User login
ONLINE EXCLUSIVE: Emergency Medicine Companies Venture into Hospital Medicine
Hollywood, Fla.-based Hospital Physician Partners (HPP) was an ED business when opportunity came knocking: Hospital administrators started asking, “Can you provide us with some hospitalists to go with our emergency-room doctors?”
Today, HPP is firmly in the HM business—and all signs point toward more hospitals hiring companies to handle both emergency care and inpatient care.
“In many ways, we expanded our efforts into hospitalist medicine as a result of requests from our hospital partners,” says Ed Weinberg, HPP’s chief operating officer. “Their needs were such that they asked us to provide hospital medicine services. So from that, it became clear that it was an area that was really growing. And that is something we are pursuing as vigorously as we are emergency medicine.”
HPP handling both emergency care and hospital medicine can help with the transition of patients from the ED to a hospital bed upstairs, he says.
“That’s where our efficiencies are, because we have physicians working who are carrying out the same philosophy,” he says.
Out of HPP’s 120 contracts, 15 are in hospital medicine. But the HM contract numbers are growing quickly, Weinberg notes.
EmCare has about 400 emergency-medicine programs and more than 50 HM programs, according to Mark Hamm, CEO of EmCare Inpatient Services. He says that it can be much more cost effective to contract with one company for both hospitalist and ED services, something hospitals find attractive.
EmCare service agreements range from completely separate emergency and HM staffs to small, rural hospitals where ED physicians also do rounds. Some hospitals “just don’t have the money for a full-time hospitalist and don’t really need one,” Hamm says.
The patient transitions tend to go more smoothly when both types of care are provided by EmCare, he adds. If they’re not, there can be slowdowns.
“Our goal is to quickly and appropriately move patients through the system,” he says. “If we have a hospitalist provider that’s not really on the same page, that can create bottlenecks. But it’s a blip. Our goal is to sit down, even if it’s not an EmCare hospitalist, to sit down with that director and say, ‘Hey look, let’s be the leader here, let’s work together and appropriately expedite these patients.’ We do the same thing on the hospitalist side.”
Inpatient care promises to be a big part of their future business, the executives agreed.
“Hospital medicine,” Weinberg says, “is growing by leaps and bounds.”
Hollywood, Fla.-based Hospital Physician Partners (HPP) was an ED business when opportunity came knocking: Hospital administrators started asking, “Can you provide us with some hospitalists to go with our emergency-room doctors?”
Today, HPP is firmly in the HM business—and all signs point toward more hospitals hiring companies to handle both emergency care and inpatient care.
“In many ways, we expanded our efforts into hospitalist medicine as a result of requests from our hospital partners,” says Ed Weinberg, HPP’s chief operating officer. “Their needs were such that they asked us to provide hospital medicine services. So from that, it became clear that it was an area that was really growing. And that is something we are pursuing as vigorously as we are emergency medicine.”
HPP handling both emergency care and hospital medicine can help with the transition of patients from the ED to a hospital bed upstairs, he says.
“That’s where our efficiencies are, because we have physicians working who are carrying out the same philosophy,” he says.
Out of HPP’s 120 contracts, 15 are in hospital medicine. But the HM contract numbers are growing quickly, Weinberg notes.
EmCare has about 400 emergency-medicine programs and more than 50 HM programs, according to Mark Hamm, CEO of EmCare Inpatient Services. He says that it can be much more cost effective to contract with one company for both hospitalist and ED services, something hospitals find attractive.
EmCare service agreements range from completely separate emergency and HM staffs to small, rural hospitals where ED physicians also do rounds. Some hospitals “just don’t have the money for a full-time hospitalist and don’t really need one,” Hamm says.
The patient transitions tend to go more smoothly when both types of care are provided by EmCare, he adds. If they’re not, there can be slowdowns.
“Our goal is to quickly and appropriately move patients through the system,” he says. “If we have a hospitalist provider that’s not really on the same page, that can create bottlenecks. But it’s a blip. Our goal is to sit down, even if it’s not an EmCare hospitalist, to sit down with that director and say, ‘Hey look, let’s be the leader here, let’s work together and appropriately expedite these patients.’ We do the same thing on the hospitalist side.”
Inpatient care promises to be a big part of their future business, the executives agreed.
“Hospital medicine,” Weinberg says, “is growing by leaps and bounds.”
Hollywood, Fla.-based Hospital Physician Partners (HPP) was an ED business when opportunity came knocking: Hospital administrators started asking, “Can you provide us with some hospitalists to go with our emergency-room doctors?”
Today, HPP is firmly in the HM business—and all signs point toward more hospitals hiring companies to handle both emergency care and inpatient care.
“In many ways, we expanded our efforts into hospitalist medicine as a result of requests from our hospital partners,” says Ed Weinberg, HPP’s chief operating officer. “Their needs were such that they asked us to provide hospital medicine services. So from that, it became clear that it was an area that was really growing. And that is something we are pursuing as vigorously as we are emergency medicine.”
HPP handling both emergency care and hospital medicine can help with the transition of patients from the ED to a hospital bed upstairs, he says.
“That’s where our efficiencies are, because we have physicians working who are carrying out the same philosophy,” he says.
Out of HPP’s 120 contracts, 15 are in hospital medicine. But the HM contract numbers are growing quickly, Weinberg notes.
EmCare has about 400 emergency-medicine programs and more than 50 HM programs, according to Mark Hamm, CEO of EmCare Inpatient Services. He says that it can be much more cost effective to contract with one company for both hospitalist and ED services, something hospitals find attractive.
EmCare service agreements range from completely separate emergency and HM staffs to small, rural hospitals where ED physicians also do rounds. Some hospitals “just don’t have the money for a full-time hospitalist and don’t really need one,” Hamm says.
The patient transitions tend to go more smoothly when both types of care are provided by EmCare, he adds. If they’re not, there can be slowdowns.
“Our goal is to quickly and appropriately move patients through the system,” he says. “If we have a hospitalist provider that’s not really on the same page, that can create bottlenecks. But it’s a blip. Our goal is to sit down, even if it’s not an EmCare hospitalist, to sit down with that director and say, ‘Hey look, let’s be the leader here, let’s work together and appropriately expedite these patients.’ We do the same thing on the hospitalist side.”
Inpatient care promises to be a big part of their future business, the executives agreed.
“Hospital medicine,” Weinberg says, “is growing by leaps and bounds.”
ONLINE EXCLUSIVE: Weighing the Costs of Palliative Care
Hospitalist David Mitchell, MD, PhD, was moonlighting in an Ohio hospital when a nurse called him about a gravely ill older patient who was experiencing shortness of breath. Should she administer the diuretic Lasix to help clear his lung congestion?
Dr. Mitchell, now a hospitalist at Sibley Memorial Hospital in Washington, D.C., and a member of SHM’s Performance Standards Committee, decided to see the patient in person and review his charts. He found that the patient had severe dementia, hadn’t walked in months, and was declining despite more than two weeks in the hospital and daily visits by three specialists.
Dr. Mitchell called the patient’s son and explained the situation, then asked whether the son thought his father would want to continue receiving aggressive therapy. “The son said, ‘Oh, no. He would never want to continue like this.’ So we stopped all the treatments, and he died by the next day,” Dr. Mitchell says.
To him, the anecdote highlights how far medicine has to go in providing personalized palliative care that honors the wishes of patients and their families. It also demonstrates how ignoring those wishes and failing to communicate can contribute to the huge costs associated with end-of-life medical care. Every day, the three specialists seeing the patient were recommending the same course of therapy. “But nobody was being the quarterback and saying, ‘Hey, listen. This is not working,’ ” Dr. Mitchell says.
Hospitalists, he says, are in an ideal position to step up and play a pivotal role in providing the kind of patient-centered care that could improve both quality and cost. So far, however, Dr. Mitchell says he’s seen wide variation in how hospitalists communicate with a patient’s family about end-of-life decisions. “For the ones who do have these conversations, the family is almost always glad that somebody finally said, ‘Do we have to do these tests? Do we have to continue to try to save his life?’ ” Dr. Mitchell says.
Time constraints, he says, are the main reason why hospitalists don’t have such conversations more often. “The communication dies when you’re busy.” And the remedy? Dr. Mitchell says the only thing that will help shift the focus from seeing as many patients as possible to making sure every encounter is a high-quality, efficient one is payment reform in the form of bundled payments to hospitals and physicians. In theory, professional standards can encourage more uniformity, he says. “But when it hits the trenches, it’s the payment that speaks.”
Hospitalist David Mitchell, MD, PhD, was moonlighting in an Ohio hospital when a nurse called him about a gravely ill older patient who was experiencing shortness of breath. Should she administer the diuretic Lasix to help clear his lung congestion?
Dr. Mitchell, now a hospitalist at Sibley Memorial Hospital in Washington, D.C., and a member of SHM’s Performance Standards Committee, decided to see the patient in person and review his charts. He found that the patient had severe dementia, hadn’t walked in months, and was declining despite more than two weeks in the hospital and daily visits by three specialists.
Dr. Mitchell called the patient’s son and explained the situation, then asked whether the son thought his father would want to continue receiving aggressive therapy. “The son said, ‘Oh, no. He would never want to continue like this.’ So we stopped all the treatments, and he died by the next day,” Dr. Mitchell says.
To him, the anecdote highlights how far medicine has to go in providing personalized palliative care that honors the wishes of patients and their families. It also demonstrates how ignoring those wishes and failing to communicate can contribute to the huge costs associated with end-of-life medical care. Every day, the three specialists seeing the patient were recommending the same course of therapy. “But nobody was being the quarterback and saying, ‘Hey, listen. This is not working,’ ” Dr. Mitchell says.
Hospitalists, he says, are in an ideal position to step up and play a pivotal role in providing the kind of patient-centered care that could improve both quality and cost. So far, however, Dr. Mitchell says he’s seen wide variation in how hospitalists communicate with a patient’s family about end-of-life decisions. “For the ones who do have these conversations, the family is almost always glad that somebody finally said, ‘Do we have to do these tests? Do we have to continue to try to save his life?’ ” Dr. Mitchell says.
Time constraints, he says, are the main reason why hospitalists don’t have such conversations more often. “The communication dies when you’re busy.” And the remedy? Dr. Mitchell says the only thing that will help shift the focus from seeing as many patients as possible to making sure every encounter is a high-quality, efficient one is payment reform in the form of bundled payments to hospitals and physicians. In theory, professional standards can encourage more uniformity, he says. “But when it hits the trenches, it’s the payment that speaks.”
Hospitalist David Mitchell, MD, PhD, was moonlighting in an Ohio hospital when a nurse called him about a gravely ill older patient who was experiencing shortness of breath. Should she administer the diuretic Lasix to help clear his lung congestion?
Dr. Mitchell, now a hospitalist at Sibley Memorial Hospital in Washington, D.C., and a member of SHM’s Performance Standards Committee, decided to see the patient in person and review his charts. He found that the patient had severe dementia, hadn’t walked in months, and was declining despite more than two weeks in the hospital and daily visits by three specialists.
Dr. Mitchell called the patient’s son and explained the situation, then asked whether the son thought his father would want to continue receiving aggressive therapy. “The son said, ‘Oh, no. He would never want to continue like this.’ So we stopped all the treatments, and he died by the next day,” Dr. Mitchell says.
To him, the anecdote highlights how far medicine has to go in providing personalized palliative care that honors the wishes of patients and their families. It also demonstrates how ignoring those wishes and failing to communicate can contribute to the huge costs associated with end-of-life medical care. Every day, the three specialists seeing the patient were recommending the same course of therapy. “But nobody was being the quarterback and saying, ‘Hey, listen. This is not working,’ ” Dr. Mitchell says.
Hospitalists, he says, are in an ideal position to step up and play a pivotal role in providing the kind of patient-centered care that could improve both quality and cost. So far, however, Dr. Mitchell says he’s seen wide variation in how hospitalists communicate with a patient’s family about end-of-life decisions. “For the ones who do have these conversations, the family is almost always glad that somebody finally said, ‘Do we have to do these tests? Do we have to continue to try to save his life?’ ” Dr. Mitchell says.
Time constraints, he says, are the main reason why hospitalists don’t have such conversations more often. “The communication dies when you’re busy.” And the remedy? Dr. Mitchell says the only thing that will help shift the focus from seeing as many patients as possible to making sure every encounter is a high-quality, efficient one is payment reform in the form of bundled payments to hospitals and physicians. In theory, professional standards can encourage more uniformity, he says. “But when it hits the trenches, it’s the payment that speaks.”
ONLINE EXCLUSIVE: Listen to Russell Holman and Ed Weinberg discuss companies' acquisition strategies
ONLINE EXCLUSIVE: Listen to Tosha Wetterneck and Keiki Hinami discuss burnout and career satisfaction
ONLINE EXCLUSIVE: Listen to David Meltzer and Scott Lundberg talk about HM efficiency
How proposed changes to personality disorders in DSM-5 will affect researchers
Crizotinib Approval Personalizes Lung Cancer Therapy
The swift approval of crizotinib capsules by the Food and Drug Administration as the first and only targeted therapy for locally advanced or metastatic ALK-positive non–small cell lung cancer represents another milestone in biomarker-driven, personalized medicine.
Crizotinib was approved, along with a companion diagnostic test, Abbott Molecular’s Vysis ALK Break Apart FISH Probe Kit, which identifies the anaplastic lymphoma kinase (ALK) fusion gene that the drug targets. Pfizer is authorized to market crizotinib as Xalkori for use in patients who test positive for the abnormality.
The ALK fusion gene – comprising portions of the EML4 (echinoderm microtubule–associated proteinlike 4) gene and the ALK gene – is present in about 3%-5% of all patients with NSCLC. Although the percentage is small, it translates to approximately 6,000-11,000 new patients annually in the United States, which is more than the numbers being diagnosed with Hodgkin’s disease, cancer of the testis, or chronic myelogenous leukemia.
"Having this number of people we can affect is really an important development in oncology," commented Dr. Mark G. Kris, chief of the thoracic oncology service at Memorial Sloan-Kettering Cancer Center in New York, during a press briefing sponsored by Pfizer.
The approval "is a delivery on the promise of personalized medicine and genomic medicine. Many folks have wondered how these concepts translate into reality. This is an example of exactly that," said Dr. Kris, a professor of medicine at Cornell University in New York.
Crizotinib is part of a paradigm shift in lung cancer management, observed Dr. Paul A. Bunn Jr., professor of medicine and the James Dudley Chair in Cancer Research at the University of Colorado at Denver. Increasingly, patients are tested for specific mutations and if they are positive, they can be treated with targeted therapies, usually pills that are safer and typically more effective than the conventional chemotherapy and radiation.
The American Society of Clinical Oncology and the National Comprehensive Cancer Network have each issued guidelines recommending that NSCLC patients be tested for mutation of the EGFR (epidermal growth factor receptor) to identify those who might benefit from tyrosine kinase inhibitors targeting EGFR. Gefitinib (Iressa) and erlotinib (Tarceva) have proved effective in clinical trials in this population, but only erlotinib is widely available in the United States.
The experimental BATTLE (Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination) trials program at the University of Texas M.D. Anderson Cancer Center in Houston has shown that it is feasible to biopsy late-stage NSCLC patients and base the choice of therapies on the results of molecular tests for abnormal KRAS, EGFR, and other genes.
Currently, the Lung Cancer Mutation Consortium is engaged in a collaborative project profiling 10 genes, including KRAS, ALK, and EGFR, in 1,000 patients at participating cancer centers. Investigators have reported that 280 (54%) of the first 516 patients were found to have at least one known driver mutation. Patients are being directed to approved targeted therapies where available, or to clinical trials of novel therapies targeting their specific mutations.
"The message is, lung cancers are not all the same. They have different molecular changes," Dr. Bunn said at the crizotinib briefing. "At the time of diagnosis, physicians need to be testing their patients for these molecular changes, and patients who have one of these changes are likely to have prolonged benefit from a pill that is more active than multiagent chemotherapy."
Indeed, Dr. Kris pointed out, in clinical trials crizotinib benefited nearly all patients with the ALK fusion gene, although the degree of benefit varied. On the other hand, withholding crizotinib from patients who test negative means they will be spared the side effects and the waste of time and resources associated with a treatment that won’t work for them. "Having a tablet makes the administration so much easier than intravenous chemotherapy," he added.
How Crizotinib Received FDA Approval
The FDA approval was based on two multicenter, single-arm studies that enrolled 255 late-stage NSCLC patients who tested positive for the ALK fusion gene before enrollment. In most cases, the patients had prior chemotherapy. Objective response rates were 50% (median duration, 42 weeks) in one study, and 61% (median duration, 48 weeks) in the other.
Among the most common side effects in the trials, the FDA listed vision disorders (visual impairment, flashes of light, blurred vision, floaters, double vision, sensitivity to light, and visual field defects), nausea, diarrhea, vomiting, edema, and constipation. Pfizer noted that grade 3 or 4 adverse events occurring in 4% or more of patients included increased ALT and neutropenia; it said that QT prolongation has also been observed.
The FDA also warned that crizotinib has been associated with potentially life-threatening pneumonitis (4 of 255 patients; 1.6%); the drug should be stopped permanently in patients with treatment-related pneumonitis, the agency said. Pregnancy is also a contraindication.
Consideration of crizotinib was expedited under the FDA’s priority review program for drugs with the potential to provide major advances in diseases for which no effective therapy exists. No survival data were reported, and the agency granted accelerated approval based on the objective response rates being reasonably likely to predict clinical benefit. Confirmatory trials are required.
Pfizer is already conducting two randomized, open-label, postmarketing phase III studies: one comparing the safety and efficacy of crizotinib with standard of care chemotherapy (pemetrexed [Alimta] or docetaxel [Taxotere]) in patients with previously treated, advanced, ALK-positive NSCLC, and the other comparing efficacy and safety of the agent to pemetrexed/cisplatin or pemetrexed/carboplatin in previously untreated patients with advanced, ALK-positive, nonsquamous NSCLC, according to a Pfizer statement.
Dr. Kris predicted that the addition of the required ALK fusion diagnostic test – at a cost of $250 – won’t be a large burden. "I think it will be very quick to add ALK testing as part of that," he said, noting the ASCO and NCCN recommendations for EGFR testing.
Because the fusion gene has been seen in patients with lung tumors other than adenocarcinomas, testing should ideally be done in all lung cancer patients, Dr. Kris added. "Based on broader experience with EGFR mutations, I think we’ve learned that there is no clinical profile that comes anywhere near the accuracy of the testing. ... I would caution against any particular kind of profile that would make one select who to test and who not to test," he said.
Recommended dosing is 250 mg taken orally twice daily with or without food. In some patients, a dosing interruption and/or dose reduction to 200 mg taken orally twice daily may be required; if further reduction is necessary, the label recommends 250 mg taken orally once daily.
Monthly treatment with crizotinib will cost $9,600. And because the tumor is driven by the mutation, the treatment is indefinite. Several patients from the trials have now been taking the drug for more than a year. "There is no stop time as long as they’re benefiting," said Dr. Mace Rothenberg, Pfizer senior vice president of clinical development and medical affairs in the oncology business unit.
Pfizer has two financial assistance programs for patients. The Pfizer First Resource Program connects eligible insured patients to specialty pharmacies to get reimbursement-support services and to obtain the medications. For uninsured and underinsured patients, the program will provide eligible patients with free medication. There is also a copay assistance program for eligible privately insured patients. Information about eligibility can be obtained by calling First Resource (1-877-744-5675), or by visiting XALKORI.com.
Dr. Kris is a consultant for Pfizer. Dr. Bunn has served as a consultant or on an advisory board for several companies in the past 2 years, but noted that he received no funding for any press discussions or research on crizotinib and has no Pfizer stock.
The swift approval of crizotinib capsules by the Food and Drug Administration as the first and only targeted therapy for locally advanced or metastatic ALK-positive non–small cell lung cancer represents another milestone in biomarker-driven, personalized medicine.
Crizotinib was approved, along with a companion diagnostic test, Abbott Molecular’s Vysis ALK Break Apart FISH Probe Kit, which identifies the anaplastic lymphoma kinase (ALK) fusion gene that the drug targets. Pfizer is authorized to market crizotinib as Xalkori for use in patients who test positive for the abnormality.
The ALK fusion gene – comprising portions of the EML4 (echinoderm microtubule–associated proteinlike 4) gene and the ALK gene – is present in about 3%-5% of all patients with NSCLC. Although the percentage is small, it translates to approximately 6,000-11,000 new patients annually in the United States, which is more than the numbers being diagnosed with Hodgkin’s disease, cancer of the testis, or chronic myelogenous leukemia.
"Having this number of people we can affect is really an important development in oncology," commented Dr. Mark G. Kris, chief of the thoracic oncology service at Memorial Sloan-Kettering Cancer Center in New York, during a press briefing sponsored by Pfizer.
The approval "is a delivery on the promise of personalized medicine and genomic medicine. Many folks have wondered how these concepts translate into reality. This is an example of exactly that," said Dr. Kris, a professor of medicine at Cornell University in New York.
Crizotinib is part of a paradigm shift in lung cancer management, observed Dr. Paul A. Bunn Jr., professor of medicine and the James Dudley Chair in Cancer Research at the University of Colorado at Denver. Increasingly, patients are tested for specific mutations and if they are positive, they can be treated with targeted therapies, usually pills that are safer and typically more effective than the conventional chemotherapy and radiation.
The American Society of Clinical Oncology and the National Comprehensive Cancer Network have each issued guidelines recommending that NSCLC patients be tested for mutation of the EGFR (epidermal growth factor receptor) to identify those who might benefit from tyrosine kinase inhibitors targeting EGFR. Gefitinib (Iressa) and erlotinib (Tarceva) have proved effective in clinical trials in this population, but only erlotinib is widely available in the United States.
The experimental BATTLE (Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination) trials program at the University of Texas M.D. Anderson Cancer Center in Houston has shown that it is feasible to biopsy late-stage NSCLC patients and base the choice of therapies on the results of molecular tests for abnormal KRAS, EGFR, and other genes.
Currently, the Lung Cancer Mutation Consortium is engaged in a collaborative project profiling 10 genes, including KRAS, ALK, and EGFR, in 1,000 patients at participating cancer centers. Investigators have reported that 280 (54%) of the first 516 patients were found to have at least one known driver mutation. Patients are being directed to approved targeted therapies where available, or to clinical trials of novel therapies targeting their specific mutations.
"The message is, lung cancers are not all the same. They have different molecular changes," Dr. Bunn said at the crizotinib briefing. "At the time of diagnosis, physicians need to be testing their patients for these molecular changes, and patients who have one of these changes are likely to have prolonged benefit from a pill that is more active than multiagent chemotherapy."
Indeed, Dr. Kris pointed out, in clinical trials crizotinib benefited nearly all patients with the ALK fusion gene, although the degree of benefit varied. On the other hand, withholding crizotinib from patients who test negative means they will be spared the side effects and the waste of time and resources associated with a treatment that won’t work for them. "Having a tablet makes the administration so much easier than intravenous chemotherapy," he added.
How Crizotinib Received FDA Approval
The FDA approval was based on two multicenter, single-arm studies that enrolled 255 late-stage NSCLC patients who tested positive for the ALK fusion gene before enrollment. In most cases, the patients had prior chemotherapy. Objective response rates were 50% (median duration, 42 weeks) in one study, and 61% (median duration, 48 weeks) in the other.
Among the most common side effects in the trials, the FDA listed vision disorders (visual impairment, flashes of light, blurred vision, floaters, double vision, sensitivity to light, and visual field defects), nausea, diarrhea, vomiting, edema, and constipation. Pfizer noted that grade 3 or 4 adverse events occurring in 4% or more of patients included increased ALT and neutropenia; it said that QT prolongation has also been observed.
