User login
Should You Report a Substance-Abusing Colleague to the State Licensing Board?
PRO
Hospitalists’ moral obligation is to protect the patient
In this era of historic budget deficits, wars, and political strife surrounding healthcare reform, one might ask if we can afford to spend valuable time and energy on the issue of reporting physicians who abuse substances.
At first glance, I certainly had skepticism about the subject, but then I dug deeper. To my surprise (and likely yours), studies indicate that physicians develop substance-abuse problems as often or more than the general population does.1 Recent reports detail horrific patient outcomes at the hands of health providers whose actions are compromised by drug use. With data showing the prevalence of substance abuse among physicians hovering around 10% to 12%, we must accept the reality that hospitalists are not exempt.2,3,4,5
As medical doctors, our promise to our patients is to provide care in an ethical manner. Even if we try to live in denial, most of us would agree that with great blessing (or power) comes great responsibility. So when the question of reporting a fellow hospitalist who is abusing substances was asked, my response was unequivocally yes.
In my opinion, this discussion can be limited to two overarching principles: First, we are compelled to put our patients first. As hospitalists, we are blessed to be caring for some of the most frail and vulnerable in our society. Fortunately, an overwhelming number of us do so with pride, skill, and integrity.
The task of providing high-quality care to an empowered patient population is difficult enough with us being physically, emotionally, and mentally exhausted. But to add substance abuse to this is just a complete and utter violation of our patients’ trust. We must agree that putting our patients’ well-being beyond reproach requires us to report any colleague who is compromised.
Second, delayed help for a colleague in trouble with substance-abuse issues could be fatal—and for more than just that single colleague. At some point, we are compelled to do more than just raise an eyebrow and shake our head. Usually at the time of discovery, months if not years of substance abuse already have gone by undetected. Deferring to the next person is just not an option. There is too much at stake. It is our moral duty to help our colleagues who are unable to realize the danger they are posing to themselves, the team, and, most importantly, the patients.
Certainly, physicians do not need another lecture about the perils of substance abuse. Whether discussing prescription drugs, alcohol, marijuana, cocaine, or the like, we all have witnessed the devastating effects of abuse. The fact is, any substance that alters our ability to perform our trusted duty must be avoided.
Colleagues, the algorithm is simple: Be vigilant, observe, confirm, and report. It is our moral and ethical imperative.
Dr. Pyke is chief medical officer of Medicus Consulting, LLC.
CON
Responsible, helpful action doesn’t always mean official involvement
Recognizing impairment in our colleagues is both difficult and ethically challenging. Despite national trends, medicine remains a largely self-regulated profession, and we have an ethical obligation to report impaired, incompetent, or unethical colleagues. Rarely are the indications for reporting or identifying a colleague clear.
As trained clinicians, we know the signs of substance abuse:6
- Frequent tardiness and absences;
- Unexplained disappearances during working hours;
- Inappropriate behavior;
- Affective lability or irritability;
- Interpersonal conflict;
- Avoidance of peers or supervisors;
- Keeping odd hours;
- Disorganized and forgetful;
- Incomplete charts and work performance;
- Heavy drinking at social functions;
- Unexplained changes in weight or energy level;
- Diminished personal hygiene;
- Slurred or rapid speech;
- Frequently dilated pupils or red, watery eyes and a runny nose;
- Defensiveness, anxiety, apathy, and manipulative behaviors; and
- Withdrawal from long-standing relationships.
Yet when it is a colleague, we are often in denial about their substance abuse. Certainly, simple seasonal allergies and allergy medications can cause a number of the above symptoms. We also are aware of and fear the potential impact of licensing board notification on a physician’s career. In fact, in a national survey of physicians, 45% of respondents who had encountered impaired or incompetent physicians had not reported them, even though 96% of those surveyed agreed that physicians should report impaired or incompetent colleagues.7
Similar to reporting child or elder abuse, you don’t want to be wrong.
At the same time, impaired physicians are disruptive. They negatively impact the lives of their patients, colleagues, and hospital staff.
It is possible to do both the responsible thing and not go directly to the licensing board. You are not responsible for diagnosing your colleagues, but rather recognizing possible impairment.
Check out the Federation of State Physician Health Programs’ website (www.fsphp.org) to identify a local physician health program. Call them and place a report of concern identifying your impaired colleague. While it’s possibly new to you, they have years of experience working with this situation. Trust these organizations, many of which are independent from licensing, to intervene responsibly and confidentially. They can evaluate your colleague and provide a treatment plan and monitoring, as needed. Their approach is rehabilitative rather than punitive, and they resist reporting to the medical board unless the physician-patient is noncompliant.
Physicians have better outcomes than the general population, with reported abstinence rates of 70% to 90% for those who complete treatment.8,9 Between 75% and 85% of physicians who complete rehabilitation and comply with close monitoring and follow-up care are able to return to work.9,10
There is hope for your impaired colleague. Contact your local physician health program.
Dr. Guerrasio is a hospitalist and director of resident and medical student remediation at the University of Colorado Denver.
References
- Hughes PH, Brandenburg N, Baldwin DC Jr., et al. Prevalence of substance use among US physicians. JAMA. 1992;267:2333-2339.
- Gold KB, Teitelbaum SA. Physicians impaired by substance abuse disorders. The Journal of Global Drug Policy and Practice website. Available at: http://www.globaldrugpolicy.org/2/2/3.php. Accessed June 27, 2011.
- Wolfgang AP. Substance abuse potential and job stress: a study of pharmacists, physicians, and nurses. J Pharm Mark Manage. 1989;3(4):97-110.
- Cicala RS. Substance abuse among physicians: What you need to know. Hosp Phys. 2003:39-46.
- Berge KH, Seppala MD, Schipper AM. Chemical dependency and the physician. Mayo Clin Proc. 2009;84(7):625-631.
- Bright RP, Krahn L. Impaired physicians: How to recognize, when to report, and where to refer. Curr Psy. 2010;9(6):11-20.
- Campbell EG, Regan S, Gruen RL, et al. Professionalism in medicine: results of a national survey of physicians. Ann Intern Med. 2007;147:795-802.
- Femino J, Nirenberg TD. Treatment outcome studies on physician impairment: a review of the literature. R I Med. 1994;77:345-350.
- Alpern F, Correnti CE, Dolan TE, Llufrio MC, Sill A. A survey of recovering Maryland physicians. Md Med J. 1992;41:301-303.
- Gallegos KV, Lubin BH, Bowers C, Blevins JW, Talbott GD, Wilson PO. Relapse and recovery: five to ten year follow-up study of chemically dependent physicians—the Georgia experience. Md Med J. 1992;41:315-319.
PRO
Hospitalists’ moral obligation is to protect the patient
In this era of historic budget deficits, wars, and political strife surrounding healthcare reform, one might ask if we can afford to spend valuable time and energy on the issue of reporting physicians who abuse substances.
At first glance, I certainly had skepticism about the subject, but then I dug deeper. To my surprise (and likely yours), studies indicate that physicians develop substance-abuse problems as often or more than the general population does.1 Recent reports detail horrific patient outcomes at the hands of health providers whose actions are compromised by drug use. With data showing the prevalence of substance abuse among physicians hovering around 10% to 12%, we must accept the reality that hospitalists are not exempt.2,3,4,5
As medical doctors, our promise to our patients is to provide care in an ethical manner. Even if we try to live in denial, most of us would agree that with great blessing (or power) comes great responsibility. So when the question of reporting a fellow hospitalist who is abusing substances was asked, my response was unequivocally yes.
In my opinion, this discussion can be limited to two overarching principles: First, we are compelled to put our patients first. As hospitalists, we are blessed to be caring for some of the most frail and vulnerable in our society. Fortunately, an overwhelming number of us do so with pride, skill, and integrity.
The task of providing high-quality care to an empowered patient population is difficult enough with us being physically, emotionally, and mentally exhausted. But to add substance abuse to this is just a complete and utter violation of our patients’ trust. We must agree that putting our patients’ well-being beyond reproach requires us to report any colleague who is compromised.
Second, delayed help for a colleague in trouble with substance-abuse issues could be fatal—and for more than just that single colleague. At some point, we are compelled to do more than just raise an eyebrow and shake our head. Usually at the time of discovery, months if not years of substance abuse already have gone by undetected. Deferring to the next person is just not an option. There is too much at stake. It is our moral duty to help our colleagues who are unable to realize the danger they are posing to themselves, the team, and, most importantly, the patients.
Certainly, physicians do not need another lecture about the perils of substance abuse. Whether discussing prescription drugs, alcohol, marijuana, cocaine, or the like, we all have witnessed the devastating effects of abuse. The fact is, any substance that alters our ability to perform our trusted duty must be avoided.
Colleagues, the algorithm is simple: Be vigilant, observe, confirm, and report. It is our moral and ethical imperative.
Dr. Pyke is chief medical officer of Medicus Consulting, LLC.
CON
Responsible, helpful action doesn’t always mean official involvement
Recognizing impairment in our colleagues is both difficult and ethically challenging. Despite national trends, medicine remains a largely self-regulated profession, and we have an ethical obligation to report impaired, incompetent, or unethical colleagues. Rarely are the indications for reporting or identifying a colleague clear.
As trained clinicians, we know the signs of substance abuse:6
- Frequent tardiness and absences;
- Unexplained disappearances during working hours;
- Inappropriate behavior;
- Affective lability or irritability;
- Interpersonal conflict;
- Avoidance of peers or supervisors;
- Keeping odd hours;
- Disorganized and forgetful;
- Incomplete charts and work performance;
- Heavy drinking at social functions;
- Unexplained changes in weight or energy level;
- Diminished personal hygiene;
- Slurred or rapid speech;
- Frequently dilated pupils or red, watery eyes and a runny nose;
- Defensiveness, anxiety, apathy, and manipulative behaviors; and
- Withdrawal from long-standing relationships.
Yet when it is a colleague, we are often in denial about their substance abuse. Certainly, simple seasonal allergies and allergy medications can cause a number of the above symptoms. We also are aware of and fear the potential impact of licensing board notification on a physician’s career. In fact, in a national survey of physicians, 45% of respondents who had encountered impaired or incompetent physicians had not reported them, even though 96% of those surveyed agreed that physicians should report impaired or incompetent colleagues.7
Similar to reporting child or elder abuse, you don’t want to be wrong.
At the same time, impaired physicians are disruptive. They negatively impact the lives of their patients, colleagues, and hospital staff.
It is possible to do both the responsible thing and not go directly to the licensing board. You are not responsible for diagnosing your colleagues, but rather recognizing possible impairment.
Check out the Federation of State Physician Health Programs’ website (www.fsphp.org) to identify a local physician health program. Call them and place a report of concern identifying your impaired colleague. While it’s possibly new to you, they have years of experience working with this situation. Trust these organizations, many of which are independent from licensing, to intervene responsibly and confidentially. They can evaluate your colleague and provide a treatment plan and monitoring, as needed. Their approach is rehabilitative rather than punitive, and they resist reporting to the medical board unless the physician-patient is noncompliant.
Physicians have better outcomes than the general population, with reported abstinence rates of 70% to 90% for those who complete treatment.8,9 Between 75% and 85% of physicians who complete rehabilitation and comply with close monitoring and follow-up care are able to return to work.9,10
There is hope for your impaired colleague. Contact your local physician health program.
Dr. Guerrasio is a hospitalist and director of resident and medical student remediation at the University of Colorado Denver.
References
- Hughes PH, Brandenburg N, Baldwin DC Jr., et al. Prevalence of substance use among US physicians. JAMA. 1992;267:2333-2339.
- Gold KB, Teitelbaum SA. Physicians impaired by substance abuse disorders. The Journal of Global Drug Policy and Practice website. Available at: http://www.globaldrugpolicy.org/2/2/3.php. Accessed June 27, 2011.
- Wolfgang AP. Substance abuse potential and job stress: a study of pharmacists, physicians, and nurses. J Pharm Mark Manage. 1989;3(4):97-110.
- Cicala RS. Substance abuse among physicians: What you need to know. Hosp Phys. 2003:39-46.
- Berge KH, Seppala MD, Schipper AM. Chemical dependency and the physician. Mayo Clin Proc. 2009;84(7):625-631.
- Bright RP, Krahn L. Impaired physicians: How to recognize, when to report, and where to refer. Curr Psy. 2010;9(6):11-20.
- Campbell EG, Regan S, Gruen RL, et al. Professionalism in medicine: results of a national survey of physicians. Ann Intern Med. 2007;147:795-802.
- Femino J, Nirenberg TD. Treatment outcome studies on physician impairment: a review of the literature. R I Med. 1994;77:345-350.
- Alpern F, Correnti CE, Dolan TE, Llufrio MC, Sill A. A survey of recovering Maryland physicians. Md Med J. 1992;41:301-303.
- Gallegos KV, Lubin BH, Bowers C, Blevins JW, Talbott GD, Wilson PO. Relapse and recovery: five to ten year follow-up study of chemically dependent physicians—the Georgia experience. Md Med J. 1992;41:315-319.
PRO
Hospitalists’ moral obligation is to protect the patient
In this era of historic budget deficits, wars, and political strife surrounding healthcare reform, one might ask if we can afford to spend valuable time and energy on the issue of reporting physicians who abuse substances.
At first glance, I certainly had skepticism about the subject, but then I dug deeper. To my surprise (and likely yours), studies indicate that physicians develop substance-abuse problems as often or more than the general population does.1 Recent reports detail horrific patient outcomes at the hands of health providers whose actions are compromised by drug use. With data showing the prevalence of substance abuse among physicians hovering around 10% to 12%, we must accept the reality that hospitalists are not exempt.2,3,4,5
As medical doctors, our promise to our patients is to provide care in an ethical manner. Even if we try to live in denial, most of us would agree that with great blessing (or power) comes great responsibility. So when the question of reporting a fellow hospitalist who is abusing substances was asked, my response was unequivocally yes.
In my opinion, this discussion can be limited to two overarching principles: First, we are compelled to put our patients first. As hospitalists, we are blessed to be caring for some of the most frail and vulnerable in our society. Fortunately, an overwhelming number of us do so with pride, skill, and integrity.
The task of providing high-quality care to an empowered patient population is difficult enough with us being physically, emotionally, and mentally exhausted. But to add substance abuse to this is just a complete and utter violation of our patients’ trust. We must agree that putting our patients’ well-being beyond reproach requires us to report any colleague who is compromised.
Second, delayed help for a colleague in trouble with substance-abuse issues could be fatal—and for more than just that single colleague. At some point, we are compelled to do more than just raise an eyebrow and shake our head. Usually at the time of discovery, months if not years of substance abuse already have gone by undetected. Deferring to the next person is just not an option. There is too much at stake. It is our moral duty to help our colleagues who are unable to realize the danger they are posing to themselves, the team, and, most importantly, the patients.
Certainly, physicians do not need another lecture about the perils of substance abuse. Whether discussing prescription drugs, alcohol, marijuana, cocaine, or the like, we all have witnessed the devastating effects of abuse. The fact is, any substance that alters our ability to perform our trusted duty must be avoided.
Colleagues, the algorithm is simple: Be vigilant, observe, confirm, and report. It is our moral and ethical imperative.
Dr. Pyke is chief medical officer of Medicus Consulting, LLC.
CON
Responsible, helpful action doesn’t always mean official involvement
Recognizing impairment in our colleagues is both difficult and ethically challenging. Despite national trends, medicine remains a largely self-regulated profession, and we have an ethical obligation to report impaired, incompetent, or unethical colleagues. Rarely are the indications for reporting or identifying a colleague clear.
As trained clinicians, we know the signs of substance abuse:6
- Frequent tardiness and absences;
- Unexplained disappearances during working hours;
- Inappropriate behavior;
- Affective lability or irritability;
- Interpersonal conflict;
- Avoidance of peers or supervisors;
- Keeping odd hours;
- Disorganized and forgetful;
- Incomplete charts and work performance;
- Heavy drinking at social functions;
- Unexplained changes in weight or energy level;
- Diminished personal hygiene;
- Slurred or rapid speech;
- Frequently dilated pupils or red, watery eyes and a runny nose;
- Defensiveness, anxiety, apathy, and manipulative behaviors; and
- Withdrawal from long-standing relationships.
Yet when it is a colleague, we are often in denial about their substance abuse. Certainly, simple seasonal allergies and allergy medications can cause a number of the above symptoms. We also are aware of and fear the potential impact of licensing board notification on a physician’s career. In fact, in a national survey of physicians, 45% of respondents who had encountered impaired or incompetent physicians had not reported them, even though 96% of those surveyed agreed that physicians should report impaired or incompetent colleagues.7
Similar to reporting child or elder abuse, you don’t want to be wrong.
At the same time, impaired physicians are disruptive. They negatively impact the lives of their patients, colleagues, and hospital staff.
It is possible to do both the responsible thing and not go directly to the licensing board. You are not responsible for diagnosing your colleagues, but rather recognizing possible impairment.
Check out the Federation of State Physician Health Programs’ website (www.fsphp.org) to identify a local physician health program. Call them and place a report of concern identifying your impaired colleague. While it’s possibly new to you, they have years of experience working with this situation. Trust these organizations, many of which are independent from licensing, to intervene responsibly and confidentially. They can evaluate your colleague and provide a treatment plan and monitoring, as needed. Their approach is rehabilitative rather than punitive, and they resist reporting to the medical board unless the physician-patient is noncompliant.
Physicians have better outcomes than the general population, with reported abstinence rates of 70% to 90% for those who complete treatment.8,9 Between 75% and 85% of physicians who complete rehabilitation and comply with close monitoring and follow-up care are able to return to work.9,10
There is hope for your impaired colleague. Contact your local physician health program.
Dr. Guerrasio is a hospitalist and director of resident and medical student remediation at the University of Colorado Denver.
References
- Hughes PH, Brandenburg N, Baldwin DC Jr., et al. Prevalence of substance use among US physicians. JAMA. 1992;267:2333-2339.
- Gold KB, Teitelbaum SA. Physicians impaired by substance abuse disorders. The Journal of Global Drug Policy and Practice website. Available at: http://www.globaldrugpolicy.org/2/2/3.php. Accessed June 27, 2011.
- Wolfgang AP. Substance abuse potential and job stress: a study of pharmacists, physicians, and nurses. J Pharm Mark Manage. 1989;3(4):97-110.
- Cicala RS. Substance abuse among physicians: What you need to know. Hosp Phys. 2003:39-46.
- Berge KH, Seppala MD, Schipper AM. Chemical dependency and the physician. Mayo Clin Proc. 2009;84(7):625-631.
- Bright RP, Krahn L. Impaired physicians: How to recognize, when to report, and where to refer. Curr Psy. 2010;9(6):11-20.
- Campbell EG, Regan S, Gruen RL, et al. Professionalism in medicine: results of a national survey of physicians. Ann Intern Med. 2007;147:795-802.
- Femino J, Nirenberg TD. Treatment outcome studies on physician impairment: a review of the literature. R I Med. 1994;77:345-350.
- Alpern F, Correnti CE, Dolan TE, Llufrio MC, Sill A. A survey of recovering Maryland physicians. Md Med J. 1992;41:301-303.
- Gallegos KV, Lubin BH, Bowers C, Blevins JW, Talbott GD, Wilson PO. Relapse and recovery: five to ten year follow-up study of chemically dependent physicians—the Georgia experience. Md Med J. 1992;41:315-319.
The Burden of Burnout
SHM’s Career Satisfaction Task Force is no longer active, but its mission—to help hospitalists and groups improve job and career satisfaction—continues with a small group of former members. Working behind the scenes, the group surveyed hospitalists across the nation and began analyzing the data, all with the goal of finding maximal approaches to preventing burnout among their peers and colleagues.
“It’s one thing to describe burnout as a problem, and it’s a second thing to say, ‘How do we minimize the risk of burnout for the individual and for the program?’ ” says Chad Whelan, MD, FHM, director of the division of hospital medicine at Loyola University Health System in Maywood, Ill.
Dr. Whelan is one of three people working on the Hospital Medicine Physician Worklife Survey project. The others are Keiki Hinami, MD, assistant professor in the division of hospital medicine at Northwestern Memorial Hospital in Chicago, and Tosha Wetterneck, MD, FACP, associate professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison.
They surveyed nearly 3,800 potential hospitalists, ultimately analyzing more than 800 responses, and Dr. Wetterneck presented results and analysis through two research abstracts at HM11. The first abstract was translated into a paper and published online in July by the Journal of General Internal Medicine.1
What they found was while 62.6% of respondents reported high satisfaction with their job and 69% with the HM specialty, there were certain satisfaction domains—such as organizational climate and personal time availability—that rated low. The authors suspect those low ratings could lead to burnout, but they also note the results provide a roadmap for HM groups looking to address the issue.
“Now we have a lot more needs and demands put upon us as a profession,” Dr. Wetterneck says. “We wanted to know what people were doing nowadays, what kind of work were they doing, and were they happy with it.”
One revealing result, she notes, is that some hospitalists are “not happy” with some of the reasons they initially chose a career in HM. For example, many physicians turn to HM because of the flexibility in scheduling and team approach to patient care and QI. Yet, survey results suggest hospitalists are unhappy with the amount of personal time they have and don’t feel like they are part of a team, she says.

—Tosha Wetterneck, MD, FACP, associate professor of medicine, University of Wisconsin School of Medicine and Public Health, Madison
Workload Worries
The JGIM article, which assessed hospitalists’ satisfaction with such aspects as workload, compensation, patient-care quality, organizational fairness, autonomy, availability of personal time, and work relationships, showed that while hospitalists rated care quality and relationships with staff and colleagues high, they ranked compensation, organizational climate, autonomy, and availability of personal time low.
“To have such low satisfaction scores with their climate and their organization is concerning,” Dr. Wetterneck says. “It’s very important for [hospitalists] to be able to feel like they’re part of a team, that they’re part of an organization, and that the work they do really matters within that organization.”
Dr. Wetterneck acknowledges schedule flexibility is a key factor in hospitalist career choice, and it worries her that a majority of hospitalists surveyed are unhappy with the amount of personal time they had.
“When I presented these findings at the meeting, I had a lot of people telling me that the field has grown so quickly and the demands on the hospitalist group have grown so much that they haven’t been able to keep pace with hiring hospitalists to meet the demands in the workplace,” she says. “So people have to work more than they thought they would in the beginning, and that’s impinging on their personal time. … The flexibility piece is lost.”
Most hospitalists asked to work more are resilient and adapt. But over time, Dr. Wetterneck says, they begin to lose the ability to balance the demands and rewards of the job, and burnout develops.
“The study that we’ve been conducting suggests that the rate of burnout among practicing hospitalists is about 30 percent, which is a significant proportion of us,” Dr. Hinami says. “[It appears] that the rate of burnout symptoms of practicing hospitalists has remained stable, or may have increased, since the last time the publication of a nationwide survey was done.”
