User login
Fast and Furious
Every May, Mayo Clinic hospitalist Jason Persoff, MD, SFHM, sheds his doctor’s gear, grabs his camera and camcorder, and heads to the Midwest in search of ferocious weather for two weeks. “My wife jokingly calls it my ‘midlife crisis prevention program,’ ” says Dr. Persoff, who works in Jacksonville, Fla.
This year, he put his doctor’s gear back on sooner than he expected.
After 20 years of chasing storms, Dr. Persoff found himself in what might have been considered an inevitable situation: helping people injured in a tornado. When a monstrous twister with winds of more than 200 mph barreled through Joplin, Mo., on May 22, Dr. Persoff was less than a mile from its path. He and a “chase partner,” Robert Balogh, MD, an Oklahoma-based internist and former hospitalist, were able to rush to the scene and assist in the aftermath.
In the moments after the fast-forming storm, Dr. Persoff hoped that the damage wouldn’t be so devastating, despite the first ominous signs he saw along the highway.
“We were dealing with a raining sky of debris,” he says. “There was Styrofoam insulation falling from the sky, papers, there was a Barbie doll in the middle of the road, but I have no idea where that came from. There were trees and twigs and leaves, so I knew that the destruction to Joplin had been significant. But I hoped that it would be very limited.”
As he traveled along another road, he saw two dozen flipped-over semi-trucks.
“There was no decision,” Dr. Balogh says. “We knew right then that the chase was over for us.”
One hospital serving the area, St. John’s Regional Medical Center, was destroyed, its roof ripped off, he learned. At press time, the tornado had killed more than 150 and caused an estimated $3 billion in damage.
Dr. Persoff checked in at the ED of another hospital, Freeman Health System, and offered his help. He spent 10 hours there, first treating trauma patients.
“We were immediately put to work because there were just so many people coming in,” he says. “The initial trauma that came in was pretty fast and furious. If somebody could be saved, and it wasn’t going to require an effort that would jeopardize resources, they did everything they could to save people. They put in chest tubes, ventilated them, [performed] other procedures.
"If somebody was dying and that was pretty obvious, it required us to rethink how we were going to approach things. And I made a diligent effort to help the dying with low doses of pain medication to help them through.”
There were amputations, impalements, eviscerations.
“We had patients who were covered in glass, and by covered I don’t mean they just had glass in their skin—they were covered with it,” he says. “When you’d examine them, there was a risk of your glove getting torn doing an exam.”
Dr. Balogh describes the patient influx as an “absolutely overwhelming” onslaught, with ambulances, cars, and pickup trucks that had rescued strangers on the roadside arriving seemingly nonstop.
It was so frantic, he says, that he was worried “if I even take time to talk to one patient .. I’ve missed the next 15.”
When the patients from St. John’s began to arrive at Freeman, Dr. Persoff treated them, too. He wrote admission orders on 24 patients.
“The patients weren’t able to provide history,” he says. “Some of the medical records fell as far as, I think, Kansas City (160 miles to the north), from the air,” he explains. “So we had no medical records. We had patients who were demented or delirious. We had patients who’d undergone routine procedures, several patients who were postoperative.”
Leaving the hospital, he said, was gut-wrenching.
“I felt like a loser. I felt like I was handing patient-care responsibilities to a completely overtaxed system because I was tired,” he says. “When I started not making good decisions, I knew that I wasn’t helping anybody and it was time for me to step aside. But that was a very hard decision to make.”
Dr. Persoff says he’ll never forget the triage nurse on duty. She was there when he arrived, about 6:30 p.m., and was perfectly orchestrating the trauma care, even though there was no way for any of the hospital staff to know what had become of their own families and homes. And she was still there when he left at 4 a.m., so efficient and fresh it was as if she’d “just come in from having showered.”
“I don’t know what she knew or where her house was or where her family was,” he says. “I just knew that she was there working like there was no tomorrow and doing it in a way that I couldn’t. That was one of the times where I was like, ‘Wow, this is really humbling.’ ”
Dr. Persoff, who writes about his hobby at Stormdoctor.blogspot.com, continued his storm chasing; he even helped provide assistance two days later, after storms near Oklahoma City exacted a human toll that was not nearly as severe. But first, he says, he had to do some soul-searching. After all, he had hoped for a tornado to form in the Joplin area.
“My chase partners and I were talking about how can the rational person want to continue storm-chasing after having seen what we’d seen. And it took me a while to sort of figure out where my own conscience was on this,” he says. “I felt very guilty for having even wanted [a tornado] earlier in the day. Then I also felt like, had the storm not formed where it did, I wouldn’t have been there, my partner Dr. Balogh wouldn’t have been there, and we would not have been able to assist in that disaster.
“So in many ways it was karma. It happened. We were there at a time when Joplin needed some help.”
After the storm, Dr. Persoff received words of thanks from the town.
Jane Culver, a floor nurse with whom he worked, told him via email: “People often say to me, ‘Doctors are just in it for the money, they really don’t really care about me.’ Well, I say they don’t know the Dr. Jason Persoffs of the world. You are a true humanitarian, and the people of Joplin are lucky you were in our midst at our hour of need.”
Stephanie Conrad, whose grandmother Clara had her broken hip cared for by Dr. Persoff, called him “the angel doctor.”
“Thank you so much for using your knowledge, skills, and expertise during this crisis,” Conrad wrote in an email. “It is physicians like you that make a difference in the lives of others. You were truly a blessing that night.”
Tom Collins is a freelance medical writer based in Florida.
Every May, Mayo Clinic hospitalist Jason Persoff, MD, SFHM, sheds his doctor’s gear, grabs his camera and camcorder, and heads to the Midwest in search of ferocious weather for two weeks. “My wife jokingly calls it my ‘midlife crisis prevention program,’ ” says Dr. Persoff, who works in Jacksonville, Fla.
This year, he put his doctor’s gear back on sooner than he expected.
After 20 years of chasing storms, Dr. Persoff found himself in what might have been considered an inevitable situation: helping people injured in a tornado. When a monstrous twister with winds of more than 200 mph barreled through Joplin, Mo., on May 22, Dr. Persoff was less than a mile from its path. He and a “chase partner,” Robert Balogh, MD, an Oklahoma-based internist and former hospitalist, were able to rush to the scene and assist in the aftermath.
In the moments after the fast-forming storm, Dr. Persoff hoped that the damage wouldn’t be so devastating, despite the first ominous signs he saw along the highway.
“We were dealing with a raining sky of debris,” he says. “There was Styrofoam insulation falling from the sky, papers, there was a Barbie doll in the middle of the road, but I have no idea where that came from. There were trees and twigs and leaves, so I knew that the destruction to Joplin had been significant. But I hoped that it would be very limited.”
As he traveled along another road, he saw two dozen flipped-over semi-trucks.
“There was no decision,” Dr. Balogh says. “We knew right then that the chase was over for us.”
One hospital serving the area, St. John’s Regional Medical Center, was destroyed, its roof ripped off, he learned. At press time, the tornado had killed more than 150 and caused an estimated $3 billion in damage.
Dr. Persoff checked in at the ED of another hospital, Freeman Health System, and offered his help. He spent 10 hours there, first treating trauma patients.
“We were immediately put to work because there were just so many people coming in,” he says. “The initial trauma that came in was pretty fast and furious. If somebody could be saved, and it wasn’t going to require an effort that would jeopardize resources, they did everything they could to save people. They put in chest tubes, ventilated them, [performed] other procedures.
"If somebody was dying and that was pretty obvious, it required us to rethink how we were going to approach things. And I made a diligent effort to help the dying with low doses of pain medication to help them through.”
There were amputations, impalements, eviscerations.
“We had patients who were covered in glass, and by covered I don’t mean they just had glass in their skin—they were covered with it,” he says. “When you’d examine them, there was a risk of your glove getting torn doing an exam.”
Dr. Balogh describes the patient influx as an “absolutely overwhelming” onslaught, with ambulances, cars, and pickup trucks that had rescued strangers on the roadside arriving seemingly nonstop.
It was so frantic, he says, that he was worried “if I even take time to talk to one patient .. I’ve missed the next 15.”
When the patients from St. John’s began to arrive at Freeman, Dr. Persoff treated them, too. He wrote admission orders on 24 patients.
“The patients weren’t able to provide history,” he says. “Some of the medical records fell as far as, I think, Kansas City (160 miles to the north), from the air,” he explains. “So we had no medical records. We had patients who were demented or delirious. We had patients who’d undergone routine procedures, several patients who were postoperative.”
Leaving the hospital, he said, was gut-wrenching.
“I felt like a loser. I felt like I was handing patient-care responsibilities to a completely overtaxed system because I was tired,” he says. “When I started not making good decisions, I knew that I wasn’t helping anybody and it was time for me to step aside. But that was a very hard decision to make.”
Dr. Persoff says he’ll never forget the triage nurse on duty. She was there when he arrived, about 6:30 p.m., and was perfectly orchestrating the trauma care, even though there was no way for any of the hospital staff to know what had become of their own families and homes. And she was still there when he left at 4 a.m., so efficient and fresh it was as if she’d “just come in from having showered.”
“I don’t know what she knew or where her house was or where her family was,” he says. “I just knew that she was there working like there was no tomorrow and doing it in a way that I couldn’t. That was one of the times where I was like, ‘Wow, this is really humbling.’ ”
Dr. Persoff, who writes about his hobby at Stormdoctor.blogspot.com, continued his storm chasing; he even helped provide assistance two days later, after storms near Oklahoma City exacted a human toll that was not nearly as severe. But first, he says, he had to do some soul-searching. After all, he had hoped for a tornado to form in the Joplin area.
“My chase partners and I were talking about how can the rational person want to continue storm-chasing after having seen what we’d seen. And it took me a while to sort of figure out where my own conscience was on this,” he says. “I felt very guilty for having even wanted [a tornado] earlier in the day. Then I also felt like, had the storm not formed where it did, I wouldn’t have been there, my partner Dr. Balogh wouldn’t have been there, and we would not have been able to assist in that disaster.
“So in many ways it was karma. It happened. We were there at a time when Joplin needed some help.”
After the storm, Dr. Persoff received words of thanks from the town.
Jane Culver, a floor nurse with whom he worked, told him via email: “People often say to me, ‘Doctors are just in it for the money, they really don’t really care about me.’ Well, I say they don’t know the Dr. Jason Persoffs of the world. You are a true humanitarian, and the people of Joplin are lucky you were in our midst at our hour of need.”
Stephanie Conrad, whose grandmother Clara had her broken hip cared for by Dr. Persoff, called him “the angel doctor.”
“Thank you so much for using your knowledge, skills, and expertise during this crisis,” Conrad wrote in an email. “It is physicians like you that make a difference in the lives of others. You were truly a blessing that night.”
Tom Collins is a freelance medical writer based in Florida.
Every May, Mayo Clinic hospitalist Jason Persoff, MD, SFHM, sheds his doctor’s gear, grabs his camera and camcorder, and heads to the Midwest in search of ferocious weather for two weeks. “My wife jokingly calls it my ‘midlife crisis prevention program,’ ” says Dr. Persoff, who works in Jacksonville, Fla.
This year, he put his doctor’s gear back on sooner than he expected.
After 20 years of chasing storms, Dr. Persoff found himself in what might have been considered an inevitable situation: helping people injured in a tornado. When a monstrous twister with winds of more than 200 mph barreled through Joplin, Mo., on May 22, Dr. Persoff was less than a mile from its path. He and a “chase partner,” Robert Balogh, MD, an Oklahoma-based internist and former hospitalist, were able to rush to the scene and assist in the aftermath.
In the moments after the fast-forming storm, Dr. Persoff hoped that the damage wouldn’t be so devastating, despite the first ominous signs he saw along the highway.
“We were dealing with a raining sky of debris,” he says. “There was Styrofoam insulation falling from the sky, papers, there was a Barbie doll in the middle of the road, but I have no idea where that came from. There were trees and twigs and leaves, so I knew that the destruction to Joplin had been significant. But I hoped that it would be very limited.”
As he traveled along another road, he saw two dozen flipped-over semi-trucks.
“There was no decision,” Dr. Balogh says. “We knew right then that the chase was over for us.”
One hospital serving the area, St. John’s Regional Medical Center, was destroyed, its roof ripped off, he learned. At press time, the tornado had killed more than 150 and caused an estimated $3 billion in damage.
Dr. Persoff checked in at the ED of another hospital, Freeman Health System, and offered his help. He spent 10 hours there, first treating trauma patients.
“We were immediately put to work because there were just so many people coming in,” he says. “The initial trauma that came in was pretty fast and furious. If somebody could be saved, and it wasn’t going to require an effort that would jeopardize resources, they did everything they could to save people. They put in chest tubes, ventilated them, [performed] other procedures.
"If somebody was dying and that was pretty obvious, it required us to rethink how we were going to approach things. And I made a diligent effort to help the dying with low doses of pain medication to help them through.”
There were amputations, impalements, eviscerations.
“We had patients who were covered in glass, and by covered I don’t mean they just had glass in their skin—they were covered with it,” he says. “When you’d examine them, there was a risk of your glove getting torn doing an exam.”
Dr. Balogh describes the patient influx as an “absolutely overwhelming” onslaught, with ambulances, cars, and pickup trucks that had rescued strangers on the roadside arriving seemingly nonstop.
It was so frantic, he says, that he was worried “if I even take time to talk to one patient .. I’ve missed the next 15.”
When the patients from St. John’s began to arrive at Freeman, Dr. Persoff treated them, too. He wrote admission orders on 24 patients.
“The patients weren’t able to provide history,” he says. “Some of the medical records fell as far as, I think, Kansas City (160 miles to the north), from the air,” he explains. “So we had no medical records. We had patients who were demented or delirious. We had patients who’d undergone routine procedures, several patients who were postoperative.”
Leaving the hospital, he said, was gut-wrenching.
“I felt like a loser. I felt like I was handing patient-care responsibilities to a completely overtaxed system because I was tired,” he says. “When I started not making good decisions, I knew that I wasn’t helping anybody and it was time for me to step aside. But that was a very hard decision to make.”
Dr. Persoff says he’ll never forget the triage nurse on duty. She was there when he arrived, about 6:30 p.m., and was perfectly orchestrating the trauma care, even though there was no way for any of the hospital staff to know what had become of their own families and homes. And she was still there when he left at 4 a.m., so efficient and fresh it was as if she’d “just come in from having showered.”
“I don’t know what she knew or where her house was or where her family was,” he says. “I just knew that she was there working like there was no tomorrow and doing it in a way that I couldn’t. That was one of the times where I was like, ‘Wow, this is really humbling.’ ”
Dr. Persoff, who writes about his hobby at Stormdoctor.blogspot.com, continued his storm chasing; he even helped provide assistance two days later, after storms near Oklahoma City exacted a human toll that was not nearly as severe. But first, he says, he had to do some soul-searching. After all, he had hoped for a tornado to form in the Joplin area.
“My chase partners and I were talking about how can the rational person want to continue storm-chasing after having seen what we’d seen. And it took me a while to sort of figure out where my own conscience was on this,” he says. “I felt very guilty for having even wanted [a tornado] earlier in the day. Then I also felt like, had the storm not formed where it did, I wouldn’t have been there, my partner Dr. Balogh wouldn’t have been there, and we would not have been able to assist in that disaster.
“So in many ways it was karma. It happened. We were there at a time when Joplin needed some help.”
After the storm, Dr. Persoff received words of thanks from the town.
Jane Culver, a floor nurse with whom he worked, told him via email: “People often say to me, ‘Doctors are just in it for the money, they really don’t really care about me.’ Well, I say they don’t know the Dr. Jason Persoffs of the world. You are a true humanitarian, and the people of Joplin are lucky you were in our midst at our hour of need.”
Stephanie Conrad, whose grandmother Clara had her broken hip cared for by Dr. Persoff, called him “the angel doctor.”
“Thank you so much for using your knowledge, skills, and expertise during this crisis,” Conrad wrote in an email. “It is physicians like you that make a difference in the lives of others. You were truly a blessing that night.”
Tom Collins is a freelance medical writer based in Florida.
Cause For Concern
When a drug is in short supply at Beth Israel Deaconess Medical Center in Boston, a message goes out to the physicians on the hospital’s intranet system. When the shortage gets close to being critically short in supply, a message will be embedded into the physician order-entry system recommending that the physicians use an alternate drug—if there is an alternate.
It’s an alert system that has been put to frequent use lately, says Joseph Li, MD, SFHM, director of the hospital medicine program at Beth Israel Deaconess, associate professor of medicine at Harvard Medical School, and president of SHM.
The rate of drug shortages has been rising steadily in recent years due to quality questions at manufacturers, consolidation in the drug-manufacturing industry, and other factors, according to data from the U.S. Food and Drug Administration and other sources.
“It does seem like there’s more today than previous years,” says Dr. Li, who was a pharmacist before he trained in internal medicine.
Some of the recent shortages at Beth Israel Deaconess have involved the diuretic furosemide, the antiemetic Compazine, and the anticoagulant heparin. “More often than not, there’s a reasonable alternative that can be chosen,” he says. “Not necessarily exactly the same drug, but usually in the same therapeutic class.”
While actual cases of patient harm due to drug shortages appear to be relatively uncommon, having drugs in short supply can lead to a safety problem hovering over a medical center and its hospitalists. In addition to the potential of simply not having an alternate to give to a patient, hospitalists and their pharmacists sometimes have to adjust to a new dosage that comes with a replacement medication.
Plus, having to manage the problem when a drug shortage hits can be a headache, with time and resources spent trying to obtain updates from drug manufacturers and find other drugs that can be used in the meantime, experts say.
With hospitalists now treating so many patients, many of them complex and on multiple medications, it is an important issue for hospitalists to stay aware of and to be prepared for, Dr. Li says. More than 90% of all medical patients at Beth Israel Deaconess are now cared for by hospitalists, he says, and it’s a similar situation for many acute-care hospitals around the country.
If a drug is in short supply, balancing availability with patient needs can be especially tricky for a hospitalist caring for patients with a multitude of demands, Dr. Li says. “There is an effort to make sure that our most vulnerable population of patients receive these treatments before the general population of patients have access to it,” he adds.
However, the very existence of hospitalists makes it easier to navigate a shortage compared to the days when hundreds of providers would be caring for a pool of patients.
“If you’re trying to notify a group of providers about shortages and have an impact on their prescribing habits, I think it’s easier today,” he says.
Troubled Waters
The FDA says it confirmed a record 178 cases of drug shortages in 2010 (www.fda.gov/drugs/drugsafety/drugshortages/default.htm). That was up from 55 shortages five years ago. And according to the University of Utah Drug Information Service, the problem is actually more pervasive than that, reporting 120 shortages in the U.S. in 2001, with a reported 211 in 2010. And through March of this year, there were 80 reported cases of shortages, on pace for another record year.
“In the past couple of years, it’s just been exponential,” says Diane Ginsburg, president of the American Society of Health-System Pharmacists and clinical professor and assistant dean for student affairs at the University of Texas’ College of Pharmacy in Austin.
According to the FDA, 77% of the shortages in 2010 involved sterile injectable drugs.
“There are fewer and fewer firms making these older sterile injectables, and they are often discontinued for newer, more profitable agents,” FDA spokeswoman Yolanda Fultz-Morris said in an email. “When one firm has a delay or a manufacturing problem, it is extremely difficult for the remaining firms to quickly increase production.”
The biggest cause for the shortages in those drugs has been product quality issues, namely microbial contamination and newly identified impurities, according to the FDA. From January to October of 2010, 42% of drug shortages were due to quality problems.
Eighteen percent were due to product discontinuation by the manufacturer and another 18% were due to delays and capacity problems. Nine percent were due to difficulties getting raw materials, and 4% of the sterile injectable shortages were due to increased demand because there was a shortage of another injectable medication. In other words, one shortage led directly to another.
Kevin Schweers, a spokesman for the National Community Pharmacists Association, says generic drugs, especially Schedule II substances, have been in short supply. But there can be problems even when one generic is available to replace another generic.
An example, he says, is when a “new generic substituted in place of the old one is made by a different manufacturer and may come in a different color or shape. That can leave patients”—including those just released from hospitals—“wondering and asking the pharmacist why their medication is different or if a mistake was made.”
Patient Safety and Communication Errors
Lalit Verma, MD, director of the hospital medicine program at Durham Regional Medical Center in North Carolina and assistant professor of medicine at the Duke University School of Medicine, is unaware of any situations in which a shortage put patients in jeopardy at his hospital. He says the pharmacy at Durham Regional, which has seen recent shortages in morphine and heparin, among other drugs, keeps doctors up to date and has adjusted doses appropriately when replacements are used.
“It’s probably been more than I’ve experienced in my 10 years as a hospitalist,” Dr. Verma says. “We have a very good pharmacy program that updates us regularly on drug shortages and offers alternatives.”
Dr. Li also says no patient’s safety has been jeopardized by a shortage.
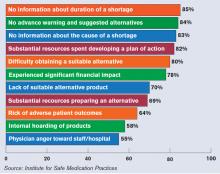
Others say patient safety has been affected, according to 1,800 healthcare practitioners who participated in a survey last year conducted by the Institute for Safe Medication Practices (ISMP), a nonprofit group. Twenty percent of the respondents said drug-shortage-related errors were made, while 32% said they had “near misses” related to drug shortages. Nineteen percent said there had been adverse patient outcomes as a result of drug shortages.
The study noted two instances in which patients died when they were switched to dilaudid because morphine was in short supply; both patients were given morphine doses instead of adjusted doses for dilaudid.
“It’s about six- or sevenfold more potent than morphine,” says Michael Cohen, ISMP president. “And so when that drug is prescribed in a morphine dose, that would be a massive overdose for some patients.”
He adds that hospitals have tried to stay on top of the drug shortage problem, but that “it’s very difficult.”
“A lot of this happens last-minute,” Cohen says. “Physicians aren’t given a chance to even realize that a certain drug isn’t available, so it causes an interruption in the whole flow of things in the hospital.” Some hospitals have had to hire staffers who handle just the inevitable daily drug shortages, he adds.
A law has been proposed in the U.S. Senate that would require drug manufacturers to notify the FDA when circumstances arise that might reasonably lead to a drug shortage (see “Senate Bill Would Require Advance Notice of Potential Shortages,” p. 41).
Cohen says another concern is that some hospitals, faced with shortages in electrolytes, such as potassium phosphate and sodium acetate, have been turning to less-regulated sterile compounding pharmacies for the products.
Dr. Verma, of Durham Regional, says perhaps the biggest challenge is staying on top of changing doses. “I think there was a learning curve for physicians in using dilaudid [rather than morphine] because the dosing is quite different, so that can cause challenges for patient care when you’re switching in and out of drug classes,” he says. “It’s not a perfect science. It doesn’t cripple us, but it does make it more challenging to fine-tune patient care.”
Ginsburg, of the ASHP, urges hospitalists to stay in close contact with the pharmacists at their hospitals and to be diligent about reporting shortages to the ASHP.
“Please work closely with the pharmacists, because we’re the ones that can really help,” she says. “We’re in it together with them, in terms of trying to provide care for their patients.” TH
Thomas R. Collins a freelance medical writer based in Florida.
When a drug is in short supply at Beth Israel Deaconess Medical Center in Boston, a message goes out to the physicians on the hospital’s intranet system. When the shortage gets close to being critically short in supply, a message will be embedded into the physician order-entry system recommending that the physicians use an alternate drug—if there is an alternate.
It’s an alert system that has been put to frequent use lately, says Joseph Li, MD, SFHM, director of the hospital medicine program at Beth Israel Deaconess, associate professor of medicine at Harvard Medical School, and president of SHM.
The rate of drug shortages has been rising steadily in recent years due to quality questions at manufacturers, consolidation in the drug-manufacturing industry, and other factors, according to data from the U.S. Food and Drug Administration and other sources.
“It does seem like there’s more today than previous years,” says Dr. Li, who was a pharmacist before he trained in internal medicine.
Some of the recent shortages at Beth Israel Deaconess have involved the diuretic furosemide, the antiemetic Compazine, and the anticoagulant heparin. “More often than not, there’s a reasonable alternative that can be chosen,” he says. “Not necessarily exactly the same drug, but usually in the same therapeutic class.”
While actual cases of patient harm due to drug shortages appear to be relatively uncommon, having drugs in short supply can lead to a safety problem hovering over a medical center and its hospitalists. In addition to the potential of simply not having an alternate to give to a patient, hospitalists and their pharmacists sometimes have to adjust to a new dosage that comes with a replacement medication.
Plus, having to manage the problem when a drug shortage hits can be a headache, with time and resources spent trying to obtain updates from drug manufacturers and find other drugs that can be used in the meantime, experts say.
With hospitalists now treating so many patients, many of them complex and on multiple medications, it is an important issue for hospitalists to stay aware of and to be prepared for, Dr. Li says. More than 90% of all medical patients at Beth Israel Deaconess are now cared for by hospitalists, he says, and it’s a similar situation for many acute-care hospitals around the country.
If a drug is in short supply, balancing availability with patient needs can be especially tricky for a hospitalist caring for patients with a multitude of demands, Dr. Li says. “There is an effort to make sure that our most vulnerable population of patients receive these treatments before the general population of patients have access to it,” he adds.
However, the very existence of hospitalists makes it easier to navigate a shortage compared to the days when hundreds of providers would be caring for a pool of patients.
“If you’re trying to notify a group of providers about shortages and have an impact on their prescribing habits, I think it’s easier today,” he says.
Troubled Waters
The FDA says it confirmed a record 178 cases of drug shortages in 2010 (www.fda.gov/drugs/drugsafety/drugshortages/default.htm). That was up from 55 shortages five years ago. And according to the University of Utah Drug Information Service, the problem is actually more pervasive than that, reporting 120 shortages in the U.S. in 2001, with a reported 211 in 2010. And through March of this year, there were 80 reported cases of shortages, on pace for another record year.
“In the past couple of years, it’s just been exponential,” says Diane Ginsburg, president of the American Society of Health-System Pharmacists and clinical professor and assistant dean for student affairs at the University of Texas’ College of Pharmacy in Austin.
According to the FDA, 77% of the shortages in 2010 involved sterile injectable drugs.
“There are fewer and fewer firms making these older sterile injectables, and they are often discontinued for newer, more profitable agents,” FDA spokeswoman Yolanda Fultz-Morris said in an email. “When one firm has a delay or a manufacturing problem, it is extremely difficult for the remaining firms to quickly increase production.”
The biggest cause for the shortages in those drugs has been product quality issues, namely microbial contamination and newly identified impurities, according to the FDA. From January to October of 2010, 42% of drug shortages were due to quality problems.
Eighteen percent were due to product discontinuation by the manufacturer and another 18% were due to delays and capacity problems. Nine percent were due to difficulties getting raw materials, and 4% of the sterile injectable shortages were due to increased demand because there was a shortage of another injectable medication. In other words, one shortage led directly to another.
Kevin Schweers, a spokesman for the National Community Pharmacists Association, says generic drugs, especially Schedule II substances, have been in short supply. But there can be problems even when one generic is available to replace another generic.
An example, he says, is when a “new generic substituted in place of the old one is made by a different manufacturer and may come in a different color or shape. That can leave patients”—including those just released from hospitals—“wondering and asking the pharmacist why their medication is different or if a mistake was made.”
Patient Safety and Communication Errors
Lalit Verma, MD, director of the hospital medicine program at Durham Regional Medical Center in North Carolina and assistant professor of medicine at the Duke University School of Medicine, is unaware of any situations in which a shortage put patients in jeopardy at his hospital. He says the pharmacy at Durham Regional, which has seen recent shortages in morphine and heparin, among other drugs, keeps doctors up to date and has adjusted doses appropriately when replacements are used.
“It’s probably been more than I’ve experienced in my 10 years as a hospitalist,” Dr. Verma says. “We have a very good pharmacy program that updates us regularly on drug shortages and offers alternatives.”
Dr. Li also says no patient’s safety has been jeopardized by a shortage.
Others say patient safety has been affected, according to 1,800 healthcare practitioners who participated in a survey last year conducted by the Institute for Safe Medication Practices (ISMP), a nonprofit group. Twenty percent of the respondents said drug-shortage-related errors were made, while 32% said they had “near misses” related to drug shortages. Nineteen percent said there had been adverse patient outcomes as a result of drug shortages.
