complete with to do's accompanied by clear plan of action and rationale. Pilot testing with trainees was conducted, including residents not involved in the study and clinical students. The tool was also reviewed by the residency program leadership, and in an effort to standardize the reporting of the items with our other evaluation forms, each item was mapped to a core competency that it was most related to. Debriefing of the instrument experience following usage was performed with 3 residents who had an interest in medical education and handoff performance.
The tool was deployed to interns following a brief educational session for interns, in which the tool was previewed and reviewed. Interns were counseled to use the form as a global performance assessment over the course of the month, in contrast to an episodic evaluation. This would also avoid the use of negative event bias by raters, in which the rater allows a single negative event to influence the perception of the person's performance, even long after the event has passed into history.
To analyze the data, descriptive statistics were used to summarize mean performance across domains. To assess whether intern performance improved over time, we split the academic year into 3 time periods of 3 months each, which we have used in earlier studies assessing intern experience.[15] Prior to analysis, postcall interns were identified by using the intern monthly call schedule located in the AMiON software program (Norwich, VT) to label the evaluation of the postcall intern. Then, all names were removed and replaced with a unique identifier for the evaluator and the evaluatee. In addition, each evaluation was also categorized as either having come from the main teaching hospital or the community hospital affiliate.
Multivariate random effects linear regression models, controlling for evaluator, evaluatee, and hospital, were used to assess the association between time (using indicator variables for season) and postcall status on intern performance. In addition, because of the skewness in the ratings, we also undertook additional analysis by transforming our data into dichotomous variables reflecting superior performance. After conducting conditional ordinal logistic regression, the main findings did not change. We also investigated within‐subject and between‐subject variation using intraclass correlation coefficients. Within‐subject intraclass correlation enabled assessment of inter‐rater reliability. Between‐subject intraclass correlation enabled the assessment of evaluator effects. Evaluator effects can encompass a variety of forms of rater bias such as leniency (in which evaluators tended to rate individuals uniformly positively), severity (rater tends to significantly avoid using positive ratings), or the halo effect (the individual being evaluated has 1 significantly positive attribute that overrides that which is being evaluated). All analyses were completed using STATA 10.0 (StataCorp, College Station, TX) with statistical significance defined as P < 0.05. This study was deemed to be exempt from institutional review board review after all data were deidentified prior to analysis.
RESULTS
From July 2009 to March 2010, 31 interns (78%) returned 60% (172/288) of the peer evaluations they received. Almost all (39/40, 98%) interns were evaluated at least once with a median of 4 ratings per intern (range, 19). Thirty‐five percent of ratings occurred when an intern was rotating at the community hospital. Ratings were very high on all domains (mean, 8.38.6). Overall sign‐out performance was rated as 8.4 (95% confidence interval [CI], 8.3‐8.5), with over 55% rating peers as 9 (maximal score). The lowest score given was 5. Individual items ranged from a low of 8.34 (95% CI, 8.21‐8.47) for updating written sign‐outs, to a high of 8.60 (95% CI, 8.50‐8.69) for collegiality (Table 1) The internal consistency of the instrument was calculated using all items and was very high, with a Cronbach = 0.98.
| ACGME Core Competency | Role | Items | Item | Mean | 95% CI | Range | % Receiving 9 as Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Patient care | Sender | Written sign‐out | Q1 | 8.34 | 8.25 to 8.48 | 69 | 53.2 |
| Sender | Updated content | Q2 | 8.35 | 8.22 to 8.47 | 59 | 54.4 | |
| Receiver | Documentation of overnight events | Q6 | 8.41 | 8.30 to 8.52 | 69 | 56.3 | |
| Medical knowledge | Sender | Anticipatory guidance | Q3 | 8.40 | 8.28 to 8.51 | 69 | 56.3 |
| Receiver | Clinical decision making during cross‐cover | Q7 | 8.45 | 8.35 to 8.55 | 69 | 56.0 | |
| Professionalism | Sender | Collegiality | Q4 | 8.60 | 8.51 to 8.68 | 69 | 65.7 |
| Receiver | Acknowledgement of professional responsibility | Q10 | 8.53 | 8.43 to 8.62 | 69 | 62.4 | |
| Receiver | Timeliness/responsiveness | Q11 | 8.50 | 8.39 to 8.60 | 69 | 61.9 | |
| Interpersonal and communication skills | Receiver | Listening behavior when receiving sign‐outs | Q8 | 8.52 | 8.42 to 8.62 | 69 | 63.6 |
| Receiver | Communication when receiving sign‐out | Q9 | 8.52 | 8.43 to 8.62 | 69 | 63.0 | |
| Systems‐based practice | Receiver | Resource use | Q12 | 8.45 | 8.35 to 8.55 | 69 | 55.6 |
| Practice‐based learning and improvement | Sender | Accepting of feedback | Q5 | 8.45 | 8.34 to 8.55 | 69 | 58.7 |
| Overall | Both | Overall sign‐out quality | Q13 | 8.44 | 8.34 to 8.54 | 69 | 55.3 |
Mean ratings for each item increased in season 2 and 3 and were statistically significant using a test for trend across ordered groups. However, in multivariate regression models, improvements remained statistically significant for only 4 items (Figure 1): 1) communication skills, 2) listening behavior, 3) accepting professional responsibility, and 4) accessing the system (Table 2). Specifically, when compared to season 1, improvements in communication skill were seen in season 2 (+0.34 [95% CI, 0.08‐0.60], P = 0.009) and were sustained in season 3 (+0.34 [95% CI, 0.06‐0.61], P = 0.018). A similar pattern was observed for listening behavior, with improvement in ratings that were similar in magnitude with increasing intern experience (season 2, +0.29 [95% CI, 0.04‐0.55], P = 0.025 compared to season 1). Although accessing the system scores showed a similar pattern of improvement with an increase in season 2 compared to season 1, the magnitude of this change was smaller (season 2, +0.21 [95% CI, 0.03‐0.39], P = 0.023). Interestingly, improvements in accepting professional responsibility rose during season 2, but the difference did not reach statistical significance until season 3 (+0.37 [95% CI, 0.08‐0.65], P = 0.012 compared to season 1).

| Outcome | |||||
|---|---|---|---|---|---|
| Coefficient (95% CI) | |||||
| Predictor | Communication Skills | Listening Behavior | Professional Responsibility | Accessing the System | Written Sign‐out Quality |
| |||||
| Season 1 | Ref | Ref | Ref | Ref | Ref |
| Season 2 | 0.29 (0.04 to 0.55)a | 0.34 (0.08 to 0.60)a | 0.24 (0.03 to 0.51) | 0.21 (0.03 to 0.39)a | 0.05 (0.25 to 0.15) |
| Season 3 | 0.29 (0.02 to 0.56)a | 0.34 (0.06 to 0.61)a | 0.37 (0.08 to 0.65)a | 0.18 (0.01 to 0.36)a | 0.08 (0.13 to 0.30) |
| Community hospital | 0.18 (0.00 to 0.37) | 0.23 (0.04 to 0.43)a | 0.06 (0.13 to 0.26) | 0.13 (0.00 to 0.25) | 0.24 (0.08 to 0.39)a |
| Postcall | 0.10 (0.25 to 0.05) | 0.04 (0.21 to 0.13) | 0.02 (0.18 to 0.13) | 0.05 (0.16 to 0.05) | 0.18 (0.31,0.05)a |
| Constant | 7.04 (6.51 to 7.58) | 6.81 (6.23 to 7.38) | 7.04 (6.50 to 7.60) | 7.02 (6.59 to 7.45) | 6.49 (6.04 to 6.94) |
In addition to increasing experience, postcall interns were rated significantly lower than nonpostcall interns in 2 items: 1) written sign‐out quality (8.21 vs 8.39, P = 0.008) and 2) accepting feedback (practice‐based learning and improvement) (8.25 vs 8.42, P = 0.006). Interestingly, when interns were at the community hospital general medicine rotation, where overall census was much lower than at the teaching hospital, peer ratings were significantly higher for overall handoff performance and 7 (written sign‐out, update content, collegiality, accepting feedback, documentation of overnight events, clinical decision making during cross‐cover, and listening behavior) of the remaining 12 specific handoff domains (P < 0.05 for all, data not shown).
Last, significant evaluator effects were observed, which contributed to the variance in ratings given. For example, using intraclass correlation coefficients (ICC), we found that there was greater within‐intern variation than between‐intern variation, highlighting that evaluator scores tended to be strongly correlated with each other (eg, ICC overall performance = 0.64) and more so than scores of multiple evaluations of the same intern (eg, ICC overall performance = 0.18).
Because ratings of handoff performance were skewed, we also conducted a sensitivity analysis using ordinal logistic regression to ascertain if our findings remained significant. Using ordinal logistic regression models, significant improvements were seen in season 3 for 3 of the above‐listed behaviors, specifically listening behavior, professional responsibility, and accessing the system. Although there was no improvement in communication, there was an improvement observed in collegiality scores that were significant in season 3.
DISCUSSION
Using an end‐of‐rotation online peer assessment of handoff skills, it is feasible to obtain ratings of intern handoff performance from peers. Although there is evidence of rater bias toward leniency and low inter‐rater reliability, peer ratings of intern performance did increase over time. In addition, peer ratings were lower for interns who were handing off their postcall service. Working on a rotation at a community affiliate with a lower census was associated with higher peer ratings of handoffs.
