User login
Have you tried these innovative alternatives to antibiotics for UTI prevention?
The authors report no financial relationships relevant to this article.
CASE: Recurrent UTI and antibiotic resistance
A 53-year-old postmenopausal woman with a history of culture-proven recurrent Escherichia coli urinary tract infections (UTIs) presents to the clinic with symptoms of UTI. She was previously treated with a postcoital regimen of trimethoprim/sulfamethoxazole, based on sensitivities identified by culture. A past work-up of her upper and lower urinary tract was negative. You send a catheterized specimen for culture; again, E. coli is identified as the pathogen but proves resistant to her current antibiotic regimen.
What treatment alternatives, aside from antibiotics, are available for this patient—and how might they affect resistance?
Increased antibiotic usage has led to greater bacterial resistance, which is perpetuated by clonal spread. Resistant strains of E. coli have been found in household members, suggesting host-host transmission as a mechanism for dissemination. Alternative treatments that reduce the use of antibiotics may minimize bacterial resistance and increase the efficacy of treatment. In the TABLE , we summarize alternative approaches to the treatment of recurrent UTI. We also describe a strategy to alleviate symptoms.
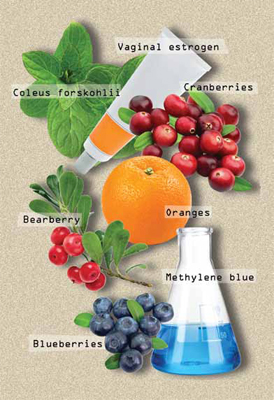
Alternatives to antibiotics in the treatment and prevention of recurrent UTI
| Category | Type | Examples and doses, if recommended |
|---|---|---|
| Vaginal estrogen | Conjugated estrogen cream Estradiol
| Premarin cream, 0.5–2 g vaginally twice weekly
|
| Nutritive agents | Cranberry juice Cranberry tablets Cystopurin Lactobacilli Blueberry products | Not recommended 1 tablet (300 to 400 mg, depending on manufacturer) twice daily Not recommended Vivag, EcoVag, 1 capsule daily by vagina for 5 days, then once weekly for 10 weeks Not recommended |
| Anti-infective drugs | Methenamine hippurate Methenamine mandelate Methylene blue | Urex or Hiprex, 1 g orally twice daily Mandelamine, 1 g orally 4 times daily Future therapy |
| Urinary acidifiers | Vitamin C/ascorbic acid | 1–3 g orally 3–4 times daily |
| Herbal remedies | Uva ursi Forskolin | Not recommended for long-term use Not recommended |
| Behavioral changes | Adequate hydration Postcoital voiding |
Vaginal estrogen is the only proven alternative to antibiotics for postmenopausal women
A lack of estrogen is a risk factor for UTI and is associated with atrophic mucosa, leading to decreased colonization with lactobacilli, increased vaginal pH, and E. coli colonization.
A randomized, double-blind, placebo-controlled trial of intravaginal estriol cream versus placebo in 93 postmenopausal women found a significant decrease in the rate of UTI among women who used the cream.1 After 8 months of follow-up, the incidence of UTI was 0.5 vs 5.9 episodes per patient-year (P <.001). Interestingly, all pretreatment cultures were negative for lactobacilli. One month after treatment, 61% of women in the estriol group were culture-positive for lactobacilli, compared with 0% of the placebo group.1
A 2008 Cochrane review of nine studies concluded that vaginal estrogen reduces the number of UTIs in postmenopausal women, with variation based on the type of estrogen and duration of use.2
Adverse effects are mild
Twenty-eight percent of the estriol group in the randomized trial described above withdrew from treatment, with 20% citing local side effects, including vaginal irritation, burning, or itching—all of which were mild and self-limited.1 Other possible adverse effects include breast tenderness, vaginal bleeding or spotting, and discharge.2
Clinical recommendations
Given the efficacy of this therapy, we recommend topical estrogen for postmenopausal patients with recurrent UTIs.
Cranberry juice may reduce UTI, but many patients withdraw
from treatment
Cranberries belong to the Vaccinium species, which contains all flavonoids, including anthocyanins and proanthocyanidins. It was previously thought that the acidification of urine produced an antibacterial effect, but several trials have documented no change in urine levels of hippuric acid when cranberry products are given, with no acidification of the urine.3 Current theory suggests that cranberries prevent bacteria from adhering to the uroepithelial cells of the walls of the bladder, by blocking expression of E. coli’s adhesion molecule, P. fimbriae, so that bacteria are unable to penetrate the mucosal surface.4,5 The major benefit of cranberry products over antibiotic prophylaxis is that they do not have the potential for resistance.4
A 2008 Cochrane review concluded that cranberry juice may reduce symptomatic UTIs, particularly among young, sexually active women—but there is a high rate of withdrawal from treatment.6 The optimal method of administration and dose remain unclear. In contrast, two recent randomized, controlled trials—published after the Cochrane review—found no difference in the rate of recurrent UTI in premenopausal women.7,8 Adverse effects in these two trials included constipation, heartburn, loose stools, vaginal itching and dryness, and migraines. Of note, there was no statistical difference in side effects between the cranberry and placebo groups.7
Vaccinium tablets may be protective in older women
Cranberry extracts of 500 mg to 1,000 mg daily have been compared with antimicrobial prophylaxis in two randomized, double-blind, controlled trials. The trials demonstrated mixed benefits. Trimethoprim/sulfamethoxazole was associated with a lower rate of UTI in younger women, compared with cranberry extracts alone (P=.02), while cranberry extracts were slightly more effective than trimethoprim alone in older women.9,10 Cranberry tablets were not associated with bacterial resistance, were cheaper, and were viewed as a more natural option. The interventions were equally well tolerated.
Overall efficacy of cranberry tablets is unclear. Side effects, albeit mild, included gastrointestinal disturbances, vaginal complaints, and rash or urticaria. There was no significant difference in the rate of adverse effects between antimicrobial treatment and cranberry tablets.9
Cystopurin has not been studied
Cystopurin is an over-the-counter (OTC) tablet containing cranberry extract and potassium citrate that is taken three times daily (3 g/dose) for 2 days. Interestingly, although a proposed mechanism for the efficacy of vitamin C and cranberry juice has been a reduction of pH, potassium citrate is an alkalizing agent that is reported to relieve burning and reduce urinary urgency and frequency. No studies have assessed this medication in the treatment of a UTI or its symptoms.
Clinical recommendations
The evidence is mixed on the use of cranberry products to reduce recurrent UTI. However, given the limited side effects associated with these products, we offer cranberry tablets to patients who have recurrent UTIs who are interested in a more natural alternative.
We generally do not recommend cranberry juice because the added fluid volume tends to exacerbate frequency and urgency symptoms.
Lactobacilli suppositories may benefit
postmenopausal women
Lactobacilli are fastidious gram-positive rods and are usually the dominant component of the vaginal flora.11 They prevent colonization and infection by more virulent bacteria by competing for adhesion receptors and nutrients as well as producing antimicrobial substances such as hydrogen peroxide and lactic acid. A decrease in lactobacilli leaves the urinary tract susceptible to infectious organisms that may colonize the vaginal mucosa and increase the risk of recurrent UTI.12-14
A 2008 review of randomized, controlled trials of oral lactobacilli and UTI was inconclusive, due to inconsistent dosing strategies and small sample sizes.15 A 2011 randomized, double-blind, placebo-controlled phase 2 trial of Lactin-V, a lactobacilli vaginal suppository, found that it reduced the rate of recurrent UTI. Lactin-V contains a hydrogen-peroxide–producing Lactobacillus crispatus developed as a probiotic that was determined to be safe and tolerable as a vaginal suppository in a phase 1 trial.16 The phase 2 trial enrolled 100 young premenopausal women with a history of recurrent UTI who took either Lactin-V or placebo daily for 5 days, then weekly for 10 weeks. Women in the Lactin-V group who had high levels of L. crispatus colonization experienced a significant reduction in the rate of UTI (15% vs 27% in the placebo group), but the effect did not reach statistical significance.14
Little difference in adverse effects
Adverse effects were reported among 56% of patients who received Lactin-V versus 50% of those given placebo. The most common of these were vaginal discharge, itching, and moderate abdominal discomfort.14 Although lactobacillus can potentially promote UTI, this phenomenon is rare.11
Regrettably, Lactin-V is not currently available in the United States. However, there are other lactobacilli vaginal suppositories on the market ( TABLE ). Given the low risk associated with their use, they should be considered as an alternative for patients who cannot or will not use estrogen.
Clinical recommendations
Probiotics such as lactobacilli are categorized as “dietary supplements”; as such, they are not regulated by the US Food and Drug Administration. We recommend the use of lactobacilli suppositories in postmenopausal women who have a contraindication to (or prefer to avoid) vaginal estrogen.
Skip blueberry products for now
Like cranberries, blueberries belong to the Vaccinium species and are thought to interfere with bacterial adhesion to the walls of the bladder. One in vitro trial suggests that blueberries also have antiproliferation effects, although no clinical studies have been performed to date to further investigate safety or efficacy.4 Consequently, we do not recommend use of these products.
Methenamine salts may benefit some populations
These anti-infective agents, including methenamine hippurate and methenamine mandelate, often are used to prevent UTI. They are found in combination OTC medications, such as Prosed DS and Urelle. Methenamine salts are bacteriostatic to all urinary tract pathogens due to their production of formaldehyde.3,16
Although methenamine produces varying concentrations of formaldehyde, depending on the acidity of the urine, there is no evidence that acidified urine enhances methenamine’s effects.16
Advantages of methenamine include the fact that it produces no changes in gut flora, poses no risk for antimicrobial resistance, and has low toxicity. It also is low in cost.17
Methenamine is contraindicated in patients with renal insufficiency or severe hepatic disease.
Adverse reactions are generally mild and include gastrointestinal disturbances, skin rashes, dysuria, and microscopic hematuria.16
Methenamine hippurate
A 2007 Cochrane review, deemed up to date in 2010, analyzed 13 randomized, controlled trials involving 2,032 participants. Subgroup analyses suggested that methenamine hippurate may be of some benefit to patients without renal tract abnormalities; these patients experienced significantly reduced symptoms after short-term treatment of 1 week or less.
Patients with spinal injury do not appear to benefit from treatment, according to a randomized, double-blind, placebo-controlled trial by Lee and colleagues.18
Methenamine mandelate
This agent is commonly used to prevent recurrent UTI, although there is a paucity of randomized, controlled trials to support its use. One such trial in patients with neurogenic bladder found that methenamine mandelate with acidification was superior to placebo (P <.02) for preventing UTI.19 Beyond this population, however, it’s difficult to assess methenamine’s efficacy in the prevention of recurrent UTI.
Methylene blue may be useful in the elderly
Methylene blue is a light-activated compound described as “photodynamic antimicrobial chemotherapy” (PACT). Once illuminated, it becomes a bactericide, causing excitation of electrons followed by one of two reactions:
- reduction oxidation
- formation of a labile singlet oxygen and then oxidation.
This makes resistance unlikely.
In an in vitro study, methylene blue was as effective as levofloxacin against Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis, Enterococcus, and Staphylococcus aureus when illuminated.
Potential benefits of this mode of treatment include local exposure and no drug interactions.
We suggest that methylene blue be placed and illuminated with a special catheter that would target UTI in the elderly population, among whom 52% of UTIs are associated with use of catheters.20 In vivo effects, adverse effects, and cost are not known, which limits current applicability of this compound.
Vitamin C may reduce UTI in pregnancy
The proposed mechanism for the efficacy of vitamin C for the treatment of UTI is the acidification of urine, which is believed to reduce the proliferation of bacteria. However, several studies have shown that vitamin C, at various doses, does not reliably reduce urine pH.15,21,22 Nonetheless, ascorbic acid was tested for its effect on UTI prevention during pregnancy.
In a single-blind trial, 110 pregnant women were divided into two groups (55 in each group):
- One group received ferrous sulfate (200 mg), folic acid (5 mg), and vitamin C (100 mg) daily for 3 months
- The other received ferrous sulfate (200 mg) and folic acid (5 mg) daily for 3 months.
Urine was cultured monthly. The incidence of UTI was significantly lower in the group receiving vitamin C (12.7%), compared with the control group (29.1%) (P=.03; odds ratio [OR], 0.35).23
Uva ursi may have a prophylactic effect
Uva ursi (UVA-E) is one of the most commonly used herbal supplements for treatment of UTI. The crude extract from Bearberry or Arctostaphylos uva-ursi has been shown to act as an antimicrobial by decreasing bacterial adherence.24 In addition, investigators have found the extract to have diuretic and anti-inflammatory properties.25,26 However, few studies have explored its efficacy. One randomized, controlled trial that included 57 women (30 allocated to UVA-E and 27 to placebo) found a statistically significant reduction in the rate of recurrence at 1 year among women taking UVA-E, compared with those who did not.27
Note that the women in this trial were given UVA-E for 1 month only. Long-term use of this herb has not been studied and may cause liver damage.
In a mouse model, forskolin reduced urinary-tract E. coli
This herb is derived from Indian coleus (Coleus forskohlii), a member of the mint family. It has been used primarily for its antiasthmatic, spasmolytic, and antihypertensive effects. It is believed to activate adenylate cyclase, increasing intracellular cyclic AMP (cAMP) concentrations and activating a number of key enzymatic pathways.28
The findings of a recent observational study have spurred the use of forskolin in the treatment of recurrent UTI.29 In the study, conducted on mice, when forskolin was injected into the bladder, intracellular E. coli decreased.
It is theorized that the incorporation of bacteria into intracellular vesicles of the bladder prevents exposure to antibiotics. When combined with an antibiotic, forskolin may increase bacterial elimination and thus lower the risk of recurrent infection. However, no randomized trials have evaluated the efficacy of this treatment.
Patients who are already taking antihypertensive medications should be cautious when using this herb, as it may lead to a drop in blood pressure.
Behavior changes are risk-free
One of the natural mechanisms that promotes bacterial elimination and prevents bacterial growth is urination. A recent review article on the subject found several contradictory studies on the effect of fluid intake on the risk of UTI.30 Although there is no definitive evidence that susceptibility to UTI is linked to fluid intake, adequate hydration may reduce the risk of recurrent infection.
Similarly, voiding shortly after sexual intercourse may prevent UTI. One case-control study found a modest protective effect in patients who voided after intercourse.31
Clinical recommendations
Given the low risk of these measures, it seems reasonable to recommend postcoital voiding and increased fluid intake to prevent recurrent UTI.
A focus on symptoms
Phenazopyridine, the chemical found in numerous OTC medications, such as Pyridium, AZO, and Uristat, was discovered by Swiss chemist Bernhard Joos in the 1950s. Its mechanism of action is still unclear, but approximately 65% of the oral dose is excreted by the kidneys, where it has a direct topical analgesic effect.32,33
Clinical recommendations
Patients should be warned that phenazopyridine will lead to orange urine discoloration.
The medication is generally well tolerated but should be used with caution in patients with acute renal failure, hemolytic anemia, or methemoglobinemia, as it may exacerbate these conditions.
Last words
Recurrent UTIs are common and impose a significant financial burden on our healthcare system. Although there are several antibiotic treatment options and dosing regimens available, increasing antibiotic resistance has made management of recurrent UTIs more difficult. Effective alternative treatments that reduce the reliance on antibiotics may minimize bacterial resistance and decrease the financial burden of this common condition.
CASE: Resolved
To reduce vaginal E. coli, the patient is started on vaginal estrogen cream. She is also advised to purchase cranberry tablets to help prevent future infections. Last, she is counseled about behavioral changes she can make and prescribed a short course of antibiotics to treat her culture-proven infection.
We want to hear from you! Tell us what you think.
URINARY PROBLEMS?
CLICK HERE to access 9 articles about treating urinary incontinence and urinary tract infections, published in OBG MANAGEMENTin 2012.
1. Raz R, Stamm W. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753-756.
2. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;2:CD005131.-
3. Mayrer AR, Andriole VT. Urinary tract antiseptics. Med Clin North Am. 1982;66(1):199-208.
4. Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51(6):738-745.
5. Salvatore S, Salvatore S, Cattoni E, et al. Urinary tract infections in women. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):131-136.
6. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;1:CD001321.-
7. Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87(2):143-150.
8. Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23-30.
9. Beerepoot MAJ, Reit G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections. Arch Intern Med. 2011;171(14):1270-1278.
10. McMurdo MET, Argo I, Phillips G, Daly F, Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother. 2009;63(2):389-395.
11. Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clinical Therapeutics. 2008;30(3):453-468.
12. Miller JL, Krieger JN. Urinary tract infections: cranberry juice underwear, and probiotics in the 21st century. Urol Clin N Am. 2002;29(3):695-699.
13. Osset J, Bartolome R, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183(3):485-491.
14. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212-1217.
15. Castello T, Girona L, Gomez MR, Mena Mur A, Garcia L. The possible value of ascorbic acid as a prophylactic agent for urinary tract infection. Spinal Cord. 1996;34(10):592-593.
16. Gleckman R, Alvarez S, Joubert DW, Matthews SJ. Drug therapy reviews: methenamine mandelate and methenamine hippurate. Am J Hosp Pharm. 1979;36(11):1509-1512.
17. Vainrub B, Musher DM. Lack of effect of methenamine in suppression of or prophylaxis against, chronic urinary tract infection. Antimicrob Agents Chemother. 1977;12(5):625-629.
18. Lee BB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;4:CD003265.-
19. Kevorkian CG, Merritt JL, Ilstrup DM. Methenamine mandelate with acidification: an effective urinary antiseptic in patients with neurogenic bladder. Mayo Clin Proc. 1984;59(8):523-529.
20. Wainwright M, Stanforth A, Jones R, Loughran C, Meegan K. Photoantimicrobials as a potential local approach to geriatric UTIs. Lett Appl Microbiol. 2010;50(5):486-492.
21. Bannwart C, Hagmaier V, Straumann E, Hofer H, Buillemier JP, Trutishauser G. Modification of urinary pH through ascorbic acid. Helv Chir Acta. 1981;48(3-4):425-428.
22. Hetey SK, Kleinberg ML, Parker WD, Johnson EW. Effect of ascorbic acid on urine pH in patients with injured spinal cords. Am J Hosp Pharm. 1980;37(2):235-237.
23. Ochoa-Brust GJ, Fernandez AR, Villanueva-Ruiz GJ, et al. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstet Gynecol Scand. 2007;86(7):783-787.
24. Turi M, Turi E, Kotjalg S, Mikelsaar M. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS. 1997;105(12):956-962.
25. Beaux D, Fleurentin J, Mortier F. Effect of extracts of Orthosiphon stamineus Benth Hieracium pilosellaL., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother Res. 1999;13(3):222-225.
26. Kubo M, Ito M, Nakata H, Matsuda H. Pharmacological studies on leaf of Arctostaphylos uva-ursi (L.) Spreng. I. Combined effect of 50% methanolic extract from Arctostaphylos uva-ursi (L.) Spreng. (bearberry leaf) and prednisolone on immuno-inflammation [article in Japanese]. Yakugaku Zasshi. 1990;110(1):59-67.
27. Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53(4):441-443.
28. Christenson JT, Thulesius D, Nazzal MM. The effect of forskolin on blood flow platelet metabolism, aggregation and ATP release. Vasa. 1995;24(1):56-61.
29. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13(5):625-630.
30. Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52-58.
31. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43(4):329-337.
32. Thomas BH, Whitehouse LW, Solomonraj G, Paul CJ. Excretion of phenazopyridine and its metabolites in the urine of humans rats, mice, and guinea pigs. J Pharm Sci. 1990;79(4):321-325.
33. Aizawa N, Wyndaele JJ. Effects of phenazopyridine on rat bladder primary afferent activity and comparison with lidocaine and acetaminophen. Neurourol Urodyn. 2010;29(8):1445-1550.
The authors report no financial relationships relevant to this article.
CASE: Recurrent UTI and antibiotic resistance
A 53-year-old postmenopausal woman with a history of culture-proven recurrent Escherichia coli urinary tract infections (UTIs) presents to the clinic with symptoms of UTI. She was previously treated with a postcoital regimen of trimethoprim/sulfamethoxazole, based on sensitivities identified by culture. A past work-up of her upper and lower urinary tract was negative. You send a catheterized specimen for culture; again, E. coli is identified as the pathogen but proves resistant to her current antibiotic regimen.
What treatment alternatives, aside from antibiotics, are available for this patient—and how might they affect resistance?
Increased antibiotic usage has led to greater bacterial resistance, which is perpetuated by clonal spread. Resistant strains of E. coli have been found in household members, suggesting host-host transmission as a mechanism for dissemination. Alternative treatments that reduce the use of antibiotics may minimize bacterial resistance and increase the efficacy of treatment. In the TABLE , we summarize alternative approaches to the treatment of recurrent UTI. We also describe a strategy to alleviate symptoms.
Alternatives to antibiotics in the treatment and prevention of recurrent UTI
| Category | Type | Examples and doses, if recommended |
|---|---|---|
| Vaginal estrogen | Conjugated estrogen cream Estradiol
| Premarin cream, 0.5–2 g vaginally twice weekly
|
| Nutritive agents | Cranberry juice Cranberry tablets Cystopurin Lactobacilli Blueberry products | Not recommended 1 tablet (300 to 400 mg, depending on manufacturer) twice daily Not recommended Vivag, EcoVag, 1 capsule daily by vagina for 5 days, then once weekly for 10 weeks Not recommended |
| Anti-infective drugs | Methenamine hippurate Methenamine mandelate Methylene blue | Urex or Hiprex, 1 g orally twice daily Mandelamine, 1 g orally 4 times daily Future therapy |
| Urinary acidifiers | Vitamin C/ascorbic acid | 1–3 g orally 3–4 times daily |
| Herbal remedies | Uva ursi Forskolin | Not recommended for long-term use Not recommended |
| Behavioral changes | Adequate hydration Postcoital voiding |
Vaginal estrogen is the only proven alternative to antibiotics for postmenopausal women
A lack of estrogen is a risk factor for UTI and is associated with atrophic mucosa, leading to decreased colonization with lactobacilli, increased vaginal pH, and E. coli colonization.
A randomized, double-blind, placebo-controlled trial of intravaginal estriol cream versus placebo in 93 postmenopausal women found a significant decrease in the rate of UTI among women who used the cream.1 After 8 months of follow-up, the incidence of UTI was 0.5 vs 5.9 episodes per patient-year (P <.001). Interestingly, all pretreatment cultures were negative for lactobacilli. One month after treatment, 61% of women in the estriol group were culture-positive for lactobacilli, compared with 0% of the placebo group.1
A 2008 Cochrane review of nine studies concluded that vaginal estrogen reduces the number of UTIs in postmenopausal women, with variation based on the type of estrogen and duration of use.2
Adverse effects are mild
Twenty-eight percent of the estriol group in the randomized trial described above withdrew from treatment, with 20% citing local side effects, including vaginal irritation, burning, or itching—all of which were mild and self-limited.1 Other possible adverse effects include breast tenderness, vaginal bleeding or spotting, and discharge.2
Clinical recommendations
Given the efficacy of this therapy, we recommend topical estrogen for postmenopausal patients with recurrent UTIs.
Cranberry juice may reduce UTI, but many patients withdraw
from treatment
Cranberries belong to the Vaccinium species, which contains all flavonoids, including anthocyanins and proanthocyanidins. It was previously thought that the acidification of urine produced an antibacterial effect, but several trials have documented no change in urine levels of hippuric acid when cranberry products are given, with no acidification of the urine.3 Current theory suggests that cranberries prevent bacteria from adhering to the uroepithelial cells of the walls of the bladder, by blocking expression of E. coli’s adhesion molecule, P. fimbriae, so that bacteria are unable to penetrate the mucosal surface.4,5 The major benefit of cranberry products over antibiotic prophylaxis is that they do not have the potential for resistance.4
A 2008 Cochrane review concluded that cranberry juice may reduce symptomatic UTIs, particularly among young, sexually active women—but there is a high rate of withdrawal from treatment.6 The optimal method of administration and dose remain unclear. In contrast, two recent randomized, controlled trials—published after the Cochrane review—found no difference in the rate of recurrent UTI in premenopausal women.7,8 Adverse effects in these two trials included constipation, heartburn, loose stools, vaginal itching and dryness, and migraines. Of note, there was no statistical difference in side effects between the cranberry and placebo groups.7
Vaccinium tablets may be protective in older women
Cranberry extracts of 500 mg to 1,000 mg daily have been compared with antimicrobial prophylaxis in two randomized, double-blind, controlled trials. The trials demonstrated mixed benefits. Trimethoprim/sulfamethoxazole was associated with a lower rate of UTI in younger women, compared with cranberry extracts alone (P=.02), while cranberry extracts were slightly more effective than trimethoprim alone in older women.9,10 Cranberry tablets were not associated with bacterial resistance, were cheaper, and were viewed as a more natural option. The interventions were equally well tolerated.
Overall efficacy of cranberry tablets is unclear. Side effects, albeit mild, included gastrointestinal disturbances, vaginal complaints, and rash or urticaria. There was no significant difference in the rate of adverse effects between antimicrobial treatment and cranberry tablets.9
Cystopurin has not been studied
Cystopurin is an over-the-counter (OTC) tablet containing cranberry extract and potassium citrate that is taken three times daily (3 g/dose) for 2 days. Interestingly, although a proposed mechanism for the efficacy of vitamin C and cranberry juice has been a reduction of pH, potassium citrate is an alkalizing agent that is reported to relieve burning and reduce urinary urgency and frequency. No studies have assessed this medication in the treatment of a UTI or its symptoms.
Clinical recommendations
The evidence is mixed on the use of cranberry products to reduce recurrent UTI. However, given the limited side effects associated with these products, we offer cranberry tablets to patients who have recurrent UTIs who are interested in a more natural alternative.
We generally do not recommend cranberry juice because the added fluid volume tends to exacerbate frequency and urgency symptoms.

Lactobacilli suppositories may benefit
postmenopausal women
Lactobacilli are fastidious gram-positive rods and are usually the dominant component of the vaginal flora.11 They prevent colonization and infection by more virulent bacteria by competing for adhesion receptors and nutrients as well as producing antimicrobial substances such as hydrogen peroxide and lactic acid. A decrease in lactobacilli leaves the urinary tract susceptible to infectious organisms that may colonize the vaginal mucosa and increase the risk of recurrent UTI.12-14
A 2008 review of randomized, controlled trials of oral lactobacilli and UTI was inconclusive, due to inconsistent dosing strategies and small sample sizes.15 A 2011 randomized, double-blind, placebo-controlled phase 2 trial of Lactin-V, a lactobacilli vaginal suppository, found that it reduced the rate of recurrent UTI. Lactin-V contains a hydrogen-peroxide–producing Lactobacillus crispatus developed as a probiotic that was determined to be safe and tolerable as a vaginal suppository in a phase 1 trial.16 The phase 2 trial enrolled 100 young premenopausal women with a history of recurrent UTI who took either Lactin-V or placebo daily for 5 days, then weekly for 10 weeks. Women in the Lactin-V group who had high levels of L. crispatus colonization experienced a significant reduction in the rate of UTI (15% vs 27% in the placebo group), but the effect did not reach statistical significance.14
Little difference in adverse effects
Adverse effects were reported among 56% of patients who received Lactin-V versus 50% of those given placebo. The most common of these were vaginal discharge, itching, and moderate abdominal discomfort.14 Although lactobacillus can potentially promote UTI, this phenomenon is rare.11
Regrettably, Lactin-V is not currently available in the United States. However, there are other lactobacilli vaginal suppositories on the market ( TABLE ). Given the low risk associated with their use, they should be considered as an alternative for patients who cannot or will not use estrogen.
Clinical recommendations
Probiotics such as lactobacilli are categorized as “dietary supplements”; as such, they are not regulated by the US Food and Drug Administration. We recommend the use of lactobacilli suppositories in postmenopausal women who have a contraindication to (or prefer to avoid) vaginal estrogen.
Skip blueberry products for now
Like cranberries, blueberries belong to the Vaccinium species and are thought to interfere with bacterial adhesion to the walls of the bladder. One in vitro trial suggests that blueberries also have antiproliferation effects, although no clinical studies have been performed to date to further investigate safety or efficacy.4 Consequently, we do not recommend use of these products.
Methenamine salts may benefit some populations
These anti-infective agents, including methenamine hippurate and methenamine mandelate, often are used to prevent UTI. They are found in combination OTC medications, such as Prosed DS and Urelle. Methenamine salts are bacteriostatic to all urinary tract pathogens due to their production of formaldehyde.3,16
Although methenamine produces varying concentrations of formaldehyde, depending on the acidity of the urine, there is no evidence that acidified urine enhances methenamine’s effects.16
Advantages of methenamine include the fact that it produces no changes in gut flora, poses no risk for antimicrobial resistance, and has low toxicity. It also is low in cost.17
Methenamine is contraindicated in patients with renal insufficiency or severe hepatic disease.
Adverse reactions are generally mild and include gastrointestinal disturbances, skin rashes, dysuria, and microscopic hematuria.16
Methenamine hippurate
A 2007 Cochrane review, deemed up to date in 2010, analyzed 13 randomized, controlled trials involving 2,032 participants. Subgroup analyses suggested that methenamine hippurate may be of some benefit to patients without renal tract abnormalities; these patients experienced significantly reduced symptoms after short-term treatment of 1 week or less.
Patients with spinal injury do not appear to benefit from treatment, according to a randomized, double-blind, placebo-controlled trial by Lee and colleagues.18
Methenamine mandelate
This agent is commonly used to prevent recurrent UTI, although there is a paucity of randomized, controlled trials to support its use. One such trial in patients with neurogenic bladder found that methenamine mandelate with acidification was superior to placebo (P <.02) for preventing UTI.19 Beyond this population, however, it’s difficult to assess methenamine’s efficacy in the prevention of recurrent UTI.
Methylene blue may be useful in the elderly
Methylene blue is a light-activated compound described as “photodynamic antimicrobial chemotherapy” (PACT). Once illuminated, it becomes a bactericide, causing excitation of electrons followed by one of two reactions:
- reduction oxidation
- formation of a labile singlet oxygen and then oxidation.
This makes resistance unlikely.
In an in vitro study, methylene blue was as effective as levofloxacin against Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis, Enterococcus, and Staphylococcus aureus when illuminated.
Potential benefits of this mode of treatment include local exposure and no drug interactions.
We suggest that methylene blue be placed and illuminated with a special catheter that would target UTI in the elderly population, among whom 52% of UTIs are associated with use of catheters.20 In vivo effects, adverse effects, and cost are not known, which limits current applicability of this compound.
Vitamin C may reduce UTI in pregnancy
The proposed mechanism for the efficacy of vitamin C for the treatment of UTI is the acidification of urine, which is believed to reduce the proliferation of bacteria. However, several studies have shown that vitamin C, at various doses, does not reliably reduce urine pH.15,21,22 Nonetheless, ascorbic acid was tested for its effect on UTI prevention during pregnancy.
In a single-blind trial, 110 pregnant women were divided into two groups (55 in each group):
- One group received ferrous sulfate (200 mg), folic acid (5 mg), and vitamin C (100 mg) daily for 3 months
- The other received ferrous sulfate (200 mg) and folic acid (5 mg) daily for 3 months.
Urine was cultured monthly. The incidence of UTI was significantly lower in the group receiving vitamin C (12.7%), compared with the control group (29.1%) (P=.03; odds ratio [OR], 0.35).23
Uva ursi may have a prophylactic effect
Uva ursi (UVA-E) is one of the most commonly used herbal supplements for treatment of UTI. The crude extract from Bearberry or Arctostaphylos uva-ursi has been shown to act as an antimicrobial by decreasing bacterial adherence.24 In addition, investigators have found the extract to have diuretic and anti-inflammatory properties.25,26 However, few studies have explored its efficacy. One randomized, controlled trial that included 57 women (30 allocated to UVA-E and 27 to placebo) found a statistically significant reduction in the rate of recurrence at 1 year among women taking UVA-E, compared with those who did not.27
Note that the women in this trial were given UVA-E for 1 month only. Long-term use of this herb has not been studied and may cause liver damage.
In a mouse model, forskolin reduced urinary-tract E. coli
This herb is derived from Indian coleus (Coleus forskohlii), a member of the mint family. It has been used primarily for its antiasthmatic, spasmolytic, and antihypertensive effects. It is believed to activate adenylate cyclase, increasing intracellular cyclic AMP (cAMP) concentrations and activating a number of key enzymatic pathways.28
The findings of a recent observational study have spurred the use of forskolin in the treatment of recurrent UTI.29 In the study, conducted on mice, when forskolin was injected into the bladder, intracellular E. coli decreased.
It is theorized that the incorporation of bacteria into intracellular vesicles of the bladder prevents exposure to antibiotics. When combined with an antibiotic, forskolin may increase bacterial elimination and thus lower the risk of recurrent infection. However, no randomized trials have evaluated the efficacy of this treatment.
Patients who are already taking antihypertensive medications should be cautious when using this herb, as it may lead to a drop in blood pressure.
Behavior changes are risk-free
One of the natural mechanisms that promotes bacterial elimination and prevents bacterial growth is urination. A recent review article on the subject found several contradictory studies on the effect of fluid intake on the risk of UTI.30 Although there is no definitive evidence that susceptibility to UTI is linked to fluid intake, adequate hydration may reduce the risk of recurrent infection.
Similarly, voiding shortly after sexual intercourse may prevent UTI. One case-control study found a modest protective effect in patients who voided after intercourse.31
Clinical recommendations
Given the low risk of these measures, it seems reasonable to recommend postcoital voiding and increased fluid intake to prevent recurrent UTI.
A focus on symptoms
Phenazopyridine, the chemical found in numerous OTC medications, such as Pyridium, AZO, and Uristat, was discovered by Swiss chemist Bernhard Joos in the 1950s. Its mechanism of action is still unclear, but approximately 65% of the oral dose is excreted by the kidneys, where it has a direct topical analgesic effect.32,33
Clinical recommendations
Patients should be warned that phenazopyridine will lead to orange urine discoloration.
The medication is generally well tolerated but should be used with caution in patients with acute renal failure, hemolytic anemia, or methemoglobinemia, as it may exacerbate these conditions.
Last words
Recurrent UTIs are common and impose a significant financial burden on our healthcare system. Although there are several antibiotic treatment options and dosing regimens available, increasing antibiotic resistance has made management of recurrent UTIs more difficult. Effective alternative treatments that reduce the reliance on antibiotics may minimize bacterial resistance and decrease the financial burden of this common condition.
CASE: Resolved
To reduce vaginal E. coli, the patient is started on vaginal estrogen cream. She is also advised to purchase cranberry tablets to help prevent future infections. Last, she is counseled about behavioral changes she can make and prescribed a short course of antibiotics to treat her culture-proven infection.
We want to hear from you! Tell us what you think.
URINARY PROBLEMS?
CLICK HERE to access 9 articles about treating urinary incontinence and urinary tract infections, published in OBG MANAGEMENTin 2012.
The authors report no financial relationships relevant to this article.
CASE: Recurrent UTI and antibiotic resistance
A 53-year-old postmenopausal woman with a history of culture-proven recurrent Escherichia coli urinary tract infections (UTIs) presents to the clinic with symptoms of UTI. She was previously treated with a postcoital regimen of trimethoprim/sulfamethoxazole, based on sensitivities identified by culture. A past work-up of her upper and lower urinary tract was negative. You send a catheterized specimen for culture; again, E. coli is identified as the pathogen but proves resistant to her current antibiotic regimen.
What treatment alternatives, aside from antibiotics, are available for this patient—and how might they affect resistance?
Increased antibiotic usage has led to greater bacterial resistance, which is perpetuated by clonal spread. Resistant strains of E. coli have been found in household members, suggesting host-host transmission as a mechanism for dissemination. Alternative treatments that reduce the use of antibiotics may minimize bacterial resistance and increase the efficacy of treatment. In the TABLE , we summarize alternative approaches to the treatment of recurrent UTI. We also describe a strategy to alleviate symptoms.
Alternatives to antibiotics in the treatment and prevention of recurrent UTI
| Category | Type | Examples and doses, if recommended |
|---|---|---|
| Vaginal estrogen | Conjugated estrogen cream Estradiol
| Premarin cream, 0.5–2 g vaginally twice weekly
|
| Nutritive agents | Cranberry juice Cranberry tablets Cystopurin Lactobacilli Blueberry products | Not recommended 1 tablet (300 to 400 mg, depending on manufacturer) twice daily Not recommended Vivag, EcoVag, 1 capsule daily by vagina for 5 days, then once weekly for 10 weeks Not recommended |
| Anti-infective drugs | Methenamine hippurate Methenamine mandelate Methylene blue | Urex or Hiprex, 1 g orally twice daily Mandelamine, 1 g orally 4 times daily Future therapy |
| Urinary acidifiers | Vitamin C/ascorbic acid | 1–3 g orally 3–4 times daily |
| Herbal remedies | Uva ursi Forskolin | Not recommended for long-term use Not recommended |
| Behavioral changes | Adequate hydration Postcoital voiding |
Vaginal estrogen is the only proven alternative to antibiotics for postmenopausal women
A lack of estrogen is a risk factor for UTI and is associated with atrophic mucosa, leading to decreased colonization with lactobacilli, increased vaginal pH, and E. coli colonization.
A randomized, double-blind, placebo-controlled trial of intravaginal estriol cream versus placebo in 93 postmenopausal women found a significant decrease in the rate of UTI among women who used the cream.1 After 8 months of follow-up, the incidence of UTI was 0.5 vs 5.9 episodes per patient-year (P <.001). Interestingly, all pretreatment cultures were negative for lactobacilli. One month after treatment, 61% of women in the estriol group were culture-positive for lactobacilli, compared with 0% of the placebo group.1
A 2008 Cochrane review of nine studies concluded that vaginal estrogen reduces the number of UTIs in postmenopausal women, with variation based on the type of estrogen and duration of use.2
Adverse effects are mild
Twenty-eight percent of the estriol group in the randomized trial described above withdrew from treatment, with 20% citing local side effects, including vaginal irritation, burning, or itching—all of which were mild and self-limited.1 Other possible adverse effects include breast tenderness, vaginal bleeding or spotting, and discharge.2
Clinical recommendations
Given the efficacy of this therapy, we recommend topical estrogen for postmenopausal patients with recurrent UTIs.
Cranberry juice may reduce UTI, but many patients withdraw
from treatment
Cranberries belong to the Vaccinium species, which contains all flavonoids, including anthocyanins and proanthocyanidins. It was previously thought that the acidification of urine produced an antibacterial effect, but several trials have documented no change in urine levels of hippuric acid when cranberry products are given, with no acidification of the urine.3 Current theory suggests that cranberries prevent bacteria from adhering to the uroepithelial cells of the walls of the bladder, by blocking expression of E. coli’s adhesion molecule, P. fimbriae, so that bacteria are unable to penetrate the mucosal surface.4,5 The major benefit of cranberry products over antibiotic prophylaxis is that they do not have the potential for resistance.4
A 2008 Cochrane review concluded that cranberry juice may reduce symptomatic UTIs, particularly among young, sexually active women—but there is a high rate of withdrawal from treatment.6 The optimal method of administration and dose remain unclear. In contrast, two recent randomized, controlled trials—published after the Cochrane review—found no difference in the rate of recurrent UTI in premenopausal women.7,8 Adverse effects in these two trials included constipation, heartburn, loose stools, vaginal itching and dryness, and migraines. Of note, there was no statistical difference in side effects between the cranberry and placebo groups.7
Vaccinium tablets may be protective in older women
Cranberry extracts of 500 mg to 1,000 mg daily have been compared with antimicrobial prophylaxis in two randomized, double-blind, controlled trials. The trials demonstrated mixed benefits. Trimethoprim/sulfamethoxazole was associated with a lower rate of UTI in younger women, compared with cranberry extracts alone (P=.02), while cranberry extracts were slightly more effective than trimethoprim alone in older women.9,10 Cranberry tablets were not associated with bacterial resistance, were cheaper, and were viewed as a more natural option. The interventions were equally well tolerated.
Overall efficacy of cranberry tablets is unclear. Side effects, albeit mild, included gastrointestinal disturbances, vaginal complaints, and rash or urticaria. There was no significant difference in the rate of adverse effects between antimicrobial treatment and cranberry tablets.9
Cystopurin has not been studied
Cystopurin is an over-the-counter (OTC) tablet containing cranberry extract and potassium citrate that is taken three times daily (3 g/dose) for 2 days. Interestingly, although a proposed mechanism for the efficacy of vitamin C and cranberry juice has been a reduction of pH, potassium citrate is an alkalizing agent that is reported to relieve burning and reduce urinary urgency and frequency. No studies have assessed this medication in the treatment of a UTI or its symptoms.
Clinical recommendations
The evidence is mixed on the use of cranberry products to reduce recurrent UTI. However, given the limited side effects associated with these products, we offer cranberry tablets to patients who have recurrent UTIs who are interested in a more natural alternative.
We generally do not recommend cranberry juice because the added fluid volume tends to exacerbate frequency and urgency symptoms.

Lactobacilli suppositories may benefit
postmenopausal women
Lactobacilli are fastidious gram-positive rods and are usually the dominant component of the vaginal flora.11 They prevent colonization and infection by more virulent bacteria by competing for adhesion receptors and nutrients as well as producing antimicrobial substances such as hydrogen peroxide and lactic acid. A decrease in lactobacilli leaves the urinary tract susceptible to infectious organisms that may colonize the vaginal mucosa and increase the risk of recurrent UTI.12-14
A 2008 review of randomized, controlled trials of oral lactobacilli and UTI was inconclusive, due to inconsistent dosing strategies and small sample sizes.15 A 2011 randomized, double-blind, placebo-controlled phase 2 trial of Lactin-V, a lactobacilli vaginal suppository, found that it reduced the rate of recurrent UTI. Lactin-V contains a hydrogen-peroxide–producing Lactobacillus crispatus developed as a probiotic that was determined to be safe and tolerable as a vaginal suppository in a phase 1 trial.16 The phase 2 trial enrolled 100 young premenopausal women with a history of recurrent UTI who took either Lactin-V or placebo daily for 5 days, then weekly for 10 weeks. Women in the Lactin-V group who had high levels of L. crispatus colonization experienced a significant reduction in the rate of UTI (15% vs 27% in the placebo group), but the effect did not reach statistical significance.14
Little difference in adverse effects
Adverse effects were reported among 56% of patients who received Lactin-V versus 50% of those given placebo. The most common of these were vaginal discharge, itching, and moderate abdominal discomfort.14 Although lactobacillus can potentially promote UTI, this phenomenon is rare.11
Regrettably, Lactin-V is not currently available in the United States. However, there are other lactobacilli vaginal suppositories on the market ( TABLE ). Given the low risk associated with their use, they should be considered as an alternative for patients who cannot or will not use estrogen.
Clinical recommendations
Probiotics such as lactobacilli are categorized as “dietary supplements”; as such, they are not regulated by the US Food and Drug Administration. We recommend the use of lactobacilli suppositories in postmenopausal women who have a contraindication to (or prefer to avoid) vaginal estrogen.
Skip blueberry products for now
Like cranberries, blueberries belong to the Vaccinium species and are thought to interfere with bacterial adhesion to the walls of the bladder. One in vitro trial suggests that blueberries also have antiproliferation effects, although no clinical studies have been performed to date to further investigate safety or efficacy.4 Consequently, we do not recommend use of these products.
Methenamine salts may benefit some populations
These anti-infective agents, including methenamine hippurate and methenamine mandelate, often are used to prevent UTI. They are found in combination OTC medications, such as Prosed DS and Urelle. Methenamine salts are bacteriostatic to all urinary tract pathogens due to their production of formaldehyde.3,16
Although methenamine produces varying concentrations of formaldehyde, depending on the acidity of the urine, there is no evidence that acidified urine enhances methenamine’s effects.16
Advantages of methenamine include the fact that it produces no changes in gut flora, poses no risk for antimicrobial resistance, and has low toxicity. It also is low in cost.17
Methenamine is contraindicated in patients with renal insufficiency or severe hepatic disease.
Adverse reactions are generally mild and include gastrointestinal disturbances, skin rashes, dysuria, and microscopic hematuria.16
Methenamine hippurate
A 2007 Cochrane review, deemed up to date in 2010, analyzed 13 randomized, controlled trials involving 2,032 participants. Subgroup analyses suggested that methenamine hippurate may be of some benefit to patients without renal tract abnormalities; these patients experienced significantly reduced symptoms after short-term treatment of 1 week or less.
Patients with spinal injury do not appear to benefit from treatment, according to a randomized, double-blind, placebo-controlled trial by Lee and colleagues.18
Methenamine mandelate
This agent is commonly used to prevent recurrent UTI, although there is a paucity of randomized, controlled trials to support its use. One such trial in patients with neurogenic bladder found that methenamine mandelate with acidification was superior to placebo (P <.02) for preventing UTI.19 Beyond this population, however, it’s difficult to assess methenamine’s efficacy in the prevention of recurrent UTI.
Methylene blue may be useful in the elderly
Methylene blue is a light-activated compound described as “photodynamic antimicrobial chemotherapy” (PACT). Once illuminated, it becomes a bactericide, causing excitation of electrons followed by one of two reactions:
- reduction oxidation
- formation of a labile singlet oxygen and then oxidation.
This makes resistance unlikely.
In an in vitro study, methylene blue was as effective as levofloxacin against Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis, Enterococcus, and Staphylococcus aureus when illuminated.
Potential benefits of this mode of treatment include local exposure and no drug interactions.
We suggest that methylene blue be placed and illuminated with a special catheter that would target UTI in the elderly population, among whom 52% of UTIs are associated with use of catheters.20 In vivo effects, adverse effects, and cost are not known, which limits current applicability of this compound.
