User login
Vaccine update 2010: Keeping up with the changes
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.
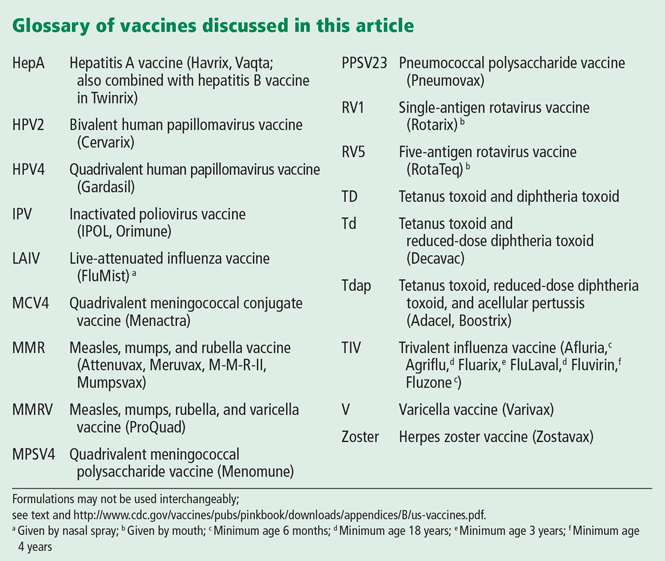
VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
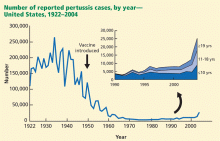
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.

VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.

VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
KEY POINTS
- New recommendations for infants and children:
- Rotavirus vaccination for infants
- Seasonal influenza vaccine yearly at ages 5–18
- Hepatitis A vaccine at age 12–23 months
- Varicella vaccine at 12–15 months and again at 4–6 years, with catch-up for others.
- New recommendations for adolescents:
- Meningococcus quadrivalent conjugate vaccine for all at age 11 or 12 and catch-up through age 18
- A shot of tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis vaccine (Tdap) at age 11 or 12 and catch-up through age 18
- Human papillomavirus vaccine (three doses) for girls at age 11 or 12 and catch-up through age 26.
- New recommendations for adults:
- One dose of Tdap instead of the next tetanus-diphtheria booster
- Herpes zoster vaccine at age 60 or older
- Pneumococcal vaccination extended to smokers and people with asthma, with a second dose 5 years after the first for people who have immune suppression, sickle cell disease, or asplenia.
Bony bridge of a bifid rib
A 21-year-old man has had cough and hemoptysis for 3 days. For the past 3 years he has smoked one pack of cigarettes a day. His medical history is unremarkable, and he has had no chest trauma or thoracic surgery.
The patient says he was born full-term, and he has never been aware of any congenital anomalies.
Q: Which is the most likely diagnosis?
- Fractured rib
- Poland syndrome
- Paget disease
- Bifid rib
A: Bifid rib, a congenital anomaly, is the correct answer (see below).
Fractured rib. The patient has no history of chest trauma or thoracic surgery, nor any evidence on chest x-ray to suggest a fractured rib. Also, he has no evidence of osteoporosis to suspect a spontaneous rib fracture. His hemoptysis is most likely due to acute bronchitis.
Poland syndrome is a unilateral deficiency of the pectoralis muscle, variably associated with ipsilateral thoracic and upper limb anomalies. Bilateral hypoplasia or aplasia of the pectoralis muscle and upper-limb defects in association with variable thoracic muscles, chest wall deformities, and lower-limb defects has been infrequently reported in the literature. The diagnosis is usually based on the physical examination (asymmetric chest) or on chest x-ray (unilateral hyperlucent lung).1 This is not the case in our patient.
Paget disease is a chronic, abnormal bone-remodeling process that leads to enlarged, less-dense, brittle bones. The spine, femur, pelvis, skull, clavicle, and humerus are most commonly affected. In the United States, the prevalence is 3% to 4% in people over age 40. Black Americans have a higher prevalence rate than black Africans, and the disease is rare in Asians.
Pain is the most common symptom, but Paget disease is usually asymptomatic. Paget disease can lead to insufficiency fractures, pathologic fractures, secondary arthritis, and nerve impingement in the spine or the base of the skull. Sarcomatous degeneration of the affected bone has been reported, but is rare.
Radiographic findings are often diagnostic. The skull and long bones typically show evidence of osteolysis from the epiphysis and advancing along the diaphysis. Radiographic findings in the sclerotic phase typically involve the axial skeleton and include trabecular coarsening and distortion and cortical thickening.
Rib abnormalities may be observed; these may either be isolated or may be a sign of multi-system malformations. However, in our patient, the radiographic finding of a bony bridge does not fit the description of Paget disease.2
BIFID RIB
The overall prevalence of bifid rib is estimated at 0.15% to 3.4% (mean 2%), and it accounts for up to 20% of all congenital rib anomalies.3 It is usually unilateral. Wattanasirichaigoon et al4 described patterns of rib defects in 47 cases, with bifid rib accounting for 28% of cases.
As with Paget disease, rib anomalies may occur in isolation or in association with multi-system malformations. Since the ribs originate from the mesoderm, it is not surprising that the costal defects are associated with malformations in other organs of the same origin, such as the heart and the kidneys.3 Bifid ribs are also seen in several genetic disorders such as Gorlin-Goltz (ie, basal cell nevus) syndrome, which affects multiple organs including bones, skin, eye, and neural system.5 Occasionally, it is encountered as a part of Jobs syndrome (ie, high levels of immunoglobulin E and recurrent infections),6 and Kindler syndrome, a rare genodermatosis.7
The literature contains little information about the clinical significance of bifid rib. Patients should undergo a thorough physical examination, including oral and cutaneous evaluation, to rule out a genetic syndrome. Physical findings such as palmar pits, subcutaneous calcifications, or odontogenic cyst warrant a more intensive radiologic and genetic investigation.5 If the physical examination is normal and if the patient is asymptomatic, additional clinical or radiologic investigation is of low yield. And as in our patient, the anomaly may go unnoticed on computed tomography of the chest.
- Allam SR, Yadav R, Meziane M, Mehta AC. A middle-aged man with asymptomatic chest wall asymmetry. Cleve Clin J Med 2006; 73:754–756.
- Hung HC, Ou HY, Huang JS, Chuang MC, Wu TJ. Tumor-associated hypercalcemia in a patient with Paget’s disease. Kaohsiung J Med Sci 2008; 24:152–156.
- Charles I, Scott J. Pectoral girdle, spine, ribs, and pelvic girdle. In:Stevenson RE, Hall JG, Goodmann RM, eds. Human Malformations and Related Anomalies, vol 2. Oxford University Press: New York, 1993:655–697.
- Wattanasirichaigoon D, Prasad C, Schneider G, Evans JA, Korf BR. Rib defects in patterns of multiple malformations: a retrospective review and phenotypic analysis of 47 cases. Am J Med Genet A 2003; 122A:63–69.
- Rai S, Gauba K. Jaw cyst-basal cell nevus-bifid rib syndrome: a case report. J Indian Soc Pedod Prev Dent 2007; 25:137–139.
- Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am 2008; 28:277–291.
- Sharma RC, Mahajan V, Sharma NL, Sharma AK. Kindler syndrome. Int J Dermatol 2003; 42:727–732.
A 21-year-old man has had cough and hemoptysis for 3 days. For the past 3 years he has smoked one pack of cigarettes a day. His medical history is unremarkable, and he has had no chest trauma or thoracic surgery.
The patient says he was born full-term, and he has never been aware of any congenital anomalies.
Q: Which is the most likely diagnosis?
- Fractured rib
- Poland syndrome
- Paget disease
- Bifid rib
A: Bifid rib, a congenital anomaly, is the correct answer (see below).
Fractured rib. The patient has no history of chest trauma or thoracic surgery, nor any evidence on chest x-ray to suggest a fractured rib. Also, he has no evidence of osteoporosis to suspect a spontaneous rib fracture. His hemoptysis is most likely due to acute bronchitis.
Poland syndrome is a unilateral deficiency of the pectoralis muscle, variably associated with ipsilateral thoracic and upper limb anomalies. Bilateral hypoplasia or aplasia of the pectoralis muscle and upper-limb defects in association with variable thoracic muscles, chest wall deformities, and lower-limb defects has been infrequently reported in the literature. The diagnosis is usually based on the physical examination (asymmetric chest) or on chest x-ray (unilateral hyperlucent lung).1 This is not the case in our patient.
Paget disease is a chronic, abnormal bone-remodeling process that leads to enlarged, less-dense, brittle bones. The spine, femur, pelvis, skull, clavicle, and humerus are most commonly affected. In the United States, the prevalence is 3% to 4% in people over age 40. Black Americans have a higher prevalence rate than black Africans, and the disease is rare in Asians.
Pain is the most common symptom, but Paget disease is usually asymptomatic. Paget disease can lead to insufficiency fractures, pathologic fractures, secondary arthritis, and nerve impingement in the spine or the base of the skull. Sarcomatous degeneration of the affected bone has been reported, but is rare.
Radiographic findings are often diagnostic. The skull and long bones typically show evidence of osteolysis from the epiphysis and advancing along the diaphysis. Radiographic findings in the sclerotic phase typically involve the axial skeleton and include trabecular coarsening and distortion and cortical thickening.
Rib abnormalities may be observed; these may either be isolated or may be a sign of multi-system malformations. However, in our patient, the radiographic finding of a bony bridge does not fit the description of Paget disease.2
BIFID RIB
The overall prevalence of bifid rib is estimated at 0.15% to 3.4% (mean 2%), and it accounts for up to 20% of all congenital rib anomalies.3 It is usually unilateral. Wattanasirichaigoon et al4 described patterns of rib defects in 47 cases, with bifid rib accounting for 28% of cases.
As with Paget disease, rib anomalies may occur in isolation or in association with multi-system malformations. Since the ribs originate from the mesoderm, it is not surprising that the costal defects are associated with malformations in other organs of the same origin, such as the heart and the kidneys.3 Bifid ribs are also seen in several genetic disorders such as Gorlin-Goltz (ie, basal cell nevus) syndrome, which affects multiple organs including bones, skin, eye, and neural system.5 Occasionally, it is encountered as a part of Jobs syndrome (ie, high levels of immunoglobulin E and recurrent infections),6 and Kindler syndrome, a rare genodermatosis.7
The literature contains little information about the clinical significance of bifid rib. Patients should undergo a thorough physical examination, including oral and cutaneous evaluation, to rule out a genetic syndrome. Physical findings such as palmar pits, subcutaneous calcifications, or odontogenic cyst warrant a more intensive radiologic and genetic investigation.5 If the physical examination is normal and if the patient is asymptomatic, additional clinical or radiologic investigation is of low yield. And as in our patient, the anomaly may go unnoticed on computed tomography of the chest.
A 21-year-old man has had cough and hemoptysis for 3 days. For the past 3 years he has smoked one pack of cigarettes a day. His medical history is unremarkable, and he has had no chest trauma or thoracic surgery.
The patient says he was born full-term, and he has never been aware of any congenital anomalies.
Q: Which is the most likely diagnosis?
- Fractured rib
- Poland syndrome
- Paget disease
- Bifid rib
A: Bifid rib, a congenital anomaly, is the correct answer (see below).
Fractured rib. The patient has no history of chest trauma or thoracic surgery, nor any evidence on chest x-ray to suggest a fractured rib. Also, he has no evidence of osteoporosis to suspect a spontaneous rib fracture. His hemoptysis is most likely due to acute bronchitis.
Poland syndrome is a unilateral deficiency of the pectoralis muscle, variably associated with ipsilateral thoracic and upper limb anomalies. Bilateral hypoplasia or aplasia of the pectoralis muscle and upper-limb defects in association with variable thoracic muscles, chest wall deformities, and lower-limb defects has been infrequently reported in the literature. The diagnosis is usually based on the physical examination (asymmetric chest) or on chest x-ray (unilateral hyperlucent lung).1 This is not the case in our patient.
Paget disease is a chronic, abnormal bone-remodeling process that leads to enlarged, less-dense, brittle bones. The spine, femur, pelvis, skull, clavicle, and humerus are most commonly affected. In the United States, the prevalence is 3% to 4% in people over age 40. Black Americans have a higher prevalence rate than black Africans, and the disease is rare in Asians.
Pain is the most common symptom, but Paget disease is usually asymptomatic. Paget disease can lead to insufficiency fractures, pathologic fractures, secondary arthritis, and nerve impingement in the spine or the base of the skull. Sarcomatous degeneration of the affected bone has been reported, but is rare.
Radiographic findings are often diagnostic. The skull and long bones typically show evidence of osteolysis from the epiphysis and advancing along the diaphysis. Radiographic findings in the sclerotic phase typically involve the axial skeleton and include trabecular coarsening and distortion and cortical thickening.
Rib abnormalities may be observed; these may either be isolated or may be a sign of multi-system malformations. However, in our patient, the radiographic finding of a bony bridge does not fit the description of Paget disease.2
BIFID RIB
The overall prevalence of bifid rib is estimated at 0.15% to 3.4% (mean 2%), and it accounts for up to 20% of all congenital rib anomalies.3 It is usually unilateral. Wattanasirichaigoon et al4 described patterns of rib defects in 47 cases, with bifid rib accounting for 28% of cases.
As with Paget disease, rib anomalies may occur in isolation or in association with multi-system malformations. Since the ribs originate from the mesoderm, it is not surprising that the costal defects are associated with malformations in other organs of the same origin, such as the heart and the kidneys.3 Bifid ribs are also seen in several genetic disorders such as Gorlin-Goltz (ie, basal cell nevus) syndrome, which affects multiple organs including bones, skin, eye, and neural system.5 Occasionally, it is encountered as a part of Jobs syndrome (ie, high levels of immunoglobulin E and recurrent infections),6 and Kindler syndrome, a rare genodermatosis.7
The literature contains little information about the clinical significance of bifid rib. Patients should undergo a thorough physical examination, including oral and cutaneous evaluation, to rule out a genetic syndrome. Physical findings such as palmar pits, subcutaneous calcifications, or odontogenic cyst warrant a more intensive radiologic and genetic investigation.5 If the physical examination is normal and if the patient is asymptomatic, additional clinical or radiologic investigation is of low yield. And as in our patient, the anomaly may go unnoticed on computed tomography of the chest.
- Allam SR, Yadav R, Meziane M, Mehta AC. A middle-aged man with asymptomatic chest wall asymmetry. Cleve Clin J Med 2006; 73:754–756.
- Hung HC, Ou HY, Huang JS, Chuang MC, Wu TJ. Tumor-associated hypercalcemia in a patient with Paget’s disease. Kaohsiung J Med Sci 2008; 24:152–156.
- Charles I, Scott J. Pectoral girdle, spine, ribs, and pelvic girdle. In:Stevenson RE, Hall JG, Goodmann RM, eds. Human Malformations and Related Anomalies, vol 2. Oxford University Press: New York, 1993:655–697.
- Wattanasirichaigoon D, Prasad C, Schneider G, Evans JA, Korf BR. Rib defects in patterns of multiple malformations: a retrospective review and phenotypic analysis of 47 cases. Am J Med Genet A 2003; 122A:63–69.
- Rai S, Gauba K. Jaw cyst-basal cell nevus-bifid rib syndrome: a case report. J Indian Soc Pedod Prev Dent 2007; 25:137–139.
- Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am 2008; 28:277–291.
- Sharma RC, Mahajan V, Sharma NL, Sharma AK. Kindler syndrome. Int J Dermatol 2003; 42:727–732.
- Allam SR, Yadav R, Meziane M, Mehta AC. A middle-aged man with asymptomatic chest wall asymmetry. Cleve Clin J Med 2006; 73:754–756.
- Hung HC, Ou HY, Huang JS, Chuang MC, Wu TJ. Tumor-associated hypercalcemia in a patient with Paget’s disease. Kaohsiung J Med Sci 2008; 24:152–156.
- Charles I, Scott J. Pectoral girdle, spine, ribs, and pelvic girdle. In:Stevenson RE, Hall JG, Goodmann RM, eds. Human Malformations and Related Anomalies, vol 2. Oxford University Press: New York, 1993:655–697.
- Wattanasirichaigoon D, Prasad C, Schneider G, Evans JA, Korf BR. Rib defects in patterns of multiple malformations: a retrospective review and phenotypic analysis of 47 cases. Am J Med Genet A 2003; 122A:63–69.
- Rai S, Gauba K. Jaw cyst-basal cell nevus-bifid rib syndrome: a case report. J Indian Soc Pedod Prev Dent 2007; 25:137–139.
- Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am 2008; 28:277–291.
- Sharma RC, Mahajan V, Sharma NL, Sharma AK. Kindler syndrome. Int J Dermatol 2003; 42:727–732.
Giant nodules on the hands
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
Q: Which is the most likely diagnosis?
- Rheumatoid arthritis
- Nodular osteoarthritis
- Tophaceous gout
- Pseudogout
- Xanthoma tuberosum
A: Tophaceous gout is the diagnosis. This patient’s serum urate level was 9 mg/dL (normal range 4.0–8.0) despite allopurinol therapy, with normal levels of lipids, urea, and creatinine. Polarized light microscopy of aspirated synovial fluid showed monosodium urate crystals, thus confirming the diagnosis.
Rheumatoid arthritis is typically polyarticular and symmetrical and spares the distal interphalangeal joints. Subcutaneous rheumatoid nodules may mimic gouty tophi.
Pseudogout shares some of the features of gout. It results from deposits of calcium pyrophosphate crystals in and around the joints. The diagnosis is made by identifying the crystals on microscopy when calcinosis is seen on x-ray. Tophaceous nodules almost never occur.
Xanthoma tuberosum is associated with hypercholesterolemia, particularly with elevated levels of low-density lipoprotein cholesterol. Lesions occur on pressure areas such as the knees or elbows and vary in size and shape from small papules to firm, lobulated tumors. They are yellow or orange, often with an erythematous halo. They are not associated with chronic proliferative arthritis.
CLINICAL PRESENTATION OF GOUT
Gout is a common metabolic disease characterized by an intermittent course of acute inflammatory arthritis initially affecting one or a few joints. Almost all patients have hyperuricemia, but serum urate levels can be normal or low during an acute attack. On the other hand, many hyperuricemic patients never have a clinical event.
If the hyperuricemia is untreated, some patients develop chronic polyarthritis and nephrolithiasis.1 Inadequate treatment of hyperuricemia may result in chronic tophaceous gout. Although tophaceous gout usually is a sign of long-standing hyperuricemia, tophi can in rare cases be a first symptom of the disorder.2
Even though our patient had been on allopurinol therapy, the dose was not high enough to achieve a serum urate level significantly below the saturation point of urate (about 6.7 mg/dL).
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.
- Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis 1997; 56:696–697.
- Thissen CA, Frank J, Lucker GP. Tophi as first clinical sign of gout. Int J Dermatol 2008; 47( suppl 1):49–51.
The pretravel visit: A ‘teaching moment’
Closer to home, general internists and other primary care providers can use the awareness of global health concerns to the health advantage of our patients, including the young and healthy, who generally eschew preventive health visits to their physicians, and busy traveling executives, who only see a doctor for (hopefully) quick resolution to intermittent problems.
In this issue of the Journal, Powell and Ford offer a general primer on travel medicine, highlighting specific concerns that should be addressed to facilitate our patients’ safe and uninterrupted travels. But often, a pretravel visit is also a good time to introduce concepts of preventive health to patients who might not otherwise be accessible or amenable.
Just as the preoperative medical consultation can provide a “teaching moment” to address smoking cessation or reversible cardiac risks to a captive audience, the visit regarding “What shots do I need to go to Thailand?” can open the door for talk about general vaccinations (see the article by Campos-Outcalt of this issue), venereal disease, air-travel-associated thrombosis, excessive alcohol use, and perhaps other wellness issues. Creating a travel advisory service within most practices will not supplant the benefits of having travelers review the CDC travel Web site or the need to refer some patients to travel medicine experts regarding specific diseases and vaccinations. But it may create the opportunity for interaction, dialogue, and even a blood pressure check with patients who might not otherwise have the time or see the need to schedule a visit with a physician in the absence of an acute medical concern.
Closer to home, general internists and other primary care providers can use the awareness of global health concerns to the health advantage of our patients, including the young and healthy, who generally eschew preventive health visits to their physicians, and busy traveling executives, who only see a doctor for (hopefully) quick resolution to intermittent problems.
In this issue of the Journal, Powell and Ford offer a general primer on travel medicine, highlighting specific concerns that should be addressed to facilitate our patients’ safe and uninterrupted travels. But often, a pretravel visit is also a good time to introduce concepts of preventive health to patients who might not otherwise be accessible or amenable.
Just as the preoperative medical consultation can provide a “teaching moment” to address smoking cessation or reversible cardiac risks to a captive audience, the visit regarding “What shots do I need to go to Thailand?” can open the door for talk about general vaccinations (see the article by Campos-Outcalt of this issue), venereal disease, air-travel-associated thrombosis, excessive alcohol use, and perhaps other wellness issues. Creating a travel advisory service within most practices will not supplant the benefits of having travelers review the CDC travel Web site or the need to refer some patients to travel medicine experts regarding specific diseases and vaccinations. But it may create the opportunity for interaction, dialogue, and even a blood pressure check with patients who might not otherwise have the time or see the need to schedule a visit with a physician in the absence of an acute medical concern.
Closer to home, general internists and other primary care providers can use the awareness of global health concerns to the health advantage of our patients, including the young and healthy, who generally eschew preventive health visits to their physicians, and busy traveling executives, who only see a doctor for (hopefully) quick resolution to intermittent problems.
In this issue of the Journal, Powell and Ford offer a general primer on travel medicine, highlighting specific concerns that should be addressed to facilitate our patients’ safe and uninterrupted travels. But often, a pretravel visit is also a good time to introduce concepts of preventive health to patients who might not otherwise be accessible or amenable.
Just as the preoperative medical consultation can provide a “teaching moment” to address smoking cessation or reversible cardiac risks to a captive audience, the visit regarding “What shots do I need to go to Thailand?” can open the door for talk about general vaccinations (see the article by Campos-Outcalt of this issue), venereal disease, air-travel-associated thrombosis, excessive alcohol use, and perhaps other wellness issues. Creating a travel advisory service within most practices will not supplant the benefits of having travelers review the CDC travel Web site or the need to refer some patients to travel medicine experts regarding specific diseases and vaccinations. But it may create the opportunity for interaction, dialogue, and even a blood pressure check with patients who might not otherwise have the time or see the need to schedule a visit with a physician in the absence of an acute medical concern.
Risks of travel, benefits of a specialist consult
Before going abroad to areas that might pose a risk to their health, most people ought to visit their primary care physicians and many should be referred to a specialist in travel medicine.
In this article, we review the key elements of the pretravel consult as it relates to the prevention and self-treatment of the most common diseases that pose health risks for travelers. We also give guidelines for when to refer patients to a specialist.
WHY PRIMARY CARE PHYSICIANS NEED TO KNOW TRAVEL MEDICINE
International travel to exotic locations is becoming more popular. In 2008, one out of five Americans traveled abroad, and 38 million visits were to developing countries where there are significant health risks for travelers.1
One third to one half of travelers to developing countries experience some kind of illness while abroad, most commonly diarrhea or upper respiratory infections, which typically lead to 3 lost days during a 2-week trip.
These illnesses are often preventable and self-treatable.2 Unfortunately, studies suggest that most travelers do not seek adequate medical advice, and that when they do they often fail to complete courses of medication.3,4
All these factors point to the need for primary care providers to become proficient in the pretravel consult and, if necessary, to refer patients to travel specialists and clinics.
WHY REFER TO A TRAVEL CLINIC?
In one study of travelers to areas of high risk for malaria or hepatitis A, 42% of those who consulted only their family doctor became ill, in contrast to 22% of those who attended a travel medicine clinic.4
As a rule of thumb, anyone traveling to an area where malaria is endemic should be referred to a specialist, as should anyone at risk of yellow fever or typhoid fever. As many as 8 per 1,000 travelers may return from areas of risk infected with malaria.5
Long-term travelers and people who will spend time in urban slums or rural or remote regions have an even greater need for referral to a travel clinic, as they are at higher risk of exposure to Japanese encephalitis, cholera, epidemic meningitis, dengue fever, and rabies.6
THE PRETRAVEL CONSULT: ESSENTIALS
A pretravel consult ought to be scheduled 4 to 6 weeks in advance of the trip, since many vaccines require that much time to induce immunity, and some require a series of shots.
Unfortunately, many patients who think of arranging a travel consult make the appointment at the last minute, and some come with an incomplete knowledge of their travel plans. However, even without enough advance notice, a consult can be beneficial.
Travelers sometimes change their itineraries in-country or engage in unanticipated risky behaviors. A good travel medicine physician tries to anticipate even these unplanned risks and changes in itinerary.
Where is the traveler going? When? For how long?
The pretravel consult starts with a detailed discussion of the patient’s itinerary. It needs to include length, dates, and location of travel, as well as anticipated activities and accommodations.
A remarkable number of travelers come to consults not knowing the names of specific countries they will visit, perhaps saying only that they are going to Africa or South America. An accurate itinerary is indispensible, as appropriate medical advice is highly specific to country and region. The incidence and geographic distribution of many travelers’ diseases change over time, and this requires physicians to consult the most current information available.
Tropical countries, in general, are risky, but each pathogen has a unique distribution that may vary between urban and rural areas or by season. Detailed, up-to-date information is available from the US Centers for Disease Control and Prevention (CDC) (www.cdc.gov) for individual countries and for specific provinces and locations within those countries. Physicians should consult the CDC whenever advising a patient preparing to travel. 7
How is the traveler’s current health?
Several immunizations cannot be given to the very young, the elderly, or those who are immunocompromised.
The greatest risk of death to travelers is not from tropical diseases but from cardiovascular disease, which according to one study is responsible for half of deaths abroad.8 Patients with heart disease or other known health concerns need to be counseled to avoid activities that will put them at further risk. The advice applies especially in situations such as remote travel or even cruises, where prompt emergency medical care may be difficult or impossible to obtain.
People infected with human immunodeficiency virus (HIV) face discriminatory travel prohibitions in 74 countries.9
Foreign-born travelers who are visiting family and friends in developing countries may have lost their immunity to local pathogens and thus can be more at risk because they are not prepared to take necessary health precautions.
Also, a significant number of travelers become infected but develop illnesses only after they return, so a posttravel visit may be necessary.6
Prescription and even over-the-the-counter drugs may be difficult or impossible to obtain in foreign countries, and ample supplies should be brought along.
Is the traveler up to date on routine immunizations?