The FDA also warned that crizotinib has been associated with potentially life-threatening pneumonitis (4 of 255 patients; 1.6%); the drug should be stopped permanently in patients with treatment-related pneumonitis, the agency said. Pregnancy is also a contraindication.
Consideration of crizotinib was expedited under the FDA’s priority review program for drugs with the potential to provide major advances in diseases for which no effective therapy exists. No survival data were reported, and the agency granted accelerated approval based on the objective response rates being reasonably likely to predict clinical benefit. Confirmatory trials are required.
Pfizer is already conducting two randomized, open-label, postmarketing phase III studies: one comparing the safety and efficacy of crizotinib with standard of care chemotherapy (pemetrexed [Alimta] or docetaxel [Taxotere]) in patients with previously treated, advanced, ALK-positive NSCLC, and the other comparing efficacy and safety of the agent to pemetrexed/cisplatin or pemetrexed/carboplatin in previously untreated patients with advanced, ALK-positive, nonsquamous NSCLC, according to a Pfizer statement.
Dr. Kris predicted that the addition of the required ALK fusion diagnostic test – at a cost of $250 – won’t be a large burden. "I think it will be very quick to add ALK testing as part of that," he said, noting the ASCO and NCCN recommendations for EGFR testing.
Because the fusion gene has been seen in patients with lung tumors other than adenocarcinomas, testing should ideally be done in all lung cancer patients, Dr. Kris added. "Based on broader experience with EGFR mutations, I think we’ve learned that there is no clinical profile that comes anywhere near the accuracy of the testing. ... I would caution against any particular kind of profile that would make one select who to test and who not to test," he said.
Recommended dosing is 250 mg taken orally twice daily with or without food. In some patients, a dosing interruption and/or dose reduction to 200 mg taken orally twice daily may be required; if further reduction is necessary, the label recommends 250 mg taken orally once daily.
Monthly treatment with crizotinib will cost $9,600. And because the tumor is driven by the mutation, the treatment is indefinite. Several patients from the trials have now been taking the drug for more than a year. "There is no stop time as long as they’re benefiting," said Dr. Mace Rothenberg, Pfizer senior vice president of clinical development and medical affairs in the oncology business unit.
Pfizer has two financial assistance programs for patients. The Pfizer First Resource Program connects eligible insured patients to specialty pharmacies to get reimbursement-support services and to obtain the medications. For uninsured and underinsured patients, the program will provide eligible patients with free medication. There is also a copay assistance program for eligible privately insured patients. Information about eligibility can be obtained by calling First Resource (1-877-744-5675), or by visiting XALKORI.com.
Dr. Kris is a consultant for Pfizer. Dr. Bunn has served as a consultant or on an advisory board for several companies in the past 2 years, but noted that he received no funding for any press discussions or research on crizotinib and has no Pfizer stock.
The swift approval of crizotinib capsules by the Food and Drug Administration as the first and only targeted therapy for locally advanced or metastatic ALK-positive non–small cell lung cancer represents another milestone in biomarker-driven, personalized medicine.
Crizotinib was approved, along with a companion diagnostic test, Abbott Molecular’s Vysis ALK Break Apart FISH Probe Kit, which identifies the anaplastic lymphoma kinase (ALK) fusion gene that the drug targets. Pfizer is authorized to market crizotinib as Xalkori for use in patients who test positive for the abnormality.
The ALK fusion gene – comprising portions of the EML4 (echinoderm microtubule–associated proteinlike 4) gene and the ALK gene – is present in about 3%-5% of all patients with NSCLC. Although the percentage is small, it translates to approximately 6,000-11,000 new patients annually in the United States, which is more than the numbers being diagnosed with Hodgkin’s disease, cancer of the testis, or chronic myelogenous leukemia.
"Having this number of people we can affect is really an important development in oncology," commented Dr. Mark G. Kris, chief of the thoracic oncology service at Memorial Sloan-Kettering Cancer Center in New York, during a press briefing sponsored by Pfizer.
The approval "is a delivery on the promise of personalized medicine and genomic medicine. Many folks have wondered how these concepts translate into reality. This is an example of exactly that," said Dr. Kris, a professor of medicine at Cornell University in New York.
Crizotinib is part of a paradigm shift in lung cancer management, observed Dr. Paul A. Bunn Jr., professor of medicine and the James Dudley Chair in Cancer Research at the University of Colorado at Denver. Increasingly, patients are tested for specific mutations and if they are positive, they can be treated with targeted therapies, usually pills that are safer and typically more effective than the conventional chemotherapy and radiation.
The American Society of Clinical Oncology and the National Comprehensive Cancer Network have each issued guidelines recommending that NSCLC patients be tested for mutation of the EGFR (epidermal growth factor receptor) to identify those who might benefit from tyrosine kinase inhibitors targeting EGFR. Gefitinib (Iressa) and erlotinib (Tarceva) have proved effective in clinical trials in this population, but only erlotinib is widely available in the United States.
The experimental BATTLE (Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination) trials program at the University of Texas M.D. Anderson Cancer Center in Houston has shown that it is feasible to biopsy late-stage NSCLC patients and base the choice of therapies on the results of molecular tests for abnormal KRAS, EGFR, and other genes.
Currently, the Lung Cancer Mutation Consortium is engaged in a collaborative project profiling 10 genes, including KRAS, ALK, and EGFR, in 1,000 patients at participating cancer centers. Investigators have reported that 280 (54%) of the first 516 patients were found to have at least one known driver mutation. Patients are being directed to approved targeted therapies where available, or to clinical trials of novel therapies targeting their specific mutations.
"The message is, lung cancers are not all the same. They have different molecular changes," Dr. Bunn said at the crizotinib briefing. "At the time of diagnosis, physicians need to be testing their patients for these molecular changes, and patients who have one of these changes are likely to have prolonged benefit from a pill that is more active than multiagent chemotherapy."
Indeed, Dr. Kris pointed out, in clinical trials crizotinib benefited nearly all patients with the ALK fusion gene, although the degree of benefit varied. On the other hand, withholding crizotinib from patients who test negative means they will be spared the side effects and the waste of time and resources associated with a treatment that won’t work for them. "Having a tablet makes the administration so much easier than intravenous chemotherapy," he added.
How Crizotinib Received FDA Approval
The FDA approval was based on two multicenter, single-arm studies that enrolled 255 late-stage NSCLC patients who tested positive for the ALK fusion gene before enrollment. In most cases, the patients had prior chemotherapy. Objective response rates were 50% (median duration, 42 weeks) in one study, and 61% (median duration, 48 weeks) in the other.
Among the most common side effects in the trials, the FDA listed vision disorders (visual impairment, flashes of light, blurred vision, floaters, double vision, sensitivity to light, and visual field defects), nausea, diarrhea, vomiting, edema, and constipation. Pfizer noted that grade 3 or 4 adverse events occurring in 4% or more of patients included increased ALT and neutropenia; it said that QT prolongation has also been observed.
The FDA also warned that crizotinib has been associated with potentially life-threatening pneumonitis (4 of 255 patients; 1.6%); the drug should be stopped permanently in patients with treatment-related pneumonitis, the agency said. Pregnancy is also a contraindication.
Consideration of crizotinib was expedited under the FDA’s priority review program for drugs with the potential to provide major advances in diseases for which no effective therapy exists. No survival data were reported, and the agency granted accelerated approval based on the objective response rates being reasonably likely to predict clinical benefit. Confirmatory trials are required.
Pfizer is already conducting two randomized, open-label, postmarketing phase III studies: one comparing the safety and efficacy of crizotinib with standard of care chemotherapy (pemetrexed [Alimta] or docetaxel [Taxotere]) in patients with previously treated, advanced, ALK-positive NSCLC, and the other comparing efficacy and safety of the agent to pemetrexed/cisplatin or pemetrexed/carboplatin in previously untreated patients with advanced, ALK-positive, nonsquamous NSCLC, according to a Pfizer statement.
Dr. Kris predicted that the addition of the required ALK fusion diagnostic test – at a cost of $250 – won’t be a large burden. "I think it will be very quick to add ALK testing as part of that," he said, noting the ASCO and NCCN recommendations for EGFR testing.
Because the fusion gene has been seen in patients with lung tumors other than adenocarcinomas, testing should ideally be done in all lung cancer patients, Dr. Kris added. "Based on broader experience with EGFR mutations, I think we’ve learned that there is no clinical profile that comes anywhere near the accuracy of the testing. ... I would caution against any particular kind of profile that would make one select who to test and who not to test," he said.
Recommended dosing is 250 mg taken orally twice daily with or without food. In some patients, a dosing interruption and/or dose reduction to 200 mg taken orally twice daily may be required; if further reduction is necessary, the label recommends 250 mg taken orally once daily.
Monthly treatment with crizotinib will cost $9,600. And because the tumor is driven by the mutation, the treatment is indefinite. Several patients from the trials have now been taking the drug for more than a year. "There is no stop time as long as they’re benefiting," said Dr. Mace Rothenberg, Pfizer senior vice president of clinical development and medical affairs in the oncology business unit.
Pfizer has two financial assistance programs for patients. The Pfizer First Resource Program connects eligible insured patients to specialty pharmacies to get reimbursement-support services and to obtain the medications. For uninsured and underinsured patients, the program will provide eligible patients with free medication. There is also a copay assistance program for eligible privately insured patients. Information about eligibility can be obtained by calling First Resource (1-877-744-5675), or by visiting XALKORI.com.
Dr. Kris is a consultant for Pfizer. Dr. Bunn has served as a consultant or on an advisory board for several companies in the past 2 years, but noted that he received no funding for any press discussions or research on crizotinib and has no Pfizer stock.
Venous thromboembolism: What to do after anticoagulation is started
Deep vein thrombosis and pulmonary embolism are collectively referred to as venous thromboembolic (VTE) disease. They affect approximately 100,000 to 300,000 patients per year in the United States.1 Although patients with deep vein thrombosis can be treated as outpatients, many are admitted for the initiation of anticoagulation. Initial anticoagulation usually requires the overlap of a parenteral anticoagulant (unfractionated heparin, low-molecular-weight heparin [LMWH] or fondaparinux) with warfarin for a minimum of 5 days and until the international normalized ratio (INR) of the prothrombin time is above 2.0 for at least 24 hours.2
Three clinical issues need to be addressed after the initiation of anticoagulation for VTE:
- Determination of the length of anticoagulation with the correct anticoagulant
- Prevention of postthrombotic syndrome
- Appropriate screening for occult malignancy.
HOW LONG SHOULD VTE BE TREATED?
The duration of anticoagulation has been a matter of debate.
The risk of recurrent VTE appears related to clinical risk factors that a patient has at the time of the initial thrombotic event. An epidemiologic study3 found that patients with VTE treated for approximately 6 months had a low rate of recurrence (0% at 2 years of follow-up) if surgery was the risk factor. The risk climbed to 9% if the risk factor was nonsurgical and to 19% if there were no provoking risk factors.
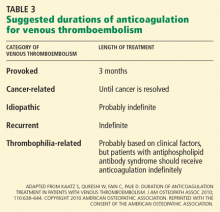
The likelihood of VTE recurrence and therefore the recommended duration of treatment depend on whether the VTE event was provoked, cancer-related, recurrent, thrombophilia-related, or idiopathic. We address each of these scenarios below.
HOW LONG TO TREAT PROVOKED VTE
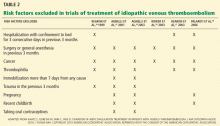
A VTE event is considered provoked if the patient had a clear inciting risk factor. As defined in various clinical trials, these risk factors include:
- Hospitalization with confinement to bed for 3 or more consecutive days in the last 3 months
- Surgery or general anesthesia in the last 3 months
- Immobilization for more than 7 days, regardless of the cause
- Trauma in the last 3 months
- Pregnancy
- Use of an oral contraceptive, regardless of which estrogen or progesterone analogue it contains
- Travel for more than 4 hours
- Recent childbirth.
However, the trials that tested different lengths of anticoagulation have varied markedly in how they defined provoked deep vein thrombosis.4–7
Recommendation: Warfarin or equivalent for 3 months
The American College of Chest Physicians (ACCP) recommends 3 months of anticoagulation with warfarin or another vitamin K antagonist for patients with VTE secondary to a transient (reversible) risk factor,2 and we agree.
HOW LONG TO TREAT CANCER-RELATED VTE
Patients with cancer are at higher risk of developing VTE. Furthermore, in one study,9 compared with other patients with VTE, patients with cancer were three times more likely to have another episode of VTE, with a cumulative rate of recurrence at 1 year of 21% vs 7%. Cancer patients were also twice as likely to suffer major bleeding complications while on anticoagulation.9
Warfarin is a difficult drug to manage because it has many interactions with foods, diseases, and other drugs. These difficulties are amplified in many cancer patients during chemotherapy.
Warfarin was compared with a LMWH in four randomized trials in cancer patients, and a meta-analysis10 found a 50% relative reduction in the rates of recurrent deep vein thrombosis and pulmonary embolism with LMWH treatment. These results were driven primarily by the CLOT trial (Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients With Cancer),11 which showed an 8% absolute risk reduction (number needed to treat 13) without an increase in major bleeding when cancer-related VTE was treated with an LMWH—ie, dalteparin (Fragmin)—for 6 months compared with warfarin.
Current thinking suggests that VTE should be treated until the cancer is resolved. However, this hypothesis has not been adequately tested, and consequently, the ACCP gives it only a level 1C recommendation.2 The largest of the four trials comparing warfarin and an LMWH lasted only 6 months. The safety of extending LMWH treatment beyond 6 months is currently unknown but is under investigation (clinicaltrials.gov identifier NCT00942968).
Recommendation: LMWH therapy for at least 6 months
The ACCP guidelines recommend LMWH therapy for 3 to 6 months, followed by warfarin or another vitamin K antagonist or continued LMWH treatment until the cancer is resolved.2
The National Comprehensive Cancer Network guidelines recommend an LMWH for 6 months as monotherapy and indefinite anticoagulation if the cancer is still active.12
The American Society of Clinical Oncology guidelines recommend an LMWH for at least 6 months and indefinite anticoagulant therapy for selected patients with active cancer.13
We agree that patients with active cancer should receive an LMWH for at least 6 months and indefinite anticoagulation until the cancer is resolved.
In our experience, many patients are reluctant to give themselves the daily injections that LMWH therapy requires, and so they need to be well-informed about the marked decrease in VTE recurrence with this less-convenient and more-expensive therapy. Many patients face insurance barriers to cover the cost of LMWH therapy; however, careful attention to preauthorization can usually overcome this obstacle.
HOW LONG TO TREAT RECURRENT VTE
It makes clinical sense that patients who have a second VTE event should be treated indefinitely. This theory was tested in a randomized clinical trial14 in which patients with provoked or unprovoked VTE were randomized after their second event to receive anticoagulation for 6 months vs indefinitely.
After 4 years of follow-up, the recurrence rate was 21% in patients assigned to 6 months of treatment and only 3% in patients who continued anticoagulation throughout the trial. On the other hand, major hemorrhage occurred in 3% of patients treated for 6 months and in 9% in patients who continued anticoagulation indefinitely.
Of note, most of the patients in this trial had unprovoked (idiopathic) VTE, so the results should not be extrapolated to patients with provoked VTE, who accounted for only 20% of the study population.14
Recommendation: Long-term anticoagulation
We agree with the ACCP recommendation2 that patients who have had a second episode of unprovoked VTE should receive long-term anticoagulation. Because of a lack of data, the duration of therapy for patients with a second episode of provoked VTE should be individualized.
HOW LONG TO TREAT THROMBOPHILIA-RELATED VTE
Inherited thrombophilias
Patients with VTE that is not related to a clear provoking risk factor or cancer frequently have testing to evaluate for a hypercoagulable state. This workup traditionally includes the most common inherited thrombophilias for gene mutations for factor V and prothrombin as well as for deficiencies in protein C, protein S, antithrombin and the acquired antiphospholipid syndrome.
The key questions that should be asked prior to embarking on this workup are:
- Will the results change the length of therapy for the patient?
- Will testing the patient help with genetic counseling and possible testing of family members?
- Will the results change the targeted INR range for warfarin or other vitamin K antagonist therapy?
Patients with inherited thrombophilia have a greater risk of developing an initial VTE event; however, these tests do not help predict the recurrence of VTE in patients with established disease more than clinical risk factors do. A prospective study demonstrated this by looking at the effect of thrombophilia and clinical factors on the recurrence of venous thrombosis and found that inherited prothrombotic abnormalities do not appear to play an important role in the risk of a recurrent event.15 On the other hand, clinical factors, such as whether the first event was idiopathic or provoked, appear more important in determining the duration of anticoagulation therapy.15 A systematic review of the common inherited thrombophilias showed the VTE recurrence rate of patients with factor V Leiden was higher than in patients without the mutation; however, the absolute rates of recurrence were not much different than what would be expected in patients with idiopathic VTE.16
A retrospective study involving a large cohort of families of patients who already had experienced a first episode of either idiopathic or provoked VTE showed high annual risks of recurrent VTE associated with hereditary deficiencies of protein S (8.4%), protein C (6.0%), and antithrombin (10%).17 However, for the more commonly occurring genetic thrombophilias, the factor V Leiden and prothrombin G20210A mutations, family members with either gene abnormality had low rates of VTE, suggesting that testing of relatives of probands is not clinically useful.16
Antiphospholipid syndrome
Antiphospholipid syndrome is an acquired thrombophilia. A patient has thrombotic antiphospholipid syndrome when there is a history of vascular thrombosis in the presence of persistently positive tests (at least 12 weeks apart) for lupus anticoagulants, anticardiolipin antibodies, or anti-beta-2 glycoprotein I. A prospective study of 412 patients with a first episode of VTE found that 15% were positive for anticardiolipin antibody at the end of 6 months of anticoagulation. The risk of recurrent VTE after 4 years was 29% in patients with antibodies and 14% in those without antibodies (relative risk 2.1; 95% confidence interval [CI] 1.3–3.3; P =.0013).18
Recent reviews advise indefinite warfarin anticoagulation in patients with VTE and persistence of antiphospholipid antibodies.19 However, the optimal duration of anticoagulation is uncertain. Until well-designed clinical trials are done, the current general consensus is to anticoagulate these patients indefinitely.20,21 Retrospective studies had suggested that patients with antiphospholipid antibodies required a higher therapeutic INR range; however, this observation was tested in two trials that found no difference in thromboembolic rates when patients were randomized to an INR of 2.0–3.0 vs 3.1–4.0,22 or 2.0–3.0 vs 3.0–4.5.23
No formal recommendations
In the absence of strong evidence, the ACCP guidelines do not include a recommendation on the duration of anticoagulation treatment specific to inherited thrombophilias. We believe that clinical factors are more important than inherited thrombophilias for deciding the duration of anticoagulation, and that testing is almost never indicated or useful. However, patients with antiphospholipid syndrome are at high risk of recurrence, and it is our practice to anticoagulate these patients indefinitely.
HOW LONG TO TREAT UNPROVOKED (IDIOPATHIC) VTE
A VTE event is thought to be idiopathic if it occurs without a clearly identified provoking factor.
In an observational study,3 patients with oral contraceptive use, transient illness, immobilization, or a history of travel had an 8.8% risk of recurrence vs 19.4% in patients with unprovoked VTE. The meta-analysis discussed above (Table 1)8 also shows that patients with these nonsurgical risk factors have a lower rate of recurrence than patients with idiopathic VTE.
The high rate of recurrence of idiopathic VTE (4% to 27% after 3 months of anticoagulation24–26) suggests that a longer duration of treatment is reasonable. However, increasing the length of therapy from 3 to 12 months delays but does not prevent recurrence, the risk of which begins to accumulate once anticoagulation is stopped.24,25
Three promising strategies to identify subgroups of patients with idiopathic VTE who are at highest risk of recurrence and who would benefit the most from prolonged anticoagulation are d-dimer testing, evaluation for residual vein thrombosis in patients who present with a deep vein thrombosis, and clinical prediction rules.
d-dimer testing
d-dimer is a degradation product of fibrin and is an indirect marker of residual thrombosis.30
In a systematic review of patients with a first episode of unprovoked VTE,31 a normal d-dimer concentration at the end of at least 3 months of anticoagulation was associated with a 3.5% annual risk of recurrence, whereas an elevated d-dimer level at that time was associated with an annual risk of 8.9%. These results were confirmed in a systematic review of individual patient data.32
In a randomized trial,28 patients with an idiopathic VTE event who received anticoagulation for at least 3 months had their d-dimer level measured 1 month after cessation of treatment. Those with an elevated level were randomized to either resume anticoagulation or not. Patients who resumed anticoagulation had an annual recurrence rate of 2%; however, those who were allocated not to restart anticoagulation had a recurrence rate of 10.9% per year. There was no difference in the rate of major bleeding between the two groups. Patients in this clinical trial who had a normal d-dimer level did not restart anticoagulation and had an annual recurrence rate of 4.4%.
Evaluation for residual thrombosis
A randomized trial34 in patients with both provoked and idiopathic deep vein thrombosis showed a reduction in recurrence when those who had residual vein thrombosis were given extended anticoagulation. In the subset of patients whose deep vein thrombosis was idiopathic, the recurrence rate was 17% per year when treatment lasted only 3 months and 10% when it was extended for up to 1 year.
Another trial35 randomized patients with provoked and idiopathic deep vein thrombosis to receive anticoagulation for the usual duration or to continue treatment until recanalization of the residual thrombus was demonstrated on follow-up Doppler ultrasonography. Patients who received this ultrasonography-tailored treatment had a lower rate of recurrence of VTE; however, the absolute reductions in recurrence rates cannot be calculated from this report for patients with idiopathic deep vein thrombosis.
A prospective observational study36 of the predictive value of d-dimer status and residual vein thrombus found that only d-dimer was an independent risk factor for recurrent VTE after vitamin K antagonist withdrawal.
A clinical prediction rule: ‘Men and HERDOO2’
A promising tool for predicting if a patient is at low risk of recurrent VTE after the first episode of proximal deep vein thrombosis or pulmonary embolism is known by the mnemonic device “Men and HERDOO2.” It is based on data prospectively derived by Rodger et al37 to identify patients with less than a 3% annual risk of recurrent VTE after their first event of idiopathic proximal deep vein thrombosis or pulmonary embolism. Risk factors for recurrent VTE were male sex (the “men” of “Men and HERDOO2”), signs of postthrombotic syndrome, including hyperpigmentation of the lower extremities, edema or redness of either leg, a d-dimer level > 250 μg/L, obesity (body mass index > 30 kg/m2, and older age (> 65 years).
Overall, one-fourth of the population were women with no risk factors or one risk factor, and their risk of recurrence was 1.6% per year. Men and women who had two or more risk factors for postthrombotic syndrome (hyperpigmentation, edema, or redness), elevated d-dimer, obesity, or older age were predicted to be at higher risk of recurrent VTE. Patients such as this should be considered for indefinite anticoagulation.
Ideally, clinical prediction rules should be validated in a separate group of patients before they are used routinely in practice,38 and this clinical prediction rule is currently being tested in the REVERSE II study. If the results are consistent, this will be an easy-to-use tool to help identify patients who likely can safely stop anticoagulation therapy after 3 to 6 months (clinicaltrials.gov Identifier: NCT00967304).