The study noted two instances in which patients died when they were switched to dilaudid because morphine was in short supply; both patients were given morphine doses instead of adjusted doses for dilaudid.
“It’s about six- or sevenfold more potent than morphine,” says Michael Cohen, ISMP president. “And so when that drug is prescribed in a morphine dose, that would be a massive overdose for some patients.”
He adds that hospitals have tried to stay on top of the drug shortage problem, but that “it’s very difficult.”
“A lot of this happens last-minute,” Cohen says. “Physicians aren’t given a chance to even realize that a certain drug isn’t available, so it causes an interruption in the whole flow of things in the hospital.” Some hospitals have had to hire staffers who handle just the inevitable daily drug shortages, he adds.
A law has been proposed in the U.S. Senate that would require drug manufacturers to notify the FDA when circumstances arise that might reasonably lead to a drug shortage (see “Senate Bill Would Require Advance Notice of Potential Shortages,” p. 41).
Cohen says another concern is that some hospitals, faced with shortages in electrolytes, such as potassium phosphate and sodium acetate, have been turning to less-regulated sterile compounding pharmacies for the products.
Dr. Verma, of Durham Regional, says perhaps the biggest challenge is staying on top of changing doses. “I think there was a learning curve for physicians in using dilaudid [rather than morphine] because the dosing is quite different, so that can cause challenges for patient care when you’re switching in and out of drug classes,” he says. “It’s not a perfect science. It doesn’t cripple us, but it does make it more challenging to fine-tune patient care.”
Ginsburg, of the ASHP, urges hospitalists to stay in close contact with the pharmacists at their hospitals and to be diligent about reporting shortages to the ASHP.
“Please work closely with the pharmacists, because we’re the ones that can really help,” she says. “We’re in it together with them, in terms of trying to provide care for their patients.” TH
Thomas R. Collins a freelance medical writer based in Florida.
When a drug is in short supply at Beth Israel Deaconess Medical Center in Boston, a message goes out to the physicians on the hospital’s intranet system. When the shortage gets close to being critically short in supply, a message will be embedded into the physician order-entry system recommending that the physicians use an alternate drug—if there is an alternate.
It’s an alert system that has been put to frequent use lately, says Joseph Li, MD, SFHM, director of the hospital medicine program at Beth Israel Deaconess, associate professor of medicine at Harvard Medical School, and president of SHM.
The rate of drug shortages has been rising steadily in recent years due to quality questions at manufacturers, consolidation in the drug-manufacturing industry, and other factors, according to data from the U.S. Food and Drug Administration and other sources.
“It does seem like there’s more today than previous years,” says Dr. Li, who was a pharmacist before he trained in internal medicine.
Some of the recent shortages at Beth Israel Deaconess have involved the diuretic furosemide, the antiemetic Compazine, and the anticoagulant heparin. “More often than not, there’s a reasonable alternative that can be chosen,” he says. “Not necessarily exactly the same drug, but usually in the same therapeutic class.”
While actual cases of patient harm due to drug shortages appear to be relatively uncommon, having drugs in short supply can lead to a safety problem hovering over a medical center and its hospitalists. In addition to the potential of simply not having an alternate to give to a patient, hospitalists and their pharmacists sometimes have to adjust to a new dosage that comes with a replacement medication.
Plus, having to manage the problem when a drug shortage hits can be a headache, with time and resources spent trying to obtain updates from drug manufacturers and find other drugs that can be used in the meantime, experts say.
With hospitalists now treating so many patients, many of them complex and on multiple medications, it is an important issue for hospitalists to stay aware of and to be prepared for, Dr. Li says. More than 90% of all medical patients at Beth Israel Deaconess are now cared for by hospitalists, he says, and it’s a similar situation for many acute-care hospitals around the country.
If a drug is in short supply, balancing availability with patient needs can be especially tricky for a hospitalist caring for patients with a multitude of demands, Dr. Li says. “There is an effort to make sure that our most vulnerable population of patients receive these treatments before the general population of patients have access to it,” he adds.
However, the very existence of hospitalists makes it easier to navigate a shortage compared to the days when hundreds of providers would be caring for a pool of patients.
“If you’re trying to notify a group of providers about shortages and have an impact on their prescribing habits, I think it’s easier today,” he says.
Troubled Waters
The FDA says it confirmed a record 178 cases of drug shortages in 2010 (www.fda.gov/drugs/drugsafety/drugshortages/default.htm). That was up from 55 shortages five years ago. And according to the University of Utah Drug Information Service, the problem is actually more pervasive than that, reporting 120 shortages in the U.S. in 2001, with a reported 211 in 2010. And through March of this year, there were 80 reported cases of shortages, on pace for another record year.
“In the past couple of years, it’s just been exponential,” says Diane Ginsburg, president of the American Society of Health-System Pharmacists and clinical professor and assistant dean for student affairs at the University of Texas’ College of Pharmacy in Austin.
According to the FDA, 77% of the shortages in 2010 involved sterile injectable drugs.
“There are fewer and fewer firms making these older sterile injectables, and they are often discontinued for newer, more profitable agents,” FDA spokeswoman Yolanda Fultz-Morris said in an email. “When one firm has a delay or a manufacturing problem, it is extremely difficult for the remaining firms to quickly increase production.”
The biggest cause for the shortages in those drugs has been product quality issues, namely microbial contamination and newly identified impurities, according to the FDA. From January to October of 2010, 42% of drug shortages were due to quality problems.
Eighteen percent were due to product discontinuation by the manufacturer and another 18% were due to delays and capacity problems. Nine percent were due to difficulties getting raw materials, and 4% of the sterile injectable shortages were due to increased demand because there was a shortage of another injectable medication. In other words, one shortage led directly to another.
Kevin Schweers, a spokesman for the National Community Pharmacists Association, says generic drugs, especially Schedule II substances, have been in short supply. But there can be problems even when one generic is available to replace another generic.
An example, he says, is when a “new generic substituted in place of the old one is made by a different manufacturer and may come in a different color or shape. That can leave patients”—including those just released from hospitals—“wondering and asking the pharmacist why their medication is different or if a mistake was made.”
Patient Safety and Communication Errors
Lalit Verma, MD, director of the hospital medicine program at Durham Regional Medical Center in North Carolina and assistant professor of medicine at the Duke University School of Medicine, is unaware of any situations in which a shortage put patients in jeopardy at his hospital. He says the pharmacy at Durham Regional, which has seen recent shortages in morphine and heparin, among other drugs, keeps doctors up to date and has adjusted doses appropriately when replacements are used.
“It’s probably been more than I’ve experienced in my 10 years as a hospitalist,” Dr. Verma says. “We have a very good pharmacy program that updates us regularly on drug shortages and offers alternatives.”
Dr. Li also says no patient’s safety has been jeopardized by a shortage.
Others say patient safety has been affected, according to 1,800 healthcare practitioners who participated in a survey last year conducted by the Institute for Safe Medication Practices (ISMP), a nonprofit group. Twenty percent of the respondents said drug-shortage-related errors were made, while 32% said they had “near misses” related to drug shortages. Nineteen percent said there had been adverse patient outcomes as a result of drug shortages.
The study noted two instances in which patients died when they were switched to dilaudid because morphine was in short supply; both patients were given morphine doses instead of adjusted doses for dilaudid.
“It’s about six- or sevenfold more potent than morphine,” says Michael Cohen, ISMP president. “And so when that drug is prescribed in a morphine dose, that would be a massive overdose for some patients.”
He adds that hospitals have tried to stay on top of the drug shortage problem, but that “it’s very difficult.”
“A lot of this happens last-minute,” Cohen says. “Physicians aren’t given a chance to even realize that a certain drug isn’t available, so it causes an interruption in the whole flow of things in the hospital.” Some hospitals have had to hire staffers who handle just the inevitable daily drug shortages, he adds.
A law has been proposed in the U.S. Senate that would require drug manufacturers to notify the FDA when circumstances arise that might reasonably lead to a drug shortage (see “Senate Bill Would Require Advance Notice of Potential Shortages,” p. 41).
Cohen says another concern is that some hospitals, faced with shortages in electrolytes, such as potassium phosphate and sodium acetate, have been turning to less-regulated sterile compounding pharmacies for the products.
Dr. Verma, of Durham Regional, says perhaps the biggest challenge is staying on top of changing doses. “I think there was a learning curve for physicians in using dilaudid [rather than morphine] because the dosing is quite different, so that can cause challenges for patient care when you’re switching in and out of drug classes,” he says. “It’s not a perfect science. It doesn’t cripple us, but it does make it more challenging to fine-tune patient care.”
Ginsburg, of the ASHP, urges hospitalists to stay in close contact with the pharmacists at their hospitals and to be diligent about reporting shortages to the ASHP.
“Please work closely with the pharmacists, because we’re the ones that can really help,” she says. “We’re in it together with them, in terms of trying to provide care for their patients.” TH
Thomas R. Collins a freelance medical writer based in Florida.
What Is Your Value?
For those of you who attended Bob Wachter’s talk at HM11 in Dallas, you learned that Bob drives a particular model of a popular SUV made by a well-known Japanese manufacturer. When he was in the market for a vehicle, he decided he wanted to buy an SUV. He acknowledged there were certainly less expensive SUVs on the market, along with more expensive alternatives.
So why did he choose to purchase that particular model? Was it the color, the seat warmers, or the keyless entry system? The answer is simple: He decided to purchase the popular SUV because he thought it was the best value for his dollar.
I have this vision of Bob, head cocked to one side, with his index finger resting against his chin and a text bubble above his head reading, “What is the quality of this vehicle and what is the price tag?”
These are decisions all of us make in our everyday lives. I make the same value judgment when I pull into the gasoline station to purchase gas (regular or premium?) or when I go to the grocery store (brand-name or generic orange juice?). But we know that higher cost doesn’t always mean higher quality. Think American-made automobiles versus Japanese-made vehicles in the 1970s and ’80s.
Along those same lines, let’s think about the U.S. healthcare system in 2011. America is trying to move its healthcare toward a value-based system. How do we receive the best healthcare for the—many times taxpayer—dollar? I am a taxpayer and I am all for higher-quality healthcare for my dollars.
At HM11, I heard from many supporters of healthcare reform, but I also heard many people vilify the government’s efforts at reforming our healthcare system. Just about everyone agreed that the future is uncertain. The current healthcare system certainly values hospitalists. It is hard to argue with the facts. In less than 15 years, our healthcare system has created jobs for more than 30,000 hospitalists, the majority of whom require nonclinical revenue from hospitals to meet expenses. The latest SHM-MGMA data show that the average hospitalist full-time equivalent (FTE) receives more than $131,500 of nonclinical revenue (primarily from hospitals) annually.
Payors of healthcare are no different than Bob when it comes to purchasing a car, or me when it comes to purchasing orange juice. Payors will pay for hospitalists as long as they perceive value in their investment.
But what is the basis of this notion that hospitalists are high-value healthcare providers, and is it justified? At HM11, I heard about the continued rise in hospitalist salaries. Higher costs mean we will have to increase quality if we hope to achieve the same value (value=quality/cost).
Don’t Worry, Share Your Data
I have listened to many presentations about healthcare value, quality, and cost. My perception is that it makes the most sense if it is personal. I live in Massachusetts, and my state government has been aggressive at helping everyone understand the quality and the cost of care being delivered at our hospitals. For example, our state government generates a massive annual report that describes the quality and cost of healthcare being delivered at individual hospitals; a PDF of the report is available at www.mass.gov. (For full disclosure, I work at Beth Israel Deaconess Medical Center [BIDMC] in Boston and I serve on a Massachusetts Department of Public Health Stroke Advisory Committee.)
The annual report shows there is not as much of a direct relationship between quality and cost as one would like to see. But I applaud Massachusetts for producing this report. Recognizing and understanding a problem is the first step in creating a solution to the problem. One cannot create a value-based system without understanding the existing quality and cost.
This is one of the reasons why, several years ago, the BIDMC leadership posted my hospital’s quality data online for public consumption (www.bidmc.org/QualityandSafety.aspx). The BIDMC website even features a short video of hospitalist Ken Sands, MD, who also happens to be the vice president of quality at BIDMC, telling you about the hospital quality data. Before the hospital posted this data online, most of our hospital staff and providers, let alone our patients and their families, were unaware of the data. BIDMC is not the only hospital who does this. I understand Cedars-Sinai Medical Center in Los Angeles and Dartmouth-Hitchcock Medical Center in New Hampshire have long shared their quality data publicly.
But the truth is, if you look hard enough, you can find these data for just about all acute-care hospitals in the country. Start with Medicare’s Hospital Compare website (www.hospitalcompare.hhs.gov). However, BIDMC and others have simply made it easier to find the data by putting it directly on their websites.
Policy of Transparency
An interesting thing happened over the past decade at BIDMC. In 1997, there were no hospitalists who cared for BIDMC patients. Today, hospitalists manage nearly 100% of the patients hospitalized on our large medical service.
When you look at the data being reported by BIDMC and the state of Massachusetts about nonsurgical conditions, doesn’t that reflect the care being provided by the hospitalists who work at BIDMC? I imagine that is what will run through my CEO and CFO’s minds when we discuss the hospitalist budget this summer. They will ask themselves, “What is the value of our hospitalists? What is the quality of their care? How much do they cost us?”
Some of you might be in a similar position. Do your hospitalists now provide the bulk of the care at your hospital? Are your hospital’s data being publicly reported? I think the answer is a resounding “yes” for many of you.
Allow me to ask this question: What are you doing to collect data to understand the quality and cost of your hospitalist program? Wouldn’t you rather know this information before your hospital or state government tells you?
As the director of my hospitalist group, I spearhead our group efforts to better understand the quality of care we provide. This proactive, introspective approach is essential, especially if hospitalist groups around the country hope to continue being perceived as “high value” providers.
I am interested in hearing from you about your efforts to understand the care being provided by your hospitalists. Feel free to email me at [email protected]. TH
Dr. Li is president of SHM.
For those of you who attended Bob Wachter’s talk at HM11 in Dallas, you learned that Bob drives a particular model of a popular SUV made by a well-known Japanese manufacturer. When he was in the market for a vehicle, he decided he wanted to buy an SUV. He acknowledged there were certainly less expensive SUVs on the market, along with more expensive alternatives.
So why did he choose to purchase that particular model? Was it the color, the seat warmers, or the keyless entry system? The answer is simple: He decided to purchase the popular SUV because he thought it was the best value for his dollar.
I have this vision of Bob, head cocked to one side, with his index finger resting against his chin and a text bubble above his head reading, “What is the quality of this vehicle and what is the price tag?”
These are decisions all of us make in our everyday lives. I make the same value judgment when I pull into the gasoline station to purchase gas (regular or premium?) or when I go to the grocery store (brand-name or generic orange juice?). But we know that higher cost doesn’t always mean higher quality. Think American-made automobiles versus Japanese-made vehicles in the 1970s and ’80s.
Along those same lines, let’s think about the U.S. healthcare system in 2011. America is trying to move its healthcare toward a value-based system. How do we receive the best healthcare for the—many times taxpayer—dollar? I am a taxpayer and I am all for higher-quality healthcare for my dollars.
At HM11, I heard from many supporters of healthcare reform, but I also heard many people vilify the government’s efforts at reforming our healthcare system. Just about everyone agreed that the future is uncertain. The current healthcare system certainly values hospitalists. It is hard to argue with the facts. In less than 15 years, our healthcare system has created jobs for more than 30,000 hospitalists, the majority of whom require nonclinical revenue from hospitals to meet expenses. The latest SHM-MGMA data show that the average hospitalist full-time equivalent (FTE) receives more than $131,500 of nonclinical revenue (primarily from hospitals) annually.
Payors of healthcare are no different than Bob when it comes to purchasing a car, or me when it comes to purchasing orange juice. Payors will pay for hospitalists as long as they perceive value in their investment.
But what is the basis of this notion that hospitalists are high-value healthcare providers, and is it justified? At HM11, I heard about the continued rise in hospitalist salaries. Higher costs mean we will have to increase quality if we hope to achieve the same value (value=quality/cost).
Don’t Worry, Share Your Data
I have listened to many presentations about healthcare value, quality, and cost. My perception is that it makes the most sense if it is personal. I live in Massachusetts, and my state government has been aggressive at helping everyone understand the quality and the cost of care being delivered at our hospitals. For example, our state government generates a massive annual report that describes the quality and cost of healthcare being delivered at individual hospitals; a PDF of the report is available at www.mass.gov. (For full disclosure, I work at Beth Israel Deaconess Medical Center [BIDMC] in Boston and I serve on a Massachusetts Department of Public Health Stroke Advisory Committee.)
The annual report shows there is not as much of a direct relationship between quality and cost as one would like to see. But I applaud Massachusetts for producing this report. Recognizing and understanding a problem is the first step in creating a solution to the problem. One cannot create a value-based system without understanding the existing quality and cost.
This is one of the reasons why, several years ago, the BIDMC leadership posted my hospital’s quality data online for public consumption (www.bidmc.org/QualityandSafety.aspx). The BIDMC website even features a short video of hospitalist Ken Sands, MD, who also happens to be the vice president of quality at BIDMC, telling you about the hospital quality data. Before the hospital posted this data online, most of our hospital staff and providers, let alone our patients and their families, were unaware of the data. BIDMC is not the only hospital who does this. I understand Cedars-Sinai Medical Center in Los Angeles and Dartmouth-Hitchcock Medical Center in New Hampshire have long shared their quality data publicly.
But the truth is, if you look hard enough, you can find these data for just about all acute-care hospitals in the country. Start with Medicare’s Hospital Compare website (www.hospitalcompare.hhs.gov). However, BIDMC and others have simply made it easier to find the data by putting it directly on their websites.
Policy of Transparency
An interesting thing happened over the past decade at BIDMC. In 1997, there were no hospitalists who cared for BIDMC patients. Today, hospitalists manage nearly 100% of the patients hospitalized on our large medical service.
When you look at the data being reported by BIDMC and the state of Massachusetts about nonsurgical conditions, doesn’t that reflect the care being provided by the hospitalists who work at BIDMC? I imagine that is what will run through my CEO and CFO’s minds when we discuss the hospitalist budget this summer. They will ask themselves, “What is the value of our hospitalists? What is the quality of their care? How much do they cost us?”
Some of you might be in a similar position. Do your hospitalists now provide the bulk of the care at your hospital? Are your hospital’s data being publicly reported? I think the answer is a resounding “yes” for many of you.
Allow me to ask this question: What are you doing to collect data to understand the quality and cost of your hospitalist program? Wouldn’t you rather know this information before your hospital or state government tells you?
As the director of my hospitalist group, I spearhead our group efforts to better understand the quality of care we provide. This proactive, introspective approach is essential, especially if hospitalist groups around the country hope to continue being perceived as “high value” providers.
I am interested in hearing from you about your efforts to understand the care being provided by your hospitalists. Feel free to email me at [email protected]. TH
Dr. Li is president of SHM.
For those of you who attended Bob Wachter’s talk at HM11 in Dallas, you learned that Bob drives a particular model of a popular SUV made by a well-known Japanese manufacturer. When he was in the market for a vehicle, he decided he wanted to buy an SUV. He acknowledged there were certainly less expensive SUVs on the market, along with more expensive alternatives.
So why did he choose to purchase that particular model? Was it the color, the seat warmers, or the keyless entry system? The answer is simple: He decided to purchase the popular SUV because he thought it was the best value for his dollar.
I have this vision of Bob, head cocked to one side, with his index finger resting against his chin and a text bubble above his head reading, “What is the quality of this vehicle and what is the price tag?”
These are decisions all of us make in our everyday lives. I make the same value judgment when I pull into the gasoline station to purchase gas (regular or premium?) or when I go to the grocery store (brand-name or generic orange juice?). But we know that higher cost doesn’t always mean higher quality. Think American-made automobiles versus Japanese-made vehicles in the 1970s and ’80s.
Along those same lines, let’s think about the U.S. healthcare system in 2011. America is trying to move its healthcare toward a value-based system. How do we receive the best healthcare for the—many times taxpayer—dollar? I am a taxpayer and I am all for higher-quality healthcare for my dollars.
At HM11, I heard from many supporters of healthcare reform, but I also heard many people vilify the government’s efforts at reforming our healthcare system. Just about everyone agreed that the future is uncertain. The current healthcare system certainly values hospitalists. It is hard to argue with the facts. In less than 15 years, our healthcare system has created jobs for more than 30,000 hospitalists, the majority of whom require nonclinical revenue from hospitals to meet expenses. The latest SHM-MGMA data show that the average hospitalist full-time equivalent (FTE) receives more than $131,500 of nonclinical revenue (primarily from hospitals) annually.
Payors of healthcare are no different than Bob when it comes to purchasing a car, or me when it comes to purchasing orange juice. Payors will pay for hospitalists as long as they perceive value in their investment.
But what is the basis of this notion that hospitalists are high-value healthcare providers, and is it justified? At HM11, I heard about the continued rise in hospitalist salaries. Higher costs mean we will have to increase quality if we hope to achieve the same value (value=quality/cost).
Don’t Worry, Share Your Data
I have listened to many presentations about healthcare value, quality, and cost. My perception is that it makes the most sense if it is personal. I live in Massachusetts, and my state government has been aggressive at helping everyone understand the quality and the cost of care being delivered at our hospitals. For example, our state government generates a massive annual report that describes the quality and cost of healthcare being delivered at individual hospitals; a PDF of the report is available at www.mass.gov. (For full disclosure, I work at Beth Israel Deaconess Medical Center [BIDMC] in Boston and I serve on a Massachusetts Department of Public Health Stroke Advisory Committee.)
The annual report shows there is not as much of a direct relationship between quality and cost as one would like to see. But I applaud Massachusetts for producing this report. Recognizing and understanding a problem is the first step in creating a solution to the problem. One cannot create a value-based system without understanding the existing quality and cost.
This is one of the reasons why, several years ago, the BIDMC leadership posted my hospital’s quality data online for public consumption (www.bidmc.org/QualityandSafety.aspx). The BIDMC website even features a short video of hospitalist Ken Sands, MD, who also happens to be the vice president of quality at BIDMC, telling you about the hospital quality data. Before the hospital posted this data online, most of our hospital staff and providers, let alone our patients and their families, were unaware of the data. BIDMC is not the only hospital who does this. I understand Cedars-Sinai Medical Center in Los Angeles and Dartmouth-Hitchcock Medical Center in New Hampshire have long shared their quality data publicly.
But the truth is, if you look hard enough, you can find these data for just about all acute-care hospitals in the country. Start with Medicare’s Hospital Compare website (www.hospitalcompare.hhs.gov). However, BIDMC and others have simply made it easier to find the data by putting it directly on their websites.
Policy of Transparency
An interesting thing happened over the past decade at BIDMC. In 1997, there were no hospitalists who cared for BIDMC patients. Today, hospitalists manage nearly 100% of the patients hospitalized on our large medical service.
When you look at the data being reported by BIDMC and the state of Massachusetts about nonsurgical conditions, doesn’t that reflect the care being provided by the hospitalists who work at BIDMC? I imagine that is what will run through my CEO and CFO’s minds when we discuss the hospitalist budget this summer. They will ask themselves, “What is the value of our hospitalists? What is the quality of their care? How much do they cost us?”
Some of you might be in a similar position. Do your hospitalists now provide the bulk of the care at your hospital? Are your hospital’s data being publicly reported? I think the answer is a resounding “yes” for many of you.
Allow me to ask this question: What are you doing to collect data to understand the quality and cost of your hospitalist program? Wouldn’t you rather know this information before your hospital or state government tells you?
As the director of my hospitalist group, I spearhead our group efforts to better understand the quality of care we provide. This proactive, introspective approach is essential, especially if hospitalist groups around the country hope to continue being perceived as “high value” providers.
I am interested in hearing from you about your efforts to understand the care being provided by your hospitalists. Feel free to email me at [email protected]. TH
Dr. Li is president of SHM.
Subsidy or Payment?
Question: Before hospitalists, who cared for hospitalized patients?
Answer: Generalists—in other words, internists, family physicians, pediatricians.
Q: How much did that system cost hospitals?
A: Nothing, or very little. In some cases, support dollars were available for weekend, night, or uninsured patient coverage, but by and large this system cost hospitals little. Physicians admitted their patients to the hospital because the alternatives (sending a hypoxic pneumonia patient home from clinic, turning out the office lights and hoping the patient survived the night, or bringing the patient home with them) offered uncomfortable ethical, malpractice, or alimony consequences. So doctors admitted these patients to the hospital and visited them daily.
Q: The average amount of support per hospitalist is $131,564, or about $1.7 million per HM group seeing adult patients. The bulk of those dollars come from the hospital. If we assume that the people running hospitals are smart, then why would those smart businesspeople pay $1.7 million for something they used to get for free?
A: Because there is something they get in return for that money. Or, perhaps, something they think they are getting in return for those dollars.
Q: What?
A: I often go through this exercise with the residents in our hospitalist training program when we discuss the drivers of the HM movement. I usually discuss the reasons why a hospital should fund these groups; it always seems like such a no-brainer to me.
Enter a recent news item from Montana. The story from the Helena Independent Record (see “Unsustainable Growth?” p. 1) noted that a multispecialty group practice in Helena announced they were no longer admitting their patients to a local hospital in protest over a new hospital policy to charge the clinic practice. The fee was to defray some of the costs of the HM program. A hospital representative was quoted as saying “physicians are responsible for obtaining hospital coverage for their own patients, not the hospital.”
I can’t really argue with the logic of that statement. Surely a clinic has responsibility to ensure that their patients get cared for while they are inpatients. If an internist is going to see a patient in the clinic and admit them to the hospital, shouldn’t an internist then see the patient in the hospital?
If I’m a hospital CEO, the answer is no.
To retrench a bit, yes, I’d want a board-certified internal-medicine (or pediatric or family medicine) physician to see the hospitalized patient. But in the process, I wouldn’t want them to only practice internal medicine. That was the model hospitals had 25 years ago—a model that cost them very little, a model that they played a large part in exterminating. The fact that most hospitals are willing to pay millions or more per year to not have that system tells me that they don’t want that system.
Q: So, what do hospitals want?
A: Hospitalists, not internists in the hospital.
What’s the difference? Well, it’s a perception issue. Many, if not most, believe that all it takes to be a great hospitalist is to show up for your shift, provide great care to your 15 patients, and go home. That is, the job is defined by the clinical effort—the internist part. Although there is tremendous benefit to this and I recognize its importance (and let’s not forget the weekend, night, and holiday coverage), this sells us short and puts our financial stability in peril.
To be great, to best help our patients, to give our hospitals what they want and need, we have to evolve from “internists in the hospital” to hospitalists. Hospitalists are defined not by our clinical effort but rather by our nonclinical effort. This is what hospitals are paying $1.7 million per year for. They had the internist in the hospital model and chose to pay more—they chose the hospitalist model.
To be a great hospitalist group means embracing the nonclinical work that envelops the clinical practice—the process and quality improvement (QI). That is, fundamentally changing the unsafe systems that surround our patients. Making them safer, more efficient and of higher quality.
This takes time.
Time = Money
It takes time to implement a QI project to reduce central line infections in the ICU. Or to develop and implement a VTE prophylaxis order set or an insulin or heparin drip protocol. Or to work closely with nursing to reduce falls on a medical unit. It takes time to be at the pneumonia core measures meeting every Monday at 7 a.m. and the hospital credentialing committee meeting every other Friday at 3 p.m. It also takes time to implement a new electronic health record or roll out the new LEAN project to reduce ED wait times.
This takes time, effort, and bandwidth—the kind that can’t be shoehorned into the average clinical day. This is work that needs to be done primarily during nonclinical hours. It’s the kind of work that defines HM as a field; the kind of work that increasingly determines your hospital’s bottom line; the kind of work that has tremendous value; the kind of work that requires remuneration.
In paying for the hospitalist model, your hospital is paying for the clinical (internist) and nonclinical (hospitalist) work you do. The $1.7 million per year is not a subsidy they pay to keep you in business. It’s the price they must pay to compensate your group for all the nonclinical work you do around quality, safety, efficiency, and leadership.