The last time a large survey measuring satisfaction among hospitalists was published was in 2001.2 It found that about 13% of hospitalists were burned out and about 25% were at risk of burnout, says Winthrop Whitcomb, MD, MHM, medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., and one of the authors of the 2001 study. Without question, burnout continues to be a major challenge for the entire field of HM, he says.
“Growth has always and will continue to fuel burnout,” says Dr. Whitcomb, cofounder and past president of SHM. “It’s a hard job, and as long as you’re growing, you’re not really getting your feet underneath you.”
The task force study found that hospitalists with burnout symptoms were much more likely to reduce work effort, leave their clinical situation, leave HM, and abandon direct patient care altogether than those without burnout symptoms.
Whereas the task force survey used a single-item question to ask hospitalists their level of burnout on a scale of 1 to 5, the 2001 study used a different scale and asked multiple questions to determine if respondents were burned out or at risk of burnout, Dr. Wetterneck explains.
“Even though it’s not a fair comparison, could it be that more hospitalists are burned out now than they were 10 years ago? I happen to think it probably is real … because of some of the satisfaction data we’re looking at,” she says.
Dr. Wetterneck’s group hasn’t analyzed if the reasons for burnout among hospitalists have changed over the years, but, anecdotally, Dr. Whelan has noticed a difference. Early hospitalists often burned out because they had to work day shifts and take night call. Today, far fewer hospitalists are always on. However, there are more hospitalists than ever before working in the hospital at off hours, which comes with different stressors, he says.
Greater Responsibility, Greater Dissatisfaction
As hospitalists’ roles expand, unpredictable interruptions are more frequent, says Sylvia McKean, MD, SFHM, FACP, a senior hospitalist at Brigham and Women’s Hospital in Boston, associate professor of medicine at Harvard Medical School, and former co-chair of the Career Satisfaction Task Force.
“For example, if you’re [scheduled] to admit patients to the hospital and you’re also on the rapid-response team and someone happens to need a rapid assessment, you can be interrupted,” she says. “If you’re a hospitalist taking care of someone who has had a subarachnoid hemorrhage and the neurosurgeon is going to come in the next morning but you’re uncertain about what to do or even to recognize a problem in that patient, those are the kinds of things that cause people to get anxious and feel more fatigued.”
As more subspecialists focus on consultations in the hospital, hospitalists are tending to see more specialty patients and, as a result, could feel overwhelmed, Dr. McKean says.
The new survey group is not yet in a position to be prescriptive about burnout, Dr. Hinami says. However, he and his colleagues hope to shed some light on possible solutions in the near future.
“What we understand about burnout is that it depends on both individual characteristics and characteristics of the work environment,” Dr. Hinami says. “We’re exploring the kind of ways in which job designs can be altered to help hospitalists—whatever their personal endowments are—to cope better with the stresses of the work.”
According to the research group, one thing is clear: Compensation is not a cure-all. One of the HM11 abstracts showed that satisfaction with compensation was correlated the least with both. “There’s only so much you can be paid more to do before it’s not enough anymore,” Dr. Wetterneck says. “There are some people who take money over a happy job, and that’s what they want to do for a couple of years. That’s not really going to grow our profession in the long run.”
Lisa Ryan is a freelance writer based in New Jersey.
Reference
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. July 2011 [epub ahead of print].
- Hoff TH, Whitcomb WF, Williams K, Nelson JR, Cheesman RA. Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851-858.
SHM’s Career Satisfaction Task Force is no longer active, but its mission—to help hospitalists and groups improve job and career satisfaction—continues with a small group of former members. Working behind the scenes, the group surveyed hospitalists across the nation and began analyzing the data, all with the goal of finding maximal approaches to preventing burnout among their peers and colleagues.
“It’s one thing to describe burnout as a problem, and it’s a second thing to say, ‘How do we minimize the risk of burnout for the individual and for the program?’ ” says Chad Whelan, MD, FHM, director of the division of hospital medicine at Loyola University Health System in Maywood, Ill.
Dr. Whelan is one of three people working on the Hospital Medicine Physician Worklife Survey project. The others are Keiki Hinami, MD, assistant professor in the division of hospital medicine at Northwestern Memorial Hospital in Chicago, and Tosha Wetterneck, MD, FACP, associate professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison.
They surveyed nearly 3,800 potential hospitalists, ultimately analyzing more than 800 responses, and Dr. Wetterneck presented results and analysis through two research abstracts at HM11. The first abstract was translated into a paper and published online in July by the Journal of General Internal Medicine.1
What they found was while 62.6% of respondents reported high satisfaction with their job and 69% with the HM specialty, there were certain satisfaction domains—such as organizational climate and personal time availability—that rated low. The authors suspect those low ratings could lead to burnout, but they also note the results provide a roadmap for HM groups looking to address the issue.
“Now we have a lot more needs and demands put upon us as a profession,” Dr. Wetterneck says. “We wanted to know what people were doing nowadays, what kind of work were they doing, and were they happy with it.”
One revealing result, she notes, is that some hospitalists are “not happy” with some of the reasons they initially chose a career in HM. For example, many physicians turn to HM because of the flexibility in scheduling and team approach to patient care and QI. Yet, survey results suggest hospitalists are unhappy with the amount of personal time they have and don’t feel like they are part of a team, she says.

—Tosha Wetterneck, MD, FACP, associate professor of medicine, University of Wisconsin School of Medicine and Public Health, Madison
Workload Worries
The JGIM article, which assessed hospitalists’ satisfaction with such aspects as workload, compensation, patient-care quality, organizational fairness, autonomy, availability of personal time, and work relationships, showed that while hospitalists rated care quality and relationships with staff and colleagues high, they ranked compensation, organizational climate, autonomy, and availability of personal time low.
“To have such low satisfaction scores with their climate and their organization is concerning,” Dr. Wetterneck says. “It’s very important for [hospitalists] to be able to feel like they’re part of a team, that they’re part of an organization, and that the work they do really matters within that organization.”
Dr. Wetterneck acknowledges schedule flexibility is a key factor in hospitalist career choice, and it worries her that a majority of hospitalists surveyed are unhappy with the amount of personal time they had.
“When I presented these findings at the meeting, I had a lot of people telling me that the field has grown so quickly and the demands on the hospitalist group have grown so much that they haven’t been able to keep pace with hiring hospitalists to meet the demands in the workplace,” she says. “So people have to work more than they thought they would in the beginning, and that’s impinging on their personal time. … The flexibility piece is lost.”
Most hospitalists asked to work more are resilient and adapt. But over time, Dr. Wetterneck says, they begin to lose the ability to balance the demands and rewards of the job, and burnout develops.
“The study that we’ve been conducting suggests that the rate of burnout among practicing hospitalists is about 30 percent, which is a significant proportion of us,” Dr. Hinami says. “[It appears] that the rate of burnout symptoms of practicing hospitalists has remained stable, or may have increased, since the last time the publication of a nationwide survey was done.”
The last time a large survey measuring satisfaction among hospitalists was published was in 2001.2 It found that about 13% of hospitalists were burned out and about 25% were at risk of burnout, says Winthrop Whitcomb, MD, MHM, medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., and one of the authors of the 2001 study. Without question, burnout continues to be a major challenge for the entire field of HM, he says.
“Growth has always and will continue to fuel burnout,” says Dr. Whitcomb, cofounder and past president of SHM. “It’s a hard job, and as long as you’re growing, you’re not really getting your feet underneath you.”
The task force study found that hospitalists with burnout symptoms were much more likely to reduce work effort, leave their clinical situation, leave HM, and abandon direct patient care altogether than those without burnout symptoms.
Whereas the task force survey used a single-item question to ask hospitalists their level of burnout on a scale of 1 to 5, the 2001 study used a different scale and asked multiple questions to determine if respondents were burned out or at risk of burnout, Dr. Wetterneck explains.
“Even though it’s not a fair comparison, could it be that more hospitalists are burned out now than they were 10 years ago? I happen to think it probably is real … because of some of the satisfaction data we’re looking at,” she says.
Dr. Wetterneck’s group hasn’t analyzed if the reasons for burnout among hospitalists have changed over the years, but, anecdotally, Dr. Whelan has noticed a difference. Early hospitalists often burned out because they had to work day shifts and take night call. Today, far fewer hospitalists are always on. However, there are more hospitalists than ever before working in the hospital at off hours, which comes with different stressors, he says.
Greater Responsibility, Greater Dissatisfaction
As hospitalists’ roles expand, unpredictable interruptions are more frequent, says Sylvia McKean, MD, SFHM, FACP, a senior hospitalist at Brigham and Women’s Hospital in Boston, associate professor of medicine at Harvard Medical School, and former co-chair of the Career Satisfaction Task Force.
“For example, if you’re [scheduled] to admit patients to the hospital and you’re also on the rapid-response team and someone happens to need a rapid assessment, you can be interrupted,” she says. “If you’re a hospitalist taking care of someone who has had a subarachnoid hemorrhage and the neurosurgeon is going to come in the next morning but you’re uncertain about what to do or even to recognize a problem in that patient, those are the kinds of things that cause people to get anxious and feel more fatigued.”
As more subspecialists focus on consultations in the hospital, hospitalists are tending to see more specialty patients and, as a result, could feel overwhelmed, Dr. McKean says.
The new survey group is not yet in a position to be prescriptive about burnout, Dr. Hinami says. However, he and his colleagues hope to shed some light on possible solutions in the near future.
“What we understand about burnout is that it depends on both individual characteristics and characteristics of the work environment,” Dr. Hinami says. “We’re exploring the kind of ways in which job designs can be altered to help hospitalists—whatever their personal endowments are—to cope better with the stresses of the work.”
According to the research group, one thing is clear: Compensation is not a cure-all. One of the HM11 abstracts showed that satisfaction with compensation was correlated the least with both. “There’s only so much you can be paid more to do before it’s not enough anymore,” Dr. Wetterneck says. “There are some people who take money over a happy job, and that’s what they want to do for a couple of years. That’s not really going to grow our profession in the long run.”
Lisa Ryan is a freelance writer based in New Jersey.
Reference
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. July 2011 [epub ahead of print].
- Hoff TH, Whitcomb WF, Williams K, Nelson JR, Cheesman RA. Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851-858.
SHM’s Career Satisfaction Task Force is no longer active, but its mission—to help hospitalists and groups improve job and career satisfaction—continues with a small group of former members. Working behind the scenes, the group surveyed hospitalists across the nation and began analyzing the data, all with the goal of finding maximal approaches to preventing burnout among their peers and colleagues.
“It’s one thing to describe burnout as a problem, and it’s a second thing to say, ‘How do we minimize the risk of burnout for the individual and for the program?’ ” says Chad Whelan, MD, FHM, director of the division of hospital medicine at Loyola University Health System in Maywood, Ill.
Dr. Whelan is one of three people working on the Hospital Medicine Physician Worklife Survey project. The others are Keiki Hinami, MD, assistant professor in the division of hospital medicine at Northwestern Memorial Hospital in Chicago, and Tosha Wetterneck, MD, FACP, associate professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison.
They surveyed nearly 3,800 potential hospitalists, ultimately analyzing more than 800 responses, and Dr. Wetterneck presented results and analysis through two research abstracts at HM11. The first abstract was translated into a paper and published online in July by the Journal of General Internal Medicine.1
What they found was while 62.6% of respondents reported high satisfaction with their job and 69% with the HM specialty, there were certain satisfaction domains—such as organizational climate and personal time availability—that rated low. The authors suspect those low ratings could lead to burnout, but they also note the results provide a roadmap for HM groups looking to address the issue.
“Now we have a lot more needs and demands put upon us as a profession,” Dr. Wetterneck says. “We wanted to know what people were doing nowadays, what kind of work were they doing, and were they happy with it.”
One revealing result, she notes, is that some hospitalists are “not happy” with some of the reasons they initially chose a career in HM. For example, many physicians turn to HM because of the flexibility in scheduling and team approach to patient care and QI. Yet, survey results suggest hospitalists are unhappy with the amount of personal time they have and don’t feel like they are part of a team, she says.

—Tosha Wetterneck, MD, FACP, associate professor of medicine, University of Wisconsin School of Medicine and Public Health, Madison
Workload Worries
The JGIM article, which assessed hospitalists’ satisfaction with such aspects as workload, compensation, patient-care quality, organizational fairness, autonomy, availability of personal time, and work relationships, showed that while hospitalists rated care quality and relationships with staff and colleagues high, they ranked compensation, organizational climate, autonomy, and availability of personal time low.
“To have such low satisfaction scores with their climate and their organization is concerning,” Dr. Wetterneck says. “It’s very important for [hospitalists] to be able to feel like they’re part of a team, that they’re part of an organization, and that the work they do really matters within that organization.”
Dr. Wetterneck acknowledges schedule flexibility is a key factor in hospitalist career choice, and it worries her that a majority of hospitalists surveyed are unhappy with the amount of personal time they had.
“When I presented these findings at the meeting, I had a lot of people telling me that the field has grown so quickly and the demands on the hospitalist group have grown so much that they haven’t been able to keep pace with hiring hospitalists to meet the demands in the workplace,” she says. “So people have to work more than they thought they would in the beginning, and that’s impinging on their personal time. … The flexibility piece is lost.”
Most hospitalists asked to work more are resilient and adapt. But over time, Dr. Wetterneck says, they begin to lose the ability to balance the demands and rewards of the job, and burnout develops.
“The study that we’ve been conducting suggests that the rate of burnout among practicing hospitalists is about 30 percent, which is a significant proportion of us,” Dr. Hinami says. “[It appears] that the rate of burnout symptoms of practicing hospitalists has remained stable, or may have increased, since the last time the publication of a nationwide survey was done.”
The last time a large survey measuring satisfaction among hospitalists was published was in 2001.2 It found that about 13% of hospitalists were burned out and about 25% were at risk of burnout, says Winthrop Whitcomb, MD, MHM, medical director of healthcare quality at Baystate Medical Center in Springfield, Mass., and one of the authors of the 2001 study. Without question, burnout continues to be a major challenge for the entire field of HM, he says.
“Growth has always and will continue to fuel burnout,” says Dr. Whitcomb, cofounder and past president of SHM. “It’s a hard job, and as long as you’re growing, you’re not really getting your feet underneath you.”
The task force study found that hospitalists with burnout symptoms were much more likely to reduce work effort, leave their clinical situation, leave HM, and abandon direct patient care altogether than those without burnout symptoms.
Whereas the task force survey used a single-item question to ask hospitalists their level of burnout on a scale of 1 to 5, the 2001 study used a different scale and asked multiple questions to determine if respondents were burned out or at risk of burnout, Dr. Wetterneck explains.
“Even though it’s not a fair comparison, could it be that more hospitalists are burned out now than they were 10 years ago? I happen to think it probably is real … because of some of the satisfaction data we’re looking at,” she says.
Dr. Wetterneck’s group hasn’t analyzed if the reasons for burnout among hospitalists have changed over the years, but, anecdotally, Dr. Whelan has noticed a difference. Early hospitalists often burned out because they had to work day shifts and take night call. Today, far fewer hospitalists are always on. However, there are more hospitalists than ever before working in the hospital at off hours, which comes with different stressors, he says.
Greater Responsibility, Greater Dissatisfaction
As hospitalists’ roles expand, unpredictable interruptions are more frequent, says Sylvia McKean, MD, SFHM, FACP, a senior hospitalist at Brigham and Women’s Hospital in Boston, associate professor of medicine at Harvard Medical School, and former co-chair of the Career Satisfaction Task Force.
“For example, if you’re [scheduled] to admit patients to the hospital and you’re also on the rapid-response team and someone happens to need a rapid assessment, you can be interrupted,” she says. “If you’re a hospitalist taking care of someone who has had a subarachnoid hemorrhage and the neurosurgeon is going to come in the next morning but you’re uncertain about what to do or even to recognize a problem in that patient, those are the kinds of things that cause people to get anxious and feel more fatigued.”
As more subspecialists focus on consultations in the hospital, hospitalists are tending to see more specialty patients and, as a result, could feel overwhelmed, Dr. McKean says.
The new survey group is not yet in a position to be prescriptive about burnout, Dr. Hinami says. However, he and his colleagues hope to shed some light on possible solutions in the near future.
“What we understand about burnout is that it depends on both individual characteristics and characteristics of the work environment,” Dr. Hinami says. “We’re exploring the kind of ways in which job designs can be altered to help hospitalists—whatever their personal endowments are—to cope better with the stresses of the work.”
According to the research group, one thing is clear: Compensation is not a cure-all. One of the HM11 abstracts showed that satisfaction with compensation was correlated the least with both. “There’s only so much you can be paid more to do before it’s not enough anymore,” Dr. Wetterneck says. “There are some people who take money over a happy job, and that’s what they want to do for a couple of years. That’s not really going to grow our profession in the long run.”
Lisa Ryan is a freelance writer based in New Jersey.
Reference
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. July 2011 [epub ahead of print].
- Hoff TH, Whitcomb WF, Williams K, Nelson JR, Cheesman RA. Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851-858.
Purposeful Visits Enhance Hospitalized Seniors’ Quality of Life
An abstract presented at HM11, “Purposeful Visits for Hospitalized Elderly Patients,” describes a service at the University of Colorado Hospital (UCH) in Denver that has shown improvements in participating patients’ mood, agitation, and orientation.
The purposeful-visit program was started, says senior author Ethan Cumbler, MD, a hospitalist at UCH and director of its Acute Care for the Elderly Service, because hospitals often are a profoundly unfriendly environment, especially for vulnerable, chronically ill patients. “It’s a social and intellectual desert where patients don’t get the stimulation they would receive at home,” he adds.
The program was established to leverage professional resources by training a core cadre of four to six volunteers in communication techniques (e.g. open-ended questioning), says the hospital’s recreational therapist, William Mramor, CTRS, MS. Charge nurses help identify patients and topics to explore, and the volunteers use a prepared script to help guide interactions, Mramor says.
“The purposeful visit directly addresses issues of patients’ feelings and promotes a patient-centered hospital experience,” he says.
Based on assessments using a five-point scale, with scores ranging from “worsening” (1 or 2) to “improving” (4 or 5), patient mood was rated 3.94 by the volunteers and 3.65 by the nurses. Slightly lower scores were recorded for patient agitation and patient orientation but in every case showed improvement.
“What distinguishes these purposeful visits is their goal of enhancing patients’ memory, decreasing their loneliness, and helping them understand the value of reconnecting to things they enjoy,” says Dr. Cumbler. —LB
Technology
New E-Pillbox Actively Monitors Med-Recon, Fights Readmissions
Electronic pillboxes are nothing new, but some hospitalists might not have seen the latest one.
Earlier this year, the FDA approved PillStation, a traditional pillbox married to a software system that uploads data to the system’s maker, SentiCare Inc., which then monitors how well a patient is following their medication regimen. The four-year-old medical firm is pitching the product to hospitals and accountable-care organizations (ACOs), among other potential clients.
And in a sales pitch practically tailored to HM, SentiCare bills itself as a medication adherence system that can help fight readmissions, particularly in cases of chronic disease or congestive heart failure. The device actually takes photographs of the pills to be taken and can record whether a patient has removed them from the device.
“Hospitals need to dramatically reduce their readmissions rates,” Yogendra Jain, chief technology officer and cofounder of SentiCare, wrote in an email to The Hospitalist. “One critical factor is medication and hospital discharge instruction adherence. Through its embedded camera, PillStation can confirm that from day one of departing the hospital... medications are loaded correctly and that the patient is taking it on time.”—RQ
Quality
Home Healthcare Has Fewer Rehospitalizations
A recent study by Avalere Health, a healthcare advisory firm based in Washington, D.C., found that providing home healthcare after hospital discharge for patients with three common conditions resulted in fewer hospital readmissions than for similar patients receiving other post-acute services. Those comparable services included long-term acute-care hospitals, inpatient rehabilitation facilities, skilled nursing facilities, and hospices.
“We tried to control for hospital DRG, severity of illness, and comorbidities,” says Emil Parker, Avalere’s director of post-acute and long-term-care practice, although he acknowledges the complexities of risk adjustment.
In comparing Medicare spending and rehospitalization rates after initial hospital visits for patients with diabetes, COPD, and congestive heart failure from 2006 to 2009, the study estimated that referrals to home healthcare resulted in $670 million in Medicare savings from 20,426 fewer readmissions.
“Hospitalists should think about the continuum of institutional support for patients discharged from the hospital with significant support needs,” Parker says. “Our study shows that in this population, provision of home healthcare is cost-effective and benefits patients by improving the continuity of their care.” —LB
Patient Safety
L.A. Hospitals Add HM for Medicaid Patients
In June, Anthem Blue Cross of Woodland Hills, Calif., began offering covered hospitalist services to its adult managed-care members covered by Medi-Cal, the Medicaid program for California residents, at 24 hospitals in Los Angeles County. The service is designed to take advantage of the existing hospitalist presence in those hospitals, which is provided by ApolloMed, a Glendale, Calif.-based medical management services company.
The hospitalist service is designed to enhance quality of care during hospitalization, reduce costs, and plan for more timely discharges and transitions to outpatient care. ApolloMed plans to add more hospitals in the region to the program, as well as additional post-discharge outpatient clinics. —LB
Technology
By the Numbers: 5.9
The percentage of total national health expenditures spent on medical devices in 2009, according to a report released in June by the Advanced Medical Technology Association.
The report highlights that while technology is washing over medicine, and HM in particular, with the adoption of electronic health records, portable ultrasounds, and tablet computing, the $147 billion spent on medical devices in 2009 represented just 5.9% of the $2.5 trillion in national health spending.
The trade group also reported that the average annual rate for medical device spending increased 7.5% in the 20-year period that ended in 2009. That outpaced the average annual rate for overall national heath expenditures, which ticked up 7% over the same time period. —RQ
An abstract presented at HM11, “Purposeful Visits for Hospitalized Elderly Patients,” describes a service at the University of Colorado Hospital (UCH) in Denver that has shown improvements in participating patients’ mood, agitation, and orientation.
The purposeful-visit program was started, says senior author Ethan Cumbler, MD, a hospitalist at UCH and director of its Acute Care for the Elderly Service, because hospitals often are a profoundly unfriendly environment, especially for vulnerable, chronically ill patients. “It’s a social and intellectual desert where patients don’t get the stimulation they would receive at home,” he adds.
The program was established to leverage professional resources by training a core cadre of four to six volunteers in communication techniques (e.g. open-ended questioning), says the hospital’s recreational therapist, William Mramor, CTRS, MS. Charge nurses help identify patients and topics to explore, and the volunteers use a prepared script to help guide interactions, Mramor says.
“The purposeful visit directly addresses issues of patients’ feelings and promotes a patient-centered hospital experience,” he says.
Based on assessments using a five-point scale, with scores ranging from “worsening” (1 or 2) to “improving” (4 or 5), patient mood was rated 3.94 by the volunteers and 3.65 by the nurses. Slightly lower scores were recorded for patient agitation and patient orientation but in every case showed improvement.