The location of the thrombosis also influences the likelihood of recurrence. Patients with isolated distal (calf) deep vein thrombosis are less likely to suffer recurrent VTE than those who present with proximal deep vein thrombosis. However, trials focusing specifically on the precise subset of idiopathic isolated distal deep vein thrombosis are lacking. In a randomized trial39 comparing 6 vs 12 weeks of anticoagulation for isolated distal deep vein thrombosis and 12 vs 24 weeks for proximal deep vein thrombosis, the annual rates of recurrence after 12 weeks of treatment were approximately 3.4% for isolated distal and 8.1% for proximal deep vein thrombosis.39
Recommendation: At least 3 months of warfarin or equivalent
We agree with the ACCP recommendation2 that patients with unprovoked VTE should receive at least 3 months of anticoagulation with a vitamin K antagonist.
If the patient has no risk factors for bleeding and good anticoagulant monitoring is achievable, we agree with long-term anticoagulation for proximal unprovoked deep vein thrombosis or pulmonary embolism, and 3 months of therapy for isolated distal unprovoked deep vein thrombosis.
Patient preferences and the risk of recurrence vs the risk of bleeding should be discussed with patients when contemplating indefinite anticoagulation.
If testing is being considered to assist in the decision to prescribe indefinite anticoagulation, we prefer using d-dimer levels rather than ultrasonography to detect residual venous thrombosis because of its ease of use and the strength of the current evidence.
PREVENTING POSTTHROMBOTIC SYNDROME
The postthrombotic (postphlebitic) syndrome is a chronic and burdensome consequence of deep vein thrombosis that occurs despite anticoagulation therapy. It is estimated to affect 23% to 60% of patients and typically manifests in the first 2 years.40 It is not only costly in clinical terms, with decreased quality of life for the patient, but health care expenditures have been estimated to range from $400 per year in a Brazilian study to $7,000 per year in a US study.40
Typical symptoms include leg pain, heaviness, swelling, and cramping. In severe cases, chronic venous ulcers can occur and are difficult to treat.41
The definition of postthrombotic syndrome has been unclear over the years, and six different scales that measure signs and symptoms have been reported.42
The Villalta scale has been proposed by the International Society of Thrombosis and Hemostasis as a diagnostic standard to define postthrombotic syndrome.42 This validated scale is based on five clinical symptoms, six clinical signs, and the presence or absence of venous ulcers. Each clinical symptom and sign is scored as mild (1 point), moderate (2 points), or severe (3 points). Symptoms include pain, cramps, heaviness, paresthesia, and pruritus; the six clinical signs are pretibial edema, skin induration, hyperpigmentation, redness, venous ectasia, and pain on calf compression.
According to the International Society of Thrombosis and Hemostasis, postthrombotic syndrome is present if the Villalta score is 5 or greater or if a venous ulcer is present in a leg with previous deep vein thrombosis. Further, using the Villalta scale, postthrombotic syndrome can be categorized as mild (score 5–9), moderate (10–14), or severe (≥ 15).
A limitation of the Villalta scale is that the presence or absence of a venous ulcer has not been assigned a score. Since a venous ulcer requires more aggressive measures, the society defines postthrombotic syndrome as severe if venous ulcers are present.42
Acute symptoms of deep vein thrombosis may take months to resolve and, indeed, acute symptoms may transition to chronic symptoms without a symptom-free interval. It is recommended that postthrombotic syndrome not be diagnosed before 3 months to avoid inappropriately attributing acute symptoms and signs of deep vein thrombosis to the postthrombotic syndrome.42
Studies of stockings
A systematic review of three randomized trials44 concluded that elastic compression stockings reduce the risk of postthrombotic syndrome (any severity) from 43% to 20% and severe postthrombotic syndrome from 15% to 7%.43
The first of these trials44 randomized patients soon after the diagnosis of deep vein thrombosis to receive made-to-order compression stockings that were rated at 30 to 40 mm Hg or to be in a control group that did not receive stockings. The second trial45 randomized patients 1 year after the index event of deep vein thrombosis to receive 20- to 30-mm Hg stockings or stockings that were two sizes too large (the control group). The third study46 randomly allocated patients to receive “off-the-shelf” stockings (30–40 mm Hg) or no stockings. Each study used its own definition of postthrombotic syndrome.
Although these studies strongly suggest compression stockings prevent postthrombotic syndrome, several methodologic issues remain:
- A standard definition of postthrombotic syndrome was not used
- The amount of compression varied between studies
- The studies were not blinded.
Lack of blinding becomes most significant when an outcome is based on subjective findings, like the symptoms that make up a large part of the diagnosis of postthrombotic syndrome.
The SOX trial, currently under way, is designed to address these methodologic issues and should be completed in 2012 (clinicaltrials.gov Identifier: NCT00143598).
Recommendation: Stockings for at least 2 years
We agree with the ACCP recommendation that a patient who has had a symptomatic proximal deep vein thrombosis should wear an elastic compression stocking with an ankle pressure gradient of 30 to 40 mm Hg as soon as possible after starting anticoagulant therapy and continuing for a minimum of 2 years.2
SCREENING FOR OCCULT MALIGNANCY
VTE can be the first manifestation of cancer.
French physician Armand Trousseau, in the 1860s, was the first to describe disseminated intravascular coagulation closely associated with adenocarcinoma. Ironically, several years later, after suffering for weeks from abdominal pain, he declared to one of his students that he had developed thrombosis, and he died of gastric cancer shortly thereafter.47
Since cancer is a well-known risk factor for VTE, it is logical to screen for cancer as an explanation for an idiopathic VTE event.48 To make an informed decision, one needs to understand the rate of occult cancer at the time VTE is diagnosed, the risk of future development of cancer, and the utility of extensive cancer screening.
The clinical efficacy, side effects, and cost-effectiveness of cancer screening in patients with idiopathic VTE are unknown. However, a systematic review47 of 34 studies found that, in patients with idiopathic VTE, cancer was diagnosed within 1 month in 6.1%, within 6 months in 8.6%, and within 1 year in 10.0% (95% CI 8.6–11.3).
A subset of studies compared two strategies for screening soon after the diagnosis of idiopathic VTE: a strategy limited to the history, physical examination, basic blood work, and chest radiography vs an extensive screening strategy that also included serum tumor markers or abdominal ultrasonography or computed tomography. Limited screening detected 49% of the prevalent cancers; extensive screening increased this rate to 70%. Stated another way, the detection rate for prevalent cancers was 5% with limited screening and 7% with extensive screening soon after the diagnosis of idiopathic VTE.47
Patients with idiopathic VTE had higher rates of cancer within 1 month of diagnosis than patients with provoked VTE (6.1% vs 1.9%), and this difference persisted at 1 year (10.0% vs 2.6%).47
Recommendation: Individualized cancer screening
Patients with idiopathic VTE have a significant risk of occult cancer within the first year after diagnosis, and cancer screening should be considered. Our practice for patients with idiopathic VTE is to perform a history and physical examination and ensure that the patient is up to date on age- and sex-specific cancer screening.
The use of additional imaging or biomarkers should be discussed with patients so they can balance the risks (radiation and potential false-positive results with their downstream consequences), costs, and potential benefits, given the lack of proven survival benefit or cost-effectiveness.
ORAL ANTICOAGULANT MANAGEMENT
Warfarin’s multiple interactions, along with the need for INR monitoring, make it a difficult medication to manage.
The Joint Commission, the US organization for health service accreditation and certification, has defined National Patient Safety Goals and quality measures for the management of anticoagulation.49 Organized anticoagulation management services, dosing algorithms, and patient self-testing using capillary INR meters or patient self-management of warfarin were recommended as tools to improve the time patients spend in the therapeutic INR range.50
Two new oral anticoagulants
The limitations of warfarin have stimulated the search for newer oral anticoagulants that do not require laboratory monitoring or have as many diet and drug interactions.
Two trials have been published with experimental oral anticoagulants that had similar efficacy and safety as warfarin in the treatment of VTE.
The study of dabigatran (Pradaxa) vs warfarin in the treatment of acute VTE (the RECOVER trial)51 randomized 2,539 patients with acute VTE to receive the oral direct thrombin inhibitor dabigatran or warfarin for approximately 6 months. Of note, each treatment group received a median of 6 days of heparin, LMWH, or fondaparinux at the beginning of blinded therapy. The rates of recurrent VTE and major bleeding were similar between the treatment arms, and overall bleeding was less with dabigatran. Dabigatran was approved in the United States in October 2010 for stroke prevention in atrial fibrillation but has yet to be approved for the treatment of VTE pending further study (clinicaltrials.gov Identifier: NCT00680186).
A study of oral rivaroxaban (Xarelto) for symptomatic VTE (the EINSTEIN-DVT trial) 52 randomized 3,449 patients with acute deep vein thrombosis to rivaroxaban or enoxaparin (Lovenox) overlapped with warfarin or another vitamin K antagonist in the usual manner. No difference was noted between the treatments in the rate of recurrence of VTE or of major bleeding. Of note, patients randomized to rivaroxaban received 15 mg twice a day for the first 3 weeks of treatment and then 20 mg per day for the remainder of their therapy and did not require parenteral anticoagulant overlap.
The long-awaited promise of easier-to-use oral anticoagulants for the treatment of VTE is drawing near and has the potential to revolutionize the treatment of this common disorder. In the meantime, close monitoring of warfarin and careful patient education regarding its use are essential. And even with the development of new drugs in the future, it is still imperative that patients with acute VTE receive the correct length of anticoagulation treatment, are prescribed stockings to prevent postthrombotic syndrome, and are updated on routine cancer screening.
- Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006; 21:722–727.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet 2003; 362:523–526.
- Schulman S, Lockner D, Juhlin-Dannfelt A. The duration of oral anticoagulation after deep vein thrombosis. A randomized study. Acta Med Scand 1985; 217:547–552.
- Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Research Committee of the British Thoracic Society. Lancet 1992; 340:873–876.
- Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995; 332:1661–1665.
- Kearon C, Ginsberg JS, Anderson DR, et al. Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J Thromb Haemost 2004; 2:743–749.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 2010; 170:1710–1716.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100:3484–3488.
- Hull RD, Pineo GF, Brant RF, et al; LITE Trial Investigators. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006; 119:1062–1072.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349:146–153.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology, Venous Thromboembolic Disease. http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed August 3, 2011.
- Lyman GH, Khorana AA, Falanga A, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Schulman S, Granqvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009; 301:2472–2485.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost 2009; 101:93–99.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med 1998; 104:332–338.
- Derksen RH, de Groot PG. Towards evidence-based treatment of thrombotic antiphospholipid syndrome. Lupus 2010; 19:470–474.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006; 295:1050–1057.
- Fonseca AG, D’Cruz DP. Controversies in the antiphospholipid syndrome: can we ever stop warfarin? J Autoimmune Dis 2008; 5:6.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005; 3:848–853.
- Agnelli G, Prandoni P, Becattini C, et al; Warfarin Optimal Duration Italian Trial Investigators. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003; 139:19–25.
- Agnelli G, Prandoni P, Santamaria MG, et al; Warfarin Optimal Duration Italian Trial Investigators. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Ridker PM, Goldhaber SZ, Glynn RJ. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:2164–2167.
- Bockenstedt P. D-dimer in venous thromboembolism. N Engl J Med 2003; 349:1203–1204.
- Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008; 149:481–490,W94.
- Douketis J, Tosetto A, Marcucci M, et al. Patient-level metaanalysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010; 153:523–531.
- Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002; 137:955–960.
- Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood 2008; 112:511–515.
- Prandoni P, Prins MH, Lensing AW, et al; AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009; 150:577–585.
- Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost 2005; 94:969–974.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000; 284:79–84.
- Pinede L, Ninet J, Duhaut P, et al; Investigators of the “Durée Optimale du Traitement AntiVitamines K” (DOTAVK) Study. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001; 103:2453–2460.
- Ashrani AA, Heit JA. Incidence and cost burden of postthrombotic syndrome. J Thromb Thrombolysis 2009; 28:465–476.
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149:698–707.
- Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009; 7:879–883.
- Kolbach DN, Sandbrink MW, Hamulyak K, Neumann HA, Prins MH. Non-pharmaceutical measures for prevention of post-thrombotic syndrome. Cochrane Database Syst Rev 2004;CD004174.
- Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997; 349:759–762.
- Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med 2001; 161:2105–2109.
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004; 141:249–256.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med 2008; 149:323–333.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Kaatz S. Impact on patient care: patient case through the continuum of care. J Thromb Thrombolysis 2010; 29:167–170.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Schulman S, Kearon C, Kakkar AK, et al; for the RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2452.
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363;2499–2510.
Deep vein thrombosis and pulmonary embolism are collectively referred to as venous thromboembolic (VTE) disease. They affect approximately 100,000 to 300,000 patients per year in the United States.1 Although patients with deep vein thrombosis can be treated as outpatients, many are admitted for the initiation of anticoagulation. Initial anticoagulation usually requires the overlap of a parenteral anticoagulant (unfractionated heparin, low-molecular-weight heparin [LMWH] or fondaparinux) with warfarin for a minimum of 5 days and until the international normalized ratio (INR) of the prothrombin time is above 2.0 for at least 24 hours.2
Three clinical issues need to be addressed after the initiation of anticoagulation for VTE:
- Determination of the length of anticoagulation with the correct anticoagulant
- Prevention of postthrombotic syndrome
- Appropriate screening for occult malignancy.
HOW LONG SHOULD VTE BE TREATED?
The duration of anticoagulation has been a matter of debate.
The risk of recurrent VTE appears related to clinical risk factors that a patient has at the time of the initial thrombotic event. An epidemiologic study3 found that patients with VTE treated for approximately 6 months had a low rate of recurrence (0% at 2 years of follow-up) if surgery was the risk factor. The risk climbed to 9% if the risk factor was nonsurgical and to 19% if there were no provoking risk factors.
The likelihood of VTE recurrence and therefore the recommended duration of treatment depend on whether the VTE event was provoked, cancer-related, recurrent, thrombophilia-related, or idiopathic. We address each of these scenarios below.
HOW LONG TO TREAT PROVOKED VTE
A VTE event is considered provoked if the patient had a clear inciting risk factor. As defined in various clinical trials, these risk factors include:
- Hospitalization with confinement to bed for 3 or more consecutive days in the last 3 months
- Surgery or general anesthesia in the last 3 months
- Immobilization for more than 7 days, regardless of the cause
- Trauma in the last 3 months
- Pregnancy
- Use of an oral contraceptive, regardless of which estrogen or progesterone analogue it contains
- Travel for more than 4 hours
- Recent childbirth.
However, the trials that tested different lengths of anticoagulation have varied markedly in how they defined provoked deep vein thrombosis.4–7
Recommendation: Warfarin or equivalent for 3 months
The American College of Chest Physicians (ACCP) recommends 3 months of anticoagulation with warfarin or another vitamin K antagonist for patients with VTE secondary to a transient (reversible) risk factor,2 and we agree.
HOW LONG TO TREAT CANCER-RELATED VTE
Patients with cancer are at higher risk of developing VTE. Furthermore, in one study,9 compared with other patients with VTE, patients with cancer were three times more likely to have another episode of VTE, with a cumulative rate of recurrence at 1 year of 21% vs 7%. Cancer patients were also twice as likely to suffer major bleeding complications while on anticoagulation.9
Warfarin is a difficult drug to manage because it has many interactions with foods, diseases, and other drugs. These difficulties are amplified in many cancer patients during chemotherapy.
Warfarin was compared with a LMWH in four randomized trials in cancer patients, and a meta-analysis10 found a 50% relative reduction in the rates of recurrent deep vein thrombosis and pulmonary embolism with LMWH treatment. These results were driven primarily by the CLOT trial (Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients With Cancer),11 which showed an 8% absolute risk reduction (number needed to treat 13) without an increase in major bleeding when cancer-related VTE was treated with an LMWH—ie, dalteparin (Fragmin)—for 6 months compared with warfarin.
Current thinking suggests that VTE should be treated until the cancer is resolved. However, this hypothesis has not been adequately tested, and consequently, the ACCP gives it only a level 1C recommendation.2 The largest of the four trials comparing warfarin and an LMWH lasted only 6 months. The safety of extending LMWH treatment beyond 6 months is currently unknown but is under investigation (clinicaltrials.gov identifier NCT00942968).
Recommendation: LMWH therapy for at least 6 months
The ACCP guidelines recommend LMWH therapy for 3 to 6 months, followed by warfarin or another vitamin K antagonist or continued LMWH treatment until the cancer is resolved.2
The National Comprehensive Cancer Network guidelines recommend an LMWH for 6 months as monotherapy and indefinite anticoagulation if the cancer is still active.12
The American Society of Clinical Oncology guidelines recommend an LMWH for at least 6 months and indefinite anticoagulant therapy for selected patients with active cancer.13
We agree that patients with active cancer should receive an LMWH for at least 6 months and indefinite anticoagulation until the cancer is resolved.
In our experience, many patients are reluctant to give themselves the daily injections that LMWH therapy requires, and so they need to be well-informed about the marked decrease in VTE recurrence with this less-convenient and more-expensive therapy. Many patients face insurance barriers to cover the cost of LMWH therapy; however, careful attention to preauthorization can usually overcome this obstacle.
HOW LONG TO TREAT RECURRENT VTE
It makes clinical sense that patients who have a second VTE event should be treated indefinitely. This theory was tested in a randomized clinical trial14 in which patients with provoked or unprovoked VTE were randomized after their second event to receive anticoagulation for 6 months vs indefinitely.
After 4 years of follow-up, the recurrence rate was 21% in patients assigned to 6 months of treatment and only 3% in patients who continued anticoagulation throughout the trial. On the other hand, major hemorrhage occurred in 3% of patients treated for 6 months and in 9% in patients who continued anticoagulation indefinitely.
Of note, most of the patients in this trial had unprovoked (idiopathic) VTE, so the results should not be extrapolated to patients with provoked VTE, who accounted for only 20% of the study population.14
Recommendation: Long-term anticoagulation
We agree with the ACCP recommendation2 that patients who have had a second episode of unprovoked VTE should receive long-term anticoagulation. Because of a lack of data, the duration of therapy for patients with a second episode of provoked VTE should be individualized.
HOW LONG TO TREAT THROMBOPHILIA-RELATED VTE
Inherited thrombophilias
Patients with VTE that is not related to a clear provoking risk factor or cancer frequently have testing to evaluate for a hypercoagulable state. This workup traditionally includes the most common inherited thrombophilias for gene mutations for factor V and prothrombin as well as for deficiencies in protein C, protein S, antithrombin and the acquired antiphospholipid syndrome.
The key questions that should be asked prior to embarking on this workup are:
- Will the results change the length of therapy for the patient?
- Will testing the patient help with genetic counseling and possible testing of family members?
- Will the results change the targeted INR range for warfarin or other vitamin K antagonist therapy?
Patients with inherited thrombophilia have a greater risk of developing an initial VTE event; however, these tests do not help predict the recurrence of VTE in patients with established disease more than clinical risk factors do. A prospective study demonstrated this by looking at the effect of thrombophilia and clinical factors on the recurrence of venous thrombosis and found that inherited prothrombotic abnormalities do not appear to play an important role in the risk of a recurrent event.15 On the other hand, clinical factors, such as whether the first event was idiopathic or provoked, appear more important in determining the duration of anticoagulation therapy.15 A systematic review of the common inherited thrombophilias showed the VTE recurrence rate of patients with factor V Leiden was higher than in patients without the mutation; however, the absolute rates of recurrence were not much different than what would be expected in patients with idiopathic VTE.16
A retrospective study involving a large cohort of families of patients who already had experienced a first episode of either idiopathic or provoked VTE showed high annual risks of recurrent VTE associated with hereditary deficiencies of protein S (8.4%), protein C (6.0%), and antithrombin (10%).17 However, for the more commonly occurring genetic thrombophilias, the factor V Leiden and prothrombin G20210A mutations, family members with either gene abnormality had low rates of VTE, suggesting that testing of relatives of probands is not clinically useful.16
Antiphospholipid syndrome
Antiphospholipid syndrome is an acquired thrombophilia. A patient has thrombotic antiphospholipid syndrome when there is a history of vascular thrombosis in the presence of persistently positive tests (at least 12 weeks apart) for lupus anticoagulants, anticardiolipin antibodies, or anti-beta-2 glycoprotein I. A prospective study of 412 patients with a first episode of VTE found that 15% were positive for anticardiolipin antibody at the end of 6 months of anticoagulation. The risk of recurrent VTE after 4 years was 29% in patients with antibodies and 14% in those without antibodies (relative risk 2.1; 95% confidence interval [CI] 1.3–3.3; P =.0013).18
Recent reviews advise indefinite warfarin anticoagulation in patients with VTE and persistence of antiphospholipid antibodies.19 However, the optimal duration of anticoagulation is uncertain. Until well-designed clinical trials are done, the current general consensus is to anticoagulate these patients indefinitely.20,21 Retrospective studies had suggested that patients with antiphospholipid antibodies required a higher therapeutic INR range; however, this observation was tested in two trials that found no difference in thromboembolic rates when patients were randomized to an INR of 2.0–3.0 vs 3.1–4.0,22 or 2.0–3.0 vs 3.0–4.5.23
No formal recommendations
In the absence of strong evidence, the ACCP guidelines do not include a recommendation on the duration of anticoagulation treatment specific to inherited thrombophilias. We believe that clinical factors are more important than inherited thrombophilias for deciding the duration of anticoagulation, and that testing is almost never indicated or useful. However, patients with antiphospholipid syndrome are at high risk of recurrence, and it is our practice to anticoagulate these patients indefinitely.
HOW LONG TO TREAT UNPROVOKED (IDIOPATHIC) VTE
A VTE event is thought to be idiopathic if it occurs without a clearly identified provoking factor.
In an observational study,3 patients with oral contraceptive use, transient illness, immobilization, or a history of travel had an 8.8% risk of recurrence vs 19.4% in patients with unprovoked VTE. The meta-analysis discussed above (Table 1)8 also shows that patients with these nonsurgical risk factors have a lower rate of recurrence than patients with idiopathic VTE.
The high rate of recurrence of idiopathic VTE (4% to 27% after 3 months of anticoagulation24–26) suggests that a longer duration of treatment is reasonable. However, increasing the length of therapy from 3 to 12 months delays but does not prevent recurrence, the risk of which begins to accumulate once anticoagulation is stopped.24,25
Three promising strategies to identify subgroups of patients with idiopathic VTE who are at highest risk of recurrence and who would benefit the most from prolonged anticoagulation are d-dimer testing, evaluation for residual vein thrombosis in patients who present with a deep vein thrombosis, and clinical prediction rules.
d-dimer testing
d-dimer is a degradation product of fibrin and is an indirect marker of residual thrombosis.30
In a systematic review of patients with a first episode of unprovoked VTE,31 a normal d-dimer concentration at the end of at least 3 months of anticoagulation was associated with a 3.5% annual risk of recurrence, whereas an elevated d-dimer level at that time was associated with an annual risk of 8.9%. These results were confirmed in a systematic review of individual patient data.32
In a randomized trial,28 patients with an idiopathic VTE event who received anticoagulation for at least 3 months had their d-dimer level measured 1 month after cessation of treatment. Those with an elevated level were randomized to either resume anticoagulation or not. Patients who resumed anticoagulation had an annual recurrence rate of 2%; however, those who were allocated not to restart anticoagulation had a recurrence rate of 10.9% per year. There was no difference in the rate of major bleeding between the two groups. Patients in this clinical trial who had a normal d-dimer level did not restart anticoagulation and had an annual recurrence rate of 4.4%.