A number of infectious diseases that have been controlled or eradicated in North America through regular childhood immunizations are still endemic in many remote areas and developing countries. All travelers should be up to date on routine immunizations, including those for measles-mumps-rubella, tetanus, polio, meningitis, and hepatitis A and B.
Polio. A one-time polio booster is recommended for adults traveling to certain countries or areas of the world.
Meningitis vaccine is now routinely given to young people, but adult patients may need it before they travel.
Hepatitis A is contracted through fecal contamination of food and water. Common sources are foods prepared in an unhygienic manner, raw fruits and vegetables, shellfish, and contaminated water.
Hepatitis B vaccine is also now routinely given to young people, but it should be offered to travelers planning to stay more than 1 month and to long-term expatriates. This vaccine is also recommended for travelers who may be exposed to blood or body fluids, who are contemplating sexual activity or tattooing in the host country, or who may require medical or dental care while traveling, as well as for adventure travelers or travelers to remote regions.
The vaccination is given in a three-dose schedule at 0, 1, and 6 months. For protection against both hepatitis A and B, the vaccine Twinrix can be used on the same schedule as for hepatitis B. An accelerated schedule of 0, 7, and 21 days with a booster at 12 months allows completion of the entire series in 4 weeks, thus putting completion of vaccination before travel in the same time frame as other vaccines in a series, such as those for rabies and Japanese encephalitis.
PREVENTIVE COUNSELING
In addition, travelers going abroad should be advised on measures to avoid diarrhea, insectvector diseases, accidents, excessive exposure to the sun, altitude sickness, and other risks their itineraries may expose them to.
Avoiding traveler’s diarrhea
Traveler’s diarrhea is by far the most common health problem experienced abroad. It is prevalent in Mexico, where 20 million visits by Americans occur each year. A quarter to half of visitors to developing countries contract traveler’s diarrhea and, on average, lose 2 to 3 days of their business trip or vacation.3,10 The disease therefore imposes not only discomfort but also financial losses on travelers, especially business travelers.
Though many pathogens may be responsible, the most common one is Escherichia coli, usually transmitted by human fecal contamination of food or drink. Preventive measures against E coli are the same as for other foodborne and waterborne infections, such as hepatitis A, cholera, and typhoid fever.
The rule for avoiding traveler’s diarrhea may be summarized by the CDC-coined phrase, “boil it, cook it, peel it, or forget it.” Simple, written advice is most likely to be followed. 6 Thorough boiling or cooking kills bacteria in contaminated food, and food should be served steaming hot. Travelers should only eat foods they know have been well cooked, declining cold dishes like salsa or casseroles. They should avoid tap water for brushing teeth or in the form of ice cubes and should stick to drinking bottled beverages, preferably carbonated ones. No matter how appetizing a salad looks, travelers should avoid eating fresh fruits and vegetables unless they are sure that they were peeled under sanitary conditions. Simply eating at a high-priced restaurant is not a guarantee of uncontaminated food. Before meals or any hand-to-mouth contact, hands should be washed in soap and water or with sanitizers.
Travelers to remote areas may wish to acquire filtering devices, chlorine, or iodine for treating water. A combination of filtering and iodine treatment is most effective.
While this advice is undoubtedly wise, the evidence shows that, in practice, most travelers fail to take all precautions, and the benefits of this counseling have been difficult to demonstrate.11 Therefore, physicians should prescribe drugs for prophylaxis and self-treatment of traveler’s diarrhea during travel.
Bismuth subsalicylate (Pepto-Bismol) taken as two tablets or 2 oz of liquid 4 times a day while traveling may reduce the risk of diarrhea by one half, though it should be avoided by patients with contraindications to aspirin.3,6
Self-treating traveler’s diarrhea
Proper hydration is crucial, since dehydration can worsen and prolong symptoms.
Ciprofloxacin (Cipro) 500 mg orally two times daily for 3 to 5 days is effective.
Azithromycin (Zithromax) 500 mg daily for 3 to 5 days may be better in some areas of Southeast Asia, where fluoroquinolone-resistant bacteria are prevalent.
Rifaximin (Xifaxan), a nonsystemic antibiotic, is another option. The dosage is 200 mg three times a day for 3 days.
Avoiding insect bites
Malaria, yellow fever, tickborne encephalitis, and dengue fever are all transmitted by insect bites. Often the best protection is to avoid being bitten.
Bites can be avoided by using insect repellants containing diethyltoluamide (DEET) or picardin. If the traveler is going to be out in the sun, he or she should apply sunscreen first, then DEET on top of that. Anopheles, which transmits malaria, is a night-biting mosquito and may be avoided by staying in screened areas at dusk and dawn and by using bed netting. Permethrin, an insecticide, can be applied to clothing and mosquito netting.
Other things to avoid
Accidents are the second most common cause of death in travelers (after cardiovascular disease), accounting for as many as one-third of deaths.9 Several studies indicate road accidents are the major cause of accidental death, but also significant are drowning and air crashes. Travelers should be advised that transportation in developing countries is often more dangerous than at home. Seaside vacationers should be aware of the dangers of riptides and other threats to swimmers and should obey warnings posted at beaches.
Sexually transmitted diseases. When appropriate, physicians should warn travelers about the dangers of contracting HIV and other sexually transmitted diseases, especially in sub-Saharan Africa.
Sunburn, dehydration. Travelers should regularly use sunscreen and should remain hydrated.
Crimes against and involving tourists are a serious threat in many places, including some popular destinations. All of the 100,000 young people traveling to Mexico each year for spring break should read the US Department of State warnings against crime and possible arrest in that country.12 Travelers who are victims of crimes in foreign countries should contact their national consulate as soon as possible. The US Department of State issues advisories on countries where there is danger to travelers because of political turmoil, crime, or other causes.13
Motion sickness and jet lag can be ameliorated by proper hydration, avoiding caffeine, and using a scopolamine patch or dimenhydrinate (Dramamine).1
When traveling to wilderness areas
Wilderness and expedition medicine is a complex subset of travel medicine.14 All travelers need to understand the risks of whatever activities they undertake.
Mountain climbers and skiers have to contend with altitude sickness and frostbite. Scuba divers have the risks of decompression sickness, barotrauma, and hazardous marine life. Travelers on expeditions may have to deal with predatory animals, exotic parasites, and ethnic or political violence. People who participate in these activities should do so only when they are properly certified and educated in the associated health risks.
Ordinary tourists should enjoy safe adventures with well-established tour agencies and venues and should be cautioned against activities that expose them to dangers they may not be prepared to confront.
Insurance, evacuation, and emergency care
Health insurance often does not pay for preventive travel medicine. Unfortunately, cost can be a factor in immunizations and other health care. The cost of most travelers’ medications and vaccinations is generally comparable to that of other immunizations. The exceptions are two specialized vaccines—ie, for Japanese encephalitis ($1,000 or more for a full course) and for rabies, which can cost considerably more. Pricing by different providers can vary widely.
Travelers, especially those who are pregnant, elderly, disabled, or immunocompromised or who have preexisting diseases, need to review their insurance policies to make certain that care in foreign countries is covered. If not, evacuation insurance can be purchased at a relatively modest cost.
TRAVEL TO AREAS OF MALARIA
While many travelers can confidently consult their primary care provider, those traveling to places where malaria is prevalent should be referred to a physician with a thorough and current knowledge of the incidence of drugresistant strains of the disease and other complex issues in travel medicine. Short-term and long-term travelers are often approached differently, but a travel medicine consult should be obtained for any patient traveling to a region with malaria risk.
Malaria kills up to 3 million each year
Malaria, caused by the Plasmodium parasite, transmitted by the night-biting Anopheles mosquito, is responsible worldwide for between 1 and 3 million deaths annually, mostly of children in sub-Saharan Africa.15 Every year about 1,500 Americans are diagnosed with malaria and, on average, 10 die.6
Nearly all cases of malaria and deaths from it are preventable. Prophylaxis is imperative for travelers to affected areas, as is preventive counseling. Based on the patient’s itinerary, the physician needs to thoroughly research potential exposure to drug-resistant strains before choosing which antimalarial regimen to prescribe.
Malaria causes symptoms of anemia, fever, or nausea and, without treatment, can lead to coma and death. Because two of the five strains, P vivax and P ovale, can remain dormant in an infected person’s liver for up to 1 year and, in rare cases, up to 4 years after travel, it is imperative that a returned traveler who experiences flu-like symptoms seek medical attention and inform the treating physician of the need to screen for malarial infection. The primary means of diagnosis is through microscopic examination of the blood.
No malaria vaccine, but prophylactic drugs are available
Unlike many of the illnesses discussed below for which vaccines are available, malaria prophylaxis requires the active participation of the patient in completing a course of medication, so noncompliance becomes a risk.
A number of prophylactic drugs are available. The choice depends on the locally resistant strains.16
Chloroquine (Aralen), the traditional malarial prophylactic drug, is still effective against many strains, primarily in Central America and some areas of the Middle East. The dosage is 500 mg once a week, started 1 week before travel and continued for 4 weeks after return to the United States.
Mefloquine (Lariam) is dosed at 250 mg weekly. The patient should be carefully screened for depression, anxiety, and other mood disorders. Even the report of bad dreams or nightmares should make a patient be considered a poor candidate for this medication. The patient should start taking this drug 3 weeks before travel to provide time to assess for adverse effects and, if necessary, to change the antimalarial regimen. Mefloquine is taken weekly while traveling and is continued for 4 weeks after return.
Doxycycline (Vibramycin) is an antibiotic. As an antimalarial prophylactic, it is taken as 100 mg daily beginning 2 days prior to travel and continuing while travelling and for 4 weeks after return.
Atovaquone-proguanil (Malarone) prevents infection at the blood stage and in the liver. It is well tolerated and is begun 2 days before travel. It is taken daily while traveling and daily for 1 week after return.
Yellow fever
Immunization is required for entry to more than 20 African nations and is recommended for those traveling to most of South America. The only physicians who can give this vaccine are those who have approval from their state health department and have been issued an official stamp, used on the World Health Organization (WHO) yellow fever vaccination card. Several countries require the card for entry from places where yellow fever is present. For any multicountry travel involving at least one area where yellow fever is endemic, the entire itinerary needs to be reviewed to make sure all legal entry requirements are met. The WHO maintains a current list of these requirements.17 If there is any doubt, it is generally best to refer and certify the traveler.
Referral should be timely. The vaccine must be given 10 days prior to entry into a country where yellow fever is endemic; it is valid for 10 years.
The yellow fever vaccine is a live-attenuated vaccine and should not be given to infants younger than 9 months old, adults over age 60 who are not properly screened and informed, or pregnant women. Immunocompromised patients are excluded from receiving this vaccine, as are patients taking immunosuppressant drugs and patients with thymus disorders such as myasthenia gravis. Patients who have had chemotherapy must wait 3 months before being vaccinated. Those on steroids (eg, prednisone 20 mg or more daily) must wait until 2 weeks after cessation of steroids to receive this vaccine. Patients who cannot be vaccinated should be advised not to travel to areas with a high risk of yellow fever.
Women contemplating pregnancy should use contraception for 28 days after yellow fever vaccination. Children younger than 9 months and the elderly are at higher risk of adverse reactions from the vaccine, either neurotropic or viscerotropic disease that mimics yellow fever infection. It is possible for physicians to write a medical waiver of contraindication to vaccination for patients who should not be immunized.
Typhoid fever
Typhoid fever can occur anywhere in the world, but it is endemic in the tropics. Worldwide, an estimated 200,000 deaths occur each year from typhoid fever, and 400 cases are reported annually in the United States, most commonly acquired by travelers to the Indian subcontinent.18 One study indicates that 95% of infected travelers had not been vaccinated, and a significant number returned with drugresistant strains.19
Typhoid fever is caused by ingestion of Salmonella typhi bacteria. It causes a febrile illness with infection of the digestive tract and reticuloendothelial system.
Prevention is the same as for traveler’s diarrhea: drink no local water and eat nothing raw. Vaccination can be provided in an intramuscular shot or a series of oral capsules. The shot is well tolerated and is valid for 3 years. The capsule provides 5 years of immunity. Vaccination is recommended for people going to areas with a high prevalence of typhoid fever, such as India, and for people planning to spend more than 2 weeks in an area where typhoid is endemic, as well as for adventurous eaters.
SOME TRAVELERS NEED MORE PROTECTION
Some travelers need more preventive measures than typical tourists or other short-term visitors. Long-term visitors or travelers to remote or other high-risk areas (eg, adventure travelers, relief workers, mission workers) may need, in addition to the measures described above, measures against Japanese encephalitis, rabies, cholera, epidemic meningitis, and dengue fever.
Japanese encephalitis
Japanese encephalitis virus is transmitted by mosquito bite. The major regions where it is endemic are rural India and Southeast Asia, most typically in areas with rice paddies and pig farms. Travelers at risk are expatriates to these areas, those planning a long stay, and remote-adventure travelers.
The vaccine JE-VAX is given as a series of three shots, on days 0, 7, and 28. Another vaccine, Ixiaro, is given in a series of two shots, on days 0 and 28.
Patients who are allergic to bee or wasp stings should not be vaccinated. The patient should remain in the office for 30 minutes after each dose to permit observation for mild anaphylactic reactions such as angioedema and urticaria, and should complete the series 10 days before travel to allow for observation for delayed reactions. Patients must weigh the risk of contracting the disease against the high cost of the vaccine.
Rabies
Rabies is a potential risk anywhere in the world except in Western Europe and Australia. Because the vaccine is costly, it is generally not given for prophylaxis except for travelers certain to have contact with animals, especially the major vectors, ie, dogs, cats, bats, and monkeys.20 Counseling about vigilance in avoiding animal contact and not promoting interaction through feeding wild animals should be part of any pretravel consult. Rabies, once acquired, is fatal.
The patient should be instructed on proper care of a bite from a potential rabies source and told to halt travel and seek medical attention. The wound should be cleaned with soap and water for 15 minutes to remove any saliva and virus from the soft tissue; this has proven to be effective in animal experiments. A virucidal such as benzalkonium chloride (Zephiran) or aqueous iodine should then be put in the wound.
Preexposure vaccination is done in a three-dose series (given on days 0, 7, and 21– 28). The patient should complete the series and adhere to the dosing schedule as closely as possible. It may be necessary to find a source of vaccine for the patient once he or she has arrived in the destination country.
If bitten, travelers without preexposure vaccination must find a source of vaccine and human rabies immune globulin (HRIG) before continuing on their trip. Postexposure treatment is 20 IU/kg of HRIG infiltrated around the wound to wall off the virus inoculation site. If the wound is in a digit or small area and not all of the HRIG can be given, then the remaining HRIG is given intramuscularly at a site distant from the vaccine site. If the patient has multiple bites, the HRIG should be diluted so it can be infiltrated around all wounds. The HRIG should be given immediately or within 7 days of beginning the vaccine series once a source is located. Later treatment than this can interfere with the patient’s ability to mount an immune reaction.
Rabies vaccine is initiated at the same time as HRIG and is given on days 0, 3, 7, 14, and 28. The CDC may soon change the schedule to allow for only four postexposure shots, but this has not yet been done as of this writing.
The patient vaccinated before exposure requires only booster doses of rabies vaccine at days 0 and 3.
Cholera
Cholera is an epidemic gastrointestinal disease historically responsible for millions of deaths. It is endemic in most tropical countries, especially in Africa and southern and southeastern Asia.21
High-risk patients, most often those working with refugees and disaster victims in endemic areas, should receive the traveler’s diarrhea and cholera vaccine Dukoral, which immunizes against Vibrio cholera and enterotoxogenic E coli. The vaccine, which is not available in the United States but is available abroad, is given as two oral doses 1 week apart for adults and three oral doses for children ages 2 to 6, and the second dose must be given 7 days before travel; this provides protection for 6 months.
At various times, the above vaccines have been in short supply. Travel medicine consults should be obtained for proper identification of the at-risk traveler for efficient use of any possibly limited vaccine.
Epidemic meningitis
The vaccine against epidemic meningitis is now routinely given in the United States to adolescents at the age of 12 or upon entry to college or the military. The fatality rate from the disease is 10%.
Meningococcal disease transmission peaks in the sub-Saharan “meningitis belt” in the dry season of December through June. Travelers to these areas at these times should be immunized. Travelers planning close contact with the local population (eg, health care workers) should be immunized. Patients traveling to Saudi Arabia for Hajj in Mecca must be immunized for meningitis for entry to the country during this time. The vaccine must be given within 3 years of entering the country and not less than 10 days before.
Dengue fever
Dengue fever is a flavivirus transmitted through the Aedes aegypti mosquito. No vaccine is available for dengue fever, so for now the only advice is to avoid insect vectors.
There are four closely related but serologically different dengue viruses that provide only weak cross-protection. In fact, previous infection with one serotype in a traveler then infected with another poses a risk of dengue hemorrhagic fever.
Because of inattention to public sanitation, this virus and its mosquito vector have reemerged in areas where they were once eliminated. The viral infection is a risk for the traveler to both urban and rural areas in the Americas, Southeast Asia, and Africa. The Pan American Health Organization has seen the number of reported dengue cases increase from 66,000 in 1980 to 700,000 in 2003.22
- US Department of Commerce International Trade Administration. Outbound overview 2008. www.tinet.ita.doc.gov/outreachpages. Accessed October 27, 2009.
- Dick L. Travel medicine: helping patients prepare for trips abroad. Am Fam Physician 1998; 58:383–398, 401–402.
- Reed JM, McIntosh IB, Powers K. Travel illness and the family practitioner: a retrospective assessment of travel-induced illness in general practice and the effect of a travel illness clinic. J Travel Med 1994 1:192–198.
- Hamer DH, Connor BA. Travel health knowledge, attitudes, and practices among United States travelers. J Travel Med 2004; 11:23–26.
- Hill DR. Health problems in a large cohort of Americans traveling to developing countries. J Travel Med 2000; 7:259–266.
- Hill DR, Ericsson CD, et al., Infectious Diseases Society of America. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1499–1539.
- US Centers for Disease Control and Prevention. Destinations. wwwn.cdc.gov/travel/destinations/list.aspx. Accessed February 11, 2010.
- Steffen R. Epidemiology: Morbidity and mortality in travelers. In:Keystone J, ed. Travel Medicine. Mosby: New York, 2004:5–12.
- Joint United Nations Programme on HIV/AIDS. HIV-related travel restrictions. www.unaids.org/en/KnowledgeCentre/Resources/FeatureStories/archive/2008/20080304_HIVrelated_travel_restrictions.asp. Accessed February 11, 2010.
- Brewster SJ, Taylor DN. Epidemiology of travelers’ diarrhea. In:Keystone J, ed. Travel Medicine. New York: Mosby, 2004:175–184.
- Ostrosky-Zeichner L, Ericsson CD. Prevention of travelers’ diarrhea. In:Keystone J, ed. Travel Medicine. New York: Mosby, 2004:185–189.
- US Department of State. Spring break in Mexico—“Know Before You Go!” http://travel.state.gov/travel/cis_pa_tw/spring_break_mexico/spring_break_mexico_2812.html. Accessed February 11, 2010.
- US Department of State. Current travel warnings. http://travel.state.gov/travel/cis_pa_tw/tw/tw_1764.html. Accessed February 11, 2010.
- Bledsoe GH, Manyak MJ, Townes DA, eds. Expedition and Wilderness Medicine. Cambridge University Press: New York, 2008.
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 2001; 64( suppl 1–2):97–106.
- US Centers for Disease Control and Prevention. The Pre-Travel Consultation: Malaria. wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/malaria.aspx. Accessed February 11, 2010.
- World Health Organization. Country list: yellow fever vaccination requirements and recommendations; and malaria situation. http://www.who.int/ith/ITH2009Countrylist.pdf. Accessed February 11, 2010.
- US Centers for Disease Control and Prevention. Typhoid Fever. www.cdc.gov/ncidod/dbmd/diseaseinfo/typhoidfever_t.htm. Accessed February 11, 2010.
- Lynch MF, Blanton EM, Bulens S, et al Typhoid fever in the United States, 1999–2006. JAMA 2009; 302:859–865.
- Plotkin SA. Rabies. Clin Infect Dis 2000; 30:4–12.
- Topps M. Oral cholera vaccine—for whom, when, and why? Travel Med Infect Dis 2006; 4:38–42.
- Petersen LR, Marfin AA. Shifting epidemiology of Flaviviridae. J Travel Med 2005; 12(suppl 1):S3–S11.
Before going abroad to areas that might pose a risk to their health, most people ought to visit their primary care physicians and many should be referred to a specialist in travel medicine.
In this article, we review the key elements of the pretravel consult as it relates to the prevention and self-treatment of the most common diseases that pose health risks for travelers. We also give guidelines for when to refer patients to a specialist.
WHY PRIMARY CARE PHYSICIANS NEED TO KNOW TRAVEL MEDICINE
International travel to exotic locations is becoming more popular. In 2008, one out of five Americans traveled abroad, and 38 million visits were to developing countries where there are significant health risks for travelers.1
One third to one half of travelers to developing countries experience some kind of illness while abroad, most commonly diarrhea or upper respiratory infections, which typically lead to 3 lost days during a 2-week trip.
These illnesses are often preventable and self-treatable.2 Unfortunately, studies suggest that most travelers do not seek adequate medical advice, and that when they do they often fail to complete courses of medication.3,4
All these factors point to the need for primary care providers to become proficient in the pretravel consult and, if necessary, to refer patients to travel specialists and clinics.
WHY REFER TO A TRAVEL CLINIC?
In one study of travelers to areas of high risk for malaria or hepatitis A, 42% of those who consulted only their family doctor became ill, in contrast to 22% of those who attended a travel medicine clinic.4
As a rule of thumb, anyone traveling to an area where malaria is endemic should be referred to a specialist, as should anyone at risk of yellow fever or typhoid fever. As many as 8 per 1,000 travelers may return from areas of risk infected with malaria.5
Long-term travelers and people who will spend time in urban slums or rural or remote regions have an even greater need for referral to a travel clinic, as they are at higher risk of exposure to Japanese encephalitis, cholera, epidemic meningitis, dengue fever, and rabies.6
THE PRETRAVEL CONSULT: ESSENTIALS
A pretravel consult ought to be scheduled 4 to 6 weeks in advance of the trip, since many vaccines require that much time to induce immunity, and some require a series of shots.
Unfortunately, many patients who think of arranging a travel consult make the appointment at the last minute, and some come with an incomplete knowledge of their travel plans. However, even without enough advance notice, a consult can be beneficial.
Travelers sometimes change their itineraries in-country or engage in unanticipated risky behaviors. A good travel medicine physician tries to anticipate even these unplanned risks and changes in itinerary.
Where is the traveler going? When? For how long?
The pretravel consult starts with a detailed discussion of the patient’s itinerary. It needs to include length, dates, and location of travel, as well as anticipated activities and accommodations.
A remarkable number of travelers come to consults not knowing the names of specific countries they will visit, perhaps saying only that they are going to Africa or South America. An accurate itinerary is indispensible, as appropriate medical advice is highly specific to country and region. The incidence and geographic distribution of many travelers’ diseases change over time, and this requires physicians to consult the most current information available.
Tropical countries, in general, are risky, but each pathogen has a unique distribution that may vary between urban and rural areas or by season. Detailed, up-to-date information is available from the US Centers for Disease Control and Prevention (CDC) (www.cdc.gov) for individual countries and for specific provinces and locations within those countries. Physicians should consult the CDC whenever advising a patient preparing to travel. 7
How is the traveler’s current health?
Several immunizations cannot be given to the very young, the elderly, or those who are immunocompromised.
The greatest risk of death to travelers is not from tropical diseases but from cardiovascular disease, which according to one study is responsible for half of deaths abroad.8 Patients with heart disease or other known health concerns need to be counseled to avoid activities that will put them at further risk. The advice applies especially in situations such as remote travel or even cruises, where prompt emergency medical care may be difficult or impossible to obtain.
People infected with human immunodeficiency virus (HIV) face discriminatory travel prohibitions in 74 countries.9
Foreign-born travelers who are visiting family and friends in developing countries may have lost their immunity to local pathogens and thus can be more at risk because they are not prepared to take necessary health precautions.
Also, a significant number of travelers become infected but develop illnesses only after they return, so a posttravel visit may be necessary.6
Prescription and even over-the-the-counter drugs may be difficult or impossible to obtain in foreign countries, and ample supplies should be brought along.
Is the traveler up to date on routine immunizations?
A number of infectious diseases that have been controlled or eradicated in North America through regular childhood immunizations are still endemic in many remote areas and developing countries. All travelers should be up to date on routine immunizations, including those for measles-mumps-rubella, tetanus, polio, meningitis, and hepatitis A and B.
Polio. A one-time polio booster is recommended for adults traveling to certain countries or areas of the world.
Meningitis vaccine is now routinely given to young people, but adult patients may need it before they travel.
Hepatitis A is contracted through fecal contamination of food and water. Common sources are foods prepared in an unhygienic manner, raw fruits and vegetables, shellfish, and contaminated water.
Hepatitis B vaccine is also now routinely given to young people, but it should be offered to travelers planning to stay more than 1 month and to long-term expatriates. This vaccine is also recommended for travelers who may be exposed to blood or body fluids, who are contemplating sexual activity or tattooing in the host country, or who may require medical or dental care while traveling, as well as for adventure travelers or travelers to remote regions.
The vaccination is given in a three-dose schedule at 0, 1, and 6 months. For protection against both hepatitis A and B, the vaccine Twinrix can be used on the same schedule as for hepatitis B. An accelerated schedule of 0, 7, and 21 days with a booster at 12 months allows completion of the entire series in 4 weeks, thus putting completion of vaccination before travel in the same time frame as other vaccines in a series, such as those for rabies and Japanese encephalitis.
PREVENTIVE COUNSELING
In addition, travelers going abroad should be advised on measures to avoid diarrhea, insectvector diseases, accidents, excessive exposure to the sun, altitude sickness, and other risks their itineraries may expose them to.
Avoiding traveler’s diarrhea
Traveler’s diarrhea is by far the most common health problem experienced abroad. It is prevalent in Mexico, where 20 million visits by Americans occur each year. A quarter to half of visitors to developing countries contract traveler’s diarrhea and, on average, lose 2 to 3 days of their business trip or vacation.3,10 The disease therefore imposes not only discomfort but also financial losses on travelers, especially business travelers.
Though many pathogens may be responsible, the most common one is Escherichia coli, usually transmitted by human fecal contamination of food or drink. Preventive measures against E coli are the same as for other foodborne and waterborne infections, such as hepatitis A, cholera, and typhoid fever.