“What distinguishes these purposeful visits is their goal of enhancing patients’ memory, decreasing their loneliness, and helping them understand the value of reconnecting to things they enjoy,” says Dr. Cumbler. —LB
Technology
New E-Pillbox Actively Monitors Med-Recon, Fights Readmissions
Electronic pillboxes are nothing new, but some hospitalists might not have seen the latest one.
Earlier this year, the FDA approved PillStation, a traditional pillbox married to a software system that uploads data to the system’s maker, SentiCare Inc., which then monitors how well a patient is following their medication regimen. The four-year-old medical firm is pitching the product to hospitals and accountable-care organizations (ACOs), among other potential clients.
And in a sales pitch practically tailored to HM, SentiCare bills itself as a medication adherence system that can help fight readmissions, particularly in cases of chronic disease or congestive heart failure. The device actually takes photographs of the pills to be taken and can record whether a patient has removed them from the device.
“Hospitals need to dramatically reduce their readmissions rates,” Yogendra Jain, chief technology officer and cofounder of SentiCare, wrote in an email to The Hospitalist. “One critical factor is medication and hospital discharge instruction adherence. Through its embedded camera, PillStation can confirm that from day one of departing the hospital... medications are loaded correctly and that the patient is taking it on time.”—RQ
Quality
Home Healthcare Has Fewer Rehospitalizations
A recent study by Avalere Health, a healthcare advisory firm based in Washington, D.C., found that providing home healthcare after hospital discharge for patients with three common conditions resulted in fewer hospital readmissions than for similar patients receiving other post-acute services. Those comparable services included long-term acute-care hospitals, inpatient rehabilitation facilities, skilled nursing facilities, and hospices.
“We tried to control for hospital DRG, severity of illness, and comorbidities,” says Emil Parker, Avalere’s director of post-acute and long-term-care practice, although he acknowledges the complexities of risk adjustment.
In comparing Medicare spending and rehospitalization rates after initial hospital visits for patients with diabetes, COPD, and congestive heart failure from 2006 to 2009, the study estimated that referrals to home healthcare resulted in $670 million in Medicare savings from 20,426 fewer readmissions.
“Hospitalists should think about the continuum of institutional support for patients discharged from the hospital with significant support needs,” Parker says. “Our study shows that in this population, provision of home healthcare is cost-effective and benefits patients by improving the continuity of their care.” —LB
Patient Safety
L.A. Hospitals Add HM for Medicaid Patients
In June, Anthem Blue Cross of Woodland Hills, Calif., began offering covered hospitalist services to its adult managed-care members covered by Medi-Cal, the Medicaid program for California residents, at 24 hospitals in Los Angeles County. The service is designed to take advantage of the existing hospitalist presence in those hospitals, which is provided by ApolloMed, a Glendale, Calif.-based medical management services company.
The hospitalist service is designed to enhance quality of care during hospitalization, reduce costs, and plan for more timely discharges and transitions to outpatient care. ApolloMed plans to add more hospitals in the region to the program, as well as additional post-discharge outpatient clinics. —LB
Technology
By the Numbers: 5.9
The percentage of total national health expenditures spent on medical devices in 2009, according to a report released in June by the Advanced Medical Technology Association.
The report highlights that while technology is washing over medicine, and HM in particular, with the adoption of electronic health records, portable ultrasounds, and tablet computing, the $147 billion spent on medical devices in 2009 represented just 5.9% of the $2.5 trillion in national health spending.
The trade group also reported that the average annual rate for medical device spending increased 7.5% in the 20-year period that ended in 2009. That outpaced the average annual rate for overall national heath expenditures, which ticked up 7% over the same time period. —RQ
An abstract presented at HM11, “Purposeful Visits for Hospitalized Elderly Patients,” describes a service at the University of Colorado Hospital (UCH) in Denver that has shown improvements in participating patients’ mood, agitation, and orientation.
The purposeful-visit program was started, says senior author Ethan Cumbler, MD, a hospitalist at UCH and director of its Acute Care for the Elderly Service, because hospitals often are a profoundly unfriendly environment, especially for vulnerable, chronically ill patients. “It’s a social and intellectual desert where patients don’t get the stimulation they would receive at home,” he adds.
The program was established to leverage professional resources by training a core cadre of four to six volunteers in communication techniques (e.g. open-ended questioning), says the hospital’s recreational therapist, William Mramor, CTRS, MS. Charge nurses help identify patients and topics to explore, and the volunteers use a prepared script to help guide interactions, Mramor says.
“The purposeful visit directly addresses issues of patients’ feelings and promotes a patient-centered hospital experience,” he says.
Based on assessments using a five-point scale, with scores ranging from “worsening” (1 or 2) to “improving” (4 or 5), patient mood was rated 3.94 by the volunteers and 3.65 by the nurses. Slightly lower scores were recorded for patient agitation and patient orientation but in every case showed improvement.
“What distinguishes these purposeful visits is their goal of enhancing patients’ memory, decreasing their loneliness, and helping them understand the value of reconnecting to things they enjoy,” says Dr. Cumbler. —LB
Technology
New E-Pillbox Actively Monitors Med-Recon, Fights Readmissions
Electronic pillboxes are nothing new, but some hospitalists might not have seen the latest one.
Earlier this year, the FDA approved PillStation, a traditional pillbox married to a software system that uploads data to the system’s maker, SentiCare Inc., which then monitors how well a patient is following their medication regimen. The four-year-old medical firm is pitching the product to hospitals and accountable-care organizations (ACOs), among other potential clients.
And in a sales pitch practically tailored to HM, SentiCare bills itself as a medication adherence system that can help fight readmissions, particularly in cases of chronic disease or congestive heart failure. The device actually takes photographs of the pills to be taken and can record whether a patient has removed them from the device.
“Hospitals need to dramatically reduce their readmissions rates,” Yogendra Jain, chief technology officer and cofounder of SentiCare, wrote in an email to The Hospitalist. “One critical factor is medication and hospital discharge instruction adherence. Through its embedded camera, PillStation can confirm that from day one of departing the hospital... medications are loaded correctly and that the patient is taking it on time.”—RQ
Quality
Home Healthcare Has Fewer Rehospitalizations
A recent study by Avalere Health, a healthcare advisory firm based in Washington, D.C., found that providing home healthcare after hospital discharge for patients with three common conditions resulted in fewer hospital readmissions than for similar patients receiving other post-acute services. Those comparable services included long-term acute-care hospitals, inpatient rehabilitation facilities, skilled nursing facilities, and hospices.
“We tried to control for hospital DRG, severity of illness, and comorbidities,” says Emil Parker, Avalere’s director of post-acute and long-term-care practice, although he acknowledges the complexities of risk adjustment.
In comparing Medicare spending and rehospitalization rates after initial hospital visits for patients with diabetes, COPD, and congestive heart failure from 2006 to 2009, the study estimated that referrals to home healthcare resulted in $670 million in Medicare savings from 20,426 fewer readmissions.
“Hospitalists should think about the continuum of institutional support for patients discharged from the hospital with significant support needs,” Parker says. “Our study shows that in this population, provision of home healthcare is cost-effective and benefits patients by improving the continuity of their care.” —LB
Patient Safety
L.A. Hospitals Add HM for Medicaid Patients
In June, Anthem Blue Cross of Woodland Hills, Calif., began offering covered hospitalist services to its adult managed-care members covered by Medi-Cal, the Medicaid program for California residents, at 24 hospitals in Los Angeles County. The service is designed to take advantage of the existing hospitalist presence in those hospitals, which is provided by ApolloMed, a Glendale, Calif.-based medical management services company.
The hospitalist service is designed to enhance quality of care during hospitalization, reduce costs, and plan for more timely discharges and transitions to outpatient care. ApolloMed plans to add more hospitals in the region to the program, as well as additional post-discharge outpatient clinics. —LB
Technology
By the Numbers: 5.9
The percentage of total national health expenditures spent on medical devices in 2009, according to a report released in June by the Advanced Medical Technology Association.
The report highlights that while technology is washing over medicine, and HM in particular, with the adoption of electronic health records, portable ultrasounds, and tablet computing, the $147 billion spent on medical devices in 2009 represented just 5.9% of the $2.5 trillion in national health spending.
The trade group also reported that the average annual rate for medical device spending increased 7.5% in the 20-year period that ended in 2009. That outpaced the average annual rate for overall national heath expenditures, which ticked up 7% over the same time period. —RQ
Conglomerate HM?
William Geers, MD, finished up his residency in 2007, then went to work for a close-knit emergency-medicine group of about 25 doctors in Daytona Beach, Fla.
“Everybody was pretty tight,” he says of his first job.
He had met his wife in residency in Daytona, but after a while, they figured it was time for a change. “We’d been in Daytona for about six years and were ready to go try someplace different,” Dr. Geers says. “Tallahassee seemed like a good match because that’s kind of right in between our families.”
He soon landed a hospitalist job at Capital Regional Medical Center, and he suddenly was a part of EmCare, one of the biggest corporations in the emergency-medicine field and, more recently, in the field of hospital medicine. EmCare provides doctors to about 400 hospitals nationwide.
Dr. Geers said the corporate affiliation didn’t factor into his decision, adding that he took more of a traditional approach when choosing a new job.
“At the time, this program was a little bit smaller, which I liked,” says Dr. Geers, who also looked at the city’s other hospital, Tallahassee Memorial. “I met some of the physicians over here. I liked them.”
But he has noticed perks.
“I think we have some advantages working with EmCare in that we do have a pretty big group that’s backing us,” he explains. “I feel a little more secure with issues like malpractice. If things like that ever come up, I really feel like I have a lot of support with EmCare.”
With the corporate presence on the rise in HM, more and more hospitalists are entering the ranks of large companies. Some are doing so straight out of residency. Some are giving up their private practices and selling them to corporations looking to expand.
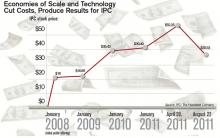
Corporations that provide hospitalists to hospitals are getting ever bigger, using sophisticated infrastructure and economies of scale, they say, to make life easier for the people who work for them, allowing the hospitalists to focus on patient care. Their efficiencies are attractive to hospitals looking to simplify.
Three years ago, North Hollywood, Calif.-based IPC: The Hospitalist Company became a publicly traded company. Its stock price has more than doubled since then.
In July, Eagle Hospital Physicians acquired North Carolina-based PrimeDoc and its 100 doctors covering seven hospitals. Similar acquisitions by larger corporations have become almost weekly news.
And, probably most significantly, Cogent Healthcare recently completed a merger with Hospitalists Management Group, a union of two of the biggest hospitalist companies in the U.S. The new company, Cogent HMG, now includes a corps of 1,000 doctors, nurses, and physician assistants (PAs), with client hospitals in 28 states.
Cogent had clients that were medium to large in size, generally in more urban areas but scattered geographically. HMG mostly served small- to medium-sized hospitals with densities in certain regions. With the merger came a recognition that the larger a company becomes, the greater the opportunity for efficiency and better services, says Rusty Holman, MD, MHM, chief clinical officer of the new company.
“The real value out of bringing these two companies together is bringing the best of different worlds together, creating new products and services for hospitals that don’t exist today, and to be able to serve a broader customer base,” says Dr. Holman, a former SHM president. “It’s also to leverage some of the infrastructure that has been built over a greater number of programs and hospitals to gain efficiency and scale that way. So that is the primary focus of the integration today.”
Cogent HMG CEO Steve Houff, MD, says the merger will mean investment in clinical support, physician recruiting, and technology, and will benefit patients and hospital partners alike.
“Both Cogent and HMG have a track record for delivering improvements in clinical quality and patient satisfaction at each of the hospitals we serve. The plan is for that to continue on a broader scale,” he wrote in an email to The Hospitalist.

—R. Jeffrey Taylor, president, chief operating officer, IPC: The Hospitalist Company, North Hollywood, Calif.
The Good, the Bad, the Oligopoly
The average size of a hospitalist group in the U.S. is about 10 full-time equivalents, according to recent survey data from SHM and MGMA. With the swelling of the size of HM’s biggest corporate players comes the question of how far the coalescing will go: Will most patient care eventually be provided by only a few groups?
R. Jeffrey Taylor, IPC’s president and chief operating officer, says the mergers and acquisitions will continue, but he doesn’t see a day when there will be just a few titans ruling all.
“I do think there will be more consolidation going forward than there is now, but I don’t see a future in which there are, you know, two or three groups that completely dominate the landscape,” he says. “There’s always that concern that that’s going to happen in the hospital industry, or that’s going to happen with payors. And there are always new entrants.”
For all the movement toward bigger companies, “this is still an unconsolidated industry,” and new physician practices will always continue to be formed, he says.
“We’re the largest group, and we’re maybe 3 1/2 percent of all the hospitals in the country. I wouldn’t consider this, today, a terribly consolidated industry,” he adds. “I do think it will move in that direction. I just don’t think it will get all the way there, because of the sort of private, entrepreneurial, independent spirit that’s common among physicians.”
Mike Tarwater, a board member of the American Hospital Association, says private hospitalist providers will only be an alternative to—and not a replacement provider for—large, self-contained systems like the Carolinas Medical Center (CMC), for which he serves as CEO. The health system has a wide spectrum of facilities—from large, urban academic centers like the 874-bed medical center in Charlotte, N.C., to 52-bed Anson Community Hospital in Wadesboro, N.C., population 5,780.
“As a system, we have the wherewithal and the recruiting expertise, and, with 1,700 physician associates across the system, we’ve kind of got critical mass,” Tarwater says. “So we will be an alternative to that in our region.”
Frank Michota, MD, FHM, director of academic affairs in the Department of Hospital Medicine at The Cleveland Clinic, says that the extensive training programs of many of the larger hospitalist groups (e.g. Cogent Academy, IPC’s extensive onboarding process and leadership conferences) could be a very good thing for the field.
“I have always thought that companies like Cogent did a very nice job in orienting their hospitalists to the patient-care goals and the process variables that were being measured,” Dr. Michota says. “I think that by making an even larger group, they have the opportunity to continue to standardize the approach to hospital care so that one hospitalist equals one hospitalist equals one hospitalist. I think that’s a positive.”
The flip side, though, is that anything that might be done wrong would be magnified in such a system.
“I think that there are some dangers in how these large companies will incentivize their hospitalists,” he adds. “If they are consistent from hospitalist to hospitalist, but if there’s a perverse adverse effect from one of their financial incentives, it will be carried out across a lot of hospitals all at the same time. “But I think it’s a little early to tell what the impact of this might be. But, at least for right now, it’s actually a positive thing because it standardizes the hospitalist.”
Tarwater says that even when larger corporations buy smaller practices, familiarity tends to remain.
“Most of what I have seen are existing groups that join through merger or acquisition, and so we already have experience with the doctors, we already have long-standing relationships with the doctors,” he says. “I think any health system or hospital would be reticent to sign up with somebody that they’ve never heard of, that doesn’t have a track record, or that they don’t know already at least some of the players.” Hospitals looking to hire a private company have to exercise caution, particularly if the company is trying to break into a new region where it isn’t known.
“Those hospitals and healthcare systems just have to be really careful who they’re signing contracts with,” he said. “It’s no different than anything else we do. You just have to know who your partners are, and what drives them and where they stand on important issues.”
Executives say patient care is not at risk, even as consolidation continues. “With or without competition, we are relentlessly trying to improve our approach to patient care, our performance, and our hospital partnerships,” Cogent HMG’s Dr. Houff says.
Money Talks
It doesn’t appear that more hospitalist companies are planning to go public—at least for now.
The largest privately held company, Cogent HMG, is not planning an initial public offering anytime soon, Dr. Houff says. The company’s goal is to “continue investing in smart growth to capture more of the hospital medicine market, expand offerings to our existing hospital clients, and provide additional support to our clinical teams on the ground,” he says. “We have a strong capital partner to help us in that effort and are not looking at the public markets at this time.”
Taking on stockholders is a tricky business—one that requires careful planning and a willingness from practice leaders and administrators to relinquish some autonomy to outside interests. And then there are the financial requirements.
“They’ve really got to be able to produce some serious revenue in order for somebody to be willing to put some money into them,” says Mark Hamm, CEO of EmCare Inpatient Services.
The lure of working for a private hospitalist company promises to continue to be an attractive one. Some are drawn by the leadership possibilities—those who “aspire to be the true alpha doctor,” as IPC’s Taylor puts it. Others are drawn by the stability of a larger company.
There also is flexibility in location, Dr. Holman notes.
“Now, with Cogent HMG, [hospitalists] have even more choices in terms of relocating within the same company,” he says. “So they can keep all of the benefits, keep all of the knowledge and familiarity of the system and philosophy of care that we employ, and just be able to transfer.”
continued below...
Dr. Houff says the majority of newly recruited physicians are coming out of residency but that the company is attracting physicians in the middle of their careers, along with physicians having backgrounds beyond internal medicine.
In Tallahassee at Capital Regional, Dr. Geers says that he feels there is support from the company that can protect his job quality, with “a little bit more room to negotiate with the hospital if the hospital wants us to take on new responsibilities.
“Whereas if we worked directly for the hospital, I don’t think we’d have much say in the matter,” he says.
He also says he is happy with the predictable schedule; he’s responsible for 7 a.m. to 7 p.m. and nothing more.
“If you’re finished rounding and you’ve seen all your patients and tied up all your loose ends, you’re not always there till 7 p.m.,” he points out. “Sometimes you can leave a little early....Once 7 p.m. comes, you’re not going to get paged in the middle of the night.”
Thomas R. Collins is a freelance medical writer based in Florida.
William Geers, MD, finished up his residency in 2007, then went to work for a close-knit emergency-medicine group of about 25 doctors in Daytona Beach, Fla.
“Everybody was pretty tight,” he says of his first job.
He had met his wife in residency in Daytona, but after a while, they figured it was time for a change. “We’d been in Daytona for about six years and were ready to go try someplace different,” Dr. Geers says. “Tallahassee seemed like a good match because that’s kind of right in between our families.”
He soon landed a hospitalist job at Capital Regional Medical Center, and he suddenly was a part of EmCare, one of the biggest corporations in the emergency-medicine field and, more recently, in the field of hospital medicine. EmCare provides doctors to about 400 hospitals nationwide.
Dr. Geers said the corporate affiliation didn’t factor into his decision, adding that he took more of a traditional approach when choosing a new job.
“At the time, this program was a little bit smaller, which I liked,” says Dr. Geers, who also looked at the city’s other hospital, Tallahassee Memorial. “I met some of the physicians over here. I liked them.”
But he has noticed perks.
“I think we have some advantages working with EmCare in that we do have a pretty big group that’s backing us,” he explains. “I feel a little more secure with issues like malpractice. If things like that ever come up, I really feel like I have a lot of support with EmCare.”
With the corporate presence on the rise in HM, more and more hospitalists are entering the ranks of large companies. Some are doing so straight out of residency. Some are giving up their private practices and selling them to corporations looking to expand.
Corporations that provide hospitalists to hospitals are getting ever bigger, using sophisticated infrastructure and economies of scale, they say, to make life easier for the people who work for them, allowing the hospitalists to focus on patient care. Their efficiencies are attractive to hospitals looking to simplify.
Three years ago, North Hollywood, Calif.-based IPC: The Hospitalist Company became a publicly traded company. Its stock price has more than doubled since then.
In July, Eagle Hospital Physicians acquired North Carolina-based PrimeDoc and its 100 doctors covering seven hospitals. Similar acquisitions by larger corporations have become almost weekly news.
And, probably most significantly, Cogent Healthcare recently completed a merger with Hospitalists Management Group, a union of two of the biggest hospitalist companies in the U.S. The new company, Cogent HMG, now includes a corps of 1,000 doctors, nurses, and physician assistants (PAs), with client hospitals in 28 states.
Cogent had clients that were medium to large in size, generally in more urban areas but scattered geographically. HMG mostly served small- to medium-sized hospitals with densities in certain regions. With the merger came a recognition that the larger a company becomes, the greater the opportunity for efficiency and better services, says Rusty Holman, MD, MHM, chief clinical officer of the new company.
“The real value out of bringing these two companies together is bringing the best of different worlds together, creating new products and services for hospitals that don’t exist today, and to be able to serve a broader customer base,” says Dr. Holman, a former SHM president. “It’s also to leverage some of the infrastructure that has been built over a greater number of programs and hospitals to gain efficiency and scale that way. So that is the primary focus of the integration today.”
Cogent HMG CEO Steve Houff, MD, says the merger will mean investment in clinical support, physician recruiting, and technology, and will benefit patients and hospital partners alike.
“Both Cogent and HMG have a track record for delivering improvements in clinical quality and patient satisfaction at each of the hospitals we serve. The plan is for that to continue on a broader scale,” he wrote in an email to The Hospitalist.

—R. Jeffrey Taylor, president, chief operating officer, IPC: The Hospitalist Company, North Hollywood, Calif.
The Good, the Bad, the Oligopoly
The average size of a hospitalist group in the U.S. is about 10 full-time equivalents, according to recent survey data from SHM and MGMA. With the swelling of the size of HM’s biggest corporate players comes the question of how far the coalescing will go: Will most patient care eventually be provided by only a few groups?
R. Jeffrey Taylor, IPC’s president and chief operating officer, says the mergers and acquisitions will continue, but he doesn’t see a day when there will be just a few titans ruling all.
“I do think there will be more consolidation going forward than there is now, but I don’t see a future in which there are, you know, two or three groups that completely dominate the landscape,” he says. “There’s always that concern that that’s going to happen in the hospital industry, or that’s going to happen with payors. And there are always new entrants.”
For all the movement toward bigger companies, “this is still an unconsolidated industry,” and new physician practices will always continue to be formed, he says.
“We’re the largest group, and we’re maybe 3 1/2 percent of all the hospitals in the country. I wouldn’t consider this, today, a terribly consolidated industry,” he adds. “I do think it will move in that direction. I just don’t think it will get all the way there, because of the sort of private, entrepreneurial, independent spirit that’s common among physicians.”
Mike Tarwater, a board member of the American Hospital Association, says private hospitalist providers will only be an alternative to—and not a replacement provider for—large, self-contained systems like the Carolinas Medical Center (CMC), for which he serves as CEO. The health system has a wide spectrum of facilities—from large, urban academic centers like the 874-bed medical center in Charlotte, N.C., to 52-bed Anson Community Hospital in Wadesboro, N.C., population 5,780.
“As a system, we have the wherewithal and the recruiting expertise, and, with 1,700 physician associates across the system, we’ve kind of got critical mass,” Tarwater says. “So we will be an alternative to that in our region.”
Frank Michota, MD, FHM, director of academic affairs in the Department of Hospital Medicine at The Cleveland Clinic, says that the extensive training programs of many of the larger hospitalist groups (e.g. Cogent Academy, IPC’s extensive onboarding process and leadership conferences) could be a very good thing for the field.
“I have always thought that companies like Cogent did a very nice job in orienting their hospitalists to the patient-care goals and the process variables that were being measured,” Dr. Michota says. “I think that by making an even larger group, they have the opportunity to continue to standardize the approach to hospital care so that one hospitalist equals one hospitalist equals one hospitalist. I think that’s a positive.”