Q: But what if my group isn’t doing these kinds of things?
A: Then your hospital funding is at risk. The Montana story addresses just such a scenario. Clearly the hospital C-suite in this instance only valued (or was presented with) clinical work. Therefore, they felt that others should subsidize the hospitalist salaries—in this case, the clinic. I don’t know the particulars of this case but deduce this because it would be ludicrous to expect the clinic to pay for the part of the hospitalists’ time spent improving the hospital’s systems of care.
Writing the Final Chapter
At the core of the HM funding model is the concept of subsidy versus compensation. If we are only providing clinical care, then the offset dollars from the hospital to support our salaries is functionally a subsidy—a dollar amount to make up for our collections shortfall. However, if it is support for the nonclinical work we are doing, then it is compensation.
As the story of hospitalist funding is written, the report from Montana should serve as a cautionary tale. Hospital financial pressures likely will focus more scrutiny on the hospitalist financial support model. And as this story plays out, HM groups will be expected to bring more to the table than patient care.
Those that do will live happily ever after.
Those that don’t will be forced to answer the tough question: What’s the difference between an internist in the hospital and a hospitalist? If the answer is nothing, that story will have a decidedly and predictably less happy ending. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Question: Before hospitalists, who cared for hospitalized patients?
Answer: Generalists—in other words, internists, family physicians, pediatricians.
Q: How much did that system cost hospitals?
A: Nothing, or very little. In some cases, support dollars were available for weekend, night, or uninsured patient coverage, but by and large this system cost hospitals little. Physicians admitted their patients to the hospital because the alternatives (sending a hypoxic pneumonia patient home from clinic, turning out the office lights and hoping the patient survived the night, or bringing the patient home with them) offered uncomfortable ethical, malpractice, or alimony consequences. So doctors admitted these patients to the hospital and visited them daily.
Q: The average amount of support per hospitalist is $131,564, or about $1.7 million per HM group seeing adult patients. The bulk of those dollars come from the hospital. If we assume that the people running hospitals are smart, then why would those smart businesspeople pay $1.7 million for something they used to get for free?
A: Because there is something they get in return for that money. Or, perhaps, something they think they are getting in return for those dollars.
Q: What?
A: I often go through this exercise with the residents in our hospitalist training program when we discuss the drivers of the HM movement. I usually discuss the reasons why a hospital should fund these groups; it always seems like such a no-brainer to me.
Enter a recent news item from Montana. The story from the Helena Independent Record (see “Unsustainable Growth?” p. 1) noted that a multispecialty group practice in Helena announced they were no longer admitting their patients to a local hospital in protest over a new hospital policy to charge the clinic practice. The fee was to defray some of the costs of the HM program. A hospital representative was quoted as saying “physicians are responsible for obtaining hospital coverage for their own patients, not the hospital.”
I can’t really argue with the logic of that statement. Surely a clinic has responsibility to ensure that their patients get cared for while they are inpatients. If an internist is going to see a patient in the clinic and admit them to the hospital, shouldn’t an internist then see the patient in the hospital?
If I’m a hospital CEO, the answer is no.
To retrench a bit, yes, I’d want a board-certified internal-medicine (or pediatric or family medicine) physician to see the hospitalized patient. But in the process, I wouldn’t want them to only practice internal medicine. That was the model hospitals had 25 years ago—a model that cost them very little, a model that they played a large part in exterminating. The fact that most hospitals are willing to pay millions or more per year to not have that system tells me that they don’t want that system.
Q: So, what do hospitals want?
A: Hospitalists, not internists in the hospital.
What’s the difference? Well, it’s a perception issue. Many, if not most, believe that all it takes to be a great hospitalist is to show up for your shift, provide great care to your 15 patients, and go home. That is, the job is defined by the clinical effort—the internist part. Although there is tremendous benefit to this and I recognize its importance (and let’s not forget the weekend, night, and holiday coverage), this sells us short and puts our financial stability in peril.
To be great, to best help our patients, to give our hospitals what they want and need, we have to evolve from “internists in the hospital” to hospitalists. Hospitalists are defined not by our clinical effort but rather by our nonclinical effort. This is what hospitals are paying $1.7 million per year for. They had the internist in the hospital model and chose to pay more—they chose the hospitalist model.
To be a great hospitalist group means embracing the nonclinical work that envelops the clinical practice—the process and quality improvement (QI). That is, fundamentally changing the unsafe systems that surround our patients. Making them safer, more efficient and of higher quality.
This takes time.
Time = Money
It takes time to implement a QI project to reduce central line infections in the ICU. Or to develop and implement a VTE prophylaxis order set or an insulin or heparin drip protocol. Or to work closely with nursing to reduce falls on a medical unit. It takes time to be at the pneumonia core measures meeting every Monday at 7 a.m. and the hospital credentialing committee meeting every other Friday at 3 p.m. It also takes time to implement a new electronic health record or roll out the new LEAN project to reduce ED wait times.
This takes time, effort, and bandwidth—the kind that can’t be shoehorned into the average clinical day. This is work that needs to be done primarily during nonclinical hours. It’s the kind of work that defines HM as a field; the kind of work that increasingly determines your hospital’s bottom line; the kind of work that has tremendous value; the kind of work that requires remuneration.
In paying for the hospitalist model, your hospital is paying for the clinical (internist) and nonclinical (hospitalist) work you do. The $1.7 million per year is not a subsidy they pay to keep you in business. It’s the price they must pay to compensate your group for all the nonclinical work you do around quality, safety, efficiency, and leadership.
Q: But what if my group isn’t doing these kinds of things?
A: Then your hospital funding is at risk. The Montana story addresses just such a scenario. Clearly the hospital C-suite in this instance only valued (or was presented with) clinical work. Therefore, they felt that others should subsidize the hospitalist salaries—in this case, the clinic. I don’t know the particulars of this case but deduce this because it would be ludicrous to expect the clinic to pay for the part of the hospitalists’ time spent improving the hospital’s systems of care.
Writing the Final Chapter
At the core of the HM funding model is the concept of subsidy versus compensation. If we are only providing clinical care, then the offset dollars from the hospital to support our salaries is functionally a subsidy—a dollar amount to make up for our collections shortfall. However, if it is support for the nonclinical work we are doing, then it is compensation.
As the story of hospitalist funding is written, the report from Montana should serve as a cautionary tale. Hospital financial pressures likely will focus more scrutiny on the hospitalist financial support model. And as this story plays out, HM groups will be expected to bring more to the table than patient care.
Those that do will live happily ever after.
Those that don’t will be forced to answer the tough question: What’s the difference between an internist in the hospital and a hospitalist? If the answer is nothing, that story will have a decidedly and predictably less happy ending. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Question: Before hospitalists, who cared for hospitalized patients?
Answer: Generalists—in other words, internists, family physicians, pediatricians.
Q: How much did that system cost hospitals?
A: Nothing, or very little. In some cases, support dollars were available for weekend, night, or uninsured patient coverage, but by and large this system cost hospitals little. Physicians admitted their patients to the hospital because the alternatives (sending a hypoxic pneumonia patient home from clinic, turning out the office lights and hoping the patient survived the night, or bringing the patient home with them) offered uncomfortable ethical, malpractice, or alimony consequences. So doctors admitted these patients to the hospital and visited them daily.
Q: The average amount of support per hospitalist is $131,564, or about $1.7 million per HM group seeing adult patients. The bulk of those dollars come from the hospital. If we assume that the people running hospitals are smart, then why would those smart businesspeople pay $1.7 million for something they used to get for free?
A: Because there is something they get in return for that money. Or, perhaps, something they think they are getting in return for those dollars.
Q: What?
A: I often go through this exercise with the residents in our hospitalist training program when we discuss the drivers of the HM movement. I usually discuss the reasons why a hospital should fund these groups; it always seems like such a no-brainer to me.
Enter a recent news item from Montana. The story from the Helena Independent Record (see “Unsustainable Growth?” p. 1) noted that a multispecialty group practice in Helena announced they were no longer admitting their patients to a local hospital in protest over a new hospital policy to charge the clinic practice. The fee was to defray some of the costs of the HM program. A hospital representative was quoted as saying “physicians are responsible for obtaining hospital coverage for their own patients, not the hospital.”
I can’t really argue with the logic of that statement. Surely a clinic has responsibility to ensure that their patients get cared for while they are inpatients. If an internist is going to see a patient in the clinic and admit them to the hospital, shouldn’t an internist then see the patient in the hospital?
If I’m a hospital CEO, the answer is no.
To retrench a bit, yes, I’d want a board-certified internal-medicine (or pediatric or family medicine) physician to see the hospitalized patient. But in the process, I wouldn’t want them to only practice internal medicine. That was the model hospitals had 25 years ago—a model that cost them very little, a model that they played a large part in exterminating. The fact that most hospitals are willing to pay millions or more per year to not have that system tells me that they don’t want that system.
Q: So, what do hospitals want?
A: Hospitalists, not internists in the hospital.
What’s the difference? Well, it’s a perception issue. Many, if not most, believe that all it takes to be a great hospitalist is to show up for your shift, provide great care to your 15 patients, and go home. That is, the job is defined by the clinical effort—the internist part. Although there is tremendous benefit to this and I recognize its importance (and let’s not forget the weekend, night, and holiday coverage), this sells us short and puts our financial stability in peril.
To be great, to best help our patients, to give our hospitals what they want and need, we have to evolve from “internists in the hospital” to hospitalists. Hospitalists are defined not by our clinical effort but rather by our nonclinical effort. This is what hospitals are paying $1.7 million per year for. They had the internist in the hospital model and chose to pay more—they chose the hospitalist model.
To be a great hospitalist group means embracing the nonclinical work that envelops the clinical practice—the process and quality improvement (QI). That is, fundamentally changing the unsafe systems that surround our patients. Making them safer, more efficient and of higher quality.
This takes time.
Time = Money
It takes time to implement a QI project to reduce central line infections in the ICU. Or to develop and implement a VTE prophylaxis order set or an insulin or heparin drip protocol. Or to work closely with nursing to reduce falls on a medical unit. It takes time to be at the pneumonia core measures meeting every Monday at 7 a.m. and the hospital credentialing committee meeting every other Friday at 3 p.m. It also takes time to implement a new electronic health record or roll out the new LEAN project to reduce ED wait times.
This takes time, effort, and bandwidth—the kind that can’t be shoehorned into the average clinical day. This is work that needs to be done primarily during nonclinical hours. It’s the kind of work that defines HM as a field; the kind of work that increasingly determines your hospital’s bottom line; the kind of work that has tremendous value; the kind of work that requires remuneration.
In paying for the hospitalist model, your hospital is paying for the clinical (internist) and nonclinical (hospitalist) work you do. The $1.7 million per year is not a subsidy they pay to keep you in business. It’s the price they must pay to compensate your group for all the nonclinical work you do around quality, safety, efficiency, and leadership.
Q: But what if my group isn’t doing these kinds of things?
A: Then your hospital funding is at risk. The Montana story addresses just such a scenario. Clearly the hospital C-suite in this instance only valued (or was presented with) clinical work. Therefore, they felt that others should subsidize the hospitalist salaries—in this case, the clinic. I don’t know the particulars of this case but deduce this because it would be ludicrous to expect the clinic to pay for the part of the hospitalists’ time spent improving the hospital’s systems of care.
Writing the Final Chapter
At the core of the HM funding model is the concept of subsidy versus compensation. If we are only providing clinical care, then the offset dollars from the hospital to support our salaries is functionally a subsidy—a dollar amount to make up for our collections shortfall. However, if it is support for the nonclinical work we are doing, then it is compensation.
As the story of hospitalist funding is written, the report from Montana should serve as a cautionary tale. Hospital financial pressures likely will focus more scrutiny on the hospitalist financial support model. And as this story plays out, HM groups will be expected to bring more to the table than patient care.
Those that do will live happily ever after.
Those that don’t will be forced to answer the tough question: What’s the difference between an internist in the hospital and a hospitalist? If the answer is nothing, that story will have a decidedly and predictably less happy ending. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
New Developments
HM11 and the publication of the SHM-MGMA survey on hospitalist productivity and compensation occur every summer, and they always provide lots of new information to get me thinking. Two things stand out this year: Hospitalist demand remains high, and hospitals are paying a lot to have hospitalist services.
Supply and Demand
Along with SHM President Joe Li and Rob Bessler, who is CEO of Sound Physicians, I had the pleasure of presenting a preview of some data from the latest SHM-MGMA survey at the annual meeting May 11 in Dallas. During the session, I asked the large crowd of hospitalists how many were from practices that are actively recruiting additional hospitalists. About 40% of the hands went up.
If 40% of HM groups are actively recruiting, some for more than one open position, that’s a lot of recruiting. But it is dramatically less than the response I got when I asked the same question just three years ago at HM08 in San Diego. At that meeting, nearly every hand in the room went up, indicating everybody was recruiting (see “We’re Hiring,” July 2008, p. 62).
Of course, my show-of-hands survey of attendees at SHM meetings is not a perfect method to assess hospitalist supply and demand. But I think the dramatic change in responses from 2008 to 2011 is meaningful; it also matches what I’m seeing in the marketplace. I hear repeatedly that the years of rapid growth in hospitalist staffing have ended in many or most major metropolitan areas. For example, in places like Seattle (where I practice), Minneapolis, and Boston, there are far fewer open positions now than just two years ago, and most are to replace a departing doctor rather than to increase the overall staffing level.
But the far more numerous smaller markets are still recruiting aggressively in an effort to increase the overall staffing of the practice (and not just replace departing doctors). And changes in resident work-hour limitations are requiring teaching hospitals to increase hospitalist staffing to offset the reduction in resident availability. But it’s possible that if the larger markets are indeed becoming somewhat saturated with hospitalists, then there will be a trickledown effect, which should make more candidates available everywhere.
What will be the side effects if indeed the supply of hospitalists catches up to the demand, or even exceeds demand, in some places? It is easy to imagine that greater competition among candidates might mean that practices are increasingly able to hire the more talented and committed doctors, which should improve the overall performance of hospitalist practices.
Although I don’t have proof, I think this phenomenon has been in play in the field of emergency medicine for many years. When I was a resident in the 1980s, ED doctors typically were not the best and brightest at their hospitals. But the way I see it, the field began to attract better candidates, and as ED residencies and practices began to “fill up,” they could be more selective in new hires. Therefore, the average talent of the average ED doctor went up.
I think the average hospitalist today is pretty talented, but I also think it could get even better if the supply of hospitalists exceeds demand. I just hope I continue to make the cut!
If typical market forces are operative for hospitalists (far from a guarantee in any healthcare enterprise), then an oversupply of hospitalists could mean a flattening of the historical trend in hospitalist incomes. To this point, in our relatively young field, incomes have risen faster than can be explained solely by inflation or increases in hospitalist productivity. A relative shortage of hospitalists might be one of the main forces pushing incomes up, and it might go away.
We’ll see.
Hospital Support Trends Up
The most remarkable number in the 2011 SHM-MGMA survey is the financial support provided to practices per FTE hospitalist annually. This support nearly always comes from a hospital, and is often colloquially, and misleadingly, referred to as the “subsidy.”
In 2001, hospital support was about $65,000 per FTE. In the 2008 and 2010 surveys, the median financial support per FTE was $97,000 and $98,000, respectively. But it jumped to $136,403 this year. That is a really huge jump in one year. (Note: The surveys changed from biannual to annual in 2010, and the new SHM-MGMA survey uses a different financial support question/methodology and has a different respondent pool than the previous SHM surveys.)
Some of the increased dollars probably went to pay rising hospitalist compensation, which rose about 3% over the prior year without any significant increase in productivity. But that 3% salary increase translates to only about $5,000 (median compensation rose from roughly $215,000 to $220,000), and could be explained in part by such factors as removing academicians from this data set. (Starting in 2010, academic hospitalists are surveyed and reported separately, so aren’t included here.) So I don’t think the change in hospitalist incomes seen in this survey has much to do with the dramatic, near-40% increase in financial support.
The survey showed that hospitalist productivity hasn’t declined, so the other most likely culprit is declining professional fee collections, which might be due to an increasing portion of hospitalized patients who are uninsured or underinsured. Many hospitals report that their “payor mix” has worsened since the economic crisis of the last few years. And because hospitals typically hold the risk for the financial performance of their hospitalists, then if the latter see more uninsured patients and collect less in professional fees, the hospital will make up the difference. This phenomenon might explain much of the increased financial support.
But I’m not satisfied that a worsening payor mix explains everything. For example, if this were the most significant reason for increasing financial support, I think we would have seen this effect in the prior survey. Why did it “hit” so suddenly in this year alone?
We will get more information about collection rates when the second part of the survey is published in September. For example, we’ll be able to compare the dollars collected per encounter or per wRVU in the current survey to the prior one. If there was a significant drop, then it will require only a little math to see how much overall collections dropped per FTE and see if it is similar to the rise in financial support provided.
Of course, it will be very informative to see what the financial support turns out to be in the next survey (check back in late spring 2012). Will it stay around $136,000 per FTE or be something very different? TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
HM11 and the publication of the SHM-MGMA survey on hospitalist productivity and compensation occur every summer, and they always provide lots of new information to get me thinking. Two things stand out this year: Hospitalist demand remains high, and hospitals are paying a lot to have hospitalist services.
Supply and Demand
Along with SHM President Joe Li and Rob Bessler, who is CEO of Sound Physicians, I had the pleasure of presenting a preview of some data from the latest SHM-MGMA survey at the annual meeting May 11 in Dallas. During the session, I asked the large crowd of hospitalists how many were from practices that are actively recruiting additional hospitalists. About 40% of the hands went up.
If 40% of HM groups are actively recruiting, some for more than one open position, that’s a lot of recruiting. But it is dramatically less than the response I got when I asked the same question just three years ago at HM08 in San Diego. At that meeting, nearly every hand in the room went up, indicating everybody was recruiting (see “We’re Hiring,” July 2008, p. 62).
Of course, my show-of-hands survey of attendees at SHM meetings is not a perfect method to assess hospitalist supply and demand. But I think the dramatic change in responses from 2008 to 2011 is meaningful; it also matches what I’m seeing in the marketplace. I hear repeatedly that the years of rapid growth in hospitalist staffing have ended in many or most major metropolitan areas. For example, in places like Seattle (where I practice), Minneapolis, and Boston, there are far fewer open positions now than just two years ago, and most are to replace a departing doctor rather than to increase the overall staffing level.
But the far more numerous smaller markets are still recruiting aggressively in an effort to increase the overall staffing of the practice (and not just replace departing doctors). And changes in resident work-hour limitations are requiring teaching hospitals to increase hospitalist staffing to offset the reduction in resident availability. But it’s possible that if the larger markets are indeed becoming somewhat saturated with hospitalists, then there will be a trickledown effect, which should make more candidates available everywhere.
What will be the side effects if indeed the supply of hospitalists catches up to the demand, or even exceeds demand, in some places? It is easy to imagine that greater competition among candidates might mean that practices are increasingly able to hire the more talented and committed doctors, which should improve the overall performance of hospitalist practices.
Although I don’t have proof, I think this phenomenon has been in play in the field of emergency medicine for many years. When I was a resident in the 1980s, ED doctors typically were not the best and brightest at their hospitals. But the way I see it, the field began to attract better candidates, and as ED residencies and practices began to “fill up,” they could be more selective in new hires. Therefore, the average talent of the average ED doctor went up.
I think the average hospitalist today is pretty talented, but I also think it could get even better if the supply of hospitalists exceeds demand. I just hope I continue to make the cut!
If typical market forces are operative for hospitalists (far from a guarantee in any healthcare enterprise), then an oversupply of hospitalists could mean a flattening of the historical trend in hospitalist incomes. To this point, in our relatively young field, incomes have risen faster than can be explained solely by inflation or increases in hospitalist productivity. A relative shortage of hospitalists might be one of the main forces pushing incomes up, and it might go away.
We’ll see.
Hospital Support Trends Up
The most remarkable number in the 2011 SHM-MGMA survey is the financial support provided to practices per FTE hospitalist annually. This support nearly always comes from a hospital, and is often colloquially, and misleadingly, referred to as the “subsidy.”
In 2001, hospital support was about $65,000 per FTE. In the 2008 and 2010 surveys, the median financial support per FTE was $97,000 and $98,000, respectively. But it jumped to $136,403 this year. That is a really huge jump in one year. (Note: The surveys changed from biannual to annual in 2010, and the new SHM-MGMA survey uses a different financial support question/methodology and has a different respondent pool than the previous SHM surveys.)
Some of the increased dollars probably went to pay rising hospitalist compensation, which rose about 3% over the prior year without any significant increase in productivity. But that 3% salary increase translates to only about $5,000 (median compensation rose from roughly $215,000 to $220,000), and could be explained in part by such factors as removing academicians from this data set. (Starting in 2010, academic hospitalists are surveyed and reported separately, so aren’t included here.) So I don’t think the change in hospitalist incomes seen in this survey has much to do with the dramatic, near-40% increase in financial support.
The survey showed that hospitalist productivity hasn’t declined, so the other most likely culprit is declining professional fee collections, which might be due to an increasing portion of hospitalized patients who are uninsured or underinsured. Many hospitals report that their “payor mix” has worsened since the economic crisis of the last few years. And because hospitals typically hold the risk for the financial performance of their hospitalists, then if the latter see more uninsured patients and collect less in professional fees, the hospital will make up the difference. This phenomenon might explain much of the increased financial support.
But I’m not satisfied that a worsening payor mix explains everything. For example, if this were the most significant reason for increasing financial support, I think we would have seen this effect in the prior survey. Why did it “hit” so suddenly in this year alone?
We will get more information about collection rates when the second part of the survey is published in September. For example, we’ll be able to compare the dollars collected per encounter or per wRVU in the current survey to the prior one. If there was a significant drop, then it will require only a little math to see how much overall collections dropped per FTE and see if it is similar to the rise in financial support provided.
Of course, it will be very informative to see what the financial support turns out to be in the next survey (check back in late spring 2012). Will it stay around $136,000 per FTE or be something very different? TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
HM11 and the publication of the SHM-MGMA survey on hospitalist productivity and compensation occur every summer, and they always provide lots of new information to get me thinking. Two things stand out this year: Hospitalist demand remains high, and hospitals are paying a lot to have hospitalist services.
Supply and Demand
Along with SHM President Joe Li and Rob Bessler, who is CEO of Sound Physicians, I had the pleasure of presenting a preview of some data from the latest SHM-MGMA survey at the annual meeting May 11 in Dallas. During the session, I asked the large crowd of hospitalists how many were from practices that are actively recruiting additional hospitalists. About 40% of the hands went up.
If 40% of HM groups are actively recruiting, some for more than one open position, that’s a lot of recruiting. But it is dramatically less than the response I got when I asked the same question just three years ago at HM08 in San Diego. At that meeting, nearly every hand in the room went up, indicating everybody was recruiting (see “We’re Hiring,” July 2008, p. 62).
Of course, my show-of-hands survey of attendees at SHM meetings is not a perfect method to assess hospitalist supply and demand. But I think the dramatic change in responses from 2008 to 2011 is meaningful; it also matches what I’m seeing in the marketplace. I hear repeatedly that the years of rapid growth in hospitalist staffing have ended in many or most major metropolitan areas. For example, in places like Seattle (where I practice), Minneapolis, and Boston, there are far fewer open positions now than just two years ago, and most are to replace a departing doctor rather than to increase the overall staffing level.
But the far more numerous smaller markets are still recruiting aggressively in an effort to increase the overall staffing of the practice (and not just replace departing doctors). And changes in resident work-hour limitations are requiring teaching hospitals to increase hospitalist staffing to offset the reduction in resident availability. But it’s possible that if the larger markets are indeed becoming somewhat saturated with hospitalists, then there will be a trickledown effect, which should make more candidates available everywhere.
What will be the side effects if indeed the supply of hospitalists catches up to the demand, or even exceeds demand, in some places? It is easy to imagine that greater competition among candidates might mean that practices are increasingly able to hire the more talented and committed doctors, which should improve the overall performance of hospitalist practices.
Although I don’t have proof, I think this phenomenon has been in play in the field of emergency medicine for many years. When I was a resident in the 1980s, ED doctors typically were not the best and brightest at their hospitals. But the way I see it, the field began to attract better candidates, and as ED residencies and practices began to “fill up,” they could be more selective in new hires. Therefore, the average talent of the average ED doctor went up.
I think the average hospitalist today is pretty talented, but I also think it could get even better if the supply of hospitalists exceeds demand. I just hope I continue to make the cut!
If typical market forces are operative for hospitalists (far from a guarantee in any healthcare enterprise), then an oversupply of hospitalists could mean a flattening of the historical trend in hospitalist incomes. To this point, in our relatively young field, incomes have risen faster than can be explained solely by inflation or increases in hospitalist productivity. A relative shortage of hospitalists might be one of the main forces pushing incomes up, and it might go away.
We’ll see.
Hospital Support Trends Up
The most remarkable number in the 2011 SHM-MGMA survey is the financial support provided to practices per FTE hospitalist annually. This support nearly always comes from a hospital, and is often colloquially, and misleadingly, referred to as the “subsidy.”
In 2001, hospital support was about $65,000 per FTE. In the 2008 and 2010 surveys, the median financial support per FTE was $97,000 and $98,000, respectively. But it jumped to $136,403 this year. That is a really huge jump in one year. (Note: The surveys changed from biannual to annual in 2010, and the new SHM-MGMA survey uses a different financial support question/methodology and has a different respondent pool than the previous SHM surveys.)
Some of the increased dollars probably went to pay rising hospitalist compensation, which rose about 3% over the prior year without any significant increase in productivity. But that 3% salary increase translates to only about $5,000 (median compensation rose from roughly $215,000 to $220,000), and could be explained in part by such factors as removing academicians from this data set. (Starting in 2010, academic hospitalists are surveyed and reported separately, so aren’t included here.) So I don’t think the change in hospitalist incomes seen in this survey has much to do with the dramatic, near-40% increase in financial support.
The survey showed that hospitalist productivity hasn’t declined, so the other most likely culprit is declining professional fee collections, which might be due to an increasing portion of hospitalized patients who are uninsured or underinsured. Many hospitals report that their “payor mix” has worsened since the economic crisis of the last few years. And because hospitals typically hold the risk for the financial performance of their hospitalists, then if the latter see more uninsured patients and collect less in professional fees, the hospital will make up the difference. This phenomenon might explain much of the increased financial support.
But I’m not satisfied that a worsening payor mix explains everything. For example, if this were the most significant reason for increasing financial support, I think we would have seen this effect in the prior survey. Why did it “hit” so suddenly in this year alone?
We will get more information about collection rates when the second part of the survey is published in September. For example, we’ll be able to compare the dollars collected per encounter or per wRVU in the current survey to the prior one. If there was a significant drop, then it will require only a little math to see how much overall collections dropped per FTE and see if it is similar to the rise in financial support provided.
Of course, it will be very informative to see what the financial support turns out to be in the next survey (check back in late spring 2012). Will it stay around $136,000 per FTE or be something very different? TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
How to assess for possible drug-drug interactions
Bronchial thermoplasty: A promising therapy, still in its infancy
Treating severe, refractory asthma is an ever-evolving challenge and a major source of frustration for patients and clinicians. Failure of inhaler treatment often results in debilitation of the patient and leads to long-term use of corticosteroids, with their insidious side effects.1–3
Most asthma research continues to focus on inhibiting the cytokine cascade to reduce inflammation. However, inflammation is not the only pathophysiologic process underlying asthma.
Bronchial thermoplasty takes a novel approach and offers reason for some optimism.4–6 The aim of this minimally invasive bronchoscopic procedure is to attenuate bronchoconstriction by reducing airway smooth muscle mass.
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Thomas Gildea and colleagues7 review the pathophysiology of asthma and the utility of decreasing airway smooth muscle via bronchial thermoplasty, its logistics, and the clinical trials that led to its approval by the US Food and Drug Administration (FDA) for the treatment of severe refractory asthma.