It is worth considering the mechanism of these findings. First, the leniency observed in peer ratings likely reflects peers unwilling to critique each other due to a desire for an esprit de corps among their classmates. The low intraclass correlation coefficient for ratings of the same intern highlight that peers do not easily converge on their ratings of the same intern. Nevertheless, the ratings on the peer evaluation did demonstrate improvements over time. This improvement could easily reflect on‐the‐job learning, as interns become more acquainted with their roles and efficient and competent in their tasks. Together, these data provide a foundation for developing milestone handoffs that reflect the natural progression of intern competence in handoffs. For example, communication appeared to improve at 3 months, whereas transfer of professional responsibility improved at 6 months after beginning internship. However, alternative explanations are also important to consider. Although it is easy and somewhat reassuring to assume that increases over time reflect a learning effect, it is also possible that interns are unwilling to critique their peers as familiarity with them increases.
There are several reasons why postcall interns could have been universally rated lower than nonpostcall interns. First, postcall interns likely had the sickest patients with the most to‐do tasks or work associated with their sign‐out because they were handing off newly admitted patients. Because the postcall sign‐out is associated with the highest workload, it may be that interns perceive that a good handoff is nothing to do, and handoffs associated with more work are not highly rated. It is also important to note that postcall interns, who in this study were at the end of a 30‐hour duty shift, were also most fatigued and overworked, which may have also affected the handoff, especially in the 2 domains of interest. Due to the time pressure to leave coupled with fatigue, they may have had less time to invest in written sign‐out quality and may not have been receptive to feedback on their performance. Likewise, performance on handoffs was rated higher when at the community hospital, which could be due to several reasons. The most plausible explanation is that the workload associated with that sign‐out is less due to lower patient census and lower patient acuity. In the community hospital, fewer residents were also geographically co‐located on a quieter ward and work room area, which may contribute to higher ratings across domains.
This study also has implications for future efforts to improve and evaluate handoff performance in residency trainees. For example, our findings suggest the importance of enhancing supervision and training for handoffs during high workload rotations or certain times of the year. In addition, evaluation systems for handoff performance that rely solely on peer evaluation will not likely yield an accurate picture of handoff performance, difficulty obtaining peer evaluations, the halo effect, and other forms of evaluator bias in ratings. Accurate handoff evaluation may require direct observation of verbal communication and faculty audit of written sign‐outs.[16, 17] Moreover, methods such as appreciative inquiry can help identify the peers with the best practices to emulate.[18] Future efforts to validate peer assessment of handoffs against these other assessment methods, such as direct observation by service attendings, are needed.
There are limitations to this study. First, although we have limited our findings to 1 residency program with 1 type of rotation, we have already expanded to a community residency program that used a float system and have disseminated our tool to several other institutions. In addition, we have a small number of participants, and our 60% return rate on monthly peer evaluations raises concerns of nonresponse bias. For example, a peer who perceived the handoff performance of an intern to be poor may be less likely to return the evaluation. Because our dataset has been deidentified per institutional review board request, we do not have any information to differentiate systematic reasons for not responding to the evaluation. Anecdotally, a critique of the tool is that it is lengthy, especially in light of the fact that 1 intern completes 3 additional handoff evaluations. It is worth understanding why the instrument had such a high internal consistency. Although the items were designed to address different competencies initially, peers may make a global assessment about someone's ability to perform a handoff and then fill out the evaluation accordingly. This speaks to the difficulty in evaluating the subcomponents of various actions related to the handoff. Because of the high internal consistency, we were able to shorten the survey to a 5‐item instrument with a Cronbach of 0.93, which we are currently using in our program and have disseminated to other programs. Although it is currently unclear if the ratings of performance on the longer peer evaluation are valid, we are investigating concurrent validity of the shorter tool by comparing peer evaluations to other measures of handoff quality as part of our current work. Last, we are only able to test associations and not make causal inferences.
CONCLUSION
Peer assessment of handoff skills is feasible via an electronic competency‐based tool. Although there is evidence of score inflation, intern performance does increase over time and is associated with various aspects of workload, such as postcall status or working on a rotation at a community affiliate with a lower census. Together, these data can provide a foundation for developing milestones handoffs that reflect the natural progression of intern competence in handoffs.
Acknowledgments
The authors thank the University of Chicago Medicine residents and chief residents, the members of the Curriculum and Housestaff Evaluation Committee, Tyrece Hunter and Amy Ice‐Gibson, and Meryl Prochaska and Laura Ruth Venable for assistance with manuscript preparation.
Disclosures
This study was funded by the University of Chicago Department of Medicine Clinical Excellence and Medical Education Award and AHRQ R03 5R03HS018278‐02 Development of and Validation of a Tool to Evaluate Hand‐off Quality.
The advent of restricted residency duty hours has thrust the safety risks of handoffs into the spotlight. More recently, the Accreditation Council of Graduate Medical Education (ACGME) has restricted hours even further to a maximum of 16 hours for first‐year residents and up to 28 hours for residents beyond their first year.[1] Although the focus on these mandates has been scheduling and staffing in residency programs, another important area of attention is for handoff education and evaluation. The Common Program Requirements for the ACGME state that all residency programs should ensure that residents are competent in handoff communications and that programs should monitor handoffs to ensure that they are safe.[2] Moreover, recent efforts have defined milestones for handoffs, specifically that by 12 months, residents should be able to effectively communicate with other caregivers to maintain continuity during transitions of care.[3] Although more detailed handoff‐specific milestones have to be flushed out, a need for evaluation instruments to assess milestones is critical. In addition, handoffs continue to represent a vulnerable time for patients in many specialties, such as surgery and pediatrics.[4, 5]
Evaluating handoffs poses specific challenges for internal medicine residency programs because handoffs are often conducted on the fly or wherever convenient, and not always at a dedicated time and place.[6] Even when evaluations could be conducted at a dedicated time and place, program faculty and leadership may not be comfortable evaluating handoffs in real time due to lack of faculty development and recent experience with handoffs. Although supervising faculty may be in the most ideal position due to their intimate knowledge of the patient and their ability to evaluate the clinical judgment of trainees, they may face additional pressures of supervision and direct patient care that prevent their attendance at the time of the handoff. For these reasons, potential people to evaluate the quality of a resident handoff may be the peers to whom they frequently handoff. Because handoffs are also conceptualized as an interactive dialogue between sender and receiver, an ideal handoff performance evaluation would capture both of these roles.[7] For these reasons, peer evaluation may be a viable modality to assist programs in evaluating handoffs. Peer evaluation has been shown to be an effective method of rating performance of medical students,[8] practicing physicians,[9] and residents.[10] Moreover, peer evaluation is now a required feature in assessing internal medicine resident performance.[11] Although enthusiasm for peer evaluation has grown in residency training, the use of it can still be limited by a variety of problems, such as reluctance to rate peers poorly, difficulty obtaining evaluations, and the utility of such evaluations. For these reasons, it is important to understand whether peer evaluation of handoffs is feasible. Therefore, the aim of this study was to assess feasibility of an online peer evaluation survey tool of handoffs in an internal medicine residency and to characterize performance over time as well and associations between workload and performance.
METHODS
From July 2009 to March 2010, all interns on the general medicine inpatient service at 2 hospitals were asked to complete an end‐of‐month anonymous peer evaluation that included 14‐items addressing all core competencies. The evaluation tool was administered electronically using New Innovations (New Innovations, Inc., Uniontown, OH). Interns signed out to each other in a cross‐cover circuit that included 3 other interns on an every fourth night call cycle.[12] Call teams included 1 resident and 1 intern who worked from 7 am on the on‐call day to noon on the postcall day. Therefore, postcall interns were expected to hand off to the next on‐call intern before noon. Although attendings and senior residents were not required to formally supervise the handoff, supervising senior residents were often present during postcall intern sign‐out to facilitate departure of the team. When interns were not postcall, they were expected to sign out before they went to the clinic in the afternoon or when their foreseeable work was complete. The interns were provided with a 45‐minute lecture on handoffs and introduced to the peer evaluation tool in July 2009 at an intern orientation. They were also prompted to complete the tool to the best of their ability after their general medicine rotation. We chose the general medicine rotation because each intern completed approximately 2 months of general medicine in their first year. This would provide ratings over time without overburdening interns to complete 3 additional evaluations after every inpatient rotation.
The peer evaluation was constructed to correspond to specific ACGME core competencies and was also linked to specific handoff behaviors that were known to be effective. The questions were adapted from prior items used in a validated direct‐observation tool previously developed by the authors (the Handoff Clinical Evaluation Exercise), which was based on literature review as well as expert opinion.[13, 14] For example, under the core competency of communication, interns were asked to rate each other on communication skills using the anchors of No questions, no acknowledgement of to do tasks, transfer of information face to face is not a priority for low unsatisfactory (1) and Appropriate use of questions, acknowledgement and read‐back of to‐do and priority tasks, face to face communication a priority for high superior (9). Items that referred to behaviors related to both giving handoff and receiving handoff were used to capture the interactive dialogue between senders and receivers that characterize ideal handoffs. In addition, specific items referring to written sign‐out and verbal sign‐out were developed to capture the specific differences. For instance, for the patient care competency in written sign‐out, low unsatisfactory (1) was defined as Incomplete written content; to do's omitted or requested with no rationale or plan, or with inadequate preparation (ie, request to transfuse but consent not obtained), and high superior (9) was defined as Content is complete with to do's accompanied by clear plan of action and rationale. Pilot testing with trainees was conducted, including residents not involved in the study and clinical students. The tool was also reviewed by the residency program leadership, and in an effort to standardize the reporting of the items with our other evaluation forms, each item was mapped to a core competency that it was most related to. Debriefing of the instrument experience following usage was performed with 3 residents who had an interest in medical education and handoff performance.