The flip side, though, is that anything that might be done wrong would be magnified in such a system.
“I think that there are some dangers in how these large companies will incentivize their hospitalists,” he adds. “If they are consistent from hospitalist to hospitalist, but if there’s a perverse adverse effect from one of their financial incentives, it will be carried out across a lot of hospitals all at the same time. “But I think it’s a little early to tell what the impact of this might be. But, at least for right now, it’s actually a positive thing because it standardizes the hospitalist.”
Tarwater says that even when larger corporations buy smaller practices, familiarity tends to remain.
“Most of what I have seen are existing groups that join through merger or acquisition, and so we already have experience with the doctors, we already have long-standing relationships with the doctors,” he says. “I think any health system or hospital would be reticent to sign up with somebody that they’ve never heard of, that doesn’t have a track record, or that they don’t know already at least some of the players.” Hospitals looking to hire a private company have to exercise caution, particularly if the company is trying to break into a new region where it isn’t known.
“Those hospitals and healthcare systems just have to be really careful who they’re signing contracts with,” he said. “It’s no different than anything else we do. You just have to know who your partners are, and what drives them and where they stand on important issues.”
Executives say patient care is not at risk, even as consolidation continues. “With or without competition, we are relentlessly trying to improve our approach to patient care, our performance, and our hospital partnerships,” Cogent HMG’s Dr. Houff says.
Money Talks
It doesn’t appear that more hospitalist companies are planning to go public—at least for now.
The largest privately held company, Cogent HMG, is not planning an initial public offering anytime soon, Dr. Houff says. The company’s goal is to “continue investing in smart growth to capture more of the hospital medicine market, expand offerings to our existing hospital clients, and provide additional support to our clinical teams on the ground,” he says. “We have a strong capital partner to help us in that effort and are not looking at the public markets at this time.”
Taking on stockholders is a tricky business—one that requires careful planning and a willingness from practice leaders and administrators to relinquish some autonomy to outside interests. And then there are the financial requirements.
“They’ve really got to be able to produce some serious revenue in order for somebody to be willing to put some money into them,” says Mark Hamm, CEO of EmCare Inpatient Services.
The lure of working for a private hospitalist company promises to continue to be an attractive one. Some are drawn by the leadership possibilities—those who “aspire to be the true alpha doctor,” as IPC’s Taylor puts it. Others are drawn by the stability of a larger company.
There also is flexibility in location, Dr. Holman notes.
“Now, with Cogent HMG, [hospitalists] have even more choices in terms of relocating within the same company,” he says. “So they can keep all of the benefits, keep all of the knowledge and familiarity of the system and philosophy of care that we employ, and just be able to transfer.”
continued below...
Dr. Houff says the majority of newly recruited physicians are coming out of residency but that the company is attracting physicians in the middle of their careers, along with physicians having backgrounds beyond internal medicine.
In Tallahassee at Capital Regional, Dr. Geers says that he feels there is support from the company that can protect his job quality, with “a little bit more room to negotiate with the hospital if the hospital wants us to take on new responsibilities.
“Whereas if we worked directly for the hospital, I don’t think we’d have much say in the matter,” he says.
He also says he is happy with the predictable schedule; he’s responsible for 7 a.m. to 7 p.m. and nothing more.
“If you’re finished rounding and you’ve seen all your patients and tied up all your loose ends, you’re not always there till 7 p.m.,” he points out. “Sometimes you can leave a little early....Once 7 p.m. comes, you’re not going to get paged in the middle of the night.”
Thomas R. Collins is a freelance medical writer based in Florida.
William Geers, MD, finished up his residency in 2007, then went to work for a close-knit emergency-medicine group of about 25 doctors in Daytona Beach, Fla.
“Everybody was pretty tight,” he says of his first job.
He had met his wife in residency in Daytona, but after a while, they figured it was time for a change. “We’d been in Daytona for about six years and were ready to go try someplace different,” Dr. Geers says. “Tallahassee seemed like a good match because that’s kind of right in between our families.”
He soon landed a hospitalist job at Capital Regional Medical Center, and he suddenly was a part of EmCare, one of the biggest corporations in the emergency-medicine field and, more recently, in the field of hospital medicine. EmCare provides doctors to about 400 hospitals nationwide.
Dr. Geers said the corporate affiliation didn’t factor into his decision, adding that he took more of a traditional approach when choosing a new job.
“At the time, this program was a little bit smaller, which I liked,” says Dr. Geers, who also looked at the city’s other hospital, Tallahassee Memorial. “I met some of the physicians over here. I liked them.”
But he has noticed perks.
“I think we have some advantages working with EmCare in that we do have a pretty big group that’s backing us,” he explains. “I feel a little more secure with issues like malpractice. If things like that ever come up, I really feel like I have a lot of support with EmCare.”
With the corporate presence on the rise in HM, more and more hospitalists are entering the ranks of large companies. Some are doing so straight out of residency. Some are giving up their private practices and selling them to corporations looking to expand.
Corporations that provide hospitalists to hospitals are getting ever bigger, using sophisticated infrastructure and economies of scale, they say, to make life easier for the people who work for them, allowing the hospitalists to focus on patient care. Their efficiencies are attractive to hospitals looking to simplify.
Three years ago, North Hollywood, Calif.-based IPC: The Hospitalist Company became a publicly traded company. Its stock price has more than doubled since then.
In July, Eagle Hospital Physicians acquired North Carolina-based PrimeDoc and its 100 doctors covering seven hospitals. Similar acquisitions by larger corporations have become almost weekly news.
And, probably most significantly, Cogent Healthcare recently completed a merger with Hospitalists Management Group, a union of two of the biggest hospitalist companies in the U.S. The new company, Cogent HMG, now includes a corps of 1,000 doctors, nurses, and physician assistants (PAs), with client hospitals in 28 states.
Cogent had clients that were medium to large in size, generally in more urban areas but scattered geographically. HMG mostly served small- to medium-sized hospitals with densities in certain regions. With the merger came a recognition that the larger a company becomes, the greater the opportunity for efficiency and better services, says Rusty Holman, MD, MHM, chief clinical officer of the new company.
“The real value out of bringing these two companies together is bringing the best of different worlds together, creating new products and services for hospitals that don’t exist today, and to be able to serve a broader customer base,” says Dr. Holman, a former SHM president. “It’s also to leverage some of the infrastructure that has been built over a greater number of programs and hospitals to gain efficiency and scale that way. So that is the primary focus of the integration today.”
Cogent HMG CEO Steve Houff, MD, says the merger will mean investment in clinical support, physician recruiting, and technology, and will benefit patients and hospital partners alike.
“Both Cogent and HMG have a track record for delivering improvements in clinical quality and patient satisfaction at each of the hospitals we serve. The plan is for that to continue on a broader scale,” he wrote in an email to The Hospitalist.

—R. Jeffrey Taylor, president, chief operating officer, IPC: The Hospitalist Company, North Hollywood, Calif.
The Good, the Bad, the Oligopoly
The average size of a hospitalist group in the U.S. is about 10 full-time equivalents, according to recent survey data from SHM and MGMA. With the swelling of the size of HM’s biggest corporate players comes the question of how far the coalescing will go: Will most patient care eventually be provided by only a few groups?
R. Jeffrey Taylor, IPC’s president and chief operating officer, says the mergers and acquisitions will continue, but he doesn’t see a day when there will be just a few titans ruling all.
“I do think there will be more consolidation going forward than there is now, but I don’t see a future in which there are, you know, two or three groups that completely dominate the landscape,” he says. “There’s always that concern that that’s going to happen in the hospital industry, or that’s going to happen with payors. And there are always new entrants.”
For all the movement toward bigger companies, “this is still an unconsolidated industry,” and new physician practices will always continue to be formed, he says.
“We’re the largest group, and we’re maybe 3 1/2 percent of all the hospitals in the country. I wouldn’t consider this, today, a terribly consolidated industry,” he adds. “I do think it will move in that direction. I just don’t think it will get all the way there, because of the sort of private, entrepreneurial, independent spirit that’s common among physicians.”
Mike Tarwater, a board member of the American Hospital Association, says private hospitalist providers will only be an alternative to—and not a replacement provider for—large, self-contained systems like the Carolinas Medical Center (CMC), for which he serves as CEO. The health system has a wide spectrum of facilities—from large, urban academic centers like the 874-bed medical center in Charlotte, N.C., to 52-bed Anson Community Hospital in Wadesboro, N.C., population 5,780.
“As a system, we have the wherewithal and the recruiting expertise, and, with 1,700 physician associates across the system, we’ve kind of got critical mass,” Tarwater says. “So we will be an alternative to that in our region.”
Frank Michota, MD, FHM, director of academic affairs in the Department of Hospital Medicine at The Cleveland Clinic, says that the extensive training programs of many of the larger hospitalist groups (e.g. Cogent Academy, IPC’s extensive onboarding process and leadership conferences) could be a very good thing for the field.
“I have always thought that companies like Cogent did a very nice job in orienting their hospitalists to the patient-care goals and the process variables that were being measured,” Dr. Michota says. “I think that by making an even larger group, they have the opportunity to continue to standardize the approach to hospital care so that one hospitalist equals one hospitalist equals one hospitalist. I think that’s a positive.”
The flip side, though, is that anything that might be done wrong would be magnified in such a system.
“I think that there are some dangers in how these large companies will incentivize their hospitalists,” he adds. “If they are consistent from hospitalist to hospitalist, but if there’s a perverse adverse effect from one of their financial incentives, it will be carried out across a lot of hospitals all at the same time. “But I think it’s a little early to tell what the impact of this might be. But, at least for right now, it’s actually a positive thing because it standardizes the hospitalist.”
Tarwater says that even when larger corporations buy smaller practices, familiarity tends to remain.
“Most of what I have seen are existing groups that join through merger or acquisition, and so we already have experience with the doctors, we already have long-standing relationships with the doctors,” he says. “I think any health system or hospital would be reticent to sign up with somebody that they’ve never heard of, that doesn’t have a track record, or that they don’t know already at least some of the players.” Hospitals looking to hire a private company have to exercise caution, particularly if the company is trying to break into a new region where it isn’t known.
“Those hospitals and healthcare systems just have to be really careful who they’re signing contracts with,” he said. “It’s no different than anything else we do. You just have to know who your partners are, and what drives them and where they stand on important issues.”
Executives say patient care is not at risk, even as consolidation continues. “With or without competition, we are relentlessly trying to improve our approach to patient care, our performance, and our hospital partnerships,” Cogent HMG’s Dr. Houff says.
Money Talks
It doesn’t appear that more hospitalist companies are planning to go public—at least for now.
The largest privately held company, Cogent HMG, is not planning an initial public offering anytime soon, Dr. Houff says. The company’s goal is to “continue investing in smart growth to capture more of the hospital medicine market, expand offerings to our existing hospital clients, and provide additional support to our clinical teams on the ground,” he says. “We have a strong capital partner to help us in that effort and are not looking at the public markets at this time.”
Taking on stockholders is a tricky business—one that requires careful planning and a willingness from practice leaders and administrators to relinquish some autonomy to outside interests. And then there are the financial requirements.
“They’ve really got to be able to produce some serious revenue in order for somebody to be willing to put some money into them,” says Mark Hamm, CEO of EmCare Inpatient Services.
The lure of working for a private hospitalist company promises to continue to be an attractive one. Some are drawn by the leadership possibilities—those who “aspire to be the true alpha doctor,” as IPC’s Taylor puts it. Others are drawn by the stability of a larger company.
There also is flexibility in location, Dr. Holman notes.
“Now, with Cogent HMG, [hospitalists] have even more choices in terms of relocating within the same company,” he says. “So they can keep all of the benefits, keep all of the knowledge and familiarity of the system and philosophy of care that we employ, and just be able to transfer.”
continued below...
Dr. Houff says the majority of newly recruited physicians are coming out of residency but that the company is attracting physicians in the middle of their careers, along with physicians having backgrounds beyond internal medicine.
In Tallahassee at Capital Regional, Dr. Geers says that he feels there is support from the company that can protect his job quality, with “a little bit more room to negotiate with the hospital if the hospital wants us to take on new responsibilities.
“Whereas if we worked directly for the hospital, I don’t think we’d have much say in the matter,” he says.
He also says he is happy with the predictable schedule; he’s responsible for 7 a.m. to 7 p.m. and nothing more.
“If you’re finished rounding and you’ve seen all your patients and tied up all your loose ends, you’re not always there till 7 p.m.,” he points out. “Sometimes you can leave a little early....Once 7 p.m. comes, you’re not going to get paged in the middle of the night.”
Thomas R. Collins is a freelance medical writer based in Florida.
Modest Gains
Hospitalists are earning a little more, working a little harder, and are less likely to switch jobs or careers, according to the 2011 State of Hospital Medicine report. The annual report, based on data collected jointly by SHM and the Medical Group Management Association (MGMA), offers more than 10,000 compensation and productivity data points for all types of hospitalists, including, for the first time, an exclusive look at academic hospitalists.
As previously reported, median adult hospitalist compensation increased to $220,619, a 2.6% increase from the $215,000 figure reported last year. “I think that’s a reflection of the market and demand for hospitalists, and the value that hospitals and other healthcare payors see that hospitalists bring,” says William “Tex” Landis, MD, FHM, medical director of Wellspan Hospitalists in York, Pa., and chair of SHM’s Practice Analysis Committee.
The compensation increases for hospitalists reported in the SHM-MGMA survey mirror results in other recent surveys. The 2011 Medical Group Compensation and Financial Survey, produced by the American Medical Group Association, found the overall average increase in physician compensation was 2.4%. Primary-care physicians (PCPs) reported a 2.6% increase in 2010, while the “hospitalist-internal medicine” category saw one of the steepest increases at 6.29%, according to the report (www.amga.org).
According to the 2011 MedScape Physician Compensation Report, 27% of the more than 15,000 physicians surveyed said their income increased from 2009 to 2010, whereas 50% said they saw no change. About 23% reported a decline in income, the report showed (www.medscape.com/features/slideshow/compensation/2011/).
Continuing a decadelong trend, the SHM-MGMA report shows hospitalists in the South make the most (median compensation $247,000, up from $235,701 in 2010) and hospitalists in the East ($212,000, up from $205,000 in 2010) lag behind the other regions (see Table 1).
The Hospitalist spoke to five members of SHM’s Practice Analysis Committee (PAC) about the survey results, and each points to continued nationwide demand as the driver of increased compensation. However, the committee also cautions HM groups and directors to be leery of trending this data, as the report is based on a volunteer survey, the survey population changes year to year, and only two years of identical survey data are available.
“As hospital medicine continues to grow, the hospitals become so dependent on the services that the hospitalists provide,” says PAC member Scarlett Blue, RN-BC, MSN, NE-BC, CPHQ, vice president of quality and clinical development for Eagle Hospital Physicians. “[Hospitals] know HM is critical and…I think that hospitalists demonstrate tremendous value, which the hospitals and the management groups recognize.”
The 2011 report, available Sept. 14, compiled data about 4,633 hospitalists in 412 groups. Eighty-five percent of the respondents classified themselves as “adult” hospitalists, 5% as “pediatric” hospitalists, and 10% as both adult and pediatric. Of note, this is the first time SHM has produced compensation and productivity data in consecutive years. In addition to compensation, the survey provides drill-down capability on productivity and reimbursement metrics, along with specific data regarding night coverage arrangements (see “Survey Insights,”), financial support payments, physician turnover, and, for the first time, a look at nonphysician providers (NPPs) in HM practice (see “Nonphysician Provider Data Available for First Time,”).
continued below...
Hospitalist Productivity
Although hospitalists are earning more, the 2011 report also shows they are producing more work relative-value units (wRVUs) than ever before. The median physician wRVU rate annually for 2011 was 4,166, a 1.4% increase over the 2010 figure.
Hospitalists in the Eastern and Midwest regions reported relatively unchanged wRVUs when compared with 2010 figures. The Southern region, which outdistanced the other regions by more than 800 wRVUs per physician, reported a 6.7% decrease in wRVUs—4,931 in 2011 compared with 5,287 in 2010. On the flipside, the Western region showed a 11.9% increase per physician (see Table 2, left).
PAC committee members agree the wRVU variance between regions is difficult to explain, but most agree the slight year-over-year increase in productivity shows the specialty is stabilizing in terms of what productivity is expected from the average hospitalist.
“Maybe it is an indication that the field is maturing and we’re settling in at some data points that we can now potentially put some stock into,” says Beth E. Hawley, MBA, FACHE, senior vice president of The Cogent Group, a consulting division of Brentwood, Tenn.-based Cogent HMG. “Before, [the figures] changed dramatically from survey to survey; I think we’re seeing more stability now from last year to this year.

—Beth E. Hawley, MBA, FACHE, senior vice president, The Cogent Group, Brentwood, Tenn., SHM Practice Analysis Committee member
“It’s helpful to be able to sort [wRVUs] by employment model, by region, by large and small hospital. We can really get some better benchmarks, in terms of what should be the expectation.”
Chris Frost, MD, FHM, national medical director of hospital medicine services for HCA, says he remains somewhat hesitant to say HM is “settling into a number” for expected wRVUs, as he routinely hears from hospital administrators looking for “additional efficiencies that we can put in place to allow and position the hospitalists to be more productive” while maintaining a high quality care delivery model. He’s also puzzled by the geographic discrepancies. “I just have to scratch my head. I haven’t entirely figured that out yet,” he says.
That said, Dr. Frost agrees the wRVU benchmarks are the most useful in terms of “billable productivity. But I also would like to see—or believe—one of the reasons compensation is going up and the work RVU is flat is hospitalists are being recognized for their value in other arenas, as it relates to the transition from the fee-for-service to pay-for-value-type models, championing effective transitions of care, leading process improvement teams, etc. Those things can’t, or don’t, necessarily lend themselves well to a work RVU equivalent.”
The Buzz: Financial Support
First reported at HM11 in May, the survey shows hospitalist support payments increased more than 39%, to $136,403 per FTE hospitalist in 2011 from $98,253 in 2010. PAC members and other hospitalist experts in practice management attribute the startling increase in support payments to more accurate reporting. Others note that the rise in support payments could be attributed to the decline in collection of professional fees, a direct result of the economic downturn.
And, according to Dr. Landis, hospitals are more willing today to fund hospitalist services than ever before.
“I think [the rise in HM support] payments is because of the evolution of healthcare in the hospital as a whole,” he says. “Hospitals are looking to hospitalists to help them provide the care that that patients and families need, expect, and want. And we’re stepping up to the plate to do it, and they’re paying us to do it. I think that’s the story.
“If you want to tie that with why there’s $136,000 going per hospitalist, [it’s] because they want us there for that rapid-response team, and rapid-response teams don’t generate a lot of RVUs necessarily.”
Hawley, the consultant, agrees the percentage increase in financial support is somewhat shocking, but she isn’t surprised by the median figure. She knows hospitals are asking more of HM groups, and all of those value-added, non-billable tasks and responsibilities come with a cost.
“It’s not totally surprising, but it’s a big leap,” she says. “I would say at least some of that has to be driven by the fact that we have hospitals and integrated delivery systems employing more and more of these physicians that responded to this survey, as well as physicians’ practices are perhaps becoming more educated as to what their finances are. There’s a better understanding of allocation of overhead, of billing fees, all of those things that go into a practice where … that may not have been as clear to folks when HM was a bit younger.”
Downward Trend: Hospitalist Turnover
In what some are calling a positive sign for the specialty, hospitalist turnover dropped to 8% in 2011, compared with a 14% turnover rate among hospitalist groups serving adults in 2010 (see Figure 1). Rates declined for both hospital-owned and non-hospital-owned groups, according to the report. Hawley, the consultant, says the decline in physician movement is “consistent” with what she sees in daily interactions with HM groups. Moreover, she considers that trend to be just as important as the overall decline in hospitalist turnover.
“[The survey indicates] more physicians are employed by a hospital or an integrated hospital system. With that, what we’re seeing on the consulting side is there are certain benefits with being employed by a hospital or healthcare system, in terms of retirement plans, things that [hospitalists] may not find as rich in a private practice or with a multispecialty group,” she says. When a hospitalist becomes employed with a hospital system, she hears them say, ‘This is where I want to live and raise my family.’ People choose this type of employment for a reason.”
That has been the experience of PAC member Tierza Stephan, MD, FACP, SFHM, who supervises more than 135 hospitalists as hospitalist district medical director for Minneapolis-based Allina Medical Clinic. Dr. Stephan currently has 15 openings at six of the eight HM groups she directs. Even so, she admits that her programs have been “blessed with low turnover.” The upshot: “We use that when we talk to the C-suite,” she says. “It’s way more costly to have one physician come in, train them, and then have them leave. The low turnover rate gets factored into the cost of what they’re paying.”
Dr. Frost says HM is becoming “less and less a stopover specialty,” as more physicians adopt HM as their career. Dr. Landis says that, although just two consecutive years of data are available, the decline in turnover rate is a good sign for the specialty.
“I think many of us suspect that as the hospitalist movement matures that there hopefully will be a stabilization of the turnover rate,” he says. “Hospitalists tend to be very portable, and when there’s a lot of open jobs and only a few hospitalists, there can be even more and more [turnover]. Typically, someone gets themselves into a situation, they feel that they’re overworked, underpaid, underappreciated, they’re going to look for another job, and those jobs are out there. As the market stabilizes, there will probably be less and less moving around.”
Interactive regional survey breakdowns
Jason Carris is editor of The Hospitalist.
Hospitalists are earning a little more, working a little harder, and are less likely to switch jobs or careers, according to the 2011 State of Hospital Medicine report. The annual report, based on data collected jointly by SHM and the Medical Group Management Association (MGMA), offers more than 10,000 compensation and productivity data points for all types of hospitalists, including, for the first time, an exclusive look at academic hospitalists.
As previously reported, median adult hospitalist compensation increased to $220,619, a 2.6% increase from the $215,000 figure reported last year. “I think that’s a reflection of the market and demand for hospitalists, and the value that hospitals and other healthcare payors see that hospitalists bring,” says William “Tex” Landis, MD, FHM, medical director of Wellspan Hospitalists in York, Pa., and chair of SHM’s Practice Analysis Committee.
The compensation increases for hospitalists reported in the SHM-MGMA survey mirror results in other recent surveys. The 2011 Medical Group Compensation and Financial Survey, produced by the American Medical Group Association, found the overall average increase in physician compensation was 2.4%. Primary-care physicians (PCPs) reported a 2.6% increase in 2010, while the “hospitalist-internal medicine” category saw one of the steepest increases at 6.29%, according to the report (www.amga.org).
According to the 2011 MedScape Physician Compensation Report, 27% of the more than 15,000 physicians surveyed said their income increased from 2009 to 2010, whereas 50% said they saw no change. About 23% reported a decline in income, the report showed (www.medscape.com/features/slideshow/compensation/2011/).
Continuing a decadelong trend, the SHM-MGMA report shows hospitalists in the South make the most (median compensation $247,000, up from $235,701 in 2010) and hospitalists in the East ($212,000, up from $205,000 in 2010) lag behind the other regions (see Table 1).