EVIDENCE FROM CLINICAL TRIALS
After studies in animals showed that bronchial thermoplasty was feasible, several randomized trials in humans—the Asthma Intervention Research (AIR) trial,6 the Research in Severe Asthma (RISA) trial,8 and the Asthma Intervention Research 2 (AIR2) trial9—found that the complication rates were acceptable, quality of life was improved, and health care utilization was reduced after the procedure during a 12- to 36-month period. These study results were essential in paving the way for FDA approval.
AIR2: A randomized controlled trial
The latest study to evaluate bronchial thermoplasty, the AIR2 trial,9 was designed with a feature that is used relatively infrequently in trials of invasive procedures: a sham control. A sham procedure can be defined as one performed on control-group participants to ensure that they experience the same incidental effects of the procedure as do participants who actually undergo the procedure.10
Thus, the patients in the control group received the same medications before and after the procedure, they were taken to the procedure room, and the bronchoscope was actually inserted into their lungs—but thermoplasty was not performed. All of this was done in a double-blind manner: neither the patients nor the physicians caring for them before and after the procedure knew which group they were in.
The aim of this exercise was to reduce bias, namely, the placebo effect, and to reinforce results that depend on subjective symptoms, such as the Asthma Quality of Life Questionnaire (AQLQ) score. Clinical trials in severe asthma are notoriously marred by the placebo effect, resulting in spurious improvements in lung function and symptoms.
The AIR2 trial found a significant reduction in severe exacerbations and emergency department visits, and a clinically meaningful improvement in AQLQ score from baseline at 6, 9, and 12 months in the bronchial thermoplasty group. However, 16 patients needed to be hospitalized after the procedure in the bronchial thermoplasty group, compared with two patients in the sham-procedure group.
The AIR2 trial, through the use of a sham-procedure control group, was able to minimize multiple forms of bias and thus provides the most reliable data for clinicians to extrapolate the good and the bad effects of bronchial thermoplasty.
THE PROCEDURE IS STILL IN ITS INFANCY
With any new therapy, we need to look at the benefits and complications not only in the short term but also the long term, ie, to determine whether the benefit is sustainable.
Long-term data on the benefits and side effects of bronchial thermoplasty have yet to be reported. However, radiofrequency ablation has been used in lung cancer therapy during the past decade, with favorable periprocedure complication profiles. Additionally, 5-year follow-up data have shown superior outcomes in stage I non-small-cell lung cancer survival rates with radiofrequency ablation compared with external-beam radiation.11
Ongoing studies will eventually provide insight on long-term outcomes of bronchial thermoplasty in asthma patients. Until such time, patients who have reached the limits of step-up therapy for severe refractory asthma should be informed that clinicians do not yet have a complete understanding of clinical benefits or sustainability of thermoplasty. Still, confidence in bronchial thermoplasty should be grounded in the simplicity of the procedure, the low short-term complication rates, and the long-term success of comparable medical procedures such as radiofrequency ablation in lung cancer, which utilizes similar technology.
Although this procedure is still in its infancy, the potential for long-term effectiveness in improving pulmonary function and quality of life in patients with severe asthma are undeniable. The body of data supporting its use will continue to evolve and hopefully point the way to better control of severe refractory asthma.
- Bollet AJ, Black R, Bunim JJ. Major undesirable side-effects resulting from prednisolone and prednisone. J Am Med Assoc 1955; 158:459–463.
- Olgaard K, Storm T, van Wowern N, et al. Glucocorticoid-induced osteoporosis in the lumbar spine, forearm, and mandible of nephrotic patients: a double-blind study on the high-dose, long-term effects of prednisone versus deflazacort. Calcif Tissue Int 1992; 50:490–497.
- Krasner AS. Glucocorticoid-induced adrenal insufficiency. JAMA 1999; 282:671–676.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Gildea TR, Khatri SB, Castro M. Bronchial thermoplasty: a new treatment for severe refractory asthma. Cleve Clin J Med 2011; 78:477–485.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Simpson JA, Weiner ESC, editors. Oxford English Dictionary. 2nd ed. New York, NY: Oxford University Press; 1989.
- Sibley GS, Jamieson TA, Marks LB, Anscher MS, Prosnitz LR. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 1998; 40:149–154.
Treating severe, refractory asthma is an ever-evolving challenge and a major source of frustration for patients and clinicians. Failure of inhaler treatment often results in debilitation of the patient and leads to long-term use of corticosteroids, with their insidious side effects.1–3
Most asthma research continues to focus on inhibiting the cytokine cascade to reduce inflammation. However, inflammation is not the only pathophysiologic process underlying asthma.
Bronchial thermoplasty takes a novel approach and offers reason for some optimism.4–6 The aim of this minimally invasive bronchoscopic procedure is to attenuate bronchoconstriction by reducing airway smooth muscle mass.
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Thomas Gildea and colleagues7 review the pathophysiology of asthma and the utility of decreasing airway smooth muscle via bronchial thermoplasty, its logistics, and the clinical trials that led to its approval by the US Food and Drug Administration (FDA) for the treatment of severe refractory asthma.
EVIDENCE FROM CLINICAL TRIALS
After studies in animals showed that bronchial thermoplasty was feasible, several randomized trials in humans—the Asthma Intervention Research (AIR) trial,6 the Research in Severe Asthma (RISA) trial,8 and the Asthma Intervention Research 2 (AIR2) trial9—found that the complication rates were acceptable, quality of life was improved, and health care utilization was reduced after the procedure during a 12- to 36-month period. These study results were essential in paving the way for FDA approval.
AIR2: A randomized controlled trial
The latest study to evaluate bronchial thermoplasty, the AIR2 trial,9 was designed with a feature that is used relatively infrequently in trials of invasive procedures: a sham control. A sham procedure can be defined as one performed on control-group participants to ensure that they experience the same incidental effects of the procedure as do participants who actually undergo the procedure.10
Thus, the patients in the control group received the same medications before and after the procedure, they were taken to the procedure room, and the bronchoscope was actually inserted into their lungs—but thermoplasty was not performed. All of this was done in a double-blind manner: neither the patients nor the physicians caring for them before and after the procedure knew which group they were in.
The aim of this exercise was to reduce bias, namely, the placebo effect, and to reinforce results that depend on subjective symptoms, such as the Asthma Quality of Life Questionnaire (AQLQ) score. Clinical trials in severe asthma are notoriously marred by the placebo effect, resulting in spurious improvements in lung function and symptoms.
The AIR2 trial found a significant reduction in severe exacerbations and emergency department visits, and a clinically meaningful improvement in AQLQ score from baseline at 6, 9, and 12 months in the bronchial thermoplasty group. However, 16 patients needed to be hospitalized after the procedure in the bronchial thermoplasty group, compared with two patients in the sham-procedure group.
The AIR2 trial, through the use of a sham-procedure control group, was able to minimize multiple forms of bias and thus provides the most reliable data for clinicians to extrapolate the good and the bad effects of bronchial thermoplasty.
THE PROCEDURE IS STILL IN ITS INFANCY
With any new therapy, we need to look at the benefits and complications not only in the short term but also the long term, ie, to determine whether the benefit is sustainable.
Long-term data on the benefits and side effects of bronchial thermoplasty have yet to be reported. However, radiofrequency ablation has been used in lung cancer therapy during the past decade, with favorable periprocedure complication profiles. Additionally, 5-year follow-up data have shown superior outcomes in stage I non-small-cell lung cancer survival rates with radiofrequency ablation compared with external-beam radiation.11
Ongoing studies will eventually provide insight on long-term outcomes of bronchial thermoplasty in asthma patients. Until such time, patients who have reached the limits of step-up therapy for severe refractory asthma should be informed that clinicians do not yet have a complete understanding of clinical benefits or sustainability of thermoplasty. Still, confidence in bronchial thermoplasty should be grounded in the simplicity of the procedure, the low short-term complication rates, and the long-term success of comparable medical procedures such as radiofrequency ablation in lung cancer, which utilizes similar technology.
Although this procedure is still in its infancy, the potential for long-term effectiveness in improving pulmonary function and quality of life in patients with severe asthma are undeniable. The body of data supporting its use will continue to evolve and hopefully point the way to better control of severe refractory asthma.
Treating severe, refractory asthma is an ever-evolving challenge and a major source of frustration for patients and clinicians. Failure of inhaler treatment often results in debilitation of the patient and leads to long-term use of corticosteroids, with their insidious side effects.1–3
Most asthma research continues to focus on inhibiting the cytokine cascade to reduce inflammation. However, inflammation is not the only pathophysiologic process underlying asthma.
Bronchial thermoplasty takes a novel approach and offers reason for some optimism.4–6 The aim of this minimally invasive bronchoscopic procedure is to attenuate bronchoconstriction by reducing airway smooth muscle mass.
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Thomas Gildea and colleagues7 review the pathophysiology of asthma and the utility of decreasing airway smooth muscle via bronchial thermoplasty, its logistics, and the clinical trials that led to its approval by the US Food and Drug Administration (FDA) for the treatment of severe refractory asthma.
EVIDENCE FROM CLINICAL TRIALS
After studies in animals showed that bronchial thermoplasty was feasible, several randomized trials in humans—the Asthma Intervention Research (AIR) trial,6 the Research in Severe Asthma (RISA) trial,8 and the Asthma Intervention Research 2 (AIR2) trial9—found that the complication rates were acceptable, quality of life was improved, and health care utilization was reduced after the procedure during a 12- to 36-month period. These study results were essential in paving the way for FDA approval.
AIR2: A randomized controlled trial
The latest study to evaluate bronchial thermoplasty, the AIR2 trial,9 was designed with a feature that is used relatively infrequently in trials of invasive procedures: a sham control. A sham procedure can be defined as one performed on control-group participants to ensure that they experience the same incidental effects of the procedure as do participants who actually undergo the procedure.10
Thus, the patients in the control group received the same medications before and after the procedure, they were taken to the procedure room, and the bronchoscope was actually inserted into their lungs—but thermoplasty was not performed. All of this was done in a double-blind manner: neither the patients nor the physicians caring for them before and after the procedure knew which group they were in.
The aim of this exercise was to reduce bias, namely, the placebo effect, and to reinforce results that depend on subjective symptoms, such as the Asthma Quality of Life Questionnaire (AQLQ) score. Clinical trials in severe asthma are notoriously marred by the placebo effect, resulting in spurious improvements in lung function and symptoms.
The AIR2 trial found a significant reduction in severe exacerbations and emergency department visits, and a clinically meaningful improvement in AQLQ score from baseline at 6, 9, and 12 months in the bronchial thermoplasty group. However, 16 patients needed to be hospitalized after the procedure in the bronchial thermoplasty group, compared with two patients in the sham-procedure group.
The AIR2 trial, through the use of a sham-procedure control group, was able to minimize multiple forms of bias and thus provides the most reliable data for clinicians to extrapolate the good and the bad effects of bronchial thermoplasty.
THE PROCEDURE IS STILL IN ITS INFANCY
With any new therapy, we need to look at the benefits and complications not only in the short term but also the long term, ie, to determine whether the benefit is sustainable.
Long-term data on the benefits and side effects of bronchial thermoplasty have yet to be reported. However, radiofrequency ablation has been used in lung cancer therapy during the past decade, with favorable periprocedure complication profiles. Additionally, 5-year follow-up data have shown superior outcomes in stage I non-small-cell lung cancer survival rates with radiofrequency ablation compared with external-beam radiation.11
Ongoing studies will eventually provide insight on long-term outcomes of bronchial thermoplasty in asthma patients. Until such time, patients who have reached the limits of step-up therapy for severe refractory asthma should be informed that clinicians do not yet have a complete understanding of clinical benefits or sustainability of thermoplasty. Still, confidence in bronchial thermoplasty should be grounded in the simplicity of the procedure, the low short-term complication rates, and the long-term success of comparable medical procedures such as radiofrequency ablation in lung cancer, which utilizes similar technology.
Although this procedure is still in its infancy, the potential for long-term effectiveness in improving pulmonary function and quality of life in patients with severe asthma are undeniable. The body of data supporting its use will continue to evolve and hopefully point the way to better control of severe refractory asthma.
- Bollet AJ, Black R, Bunim JJ. Major undesirable side-effects resulting from prednisolone and prednisone. J Am Med Assoc 1955; 158:459–463.
- Olgaard K, Storm T, van Wowern N, et al. Glucocorticoid-induced osteoporosis in the lumbar spine, forearm, and mandible of nephrotic patients: a double-blind study on the high-dose, long-term effects of prednisone versus deflazacort. Calcif Tissue Int 1992; 50:490–497.
- Krasner AS. Glucocorticoid-induced adrenal insufficiency. JAMA 1999; 282:671–676.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Gildea TR, Khatri SB, Castro M. Bronchial thermoplasty: a new treatment for severe refractory asthma. Cleve Clin J Med 2011; 78:477–485.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Simpson JA, Weiner ESC, editors. Oxford English Dictionary. 2nd ed. New York, NY: Oxford University Press; 1989.
- Sibley GS, Jamieson TA, Marks LB, Anscher MS, Prosnitz LR. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 1998; 40:149–154.
- Bollet AJ, Black R, Bunim JJ. Major undesirable side-effects resulting from prednisolone and prednisone. J Am Med Assoc 1955; 158:459–463.
- Olgaard K, Storm T, van Wowern N, et al. Glucocorticoid-induced osteoporosis in the lumbar spine, forearm, and mandible of nephrotic patients: a double-blind study on the high-dose, long-term effects of prednisone versus deflazacort. Calcif Tissue Int 1992; 50:490–497.
- Krasner AS. Glucocorticoid-induced adrenal insufficiency. JAMA 1999; 282:671–676.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Gildea TR, Khatri SB, Castro M. Bronchial thermoplasty: a new treatment for severe refractory asthma. Cleve Clin J Med 2011; 78:477–485.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Simpson JA, Weiner ESC, editors. Oxford English Dictionary. 2nd ed. New York, NY: Oxford University Press; 1989.
- Sibley GS, Jamieson TA, Marks LB, Anscher MS, Prosnitz LR. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 1998; 40:149–154.
Bronchial thermoplasty: A new treatment for severe refractory asthma
Asthma now has a new treatment, but it isn’t for everybody. Called bronchial thermoplasty, it is reserved for patients whose asthma is severe and refractory, as it involves three sessions of bronchoscopy, each lasting up to 1 hour, during which the smooth muscle layer is methodically ablated from the airway using radiofrequency energy.1,2
The US Food and Drug Administration (FDA) has approved bronchial thermoplasty,3 and although it does not cure asthma or completely eliminate its symptoms, patients with severe asthma that was not well controlled with medical therapy who underwent this procedure in clinical trials subsequently had fewer symptoms, enjoyed better quality of life, and needed less intensive health care (such as emergency room visits) than patients who did not undergo the procedure.4–6
Here, we present an overview of the pathophysiology of severe refractory asthma and the clinical trials of bronchial thermoplasty, its current protocols, and the status of this new treatment.
WHAT IS SEVERE REFRACTORY ASTHMA?
Asthma is a chronic inflammatory condition of the airways characterized by episodic symptoms of breathlessness, cough, and wheezing, which can wax and wane over time. Approximately 8.2% of the general population is affected.7
Our understanding of the pathophysiology of asthma has improved over the past 20 years, and with the publication of clinical guidelines from the National Asthma Education and Prevention Program in 1991,8 1997,9 and 2002,10 outcomes have improved. Most people with asthma can control their symptoms if they adhere to anti-inflammatory therapies and avoid triggers. Yet 5% to 10% of asthma patients have severe refractory disease, and asthma accounts for nearly half a million hospitalizations every year.11
The latest guidelines, published in 2007, emphasize the importance of assessing the severity of asthma, including the patient’s impairment (symptoms and limitations) and risk (likelihood of exacerbations).12
Workshop consensus definition of severe refractory asthma
A consensus group convened by the American Thoracic Society13 defined asthma as severe and refractory if the patient meets at least one of the following major criteria:
- Takes oral corticosteroids continuously or nearly continuously (> 50% of year)
- Takes high-dose inhaled corticosteroids.
In addition, the patient must meet at least two minor criteria, ie:
- Takes a controller medication such as a long-acting beta-agonist, theophylline, or a leukotriene antagonist every day
- Takes a short-acting beta agonist every day or nearly every day
- Has persistent airway obstruction, ie, a forced expiratory volume in 1 second (FEV1) less than 80% of predicted, or a peak expiratory flow that has a diurnal variability greater than 20%
- Has one or more urgent care visits for asthma per year
- Needs three or more oral corticosteroid “bursts” per year
- Has prompt deterioration when the dose of oral or inhaled corticosteroid is reduced by 25% or less
- Has had a near-fatal asthma event in the past.
Compared with people with mild asthma, people who have severe refractory asthma tend to be older, have fewer allergies, and make more use of intensive and urgent health care.14
Asthma is due to both inflammation and bronchoconstriction
The pathophysiology of asthma involves both chronic airway inflammation and bronchoconstriction, the latter characterized by a greater response to methacholine. Histologic findings include excessive mucus secretion, epithelial cell injury, and smooth muscle hypertrophy. These changes can lead to persistent airflow obstruction that can be difficult to control with medical therapies.12
Bronchoconstriction can be reversed temporarily with bronchodilators, but no longlasting therapy to reduce it has been available until now. Bronchial thermoplasty targets this gap in asthma management.
STUDIES OF BRONCHIAL THERMOPLASTY
Radiofrequency ablation has been used to treat other medical conditions such as lung cancer and cardiac arrhythmias.15,16 Its use to treat asthma by eradicating smooth muscle cells from the airway wall began with studies in animals.1 Later, studies were done in people without asthma,2 then in patients with mild to moderate asthma,17 and finally in patients with moderate to severe refractory asthma.4–6
These studies helped clarify which type of patients would be appropriate candidates and the outcomes to be anticipated, including adverse events.
Early studies
Danek et al,1 in a study in nonasthmatic dogs, found that thermoplasty at 65°C or 75°C (149°F or 167°F) attenuated the airway’s response to methacholine up to 3 years after treatment. As early as 1 week after treatment, airway smooth muscle was seen to be degenerating or absent, and the effect was inversely proportional to airway responsiveness.
Adverse effects of the procedure were cough, inflammatory edema of the airway wall, retained mucus, and blanching of the airway wall at the site of catheter contact. Three years later, there was no evidence of smooth muscle regeneration.
Miller et al2 next performed a feasibility study in eight patients, mean age 58 ± 8.3 years, who were scheduled to undergo lung resection for lung cancer. Five to 20 days before surgery, the investigators performed thermoplasty at 55°C or 65°C (131°F or 149°F) in three to nine sites per patient, 1 cm from known tumors but within areas to be resected. There were no significant adverse events such as hemoptysis, respiratory infections, or excess bronchial irritation.
A pilot study in mild to moderate asthma
Cox et al17 performed the first study of bronchial thermoplasty in patients with mild to moderate asthma. This was a prospective observational study in 16 patients who were younger than the patients in the previous study, with an average age of 30 years (range 24–58). They were given prednisone 30 or 50 mg the day before the procedure and on the day of the procedure. Three treatments were done, 3 weeks apart. The right middle lobe was not treated because the bronchus leading to it is relatively long and narrow, raising concern about damaging it.18
Results. The most frequent side effects were symptoms of airway irritation such as cough, dyspnea, wheezing, and bronchospasm. The mean time to onset was less than 1.7 days, and the mean time to resolution was 4.6 days after the most recent procedure. None of the patients needed to be hospitalized in the immediate postprocedure period.
In the 2 years after the procedure, there were 312 adverse events, mainly mild. Three (1%) of the adverse events were reported as severe, but they were deemed not related to the procedure. Yearly computed tomographic scans of the chest showed no structural changes such as bronchiectasis in the parenchyma or bronchial wall.
The FEV1 was higher at 12 weeks and at 1 year after thermoplasty than at baseline but was not significantly different from baseline at 2 years.
At baseline, the patients reported that 50% of their days were symptom-free; this increased to 73% at 12 weeks (P = .015).
In addition, airway hyperresponsiveness decreased significantly, and the effect persisted over 2 years. The provocative concentration of methacholine that caused a 20% reduction in FEV1 (the PC20) was:
- 0.92 mg/mL at baseline (95% confidence interval 0.42–1.99)
- 4.75 mg/mL at 12 weeks (2.51–8.85)
- 5.45 mg/mL at 1 year (1.54–19.32)
- 3.40 mg/mL at 2 years (1.35–8.52).
Limitations of this study include the relatively small number of patients enrolled and their relatively stable asthma.
The AIR trial: A randomized trial in moderate or persistent asthma
The first large multicenter trial of bronchial thermoplasty, the Asthma Intervention Research (AIR) trial,4 was prospective and randomized but not blinded. The aim was to determine whether bronchial thermoplasty would improve asthma control after long-acting beta agonists were discontinued.
Patients could be enrolled if they were 18 to 65 years old, had moderate or persistent asthma, and needed to take an inhaled corticosteroid (beclomethasone [Qvar] 200 μg or more or an equivalent drug) and a long-acting beta agonist (salmeterol [Serevent] 100 μg or more or an equivalent drug) every day. They also needed to have FEV1 values of 60% to 85% of predicted and airway reactivity (PC20 < 8 mg/mL), and their asthma had to have been stable for 6 weeks.
At baseline, the long-acting beta agonist was withdrawn temporarily; the final criterion for entry was that their asthma had to become worse when this was done.
Then, 112 patients were randomized to receive either bronchial thermoplasty with medical care (inhaled corticosteroids and long-acting beta agonists) or usual care, ie, medical therapy alone. Treatments were done in three sessions over 9 weeks, followed by attempts to discontinue their long-acting beta agonists at 3, 6, and 9 months after the procedure without exacerbations.
An exacerbation was defined as at least one of the following for 2 consecutive days: a reduction of peak flow by 20% of baseline average, the need for more than three additional puffs of rescue inhaler, or nocturnal awakenings caused by asthma symptoms. The patients kept a daily diary of their symptoms and rescue inhaler use, and they completed the Asthma Quality of Life Questionnaire (AQLQ) and the Asthma Control Questionnaire (ACQ).
Results. The number of mild (but not severe) exacerbations per week was significantly lower at 3 and 12 months than at baseline in the thermoplasty group, with 10 fewer mild exacerbations per patient per year, but was unchanged in the control group. There were significantly greater improvements in morning peak flow at 3, 6, and 12 months from baseline in the treatment group than in the usual-care group. Rescue medication use was also significantly less at 3 and 12 months. Symptom scores, AQLQ scores, and ACQ scores were all significantly better than at baseline as well.
Not surprisingly, in this cohort with unstable asthma, there were 407 adverse events in the treatment group and 106 adverse events in the control group. Most of these occurred within 1 day and resolved within 7 days after the procedure. There were more hospitalizations in the treatment group as well, for reasons that included exacerbations of asthma, collapse of the left lower lobe, and pleurisy.4
Therefore, this trial found that thermoplasty improved asthma symptoms within 3 months and that the effect lasted 1 year, with an encouraging reduction in the number of mild exacerbations. However, it was not blinded, and there is a strong placebo effect in asthma. Needed was a randomized trial in which the control group would undergo a sham treatment.
The RISA trial: A randomized trial in severe asthma
The Research in Severe Asthma (RISA) trial5 included patients with more severe asthma than those in the AIR trial. Entry criteria were:
- Taking high doses of an inhaled corticosteroid (> 750 μg of fluticasone or its equivalent per day)
- Taking prednisone (≤ 30 mg/day)
- An FEV1 of at least 50% of predicted without a bronchodilator
- A positive methacholine test.
Seventeen patients were randomized to undergo bronchial thermoplasty, and another 17 were randomized to receive medical treatment.
After a 2-week run-in period, the thermoplasty patients underwent three treatments, performed 3 weeks apart. For the next 16 weeks, the corticosteroid doses were kept stable in both groups, followed by a 14-week corticosteroid-weaning phase and then a 16-week reduced-corticosteroid phase. During this time, attempts were made to decrease the oral or inhaled corticosteroid doses according to a protocol (eg, a 20%–25% reduction every 2–4 weeks) unless there were mild exacerbations lasting more than 7 days.
Results. There were more adverse events in the thermoplasty group than in the medical management group in the treatment period, including seven hospitalizations for exacerbations of asthma and a partial collapse of the left lower lobe. There were no significant differences in adverse events between groups in the posttreatment period (up to 6 weeks after the last treatment). Forty-nine percent of the events were mild in each group; 10% of the events were severe in the thermoplasty group vs 4% in the control group.
During the steroid-stable phase, patients in the thermoplasty group used rescue inhalers significantly less than those in the control group, and their prebronchodilator FEV1 and AQLQ and ACQ scores were better. The differences in rescue inhaler use and questionnaire scores remained significant at 1 year.
Comment. As expected, serious adverse events occurred more often in patients with severe asthma in the treatment group than in the control group. However, 1 year after the procedure, the adverse-event rates were similar in the treatment and control groups, suggesting that this procedure can be safely performed in similar patient populations. Although there was significant potential for a placebo effect, these patients with severe persistent asthma showed significant improvement in clinical measures of asthma compared with the control group.
AIR2: A randomized, double-blind trial
The latest trial of this new therapy in severe asthma was the AIR2 trial.6 A major difference in its design compared with the earlier ones was that the control group underwent sham thermoplasty, allowing the trial to be truly double-blinded. (The bronchoscopy team knew which patients got which treatment, but the patients and the study physicians following them did not).
The primary outcome was the change in AQLQ score from baseline at 6, 9, and 12 months. Secondary outcomes included absolute changes in the asthma control scores, symptom scores, peak flows, rescue medication use, and FEV1.
The randomized groups (196 patients in the thermoplasty group and 101 in the sham treatment group) were well matched, and more than 80% in each group met the American Thoracic Society criteria for severe refractory asthma.
On the AQLQ, a change of more than 0.5 is considered clinically meaningful. Interestingly, there was a significant and clinically meaningful improvement in AQLQ in 64% of the sham treatment group, highlighting the placebo effect in asthma treatment.19 However, a larger proportion (79%) of the treated group had a clinically meaningful improvement on the AQLQ than in the sham treatment group.
Adverse events occurred in both groups; however, during the treatment phase, 16 patients in the bronchial thermoplasty group needed to be hospitalized for respiratory symptoms including worsening asthma, atelectasis, lower respiratory tract infections, decreased FEV1, and an aspirated tooth. One episode of hemoptysis required bronchial artery embolization. In contrast, only two patients in the sham treatment group needed hospitalization.
Therefore, this trial showed that patients with severe asthma treated with bronchial thermoplasty had a long-term improvement in quality of life and needed less health care.6
Translating these trials into practice
To summarize, these clinical trials showed that bronchial thermoplasty was feasible, was relatively safe, and produced better clinical outcomes in patients with severe asthma when medical therapies did not control their symptoms.
In practice, patient selection is likely to be important. A key question will be, Does the patient truly have severe refractory asthma, or is the patient not taking his or her medication? Adherence to therapy should be evaluated.
In addition, patients need to be observed and monitored closely during and after the treatment period, as airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure. About 80% of all study patients had multiple symptoms of asthma and other symptoms in the treatment period. Rarely did these symptoms result in hospitalization, but they were more common in the treatment group in the AIR2 trial.
Long-term studies have evaluated the duration of effect and the safety of bronchial thermoplasty, and outcomes appear favorable.20,21
WHY DOES IT WORK?