The tool was deployed to interns following a brief educational session for interns, in which the tool was previewed and reviewed. Interns were counseled to use the form as a global performance assessment over the course of the month, in contrast to an episodic evaluation. This would also avoid the use of negative event bias by raters, in which the rater allows a single negative event to influence the perception of the person's performance, even long after the event has passed into history.
To analyze the data, descriptive statistics were used to summarize mean performance across domains. To assess whether intern performance improved over time, we split the academic year into 3 time periods of 3 months each, which we have used in earlier studies assessing intern experience.[15] Prior to analysis, postcall interns were identified by using the intern monthly call schedule located in the AMiON software program (Norwich, VT) to label the evaluation of the postcall intern. Then, all names were removed and replaced with a unique identifier for the evaluator and the evaluatee. In addition, each evaluation was also categorized as either having come from the main teaching hospital or the community hospital affiliate.
Multivariate random effects linear regression models, controlling for evaluator, evaluatee, and hospital, were used to assess the association between time (using indicator variables for season) and postcall status on intern performance. In addition, because of the skewness in the ratings, we also undertook additional analysis by transforming our data into dichotomous variables reflecting superior performance. After conducting conditional ordinal logistic regression, the main findings did not change. We also investigated within‐subject and between‐subject variation using intraclass correlation coefficients. Within‐subject intraclass correlation enabled assessment of inter‐rater reliability. Between‐subject intraclass correlation enabled the assessment of evaluator effects. Evaluator effects can encompass a variety of forms of rater bias such as leniency (in which evaluators tended to rate individuals uniformly positively), severity (rater tends to significantly avoid using positive ratings), or the halo effect (the individual being evaluated has 1 significantly positive attribute that overrides that which is being evaluated). All analyses were completed using STATA 10.0 (StataCorp, College Station, TX) with statistical significance defined as P < 0.05. This study was deemed to be exempt from institutional review board review after all data were deidentified prior to analysis.
RESULTS
From July 2009 to March 2010, 31 interns (78%) returned 60% (172/288) of the peer evaluations they received. Almost all (39/40, 98%) interns were evaluated at least once with a median of 4 ratings per intern (range, 19). Thirty‐five percent of ratings occurred when an intern was rotating at the community hospital. Ratings were very high on all domains (mean, 8.38.6). Overall sign‐out performance was rated as 8.4 (95% confidence interval [CI], 8.3‐8.5), with over 55% rating peers as 9 (maximal score). The lowest score given was 5. Individual items ranged from a low of 8.34 (95% CI, 8.21‐8.47) for updating written sign‐outs, to a high of 8.60 (95% CI, 8.50‐8.69) for collegiality (Table 1) The internal consistency of the instrument was calculated using all items and was very high, with a Cronbach = 0.98.
| ACGME Core Competency | Role | Items | Item | Mean | 95% CI | Range | % Receiving 9 as Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Patient care | Sender | Written sign‐out | Q1 | 8.34 | 8.25 to 8.48 | 69 | 53.2 |
| Sender | Updated content | Q2 | 8.35 | 8.22 to 8.47 | 59 | 54.4 | |
| Receiver | Documentation of overnight events | Q6 | 8.41 | 8.30 to 8.52 | 69 | 56.3 | |
| Medical knowledge | Sender | Anticipatory guidance | Q3 | 8.40 | 8.28 to 8.51 | 69 | 56.3 |
| Receiver | Clinical decision making during cross‐cover | Q7 | 8.45 | 8.35 to 8.55 | 69 | 56.0 | |
| Professionalism | Sender | Collegiality | Q4 | 8.60 | 8.51 to 8.68 | 69 | 65.7 |
| Receiver | Acknowledgement of professional responsibility | Q10 | 8.53 | 8.43 to 8.62 | 69 | 62.4 | |
| Receiver | Timeliness/responsiveness | Q11 | 8.50 | 8.39 to 8.60 | 69 | 61.9 | |
| Interpersonal and communication skills | Receiver | Listening behavior when receiving sign‐outs | Q8 | 8.52 | 8.42 to 8.62 | 69 | 63.6 |
| Receiver | Communication when receiving sign‐out | Q9 | 8.52 | 8.43 to 8.62 | 69 | 63.0 | |
| Systems‐based practice | Receiver | Resource use | Q12 | 8.45 | 8.35 to 8.55 | 69 | 55.6 |
| Practice‐based learning and improvement | Sender | Accepting of feedback | Q5 | 8.45 | 8.34 to 8.55 | 69 | 58.7 |
| Overall | Both | Overall sign‐out quality | Q13 | 8.44 | 8.34 to 8.54 | 69 | 55.3 |
Mean ratings for each item increased in season 2 and 3 and were statistically significant using a test for trend across ordered groups. However, in multivariate regression models, improvements remained statistically significant for only 4 items (Figure 1): 1) communication skills, 2) listening behavior, 3) accepting professional responsibility, and 4) accessing the system (Table 2). Specifically, when compared to season 1, improvements in communication skill were seen in season 2 (+0.34 [95% CI, 0.08‐0.60], P = 0.009) and were sustained in season 3 (+0.34 [95% CI, 0.06‐0.61], P = 0.018). A similar pattern was observed for listening behavior, with improvement in ratings that were similar in magnitude with increasing intern experience (season 2, +0.29 [95% CI, 0.04‐0.55], P = 0.025 compared to season 1). Although accessing the system scores showed a similar pattern of improvement with an increase in season 2 compared to season 1, the magnitude of this change was smaller (season 2, +0.21 [95% CI, 0.03‐0.39], P = 0.023). Interestingly, improvements in accepting professional responsibility rose during season 2, but the difference did not reach statistical significance until season 3 (+0.37 [95% CI, 0.08‐0.65], P = 0.012 compared to season 1).

| Outcome | |||||
|---|---|---|---|---|---|
| Coefficient (95% CI) | |||||
| Predictor | Communication Skills | Listening Behavior | Professional Responsibility | Accessing the System | Written Sign‐out Quality |
| |||||
| Season 1 | Ref | Ref | Ref | Ref | Ref |
| Season 2 | 0.29 (0.04 to 0.55)a | 0.34 (0.08 to 0.60)a | 0.24 (0.03 to 0.51) | 0.21 (0.03 to 0.39)a | 0.05 (0.25 to 0.15) |
| Season 3 | 0.29 (0.02 to 0.56)a | 0.34 (0.06 to 0.61)a | 0.37 (0.08 to 0.65)a | 0.18 (0.01 to 0.36)a | 0.08 (0.13 to 0.30) |
| Community hospital | 0.18 (0.00 to 0.37) | 0.23 (0.04 to 0.43)a | 0.06 (0.13 to 0.26) | 0.13 (0.00 to 0.25) | 0.24 (0.08 to 0.39)a |
| Postcall | 0.10 (0.25 to 0.05) | 0.04 (0.21 to 0.13) | 0.02 (0.18 to 0.13) | 0.05 (0.16 to 0.05) | 0.18 (0.31,0.05)a |
| Constant | 7.04 (6.51 to 7.58) | 6.81 (6.23 to 7.38) | 7.04 (6.50 to 7.60) | 7.02 (6.59 to 7.45) | 6.49 (6.04 to 6.94) |
In addition to increasing experience, postcall interns were rated significantly lower than nonpostcall interns in 2 items: 1) written sign‐out quality (8.21 vs 8.39, P = 0.008) and 2) accepting feedback (practice‐based learning and improvement) (8.25 vs 8.42, P = 0.006). Interestingly, when interns were at the community hospital general medicine rotation, where overall census was much lower than at the teaching hospital, peer ratings were significantly higher for overall handoff performance and 7 (written sign‐out, update content, collegiality, accepting feedback, documentation of overnight events, clinical decision making during cross‐cover, and listening behavior) of the remaining 12 specific handoff domains (P < 0.05 for all, data not shown).
Last, significant evaluator effects were observed, which contributed to the variance in ratings given. For example, using intraclass correlation coefficients (ICC), we found that there was greater within‐intern variation than between‐intern variation, highlighting that evaluator scores tended to be strongly correlated with each other (eg, ICC overall performance = 0.64) and more so than scores of multiple evaluations of the same intern (eg, ICC overall performance = 0.18).
Because ratings of handoff performance were skewed, we also conducted a sensitivity analysis using ordinal logistic regression to ascertain if our findings remained significant. Using ordinal logistic regression models, significant improvements were seen in season 3 for 3 of the above‐listed behaviors, specifically listening behavior, professional responsibility, and accessing the system. Although there was no improvement in communication, there was an improvement observed in collegiality scores that were significant in season 3.
DISCUSSION
Using an end‐of‐rotation online peer assessment of handoff skills, it is feasible to obtain ratings of intern handoff performance from peers. Although there is evidence of rater bias toward leniency and low inter‐rater reliability, peer ratings of intern performance did increase over time. In addition, peer ratings were lower for interns who were handing off their postcall service. Working on a rotation at a community affiliate with a lower census was associated with higher peer ratings of handoffs.
It is worth considering the mechanism of these findings. First, the leniency observed in peer ratings likely reflects peers unwilling to critique each other due to a desire for an esprit de corps among their classmates. The low intraclass correlation coefficient for ratings of the same intern highlight that peers do not easily converge on their ratings of the same intern. Nevertheless, the ratings on the peer evaluation did demonstrate improvements over time. This improvement could easily reflect on‐the‐job learning, as interns become more acquainted with their roles and efficient and competent in their tasks. Together, these data provide a foundation for developing milestone handoffs that reflect the natural progression of intern competence in handoffs. For example, communication appeared to improve at 3 months, whereas transfer of professional responsibility improved at 6 months after beginning internship. However, alternative explanations are also important to consider. Although it is easy and somewhat reassuring to assume that increases over time reflect a learning effect, it is also possible that interns are unwilling to critique their peers as familiarity with them increases.
