User login
Quality over Quantity
The Mayo Clinic is technically one. So are Pennsylvania’s Geisinger Health System, California-based Kaiser Permanente, and the Cleveland Clinic. Beyond the handful of long-established and well-integrated sites being labeled as de facto accountable care organizations (ACOs), advocates are seizing the moment and pushing for a bold vision of what role ACOs will play in the movement to reform the healthcare payment system across the country. In at least two major pilot projects in the works, hospitalists are expected to be front and center in leading the transition.
An ACO is an agreed-upon group of providers bands together to assume joint responsibility for both the quality and cost of healthcare for a specific population of beneficiaries. “What an ACO is trying to do is defragment healthcare,” says Mark Werner, MD, chief medical officer for southwest Virginia’s Carilion Clinic. As long as the group meets defined quality benchmarks, its providers can share in any financial rewards that spring from cost savings. But the providers also share in the collective risk of penalties for poor performance. Using the buzzwords of the moment, an “alignment of incentives” could help “bend the curve” of the sharp upturn in healthcare delivery costs.
ACO advocates argue that by pushing quantity over quality, the current fee-for-service payment system actually punishes providers that coordinate care or promote greater efficiencies; policy analysts are nearly unanimous in decreeing that the current model is fundamentally broken and must be replaced. “Well, actually, it’s not broken,” says Alfred Tallia, MD, MPH, professor and chair of the department of family medicine at the Robert Wood Johnson Medical School in New Brunswick, N.J. “It’s working very well for delivering what we’ve got now, which is not what we need, unfortunately.”
—Ralph Whatley, MD, chair, department of medicine, Carilion Clinic, Roanoke, Va.
Perfect Timing
The current push for healthcare reform offers the opportunity to make the case for a more equitable, outcome-oriented payment system as a necessary component of any structure that emerges. Many reform advocates in Massachusetts already have moved from asking how to provide more healthcare coverage to asking how the government can afford it, and ACOs have become a favored mechanism for controlling costs.
The general ACO concept has been backed by the nonpartisan Medicare Payment Advisory Commission, and received another boost when the Accountable Care Promotion Act, initially co-sponsored by Rep. Peter Welch (D-Vt.) and Rep. Earl Pomeroy (D-N.D.) in May, was incorporated in its entirety into the healthcare reform bill introduced in the House of Representatives. The bill would launch a pilot program for ACOs for Medicare beneficiaries, while similar provisions within the Senate healthcare reform bill would set up pilot projects for both Medicare beneficiaries and pediatric beneficiaries of Medicaid or the Children’s Health Insurance Program.
Among the pilot projects already planned, healthcare officials at Robert Wood Johnson are hoping to create an academic-health-center-related ACO to link the disparate elements of healthcare delivery across a large swath. “Our vision is really to build the finest 21st-century integrated delivery system for New Jersey,” Dr. Tallia says. “And that would include everything from advanced, personalized in-home and outpatient primary care to high-tech, leading-edge inpatient quaternary care—and everything in between.”
Virginia’s Carilion Clinic was the first to announce its participation in a separate pilot involving the Engelberg Center for Health Care Reform at the Brookings Institution in Washington, D.C., and the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H. Both institutions have been heavyweights in championing the ACO cause. Dr. Werner says Carilion actually began transforming itself into a more coordinated and integrated organization about three years ago, well before the current ACO buzz began. “We always said from the beginning that we were creating an accountable physicians group, where the physician group had accountability for all of the outcomes important in healthcare,” he says.
The Nitty-Gritty
So how would such organizations actually work? Dr. Tallia sees three absolutes: local accountability, shared savings, and performance measurements. Beyond those necessities, the details begin to blur. The bad taste left by the widely despised capitation payment systems of the 1980s and ’90s has made experts wary of dwelling on the similarities between ACOs and fixed, prepaid capitation plans. Any mention of the C-word, in fact, is followed almost immediately by a caveat: This is a flexible, big-tent strategy that avoids any one-size-fits-all payment prescriptions. And most advocates are emphasizing that ACOs should be voluntary.
Analyses have suggested that in order to succeed, an ACO should enroll 5,000 or more Medicare beneficiaries, or at least 15,000 privately insured patients. Which combination of patients and providers should be included has been left vague to allow emerging networks to tailor the model to their own needs. Some experts differ as to whether hospitals are a necessary component, though almost all agree on the need to include primary-care providers.
Dr. Tallia envisions his medical-school-based linkup as a marriage between New Jersey’s largest multispecialty medical network, the Robert Wood Johnson Medical Group, and the 30% to 40% of primary-care practices in the state that already have relationships with the school. “If you marry the primary-care relationships to the subspecialty care in the Robert Wood Johnson Medical Group and then tie in the area hospitals, by golly, you’ve got an ACO,” he says.
Robert Wood Johnson University Hospital is building an inpatient hospitalist service that will become an integral part of that mission, he says, with its focus on increasing efficiency, reducing the length of hospital stays, appropriate testing and handoffs, and proper communication with other care providers prior to hospital discharges.
ACO Outreach
But any system in which success leads to fewer hospitalizations also needs buy-in from those who stand to lose business. In short, hospitalists and other specialists will need financial incentives, too. That reward system, in turn, requires the right formula for setting and regularly measuring quality standards.
Based on initial savings estimates, however, Dr. Tallia isn’t worried about anyone missing out on a slice of the pie. “We’re looking at somewhere between 15% and 25% cost reductions,” he says, adding participants should gain sizable rewards. Initially, he says, he hopes to start with 5,000 to 10,000 enrollees and launch demonstration projects targeting patient subsets like Medicare beneficiaries and those insured by large employer groups. Ultimately, he’d love to have half of the state’s insured population.
From its own database models, Virginia’s Carilion Clinic estimates that its doctor group takes care of as many as 60,000 Medicare patients per year, with a strong tilt toward primary-care providers. For the past six months, the clinic has been working to identify the geographical scope and specific subset of beneficiaries that would work best for the pilot.
Once it settles on the best combination, Dr. Werner says, the clinic can look at that group’s historical spend rate over the past few years, then agree on a reduction in the rate of growth by, say, 1.5%. “If we’re able to have reductions that exceed 1.5 percent, we would have an opportunity to share in those reductions,” he says.
HM Front and Center
If all goes well, the first pieces of the Carilion ACO will be in place by Jan. 1, and Ralph Whatley, MD, chair of the department of medicine, says the hospitalist program will be “ground zero” in helping to smooth the transition through the proper handling of admissions, discharges, and handoffs of care. “If we do our job as an accountable care organization well, one of the things we should see is that we have less admissions to our hospitalist service,” Dr. Whatley says, especially as the management of such conditions as chronic diseases moves to outpatient settings. Nevertheless, “we can have our hospitalists front and center in the efforts to make the acute management of illness that requires the inpatient setting more efficient, less costly, and with better outcomes.”
Carilion’s hospitalists have played prominent roles in many of the clinic’s quality, safety, and efficiency initiatives. “I would have difficulty imagining that a health system that didn’t have a widespread, cohesive hospitalist service could pull off the kind of inpatient management efficiency, even preventive medicine, that a hospitalist model like ours is going to be able to do,” Dr. Whatley says.
Similarly, he has difficulty imagining how an organization could pull off a successful ACO without ready access to patient information through electronic health records, as Carilion now does. Unsurprisingly, many healthcare payment reform advocates are pushing for the technology needed for ACO-style startups to flourish.
As Dr. Werner says, “You need to give the group of physicians that are going to be part of an accountable group the necessary infrastructure and tools to be able to provide care together.” TH
Bryn Nelson is a freelance writer based in Seattle.
The Mayo Clinic is technically one. So are Pennsylvania’s Geisinger Health System, California-based Kaiser Permanente, and the Cleveland Clinic. Beyond the handful of long-established and well-integrated sites being labeled as de facto accountable care organizations (ACOs), advocates are seizing the moment and pushing for a bold vision of what role ACOs will play in the movement to reform the healthcare payment system across the country. In at least two major pilot projects in the works, hospitalists are expected to be front and center in leading the transition.
An ACO is an agreed-upon group of providers bands together to assume joint responsibility for both the quality and cost of healthcare for a specific population of beneficiaries. “What an ACO is trying to do is defragment healthcare,” says Mark Werner, MD, chief medical officer for southwest Virginia’s Carilion Clinic. As long as the group meets defined quality benchmarks, its providers can share in any financial rewards that spring from cost savings. But the providers also share in the collective risk of penalties for poor performance. Using the buzzwords of the moment, an “alignment of incentives” could help “bend the curve” of the sharp upturn in healthcare delivery costs.
ACO advocates argue that by pushing quantity over quality, the current fee-for-service payment system actually punishes providers that coordinate care or promote greater efficiencies; policy analysts are nearly unanimous in decreeing that the current model is fundamentally broken and must be replaced. “Well, actually, it’s not broken,” says Alfred Tallia, MD, MPH, professor and chair of the department of family medicine at the Robert Wood Johnson Medical School in New Brunswick, N.J. “It’s working very well for delivering what we’ve got now, which is not what we need, unfortunately.”
—Ralph Whatley, MD, chair, department of medicine, Carilion Clinic, Roanoke, Va.
Perfect Timing
The current push for healthcare reform offers the opportunity to make the case for a more equitable, outcome-oriented payment system as a necessary component of any structure that emerges. Many reform advocates in Massachusetts already have moved from asking how to provide more healthcare coverage to asking how the government can afford it, and ACOs have become a favored mechanism for controlling costs.
The general ACO concept has been backed by the nonpartisan Medicare Payment Advisory Commission, and received another boost when the Accountable Care Promotion Act, initially co-sponsored by Rep. Peter Welch (D-Vt.) and Rep. Earl Pomeroy (D-N.D.) in May, was incorporated in its entirety into the healthcare reform bill introduced in the House of Representatives. The bill would launch a pilot program for ACOs for Medicare beneficiaries, while similar provisions within the Senate healthcare reform bill would set up pilot projects for both Medicare beneficiaries and pediatric beneficiaries of Medicaid or the Children’s Health Insurance Program.
Among the pilot projects already planned, healthcare officials at Robert Wood Johnson are hoping to create an academic-health-center-related ACO to link the disparate elements of healthcare delivery across a large swath. “Our vision is really to build the finest 21st-century integrated delivery system for New Jersey,” Dr. Tallia says. “And that would include everything from advanced, personalized in-home and outpatient primary care to high-tech, leading-edge inpatient quaternary care—and everything in between.”
Virginia’s Carilion Clinic was the first to announce its participation in a separate pilot involving the Engelberg Center for Health Care Reform at the Brookings Institution in Washington, D.C., and the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H. Both institutions have been heavyweights in championing the ACO cause. Dr. Werner says Carilion actually began transforming itself into a more coordinated and integrated organization about three years ago, well before the current ACO buzz began. “We always said from the beginning that we were creating an accountable physicians group, where the physician group had accountability for all of the outcomes important in healthcare,” he says.
The Nitty-Gritty
So how would such organizations actually work? Dr. Tallia sees three absolutes: local accountability, shared savings, and performance measurements. Beyond those necessities, the details begin to blur. The bad taste left by the widely despised capitation payment systems of the 1980s and ’90s has made experts wary of dwelling on the similarities between ACOs and fixed, prepaid capitation plans. Any mention of the C-word, in fact, is followed almost immediately by a caveat: This is a flexible, big-tent strategy that avoids any one-size-fits-all payment prescriptions. And most advocates are emphasizing that ACOs should be voluntary.
Analyses have suggested that in order to succeed, an ACO should enroll 5,000 or more Medicare beneficiaries, or at least 15,000 privately insured patients. Which combination of patients and providers should be included has been left vague to allow emerging networks to tailor the model to their own needs. Some experts differ as to whether hospitals are a necessary component, though almost all agree on the need to include primary-care providers.
Dr. Tallia envisions his medical-school-based linkup as a marriage between New Jersey’s largest multispecialty medical network, the Robert Wood Johnson Medical Group, and the 30% to 40% of primary-care practices in the state that already have relationships with the school. “If you marry the primary-care relationships to the subspecialty care in the Robert Wood Johnson Medical Group and then tie in the area hospitals, by golly, you’ve got an ACO,” he says.
Robert Wood Johnson University Hospital is building an inpatient hospitalist service that will become an integral part of that mission, he says, with its focus on increasing efficiency, reducing the length of hospital stays, appropriate testing and handoffs, and proper communication with other care providers prior to hospital discharges.
ACO Outreach
But any system in which success leads to fewer hospitalizations also needs buy-in from those who stand to lose business. In short, hospitalists and other specialists will need financial incentives, too. That reward system, in turn, requires the right formula for setting and regularly measuring quality standards.
Based on initial savings estimates, however, Dr. Tallia isn’t worried about anyone missing out on a slice of the pie. “We’re looking at somewhere between 15% and 25% cost reductions,” he says, adding participants should gain sizable rewards. Initially, he says, he hopes to start with 5,000 to 10,000 enrollees and launch demonstration projects targeting patient subsets like Medicare beneficiaries and those insured by large employer groups. Ultimately, he’d love to have half of the state’s insured population.
From its own database models, Virginia’s Carilion Clinic estimates that its doctor group takes care of as many as 60,000 Medicare patients per year, with a strong tilt toward primary-care providers. For the past six months, the clinic has been working to identify the geographical scope and specific subset of beneficiaries that would work best for the pilot.
Once it settles on the best combination, Dr. Werner says, the clinic can look at that group’s historical spend rate over the past few years, then agree on a reduction in the rate of growth by, say, 1.5%. “If we’re able to have reductions that exceed 1.5 percent, we would have an opportunity to share in those reductions,” he says.
HM Front and Center
If all goes well, the first pieces of the Carilion ACO will be in place by Jan. 1, and Ralph Whatley, MD, chair of the department of medicine, says the hospitalist program will be “ground zero” in helping to smooth the transition through the proper handling of admissions, discharges, and handoffs of care. “If we do our job as an accountable care organization well, one of the things we should see is that we have less admissions to our hospitalist service,” Dr. Whatley says, especially as the management of such conditions as chronic diseases moves to outpatient settings. Nevertheless, “we can have our hospitalists front and center in the efforts to make the acute management of illness that requires the inpatient setting more efficient, less costly, and with better outcomes.”
Carilion’s hospitalists have played prominent roles in many of the clinic’s quality, safety, and efficiency initiatives. “I would have difficulty imagining that a health system that didn’t have a widespread, cohesive hospitalist service could pull off the kind of inpatient management efficiency, even preventive medicine, that a hospitalist model like ours is going to be able to do,” Dr. Whatley says.
Similarly, he has difficulty imagining how an organization could pull off a successful ACO without ready access to patient information through electronic health records, as Carilion now does. Unsurprisingly, many healthcare payment reform advocates are pushing for the technology needed for ACO-style startups to flourish.
As Dr. Werner says, “You need to give the group of physicians that are going to be part of an accountable group the necessary infrastructure and tools to be able to provide care together.” TH
Bryn Nelson is a freelance writer based in Seattle.
The Mayo Clinic is technically one. So are Pennsylvania’s Geisinger Health System, California-based Kaiser Permanente, and the Cleveland Clinic. Beyond the handful of long-established and well-integrated sites being labeled as de facto accountable care organizations (ACOs), advocates are seizing the moment and pushing for a bold vision of what role ACOs will play in the movement to reform the healthcare payment system across the country. In at least two major pilot projects in the works, hospitalists are expected to be front and center in leading the transition.
An ACO is an agreed-upon group of providers bands together to assume joint responsibility for both the quality and cost of healthcare for a specific population of beneficiaries. “What an ACO is trying to do is defragment healthcare,” says Mark Werner, MD, chief medical officer for southwest Virginia’s Carilion Clinic. As long as the group meets defined quality benchmarks, its providers can share in any financial rewards that spring from cost savings. But the providers also share in the collective risk of penalties for poor performance. Using the buzzwords of the moment, an “alignment of incentives” could help “bend the curve” of the sharp upturn in healthcare delivery costs.
ACO advocates argue that by pushing quantity over quality, the current fee-for-service payment system actually punishes providers that coordinate care or promote greater efficiencies; policy analysts are nearly unanimous in decreeing that the current model is fundamentally broken and must be replaced. “Well, actually, it’s not broken,” says Alfred Tallia, MD, MPH, professor and chair of the department of family medicine at the Robert Wood Johnson Medical School in New Brunswick, N.J. “It’s working very well for delivering what we’ve got now, which is not what we need, unfortunately.”
—Ralph Whatley, MD, chair, department of medicine, Carilion Clinic, Roanoke, Va.
Perfect Timing
The current push for healthcare reform offers the opportunity to make the case for a more equitable, outcome-oriented payment system as a necessary component of any structure that emerges. Many reform advocates in Massachusetts already have moved from asking how to provide more healthcare coverage to asking how the government can afford it, and ACOs have become a favored mechanism for controlling costs.
The general ACO concept has been backed by the nonpartisan Medicare Payment Advisory Commission, and received another boost when the Accountable Care Promotion Act, initially co-sponsored by Rep. Peter Welch (D-Vt.) and Rep. Earl Pomeroy (D-N.D.) in May, was incorporated in its entirety into the healthcare reform bill introduced in the House of Representatives. The bill would launch a pilot program for ACOs for Medicare beneficiaries, while similar provisions within the Senate healthcare reform bill would set up pilot projects for both Medicare beneficiaries and pediatric beneficiaries of Medicaid or the Children’s Health Insurance Program.
Among the pilot projects already planned, healthcare officials at Robert Wood Johnson are hoping to create an academic-health-center-related ACO to link the disparate elements of healthcare delivery across a large swath. “Our vision is really to build the finest 21st-century integrated delivery system for New Jersey,” Dr. Tallia says. “And that would include everything from advanced, personalized in-home and outpatient primary care to high-tech, leading-edge inpatient quaternary care—and everything in between.”
Virginia’s Carilion Clinic was the first to announce its participation in a separate pilot involving the Engelberg Center for Health Care Reform at the Brookings Institution in Washington, D.C., and the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H. Both institutions have been heavyweights in championing the ACO cause. Dr. Werner says Carilion actually began transforming itself into a more coordinated and integrated organization about three years ago, well before the current ACO buzz began. “We always said from the beginning that we were creating an accountable physicians group, where the physician group had accountability for all of the outcomes important in healthcare,” he says.
The Nitty-Gritty
So how would such organizations actually work? Dr. Tallia sees three absolutes: local accountability, shared savings, and performance measurements. Beyond those necessities, the details begin to blur. The bad taste left by the widely despised capitation payment systems of the 1980s and ’90s has made experts wary of dwelling on the similarities between ACOs and fixed, prepaid capitation plans. Any mention of the C-word, in fact, is followed almost immediately by a caveat: This is a flexible, big-tent strategy that avoids any one-size-fits-all payment prescriptions. And most advocates are emphasizing that ACOs should be voluntary.
Analyses have suggested that in order to succeed, an ACO should enroll 5,000 or more Medicare beneficiaries, or at least 15,000 privately insured patients. Which combination of patients and providers should be included has been left vague to allow emerging networks to tailor the model to their own needs. Some experts differ as to whether hospitals are a necessary component, though almost all agree on the need to include primary-care providers.
Dr. Tallia envisions his medical-school-based linkup as a marriage between New Jersey’s largest multispecialty medical network, the Robert Wood Johnson Medical Group, and the 30% to 40% of primary-care practices in the state that already have relationships with the school. “If you marry the primary-care relationships to the subspecialty care in the Robert Wood Johnson Medical Group and then tie in the area hospitals, by golly, you’ve got an ACO,” he says.
Robert Wood Johnson University Hospital is building an inpatient hospitalist service that will become an integral part of that mission, he says, with its focus on increasing efficiency, reducing the length of hospital stays, appropriate testing and handoffs, and proper communication with other care providers prior to hospital discharges.
ACO Outreach
But any system in which success leads to fewer hospitalizations also needs buy-in from those who stand to lose business. In short, hospitalists and other specialists will need financial incentives, too. That reward system, in turn, requires the right formula for setting and regularly measuring quality standards.
Based on initial savings estimates, however, Dr. Tallia isn’t worried about anyone missing out on a slice of the pie. “We’re looking at somewhere between 15% and 25% cost reductions,” he says, adding participants should gain sizable rewards. Initially, he says, he hopes to start with 5,000 to 10,000 enrollees and launch demonstration projects targeting patient subsets like Medicare beneficiaries and those insured by large employer groups. Ultimately, he’d love to have half of the state’s insured population.
From its own database models, Virginia’s Carilion Clinic estimates that its doctor group takes care of as many as 60,000 Medicare patients per year, with a strong tilt toward primary-care providers. For the past six months, the clinic has been working to identify the geographical scope and specific subset of beneficiaries that would work best for the pilot.
Once it settles on the best combination, Dr. Werner says, the clinic can look at that group’s historical spend rate over the past few years, then agree on a reduction in the rate of growth by, say, 1.5%. “If we’re able to have reductions that exceed 1.5 percent, we would have an opportunity to share in those reductions,” he says.
HM Front and Center
If all goes well, the first pieces of the Carilion ACO will be in place by Jan. 1, and Ralph Whatley, MD, chair of the department of medicine, says the hospitalist program will be “ground zero” in helping to smooth the transition through the proper handling of admissions, discharges, and handoffs of care. “If we do our job as an accountable care organization well, one of the things we should see is that we have less admissions to our hospitalist service,” Dr. Whatley says, especially as the management of such conditions as chronic diseases moves to outpatient settings. Nevertheless, “we can have our hospitalists front and center in the efforts to make the acute management of illness that requires the inpatient setting more efficient, less costly, and with better outcomes.”
Carilion’s hospitalists have played prominent roles in many of the clinic’s quality, safety, and efficiency initiatives. “I would have difficulty imagining that a health system that didn’t have a widespread, cohesive hospitalist service could pull off the kind of inpatient management efficiency, even preventive medicine, that a hospitalist model like ours is going to be able to do,” Dr. Whatley says.
Similarly, he has difficulty imagining how an organization could pull off a successful ACO without ready access to patient information through electronic health records, as Carilion now does. Unsurprisingly, many healthcare payment reform advocates are pushing for the technology needed for ACO-style startups to flourish.
As Dr. Werner says, “You need to give the group of physicians that are going to be part of an accountable group the necessary infrastructure and tools to be able to provide care together.” TH
Bryn Nelson is a freelance writer based in Seattle.
Submission Support
Physicians receive requests for documentation on a daily basis. Insurer requests need particular attention, as they can be directly related to reimbursement. If the documentation supports the service, payment is rendered (pre-payment request) or maintained (post-payment request). If the documentation is not supportive, payment is denied (pre-payment request) or refunded (post-payment request).
The two most common reasons submitted documentation is not supportive: It lacks information or only a portion of the documentation was submitted.
Not Enough Documentation
“Insufficient documentation” can take many forms. Each visit category (e.g., initial hospital care or subsequent hospital care) and level of service (e.g., 99221-99233) has corresponding documentation requirements. A full list of requirements is available on the Centers for Medicare and Medicaid Services Web site (www.cms.hhs.gov/MLNProducts/Downloads/1995dg.pdf). Selecting an evaluation and management (E/M) level is focused on either upon the content of three key components: history, exam, and decision-making; time can also be a consideration but only when counseling or coordination of care dominate more than 50% of the physician’s total visit time.1 Failure to document any essential element in a given visit level (e.g., family history required but missing for 99222 and 99223) might result in a reviewer down-coding or denying the service.
Dates and signatures are vital to each encounter. The reviewer must be able to identify each individual who performs, documents, and bills for a service, as well as when the service occurred. Notes that lack dates or signatures are not considered in support of a billed service. Notes that contain an illegible signature are equally problematic. If the legibility of a signature prevents the reviewer from correctly identifying the rendering physician, the service can be denied.
It is advisable for the physician to print their name alongside the signature on the encounter note, or include a separate signature sheet with the requested documentation to assist the reviewer in deciphering the physician’s scrawl. Keep in mind that stamped signatures are not acceptable. Medicare accepts handwritten signatures, electronic signatures, or facsimiles of original written or electronic signatures.2
A service is questioned when two different sets of handwriting appear on a note and only one signature is provided. Because the reviewer cannot confirm the credentials of the unidentified individual and cannot be sure which portion belongs to the identified individual, the entire note is disregarded.
Incomplete Submission
Many times, an encounter note does not contain the cumulative information representing the reported service. For example, other pieces of pertinent information might be included in the data section or order section of the chart. If the individual responsible for gathering the requested documentation does not review the information before submitting it, those other important entries could be missed, and the complexity of the billed service might not be justified.
To avoid this, have the designated individual review the note for specific references to information housed in different areas of the chart. The provider should submit any entry with the same date as the requested documentation: labs, diagnostic testing, physician orders, patient instructions, nursing notes, resident notes, notes by other physicians in the same group, discharge summaries, etc.
Legibility is crucial when the documentation is sent for review. Note that the reviewer will not contact the provider if the information is not readable. Most reviewers seek another reviewer’s assistance in translating the handwriting, but they are not obligated to do this. If the note is deemed incomprehensible, the service is denied.
Electronic health records (EHR) are assisting physicians and other providers with legibility issues, and can help take the guesswork out of the note’s content. If a physician is still writing notes by hand, a transcription could be sent along with the documentation to prevent unnecessary denials. It is not advisable to do this for all requests, but only for requests involving providers who have particularly problematic handwriting.
Timeliness of Response
Once the documentation request is received, the physician has a small window of opportunity to review the request, collect the information, and issue a response. A lack of physician response always results in a service denial or a refund request. Once denied, the physician must go through the proper channels of appeal (with a different insurer reviewing department). Requests for refunds are more difficult to overturn. It is difficult to “open” a case that has been “closed.” Denials resulting from a failure to respond to a pre-payment request are a bit easier to resolve because the resulting denial is typically the payor’s initial determination of the claim. The physician usually is allowed an appeal of the payor’s initial determination. However, it is not a cost-effective process to handle prepayment requests in this manner. Always attempt to respond to the initial request within the designated time frame. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Pohlig, C. Documentation and Coding Evaluation and Management Services. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians, 2008;79-109.
- Centers for Medicare and Medicaid Services: CR 5971 Clarification-Signature Requirements. Medicare Learning Network Web site. Available at: www.cms.hhs.gov/MLNMattersArticles/downloads/SE0829.pdf. Accessed Sept. 1, 2009.
- Pohlig C. Evaluation and Management Services: An Overview. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians, 2008; 65-78.
Physicians receive requests for documentation on a daily basis. Insurer requests need particular attention, as they can be directly related to reimbursement. If the documentation supports the service, payment is rendered (pre-payment request) or maintained (post-payment request). If the documentation is not supportive, payment is denied (pre-payment request) or refunded (post-payment request).
The two most common reasons submitted documentation is not supportive: It lacks information or only a portion of the documentation was submitted.
Not Enough Documentation
“Insufficient documentation” can take many forms. Each visit category (e.g., initial hospital care or subsequent hospital care) and level of service (e.g., 99221-99233) has corresponding documentation requirements. A full list of requirements is available on the Centers for Medicare and Medicaid Services Web site (www.cms.hhs.gov/MLNProducts/Downloads/1995dg.pdf). Selecting an evaluation and management (E/M) level is focused on either upon the content of three key components: history, exam, and decision-making; time can also be a consideration but only when counseling or coordination of care dominate more than 50% of the physician’s total visit time.1 Failure to document any essential element in a given visit level (e.g., family history required but missing for 99222 and 99223) might result in a reviewer down-coding or denying the service.