The role of airway smooth muscle in asthma is yet to be fully elucidated. The trials outlined here showed that although asthma is a disease of the airways, including the small airways, treatment of airways 3 mm or larger improves asthma symptoms, quality of life, and health care utilization.6 Thus, the role of airway smooth muscle in asthma and as a target of therapy has not previously been fully realized.21
Early investigations into the mechanisms of airflow obstruction and airway resistance found that 75% of postnasal resistance occurs in the first six to eight generations (ie, branchings) of the airways, indicating that larger airways are involved.22 (The number of generations varies depending on the size of the person but it typically is 10 to 12.) Findings from the study in dogs introduced the idea that smooth muscle alterations contributed to the changes in airway resistance, and that subtle changes in airway smooth muscle could clinically benefit asthma patients.1
The speculated purpose of the airway smooth muscle layer is to support the airway, allow gas exchange, propel mucus for clearance, defend the airway, enhance cough, and promote lymphatic flow. However, the airway smooth muscle layer may also be vestigial. In asthma, airway smooth muscle adds to bronchoconstriction and hyperresponsiveness, and has a role in mediating inflammation and airway remodeling.21 No definitive studies have shown that eliminating airway smooth muscle greatly inhibits normal airway function.18
What exactly does thermoplasty do to the smooth muscle? Studies in smooth muscle from cows showed that high temperatures directly disrupt the actin-myosin interaction, likely through denaturation of motor proteins.23 This immediate loss of muscle cell function is not likely to be the result of apoptosis, autophagy, or necrosis, or mediated by heat-shock proteins, in view of the relatively quick muscle response and lack of progressive changes. Tissue responsiveness is substantially reduced a few seconds after application of 60°C of heat and is subsequently abolished within 5 minutes after treatment.23
The intervention appears to be dose-dependent. Responsiveness to cholinergic stimulation is lessened by treatment, and the desired effect is seen within seconds and does not progress.
Therefore, we can surmise that disruption of myosin function is likely the mechanism of the therapeutic effect, breaking the cascade of airway smooth muscle spasm. Now that we know about the airway smooth muscle as a possible target of therapy, and that it may play only a vestigial role, we can think about other therapies that focus on it.18,23
BRONCHIAL THERMOPLASTY PROTOCOLS
Patients are assessed before and on the day of the procedure to make sure their disease is stable (ie, their postbronchodilator FEV1 is within 15% of baseline values, and they have no evidence of asthma exacerbation or active infection), similar to the protocol used in the AIR2 trial,6 before proceeding with the treatment.
Patients are given 50 mg of prednisone 3 days before and again on the day of the procedure. Nebulized albuterol (2.5–5.0 mg) is given before the patients undergo screening spirometry and again before the procedure. If the preprocedure FEV1 is lower than 15% below baseline, we postpone the procedure to another day.
The procedure is performed with the patient under moderate conscious sedation, typically using fentanyl (Sublimaze), midazolam (Versed), and topical lidocaine in a monitored environment. The bronchoscope is inserted via either the mouth or nose, and supplemental oxygen is provided.
Thermoplasty is performed with the Alair system (Asthmatx, Inc., Sunnyvale, CA), which delivers a specific amount of radiofrequency (thermal) energy through a dedicated catheter. The catheter is deployed through a 2.0-mm channel of a flexible bronchoscope, starting in distal airways as small as 3 mm in diameter and working proximally to sequentially treat all airways to the mainstem lobar bronchi. The sites treated are meticulously recorded on a bronchial airway map to ensure that treatment sites are not skipped or overlapped (FIGURE 1).
An array of four electrodes is manually expanded to make contact with the airway walls; each electrode has 5 mm of exposed wire. As the energy is delivered, the control unit measures electrical resistance converted to thermal energy and turns off the current when an appropriate dosage is given. This thermal energy is what is responsible for altering the airway smooth muscle.
A full course of treatment requires three separate bronchoscopy sessions, each separated by 2 to 3 weeks. The left lower lobe and the right lower lobe are treated in separate procedures, and then both upper lobes are treated in a third procedure to minimize any respiratory symptoms. Each procedure usually requires 50 to 75 activations of the device and takes up to 60 minutes.
After each procedure the patient should be observed for 3 to 4 hours, and spirometry should be repeated to make sure the FEV1 (percent predicted) is within 20% of the baseline value. An additional 50-mg dose of prednisone is prescribed for the day after the procedure.24
FDA CLEARANCE AND LONG-TERM FOLLOW-UP
The FDA approved the Alair device for treating severe refractory asthma in early 2010.3 The indications for it are based on the study populations in the published trials. Patients can be evaluated for this treatment if they have well-documented severe persistent asthma not well controlled on inhaled corticosteroids and long-acting beta agonists and have no significant contraindications to bronchoscopy.
As part of the conditions of approval, the FDA required a postapproval study based on the long-term follow-up of the AIR2 trial. They specifically wanted to compare patients who have desirable long-term outcomes and those in whom any treatment effect wanes with time. Since we have only a few years of follow-up data, we still do not know all the possible late effects of the treatment; we have an opportunity to learn more.
Another question that needs to be studied is whether thermoplasty will help other forms of bronchospastic lung disease, such as chronic obstructive pulmonary disease.
A second postapproval study will be a prospective, open-label, single-arm, multicenter study conducted in the United States to assess the treatment effect and short-term and long-term safety profile of thermoplasty in asthma.
As experience with the procedure increases, we will be better able to characterize which patients may benefit from it. In addition, the knowledge gained by the longer-term study of airway smooth muscle function alterations will potentially drive the discovery of other innovative therapies for severe asthma.
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004; 97:1946–1953.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- US Food and Drug Administration (FDA). Approval of Alair Bronchial Thermoplasty System: Alair Catheter and Alair RF Controller. 2010. www.accessdata.fda.gov/cdrh_docs/pdf8/P080032a.pdf. Accessed June 1, 2011.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education—United States, 2001–2009. MMWR Morb Mortal Wkly Rep 2011; 60( 17):547–552.
- Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. National Asthma Education Program. Expert Panel Report. J Allergy Clin Immunol 1991; 88:425–534.
- US Department of Health and Human Services. Expert panel report 2 (EPR-2): Guidelines for the diagnosis and management of asthma, 1997. www.nhlbi.nih.gov/guidelines/archives/epr-2/index.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report: Guidelines for the diagnosis and management of asthma—Update on selected topics 2002. www.nhlbi.nih.gov/guidelines/archives/epr-2_upd/index.htm. Accessed June 1, 2011.
- Akinbami L. Asthma prevalence, health care use and mortality: United States 2003–05, CDC National Center for Health Statistics, 2006. www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma full report, 2007. www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 1, 2011.
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society Am J Respir Crit Care Med 2000; 162:2341–2351.
- Moore WC, Bleecker ER, Curran-Everett D, et al; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119:405–413.
- Ambrogi MC, Fanucchi O, Lencioni R, Cioni R, Mussi A. Pulmonary radiofrequency ablation in a single lung patient. Thorax 2006; 61:828–829.
- Benussi S, Cini R, Gaynor SL, Alfieri O, Calafiore AM. Bipolar radiofrequency maze procedure through a transseptal approach. Ann Thorac Surg 2010; 90:1025–1027.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004; 24:659–663.
- Wise RA, Bartlett SJ, Brown ED, et al; American Lung Association Asthma Clinical Research Centers. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol 2009; 124:436–444.
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G; AIR2 Trial Study Group. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011. doi: 10.1016/j.anai.2011.03.005.
- Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011; 11:8.
- Solway J, Irvin CG. Airway smooth muscle as a target for asthma therapy. N Engl J Med 2007; 356:1367–1369.
- Ingram RH, McFadden ER. Localization and mechanisms of airway responses. N Engl J Med 1977; 297:596–600.
- Dyrda P, Tazzeo T, DoHarris L, et al. Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol 2011; 44:213–221.
- Mayse ML, Laviolette M, Rubin AS, et al. Clinical pearls for bronchial thermoplasty. J Bronchol 2007; 14:115–123.
Asthma now has a new treatment, but it isn’t for everybody. Called bronchial thermoplasty, it is reserved for patients whose asthma is severe and refractory, as it involves three sessions of bronchoscopy, each lasting up to 1 hour, during which the smooth muscle layer is methodically ablated from the airway using radiofrequency energy.1,2
The US Food and Drug Administration (FDA) has approved bronchial thermoplasty,3 and although it does not cure asthma or completely eliminate its symptoms, patients with severe asthma that was not well controlled with medical therapy who underwent this procedure in clinical trials subsequently had fewer symptoms, enjoyed better quality of life, and needed less intensive health care (such as emergency room visits) than patients who did not undergo the procedure.4–6
Here, we present an overview of the pathophysiology of severe refractory asthma and the clinical trials of bronchial thermoplasty, its current protocols, and the status of this new treatment.
WHAT IS SEVERE REFRACTORY ASTHMA?
Asthma is a chronic inflammatory condition of the airways characterized by episodic symptoms of breathlessness, cough, and wheezing, which can wax and wane over time. Approximately 8.2% of the general population is affected.7
Our understanding of the pathophysiology of asthma has improved over the past 20 years, and with the publication of clinical guidelines from the National Asthma Education and Prevention Program in 1991,8 1997,9 and 2002,10 outcomes have improved. Most people with asthma can control their symptoms if they adhere to anti-inflammatory therapies and avoid triggers. Yet 5% to 10% of asthma patients have severe refractory disease, and asthma accounts for nearly half a million hospitalizations every year.11
The latest guidelines, published in 2007, emphasize the importance of assessing the severity of asthma, including the patient’s impairment (symptoms and limitations) and risk (likelihood of exacerbations).12
Workshop consensus definition of severe refractory asthma
A consensus group convened by the American Thoracic Society13 defined asthma as severe and refractory if the patient meets at least one of the following major criteria:
- Takes oral corticosteroids continuously or nearly continuously (> 50% of year)
- Takes high-dose inhaled corticosteroids.
In addition, the patient must meet at least two minor criteria, ie:
- Takes a controller medication such as a long-acting beta-agonist, theophylline, or a leukotriene antagonist every day
- Takes a short-acting beta agonist every day or nearly every day
- Has persistent airway obstruction, ie, a forced expiratory volume in 1 second (FEV1) less than 80% of predicted, or a peak expiratory flow that has a diurnal variability greater than 20%
- Has one or more urgent care visits for asthma per year
- Needs three or more oral corticosteroid “bursts” per year
- Has prompt deterioration when the dose of oral or inhaled corticosteroid is reduced by 25% or less
- Has had a near-fatal asthma event in the past.
Compared with people with mild asthma, people who have severe refractory asthma tend to be older, have fewer allergies, and make more use of intensive and urgent health care.14
Asthma is due to both inflammation and bronchoconstriction
The pathophysiology of asthma involves both chronic airway inflammation and bronchoconstriction, the latter characterized by a greater response to methacholine. Histologic findings include excessive mucus secretion, epithelial cell injury, and smooth muscle hypertrophy. These changes can lead to persistent airflow obstruction that can be difficult to control with medical therapies.12
Bronchoconstriction can be reversed temporarily with bronchodilators, but no longlasting therapy to reduce it has been available until now. Bronchial thermoplasty targets this gap in asthma management.
STUDIES OF BRONCHIAL THERMOPLASTY
Radiofrequency ablation has been used to treat other medical conditions such as lung cancer and cardiac arrhythmias.15,16 Its use to treat asthma by eradicating smooth muscle cells from the airway wall began with studies in animals.1 Later, studies were done in people without asthma,2 then in patients with mild to moderate asthma,17 and finally in patients with moderate to severe refractory asthma.4–6
These studies helped clarify which type of patients would be appropriate candidates and the outcomes to be anticipated, including adverse events.
Early studies
Danek et al,1 in a study in nonasthmatic dogs, found that thermoplasty at 65°C or 75°C (149°F or 167°F) attenuated the airway’s response to methacholine up to 3 years after treatment. As early as 1 week after treatment, airway smooth muscle was seen to be degenerating or absent, and the effect was inversely proportional to airway responsiveness.
Adverse effects of the procedure were cough, inflammatory edema of the airway wall, retained mucus, and blanching of the airway wall at the site of catheter contact. Three years later, there was no evidence of smooth muscle regeneration.
Miller et al2 next performed a feasibility study in eight patients, mean age 58 ± 8.3 years, who were scheduled to undergo lung resection for lung cancer. Five to 20 days before surgery, the investigators performed thermoplasty at 55°C or 65°C (131°F or 149°F) in three to nine sites per patient, 1 cm from known tumors but within areas to be resected. There were no significant adverse events such as hemoptysis, respiratory infections, or excess bronchial irritation.
A pilot study in mild to moderate asthma
Cox et al17 performed the first study of bronchial thermoplasty in patients with mild to moderate asthma. This was a prospective observational study in 16 patients who were younger than the patients in the previous study, with an average age of 30 years (range 24–58). They were given prednisone 30 or 50 mg the day before the procedure and on the day of the procedure. Three treatments were done, 3 weeks apart. The right middle lobe was not treated because the bronchus leading to it is relatively long and narrow, raising concern about damaging it.18
Results. The most frequent side effects were symptoms of airway irritation such as cough, dyspnea, wheezing, and bronchospasm. The mean time to onset was less than 1.7 days, and the mean time to resolution was 4.6 days after the most recent procedure. None of the patients needed to be hospitalized in the immediate postprocedure period.
In the 2 years after the procedure, there were 312 adverse events, mainly mild. Three (1%) of the adverse events were reported as severe, but they were deemed not related to the procedure. Yearly computed tomographic scans of the chest showed no structural changes such as bronchiectasis in the parenchyma or bronchial wall.
The FEV1 was higher at 12 weeks and at 1 year after thermoplasty than at baseline but was not significantly different from baseline at 2 years.
At baseline, the patients reported that 50% of their days were symptom-free; this increased to 73% at 12 weeks (P = .015).
In addition, airway hyperresponsiveness decreased significantly, and the effect persisted over 2 years. The provocative concentration of methacholine that caused a 20% reduction in FEV1 (the PC20) was:
- 0.92 mg/mL at baseline (95% confidence interval 0.42–1.99)
- 4.75 mg/mL at 12 weeks (2.51–8.85)
- 5.45 mg/mL at 1 year (1.54–19.32)
- 3.40 mg/mL at 2 years (1.35–8.52).
Limitations of this study include the relatively small number of patients enrolled and their relatively stable asthma.
The AIR trial: A randomized trial in moderate or persistent asthma
The first large multicenter trial of bronchial thermoplasty, the Asthma Intervention Research (AIR) trial,4 was prospective and randomized but not blinded. The aim was to determine whether bronchial thermoplasty would improve asthma control after long-acting beta agonists were discontinued.
Patients could be enrolled if they were 18 to 65 years old, had moderate or persistent asthma, and needed to take an inhaled corticosteroid (beclomethasone [Qvar] 200 μg or more or an equivalent drug) and a long-acting beta agonist (salmeterol [Serevent] 100 μg or more or an equivalent drug) every day. They also needed to have FEV1 values of 60% to 85% of predicted and airway reactivity (PC20 < 8 mg/mL), and their asthma had to have been stable for 6 weeks.
At baseline, the long-acting beta agonist was withdrawn temporarily; the final criterion for entry was that their asthma had to become worse when this was done.
Then, 112 patients were randomized to receive either bronchial thermoplasty with medical care (inhaled corticosteroids and long-acting beta agonists) or usual care, ie, medical therapy alone. Treatments were done in three sessions over 9 weeks, followed by attempts to discontinue their long-acting beta agonists at 3, 6, and 9 months after the procedure without exacerbations.
An exacerbation was defined as at least one of the following for 2 consecutive days: a reduction of peak flow by 20% of baseline average, the need for more than three additional puffs of rescue inhaler, or nocturnal awakenings caused by asthma symptoms. The patients kept a daily diary of their symptoms and rescue inhaler use, and they completed the Asthma Quality of Life Questionnaire (AQLQ) and the Asthma Control Questionnaire (ACQ).
Results. The number of mild (but not severe) exacerbations per week was significantly lower at 3 and 12 months than at baseline in the thermoplasty group, with 10 fewer mild exacerbations per patient per year, but was unchanged in the control group. There were significantly greater improvements in morning peak flow at 3, 6, and 12 months from baseline in the treatment group than in the usual-care group. Rescue medication use was also significantly less at 3 and 12 months. Symptom scores, AQLQ scores, and ACQ scores were all significantly better than at baseline as well.
Not surprisingly, in this cohort with unstable asthma, there were 407 adverse events in the treatment group and 106 adverse events in the control group. Most of these occurred within 1 day and resolved within 7 days after the procedure. There were more hospitalizations in the treatment group as well, for reasons that included exacerbations of asthma, collapse of the left lower lobe, and pleurisy.4
Therefore, this trial found that thermoplasty improved asthma symptoms within 3 months and that the effect lasted 1 year, with an encouraging reduction in the number of mild exacerbations. However, it was not blinded, and there is a strong placebo effect in asthma. Needed was a randomized trial in which the control group would undergo a sham treatment.
The RISA trial: A randomized trial in severe asthma
The Research in Severe Asthma (RISA) trial5 included patients with more severe asthma than those in the AIR trial. Entry criteria were:
- Taking high doses of an inhaled corticosteroid (> 750 μg of fluticasone or its equivalent per day)
- Taking prednisone (≤ 30 mg/day)
- An FEV1 of at least 50% of predicted without a bronchodilator
- A positive methacholine test.
Seventeen patients were randomized to undergo bronchial thermoplasty, and another 17 were randomized to receive medical treatment.
After a 2-week run-in period, the thermoplasty patients underwent three treatments, performed 3 weeks apart. For the next 16 weeks, the corticosteroid doses were kept stable in both groups, followed by a 14-week corticosteroid-weaning phase and then a 16-week reduced-corticosteroid phase. During this time, attempts were made to decrease the oral or inhaled corticosteroid doses according to a protocol (eg, a 20%–25% reduction every 2–4 weeks) unless there were mild exacerbations lasting more than 7 days.
Results. There were more adverse events in the thermoplasty group than in the medical management group in the treatment period, including seven hospitalizations for exacerbations of asthma and a partial collapse of the left lower lobe. There were no significant differences in adverse events between groups in the posttreatment period (up to 6 weeks after the last treatment). Forty-nine percent of the events were mild in each group; 10% of the events were severe in the thermoplasty group vs 4% in the control group.
During the steroid-stable phase, patients in the thermoplasty group used rescue inhalers significantly less than those in the control group, and their prebronchodilator FEV1 and AQLQ and ACQ scores were better. The differences in rescue inhaler use and questionnaire scores remained significant at 1 year.
Comment. As expected, serious adverse events occurred more often in patients with severe asthma in the treatment group than in the control group. However, 1 year after the procedure, the adverse-event rates were similar in the treatment and control groups, suggesting that this procedure can be safely performed in similar patient populations. Although there was significant potential for a placebo effect, these patients with severe persistent asthma showed significant improvement in clinical measures of asthma compared with the control group.
AIR2: A randomized, double-blind trial
The latest trial of this new therapy in severe asthma was the AIR2 trial.6 A major difference in its design compared with the earlier ones was that the control group underwent sham thermoplasty, allowing the trial to be truly double-blinded. (The bronchoscopy team knew which patients got which treatment, but the patients and the study physicians following them did not).
The primary outcome was the change in AQLQ score from baseline at 6, 9, and 12 months. Secondary outcomes included absolute changes in the asthma control scores, symptom scores, peak flows, rescue medication use, and FEV1.
The randomized groups (196 patients in the thermoplasty group and 101 in the sham treatment group) were well matched, and more than 80% in each group met the American Thoracic Society criteria for severe refractory asthma.
On the AQLQ, a change of more than 0.5 is considered clinically meaningful. Interestingly, there was a significant and clinically meaningful improvement in AQLQ in 64% of the sham treatment group, highlighting the placebo effect in asthma treatment.19 However, a larger proportion (79%) of the treated group had a clinically meaningful improvement on the AQLQ than in the sham treatment group.
Adverse events occurred in both groups; however, during the treatment phase, 16 patients in the bronchial thermoplasty group needed to be hospitalized for respiratory symptoms including worsening asthma, atelectasis, lower respiratory tract infections, decreased FEV1, and an aspirated tooth. One episode of hemoptysis required bronchial artery embolization. In contrast, only two patients in the sham treatment group needed hospitalization.
Therefore, this trial showed that patients with severe asthma treated with bronchial thermoplasty had a long-term improvement in quality of life and needed less health care.6
Translating these trials into practice
To summarize, these clinical trials showed that bronchial thermoplasty was feasible, was relatively safe, and produced better clinical outcomes in patients with severe asthma when medical therapies did not control their symptoms.
In practice, patient selection is likely to be important. A key question will be, Does the patient truly have severe refractory asthma, or is the patient not taking his or her medication? Adherence to therapy should be evaluated.
In addition, patients need to be observed and monitored closely during and after the treatment period, as airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure. About 80% of all study patients had multiple symptoms of asthma and other symptoms in the treatment period. Rarely did these symptoms result in hospitalization, but they were more common in the treatment group in the AIR2 trial.
Long-term studies have evaluated the duration of effect and the safety of bronchial thermoplasty, and outcomes appear favorable.20,21
WHY DOES IT WORK?
The role of airway smooth muscle in asthma is yet to be fully elucidated. The trials outlined here showed that although asthma is a disease of the airways, including the small airways, treatment of airways 3 mm or larger improves asthma symptoms, quality of life, and health care utilization.6 Thus, the role of airway smooth muscle in asthma and as a target of therapy has not previously been fully realized.21
Early investigations into the mechanisms of airflow obstruction and airway resistance found that 75% of postnasal resistance occurs in the first six to eight generations (ie, branchings) of the airways, indicating that larger airways are involved.22 (The number of generations varies depending on the size of the person but it typically is 10 to 12.) Findings from the study in dogs introduced the idea that smooth muscle alterations contributed to the changes in airway resistance, and that subtle changes in airway smooth muscle could clinically benefit asthma patients.1
The speculated purpose of the airway smooth muscle layer is to support the airway, allow gas exchange, propel mucus for clearance, defend the airway, enhance cough, and promote lymphatic flow. However, the airway smooth muscle layer may also be vestigial. In asthma, airway smooth muscle adds to bronchoconstriction and hyperresponsiveness, and has a role in mediating inflammation and airway remodeling.21 No definitive studies have shown that eliminating airway smooth muscle greatly inhibits normal airway function.18
What exactly does thermoplasty do to the smooth muscle? Studies in smooth muscle from cows showed that high temperatures directly disrupt the actin-myosin interaction, likely through denaturation of motor proteins.23 This immediate loss of muscle cell function is not likely to be the result of apoptosis, autophagy, or necrosis, or mediated by heat-shock proteins, in view of the relatively quick muscle response and lack of progressive changes. Tissue responsiveness is substantially reduced a few seconds after application of 60°C of heat and is subsequently abolished within 5 minutes after treatment.23
The intervention appears to be dose-dependent. Responsiveness to cholinergic stimulation is lessened by treatment, and the desired effect is seen within seconds and does not progress.
Therefore, we can surmise that disruption of myosin function is likely the mechanism of the therapeutic effect, breaking the cascade of airway smooth muscle spasm. Now that we know about the airway smooth muscle as a possible target of therapy, and that it may play only a vestigial role, we can think about other therapies that focus on it.18,23
BRONCHIAL THERMOPLASTY PROTOCOLS
Patients are assessed before and on the day of the procedure to make sure their disease is stable (ie, their postbronchodilator FEV1 is within 15% of baseline values, and they have no evidence of asthma exacerbation or active infection), similar to the protocol used in the AIR2 trial,6 before proceeding with the treatment.
Patients are given 50 mg of prednisone 3 days before and again on the day of the procedure. Nebulized albuterol (2.5–5.0 mg) is given before the patients undergo screening spirometry and again before the procedure. If the preprocedure FEV1 is lower than 15% below baseline, we postpone the procedure to another day.
The procedure is performed with the patient under moderate conscious sedation, typically using fentanyl (Sublimaze), midazolam (Versed), and topical lidocaine in a monitored environment. The bronchoscope is inserted via either the mouth or nose, and supplemental oxygen is provided.
Thermoplasty is performed with the Alair system (Asthmatx, Inc., Sunnyvale, CA), which delivers a specific amount of radiofrequency (thermal) energy through a dedicated catheter. The catheter is deployed through a 2.0-mm channel of a flexible bronchoscope, starting in distal airways as small as 3 mm in diameter and working proximally to sequentially treat all airways to the mainstem lobar bronchi. The sites treated are meticulously recorded on a bronchial airway map to ensure that treatment sites are not skipped or overlapped (FIGURE 1).
An array of four electrodes is manually expanded to make contact with the airway walls; each electrode has 5 mm of exposed wire. As the energy is delivered, the control unit measures electrical resistance converted to thermal energy and turns off the current when an appropriate dosage is given. This thermal energy is what is responsible for altering the airway smooth muscle.
A full course of treatment requires three separate bronchoscopy sessions, each separated by 2 to 3 weeks. The left lower lobe and the right lower lobe are treated in separate procedures, and then both upper lobes are treated in a third procedure to minimize any respiratory symptoms. Each procedure usually requires 50 to 75 activations of the device and takes up to 60 minutes.
After each procedure the patient should be observed for 3 to 4 hours, and spirometry should be repeated to make sure the FEV1 (percent predicted) is within 20% of the baseline value. An additional 50-mg dose of prednisone is prescribed for the day after the procedure.24
FDA CLEARANCE AND LONG-TERM FOLLOW-UP
The FDA approved the Alair device for treating severe refractory asthma in early 2010.3 The indications for it are based on the study populations in the published trials. Patients can be evaluated for this treatment if they have well-documented severe persistent asthma not well controlled on inhaled corticosteroids and long-acting beta agonists and have no significant contraindications to bronchoscopy.
As part of the conditions of approval, the FDA required a postapproval study based on the long-term follow-up of the AIR2 trial. They specifically wanted to compare patients who have desirable long-term outcomes and those in whom any treatment effect wanes with time. Since we have only a few years of follow-up data, we still do not know all the possible late effects of the treatment; we have an opportunity to learn more.
Another question that needs to be studied is whether thermoplasty will help other forms of bronchospastic lung disease, such as chronic obstructive pulmonary disease.
A second postapproval study will be a prospective, open-label, single-arm, multicenter study conducted in the United States to assess the treatment effect and short-term and long-term safety profile of thermoplasty in asthma.
As experience with the procedure increases, we will be better able to characterize which patients may benefit from it. In addition, the knowledge gained by the longer-term study of airway smooth muscle function alterations will potentially drive the discovery of other innovative therapies for severe asthma.
Asthma now has a new treatment, but it isn’t for everybody. Called bronchial thermoplasty, it is reserved for patients whose asthma is severe and refractory, as it involves three sessions of bronchoscopy, each lasting up to 1 hour, during which the smooth muscle layer is methodically ablated from the airway using radiofrequency energy.1,2
The US Food and Drug Administration (FDA) has approved bronchial thermoplasty,3 and although it does not cure asthma or completely eliminate its symptoms, patients with severe asthma that was not well controlled with medical therapy who underwent this procedure in clinical trials subsequently had fewer symptoms, enjoyed better quality of life, and needed less intensive health care (such as emergency room visits) than patients who did not undergo the procedure.4–6
Here, we present an overview of the pathophysiology of severe refractory asthma and the clinical trials of bronchial thermoplasty, its current protocols, and the status of this new treatment.
WHAT IS SEVERE REFRACTORY ASTHMA?
Asthma is a chronic inflammatory condition of the airways characterized by episodic symptoms of breathlessness, cough, and wheezing, which can wax and wane over time. Approximately 8.2% of the general population is affected.7
Our understanding of the pathophysiology of asthma has improved over the past 20 years, and with the publication of clinical guidelines from the National Asthma Education and Prevention Program in 1991,8 1997,9 and 2002,10 outcomes have improved. Most people with asthma can control their symptoms if they adhere to anti-inflammatory therapies and avoid triggers. Yet 5% to 10% of asthma patients have severe refractory disease, and asthma accounts for nearly half a million hospitalizations every year.11
The latest guidelines, published in 2007, emphasize the importance of assessing the severity of asthma, including the patient’s impairment (symptoms and limitations) and risk (likelihood of exacerbations).12
Workshop consensus definition of severe refractory asthma
A consensus group convened by the American Thoracic Society13 defined asthma as severe and refractory if the patient meets at least one of the following major criteria:
- Takes oral corticosteroids continuously or nearly continuously (> 50% of year)
- Takes high-dose inhaled corticosteroids.
In addition, the patient must meet at least two minor criteria, ie:
- Takes a controller medication such as a long-acting beta-agonist, theophylline, or a leukotriene antagonist every day
- Takes a short-acting beta agonist every day or nearly every day
- Has persistent airway obstruction, ie, a forced expiratory volume in 1 second (FEV1) less than 80% of predicted, or a peak expiratory flow that has a diurnal variability greater than 20%
- Has one or more urgent care visits for asthma per year
- Needs three or more oral corticosteroid “bursts” per year
- Has prompt deterioration when the dose of oral or inhaled corticosteroid is reduced by 25% or less
- Has had a near-fatal asthma event in the past.
