User login
Triceps Tendon Fascia for Collateral Ligament Reconstruction About the Elbow: A Clinical and Biomechanical Evaluation
Tumoral Calcinosis: What Is the Treatment? Report of Two Cases of Different Types and Review of the Literature
From Wall Graft to Roof Graft: Reassessment of Femoral Posterior Cruciate Ligament Positioning
New Products/Product News
What's Eating You? Oak Leaf Itch Mite (Pyemotes herfsi)
Ready for flu season? The 2011-2012 ACIP recommendations
The Centers for Disease Control and Prevention (CDC) has released recommendations made by the Advisory Committee for Immunization Practices (ACIP) for using influenza vaccine for the upcoming influenza season.1 The recommendations, which are easier to follow than in past years, continue to advise that patients older than 6 months of age (without a contraindication) be vaccinated annually. But there are some newer recommendations, as well, and they are reviewed here.
2011-2012 vaccine choices include a new product
Although the virus strains in the 2011-2012 vaccines are the same as in 2010-20112—A/California/7/2009 (H1N1)-like, A/Perth/16/ 2009 (H3N2)-like, and B/Brisbane/60/2008-like antigens—individuals vaccinated last year should receive the vaccine again this year. Over the course of a year, antibodies that developed in response to an influenza vaccine decline, and it is believed that even if the vaccine strains have not changed, annual vaccination confers optimal protection.
Although all influenza vaccine products available in the United States contain the same virus strains, the products contain either killed virus (trivalent influenza vaccine [TIV]) or live virus (live attenuated influenza vaccine [LAIV]). The only LAIV vaccine available is the intranasally administered FluMist (MedImmune). It is licensed for use in those between the ages of 2 and 49 years who are healthy, nonpregnant, and without high-risk medical conditions. The CDC does not state a preference for LAIV or TIV in this age group.
A new intradermally administered TIV, Fluzone Intradermal (Sanofi Pasteur),3 was licensed in May 2011 for use in individuals ages 18 through 64 years. It contains less antigen than intramuscular TIV options and is administered in a smaller volume (0.1 rather than 0.5 mL). The preferred site of administration is over the deltoid muscle. Injection-site erythema, induration, swelling, and pruritus occur more frequently than with intramuscular vaccine, but usually these reactions are self-limited, resolving within 3 to 7 days.
Again this coming season, a higher antigen product, Fluzone High-Dose (Sanofi Pasteur), will be available for adults ages 65 and older.4 Fluzone High-Dose contains 4 times the amount of influenza antigen as other TIV options. Ongoing studies are comparing this product with others for effectiveness and rates of adverse reactions. At this time, however, ACIP has not identified a preferred TIV product for this age group.
Easier decision making with young children
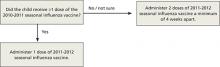
How to determine the number of doses needed by a child younger than 9 years has been simplified. If the child received 1 or more doses of vaccine last season, only 1 dose is needed this year. If no vaccine was received last year or if that status is unknown, 2 doses are recommended (FIGURE).1
FIGURE
How many doses of flu vaccine for children 6 months through 8 years of age?1
Egg allergy does not necessarily prohibit vaccination
The last recommendation change this season is that a history of egg allergy is no longer an automatic contraindication to influenza vaccine.1 The only contraindication to receiving the vaccine is a prior severe allergic reaction to influenza vaccine. ACIP now states that individuals who have experienced only hives after exposure to egg should receive the vaccine, but only a TIV product and only from a health care provider who is familiar with the potential manifestations of egg allergy. Additionally, those who receive the vaccine should be observed for at least 30 minutes for signs of a reaction.
In the past, some providers have used a 2-step approach (giving a small proportion as a dose first; then, if no reaction occurs, administering the remaining portion). Others have recommended skin testing with vaccine before administration. Neither of these approaches is necessary, according to ACIP, which cites studies that showed skin prick testing with vaccine is poorly predictive of allergic reactions and administration of both full doses and 2-step doses have been well tolerated.1
Many people reporting egg allergy will not have a reaction to influenza vaccine.1 In addition, current influenza vaccine products contain very low levels of egg protein. Individuals more likely to have a serious reaction are those who have had severe reactions to egg—eg, angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or other emergency medical interventions. Such people should be referred to a physician with expertise in the management of allergic conditions for further risk assessment.1 As another precaution, the ACIP recommendations state that all vaccines should be administered in settings where equipment is on hand for treatment of anaphylaxis and where providers are trained in the recognition and treatment of allergic reactions.
1. Centers for Disease Control and Prevention Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011 [early release]. MMWR Morb Mortal Wkly Rep. 2011;60:1-5.
2. Centers for Disease Control and Prevention Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2011;60:705-712.
3. Johnson DR. Fluzone Intradermal (influenza virus vaccine): a new option for influenza prevention. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-5-flu-intradermal.pdf. Accessed July 27, 2011.
4. Johnson DR. Fluzone High-Dose vaccine: one year post-approval: experience during the 2010-2011 influenza season. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-4-flu-high-dose.pdf. Accessed July 27, 2011.
The Centers for Disease Control and Prevention (CDC) has released recommendations made by the Advisory Committee for Immunization Practices (ACIP) for using influenza vaccine for the upcoming influenza season.1 The recommendations, which are easier to follow than in past years, continue to advise that patients older than 6 months of age (without a contraindication) be vaccinated annually. But there are some newer recommendations, as well, and they are reviewed here.
2011-2012 vaccine choices include a new product
Although the virus strains in the 2011-2012 vaccines are the same as in 2010-20112—A/California/7/2009 (H1N1)-like, A/Perth/16/ 2009 (H3N2)-like, and B/Brisbane/60/2008-like antigens—individuals vaccinated last year should receive the vaccine again this year. Over the course of a year, antibodies that developed in response to an influenza vaccine decline, and it is believed that even if the vaccine strains have not changed, annual vaccination confers optimal protection.
Although all influenza vaccine products available in the United States contain the same virus strains, the products contain either killed virus (trivalent influenza vaccine [TIV]) or live virus (live attenuated influenza vaccine [LAIV]). The only LAIV vaccine available is the intranasally administered FluMist (MedImmune). It is licensed for use in those between the ages of 2 and 49 years who are healthy, nonpregnant, and without high-risk medical conditions. The CDC does not state a preference for LAIV or TIV in this age group.
A new intradermally administered TIV, Fluzone Intradermal (Sanofi Pasteur),3 was licensed in May 2011 for use in individuals ages 18 through 64 years. It contains less antigen than intramuscular TIV options and is administered in a smaller volume (0.1 rather than 0.5 mL). The preferred site of administration is over the deltoid muscle. Injection-site erythema, induration, swelling, and pruritus occur more frequently than with intramuscular vaccine, but usually these reactions are self-limited, resolving within 3 to 7 days.
Again this coming season, a higher antigen product, Fluzone High-Dose (Sanofi Pasteur), will be available for adults ages 65 and older.4 Fluzone High-Dose contains 4 times the amount of influenza antigen as other TIV options. Ongoing studies are comparing this product with others for effectiveness and rates of adverse reactions. At this time, however, ACIP has not identified a preferred TIV product for this age group.
Easier decision making with young children
How to determine the number of doses needed by a child younger than 9 years has been simplified. If the child received 1 or more doses of vaccine last season, only 1 dose is needed this year. If no vaccine was received last year or if that status is unknown, 2 doses are recommended (FIGURE).1
FIGURE
How many doses of flu vaccine for children 6 months through 8 years of age?1
Egg allergy does not necessarily prohibit vaccination
The last recommendation change this season is that a history of egg allergy is no longer an automatic contraindication to influenza vaccine.1 The only contraindication to receiving the vaccine is a prior severe allergic reaction to influenza vaccine. ACIP now states that individuals who have experienced only hives after exposure to egg should receive the vaccine, but only a TIV product and only from a health care provider who is familiar with the potential manifestations of egg allergy. Additionally, those who receive the vaccine should be observed for at least 30 minutes for signs of a reaction.
In the past, some providers have used a 2-step approach (giving a small proportion as a dose first; then, if no reaction occurs, administering the remaining portion). Others have recommended skin testing with vaccine before administration. Neither of these approaches is necessary, according to ACIP, which cites studies that showed skin prick testing with vaccine is poorly predictive of allergic reactions and administration of both full doses and 2-step doses have been well tolerated.1
Many people reporting egg allergy will not have a reaction to influenza vaccine.1 In addition, current influenza vaccine products contain very low levels of egg protein. Individuals more likely to have a serious reaction are those who have had severe reactions to egg—eg, angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or other emergency medical interventions. Such people should be referred to a physician with expertise in the management of allergic conditions for further risk assessment.1 As another precaution, the ACIP recommendations state that all vaccines should be administered in settings where equipment is on hand for treatment of anaphylaxis and where providers are trained in the recognition and treatment of allergic reactions.
The Centers for Disease Control and Prevention (CDC) has released recommendations made by the Advisory Committee for Immunization Practices (ACIP) for using influenza vaccine for the upcoming influenza season.1 The recommendations, which are easier to follow than in past years, continue to advise that patients older than 6 months of age (without a contraindication) be vaccinated annually. But there are some newer recommendations, as well, and they are reviewed here.
2011-2012 vaccine choices include a new product
Although the virus strains in the 2011-2012 vaccines are the same as in 2010-20112—A/California/7/2009 (H1N1)-like, A/Perth/16/ 2009 (H3N2)-like, and B/Brisbane/60/2008-like antigens—individuals vaccinated last year should receive the vaccine again this year. Over the course of a year, antibodies that developed in response to an influenza vaccine decline, and it is believed that even if the vaccine strains have not changed, annual vaccination confers optimal protection.
Although all influenza vaccine products available in the United States contain the same virus strains, the products contain either killed virus (trivalent influenza vaccine [TIV]) or live virus (live attenuated influenza vaccine [LAIV]). The only LAIV vaccine available is the intranasally administered FluMist (MedImmune). It is licensed for use in those between the ages of 2 and 49 years who are healthy, nonpregnant, and without high-risk medical conditions. The CDC does not state a preference for LAIV or TIV in this age group.
A new intradermally administered TIV, Fluzone Intradermal (Sanofi Pasteur),3 was licensed in May 2011 for use in individuals ages 18 through 64 years. It contains less antigen than intramuscular TIV options and is administered in a smaller volume (0.1 rather than 0.5 mL). The preferred site of administration is over the deltoid muscle. Injection-site erythema, induration, swelling, and pruritus occur more frequently than with intramuscular vaccine, but usually these reactions are self-limited, resolving within 3 to 7 days.
Again this coming season, a higher antigen product, Fluzone High-Dose (Sanofi Pasteur), will be available for adults ages 65 and older.4 Fluzone High-Dose contains 4 times the amount of influenza antigen as other TIV options. Ongoing studies are comparing this product with others for effectiveness and rates of adverse reactions. At this time, however, ACIP has not identified a preferred TIV product for this age group.
Easier decision making with young children
How to determine the number of doses needed by a child younger than 9 years has been simplified. If the child received 1 or more doses of vaccine last season, only 1 dose is needed this year. If no vaccine was received last year or if that status is unknown, 2 doses are recommended (FIGURE).1
FIGURE
How many doses of flu vaccine for children 6 months through 8 years of age?1
Egg allergy does not necessarily prohibit vaccination
The last recommendation change this season is that a history of egg allergy is no longer an automatic contraindication to influenza vaccine.1 The only contraindication to receiving the vaccine is a prior severe allergic reaction to influenza vaccine. ACIP now states that individuals who have experienced only hives after exposure to egg should receive the vaccine, but only a TIV product and only from a health care provider who is familiar with the potential manifestations of egg allergy. Additionally, those who receive the vaccine should be observed for at least 30 minutes for signs of a reaction.
In the past, some providers have used a 2-step approach (giving a small proportion as a dose first; then, if no reaction occurs, administering the remaining portion). Others have recommended skin testing with vaccine before administration. Neither of these approaches is necessary, according to ACIP, which cites studies that showed skin prick testing with vaccine is poorly predictive of allergic reactions and administration of both full doses and 2-step doses have been well tolerated.1
Many people reporting egg allergy will not have a reaction to influenza vaccine.1 In addition, current influenza vaccine products contain very low levels of egg protein. Individuals more likely to have a serious reaction are those who have had severe reactions to egg—eg, angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or other emergency medical interventions. Such people should be referred to a physician with expertise in the management of allergic conditions for further risk assessment.1 As another precaution, the ACIP recommendations state that all vaccines should be administered in settings where equipment is on hand for treatment of anaphylaxis and where providers are trained in the recognition and treatment of allergic reactions.
1. Centers for Disease Control and Prevention Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011 [early release]. MMWR Morb Mortal Wkly Rep. 2011;60:1-5.
2. Centers for Disease Control and Prevention Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2011;60:705-712.
3. Johnson DR. Fluzone Intradermal (influenza virus vaccine): a new option for influenza prevention. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-5-flu-intradermal.pdf. Accessed July 27, 2011.
4. Johnson DR. Fluzone High-Dose vaccine: one year post-approval: experience during the 2010-2011 influenza season. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-4-flu-high-dose.pdf. Accessed July 27, 2011.
1. Centers for Disease Control and Prevention Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011 [early release]. MMWR Morb Mortal Wkly Rep. 2011;60:1-5.
2. Centers for Disease Control and Prevention Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2011;60:705-712.
3. Johnson DR. Fluzone Intradermal (influenza virus vaccine): a new option for influenza prevention. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-5-flu-intradermal.pdf. Accessed July 27, 2011.
4. Johnson DR. Fluzone High-Dose vaccine: one year post-approval: experience during the 2010-2011 influenza season. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-4-flu-high-dose.pdf. Accessed July 27, 2011.
Correcting pelvic organ prolapse with robotic sacrocolpopexy
- Promontory dissection and creation of the retroperitoneal tunnel
- Dissection of the rectovaginal and vesicovaginal spaces
- Attachment of y-mesh to vagina: Start anteriorly
- Posterior y-mesh attachment
- Attachment of mesh to sacral promontory
These videos were provided by Catherine A. Matthews, MD.
Recent years have seen growing recognition that adequate support of the vaginal apex is an essential component of durable surgical repair of pelvic organ prolapse.1,2 Sacrocolpopexy is now considered the gold standard for repair of Level-1 defects of pelvic support, providing excellent long-term results.3-5
A recent randomized, controlled trial demonstrated the superior efficacy of laparoscopic sacrocolpopexy to a total vaginal mesh procedure in women who have vaginal vault prolapse—further evidence that sacrocolpopexy is the procedure of choice for these patients.6
The advantages of sacrocolpopexy include:
- reduced risk of mesh exposure, compared to insertion of vaginal mesh
- preservation of vaginal length
- reduced risk of re-operation for symptomatic recurrent prolapse
- reduced risk of de novo dyspareunia secondary to contraction of mesh.
Obstacles. Although a small number of surgeons are able to accomplish sacrocolpopexy using standard laparoscopic techniques, most of these procedures are still performed by laparotomy because the extensive suturing and knot-tying present a surgical challenge. Open sacrocolpopexy has disadvantages, too, including more pain, longer recovery, and longer length of stay.7-9
With the introduction of the da Vinci robot (Intuitive Surgical), the feasibility of having more surgeons perform this operation using a reproducible, minimally-invasive technique is much greater. The steep learning curve associated with standard laparoscopy in regard to mastering intracorporeal knot-tying and suturing is greatly diminished by articulating instruments. This makes robotic sacrocolpopexy an accessible option for all gynecologic surgeons who treat women with pelvic organ prolapse.
In this article, I detail the steps—with tips and tricks from my experience—to completing an efficient robotic-assisted sacrocolpopexy—modeled exactly after the open technique—that utilizes a y-shaped polypropylene mesh graft. Included is capsule advice from OBG Management’s coding consultant on obtaining reimbursement for robotic procedures (see “ Coding tips for robotic sacrocolpopexy”).
- Two proficient tableside assistants are needed
- Use steep Trendelenburg to remove the bowel from the operative field
- A fan retractor is necessary in some cases to gain access to the promontory
- Correct identification of the sacral promontory is key
- In the absence of haptic feedback, novice surgeons must be aware of the potential danger in dissecting too far laterally and entering the common iliac vessels
- Y-shaped grafts should be fashioned individually
- Know the exit point of the needle at the promontory
- Adequate spacing between the robotic arms is essential to avoiding interference among instruments during the procedure.
Details of the procedure
1. Surgical preparation, set-up
The patient completes a bowel prep using two bottles of magnesium citrate and taking only clear liquids 1 day before surgery. Although mechanical bowel cleansing has not been shown to decrease operative morbidity, manipulation and retraction of the sigmoid colon may be easier with the bowel empty.
Perioperative antibiotics are administered 30 minutes prior to the procedure. Heparin, 5,000 U, is injected subcutaneously for thromboprophylaxis as the patient is en route to the operating suite.
The patient is placed in the dorsal lithotomy position, buttocks extending one inch over the end of the operating table. The table should be covered with egg-crate foam to avoid having her slip down while in steep Trendelenburg position.
After the patient is prepped and draped, a Foley catheter is placed into the bladder. EEA sizers (Covidien) are inserted into the vagina and rectum.
Two experienced surgical assistants are necessary:
- One on the patient’s right side to assist with tissue retraction and introduction of suture material
- Another seated between the patient’s legs to provide adequate vaginal and rectal manipulation during surgery.
2. Port placement, docking, and instrumentation
Pneumoperitoneum is obtained with a Veress needle. Five trocars are then placed (FIGURE 1).
Careful port placement is integral to the success of this procedure because:
- Inadequate distance between robotic arms and the camera results in arm collisions and interference
- Visualization and access to the sacral promontory may be compromised if the camera is inserted too low on the anterior abdominal wall
- Bowel retraction may be compromised if the fourth arm of the robot isn’t at least 3 cm above the anterior superior iliac crest.
My experience evaluating the abdomen before trocar insertion is that at least 15 cm is required between the pubic bone and the umbilicus to rely on this landmark for locating the 12-mm camera port.10 If this distance is shorter (as it is in many obese women), insertion above the umbilicus is necessary.
An accessory 12-mm port, used to introduce sutures and the mesh graft, is placed approximately 10 cm lateral and 4 cm cephalad to the camera in the right-upper quadrant.
An 8-mm robotic port is placed in the right lower quadrant, 10 cm lateral to the accessory port and approximately 3 cm above the anterior superior iliac crest.
The third and fourth robotic arms are placed 10 cm apart in the left lower quadrant, with the fourth arm typically as far lateral as possible.
Docking. After the patient has been placed in steep Trendelenburg position and the table is locked, the robot is docked from the patient’s left side at a 45° angle to the table. Side-docking permits easy access to the vagina to 1) evaluate graft tension and 2) complete cystoscopy to ensure ureteral and bladder integrity.
TIP Take care to ensure that the spine of the robot is positioned right next to the bed at the level of the patient’s hip; driving it up too high in relation to the abdomen can compromise the mobility of the fourth arm. In addition, if the robot is not close enough to the bed, the reach of the first (right) arm may be limited.
Next, introduce monopolar scissors through the right arm; a bipolar PK Dissector (Intuitive Surgical) through the left arm; and an atraumatic bowel grasper, such as Cadiere Forceps (Intuitive Surgical), through the fourth arm.
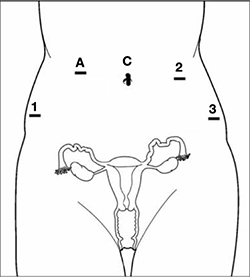
FIGURE 1 Placement of 5 ports for robotic sacrocolpopexy
Key: C, camera; A, accessory port; 1, right arm (monopolar shears); 2, left arm (PK Dissector); 3, fourth arm (Cadiere Forceps).
3. Dissect the sacral promontory and create a retroperitoneal tunnel
With the use of a 0° scope or 30° down-scope, retract the sigmoid colon laterally using Cadiere forceps and identify the right ureter.
TIP When you attempt robotic sacrocolpopexy for the first time, it may help to identify the sacral promontory, using a standard laparoscopic instrument with haptic feedback, before you dock the robot.
Elevate the peritoneum overlying the sacral promontory and open it using monopolar cautery. Expose the fat pad that overlays the anterior longitudinal ligament and gently dissect it away (FIGURE 2; VIDEO 1). Often, the middle sacral artery is visualized; it can be coagulated using the PK Dissector if necessary.
TIP In a case in which the promontory is difficult to find, dissecting the retrorectal space is a simple way to mobilize the bowel away from the sacrum, thus exposing the promontory.
TRICK Instead of opening the peritoneum from the sacrum to the cul-de-sac, I create a retroperitoneal tunnel along that right paracolic gutter, from the promontory to just medial to the right uterosacral ligament (VIDEO 1). Doing so has three benefits:
- It is quicker and less bloody
- It allows the mesh to lay flat in the hollow of the sacrum when you bring the sacral arm up to the promontory
- There is much less peritoneum to close over the mesh at the end of the procedure.
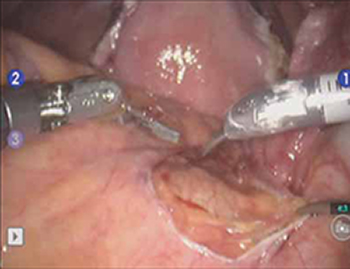
FIGURE 2 Entering the peritoneum
Open the peritoneum at the sacral promontory and dissect the fat pad. This reveals the anterior longitudinal ligament.
4. Dissect the vesicovaginal and rectovaginal spaces
Effective vaginal and rectal manipulation is critical to complete this part of the procedure safely. To gain access to the rectovaginal space, the vaginal assistant needs to push the vagina all the way in and up toward the anterior abdominal wall (the handle of the EEA sizer will be pushing hard up against the perineum) while simultaneously pushing the rectal probe downward (effectively scissoring the two apart).
From the exit point of the retroperitoneal tunnel that was created at the beginning of the case, then extend the peritoneal incision transversely in the shape of a “T” to expose the posterior vaginal wall (FIGURE 3, VIDEO 2). If indicated, dissect the rectovaginal space all the way down to the perineal body.
Deviate the vagina posteriorly to facilitate dissection of the bladder from the anterior vaginal wall. Use sharp dissection with scissors and short bursts of energy with monopolar cautery.
TIP If you encounter significant scarring between the bladder and vagina, retrograde-fill the bladder with 300 mL of saline mixed with methylene blue dye to identify the surgical plane.
Expose approximately 4 to 6 cm of anterior vaginal wall, depending on the degree of anterior vaginal wall prolapse. Try to leave the peritoneum intact at the apex of the vagina to reduce the chance that mesh will erode.
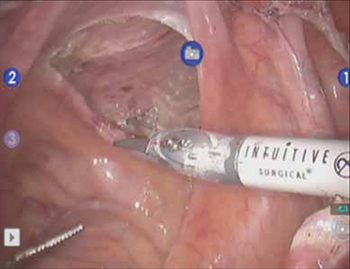
FIGURE 3 The peritoneal incision
Extend the peritoneal incision along the cul-de-sac to the posterior vaginal wall in a T-shaped configuration to gain access the rectovaginal space. When perorming cervicosacropexy, it is easiest to develop this surgical plane before amputating the cervix.
5. Attach the y-mesh to the vagina
Several mesh options exist: IntePro (American Medical Systems), Alyte (Bard Medical), and Restorelle Y (Mpathy Medical) are preformed Type-1 polypropylene mesh products. A correctly sized mesh can easily be fashioned by suturing together two strips of Gynemesh (Ethicon) that are approximately 3 cm wide.
Because there can be significant variability in the relative dimensions of the anterior and posterior segments of mesh, I recommend fashioning the graft after dissection is complete: When posterior wall prolapse is extensive, for example, preformed y-mesh strips may not be long enough to reach all the way down to the perineal body. After having assessed the differences in graft placement and manipulation when the two arms are sutured together 1) before the grafts are placed intracorporeally and 2) after they are placed, I’ve concluded that the first method—suturing before placement—is far easier.
Introduce the mesh graft through the accessory port after exchanging the scissors and PK dissector for a suture cutter and a large needle driver. Retract the bladder using the fourth arm, and place the anterior mesh arm over the anterior vaginal wall; suture it in place using 2-0 Gore-Tex sutures on CT-2 needles that are each cut to 6 inches long.
For greatest efficiency, anchor the two distal corners first (FIGURE 4; VIDEO 3), then place a series of interrupted stitches towards the vaginal apex. Tie the knots using 2 surgeon’s knots, followed by 2 half-hitches. Attempt to achieve healthy bites through the vaginal muscularis without perforating the epithelium.
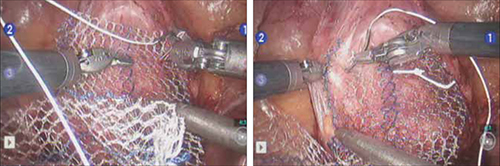
FIGURE 4 Suturing the mesh graft to the vaginal wall
Left and right: Suture the y-shaped polypropylene mesh graft to the anterior vaginal wall first, starting at the distal corners. The bladder is retracted cephalad using the fourth arm.After you’ve adequately secured the anterior mesh arm, deviate the vagina anteriorly and drape the posterior mesh arm over the posterior vaginal wall with the assistance of the fourth robotic arm that can hold upward traction on the sacral end of the mesh graft. Starting at the vaginal apex, place 6 to 8 interrupted sutures to secure the mesh to the posterior vaginal wall (FIGURE 5; VIDEO 4). If necessary, exchange the 0° scope for a 30° up-scope so that you can fully visualize the rectovaginal space.
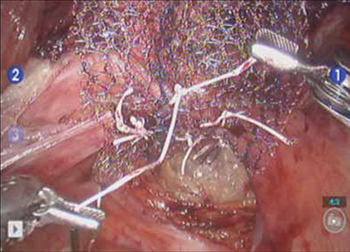
FIGURE 5 Attachment of the posterior arm of the mesh
The fourth arm of the robot provides upward traction on the sacral portion of the mesh graft.
6. Attach the graft to the sacrum
Again retract the sigmoid laterally to expose the promontory dissection. Retrieve the sacral arm of the mesh through the retroperitoneal tunnel and pull it up toward the promontory. Deviate the vagina toward the sacrum and, ensuring that there is no excessive tension, suture the sacral portion of the mesh graft to the anterior longitudinal ligament at the promontory, using 2 or 3 interrupted sutures (FIGURE 6, VIDEO 5).
When placing the needle during this critical juncture, it is important to rotate through the ligament along the curvature of the needle—as opposed to driving the needle forward and risking exiting farther laterally than expected.
TIP Because of the slight traction that exists on the mesh, a slip-knot is preferred instead of a surgeon’s knot when suturing the sacral portion of the mesh graft to the anterior longitudinal ligament. Take care to visualize the middle sacral artery and either suture around it or cauterize it.
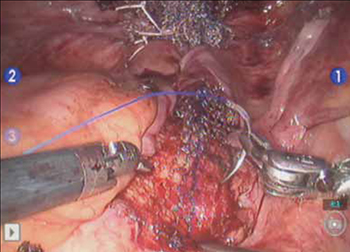
FIGURE 6 Suture the mesh directly to the anterior longitudinal ligament
Use two or three stitches, secured with slip-knots. Take care not to create excessive tension on the mesh graft.
7. Extraperitonealize the mesh
I no longer routinely close the peritoneum over the mesh at the vaginal apex because I have not had a single case of small-bowel obstruction since I began performing this procedure laparoscopically. You should close the peritoneal window at the promontory, however, if the mesh is tented up at all, because tenting creates the potential for bowel to get caught beneath the mesh. Perform this closure using 2-0 monofilament suture or 2-0 Vicryl suture (Ethicon) cut to 8 inches (FIGURE 7). It is always easiest to start distally and suture towards the camera and operative instruments.
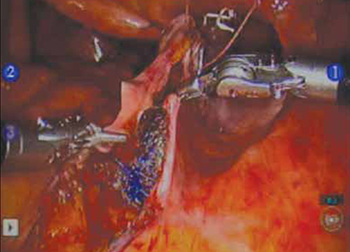
FIGURE 7 Extraperitonealize the mesh
Close the peritoneum from the apex of the vagina with a purse-string—like stitch, continuing it to the promontory in running fashion.
8. Ensure that mesh is not in the bladder or rectum
Perform cystoscopy and a rectal examination at the end of each case to confirm that no sutures or mesh material are within either viscus. It is much easier to remove these before leaving the operating room.
Modifying the procedure for uterovaginal prolapse
If the patient has an intact uterus and benign cervical cytology, perform supracervical hysterectomy before proceeding with Steps 1–8 above.
TIP Leaving the cervix in situ may reduce the chance of mesh erosion and provides an excellent platform for mesh attachment.
TRICK I find it most helpful to fully dissect the anterior and posterior vaginal walls before cervical amputation because upward traction on the corpus improves visualization of the surgical planes.
Once the cervix is amputated, however, effective vaginal manipulation can present a surgical challenge. Some surgeons use a tenaculum attached to the fourth arm of the robot to apply traction on the cervix, but this eliminates this arm from performing other necessary tasks. Malleable or Breisky-Navratil retractors can be used to delineate the anterior and posterior vaginal fornices, but are not always satisfactory—especially if an assistant isn’t seated between the legs.
TIP A useful and inexpensive instrument is the Colpo-Probe vaginal fornix delineator (Cooper Surgical) (FIGURE 8), which not only assists in dissecting the vagina from the bladder and rectum but also provides a stable surface during attachment of mesh.