Dates and signatures are vital to each encounter. The reviewer must be able to identify each individual who performs, documents, and bills for a service, as well as when the service occurred. Notes that lack dates or signatures are not considered in support of a billed service. Notes that contain an illegible signature are equally problematic. If the legibility of a signature prevents the reviewer from correctly identifying the rendering physician, the service can be denied.
It is advisable for the physician to print their name alongside the signature on the encounter note, or include a separate signature sheet with the requested documentation to assist the reviewer in deciphering the physician’s scrawl. Keep in mind that stamped signatures are not acceptable. Medicare accepts handwritten signatures, electronic signatures, or facsimiles of original written or electronic signatures.2
A service is questioned when two different sets of handwriting appear on a note and only one signature is provided. Because the reviewer cannot confirm the credentials of the unidentified individual and cannot be sure which portion belongs to the identified individual, the entire note is disregarded.
Incomplete Submission
Many times, an encounter note does not contain the cumulative information representing the reported service. For example, other pieces of pertinent information might be included in the data section or order section of the chart. If the individual responsible for gathering the requested documentation does not review the information before submitting it, those other important entries could be missed, and the complexity of the billed service might not be justified.
To avoid this, have the designated individual review the note for specific references to information housed in different areas of the chart. The provider should submit any entry with the same date as the requested documentation: labs, diagnostic testing, physician orders, patient instructions, nursing notes, resident notes, notes by other physicians in the same group, discharge summaries, etc.
Legibility is crucial when the documentation is sent for review. Note that the reviewer will not contact the provider if the information is not readable. Most reviewers seek another reviewer’s assistance in translating the handwriting, but they are not obligated to do this. If the note is deemed incomprehensible, the service is denied.
Electronic health records (EHR) are assisting physicians and other providers with legibility issues, and can help take the guesswork out of the note’s content. If a physician is still writing notes by hand, a transcription could be sent along with the documentation to prevent unnecessary denials. It is not advisable to do this for all requests, but only for requests involving providers who have particularly problematic handwriting.
Timeliness of Response
Once the documentation request is received, the physician has a small window of opportunity to review the request, collect the information, and issue a response. A lack of physician response always results in a service denial or a refund request. Once denied, the physician must go through the proper channels of appeal (with a different insurer reviewing department). Requests for refunds are more difficult to overturn. It is difficult to “open” a case that has been “closed.” Denials resulting from a failure to respond to a pre-payment request are a bit easier to resolve because the resulting denial is typically the payor’s initial determination of the claim. The physician usually is allowed an appeal of the payor’s initial determination. However, it is not a cost-effective process to handle prepayment requests in this manner. Always attempt to respond to the initial request within the designated time frame. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Pohlig, C. Documentation and Coding Evaluation and Management Services. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians, 2008;79-109.
- Centers for Medicare and Medicaid Services: CR 5971 Clarification-Signature Requirements. Medicare Learning Network Web site. Available at: www.cms.hhs.gov/MLNMattersArticles/downloads/SE0829.pdf. Accessed Sept. 1, 2009.
- Pohlig C. Evaluation and Management Services: An Overview. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians, 2008; 65-78.
Physicians receive requests for documentation on a daily basis. Insurer requests need particular attention, as they can be directly related to reimbursement. If the documentation supports the service, payment is rendered (pre-payment request) or maintained (post-payment request). If the documentation is not supportive, payment is denied (pre-payment request) or refunded (post-payment request).
The two most common reasons submitted documentation is not supportive: It lacks information or only a portion of the documentation was submitted.
Not Enough Documentation
“Insufficient documentation” can take many forms. Each visit category (e.g., initial hospital care or subsequent hospital care) and level of service (e.g., 99221-99233) has corresponding documentation requirements. A full list of requirements is available on the Centers for Medicare and Medicaid Services Web site (www.cms.hhs.gov/MLNProducts/Downloads/1995dg.pdf). Selecting an evaluation and management (E/M) level is focused on either upon the content of three key components: history, exam, and decision-making; time can also be a consideration but only when counseling or coordination of care dominate more than 50% of the physician’s total visit time.1 Failure to document any essential element in a given visit level (e.g., family history required but missing for 99222 and 99223) might result in a reviewer down-coding or denying the service.
Dates and signatures are vital to each encounter. The reviewer must be able to identify each individual who performs, documents, and bills for a service, as well as when the service occurred. Notes that lack dates or signatures are not considered in support of a billed service. Notes that contain an illegible signature are equally problematic. If the legibility of a signature prevents the reviewer from correctly identifying the rendering physician, the service can be denied.
It is advisable for the physician to print their name alongside the signature on the encounter note, or include a separate signature sheet with the requested documentation to assist the reviewer in deciphering the physician’s scrawl. Keep in mind that stamped signatures are not acceptable. Medicare accepts handwritten signatures, electronic signatures, or facsimiles of original written or electronic signatures.2
A service is questioned when two different sets of handwriting appear on a note and only one signature is provided. Because the reviewer cannot confirm the credentials of the unidentified individual and cannot be sure which portion belongs to the identified individual, the entire note is disregarded.
Incomplete Submission
Many times, an encounter note does not contain the cumulative information representing the reported service. For example, other pieces of pertinent information might be included in the data section or order section of the chart. If the individual responsible for gathering the requested documentation does not review the information before submitting it, those other important entries could be missed, and the complexity of the billed service might not be justified.
To avoid this, have the designated individual review the note for specific references to information housed in different areas of the chart. The provider should submit any entry with the same date as the requested documentation: labs, diagnostic testing, physician orders, patient instructions, nursing notes, resident notes, notes by other physicians in the same group, discharge summaries, etc.
Legibility is crucial when the documentation is sent for review. Note that the reviewer will not contact the provider if the information is not readable. Most reviewers seek another reviewer’s assistance in translating the handwriting, but they are not obligated to do this. If the note is deemed incomprehensible, the service is denied.
Electronic health records (EHR) are assisting physicians and other providers with legibility issues, and can help take the guesswork out of the note’s content. If a physician is still writing notes by hand, a transcription could be sent along with the documentation to prevent unnecessary denials. It is not advisable to do this for all requests, but only for requests involving providers who have particularly problematic handwriting.
Timeliness of Response
Once the documentation request is received, the physician has a small window of opportunity to review the request, collect the information, and issue a response. A lack of physician response always results in a service denial or a refund request. Once denied, the physician must go through the proper channels of appeal (with a different insurer reviewing department). Requests for refunds are more difficult to overturn. It is difficult to “open” a case that has been “closed.” Denials resulting from a failure to respond to a pre-payment request are a bit easier to resolve because the resulting denial is typically the payor’s initial determination of the claim. The physician usually is allowed an appeal of the payor’s initial determination. However, it is not a cost-effective process to handle prepayment requests in this manner. Always attempt to respond to the initial request within the designated time frame. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Pohlig, C. Documentation and Coding Evaluation and Management Services. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians, 2008;79-109.
- Centers for Medicare and Medicaid Services: CR 5971 Clarification-Signature Requirements. Medicare Learning Network Web site. Available at: www.cms.hhs.gov/MLNMattersArticles/downloads/SE0829.pdf. Accessed Sept. 1, 2009.
- Pohlig C. Evaluation and Management Services: An Overview. In: Coding for Chest Medicine 2009. Northbrook, Ill.: American College of Chest Physicians, 2008; 65-78.
What is the best initial treatment of an adult patient with healthcare-associated pneumonia?
Case
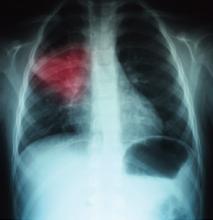
A 68-year-old man with hypertension, diabetes, and recent hip fracture with poor functional status presents from a nursing home with a productive cough, shortness of breath, and chills of two-day duration. He finished a five-day course of cephalexin for a urinary tract infection one week ago. His vital signs reveal a blood pressure of 162/80 mm/Hg, temperature of 101.9°F, respirations of 26 breaths per minute, and oxygen saturation of 88% on room air. Coarse breath sounds are noted in the right lung field and his chest X-ray reveals a right-middle-lobe infiltrate.
He is admitted to the hospital with a diagnosis of healthcare-associated pneumonia. What is the best empiric antibiotic coverage for this patient?
Overview
Modern medicine exists over a continuum of care that is delivered in a manifold of different settings. Patients routinely receive complex medical care at home, including wound care and infusion of intravenous antibiotics. Additionally, many patients are interfacing with the healthcare system on a regular basis via hemodialysis centers or sub-acute rehabilitation centers. As a result of these interactions, patients are exposed to—and colonized by—different bacterial pathogens that can result in a variety of infections.1
While patients with healthcare-associated pneumonia (HCAP) can present similarly to those with community-acquired pneumonia (CAP)—patients with CAP normally present with a lower-respiratory-tract infection—the differences in the likely etiological pathogens dictate that these patients be considered for broader-spectrum empiric antibiotics. Hospitalists will continue to be responsible for choosing the initial antibiotic regimen for these patients, and they need to be able to recognize this disease process in order to treat it appropriately.
The joint American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines released in 2005 emphasize that certain clinical HCAP risk factors center on increased interactions and encounters with healthcare facilities.2 These risk factors are evolving over time to include a patient’s functional status, recent antibiotic use, and clinical severity.
Review of the Data
Differences between HCAP and CAP
HCAP represents a diagnostic category of pneumonia created to differentiate patients with infections caused by a different microbiological subset of bacteria, including possible multi-drug-resistant (MDR) organisms, from patients with CAP. Thus far, culture data support this dichotomy.3,4
Kollef and colleagues performed a multicenter, retrospective cohort study of 4,543 patients with bacterial respiratory culture-positive pneumonia between 2002 and 2003. The study examined the bacteriological differences between CAP and HCAP. In this study, HCAP patients were defined as having: transfer from another healthcare facility; long-term hemodialysis; or prior hospitalization within 30 days in which they had non-ventilator-associated pneumonia (VAP). CAP patients were defined as having non-VAP and non-HCAP.
The study showed that the frequency of Pseudomonas aeurginosa (25% HCAP vs. 17% CAP) and Staphylococcus aureus (46% vs. 25%), which included methicillin-resistant Staphylococcus aureus (MRSA) (18% vs. 6%), was significantly higher in patients with HCAP than those with CAP. Additionally, frequency of Streptococcus pneumoniae (5% vs. 16%) and Haemophilus influenza (5% vs. 16%) infections were noted as significantly lower.3
A single-center, retrospective cohort analysis of 639 patients done by Micek et al yielded similar culture differences between CAP and HCAP patients. In this study, criteria for HCAP were defined as hospitalization in the past year, immunosuppression, nursing-home resident, or hemodialysis. The study authors found that a significantly higher percentage of HCAP patients were infected with MRSA (30% vs. 12%), Pseudomonas aeurginosa (25% vs. 4%), and other non-fermenting gram-negative rods (GNR) (10% vs. 2%). HCAP patients again were noted as having significantly fewer infections with S. pneumoniae (10% vs. 40%) and Haemophilus influenza (4% vs. 17%).
In addition to showing a difference in the bacteriology of CAP and HCAP, the Kollef study also evaluated mortality rates, length of stay, and hospital charges. Mortality rates for HCAP (19.8%) were similar to those of hospital-acquired pneumonia (HAP) (18.8%), and both of these were significantly higher than CAP (10%). Length of stay and hospital cost increased across the spectrum, from CAP to HCAP to HAP, with significant differences between each.3
ATS/IDSA Guidelines
In 2005, a joint committee of the ATS and ISDA updated its initial 1996 nosocomial pneumonia guidelines. The guideline update included the new HCAP category.2 The No. 1 goal of these guidelines was to emphasize early and appropriate antibiotics, followed by tailoring of the treatment regimen based upon culture and clinical data. To this end, HCAP risk factors were developed via extrapolation from observational data generated from HAP and VAP patients.5,6,7
The risk factors are summarized in Table 1 (see p. 19).2 Guidelines dictated that the identification of any of these risk factors in pneumonia patients at the time of admission indicates increased risk for infection with an MDR organism. These high-risk patients require placement into the diagnostic category of HCAP.
Once a patient has been diagnosed with HCAP, the guidelines recommended obtaining lower-respiratory-tract cultures and initiating broad-spectrum antibiotic therapy. Appropriate empiric antibiotic therapy was suggested to be the same as for HAP. This regimen requires coverage with two anti-pseudomonal agents, as well as an agent with activity against MRSA.
The rationale behind initial coverage with two anti-pseudomonal agents stems from the finding that pseudomonas has a high rate of resistance to many antibiotics, and that if two agents are empirically started, chances of appropriate coverage increase from the outset. This is important, as timely administration of appropriate antibiotics has been shown to decrease mortality in infections.8
Additional considerations for empiric antibiotic treatment include sensitivities of local microbiologic data, as well as any recent antibiotic regimens given to the patient. Following this broad primary antibiotic coverage, de-escalation was recommended based on results of lower respiratory cultures and clinical improvement.2
Evolution of Diagnostic Criteria and Empiric Antibiotic Coverage
Since the publication of the 2005 ATS/IDSA guidelines, the aforementioned risk factors for HCAP have been brought into question, as they have yet to be validated by prospective trials. There is a growing concern that these criteria may not be adequately specific and, therefore, might call for too many patients to be treated with a broader spectrum of antibiotic coverage, thereby increasing the likelihood of developing MDR bacteria.
In order to further analyze HCAP criteria, Poch and Ost wrote a review earlier this year examining the data behind each of the risk factors cited in the ATS/IDSA guidelines; they found considerable heterogeneity in magnitude of MDR infection risk for these criteria.9 The authors also reviewed studies looking at other risk factors for MDR infections in patients living in nursing homes or afflicted with CAP. They proposed that such additional factors as patient specific risks (including functional status and previous antibiotic exposure) and contextual risks (including nurse-to-patient ratio) be evaluated and possibly incorporated into criteria.
Of all the patients with HCAP criteria, residents in nursing homes have been studied the best. Loeb et al, while looking for a way to decrease hospitalizations for nursing-home residents, showed that patients who get pneumonia (by guideline definition HCAP) can be effectively treated as outpatients with a single antibiotic agent.10 This randomized controlled trial of 680 patients, all with HCAP, were treated with oral levofloxacin at the nursing home or admitted to the hospital. There were no significant differences between mortality (8% vs. 9%) and quality-of-life measures between the two groups. Furthermore, analysis of data from the 1980s showed that nursing-home-acquired pneumonia could be treated effectively with single agents.11,12
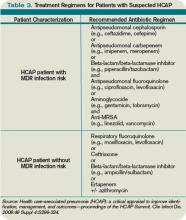
To address some of the questions regarding HCAP, national infectious-disease leaders were brought together to respond to a number of HCAP questions.13 One of the questions centered on the recommended empiric coverage for HCAP. Given the above noted studies in nursing-home patients, disagreement emerged about the need to empirically treat all HCAP patients with broad-spectrum antibiotics. Therefore, another assessment of risk factors for MDR infections was proposed (see Table 2, p. 20) and a consensus was reached, resulting in the current recommendations. The current guidelines state that once a patient has met HCAP criteria, if they have additional MDR risk factors, then broad antibiotic coverage is recommended; however, if no additional MDR risk is found, then more conservative, narrower coverage could be given (see Table 3, p. 31).13
Additional considerations
More studies are needed to refine and validate the specific diagnostic criteria for HCAP, as well as the MDR infectious risk factors. Moreover, current recommendations are for lower respiratory cultures to be obtained on all patients with pneumonia and antibiotic coverage to be titrated according to these results. This practice, however, appears to be uncommon. More data are needed to further guide treatment following initiation of empiric antibiotic coverage without the guidance of culture data, with reliance upon clinical parameters instead.
Back to the Case
This patient met initial criteria for HCAP because he was a nursing home resident, and was found to have additional MDR risk factors (poor functional status and a recent course of antibiotics). Therefore, lower respiratory cultures were obtained, supplemental oxygen was started, and piperacillin/tazobactam plus levofloxacin and vancomycin (with consideration made for local resistance patterns) was administered. He clinically improved over the next two days. His sputum cultures grew Pseudomonas aeuroginosa, which was sensitive to piperacillin/tazobactam but resistant to levofloxacin.
The vancomycin and levofloxacin were discontinued, and he was treated with a seven-day course of piperacillin/tazobactam.
Bottom Line
For adults who present with pneumonia from the community, special attention must be paid to certain parts of the patient’s history to determine if they have HCAP.
Patients who have HCAP can benefit from broad-spectrum empiric antibiotic coverage, which current expert consensus believes is dependent upon further MDR infection risk factors. TH
Dr. Rohde is medicine faculty hospitalist at the University of Michigan in Ann Arbor.
References
- Jernigan JA, Pullen AL, Flowers L, Bell M, Jarvis WR. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol. 2003;24(6):409-414.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(5):3854-3862.
- Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51(10):3568-3573.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903.
- Celis R, Torres A, Gatell JM, Almela M, Rodríguez-Roisin R, Augustí-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93(2):318-324.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001;18(2):362-368.
- Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31 Supple 4:S131-S138.
- Poch DS, Ost DE. What are the important risk factors for healthcare-associated pneumonia? Semin Respir Crit Care Med. 2009;30(1):26-35.
- Loeb M, Carusone SC, Goeree R, et al. Effect of clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503-2510.
- Peterson PK, Stein D, Guay DR, et al. Prospective study of lower respiratory tract infections in an extended-care nursing home program: potential role of oral ciprofloxacin. Am J Med. 1988;85(2):164-171.
- Trenholme GM, Schmitt BA, Spear J, Gvazdinskas LC, Levin S. Randomized study of intravenous/oral ciprofloxacin versus ceftazidime in the treatment of hospital and nursing home patients with lower respiratory tract infections. Am J Med. 1989(5A);87:116S-118S.
- Kollef MH, Morrow LE, Baughman RP, et al. Healthcare-associated pneumonia (HCAP): a critical appraisal to improve identification, management and outcomes—proceedings of the HCAP summit. Clin Infect Dis. 2008;46 Suppl 4:S296-S334.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919.
- El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39(4):474-480.
If you are interested in joining our reader-involvement program, e-mail Editor Jason Carris at [email protected].
Case
A 68-year-old man with hypertension, diabetes, and recent hip fracture with poor functional status presents from a nursing home with a productive cough, shortness of breath, and chills of two-day duration. He finished a five-day course of cephalexin for a urinary tract infection one week ago. His vital signs reveal a blood pressure of 162/80 mm/Hg, temperature of 101.9°F, respirations of 26 breaths per minute, and oxygen saturation of 88% on room air. Coarse breath sounds are noted in the right lung field and his chest X-ray reveals a right-middle-lobe infiltrate.
He is admitted to the hospital with a diagnosis of healthcare-associated pneumonia. What is the best empiric antibiotic coverage for this patient?
Overview
Modern medicine exists over a continuum of care that is delivered in a manifold of different settings. Patients routinely receive complex medical care at home, including wound care and infusion of intravenous antibiotics. Additionally, many patients are interfacing with the healthcare system on a regular basis via hemodialysis centers or sub-acute rehabilitation centers. As a result of these interactions, patients are exposed to—and colonized by—different bacterial pathogens that can result in a variety of infections.1
While patients with healthcare-associated pneumonia (HCAP) can present similarly to those with community-acquired pneumonia (CAP)—patients with CAP normally present with a lower-respiratory-tract infection—the differences in the likely etiological pathogens dictate that these patients be considered for broader-spectrum empiric antibiotics. Hospitalists will continue to be responsible for choosing the initial antibiotic regimen for these patients, and they need to be able to recognize this disease process in order to treat it appropriately.
The joint American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines released in 2005 emphasize that certain clinical HCAP risk factors center on increased interactions and encounters with healthcare facilities.2 These risk factors are evolving over time to include a patient’s functional status, recent antibiotic use, and clinical severity.
Review of the Data
Differences between HCAP and CAP
HCAP represents a diagnostic category of pneumonia created to differentiate patients with infections caused by a different microbiological subset of bacteria, including possible multi-drug-resistant (MDR) organisms, from patients with CAP. Thus far, culture data support this dichotomy.3,4
Kollef and colleagues performed a multicenter, retrospective cohort study of 4,543 patients with bacterial respiratory culture-positive pneumonia between 2002 and 2003. The study examined the bacteriological differences between CAP and HCAP. In this study, HCAP patients were defined as having: transfer from another healthcare facility; long-term hemodialysis; or prior hospitalization within 30 days in which they had non-ventilator-associated pneumonia (VAP). CAP patients were defined as having non-VAP and non-HCAP.
The study showed that the frequency of Pseudomonas aeurginosa (25% HCAP vs. 17% CAP) and Staphylococcus aureus (46% vs. 25%), which included methicillin-resistant Staphylococcus aureus (MRSA) (18% vs. 6%), was significantly higher in patients with HCAP than those with CAP. Additionally, frequency of Streptococcus pneumoniae (5% vs. 16%) and Haemophilus influenza (5% vs. 16%) infections were noted as significantly lower.3
A single-center, retrospective cohort analysis of 639 patients done by Micek et al yielded similar culture differences between CAP and HCAP patients. In this study, criteria for HCAP were defined as hospitalization in the past year, immunosuppression, nursing-home resident, or hemodialysis. The study authors found that a significantly higher percentage of HCAP patients were infected with MRSA (30% vs. 12%), Pseudomonas aeurginosa (25% vs. 4%), and other non-fermenting gram-negative rods (GNR) (10% vs. 2%). HCAP patients again were noted as having significantly fewer infections with S. pneumoniae (10% vs. 40%) and Haemophilus influenza (4% vs. 17%).
In addition to showing a difference in the bacteriology of CAP and HCAP, the Kollef study also evaluated mortality rates, length of stay, and hospital charges. Mortality rates for HCAP (19.8%) were similar to those of hospital-acquired pneumonia (HAP) (18.8%), and both of these were significantly higher than CAP (10%). Length of stay and hospital cost increased across the spectrum, from CAP to HCAP to HAP, with significant differences between each.3
ATS/IDSA Guidelines
In 2005, a joint committee of the ATS and ISDA updated its initial 1996 nosocomial pneumonia guidelines. The guideline update included the new HCAP category.2 The No. 1 goal of these guidelines was to emphasize early and appropriate antibiotics, followed by tailoring of the treatment regimen based upon culture and clinical data. To this end, HCAP risk factors were developed via extrapolation from observational data generated from HAP and VAP patients.5,6,7
The risk factors are summarized in Table 1 (see p. 19).2 Guidelines dictated that the identification of any of these risk factors in pneumonia patients at the time of admission indicates increased risk for infection with an MDR organism. These high-risk patients require placement into the diagnostic category of HCAP.
Once a patient has been diagnosed with HCAP, the guidelines recommended obtaining lower-respiratory-tract cultures and initiating broad-spectrum antibiotic therapy. Appropriate empiric antibiotic therapy was suggested to be the same as for HAP. This regimen requires coverage with two anti-pseudomonal agents, as well as an agent with activity against MRSA.
The rationale behind initial coverage with two anti-pseudomonal agents stems from the finding that pseudomonas has a high rate of resistance to many antibiotics, and that if two agents are empirically started, chances of appropriate coverage increase from the outset. This is important, as timely administration of appropriate antibiotics has been shown to decrease mortality in infections.8
Additional considerations for empiric antibiotic treatment include sensitivities of local microbiologic data, as well as any recent antibiotic regimens given to the patient. Following this broad primary antibiotic coverage, de-escalation was recommended based on results of lower respiratory cultures and clinical improvement.2
Evolution of Diagnostic Criteria and Empiric Antibiotic Coverage
Since the publication of the 2005 ATS/IDSA guidelines, the aforementioned risk factors for HCAP have been brought into question, as they have yet to be validated by prospective trials. There is a growing concern that these criteria may not be adequately specific and, therefore, might call for too many patients to be treated with a broader spectrum of antibiotic coverage, thereby increasing the likelihood of developing MDR bacteria.
In order to further analyze HCAP criteria, Poch and Ost wrote a review earlier this year examining the data behind each of the risk factors cited in the ATS/IDSA guidelines; they found considerable heterogeneity in magnitude of MDR infection risk for these criteria.9 The authors also reviewed studies looking at other risk factors for MDR infections in patients living in nursing homes or afflicted with CAP. They proposed that such additional factors as patient specific risks (including functional status and previous antibiotic exposure) and contextual risks (including nurse-to-patient ratio) be evaluated and possibly incorporated into criteria.
Of all the patients with HCAP criteria, residents in nursing homes have been studied the best. Loeb et al, while looking for a way to decrease hospitalizations for nursing-home residents, showed that patients who get pneumonia (by guideline definition HCAP) can be effectively treated as outpatients with a single antibiotic agent.10 This randomized controlled trial of 680 patients, all with HCAP, were treated with oral levofloxacin at the nursing home or admitted to the hospital. There were no significant differences between mortality (8% vs. 9%) and quality-of-life measures between the two groups. Furthermore, analysis of data from the 1980s showed that nursing-home-acquired pneumonia could be treated effectively with single agents.11,12
To address some of the questions regarding HCAP, national infectious-disease leaders were brought together to respond to a number of HCAP questions.13 One of the questions centered on the recommended empiric coverage for HCAP. Given the above noted studies in nursing-home patients, disagreement emerged about the need to empirically treat all HCAP patients with broad-spectrum antibiotics. Therefore, another assessment of risk factors for MDR infections was proposed (see Table 2, p. 20) and a consensus was reached, resulting in the current recommendations. The current guidelines state that once a patient has met HCAP criteria, if they have additional MDR risk factors, then broad antibiotic coverage is recommended; however, if no additional MDR risk is found, then more conservative, narrower coverage could be given (see Table 3, p. 31).13
Additional considerations
More studies are needed to refine and validate the specific diagnostic criteria for HCAP, as well as the MDR infectious risk factors. Moreover, current recommendations are for lower respiratory cultures to be obtained on all patients with pneumonia and antibiotic coverage to be titrated according to these results. This practice, however, appears to be uncommon. More data are needed to further guide treatment following initiation of empiric antibiotic coverage without the guidance of culture data, with reliance upon clinical parameters instead.
Back to the Case
This patient met initial criteria for HCAP because he was a nursing home resident, and was found to have additional MDR risk factors (poor functional status and a recent course of antibiotics). Therefore, lower respiratory cultures were obtained, supplemental oxygen was started, and piperacillin/tazobactam plus levofloxacin and vancomycin (with consideration made for local resistance patterns) was administered. He clinically improved over the next two days. His sputum cultures grew Pseudomonas aeuroginosa, which was sensitive to piperacillin/tazobactam but resistant to levofloxacin.
The vancomycin and levofloxacin were discontinued, and he was treated with a seven-day course of piperacillin/tazobactam.
Bottom Line
For adults who present with pneumonia from the community, special attention must be paid to certain parts of the patient’s history to determine if they have HCAP.
Patients who have HCAP can benefit from broad-spectrum empiric antibiotic coverage, which current expert consensus believes is dependent upon further MDR infection risk factors. TH
Dr. Rohde is medicine faculty hospitalist at the University of Michigan in Ann Arbor.
References
- Jernigan JA, Pullen AL, Flowers L, Bell M, Jarvis WR. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol. 2003;24(6):409-414.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(5):3854-3862.
- Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51(10):3568-3573.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903.
- Celis R, Torres A, Gatell JM, Almela M, Rodríguez-Roisin R, Augustí-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93(2):318-324.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001;18(2):362-368.
- Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31 Supple 4:S131-S138.
- Poch DS, Ost DE. What are the important risk factors for healthcare-associated pneumonia? Semin Respir Crit Care Med. 2009;30(1):26-35.
- Loeb M, Carusone SC, Goeree R, et al. Effect of clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503-2510.