Compared with people with mild asthma, people who have severe refractory asthma tend to be older, have fewer allergies, and make more use of intensive and urgent health care.14
Asthma is due to both inflammation and bronchoconstriction
The pathophysiology of asthma involves both chronic airway inflammation and bronchoconstriction, the latter characterized by a greater response to methacholine. Histologic findings include excessive mucus secretion, epithelial cell injury, and smooth muscle hypertrophy. These changes can lead to persistent airflow obstruction that can be difficult to control with medical therapies.12
Bronchoconstriction can be reversed temporarily with bronchodilators, but no longlasting therapy to reduce it has been available until now. Bronchial thermoplasty targets this gap in asthma management.
STUDIES OF BRONCHIAL THERMOPLASTY
Radiofrequency ablation has been used to treat other medical conditions such as lung cancer and cardiac arrhythmias.15,16 Its use to treat asthma by eradicating smooth muscle cells from the airway wall began with studies in animals.1 Later, studies were done in people without asthma,2 then in patients with mild to moderate asthma,17 and finally in patients with moderate to severe refractory asthma.4–6
These studies helped clarify which type of patients would be appropriate candidates and the outcomes to be anticipated, including adverse events.
Early studies
Danek et al,1 in a study in nonasthmatic dogs, found that thermoplasty at 65°C or 75°C (149°F or 167°F) attenuated the airway’s response to methacholine up to 3 years after treatment. As early as 1 week after treatment, airway smooth muscle was seen to be degenerating or absent, and the effect was inversely proportional to airway responsiveness.
Adverse effects of the procedure were cough, inflammatory edema of the airway wall, retained mucus, and blanching of the airway wall at the site of catheter contact. Three years later, there was no evidence of smooth muscle regeneration.
Miller et al2 next performed a feasibility study in eight patients, mean age 58 ± 8.3 years, who were scheduled to undergo lung resection for lung cancer. Five to 20 days before surgery, the investigators performed thermoplasty at 55°C or 65°C (131°F or 149°F) in three to nine sites per patient, 1 cm from known tumors but within areas to be resected. There were no significant adverse events such as hemoptysis, respiratory infections, or excess bronchial irritation.
A pilot study in mild to moderate asthma
Cox et al17 performed the first study of bronchial thermoplasty in patients with mild to moderate asthma. This was a prospective observational study in 16 patients who were younger than the patients in the previous study, with an average age of 30 years (range 24–58). They were given prednisone 30 or 50 mg the day before the procedure and on the day of the procedure. Three treatments were done, 3 weeks apart. The right middle lobe was not treated because the bronchus leading to it is relatively long and narrow, raising concern about damaging it.18
Results. The most frequent side effects were symptoms of airway irritation such as cough, dyspnea, wheezing, and bronchospasm. The mean time to onset was less than 1.7 days, and the mean time to resolution was 4.6 days after the most recent procedure. None of the patients needed to be hospitalized in the immediate postprocedure period.
In the 2 years after the procedure, there were 312 adverse events, mainly mild. Three (1%) of the adverse events were reported as severe, but they were deemed not related to the procedure. Yearly computed tomographic scans of the chest showed no structural changes such as bronchiectasis in the parenchyma or bronchial wall.
The FEV1 was higher at 12 weeks and at 1 year after thermoplasty than at baseline but was not significantly different from baseline at 2 years.
At baseline, the patients reported that 50% of their days were symptom-free; this increased to 73% at 12 weeks (P = .015).
In addition, airway hyperresponsiveness decreased significantly, and the effect persisted over 2 years. The provocative concentration of methacholine that caused a 20% reduction in FEV1 (the PC20) was:
- 0.92 mg/mL at baseline (95% confidence interval 0.42–1.99)
- 4.75 mg/mL at 12 weeks (2.51–8.85)
- 5.45 mg/mL at 1 year (1.54–19.32)
- 3.40 mg/mL at 2 years (1.35–8.52).
Limitations of this study include the relatively small number of patients enrolled and their relatively stable asthma.
The AIR trial: A randomized trial in moderate or persistent asthma
The first large multicenter trial of bronchial thermoplasty, the Asthma Intervention Research (AIR) trial,4 was prospective and randomized but not blinded. The aim was to determine whether bronchial thermoplasty would improve asthma control after long-acting beta agonists were discontinued.
Patients could be enrolled if they were 18 to 65 years old, had moderate or persistent asthma, and needed to take an inhaled corticosteroid (beclomethasone [Qvar] 200 μg or more or an equivalent drug) and a long-acting beta agonist (salmeterol [Serevent] 100 μg or more or an equivalent drug) every day. They also needed to have FEV1 values of 60% to 85% of predicted and airway reactivity (PC20 < 8 mg/mL), and their asthma had to have been stable for 6 weeks.
At baseline, the long-acting beta agonist was withdrawn temporarily; the final criterion for entry was that their asthma had to become worse when this was done.
Then, 112 patients were randomized to receive either bronchial thermoplasty with medical care (inhaled corticosteroids and long-acting beta agonists) or usual care, ie, medical therapy alone. Treatments were done in three sessions over 9 weeks, followed by attempts to discontinue their long-acting beta agonists at 3, 6, and 9 months after the procedure without exacerbations.
An exacerbation was defined as at least one of the following for 2 consecutive days: a reduction of peak flow by 20% of baseline average, the need for more than three additional puffs of rescue inhaler, or nocturnal awakenings caused by asthma symptoms. The patients kept a daily diary of their symptoms and rescue inhaler use, and they completed the Asthma Quality of Life Questionnaire (AQLQ) and the Asthma Control Questionnaire (ACQ).
Results. The number of mild (but not severe) exacerbations per week was significantly lower at 3 and 12 months than at baseline in the thermoplasty group, with 10 fewer mild exacerbations per patient per year, but was unchanged in the control group. There were significantly greater improvements in morning peak flow at 3, 6, and 12 months from baseline in the treatment group than in the usual-care group. Rescue medication use was also significantly less at 3 and 12 months. Symptom scores, AQLQ scores, and ACQ scores were all significantly better than at baseline as well.
Not surprisingly, in this cohort with unstable asthma, there were 407 adverse events in the treatment group and 106 adverse events in the control group. Most of these occurred within 1 day and resolved within 7 days after the procedure. There were more hospitalizations in the treatment group as well, for reasons that included exacerbations of asthma, collapse of the left lower lobe, and pleurisy.4
Therefore, this trial found that thermoplasty improved asthma symptoms within 3 months and that the effect lasted 1 year, with an encouraging reduction in the number of mild exacerbations. However, it was not blinded, and there is a strong placebo effect in asthma. Needed was a randomized trial in which the control group would undergo a sham treatment.
The RISA trial: A randomized trial in severe asthma
The Research in Severe Asthma (RISA) trial5 included patients with more severe asthma than those in the AIR trial. Entry criteria were:
- Taking high doses of an inhaled corticosteroid (> 750 μg of fluticasone or its equivalent per day)
- Taking prednisone (≤ 30 mg/day)
- An FEV1 of at least 50% of predicted without a bronchodilator
- A positive methacholine test.
Seventeen patients were randomized to undergo bronchial thermoplasty, and another 17 were randomized to receive medical treatment.
After a 2-week run-in period, the thermoplasty patients underwent three treatments, performed 3 weeks apart. For the next 16 weeks, the corticosteroid doses were kept stable in both groups, followed by a 14-week corticosteroid-weaning phase and then a 16-week reduced-corticosteroid phase. During this time, attempts were made to decrease the oral or inhaled corticosteroid doses according to a protocol (eg, a 20%–25% reduction every 2–4 weeks) unless there were mild exacerbations lasting more than 7 days.
Results. There were more adverse events in the thermoplasty group than in the medical management group in the treatment period, including seven hospitalizations for exacerbations of asthma and a partial collapse of the left lower lobe. There were no significant differences in adverse events between groups in the posttreatment period (up to 6 weeks after the last treatment). Forty-nine percent of the events were mild in each group; 10% of the events were severe in the thermoplasty group vs 4% in the control group.
During the steroid-stable phase, patients in the thermoplasty group used rescue inhalers significantly less than those in the control group, and their prebronchodilator FEV1 and AQLQ and ACQ scores were better. The differences in rescue inhaler use and questionnaire scores remained significant at 1 year.
Comment. As expected, serious adverse events occurred more often in patients with severe asthma in the treatment group than in the control group. However, 1 year after the procedure, the adverse-event rates were similar in the treatment and control groups, suggesting that this procedure can be safely performed in similar patient populations. Although there was significant potential for a placebo effect, these patients with severe persistent asthma showed significant improvement in clinical measures of asthma compared with the control group.
AIR2: A randomized, double-blind trial
The latest trial of this new therapy in severe asthma was the AIR2 trial.6 A major difference in its design compared with the earlier ones was that the control group underwent sham thermoplasty, allowing the trial to be truly double-blinded. (The bronchoscopy team knew which patients got which treatment, but the patients and the study physicians following them did not).
The primary outcome was the change in AQLQ score from baseline at 6, 9, and 12 months. Secondary outcomes included absolute changes in the asthma control scores, symptom scores, peak flows, rescue medication use, and FEV1.
The randomized groups (196 patients in the thermoplasty group and 101 in the sham treatment group) were well matched, and more than 80% in each group met the American Thoracic Society criteria for severe refractory asthma.
On the AQLQ, a change of more than 0.5 is considered clinically meaningful. Interestingly, there was a significant and clinically meaningful improvement in AQLQ in 64% of the sham treatment group, highlighting the placebo effect in asthma treatment.19 However, a larger proportion (79%) of the treated group had a clinically meaningful improvement on the AQLQ than in the sham treatment group.
Adverse events occurred in both groups; however, during the treatment phase, 16 patients in the bronchial thermoplasty group needed to be hospitalized for respiratory symptoms including worsening asthma, atelectasis, lower respiratory tract infections, decreased FEV1, and an aspirated tooth. One episode of hemoptysis required bronchial artery embolization. In contrast, only two patients in the sham treatment group needed hospitalization.
Therefore, this trial showed that patients with severe asthma treated with bronchial thermoplasty had a long-term improvement in quality of life and needed less health care.6
Translating these trials into practice
To summarize, these clinical trials showed that bronchial thermoplasty was feasible, was relatively safe, and produced better clinical outcomes in patients with severe asthma when medical therapies did not control their symptoms.
In practice, patient selection is likely to be important. A key question will be, Does the patient truly have severe refractory asthma, or is the patient not taking his or her medication? Adherence to therapy should be evaluated.
In addition, patients need to be observed and monitored closely during and after the treatment period, as airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure. About 80% of all study patients had multiple symptoms of asthma and other symptoms in the treatment period. Rarely did these symptoms result in hospitalization, but they were more common in the treatment group in the AIR2 trial.
Long-term studies have evaluated the duration of effect and the safety of bronchial thermoplasty, and outcomes appear favorable.20,21
WHY DOES IT WORK?
The role of airway smooth muscle in asthma is yet to be fully elucidated. The trials outlined here showed that although asthma is a disease of the airways, including the small airways, treatment of airways 3 mm or larger improves asthma symptoms, quality of life, and health care utilization.6 Thus, the role of airway smooth muscle in asthma and as a target of therapy has not previously been fully realized.21
Early investigations into the mechanisms of airflow obstruction and airway resistance found that 75% of postnasal resistance occurs in the first six to eight generations (ie, branchings) of the airways, indicating that larger airways are involved.22 (The number of generations varies depending on the size of the person but it typically is 10 to 12.) Findings from the study in dogs introduced the idea that smooth muscle alterations contributed to the changes in airway resistance, and that subtle changes in airway smooth muscle could clinically benefit asthma patients.1
The speculated purpose of the airway smooth muscle layer is to support the airway, allow gas exchange, propel mucus for clearance, defend the airway, enhance cough, and promote lymphatic flow. However, the airway smooth muscle layer may also be vestigial. In asthma, airway smooth muscle adds to bronchoconstriction and hyperresponsiveness, and has a role in mediating inflammation and airway remodeling.21 No definitive studies have shown that eliminating airway smooth muscle greatly inhibits normal airway function.18
What exactly does thermoplasty do to the smooth muscle? Studies in smooth muscle from cows showed that high temperatures directly disrupt the actin-myosin interaction, likely through denaturation of motor proteins.23 This immediate loss of muscle cell function is not likely to be the result of apoptosis, autophagy, or necrosis, or mediated by heat-shock proteins, in view of the relatively quick muscle response and lack of progressive changes. Tissue responsiveness is substantially reduced a few seconds after application of 60°C of heat and is subsequently abolished within 5 minutes after treatment.23
The intervention appears to be dose-dependent. Responsiveness to cholinergic stimulation is lessened by treatment, and the desired effect is seen within seconds and does not progress.
Therefore, we can surmise that disruption of myosin function is likely the mechanism of the therapeutic effect, breaking the cascade of airway smooth muscle spasm. Now that we know about the airway smooth muscle as a possible target of therapy, and that it may play only a vestigial role, we can think about other therapies that focus on it.18,23
BRONCHIAL THERMOPLASTY PROTOCOLS
Patients are assessed before and on the day of the procedure to make sure their disease is stable (ie, their postbronchodilator FEV1 is within 15% of baseline values, and they have no evidence of asthma exacerbation or active infection), similar to the protocol used in the AIR2 trial,6 before proceeding with the treatment.
Patients are given 50 mg of prednisone 3 days before and again on the day of the procedure. Nebulized albuterol (2.5–5.0 mg) is given before the patients undergo screening spirometry and again before the procedure. If the preprocedure FEV1 is lower than 15% below baseline, we postpone the procedure to another day.
The procedure is performed with the patient under moderate conscious sedation, typically using fentanyl (Sublimaze), midazolam (Versed), and topical lidocaine in a monitored environment. The bronchoscope is inserted via either the mouth or nose, and supplemental oxygen is provided.
Thermoplasty is performed with the Alair system (Asthmatx, Inc., Sunnyvale, CA), which delivers a specific amount of radiofrequency (thermal) energy through a dedicated catheter. The catheter is deployed through a 2.0-mm channel of a flexible bronchoscope, starting in distal airways as small as 3 mm in diameter and working proximally to sequentially treat all airways to the mainstem lobar bronchi. The sites treated are meticulously recorded on a bronchial airway map to ensure that treatment sites are not skipped or overlapped (FIGURE 1).
An array of four electrodes is manually expanded to make contact with the airway walls; each electrode has 5 mm of exposed wire. As the energy is delivered, the control unit measures electrical resistance converted to thermal energy and turns off the current when an appropriate dosage is given. This thermal energy is what is responsible for altering the airway smooth muscle.
A full course of treatment requires three separate bronchoscopy sessions, each separated by 2 to 3 weeks. The left lower lobe and the right lower lobe are treated in separate procedures, and then both upper lobes are treated in a third procedure to minimize any respiratory symptoms. Each procedure usually requires 50 to 75 activations of the device and takes up to 60 minutes.
After each procedure the patient should be observed for 3 to 4 hours, and spirometry should be repeated to make sure the FEV1 (percent predicted) is within 20% of the baseline value. An additional 50-mg dose of prednisone is prescribed for the day after the procedure.24
FDA CLEARANCE AND LONG-TERM FOLLOW-UP
The FDA approved the Alair device for treating severe refractory asthma in early 2010.3 The indications for it are based on the study populations in the published trials. Patients can be evaluated for this treatment if they have well-documented severe persistent asthma not well controlled on inhaled corticosteroids and long-acting beta agonists and have no significant contraindications to bronchoscopy.
As part of the conditions of approval, the FDA required a postapproval study based on the long-term follow-up of the AIR2 trial. They specifically wanted to compare patients who have desirable long-term outcomes and those in whom any treatment effect wanes with time. Since we have only a few years of follow-up data, we still do not know all the possible late effects of the treatment; we have an opportunity to learn more.
Another question that needs to be studied is whether thermoplasty will help other forms of bronchospastic lung disease, such as chronic obstructive pulmonary disease.
A second postapproval study will be a prospective, open-label, single-arm, multicenter study conducted in the United States to assess the treatment effect and short-term and long-term safety profile of thermoplasty in asthma.
As experience with the procedure increases, we will be better able to characterize which patients may benefit from it. In addition, the knowledge gained by the longer-term study of airway smooth muscle function alterations will potentially drive the discovery of other innovative therapies for severe asthma.
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004; 97:1946–1953.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- US Food and Drug Administration (FDA). Approval of Alair Bronchial Thermoplasty System: Alair Catheter and Alair RF Controller. 2010. www.accessdata.fda.gov/cdrh_docs/pdf8/P080032a.pdf. Accessed June 1, 2011.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education—United States, 2001–2009. MMWR Morb Mortal Wkly Rep 2011; 60( 17):547–552.
- Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. National Asthma Education Program. Expert Panel Report. J Allergy Clin Immunol 1991; 88:425–534.
- US Department of Health and Human Services. Expert panel report 2 (EPR-2): Guidelines for the diagnosis and management of asthma, 1997. www.nhlbi.nih.gov/guidelines/archives/epr-2/index.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report: Guidelines for the diagnosis and management of asthma—Update on selected topics 2002. www.nhlbi.nih.gov/guidelines/archives/epr-2_upd/index.htm. Accessed June 1, 2011.
- Akinbami L. Asthma prevalence, health care use and mortality: United States 2003–05, CDC National Center for Health Statistics, 2006. www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma full report, 2007. www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 1, 2011.
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society Am J Respir Crit Care Med 2000; 162:2341–2351.
- Moore WC, Bleecker ER, Curran-Everett D, et al; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119:405–413.
- Ambrogi MC, Fanucchi O, Lencioni R, Cioni R, Mussi A. Pulmonary radiofrequency ablation in a single lung patient. Thorax 2006; 61:828–829.
- Benussi S, Cini R, Gaynor SL, Alfieri O, Calafiore AM. Bipolar radiofrequency maze procedure through a transseptal approach. Ann Thorac Surg 2010; 90:1025–1027.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004; 24:659–663.
- Wise RA, Bartlett SJ, Brown ED, et al; American Lung Association Asthma Clinical Research Centers. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol 2009; 124:436–444.
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G; AIR2 Trial Study Group. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011. doi: 10.1016/j.anai.2011.03.005.
- Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011; 11:8.
- Solway J, Irvin CG. Airway smooth muscle as a target for asthma therapy. N Engl J Med 2007; 356:1367–1369.
- Ingram RH, McFadden ER. Localization and mechanisms of airway responses. N Engl J Med 1977; 297:596–600.
- Dyrda P, Tazzeo T, DoHarris L, et al. Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol 2011; 44:213–221.
- Mayse ML, Laviolette M, Rubin AS, et al. Clinical pearls for bronchial thermoplasty. J Bronchol 2007; 14:115–123.
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004; 97:1946–1953.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- US Food and Drug Administration (FDA). Approval of Alair Bronchial Thermoplasty System: Alair Catheter and Alair RF Controller. 2010. www.accessdata.fda.gov/cdrh_docs/pdf8/P080032a.pdf. Accessed June 1, 2011.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education—United States, 2001–2009. MMWR Morb Mortal Wkly Rep 2011; 60( 17):547–552.
- Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. National Asthma Education Program. Expert Panel Report. J Allergy Clin Immunol 1991; 88:425–534.
- US Department of Health and Human Services. Expert panel report 2 (EPR-2): Guidelines for the diagnosis and management of asthma, 1997. www.nhlbi.nih.gov/guidelines/archives/epr-2/index.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report: Guidelines for the diagnosis and management of asthma—Update on selected topics 2002. www.nhlbi.nih.gov/guidelines/archives/epr-2_upd/index.htm. Accessed June 1, 2011.
- Akinbami L. Asthma prevalence, health care use and mortality: United States 2003–05, CDC National Center for Health Statistics, 2006. www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma full report, 2007. www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 1, 2011.
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society Am J Respir Crit Care Med 2000; 162:2341–2351.
- Moore WC, Bleecker ER, Curran-Everett D, et al; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119:405–413.
- Ambrogi MC, Fanucchi O, Lencioni R, Cioni R, Mussi A. Pulmonary radiofrequency ablation in a single lung patient. Thorax 2006; 61:828–829.
- Benussi S, Cini R, Gaynor SL, Alfieri O, Calafiore AM. Bipolar radiofrequency maze procedure through a transseptal approach. Ann Thorac Surg 2010; 90:1025–1027.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004; 24:659–663.
- Wise RA, Bartlett SJ, Brown ED, et al; American Lung Association Asthma Clinical Research Centers. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol 2009; 124:436–444.
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G; AIR2 Trial Study Group. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011. doi: 10.1016/j.anai.2011.03.005.
- Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011; 11:8.
- Solway J, Irvin CG. Airway smooth muscle as a target for asthma therapy. N Engl J Med 2007; 356:1367–1369.
- Ingram RH, McFadden ER. Localization and mechanisms of airway responses. N Engl J Med 1977; 297:596–600.
- Dyrda P, Tazzeo T, DoHarris L, et al. Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol 2011; 44:213–221.
- Mayse ML, Laviolette M, Rubin AS, et al. Clinical pearls for bronchial thermoplasty. J Bronchol 2007; 14:115–123.
KEY POINTS
- Bronchial thermoplasty involves the application of radiofrequency energy to the airways distal to the mainstem bronchi down to airways as small as 3 mm in diameter.
- Treatments are done in three separate sessions, with careful monitoring before and after for respiratory complications that can occur in severe asthma. Airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure, thus requiring close patient follow-up.
- In clinical trials, including a randomized trial in which the control group underwent sham thermoplasty, bronchial thermoplasty had an acceptable safety profile while improving asthma quality-of-life scores, symptoms, and health care utilization.
Vancomycin: A 50-something-year-old antibiotic we still don’t understand
In the past half-century, vancomycin has gone from near-orphan status to being one of the most often used antibiotics in our formulary. The driving force for its use is clear: the evolution of Staphylococcus aureus. At first, vancomycin was used to treat infections caused by penicillin-resistant strains. However, the discovery of methicillin curbed its use for more than 2 decades.1
Then, as methicillin-resistant S aureus (MRSA) began to spread in the 1980s, the use of vancomycin began to increase, and with the rise in community-associated MRSA infections in the 1990s, it became even more widely prescribed. The recent Infectious Diseases Society of America (IDSA) guidelines for treatment of infections due to MRSA are replete with references to the use of vancomycin.2
Another factor driving the use of vancomycin is the increased prevalence of device-associated infections, many of which are caused by coagulase-negative staphylococci and other organisms that colonize the skin.3 Many of these bacteria are susceptible only to vancomycin; they may be associated with infections of vascular catheters, cardiac valves, pacemakers, implantable cardioverter-defibrillators, orthopedic implants, neurosurgical devices, and other devices.
To use vancomycin appropriately, we need to recognize the changing minimum inhibitory concentrations (MICs), to select proper doses and dosing intervals, and to know how to monitor its use. Despite more than 50 years of experience with vancomycin, we sometimes find ourselves with more questions than answers about its optimal use.
WHAT IS VANCOMYCIN?
Vancomycin is a glycopeptide antibiotic isolated from a strain of Streptomyces orientalis discovered in a soil sample from Borneo in the mid-1950s.1 It exerts its action by binding to a d-alanyl-d-alanine cell wall precursor necessary for peptidoglycan cross-linking and, therefore, for inhibiting bacterial cell wall synthesis.
Vancomycin is bactericidal against most gram-positive species, including streptococci and staphylococci, with the exception of Enterococcus species, for which it is bacteriostatic. Though it is bactericidal, it appears to kill bacteria more slowly than beta-lactam antibiotics, and therefore it may take longer to clear bacteremia.4
WHAT IS THE BEST WAY TO DOSE VANCOMYCIN?
Vancomycin is widely distributed to most tissues, with an approximate volume of distribution of 0.4 to 1 L/kg; 50% to 55% is protein-bound. Because of this large volume of distribution, vancomycin’s dosing is based on actual body weight.
Vancomycin is not metabolized and is primarily excreted unchanged in the urine via glomerular filtration. It therefore requires dosage adjustments for renal insufficiency.
Vancomycin’s molecular weight is 1,485.73 Da, making it less susceptible to removal by dialysis than smaller molecules. Dosing of vancomycin in patients on hemodialysis depends on many factors specific to the dialysis center, including but not limited to the type of filter used, the duration of filtration, and whether high-flux filtration is used.
Is continuous intravenous infusion better than standard dosing?
Giving vancomycin by continuous infusion has been suggested as a way to optimize its serum concentration and improve its clinical effectiveness.
Wysocki et al5 conducted a multicenter, prospective, randomized study comparing continuous and intermittent intravenous infusions of vancomycin (the latter every 12 hours) to treat severe hospital-acquired MRSA infections, including bloodstream infections and pneumonia. Although blood concentrations above 10 μg/mL were reached more than 30 hours faster with continuous infusions than with intermittent ones, the microbiologic and clinical outcomes were similar with either method.
James et al6 compared the pharmacodynamics of conventional dosing of vancomycin (ie, 1 g every 12 hours) and continuous infusion in 10 patients with suspected or documented gram-positive infections in a prospective, randomized, crossover study. While no adverse effects were observed, the authors also found no statistically significant difference between the treatment groups in the pharmacodynamic variables investigated, including the area under the curve (AUC) divided by the MIC (the AUC-MIC ratio).
In view of the currently available data, the guidelines for monitoring vancomycin therapy note that there does not appear to be any difference in patient outcomes with continuous infusion vs intermittent dosing.7
Should a loading dose be given?
Another proposed strategy for optimizing vancomycin’s effectiveness is to give a higher initial dose, ie, a loading dose.
Wang et al8 performed a single-center study in 28 patients who received a 25 mg/kg loading dose at a rate of 500 mg/hour. This loading dose was safe, but the authors did not evaluate its efficacy.
Mohammedi et al9 compared loading doses of 500 mg and 15 mg/kg in critically ill patients receiving vancomycin by continuous infusion. The weight-based loading dose produced higher post-dose levels and a significantly higher rate of clinical cure, but there was no significant difference in the rate of survival to discharge from the intensive care unit.
While the use of a loading dose appears to be safe and likely leads to more rapid attainment of therapeutic blood levels, we lack data on whether it improves clinical outcomes, and further study is needed to determine its role.
WHAT IS THE BEST WAY TO MONITOR VANCOMYCIN THERAPY?
Whether and how to use the serum vancomycin concentration to adjust the dosing has been a matter of debate for many years. Convincing evidence that vancomycin levels predict clinical outcomes or that measuring them prevents toxicity is lacking.7
A consensus statement from the American Society of Health-System Pharmacists, the IDSA, and the Society of Infectious Diseases Pharmacists7 contains recommendations for monitoring vancomycin therapy, based on a critical evaluation of the available scientific evidence. Their recommendations:
- Vancomycin serum concentrations should be checked to optimize therapy and used as a surrogate marker of effectiveness.
- Trough, rather than peak, levels should be monitored.
- Trough levels should be checked just before the fourth dose, when steady-state levels are likely to have been achieved. More frequent monitoring may be considered in patients with fluctuating renal function.
- Trough levels should be higher than 10 mg/L to prevent the development of resistance.
- To improve antibiotic penetration and optimize the likelihood of achieving pharmacokinetic and pharmacodynamic targets, trough levels of 15 to 20 mg/L are recommended for pathogens with a vancomycin MIC of 1 mg/L or higher and for complicated infections such as endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia.
- For prolonged courses, it is appropriate to check vancomycin levels weekly in hemodynamically stable patients and more often in those who are not hemodynamically stable.
IS VANCOMYCIN NEPHROTOXIC?
In the 1950s, vancomycin formulations were sometimes called “Mississippi mud” because of the many impurities they contained.1 These impurities were associated with significant nephrotoxicity. Better purification methods used in the manufacture of current formulations mitigate this problem, resulting in a lower incidence of nephrotoxicity.