There are several reasons why postcall interns could have been universally rated lower than nonpostcall interns. First, postcall interns likely had the sickest patients with the most to‐do tasks or work associated with their sign‐out because they were handing off newly admitted patients. Because the postcall sign‐out is associated with the highest workload, it may be that interns perceive that a good handoff is nothing to do, and handoffs associated with more work are not highly rated. It is also important to note that postcall interns, who in this study were at the end of a 30‐hour duty shift, were also most fatigued and overworked, which may have also affected the handoff, especially in the 2 domains of interest. Due to the time pressure to leave coupled with fatigue, they may have had less time to invest in written sign‐out quality and may not have been receptive to feedback on their performance. Likewise, performance on handoffs was rated higher when at the community hospital, which could be due to several reasons. The most plausible explanation is that the workload associated with that sign‐out is less due to lower patient census and lower patient acuity. In the community hospital, fewer residents were also geographically co‐located on a quieter ward and work room area, which may contribute to higher ratings across domains.
This study also has implications for future efforts to improve and evaluate handoff performance in residency trainees. For example, our findings suggest the importance of enhancing supervision and training for handoffs during high workload rotations or certain times of the year. In addition, evaluation systems for handoff performance that rely solely on peer evaluation will not likely yield an accurate picture of handoff performance, difficulty obtaining peer evaluations, the halo effect, and other forms of evaluator bias in ratings. Accurate handoff evaluation may require direct observation of verbal communication and faculty audit of written sign‐outs.[16, 17] Moreover, methods such as appreciative inquiry can help identify the peers with the best practices to emulate.[18] Future efforts to validate peer assessment of handoffs against these other assessment methods, such as direct observation by service attendings, are needed.
There are limitations to this study. First, although we have limited our findings to 1 residency program with 1 type of rotation, we have already expanded to a community residency program that used a float system and have disseminated our tool to several other institutions. In addition, we have a small number of participants, and our 60% return rate on monthly peer evaluations raises concerns of nonresponse bias. For example, a peer who perceived the handoff performance of an intern to be poor may be less likely to return the evaluation. Because our dataset has been deidentified per institutional review board request, we do not have any information to differentiate systematic reasons for not responding to the evaluation. Anecdotally, a critique of the tool is that it is lengthy, especially in light of the fact that 1 intern completes 3 additional handoff evaluations. It is worth understanding why the instrument had such a high internal consistency. Although the items were designed to address different competencies initially, peers may make a global assessment about someone's ability to perform a handoff and then fill out the evaluation accordingly. This speaks to the difficulty in evaluating the subcomponents of various actions related to the handoff. Because of the high internal consistency, we were able to shorten the survey to a 5‐item instrument with a Cronbach of 0.93, which we are currently using in our program and have disseminated to other programs. Although it is currently unclear if the ratings of performance on the longer peer evaluation are valid, we are investigating concurrent validity of the shorter tool by comparing peer evaluations to other measures of handoff quality as part of our current work. Last, we are only able to test associations and not make causal inferences.
CONCLUSION
Peer assessment of handoff skills is feasible via an electronic competency‐based tool. Although there is evidence of score inflation, intern performance does increase over time and is associated with various aspects of workload, such as postcall status or working on a rotation at a community affiliate with a lower census. Together, these data can provide a foundation for developing milestones handoffs that reflect the natural progression of intern competence in handoffs.
Acknowledgments
The authors thank the University of Chicago Medicine residents and chief residents, the members of the Curriculum and Housestaff Evaluation Committee, Tyrece Hunter and Amy Ice‐Gibson, and Meryl Prochaska and Laura Ruth Venable for assistance with manuscript preparation.
Disclosures
This study was funded by the University of Chicago Department of Medicine Clinical Excellence and Medical Education Award and AHRQ R03 5R03HS018278‐02 Development of and Validation of a Tool to Evaluate Hand‐off Quality.
- , , ; the ACGME Duty Hour Task Force. The new recommendations on duty hours from the ACGME Task Force. N Engl J Med. 2010; 363.
- Common program requirements. Available at: http://acgme‐2010standards.org/pdf/Common_Program_Requirements_07012011.pdf. Accessed December 10, 2012.
- , , , et al. Charting the road to competence: developmental milestones for internal medicine residency training. J Grad Med Educ. 2009;1(1):5–20.
- , , , et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204(4):533–540.
- , , , . Patient handoffs: pediatric resident experiences and lessons learned. Clin Pediatr (Phila). 2011;50(1):57–63.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , , . Communication, communication, communication: the art of the handoff. Ann Emerg Med. 2010;55(2):181–183.
- , , , , . Use of peer evaluation in the assessment of medical students. J Med Educ. 1981;56:35–42.
- , , , , , . Use of peer ratings to evaluate physician performance. JAMA. 1993;269:1655–1660.
- , , . A pilot study of peer review in residency training. J Gen Intern Med. 1999;14(9):551–554.
- ACGME Program Requirements for Graduate Medical Education in Internal Medicine Effective July 1, 2009. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/140_internal_medicine_07012009.pdf. Accessed December 10, 2012.
- , , , , , . The effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Hand‐off education and evaluation: piloting the observed simulated hand‐off experience (OSHE). J Gen Intern Med. 2010;25(2):129–134.
- , , , , , . Validation of a handoff assessment tool: the Handoff CEX [published online ahead of print June 7, 2012]. J Clin Nurs. doi: 10.1111/j.1365‐2702.2012.04131.x.
- , , , et al. Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities. JAMA. 2008;300(10):1146–1153.
- , . Using direct observation, formal evaluation, and an interactive curriculum to improve the sign‐out practices of internal medicine interns. Acad Med. 2010;85(7):1182–1188.
- , , , . Faculty member review and feedback using a sign‐out checklist: improving intern written sign‐out. Acad Med. 2012;87(8):1125–1131.
- , , , et al. Use of an appreciative inquiry approach to improve resident sign‐out in an era of multiple shift changes. J Gen Intern Med. 2012;27(3):287–291.
- , , ; the ACGME Duty Hour Task Force. The new recommendations on duty hours from the ACGME Task Force. N Engl J Med. 2010; 363.
- Common program requirements. Available at: http://acgme‐2010standards.org/pdf/Common_Program_Requirements_07012011.pdf. Accessed December 10, 2012.
- , , , et al. Charting the road to competence: developmental milestones for internal medicine residency training. J Grad Med Educ. 2009;1(1):5–20.
- , , , et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204(4):533–540.
- , , , . Patient handoffs: pediatric resident experiences and lessons learned. Clin Pediatr (Phila). 2011;50(1):57–63.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , , . Communication, communication, communication: the art of the handoff. Ann Emerg Med. 2010;55(2):181–183.
- , , , , . Use of peer evaluation in the assessment of medical students. J Med Educ. 1981;56:35–42.
- , , , , , . Use of peer ratings to evaluate physician performance. JAMA. 1993;269:1655–1660.
- , , . A pilot study of peer review in residency training. J Gen Intern Med. 1999;14(9):551–554.
- ACGME Program Requirements for Graduate Medical Education in Internal Medicine Effective July 1, 2009. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/ProgramRequirements/140_internal_medicine_07012009.pdf. Accessed December 10, 2012.
- , , , , , . The effects of on‐duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798.
- , , , et al. Hand‐off education and evaluation: piloting the observed simulated hand‐off experience (OSHE). J Gen Intern Med. 2010;25(2):129–134.
- , , , , , . Validation of a handoff assessment tool: the Handoff CEX [published online ahead of print June 7, 2012]. J Clin Nurs. doi: 10.1111/j.1365‐2702.2012.04131.x.
- , , , et al. Association of workload of on‐call medical interns with on‐call sleep duration, shift duration, and participation in educational activities. JAMA. 2008;300(10):1146–1153.
- , . Using direct observation, formal evaluation, and an interactive curriculum to improve the sign‐out practices of internal medicine interns. Acad Med. 2010;85(7):1182–1188.
- , , , . Faculty member review and feedback using a sign‐out checklist: improving intern written sign‐out. Acad Med. 2012;87(8):1125–1131.
- , , , et al. Use of an appreciative inquiry approach to improve resident sign‐out in an era of multiple shift changes. J Gen Intern Med. 2012;27(3):287–291.
Copyright © 2012 Society of Hospital Medicine
Medicare Funding May Become Enormous Burden for Generations of Future Taxpayers
February 2033
Dear sons:
Now that most of my baby boomer friends are 80 or 90 years old and are still hanging on, I wanted to apologize for leaving you in such a mess. Looking back, we all should have made some tough choices back in 2013, when some thoughtful belt-tightening would have created a fiscally sound ability for our country to provide healthcare and a safety net, not only to our senior citizens, but to all Americans. After today’s riots across the country, I felt I had to reach out to you and beg you to let rational minds prevail.
My fellow seniors, who paid into the Medicare and Social Security programs through our payroll taxes during the 30 to 40 years we worked in American industries, believe we are entitled to live forever with unlimited healthcare paid for by you. We are lined up almost every day at one doctor’s office or another to have our fourth joint replacement or our monthly MRI. Even though the actuaries tell us we all blew through our own contributions to Medicare sometime around our 75th birthdays, the general thinking of my friends on the golf course is that we paid for our parents’ healthcare and retirement, and you should just suck it up and stop whining.