The rule for avoiding traveler’s diarrhea may be summarized by the CDC-coined phrase, “boil it, cook it, peel it, or forget it.” Simple, written advice is most likely to be followed. 6 Thorough boiling or cooking kills bacteria in contaminated food, and food should be served steaming hot. Travelers should only eat foods they know have been well cooked, declining cold dishes like salsa or casseroles. They should avoid tap water for brushing teeth or in the form of ice cubes and should stick to drinking bottled beverages, preferably carbonated ones. No matter how appetizing a salad looks, travelers should avoid eating fresh fruits and vegetables unless they are sure that they were peeled under sanitary conditions. Simply eating at a high-priced restaurant is not a guarantee of uncontaminated food. Before meals or any hand-to-mouth contact, hands should be washed in soap and water or with sanitizers.
Travelers to remote areas may wish to acquire filtering devices, chlorine, or iodine for treating water. A combination of filtering and iodine treatment is most effective.
While this advice is undoubtedly wise, the evidence shows that, in practice, most travelers fail to take all precautions, and the benefits of this counseling have been difficult to demonstrate.11 Therefore, physicians should prescribe drugs for prophylaxis and self-treatment of traveler’s diarrhea during travel.
Bismuth subsalicylate (Pepto-Bismol) taken as two tablets or 2 oz of liquid 4 times a day while traveling may reduce the risk of diarrhea by one half, though it should be avoided by patients with contraindications to aspirin.3,6
Self-treating traveler’s diarrhea
Proper hydration is crucial, since dehydration can worsen and prolong symptoms.
Ciprofloxacin (Cipro) 500 mg orally two times daily for 3 to 5 days is effective.
Azithromycin (Zithromax) 500 mg daily for 3 to 5 days may be better in some areas of Southeast Asia, where fluoroquinolone-resistant bacteria are prevalent.
Rifaximin (Xifaxan), a nonsystemic antibiotic, is another option. The dosage is 200 mg three times a day for 3 days.
Avoiding insect bites
Malaria, yellow fever, tickborne encephalitis, and dengue fever are all transmitted by insect bites. Often the best protection is to avoid being bitten.
Bites can be avoided by using insect repellants containing diethyltoluamide (DEET) or picardin. If the traveler is going to be out in the sun, he or she should apply sunscreen first, then DEET on top of that. Anopheles, which transmits malaria, is a night-biting mosquito and may be avoided by staying in screened areas at dusk and dawn and by using bed netting. Permethrin, an insecticide, can be applied to clothing and mosquito netting.
Other things to avoid
Accidents are the second most common cause of death in travelers (after cardiovascular disease), accounting for as many as one-third of deaths.9 Several studies indicate road accidents are the major cause of accidental death, but also significant are drowning and air crashes. Travelers should be advised that transportation in developing countries is often more dangerous than at home. Seaside vacationers should be aware of the dangers of riptides and other threats to swimmers and should obey warnings posted at beaches.
Sexually transmitted diseases. When appropriate, physicians should warn travelers about the dangers of contracting HIV and other sexually transmitted diseases, especially in sub-Saharan Africa.
Sunburn, dehydration. Travelers should regularly use sunscreen and should remain hydrated.
Crimes against and involving tourists are a serious threat in many places, including some popular destinations. All of the 100,000 young people traveling to Mexico each year for spring break should read the US Department of State warnings against crime and possible arrest in that country.12 Travelers who are victims of crimes in foreign countries should contact their national consulate as soon as possible. The US Department of State issues advisories on countries where there is danger to travelers because of political turmoil, crime, or other causes.13
Motion sickness and jet lag can be ameliorated by proper hydration, avoiding caffeine, and using a scopolamine patch or dimenhydrinate (Dramamine).1
When traveling to wilderness areas
Wilderness and expedition medicine is a complex subset of travel medicine.14 All travelers need to understand the risks of whatever activities they undertake.
Mountain climbers and skiers have to contend with altitude sickness and frostbite. Scuba divers have the risks of decompression sickness, barotrauma, and hazardous marine life. Travelers on expeditions may have to deal with predatory animals, exotic parasites, and ethnic or political violence. People who participate in these activities should do so only when they are properly certified and educated in the associated health risks.
Ordinary tourists should enjoy safe adventures with well-established tour agencies and venues and should be cautioned against activities that expose them to dangers they may not be prepared to confront.
Insurance, evacuation, and emergency care
Health insurance often does not pay for preventive travel medicine. Unfortunately, cost can be a factor in immunizations and other health care. The cost of most travelers’ medications and vaccinations is generally comparable to that of other immunizations. The exceptions are two specialized vaccines—ie, for Japanese encephalitis ($1,000 or more for a full course) and for rabies, which can cost considerably more. Pricing by different providers can vary widely.
Travelers, especially those who are pregnant, elderly, disabled, or immunocompromised or who have preexisting diseases, need to review their insurance policies to make certain that care in foreign countries is covered. If not, evacuation insurance can be purchased at a relatively modest cost.
TRAVEL TO AREAS OF MALARIA
While many travelers can confidently consult their primary care provider, those traveling to places where malaria is prevalent should be referred to a physician with a thorough and current knowledge of the incidence of drugresistant strains of the disease and other complex issues in travel medicine. Short-term and long-term travelers are often approached differently, but a travel medicine consult should be obtained for any patient traveling to a region with malaria risk.
Malaria kills up to 3 million each year
Malaria, caused by the Plasmodium parasite, transmitted by the night-biting Anopheles mosquito, is responsible worldwide for between 1 and 3 million deaths annually, mostly of children in sub-Saharan Africa.15 Every year about 1,500 Americans are diagnosed with malaria and, on average, 10 die.6
Nearly all cases of malaria and deaths from it are preventable. Prophylaxis is imperative for travelers to affected areas, as is preventive counseling. Based on the patient’s itinerary, the physician needs to thoroughly research potential exposure to drug-resistant strains before choosing which antimalarial regimen to prescribe.
Malaria causes symptoms of anemia, fever, or nausea and, without treatment, can lead to coma and death. Because two of the five strains, P vivax and P ovale, can remain dormant in an infected person’s liver for up to 1 year and, in rare cases, up to 4 years after travel, it is imperative that a returned traveler who experiences flu-like symptoms seek medical attention and inform the treating physician of the need to screen for malarial infection. The primary means of diagnosis is through microscopic examination of the blood.
No malaria vaccine, but prophylactic drugs are available
Unlike many of the illnesses discussed below for which vaccines are available, malaria prophylaxis requires the active participation of the patient in completing a course of medication, so noncompliance becomes a risk.
A number of prophylactic drugs are available. The choice depends on the locally resistant strains.16
Chloroquine (Aralen), the traditional malarial prophylactic drug, is still effective against many strains, primarily in Central America and some areas of the Middle East. The dosage is 500 mg once a week, started 1 week before travel and continued for 4 weeks after return to the United States.
Mefloquine (Lariam) is dosed at 250 mg weekly. The patient should be carefully screened for depression, anxiety, and other mood disorders. Even the report of bad dreams or nightmares should make a patient be considered a poor candidate for this medication. The patient should start taking this drug 3 weeks before travel to provide time to assess for adverse effects and, if necessary, to change the antimalarial regimen. Mefloquine is taken weekly while traveling and is continued for 4 weeks after return.
Doxycycline (Vibramycin) is an antibiotic. As an antimalarial prophylactic, it is taken as 100 mg daily beginning 2 days prior to travel and continuing while travelling and for 4 weeks after return.
Atovaquone-proguanil (Malarone) prevents infection at the blood stage and in the liver. It is well tolerated and is begun 2 days before travel. It is taken daily while traveling and daily for 1 week after return.
Yellow fever
Immunization is required for entry to more than 20 African nations and is recommended for those traveling to most of South America. The only physicians who can give this vaccine are those who have approval from their state health department and have been issued an official stamp, used on the World Health Organization (WHO) yellow fever vaccination card. Several countries require the card for entry from places where yellow fever is present. For any multicountry travel involving at least one area where yellow fever is endemic, the entire itinerary needs to be reviewed to make sure all legal entry requirements are met. The WHO maintains a current list of these requirements.17 If there is any doubt, it is generally best to refer and certify the traveler.
Referral should be timely. The vaccine must be given 10 days prior to entry into a country where yellow fever is endemic; it is valid for 10 years.
The yellow fever vaccine is a live-attenuated vaccine and should not be given to infants younger than 9 months old, adults over age 60 who are not properly screened and informed, or pregnant women. Immunocompromised patients are excluded from receiving this vaccine, as are patients taking immunosuppressant drugs and patients with thymus disorders such as myasthenia gravis. Patients who have had chemotherapy must wait 3 months before being vaccinated. Those on steroids (eg, prednisone 20 mg or more daily) must wait until 2 weeks after cessation of steroids to receive this vaccine. Patients who cannot be vaccinated should be advised not to travel to areas with a high risk of yellow fever.
Women contemplating pregnancy should use contraception for 28 days after yellow fever vaccination. Children younger than 9 months and the elderly are at higher risk of adverse reactions from the vaccine, either neurotropic or viscerotropic disease that mimics yellow fever infection. It is possible for physicians to write a medical waiver of contraindication to vaccination for patients who should not be immunized.
Typhoid fever
Typhoid fever can occur anywhere in the world, but it is endemic in the tropics. Worldwide, an estimated 200,000 deaths occur each year from typhoid fever, and 400 cases are reported annually in the United States, most commonly acquired by travelers to the Indian subcontinent.18 One study indicates that 95% of infected travelers had not been vaccinated, and a significant number returned with drugresistant strains.19
Typhoid fever is caused by ingestion of Salmonella typhi bacteria. It causes a febrile illness with infection of the digestive tract and reticuloendothelial system.
Prevention is the same as for traveler’s diarrhea: drink no local water and eat nothing raw. Vaccination can be provided in an intramuscular shot or a series of oral capsules. The shot is well tolerated and is valid for 3 years. The capsule provides 5 years of immunity. Vaccination is recommended for people going to areas with a high prevalence of typhoid fever, such as India, and for people planning to spend more than 2 weeks in an area where typhoid is endemic, as well as for adventurous eaters.
SOME TRAVELERS NEED MORE PROTECTION
Some travelers need more preventive measures than typical tourists or other short-term visitors. Long-term visitors or travelers to remote or other high-risk areas (eg, adventure travelers, relief workers, mission workers) may need, in addition to the measures described above, measures against Japanese encephalitis, rabies, cholera, epidemic meningitis, and dengue fever.
Japanese encephalitis
Japanese encephalitis virus is transmitted by mosquito bite. The major regions where it is endemic are rural India and Southeast Asia, most typically in areas with rice paddies and pig farms. Travelers at risk are expatriates to these areas, those planning a long stay, and remote-adventure travelers.
The vaccine JE-VAX is given as a series of three shots, on days 0, 7, and 28. Another vaccine, Ixiaro, is given in a series of two shots, on days 0 and 28.
Patients who are allergic to bee or wasp stings should not be vaccinated. The patient should remain in the office for 30 minutes after each dose to permit observation for mild anaphylactic reactions such as angioedema and urticaria, and should complete the series 10 days before travel to allow for observation for delayed reactions. Patients must weigh the risk of contracting the disease against the high cost of the vaccine.
Rabies
Rabies is a potential risk anywhere in the world except in Western Europe and Australia. Because the vaccine is costly, it is generally not given for prophylaxis except for travelers certain to have contact with animals, especially the major vectors, ie, dogs, cats, bats, and monkeys.20 Counseling about vigilance in avoiding animal contact and not promoting interaction through feeding wild animals should be part of any pretravel consult. Rabies, once acquired, is fatal.
The patient should be instructed on proper care of a bite from a potential rabies source and told to halt travel and seek medical attention. The wound should be cleaned with soap and water for 15 minutes to remove any saliva and virus from the soft tissue; this has proven to be effective in animal experiments. A virucidal such as benzalkonium chloride (Zephiran) or aqueous iodine should then be put in the wound.
Preexposure vaccination is done in a three-dose series (given on days 0, 7, and 21– 28). The patient should complete the series and adhere to the dosing schedule as closely as possible. It may be necessary to find a source of vaccine for the patient once he or she has arrived in the destination country.
If bitten, travelers without preexposure vaccination must find a source of vaccine and human rabies immune globulin (HRIG) before continuing on their trip. Postexposure treatment is 20 IU/kg of HRIG infiltrated around the wound to wall off the virus inoculation site. If the wound is in a digit or small area and not all of the HRIG can be given, then the remaining HRIG is given intramuscularly at a site distant from the vaccine site. If the patient has multiple bites, the HRIG should be diluted so it can be infiltrated around all wounds. The HRIG should be given immediately or within 7 days of beginning the vaccine series once a source is located. Later treatment than this can interfere with the patient’s ability to mount an immune reaction.
Rabies vaccine is initiated at the same time as HRIG and is given on days 0, 3, 7, 14, and 28. The CDC may soon change the schedule to allow for only four postexposure shots, but this has not yet been done as of this writing.
The patient vaccinated before exposure requires only booster doses of rabies vaccine at days 0 and 3.
Cholera
Cholera is an epidemic gastrointestinal disease historically responsible for millions of deaths. It is endemic in most tropical countries, especially in Africa and southern and southeastern Asia.21
High-risk patients, most often those working with refugees and disaster victims in endemic areas, should receive the traveler’s diarrhea and cholera vaccine Dukoral, which immunizes against Vibrio cholera and enterotoxogenic E coli. The vaccine, which is not available in the United States but is available abroad, is given as two oral doses 1 week apart for adults and three oral doses for children ages 2 to 6, and the second dose must be given 7 days before travel; this provides protection for 6 months.
At various times, the above vaccines have been in short supply. Travel medicine consults should be obtained for proper identification of the at-risk traveler for efficient use of any possibly limited vaccine.
Epidemic meningitis
The vaccine against epidemic meningitis is now routinely given in the United States to adolescents at the age of 12 or upon entry to college or the military. The fatality rate from the disease is 10%.
Meningococcal disease transmission peaks in the sub-Saharan “meningitis belt” in the dry season of December through June. Travelers to these areas at these times should be immunized. Travelers planning close contact with the local population (eg, health care workers) should be immunized. Patients traveling to Saudi Arabia for Hajj in Mecca must be immunized for meningitis for entry to the country during this time. The vaccine must be given within 3 years of entering the country and not less than 10 days before.
Dengue fever
Dengue fever is a flavivirus transmitted through the Aedes aegypti mosquito. No vaccine is available for dengue fever, so for now the only advice is to avoid insect vectors.
There are four closely related but serologically different dengue viruses that provide only weak cross-protection. In fact, previous infection with one serotype in a traveler then infected with another poses a risk of dengue hemorrhagic fever.
Because of inattention to public sanitation, this virus and its mosquito vector have reemerged in areas where they were once eliminated. The viral infection is a risk for the traveler to both urban and rural areas in the Americas, Southeast Asia, and Africa. The Pan American Health Organization has seen the number of reported dengue cases increase from 66,000 in 1980 to 700,000 in 2003.22
Before going abroad to areas that might pose a risk to their health, most people ought to visit their primary care physicians and many should be referred to a specialist in travel medicine.
In this article, we review the key elements of the pretravel consult as it relates to the prevention and self-treatment of the most common diseases that pose health risks for travelers. We also give guidelines for when to refer patients to a specialist.
WHY PRIMARY CARE PHYSICIANS NEED TO KNOW TRAVEL MEDICINE
International travel to exotic locations is becoming more popular. In 2008, one out of five Americans traveled abroad, and 38 million visits were to developing countries where there are significant health risks for travelers.1
One third to one half of travelers to developing countries experience some kind of illness while abroad, most commonly diarrhea or upper respiratory infections, which typically lead to 3 lost days during a 2-week trip.
These illnesses are often preventable and self-treatable.2 Unfortunately, studies suggest that most travelers do not seek adequate medical advice, and that when they do they often fail to complete courses of medication.3,4
All these factors point to the need for primary care providers to become proficient in the pretravel consult and, if necessary, to refer patients to travel specialists and clinics.
WHY REFER TO A TRAVEL CLINIC?
In one study of travelers to areas of high risk for malaria or hepatitis A, 42% of those who consulted only their family doctor became ill, in contrast to 22% of those who attended a travel medicine clinic.4
As a rule of thumb, anyone traveling to an area where malaria is endemic should be referred to a specialist, as should anyone at risk of yellow fever or typhoid fever. As many as 8 per 1,000 travelers may return from areas of risk infected with malaria.5
Long-term travelers and people who will spend time in urban slums or rural or remote regions have an even greater need for referral to a travel clinic, as they are at higher risk of exposure to Japanese encephalitis, cholera, epidemic meningitis, dengue fever, and rabies.6
THE PRETRAVEL CONSULT: ESSENTIALS
A pretravel consult ought to be scheduled 4 to 6 weeks in advance of the trip, since many vaccines require that much time to induce immunity, and some require a series of shots.
Unfortunately, many patients who think of arranging a travel consult make the appointment at the last minute, and some come with an incomplete knowledge of their travel plans. However, even without enough advance notice, a consult can be beneficial.
Travelers sometimes change their itineraries in-country or engage in unanticipated risky behaviors. A good travel medicine physician tries to anticipate even these unplanned risks and changes in itinerary.
Where is the traveler going? When? For how long?
The pretravel consult starts with a detailed discussion of the patient’s itinerary. It needs to include length, dates, and location of travel, as well as anticipated activities and accommodations.
A remarkable number of travelers come to consults not knowing the names of specific countries they will visit, perhaps saying only that they are going to Africa or South America. An accurate itinerary is indispensible, as appropriate medical advice is highly specific to country and region. The incidence and geographic distribution of many travelers’ diseases change over time, and this requires physicians to consult the most current information available.
Tropical countries, in general, are risky, but each pathogen has a unique distribution that may vary between urban and rural areas or by season. Detailed, up-to-date information is available from the US Centers for Disease Control and Prevention (CDC) (www.cdc.gov) for individual countries and for specific provinces and locations within those countries. Physicians should consult the CDC whenever advising a patient preparing to travel. 7
How is the traveler’s current health?
Several immunizations cannot be given to the very young, the elderly, or those who are immunocompromised.
The greatest risk of death to travelers is not from tropical diseases but from cardiovascular disease, which according to one study is responsible for half of deaths abroad.8 Patients with heart disease or other known health concerns need to be counseled to avoid activities that will put them at further risk. The advice applies especially in situations such as remote travel or even cruises, where prompt emergency medical care may be difficult or impossible to obtain.
People infected with human immunodeficiency virus (HIV) face discriminatory travel prohibitions in 74 countries.9
Foreign-born travelers who are visiting family and friends in developing countries may have lost their immunity to local pathogens and thus can be more at risk because they are not prepared to take necessary health precautions.
Also, a significant number of travelers become infected but develop illnesses only after they return, so a posttravel visit may be necessary.6
Prescription and even over-the-the-counter drugs may be difficult or impossible to obtain in foreign countries, and ample supplies should be brought along.
Is the traveler up to date on routine immunizations?
A number of infectious diseases that have been controlled or eradicated in North America through regular childhood immunizations are still endemic in many remote areas and developing countries. All travelers should be up to date on routine immunizations, including those for measles-mumps-rubella, tetanus, polio, meningitis, and hepatitis A and B.
Polio. A one-time polio booster is recommended for adults traveling to certain countries or areas of the world.
Meningitis vaccine is now routinely given to young people, but adult patients may need it before they travel.
Hepatitis A is contracted through fecal contamination of food and water. Common sources are foods prepared in an unhygienic manner, raw fruits and vegetables, shellfish, and contaminated water.
Hepatitis B vaccine is also now routinely given to young people, but it should be offered to travelers planning to stay more than 1 month and to long-term expatriates. This vaccine is also recommended for travelers who may be exposed to blood or body fluids, who are contemplating sexual activity or tattooing in the host country, or who may require medical or dental care while traveling, as well as for adventure travelers or travelers to remote regions.
The vaccination is given in a three-dose schedule at 0, 1, and 6 months. For protection against both hepatitis A and B, the vaccine Twinrix can be used on the same schedule as for hepatitis B. An accelerated schedule of 0, 7, and 21 days with a booster at 12 months allows completion of the entire series in 4 weeks, thus putting completion of vaccination before travel in the same time frame as other vaccines in a series, such as those for rabies and Japanese encephalitis.
PREVENTIVE COUNSELING
In addition, travelers going abroad should be advised on measures to avoid diarrhea, insectvector diseases, accidents, excessive exposure to the sun, altitude sickness, and other risks their itineraries may expose them to.
Avoiding traveler’s diarrhea
Traveler’s diarrhea is by far the most common health problem experienced abroad. It is prevalent in Mexico, where 20 million visits by Americans occur each year. A quarter to half of visitors to developing countries contract traveler’s diarrhea and, on average, lose 2 to 3 days of their business trip or vacation.3,10 The disease therefore imposes not only discomfort but also financial losses on travelers, especially business travelers.
Though many pathogens may be responsible, the most common one is Escherichia coli, usually transmitted by human fecal contamination of food or drink. Preventive measures against E coli are the same as for other foodborne and waterborne infections, such as hepatitis A, cholera, and typhoid fever.
The rule for avoiding traveler’s diarrhea may be summarized by the CDC-coined phrase, “boil it, cook it, peel it, or forget it.” Simple, written advice is most likely to be followed. 6 Thorough boiling or cooking kills bacteria in contaminated food, and food should be served steaming hot. Travelers should only eat foods they know have been well cooked, declining cold dishes like salsa or casseroles. They should avoid tap water for brushing teeth or in the form of ice cubes and should stick to drinking bottled beverages, preferably carbonated ones. No matter how appetizing a salad looks, travelers should avoid eating fresh fruits and vegetables unless they are sure that they were peeled under sanitary conditions. Simply eating at a high-priced restaurant is not a guarantee of uncontaminated food. Before meals or any hand-to-mouth contact, hands should be washed in soap and water or with sanitizers.
Travelers to remote areas may wish to acquire filtering devices, chlorine, or iodine for treating water. A combination of filtering and iodine treatment is most effective.
While this advice is undoubtedly wise, the evidence shows that, in practice, most travelers fail to take all precautions, and the benefits of this counseling have been difficult to demonstrate.11 Therefore, physicians should prescribe drugs for prophylaxis and self-treatment of traveler’s diarrhea during travel.
Bismuth subsalicylate (Pepto-Bismol) taken as two tablets or 2 oz of liquid 4 times a day while traveling may reduce the risk of diarrhea by one half, though it should be avoided by patients with contraindications to aspirin.3,6
Self-treating traveler’s diarrhea
Proper hydration is crucial, since dehydration can worsen and prolong symptoms.
Ciprofloxacin (Cipro) 500 mg orally two times daily for 3 to 5 days is effective.
Azithromycin (Zithromax) 500 mg daily for 3 to 5 days may be better in some areas of Southeast Asia, where fluoroquinolone-resistant bacteria are prevalent.
Rifaximin (Xifaxan), a nonsystemic antibiotic, is another option. The dosage is 200 mg three times a day for 3 days.
Avoiding insect bites
Malaria, yellow fever, tickborne encephalitis, and dengue fever are all transmitted by insect bites. Often the best protection is to avoid being bitten.
Bites can be avoided by using insect repellants containing diethyltoluamide (DEET) or picardin. If the traveler is going to be out in the sun, he or she should apply sunscreen first, then DEET on top of that. Anopheles, which transmits malaria, is a night-biting mosquito and may be avoided by staying in screened areas at dusk and dawn and by using bed netting. Permethrin, an insecticide, can be applied to clothing and mosquito netting.
Other things to avoid
Accidents are the second most common cause of death in travelers (after cardiovascular disease), accounting for as many as one-third of deaths.9 Several studies indicate road accidents are the major cause of accidental death, but also significant are drowning and air crashes. Travelers should be advised that transportation in developing countries is often more dangerous than at home. Seaside vacationers should be aware of the dangers of riptides and other threats to swimmers and should obey warnings posted at beaches.
Sexually transmitted diseases. When appropriate, physicians should warn travelers about the dangers of contracting HIV and other sexually transmitted diseases, especially in sub-Saharan Africa.
Sunburn, dehydration. Travelers should regularly use sunscreen and should remain hydrated.
Crimes against and involving tourists are a serious threat in many places, including some popular destinations. All of the 100,000 young people traveling to Mexico each year for spring break should read the US Department of State warnings against crime and possible arrest in that country.12 Travelers who are victims of crimes in foreign countries should contact their national consulate as soon as possible. The US Department of State issues advisories on countries where there is danger to travelers because of political turmoil, crime, or other causes.13
Motion sickness and jet lag can be ameliorated by proper hydration, avoiding caffeine, and using a scopolamine patch or dimenhydrinate (Dramamine).1
When traveling to wilderness areas
Wilderness and expedition medicine is a complex subset of travel medicine.14 All travelers need to understand the risks of whatever activities they undertake.
Mountain climbers and skiers have to contend with altitude sickness and frostbite. Scuba divers have the risks of decompression sickness, barotrauma, and hazardous marine life. Travelers on expeditions may have to deal with predatory animals, exotic parasites, and ethnic or political violence. People who participate in these activities should do so only when they are properly certified and educated in the associated health risks.
Ordinary tourists should enjoy safe adventures with well-established tour agencies and venues and should be cautioned against activities that expose them to dangers they may not be prepared to confront.
Insurance, evacuation, and emergency care
Health insurance often does not pay for preventive travel medicine. Unfortunately, cost can be a factor in immunizations and other health care. The cost of most travelers’ medications and vaccinations is generally comparable to that of other immunizations. The exceptions are two specialized vaccines—ie, for Japanese encephalitis ($1,000 or more for a full course) and for rabies, which can cost considerably more. Pricing by different providers can vary widely.
Travelers, especially those who are pregnant, elderly, disabled, or immunocompromised or who have preexisting diseases, need to review their insurance policies to make certain that care in foreign countries is covered. If not, evacuation insurance can be purchased at a relatively modest cost.
TRAVEL TO AREAS OF MALARIA
While many travelers can confidently consult their primary care provider, those traveling to places where malaria is prevalent should be referred to a physician with a thorough and current knowledge of the incidence of drugresistant strains of the disease and other complex issues in travel medicine. Short-term and long-term travelers are often approached differently, but a travel medicine consult should be obtained for any patient traveling to a region with malaria risk.
Malaria kills up to 3 million each year
Malaria, caused by the Plasmodium parasite, transmitted by the night-biting Anopheles mosquito, is responsible worldwide for between 1 and 3 million deaths annually, mostly of children in sub-Saharan Africa.15 Every year about 1,500 Americans are diagnosed with malaria and, on average, 10 die.6
Nearly all cases of malaria and deaths from it are preventable. Prophylaxis is imperative for travelers to affected areas, as is preventive counseling. Based on the patient’s itinerary, the physician needs to thoroughly research potential exposure to drug-resistant strains before choosing which antimalarial regimen to prescribe.