Evaluation for residual thrombosis
A randomized trial34 in patients with both provoked and idiopathic deep vein thrombosis showed a reduction in recurrence when those who had residual vein thrombosis were given extended anticoagulation. In the subset of patients whose deep vein thrombosis was idiopathic, the recurrence rate was 17% per year when treatment lasted only 3 months and 10% when it was extended for up to 1 year.
Another trial35 randomized patients with provoked and idiopathic deep vein thrombosis to receive anticoagulation for the usual duration or to continue treatment until recanalization of the residual thrombus was demonstrated on follow-up Doppler ultrasonography. Patients who received this ultrasonography-tailored treatment had a lower rate of recurrence of VTE; however, the absolute reductions in recurrence rates cannot be calculated from this report for patients with idiopathic deep vein thrombosis.
A prospective observational study36 of the predictive value of d-dimer status and residual vein thrombus found that only d-dimer was an independent risk factor for recurrent VTE after vitamin K antagonist withdrawal.
A clinical prediction rule: ‘Men and HERDOO2’
A promising tool for predicting if a patient is at low risk of recurrent VTE after the first episode of proximal deep vein thrombosis or pulmonary embolism is known by the mnemonic device “Men and HERDOO2.” It is based on data prospectively derived by Rodger et al37 to identify patients with less than a 3% annual risk of recurrent VTE after their first event of idiopathic proximal deep vein thrombosis or pulmonary embolism. Risk factors for recurrent VTE were male sex (the “men” of “Men and HERDOO2”), signs of postthrombotic syndrome, including hyperpigmentation of the lower extremities, edema or redness of either leg, a d-dimer level > 250 μg/L, obesity (body mass index > 30 kg/m2, and older age (> 65 years).
Overall, one-fourth of the population were women with no risk factors or one risk factor, and their risk of recurrence was 1.6% per year. Men and women who had two or more risk factors for postthrombotic syndrome (hyperpigmentation, edema, or redness), elevated d-dimer, obesity, or older age were predicted to be at higher risk of recurrent VTE. Patients such as this should be considered for indefinite anticoagulation.
Ideally, clinical prediction rules should be validated in a separate group of patients before they are used routinely in practice,38 and this clinical prediction rule is currently being tested in the REVERSE II study. If the results are consistent, this will be an easy-to-use tool to help identify patients who likely can safely stop anticoagulation therapy after 3 to 6 months (clinicaltrials.gov Identifier: NCT00967304).
The location of the thrombosis also influences the likelihood of recurrence. Patients with isolated distal (calf) deep vein thrombosis are less likely to suffer recurrent VTE than those who present with proximal deep vein thrombosis. However, trials focusing specifically on the precise subset of idiopathic isolated distal deep vein thrombosis are lacking. In a randomized trial39 comparing 6 vs 12 weeks of anticoagulation for isolated distal deep vein thrombosis and 12 vs 24 weeks for proximal deep vein thrombosis, the annual rates of recurrence after 12 weeks of treatment were approximately 3.4% for isolated distal and 8.1% for proximal deep vein thrombosis.39
Recommendation: At least 3 months of warfarin or equivalent
We agree with the ACCP recommendation2 that patients with unprovoked VTE should receive at least 3 months of anticoagulation with a vitamin K antagonist.
If the patient has no risk factors for bleeding and good anticoagulant monitoring is achievable, we agree with long-term anticoagulation for proximal unprovoked deep vein thrombosis or pulmonary embolism, and 3 months of therapy for isolated distal unprovoked deep vein thrombosis.
Patient preferences and the risk of recurrence vs the risk of bleeding should be discussed with patients when contemplating indefinite anticoagulation.
If testing is being considered to assist in the decision to prescribe indefinite anticoagulation, we prefer using d-dimer levels rather than ultrasonography to detect residual venous thrombosis because of its ease of use and the strength of the current evidence.
PREVENTING POSTTHROMBOTIC SYNDROME
The postthrombotic (postphlebitic) syndrome is a chronic and burdensome consequence of deep vein thrombosis that occurs despite anticoagulation therapy. It is estimated to affect 23% to 60% of patients and typically manifests in the first 2 years.40 It is not only costly in clinical terms, with decreased quality of life for the patient, but health care expenditures have been estimated to range from $400 per year in a Brazilian study to $7,000 per year in a US study.40
Typical symptoms include leg pain, heaviness, swelling, and cramping. In severe cases, chronic venous ulcers can occur and are difficult to treat.41
The definition of postthrombotic syndrome has been unclear over the years, and six different scales that measure signs and symptoms have been reported.42
The Villalta scale has been proposed by the International Society of Thrombosis and Hemostasis as a diagnostic standard to define postthrombotic syndrome.42 This validated scale is based on five clinical symptoms, six clinical signs, and the presence or absence of venous ulcers. Each clinical symptom and sign is scored as mild (1 point), moderate (2 points), or severe (3 points). Symptoms include pain, cramps, heaviness, paresthesia, and pruritus; the six clinical signs are pretibial edema, skin induration, hyperpigmentation, redness, venous ectasia, and pain on calf compression.
According to the International Society of Thrombosis and Hemostasis, postthrombotic syndrome is present if the Villalta score is 5 or greater or if a venous ulcer is present in a leg with previous deep vein thrombosis. Further, using the Villalta scale, postthrombotic syndrome can be categorized as mild (score 5–9), moderate (10–14), or severe (≥ 15).
A limitation of the Villalta scale is that the presence or absence of a venous ulcer has not been assigned a score. Since a venous ulcer requires more aggressive measures, the society defines postthrombotic syndrome as severe if venous ulcers are present.42
Acute symptoms of deep vein thrombosis may take months to resolve and, indeed, acute symptoms may transition to chronic symptoms without a symptom-free interval. It is recommended that postthrombotic syndrome not be diagnosed before 3 months to avoid inappropriately attributing acute symptoms and signs of deep vein thrombosis to the postthrombotic syndrome.42
Studies of stockings
A systematic review of three randomized trials44 concluded that elastic compression stockings reduce the risk of postthrombotic syndrome (any severity) from 43% to 20% and severe postthrombotic syndrome from 15% to 7%.43
The first of these trials44 randomized patients soon after the diagnosis of deep vein thrombosis to receive made-to-order compression stockings that were rated at 30 to 40 mm Hg or to be in a control group that did not receive stockings. The second trial45 randomized patients 1 year after the index event of deep vein thrombosis to receive 20- to 30-mm Hg stockings or stockings that were two sizes too large (the control group). The third study46 randomly allocated patients to receive “off-the-shelf” stockings (30–40 mm Hg) or no stockings. Each study used its own definition of postthrombotic syndrome.
Although these studies strongly suggest compression stockings prevent postthrombotic syndrome, several methodologic issues remain:
- A standard definition of postthrombotic syndrome was not used
- The amount of compression varied between studies
- The studies were not blinded.
Lack of blinding becomes most significant when an outcome is based on subjective findings, like the symptoms that make up a large part of the diagnosis of postthrombotic syndrome.
The SOX trial, currently under way, is designed to address these methodologic issues and should be completed in 2012 (clinicaltrials.gov Identifier: NCT00143598).
Recommendation: Stockings for at least 2 years
We agree with the ACCP recommendation that a patient who has had a symptomatic proximal deep vein thrombosis should wear an elastic compression stocking with an ankle pressure gradient of 30 to 40 mm Hg as soon as possible after starting anticoagulant therapy and continuing for a minimum of 2 years.2
SCREENING FOR OCCULT MALIGNANCY
VTE can be the first manifestation of cancer.
French physician Armand Trousseau, in the 1860s, was the first to describe disseminated intravascular coagulation closely associated with adenocarcinoma. Ironically, several years later, after suffering for weeks from abdominal pain, he declared to one of his students that he had developed thrombosis, and he died of gastric cancer shortly thereafter.47
Since cancer is a well-known risk factor for VTE, it is logical to screen for cancer as an explanation for an idiopathic VTE event.48 To make an informed decision, one needs to understand the rate of occult cancer at the time VTE is diagnosed, the risk of future development of cancer, and the utility of extensive cancer screening.
The clinical efficacy, side effects, and cost-effectiveness of cancer screening in patients with idiopathic VTE are unknown. However, a systematic review47 of 34 studies found that, in patients with idiopathic VTE, cancer was diagnosed within 1 month in 6.1%, within 6 months in 8.6%, and within 1 year in 10.0% (95% CI 8.6–11.3).
A subset of studies compared two strategies for screening soon after the diagnosis of idiopathic VTE: a strategy limited to the history, physical examination, basic blood work, and chest radiography vs an extensive screening strategy that also included serum tumor markers or abdominal ultrasonography or computed tomography. Limited screening detected 49% of the prevalent cancers; extensive screening increased this rate to 70%. Stated another way, the detection rate for prevalent cancers was 5% with limited screening and 7% with extensive screening soon after the diagnosis of idiopathic VTE.47
Patients with idiopathic VTE had higher rates of cancer within 1 month of diagnosis than patients with provoked VTE (6.1% vs 1.9%), and this difference persisted at 1 year (10.0% vs 2.6%).47
Recommendation: Individualized cancer screening
Patients with idiopathic VTE have a significant risk of occult cancer within the first year after diagnosis, and cancer screening should be considered. Our practice for patients with idiopathic VTE is to perform a history and physical examination and ensure that the patient is up to date on age- and sex-specific cancer screening.
The use of additional imaging or biomarkers should be discussed with patients so they can balance the risks (radiation and potential false-positive results with their downstream consequences), costs, and potential benefits, given the lack of proven survival benefit or cost-effectiveness.
ORAL ANTICOAGULANT MANAGEMENT
Warfarin’s multiple interactions, along with the need for INR monitoring, make it a difficult medication to manage.
The Joint Commission, the US organization for health service accreditation and certification, has defined National Patient Safety Goals and quality measures for the management of anticoagulation.49 Organized anticoagulation management services, dosing algorithms, and patient self-testing using capillary INR meters or patient self-management of warfarin were recommended as tools to improve the time patients spend in the therapeutic INR range.50
Two new oral anticoagulants
The limitations of warfarin have stimulated the search for newer oral anticoagulants that do not require laboratory monitoring or have as many diet and drug interactions.
Two trials have been published with experimental oral anticoagulants that had similar efficacy and safety as warfarin in the treatment of VTE.
The study of dabigatran (Pradaxa) vs warfarin in the treatment of acute VTE (the RECOVER trial)51 randomized 2,539 patients with acute VTE to receive the oral direct thrombin inhibitor dabigatran or warfarin for approximately 6 months. Of note, each treatment group received a median of 6 days of heparin, LMWH, or fondaparinux at the beginning of blinded therapy. The rates of recurrent VTE and major bleeding were similar between the treatment arms, and overall bleeding was less with dabigatran. Dabigatran was approved in the United States in October 2010 for stroke prevention in atrial fibrillation but has yet to be approved for the treatment of VTE pending further study (clinicaltrials.gov Identifier: NCT00680186).
A study of oral rivaroxaban (Xarelto) for symptomatic VTE (the EINSTEIN-DVT trial) 52 randomized 3,449 patients with acute deep vein thrombosis to rivaroxaban or enoxaparin (Lovenox) overlapped with warfarin or another vitamin K antagonist in the usual manner. No difference was noted between the treatments in the rate of recurrence of VTE or of major bleeding. Of note, patients randomized to rivaroxaban received 15 mg twice a day for the first 3 weeks of treatment and then 20 mg per day for the remainder of their therapy and did not require parenteral anticoagulant overlap.
The long-awaited promise of easier-to-use oral anticoagulants for the treatment of VTE is drawing near and has the potential to revolutionize the treatment of this common disorder. In the meantime, close monitoring of warfarin and careful patient education regarding its use are essential. And even with the development of new drugs in the future, it is still imperative that patients with acute VTE receive the correct length of anticoagulation treatment, are prescribed stockings to prevent postthrombotic syndrome, and are updated on routine cancer screening.
Deep vein thrombosis and pulmonary embolism are collectively referred to as venous thromboembolic (VTE) disease. They affect approximately 100,000 to 300,000 patients per year in the United States.1 Although patients with deep vein thrombosis can be treated as outpatients, many are admitted for the initiation of anticoagulation. Initial anticoagulation usually requires the overlap of a parenteral anticoagulant (unfractionated heparin, low-molecular-weight heparin [LMWH] or fondaparinux) with warfarin for a minimum of 5 days and until the international normalized ratio (INR) of the prothrombin time is above 2.0 for at least 24 hours.2
Three clinical issues need to be addressed after the initiation of anticoagulation for VTE:
- Determination of the length of anticoagulation with the correct anticoagulant
- Prevention of postthrombotic syndrome
- Appropriate screening for occult malignancy.
HOW LONG SHOULD VTE BE TREATED?
The duration of anticoagulation has been a matter of debate.
The risk of recurrent VTE appears related to clinical risk factors that a patient has at the time of the initial thrombotic event. An epidemiologic study3 found that patients with VTE treated for approximately 6 months had a low rate of recurrence (0% at 2 years of follow-up) if surgery was the risk factor. The risk climbed to 9% if the risk factor was nonsurgical and to 19% if there were no provoking risk factors.
The likelihood of VTE recurrence and therefore the recommended duration of treatment depend on whether the VTE event was provoked, cancer-related, recurrent, thrombophilia-related, or idiopathic. We address each of these scenarios below.
HOW LONG TO TREAT PROVOKED VTE
A VTE event is considered provoked if the patient had a clear inciting risk factor. As defined in various clinical trials, these risk factors include:
- Hospitalization with confinement to bed for 3 or more consecutive days in the last 3 months
- Surgery or general anesthesia in the last 3 months
- Immobilization for more than 7 days, regardless of the cause
- Trauma in the last 3 months
- Pregnancy
- Use of an oral contraceptive, regardless of which estrogen or progesterone analogue it contains
- Travel for more than 4 hours
- Recent childbirth.
However, the trials that tested different lengths of anticoagulation have varied markedly in how they defined provoked deep vein thrombosis.4–7
Recommendation: Warfarin or equivalent for 3 months
The American College of Chest Physicians (ACCP) recommends 3 months of anticoagulation with warfarin or another vitamin K antagonist for patients with VTE secondary to a transient (reversible) risk factor,2 and we agree.
HOW LONG TO TREAT CANCER-RELATED VTE
Patients with cancer are at higher risk of developing VTE. Furthermore, in one study,9 compared with other patients with VTE, patients with cancer were three times more likely to have another episode of VTE, with a cumulative rate of recurrence at 1 year of 21% vs 7%. Cancer patients were also twice as likely to suffer major bleeding complications while on anticoagulation.9
Warfarin is a difficult drug to manage because it has many interactions with foods, diseases, and other drugs. These difficulties are amplified in many cancer patients during chemotherapy.
Warfarin was compared with a LMWH in four randomized trials in cancer patients, and a meta-analysis10 found a 50% relative reduction in the rates of recurrent deep vein thrombosis and pulmonary embolism with LMWH treatment. These results were driven primarily by the CLOT trial (Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients With Cancer),11 which showed an 8% absolute risk reduction (number needed to treat 13) without an increase in major bleeding when cancer-related VTE was treated with an LMWH—ie, dalteparin (Fragmin)—for 6 months compared with warfarin.
Current thinking suggests that VTE should be treated until the cancer is resolved. However, this hypothesis has not been adequately tested, and consequently, the ACCP gives it only a level 1C recommendation.2 The largest of the four trials comparing warfarin and an LMWH lasted only 6 months. The safety of extending LMWH treatment beyond 6 months is currently unknown but is under investigation (clinicaltrials.gov identifier NCT00942968).
Recommendation: LMWH therapy for at least 6 months
The ACCP guidelines recommend LMWH therapy for 3 to 6 months, followed by warfarin or another vitamin K antagonist or continued LMWH treatment until the cancer is resolved.2
The National Comprehensive Cancer Network guidelines recommend an LMWH for 6 months as monotherapy and indefinite anticoagulation if the cancer is still active.12
The American Society of Clinical Oncology guidelines recommend an LMWH for at least 6 months and indefinite anticoagulant therapy for selected patients with active cancer.13
We agree that patients with active cancer should receive an LMWH for at least 6 months and indefinite anticoagulation until the cancer is resolved.
In our experience, many patients are reluctant to give themselves the daily injections that LMWH therapy requires, and so they need to be well-informed about the marked decrease in VTE recurrence with this less-convenient and more-expensive therapy. Many patients face insurance barriers to cover the cost of LMWH therapy; however, careful attention to preauthorization can usually overcome this obstacle.
HOW LONG TO TREAT RECURRENT VTE
It makes clinical sense that patients who have a second VTE event should be treated indefinitely. This theory was tested in a randomized clinical trial14 in which patients with provoked or unprovoked VTE were randomized after their second event to receive anticoagulation for 6 months vs indefinitely.
After 4 years of follow-up, the recurrence rate was 21% in patients assigned to 6 months of treatment and only 3% in patients who continued anticoagulation throughout the trial. On the other hand, major hemorrhage occurred in 3% of patients treated for 6 months and in 9% in patients who continued anticoagulation indefinitely.
Of note, most of the patients in this trial had unprovoked (idiopathic) VTE, so the results should not be extrapolated to patients with provoked VTE, who accounted for only 20% of the study population.14
Recommendation: Long-term anticoagulation
We agree with the ACCP recommendation2 that patients who have had a second episode of unprovoked VTE should receive long-term anticoagulation. Because of a lack of data, the duration of therapy for patients with a second episode of provoked VTE should be individualized.
HOW LONG TO TREAT THROMBOPHILIA-RELATED VTE
Inherited thrombophilias
Patients with VTE that is not related to a clear provoking risk factor or cancer frequently have testing to evaluate for a hypercoagulable state. This workup traditionally includes the most common inherited thrombophilias for gene mutations for factor V and prothrombin as well as for deficiencies in protein C, protein S, antithrombin and the acquired antiphospholipid syndrome.
The key questions that should be asked prior to embarking on this workup are:
- Will the results change the length of therapy for the patient?
- Will testing the patient help with genetic counseling and possible testing of family members?
- Will the results change the targeted INR range for warfarin or other vitamin K antagonist therapy?
Patients with inherited thrombophilia have a greater risk of developing an initial VTE event; however, these tests do not help predict the recurrence of VTE in patients with established disease more than clinical risk factors do. A prospective study demonstrated this by looking at the effect of thrombophilia and clinical factors on the recurrence of venous thrombosis and found that inherited prothrombotic abnormalities do not appear to play an important role in the risk of a recurrent event.15 On the other hand, clinical factors, such as whether the first event was idiopathic or provoked, appear more important in determining the duration of anticoagulation therapy.15 A systematic review of the common inherited thrombophilias showed the VTE recurrence rate of patients with factor V Leiden was higher than in patients without the mutation; however, the absolute rates of recurrence were not much different than what would be expected in patients with idiopathic VTE.16
A retrospective study involving a large cohort of families of patients who already had experienced a first episode of either idiopathic or provoked VTE showed high annual risks of recurrent VTE associated with hereditary deficiencies of protein S (8.4%), protein C (6.0%), and antithrombin (10%).17 However, for the more commonly occurring genetic thrombophilias, the factor V Leiden and prothrombin G20210A mutations, family members with either gene abnormality had low rates of VTE, suggesting that testing of relatives of probands is not clinically useful.16
Antiphospholipid syndrome
Antiphospholipid syndrome is an acquired thrombophilia. A patient has thrombotic antiphospholipid syndrome when there is a history of vascular thrombosis in the presence of persistently positive tests (at least 12 weeks apart) for lupus anticoagulants, anticardiolipin antibodies, or anti-beta-2 glycoprotein I. A prospective study of 412 patients with a first episode of VTE found that 15% were positive for anticardiolipin antibody at the end of 6 months of anticoagulation. The risk of recurrent VTE after 4 years was 29% in patients with antibodies and 14% in those without antibodies (relative risk 2.1; 95% confidence interval [CI] 1.3–3.3; P =.0013).18
Recent reviews advise indefinite warfarin anticoagulation in patients with VTE and persistence of antiphospholipid antibodies.19 However, the optimal duration of anticoagulation is uncertain. Until well-designed clinical trials are done, the current general consensus is to anticoagulate these patients indefinitely.20,21 Retrospective studies had suggested that patients with antiphospholipid antibodies required a higher therapeutic INR range; however, this observation was tested in two trials that found no difference in thromboembolic rates when patients were randomized to an INR of 2.0–3.0 vs 3.1–4.0,22 or 2.0–3.0 vs 3.0–4.5.23
No formal recommendations
In the absence of strong evidence, the ACCP guidelines do not include a recommendation on the duration of anticoagulation treatment specific to inherited thrombophilias. We believe that clinical factors are more important than inherited thrombophilias for deciding the duration of anticoagulation, and that testing is almost never indicated or useful. However, patients with antiphospholipid syndrome are at high risk of recurrence, and it is our practice to anticoagulate these patients indefinitely.
HOW LONG TO TREAT UNPROVOKED (IDIOPATHIC) VTE
A VTE event is thought to be idiopathic if it occurs without a clearly identified provoking factor.
In an observational study,3 patients with oral contraceptive use, transient illness, immobilization, or a history of travel had an 8.8% risk of recurrence vs 19.4% in patients with unprovoked VTE. The meta-analysis discussed above (Table 1)8 also shows that patients with these nonsurgical risk factors have a lower rate of recurrence than patients with idiopathic VTE.
The high rate of recurrence of idiopathic VTE (4% to 27% after 3 months of anticoagulation24–26) suggests that a longer duration of treatment is reasonable. However, increasing the length of therapy from 3 to 12 months delays but does not prevent recurrence, the risk of which begins to accumulate once anticoagulation is stopped.24,25
Three promising strategies to identify subgroups of patients with idiopathic VTE who are at highest risk of recurrence and who would benefit the most from prolonged anticoagulation are d-dimer testing, evaluation for residual vein thrombosis in patients who present with a deep vein thrombosis, and clinical prediction rules.
d-dimer testing
d-dimer is a degradation product of fibrin and is an indirect marker of residual thrombosis.30
In a systematic review of patients with a first episode of unprovoked VTE,31 a normal d-dimer concentration at the end of at least 3 months of anticoagulation was associated with a 3.5% annual risk of recurrence, whereas an elevated d-dimer level at that time was associated with an annual risk of 8.9%. These results were confirmed in a systematic review of individual patient data.32
In a randomized trial,28 patients with an idiopathic VTE event who received anticoagulation for at least 3 months had their d-dimer level measured 1 month after cessation of treatment. Those with an elevated level were randomized to either resume anticoagulation or not. Patients who resumed anticoagulation had an annual recurrence rate of 2%; however, those who were allocated not to restart anticoagulation had a recurrence rate of 10.9% per year. There was no difference in the rate of major bleeding between the two groups. Patients in this clinical trial who had a normal d-dimer level did not restart anticoagulation and had an annual recurrence rate of 4.4%.
Evaluation for residual thrombosis
A randomized trial34 in patients with both provoked and idiopathic deep vein thrombosis showed a reduction in recurrence when those who had residual vein thrombosis were given extended anticoagulation. In the subset of patients whose deep vein thrombosis was idiopathic, the recurrence rate was 17% per year when treatment lasted only 3 months and 10% when it was extended for up to 1 year.
Another trial35 randomized patients with provoked and idiopathic deep vein thrombosis to receive anticoagulation for the usual duration or to continue treatment until recanalization of the residual thrombus was demonstrated on follow-up Doppler ultrasonography. Patients who received this ultrasonography-tailored treatment had a lower rate of recurrence of VTE; however, the absolute reductions in recurrence rates cannot be calculated from this report for patients with idiopathic deep vein thrombosis.