FIGURE 8 Colpo-Probe
This device delineates the anterior and posterior vaginal fornices. It provides a stable platform against which to suture the mesh graft.
Tips and tricks for managing hemorrhage during sacrocolpopexy
Four potential areas of bleeding danger exist:
- In trying to find the sacral promontory, you risk entering the right common iliac vein if dissection is too far cephalad and lateral.
TIP I strongly advise novice robotic surgeons to try to identify the site of the promontory with a standard laparoscopic instrument with haptic feedback before moving to the surgical console.
TRICK Another trick that can help with safe identification of the promontory is mobilization of the sigmoid colon away from the sacrum by developing the retrorectal space. - During dissection of the fat pad from the promontory, you can encounter the middle sacral artery.
TRICK Spreading carefully in a caudal–cephalad direction until the level of the ligament is reached, instead of spreading in a lateral dimension, can decrease the chances of lacerating of this vessel. - A dangerous plexus of veins traverses the hollow of the sacrum. If you are trying to affix mesh at the level of S2-3, therefore, you may encounter significant bleeding.
TIP Work above the level of S1 to avoid these veins completely. - In securing the mesh to the sacral promontory, you can puncture the left common iliac vein if you are not aware of the exit point of the needle and it traverses too far medially.
TIP If you encounter bleeding, introduce a RAY-TEC sponge (Johnson & Johnson) through the accessory port. Apply manual compression for at least 5 minutes. If bleeding persists, I recommend Floseal Hemostatic Matrix (Baxter) to control hemorrhage that arises from arterial and venous sources.
TRICK As last resort, perform emergency undocking in rapid fashion while the bedside assistant maintains pressure through the accessory port.
Conclusion
The da Vinci robotic surgical system facilitates completion of sacrocolpopexy and cervicosacropexy, in a manner identical to the open technique, by surgeons who may not possess advanced laparoscopic skills. Full knowledge of the relevant anatomy is critical; there is the potential for significant morbidity during the procedure if surgical planes are created incorrectly.
Robotic surgery in on the rise, but coding for robotic procedures is still limited to the basic, conventional procedure. Why? Because insurers reimburse the hospital for use of the equipment but still refuse to reimburse the surgeon any additional amount for incorporating the robot into the surgical technique.
Coding for your work when performing robotic sacrocolpopexy is straightforward: Report laparoscopic code 57425 (laparoscopy, surgical, colpopexy [suspension of vaginal apex]) for the procedure. Mesh that might be used as part of the repair is not reported separately.
Blue Cross/Blue Shield (BC/BS) added the Healthcare Common Procedure Coding System Level-II code S2900 (surgical techniques requiring use of robotic surgical system) to the national code set a few years ago. Although BC/BS and some other payers accept this code on the claim, there is no reimbursement attached: It was developed for informational purposes only.
Remember, however, that coding is complete only when you have an appropriate linking diagnosis to establish the medical necessity of laparoscopic sacrocolpopexy. Diagnostic coding options for this surgical procedure include documentation of uterovaginal prolapse (incomplete prolapse is 618.2; complete prolapse is 618.3); vaginal vault prolapse after hysterectomy (618.5); and uterine prolapse without vaginal wall prolapse (618.1).
—MELANIE WITT, RN, CPC, COBGC, MA
We want to hear from you! Tell us what you think.
1. Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438-1443.
2. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;(3):CD004014.-
3. Sullivan ES, Longaker CJ, Lee PY. Total pelvic mesh repair: a ten-year experience. Dis Colon Rectum. 2001;44(6):857-863.
4. Culligan PJ, Murphy M, Blackwell L, Hammons G, Graham C, Heit MH. Long-term success of abdominal sacral colpopexy using synthetic mesh. Am J Obstet Gynecol. 2002;187(6):1473-1482.
5. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805-823.
6. Maher CF, Feiner B, DeCuyper EM, Nichlos CJ, Hickey KV, O’Rourke P. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol. 2011;204(4):360.e1-7.
7. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol. 1994;84(5):885-888.
8. Ross JW. Techniques of laparoscopic repair of total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4(2):173-183.
9. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy hysterectomy, and burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6(2):115-119.
10. Matthews CA, Schubert CM, Woodward AP, Gill EJ. Variance in abdominal wall anatomy and port placement in women undergoing robotic gynecologic surgery. J Minim Invasive Gynecol. 2010;17(5):583-586.
- Promontory dissection and creation of the retroperitoneal tunnel
- Dissection of the rectovaginal and vesicovaginal spaces
- Attachment of y-mesh to vagina: Start anteriorly
- Posterior y-mesh attachment
- Attachment of mesh to sacral promontory
These videos were provided by Catherine A. Matthews, MD.
Recent years have seen growing recognition that adequate support of the vaginal apex is an essential component of durable surgical repair of pelvic organ prolapse.1,2 Sacrocolpopexy is now considered the gold standard for repair of Level-1 defects of pelvic support, providing excellent long-term results.3-5
A recent randomized, controlled trial demonstrated the superior efficacy of laparoscopic sacrocolpopexy to a total vaginal mesh procedure in women who have vaginal vault prolapse—further evidence that sacrocolpopexy is the procedure of choice for these patients.6
The advantages of sacrocolpopexy include:
- reduced risk of mesh exposure, compared to insertion of vaginal mesh
- preservation of vaginal length
- reduced risk of re-operation for symptomatic recurrent prolapse
- reduced risk of de novo dyspareunia secondary to contraction of mesh.
Obstacles. Although a small number of surgeons are able to accomplish sacrocolpopexy using standard laparoscopic techniques, most of these procedures are still performed by laparotomy because the extensive suturing and knot-tying present a surgical challenge. Open sacrocolpopexy has disadvantages, too, including more pain, longer recovery, and longer length of stay.7-9
With the introduction of the da Vinci robot (Intuitive Surgical), the feasibility of having more surgeons perform this operation using a reproducible, minimally-invasive technique is much greater. The steep learning curve associated with standard laparoscopy in regard to mastering intracorporeal knot-tying and suturing is greatly diminished by articulating instruments. This makes robotic sacrocolpopexy an accessible option for all gynecologic surgeons who treat women with pelvic organ prolapse.
In this article, I detail the steps—with tips and tricks from my experience—to completing an efficient robotic-assisted sacrocolpopexy—modeled exactly after the open technique—that utilizes a y-shaped polypropylene mesh graft. Included is capsule advice from OBG Management’s coding consultant on obtaining reimbursement for robotic procedures (see “ Coding tips for robotic sacrocolpopexy”).
- Two proficient tableside assistants are needed
- Use steep Trendelenburg to remove the bowel from the operative field
- A fan retractor is necessary in some cases to gain access to the promontory
- Correct identification of the sacral promontory is key
- In the absence of haptic feedback, novice surgeons must be aware of the potential danger in dissecting too far laterally and entering the common iliac vessels
- Y-shaped grafts should be fashioned individually
- Know the exit point of the needle at the promontory
- Adequate spacing between the robotic arms is essential to avoiding interference among instruments during the procedure.
Details of the procedure
1. Surgical preparation, set-up
The patient completes a bowel prep using two bottles of magnesium citrate and taking only clear liquids 1 day before surgery. Although mechanical bowel cleansing has not been shown to decrease operative morbidity, manipulation and retraction of the sigmoid colon may be easier with the bowel empty.
Perioperative antibiotics are administered 30 minutes prior to the procedure. Heparin, 5,000 U, is injected subcutaneously for thromboprophylaxis as the patient is en route to the operating suite.
The patient is placed in the dorsal lithotomy position, buttocks extending one inch over the end of the operating table. The table should be covered with egg-crate foam to avoid having her slip down while in steep Trendelenburg position.
After the patient is prepped and draped, a Foley catheter is placed into the bladder. EEA sizers (Covidien) are inserted into the vagina and rectum.
Two experienced surgical assistants are necessary:
- One on the patient’s right side to assist with tissue retraction and introduction of suture material
- Another seated between the patient’s legs to provide adequate vaginal and rectal manipulation during surgery.
2. Port placement, docking, and instrumentation
Pneumoperitoneum is obtained with a Veress needle. Five trocars are then placed (FIGURE 1).
Careful port placement is integral to the success of this procedure because:
- Inadequate distance between robotic arms and the camera results in arm collisions and interference
- Visualization and access to the sacral promontory may be compromised if the camera is inserted too low on the anterior abdominal wall
- Bowel retraction may be compromised if the fourth arm of the robot isn’t at least 3 cm above the anterior superior iliac crest.
My experience evaluating the abdomen before trocar insertion is that at least 15 cm is required between the pubic bone and the umbilicus to rely on this landmark for locating the 12-mm camera port.10 If this distance is shorter (as it is in many obese women), insertion above the umbilicus is necessary.
An accessory 12-mm port, used to introduce sutures and the mesh graft, is placed approximately 10 cm lateral and 4 cm cephalad to the camera in the right-upper quadrant.
An 8-mm robotic port is placed in the right lower quadrant, 10 cm lateral to the accessory port and approximately 3 cm above the anterior superior iliac crest.
The third and fourth robotic arms are placed 10 cm apart in the left lower quadrant, with the fourth arm typically as far lateral as possible.
Docking. After the patient has been placed in steep Trendelenburg position and the table is locked, the robot is docked from the patient’s left side at a 45° angle to the table. Side-docking permits easy access to the vagina to 1) evaluate graft tension and 2) complete cystoscopy to ensure ureteral and bladder integrity.
TIP Take care to ensure that the spine of the robot is positioned right next to the bed at the level of the patient’s hip; driving it up too high in relation to the abdomen can compromise the mobility of the fourth arm. In addition, if the robot is not close enough to the bed, the reach of the first (right) arm may be limited.
Next, introduce monopolar scissors through the right arm; a bipolar PK Dissector (Intuitive Surgical) through the left arm; and an atraumatic bowel grasper, such as Cadiere Forceps (Intuitive Surgical), through the fourth arm.