The Hospitalist spoke to five members of SHM’s Practice Analysis Committee (PAC) about the survey results, and each points to continued nationwide demand as the driver of increased compensation. However, the committee also cautions HM groups and directors to be leery of trending this data, as the report is based on a volunteer survey, the survey population changes year to year, and only two years of identical survey data are available.
“As hospital medicine continues to grow, the hospitals become so dependent on the services that the hospitalists provide,” says PAC member Scarlett Blue, RN-BC, MSN, NE-BC, CPHQ, vice president of quality and clinical development for Eagle Hospital Physicians. “[Hospitals] know HM is critical and…I think that hospitalists demonstrate tremendous value, which the hospitals and the management groups recognize.”
The 2011 report, available Sept. 14, compiled data about 4,633 hospitalists in 412 groups. Eighty-five percent of the respondents classified themselves as “adult” hospitalists, 5% as “pediatric” hospitalists, and 10% as both adult and pediatric. Of note, this is the first time SHM has produced compensation and productivity data in consecutive years. In addition to compensation, the survey provides drill-down capability on productivity and reimbursement metrics, along with specific data regarding night coverage arrangements (see “Survey Insights,”), financial support payments, physician turnover, and, for the first time, a look at nonphysician providers (NPPs) in HM practice (see “Nonphysician Provider Data Available for First Time,”).
continued below...
Hospitalist Productivity
Although hospitalists are earning more, the 2011 report also shows they are producing more work relative-value units (wRVUs) than ever before. The median physician wRVU rate annually for 2011 was 4,166, a 1.4% increase over the 2010 figure.
Hospitalists in the Eastern and Midwest regions reported relatively unchanged wRVUs when compared with 2010 figures. The Southern region, which outdistanced the other regions by more than 800 wRVUs per physician, reported a 6.7% decrease in wRVUs—4,931 in 2011 compared with 5,287 in 2010. On the flipside, the Western region showed a 11.9% increase per physician (see Table 2, left).
PAC committee members agree the wRVU variance between regions is difficult to explain, but most agree the slight year-over-year increase in productivity shows the specialty is stabilizing in terms of what productivity is expected from the average hospitalist.
“Maybe it is an indication that the field is maturing and we’re settling in at some data points that we can now potentially put some stock into,” says Beth E. Hawley, MBA, FACHE, senior vice president of The Cogent Group, a consulting division of Brentwood, Tenn.-based Cogent HMG. “Before, [the figures] changed dramatically from survey to survey; I think we’re seeing more stability now from last year to this year.

—Beth E. Hawley, MBA, FACHE, senior vice president, The Cogent Group, Brentwood, Tenn., SHM Practice Analysis Committee member
“It’s helpful to be able to sort [wRVUs] by employment model, by region, by large and small hospital. We can really get some better benchmarks, in terms of what should be the expectation.”
Chris Frost, MD, FHM, national medical director of hospital medicine services for HCA, says he remains somewhat hesitant to say HM is “settling into a number” for expected wRVUs, as he routinely hears from hospital administrators looking for “additional efficiencies that we can put in place to allow and position the hospitalists to be more productive” while maintaining a high quality care delivery model. He’s also puzzled by the geographic discrepancies. “I just have to scratch my head. I haven’t entirely figured that out yet,” he says.
That said, Dr. Frost agrees the wRVU benchmarks are the most useful in terms of “billable productivity. But I also would like to see—or believe—one of the reasons compensation is going up and the work RVU is flat is hospitalists are being recognized for their value in other arenas, as it relates to the transition from the fee-for-service to pay-for-value-type models, championing effective transitions of care, leading process improvement teams, etc. Those things can’t, or don’t, necessarily lend themselves well to a work RVU equivalent.”
The Buzz: Financial Support
First reported at HM11 in May, the survey shows hospitalist support payments increased more than 39%, to $136,403 per FTE hospitalist in 2011 from $98,253 in 2010. PAC members and other hospitalist experts in practice management attribute the startling increase in support payments to more accurate reporting. Others note that the rise in support payments could be attributed to the decline in collection of professional fees, a direct result of the economic downturn.
And, according to Dr. Landis, hospitals are more willing today to fund hospitalist services than ever before.
“I think [the rise in HM support] payments is because of the evolution of healthcare in the hospital as a whole,” he says. “Hospitals are looking to hospitalists to help them provide the care that that patients and families need, expect, and want. And we’re stepping up to the plate to do it, and they’re paying us to do it. I think that’s the story.
“If you want to tie that with why there’s $136,000 going per hospitalist, [it’s] because they want us there for that rapid-response team, and rapid-response teams don’t generate a lot of RVUs necessarily.”
Hawley, the consultant, agrees the percentage increase in financial support is somewhat shocking, but she isn’t surprised by the median figure. She knows hospitals are asking more of HM groups, and all of those value-added, non-billable tasks and responsibilities come with a cost.
“It’s not totally surprising, but it’s a big leap,” she says. “I would say at least some of that has to be driven by the fact that we have hospitals and integrated delivery systems employing more and more of these physicians that responded to this survey, as well as physicians’ practices are perhaps becoming more educated as to what their finances are. There’s a better understanding of allocation of overhead, of billing fees, all of those things that go into a practice where … that may not have been as clear to folks when HM was a bit younger.”
Downward Trend: Hospitalist Turnover
In what some are calling a positive sign for the specialty, hospitalist turnover dropped to 8% in 2011, compared with a 14% turnover rate among hospitalist groups serving adults in 2010 (see Figure 1). Rates declined for both hospital-owned and non-hospital-owned groups, according to the report. Hawley, the consultant, says the decline in physician movement is “consistent” with what she sees in daily interactions with HM groups. Moreover, she considers that trend to be just as important as the overall decline in hospitalist turnover.
“[The survey indicates] more physicians are employed by a hospital or an integrated hospital system. With that, what we’re seeing on the consulting side is there are certain benefits with being employed by a hospital or healthcare system, in terms of retirement plans, things that [hospitalists] may not find as rich in a private practice or with a multispecialty group,” she says. When a hospitalist becomes employed with a hospital system, she hears them say, ‘This is where I want to live and raise my family.’ People choose this type of employment for a reason.”
That has been the experience of PAC member Tierza Stephan, MD, FACP, SFHM, who supervises more than 135 hospitalists as hospitalist district medical director for Minneapolis-based Allina Medical Clinic. Dr. Stephan currently has 15 openings at six of the eight HM groups she directs. Even so, she admits that her programs have been “blessed with low turnover.” The upshot: “We use that when we talk to the C-suite,” she says. “It’s way more costly to have one physician come in, train them, and then have them leave. The low turnover rate gets factored into the cost of what they’re paying.”
Dr. Frost says HM is becoming “less and less a stopover specialty,” as more physicians adopt HM as their career. Dr. Landis says that, although just two consecutive years of data are available, the decline in turnover rate is a good sign for the specialty.
“I think many of us suspect that as the hospitalist movement matures that there hopefully will be a stabilization of the turnover rate,” he says. “Hospitalists tend to be very portable, and when there’s a lot of open jobs and only a few hospitalists, there can be even more and more [turnover]. Typically, someone gets themselves into a situation, they feel that they’re overworked, underpaid, underappreciated, they’re going to look for another job, and those jobs are out there. As the market stabilizes, there will probably be less and less moving around.”
Interactive regional survey breakdowns
Jason Carris is editor of The Hospitalist.
Hospitalists are earning a little more, working a little harder, and are less likely to switch jobs or careers, according to the 2011 State of Hospital Medicine report. The annual report, based on data collected jointly by SHM and the Medical Group Management Association (MGMA), offers more than 10,000 compensation and productivity data points for all types of hospitalists, including, for the first time, an exclusive look at academic hospitalists.
As previously reported, median adult hospitalist compensation increased to $220,619, a 2.6% increase from the $215,000 figure reported last year. “I think that’s a reflection of the market and demand for hospitalists, and the value that hospitals and other healthcare payors see that hospitalists bring,” says William “Tex” Landis, MD, FHM, medical director of Wellspan Hospitalists in York, Pa., and chair of SHM’s Practice Analysis Committee.
The compensation increases for hospitalists reported in the SHM-MGMA survey mirror results in other recent surveys. The 2011 Medical Group Compensation and Financial Survey, produced by the American Medical Group Association, found the overall average increase in physician compensation was 2.4%. Primary-care physicians (PCPs) reported a 2.6% increase in 2010, while the “hospitalist-internal medicine” category saw one of the steepest increases at 6.29%, according to the report (www.amga.org).
According to the 2011 MedScape Physician Compensation Report, 27% of the more than 15,000 physicians surveyed said their income increased from 2009 to 2010, whereas 50% said they saw no change. About 23% reported a decline in income, the report showed (www.medscape.com/features/slideshow/compensation/2011/).
Continuing a decadelong trend, the SHM-MGMA report shows hospitalists in the South make the most (median compensation $247,000, up from $235,701 in 2010) and hospitalists in the East ($212,000, up from $205,000 in 2010) lag behind the other regions (see Table 1).
The Hospitalist spoke to five members of SHM’s Practice Analysis Committee (PAC) about the survey results, and each points to continued nationwide demand as the driver of increased compensation. However, the committee also cautions HM groups and directors to be leery of trending this data, as the report is based on a volunteer survey, the survey population changes year to year, and only two years of identical survey data are available.
“As hospital medicine continues to grow, the hospitals become so dependent on the services that the hospitalists provide,” says PAC member Scarlett Blue, RN-BC, MSN, NE-BC, CPHQ, vice president of quality and clinical development for Eagle Hospital Physicians. “[Hospitals] know HM is critical and…I think that hospitalists demonstrate tremendous value, which the hospitals and the management groups recognize.”
The 2011 report, available Sept. 14, compiled data about 4,633 hospitalists in 412 groups. Eighty-five percent of the respondents classified themselves as “adult” hospitalists, 5% as “pediatric” hospitalists, and 10% as both adult and pediatric. Of note, this is the first time SHM has produced compensation and productivity data in consecutive years. In addition to compensation, the survey provides drill-down capability on productivity and reimbursement metrics, along with specific data regarding night coverage arrangements (see “Survey Insights,”), financial support payments, physician turnover, and, for the first time, a look at nonphysician providers (NPPs) in HM practice (see “Nonphysician Provider Data Available for First Time,”).
continued below...
Hospitalist Productivity
Although hospitalists are earning more, the 2011 report also shows they are producing more work relative-value units (wRVUs) than ever before. The median physician wRVU rate annually for 2011 was 4,166, a 1.4% increase over the 2010 figure.
Hospitalists in the Eastern and Midwest regions reported relatively unchanged wRVUs when compared with 2010 figures. The Southern region, which outdistanced the other regions by more than 800 wRVUs per physician, reported a 6.7% decrease in wRVUs—4,931 in 2011 compared with 5,287 in 2010. On the flipside, the Western region showed a 11.9% increase per physician (see Table 2, left).
PAC committee members agree the wRVU variance between regions is difficult to explain, but most agree the slight year-over-year increase in productivity shows the specialty is stabilizing in terms of what productivity is expected from the average hospitalist.
“Maybe it is an indication that the field is maturing and we’re settling in at some data points that we can now potentially put some stock into,” says Beth E. Hawley, MBA, FACHE, senior vice president of The Cogent Group, a consulting division of Brentwood, Tenn.-based Cogent HMG. “Before, [the figures] changed dramatically from survey to survey; I think we’re seeing more stability now from last year to this year.

—Beth E. Hawley, MBA, FACHE, senior vice president, The Cogent Group, Brentwood, Tenn., SHM Practice Analysis Committee member
“It’s helpful to be able to sort [wRVUs] by employment model, by region, by large and small hospital. We can really get some better benchmarks, in terms of what should be the expectation.”
Chris Frost, MD, FHM, national medical director of hospital medicine services for HCA, says he remains somewhat hesitant to say HM is “settling into a number” for expected wRVUs, as he routinely hears from hospital administrators looking for “additional efficiencies that we can put in place to allow and position the hospitalists to be more productive” while maintaining a high quality care delivery model. He’s also puzzled by the geographic discrepancies. “I just have to scratch my head. I haven’t entirely figured that out yet,” he says.
That said, Dr. Frost agrees the wRVU benchmarks are the most useful in terms of “billable productivity. But I also would like to see—or believe—one of the reasons compensation is going up and the work RVU is flat is hospitalists are being recognized for their value in other arenas, as it relates to the transition from the fee-for-service to pay-for-value-type models, championing effective transitions of care, leading process improvement teams, etc. Those things can’t, or don’t, necessarily lend themselves well to a work RVU equivalent.”
The Buzz: Financial Support
First reported at HM11 in May, the survey shows hospitalist support payments increased more than 39%, to $136,403 per FTE hospitalist in 2011 from $98,253 in 2010. PAC members and other hospitalist experts in practice management attribute the startling increase in support payments to more accurate reporting. Others note that the rise in support payments could be attributed to the decline in collection of professional fees, a direct result of the economic downturn.
And, according to Dr. Landis, hospitals are more willing today to fund hospitalist services than ever before.
“I think [the rise in HM support] payments is because of the evolution of healthcare in the hospital as a whole,” he says. “Hospitals are looking to hospitalists to help them provide the care that that patients and families need, expect, and want. And we’re stepping up to the plate to do it, and they’re paying us to do it. I think that’s the story.
“If you want to tie that with why there’s $136,000 going per hospitalist, [it’s] because they want us there for that rapid-response team, and rapid-response teams don’t generate a lot of RVUs necessarily.”
Hawley, the consultant, agrees the percentage increase in financial support is somewhat shocking, but she isn’t surprised by the median figure. She knows hospitals are asking more of HM groups, and all of those value-added, non-billable tasks and responsibilities come with a cost.
“It’s not totally surprising, but it’s a big leap,” she says. “I would say at least some of that has to be driven by the fact that we have hospitals and integrated delivery systems employing more and more of these physicians that responded to this survey, as well as physicians’ practices are perhaps becoming more educated as to what their finances are. There’s a better understanding of allocation of overhead, of billing fees, all of those things that go into a practice where … that may not have been as clear to folks when HM was a bit younger.”
Downward Trend: Hospitalist Turnover
In what some are calling a positive sign for the specialty, hospitalist turnover dropped to 8% in 2011, compared with a 14% turnover rate among hospitalist groups serving adults in 2010 (see Figure 1). Rates declined for both hospital-owned and non-hospital-owned groups, according to the report. Hawley, the consultant, says the decline in physician movement is “consistent” with what she sees in daily interactions with HM groups. Moreover, she considers that trend to be just as important as the overall decline in hospitalist turnover.
“[The survey indicates] more physicians are employed by a hospital or an integrated hospital system. With that, what we’re seeing on the consulting side is there are certain benefits with being employed by a hospital or healthcare system, in terms of retirement plans, things that [hospitalists] may not find as rich in a private practice or with a multispecialty group,” she says. When a hospitalist becomes employed with a hospital system, she hears them say, ‘This is where I want to live and raise my family.’ People choose this type of employment for a reason.”
That has been the experience of PAC member Tierza Stephan, MD, FACP, SFHM, who supervises more than 135 hospitalists as hospitalist district medical director for Minneapolis-based Allina Medical Clinic. Dr. Stephan currently has 15 openings at six of the eight HM groups she directs. Even so, she admits that her programs have been “blessed with low turnover.” The upshot: “We use that when we talk to the C-suite,” she says. “It’s way more costly to have one physician come in, train them, and then have them leave. The low turnover rate gets factored into the cost of what they’re paying.”
Dr. Frost says HM is becoming “less and less a stopover specialty,” as more physicians adopt HM as their career. Dr. Landis says that, although just two consecutive years of data are available, the decline in turnover rate is a good sign for the specialty.
“I think many of us suspect that as the hospitalist movement matures that there hopefully will be a stabilization of the turnover rate,” he says. “Hospitalists tend to be very portable, and when there’s a lot of open jobs and only a few hospitalists, there can be even more and more [turnover]. Typically, someone gets themselves into a situation, they feel that they’re overworked, underpaid, underappreciated, they’re going to look for another job, and those jobs are out there. As the market stabilizes, there will probably be less and less moving around.”
Interactive regional survey breakdowns
Jason Carris is editor of The Hospitalist.
The Bigger Picture
For those of you who were kind enough to pick up my column in The Hospitalist last month (see “A Critical First Step,” p. 56), you spent a few minutes reading my thoughts on the value of hospitalists. I mentioned the fact that the U.S. is moving rapidly toward a value-based system to purchase healthcare and that all healthcare providers, including hospitalists, will be increasingly judged on the value of care they deliver to their patients and the healthcare system. (Remember, value=quality÷cost.)
I believe that the hospitalist programs that are going to be the most successful are those that are able to continually measure their quality and costs, allowing them to make improvements. These are the groups that will understand their own performance well before others make their “value” judgments.
History Lesson
In his famous book “The Wealth of Nations,” 18th-century economist Adam Smith used the example of a pin factory to show how specialization improved human productivity. The process of producing a pin was broken down into many small tasks, each done by a different “specialist.” This resulted not only in increased efficiency, but also increased productivity. The factory was then able to reinvest the profits in even more efficient machinery, which was able to reduce labor costs. The lower cost for pins was a benefit, and it was spread across the entire population.
We have seen similar examples in American healthcare. In many American hospitals, the images of CT scans performed during nighttime hours are transmitted to India and other Asian countries where highly trained radiologists interpret the scans and transmit their interpretations back to the physicians caring for the patients. Like the pin factory workers, these radiologists are specialists with unique skills; they operate specialized machinery to make the system more efficient with resultant lower costs. At the level of the individual patient, this system means getting test results back in a more timely fashion. Increased quality and lower costs: These are high-value providers.
It should be obvious to us that hospitalists are “specialists.” While most hospitalists are trained as general internists, pediatricians, and family physicians, you and I are “specialists” who focus our efforts on the care of hospitalized patients. In the late 1990s, much was made of the fact that hospitalists were able to reduce hospital costs because of decreased length of stay (LOS) for patients, without any adverse effects on clinical outcomes. Today, hospitalists number more than 30,000 nationwide, and virtually all American hospitals with more than 200 beds have hospitalists.
Hospitals hired hospitalists in droves because they were perceived as “inpatient specialists” who were able to reduce the cost of healthcare delivery. Like lower pin costs, this economic benefit was spread across the entire population. Hospitalist “value” went up because of lower costs.
But an interesting thing changed over the past decade: It seems that fewer and fewer people are talking about hospitalists reducing LOS. They just expect it. LOS is raised as an issue only if it goes up. In many hospitals, the budget now takes into account the average LOS based on hospitalist care. (I suspect that in 18th-century Scotland, people also grew accustomed to the lower cost of pins, and grew to expect it, and the cost of pins was raised as an issue only if the price went up.)
But has anyone spoken to the hospitalists? Has anyone asked us about the benefits of our profession? Many of the hospitalists I meet mention “reduction of length of stay” as a main reason to have hospitalists. I think that response was appropriate more often than not over the past 15 years.
But today, ask any hospital administrator that same question, and what do you expect the answer to be? It should not be surprising to hospitalists that most hospitals expect much more from their hospitalist programs than “just reducing the length of stay.” These are the same hospitals that often support—to the tune of more than $100,000 per hospitalist full-time equivalent—your HM program. If your hospitalist program is anything like mine at Beth Israel Deaconess Medical Center in Boston, this represents millions of dollars to the hospitalist program budget.
Increased Pressures
The fact that hospitals’ expectations of hospitalists have changed should not come as a surprise. Americans’ expectations of hospitals have changed markedly over the past 15 years. In the 1990s, when hospital medicine was “born,” there was little mention of quality and patient satisfaction when it came to healthcare. Who would argue that improving quality and patient satisfaction is a bad thing?
Over the past decade, we’ve seen the development of Medicare core measures and the link between patient outcomes and Medicare reimbursement. Hospitals could not have achieved many of their performance improvements without their partnerships with hospitalists.
Hospitals are under increasing pressure to not only decrease costs, but also improve quality. It is understandable that they turn to the “inpatient specialists”—the hospitalists—to help them meet societal expectations. But as hospitalists, this puts pressure on us to continually improve our game—or face the consequences. A pin factory in Scotland can only survive if it produces higher-quality pins at a lower cost than its competitor.
Hospitals and our American healthcare system expect much more today from hospitalists, and they should; patients’ lives are at stake. It should not be a surprise that hospitalist programs that struggle are those that fail to meet expectations. Successful hospitalist programs, the ones that are able to demonstrate their “value,” do so well beyond knowing their patient’s average length of stay.
I am interested in learning about your efforts to demonstrate the “value” of your hospitalist group. Feel free to email me at [email protected].
Dr. Li is president of SHM.
For those of you who were kind enough to pick up my column in The Hospitalist last month (see “A Critical First Step,” p. 56), you spent a few minutes reading my thoughts on the value of hospitalists. I mentioned the fact that the U.S. is moving rapidly toward a value-based system to purchase healthcare and that all healthcare providers, including hospitalists, will be increasingly judged on the value of care they deliver to their patients and the healthcare system. (Remember, value=quality÷cost.)
I believe that the hospitalist programs that are going to be the most successful are those that are able to continually measure their quality and costs, allowing them to make improvements. These are the groups that will understand their own performance well before others make their “value” judgments.
History Lesson
In his famous book “The Wealth of Nations,” 18th-century economist Adam Smith used the example of a pin factory to show how specialization improved human productivity. The process of producing a pin was broken down into many small tasks, each done by a different “specialist.” This resulted not only in increased efficiency, but also increased productivity. The factory was then able to reinvest the profits in even more efficient machinery, which was able to reduce labor costs. The lower cost for pins was a benefit, and it was spread across the entire population.
We have seen similar examples in American healthcare. In many American hospitals, the images of CT scans performed during nighttime hours are transmitted to India and other Asian countries where highly trained radiologists interpret the scans and transmit their interpretations back to the physicians caring for the patients. Like the pin factory workers, these radiologists are specialists with unique skills; they operate specialized machinery to make the system more efficient with resultant lower costs. At the level of the individual patient, this system means getting test results back in a more timely fashion. Increased quality and lower costs: These are high-value providers.
It should be obvious to us that hospitalists are “specialists.” While most hospitalists are trained as general internists, pediatricians, and family physicians, you and I are “specialists” who focus our efforts on the care of hospitalized patients. In the late 1990s, much was made of the fact that hospitalists were able to reduce hospital costs because of decreased length of stay (LOS) for patients, without any adverse effects on clinical outcomes. Today, hospitalists number more than 30,000 nationwide, and virtually all American hospitals with more than 200 beds have hospitalists.
Hospitals hired hospitalists in droves because they were perceived as “inpatient specialists” who were able to reduce the cost of healthcare delivery. Like lower pin costs, this economic benefit was spread across the entire population. Hospitalist “value” went up because of lower costs.