Malaria causes symptoms of anemia, fever, or nausea and, without treatment, can lead to coma and death. Because two of the five strains, P vivax and P ovale, can remain dormant in an infected person’s liver for up to 1 year and, in rare cases, up to 4 years after travel, it is imperative that a returned traveler who experiences flu-like symptoms seek medical attention and inform the treating physician of the need to screen for malarial infection. The primary means of diagnosis is through microscopic examination of the blood.
No malaria vaccine, but prophylactic drugs are available
Unlike many of the illnesses discussed below for which vaccines are available, malaria prophylaxis requires the active participation of the patient in completing a course of medication, so noncompliance becomes a risk.
A number of prophylactic drugs are available. The choice depends on the locally resistant strains.16
Chloroquine (Aralen), the traditional malarial prophylactic drug, is still effective against many strains, primarily in Central America and some areas of the Middle East. The dosage is 500 mg once a week, started 1 week before travel and continued for 4 weeks after return to the United States.
Mefloquine (Lariam) is dosed at 250 mg weekly. The patient should be carefully screened for depression, anxiety, and other mood disorders. Even the report of bad dreams or nightmares should make a patient be considered a poor candidate for this medication. The patient should start taking this drug 3 weeks before travel to provide time to assess for adverse effects and, if necessary, to change the antimalarial regimen. Mefloquine is taken weekly while traveling and is continued for 4 weeks after return.
Doxycycline (Vibramycin) is an antibiotic. As an antimalarial prophylactic, it is taken as 100 mg daily beginning 2 days prior to travel and continuing while travelling and for 4 weeks after return.
Atovaquone-proguanil (Malarone) prevents infection at the blood stage and in the liver. It is well tolerated and is begun 2 days before travel. It is taken daily while traveling and daily for 1 week after return.
Yellow fever
Immunization is required for entry to more than 20 African nations and is recommended for those traveling to most of South America. The only physicians who can give this vaccine are those who have approval from their state health department and have been issued an official stamp, used on the World Health Organization (WHO) yellow fever vaccination card. Several countries require the card for entry from places where yellow fever is present. For any multicountry travel involving at least one area where yellow fever is endemic, the entire itinerary needs to be reviewed to make sure all legal entry requirements are met. The WHO maintains a current list of these requirements.17 If there is any doubt, it is generally best to refer and certify the traveler.
Referral should be timely. The vaccine must be given 10 days prior to entry into a country where yellow fever is endemic; it is valid for 10 years.
The yellow fever vaccine is a live-attenuated vaccine and should not be given to infants younger than 9 months old, adults over age 60 who are not properly screened and informed, or pregnant women. Immunocompromised patients are excluded from receiving this vaccine, as are patients taking immunosuppressant drugs and patients with thymus disorders such as myasthenia gravis. Patients who have had chemotherapy must wait 3 months before being vaccinated. Those on steroids (eg, prednisone 20 mg or more daily) must wait until 2 weeks after cessation of steroids to receive this vaccine. Patients who cannot be vaccinated should be advised not to travel to areas with a high risk of yellow fever.
Women contemplating pregnancy should use contraception for 28 days after yellow fever vaccination. Children younger than 9 months and the elderly are at higher risk of adverse reactions from the vaccine, either neurotropic or viscerotropic disease that mimics yellow fever infection. It is possible for physicians to write a medical waiver of contraindication to vaccination for patients who should not be immunized.
Typhoid fever
Typhoid fever can occur anywhere in the world, but it is endemic in the tropics. Worldwide, an estimated 200,000 deaths occur each year from typhoid fever, and 400 cases are reported annually in the United States, most commonly acquired by travelers to the Indian subcontinent.18 One study indicates that 95% of infected travelers had not been vaccinated, and a significant number returned with drugresistant strains.19
Typhoid fever is caused by ingestion of Salmonella typhi bacteria. It causes a febrile illness with infection of the digestive tract and reticuloendothelial system.
Prevention is the same as for traveler’s diarrhea: drink no local water and eat nothing raw. Vaccination can be provided in an intramuscular shot or a series of oral capsules. The shot is well tolerated and is valid for 3 years. The capsule provides 5 years of immunity. Vaccination is recommended for people going to areas with a high prevalence of typhoid fever, such as India, and for people planning to spend more than 2 weeks in an area where typhoid is endemic, as well as for adventurous eaters.
SOME TRAVELERS NEED MORE PROTECTION
Some travelers need more preventive measures than typical tourists or other short-term visitors. Long-term visitors or travelers to remote or other high-risk areas (eg, adventure travelers, relief workers, mission workers) may need, in addition to the measures described above, measures against Japanese encephalitis, rabies, cholera, epidemic meningitis, and dengue fever.
Japanese encephalitis
Japanese encephalitis virus is transmitted by mosquito bite. The major regions where it is endemic are rural India and Southeast Asia, most typically in areas with rice paddies and pig farms. Travelers at risk are expatriates to these areas, those planning a long stay, and remote-adventure travelers.
The vaccine JE-VAX is given as a series of three shots, on days 0, 7, and 28. Another vaccine, Ixiaro, is given in a series of two shots, on days 0 and 28.
Patients who are allergic to bee or wasp stings should not be vaccinated. The patient should remain in the office for 30 minutes after each dose to permit observation for mild anaphylactic reactions such as angioedema and urticaria, and should complete the series 10 days before travel to allow for observation for delayed reactions. Patients must weigh the risk of contracting the disease against the high cost of the vaccine.
Rabies
Rabies is a potential risk anywhere in the world except in Western Europe and Australia. Because the vaccine is costly, it is generally not given for prophylaxis except for travelers certain to have contact with animals, especially the major vectors, ie, dogs, cats, bats, and monkeys.20 Counseling about vigilance in avoiding animal contact and not promoting interaction through feeding wild animals should be part of any pretravel consult. Rabies, once acquired, is fatal.
The patient should be instructed on proper care of a bite from a potential rabies source and told to halt travel and seek medical attention. The wound should be cleaned with soap and water for 15 minutes to remove any saliva and virus from the soft tissue; this has proven to be effective in animal experiments. A virucidal such as benzalkonium chloride (Zephiran) or aqueous iodine should then be put in the wound.
Preexposure vaccination is done in a three-dose series (given on days 0, 7, and 21– 28). The patient should complete the series and adhere to the dosing schedule as closely as possible. It may be necessary to find a source of vaccine for the patient once he or she has arrived in the destination country.
If bitten, travelers without preexposure vaccination must find a source of vaccine and human rabies immune globulin (HRIG) before continuing on their trip. Postexposure treatment is 20 IU/kg of HRIG infiltrated around the wound to wall off the virus inoculation site. If the wound is in a digit or small area and not all of the HRIG can be given, then the remaining HRIG is given intramuscularly at a site distant from the vaccine site. If the patient has multiple bites, the HRIG should be diluted so it can be infiltrated around all wounds. The HRIG should be given immediately or within 7 days of beginning the vaccine series once a source is located. Later treatment than this can interfere with the patient’s ability to mount an immune reaction.
Rabies vaccine is initiated at the same time as HRIG and is given on days 0, 3, 7, 14, and 28. The CDC may soon change the schedule to allow for only four postexposure shots, but this has not yet been done as of this writing.
The patient vaccinated before exposure requires only booster doses of rabies vaccine at days 0 and 3.
Cholera
Cholera is an epidemic gastrointestinal disease historically responsible for millions of deaths. It is endemic in most tropical countries, especially in Africa and southern and southeastern Asia.21
High-risk patients, most often those working with refugees and disaster victims in endemic areas, should receive the traveler’s diarrhea and cholera vaccine Dukoral, which immunizes against Vibrio cholera and enterotoxogenic E coli. The vaccine, which is not available in the United States but is available abroad, is given as two oral doses 1 week apart for adults and three oral doses for children ages 2 to 6, and the second dose must be given 7 days before travel; this provides protection for 6 months.
At various times, the above vaccines have been in short supply. Travel medicine consults should be obtained for proper identification of the at-risk traveler for efficient use of any possibly limited vaccine.
Epidemic meningitis
The vaccine against epidemic meningitis is now routinely given in the United States to adolescents at the age of 12 or upon entry to college or the military. The fatality rate from the disease is 10%.
Meningococcal disease transmission peaks in the sub-Saharan “meningitis belt” in the dry season of December through June. Travelers to these areas at these times should be immunized. Travelers planning close contact with the local population (eg, health care workers) should be immunized. Patients traveling to Saudi Arabia for Hajj in Mecca must be immunized for meningitis for entry to the country during this time. The vaccine must be given within 3 years of entering the country and not less than 10 days before.
Dengue fever
Dengue fever is a flavivirus transmitted through the Aedes aegypti mosquito. No vaccine is available for dengue fever, so for now the only advice is to avoid insect vectors.
There are four closely related but serologically different dengue viruses that provide only weak cross-protection. In fact, previous infection with one serotype in a traveler then infected with another poses a risk of dengue hemorrhagic fever.
Because of inattention to public sanitation, this virus and its mosquito vector have reemerged in areas where they were once eliminated. The viral infection is a risk for the traveler to both urban and rural areas in the Americas, Southeast Asia, and Africa. The Pan American Health Organization has seen the number of reported dengue cases increase from 66,000 in 1980 to 700,000 in 2003.22
- US Department of Commerce International Trade Administration. Outbound overview 2008. www.tinet.ita.doc.gov/outreachpages. Accessed October 27, 2009.
- Dick L. Travel medicine: helping patients prepare for trips abroad. Am Fam Physician 1998; 58:383–398, 401–402.
- Reed JM, McIntosh IB, Powers K. Travel illness and the family practitioner: a retrospective assessment of travel-induced illness in general practice and the effect of a travel illness clinic. J Travel Med 1994 1:192–198.
- Hamer DH, Connor BA. Travel health knowledge, attitudes, and practices among United States travelers. J Travel Med 2004; 11:23–26.
- Hill DR. Health problems in a large cohort of Americans traveling to developing countries. J Travel Med 2000; 7:259–266.
- Hill DR, Ericsson CD, et al., Infectious Diseases Society of America. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1499–1539.
- US Centers for Disease Control and Prevention. Destinations. wwwn.cdc.gov/travel/destinations/list.aspx. Accessed February 11, 2010.
- Steffen R. Epidemiology: Morbidity and mortality in travelers. In:Keystone J, ed. Travel Medicine. Mosby: New York, 2004:5–12.
- Joint United Nations Programme on HIV/AIDS. HIV-related travel restrictions. www.unaids.org/en/KnowledgeCentre/Resources/FeatureStories/archive/2008/20080304_HIVrelated_travel_restrictions.asp. Accessed February 11, 2010.
- Brewster SJ, Taylor DN. Epidemiology of travelers’ diarrhea. In:Keystone J, ed. Travel Medicine. New York: Mosby, 2004:175–184.
- Ostrosky-Zeichner L, Ericsson CD. Prevention of travelers’ diarrhea. In:Keystone J, ed. Travel Medicine. New York: Mosby, 2004:185–189.
- US Department of State. Spring break in Mexico—“Know Before You Go!” http://travel.state.gov/travel/cis_pa_tw/spring_break_mexico/spring_break_mexico_2812.html. Accessed February 11, 2010.
- US Department of State. Current travel warnings. http://travel.state.gov/travel/cis_pa_tw/tw/tw_1764.html. Accessed February 11, 2010.
- Bledsoe GH, Manyak MJ, Townes DA, eds. Expedition and Wilderness Medicine. Cambridge University Press: New York, 2008.
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 2001; 64( suppl 1–2):97–106.
- US Centers for Disease Control and Prevention. The Pre-Travel Consultation: Malaria. wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/malaria.aspx. Accessed February 11, 2010.
- World Health Organization. Country list: yellow fever vaccination requirements and recommendations; and malaria situation. http://www.who.int/ith/ITH2009Countrylist.pdf. Accessed February 11, 2010.
- US Centers for Disease Control and Prevention. Typhoid Fever. www.cdc.gov/ncidod/dbmd/diseaseinfo/typhoidfever_t.htm. Accessed February 11, 2010.
- Lynch MF, Blanton EM, Bulens S, et al Typhoid fever in the United States, 1999–2006. JAMA 2009; 302:859–865.
- Plotkin SA. Rabies. Clin Infect Dis 2000; 30:4–12.
- Topps M. Oral cholera vaccine—for whom, when, and why? Travel Med Infect Dis 2006; 4:38–42.
- Petersen LR, Marfin AA. Shifting epidemiology of Flaviviridae. J Travel Med 2005; 12(suppl 1):S3–S11.
- US Department of Commerce International Trade Administration. Outbound overview 2008. www.tinet.ita.doc.gov/outreachpages. Accessed October 27, 2009.
- Dick L. Travel medicine: helping patients prepare for trips abroad. Am Fam Physician 1998; 58:383–398, 401–402.
- Reed JM, McIntosh IB, Powers K. Travel illness and the family practitioner: a retrospective assessment of travel-induced illness in general practice and the effect of a travel illness clinic. J Travel Med 1994 1:192–198.
- Hamer DH, Connor BA. Travel health knowledge, attitudes, and practices among United States travelers. J Travel Med 2004; 11:23–26.
- Hill DR. Health problems in a large cohort of Americans traveling to developing countries. J Travel Med 2000; 7:259–266.
- Hill DR, Ericsson CD, et al., Infectious Diseases Society of America. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1499–1539.
- US Centers for Disease Control and Prevention. Destinations. wwwn.cdc.gov/travel/destinations/list.aspx. Accessed February 11, 2010.
- Steffen R. Epidemiology: Morbidity and mortality in travelers. In:Keystone J, ed. Travel Medicine. Mosby: New York, 2004:5–12.
- Joint United Nations Programme on HIV/AIDS. HIV-related travel restrictions. www.unaids.org/en/KnowledgeCentre/Resources/FeatureStories/archive/2008/20080304_HIVrelated_travel_restrictions.asp. Accessed February 11, 2010.
- Brewster SJ, Taylor DN. Epidemiology of travelers’ diarrhea. In:Keystone J, ed. Travel Medicine. New York: Mosby, 2004:175–184.
- Ostrosky-Zeichner L, Ericsson CD. Prevention of travelers’ diarrhea. In:Keystone J, ed. Travel Medicine. New York: Mosby, 2004:185–189.
- US Department of State. Spring break in Mexico—“Know Before You Go!” http://travel.state.gov/travel/cis_pa_tw/spring_break_mexico/spring_break_mexico_2812.html. Accessed February 11, 2010.
- US Department of State. Current travel warnings. http://travel.state.gov/travel/cis_pa_tw/tw/tw_1764.html. Accessed February 11, 2010.
- Bledsoe GH, Manyak MJ, Townes DA, eds. Expedition and Wilderness Medicine. Cambridge University Press: New York, 2008.
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 2001; 64( suppl 1–2):97–106.
- US Centers for Disease Control and Prevention. The Pre-Travel Consultation: Malaria. wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/malaria.aspx. Accessed February 11, 2010.
- World Health Organization. Country list: yellow fever vaccination requirements and recommendations; and malaria situation. http://www.who.int/ith/ITH2009Countrylist.pdf. Accessed February 11, 2010.
- US Centers for Disease Control and Prevention. Typhoid Fever. www.cdc.gov/ncidod/dbmd/diseaseinfo/typhoidfever_t.htm. Accessed February 11, 2010.
- Lynch MF, Blanton EM, Bulens S, et al Typhoid fever in the United States, 1999–2006. JAMA 2009; 302:859–865.
- Plotkin SA. Rabies. Clin Infect Dis 2000; 30:4–12.
- Topps M. Oral cholera vaccine—for whom, when, and why? Travel Med Infect Dis 2006; 4:38–42.
- Petersen LR, Marfin AA. Shifting epidemiology of Flaviviridae. J Travel Med 2005; 12(suppl 1):S3–S11.
KEY POINTS
- Primary care physicians should be proficient in the basic pretravel consult, including advice on immunizations and travel-related health problems.
- Based on the traveler’s itinerary, the physician should consult current government recommendations for pretravel preparation at www.cdc.gov/travel/destinations/list.aspx.
- Drug-resistant malarial strains are on the rise in many areas of the world.
- To enter many African and South American countries, travelers need official certification by a specialist that they have been vaccinated against yellow fever.
Grand Rounds: Five-Day-Old Infant With Hip "Clunk"
A 5-day-old infant was referred to the pediatric orthopedic clinic for evaluation of a left hip “clunk.” She is a firstborn child, born at full term (39 weeks) via cesarean delivery secondary to breech presentation. Her weight at birth was 7 lb 6 oz. The infant was noted to have a left hip clunk during a routine physical examination by her pediatrician, who made a referral to the pediatric orthopedic clinic for possible hip dysplasia. This is the patient’s first visit to the clinic.
There is no family history of hip dysplasia or other orthopedic abnormalities. The infant is a well-appearing, alert female measuring 20.5” in length and weighing 7 lb 4 oz. Vital signs are stable with no abnormality detected. The heart is regular in rate and rhythm, and the chest is clear bilaterally.
No cutaneous abnormalities are noted. The patient is able to move all her extremities spontaneously, and her spine is straight and normal with no evidence of spinal dysraphism. Her feet are normal bilaterally, with full range of motion and no equinovarus or metatarsus adductus deformity.
The neurologic examination is also unremarkable, with normal neonatal reflexes and excellent muscle tone throughout.
Examination of the infant’s hips reveals a positive result on the Barlow test on the left side (the hip can be dislocated). There is also a positive Ortolani sign (the hip can be reduced), with asymmetric thigh skin folds noted (see Figures 1A and 1B, respectively).
Based on these positive physical examination findings, the patient was diagnosed with developmental dysplasia of the hip (DDH). Initial ultrasonography to confirm the diagnosis was not considered necessary, as the physical examination demonstrated obvious instability.1 The infant was placed in a Pavlik harness, which her parents were instructed should be worn full-time (see Figures 2A and 2B). She was scheduled for weekly follow-up visits for adjustments to the harness and serial hip examinations.
At the second follow-up visit, ultrasonography was performed, confirming the presence of dysplasia with decreased femoral head coverage and a steep socket (acetabulum). Use of the Pavlik harness was continued full-time for six weeks.
At age 6 weeks, the infant underwent a follow-up ultrasound to assess for improvement in the degree of dysplasia. The test revealed normal hips bilaterally with no evidence of DDH. Therefore, use of the Pavlik harness was discontinued. The parents were instructed to bring the child back in six months for a repeat clinical examination and an anteroposterior x-ray of the pelvis.1
Discussion
The term developmental dysplasia of the hip (DDH) has replaced the more traditional term congenital hip dislocation because DDH more accurately reflects the variable characteristics that can be seen with this condition. As DDH may not be present at birth, the term congenital is misleading. We now know that DDH may occur in utero, perinatally, or during infancy and childhood.2,3
Generally, DDH is used to describe an abnormal relationship between the femoral head and the acetabulum (see Figure 34). The term represents a wide spectrum of abnormality, as shown in the Graf classification of hips in infants: type I refers to a normal hip; type II, immature development to mild dysplasia; type III, subluxation of the femoral head; and type 4, frank dislocation with severe instability.5
Diagnosing and managing DDH correctly requires the clinician to have a thorough understanding of the normal growth and development that occurs in the hip joint. Embryologically, the joint (including the femoral head and acetabulum) develops from the same primitive mesenchymal cells.6 By 11 to 12 weeks’ gestation, the initial structures of the hip joint are fully formed; theoretically, this is the earliest time at which a dislocation can occur.2,7 DDH that develops at this stage would be called teratologic; this condition is seen most frequently in patients who have underlying neuromuscular conditions, such as myelodysplasia (spina bifida) or arthrogryposis. A typical dislocation takes place during the perinatal period in an infant who is otherwise healthy.2
Etiology
DDH occurs in about 11 of every 1,000 infants, with frank dislocations occurring in one to two infants per 10,000.8 The left hip is involved in approximately 60% of cases, the right in 20%, and both hips in about 20%. In the most common intrauterine fetal position, the left hip is lower than the right (usually abutting the mother’s sacrum) and is often in adduction. This is likely the reason that the left hip is more commonly affected by DDH.
DDH is believed to be multifactorial, with physiologic, genetic, and mechanical factors implicated in the etiology.3 The incidence of DDH varies with factors such as the patient’s age, race, and gender, the experience and training of the examiner, and the diagnostic criteria that are used.
Known risk factors for a positive newborn screening are shown in the table.9,10 It is often helpful for clinicians to remember the “4F” mnemonic associated with DDH: female, firstborn, foot first, and family history.9
There is also an increased risk for DDH in patients with other conditions that are associated with intrauterine crowding. These include congenital muscular torticollis, metatarsus adductus, and congenital dislocation of the knee.2
Physical Examination
All newborn infants should be screened for DDH as part of the initial physical examination, with ultrasonography recommended for infants deemed at high risk for DDH and for those with inconclusive results on examination.1,10,11 Providers should be aware that the newborn hip examination requires a considerable amount of practice and expertise.
A thorough medical history should always be obtained first, including gestational age, presentation (breech vs vertex), type of delivery (cesarean vs vaginal), gender, birth order, family history of DDH, ligamentous laxity, or myopathy.8
The examining clinician begins by placing the infant on a firm, flat surface. The infant should be as relaxed as possible. Next, the clinician observes both lower extremities for asymmetric thigh or buttock skin folds. Bilateral DDH can be very difficult to diagnose on the basis of this examination due to the lack of asymmetry (hips will have symmetric abnormality).
The Galeazzi sign is elicited by placing the infant supine with the hips and knees flexed to 90°.12 With the hips in neutral abduction, the provider should determine whether the knees are at the same height. Unequal knee heights—a positive result for the Galeazzi sign—suggest femoral shortening (apparent leg length discrepancy), which may be explained by a hip dislocation. If both hips are dislocated, a false-negative result will often occur, since both will appear short and there will be no discrepancy.2,12
Among physical examination techniques, the Ortolani and Barlow maneuvers are considered most reliable to detect hip instability in newborns and infants younger than 6 months2,13,14 (review Figures 1A and 1B). The Ortolani test is used to detect the sensation of the dislocated hip reducing into the acetabulum, and the Barlow test elicits the unstable hip dislocating.2 A palpable and occasionally audible clunk is considered a positive result on the Barlow test and usually indicates a diagnosis of DDH.14 High-pitched clicks or snaps frequently occur with hip range-of-motion maneuvers and during Ortolani and Barlow testing. These sounds are often attributed to snapping of the iliotibial band over the greater trochanter and do not usually signify dysplasia.15
Because DDH is a dynamic and evolving process, the physical findings on clinical examination change significantly, depending on the age of the infant or child. As an infant approaches age 3 months, limited hip abduction (especially when asymmetric) is often the most reliable physical examination finding in patients with DDH.12 After age 3 to 4 months, Ortolani and Barlow testing will often produce negative results as progressive soft tissue contractures evolve.
Once a child begins to walk, gait abnormalities (eg, a short-limbed or waddling gait pattern) may raise suspicion for a diagnosis of DDH.7 It has been recommended that evaluation for DDH be performed at each routine office examination until the child is 12 months of age.1
Treatment
The Pavlik harness is considered first-line treatment for DDH in infants younger than 6 months. The harness is a dynamic splint that allows the infant to engage in a sphere of active motion that encourages stabilization and deepening of the socket. The harness is applied with the knees flexed to about 90° and the hips in about 70° of abduction and 100° to 110° of flexion (as shown in Figures 2A and 2B).9
The duration of treatment depends on the infant’s age at presentation and the severity of DDH. Progress is judged by serial examinations and dynamic ultrasounds. The harness is worn full-time until clinical and radiographic examinations both yield normal results. After six weeks of treatment, the hips are examined out of the harness, and a repeat ultrasound is usually obtained. If findings are normal, use of the harness is ordinarily discontinued. Some patients will require harness use for a longer period in cases of delayed development of the acetabulum and/or severe laxity of the ligaments.9
The Pavlik harness is successful more than 90% of the time in newborns with DDH.8 Success rates have been reported as greatest in infants younger than 8 weeks at the time of treatment initiation, those with only one affected hip, and those with less severe disease (Graf types II or III).16
If ultrasonography shows no improvement after two to three weeks, it is usually recommended that the harness be discontinued; most orthopedic surgeons will then proceed with a closed or open reduction and spica body casting. Similarly, when the diagnosis of DDH is delayed until after ages 6 to 8 months, a closed reduction under anesthesia and placement of a spica body cast is usually the recommended treatment to maintain the hip in the reduced position.17,18 Some older children (ages 1 to 5 years) may require bracing, traction, open reduction, and/or femoral or pelvic osteotomy.17,18 It is believed that undiagnosed, untreated DDH can lead to early-onset degenerative hip disease (arthritis).1
Patient/Family Education
The Pavlik harness is most effective when a consistent support system exists to educate parents about the importance of the harness, its care and maintenance, and the consequences of failure. Close monitoring of the infant’s progress is also essential to promoting adherence. Application and removal of the harness should be demonstrated to the parent or caregiver, as well as diapering, dressing, and undressing the infant; they should then be encouraged to practice immediately in the clinic or office.
During visits for harness adjustment, the strap position should be marked with indelible ink, allowing parents to reapply the device correctly, should removal be required (eg, for bathing).9 Ten percent of parents reportedly find reapplying the harness difficult during the first weeks of use. Difficulty in dressing and carrying an infant in a harness, feet slipping out of the harness, and skin irritation have been reported by about one-third of parents.19
Treatment adherence and subsequent success with the Pavlik harness is reported greatest (95%) in patients whose parents engage in demonstrations of harness use and follow instructions precisely.19 By providing a contact name and office number and following up with a phone call a few days after the harness is first applied, clinicians can significantly decrease parents’ anxiety and increase overall compliance.9
Conclusion
Despite recent increased awareness of DDH and the importance of thorough screening programs, hip dysplasia continues to be a frequently missed diagnosis in pediatrics. It is often up to the primary care clinician to screen for, assess, and potentially diagnose DDH. Therefore, a thorough understanding of this condition can promote early detection and diagnosis, with less invasive treatment and a more favorable outcome.
A proper hip examination should be a standard component of all newborn and infant well-child examinations. If DDH is suspected, appropriate referral to a pediatric orthopedic surgeon must be made so that timely treatment can be initiated. Early use of the Pavlik harness is significantly easier than the invasive surgery and prolonged immobilization necessitated by a delayed diagnosis. Whatever the course of treatment required, it is important for clinicians to support the patient and family: training and anticipatory guidance are essential components of DDH management.