- Peterson PK, Stein D, Guay DR, et al. Prospective study of lower respiratory tract infections in an extended-care nursing home program: potential role of oral ciprofloxacin. Am J Med. 1988;85(2):164-171.
- Trenholme GM, Schmitt BA, Spear J, Gvazdinskas LC, Levin S. Randomized study of intravenous/oral ciprofloxacin versus ceftazidime in the treatment of hospital and nursing home patients with lower respiratory tract infections. Am J Med. 1989(5A);87:116S-118S.
- Kollef MH, Morrow LE, Baughman RP, et al. Healthcare-associated pneumonia (HCAP): a critical appraisal to improve identification, management and outcomes—proceedings of the HCAP summit. Clin Infect Dis. 2008;46 Suppl 4:S296-S334.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919.
- El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39(4):474-480.
If you are interested in joining our reader-involvement program, e-mail Editor Jason Carris at [email protected].
Case
A 68-year-old man with hypertension, diabetes, and recent hip fracture with poor functional status presents from a nursing home with a productive cough, shortness of breath, and chills of two-day duration. He finished a five-day course of cephalexin for a urinary tract infection one week ago. His vital signs reveal a blood pressure of 162/80 mm/Hg, temperature of 101.9°F, respirations of 26 breaths per minute, and oxygen saturation of 88% on room air. Coarse breath sounds are noted in the right lung field and his chest X-ray reveals a right-middle-lobe infiltrate.
He is admitted to the hospital with a diagnosis of healthcare-associated pneumonia. What is the best empiric antibiotic coverage for this patient?
Overview
Modern medicine exists over a continuum of care that is delivered in a manifold of different settings. Patients routinely receive complex medical care at home, including wound care and infusion of intravenous antibiotics. Additionally, many patients are interfacing with the healthcare system on a regular basis via hemodialysis centers or sub-acute rehabilitation centers. As a result of these interactions, patients are exposed to—and colonized by—different bacterial pathogens that can result in a variety of infections.1
While patients with healthcare-associated pneumonia (HCAP) can present similarly to those with community-acquired pneumonia (CAP)—patients with CAP normally present with a lower-respiratory-tract infection—the differences in the likely etiological pathogens dictate that these patients be considered for broader-spectrum empiric antibiotics. Hospitalists will continue to be responsible for choosing the initial antibiotic regimen for these patients, and they need to be able to recognize this disease process in order to treat it appropriately.
The joint American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines released in 2005 emphasize that certain clinical HCAP risk factors center on increased interactions and encounters with healthcare facilities.2 These risk factors are evolving over time to include a patient’s functional status, recent antibiotic use, and clinical severity.
Review of the Data
Differences between HCAP and CAP
HCAP represents a diagnostic category of pneumonia created to differentiate patients with infections caused by a different microbiological subset of bacteria, including possible multi-drug-resistant (MDR) organisms, from patients with CAP. Thus far, culture data support this dichotomy.3,4
Kollef and colleagues performed a multicenter, retrospective cohort study of 4,543 patients with bacterial respiratory culture-positive pneumonia between 2002 and 2003. The study examined the bacteriological differences between CAP and HCAP. In this study, HCAP patients were defined as having: transfer from another healthcare facility; long-term hemodialysis; or prior hospitalization within 30 days in which they had non-ventilator-associated pneumonia (VAP). CAP patients were defined as having non-VAP and non-HCAP.
The study showed that the frequency of Pseudomonas aeurginosa (25% HCAP vs. 17% CAP) and Staphylococcus aureus (46% vs. 25%), which included methicillin-resistant Staphylococcus aureus (MRSA) (18% vs. 6%), was significantly higher in patients with HCAP than those with CAP. Additionally, frequency of Streptococcus pneumoniae (5% vs. 16%) and Haemophilus influenza (5% vs. 16%) infections were noted as significantly lower.3
A single-center, retrospective cohort analysis of 639 patients done by Micek et al yielded similar culture differences between CAP and HCAP patients. In this study, criteria for HCAP were defined as hospitalization in the past year, immunosuppression, nursing-home resident, or hemodialysis. The study authors found that a significantly higher percentage of HCAP patients were infected with MRSA (30% vs. 12%), Pseudomonas aeurginosa (25% vs. 4%), and other non-fermenting gram-negative rods (GNR) (10% vs. 2%). HCAP patients again were noted as having significantly fewer infections with S. pneumoniae (10% vs. 40%) and Haemophilus influenza (4% vs. 17%).
In addition to showing a difference in the bacteriology of CAP and HCAP, the Kollef study also evaluated mortality rates, length of stay, and hospital charges. Mortality rates for HCAP (19.8%) were similar to those of hospital-acquired pneumonia (HAP) (18.8%), and both of these were significantly higher than CAP (10%). Length of stay and hospital cost increased across the spectrum, from CAP to HCAP to HAP, with significant differences between each.3
ATS/IDSA Guidelines
In 2005, a joint committee of the ATS and ISDA updated its initial 1996 nosocomial pneumonia guidelines. The guideline update included the new HCAP category.2 The No. 1 goal of these guidelines was to emphasize early and appropriate antibiotics, followed by tailoring of the treatment regimen based upon culture and clinical data. To this end, HCAP risk factors were developed via extrapolation from observational data generated from HAP and VAP patients.5,6,7
The risk factors are summarized in Table 1 (see p. 19).2 Guidelines dictated that the identification of any of these risk factors in pneumonia patients at the time of admission indicates increased risk for infection with an MDR organism. These high-risk patients require placement into the diagnostic category of HCAP.
Once a patient has been diagnosed with HCAP, the guidelines recommended obtaining lower-respiratory-tract cultures and initiating broad-spectrum antibiotic therapy. Appropriate empiric antibiotic therapy was suggested to be the same as for HAP. This regimen requires coverage with two anti-pseudomonal agents, as well as an agent with activity against MRSA.
The rationale behind initial coverage with two anti-pseudomonal agents stems from the finding that pseudomonas has a high rate of resistance to many antibiotics, and that if two agents are empirically started, chances of appropriate coverage increase from the outset. This is important, as timely administration of appropriate antibiotics has been shown to decrease mortality in infections.8
Additional considerations for empiric antibiotic treatment include sensitivities of local microbiologic data, as well as any recent antibiotic regimens given to the patient. Following this broad primary antibiotic coverage, de-escalation was recommended based on results of lower respiratory cultures and clinical improvement.2
Evolution of Diagnostic Criteria and Empiric Antibiotic Coverage
Since the publication of the 2005 ATS/IDSA guidelines, the aforementioned risk factors for HCAP have been brought into question, as they have yet to be validated by prospective trials. There is a growing concern that these criteria may not be adequately specific and, therefore, might call for too many patients to be treated with a broader spectrum of antibiotic coverage, thereby increasing the likelihood of developing MDR bacteria.
In order to further analyze HCAP criteria, Poch and Ost wrote a review earlier this year examining the data behind each of the risk factors cited in the ATS/IDSA guidelines; they found considerable heterogeneity in magnitude of MDR infection risk for these criteria.9 The authors also reviewed studies looking at other risk factors for MDR infections in patients living in nursing homes or afflicted with CAP. They proposed that such additional factors as patient specific risks (including functional status and previous antibiotic exposure) and contextual risks (including nurse-to-patient ratio) be evaluated and possibly incorporated into criteria.
Of all the patients with HCAP criteria, residents in nursing homes have been studied the best. Loeb et al, while looking for a way to decrease hospitalizations for nursing-home residents, showed that patients who get pneumonia (by guideline definition HCAP) can be effectively treated as outpatients with a single antibiotic agent.10 This randomized controlled trial of 680 patients, all with HCAP, were treated with oral levofloxacin at the nursing home or admitted to the hospital. There were no significant differences between mortality (8% vs. 9%) and quality-of-life measures between the two groups. Furthermore, analysis of data from the 1980s showed that nursing-home-acquired pneumonia could be treated effectively with single agents.11,12
To address some of the questions regarding HCAP, national infectious-disease leaders were brought together to respond to a number of HCAP questions.13 One of the questions centered on the recommended empiric coverage for HCAP. Given the above noted studies in nursing-home patients, disagreement emerged about the need to empirically treat all HCAP patients with broad-spectrum antibiotics. Therefore, another assessment of risk factors for MDR infections was proposed (see Table 2, p. 20) and a consensus was reached, resulting in the current recommendations. The current guidelines state that once a patient has met HCAP criteria, if they have additional MDR risk factors, then broad antibiotic coverage is recommended; however, if no additional MDR risk is found, then more conservative, narrower coverage could be given (see Table 3, p. 31).13
Additional considerations
More studies are needed to refine and validate the specific diagnostic criteria for HCAP, as well as the MDR infectious risk factors. Moreover, current recommendations are for lower respiratory cultures to be obtained on all patients with pneumonia and antibiotic coverage to be titrated according to these results. This practice, however, appears to be uncommon. More data are needed to further guide treatment following initiation of empiric antibiotic coverage without the guidance of culture data, with reliance upon clinical parameters instead.
Back to the Case
This patient met initial criteria for HCAP because he was a nursing home resident, and was found to have additional MDR risk factors (poor functional status and a recent course of antibiotics). Therefore, lower respiratory cultures were obtained, supplemental oxygen was started, and piperacillin/tazobactam plus levofloxacin and vancomycin (with consideration made for local resistance patterns) was administered. He clinically improved over the next two days. His sputum cultures grew Pseudomonas aeuroginosa, which was sensitive to piperacillin/tazobactam but resistant to levofloxacin.
The vancomycin and levofloxacin were discontinued, and he was treated with a seven-day course of piperacillin/tazobactam.
Bottom Line
For adults who present with pneumonia from the community, special attention must be paid to certain parts of the patient’s history to determine if they have HCAP.
Patients who have HCAP can benefit from broad-spectrum empiric antibiotic coverage, which current expert consensus believes is dependent upon further MDR infection risk factors. TH
Dr. Rohde is medicine faculty hospitalist at the University of Michigan in Ann Arbor.
References
- Jernigan JA, Pullen AL, Flowers L, Bell M, Jarvis WR. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol. 2003;24(6):409-414.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416.
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(5):3854-3862.
- Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51(10):3568-3573.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903.
- Celis R, Torres A, Gatell JM, Almela M, Rodríguez-Roisin R, Augustí-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93(2):318-324.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001;18(2):362-368.
- Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31 Supple 4:S131-S138.
- Poch DS, Ost DE. What are the important risk factors for healthcare-associated pneumonia? Semin Respir Crit Care Med. 2009;30(1):26-35.
- Loeb M, Carusone SC, Goeree R, et al. Effect of clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503-2510.
- Peterson PK, Stein D, Guay DR, et al. Prospective study of lower respiratory tract infections in an extended-care nursing home program: potential role of oral ciprofloxacin. Am J Med. 1988;85(2):164-171.
- Trenholme GM, Schmitt BA, Spear J, Gvazdinskas LC, Levin S. Randomized study of intravenous/oral ciprofloxacin versus ceftazidime in the treatment of hospital and nursing home patients with lower respiratory tract infections. Am J Med. 1989(5A);87:116S-118S.
- Kollef MH, Morrow LE, Baughman RP, et al. Healthcare-associated pneumonia (HCAP): a critical appraisal to improve identification, management and outcomes—proceedings of the HCAP summit. Clin Infect Dis. 2008;46 Suppl 4:S296-S334.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919.
- El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39(4):474-480.
If you are interested in joining our reader-involvement program, e-mail Editor Jason Carris at [email protected].
Should group directors continue clinical practice?
PRO
Clinical practice is beneficial to patients, the group, and your career
Finding a balance between clinical care and leadership duties truly is a challenge for hospitalist directors. Changes in the landscape of inpatient care delivery, rapid growth of HM groups, and expansion of hospitalist roles have resulted in a substantial increase in a director’s responsibilities. Today’s hospitalist leader squarely faces the dilemma of continuing clinical practice and performing administrative efforts while demonstrating competence in each. To be effective, this is precisely what physician leaders must strive to do.
Maintaining clinical practice alongside directorship duties conveys advantages in critical leadership areas. You must consider the benefits to your patient, your career, and the hospitalist group.
The Patient, Director, Group
Physician leaders offer clinical experience combined with a unique perspective on systems of care, or “the big picture.”
Likewise, caring for patients provides the opportunity to interact with and listen to the customer, which is necessary for important outcomes, such as patient satisfaction. It reminds us that we are here to care for and about patients, keeping our efforts patient-centered.
Direct patient care refocuses directors on the fundamental reason they are in leadership. It offers intrinsic professional rewards and intellectual satisfaction that will sustain and strengthen the leadership role. The effective leader strategically finds balance by delegating, prioritizing, and focusing on time management.
Continuing your clinical practice affords physician leaders leverage with their constituents—the hospitalists. Working in the trenches, especially during critical times, yields legitimacy and credibility. It also allows the leader to identify with and respond to concerns raised by members. This can connect the leader to the group, avoiding the “suit vs. white coat” dynamic. The same principle extends to other stakeholders who are part of the care team, such as nurses and referring physicians.
Other Factors
Maintaining clinical aptitude ensures that leaders stay apprised of current practices, and are aware of the latest techniques, data, and evidence. This is critical for ensuring group performance in quality initiatives, and for setting standards of clinical excellence in the group practice.
In academic centers, ward teaching allows leaders to train future physicians, pass on knowledge, and gain an understanding of the next generation and its priorities, thus keeping an eye on the future and having a clear vision.
Perhaps the most important benefit direct patient care provides in leadership is the ability to accomplish the group’s mission. A firsthand experience brings understanding of issues around workflow, efficiency, and career satisfaction. It allows leaders to audit best practices. It inspires innovative ideas for healthcare delivery and processing improvement changes.
The model of successful physician leadership is based on clinical excellence. The construct of a separation between clinical and administrative roles is a false dichotomy; the two are interdependent. HM directors have a duty to perform both, as it is the combination that makes leaders successful. TH
Dr. Wright is associate clinical professor and chief of the division of hospital medicine at the University of Wisconsin School of Medicine and Public Health in Madison.
CON
Physician leaders should relinquish clinical practice, focus on leading
I believe the vast majority of hospitalists agree with the “pro” side of this debate, but I also believe that this kind of knee-jerk reaction reflects the core deficiency that plagues physicians’ thinking regarding leadership.
The way medicine is being practiced and delivered in the hospital setting is rapidly changing. In fact, our specialty is based on this premise. Yet hospitalists still have a stone-age mentality when it comes to physician leaders. The concept of leadership, in most cases, is an afterthought.
Our Role as Leaders
The HM leader is expected to act as a caretaker: set the schedule, organize and implement QI programs, and represent the hospitalists to administration. Most HM directors are accidental leaders who sheepishly step into a position when the opportunity presents itself. This usually happens as programs grow. Few industries would accept this business model. Leadership should be considered critical and given its due respect in terms of resources, training, and experience. Rarely are supervisory positions rewarded to accidental, part-time volunteers. Leaders are chosen, groomed, and given the sufficient time and resources to carry out their mandate.
When HM programs become dysfunctional, hospitalists are quick to blame the administration—some refer to it as the “evil empire” or “the dark side.” But interesting research by Gallup Inc. has shown that the majority of employees who leave their jobs actually are leaving their manager.1
Wants vs. Needs
Leaders face dilemmas every work day. For instance, leaders need to communicate the administration’s goals and weave them into HM department systems and policies. Conversely, HM leaders have to negotiate with administration to secure the resources they need to execute those goals. Technologies are mere facilitators; people actually produce results. Yet many administrators and HM leaders are fixated on the latest software without giving much thought about how staff will implement the changes.
HM leaders need time and resources to be effective. As hospitalists, we’ve been bombarded by the evidence-based medicine mantra. But most hospitalists have never heard of, or they laugh at, evidence-based techniques that were first documented in the 1970s.2 Data is available regarding management skills that can be used to effect positive organization behavior.
We also need to be authentic leaders to combat internal disruptions from medical staff. Gallup Management research has shown that 42% of physicians on medical staffs are actively disengaged.3 Physicians not only are distant, they also actively sabotage and poison new efforts introduced by administration or physician leaders.
The hospitalist leader should only perform clinical responsibilities if they are absolutely necessary. The HM director should be given all the time, resources, due respect, and training to be a dynamic leader. The hospitalist movement would be better for it. TH
References
- Buckingham, M, Coffman C. First, Break All the Rules: How Managers Trump Companies. 1999. New York City: Simon & Schuster.
- Luthans F. Organizational Behavior. 1973. New York City: McGraw-Hill.
- Paller D. What the doctor ordered. Gallup Management Web site. Available at: http://gmj.gallup.com/content/18361/What-Doctor-Ordered.aspx. Accessed Nov. 9, 2009.
Dr. Yu is medical director of hospitalist services at Decatur (Ill.) Memorial Hospital.
PRO
Clinical practice is beneficial to patients, the group, and your career
Finding a balance between clinical care and leadership duties truly is a challenge for hospitalist directors. Changes in the landscape of inpatient care delivery, rapid growth of HM groups, and expansion of hospitalist roles have resulted in a substantial increase in a director’s responsibilities. Today’s hospitalist leader squarely faces the dilemma of continuing clinical practice and performing administrative efforts while demonstrating competence in each. To be effective, this is precisely what physician leaders must strive to do.
Maintaining clinical practice alongside directorship duties conveys advantages in critical leadership areas. You must consider the benefits to your patient, your career, and the hospitalist group.
The Patient, Director, Group
Physician leaders offer clinical experience combined with a unique perspective on systems of care, or “the big picture.”
Likewise, caring for patients provides the opportunity to interact with and listen to the customer, which is necessary for important outcomes, such as patient satisfaction. It reminds us that we are here to care for and about patients, keeping our efforts patient-centered.
Direct patient care refocuses directors on the fundamental reason they are in leadership. It offers intrinsic professional rewards and intellectual satisfaction that will sustain and strengthen the leadership role. The effective leader strategically finds balance by delegating, prioritizing, and focusing on time management.
Continuing your clinical practice affords physician leaders leverage with their constituents—the hospitalists. Working in the trenches, especially during critical times, yields legitimacy and credibility. It also allows the leader to identify with and respond to concerns raised by members. This can connect the leader to the group, avoiding the “suit vs. white coat” dynamic. The same principle extends to other stakeholders who are part of the care team, such as nurses and referring physicians.
Other Factors
Maintaining clinical aptitude ensures that leaders stay apprised of current practices, and are aware of the latest techniques, data, and evidence. This is critical for ensuring group performance in quality initiatives, and for setting standards of clinical excellence in the group practice.
In academic centers, ward teaching allows leaders to train future physicians, pass on knowledge, and gain an understanding of the next generation and its priorities, thus keeping an eye on the future and having a clear vision.
Perhaps the most important benefit direct patient care provides in leadership is the ability to accomplish the group’s mission. A firsthand experience brings understanding of issues around workflow, efficiency, and career satisfaction. It allows leaders to audit best practices. It inspires innovative ideas for healthcare delivery and processing improvement changes.
The model of successful physician leadership is based on clinical excellence. The construct of a separation between clinical and administrative roles is a false dichotomy; the two are interdependent. HM directors have a duty to perform both, as it is the combination that makes leaders successful. TH
Dr. Wright is associate clinical professor and chief of the division of hospital medicine at the University of Wisconsin School of Medicine and Public Health in Madison.
CON
Physician leaders should relinquish clinical practice, focus on leading
I believe the vast majority of hospitalists agree with the “pro” side of this debate, but I also believe that this kind of knee-jerk reaction reflects the core deficiency that plagues physicians’ thinking regarding leadership.
The way medicine is being practiced and delivered in the hospital setting is rapidly changing. In fact, our specialty is based on this premise. Yet hospitalists still have a stone-age mentality when it comes to physician leaders. The concept of leadership, in most cases, is an afterthought.
Our Role as Leaders
The HM leader is expected to act as a caretaker: set the schedule, organize and implement QI programs, and represent the hospitalists to administration. Most HM directors are accidental leaders who sheepishly step into a position when the opportunity presents itself. This usually happens as programs grow. Few industries would accept this business model. Leadership should be considered critical and given its due respect in terms of resources, training, and experience. Rarely are supervisory positions rewarded to accidental, part-time volunteers. Leaders are chosen, groomed, and given the sufficient time and resources to carry out their mandate.
When HM programs become dysfunctional, hospitalists are quick to blame the administration—some refer to it as the “evil empire” or “the dark side.” But interesting research by Gallup Inc. has shown that the majority of employees who leave their jobs actually are leaving their manager.1
Wants vs. Needs
Leaders face dilemmas every work day. For instance, leaders need to communicate the administration’s goals and weave them into HM department systems and policies. Conversely, HM leaders have to negotiate with administration to secure the resources they need to execute those goals. Technologies are mere facilitators; people actually produce results. Yet many administrators and HM leaders are fixated on the latest software without giving much thought about how staff will implement the changes.
HM leaders need time and resources to be effective. As hospitalists, we’ve been bombarded by the evidence-based medicine mantra. But most hospitalists have never heard of, or they laugh at, evidence-based techniques that were first documented in the 1970s.2 Data is available regarding management skills that can be used to effect positive organization behavior.
We also need to be authentic leaders to combat internal disruptions from medical staff. Gallup Management research has shown that 42% of physicians on medical staffs are actively disengaged.3 Physicians not only are distant, they also actively sabotage and poison new efforts introduced by administration or physician leaders.
The hospitalist leader should only perform clinical responsibilities if they are absolutely necessary. The HM director should be given all the time, resources, due respect, and training to be a dynamic leader. The hospitalist movement would be better for it. TH
References
- Buckingham, M, Coffman C. First, Break All the Rules: How Managers Trump Companies. 1999. New York City: Simon & Schuster.
- Luthans F. Organizational Behavior. 1973. New York City: McGraw-Hill.
- Paller D. What the doctor ordered. Gallup Management Web site. Available at: http://gmj.gallup.com/content/18361/What-Doctor-Ordered.aspx. Accessed Nov. 9, 2009.
Dr. Yu is medical director of hospitalist services at Decatur (Ill.) Memorial Hospital.
PRO
Clinical practice is beneficial to patients, the group, and your career
Finding a balance between clinical care and leadership duties truly is a challenge for hospitalist directors. Changes in the landscape of inpatient care delivery, rapid growth of HM groups, and expansion of hospitalist roles have resulted in a substantial increase in a director’s responsibilities. Today’s hospitalist leader squarely faces the dilemma of continuing clinical practice and performing administrative efforts while demonstrating competence in each. To be effective, this is precisely what physician leaders must strive to do.
Maintaining clinical practice alongside directorship duties conveys advantages in critical leadership areas. You must consider the benefits to your patient, your career, and the hospitalist group.
The Patient, Director, Group
Physician leaders offer clinical experience combined with a unique perspective on systems of care, or “the big picture.”
Likewise, caring for patients provides the opportunity to interact with and listen to the customer, which is necessary for important outcomes, such as patient satisfaction. It reminds us that we are here to care for and about patients, keeping our efforts patient-centered.
Direct patient care refocuses directors on the fundamental reason they are in leadership. It offers intrinsic professional rewards and intellectual satisfaction that will sustain and strengthen the leadership role. The effective leader strategically finds balance by delegating, prioritizing, and focusing on time management.
Continuing your clinical practice affords physician leaders leverage with their constituents—the hospitalists. Working in the trenches, especially during critical times, yields legitimacy and credibility. It also allows the leader to identify with and respond to concerns raised by members. This can connect the leader to the group, avoiding the “suit vs. white coat” dynamic. The same principle extends to other stakeholders who are part of the care team, such as nurses and referring physicians.
Other Factors
Maintaining clinical aptitude ensures that leaders stay apprised of current practices, and are aware of the latest techniques, data, and evidence. This is critical for ensuring group performance in quality initiatives, and for setting standards of clinical excellence in the group practice.
In academic centers, ward teaching allows leaders to train future physicians, pass on knowledge, and gain an understanding of the next generation and its priorities, thus keeping an eye on the future and having a clear vision.
Perhaps the most important benefit direct patient care provides in leadership is the ability to accomplish the group’s mission. A firsthand experience brings understanding of issues around workflow, efficiency, and career satisfaction. It allows leaders to audit best practices. It inspires innovative ideas for healthcare delivery and processing improvement changes.
The model of successful physician leadership is based on clinical excellence. The construct of a separation between clinical and administrative roles is a false dichotomy; the two are interdependent. HM directors have a duty to perform both, as it is the combination that makes leaders successful. TH
Dr. Wright is associate clinical professor and chief of the division of hospital medicine at the University of Wisconsin School of Medicine and Public Health in Madison.
CON
Physician leaders should relinquish clinical practice, focus on leading
I believe the vast majority of hospitalists agree with the “pro” side of this debate, but I also believe that this kind of knee-jerk reaction reflects the core deficiency that plagues physicians’ thinking regarding leadership.
The way medicine is being practiced and delivered in the hospital setting is rapidly changing. In fact, our specialty is based on this premise. Yet hospitalists still have a stone-age mentality when it comes to physician leaders. The concept of leadership, in most cases, is an afterthought.
Our Role as Leaders
The HM leader is expected to act as a caretaker: set the schedule, organize and implement QI programs, and represent the hospitalists to administration. Most HM directors are accidental leaders who sheepishly step into a position when the opportunity presents itself. This usually happens as programs grow. Few industries would accept this business model. Leadership should be considered critical and given its due respect in terms of resources, training, and experience. Rarely are supervisory positions rewarded to accidental, part-time volunteers. Leaders are chosen, groomed, and given the sufficient time and resources to carry out their mandate.
When HM programs become dysfunctional, hospitalists are quick to blame the administration—some refer to it as the “evil empire” or “the dark side.” But interesting research by Gallup Inc. has shown that the majority of employees who leave their jobs actually are leaving their manager.1
Wants vs. Needs
Leaders face dilemmas every work day. For instance, leaders need to communicate the administration’s goals and weave them into HM department systems and policies. Conversely, HM leaders have to negotiate with administration to secure the resources they need to execute those goals. Technologies are mere facilitators; people actually produce results. Yet many administrators and HM leaders are fixated on the latest software without giving much thought about how staff will implement the changes.
HM leaders need time and resources to be effective. As hospitalists, we’ve been bombarded by the evidence-based medicine mantra. But most hospitalists have never heard of, or they laugh at, evidence-based techniques that were first documented in the 1970s.2 Data is available regarding management skills that can be used to effect positive organization behavior.
We also need to be authentic leaders to combat internal disruptions from medical staff. Gallup Management research has shown that 42% of physicians on medical staffs are actively disengaged.3 Physicians not only are distant, they also actively sabotage and poison new efforts introduced by administration or physician leaders.
The hospitalist leader should only perform clinical responsibilities if they are absolutely necessary. The HM director should be given all the time, resources, due respect, and training to be a dynamic leader. The hospitalist movement would be better for it. TH
References
- Buckingham, M, Coffman C. First, Break All the Rules: How Managers Trump Companies. 1999. New York City: Simon & Schuster.
- Luthans F. Organizational Behavior. 1973. New York City: McGraw-Hill.
- Paller D. What the doctor ordered. Gallup Management Web site. Available at: http://gmj.gallup.com/content/18361/What-Doctor-Ordered.aspx. Accessed Nov. 9, 2009.
Dr. Yu is medical director of hospitalist services at Decatur (Ill.) Memorial Hospital.
In the Literature
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk of VTE with travel
- Hyponatremia and mortality
- Clopidogrel and aspirin for atrial fibrillation
- Cost-effective evaluation of syncope
- Early vs. delayed intervention in STEMI patients receiving fibrinolytics
- Predictors of prolonged SSU length of stay
- Rates of survival for in-hospital CPR
- Hospitalists and hospital quality measures
Travel Increases Risk for Venous Thromboembolism in a Dose-Response Relationship
Clinical question: What is the association between travel and the risk of venous thromboembolism (VTE)?