Vitamin C may reduce UTI in pregnancy
The proposed mechanism for the efficacy of vitamin C for the treatment of UTI is the acidification of urine, which is believed to reduce the proliferation of bacteria. However, several studies have shown that vitamin C, at various doses, does not reliably reduce urine pH.15,21,22 Nonetheless, ascorbic acid was tested for its effect on UTI prevention during pregnancy.
In a single-blind trial, 110 pregnant women were divided into two groups (55 in each group):
- One group received ferrous sulfate (200 mg), folic acid (5 mg), and vitamin C (100 mg) daily for 3 months
- The other received ferrous sulfate (200 mg) and folic acid (5 mg) daily for 3 months.
Urine was cultured monthly. The incidence of UTI was significantly lower in the group receiving vitamin C (12.7%), compared with the control group (29.1%) (P=.03; odds ratio [OR], 0.35).23
Uva ursi may have a prophylactic effect
Uva ursi (UVA-E) is one of the most commonly used herbal supplements for treatment of UTI. The crude extract from Bearberry or Arctostaphylos uva-ursi has been shown to act as an antimicrobial by decreasing bacterial adherence.24 In addition, investigators have found the extract to have diuretic and anti-inflammatory properties.25,26 However, few studies have explored its efficacy. One randomized, controlled trial that included 57 women (30 allocated to UVA-E and 27 to placebo) found a statistically significant reduction in the rate of recurrence at 1 year among women taking UVA-E, compared with those who did not.27
Note that the women in this trial were given UVA-E for 1 month only. Long-term use of this herb has not been studied and may cause liver damage.
In a mouse model, forskolin reduced urinary-tract E. coli
This herb is derived from Indian coleus (Coleus forskohlii), a member of the mint family. It has been used primarily for its antiasthmatic, spasmolytic, and antihypertensive effects. It is believed to activate adenylate cyclase, increasing intracellular cyclic AMP (cAMP) concentrations and activating a number of key enzymatic pathways.28
The findings of a recent observational study have spurred the use of forskolin in the treatment of recurrent UTI.29 In the study, conducted on mice, when forskolin was injected into the bladder, intracellular E. coli decreased.
It is theorized that the incorporation of bacteria into intracellular vesicles of the bladder prevents exposure to antibiotics. When combined with an antibiotic, forskolin may increase bacterial elimination and thus lower the risk of recurrent infection. However, no randomized trials have evaluated the efficacy of this treatment.
Patients who are already taking antihypertensive medications should be cautious when using this herb, as it may lead to a drop in blood pressure.
Behavior changes are risk-free
One of the natural mechanisms that promotes bacterial elimination and prevents bacterial growth is urination. A recent review article on the subject found several contradictory studies on the effect of fluid intake on the risk of UTI.30 Although there is no definitive evidence that susceptibility to UTI is linked to fluid intake, adequate hydration may reduce the risk of recurrent infection.
Similarly, voiding shortly after sexual intercourse may prevent UTI. One case-control study found a modest protective effect in patients who voided after intercourse.31
Clinical recommendations
Given the low risk of these measures, it seems reasonable to recommend postcoital voiding and increased fluid intake to prevent recurrent UTI.
A focus on symptoms
Phenazopyridine, the chemical found in numerous OTC medications, such as Pyridium, AZO, and Uristat, was discovered by Swiss chemist Bernhard Joos in the 1950s. Its mechanism of action is still unclear, but approximately 65% of the oral dose is excreted by the kidneys, where it has a direct topical analgesic effect.32,33
Clinical recommendations
Patients should be warned that phenazopyridine will lead to orange urine discoloration.
The medication is generally well tolerated but should be used with caution in patients with acute renal failure, hemolytic anemia, or methemoglobinemia, as it may exacerbate these conditions.
Last words
Recurrent UTIs are common and impose a significant financial burden on our healthcare system. Although there are several antibiotic treatment options and dosing regimens available, increasing antibiotic resistance has made management of recurrent UTIs more difficult. Effective alternative treatments that reduce the reliance on antibiotics may minimize bacterial resistance and decrease the financial burden of this common condition.
CASE: Resolved
To reduce vaginal E. coli, the patient is started on vaginal estrogen cream. She is also advised to purchase cranberry tablets to help prevent future infections. Last, she is counseled about behavioral changes she can make and prescribed a short course of antibiotics to treat her culture-proven infection.
We want to hear from you! Tell us what you think.
URINARY PROBLEMS?
CLICK HERE to access 9 articles about treating urinary incontinence and urinary tract infections, published in OBG MANAGEMENTin 2012.
1. Raz R, Stamm W. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753-756.
2. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;2:CD005131.-
3. Mayrer AR, Andriole VT. Urinary tract antiseptics. Med Clin North Am. 1982;66(1):199-208.
4. Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51(6):738-745.
5. Salvatore S, Salvatore S, Cattoni E, et al. Urinary tract infections in women. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):131-136.
6. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;1:CD001321.-
7. Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87(2):143-150.
8. Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23-30.
9. Beerepoot MAJ, Reit G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections. Arch Intern Med. 2011;171(14):1270-1278.
10. McMurdo MET, Argo I, Phillips G, Daly F, Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother. 2009;63(2):389-395.
11. Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clinical Therapeutics. 2008;30(3):453-468.
12. Miller JL, Krieger JN. Urinary tract infections: cranberry juice underwear, and probiotics in the 21st century. Urol Clin N Am. 2002;29(3):695-699.
13. Osset J, Bartolome R, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183(3):485-491.
14. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212-1217.
15. Castello T, Girona L, Gomez MR, Mena Mur A, Garcia L. The possible value of ascorbic acid as a prophylactic agent for urinary tract infection. Spinal Cord. 1996;34(10):592-593.
16. Gleckman R, Alvarez S, Joubert DW, Matthews SJ. Drug therapy reviews: methenamine mandelate and methenamine hippurate. Am J Hosp Pharm. 1979;36(11):1509-1512.
17. Vainrub B, Musher DM. Lack of effect of methenamine in suppression of or prophylaxis against, chronic urinary tract infection. Antimicrob Agents Chemother. 1977;12(5):625-629.
18. Lee BB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;4:CD003265.-
19. Kevorkian CG, Merritt JL, Ilstrup DM. Methenamine mandelate with acidification: an effective urinary antiseptic in patients with neurogenic bladder. Mayo Clin Proc. 1984;59(8):523-529.
20. Wainwright M, Stanforth A, Jones R, Loughran C, Meegan K. Photoantimicrobials as a potential local approach to geriatric UTIs. Lett Appl Microbiol. 2010;50(5):486-492.
21. Bannwart C, Hagmaier V, Straumann E, Hofer H, Buillemier JP, Trutishauser G. Modification of urinary pH through ascorbic acid. Helv Chir Acta. 1981;48(3-4):425-428.
22. Hetey SK, Kleinberg ML, Parker WD, Johnson EW. Effect of ascorbic acid on urine pH in patients with injured spinal cords. Am J Hosp Pharm. 1980;37(2):235-237.
23. Ochoa-Brust GJ, Fernandez AR, Villanueva-Ruiz GJ, et al. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstet Gynecol Scand. 2007;86(7):783-787.
24. Turi M, Turi E, Kotjalg S, Mikelsaar M. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS. 1997;105(12):956-962.
25. Beaux D, Fleurentin J, Mortier F. Effect of extracts of Orthosiphon stamineus Benth Hieracium pilosellaL., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother Res. 1999;13(3):222-225.
26. Kubo M, Ito M, Nakata H, Matsuda H. Pharmacological studies on leaf of Arctostaphylos uva-ursi (L.) Spreng. I. Combined effect of 50% methanolic extract from Arctostaphylos uva-ursi (L.) Spreng. (bearberry leaf) and prednisolone on immuno-inflammation [article in Japanese]. Yakugaku Zasshi. 1990;110(1):59-67.
27. Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53(4):441-443.
28. Christenson JT, Thulesius D, Nazzal MM. The effect of forskolin on blood flow platelet metabolism, aggregation and ATP release. Vasa. 1995;24(1):56-61.
29. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13(5):625-630.
30. Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52-58.
31. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43(4):329-337.
32. Thomas BH, Whitehouse LW, Solomonraj G, Paul CJ. Excretion of phenazopyridine and its metabolites in the urine of humans rats, mice, and guinea pigs. J Pharm Sci. 1990;79(4):321-325.
33. Aizawa N, Wyndaele JJ. Effects of phenazopyridine on rat bladder primary afferent activity and comparison with lidocaine and acetaminophen. Neurourol Urodyn. 2010;29(8):1445-1550.
1. Raz R, Stamm W. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329(11):753-756.
2. Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;2:CD005131.-
3. Mayrer AR, Andriole VT. Urinary tract antiseptics. Med Clin North Am. 1982;66(1):199-208.
4. Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51(6):738-745.
5. Salvatore S, Salvatore S, Cattoni E, et al. Urinary tract infections in women. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):131-136.
6. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;1:CD001321.-
7. Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87(2):143-150.
8. Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23-30.
9. Beerepoot MAJ, Reit G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections. Arch Intern Med. 2011;171(14):1270-1278.
10. McMurdo MET, Argo I, Phillips G, Daly F, Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother. 2009;63(2):389-395.
11. Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clinical Therapeutics. 2008;30(3):453-468.
12. Miller JL, Krieger JN. Urinary tract infections: cranberry juice underwear, and probiotics in the 21st century. Urol Clin N Am. 2002;29(3):695-699.
13. Osset J, Bartolome R, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183(3):485-491.
14. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212-1217.
15. Castello T, Girona L, Gomez MR, Mena Mur A, Garcia L. The possible value of ascorbic acid as a prophylactic agent for urinary tract infection. Spinal Cord. 1996;34(10):592-593.
16. Gleckman R, Alvarez S, Joubert DW, Matthews SJ. Drug therapy reviews: methenamine mandelate and methenamine hippurate. Am J Hosp Pharm. 1979;36(11):1509-1512.
17. Vainrub B, Musher DM. Lack of effect of methenamine in suppression of or prophylaxis against, chronic urinary tract infection. Antimicrob Agents Chemother. 1977;12(5):625-629.
18. Lee BB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;4:CD003265.-
19. Kevorkian CG, Merritt JL, Ilstrup DM. Methenamine mandelate with acidification: an effective urinary antiseptic in patients with neurogenic bladder. Mayo Clin Proc. 1984;59(8):523-529.
20. Wainwright M, Stanforth A, Jones R, Loughran C, Meegan K. Photoantimicrobials as a potential local approach to geriatric UTIs. Lett Appl Microbiol. 2010;50(5):486-492.
21. Bannwart C, Hagmaier V, Straumann E, Hofer H, Buillemier JP, Trutishauser G. Modification of urinary pH through ascorbic acid. Helv Chir Acta. 1981;48(3-4):425-428.
22. Hetey SK, Kleinberg ML, Parker WD, Johnson EW. Effect of ascorbic acid on urine pH in patients with injured spinal cords. Am J Hosp Pharm. 1980;37(2):235-237.
23. Ochoa-Brust GJ, Fernandez AR, Villanueva-Ruiz GJ, et al. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstet Gynecol Scand. 2007;86(7):783-787.
24. Turi M, Turi E, Kotjalg S, Mikelsaar M. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS. 1997;105(12):956-962.
25. Beaux D, Fleurentin J, Mortier F. Effect of extracts of Orthosiphon stamineus Benth Hieracium pilosellaL., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother Res. 1999;13(3):222-225.
26. Kubo M, Ito M, Nakata H, Matsuda H. Pharmacological studies on leaf of Arctostaphylos uva-ursi (L.) Spreng. I. Combined effect of 50% methanolic extract from Arctostaphylos uva-ursi (L.) Spreng. (bearberry leaf) and prednisolone on immuno-inflammation [article in Japanese]. Yakugaku Zasshi. 1990;110(1):59-67.
27. Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53(4):441-443.
28. Christenson JT, Thulesius D, Nazzal MM. The effect of forskolin on blood flow platelet metabolism, aggregation and ATP release. Vasa. 1995;24(1):56-61.
29. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13(5):625-630.
30. Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52-58.
31. Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43(4):329-337.
32. Thomas BH, Whitehouse LW, Solomonraj G, Paul CJ. Excretion of phenazopyridine and its metabolites in the urine of humans rats, mice, and guinea pigs. J Pharm Sci. 1990;79(4):321-325.
33. Aizawa N, Wyndaele JJ. Effects of phenazopyridine on rat bladder primary afferent activity and comparison with lidocaine and acetaminophen. Neurourol Urodyn. 2010;29(8):1445-1550.
How reliable is self-testing for gonorrhea and chlamydia among men who have sex with men?
Background Recent studies have demonstrated a high prevalence of pharyngeal (P) and rectal (R) Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) infections among men who have sex with men (MSM). Guidelines by the Centers for Disease Control and Prevention recommend testing at least annually. But surveys of medical providers suggest that adherence to these guidelines is minimal as a result of limited time and staff. Because of these concerns, we evaluated the feasibility and accuracy of patient self-testing.
Methods Three-hundred seventy-four patients at a Washington, DC clinic who identified themselves as MSM and requested testing for sexually transmitted infections (STIs) participated in the study. Patients performed self-screening using the Gen-Probe APTIMA Combo 2 (AC2) kit after viewing written and pictorial instructions. Trained providers also screened patients. We randomized the order in which patients or providers performed testing.
Results Among those receiving specific tests, 8% of patients tested positive for R-GC, 9.3% for P-GC, 12.7% for R-CT, and 1.3% for P-CT. We performed McNemar tests, stratified by infection type and anatomic site to evaluate concordance. Self-administered testing was significantly better at identifying P-GC (discordant: 3%) and R-GC (discordant: 2.9%) (P≤.01), and had results similar to provider- administered testing for P-CT (discordant: 0.5%) and R-CT (discordant: 1.1%) detection.
Conclusions The equivalent or better detection rates for rectal and oral gonorrhea and chlamydia among patients suggest that patients are capable of performing their own screening for STIs, which may increase infection detection and treatment.
The prevalence of Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) infections among men who have sex with men (MSM) are common but unfortunately difficult to identify as a result of their anatomic location and lack of symptoms. In recent studies, 3.7% to 14.9% of MSM tested positive for GC, and 1.7% to 10.7% tested positive for CT on a first screening.1-4 Even more striking, however, was that many of these infections were extragenital and asymptomatic, with rectal GC infections 5 times more common than urethral in one study,3 and more than 80% of rectal GC and CT infections reportedly asymptomatic in another.2
Given the prevalence of undetected infection, the Centers for Disease Control and Prevention (CDC) recommends screening all sexually active MSM at least yearly for rectal GC and CT and pharyngeal GC infections in addition to urethral infections.5 However, research suggests that relatively few physicians follow these recommendations. In a survey of 3509 physicians, less than 14% reported screening their male patients for gonorrhea and chlamydia.6 In another survey, approximately one-third of providers reported not having enough staff to talk with patients about sexually transmitted infections (STIs) and testing, not having enough time in patient visits, and having difficulty keeping up with guidelines for caring for high-risk patients, including MSM.7
These studies raise concern that GC and CT infections will go undiagnosed; failure to detect these infections in MSM is particularly dangerous, given the STIs’ relationship to human immunodeficiency virus (HIV) transmission.8 Urethral GC infections have been shown to increase shedding of HIV in semen,9 and a recent study of MSM also demonstrated that having had multiple rectal GC or CT infections within a 2-year period made HIV seroconversion more likely.10
Due to the importance of identifying GC and CT infections and providers’ concerns about lack of time to do so, studies have explored the possibility of patient-administered testing, which has numerous potential benefits. It decreases the time that a health care provider has to spend on STI testing, and it could lead to screening of larger numbers of patients. It may also enable providers to reach patient populations that are often not appropriately screened, including MSM, prison inmates, homeless patients, drug users, adolescents, and patients in rural or disadvantaged areas.11-19
Comparisons of patient- and clinician-collected samples have yielded encouraging results about the ability of MSM to perform self-administered testing. There was 98% concordance between patient and provider results for rectal GC/CT swabs in one study.20 And another study found that self-collected rectal swabs had equivalent or better sensitivity and specificity for GC/CT detection than provider-collected swabs.21
Only one study has explored both pharyngeal and rectal self-testing of MSM patients, and it found that patient and provider results were concordant for 91.6% of rectal specimens and 93.6% of pharyngeal specimens. Most discordant cases involved patient-identified positives where the provider test was negative. The study authors considered these likely false positives; most occurred in patients who were positive for GC at another site, making cross-contamination probable.22 It is important to investigate further, however, because it is also possible that patients were identifying cases that providers missed.
The aim of our study, therefore, was to explore the ability of MSM patients to perform both pharyngeal and rectal testing for gonorrhea and chlamydia by evaluating the concordance of patient- and provider- administered testing results. We designed the study so that both patients and trained providers performed testing using Gen-Probe’s APTIMA Combo 2 (AC2) kits at pharyngeal, rectal, or both sites, depending on the patient’s recent sexual practices. As discussed, prior studies suggested that concordance would be good for rectal swab specimens. But only one study had examined pharyngeal swab concordance in addition to rectal, and it led to concerns about patient-generated false positives. We wanted our study to evaluate whether patient-administered testing produced accurate results for both pharyngeal and rectal specimens, and whether patient testing behaviors led to cross-contamination and subsequent false-positives when performing both tests.
METHODS
Patient recruitment and eligibility
We recruited patients from STI testing clinics and from primary and HIV care clinics at the Whitman-Walker Clinic in Washington, DC from September 15, 2009 to April 19, 2011. Eligible participants were men who’d had sex with men in the last 6 months and who wanted testing for gonorrhea and chlamydia. The presence of symptoms, reports of condom use during sex, and HIV status did not affect eligibility.
Interested patients signed a consent form and completed a behavioral questionnaire, which included questions about demographics and sexual behavior. A patient’s responses to this questionnaire determined whether he was eligible to participate and the type of testing needed. For example, if a patient had had only oral or anal sex but not both, we limited testing to either pharyngeal or rectal swabbing.
Testing procedures
We randomized patients to either perform self-testing first or to have provider-administered testing first. When patients were ready to self-administer the swabs, we gave them placards that explained how to properly collect samples23 and Gen-Probe AC2 testing kits (Hologic Gen-Probe, San Diego, Calif) for each test they needed to perform. The provider remained in the room while the patient performed the testing to ensure that the patient made an attempt at screening and to identify any common problems with the instructions. If the provider observed problems with how the patient performed the screening, he or she recorded that on a spreadsheet after the patient left.
The 4 providers who performed testing (a nursing student, a medical student, and 2 clinical research assistants) had all been previously trained in STI testing techniques by clinic MDs. When these providers performed testing, they swabbed the patient twice at each site. One of these swabs was stored for research testing, and the other went immediately to the clinic laboratory for testing so that the patient could receive the standard of care antibiotic treatment if the test was positive. The designated research samples were tested in a Gen-Probe laboratory in California.
Statistical methods
Based on a previous study by Lampinen et al that examined concordance between patient- and provider-obtained rectal swabs, we planned on a total sample of 360 patients to achieve 80% power in detecting a difference in positive tests of 5% between patients and clinicians at a one-sided 5% significance level.24 This plan assumed that the percentage of discordant pairs would be 15%, where one pair consists of one test result each from a clinician and a patient at the same site. We performed sample-size calculations in nQuery Advisor version 6.0 (Statistical Solutions, Saugus, Mass). Ultimately, we enrolled 374 men in the study. We tested 5 patients twice, but only their first screening was included in the primary analyses.
We entered demographic and behavioral data from the patient questionnaires into an online database. We also generated descriptive statistics for demographics and baseline characteristics from the questionnaires.
We performed an exact McNemar’s test for each anatomic site and STI to evaluate whether there were significant differences between patient- and provider-performed swabs. We also calculated Kappa coefficients for each site and STI as a measure of concordance. We regarded as positive any test results that were equivocal, because in clinical practice a provider would likely provide treatment. However, we also performed McNemar testing and Kappa calculations with these results excluded and classified as negative to ensure that no significant differences resulted. We evaluated statistical significance at the 0.05 level (2-sided), and performed all analyses in SAS software version 9.2 (SAS Institute, Cary, NC).
RESULTS
The patients who enrolled in the study represented a wide range of ages and ethnic backgrounds, with a median age of 33 years and with Caucasian patients accounting for 54.8%. Patients who had had sex with only men in the past year accounted for 89.8% of the sample, while 8.6% had both male and female partners (the final 1.6% had either no sex partners or missing data). The average number of male partners in the last 2 months was 2. As for sexual practice, 86.9% had insertive oral intercourse in the past year, 70.8% had insertive anal intercourse, 87.2% had receptive oral intercourse, and 67.9% had receptive anal intercourse (TABLE 1).
Considering only provider-identified positives, 5.1% of the patients tested positive for rectal gonorrhea, 11.6% for rectal chlamydia, 6.3% for pharyngeal gonorrhea, and 0.8% for pharyngeal chlamydia (TABLE 2). Considering both provider- and patient-identified positives, 8.0% of patients tested positive for rectal gonorrhea, 9.3% for pharyngeal gonorrhea, 12.7% for rectal chlamydia, and 1.3% for pharyngeal chlamydia. Five equivocal results were identified—2 for a patient rectal gonorrhea test, one for a patient pharyngeal gonorrhea test, one for a provider pharyngeal gonorrhea test, and one for a provider rectal chlamydia test.
In only one case did a provider identify a positive result when the patient’s result was negative. In 23 cases, however, the patient identified a positive result when the provider’s result was negative. Patients identified significantly more positives for rectal and pharyngeal gonorrhea than providers, but there were no significant differences in patient and provider results for the chlamydia tests.
Even with these 24 discordant results, there was ≥75% concordance between patient and provider results on all tests, with very strong concordance (95%) for rectal chlamydia results (TABLE 2). When we re-ran the McNemar tests and the Kappa coefficients with equivocal results considered missing and negative, there were no statistically significant differences when compared with the calculations done with equivocal results considered positive.
Some difficulties observed with self-testing. Observing providers noted anecdotally that there were minor difficulties with the self-administered testing instructions. Three patients spilled the preservative liquid in which swabs are placed, 5 patients used the incorrect swab to perform testing, and 2 other patients had samples that were noted as compromised by the provider. None of these documented problems involved the 24 discordant cases.
TABLE 1
Characteristics of men participating in the study
| Demographic | N=374 |
|---|---|
| Age, y | |
| Median | 33 |
| Range | 18-70 |
| n (%) | |
| Race/ethnicity | |
| White/non-Hispanic | 205 (54.8) |
| Black/non-Hispanic | 85 (22.7) |
| Latino/Hispanic | 40 (10.7) |
| Asian | 13 (3.5) |
| Mixed | 8 (2.1) |
| Other | 19 (5.1) |
| Missing data | 4 (1.1) |
| Modes of GC/CT testing | |
| Rectal and pharyngeal | 272 (72.7) |
| Rectal only | 5 (1.3) |
| Pharyngeal only | 97 (25.9) |
| Sexual partner(s) in the last 12 months | |
| Men | 336 (89.8) |
| Men and women | 32 (8.6) |
| None | 1 (0.3) |
| Missing data | 5 (1.3) |
| Number of male sexual partners in the past 30 days | |
| 0-1 | 178 (47.6) |
| 2-3 | 130 (34.8) |
| 4 or more | 64 (17.1) |
| Missing data | 2 (0.5) |
| Number of male sexual partners in the past 60 days | |
| 0-1 | 104 (27.8) |
| 2-3 | 135 (36.1) |
| 4 or more | 133 (35.6) |
| Missing data | 2 (0.5) |
| Practiced insertive oral intercourse in past 12 months | |
| Yes | 325 (86.9) |
| No | 20 (5.4) |
| Missing data | 29 (7.7) |
| Practiced insertive anal intercourse in past 12 months | |
| Yes | 265 (70.8) |
| No | 78 (20.9) |
| Missing data | 31 (8.3) |
| Practiced receptive oral intercourse in past 12 months | |
| Yes | 326 (87.2) |
| No | 20 (5.3) |
| Missing data | 28 (7.5) |
| Practiced receptive anal intercourse in past 12 months | |
| Yes | 254 (67.9) |
| No | 90 (24.1) |
| Missing data | 30 (8.0) |
| CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae. | |
TABLE 2
Comparison of provider and patient testing results for GC/CT by anatomic site reveals ≥75% concordance on all tests
| Patient test result | Total tests, N | Provider positive test result, n (%) | Provider negative test result, n (%) | P value | Kappa coefficient |
|---|---|---|---|---|---|
| Rectal GC Patient positive test result Patient negative test result | 276 | 14 (5.1) 0 | 8 (2.9)* 254 (92) | <.01 | 0.76 |
| Pharyngeal GC Patient positive test result Patient negative test result | 367 | 23 (6.3) 1 (0.3)* | 10 (2.7)* 333 (90.7) | .01 | 0.79 |
| Rectal CT Patient positive test result Patient negative test result | 276 | 32 (11.6) 0 | 3 (1.1)* 241 (87.3) | .25 | 0.95 |
| Pharyngeal CT Patient positive test result Patient negative test result | 367 | 3 (0.8) 0 | 2 (0.5)* 362 (98.7) | .50 | 0.75 |
| CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae. *Discordant patient-provider test results. | |||||
DISCUSSION
The prevalence of gonorrhea and chlamydia in this study population was similar to what has been observed in previous studies of MSM,1-4 which confirms the need for improved detection of infections. Self- administered testing is one possible means of increasing the number of patients who are screened and treated, as the results of this study suggest it is equally or more accurate than provider-administered testing at detecting cases of gonorrhea and chlamydia.
Patient and provider results were equivalent for chlamydia detection, and patients appeared to identify more cases of gonorrhea, although interpretation of the significance of this finding is difficult given the relatively small number of cases. However, it is likely that the 23 cases in which patients identified positives that providers did not were true positives, given the sensitivity (84%-100%) and specificity (≥99.4%) of the AC2 test for detecting GC and CT.25
Assuming that our population has a similar prevalence of these STIs as prior studies, we would expect positive predictive values of approximately 100% for rectal GC, 95% for pharyngeal GC, 86.4% for rectal CT, and 71.4% for pharyngeal CT.26
Possible explanations for patients achieving better results than providers in some cases. The most likely explanation for better patient performance on some of the tests is that patients swabbed more meticulously and contacted more surface area.
This was also seen in a study that compared the ability of patients and physicians to identify human papillomavirus infection in swabs taken from areas where skin scraping had been performed. Patients were found to collect an appropriate sample significantly more often, which was thought to be due to physician hesitation to thoroughly scrape the patient’s skin.27 In our study, patients were anecdotally observed to be less likely to gag on a self-administered throat swab and to have less visible tensing of the rectal sphincter on self-administered rectal swab, which could have contributed to improved results.
Potential changes to the patient instructions before clinical implementation. These results suggest that self-administered testing could be substituted for clinician-administered testing, potentially improving detection. But widespread implementation would require a few modifications to the testing instructions based on the trained providers’ observations. Although the evidence is anecdotal, providers noted that several patients had difficulty with the instructions. For example, the placard said to use only the swab with the “blue shaft,” but 5 patients got confused and used the white swab either in addition to or instead of the blue swab. (The additional white swab was intended for use in making wet preps, when women are tested.) Many more patients ultimately used the correct swab, but appeared confused by the presence of the additional swab in the kit.
The confusion in these situations might have been avoided if the instructions had told men to throw one swab away and to use only the “blue swab” or the swab with the “blue handle” to avoid potential confusion over the word “shaft.” Another helpful modification to the instructions would be to alert men to the presence of the preservative liquid in the tubes. Several patients laid the tube horizontally with the cap off and spilled much of the liquid. It was still possible to test these samples, and there were no discrepancies between patient and provider results involving them. But ensuring standardization of the test tube contents should still be a priority in editing the instructions.
Incorporating self-testing into clinical practice. While the results of this study suggest that self-testing could be used with a few modifications to the instructions, how best to incorporate self-testing into the clinical setting still needs to be addressed. It might be possible for patients to simply perform testing after a physical exam; for patients to come in when it is convenient for them and leave a sample in the lab; or even for patients to perform testing at home and bring the swabs back to their clinic at a later time.
We obtained the results in this study when patients performed testing in a clinic with a trained provider in the room. One concern in implementing widespread self-testing would be that a provider’s presence in our study might have made patients more likely to spend the time needed to read instructions thoroughly and to put effort into performing the test correctly. However, it is also possible that knowing a provider will be duplicating the testing could lead to decreased patient effort. It may be that having responsibility for one’s own test results is what ensures adequate performance.
Further studies could explore the impact of a provider in the room, but studies have already examined testing done in private and yielded similar results for concordance, which suggests that any impact of the provider’s presence was relatively minimal. A 2009-2010 study at a London clinic, for example, allowed patients to perform testing privately following instructions from a nurse and identified a 9.8% prevalence of rectal CT infection and a 4.2% prevalence of rectal GC infection. These results are similar to those seen in our study and therefore suggest that patients were appropriately identifying infections without observation.28
Another study published in 2012 demonstrated that GC/CT testing swabs sent through the mail without any accompanying transport medium yielded results equivalent to those for swabs shipped in the liquid medium. This approach would make testing at home significantly easier, as patients would not have to be concerned about appropriate storage of their samples before returning them to a clinic.29
Applicability to female patients. Implementation of self-administered testing should be considered for female patients. In fact, most prior studies on the feasibility of self-testing have focused on women, as detection of their chlamydia and gonorrhea infections is equally critical. CDC guidelines stress the importance of yearly chlamydia screening for all women <25 years and for women >25 years who are at high risk (ie, multiple partners); and yearly gonorrhea screening for women at high risk, given the risk of medical complications including pelvic inflammatory disease and infertility if infections are missed.5
Researchers have found that after women participate in self-testing, they report that they would get STI testing more regularly if they could perform it themselves.19,30 Additionally, in studies similar to ours, women performing testing for gonorrhea and chlamydia using self-collected vaginal swabs have achieved similar sensitivity and specificity results to provider-performed testing.12,15,31
Study limitations. This was a self- selected MSM sample seeking STI testing from an urban community health center. Thus, one might assume that the detection of pharyngeal and rectal gonorrhea and chlamydia infections was higher in this sample compared with the general MSM population. That seems unlikely, however, given the similarity (noted earlier) between our findings and data reported in previous studies.1-4 Additionally, the presence of a provider in the room may have influenced patient performance, as we discussed earlier.
It therefore appears that self-administered testing could have universal applicability. Once optimal ways to incorporate self-testing for both men and women are identified, providers should be able to comply with the CDC testing guidelines without an increase in time or staff needed, thereby leading to increased detection and treatment of STIs and benefits for both patients and providers.
CORRESPONDENCE
Michael W. Plankey, PhD, Georgetown University Medical Center, Department of Medicine, Division of Infectious Diseases, 2115 Wisconsin Avenue, NW, Suite 130, Washington, DC 20007; [email protected]
1. Baker J, Plankey M, Josayma Y, et al. The prevalence of rectal, urethral, and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis among asymptomatic men who have sex with men in a prospective cohort in Washington, D.C. AIDS Patient Care STDs. 2009;23:585-588.
2. Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41:67-74.
3. Mimiaga MJ, Helms DJ, Reisner SL, et al. Gonococcal, chlamydia, and syphilis infection positivity among MSM attending a large primary care clinic, Boston, 2003 to 2004. Sex Transm Dis. 2009;36:507-511.
4. Park J, Marcus JL, Pandori M, et al. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis. 2012;39:482-484.
5. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
6. St Lawrence JS, Montano DE, Kasprzyk D, et al. STD screening, testing, case reporting, and clinical and partner notification practices: a national survey of US physicians. Am J Public Health. 2002;92:1784-1788.
7. Mark H, Irwin K, Sternberg M, et al. Providers’ perceived barriers to sexually transmitted disease care in 2 large health maintenance organizations. Sex Transm Dis. 2008;35:184-189.
8. Xu F, Sternberg MR, Markowitz LE. Men who have sex with men in the United States: demographic and behavioral characteristics and prevalence of HIV and HSV-2 infection: results from National Health and Nutrition Examination Survey 2001-2006. Sex Transm Dis. 2010;37:399-405.
9. Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868-1873.
10. Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537-543.
11. Bradshaw CS, Pierce LI, Tabrizi SN, et al. Screening injecting drug users for sexually transmitted infections and blood borne viruses using street outreach and self collected sampling. Sex Transm Infect. 2005;81:53-58.
12. Fang J, Husman C, DeSilva L, et al. Evaluation of self-collected vaginal swab, first void urine, and endocervical swab specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in adolescent females. J Pediatr Adolesc Gynecol. 2008;21:355-360.
13. Gaydos CA. Nucleic acid amplification tests for gonorrhea and chlamydia: practice and applications. Infect Dis Clin North Am. 2005;19:367-386 ix.
14. Hobbs MM, van der Pol B, Totten P, et al. From the NIH: proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis. 2008;35:8-13.
15. Knox J, Tabrizi SN, Miller P, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis. 2002;29:647-654.
16. Lippman SA, Jones HE, Luppi CG, et al. Home-based self-sampling and self-testing for sexually transmitted infections: acceptable and feasible alternatives to provider-based screening in low-income women in Sao Paulo, Brazil. Sex Transm Dis. 2007;34:421-428.
17. Newman SB, Nelson MB, Gaydos CA, et al. Female prisoners’ p of collection methods for testing for Chlamydia trachomatis and Neisseria gonorrhoeae infection. Sex Transm Dis. 2003;30:306-309.
18. Richardson E, Sellors JW, Mackinnon S, et al. Prevalence of Chlamydia trachomatis infections and specimen collection preference among women, using self-collected vaginal swabs in community settings. Sex Transm Dis. 2003;30:880-885.
19. Wiesenfeld HC, Lowry DL, Heine RP, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis. 2001;28:321-325.
20. van der Helm JJ, Hoebe CJ, van Rooijen MS, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis. 2009;36:493-497.
21. Moncada J, Schachter J, Liska S, et al. Evaluation of self-collected glans and rectal swabs from men who have sex with men for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of nucleic acid amplification tests. J Clin Microbiol. 2009;47:1657-1662.
22. Alexander S, Ison C, Parry J, et al. Self-taken pharyngeal and rectal swabs are appropriate for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in asymptomatic men who have sex with men. Sex Transm Infect. 2008;84:488-492.
23. San Francisco City Clinic. Patient instructions for self-collected specimens: pharyngeal and rectal. Available at: http://www.sfcityclinic.org/providers. Accessed December 15 2011.
24. Lampinen TM, Latulippe L, van Niekerk D, et al. Illustrated instructions for self-collection of anorectal swab specimens and their adequacy for cytological examination. Sex Transm Dis. 2006;33:386-388.
25. Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis. 2008;35:637-642.
26. Ota KV, Tamari IE, Smieja M, et al. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima Combo 2 assay and culture. Sex Transm Infect. 2009;85:182-186.
27. Hernandez BY, McDuffie K, Goodman MT, et al. Comparison of physician- and self-collected genital specimens for detection of human papillomavirus in men. J Clin Microbiol. 2006;44:513-517.
28. Soni S, White JA. Self-screening for Neisseria gonorrhoeae and Chlamydia trachomatis in the human immunodeficiency virus clinic—high yields and high acceptability. Sex Transm Dis. 2011;38:1107-1109.
29. Gaydos CA, Farshy C, Barnes M, et al. Can mailed swab samples be dry-shipped for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by nucleic acid amplification tests? Diagn Microbiol Infect Dis. 2012;73:16-20.
30. Chernesky MA, Hook EW, 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005;32:729-733.
31. Polaneczky M, Quigley C, Pollock L, et al. Use of self-collected vaginal specimens for detection of Chlamydia trachomatis infection. Obstet Gynecol. 1998;91:375-378.
Background Recent studies have demonstrated a high prevalence of pharyngeal (P) and rectal (R) Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) infections among men who have sex with men (MSM). Guidelines by the Centers for Disease Control and Prevention recommend testing at least annually. But surveys of medical providers suggest that adherence to these guidelines is minimal as a result of limited time and staff. Because of these concerns, we evaluated the feasibility and accuracy of patient self-testing.
Methods Three-hundred seventy-four patients at a Washington, DC clinic who identified themselves as MSM and requested testing for sexually transmitted infections (STIs) participated in the study. Patients performed self-screening using the Gen-Probe APTIMA Combo 2 (AC2) kit after viewing written and pictorial instructions. Trained providers also screened patients. We randomized the order in which patients or providers performed testing.
Results Among those receiving specific tests, 8% of patients tested positive for R-GC, 9.3% for P-GC, 12.7% for R-CT, and 1.3% for P-CT. We performed McNemar tests, stratified by infection type and anatomic site to evaluate concordance. Self-administered testing was significantly better at identifying P-GC (discordant: 3%) and R-GC (discordant: 2.9%) (P≤.01), and had results similar to provider- administered testing for P-CT (discordant: 0.5%) and R-CT (discordant: 1.1%) detection.
Conclusions The equivalent or better detection rates for rectal and oral gonorrhea and chlamydia among patients suggest that patients are capable of performing their own screening for STIs, which may increase infection detection and treatment.
The prevalence of Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) infections among men who have sex with men (MSM) are common but unfortunately difficult to identify as a result of their anatomic location and lack of symptoms. In recent studies, 3.7% to 14.9% of MSM tested positive for GC, and 1.7% to 10.7% tested positive for CT on a first screening.1-4 Even more striking, however, was that many of these infections were extragenital and asymptomatic, with rectal GC infections 5 times more common than urethral in one study,3 and more than 80% of rectal GC and CT infections reportedly asymptomatic in another.2
Given the prevalence of undetected infection, the Centers for Disease Control and Prevention (CDC) recommends screening all sexually active MSM at least yearly for rectal GC and CT and pharyngeal GC infections in addition to urethral infections.5 However, research suggests that relatively few physicians follow these recommendations. In a survey of 3509 physicians, less than 14% reported screening their male patients for gonorrhea and chlamydia.6 In another survey, approximately one-third of providers reported not having enough staff to talk with patients about sexually transmitted infections (STIs) and testing, not having enough time in patient visits, and having difficulty keeping up with guidelines for caring for high-risk patients, including MSM.7
These studies raise concern that GC and CT infections will go undiagnosed; failure to detect these infections in MSM is particularly dangerous, given the STIs’ relationship to human immunodeficiency virus (HIV) transmission.8 Urethral GC infections have been shown to increase shedding of HIV in semen,9 and a recent study of MSM also demonstrated that having had multiple rectal GC or CT infections within a 2-year period made HIV seroconversion more likely.10
Due to the importance of identifying GC and CT infections and providers’ concerns about lack of time to do so, studies have explored the possibility of patient-administered testing, which has numerous potential benefits. It decreases the time that a health care provider has to spend on STI testing, and it could lead to screening of larger numbers of patients. It may also enable providers to reach patient populations that are often not appropriately screened, including MSM, prison inmates, homeless patients, drug users, adolescents, and patients in rural or disadvantaged areas.11-19
Comparisons of patient- and clinician-collected samples have yielded encouraging results about the ability of MSM to perform self-administered testing. There was 98% concordance between patient and provider results for rectal GC/CT swabs in one study.20 And another study found that self-collected rectal swabs had equivalent or better sensitivity and specificity for GC/CT detection than provider-collected swabs.21
Only one study has explored both pharyngeal and rectal self-testing of MSM patients, and it found that patient and provider results were concordant for 91.6% of rectal specimens and 93.6% of pharyngeal specimens. Most discordant cases involved patient-identified positives where the provider test was negative. The study authors considered these likely false positives; most occurred in patients who were positive for GC at another site, making cross-contamination probable.22 It is important to investigate further, however, because it is also possible that patients were identifying cases that providers missed.
The aim of our study, therefore, was to explore the ability of MSM patients to perform both pharyngeal and rectal testing for gonorrhea and chlamydia by evaluating the concordance of patient- and provider- administered testing results. We designed the study so that both patients and trained providers performed testing using Gen-Probe’s APTIMA Combo 2 (AC2) kits at pharyngeal, rectal, or both sites, depending on the patient’s recent sexual practices. As discussed, prior studies suggested that concordance would be good for rectal swab specimens. But only one study had examined pharyngeal swab concordance in addition to rectal, and it led to concerns about patient-generated false positives. We wanted our study to evaluate whether patient-administered testing produced accurate results for both pharyngeal and rectal specimens, and whether patient testing behaviors led to cross-contamination and subsequent false-positives when performing both tests.
METHODS
Patient recruitment and eligibility
We recruited patients from STI testing clinics and from primary and HIV care clinics at the Whitman-Walker Clinic in Washington, DC from September 15, 2009 to April 19, 2011. Eligible participants were men who’d had sex with men in the last 6 months and who wanted testing for gonorrhea and chlamydia. The presence of symptoms, reports of condom use during sex, and HIV status did not affect eligibility.
Interested patients signed a consent form and completed a behavioral questionnaire, which included questions about demographics and sexual behavior. A patient’s responses to this questionnaire determined whether he was eligible to participate and the type of testing needed. For example, if a patient had had only oral or anal sex but not both, we limited testing to either pharyngeal or rectal swabbing.
Testing procedures
We randomized patients to either perform self-testing first or to have provider-administered testing first. When patients were ready to self-administer the swabs, we gave them placards that explained how to properly collect samples23 and Gen-Probe AC2 testing kits (Hologic Gen-Probe, San Diego, Calif) for each test they needed to perform. The provider remained in the room while the patient performed the testing to ensure that the patient made an attempt at screening and to identify any common problems with the instructions. If the provider observed problems with how the patient performed the screening, he or she recorded that on a spreadsheet after the patient left.
The 4 providers who performed testing (a nursing student, a medical student, and 2 clinical research assistants) had all been previously trained in STI testing techniques by clinic MDs. When these providers performed testing, they swabbed the patient twice at each site. One of these swabs was stored for research testing, and the other went immediately to the clinic laboratory for testing so that the patient could receive the standard of care antibiotic treatment if the test was positive. The designated research samples were tested in a Gen-Probe laboratory in California.
Statistical methods
Based on a previous study by Lampinen et al that examined concordance between patient- and provider-obtained rectal swabs, we planned on a total sample of 360 patients to achieve 80% power in detecting a difference in positive tests of 5% between patients and clinicians at a one-sided 5% significance level.24 This plan assumed that the percentage of discordant pairs would be 15%, where one pair consists of one test result each from a clinician and a patient at the same site. We performed sample-size calculations in nQuery Advisor version 6.0 (Statistical Solutions, Saugus, Mass). Ultimately, we enrolled 374 men in the study. We tested 5 patients twice, but only their first screening was included in the primary analyses.
We entered demographic and behavioral data from the patient questionnaires into an online database. We also generated descriptive statistics for demographics and baseline characteristics from the questionnaires.
We performed an exact McNemar’s test for each anatomic site and STI to evaluate whether there were significant differences between patient- and provider-performed swabs. We also calculated Kappa coefficients for each site and STI as a measure of concordance. We regarded as positive any test results that were equivocal, because in clinical practice a provider would likely provide treatment. However, we also performed McNemar testing and Kappa calculations with these results excluded and classified as negative to ensure that no significant differences resulted. We evaluated statistical significance at the 0.05 level (2-sided), and performed all analyses in SAS software version 9.2 (SAS Institute, Cary, NC).
RESULTS
The patients who enrolled in the study represented a wide range of ages and ethnic backgrounds, with a median age of 33 years and with Caucasian patients accounting for 54.8%. Patients who had had sex with only men in the past year accounted for 89.8% of the sample, while 8.6% had both male and female partners (the final 1.6% had either no sex partners or missing data). The average number of male partners in the last 2 months was 2. As for sexual practice, 86.9% had insertive oral intercourse in the past year, 70.8% had insertive anal intercourse, 87.2% had receptive oral intercourse, and 67.9% had receptive anal intercourse (TABLE 1).
Considering only provider-identified positives, 5.1% of the patients tested positive for rectal gonorrhea, 11.6% for rectal chlamydia, 6.3% for pharyngeal gonorrhea, and 0.8% for pharyngeal chlamydia (TABLE 2). Considering both provider- and patient-identified positives, 8.0% of patients tested positive for rectal gonorrhea, 9.3% for pharyngeal gonorrhea, 12.7% for rectal chlamydia, and 1.3% for pharyngeal chlamydia. Five equivocal results were identified—2 for a patient rectal gonorrhea test, one for a patient pharyngeal gonorrhea test, one for a provider pharyngeal gonorrhea test, and one for a provider rectal chlamydia test.
In only one case did a provider identify a positive result when the patient’s result was negative. In 23 cases, however, the patient identified a positive result when the provider’s result was negative. Patients identified significantly more positives for rectal and pharyngeal gonorrhea than providers, but there were no significant differences in patient and provider results for the chlamydia tests.
Even with these 24 discordant results, there was ≥75% concordance between patient and provider results on all tests, with very strong concordance (95%) for rectal chlamydia results (TABLE 2). When we re-ran the McNemar tests and the Kappa coefficients with equivocal results considered missing and negative, there were no statistically significant differences when compared with the calculations done with equivocal results considered positive.
Some difficulties observed with self-testing. Observing providers noted anecdotally that there were minor difficulties with the self-administered testing instructions. Three patients spilled the preservative liquid in which swabs are placed, 5 patients used the incorrect swab to perform testing, and 2 other patients had samples that were noted as compromised by the provider. None of these documented problems involved the 24 discordant cases.