FIGURE 1 Placement of 5 ports for robotic sacrocolpopexy
Key: C, camera; A, accessory port; 1, right arm (monopolar shears); 2, left arm (PK Dissector); 3, fourth arm (Cadiere Forceps).
3. Dissect the sacral promontory and create a retroperitoneal tunnel
With the use of a 0° scope or 30° down-scope, retract the sigmoid colon laterally using Cadiere forceps and identify the right ureter.
TIP When you attempt robotic sacrocolpopexy for the first time, it may help to identify the sacral promontory, using a standard laparoscopic instrument with haptic feedback, before you dock the robot.
Elevate the peritoneum overlying the sacral promontory and open it using monopolar cautery. Expose the fat pad that overlays the anterior longitudinal ligament and gently dissect it away (FIGURE 2; VIDEO 1). Often, the middle sacral artery is visualized; it can be coagulated using the PK Dissector if necessary.
TIP In a case in which the promontory is difficult to find, dissecting the retrorectal space is a simple way to mobilize the bowel away from the sacrum, thus exposing the promontory.
TRICK Instead of opening the peritoneum from the sacrum to the cul-de-sac, I create a retroperitoneal tunnel along that right paracolic gutter, from the promontory to just medial to the right uterosacral ligament (VIDEO 1). Doing so has three benefits:
- It is quicker and less bloody
- It allows the mesh to lay flat in the hollow of the sacrum when you bring the sacral arm up to the promontory
- There is much less peritoneum to close over the mesh at the end of the procedure.

FIGURE 2 Entering the peritoneum
Open the peritoneum at the sacral promontory and dissect the fat pad. This reveals the anterior longitudinal ligament.
4. Dissect the vesicovaginal and rectovaginal spaces
Effective vaginal and rectal manipulation is critical to complete this part of the procedure safely. To gain access to the rectovaginal space, the vaginal assistant needs to push the vagina all the way in and up toward the anterior abdominal wall (the handle of the EEA sizer will be pushing hard up against the perineum) while simultaneously pushing the rectal probe downward (effectively scissoring the two apart).
From the exit point of the retroperitoneal tunnel that was created at the beginning of the case, then extend the peritoneal incision transversely in the shape of a “T” to expose the posterior vaginal wall (FIGURE 3, VIDEO 2). If indicated, dissect the rectovaginal space all the way down to the perineal body.
Deviate the vagina posteriorly to facilitate dissection of the bladder from the anterior vaginal wall. Use sharp dissection with scissors and short bursts of energy with monopolar cautery.
TIP If you encounter significant scarring between the bladder and vagina, retrograde-fill the bladder with 300 mL of saline mixed with methylene blue dye to identify the surgical plane.
Expose approximately 4 to 6 cm of anterior vaginal wall, depending on the degree of anterior vaginal wall prolapse. Try to leave the peritoneum intact at the apex of the vagina to reduce the chance that mesh will erode.

FIGURE 3 The peritoneal incision
Extend the peritoneal incision along the cul-de-sac to the posterior vaginal wall in a T-shaped configuration to gain access the rectovaginal space. When perorming cervicosacropexy, it is easiest to develop this surgical plane before amputating the cervix.
5. Attach the y-mesh to the vagina
Several mesh options exist: IntePro (American Medical Systems), Alyte (Bard Medical), and Restorelle Y (Mpathy Medical) are preformed Type-1 polypropylene mesh products. A correctly sized mesh can easily be fashioned by suturing together two strips of Gynemesh (Ethicon) that are approximately 3 cm wide.
Because there can be significant variability in the relative dimensions of the anterior and posterior segments of mesh, I recommend fashioning the graft after dissection is complete: When posterior wall prolapse is extensive, for example, preformed y-mesh strips may not be long enough to reach all the way down to the perineal body. After having assessed the differences in graft placement and manipulation when the two arms are sutured together 1) before the grafts are placed intracorporeally and 2) after they are placed, I’ve concluded that the first method—suturing before placement—is far easier.
Introduce the mesh graft through the accessory port after exchanging the scissors and PK dissector for a suture cutter and a large needle driver. Retract the bladder using the fourth arm, and place the anterior mesh arm over the anterior vaginal wall; suture it in place using 2-0 Gore-Tex sutures on CT-2 needles that are each cut to 6 inches long.
For greatest efficiency, anchor the two distal corners first (FIGURE 4; VIDEO 3), then place a series of interrupted stitches towards the vaginal apex. Tie the knots using 2 surgeon’s knots, followed by 2 half-hitches. Attempt to achieve healthy bites through the vaginal muscularis without perforating the epithelium.

FIGURE 4 Suturing the mesh graft to the vaginal wall
Left and right: Suture the y-shaped polypropylene mesh graft to the anterior vaginal wall first, starting at the distal corners. The bladder is retracted cephalad using the fourth arm.After you’ve adequately secured the anterior mesh arm, deviate the vagina anteriorly and drape the posterior mesh arm over the posterior vaginal wall with the assistance of the fourth robotic arm that can hold upward traction on the sacral end of the mesh graft. Starting at the vaginal apex, place 6 to 8 interrupted sutures to secure the mesh to the posterior vaginal wall (FIGURE 5; VIDEO 4). If necessary, exchange the 0° scope for a 30° up-scope so that you can fully visualize the rectovaginal space.

FIGURE 5 Attachment of the posterior arm of the mesh
The fourth arm of the robot provides upward traction on the sacral portion of the mesh graft.
6. Attach the graft to the sacrum
Again retract the sigmoid laterally to expose the promontory dissection. Retrieve the sacral arm of the mesh through the retroperitoneal tunnel and pull it up toward the promontory. Deviate the vagina toward the sacrum and, ensuring that there is no excessive tension, suture the sacral portion of the mesh graft to the anterior longitudinal ligament at the promontory, using 2 or 3 interrupted sutures (FIGURE 6, VIDEO 5).
When placing the needle during this critical juncture, it is important to rotate through the ligament along the curvature of the needle—as opposed to driving the needle forward and risking exiting farther laterally than expected.
TIP Because of the slight traction that exists on the mesh, a slip-knot is preferred instead of a surgeon’s knot when suturing the sacral portion of the mesh graft to the anterior longitudinal ligament. Take care to visualize the middle sacral artery and either suture around it or cauterize it.

FIGURE 6 Suture the mesh directly to the anterior longitudinal ligament
Use two or three stitches, secured with slip-knots. Take care not to create excessive tension on the mesh graft.
7. Extraperitonealize the mesh
I no longer routinely close the peritoneum over the mesh at the vaginal apex because I have not had a single case of small-bowel obstruction since I began performing this procedure laparoscopically. You should close the peritoneal window at the promontory, however, if the mesh is tented up at all, because tenting creates the potential for bowel to get caught beneath the mesh. Perform this closure using 2-0 monofilament suture or 2-0 Vicryl suture (Ethicon) cut to 8 inches (FIGURE 7). It is always easiest to start distally and suture towards the camera and operative instruments.

FIGURE 7 Extraperitonealize the mesh
Close the peritoneum from the apex of the vagina with a purse-string—like stitch, continuing it to the promontory in running fashion.
8. Ensure that mesh is not in the bladder or rectum
Perform cystoscopy and a rectal examination at the end of each case to confirm that no sutures or mesh material are within either viscus. It is much easier to remove these before leaving the operating room.
Modifying the procedure for uterovaginal prolapse
If the patient has an intact uterus and benign cervical cytology, perform supracervical hysterectomy before proceeding with Steps 1–8 above.
TIP Leaving the cervix in situ may reduce the chance of mesh erosion and provides an excellent platform for mesh attachment.
TRICK I find it most helpful to fully dissect the anterior and posterior vaginal walls before cervical amputation because upward traction on the corpus improves visualization of the surgical planes.
Once the cervix is amputated, however, effective vaginal manipulation can present a surgical challenge. Some surgeons use a tenaculum attached to the fourth arm of the robot to apply traction on the cervix, but this eliminates this arm from performing other necessary tasks. Malleable or Breisky-Navratil retractors can be used to delineate the anterior and posterior vaginal fornices, but are not always satisfactory—especially if an assistant isn’t seated between the legs.
TIP A useful and inexpensive instrument is the Colpo-Probe vaginal fornix delineator (Cooper Surgical) (FIGURE 8), which not only assists in dissecting the vagina from the bladder and rectum but also provides a stable surface during attachment of mesh.