Background: Previous studies evaluating the relationship between long-distance travel and VTE have been heterogeneous and inconclusive. Though a relationship is often discussed, only about half of prior investigations have identified an elevated VTE risk in those who travel, and the impact of duration on VTE risk is unclear.
Study design: Meta-analysis.
Setting: Western countries.
Synopsis: Studies were included if they investigated the association between travel and VTE for persons using any mode of transportation and if nontraveling persons were included for comparison. Fourteen studies met the criteria, and included 4,055 patients with VTE. Compared with nontravelers, the overall pooled relative risk for VTE in travelers was 2.0 (95% CI, 1.5-2.7).
Significant heterogeneity was present among these 14 studies, specifically with regard to the method used for selecting control participants. Six case-control studies used control patients who had been referred for VTE evaluation. When these studies were excluded, the pooled relative risk for VTE in travelers was 2.8 (95% CI, 2.2-3.7).
A dose-response relationship was identified. There was an 18% higher risk for VTE for each two-hour increase in duration of travel among all modes of transportation (P=0.010). When studies evaluating only air travel were analyzed, a 26% higher risk was found for every two-hour increase in air travel (P=0.005).
Bottom line: Travel is associated with a three-fold increase in the risk for VTE, and for each two-hour increase in travel duration, the risk increases approximately 18%.
Citation: Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151(3):180-190.
Hyponatremia in Hospitalized Patients is Associated with Increased Mortality
Clinical question: Is hyponatremia in hospitalized patients associated with increased mortality?
Background: Hyponatremia is the most common electrolyte abnormality in hospitalized patients. Patients admitted with hyponatremia have increased in-hospital mortality. Long-term mortality in hospitalized patients with hyponatremia is not known. Further, the effects of the degree of hyponatremia on mortality are not known.
Study design: Prospective cohort.
Setting: Two teaching hospitals in Boston.
Synopsis: The study identified 14,290 patients with hyponatremia (serum sodium <135 mEq/L) at admission (14.5%) and an additional 5,093 patients (19,383 total patients, or 19.7% of the 98,411 study patients) with hyponatremia at some point during their hospital stay. After multivariable adjustments and correction for hyperglycemia, patients with hyponatremia had increased mortality in the hospital (OR 1.47, 95% CI, 1.33-1.62), at one year (HR 1.38, 95% CI, 1.32-1.46), and at five years (HR 1.25, 95% CI, 1.21-1.30) compared with normonatremic patients. These mortality differences were seen in patients with mild, moderate, and moderately severe hyponatremia (serum sodium concentrations 130-134, 125-129, and 120-124 mEq/L, respectively), but not in patients with severe hyponatremia (serum sodium <120 mEq/L).
This study is limited by its post-hoc identification and classification of patients using ICD-9-CM codes, which could have resulted in some misclassification. Also, this study includes only two teaching hospitals in an urban setting; the prevalence of hyponatremia might differ in other settings. Causality cannot be determined based on these results.
Bottom line: Hospitalized patients with hyponatremia have increased in-hospital and long-term mortality.
Citation: Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857-865.
Clopidogrel Plus Aspirin in Patients with Atrial Fibrillation Reduces Risk of Major Vascular Events
Clinical question: Does the addition of clopidogrel to aspirin therapy reduce the risk of major vascular events in patients with atrial fibrillation for whom vitamin K antagonists (VKAs) are unsuitable?
Background: Although VKAs reduce the risk of stroke in atrial fibrillation, many patients are unable to use VKAs and are treated with aspirin instead. The potential benefits of adding clopidogrel to aspirin therapy in this population are unknown.
Study design: Randomized controlled trial.
Setting: Five hundred eighty medical centers in 33 countries.
Synopsis: More than 7,500 patients with atrial fibrillation who were also at high risk for stroke were randomly assigned to receive either clopidogrel or placebo once daily. All patients also received aspirin at a dose of 75 mg to 100 mg daily. A major vascular event occurred in 6.8% of patients per year who received clopidogrel and in 7.6% of patients per year who received placebo (RR 0.89, 95% CI, 0.89-0.98, P=0.01). This reduction primarily was due to a reduction in stroke, which occurred in 2.4% of patients per year who received clopidogrel, compared with 3.3% of patients per year who received placebo (RR 0.72, 95% CI, 0.62-0.83, P<0.001).
Major bleeding occurred in 2% of patients per year who received clopidogrel and in 1.3% of patients per year who received placebo (RR 1.57, 95% CI, 1.29-1.92, P<0.001).
Bottom line: Adding clopidogrel to aspirin in patients with atrial fibrillation who are not eligible for VKAs decreases the risk of major vascular events, including stroke, but increases risk of major hemorrhage compared with aspirin alone.
Citation: ACTIVE Investigators, Connolly SJ, Pogue J, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360(20):2066-2078.
Prioritize Syncope Testing by Diagnostic Yield and Cost Effectiveness
Clinical question: What are the utilization, yield, and cost effectiveness of tests used for evaluation of syncope in older patients?
Background: Clinicians utilize multiple diagnostic tests to help delineate the cause of syncope, but the yield and cost effectiveness of many of these tests are unclear. Further, it is unknown if considering patient characteristics, as in the San Francisco syncope rule (SFSR), can improve the yield of diagnostic tests.
Study design: Retrospective cohort.
Setting: Single acute-care hospital.
Synopsis: Review of 2,106 admissions in patients 65 and older with syncope revealed that the most common tests were electrocardiogram (99%), telemetry (95%), cardiac enzymes (95%), and head computed tomography (CT) scan (63%). The majority of tests did not affect diagnosis or management.
Postural blood pressure (BP) reading was infrequently recorded (38%) but had the highest yield. BP influenced diagnosis at least 18% of the time and management at least 25% of the time. Tests with the lowest likelihood of affecting diagnosis and management were head CT, carotid ultrasound, electroencephalography (EEG), and cardiac enzymes.
EEG had the highest cost per test affecting the diagnosis or management ($32,973), followed by head CT. The cost per test affecting diagnosis or management for postural BP was $17. Cardiac testing, including telemetry, echocardiogram, and cardiac enzymes, had significantly better yield in patients who met SFSR criteria.
Bottom line: In patients with syncope, the history and exam should guide evaluation, and tests with high yield and low cost per test, such as postural BP, should be prioritized.
Citation: Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14): 1299-1305.
Early PCI is Superior to Delayed Intervention in Patients with STEMI Receiving Fibrinolytic Therapy
Clinical question: Does early percutaneous coronary intervention (PCI) improve clinical outcomes compared with standard management in patients with ST elevation myocardial infarction (STEMI) who receive fibrinolysis?
Background: Prior research has demonstrated the benefit of timely PCI in the management of acute coronary syndrome, specifically with ST elevation. However, many hospitals do not have this capability and utilize fibrinolysis as a standard alternative. The optimal timing of subsequent invasive intervention following fibrinolysis has not been established.
Study design: Multicenter randomized trial.
Setting: Fifty-two sites in three provinces in Canada.
Synopsis: This study randomized 1,059 patients presenting with STEMI and receiving fibrinolysis to early intervention (immediate transfer to another hospital with PCI less than six hours after fibrinolysis) versus standard intervention (rescue PCI if needed, or delayed angiography at more than 24 hours). The primary outcome was the composite of death, reinfarction, recurrent ischemia, new or worsening congestive heart failure, or cardiogenic shock within 30 days.
The primary outcome occurred in 11% of patients in the early intervention group, compared with 17.2% of patients randomized to standard intervention (RR 0.64, 95% CI, 0.47-0.87, P=0.004). Urgent catheterization was performed within 12 hours of fibrinolysis in 34.9% of patients randomized to the standard treatment group.
This study was not powered to detect differences in mortality and other individual components of the primary endpoint.
Bottom line: STEMI patients who received fibrinolysis had a lower risk of adverse outcomes when receiving transfer and PCI within six hours, compared with standard delayed intervention.
Citation: Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360(26):2705-2718.
Specialty Consultation and Limited Access Tests Predict Unsuccessful SSU Admissions
Clinical question: In patients admitted to short-stay units (SSUs), what characteristics are associated with unsuccessful SSU admission?
Background: Short-stay units have become prevalent in U.S. hospitals, but it is unclear which patient populations are best served by SSUs.
Study design: Prospective cohort.
Setting: Fourteen-bed SSU in a 500-bed public teaching hospital in Chicago.
Synopsis: More than 700 patients admitted to the Cook County Hospital SSU over a four-month period were interviewed and examined, and their ED and inpatient records were reviewed. An SSU admission was defined as “successful” if the length of stay (LOS) was less than 72 hours and the patient was discharged directly from the SSU.
Overall, 79% of patients had a successful SSU admission. In multivariate analysis, the strongest predictors of an unsuccessful SSU stay were subspecialty consultation (OR 8.1, P<0.001), a provisional diagnosis of heart failure (OR 1.9, P=0.02), and limited availability of a diagnostic test (OR 2.5, P<0.001).
The study was limited primarily to patients with cardiovascular diagnoses.
Bottom line: Patients admitted to SSUs who receive specialty consultation, carry a diagnosis of heart failure, or require diagnostic testing that is not readily available might have a longer LOS or eventual inpatient admission.
Citation: Lucas BP, Kumapley R, Mba B, et al. A hospitalist-run short-stay unit: features that predict length-of-stay and eventual admission to traditional inpatient services. J Hosp Med. 2009;4(5):276-284.
Lack of Significant Gains in Survival Rates Following In-Hospital CPR
Clinical question: Is survival after in-hospital CPR improving over time, and what are the factors associated with survival?
Background: Advances in out-of-hospital CPR have improved outcomes. However, it is unknown whether the survival rate after in-hospital CPR is improving over time, and it is unclear which patient and/or hospital characteristics predict post-CPR survival.
Study design: Retrospective cohort.
Setting: Inpatient Medicare beneficiaries from 1992 to 2005.
Synopsis: The study examined more than 150 million Medicare admissions, 433,985 of which underwent in-hospital CPR. Survival to discharge occurred in 18.3% of CPR events and did not change significantly from 1992 to 2005. The cumulative incidence of in-hospital CPR events was 2.73 per 1,000 admissions; it did not change substantially over time.
The survival rate was lower among black patients (OR 0.76, 95% CI, 0.74-0.79), which is partially explained due to the fact they tended to receive CPR at hospitals with lower post-CPR survival. Gender (specifically male), older age, race (specifically other nonwhite patients), higher burden of chronic illness, and admission from a skilled nursing facility were significantly associated with decreased survival to hospital discharge following CPR.
Limitations of this study included the identification of CPR by ICD-9 codes, which have not been validated for this purpose and could vary among hospitals. Other factors that might explain variations in survival were not available, including severity of acute illness and the presence (or absence) of a shockable rhythm at initial presentation.
Bottom line: Rates of survival to hospital discharge among Medicare beneficiaries receiving in-hospital CPR have remained constant over time, with poorer survival rates among blacks and other nonwhite patients.
Citation: Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22-31.
Hospitalists Are Associated with Improved Performance on Quality Metrics
Clinical question: Is the presence of hospitalist physicians associated with improved performance on standard quality measures for acute myocardial infarction (AMI), congestive heart failure (CHF), and pneumonia?
Background: Previous investigations have demonstrated significant improvements in cost and LOS for patients under the care of hospitalists compared with other inpatient providers. The association between hospitalist prevalence and quality of care, as measured by standard quality process measures, is unknown.
Study design: Cross-sectional.
Setting: More than 3,600 hospitals participating in the Health Quality Alliance (HQA) program.
Synopsis: Investigators looked at a large sample of HQA hospitals in the American Hospital Association survey, and identified facilities with hospitalist services and those without. The primary endpoint was the adherence to composites of standard quality process measures across three disease categories (AMI, CHF, and pneumonia) and two domains of care (disease treatment/diagnosis and counseling/prevention).
Multivariable analyses revealed a statistically significant association between the presence of hospitalists and adherence to composite quality measures for AMI and pneumonia. This association was demonstrated for both treatment and counseling domains.
The study is cross-sectional, so conclusions cannot be drawn about causality. Also, there are likely unmeasured differences between hospitals that utilize hospitalists compared with those that do not, which could further confound the relationship between the presence of hospitalists and adherence to quality measures.
Finally, this study only evaluated hospital-level performance, and it cannot offer insight on the quality of individual patient care by hospitalist providers.
Bottom line: The presence of hospitalists is associated with improvement in adherence to quality measures for both AMI and pneumonia, and across clinical domains of treatment and counseling.
Citation: López L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk of VTE with travel
- Hyponatremia and mortality
- Clopidogrel and aspirin for atrial fibrillation
- Cost-effective evaluation of syncope
- Early vs. delayed intervention in STEMI patients receiving fibrinolytics
- Predictors of prolonged SSU length of stay
- Rates of survival for in-hospital CPR
- Hospitalists and hospital quality measures
Travel Increases Risk for Venous Thromboembolism in a Dose-Response Relationship
Clinical question: What is the association between travel and the risk of venous thromboembolism (VTE)?
Background: Previous studies evaluating the relationship between long-distance travel and VTE have been heterogeneous and inconclusive. Though a relationship is often discussed, only about half of prior investigations have identified an elevated VTE risk in those who travel, and the impact of duration on VTE risk is unclear.
Study design: Meta-analysis.
Setting: Western countries.
Synopsis: Studies were included if they investigated the association between travel and VTE for persons using any mode of transportation and if nontraveling persons were included for comparison. Fourteen studies met the criteria, and included 4,055 patients with VTE. Compared with nontravelers, the overall pooled relative risk for VTE in travelers was 2.0 (95% CI, 1.5-2.7).
Significant heterogeneity was present among these 14 studies, specifically with regard to the method used for selecting control participants. Six case-control studies used control patients who had been referred for VTE evaluation. When these studies were excluded, the pooled relative risk for VTE in travelers was 2.8 (95% CI, 2.2-3.7).
A dose-response relationship was identified. There was an 18% higher risk for VTE for each two-hour increase in duration of travel among all modes of transportation (P=0.010). When studies evaluating only air travel were analyzed, a 26% higher risk was found for every two-hour increase in air travel (P=0.005).
Bottom line: Travel is associated with a three-fold increase in the risk for VTE, and for each two-hour increase in travel duration, the risk increases approximately 18%.
Citation: Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151(3):180-190.
Hyponatremia in Hospitalized Patients is Associated with Increased Mortality
Clinical question: Is hyponatremia in hospitalized patients associated with increased mortality?
Background: Hyponatremia is the most common electrolyte abnormality in hospitalized patients. Patients admitted with hyponatremia have increased in-hospital mortality. Long-term mortality in hospitalized patients with hyponatremia is not known. Further, the effects of the degree of hyponatremia on mortality are not known.
Study design: Prospective cohort.
Setting: Two teaching hospitals in Boston.
Synopsis: The study identified 14,290 patients with hyponatremia (serum sodium <135 mEq/L) at admission (14.5%) and an additional 5,093 patients (19,383 total patients, or 19.7% of the 98,411 study patients) with hyponatremia at some point during their hospital stay. After multivariable adjustments and correction for hyperglycemia, patients with hyponatremia had increased mortality in the hospital (OR 1.47, 95% CI, 1.33-1.62), at one year (HR 1.38, 95% CI, 1.32-1.46), and at five years (HR 1.25, 95% CI, 1.21-1.30) compared with normonatremic patients. These mortality differences were seen in patients with mild, moderate, and moderately severe hyponatremia (serum sodium concentrations 130-134, 125-129, and 120-124 mEq/L, respectively), but not in patients with severe hyponatremia (serum sodium <120 mEq/L).
This study is limited by its post-hoc identification and classification of patients using ICD-9-CM codes, which could have resulted in some misclassification. Also, this study includes only two teaching hospitals in an urban setting; the prevalence of hyponatremia might differ in other settings. Causality cannot be determined based on these results.
Bottom line: Hospitalized patients with hyponatremia have increased in-hospital and long-term mortality.
Citation: Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857-865.
Clopidogrel Plus Aspirin in Patients with Atrial Fibrillation Reduces Risk of Major Vascular Events
Clinical question: Does the addition of clopidogrel to aspirin therapy reduce the risk of major vascular events in patients with atrial fibrillation for whom vitamin K antagonists (VKAs) are unsuitable?
Background: Although VKAs reduce the risk of stroke in atrial fibrillation, many patients are unable to use VKAs and are treated with aspirin instead. The potential benefits of adding clopidogrel to aspirin therapy in this population are unknown.
Study design: Randomized controlled trial.
Setting: Five hundred eighty medical centers in 33 countries.
Synopsis: More than 7,500 patients with atrial fibrillation who were also at high risk for stroke were randomly assigned to receive either clopidogrel or placebo once daily. All patients also received aspirin at a dose of 75 mg to 100 mg daily. A major vascular event occurred in 6.8% of patients per year who received clopidogrel and in 7.6% of patients per year who received placebo (RR 0.89, 95% CI, 0.89-0.98, P=0.01). This reduction primarily was due to a reduction in stroke, which occurred in 2.4% of patients per year who received clopidogrel, compared with 3.3% of patients per year who received placebo (RR 0.72, 95% CI, 0.62-0.83, P<0.001).
Major bleeding occurred in 2% of patients per year who received clopidogrel and in 1.3% of patients per year who received placebo (RR 1.57, 95% CI, 1.29-1.92, P<0.001).
Bottom line: Adding clopidogrel to aspirin in patients with atrial fibrillation who are not eligible for VKAs decreases the risk of major vascular events, including stroke, but increases risk of major hemorrhage compared with aspirin alone.
Citation: ACTIVE Investigators, Connolly SJ, Pogue J, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360(20):2066-2078.
Prioritize Syncope Testing by Diagnostic Yield and Cost Effectiveness
Clinical question: What are the utilization, yield, and cost effectiveness of tests used for evaluation of syncope in older patients?
Background: Clinicians utilize multiple diagnostic tests to help delineate the cause of syncope, but the yield and cost effectiveness of many of these tests are unclear. Further, it is unknown if considering patient characteristics, as in the San Francisco syncope rule (SFSR), can improve the yield of diagnostic tests.
Study design: Retrospective cohort.
Setting: Single acute-care hospital.
Synopsis: Review of 2,106 admissions in patients 65 and older with syncope revealed that the most common tests were electrocardiogram (99%), telemetry (95%), cardiac enzymes (95%), and head computed tomography (CT) scan (63%). The majority of tests did not affect diagnosis or management.
Postural blood pressure (BP) reading was infrequently recorded (38%) but had the highest yield. BP influenced diagnosis at least 18% of the time and management at least 25% of the time. Tests with the lowest likelihood of affecting diagnosis and management were head CT, carotid ultrasound, electroencephalography (EEG), and cardiac enzymes.
EEG had the highest cost per test affecting the diagnosis or management ($32,973), followed by head CT. The cost per test affecting diagnosis or management for postural BP was $17. Cardiac testing, including telemetry, echocardiogram, and cardiac enzymes, had significantly better yield in patients who met SFSR criteria.
Bottom line: In patients with syncope, the history and exam should guide evaluation, and tests with high yield and low cost per test, such as postural BP, should be prioritized.
Citation: Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14): 1299-1305.
Early PCI is Superior to Delayed Intervention in Patients with STEMI Receiving Fibrinolytic Therapy
Clinical question: Does early percutaneous coronary intervention (PCI) improve clinical outcomes compared with standard management in patients with ST elevation myocardial infarction (STEMI) who receive fibrinolysis?
Background: Prior research has demonstrated the benefit of timely PCI in the management of acute coronary syndrome, specifically with ST elevation. However, many hospitals do not have this capability and utilize fibrinolysis as a standard alternative. The optimal timing of subsequent invasive intervention following fibrinolysis has not been established.
Study design: Multicenter randomized trial.
Setting: Fifty-two sites in three provinces in Canada.
Synopsis: This study randomized 1,059 patients presenting with STEMI and receiving fibrinolysis to early intervention (immediate transfer to another hospital with PCI less than six hours after fibrinolysis) versus standard intervention (rescue PCI if needed, or delayed angiography at more than 24 hours). The primary outcome was the composite of death, reinfarction, recurrent ischemia, new or worsening congestive heart failure, or cardiogenic shock within 30 days.
The primary outcome occurred in 11% of patients in the early intervention group, compared with 17.2% of patients randomized to standard intervention (RR 0.64, 95% CI, 0.47-0.87, P=0.004). Urgent catheterization was performed within 12 hours of fibrinolysis in 34.9% of patients randomized to the standard treatment group.
This study was not powered to detect differences in mortality and other individual components of the primary endpoint.
Bottom line: STEMI patients who received fibrinolysis had a lower risk of adverse outcomes when receiving transfer and PCI within six hours, compared with standard delayed intervention.
Citation: Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360(26):2705-2718.
Specialty Consultation and Limited Access Tests Predict Unsuccessful SSU Admissions
Clinical question: In patients admitted to short-stay units (SSUs), what characteristics are associated with unsuccessful SSU admission?
Background: Short-stay units have become prevalent in U.S. hospitals, but it is unclear which patient populations are best served by SSUs.
Study design: Prospective cohort.
Setting: Fourteen-bed SSU in a 500-bed public teaching hospital in Chicago.
Synopsis: More than 700 patients admitted to the Cook County Hospital SSU over a four-month period were interviewed and examined, and their ED and inpatient records were reviewed. An SSU admission was defined as “successful” if the length of stay (LOS) was less than 72 hours and the patient was discharged directly from the SSU.
Overall, 79% of patients had a successful SSU admission. In multivariate analysis, the strongest predictors of an unsuccessful SSU stay were subspecialty consultation (OR 8.1, P<0.001), a provisional diagnosis of heart failure (OR 1.9, P=0.02), and limited availability of a diagnostic test (OR 2.5, P<0.001).
The study was limited primarily to patients with cardiovascular diagnoses.
Bottom line: Patients admitted to SSUs who receive specialty consultation, carry a diagnosis of heart failure, or require diagnostic testing that is not readily available might have a longer LOS or eventual inpatient admission.
Citation: Lucas BP, Kumapley R, Mba B, et al. A hospitalist-run short-stay unit: features that predict length-of-stay and eventual admission to traditional inpatient services. J Hosp Med. 2009;4(5):276-284.
Lack of Significant Gains in Survival Rates Following In-Hospital CPR
Clinical question: Is survival after in-hospital CPR improving over time, and what are the factors associated with survival?
Background: Advances in out-of-hospital CPR have improved outcomes. However, it is unknown whether the survival rate after in-hospital CPR is improving over time, and it is unclear which patient and/or hospital characteristics predict post-CPR survival.
Study design: Retrospective cohort.
Setting: Inpatient Medicare beneficiaries from 1992 to 2005.
Synopsis: The study examined more than 150 million Medicare admissions, 433,985 of which underwent in-hospital CPR. Survival to discharge occurred in 18.3% of CPR events and did not change significantly from 1992 to 2005. The cumulative incidence of in-hospital CPR events was 2.73 per 1,000 admissions; it did not change substantially over time.
The survival rate was lower among black patients (OR 0.76, 95% CI, 0.74-0.79), which is partially explained due to the fact they tended to receive CPR at hospitals with lower post-CPR survival. Gender (specifically male), older age, race (specifically other nonwhite patients), higher burden of chronic illness, and admission from a skilled nursing facility were significantly associated with decreased survival to hospital discharge following CPR.
Limitations of this study included the identification of CPR by ICD-9 codes, which have not been validated for this purpose and could vary among hospitals. Other factors that might explain variations in survival were not available, including severity of acute illness and the presence (or absence) of a shockable rhythm at initial presentation.
Bottom line: Rates of survival to hospital discharge among Medicare beneficiaries receiving in-hospital CPR have remained constant over time, with poorer survival rates among blacks and other nonwhite patients.
Citation: Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22-31.
Hospitalists Are Associated with Improved Performance on Quality Metrics
Clinical question: Is the presence of hospitalist physicians associated with improved performance on standard quality measures for acute myocardial infarction (AMI), congestive heart failure (CHF), and pneumonia?
Background: Previous investigations have demonstrated significant improvements in cost and LOS for patients under the care of hospitalists compared with other inpatient providers. The association between hospitalist prevalence and quality of care, as measured by standard quality process measures, is unknown.
Study design: Cross-sectional.
Setting: More than 3,600 hospitals participating in the Health Quality Alliance (HQA) program.
Synopsis: Investigators looked at a large sample of HQA hospitals in the American Hospital Association survey, and identified facilities with hospitalist services and those without. The primary endpoint was the adherence to composites of standard quality process measures across three disease categories (AMI, CHF, and pneumonia) and two domains of care (disease treatment/diagnosis and counseling/prevention).
Multivariable analyses revealed a statistically significant association between the presence of hospitalists and adherence to composite quality measures for AMI and pneumonia. This association was demonstrated for both treatment and counseling domains.
The study is cross-sectional, so conclusions cannot be drawn about causality. Also, there are likely unmeasured differences between hospitals that utilize hospitalists compared with those that do not, which could further confound the relationship between the presence of hospitalists and adherence to quality measures.
Finally, this study only evaluated hospital-level performance, and it cannot offer insight on the quality of individual patient care by hospitalist providers.
Bottom line: The presence of hospitalists is associated with improvement in adherence to quality measures for both AMI and pneumonia, and across clinical domains of treatment and counseling.
Citation: López L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk of VTE with travel
- Hyponatremia and mortality
- Clopidogrel and aspirin for atrial fibrillation
- Cost-effective evaluation of syncope
- Early vs. delayed intervention in STEMI patients receiving fibrinolytics
- Predictors of prolonged SSU length of stay
- Rates of survival for in-hospital CPR
- Hospitalists and hospital quality measures
Travel Increases Risk for Venous Thromboembolism in a Dose-Response Relationship
Clinical question: What is the association between travel and the risk of venous thromboembolism (VTE)?
Background: Previous studies evaluating the relationship between long-distance travel and VTE have been heterogeneous and inconclusive. Though a relationship is often discussed, only about half of prior investigations have identified an elevated VTE risk in those who travel, and the impact of duration on VTE risk is unclear.
Study design: Meta-analysis.
Setting: Western countries.
Synopsis: Studies were included if they investigated the association between travel and VTE for persons using any mode of transportation and if nontraveling persons were included for comparison. Fourteen studies met the criteria, and included 4,055 patients with VTE. Compared with nontravelers, the overall pooled relative risk for VTE in travelers was 2.0 (95% CI, 1.5-2.7).
Significant heterogeneity was present among these 14 studies, specifically with regard to the method used for selecting control participants. Six case-control studies used control patients who had been referred for VTE evaluation. When these studies were excluded, the pooled relative risk for VTE in travelers was 2.8 (95% CI, 2.2-3.7).
A dose-response relationship was identified. There was an 18% higher risk for VTE for each two-hour increase in duration of travel among all modes of transportation (P=0.010). When studies evaluating only air travel were analyzed, a 26% higher risk was found for every two-hour increase in air travel (P=0.005).
Bottom line: Travel is associated with a three-fold increase in the risk for VTE, and for each two-hour increase in travel duration, the risk increases approximately 18%.
Citation: Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151(3):180-190.
Hyponatremia in Hospitalized Patients is Associated with Increased Mortality
Clinical question: Is hyponatremia in hospitalized patients associated with increased mortality?
Background: Hyponatremia is the most common electrolyte abnormality in hospitalized patients. Patients admitted with hyponatremia have increased in-hospital mortality. Long-term mortality in hospitalized patients with hyponatremia is not known. Further, the effects of the degree of hyponatremia on mortality are not known.