But an interesting thing changed over the past decade: It seems that fewer and fewer people are talking about hospitalists reducing LOS. They just expect it. LOS is raised as an issue only if it goes up. In many hospitals, the budget now takes into account the average LOS based on hospitalist care. (I suspect that in 18th-century Scotland, people also grew accustomed to the lower cost of pins, and grew to expect it, and the cost of pins was raised as an issue only if the price went up.)
But has anyone spoken to the hospitalists? Has anyone asked us about the benefits of our profession? Many of the hospitalists I meet mention “reduction of length of stay” as a main reason to have hospitalists. I think that response was appropriate more often than not over the past 15 years.
But today, ask any hospital administrator that same question, and what do you expect the answer to be? It should not be surprising to hospitalists that most hospitals expect much more from their hospitalist programs than “just reducing the length of stay.” These are the same hospitals that often support—to the tune of more than $100,000 per hospitalist full-time equivalent—your HM program. If your hospitalist program is anything like mine at Beth Israel Deaconess Medical Center in Boston, this represents millions of dollars to the hospitalist program budget.
Increased Pressures
The fact that hospitals’ expectations of hospitalists have changed should not come as a surprise. Americans’ expectations of hospitals have changed markedly over the past 15 years. In the 1990s, when hospital medicine was “born,” there was little mention of quality and patient satisfaction when it came to healthcare. Who would argue that improving quality and patient satisfaction is a bad thing?
Over the past decade, we’ve seen the development of Medicare core measures and the link between patient outcomes and Medicare reimbursement. Hospitals could not have achieved many of their performance improvements without their partnerships with hospitalists.
Hospitals are under increasing pressure to not only decrease costs, but also improve quality. It is understandable that they turn to the “inpatient specialists”—the hospitalists—to help them meet societal expectations. But as hospitalists, this puts pressure on us to continually improve our game—or face the consequences. A pin factory in Scotland can only survive if it produces higher-quality pins at a lower cost than its competitor.
Hospitals and our American healthcare system expect much more today from hospitalists, and they should; patients’ lives are at stake. It should not be a surprise that hospitalist programs that struggle are those that fail to meet expectations. Successful hospitalist programs, the ones that are able to demonstrate their “value,” do so well beyond knowing their patient’s average length of stay.
I am interested in learning about your efforts to demonstrate the “value” of your hospitalist group. Feel free to email me at [email protected].
Dr. Li is president of SHM.
For those of you who were kind enough to pick up my column in The Hospitalist last month (see “A Critical First Step,” p. 56), you spent a few minutes reading my thoughts on the value of hospitalists. I mentioned the fact that the U.S. is moving rapidly toward a value-based system to purchase healthcare and that all healthcare providers, including hospitalists, will be increasingly judged on the value of care they deliver to their patients and the healthcare system. (Remember, value=quality÷cost.)
I believe that the hospitalist programs that are going to be the most successful are those that are able to continually measure their quality and costs, allowing them to make improvements. These are the groups that will understand their own performance well before others make their “value” judgments.
History Lesson
In his famous book “The Wealth of Nations,” 18th-century economist Adam Smith used the example of a pin factory to show how specialization improved human productivity. The process of producing a pin was broken down into many small tasks, each done by a different “specialist.” This resulted not only in increased efficiency, but also increased productivity. The factory was then able to reinvest the profits in even more efficient machinery, which was able to reduce labor costs. The lower cost for pins was a benefit, and it was spread across the entire population.
We have seen similar examples in American healthcare. In many American hospitals, the images of CT scans performed during nighttime hours are transmitted to India and other Asian countries where highly trained radiologists interpret the scans and transmit their interpretations back to the physicians caring for the patients. Like the pin factory workers, these radiologists are specialists with unique skills; they operate specialized machinery to make the system more efficient with resultant lower costs. At the level of the individual patient, this system means getting test results back in a more timely fashion. Increased quality and lower costs: These are high-value providers.
It should be obvious to us that hospitalists are “specialists.” While most hospitalists are trained as general internists, pediatricians, and family physicians, you and I are “specialists” who focus our efforts on the care of hospitalized patients. In the late 1990s, much was made of the fact that hospitalists were able to reduce hospital costs because of decreased length of stay (LOS) for patients, without any adverse effects on clinical outcomes. Today, hospitalists number more than 30,000 nationwide, and virtually all American hospitals with more than 200 beds have hospitalists.
Hospitals hired hospitalists in droves because they were perceived as “inpatient specialists” who were able to reduce the cost of healthcare delivery. Like lower pin costs, this economic benefit was spread across the entire population. Hospitalist “value” went up because of lower costs.
But an interesting thing changed over the past decade: It seems that fewer and fewer people are talking about hospitalists reducing LOS. They just expect it. LOS is raised as an issue only if it goes up. In many hospitals, the budget now takes into account the average LOS based on hospitalist care. (I suspect that in 18th-century Scotland, people also grew accustomed to the lower cost of pins, and grew to expect it, and the cost of pins was raised as an issue only if the price went up.)
But has anyone spoken to the hospitalists? Has anyone asked us about the benefits of our profession? Many of the hospitalists I meet mention “reduction of length of stay” as a main reason to have hospitalists. I think that response was appropriate more often than not over the past 15 years.
But today, ask any hospital administrator that same question, and what do you expect the answer to be? It should not be surprising to hospitalists that most hospitals expect much more from their hospitalist programs than “just reducing the length of stay.” These are the same hospitals that often support—to the tune of more than $100,000 per hospitalist full-time equivalent—your HM program. If your hospitalist program is anything like mine at Beth Israel Deaconess Medical Center in Boston, this represents millions of dollars to the hospitalist program budget.
Increased Pressures
The fact that hospitals’ expectations of hospitalists have changed should not come as a surprise. Americans’ expectations of hospitals have changed markedly over the past 15 years. In the 1990s, when hospital medicine was “born,” there was little mention of quality and patient satisfaction when it came to healthcare. Who would argue that improving quality and patient satisfaction is a bad thing?
Over the past decade, we’ve seen the development of Medicare core measures and the link between patient outcomes and Medicare reimbursement. Hospitals could not have achieved many of their performance improvements without their partnerships with hospitalists.
Hospitals are under increasing pressure to not only decrease costs, but also improve quality. It is understandable that they turn to the “inpatient specialists”—the hospitalists—to help them meet societal expectations. But as hospitalists, this puts pressure on us to continually improve our game—or face the consequences. A pin factory in Scotland can only survive if it produces higher-quality pins at a lower cost than its competitor.
Hospitals and our American healthcare system expect much more today from hospitalists, and they should; patients’ lives are at stake. It should not be a surprise that hospitalist programs that struggle are those that fail to meet expectations. Successful hospitalist programs, the ones that are able to demonstrate their “value,” do so well beyond knowing their patient’s average length of stay.
I am interested in learning about your efforts to demonstrate the “value” of your hospitalist group. Feel free to email me at [email protected].
Dr. Li is president of SHM.
Fiddling As HM Burns
It’s been a hectic week, as the Annals of Internal Medicine paper regarding hospitalist outcomes was published.1 I cannot escape the fallout of the paper showing that the hospitalist model is associated with increased costs of care. The Internet, the phone, my email, the radio, the hallways all are abuzz with excitement about the implications of this paper. Everyone, it seems, has an opinion. The viewpoints range from “the article is methodologically flawed” to “yeah, but that data is old and things are different now,” to “I knew the model was bunk” to “it’s time to bring back the traditional model of care.”
Moreover, nobody is afraid to share.
Wherever you stand on this continuum, it isn’t hard to find a supporting opinion. NPR covered it, newspapers reported it, and bloggers blogged it. Thousands of words were typed, printed, tweeted, spoken. However, one word seemed conspicuously absent. That word? Thanks.
The Study
The study authors examined more than 58,000 admissions at 454 hospitals and compared the impact of hospitalist versus PCP care on in-hospital and post-discharge outcomes. Predictably, hospital length of stay (LOS) was shorter (0.64 days) and less costly ($282) with the hospitalist model. This has been shown, albeit generally with more robust outcomes, in nearly all-previous studies. Importantly, however, this study lifted the veil on what happens after discharge—and the findings have rocked the foundation of the HM field.
Hospitalist patients were less likely to follow up with their PCP, more likely to go to a skilled nursing facility, more likely to return to the ED, and had higher rates of 30-day readmission. All of this post-discharge care came with a price tag—$332 more than the PCP model—making the bundled in- and outpatient costs of care about $50 more per patient in the hospitalist model.
And this is where the controversy—and the words—begins. Connecting the earlier discharge, the added SNF utilization, and the higher readmission rate could only mean only one thing to those that favor the traditional model—a cost shift. Clearly hospitalists, motivated by saving money, are shifting the financial costs just beyond the hospital confines, discharging patients so early that they require nursing home and, ultimately, more ED visits and hospital care.
On the other side of the ledger, HM supporters have pointed out that the patients in the two arms were not the same. The HM patients were more likely to be admitted from a nursing home, more comorbid, poorer, and more likely to be admitted on a weekend—all valid points, which are hard to control for in an observational study. They argue that patients in an SNF are, of course, less likely to see their PCP than patients at home and, therefore, more likely to be sent to an ED (and admitted) when issues arise. Perhaps, the argument goes, in this scenario the system is actually working. Without indicators of quality of life and functional status, it’s hard to know that HM patients didn’t do better. Sure, there were more readmissions and it cost more, but perhaps that’s the cost of better, longer-term outcomes.
My take: Let’s move beyond debating the study merits and its implications. HM is here to stay. No matter how much we conjure Osler, we aren’t going back to the traditional model. In the debate we miss the point. Rome is afire; it’s time to stop fiddling.
So, let’s put our preconceived biases, the potential methodological flaws, the conspiratorial overtones, the vitriolic banter, and the fruitless debates behind us. This study was generally well done. It focuses on a (perhaps, the) crucial issue for HM. And its findings are plausible. For that I say “thanks”: for exposing this issue so we can tackle it head on by moving in at least three distinct directions—quality, training, and retraining.
Now let’s put down the fiddle and pick up the extinguisher.
$50: The Price of Quality?
First, I hope this study finally pushes our field beyond the cost discussion. We simply can and should not be a field that is about saving money. Yes, it is great to save money. But more important, we have to enhance the quality and safety of hospital (and post-hospital) care.
The piece missing from the Annals paper is any significant look at quality metrics, beyond perhaps readmission. It is possible, and maybe probable, that hospitalists in this study reduced complications, avoided harm, and improved inpatient mortality. Perhaps this, and not a zeal for too-early discharges, is what fueled the lower hospital cost and shorter LOS.
How much is that worth? It’s hard to say, but I’d venture much more than the $50 more per patient associated with the hospitalist model. Quality, even at higher costs, needs to be our primary focus moving forward. We must improve the quality of care to levels that, if necessary, Medicare would happily pay more for. This must be our singular goal. I’d also argue that we include the post-discharge period in our quality reach.
More QI, Less MEN
We cannot continue to train square pegs and struggle to cram them into round holes. We need more systems thinkers. We do a tremendous job teaching our students and residents about the interplay of pathophysiology and pharmacology, but spend very little time with the interplay between our patients and the system. We simply must triage process improvement, quality, safety, and efficiency training closer to the top of our medical curricula.
As such, it shouldn’t be that surprising that two groups of providers with the exact same background and training should have similar outcomes as seen in this study. The reality is that these providers came from very similar training backgrounds. Yes, they have chosen different practice models, but we all learned how to treat pneumonia and heart failure. It’s not about the model as much as what you do with the model. Cohorting patients to providers who just care for hospitalized patients will lead to efficiencies, but if we want to fundamentally improve patient outcomes, both during and after their hospital stay, we need to train hospitalists to transform that model through systematic process improvement.
And I firmly believe that hospitalists should lead this sea change. Our teaching brethren are perfectly positioned to develop hospitalist-focused training models that better prepare future hospitalists to fundamentally improve not just transitions of care but indeed all systems of care. Training that emphasizes systems thinking, mentored process improvement, and patient safety across the continuum of care.
BOOSTing Outcomes
This paper highlights HM’s Achilles’ heel. It has always been transitions of care—specifically, communication with PCPs. HM is by its very nature a fractured care model. And that discontinuity results in information drop on transitions. A PCP who knows a patient and admits and follows them after discharge is better positioned to reduce readmissions because there is no information drop in that model. The success of the HM model hinges on hospitalists efficiently and effectively approximating that level of knowledge transfer to PCPs. And to be honest, we don’t need an NIH-funded study to tell us that we have not been doing a great job with this.
This is not necessarily from a lack of effort but rather because we lack systems that simplify information transfer on transitions. It is incumbent these systems be built. It is incumbent we lead this. Whether you choose Project BOOST, Project RED, or a homegrown solution, it is no longer acceptable to ignore the transitions of care issue.
And with that I’ve just typed my 1,319th word about an article that has already commanded too many words. Don’t get me wrong, the discussion is important, and for that we must thank the study’s authors. But it’s time to move beyond the discussion, the debate, the words.
It’s time to turn words into deeds.
Dr. Glasheen is physician editor of The Hospitalist.
Reference
It’s been a hectic week, as the Annals of Internal Medicine paper regarding hospitalist outcomes was published.1 I cannot escape the fallout of the paper showing that the hospitalist model is associated with increased costs of care. The Internet, the phone, my email, the radio, the hallways all are abuzz with excitement about the implications of this paper. Everyone, it seems, has an opinion. The viewpoints range from “the article is methodologically flawed” to “yeah, but that data is old and things are different now,” to “I knew the model was bunk” to “it’s time to bring back the traditional model of care.”
Moreover, nobody is afraid to share.
Wherever you stand on this continuum, it isn’t hard to find a supporting opinion. NPR covered it, newspapers reported it, and bloggers blogged it. Thousands of words were typed, printed, tweeted, spoken. However, one word seemed conspicuously absent. That word? Thanks.
The Study
The study authors examined more than 58,000 admissions at 454 hospitals and compared the impact of hospitalist versus PCP care on in-hospital and post-discharge outcomes. Predictably, hospital length of stay (LOS) was shorter (0.64 days) and less costly ($282) with the hospitalist model. This has been shown, albeit generally with more robust outcomes, in nearly all-previous studies. Importantly, however, this study lifted the veil on what happens after discharge—and the findings have rocked the foundation of the HM field.
Hospitalist patients were less likely to follow up with their PCP, more likely to go to a skilled nursing facility, more likely to return to the ED, and had higher rates of 30-day readmission. All of this post-discharge care came with a price tag—$332 more than the PCP model—making the bundled in- and outpatient costs of care about $50 more per patient in the hospitalist model.
And this is where the controversy—and the words—begins. Connecting the earlier discharge, the added SNF utilization, and the higher readmission rate could only mean only one thing to those that favor the traditional model—a cost shift. Clearly hospitalists, motivated by saving money, are shifting the financial costs just beyond the hospital confines, discharging patients so early that they require nursing home and, ultimately, more ED visits and hospital care.
On the other side of the ledger, HM supporters have pointed out that the patients in the two arms were not the same. The HM patients were more likely to be admitted from a nursing home, more comorbid, poorer, and more likely to be admitted on a weekend—all valid points, which are hard to control for in an observational study. They argue that patients in an SNF are, of course, less likely to see their PCP than patients at home and, therefore, more likely to be sent to an ED (and admitted) when issues arise. Perhaps, the argument goes, in this scenario the system is actually working. Without indicators of quality of life and functional status, it’s hard to know that HM patients didn’t do better. Sure, there were more readmissions and it cost more, but perhaps that’s the cost of better, longer-term outcomes.
My take: Let’s move beyond debating the study merits and its implications. HM is here to stay. No matter how much we conjure Osler, we aren’t going back to the traditional model. In the debate we miss the point. Rome is afire; it’s time to stop fiddling.
So, let’s put our preconceived biases, the potential methodological flaws, the conspiratorial overtones, the vitriolic banter, and the fruitless debates behind us. This study was generally well done. It focuses on a (perhaps, the) crucial issue for HM. And its findings are plausible. For that I say “thanks”: for exposing this issue so we can tackle it head on by moving in at least three distinct directions—quality, training, and retraining.
Now let’s put down the fiddle and pick up the extinguisher.
$50: The Price of Quality?
First, I hope this study finally pushes our field beyond the cost discussion. We simply can and should not be a field that is about saving money. Yes, it is great to save money. But more important, we have to enhance the quality and safety of hospital (and post-hospital) care.
The piece missing from the Annals paper is any significant look at quality metrics, beyond perhaps readmission. It is possible, and maybe probable, that hospitalists in this study reduced complications, avoided harm, and improved inpatient mortality. Perhaps this, and not a zeal for too-early discharges, is what fueled the lower hospital cost and shorter LOS.
How much is that worth? It’s hard to say, but I’d venture much more than the $50 more per patient associated with the hospitalist model. Quality, even at higher costs, needs to be our primary focus moving forward. We must improve the quality of care to levels that, if necessary, Medicare would happily pay more for. This must be our singular goal. I’d also argue that we include the post-discharge period in our quality reach.
More QI, Less MEN
We cannot continue to train square pegs and struggle to cram them into round holes. We need more systems thinkers. We do a tremendous job teaching our students and residents about the interplay of pathophysiology and pharmacology, but spend very little time with the interplay between our patients and the system. We simply must triage process improvement, quality, safety, and efficiency training closer to the top of our medical curricula.
As such, it shouldn’t be that surprising that two groups of providers with the exact same background and training should have similar outcomes as seen in this study. The reality is that these providers came from very similar training backgrounds. Yes, they have chosen different practice models, but we all learned how to treat pneumonia and heart failure. It’s not about the model as much as what you do with the model. Cohorting patients to providers who just care for hospitalized patients will lead to efficiencies, but if we want to fundamentally improve patient outcomes, both during and after their hospital stay, we need to train hospitalists to transform that model through systematic process improvement.
And I firmly believe that hospitalists should lead this sea change. Our teaching brethren are perfectly positioned to develop hospitalist-focused training models that better prepare future hospitalists to fundamentally improve not just transitions of care but indeed all systems of care. Training that emphasizes systems thinking, mentored process improvement, and patient safety across the continuum of care.
BOOSTing Outcomes
This paper highlights HM’s Achilles’ heel. It has always been transitions of care—specifically, communication with PCPs. HM is by its very nature a fractured care model. And that discontinuity results in information drop on transitions. A PCP who knows a patient and admits and follows them after discharge is better positioned to reduce readmissions because there is no information drop in that model. The success of the HM model hinges on hospitalists efficiently and effectively approximating that level of knowledge transfer to PCPs. And to be honest, we don’t need an NIH-funded study to tell us that we have not been doing a great job with this.
This is not necessarily from a lack of effort but rather because we lack systems that simplify information transfer on transitions. It is incumbent these systems be built. It is incumbent we lead this. Whether you choose Project BOOST, Project RED, or a homegrown solution, it is no longer acceptable to ignore the transitions of care issue.
And with that I’ve just typed my 1,319th word about an article that has already commanded too many words. Don’t get me wrong, the discussion is important, and for that we must thank the study’s authors. But it’s time to move beyond the discussion, the debate, the words.
It’s time to turn words into deeds.
Dr. Glasheen is physician editor of The Hospitalist.
Reference
It’s been a hectic week, as the Annals of Internal Medicine paper regarding hospitalist outcomes was published.1 I cannot escape the fallout of the paper showing that the hospitalist model is associated with increased costs of care. The Internet, the phone, my email, the radio, the hallways all are abuzz with excitement about the implications of this paper. Everyone, it seems, has an opinion. The viewpoints range from “the article is methodologically flawed” to “yeah, but that data is old and things are different now,” to “I knew the model was bunk” to “it’s time to bring back the traditional model of care.”
Moreover, nobody is afraid to share.
Wherever you stand on this continuum, it isn’t hard to find a supporting opinion. NPR covered it, newspapers reported it, and bloggers blogged it. Thousands of words were typed, printed, tweeted, spoken. However, one word seemed conspicuously absent. That word? Thanks.
The Study
The study authors examined more than 58,000 admissions at 454 hospitals and compared the impact of hospitalist versus PCP care on in-hospital and post-discharge outcomes. Predictably, hospital length of stay (LOS) was shorter (0.64 days) and less costly ($282) with the hospitalist model. This has been shown, albeit generally with more robust outcomes, in nearly all-previous studies. Importantly, however, this study lifted the veil on what happens after discharge—and the findings have rocked the foundation of the HM field.
Hospitalist patients were less likely to follow up with their PCP, more likely to go to a skilled nursing facility, more likely to return to the ED, and had higher rates of 30-day readmission. All of this post-discharge care came with a price tag—$332 more than the PCP model—making the bundled in- and outpatient costs of care about $50 more per patient in the hospitalist model.
And this is where the controversy—and the words—begins. Connecting the earlier discharge, the added SNF utilization, and the higher readmission rate could only mean only one thing to those that favor the traditional model—a cost shift. Clearly hospitalists, motivated by saving money, are shifting the financial costs just beyond the hospital confines, discharging patients so early that they require nursing home and, ultimately, more ED visits and hospital care.
On the other side of the ledger, HM supporters have pointed out that the patients in the two arms were not the same. The HM patients were more likely to be admitted from a nursing home, more comorbid, poorer, and more likely to be admitted on a weekend—all valid points, which are hard to control for in an observational study. They argue that patients in an SNF are, of course, less likely to see their PCP than patients at home and, therefore, more likely to be sent to an ED (and admitted) when issues arise. Perhaps, the argument goes, in this scenario the system is actually working. Without indicators of quality of life and functional status, it’s hard to know that HM patients didn’t do better. Sure, there were more readmissions and it cost more, but perhaps that’s the cost of better, longer-term outcomes.
My take: Let’s move beyond debating the study merits and its implications. HM is here to stay. No matter how much we conjure Osler, we aren’t going back to the traditional model. In the debate we miss the point. Rome is afire; it’s time to stop fiddling.
So, let’s put our preconceived biases, the potential methodological flaws, the conspiratorial overtones, the vitriolic banter, and the fruitless debates behind us. This study was generally well done. It focuses on a (perhaps, the) crucial issue for HM. And its findings are plausible. For that I say “thanks”: for exposing this issue so we can tackle it head on by moving in at least three distinct directions—quality, training, and retraining.
Now let’s put down the fiddle and pick up the extinguisher.
$50: The Price of Quality?
First, I hope this study finally pushes our field beyond the cost discussion. We simply can and should not be a field that is about saving money. Yes, it is great to save money. But more important, we have to enhance the quality and safety of hospital (and post-hospital) care.
The piece missing from the Annals paper is any significant look at quality metrics, beyond perhaps readmission. It is possible, and maybe probable, that hospitalists in this study reduced complications, avoided harm, and improved inpatient mortality. Perhaps this, and not a zeal for too-early discharges, is what fueled the lower hospital cost and shorter LOS.
How much is that worth? It’s hard to say, but I’d venture much more than the $50 more per patient associated with the hospitalist model. Quality, even at higher costs, needs to be our primary focus moving forward. We must improve the quality of care to levels that, if necessary, Medicare would happily pay more for. This must be our singular goal. I’d also argue that we include the post-discharge period in our quality reach.