TABLE 1
Characteristics of men participating in the study
| Demographic | N=374 |
|---|---|
| Age, y | |
| Median | 33 |
| Range | 18-70 |
| n (%) | |
| Race/ethnicity | |
| White/non-Hispanic | 205 (54.8) |
| Black/non-Hispanic | 85 (22.7) |
| Latino/Hispanic | 40 (10.7) |
| Asian | 13 (3.5) |
| Mixed | 8 (2.1) |
| Other | 19 (5.1) |
| Missing data | 4 (1.1) |
| Modes of GC/CT testing | |
| Rectal and pharyngeal | 272 (72.7) |
| Rectal only | 5 (1.3) |
| Pharyngeal only | 97 (25.9) |
| Sexual partner(s) in the last 12 months | |
| Men | 336 (89.8) |
| Men and women | 32 (8.6) |
| None | 1 (0.3) |
| Missing data | 5 (1.3) |
| Number of male sexual partners in the past 30 days | |
| 0-1 | 178 (47.6) |
| 2-3 | 130 (34.8) |
| 4 or more | 64 (17.1) |
| Missing data | 2 (0.5) |
| Number of male sexual partners in the past 60 days | |
| 0-1 | 104 (27.8) |
| 2-3 | 135 (36.1) |
| 4 or more | 133 (35.6) |
| Missing data | 2 (0.5) |
| Practiced insertive oral intercourse in past 12 months | |
| Yes | 325 (86.9) |
| No | 20 (5.4) |
| Missing data | 29 (7.7) |
| Practiced insertive anal intercourse in past 12 months | |
| Yes | 265 (70.8) |
| No | 78 (20.9) |
| Missing data | 31 (8.3) |
| Practiced receptive oral intercourse in past 12 months | |
| Yes | 326 (87.2) |
| No | 20 (5.3) |
| Missing data | 28 (7.5) |
| Practiced receptive anal intercourse in past 12 months | |
| Yes | 254 (67.9) |
| No | 90 (24.1) |
| Missing data | 30 (8.0) |
| CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae. | |
TABLE 2
Comparison of provider and patient testing results for GC/CT by anatomic site reveals ≥75% concordance on all tests
| Patient test result | Total tests, N | Provider positive test result, n (%) | Provider negative test result, n (%) | P value | Kappa coefficient |
|---|---|---|---|---|---|
| Rectal GC Patient positive test result Patient negative test result | 276 | 14 (5.1) 0 | 8 (2.9)* 254 (92) | <.01 | 0.76 |
| Pharyngeal GC Patient positive test result Patient negative test result | 367 | 23 (6.3) 1 (0.3)* | 10 (2.7)* 333 (90.7) | .01 | 0.79 |
| Rectal CT Patient positive test result Patient negative test result | 276 | 32 (11.6) 0 | 3 (1.1)* 241 (87.3) | .25 | 0.95 |
| Pharyngeal CT Patient positive test result Patient negative test result | 367 | 3 (0.8) 0 | 2 (0.5)* 362 (98.7) | .50 | 0.75 |
| CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae. *Discordant patient-provider test results. | |||||
DISCUSSION
The prevalence of gonorrhea and chlamydia in this study population was similar to what has been observed in previous studies of MSM,1-4 which confirms the need for improved detection of infections. Self- administered testing is one possible means of increasing the number of patients who are screened and treated, as the results of this study suggest it is equally or more accurate than provider-administered testing at detecting cases of gonorrhea and chlamydia.
Patient and provider results were equivalent for chlamydia detection, and patients appeared to identify more cases of gonorrhea, although interpretation of the significance of this finding is difficult given the relatively small number of cases. However, it is likely that the 23 cases in which patients identified positives that providers did not were true positives, given the sensitivity (84%-100%) and specificity (≥99.4%) of the AC2 test for detecting GC and CT.25
Assuming that our population has a similar prevalence of these STIs as prior studies, we would expect positive predictive values of approximately 100% for rectal GC, 95% for pharyngeal GC, 86.4% for rectal CT, and 71.4% for pharyngeal CT.26
Possible explanations for patients achieving better results than providers in some cases. The most likely explanation for better patient performance on some of the tests is that patients swabbed more meticulously and contacted more surface area.
This was also seen in a study that compared the ability of patients and physicians to identify human papillomavirus infection in swabs taken from areas where skin scraping had been performed. Patients were found to collect an appropriate sample significantly more often, which was thought to be due to physician hesitation to thoroughly scrape the patient’s skin.27 In our study, patients were anecdotally observed to be less likely to gag on a self-administered throat swab and to have less visible tensing of the rectal sphincter on self-administered rectal swab, which could have contributed to improved results.
Potential changes to the patient instructions before clinical implementation. These results suggest that self-administered testing could be substituted for clinician-administered testing, potentially improving detection. But widespread implementation would require a few modifications to the testing instructions based on the trained providers’ observations. Although the evidence is anecdotal, providers noted that several patients had difficulty with the instructions. For example, the placard said to use only the swab with the “blue shaft,” but 5 patients got confused and used the white swab either in addition to or instead of the blue swab. (The additional white swab was intended for use in making wet preps, when women are tested.) Many more patients ultimately used the correct swab, but appeared confused by the presence of the additional swab in the kit.
The confusion in these situations might have been avoided if the instructions had told men to throw one swab away and to use only the “blue swab” or the swab with the “blue handle” to avoid potential confusion over the word “shaft.” Another helpful modification to the instructions would be to alert men to the presence of the preservative liquid in the tubes. Several patients laid the tube horizontally with the cap off and spilled much of the liquid. It was still possible to test these samples, and there were no discrepancies between patient and provider results involving them. But ensuring standardization of the test tube contents should still be a priority in editing the instructions.
Incorporating self-testing into clinical practice. While the results of this study suggest that self-testing could be used with a few modifications to the instructions, how best to incorporate self-testing into the clinical setting still needs to be addressed. It might be possible for patients to simply perform testing after a physical exam; for patients to come in when it is convenient for them and leave a sample in the lab; or even for patients to perform testing at home and bring the swabs back to their clinic at a later time.
We obtained the results in this study when patients performed testing in a clinic with a trained provider in the room. One concern in implementing widespread self-testing would be that a provider’s presence in our study might have made patients more likely to spend the time needed to read instructions thoroughly and to put effort into performing the test correctly. However, it is also possible that knowing a provider will be duplicating the testing could lead to decreased patient effort. It may be that having responsibility for one’s own test results is what ensures adequate performance.
Further studies could explore the impact of a provider in the room, but studies have already examined testing done in private and yielded similar results for concordance, which suggests that any impact of the provider’s presence was relatively minimal. A 2009-2010 study at a London clinic, for example, allowed patients to perform testing privately following instructions from a nurse and identified a 9.8% prevalence of rectal CT infection and a 4.2% prevalence of rectal GC infection. These results are similar to those seen in our study and therefore suggest that patients were appropriately identifying infections without observation.28
Another study published in 2012 demonstrated that GC/CT testing swabs sent through the mail without any accompanying transport medium yielded results equivalent to those for swabs shipped in the liquid medium. This approach would make testing at home significantly easier, as patients would not have to be concerned about appropriate storage of their samples before returning them to a clinic.29
Applicability to female patients. Implementation of self-administered testing should be considered for female patients. In fact, most prior studies on the feasibility of self-testing have focused on women, as detection of their chlamydia and gonorrhea infections is equally critical. CDC guidelines stress the importance of yearly chlamydia screening for all women <25 years and for women >25 years who are at high risk (ie, multiple partners); and yearly gonorrhea screening for women at high risk, given the risk of medical complications including pelvic inflammatory disease and infertility if infections are missed.5
Researchers have found that after women participate in self-testing, they report that they would get STI testing more regularly if they could perform it themselves.19,30 Additionally, in studies similar to ours, women performing testing for gonorrhea and chlamydia using self-collected vaginal swabs have achieved similar sensitivity and specificity results to provider-performed testing.12,15,31
Study limitations. This was a self- selected MSM sample seeking STI testing from an urban community health center. Thus, one might assume that the detection of pharyngeal and rectal gonorrhea and chlamydia infections was higher in this sample compared with the general MSM population. That seems unlikely, however, given the similarity (noted earlier) between our findings and data reported in previous studies.1-4 Additionally, the presence of a provider in the room may have influenced patient performance, as we discussed earlier.
It therefore appears that self-administered testing could have universal applicability. Once optimal ways to incorporate self-testing for both men and women are identified, providers should be able to comply with the CDC testing guidelines without an increase in time or staff needed, thereby leading to increased detection and treatment of STIs and benefits for both patients and providers.
CORRESPONDENCE
Michael W. Plankey, PhD, Georgetown University Medical Center, Department of Medicine, Division of Infectious Diseases, 2115 Wisconsin Avenue, NW, Suite 130, Washington, DC 20007; [email protected]
Background Recent studies have demonstrated a high prevalence of pharyngeal (P) and rectal (R) Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) infections among men who have sex with men (MSM). Guidelines by the Centers for Disease Control and Prevention recommend testing at least annually. But surveys of medical providers suggest that adherence to these guidelines is minimal as a result of limited time and staff. Because of these concerns, we evaluated the feasibility and accuracy of patient self-testing.
Methods Three-hundred seventy-four patients at a Washington, DC clinic who identified themselves as MSM and requested testing for sexually transmitted infections (STIs) participated in the study. Patients performed self-screening using the Gen-Probe APTIMA Combo 2 (AC2) kit after viewing written and pictorial instructions. Trained providers also screened patients. We randomized the order in which patients or providers performed testing.
Results Among those receiving specific tests, 8% of patients tested positive for R-GC, 9.3% for P-GC, 12.7% for R-CT, and 1.3% for P-CT. We performed McNemar tests, stratified by infection type and anatomic site to evaluate concordance. Self-administered testing was significantly better at identifying P-GC (discordant: 3%) and R-GC (discordant: 2.9%) (P≤.01), and had results similar to provider- administered testing for P-CT (discordant: 0.5%) and R-CT (discordant: 1.1%) detection.
Conclusions The equivalent or better detection rates for rectal and oral gonorrhea and chlamydia among patients suggest that patients are capable of performing their own screening for STIs, which may increase infection detection and treatment.
The prevalence of Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) infections among men who have sex with men (MSM) are common but unfortunately difficult to identify as a result of their anatomic location and lack of symptoms. In recent studies, 3.7% to 14.9% of MSM tested positive for GC, and 1.7% to 10.7% tested positive for CT on a first screening.1-4 Even more striking, however, was that many of these infections were extragenital and asymptomatic, with rectal GC infections 5 times more common than urethral in one study,3 and more than 80% of rectal GC and CT infections reportedly asymptomatic in another.2
Given the prevalence of undetected infection, the Centers for Disease Control and Prevention (CDC) recommends screening all sexually active MSM at least yearly for rectal GC and CT and pharyngeal GC infections in addition to urethral infections.5 However, research suggests that relatively few physicians follow these recommendations. In a survey of 3509 physicians, less than 14% reported screening their male patients for gonorrhea and chlamydia.6 In another survey, approximately one-third of providers reported not having enough staff to talk with patients about sexually transmitted infections (STIs) and testing, not having enough time in patient visits, and having difficulty keeping up with guidelines for caring for high-risk patients, including MSM.7
These studies raise concern that GC and CT infections will go undiagnosed; failure to detect these infections in MSM is particularly dangerous, given the STIs’ relationship to human immunodeficiency virus (HIV) transmission.8 Urethral GC infections have been shown to increase shedding of HIV in semen,9 and a recent study of MSM also demonstrated that having had multiple rectal GC or CT infections within a 2-year period made HIV seroconversion more likely.10
Due to the importance of identifying GC and CT infections and providers’ concerns about lack of time to do so, studies have explored the possibility of patient-administered testing, which has numerous potential benefits. It decreases the time that a health care provider has to spend on STI testing, and it could lead to screening of larger numbers of patients. It may also enable providers to reach patient populations that are often not appropriately screened, including MSM, prison inmates, homeless patients, drug users, adolescents, and patients in rural or disadvantaged areas.11-19
Comparisons of patient- and clinician-collected samples have yielded encouraging results about the ability of MSM to perform self-administered testing. There was 98% concordance between patient and provider results for rectal GC/CT swabs in one study.20 And another study found that self-collected rectal swabs had equivalent or better sensitivity and specificity for GC/CT detection than provider-collected swabs.21
Only one study has explored both pharyngeal and rectal self-testing of MSM patients, and it found that patient and provider results were concordant for 91.6% of rectal specimens and 93.6% of pharyngeal specimens. Most discordant cases involved patient-identified positives where the provider test was negative. The study authors considered these likely false positives; most occurred in patients who were positive for GC at another site, making cross-contamination probable.22 It is important to investigate further, however, because it is also possible that patients were identifying cases that providers missed.
The aim of our study, therefore, was to explore the ability of MSM patients to perform both pharyngeal and rectal testing for gonorrhea and chlamydia by evaluating the concordance of patient- and provider- administered testing results. We designed the study so that both patients and trained providers performed testing using Gen-Probe’s APTIMA Combo 2 (AC2) kits at pharyngeal, rectal, or both sites, depending on the patient’s recent sexual practices. As discussed, prior studies suggested that concordance would be good for rectal swab specimens. But only one study had examined pharyngeal swab concordance in addition to rectal, and it led to concerns about patient-generated false positives. We wanted our study to evaluate whether patient-administered testing produced accurate results for both pharyngeal and rectal specimens, and whether patient testing behaviors led to cross-contamination and subsequent false-positives when performing both tests.
METHODS
Patient recruitment and eligibility
We recruited patients from STI testing clinics and from primary and HIV care clinics at the Whitman-Walker Clinic in Washington, DC from September 15, 2009 to April 19, 2011. Eligible participants were men who’d had sex with men in the last 6 months and who wanted testing for gonorrhea and chlamydia. The presence of symptoms, reports of condom use during sex, and HIV status did not affect eligibility.
Interested patients signed a consent form and completed a behavioral questionnaire, which included questions about demographics and sexual behavior. A patient’s responses to this questionnaire determined whether he was eligible to participate and the type of testing needed. For example, if a patient had had only oral or anal sex but not both, we limited testing to either pharyngeal or rectal swabbing.
Testing procedures
We randomized patients to either perform self-testing first or to have provider-administered testing first. When patients were ready to self-administer the swabs, we gave them placards that explained how to properly collect samples23 and Gen-Probe AC2 testing kits (Hologic Gen-Probe, San Diego, Calif) for each test they needed to perform. The provider remained in the room while the patient performed the testing to ensure that the patient made an attempt at screening and to identify any common problems with the instructions. If the provider observed problems with how the patient performed the screening, he or she recorded that on a spreadsheet after the patient left.
The 4 providers who performed testing (a nursing student, a medical student, and 2 clinical research assistants) had all been previously trained in STI testing techniques by clinic MDs. When these providers performed testing, they swabbed the patient twice at each site. One of these swabs was stored for research testing, and the other went immediately to the clinic laboratory for testing so that the patient could receive the standard of care antibiotic treatment if the test was positive. The designated research samples were tested in a Gen-Probe laboratory in California.
Statistical methods
Based on a previous study by Lampinen et al that examined concordance between patient- and provider-obtained rectal swabs, we planned on a total sample of 360 patients to achieve 80% power in detecting a difference in positive tests of 5% between patients and clinicians at a one-sided 5% significance level.24 This plan assumed that the percentage of discordant pairs would be 15%, where one pair consists of one test result each from a clinician and a patient at the same site. We performed sample-size calculations in nQuery Advisor version 6.0 (Statistical Solutions, Saugus, Mass). Ultimately, we enrolled 374 men in the study. We tested 5 patients twice, but only their first screening was included in the primary analyses.
We entered demographic and behavioral data from the patient questionnaires into an online database. We also generated descriptive statistics for demographics and baseline characteristics from the questionnaires.
We performed an exact McNemar’s test for each anatomic site and STI to evaluate whether there were significant differences between patient- and provider-performed swabs. We also calculated Kappa coefficients for each site and STI as a measure of concordance. We regarded as positive any test results that were equivocal, because in clinical practice a provider would likely provide treatment. However, we also performed McNemar testing and Kappa calculations with these results excluded and classified as negative to ensure that no significant differences resulted. We evaluated statistical significance at the 0.05 level (2-sided), and performed all analyses in SAS software version 9.2 (SAS Institute, Cary, NC).
RESULTS
The patients who enrolled in the study represented a wide range of ages and ethnic backgrounds, with a median age of 33 years and with Caucasian patients accounting for 54.8%. Patients who had had sex with only men in the past year accounted for 89.8% of the sample, while 8.6% had both male and female partners (the final 1.6% had either no sex partners or missing data). The average number of male partners in the last 2 months was 2. As for sexual practice, 86.9% had insertive oral intercourse in the past year, 70.8% had insertive anal intercourse, 87.2% had receptive oral intercourse, and 67.9% had receptive anal intercourse (TABLE 1).
Considering only provider-identified positives, 5.1% of the patients tested positive for rectal gonorrhea, 11.6% for rectal chlamydia, 6.3% for pharyngeal gonorrhea, and 0.8% for pharyngeal chlamydia (TABLE 2). Considering both provider- and patient-identified positives, 8.0% of patients tested positive for rectal gonorrhea, 9.3% for pharyngeal gonorrhea, 12.7% for rectal chlamydia, and 1.3% for pharyngeal chlamydia. Five equivocal results were identified—2 for a patient rectal gonorrhea test, one for a patient pharyngeal gonorrhea test, one for a provider pharyngeal gonorrhea test, and one for a provider rectal chlamydia test.
In only one case did a provider identify a positive result when the patient’s result was negative. In 23 cases, however, the patient identified a positive result when the provider’s result was negative. Patients identified significantly more positives for rectal and pharyngeal gonorrhea than providers, but there were no significant differences in patient and provider results for the chlamydia tests.
Even with these 24 discordant results, there was ≥75% concordance between patient and provider results on all tests, with very strong concordance (95%) for rectal chlamydia results (TABLE 2). When we re-ran the McNemar tests and the Kappa coefficients with equivocal results considered missing and negative, there were no statistically significant differences when compared with the calculations done with equivocal results considered positive.
Some difficulties observed with self-testing. Observing providers noted anecdotally that there were minor difficulties with the self-administered testing instructions. Three patients spilled the preservative liquid in which swabs are placed, 5 patients used the incorrect swab to perform testing, and 2 other patients had samples that were noted as compromised by the provider. None of these documented problems involved the 24 discordant cases.
TABLE 1
Characteristics of men participating in the study
| Demographic | N=374 |
|---|---|
| Age, y | |
| Median | 33 |
| Range | 18-70 |
| n (%) | |
| Race/ethnicity | |
| White/non-Hispanic | 205 (54.8) |
| Black/non-Hispanic | 85 (22.7) |
| Latino/Hispanic | 40 (10.7) |
| Asian | 13 (3.5) |
| Mixed | 8 (2.1) |
| Other | 19 (5.1) |
| Missing data | 4 (1.1) |
| Modes of GC/CT testing | |
| Rectal and pharyngeal | 272 (72.7) |
| Rectal only | 5 (1.3) |
| Pharyngeal only | 97 (25.9) |
| Sexual partner(s) in the last 12 months | |
| Men | 336 (89.8) |
| Men and women | 32 (8.6) |
| None | 1 (0.3) |
| Missing data | 5 (1.3) |
| Number of male sexual partners in the past 30 days | |
| 0-1 | 178 (47.6) |
| 2-3 | 130 (34.8) |
| 4 or more | 64 (17.1) |
| Missing data | 2 (0.5) |
| Number of male sexual partners in the past 60 days | |
| 0-1 | 104 (27.8) |
| 2-3 | 135 (36.1) |
| 4 or more | 133 (35.6) |
| Missing data | 2 (0.5) |
| Practiced insertive oral intercourse in past 12 months | |
| Yes | 325 (86.9) |
| No | 20 (5.4) |
| Missing data | 29 (7.7) |
| Practiced insertive anal intercourse in past 12 months | |
| Yes | 265 (70.8) |
| No | 78 (20.9) |
| Missing data | 31 (8.3) |
| Practiced receptive oral intercourse in past 12 months | |
| Yes | 326 (87.2) |
| No | 20 (5.3) |
| Missing data | 28 (7.5) |
| Practiced receptive anal intercourse in past 12 months | |
| Yes | 254 (67.9) |
| No | 90 (24.1) |
| Missing data | 30 (8.0) |
| CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae. | |
TABLE 2
Comparison of provider and patient testing results for GC/CT by anatomic site reveals ≥75% concordance on all tests
| Patient test result | Total tests, N | Provider positive test result, n (%) | Provider negative test result, n (%) | P value | Kappa coefficient |
|---|---|---|---|---|---|
| Rectal GC Patient positive test result Patient negative test result | 276 | 14 (5.1) 0 | 8 (2.9)* 254 (92) | <.01 | 0.76 |
| Pharyngeal GC Patient positive test result Patient negative test result | 367 | 23 (6.3) 1 (0.3)* | 10 (2.7)* 333 (90.7) | .01 | 0.79 |
| Rectal CT Patient positive test result Patient negative test result | 276 | 32 (11.6) 0 | 3 (1.1)* 241 (87.3) | .25 | 0.95 |
| Pharyngeal CT Patient positive test result Patient negative test result | 367 | 3 (0.8) 0 | 2 (0.5)* 362 (98.7) | .50 | 0.75 |
| CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae. *Discordant patient-provider test results. | |||||
DISCUSSION
The prevalence of gonorrhea and chlamydia in this study population was similar to what has been observed in previous studies of MSM,1-4 which confirms the need for improved detection of infections. Self- administered testing is one possible means of increasing the number of patients who are screened and treated, as the results of this study suggest it is equally or more accurate than provider-administered testing at detecting cases of gonorrhea and chlamydia.
Patient and provider results were equivalent for chlamydia detection, and patients appeared to identify more cases of gonorrhea, although interpretation of the significance of this finding is difficult given the relatively small number of cases. However, it is likely that the 23 cases in which patients identified positives that providers did not were true positives, given the sensitivity (84%-100%) and specificity (≥99.4%) of the AC2 test for detecting GC and CT.25
Assuming that our population has a similar prevalence of these STIs as prior studies, we would expect positive predictive values of approximately 100% for rectal GC, 95% for pharyngeal GC, 86.4% for rectal CT, and 71.4% for pharyngeal CT.26
Possible explanations for patients achieving better results than providers in some cases. The most likely explanation for better patient performance on some of the tests is that patients swabbed more meticulously and contacted more surface area.
This was also seen in a study that compared the ability of patients and physicians to identify human papillomavirus infection in swabs taken from areas where skin scraping had been performed. Patients were found to collect an appropriate sample significantly more often, which was thought to be due to physician hesitation to thoroughly scrape the patient’s skin.27 In our study, patients were anecdotally observed to be less likely to gag on a self-administered throat swab and to have less visible tensing of the rectal sphincter on self-administered rectal swab, which could have contributed to improved results.
Potential changes to the patient instructions before clinical implementation. These results suggest that self-administered testing could be substituted for clinician-administered testing, potentially improving detection. But widespread implementation would require a few modifications to the testing instructions based on the trained providers’ observations. Although the evidence is anecdotal, providers noted that several patients had difficulty with the instructions. For example, the placard said to use only the swab with the “blue shaft,” but 5 patients got confused and used the white swab either in addition to or instead of the blue swab. (The additional white swab was intended for use in making wet preps, when women are tested.) Many more patients ultimately used the correct swab, but appeared confused by the presence of the additional swab in the kit.
The confusion in these situations might have been avoided if the instructions had told men to throw one swab away and to use only the “blue swab” or the swab with the “blue handle” to avoid potential confusion over the word “shaft.” Another helpful modification to the instructions would be to alert men to the presence of the preservative liquid in the tubes. Several patients laid the tube horizontally with the cap off and spilled much of the liquid. It was still possible to test these samples, and there were no discrepancies between patient and provider results involving them. But ensuring standardization of the test tube contents should still be a priority in editing the instructions.
Incorporating self-testing into clinical practice. While the results of this study suggest that self-testing could be used with a few modifications to the instructions, how best to incorporate self-testing into the clinical setting still needs to be addressed. It might be possible for patients to simply perform testing after a physical exam; for patients to come in when it is convenient for them and leave a sample in the lab; or even for patients to perform testing at home and bring the swabs back to their clinic at a later time.
We obtained the results in this study when patients performed testing in a clinic with a trained provider in the room. One concern in implementing widespread self-testing would be that a provider’s presence in our study might have made patients more likely to spend the time needed to read instructions thoroughly and to put effort into performing the test correctly. However, it is also possible that knowing a provider will be duplicating the testing could lead to decreased patient effort. It may be that having responsibility for one’s own test results is what ensures adequate performance.
Further studies could explore the impact of a provider in the room, but studies have already examined testing done in private and yielded similar results for concordance, which suggests that any impact of the provider’s presence was relatively minimal. A 2009-2010 study at a London clinic, for example, allowed patients to perform testing privately following instructions from a nurse and identified a 9.8% prevalence of rectal CT infection and a 4.2% prevalence of rectal GC infection. These results are similar to those seen in our study and therefore suggest that patients were appropriately identifying infections without observation.28
Another study published in 2012 demonstrated that GC/CT testing swabs sent through the mail without any accompanying transport medium yielded results equivalent to those for swabs shipped in the liquid medium. This approach would make testing at home significantly easier, as patients would not have to be concerned about appropriate storage of their samples before returning them to a clinic.29
Applicability to female patients. Implementation of self-administered testing should be considered for female patients. In fact, most prior studies on the feasibility of self-testing have focused on women, as detection of their chlamydia and gonorrhea infections is equally critical. CDC guidelines stress the importance of yearly chlamydia screening for all women <25 years and for women >25 years who are at high risk (ie, multiple partners); and yearly gonorrhea screening for women at high risk, given the risk of medical complications including pelvic inflammatory disease and infertility if infections are missed.5
Researchers have found that after women participate in self-testing, they report that they would get STI testing more regularly if they could perform it themselves.19,30 Additionally, in studies similar to ours, women performing testing for gonorrhea and chlamydia using self-collected vaginal swabs have achieved similar sensitivity and specificity results to provider-performed testing.12,15,31
Study limitations. This was a self- selected MSM sample seeking STI testing from an urban community health center. Thus, one might assume that the detection of pharyngeal and rectal gonorrhea and chlamydia infections was higher in this sample compared with the general MSM population. That seems unlikely, however, given the similarity (noted earlier) between our findings and data reported in previous studies.1-4 Additionally, the presence of a provider in the room may have influenced patient performance, as we discussed earlier.
It therefore appears that self-administered testing could have universal applicability. Once optimal ways to incorporate self-testing for both men and women are identified, providers should be able to comply with the CDC testing guidelines without an increase in time or staff needed, thereby leading to increased detection and treatment of STIs and benefits for both patients and providers.
CORRESPONDENCE
Michael W. Plankey, PhD, Georgetown University Medical Center, Department of Medicine, Division of Infectious Diseases, 2115 Wisconsin Avenue, NW, Suite 130, Washington, DC 20007; [email protected]
1. Baker J, Plankey M, Josayma Y, et al. The prevalence of rectal, urethral, and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis among asymptomatic men who have sex with men in a prospective cohort in Washington, D.C. AIDS Patient Care STDs. 2009;23:585-588.
2. Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41:67-74.
3. Mimiaga MJ, Helms DJ, Reisner SL, et al. Gonococcal, chlamydia, and syphilis infection positivity among MSM attending a large primary care clinic, Boston, 2003 to 2004. Sex Transm Dis. 2009;36:507-511.
4. Park J, Marcus JL, Pandori M, et al. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis. 2012;39:482-484.
5. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
6. St Lawrence JS, Montano DE, Kasprzyk D, et al. STD screening, testing, case reporting, and clinical and partner notification practices: a national survey of US physicians. Am J Public Health. 2002;92:1784-1788.
7. Mark H, Irwin K, Sternberg M, et al. Providers’ perceived barriers to sexually transmitted disease care in 2 large health maintenance organizations. Sex Transm Dis. 2008;35:184-189.
8. Xu F, Sternberg MR, Markowitz LE. Men who have sex with men in the United States: demographic and behavioral characteristics and prevalence of HIV and HSV-2 infection: results from National Health and Nutrition Examination Survey 2001-2006. Sex Transm Dis. 2010;37:399-405.
9. Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868-1873.
10. Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537-543.
11. Bradshaw CS, Pierce LI, Tabrizi SN, et al. Screening injecting drug users for sexually transmitted infections and blood borne viruses using street outreach and self collected sampling. Sex Transm Infect. 2005;81:53-58.
12. Fang J, Husman C, DeSilva L, et al. Evaluation of self-collected vaginal swab, first void urine, and endocervical swab specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in adolescent females. J Pediatr Adolesc Gynecol. 2008;21:355-360.
13. Gaydos CA. Nucleic acid amplification tests for gonorrhea and chlamydia: practice and applications. Infect Dis Clin North Am. 2005;19:367-386 ix.
14. Hobbs MM, van der Pol B, Totten P, et al. From the NIH: proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis. 2008;35:8-13.
15. Knox J, Tabrizi SN, Miller P, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis. 2002;29:647-654.
16. Lippman SA, Jones HE, Luppi CG, et al. Home-based self-sampling and self-testing for sexually transmitted infections: acceptable and feasible alternatives to provider-based screening in low-income women in Sao Paulo, Brazil. Sex Transm Dis. 2007;34:421-428.
17. Newman SB, Nelson MB, Gaydos CA, et al. Female prisoners’ p of collection methods for testing for Chlamydia trachomatis and Neisseria gonorrhoeae infection. Sex Transm Dis. 2003;30:306-309.
18. Richardson E, Sellors JW, Mackinnon S, et al. Prevalence of Chlamydia trachomatis infections and specimen collection preference among women, using self-collected vaginal swabs in community settings. Sex Transm Dis. 2003;30:880-885.
19. Wiesenfeld HC, Lowry DL, Heine RP, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis. 2001;28:321-325.
20. van der Helm JJ, Hoebe CJ, van Rooijen MS, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis. 2009;36:493-497.
21. Moncada J, Schachter J, Liska S, et al. Evaluation of self-collected glans and rectal swabs from men who have sex with men for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of nucleic acid amplification tests. J Clin Microbiol. 2009;47:1657-1662.
22. Alexander S, Ison C, Parry J, et al. Self-taken pharyngeal and rectal swabs are appropriate for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in asymptomatic men who have sex with men. Sex Transm Infect. 2008;84:488-492.
23. San Francisco City Clinic. Patient instructions for self-collected specimens: pharyngeal and rectal. Available at: http://www.sfcityclinic.org/providers. Accessed December 15 2011.
24. Lampinen TM, Latulippe L, van Niekerk D, et al. Illustrated instructions for self-collection of anorectal swab specimens and their adequacy for cytological examination. Sex Transm Dis. 2006;33:386-388.
25. Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis. 2008;35:637-642.
26. Ota KV, Tamari IE, Smieja M, et al. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima Combo 2 assay and culture. Sex Transm Infect. 2009;85:182-186.
27. Hernandez BY, McDuffie K, Goodman MT, et al. Comparison of physician- and self-collected genital specimens for detection of human papillomavirus in men. J Clin Microbiol. 2006;44:513-517.
28. Soni S, White JA. Self-screening for Neisseria gonorrhoeae and Chlamydia trachomatis in the human immunodeficiency virus clinic—high yields and high acceptability. Sex Transm Dis. 2011;38:1107-1109.
29. Gaydos CA, Farshy C, Barnes M, et al. Can mailed swab samples be dry-shipped for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by nucleic acid amplification tests? Diagn Microbiol Infect Dis. 2012;73:16-20.
30. Chernesky MA, Hook EW, 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005;32:729-733.
31. Polaneczky M, Quigley C, Pollock L, et al. Use of self-collected vaginal specimens for detection of Chlamydia trachomatis infection. Obstet Gynecol. 1998;91:375-378.
1. Baker J, Plankey M, Josayma Y, et al. The prevalence of rectal, urethral, and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis among asymptomatic men who have sex with men in a prospective cohort in Washington, D.C. AIDS Patient Care STDs. 2009;23:585-588.
2. Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41:67-74.
3. Mimiaga MJ, Helms DJ, Reisner SL, et al. Gonococcal, chlamydia, and syphilis infection positivity among MSM attending a large primary care clinic, Boston, 2003 to 2004. Sex Transm Dis. 2009;36:507-511.
4. Park J, Marcus JL, Pandori M, et al. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis. 2012;39:482-484.
5. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
6. St Lawrence JS, Montano DE, Kasprzyk D, et al. STD screening, testing, case reporting, and clinical and partner notification practices: a national survey of US physicians. Am J Public Health. 2002;92:1784-1788.
7. Mark H, Irwin K, Sternberg M, et al. Providers’ perceived barriers to sexually transmitted disease care in 2 large health maintenance organizations. Sex Transm Dis. 2008;35:184-189.
8. Xu F, Sternberg MR, Markowitz LE. Men who have sex with men in the United States: demographic and behavioral characteristics and prevalence of HIV and HSV-2 infection: results from National Health and Nutrition Examination Survey 2001-2006. Sex Transm Dis. 2010;37:399-405.
9. Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868-1873.
10. Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537-543.
11. Bradshaw CS, Pierce LI, Tabrizi SN, et al. Screening injecting drug users for sexually transmitted infections and blood borne viruses using street outreach and self collected sampling. Sex Transm Infect. 2005;81:53-58.
12. Fang J, Husman C, DeSilva L, et al. Evaluation of self-collected vaginal swab, first void urine, and endocervical swab specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in adolescent females. J Pediatr Adolesc Gynecol. 2008;21:355-360.
13. Gaydos CA. Nucleic acid amplification tests for gonorrhea and chlamydia: practice and applications. Infect Dis Clin North Am. 2005;19:367-386 ix.
14. Hobbs MM, van der Pol B, Totten P, et al. From the NIH: proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis. 2008;35:8-13.
15. Knox J, Tabrizi SN, Miller P, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis. 2002;29:647-654.
16. Lippman SA, Jones HE, Luppi CG, et al. Home-based self-sampling and self-testing for sexually transmitted infections: acceptable and feasible alternatives to provider-based screening in low-income women in Sao Paulo, Brazil. Sex Transm Dis. 2007;34:421-428.
17. Newman SB, Nelson MB, Gaydos CA, et al. Female prisoners’ p of collection methods for testing for Chlamydia trachomatis and Neisseria gonorrhoeae infection. Sex Transm Dis. 2003;30:306-309.
18. Richardson E, Sellors JW, Mackinnon S, et al. Prevalence of Chlamydia trachomatis infections and specimen collection preference among women, using self-collected vaginal swabs in community settings. Sex Transm Dis. 2003;30:880-885.
19. Wiesenfeld HC, Lowry DL, Heine RP, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis. 2001;28:321-325.
20. van der Helm JJ, Hoebe CJ, van Rooijen MS, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis. 2009;36:493-497.
21. Moncada J, Schachter J, Liska S, et al. Evaluation of self-collected glans and rectal swabs from men who have sex with men for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of nucleic acid amplification tests. J Clin Microbiol. 2009;47:1657-1662.
22. Alexander S, Ison C, Parry J, et al. Self-taken pharyngeal and rectal swabs are appropriate for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in asymptomatic men who have sex with men. Sex Transm Infect. 2008;84:488-492.
23. San Francisco City Clinic. Patient instructions for self-collected specimens: pharyngeal and rectal. Available at: http://www.sfcityclinic.org/providers. Accessed December 15 2011.
24. Lampinen TM, Latulippe L, van Niekerk D, et al. Illustrated instructions for self-collection of anorectal swab specimens and their adequacy for cytological examination. Sex Transm Dis. 2006;33:386-388.
25. Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis. 2008;35:637-642.
26. Ota KV, Tamari IE, Smieja M, et al. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima Combo 2 assay and culture. Sex Transm Infect. 2009;85:182-186.
27. Hernandez BY, McDuffie K, Goodman MT, et al. Comparison of physician- and self-collected genital specimens for detection of human papillomavirus in men. J Clin Microbiol. 2006;44:513-517.
28. Soni S, White JA. Self-screening for Neisseria gonorrhoeae and Chlamydia trachomatis in the human immunodeficiency virus clinic—high yields and high acceptability. Sex Transm Dis. 2011;38:1107-1109.
29. Gaydos CA, Farshy C, Barnes M, et al. Can mailed swab samples be dry-shipped for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by nucleic acid amplification tests? Diagn Microbiol Infect Dis. 2012;73:16-20.
30. Chernesky MA, Hook EW, 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005;32:729-733.
31. Polaneczky M, Quigley C, Pollock L, et al. Use of self-collected vaginal specimens for detection of Chlamydia trachomatis infection. Obstet Gynecol. 1998;91:375-378.
Top gynecologic surgeons gather for 2012 PAGS
Mark Walters, MD
![]()
Susan B. Levy, MD
![]()
![]()
Tomasso Falcone, MD
![]()
Amy Garcia, MD
More than 300 physicians attended the 15th annual Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium December 13–15, 2012, in Las Vegas. One likely reason was an abundance of offerings, including:
- a laparoscopist’s view of pelvic and abdominal anatomy
- case-based discussion of the evaluation of female pelvic floor disorders
- a surgical video fest with expert discussion and audience participation
- an in-depth look at fibroid management
- a focus on hysterectomy, from the vaginal approach to single-port laparoscopy and robotics
- a panel discussion of pelvic pain and its management
- tips on avoiding and managing laparoscopic and other complications
- a breakout session on endometriosis surgery
- the latest on evaluation and management of fetal incontinence.
Here are a few additional highlights of the 2012 program:
Surgery for stress incontinence: Which sling is for which patient?
When it comes to slings, one size does not fit all. That point was emphasized by Mark Walters, MD, in a comprehensive session that described the surgical techniques behind various bladder-neck and midurethral sling procedures, as well as the associated cure rates, complications, and pros and cons. To watch a 7-minute video in which Dr. Walters elaborates on patient-selection criteria, CLICK HERE .
Surgical approach to prolapse—what I do and why I do it
“That’s something you have to come to grips with in your own practice—what’s best in your hands?” he said.
While showing videos of actual surgeries, he described specific techniques, pearls, and pitfalls, and emphasized the importance of cystoscopy to rule out bladder injury.
Keynote address: The economics of surgical gynecology
She also described the current payment environment, explained why the current trend in health-care spending is unsustainable, and stressed the need to find areas in surgical gynecologic practice that may benefit from improvements in health-care delivery. CLICK HERE for Dr. Levy’s overview of the issues on video.
After Dr. Levy’s keynote address on the economics of surgical gynecology, OBG Management gathered the opinions of four participants: Gary Bostrom, MD, of California; Richard Robinson, MD, of Georgia; Timothy Hall, MD, of North Carolina; and Todd Slater, MD, of Ohio. To hear their points of view, CLICK HERE .
Myomectomy: Open to robotic approaches
“Myomectomy is not a dying art by any stretch,” said PAGS Co-Chair Tommaso Falcone, MD, in opening this session. “In fact, it’s expected to increase,” he added, as more women seek to preserve their uterus.
He then proceeded to describe management approaches (including watchful waiting), indications for myomectomy, and surgical options, including data on both perioperative and reproductive outcomes.
CLICK HERE for a video summary of Dr. Falcone’s talk.
Laparoscopic supracervical hysterectomy
As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common. Amy Garcia, MD, described the indications, technique, benefits, and risks associated with this procedure. CLICK HERE to hear Dr. Garcia highlight the key points of her talk.
Join me in Las Vegas for FUUS 2013!
“This is a unique meeting,” says Dr. Karram, “as it addresses both urologic and gynecologic issues related to female pelvic medicine and reconstructive surgery.” It’s also timely—with the first board exam for the subspecialty of female pelvic medicine and reconstructive surgery being held in June 2013. Prepare yourself to meet the demand for physicians who have the expertise to evaluate pelvic floor disorders.
“The meeting is attended by 50% gynecologists and 50% urologists, has many breakout sessions, and covers a variety of topics—everything from vaginal surgery for prolapse, voiding dysfunction, and types of reconstructive procedures with laparoscopic and robotic approaches,” says Dr. Karram, who is excited for this year’s special symposium by Karl J. Kreder, Jr, MD, on April 20 that addresses pelvic pain syndromes. For a complete agenda and registration details, visit www.fuus-cme.org.
Mark Walters, MD
![]()
Susan B. Levy, MD
![]()
![]()
Tomasso Falcone, MD
![]()
Amy Garcia, MD
More than 300 physicians attended the 15th annual Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium December 13–15, 2012, in Las Vegas. One likely reason was an abundance of offerings, including:
- a laparoscopist’s view of pelvic and abdominal anatomy
- case-based discussion of the evaluation of female pelvic floor disorders
- a surgical video fest with expert discussion and audience participation
- an in-depth look at fibroid management
- a focus on hysterectomy, from the vaginal approach to single-port laparoscopy and robotics
- a panel discussion of pelvic pain and its management
- tips on avoiding and managing laparoscopic and other complications
- a breakout session on endometriosis surgery
- the latest on evaluation and management of fetal incontinence.
Here are a few additional highlights of the 2012 program:
Surgery for stress incontinence: Which sling is for which patient?
When it comes to slings, one size does not fit all. That point was emphasized by Mark Walters, MD, in a comprehensive session that described the surgical techniques behind various bladder-neck and midurethral sling procedures, as well as the associated cure rates, complications, and pros and cons. To watch a 7-minute video in which Dr. Walters elaborates on patient-selection criteria, CLICK HERE .
Surgical approach to prolapse—what I do and why I do it
“That’s something you have to come to grips with in your own practice—what’s best in your hands?” he said.
While showing videos of actual surgeries, he described specific techniques, pearls, and pitfalls, and emphasized the importance of cystoscopy to rule out bladder injury.
Keynote address: The economics of surgical gynecology
She also described the current payment environment, explained why the current trend in health-care spending is unsustainable, and stressed the need to find areas in surgical gynecologic practice that may benefit from improvements in health-care delivery. CLICK HERE for Dr. Levy’s overview of the issues on video.
After Dr. Levy’s keynote address on the economics of surgical gynecology, OBG Management gathered the opinions of four participants: Gary Bostrom, MD, of California; Richard Robinson, MD, of Georgia; Timothy Hall, MD, of North Carolina; and Todd Slater, MD, of Ohio. To hear their points of view, CLICK HERE .
Myomectomy: Open to robotic approaches
“Myomectomy is not a dying art by any stretch,” said PAGS Co-Chair Tommaso Falcone, MD, in opening this session. “In fact, it’s expected to increase,” he added, as more women seek to preserve their uterus.
He then proceeded to describe management approaches (including watchful waiting), indications for myomectomy, and surgical options, including data on both perioperative and reproductive outcomes.
CLICK HERE for a video summary of Dr. Falcone’s talk.
Laparoscopic supracervical hysterectomy
As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common. Amy Garcia, MD, described the indications, technique, benefits, and risks associated with this procedure. CLICK HERE to hear Dr. Garcia highlight the key points of her talk.
Join me in Las Vegas for FUUS 2013!
“This is a unique meeting,” says Dr. Karram, “as it addresses both urologic and gynecologic issues related to female pelvic medicine and reconstructive surgery.” It’s also timely—with the first board exam for the subspecialty of female pelvic medicine and reconstructive surgery being held in June 2013. Prepare yourself to meet the demand for physicians who have the expertise to evaluate pelvic floor disorders.
“The meeting is attended by 50% gynecologists and 50% urologists, has many breakout sessions, and covers a variety of topics—everything from vaginal surgery for prolapse, voiding dysfunction, and types of reconstructive procedures with laparoscopic and robotic approaches,” says Dr. Karram, who is excited for this year’s special symposium by Karl J. Kreder, Jr, MD, on April 20 that addresses pelvic pain syndromes. For a complete agenda and registration details, visit www.fuus-cme.org.
Mark Walters, MD
![]()
Susan B. Levy, MD
![]()
![]()
Tomasso Falcone, MD
![]()
Amy Garcia, MD
More than 300 physicians attended the 15th annual Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium December 13–15, 2012, in Las Vegas. One likely reason was an abundance of offerings, including:
- a laparoscopist’s view of pelvic and abdominal anatomy
- case-based discussion of the evaluation of female pelvic floor disorders
- a surgical video fest with expert discussion and audience participation
- an in-depth look at fibroid management
- a focus on hysterectomy, from the vaginal approach to single-port laparoscopy and robotics
- a panel discussion of pelvic pain and its management
- tips on avoiding and managing laparoscopic and other complications
- a breakout session on endometriosis surgery
- the latest on evaluation and management of fetal incontinence.
Here are a few additional highlights of the 2012 program:
Surgery for stress incontinence: Which sling is for which patient?
When it comes to slings, one size does not fit all. That point was emphasized by Mark Walters, MD, in a comprehensive session that described the surgical techniques behind various bladder-neck and midurethral sling procedures, as well as the associated cure rates, complications, and pros and cons. To watch a 7-minute video in which Dr. Walters elaborates on patient-selection criteria, CLICK HERE .
Surgical approach to prolapse—what I do and why I do it
“That’s something you have to come to grips with in your own practice—what’s best in your hands?” he said.
While showing videos of actual surgeries, he described specific techniques, pearls, and pitfalls, and emphasized the importance of cystoscopy to rule out bladder injury.
Keynote address: The economics of surgical gynecology
She also described the current payment environment, explained why the current trend in health-care spending is unsustainable, and stressed the need to find areas in surgical gynecologic practice that may benefit from improvements in health-care delivery. CLICK HERE for Dr. Levy’s overview of the issues on video.
After Dr. Levy’s keynote address on the economics of surgical gynecology, OBG Management gathered the opinions of four participants: Gary Bostrom, MD, of California; Richard Robinson, MD, of Georgia; Timothy Hall, MD, of North Carolina; and Todd Slater, MD, of Ohio. To hear their points of view, CLICK HERE .
Myomectomy: Open to robotic approaches
“Myomectomy is not a dying art by any stretch,” said PAGS Co-Chair Tommaso Falcone, MD, in opening this session. “In fact, it’s expected to increase,” he added, as more women seek to preserve their uterus.
He then proceeded to describe management approaches (including watchful waiting), indications for myomectomy, and surgical options, including data on both perioperative and reproductive outcomes.
CLICK HERE for a video summary of Dr. Falcone’s talk.