Study design: Prospective cohort.
Setting: Two teaching hospitals in Boston.
Synopsis: The study identified 14,290 patients with hyponatremia (serum sodium <135 mEq/L) at admission (14.5%) and an additional 5,093 patients (19,383 total patients, or 19.7% of the 98,411 study patients) with hyponatremia at some point during their hospital stay. After multivariable adjustments and correction for hyperglycemia, patients with hyponatremia had increased mortality in the hospital (OR 1.47, 95% CI, 1.33-1.62), at one year (HR 1.38, 95% CI, 1.32-1.46), and at five years (HR 1.25, 95% CI, 1.21-1.30) compared with normonatremic patients. These mortality differences were seen in patients with mild, moderate, and moderately severe hyponatremia (serum sodium concentrations 130-134, 125-129, and 120-124 mEq/L, respectively), but not in patients with severe hyponatremia (serum sodium <120 mEq/L).
This study is limited by its post-hoc identification and classification of patients using ICD-9-CM codes, which could have resulted in some misclassification. Also, this study includes only two teaching hospitals in an urban setting; the prevalence of hyponatremia might differ in other settings. Causality cannot be determined based on these results.
Bottom line: Hospitalized patients with hyponatremia have increased in-hospital and long-term mortality.
Citation: Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857-865.
Clopidogrel Plus Aspirin in Patients with Atrial Fibrillation Reduces Risk of Major Vascular Events
Clinical question: Does the addition of clopidogrel to aspirin therapy reduce the risk of major vascular events in patients with atrial fibrillation for whom vitamin K antagonists (VKAs) are unsuitable?
Background: Although VKAs reduce the risk of stroke in atrial fibrillation, many patients are unable to use VKAs and are treated with aspirin instead. The potential benefits of adding clopidogrel to aspirin therapy in this population are unknown.
Study design: Randomized controlled trial.
Setting: Five hundred eighty medical centers in 33 countries.
Synopsis: More than 7,500 patients with atrial fibrillation who were also at high risk for stroke were randomly assigned to receive either clopidogrel or placebo once daily. All patients also received aspirin at a dose of 75 mg to 100 mg daily. A major vascular event occurred in 6.8% of patients per year who received clopidogrel and in 7.6% of patients per year who received placebo (RR 0.89, 95% CI, 0.89-0.98, P=0.01). This reduction primarily was due to a reduction in stroke, which occurred in 2.4% of patients per year who received clopidogrel, compared with 3.3% of patients per year who received placebo (RR 0.72, 95% CI, 0.62-0.83, P<0.001).
Major bleeding occurred in 2% of patients per year who received clopidogrel and in 1.3% of patients per year who received placebo (RR 1.57, 95% CI, 1.29-1.92, P<0.001).
Bottom line: Adding clopidogrel to aspirin in patients with atrial fibrillation who are not eligible for VKAs decreases the risk of major vascular events, including stroke, but increases risk of major hemorrhage compared with aspirin alone.
Citation: ACTIVE Investigators, Connolly SJ, Pogue J, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360(20):2066-2078.
Prioritize Syncope Testing by Diagnostic Yield and Cost Effectiveness
Clinical question: What are the utilization, yield, and cost effectiveness of tests used for evaluation of syncope in older patients?
Background: Clinicians utilize multiple diagnostic tests to help delineate the cause of syncope, but the yield and cost effectiveness of many of these tests are unclear. Further, it is unknown if considering patient characteristics, as in the San Francisco syncope rule (SFSR), can improve the yield of diagnostic tests.
Study design: Retrospective cohort.
Setting: Single acute-care hospital.
Synopsis: Review of 2,106 admissions in patients 65 and older with syncope revealed that the most common tests were electrocardiogram (99%), telemetry (95%), cardiac enzymes (95%), and head computed tomography (CT) scan (63%). The majority of tests did not affect diagnosis or management.
Postural blood pressure (BP) reading was infrequently recorded (38%) but had the highest yield. BP influenced diagnosis at least 18% of the time and management at least 25% of the time. Tests with the lowest likelihood of affecting diagnosis and management were head CT, carotid ultrasound, electroencephalography (EEG), and cardiac enzymes.
EEG had the highest cost per test affecting the diagnosis or management ($32,973), followed by head CT. The cost per test affecting diagnosis or management for postural BP was $17. Cardiac testing, including telemetry, echocardiogram, and cardiac enzymes, had significantly better yield in patients who met SFSR criteria.
Bottom line: In patients with syncope, the history and exam should guide evaluation, and tests with high yield and low cost per test, such as postural BP, should be prioritized.
Citation: Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14): 1299-1305.
Early PCI is Superior to Delayed Intervention in Patients with STEMI Receiving Fibrinolytic Therapy
Clinical question: Does early percutaneous coronary intervention (PCI) improve clinical outcomes compared with standard management in patients with ST elevation myocardial infarction (STEMI) who receive fibrinolysis?
Background: Prior research has demonstrated the benefit of timely PCI in the management of acute coronary syndrome, specifically with ST elevation. However, many hospitals do not have this capability and utilize fibrinolysis as a standard alternative. The optimal timing of subsequent invasive intervention following fibrinolysis has not been established.
Study design: Multicenter randomized trial.
Setting: Fifty-two sites in three provinces in Canada.
Synopsis: This study randomized 1,059 patients presenting with STEMI and receiving fibrinolysis to early intervention (immediate transfer to another hospital with PCI less than six hours after fibrinolysis) versus standard intervention (rescue PCI if needed, or delayed angiography at more than 24 hours). The primary outcome was the composite of death, reinfarction, recurrent ischemia, new or worsening congestive heart failure, or cardiogenic shock within 30 days.
The primary outcome occurred in 11% of patients in the early intervention group, compared with 17.2% of patients randomized to standard intervention (RR 0.64, 95% CI, 0.47-0.87, P=0.004). Urgent catheterization was performed within 12 hours of fibrinolysis in 34.9% of patients randomized to the standard treatment group.
This study was not powered to detect differences in mortality and other individual components of the primary endpoint.
Bottom line: STEMI patients who received fibrinolysis had a lower risk of adverse outcomes when receiving transfer and PCI within six hours, compared with standard delayed intervention.
Citation: Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360(26):2705-2718.
Specialty Consultation and Limited Access Tests Predict Unsuccessful SSU Admissions
Clinical question: In patients admitted to short-stay units (SSUs), what characteristics are associated with unsuccessful SSU admission?
Background: Short-stay units have become prevalent in U.S. hospitals, but it is unclear which patient populations are best served by SSUs.
Study design: Prospective cohort.
Setting: Fourteen-bed SSU in a 500-bed public teaching hospital in Chicago.
Synopsis: More than 700 patients admitted to the Cook County Hospital SSU over a four-month period were interviewed and examined, and their ED and inpatient records were reviewed. An SSU admission was defined as “successful” if the length of stay (LOS) was less than 72 hours and the patient was discharged directly from the SSU.
Overall, 79% of patients had a successful SSU admission. In multivariate analysis, the strongest predictors of an unsuccessful SSU stay were subspecialty consultation (OR 8.1, P<0.001), a provisional diagnosis of heart failure (OR 1.9, P=0.02), and limited availability of a diagnostic test (OR 2.5, P<0.001).
The study was limited primarily to patients with cardiovascular diagnoses.
Bottom line: Patients admitted to SSUs who receive specialty consultation, carry a diagnosis of heart failure, or require diagnostic testing that is not readily available might have a longer LOS or eventual inpatient admission.
Citation: Lucas BP, Kumapley R, Mba B, et al. A hospitalist-run short-stay unit: features that predict length-of-stay and eventual admission to traditional inpatient services. J Hosp Med. 2009;4(5):276-284.
Lack of Significant Gains in Survival Rates Following In-Hospital CPR
Clinical question: Is survival after in-hospital CPR improving over time, and what are the factors associated with survival?
Background: Advances in out-of-hospital CPR have improved outcomes. However, it is unknown whether the survival rate after in-hospital CPR is improving over time, and it is unclear which patient and/or hospital characteristics predict post-CPR survival.
Study design: Retrospective cohort.
Setting: Inpatient Medicare beneficiaries from 1992 to 2005.
Synopsis: The study examined more than 150 million Medicare admissions, 433,985 of which underwent in-hospital CPR. Survival to discharge occurred in 18.3% of CPR events and did not change significantly from 1992 to 2005. The cumulative incidence of in-hospital CPR events was 2.73 per 1,000 admissions; it did not change substantially over time.
The survival rate was lower among black patients (OR 0.76, 95% CI, 0.74-0.79), which is partially explained due to the fact they tended to receive CPR at hospitals with lower post-CPR survival. Gender (specifically male), older age, race (specifically other nonwhite patients), higher burden of chronic illness, and admission from a skilled nursing facility were significantly associated with decreased survival to hospital discharge following CPR.
Limitations of this study included the identification of CPR by ICD-9 codes, which have not been validated for this purpose and could vary among hospitals. Other factors that might explain variations in survival were not available, including severity of acute illness and the presence (or absence) of a shockable rhythm at initial presentation.
Bottom line: Rates of survival to hospital discharge among Medicare beneficiaries receiving in-hospital CPR have remained constant over time, with poorer survival rates among blacks and other nonwhite patients.
Citation: Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22-31.
Hospitalists Are Associated with Improved Performance on Quality Metrics
Clinical question: Is the presence of hospitalist physicians associated with improved performance on standard quality measures for acute myocardial infarction (AMI), congestive heart failure (CHF), and pneumonia?
Background: Previous investigations have demonstrated significant improvements in cost and LOS for patients under the care of hospitalists compared with other inpatient providers. The association between hospitalist prevalence and quality of care, as measured by standard quality process measures, is unknown.
Study design: Cross-sectional.
Setting: More than 3,600 hospitals participating in the Health Quality Alliance (HQA) program.
Synopsis: Investigators looked at a large sample of HQA hospitals in the American Hospital Association survey, and identified facilities with hospitalist services and those without. The primary endpoint was the adherence to composites of standard quality process measures across three disease categories (AMI, CHF, and pneumonia) and two domains of care (disease treatment/diagnosis and counseling/prevention).
Multivariable analyses revealed a statistically significant association between the presence of hospitalists and adherence to composite quality measures for AMI and pneumonia. This association was demonstrated for both treatment and counseling domains.
The study is cross-sectional, so conclusions cannot be drawn about causality. Also, there are likely unmeasured differences between hospitals that utilize hospitalists compared with those that do not, which could further confound the relationship between the presence of hospitalists and adherence to quality measures.
Finally, this study only evaluated hospital-level performance, and it cannot offer insight on the quality of individual patient care by hospitalist providers.
Bottom line: The presence of hospitalists is associated with improvement in adherence to quality measures for both AMI and pneumonia, and across clinical domains of treatment and counseling.
Citation: López L, Hicks LS, Cohen AP, McKean S, Weissman JS. Hospitalists and the quality of care in hospitals. Arch Intern Med. 2009;169(15):1389-1394. TH
Market Watch
New Generics
- Nateglinide (generic Starlix) tablets1
New Drugs, Indications, and Dosage Forms
- Abatacept (Orencia), a selective costimulation modulator used in treating moderate to severe juvenile idiopathic arthritis and rheumatoid arthritis (RA), has undergone a label change regarding earlier use in methotrexate-naïve patients with moderate to severe RA of less than two years’ disease duration.2,3
- Aliskiren/valsartan (Valturna) has been approved by the FDA for treating hypertension in patients with inadequate hypertension control using aliskiren or valsartan alone. It’s also approved for first-line treatment of patients who are likely to need multiple agents to manage their hypertension.4
- Cethromycin (Restanza) has been granted orphan drug approval as a once-daily agent for the prophylaxis of anthrax, tularemia, and the plague. Studies are being conducted on the drug as a potential bioterrorism countermeasure agent through a Department of Defense contract.5
- Ganciclovir ophthalmic gel 0.15% (Zirgan) has been approved by the FDA for treating acute herpetic keratitis. It held orphan drug status for this indication since April 2007. Comparable clinical resolution of herpetic keratitis was obtained compared with acyclovir at day seven in an open-label, multicenter study of 213 patients (77% ganciclovir; 72% acyclovir). The most common adverse effects in clinical trials were blurred vision, eye irritation, punctate keratitis, and conjunctival hyperemia. Dosing recommendations are to instill one drop of ganciclovir in the affected eye five times daily until the ulcer heals, then instill one drop three times daily for seven days. It is anticipated that this product will be available in a 5-g tube in early 2010.6
- Glycerol phenylbutyrate (HPN-100), an experimental intermittent or chronic treatment for patients with cirrhosis and hepatic encephalopathy, has received orphan drug status. A phase-2 trial is planned for late 2009 or early 2010.7 Glycerol phenylbutyrate is a pre-pro-drug of phenylacetic acid, the active component of buphenyl (approved by the FDA to treat urea cycle disorders). Glycerol phenylbutyrate is administered in liquid form and also has orphan drug status for treating urea cycle disorders.
- Guanfacine extended-release tablets (Intuiv), a once-daily, nonstimulant treatment for attention deficit hyperactivity disorder (ADHD), has been approved by the FDA for treating patients 6 to 17 years old. Because guanfacine is not a controlled substance, a 90-day supply can be prescribed.8
- Pancrelipase (Zenpep), a delayed-release pancrelipase enzyme product, has been approved by the FDA for treating adults and children (ages 1 to 12) with cystic fibrosis. The most common adverse effects reported in clinical trials were flatulence, abdominal pain, headache, and cough. The product is available in four prescription strengths: “Eurand 5” is 5,000 USP units of lipase, 17,000 USP units of protease, and 27,000 USP units of amylase; “Eurand 10” is 10,000 units lipase, 34,000 units protease, and 55,000 units amylase; “Eurand 15” is 15,000 units lipase, 51,000 units protease, and 82,000 units amylase; and “Eurand 20” is 20,000 units lipase, 68,000 units protease, and 109,000 units amylase.9,10
- Vigabatrin (Sabril) has been approved by the FDA in an oral solution as monotherapy for treating infantile spasms in children ages one month to 2 years. The tablets also are approved for adjunctive therapy for refractory complex partial seizures in adults who have not adequately responded to other treatments. It is available in 500-mg powder packets for oral solution preparation and 500-mg tablets.11 One severe adverse effect is progressive peripheral vision loss with the potential to decrease visual acuity. Due to this risk of permanent vision loss, vigabatrin is available only through a restricted distribution program.
Pipeline
- Human papillomavirus quadrivalent (Types 6, 11, 16 and 18; Gardasil) has been recommended for approval to prevent genital warts in boys and young men 9 to 26 years old. The FDA is expected to make a decision by the end of 2009.12,13 This vaccine already is approved for use in men in 112 countries.
- Oral insulin (Ora-Lyn) is a proprietary formulation that delivers insulin spray through the buccal mucosa.14 In September, Ora-Lyn was approved under the FDA’s Treatment Investigational New Drug (IND) program for both Type 1 and Type 2 diabetes mellitus. This program allows manufacturers to provide early medication access to investigational drugs for patients with life-threatening or other serious conditions for which there are no satisfactory treatment alternatives. Doctors must register with the IND program to obtain the medication for their patients.15Ora-Lyn already is approved abroad.
Drug Information
- On Sept. 22, the FDA banned candy- and fruit-flavored cigarettes under the Family Smoking Prevention and Tobacco Control Act. The goal is to reduce smoking in America.16 Menthol cigarettes and flavored tobacco products are not part of this ban, but they are being evaluated as many of these products are seen as a gateway for children and young adults to begin smoking. More information is available at www.fda.gov/flavoredtobacco. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
References
- Par Pharma to begin marketing Starlix generic. Pharmaceutical-Technology.com Web site. Available at: www.pharmaceutical-technology.com/news/news64185.html. Accessed Sept. 23, 2009.
- Highlights of prescribing information. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/125118s0086lbl.pdf Accessed Sept. 23, 2009.
- Bratulic A. Bristol Myers Squibb: Orencia label updated to support earlier use in adults with RA. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=DA78CDE71605485C9BC1B3B40392B1C0&logRowId=323904. Accessed Sept. 23, 2009.
- Bratulic A. FDA approves Novartis’ Valturna for hypertension. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=4D45881D26B8447D950A4D63E80B806C&logRowId=327307. Accessed Sept. 23, 2009.
- Advanced Life Sciences’ Restanza could treat plague and anthrax. Pharmaceutical-Technology.com Web site. Available at: www.pharmaceutical-technology.com/News/News64553.html. Accessed Sept. 23, 2009.
- FDA approves Zirgan. Drugs.com Web site. Available at: www.drugs.com/newdrugs/sirion-therapeutics-announces-fda-approval-zirgan-ganciclovir-ophthalmic-gel-0-15-herpetic-keratitis-1657.html. Accessed Sept. 23, 2009.
- 7. Hyperion Therapeutics receives orphan drug designation for HPN-100 for the treatment of hepatic encephalopathy. Hyperion Therapeutics Web site. Available at: www.hyperiontx.com/press/release/pr_1253144476. Accessed Sept. 23, 2009.
- George J. FDA approves nonstimulant Shire ADHD drug. Philadelphia Business Journal Web site. Available at: philadelphia.bizjournals.com/philadelphia/stories/2009/08/31/daily40.html?surround=etf&ana=e_article. Accessed Sept. 23, 2009.
- Petrochko C. FDA approves first EPI drug for kids. Medpage Today Web site. Available at: www.medpagetoday.com/Gastroenterology/GeneralGastroenterology/15734. Accessed Sept. 23, 2009.
- Highlights of prescribing information. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/022210s000lbl.pdf. Accessed Sept. 23, 2009.
- Sabril approved for infantile spasms and adult epileptic seizures. Monthly Prescribing Reference Web site. Available at: www.empr.com/Sabril-approved-for-infantile-spasms-and-adult-epileptic-seizures/article/147148/. Accessed Sept. 23, 2009.
- FDA advisory committee recommends approval for use of Gardasil in boys and men. Merck Web site. Available at: www.merck.com/newsroom/press_releases/product/2009_0909.html. Accessed Sept. 23, 2009.
- Bratulic A. Merck & Co.’s Gardasil recommended by FDA panel for use in boys and men. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=352E8E6109E14925B0168FF465E27C1F&logRowId=325991. Accessed Sept. 23, 2009.
- Generex technology. Generex Biotechnology Web site. Available at: www.generex.com/technology.php. Accessed Sept. 23, 2009.
- Reidy C. Generex Drug is OK’d under special FDA program. The Boston Globe Web site. www.generex.com/fckuploads/file/Boston_Globe_09_10_09.pdf. Accessed Sept. 23, 2009.
- Quinn K. Candy and fruit flavored cigarettes now illegal in United States; step is first under new tobacco law. Food and Drug Administration Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183211.htm. Accessed Sept. 23, 2009.
New Generics
- Nateglinide (generic Starlix) tablets1
New Drugs, Indications, and Dosage Forms
- Abatacept (Orencia), a selective costimulation modulator used in treating moderate to severe juvenile idiopathic arthritis and rheumatoid arthritis (RA), has undergone a label change regarding earlier use in methotrexate-naïve patients with moderate to severe RA of less than two years’ disease duration.2,3
- Aliskiren/valsartan (Valturna) has been approved by the FDA for treating hypertension in patients with inadequate hypertension control using aliskiren or valsartan alone. It’s also approved for first-line treatment of patients who are likely to need multiple agents to manage their hypertension.4
- Cethromycin (Restanza) has been granted orphan drug approval as a once-daily agent for the prophylaxis of anthrax, tularemia, and the plague. Studies are being conducted on the drug as a potential bioterrorism countermeasure agent through a Department of Defense contract.5
- Ganciclovir ophthalmic gel 0.15% (Zirgan) has been approved by the FDA for treating acute herpetic keratitis. It held orphan drug status for this indication since April 2007. Comparable clinical resolution of herpetic keratitis was obtained compared with acyclovir at day seven in an open-label, multicenter study of 213 patients (77% ganciclovir; 72% acyclovir). The most common adverse effects in clinical trials were blurred vision, eye irritation, punctate keratitis, and conjunctival hyperemia. Dosing recommendations are to instill one drop of ganciclovir in the affected eye five times daily until the ulcer heals, then instill one drop three times daily for seven days. It is anticipated that this product will be available in a 5-g tube in early 2010.6
- Glycerol phenylbutyrate (HPN-100), an experimental intermittent or chronic treatment for patients with cirrhosis and hepatic encephalopathy, has received orphan drug status. A phase-2 trial is planned for late 2009 or early 2010.7 Glycerol phenylbutyrate is a pre-pro-drug of phenylacetic acid, the active component of buphenyl (approved by the FDA to treat urea cycle disorders). Glycerol phenylbutyrate is administered in liquid form and also has orphan drug status for treating urea cycle disorders.
- Guanfacine extended-release tablets (Intuiv), a once-daily, nonstimulant treatment for attention deficit hyperactivity disorder (ADHD), has been approved by the FDA for treating patients 6 to 17 years old. Because guanfacine is not a controlled substance, a 90-day supply can be prescribed.8
- Pancrelipase (Zenpep), a delayed-release pancrelipase enzyme product, has been approved by the FDA for treating adults and children (ages 1 to 12) with cystic fibrosis. The most common adverse effects reported in clinical trials were flatulence, abdominal pain, headache, and cough. The product is available in four prescription strengths: “Eurand 5” is 5,000 USP units of lipase, 17,000 USP units of protease, and 27,000 USP units of amylase; “Eurand 10” is 10,000 units lipase, 34,000 units protease, and 55,000 units amylase; “Eurand 15” is 15,000 units lipase, 51,000 units protease, and 82,000 units amylase; and “Eurand 20” is 20,000 units lipase, 68,000 units protease, and 109,000 units amylase.9,10
- Vigabatrin (Sabril) has been approved by the FDA in an oral solution as monotherapy for treating infantile spasms in children ages one month to 2 years. The tablets also are approved for adjunctive therapy for refractory complex partial seizures in adults who have not adequately responded to other treatments. It is available in 500-mg powder packets for oral solution preparation and 500-mg tablets.11 One severe adverse effect is progressive peripheral vision loss with the potential to decrease visual acuity. Due to this risk of permanent vision loss, vigabatrin is available only through a restricted distribution program.
Pipeline
- Human papillomavirus quadrivalent (Types 6, 11, 16 and 18; Gardasil) has been recommended for approval to prevent genital warts in boys and young men 9 to 26 years old. The FDA is expected to make a decision by the end of 2009.12,13 This vaccine already is approved for use in men in 112 countries.
- Oral insulin (Ora-Lyn) is a proprietary formulation that delivers insulin spray through the buccal mucosa.14 In September, Ora-Lyn was approved under the FDA’s Treatment Investigational New Drug (IND) program for both Type 1 and Type 2 diabetes mellitus. This program allows manufacturers to provide early medication access to investigational drugs for patients with life-threatening or other serious conditions for which there are no satisfactory treatment alternatives. Doctors must register with the IND program to obtain the medication for their patients.15Ora-Lyn already is approved abroad.
Drug Information
- On Sept. 22, the FDA banned candy- and fruit-flavored cigarettes under the Family Smoking Prevention and Tobacco Control Act. The goal is to reduce smoking in America.16 Menthol cigarettes and flavored tobacco products are not part of this ban, but they are being evaluated as many of these products are seen as a gateway for children and young adults to begin smoking. More information is available at www.fda.gov/flavoredtobacco. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
References
- Par Pharma to begin marketing Starlix generic. Pharmaceutical-Technology.com Web site. Available at: www.pharmaceutical-technology.com/news/news64185.html. Accessed Sept. 23, 2009.
- Highlights of prescribing information. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/125118s0086lbl.pdf Accessed Sept. 23, 2009.
- Bratulic A. Bristol Myers Squibb: Orencia label updated to support earlier use in adults with RA. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=DA78CDE71605485C9BC1B3B40392B1C0&logRowId=323904. Accessed Sept. 23, 2009.
- Bratulic A. FDA approves Novartis’ Valturna for hypertension. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=4D45881D26B8447D950A4D63E80B806C&logRowId=327307. Accessed Sept. 23, 2009.
- Advanced Life Sciences’ Restanza could treat plague and anthrax. Pharmaceutical-Technology.com Web site. Available at: www.pharmaceutical-technology.com/News/News64553.html. Accessed Sept. 23, 2009.
- FDA approves Zirgan. Drugs.com Web site. Available at: www.drugs.com/newdrugs/sirion-therapeutics-announces-fda-approval-zirgan-ganciclovir-ophthalmic-gel-0-15-herpetic-keratitis-1657.html. Accessed Sept. 23, 2009.
- 7. Hyperion Therapeutics receives orphan drug designation for HPN-100 for the treatment of hepatic encephalopathy. Hyperion Therapeutics Web site. Available at: www.hyperiontx.com/press/release/pr_1253144476. Accessed Sept. 23, 2009.
- George J. FDA approves nonstimulant Shire ADHD drug. Philadelphia Business Journal Web site. Available at: philadelphia.bizjournals.com/philadelphia/stories/2009/08/31/daily40.html?surround=etf&ana=e_article. Accessed Sept. 23, 2009.
- Petrochko C. FDA approves first EPI drug for kids. Medpage Today Web site. Available at: www.medpagetoday.com/Gastroenterology/GeneralGastroenterology/15734. Accessed Sept. 23, 2009.
- Highlights of prescribing information. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/022210s000lbl.pdf. Accessed Sept. 23, 2009.
- Sabril approved for infantile spasms and adult epileptic seizures. Monthly Prescribing Reference Web site. Available at: www.empr.com/Sabril-approved-for-infantile-spasms-and-adult-epileptic-seizures/article/147148/. Accessed Sept. 23, 2009.
- FDA advisory committee recommends approval for use of Gardasil in boys and men. Merck Web site. Available at: www.merck.com/newsroom/press_releases/product/2009_0909.html. Accessed Sept. 23, 2009.
- Bratulic A. Merck & Co.’s Gardasil recommended by FDA panel for use in boys and men. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=352E8E6109E14925B0168FF465E27C1F&logRowId=325991. Accessed Sept. 23, 2009.
- Generex technology. Generex Biotechnology Web site. Available at: www.generex.com/technology.php. Accessed Sept. 23, 2009.
- Reidy C. Generex Drug is OK’d under special FDA program. The Boston Globe Web site. www.generex.com/fckuploads/file/Boston_Globe_09_10_09.pdf. Accessed Sept. 23, 2009.
- Quinn K. Candy and fruit flavored cigarettes now illegal in United States; step is first under new tobacco law. Food and Drug Administration Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183211.htm. Accessed Sept. 23, 2009.
New Generics
- Nateglinide (generic Starlix) tablets1
New Drugs, Indications, and Dosage Forms
- Abatacept (Orencia), a selective costimulation modulator used in treating moderate to severe juvenile idiopathic arthritis and rheumatoid arthritis (RA), has undergone a label change regarding earlier use in methotrexate-naïve patients with moderate to severe RA of less than two years’ disease duration.2,3
- Aliskiren/valsartan (Valturna) has been approved by the FDA for treating hypertension in patients with inadequate hypertension control using aliskiren or valsartan alone. It’s also approved for first-line treatment of patients who are likely to need multiple agents to manage their hypertension.4
- Cethromycin (Restanza) has been granted orphan drug approval as a once-daily agent for the prophylaxis of anthrax, tularemia, and the plague. Studies are being conducted on the drug as a potential bioterrorism countermeasure agent through a Department of Defense contract.5
- Ganciclovir ophthalmic gel 0.15% (Zirgan) has been approved by the FDA for treating acute herpetic keratitis. It held orphan drug status for this indication since April 2007. Comparable clinical resolution of herpetic keratitis was obtained compared with acyclovir at day seven in an open-label, multicenter study of 213 patients (77% ganciclovir; 72% acyclovir). The most common adverse effects in clinical trials were blurred vision, eye irritation, punctate keratitis, and conjunctival hyperemia. Dosing recommendations are to instill one drop of ganciclovir in the affected eye five times daily until the ulcer heals, then instill one drop three times daily for seven days. It is anticipated that this product will be available in a 5-g tube in early 2010.6
- Glycerol phenylbutyrate (HPN-100), an experimental intermittent or chronic treatment for patients with cirrhosis and hepatic encephalopathy, has received orphan drug status. A phase-2 trial is planned for late 2009 or early 2010.7 Glycerol phenylbutyrate is a pre-pro-drug of phenylacetic acid, the active component of buphenyl (approved by the FDA to treat urea cycle disorders). Glycerol phenylbutyrate is administered in liquid form and also has orphan drug status for treating urea cycle disorders.