More QI, Less MEN
We cannot continue to train square pegs and struggle to cram them into round holes. We need more systems thinkers. We do a tremendous job teaching our students and residents about the interplay of pathophysiology and pharmacology, but spend very little time with the interplay between our patients and the system. We simply must triage process improvement, quality, safety, and efficiency training closer to the top of our medical curricula.
As such, it shouldn’t be that surprising that two groups of providers with the exact same background and training should have similar outcomes as seen in this study. The reality is that these providers came from very similar training backgrounds. Yes, they have chosen different practice models, but we all learned how to treat pneumonia and heart failure. It’s not about the model as much as what you do with the model. Cohorting patients to providers who just care for hospitalized patients will lead to efficiencies, but if we want to fundamentally improve patient outcomes, both during and after their hospital stay, we need to train hospitalists to transform that model through systematic process improvement.
And I firmly believe that hospitalists should lead this sea change. Our teaching brethren are perfectly positioned to develop hospitalist-focused training models that better prepare future hospitalists to fundamentally improve not just transitions of care but indeed all systems of care. Training that emphasizes systems thinking, mentored process improvement, and patient safety across the continuum of care.
BOOSTing Outcomes
This paper highlights HM’s Achilles’ heel. It has always been transitions of care—specifically, communication with PCPs. HM is by its very nature a fractured care model. And that discontinuity results in information drop on transitions. A PCP who knows a patient and admits and follows them after discharge is better positioned to reduce readmissions because there is no information drop in that model. The success of the HM model hinges on hospitalists efficiently and effectively approximating that level of knowledge transfer to PCPs. And to be honest, we don’t need an NIH-funded study to tell us that we have not been doing a great job with this.
This is not necessarily from a lack of effort but rather because we lack systems that simplify information transfer on transitions. It is incumbent these systems be built. It is incumbent we lead this. Whether you choose Project BOOST, Project RED, or a homegrown solution, it is no longer acceptable to ignore the transitions of care issue.
And with that I’ve just typed my 1,319th word about an article that has already commanded too many words. Don’t get me wrong, the discussion is important, and for that we must thank the study’s authors. But it’s time to move beyond the discussion, the debate, the words.
It’s time to turn words into deeds.
Dr. Glasheen is physician editor of The Hospitalist.
Reference
Hospital-Focused Practice
As regular readers of The Hospitalist are aware, essentially every specialty in medicine is adopting the hospitalist model to some degree. After the “legacy” specialties of medicine and pediatrics, the model has more recently been embraced enthusiastically by neurologists, obstetricians, and general surgeons. But even fields like dermatology and ENT have put a hospitalist version of their specialties in place in at least a few places.
Did you know there is a Society for Dermatology Hospitalists? Did you know that the Neurohospitalist Society has its own journal? Did you know OB hospitalists have a really neat website, and the Society of OB/GYN Hospitalists is scheduled to have its first annual meeting in Boulder, Colo., Sept. 23-25?
It’ll make your head spin if you think about it too long. All of this raises a number of issues, including the need for more precise terminology to describe these fields and their practitioners.
The Need for Better Terminology
For example, now that we have neurohospitalists and psychiatric hospitalists, is it time to start attaching a modifier or prefix every time we use the word “hospitalist,” including when referring to “medical” hospitalists? I don’t think so. For the time being, I propose that when used alone, the word “hospitalist” still refers to a doctor who provides general medical care for adult inpatients. But I think any other use of the word does require a modifier, as in “peds hospitalist” or “GI hospitalist.”
(I think my view makes sense, but then, I’ve tried for years to ensure nocternist, with an E—NOCTernal intERNIST)—is the preferred spelling over nocternist, with a U. But Google returns nine hits for the former and 365,000 for the latter. Looks like I lost that one.)
Terminology for general and trauma surgeons is tricky. There is an emerging field of acute-care surgery, distinct from general surgery, which some argue passionately is nothing like a hospitalist model, and they tend to be offended if one uses the latter term. So, for now, we’ll need to use both “acute-care surgeon” and “surgical hospitalist” carefully. Although there are meaningful distinctions between acute-care surgery and a “standard” general surgery practice devoted to the hospital, there is an awful lot of overlap in the Venn diagrams of their expertise and what they do. But for now, it looks like we should expect both “acute-care surgeon” and “surgical hospitalist” to appear commonly, and the context will determine whether the terms could be used interchangeably.
While “obstetric hospitalist,” or “OB hospitalist,” is a perfectly useful term, I think it is great when laborist is substituted, at least in informal communication.
We still need a way to speak of all of these clinical roles (I don’t think we can properly call them specialties yet). I propose that we refer to all of them as specialties within the realm of “hospital-focused practice.” I’ve borrowed this term from the American Board of Internal Medicine’s Recognition of Focused Practice in Hospital Medicine, the new pathway to Maintenance of Certification.
And what about those doctors in each specialty who continue to practice in the traditional inpatient and outpatient model? Let’s call them “traditionalists.”
Hospital-Focused Practice
A rational vocabulary is only one of many significant issues raised by the growth of hospital-focused disciplines. In January, I participated in an SHM-convened, and AHA-supported, meeting of 11 practitioners who were hospitalists in neurology, obstetrics, general surgery, medicine, pediatrics, and ENT. (Sadly, the invited dermatology hospitalist couldn’t make it.) The meeting was filled with interest and sharing of lessons learned in each field. We discussed questions, and I have provided a very brief answer to each based on the conversation during the meeting and my own work with practices across many different specialties that have adopted the hospitalist model:
What are the reasons each specialty is turning to this model, and what is its prevalence? Hospitalists have appeared in a specialty largely to fill the void left by the traditionalists who no longer want to care for unattached patients admitted through the ED, or who want to leave the hospital altogether for a solely outpatient practice.
What are typical staffing models, night coverage arrangements, and provider career sustainability? These vary a lot by specialty, but laborists typically work 24-hour, in-house shifts. Surgical hospitalists usually work 12-hour shifts if they are in-house all the time, or 24-hour shifts if they take call from home. Neurohospitalists essentially always take call from home (did you even have to ask?).
Career longevity is still a matter of speculation, but the majority of those who have transitioned from traditional to hospitalist practice in their field are convinced they will have a longer career than if they hadn’t made the switch.
What are the effects of this practice model on clinical quality, patient outcomes, healthcare economics, and liability? It will be really difficult to get convincing research data on the quality effects of the hospitalist model in many fields. After more than 15 years in operation, research about the quality effects of the medical hospitalist model is not robust enough to satisfy some. But OB hospitalists may be the exception here. There is hope that their continuous, on-site presence will reduce complications from emergencies, and in doing so might reduce malpractice risk.
What is the prevalent financial model? The experience across a lot of healthcare settings to this point is that professional fee revenue alone usually is not enough to support a hospitalist practice model in any specialty. Just like medical and pediatric hospitalist models, the hospital in which the doctors practice usually provides additional financial support.
Hospitals usually are willing to do this because they are able to reallocate dollars spent paying for numerous specialty doctors to take ED call with poor performance, and instead use those dollars to support a hospitalist practice in that specialty that promises a better return on the investment.
Join us in November for a meeting to understand the implications of hospital-focused practice. Those of us at the January meeting of specialty hospitalists thought that it would be valuable to convene a much larger meeting to think about issues like those above and others. At the Nov. 4 meeting in Las Vegas, we plan to hear from such national figures as CMS’ chief medical officer, physicians practicing in a hospitalist model, and hospital and healthcare executives. The meeting will be structured to promote interaction and communication from attendees.
I hope to see you in Las Vegas. We have a lot to learn from one another.
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
As regular readers of The Hospitalist are aware, essentially every specialty in medicine is adopting the hospitalist model to some degree. After the “legacy” specialties of medicine and pediatrics, the model has more recently been embraced enthusiastically by neurologists, obstetricians, and general surgeons. But even fields like dermatology and ENT have put a hospitalist version of their specialties in place in at least a few places.
Did you know there is a Society for Dermatology Hospitalists? Did you know that the Neurohospitalist Society has its own journal? Did you know OB hospitalists have a really neat website, and the Society of OB/GYN Hospitalists is scheduled to have its first annual meeting in Boulder, Colo., Sept. 23-25?
It’ll make your head spin if you think about it too long. All of this raises a number of issues, including the need for more precise terminology to describe these fields and their practitioners.
The Need for Better Terminology
For example, now that we have neurohospitalists and psychiatric hospitalists, is it time to start attaching a modifier or prefix every time we use the word “hospitalist,” including when referring to “medical” hospitalists? I don’t think so. For the time being, I propose that when used alone, the word “hospitalist” still refers to a doctor who provides general medical care for adult inpatients. But I think any other use of the word does require a modifier, as in “peds hospitalist” or “GI hospitalist.”
(I think my view makes sense, but then, I’ve tried for years to ensure nocternist, with an E—NOCTernal intERNIST)—is the preferred spelling over nocternist, with a U. But Google returns nine hits for the former and 365,000 for the latter. Looks like I lost that one.)
Terminology for general and trauma surgeons is tricky. There is an emerging field of acute-care surgery, distinct from general surgery, which some argue passionately is nothing like a hospitalist model, and they tend to be offended if one uses the latter term. So, for now, we’ll need to use both “acute-care surgeon” and “surgical hospitalist” carefully. Although there are meaningful distinctions between acute-care surgery and a “standard” general surgery practice devoted to the hospital, there is an awful lot of overlap in the Venn diagrams of their expertise and what they do. But for now, it looks like we should expect both “acute-care surgeon” and “surgical hospitalist” to appear commonly, and the context will determine whether the terms could be used interchangeably.
While “obstetric hospitalist,” or “OB hospitalist,” is a perfectly useful term, I think it is great when laborist is substituted, at least in informal communication.
We still need a way to speak of all of these clinical roles (I don’t think we can properly call them specialties yet). I propose that we refer to all of them as specialties within the realm of “hospital-focused practice.” I’ve borrowed this term from the American Board of Internal Medicine’s Recognition of Focused Practice in Hospital Medicine, the new pathway to Maintenance of Certification.
And what about those doctors in each specialty who continue to practice in the traditional inpatient and outpatient model? Let’s call them “traditionalists.”
Hospital-Focused Practice
A rational vocabulary is only one of many significant issues raised by the growth of hospital-focused disciplines. In January, I participated in an SHM-convened, and AHA-supported, meeting of 11 practitioners who were hospitalists in neurology, obstetrics, general surgery, medicine, pediatrics, and ENT. (Sadly, the invited dermatology hospitalist couldn’t make it.) The meeting was filled with interest and sharing of lessons learned in each field. We discussed questions, and I have provided a very brief answer to each based on the conversation during the meeting and my own work with practices across many different specialties that have adopted the hospitalist model:
What are the reasons each specialty is turning to this model, and what is its prevalence? Hospitalists have appeared in a specialty largely to fill the void left by the traditionalists who no longer want to care for unattached patients admitted through the ED, or who want to leave the hospital altogether for a solely outpatient practice.
What are typical staffing models, night coverage arrangements, and provider career sustainability? These vary a lot by specialty, but laborists typically work 24-hour, in-house shifts. Surgical hospitalists usually work 12-hour shifts if they are in-house all the time, or 24-hour shifts if they take call from home. Neurohospitalists essentially always take call from home (did you even have to ask?).
Career longevity is still a matter of speculation, but the majority of those who have transitioned from traditional to hospitalist practice in their field are convinced they will have a longer career than if they hadn’t made the switch.
What are the effects of this practice model on clinical quality, patient outcomes, healthcare economics, and liability? It will be really difficult to get convincing research data on the quality effects of the hospitalist model in many fields. After more than 15 years in operation, research about the quality effects of the medical hospitalist model is not robust enough to satisfy some. But OB hospitalists may be the exception here. There is hope that their continuous, on-site presence will reduce complications from emergencies, and in doing so might reduce malpractice risk.
What is the prevalent financial model? The experience across a lot of healthcare settings to this point is that professional fee revenue alone usually is not enough to support a hospitalist practice model in any specialty. Just like medical and pediatric hospitalist models, the hospital in which the doctors practice usually provides additional financial support.
Hospitals usually are willing to do this because they are able to reallocate dollars spent paying for numerous specialty doctors to take ED call with poor performance, and instead use those dollars to support a hospitalist practice in that specialty that promises a better return on the investment.
Join us in November for a meeting to understand the implications of hospital-focused practice. Those of us at the January meeting of specialty hospitalists thought that it would be valuable to convene a much larger meeting to think about issues like those above and others. At the Nov. 4 meeting in Las Vegas, we plan to hear from such national figures as CMS’ chief medical officer, physicians practicing in a hospitalist model, and hospital and healthcare executives. The meeting will be structured to promote interaction and communication from attendees.
I hope to see you in Las Vegas. We have a lot to learn from one another.
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
As regular readers of The Hospitalist are aware, essentially every specialty in medicine is adopting the hospitalist model to some degree. After the “legacy” specialties of medicine and pediatrics, the model has more recently been embraced enthusiastically by neurologists, obstetricians, and general surgeons. But even fields like dermatology and ENT have put a hospitalist version of their specialties in place in at least a few places.
Did you know there is a Society for Dermatology Hospitalists? Did you know that the Neurohospitalist Society has its own journal? Did you know OB hospitalists have a really neat website, and the Society of OB/GYN Hospitalists is scheduled to have its first annual meeting in Boulder, Colo., Sept. 23-25?
It’ll make your head spin if you think about it too long. All of this raises a number of issues, including the need for more precise terminology to describe these fields and their practitioners.
The Need for Better Terminology
For example, now that we have neurohospitalists and psychiatric hospitalists, is it time to start attaching a modifier or prefix every time we use the word “hospitalist,” including when referring to “medical” hospitalists? I don’t think so. For the time being, I propose that when used alone, the word “hospitalist” still refers to a doctor who provides general medical care for adult inpatients. But I think any other use of the word does require a modifier, as in “peds hospitalist” or “GI hospitalist.”
(I think my view makes sense, but then, I’ve tried for years to ensure nocternist, with an E—NOCTernal intERNIST)—is the preferred spelling over nocternist, with a U. But Google returns nine hits for the former and 365,000 for the latter. Looks like I lost that one.)
Terminology for general and trauma surgeons is tricky. There is an emerging field of acute-care surgery, distinct from general surgery, which some argue passionately is nothing like a hospitalist model, and they tend to be offended if one uses the latter term. So, for now, we’ll need to use both “acute-care surgeon” and “surgical hospitalist” carefully. Although there are meaningful distinctions between acute-care surgery and a “standard” general surgery practice devoted to the hospital, there is an awful lot of overlap in the Venn diagrams of their expertise and what they do. But for now, it looks like we should expect both “acute-care surgeon” and “surgical hospitalist” to appear commonly, and the context will determine whether the terms could be used interchangeably.
While “obstetric hospitalist,” or “OB hospitalist,” is a perfectly useful term, I think it is great when laborist is substituted, at least in informal communication.
We still need a way to speak of all of these clinical roles (I don’t think we can properly call them specialties yet). I propose that we refer to all of them as specialties within the realm of “hospital-focused practice.” I’ve borrowed this term from the American Board of Internal Medicine’s Recognition of Focused Practice in Hospital Medicine, the new pathway to Maintenance of Certification.
And what about those doctors in each specialty who continue to practice in the traditional inpatient and outpatient model? Let’s call them “traditionalists.”
Hospital-Focused Practice
A rational vocabulary is only one of many significant issues raised by the growth of hospital-focused disciplines. In January, I participated in an SHM-convened, and AHA-supported, meeting of 11 practitioners who were hospitalists in neurology, obstetrics, general surgery, medicine, pediatrics, and ENT. (Sadly, the invited dermatology hospitalist couldn’t make it.) The meeting was filled with interest and sharing of lessons learned in each field. We discussed questions, and I have provided a very brief answer to each based on the conversation during the meeting and my own work with practices across many different specialties that have adopted the hospitalist model:
What are the reasons each specialty is turning to this model, and what is its prevalence? Hospitalists have appeared in a specialty largely to fill the void left by the traditionalists who no longer want to care for unattached patients admitted through the ED, or who want to leave the hospital altogether for a solely outpatient practice.
What are typical staffing models, night coverage arrangements, and provider career sustainability? These vary a lot by specialty, but laborists typically work 24-hour, in-house shifts. Surgical hospitalists usually work 12-hour shifts if they are in-house all the time, or 24-hour shifts if they take call from home. Neurohospitalists essentially always take call from home (did you even have to ask?).
Career longevity is still a matter of speculation, but the majority of those who have transitioned from traditional to hospitalist practice in their field are convinced they will have a longer career than if they hadn’t made the switch.
What are the effects of this practice model on clinical quality, patient outcomes, healthcare economics, and liability? It will be really difficult to get convincing research data on the quality effects of the hospitalist model in many fields. After more than 15 years in operation, research about the quality effects of the medical hospitalist model is not robust enough to satisfy some. But OB hospitalists may be the exception here. There is hope that their continuous, on-site presence will reduce complications from emergencies, and in doing so might reduce malpractice risk.
What is the prevalent financial model? The experience across a lot of healthcare settings to this point is that professional fee revenue alone usually is not enough to support a hospitalist practice model in any specialty. Just like medical and pediatric hospitalist models, the hospital in which the doctors practice usually provides additional financial support.
Hospitals usually are willing to do this because they are able to reallocate dollars spent paying for numerous specialty doctors to take ED call with poor performance, and instead use those dollars to support a hospitalist practice in that specialty that promises a better return on the investment.
Join us in November for a meeting to understand the implications of hospital-focused practice. Those of us at the January meeting of specialty hospitalists thought that it would be valuable to convene a much larger meeting to think about issues like those above and others. At the Nov. 4 meeting in Las Vegas, we plan to hear from such national figures as CMS’ chief medical officer, physicians practicing in a hospitalist model, and hospital and healthcare executives. The meeting will be structured to promote interaction and communication from attendees.
I hope to see you in Las Vegas. We have a lot to learn from one another.
Dr. Nelson has been a practicing hospitalist since 1988 and is cofounder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course codirector and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Pediatric HM Highlights
It’s been a while since I went to a buffet and stuffed myself silly, but that’s how my mind felt on the flight home from Kansas City, Mo. After four incredibly packed days at Pediatric Hospital Medicine (PHM) 2011, I was one “wafer-thin mint” away from an explosion. What I wanted was some kind of a mental digestif. What I got instead was a light beer. It helped a little bit, but when I awoke during the landing in Austin, Texas, I realized that I remained in need of a better way to distill the thousand points of information from the conference into something more manageable.
Buoyed by Michelle Marks and Joel Tieder’s Top 10 Articles of the Year luncheon, specifically the piece on neurosurgeons and Kangaroo Care, I thought I might try my own version of a decompressive operation.
Without further delay, here are the top 10 things that I learned at Pediatric Hospital Medicine 2011:
10. We continue to grow as a field. Although the exact number of pediatric hospitalists in the U.S. remains somewhat unclear (but is probably between 1,000 and 2,000), what is known is that attendance at our annual meeting grows every year. Our tripartite meeting, sponsored by the American Academy of Pediatrics (AAP), SHM, and the Academic Pediatric Association (APA), hit a record 450 attendees this year. Beyond the physical numbers, it is quite clear that we are growing in many other domains as well.
9. It is time to re-evaluate the impact of CME on physician practice and outcomes. The literature on continuing professional education has been quite sobering to date, with nothing to show for the thousands of dollars spent per individual. But most of those studies were performed in the last century (think eight hours of lecture a day followed by dinners with big pharma) and I’ll bet that there was not the focus on learner-centered education that was evident in Kansas City.
With a dizzying array of workshops and interactive small group sessions spread amongst seven different tracks, it was difficult, if not impossible, to be a passive participant in the process. And since learning retention rates are generally proportionate to how active a role adults play in their own education, I am going to guess that many other attendees’ brains still have that “I’m thinking” hourglass icon over them. We have the conference planning committee to thank for this.
8. The JCPHM (Joint Council of Pediatric Hospital Medicine) will be increasingly important as we develop. Much like the constant stream of unfamiliar new vaccine names that have appeared in recent years, this proposed new committee comes with another long set of initials and an unfamiliar indication.
The JCPHM will function as a coordinating body, ensuring that work done through the AAP, SHM, and AAP are aligned to provide maximal benefit to pediatric hospitalists as a whole. And thus, similar to immunizations, the benefits will be most evident if we remain healthy as we grow in the context of an increasingly complex environment.
7. Our collective research accomplishments merit national recognition. It was not more than just a few years ago that we were in our research infancy. Posters at our meetings largely represented single-site descriptive studies, typically using survey methodology. This year we had four research breakout tracks, in addition to the plenary and three poster sessions.
Pediatric Research in Inpatient Settings (PRIS) leads the way with a Forbes-like listing of million-dollar grants and a partnership with Child Health Corporation of America’s (CHCA) uber-powerful Pediatric Health Information Systems (PHIS) database. Expect some landmark studies in the near future.
In the meantime, it is clear that the rest of the field is not languishing from smaller budgets. From clear outcome and process measurements of family-centered rounds to studying the spectrum of transitions of care to the impact of early warning systems, there was a predominant focus on quality and safety.
In fact, the tone was set at the opening keynote address, as Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ), described creative and innovative ways to study and translate work in this area to improved patient care.
6. We are poised to develop effective training systems for our future workforce. From the use of our core competencies in sessions to a full complement of workshops on education (from individual to team and from student to fellow), it is clear that thoughtful deliberation has paved the way for our future. We possess expertise in education that dovetails nicely with our need to grow and sustain an experienced, well-trained workforce.
Intrinsically, we know that we possess a unique body of skills, knowledge, and attitudes. The explicit articulation of this into longitudinal curricula will headline our evolution as a field.
5. Our new peer-reviewed journal has a bright future. Kudos to Shawn Ralston and the rest of the editorial board for publishing the first peer-reviewed edition of Hospital Pediatrics. Original research, evidence-based content, and practical commentary grace the pages with a little bit of something for everyone. The AAP has demonstrated a healthy level of support for this endeavor, as they sent out an introductory email announcing the journal to all of their membership over the weekend. I am confident that support from our pediatric hospitalist community will follow in the form of an exponential increase in quality submissions. Look no further than the PHM 2011 abstract book to get a preview of what our journal will highlight in the near future.
4. We connect with each other through a language of quality and value in our work. Quality spanned the continuum from conversation to collaboration, as like-minded souls shared ideas and passions amidst the sessions. The improved outcomes demonstrated by the Value in Inpatient Pediatrics (VIP) network are a testament to the positive change that can arise from such efforts. VIP, with its focus on inclusive and front-line collaboratives, also announced an upcoming merger with the AAP’s Quality Improvement and Innovation Network (QuIIN), approved by the AAP board in May.
Perhaps more impressive was that more than 12% of the attendees at PHM 2011 attended the annual VIP dinner and similar numbers signed up to participate in future efforts. At this pace, widespread improvement and value are easily within our sights.