Laparoscopic supracervical hysterectomy
As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common. Amy Garcia, MD, described the indications, technique, benefits, and risks associated with this procedure. CLICK HERE to hear Dr. Garcia highlight the key points of her talk.
Join me in Las Vegas for FUUS 2013!
“This is a unique meeting,” says Dr. Karram, “as it addresses both urologic and gynecologic issues related to female pelvic medicine and reconstructive surgery.” It’s also timely—with the first board exam for the subspecialty of female pelvic medicine and reconstructive surgery being held in June 2013. Prepare yourself to meet the demand for physicians who have the expertise to evaluate pelvic floor disorders.
“The meeting is attended by 50% gynecologists and 50% urologists, has many breakout sessions, and covers a variety of topics—everything from vaginal surgery for prolapse, voiding dysfunction, and types of reconstructive procedures with laparoscopic and robotic approaches,” says Dr. Karram, who is excited for this year’s special symposium by Karl J. Kreder, Jr, MD, on April 20 that addresses pelvic pain syndromes. For a complete agenda and registration details, visit www.fuus-cme.org.
Failure to diagnose preeclampsia … and more
A MOTHER CALLED HER OBGYN at 34 weeks’ gestation with complaints of a headache, swelling, and weight gain. The ObGyn prescribed Tylenol. The next morning, the mother was found unconscious on her kitchen floor. She was taken to the emergency department (ED), where she underwent a cesarean delivery and brain surgery. The child, born prematurely, suffered a stroke that resulted in brain damage and cerebral palsy (CP).
PARENTS’ CLAIM The ObGyn should have immediately evaluated the mother when she called with a headache. Failure to recognize eclampsia led to severe hypertension.
PHYSICIAN’S DEFENSE When the mother called the ObGyn, she reported a headache and diarrhea, and asked if it was all right to take Tylenol. The ObGyn claimed she asked the mother several questions and the mother’s answers included that the headache was not severe and that she’d had it for a few hours. The mother denied blurred vision, abdominal or uterine pain, and reported that she was not vomiting. The ObGyn believed that the mother had a virus and recommended Tylenol. The fetus’ stroke had occurred the day prior to the mother’s eclamptic episode.
VERDICT At first, a Pennsylvania defense verdict was returned. After an appeal, the second trial resulted in a $3.75 million verdict.
Spontaneous home birth goes badly awry
A WOMAN SPONTANEOUSLY DELIVERED her fourth baby at home. An ambulance transported the mother and child to the ED. Upon arrival, the child had depressed breathing. The pediatrician ordered a chest x-ray, which indicated a collapsed lung. A chest tube was inserted. The infant was monitored for the next 2 hours, when transfer to another hospital was arranged because her condition worsened. She sustained brain damage from the respiratory problems and died 2 days after birth.
PARENTS’ CLAIM The pediatrician failed to establish an airway and place a central line.
PHYSICIAN’S DEFENSE The newborn’s breathing difficulties were due to aspiration of meconium. The fetus suffered an in-utero hypoxic event due to a small placenta.
VERDICT A Kentucky defense verdict was returned.
NICU team not called early enough
AN INFANT’S HEART RATE was 100 bpm at birth. She was blue and not breathing, and suffered seizures in the first 24 hours of life. She was found to have brain damage, CP, and spastic quadriplegia. She requires a feeding tube and is unable to speak or walk.
PARENTS’ CLAIM The nurse should have called the NICU team before the baby’s birth because fetal distress was evident. The team arrived and began resuscitation 5 minutes after birth. The delay allowed for a lack of oxygen, which caused brain damage.
DEFENDANTS’ DEFENSE A placental infection caused the baby’s distress.
VERDICT A $8,583,000 Ohio verdict was returned against the hospital.
Woman not told cancer had spread to nodes
A 56-YEAR-OLD WOMAN underwent right breast mastectomy. The surgeon did not remove any lymph nodes despite radiologic evidence of possible nodal involvement. After the mastectomy, the surgeon advised the patient to see an oncologist.
The patient could not get an appointment with the oncologist for 6 months. During that visit, the oncologist told her that cancer had invaded lymph nodes that had not been removed. The cancer metastasized to a lung. Despite surgery, she was told that recurrence was inevitable.
PATIENT’S CLAIM Metastasis could have been avoided if the lymph nodes had been removed at mastectomy. The surgeon had not told her about lymph node involvement, which contributed to the delay in seeing the oncologist.
PHYSICIAN’S DEFENSE Removal of the lymph nodes was not necessary—immediate chemotherapy could have effectively addressed the cancer. The patient was told of the lymph node involvement and clearly advised that prompt chemotherapy was necessary.
VERDICT A $500,000 New York verdict was returned for past pain and suffering. Defense posttrial motions were denied. The judge granted the patient’s motion for future pain and suffering and awarded $500,000.
A WOMAN WENT TO THE HOSPITAL FOR THE BIRTH of her eighth child. She had received no prenatal care, although she had a history of preeclampsia. Upon arrival at the ED, she had decreased blood pressure. Two on-call ObGyns delivered the baby. Shoulder dystocia was encountered, and after several unsuccessful attempts were made to dislodge the shoulder, a rescue cesarean delivery was performed. The child has a brachial plexus injury.
PARENTS’ CLAIM The ObGyns failed to perform a cesarean delivery in a timely manner, and used excessive force in attempting to free the baby’s shoulder.
PHYSICIAN’S DEFENSE All appropriate measures were taken in an effort to facilitate a prompt and injury-free delivery.
VERDICT A $1,250,000 Ohio verdict was returned.
Failure to detect fetal growth restriction
A CHILD WAS DELIVERED BY AN OBGYN and a neonatologist. The child has CP with developmental delays and spastic quadriplegia. She requires constant care.
PARENTS’ CLAIM The child’s CP was caused by an hypoxic event that occurred 3 hours before delivery. The fetus was extremely small, which increased the susceptibility to hypoxic events. The ObGyn was negligent in failing to diagnose fetal growth restriction caused by placental insufficiency. The fetal monitor showed an abnormal heart rate during that 3-hour span. Fetal distress should have prompted action by the ObGyn; a cesarean delivery could have avoided the injury.
DEFENDANTS’ DEFENSE Prenatal tests, including ultrasonography, indicated that the fetus had grown appropriately. Fetal heart-rate monitors did not reveal problematic heart function. The child’s CP was due to chronic hypoxia that could not have been detected or prevented.
VERDICT A $6.5 million New York settlement was reached.
Emergency cesarean after fetal distress
AFTER A NORMAL PREGNANCY, an emergency cesarean delivery was performed when the fetal monitor indicated fetal distress. The child suffered hypoxic ischemic encephalopathy resulting in permanent neurologic deficits.
PARENTS’ CLAIM The nurse failed to timely alert the physician of decelerations shown on the fetal heart-rate monitor. A cesarean should have been performed earlier.
DEFENDANT’S DEFENSE The cesarean was performed when fetal distress was evident.
VERDICT A Massachusetts defense verdict was returned.
Pelvic abscess after hysterectomy
A WOMAN UNDERWENT a total vaginal hysterectomy without prophylactic antibiotics. Six days after discharge, she went to the ED with fever, chills, abdominal pain, and diarrhea. She was given antibiotics and admitted after a CT scan and physical examination suggested an infection. At discharge 6 days later, antibiotics were not prescribed because she had been afebrile for over 48 hours. She continued to have abdominal distention, and returned to the hospital the next day with an ultrasound taken elsewhere that revealed a 9-cm pelvic abscess. She underwent bilateral salpingo-oophorectomy and was discharged after 4 days, this time with antibiotics. She continued to have diarrhea, severe abdominal pain, and weight loss for a year.
PATIENT’S CLAIM Prophylactic antibiotics should have been prescribed prior to surgery, and continued when she left the hospital the first time.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A confidential Utah settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska ( www.verdictslaska.com ). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
A MOTHER CALLED HER OBGYN at 34 weeks’ gestation with complaints of a headache, swelling, and weight gain. The ObGyn prescribed Tylenol. The next morning, the mother was found unconscious on her kitchen floor. She was taken to the emergency department (ED), where she underwent a cesarean delivery and brain surgery. The child, born prematurely, suffered a stroke that resulted in brain damage and cerebral palsy (CP).
PARENTS’ CLAIM The ObGyn should have immediately evaluated the mother when she called with a headache. Failure to recognize eclampsia led to severe hypertension.
PHYSICIAN’S DEFENSE When the mother called the ObGyn, she reported a headache and diarrhea, and asked if it was all right to take Tylenol. The ObGyn claimed she asked the mother several questions and the mother’s answers included that the headache was not severe and that she’d had it for a few hours. The mother denied blurred vision, abdominal or uterine pain, and reported that she was not vomiting. The ObGyn believed that the mother had a virus and recommended Tylenol. The fetus’ stroke had occurred the day prior to the mother’s eclamptic episode.
VERDICT At first, a Pennsylvania defense verdict was returned. After an appeal, the second trial resulted in a $3.75 million verdict.
Spontaneous home birth goes badly awry
A WOMAN SPONTANEOUSLY DELIVERED her fourth baby at home. An ambulance transported the mother and child to the ED. Upon arrival, the child had depressed breathing. The pediatrician ordered a chest x-ray, which indicated a collapsed lung. A chest tube was inserted. The infant was monitored for the next 2 hours, when transfer to another hospital was arranged because her condition worsened. She sustained brain damage from the respiratory problems and died 2 days after birth.
PARENTS’ CLAIM The pediatrician failed to establish an airway and place a central line.
PHYSICIAN’S DEFENSE The newborn’s breathing difficulties were due to aspiration of meconium. The fetus suffered an in-utero hypoxic event due to a small placenta.
VERDICT A Kentucky defense verdict was returned.
NICU team not called early enough
AN INFANT’S HEART RATE was 100 bpm at birth. She was blue and not breathing, and suffered seizures in the first 24 hours of life. She was found to have brain damage, CP, and spastic quadriplegia. She requires a feeding tube and is unable to speak or walk.
PARENTS’ CLAIM The nurse should have called the NICU team before the baby’s birth because fetal distress was evident. The team arrived and began resuscitation 5 minutes after birth. The delay allowed for a lack of oxygen, which caused brain damage.
DEFENDANTS’ DEFENSE A placental infection caused the baby’s distress.
VERDICT A $8,583,000 Ohio verdict was returned against the hospital.
Woman not told cancer had spread to nodes
A 56-YEAR-OLD WOMAN underwent right breast mastectomy. The surgeon did not remove any lymph nodes despite radiologic evidence of possible nodal involvement. After the mastectomy, the surgeon advised the patient to see an oncologist.
The patient could not get an appointment with the oncologist for 6 months. During that visit, the oncologist told her that cancer had invaded lymph nodes that had not been removed. The cancer metastasized to a lung. Despite surgery, she was told that recurrence was inevitable.
PATIENT’S CLAIM Metastasis could have been avoided if the lymph nodes had been removed at mastectomy. The surgeon had not told her about lymph node involvement, which contributed to the delay in seeing the oncologist.
PHYSICIAN’S DEFENSE Removal of the lymph nodes was not necessary—immediate chemotherapy could have effectively addressed the cancer. The patient was told of the lymph node involvement and clearly advised that prompt chemotherapy was necessary.
VERDICT A $500,000 New York verdict was returned for past pain and suffering. Defense posttrial motions were denied. The judge granted the patient’s motion for future pain and suffering and awarded $500,000.
A WOMAN WENT TO THE HOSPITAL FOR THE BIRTH of her eighth child. She had received no prenatal care, although she had a history of preeclampsia. Upon arrival at the ED, she had decreased blood pressure. Two on-call ObGyns delivered the baby. Shoulder dystocia was encountered, and after several unsuccessful attempts were made to dislodge the shoulder, a rescue cesarean delivery was performed. The child has a brachial plexus injury.
PARENTS’ CLAIM The ObGyns failed to perform a cesarean delivery in a timely manner, and used excessive force in attempting to free the baby’s shoulder.
PHYSICIAN’S DEFENSE All appropriate measures were taken in an effort to facilitate a prompt and injury-free delivery.
VERDICT A $1,250,000 Ohio verdict was returned.
Failure to detect fetal growth restriction
A CHILD WAS DELIVERED BY AN OBGYN and a neonatologist. The child has CP with developmental delays and spastic quadriplegia. She requires constant care.
PARENTS’ CLAIM The child’s CP was caused by an hypoxic event that occurred 3 hours before delivery. The fetus was extremely small, which increased the susceptibility to hypoxic events. The ObGyn was negligent in failing to diagnose fetal growth restriction caused by placental insufficiency. The fetal monitor showed an abnormal heart rate during that 3-hour span. Fetal distress should have prompted action by the ObGyn; a cesarean delivery could have avoided the injury.
DEFENDANTS’ DEFENSE Prenatal tests, including ultrasonography, indicated that the fetus had grown appropriately. Fetal heart-rate monitors did not reveal problematic heart function. The child’s CP was due to chronic hypoxia that could not have been detected or prevented.
VERDICT A $6.5 million New York settlement was reached.
Emergency cesarean after fetal distress
AFTER A NORMAL PREGNANCY, an emergency cesarean delivery was performed when the fetal monitor indicated fetal distress. The child suffered hypoxic ischemic encephalopathy resulting in permanent neurologic deficits.
PARENTS’ CLAIM The nurse failed to timely alert the physician of decelerations shown on the fetal heart-rate monitor. A cesarean should have been performed earlier.
DEFENDANT’S DEFENSE The cesarean was performed when fetal distress was evident.
VERDICT A Massachusetts defense verdict was returned.
Pelvic abscess after hysterectomy
A WOMAN UNDERWENT a total vaginal hysterectomy without prophylactic antibiotics. Six days after discharge, she went to the ED with fever, chills, abdominal pain, and diarrhea. She was given antibiotics and admitted after a CT scan and physical examination suggested an infection. At discharge 6 days later, antibiotics were not prescribed because she had been afebrile for over 48 hours. She continued to have abdominal distention, and returned to the hospital the next day with an ultrasound taken elsewhere that revealed a 9-cm pelvic abscess. She underwent bilateral salpingo-oophorectomy and was discharged after 4 days, this time with antibiotics. She continued to have diarrhea, severe abdominal pain, and weight loss for a year.
PATIENT’S CLAIM Prophylactic antibiotics should have been prescribed prior to surgery, and continued when she left the hospital the first time.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A confidential Utah settlement was reached.
A MOTHER CALLED HER OBGYN at 34 weeks’ gestation with complaints of a headache, swelling, and weight gain. The ObGyn prescribed Tylenol. The next morning, the mother was found unconscious on her kitchen floor. She was taken to the emergency department (ED), where she underwent a cesarean delivery and brain surgery. The child, born prematurely, suffered a stroke that resulted in brain damage and cerebral palsy (CP).
PARENTS’ CLAIM The ObGyn should have immediately evaluated the mother when she called with a headache. Failure to recognize eclampsia led to severe hypertension.
PHYSICIAN’S DEFENSE When the mother called the ObGyn, she reported a headache and diarrhea, and asked if it was all right to take Tylenol. The ObGyn claimed she asked the mother several questions and the mother’s answers included that the headache was not severe and that she’d had it for a few hours. The mother denied blurred vision, abdominal or uterine pain, and reported that she was not vomiting. The ObGyn believed that the mother had a virus and recommended Tylenol. The fetus’ stroke had occurred the day prior to the mother’s eclamptic episode.
VERDICT At first, a Pennsylvania defense verdict was returned. After an appeal, the second trial resulted in a $3.75 million verdict.
Spontaneous home birth goes badly awry
A WOMAN SPONTANEOUSLY DELIVERED her fourth baby at home. An ambulance transported the mother and child to the ED. Upon arrival, the child had depressed breathing. The pediatrician ordered a chest x-ray, which indicated a collapsed lung. A chest tube was inserted. The infant was monitored for the next 2 hours, when transfer to another hospital was arranged because her condition worsened. She sustained brain damage from the respiratory problems and died 2 days after birth.
PARENTS’ CLAIM The pediatrician failed to establish an airway and place a central line.
PHYSICIAN’S DEFENSE The newborn’s breathing difficulties were due to aspiration of meconium. The fetus suffered an in-utero hypoxic event due to a small placenta.
VERDICT A Kentucky defense verdict was returned.
NICU team not called early enough
AN INFANT’S HEART RATE was 100 bpm at birth. She was blue and not breathing, and suffered seizures in the first 24 hours of life. She was found to have brain damage, CP, and spastic quadriplegia. She requires a feeding tube and is unable to speak or walk.
PARENTS’ CLAIM The nurse should have called the NICU team before the baby’s birth because fetal distress was evident. The team arrived and began resuscitation 5 minutes after birth. The delay allowed for a lack of oxygen, which caused brain damage.
DEFENDANTS’ DEFENSE A placental infection caused the baby’s distress.
VERDICT A $8,583,000 Ohio verdict was returned against the hospital.
Woman not told cancer had spread to nodes
A 56-YEAR-OLD WOMAN underwent right breast mastectomy. The surgeon did not remove any lymph nodes despite radiologic evidence of possible nodal involvement. After the mastectomy, the surgeon advised the patient to see an oncologist.
The patient could not get an appointment with the oncologist for 6 months. During that visit, the oncologist told her that cancer had invaded lymph nodes that had not been removed. The cancer metastasized to a lung. Despite surgery, she was told that recurrence was inevitable.
PATIENT’S CLAIM Metastasis could have been avoided if the lymph nodes had been removed at mastectomy. The surgeon had not told her about lymph node involvement, which contributed to the delay in seeing the oncologist.
PHYSICIAN’S DEFENSE Removal of the lymph nodes was not necessary—immediate chemotherapy could have effectively addressed the cancer. The patient was told of the lymph node involvement and clearly advised that prompt chemotherapy was necessary.
VERDICT A $500,000 New York verdict was returned for past pain and suffering. Defense posttrial motions were denied. The judge granted the patient’s motion for future pain and suffering and awarded $500,000.
A WOMAN WENT TO THE HOSPITAL FOR THE BIRTH of her eighth child. She had received no prenatal care, although she had a history of preeclampsia. Upon arrival at the ED, she had decreased blood pressure. Two on-call ObGyns delivered the baby. Shoulder dystocia was encountered, and after several unsuccessful attempts were made to dislodge the shoulder, a rescue cesarean delivery was performed. The child has a brachial plexus injury.
PARENTS’ CLAIM The ObGyns failed to perform a cesarean delivery in a timely manner, and used excessive force in attempting to free the baby’s shoulder.
PHYSICIAN’S DEFENSE All appropriate measures were taken in an effort to facilitate a prompt and injury-free delivery.
VERDICT A $1,250,000 Ohio verdict was returned.
Failure to detect fetal growth restriction
A CHILD WAS DELIVERED BY AN OBGYN and a neonatologist. The child has CP with developmental delays and spastic quadriplegia. She requires constant care.
PARENTS’ CLAIM The child’s CP was caused by an hypoxic event that occurred 3 hours before delivery. The fetus was extremely small, which increased the susceptibility to hypoxic events. The ObGyn was negligent in failing to diagnose fetal growth restriction caused by placental insufficiency. The fetal monitor showed an abnormal heart rate during that 3-hour span. Fetal distress should have prompted action by the ObGyn; a cesarean delivery could have avoided the injury.
DEFENDANTS’ DEFENSE Prenatal tests, including ultrasonography, indicated that the fetus had grown appropriately. Fetal heart-rate monitors did not reveal problematic heart function. The child’s CP was due to chronic hypoxia that could not have been detected or prevented.
VERDICT A $6.5 million New York settlement was reached.
Emergency cesarean after fetal distress
AFTER A NORMAL PREGNANCY, an emergency cesarean delivery was performed when the fetal monitor indicated fetal distress. The child suffered hypoxic ischemic encephalopathy resulting in permanent neurologic deficits.
PARENTS’ CLAIM The nurse failed to timely alert the physician of decelerations shown on the fetal heart-rate monitor. A cesarean should have been performed earlier.
DEFENDANT’S DEFENSE The cesarean was performed when fetal distress was evident.
VERDICT A Massachusetts defense verdict was returned.
Pelvic abscess after hysterectomy
A WOMAN UNDERWENT a total vaginal hysterectomy without prophylactic antibiotics. Six days after discharge, she went to the ED with fever, chills, abdominal pain, and diarrhea. She was given antibiotics and admitted after a CT scan and physical examination suggested an infection. At discharge 6 days later, antibiotics were not prescribed because she had been afebrile for over 48 hours. She continued to have abdominal distention, and returned to the hospital the next day with an ultrasound taken elsewhere that revealed a 9-cm pelvic abscess. She underwent bilateral salpingo-oophorectomy and was discharged after 4 days, this time with antibiotics. She continued to have diarrhea, severe abdominal pain, and weight loss for a year.
PATIENT’S CLAIM Prophylactic antibiotics should have been prescribed prior to surgery, and continued when she left the hospital the first time.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A confidential Utah settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska ( www.verdictslaska.com ). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska ( www.verdictslaska.com ). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
Delayed MI diagnosis ends in disability, huge ($126M) verdict … more
Delayed heart attack diagnosis ends in disability and a huge verdict
DESPITE FEELING ILL ON AWAKENING, a 50-year-old woman went to work, where she suffered crushing chest pain radiating down her left arm and up to her jaw. Her coworker (and husband at the time) recognized the symptoms of a heart attack and drove her to the emergency department (ED).
An electrocardiogram (EKG) performed more than 4 hours later was read as not indicating a heart attack. The patient was given pain medication and an antianxiety drug because she had a history of anxiety. She spent the night at the hospital, lying on a gurney in a hallway at times.
In the morning, her husband called his own cardiologist, whose office was across the street from the hospital. The cardiologist came to the ED and immediately arranged to have the patient transferred by ambulance to the intensive care unit at another hospital.
Upon arrival, the patient was immediately sent to the hospital’s cardiac catheterization lab, where a heart attack was diagnosed. She underwent immediate surgery, during which she suffered dissection of an artery. Because of damage to her heart, she couldn’t return to work.
PLAINTIFF’S CLAIM The patient lost 70% of her heart’s pumping capacity and would require a heart transplant eventually. A cardiologist should have evaluated the patient immediately upon her arrival at the first hospital; the EKG done at that hospital was misread. On the catheterization film taken before surgery at the second hospital, the front portion of the patient’s heart was motionless.
THE DEFENSE The dissection during surgery caused the patient’s injuries.
VERDICT $126.6 million New York verdict.
COMMENT I do some malpractice case review and have seen 2 cases just like this one. If it sounds like a horse (myocardial infarction), it is a horse until proven otherwise. I’ve heard of men in their 40s seeking urgent care, being diagnosed with dyspepsia, and dying within 2 days.
Inadequate INR monitoring implicated in woman's death
A 59-YEAR-OLD WOMAN was diagnosed with atrial fibrillation and heart failure by a cardiologist and put on warfarin, which the cardiologist discontinued after a few days. Warfarin was resumed when the patient underwent surgery to place a mechanical heart valve.
The patient’s international normalized ratio (INR) was tested daily while she was in the hospital, and warfarin was stopped several times. She was discharged with a prescription for 2 mg warfarin because her INR was 2.2, below the therapeutic range.
At a follow-up visit, the cardiologist checked the INR, which was 3.1. He saw the patient in the office again 8 days later, and 6 days after that a call was made to him, but no further blood tests were performed.
Eight days after the call, the patient was found unresponsive, with indications of gastrointestinal (GI) bleeding, and taken to the emergency department. Her INR level was at least 24.4, the highest the equipment could measure. In addition to GI bleeding, she had bleeding in her lungs. She died the next day.
PLAINTIFF’S CLAIM The defendants didn’t monitor INR properly; the doctor knew the importance of monitoring INR while the patient was taking warfarin.
THE DEFENSE The INR level was normal at the posthospital visit. That measurement, along with the monitoring done while the patient was hospitalized, was appropriate monitoring. The patient died of sepsis, not exsanguination.
VERDICT $386,648 net California verdict.
COMMENT This could have happened to any of us. If you monitor warfarin in your practice, make sure the follow-up system is water tight. Use a registry and double checking system. Be sure you know who is responsible during care transitions.
Delayed heart attack diagnosis ends in disability and a huge verdict
DESPITE FEELING ILL ON AWAKENING, a 50-year-old woman went to work, where she suffered crushing chest pain radiating down her left arm and up to her jaw. Her coworker (and husband at the time) recognized the symptoms of a heart attack and drove her to the emergency department (ED).
An electrocardiogram (EKG) performed more than 4 hours later was read as not indicating a heart attack. The patient was given pain medication and an antianxiety drug because she had a history of anxiety. She spent the night at the hospital, lying on a gurney in a hallway at times.
In the morning, her husband called his own cardiologist, whose office was across the street from the hospital. The cardiologist came to the ED and immediately arranged to have the patient transferred by ambulance to the intensive care unit at another hospital.
Upon arrival, the patient was immediately sent to the hospital’s cardiac catheterization lab, where a heart attack was diagnosed. She underwent immediate surgery, during which she suffered dissection of an artery. Because of damage to her heart, she couldn’t return to work.
PLAINTIFF’S CLAIM The patient lost 70% of her heart’s pumping capacity and would require a heart transplant eventually. A cardiologist should have evaluated the patient immediately upon her arrival at the first hospital; the EKG done at that hospital was misread. On the catheterization film taken before surgery at the second hospital, the front portion of the patient’s heart was motionless.
THE DEFENSE The dissection during surgery caused the patient’s injuries.
VERDICT $126.6 million New York verdict.
COMMENT I do some malpractice case review and have seen 2 cases just like this one. If it sounds like a horse (myocardial infarction), it is a horse until proven otherwise. I’ve heard of men in their 40s seeking urgent care, being diagnosed with dyspepsia, and dying within 2 days.
Inadequate INR monitoring implicated in woman's death
A 59-YEAR-OLD WOMAN was diagnosed with atrial fibrillation and heart failure by a cardiologist and put on warfarin, which the cardiologist discontinued after a few days. Warfarin was resumed when the patient underwent surgery to place a mechanical heart valve.
The patient’s international normalized ratio (INR) was tested daily while she was in the hospital, and warfarin was stopped several times. She was discharged with a prescription for 2 mg warfarin because her INR was 2.2, below the therapeutic range.
At a follow-up visit, the cardiologist checked the INR, which was 3.1. He saw the patient in the office again 8 days later, and 6 days after that a call was made to him, but no further blood tests were performed.
Eight days after the call, the patient was found unresponsive, with indications of gastrointestinal (GI) bleeding, and taken to the emergency department. Her INR level was at least 24.4, the highest the equipment could measure. In addition to GI bleeding, she had bleeding in her lungs. She died the next day.
PLAINTIFF’S CLAIM The defendants didn’t monitor INR properly; the doctor knew the importance of monitoring INR while the patient was taking warfarin.
THE DEFENSE The INR level was normal at the posthospital visit. That measurement, along with the monitoring done while the patient was hospitalized, was appropriate monitoring. The patient died of sepsis, not exsanguination.
VERDICT $386,648 net California verdict.
COMMENT This could have happened to any of us. If you monitor warfarin in your practice, make sure the follow-up system is water tight. Use a registry and double checking system. Be sure you know who is responsible during care transitions.
Delayed heart attack diagnosis ends in disability and a huge verdict
DESPITE FEELING ILL ON AWAKENING, a 50-year-old woman went to work, where she suffered crushing chest pain radiating down her left arm and up to her jaw. Her coworker (and husband at the time) recognized the symptoms of a heart attack and drove her to the emergency department (ED).
An electrocardiogram (EKG) performed more than 4 hours later was read as not indicating a heart attack. The patient was given pain medication and an antianxiety drug because she had a history of anxiety. She spent the night at the hospital, lying on a gurney in a hallway at times.
In the morning, her husband called his own cardiologist, whose office was across the street from the hospital. The cardiologist came to the ED and immediately arranged to have the patient transferred by ambulance to the intensive care unit at another hospital.
Upon arrival, the patient was immediately sent to the hospital’s cardiac catheterization lab, where a heart attack was diagnosed. She underwent immediate surgery, during which she suffered dissection of an artery. Because of damage to her heart, she couldn’t return to work.
PLAINTIFF’S CLAIM The patient lost 70% of her heart’s pumping capacity and would require a heart transplant eventually. A cardiologist should have evaluated the patient immediately upon her arrival at the first hospital; the EKG done at that hospital was misread. On the catheterization film taken before surgery at the second hospital, the front portion of the patient’s heart was motionless.
THE DEFENSE The dissection during surgery caused the patient’s injuries.
VERDICT $126.6 million New York verdict.
COMMENT I do some malpractice case review and have seen 2 cases just like this one. If it sounds like a horse (myocardial infarction), it is a horse until proven otherwise. I’ve heard of men in their 40s seeking urgent care, being diagnosed with dyspepsia, and dying within 2 days.
Inadequate INR monitoring implicated in woman's death
A 59-YEAR-OLD WOMAN was diagnosed with atrial fibrillation and heart failure by a cardiologist and put on warfarin, which the cardiologist discontinued after a few days. Warfarin was resumed when the patient underwent surgery to place a mechanical heart valve.
The patient’s international normalized ratio (INR) was tested daily while she was in the hospital, and warfarin was stopped several times. She was discharged with a prescription for 2 mg warfarin because her INR was 2.2, below the therapeutic range.
At a follow-up visit, the cardiologist checked the INR, which was 3.1. He saw the patient in the office again 8 days later, and 6 days after that a call was made to him, but no further blood tests were performed.
Eight days after the call, the patient was found unresponsive, with indications of gastrointestinal (GI) bleeding, and taken to the emergency department. Her INR level was at least 24.4, the highest the equipment could measure. In addition to GI bleeding, she had bleeding in her lungs. She died the next day.
PLAINTIFF’S CLAIM The defendants didn’t monitor INR properly; the doctor knew the importance of monitoring INR while the patient was taking warfarin.
THE DEFENSE The INR level was normal at the posthospital visit. That measurement, along with the monitoring done while the patient was hospitalized, was appropriate monitoring. The patient died of sepsis, not exsanguination.
VERDICT $386,648 net California verdict.
COMMENT This could have happened to any of us. If you monitor warfarin in your practice, make sure the follow-up system is water tight. Use a registry and double checking system. Be sure you know who is responsible during care transitions.
Do drug treatment POEMs report data in clinically useful ways?
Purpose To provide the best care, physicians must determine what published information is relevant, valid, and clinically useful. Patient-Oriented Evidence that Matters (POEMs) defines relevance as information that addresses clinical questions, measures clinical outcomes, and has the potential to change practice. The most useful clinical information is presented in terms of absolute risk reduction (ARR), number needed to treat (NNT), and number needed to harm (NNH). The purpose of this study was to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or NNH.
Methods We independently reviewed all drug treatment articles in 7 journals during a 6-month period for relevance, validity, and clinical usefulness. (Journals included Journal of the American Medical Association [JAMA], Archives of Internal Medicine [Arch Intern Med], British Medical Journal [BMJ], New England Journal of Medicine, Lancet, Obstetrics and Gynecology [Obstet Gynecol], and Pediatrics.) We assessed clinical usefulness by recording whether the articles reported ARR, NNT, or NNH.
Results Of the 995 articles we reviewed, only 2.4% met relevance criteria. Fewer than 1% of all drug therapy articles were POEMs with calculated ARR, NNT, or NNH. Arch Intern Med, JAMA, and BMJ published the most drug therapy POEMs: 33%, 20%, and 17%, respectively. JAMA, BMJ, and Obstet Gynecol were the only journals that published POEMs with clinically useful information.
Conclusions Most major journals that address primary care issues do not publish drug therapy POEMs; those that do rarely present information in a clinically useful manner. Editors should require authors to provide ARRs, NNTs, and NNHs to help clinicians provide the best medical care for their patients.
Medical professionals are inundated by new information, which some have described as an “information jungle.”1 Thousands of articles are published each year in hundreds of journals,2 adding to an ever-expanding knowledge base. One study suggests that the experienced primary care physician uses up to 2 million pieces of information each year to manage patients.3 To provide appropriate patient care, physicians must stay abreast of current medical knowledge.4 However, busy clinicians have little time to navigate the information jungle and sift through all of the data to determine what is relevant and clinically useful.
More than a decade has passed since Allen Shaughnessy and David Slawson developed the concept of Patient-Oriented Evidence that Matters (POEMs), “a summary of a valid piece of research that carries information that is important to patients and so to their doctors.”5 They developed a formula to classify research as a POEM: U=R×V/W, where U=usefulness of the information to doctors, R=relevance of the information to doctors, V=validity of the information, and W=work to access the information.1 The most useful information is both relevant and valid and takes little work to access. In 2002, the British Medical Journal (BMJ) proposed publishing one POEM a week based on the following criteria:
- It addresses a question that doctors encounter.
- It measures outcomes that doctors and their patients care about: symptoms, morbidity, quality of life, and mortality.
- It has the potential to change the way doctors practice.5
Even with the advent of POEMs, the true benefit of research and its application to clinical practice has yet to be determined. Physicians still have to decide which studies are valid, interpret the outcomes, and determine how they affect individual practice.
Research shows that clinicians, patients, and policy makers are more impressed by larger percentage differences than smaller ones.6 This fact is evident in the way trial results are presented in the news, by pharmaceutical representatives, and in journal articles. The relative risk reduction (RRR) is touted as suggesting either benefit or reduced harm, and the absolute numbers are largely under-reported. One study found that treatment effectiveness was perceived to be lower when the absolute risk reduction (ARR) rather than the RRR was reported.7 Perception of effectiveness decreased further when the number needed to treat (NNT) was presented.
The authors of the study concluded that ARR and NNT provide more concrete information than RRR about an intervention because they express efficacy “in a way which incorporates both the magnitude of the reduction of risk and the baseline risk without treatment.” They note that “because the exclusive reporting of relative risk may overstate the effectiveness of a treatment, actual event rates and absolute changes in risk should be reported.”7
These numbers are rarely found in journal articles and, when present, rarely appear in abstracts, tables, or graphs, where the busy clinician looks to find information quickly. Our study sought to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or number needed to harm (NNH), as demonstrated in TABLE 1.
TABLE 1
How to calculate RRR, ARR, and NNT
| Example: The rate of myocardial infarction in the control group is 4% and the rate in the treatment group is 2% | |
|---|---|
| RRR=event rate of control group - event rate of treatment group/event rate of control group | RRR=[4–2]/4=50% |
| ARR=event rate of control group - event rate of treatment group | ARR=4–2=2% |
| NNT*=100/ARR | NNT=100/2=50 |
| ARR, absolute risk reduction; NNT, number needed to treat; RRR, relative risk reduction. *This calculation is the same for the number needed to harm. | |
Methods
Pilot study
We first performed a pilot study that retrospectively reviewed all drug therapy articles published in the Journal of the American Medical Association (JAMA) from April 1, 2008, through April 1, 2009. Its purpose was to ensure concurrence in data gathering, rule out any measurement bias, and refine the analysis tool.
We applied an algorithmic approach to the review and used an Excel spreadsheet as a record-keeping tool, giving each article an abbreviated name and recording the issue, year, volume, and page numbers. We excluded case reports, review articles that were not systematic reviews or meta-analyses, letters, and editorials. We also excluded articles on cancer chemotherapy because, although family physicians need to have a working knowledge of antineoplastic drugs, they do not routinely prescribe them. Moreover, family physicians rarely write the first prescription for such a drug.
We reviewed the drug treatment articles to determine relevance—that is, whether they met POEMs criteria: addressed a question that most family medicine doctors encounter in a typical 6-month period, measured an outcome that family physicians and patients care about—such as morbidity, mortality, quality of life, or effect on clinical events—and had the potential to change clinical practice.8 Articles that met all 3 criteria were included in our analysis; articles that did not were recorded but excluded from further examination.
We analyzed articles that met relevance criteria for validity and clinical usefulness. We assessed validity based on whether the article was a randomized, controlled, double-blinded trial and whether allocation was concealed, follow-up was complete, information was analyzed on an intention-to-treat basis, and the results were statistically significant. We assessed clinical usefulness by recording whether the study reported RRR, ARR, NNT, or NNH, and if so, whether it recorded the information in free text, including the abstract, or in a graph or chart.
Review of articles in 7 journals
After the pilot study, we reviewed articles in JAMA and 6 other journals during a 6-month period from April 1, 2008 through September 30, 2008. We applied the same algorithmic analysis as in the pilot study to drug therapy articles in Archives of Internal Medicine (Arch Intern Med), BMJ, New England Journal of Medicine (N Engl J Med), Lancet, Obstetrics and Gynecology (Obstet Gynecol), and Pediatrics. We met regularly and settled disagreements about relevance, validity, or clinical usefulness by re-reviewing the article.
We recorded the total number of drug therapy articles for each journal, then tallied the number of POEMs and the total number of calculations of RRR, ARR, NNT, and NNH in articles that met POEMs criteria. We used these numbers to determine the percentage of POEMs and POEMs with clinically useful information for each journal.
Results
We identified a total of 995 articles in the 7 journals during the 6-month study period. Of these, 24 (2.4%) were classified as drug therapy POEMs and 6 (0.6%) were POEMs with clinically useful information.
The journals that published the most POEMs were Arch Intern Med, N Engl J Med, BMJ, and JAMA. Arch Intern Med had the highest percentage (33%), followed by JAMA (20%), and BMJ (17%) (TABLE 2).
When we analyzed the POEMs for clinical usefulness based on whether they provided calculated ARR, NNT, or NNH, only 3 journals published POEMs with clinically useful information: JAMA published the most (15%), followed by BMJ (9%) and Obstet Gynecol (4%) (FIGURE).
FIGURE
Percentage of POEMs with clinically useful information*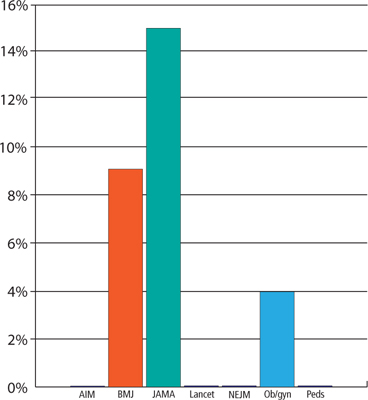
AIM, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; Ob/gyn, Obstetrics and Gynecology; Peds, Pediatrics; POEMs, Patient-Oriented Evidence that Matters.
*Clinically useful information is defined as information that includes the absolute risk reduction, number needed to treat, or number needed to harm.
TABLE 2
Few drug therapy articles are POEMs
| Journal | Articles published in 6 months, n | Drug therapy articles, n | Drug therapy articles that are POEMs, n (%) |
|---|---|---|---|
| Arch Intern Med | 123 | 24 | 8 (33) |
| BMJ | 127 | 23 | 4 (17) |
| JAMA | 85 | 20 | 4 (20) |
| Lancet | 92 | 33 | 1 (3) |
| N Engl J Med | 128 | 39 | 4 (10) |
| 0bstet Gynecol | 115 | 24 | 2 (8) |
| Pediatrics | 325 | 50 | 1 (2) |
| Arch Intern Med, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; N Engl J Med, New England Journal of Medicine; Obstet Gynecol, Obstetrics and Gynecology; POEMs, Patient-Oriented Evidence that Matters. | |||
Discussion
Our study findings are consistent with the relevance data from a previous study published in 1999.8 After more than a decade, medical journals still are not publishing drug therapy POEMs.
A disturbing scarcity of useful information. The paucity of drug therapy POEMs with clinically useful information is alarming. Based on our data we estimate that a physician would have to read on average 36 drug therapy articles to find one clinically helpful drug therapy POEM. This finding suggests that the medical literature is not helping clinicians provide the best patient care or, when it does, the busy clinician is forced to spend what little time is available in calculations to determine what can actually affect practice in positive ways.
Study limitations. Our study has a number of limitations. The study settings in the articles we reviewed ultimately determined what information was important and could potentially change clinical practice. Some studies, for example, were performed in developing countries, where the therapy being tested was not commonly used and would alter practice. In the United States, however, the same treatment would not affect clinical practice because it was either common practice or standard of care.
We reviewed only 7 major medical journals. Our results cannot necessarily be extrapolated to other major journals, although they do suggest that the findings are not limited to a few publications. Moreover, we reviewed only 2 specialty journals. It is possible that other such journals are publishing more POEMs than we observed and providing more concrete numbers that specialists can use to quickly and easily adjust their practice patterns than general journals.
We did not analyze any family medicine journals for the following reasons: American Family Physician publishes only review articles; The Journal of Family Practice does not routinely publish original research; and Annals of Family Medicine (the research journal of the discipline) is less well established than the other journals we selected for this review, having been launched in 2003.
In addition, 6 months may not have been long enough to accurately calculate the percentage of POEMs or clinically useful information in the journals we reviewed. During the pilot study, in which we analyzed a full year of JAMA, only one POEM was published in the second 6 months, and it did not contain clinically useful information. Moreover, we reviewed most journals over 6 consecutive months rather than 6 randomly chosen months.
Finally, we reviewed only drug therapy articles. Future studies could examine surgical, diagnostic, or prognostic studies.
Toward more, and more useful, POEMs
Despite the scarcity of POEMs that provide clinically useful information in major medical journals, it is important that physicians continue to practice evidence-based medicine, sifting through the available information and even calculating ARR, NNT, and NNH themselves, which most busy clinicians do not have the time or inclination to do.
How can we improve the clinical usefulness of published data? One way is for journal editors to require that authors provide ARRs and NNTs or NNHs. Another is for authors to include these calculations on their own initiative. Either way, the goal is better clinical practice and optimal patient care.
CORRESPONDENCE Adrienne Z. Ables, PharmD, Edward Via College of Osteopathic Medicine, Carolinas Campus, 350 Howard Street, Spartanburg, SC 29303; [email protected]
1. Shaughnessy AF, Slawson DC, Bennett JH. Becoming an information master: a guidebook to the medical information jungle. J Fam Pract. 1994;39:489-499.
2. Shaughnessy AF. Evaluating and understanding articles about treatment. Am Fam Physician. 2009;79:668-670.
3. Wyatt J. Uses and sources of medical knowledge. Lancet. 1991;338:1368-1372.
4. Gonzáles-Gonzáles AI, Dawes M, Sánchez-Mateos J, et al. Information needs and information-seeking behavior of primary care physicians. Ann Fam Med. 2007;5:345-352.
5. Smith R. A POEM a week for the BMJ. BMJ. 2002;325:983.-
6. Naylor CD, Chen E, Strauss B. Measured enthusiasm: Does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992;117:916-921.
7. Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ. 1994;309:761-764.
8. Ebell MH, Barry HC, Slawson DC, et al. Finding POEMs in the Medical Literature. J Fam Pract 1999;48:350-355.
Purpose To provide the best care, physicians must determine what published information is relevant, valid, and clinically useful. Patient-Oriented Evidence that Matters (POEMs) defines relevance as information that addresses clinical questions, measures clinical outcomes, and has the potential to change practice. The most useful clinical information is presented in terms of absolute risk reduction (ARR), number needed to treat (NNT), and number needed to harm (NNH). The purpose of this study was to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or NNH.
Methods We independently reviewed all drug treatment articles in 7 journals during a 6-month period for relevance, validity, and clinical usefulness. (Journals included Journal of the American Medical Association [JAMA], Archives of Internal Medicine [Arch Intern Med], British Medical Journal [BMJ], New England Journal of Medicine, Lancet, Obstetrics and Gynecology [Obstet Gynecol], and Pediatrics.) We assessed clinical usefulness by recording whether the articles reported ARR, NNT, or NNH.
Results Of the 995 articles we reviewed, only 2.4% met relevance criteria. Fewer than 1% of all drug therapy articles were POEMs with calculated ARR, NNT, or NNH. Arch Intern Med, JAMA, and BMJ published the most drug therapy POEMs: 33%, 20%, and 17%, respectively. JAMA, BMJ, and Obstet Gynecol were the only journals that published POEMs with clinically useful information.
Conclusions Most major journals that address primary care issues do not publish drug therapy POEMs; those that do rarely present information in a clinically useful manner. Editors should require authors to provide ARRs, NNTs, and NNHs to help clinicians provide the best medical care for their patients.
Medical professionals are inundated by new information, which some have described as an “information jungle.”1 Thousands of articles are published each year in hundreds of journals,2 adding to an ever-expanding knowledge base. One study suggests that the experienced primary care physician uses up to 2 million pieces of information each year to manage patients.3 To provide appropriate patient care, physicians must stay abreast of current medical knowledge.4 However, busy clinicians have little time to navigate the information jungle and sift through all of the data to determine what is relevant and clinically useful.
More than a decade has passed since Allen Shaughnessy and David Slawson developed the concept of Patient-Oriented Evidence that Matters (POEMs), “a summary of a valid piece of research that carries information that is important to patients and so to their doctors.”5 They developed a formula to classify research as a POEM: U=R×V/W, where U=usefulness of the information to doctors, R=relevance of the information to doctors, V=validity of the information, and W=work to access the information.1 The most useful information is both relevant and valid and takes little work to access. In 2002, the British Medical Journal (BMJ) proposed publishing one POEM a week based on the following criteria:
- It addresses a question that doctors encounter.
- It measures outcomes that doctors and their patients care about: symptoms, morbidity, quality of life, and mortality.
- It has the potential to change the way doctors practice.5
Even with the advent of POEMs, the true benefit of research and its application to clinical practice has yet to be determined. Physicians still have to decide which studies are valid, interpret the outcomes, and determine how they affect individual practice.
Research shows that clinicians, patients, and policy makers are more impressed by larger percentage differences than smaller ones.6 This fact is evident in the way trial results are presented in the news, by pharmaceutical representatives, and in journal articles. The relative risk reduction (RRR) is touted as suggesting either benefit or reduced harm, and the absolute numbers are largely under-reported. One study found that treatment effectiveness was perceived to be lower when the absolute risk reduction (ARR) rather than the RRR was reported.7 Perception of effectiveness decreased further when the number needed to treat (NNT) was presented.