Now I do admit that my friends tend to overlook the fact that when we were just in our 50s, like you are now, there were eight or nine workers (i.e. taxpayers) for every retiree. Now it seems it is one taxpayer working to support one retiree. The math just doesn’t work anymore. No wonder your tax burden is so suffocating that young workers can’t afford a home or a second car or even a vacation. I can see why there is talk by some of rationing care, but some of the rhetoric is kind of frightening.
Yes, there are more 90-year-olds with severe dementia on chronic dialysis than I would like to see. I don’t necessarily agree that everyone has a right to die with a normal BUN. Our generation did some great things with immunizations, cancer prevention, reducing the risks of coronary heart disease, and stroke prevention and treatment. The end result is that many of those who would have died earlier have lived beyond our country’s means to provide for them. For heaven’s sake, there are more than 1 million Americans over the age of 100 today. Once a woman gets past 65, it seems they are destined to live indefinitely.
Believe it or not, I was around in the 1960s when Medicare was first discussed and people were looking at life expectancies in the early 70s. No one saw the advent of so much expensive technology in diagnostic testing and surgical intervention. Despite more bipartisan national commissions and reports than I care to remember, no president or Congress has had the cojones to make the tough choices to provide the basic health needs for seniors in a fiscally sound system that doesn’t overwhelm the workforce.
I know the slogans urge a move from Medicare to “MediCan’t.” I know some want to bar seniors from getting flu shots and want to have pneumonia be the old man’s friend again. I sense a feeling that the elderly are becoming the enemy of the working class. I hear the rants that most of our nation’s wealth is held by those over 65, yet my generation wants more and more, feeling we paid for this and we deserve everything we have coming to us.
Once again, sorry this all had to fall on you, but I have got to run. I am going to see your grandmother. I can’t believe how well she is recovering from arthroscopic surgery. Pretty amazing for someone who is 105 years old.
Love,
Dad
Dr. Wellikson is CEO of SHM.
February 2033
Dear sons:
Now that most of my baby boomer friends are 80 or 90 years old and are still hanging on, I wanted to apologize for leaving you in such a mess. Looking back, we all should have made some tough choices back in 2013, when some thoughtful belt-tightening would have created a fiscally sound ability for our country to provide healthcare and a safety net, not only to our senior citizens, but to all Americans. After today’s riots across the country, I felt I had to reach out to you and beg you to let rational minds prevail.
My fellow seniors, who paid into the Medicare and Social Security programs through our payroll taxes during the 30 to 40 years we worked in American industries, believe we are entitled to live forever with unlimited healthcare paid for by you. We are lined up almost every day at one doctor’s office or another to have our fourth joint replacement or our monthly MRI. Even though the actuaries tell us we all blew through our own contributions to Medicare sometime around our 75th birthdays, the general thinking of my friends on the golf course is that we paid for our parents’ healthcare and retirement, and you should just suck it up and stop whining.
Now I do admit that my friends tend to overlook the fact that when we were just in our 50s, like you are now, there were eight or nine workers (i.e. taxpayers) for every retiree. Now it seems it is one taxpayer working to support one retiree. The math just doesn’t work anymore. No wonder your tax burden is so suffocating that young workers can’t afford a home or a second car or even a vacation. I can see why there is talk by some of rationing care, but some of the rhetoric is kind of frightening.
Yes, there are more 90-year-olds with severe dementia on chronic dialysis than I would like to see. I don’t necessarily agree that everyone has a right to die with a normal BUN. Our generation did some great things with immunizations, cancer prevention, reducing the risks of coronary heart disease, and stroke prevention and treatment. The end result is that many of those who would have died earlier have lived beyond our country’s means to provide for them. For heaven’s sake, there are more than 1 million Americans over the age of 100 today. Once a woman gets past 65, it seems they are destined to live indefinitely.
Believe it or not, I was around in the 1960s when Medicare was first discussed and people were looking at life expectancies in the early 70s. No one saw the advent of so much expensive technology in diagnostic testing and surgical intervention. Despite more bipartisan national commissions and reports than I care to remember, no president or Congress has had the cojones to make the tough choices to provide the basic health needs for seniors in a fiscally sound system that doesn’t overwhelm the workforce.
I know the slogans urge a move from Medicare to “MediCan’t.” I know some want to bar seniors from getting flu shots and want to have pneumonia be the old man’s friend again. I sense a feeling that the elderly are becoming the enemy of the working class. I hear the rants that most of our nation’s wealth is held by those over 65, yet my generation wants more and more, feeling we paid for this and we deserve everything we have coming to us.
Once again, sorry this all had to fall on you, but I have got to run. I am going to see your grandmother. I can’t believe how well she is recovering from arthroscopic surgery. Pretty amazing for someone who is 105 years old.
Love,
Dad
Dr. Wellikson is CEO of SHM.
February 2033
Dear sons:
Now that most of my baby boomer friends are 80 or 90 years old and are still hanging on, I wanted to apologize for leaving you in such a mess. Looking back, we all should have made some tough choices back in 2013, when some thoughtful belt-tightening would have created a fiscally sound ability for our country to provide healthcare and a safety net, not only to our senior citizens, but to all Americans. After today’s riots across the country, I felt I had to reach out to you and beg you to let rational minds prevail.
My fellow seniors, who paid into the Medicare and Social Security programs through our payroll taxes during the 30 to 40 years we worked in American industries, believe we are entitled to live forever with unlimited healthcare paid for by you. We are lined up almost every day at one doctor’s office or another to have our fourth joint replacement or our monthly MRI. Even though the actuaries tell us we all blew through our own contributions to Medicare sometime around our 75th birthdays, the general thinking of my friends on the golf course is that we paid for our parents’ healthcare and retirement, and you should just suck it up and stop whining.
Now I do admit that my friends tend to overlook the fact that when we were just in our 50s, like you are now, there were eight or nine workers (i.e. taxpayers) for every retiree. Now it seems it is one taxpayer working to support one retiree. The math just doesn’t work anymore. No wonder your tax burden is so suffocating that young workers can’t afford a home or a second car or even a vacation. I can see why there is talk by some of rationing care, but some of the rhetoric is kind of frightening.
Yes, there are more 90-year-olds with severe dementia on chronic dialysis than I would like to see. I don’t necessarily agree that everyone has a right to die with a normal BUN. Our generation did some great things with immunizations, cancer prevention, reducing the risks of coronary heart disease, and stroke prevention and treatment. The end result is that many of those who would have died earlier have lived beyond our country’s means to provide for them. For heaven’s sake, there are more than 1 million Americans over the age of 100 today. Once a woman gets past 65, it seems they are destined to live indefinitely.
Believe it or not, I was around in the 1960s when Medicare was first discussed and people were looking at life expectancies in the early 70s. No one saw the advent of so much expensive technology in diagnostic testing and surgical intervention. Despite more bipartisan national commissions and reports than I care to remember, no president or Congress has had the cojones to make the tough choices to provide the basic health needs for seniors in a fiscally sound system that doesn’t overwhelm the workforce.
I know the slogans urge a move from Medicare to “MediCan’t.” I know some want to bar seniors from getting flu shots and want to have pneumonia be the old man’s friend again. I sense a feeling that the elderly are becoming the enemy of the working class. I hear the rants that most of our nation’s wealth is held by those over 65, yet my generation wants more and more, feeling we paid for this and we deserve everything we have coming to us.
Once again, sorry this all had to fall on you, but I have got to run. I am going to see your grandmother. I can’t believe how well she is recovering from arthroscopic surgery. Pretty amazing for someone who is 105 years old.
Love,
Dad
Dr. Wellikson is CEO of SHM.
Southern Hospital Medicine Conference Drives Home the Value of Hospitalists
More than 300 hospitalists and other clinicians recently attended the 13th annual Southern Hospital Medicine Conference in Atlanta. The conference is a joint collaboration between the Emory University School of Medicine in Atlanta and Ochsner Health System New Orleans. The meeting site has alternated between the two cities each year since 2005.
The prevailing conference theme in 2012 was “Value and Values in Hospital Medicine,” alluding to the value that hospitalists bring to the medical community and hospitals and the values shared by hospitalists. The conference offered five pre-courses and more than 50 sessions focused on educating hospitalists on current best practices within core topic areas, including clinical care, quality improvement, healthcare information technology, innovative care models, systems of care, and transitions of care. A judged poster competition featured research and clinical vignettes abstracts, with interesting clinical cases as well as new research in hospital medicine.
One of the highlights of this year’s conference was the keynote address delivered by Dr. William A. Bornstein, chief quality and medical officer of Emory Healthcare. Dr. Bornstein discussed the various aspects of quality and cost in hospital care. He described the challenges in defining quality and measuring cost when trying to calculate the “value” equation in medicine (value=quality/cost). He outlined the Institute of Medicine’s previously described STEEEP (safe, timely, effective, efficient, equitable, patient-centered) aims of quality in 2001.
Dr. Bornstein’s own definition for quality is “partnering with patients and families to reliably, 100% of the time, deliver when, where, and how they want it—and with minimal waste—care based on the best available evidence and consistent with patient and family values and preferences.” To measure outcome, he said, we need to address system structure (what’s in place before the patient arrives), process (what we do for the patient), and culture (how we can get the buy-in from all stakeholders). The sum of these factors achieves outcome, which requires risk adjustment and, ideally, long-term follow-up data, he said.
Dr. Bornstein also discussed the need to develop standard processes whereby equivalent clinicians can follow similar processes to achieve the same results. When physicians “do it the same” (i.e. standardized protocols), error rates and cost decrease, he explained.
Dr. Bornstein also focused on transformative solutions to address problems in healthcare as a whole, rather than attempting to fix problems piecemeal.