A prospective observational study36 of the predictive value of d-dimer status and residual vein thrombus found that only d-dimer was an independent risk factor for recurrent VTE after vitamin K antagonist withdrawal.
A clinical prediction rule: ‘Men and HERDOO2’
A promising tool for predicting if a patient is at low risk of recurrent VTE after the first episode of proximal deep vein thrombosis or pulmonary embolism is known by the mnemonic device “Men and HERDOO2.” It is based on data prospectively derived by Rodger et al37 to identify patients with less than a 3% annual risk of recurrent VTE after their first event of idiopathic proximal deep vein thrombosis or pulmonary embolism. Risk factors for recurrent VTE were male sex (the “men” of “Men and HERDOO2”), signs of postthrombotic syndrome, including hyperpigmentation of the lower extremities, edema or redness of either leg, a d-dimer level > 250 μg/L, obesity (body mass index > 30 kg/m2, and older age (> 65 years).
Overall, one-fourth of the population were women with no risk factors or one risk factor, and their risk of recurrence was 1.6% per year. Men and women who had two or more risk factors for postthrombotic syndrome (hyperpigmentation, edema, or redness), elevated d-dimer, obesity, or older age were predicted to be at higher risk of recurrent VTE. Patients such as this should be considered for indefinite anticoagulation.
Ideally, clinical prediction rules should be validated in a separate group of patients before they are used routinely in practice,38 and this clinical prediction rule is currently being tested in the REVERSE II study. If the results are consistent, this will be an easy-to-use tool to help identify patients who likely can safely stop anticoagulation therapy after 3 to 6 months (clinicaltrials.gov Identifier: NCT00967304).
The location of the thrombosis also influences the likelihood of recurrence. Patients with isolated distal (calf) deep vein thrombosis are less likely to suffer recurrent VTE than those who present with proximal deep vein thrombosis. However, trials focusing specifically on the precise subset of idiopathic isolated distal deep vein thrombosis are lacking. In a randomized trial39 comparing 6 vs 12 weeks of anticoagulation for isolated distal deep vein thrombosis and 12 vs 24 weeks for proximal deep vein thrombosis, the annual rates of recurrence after 12 weeks of treatment were approximately 3.4% for isolated distal and 8.1% for proximal deep vein thrombosis.39
Recommendation: At least 3 months of warfarin or equivalent
We agree with the ACCP recommendation2 that patients with unprovoked VTE should receive at least 3 months of anticoagulation with a vitamin K antagonist.
If the patient has no risk factors for bleeding and good anticoagulant monitoring is achievable, we agree with long-term anticoagulation for proximal unprovoked deep vein thrombosis or pulmonary embolism, and 3 months of therapy for isolated distal unprovoked deep vein thrombosis.
Patient preferences and the risk of recurrence vs the risk of bleeding should be discussed with patients when contemplating indefinite anticoagulation.
If testing is being considered to assist in the decision to prescribe indefinite anticoagulation, we prefer using d-dimer levels rather than ultrasonography to detect residual venous thrombosis because of its ease of use and the strength of the current evidence.
PREVENTING POSTTHROMBOTIC SYNDROME
The postthrombotic (postphlebitic) syndrome is a chronic and burdensome consequence of deep vein thrombosis that occurs despite anticoagulation therapy. It is estimated to affect 23% to 60% of patients and typically manifests in the first 2 years.40 It is not only costly in clinical terms, with decreased quality of life for the patient, but health care expenditures have been estimated to range from $400 per year in a Brazilian study to $7,000 per year in a US study.40
Typical symptoms include leg pain, heaviness, swelling, and cramping. In severe cases, chronic venous ulcers can occur and are difficult to treat.41
The definition of postthrombotic syndrome has been unclear over the years, and six different scales that measure signs and symptoms have been reported.42
The Villalta scale has been proposed by the International Society of Thrombosis and Hemostasis as a diagnostic standard to define postthrombotic syndrome.42 This validated scale is based on five clinical symptoms, six clinical signs, and the presence or absence of venous ulcers. Each clinical symptom and sign is scored as mild (1 point), moderate (2 points), or severe (3 points). Symptoms include pain, cramps, heaviness, paresthesia, and pruritus; the six clinical signs are pretibial edema, skin induration, hyperpigmentation, redness, venous ectasia, and pain on calf compression.
According to the International Society of Thrombosis and Hemostasis, postthrombotic syndrome is present if the Villalta score is 5 or greater or if a venous ulcer is present in a leg with previous deep vein thrombosis. Further, using the Villalta scale, postthrombotic syndrome can be categorized as mild (score 5–9), moderate (10–14), or severe (≥ 15).
A limitation of the Villalta scale is that the presence or absence of a venous ulcer has not been assigned a score. Since a venous ulcer requires more aggressive measures, the society defines postthrombotic syndrome as severe if venous ulcers are present.42
Acute symptoms of deep vein thrombosis may take months to resolve and, indeed, acute symptoms may transition to chronic symptoms without a symptom-free interval. It is recommended that postthrombotic syndrome not be diagnosed before 3 months to avoid inappropriately attributing acute symptoms and signs of deep vein thrombosis to the postthrombotic syndrome.42
Studies of stockings
A systematic review of three randomized trials44 concluded that elastic compression stockings reduce the risk of postthrombotic syndrome (any severity) from 43% to 20% and severe postthrombotic syndrome from 15% to 7%.43
The first of these trials44 randomized patients soon after the diagnosis of deep vein thrombosis to receive made-to-order compression stockings that were rated at 30 to 40 mm Hg or to be in a control group that did not receive stockings. The second trial45 randomized patients 1 year after the index event of deep vein thrombosis to receive 20- to 30-mm Hg stockings or stockings that were two sizes too large (the control group). The third study46 randomly allocated patients to receive “off-the-shelf” stockings (30–40 mm Hg) or no stockings. Each study used its own definition of postthrombotic syndrome.
Although these studies strongly suggest compression stockings prevent postthrombotic syndrome, several methodologic issues remain:
- A standard definition of postthrombotic syndrome was not used
- The amount of compression varied between studies
- The studies were not blinded.
Lack of blinding becomes most significant when an outcome is based on subjective findings, like the symptoms that make up a large part of the diagnosis of postthrombotic syndrome.
The SOX trial, currently under way, is designed to address these methodologic issues and should be completed in 2012 (clinicaltrials.gov Identifier: NCT00143598).
Recommendation: Stockings for at least 2 years
We agree with the ACCP recommendation that a patient who has had a symptomatic proximal deep vein thrombosis should wear an elastic compression stocking with an ankle pressure gradient of 30 to 40 mm Hg as soon as possible after starting anticoagulant therapy and continuing for a minimum of 2 years.2
SCREENING FOR OCCULT MALIGNANCY
VTE can be the first manifestation of cancer.
French physician Armand Trousseau, in the 1860s, was the first to describe disseminated intravascular coagulation closely associated with adenocarcinoma. Ironically, several years later, after suffering for weeks from abdominal pain, he declared to one of his students that he had developed thrombosis, and he died of gastric cancer shortly thereafter.47
Since cancer is a well-known risk factor for VTE, it is logical to screen for cancer as an explanation for an idiopathic VTE event.48 To make an informed decision, one needs to understand the rate of occult cancer at the time VTE is diagnosed, the risk of future development of cancer, and the utility of extensive cancer screening.
The clinical efficacy, side effects, and cost-effectiveness of cancer screening in patients with idiopathic VTE are unknown. However, a systematic review47 of 34 studies found that, in patients with idiopathic VTE, cancer was diagnosed within 1 month in 6.1%, within 6 months in 8.6%, and within 1 year in 10.0% (95% CI 8.6–11.3).
A subset of studies compared two strategies for screening soon after the diagnosis of idiopathic VTE: a strategy limited to the history, physical examination, basic blood work, and chest radiography vs an extensive screening strategy that also included serum tumor markers or abdominal ultrasonography or computed tomography. Limited screening detected 49% of the prevalent cancers; extensive screening increased this rate to 70%. Stated another way, the detection rate for prevalent cancers was 5% with limited screening and 7% with extensive screening soon after the diagnosis of idiopathic VTE.47
Patients with idiopathic VTE had higher rates of cancer within 1 month of diagnosis than patients with provoked VTE (6.1% vs 1.9%), and this difference persisted at 1 year (10.0% vs 2.6%).47
Recommendation: Individualized cancer screening
Patients with idiopathic VTE have a significant risk of occult cancer within the first year after diagnosis, and cancer screening should be considered. Our practice for patients with idiopathic VTE is to perform a history and physical examination and ensure that the patient is up to date on age- and sex-specific cancer screening.
The use of additional imaging or biomarkers should be discussed with patients so they can balance the risks (radiation and potential false-positive results with their downstream consequences), costs, and potential benefits, given the lack of proven survival benefit or cost-effectiveness.
ORAL ANTICOAGULANT MANAGEMENT
Warfarin’s multiple interactions, along with the need for INR monitoring, make it a difficult medication to manage.
The Joint Commission, the US organization for health service accreditation and certification, has defined National Patient Safety Goals and quality measures for the management of anticoagulation.49 Organized anticoagulation management services, dosing algorithms, and patient self-testing using capillary INR meters or patient self-management of warfarin were recommended as tools to improve the time patients spend in the therapeutic INR range.50
Two new oral anticoagulants
The limitations of warfarin have stimulated the search for newer oral anticoagulants that do not require laboratory monitoring or have as many diet and drug interactions.
Two trials have been published with experimental oral anticoagulants that had similar efficacy and safety as warfarin in the treatment of VTE.
The study of dabigatran (Pradaxa) vs warfarin in the treatment of acute VTE (the RECOVER trial)51 randomized 2,539 patients with acute VTE to receive the oral direct thrombin inhibitor dabigatran or warfarin for approximately 6 months. Of note, each treatment group received a median of 6 days of heparin, LMWH, or fondaparinux at the beginning of blinded therapy. The rates of recurrent VTE and major bleeding were similar between the treatment arms, and overall bleeding was less with dabigatran. Dabigatran was approved in the United States in October 2010 for stroke prevention in atrial fibrillation but has yet to be approved for the treatment of VTE pending further study (clinicaltrials.gov Identifier: NCT00680186).
A study of oral rivaroxaban (Xarelto) for symptomatic VTE (the EINSTEIN-DVT trial) 52 randomized 3,449 patients with acute deep vein thrombosis to rivaroxaban or enoxaparin (Lovenox) overlapped with warfarin or another vitamin K antagonist in the usual manner. No difference was noted between the treatments in the rate of recurrence of VTE or of major bleeding. Of note, patients randomized to rivaroxaban received 15 mg twice a day for the first 3 weeks of treatment and then 20 mg per day for the remainder of their therapy and did not require parenteral anticoagulant overlap.
The long-awaited promise of easier-to-use oral anticoagulants for the treatment of VTE is drawing near and has the potential to revolutionize the treatment of this common disorder. In the meantime, close monitoring of warfarin and careful patient education regarding its use are essential. And even with the development of new drugs in the future, it is still imperative that patients with acute VTE receive the correct length of anticoagulation treatment, are prescribed stockings to prevent postthrombotic syndrome, and are updated on routine cancer screening.
- Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006; 21:722–727.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet 2003; 362:523–526.
- Schulman S, Lockner D, Juhlin-Dannfelt A. The duration of oral anticoagulation after deep vein thrombosis. A randomized study. Acta Med Scand 1985; 217:547–552.
- Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Research Committee of the British Thoracic Society. Lancet 1992; 340:873–876.
- Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995; 332:1661–1665.
- Kearon C, Ginsberg JS, Anderson DR, et al. Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J Thromb Haemost 2004; 2:743–749.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 2010; 170:1710–1716.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100:3484–3488.
- Hull RD, Pineo GF, Brant RF, et al; LITE Trial Investigators. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006; 119:1062–1072.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349:146–153.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology, Venous Thromboembolic Disease. http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed August 3, 2011.
- Lyman GH, Khorana AA, Falanga A, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Schulman S, Granqvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009; 301:2472–2485.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost 2009; 101:93–99.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med 1998; 104:332–338.
- Derksen RH, de Groot PG. Towards evidence-based treatment of thrombotic antiphospholipid syndrome. Lupus 2010; 19:470–474.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006; 295:1050–1057.
- Fonseca AG, D’Cruz DP. Controversies in the antiphospholipid syndrome: can we ever stop warfarin? J Autoimmune Dis 2008; 5:6.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005; 3:848–853.
- Agnelli G, Prandoni P, Becattini C, et al; Warfarin Optimal Duration Italian Trial Investigators. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003; 139:19–25.
- Agnelli G, Prandoni P, Santamaria MG, et al; Warfarin Optimal Duration Italian Trial Investigators. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Ridker PM, Goldhaber SZ, Glynn RJ. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:2164–2167.
- Bockenstedt P. D-dimer in venous thromboembolism. N Engl J Med 2003; 349:1203–1204.
- Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008; 149:481–490,W94.
- Douketis J, Tosetto A, Marcucci M, et al. Patient-level metaanalysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010; 153:523–531.
- Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002; 137:955–960.
- Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood 2008; 112:511–515.
- Prandoni P, Prins MH, Lensing AW, et al; AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009; 150:577–585.
- Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost 2005; 94:969–974.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000; 284:79–84.
- Pinede L, Ninet J, Duhaut P, et al; Investigators of the “Durée Optimale du Traitement AntiVitamines K” (DOTAVK) Study. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001; 103:2453–2460.
- Ashrani AA, Heit JA. Incidence and cost burden of postthrombotic syndrome. J Thromb Thrombolysis 2009; 28:465–476.
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149:698–707.
- Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009; 7:879–883.
- Kolbach DN, Sandbrink MW, Hamulyak K, Neumann HA, Prins MH. Non-pharmaceutical measures for prevention of post-thrombotic syndrome. Cochrane Database Syst Rev 2004;CD004174.
- Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997; 349:759–762.
- Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med 2001; 161:2105–2109.
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004; 141:249–256.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med 2008; 149:323–333.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Kaatz S. Impact on patient care: patient case through the continuum of care. J Thromb Thrombolysis 2010; 29:167–170.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Schulman S, Kearon C, Kakkar AK, et al; for the RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2452.
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363;2499–2510.
- Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006; 21:722–727.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet 2003; 362:523–526.
- Schulman S, Lockner D, Juhlin-Dannfelt A. The duration of oral anticoagulation after deep vein thrombosis. A randomized study. Acta Med Scand 1985; 217:547–552.
- Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Research Committee of the British Thoracic Society. Lancet 1992; 340:873–876.
- Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995; 332:1661–1665.
- Kearon C, Ginsberg JS, Anderson DR, et al. Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J Thromb Haemost 2004; 2:743–749.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 2010; 170:1710–1716.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100:3484–3488.
- Hull RD, Pineo GF, Brant RF, et al; LITE Trial Investigators. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006; 119:1062–1072.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349:146–153.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology, Venous Thromboembolic Disease. http://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed August 3, 2011.
- Lyman GH, Khorana AA, Falanga A, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Schulman S, Granqvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009; 301:2472–2485.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost 2009; 101:93–99.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med 1998; 104:332–338.
- Derksen RH, de Groot PG. Towards evidence-based treatment of thrombotic antiphospholipid syndrome. Lupus 2010; 19:470–474.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006; 295:1050–1057.
- Fonseca AG, D’Cruz DP. Controversies in the antiphospholipid syndrome: can we ever stop warfarin? J Autoimmune Dis 2008; 5:6.
- Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138.
- Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005; 3:848–853.
- Agnelli G, Prandoni P, Becattini C, et al; Warfarin Optimal Duration Italian Trial Investigators. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003; 139:19–25.
- Agnelli G, Prandoni P, Santamaria MG, et al; Warfarin Optimal Duration Italian Trial Investigators. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Kearon C, Ginsberg JS, Kovacs MJ, et al; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:631–639.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Ridker PM, Goldhaber SZ, Glynn RJ. Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349:2164–2167.
- Bockenstedt P. D-dimer in venous thromboembolism. N Engl J Med 2003; 349:1203–1204.
- Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008; 149:481–490,W94.
- Douketis J, Tosetto A, Marcucci M, et al. Patient-level metaanalysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010; 153:523–531.
- Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002; 137:955–960.
- Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood 2008; 112:511–515.
- Prandoni P, Prins MH, Lensing AW, et al; AESOPUS Investigators. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009; 150:577–585.
- Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost 2005; 94:969–974.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000; 284:79–84.
- Pinede L, Ninet J, Duhaut P, et al; Investigators of the “Durée Optimale du Traitement AntiVitamines K” (DOTAVK) Study. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001; 103:2453–2460.
- Ashrani AA, Heit JA. Incidence and cost burden of postthrombotic syndrome. J Thromb Thrombolysis 2009; 28:465–476.
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149:698–707.
- Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009; 7:879–883.
- Kolbach DN, Sandbrink MW, Hamulyak K, Neumann HA, Prins MH. Non-pharmaceutical measures for prevention of post-thrombotic syndrome. Cochrane Database Syst Rev 2004;CD004174.
- Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997; 349:759–762.
- Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med 2001; 161:2105–2109.
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004; 141:249–256.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med 2008; 149:323–333.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Kaatz S. Impact on patient care: patient case through the continuum of care. J Thromb Thrombolysis 2010; 29:167–170.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):160S–198S.
- Schulman S, Kearon C, Kakkar AK, et al; for the RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2452.
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363;2499–2510.
KEY POINTS
- A low-molecular-weight heparin for at least 6 months is the treatment of choice for cancer-related VTE.
- We recommend 3 months of anticoagulation for VTE caused by a reversible risk factor and indefinite treatment for idiopathic VTE in patients without risk factors for bleeding who can get anticoagulation monitoring.
- Clinical factors are more important in deciding the duration of anticoagulation therapy than evidence of an inherited thrombophilic state.
- Elastic compression stockings reduce the risk of postthrombotic syndrome substantially.
- Patients with idiopathic VTE should have a basic screening for malignancy.
Unmasking gastric cancer
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
Hepatic encephalopathy: Suspect it early in patients with cirrhosis
Hepatic encephalopathy is a serious but often reversible complication that arises when the liver cannot detoxify the portal venous blood (Table 1).1
PROPOSED PATHOGENETIC FACTORS
About 5.5 million cases of chronic liver disease and cirrhosis were reported in the United States in 2001. Hepatic encephalopathy is becoming more common as the prevalence of cirrhosis increases,2 and this will have important economic repercussions; in 2001, charges from hospitalizations because of hepatic encephalopathy were estimated at $932 million.3
Hepatic encephalopathy develops as cirrhosis progresses or as a result of portosystemic shunting, so that the liver cannot detoxify the portal venous blood. Several neurotoxins (notably ammonia) and inflammatory mediators play key roles in its pathogenesis, inducing low-grade brain edema and producing a wide spectrum of neuropsychiatric manifestations.4 Yet its pathogenesis is not entirely understood, impeding advances in its diagnosis and therapy.
Several hypotheses about the pathogenesis of hepatic encephalopathy have emerged in the last few years, and a number of factors are reported to directly or indirectly affect brain function in this condition. Ammonia and glutamine are the neurotoxins most often implicated in this syndrome5; others include inflammatory mediators, certain amino acids, and manganese.5,6
Ammonia causes brain swelling
Ammonia is primarily the byproduct of bacterial metabolism of protein and nitrogenous compounds in the colon and of glutamine metabolism in enterocytes.7
Normally, gut-absorbed ammonia is delivered via the portal vein to the liver, where most of it is metabolized into urea, leaving a small amount to be metabolized in the muscles, heart, brain, and kidneys. In cirrhosis and other conditions associated with hepatic encephalopathy, less ammonia is metabolized into urea and more of it reaches the astrocytes in the brain. The brain lacks a urea cycle but metabolizes ammonia to glutamine via glutamine synthase, an enzyme unique to astrocytes.
Ammonia causes swelling of astrocytes and brain edema via generation of glutamine, an osmotically active substance.
Glutamine causes swelling, oxidative stress
Glutamine draws water into astrocytes and induces changes of type II astrocytosis (also called Alzheimer type II astrocytosis)5 characterized by swelling, enlarged and pale nuclei, and displacement of chromatin to the periphery of the cell. Inhibition of glutamine synthase prevents astrocyte swelling in animals.8
Glutamine also enhances the activation of several receptors, including N-methyl-d-aspartate (NMDA) receptors,9,10 gammaaminobutyric acid (GABA) receptors, and peripheral-type benzodiazepine receptors on the mitochondrial membrane.10–12 A state of oxidative stress ensues, and this affects oxidation of protein and RNA, neurotransmitter synthesis, and neurotransmission at the neuronal junction.13 Reactive nitrogen and oxide radicals induce the release of inflammatory mediators such as interleukins 1 and 6, tumor necrosis factor, interferons, and neurosteroids, and contribute to edema and neurotoxicity.6,10 Neurosteroids are byproducts of mitochondrial metabolism of steroid hormones in the astrocyte.
Manganese enhances neurosteroid synthesis
Manganese enhances neurosteroid synthesis via activation of translocator proteins on the astrocyte membrane. It was first recognized as a factor in hepatic encephalopathy when cirrhotic patients experiencing extrapyramidal symptoms were found to have deposits of manganese in the caudate nucleus and in the globus pallidus on magnetic resonance imaging (MRI). Such deposits were also seen in specimens of brain tissue on autopsy of these patients. When the encephalopathy resolved, so did the abnormalities on MRI.14,15
Changes in the blood-brain barrier
Astrocytes contribute to the selective permeability of the blood-brain barrier. Disruptions in the permeability of the blood-brain barrier underlie hepatic encephalopathy, with poor diffusion of molecules out of astrocytes.
For instance, zinc, which plays a regulatory role in gene transcription and synaptic plasticity, accumulates in the astrocytes, causing relative zinc deficiency and further affecting neurotransmitter synthesis and neurotransmission at the neuronal synapse.6,16
Hyponatremia
Hyponatremia (a serum sodium concentration < 130 mmol/L) is increasingly being recognized as an independent predictor of overt hepatic encephalopathy and is reported to increase the risk by a factor of eight.17
Neuronal dysfunction
Astrocytes are integral to the physiologic functioning of the neurons, and it is becoming clear that both neurons and astrocytes are affected in hepatic encephalopathy.
Additionally, neuroinflammation and a decrease in energy metabolism by the brain are described during episodes of hepatic encephalopathy.18
Amino acid imbalance
An imbalance between aromatic amino acids (ie, high levels of tyrosine and phenylalanine) and branched-chain amino acids (leucine, isoleucine, and valine) has been linked with encephalopathy in patients with liver disease, 19–21 but it is not totally clear whether this imbalance contributes to hepatic encephalopathy or is a consequence of it.
Low-grade brain edema
Edema of the brain occurs in all forms of hepatic encephalopathy, but in cirrhosis it is characteristically of low grade. The mechanism behind this low-grade edema is not clear. Studies have shown that swelling of astrocytes is not global but involves certain areas of the brain and is associated with compensatory extrusion of intracellular myoinositol.22 This, in combination with a mild degree of brain atrophy23 observed in patients with chronic liver disease, is thought to keep the brain from extreme swelling and herniation, a phenomenon usually seen in acute hepatic failure.24,25
Transjugular intrahepatic portosystemic shunting and encephalopathy
The incidence rate of hepatic encephalopathy after placement of a portosystemic shunt to treat portal hypertension ranges from 30% to 55% and is similar to the rate in cirrhotic patients without a shunt.26 In 5% to 8% of patients, the hepatic encephalopathy is refractory and requires intentional occlusion of the shunt.26,27 An elevated serum creatinine level appears to be a risk factor for refractory hepatic encephalopathy in patients with a portosystemic shunt.26
In one study,28 when transjugular intrahepatic portosystemic shunting was done early in the treatment of cirrhotic patients with acute variceal bleeding, the rates of treatment failure and death were significantly less than in a control group that received endoscopic therapy, and no significant difference was noted in the rate of encephalopathy or of serious adverse effects between the groups.