Over the last several years, organizations such as the American Thoracic Society and the IDSA have recommended targeting higher vancomycin trough concentrations.10 The consequent widespread use of higher doses has renewed interest in vancomycin’s potential nephrotoxicity.
Lodise et al,11 in a cohort study, examined the incidence of nephrotoxicity with higher daily doses of vancomycin (≥ 4 g/day), lower daily doses (< 4 g/day), and linezolid (Zyvox). They defined nephrotoxicity as an increase in serum creatinine of 0.5 mg/dL or a decrease in calculated creatinine clearance of 50% from baseline on 2 consecutive days.
The incidence of nephrotoxicity was significantly higher in the high-dose vancomycin group (34.6%) than in the low-dose vancomycin group (10.9%) and in the linezolid group (6.7%) (P = .001). Additional factors associated with nephrotoxicity in this study included baseline creatinine clearance less than 86.6 mL/minute, weight greater than 101.4 kg (223.5 lb), and being in an intensive care unit.
Hidayat et al12 investigated outcomes in patients with high vs low vancomycin trough levels (≥ 15 mg/L vs < 15 mg/L) in a prospective cohort study. Sixty-three patients achieved an average vancomycin trough of 15 to 20 mg/L, and of these, 11 developed nephrotoxicity, compared with no patients in the low-trough group (P = .01). Of the 11 who developed nephrotoxicity, 10 were concomitantly taking other potentially nephrotoxic agents.
Comment. The data on vancomycin and nephrotoxicity are mostly from studies that had limitations such as small numbers of patients, retrospective design, and variable definitions of nephrotoxicity. Many of the patients in these studies had additional factors contributing to nephrotoxicity, including hemodynamic instability and concomitant exposure to other nephrotoxins. Additionally, the sequence of events (nephrotoxicity leading to elevated vancomycin levels vs elevated vancomycin levels causing nephrotoxicity) is still debatable.
The incidence of nephrotoxicity associated with vancomycin therapy is difficult to determine. However, based on current information, the incidence of nephrotoxicity appears to be low when vancomycin is used as monotherapy.
IS S AUREUS BECOMING RESISTANT TO VANCOMYCIN?
An issue of increasing importance in health care settings is the emergence of vancomycin-intermediate S aureus (VISA) and vancomycin-resistant S aureus (VRSA). Eleven cases of VRSA were identified in the United States from 2002 to 2005.13 All cases of VRSA in the United States have involved the incorporation of enterococcal vanA cassette into the S aureus genome.14 While true VRSA isolates remain rare, VISA isolates are becoming more common.
Heteroresistant VISA: An emerging subpopulation of MRSA
Another population of S aureus that has emerged is heteroresistant vancomycin-intermediate S aureus (hVISA). It is defined as the presence of subpopulations of VISA within a population of MRSA at a rate of one organism per 105 to 106 organisms. With traditional testing methods, the vancomycin MIC for the entire population of the strain is within the susceptible range.15 These hVISA populations are thought to be precursors to the development of VISA.16
The resistance to vancomycin in hVISA and VISA populations is due to increased cell wall thickness, altered penicillin-binding protein profiles, and decreased cell wall autolysis.
While the true prevalence of hVISA is difficult to predict because of challenges in microbiological detection and probably varies between geographic regions and individual institutions, different studies have reported hVISA rates between 2% and 13% of all MRSA isolates.15–17
Reduced vancomycin susceptibility can develop regardless of methicillin susceptibility.18
While hVISA is not common, its presence is thought to be a predictor of failing vancomycin therapy.15
Factors associated with hVISA bacteremia include high-bacterial-load infections, treatment failure (including persistent bacteremia for more than 7 days), and initially low serum vancomycin levels.15
‘MIC creep’: Is it real?
Also worrisome, the average vancomycin MIC for S aureus has been shifting upward, based on reports from several institutions, although it is still within the susceptible range.19,20 However, this “MIC creep” likely reflects, at least in part, differences in MIC testing and varying methods used to analyze the data.19,20
Holmes and Jorgensen,21 in a single-institution study of MRSA isolates recovered from bacteremic patients from 1999 to 2006, determined that no MIC creep existed when they tested vancomycin MICs using the broth microdilution method. The authors found the MIC90 (ie, the MIC in at least 90% of the isolates) remained less than 1 mg/L during each year of the study.
Sader et al,22 in a multicenter study, evaluated 1,800 MRSA bloodstream isolates from nine hospitals across the United States from 2002 to 2006. Vancomycin MICs were again measured by broth microdilution methods. The mode MIC remained stable at 0.625 mg/L during the study period, and the authors did not detect a trend of rising MICs.
The inconsistency between reports of MIC creep at single institutions and the absence of this phenomenon in large, multicenter studies seems to imply that vancomycin MIC creep is not occurring on a grand scale.
Vancomycin tolerance
Another troubling matter with S aureus and vancomycin is the issue of tolerance. Vancomycin tolerance, defined in terms of increased minimum bactericidal concentration, represents a loss of bactericidal activity. Tolerance to vancomycin can occur even if the MIC remains in the susceptible range.23
Safdar and Rolston,24 in an observational study from a cancer center, reported that of eight cases of bacteremia that was resistant to vancomycin therapy, three were caused by S aureus.
Sakoulas et al25 found that higher levels of vancomycin bactericidal activity were associated with higher rates of clinical success; however, they found no effect on the mortality rate.
The issue of vancomycin tolerance remains controversial, and because testing for it is impractical in clinical microbiology laboratories, its implications outside the research arena are difficult to ascertain at present.
IS VANCOMYCIN STILL THE BEST DRUG FOR S AUREUS?
MIC break points have been lowered
In 2006, the Clinical Laboratories and Standards Institute lowered its break points for vancomycin MIC categories for S aureus:
- Susceptible: ≤ 2 mg/L (formerly ≤ 4 mg/L)
- Intermediate: 4–8 mg/L (formerly 8–16 mg/L)
- Resistant: ≥ 16 mg/L (formerly ≥ 32 mg/L).
The rationales for these changes were that the lower break points would better detect hVISA, and that cases have been reported of clinical treatment failure of S aureus infections in which the MICs for vancomycin were 4 mg/L.26
Since 2006, the question has been raised whether to lower the break points even further. A reason for this proposal comes from an enhanced understanding of the pharmacokinetics and pharmacodynamics of vancomycin.
The variable most closely associated with clinical response to vancomycin is the AUC-MIC ratio. An AUC-MIC ratio of 400 or higher may be associated with better outcomes in patients with serious S aureus infection. A study of 108 patients with S aureus infection of the lower respiratory tract indicated that organism eradication was more likely if the AUC-MIC ratio was 400 or greater compared with values less than 400, and this was statistically significant.27 However, in cases of S aureus infection with a vancomycin MIC of 2 mg/L or higher, this ratio may not be achievable.
A prospective study of 414 MRSA bacteremia episodes found a vancomycin MIC of 2 mg/L to be a predictor of death.28 The authors concluded that vancomycin may not be the optimal treatment for MRSA with a vancomycin MIC of 2 mg/L.28 Additional studies have also suggested a possible decrease in response to vancomycin in MRSA isolates with elevated MICs within the susceptible range.25,29
Recent guidelines from the IDSA recommend using the clinical response, regardless of the MIC, to guide antimicrobial selection for isolates with MICs in the susceptible range.2
Combination therapy with vancomycin
As vancomycin use has increased, therapeutic failures with vancomycin have become apparent. Combination therapy has been suggested as an option to increase the efficacy of vancomycin when treating complicated infections.
Rifampin plus vancomycin is controversial.30 The combination is theoretically beneficial, especially in infections associated with prosthetic devices. However, clinical studies have failed to convincingly support its use, and some have suggested that it might prolong bacteremia. In addition, it has numerous drug interactions to consider and adverse effects.31
Gentamicin plus vancomycin. The evidence supporting the use of this combination is weak at best. It appears that clinicians may have extrapolated from the success reported by Korzeniowski and Sande,32 who found that methicillin-susceptible S aureus bacteremia was cleared faster if gentamicin was added to nafcillin. A more recent study33 that compared daptomycin (Cubicin) monotherapy with combined vancomycin and gentamicin to treat MRSA bacteremia and endocarditis showed a better overall success rate with daptomycin (44% vs 32.6%), but the difference was not statistically significant.
Gentamicin has some toxicity. Even short-term use (for the first 4 days of therapy) at low doses for bacteremia and endocarditis due to staphylococci has been associated with a higher rate of renal adverse events, including a significant decrease in creatinine clearance.34
Clindamycin or linezolid plus vancomycin is used to decrease toxin production by S aureus.30
While combination therapy with vancomycin is recommended in specific clinical situations, and the combinations are synergistic in vitro, information is lacking about clinical outcomes to support their use.
Don’t use vancomycin when another drug would be better
Vancomycin continues to be the drug of choice in many circumstances, but in some instances its role is under scrutiny and another drug might be better.
Beta-lactams. In patients with infection due to methicillin-susceptible S aureus, failure rates are higher with vancomycin than with beta-lactam therapy, specifically nafcillin.35–37 Beta-lactam antibiotics are thus the drugs of choice for treating infection with beta-lactam-susceptible strains of S aureus.
Linezolid. In theory, linezolid’s ability to decrease production of the S aureus Panton-Valentine leukocidin (PVL) toxin may be an advantage over vancomycin for treating necrotizing pneumonias. For the treatment of MRSA pneumonia, however, controversy exists as to whether linezolid is superior to vancomycin. An analysis of two prospective, randomized, double-blind studies of patients with MRSA pneumonia suggested that initial therapy with linezolid was associated with better survival and clinical cure rates,38 but a subsequent meta-analysis did not substantiate this finding.39 An additional comparative study has been completed, and analysis of the results is in progress.
Daptomycin, approved for skin and soft-tissue infections and bacteremias, including those with right-sided endocarditis, is a lipopeptide antibiotic with a spectrum of action similar to that of vancomycin.40 Daptomycin is also active against many strains of vancomycin-resistant enterococci. As noted above, in the MRSA subgroup of the pivotal comparative study of treatment for S aureus bacteremia and endocarditis, the success rate for daptomycin-treated patients (44.4%) was better than that for patients treated with vancomycin plus gentamicin (32.6%), but the difference was not statistically significant.33,41
The creatine phosphokinase concentration should be monitored weekly in patients on daptomycin.42 Daptomycin is inactivated by lung surfactant and should not be used to treat pneumonia.
Other treatment options approved by the US Food and Drug Administration (FDA) for MRSA infections include tigecycline (Tygacil), quinupristin-dalfopristin (Synercid), telavancin (Vibativ), and ceftaroline (Teflaro).
Tigecycline is a glycylcycline with bacteriostatic activity against S aureus and wide distribution to the tissues.43
Quinupristin-dalfopristin, a streptogramin antibiotic, has activity against S aureus. Its use may be associated with severe myalgias, sometimes leading patients to stop taking it.
Telavancin, recently approved by the FDA, is a lipoglycopeptide antibiotic.44 It is currently approved to treat complicated skin and skin structure infections and was found to be not inferior to vancomycin. An important side effect of this agent is nephrotoxicity. A negative pregnancy test is required before using this agent in women of childbearing potential.
Ceftaroline, a fifth-generation cephalosporin active against MRSA, has been approved by the FDA for the treatment of skin and skin structure infections and community-acquired pneumonia.45
- Murray BE, Nannini EC. Glycopeptides (vancomycin and teicoplanin), streptogramins (quinupristin-dalfopristin), and lipopeptides (daptomycin). In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010:449–468.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:285–292.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001; 45:2460–2467.
- James JK, Palmer SM, Levine DP, Rybak MJ. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996; 40:696–700.
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98.
- Wang JT, Fang CT, Chen YC, Chang SC. Necessity of a loading dose when using vancomycin in critically ill patients (letter). J Antimicrob Chemother 2001; 47:246.
- Mohammedi I, Descloux E, Argaud L, Le Scanff J, Robert D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrob Agents 2006; 27:259–262.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52:1330–1336.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Centers for Disease Control and Prevention. CDC reminds clinical laboratories and healthcare infection preventionists of their role in the search and containment of vancomycin-resistant Staphylococcus aureus (VRSA), May 2010. http://emergency.cdc.gov/coca/reminders/2010/2010may06.asp. Accessed June 7, 2011.
- Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 2008; 46:668–674.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sader HS, Jones RN, Rossi KL, Rybak MJ. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine USA hospitals. J Antimicrob Chemother 2009; 64:1024–1028.
- Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 2009; 49:1169–1174.
- Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 2006; 44:3883–3886.
- Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in nonvancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother 2007; 60:788–794.
- Holmes RL, Jorgensen JH. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother 2008; 52:757–760.
- Sader HS, Fey PD, Limaye AP, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother 2009; 53:4127–4132.
- May J, Shannon K, King A, French G. Glycopeptide tolerance in Staphylococcus aureus. J Antimicrob Chemother 1998; 42:189–197.
- Safdar A, Rolston KV. Vancomycin tolerance, a potential mechanism for refractory gram-positive bacteremia observational study in patients with cancer. Cancer 2006; 106:1815–1820.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007; 44:1208–1215.
- Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43:925–942.
- Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:193–200.
- Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008; 52:3315–3320.
- Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2009; 49:1072–1079.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Rehm SJ, Boucher H, Levine D, et al. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother 2008; 62:1413–1421.
- Cosgrove SE, Vigliani GA, Fowler VG, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48:713–721.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003; 124:1789–1797.
- Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 2010; 38:1802–1808.
- Kosmidis C, Levine DP. Daptomycin: pharmacology and clinical use. Expert Opin Pharmacother 2010; 11:615–625.
- Fowler VG, Boucher HW, Corey GR, et al; S aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Daptomycin package insert. Lexington, MA. Cubist Pharmaceuticals, Inc. November 2010. www.cubicin.com/pdf/PrescribingInformation.pdf. Accessed June 7, 2011.
- Peterson LR. A review of tigecycline—the first glycylcycline. Int J Antimicrob Agents 2008; 32(suppl 4):S215–S222.
- Saravolatz LD, Stein GE, Johnson LB. Telavancin: a novel lipoglycopeptide. Clin Infect Dis 2009; 49:1908–1914.
- Ceftaroline package insert. St. Louis, MO. Forest Pharmaceuticals. October 2010.
In the past half-century, vancomycin has gone from near-orphan status to being one of the most often used antibiotics in our formulary. The driving force for its use is clear: the evolution of Staphylococcus aureus. At first, vancomycin was used to treat infections caused by penicillin-resistant strains. However, the discovery of methicillin curbed its use for more than 2 decades.1
Then, as methicillin-resistant S aureus (MRSA) began to spread in the 1980s, the use of vancomycin began to increase, and with the rise in community-associated MRSA infections in the 1990s, it became even more widely prescribed. The recent Infectious Diseases Society of America (IDSA) guidelines for treatment of infections due to MRSA are replete with references to the use of vancomycin.2
Another factor driving the use of vancomycin is the increased prevalence of device-associated infections, many of which are caused by coagulase-negative staphylococci and other organisms that colonize the skin.3 Many of these bacteria are susceptible only to vancomycin; they may be associated with infections of vascular catheters, cardiac valves, pacemakers, implantable cardioverter-defibrillators, orthopedic implants, neurosurgical devices, and other devices.
To use vancomycin appropriately, we need to recognize the changing minimum inhibitory concentrations (MICs), to select proper doses and dosing intervals, and to know how to monitor its use. Despite more than 50 years of experience with vancomycin, we sometimes find ourselves with more questions than answers about its optimal use.
WHAT IS VANCOMYCIN?
Vancomycin is a glycopeptide antibiotic isolated from a strain of Streptomyces orientalis discovered in a soil sample from Borneo in the mid-1950s.1 It exerts its action by binding to a d-alanyl-d-alanine cell wall precursor necessary for peptidoglycan cross-linking and, therefore, for inhibiting bacterial cell wall synthesis.
Vancomycin is bactericidal against most gram-positive species, including streptococci and staphylococci, with the exception of Enterococcus species, for which it is bacteriostatic. Though it is bactericidal, it appears to kill bacteria more slowly than beta-lactam antibiotics, and therefore it may take longer to clear bacteremia.4
WHAT IS THE BEST WAY TO DOSE VANCOMYCIN?
Vancomycin is widely distributed to most tissues, with an approximate volume of distribution of 0.4 to 1 L/kg; 50% to 55% is protein-bound. Because of this large volume of distribution, vancomycin’s dosing is based on actual body weight.
Vancomycin is not metabolized and is primarily excreted unchanged in the urine via glomerular filtration. It therefore requires dosage adjustments for renal insufficiency.
Vancomycin’s molecular weight is 1,485.73 Da, making it less susceptible to removal by dialysis than smaller molecules. Dosing of vancomycin in patients on hemodialysis depends on many factors specific to the dialysis center, including but not limited to the type of filter used, the duration of filtration, and whether high-flux filtration is used.
Is continuous intravenous infusion better than standard dosing?
Giving vancomycin by continuous infusion has been suggested as a way to optimize its serum concentration and improve its clinical effectiveness.
Wysocki et al5 conducted a multicenter, prospective, randomized study comparing continuous and intermittent intravenous infusions of vancomycin (the latter every 12 hours) to treat severe hospital-acquired MRSA infections, including bloodstream infections and pneumonia. Although blood concentrations above 10 μg/mL were reached more than 30 hours faster with continuous infusions than with intermittent ones, the microbiologic and clinical outcomes were similar with either method.
James et al6 compared the pharmacodynamics of conventional dosing of vancomycin (ie, 1 g every 12 hours) and continuous infusion in 10 patients with suspected or documented gram-positive infections in a prospective, randomized, crossover study. While no adverse effects were observed, the authors also found no statistically significant difference between the treatment groups in the pharmacodynamic variables investigated, including the area under the curve (AUC) divided by the MIC (the AUC-MIC ratio).
In view of the currently available data, the guidelines for monitoring vancomycin therapy note that there does not appear to be any difference in patient outcomes with continuous infusion vs intermittent dosing.7
Should a loading dose be given?
Another proposed strategy for optimizing vancomycin’s effectiveness is to give a higher initial dose, ie, a loading dose.
Wang et al8 performed a single-center study in 28 patients who received a 25 mg/kg loading dose at a rate of 500 mg/hour. This loading dose was safe, but the authors did not evaluate its efficacy.
Mohammedi et al9 compared loading doses of 500 mg and 15 mg/kg in critically ill patients receiving vancomycin by continuous infusion. The weight-based loading dose produced higher post-dose levels and a significantly higher rate of clinical cure, but there was no significant difference in the rate of survival to discharge from the intensive care unit.
While the use of a loading dose appears to be safe and likely leads to more rapid attainment of therapeutic blood levels, we lack data on whether it improves clinical outcomes, and further study is needed to determine its role.
WHAT IS THE BEST WAY TO MONITOR VANCOMYCIN THERAPY?
Whether and how to use the serum vancomycin concentration to adjust the dosing has been a matter of debate for many years. Convincing evidence that vancomycin levels predict clinical outcomes or that measuring them prevents toxicity is lacking.7
A consensus statement from the American Society of Health-System Pharmacists, the IDSA, and the Society of Infectious Diseases Pharmacists7 contains recommendations for monitoring vancomycin therapy, based on a critical evaluation of the available scientific evidence. Their recommendations:
- Vancomycin serum concentrations should be checked to optimize therapy and used as a surrogate marker of effectiveness.
- Trough, rather than peak, levels should be monitored.
- Trough levels should be checked just before the fourth dose, when steady-state levels are likely to have been achieved. More frequent monitoring may be considered in patients with fluctuating renal function.
- Trough levels should be higher than 10 mg/L to prevent the development of resistance.
- To improve antibiotic penetration and optimize the likelihood of achieving pharmacokinetic and pharmacodynamic targets, trough levels of 15 to 20 mg/L are recommended for pathogens with a vancomycin MIC of 1 mg/L or higher and for complicated infections such as endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia.
- For prolonged courses, it is appropriate to check vancomycin levels weekly in hemodynamically stable patients and more often in those who are not hemodynamically stable.
IS VANCOMYCIN NEPHROTOXIC?
In the 1950s, vancomycin formulations were sometimes called “Mississippi mud” because of the many impurities they contained.1 These impurities were associated with significant nephrotoxicity. Better purification methods used in the manufacture of current formulations mitigate this problem, resulting in a lower incidence of nephrotoxicity.
Over the last several years, organizations such as the American Thoracic Society and the IDSA have recommended targeting higher vancomycin trough concentrations.10 The consequent widespread use of higher doses has renewed interest in vancomycin’s potential nephrotoxicity.
Lodise et al,11 in a cohort study, examined the incidence of nephrotoxicity with higher daily doses of vancomycin (≥ 4 g/day), lower daily doses (< 4 g/day), and linezolid (Zyvox). They defined nephrotoxicity as an increase in serum creatinine of 0.5 mg/dL or a decrease in calculated creatinine clearance of 50% from baseline on 2 consecutive days.
The incidence of nephrotoxicity was significantly higher in the high-dose vancomycin group (34.6%) than in the low-dose vancomycin group (10.9%) and in the linezolid group (6.7%) (P = .001). Additional factors associated with nephrotoxicity in this study included baseline creatinine clearance less than 86.6 mL/minute, weight greater than 101.4 kg (223.5 lb), and being in an intensive care unit.
Hidayat et al12 investigated outcomes in patients with high vs low vancomycin trough levels (≥ 15 mg/L vs < 15 mg/L) in a prospective cohort study. Sixty-three patients achieved an average vancomycin trough of 15 to 20 mg/L, and of these, 11 developed nephrotoxicity, compared with no patients in the low-trough group (P = .01). Of the 11 who developed nephrotoxicity, 10 were concomitantly taking other potentially nephrotoxic agents.
Comment. The data on vancomycin and nephrotoxicity are mostly from studies that had limitations such as small numbers of patients, retrospective design, and variable definitions of nephrotoxicity. Many of the patients in these studies had additional factors contributing to nephrotoxicity, including hemodynamic instability and concomitant exposure to other nephrotoxins. Additionally, the sequence of events (nephrotoxicity leading to elevated vancomycin levels vs elevated vancomycin levels causing nephrotoxicity) is still debatable.
The incidence of nephrotoxicity associated with vancomycin therapy is difficult to determine. However, based on current information, the incidence of nephrotoxicity appears to be low when vancomycin is used as monotherapy.
IS S AUREUS BECOMING RESISTANT TO VANCOMYCIN?
An issue of increasing importance in health care settings is the emergence of vancomycin-intermediate S aureus (VISA) and vancomycin-resistant S aureus (VRSA). Eleven cases of VRSA were identified in the United States from 2002 to 2005.13 All cases of VRSA in the United States have involved the incorporation of enterococcal vanA cassette into the S aureus genome.14 While true VRSA isolates remain rare, VISA isolates are becoming more common.
Heteroresistant VISA: An emerging subpopulation of MRSA
Another population of S aureus that has emerged is heteroresistant vancomycin-intermediate S aureus (hVISA). It is defined as the presence of subpopulations of VISA within a population of MRSA at a rate of one organism per 105 to 106 organisms. With traditional testing methods, the vancomycin MIC for the entire population of the strain is within the susceptible range.15 These hVISA populations are thought to be precursors to the development of VISA.16
The resistance to vancomycin in hVISA and VISA populations is due to increased cell wall thickness, altered penicillin-binding protein profiles, and decreased cell wall autolysis.
While the true prevalence of hVISA is difficult to predict because of challenges in microbiological detection and probably varies between geographic regions and individual institutions, different studies have reported hVISA rates between 2% and 13% of all MRSA isolates.15–17
Reduced vancomycin susceptibility can develop regardless of methicillin susceptibility.18
While hVISA is not common, its presence is thought to be a predictor of failing vancomycin therapy.15
Factors associated with hVISA bacteremia include high-bacterial-load infections, treatment failure (including persistent bacteremia for more than 7 days), and initially low serum vancomycin levels.15
‘MIC creep’: Is it real?
Also worrisome, the average vancomycin MIC for S aureus has been shifting upward, based on reports from several institutions, although it is still within the susceptible range.19,20 However, this “MIC creep” likely reflects, at least in part, differences in MIC testing and varying methods used to analyze the data.19,20
Holmes and Jorgensen,21 in a single-institution study of MRSA isolates recovered from bacteremic patients from 1999 to 2006, determined that no MIC creep existed when they tested vancomycin MICs using the broth microdilution method. The authors found the MIC90 (ie, the MIC in at least 90% of the isolates) remained less than 1 mg/L during each year of the study.
Sader et al,22 in a multicenter study, evaluated 1,800 MRSA bloodstream isolates from nine hospitals across the United States from 2002 to 2006. Vancomycin MICs were again measured by broth microdilution methods. The mode MIC remained stable at 0.625 mg/L during the study period, and the authors did not detect a trend of rising MICs.
The inconsistency between reports of MIC creep at single institutions and the absence of this phenomenon in large, multicenter studies seems to imply that vancomycin MIC creep is not occurring on a grand scale.
Vancomycin tolerance
Another troubling matter with S aureus and vancomycin is the issue of tolerance. Vancomycin tolerance, defined in terms of increased minimum bactericidal concentration, represents a loss of bactericidal activity. Tolerance to vancomycin can occur even if the MIC remains in the susceptible range.23
Safdar and Rolston,24 in an observational study from a cancer center, reported that of eight cases of bacteremia that was resistant to vancomycin therapy, three were caused by S aureus.
Sakoulas et al25 found that higher levels of vancomycin bactericidal activity were associated with higher rates of clinical success; however, they found no effect on the mortality rate.
The issue of vancomycin tolerance remains controversial, and because testing for it is impractical in clinical microbiology laboratories, its implications outside the research arena are difficult to ascertain at present.
IS VANCOMYCIN STILL THE BEST DRUG FOR S AUREUS?
MIC break points have been lowered
In 2006, the Clinical Laboratories and Standards Institute lowered its break points for vancomycin MIC categories for S aureus:
- Susceptible: ≤ 2 mg/L (formerly ≤ 4 mg/L)
- Intermediate: 4–8 mg/L (formerly 8–16 mg/L)
- Resistant: ≥ 16 mg/L (formerly ≥ 32 mg/L).
The rationales for these changes were that the lower break points would better detect hVISA, and that cases have been reported of clinical treatment failure of S aureus infections in which the MICs for vancomycin were 4 mg/L.26
Since 2006, the question has been raised whether to lower the break points even further. A reason for this proposal comes from an enhanced understanding of the pharmacokinetics and pharmacodynamics of vancomycin.
The variable most closely associated with clinical response to vancomycin is the AUC-MIC ratio. An AUC-MIC ratio of 400 or higher may be associated with better outcomes in patients with serious S aureus infection. A study of 108 patients with S aureus infection of the lower respiratory tract indicated that organism eradication was more likely if the AUC-MIC ratio was 400 or greater compared with values less than 400, and this was statistically significant.27 However, in cases of S aureus infection with a vancomycin MIC of 2 mg/L or higher, this ratio may not be achievable.
A prospective study of 414 MRSA bacteremia episodes found a vancomycin MIC of 2 mg/L to be a predictor of death.28 The authors concluded that vancomycin may not be the optimal treatment for MRSA with a vancomycin MIC of 2 mg/L.28 Additional studies have also suggested a possible decrease in response to vancomycin in MRSA isolates with elevated MICs within the susceptible range.25,29
Recent guidelines from the IDSA recommend using the clinical response, regardless of the MIC, to guide antimicrobial selection for isolates with MICs in the susceptible range.2
Combination therapy with vancomycin
As vancomycin use has increased, therapeutic failures with vancomycin have become apparent. Combination therapy has been suggested as an option to increase the efficacy of vancomycin when treating complicated infections.
Rifampin plus vancomycin is controversial.30 The combination is theoretically beneficial, especially in infections associated with prosthetic devices. However, clinical studies have failed to convincingly support its use, and some have suggested that it might prolong bacteremia. In addition, it has numerous drug interactions to consider and adverse effects.31
Gentamicin plus vancomycin. The evidence supporting the use of this combination is weak at best. It appears that clinicians may have extrapolated from the success reported by Korzeniowski and Sande,32 who found that methicillin-susceptible S aureus bacteremia was cleared faster if gentamicin was added to nafcillin. A more recent study33 that compared daptomycin (Cubicin) monotherapy with combined vancomycin and gentamicin to treat MRSA bacteremia and endocarditis showed a better overall success rate with daptomycin (44% vs 32.6%), but the difference was not statistically significant.