Jason Stein, MD, SFHM, offered another conference highlight: a pre-conference program and plenary session on an innovative approach to improve hospital outcomes through implementation of the accountable-care unit (ACU). Dr. Stein, director of the clinical research program at Emory School of Medicine, described the current state of hospital care as asynchronous, with various providers caring for the patient without much coordination. For example, the physician sees the patient at 9 a.m., followed by the nurse at 10 a.m., and then finally the visiting family at 11 a.m. The ACU model of care would involve all the providers rounding with the patient and family at a scheduled time daily to provide synchronous care.
Dr. Stein described an ACU as a geographic inpatient area consistently responsible for the clinical, service, and cost outcomes it produces. Features of this unit include:
- Assignment of physicians by units to enhance predictability;
- Cohesiveness and communication;
- Structured interdisciplinary bedside rounds to consistently deliver evidence-based, patient-centered care;
- Evaluation of performance data by unit instead of facility or service line; and
- A dyad partnership involving a nurse unit director and a physician unit medical director.
ACU implementation at Emory has led to decreased mortality, reduced length of stay, and improved patient satisfaction compared to traditional units, according to Dr. Stein. While the ACU might not be suited for all, he said, all hospitals can learn from various components of this innovative approach to deliver better patient care.
The ever-changing state of HM in the U.S. remains a challenge, but it continues to generate innovation and excitement. The high number of engaged participants from 30 different states attending the 13th annual Southern Hospital Medicine Conference demonstrates that hospitalists are eager to learn and ready to improve their practice in order to provide high-value healthcare in U.S. hospitals today.
Dr. Lee is vice chairman in the department of hospital medicine at Ochsner Health System. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory University. Dr. Deitelzweig is system chairman in the department of hospital medicine and medical director for regional business development at Ochsner Health System. Dr. Wang is the division director of hospital medicine at Emory University. Dr. Dressler is director for education in the division of hospital medicine and an associate program director for the J. Willis Hurst Internal Medicine Residency Program at Emory University.
More than 300 hospitalists and other clinicians recently attended the 13th annual Southern Hospital Medicine Conference in Atlanta. The conference is a joint collaboration between the Emory University School of Medicine in Atlanta and Ochsner Health System New Orleans. The meeting site has alternated between the two cities each year since 2005.
The prevailing conference theme in 2012 was “Value and Values in Hospital Medicine,” alluding to the value that hospitalists bring to the medical community and hospitals and the values shared by hospitalists. The conference offered five pre-courses and more than 50 sessions focused on educating hospitalists on current best practices within core topic areas, including clinical care, quality improvement, healthcare information technology, innovative care models, systems of care, and transitions of care. A judged poster competition featured research and clinical vignettes abstracts, with interesting clinical cases as well as new research in hospital medicine.
One of the highlights of this year’s conference was the keynote address delivered by Dr. William A. Bornstein, chief quality and medical officer of Emory Healthcare. Dr. Bornstein discussed the various aspects of quality and cost in hospital care. He described the challenges in defining quality and measuring cost when trying to calculate the “value” equation in medicine (value=quality/cost). He outlined the Institute of Medicine’s previously described STEEEP (safe, timely, effective, efficient, equitable, patient-centered) aims of quality in 2001.
Dr. Bornstein’s own definition for quality is “partnering with patients and families to reliably, 100% of the time, deliver when, where, and how they want it—and with minimal waste—care based on the best available evidence and consistent with patient and family values and preferences.” To measure outcome, he said, we need to address system structure (what’s in place before the patient arrives), process (what we do for the patient), and culture (how we can get the buy-in from all stakeholders). The sum of these factors achieves outcome, which requires risk adjustment and, ideally, long-term follow-up data, he said.
Dr. Bornstein also discussed the need to develop standard processes whereby equivalent clinicians can follow similar processes to achieve the same results. When physicians “do it the same” (i.e. standardized protocols), error rates and cost decrease, he explained.
Dr. Bornstein also focused on transformative solutions to address problems in healthcare as a whole, rather than attempting to fix problems piecemeal.
Jason Stein, MD, SFHM, offered another conference highlight: a pre-conference program and plenary session on an innovative approach to improve hospital outcomes through implementation of the accountable-care unit (ACU). Dr. Stein, director of the clinical research program at Emory School of Medicine, described the current state of hospital care as asynchronous, with various providers caring for the patient without much coordination. For example, the physician sees the patient at 9 a.m., followed by the nurse at 10 a.m., and then finally the visiting family at 11 a.m. The ACU model of care would involve all the providers rounding with the patient and family at a scheduled time daily to provide synchronous care.
Dr. Stein described an ACU as a geographic inpatient area consistently responsible for the clinical, service, and cost outcomes it produces. Features of this unit include:
- Assignment of physicians by units to enhance predictability;
- Cohesiveness and communication;
- Structured interdisciplinary bedside rounds to consistently deliver evidence-based, patient-centered care;
- Evaluation of performance data by unit instead of facility or service line; and
- A dyad partnership involving a nurse unit director and a physician unit medical director.
ACU implementation at Emory has led to decreased mortality, reduced length of stay, and improved patient satisfaction compared to traditional units, according to Dr. Stein. While the ACU might not be suited for all, he said, all hospitals can learn from various components of this innovative approach to deliver better patient care.
The ever-changing state of HM in the U.S. remains a challenge, but it continues to generate innovation and excitement. The high number of engaged participants from 30 different states attending the 13th annual Southern Hospital Medicine Conference demonstrates that hospitalists are eager to learn and ready to improve their practice in order to provide high-value healthcare in U.S. hospitals today.
Dr. Lee is vice chairman in the department of hospital medicine at Ochsner Health System. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory University. Dr. Deitelzweig is system chairman in the department of hospital medicine and medical director for regional business development at Ochsner Health System. Dr. Wang is the division director of hospital medicine at Emory University. Dr. Dressler is director for education in the division of hospital medicine and an associate program director for the J. Willis Hurst Internal Medicine Residency Program at Emory University.
More than 300 hospitalists and other clinicians recently attended the 13th annual Southern Hospital Medicine Conference in Atlanta. The conference is a joint collaboration between the Emory University School of Medicine in Atlanta and Ochsner Health System New Orleans. The meeting site has alternated between the two cities each year since 2005.
The prevailing conference theme in 2012 was “Value and Values in Hospital Medicine,” alluding to the value that hospitalists bring to the medical community and hospitals and the values shared by hospitalists. The conference offered five pre-courses and more than 50 sessions focused on educating hospitalists on current best practices within core topic areas, including clinical care, quality improvement, healthcare information technology, innovative care models, systems of care, and transitions of care. A judged poster competition featured research and clinical vignettes abstracts, with interesting clinical cases as well as new research in hospital medicine.
One of the highlights of this year’s conference was the keynote address delivered by Dr. William A. Bornstein, chief quality and medical officer of Emory Healthcare. Dr. Bornstein discussed the various aspects of quality and cost in hospital care. He described the challenges in defining quality and measuring cost when trying to calculate the “value” equation in medicine (value=quality/cost). He outlined the Institute of Medicine’s previously described STEEEP (safe, timely, effective, efficient, equitable, patient-centered) aims of quality in 2001.
Dr. Bornstein’s own definition for quality is “partnering with patients and families to reliably, 100% of the time, deliver when, where, and how they want it—and with minimal waste—care based on the best available evidence and consistent with patient and family values and preferences.” To measure outcome, he said, we need to address system structure (what’s in place before the patient arrives), process (what we do for the patient), and culture (how we can get the buy-in from all stakeholders). The sum of these factors achieves outcome, which requires risk adjustment and, ideally, long-term follow-up data, he said.
Dr. Bornstein also discussed the need to develop standard processes whereby equivalent clinicians can follow similar processes to achieve the same results. When physicians “do it the same” (i.e. standardized protocols), error rates and cost decrease, he explained.
Dr. Bornstein also focused on transformative solutions to address problems in healthcare as a whole, rather than attempting to fix problems piecemeal.
Jason Stein, MD, SFHM, offered another conference highlight: a pre-conference program and plenary session on an innovative approach to improve hospital outcomes through implementation of the accountable-care unit (ACU). Dr. Stein, director of the clinical research program at Emory School of Medicine, described the current state of hospital care as asynchronous, with various providers caring for the patient without much coordination. For example, the physician sees the patient at 9 a.m., followed by the nurse at 10 a.m., and then finally the visiting family at 11 a.m. The ACU model of care would involve all the providers rounding with the patient and family at a scheduled time daily to provide synchronous care.
Dr. Stein described an ACU as a geographic inpatient area consistently responsible for the clinical, service, and cost outcomes it produces. Features of this unit include:
- Assignment of physicians by units to enhance predictability;
- Cohesiveness and communication;
- Structured interdisciplinary bedside rounds to consistently deliver evidence-based, patient-centered care;
- Evaluation of performance data by unit instead of facility or service line; and
- A dyad partnership involving a nurse unit director and a physician unit medical director.
ACU implementation at Emory has led to decreased mortality, reduced length of stay, and improved patient satisfaction compared to traditional units, according to Dr. Stein. While the ACU might not be suited for all, he said, all hospitals can learn from various components of this innovative approach to deliver better patient care.
The ever-changing state of HM in the U.S. remains a challenge, but it continues to generate innovation and excitement. The high number of engaged participants from 30 different states attending the 13th annual Southern Hospital Medicine Conference demonstrates that hospitalists are eager to learn and ready to improve their practice in order to provide high-value healthcare in U.S. hospitals today.