Whether to place a portosystemic shunt in a patient with cirrhosis and a history of hepatic encephalopathy depends on the possible underlying causes of the encephalopathy. For example, if encephalopathy was precipitated by variceal bleeding, shunt placement will prevent further bleeding and will make a recurrence of encephalopathy less likely. However, if the encephalopathy is persistent and uncontrollable, then shunt placement is contraindicated.27
A SPECTRUM OF SYMPTOMS
The spectrum of symptoms extends from a subclinical syndrome that may not be clinically apparent (early-stage or “minimal” hepatic encephalopathy) to full-blown neuropsychiatric manifestations such as cognitive impairment, confusion, slow speech, loss of fine motor skills, asterixis, peripheral neuropathy, clonus, the Babinski sign, decerebrate and decorticate posturing, seizures, extrapyramidal symptoms, and coma.4 The clinical manifestations are usually reversible with prompt treatment, but recurrence is common, typically induced by an event such as gastrointestinal bleeding or an infection.
Minimal hepatic encephalopathy is important to recognize
Although this subclinical syndrome is a very early stage, it is nevertheless associated with higher rates of morbidity and can affect quality of life, including the patient’s ability to drive a car.29,30
Abnormal changes in the brain begin at this stage and eventually progress to more damage and to the development of overt clinical symptoms.
The exact prevalence of minimal hepatic encephalopathy is not known because it is difficult to diagnose, but reported rates range between 30% and 84% of patients with cirrhosis.31 Progression from minimal to overt hepatic encephalopathy is 3.7 times more likely than in patients without the diagnosis of minimal hepatic encephalopathy.32
Thus, minimal hepatic encephalopathy is important to identify,29 so that treatment can be started.
Overt encephalopathy and survival
The prevalence of overt encephalopathy in cirrhosis ranges from 30% to 40% and is even higher in the advanced stages. Once encephalopathy develops, the prognosis worsens rapidly. In patients who do not undergo liver transplantation, the survival rate at 1 year is 42%, and the survival rate at 3 years is 23%.33
These rates are worse than those after liver transplantation, and the American Association for the Study of Liver Diseases recommends that patients with cirrhosis who develop a first episode of encephalopathy be considered for liver transplantation and be referred to a transplantation center.34
CHALLENGES IN DIAGNOSIS
Since the symptoms of hepatic encephalopathy are not specific and can be subtle in the early stage, its diagnosis may be a challenge. It is important to recognize that this neuropsychiatric complication occurs in people with severe comorbidities and requires dedicated time for evaluation and management.
Special tests may be needed to detect subclinical hepatic encephalopathy
In subclinical hepatic encephalopathy, the apparent lack of manifestations poses a great diagnostic challenge, but a thorough history may uncover poor social interaction, personality changes, poor performance at work, and recent traffic violations or motor vehicle accidents. Primary care physicians are usually the first to suspect the condition because they are familiar with the patient’s baseline mental and physical conditions.
For example, the primary care physician may notice decreased attention and worsening memory during a follow-up visit, or the physician may ask whether the patient has difficulty with work performance and handwork (psychomotor and fine motor skills), and whether there have been traffic violations or car accidents (visuospatial skills). Such clues, although not restrictive, may help identify patients with minimal hepatic encephalopathy and prompt referral for neuropsychiatric testing.
Neurologic deficits described in the subclinical form are in the domains of attention and concentration, working memory, visuospatial ability, and fine motor skills; communication skills remain intact.35 These deficits are not reliably detected on standard clinical evaluation but can be detected by neuropsychiatric and neurophysiologic testing.
While several tests for minimal hepatic encephalopathy have been developed, they need to be validated in large trials in the United States.
Neurophysiologic tests include electroencephalography and auditory or visual event-related P300 (evoked potential) testing.
Neuropsychiatric tests traditionally involved several batteries administered and interpreted by specialized personnel. They were time-consuming and were not practical in a typical office setting. They were later refined into the Psychometric Hepatic Encephalopathy Score test (ie, the PSE syndrome test).36 This combines a digit symbol test, a serial dotting test, a line-tracing test, and a number-connection or figure-connection test. An abnormal result in at least three of the four subtests constitutes an overall abnormal PSE syndrome test.
The PSE syndrome test has been validated for standard use in Germany, Spain, Italy, the United Kingdom, and India.35 In 1999, the Working Group on Hepatic Encephalopathy designated it as the official test for minimal hepatic encephalopathy.1 But the test has not been validated for use in the United States. Other tests have been developed, but their use is also limited by a lack of validation and by copyright laws. These factors constitute major obstacles to the diagnosis of subclinical hepatic encephalopathy in the United States. Nonetheless, physicians who suspect minimal hepatic encephalopathy may start lactulose therapy37 and schedule frequent follow-up visits to address and manage potential precipitating factors for overt hepatic encephalopathy.
Staging the severity of the encephalopathy
It is essential to exclude stroke, cerebral bleeding, and brain tumor before making a diagnosis of a first episode of hepatic encephalopathy. Thereafter, such exclusion must be guided by whether the patient has risk factors for these conditions or persistent symptoms of encephalopathy that do not respond to medical therapy.
Laboratory tests can identify metabolic derangements
Although laboratory tests are not diagnostic for hepatic encephalopathy, they can identify metabolic derangements that could contribute to it.
Imaging can help exclude other diagnoses
Neurophysiologic imaging studies such as magnetic resonance spectroscopy, magnetic transfer imaging, and water-mapping techniques have helped elucidate pathologic mechanisms of hepatic encephalopathy and are available in research centers, but they are not currently considered for diagnosis.
SEVERAL LINES OF TREATMENT
Treatment of hepatic encephalopathy involves a preemptive approach to address potential precipitating factors, medical therapy to reduce the production and absorption of ammonia from the gut, and surgical or interventional therapies. A multidisciplinary approach for testing the severity of neurologic impairment and response to therapy is needed to help determine if and when liver transplantation is required.
Prevent potential precipitating factors
An important concept in managing hepatic encephalopathy is to recognize that every cirrhotic patient is at risk and to make an effort to address potential precipitating factors during regular clinic visits. This includes reviewing medication dosing and adverse effects, emphasizing abstinence from alcohol and other toxic substances, and preventing bleeding from esophageal varices with endoscopic band ligation.
Diet therapy
The prevalence of malnutrition in cirrhosis may be as high as 100%. Vitamin and nutritional deficiencies should be evaluated by a nutrition specialist, and nutritional needs should be reassessed on a regular basis. Protein restriction is no longer recommended and may even be harmful.
Guidelines of the European Society of Parenteral and Enteric Nutrition in 2006 recommended that patients with liver disease should have an energy intake of 35 to 40 kcal/kg of body weight daily, with a total daily protein intake of 1.2 to 1.5 mg/kg of body weight.41 Frequent meals and bedtime snacks are encouraged to avoid periods of prolonged fasting and catabolism of muscle protein and to improve nitrogen balance. Branched-chain amino acids and vegetable protein supplements are suggested to help meet the daily requirements.42
Drug therapy to reduce neurotoxins
Drug treatment is directed at reducing the neurotoxins that accumulate in cirrhosis. A variety of agents have been used.
Lactulose (Kristalose) is approved by the US Food and Drug Administration (FDA) as a first-line treatment. It has been shown to improve quality of life and cognitive function in patients with cirrhosis and minimal hepatic encephalopathy, although it has failed to improve mortality rates.37
Lactulose, a cathartic disaccharide, is metabolized by colonic bacteria into short-chain fatty acids. The acidic microenvironment has three major effects:
- It aids the transformation of ammonia to ammonium (NH4+), which is then trapped in the stool, leaving less ammonia to be absorbed
- It has a cathartic effect
- It reduces the breakdown of nitrogenous compounds into ammonia.43
Lactulose has an excessively sweet taste. Its side effects include flatulence, abdominal discomfort, and diarrhea. The usual oral dose is 15 to 45 mL/day given in multiple doses to induce two to three soft bowel movements daily. At this dosage, the monthly cost varies between $60 and $120.
Lactilol, a nonabsorbable disaccharide, is as effective as lactulose but with fewer side effects. It is not available in the United States.
Rifaximin (Xifaxan), a derivative of rifamycin, is FDA-approved for the maintenance of remission of hepatic encephalopathy but is not recommended as a first-line agent. It inhibits bacterial RNA synthesis in the gut. Less than 0.4% of an oral dose is absorbed.44
In a randomized, double-blind, placebo-controlled trial in patients who had had at least two episodes of hepatic encephalopathy while on lactulose therapy, taking rifaximin 550 mg twice a day for 6 months provided a prolonged remission from recurrences of encephalopathy compared with placebo.45 Side effects included nausea, vomiting, abdominal pain, weight loss, and Clostridium difficile colitis, which was reported in two cases in the study.45
Unfortunately, the effects of this drug beyond 6 months of therapy have not been studied. In addition, the drug is expensive: 1 month of treatment with rifaximin can cost between $700 and $1,500. Combining lactulose and rifaximin adds to the costs and the side effects, and contributes to poor adherence to therapy.
Other antibiotics such as metronidazole (Flagyl), vancomycin, and neomycin have been used as alternatives to lactulose, based on the principle that they reduce ammonia-producing bacteria in the gut. However, their efficacy in hepatic encephalopathy remains to be determined.
In controlled trials, neomycin combined with sorbitol, magnesium sulfate, or lactulose was as effective as lactulose, but when used alone, neomycin was no better than placebo.46,47 Neomycin was approved many years ago as an adjunct in the management of hepatic coma, but it has since fallen out of favor in the management of hepatic encephalopathy because of poor trial results and because of neurotoxicity and ototoxicity.
Branched-chain amino acids (leucine, isoleucine, and valine)48 are reported to increase ammonia intake in muscle and to improve cognitive functions on the PSE scale in minimal hepatic encephalopathy,49,50 but they did not decrease the rate of recurrence of hepatic encephalopathy.51 While debate continues over their efficacy in the management of hepatic encephalopathy, branched-chain amino acids may be used to improve nutritional status and muscle mass of patients with cirrhosis. However, the dosing is not standardized, and long-term compliance may be problematic.
Other medical therapies include zinc,16 sodium benzoate,50 and l-ornithine-l-aspartate52,53 to stimulate residual urea cycle activities; probiotics (which pose a risk of sepsis from fungi and lactobacilli); and laxatives.
Liver dialysis
Adsorbing toxins from the blood via liver dialysis or using a non-cell-based liver support system such as MARS (Molecular Adsorbent Recirculating System, Gambro, Inc.) appears to improve the amino acid profile in hepatic encephalopathy, but its role has not been clarified, and its use is limited to clinical trials.54,55
Transjugular intrahepatic shunts and large portosystemic shunts may need to be closed in order to reverse encephalopathy refractory to drug therapy.26,27,56
Liver transplantation
The current scoring system for end-stage liver disease does not include hepatic encephalopathy as a criterion for prioritizing patients on the transplantation list because it was originally developed to assess short-term prognosis in patients undergoing transjugular intrahepatic shunting. As a consequence, patients with end-stage liver disease are at increased risk of repeated episodes of encephalopathy, hospital readmission, and death. Therefore, the American Association for the Study of Liver Diseases recommends referral to a transplantation center when the patient experiences a first episode of overt hepatic encephalopathy to initiate a workup for liver transplantation.34
Liver transplantation improves survival in patients with severe hepatic dysfunction, but the presence of neurologic deficits may result in significant morbidity and in death.57,58 After transplantation, resolution of cognitive dysfunction, brain edema, and white-matter changes have been reported,59 but neuronal cell death and persistent cognitive impairment after resolution of overt hepatic encephalopathy are also described.60–63
Whether neurologic impairment will resolve after liver transplantation depends on a number of factors: the severity of encephalopathy before transplantation; the nature of the neurologic deficits; advanced age; history of alcohol abuse and the presence of alcoholic brain damage; persistence of portosystemic shunts after transplant; emergency transplantation; complications during surgery; and side effects of immunosuppressive drugs.57,58,64
The optimal timing of liver transplantation is not clearly defined for patients who have had bouts of hepatic encephalopathy, and more study is needed to determine the reversibility of clinical symptoms and brain damage. It is in these situations that neuropsychiatric testing and advanced neuroimaging can help determine the efficacy of therapeutic interventions, and it should be considered part of the pretransplantation evaluation.
Managing sleep disturbances
Insomnia and other changes in sleep-wake patterns are common in patients with cirrhosis, especially advanced cirrhosis.65 It is not known whether these changes represent early stages of hepatic encephalopathy.66 Patients often complain of fatigue, the need for frequent naps, and lethargy during the day and restlessness and inability to sleep at night. This affects the patient’s behavior and daytime functioning, and it also burdens household members and caregivers.
Long-acting benzodiazepines should be avoided when treating sleep disorders in cirrhosis because they may precipitate the encephalopathy. In a randomized controlled trial, hydroxyzine (Vistaril) at a dose of 25 mg at bedtime improved sleep behavior in 40% of patients with cirrhosis and subclinical hepatic encephalopathy, but 1 of 17 patients developed acute encephalopathy, which reversed with cessation of the hydroxyzine.66 Clearly, caution and close monitoring are required when giving hydroxyzine for sleep disorders in cirrhotic patients.
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35:716–721.
- Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: a general population-based study. J Hepatol 2008; 49:732–738.
- Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25(suppl 1):3–9.
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009; 50:2014–2021.
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 2007; 22:219–234.
- Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care 2010; 13:87–92.
- Romero-Gómez M, Ramos-Guerrero R, Grande L, et al. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 2004; 41:49–54.
- Tanigami H, Rebel A, Martin LJ, et al. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience 2005; 131:437–449.
- Llansola M, Rodrigo R, Monfort P, et al. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab Brain Dis 2007; 22:321–335.
- Montoliu C, Piedrafita B, Serra MA, et al. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol 2009; 43:272–279.
- Desjardins P, Butterworth RF. The “peripheral-type” benzodiazepine (omega 3) receptor in hyperammonemic disorders. Neurochem Int 2002; 41:109–114.
- Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut 2008; 57:1156–1165.
- Cauli O, Rodrigo R, Llansola M, et al. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 2009; 24:69–80.
- Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet 1995; 346:270–274.
- Pomier-Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 1995; 345:735.
- Schliess F, Görg B, Häussinger D. RNA oxidation and zinc in hepatic encephalopathy and hyperammonemia. Metab Brain Dis 2009; 24:119–134.
- Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol 2009; 104:1382–1389.
- Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis 2007; 22:199–218.
- Marchesini G, Zoli M, Dondi C, et al. Prevalence of subclinical hepatic encephalopathy in cirrhotics and relationship to plasma amino acid imbalance. Dig Dis Sci 1980; 25:763–768.
- Morgan MY, Milsom JP, Sherlock S. Plasma ratio of valine, leucine and isoleucine to phenylalanine and tyrosine in liver disease. Gut 1978; 19:1068–1073.
- Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, Soeters PB. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976; 80:77–91.
- Poveda MJ, Bernabeu A, Concepción L, et al. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage 2010; 52:481–487.
- Bernthal P, Hays A, Tarter RE, Van Thiel D, Lecky J, Hegedus A. Cerebral CT scan abnormalities in cholestatic and hepatocellular disease and their relationship to neuropsychologic test performance. Hepatology 1987; 7:107–114.
- Sugimoto R, Iwasa M, Maeda M, et al. Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 2008; 103:1413–1420.
- Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2006; 43:1187–1190.
- Masson S, Mardini HA, Rose JD, Record CO. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM 2008; 101:493–501.
- Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010; 51:306.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Kircheis G, Knoche A, Hilger N, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009; 137:1706–1715.e1–9.
- Bajaj JS, Saeian K, Schubert CM, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009; 50:1175–1183.
- Hartmann IJ, Groeneweg M, Quero JC, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol 2000; 95:2029–2034.
- Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 2001; 96:2718–2723.
- Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30:890–895.
- Murray KF, Carithers RL jR; AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis 2004; 19:253–267.
- Weissenborn K. PHES: one label, different goods?! J Hepatol 2008; 49:308–312.
- Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007; 45:549–559.
- Parsons-Smith BG, Summerskill WHJ, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957; 2:867–871.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84.
- Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 2008; 29:1612–1621.
- Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006; 25:285–294.
- Gheorghe L, Iacob R, Vadan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol 2005; 14:231–238.
- Weber FL. Effects of lactulose on nitrogen metabolism. Scand J Gastroenterol Suppl 1997; 222:83–87.
- Ojetti V, Lauritano EC, Barbaro F, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol 2009; 5:675–682.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362:1071–1081.
- Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol 2001; 96:1968–1976.
- Rothenberg ME, Keeffe EB. Antibiotics in the management of hepatic encephalopathy: an evidence-based review. Rev Gastroenterol Disord 2005; 5(suppl 3):26–35.
- Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr 2006; 136(suppl 1):295S–298S.
- Egberts EH, Schomerus H, Hamster W, Jürgens P. [Branched-chain amino acids in the treatment of latent porto-systemic encephalopathy. A placebo-controlled double-blind cross-over study] [in German]. Z Ernahrungswiss 1986; 25:9–28.
- Plauth M, Egberts EH, Hamster W, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol 1993; 17:308–314.
- Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 2011; 106:1081–1088.
- Efrati C, Masini A, Merli M, Valeriano V, Riggio O. Effect of sodium benzoate on blood ammonia response to oral glutamine challenge in cirrhotic patients: a note of caution. Am J Gastroenterol 2000; 95:3574–3578.
- Schmid M, Peck-Radosavljevic M, König F, Mittermaier C, Gangl A, Ferenci P. A double-blind, randomized, placebo-controlled trial of intravenous L-ornithine-L-aspartate on postural control in patients with cirrhosis. Liver Int 2010; 30:574–582.
- Blei AT. MARS and treatment of hepatic encephalopathy [in Spanish). Gastroenterol Hepatol 2005; 28:100–104.
- Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002; 36:949–958.
- Zidi SH, Zanditenas D, Gelu-Siméon M, et al. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int 2007; 27:1389–1393.
- Dhar R, Young GB, Marotta P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care 2008; 8:253–258.
- Teperman LW, Peyregne VP. Considerations on the impact of hepatic encephalopathy treatments in the pretransplant setting. Transplantation 2010; 89:771–778.
- Rovira A, Córdoba J, Sanpedro F, Grivé E, Rovira-Gols A, Alonso J. Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology 2002; 59:335–341.
- Senzolo M, Pizzolato G, Ferronato C, et al. Long-term evaluation of cognitive function and cerebral metabolism in liver transplanted patients. Transplant Proc 2009; 41:1295–1296.
- Butterworth RF. Neuronal cell death in hepatic encephalopathy. Metab Brain Dis 2007; 22:309–320.
- DiMartini A, Chopra K. The importance of hepatic encephalopathy: pre-transplant and post-transplant. Liver Transpl 2009; 15:121–123.
- Saner FH, Nadalin S, Radtke A, Sotiropoulos GC, Kaiser GM, Paul A. Liver transplantation and neurological side effects. Metab Brain Dis 2009; 24:183–187.
- Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl 2009; 15:184–192.
- Montagnese S, Middleton B, Skene DJ, Morgan MY. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int 2009; 29:1372–1382.
- Spahr L, Coeytaux A, Giostra E, Hadengue A, Annoni JM. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol 2007; 102:744–753.
Hepatic encephalopathy is a serious but often reversible complication that arises when the liver cannot detoxify the portal venous blood (Table 1).1
PROPOSED PATHOGENETIC FACTORS
About 5.5 million cases of chronic liver disease and cirrhosis were reported in the United States in 2001. Hepatic encephalopathy is becoming more common as the prevalence of cirrhosis increases,2 and this will have important economic repercussions; in 2001, charges from hospitalizations because of hepatic encephalopathy were estimated at $932 million.3
Hepatic encephalopathy develops as cirrhosis progresses or as a result of portosystemic shunting, so that the liver cannot detoxify the portal venous blood. Several neurotoxins (notably ammonia) and inflammatory mediators play key roles in its pathogenesis, inducing low-grade brain edema and producing a wide spectrum of neuropsychiatric manifestations.4 Yet its pathogenesis is not entirely understood, impeding advances in its diagnosis and therapy.
Several hypotheses about the pathogenesis of hepatic encephalopathy have emerged in the last few years, and a number of factors are reported to directly or indirectly affect brain function in this condition. Ammonia and glutamine are the neurotoxins most often implicated in this syndrome5; others include inflammatory mediators, certain amino acids, and manganese.5,6
Ammonia causes brain swelling
Ammonia is primarily the byproduct of bacterial metabolism of protein and nitrogenous compounds in the colon and of glutamine metabolism in enterocytes.7
Normally, gut-absorbed ammonia is delivered via the portal vein to the liver, where most of it is metabolized into urea, leaving a small amount to be metabolized in the muscles, heart, brain, and kidneys. In cirrhosis and other conditions associated with hepatic encephalopathy, less ammonia is metabolized into urea and more of it reaches the astrocytes in the brain. The brain lacks a urea cycle but metabolizes ammonia to glutamine via glutamine synthase, an enzyme unique to astrocytes.
Ammonia causes swelling of astrocytes and brain edema via generation of glutamine, an osmotically active substance.
Glutamine causes swelling, oxidative stress
Glutamine draws water into astrocytes and induces changes of type II astrocytosis (also called Alzheimer type II astrocytosis)5 characterized by swelling, enlarged and pale nuclei, and displacement of chromatin to the periphery of the cell. Inhibition of glutamine synthase prevents astrocyte swelling in animals.8
Glutamine also enhances the activation of several receptors, including N-methyl-d-aspartate (NMDA) receptors,9,10 gammaaminobutyric acid (GABA) receptors, and peripheral-type benzodiazepine receptors on the mitochondrial membrane.10–12 A state of oxidative stress ensues, and this affects oxidation of protein and RNA, neurotransmitter synthesis, and neurotransmission at the neuronal junction.13 Reactive nitrogen and oxide radicals induce the release of inflammatory mediators such as interleukins 1 and 6, tumor necrosis factor, interferons, and neurosteroids, and contribute to edema and neurotoxicity.6,10 Neurosteroids are byproducts of mitochondrial metabolism of steroid hormones in the astrocyte.
Manganese enhances neurosteroid synthesis
Manganese enhances neurosteroid synthesis via activation of translocator proteins on the astrocyte membrane. It was first recognized as a factor in hepatic encephalopathy when cirrhotic patients experiencing extrapyramidal symptoms were found to have deposits of manganese in the caudate nucleus and in the globus pallidus on magnetic resonance imaging (MRI). Such deposits were also seen in specimens of brain tissue on autopsy of these patients. When the encephalopathy resolved, so did the abnormalities on MRI.14,15
Changes in the blood-brain barrier
Astrocytes contribute to the selective permeability of the blood-brain barrier. Disruptions in the permeability of the blood-brain barrier underlie hepatic encephalopathy, with poor diffusion of molecules out of astrocytes.
For instance, zinc, which plays a regulatory role in gene transcription and synaptic plasticity, accumulates in the astrocytes, causing relative zinc deficiency and further affecting neurotransmitter synthesis and neurotransmission at the neuronal synapse.6,16
Hyponatremia
Hyponatremia (a serum sodium concentration < 130 mmol/L) is increasingly being recognized as an independent predictor of overt hepatic encephalopathy and is reported to increase the risk by a factor of eight.17
Neuronal dysfunction
Astrocytes are integral to the physiologic functioning of the neurons, and it is becoming clear that both neurons and astrocytes are affected in hepatic encephalopathy.