FIGURE 8 Colpo-Probe
This device delineates the anterior and posterior vaginal fornices. It provides a stable platform against which to suture the mesh graft.
Tips and tricks for managing hemorrhage during sacrocolpopexy
Four potential areas of bleeding danger exist:
- In trying to find the sacral promontory, you risk entering the right common iliac vein if dissection is too far cephalad and lateral.
TIP I strongly advise novice robotic surgeons to try to identify the site of the promontory with a standard laparoscopic instrument with haptic feedback before moving to the surgical console.
TRICK Another trick that can help with safe identification of the promontory is mobilization of the sigmoid colon away from the sacrum by developing the retrorectal space. - During dissection of the fat pad from the promontory, you can encounter the middle sacral artery.
TRICK Spreading carefully in a caudal–cephalad direction until the level of the ligament is reached, instead of spreading in a lateral dimension, can decrease the chances of lacerating of this vessel. - A dangerous plexus of veins traverses the hollow of the sacrum. If you are trying to affix mesh at the level of S2-3, therefore, you may encounter significant bleeding.
TIP Work above the level of S1 to avoid these veins completely. - In securing the mesh to the sacral promontory, you can puncture the left common iliac vein if you are not aware of the exit point of the needle and it traverses too far medially.
TIP If you encounter bleeding, introduce a RAY-TEC sponge (Johnson & Johnson) through the accessory port. Apply manual compression for at least 5 minutes. If bleeding persists, I recommend Floseal Hemostatic Matrix (Baxter) to control hemorrhage that arises from arterial and venous sources.
TRICK As last resort, perform emergency undocking in rapid fashion while the bedside assistant maintains pressure through the accessory port.
Conclusion
The da Vinci robotic surgical system facilitates completion of sacrocolpopexy and cervicosacropexy, in a manner identical to the open technique, by surgeons who may not possess advanced laparoscopic skills. Full knowledge of the relevant anatomy is critical; there is the potential for significant morbidity during the procedure if surgical planes are created incorrectly.
Robotic surgery in on the rise, but coding for robotic procedures is still limited to the basic, conventional procedure. Why? Because insurers reimburse the hospital for use of the equipment but still refuse to reimburse the surgeon any additional amount for incorporating the robot into the surgical technique.
Coding for your work when performing robotic sacrocolpopexy is straightforward: Report laparoscopic code 57425 (laparoscopy, surgical, colpopexy [suspension of vaginal apex]) for the procedure. Mesh that might be used as part of the repair is not reported separately.
Blue Cross/Blue Shield (BC/BS) added the Healthcare Common Procedure Coding System Level-II code S2900 (surgical techniques requiring use of robotic surgical system) to the national code set a few years ago. Although BC/BS and some other payers accept this code on the claim, there is no reimbursement attached: It was developed for informational purposes only.
Remember, however, that coding is complete only when you have an appropriate linking diagnosis to establish the medical necessity of laparoscopic sacrocolpopexy. Diagnostic coding options for this surgical procedure include documentation of uterovaginal prolapse (incomplete prolapse is 618.2; complete prolapse is 618.3); vaginal vault prolapse after hysterectomy (618.5); and uterine prolapse without vaginal wall prolapse (618.1).
—MELANIE WITT, RN, CPC, COBGC, MA
We want to hear from you! Tell us what you think.
- Promontory dissection and creation of the retroperitoneal tunnel
- Dissection of the rectovaginal and vesicovaginal spaces
- Attachment of y-mesh to vagina: Start anteriorly
- Posterior y-mesh attachment
- Attachment of mesh to sacral promontory
These videos were provided by Catherine A. Matthews, MD.
Recent years have seen growing recognition that adequate support of the vaginal apex is an essential component of durable surgical repair of pelvic organ prolapse.1,2 Sacrocolpopexy is now considered the gold standard for repair of Level-1 defects of pelvic support, providing excellent long-term results.3-5
A recent randomized, controlled trial demonstrated the superior efficacy of laparoscopic sacrocolpopexy to a total vaginal mesh procedure in women who have vaginal vault prolapse—further evidence that sacrocolpopexy is the procedure of choice for these patients.6
The advantages of sacrocolpopexy include:
- reduced risk of mesh exposure, compared to insertion of vaginal mesh
- preservation of vaginal length
- reduced risk of re-operation for symptomatic recurrent prolapse
- reduced risk of de novo dyspareunia secondary to contraction of mesh.
Obstacles. Although a small number of surgeons are able to accomplish sacrocolpopexy using standard laparoscopic techniques, most of these procedures are still performed by laparotomy because the extensive suturing and knot-tying present a surgical challenge. Open sacrocolpopexy has disadvantages, too, including more pain, longer recovery, and longer length of stay.7-9
With the introduction of the da Vinci robot (Intuitive Surgical), the feasibility of having more surgeons perform this operation using a reproducible, minimally-invasive technique is much greater. The steep learning curve associated with standard laparoscopy in regard to mastering intracorporeal knot-tying and suturing is greatly diminished by articulating instruments. This makes robotic sacrocolpopexy an accessible option for all gynecologic surgeons who treat women with pelvic organ prolapse.
In this article, I detail the steps—with tips and tricks from my experience—to completing an efficient robotic-assisted sacrocolpopexy—modeled exactly after the open technique—that utilizes a y-shaped polypropylene mesh graft. Included is capsule advice from OBG Management’s coding consultant on obtaining reimbursement for robotic procedures (see “ Coding tips for robotic sacrocolpopexy”).
- Two proficient tableside assistants are needed
- Use steep Trendelenburg to remove the bowel from the operative field
- A fan retractor is necessary in some cases to gain access to the promontory
- Correct identification of the sacral promontory is key
- In the absence of haptic feedback, novice surgeons must be aware of the potential danger in dissecting too far laterally and entering the common iliac vessels
- Y-shaped grafts should be fashioned individually
- Know the exit point of the needle at the promontory
- Adequate spacing between the robotic arms is essential to avoiding interference among instruments during the procedure.
Details of the procedure
1. Surgical preparation, set-up
The patient completes a bowel prep using two bottles of magnesium citrate and taking only clear liquids 1 day before surgery. Although mechanical bowel cleansing has not been shown to decrease operative morbidity, manipulation and retraction of the sigmoid colon may be easier with the bowel empty.
Perioperative antibiotics are administered 30 minutes prior to the procedure. Heparin, 5,000 U, is injected subcutaneously for thromboprophylaxis as the patient is en route to the operating suite.
The patient is placed in the dorsal lithotomy position, buttocks extending one inch over the end of the operating table. The table should be covered with egg-crate foam to avoid having her slip down while in steep Trendelenburg position.
After the patient is prepped and draped, a Foley catheter is placed into the bladder. EEA sizers (Covidien) are inserted into the vagina and rectum.
Two experienced surgical assistants are necessary:
- One on the patient’s right side to assist with tissue retraction and introduction of suture material
- Another seated between the patient’s legs to provide adequate vaginal and rectal manipulation during surgery.
2. Port placement, docking, and instrumentation
Pneumoperitoneum is obtained with a Veress needle. Five trocars are then placed (FIGURE 1).
Careful port placement is integral to the success of this procedure because:
- Inadequate distance between robotic arms and the camera results in arm collisions and interference
- Visualization and access to the sacral promontory may be compromised if the camera is inserted too low on the anterior abdominal wall
- Bowel retraction may be compromised if the fourth arm of the robot isn’t at least 3 cm above the anterior superior iliac crest.
My experience evaluating the abdomen before trocar insertion is that at least 15 cm is required between the pubic bone and the umbilicus to rely on this landmark for locating the 12-mm camera port.10 If this distance is shorter (as it is in many obese women), insertion above the umbilicus is necessary.
An accessory 12-mm port, used to introduce sutures and the mesh graft, is placed approximately 10 cm lateral and 4 cm cephalad to the camera in the right-upper quadrant.
An 8-mm robotic port is placed in the right lower quadrant, 10 cm lateral to the accessory port and approximately 3 cm above the anterior superior iliac crest.
The third and fourth robotic arms are placed 10 cm apart in the left lower quadrant, with the fourth arm typically as far lateral as possible.
Docking. After the patient has been placed in steep Trendelenburg position and the table is locked, the robot is docked from the patient’s left side at a 45° angle to the table. Side-docking permits easy access to the vagina to 1) evaluate graft tension and 2) complete cystoscopy to ensure ureteral and bladder integrity.
TIP Take care to ensure that the spine of the robot is positioned right next to the bed at the level of the patient’s hip; driving it up too high in relation to the abdomen can compromise the mobility of the fourth arm. In addition, if the robot is not close enough to the bed, the reach of the first (right) arm may be limited.
Next, introduce monopolar scissors through the right arm; a bipolar PK Dissector (Intuitive Surgical) through the left arm; and an atraumatic bowel grasper, such as Cadiere Forceps (Intuitive Surgical), through the fourth arm.

FIGURE 1 Placement of 5 ports for robotic sacrocolpopexy
Key: C, camera; A, accessory port; 1, right arm (monopolar shears); 2, left arm (PK Dissector); 3, fourth arm (Cadiere Forceps).
3. Dissect the sacral promontory and create a retroperitoneal tunnel
With the use of a 0° scope or 30° down-scope, retract the sigmoid colon laterally using Cadiere forceps and identify the right ureter.
TIP When you attempt robotic sacrocolpopexy for the first time, it may help to identify the sacral promontory, using a standard laparoscopic instrument with haptic feedback, before you dock the robot.
Elevate the peritoneum overlying the sacral promontory and open it using monopolar cautery. Expose the fat pad that overlays the anterior longitudinal ligament and gently dissect it away (FIGURE 2; VIDEO 1). Often, the middle sacral artery is visualized; it can be coagulated using the PK Dissector if necessary.
TIP In a case in which the promontory is difficult to find, dissecting the retrorectal space is a simple way to mobilize the bowel away from the sacrum, thus exposing the promontory.
TRICK Instead of opening the peritoneum from the sacrum to the cul-de-sac, I create a retroperitoneal tunnel along that right paracolic gutter, from the promontory to just medial to the right uterosacral ligament (VIDEO 1). Doing so has three benefits:
- It is quicker and less bloody
- It allows the mesh to lay flat in the hollow of the sacrum when you bring the sacral arm up to the promontory
- There is much less peritoneum to close over the mesh at the end of the procedure.

FIGURE 2 Entering the peritoneum
Open the peritoneum at the sacral promontory and dissect the fat pad. This reveals the anterior longitudinal ligament.
4. Dissect the vesicovaginal and rectovaginal spaces
Effective vaginal and rectal manipulation is critical to complete this part of the procedure safely. To gain access to the rectovaginal space, the vaginal assistant needs to push the vagina all the way in and up toward the anterior abdominal wall (the handle of the EEA sizer will be pushing hard up against the perineum) while simultaneously pushing the rectal probe downward (effectively scissoring the two apart).
From the exit point of the retroperitoneal tunnel that was created at the beginning of the case, then extend the peritoneal incision transversely in the shape of a “T” to expose the posterior vaginal wall (FIGURE 3, VIDEO 2). If indicated, dissect the rectovaginal space all the way down to the perineal body.
Deviate the vagina posteriorly to facilitate dissection of the bladder from the anterior vaginal wall. Use sharp dissection with scissors and short bursts of energy with monopolar cautery.
TIP If you encounter significant scarring between the bladder and vagina, retrograde-fill the bladder with 300 mL of saline mixed with methylene blue dye to identify the surgical plane.
Expose approximately 4 to 6 cm of anterior vaginal wall, depending on the degree of anterior vaginal wall prolapse. Try to leave the peritoneum intact at the apex of the vagina to reduce the chance that mesh will erode.

FIGURE 3 The peritoneal incision
Extend the peritoneal incision along the cul-de-sac to the posterior vaginal wall in a T-shaped configuration to gain access the rectovaginal space. When perorming cervicosacropexy, it is easiest to develop this surgical plane before amputating the cervix.
5. Attach the y-mesh to the vagina
Several mesh options exist: IntePro (American Medical Systems), Alyte (Bard Medical), and Restorelle Y (Mpathy Medical) are preformed Type-1 polypropylene mesh products. A correctly sized mesh can easily be fashioned by suturing together two strips of Gynemesh (Ethicon) that are approximately 3 cm wide.
Because there can be significant variability in the relative dimensions of the anterior and posterior segments of mesh, I recommend fashioning the graft after dissection is complete: When posterior wall prolapse is extensive, for example, preformed y-mesh strips may not be long enough to reach all the way down to the perineal body. After having assessed the differences in graft placement and manipulation when the two arms are sutured together 1) before the grafts are placed intracorporeally and 2) after they are placed, I’ve concluded that the first method—suturing before placement—is far easier.
Introduce the mesh graft through the accessory port after exchanging the scissors and PK dissector for a suture cutter and a large needle driver. Retract the bladder using the fourth arm, and place the anterior mesh arm over the anterior vaginal wall; suture it in place using 2-0 Gore-Tex sutures on CT-2 needles that are each cut to 6 inches long.
For greatest efficiency, anchor the two distal corners first (FIGURE 4; VIDEO 3), then place a series of interrupted stitches towards the vaginal apex. Tie the knots using 2 surgeon’s knots, followed by 2 half-hitches. Attempt to achieve healthy bites through the vaginal muscularis without perforating the epithelium.

FIGURE 4 Suturing the mesh graft to the vaginal wall
Left and right: Suture the y-shaped polypropylene mesh graft to the anterior vaginal wall first, starting at the distal corners. The bladder is retracted cephalad using the fourth arm.After you’ve adequately secured the anterior mesh arm, deviate the vagina anteriorly and drape the posterior mesh arm over the posterior vaginal wall with the assistance of the fourth robotic arm that can hold upward traction on the sacral end of the mesh graft. Starting at the vaginal apex, place 6 to 8 interrupted sutures to secure the mesh to the posterior vaginal wall (FIGURE 5; VIDEO 4). If necessary, exchange the 0° scope for a 30° up-scope so that you can fully visualize the rectovaginal space.

FIGURE 5 Attachment of the posterior arm of the mesh
The fourth arm of the robot provides upward traction on the sacral portion of the mesh graft.
6. Attach the graft to the sacrum
Again retract the sigmoid laterally to expose the promontory dissection. Retrieve the sacral arm of the mesh through the retroperitoneal tunnel and pull it up toward the promontory. Deviate the vagina toward the sacrum and, ensuring that there is no excessive tension, suture the sacral portion of the mesh graft to the anterior longitudinal ligament at the promontory, using 2 or 3 interrupted sutures (FIGURE 6, VIDEO 5).
When placing the needle during this critical juncture, it is important to rotate through the ligament along the curvature of the needle—as opposed to driving the needle forward and risking exiting farther laterally than expected.
TIP Because of the slight traction that exists on the mesh, a slip-knot is preferred instead of a surgeon’s knot when suturing the sacral portion of the mesh graft to the anterior longitudinal ligament. Take care to visualize the middle sacral artery and either suture around it or cauterize it.

FIGURE 6 Suture the mesh directly to the anterior longitudinal ligament
Use two or three stitches, secured with slip-knots. Take care not to create excessive tension on the mesh graft.
7. Extraperitonealize the mesh
I no longer routinely close the peritoneum over the mesh at the vaginal apex because I have not had a single case of small-bowel obstruction since I began performing this procedure laparoscopically. You should close the peritoneal window at the promontory, however, if the mesh is tented up at all, because tenting creates the potential for bowel to get caught beneath the mesh. Perform this closure using 2-0 monofilament suture or 2-0 Vicryl suture (Ethicon) cut to 8 inches (FIGURE 7). It is always easiest to start distally and suture towards the camera and operative instruments.

FIGURE 7 Extraperitonealize the mesh
Close the peritoneum from the apex of the vagina with a purse-string—like stitch, continuing it to the promontory in running fashion.
8. Ensure that mesh is not in the bladder or rectum
Perform cystoscopy and a rectal examination at the end of each case to confirm that no sutures or mesh material are within either viscus. It is much easier to remove these before leaving the operating room.
Modifying the procedure for uterovaginal prolapse
If the patient has an intact uterus and benign cervical cytology, perform supracervical hysterectomy before proceeding with Steps 1–8 above.
TIP Leaving the cervix in situ may reduce the chance of mesh erosion and provides an excellent platform for mesh attachment.
TRICK I find it most helpful to fully dissect the anterior and posterior vaginal walls before cervical amputation because upward traction on the corpus improves visualization of the surgical planes.
Once the cervix is amputated, however, effective vaginal manipulation can present a surgical challenge. Some surgeons use a tenaculum attached to the fourth arm of the robot to apply traction on the cervix, but this eliminates this arm from performing other necessary tasks. Malleable or Breisky-Navratil retractors can be used to delineate the anterior and posterior vaginal fornices, but are not always satisfactory—especially if an assistant isn’t seated between the legs.
TIP A useful and inexpensive instrument is the Colpo-Probe vaginal fornix delineator (Cooper Surgical) (FIGURE 8), which not only assists in dissecting the vagina from the bladder and rectum but also provides a stable surface during attachment of mesh.