Dr. Lee is vice chairman in the department of hospital medicine at Ochsner Health System. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory University. Dr. Deitelzweig is system chairman in the department of hospital medicine and medical director for regional business development at Ochsner Health System. Dr. Wang is the division director of hospital medicine at Emory University. Dr. Dressler is director for education in the division of hospital medicine and an associate program director for the J. Willis Hurst Internal Medicine Residency Program at Emory University.
Tips to Help Hospital Medicine Group Leaders Know When to Grow Their Service
SHM board member Burke Kealey, MD, SFHM, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., says there is no easy way to know when it is the right time to grow. He offers four tips to hospitalist group leaders grappling with the question:
- Benchmark: Use the SHM survey, MGMA data, or local analyses to determine best practices. But don’t be a slave to data that don’t account for the particulars of your payor mix, patient population, etc.
- Network: Meet with group leaders in nearby practices. Talk to administrators. Understand the competitive set for your hospital and know what their data sets are.
- Communicate: Talk to doctors, C-suite executives, and everyone in between. Front-line physicians and nurses often know better than practice heads which resources are needed, and where.
- Stay flexible: Don’t be wedded to needing to grow. Maybe a group has physicians who want extra shifts to handle a new schedule. Maybe the installation of new technology will improve efficiency and eliminate the need for a new physician.
SHM board member Burke Kealey, MD, SFHM, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., says there is no easy way to know when it is the right time to grow. He offers four tips to hospitalist group leaders grappling with the question:
- Benchmark: Use the SHM survey, MGMA data, or local analyses to determine best practices. But don’t be a slave to data that don’t account for the particulars of your payor mix, patient population, etc.
- Network: Meet with group leaders in nearby practices. Talk to administrators. Understand the competitive set for your hospital and know what their data sets are.
- Communicate: Talk to doctors, C-suite executives, and everyone in between. Front-line physicians and nurses often know better than practice heads which resources are needed, and where.
- Stay flexible: Don’t be wedded to needing to grow. Maybe a group has physicians who want extra shifts to handle a new schedule. Maybe the installation of new technology will improve efficiency and eliminate the need for a new physician.
SHM board member Burke Kealey, MD, SFHM, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., says there is no easy way to know when it is the right time to grow. He offers four tips to hospitalist group leaders grappling with the question:
- Benchmark: Use the SHM survey, MGMA data, or local analyses to determine best practices. But don’t be a slave to data that don’t account for the particulars of your payor mix, patient population, etc.
- Network: Meet with group leaders in nearby practices. Talk to administrators. Understand the competitive set for your hospital and know what their data sets are.
- Communicate: Talk to doctors, C-suite executives, and everyone in between. Front-line physicians and nurses often know better than practice heads which resources are needed, and where.
- Stay flexible: Don’t be wedded to needing to grow. Maybe a group has physicians who want extra shifts to handle a new schedule. Maybe the installation of new technology will improve efficiency and eliminate the need for a new physician.
Fundamentals of Highly Reliable Organizations Could Benefit Hospitalists
Reliability. This sounds like a decent trait. Who wouldn’t want to be described as “reliable”? It sounds reputable whether you’re a person, a car, or a dishwasher. So how does one become or emulate the trait of being reliable, one who is predictable, punctua—“reproducible,” if you will?
Organizational reliability has received a fair bit of press these days. The industries that have come to embrace reliability concepts are those in which failure is easy to come by, and those in which failure is likely to be catastrophic if it occurs. In the medical industry, failure occurs to people, not widgets or machines, so by definition it tends to be catastrophic. These failures generally come in three flavors:
- The expected fails to occur (i.e. a patient with pneumonia does not receive their antibiotics on time);
- The unexpected occurs (i.e. a patient falls and breaks their hip); or
- The unexpected was not previously thought of (i.e. low-risk patient has a PEA arrest).
A fair bit of research has been done on how organizations can become more reliable. In their book “Managing the Unexpected: Assuring High Performance in an Age of Complexity,”1 Karl Weick and Kathleen Sutcliffe studied firefighting, workers on aircraft carriers, and nuclear power plant employees. They all have in common the fundamental similarity that failure in their workplace is catastrophically dangerous, and that they must continuously strive to reduce the risk and/or mitigate effectively. The Agency for Healthcare Research and Quality (AHRQ) specifically studied, through case studies and site visits, how some healthcare organizations have achieved some success in the different domains of reliability.2
What both studies found is that there are five prerequisites that, if done well, lead to an organizational “state of mindfulness.” What they and others have found in their research of highly reliable organizations (HROs) is not that they have failure-free operations, but that they continuously and “mindfully” think about how to be failure-free. Inattention and complacency are the biggest threats to reliability.
The Fundamentals
The first prerequisite is sensitivity to operations. This refers to actively seeking information on how things actually are working, instead of how they are supposed to be working. It is being acutely aware of all operations, including the smallest details: Does the patient have an armband on? Is the nurse washing their hands? Is the whiteboard information correct? Is the bed alarm enabled? It is the state of mind when everyone knows how things should work, look, feel, sound, and can recognize when something is out of bounds.
The next prerequisite is a preoccupation with failure. This refers to a system in which failure and near-misses are completely transparent, and openly and honestly discussed (without inciting individual blame or punitive action), and learned from communally. This “group thought” continually reaffirms the fact that systems, and everyone in them, are completely fallible to errors. It is the complete opposite of inattention and complacency. It is continuously asking “What can go wrong, how can it go wrong, when will it go wrong, and how can I stop it?”
The next prerequisite is reluctance to oversimplify. This does not imply that simplicity is bad, but that oversimplicity is lethal. It forces people and organizations to avoid shortcuts and to not rely on simplistic explanations for situations that need to be complicated. Think of this as making a complicated soufflé; if you leave out a step or an ingredient, the product will be far from a soufflé.
The next prerequisite is deference to expertise. This principle recognizes that authority and/or rank are not equivalent to expertise. This assumes that people and organizations are willing and able to defer decision-making to the person who will make the best decision, not to who ranks highest in the organizational chart. A junior hospitalist might be much more likely to make a good decision on building a new order set than the hospitalist director is.
The last prerequisite is resilience. Webster’s defines resilience as “the capability of a strained body to recover its size and shape after deformation caused especially by compressive stress … an ability to recover from or adjust easily to misfortune or change.” The “compressive stresses” and “misfortune or change” can present in a number of different ways, including bad patient outcomes, bad national press, or bad hospital rankings. A good HRO is not one that does not experience unexpected events, but is one that is not disabled by them. They routinely train and practice for worst-case scenarios. It is easy to “audit” resilience by looking at the organizational response to unexpected events. Are they handled with grace, ease, and speed, or with panic, anxiety, and ongoing uncertainty? Resilience involves adequately functioning despite adversity, recovering well, and learning from the experience.
Take-Home Message
The first three principles relate to how organizations can anticipate and reduce the risk of failure; the last two principles relate to how organizations mitigate the extent or severity of failure when it occurs. Together, they create the state of mindfulness, in which all senses are open and alert for signs of aberrations in the system, and where there is continuous learning of how to make the system function better.
What does this mean for a hospitalist to function in an HRO? Most hospitalists are on the front lines, where they routinely see where and how things can fail. They need to resist the urge to become complacent and remain continuously alert to signals that the system is not functioning for the safety of the patient. And when things do go awry, they need to be part of the resilience plan, to work with their teams to swiftly and effectively mitigate ongoing risks, and defer decision to expertise and not necessarily authority.
It also requires that each of us work within multidisciplinary teams in which all members add to the “state of mindfulness,” including the patient and their families (who very often note “aberrancies” before anyone else does). Think of your hospital as ascribed by Gordon Bethune, the former CEO of Continental Airlines. When asked why all employees received a bonus for on-time departure (instead of only employees on the front line), he held up his wristwatch and said, “What part of this watch don’t you think we need?”
Hospitalists can be powerful motivators for a culture change that empowers all hospital employees to be engaged in anticipating and managing failures—just by being mindful. This is a great place to start.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Weick KE, Sutcliffe KM. Managing the unexpected: Resilient performance in an age of uncertainty, 2nd ed. 2007: Hoboken, NJ: John Wiley & Sons Inc.
- Agency for Healthcare Research and Quality. Becoming a high reliability organization: operational advice for hospital leaders. Agency for Healthcare Research and Quality website. Available at: http://www.ahrq.gov/qual/hroadvice/. Accessed Dec. 10, 2012.
Reliability. This sounds like a decent trait. Who wouldn’t want to be described as “reliable”? It sounds reputable whether you’re a person, a car, or a dishwasher. So how does one become or emulate the trait of being reliable, one who is predictable, punctua—“reproducible,” if you will?
Organizational reliability has received a fair bit of press these days. The industries that have come to embrace reliability concepts are those in which failure is easy to come by, and those in which failure is likely to be catastrophic if it occurs. In the medical industry, failure occurs to people, not widgets or machines, so by definition it tends to be catastrophic. These failures generally come in three flavors:
- The expected fails to occur (i.e. a patient with pneumonia does not receive their antibiotics on time);
- The unexpected occurs (i.e. a patient falls and breaks their hip); or
- The unexpected was not previously thought of (i.e. low-risk patient has a PEA arrest).
A fair bit of research has been done on how organizations can become more reliable. In their book “Managing the Unexpected: Assuring High Performance in an Age of Complexity,”1 Karl Weick and Kathleen Sutcliffe studied firefighting, workers on aircraft carriers, and nuclear power plant employees. They all have in common the fundamental similarity that failure in their workplace is catastrophically dangerous, and that they must continuously strive to reduce the risk and/or mitigate effectively. The Agency for Healthcare Research and Quality (AHRQ) specifically studied, through case studies and site visits, how some healthcare organizations have achieved some success in the different domains of reliability.2
What both studies found is that there are five prerequisites that, if done well, lead to an organizational “state of mindfulness.” What they and others have found in their research of highly reliable organizations (HROs) is not that they have failure-free operations, but that they continuously and “mindfully” think about how to be failure-free. Inattention and complacency are the biggest threats to reliability.