The authors of the study concluded that ARR and NNT provide more concrete information than RRR about an intervention because they express efficacy “in a way which incorporates both the magnitude of the reduction of risk and the baseline risk without treatment.” They note that “because the exclusive reporting of relative risk may overstate the effectiveness of a treatment, actual event rates and absolute changes in risk should be reported.”7
These numbers are rarely found in journal articles and, when present, rarely appear in abstracts, tables, or graphs, where the busy clinician looks to find information quickly. Our study sought to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or number needed to harm (NNH), as demonstrated in TABLE 1.
TABLE 1
How to calculate RRR, ARR, and NNT
| Example: The rate of myocardial infarction in the control group is 4% and the rate in the treatment group is 2% | |
|---|---|
| RRR=event rate of control group - event rate of treatment group/event rate of control group | RRR=[4–2]/4=50% |
| ARR=event rate of control group - event rate of treatment group | ARR=4–2=2% |
| NNT*=100/ARR | NNT=100/2=50 |
| ARR, absolute risk reduction; NNT, number needed to treat; RRR, relative risk reduction. *This calculation is the same for the number needed to harm. | |
Methods
Pilot study
We first performed a pilot study that retrospectively reviewed all drug therapy articles published in the Journal of the American Medical Association (JAMA) from April 1, 2008, through April 1, 2009. Its purpose was to ensure concurrence in data gathering, rule out any measurement bias, and refine the analysis tool.
We applied an algorithmic approach to the review and used an Excel spreadsheet as a record-keeping tool, giving each article an abbreviated name and recording the issue, year, volume, and page numbers. We excluded case reports, review articles that were not systematic reviews or meta-analyses, letters, and editorials. We also excluded articles on cancer chemotherapy because, although family physicians need to have a working knowledge of antineoplastic drugs, they do not routinely prescribe them. Moreover, family physicians rarely write the first prescription for such a drug.
We reviewed the drug treatment articles to determine relevance—that is, whether they met POEMs criteria: addressed a question that most family medicine doctors encounter in a typical 6-month period, measured an outcome that family physicians and patients care about—such as morbidity, mortality, quality of life, or effect on clinical events—and had the potential to change clinical practice.8 Articles that met all 3 criteria were included in our analysis; articles that did not were recorded but excluded from further examination.
We analyzed articles that met relevance criteria for validity and clinical usefulness. We assessed validity based on whether the article was a randomized, controlled, double-blinded trial and whether allocation was concealed, follow-up was complete, information was analyzed on an intention-to-treat basis, and the results were statistically significant. We assessed clinical usefulness by recording whether the study reported RRR, ARR, NNT, or NNH, and if so, whether it recorded the information in free text, including the abstract, or in a graph or chart.
Review of articles in 7 journals
After the pilot study, we reviewed articles in JAMA and 6 other journals during a 6-month period from April 1, 2008 through September 30, 2008. We applied the same algorithmic analysis as in the pilot study to drug therapy articles in Archives of Internal Medicine (Arch Intern Med), BMJ, New England Journal of Medicine (N Engl J Med), Lancet, Obstetrics and Gynecology (Obstet Gynecol), and Pediatrics. We met regularly and settled disagreements about relevance, validity, or clinical usefulness by re-reviewing the article.
We recorded the total number of drug therapy articles for each journal, then tallied the number of POEMs and the total number of calculations of RRR, ARR, NNT, and NNH in articles that met POEMs criteria. We used these numbers to determine the percentage of POEMs and POEMs with clinically useful information for each journal.
Results
We identified a total of 995 articles in the 7 journals during the 6-month study period. Of these, 24 (2.4%) were classified as drug therapy POEMs and 6 (0.6%) were POEMs with clinically useful information.
The journals that published the most POEMs were Arch Intern Med, N Engl J Med, BMJ, and JAMA. Arch Intern Med had the highest percentage (33%), followed by JAMA (20%), and BMJ (17%) (TABLE 2).
When we analyzed the POEMs for clinical usefulness based on whether they provided calculated ARR, NNT, or NNH, only 3 journals published POEMs with clinically useful information: JAMA published the most (15%), followed by BMJ (9%) and Obstet Gynecol (4%) (FIGURE).
FIGURE
Percentage of POEMs with clinically useful information*
AIM, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; Ob/gyn, Obstetrics and Gynecology; Peds, Pediatrics; POEMs, Patient-Oriented Evidence that Matters.
*Clinically useful information is defined as information that includes the absolute risk reduction, number needed to treat, or number needed to harm.
TABLE 2
Few drug therapy articles are POEMs
| Journal | Articles published in 6 months, n | Drug therapy articles, n | Drug therapy articles that are POEMs, n (%) |
|---|---|---|---|
| Arch Intern Med | 123 | 24 | 8 (33) |
| BMJ | 127 | 23 | 4 (17) |
| JAMA | 85 | 20 | 4 (20) |
| Lancet | 92 | 33 | 1 (3) |
| N Engl J Med | 128 | 39 | 4 (10) |
| 0bstet Gynecol | 115 | 24 | 2 (8) |
| Pediatrics | 325 | 50 | 1 (2) |
| Arch Intern Med, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; N Engl J Med, New England Journal of Medicine; Obstet Gynecol, Obstetrics and Gynecology; POEMs, Patient-Oriented Evidence that Matters. | |||
Discussion
Our study findings are consistent with the relevance data from a previous study published in 1999.8 After more than a decade, medical journals still are not publishing drug therapy POEMs.
A disturbing scarcity of useful information. The paucity of drug therapy POEMs with clinically useful information is alarming. Based on our data we estimate that a physician would have to read on average 36 drug therapy articles to find one clinically helpful drug therapy POEM. This finding suggests that the medical literature is not helping clinicians provide the best patient care or, when it does, the busy clinician is forced to spend what little time is available in calculations to determine what can actually affect practice in positive ways.
Study limitations. Our study has a number of limitations. The study settings in the articles we reviewed ultimately determined what information was important and could potentially change clinical practice. Some studies, for example, were performed in developing countries, where the therapy being tested was not commonly used and would alter practice. In the United States, however, the same treatment would not affect clinical practice because it was either common practice or standard of care.
We reviewed only 7 major medical journals. Our results cannot necessarily be extrapolated to other major journals, although they do suggest that the findings are not limited to a few publications. Moreover, we reviewed only 2 specialty journals. It is possible that other such journals are publishing more POEMs than we observed and providing more concrete numbers that specialists can use to quickly and easily adjust their practice patterns than general journals.
We did not analyze any family medicine journals for the following reasons: American Family Physician publishes only review articles; The Journal of Family Practice does not routinely publish original research; and Annals of Family Medicine (the research journal of the discipline) is less well established than the other journals we selected for this review, having been launched in 2003.
In addition, 6 months may not have been long enough to accurately calculate the percentage of POEMs or clinically useful information in the journals we reviewed. During the pilot study, in which we analyzed a full year of JAMA, only one POEM was published in the second 6 months, and it did not contain clinically useful information. Moreover, we reviewed most journals over 6 consecutive months rather than 6 randomly chosen months.
Finally, we reviewed only drug therapy articles. Future studies could examine surgical, diagnostic, or prognostic studies.
Toward more, and more useful, POEMs
Despite the scarcity of POEMs that provide clinically useful information in major medical journals, it is important that physicians continue to practice evidence-based medicine, sifting through the available information and even calculating ARR, NNT, and NNH themselves, which most busy clinicians do not have the time or inclination to do.
How can we improve the clinical usefulness of published data? One way is for journal editors to require that authors provide ARRs and NNTs or NNHs. Another is for authors to include these calculations on their own initiative. Either way, the goal is better clinical practice and optimal patient care.
CORRESPONDENCE Adrienne Z. Ables, PharmD, Edward Via College of Osteopathic Medicine, Carolinas Campus, 350 Howard Street, Spartanburg, SC 29303; [email protected]
Purpose To provide the best care, physicians must determine what published information is relevant, valid, and clinically useful. Patient-Oriented Evidence that Matters (POEMs) defines relevance as information that addresses clinical questions, measures clinical outcomes, and has the potential to change practice. The most useful clinical information is presented in terms of absolute risk reduction (ARR), number needed to treat (NNT), and number needed to harm (NNH). The purpose of this study was to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or NNH.
Methods We independently reviewed all drug treatment articles in 7 journals during a 6-month period for relevance, validity, and clinical usefulness. (Journals included Journal of the American Medical Association [JAMA], Archives of Internal Medicine [Arch Intern Med], British Medical Journal [BMJ], New England Journal of Medicine, Lancet, Obstetrics and Gynecology [Obstet Gynecol], and Pediatrics.) We assessed clinical usefulness by recording whether the articles reported ARR, NNT, or NNH.
Results Of the 995 articles we reviewed, only 2.4% met relevance criteria. Fewer than 1% of all drug therapy articles were POEMs with calculated ARR, NNT, or NNH. Arch Intern Med, JAMA, and BMJ published the most drug therapy POEMs: 33%, 20%, and 17%, respectively. JAMA, BMJ, and Obstet Gynecol were the only journals that published POEMs with clinically useful information.
Conclusions Most major journals that address primary care issues do not publish drug therapy POEMs; those that do rarely present information in a clinically useful manner. Editors should require authors to provide ARRs, NNTs, and NNHs to help clinicians provide the best medical care for their patients.
Medical professionals are inundated by new information, which some have described as an “information jungle.”1 Thousands of articles are published each year in hundreds of journals,2 adding to an ever-expanding knowledge base. One study suggests that the experienced primary care physician uses up to 2 million pieces of information each year to manage patients.3 To provide appropriate patient care, physicians must stay abreast of current medical knowledge.4 However, busy clinicians have little time to navigate the information jungle and sift through all of the data to determine what is relevant and clinically useful.
More than a decade has passed since Allen Shaughnessy and David Slawson developed the concept of Patient-Oriented Evidence that Matters (POEMs), “a summary of a valid piece of research that carries information that is important to patients and so to their doctors.”5 They developed a formula to classify research as a POEM: U=R×V/W, where U=usefulness of the information to doctors, R=relevance of the information to doctors, V=validity of the information, and W=work to access the information.1 The most useful information is both relevant and valid and takes little work to access. In 2002, the British Medical Journal (BMJ) proposed publishing one POEM a week based on the following criteria:
- It addresses a question that doctors encounter.
- It measures outcomes that doctors and their patients care about: symptoms, morbidity, quality of life, and mortality.
- It has the potential to change the way doctors practice.5
Even with the advent of POEMs, the true benefit of research and its application to clinical practice has yet to be determined. Physicians still have to decide which studies are valid, interpret the outcomes, and determine how they affect individual practice.
Research shows that clinicians, patients, and policy makers are more impressed by larger percentage differences than smaller ones.6 This fact is evident in the way trial results are presented in the news, by pharmaceutical representatives, and in journal articles. The relative risk reduction (RRR) is touted as suggesting either benefit or reduced harm, and the absolute numbers are largely under-reported. One study found that treatment effectiveness was perceived to be lower when the absolute risk reduction (ARR) rather than the RRR was reported.7 Perception of effectiveness decreased further when the number needed to treat (NNT) was presented.
The authors of the study concluded that ARR and NNT provide more concrete information than RRR about an intervention because they express efficacy “in a way which incorporates both the magnitude of the reduction of risk and the baseline risk without treatment.” They note that “because the exclusive reporting of relative risk may overstate the effectiveness of a treatment, actual event rates and absolute changes in risk should be reported.”7
These numbers are rarely found in journal articles and, when present, rarely appear in abstracts, tables, or graphs, where the busy clinician looks to find information quickly. Our study sought to estimate the percentage of drug treatment articles published in major medical journals that provide a calculated ARR, NNT, or number needed to harm (NNH), as demonstrated in TABLE 1.
TABLE 1
How to calculate RRR, ARR, and NNT
| Example: The rate of myocardial infarction in the control group is 4% and the rate in the treatment group is 2% | |
|---|---|
| RRR=event rate of control group - event rate of treatment group/event rate of control group | RRR=[4–2]/4=50% |
| ARR=event rate of control group - event rate of treatment group | ARR=4–2=2% |
| NNT*=100/ARR | NNT=100/2=50 |
| ARR, absolute risk reduction; NNT, number needed to treat; RRR, relative risk reduction. *This calculation is the same for the number needed to harm. | |
Methods
Pilot study
We first performed a pilot study that retrospectively reviewed all drug therapy articles published in the Journal of the American Medical Association (JAMA) from April 1, 2008, through April 1, 2009. Its purpose was to ensure concurrence in data gathering, rule out any measurement bias, and refine the analysis tool.
We applied an algorithmic approach to the review and used an Excel spreadsheet as a record-keeping tool, giving each article an abbreviated name and recording the issue, year, volume, and page numbers. We excluded case reports, review articles that were not systematic reviews or meta-analyses, letters, and editorials. We also excluded articles on cancer chemotherapy because, although family physicians need to have a working knowledge of antineoplastic drugs, they do not routinely prescribe them. Moreover, family physicians rarely write the first prescription for such a drug.
We reviewed the drug treatment articles to determine relevance—that is, whether they met POEMs criteria: addressed a question that most family medicine doctors encounter in a typical 6-month period, measured an outcome that family physicians and patients care about—such as morbidity, mortality, quality of life, or effect on clinical events—and had the potential to change clinical practice.8 Articles that met all 3 criteria were included in our analysis; articles that did not were recorded but excluded from further examination.
We analyzed articles that met relevance criteria for validity and clinical usefulness. We assessed validity based on whether the article was a randomized, controlled, double-blinded trial and whether allocation was concealed, follow-up was complete, information was analyzed on an intention-to-treat basis, and the results were statistically significant. We assessed clinical usefulness by recording whether the study reported RRR, ARR, NNT, or NNH, and if so, whether it recorded the information in free text, including the abstract, or in a graph or chart.
Review of articles in 7 journals
After the pilot study, we reviewed articles in JAMA and 6 other journals during a 6-month period from April 1, 2008 through September 30, 2008. We applied the same algorithmic analysis as in the pilot study to drug therapy articles in Archives of Internal Medicine (Arch Intern Med), BMJ, New England Journal of Medicine (N Engl J Med), Lancet, Obstetrics and Gynecology (Obstet Gynecol), and Pediatrics. We met regularly and settled disagreements about relevance, validity, or clinical usefulness by re-reviewing the article.
We recorded the total number of drug therapy articles for each journal, then tallied the number of POEMs and the total number of calculations of RRR, ARR, NNT, and NNH in articles that met POEMs criteria. We used these numbers to determine the percentage of POEMs and POEMs with clinically useful information for each journal.
Results
We identified a total of 995 articles in the 7 journals during the 6-month study period. Of these, 24 (2.4%) were classified as drug therapy POEMs and 6 (0.6%) were POEMs with clinically useful information.
The journals that published the most POEMs were Arch Intern Med, N Engl J Med, BMJ, and JAMA. Arch Intern Med had the highest percentage (33%), followed by JAMA (20%), and BMJ (17%) (TABLE 2).
When we analyzed the POEMs for clinical usefulness based on whether they provided calculated ARR, NNT, or NNH, only 3 journals published POEMs with clinically useful information: JAMA published the most (15%), followed by BMJ (9%) and Obstet Gynecol (4%) (FIGURE).
FIGURE
Percentage of POEMs with clinically useful information*
AIM, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; Ob/gyn, Obstetrics and Gynecology; Peds, Pediatrics; POEMs, Patient-Oriented Evidence that Matters.
*Clinically useful information is defined as information that includes the absolute risk reduction, number needed to treat, or number needed to harm.
TABLE 2
Few drug therapy articles are POEMs
| Journal | Articles published in 6 months, n | Drug therapy articles, n | Drug therapy articles that are POEMs, n (%) |
|---|---|---|---|
| Arch Intern Med | 123 | 24 | 8 (33) |
| BMJ | 127 | 23 | 4 (17) |
| JAMA | 85 | 20 | 4 (20) |
| Lancet | 92 | 33 | 1 (3) |
| N Engl J Med | 128 | 39 | 4 (10) |
| 0bstet Gynecol | 115 | 24 | 2 (8) |
| Pediatrics | 325 | 50 | 1 (2) |
| Arch Intern Med, Archives of Internal Medicine; BMJ, British Medical Journal; JAMA, Journal of the American Medical Association; N Engl J Med, New England Journal of Medicine; Obstet Gynecol, Obstetrics and Gynecology; POEMs, Patient-Oriented Evidence that Matters. | |||
Discussion
Our study findings are consistent with the relevance data from a previous study published in 1999.8 After more than a decade, medical journals still are not publishing drug therapy POEMs.
A disturbing scarcity of useful information. The paucity of drug therapy POEMs with clinically useful information is alarming. Based on our data we estimate that a physician would have to read on average 36 drug therapy articles to find one clinically helpful drug therapy POEM. This finding suggests that the medical literature is not helping clinicians provide the best patient care or, when it does, the busy clinician is forced to spend what little time is available in calculations to determine what can actually affect practice in positive ways.
Study limitations. Our study has a number of limitations. The study settings in the articles we reviewed ultimately determined what information was important and could potentially change clinical practice. Some studies, for example, were performed in developing countries, where the therapy being tested was not commonly used and would alter practice. In the United States, however, the same treatment would not affect clinical practice because it was either common practice or standard of care.
We reviewed only 7 major medical journals. Our results cannot necessarily be extrapolated to other major journals, although they do suggest that the findings are not limited to a few publications. Moreover, we reviewed only 2 specialty journals. It is possible that other such journals are publishing more POEMs than we observed and providing more concrete numbers that specialists can use to quickly and easily adjust their practice patterns than general journals.
We did not analyze any family medicine journals for the following reasons: American Family Physician publishes only review articles; The Journal of Family Practice does not routinely publish original research; and Annals of Family Medicine (the research journal of the discipline) is less well established than the other journals we selected for this review, having been launched in 2003.
In addition, 6 months may not have been long enough to accurately calculate the percentage of POEMs or clinically useful information in the journals we reviewed. During the pilot study, in which we analyzed a full year of JAMA, only one POEM was published in the second 6 months, and it did not contain clinically useful information. Moreover, we reviewed most journals over 6 consecutive months rather than 6 randomly chosen months.
Finally, we reviewed only drug therapy articles. Future studies could examine surgical, diagnostic, or prognostic studies.
Toward more, and more useful, POEMs
Despite the scarcity of POEMs that provide clinically useful information in major medical journals, it is important that physicians continue to practice evidence-based medicine, sifting through the available information and even calculating ARR, NNT, and NNH themselves, which most busy clinicians do not have the time or inclination to do.
How can we improve the clinical usefulness of published data? One way is for journal editors to require that authors provide ARRs and NNTs or NNHs. Another is for authors to include these calculations on their own initiative. Either way, the goal is better clinical practice and optimal patient care.
CORRESPONDENCE Adrienne Z. Ables, PharmD, Edward Via College of Osteopathic Medicine, Carolinas Campus, 350 Howard Street, Spartanburg, SC 29303; [email protected]
1. Shaughnessy AF, Slawson DC, Bennett JH. Becoming an information master: a guidebook to the medical information jungle. J Fam Pract. 1994;39:489-499.
2. Shaughnessy AF. Evaluating and understanding articles about treatment. Am Fam Physician. 2009;79:668-670.
3. Wyatt J. Uses and sources of medical knowledge. Lancet. 1991;338:1368-1372.
4. Gonzáles-Gonzáles AI, Dawes M, Sánchez-Mateos J, et al. Information needs and information-seeking behavior of primary care physicians. Ann Fam Med. 2007;5:345-352.
5. Smith R. A POEM a week for the BMJ. BMJ. 2002;325:983.-
6. Naylor CD, Chen E, Strauss B. Measured enthusiasm: Does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992;117:916-921.
7. Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ. 1994;309:761-764.
8. Ebell MH, Barry HC, Slawson DC, et al. Finding POEMs in the Medical Literature. J Fam Pract 1999;48:350-355.
1. Shaughnessy AF, Slawson DC, Bennett JH. Becoming an information master: a guidebook to the medical information jungle. J Fam Pract. 1994;39:489-499.
2. Shaughnessy AF. Evaluating and understanding articles about treatment. Am Fam Physician. 2009;79:668-670.
3. Wyatt J. Uses and sources of medical knowledge. Lancet. 1991;338:1368-1372.
4. Gonzáles-Gonzáles AI, Dawes M, Sánchez-Mateos J, et al. Information needs and information-seeking behavior of primary care physicians. Ann Fam Med. 2007;5:345-352.
5. Smith R. A POEM a week for the BMJ. BMJ. 2002;325:983.-
6. Naylor CD, Chen E, Strauss B. Measured enthusiasm: Does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992;117:916-921.
7. Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ. 1994;309:761-764.
8. Ebell MH, Barry HC, Slawson DC, et al. Finding POEMs in the Medical Literature. J Fam Pract 1999;48:350-355.
Patient abusing alcohol or drugs? Help starts with a single question
• Screen patients for substance use disorders, with a single (validated) question for alcohol and another for drugs. A
• Follow a positive screen for alcohol with an assessment to distinguish between hazardous drinking and drinking that is indicative of alcohol dependence. C
• Approach a substance use disorder as you would any chronic medical condition, seeking to engage the patient to encourage behavior change. Motivational interviewing is a useful tool. C
• Consider pharmacotherapeutic options for patients with alcohol or drug dependence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Episodic heavy drinking, like alcoholism and drug addiction, is increasingly recognized as a medical problem that primary care physicians can, and should, address.1 But it is rarely the chief reason for an office visit. Nor is it a subject patients are likely to bring up.
However, patients are generally willing to talk to a trusted doctor who asks about their use (or misuse) of alcohol or other substances. And primary care physicians can do much to help—with brief interventions, a growing armamentarium of pharmacotherapy, and referrals as needed. In the pages that follow, you’ll find easy-to-use screening tools and effective intervention strategies.
SCREENING NEEDN'T BE TIME-CONSUMING
Screening for substance use isn’t difficult. In fact, it can usually be accomplished with 2 targeted questions—one for alcohol use and one for drugs.
Alcohol. Two single-question screens to detect hazardous drinking have been validated, despite having different parameters. Ask either:
Q: When was the last time you had more than ____ drinks (4 for women and 5 for men) in one day?
Q: How many times in the past year have you had ___ or more drinks (4 for women and 5 for men) in one day?
For the first question, any answer within the past 3 months is a positive screen for hazardous drinking.2 For the second, anything other than zero is positive.3,4
Initial screening can also be done with the AUDIT-C (TABLE), a validated short (3-question) version of the Alcohol Use Disorders Identification Test that can be self-administered.5,6
Drugs. Only one single-question screen for drug use has been validated:
Q: How many times in the past year have you used an illegal drug or taken a prescription medication for nonmedical reasons?
Any answer other than never is a positive screen for hazardous drug use.7
CASE Jason F, a healthy and fit 28-year-old, has been your patient, along with his family, for years. He’s in your office because of a knee injury he incurred while running, and you take a moment to ask him, for the first time, how much he drinks and whether he takes drugs. His answer—that he drinks 3 or 4 times a week and often has multiple drinks at parties or nights out with the guys—takes you a bit by surprise.
Now what?
TABLE
3-question AUDIT-C screen for alcohol dependence5,6
| 1. How often do you have a drink containing alcohol? | ||
| 0 Never | 3 2 to 3 times a week | |
| 1 Monthly or less | 4 4 or more times a week | |
| 2 2 to 4 times a month | ||
| 2. How many drinks containing alcohol do you have on a typical day when you are drinking? | ||
| 0 I don’t drink. | 2 5 or 6 | |
| 0 1 or 2 | 3 7 to 9 | |
| 1 3 or 4 | 4 10 or more | |
| 3. How often do you have 6 or more drinks on one occasion? | ||
| 0 Never | 3 Weekly | |
| 1 Less than monthly | 4 Daily or almost daily | |
| 2 Monthly | ||
| Scoring the AUDIT-C | ||
| Alcohol dependence | ||
| Men | Women | |
| Threshold score | 5 | 4 |
| Sensitivity (%) | 80 | 76 |
| Specificity (%) | 74 | 78 |
| AROC | 0.769 | 0.767 |
| AROC, area under the receiver operating characteristic curve; AUDIT, Alcohol Use Disorders Identification Test. | ||
TELL ME MORE ABOUT IT
It is important to respond to a positive screen by requesting more information. In the conversation that ensues, the patient may provide the details you need to determine whether the drinking or drug use is indicative of a diagnosable substance use disorder, an umbrella term for alcohol or drug abuse and alcohol or drug dependence.
Alcohol or drug abuse vs dependence. Criteria for alcohol or drug abuse in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision (DSM-IV-TR) include risky behavior, such as drinking and driving; problems with work and/or close relationships; or run-ins with the law, such as an arrest for driving while intoxicated. Criteria for alcohol or drug dependence include the inability to cut down or stop using the substance; evidence of tolerance and withdrawal; spending more time ingesting the substance; increasing attention to substance use while interest in other activities diminishes; and continued use despite recurrent problems.8
Tools zero in on extent of problem
To learn more about your patient’s situation, consider using the following criteria and tools:
DSM criteria. Ask the following 2 questions, which are among DSM-IV-TR criteria for alcohol dependence:
- How many times in the last year have you had a lot more to drink than you intended?
- How many times in the last year have you been drinking in situations where it could have been hazardous, where you could have caused an accident or gotten hurt?
Any answer other than zero to either question is suggestive of a substance use disorder. In exploratory analyses, this approach had positive likelihood ratios of 4.7 to 16 and negative likelihood ratios of 0.05 to 0.30.9,10
Although the above questions refer to alcohol use, they could be revised to learn more about a patient’s use of marijuana or other drugs, as well. (There are few tools for the assessment of drug use, because any illegal or nonmedical use of controlled substances has clear risks of major harm.)
CAGE. Another tool that is effective in assessing alcohol use is the 4-question CAGE—an acronym for Cut down, Annoyed, Guilty, and Eye opener:
- Have you ever felt that you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt bad or guilty about your drinking?
- Have you ever had a drink in the morning to get rid of a hangover?
One meta-analysis found that a positive CAGE test—ie, a positive response to one or more of the questions—had a sensitivity of 0.85 and a specificity of 0.78 in identifying alcohol dependence in a primary care setting (using DSM criteria as the gold standard).11
AUDIT. This 10-item tool, a longer version of the AUDIT-C (available at http://www.medstudentlearning.com/node/6556), can also be used to determine the extent of alcohol use. This test provides detailed information about the quantity and frequency of alcohol use; however, it does not clearly distinguish between hazardous drinking and alcohol use disorders.12
If the patient is a teen
Assessment methods can be adjusted without difficulty to fit the age of the patient. The National Institute on Alcohol Abuse and Alcoholism has published the Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide, available at http://www.niaaa.nih.gov/Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. The 6-question CRAFFT (for Car, Relax, Alone, Forget, Friends, Trouble) is a validated tool designed to assess adolescents’ use of both alcohol and drugs (http://www.ceasar-boston.org/clinicians/crafft.php).13,14
CASE You give Mr. F the CAGE test, and he answers No to all 4 questions. You conclude that while his drinking may be hazardous, he does not appear to have alcohol abuse or dependence.
FOLLOW UP WITH A BRIEF INTERVENTION
For decades, evidence has shown that brief interventions are often effective in helping hazardous drinkers like Mr. F cut back to safer levels.15-18 In some cases, the impact has been great enough to reduce health care and societal costs for up to 4 years19 and to cut the risk of alcohol-related death by about half.20 As a result, the US Preventive Services Task Force has given a B rating to counseling to reduce alcohol misuse by primary care providers.21 (There is less evidence that brief interventions are effective for drug problems,22 or in settings other than primary care.23)
Treat drug/alcohol problems
If you determine that your patient is engaging in hazardous alcohol or drug use or has a diagnosable substance use disorder, you do not have to drop everything else or treat it as an acute event. What matters is long-term success, which is best achieved by partnering with the patient.
Start by approaching drug and alcohol problems as you would a case of newly elevated blood pressure. Bring up the problem, seeking to engage the patient in addres- sing it.
If he or she does not agree to quit or cut back on drinking the first time you broach the subject, don’t be surprised or discouraged. Keep in mind that patients do not always respond positively to advice about handling chronic medical conditions either, particularly at first, and that you’ll be working together over time. What’s important, in the jargon of the Stages of Change model,24 is to help the patient move from precontemplation to contemplation, and perhaps beyond that to planning or action.
Use motivational interviewing to partner with patients
Motivational interviewing is useful in helping patients change health-related behaviors. The technique, which is not hard to learn or apply, is based on the recognition that a simple shift in style toward a guiding (rather than directive) approach can often reap benefits that are immediately apparent. 25-28
Motivational Interviewing: Helping People Change (3rd ed, by William R. Miller and Stephen Rollnick; Guilford Press, 2013) is an excellent resource for clinicians who wish to master this technique. An online tutorial in screening and brief intervention for alcohol or drug misuse is available free at https://adept.missouri.edu. Video demonstrations of motivational interviewing to address these issues are also available here ; to access them, click on “Training”, then on “Go to SBIRT videos”).
CASE Before Mr. F’s visit is concluded, you initiate a conversation about alcohol use, stating: “As your doctor, I’m concerned that the amount of alcohol you’re drinking could be hazardous to your health. I recommend that you cut down to no more than 4 drinks in any one day and to no more than 14 drinks a week.” You make it clear that change is up to him, and ask what he thinks about what you’ve said.
You also schedule a return visit in one month, at which time you will continue the conversation.
PHARMACOTHERAPY IS A USEFUL TOOL
Increasingly, alcohol and drug dependence—like other chronic conditions—can be effectively addressed with medication.
Drugs to treat alcohol dependence
Naltrexone. A daily dose of naltrexone, starting at 25 mg daily for a few days and going as high as 100 mg/d, can help patients with alcohol dependence limit their drinking to safe levels (number needed to treat [NNT]=9).29 This will reduce the risk of alcohol-related harm while the patient considers quitting.
The most common adverse effect is nausea, but a low starting dose may alleviate it. Naltrexone, also available as a 380-mg intramuscular (IM) depot injection once every 4 weeks, is an opioid antagonist and should not be given to any patient who’s taking opioids.
A 2010 Cochrane review found only 4 trials of naltrexone IM, and failed to show significant reductions in drinking.29 But post hoc analyses of trials of both oral and IM naltrexone found that those in which compliance was assured (either by direct observation or IM administration) had better outcomes than those in which it was not.30 Another post hoc analysis found that patients whose alcohol dependence was more severe derived greater benefits from the drug than those who were less severely affected.31
Acamprosate. Two 333-mg pills tid can help newly abstinent drinkers remain alcohol-free (NNT=9).32,33 The most common adverse effect is diarrhea, which may subside with continued use.
Combining acamprosate and naltrexone does not appear to be more effective than either drug alone. In a recently published meta-analysis comparing the 2 drugs, those taking acamprosate had slightly better rates of abstinence from alcohol, while naltrexone was slightly better in reducing heavy drinking.34
Disulfiram Unlike naltrexone and acamprosate, which work by altering the brain’s reward circuits, disulfiram blocks metabolism of ethanol, leading to the accumulation of a toxic metabolite and its punishing syndrome. The major problem with the drug is noncompliance, which can be addressed by enlisting the help of a caregiver or partner to ensure that it is taken daily. 35-37
Other medications that have been tested (though not approved by the US Food and Drug Administration [FDA]) as a treatment for alcohol dependence include:
- topiramate, which has been found to have modest efficacy in increasing the number of abstinent days and decreasing heavy-drinking days;38
- baclofen, which has shown efficacy in small clinical trials;39 and
- ondansetron, which has been shown to effectively treat early-onset alcohol dependence.40,41
For patients with depression and alcohol dependence, the combination of naltrexone and sertraline has been found to be superior to either drug by itself—and to have fewer adverse effects. 42 Gabapentin and lorazepam have been compared in treating alcohol withdrawal, with gabapentin resulting in greater efficacy and fewer adverse effects than lorazepam.43,44
Pharmacotherapy for drug abuse, dependence
For methamphetamine abuse and dependence. Two randomized clinical trials have studied medications for methamphetamine abuse and dependence. In one small study, topiramate did not increase the proportion of patients who achieved abstinence, but in a post hoc subgroup analysis, it did appear to help newly abstinent patients avoid relapse.45
In another study, mirtazapine significantly decreased the proportion of patients whose weekly urine tests were positive for methamphetamine (from 73% to 44%); no significant change was found among those on placebo.46
Both drugs were well tolerated, but compliance was low in both trials despite weekly counseling. Each has only one clinical trial to support its use, and neither has FDA approval for addiction treatment.
For marijuana dependence. In a study of 50 people seeking treatment for marijuana dependence, gabapentin 400 mg 3 times a day significantly improved the proportion reporting no cannabis use and whose urine tested negative for the drug.47
Another recent trial randomized adolescents dependent on cannabis to placebo or N-acetylcysteine 1200 mg twice a day. Those on the active drug were 2.4 times more likely than those on placebo to have negative urine tests with a number needed to treat of 7.48 Both trials ran for about 3 months. Neither drug is FDA approved to treat marijuana dependence.
For opioid dependence. As maintenance medication for patients dependent on opioids, both methadone and buprenorphine have been shown to reduce the use of illicit opioids, lower mortality, and improve retention compared with treatment without medication.49 Methadone would be a better choice than buprenorphine, which is a partial agonist with a ceiling on both its good (eg, stopping craving) and bad (eg, overdose risk) effects. In an open-label observational study of patients’ preferences, those who chose methadone maintenance over buprenorphine were twice as likely to remain in treatment.50 Both drugs are FDA-approved for treating opioid dependence.
Methadone is a full agonist that can be given for opioid dependence only in a federally licensed methadone maintenance clinic. It has been shown to reduce the use of other opioids, reduce criminal behaviors,51 improve function in many areas,52 and reduce mortality.53 In a cohort study of Massachusetts Medicaid data, methadone reduced mortality, which was 75% higher among those receiving abstinence-based treatment.52,54
Buprenorphine (alone and in combination with naloxone), effectively reduces the use of illicit opioids and improves functional status.55-57
Naltrexone, an opioid antagonist, may be effective in the treatment of opioid dependence. As with disulfiram for alcohol dependence, a major limitation of naltrexone for opioid dependence is noncompliance. But once a patient has been on oral naltrexone, he or she can be switched to naltrexone IM, which can be administered every 4 weeks. A Cochrane review published in 2011 found no evidence that naltrexone was superior to placebo, 58 but since then another randomized clinical trial has been published that found naltrexone 380 mg IM every 4 weeks was superior to IM placebo. Patients on naltrexone were more likely to remain in treatment and have opioid-free urine tests, and reported less craving for opioids.59 Both oral and depot injection naltrexone are FDA approved for treatment of opioid dependence. No comparisons of naltrexone vs either methadone or buprenorphine have been published.
To learn more about pharmacotherapy for opioid dependence, see “Diagnosing and treating opioid dependence” (J Fam Pract. 2012;61:588-596).
DEALING WITH THE CHALLENGES
As noted earlier, some patients with substance use disorders, like some patients with depression or hypertension, respond well to care and counseling, and some do not. Just as with other conditions, consultation with a specialist often helps.
A major difference in arranging consultations for patients with substance use disorders, however, is that clinicians who specialize in substance abuse and dependence often work in health care systems that are largely, or entirely, separate from those in which primary care physicians typically work. This, plus the stigma that surrounds problems with substance use, presents barriers to patients, who may shy away from going across town or to another city to see a provider they don’t know for a problem they’re either resistant to “owning” or ashamed of.
Yet it is possible to reach across this divide and make it easier for patients. One way to do that might be to partner with a local alcohol- and drug-treatment program so that your patients are referred, not to a faceless agency, but rather to a specific clinician; you might even call the provider while the patient is in your office so they can “meet.” Another approach, taken by some multispecialty practices, is to add psychotherapists to the staff so that patients can simply walk down the hall to obtain the mental health care they need.
Reaching across this divide is also a useful strategy for primary care physicians, who may welcome opportunities to meet with someone from a local treatment agency, not just for referrals but to learn more about treating patients with substance use problems. The Patient Protection and Affordable Care Act, which cites substance use disorders as one of 6 chronic health conditions that primary care medical homes are expected to address, may lead to better integration of health care systems that address physical health, as well as mental health and substance use disorders.
CORRESPONDENCE
Daniel C. Vinson, MD, MSPH, MA306E Health Sciences, Family and Community Medicine, University of Missouri, Columbia, MO 652312; [email protected]
- Friedman PD. Alcohol use in adults. N Engl J Med. 2013;368:365-373.
- Williams RH, Vinson DC. Validation of a single question screen for problem drinking. J Fam Pract. 2001;50:307-312.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;74:783-788.
- Dawson DA, Pulay AJ, Grant BF. A comparison of two single-item screeners for hazardous drinking and alcohol use disorder. Alcohol Clin Exp Res. 2010;34:364-374.
- Bush K, Kivlahan DR, Mcdonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789-1795.
- Dawson DA, Grant BF, Stinson FS, et al. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29:844-854.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
- American Psychiatric Asociation. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text rev. (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82-92.
- Vinson DC, Kruse RL, Seale JP. Simplifying alcohol assessment:two questions to identify alcohol use disorders. Alcohol Clin Exp Res. 2007;31:1392-139.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
- Johnson JA, Lee A, Vinson DC, et al. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2012;July 26 [Epub ahead of print].
- Center for Adolescent Substance Abuse Research. The CRAFFT Screening Tool. Boston MA:2009 [updated 2012). Available at: http://www.ceasar-boston.org/clinicians/crafft.php. Accessed January 15, 2013.
- Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27:67-734.
- Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ. 1988;297:663-668.
- Fleming MF, Barry KL, Manwell LB, et al. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1039-1044.
- Bertholet N, Daeppen JB, Wietlisbach V, et al. Brief alcohol intervention in primary care reduces alcohol consumption: Systematic review and meta-analysis. Arch Intern Med. 2005;165:986-995.
- Kaner EFS, Dickinson HO, Beyer F, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007(2);CD004148.-
- Fleming MF, Mundt MP, French MT, et al. Brief physician advice for problem drinkers: long-term efficacy and benefit-cost analysis. Alcohol Clin Exp Res. 2002;26:36-43.
- Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99:839-845.
- U.S.Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
- Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention ffor unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4:131-136.
- Field CA, Baird J, Saitz R, et al. The mixed evidence for brief intervention in emergency departments, trauma care centers, and inpatient hospital settings: what should we do? Alcohol Clin Exp Res. 2010;34:2004-2010.
- DiClemente CC, Prochaska JO. Toward a comprehensive transtheoretical model of change: stages of change and addictive behaviors. In: Miller WR, Heather N, eds. Treating Addictive Behaviors. New York, NY:Plenum Press;1998:3–24.
- Mason P, Butler CC. Health Behavior Change: A Guide for Practitioners. 2nd ed. London UK:Elsevier; 2010.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York NY: Guilford Press;2002.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
- Rollnick S, Butler CC, Kinnersley P, et al. Motivational interviewing. BMJ. 2010;340:c1900.-
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;(12):CD001867.-
- Swift R, Oslin DW, Alexander M, et al. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012-1018.
- Pettinati HM, Silverman BL, Battisti JJ, et al. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin Exp Res. 2011;35:1804-1811.
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51-63.
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010(9);CD004332.-
- Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2012;October 17 [Epub ahead of print].
- Azrin NH. Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther. 1976;14:339-348.
- Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. J Behav Ther Exp Psychiatr. 1982;13:105-112.
- Keane TM, Foy DW, Nunn B, et al. Spouse contracting to increase antabuse compliance in alcoholic veterans. J Clin Psychol. 1984;40:340-344.
- Arbaizar B, Diersen-Sotos T, Gomez-Acebo I, et al. Topiramate in the treatment of alcohol dependence: a meta-analysis. Actas Espanolas de Psiquiatria. 2010;38:8-12.
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34-56.
- Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963-971.
- Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatr. 2011;168:265-275.
- Pettatini HM, Oslin D, Lampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatr. 2010;167:668-675.
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatr. 2011;168:709-717.
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582-1588.
- Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297-1306.
- Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatr. 2011;68:1168-1175.
- Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
- Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatr. 2012;169:805-812.
- Farrell M, Wodak A, Gowing L. Maintenance drugs to treat opioid dependence. BMJ. 2012;344.-
- Pinto H, Maskrey V, Swift L, et al. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39:340-352.
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515-532.
- Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303-1310.
- Clausen T, Anchersen K, Waal H. Mortality prior to during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151-157.
- Clark RE, Samnaliev M, Baxter JD, et al. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Affairs. 2011;30:1425-1433.
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Improvement Protocol (TIP) Series 40. Rockville Md: Substance Abuse and Mental Health Services Administration; 2004.
- Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
- Fiellin DA. Buprenorphine: effective treatment of opioid addiction starts in the office. Am Fam Physician. 2006;73:1513.-
- Minozzi S, Amato L, Vecchi S, et al. Oral naltrexone maintainance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;(4):CD001333.-
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513.
• Screen patients for substance use disorders, with a single (validated) question for alcohol and another for drugs. A
• Follow a positive screen for alcohol with an assessment to distinguish between hazardous drinking and drinking that is indicative of alcohol dependence. C
• Approach a substance use disorder as you would any chronic medical condition, seeking to engage the patient to encourage behavior change. Motivational interviewing is a useful tool. C
• Consider pharmacotherapeutic options for patients with alcohol or drug dependence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Episodic heavy drinking, like alcoholism and drug addiction, is increasingly recognized as a medical problem that primary care physicians can, and should, address.1 But it is rarely the chief reason for an office visit. Nor is it a subject patients are likely to bring up.
However, patients are generally willing to talk to a trusted doctor who asks about their use (or misuse) of alcohol or other substances. And primary care physicians can do much to help—with brief interventions, a growing armamentarium of pharmacotherapy, and referrals as needed. In the pages that follow, you’ll find easy-to-use screening tools and effective intervention strategies.
SCREENING NEEDN'T BE TIME-CONSUMING
Screening for substance use isn’t difficult. In fact, it can usually be accomplished with 2 targeted questions—one for alcohol use and one for drugs.
Alcohol. Two single-question screens to detect hazardous drinking have been validated, despite having different parameters. Ask either:
Q: When was the last time you had more than ____ drinks (4 for women and 5 for men) in one day?
Q: How many times in the past year have you had ___ or more drinks (4 for women and 5 for men) in one day?
For the first question, any answer within the past 3 months is a positive screen for hazardous drinking.2 For the second, anything other than zero is positive.3,4
Initial screening can also be done with the AUDIT-C (TABLE), a validated short (3-question) version of the Alcohol Use Disorders Identification Test that can be self-administered.5,6
Drugs. Only one single-question screen for drug use has been validated:
Q: How many times in the past year have you used an illegal drug or taken a prescription medication for nonmedical reasons?
Any answer other than never is a positive screen for hazardous drug use.7
CASE Jason F, a healthy and fit 28-year-old, has been your patient, along with his family, for years. He’s in your office because of a knee injury he incurred while running, and you take a moment to ask him, for the first time, how much he drinks and whether he takes drugs. His answer—that he drinks 3 or 4 times a week and often has multiple drinks at parties or nights out with the guys—takes you a bit by surprise.
Now what?
TABLE
3-question AUDIT-C screen for alcohol dependence5,6
| 1. How often do you have a drink containing alcohol? | ||
| 0 Never | 3 2 to 3 times a week | |
| 1 Monthly or less | 4 4 or more times a week | |
| 2 2 to 4 times a month | ||
| 2. How many drinks containing alcohol do you have on a typical day when you are drinking? | ||
| 0 I don’t drink. | 2 5 or 6 | |
| 0 1 or 2 | 3 7 to 9 | |
| 1 3 or 4 | 4 10 or more | |
| 3. How often do you have 6 or more drinks on one occasion? | ||
| 0 Never | 3 Weekly | |
| 1 Less than monthly | 4 Daily or almost daily | |
| 2 Monthly | ||
| Scoring the AUDIT-C | ||
| Alcohol dependence | ||
| Men | Women | |
| Threshold score | 5 | 4 |
| Sensitivity (%) | 80 | 76 |
| Specificity (%) | 74 | 78 |
| AROC | 0.769 | 0.767 |
| AROC, area under the receiver operating characteristic curve; AUDIT, Alcohol Use Disorders Identification Test. | ||
TELL ME MORE ABOUT IT
It is important to respond to a positive screen by requesting more information. In the conversation that ensues, the patient may provide the details you need to determine whether the drinking or drug use is indicative of a diagnosable substance use disorder, an umbrella term for alcohol or drug abuse and alcohol or drug dependence.
Alcohol or drug abuse vs dependence. Criteria for alcohol or drug abuse in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision (DSM-IV-TR) include risky behavior, such as drinking and driving; problems with work and/or close relationships; or run-ins with the law, such as an arrest for driving while intoxicated. Criteria for alcohol or drug dependence include the inability to cut down or stop using the substance; evidence of tolerance and withdrawal; spending more time ingesting the substance; increasing attention to substance use while interest in other activities diminishes; and continued use despite recurrent problems.8
Tools zero in on extent of problem
To learn more about your patient’s situation, consider using the following criteria and tools:
DSM criteria. Ask the following 2 questions, which are among DSM-IV-TR criteria for alcohol dependence:
- How many times in the last year have you had a lot more to drink than you intended?
- How many times in the last year have you been drinking in situations where it could have been hazardous, where you could have caused an accident or gotten hurt?
Any answer other than zero to either question is suggestive of a substance use disorder. In exploratory analyses, this approach had positive likelihood ratios of 4.7 to 16 and negative likelihood ratios of 0.05 to 0.30.9,10
Although the above questions refer to alcohol use, they could be revised to learn more about a patient’s use of marijuana or other drugs, as well. (There are few tools for the assessment of drug use, because any illegal or nonmedical use of controlled substances has clear risks of major harm.)