FIGURE 8 Colpo-Probe
This device delineates the anterior and posterior vaginal fornices. It provides a stable platform against which to suture the mesh graft.
Tips and tricks for managing hemorrhage during sacrocolpopexy
Four potential areas of bleeding danger exist:
- In trying to find the sacral promontory, you risk entering the right common iliac vein if dissection is too far cephalad and lateral.
TIP I strongly advise novice robotic surgeons to try to identify the site of the promontory with a standard laparoscopic instrument with haptic feedback before moving to the surgical console.
TRICK Another trick that can help with safe identification of the promontory is mobilization of the sigmoid colon away from the sacrum by developing the retrorectal space. - During dissection of the fat pad from the promontory, you can encounter the middle sacral artery.
TRICK Spreading carefully in a caudal–cephalad direction until the level of the ligament is reached, instead of spreading in a lateral dimension, can decrease the chances of lacerating of this vessel. - A dangerous plexus of veins traverses the hollow of the sacrum. If you are trying to affix mesh at the level of S2-3, therefore, you may encounter significant bleeding.
TIP Work above the level of S1 to avoid these veins completely. - In securing the mesh to the sacral promontory, you can puncture the left common iliac vein if you are not aware of the exit point of the needle and it traverses too far medially.
TIP If you encounter bleeding, introduce a RAY-TEC sponge (Johnson & Johnson) through the accessory port. Apply manual compression for at least 5 minutes. If bleeding persists, I recommend Floseal Hemostatic Matrix (Baxter) to control hemorrhage that arises from arterial and venous sources.
TRICK As last resort, perform emergency undocking in rapid fashion while the bedside assistant maintains pressure through the accessory port.
Conclusion
The da Vinci robotic surgical system facilitates completion of sacrocolpopexy and cervicosacropexy, in a manner identical to the open technique, by surgeons who may not possess advanced laparoscopic skills. Full knowledge of the relevant anatomy is critical; there is the potential for significant morbidity during the procedure if surgical planes are created incorrectly.
Robotic surgery in on the rise, but coding for robotic procedures is still limited to the basic, conventional procedure. Why? Because insurers reimburse the hospital for use of the equipment but still refuse to reimburse the surgeon any additional amount for incorporating the robot into the surgical technique.
Coding for your work when performing robotic sacrocolpopexy is straightforward: Report laparoscopic code 57425 (laparoscopy, surgical, colpopexy [suspension of vaginal apex]) for the procedure. Mesh that might be used as part of the repair is not reported separately.
Blue Cross/Blue Shield (BC/BS) added the Healthcare Common Procedure Coding System Level-II code S2900 (surgical techniques requiring use of robotic surgical system) to the national code set a few years ago. Although BC/BS and some other payers accept this code on the claim, there is no reimbursement attached: It was developed for informational purposes only.
Remember, however, that coding is complete only when you have an appropriate linking diagnosis to establish the medical necessity of laparoscopic sacrocolpopexy. Diagnostic coding options for this surgical procedure include documentation of uterovaginal prolapse (incomplete prolapse is 618.2; complete prolapse is 618.3); vaginal vault prolapse after hysterectomy (618.5); and uterine prolapse without vaginal wall prolapse (618.1).
—MELANIE WITT, RN, CPC, COBGC, MA
We want to hear from you! Tell us what you think.
1. Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438-1443.
2. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;(3):CD004014.-
3. Sullivan ES, Longaker CJ, Lee PY. Total pelvic mesh repair: a ten-year experience. Dis Colon Rectum. 2001;44(6):857-863.
4. Culligan PJ, Murphy M, Blackwell L, Hammons G, Graham C, Heit MH. Long-term success of abdominal sacral colpopexy using synthetic mesh. Am J Obstet Gynecol. 2002;187(6):1473-1482.
5. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805-823.
6. Maher CF, Feiner B, DeCuyper EM, Nichlos CJ, Hickey KV, O’Rourke P. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol. 2011;204(4):360.e1-7.
7. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol. 1994;84(5):885-888.
8. Ross JW. Techniques of laparoscopic repair of total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4(2):173-183.
9. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy hysterectomy, and burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6(2):115-119.
10. Matthews CA, Schubert CM, Woodward AP, Gill EJ. Variance in abdominal wall anatomy and port placement in women undergoing robotic gynecologic surgery. J Minim Invasive Gynecol. 2010;17(5):583-586.
1. Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438-1443.
2. Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;(3):CD004014.-
3. Sullivan ES, Longaker CJ, Lee PY. Total pelvic mesh repair: a ten-year experience. Dis Colon Rectum. 2001;44(6):857-863.
4. Culligan PJ, Murphy M, Blackwell L, Hammons G, Graham C, Heit MH. Long-term success of abdominal sacral colpopexy using synthetic mesh. Am J Obstet Gynecol. 2002;187(6):1473-1482.
5. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805-823.
6. Maher CF, Feiner B, DeCuyper EM, Nichlos CJ, Hickey KV, O’Rourke P. Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol. 2011;204(4):360.e1-7.
7. Nezhat CH, Nezhat F, Nezhat C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol. 1994;84(5):885-888.
8. Ross JW. Techniques of laparoscopic repair of total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4(2):173-183.
9. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy hysterectomy, and burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6(2):115-119.
10. Matthews CA, Schubert CM, Woodward AP, Gill EJ. Variance in abdominal wall anatomy and port placement in women undergoing robotic gynecologic surgery. J Minim Invasive Gynecol. 2010;17(5):583-586.
"All in his head" Dx leaves boy limping for more than a year … When a migraine isn't a migraine ... more
“All in his head” Dx leaves boy limping for more than a year
A 9-YEAR-OLD BOY developed pain in his ankle and a resulting limp. Despite several visits to his pediatrician at a local clinic and consultations with specialists, the limp became worse. A work-up in the emergency department (ED) led to a diagnosis of dystonia and a follow-up visit with a specialist.
The specialist, whose area of expertise wasn’t dystonia, concluded that the symptoms were “in the boy’s head” and changed the diagnosis to conversion disorder without consulting the ED records or the physician who diagnosed dystonia. The boy was admitted to a rehabilitation hospital, where, according to his parents, he underwent a bizarre and punitive behavior regimen. The physician in charge at the hospital ordered removal of the crutches the patient needed to walk and directed that the boy do sit-ups and push-ups whenever he fell or lost his balance.
When the boy hadn’t improved after 30 days in the rehabilitation hospital, the treatment team ordered that he return to school on the condition that the school be informed that the child had a psychiatric condition and could walk normally if he wanted to. The school staff was instructed to forbid the boy to use crutches and not to help him up if he fell.
The situation continued for a year despite repeated questions from the boy’s parents and visits to the clinic. The family was dissuaded from seeking additional testing on the grounds that it would further “medicalize” his condition. A blood test done more than a year after the limp started confirmed the original diagnosis of dystonia.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $890,000 Ohio verdict
COMMENT Although many unusual symptoms do have a psychiatric basis, in this case, poor communication and follow-up resulted in an almost $900,000 verdict.
When a migraine isn’t a migraine
WEAKNESS, LOSS OF BALANCE, AND HEARING LOSS prompted a 45-year-old woman to visit the emergency department (ED). An ED physician diagnosed a migraine headache and discharged her.
Five days later the woman returned to the ED with similar complaints, including imbalance, facial droop, dizziness, and weakness in the left arm. She was admitted to the hospital, where she had a stroke and died 5 days later.
PLAINTIFF’S CLAIM The ED doctor diagnosed a migraine headache and discharged the patient from the hospital when she really had a transient ischemic attack. The patient should have been referred for a neurologic evaluation, which would have revealed cardiomyopathy, which often shows no symptoms before precipitating a massive stroke.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Illinois settlement.
COMMENT Faced with the hectic pace of practice, we need to carefully evaluate even the most routine complaints such as headache and perform a careful general physical, which in this case might have disclosed a murmur and raised the index of suspicion.
Confusion over warfarin Rx ends badly
A 48-YEAR-OLD MAN who had suffered a patellar tendon rupture to the left knee underwent bilateral patellar tendon repair by an orthopedic surgeon; long leg cylinder casts were applied to both legs. The patient started taking 5 mg warfarin the following day.
Two days later he was transferred to a skilled nursing facility for physical therapy and warfarin adjustment and assigned a primary care physician. During his stay in the nursing facility, the patient’s blood tests never showed a therapeutic warfarin level. He saw the orthopedist, who prescribed 4 to 6 more weeks of warfarin therapy and scheduled a return appointment for 2 weeks later.
The day after the patient saw the orthopedist, his primary care physician increased the warfarin dose to 6 mg. When a blood test 3 days later showed a nontherapeutic level, she increased the dose to 7 mg.
Twelve days later, the leg casts were removed and knee immobilizers applied. The doctor who removed the casts recommended that the patient keep taking warfarin for at least 6 more weeks until removal of the knee immobilizers and the start of range of motion exercises. The patient was given a prescription to take to the skilled nursing facility to continue warfarin at the discretion of the primary care physician. That same day, the primary care doctor ordered by telephone that the patient continue to receive the same dose of warfarin.
The patient was discharged home 2 days later with orders for physical therapy and a blood draw for prothrombin time/international normalized ratio (INR). Physical therapy began 3 days before the blood draw was to be performed. The blood draw was actually done a day later than ordered and one day after the patient had taken his last dose of warfarin.
The home health nurse notified the orthopedist that the patient had taken his last dose of warfarin and faxed him the results of the blood test, showing an INR of 1.3. Six days later, the nurse contacted the orthopedist again about the exhausted warfarin supply. The orthopedist told the nurse to get in touch with the primary care physician who had followed the patient during his stay at the skilled nursing facility. The nurse left a voice-mail message on the phone of the primary care physician’s nurse. Twenty-five days later, the patient suffered an embolism in his main pulmonary artery and died.
PLAINTIFF’S CLAIM The home health agency and physicians were negligent in failing to properly monitor the patient’s warfarin therapy.
THE DEFENSE The home health nurse acted properly in contacting the doctor. The orthopedist claimed that he had no duty to monitor the patient’s warfarin therapy because that was the responsibility of an internist. The primary care physician claimed that she wasn’t responsible for monitoring the warfarin after the patient was discharged from the skilled nursing facility.
VERDICT $76,760.12 net California verdict against the primary care physician with confidential post-trial settlement. The orthopedist received a defense verdict.
COMMENT Another example of lack of coordination of care, noncompliance, and inadequate follow-up. Although we can only partially improve adherence, we should shoulder responsibility for coordinated care!
“All in his head” Dx leaves boy limping for more than a year
A 9-YEAR-OLD BOY developed pain in his ankle and a resulting limp. Despite several visits to his pediatrician at a local clinic and consultations with specialists, the limp became worse. A work-up in the emergency department (ED) led to a diagnosis of dystonia and a follow-up visit with a specialist.
The specialist, whose area of expertise wasn’t dystonia, concluded that the symptoms were “in the boy’s head” and changed the diagnosis to conversion disorder without consulting the ED records or the physician who diagnosed dystonia. The boy was admitted to a rehabilitation hospital, where, according to his parents, he underwent a bizarre and punitive behavior regimen. The physician in charge at the hospital ordered removal of the crutches the patient needed to walk and directed that the boy do sit-ups and push-ups whenever he fell or lost his balance.
When the boy hadn’t improved after 30 days in the rehabilitation hospital, the treatment team ordered that he return to school on the condition that the school be informed that the child had a psychiatric condition and could walk normally if he wanted to. The school staff was instructed to forbid the boy to use crutches and not to help him up if he fell.
The situation continued for a year despite repeated questions from the boy’s parents and visits to the clinic. The family was dissuaded from seeking additional testing on the grounds that it would further “medicalize” his condition. A blood test done more than a year after the limp started confirmed the original diagnosis of dystonia.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $890,000 Ohio verdict
COMMENT Although many unusual symptoms do have a psychiatric basis, in this case, poor communication and follow-up resulted in an almost $900,000 verdict.
When a migraine isn’t a migraine
WEAKNESS, LOSS OF BALANCE, AND HEARING LOSS prompted a 45-year-old woman to visit the emergency department (ED). An ED physician diagnosed a migraine headache and discharged her.
Five days later the woman returned to the ED with similar complaints, including imbalance, facial droop, dizziness, and weakness in the left arm. She was admitted to the hospital, where she had a stroke and died 5 days later.
PLAINTIFF’S CLAIM The ED doctor diagnosed a migraine headache and discharged the patient from the hospital when she really had a transient ischemic attack. The patient should have been referred for a neurologic evaluation, which would have revealed cardiomyopathy, which often shows no symptoms before precipitating a massive stroke.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Illinois settlement.
COMMENT Faced with the hectic pace of practice, we need to carefully evaluate even the most routine complaints such as headache and perform a careful general physical, which in this case might have disclosed a murmur and raised the index of suspicion.
Confusion over warfarin Rx ends badly
A 48-YEAR-OLD MAN who had suffered a patellar tendon rupture to the left knee underwent bilateral patellar tendon repair by an orthopedic surgeon; long leg cylinder casts were applied to both legs. The patient started taking 5 mg warfarin the following day.
Two days later he was transferred to a skilled nursing facility for physical therapy and warfarin adjustment and assigned a primary care physician. During his stay in the nursing facility, the patient’s blood tests never showed a therapeutic warfarin level. He saw the orthopedist, who prescribed 4 to 6 more weeks of warfarin therapy and scheduled a return appointment for 2 weeks later.
The day after the patient saw the orthopedist, his primary care physician increased the warfarin dose to 6 mg. When a blood test 3 days later showed a nontherapeutic level, she increased the dose to 7 mg.
Twelve days later, the leg casts were removed and knee immobilizers applied. The doctor who removed the casts recommended that the patient keep taking warfarin for at least 6 more weeks until removal of the knee immobilizers and the start of range of motion exercises. The patient was given a prescription to take to the skilled nursing facility to continue warfarin at the discretion of the primary care physician. That same day, the primary care doctor ordered by telephone that the patient continue to receive the same dose of warfarin.
The patient was discharged home 2 days later with orders for physical therapy and a blood draw for prothrombin time/international normalized ratio (INR). Physical therapy began 3 days before the blood draw was to be performed. The blood draw was actually done a day later than ordered and one day after the patient had taken his last dose of warfarin.
The home health nurse notified the orthopedist that the patient had taken his last dose of warfarin and faxed him the results of the blood test, showing an INR of 1.3. Six days later, the nurse contacted the orthopedist again about the exhausted warfarin supply. The orthopedist told the nurse to get in touch with the primary care physician who had followed the patient during his stay at the skilled nursing facility. The nurse left a voice-mail message on the phone of the primary care physician’s nurse. Twenty-five days later, the patient suffered an embolism in his main pulmonary artery and died.
PLAINTIFF’S CLAIM The home health agency and physicians were negligent in failing to properly monitor the patient’s warfarin therapy.
THE DEFENSE The home health nurse acted properly in contacting the doctor. The orthopedist claimed that he had no duty to monitor the patient’s warfarin therapy because that was the responsibility of an internist. The primary care physician claimed that she wasn’t responsible for monitoring the warfarin after the patient was discharged from the skilled nursing facility.
VERDICT $76,760.12 net California verdict against the primary care physician with confidential post-trial settlement. The orthopedist received a defense verdict.
COMMENT Another example of lack of coordination of care, noncompliance, and inadequate follow-up. Although we can only partially improve adherence, we should shoulder responsibility for coordinated care!
“All in his head” Dx leaves boy limping for more than a year
A 9-YEAR-OLD BOY developed pain in his ankle and a resulting limp. Despite several visits to his pediatrician at a local clinic and consultations with specialists, the limp became worse. A work-up in the emergency department (ED) led to a diagnosis of dystonia and a follow-up visit with a specialist.
The specialist, whose area of expertise wasn’t dystonia, concluded that the symptoms were “in the boy’s head” and changed the diagnosis to conversion disorder without consulting the ED records or the physician who diagnosed dystonia. The boy was admitted to a rehabilitation hospital, where, according to his parents, he underwent a bizarre and punitive behavior regimen. The physician in charge at the hospital ordered removal of the crutches the patient needed to walk and directed that the boy do sit-ups and push-ups whenever he fell or lost his balance.