1. Karmazyn BK, Gunderman RB, Coley BD, et al; American College of Radiology. ACR appropriateness criteria on developmental dysplasia of the hip—child. J Am Coll Radiol. 2009;6(8):551-557.
2. American Academy of Pediatrics, Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4 pt 1):896-905.
3. Mencio GA. Developmental dysplasia of the hip. In: Sponseller PD, ed. Orthopaedic Knowledge Update: Pediatrics–2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2002:161-171.
4. Children’s Hospital at Westmead. Developmental dysplasia of the hip (DDH). www.chw.edu.au/parents/factsheets/developj.htm. Accessed March 26, 2010.
5. Graf R. Classification of hip joint dysplasia by means of sonography. Arch Orthop Trauma Surg. 1984; 102:248-255.
6. Weinstein SL. Developmental hip dysplasia and dislocation. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:905-956.
7. Aronsson DD, Goldberg MJ, Kling TF Jr, Roy DR. Developmental dysplasia of the hip. Pediatrics. 1994; 94(2 pt 1):201-208.
8. Guille JT, Pizzutillo PD, MacEwan GD. Developmental dysplasia of the hip from birth to six months. J Am Acad Orthop Surg. 2000;8(4):232-242.
9. Hart ES, Albright MB, Rebello GN, Grottkau BE. Developmental dysplasia of the hip: nursing implications and anticipatory guidelines for parents. Orthop Nurs. 2006;25(2):100-109.
10. Dogruel H, Atalar H, Yavus OY, Sayli U. Clinical examination versus ultrasonography in detecting developmental dysplasia of the hip. Int Orthop. 2008; 32(3):415-419.
11. Mahan ST, Katz JN, Kim YJ. To screen or not to screen? A decision analysis of the utility of screening for developmental dysplasia of the hip. J Bone Joint Surg Am. 2009;91(7);1705-1719.
12. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
13. Ortolani M. Congenital hip dysplasia in the light of early and very early diagnosis. Clin Orthop Relat Res. 1976;119(1):6-10.
14. Barlow TG. Congenital dislocation of the hip in the newborn. Proc R Soc Med. 1966;59(11 part 1):1103-1106.
15. Bond CD, Hennrikus WL, DellaMaggiore ED. Prospective evaluation of newborn soft-tissue “clicks” with ultrasound. J Pediatr Orthop. 1997;17(2):199-201.
16. Atalar H, Sayli U, Yavuz OY, et al. Indicators of successful use of the Pavlik harness in infants with developmental dysplasia of the hip. Int Orthop. 2007; 31(2):145-150.
17. Rampal V, Sabourin M, Erdeneshoo E, et al. Closed reduction with traction for developmental dysplasia of the hip in children aged between one and five years. J Bone Joint Surg Br. 2008;90-B(7):858-863.
18. Clarke NMP, Sakthivel K. The diagnosis and management of congenital dislocation of the hip. Paediatr Child Health. 2008;18(6):268-271.
19. Hassan FA. Compliance of parents with regard to Pavlik harness treatment in developmental dysplasia of the hip. J Pediatr Orthop. 2009;18(3):111-115.
A 5-day-old infant was referred to the pediatric orthopedic clinic for evaluation of a left hip “clunk.” She is a firstborn child, born at full term (39 weeks) via cesarean delivery secondary to breech presentation. Her weight at birth was 7 lb 6 oz. The infant was noted to have a left hip clunk during a routine physical examination by her pediatrician, who made a referral to the pediatric orthopedic clinic for possible hip dysplasia. This is the patient’s first visit to the clinic.
There is no family history of hip dysplasia or other orthopedic abnormalities. The infant is a well-appearing, alert female measuring 20.5” in length and weighing 7 lb 4 oz. Vital signs are stable with no abnormality detected. The heart is regular in rate and rhythm, and the chest is clear bilaterally.
No cutaneous abnormalities are noted. The patient is able to move all her extremities spontaneously, and her spine is straight and normal with no evidence of spinal dysraphism. Her feet are normal bilaterally, with full range of motion and no equinovarus or metatarsus adductus deformity.
The neurologic examination is also unremarkable, with normal neonatal reflexes and excellent muscle tone throughout.
Examination of the infant’s hips reveals a positive result on the Barlow test on the left side (the hip can be dislocated). There is also a positive Ortolani sign (the hip can be reduced), with asymmetric thigh skin folds noted (see Figures 1A and 1B, respectively).
Based on these positive physical examination findings, the patient was diagnosed with developmental dysplasia of the hip (DDH). Initial ultrasonography to confirm the diagnosis was not considered necessary, as the physical examination demonstrated obvious instability.1 The infant was placed in a Pavlik harness, which her parents were instructed should be worn full-time (see Figures 2A and 2B). She was scheduled for weekly follow-up visits for adjustments to the harness and serial hip examinations.
At the second follow-up visit, ultrasonography was performed, confirming the presence of dysplasia with decreased femoral head coverage and a steep socket (acetabulum). Use of the Pavlik harness was continued full-time for six weeks.
At age 6 weeks, the infant underwent a follow-up ultrasound to assess for improvement in the degree of dysplasia. The test revealed normal hips bilaterally with no evidence of DDH. Therefore, use of the Pavlik harness was discontinued. The parents were instructed to bring the child back in six months for a repeat clinical examination and an anteroposterior x-ray of the pelvis.1
Discussion
The term developmental dysplasia of the hip (DDH) has replaced the more traditional term congenital hip dislocation because DDH more accurately reflects the variable characteristics that can be seen with this condition. As DDH may not be present at birth, the term congenital is misleading. We now know that DDH may occur in utero, perinatally, or during infancy and childhood.2,3
Generally, DDH is used to describe an abnormal relationship between the femoral head and the acetabulum (see Figure 34). The term represents a wide spectrum of abnormality, as shown in the Graf classification of hips in infants: type I refers to a normal hip; type II, immature development to mild dysplasia; type III, subluxation of the femoral head; and type 4, frank dislocation with severe instability.5
Diagnosing and managing DDH correctly requires the clinician to have a thorough understanding of the normal growth and development that occurs in the hip joint. Embryologically, the joint (including the femoral head and acetabulum) develops from the same primitive mesenchymal cells.6 By 11 to 12 weeks’ gestation, the initial structures of the hip joint are fully formed; theoretically, this is the earliest time at which a dislocation can occur.2,7 DDH that develops at this stage would be called teratologic; this condition is seen most frequently in patients who have underlying neuromuscular conditions, such as myelodysplasia (spina bifida) or arthrogryposis. A typical dislocation takes place during the perinatal period in an infant who is otherwise healthy.2
Etiology
DDH occurs in about 11 of every 1,000 infants, with frank dislocations occurring in one to two infants per 10,000.8 The left hip is involved in approximately 60% of cases, the right in 20%, and both hips in about 20%. In the most common intrauterine fetal position, the left hip is lower than the right (usually abutting the mother’s sacrum) and is often in adduction. This is likely the reason that the left hip is more commonly affected by DDH.
DDH is believed to be multifactorial, with physiologic, genetic, and mechanical factors implicated in the etiology.3 The incidence of DDH varies with factors such as the patient’s age, race, and gender, the experience and training of the examiner, and the diagnostic criteria that are used.
Known risk factors for a positive newborn screening are shown in the table.9,10 It is often helpful for clinicians to remember the “4F” mnemonic associated with DDH: female, firstborn, foot first, and family history.9
There is also an increased risk for DDH in patients with other conditions that are associated with intrauterine crowding. These include congenital muscular torticollis, metatarsus adductus, and congenital dislocation of the knee.2
Physical Examination
All newborn infants should be screened for DDH as part of the initial physical examination, with ultrasonography recommended for infants deemed at high risk for DDH and for those with inconclusive results on examination.1,10,11 Providers should be aware that the newborn hip examination requires a considerable amount of practice and expertise.
A thorough medical history should always be obtained first, including gestational age, presentation (breech vs vertex), type of delivery (cesarean vs vaginal), gender, birth order, family history of DDH, ligamentous laxity, or myopathy.8
The examining clinician begins by placing the infant on a firm, flat surface. The infant should be as relaxed as possible. Next, the clinician observes both lower extremities for asymmetric thigh or buttock skin folds. Bilateral DDH can be very difficult to diagnose on the basis of this examination due to the lack of asymmetry (hips will have symmetric abnormality).
The Galeazzi sign is elicited by placing the infant supine with the hips and knees flexed to 90°.12 With the hips in neutral abduction, the provider should determine whether the knees are at the same height. Unequal knee heights—a positive result for the Galeazzi sign—suggest femoral shortening (apparent leg length discrepancy), which may be explained by a hip dislocation. If both hips are dislocated, a false-negative result will often occur, since both will appear short and there will be no discrepancy.2,12
Among physical examination techniques, the Ortolani and Barlow maneuvers are considered most reliable to detect hip instability in newborns and infants younger than 6 months2,13,14 (review Figures 1A and 1B). The Ortolani test is used to detect the sensation of the dislocated hip reducing into the acetabulum, and the Barlow test elicits the unstable hip dislocating.2 A palpable and occasionally audible clunk is considered a positive result on the Barlow test and usually indicates a diagnosis of DDH.14 High-pitched clicks or snaps frequently occur with hip range-of-motion maneuvers and during Ortolani and Barlow testing. These sounds are often attributed to snapping of the iliotibial band over the greater trochanter and do not usually signify dysplasia.15
Because DDH is a dynamic and evolving process, the physical findings on clinical examination change significantly, depending on the age of the infant or child. As an infant approaches age 3 months, limited hip abduction (especially when asymmetric) is often the most reliable physical examination finding in patients with DDH.12 After age 3 to 4 months, Ortolani and Barlow testing will often produce negative results as progressive soft tissue contractures evolve.
Once a child begins to walk, gait abnormalities (eg, a short-limbed or waddling gait pattern) may raise suspicion for a diagnosis of DDH.7 It has been recommended that evaluation for DDH be performed at each routine office examination until the child is 12 months of age.1
Treatment
The Pavlik harness is considered first-line treatment for DDH in infants younger than 6 months. The harness is a dynamic splint that allows the infant to engage in a sphere of active motion that encourages stabilization and deepening of the socket. The harness is applied with the knees flexed to about 90° and the hips in about 70° of abduction and 100° to 110° of flexion (as shown in Figures 2A and 2B).9
The duration of treatment depends on the infant’s age at presentation and the severity of DDH. Progress is judged by serial examinations and dynamic ultrasounds. The harness is worn full-time until clinical and radiographic examinations both yield normal results. After six weeks of treatment, the hips are examined out of the harness, and a repeat ultrasound is usually obtained. If findings are normal, use of the harness is ordinarily discontinued. Some patients will require harness use for a longer period in cases of delayed development of the acetabulum and/or severe laxity of the ligaments.9
The Pavlik harness is successful more than 90% of the time in newborns with DDH.8 Success rates have been reported as greatest in infants younger than 8 weeks at the time of treatment initiation, those with only one affected hip, and those with less severe disease (Graf types II or III).16
If ultrasonography shows no improvement after two to three weeks, it is usually recommended that the harness be discontinued; most orthopedic surgeons will then proceed with a closed or open reduction and spica body casting. Similarly, when the diagnosis of DDH is delayed until after ages 6 to 8 months, a closed reduction under anesthesia and placement of a spica body cast is usually the recommended treatment to maintain the hip in the reduced position.17,18 Some older children (ages 1 to 5 years) may require bracing, traction, open reduction, and/or femoral or pelvic osteotomy.17,18 It is believed that undiagnosed, untreated DDH can lead to early-onset degenerative hip disease (arthritis).1
Patient/Family Education
The Pavlik harness is most effective when a consistent support system exists to educate parents about the importance of the harness, its care and maintenance, and the consequences of failure. Close monitoring of the infant’s progress is also essential to promoting adherence. Application and removal of the harness should be demonstrated to the parent or caregiver, as well as diapering, dressing, and undressing the infant; they should then be encouraged to practice immediately in the clinic or office.
During visits for harness adjustment, the strap position should be marked with indelible ink, allowing parents to reapply the device correctly, should removal be required (eg, for bathing).9 Ten percent of parents reportedly find reapplying the harness difficult during the first weeks of use. Difficulty in dressing and carrying an infant in a harness, feet slipping out of the harness, and skin irritation have been reported by about one-third of parents.19
Treatment adherence and subsequent success with the Pavlik harness is reported greatest (95%) in patients whose parents engage in demonstrations of harness use and follow instructions precisely.19 By providing a contact name and office number and following up with a phone call a few days after the harness is first applied, clinicians can significantly decrease parents’ anxiety and increase overall compliance.9
Conclusion
Despite recent increased awareness of DDH and the importance of thorough screening programs, hip dysplasia continues to be a frequently missed diagnosis in pediatrics. It is often up to the primary care clinician to screen for, assess, and potentially diagnose DDH. Therefore, a thorough understanding of this condition can promote early detection and diagnosis, with less invasive treatment and a more favorable outcome.
A proper hip examination should be a standard component of all newborn and infant well-child examinations. If DDH is suspected, appropriate referral to a pediatric orthopedic surgeon must be made so that timely treatment can be initiated. Early use of the Pavlik harness is significantly easier than the invasive surgery and prolonged immobilization necessitated by a delayed diagnosis. Whatever the course of treatment required, it is important for clinicians to support the patient and family: training and anticipatory guidance are essential components of DDH management.
A 5-day-old infant was referred to the pediatric orthopedic clinic for evaluation of a left hip “clunk.” She is a firstborn child, born at full term (39 weeks) via cesarean delivery secondary to breech presentation. Her weight at birth was 7 lb 6 oz. The infant was noted to have a left hip clunk during a routine physical examination by her pediatrician, who made a referral to the pediatric orthopedic clinic for possible hip dysplasia. This is the patient’s first visit to the clinic.
There is no family history of hip dysplasia or other orthopedic abnormalities. The infant is a well-appearing, alert female measuring 20.5” in length and weighing 7 lb 4 oz. Vital signs are stable with no abnormality detected. The heart is regular in rate and rhythm, and the chest is clear bilaterally.
No cutaneous abnormalities are noted. The patient is able to move all her extremities spontaneously, and her spine is straight and normal with no evidence of spinal dysraphism. Her feet are normal bilaterally, with full range of motion and no equinovarus or metatarsus adductus deformity.
The neurologic examination is also unremarkable, with normal neonatal reflexes and excellent muscle tone throughout.
Examination of the infant’s hips reveals a positive result on the Barlow test on the left side (the hip can be dislocated). There is also a positive Ortolani sign (the hip can be reduced), with asymmetric thigh skin folds noted (see Figures 1A and 1B, respectively).
Based on these positive physical examination findings, the patient was diagnosed with developmental dysplasia of the hip (DDH). Initial ultrasonography to confirm the diagnosis was not considered necessary, as the physical examination demonstrated obvious instability.1 The infant was placed in a Pavlik harness, which her parents were instructed should be worn full-time (see Figures 2A and 2B). She was scheduled for weekly follow-up visits for adjustments to the harness and serial hip examinations.
At the second follow-up visit, ultrasonography was performed, confirming the presence of dysplasia with decreased femoral head coverage and a steep socket (acetabulum). Use of the Pavlik harness was continued full-time for six weeks.
At age 6 weeks, the infant underwent a follow-up ultrasound to assess for improvement in the degree of dysplasia. The test revealed normal hips bilaterally with no evidence of DDH. Therefore, use of the Pavlik harness was discontinued. The parents were instructed to bring the child back in six months for a repeat clinical examination and an anteroposterior x-ray of the pelvis.1
Discussion
The term developmental dysplasia of the hip (DDH) has replaced the more traditional term congenital hip dislocation because DDH more accurately reflects the variable characteristics that can be seen with this condition. As DDH may not be present at birth, the term congenital is misleading. We now know that DDH may occur in utero, perinatally, or during infancy and childhood.2,3
Generally, DDH is used to describe an abnormal relationship between the femoral head and the acetabulum (see Figure 34). The term represents a wide spectrum of abnormality, as shown in the Graf classification of hips in infants: type I refers to a normal hip; type II, immature development to mild dysplasia; type III, subluxation of the femoral head; and type 4, frank dislocation with severe instability.5
Diagnosing and managing DDH correctly requires the clinician to have a thorough understanding of the normal growth and development that occurs in the hip joint. Embryologically, the joint (including the femoral head and acetabulum) develops from the same primitive mesenchymal cells.6 By 11 to 12 weeks’ gestation, the initial structures of the hip joint are fully formed; theoretically, this is the earliest time at which a dislocation can occur.2,7 DDH that develops at this stage would be called teratologic; this condition is seen most frequently in patients who have underlying neuromuscular conditions, such as myelodysplasia (spina bifida) or arthrogryposis. A typical dislocation takes place during the perinatal period in an infant who is otherwise healthy.2
Etiology
DDH occurs in about 11 of every 1,000 infants, with frank dislocations occurring in one to two infants per 10,000.8 The left hip is involved in approximately 60% of cases, the right in 20%, and both hips in about 20%. In the most common intrauterine fetal position, the left hip is lower than the right (usually abutting the mother’s sacrum) and is often in adduction. This is likely the reason that the left hip is more commonly affected by DDH.
DDH is believed to be multifactorial, with physiologic, genetic, and mechanical factors implicated in the etiology.3 The incidence of DDH varies with factors such as the patient’s age, race, and gender, the experience and training of the examiner, and the diagnostic criteria that are used.
Known risk factors for a positive newborn screening are shown in the table.9,10 It is often helpful for clinicians to remember the “4F” mnemonic associated with DDH: female, firstborn, foot first, and family history.9
There is also an increased risk for DDH in patients with other conditions that are associated with intrauterine crowding. These include congenital muscular torticollis, metatarsus adductus, and congenital dislocation of the knee.2
Physical Examination
All newborn infants should be screened for DDH as part of the initial physical examination, with ultrasonography recommended for infants deemed at high risk for DDH and for those with inconclusive results on examination.1,10,11 Providers should be aware that the newborn hip examination requires a considerable amount of practice and expertise.
A thorough medical history should always be obtained first, including gestational age, presentation (breech vs vertex), type of delivery (cesarean vs vaginal), gender, birth order, family history of DDH, ligamentous laxity, or myopathy.8
The examining clinician begins by placing the infant on a firm, flat surface. The infant should be as relaxed as possible. Next, the clinician observes both lower extremities for asymmetric thigh or buttock skin folds. Bilateral DDH can be very difficult to diagnose on the basis of this examination due to the lack of asymmetry (hips will have symmetric abnormality).
The Galeazzi sign is elicited by placing the infant supine with the hips and knees flexed to 90°.12 With the hips in neutral abduction, the provider should determine whether the knees are at the same height. Unequal knee heights—a positive result for the Galeazzi sign—suggest femoral shortening (apparent leg length discrepancy), which may be explained by a hip dislocation. If both hips are dislocated, a false-negative result will often occur, since both will appear short and there will be no discrepancy.2,12
Among physical examination techniques, the Ortolani and Barlow maneuvers are considered most reliable to detect hip instability in newborns and infants younger than 6 months2,13,14 (review Figures 1A and 1B). The Ortolani test is used to detect the sensation of the dislocated hip reducing into the acetabulum, and the Barlow test elicits the unstable hip dislocating.2 A palpable and occasionally audible clunk is considered a positive result on the Barlow test and usually indicates a diagnosis of DDH.14 High-pitched clicks or snaps frequently occur with hip range-of-motion maneuvers and during Ortolani and Barlow testing. These sounds are often attributed to snapping of the iliotibial band over the greater trochanter and do not usually signify dysplasia.15
Because DDH is a dynamic and evolving process, the physical findings on clinical examination change significantly, depending on the age of the infant or child. As an infant approaches age 3 months, limited hip abduction (especially when asymmetric) is often the most reliable physical examination finding in patients with DDH.12 After age 3 to 4 months, Ortolani and Barlow testing will often produce negative results as progressive soft tissue contractures evolve.
Once a child begins to walk, gait abnormalities (eg, a short-limbed or waddling gait pattern) may raise suspicion for a diagnosis of DDH.7 It has been recommended that evaluation for DDH be performed at each routine office examination until the child is 12 months of age.1
Treatment
The Pavlik harness is considered first-line treatment for DDH in infants younger than 6 months. The harness is a dynamic splint that allows the infant to engage in a sphere of active motion that encourages stabilization and deepening of the socket. The harness is applied with the knees flexed to about 90° and the hips in about 70° of abduction and 100° to 110° of flexion (as shown in Figures 2A and 2B).9
The duration of treatment depends on the infant’s age at presentation and the severity of DDH. Progress is judged by serial examinations and dynamic ultrasounds. The harness is worn full-time until clinical and radiographic examinations both yield normal results. After six weeks of treatment, the hips are examined out of the harness, and a repeat ultrasound is usually obtained. If findings are normal, use of the harness is ordinarily discontinued. Some patients will require harness use for a longer period in cases of delayed development of the acetabulum and/or severe laxity of the ligaments.9
The Pavlik harness is successful more than 90% of the time in newborns with DDH.8 Success rates have been reported as greatest in infants younger than 8 weeks at the time of treatment initiation, those with only one affected hip, and those with less severe disease (Graf types II or III).16
If ultrasonography shows no improvement after two to three weeks, it is usually recommended that the harness be discontinued; most orthopedic surgeons will then proceed with a closed or open reduction and spica body casting. Similarly, when the diagnosis of DDH is delayed until after ages 6 to 8 months, a closed reduction under anesthesia and placement of a spica body cast is usually the recommended treatment to maintain the hip in the reduced position.17,18 Some older children (ages 1 to 5 years) may require bracing, traction, open reduction, and/or femoral or pelvic osteotomy.17,18 It is believed that undiagnosed, untreated DDH can lead to early-onset degenerative hip disease (arthritis).1
Patient/Family Education
The Pavlik harness is most effective when a consistent support system exists to educate parents about the importance of the harness, its care and maintenance, and the consequences of failure. Close monitoring of the infant’s progress is also essential to promoting adherence. Application and removal of the harness should be demonstrated to the parent or caregiver, as well as diapering, dressing, and undressing the infant; they should then be encouraged to practice immediately in the clinic or office.
During visits for harness adjustment, the strap position should be marked with indelible ink, allowing parents to reapply the device correctly, should removal be required (eg, for bathing).9 Ten percent of parents reportedly find reapplying the harness difficult during the first weeks of use. Difficulty in dressing and carrying an infant in a harness, feet slipping out of the harness, and skin irritation have been reported by about one-third of parents.19
Treatment adherence and subsequent success with the Pavlik harness is reported greatest (95%) in patients whose parents engage in demonstrations of harness use and follow instructions precisely.19 By providing a contact name and office number and following up with a phone call a few days after the harness is first applied, clinicians can significantly decrease parents’ anxiety and increase overall compliance.9
Conclusion
Despite recent increased awareness of DDH and the importance of thorough screening programs, hip dysplasia continues to be a frequently missed diagnosis in pediatrics. It is often up to the primary care clinician to screen for, assess, and potentially diagnose DDH. Therefore, a thorough understanding of this condition can promote early detection and diagnosis, with less invasive treatment and a more favorable outcome.
A proper hip examination should be a standard component of all newborn and infant well-child examinations. If DDH is suspected, appropriate referral to a pediatric orthopedic surgeon must be made so that timely treatment can be initiated. Early use of the Pavlik harness is significantly easier than the invasive surgery and prolonged immobilization necessitated by a delayed diagnosis. Whatever the course of treatment required, it is important for clinicians to support the patient and family: training and anticipatory guidance are essential components of DDH management.
1. Karmazyn BK, Gunderman RB, Coley BD, et al; American College of Radiology. ACR appropriateness criteria on developmental dysplasia of the hip—child. J Am Coll Radiol. 2009;6(8):551-557.
2. American Academy of Pediatrics, Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4 pt 1):896-905.
3. Mencio GA. Developmental dysplasia of the hip. In: Sponseller PD, ed. Orthopaedic Knowledge Update: Pediatrics–2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2002:161-171.
4. Children’s Hospital at Westmead. Developmental dysplasia of the hip (DDH). www.chw.edu.au/parents/factsheets/developj.htm. Accessed March 26, 2010.
5. Graf R. Classification of hip joint dysplasia by means of sonography. Arch Orthop Trauma Surg. 1984; 102:248-255.
6. Weinstein SL. Developmental hip dysplasia and dislocation. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:905-956.
7. Aronsson DD, Goldberg MJ, Kling TF Jr, Roy DR. Developmental dysplasia of the hip. Pediatrics. 1994; 94(2 pt 1):201-208.
8. Guille JT, Pizzutillo PD, MacEwan GD. Developmental dysplasia of the hip from birth to six months. J Am Acad Orthop Surg. 2000;8(4):232-242.
9. Hart ES, Albright MB, Rebello GN, Grottkau BE. Developmental dysplasia of the hip: nursing implications and anticipatory guidelines for parents. Orthop Nurs. 2006;25(2):100-109.
10. Dogruel H, Atalar H, Yavus OY, Sayli U. Clinical examination versus ultrasonography in detecting developmental dysplasia of the hip. Int Orthop. 2008; 32(3):415-419.
11. Mahan ST, Katz JN, Kim YJ. To screen or not to screen? A decision analysis of the utility of screening for developmental dysplasia of the hip. J Bone Joint Surg Am. 2009;91(7);1705-1719.
12. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
13. Ortolani M. Congenital hip dysplasia in the light of early and very early diagnosis. Clin Orthop Relat Res. 1976;119(1):6-10.
14. Barlow TG. Congenital dislocation of the hip in the newborn. Proc R Soc Med. 1966;59(11 part 1):1103-1106.
15. Bond CD, Hennrikus WL, DellaMaggiore ED. Prospective evaluation of newborn soft-tissue “clicks” with ultrasound. J Pediatr Orthop. 1997;17(2):199-201.
16. Atalar H, Sayli U, Yavuz OY, et al. Indicators of successful use of the Pavlik harness in infants with developmental dysplasia of the hip. Int Orthop. 2007; 31(2):145-150.
17. Rampal V, Sabourin M, Erdeneshoo E, et al. Closed reduction with traction for developmental dysplasia of the hip in children aged between one and five years. J Bone Joint Surg Br. 2008;90-B(7):858-863.
18. Clarke NMP, Sakthivel K. The diagnosis and management of congenital dislocation of the hip. Paediatr Child Health. 2008;18(6):268-271.
19. Hassan FA. Compliance of parents with regard to Pavlik harness treatment in developmental dysplasia of the hip. J Pediatr Orthop. 2009;18(3):111-115.