Gentamicin has some toxicity. Even short-term use (for the first 4 days of therapy) at low doses for bacteremia and endocarditis due to staphylococci has been associated with a higher rate of renal adverse events, including a significant decrease in creatinine clearance.34
Clindamycin or linezolid plus vancomycin is used to decrease toxin production by S aureus.30
While combination therapy with vancomycin is recommended in specific clinical situations, and the combinations are synergistic in vitro, information is lacking about clinical outcomes to support their use.
Don’t use vancomycin when another drug would be better
Vancomycin continues to be the drug of choice in many circumstances, but in some instances its role is under scrutiny and another drug might be better.
Beta-lactams. In patients with infection due to methicillin-susceptible S aureus, failure rates are higher with vancomycin than with beta-lactam therapy, specifically nafcillin.35–37 Beta-lactam antibiotics are thus the drugs of choice for treating infection with beta-lactam-susceptible strains of S aureus.
Linezolid. In theory, linezolid’s ability to decrease production of the S aureus Panton-Valentine leukocidin (PVL) toxin may be an advantage over vancomycin for treating necrotizing pneumonias. For the treatment of MRSA pneumonia, however, controversy exists as to whether linezolid is superior to vancomycin. An analysis of two prospective, randomized, double-blind studies of patients with MRSA pneumonia suggested that initial therapy with linezolid was associated with better survival and clinical cure rates,38 but a subsequent meta-analysis did not substantiate this finding.39 An additional comparative study has been completed, and analysis of the results is in progress.
Daptomycin, approved for skin and soft-tissue infections and bacteremias, including those with right-sided endocarditis, is a lipopeptide antibiotic with a spectrum of action similar to that of vancomycin.40 Daptomycin is also active against many strains of vancomycin-resistant enterococci. As noted above, in the MRSA subgroup of the pivotal comparative study of treatment for S aureus bacteremia and endocarditis, the success rate for daptomycin-treated patients (44.4%) was better than that for patients treated with vancomycin plus gentamicin (32.6%), but the difference was not statistically significant.33,41
The creatine phosphokinase concentration should be monitored weekly in patients on daptomycin.42 Daptomycin is inactivated by lung surfactant and should not be used to treat pneumonia.
Other treatment options approved by the US Food and Drug Administration (FDA) for MRSA infections include tigecycline (Tygacil), quinupristin-dalfopristin (Synercid), telavancin (Vibativ), and ceftaroline (Teflaro).
Tigecycline is a glycylcycline with bacteriostatic activity against S aureus and wide distribution to the tissues.43
Quinupristin-dalfopristin, a streptogramin antibiotic, has activity against S aureus. Its use may be associated with severe myalgias, sometimes leading patients to stop taking it.
Telavancin, recently approved by the FDA, is a lipoglycopeptide antibiotic.44 It is currently approved to treat complicated skin and skin structure infections and was found to be not inferior to vancomycin. An important side effect of this agent is nephrotoxicity. A negative pregnancy test is required before using this agent in women of childbearing potential.
Ceftaroline, a fifth-generation cephalosporin active against MRSA, has been approved by the FDA for the treatment of skin and skin structure infections and community-acquired pneumonia.45
In the past half-century, vancomycin has gone from near-orphan status to being one of the most often used antibiotics in our formulary. The driving force for its use is clear: the evolution of Staphylococcus aureus. At first, vancomycin was used to treat infections caused by penicillin-resistant strains. However, the discovery of methicillin curbed its use for more than 2 decades.1
Then, as methicillin-resistant S aureus (MRSA) began to spread in the 1980s, the use of vancomycin began to increase, and with the rise in community-associated MRSA infections in the 1990s, it became even more widely prescribed. The recent Infectious Diseases Society of America (IDSA) guidelines for treatment of infections due to MRSA are replete with references to the use of vancomycin.2
Another factor driving the use of vancomycin is the increased prevalence of device-associated infections, many of which are caused by coagulase-negative staphylococci and other organisms that colonize the skin.3 Many of these bacteria are susceptible only to vancomycin; they may be associated with infections of vascular catheters, cardiac valves, pacemakers, implantable cardioverter-defibrillators, orthopedic implants, neurosurgical devices, and other devices.
To use vancomycin appropriately, we need to recognize the changing minimum inhibitory concentrations (MICs), to select proper doses and dosing intervals, and to know how to monitor its use. Despite more than 50 years of experience with vancomycin, we sometimes find ourselves with more questions than answers about its optimal use.
WHAT IS VANCOMYCIN?
Vancomycin is a glycopeptide antibiotic isolated from a strain of Streptomyces orientalis discovered in a soil sample from Borneo in the mid-1950s.1 It exerts its action by binding to a d-alanyl-d-alanine cell wall precursor necessary for peptidoglycan cross-linking and, therefore, for inhibiting bacterial cell wall synthesis.
Vancomycin is bactericidal against most gram-positive species, including streptococci and staphylococci, with the exception of Enterococcus species, for which it is bacteriostatic. Though it is bactericidal, it appears to kill bacteria more slowly than beta-lactam antibiotics, and therefore it may take longer to clear bacteremia.4
WHAT IS THE BEST WAY TO DOSE VANCOMYCIN?
Vancomycin is widely distributed to most tissues, with an approximate volume of distribution of 0.4 to 1 L/kg; 50% to 55% is protein-bound. Because of this large volume of distribution, vancomycin’s dosing is based on actual body weight.
Vancomycin is not metabolized and is primarily excreted unchanged in the urine via glomerular filtration. It therefore requires dosage adjustments for renal insufficiency.
Vancomycin’s molecular weight is 1,485.73 Da, making it less susceptible to removal by dialysis than smaller molecules. Dosing of vancomycin in patients on hemodialysis depends on many factors specific to the dialysis center, including but not limited to the type of filter used, the duration of filtration, and whether high-flux filtration is used.
Is continuous intravenous infusion better than standard dosing?
Giving vancomycin by continuous infusion has been suggested as a way to optimize its serum concentration and improve its clinical effectiveness.
Wysocki et al5 conducted a multicenter, prospective, randomized study comparing continuous and intermittent intravenous infusions of vancomycin (the latter every 12 hours) to treat severe hospital-acquired MRSA infections, including bloodstream infections and pneumonia. Although blood concentrations above 10 μg/mL were reached more than 30 hours faster with continuous infusions than with intermittent ones, the microbiologic and clinical outcomes were similar with either method.
James et al6 compared the pharmacodynamics of conventional dosing of vancomycin (ie, 1 g every 12 hours) and continuous infusion in 10 patients with suspected or documented gram-positive infections in a prospective, randomized, crossover study. While no adverse effects were observed, the authors also found no statistically significant difference between the treatment groups in the pharmacodynamic variables investigated, including the area under the curve (AUC) divided by the MIC (the AUC-MIC ratio).
In view of the currently available data, the guidelines for monitoring vancomycin therapy note that there does not appear to be any difference in patient outcomes with continuous infusion vs intermittent dosing.7
Should a loading dose be given?
Another proposed strategy for optimizing vancomycin’s effectiveness is to give a higher initial dose, ie, a loading dose.
Wang et al8 performed a single-center study in 28 patients who received a 25 mg/kg loading dose at a rate of 500 mg/hour. This loading dose was safe, but the authors did not evaluate its efficacy.
Mohammedi et al9 compared loading doses of 500 mg and 15 mg/kg in critically ill patients receiving vancomycin by continuous infusion. The weight-based loading dose produced higher post-dose levels and a significantly higher rate of clinical cure, but there was no significant difference in the rate of survival to discharge from the intensive care unit.
While the use of a loading dose appears to be safe and likely leads to more rapid attainment of therapeutic blood levels, we lack data on whether it improves clinical outcomes, and further study is needed to determine its role.
WHAT IS THE BEST WAY TO MONITOR VANCOMYCIN THERAPY?
Whether and how to use the serum vancomycin concentration to adjust the dosing has been a matter of debate for many years. Convincing evidence that vancomycin levels predict clinical outcomes or that measuring them prevents toxicity is lacking.7
A consensus statement from the American Society of Health-System Pharmacists, the IDSA, and the Society of Infectious Diseases Pharmacists7 contains recommendations for monitoring vancomycin therapy, based on a critical evaluation of the available scientific evidence. Their recommendations:
- Vancomycin serum concentrations should be checked to optimize therapy and used as a surrogate marker of effectiveness.
- Trough, rather than peak, levels should be monitored.
- Trough levels should be checked just before the fourth dose, when steady-state levels are likely to have been achieved. More frequent monitoring may be considered in patients with fluctuating renal function.
- Trough levels should be higher than 10 mg/L to prevent the development of resistance.
- To improve antibiotic penetration and optimize the likelihood of achieving pharmacokinetic and pharmacodynamic targets, trough levels of 15 to 20 mg/L are recommended for pathogens with a vancomycin MIC of 1 mg/L or higher and for complicated infections such as endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia.
- For prolonged courses, it is appropriate to check vancomycin levels weekly in hemodynamically stable patients and more often in those who are not hemodynamically stable.
IS VANCOMYCIN NEPHROTOXIC?
In the 1950s, vancomycin formulations were sometimes called “Mississippi mud” because of the many impurities they contained.1 These impurities were associated with significant nephrotoxicity. Better purification methods used in the manufacture of current formulations mitigate this problem, resulting in a lower incidence of nephrotoxicity.
Over the last several years, organizations such as the American Thoracic Society and the IDSA have recommended targeting higher vancomycin trough concentrations.10 The consequent widespread use of higher doses has renewed interest in vancomycin’s potential nephrotoxicity.
Lodise et al,11 in a cohort study, examined the incidence of nephrotoxicity with higher daily doses of vancomycin (≥ 4 g/day), lower daily doses (< 4 g/day), and linezolid (Zyvox). They defined nephrotoxicity as an increase in serum creatinine of 0.5 mg/dL or a decrease in calculated creatinine clearance of 50% from baseline on 2 consecutive days.
The incidence of nephrotoxicity was significantly higher in the high-dose vancomycin group (34.6%) than in the low-dose vancomycin group (10.9%) and in the linezolid group (6.7%) (P = .001). Additional factors associated with nephrotoxicity in this study included baseline creatinine clearance less than 86.6 mL/minute, weight greater than 101.4 kg (223.5 lb), and being in an intensive care unit.
Hidayat et al12 investigated outcomes in patients with high vs low vancomycin trough levels (≥ 15 mg/L vs < 15 mg/L) in a prospective cohort study. Sixty-three patients achieved an average vancomycin trough of 15 to 20 mg/L, and of these, 11 developed nephrotoxicity, compared with no patients in the low-trough group (P = .01). Of the 11 who developed nephrotoxicity, 10 were concomitantly taking other potentially nephrotoxic agents.
Comment. The data on vancomycin and nephrotoxicity are mostly from studies that had limitations such as small numbers of patients, retrospective design, and variable definitions of nephrotoxicity. Many of the patients in these studies had additional factors contributing to nephrotoxicity, including hemodynamic instability and concomitant exposure to other nephrotoxins. Additionally, the sequence of events (nephrotoxicity leading to elevated vancomycin levels vs elevated vancomycin levels causing nephrotoxicity) is still debatable.
The incidence of nephrotoxicity associated with vancomycin therapy is difficult to determine. However, based on current information, the incidence of nephrotoxicity appears to be low when vancomycin is used as monotherapy.
IS S AUREUS BECOMING RESISTANT TO VANCOMYCIN?
An issue of increasing importance in health care settings is the emergence of vancomycin-intermediate S aureus (VISA) and vancomycin-resistant S aureus (VRSA). Eleven cases of VRSA were identified in the United States from 2002 to 2005.13 All cases of VRSA in the United States have involved the incorporation of enterococcal vanA cassette into the S aureus genome.14 While true VRSA isolates remain rare, VISA isolates are becoming more common.
Heteroresistant VISA: An emerging subpopulation of MRSA
Another population of S aureus that has emerged is heteroresistant vancomycin-intermediate S aureus (hVISA). It is defined as the presence of subpopulations of VISA within a population of MRSA at a rate of one organism per 105 to 106 organisms. With traditional testing methods, the vancomycin MIC for the entire population of the strain is within the susceptible range.15 These hVISA populations are thought to be precursors to the development of VISA.16
The resistance to vancomycin in hVISA and VISA populations is due to increased cell wall thickness, altered penicillin-binding protein profiles, and decreased cell wall autolysis.
While the true prevalence of hVISA is difficult to predict because of challenges in microbiological detection and probably varies between geographic regions and individual institutions, different studies have reported hVISA rates between 2% and 13% of all MRSA isolates.15–17
Reduced vancomycin susceptibility can develop regardless of methicillin susceptibility.18
While hVISA is not common, its presence is thought to be a predictor of failing vancomycin therapy.15
Factors associated with hVISA bacteremia include high-bacterial-load infections, treatment failure (including persistent bacteremia for more than 7 days), and initially low serum vancomycin levels.15
‘MIC creep’: Is it real?
Also worrisome, the average vancomycin MIC for S aureus has been shifting upward, based on reports from several institutions, although it is still within the susceptible range.19,20 However, this “MIC creep” likely reflects, at least in part, differences in MIC testing and varying methods used to analyze the data.19,20
Holmes and Jorgensen,21 in a single-institution study of MRSA isolates recovered from bacteremic patients from 1999 to 2006, determined that no MIC creep existed when they tested vancomycin MICs using the broth microdilution method. The authors found the MIC90 (ie, the MIC in at least 90% of the isolates) remained less than 1 mg/L during each year of the study.
Sader et al,22 in a multicenter study, evaluated 1,800 MRSA bloodstream isolates from nine hospitals across the United States from 2002 to 2006. Vancomycin MICs were again measured by broth microdilution methods. The mode MIC remained stable at 0.625 mg/L during the study period, and the authors did not detect a trend of rising MICs.
The inconsistency between reports of MIC creep at single institutions and the absence of this phenomenon in large, multicenter studies seems to imply that vancomycin MIC creep is not occurring on a grand scale.
Vancomycin tolerance
Another troubling matter with S aureus and vancomycin is the issue of tolerance. Vancomycin tolerance, defined in terms of increased minimum bactericidal concentration, represents a loss of bactericidal activity. Tolerance to vancomycin can occur even if the MIC remains in the susceptible range.23
Safdar and Rolston,24 in an observational study from a cancer center, reported that of eight cases of bacteremia that was resistant to vancomycin therapy, three were caused by S aureus.
Sakoulas et al25 found that higher levels of vancomycin bactericidal activity were associated with higher rates of clinical success; however, they found no effect on the mortality rate.
The issue of vancomycin tolerance remains controversial, and because testing for it is impractical in clinical microbiology laboratories, its implications outside the research arena are difficult to ascertain at present.
IS VANCOMYCIN STILL THE BEST DRUG FOR S AUREUS?
MIC break points have been lowered
In 2006, the Clinical Laboratories and Standards Institute lowered its break points for vancomycin MIC categories for S aureus:
- Susceptible: ≤ 2 mg/L (formerly ≤ 4 mg/L)
- Intermediate: 4–8 mg/L (formerly 8–16 mg/L)
- Resistant: ≥ 16 mg/L (formerly ≥ 32 mg/L).
The rationales for these changes were that the lower break points would better detect hVISA, and that cases have been reported of clinical treatment failure of S aureus infections in which the MICs for vancomycin were 4 mg/L.26
Since 2006, the question has been raised whether to lower the break points even further. A reason for this proposal comes from an enhanced understanding of the pharmacokinetics and pharmacodynamics of vancomycin.
The variable most closely associated with clinical response to vancomycin is the AUC-MIC ratio. An AUC-MIC ratio of 400 or higher may be associated with better outcomes in patients with serious S aureus infection. A study of 108 patients with S aureus infection of the lower respiratory tract indicated that organism eradication was more likely if the AUC-MIC ratio was 400 or greater compared with values less than 400, and this was statistically significant.27 However, in cases of S aureus infection with a vancomycin MIC of 2 mg/L or higher, this ratio may not be achievable.
A prospective study of 414 MRSA bacteremia episodes found a vancomycin MIC of 2 mg/L to be a predictor of death.28 The authors concluded that vancomycin may not be the optimal treatment for MRSA with a vancomycin MIC of 2 mg/L.28 Additional studies have also suggested a possible decrease in response to vancomycin in MRSA isolates with elevated MICs within the susceptible range.25,29
Recent guidelines from the IDSA recommend using the clinical response, regardless of the MIC, to guide antimicrobial selection for isolates with MICs in the susceptible range.2
Combination therapy with vancomycin
As vancomycin use has increased, therapeutic failures with vancomycin have become apparent. Combination therapy has been suggested as an option to increase the efficacy of vancomycin when treating complicated infections.
Rifampin plus vancomycin is controversial.30 The combination is theoretically beneficial, especially in infections associated with prosthetic devices. However, clinical studies have failed to convincingly support its use, and some have suggested that it might prolong bacteremia. In addition, it has numerous drug interactions to consider and adverse effects.31
Gentamicin plus vancomycin. The evidence supporting the use of this combination is weak at best. It appears that clinicians may have extrapolated from the success reported by Korzeniowski and Sande,32 who found that methicillin-susceptible S aureus bacteremia was cleared faster if gentamicin was added to nafcillin. A more recent study33 that compared daptomycin (Cubicin) monotherapy with combined vancomycin and gentamicin to treat MRSA bacteremia and endocarditis showed a better overall success rate with daptomycin (44% vs 32.6%), but the difference was not statistically significant.
Gentamicin has some toxicity. Even short-term use (for the first 4 days of therapy) at low doses for bacteremia and endocarditis due to staphylococci has been associated with a higher rate of renal adverse events, including a significant decrease in creatinine clearance.34
Clindamycin or linezolid plus vancomycin is used to decrease toxin production by S aureus.30
While combination therapy with vancomycin is recommended in specific clinical situations, and the combinations are synergistic in vitro, information is lacking about clinical outcomes to support their use.
Don’t use vancomycin when another drug would be better
Vancomycin continues to be the drug of choice in many circumstances, but in some instances its role is under scrutiny and another drug might be better.
Beta-lactams. In patients with infection due to methicillin-susceptible S aureus, failure rates are higher with vancomycin than with beta-lactam therapy, specifically nafcillin.35–37 Beta-lactam antibiotics are thus the drugs of choice for treating infection with beta-lactam-susceptible strains of S aureus.
Linezolid. In theory, linezolid’s ability to decrease production of the S aureus Panton-Valentine leukocidin (PVL) toxin may be an advantage over vancomycin for treating necrotizing pneumonias. For the treatment of MRSA pneumonia, however, controversy exists as to whether linezolid is superior to vancomycin. An analysis of two prospective, randomized, double-blind studies of patients with MRSA pneumonia suggested that initial therapy with linezolid was associated with better survival and clinical cure rates,38 but a subsequent meta-analysis did not substantiate this finding.39 An additional comparative study has been completed, and analysis of the results is in progress.
Daptomycin, approved for skin and soft-tissue infections and bacteremias, including those with right-sided endocarditis, is a lipopeptide antibiotic with a spectrum of action similar to that of vancomycin.40 Daptomycin is also active against many strains of vancomycin-resistant enterococci. As noted above, in the MRSA subgroup of the pivotal comparative study of treatment for S aureus bacteremia and endocarditis, the success rate for daptomycin-treated patients (44.4%) was better than that for patients treated with vancomycin plus gentamicin (32.6%), but the difference was not statistically significant.33,41
The creatine phosphokinase concentration should be monitored weekly in patients on daptomycin.42 Daptomycin is inactivated by lung surfactant and should not be used to treat pneumonia.
Other treatment options approved by the US Food and Drug Administration (FDA) for MRSA infections include tigecycline (Tygacil), quinupristin-dalfopristin (Synercid), telavancin (Vibativ), and ceftaroline (Teflaro).
Tigecycline is a glycylcycline with bacteriostatic activity against S aureus and wide distribution to the tissues.43
Quinupristin-dalfopristin, a streptogramin antibiotic, has activity against S aureus. Its use may be associated with severe myalgias, sometimes leading patients to stop taking it.
Telavancin, recently approved by the FDA, is a lipoglycopeptide antibiotic.44 It is currently approved to treat complicated skin and skin structure infections and was found to be not inferior to vancomycin. An important side effect of this agent is nephrotoxicity. A negative pregnancy test is required before using this agent in women of childbearing potential.
Ceftaroline, a fifth-generation cephalosporin active against MRSA, has been approved by the FDA for the treatment of skin and skin structure infections and community-acquired pneumonia.45
- Murray BE, Nannini EC. Glycopeptides (vancomycin and teicoplanin), streptogramins (quinupristin-dalfopristin), and lipopeptides (daptomycin). In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010:449–468.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:285–292.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001; 45:2460–2467.
- James JK, Palmer SM, Levine DP, Rybak MJ. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996; 40:696–700.
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98.
- Wang JT, Fang CT, Chen YC, Chang SC. Necessity of a loading dose when using vancomycin in critically ill patients (letter). J Antimicrob Chemother 2001; 47:246.
- Mohammedi I, Descloux E, Argaud L, Le Scanff J, Robert D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrob Agents 2006; 27:259–262.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52:1330–1336.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Centers for Disease Control and Prevention. CDC reminds clinical laboratories and healthcare infection preventionists of their role in the search and containment of vancomycin-resistant Staphylococcus aureus (VRSA), May 2010. http://emergency.cdc.gov/coca/reminders/2010/2010may06.asp. Accessed June 7, 2011.
- Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 2008; 46:668–674.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sader HS, Jones RN, Rossi KL, Rybak MJ. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine USA hospitals. J Antimicrob Chemother 2009; 64:1024–1028.
- Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 2009; 49:1169–1174.
- Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 2006; 44:3883–3886.
- Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in nonvancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother 2007; 60:788–794.
- Holmes RL, Jorgensen JH. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother 2008; 52:757–760.
- Sader HS, Fey PD, Limaye AP, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother 2009; 53:4127–4132.
- May J, Shannon K, King A, French G. Glycopeptide tolerance in Staphylococcus aureus. J Antimicrob Chemother 1998; 42:189–197.
- Safdar A, Rolston KV. Vancomycin tolerance, a potential mechanism for refractory gram-positive bacteremia observational study in patients with cancer. Cancer 2006; 106:1815–1820.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007; 44:1208–1215.
- Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43:925–942.
- Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:193–200.
- Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008; 52:3315–3320.
- Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2009; 49:1072–1079.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Rehm SJ, Boucher H, Levine D, et al. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother 2008; 62:1413–1421.
- Cosgrove SE, Vigliani GA, Fowler VG, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48:713–721.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003; 124:1789–1797.
- Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 2010; 38:1802–1808.
- Kosmidis C, Levine DP. Daptomycin: pharmacology and clinical use. Expert Opin Pharmacother 2010; 11:615–625.
- Fowler VG, Boucher HW, Corey GR, et al; S aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Daptomycin package insert. Lexington, MA. Cubist Pharmaceuticals, Inc. November 2010. www.cubicin.com/pdf/PrescribingInformation.pdf. Accessed June 7, 2011.
- Peterson LR. A review of tigecycline—the first glycylcycline. Int J Antimicrob Agents 2008; 32(suppl 4):S215–S222.
- Saravolatz LD, Stein GE, Johnson LB. Telavancin: a novel lipoglycopeptide. Clin Infect Dis 2009; 49:1908–1914.
- Ceftaroline package insert. St. Louis, MO. Forest Pharmaceuticals. October 2010.
- Murray BE, Nannini EC. Glycopeptides (vancomycin and teicoplanin), streptogramins (quinupristin-dalfopristin), and lipopeptides (daptomycin). In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010:449–468.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:285–292.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001; 45:2460–2467.
- James JK, Palmer SM, Levine DP, Rybak MJ. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996; 40:696–700.
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98.
- Wang JT, Fang CT, Chen YC, Chang SC. Necessity of a loading dose when using vancomycin in critically ill patients (letter). J Antimicrob Chemother 2001; 47:246.
- Mohammedi I, Descloux E, Argaud L, Le Scanff J, Robert D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrob Agents 2006; 27:259–262.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52:1330–1336.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Centers for Disease Control and Prevention. CDC reminds clinical laboratories and healthcare infection preventionists of their role in the search and containment of vancomycin-resistant Staphylococcus aureus (VRSA), May 2010. http://emergency.cdc.gov/coca/reminders/2010/2010may06.asp. Accessed June 7, 2011.
- Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 2008; 46:668–674.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sader HS, Jones RN, Rossi KL, Rybak MJ. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine USA hospitals. J Antimicrob Chemother 2009; 64:1024–1028.
- Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 2009; 49:1169–1174.
- Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 2006; 44:3883–3886.
- Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in nonvancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother 2007; 60:788–794.
- Holmes RL, Jorgensen JH. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother 2008; 52:757–760.
- Sader HS, Fey PD, Limaye AP, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother 2009; 53:4127–4132.
- May J, Shannon K, King A, French G. Glycopeptide tolerance in Staphylococcus aureus. J Antimicrob Chemother 1998; 42:189–197.
- Safdar A, Rolston KV. Vancomycin tolerance, a potential mechanism for refractory gram-positive bacteremia observational study in patients with cancer. Cancer 2006; 106:1815–1820.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007; 44:1208–1215.
- Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43:925–942.
- Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:193–200.
- Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008; 52:3315–3320.
- Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2009; 49:1072–1079.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Rehm SJ, Boucher H, Levine D, et al. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother 2008; 62:1413–1421.
- Cosgrove SE, Vigliani GA, Fowler VG, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48:713–721.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003; 124:1789–1797.
- Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 2010; 38:1802–1808.
- Kosmidis C, Levine DP. Daptomycin: pharmacology and clinical use. Expert Opin Pharmacother 2010; 11:615–625.
- Fowler VG, Boucher HW, Corey GR, et al; S aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Daptomycin package insert. Lexington, MA. Cubist Pharmaceuticals, Inc. November 2010. www.cubicin.com/pdf/PrescribingInformation.pdf. Accessed June 7, 2011.
- Peterson LR. A review of tigecycline—the first glycylcycline. Int J Antimicrob Agents 2008; 32(suppl 4):S215–S222.
- Saravolatz LD, Stein GE, Johnson LB. Telavancin: a novel lipoglycopeptide. Clin Infect Dis 2009; 49:1908–1914.
- Ceftaroline package insert. St. Louis, MO. Forest Pharmaceuticals. October 2010.
KEY POINTS
- Giving vancomycin by continuous infusion appears to offer no advantage over giving it every 12 hours.
- Therapeutic blood levels can be reached more quickly if a loading dose is given, but whether this offers a clinical advantage is unclear.
- The trough vancomycin serum concentration should be greater than 10 mg/L to prevent the development of resistance, and trough levels of 15 to 20 mg/L are recommended if the minimum inhibitory concentration (MIC) is 1 mg/L or higher.
- Whether S aureus is becoming resistant to vancomycin is not clear.
- The variable most closely associated with clinical response to vancomycin is the area under the curve (AUC) divided by the MIC (the AUC-MIC ratio), which should be greater than 400.
Hypothermia after cardiac arrest: Beneficial, but slow to be adopted
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
KEY POINTS
- This treatment is indicated for comatose adult patients who have had a witnessed cardiac arrest, whose initial cardiac rhythm is ventricular fibrillation or pulseless ventricular tachycardia, and who have return of spontaneous circulation with basic and advanced cardiac life support.
- Contraindications include hemorrhagic stroke, a Glasgow Coma Scale score of 8 or higher, cardiac arrest due to drug overdose, and preexisting hypothermia. Relative contraindications include baseline coagulopathy and severe hypotension (mean arterial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
- Adverse effects have included hypokalemia, bradyarrhythmia, ventricular tachycardia, hypotension, seizures, hyperglycemia, a transient decrease in the glomerular filtration rate, abnormal coagulation studies, and an increased incidence of pneumonia and sepsis.