- Guanfacine extended-release tablets (Intuiv), a once-daily, nonstimulant treatment for attention deficit hyperactivity disorder (ADHD), has been approved by the FDA for treating patients 6 to 17 years old. Because guanfacine is not a controlled substance, a 90-day supply can be prescribed.8
- Pancrelipase (Zenpep), a delayed-release pancrelipase enzyme product, has been approved by the FDA for treating adults and children (ages 1 to 12) with cystic fibrosis. The most common adverse effects reported in clinical trials were flatulence, abdominal pain, headache, and cough. The product is available in four prescription strengths: “Eurand 5” is 5,000 USP units of lipase, 17,000 USP units of protease, and 27,000 USP units of amylase; “Eurand 10” is 10,000 units lipase, 34,000 units protease, and 55,000 units amylase; “Eurand 15” is 15,000 units lipase, 51,000 units protease, and 82,000 units amylase; and “Eurand 20” is 20,000 units lipase, 68,000 units protease, and 109,000 units amylase.9,10
- Vigabatrin (Sabril) has been approved by the FDA in an oral solution as monotherapy for treating infantile spasms in children ages one month to 2 years. The tablets also are approved for adjunctive therapy for refractory complex partial seizures in adults who have not adequately responded to other treatments. It is available in 500-mg powder packets for oral solution preparation and 500-mg tablets.11 One severe adverse effect is progressive peripheral vision loss with the potential to decrease visual acuity. Due to this risk of permanent vision loss, vigabatrin is available only through a restricted distribution program.
Pipeline
- Human papillomavirus quadrivalent (Types 6, 11, 16 and 18; Gardasil) has been recommended for approval to prevent genital warts in boys and young men 9 to 26 years old. The FDA is expected to make a decision by the end of 2009.12,13 This vaccine already is approved for use in men in 112 countries.
- Oral insulin (Ora-Lyn) is a proprietary formulation that delivers insulin spray through the buccal mucosa.14 In September, Ora-Lyn was approved under the FDA’s Treatment Investigational New Drug (IND) program for both Type 1 and Type 2 diabetes mellitus. This program allows manufacturers to provide early medication access to investigational drugs for patients with life-threatening or other serious conditions for which there are no satisfactory treatment alternatives. Doctors must register with the IND program to obtain the medication for their patients.15Ora-Lyn already is approved abroad.
Drug Information
- On Sept. 22, the FDA banned candy- and fruit-flavored cigarettes under the Family Smoking Prevention and Tobacco Control Act. The goal is to reduce smoking in America.16 Menthol cigarettes and flavored tobacco products are not part of this ban, but they are being evaluated as many of these products are seen as a gateway for children and young adults to begin smoking. More information is available at www.fda.gov/flavoredtobacco. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
References
- Par Pharma to begin marketing Starlix generic. Pharmaceutical-Technology.com Web site. Available at: www.pharmaceutical-technology.com/news/news64185.html. Accessed Sept. 23, 2009.
- Highlights of prescribing information. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/125118s0086lbl.pdf Accessed Sept. 23, 2009.
- Bratulic A. Bristol Myers Squibb: Orencia label updated to support earlier use in adults with RA. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=DA78CDE71605485C9BC1B3B40392B1C0&logRowId=323904. Accessed Sept. 23, 2009.
- Bratulic A. FDA approves Novartis’ Valturna for hypertension. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=4D45881D26B8447D950A4D63E80B806C&logRowId=327307. Accessed Sept. 23, 2009.
- Advanced Life Sciences’ Restanza could treat plague and anthrax. Pharmaceutical-Technology.com Web site. Available at: www.pharmaceutical-technology.com/News/News64553.html. Accessed Sept. 23, 2009.
- FDA approves Zirgan. Drugs.com Web site. Available at: www.drugs.com/newdrugs/sirion-therapeutics-announces-fda-approval-zirgan-ganciclovir-ophthalmic-gel-0-15-herpetic-keratitis-1657.html. Accessed Sept. 23, 2009.
- 7. Hyperion Therapeutics receives orphan drug designation for HPN-100 for the treatment of hepatic encephalopathy. Hyperion Therapeutics Web site. Available at: www.hyperiontx.com/press/release/pr_1253144476. Accessed Sept. 23, 2009.
- George J. FDA approves nonstimulant Shire ADHD drug. Philadelphia Business Journal Web site. Available at: philadelphia.bizjournals.com/philadelphia/stories/2009/08/31/daily40.html?surround=etf&ana=e_article. Accessed Sept. 23, 2009.
- Petrochko C. FDA approves first EPI drug for kids. Medpage Today Web site. Available at: www.medpagetoday.com/Gastroenterology/GeneralGastroenterology/15734. Accessed Sept. 23, 2009.
- Highlights of prescribing information. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/022210s000lbl.pdf. Accessed Sept. 23, 2009.
- Sabril approved for infantile spasms and adult epileptic seizures. Monthly Prescribing Reference Web site. Available at: www.empr.com/Sabril-approved-for-infantile-spasms-and-adult-epileptic-seizures/article/147148/. Accessed Sept. 23, 2009.
- FDA advisory committee recommends approval for use of Gardasil in boys and men. Merck Web site. Available at: www.merck.com/newsroom/press_releases/product/2009_0909.html. Accessed Sept. 23, 2009.
- Bratulic A. Merck & Co.’s Gardasil recommended by FDA panel for use in boys and men. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=352E8E6109E14925B0168FF465E27C1F&logRowId=325991. Accessed Sept. 23, 2009.
- Generex technology. Generex Biotechnology Web site. Available at: www.generex.com/technology.php. Accessed Sept. 23, 2009.
- Reidy C. Generex Drug is OK’d under special FDA program. The Boston Globe Web site. www.generex.com/fckuploads/file/Boston_Globe_09_10_09.pdf. Accessed Sept. 23, 2009.
- Quinn K. Candy and fruit flavored cigarettes now illegal in United States; step is first under new tobacco law. Food and Drug Administration Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183211.htm. Accessed Sept. 23, 2009.
2009: Year in Review
From continued membership growth to increased visibility in the national media, SHM and its members have been influencing healthcare for more than a decade. But even by the highest of standards, 2009 has been a landmark year—one that demonstrated hospitalists’ collective ability to transform healthcare and improve care to the hospitalized patient.
“The momentum of the hospital medicine movement has been growing for years, and 2009 has been no exception,” says Scott Flanders, MD, FHM, president of SHM. “This year built on the successes of the past and plainly illustrated the impact that hospital medicine will have on the future of healthcare.”
Groundbreaking QI Programs Go Nationwide
This year, SHM and its members began to tackle some of the most pressing QI issues in healthcare: reducing readmissions to the hospital and glycemic control. New research in the New England Journal of Medicine couldn’t have made the need for reducing readmissions any clearer: Unplanned hospital readmissions cost Medicare $17.4 billion annually.1
SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) helps hospitals implement customized programs to reduce readmissions through improved discharge processes. Hospitalists who enroll in the yearlong program take advantage of a one-on-one mentorship arrangement with experts in the field. Participants can also access the Project BOOST resource toolkit.
Project BOOST began in six pilot hospital sites in 2008 and added 24 new sites in March 2009. The program’s leaders are looking forward to further expansion in 2010. “The response to Project BOOST has been overwhelmingly positive. Given today’s healthcare climate, we know its impact will be even greater in years to come,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives. “There is a very serious need to improve discharge processes in hospitals across the country. With Project BOOST, hospitalists are taking the initiative to ensure a smooth transition from hospital to home.”
SHM also launched the Glycemic Control Mentored Implementation (GCMI) program. Like Project BOOST, GCMI uses a combination of one-on-one mentorships and customized resources to assist hospitalists with QI program implementation.
GCMI takes on another common chronic issue hospitalists face daily: managing glycemic levels in hospitalized patients. The GCMI program is currently in 30 sites across the country.

—Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives
HM09 Draws Capacity Crowd in Chicago
In an economic climate that forced many industries’ annual meetings to be canceled, delayed, or scaled back, Hospital Medicine 2009 (HM09) in Chicago exceeded expectations. SHM had expected about 1,500 participants in the annual conference; organizers were pleasantly surprised to receive more than 2,000 registrations for the May event. The demand for exhibition space also surpassed projections.
“We’ve long known that hospitalists see real value in a meeting specifically designed for them, with relevant educational sessions and plenty of time for networking,” says Geri Barnes, SHM’s senior director of education and meetings. “Each year, we’ve received more and more interest in the annual conference, but the response to our 2009 conference was unprecedented.”
HM10 is April 8-11 at the Gaylord National Hotel and Convention Center in Washington, D.C.
SHM, MGMA Form Research Partnership
Beginning in 2010, SHM and the Medical Group Management Association (MGMA) will team up to give hospitalists and healthcare executives an even clearer picture on hospitalist compensation and productivity.
Prior to the partnership, SHM had conducted its own research. Now, hospitals and HM managers will have new data at their fingertips, and additional analysis and name-brand recognition of one of the leaders in medical practice research. The first round of research will be available in summer 2010. SHM and MGMA already have collaborated on educational webinars for hospitalists, and SHM is offering books published by MGMA on its Web site.
“This new alliance will pay dividends for years to come,” says Leslie Flores, the director of SHM’s Practice Management Institute. “The information from our compensation and productivity surveys has always been valuable to hospitals. Having the MGMA name attached to next year’s product will only increase its significance and usefulness.”
Hospitalists will receive the joint survey questionnaire from SHM and MGMA in January.
HM Fellows
Three letters can mean a lot, especially for hospitalists looking for ways to demonstrate their commitment to the specialty. This year was the first in which qualified hospitalists could earn the Fellow in Hospital Medicine (FHM) designation. The first class of more than 500 FHM designees was introduced in an on-stage ceremony at HM09.
“This is a special way for SHM—and the healthcare industry as a whole—to recognize the unique achievements and dedication that hospital medicine requires,” says Todd Von Deak, MBA, CAE, SHM’s vice president for marketing and membership. “As the specialty grows in number and influence, so will the fellows program.”
In 2010, SHM will induct the first class of Senior Fellows in Hospital Medicine (SFHM). While the process for applying for the senior designation will be similar to the FHM designation, the SFHM will require additional years of practice and leadership in the specialty.
The fellows program also features the Master in Hospital Medicine (MHM) designation, the highest level of recognition available. The MHM will be available in 2011, and the nomination process will be invitation-only.
Outside Recognition
SHM isn’t the only group recognizing the impact hospitalists are making on healthcare. In September, the American Board of Internal Medicine (ABIM) announced that hospitalists will be able to apply for Recognition of Focused Practice (RFP) in Hospital Medicine as part of ABIM’s maintenance of certification (MOC) program. The application process will be available as early as next month.
SHM will be assisting hospitalists in the application process through online resources and the MOC pre-course, which will be offered before HM10. Hospitalists with three years of experience in the field can apply for the RFP program. Although most physicians are required to recertify every 10 years, hospitalists won’t have to wait until their certification is up to apply for focused recognition. For more information about the RFP in HM program, visit www.abim.org.
Hospital-Provider Partners
Treating hospitalized patients has always been a team sport. From caseworkers and pharmacists to physicians and critical-care nurses, the diverse and specialized needs of hospital care demand collaboration and coordination.
That’s the idea behind the Hospital Care Collaborative (HCC), which convened for the first time in 2009. The group is made up of six national organizations that represent hundreds of thousands of care providers. The HCC has developed and published “Common Principles for Team-Based Healthcare.” The principles emphasize the need for teamwork within the hospital setting and a focus on the patient.
As part of its goals for the future, the HCC will identify best practices in teamwork and promote educational programs that encourage interdisciplinary teams.
Look Back, Look Forward
For SHM CEO Larry Wellikson, MD, FHM, the end of 2009 is an opportunity to look forward to 2010 and beyond. “Ten years ago, hospital medicine was little more than an idea,” he says. “Today, it is a growing medical specialty, recognized by leaders in healthcare and public policy, with thousands of experienced and enthusiastic hospitalists throughout the country.
“I am confident that when we look back ten years from now, we will see a hospital landscape transformed for the better, and that hospitalists and the rest of the new healthcare team will have played an important role.” TH
Brendon Shank is a freelance writer based in Philadelphia.
Reference
- Jencks SF, Williams MV, Coleman A. Rehospitaliza- tions among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
From continued membership growth to increased visibility in the national media, SHM and its members have been influencing healthcare for more than a decade. But even by the highest of standards, 2009 has been a landmark year—one that demonstrated hospitalists’ collective ability to transform healthcare and improve care to the hospitalized patient.
“The momentum of the hospital medicine movement has been growing for years, and 2009 has been no exception,” says Scott Flanders, MD, FHM, president of SHM. “This year built on the successes of the past and plainly illustrated the impact that hospital medicine will have on the future of healthcare.”
Groundbreaking QI Programs Go Nationwide
This year, SHM and its members began to tackle some of the most pressing QI issues in healthcare: reducing readmissions to the hospital and glycemic control. New research in the New England Journal of Medicine couldn’t have made the need for reducing readmissions any clearer: Unplanned hospital readmissions cost Medicare $17.4 billion annually.1
SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) helps hospitals implement customized programs to reduce readmissions through improved discharge processes. Hospitalists who enroll in the yearlong program take advantage of a one-on-one mentorship arrangement with experts in the field. Participants can also access the Project BOOST resource toolkit.
Project BOOST began in six pilot hospital sites in 2008 and added 24 new sites in March 2009. The program’s leaders are looking forward to further expansion in 2010. “The response to Project BOOST has been overwhelmingly positive. Given today’s healthcare climate, we know its impact will be even greater in years to come,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives. “There is a very serious need to improve discharge processes in hospitals across the country. With Project BOOST, hospitalists are taking the initiative to ensure a smooth transition from hospital to home.”
SHM also launched the Glycemic Control Mentored Implementation (GCMI) program. Like Project BOOST, GCMI uses a combination of one-on-one mentorships and customized resources to assist hospitalists with QI program implementation.
GCMI takes on another common chronic issue hospitalists face daily: managing glycemic levels in hospitalized patients. The GCMI program is currently in 30 sites across the country.

—Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives
HM09 Draws Capacity Crowd in Chicago
In an economic climate that forced many industries’ annual meetings to be canceled, delayed, or scaled back, Hospital Medicine 2009 (HM09) in Chicago exceeded expectations. SHM had expected about 1,500 participants in the annual conference; organizers were pleasantly surprised to receive more than 2,000 registrations for the May event. The demand for exhibition space also surpassed projections.
“We’ve long known that hospitalists see real value in a meeting specifically designed for them, with relevant educational sessions and plenty of time for networking,” says Geri Barnes, SHM’s senior director of education and meetings. “Each year, we’ve received more and more interest in the annual conference, but the response to our 2009 conference was unprecedented.”
HM10 is April 8-11 at the Gaylord National Hotel and Convention Center in Washington, D.C.
SHM, MGMA Form Research Partnership
Beginning in 2010, SHM and the Medical Group Management Association (MGMA) will team up to give hospitalists and healthcare executives an even clearer picture on hospitalist compensation and productivity.
Prior to the partnership, SHM had conducted its own research. Now, hospitals and HM managers will have new data at their fingertips, and additional analysis and name-brand recognition of one of the leaders in medical practice research. The first round of research will be available in summer 2010. SHM and MGMA already have collaborated on educational webinars for hospitalists, and SHM is offering books published by MGMA on its Web site.
“This new alliance will pay dividends for years to come,” says Leslie Flores, the director of SHM’s Practice Management Institute. “The information from our compensation and productivity surveys has always been valuable to hospitals. Having the MGMA name attached to next year’s product will only increase its significance and usefulness.”
Hospitalists will receive the joint survey questionnaire from SHM and MGMA in January.
HM Fellows
Three letters can mean a lot, especially for hospitalists looking for ways to demonstrate their commitment to the specialty. This year was the first in which qualified hospitalists could earn the Fellow in Hospital Medicine (FHM) designation. The first class of more than 500 FHM designees was introduced in an on-stage ceremony at HM09.
“This is a special way for SHM—and the healthcare industry as a whole—to recognize the unique achievements and dedication that hospital medicine requires,” says Todd Von Deak, MBA, CAE, SHM’s vice president for marketing and membership. “As the specialty grows in number and influence, so will the fellows program.”
In 2010, SHM will induct the first class of Senior Fellows in Hospital Medicine (SFHM). While the process for applying for the senior designation will be similar to the FHM designation, the SFHM will require additional years of practice and leadership in the specialty.
The fellows program also features the Master in Hospital Medicine (MHM) designation, the highest level of recognition available. The MHM will be available in 2011, and the nomination process will be invitation-only.
Outside Recognition
SHM isn’t the only group recognizing the impact hospitalists are making on healthcare. In September, the American Board of Internal Medicine (ABIM) announced that hospitalists will be able to apply for Recognition of Focused Practice (RFP) in Hospital Medicine as part of ABIM’s maintenance of certification (MOC) program. The application process will be available as early as next month.
SHM will be assisting hospitalists in the application process through online resources and the MOC pre-course, which will be offered before HM10. Hospitalists with three years of experience in the field can apply for the RFP program. Although most physicians are required to recertify every 10 years, hospitalists won’t have to wait until their certification is up to apply for focused recognition. For more information about the RFP in HM program, visit www.abim.org.
Hospital-Provider Partners
Treating hospitalized patients has always been a team sport. From caseworkers and pharmacists to physicians and critical-care nurses, the diverse and specialized needs of hospital care demand collaboration and coordination.
That’s the idea behind the Hospital Care Collaborative (HCC), which convened for the first time in 2009. The group is made up of six national organizations that represent hundreds of thousands of care providers. The HCC has developed and published “Common Principles for Team-Based Healthcare.” The principles emphasize the need for teamwork within the hospital setting and a focus on the patient.
As part of its goals for the future, the HCC will identify best practices in teamwork and promote educational programs that encourage interdisciplinary teams.
Look Back, Look Forward
For SHM CEO Larry Wellikson, MD, FHM, the end of 2009 is an opportunity to look forward to 2010 and beyond. “Ten years ago, hospital medicine was little more than an idea,” he says. “Today, it is a growing medical specialty, recognized by leaders in healthcare and public policy, with thousands of experienced and enthusiastic hospitalists throughout the country.
“I am confident that when we look back ten years from now, we will see a hospital landscape transformed for the better, and that hospitalists and the rest of the new healthcare team will have played an important role.” TH
Brendon Shank is a freelance writer based in Philadelphia.
Reference
- Jencks SF, Williams MV, Coleman A. Rehospitaliza- tions among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
From continued membership growth to increased visibility in the national media, SHM and its members have been influencing healthcare for more than a decade. But even by the highest of standards, 2009 has been a landmark year—one that demonstrated hospitalists’ collective ability to transform healthcare and improve care to the hospitalized patient.
“The momentum of the hospital medicine movement has been growing for years, and 2009 has been no exception,” says Scott Flanders, MD, FHM, president of SHM. “This year built on the successes of the past and plainly illustrated the impact that hospital medicine will have on the future of healthcare.”
Groundbreaking QI Programs Go Nationwide
This year, SHM and its members began to tackle some of the most pressing QI issues in healthcare: reducing readmissions to the hospital and glycemic control. New research in the New England Journal of Medicine couldn’t have made the need for reducing readmissions any clearer: Unplanned hospital readmissions cost Medicare $17.4 billion annually.1
SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) helps hospitals implement customized programs to reduce readmissions through improved discharge processes. Hospitalists who enroll in the yearlong program take advantage of a one-on-one mentorship arrangement with experts in the field. Participants can also access the Project BOOST resource toolkit.
Project BOOST began in six pilot hospital sites in 2008 and added 24 new sites in March 2009. The program’s leaders are looking forward to further expansion in 2010. “The response to Project BOOST has been overwhelmingly positive. Given today’s healthcare climate, we know its impact will be even greater in years to come,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives. “There is a very serious need to improve discharge processes in hospitals across the country. With Project BOOST, hospitalists are taking the initiative to ensure a smooth transition from hospital to home.”
SHM also launched the Glycemic Control Mentored Implementation (GCMI) program. Like Project BOOST, GCMI uses a combination of one-on-one mentorships and customized resources to assist hospitalists with QI program implementation.
GCMI takes on another common chronic issue hospitalists face daily: managing glycemic levels in hospitalized patients. The GCMI program is currently in 30 sites across the country.

—Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives
HM09 Draws Capacity Crowd in Chicago
In an economic climate that forced many industries’ annual meetings to be canceled, delayed, or scaled back, Hospital Medicine 2009 (HM09) in Chicago exceeded expectations. SHM had expected about 1,500 participants in the annual conference; organizers were pleasantly surprised to receive more than 2,000 registrations for the May event. The demand for exhibition space also surpassed projections.
“We’ve long known that hospitalists see real value in a meeting specifically designed for them, with relevant educational sessions and plenty of time for networking,” says Geri Barnes, SHM’s senior director of education and meetings. “Each year, we’ve received more and more interest in the annual conference, but the response to our 2009 conference was unprecedented.”
HM10 is April 8-11 at the Gaylord National Hotel and Convention Center in Washington, D.C.
SHM, MGMA Form Research Partnership
Beginning in 2010, SHM and the Medical Group Management Association (MGMA) will team up to give hospitalists and healthcare executives an even clearer picture on hospitalist compensation and productivity.
Prior to the partnership, SHM had conducted its own research. Now, hospitals and HM managers will have new data at their fingertips, and additional analysis and name-brand recognition of one of the leaders in medical practice research. The first round of research will be available in summer 2010. SHM and MGMA already have collaborated on educational webinars for hospitalists, and SHM is offering books published by MGMA on its Web site.
“This new alliance will pay dividends for years to come,” says Leslie Flores, the director of SHM’s Practice Management Institute. “The information from our compensation and productivity surveys has always been valuable to hospitals. Having the MGMA name attached to next year’s product will only increase its significance and usefulness.”
Hospitalists will receive the joint survey questionnaire from SHM and MGMA in January.
HM Fellows
Three letters can mean a lot, especially for hospitalists looking for ways to demonstrate their commitment to the specialty. This year was the first in which qualified hospitalists could earn the Fellow in Hospital Medicine (FHM) designation. The first class of more than 500 FHM designees was introduced in an on-stage ceremony at HM09.
“This is a special way for SHM—and the healthcare industry as a whole—to recognize the unique achievements and dedication that hospital medicine requires,” says Todd Von Deak, MBA, CAE, SHM’s vice president for marketing and membership. “As the specialty grows in number and influence, so will the fellows program.”
In 2010, SHM will induct the first class of Senior Fellows in Hospital Medicine (SFHM). While the process for applying for the senior designation will be similar to the FHM designation, the SFHM will require additional years of practice and leadership in the specialty.
The fellows program also features the Master in Hospital Medicine (MHM) designation, the highest level of recognition available. The MHM will be available in 2011, and the nomination process will be invitation-only.
Outside Recognition
SHM isn’t the only group recognizing the impact hospitalists are making on healthcare. In September, the American Board of Internal Medicine (ABIM) announced that hospitalists will be able to apply for Recognition of Focused Practice (RFP) in Hospital Medicine as part of ABIM’s maintenance of certification (MOC) program. The application process will be available as early as next month.
SHM will be assisting hospitalists in the application process through online resources and the MOC pre-course, which will be offered before HM10. Hospitalists with three years of experience in the field can apply for the RFP program. Although most physicians are required to recertify every 10 years, hospitalists won’t have to wait until their certification is up to apply for focused recognition. For more information about the RFP in HM program, visit www.abim.org.
Hospital-Provider Partners
Treating hospitalized patients has always been a team sport. From caseworkers and pharmacists to physicians and critical-care nurses, the diverse and specialized needs of hospital care demand collaboration and coordination.
That’s the idea behind the Hospital Care Collaborative (HCC), which convened for the first time in 2009. The group is made up of six national organizations that represent hundreds of thousands of care providers. The HCC has developed and published “Common Principles for Team-Based Healthcare.” The principles emphasize the need for teamwork within the hospital setting and a focus on the patient.
As part of its goals for the future, the HCC will identify best practices in teamwork and promote educational programs that encourage interdisciplinary teams.
Look Back, Look Forward
For SHM CEO Larry Wellikson, MD, FHM, the end of 2009 is an opportunity to look forward to 2010 and beyond. “Ten years ago, hospital medicine was little more than an idea,” he says. “Today, it is a growing medical specialty, recognized by leaders in healthcare and public policy, with thousands of experienced and enthusiastic hospitalists throughout the country.
“I am confident that when we look back ten years from now, we will see a hospital landscape transformed for the better, and that hospitalists and the rest of the new healthcare team will have played an important role.” TH
Brendon Shank is a freelance writer based in Philadelphia.
Reference
- Jencks SF, Williams MV, Coleman A. Rehospitaliza- tions among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
Spotlight on Stroke
Ethan Cumbler, MD, is board-certified in internal medicine and pediatrics, and has practiced hospital medicine for six years, first at a community hospital and now at the University of Colorado Denver (UCD), where he directs the Acute Care for the Elderly service. The prevalence of stroke in his practice and the daily challenges of managing stroke patients led Dr. Cumbler to seek additional training in stroke care. He is the hospitalist representative to the UCD stroke council, a researcher in the arena of acute stroke care, and is helping UCD become a Joint Commission-certified stroke center.
“There are a variety of roles for the hospitalist in stroke care,” Dr. Cumbler says, explaining that HM physicians can be admitting attendings for stroke patients or part of acute stroke teams, and participate in decisions to start such treatments as intravenous recombinant tissue plasminogen activator (t-PA), the Food and Drug Administration-approved clot-busting therapy. “[Hospitalists] can be medical consultants on stroke patients admitted to other hospital services, managing common comorbid conditions such as blood pressure and glucose levels, which have particular character for patients immediately post-stroke.”
Stroke is the third-leading cause of death in the U.S., as well as a leading cause of serious, long-term disability. How many stroke patients are seen by hospitalists is not known, but it is reasonable to assume that a majority of hospitalized stroke patients will encounter a hospitalist, if not for acute treatment, then for ongoing medical management.
Some hospitalists think stroke and transient ischemic attacks (TIAs)—temporary neurological deficits sometimes called “mini-strokes,” and a major risk factor for full-blown strokes—are among the most common diseases seen by hospitalists.1 Acute stroke care is a growing part of HM practice because neurologist availability in emergent situations varies widely between hospitals. The rapid evolution of stroke treatment and the time-sensitive needs of stroke patients represents a huge opportunity for hospitalists to fill that void for their hospitals—whether they want to or not.
“I think hospitalists are fully capable of learning and mastering stroke care, but it requires both interest and training,” Dr. Cumbler says.