Additionally, neuroinflammation and a decrease in energy metabolism by the brain are described during episodes of hepatic encephalopathy.18
Amino acid imbalance
An imbalance between aromatic amino acids (ie, high levels of tyrosine and phenylalanine) and branched-chain amino acids (leucine, isoleucine, and valine) has been linked with encephalopathy in patients with liver disease, 19–21 but it is not totally clear whether this imbalance contributes to hepatic encephalopathy or is a consequence of it.
Low-grade brain edema
Edema of the brain occurs in all forms of hepatic encephalopathy, but in cirrhosis it is characteristically of low grade. The mechanism behind this low-grade edema is not clear. Studies have shown that swelling of astrocytes is not global but involves certain areas of the brain and is associated with compensatory extrusion of intracellular myoinositol.22 This, in combination with a mild degree of brain atrophy23 observed in patients with chronic liver disease, is thought to keep the brain from extreme swelling and herniation, a phenomenon usually seen in acute hepatic failure.24,25
Transjugular intrahepatic portosystemic shunting and encephalopathy
The incidence rate of hepatic encephalopathy after placement of a portosystemic shunt to treat portal hypertension ranges from 30% to 55% and is similar to the rate in cirrhotic patients without a shunt.26 In 5% to 8% of patients, the hepatic encephalopathy is refractory and requires intentional occlusion of the shunt.26,27 An elevated serum creatinine level appears to be a risk factor for refractory hepatic encephalopathy in patients with a portosystemic shunt.26
In one study,28 when transjugular intrahepatic portosystemic shunting was done early in the treatment of cirrhotic patients with acute variceal bleeding, the rates of treatment failure and death were significantly less than in a control group that received endoscopic therapy, and no significant difference was noted in the rate of encephalopathy or of serious adverse effects between the groups.
Whether to place a portosystemic shunt in a patient with cirrhosis and a history of hepatic encephalopathy depends on the possible underlying causes of the encephalopathy. For example, if encephalopathy was precipitated by variceal bleeding, shunt placement will prevent further bleeding and will make a recurrence of encephalopathy less likely. However, if the encephalopathy is persistent and uncontrollable, then shunt placement is contraindicated.27
A SPECTRUM OF SYMPTOMS
The spectrum of symptoms extends from a subclinical syndrome that may not be clinically apparent (early-stage or “minimal” hepatic encephalopathy) to full-blown neuropsychiatric manifestations such as cognitive impairment, confusion, slow speech, loss of fine motor skills, asterixis, peripheral neuropathy, clonus, the Babinski sign, decerebrate and decorticate posturing, seizures, extrapyramidal symptoms, and coma.4 The clinical manifestations are usually reversible with prompt treatment, but recurrence is common, typically induced by an event such as gastrointestinal bleeding or an infection.
Minimal hepatic encephalopathy is important to recognize
Although this subclinical syndrome is a very early stage, it is nevertheless associated with higher rates of morbidity and can affect quality of life, including the patient’s ability to drive a car.29,30
Abnormal changes in the brain begin at this stage and eventually progress to more damage and to the development of overt clinical symptoms.
The exact prevalence of minimal hepatic encephalopathy is not known because it is difficult to diagnose, but reported rates range between 30% and 84% of patients with cirrhosis.31 Progression from minimal to overt hepatic encephalopathy is 3.7 times more likely than in patients without the diagnosis of minimal hepatic encephalopathy.32
Thus, minimal hepatic encephalopathy is important to identify,29 so that treatment can be started.
Overt encephalopathy and survival
The prevalence of overt encephalopathy in cirrhosis ranges from 30% to 40% and is even higher in the advanced stages. Once encephalopathy develops, the prognosis worsens rapidly. In patients who do not undergo liver transplantation, the survival rate at 1 year is 42%, and the survival rate at 3 years is 23%.33
These rates are worse than those after liver transplantation, and the American Association for the Study of Liver Diseases recommends that patients with cirrhosis who develop a first episode of encephalopathy be considered for liver transplantation and be referred to a transplantation center.34
CHALLENGES IN DIAGNOSIS
Since the symptoms of hepatic encephalopathy are not specific and can be subtle in the early stage, its diagnosis may be a challenge. It is important to recognize that this neuropsychiatric complication occurs in people with severe comorbidities and requires dedicated time for evaluation and management.
Special tests may be needed to detect subclinical hepatic encephalopathy
In subclinical hepatic encephalopathy, the apparent lack of manifestations poses a great diagnostic challenge, but a thorough history may uncover poor social interaction, personality changes, poor performance at work, and recent traffic violations or motor vehicle accidents. Primary care physicians are usually the first to suspect the condition because they are familiar with the patient’s baseline mental and physical conditions.
For example, the primary care physician may notice decreased attention and worsening memory during a follow-up visit, or the physician may ask whether the patient has difficulty with work performance and handwork (psychomotor and fine motor skills), and whether there have been traffic violations or car accidents (visuospatial skills). Such clues, although not restrictive, may help identify patients with minimal hepatic encephalopathy and prompt referral for neuropsychiatric testing.
Neurologic deficits described in the subclinical form are in the domains of attention and concentration, working memory, visuospatial ability, and fine motor skills; communication skills remain intact.35 These deficits are not reliably detected on standard clinical evaluation but can be detected by neuropsychiatric and neurophysiologic testing.
While several tests for minimal hepatic encephalopathy have been developed, they need to be validated in large trials in the United States.
Neurophysiologic tests include electroencephalography and auditory or visual event-related P300 (evoked potential) testing.
Neuropsychiatric tests traditionally involved several batteries administered and interpreted by specialized personnel. They were time-consuming and were not practical in a typical office setting. They were later refined into the Psychometric Hepatic Encephalopathy Score test (ie, the PSE syndrome test).36 This combines a digit symbol test, a serial dotting test, a line-tracing test, and a number-connection or figure-connection test. An abnormal result in at least three of the four subtests constitutes an overall abnormal PSE syndrome test.
The PSE syndrome test has been validated for standard use in Germany, Spain, Italy, the United Kingdom, and India.35 In 1999, the Working Group on Hepatic Encephalopathy designated it as the official test for minimal hepatic encephalopathy.1 But the test has not been validated for use in the United States. Other tests have been developed, but their use is also limited by a lack of validation and by copyright laws. These factors constitute major obstacles to the diagnosis of subclinical hepatic encephalopathy in the United States. Nonetheless, physicians who suspect minimal hepatic encephalopathy may start lactulose therapy37 and schedule frequent follow-up visits to address and manage potential precipitating factors for overt hepatic encephalopathy.
Staging the severity of the encephalopathy
It is essential to exclude stroke, cerebral bleeding, and brain tumor before making a diagnosis of a first episode of hepatic encephalopathy. Thereafter, such exclusion must be guided by whether the patient has risk factors for these conditions or persistent symptoms of encephalopathy that do not respond to medical therapy.
Laboratory tests can identify metabolic derangements
Although laboratory tests are not diagnostic for hepatic encephalopathy, they can identify metabolic derangements that could contribute to it.
Imaging can help exclude other diagnoses
Neurophysiologic imaging studies such as magnetic resonance spectroscopy, magnetic transfer imaging, and water-mapping techniques have helped elucidate pathologic mechanisms of hepatic encephalopathy and are available in research centers, but they are not currently considered for diagnosis.
SEVERAL LINES OF TREATMENT
Treatment of hepatic encephalopathy involves a preemptive approach to address potential precipitating factors, medical therapy to reduce the production and absorption of ammonia from the gut, and surgical or interventional therapies. A multidisciplinary approach for testing the severity of neurologic impairment and response to therapy is needed to help determine if and when liver transplantation is required.
Prevent potential precipitating factors
An important concept in managing hepatic encephalopathy is to recognize that every cirrhotic patient is at risk and to make an effort to address potential precipitating factors during regular clinic visits. This includes reviewing medication dosing and adverse effects, emphasizing abstinence from alcohol and other toxic substances, and preventing bleeding from esophageal varices with endoscopic band ligation.
Diet therapy
The prevalence of malnutrition in cirrhosis may be as high as 100%. Vitamin and nutritional deficiencies should be evaluated by a nutrition specialist, and nutritional needs should be reassessed on a regular basis. Protein restriction is no longer recommended and may even be harmful.
Guidelines of the European Society of Parenteral and Enteric Nutrition in 2006 recommended that patients with liver disease should have an energy intake of 35 to 40 kcal/kg of body weight daily, with a total daily protein intake of 1.2 to 1.5 mg/kg of body weight.41 Frequent meals and bedtime snacks are encouraged to avoid periods of prolonged fasting and catabolism of muscle protein and to improve nitrogen balance. Branched-chain amino acids and vegetable protein supplements are suggested to help meet the daily requirements.42
Drug therapy to reduce neurotoxins
Drug treatment is directed at reducing the neurotoxins that accumulate in cirrhosis. A variety of agents have been used.
Lactulose (Kristalose) is approved by the US Food and Drug Administration (FDA) as a first-line treatment. It has been shown to improve quality of life and cognitive function in patients with cirrhosis and minimal hepatic encephalopathy, although it has failed to improve mortality rates.37
Lactulose, a cathartic disaccharide, is metabolized by colonic bacteria into short-chain fatty acids. The acidic microenvironment has three major effects:
- It aids the transformation of ammonia to ammonium (NH4+), which is then trapped in the stool, leaving less ammonia to be absorbed
- It has a cathartic effect
- It reduces the breakdown of nitrogenous compounds into ammonia.43
Lactulose has an excessively sweet taste. Its side effects include flatulence, abdominal discomfort, and diarrhea. The usual oral dose is 15 to 45 mL/day given in multiple doses to induce two to three soft bowel movements daily. At this dosage, the monthly cost varies between $60 and $120.
Lactilol, a nonabsorbable disaccharide, is as effective as lactulose but with fewer side effects. It is not available in the United States.
Rifaximin (Xifaxan), a derivative of rifamycin, is FDA-approved for the maintenance of remission of hepatic encephalopathy but is not recommended as a first-line agent. It inhibits bacterial RNA synthesis in the gut. Less than 0.4% of an oral dose is absorbed.44
In a randomized, double-blind, placebo-controlled trial in patients who had had at least two episodes of hepatic encephalopathy while on lactulose therapy, taking rifaximin 550 mg twice a day for 6 months provided a prolonged remission from recurrences of encephalopathy compared with placebo.45 Side effects included nausea, vomiting, abdominal pain, weight loss, and Clostridium difficile colitis, which was reported in two cases in the study.45
Unfortunately, the effects of this drug beyond 6 months of therapy have not been studied. In addition, the drug is expensive: 1 month of treatment with rifaximin can cost between $700 and $1,500. Combining lactulose and rifaximin adds to the costs and the side effects, and contributes to poor adherence to therapy.
Other antibiotics such as metronidazole (Flagyl), vancomycin, and neomycin have been used as alternatives to lactulose, based on the principle that they reduce ammonia-producing bacteria in the gut. However, their efficacy in hepatic encephalopathy remains to be determined.
In controlled trials, neomycin combined with sorbitol, magnesium sulfate, or lactulose was as effective as lactulose, but when used alone, neomycin was no better than placebo.46,47 Neomycin was approved many years ago as an adjunct in the management of hepatic coma, but it has since fallen out of favor in the management of hepatic encephalopathy because of poor trial results and because of neurotoxicity and ototoxicity.
Branched-chain amino acids (leucine, isoleucine, and valine)48 are reported to increase ammonia intake in muscle and to improve cognitive functions on the PSE scale in minimal hepatic encephalopathy,49,50 but they did not decrease the rate of recurrence of hepatic encephalopathy.51 While debate continues over their efficacy in the management of hepatic encephalopathy, branched-chain amino acids may be used to improve nutritional status and muscle mass of patients with cirrhosis. However, the dosing is not standardized, and long-term compliance may be problematic.
Other medical therapies include zinc,16 sodium benzoate,50 and l-ornithine-l-aspartate52,53 to stimulate residual urea cycle activities; probiotics (which pose a risk of sepsis from fungi and lactobacilli); and laxatives.
Liver dialysis
Adsorbing toxins from the blood via liver dialysis or using a non-cell-based liver support system such as MARS (Molecular Adsorbent Recirculating System, Gambro, Inc.) appears to improve the amino acid profile in hepatic encephalopathy, but its role has not been clarified, and its use is limited to clinical trials.54,55
Transjugular intrahepatic shunts and large portosystemic shunts may need to be closed in order to reverse encephalopathy refractory to drug therapy.26,27,56
Liver transplantation
The current scoring system for end-stage liver disease does not include hepatic encephalopathy as a criterion for prioritizing patients on the transplantation list because it was originally developed to assess short-term prognosis in patients undergoing transjugular intrahepatic shunting. As a consequence, patients with end-stage liver disease are at increased risk of repeated episodes of encephalopathy, hospital readmission, and death. Therefore, the American Association for the Study of Liver Diseases recommends referral to a transplantation center when the patient experiences a first episode of overt hepatic encephalopathy to initiate a workup for liver transplantation.34
Liver transplantation improves survival in patients with severe hepatic dysfunction, but the presence of neurologic deficits may result in significant morbidity and in death.57,58 After transplantation, resolution of cognitive dysfunction, brain edema, and white-matter changes have been reported,59 but neuronal cell death and persistent cognitive impairment after resolution of overt hepatic encephalopathy are also described.60–63
Whether neurologic impairment will resolve after liver transplantation depends on a number of factors: the severity of encephalopathy before transplantation; the nature of the neurologic deficits; advanced age; history of alcohol abuse and the presence of alcoholic brain damage; persistence of portosystemic shunts after transplant; emergency transplantation; complications during surgery; and side effects of immunosuppressive drugs.57,58,64
The optimal timing of liver transplantation is not clearly defined for patients who have had bouts of hepatic encephalopathy, and more study is needed to determine the reversibility of clinical symptoms and brain damage. It is in these situations that neuropsychiatric testing and advanced neuroimaging can help determine the efficacy of therapeutic interventions, and it should be considered part of the pretransplantation evaluation.
Managing sleep disturbances
Insomnia and other changes in sleep-wake patterns are common in patients with cirrhosis, especially advanced cirrhosis.65 It is not known whether these changes represent early stages of hepatic encephalopathy.66 Patients often complain of fatigue, the need for frequent naps, and lethargy during the day and restlessness and inability to sleep at night. This affects the patient’s behavior and daytime functioning, and it also burdens household members and caregivers.
Long-acting benzodiazepines should be avoided when treating sleep disorders in cirrhosis because they may precipitate the encephalopathy. In a randomized controlled trial, hydroxyzine (Vistaril) at a dose of 25 mg at bedtime improved sleep behavior in 40% of patients with cirrhosis and subclinical hepatic encephalopathy, but 1 of 17 patients developed acute encephalopathy, which reversed with cessation of the hydroxyzine.66 Clearly, caution and close monitoring are required when giving hydroxyzine for sleep disorders in cirrhotic patients.
Hepatic encephalopathy is a serious but often reversible complication that arises when the liver cannot detoxify the portal venous blood (Table 1).1
PROPOSED PATHOGENETIC FACTORS
About 5.5 million cases of chronic liver disease and cirrhosis were reported in the United States in 2001. Hepatic encephalopathy is becoming more common as the prevalence of cirrhosis increases,2 and this will have important economic repercussions; in 2001, charges from hospitalizations because of hepatic encephalopathy were estimated at $932 million.3
Hepatic encephalopathy develops as cirrhosis progresses or as a result of portosystemic shunting, so that the liver cannot detoxify the portal venous blood. Several neurotoxins (notably ammonia) and inflammatory mediators play key roles in its pathogenesis, inducing low-grade brain edema and producing a wide spectrum of neuropsychiatric manifestations.4 Yet its pathogenesis is not entirely understood, impeding advances in its diagnosis and therapy.
Several hypotheses about the pathogenesis of hepatic encephalopathy have emerged in the last few years, and a number of factors are reported to directly or indirectly affect brain function in this condition. Ammonia and glutamine are the neurotoxins most often implicated in this syndrome5; others include inflammatory mediators, certain amino acids, and manganese.5,6
Ammonia causes brain swelling
Ammonia is primarily the byproduct of bacterial metabolism of protein and nitrogenous compounds in the colon and of glutamine metabolism in enterocytes.7
Normally, gut-absorbed ammonia is delivered via the portal vein to the liver, where most of it is metabolized into urea, leaving a small amount to be metabolized in the muscles, heart, brain, and kidneys. In cirrhosis and other conditions associated with hepatic encephalopathy, less ammonia is metabolized into urea and more of it reaches the astrocytes in the brain. The brain lacks a urea cycle but metabolizes ammonia to glutamine via glutamine synthase, an enzyme unique to astrocytes.
Ammonia causes swelling of astrocytes and brain edema via generation of glutamine, an osmotically active substance.
Glutamine causes swelling, oxidative stress
Glutamine draws water into astrocytes and induces changes of type II astrocytosis (also called Alzheimer type II astrocytosis)5 characterized by swelling, enlarged and pale nuclei, and displacement of chromatin to the periphery of the cell. Inhibition of glutamine synthase prevents astrocyte swelling in animals.8
Glutamine also enhances the activation of several receptors, including N-methyl-d-aspartate (NMDA) receptors,9,10 gammaaminobutyric acid (GABA) receptors, and peripheral-type benzodiazepine receptors on the mitochondrial membrane.10–12 A state of oxidative stress ensues, and this affects oxidation of protein and RNA, neurotransmitter synthesis, and neurotransmission at the neuronal junction.13 Reactive nitrogen and oxide radicals induce the release of inflammatory mediators such as interleukins 1 and 6, tumor necrosis factor, interferons, and neurosteroids, and contribute to edema and neurotoxicity.6,10 Neurosteroids are byproducts of mitochondrial metabolism of steroid hormones in the astrocyte.
Manganese enhances neurosteroid synthesis
Manganese enhances neurosteroid synthesis via activation of translocator proteins on the astrocyte membrane. It was first recognized as a factor in hepatic encephalopathy when cirrhotic patients experiencing extrapyramidal symptoms were found to have deposits of manganese in the caudate nucleus and in the globus pallidus on magnetic resonance imaging (MRI). Such deposits were also seen in specimens of brain tissue on autopsy of these patients. When the encephalopathy resolved, so did the abnormalities on MRI.14,15
Changes in the blood-brain barrier
Astrocytes contribute to the selective permeability of the blood-brain barrier. Disruptions in the permeability of the blood-brain barrier underlie hepatic encephalopathy, with poor diffusion of molecules out of astrocytes.
For instance, zinc, which plays a regulatory role in gene transcription and synaptic plasticity, accumulates in the astrocytes, causing relative zinc deficiency and further affecting neurotransmitter synthesis and neurotransmission at the neuronal synapse.6,16
Hyponatremia
Hyponatremia (a serum sodium concentration < 130 mmol/L) is increasingly being recognized as an independent predictor of overt hepatic encephalopathy and is reported to increase the risk by a factor of eight.17
Neuronal dysfunction
Astrocytes are integral to the physiologic functioning of the neurons, and it is becoming clear that both neurons and astrocytes are affected in hepatic encephalopathy.
Additionally, neuroinflammation and a decrease in energy metabolism by the brain are described during episodes of hepatic encephalopathy.18
Amino acid imbalance
An imbalance between aromatic amino acids (ie, high levels of tyrosine and phenylalanine) and branched-chain amino acids (leucine, isoleucine, and valine) has been linked with encephalopathy in patients with liver disease, 19–21 but it is not totally clear whether this imbalance contributes to hepatic encephalopathy or is a consequence of it.
Low-grade brain edema
Edema of the brain occurs in all forms of hepatic encephalopathy, but in cirrhosis it is characteristically of low grade. The mechanism behind this low-grade edema is not clear. Studies have shown that swelling of astrocytes is not global but involves certain areas of the brain and is associated with compensatory extrusion of intracellular myoinositol.22 This, in combination with a mild degree of brain atrophy23 observed in patients with chronic liver disease, is thought to keep the brain from extreme swelling and herniation, a phenomenon usually seen in acute hepatic failure.24,25
Transjugular intrahepatic portosystemic shunting and encephalopathy
The incidence rate of hepatic encephalopathy after placement of a portosystemic shunt to treat portal hypertension ranges from 30% to 55% and is similar to the rate in cirrhotic patients without a shunt.26 In 5% to 8% of patients, the hepatic encephalopathy is refractory and requires intentional occlusion of the shunt.26,27 An elevated serum creatinine level appears to be a risk factor for refractory hepatic encephalopathy in patients with a portosystemic shunt.26
In one study,28 when transjugular intrahepatic portosystemic shunting was done early in the treatment of cirrhotic patients with acute variceal bleeding, the rates of treatment failure and death were significantly less than in a control group that received endoscopic therapy, and no significant difference was noted in the rate of encephalopathy or of serious adverse effects between the groups.
Whether to place a portosystemic shunt in a patient with cirrhosis and a history of hepatic encephalopathy depends on the possible underlying causes of the encephalopathy. For example, if encephalopathy was precipitated by variceal bleeding, shunt placement will prevent further bleeding and will make a recurrence of encephalopathy less likely. However, if the encephalopathy is persistent and uncontrollable, then shunt placement is contraindicated.27
A SPECTRUM OF SYMPTOMS
The spectrum of symptoms extends from a subclinical syndrome that may not be clinically apparent (early-stage or “minimal” hepatic encephalopathy) to full-blown neuropsychiatric manifestations such as cognitive impairment, confusion, slow speech, loss of fine motor skills, asterixis, peripheral neuropathy, clonus, the Babinski sign, decerebrate and decorticate posturing, seizures, extrapyramidal symptoms, and coma.4 The clinical manifestations are usually reversible with prompt treatment, but recurrence is common, typically induced by an event such as gastrointestinal bleeding or an infection.
Minimal hepatic encephalopathy is important to recognize
Although this subclinical syndrome is a very early stage, it is nevertheless associated with higher rates of morbidity and can affect quality of life, including the patient’s ability to drive a car.29,30
Abnormal changes in the brain begin at this stage and eventually progress to more damage and to the development of overt clinical symptoms.
The exact prevalence of minimal hepatic encephalopathy is not known because it is difficult to diagnose, but reported rates range between 30% and 84% of patients with cirrhosis.31 Progression from minimal to overt hepatic encephalopathy is 3.7 times more likely than in patients without the diagnosis of minimal hepatic encephalopathy.32
Thus, minimal hepatic encephalopathy is important to identify,29 so that treatment can be started.
Overt encephalopathy and survival
The prevalence of overt encephalopathy in cirrhosis ranges from 30% to 40% and is even higher in the advanced stages. Once encephalopathy develops, the prognosis worsens rapidly. In patients who do not undergo liver transplantation, the survival rate at 1 year is 42%, and the survival rate at 3 years is 23%.33
These rates are worse than those after liver transplantation, and the American Association for the Study of Liver Diseases recommends that patients with cirrhosis who develop a first episode of encephalopathy be considered for liver transplantation and be referred to a transplantation center.34
CHALLENGES IN DIAGNOSIS
Since the symptoms of hepatic encephalopathy are not specific and can be subtle in the early stage, its diagnosis may be a challenge. It is important to recognize that this neuropsychiatric complication occurs in people with severe comorbidities and requires dedicated time for evaluation and management.