1. Karmazyn BK, Gunderman RB, Coley BD, et al; American College of Radiology. ACR appropriateness criteria on developmental dysplasia of the hip—child. J Am Coll Radiol. 2009;6(8):551-557.
2. American Academy of Pediatrics, Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4 pt 1):896-905.
3. Mencio GA. Developmental dysplasia of the hip. In: Sponseller PD, ed. Orthopaedic Knowledge Update: Pediatrics–2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2002:161-171.
4. Children’s Hospital at Westmead. Developmental dysplasia of the hip (DDH). www.chw.edu.au/parents/factsheets/developj.htm. Accessed March 26, 2010.
5. Graf R. Classification of hip joint dysplasia by means of sonography. Arch Orthop Trauma Surg. 1984; 102:248-255.
6. Weinstein SL. Developmental hip dysplasia and dislocation. In: Morrissy RT, Weinstein SL, eds. Lovell and Winter’s Pediatric Orthopaedics. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:905-956.
7. Aronsson DD, Goldberg MJ, Kling TF Jr, Roy DR. Developmental dysplasia of the hip. Pediatrics. 1994; 94(2 pt 1):201-208.
8. Guille JT, Pizzutillo PD, MacEwan GD. Developmental dysplasia of the hip from birth to six months. J Am Acad Orthop Surg. 2000;8(4):232-242.
9. Hart ES, Albright MB, Rebello GN, Grottkau BE. Developmental dysplasia of the hip: nursing implications and anticipatory guidelines for parents. Orthop Nurs. 2006;25(2):100-109.
10. Dogruel H, Atalar H, Yavus OY, Sayli U. Clinical examination versus ultrasonography in detecting developmental dysplasia of the hip. Int Orthop. 2008; 32(3):415-419.
11. Mahan ST, Katz JN, Kim YJ. To screen or not to screen? A decision analysis of the utility of screening for developmental dysplasia of the hip. J Bone Joint Surg Am. 2009;91(7);1705-1719.
12. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
13. Ortolani M. Congenital hip dysplasia in the light of early and very early diagnosis. Clin Orthop Relat Res. 1976;119(1):6-10.
14. Barlow TG. Congenital dislocation of the hip in the newborn. Proc R Soc Med. 1966;59(11 part 1):1103-1106.
15. Bond CD, Hennrikus WL, DellaMaggiore ED. Prospective evaluation of newborn soft-tissue “clicks” with ultrasound. J Pediatr Orthop. 1997;17(2):199-201.
16. Atalar H, Sayli U, Yavuz OY, et al. Indicators of successful use of the Pavlik harness in infants with developmental dysplasia of the hip. Int Orthop. 2007; 31(2):145-150.
17. Rampal V, Sabourin M, Erdeneshoo E, et al. Closed reduction with traction for developmental dysplasia of the hip in children aged between one and five years. J Bone Joint Surg Br. 2008;90-B(7):858-863.
18. Clarke NMP, Sakthivel K. The diagnosis and management of congenital dislocation of the hip. Paediatr Child Health. 2008;18(6):268-271.
19. Hassan FA. Compliance of parents with regard to Pavlik harness treatment in developmental dysplasia of the hip. J Pediatr Orthop. 2009;18(3):111-115.
Howel-Evans Syndrome: A Variant of Ectodermal Dysplasia
Botanical Briefs: Poisonwood (Metopium toxiferum)
How to treat PTSD in patients with comorbid mood disorders
Major depressive disorder (MDD) and bipolar spectrum disorders are associated with some symptoms of—and fully defined—posttraumatic stress disorder (PTSD). Many traumatic experiences can lead to this comorbidity, the most common being exposure to or witnessing combat for men and rape and sexual molestation for women.1
Trauma has major prognostic and treatment implications for affectively ill patients, including those whose symptoms do not meet PTSD’s full diagnostic criteria. This article aims to help clinicians by:
- presenting evidence characterizing the overlap between affective disorders and PTSD
- reviewing evidence that the bipolar spectrum may be broader than generally thought, an insight that affects PTSD treatment
- making a case for routine PTSD screening for all patients with affective illnesses
- recommending PTSD treatments tailored to the patient’s comorbid affective disorder.
Overlap of trauma and affective illness
PTSD is remarkably comorbid with mood disorders. Americans with MDD and bipolar disorder (BPD) are 7 and 9.4 times, respectively, more likely to meet criteria for PTSD than persons in the general population, according to odds ratios Kessler et al2 calculated from the National Comorbidity Survey database.
I have never seen a patient with PTSD who did not also meet criteria for an affective disorder. The concurrence of PTSD and MDD is not the product of overlapping diagnostic criteria. Rather, evidence indicates these are distinct diagnostic entities.3 A review of diagnostic criteria for PTSD and hypomania/mania leads to the same conclusion.
Bipolar spectrum disorders
DSM-IV-TR assumes that mood disorders fall neatly into boxes. Other data (Table 1)4–8 indicate that these disorders fall along a continuum or—more conservatively—that the scope of bipolarity is much wider than DSM-IV-TR recognizes. This is a controversial topic, and the individual clinician’s position could impact how one manages PTSD patients.
Table 1
Evidence of bipolar spectrum features in major depressive episodes
| Study | Design | Conclusion |
|---|---|---|
| Akiskal and Mallya, 19874 | 200 community mental health clinic patients diagnosed as having MDD | 50% could be classified as having a bipolar disorder |
| Benazzi, 19975 | 203 consecutively presenting patients with depression | 45% met criteria for bipolar II disorder |
| Akiskal and Benazzi, 20056 | 563 consecutive patients presenting with a DSM-IV-diagnosed MDE | 58% showed features of bipolar II disorder |
| Akiskal et al, 20067 | 493 patients in a French national study presenting with MDE | 65% were determined to fall along the ‘bipolar spectrum’ |
| Rabakowski et al, 20058 | 880 Polish outpatients presenting with MDE | 40% met criteria for bipolar disorder |
| MDD: major depressive disorder; MDE: major depressive episode | ||
In this article, I include bipolar I disorder, bipolar II disorder, and mixed depression within the “bipolar spectrum disorders.” If one accepts this—and I do—it follows that 50% to 70% of all major depressive episodes (MDEs) are bipolar in nature.4–9 Depending on your practice setting, you may see a higher or lower base rate of bipolar spectrum disorders.
Mixed depression is not recognized in DSM-IV-TR, and the purpose of this article is not to defend its inclusion as a bipolar spectrum phenomenon. A proposed definition of mixed depression9 requires the presence of an MDE contaminated by ≥3 features of hypomania or mania, without euphoria or inflated self-esteem/grandiosity (Table 2).10
Some experts believe episodes of hypomania and mania frequently occur in the illness course of persons with mixed depression; indeed, mixed depression is a predictor of a bipolar course. It is observed in outpatient9 and inpatient settings.11 Common forms of mixed depression feature combinations of irritability, psychomotor agitation (mild to severe), increased talkativeness (which may fall short of frank pressured speech), racing or “crowded” thoughts (or “mental overactivity”), and distractibility. Other than increased self-esteem/grandiosity, any symptoms within DSM-IV-TR criterion B for a hypomanic or manic episode may be seen in mixed depression. Psychosis is an exclusion criterion for mixed depression.
Mixed depression responds poorly to antidepressant monotherapy. Validation studies suggest that mixed depression is a bipolar variant, as determined by its capacity to predict a bipolar course and its association with a family history of bipolar disorder and age of onset.9
Table 2
Diagnostic characteristics of a hypomanic episode, DSM-IV-TR criteria A and B
| A. A distinct period of persistently elevated, expansive, or irritable mood, lasting throughout at least 4 days, that is clearly different from the usual nondepressed mood. |
| B. During the period of mood disturbance, 3 or more of the following symptoms have persisted (4 if the mood is only irritable) and have been present to a significant degree: 1) inflated self-esteem or grandiosity 2) decreased need for sleep (eg, feels rested after only 3 hours of sleep) 3) more talkative than usual or pressure to keep talking 4) flight of ideas or subjective experience that thoughts are racing 5) distractibility (ie, attention too easily drawn to unimportant or irrelevant external stimuli) 6) increase in goal-directed activity (either socially, at work or school, or sexually) or psychomotor agitation 7) excessive involvement in pleasurable activities that have a high potential for painful consequences (eg, the person engages in unrestrained buying sprees, sexual indiscretions, or foolish business investments). |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
PTSD risk in affective illness
An adolescent sample. A preliminary cross-sectional study conducted by our group indicates that adolescents with affective disorders may have a much higher risk of developing PTSD than psychiatric comparison subjects.12 We used modules from the Structured Clinical Interview for DSM-IV (SCID) to screen for intra-episode psychopathology (as opposed to lifetime prevalence of disorders) in 79 adolescents with MDD, 34 with BPD as defined in the DSM-IV-TR, and 26 with neither affective disorder (psychiatric controls). We found:
- 38.2% of subjects with BPD met criteria for PTSD, compared with 13.9% of those with MDD (OR 4.9; P =.001)
- 3.8% of adolescents without a mood disorder met criteria for PTSD.
We also found that comorbid PTSD was associated with a 4.5-fold higher risk of a suicide attempt, even after we controlled for BPD diagnosis. When we controlled for the presence of other concurrent anxiety disorders, the likelihood of an adolescent with PTSD having attempted suicide remained significant (OR 3.4; P=.023). This finding suggests that PTSD is an independent risk factor for a suicide attempt.
An adult sample. We then focused on adults meeting criteria for MDD or BPD. In a study of 187 consecutively presenting affectively ill patients, we used the SCID to screen for multiple anxiety disorders including PTSD.13 Lifetime—as opposed to intra-episode—PTSD prevalence was 23.8% among the 118 patients with MDD and 62.3% among the 69 patients with BPD. A patient with BPD was 5 times more likely to have PTSD than a patient with MDD (OR 5.3; P < .0001). The most common cause of trauma leading to PTSD was sexual molestation or rape as a child or adolescent in this predominantly female Latino population.
Populations at risk for PTSD
The prevalence of PTSD in clinical samples varies, depending on the population studied. For instance, women are at much higher risk for developing PTSD than men, even in comparisons where men are exposed to a greater number of traumatic events and analyses control for differences in the prevalence of sexual abuse. The gender difference is greater if the trauma occurs during childhood.14 Essentially all patients in our adolescent and adult studies developed PTSD in response to childhood or adolescent sexual trauma.12,13
A population exposed to a high rate of violent crime would be expected to show a higher PTSD prevalence than one exposed to substantially less violence. The base rate of PTSD also is much higher in affectively ill patients than in the general population.
An analysis by Otto et al15 found a 16% lifetime prevalence of concomitant PTSD in 1,214 persons with BPD (not the manifold forms within the bipolar spectrum). Oquendo et al16 reported a 25.7% lifetime prevalence of PTSD in 230 patients with a history of MDD. Other epidemiologic2 and clinical studies12,13 suggest a considerably higher base rate of PTSD among persons with bipolar disorders than those with MDD.
The method of ascertaining the presence of this disorder may be another variable affecting the reported PTSD prevalence. Persistent avoidance—including “efforts to avoid thoughts, feelings, or conversations associated with the trauma”—is a diagnostic feature of PTSD.10 Researchers and clinicians who do not intentionally screen patients for PTSD are not likely to detect it. Determining the true prevalence of PTSD requires empathic inquiry about exposure to traumatic events.
PTSD screening
Humans are remarkably resilient, and most persons exposed to major trauma are thought not to develop PTSD. However, in my experience, because PTSD appears to be common among persons with affective illness, determining whether such patients have been traumatized is important for prognosis and treatment selection.
To get started, you could create a 1-page form to record traumatic events and identify features of PTSD according to DSM-IV-TR criteria (Checklist).10 PTSD screening without a form can become second nature with practice; an experienced clinician can screen a traumatized patient for the disorder within 3 to 5 minutes.
When screening for a history of trauma, ask patients in a straightforward manner if they have:
- been victims of violent crimes
- witnessed violent crimes
- been exposed to events in which people could have suffered grave injury
- experienced emotional, physical, or sexual abuse.
A person who has experienced emotional abuse but not physical or sexual abuse cannot meet DSM-IV-TR criterion A and therefore does not meet full criteria for PTSD. Many emotionally abused persons meet criteria B through F, however, and they are most reasonably managed similarly to persons who also meet criterion A. When formulating a treatment plan, I recommend using clinical judgment rather than rigid adherence to DSM-IV-TR.
Checklist
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
| Criterion A. The person has been exposed to a traumatic event in which both of the following have been present: | |
| □ | 1. The person has experienced, witnessed, or been confronted with an event or events that involve actual or threatened death or serious injury, or a threat to the physical integrity of oneself or others |
| □ | 2. The person’s response involved intense fear, helplessness, or horror |
| Criterion B. The traumatic event is persistently re-experienced in at least 1 of the following ways: | |
| □ | 1. Recurrent and intrusive distressing recollections of the event, including images, thoughts, or perceptions |
| □ | 2. Recurrent distressing dreams of the event |
| □ | 3. Acting or feeling as if the traumatic event were recurring (includes a sense of reliving the experience, illusions, hallucinations, and dissociative flashback episodes, including those that occur upon awakening or when intoxicated) |
| □ | 4. Intense psychological distress at exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event |
| □ | 5. Physiologic reactivity upon exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event |
| Criterion C. Persistent avoidance of stimuli associated with the trauma and numbing of general responsiveness (not present before the trauma), as indicated by at least 3 of the following: | |
| □ | 1. Efforts to avoid thoughts, feelings, or conversations associated with the trauma |
| □ | 2. Efforts to avoid activities, places, or people that arouse recollections of the trauma |
| □ | 3. Inability to recall an important aspect of the trauma |
| □ | 4. Markedly diminished interest or participation in significant activities |
| □ | 5. Feeling of detachment or estrangement from others |
| □ | 6. Restricted range of affect |
| □ | 7. Sense of foreshortened future |
| Criterion D. Persistent symptoms of increasing arousal (not present before the trauma), indicated by at least 2 of the following: | |
| □ | 1. Difficulty falling or staying asleep |
| □ | 2. Irritability or outbursts of anger |
| □ | 3. Difficulty concentrating |
| □ | 4. Hypervigilance |
| □ | 5. Exaggerated startle response |
| □ | Criterion E. Duration of disturbance (symptoms in B, C, and D) is >1 month |
| □ | Criterion F. Disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Source: Adapted from Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
Treating PTSD in depression
Pharmacotherapy and psychotherapeutic interventions are important to PTSD patients’ recovery. Limited resources often prevent these patients from receiving expert psychotherapeutic intervention, however, leaving pharmacotherapy as the mainstay of treatment. This is unfortunate, because psychological interventions may be sufficient and preferred in some instances (Box).17–20
Pharmacotherapy for comorbid MDD. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine are first-line interventions for PTSD in depressed patients who do not meet criteria for a bipolar spectrum disorder. Placebo-controlled studies suggest that sertraline,21,22 fluoxetine,23 and paroxetine,24 are effective; doses higher than those used to treat depression may be required. Extended-release venlafaxine25 in dosages similar to those needed for depressive disorders also can be effective. Bupropion does not appear to be beneficial in treating PTSD.
The monoamine oxidase inhibitor phenelzine was long used successfully in treating PTSD but for the most part has been replaced by SSRIs. Because of its associated dietary restrictions, risk of hypertensive crises, and other side effects, phenelzine probably is best reserved for patients who do not respond to treatment with SSRIs or venlafaxine.
Pharmacotherapy for comorbid bipolar spectrum. If one accepts that most patients meeting criteria for MDE have a bipolar spectrum disorder, then most affectively ill patients with PTSD would need to be treated as if they have bipolar disorder. Oddly enough, this creates difficulties for the use of not only antidepressants and benzodiazepines, but also mood stabilizers:
- Patients with BPD and comorbid anxiety disorders, including PTSD, may be resistant to mood stabilizers.26,27
- Antidepressants can precipitate hypomanic or manic switches or onset of mixed hypomania, a mixed state, or rapid cycling in patients with a bipolar spectrum disorder.28–30
- Benzodiazepines do not appear to relieve acute or chronic PTSD-related distress, and discontinuation could cause rebound symptoms.31
Because no outcome studies have addressed PTSD management in patients with bipolar spectrum disorders, clinicians must rely on their judgment when formulating treatment plans. One strategy is to treat patients with mood stabilizers, then leave well enough alone if both the mood and anxiety symptoms remit (which is possible but unlikely in my experience). I often start treatment for the bipolar spectrum disorder and co-existing PTSD using mood stabilizers (including atypical antipsychotics) and prazosin, an α-1antagonist originally used for treating hypertension.
Prazosin can help diminish nightmares, dreams, and other painful recollections of trauma.32,33 The drug does not affect time to sleep onset. It also has been reported to reduce avoidance behavior and hyperarousal, such as irritability and anger.34 This has been my experience.
Cognitive-behavioral therapy (CBT) involving prolonged exposure (PE) to trauma-related stimuli has been shown to be effective for posttraumatic stress disorder (PTSD) in controlled studies.17,18 PE is an individual CBT designed to help patients process traumatic events and reduce psychological distress. It involves education about reactions to trauma, relaxation techniques, imaginal reliving of the trauma, exposure to cues associated with the trauma, and cognitive restructuring.
Administering D-cycloserine before behavioral treatment sessions facilitates fear extinction, and its use to enhance prolonged PE constitutes state-of-the-art treatment.19 Eye movement desensitization and reprocessing also may be effective.18,20
PE is a reasonable first-line treatment for PTSD patients with comorbid bipolar spectrum disorders when PTSD symptoms persist after pharmacologic treatment for the bipolar spectrum disorder. PE also is a first-line treatment for PTSD in patients with comorbid major depressive disorder. Barriers to PE treatment include its cost and finding professionals who are expert in its use.
Prazosin to treat PTSD-related symptoms in children or adolescents has not been studied, but it can be useful in adults over a wide range of doses. As little as 1 mg at bedtime may confer benefit, although the mean prazosin dose in an 8-week, placebo-controlled study of 40 combat veterans was 13.3 mg in the evening.33
I often initiate prazosin treatment as follows:
- 1 mg on the first night of treatment
- 2 mg on the second night
- 3 mg on the third night
- then, if tolerated, 1 mg upon waking, 1 mg 8 hours later, and 3 mg at bedtime. I then slowly adjust the dose schedule based on the patient’s needs, such as minimizing painful re-experiencing of the trauma. Reducing avoidance and hyperarousal also are reasonable targets. For example, when using prazosin to treat extremely angry men with PTSD stemming from exposure to violent crimes, I have observed that even “murderous” rage ceases with prazosin treatment, only to reappear when prazosin is discontinued.
In treating approximately 100 patients with prazosin, I have not exceeded 16 mg/d. Dosages used for treating hypertension usually are 5 to 20 mg/d. When using prazosin, I always:
- warn patients that faintness or fainting is a side effect and record this caveat in their chart
- obtain sitting and standing blood pressure and pulse before starting treatment and subsequently
- ask patients if they feel dizzy when changing posture before and after initiating treatment.
Most of my PTSD patients are suffering so much that they are willing to accept the risk of fainting associated with prazosin use. For PTSD comorbid with severe panic disorder,12,13 I find that a benzodiazepine with antipanic properties such as alprazolam or clonazepam often works well in conjunction with prazosin.
Some patients with bipolar spectrum disorders might benefit from the addition of an SSRI after mood stabilizer treatment proves effective. However, I have never managed a patient in this manner, and like my own treatment strategy, this has not been subjected to rigorous empiric inquiry. In my view, psychological treatment is much preferred to antidepressant therapy.
Related resource
- Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet. 2007;369:935-945.
Drug brand names
- Alprazolam • Xanax
- Bupropion • Wellbutrin
- Clonazepam • Klonopin
- D-cycloserine • Seromycin
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Phenelzine • Nardil
- Prazosin • Minipress
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Dilsaver reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity-Replication (NCS-R). JAMA. 2003;289:3095-3105.
2. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048-1060.
3. Franklin CL, Zimmerman M. Posttraumatic stress disorder and major depressive disorder: investigating the role of overlapping symptoms in diagnostic comorbidity. J Nerv Ment Dis. 2001;189:548-551.
4. Akiskal HS, Mallya G. Criteria for the “soft” bipolar spectrum: treatment implications. Psychopharmacol Bull. 1987;23:68-73.
5. Benazzi F. Prevalence of bipolar II disorder in outpatient depression: a 203-case study in a private practice. J Affect Disord. 1997;43:163-164.
6. Akiskal HS, Benazzi F. Optimizing the detection of bipolar II in outpatient private practice: toward a systematization of clinical diagnostic wisdom. J Clin Psychiatry. 2005;66:914-921.
7. Akiskal HS, Akiskal KK, Lancrenon S, et al. Validating the soft bipolar spectrum in the French National EPIDEP study: the prominence of BP-II. J Affect Disord. 2006;96:207-213.
8. Rabakowski JK, Suwalska D, Lojko D, et al. Bipolar disorders among Polish psychiatric outpatients treated for major depression. J Affect Disord. 2005;84:141-147.
9. Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet. 2007;369:935-945.
10. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
11. Maj M, Pirozzi R, Magliano, et al. Agitated ‘unipolar’ major depression: prevalence, phenomenology, and outcome. J Clin Psychiatry. 2006;67:712-719.
12. Dilsaver SC, Benazzi F, Akiskal HS, et al. Post-traumatic stress disorder among adolescents with bipolar disorder and its relationship to suicidality. Bipolar Disord. 2007;9:649-655.
13. Dilsaver SC, Benazzi F, Akiskal KK, et al. Differential patterns of lifetime multiple anxiety disorder comorbidity between Latino adults with bipolar I and major depressive disorders. Bull Menninger Clinic. 2008;72:130-148.
14. Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38:619-628.
15. Otto MW, Perlman CA, Wernicke R, et al. Posttraumatic stress disorder in patients with bipolar disorder: a review of prevalence, correlates, and treatment strategies. Bipolar Disord. 2004;6:470-479.
16. Oquendo M, Brent DA, Birmaher B, et al. Posttraumatic stress disorder comorbid with major depression: factors mediating the association with suicidal behavior. Am J Psychiatry. 2005;162:560-566.
17. Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized-controlled trial. JAMA. 2007;297:820-830.
18. Bisson J, Andrew M. Psychological treatment for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2005;CD003388.-
19. Cukor J, Spitalnick J, Difede J, et al. Emerging treatments for PTSD. Clin Psychol Rev. 2009;29(8):715-726.
20. Hogberg G, Pagani M, Sundin O, et al. Treatment of posttraumatic stress disorder with eye movement desensitization and reprocessing: outcome is stable in 35-month follow-up. Psychiatry Res. 2008;159(1-2):101-108.
21. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837-1844.
22. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68:711-720.
23. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63:199-206.
24. Tucker P, Zaninelli R, Yehuda R, et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:860-868.
25. Pae CU, Lim HK, Ajwani N, et al. Extended-release formulation of venlafaxine in the treatment of post-traumatic stress disorder. Expert Rev Neurother. 2007;7:603-615.
26. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from the STEP-BD. J Clin Psychopharmacol. 2004;24(5):512-520.
27. Quarantini LC, Miranda-Scippa A, Nery-Fernandes F, et al. The impact of comorbid posttraumatic stress disorder on bipolar patients. Affect Disord. 2009; [Epub ahead of print].
28. Henry C, Sorbara F, Lacoste J, et al. Antidepressant induced mania in bipolar patients: identification and risk factors. J Clin Psychiatry. 2001;62:249-255.
29. Gao K, Kemp DE, Gonocy SJ, et al. Treatment-emergent mania/hypomania during antidepressant monotherapy in patients with rapid cycling bipolar disorder. Bipolar Disord. 2008;10:907-915.
30. Dilsaver SC, Swann AC. Mixed mania: apparent induction by a tricyclic antidepressant in five consecutively treated patients with bipolar depression. Biol Psychiatry. 1995;1:60-62.
31. Braun P, Greenberg D, Dasberg H, et al. Core symptoms of posttraumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry. 1990;51:236-238.
32. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
33. Raskind MA, Perskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with posttraumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
34. Taylor FB, Lowe K, Thompson C, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59:577-581.
Major depressive disorder (MDD) and bipolar spectrum disorders are associated with some symptoms of—and fully defined—posttraumatic stress disorder (PTSD). Many traumatic experiences can lead to this comorbidity, the most common being exposure to or witnessing combat for men and rape and sexual molestation for women.1
Trauma has major prognostic and treatment implications for affectively ill patients, including those whose symptoms do not meet PTSD’s full diagnostic criteria. This article aims to help clinicians by:
- presenting evidence characterizing the overlap between affective disorders and PTSD
- reviewing evidence that the bipolar spectrum may be broader than generally thought, an insight that affects PTSD treatment
- making a case for routine PTSD screening for all patients with affective illnesses
- recommending PTSD treatments tailored to the patient’s comorbid affective disorder.
Overlap of trauma and affective illness
PTSD is remarkably comorbid with mood disorders. Americans with MDD and bipolar disorder (BPD) are 7 and 9.4 times, respectively, more likely to meet criteria for PTSD than persons in the general population, according to odds ratios Kessler et al2 calculated from the National Comorbidity Survey database.
I have never seen a patient with PTSD who did not also meet criteria for an affective disorder. The concurrence of PTSD and MDD is not the product of overlapping diagnostic criteria. Rather, evidence indicates these are distinct diagnostic entities.3 A review of diagnostic criteria for PTSD and hypomania/mania leads to the same conclusion.
Bipolar spectrum disorders
DSM-IV-TR assumes that mood disorders fall neatly into boxes. Other data (Table 1)4–8 indicate that these disorders fall along a continuum or—more conservatively—that the scope of bipolarity is much wider than DSM-IV-TR recognizes. This is a controversial topic, and the individual clinician’s position could impact how one manages PTSD patients.
Table 1
Evidence of bipolar spectrum features in major depressive episodes
| Study | Design | Conclusion |
|---|---|---|
| Akiskal and Mallya, 19874 | 200 community mental health clinic patients diagnosed as having MDD | 50% could be classified as having a bipolar disorder |
| Benazzi, 19975 | 203 consecutively presenting patients with depression | 45% met criteria for bipolar II disorder |
| Akiskal and Benazzi, 20056 | 563 consecutive patients presenting with a DSM-IV-diagnosed MDE | 58% showed features of bipolar II disorder |
| Akiskal et al, 20067 | 493 patients in a French national study presenting with MDE | 65% were determined to fall along the ‘bipolar spectrum’ |
| Rabakowski et al, 20058 | 880 Polish outpatients presenting with MDE | 40% met criteria for bipolar disorder |
| MDD: major depressive disorder; MDE: major depressive episode | ||
In this article, I include bipolar I disorder, bipolar II disorder, and mixed depression within the “bipolar spectrum disorders.” If one accepts this—and I do—it follows that 50% to 70% of all major depressive episodes (MDEs) are bipolar in nature.4–9 Depending on your practice setting, you may see a higher or lower base rate of bipolar spectrum disorders.