HM Can Help Fill a Void
According to the American Heart Association (AHA), there are four neurologists per 100,000 Americans, and not all of those neurologists specialize in stroke care.2 The scarcity of neurological specialists means that in many hospitals, a neurologist won’t be available for the critical assessment and treatment decisions required in the first few hours after a stroke is diagnosed. Yet many hospitalists complain that their preparation during internal-medicine residency did not equip them to care for acute stroke patients.3
S. Andrew Josephson, MD, a neurovascular physician and director of the neurohospitalist program at the University of California at San Francisco Medical Center, says the number of hospitalists on the front lines of acute stroke care is growing every day. “A new stroke is a very treatable neurological emergency that requires ultra-fast intervention,”7 Dr. Josephson says, “and hospitalists, increasingly, are the people who matter most in that intervention.” The reason, in most cases, is hospitalists are available at all times, and neurologists aren’t.
Given variable access to neurologists at the time of urgent need in many hospitals, the actions hospitalists can take in acute stroke management include:
- Become better trained in stroke care. Sessions on stroke management are included in numerous HM educational programs, including SHM conferences and in continuing medical education (CME) offerings from such groups as the American Academy of Neurology (see “Stroke Training, Resources, and Opportunities,” p. 30).
- Partner with neurologists in your hospital. One trend is to develop a neurohospitalist practice.
- Push for increased organization and response times for stroke patients. Given HM’s focus on quality and patient safety, hospitalists are natural champions for improving systems of care for stroke. Hospitalists can work with neurologists, radiologists, pharmacists, and other providers to develop stroke treatment protocols and rapid response capabilities.
- Help develop a stroke team, and seek certification as a primary stroke center. The Joint Commission certifies stroke centers (www.jointcommission.org/CertificationPrograms/PrimaryStroke Centers) based on demonstrated compliance with disease-based standards, effective use of clinical practice guidelines, and performance-improvement activities.
- Establish a collaborative relationship with a regional stroke center or tertiary hospital. This could manifest as a telemedicine link to aid in stroke assessment and treatment decisions (see “Rural Response: The ‘Drip and Ship’ Method,” p. 28).
- Refine approaches to more rapidly identify and work up patients who experience a stroke while they are in the hospital.

—S. Andrew Josephson, MD, director, neurohospitalist program, University of California at San Francisco Medical Center
Streamline In-Hospital Stroke Response
From 6.5% to 15% of stroke patients experience their stroke while they are in the hospital.4 “Hospitals are not always geared up to deal with neurological emergencies, and yet these patients are firmly within our domain,” Dr. Cumbler says. “We found that it took three times longer in our hospital to complete the evaluation when the stroke happened in the hospital than for strokes presenting in the emergency department.”
Through a hospitalwide quality-improvement (QI) project, UCD’s in-hospital stroke response time was reduced to 37 minutes from 70 minutes.
A comprehensive approach to stroke QI should include training first witnesses in the hospital (e.g., nurses, physical therapists, and housekeepers) to recognize potential stroke symptoms; creating a rapid response capability from personnel who understand how to evaluate and treat suspected stroke and are able to respond quickly; and making suspected stroke a top priority in the radiology lab.
Stroke patient management processes need to be improved and provider roles better defined. Hospitalists can help on the frontlines, and should advocate for quality and patient safety measures.
“Stroke has so many facets: the need to reduce risk, to educate the public about the need for prompt response, the appropriate evaluation of risks and benefits of treatment,” Dr. Cumbler says. “How do you achieve a system in the hospital where patients are fully able to realize benefits of all these advances? I think there’s something in stroke treatment for every hospitalist and, for those with a particular interest, opportunities to play leadership roles.”
New Era in Stroke Care
Many compare the evolution of stroke care to that of more common conditions, and hospitalists have a buffet of new and improved treatments and technologies at their disposal. “This is an interesting time in the treatment of stroke,” Dr. Cumbler says. “We are at the cusp of a new era. Previously, stroke was one of the classic neurologic issues in hospital medicine, but we did not have much to offer. Now, as with heart attack, we have a growing array of urgent and effective treatment options, and new imaging techniques to determine whether to treat and with what type of treatment.”
New and emerging treatment approaches include:
- Induced hypothermia, to protect the brain;
- Enhanced thrombolytics by ultrasound;
- Perfusion-based treatment time windows;
- Recanalization;
- Extended cardiac telemetry targeting atrial fibrillation;
- Neuroprotective agents; and
- Pressor usage to raise blood pressure in the post-stroke patient.
Interventional strategies seek to combine intravenous t-PA with localized techniques to open occluded vessels. While these are cutting-edge and not yet integrated into medical routine, “they illustrate why stroke management is so exciting right now,” Dr. Cumbler says.
As stroke treatment becomes more standardized, hospitals will expect HM physicians to be thoroughly versed in optimal stroke care, says David Yu, MD, MBA, FACP, medical director of hospitalist services at Decatur Memorial Hospital in Illinois and a member of Team Hospitalist. “There will be a shift in hospital medicine, with the practice of neurology becoming more open to non-neurologists,” he says. “As opportunities for stroke treatment increase, more responsibility will fall on hospitalists. It is part of the evolution of our field.”
That evolution is reflected in Medicare’s decision in 2005 to begin paying hospitals a higher diagnostic-related grouping (DRG) rate for administering intravenous t-PA.5 DRG 559 pays a hospital about $6,000 more, regionally adjusted, for stroke treatment that includes intravenous t-PA, compared with stroke care without it. That differential creates incentives for the hospital to invest in infrastructure, staffing, and training.
The Neurohospitalist
Recent journal articles have explored the emergence of neurohospitalists—hybrid physicians who are loosely defined as neurologists whose primary focus is the care of hospitalized patients. The neurohospitalist trend is spurred by the same time and fiscal constraints that drove the HM movement, says William Freeman, MD, neurologist at the Mayo Clinic in Jacksonville, Fla., and coauthor of one of those articles.6
Office-based neurologists increasingly are unavailable to respond to neurological emergencies in the hospital. Depending on the size of the hospital and its need for specialist access, an organized neurohospitalist group covering a schedule in the hospital could make significant contributions to quality of care, length of stay, and other stroke outcomes, Dr. Freeman says. “This field is starting to gel and crystallize, as more neurologists find themselves focusing their practice on site of care,” he notes.
Although not all experts agree, Dr. Freeman says that general hospitalists could become neurohospitalists, and vice versa. Neurologists could learn more internal medicine, and the two groups could work together more closely, he says.
Dr. Josephson of the University of California at San Francisco Medical Center reserves the term “neurohospitalist” for neurologists, but adds that medical hospitalists can manage neurologic disorders. He also sees potential for joint research on the management of hospitalized neurologic patients.
Drs. Freeman and Josephson have led discussions of the neurohospitalist model, both within AAN and in a recent conference call with SHM representatives. Data are limited on the numbers of physicians practicing this specialty, but job postings are growing and a neurohospitalist listserv sponsored by AAN grew to 250 members from 50 within six months. The University of California at San Francisco Medical Center established the first neurohospitalist fellowship in 2008, and a neurohospitalist journal is in development. “Most stroke patients are not seen by neurologists. I keep saying that at stroke conventions,” Dr. Josephson explains. “Hospitalists are going to continue to be out front on stroke management. Some will have a neurologist available. More likely, the hospitalist and neurologist will be participating in acute stroke management as part of some system of care with the emergency department or critical care.” TH
Larry Beresford is a freelance writer based in Oakland, Calif.
References
- Glasheen J, Cumbler E, Tailoring internal medicine training to improve hospitalist outcomes. Arch Intern Med. 2009;169:204-205.
- Telemedicine helps experts treat stroke from afar. National Stroke Association Web site. Available at: http://www.stroke.org/site/News2?page=NewsArticle&id=8208&news_iv_ctrl=1221. Accessed Nov. 4, 2009.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3)247-254.
- Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
- Demaerschalk BM, Durocher DL. How diagnosis-related group 559 will change the US Medicare cost reimbursement ratio for stroke centers. Stroke. 2007;38:1309-1312.
- Freeman WD, Gronseth G, Eidelman BH. Is it time for neurohospitalists? Neurology. 2009;72:476-477.
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329.
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945-2948.
- Lyden P. Thrombolytic therapy for acute stroke—not a moment to lose. N Engl J Med. 2008;359:1393-1397.
- Doheny K. Few stroke patients get clot-busting drug. Business Week Web site. Available at: http://www.businessweek.com/lifestyle/content/healthday/624280.html. Accessed Sept. 23, 2009.
- Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent strokes. N Engl J Med. 2008;359:1238-1251.
- Cumbler E, Glasheen J. Risk stratification tools for TIA: Which patients require hospital admission? J Hosp Med. 2009;4:247-251.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007; 369:283-292.
- Cumbler E, Glasheen J. Management of blood pressure after acute ischemic stroke: An evidence-based guide for the hospitalist. J Hosp Med. 2007;2:261-267.
Image Source: FORESTPATH/ISTOCKPHOTO.COM
Ethan Cumbler, MD, is board-certified in internal medicine and pediatrics, and has practiced hospital medicine for six years, first at a community hospital and now at the University of Colorado Denver (UCD), where he directs the Acute Care for the Elderly service. The prevalence of stroke in his practice and the daily challenges of managing stroke patients led Dr. Cumbler to seek additional training in stroke care. He is the hospitalist representative to the UCD stroke council, a researcher in the arena of acute stroke care, and is helping UCD become a Joint Commission-certified stroke center.
“There are a variety of roles for the hospitalist in stroke care,” Dr. Cumbler says, explaining that HM physicians can be admitting attendings for stroke patients or part of acute stroke teams, and participate in decisions to start such treatments as intravenous recombinant tissue plasminogen activator (t-PA), the Food and Drug Administration-approved clot-busting therapy. “[Hospitalists] can be medical consultants on stroke patients admitted to other hospital services, managing common comorbid conditions such as blood pressure and glucose levels, which have particular character for patients immediately post-stroke.”
Stroke is the third-leading cause of death in the U.S., as well as a leading cause of serious, long-term disability. How many stroke patients are seen by hospitalists is not known, but it is reasonable to assume that a majority of hospitalized stroke patients will encounter a hospitalist, if not for acute treatment, then for ongoing medical management.
Some hospitalists think stroke and transient ischemic attacks (TIAs)—temporary neurological deficits sometimes called “mini-strokes,” and a major risk factor for full-blown strokes—are among the most common diseases seen by hospitalists.1 Acute stroke care is a growing part of HM practice because neurologist availability in emergent situations varies widely between hospitals. The rapid evolution of stroke treatment and the time-sensitive needs of stroke patients represents a huge opportunity for hospitalists to fill that void for their hospitals—whether they want to or not.
“I think hospitalists are fully capable of learning and mastering stroke care, but it requires both interest and training,” Dr. Cumbler says.
HM Can Help Fill a Void
According to the American Heart Association (AHA), there are four neurologists per 100,000 Americans, and not all of those neurologists specialize in stroke care.2 The scarcity of neurological specialists means that in many hospitals, a neurologist won’t be available for the critical assessment and treatment decisions required in the first few hours after a stroke is diagnosed. Yet many hospitalists complain that their preparation during internal-medicine residency did not equip them to care for acute stroke patients.3
S. Andrew Josephson, MD, a neurovascular physician and director of the neurohospitalist program at the University of California at San Francisco Medical Center, says the number of hospitalists on the front lines of acute stroke care is growing every day. “A new stroke is a very treatable neurological emergency that requires ultra-fast intervention,”7 Dr. Josephson says, “and hospitalists, increasingly, are the people who matter most in that intervention.” The reason, in most cases, is hospitalists are available at all times, and neurologists aren’t.
Given variable access to neurologists at the time of urgent need in many hospitals, the actions hospitalists can take in acute stroke management include:
- Become better trained in stroke care. Sessions on stroke management are included in numerous HM educational programs, including SHM conferences and in continuing medical education (CME) offerings from such groups as the American Academy of Neurology (see “Stroke Training, Resources, and Opportunities,” p. 30).
- Partner with neurologists in your hospital. One trend is to develop a neurohospitalist practice.
- Push for increased organization and response times for stroke patients. Given HM’s focus on quality and patient safety, hospitalists are natural champions for improving systems of care for stroke. Hospitalists can work with neurologists, radiologists, pharmacists, and other providers to develop stroke treatment protocols and rapid response capabilities.
- Help develop a stroke team, and seek certification as a primary stroke center. The Joint Commission certifies stroke centers (www.jointcommission.org/CertificationPrograms/PrimaryStroke Centers) based on demonstrated compliance with disease-based standards, effective use of clinical practice guidelines, and performance-improvement activities.
- Establish a collaborative relationship with a regional stroke center or tertiary hospital. This could manifest as a telemedicine link to aid in stroke assessment and treatment decisions (see “Rural Response: The ‘Drip and Ship’ Method,” p. 28).
- Refine approaches to more rapidly identify and work up patients who experience a stroke while they are in the hospital.

—S. Andrew Josephson, MD, director, neurohospitalist program, University of California at San Francisco Medical Center
Streamline In-Hospital Stroke Response
From 6.5% to 15% of stroke patients experience their stroke while they are in the hospital.4 “Hospitals are not always geared up to deal with neurological emergencies, and yet these patients are firmly within our domain,” Dr. Cumbler says. “We found that it took three times longer in our hospital to complete the evaluation when the stroke happened in the hospital than for strokes presenting in the emergency department.”
Through a hospitalwide quality-improvement (QI) project, UCD’s in-hospital stroke response time was reduced to 37 minutes from 70 minutes.
A comprehensive approach to stroke QI should include training first witnesses in the hospital (e.g., nurses, physical therapists, and housekeepers) to recognize potential stroke symptoms; creating a rapid response capability from personnel who understand how to evaluate and treat suspected stroke and are able to respond quickly; and making suspected stroke a top priority in the radiology lab.
Stroke patient management processes need to be improved and provider roles better defined. Hospitalists can help on the frontlines, and should advocate for quality and patient safety measures.
“Stroke has so many facets: the need to reduce risk, to educate the public about the need for prompt response, the appropriate evaluation of risks and benefits of treatment,” Dr. Cumbler says. “How do you achieve a system in the hospital where patients are fully able to realize benefits of all these advances? I think there’s something in stroke treatment for every hospitalist and, for those with a particular interest, opportunities to play leadership roles.”
New Era in Stroke Care
Many compare the evolution of stroke care to that of more common conditions, and hospitalists have a buffet of new and improved treatments and technologies at their disposal. “This is an interesting time in the treatment of stroke,” Dr. Cumbler says. “We are at the cusp of a new era. Previously, stroke was one of the classic neurologic issues in hospital medicine, but we did not have much to offer. Now, as with heart attack, we have a growing array of urgent and effective treatment options, and new imaging techniques to determine whether to treat and with what type of treatment.”
New and emerging treatment approaches include:
- Induced hypothermia, to protect the brain;
- Enhanced thrombolytics by ultrasound;
- Perfusion-based treatment time windows;
- Recanalization;
- Extended cardiac telemetry targeting atrial fibrillation;
- Neuroprotective agents; and
- Pressor usage to raise blood pressure in the post-stroke patient.
Interventional strategies seek to combine intravenous t-PA with localized techniques to open occluded vessels. While these are cutting-edge and not yet integrated into medical routine, “they illustrate why stroke management is so exciting right now,” Dr. Cumbler says.
As stroke treatment becomes more standardized, hospitals will expect HM physicians to be thoroughly versed in optimal stroke care, says David Yu, MD, MBA, FACP, medical director of hospitalist services at Decatur Memorial Hospital in Illinois and a member of Team Hospitalist. “There will be a shift in hospital medicine, with the practice of neurology becoming more open to non-neurologists,” he says. “As opportunities for stroke treatment increase, more responsibility will fall on hospitalists. It is part of the evolution of our field.”
That evolution is reflected in Medicare’s decision in 2005 to begin paying hospitals a higher diagnostic-related grouping (DRG) rate for administering intravenous t-PA.5 DRG 559 pays a hospital about $6,000 more, regionally adjusted, for stroke treatment that includes intravenous t-PA, compared with stroke care without it. That differential creates incentives for the hospital to invest in infrastructure, staffing, and training.
The Neurohospitalist
Recent journal articles have explored the emergence of neurohospitalists—hybrid physicians who are loosely defined as neurologists whose primary focus is the care of hospitalized patients. The neurohospitalist trend is spurred by the same time and fiscal constraints that drove the HM movement, says William Freeman, MD, neurologist at the Mayo Clinic in Jacksonville, Fla., and coauthor of one of those articles.6
Office-based neurologists increasingly are unavailable to respond to neurological emergencies in the hospital. Depending on the size of the hospital and its need for specialist access, an organized neurohospitalist group covering a schedule in the hospital could make significant contributions to quality of care, length of stay, and other stroke outcomes, Dr. Freeman says. “This field is starting to gel and crystallize, as more neurologists find themselves focusing their practice on site of care,” he notes.
Although not all experts agree, Dr. Freeman says that general hospitalists could become neurohospitalists, and vice versa. Neurologists could learn more internal medicine, and the two groups could work together more closely, he says.
Dr. Josephson of the University of California at San Francisco Medical Center reserves the term “neurohospitalist” for neurologists, but adds that medical hospitalists can manage neurologic disorders. He also sees potential for joint research on the management of hospitalized neurologic patients.
Drs. Freeman and Josephson have led discussions of the neurohospitalist model, both within AAN and in a recent conference call with SHM representatives. Data are limited on the numbers of physicians practicing this specialty, but job postings are growing and a neurohospitalist listserv sponsored by AAN grew to 250 members from 50 within six months. The University of California at San Francisco Medical Center established the first neurohospitalist fellowship in 2008, and a neurohospitalist journal is in development. “Most stroke patients are not seen by neurologists. I keep saying that at stroke conventions,” Dr. Josephson explains. “Hospitalists are going to continue to be out front on stroke management. Some will have a neurologist available. More likely, the hospitalist and neurologist will be participating in acute stroke management as part of some system of care with the emergency department or critical care.” TH
Larry Beresford is a freelance writer based in Oakland, Calif.
References
- Glasheen J, Cumbler E, Tailoring internal medicine training to improve hospitalist outcomes. Arch Intern Med. 2009;169:204-205.
- Telemedicine helps experts treat stroke from afar. National Stroke Association Web site. Available at: http://www.stroke.org/site/News2?page=NewsArticle&id=8208&news_iv_ctrl=1221. Accessed Nov. 4, 2009.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3)247-254.
- Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
- Demaerschalk BM, Durocher DL. How diagnosis-related group 559 will change the US Medicare cost reimbursement ratio for stroke centers. Stroke. 2007;38:1309-1312.
- Freeman WD, Gronseth G, Eidelman BH. Is it time for neurohospitalists? Neurology. 2009;72:476-477.
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329.
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945-2948.
- Lyden P. Thrombolytic therapy for acute stroke—not a moment to lose. N Engl J Med. 2008;359:1393-1397.
- Doheny K. Few stroke patients get clot-busting drug. Business Week Web site. Available at: http://www.businessweek.com/lifestyle/content/healthday/624280.html. Accessed Sept. 23, 2009.
- Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent strokes. N Engl J Med. 2008;359:1238-1251.
- Cumbler E, Glasheen J. Risk stratification tools for TIA: Which patients require hospital admission? J Hosp Med. 2009;4:247-251.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007; 369:283-292.
- Cumbler E, Glasheen J. Management of blood pressure after acute ischemic stroke: An evidence-based guide for the hospitalist. J Hosp Med. 2007;2:261-267.
Image Source: FORESTPATH/ISTOCKPHOTO.COM
Ethan Cumbler, MD, is board-certified in internal medicine and pediatrics, and has practiced hospital medicine for six years, first at a community hospital and now at the University of Colorado Denver (UCD), where he directs the Acute Care for the Elderly service. The prevalence of stroke in his practice and the daily challenges of managing stroke patients led Dr. Cumbler to seek additional training in stroke care. He is the hospitalist representative to the UCD stroke council, a researcher in the arena of acute stroke care, and is helping UCD become a Joint Commission-certified stroke center.
“There are a variety of roles for the hospitalist in stroke care,” Dr. Cumbler says, explaining that HM physicians can be admitting attendings for stroke patients or part of acute stroke teams, and participate in decisions to start such treatments as intravenous recombinant tissue plasminogen activator (t-PA), the Food and Drug Administration-approved clot-busting therapy. “[Hospitalists] can be medical consultants on stroke patients admitted to other hospital services, managing common comorbid conditions such as blood pressure and glucose levels, which have particular character for patients immediately post-stroke.”
Stroke is the third-leading cause of death in the U.S., as well as a leading cause of serious, long-term disability. How many stroke patients are seen by hospitalists is not known, but it is reasonable to assume that a majority of hospitalized stroke patients will encounter a hospitalist, if not for acute treatment, then for ongoing medical management.
Some hospitalists think stroke and transient ischemic attacks (TIAs)—temporary neurological deficits sometimes called “mini-strokes,” and a major risk factor for full-blown strokes—are among the most common diseases seen by hospitalists.1 Acute stroke care is a growing part of HM practice because neurologist availability in emergent situations varies widely between hospitals. The rapid evolution of stroke treatment and the time-sensitive needs of stroke patients represents a huge opportunity for hospitalists to fill that void for their hospitals—whether they want to or not.
“I think hospitalists are fully capable of learning and mastering stroke care, but it requires both interest and training,” Dr. Cumbler says.
HM Can Help Fill a Void
According to the American Heart Association (AHA), there are four neurologists per 100,000 Americans, and not all of those neurologists specialize in stroke care.2 The scarcity of neurological specialists means that in many hospitals, a neurologist won’t be available for the critical assessment and treatment decisions required in the first few hours after a stroke is diagnosed. Yet many hospitalists complain that their preparation during internal-medicine residency did not equip them to care for acute stroke patients.3
S. Andrew Josephson, MD, a neurovascular physician and director of the neurohospitalist program at the University of California at San Francisco Medical Center, says the number of hospitalists on the front lines of acute stroke care is growing every day. “A new stroke is a very treatable neurological emergency that requires ultra-fast intervention,”7 Dr. Josephson says, “and hospitalists, increasingly, are the people who matter most in that intervention.” The reason, in most cases, is hospitalists are available at all times, and neurologists aren’t.
Given variable access to neurologists at the time of urgent need in many hospitals, the actions hospitalists can take in acute stroke management include:
- Become better trained in stroke care. Sessions on stroke management are included in numerous HM educational programs, including SHM conferences and in continuing medical education (CME) offerings from such groups as the American Academy of Neurology (see “Stroke Training, Resources, and Opportunities,” p. 30).
- Partner with neurologists in your hospital. One trend is to develop a neurohospitalist practice.
- Push for increased organization and response times for stroke patients. Given HM’s focus on quality and patient safety, hospitalists are natural champions for improving systems of care for stroke. Hospitalists can work with neurologists, radiologists, pharmacists, and other providers to develop stroke treatment protocols and rapid response capabilities.
- Help develop a stroke team, and seek certification as a primary stroke center. The Joint Commission certifies stroke centers (www.jointcommission.org/CertificationPrograms/PrimaryStroke Centers) based on demonstrated compliance with disease-based standards, effective use of clinical practice guidelines, and performance-improvement activities.
- Establish a collaborative relationship with a regional stroke center or tertiary hospital. This could manifest as a telemedicine link to aid in stroke assessment and treatment decisions (see “Rural Response: The ‘Drip and Ship’ Method,” p. 28).
- Refine approaches to more rapidly identify and work up patients who experience a stroke while they are in the hospital.

—S. Andrew Josephson, MD, director, neurohospitalist program, University of California at San Francisco Medical Center
Streamline In-Hospital Stroke Response
From 6.5% to 15% of stroke patients experience their stroke while they are in the hospital.4 “Hospitals are not always geared up to deal with neurological emergencies, and yet these patients are firmly within our domain,” Dr. Cumbler says. “We found that it took three times longer in our hospital to complete the evaluation when the stroke happened in the hospital than for strokes presenting in the emergency department.”
Through a hospitalwide quality-improvement (QI) project, UCD’s in-hospital stroke response time was reduced to 37 minutes from 70 minutes.
A comprehensive approach to stroke QI should include training first witnesses in the hospital (e.g., nurses, physical therapists, and housekeepers) to recognize potential stroke symptoms; creating a rapid response capability from personnel who understand how to evaluate and treat suspected stroke and are able to respond quickly; and making suspected stroke a top priority in the radiology lab.
Stroke patient management processes need to be improved and provider roles better defined. Hospitalists can help on the frontlines, and should advocate for quality and patient safety measures.
“Stroke has so many facets: the need to reduce risk, to educate the public about the need for prompt response, the appropriate evaluation of risks and benefits of treatment,” Dr. Cumbler says. “How do you achieve a system in the hospital where patients are fully able to realize benefits of all these advances? I think there’s something in stroke treatment for every hospitalist and, for those with a particular interest, opportunities to play leadership roles.”
New Era in Stroke Care
Many compare the evolution of stroke care to that of more common conditions, and hospitalists have a buffet of new and improved treatments and technologies at their disposal. “This is an interesting time in the treatment of stroke,” Dr. Cumbler says. “We are at the cusp of a new era. Previously, stroke was one of the classic neurologic issues in hospital medicine, but we did not have much to offer. Now, as with heart attack, we have a growing array of urgent and effective treatment options, and new imaging techniques to determine whether to treat and with what type of treatment.”
New and emerging treatment approaches include:
- Induced hypothermia, to protect the brain;
- Enhanced thrombolytics by ultrasound;
- Perfusion-based treatment time windows;
- Recanalization;
- Extended cardiac telemetry targeting atrial fibrillation;
- Neuroprotective agents; and
- Pressor usage to raise blood pressure in the post-stroke patient.
Interventional strategies seek to combine intravenous t-PA with localized techniques to open occluded vessels. While these are cutting-edge and not yet integrated into medical routine, “they illustrate why stroke management is so exciting right now,” Dr. Cumbler says.
As stroke treatment becomes more standardized, hospitals will expect HM physicians to be thoroughly versed in optimal stroke care, says David Yu, MD, MBA, FACP, medical director of hospitalist services at Decatur Memorial Hospital in Illinois and a member of Team Hospitalist. “There will be a shift in hospital medicine, with the practice of neurology becoming more open to non-neurologists,” he says. “As opportunities for stroke treatment increase, more responsibility will fall on hospitalists. It is part of the evolution of our field.”
That evolution is reflected in Medicare’s decision in 2005 to begin paying hospitals a higher diagnostic-related grouping (DRG) rate for administering intravenous t-PA.5 DRG 559 pays a hospital about $6,000 more, regionally adjusted, for stroke treatment that includes intravenous t-PA, compared with stroke care without it. That differential creates incentives for the hospital to invest in infrastructure, staffing, and training.
The Neurohospitalist
Recent journal articles have explored the emergence of neurohospitalists—hybrid physicians who are loosely defined as neurologists whose primary focus is the care of hospitalized patients. The neurohospitalist trend is spurred by the same time and fiscal constraints that drove the HM movement, says William Freeman, MD, neurologist at the Mayo Clinic in Jacksonville, Fla., and coauthor of one of those articles.6
Office-based neurologists increasingly are unavailable to respond to neurological emergencies in the hospital. Depending on the size of the hospital and its need for specialist access, an organized neurohospitalist group covering a schedule in the hospital could make significant contributions to quality of care, length of stay, and other stroke outcomes, Dr. Freeman says. “This field is starting to gel and crystallize, as more neurologists find themselves focusing their practice on site of care,” he notes.
Although not all experts agree, Dr. Freeman says that general hospitalists could become neurohospitalists, and vice versa. Neurologists could learn more internal medicine, and the two groups could work together more closely, he says.