CAGE. Another tool that is effective in assessing alcohol use is the 4-question CAGE—an acronym for Cut down, Annoyed, Guilty, and Eye opener:
- Have you ever felt that you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt bad or guilty about your drinking?
- Have you ever had a drink in the morning to get rid of a hangover?
One meta-analysis found that a positive CAGE test—ie, a positive response to one or more of the questions—had a sensitivity of 0.85 and a specificity of 0.78 in identifying alcohol dependence in a primary care setting (using DSM criteria as the gold standard).11
AUDIT. This 10-item tool, a longer version of the AUDIT-C (available at http://www.medstudentlearning.com/node/6556), can also be used to determine the extent of alcohol use. This test provides detailed information about the quantity and frequency of alcohol use; however, it does not clearly distinguish between hazardous drinking and alcohol use disorders.12
If the patient is a teen
Assessment methods can be adjusted without difficulty to fit the age of the patient. The National Institute on Alcohol Abuse and Alcoholism has published the Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide, available at http://www.niaaa.nih.gov/Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. The 6-question CRAFFT (for Car, Relax, Alone, Forget, Friends, Trouble) is a validated tool designed to assess adolescents’ use of both alcohol and drugs (http://www.ceasar-boston.org/clinicians/crafft.php).13,14
CASE You give Mr. F the CAGE test, and he answers No to all 4 questions. You conclude that while his drinking may be hazardous, he does not appear to have alcohol abuse or dependence.
FOLLOW UP WITH A BRIEF INTERVENTION
For decades, evidence has shown that brief interventions are often effective in helping hazardous drinkers like Mr. F cut back to safer levels.15-18 In some cases, the impact has been great enough to reduce health care and societal costs for up to 4 years19 and to cut the risk of alcohol-related death by about half.20 As a result, the US Preventive Services Task Force has given a B rating to counseling to reduce alcohol misuse by primary care providers.21 (There is less evidence that brief interventions are effective for drug problems,22 or in settings other than primary care.23)
Treat drug/alcohol problems
If you determine that your patient is engaging in hazardous alcohol or drug use or has a diagnosable substance use disorder, you do not have to drop everything else or treat it as an acute event. What matters is long-term success, which is best achieved by partnering with the patient.
Start by approaching drug and alcohol problems as you would a case of newly elevated blood pressure. Bring up the problem, seeking to engage the patient in addres- sing it.
If he or she does not agree to quit or cut back on drinking the first time you broach the subject, don’t be surprised or discouraged. Keep in mind that patients do not always respond positively to advice about handling chronic medical conditions either, particularly at first, and that you’ll be working together over time. What’s important, in the jargon of the Stages of Change model,24 is to help the patient move from precontemplation to contemplation, and perhaps beyond that to planning or action.
Use motivational interviewing to partner with patients
Motivational interviewing is useful in helping patients change health-related behaviors. The technique, which is not hard to learn or apply, is based on the recognition that a simple shift in style toward a guiding (rather than directive) approach can often reap benefits that are immediately apparent. 25-28
Motivational Interviewing: Helping People Change (3rd ed, by William R. Miller and Stephen Rollnick; Guilford Press, 2013) is an excellent resource for clinicians who wish to master this technique. An online tutorial in screening and brief intervention for alcohol or drug misuse is available free at https://adept.missouri.edu. Video demonstrations of motivational interviewing to address these issues are also available here ; to access them, click on “Training”, then on “Go to SBIRT videos”).
CASE Before Mr. F’s visit is concluded, you initiate a conversation about alcohol use, stating: “As your doctor, I’m concerned that the amount of alcohol you’re drinking could be hazardous to your health. I recommend that you cut down to no more than 4 drinks in any one day and to no more than 14 drinks a week.” You make it clear that change is up to him, and ask what he thinks about what you’ve said.
You also schedule a return visit in one month, at which time you will continue the conversation.
PHARMACOTHERAPY IS A USEFUL TOOL
Increasingly, alcohol and drug dependence—like other chronic conditions—can be effectively addressed with medication.
Drugs to treat alcohol dependence
Naltrexone. A daily dose of naltrexone, starting at 25 mg daily for a few days and going as high as 100 mg/d, can help patients with alcohol dependence limit their drinking to safe levels (number needed to treat [NNT]=9).29 This will reduce the risk of alcohol-related harm while the patient considers quitting.
The most common adverse effect is nausea, but a low starting dose may alleviate it. Naltrexone, also available as a 380-mg intramuscular (IM) depot injection once every 4 weeks, is an opioid antagonist and should not be given to any patient who’s taking opioids.
A 2010 Cochrane review found only 4 trials of naltrexone IM, and failed to show significant reductions in drinking.29 But post hoc analyses of trials of both oral and IM naltrexone found that those in which compliance was assured (either by direct observation or IM administration) had better outcomes than those in which it was not.30 Another post hoc analysis found that patients whose alcohol dependence was more severe derived greater benefits from the drug than those who were less severely affected.31
Acamprosate. Two 333-mg pills tid can help newly abstinent drinkers remain alcohol-free (NNT=9).32,33 The most common adverse effect is diarrhea, which may subside with continued use.
Combining acamprosate and naltrexone does not appear to be more effective than either drug alone. In a recently published meta-analysis comparing the 2 drugs, those taking acamprosate had slightly better rates of abstinence from alcohol, while naltrexone was slightly better in reducing heavy drinking.34
Disulfiram Unlike naltrexone and acamprosate, which work by altering the brain’s reward circuits, disulfiram blocks metabolism of ethanol, leading to the accumulation of a toxic metabolite and its punishing syndrome. The major problem with the drug is noncompliance, which can be addressed by enlisting the help of a caregiver or partner to ensure that it is taken daily. 35-37
Other medications that have been tested (though not approved by the US Food and Drug Administration [FDA]) as a treatment for alcohol dependence include:
- topiramate, which has been found to have modest efficacy in increasing the number of abstinent days and decreasing heavy-drinking days;38
- baclofen, which has shown efficacy in small clinical trials;39 and
- ondansetron, which has been shown to effectively treat early-onset alcohol dependence.40,41
For patients with depression and alcohol dependence, the combination of naltrexone and sertraline has been found to be superior to either drug by itself—and to have fewer adverse effects. 42 Gabapentin and lorazepam have been compared in treating alcohol withdrawal, with gabapentin resulting in greater efficacy and fewer adverse effects than lorazepam.43,44
Pharmacotherapy for drug abuse, dependence
For methamphetamine abuse and dependence. Two randomized clinical trials have studied medications for methamphetamine abuse and dependence. In one small study, topiramate did not increase the proportion of patients who achieved abstinence, but in a post hoc subgroup analysis, it did appear to help newly abstinent patients avoid relapse.45
In another study, mirtazapine significantly decreased the proportion of patients whose weekly urine tests were positive for methamphetamine (from 73% to 44%); no significant change was found among those on placebo.46
Both drugs were well tolerated, but compliance was low in both trials despite weekly counseling. Each has only one clinical trial to support its use, and neither has FDA approval for addiction treatment.
For marijuana dependence. In a study of 50 people seeking treatment for marijuana dependence, gabapentin 400 mg 3 times a day significantly improved the proportion reporting no cannabis use and whose urine tested negative for the drug.47
Another recent trial randomized adolescents dependent on cannabis to placebo or N-acetylcysteine 1200 mg twice a day. Those on the active drug were 2.4 times more likely than those on placebo to have negative urine tests with a number needed to treat of 7.48 Both trials ran for about 3 months. Neither drug is FDA approved to treat marijuana dependence.
For opioid dependence. As maintenance medication for patients dependent on opioids, both methadone and buprenorphine have been shown to reduce the use of illicit opioids, lower mortality, and improve retention compared with treatment without medication.49 Methadone would be a better choice than buprenorphine, which is a partial agonist with a ceiling on both its good (eg, stopping craving) and bad (eg, overdose risk) effects. In an open-label observational study of patients’ preferences, those who chose methadone maintenance over buprenorphine were twice as likely to remain in treatment.50 Both drugs are FDA-approved for treating opioid dependence.
Methadone is a full agonist that can be given for opioid dependence only in a federally licensed methadone maintenance clinic. It has been shown to reduce the use of other opioids, reduce criminal behaviors,51 improve function in many areas,52 and reduce mortality.53 In a cohort study of Massachusetts Medicaid data, methadone reduced mortality, which was 75% higher among those receiving abstinence-based treatment.52,54
Buprenorphine (alone and in combination with naloxone), effectively reduces the use of illicit opioids and improves functional status.55-57
Naltrexone, an opioid antagonist, may be effective in the treatment of opioid dependence. As with disulfiram for alcohol dependence, a major limitation of naltrexone for opioid dependence is noncompliance. But once a patient has been on oral naltrexone, he or she can be switched to naltrexone IM, which can be administered every 4 weeks. A Cochrane review published in 2011 found no evidence that naltrexone was superior to placebo, 58 but since then another randomized clinical trial has been published that found naltrexone 380 mg IM every 4 weeks was superior to IM placebo. Patients on naltrexone were more likely to remain in treatment and have opioid-free urine tests, and reported less craving for opioids.59 Both oral and depot injection naltrexone are FDA approved for treatment of opioid dependence. No comparisons of naltrexone vs either methadone or buprenorphine have been published.
To learn more about pharmacotherapy for opioid dependence, see “Diagnosing and treating opioid dependence” (J Fam Pract. 2012;61:588-596).
DEALING WITH THE CHALLENGES
As noted earlier, some patients with substance use disorders, like some patients with depression or hypertension, respond well to care and counseling, and some do not. Just as with other conditions, consultation with a specialist often helps.
A major difference in arranging consultations for patients with substance use disorders, however, is that clinicians who specialize in substance abuse and dependence often work in health care systems that are largely, or entirely, separate from those in which primary care physicians typically work. This, plus the stigma that surrounds problems with substance use, presents barriers to patients, who may shy away from going across town or to another city to see a provider they don’t know for a problem they’re either resistant to “owning” or ashamed of.
Yet it is possible to reach across this divide and make it easier for patients. One way to do that might be to partner with a local alcohol- and drug-treatment program so that your patients are referred, not to a faceless agency, but rather to a specific clinician; you might even call the provider while the patient is in your office so they can “meet.” Another approach, taken by some multispecialty practices, is to add psychotherapists to the staff so that patients can simply walk down the hall to obtain the mental health care they need.
Reaching across this divide is also a useful strategy for primary care physicians, who may welcome opportunities to meet with someone from a local treatment agency, not just for referrals but to learn more about treating patients with substance use problems. The Patient Protection and Affordable Care Act, which cites substance use disorders as one of 6 chronic health conditions that primary care medical homes are expected to address, may lead to better integration of health care systems that address physical health, as well as mental health and substance use disorders.
CORRESPONDENCE
Daniel C. Vinson, MD, MSPH, MA306E Health Sciences, Family and Community Medicine, University of Missouri, Columbia, MO 652312; [email protected]
• Screen patients for substance use disorders, with a single (validated) question for alcohol and another for drugs. A
• Follow a positive screen for alcohol with an assessment to distinguish between hazardous drinking and drinking that is indicative of alcohol dependence. C
• Approach a substance use disorder as you would any chronic medical condition, seeking to engage the patient to encourage behavior change. Motivational interviewing is a useful tool. C
• Consider pharmacotherapeutic options for patients with alcohol or drug dependence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Episodic heavy drinking, like alcoholism and drug addiction, is increasingly recognized as a medical problem that primary care physicians can, and should, address.1 But it is rarely the chief reason for an office visit. Nor is it a subject patients are likely to bring up.
However, patients are generally willing to talk to a trusted doctor who asks about their use (or misuse) of alcohol or other substances. And primary care physicians can do much to help—with brief interventions, a growing armamentarium of pharmacotherapy, and referrals as needed. In the pages that follow, you’ll find easy-to-use screening tools and effective intervention strategies.
SCREENING NEEDN'T BE TIME-CONSUMING
Screening for substance use isn’t difficult. In fact, it can usually be accomplished with 2 targeted questions—one for alcohol use and one for drugs.
Alcohol. Two single-question screens to detect hazardous drinking have been validated, despite having different parameters. Ask either:
Q: When was the last time you had more than ____ drinks (4 for women and 5 for men) in one day?
Q: How many times in the past year have you had ___ or more drinks (4 for women and 5 for men) in one day?
For the first question, any answer within the past 3 months is a positive screen for hazardous drinking.2 For the second, anything other than zero is positive.3,4
Initial screening can also be done with the AUDIT-C (TABLE), a validated short (3-question) version of the Alcohol Use Disorders Identification Test that can be self-administered.5,6
Drugs. Only one single-question screen for drug use has been validated:
Q: How many times in the past year have you used an illegal drug or taken a prescription medication for nonmedical reasons?
Any answer other than never is a positive screen for hazardous drug use.7
CASE Jason F, a healthy and fit 28-year-old, has been your patient, along with his family, for years. He’s in your office because of a knee injury he incurred while running, and you take a moment to ask him, for the first time, how much he drinks and whether he takes drugs. His answer—that he drinks 3 or 4 times a week and often has multiple drinks at parties or nights out with the guys—takes you a bit by surprise.
Now what?
TABLE
3-question AUDIT-C screen for alcohol dependence5,6
| 1. How often do you have a drink containing alcohol? | ||
| 0 Never | 3 2 to 3 times a week | |
| 1 Monthly or less | 4 4 or more times a week | |
| 2 2 to 4 times a month | ||
| 2. How many drinks containing alcohol do you have on a typical day when you are drinking? | ||
| 0 I don’t drink. | 2 5 or 6 | |
| 0 1 or 2 | 3 7 to 9 | |
| 1 3 or 4 | 4 10 or more | |
| 3. How often do you have 6 or more drinks on one occasion? | ||
| 0 Never | 3 Weekly | |
| 1 Less than monthly | 4 Daily or almost daily | |
| 2 Monthly | ||
| Scoring the AUDIT-C | ||
| Alcohol dependence | ||
| Men | Women | |
| Threshold score | 5 | 4 |
| Sensitivity (%) | 80 | 76 |
| Specificity (%) | 74 | 78 |
| AROC | 0.769 | 0.767 |
| AROC, area under the receiver operating characteristic curve; AUDIT, Alcohol Use Disorders Identification Test. | ||
TELL ME MORE ABOUT IT
It is important to respond to a positive screen by requesting more information. In the conversation that ensues, the patient may provide the details you need to determine whether the drinking or drug use is indicative of a diagnosable substance use disorder, an umbrella term for alcohol or drug abuse and alcohol or drug dependence.
Alcohol or drug abuse vs dependence. Criteria for alcohol or drug abuse in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision (DSM-IV-TR) include risky behavior, such as drinking and driving; problems with work and/or close relationships; or run-ins with the law, such as an arrest for driving while intoxicated. Criteria for alcohol or drug dependence include the inability to cut down or stop using the substance; evidence of tolerance and withdrawal; spending more time ingesting the substance; increasing attention to substance use while interest in other activities diminishes; and continued use despite recurrent problems.8
Tools zero in on extent of problem
To learn more about your patient’s situation, consider using the following criteria and tools:
DSM criteria. Ask the following 2 questions, which are among DSM-IV-TR criteria for alcohol dependence:
- How many times in the last year have you had a lot more to drink than you intended?
- How many times in the last year have you been drinking in situations where it could have been hazardous, where you could have caused an accident or gotten hurt?
Any answer other than zero to either question is suggestive of a substance use disorder. In exploratory analyses, this approach had positive likelihood ratios of 4.7 to 16 and negative likelihood ratios of 0.05 to 0.30.9,10
Although the above questions refer to alcohol use, they could be revised to learn more about a patient’s use of marijuana or other drugs, as well. (There are few tools for the assessment of drug use, because any illegal or nonmedical use of controlled substances has clear risks of major harm.)
CAGE. Another tool that is effective in assessing alcohol use is the 4-question CAGE—an acronym for Cut down, Annoyed, Guilty, and Eye opener:
- Have you ever felt that you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt bad or guilty about your drinking?
- Have you ever had a drink in the morning to get rid of a hangover?
One meta-analysis found that a positive CAGE test—ie, a positive response to one or more of the questions—had a sensitivity of 0.85 and a specificity of 0.78 in identifying alcohol dependence in a primary care setting (using DSM criteria as the gold standard).11
AUDIT. This 10-item tool, a longer version of the AUDIT-C (available at http://www.medstudentlearning.com/node/6556), can also be used to determine the extent of alcohol use. This test provides detailed information about the quantity and frequency of alcohol use; however, it does not clearly distinguish between hazardous drinking and alcohol use disorders.12
If the patient is a teen
Assessment methods can be adjusted without difficulty to fit the age of the patient. The National Institute on Alcohol Abuse and Alcoholism has published the Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide, available at http://www.niaaa.nih.gov/Publications/EducationTrainingMaterials/Pages/YouthGuide.aspx. The 6-question CRAFFT (for Car, Relax, Alone, Forget, Friends, Trouble) is a validated tool designed to assess adolescents’ use of both alcohol and drugs (http://www.ceasar-boston.org/clinicians/crafft.php).13,14
CASE You give Mr. F the CAGE test, and he answers No to all 4 questions. You conclude that while his drinking may be hazardous, he does not appear to have alcohol abuse or dependence.
FOLLOW UP WITH A BRIEF INTERVENTION
For decades, evidence has shown that brief interventions are often effective in helping hazardous drinkers like Mr. F cut back to safer levels.15-18 In some cases, the impact has been great enough to reduce health care and societal costs for up to 4 years19 and to cut the risk of alcohol-related death by about half.20 As a result, the US Preventive Services Task Force has given a B rating to counseling to reduce alcohol misuse by primary care providers.21 (There is less evidence that brief interventions are effective for drug problems,22 or in settings other than primary care.23)
Treat drug/alcohol problems
If you determine that your patient is engaging in hazardous alcohol or drug use or has a diagnosable substance use disorder, you do not have to drop everything else or treat it as an acute event. What matters is long-term success, which is best achieved by partnering with the patient.
Start by approaching drug and alcohol problems as you would a case of newly elevated blood pressure. Bring up the problem, seeking to engage the patient in addres- sing it.
If he or she does not agree to quit or cut back on drinking the first time you broach the subject, don’t be surprised or discouraged. Keep in mind that patients do not always respond positively to advice about handling chronic medical conditions either, particularly at first, and that you’ll be working together over time. What’s important, in the jargon of the Stages of Change model,24 is to help the patient move from precontemplation to contemplation, and perhaps beyond that to planning or action.
Use motivational interviewing to partner with patients
Motivational interviewing is useful in helping patients change health-related behaviors. The technique, which is not hard to learn or apply, is based on the recognition that a simple shift in style toward a guiding (rather than directive) approach can often reap benefits that are immediately apparent. 25-28
Motivational Interviewing: Helping People Change (3rd ed, by William R. Miller and Stephen Rollnick; Guilford Press, 2013) is an excellent resource for clinicians who wish to master this technique. An online tutorial in screening and brief intervention for alcohol or drug misuse is available free at https://adept.missouri.edu. Video demonstrations of motivational interviewing to address these issues are also available here ; to access them, click on “Training”, then on “Go to SBIRT videos”).
CASE Before Mr. F’s visit is concluded, you initiate a conversation about alcohol use, stating: “As your doctor, I’m concerned that the amount of alcohol you’re drinking could be hazardous to your health. I recommend that you cut down to no more than 4 drinks in any one day and to no more than 14 drinks a week.” You make it clear that change is up to him, and ask what he thinks about what you’ve said.
You also schedule a return visit in one month, at which time you will continue the conversation.
PHARMACOTHERAPY IS A USEFUL TOOL
Increasingly, alcohol and drug dependence—like other chronic conditions—can be effectively addressed with medication.
Drugs to treat alcohol dependence
Naltrexone. A daily dose of naltrexone, starting at 25 mg daily for a few days and going as high as 100 mg/d, can help patients with alcohol dependence limit their drinking to safe levels (number needed to treat [NNT]=9).29 This will reduce the risk of alcohol-related harm while the patient considers quitting.
The most common adverse effect is nausea, but a low starting dose may alleviate it. Naltrexone, also available as a 380-mg intramuscular (IM) depot injection once every 4 weeks, is an opioid antagonist and should not be given to any patient who’s taking opioids.
A 2010 Cochrane review found only 4 trials of naltrexone IM, and failed to show significant reductions in drinking.29 But post hoc analyses of trials of both oral and IM naltrexone found that those in which compliance was assured (either by direct observation or IM administration) had better outcomes than those in which it was not.30 Another post hoc analysis found that patients whose alcohol dependence was more severe derived greater benefits from the drug than those who were less severely affected.31
Acamprosate. Two 333-mg pills tid can help newly abstinent drinkers remain alcohol-free (NNT=9).32,33 The most common adverse effect is diarrhea, which may subside with continued use.
Combining acamprosate and naltrexone does not appear to be more effective than either drug alone. In a recently published meta-analysis comparing the 2 drugs, those taking acamprosate had slightly better rates of abstinence from alcohol, while naltrexone was slightly better in reducing heavy drinking.34
Disulfiram Unlike naltrexone and acamprosate, which work by altering the brain’s reward circuits, disulfiram blocks metabolism of ethanol, leading to the accumulation of a toxic metabolite and its punishing syndrome. The major problem with the drug is noncompliance, which can be addressed by enlisting the help of a caregiver or partner to ensure that it is taken daily. 35-37
Other medications that have been tested (though not approved by the US Food and Drug Administration [FDA]) as a treatment for alcohol dependence include:
- topiramate, which has been found to have modest efficacy in increasing the number of abstinent days and decreasing heavy-drinking days;38
- baclofen, which has shown efficacy in small clinical trials;39 and
- ondansetron, which has been shown to effectively treat early-onset alcohol dependence.40,41
For patients with depression and alcohol dependence, the combination of naltrexone and sertraline has been found to be superior to either drug by itself—and to have fewer adverse effects. 42 Gabapentin and lorazepam have been compared in treating alcohol withdrawal, with gabapentin resulting in greater efficacy and fewer adverse effects than lorazepam.43,44
Pharmacotherapy for drug abuse, dependence
For methamphetamine abuse and dependence. Two randomized clinical trials have studied medications for methamphetamine abuse and dependence. In one small study, topiramate did not increase the proportion of patients who achieved abstinence, but in a post hoc subgroup analysis, it did appear to help newly abstinent patients avoid relapse.45
In another study, mirtazapine significantly decreased the proportion of patients whose weekly urine tests were positive for methamphetamine (from 73% to 44%); no significant change was found among those on placebo.46
Both drugs were well tolerated, but compliance was low in both trials despite weekly counseling. Each has only one clinical trial to support its use, and neither has FDA approval for addiction treatment.
For marijuana dependence. In a study of 50 people seeking treatment for marijuana dependence, gabapentin 400 mg 3 times a day significantly improved the proportion reporting no cannabis use and whose urine tested negative for the drug.47
Another recent trial randomized adolescents dependent on cannabis to placebo or N-acetylcysteine 1200 mg twice a day. Those on the active drug were 2.4 times more likely than those on placebo to have negative urine tests with a number needed to treat of 7.48 Both trials ran for about 3 months. Neither drug is FDA approved to treat marijuana dependence.
For opioid dependence. As maintenance medication for patients dependent on opioids, both methadone and buprenorphine have been shown to reduce the use of illicit opioids, lower mortality, and improve retention compared with treatment without medication.49 Methadone would be a better choice than buprenorphine, which is a partial agonist with a ceiling on both its good (eg, stopping craving) and bad (eg, overdose risk) effects. In an open-label observational study of patients’ preferences, those who chose methadone maintenance over buprenorphine were twice as likely to remain in treatment.50 Both drugs are FDA-approved for treating opioid dependence.
Methadone is a full agonist that can be given for opioid dependence only in a federally licensed methadone maintenance clinic. It has been shown to reduce the use of other opioids, reduce criminal behaviors,51 improve function in many areas,52 and reduce mortality.53 In a cohort study of Massachusetts Medicaid data, methadone reduced mortality, which was 75% higher among those receiving abstinence-based treatment.52,54
Buprenorphine (alone and in combination with naloxone), effectively reduces the use of illicit opioids and improves functional status.55-57
Naltrexone, an opioid antagonist, may be effective in the treatment of opioid dependence. As with disulfiram for alcohol dependence, a major limitation of naltrexone for opioid dependence is noncompliance. But once a patient has been on oral naltrexone, he or she can be switched to naltrexone IM, which can be administered every 4 weeks. A Cochrane review published in 2011 found no evidence that naltrexone was superior to placebo, 58 but since then another randomized clinical trial has been published that found naltrexone 380 mg IM every 4 weeks was superior to IM placebo. Patients on naltrexone were more likely to remain in treatment and have opioid-free urine tests, and reported less craving for opioids.59 Both oral and depot injection naltrexone are FDA approved for treatment of opioid dependence. No comparisons of naltrexone vs either methadone or buprenorphine have been published.
To learn more about pharmacotherapy for opioid dependence, see “Diagnosing and treating opioid dependence” (J Fam Pract. 2012;61:588-596).
DEALING WITH THE CHALLENGES
As noted earlier, some patients with substance use disorders, like some patients with depression or hypertension, respond well to care and counseling, and some do not. Just as with other conditions, consultation with a specialist often helps.
A major difference in arranging consultations for patients with substance use disorders, however, is that clinicians who specialize in substance abuse and dependence often work in health care systems that are largely, or entirely, separate from those in which primary care physicians typically work. This, plus the stigma that surrounds problems with substance use, presents barriers to patients, who may shy away from going across town or to another city to see a provider they don’t know for a problem they’re either resistant to “owning” or ashamed of.
Yet it is possible to reach across this divide and make it easier for patients. One way to do that might be to partner with a local alcohol- and drug-treatment program so that your patients are referred, not to a faceless agency, but rather to a specific clinician; you might even call the provider while the patient is in your office so they can “meet.” Another approach, taken by some multispecialty practices, is to add psychotherapists to the staff so that patients can simply walk down the hall to obtain the mental health care they need.
Reaching across this divide is also a useful strategy for primary care physicians, who may welcome opportunities to meet with someone from a local treatment agency, not just for referrals but to learn more about treating patients with substance use problems. The Patient Protection and Affordable Care Act, which cites substance use disorders as one of 6 chronic health conditions that primary care medical homes are expected to address, may lead to better integration of health care systems that address physical health, as well as mental health and substance use disorders.
CORRESPONDENCE
Daniel C. Vinson, MD, MSPH, MA306E Health Sciences, Family and Community Medicine, University of Missouri, Columbia, MO 652312; [email protected]
- Friedman PD. Alcohol use in adults. N Engl J Med. 2013;368:365-373.
- Williams RH, Vinson DC. Validation of a single question screen for problem drinking. J Fam Pract. 2001;50:307-312.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;74:783-788.
- Dawson DA, Pulay AJ, Grant BF. A comparison of two single-item screeners for hazardous drinking and alcohol use disorder. Alcohol Clin Exp Res. 2010;34:364-374.
- Bush K, Kivlahan DR, Mcdonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789-1795.
- Dawson DA, Grant BF, Stinson FS, et al. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29:844-854.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
- American Psychiatric Asociation. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text rev. (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82-92.
- Vinson DC, Kruse RL, Seale JP. Simplifying alcohol assessment:two questions to identify alcohol use disorders. Alcohol Clin Exp Res. 2007;31:1392-139.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
- Johnson JA, Lee A, Vinson DC, et al. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2012;July 26 [Epub ahead of print].
- Center for Adolescent Substance Abuse Research. The CRAFFT Screening Tool. Boston MA:2009 [updated 2012). Available at: http://www.ceasar-boston.org/clinicians/crafft.php. Accessed January 15, 2013.
- Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27:67-734.
- Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ. 1988;297:663-668.
- Fleming MF, Barry KL, Manwell LB, et al. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1039-1044.
- Bertholet N, Daeppen JB, Wietlisbach V, et al. Brief alcohol intervention in primary care reduces alcohol consumption: Systematic review and meta-analysis. Arch Intern Med. 2005;165:986-995.
- Kaner EFS, Dickinson HO, Beyer F, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007(2);CD004148.-
- Fleming MF, Mundt MP, French MT, et al. Brief physician advice for problem drinkers: long-term efficacy and benefit-cost analysis. Alcohol Clin Exp Res. 2002;26:36-43.
- Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99:839-845.
- U.S.Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
- Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention ffor unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4:131-136.
- Field CA, Baird J, Saitz R, et al. The mixed evidence for brief intervention in emergency departments, trauma care centers, and inpatient hospital settings: what should we do? Alcohol Clin Exp Res. 2010;34:2004-2010.
- DiClemente CC, Prochaska JO. Toward a comprehensive transtheoretical model of change: stages of change and addictive behaviors. In: Miller WR, Heather N, eds. Treating Addictive Behaviors. New York, NY:Plenum Press;1998:3–24.
- Mason P, Butler CC. Health Behavior Change: A Guide for Practitioners. 2nd ed. London UK:Elsevier; 2010.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York NY: Guilford Press;2002.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
- Rollnick S, Butler CC, Kinnersley P, et al. Motivational interviewing. BMJ. 2010;340:c1900.-
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;(12):CD001867.-
- Swift R, Oslin DW, Alexander M, et al. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012-1018.
- Pettinati HM, Silverman BL, Battisti JJ, et al. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin Exp Res. 2011;35:1804-1811.
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51-63.
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010(9);CD004332.-
- Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2012;October 17 [Epub ahead of print].
- Azrin NH. Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther. 1976;14:339-348.
- Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. J Behav Ther Exp Psychiatr. 1982;13:105-112.
- Keane TM, Foy DW, Nunn B, et al. Spouse contracting to increase antabuse compliance in alcoholic veterans. J Clin Psychol. 1984;40:340-344.
- Arbaizar B, Diersen-Sotos T, Gomez-Acebo I, et al. Topiramate in the treatment of alcohol dependence: a meta-analysis. Actas Espanolas de Psiquiatria. 2010;38:8-12.
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34-56.
- Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963-971.
- Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatr. 2011;168:265-275.
- Pettatini HM, Oslin D, Lampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatr. 2010;167:668-675.
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatr. 2011;168:709-717.
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582-1588.
- Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297-1306.
- Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatr. 2011;68:1168-1175.
- Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
- Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatr. 2012;169:805-812.
- Farrell M, Wodak A, Gowing L. Maintenance drugs to treat opioid dependence. BMJ. 2012;344.-
- Pinto H, Maskrey V, Swift L, et al. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39:340-352.
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515-532.
- Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303-1310.
- Clausen T, Anchersen K, Waal H. Mortality prior to during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151-157.
- Clark RE, Samnaliev M, Baxter JD, et al. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Affairs. 2011;30:1425-1433.
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Improvement Protocol (TIP) Series 40. Rockville Md: Substance Abuse and Mental Health Services Administration; 2004.
- Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
- Fiellin DA. Buprenorphine: effective treatment of opioid addiction starts in the office. Am Fam Physician. 2006;73:1513.-
- Minozzi S, Amato L, Vecchi S, et al. Oral naltrexone maintainance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;(4):CD001333.-
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513.
- Friedman PD. Alcohol use in adults. N Engl J Med. 2013;368:365-373.
- Williams RH, Vinson DC. Validation of a single question screen for problem drinking. J Fam Pract. 2001;50:307-312.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;74:783-788.
- Dawson DA, Pulay AJ, Grant BF. A comparison of two single-item screeners for hazardous drinking and alcohol use disorder. Alcohol Clin Exp Res. 2010;34:364-374.
- Bush K, Kivlahan DR, Mcdonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789-1795.
- Dawson DA, Grant BF, Stinson FS, et al. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29:844-854.
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
- American Psychiatric Asociation. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text rev. (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
- Saha TD, Stinson FS, Grant BF. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend. 2007;89:82-92.
- Vinson DC, Kruse RL, Seale JP. Simplifying alcohol assessment:two questions to identify alcohol use disorders. Alcohol Clin Exp Res. 2007;31:1392-139.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
- Johnson JA, Lee A, Vinson DC, et al. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2012;July 26 [Epub ahead of print].
- Center for Adolescent Substance Abuse Research. The CRAFFT Screening Tool. Boston MA:2009 [updated 2012). Available at: http://www.ceasar-boston.org/clinicians/crafft.php. Accessed January 15, 2013.
- Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27:67-734.
- Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ. 1988;297:663-668.
- Fleming MF, Barry KL, Manwell LB, et al. Brief physician advice for problem alcohol drinkers: a randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1039-1044.
- Bertholet N, Daeppen JB, Wietlisbach V, et al. Brief alcohol intervention in primary care reduces alcohol consumption: Systematic review and meta-analysis. Arch Intern Med. 2005;165:986-995.
- Kaner EFS, Dickinson HO, Beyer F, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007(2);CD004148.-
- Fleming MF, Mundt MP, French MT, et al. Brief physician advice for problem drinkers: long-term efficacy and benefit-cost analysis. Alcohol Clin Exp Res. 2002;26:36-43.
- Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99:839-845.
- U.S.Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
- Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention ffor unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4:131-136.
- Field CA, Baird J, Saitz R, et al. The mixed evidence for brief intervention in emergency departments, trauma care centers, and inpatient hospital settings: what should we do? Alcohol Clin Exp Res. 2010;34:2004-2010.
- DiClemente CC, Prochaska JO. Toward a comprehensive transtheoretical model of change: stages of change and addictive behaviors. In: Miller WR, Heather N, eds. Treating Addictive Behaviors. New York, NY:Plenum Press;1998:3–24.
- Mason P, Butler CC. Health Behavior Change: A Guide for Practitioners. 2nd ed. London UK:Elsevier; 2010.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York NY: Guilford Press;2002.
- Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psych. 2005;1:91-111.
- Rollnick S, Butler CC, Kinnersley P, et al. Motivational interviewing. BMJ. 2010;340:c1900.-
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;(12):CD001867.-
- Swift R, Oslin DW, Alexander M, et al. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012-1018.
- Pettinati HM, Silverman BL, Battisti JJ, et al. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin Exp Res. 2011;35:1804-1811.
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51-63.
- Rosner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010(9);CD004332.-
- Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2012;October 17 [Epub ahead of print].
- Azrin NH. Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther. 1976;14:339-348.
- Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. J Behav Ther Exp Psychiatr. 1982;13:105-112.
- Keane TM, Foy DW, Nunn B, et al. Spouse contracting to increase antabuse compliance in alcoholic veterans. J Clin Psychol. 1984;40:340-344.
- Arbaizar B, Diersen-Sotos T, Gomez-Acebo I, et al. Topiramate in the treatment of alcohol dependence: a meta-analysis. Actas Espanolas de Psiquiatria. 2010;38:8-12.
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34-56.
- Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963-971.
- Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatr. 2011;168:265-275.
- Pettatini HM, Oslin D, Lampman KM, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatr. 2010;167:668-675.
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatr. 2011;168:709-717.
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582-1588.
- Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297-1306.
- Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatr. 2011;68:1168-1175.
- Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
- Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatr. 2012;169:805-812.
- Farrell M, Wodak A, Gowing L. Maintenance drugs to treat opioid dependence. BMJ. 2012;344.-
- Pinto H, Maskrey V, Swift L, et al. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39:340-352.
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515-532.
- Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303-1310.
- Clausen T, Anchersen K, Waal H. Mortality prior to during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151-157.
- Clark RE, Samnaliev M, Baxter JD, et al. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Affairs. 2011;30:1425-1433.
- Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction: Treatment Improvement Protocol (TIP) Series 40. Rockville Md: Substance Abuse and Mental Health Services Administration; 2004.
- Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949-958.
- Fiellin DA. Buprenorphine: effective treatment of opioid addiction starts in the office. Am Fam Physician. 2006;73:1513.-
- Minozzi S, Amato L, Vecchi S, et al. Oral naltrexone maintainance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;(4):CD001333.-
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513.
The ABCDEs of obstructive sleep apnea
Symptoms of sleep-disordered breathing range from primary snoring and upper airway resistance to obstructive sleep apnea (OSA). Psychiatric disorders and OSA frequently are comorbid. In a study of veterans with OSA, 22% had depression, 17% had anxiety, 12% had posttraumatic stress disorder, and 5% had psychosis.1 Treatments for OSA include dental devices, positive airway pressure ventilation, and surgery. Treating OSA often improves comorbid psychiatric disorders.2 However, medication-induced weight gain (eg, from antipsychotics) and hypnotics can worsen OSA. The mnemonic ABCDE can help you remember precipitating factors of OSA, associated sleep patterns, and complications of untreated OSA.
Precipitating factors
Age, gender, and race. OSA has a higher prevalence among middle-age men and the incidence of OSA gradually increases in postmenopausal women. African American patients also are at increased risk.
Bulkiness. Obesity is a significant risk factor for OSA, especially among middle-age men. Secondary fat deposition around the neck and decreased muscle tone and lung volume may lead to OSA.
Circumference of the neck. A neck circumference of >16 inches in women and >17 inches in men indicates a greater risk of developing OSA.3
Disrupted air flow. Airway narrowing can be present in patients with a small oropharynx, large tongue or uvula, backward tongue displacement, nasal obstruction, or craniofacial abnormalities.4 Certain medications (eg, muscle relaxants), alcohol, or hypothyroidism can reduce muscle tone and lead to OSA.5 Gastroesophageal reflux, asthma, pregnancy, stroke, and neuromuscular disease increase susceptibility to OSA. Patients with cardiac failure often have associated central sleep apnea.4
Extended family members. Patients with first-degree relatives who have OSA are at an increased risk of developing it themselves.5
Associated sleep patterns
Arousals. Intermittent nighttime sleep, non-restorative sleep, restless sleep, and insomnia are common among patients with OSA.5
Blocked airway and snoring. Snoring is common in OSA and signifies partial airway obstruction.
Choking, coughing, and gasping for air. As a result of decreased oxygenation, OSA patients usually wake up gasping for air. Associated gastroesophageal reflux also can cause cough.
Dry and/or open mouth. Most OSA patients breathe through their mouth because of obstruction in the upper airway.6 Patients often complain of dry mouth and morning thirst.
Excessive daytime sleepiness. Because of lack of nighttime sleep, it is common for individuals with OSA to feel tired during the day or want to nap.
Complications of untreated OSA
Anxiety and depression. There is a strong relationship between untreated OSA and psychiatric disorders, especially anxiety and depression in adults.1
Body mass index elevation or obesity. Frequent apneas are linked to an increase in leptin and ghrelin levels, which leads to increased appetite.4,5
Cardiovascular complications. Increased incidences of pulmonary or systemic hypertension, cardiac arrhythmias, myocardial infarctions, and strokes have been associated with untreated OSA.5
Daytime tiredness and sleepiness. Attention problems, tardiness, and accidents are common among patients with OSA.
Endocrine abnormalities. Individuals with moderate to severe OSA have a higher risk of developing diabetes mellitus and hypercholesterolemia.4
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
2. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.-
3. Victor LD. Obstructive sleep apnea. Am Fam Physician. 1999;60(8):2279-2286.
4. Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 5th ed. Philadelphia PA: Elsevier Saunders; 2010.
5. Al Lawati NM, Patel SR, Ayas NT. Epidemiology risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51(4):285-293.
6. Oksenberg A, Froom P, Melamed S. Dry mouth upon awakening in obstructive sleep apnea. J Sleep Res. 2006;15(3):317-320.
Symptoms of sleep-disordered breathing range from primary snoring and upper airway resistance to obstructive sleep apnea (OSA). Psychiatric disorders and OSA frequently are comorbid. In a study of veterans with OSA, 22% had depression, 17% had anxiety, 12% had posttraumatic stress disorder, and 5% had psychosis.1 Treatments for OSA include dental devices, positive airway pressure ventilation, and surgery. Treating OSA often improves comorbid psychiatric disorders.2 However, medication-induced weight gain (eg, from antipsychotics) and hypnotics can worsen OSA. The mnemonic ABCDE can help you remember precipitating factors of OSA, associated sleep patterns, and complications of untreated OSA.
Precipitating factors
Age, gender, and race. OSA has a higher prevalence among middle-age men and the incidence of OSA gradually increases in postmenopausal women. African American patients also are at increased risk.
Bulkiness. Obesity is a significant risk factor for OSA, especially among middle-age men. Secondary fat deposition around the neck and decreased muscle tone and lung volume may lead to OSA.
Circumference of the neck. A neck circumference of >16 inches in women and >17 inches in men indicates a greater risk of developing OSA.3
Disrupted air flow. Airway narrowing can be present in patients with a small oropharynx, large tongue or uvula, backward tongue displacement, nasal obstruction, or craniofacial abnormalities.4 Certain medications (eg, muscle relaxants), alcohol, or hypothyroidism can reduce muscle tone and lead to OSA.5 Gastroesophageal reflux, asthma, pregnancy, stroke, and neuromuscular disease increase susceptibility to OSA. Patients with cardiac failure often have associated central sleep apnea.4
Extended family members. Patients with first-degree relatives who have OSA are at an increased risk of developing it themselves.5
Associated sleep patterns
Arousals. Intermittent nighttime sleep, non-restorative sleep, restless sleep, and insomnia are common among patients with OSA.5
Blocked airway and snoring. Snoring is common in OSA and signifies partial airway obstruction.
Choking, coughing, and gasping for air. As a result of decreased oxygenation, OSA patients usually wake up gasping for air. Associated gastroesophageal reflux also can cause cough.
Dry and/or open mouth. Most OSA patients breathe through their mouth because of obstruction in the upper airway.6 Patients often complain of dry mouth and morning thirst.
Excessive daytime sleepiness. Because of lack of nighttime sleep, it is common for individuals with OSA to feel tired during the day or want to nap.
Complications of untreated OSA
Anxiety and depression. There is a strong relationship between untreated OSA and psychiatric disorders, especially anxiety and depression in adults.1
Body mass index elevation or obesity. Frequent apneas are linked to an increase in leptin and ghrelin levels, which leads to increased appetite.4,5
Cardiovascular complications. Increased incidences of pulmonary or systemic hypertension, cardiac arrhythmias, myocardial infarctions, and strokes have been associated with untreated OSA.5
Daytime tiredness and sleepiness. Attention problems, tardiness, and accidents are common among patients with OSA.
Endocrine abnormalities. Individuals with moderate to severe OSA have a higher risk of developing diabetes mellitus and hypercholesterolemia.4
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Symptoms of sleep-disordered breathing range from primary snoring and upper airway resistance to obstructive sleep apnea (OSA). Psychiatric disorders and OSA frequently are comorbid. In a study of veterans with OSA, 22% had depression, 17% had anxiety, 12% had posttraumatic stress disorder, and 5% had psychosis.1 Treatments for OSA include dental devices, positive airway pressure ventilation, and surgery. Treating OSA often improves comorbid psychiatric disorders.2 However, medication-induced weight gain (eg, from antipsychotics) and hypnotics can worsen OSA. The mnemonic ABCDE can help you remember precipitating factors of OSA, associated sleep patterns, and complications of untreated OSA.
Precipitating factors
Age, gender, and race. OSA has a higher prevalence among middle-age men and the incidence of OSA gradually increases in postmenopausal women. African American patients also are at increased risk.
Bulkiness. Obesity is a significant risk factor for OSA, especially among middle-age men. Secondary fat deposition around the neck and decreased muscle tone and lung volume may lead to OSA.
Circumference of the neck. A neck circumference of >16 inches in women and >17 inches in men indicates a greater risk of developing OSA.3
Disrupted air flow. Airway narrowing can be present in patients with a small oropharynx, large tongue or uvula, backward tongue displacement, nasal obstruction, or craniofacial abnormalities.4 Certain medications (eg, muscle relaxants), alcohol, or hypothyroidism can reduce muscle tone and lead to OSA.5 Gastroesophageal reflux, asthma, pregnancy, stroke, and neuromuscular disease increase susceptibility to OSA. Patients with cardiac failure often have associated central sleep apnea.4
Extended family members. Patients with first-degree relatives who have OSA are at an increased risk of developing it themselves.5
Associated sleep patterns
Arousals. Intermittent nighttime sleep, non-restorative sleep, restless sleep, and insomnia are common among patients with OSA.5
Blocked airway and snoring. Snoring is common in OSA and signifies partial airway obstruction.
Choking, coughing, and gasping for air. As a result of decreased oxygenation, OSA patients usually wake up gasping for air. Associated gastroesophageal reflux also can cause cough.
Dry and/or open mouth. Most OSA patients breathe through their mouth because of obstruction in the upper airway.6 Patients often complain of dry mouth and morning thirst.
Excessive daytime sleepiness. Because of lack of nighttime sleep, it is common for individuals with OSA to feel tired during the day or want to nap.
Complications of untreated OSA
Anxiety and depression. There is a strong relationship between untreated OSA and psychiatric disorders, especially anxiety and depression in adults.1
Body mass index elevation or obesity. Frequent apneas are linked to an increase in leptin and ghrelin levels, which leads to increased appetite.4,5
Cardiovascular complications. Increased incidences of pulmonary or systemic hypertension, cardiac arrhythmias, myocardial infarctions, and strokes have been associated with untreated OSA.5
Daytime tiredness and sleepiness. Attention problems, tardiness, and accidents are common among patients with OSA.
Endocrine abnormalities. Individuals with moderate to severe OSA have a higher risk of developing diabetes mellitus and hypercholesterolemia.4
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
2. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.-
3. Victor LD. Obstructive sleep apnea. Am Fam Physician. 1999;60(8):2279-2286.
4. Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 5th ed. Philadelphia PA: Elsevier Saunders; 2010.
5. Al Lawati NM, Patel SR, Ayas NT. Epidemiology risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51(4):285-293.
6. Oksenberg A, Froom P, Melamed S. Dry mouth upon awakening in obstructive sleep apnea. J Sleep Res. 2006;15(3):317-320.
1. Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411.
2. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.-
3. Victor LD. Obstructive sleep apnea. Am Fam Physician. 1999;60(8):2279-2286.
4. Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 5th ed. Philadelphia PA: Elsevier Saunders; 2010.
5. Al Lawati NM, Patel SR, Ayas NT. Epidemiology risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51(4):285-293.
6. Oksenberg A, Froom P, Melamed S. Dry mouth upon awakening in obstructive sleep apnea. J Sleep Res. 2006;15(3):317-320.
Antipsychotics for nonpsychotic illness
Second-generation antipsychotics (SGAs) represent 5% of all U.S. drug expenditures.1 Their use for indications not approved by the FDA (“off-label” use) increased to a total of $6 billion in 2008, $5.4 billion of which was for uses with limited or uncertain evidence.1
Off-label use of antipsychotics usually is based on novel applications of known receptor binding affinities (Table 1).2-5 For example, antipsychotics with strong antihistamine effects may promote sedation and could be used to treat insomnia. Clinicians also might use antipsychotics to treat a specific symptom of an illness when other treatment options are limited6 or when patients do not respond to standard treatments.
Table 1
Possible rationales for antipsychotic use for nonpsychotic conditions
| Condition | Possible rationale |
|---|---|
| Insomnia2 | Effects on H1 α-1 adrenergic and muscarinic cholinergic receptors. 5-HT2 antagonism activity also has been implicated |
| Tics of Tourette’s disorder3 | By blocking dopamine receptors antipsychotics decrease the primarily dopaminergic input from the substantia nigra and ventral tegmentum to the basal ganglia |
| Delirium4 | Patients have reversible impairment of cerebral oxidative metabolism and multiple neurotransmitter abnormalities (dopamine acetylcholine CNS γ-aminobutyric acid and serotonin). Other hypotheses include inflammatory reactions damage to certain structural pathways and disruption of cortisol and β-endorphin circadian rhythms |
| Stuttering5 | Stutterers have a marked increase in dopaminergic afferent activity in the tail of the left caudate nucleus compared with healthy controls |
| H1: histamine | |
To safely use any medication off-label, clinicians should become familiar with literature on the proposed use. Clinicians should consider off-label use only after carefully weighing the potential therapeutic benefits against the risks. Patients should be aware that the prescribed use is not FDA-approved and informed consent should include a discussion of alternative treatments. The high cost of SGAs may be a limiting factor and should be discussed with patients.
This article reviews the evidence for using antipsychotics to treat insomnia, tics, delirium, and stuttering (Table 2). Click here for a review of the evidence supporting antipsychotics for treating migraine and cluster headaches and nausea
Table 2
Antipsychotics for nonpsychotic disorders: Strength of the evidence
| Condition | Strength of evidencea |
|---|---|
| Insomnia | Weak to intermediate: Haloperidol olanzapine quetiapine risperidone ziprasidone |
| Tics of Tourette’s disorder | Strong: Haloperidol pimozide |
| Intermediate: Chlorpromazine fluphenazine penfluridol perphenazine thioridazine trifluoperazine | |
| Weak: Risperidone | |
| Very weak: Aripiprazole olanzapine quetiapine ziprasidone | |
| Not effective: Clozapine | |
| Delirium | Intermediate: Haloperidol |
| Weak: Olanzapine quetiapine risperidone | |
| Very weak: Aripiprazole ziprasidone | |
| Stuttering | Very weak: Chlorpromazine haloperidol olanzapine risperidone |
| aStrong: Multiple well-designed RCTs directly relevant to the recommendation yielding consistent findings Intermediate: Some evidence from RCTs that support the recommendation but the scientific support was not optimal Weak: Consensus recommendation in the absence of relevant RCTs and better evidence than case report or series Very weak: Case reports case series or preliminary studies RCTs: randomized controlled trials INSOMNIA Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233-246. Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111-123. Cohrs S, Meier A, Neumann AC, et al. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66(8):989-996. Cohrs S, Rodenbeck A, Guan Z, et al. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology. 2004;174(3):421-429. Juri C, Chaná P, Tapia J, et al. Quetiapine for insomnia in Parkinson’s disease: results from an open-label trial. Clin Neuropharmacol. 2005;28(4):185-187. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161. Sharpley AL, Vassallo CM, Cowen PJ. Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry. 2000;47(5):468-470. Terán A, Majadas S, Galan J. Quetiapine in the treatment of sleep disturbances associated with addictive conditions: a retrospective study. Subst Use Misuse. 2008;43(14):2169-2171. Wiegand MH, Landry F, Brückner T, et al. Quetiapine in primary insomnia: a pilot study. Psychopharmacology (Berl). 2008;196(2):337-338. TICS OF TOURETTE’S DISORDER Abuzzahab FS, Anderson FO. Gilles de la Tourette’s syndrome: international registry. Minn Med. 1973;56(6):492-496. Borison RL, Ang L, Chang S, et al. New pharmacological approaches in the treatment of Tourette’s syndrome. Adv Neurol. 1982;35:377-382. Bubl E, Perlov E, Tebartz Van Elst L. Aripiprazole in patients with Tourette syndrome. World Biol J Psychiatry. 2006;7(2):123-125. Caine ED, Polinsky RJ, Kartzinel R, et al. The trial use of clozapine for abnormal involuntary movement disorders. Am J Psychiatry. 1979;136(3):317-320. Dion Y, Annable L, Sabdor P, et al. Risperidone in the treatment of Tourette’s syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22(1):31-39. McCracken JT, Suddath R, Chang S, et al. Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette’s syndrome. J Child Adolesc Psychopharmacol. 2008;18(5):501-508. Mikkelsen EJ, Detlor J, Cohen DJ. School avoidance and social phobia triggered by haloperidol in patients with Tourette’s disorder. Am J Psychiatry. 1981;138(12):1572-1576. Murphy TK, Bengston MA, Soto O, et al. Case series on the use of aripiprazole for Tourette syndrome. Int J Neuropsychopharmacol. 2005;8(3):489-490. Párraga HC, Párraga M, Woodward R, et al. Quetiapine treatment of children with Tourette’s syndrome: report of two cases. J Child Adolesc Psychopharmacol. 2001;11(2):187-191. Regeur L, Pakkenberg B, Fog R, et al. Clinical features and long-term treatment with pimozide in 65 patients with Gilles de la Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 1986;49(7):791-795. Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299. Sallee FR, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154(8):1057-1062. Scahill L, Leckman JF, Schultz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003; 60(7):1130-1135. Shapiro AK, Shapiro E, Eisenkraft GJ. Treatment of Tourette’s disorder with penfluridol. Compr Psychiatry. 1983;24(4): 327-331. Shapiro AK, Shapiro E, Wayne HL. Treatment of Tourette’s syndrome with haloperidol: review of 34 cases. Arch Gen Psychiatry. 1973;28(1):92-96. Shapiro AK, Shapiro E, Young JG, et al. Gilles de la Tourette’s syndrome. 2nd ed. New York, NY: Raven Press; 1998:387-390. Stephens RJ, Bassel C, Sandor P. Olanzapine in the treatment of aggression and tics in children with Tourette’s syndrome-a pilot study. J Child Adolesc Psychopharmacol. 2004;14(2):255-266. DELIRIUM Alao AO, Moskowitz L. Aripiprazole and delirium. Ann Clin Psychiatry. 2006;18(4):267-269. Al-Samarrai S, Dunn J, Newmark T, et al. Quetiapine for treatment-resistant delirium. Psychosomatics. 2003;44(4): 350-351. Bourgeois JA, Hilty DM. Prolonged delirium managed with risperidone. Psychosomatics. 2005;46(1):90-91. Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43(3):175-182. Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419-427. Hans CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment for delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry. 2003;25(4):289-292. Hu H, Deng W, Yang H. A prospective random control study comparison of olanzapine and haloperidol in senile delirium [in Chinese]. Chong’qing Medical Journal. 2004;8:1234-1237. Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40(11):1966-1973. Leso L, Schwartz TL. Ziprasidone treatment of delirium. Psychosomatics. 2002;43(1):61-62. Parellada E, Baeza I, de Pablo J, et al. Risperidone in the treatment of patients with delirium. J Clin Psychiatry. 2004;65(3):348-353. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64(11):1316-1321. Sipahimalani A, Masand PS. Olanzapine in the treatment of delirium. Psychosomatics. 1998;39(5):422-430. Sipahimalani A, Masand PS. Use of risperidone in delirium: case reports. Ann Clin Psychiatry. 1997;9(2):105-107. Straker DA, Shapiro PA, Muskin PR. Aripiprazole in the treatment of delirium. Psychosomatics. 2006;47(5):385-391. Young CC, Lujan E. Intravenous ziprasidone for treatment of delirium in the intensive care unit. Anesthesiology. 2004;101(3): 794-795. STUTTERING Burr HG, Mullendore JM. Recent investigations on tranquilizers and stuttering. J Speech Hear Disord. 1960;25;33-37. Lavid N, Franklin DL, Maguire GA. Management of child and adolescent stuttering with olanzapine: three case reports. Ann Clin Psychiatry. 1999;11(4):233-236. Tapia F. Haldol in the treatment of children with tics and stutterers and an incidental finding. Behav Neuropsychiatry. 1969;1(3):28. van Wattum PJ. Stuttering improved with risperidone. J Am Acad Child Adolesc Psychiatry. 2006;45(2):133. | |
Current use of antipsychotics
Antipsychotics are divided into 2 major classes—first-generation antipsychotics (FGAs) and SGAs—and principally are FDA-approved for treating schizophrenia. Some antipsychotics have received FDA approval for maintenance treatment of schizophrenia and bipolar disorder (BD), and others have been approved to treat tic disorders (haloperidol and pimozide).
To varying degrees, all antipsychotics block D2 receptors, which is thought to be necessary for treating psychosis. However, some SGAs have significant affinity at other receptors—such as 5-HT2A and 5-HT1A—that confer additional properties that are not fully understood (Table 3). For example, it is believed that 5-HT2A blockade in the striatum reduces the potential for extrapyramidal symptoms (EPS).
Each antipsychotic blocks a unique set of receptors in the brain, leading to a specific set of intended and potentially untoward effects. For example, olanzapine’s effect on psychosis largely stems from its action at the D2 receptor, whereas its sedative and anticholinergic properties are a result of activity at histamine (H1) receptors and muscarinic receptors, respectively. Clinicians can make rational use of unintended effects by carefully selecting a medication based on receptor binding profile (eg, using an antipsychotic with sedating properties in a patient who has psychosis and insomnia). This approach can limit use of multiple medications and maximize a medication’s known effects while attempting to minimize side effects.
Table 3
Antipsychotics: Receptor pharmacology and common side effects
| Antipsychotic | Pharmacology | Common side effectsa |
|---|---|---|
| Prochlorperazinea,b | D2 receptor antagonist and α-1 adrenergic receptor antagonism | EPS, akathisia, prolactinemia, orthostatic hypotension, altered cardiac conduction, agranulocytosis, sexual dysfunction |
| Chlorpromazinea,b | D2 receptor antagonist. Also binds to H1 and cholinergic M1 | EPS, akathisia, prolactinemia, orthostatic hypotension, urinary retention, non-specific QT changes, agranulocytosis, sexual dysfunction |
| Droperidola,b | D2 receptor antagonist and antagonist at peripheral α-1 activity | EPS, akathisia, prolactinemia, orthostatic hypotension, urinary retention, QT changes (dose dependent) |
| Haloperidola,b | D2 receptor antagonist. Also binds to D1, 5-HT2, H1, and α-2 adrenergic receptors | EPS, akathisia, prolactinemia, QT changes (dose dependent) |
| Aripiprazolea,c,d | D2 and 5-HT1A partial agonism, 5-HT2A antagonism | Akathisia, EPS, sedation, restlessness, insomnia, tremor, anxiety, nausea, vomiting, possible weight gain (20% to 30%) |
| Clozapinea,c,e | 5-HT2, D1, D2, D3, D4, M1, H1, α-1, and α-2 antagonism | Sedation, dizziness, tachycardia, weight gain, nausea, vomiting, constipation |
| Olanzapinea,c | 5-HT2A, 5-HT2C, D1, D2, D3, D4, M1-5, H1, and α1- antagonism | Sedation, EPS, prolactinemia, weight gain, constipation |
| Quetiapinea,c,d | D1, D2, 5-HT2A, 5-HT1A, H1, α-1, and α-2 antagonism | Sedation, orthostatic hypotension, weight gain, triglyceride abnormalities, hypertension (frequently diastolic), constipation |
| Risperidonea,c | 5-HT2, D2, H1, α-1, and α-2 antagonism | Sedation, akathisia, EPS, prolactinemia, weight gain, tremor |
| Ziprasidonea,c | D2, D3, 5-HT2A, 5-HT2C, 5-HT1D, and α-1 antagonism; moderate inhibition of 5-HT and NE reuptake; 5-HT1A agonism | EPS, sedation, headache, dizziness, nausea |
| aSide effects and their prominence usually are based on receptor binding profile. All antipsychotics to varying degrees share the following symptoms: EPS, neuroleptic malignant syndrome, QTc prolongation, anticholinergic side effects (urinary retention, decreased gastrointestinal motility, xerostomia), sedation, orthostatic hypotension, blood dyscrasias, and problems with temperature regulation. The class as a whole also carries a “black-box” warning regarding increased mortality when treating geriatric patients with psychosis related to dementia bNo frequencies were available cOnly side effects with frequency >10% listed d”Black-box” warning for suicidal ideation and behavior in children, adolescents, and young adults (age 18 to 24) with major depressive disorder and other psychiatric disorders e”Black-box” warnings for agranulocytosis, myocarditis, orthostatic hypotension, seizure risk EPS: extrapyramidal symptoms; H1: histamine; M1: muscarinic; NE: norepinephrine | ||
Insomnia
Clinicians use FGAs and SGAs to treat insomnia because of their sedating effects, although evidence supporting this use is questionable. Among the FGAs, chlorpromazine produces moderate to severe sedation, whereas haloperidol is only mildly sedating. Clozapine is believed to be the most sedating SGA, whereas quetiapine and olanzapine produce moderate sedation.7
Most data on antipsychotics’ sedating effects comes from studies completed for schizophrenia or BD. Few studies have evaluated using antipsychotics to treat primary insomnia or other sleep disorders in otherwise healthy patients.2 However, data from phase I studies of antipsychotics has shown that schizophrenia patients tolerate a higher maximum dose compared with healthy volunteers, who often experience more sedation.
An antipsychotic’s potential for sedation is directly related to its affinity at H1 receptors and total drug concentration at the H1 receptor binding site. Because drugs with lower affinity for D2 receptors typically are prescribed at higher doses when treating psychiatric illness, the corresponding concentration at H1 receptors can lead to greater sedation compared with equivalent doses of higher-potency agents.
The same phenomenon is seen with high-potency agents. Haloperidol has a relatively weak binding affinity to the H1 receptor,8 but causes more sedation at higher doses. Haloperidol, 20 mg/d, produces sedation in more patients than a moderate dose of risperidone, 2 to 10 mg/d.8 These observations correlate with “the high milligram-low-potency” spectrum seen with FGAs.7
Among SGAs, a double-blind, placebo-controlled, crossover study of the effects of ziprasidone, 40 mg/d, on sleep in a group of healthy volunteers found a significant increase in total sleep time and sleep efficiency.9 A double-blind trial compared patients taking low, medium, or high daily doses of olanzapine with patients receiving haloperidol or placebo.10 Sedation was reported in 20% of patients taking low doses of olanzapine (5 ± 2.5 mg/d) compared with 29.7% on medium doses (10 ± 2.5 mg/d) and 39.1% on high doses (15 ± 2.5 mg/d).10
A double-blind, placebo-controlled, crossover study demonstrated that olanzapine produced significant increases in sleep continuity, slow wave sleep, and subjective ratings of sleep quality in healthy men.11 Similarly, a study comparing haloperidol, 12 mg/d, and quetiapine, 75 to 750 mg/d, for treating acute schizophrenia found an 8% to 11% incidence of somnolence in the quetiapine group compared with 6% and 8% in the haloperidol and placebo groups, respectively.12 Somnolence was reported as an adverse event in these studies, which were designed to examine the drug’s effect on acute schizophrenia and did not evaluate its effect on sleep.
A double-blind, placebo-controlled, crossover study examining quetiapine’s effects on sleep in 14 healthy patients demonstrated a significant difference in total sleep time, sleep period time, and sleep efficiency.13 Similarly, an open-label pilot study of quetiapine’s effect on primary insomnia showed significant improvement in total sleep time and sleep efficiency.14
Studies examining quetiapine’s effects on insomnia in patients with substance abuse15 and women with localized breast cancer16 showed improved sleep scores on multiple assessment tools, while an open-label study of quetiapine for Parkinson’s disease demonstrated decreased sleep latency.17 Adjunctive quetiapine administered over a 6-week, open-label trial in veterans with posttraumatic stress disorder revealed significant improvement from baseline in sleep quality and duration and diminished dreaming.18
Sedating antipsychotics such as thioridazine and chlorpromazine historically were used off-label for insomnia, but fell out of favor because of their associated cardiac risks. More recently, clinicians have been using SGAs in a similar manner19 even though SGAs are costly and have significant risks such as metabolic problems.
Studies supporting the use of SGAs for the short-term or long-term treatment of insomnia are limited by small sample sizes or open-label designs.20 In 2005 the National Institutes of Health State-of-the-Science Conference Panel did not recommend using SGAs for treating chronic insomnia.21
Tics in Tourette’s disorder
FGAs and SGAs have been used to treat tics associated with Tourette’s disorder (TD).22 Haloperidol is FDA-approved for treating tics in adult and pediatric patients with TD. Many studies have reported the efficacy of haloperidol in this population; however, cognitive blunting, weight gain, lethargy, and akathisia limit its use.23
Pimozide, the most widely used alternative to haloperidol for treating TD, can cause clinically significant QTc prolongation and sudden death. Penfluridol demonstrated significant symptomatic improvement compared with haloperidol in 1 study, but its carcinogenic potential limits its use.24
A double-blind, placebo-controlled study comparing fluphenazine and trifluoperazine with haloperidol for treating TD showed that both are significantly more effective than placebo, but none was more effective than the others.25 Studies show chlorpromazine, perphenazine, and thioridazine are less effective than haloperidol and their use is limited by photosensitivity, dermatitis, EPS, and blood and liver dyscrasias.26
Risperidone is superior to placebo for treating tics associated with TD.27 A placebo-controlled trial of ziprasidone showed the drug has efficacy similar to risperidone in reducing tics in children and adolescents with TD.28 However, ziprasidone is not FDA-approved for this use.
Evidence supporting the use of other SGAs for treating TD is more limited. Several small studies of olanzapine and aripiprazole had limited but favorable results. Quetiapine has not been studied for treating TD, but several case reports have indicated a positive response. In a double-blind, placebo-controlled trial, clozapine showed no therapeutic benefit for TD.29
Delirium
American Psychiatric Association practice guidelines suggest using psychotropic medications to treat neuropsychiatric symptoms of delirium.30 Antipsychotics are considered first-line agents that lower hospital mortality rates, decrease lengths of hospital stays, and improve delirium symptoms, in some cases before the underlying medical etiologies resolve.30,31 Available in liquid, oral, IM, and IV formulations, haloperidol is the mainstay of symptomatic treatment of delirium.31 Although not FDA-approved, it is recommended by the Society of Critical Care Medicine as a safe, cost-effective, and efficacious therapy for the psychiatric symptoms associated with delirium.
The most extensively studied SGA for treating delirium, risperidone often is used as an alternative to haloperidol. Case reports describe its potential efficacy.32 In a head-to-head study, risperidone was as effective as low-dose haloperidol for acute delirium treatment.33
Olanzapine was effective in managing delirium in several case studies.34 Also, in a 7-day, randomized, placebo-controlled study, olanzapine and haloperidol showed significantly greater and relatively equivalent improvement compared with placebo; patients treated with olanzapine experienced more rapid improvement in 1 study.35
Case reports and prospective studies also have described quetiapine as effective for treating delirium.36,37 In a prospective, double-blind, placebo-controlled study, patients taking quetiapine had a faster resolution of delirium with reduced overall duration and less agitation than those taking placebo.37 Mortality, intensive care unit length of stay, and incidence of QTc prolongation did not differ, but patients treated with quetiapine were more likely to have increased somnolence and were more frequently discharged to home or rehabilitation centers. One limitation of the study is that concomitant haloperidol use on an “as needed” basis was permitted.38
Evidence supporting the efficacy of ziprasidone for delirium is limited to case reports.39 In 1 case report, a patient with chronic HIV infection and acute cryptococcal meningitis experienced significant improvement of delirium symptoms but could not continue ziprasidone because of fluctuating QTc intervals.40
In 2 patients with delirium, aripiprazole, 15 and 30 mg/d, improved confusion, disorientation, and agitation within 7 days.41 In another study of delirium, 13 of 14 patients on flexibly dosed aripiprazole (5 to 15 mg/d) showed improvement in Clinical Global Impressions Scale scores, although 3 patients developed prolonged QTc intervals.42
Stuttering or stammering
Stuttering or stammering are age-inappropriate disturbances in normal fluency and time patterning of speech. The evidence for antipsychotics to treat stuttering or stammering speech mainly consists of case reports and does not include disfluency frequency data, which makes it difficult to accept claims of efficacy. Disfluency frequency data describe how often a patient has specific disfluencies (blocks, prolongations, interjection, and repetition of syllables, words, or phrases).
Two FGAs (chlorpromazine and haloperidol) and 2 SGAs (risperidone and olanzapine) have been evaluated for treating stuttering. Children were 2.5 times more likely to demonstrate significant improvement when taking chlorpromazine vs placebo.43 An open-label study of haloperidol lacked disfluency frequency data, therefore casting doubts on haloperidol’s reported efficacy in the study.44
In a case report, a 4-year-old boy with severe behavioral dyscontrol showed complete remission of stammering after 1 day of risperidone, 0.25 mg/d.45 The patient’s symptoms reappeared several days after the drug was stopped. In a case series of 2 patients with developmental stuttering, 1 patient reported significant improvement in fluency with olanzapine, 2.5 mg/d, and the other showed marked improvement in fluency with 5 mg/d.46
Related Resources
- Sipahimalani A, Masand PS. Use of risperidone in delirium: case reports. Ann Clin Psychiatry. 1997;9(2):105-107.
- Shapiro AK, Shapiro E, Wayne HL. Treatment of Tourette’s syndrome with haloperidol: review of 34 cases. Arch Gen Psychiatry. 1973;28(1):92-96.
- Sipahimalani A, Masand PS. Olanzapine in the treatment of delirium. Psychosomatics. 1998;39(5):422-430.
Drug Brand Names
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Permitil, Prolixin
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Pimozide • Orap
- Prochlorperazine • Compazine
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Trifluoperazine • Stelazine
- Ziprasidone • Geodon
Disclosure
Dr. Macaluso has received grant or research support from EnVivo Pharmaceuticals, Janssen, L.P., and Pfizer, Inc.
Dr. Tripathi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. DeMartinis N, Winokur A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord Drug Targets. 2007;6(1):17-29.
3. Leckman JF, Bloch MH, Smith ME, et al. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):237-247.
4. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
5. Wu JC, Maguire G, Riley G, et al. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8(3):767-770.
6. Devulapalli K, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
7. Miller DD. Atypical antipsychotics: sleep sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):3-7.
8. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
9. Cohrs S, Meier A, Neumann AC, et al. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66(8):989-996.
10. Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111-123.
11. Sharpley AL, Vassallo CM, Cowen PJ. Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry. 2000;47(5):468-470.
12. Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233-246.
13. Cohrs S, Rodenbeck A, Guan Z, et al. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology. 2004;174(3):421-429.
14. Wiegand MH, Landry F, Brückner T, et al. Quetiapine in primary insomnia: a pilot study. Psychopharmacology (Berl). 2008;196(2):337-338.
15. Terán A, Majadas S, Galan J. Quetiapine in the treatment of sleep disturbances associated with addictive conditions: a retrospective study. Subst Use Misuse. 2008;43(14):2169-2171.
16. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
17. Juri C, Chaná P, Tapia J, et al. Quetiapine for insomnia in Parkinson’s disease: results from an open-label trial. Clin Neuropharmacol. 2005;28(4):185-187.
18. Robert S, Hamner MB, Kose S, et al. Quetiapine improves sleep disturbances in combat veterans with PTSD: sleep data from a prospective, open-label study. J Clin Psychopharmacol. 2005;25(4):387-388.
19. Wilson S, Nutt D. Management of insomnia: treatments and mechanisms. Br J Psychiatry. 2007;191:195-197.
20. Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129-1141.
21. National Institutes of Health. National Institutes of Health State of the Science Conference statement on manifestations and management of chronic insomnia in adults June 13-15, 2005. Sleep. 2005;28(9):1049-1057.
22. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
23. Mikkelsen EJ, Detlor J, Cohen DJ. School avoidance and social phobia triggered by haloperidol in patients with Tourette’s disorder. Am J Psychiatry. 1981;138(12):1572-1576.
24. Shapiro AK, Shapiro E, Eisenkraft GJ. Treatment of Tourette’s disorder with penfluridol. Compr Psychiatry. 1983;24(4):327-331.
25. Borison RL, Ang L, Chang S, et al. New pharmacological approaches in the treatment of Tourette’s syndrome. Adv Neurol. 1982;35:377-382.
26. Shapiro AK, Shapiro E, Young JG, et al. Gilles de la Tourette’s syndrome. 2nd ed. New York, NY: Raven Press; 1998:387–390.
27. Dion Y, Annable L, Sabdor P, et al. Risperidone in the treatment of Tourette’s syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22(1):31-39.
28. Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
29. Caine ED, Polinsky RJ, Kartzinel R, et al. The trial use of clozapine for abnormal involuntary movement disorders. Am J Psychiatry. 1979;136(3):317-320.
30. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
31. Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40(11):1966-1973.
32. Parellada E, Baeza I, de Pablo J, et al. Risperidone in the treatment of patients with delirium. J Clin Psychiatry. 2004;65(3):348-353.
33. Hans CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
34. Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43(3):175-182.
35. Hu H, Deng W, Yang H. A prospective random control study comparison of olanzapine and haloperidol in senile delirium [in Chinese]. Chong’qing Medical Journal. 2004;8:1234-1237.
36. Al-Samarrai S, Dunn J, Newmark T, et al. Quetiapine for treatment-resistant delirium. Psychosomatics. 2003;44(4):350-351.
37. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64(11):1316-1321.
38. Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419-427.
39. Young CC, Lujan E. Intravenous ziprasidone for treatment of delirium in the intensive care unit. Anesthesiology. 2004;101(3):794-795.
40. Leso L, Schwartz TL. Ziprasidone treatment of delirium. Psychosomatics. 2002;43(1):61-62.
41. Alao AO, Moskowitz L. Aripiprazole and delirium. Ann Clin Psychiatry. 2006;18(4):267-269.
42. Straker DA, Shapiro PA, Muskin PR. Aripiprazole in the treatment of delirium. Psychosomatics. 2006;47(5):385-391.
43. Burr HG, Mullendore JM. Recent investigations on tranquilizers and stuttering. J Speech Hear Disord. 1960;25:33-37.
44. Tapia F. Haldol in the treatment of children with tics and stutterers and an incidental finding. Behav Neuropsychiatry. 1969;1(3):28.-
45. van Wattum PJ. Stuttering improved with risperidone. J Am Acad Child Adolesc Psychiatry. 2006;45(2):133.-
46. Lavid N, Franklin DL, Maguire GA. Management of child and adolescent stuttering with olanzapine: three case reports. Ann Clin Psychiatry. 1999;11(4):233-236.
Second-generation antipsychotics (SGAs) represent 5% of all U.S. drug expenditures.1 Their use for indications not approved by the FDA (“off-label” use) increased to a total of $6 billion in 2008, $5.4 billion of which was for uses with limited or uncertain evidence.1
Off-label use of antipsychotics usually is based on novel applications of known receptor binding affinities (Table 1).2-5 For example, antipsychotics with strong antihistamine effects may promote sedation and could be used to treat insomnia. Clinicians also might use antipsychotics to treat a specific symptom of an illness when other treatment options are limited6 or when patients do not respond to standard treatments.
Table 1
Possible rationales for antipsychotic use for nonpsychotic conditions
| Condition | Possible rationale |
|---|---|
| Insomnia2 | Effects on H1 α-1 adrenergic and muscarinic cholinergic receptors. 5-HT2 antagonism activity also has been implicated |
| Tics of Tourette’s disorder3 | By blocking dopamine receptors antipsychotics decrease the primarily dopaminergic input from the substantia nigra and ventral tegmentum to the basal ganglia |
| Delirium4 | Patients have reversible impairment of cerebral oxidative metabolism and multiple neurotransmitter abnormalities (dopamine acetylcholine CNS γ-aminobutyric acid and serotonin). Other hypotheses include inflammatory reactions damage to certain structural pathways and disruption of cortisol and β-endorphin circadian rhythms |
| Stuttering5 | Stutterers have a marked increase in dopaminergic afferent activity in the tail of the left caudate nucleus compared with healthy controls |
| H1: histamine | |
To safely use any medication off-label, clinicians should become familiar with literature on the proposed use. Clinicians should consider off-label use only after carefully weighing the potential therapeutic benefits against the risks. Patients should be aware that the prescribed use is not FDA-approved and informed consent should include a discussion of alternative treatments. The high cost of SGAs may be a limiting factor and should be discussed with patients.
This article reviews the evidence for using antipsychotics to treat insomnia, tics, delirium, and stuttering (Table 2). Click here for a review of the evidence supporting antipsychotics for treating migraine and cluster headaches and nausea
Table 2
Antipsychotics for nonpsychotic disorders: Strength of the evidence
| Condition | Strength of evidencea |
|---|---|
| Insomnia | Weak to intermediate: Haloperidol olanzapine quetiapine risperidone ziprasidone |
| Tics of Tourette’s disorder | Strong: Haloperidol pimozide |
| Intermediate: Chlorpromazine fluphenazine penfluridol perphenazine thioridazine trifluoperazine | |
| Weak: Risperidone | |
| Very weak: Aripiprazole olanzapine quetiapine ziprasidone | |
| Not effective: Clozapine | |
| Delirium | Intermediate: Haloperidol |
| Weak: Olanzapine quetiapine risperidone | |
| Very weak: Aripiprazole ziprasidone | |
| Stuttering | Very weak: Chlorpromazine haloperidol olanzapine risperidone |
| aStrong: Multiple well-designed RCTs directly relevant to the recommendation yielding consistent findings Intermediate: Some evidence from RCTs that support the recommendation but the scientific support was not optimal Weak: Consensus recommendation in the absence of relevant RCTs and better evidence than case report or series Very weak: Case reports case series or preliminary studies RCTs: randomized controlled trials INSOMNIA Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233-246. Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111-123. Cohrs S, Meier A, Neumann AC, et al. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66(8):989-996. Cohrs S, Rodenbeck A, Guan Z, et al. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology. 2004;174(3):421-429. Juri C, Chaná P, Tapia J, et al. Quetiapine for insomnia in Parkinson’s disease: results from an open-label trial. Clin Neuropharmacol. 2005;28(4):185-187. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161. Sharpley AL, Vassallo CM, Cowen PJ. Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry. 2000;47(5):468-470. Terán A, Majadas S, Galan J. Quetiapine in the treatment of sleep disturbances associated with addictive conditions: a retrospective study. Subst Use Misuse. 2008;43(14):2169-2171. Wiegand MH, Landry F, Brückner T, et al. Quetiapine in primary insomnia: a pilot study. Psychopharmacology (Berl). 2008;196(2):337-338. TICS OF TOURETTE’S DISORDER Abuzzahab FS, Anderson FO. Gilles de la Tourette’s syndrome: international registry. Minn Med. 1973;56(6):492-496. Borison RL, Ang L, Chang S, et al. New pharmacological approaches in the treatment of Tourette’s syndrome. Adv Neurol. 1982;35:377-382. Bubl E, Perlov E, Tebartz Van Elst L. Aripiprazole in patients with Tourette syndrome. World Biol J Psychiatry. 2006;7(2):123-125. Caine ED, Polinsky RJ, Kartzinel R, et al. The trial use of clozapine for abnormal involuntary movement disorders. Am J Psychiatry. 1979;136(3):317-320. Dion Y, Annable L, Sabdor P, et al. Risperidone in the treatment of Tourette’s syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22(1):31-39. McCracken JT, Suddath R, Chang S, et al. Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette’s syndrome. J Child Adolesc Psychopharmacol. 2008;18(5):501-508. Mikkelsen EJ, Detlor J, Cohen DJ. School avoidance and social phobia triggered by haloperidol in patients with Tourette’s disorder. Am J Psychiatry. 1981;138(12):1572-1576. Murphy TK, Bengston MA, Soto O, et al. Case series on the use of aripiprazole for Tourette syndrome. Int J Neuropsychopharmacol. 2005;8(3):489-490. Párraga HC, Párraga M, Woodward R, et al. Quetiapine treatment of children with Tourette’s syndrome: report of two cases. J Child Adolesc Psychopharmacol. 2001;11(2):187-191. Regeur L, Pakkenberg B, Fog R, et al. Clinical features and long-term treatment with pimozide in 65 patients with Gilles de la Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 1986;49(7):791-795. Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299. Sallee FR, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154(8):1057-1062. Scahill L, Leckman JF, Schultz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003; 60(7):1130-1135. Shapiro AK, Shapiro E, Eisenkraft GJ. Treatment of Tourette’s disorder with penfluridol. Compr Psychiatry. 1983;24(4): 327-331. Shapiro AK, Shapiro E, Wayne HL. Treatment of Tourette’s syndrome with haloperidol: review of 34 cases. Arch Gen Psychiatry. 1973;28(1):92-96. Shapiro AK, Shapiro E, Young JG, et al. Gilles de la Tourette’s syndrome. 2nd ed. New York, NY: Raven Press; 1998:387-390. Stephens RJ, Bassel C, Sandor P. Olanzapine in the treatment of aggression and tics in children with Tourette’s syndrome-a pilot study. J Child Adolesc Psychopharmacol. 2004;14(2):255-266. DELIRIUM Alao AO, Moskowitz L. Aripiprazole and delirium. Ann Clin Psychiatry. 2006;18(4):267-269. Al-Samarrai S, Dunn J, Newmark T, et al. Quetiapine for treatment-resistant delirium. Psychosomatics. 2003;44(4): 350-351. Bourgeois JA, Hilty DM. Prolonged delirium managed with risperidone. Psychosomatics. 2005;46(1):90-91. Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43(3):175-182. Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419-427. Hans CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment for delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry. 2003;25(4):289-292. Hu H, Deng W, Yang H. A prospective random control study comparison of olanzapine and haloperidol in senile delirium [in Chinese]. Chong’qing Medical Journal. 2004;8:1234-1237. Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40(11):1966-1973. Leso L, Schwartz TL. Ziprasidone treatment of delirium. Psychosomatics. 2002;43(1):61-62. Parellada E, Baeza I, de Pablo J, et al. Risperidone in the treatment of patients with delirium. J Clin Psychiatry. 2004;65(3):348-353. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64(11):1316-1321. Sipahimalani A, Masand PS. Olanzapine in the treatment of delirium. Psychosomatics. 1998;39(5):422-430. Sipahimalani A, Masand PS. Use of risperidone in delirium: case reports. Ann Clin Psychiatry. 1997;9(2):105-107. Straker DA, Shapiro PA, Muskin PR. Aripiprazole in the treatment of delirium. Psychosomatics. 2006;47(5):385-391. Young CC, Lujan E. Intravenous ziprasidone for treatment of delirium in the intensive care unit. Anesthesiology. 2004;101(3): 794-795. STUTTERING Burr HG, Mullendore JM. Recent investigations on tranquilizers and stuttering. J Speech Hear Disord. 1960;25;33-37. Lavid N, Franklin DL, Maguire GA. Management of child and adolescent stuttering with olanzapine: three case reports. Ann Clin Psychiatry. 1999;11(4):233-236. Tapia F. Haldol in the treatment of children with tics and stutterers and an incidental finding. Behav Neuropsychiatry. 1969;1(3):28. van Wattum PJ. Stuttering improved with risperidone. J Am Acad Child Adolesc Psychiatry. 2006;45(2):133. | |
Current use of antipsychotics
Antipsychotics are divided into 2 major classes—first-generation antipsychotics (FGAs) and SGAs—and principally are FDA-approved for treating schizophrenia. Some antipsychotics have received FDA approval for maintenance treatment of schizophrenia and bipolar disorder (BD), and others have been approved to treat tic disorders (haloperidol and pimozide).
To varying degrees, all antipsychotics block D2 receptors, which is thought to be necessary for treating psychosis. However, some SGAs have significant affinity at other receptors—such as 5-HT2A and 5-HT1A—that confer additional properties that are not fully understood (Table 3). For example, it is believed that 5-HT2A blockade in the striatum reduces the potential for extrapyramidal symptoms (EPS).
Each antipsychotic blocks a unique set of receptors in the brain, leading to a specific set of intended and potentially untoward effects. For example, olanzapine’s effect on psychosis largely stems from its action at the D2 receptor, whereas its sedative and anticholinergic properties are a result of activity at histamine (H1) receptors and muscarinic receptors, respectively. Clinicians can make rational use of unintended effects by carefully selecting a medication based on receptor binding profile (eg, using an antipsychotic with sedating properties in a patient who has psychosis and insomnia). This approach can limit use of multiple medications and maximize a medication’s known effects while attempting to minimize side effects.
Table 3
Antipsychotics: Receptor pharmacology and common side effects
| Antipsychotic | Pharmacology | Common side effectsa |
|---|---|---|
| Prochlorperazinea,b | D2 receptor antagonist and α-1 adrenergic receptor antagonism | EPS, akathisia, prolactinemia, orthostatic hypotension, altered cardiac conduction, agranulocytosis, sexual dysfunction |
| Chlorpromazinea,b | D2 receptor antagonist. Also binds to H1 and cholinergic M1 | EPS, akathisia, prolactinemia, orthostatic hypotension, urinary retention, non-specific QT changes, agranulocytosis, sexual dysfunction |
| Droperidola,b | D2 receptor antagonist and antagonist at peripheral α-1 activity | EPS, akathisia, prolactinemia, orthostatic hypotension, urinary retention, QT changes (dose dependent) |
| Haloperidola,b | D2 receptor antagonist. Also binds to D1, 5-HT2, H1, and α-2 adrenergic receptors | EPS, akathisia, prolactinemia, QT changes (dose dependent) |
| Aripiprazolea,c,d | D2 and 5-HT1A partial agonism, 5-HT2A antagonism | Akathisia, EPS, sedation, restlessness, insomnia, tremor, anxiety, nausea, vomiting, possible weight gain (20% to 30%) |
| Clozapinea,c,e | 5-HT2, D1, D2, D3, D4, M1, H1, α-1, and α-2 antagonism | Sedation, dizziness, tachycardia, weight gain, nausea, vomiting, constipation |
| Olanzapinea,c | 5-HT2A, 5-HT2C, D1, D2, D3, D4, M1-5, H1, and α1- antagonism | Sedation, EPS, prolactinemia, weight gain, constipation |
| Quetiapinea,c,d | D1, D2, 5-HT2A, 5-HT1A, H1, α-1, and α-2 antagonism | Sedation, orthostatic hypotension, weight gain, triglyceride abnormalities, hypertension (frequently diastolic), constipation |
| Risperidonea,c | 5-HT2, D2, H1, α-1, and α-2 antagonism | Sedation, akathisia, EPS, prolactinemia, weight gain, tremor |
| Ziprasidonea,c | D2, D3, 5-HT2A, 5-HT2C, 5-HT1D, and α-1 antagonism; moderate inhibition of 5-HT and NE reuptake; 5-HT1A agonism | EPS, sedation, headache, dizziness, nausea |
| aSide effects and their prominence usually are based on receptor binding profile. All antipsychotics to varying degrees share the following symptoms: EPS, neuroleptic malignant syndrome, QTc prolongation, anticholinergic side effects (urinary retention, decreased gastrointestinal motility, xerostomia), sedation, orthostatic hypotension, blood dyscrasias, and problems with temperature regulation. The class as a whole also carries a “black-box” warning regarding increased mortality when treating geriatric patients with psychosis related to dementia bNo frequencies were available cOnly side effects with frequency >10% listed d”Black-box” warning for suicidal ideation and behavior in children, adolescents, and young adults (age 18 to 24) with major depressive disorder and other psychiatric disorders e”Black-box” warnings for agranulocytosis, myocarditis, orthostatic hypotension, seizure risk EPS: extrapyramidal symptoms; H1: histamine; M1: muscarinic; NE: norepinephrine | ||
Insomnia
Clinicians use FGAs and SGAs to treat insomnia because of their sedating effects, although evidence supporting this use is questionable. Among the FGAs, chlorpromazine produces moderate to severe sedation, whereas haloperidol is only mildly sedating. Clozapine is believed to be the most sedating SGA, whereas quetiapine and olanzapine produce moderate sedation.7
Most data on antipsychotics’ sedating effects comes from studies completed for schizophrenia or BD. Few studies have evaluated using antipsychotics to treat primary insomnia or other sleep disorders in otherwise healthy patients.2 However, data from phase I studies of antipsychotics has shown that schizophrenia patients tolerate a higher maximum dose compared with healthy volunteers, who often experience more sedation.
An antipsychotic’s potential for sedation is directly related to its affinity at H1 receptors and total drug concentration at the H1 receptor binding site. Because drugs with lower affinity for D2 receptors typically are prescribed at higher doses when treating psychiatric illness, the corresponding concentration at H1 receptors can lead to greater sedation compared with equivalent doses of higher-potency agents.
The same phenomenon is seen with high-potency agents. Haloperidol has a relatively weak binding affinity to the H1 receptor,8 but causes more sedation at higher doses. Haloperidol, 20 mg/d, produces sedation in more patients than a moderate dose of risperidone, 2 to 10 mg/d.8 These observations correlate with “the high milligram-low-potency” spectrum seen with FGAs.7
Among SGAs, a double-blind, placebo-controlled, crossover study of the effects of ziprasidone, 40 mg/d, on sleep in a group of healthy volunteers found a significant increase in total sleep time and sleep efficiency.9 A double-blind trial compared patients taking low, medium, or high daily doses of olanzapine with patients receiving haloperidol or placebo.10 Sedation was reported in 20% of patients taking low doses of olanzapine (5 ± 2.5 mg/d) compared with 29.7% on medium doses (10 ± 2.5 mg/d) and 39.1% on high doses (15 ± 2.5 mg/d).10
A double-blind, placebo-controlled, crossover study demonstrated that olanzapine produced significant increases in sleep continuity, slow wave sleep, and subjective ratings of sleep quality in healthy men.11 Similarly, a study comparing haloperidol, 12 mg/d, and quetiapine, 75 to 750 mg/d, for treating acute schizophrenia found an 8% to 11% incidence of somnolence in the quetiapine group compared with 6% and 8% in the haloperidol and placebo groups, respectively.12 Somnolence was reported as an adverse event in these studies, which were designed to examine the drug’s effect on acute schizophrenia and did not evaluate its effect on sleep.
A double-blind, placebo-controlled, crossover study examining quetiapine’s effects on sleep in 14 healthy patients demonstrated a significant difference in total sleep time, sleep period time, and sleep efficiency.13 Similarly, an open-label pilot study of quetiapine’s effect on primary insomnia showed significant improvement in total sleep time and sleep efficiency.14
Studies examining quetiapine’s effects on insomnia in patients with substance abuse15 and women with localized breast cancer16 showed improved sleep scores on multiple assessment tools, while an open-label study of quetiapine for Parkinson’s disease demonstrated decreased sleep latency.17 Adjunctive quetiapine administered over a 6-week, open-label trial in veterans with posttraumatic stress disorder revealed significant improvement from baseline in sleep quality and duration and diminished dreaming.18
Sedating antipsychotics such as thioridazine and chlorpromazine historically were used off-label for insomnia, but fell out of favor because of their associated cardiac risks. More recently, clinicians have been using SGAs in a similar manner19 even though SGAs are costly and have significant risks such as metabolic problems.
Studies supporting the use of SGAs for the short-term or long-term treatment of insomnia are limited by small sample sizes or open-label designs.20 In 2005 the National Institutes of Health State-of-the-Science Conference Panel did not recommend using SGAs for treating chronic insomnia.21
Tics in Tourette’s disorder
FGAs and SGAs have been used to treat tics associated with Tourette’s disorder (TD).22 Haloperidol is FDA-approved for treating tics in adult and pediatric patients with TD. Many studies have reported the efficacy of haloperidol in this population; however, cognitive blunting, weight gain, lethargy, and akathisia limit its use.23
Pimozide, the most widely used alternative to haloperidol for treating TD, can cause clinically significant QTc prolongation and sudden death. Penfluridol demonstrated significant symptomatic improvement compared with haloperidol in 1 study, but its carcinogenic potential limits its use.24
A double-blind, placebo-controlled study comparing fluphenazine and trifluoperazine with haloperidol for treating TD showed that both are significantly more effective than placebo, but none was more effective than the others.25 Studies show chlorpromazine, perphenazine, and thioridazine are less effective than haloperidol and their use is limited by photosensitivity, dermatitis, EPS, and blood and liver dyscrasias.26
Risperidone is superior to placebo for treating tics associated with TD.27 A placebo-controlled trial of ziprasidone showed the drug has efficacy similar to risperidone in reducing tics in children and adolescents with TD.28 However, ziprasidone is not FDA-approved for this use.