Mixed depression is not recognized in DSM-IV-TR, and the purpose of this article is not to defend its inclusion as a bipolar spectrum phenomenon. A proposed definition of mixed depression9 requires the presence of an MDE contaminated by ≥3 features of hypomania or mania, without euphoria or inflated self-esteem/grandiosity (Table 2).10
Some experts believe episodes of hypomania and mania frequently occur in the illness course of persons with mixed depression; indeed, mixed depression is a predictor of a bipolar course. It is observed in outpatient9 and inpatient settings.11 Common forms of mixed depression feature combinations of irritability, psychomotor agitation (mild to severe), increased talkativeness (which may fall short of frank pressured speech), racing or “crowded” thoughts (or “mental overactivity”), and distractibility. Other than increased self-esteem/grandiosity, any symptoms within DSM-IV-TR criterion B for a hypomanic or manic episode may be seen in mixed depression. Psychosis is an exclusion criterion for mixed depression.
Mixed depression responds poorly to antidepressant monotherapy. Validation studies suggest that mixed depression is a bipolar variant, as determined by its capacity to predict a bipolar course and its association with a family history of bipolar disorder and age of onset.9
Table 2
Diagnostic characteristics of a hypomanic episode, DSM-IV-TR criteria A and B
| A. A distinct period of persistently elevated, expansive, or irritable mood, lasting throughout at least 4 days, that is clearly different from the usual nondepressed mood. |
| B. During the period of mood disturbance, 3 or more of the following symptoms have persisted (4 if the mood is only irritable) and have been present to a significant degree: 1) inflated self-esteem or grandiosity 2) decreased need for sleep (eg, feels rested after only 3 hours of sleep) 3) more talkative than usual or pressure to keep talking 4) flight of ideas or subjective experience that thoughts are racing 5) distractibility (ie, attention too easily drawn to unimportant or irrelevant external stimuli) 6) increase in goal-directed activity (either socially, at work or school, or sexually) or psychomotor agitation 7) excessive involvement in pleasurable activities that have a high potential for painful consequences (eg, the person engages in unrestrained buying sprees, sexual indiscretions, or foolish business investments). |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
PTSD risk in affective illness
An adolescent sample. A preliminary cross-sectional study conducted by our group indicates that adolescents with affective disorders may have a much higher risk of developing PTSD than psychiatric comparison subjects.12 We used modules from the Structured Clinical Interview for DSM-IV (SCID) to screen for intra-episode psychopathology (as opposed to lifetime prevalence of disorders) in 79 adolescents with MDD, 34 with BPD as defined in the DSM-IV-TR, and 26 with neither affective disorder (psychiatric controls). We found:
- 38.2% of subjects with BPD met criteria for PTSD, compared with 13.9% of those with MDD (OR 4.9; P =.001)
- 3.8% of adolescents without a mood disorder met criteria for PTSD.
We also found that comorbid PTSD was associated with a 4.5-fold higher risk of a suicide attempt, even after we controlled for BPD diagnosis. When we controlled for the presence of other concurrent anxiety disorders, the likelihood of an adolescent with PTSD having attempted suicide remained significant (OR 3.4; P=.023). This finding suggests that PTSD is an independent risk factor for a suicide attempt.
An adult sample. We then focused on adults meeting criteria for MDD or BPD. In a study of 187 consecutively presenting affectively ill patients, we used the SCID to screen for multiple anxiety disorders including PTSD.13 Lifetime—as opposed to intra-episode—PTSD prevalence was 23.8% among the 118 patients with MDD and 62.3% among the 69 patients with BPD. A patient with BPD was 5 times more likely to have PTSD than a patient with MDD (OR 5.3; P < .0001). The most common cause of trauma leading to PTSD was sexual molestation or rape as a child or adolescent in this predominantly female Latino population.
Populations at risk for PTSD
The prevalence of PTSD in clinical samples varies, depending on the population studied. For instance, women are at much higher risk for developing PTSD than men, even in comparisons where men are exposed to a greater number of traumatic events and analyses control for differences in the prevalence of sexual abuse. The gender difference is greater if the trauma occurs during childhood.14 Essentially all patients in our adolescent and adult studies developed PTSD in response to childhood or adolescent sexual trauma.12,13
A population exposed to a high rate of violent crime would be expected to show a higher PTSD prevalence than one exposed to substantially less violence. The base rate of PTSD also is much higher in affectively ill patients than in the general population.
An analysis by Otto et al15 found a 16% lifetime prevalence of concomitant PTSD in 1,214 persons with BPD (not the manifold forms within the bipolar spectrum). Oquendo et al16 reported a 25.7% lifetime prevalence of PTSD in 230 patients with a history of MDD. Other epidemiologic2 and clinical studies12,13 suggest a considerably higher base rate of PTSD among persons with bipolar disorders than those with MDD.
The method of ascertaining the presence of this disorder may be another variable affecting the reported PTSD prevalence. Persistent avoidance—including “efforts to avoid thoughts, feelings, or conversations associated with the trauma”—is a diagnostic feature of PTSD.10 Researchers and clinicians who do not intentionally screen patients for PTSD are not likely to detect it. Determining the true prevalence of PTSD requires empathic inquiry about exposure to traumatic events.
PTSD screening
Humans are remarkably resilient, and most persons exposed to major trauma are thought not to develop PTSD. However, in my experience, because PTSD appears to be common among persons with affective illness, determining whether such patients have been traumatized is important for prognosis and treatment selection.
To get started, you could create a 1-page form to record traumatic events and identify features of PTSD according to DSM-IV-TR criteria (Checklist).10 PTSD screening without a form can become second nature with practice; an experienced clinician can screen a traumatized patient for the disorder within 3 to 5 minutes.
When screening for a history of trauma, ask patients in a straightforward manner if they have:
- been victims of violent crimes
- witnessed violent crimes
- been exposed to events in which people could have suffered grave injury
- experienced emotional, physical, or sexual abuse.
A person who has experienced emotional abuse but not physical or sexual abuse cannot meet DSM-IV-TR criterion A and therefore does not meet full criteria for PTSD. Many emotionally abused persons meet criteria B through F, however, and they are most reasonably managed similarly to persons who also meet criterion A. When formulating a treatment plan, I recommend using clinical judgment rather than rigid adherence to DSM-IV-TR.
Checklist
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
| Criterion A. The person has been exposed to a traumatic event in which both of the following have been present: | |
| □ | 1. The person has experienced, witnessed, or been confronted with an event or events that involve actual or threatened death or serious injury, or a threat to the physical integrity of oneself or others |
| □ | 2. The person’s response involved intense fear, helplessness, or horror |
| Criterion B. The traumatic event is persistently re-experienced in at least 1 of the following ways: | |
| □ | 1. Recurrent and intrusive distressing recollections of the event, including images, thoughts, or perceptions |
| □ | 2. Recurrent distressing dreams of the event |
| □ | 3. Acting or feeling as if the traumatic event were recurring (includes a sense of reliving the experience, illusions, hallucinations, and dissociative flashback episodes, including those that occur upon awakening or when intoxicated) |
| □ | 4. Intense psychological distress at exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event |
| □ | 5. Physiologic reactivity upon exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event |
| Criterion C. Persistent avoidance of stimuli associated with the trauma and numbing of general responsiveness (not present before the trauma), as indicated by at least 3 of the following: | |
| □ | 1. Efforts to avoid thoughts, feelings, or conversations associated with the trauma |
| □ | 2. Efforts to avoid activities, places, or people that arouse recollections of the trauma |
| □ | 3. Inability to recall an important aspect of the trauma |
| □ | 4. Markedly diminished interest or participation in significant activities |
| □ | 5. Feeling of detachment or estrangement from others |
| □ | 6. Restricted range of affect |
| □ | 7. Sense of foreshortened future |
| Criterion D. Persistent symptoms of increasing arousal (not present before the trauma), indicated by at least 2 of the following: | |
| □ | 1. Difficulty falling or staying asleep |
| □ | 2. Irritability or outbursts of anger |
| □ | 3. Difficulty concentrating |
| □ | 4. Hypervigilance |
| □ | 5. Exaggerated startle response |
| □ | Criterion E. Duration of disturbance (symptoms in B, C, and D) is >1 month |
| □ | Criterion F. Disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Source: Adapted from Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
Treating PTSD in depression
Pharmacotherapy and psychotherapeutic interventions are important to PTSD patients’ recovery. Limited resources often prevent these patients from receiving expert psychotherapeutic intervention, however, leaving pharmacotherapy as the mainstay of treatment. This is unfortunate, because psychological interventions may be sufficient and preferred in some instances (Box).17–20
Pharmacotherapy for comorbid MDD. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine are first-line interventions for PTSD in depressed patients who do not meet criteria for a bipolar spectrum disorder. Placebo-controlled studies suggest that sertraline,21,22 fluoxetine,23 and paroxetine,24 are effective; doses higher than those used to treat depression may be required. Extended-release venlafaxine25 in dosages similar to those needed for depressive disorders also can be effective. Bupropion does not appear to be beneficial in treating PTSD.
The monoamine oxidase inhibitor phenelzine was long used successfully in treating PTSD but for the most part has been replaced by SSRIs. Because of its associated dietary restrictions, risk of hypertensive crises, and other side effects, phenelzine probably is best reserved for patients who do not respond to treatment with SSRIs or venlafaxine.
Pharmacotherapy for comorbid bipolar spectrum. If one accepts that most patients meeting criteria for MDE have a bipolar spectrum disorder, then most affectively ill patients with PTSD would need to be treated as if they have bipolar disorder. Oddly enough, this creates difficulties for the use of not only antidepressants and benzodiazepines, but also mood stabilizers:
- Patients with BPD and comorbid anxiety disorders, including PTSD, may be resistant to mood stabilizers.26,27
- Antidepressants can precipitate hypomanic or manic switches or onset of mixed hypomania, a mixed state, or rapid cycling in patients with a bipolar spectrum disorder.28–30
- Benzodiazepines do not appear to relieve acute or chronic PTSD-related distress, and discontinuation could cause rebound symptoms.31
Because no outcome studies have addressed PTSD management in patients with bipolar spectrum disorders, clinicians must rely on their judgment when formulating treatment plans. One strategy is to treat patients with mood stabilizers, then leave well enough alone if both the mood and anxiety symptoms remit (which is possible but unlikely in my experience). I often start treatment for the bipolar spectrum disorder and co-existing PTSD using mood stabilizers (including atypical antipsychotics) and prazosin, an α-1antagonist originally used for treating hypertension.
Prazosin can help diminish nightmares, dreams, and other painful recollections of trauma.32,33 The drug does not affect time to sleep onset. It also has been reported to reduce avoidance behavior and hyperarousal, such as irritability and anger.34 This has been my experience.
Cognitive-behavioral therapy (CBT) involving prolonged exposure (PE) to trauma-related stimuli has been shown to be effective for posttraumatic stress disorder (PTSD) in controlled studies.17,18 PE is an individual CBT designed to help patients process traumatic events and reduce psychological distress. It involves education about reactions to trauma, relaxation techniques, imaginal reliving of the trauma, exposure to cues associated with the trauma, and cognitive restructuring.
Administering D-cycloserine before behavioral treatment sessions facilitates fear extinction, and its use to enhance prolonged PE constitutes state-of-the-art treatment.19 Eye movement desensitization and reprocessing also may be effective.18,20
PE is a reasonable first-line treatment for PTSD patients with comorbid bipolar spectrum disorders when PTSD symptoms persist after pharmacologic treatment for the bipolar spectrum disorder. PE also is a first-line treatment for PTSD in patients with comorbid major depressive disorder. Barriers to PE treatment include its cost and finding professionals who are expert in its use.
Prazosin to treat PTSD-related symptoms in children or adolescents has not been studied, but it can be useful in adults over a wide range of doses. As little as 1 mg at bedtime may confer benefit, although the mean prazosin dose in an 8-week, placebo-controlled study of 40 combat veterans was 13.3 mg in the evening.33
I often initiate prazosin treatment as follows:
- 1 mg on the first night of treatment
- 2 mg on the second night
- 3 mg on the third night
- then, if tolerated, 1 mg upon waking, 1 mg 8 hours later, and 3 mg at bedtime. I then slowly adjust the dose schedule based on the patient’s needs, such as minimizing painful re-experiencing of the trauma. Reducing avoidance and hyperarousal also are reasonable targets. For example, when using prazosin to treat extremely angry men with PTSD stemming from exposure to violent crimes, I have observed that even “murderous” rage ceases with prazosin treatment, only to reappear when prazosin is discontinued.
In treating approximately 100 patients with prazosin, I have not exceeded 16 mg/d. Dosages used for treating hypertension usually are 5 to 20 mg/d. When using prazosin, I always:
- warn patients that faintness or fainting is a side effect and record this caveat in their chart
- obtain sitting and standing blood pressure and pulse before starting treatment and subsequently
- ask patients if they feel dizzy when changing posture before and after initiating treatment.
Most of my PTSD patients are suffering so much that they are willing to accept the risk of fainting associated with prazosin use. For PTSD comorbid with severe panic disorder,12,13 I find that a benzodiazepine with antipanic properties such as alprazolam or clonazepam often works well in conjunction with prazosin.
Some patients with bipolar spectrum disorders might benefit from the addition of an SSRI after mood stabilizer treatment proves effective. However, I have never managed a patient in this manner, and like my own treatment strategy, this has not been subjected to rigorous empiric inquiry. In my view, psychological treatment is much preferred to antidepressant therapy.
Related resource
- Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet. 2007;369:935-945.
Drug brand names
- Alprazolam • Xanax
- Bupropion • Wellbutrin
- Clonazepam • Klonopin
- D-cycloserine • Seromycin
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Phenelzine • Nardil
- Prazosin • Minipress
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Dilsaver reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Major depressive disorder (MDD) and bipolar spectrum disorders are associated with some symptoms of—and fully defined—posttraumatic stress disorder (PTSD). Many traumatic experiences can lead to this comorbidity, the most common being exposure to or witnessing combat for men and rape and sexual molestation for women.1
Trauma has major prognostic and treatment implications for affectively ill patients, including those whose symptoms do not meet PTSD’s full diagnostic criteria. This article aims to help clinicians by:
- presenting evidence characterizing the overlap between affective disorders and PTSD
- reviewing evidence that the bipolar spectrum may be broader than generally thought, an insight that affects PTSD treatment
- making a case for routine PTSD screening for all patients with affective illnesses
- recommending PTSD treatments tailored to the patient’s comorbid affective disorder.
Overlap of trauma and affective illness
PTSD is remarkably comorbid with mood disorders. Americans with MDD and bipolar disorder (BPD) are 7 and 9.4 times, respectively, more likely to meet criteria for PTSD than persons in the general population, according to odds ratios Kessler et al2 calculated from the National Comorbidity Survey database.
I have never seen a patient with PTSD who did not also meet criteria for an affective disorder. The concurrence of PTSD and MDD is not the product of overlapping diagnostic criteria. Rather, evidence indicates these are distinct diagnostic entities.3 A review of diagnostic criteria for PTSD and hypomania/mania leads to the same conclusion.
Bipolar spectrum disorders
DSM-IV-TR assumes that mood disorders fall neatly into boxes. Other data (Table 1)4–8 indicate that these disorders fall along a continuum or—more conservatively—that the scope of bipolarity is much wider than DSM-IV-TR recognizes. This is a controversial topic, and the individual clinician’s position could impact how one manages PTSD patients.
Table 1
Evidence of bipolar spectrum features in major depressive episodes
| Study | Design | Conclusion |
|---|---|---|
| Akiskal and Mallya, 19874 | 200 community mental health clinic patients diagnosed as having MDD | 50% could be classified as having a bipolar disorder |
| Benazzi, 19975 | 203 consecutively presenting patients with depression | 45% met criteria for bipolar II disorder |
| Akiskal and Benazzi, 20056 | 563 consecutive patients presenting with a DSM-IV-diagnosed MDE | 58% showed features of bipolar II disorder |
| Akiskal et al, 20067 | 493 patients in a French national study presenting with MDE | 65% were determined to fall along the ‘bipolar spectrum’ |
| Rabakowski et al, 20058 | 880 Polish outpatients presenting with MDE | 40% met criteria for bipolar disorder |
| MDD: major depressive disorder; MDE: major depressive episode | ||
In this article, I include bipolar I disorder, bipolar II disorder, and mixed depression within the “bipolar spectrum disorders.” If one accepts this—and I do—it follows that 50% to 70% of all major depressive episodes (MDEs) are bipolar in nature.4–9 Depending on your practice setting, you may see a higher or lower base rate of bipolar spectrum disorders.
Mixed depression is not recognized in DSM-IV-TR, and the purpose of this article is not to defend its inclusion as a bipolar spectrum phenomenon. A proposed definition of mixed depression9 requires the presence of an MDE contaminated by ≥3 features of hypomania or mania, without euphoria or inflated self-esteem/grandiosity (Table 2).10
Some experts believe episodes of hypomania and mania frequently occur in the illness course of persons with mixed depression; indeed, mixed depression is a predictor of a bipolar course. It is observed in outpatient9 and inpatient settings.11 Common forms of mixed depression feature combinations of irritability, psychomotor agitation (mild to severe), increased talkativeness (which may fall short of frank pressured speech), racing or “crowded” thoughts (or “mental overactivity”), and distractibility. Other than increased self-esteem/grandiosity, any symptoms within DSM-IV-TR criterion B for a hypomanic or manic episode may be seen in mixed depression. Psychosis is an exclusion criterion for mixed depression.
Mixed depression responds poorly to antidepressant monotherapy. Validation studies suggest that mixed depression is a bipolar variant, as determined by its capacity to predict a bipolar course and its association with a family history of bipolar disorder and age of onset.9
Table 2
Diagnostic characteristics of a hypomanic episode, DSM-IV-TR criteria A and B
| A. A distinct period of persistently elevated, expansive, or irritable mood, lasting throughout at least 4 days, that is clearly different from the usual nondepressed mood. |
| B. During the period of mood disturbance, 3 or more of the following symptoms have persisted (4 if the mood is only irritable) and have been present to a significant degree: 1) inflated self-esteem or grandiosity 2) decreased need for sleep (eg, feels rested after only 3 hours of sleep) 3) more talkative than usual or pressure to keep talking 4) flight of ideas or subjective experience that thoughts are racing 5) distractibility (ie, attention too easily drawn to unimportant or irrelevant external stimuli) 6) increase in goal-directed activity (either socially, at work or school, or sexually) or psychomotor agitation 7) excessive involvement in pleasurable activities that have a high potential for painful consequences (eg, the person engages in unrestrained buying sprees, sexual indiscretions, or foolish business investments). |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 |
PTSD risk in affective illness
An adolescent sample. A preliminary cross-sectional study conducted by our group indicates that adolescents with affective disorders may have a much higher risk of developing PTSD than psychiatric comparison subjects.12 We used modules from the Structured Clinical Interview for DSM-IV (SCID) to screen for intra-episode psychopathology (as opposed to lifetime prevalence of disorders) in 79 adolescents with MDD, 34 with BPD as defined in the DSM-IV-TR, and 26 with neither affective disorder (psychiatric controls). We found:
- 38.2% of subjects with BPD met criteria for PTSD, compared with 13.9% of those with MDD (OR 4.9; P =.001)
- 3.8% of adolescents without a mood disorder met criteria for PTSD.
We also found that comorbid PTSD was associated with a 4.5-fold higher risk of a suicide attempt, even after we controlled for BPD diagnosis. When we controlled for the presence of other concurrent anxiety disorders, the likelihood of an adolescent with PTSD having attempted suicide remained significant (OR 3.4; P=.023). This finding suggests that PTSD is an independent risk factor for a suicide attempt.
An adult sample. We then focused on adults meeting criteria for MDD or BPD. In a study of 187 consecutively presenting affectively ill patients, we used the SCID to screen for multiple anxiety disorders including PTSD.13 Lifetime—as opposed to intra-episode—PTSD prevalence was 23.8% among the 118 patients with MDD and 62.3% among the 69 patients with BPD. A patient with BPD was 5 times more likely to have PTSD than a patient with MDD (OR 5.3; P < .0001). The most common cause of trauma leading to PTSD was sexual molestation or rape as a child or adolescent in this predominantly female Latino population.
Populations at risk for PTSD
The prevalence of PTSD in clinical samples varies, depending on the population studied. For instance, women are at much higher risk for developing PTSD than men, even in comparisons where men are exposed to a greater number of traumatic events and analyses control for differences in the prevalence of sexual abuse. The gender difference is greater if the trauma occurs during childhood.14 Essentially all patients in our adolescent and adult studies developed PTSD in response to childhood or adolescent sexual trauma.12,13
A population exposed to a high rate of violent crime would be expected to show a higher PTSD prevalence than one exposed to substantially less violence. The base rate of PTSD also is much higher in affectively ill patients than in the general population.
An analysis by Otto et al15 found a 16% lifetime prevalence of concomitant PTSD in 1,214 persons with BPD (not the manifold forms within the bipolar spectrum). Oquendo et al16 reported a 25.7% lifetime prevalence of PTSD in 230 patients with a history of MDD. Other epidemiologic2 and clinical studies12,13 suggest a considerably higher base rate of PTSD among persons with bipolar disorders than those with MDD.
The method of ascertaining the presence of this disorder may be another variable affecting the reported PTSD prevalence. Persistent avoidance—including “efforts to avoid thoughts, feelings, or conversations associated with the trauma”—is a diagnostic feature of PTSD.10 Researchers and clinicians who do not intentionally screen patients for PTSD are not likely to detect it. Determining the true prevalence of PTSD requires empathic inquiry about exposure to traumatic events.
PTSD screening
Humans are remarkably resilient, and most persons exposed to major trauma are thought not to develop PTSD. However, in my experience, because PTSD appears to be common among persons with affective illness, determining whether such patients have been traumatized is important for prognosis and treatment selection.
To get started, you could create a 1-page form to record traumatic events and identify features of PTSD according to DSM-IV-TR criteria (Checklist).10 PTSD screening without a form can become second nature with practice; an experienced clinician can screen a traumatized patient for the disorder within 3 to 5 minutes.
When screening for a history of trauma, ask patients in a straightforward manner if they have:
- been victims of violent crimes
- witnessed violent crimes
- been exposed to events in which people could have suffered grave injury
- experienced emotional, physical, or sexual abuse.
A person who has experienced emotional abuse but not physical or sexual abuse cannot meet DSM-IV-TR criterion A and therefore does not meet full criteria for PTSD. Many emotionally abused persons meet criteria B through F, however, and they are most reasonably managed similarly to persons who also meet criterion A. When formulating a treatment plan, I recommend using clinical judgment rather than rigid adherence to DSM-IV-TR.
Checklist
DSM-IV-TR diagnostic criteria for posttraumatic stress disorder
| Criterion A. The person has been exposed to a traumatic event in which both of the following have been present: | |
| □ | 1. The person has experienced, witnessed, or been confronted with an event or events that involve actual or threatened death or serious injury, or a threat to the physical integrity of oneself or others |
| □ | 2. The person’s response involved intense fear, helplessness, or horror |
| Criterion B. The traumatic event is persistently re-experienced in at least 1 of the following ways: | |
| □ | 1. Recurrent and intrusive distressing recollections of the event, including images, thoughts, or perceptions |
| □ | 2. Recurrent distressing dreams of the event |
| □ | 3. Acting or feeling as if the traumatic event were recurring (includes a sense of reliving the experience, illusions, hallucinations, and dissociative flashback episodes, including those that occur upon awakening or when intoxicated) |
| □ | 4. Intense psychological distress at exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event |
| □ | 5. Physiologic reactivity upon exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event |
| Criterion C. Persistent avoidance of stimuli associated with the trauma and numbing of general responsiveness (not present before the trauma), as indicated by at least 3 of the following: | |
| □ | 1. Efforts to avoid thoughts, feelings, or conversations associated with the trauma |
| □ | 2. Efforts to avoid activities, places, or people that arouse recollections of the trauma |
| □ | 3. Inability to recall an important aspect of the trauma |
| □ | 4. Markedly diminished interest or participation in significant activities |
| □ | 5. Feeling of detachment or estrangement from others |
| □ | 6. Restricted range of affect |
| □ | 7. Sense of foreshortened future |
| Criterion D. Persistent symptoms of increasing arousal (not present before the trauma), indicated by at least 2 of the following: | |
| □ | 1. Difficulty falling or staying asleep |
| □ | 2. Irritability or outbursts of anger |
| □ | 3. Difficulty concentrating |
| □ | 4. Hypervigilance |
| □ | 5. Exaggerated startle response |
| □ | Criterion E. Duration of disturbance (symptoms in B, C, and D) is >1 month |
| □ | Criterion F. Disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Source: Adapted from Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
Treating PTSD in depression
Pharmacotherapy and psychotherapeutic interventions are important to PTSD patients’ recovery. Limited resources often prevent these patients from receiving expert psychotherapeutic intervention, however, leaving pharmacotherapy as the mainstay of treatment. This is unfortunate, because psychological interventions may be sufficient and preferred in some instances (Box).17–20
Pharmacotherapy for comorbid MDD. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine are first-line interventions for PTSD in depressed patients who do not meet criteria for a bipolar spectrum disorder. Placebo-controlled studies suggest that sertraline,21,22 fluoxetine,23 and paroxetine,24 are effective; doses higher than those used to treat depression may be required. Extended-release venlafaxine25 in dosages similar to those needed for depressive disorders also can be effective. Bupropion does not appear to be beneficial in treating PTSD.
The monoamine oxidase inhibitor phenelzine was long used successfully in treating PTSD but for the most part has been replaced by SSRIs. Because of its associated dietary restrictions, risk of hypertensive crises, and other side effects, phenelzine probably is best reserved for patients who do not respond to treatment with SSRIs or venlafaxine.