Special tests may be needed to detect subclinical hepatic encephalopathy
In subclinical hepatic encephalopathy, the apparent lack of manifestations poses a great diagnostic challenge, but a thorough history may uncover poor social interaction, personality changes, poor performance at work, and recent traffic violations or motor vehicle accidents. Primary care physicians are usually the first to suspect the condition because they are familiar with the patient’s baseline mental and physical conditions.
For example, the primary care physician may notice decreased attention and worsening memory during a follow-up visit, or the physician may ask whether the patient has difficulty with work performance and handwork (psychomotor and fine motor skills), and whether there have been traffic violations or car accidents (visuospatial skills). Such clues, although not restrictive, may help identify patients with minimal hepatic encephalopathy and prompt referral for neuropsychiatric testing.
Neurologic deficits described in the subclinical form are in the domains of attention and concentration, working memory, visuospatial ability, and fine motor skills; communication skills remain intact.35 These deficits are not reliably detected on standard clinical evaluation but can be detected by neuropsychiatric and neurophysiologic testing.
While several tests for minimal hepatic encephalopathy have been developed, they need to be validated in large trials in the United States.
Neurophysiologic tests include electroencephalography and auditory or visual event-related P300 (evoked potential) testing.
Neuropsychiatric tests traditionally involved several batteries administered and interpreted by specialized personnel. They were time-consuming and were not practical in a typical office setting. They were later refined into the Psychometric Hepatic Encephalopathy Score test (ie, the PSE syndrome test).36 This combines a digit symbol test, a serial dotting test, a line-tracing test, and a number-connection or figure-connection test. An abnormal result in at least three of the four subtests constitutes an overall abnormal PSE syndrome test.
The PSE syndrome test has been validated for standard use in Germany, Spain, Italy, the United Kingdom, and India.35 In 1999, the Working Group on Hepatic Encephalopathy designated it as the official test for minimal hepatic encephalopathy.1 But the test has not been validated for use in the United States. Other tests have been developed, but their use is also limited by a lack of validation and by copyright laws. These factors constitute major obstacles to the diagnosis of subclinical hepatic encephalopathy in the United States. Nonetheless, physicians who suspect minimal hepatic encephalopathy may start lactulose therapy37 and schedule frequent follow-up visits to address and manage potential precipitating factors for overt hepatic encephalopathy.
Staging the severity of the encephalopathy
It is essential to exclude stroke, cerebral bleeding, and brain tumor before making a diagnosis of a first episode of hepatic encephalopathy. Thereafter, such exclusion must be guided by whether the patient has risk factors for these conditions or persistent symptoms of encephalopathy that do not respond to medical therapy.
Laboratory tests can identify metabolic derangements
Although laboratory tests are not diagnostic for hepatic encephalopathy, they can identify metabolic derangements that could contribute to it.
Imaging can help exclude other diagnoses
Neurophysiologic imaging studies such as magnetic resonance spectroscopy, magnetic transfer imaging, and water-mapping techniques have helped elucidate pathologic mechanisms of hepatic encephalopathy and are available in research centers, but they are not currently considered for diagnosis.
SEVERAL LINES OF TREATMENT
Treatment of hepatic encephalopathy involves a preemptive approach to address potential precipitating factors, medical therapy to reduce the production and absorption of ammonia from the gut, and surgical or interventional therapies. A multidisciplinary approach for testing the severity of neurologic impairment and response to therapy is needed to help determine if and when liver transplantation is required.
Prevent potential precipitating factors
An important concept in managing hepatic encephalopathy is to recognize that every cirrhotic patient is at risk and to make an effort to address potential precipitating factors during regular clinic visits. This includes reviewing medication dosing and adverse effects, emphasizing abstinence from alcohol and other toxic substances, and preventing bleeding from esophageal varices with endoscopic band ligation.
Diet therapy
The prevalence of malnutrition in cirrhosis may be as high as 100%. Vitamin and nutritional deficiencies should be evaluated by a nutrition specialist, and nutritional needs should be reassessed on a regular basis. Protein restriction is no longer recommended and may even be harmful.
Guidelines of the European Society of Parenteral and Enteric Nutrition in 2006 recommended that patients with liver disease should have an energy intake of 35 to 40 kcal/kg of body weight daily, with a total daily protein intake of 1.2 to 1.5 mg/kg of body weight.41 Frequent meals and bedtime snacks are encouraged to avoid periods of prolonged fasting and catabolism of muscle protein and to improve nitrogen balance. Branched-chain amino acids and vegetable protein supplements are suggested to help meet the daily requirements.42
Drug therapy to reduce neurotoxins
Drug treatment is directed at reducing the neurotoxins that accumulate in cirrhosis. A variety of agents have been used.
Lactulose (Kristalose) is approved by the US Food and Drug Administration (FDA) as a first-line treatment. It has been shown to improve quality of life and cognitive function in patients with cirrhosis and minimal hepatic encephalopathy, although it has failed to improve mortality rates.37
Lactulose, a cathartic disaccharide, is metabolized by colonic bacteria into short-chain fatty acids. The acidic microenvironment has three major effects:
- It aids the transformation of ammonia to ammonium (NH4+), which is then trapped in the stool, leaving less ammonia to be absorbed
- It has a cathartic effect
- It reduces the breakdown of nitrogenous compounds into ammonia.43
Lactulose has an excessively sweet taste. Its side effects include flatulence, abdominal discomfort, and diarrhea. The usual oral dose is 15 to 45 mL/day given in multiple doses to induce two to three soft bowel movements daily. At this dosage, the monthly cost varies between $60 and $120.
Lactilol, a nonabsorbable disaccharide, is as effective as lactulose but with fewer side effects. It is not available in the United States.
Rifaximin (Xifaxan), a derivative of rifamycin, is FDA-approved for the maintenance of remission of hepatic encephalopathy but is not recommended as a first-line agent. It inhibits bacterial RNA synthesis in the gut. Less than 0.4% of an oral dose is absorbed.44
In a randomized, double-blind, placebo-controlled trial in patients who had had at least two episodes of hepatic encephalopathy while on lactulose therapy, taking rifaximin 550 mg twice a day for 6 months provided a prolonged remission from recurrences of encephalopathy compared with placebo.45 Side effects included nausea, vomiting, abdominal pain, weight loss, and Clostridium difficile colitis, which was reported in two cases in the study.45
Unfortunately, the effects of this drug beyond 6 months of therapy have not been studied. In addition, the drug is expensive: 1 month of treatment with rifaximin can cost between $700 and $1,500. Combining lactulose and rifaximin adds to the costs and the side effects, and contributes to poor adherence to therapy.
Other antibiotics such as metronidazole (Flagyl), vancomycin, and neomycin have been used as alternatives to lactulose, based on the principle that they reduce ammonia-producing bacteria in the gut. However, their efficacy in hepatic encephalopathy remains to be determined.
In controlled trials, neomycin combined with sorbitol, magnesium sulfate, or lactulose was as effective as lactulose, but when used alone, neomycin was no better than placebo.46,47 Neomycin was approved many years ago as an adjunct in the management of hepatic coma, but it has since fallen out of favor in the management of hepatic encephalopathy because of poor trial results and because of neurotoxicity and ototoxicity.
Branched-chain amino acids (leucine, isoleucine, and valine)48 are reported to increase ammonia intake in muscle and to improve cognitive functions on the PSE scale in minimal hepatic encephalopathy,49,50 but they did not decrease the rate of recurrence of hepatic encephalopathy.51 While debate continues over their efficacy in the management of hepatic encephalopathy, branched-chain amino acids may be used to improve nutritional status and muscle mass of patients with cirrhosis. However, the dosing is not standardized, and long-term compliance may be problematic.
Other medical therapies include zinc,16 sodium benzoate,50 and l-ornithine-l-aspartate52,53 to stimulate residual urea cycle activities; probiotics (which pose a risk of sepsis from fungi and lactobacilli); and laxatives.
Liver dialysis
Adsorbing toxins from the blood via liver dialysis or using a non-cell-based liver support system such as MARS (Molecular Adsorbent Recirculating System, Gambro, Inc.) appears to improve the amino acid profile in hepatic encephalopathy, but its role has not been clarified, and its use is limited to clinical trials.54,55
Transjugular intrahepatic shunts and large portosystemic shunts may need to be closed in order to reverse encephalopathy refractory to drug therapy.26,27,56
Liver transplantation
The current scoring system for end-stage liver disease does not include hepatic encephalopathy as a criterion for prioritizing patients on the transplantation list because it was originally developed to assess short-term prognosis in patients undergoing transjugular intrahepatic shunting. As a consequence, patients with end-stage liver disease are at increased risk of repeated episodes of encephalopathy, hospital readmission, and death. Therefore, the American Association for the Study of Liver Diseases recommends referral to a transplantation center when the patient experiences a first episode of overt hepatic encephalopathy to initiate a workup for liver transplantation.34
Liver transplantation improves survival in patients with severe hepatic dysfunction, but the presence of neurologic deficits may result in significant morbidity and in death.57,58 After transplantation, resolution of cognitive dysfunction, brain edema, and white-matter changes have been reported,59 but neuronal cell death and persistent cognitive impairment after resolution of overt hepatic encephalopathy are also described.60–63
Whether neurologic impairment will resolve after liver transplantation depends on a number of factors: the severity of encephalopathy before transplantation; the nature of the neurologic deficits; advanced age; history of alcohol abuse and the presence of alcoholic brain damage; persistence of portosystemic shunts after transplant; emergency transplantation; complications during surgery; and side effects of immunosuppressive drugs.57,58,64
The optimal timing of liver transplantation is not clearly defined for patients who have had bouts of hepatic encephalopathy, and more study is needed to determine the reversibility of clinical symptoms and brain damage. It is in these situations that neuropsychiatric testing and advanced neuroimaging can help determine the efficacy of therapeutic interventions, and it should be considered part of the pretransplantation evaluation.
Managing sleep disturbances
Insomnia and other changes in sleep-wake patterns are common in patients with cirrhosis, especially advanced cirrhosis.65 It is not known whether these changes represent early stages of hepatic encephalopathy.66 Patients often complain of fatigue, the need for frequent naps, and lethargy during the day and restlessness and inability to sleep at night. This affects the patient’s behavior and daytime functioning, and it also burdens household members and caregivers.
Long-acting benzodiazepines should be avoided when treating sleep disorders in cirrhosis because they may precipitate the encephalopathy. In a randomized controlled trial, hydroxyzine (Vistaril) at a dose of 25 mg at bedtime improved sleep behavior in 40% of patients with cirrhosis and subclinical hepatic encephalopathy, but 1 of 17 patients developed acute encephalopathy, which reversed with cessation of the hydroxyzine.66 Clearly, caution and close monitoring are required when giving hydroxyzine for sleep disorders in cirrhotic patients.
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35:716–721.
- Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: a general population-based study. J Hepatol 2008; 49:732–738.
- Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25(suppl 1):3–9.
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009; 50:2014–2021.
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 2007; 22:219–234.
- Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care 2010; 13:87–92.
- Romero-Gómez M, Ramos-Guerrero R, Grande L, et al. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 2004; 41:49–54.
- Tanigami H, Rebel A, Martin LJ, et al. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience 2005; 131:437–449.
- Llansola M, Rodrigo R, Monfort P, et al. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab Brain Dis 2007; 22:321–335.
- Montoliu C, Piedrafita B, Serra MA, et al. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol 2009; 43:272–279.
- Desjardins P, Butterworth RF. The “peripheral-type” benzodiazepine (omega 3) receptor in hyperammonemic disorders. Neurochem Int 2002; 41:109–114.
- Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut 2008; 57:1156–1165.
- Cauli O, Rodrigo R, Llansola M, et al. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 2009; 24:69–80.
- Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet 1995; 346:270–274.
- Pomier-Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 1995; 345:735.
- Schliess F, Görg B, Häussinger D. RNA oxidation and zinc in hepatic encephalopathy and hyperammonemia. Metab Brain Dis 2009; 24:119–134.
- Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol 2009; 104:1382–1389.
- Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis 2007; 22:199–218.
- Marchesini G, Zoli M, Dondi C, et al. Prevalence of subclinical hepatic encephalopathy in cirrhotics and relationship to plasma amino acid imbalance. Dig Dis Sci 1980; 25:763–768.
- Morgan MY, Milsom JP, Sherlock S. Plasma ratio of valine, leucine and isoleucine to phenylalanine and tyrosine in liver disease. Gut 1978; 19:1068–1073.
- Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, Soeters PB. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976; 80:77–91.
- Poveda MJ, Bernabeu A, Concepción L, et al. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage 2010; 52:481–487.
- Bernthal P, Hays A, Tarter RE, Van Thiel D, Lecky J, Hegedus A. Cerebral CT scan abnormalities in cholestatic and hepatocellular disease and their relationship to neuropsychologic test performance. Hepatology 1987; 7:107–114.
- Sugimoto R, Iwasa M, Maeda M, et al. Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 2008; 103:1413–1420.
- Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2006; 43:1187–1190.
- Masson S, Mardini HA, Rose JD, Record CO. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM 2008; 101:493–501.
- Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010; 51:306.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Kircheis G, Knoche A, Hilger N, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009; 137:1706–1715.e1–9.
- Bajaj JS, Saeian K, Schubert CM, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009; 50:1175–1183.
- Hartmann IJ, Groeneweg M, Quero JC, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol 2000; 95:2029–2034.
- Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 2001; 96:2718–2723.
- Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30:890–895.
- Murray KF, Carithers RL jR; AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis 2004; 19:253–267.
- Weissenborn K. PHES: one label, different goods?! J Hepatol 2008; 49:308–312.
- Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007; 45:549–559.
- Parsons-Smith BG, Summerskill WHJ, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957; 2:867–871.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84.
- Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 2008; 29:1612–1621.
- Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006; 25:285–294.
- Gheorghe L, Iacob R, Vadan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol 2005; 14:231–238.
- Weber FL. Effects of lactulose on nitrogen metabolism. Scand J Gastroenterol Suppl 1997; 222:83–87.
- Ojetti V, Lauritano EC, Barbaro F, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol 2009; 5:675–682.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362:1071–1081.
- Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol 2001; 96:1968–1976.
- Rothenberg ME, Keeffe EB. Antibiotics in the management of hepatic encephalopathy: an evidence-based review. Rev Gastroenterol Disord 2005; 5(suppl 3):26–35.
- Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr 2006; 136(suppl 1):295S–298S.
- Egberts EH, Schomerus H, Hamster W, Jürgens P. [Branched-chain amino acids in the treatment of latent porto-systemic encephalopathy. A placebo-controlled double-blind cross-over study] [in German]. Z Ernahrungswiss 1986; 25:9–28.
- Plauth M, Egberts EH, Hamster W, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol 1993; 17:308–314.
- Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 2011; 106:1081–1088.
- Efrati C, Masini A, Merli M, Valeriano V, Riggio O. Effect of sodium benzoate on blood ammonia response to oral glutamine challenge in cirrhotic patients: a note of caution. Am J Gastroenterol 2000; 95:3574–3578.
- Schmid M, Peck-Radosavljevic M, König F, Mittermaier C, Gangl A, Ferenci P. A double-blind, randomized, placebo-controlled trial of intravenous L-ornithine-L-aspartate on postural control in patients with cirrhosis. Liver Int 2010; 30:574–582.
- Blei AT. MARS and treatment of hepatic encephalopathy [in Spanish). Gastroenterol Hepatol 2005; 28:100–104.
- Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002; 36:949–958.
- Zidi SH, Zanditenas D, Gelu-Siméon M, et al. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int 2007; 27:1389–1393.
- Dhar R, Young GB, Marotta P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care 2008; 8:253–258.
- Teperman LW, Peyregne VP. Considerations on the impact of hepatic encephalopathy treatments in the pretransplant setting. Transplantation 2010; 89:771–778.
- Rovira A, Córdoba J, Sanpedro F, Grivé E, Rovira-Gols A, Alonso J. Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology 2002; 59:335–341.
- Senzolo M, Pizzolato G, Ferronato C, et al. Long-term evaluation of cognitive function and cerebral metabolism in liver transplanted patients. Transplant Proc 2009; 41:1295–1296.
- Butterworth RF. Neuronal cell death in hepatic encephalopathy. Metab Brain Dis 2007; 22:309–320.
- DiMartini A, Chopra K. The importance of hepatic encephalopathy: pre-transplant and post-transplant. Liver Transpl 2009; 15:121–123.
- Saner FH, Nadalin S, Radtke A, Sotiropoulos GC, Kaiser GM, Paul A. Liver transplantation and neurological side effects. Metab Brain Dis 2009; 24:183–187.
- Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl 2009; 15:184–192.
- Montagnese S, Middleton B, Skene DJ, Morgan MY. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int 2009; 29:1372–1382.
- Spahr L, Coeytaux A, Giostra E, Hadengue A, Annoni JM. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol 2007; 102:744–753.
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35:716–721.
- Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: a general population-based study. J Hepatol 2008; 49:732–738.
- Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25(suppl 1):3–9.
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009; 50:2014–2021.
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 2007; 22:219–234.
- Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care 2010; 13:87–92.
- Romero-Gómez M, Ramos-Guerrero R, Grande L, et al. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J Hepatol 2004; 41:49–54.
- Tanigami H, Rebel A, Martin LJ, et al. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience 2005; 131:437–449.
- Llansola M, Rodrigo R, Monfort P, et al. NMDA receptors in hyperammonemia and hepatic encephalopathy. Metab Brain Dis 2007; 22:321–335.
- Montoliu C, Piedrafita B, Serra MA, et al. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol 2009; 43:272–279.
- Desjardins P, Butterworth RF. The “peripheral-type” benzodiazepine (omega 3) receptor in hyperammonemic disorders. Neurochem Int 2002; 41:109–114.
- Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut 2008; 57:1156–1165.
- Cauli O, Rodrigo R, Llansola M, et al. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 2009; 24:69–80.
- Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet 1995; 346:270–274.
- Pomier-Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 1995; 345:735.
- Schliess F, Görg B, Häussinger D. RNA oxidation and zinc in hepatic encephalopathy and hyperammonemia. Metab Brain Dis 2009; 24:119–134.
- Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol 2009; 104:1382–1389.
- Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis 2007; 22:199–218.
- Marchesini G, Zoli M, Dondi C, et al. Prevalence of subclinical hepatic encephalopathy in cirrhotics and relationship to plasma amino acid imbalance. Dig Dis Sci 1980; 25:763–768.
- Morgan MY, Milsom JP, Sherlock S. Plasma ratio of valine, leucine and isoleucine to phenylalanine and tyrosine in liver disease. Gut 1978; 19:1068–1073.
- Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, Soeters PB. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976; 80:77–91.
- Poveda MJ, Bernabeu A, Concepción L, et al. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage 2010; 52:481–487.
- Bernthal P, Hays A, Tarter RE, Van Thiel D, Lecky J, Hegedus A. Cerebral CT scan abnormalities in cholestatic and hepatocellular disease and their relationship to neuropsychologic test performance. Hepatology 1987; 7:107–114.
- Sugimoto R, Iwasa M, Maeda M, et al. Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol 2008; 103:1413–1420.
- Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2006; 43:1187–1190.
- Masson S, Mardini HA, Rose JD, Record CO. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM 2008; 101:493–501.
- Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010; 51:306.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Kircheis G, Knoche A, Hilger N, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009; 137:1706–1715.e1–9.
- Bajaj JS, Saeian K, Schubert CM, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009; 50:1175–1183.
- Hartmann IJ, Groeneweg M, Quero JC, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol 2000; 95:2029–2034.
- Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 2001; 96:2718–2723.
- Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30:890–895.
- Murray KF, Carithers RL jR; AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology 2005; 41:1407–1432.
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis 2004; 19:253–267.
- Weissenborn K. PHES: one label, different goods?! J Hepatol 2008; 49:308–312.
- Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007; 45:549–559.
- Parsons-Smith BG, Summerskill WHJ, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957; 2:867–871.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84.
- Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 2008; 29:1612–1621.
- Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 2006; 25:285–294.
- Gheorghe L, Iacob R, Vadan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol 2005; 14:231–238.
- Weber FL. Effects of lactulose on nitrogen metabolism. Scand J Gastroenterol Suppl 1997; 222:83–87.
- Ojetti V, Lauritano EC, Barbaro F, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol 2009; 5:675–682.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362:1071–1081.
- Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol 2001; 96:1968–1976.
- Rothenberg ME, Keeffe EB. Antibiotics in the management of hepatic encephalopathy: an evidence-based review. Rev Gastroenterol Disord 2005; 5(suppl 3):26–35.
- Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr 2006; 136(suppl 1):295S–298S.
- Egberts EH, Schomerus H, Hamster W, Jürgens P. [Branched-chain amino acids in the treatment of latent porto-systemic encephalopathy. A placebo-controlled double-blind cross-over study] [in German]. Z Ernahrungswiss 1986; 25:9–28.
- Plauth M, Egberts EH, Hamster W, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol 1993; 17:308–314.
- Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 2011; 106:1081–1088.
- Efrati C, Masini A, Merli M, Valeriano V, Riggio O. Effect of sodium benzoate on blood ammonia response to oral glutamine challenge in cirrhotic patients: a note of caution. Am J Gastroenterol 2000; 95:3574–3578.
- Schmid M, Peck-Radosavljevic M, König F, Mittermaier C, Gangl A, Ferenci P. A double-blind, randomized, placebo-controlled trial of intravenous L-ornithine-L-aspartate on postural control in patients with cirrhosis. Liver Int 2010; 30:574–582.
- Blei AT. MARS and treatment of hepatic encephalopathy [in Spanish). Gastroenterol Hepatol 2005; 28:100–104.
- Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002; 36:949–958.
- Zidi SH, Zanditenas D, Gelu-Siméon M, et al. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int 2007; 27:1389–1393.
- Dhar R, Young GB, Marotta P. Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care 2008; 8:253–258.
- Teperman LW, Peyregne VP. Considerations on the impact of hepatic encephalopathy treatments in the pretransplant setting. Transplantation 2010; 89:771–778.
- Rovira A, Córdoba J, Sanpedro F, Grivé E, Rovira-Gols A, Alonso J. Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology 2002; 59:335–341.
- Senzolo M, Pizzolato G, Ferronato C, et al. Long-term evaluation of cognitive function and cerebral metabolism in liver transplanted patients. Transplant Proc 2009; 41:1295–1296.
- Butterworth RF. Neuronal cell death in hepatic encephalopathy. Metab Brain Dis 2007; 22:309–320.
- DiMartini A, Chopra K. The importance of hepatic encephalopathy: pre-transplant and post-transplant. Liver Transpl 2009; 15:121–123.
- Saner FH, Nadalin S, Radtke A, Sotiropoulos GC, Kaiser GM, Paul A. Liver transplantation and neurological side effects. Metab Brain Dis 2009; 24:183–187.
- Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl 2009; 15:184–192.
- Montagnese S, Middleton B, Skene DJ, Morgan MY. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int 2009; 29:1372–1382.
- Spahr L, Coeytaux A, Giostra E, Hadengue A, Annoni JM. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol 2007; 102:744–753.
KEY POINTS
- Hepatic encephalopathy should be considered in any patient with cirrhosis who presents with neuropsychiatric manifestations in the absence of another brain disorder, such as stroke or brain tumor.
- “Minimal” hepatic encephalopathy may not be obvious on clinical examination but can be detected with neurophysiologic and neuropsychiatric testing.
- Every cirrhotic patient is at risk; potential precipitating factors should be addressed during regular clinic visits.
- Management requires prompt identification of precipitating factors and initiation of empiric medical therapy. Current treatments include drugs to prevent ammonia generation in the colon.
- Long-acting benzodiazepines should not be used to treat sleep disorders in patients with cirrhosis, as they may precipitate encephalopathy.