When the boy hadn’t improved after 30 days in the rehabilitation hospital, the treatment team ordered that he return to school on the condition that the school be informed that the child had a psychiatric condition and could walk normally if he wanted to. The school staff was instructed to forbid the boy to use crutches and not to help him up if he fell.
The situation continued for a year despite repeated questions from the boy’s parents and visits to the clinic. The family was dissuaded from seeking additional testing on the grounds that it would further “medicalize” his condition. A blood test done more than a year after the limp started confirmed the original diagnosis of dystonia.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $890,000 Ohio verdict
COMMENT Although many unusual symptoms do have a psychiatric basis, in this case, poor communication and follow-up resulted in an almost $900,000 verdict.
When a migraine isn’t a migraine
WEAKNESS, LOSS OF BALANCE, AND HEARING LOSS prompted a 45-year-old woman to visit the emergency department (ED). An ED physician diagnosed a migraine headache and discharged her.
Five days later the woman returned to the ED with similar complaints, including imbalance, facial droop, dizziness, and weakness in the left arm. She was admitted to the hospital, where she had a stroke and died 5 days later.
PLAINTIFF’S CLAIM The ED doctor diagnosed a migraine headache and discharged the patient from the hospital when she really had a transient ischemic attack. The patient should have been referred for a neurologic evaluation, which would have revealed cardiomyopathy, which often shows no symptoms before precipitating a massive stroke.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Illinois settlement.
COMMENT Faced with the hectic pace of practice, we need to carefully evaluate even the most routine complaints such as headache and perform a careful general physical, which in this case might have disclosed a murmur and raised the index of suspicion.
Confusion over warfarin Rx ends badly
A 48-YEAR-OLD MAN who had suffered a patellar tendon rupture to the left knee underwent bilateral patellar tendon repair by an orthopedic surgeon; long leg cylinder casts were applied to both legs. The patient started taking 5 mg warfarin the following day.
Two days later he was transferred to a skilled nursing facility for physical therapy and warfarin adjustment and assigned a primary care physician. During his stay in the nursing facility, the patient’s blood tests never showed a therapeutic warfarin level. He saw the orthopedist, who prescribed 4 to 6 more weeks of warfarin therapy and scheduled a return appointment for 2 weeks later.
The day after the patient saw the orthopedist, his primary care physician increased the warfarin dose to 6 mg. When a blood test 3 days later showed a nontherapeutic level, she increased the dose to 7 mg.
Twelve days later, the leg casts were removed and knee immobilizers applied. The doctor who removed the casts recommended that the patient keep taking warfarin for at least 6 more weeks until removal of the knee immobilizers and the start of range of motion exercises. The patient was given a prescription to take to the skilled nursing facility to continue warfarin at the discretion of the primary care physician. That same day, the primary care doctor ordered by telephone that the patient continue to receive the same dose of warfarin.
The patient was discharged home 2 days later with orders for physical therapy and a blood draw for prothrombin time/international normalized ratio (INR). Physical therapy began 3 days before the blood draw was to be performed. The blood draw was actually done a day later than ordered and one day after the patient had taken his last dose of warfarin.
The home health nurse notified the orthopedist that the patient had taken his last dose of warfarin and faxed him the results of the blood test, showing an INR of 1.3. Six days later, the nurse contacted the orthopedist again about the exhausted warfarin supply. The orthopedist told the nurse to get in touch with the primary care physician who had followed the patient during his stay at the skilled nursing facility. The nurse left a voice-mail message on the phone of the primary care physician’s nurse. Twenty-five days later, the patient suffered an embolism in his main pulmonary artery and died.
PLAINTIFF’S CLAIM The home health agency and physicians were negligent in failing to properly monitor the patient’s warfarin therapy.
THE DEFENSE The home health nurse acted properly in contacting the doctor. The orthopedist claimed that he had no duty to monitor the patient’s warfarin therapy because that was the responsibility of an internist. The primary care physician claimed that she wasn’t responsible for monitoring the warfarin after the patient was discharged from the skilled nursing facility.
VERDICT $76,760.12 net California verdict against the primary care physician with confidential post-trial settlement. The orthopedist received a defense verdict.
COMMENT Another example of lack of coordination of care, noncompliance, and inadequate follow-up. Although we can only partially improve adherence, we should shoulder responsibility for coordinated care!
Statins for patients with nonalcoholic fatty liver?
Treat patients with hyperlipidemia and presumed nonalcoholic fatty liver disease with atorvastatin to reduce the risk of cardiovascular events.1
STRENGTH OF RECOMMENDATION
B: Based on a single prospective randomized controlled trial (RCT).
Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post hoc analysis. Lancet. 2010; 376:1916-1922.
ILLUSTRATIVE CASE
An obese 58-year-old man with type 2 diabetes comes to your office for follow-up. His low-density lipoprotein cholesterol (LDL-C) is 130 mg/dL; triglycerides, 300 mg/dL; alanine transaminase (ALT), 110 units/L; and aspartate transaminase (AST), 120 units/L. The patient’s work-up for chronic hepatitis B and C, autoimmune hepatitis, hemochromatosis, and Wilson’s disease are negative, and you rule out alcohol misuse based on his medical history. An ultrasound of the patient’s liver reveals hepatic steatosis, and you diagnose nonalcoholic fatty liver disease (NAFLD). Should you start him on a statin?
Patients with central obesity, diabetes, hypertension, hyperlipidemia, and metabolic syndrome are at high risk of developing NAFLD. These conditions have increased in prevalence, and NAFLD is now the most common cause of liver disease in the United States.2 In Western industrialized countries, approximately 30% of the general population and 70% to 90% of patients with diabetes will develop NAFLD.3 Although most patients are asymptomatic, their liver enzymes are elevated. To diagnose NAFLD, it is necessary to rule out alcoholic hepatitis with a medical history, and viral hepatitis, hereditary hemochromatosis, Wilson’s disease, and autoimmune hepatitis with laboratory testing. Ultrasound reveals fat accumulation in the liver.
Treatment for NAFLD has little evidence of benefit
Patients with NAFLD have a much higher mortality rate than that of the general public, primarily because of cardiovascular disease.4-6 Increased physical activity and weight loss is the only therapy that has solid evidence of a benefit,7 although other treatments, such as insulin-sensitizing drugs (metformin or pioglitazone), may be beneficial.8 Surprisingly, atorvastatin has been found to reduce aminotransferase levels in patients with NAFLD,9,10 but clinicians are often concerned about prescribing a statin for patients with elevated liver enzymes. In one study, 50% of primary care physicians said they would not prescribe statins for patients whose liver enzymes are 1.5× the upper limit of normal (ULN).11
STUDY SUMMARY: Statins lower risk of cardiovascular morbidity and mortality
The Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study was a randomized, prospective open-label, intention-to-treat trial involving 1600 patients. All had established coronary heart disease (CHD), were younger than 75 years, and had triglycerides <400 mg/dL and LDL-C >100 mg/dL. The study reviewed here—evaluating the risk-to-benefit ratio of using a statin to treat hyperlipidemia in patients with NAFLD—was a post hoc analysis of the GREACE study.1
Participants were randomized to either usual care or structured care with atorvastatin, starting at 10 mg/d and adjusted to 80 mg/d to bring the LDL-C level below 100 mg/dL. In the usual care group, treatment included lifestyle changes plus necessary drug treatments (only 30% of those in the usual care group received hyperlipidemia drugs). Patients were followed after medication dose titration, then every 6 months for 3 years. Serum ALT and AST were measured at baseline, at 6 weeks, and every 6 months.
At baseline, mild-to-moderate increases (<3× ULN) in ALT/AST were noted in 437 of the 1600 patients. For these patients, alcoholic hepatitis, chronic hepatitis B and C, Wilson’s disease, and autoimmune hepatitis were excluded by history, laboratory tests, and ultrasound, and the elevated liver enzymes were attributed to NAFLD.
The primary endpoints were the first occurrence of any cardiovascular event, including nonfatal myocardial infarction, revascularization, unstable angina, heart failure, and stroke; all-cause mortality; and CHD mortality. The relative risk (RR) for such events was calculated for the 437 patients with elevated liver enzymes, compared with that of patients without abnormal liver tests. Elevated liver enzymes and liver-related adverse events were secondary endpoints.
A cardiovascular event occurred in 10% (22/227) of the patients with elevated liver enzymes who received a statin, and 30% (63/210) of patients who had elevated liver enzymes but did not receive a statin.
There were 3.2 events per 100 patient-years in the atorvastatin group, compared with 10 events per 100 patient-years in those not on atorvastatin, a 68% reduction in RR (P<.0001) and an NNT of 15 per year to prevent one cardiovascular event. The risk reduction in cardiovascular events was greater in patients with NAFLD (68%) than in patients with normal liver tests (39%).
An added benefit was the reduction in ALT/AST levels during treatment for patients with NAFLD who were taking a statin, an average decrease of 47% in AST levels and 35% in ALT levels. In addition, 89% of the patients in the statin group had normal ALT, AST, and gamma-glutamyl transferase levels by the end of the 3-year follow-up. Patients with NAFLD who did not receive statins had a 12% increase in AST and ALT by the end of the 3-year study.
Only 10 of 880 patients taking statins developed liver enzymes more than 3× ULN. In 3 of these patients, dose adjustments brought the liver enzymes back to normal. Only 7 (<1%) patients who received a statin had to discontinue therapy because of liver-related adverse effects.
WHAT’S NEW: Liver enzymes improve, with few adverse effects
Preliminary studies have shown an improvement in liver enzymes in patients with NAFLD treated with a statin.9,10 This is the first study to show survival benefits and significant reduction in major cardiovascular morbidity for such patients, as well.
This is also the first large-scale study that shows that treating NAFLD patients with a statin decreases liver enzyme levels, with minimal adverse effects.
CAVEATS: Differences in groups, few women could skew results
This study cannot be considered the final word on this topic. Patients in the “structured care” group were followed at a university clinic, while those in the “usual care” group were followed by either a family physician or a cardiologist outside the hospital, based on their choice. There may have been other differences in the care received by the 2 groups that could account for the difference in mortality and morbidity reduction.
In addition, study participants had coronary artery disease, and atorvastatin was not used for primary prevention. Moreover, nearly 80% of the study participants were male, which raises the question of generalizability. And this study was a post hoc analysis of the larger GREACE study, which also raises concerns about the validity of findings.
In the absence of a larger prospective RCT, however, this is the best available evidence to support the use of statins in this population, and suggests that treating patients with NAFLD with statins is safe and effective.
CHALLENGES TO IMPLEMENTATION: Extensive Dx tests are costly
Study participants were evaluated to rule out other causes of their abnormal liver tests, with extensive laboratory tests and an ultrasound evaluation of the liver. Such extensive testing may be cost prohibitive in some situations.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post hoc analysis. Lancet. 2010;376:1916-1922.
2. Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75:721-728.
3. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231.
4. Adams LA, LympJ F, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121.
5. Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602.
6. Targher G, Day CP, Bonora E. Risk of cardiovascular diseases in patients with nonalcoholic fatty liver. N Engl J Med. 2010;363:1341-1350.
7. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129.
8. Angelico F, Burattin M, Alessandri C, et al. Drugs improving insulin resistance for nonalcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;(1):CD005166.-
9. Hyogo H, Tazuma S, Arihiro K, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711-1718.
10. Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH). Are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis. 2007;16:39-46.
11. Rzouq FS, Volk ML, Hatoum HH, et al. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. Am J Med Sci. 2010;340:89-93.
Treat patients with hyperlipidemia and presumed nonalcoholic fatty liver disease with atorvastatin to reduce the risk of cardiovascular events.1
STRENGTH OF RECOMMENDATION
B: Based on a single prospective randomized controlled trial (RCT).
Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post hoc analysis. Lancet. 2010; 376:1916-1922.
ILLUSTRATIVE CASE
An obese 58-year-old man with type 2 diabetes comes to your office for follow-up. His low-density lipoprotein cholesterol (LDL-C) is 130 mg/dL; triglycerides, 300 mg/dL; alanine transaminase (ALT), 110 units/L; and aspartate transaminase (AST), 120 units/L. The patient’s work-up for chronic hepatitis B and C, autoimmune hepatitis, hemochromatosis, and Wilson’s disease are negative, and you rule out alcohol misuse based on his medical history. An ultrasound of the patient’s liver reveals hepatic steatosis, and you diagnose nonalcoholic fatty liver disease (NAFLD). Should you start him on a statin?
Patients with central obesity, diabetes, hypertension, hyperlipidemia, and metabolic syndrome are at high risk of developing NAFLD. These conditions have increased in prevalence, and NAFLD is now the most common cause of liver disease in the United States.2 In Western industrialized countries, approximately 30% of the general population and 70% to 90% of patients with diabetes will develop NAFLD.3 Although most patients are asymptomatic, their liver enzymes are elevated. To diagnose NAFLD, it is necessary to rule out alcoholic hepatitis with a medical history, and viral hepatitis, hereditary hemochromatosis, Wilson’s disease, and autoimmune hepatitis with laboratory testing. Ultrasound reveals fat accumulation in the liver.
Treatment for NAFLD has little evidence of benefit
Patients with NAFLD have a much higher mortality rate than that of the general public, primarily because of cardiovascular disease.4-6 Increased physical activity and weight loss is the only therapy that has solid evidence of a benefit,7 although other treatments, such as insulin-sensitizing drugs (metformin or pioglitazone), may be beneficial.8 Surprisingly, atorvastatin has been found to reduce aminotransferase levels in patients with NAFLD,9,10 but clinicians are often concerned about prescribing a statin for patients with elevated liver enzymes. In one study, 50% of primary care physicians said they would not prescribe statins for patients whose liver enzymes are 1.5× the upper limit of normal (ULN).11
STUDY SUMMARY: Statins lower risk of cardiovascular morbidity and mortality
The Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study was a randomized, prospective open-label, intention-to-treat trial involving 1600 patients. All had established coronary heart disease (CHD), were younger than 75 years, and had triglycerides <400 mg/dL and LDL-C >100 mg/dL. The study reviewed here—evaluating the risk-to-benefit ratio of using a statin to treat hyperlipidemia in patients with NAFLD—was a post hoc analysis of the GREACE study.1
Participants were randomized to either usual care or structured care with atorvastatin, starting at 10 mg/d and adjusted to 80 mg/d to bring the LDL-C level below 100 mg/dL. In the usual care group, treatment included lifestyle changes plus necessary drug treatments (only 30% of those in the usual care group received hyperlipidemia drugs). Patients were followed after medication dose titration, then every 6 months for 3 years. Serum ALT and AST were measured at baseline, at 6 weeks, and every 6 months.
At baseline, mild-to-moderate increases (<3× ULN) in ALT/AST were noted in 437 of the 1600 patients. For these patients, alcoholic hepatitis, chronic hepatitis B and C, Wilson’s disease, and autoimmune hepatitis were excluded by history, laboratory tests, and ultrasound, and the elevated liver enzymes were attributed to NAFLD.
The primary endpoints were the first occurrence of any cardiovascular event, including nonfatal myocardial infarction, revascularization, unstable angina, heart failure, and stroke; all-cause mortality; and CHD mortality. The relative risk (RR) for such events was calculated for the 437 patients with elevated liver enzymes, compared with that of patients without abnormal liver tests. Elevated liver enzymes and liver-related adverse events were secondary endpoints.
A cardiovascular event occurred in 10% (22/227) of the patients with elevated liver enzymes who received a statin, and 30% (63/210) of patients who had elevated liver enzymes but did not receive a statin.
There were 3.2 events per 100 patient-years in the atorvastatin group, compared with 10 events per 100 patient-years in those not on atorvastatin, a 68% reduction in RR (P<.0001) and an NNT of 15 per year to prevent one cardiovascular event. The risk reduction in cardiovascular events was greater in patients with NAFLD (68%) than in patients with normal liver tests (39%).
An added benefit was the reduction in ALT/AST levels during treatment for patients with NAFLD who were taking a statin, an average decrease of 47% in AST levels and 35% in ALT levels. In addition, 89% of the patients in the statin group had normal ALT, AST, and gamma-glutamyl transferase levels by the end of the 3-year follow-up. Patients with NAFLD who did not receive statins had a 12% increase in AST and ALT by the end of the 3-year study.