The Fundamentals
The first prerequisite is sensitivity to operations. This refers to actively seeking information on how things actually are working, instead of how they are supposed to be working. It is being acutely aware of all operations, including the smallest details: Does the patient have an armband on? Is the nurse washing their hands? Is the whiteboard information correct? Is the bed alarm enabled? It is the state of mind when everyone knows how things should work, look, feel, sound, and can recognize when something is out of bounds.
The next prerequisite is a preoccupation with failure. This refers to a system in which failure and near-misses are completely transparent, and openly and honestly discussed (without inciting individual blame or punitive action), and learned from communally. This “group thought” continually reaffirms the fact that systems, and everyone in them, are completely fallible to errors. It is the complete opposite of inattention and complacency. It is continuously asking “What can go wrong, how can it go wrong, when will it go wrong, and how can I stop it?”
The next prerequisite is reluctance to oversimplify. This does not imply that simplicity is bad, but that oversimplicity is lethal. It forces people and organizations to avoid shortcuts and to not rely on simplistic explanations for situations that need to be complicated. Think of this as making a complicated soufflé; if you leave out a step or an ingredient, the product will be far from a soufflé.
The next prerequisite is deference to expertise. This principle recognizes that authority and/or rank are not equivalent to expertise. This assumes that people and organizations are willing and able to defer decision-making to the person who will make the best decision, not to who ranks highest in the organizational chart. A junior hospitalist might be much more likely to make a good decision on building a new order set than the hospitalist director is.
The last prerequisite is resilience. Webster’s defines resilience as “the capability of a strained body to recover its size and shape after deformation caused especially by compressive stress … an ability to recover from or adjust easily to misfortune or change.” The “compressive stresses” and “misfortune or change” can present in a number of different ways, including bad patient outcomes, bad national press, or bad hospital rankings. A good HRO is not one that does not experience unexpected events, but is one that is not disabled by them. They routinely train and practice for worst-case scenarios. It is easy to “audit” resilience by looking at the organizational response to unexpected events. Are they handled with grace, ease, and speed, or with panic, anxiety, and ongoing uncertainty? Resilience involves adequately functioning despite adversity, recovering well, and learning from the experience.
Take-Home Message
The first three principles relate to how organizations can anticipate and reduce the risk of failure; the last two principles relate to how organizations mitigate the extent or severity of failure when it occurs. Together, they create the state of mindfulness, in which all senses are open and alert for signs of aberrations in the system, and where there is continuous learning of how to make the system function better.
What does this mean for a hospitalist to function in an HRO? Most hospitalists are on the front lines, where they routinely see where and how things can fail. They need to resist the urge to become complacent and remain continuously alert to signals that the system is not functioning for the safety of the patient. And when things do go awry, they need to be part of the resilience plan, to work with their teams to swiftly and effectively mitigate ongoing risks, and defer decision to expertise and not necessarily authority.
It also requires that each of us work within multidisciplinary teams in which all members add to the “state of mindfulness,” including the patient and their families (who very often note “aberrancies” before anyone else does). Think of your hospital as ascribed by Gordon Bethune, the former CEO of Continental Airlines. When asked why all employees received a bonus for on-time departure (instead of only employees on the front line), he held up his wristwatch and said, “What part of this watch don’t you think we need?”
Hospitalists can be powerful motivators for a culture change that empowers all hospital employees to be engaged in anticipating and managing failures—just by being mindful. This is a great place to start.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Weick KE, Sutcliffe KM. Managing the unexpected: Resilient performance in an age of uncertainty, 2nd ed. 2007: Hoboken, NJ: John Wiley & Sons Inc.
- Agency for Healthcare Research and Quality. Becoming a high reliability organization: operational advice for hospital leaders. Agency for Healthcare Research and Quality website. Available at: http://www.ahrq.gov/qual/hroadvice/. Accessed Dec. 10, 2012.
Reliability. This sounds like a decent trait. Who wouldn’t want to be described as “reliable”? It sounds reputable whether you’re a person, a car, or a dishwasher. So how does one become or emulate the trait of being reliable, one who is predictable, punctua—“reproducible,” if you will?
Organizational reliability has received a fair bit of press these days. The industries that have come to embrace reliability concepts are those in which failure is easy to come by, and those in which failure is likely to be catastrophic if it occurs. In the medical industry, failure occurs to people, not widgets or machines, so by definition it tends to be catastrophic. These failures generally come in three flavors:
- The expected fails to occur (i.e. a patient with pneumonia does not receive their antibiotics on time);
- The unexpected occurs (i.e. a patient falls and breaks their hip); or
- The unexpected was not previously thought of (i.e. low-risk patient has a PEA arrest).
A fair bit of research has been done on how organizations can become more reliable. In their book “Managing the Unexpected: Assuring High Performance in an Age of Complexity,”1 Karl Weick and Kathleen Sutcliffe studied firefighting, workers on aircraft carriers, and nuclear power plant employees. They all have in common the fundamental similarity that failure in their workplace is catastrophically dangerous, and that they must continuously strive to reduce the risk and/or mitigate effectively. The Agency for Healthcare Research and Quality (AHRQ) specifically studied, through case studies and site visits, how some healthcare organizations have achieved some success in the different domains of reliability.2
What both studies found is that there are five prerequisites that, if done well, lead to an organizational “state of mindfulness.” What they and others have found in their research of highly reliable organizations (HROs) is not that they have failure-free operations, but that they continuously and “mindfully” think about how to be failure-free. Inattention and complacency are the biggest threats to reliability.
The Fundamentals
The first prerequisite is sensitivity to operations. This refers to actively seeking information on how things actually are working, instead of how they are supposed to be working. It is being acutely aware of all operations, including the smallest details: Does the patient have an armband on? Is the nurse washing their hands? Is the whiteboard information correct? Is the bed alarm enabled? It is the state of mind when everyone knows how things should work, look, feel, sound, and can recognize when something is out of bounds.
The next prerequisite is a preoccupation with failure. This refers to a system in which failure and near-misses are completely transparent, and openly and honestly discussed (without inciting individual blame or punitive action), and learned from communally. This “group thought” continually reaffirms the fact that systems, and everyone in them, are completely fallible to errors. It is the complete opposite of inattention and complacency. It is continuously asking “What can go wrong, how can it go wrong, when will it go wrong, and how can I stop it?”
The next prerequisite is reluctance to oversimplify. This does not imply that simplicity is bad, but that oversimplicity is lethal. It forces people and organizations to avoid shortcuts and to not rely on simplistic explanations for situations that need to be complicated. Think of this as making a complicated soufflé; if you leave out a step or an ingredient, the product will be far from a soufflé.
The next prerequisite is deference to expertise. This principle recognizes that authority and/or rank are not equivalent to expertise. This assumes that people and organizations are willing and able to defer decision-making to the person who will make the best decision, not to who ranks highest in the organizational chart. A junior hospitalist might be much more likely to make a good decision on building a new order set than the hospitalist director is.
The last prerequisite is resilience. Webster’s defines resilience as “the capability of a strained body to recover its size and shape after deformation caused especially by compressive stress … an ability to recover from or adjust easily to misfortune or change.” The “compressive stresses” and “misfortune or change” can present in a number of different ways, including bad patient outcomes, bad national press, or bad hospital rankings. A good HRO is not one that does not experience unexpected events, but is one that is not disabled by them. They routinely train and practice for worst-case scenarios. It is easy to “audit” resilience by looking at the organizational response to unexpected events. Are they handled with grace, ease, and speed, or with panic, anxiety, and ongoing uncertainty? Resilience involves adequately functioning despite adversity, recovering well, and learning from the experience.
Take-Home Message
The first three principles relate to how organizations can anticipate and reduce the risk of failure; the last two principles relate to how organizations mitigate the extent or severity of failure when it occurs. Together, they create the state of mindfulness, in which all senses are open and alert for signs of aberrations in the system, and where there is continuous learning of how to make the system function better.
What does this mean for a hospitalist to function in an HRO? Most hospitalists are on the front lines, where they routinely see where and how things can fail. They need to resist the urge to become complacent and remain continuously alert to signals that the system is not functioning for the safety of the patient. And when things do go awry, they need to be part of the resilience plan, to work with their teams to swiftly and effectively mitigate ongoing risks, and defer decision to expertise and not necessarily authority.
It also requires that each of us work within multidisciplinary teams in which all members add to the “state of mindfulness,” including the patient and their families (who very often note “aberrancies” before anyone else does). Think of your hospital as ascribed by Gordon Bethune, the former CEO of Continental Airlines. When asked why all employees received a bonus for on-time departure (instead of only employees on the front line), he held up his wristwatch and said, “What part of this watch don’t you think we need?”
Hospitalists can be powerful motivators for a culture change that empowers all hospital employees to be engaged in anticipating and managing failures—just by being mindful. This is a great place to start.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Weick KE, Sutcliffe KM. Managing the unexpected: Resilient performance in an age of uncertainty, 2nd ed. 2007: Hoboken, NJ: John Wiley & Sons Inc.
- Agency for Healthcare Research and Quality. Becoming a high reliability organization: operational advice for hospital leaders. Agency for Healthcare Research and Quality website. Available at: http://www.ahrq.gov/qual/hroadvice/. Accessed Dec. 10, 2012.