Evidence supporting the use of other SGAs for treating TD is more limited. Several small studies of olanzapine and aripiprazole had limited but favorable results. Quetiapine has not been studied for treating TD, but several case reports have indicated a positive response. In a double-blind, placebo-controlled trial, clozapine showed no therapeutic benefit for TD.29
Delirium
American Psychiatric Association practice guidelines suggest using psychotropic medications to treat neuropsychiatric symptoms of delirium.30 Antipsychotics are considered first-line agents that lower hospital mortality rates, decrease lengths of hospital stays, and improve delirium symptoms, in some cases before the underlying medical etiologies resolve.30,31 Available in liquid, oral, IM, and IV formulations, haloperidol is the mainstay of symptomatic treatment of delirium.31 Although not FDA-approved, it is recommended by the Society of Critical Care Medicine as a safe, cost-effective, and efficacious therapy for the psychiatric symptoms associated with delirium.
The most extensively studied SGA for treating delirium, risperidone often is used as an alternative to haloperidol. Case reports describe its potential efficacy.32 In a head-to-head study, risperidone was as effective as low-dose haloperidol for acute delirium treatment.33
Olanzapine was effective in managing delirium in several case studies.34 Also, in a 7-day, randomized, placebo-controlled study, olanzapine and haloperidol showed significantly greater and relatively equivalent improvement compared with placebo; patients treated with olanzapine experienced more rapid improvement in 1 study.35
Case reports and prospective studies also have described quetiapine as effective for treating delirium.36,37 In a prospective, double-blind, placebo-controlled study, patients taking quetiapine had a faster resolution of delirium with reduced overall duration and less agitation than those taking placebo.37 Mortality, intensive care unit length of stay, and incidence of QTc prolongation did not differ, but patients treated with quetiapine were more likely to have increased somnolence and were more frequently discharged to home or rehabilitation centers. One limitation of the study is that concomitant haloperidol use on an “as needed” basis was permitted.38
Evidence supporting the efficacy of ziprasidone for delirium is limited to case reports.39 In 1 case report, a patient with chronic HIV infection and acute cryptococcal meningitis experienced significant improvement of delirium symptoms but could not continue ziprasidone because of fluctuating QTc intervals.40
In 2 patients with delirium, aripiprazole, 15 and 30 mg/d, improved confusion, disorientation, and agitation within 7 days.41 In another study of delirium, 13 of 14 patients on flexibly dosed aripiprazole (5 to 15 mg/d) showed improvement in Clinical Global Impressions Scale scores, although 3 patients developed prolonged QTc intervals.42
Stuttering or stammering
Stuttering or stammering are age-inappropriate disturbances in normal fluency and time patterning of speech. The evidence for antipsychotics to treat stuttering or stammering speech mainly consists of case reports and does not include disfluency frequency data, which makes it difficult to accept claims of efficacy. Disfluency frequency data describe how often a patient has specific disfluencies (blocks, prolongations, interjection, and repetition of syllables, words, or phrases).
Two FGAs (chlorpromazine and haloperidol) and 2 SGAs (risperidone and olanzapine) have been evaluated for treating stuttering. Children were 2.5 times more likely to demonstrate significant improvement when taking chlorpromazine vs placebo.43 An open-label study of haloperidol lacked disfluency frequency data, therefore casting doubts on haloperidol’s reported efficacy in the study.44
In a case report, a 4-year-old boy with severe behavioral dyscontrol showed complete remission of stammering after 1 day of risperidone, 0.25 mg/d.45 The patient’s symptoms reappeared several days after the drug was stopped. In a case series of 2 patients with developmental stuttering, 1 patient reported significant improvement in fluency with olanzapine, 2.5 mg/d, and the other showed marked improvement in fluency with 5 mg/d.46
Related Resources
- Sipahimalani A, Masand PS. Use of risperidone in delirium: case reports. Ann Clin Psychiatry. 1997;9(2):105-107.
- Shapiro AK, Shapiro E, Wayne HL. Treatment of Tourette’s syndrome with haloperidol: review of 34 cases. Arch Gen Psychiatry. 1973;28(1):92-96.
- Sipahimalani A, Masand PS. Olanzapine in the treatment of delirium. Psychosomatics. 1998;39(5):422-430.
Drug Brand Names
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Permitil, Prolixin
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Pimozide • Orap
- Prochlorperazine • Compazine
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Trifluoperazine • Stelazine
- Ziprasidone • Geodon
Disclosure
Dr. Macaluso has received grant or research support from EnVivo Pharmaceuticals, Janssen, L.P., and Pfizer, Inc.
Dr. Tripathi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Second-generation antipsychotics (SGAs) represent 5% of all U.S. drug expenditures.1 Their use for indications not approved by the FDA (“off-label” use) increased to a total of $6 billion in 2008, $5.4 billion of which was for uses with limited or uncertain evidence.1
Off-label use of antipsychotics usually is based on novel applications of known receptor binding affinities (Table 1).2-5 For example, antipsychotics with strong antihistamine effects may promote sedation and could be used to treat insomnia. Clinicians also might use antipsychotics to treat a specific symptom of an illness when other treatment options are limited6 or when patients do not respond to standard treatments.
Table 1
Possible rationales for antipsychotic use for nonpsychotic conditions
| Condition | Possible rationale |
|---|---|
| Insomnia2 | Effects on H1 α-1 adrenergic and muscarinic cholinergic receptors. 5-HT2 antagonism activity also has been implicated |
| Tics of Tourette’s disorder3 | By blocking dopamine receptors antipsychotics decrease the primarily dopaminergic input from the substantia nigra and ventral tegmentum to the basal ganglia |
| Delirium4 | Patients have reversible impairment of cerebral oxidative metabolism and multiple neurotransmitter abnormalities (dopamine acetylcholine CNS γ-aminobutyric acid and serotonin). Other hypotheses include inflammatory reactions damage to certain structural pathways and disruption of cortisol and β-endorphin circadian rhythms |
| Stuttering5 | Stutterers have a marked increase in dopaminergic afferent activity in the tail of the left caudate nucleus compared with healthy controls |
| H1: histamine | |
To safely use any medication off-label, clinicians should become familiar with literature on the proposed use. Clinicians should consider off-label use only after carefully weighing the potential therapeutic benefits against the risks. Patients should be aware that the prescribed use is not FDA-approved and informed consent should include a discussion of alternative treatments. The high cost of SGAs may be a limiting factor and should be discussed with patients.
This article reviews the evidence for using antipsychotics to treat insomnia, tics, delirium, and stuttering (Table 2). Click here for a review of the evidence supporting antipsychotics for treating migraine and cluster headaches and nausea
Table 2
Antipsychotics for nonpsychotic disorders: Strength of the evidence
| Condition | Strength of evidencea |
|---|---|
| Insomnia | Weak to intermediate: Haloperidol olanzapine quetiapine risperidone ziprasidone |
| Tics of Tourette’s disorder | Strong: Haloperidol pimozide |
| Intermediate: Chlorpromazine fluphenazine penfluridol perphenazine thioridazine trifluoperazine | |
| Weak: Risperidone | |
| Very weak: Aripiprazole olanzapine quetiapine ziprasidone | |
| Not effective: Clozapine | |
| Delirium | Intermediate: Haloperidol |
| Weak: Olanzapine quetiapine risperidone | |
| Very weak: Aripiprazole ziprasidone | |
| Stuttering | Very weak: Chlorpromazine haloperidol olanzapine risperidone |
| aStrong: Multiple well-designed RCTs directly relevant to the recommendation yielding consistent findings Intermediate: Some evidence from RCTs that support the recommendation but the scientific support was not optimal Weak: Consensus recommendation in the absence of relevant RCTs and better evidence than case report or series Very weak: Case reports case series or preliminary studies RCTs: randomized controlled trials INSOMNIA Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233-246. Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111-123. Cohrs S, Meier A, Neumann AC, et al. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66(8):989-996. Cohrs S, Rodenbeck A, Guan Z, et al. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology. 2004;174(3):421-429. Juri C, Chaná P, Tapia J, et al. Quetiapine for insomnia in Parkinson’s disease: results from an open-label trial. Clin Neuropharmacol. 2005;28(4):185-187. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161. Sharpley AL, Vassallo CM, Cowen PJ. Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry. 2000;47(5):468-470. Terán A, Majadas S, Galan J. Quetiapine in the treatment of sleep disturbances associated with addictive conditions: a retrospective study. Subst Use Misuse. 2008;43(14):2169-2171. Wiegand MH, Landry F, Brückner T, et al. Quetiapine in primary insomnia: a pilot study. Psychopharmacology (Berl). 2008;196(2):337-338. TICS OF TOURETTE’S DISORDER Abuzzahab FS, Anderson FO. Gilles de la Tourette’s syndrome: international registry. Minn Med. 1973;56(6):492-496. Borison RL, Ang L, Chang S, et al. New pharmacological approaches in the treatment of Tourette’s syndrome. Adv Neurol. 1982;35:377-382. Bubl E, Perlov E, Tebartz Van Elst L. Aripiprazole in patients with Tourette syndrome. World Biol J Psychiatry. 2006;7(2):123-125. Caine ED, Polinsky RJ, Kartzinel R, et al. The trial use of clozapine for abnormal involuntary movement disorders. Am J Psychiatry. 1979;136(3):317-320. Dion Y, Annable L, Sabdor P, et al. Risperidone in the treatment of Tourette’s syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22(1):31-39. McCracken JT, Suddath R, Chang S, et al. Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette’s syndrome. J Child Adolesc Psychopharmacol. 2008;18(5):501-508. Mikkelsen EJ, Detlor J, Cohen DJ. School avoidance and social phobia triggered by haloperidol in patients with Tourette’s disorder. Am J Psychiatry. 1981;138(12):1572-1576. Murphy TK, Bengston MA, Soto O, et al. Case series on the use of aripiprazole for Tourette syndrome. Int J Neuropsychopharmacol. 2005;8(3):489-490. Párraga HC, Párraga M, Woodward R, et al. Quetiapine treatment of children with Tourette’s syndrome: report of two cases. J Child Adolesc Psychopharmacol. 2001;11(2):187-191. Regeur L, Pakkenberg B, Fog R, et al. Clinical features and long-term treatment with pimozide in 65 patients with Gilles de la Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 1986;49(7):791-795. Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299. Sallee FR, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry. 1997;154(8):1057-1062. Scahill L, Leckman JF, Schultz RT, et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003; 60(7):1130-1135. Shapiro AK, Shapiro E, Eisenkraft GJ. Treatment of Tourette’s disorder with penfluridol. Compr Psychiatry. 1983;24(4): 327-331. Shapiro AK, Shapiro E, Wayne HL. Treatment of Tourette’s syndrome with haloperidol: review of 34 cases. Arch Gen Psychiatry. 1973;28(1):92-96. Shapiro AK, Shapiro E, Young JG, et al. Gilles de la Tourette’s syndrome. 2nd ed. New York, NY: Raven Press; 1998:387-390. Stephens RJ, Bassel C, Sandor P. Olanzapine in the treatment of aggression and tics in children with Tourette’s syndrome-a pilot study. J Child Adolesc Psychopharmacol. 2004;14(2):255-266. DELIRIUM Alao AO, Moskowitz L. Aripiprazole and delirium. Ann Clin Psychiatry. 2006;18(4):267-269. Al-Samarrai S, Dunn J, Newmark T, et al. Quetiapine for treatment-resistant delirium. Psychosomatics. 2003;44(4): 350-351. Bourgeois JA, Hilty DM. Prolonged delirium managed with risperidone. Psychosomatics. 2005;46(1):90-91. Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43(3):175-182. Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419-427. Hans CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment for delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry. 2003;25(4):289-292. Hu H, Deng W, Yang H. A prospective random control study comparison of olanzapine and haloperidol in senile delirium [in Chinese]. Chong’qing Medical Journal. 2004;8:1234-1237. Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40(11):1966-1973. Leso L, Schwartz TL. Ziprasidone treatment of delirium. Psychosomatics. 2002;43(1):61-62. Parellada E, Baeza I, de Pablo J, et al. Risperidone in the treatment of patients with delirium. J Clin Psychiatry. 2004;65(3):348-353. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64(11):1316-1321. Sipahimalani A, Masand PS. Olanzapine in the treatment of delirium. Psychosomatics. 1998;39(5):422-430. Sipahimalani A, Masand PS. Use of risperidone in delirium: case reports. Ann Clin Psychiatry. 1997;9(2):105-107. Straker DA, Shapiro PA, Muskin PR. Aripiprazole in the treatment of delirium. Psychosomatics. 2006;47(5):385-391. Young CC, Lujan E. Intravenous ziprasidone for treatment of delirium in the intensive care unit. Anesthesiology. 2004;101(3): 794-795. STUTTERING Burr HG, Mullendore JM. Recent investigations on tranquilizers and stuttering. J Speech Hear Disord. 1960;25;33-37. Lavid N, Franklin DL, Maguire GA. Management of child and adolescent stuttering with olanzapine: three case reports. Ann Clin Psychiatry. 1999;11(4):233-236. Tapia F. Haldol in the treatment of children with tics and stutterers and an incidental finding. Behav Neuropsychiatry. 1969;1(3):28. van Wattum PJ. Stuttering improved with risperidone. J Am Acad Child Adolesc Psychiatry. 2006;45(2):133. | |
Current use of antipsychotics
Antipsychotics are divided into 2 major classes—first-generation antipsychotics (FGAs) and SGAs—and principally are FDA-approved for treating schizophrenia. Some antipsychotics have received FDA approval for maintenance treatment of schizophrenia and bipolar disorder (BD), and others have been approved to treat tic disorders (haloperidol and pimozide).
To varying degrees, all antipsychotics block D2 receptors, which is thought to be necessary for treating psychosis. However, some SGAs have significant affinity at other receptors—such as 5-HT2A and 5-HT1A—that confer additional properties that are not fully understood (Table 3). For example, it is believed that 5-HT2A blockade in the striatum reduces the potential for extrapyramidal symptoms (EPS).
Each antipsychotic blocks a unique set of receptors in the brain, leading to a specific set of intended and potentially untoward effects. For example, olanzapine’s effect on psychosis largely stems from its action at the D2 receptor, whereas its sedative and anticholinergic properties are a result of activity at histamine (H1) receptors and muscarinic receptors, respectively. Clinicians can make rational use of unintended effects by carefully selecting a medication based on receptor binding profile (eg, using an antipsychotic with sedating properties in a patient who has psychosis and insomnia). This approach can limit use of multiple medications and maximize a medication’s known effects while attempting to minimize side effects.
Table 3
Antipsychotics: Receptor pharmacology and common side effects
| Antipsychotic | Pharmacology | Common side effectsa |
|---|---|---|
| Prochlorperazinea,b | D2 receptor antagonist and α-1 adrenergic receptor antagonism | EPS, akathisia, prolactinemia, orthostatic hypotension, altered cardiac conduction, agranulocytosis, sexual dysfunction |
| Chlorpromazinea,b | D2 receptor antagonist. Also binds to H1 and cholinergic M1 | EPS, akathisia, prolactinemia, orthostatic hypotension, urinary retention, non-specific QT changes, agranulocytosis, sexual dysfunction |
| Droperidola,b | D2 receptor antagonist and antagonist at peripheral α-1 activity | EPS, akathisia, prolactinemia, orthostatic hypotension, urinary retention, QT changes (dose dependent) |
| Haloperidola,b | D2 receptor antagonist. Also binds to D1, 5-HT2, H1, and α-2 adrenergic receptors | EPS, akathisia, prolactinemia, QT changes (dose dependent) |
| Aripiprazolea,c,d | D2 and 5-HT1A partial agonism, 5-HT2A antagonism | Akathisia, EPS, sedation, restlessness, insomnia, tremor, anxiety, nausea, vomiting, possible weight gain (20% to 30%) |
| Clozapinea,c,e | 5-HT2, D1, D2, D3, D4, M1, H1, α-1, and α-2 antagonism | Sedation, dizziness, tachycardia, weight gain, nausea, vomiting, constipation |
| Olanzapinea,c | 5-HT2A, 5-HT2C, D1, D2, D3, D4, M1-5, H1, and α1- antagonism | Sedation, EPS, prolactinemia, weight gain, constipation |
| Quetiapinea,c,d | D1, D2, 5-HT2A, 5-HT1A, H1, α-1, and α-2 antagonism | Sedation, orthostatic hypotension, weight gain, triglyceride abnormalities, hypertension (frequently diastolic), constipation |
| Risperidonea,c | 5-HT2, D2, H1, α-1, and α-2 antagonism | Sedation, akathisia, EPS, prolactinemia, weight gain, tremor |
| Ziprasidonea,c | D2, D3, 5-HT2A, 5-HT2C, 5-HT1D, and α-1 antagonism; moderate inhibition of 5-HT and NE reuptake; 5-HT1A agonism | EPS, sedation, headache, dizziness, nausea |
| aSide effects and their prominence usually are based on receptor binding profile. All antipsychotics to varying degrees share the following symptoms: EPS, neuroleptic malignant syndrome, QTc prolongation, anticholinergic side effects (urinary retention, decreased gastrointestinal motility, xerostomia), sedation, orthostatic hypotension, blood dyscrasias, and problems with temperature regulation. The class as a whole also carries a “black-box” warning regarding increased mortality when treating geriatric patients with psychosis related to dementia bNo frequencies were available cOnly side effects with frequency >10% listed d”Black-box” warning for suicidal ideation and behavior in children, adolescents, and young adults (age 18 to 24) with major depressive disorder and other psychiatric disorders e”Black-box” warnings for agranulocytosis, myocarditis, orthostatic hypotension, seizure risk EPS: extrapyramidal symptoms; H1: histamine; M1: muscarinic; NE: norepinephrine | ||
Insomnia
Clinicians use FGAs and SGAs to treat insomnia because of their sedating effects, although evidence supporting this use is questionable. Among the FGAs, chlorpromazine produces moderate to severe sedation, whereas haloperidol is only mildly sedating. Clozapine is believed to be the most sedating SGA, whereas quetiapine and olanzapine produce moderate sedation.7
Most data on antipsychotics’ sedating effects comes from studies completed for schizophrenia or BD. Few studies have evaluated using antipsychotics to treat primary insomnia or other sleep disorders in otherwise healthy patients.2 However, data from phase I studies of antipsychotics has shown that schizophrenia patients tolerate a higher maximum dose compared with healthy volunteers, who often experience more sedation.
An antipsychotic’s potential for sedation is directly related to its affinity at H1 receptors and total drug concentration at the H1 receptor binding site. Because drugs with lower affinity for D2 receptors typically are prescribed at higher doses when treating psychiatric illness, the corresponding concentration at H1 receptors can lead to greater sedation compared with equivalent doses of higher-potency agents.
The same phenomenon is seen with high-potency agents. Haloperidol has a relatively weak binding affinity to the H1 receptor,8 but causes more sedation at higher doses. Haloperidol, 20 mg/d, produces sedation in more patients than a moderate dose of risperidone, 2 to 10 mg/d.8 These observations correlate with “the high milligram-low-potency” spectrum seen with FGAs.7
Among SGAs, a double-blind, placebo-controlled, crossover study of the effects of ziprasidone, 40 mg/d, on sleep in a group of healthy volunteers found a significant increase in total sleep time and sleep efficiency.9 A double-blind trial compared patients taking low, medium, or high daily doses of olanzapine with patients receiving haloperidol or placebo.10 Sedation was reported in 20% of patients taking low doses of olanzapine (5 ± 2.5 mg/d) compared with 29.7% on medium doses (10 ± 2.5 mg/d) and 39.1% on high doses (15 ± 2.5 mg/d).10
A double-blind, placebo-controlled, crossover study demonstrated that olanzapine produced significant increases in sleep continuity, slow wave sleep, and subjective ratings of sleep quality in healthy men.11 Similarly, a study comparing haloperidol, 12 mg/d, and quetiapine, 75 to 750 mg/d, for treating acute schizophrenia found an 8% to 11% incidence of somnolence in the quetiapine group compared with 6% and 8% in the haloperidol and placebo groups, respectively.12 Somnolence was reported as an adverse event in these studies, which were designed to examine the drug’s effect on acute schizophrenia and did not evaluate its effect on sleep.
A double-blind, placebo-controlled, crossover study examining quetiapine’s effects on sleep in 14 healthy patients demonstrated a significant difference in total sleep time, sleep period time, and sleep efficiency.13 Similarly, an open-label pilot study of quetiapine’s effect on primary insomnia showed significant improvement in total sleep time and sleep efficiency.14
Studies examining quetiapine’s effects on insomnia in patients with substance abuse15 and women with localized breast cancer16 showed improved sleep scores on multiple assessment tools, while an open-label study of quetiapine for Parkinson’s disease demonstrated decreased sleep latency.17 Adjunctive quetiapine administered over a 6-week, open-label trial in veterans with posttraumatic stress disorder revealed significant improvement from baseline in sleep quality and duration and diminished dreaming.18
Sedating antipsychotics such as thioridazine and chlorpromazine historically were used off-label for insomnia, but fell out of favor because of their associated cardiac risks. More recently, clinicians have been using SGAs in a similar manner19 even though SGAs are costly and have significant risks such as metabolic problems.
Studies supporting the use of SGAs for the short-term or long-term treatment of insomnia are limited by small sample sizes or open-label designs.20 In 2005 the National Institutes of Health State-of-the-Science Conference Panel did not recommend using SGAs for treating chronic insomnia.21
Tics in Tourette’s disorder
FGAs and SGAs have been used to treat tics associated with Tourette’s disorder (TD).22 Haloperidol is FDA-approved for treating tics in adult and pediatric patients with TD. Many studies have reported the efficacy of haloperidol in this population; however, cognitive blunting, weight gain, lethargy, and akathisia limit its use.23
Pimozide, the most widely used alternative to haloperidol for treating TD, can cause clinically significant QTc prolongation and sudden death. Penfluridol demonstrated significant symptomatic improvement compared with haloperidol in 1 study, but its carcinogenic potential limits its use.24
A double-blind, placebo-controlled study comparing fluphenazine and trifluoperazine with haloperidol for treating TD showed that both are significantly more effective than placebo, but none was more effective than the others.25 Studies show chlorpromazine, perphenazine, and thioridazine are less effective than haloperidol and their use is limited by photosensitivity, dermatitis, EPS, and blood and liver dyscrasias.26
Risperidone is superior to placebo for treating tics associated with TD.27 A placebo-controlled trial of ziprasidone showed the drug has efficacy similar to risperidone in reducing tics in children and adolescents with TD.28 However, ziprasidone is not FDA-approved for this use.
Evidence supporting the use of other SGAs for treating TD is more limited. Several small studies of olanzapine and aripiprazole had limited but favorable results. Quetiapine has not been studied for treating TD, but several case reports have indicated a positive response. In a double-blind, placebo-controlled trial, clozapine showed no therapeutic benefit for TD.29
Delirium
American Psychiatric Association practice guidelines suggest using psychotropic medications to treat neuropsychiatric symptoms of delirium.30 Antipsychotics are considered first-line agents that lower hospital mortality rates, decrease lengths of hospital stays, and improve delirium symptoms, in some cases before the underlying medical etiologies resolve.30,31 Available in liquid, oral, IM, and IV formulations, haloperidol is the mainstay of symptomatic treatment of delirium.31 Although not FDA-approved, it is recommended by the Society of Critical Care Medicine as a safe, cost-effective, and efficacious therapy for the psychiatric symptoms associated with delirium.
The most extensively studied SGA for treating delirium, risperidone often is used as an alternative to haloperidol. Case reports describe its potential efficacy.32 In a head-to-head study, risperidone was as effective as low-dose haloperidol for acute delirium treatment.33
Olanzapine was effective in managing delirium in several case studies.34 Also, in a 7-day, randomized, placebo-controlled study, olanzapine and haloperidol showed significantly greater and relatively equivalent improvement compared with placebo; patients treated with olanzapine experienced more rapid improvement in 1 study.35
Case reports and prospective studies also have described quetiapine as effective for treating delirium.36,37 In a prospective, double-blind, placebo-controlled study, patients taking quetiapine had a faster resolution of delirium with reduced overall duration and less agitation than those taking placebo.37 Mortality, intensive care unit length of stay, and incidence of QTc prolongation did not differ, but patients treated with quetiapine were more likely to have increased somnolence and were more frequently discharged to home or rehabilitation centers. One limitation of the study is that concomitant haloperidol use on an “as needed” basis was permitted.38
Evidence supporting the efficacy of ziprasidone for delirium is limited to case reports.39 In 1 case report, a patient with chronic HIV infection and acute cryptococcal meningitis experienced significant improvement of delirium symptoms but could not continue ziprasidone because of fluctuating QTc intervals.40
In 2 patients with delirium, aripiprazole, 15 and 30 mg/d, improved confusion, disorientation, and agitation within 7 days.41 In another study of delirium, 13 of 14 patients on flexibly dosed aripiprazole (5 to 15 mg/d) showed improvement in Clinical Global Impressions Scale scores, although 3 patients developed prolonged QTc intervals.42
Stuttering or stammering
Stuttering or stammering are age-inappropriate disturbances in normal fluency and time patterning of speech. The evidence for antipsychotics to treat stuttering or stammering speech mainly consists of case reports and does not include disfluency frequency data, which makes it difficult to accept claims of efficacy. Disfluency frequency data describe how often a patient has specific disfluencies (blocks, prolongations, interjection, and repetition of syllables, words, or phrases).
Two FGAs (chlorpromazine and haloperidol) and 2 SGAs (risperidone and olanzapine) have been evaluated for treating stuttering. Children were 2.5 times more likely to demonstrate significant improvement when taking chlorpromazine vs placebo.43 An open-label study of haloperidol lacked disfluency frequency data, therefore casting doubts on haloperidol’s reported efficacy in the study.44
In a case report, a 4-year-old boy with severe behavioral dyscontrol showed complete remission of stammering after 1 day of risperidone, 0.25 mg/d.45 The patient’s symptoms reappeared several days after the drug was stopped. In a case series of 2 patients with developmental stuttering, 1 patient reported significant improvement in fluency with olanzapine, 2.5 mg/d, and the other showed marked improvement in fluency with 5 mg/d.46
Related Resources
- Sipahimalani A, Masand PS. Use of risperidone in delirium: case reports. Ann Clin Psychiatry. 1997;9(2):105-107.
- Shapiro AK, Shapiro E, Wayne HL. Treatment of Tourette’s syndrome with haloperidol: review of 34 cases. Arch Gen Psychiatry. 1973;28(1):92-96.
- Sipahimalani A, Masand PS. Olanzapine in the treatment of delirium. Psychosomatics. 1998;39(5):422-430.
Drug Brand Names
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Permitil, Prolixin
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Pimozide • Orap
- Prochlorperazine • Compazine
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Trifluoperazine • Stelazine
- Ziprasidone • Geodon
Disclosure
Dr. Macaluso has received grant or research support from EnVivo Pharmaceuticals, Janssen, L.P., and Pfizer, Inc.
Dr. Tripathi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. DeMartinis N, Winokur A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord Drug Targets. 2007;6(1):17-29.
3. Leckman JF, Bloch MH, Smith ME, et al. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):237-247.
4. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
5. Wu JC, Maguire G, Riley G, et al. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8(3):767-770.
6. Devulapalli K, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
7. Miller DD. Atypical antipsychotics: sleep sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):3-7.
8. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
9. Cohrs S, Meier A, Neumann AC, et al. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66(8):989-996.
10. Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111-123.
11. Sharpley AL, Vassallo CM, Cowen PJ. Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry. 2000;47(5):468-470.
12. Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233-246.
13. Cohrs S, Rodenbeck A, Guan Z, et al. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology. 2004;174(3):421-429.
14. Wiegand MH, Landry F, Brückner T, et al. Quetiapine in primary insomnia: a pilot study. Psychopharmacology (Berl). 2008;196(2):337-338.
15. Terán A, Majadas S, Galan J. Quetiapine in the treatment of sleep disturbances associated with addictive conditions: a retrospective study. Subst Use Misuse. 2008;43(14):2169-2171.
16. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
17. Juri C, Chaná P, Tapia J, et al. Quetiapine for insomnia in Parkinson’s disease: results from an open-label trial. Clin Neuropharmacol. 2005;28(4):185-187.
18. Robert S, Hamner MB, Kose S, et al. Quetiapine improves sleep disturbances in combat veterans with PTSD: sleep data from a prospective, open-label study. J Clin Psychopharmacol. 2005;25(4):387-388.
19. Wilson S, Nutt D. Management of insomnia: treatments and mechanisms. Br J Psychiatry. 2007;191:195-197.
20. Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129-1141.
21. National Institutes of Health. National Institutes of Health State of the Science Conference statement on manifestations and management of chronic insomnia in adults June 13-15, 2005. Sleep. 2005;28(9):1049-1057.
22. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
23. Mikkelsen EJ, Detlor J, Cohen DJ. School avoidance and social phobia triggered by haloperidol in patients with Tourette’s disorder. Am J Psychiatry. 1981;138(12):1572-1576.
24. Shapiro AK, Shapiro E, Eisenkraft GJ. Treatment of Tourette’s disorder with penfluridol. Compr Psychiatry. 1983;24(4):327-331.
25. Borison RL, Ang L, Chang S, et al. New pharmacological approaches in the treatment of Tourette’s syndrome. Adv Neurol. 1982;35:377-382.
26. Shapiro AK, Shapiro E, Young JG, et al. Gilles de la Tourette’s syndrome. 2nd ed. New York, NY: Raven Press; 1998:387–390.
27. Dion Y, Annable L, Sabdor P, et al. Risperidone in the treatment of Tourette’s syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22(1):31-39.
28. Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
29. Caine ED, Polinsky RJ, Kartzinel R, et al. The trial use of clozapine for abnormal involuntary movement disorders. Am J Psychiatry. 1979;136(3):317-320.
30. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
31. Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40(11):1966-1973.
32. Parellada E, Baeza I, de Pablo J, et al. Risperidone in the treatment of patients with delirium. J Clin Psychiatry. 2004;65(3):348-353.
33. Hans CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
34. Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43(3):175-182.
35. Hu H, Deng W, Yang H. A prospective random control study comparison of olanzapine and haloperidol in senile delirium [in Chinese]. Chong’qing Medical Journal. 2004;8:1234-1237.
36. Al-Samarrai S, Dunn J, Newmark T, et al. Quetiapine for treatment-resistant delirium. Psychosomatics. 2003;44(4):350-351.
37. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64(11):1316-1321.
38. Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419-427.
39. Young CC, Lujan E. Intravenous ziprasidone for treatment of delirium in the intensive care unit. Anesthesiology. 2004;101(3):794-795.
40. Leso L, Schwartz TL. Ziprasidone treatment of delirium. Psychosomatics. 2002;43(1):61-62.
41. Alao AO, Moskowitz L. Aripiprazole and delirium. Ann Clin Psychiatry. 2006;18(4):267-269.
42. Straker DA, Shapiro PA, Muskin PR. Aripiprazole in the treatment of delirium. Psychosomatics. 2006;47(5):385-391.
43. Burr HG, Mullendore JM. Recent investigations on tranquilizers and stuttering. J Speech Hear Disord. 1960;25:33-37.
44. Tapia F. Haldol in the treatment of children with tics and stutterers and an incidental finding. Behav Neuropsychiatry. 1969;1(3):28.-
45. van Wattum PJ. Stuttering improved with risperidone. J Am Acad Child Adolesc Psychiatry. 2006;45(2):133.-
46. Lavid N, Franklin DL, Maguire GA. Management of child and adolescent stuttering with olanzapine: three case reports. Ann Clin Psychiatry. 1999;11(4):233-236.
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. DeMartinis N, Winokur A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord Drug Targets. 2007;6(1):17-29.
3. Leckman JF, Bloch MH, Smith ME, et al. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):237-247.
4. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856.
5. Wu JC, Maguire G, Riley G, et al. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8(3):767-770.
6. Devulapalli K, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
7. Miller DD. Atypical antipsychotics: sleep sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):3-7.
8. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
9. Cohrs S, Meier A, Neumann AC, et al. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66(8):989-996.
10. Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111-123.
11. Sharpley AL, Vassallo CM, Cowen PJ. Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry. 2000;47(5):468-470.
12. Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233-246.
13. Cohrs S, Rodenbeck A, Guan Z, et al. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology. 2004;174(3):421-429.
14. Wiegand MH, Landry F, Brückner T, et al. Quetiapine in primary insomnia: a pilot study. Psychopharmacology (Berl). 2008;196(2):337-338.
15. Terán A, Majadas S, Galan J. Quetiapine in the treatment of sleep disturbances associated with addictive conditions: a retrospective study. Subst Use Misuse. 2008;43(14):2169-2171.
16. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
17. Juri C, Chaná P, Tapia J, et al. Quetiapine for insomnia in Parkinson’s disease: results from an open-label trial. Clin Neuropharmacol. 2005;28(4):185-187.
18. Robert S, Hamner MB, Kose S, et al. Quetiapine improves sleep disturbances in combat veterans with PTSD: sleep data from a prospective, open-label study. J Clin Psychopharmacol. 2005;25(4):387-388.
19. Wilson S, Nutt D. Management of insomnia: treatments and mechanisms. Br J Psychiatry. 2007;191:195-197.
20. Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129-1141.
21. National Institutes of Health. National Institutes of Health State of the Science Conference statement on manifestations and management of chronic insomnia in adults June 13-15, 2005. Sleep. 2005;28(9):1049-1057.
22. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
23. Mikkelsen EJ, Detlor J, Cohen DJ. School avoidance and social phobia triggered by haloperidol in patients with Tourette’s disorder. Am J Psychiatry. 1981;138(12):1572-1576.
24. Shapiro AK, Shapiro E, Eisenkraft GJ. Treatment of Tourette’s disorder with penfluridol. Compr Psychiatry. 1983;24(4):327-331.
25. Borison RL, Ang L, Chang S, et al. New pharmacological approaches in the treatment of Tourette’s syndrome. Adv Neurol. 1982;35:377-382.
26. Shapiro AK, Shapiro E, Young JG, et al. Gilles de la Tourette’s syndrome. 2nd ed. New York, NY: Raven Press; 1998:387–390.
27. Dion Y, Annable L, Sabdor P, et al. Risperidone in the treatment of Tourette’s syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22(1):31-39.
28. Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
29. Caine ED, Polinsky RJ, Kartzinel R, et al. The trial use of clozapine for abnormal involuntary movement disorders. Am J Psychiatry. 1979;136(3):317-320.
30. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
31. Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40(11):1966-1973.
32. Parellada E, Baeza I, de Pablo J, et al. Risperidone in the treatment of patients with delirium. J Clin Psychiatry. 2004;65(3):348-353.
33. Hans CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297-301.
34. Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43(3):175-182.
35. Hu H, Deng W, Yang H. A prospective random control study comparison of olanzapine and haloperidol in senile delirium [in Chinese]. Chong’qing Medical Journal. 2004;8:1234-1237.
36. Al-Samarrai S, Dunn J, Newmark T, et al. Quetiapine for treatment-resistant delirium. Psychosomatics. 2003;44(4):350-351.
37. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64(11):1316-1321.
38. Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419-427.
39. Young CC, Lujan E. Intravenous ziprasidone for treatment of delirium in the intensive care unit. Anesthesiology. 2004;101(3):794-795.
40. Leso L, Schwartz TL. Ziprasidone treatment of delirium. Psychosomatics. 2002;43(1):61-62.
41. Alao AO, Moskowitz L. Aripiprazole and delirium. Ann Clin Psychiatry. 2006;18(4):267-269.
42. Straker DA, Shapiro PA, Muskin PR. Aripiprazole in the treatment of delirium. Psychosomatics. 2006;47(5):385-391.
43. Burr HG, Mullendore JM. Recent investigations on tranquilizers and stuttering. J Speech Hear Disord. 1960;25:33-37.
44. Tapia F. Haldol in the treatment of children with tics and stutterers and an incidental finding. Behav Neuropsychiatry. 1969;1(3):28.-
45. van Wattum PJ. Stuttering improved with risperidone. J Am Acad Child Adolesc Psychiatry. 2006;45(2):133.-
46. Lavid N, Franklin DL, Maguire GA. Management of child and adolescent stuttering with olanzapine: three case reports. Ann Clin Psychiatry. 1999;11(4):233-236.
An app to help your patient with chronic pelvic pain
In this series, I review what I call prescription apps—apps that you might consider recommending to your patient to enhance her medical care. Many patients are already looking at medical apps and want to hear your opinion. Often, the free apps I recommend to patients are downloaded before they leave my office. When recommending apps, their cost (not necessarily a measure of quality or utility) and platform (device that the app has been designed for) should be taken into account. It is helpful to know whether the app you are recommending is supported by your patient’s smartphone.
Chronic pelvic pain: multifactorial
Chronic pelvic pain, like most chronic pain conditions, is multifactorial in nature. It is not surprising then that most women with chronic pelvic pain do best with a multidisciplinary management approach that addresses both physical and emotional well-being, including the mind-body aspect of chronic pain (how mood and emotions affect pain), exercise, pacing of activities, attention to sleep hygiene, and the role of dysfunctional eating patterns. However, a patient’s access to formal mind-body programs or even a pain psychologist can be hard to come by for a variety of reasons.

An app that tracks pain and treatment
WebMD Pain Coach is a mobile mind-body program and pain coach all rolled into one. While specifically designed for nongynecologic pain conditions (fibromyalgia, migraine, back pain), the app works just as well for pelvic pain. Pain conditions, such as pelvic pain, that are not preloaded into the app are easy to add.1,2
WebMD Pain Coach provides a way for the user to journal as well as track her pain scores, pain triggers, mood, sleep, diet, and response to therapies. It can provide a snapshot, yearly for instance, of tracked pain levels and is preloaded with goals that a user can customize easily. The app also is loaded with excellent pain management tips, videos, and slide shows. There are more than 300 patient-focused articles from the archives of WebMD and other sources that have been reviewed by experts. Progress and notes can be converted into a PDF for use at home or with a health-care provider—a very helpful tool as it can be hard to arrange the many domains of food, rest, exercise, mood, treatments, and pain scores in an organized fashion.1,2
Pros: With a multidisciplinary approach to managing chronic pain, it can be very helpful for patients to track their daily activity, pain triggers, pain levels, and tried therapies. The app provides an opportunity to learn more about the mind–body connection, which is a core component of effective pain management. This app also has excellent medical information and useful strategies for managing chronic pain. It’s easy to use as a source of information, a journal, and a pocket coach.
Cons: This is a free app for iPhone, iTouch, and the iPad—but currently only available for Apple products.
Verdict: This is a great tool on many levels. It would be useful for someone who just wants to track their pain and triggers, but also helpful for the patient who wants to obtain more control and learn more about managing pain. This app would be complementary for someone already engaged in mind–body work, but also be useful for someone who does not have access to those services.
- Why (and how) you should encourage your patients’ search for health information on the Web (December 2011)
- Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception? (Guest Editorial, October 2012)
- To blog or not to blog? What’s the answer for you and your practice? (August 2011)
- For better or, maybe worse, patients are judging your care online (March 2011)
- Twitter 101 for ObGyns: Pearls, pitfalls, and potential (September 2010)
We want to hear from you! Tell us what you think.
1. WebMD. WebMD Pain Coach: A Better Day Starts Here. http://www.webmd.com/webmdpaincoachapp. Accessed January 17, 2013.
2. WebMD. WebMD Pain Coach. iTunes Preview. Apple, Inc. https://itunes.apple.com/us/app/webmd-pain-coach/id536303342?mt=8. Released September 17, 2012. Accessed January 17, 2013.
In this series, I review what I call prescription apps—apps that you might consider recommending to your patient to enhance her medical care. Many patients are already looking at medical apps and want to hear your opinion. Often, the free apps I recommend to patients are downloaded before they leave my office. When recommending apps, their cost (not necessarily a measure of quality or utility) and platform (device that the app has been designed for) should be taken into account. It is helpful to know whether the app you are recommending is supported by your patient’s smartphone.
Chronic pelvic pain: multifactorial
Chronic pelvic pain, like most chronic pain conditions, is multifactorial in nature. It is not surprising then that most women with chronic pelvic pain do best with a multidisciplinary management approach that addresses both physical and emotional well-being, including the mind-body aspect of chronic pain (how mood and emotions affect pain), exercise, pacing of activities, attention to sleep hygiene, and the role of dysfunctional eating patterns. However, a patient’s access to formal mind-body programs or even a pain psychologist can be hard to come by for a variety of reasons.

An app that tracks pain and treatment
WebMD Pain Coach is a mobile mind-body program and pain coach all rolled into one. While specifically designed for nongynecologic pain conditions (fibromyalgia, migraine, back pain), the app works just as well for pelvic pain. Pain conditions, such as pelvic pain, that are not preloaded into the app are easy to add.1,2
WebMD Pain Coach provides a way for the user to journal as well as track her pain scores, pain triggers, mood, sleep, diet, and response to therapies. It can provide a snapshot, yearly for instance, of tracked pain levels and is preloaded with goals that a user can customize easily. The app also is loaded with excellent pain management tips, videos, and slide shows. There are more than 300 patient-focused articles from the archives of WebMD and other sources that have been reviewed by experts. Progress and notes can be converted into a PDF for use at home or with a health-care provider—a very helpful tool as it can be hard to arrange the many domains of food, rest, exercise, mood, treatments, and pain scores in an organized fashion.1,2
Pros: With a multidisciplinary approach to managing chronic pain, it can be very helpful for patients to track their daily activity, pain triggers, pain levels, and tried therapies. The app provides an opportunity to learn more about the mind–body connection, which is a core component of effective pain management. This app also has excellent medical information and useful strategies for managing chronic pain. It’s easy to use as a source of information, a journal, and a pocket coach.
Cons: This is a free app for iPhone, iTouch, and the iPad—but currently only available for Apple products.
Verdict: This is a great tool on many levels. It would be useful for someone who just wants to track their pain and triggers, but also helpful for the patient who wants to obtain more control and learn more about managing pain. This app would be complementary for someone already engaged in mind–body work, but also be useful for someone who does not have access to those services.
- Why (and how) you should encourage your patients’ search for health information on the Web (December 2011)
- Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception? (Guest Editorial, October 2012)
- To blog or not to blog? What’s the answer for you and your practice? (August 2011)
- For better or, maybe worse, patients are judging your care online (March 2011)
- Twitter 101 for ObGyns: Pearls, pitfalls, and potential (September 2010)
We want to hear from you! Tell us what you think.
In this series, I review what I call prescription apps—apps that you might consider recommending to your patient to enhance her medical care. Many patients are already looking at medical apps and want to hear your opinion. Often, the free apps I recommend to patients are downloaded before they leave my office. When recommending apps, their cost (not necessarily a measure of quality or utility) and platform (device that the app has been designed for) should be taken into account. It is helpful to know whether the app you are recommending is supported by your patient’s smartphone.
Chronic pelvic pain: multifactorial
Chronic pelvic pain, like most chronic pain conditions, is multifactorial in nature. It is not surprising then that most women with chronic pelvic pain do best with a multidisciplinary management approach that addresses both physical and emotional well-being, including the mind-body aspect of chronic pain (how mood and emotions affect pain), exercise, pacing of activities, attention to sleep hygiene, and the role of dysfunctional eating patterns. However, a patient’s access to formal mind-body programs or even a pain psychologist can be hard to come by for a variety of reasons.

An app that tracks pain and treatment
WebMD Pain Coach is a mobile mind-body program and pain coach all rolled into one. While specifically designed for nongynecologic pain conditions (fibromyalgia, migraine, back pain), the app works just as well for pelvic pain. Pain conditions, such as pelvic pain, that are not preloaded into the app are easy to add.1,2
WebMD Pain Coach provides a way for the user to journal as well as track her pain scores, pain triggers, mood, sleep, diet, and response to therapies. It can provide a snapshot, yearly for instance, of tracked pain levels and is preloaded with goals that a user can customize easily. The app also is loaded with excellent pain management tips, videos, and slide shows. There are more than 300 patient-focused articles from the archives of WebMD and other sources that have been reviewed by experts. Progress and notes can be converted into a PDF for use at home or with a health-care provider—a very helpful tool as it can be hard to arrange the many domains of food, rest, exercise, mood, treatments, and pain scores in an organized fashion.1,2
Pros: With a multidisciplinary approach to managing chronic pain, it can be very helpful for patients to track their daily activity, pain triggers, pain levels, and tried therapies. The app provides an opportunity to learn more about the mind–body connection, which is a core component of effective pain management. This app also has excellent medical information and useful strategies for managing chronic pain. It’s easy to use as a source of information, a journal, and a pocket coach.
Cons: This is a free app for iPhone, iTouch, and the iPad—but currently only available for Apple products.
Verdict: This is a great tool on many levels. It would be useful for someone who just wants to track their pain and triggers, but also helpful for the patient who wants to obtain more control and learn more about managing pain. This app would be complementary for someone already engaged in mind–body work, but also be useful for someone who does not have access to those services.
- Why (and how) you should encourage your patients’ search for health information on the Web (December 2011)
- Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception? (Guest Editorial, October 2012)
- To blog or not to blog? What’s the answer for you and your practice? (August 2011)
- For better or, maybe worse, patients are judging your care online (March 2011)
- Twitter 101 for ObGyns: Pearls, pitfalls, and potential (September 2010)
We want to hear from you! Tell us what you think.
1. WebMD. WebMD Pain Coach: A Better Day Starts Here. http://www.webmd.com/webmdpaincoachapp. Accessed January 17, 2013.
2. WebMD. WebMD Pain Coach. iTunes Preview. Apple, Inc. https://itunes.apple.com/us/app/webmd-pain-coach/id536303342?mt=8. Released September 17, 2012. Accessed January 17, 2013.
1. WebMD. WebMD Pain Coach: A Better Day Starts Here. http://www.webmd.com/webmdpaincoachapp. Accessed January 17, 2013.
2. WebMD. WebMD Pain Coach. iTunes Preview. Apple, Inc. https://itunes.apple.com/us/app/webmd-pain-coach/id536303342?mt=8. Released September 17, 2012. Accessed January 17, 2013.