Only 10 of 880 patients taking statins developed liver enzymes more than 3× ULN. In 3 of these patients, dose adjustments brought the liver enzymes back to normal. Only 7 (<1%) patients who received a statin had to discontinue therapy because of liver-related adverse effects.
WHAT’S NEW: Liver enzymes improve, with few adverse effects
Preliminary studies have shown an improvement in liver enzymes in patients with NAFLD treated with a statin.9,10 This is the first study to show survival benefits and significant reduction in major cardiovascular morbidity for such patients, as well.
This is also the first large-scale study that shows that treating NAFLD patients with a statin decreases liver enzyme levels, with minimal adverse effects.
CAVEATS: Differences in groups, few women could skew results
This study cannot be considered the final word on this topic. Patients in the “structured care” group were followed at a university clinic, while those in the “usual care” group were followed by either a family physician or a cardiologist outside the hospital, based on their choice. There may have been other differences in the care received by the 2 groups that could account for the difference in mortality and morbidity reduction.
In addition, study participants had coronary artery disease, and atorvastatin was not used for primary prevention. Moreover, nearly 80% of the study participants were male, which raises the question of generalizability. And this study was a post hoc analysis of the larger GREACE study, which also raises concerns about the validity of findings.
In the absence of a larger prospective RCT, however, this is the best available evidence to support the use of statins in this population, and suggests that treating patients with NAFLD with statins is safe and effective.
CHALLENGES TO IMPLEMENTATION: Extensive Dx tests are costly
Study participants were evaluated to rule out other causes of their abnormal liver tests, with extensive laboratory tests and an ultrasound evaluation of the liver. Such extensive testing may be cost prohibitive in some situations.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
Treat patients with hyperlipidemia and presumed nonalcoholic fatty liver disease with atorvastatin to reduce the risk of cardiovascular events.1
STRENGTH OF RECOMMENDATION
B: Based on a single prospective randomized controlled trial (RCT).
Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post hoc analysis. Lancet. 2010; 376:1916-1922.
ILLUSTRATIVE CASE
An obese 58-year-old man with type 2 diabetes comes to your office for follow-up. His low-density lipoprotein cholesterol (LDL-C) is 130 mg/dL; triglycerides, 300 mg/dL; alanine transaminase (ALT), 110 units/L; and aspartate transaminase (AST), 120 units/L. The patient’s work-up for chronic hepatitis B and C, autoimmune hepatitis, hemochromatosis, and Wilson’s disease are negative, and you rule out alcohol misuse based on his medical history. An ultrasound of the patient’s liver reveals hepatic steatosis, and you diagnose nonalcoholic fatty liver disease (NAFLD). Should you start him on a statin?
Patients with central obesity, diabetes, hypertension, hyperlipidemia, and metabolic syndrome are at high risk of developing NAFLD. These conditions have increased in prevalence, and NAFLD is now the most common cause of liver disease in the United States.2 In Western industrialized countries, approximately 30% of the general population and 70% to 90% of patients with diabetes will develop NAFLD.3 Although most patients are asymptomatic, their liver enzymes are elevated. To diagnose NAFLD, it is necessary to rule out alcoholic hepatitis with a medical history, and viral hepatitis, hereditary hemochromatosis, Wilson’s disease, and autoimmune hepatitis with laboratory testing. Ultrasound reveals fat accumulation in the liver.
Treatment for NAFLD has little evidence of benefit
Patients with NAFLD have a much higher mortality rate than that of the general public, primarily because of cardiovascular disease.4-6 Increased physical activity and weight loss is the only therapy that has solid evidence of a benefit,7 although other treatments, such as insulin-sensitizing drugs (metformin or pioglitazone), may be beneficial.8 Surprisingly, atorvastatin has been found to reduce aminotransferase levels in patients with NAFLD,9,10 but clinicians are often concerned about prescribing a statin for patients with elevated liver enzymes. In one study, 50% of primary care physicians said they would not prescribe statins for patients whose liver enzymes are 1.5× the upper limit of normal (ULN).11
STUDY SUMMARY: Statins lower risk of cardiovascular morbidity and mortality
The Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study was a randomized, prospective open-label, intention-to-treat trial involving 1600 patients. All had established coronary heart disease (CHD), were younger than 75 years, and had triglycerides <400 mg/dL and LDL-C >100 mg/dL. The study reviewed here—evaluating the risk-to-benefit ratio of using a statin to treat hyperlipidemia in patients with NAFLD—was a post hoc analysis of the GREACE study.1
Participants were randomized to either usual care or structured care with atorvastatin, starting at 10 mg/d and adjusted to 80 mg/d to bring the LDL-C level below 100 mg/dL. In the usual care group, treatment included lifestyle changes plus necessary drug treatments (only 30% of those in the usual care group received hyperlipidemia drugs). Patients were followed after medication dose titration, then every 6 months for 3 years. Serum ALT and AST were measured at baseline, at 6 weeks, and every 6 months.
At baseline, mild-to-moderate increases (<3× ULN) in ALT/AST were noted in 437 of the 1600 patients. For these patients, alcoholic hepatitis, chronic hepatitis B and C, Wilson’s disease, and autoimmune hepatitis were excluded by history, laboratory tests, and ultrasound, and the elevated liver enzymes were attributed to NAFLD.
The primary endpoints were the first occurrence of any cardiovascular event, including nonfatal myocardial infarction, revascularization, unstable angina, heart failure, and stroke; all-cause mortality; and CHD mortality. The relative risk (RR) for such events was calculated for the 437 patients with elevated liver enzymes, compared with that of patients without abnormal liver tests. Elevated liver enzymes and liver-related adverse events were secondary endpoints.
A cardiovascular event occurred in 10% (22/227) of the patients with elevated liver enzymes who received a statin, and 30% (63/210) of patients who had elevated liver enzymes but did not receive a statin.
There were 3.2 events per 100 patient-years in the atorvastatin group, compared with 10 events per 100 patient-years in those not on atorvastatin, a 68% reduction in RR (P<.0001) and an NNT of 15 per year to prevent one cardiovascular event. The risk reduction in cardiovascular events was greater in patients with NAFLD (68%) than in patients with normal liver tests (39%).
An added benefit was the reduction in ALT/AST levels during treatment for patients with NAFLD who were taking a statin, an average decrease of 47% in AST levels and 35% in ALT levels. In addition, 89% of the patients in the statin group had normal ALT, AST, and gamma-glutamyl transferase levels by the end of the 3-year follow-up. Patients with NAFLD who did not receive statins had a 12% increase in AST and ALT by the end of the 3-year study.
Only 10 of 880 patients taking statins developed liver enzymes more than 3× ULN. In 3 of these patients, dose adjustments brought the liver enzymes back to normal. Only 7 (<1%) patients who received a statin had to discontinue therapy because of liver-related adverse effects.
WHAT’S NEW: Liver enzymes improve, with few adverse effects
Preliminary studies have shown an improvement in liver enzymes in patients with NAFLD treated with a statin.9,10 This is the first study to show survival benefits and significant reduction in major cardiovascular morbidity for such patients, as well.
This is also the first large-scale study that shows that treating NAFLD patients with a statin decreases liver enzyme levels, with minimal adverse effects.
CAVEATS: Differences in groups, few women could skew results
This study cannot be considered the final word on this topic. Patients in the “structured care” group were followed at a university clinic, while those in the “usual care” group were followed by either a family physician or a cardiologist outside the hospital, based on their choice. There may have been other differences in the care received by the 2 groups that could account for the difference in mortality and morbidity reduction.
In addition, study participants had coronary artery disease, and atorvastatin was not used for primary prevention. Moreover, nearly 80% of the study participants were male, which raises the question of generalizability. And this study was a post hoc analysis of the larger GREACE study, which also raises concerns about the validity of findings.
In the absence of a larger prospective RCT, however, this is the best available evidence to support the use of statins in this population, and suggests that treating patients with NAFLD with statins is safe and effective.
CHALLENGES TO IMPLEMENTATION: Extensive Dx tests are costly
Study participants were evaluated to rule out other causes of their abnormal liver tests, with extensive laboratory tests and an ultrasound evaluation of the liver. Such extensive testing may be cost prohibitive in some situations.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post hoc analysis. Lancet. 2010;376:1916-1922.
2. Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75:721-728.
3. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231.
4. Adams LA, LympJ F, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121.
5. Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602.
6. Targher G, Day CP, Bonora E. Risk of cardiovascular diseases in patients with nonalcoholic fatty liver. N Engl J Med. 2010;363:1341-1350.
7. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129.
8. Angelico F, Burattin M, Alessandri C, et al. Drugs improving insulin resistance for nonalcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;(1):CD005166.-
9. Hyogo H, Tazuma S, Arihiro K, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711-1718.
10. Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH). Are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis. 2007;16:39-46.
11. Rzouq FS, Volk ML, Hatoum HH, et al. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. Am J Med Sci. 2010;340:89-93.
1. Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post hoc analysis. Lancet. 2010;376:1916-1922.
2. Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75:721-728.
3. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231.
4. Adams LA, LympJ F, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121.
5. Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602.
6. Targher G, Day CP, Bonora E. Risk of cardiovascular diseases in patients with nonalcoholic fatty liver. N Engl J Med. 2010;363:1341-1350.
7. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129.
8. Angelico F, Burattin M, Alessandri C, et al. Drugs improving insulin resistance for nonalcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;(1):CD005166.-
9. Hyogo H, Tazuma S, Arihiro K, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711-1718.
10. Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH). Are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis. 2007;16:39-46.
11. Rzouq FS, Volk ML, Hatoum HH, et al. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. Am J Med Sci. 2010;340:89-93.
Copyright © 2011 The Family Physicians Inquiries Network.
All rights reserved.
A stroke—or something else?
A 54-year-old white woman with a history of a cerebrovascular accident (CVA) a year earlier sought care at the local emergency department for numbness and weakness in her right foot. She reported no other neurologic symptoms. She had mild weakness in her right leg and a mildly unsteady gait. Her neurologic examination was otherwise normal.
Initial testing included a complete blood count (CBC), renal profile, and thyroid-stimulating hormone measurement. All results were normal. A noncontrast computed tomography (CT) scan of the head was normal. We admitted her for further evaluation of probable acute ischemic stroke.
By the following day, the patient’s leg weakness and unsteadiness had worsened. A magnetic resonance imaging (MRI) scan of her head showed a prior left pontine infarct, but no new findings. She developed right arm weakness, and an MRI scan of her spine (FIGURE 1) showed multiple intradural lesions. A lumbar puncture showed elevated protein and oligoclonal bands. CT scans of the chest, abdomen, and pelvis were unremarkable. Two lumbar punctures for cytology and culture evaluations yielded negative results. A full-body positron-emission tomography (PET) scan showed diffuse small inguinal adenopathy bilaterally, suggestive of metastatic disease or lymphoma.
FIGURE 1
MRI of the spine
This MRI scan with contrast of the patient’s spine shows diffuse thoracic extramedullary, intradural lesions.
What is your presumptive diagnosis?
Diagnosis: Sarcoidosis
Findings from the full-body PET scan (FIGURE 2) prompted a biopsy of a right inguinal node, which showed a noncaseating granuloma—a hallmark finding of sarcoidosis.
Sarcoidosis is a multisystem disease of unknown cause. The exact prevalence in the general population is estimated at 10 to 20 cases per 100,000.1 A higher incidence occurs in blacks in the United States, with a 2.4% lifetime risk compared with 0.85% of whites.2 Sarcoidosis usually appears in patients ages 20 to 40 years, and although this systemic disease usually affects the lungs, 5% to 10% of patients will have nervous system involvement.3,4
FIGURE 2
Full-body PET scan
This PET scan shows diffuse hypermetabolic adenopathy with bilateral iliac adenopathy, small hypermetabolic bilateral cervical lymph nodes, a hypermetabolic left axillary node, and a large hypermetabolic portacaval node.
What you’ll see
The most common presenting symptoms of systemic sarcoidosis are chronic cough, shortness of breath, and chest pain. Fatigue, weight loss, and myalgias are also frequently part of the initial presentation.
Patients with sarcoidosis can present with neurologic symptoms suggestive of many diseases (TABLE 1), and in the absence of systemic symptoms the diagnosis of neurosarcoidosis is easily confused with CVA. Most patients with neurosarcoidosis have cranial nerve involvement (50%-75%).1 Other common presentations include seizures, meningitis, psychiatric symptoms, mass lesions, or endocrine abnormalities.
TABLE 1
Differential diagnosis of an acute neurologic event
| Infectious Encephalitis Helminthic infection HIV Lyme disease Meningitis Progressive multifocal leukoencephalopathy Syphilis Tuberculosis |
| Neoplastic CNS lymphoma Meningioma/glioma Metastatic disease |
| Neurologic CNS vasculitis Cranial nerve palsy Ischemic or hemorrhagic stroke Meningitis/encephalitis Multiple sclerosis Neurosarcoidosis Peripheral neuropathy Seizure |
| Psychiatric Depression Malingering Pseudoseizures Somatoform disorder |
| Rheumatologic Lupus erythematosus |
| CNS, central nervous system; HIV, human immunodeficiency virus |
Useful studies in the clinical evaluation
Consider a diagnosis of sarcoidosis involving the nervous system when an initial work-up for CVA is negative. In addition to asking about systemic symptoms, perform a complete neurologic exam and skin exam, search for lymphadenopathy, and conduct an ophthalmologic evaluation. After the initial evaluation, a neurology consult will likely be needed to guide further testing.
Choice of serum studies will vary depending on presenting symptoms, but they usually include tests for infection (CBC, cultures, Lyme titers, rapid plasma reagin, tuberculin skin test), rheumatologic disorders (antinuclear antibodies, erythrocyte sedimentation rate, C-reactive protein), and neoplastic diseases (lactate dehydrogenase, peripheral smear).5 Serum angiotensin-converting enzyme (ACE) may be useful in the diagnosis of systemic sarcoidosis, with positive results seen in approximately 75% of cases.3
Examination of cerebrospinal fluid often reveals an elevated total protein with oligoclonal bands, normal to low glucose, and possibly mild pleocytosis of monocytic or lymphocytic predominance.3 Spinal fluid ACE is neither sensitive nor specific for neurosarcoidosis, as it may be elevated in infectious or malignant processes.3
Imaging studies should include contrast-enhanced brain MRI, which may reveal multiple white matter lesions.6 Although the specificity of PET for neurosarcoidosis is poor—with positive results being seen also in infectious and neoplastic processes—the scan may help in identifying extraneural sites for biopsy. Histology will generally show the classic noncaseating granuloma with surrounding lymphocytes, plasma cells, and mast cells.
Treat with high-dose steroids
The mainstay of treatment, based largely on expert opinion, is high-dose steroids that are gradually tapered over weeks (TABLE 2). Other agents may be added if the condition is poorly controlled with steroids alone, or may be given if symptoms recur while tapering the steroid dose. Recurrence of sarcoidosis is common after doses of <10 to 20 mg/d. Prophylactic measures to counteract the adverse effects of long-term steroid use include weight-bearing exercise programs; administration of calcium, vitamin D, and bisphosphonates; and resorting to a stress-dose steroid regimen in times of illness.
The prognosis with sarcoidosis can vary widely. Case studies show that two-thirds of patients may have a nonrecurring illness. Among the remaining one-third, the disease course may be relapsing-remitting or progressive. When confronted with an acute neurologic event, consider recurrent sarcoidosis and coordinate care between specialists. Also, take steps to prevent complications related to prolonged steroid use.
TABLE 2
Treatment of neurosarcoidosis3
| Medication* | Side effects | Comments |
|---|---|---|
| Methylprednisolone | Hyperglycemia | |
| Prednisone | Osteoporosis, hyperglycemia, hypertension, diabetes, glaucoma, cataracts, psychosis, Cushing’s syndrome | Taper as able. Concomitant use of cytotoxic agents may facilitate taper. Monitor glucose and give calcium/vitamin D prophylaxis |
| Methotrexate | Anemia, neutropenia, liver damage | Weekly dosing well tolerated. Give folic acid 1 mg/d. Monitor liver function tests periodically |
| Cyclosporine | Renal insufficiency, hypertension | |
| Azathioprine | Anemia, neutropenia, liver damage | |
| Cyclophosphamide | Cystitis, neutropenia | Monitor urine monthly for microscopic hematuria |
| Hydroxychloroquine | Retinopathy, hypoglycemia, ototoxicity, myopathy, cardiomyopathy, neuropathy | Refer for eye exams every 3-6 months. May be useful to counteract hyperglycemic effect of steroids |
| Infliximab | Fever, headache, dizziness, flushing, abdominal pain, dyspepsia, myalgia, arthralgia, polyneuropathy | Screen for tuberculosis before starting treatment. Contraindicated in patients with congestive heart failure |