3. Complex care is the new family-centered rounds. Atul Gawande’s recent New Yorker article about “Hot Spotters” could very well have been referring to the body of work that is represented by pediatric generalists (to include a fair number of hospitalists) over the past few years. Closing plenary speakers Robert Lyle and Patrick Casey wowed the audience as they described their medical home for medically complex children—and an estimated savings to Arkansas Medicaid of nearly $3 million per year.
As hospitals and hospital systems look to create accountable care organizations (ACOs), this kind of work will be increasingly prioritized, as it has the potential to generate the biggest gains in valued care.
2. STP, yeah, you know me. Chris Maloney and Suzanne Swanson Mendez brought down the house at the PHM Roundtable update as they presented preliminary results of their large and representative STP (strategic planning) Committee, which is mapping out future certification options for pediatric hospitalists. A lively debate ensued as questions surrounding how to best notify and involve pediatric hospitalists in these decisions came to the forefront. Are we a democracy? Are we a republic? Is there a better model for this decision?
Despite the lack of consensus on how to best move toward a decision, the discussion remained open and engaging. In contrast to recent certification decisions from other organizations, the audience clearly relished the opportunity to provide input, and the STP committee continues to look for able and willing participants.
1. A top-10 list is not enough to cover everything from PHM 2011. From clinical and practice conundrums galore to late nights at Spectators to Kevin Powell’s mad acting skills, a 1,500-word-top-10 list simply does not do the meeting justice! I place full blame on the planning committee for this overindulgent buffet and the unfortunate omission of many other meaningful lessons.
Thank you: Erin Stucky Fisher (chair), Brian Pate, Allison Ballantine, Matt Garber, Jeff Simmons, Doug Carlson and Tamara Simon.
If you’re feeling like you missed out, or have already digested and want more, PHM 2012 will be here soon enough to fill your appetite. Cincinnati, look out.
Dr. Shen is pediatric editor of The Hospitalist.
It’s been a while since I went to a buffet and stuffed myself silly, but that’s how my mind felt on the flight home from Kansas City, Mo. After four incredibly packed days at Pediatric Hospital Medicine (PHM) 2011, I was one “wafer-thin mint” away from an explosion. What I wanted was some kind of a mental digestif. What I got instead was a light beer. It helped a little bit, but when I awoke during the landing in Austin, Texas, I realized that I remained in need of a better way to distill the thousand points of information from the conference into something more manageable.
Buoyed by Michelle Marks and Joel Tieder’s Top 10 Articles of the Year luncheon, specifically the piece on neurosurgeons and Kangaroo Care, I thought I might try my own version of a decompressive operation.
Without further delay, here are the top 10 things that I learned at Pediatric Hospital Medicine 2011:
10. We continue to grow as a field. Although the exact number of pediatric hospitalists in the U.S. remains somewhat unclear (but is probably between 1,000 and 2,000), what is known is that attendance at our annual meeting grows every year. Our tripartite meeting, sponsored by the American Academy of Pediatrics (AAP), SHM, and the Academic Pediatric Association (APA), hit a record 450 attendees this year. Beyond the physical numbers, it is quite clear that we are growing in many other domains as well.
9. It is time to re-evaluate the impact of CME on physician practice and outcomes. The literature on continuing professional education has been quite sobering to date, with nothing to show for the thousands of dollars spent per individual. But most of those studies were performed in the last century (think eight hours of lecture a day followed by dinners with big pharma) and I’ll bet that there was not the focus on learner-centered education that was evident in Kansas City.
With a dizzying array of workshops and interactive small group sessions spread amongst seven different tracks, it was difficult, if not impossible, to be a passive participant in the process. And since learning retention rates are generally proportionate to how active a role adults play in their own education, I am going to guess that many other attendees’ brains still have that “I’m thinking” hourglass icon over them. We have the conference planning committee to thank for this.
8. The JCPHM (Joint Council of Pediatric Hospital Medicine) will be increasingly important as we develop. Much like the constant stream of unfamiliar new vaccine names that have appeared in recent years, this proposed new committee comes with another long set of initials and an unfamiliar indication.
The JCPHM will function as a coordinating body, ensuring that work done through the AAP, SHM, and AAP are aligned to provide maximal benefit to pediatric hospitalists as a whole. And thus, similar to immunizations, the benefits will be most evident if we remain healthy as we grow in the context of an increasingly complex environment.
7. Our collective research accomplishments merit national recognition. It was not more than just a few years ago that we were in our research infancy. Posters at our meetings largely represented single-site descriptive studies, typically using survey methodology. This year we had four research breakout tracks, in addition to the plenary and three poster sessions.
Pediatric Research in Inpatient Settings (PRIS) leads the way with a Forbes-like listing of million-dollar grants and a partnership with Child Health Corporation of America’s (CHCA) uber-powerful Pediatric Health Information Systems (PHIS) database. Expect some landmark studies in the near future.
In the meantime, it is clear that the rest of the field is not languishing from smaller budgets. From clear outcome and process measurements of family-centered rounds to studying the spectrum of transitions of care to the impact of early warning systems, there was a predominant focus on quality and safety.
In fact, the tone was set at the opening keynote address, as Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ), described creative and innovative ways to study and translate work in this area to improved patient care.
6. We are poised to develop effective training systems for our future workforce. From the use of our core competencies in sessions to a full complement of workshops on education (from individual to team and from student to fellow), it is clear that thoughtful deliberation has paved the way for our future. We possess expertise in education that dovetails nicely with our need to grow and sustain an experienced, well-trained workforce.
Intrinsically, we know that we possess a unique body of skills, knowledge, and attitudes. The explicit articulation of this into longitudinal curricula will headline our evolution as a field.
5. Our new peer-reviewed journal has a bright future. Kudos to Shawn Ralston and the rest of the editorial board for publishing the first peer-reviewed edition of Hospital Pediatrics. Original research, evidence-based content, and practical commentary grace the pages with a little bit of something for everyone. The AAP has demonstrated a healthy level of support for this endeavor, as they sent out an introductory email announcing the journal to all of their membership over the weekend. I am confident that support from our pediatric hospitalist community will follow in the form of an exponential increase in quality submissions. Look no further than the PHM 2011 abstract book to get a preview of what our journal will highlight in the near future.
4. We connect with each other through a language of quality and value in our work. Quality spanned the continuum from conversation to collaboration, as like-minded souls shared ideas and passions amidst the sessions. The improved outcomes demonstrated by the Value in Inpatient Pediatrics (VIP) network are a testament to the positive change that can arise from such efforts. VIP, with its focus on inclusive and front-line collaboratives, also announced an upcoming merger with the AAP’s Quality Improvement and Innovation Network (QuIIN), approved by the AAP board in May.
Perhaps more impressive was that more than 12% of the attendees at PHM 2011 attended the annual VIP dinner and similar numbers signed up to participate in future efforts. At this pace, widespread improvement and value are easily within our sights.
3. Complex care is the new family-centered rounds. Atul Gawande’s recent New Yorker article about “Hot Spotters” could very well have been referring to the body of work that is represented by pediatric generalists (to include a fair number of hospitalists) over the past few years. Closing plenary speakers Robert Lyle and Patrick Casey wowed the audience as they described their medical home for medically complex children—and an estimated savings to Arkansas Medicaid of nearly $3 million per year.
As hospitals and hospital systems look to create accountable care organizations (ACOs), this kind of work will be increasingly prioritized, as it has the potential to generate the biggest gains in valued care.
2. STP, yeah, you know me. Chris Maloney and Suzanne Swanson Mendez brought down the house at the PHM Roundtable update as they presented preliminary results of their large and representative STP (strategic planning) Committee, which is mapping out future certification options for pediatric hospitalists. A lively debate ensued as questions surrounding how to best notify and involve pediatric hospitalists in these decisions came to the forefront. Are we a democracy? Are we a republic? Is there a better model for this decision?
Despite the lack of consensus on how to best move toward a decision, the discussion remained open and engaging. In contrast to recent certification decisions from other organizations, the audience clearly relished the opportunity to provide input, and the STP committee continues to look for able and willing participants.
1. A top-10 list is not enough to cover everything from PHM 2011. From clinical and practice conundrums galore to late nights at Spectators to Kevin Powell’s mad acting skills, a 1,500-word-top-10 list simply does not do the meeting justice! I place full blame on the planning committee for this overindulgent buffet and the unfortunate omission of many other meaningful lessons.
Thank you: Erin Stucky Fisher (chair), Brian Pate, Allison Ballantine, Matt Garber, Jeff Simmons, Doug Carlson and Tamara Simon.
If you’re feeling like you missed out, or have already digested and want more, PHM 2012 will be here soon enough to fill your appetite. Cincinnati, look out.
Dr. Shen is pediatric editor of The Hospitalist.
It’s been a while since I went to a buffet and stuffed myself silly, but that’s how my mind felt on the flight home from Kansas City, Mo. After four incredibly packed days at Pediatric Hospital Medicine (PHM) 2011, I was one “wafer-thin mint” away from an explosion. What I wanted was some kind of a mental digestif. What I got instead was a light beer. It helped a little bit, but when I awoke during the landing in Austin, Texas, I realized that I remained in need of a better way to distill the thousand points of information from the conference into something more manageable.
Buoyed by Michelle Marks and Joel Tieder’s Top 10 Articles of the Year luncheon, specifically the piece on neurosurgeons and Kangaroo Care, I thought I might try my own version of a decompressive operation.
Without further delay, here are the top 10 things that I learned at Pediatric Hospital Medicine 2011:
10. We continue to grow as a field. Although the exact number of pediatric hospitalists in the U.S. remains somewhat unclear (but is probably between 1,000 and 2,000), what is known is that attendance at our annual meeting grows every year. Our tripartite meeting, sponsored by the American Academy of Pediatrics (AAP), SHM, and the Academic Pediatric Association (APA), hit a record 450 attendees this year. Beyond the physical numbers, it is quite clear that we are growing in many other domains as well.
9. It is time to re-evaluate the impact of CME on physician practice and outcomes. The literature on continuing professional education has been quite sobering to date, with nothing to show for the thousands of dollars spent per individual. But most of those studies were performed in the last century (think eight hours of lecture a day followed by dinners with big pharma) and I’ll bet that there was not the focus on learner-centered education that was evident in Kansas City.
With a dizzying array of workshops and interactive small group sessions spread amongst seven different tracks, it was difficult, if not impossible, to be a passive participant in the process. And since learning retention rates are generally proportionate to how active a role adults play in their own education, I am going to guess that many other attendees’ brains still have that “I’m thinking” hourglass icon over them. We have the conference planning committee to thank for this.
8. The JCPHM (Joint Council of Pediatric Hospital Medicine) will be increasingly important as we develop. Much like the constant stream of unfamiliar new vaccine names that have appeared in recent years, this proposed new committee comes with another long set of initials and an unfamiliar indication.
The JCPHM will function as a coordinating body, ensuring that work done through the AAP, SHM, and AAP are aligned to provide maximal benefit to pediatric hospitalists as a whole. And thus, similar to immunizations, the benefits will be most evident if we remain healthy as we grow in the context of an increasingly complex environment.
7. Our collective research accomplishments merit national recognition. It was not more than just a few years ago that we were in our research infancy. Posters at our meetings largely represented single-site descriptive studies, typically using survey methodology. This year we had four research breakout tracks, in addition to the plenary and three poster sessions.
Pediatric Research in Inpatient Settings (PRIS) leads the way with a Forbes-like listing of million-dollar grants and a partnership with Child Health Corporation of America’s (CHCA) uber-powerful Pediatric Health Information Systems (PHIS) database. Expect some landmark studies in the near future.
In the meantime, it is clear that the rest of the field is not languishing from smaller budgets. From clear outcome and process measurements of family-centered rounds to studying the spectrum of transitions of care to the impact of early warning systems, there was a predominant focus on quality and safety.
In fact, the tone was set at the opening keynote address, as Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ), described creative and innovative ways to study and translate work in this area to improved patient care.
6. We are poised to develop effective training systems for our future workforce. From the use of our core competencies in sessions to a full complement of workshops on education (from individual to team and from student to fellow), it is clear that thoughtful deliberation has paved the way for our future. We possess expertise in education that dovetails nicely with our need to grow and sustain an experienced, well-trained workforce.
Intrinsically, we know that we possess a unique body of skills, knowledge, and attitudes. The explicit articulation of this into longitudinal curricula will headline our evolution as a field.
5. Our new peer-reviewed journal has a bright future. Kudos to Shawn Ralston and the rest of the editorial board for publishing the first peer-reviewed edition of Hospital Pediatrics. Original research, evidence-based content, and practical commentary grace the pages with a little bit of something for everyone. The AAP has demonstrated a healthy level of support for this endeavor, as they sent out an introductory email announcing the journal to all of their membership over the weekend. I am confident that support from our pediatric hospitalist community will follow in the form of an exponential increase in quality submissions. Look no further than the PHM 2011 abstract book to get a preview of what our journal will highlight in the near future.
4. We connect with each other through a language of quality and value in our work. Quality spanned the continuum from conversation to collaboration, as like-minded souls shared ideas and passions amidst the sessions. The improved outcomes demonstrated by the Value in Inpatient Pediatrics (VIP) network are a testament to the positive change that can arise from such efforts. VIP, with its focus on inclusive and front-line collaboratives, also announced an upcoming merger with the AAP’s Quality Improvement and Innovation Network (QuIIN), approved by the AAP board in May.
Perhaps more impressive was that more than 12% of the attendees at PHM 2011 attended the annual VIP dinner and similar numbers signed up to participate in future efforts. At this pace, widespread improvement and value are easily within our sights.
3. Complex care is the new family-centered rounds. Atul Gawande’s recent New Yorker article about “Hot Spotters” could very well have been referring to the body of work that is represented by pediatric generalists (to include a fair number of hospitalists) over the past few years. Closing plenary speakers Robert Lyle and Patrick Casey wowed the audience as they described their medical home for medically complex children—and an estimated savings to Arkansas Medicaid of nearly $3 million per year.
As hospitals and hospital systems look to create accountable care organizations (ACOs), this kind of work will be increasingly prioritized, as it has the potential to generate the biggest gains in valued care.
2. STP, yeah, you know me. Chris Maloney and Suzanne Swanson Mendez brought down the house at the PHM Roundtable update as they presented preliminary results of their large and representative STP (strategic planning) Committee, which is mapping out future certification options for pediatric hospitalists. A lively debate ensued as questions surrounding how to best notify and involve pediatric hospitalists in these decisions came to the forefront. Are we a democracy? Are we a republic? Is there a better model for this decision?
Despite the lack of consensus on how to best move toward a decision, the discussion remained open and engaging. In contrast to recent certification decisions from other organizations, the audience clearly relished the opportunity to provide input, and the STP committee continues to look for able and willing participants.
1. A top-10 list is not enough to cover everything from PHM 2011. From clinical and practice conundrums galore to late nights at Spectators to Kevin Powell’s mad acting skills, a 1,500-word-top-10 list simply does not do the meeting justice! I place full blame on the planning committee for this overindulgent buffet and the unfortunate omission of many other meaningful lessons.
Thank you: Erin Stucky Fisher (chair), Brian Pate, Allison Ballantine, Matt Garber, Jeff Simmons, Doug Carlson and Tamara Simon.
If you’re feeling like you missed out, or have already digested and want more, PHM 2012 will be here soon enough to fill your appetite. Cincinnati, look out.
Dr. Shen is pediatric editor of The Hospitalist.
Dr. Hospitalist
What I am curious about are the current issues around using length of stay (LOS) or cost/case or the like as part of compensation packages. I have had discussions with several other folks, and I think I am getting the picture. However, it sounds like there has been some new interpretation of the laws around gainsharing, and that is what I am curious about.
K.S., Ohio
Dr. Hospitalist responds:
Tough question, and let me start by saying that I’m not a healthcare lawyer: This stuff is tricky. I’ll do my best to explain the current situation as I understand it, but I’m no expert on this.
So gainsharing, as generally defined in healthcare, is where a hospital and a group of physicians design a contract around services for which the two sides can share in any savings. Physicians are paid fee-for-service by Medicare, thus they are reimbursed per unit of work, with no incentive for cost control. Hospitals are paid on a per-case (or per-procedure) basis, so cost control means a lot to them: Because they get a set payment, any savings generated, they get to keep. Ideally, this means that better performance leads to more efficient care, less waste, and better outcomes. Unfortunately, that’s not always what happens, especially in the view of the federal government (you know, the guys who issue the bright orange jumpsuits for you to wear when you break the law).
Gainsharing has an interesting history as interpreted by the Office of the Inspector General (OIG) and the Centers for Medicare & Medicaid Services (CMS). Back in 1999, the OIG explicitly stopped any gainsharing models between physicians and hospitals based on concerns that these contracts might reduce the care provided to patients. The opinion was that there might be a “race to the bottom” in terms of cutting expenses (read: services).
Since then, there has been only incremental movement forward in the form of demonstration projects. One project looked at two very specific procedures: cardiac catheterization and coronary artery bypass grafting (CABG). The results showed that gainsharing could be beneficial to the hospital-physician relationship, and, more important, not harmful to the patient. There has since been some movement toward gainsharing, but only in the context of specific procedures, with very clear safeguards around it, including an independent auditor. Nothing to this point has suggested that a cost per case or adjusted LOS gainsharing agreement would pass muster with the OIG.
So, at this point in time, I would caution against any contract that contained explicit references connecting compensation to a change in hospital costs, such as reducing LOS or cost per case. The new accountable-care organization (ACO) model might be a different prism through which to view this, but it’s a world apart from an individual physician or hospitalist group contract (see “A Chilly Reception,” August 2011, p. 23).
For contractual compensation, I think that quality metrics can fill a need, and there are lots of ways to be creative here. You could set a target around something measurable (appropriate DVT prophylaxis is just one example) and tie dollars to that specific performance. The key is avoiding any language that would imply additional physician compensation for a reduction in patient services.
Might things change in the future? Your guess is as good as mine.
What I am curious about are the current issues around using length of stay (LOS) or cost/case or the like as part of compensation packages. I have had discussions with several other folks, and I think I am getting the picture. However, it sounds like there has been some new interpretation of the laws around gainsharing, and that is what I am curious about.
K.S., Ohio
Dr. Hospitalist responds:
Tough question, and let me start by saying that I’m not a healthcare lawyer: This stuff is tricky. I’ll do my best to explain the current situation as I understand it, but I’m no expert on this.
So gainsharing, as generally defined in healthcare, is where a hospital and a group of physicians design a contract around services for which the two sides can share in any savings. Physicians are paid fee-for-service by Medicare, thus they are reimbursed per unit of work, with no incentive for cost control. Hospitals are paid on a per-case (or per-procedure) basis, so cost control means a lot to them: Because they get a set payment, any savings generated, they get to keep. Ideally, this means that better performance leads to more efficient care, less waste, and better outcomes. Unfortunately, that’s not always what happens, especially in the view of the federal government (you know, the guys who issue the bright orange jumpsuits for you to wear when you break the law).
Gainsharing has an interesting history as interpreted by the Office of the Inspector General (OIG) and the Centers for Medicare & Medicaid Services (CMS). Back in 1999, the OIG explicitly stopped any gainsharing models between physicians and hospitals based on concerns that these contracts might reduce the care provided to patients. The opinion was that there might be a “race to the bottom” in terms of cutting expenses (read: services).
Since then, there has been only incremental movement forward in the form of demonstration projects. One project looked at two very specific procedures: cardiac catheterization and coronary artery bypass grafting (CABG). The results showed that gainsharing could be beneficial to the hospital-physician relationship, and, more important, not harmful to the patient. There has since been some movement toward gainsharing, but only in the context of specific procedures, with very clear safeguards around it, including an independent auditor. Nothing to this point has suggested that a cost per case or adjusted LOS gainsharing agreement would pass muster with the OIG.
So, at this point in time, I would caution against any contract that contained explicit references connecting compensation to a change in hospital costs, such as reducing LOS or cost per case. The new accountable-care organization (ACO) model might be a different prism through which to view this, but it’s a world apart from an individual physician or hospitalist group contract (see “A Chilly Reception,” August 2011, p. 23).
For contractual compensation, I think that quality metrics can fill a need, and there are lots of ways to be creative here. You could set a target around something measurable (appropriate DVT prophylaxis is just one example) and tie dollars to that specific performance. The key is avoiding any language that would imply additional physician compensation for a reduction in patient services.
Might things change in the future? Your guess is as good as mine.
What I am curious about are the current issues around using length of stay (LOS) or cost/case or the like as part of compensation packages. I have had discussions with several other folks, and I think I am getting the picture. However, it sounds like there has been some new interpretation of the laws around gainsharing, and that is what I am curious about.
K.S., Ohio
Dr. Hospitalist responds:
Tough question, and let me start by saying that I’m not a healthcare lawyer: This stuff is tricky. I’ll do my best to explain the current situation as I understand it, but I’m no expert on this.
So gainsharing, as generally defined in healthcare, is where a hospital and a group of physicians design a contract around services for which the two sides can share in any savings. Physicians are paid fee-for-service by Medicare, thus they are reimbursed per unit of work, with no incentive for cost control. Hospitals are paid on a per-case (or per-procedure) basis, so cost control means a lot to them: Because they get a set payment, any savings generated, they get to keep. Ideally, this means that better performance leads to more efficient care, less waste, and better outcomes. Unfortunately, that’s not always what happens, especially in the view of the federal government (you know, the guys who issue the bright orange jumpsuits for you to wear when you break the law).
Gainsharing has an interesting history as interpreted by the Office of the Inspector General (OIG) and the Centers for Medicare & Medicaid Services (CMS). Back in 1999, the OIG explicitly stopped any gainsharing models between physicians and hospitals based on concerns that these contracts might reduce the care provided to patients. The opinion was that there might be a “race to the bottom” in terms of cutting expenses (read: services).
Since then, there has been only incremental movement forward in the form of demonstration projects. One project looked at two very specific procedures: cardiac catheterization and coronary artery bypass grafting (CABG). The results showed that gainsharing could be beneficial to the hospital-physician relationship, and, more important, not harmful to the patient. There has since been some movement toward gainsharing, but only in the context of specific procedures, with very clear safeguards around it, including an independent auditor. Nothing to this point has suggested that a cost per case or adjusted LOS gainsharing agreement would pass muster with the OIG.
So, at this point in time, I would caution against any contract that contained explicit references connecting compensation to a change in hospital costs, such as reducing LOS or cost per case. The new accountable-care organization (ACO) model might be a different prism through which to view this, but it’s a world apart from an individual physician or hospitalist group contract (see “A Chilly Reception,” August 2011, p. 23).
For contractual compensation, I think that quality metrics can fill a need, and there are lots of ways to be creative here. You could set a target around something measurable (appropriate DVT prophylaxis is just one example) and tie dollars to that specific performance. The key is avoiding any language that would imply additional physician compensation for a reduction in patient services.
Might things change in the future? Your guess is as good as mine.