Pharmacotherapy for comorbid bipolar spectrum. If one accepts that most patients meeting criteria for MDE have a bipolar spectrum disorder, then most affectively ill patients with PTSD would need to be treated as if they have bipolar disorder. Oddly enough, this creates difficulties for the use of not only antidepressants and benzodiazepines, but also mood stabilizers:
- Patients with BPD and comorbid anxiety disorders, including PTSD, may be resistant to mood stabilizers.26,27
- Antidepressants can precipitate hypomanic or manic switches or onset of mixed hypomania, a mixed state, or rapid cycling in patients with a bipolar spectrum disorder.28–30
- Benzodiazepines do not appear to relieve acute or chronic PTSD-related distress, and discontinuation could cause rebound symptoms.31
Because no outcome studies have addressed PTSD management in patients with bipolar spectrum disorders, clinicians must rely on their judgment when formulating treatment plans. One strategy is to treat patients with mood stabilizers, then leave well enough alone if both the mood and anxiety symptoms remit (which is possible but unlikely in my experience). I often start treatment for the bipolar spectrum disorder and co-existing PTSD using mood stabilizers (including atypical antipsychotics) and prazosin, an α-1antagonist originally used for treating hypertension.
Prazosin can help diminish nightmares, dreams, and other painful recollections of trauma.32,33 The drug does not affect time to sleep onset. It also has been reported to reduce avoidance behavior and hyperarousal, such as irritability and anger.34 This has been my experience.
Cognitive-behavioral therapy (CBT) involving prolonged exposure (PE) to trauma-related stimuli has been shown to be effective for posttraumatic stress disorder (PTSD) in controlled studies.17,18 PE is an individual CBT designed to help patients process traumatic events and reduce psychological distress. It involves education about reactions to trauma, relaxation techniques, imaginal reliving of the trauma, exposure to cues associated with the trauma, and cognitive restructuring.
Administering D-cycloserine before behavioral treatment sessions facilitates fear extinction, and its use to enhance prolonged PE constitutes state-of-the-art treatment.19 Eye movement desensitization and reprocessing also may be effective.18,20
PE is a reasonable first-line treatment for PTSD patients with comorbid bipolar spectrum disorders when PTSD symptoms persist after pharmacologic treatment for the bipolar spectrum disorder. PE also is a first-line treatment for PTSD in patients with comorbid major depressive disorder. Barriers to PE treatment include its cost and finding professionals who are expert in its use.
Prazosin to treat PTSD-related symptoms in children or adolescents has not been studied, but it can be useful in adults over a wide range of doses. As little as 1 mg at bedtime may confer benefit, although the mean prazosin dose in an 8-week, placebo-controlled study of 40 combat veterans was 13.3 mg in the evening.33
I often initiate prazosin treatment as follows:
- 1 mg on the first night of treatment
- 2 mg on the second night
- 3 mg on the third night
- then, if tolerated, 1 mg upon waking, 1 mg 8 hours later, and 3 mg at bedtime. I then slowly adjust the dose schedule based on the patient’s needs, such as minimizing painful re-experiencing of the trauma. Reducing avoidance and hyperarousal also are reasonable targets. For example, when using prazosin to treat extremely angry men with PTSD stemming from exposure to violent crimes, I have observed that even “murderous” rage ceases with prazosin treatment, only to reappear when prazosin is discontinued.
In treating approximately 100 patients with prazosin, I have not exceeded 16 mg/d. Dosages used for treating hypertension usually are 5 to 20 mg/d. When using prazosin, I always:
- warn patients that faintness or fainting is a side effect and record this caveat in their chart
- obtain sitting and standing blood pressure and pulse before starting treatment and subsequently
- ask patients if they feel dizzy when changing posture before and after initiating treatment.
Most of my PTSD patients are suffering so much that they are willing to accept the risk of fainting associated with prazosin use. For PTSD comorbid with severe panic disorder,12,13 I find that a benzodiazepine with antipanic properties such as alprazolam or clonazepam often works well in conjunction with prazosin.
Some patients with bipolar spectrum disorders might benefit from the addition of an SSRI after mood stabilizer treatment proves effective. However, I have never managed a patient in this manner, and like my own treatment strategy, this has not been subjected to rigorous empiric inquiry. In my view, psychological treatment is much preferred to antidepressant therapy.
Related resource
- Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet. 2007;369:935-945.
Drug brand names
- Alprazolam • Xanax
- Bupropion • Wellbutrin
- Clonazepam • Klonopin
- D-cycloserine • Seromycin
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Phenelzine • Nardil
- Prazosin • Minipress
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Dr. Dilsaver reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity-Replication (NCS-R). JAMA. 2003;289:3095-3105.
2. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048-1060.
3. Franklin CL, Zimmerman M. Posttraumatic stress disorder and major depressive disorder: investigating the role of overlapping symptoms in diagnostic comorbidity. J Nerv Ment Dis. 2001;189:548-551.
4. Akiskal HS, Mallya G. Criteria for the “soft” bipolar spectrum: treatment implications. Psychopharmacol Bull. 1987;23:68-73.
5. Benazzi F. Prevalence of bipolar II disorder in outpatient depression: a 203-case study in a private practice. J Affect Disord. 1997;43:163-164.
6. Akiskal HS, Benazzi F. Optimizing the detection of bipolar II in outpatient private practice: toward a systematization of clinical diagnostic wisdom. J Clin Psychiatry. 2005;66:914-921.
7. Akiskal HS, Akiskal KK, Lancrenon S, et al. Validating the soft bipolar spectrum in the French National EPIDEP study: the prominence of BP-II. J Affect Disord. 2006;96:207-213.
8. Rabakowski JK, Suwalska D, Lojko D, et al. Bipolar disorders among Polish psychiatric outpatients treated for major depression. J Affect Disord. 2005;84:141-147.
9. Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet. 2007;369:935-945.
10. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
11. Maj M, Pirozzi R, Magliano, et al. Agitated ‘unipolar’ major depression: prevalence, phenomenology, and outcome. J Clin Psychiatry. 2006;67:712-719.
12. Dilsaver SC, Benazzi F, Akiskal HS, et al. Post-traumatic stress disorder among adolescents with bipolar disorder and its relationship to suicidality. Bipolar Disord. 2007;9:649-655.
13. Dilsaver SC, Benazzi F, Akiskal KK, et al. Differential patterns of lifetime multiple anxiety disorder comorbidity between Latino adults with bipolar I and major depressive disorders. Bull Menninger Clinic. 2008;72:130-148.
14. Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38:619-628.
15. Otto MW, Perlman CA, Wernicke R, et al. Posttraumatic stress disorder in patients with bipolar disorder: a review of prevalence, correlates, and treatment strategies. Bipolar Disord. 2004;6:470-479.
16. Oquendo M, Brent DA, Birmaher B, et al. Posttraumatic stress disorder comorbid with major depression: factors mediating the association with suicidal behavior. Am J Psychiatry. 2005;162:560-566.
17. Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized-controlled trial. JAMA. 2007;297:820-830.
18. Bisson J, Andrew M. Psychological treatment for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2005;CD003388.-
19. Cukor J, Spitalnick J, Difede J, et al. Emerging treatments for PTSD. Clin Psychol Rev. 2009;29(8):715-726.
20. Hogberg G, Pagani M, Sundin O, et al. Treatment of posttraumatic stress disorder with eye movement desensitization and reprocessing: outcome is stable in 35-month follow-up. Psychiatry Res. 2008;159(1-2):101-108.
21. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837-1844.
22. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68:711-720.
23. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63:199-206.
24. Tucker P, Zaninelli R, Yehuda R, et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:860-868.
25. Pae CU, Lim HK, Ajwani N, et al. Extended-release formulation of venlafaxine in the treatment of post-traumatic stress disorder. Expert Rev Neurother. 2007;7:603-615.
26. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from the STEP-BD. J Clin Psychopharmacol. 2004;24(5):512-520.
27. Quarantini LC, Miranda-Scippa A, Nery-Fernandes F, et al. The impact of comorbid posttraumatic stress disorder on bipolar patients. Affect Disord. 2009; [Epub ahead of print].
28. Henry C, Sorbara F, Lacoste J, et al. Antidepressant induced mania in bipolar patients: identification and risk factors. J Clin Psychiatry. 2001;62:249-255.
29. Gao K, Kemp DE, Gonocy SJ, et al. Treatment-emergent mania/hypomania during antidepressant monotherapy in patients with rapid cycling bipolar disorder. Bipolar Disord. 2008;10:907-915.
30. Dilsaver SC, Swann AC. Mixed mania: apparent induction by a tricyclic antidepressant in five consecutively treated patients with bipolar depression. Biol Psychiatry. 1995;1:60-62.
31. Braun P, Greenberg D, Dasberg H, et al. Core symptoms of posttraumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry. 1990;51:236-238.
32. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
33. Raskind MA, Perskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with posttraumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
34. Taylor FB, Lowe K, Thompson C, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59:577-581.
1. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity-Replication (NCS-R). JAMA. 2003;289:3095-3105.
2. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048-1060.
3. Franklin CL, Zimmerman M. Posttraumatic stress disorder and major depressive disorder: investigating the role of overlapping symptoms in diagnostic comorbidity. J Nerv Ment Dis. 2001;189:548-551.
4. Akiskal HS, Mallya G. Criteria for the “soft” bipolar spectrum: treatment implications. Psychopharmacol Bull. 1987;23:68-73.
5. Benazzi F. Prevalence of bipolar II disorder in outpatient depression: a 203-case study in a private practice. J Affect Disord. 1997;43:163-164.
6. Akiskal HS, Benazzi F. Optimizing the detection of bipolar II in outpatient private practice: toward a systematization of clinical diagnostic wisdom. J Clin Psychiatry. 2005;66:914-921.
7. Akiskal HS, Akiskal KK, Lancrenon S, et al. Validating the soft bipolar spectrum in the French National EPIDEP study: the prominence of BP-II. J Affect Disord. 2006;96:207-213.
8. Rabakowski JK, Suwalska D, Lojko D, et al. Bipolar disorders among Polish psychiatric outpatients treated for major depression. J Affect Disord. 2005;84:141-147.
9. Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet. 2007;369:935-945.
10. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
11. Maj M, Pirozzi R, Magliano, et al. Agitated ‘unipolar’ major depression: prevalence, phenomenology, and outcome. J Clin Psychiatry. 2006;67:712-719.
12. Dilsaver SC, Benazzi F, Akiskal HS, et al. Post-traumatic stress disorder among adolescents with bipolar disorder and its relationship to suicidality. Bipolar Disord. 2007;9:649-655.
13. Dilsaver SC, Benazzi F, Akiskal KK, et al. Differential patterns of lifetime multiple anxiety disorder comorbidity between Latino adults with bipolar I and major depressive disorders. Bull Menninger Clinic. 2008;72:130-148.
14. Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38:619-628.
15. Otto MW, Perlman CA, Wernicke R, et al. Posttraumatic stress disorder in patients with bipolar disorder: a review of prevalence, correlates, and treatment strategies. Bipolar Disord. 2004;6:470-479.
16. Oquendo M, Brent DA, Birmaher B, et al. Posttraumatic stress disorder comorbid with major depression: factors mediating the association with suicidal behavior. Am J Psychiatry. 2005;162:560-566.
17. Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized-controlled trial. JAMA. 2007;297:820-830.
18. Bisson J, Andrew M. Psychological treatment for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2005;CD003388.-
19. Cukor J, Spitalnick J, Difede J, et al. Emerging treatments for PTSD. Clin Psychol Rev. 2009;29(8):715-726.
20. Hogberg G, Pagani M, Sundin O, et al. Treatment of posttraumatic stress disorder with eye movement desensitization and reprocessing: outcome is stable in 35-month follow-up. Psychiatry Res. 2008;159(1-2):101-108.
21. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837-1844.
22. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68:711-720.
23. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63:199-206.
24. Tucker P, Zaninelli R, Yehuda R, et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:860-868.
25. Pae CU, Lim HK, Ajwani N, et al. Extended-release formulation of venlafaxine in the treatment of post-traumatic stress disorder. Expert Rev Neurother. 2007;7:603-615.
26. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from the STEP-BD. J Clin Psychopharmacol. 2004;24(5):512-520.
27. Quarantini LC, Miranda-Scippa A, Nery-Fernandes F, et al. The impact of comorbid posttraumatic stress disorder on bipolar patients. Affect Disord. 2009; [Epub ahead of print].
28. Henry C, Sorbara F, Lacoste J, et al. Antidepressant induced mania in bipolar patients: identification and risk factors. J Clin Psychiatry. 2001;62:249-255.
29. Gao K, Kemp DE, Gonocy SJ, et al. Treatment-emergent mania/hypomania during antidepressant monotherapy in patients with rapid cycling bipolar disorder. Bipolar Disord. 2008;10:907-915.
30. Dilsaver SC, Swann AC. Mixed mania: apparent induction by a tricyclic antidepressant in five consecutively treated patients with bipolar depression. Biol Psychiatry. 1995;1:60-62.
31. Braun P, Greenberg D, Dasberg H, et al. Core symptoms of posttraumatic stress disorder unimproved by alprazolam treatment. J Clin Psychiatry. 1990;51:236-238.
32. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
33. Raskind MA, Perskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with posttraumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
34. Taylor FB, Lowe K, Thompson C, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59:577-581.
Stubborn pneumonia turns out to be cancer ... Iodine contrast media kills man with known shellfish allergy...more
Stubborn pneumonia turns out to be cancer
AFTER RECEIVING ANTIBIOTICS FOR PNEUMONIA, a 37-year-old man improved but didn’t fully recover; his radiographs didn’t return to normal. He’d never smoked cigarettes.
During the several months after the pneumonia, the patient’s doctor ordered repeat radiographs and prescribed antibiotics and pain medication. When the patient’s spine collapsed, the doctor diagnosed metastatic lung cancer. The patient received palliative treatment and ultimately died.
PLAINTIFF’S CLAIM The doctor was negligent in failing to change the patient’s treatment after 2 or 3 months and failing to order a computed tomography (CT) scan or refer the patient to a pulmonologist.
THE DEFENSE No information about the doctor’s defense is available.
VERDICT $1.25 million Washington settlement.
COMMENT I’d like a nickel for every case of delayed diagnosis of lung cancer based on clearly abnormal chest radiographs. We can argue about whether diagnosis would make a difference, but we need to follow up assiduously on abnormal radiographs and document our actions.
Rapidly raised serum sodium leads to osmotic demyelination
A 60-YEAR-OLD WOMAN went to her local medical center complaining of a cough for the previous 2 weeks, decreased appetite and oral intake, and generalized body aches. She first went to urgent care, where laboratory studies showed critically low levels of sodium and potassium. Based on these results, the woman was told to go to the facility’s emergency department (ED).
In the ED, she reported feeling very weak and tired and having body aches and pain. When laboratory tests showed that her sodium and potassium levels had fallen further, she was admitted to the intensive care unit (ICU).
The doctor who saw the patient in the ICU ordered intravenous fluids with normal saline and potassium supplements. He then had the patient admitted to the ICU at another hospital. The physician at that hospital continued to prescribe IV sodium and potassium until the patient was discharged with diagnoses that included hyponatremia and hypokalemia.
Ten days later, the patient returned to the ED complaining of slurred speech for the previous 2 days. A CT scan of her head showed a possible basilar tip aneurysm. Subsequent magnetic resonance imaging with and without contrast and intracranial magnetic resonance angiography confirmed a basilar tip aneurysm and showed findings suggestive of osmotic demyelination. Neurologic examination revealed dysarthria, right upper extremity weakness without spasticity, and periods of confusion interspersed with lucid intervals.
A subsequent neurologic consultation confirmed osmotic demyelination syndrome (formerly known as central pontine myelinolysis). Neurologic examination at that time found continued mild dysarthria, problems standing, inability to walk unsupported, mild oral and pharyngeal dysphagia, and language and writing deficits.
PLAINTIFF’S CLAIM The patient’s sodium level was increased at an inappropriately rapid rate, which caused neurologically devastating osmotic demyelination. Serum sodium should have been monitored every 4 hours during the first 24 hours of treatment. The plaintiff also alleged negligence in continuing normal saline after the patient’s serum sodium was measured at 112 mEq/L.
THE DEFENSE The treatment provided was appropriate.
VERDICT $550,000 California settlement.
COMMENT Avoiding osmotic demyelination syndrome requires careful treatment and monitoring. I have independently reviewed several allegations of malpractice involving this uncommon, but devastating condition. Two recent articles summarize the treatment of this disorder: Sterns RH, Silver S, Klein-schmidt-DeMasters BK, et al. Current perspectives in the management of hyponatremia: prevention of CPM. Expert Rev Neurother. 2007;7:1791-1797; and Lien YH, Shapiro JI. Hyponatremia: clinical diagnosis and management. Am J Med. 2007;120:653-658.
Iodine contrast media kills man with known shellfish allergy
A 41-YEAR-OLD MAN WITH CHEST PAIN was admitted to his local hospital, where he received a diagnosis of acute coronary syndrome. After treatment in the emergency department, the patient was admitted to the telemetry unit by an internist, the partner of the patient’s primary care physician. The patient’s admission records noted that he had an allergy to shellfish.
The next morning, a cardiologist was called in. The cardiologist then called in an interventional cardiologist, who scheduled a cardiac catheterization. The interventional cardiologist ordered 1 dose of steroids, followed a few minutes later by contrast iodine. The patient immediately suffered a severe allergic reaction and died.
PLAINTIFF’S CLAIM The internist who admitted the patient to the telemetry unit took an incomplete history regarding the patient’s allergies (although the admission records contained that information). No information about the claims against the 2 cardiologists is available.
THE DEFENSE No information about the defense is available.
VERDICT $4.7 million gross verdict in Florida.
COMMENT In addition to considering the risk of dye loads and carefully checking renal function, remember to assess for allergy when administering contrast agents. Failure to do so in this case led to the death of the patient and a multimillion-dollar verdict.
Stubborn pneumonia turns out to be cancer
AFTER RECEIVING ANTIBIOTICS FOR PNEUMONIA, a 37-year-old man improved but didn’t fully recover; his radiographs didn’t return to normal. He’d never smoked cigarettes.
During the several months after the pneumonia, the patient’s doctor ordered repeat radiographs and prescribed antibiotics and pain medication. When the patient’s spine collapsed, the doctor diagnosed metastatic lung cancer. The patient received palliative treatment and ultimately died.
PLAINTIFF’S CLAIM The doctor was negligent in failing to change the patient’s treatment after 2 or 3 months and failing to order a computed tomography (CT) scan or refer the patient to a pulmonologist.
THE DEFENSE No information about the doctor’s defense is available.
VERDICT $1.25 million Washington settlement.
COMMENT I’d like a nickel for every case of delayed diagnosis of lung cancer based on clearly abnormal chest radiographs. We can argue about whether diagnosis would make a difference, but we need to follow up assiduously on abnormal radiographs and document our actions.
Rapidly raised serum sodium leads to osmotic demyelination
A 60-YEAR-OLD WOMAN went to her local medical center complaining of a cough for the previous 2 weeks, decreased appetite and oral intake, and generalized body aches. She first went to urgent care, where laboratory studies showed critically low levels of sodium and potassium. Based on these results, the woman was told to go to the facility’s emergency department (ED).
In the ED, she reported feeling very weak and tired and having body aches and pain. When laboratory tests showed that her sodium and potassium levels had fallen further, she was admitted to the intensive care unit (ICU).
The doctor who saw the patient in the ICU ordered intravenous fluids with normal saline and potassium supplements. He then had the patient admitted to the ICU at another hospital. The physician at that hospital continued to prescribe IV sodium and potassium until the patient was discharged with diagnoses that included hyponatremia and hypokalemia.
Ten days later, the patient returned to the ED complaining of slurred speech for the previous 2 days. A CT scan of her head showed a possible basilar tip aneurysm. Subsequent magnetic resonance imaging with and without contrast and intracranial magnetic resonance angiography confirmed a basilar tip aneurysm and showed findings suggestive of osmotic demyelination. Neurologic examination revealed dysarthria, right upper extremity weakness without spasticity, and periods of confusion interspersed with lucid intervals.
A subsequent neurologic consultation confirmed osmotic demyelination syndrome (formerly known as central pontine myelinolysis). Neurologic examination at that time found continued mild dysarthria, problems standing, inability to walk unsupported, mild oral and pharyngeal dysphagia, and language and writing deficits.
PLAINTIFF’S CLAIM The patient’s sodium level was increased at an inappropriately rapid rate, which caused neurologically devastating osmotic demyelination. Serum sodium should have been monitored every 4 hours during the first 24 hours of treatment. The plaintiff also alleged negligence in continuing normal saline after the patient’s serum sodium was measured at 112 mEq/L.
THE DEFENSE The treatment provided was appropriate.
VERDICT $550,000 California settlement.
COMMENT Avoiding osmotic demyelination syndrome requires careful treatment and monitoring. I have independently reviewed several allegations of malpractice involving this uncommon, but devastating condition. Two recent articles summarize the treatment of this disorder: Sterns RH, Silver S, Klein-schmidt-DeMasters BK, et al. Current perspectives in the management of hyponatremia: prevention of CPM. Expert Rev Neurother. 2007;7:1791-1797; and Lien YH, Shapiro JI. Hyponatremia: clinical diagnosis and management. Am J Med. 2007;120:653-658.
Iodine contrast media kills man with known shellfish allergy
A 41-YEAR-OLD MAN WITH CHEST PAIN was admitted to his local hospital, where he received a diagnosis of acute coronary syndrome. After treatment in the emergency department, the patient was admitted to the telemetry unit by an internist, the partner of the patient’s primary care physician. The patient’s admission records noted that he had an allergy to shellfish.
The next morning, a cardiologist was called in. The cardiologist then called in an interventional cardiologist, who scheduled a cardiac catheterization. The interventional cardiologist ordered 1 dose of steroids, followed a few minutes later by contrast iodine. The patient immediately suffered a severe allergic reaction and died.
PLAINTIFF’S CLAIM The internist who admitted the patient to the telemetry unit took an incomplete history regarding the patient’s allergies (although the admission records contained that information). No information about the claims against the 2 cardiologists is available.
THE DEFENSE No information about the defense is available.
VERDICT $4.7 million gross verdict in Florida.
COMMENT In addition to considering the risk of dye loads and carefully checking renal function, remember to assess for allergy when administering contrast agents. Failure to do so in this case led to the death of the patient and a multimillion-dollar verdict.
Stubborn pneumonia turns out to be cancer
AFTER RECEIVING ANTIBIOTICS FOR PNEUMONIA, a 37-year-old man improved but didn’t fully recover; his radiographs didn’t return to normal. He’d never smoked cigarettes.
During the several months after the pneumonia, the patient’s doctor ordered repeat radiographs and prescribed antibiotics and pain medication. When the patient’s spine collapsed, the doctor diagnosed metastatic lung cancer. The patient received palliative treatment and ultimately died.
PLAINTIFF’S CLAIM The doctor was negligent in failing to change the patient’s treatment after 2 or 3 months and failing to order a computed tomography (CT) scan or refer the patient to a pulmonologist.
THE DEFENSE No information about the doctor’s defense is available.
VERDICT $1.25 million Washington settlement.
COMMENT I’d like a nickel for every case of delayed diagnosis of lung cancer based on clearly abnormal chest radiographs. We can argue about whether diagnosis would make a difference, but we need to follow up assiduously on abnormal radiographs and document our actions.
Rapidly raised serum sodium leads to osmotic demyelination
A 60-YEAR-OLD WOMAN went to her local medical center complaining of a cough for the previous 2 weeks, decreased appetite and oral intake, and generalized body aches. She first went to urgent care, where laboratory studies showed critically low levels of sodium and potassium. Based on these results, the woman was told to go to the facility’s emergency department (ED).
In the ED, she reported feeling very weak and tired and having body aches and pain. When laboratory tests showed that her sodium and potassium levels had fallen further, she was admitted to the intensive care unit (ICU).
The doctor who saw the patient in the ICU ordered intravenous fluids with normal saline and potassium supplements. He then had the patient admitted to the ICU at another hospital. The physician at that hospital continued to prescribe IV sodium and potassium until the patient was discharged with diagnoses that included hyponatremia and hypokalemia.
Ten days later, the patient returned to the ED complaining of slurred speech for the previous 2 days. A CT scan of her head showed a possible basilar tip aneurysm. Subsequent magnetic resonance imaging with and without contrast and intracranial magnetic resonance angiography confirmed a basilar tip aneurysm and showed findings suggestive of osmotic demyelination. Neurologic examination revealed dysarthria, right upper extremity weakness without spasticity, and periods of confusion interspersed with lucid intervals.
A subsequent neurologic consultation confirmed osmotic demyelination syndrome (formerly known as central pontine myelinolysis). Neurologic examination at that time found continued mild dysarthria, problems standing, inability to walk unsupported, mild oral and pharyngeal dysphagia, and language and writing deficits.
PLAINTIFF’S CLAIM The patient’s sodium level was increased at an inappropriately rapid rate, which caused neurologically devastating osmotic demyelination. Serum sodium should have been monitored every 4 hours during the first 24 hours of treatment. The plaintiff also alleged negligence in continuing normal saline after the patient’s serum sodium was measured at 112 mEq/L.
THE DEFENSE The treatment provided was appropriate.
VERDICT $550,000 California settlement.
COMMENT Avoiding osmotic demyelination syndrome requires careful treatment and monitoring. I have independently reviewed several allegations of malpractice involving this uncommon, but devastating condition. Two recent articles summarize the treatment of this disorder: Sterns RH, Silver S, Klein-schmidt-DeMasters BK, et al. Current perspectives in the management of hyponatremia: prevention of CPM. Expert Rev Neurother. 2007;7:1791-1797; and Lien YH, Shapiro JI. Hyponatremia: clinical diagnosis and management. Am J Med. 2007;120:653-658.
Iodine contrast media kills man with known shellfish allergy
A 41-YEAR-OLD MAN WITH CHEST PAIN was admitted to his local hospital, where he received a diagnosis of acute coronary syndrome. After treatment in the emergency department, the patient was admitted to the telemetry unit by an internist, the partner of the patient’s primary care physician. The patient’s admission records noted that he had an allergy to shellfish.
The next morning, a cardiologist was called in. The cardiologist then called in an interventional cardiologist, who scheduled a cardiac catheterization. The interventional cardiologist ordered 1 dose of steroids, followed a few minutes later by contrast iodine. The patient immediately suffered a severe allergic reaction and died.
PLAINTIFF’S CLAIM The internist who admitted the patient to the telemetry unit took an incomplete history regarding the patient’s allergies (although the admission records contained that information). No information about the claims against the 2 cardiologists is available.
THE DEFENSE No information about the defense is available.
VERDICT $4.7 million gross verdict in Florida.
COMMENT In addition to considering the risk of dye loads and carefully checking renal function, remember to assess for allergy when administering contrast agents. Failure to do so in this case led to the death of the patient and a multimillion-dollar verdict.