| *For dosing details, consult a neurologist or rheumatologist | ||
Improvement for our patient
Based on cerebrospinal fluid study results, a positive peripheral lymph node biopsy, and the exclusion of other diagnoses, we regarded the diagnosis of sarcoidosis as highly probable and initiated high-dose intravenous corticosteroids. Over several weeks, our patient gradually improved with physical therapy and was walking unassisted at the time of discharge from a hospital-based rehabilitation unit. Repeat MRI scans showed a reduction in the size of her intradural lesions, and we slowly tapered her steroids.
CORRESPONDENCE
Hillary R. Mount, MD, 2123 Auburn Avenue,#340, Cincinnati, OH 45219; [email protected]
1. Joseph FG, Scolding NJ. Sarcoidosis of the nervous system. Pract Neurol. 2007;7:234-244.
2. Burns TM. Neurosarcoidosis. Arch Neurol. 2003;60:1166-1168.
3. Hoitsma E, Drent M, Sharma OP. A pragmatic approach to diagnosing and treating neurosarcoidosis in the 21st century. Curr Opin Pulm Med. 2010;16:472-479.
4. Habersberger J, Manins V, Taylor AJ. Cardiac sarcoidosis. Intern Med J. 2008;38:270-277.
5. Vargas DL, Stern BJ. Neurosarcoidosis: diagnosis and management. Semin Respir Crit Care Med. 2010;31:419-427.
6. Cavazza A, Harari S, Caminati A, et al. The histology of pulmonary sarcoidosis: a review with particular emphasis on unusual and underrecognized features. Int J Surg Pathol. 2009;17:219-230.
A 54-year-old white woman with a history of a cerebrovascular accident (CVA) a year earlier sought care at the local emergency department for numbness and weakness in her right foot. She reported no other neurologic symptoms. She had mild weakness in her right leg and a mildly unsteady gait. Her neurologic examination was otherwise normal.
Initial testing included a complete blood count (CBC), renal profile, and thyroid-stimulating hormone measurement. All results were normal. A noncontrast computed tomography (CT) scan of the head was normal. We admitted her for further evaluation of probable acute ischemic stroke.
By the following day, the patient’s leg weakness and unsteadiness had worsened. A magnetic resonance imaging (MRI) scan of her head showed a prior left pontine infarct, but no new findings. She developed right arm weakness, and an MRI scan of her spine (FIGURE 1) showed multiple intradural lesions. A lumbar puncture showed elevated protein and oligoclonal bands. CT scans of the chest, abdomen, and pelvis were unremarkable. Two lumbar punctures for cytology and culture evaluations yielded negative results. A full-body positron-emission tomography (PET) scan showed diffuse small inguinal adenopathy bilaterally, suggestive of metastatic disease or lymphoma.
FIGURE 1
MRI of the spine
This MRI scan with contrast of the patient’s spine shows diffuse thoracic extramedullary, intradural lesions.
What is your presumptive diagnosis?
Diagnosis: Sarcoidosis
Findings from the full-body PET scan (FIGURE 2) prompted a biopsy of a right inguinal node, which showed a noncaseating granuloma—a hallmark finding of sarcoidosis.
Sarcoidosis is a multisystem disease of unknown cause. The exact prevalence in the general population is estimated at 10 to 20 cases per 100,000.1 A higher incidence occurs in blacks in the United States, with a 2.4% lifetime risk compared with 0.85% of whites.2 Sarcoidosis usually appears in patients ages 20 to 40 years, and although this systemic disease usually affects the lungs, 5% to 10% of patients will have nervous system involvement.3,4
FIGURE 2
Full-body PET scan
This PET scan shows diffuse hypermetabolic adenopathy with bilateral iliac adenopathy, small hypermetabolic bilateral cervical lymph nodes, a hypermetabolic left axillary node, and a large hypermetabolic portacaval node.
What you’ll see
The most common presenting symptoms of systemic sarcoidosis are chronic cough, shortness of breath, and chest pain. Fatigue, weight loss, and myalgias are also frequently part of the initial presentation.
Patients with sarcoidosis can present with neurologic symptoms suggestive of many diseases (TABLE 1), and in the absence of systemic symptoms the diagnosis of neurosarcoidosis is easily confused with CVA. Most patients with neurosarcoidosis have cranial nerve involvement (50%-75%).1 Other common presentations include seizures, meningitis, psychiatric symptoms, mass lesions, or endocrine abnormalities.
TABLE 1
Differential diagnosis of an acute neurologic event
| Infectious Encephalitis Helminthic infection HIV Lyme disease Meningitis Progressive multifocal leukoencephalopathy Syphilis Tuberculosis |
| Neoplastic CNS lymphoma Meningioma/glioma Metastatic disease |
| Neurologic CNS vasculitis Cranial nerve palsy Ischemic or hemorrhagic stroke Meningitis/encephalitis Multiple sclerosis Neurosarcoidosis Peripheral neuropathy Seizure |
| Psychiatric Depression Malingering Pseudoseizures Somatoform disorder |
| Rheumatologic Lupus erythematosus |
| CNS, central nervous system; HIV, human immunodeficiency virus |
Useful studies in the clinical evaluation
Consider a diagnosis of sarcoidosis involving the nervous system when an initial work-up for CVA is negative. In addition to asking about systemic symptoms, perform a complete neurologic exam and skin exam, search for lymphadenopathy, and conduct an ophthalmologic evaluation. After the initial evaluation, a neurology consult will likely be needed to guide further testing.
Choice of serum studies will vary depending on presenting symptoms, but they usually include tests for infection (CBC, cultures, Lyme titers, rapid plasma reagin, tuberculin skin test), rheumatologic disorders (antinuclear antibodies, erythrocyte sedimentation rate, C-reactive protein), and neoplastic diseases (lactate dehydrogenase, peripheral smear).5 Serum angiotensin-converting enzyme (ACE) may be useful in the diagnosis of systemic sarcoidosis, with positive results seen in approximately 75% of cases.3
Examination of cerebrospinal fluid often reveals an elevated total protein with oligoclonal bands, normal to low glucose, and possibly mild pleocytosis of monocytic or lymphocytic predominance.3 Spinal fluid ACE is neither sensitive nor specific for neurosarcoidosis, as it may be elevated in infectious or malignant processes.3
Imaging studies should include contrast-enhanced brain MRI, which may reveal multiple white matter lesions.6 Although the specificity of PET for neurosarcoidosis is poor—with positive results being seen also in infectious and neoplastic processes—the scan may help in identifying extraneural sites for biopsy. Histology will generally show the classic noncaseating granuloma with surrounding lymphocytes, plasma cells, and mast cells.
Treat with high-dose steroids
The mainstay of treatment, based largely on expert opinion, is high-dose steroids that are gradually tapered over weeks (TABLE 2). Other agents may be added if the condition is poorly controlled with steroids alone, or may be given if symptoms recur while tapering the steroid dose. Recurrence of sarcoidosis is common after doses of <10 to 20 mg/d. Prophylactic measures to counteract the adverse effects of long-term steroid use include weight-bearing exercise programs; administration of calcium, vitamin D, and bisphosphonates; and resorting to a stress-dose steroid regimen in times of illness.
The prognosis with sarcoidosis can vary widely. Case studies show that two-thirds of patients may have a nonrecurring illness. Among the remaining one-third, the disease course may be relapsing-remitting or progressive. When confronted with an acute neurologic event, consider recurrent sarcoidosis and coordinate care between specialists. Also, take steps to prevent complications related to prolonged steroid use.
TABLE 2
Treatment of neurosarcoidosis3
| Medication* | Side effects | Comments |
|---|---|---|
| Methylprednisolone | Hyperglycemia | |
| Prednisone | Osteoporosis, hyperglycemia, hypertension, diabetes, glaucoma, cataracts, psychosis, Cushing’s syndrome | Taper as able. Concomitant use of cytotoxic agents may facilitate taper. Monitor glucose and give calcium/vitamin D prophylaxis |
| Methotrexate | Anemia, neutropenia, liver damage | Weekly dosing well tolerated. Give folic acid 1 mg/d. Monitor liver function tests periodically |
| Cyclosporine | Renal insufficiency, hypertension | |
| Azathioprine | Anemia, neutropenia, liver damage | |
| Cyclophosphamide | Cystitis, neutropenia | Monitor urine monthly for microscopic hematuria |
| Hydroxychloroquine | Retinopathy, hypoglycemia, ototoxicity, myopathy, cardiomyopathy, neuropathy | Refer for eye exams every 3-6 months. May be useful to counteract hyperglycemic effect of steroids |
| Infliximab | Fever, headache, dizziness, flushing, abdominal pain, dyspepsia, myalgia, arthralgia, polyneuropathy | Screen for tuberculosis before starting treatment. Contraindicated in patients with congestive heart failure |
| *For dosing details, consult a neurologist or rheumatologist | ||
Improvement for our patient
Based on cerebrospinal fluid study results, a positive peripheral lymph node biopsy, and the exclusion of other diagnoses, we regarded the diagnosis of sarcoidosis as highly probable and initiated high-dose intravenous corticosteroids. Over several weeks, our patient gradually improved with physical therapy and was walking unassisted at the time of discharge from a hospital-based rehabilitation unit. Repeat MRI scans showed a reduction in the size of her intradural lesions, and we slowly tapered her steroids.
CORRESPONDENCE
Hillary R. Mount, MD, 2123 Auburn Avenue,#340, Cincinnati, OH 45219; [email protected]
A 54-year-old white woman with a history of a cerebrovascular accident (CVA) a year earlier sought care at the local emergency department for numbness and weakness in her right foot. She reported no other neurologic symptoms. She had mild weakness in her right leg and a mildly unsteady gait. Her neurologic examination was otherwise normal.
Initial testing included a complete blood count (CBC), renal profile, and thyroid-stimulating hormone measurement. All results were normal. A noncontrast computed tomography (CT) scan of the head was normal. We admitted her for further evaluation of probable acute ischemic stroke.
By the following day, the patient’s leg weakness and unsteadiness had worsened. A magnetic resonance imaging (MRI) scan of her head showed a prior left pontine infarct, but no new findings. She developed right arm weakness, and an MRI scan of her spine (FIGURE 1) showed multiple intradural lesions. A lumbar puncture showed elevated protein and oligoclonal bands. CT scans of the chest, abdomen, and pelvis were unremarkable. Two lumbar punctures for cytology and culture evaluations yielded negative results. A full-body positron-emission tomography (PET) scan showed diffuse small inguinal adenopathy bilaterally, suggestive of metastatic disease or lymphoma.
FIGURE 1
MRI of the spine
This MRI scan with contrast of the patient’s spine shows diffuse thoracic extramedullary, intradural lesions.
What is your presumptive diagnosis?
Diagnosis: Sarcoidosis
Findings from the full-body PET scan (FIGURE 2) prompted a biopsy of a right inguinal node, which showed a noncaseating granuloma—a hallmark finding of sarcoidosis.
Sarcoidosis is a multisystem disease of unknown cause. The exact prevalence in the general population is estimated at 10 to 20 cases per 100,000.1 A higher incidence occurs in blacks in the United States, with a 2.4% lifetime risk compared with 0.85% of whites.2 Sarcoidosis usually appears in patients ages 20 to 40 years, and although this systemic disease usually affects the lungs, 5% to 10% of patients will have nervous system involvement.3,4
FIGURE 2
Full-body PET scan
This PET scan shows diffuse hypermetabolic adenopathy with bilateral iliac adenopathy, small hypermetabolic bilateral cervical lymph nodes, a hypermetabolic left axillary node, and a large hypermetabolic portacaval node.
What you’ll see
The most common presenting symptoms of systemic sarcoidosis are chronic cough, shortness of breath, and chest pain. Fatigue, weight loss, and myalgias are also frequently part of the initial presentation.
Patients with sarcoidosis can present with neurologic symptoms suggestive of many diseases (TABLE 1), and in the absence of systemic symptoms the diagnosis of neurosarcoidosis is easily confused with CVA. Most patients with neurosarcoidosis have cranial nerve involvement (50%-75%).1 Other common presentations include seizures, meningitis, psychiatric symptoms, mass lesions, or endocrine abnormalities.
TABLE 1
Differential diagnosis of an acute neurologic event
| Infectious Encephalitis Helminthic infection HIV Lyme disease Meningitis Progressive multifocal leukoencephalopathy Syphilis Tuberculosis |
| Neoplastic CNS lymphoma Meningioma/glioma Metastatic disease |
| Neurologic CNS vasculitis Cranial nerve palsy Ischemic or hemorrhagic stroke Meningitis/encephalitis Multiple sclerosis Neurosarcoidosis Peripheral neuropathy Seizure |
| Psychiatric Depression Malingering Pseudoseizures Somatoform disorder |
| Rheumatologic Lupus erythematosus |
| CNS, central nervous system; HIV, human immunodeficiency virus |
Useful studies in the clinical evaluation
Consider a diagnosis of sarcoidosis involving the nervous system when an initial work-up for CVA is negative. In addition to asking about systemic symptoms, perform a complete neurologic exam and skin exam, search for lymphadenopathy, and conduct an ophthalmologic evaluation. After the initial evaluation, a neurology consult will likely be needed to guide further testing.
Choice of serum studies will vary depending on presenting symptoms, but they usually include tests for infection (CBC, cultures, Lyme titers, rapid plasma reagin, tuberculin skin test), rheumatologic disorders (antinuclear antibodies, erythrocyte sedimentation rate, C-reactive protein), and neoplastic diseases (lactate dehydrogenase, peripheral smear).5 Serum angiotensin-converting enzyme (ACE) may be useful in the diagnosis of systemic sarcoidosis, with positive results seen in approximately 75% of cases.3
Examination of cerebrospinal fluid often reveals an elevated total protein with oligoclonal bands, normal to low glucose, and possibly mild pleocytosis of monocytic or lymphocytic predominance.3 Spinal fluid ACE is neither sensitive nor specific for neurosarcoidosis, as it may be elevated in infectious or malignant processes.3
Imaging studies should include contrast-enhanced brain MRI, which may reveal multiple white matter lesions.6 Although the specificity of PET for neurosarcoidosis is poor—with positive results being seen also in infectious and neoplastic processes—the scan may help in identifying extraneural sites for biopsy. Histology will generally show the classic noncaseating granuloma with surrounding lymphocytes, plasma cells, and mast cells.
Treat with high-dose steroids
The mainstay of treatment, based largely on expert opinion, is high-dose steroids that are gradually tapered over weeks (TABLE 2). Other agents may be added if the condition is poorly controlled with steroids alone, or may be given if symptoms recur while tapering the steroid dose. Recurrence of sarcoidosis is common after doses of <10 to 20 mg/d. Prophylactic measures to counteract the adverse effects of long-term steroid use include weight-bearing exercise programs; administration of calcium, vitamin D, and bisphosphonates; and resorting to a stress-dose steroid regimen in times of illness.
The prognosis with sarcoidosis can vary widely. Case studies show that two-thirds of patients may have a nonrecurring illness. Among the remaining one-third, the disease course may be relapsing-remitting or progressive. When confronted with an acute neurologic event, consider recurrent sarcoidosis and coordinate care between specialists. Also, take steps to prevent complications related to prolonged steroid use.
TABLE 2
Treatment of neurosarcoidosis3
| Medication* | Side effects | Comments |
|---|---|---|
| Methylprednisolone | Hyperglycemia | |
| Prednisone | Osteoporosis, hyperglycemia, hypertension, diabetes, glaucoma, cataracts, psychosis, Cushing’s syndrome | Taper as able. Concomitant use of cytotoxic agents may facilitate taper. Monitor glucose and give calcium/vitamin D prophylaxis |
| Methotrexate | Anemia, neutropenia, liver damage | Weekly dosing well tolerated. Give folic acid 1 mg/d. Monitor liver function tests periodically |
| Cyclosporine | Renal insufficiency, hypertension | |
| Azathioprine | Anemia, neutropenia, liver damage | |
| Cyclophosphamide | Cystitis, neutropenia | Monitor urine monthly for microscopic hematuria |
| Hydroxychloroquine | Retinopathy, hypoglycemia, ototoxicity, myopathy, cardiomyopathy, neuropathy | Refer for eye exams every 3-6 months. May be useful to counteract hyperglycemic effect of steroids |
| Infliximab | Fever, headache, dizziness, flushing, abdominal pain, dyspepsia, myalgia, arthralgia, polyneuropathy | Screen for tuberculosis before starting treatment. Contraindicated in patients with congestive heart failure |
| *For dosing details, consult a neurologist or rheumatologist | ||
Improvement for our patient
Based on cerebrospinal fluid study results, a positive peripheral lymph node biopsy, and the exclusion of other diagnoses, we regarded the diagnosis of sarcoidosis as highly probable and initiated high-dose intravenous corticosteroids. Over several weeks, our patient gradually improved with physical therapy and was walking unassisted at the time of discharge from a hospital-based rehabilitation unit. Repeat MRI scans showed a reduction in the size of her intradural lesions, and we slowly tapered her steroids.
CORRESPONDENCE
Hillary R. Mount, MD, 2123 Auburn Avenue,#340, Cincinnati, OH 45219; [email protected]
1. Joseph FG, Scolding NJ. Sarcoidosis of the nervous system. Pract Neurol. 2007;7:234-244.
2. Burns TM. Neurosarcoidosis. Arch Neurol. 2003;60:1166-1168.
3. Hoitsma E, Drent M, Sharma OP. A pragmatic approach to diagnosing and treating neurosarcoidosis in the 21st century. Curr Opin Pulm Med. 2010;16:472-479.
4. Habersberger J, Manins V, Taylor AJ. Cardiac sarcoidosis. Intern Med J. 2008;38:270-277.
5. Vargas DL, Stern BJ. Neurosarcoidosis: diagnosis and management. Semin Respir Crit Care Med. 2010;31:419-427.
6. Cavazza A, Harari S, Caminati A, et al. The histology of pulmonary sarcoidosis: a review with particular emphasis on unusual and underrecognized features. Int J Surg Pathol. 2009;17:219-230.
1. Joseph FG, Scolding NJ. Sarcoidosis of the nervous system. Pract Neurol. 2007;7:234-244.
2. Burns TM. Neurosarcoidosis. Arch Neurol. 2003;60:1166-1168.
3. Hoitsma E, Drent M, Sharma OP. A pragmatic approach to diagnosing and treating neurosarcoidosis in the 21st century. Curr Opin Pulm Med. 2010;16:472-479.
4. Habersberger J, Manins V, Taylor AJ. Cardiac sarcoidosis. Intern Med J. 2008;38:270-277.
5. Vargas DL, Stern BJ. Neurosarcoidosis: diagnosis and management. Semin Respir Crit Care Med. 2010;31:419-427.
6. Cavazza A, Harari S, Caminati A, et al. The histology of pulmonary sarcoidosis: a review with particular emphasis on unusual and underrecognized features. Int J Surg Pathol. 2009;17:219-230.