Dr. Josephson of the University of California at San Francisco Medical Center reserves the term “neurohospitalist” for neurologists, but adds that medical hospitalists can manage neurologic disorders. He also sees potential for joint research on the management of hospitalized neurologic patients.
Drs. Freeman and Josephson have led discussions of the neurohospitalist model, both within AAN and in a recent conference call with SHM representatives. Data are limited on the numbers of physicians practicing this specialty, but job postings are growing and a neurohospitalist listserv sponsored by AAN grew to 250 members from 50 within six months. The University of California at San Francisco Medical Center established the first neurohospitalist fellowship in 2008, and a neurohospitalist journal is in development. “Most stroke patients are not seen by neurologists. I keep saying that at stroke conventions,” Dr. Josephson explains. “Hospitalists are going to continue to be out front on stroke management. Some will have a neurologist available. More likely, the hospitalist and neurologist will be participating in acute stroke management as part of some system of care with the emergency department or critical care.” TH
Larry Beresford is a freelance writer based in Oakland, Calif.
References
- Glasheen J, Cumbler E, Tailoring internal medicine training to improve hospitalist outcomes. Arch Intern Med. 2009;169:204-205.
- Telemedicine helps experts treat stroke from afar. National Stroke Association Web site. Available at: http://www.stroke.org/site/News2?page=NewsArticle&id=8208&news_iv_ctrl=1221. Accessed Nov. 4, 2009.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3)247-254.
- Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
- Demaerschalk BM, Durocher DL. How diagnosis-related group 559 will change the US Medicare cost reimbursement ratio for stroke centers. Stroke. 2007;38:1309-1312.
- Freeman WD, Gronseth G, Eidelman BH. Is it time for neurohospitalists? Neurology. 2009;72:476-477.
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329.
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945-2948.
- Lyden P. Thrombolytic therapy for acute stroke—not a moment to lose. N Engl J Med. 2008;359:1393-1397.
- Doheny K. Few stroke patients get clot-busting drug. Business Week Web site. Available at: http://www.businessweek.com/lifestyle/content/healthday/624280.html. Accessed Sept. 23, 2009.
- Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent strokes. N Engl J Med. 2008;359:1238-1251.
- Cumbler E, Glasheen J. Risk stratification tools for TIA: Which patients require hospital admission? J Hosp Med. 2009;4:247-251.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007; 369:283-292.
- Cumbler E, Glasheen J. Management of blood pressure after acute ischemic stroke: An evidence-based guide for the hospitalist. J Hosp Med. 2007;2:261-267.
Image Source: FORESTPATH/ISTOCKPHOTO.COM
ONLINE EXCLUSIVE: Audio interview with Robert Wachter, MD, FHM
Listen to HM pioneer Bob Wachter recap his HM09 keynote address about the quality and patient safety revolution
Listen to HM pioneer Bob Wachter recap his HM09 keynote address about the quality and patient safety revolution
Listen to HM pioneer Bob Wachter recap his HM09 keynote address about the quality and patient safety revolution
All Grown Up
There are times when Dan Hale, MD, FAAP, wishes he had more standardized tools to use when he leads a team of four full-time and four part-time pediatric hospitalists at Central Maine Medical Center (CMMC) in Lewiston. Even after five years at the community hospital, the pediatric HM program still is searching for the best way to hand off patients who are leaving the hospital to their primary-care physicians (PCPs).
It also would be beneficial to have markers against which CMMC could compare itself with similarly sized pediatric HM programs around the country, says Dr. Hale, chief of pediatrics at the medical center. CMMC, which averages about 4,000 patient encounters per year, is one of three hospitals in the state with a pediatric HM program. “It would be nice to see progress being made in these areas,” he says.
Dr. Hale might not have to wait long to see his wishes granted. More than 20 pediatric hospitalists from across the nation met in Chicago earlier this year, intent on developing a strategic framework for pediatric HM (PHM). About 10% of the 30,000-plus hospitalists practicing in the U.S. focus exclusively on pediatrics, according to SHM’s 2007-2008 “Bi-Annual Survey on the State of the Hospital Medicine Movement.” Like the hospitalist movement in general, PHM is growing in number and influence as pediatric hospitalists take on leadership roles and develop working relationships with hospital administrators. The time has come to clearly define the discipline for other physicians, as well as patients and their families, and leverage PHM’s growth and usefulness to improve medical care for children, says Erin Stucky, MD, FHM, a pediatric hospitalist at Rady Children’s Hospital and Health Center in San Diego.

—Jennifer Daru, MD, FAAP, FHM, chief, division of pediatric hospital medicine, California Pacific Medical Center, San Francisco
“It’s a little bit of pie in the sky, a little bit of rose-colored glasses, but it’s good to aim high,” she says.
Some PHM leaders think the subspecialty has advanced enough in recent years to apply its collective knowledge and influence on a broader stage. “We have gone through our adolescence, and now we are a big community,” says Jack Percelay, MD, MPH, FHM, a pediatric hospitalist at Saint Barnabas Medical Center in New York City and SHM board member. “We’re active at almost all the major medical centers and we need to step up to the plate. We need to start the hard work of bringing our vision to fruition.”
Definition and Strategy
Drs. Stucky and Percelay attended the Pediatric Hospital Medicine (PHM) Strategic Planning Roundtable and serve on the roundtable’s planning committee. SHM, the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP) sponsored the gathering, which included young and veteran pediatric hospitalists, clinicians, researchers, and hospitalists from academic, children’s, and community hospitals. The net was cast far and wide to gather information from a broad cross-section of stakeholders.

—Jack Percelay, MD, MPH, FHM, pediatric hospitalist, Saint Barnabas Medical Center, New York City, SHM board member
As pediatric hospitalists strive to better demonstrate how they can help hospitals improve the quality of patient care and safety while decreasing its cost, the roundtable is charged with defining and educating healthcare professionals on the key issues. Also in the crosshairs: simultaneously advancing evidence-based medicine and family-based care.
“We need to distinguish that we are not just house physicians, but really establish ourselves as content-area knowledge experts,” Dr. Percelay says. In other words, pediatric hospitalists are physicians who specialize in effective and efficient medicine in resource-intensive facilities.
Pediatric hospitalists also grapple with how to enhance career satisfaction and sustainability at a time when many PHM programs require a burdensome clinical load that fosters burnout. Many PHM leaders also think pediatric hospitalists need extra training but fear they will lose those physicians to fellowships. And as the PHM ranks fill with physicians who have little or no outpatient training, there is the challenge of explaining the capabilities and limitations pediatric hospitalists and primary-care physicians (PCPs) have in order to avoid unrealistic expectations and friction.
Participants in the strategic roundtable aim to address several broad goals outlined in an executive summary, which can be viewed in the “Section on Hospital Medicine” on the AAP Web site (www.aap.org/sections/hospcare/default.cfm). The following are some of the goals:
- Ensure care for hospitalized children is fully integrated and includes the medical home;
- Design and support systems for children that eliminate harm associated with hospital care;
- Develop a skilled and stable workforce that provides expert care for hospitalized children;
- Use collaborative research models to answer questions of clinical efficacy, comparative effectiveness, and quality improvement inclusive of patient safety, and deliver care based on that knowledge;
- Provide the expertise that supports innovative continuing education in the care of the hospitalized child for pediatric hospitalists, trainees, midlevel providers, and hospital staff;
- Create value and provide academic and systems leadership for patients and organizations based on pediatric hospitalists’ unique expertise in PHM clinical care, research, and education; and
- Be leaders and influential agents in local, state, and national healthcare policies that affect hospital care.
Although it was discussed, the roundtable decided against the establishment of a professional organization for pediatric hospitalists. Instead, the group agreed to continue to utilize the resources and organizational support provided by SHM, APA, and AAP. All three groups contributed money to the roundtable, sent representatives to the meeting, and are interested in the results.
“The Academic Pediatric Association has been involved with pediatric hospital medicine from the beginning, and we plan on continuing our involvement,” says Daniel Rauch, MD, FHM, associate director of pediatrics at Elmhurst Hospital Center in New York City and co-chair of the APA’s Hospital Medicine Special Interest Group, which is paying close attention to PHM education and research issues.
Strategic Initiatives
The roundtable established four workgroups: clinical practice/workforce, quality and safety, research, and education. The workgroups are directed to create strategic initiative projects focused on advancing the goals laid out at the roundtable meeting and complete most of the projects no later than the July 2010 PHM Conference in Minneapolis (see “A Closer Look at the Pediatric Hospital Medicine Initiatives,” p. 7). At the 2009 PHM conference in Tampa, Fla., roundtable participants reported on some of the initiatives’ preliminary results.
“I walked away … energized and ready to help change the world, which is a pretty great feeling,” says Jennifer Daru, MD, FAAP, FHM, chief of the division of pediatric hospital medicine at California Pacific Medical Center in San Francisco and co-leader of the roundtable’s clinical practice/workforce workgroup.
One of Dr. Daru’s workgroup’s strategic initiative projects should make Dr. Hale and his pediatric hospitalists at Central Maine Medical Center happy. Dr. Daru’s group is creating a clinical practice dashboard template that PHM programs can use to internally track patient care and compare themselves with other programs and national standards.
“I think very few programs have a dashboard, because it’s a relatively newer thing for pediatric hospital medicine,” Dr. Daru says. “With this dashboard, we want to be able to say, ‘Here are the things you should look at to ensure quality care for your kids, and as you look at them, you should probably track them over time.’ ”
Steve Narang, MD, medical director of quality/safety and pediatric emergency services at Our Lady of the Lake Regional Medical Center and Children’s Hospital in Baton Rouge, La., is leading the quality and safety workgroup, which is focused on patient identification, patient handoffs between pediatric hospitalists and PCPs, and clinical outcomes for common pediatric diagnoses.
“Most doctors don’t like standardized forms or cookbook medicine, but they do understand good care. Hopefully, we will show success in these initiatives and they will serve as a launching pad to other initiatives,” Dr. Narang says.
Dr. Hale, for one, is excited by the initiatives and workgroups, and optimistic the strategic projects will help his program. In recent years, the PHM community has talked about these kinds of advances, and he’s encouraged to see them moving forward. “These initiatives contribute to the strength of our field,” says Dr. Hale, who also serves on the executive board of AAP’s Maine chapter.
About 80 pediatric hospitalists have volunteered to help with the strategic initiatives. Earlier this year, a request for help was broadcast over the Section on Hospital Medicine listserv run by the AAP. It was announced at HM09 in Chicago and the PHM conference in Tampa. Everyone who submitted a resume or CV, references, and a statement of interest is included, Dr. Percelay says. “This is not supposed to be some exclusive club that no one can get into,” he says. “We are committed to a transparent process.”
While the application deadline has passed, organizers expect additional calls for volunteers in the future as strategic projects move forward, projects are added, and current volunteers depart (see “How to Get Involved,” above).
“They will be the next volunteer go-tos. We will essentially build them into new projects that come up or if gaps emerge,” Dr. Daru says. “We want to have as many people as possible who are really motivated.”
Group Effort
You don’t necessarily have to volunteer for workgroups to be a part of the broader effort. You can read and comment on draft reports released by some of the project teams, or review the roundtable’s executive summary and find ways to apply the vision and goals to your own PHM program, says Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas, and pediatric editor of The Hospitalist.
“If each pediatric hospitalist set strategic initiatives for their own group or hospital, chances are they would find remarkable similarity between what they came up with and what the strategic planning roundtable came up with,” says Dr. Shen, who is directing one of the quality and safety workgroup’s initiatives. “There are plenty of ways to think globally and act locally.” TH
Lisa Ryan is a freelance writer based in New Jersey.
Top Image Source: HOMER SYKES/ALAMY
There are times when Dan Hale, MD, FAAP, wishes he had more standardized tools to use when he leads a team of four full-time and four part-time pediatric hospitalists at Central Maine Medical Center (CMMC) in Lewiston. Even after five years at the community hospital, the pediatric HM program still is searching for the best way to hand off patients who are leaving the hospital to their primary-care physicians (PCPs).
It also would be beneficial to have markers against which CMMC could compare itself with similarly sized pediatric HM programs around the country, says Dr. Hale, chief of pediatrics at the medical center. CMMC, which averages about 4,000 patient encounters per year, is one of three hospitals in the state with a pediatric HM program. “It would be nice to see progress being made in these areas,” he says.
Dr. Hale might not have to wait long to see his wishes granted. More than 20 pediatric hospitalists from across the nation met in Chicago earlier this year, intent on developing a strategic framework for pediatric HM (PHM). About 10% of the 30,000-plus hospitalists practicing in the U.S. focus exclusively on pediatrics, according to SHM’s 2007-2008 “Bi-Annual Survey on the State of the Hospital Medicine Movement.” Like the hospitalist movement in general, PHM is growing in number and influence as pediatric hospitalists take on leadership roles and develop working relationships with hospital administrators. The time has come to clearly define the discipline for other physicians, as well as patients and their families, and leverage PHM’s growth and usefulness to improve medical care for children, says Erin Stucky, MD, FHM, a pediatric hospitalist at Rady Children’s Hospital and Health Center in San Diego.

—Jennifer Daru, MD, FAAP, FHM, chief, division of pediatric hospital medicine, California Pacific Medical Center, San Francisco
“It’s a little bit of pie in the sky, a little bit of rose-colored glasses, but it’s good to aim high,” she says.
Some PHM leaders think the subspecialty has advanced enough in recent years to apply its collective knowledge and influence on a broader stage. “We have gone through our adolescence, and now we are a big community,” says Jack Percelay, MD, MPH, FHM, a pediatric hospitalist at Saint Barnabas Medical Center in New York City and SHM board member. “We’re active at almost all the major medical centers and we need to step up to the plate. We need to start the hard work of bringing our vision to fruition.”
Definition and Strategy
Drs. Stucky and Percelay attended the Pediatric Hospital Medicine (PHM) Strategic Planning Roundtable and serve on the roundtable’s planning committee. SHM, the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP) sponsored the gathering, which included young and veteran pediatric hospitalists, clinicians, researchers, and hospitalists from academic, children’s, and community hospitals. The net was cast far and wide to gather information from a broad cross-section of stakeholders.

—Jack Percelay, MD, MPH, FHM, pediatric hospitalist, Saint Barnabas Medical Center, New York City, SHM board member
As pediatric hospitalists strive to better demonstrate how they can help hospitals improve the quality of patient care and safety while decreasing its cost, the roundtable is charged with defining and educating healthcare professionals on the key issues. Also in the crosshairs: simultaneously advancing evidence-based medicine and family-based care.
“We need to distinguish that we are not just house physicians, but really establish ourselves as content-area knowledge experts,” Dr. Percelay says. In other words, pediatric hospitalists are physicians who specialize in effective and efficient medicine in resource-intensive facilities.
Pediatric hospitalists also grapple with how to enhance career satisfaction and sustainability at a time when many PHM programs require a burdensome clinical load that fosters burnout. Many PHM leaders also think pediatric hospitalists need extra training but fear they will lose those physicians to fellowships. And as the PHM ranks fill with physicians who have little or no outpatient training, there is the challenge of explaining the capabilities and limitations pediatric hospitalists and primary-care physicians (PCPs) have in order to avoid unrealistic expectations and friction.
Participants in the strategic roundtable aim to address several broad goals outlined in an executive summary, which can be viewed in the “Section on Hospital Medicine” on the AAP Web site (www.aap.org/sections/hospcare/default.cfm). The following are some of the goals:
- Ensure care for hospitalized children is fully integrated and includes the medical home;
- Design and support systems for children that eliminate harm associated with hospital care;
- Develop a skilled and stable workforce that provides expert care for hospitalized children;
- Use collaborative research models to answer questions of clinical efficacy, comparative effectiveness, and quality improvement inclusive of patient safety, and deliver care based on that knowledge;
- Provide the expertise that supports innovative continuing education in the care of the hospitalized child for pediatric hospitalists, trainees, midlevel providers, and hospital staff;
- Create value and provide academic and systems leadership for patients and organizations based on pediatric hospitalists’ unique expertise in PHM clinical care, research, and education; and
- Be leaders and influential agents in local, state, and national healthcare policies that affect hospital care.
Although it was discussed, the roundtable decided against the establishment of a professional organization for pediatric hospitalists. Instead, the group agreed to continue to utilize the resources and organizational support provided by SHM, APA, and AAP. All three groups contributed money to the roundtable, sent representatives to the meeting, and are interested in the results.
“The Academic Pediatric Association has been involved with pediatric hospital medicine from the beginning, and we plan on continuing our involvement,” says Daniel Rauch, MD, FHM, associate director of pediatrics at Elmhurst Hospital Center in New York City and co-chair of the APA’s Hospital Medicine Special Interest Group, which is paying close attention to PHM education and research issues.
Strategic Initiatives
The roundtable established four workgroups: clinical practice/workforce, quality and safety, research, and education. The workgroups are directed to create strategic initiative projects focused on advancing the goals laid out at the roundtable meeting and complete most of the projects no later than the July 2010 PHM Conference in Minneapolis (see “A Closer Look at the Pediatric Hospital Medicine Initiatives,” p. 7). At the 2009 PHM conference in Tampa, Fla., roundtable participants reported on some of the initiatives’ preliminary results.
“I walked away … energized and ready to help change the world, which is a pretty great feeling,” says Jennifer Daru, MD, FAAP, FHM, chief of the division of pediatric hospital medicine at California Pacific Medical Center in San Francisco and co-leader of the roundtable’s clinical practice/workforce workgroup.
One of Dr. Daru’s workgroup’s strategic initiative projects should make Dr. Hale and his pediatric hospitalists at Central Maine Medical Center happy. Dr. Daru’s group is creating a clinical practice dashboard template that PHM programs can use to internally track patient care and compare themselves with other programs and national standards.
“I think very few programs have a dashboard, because it’s a relatively newer thing for pediatric hospital medicine,” Dr. Daru says. “With this dashboard, we want to be able to say, ‘Here are the things you should look at to ensure quality care for your kids, and as you look at them, you should probably track them over time.’ ”
Steve Narang, MD, medical director of quality/safety and pediatric emergency services at Our Lady of the Lake Regional Medical Center and Children’s Hospital in Baton Rouge, La., is leading the quality and safety workgroup, which is focused on patient identification, patient handoffs between pediatric hospitalists and PCPs, and clinical outcomes for common pediatric diagnoses.
“Most doctors don’t like standardized forms or cookbook medicine, but they do understand good care. Hopefully, we will show success in these initiatives and they will serve as a launching pad to other initiatives,” Dr. Narang says.
Dr. Hale, for one, is excited by the initiatives and workgroups, and optimistic the strategic projects will help his program. In recent years, the PHM community has talked about these kinds of advances, and he’s encouraged to see them moving forward. “These initiatives contribute to the strength of our field,” says Dr. Hale, who also serves on the executive board of AAP’s Maine chapter.
About 80 pediatric hospitalists have volunteered to help with the strategic initiatives. Earlier this year, a request for help was broadcast over the Section on Hospital Medicine listserv run by the AAP. It was announced at HM09 in Chicago and the PHM conference in Tampa. Everyone who submitted a resume or CV, references, and a statement of interest is included, Dr. Percelay says. “This is not supposed to be some exclusive club that no one can get into,” he says. “We are committed to a transparent process.”
While the application deadline has passed, organizers expect additional calls for volunteers in the future as strategic projects move forward, projects are added, and current volunteers depart (see “How to Get Involved,” above).
“They will be the next volunteer go-tos. We will essentially build them into new projects that come up or if gaps emerge,” Dr. Daru says. “We want to have as many people as possible who are really motivated.”
Group Effort
You don’t necessarily have to volunteer for workgroups to be a part of the broader effort. You can read and comment on draft reports released by some of the project teams, or review the roundtable’s executive summary and find ways to apply the vision and goals to your own PHM program, says Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas, and pediatric editor of The Hospitalist.
“If each pediatric hospitalist set strategic initiatives for their own group or hospital, chances are they would find remarkable similarity between what they came up with and what the strategic planning roundtable came up with,” says Dr. Shen, who is directing one of the quality and safety workgroup’s initiatives. “There are plenty of ways to think globally and act locally.” TH
Lisa Ryan is a freelance writer based in New Jersey.
Top Image Source: HOMER SYKES/ALAMY
There are times when Dan Hale, MD, FAAP, wishes he had more standardized tools to use when he leads a team of four full-time and four part-time pediatric hospitalists at Central Maine Medical Center (CMMC) in Lewiston. Even after five years at the community hospital, the pediatric HM program still is searching for the best way to hand off patients who are leaving the hospital to their primary-care physicians (PCPs).
It also would be beneficial to have markers against which CMMC could compare itself with similarly sized pediatric HM programs around the country, says Dr. Hale, chief of pediatrics at the medical center. CMMC, which averages about 4,000 patient encounters per year, is one of three hospitals in the state with a pediatric HM program. “It would be nice to see progress being made in these areas,” he says.
Dr. Hale might not have to wait long to see his wishes granted. More than 20 pediatric hospitalists from across the nation met in Chicago earlier this year, intent on developing a strategic framework for pediatric HM (PHM). About 10% of the 30,000-plus hospitalists practicing in the U.S. focus exclusively on pediatrics, according to SHM’s 2007-2008 “Bi-Annual Survey on the State of the Hospital Medicine Movement.” Like the hospitalist movement in general, PHM is growing in number and influence as pediatric hospitalists take on leadership roles and develop working relationships with hospital administrators. The time has come to clearly define the discipline for other physicians, as well as patients and their families, and leverage PHM’s growth and usefulness to improve medical care for children, says Erin Stucky, MD, FHM, a pediatric hospitalist at Rady Children’s Hospital and Health Center in San Diego.

—Jennifer Daru, MD, FAAP, FHM, chief, division of pediatric hospital medicine, California Pacific Medical Center, San Francisco
“It’s a little bit of pie in the sky, a little bit of rose-colored glasses, but it’s good to aim high,” she says.
Some PHM leaders think the subspecialty has advanced enough in recent years to apply its collective knowledge and influence on a broader stage. “We have gone through our adolescence, and now we are a big community,” says Jack Percelay, MD, MPH, FHM, a pediatric hospitalist at Saint Barnabas Medical Center in New York City and SHM board member. “We’re active at almost all the major medical centers and we need to step up to the plate. We need to start the hard work of bringing our vision to fruition.”
Definition and Strategy
Drs. Stucky and Percelay attended the Pediatric Hospital Medicine (PHM) Strategic Planning Roundtable and serve on the roundtable’s planning committee. SHM, the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP) sponsored the gathering, which included young and veteran pediatric hospitalists, clinicians, researchers, and hospitalists from academic, children’s, and community hospitals. The net was cast far and wide to gather information from a broad cross-section of stakeholders.

—Jack Percelay, MD, MPH, FHM, pediatric hospitalist, Saint Barnabas Medical Center, New York City, SHM board member
As pediatric hospitalists strive to better demonstrate how they can help hospitals improve the quality of patient care and safety while decreasing its cost, the roundtable is charged with defining and educating healthcare professionals on the key issues. Also in the crosshairs: simultaneously advancing evidence-based medicine and family-based care.
“We need to distinguish that we are not just house physicians, but really establish ourselves as content-area knowledge experts,” Dr. Percelay says. In other words, pediatric hospitalists are physicians who specialize in effective and efficient medicine in resource-intensive facilities.
Pediatric hospitalists also grapple with how to enhance career satisfaction and sustainability at a time when many PHM programs require a burdensome clinical load that fosters burnout. Many PHM leaders also think pediatric hospitalists need extra training but fear they will lose those physicians to fellowships. And as the PHM ranks fill with physicians who have little or no outpatient training, there is the challenge of explaining the capabilities and limitations pediatric hospitalists and primary-care physicians (PCPs) have in order to avoid unrealistic expectations and friction.
Participants in the strategic roundtable aim to address several broad goals outlined in an executive summary, which can be viewed in the “Section on Hospital Medicine” on the AAP Web site (www.aap.org/sections/hospcare/default.cfm). The following are some of the goals:
- Ensure care for hospitalized children is fully integrated and includes the medical home;
- Design and support systems for children that eliminate harm associated with hospital care;
- Develop a skilled and stable workforce that provides expert care for hospitalized children;
- Use collaborative research models to answer questions of clinical efficacy, comparative effectiveness, and quality improvement inclusive of patient safety, and deliver care based on that knowledge;
- Provide the expertise that supports innovative continuing education in the care of the hospitalized child for pediatric hospitalists, trainees, midlevel providers, and hospital staff;
- Create value and provide academic and systems leadership for patients and organizations based on pediatric hospitalists’ unique expertise in PHM clinical care, research, and education; and
- Be leaders and influential agents in local, state, and national healthcare policies that affect hospital care.
Although it was discussed, the roundtable decided against the establishment of a professional organization for pediatric hospitalists. Instead, the group agreed to continue to utilize the resources and organizational support provided by SHM, APA, and AAP. All three groups contributed money to the roundtable, sent representatives to the meeting, and are interested in the results.
“The Academic Pediatric Association has been involved with pediatric hospital medicine from the beginning, and we plan on continuing our involvement,” says Daniel Rauch, MD, FHM, associate director of pediatrics at Elmhurst Hospital Center in New York City and co-chair of the APA’s Hospital Medicine Special Interest Group, which is paying close attention to PHM education and research issues.
Strategic Initiatives
The roundtable established four workgroups: clinical practice/workforce, quality and safety, research, and education. The workgroups are directed to create strategic initiative projects focused on advancing the goals laid out at the roundtable meeting and complete most of the projects no later than the July 2010 PHM Conference in Minneapolis (see “A Closer Look at the Pediatric Hospital Medicine Initiatives,” p. 7). At the 2009 PHM conference in Tampa, Fla., roundtable participants reported on some of the initiatives’ preliminary results.
“I walked away … energized and ready to help change the world, which is a pretty great feeling,” says Jennifer Daru, MD, FAAP, FHM, chief of the division of pediatric hospital medicine at California Pacific Medical Center in San Francisco and co-leader of the roundtable’s clinical practice/workforce workgroup.
One of Dr. Daru’s workgroup’s strategic initiative projects should make Dr. Hale and his pediatric hospitalists at Central Maine Medical Center happy. Dr. Daru’s group is creating a clinical practice dashboard template that PHM programs can use to internally track patient care and compare themselves with other programs and national standards.
“I think very few programs have a dashboard, because it’s a relatively newer thing for pediatric hospital medicine,” Dr. Daru says. “With this dashboard, we want to be able to say, ‘Here are the things you should look at to ensure quality care for your kids, and as you look at them, you should probably track them over time.’ ”
Steve Narang, MD, medical director of quality/safety and pediatric emergency services at Our Lady of the Lake Regional Medical Center and Children’s Hospital in Baton Rouge, La., is leading the quality and safety workgroup, which is focused on patient identification, patient handoffs between pediatric hospitalists and PCPs, and clinical outcomes for common pediatric diagnoses.
“Most doctors don’t like standardized forms or cookbook medicine, but they do understand good care. Hopefully, we will show success in these initiatives and they will serve as a launching pad to other initiatives,” Dr. Narang says.
Dr. Hale, for one, is excited by the initiatives and workgroups, and optimistic the strategic projects will help his program. In recent years, the PHM community has talked about these kinds of advances, and he’s encouraged to see them moving forward. “These initiatives contribute to the strength of our field,” says Dr. Hale, who also serves on the executive board of AAP’s Maine chapter.
About 80 pediatric hospitalists have volunteered to help with the strategic initiatives. Earlier this year, a request for help was broadcast over the Section on Hospital Medicine listserv run by the AAP. It was announced at HM09 in Chicago and the PHM conference in Tampa. Everyone who submitted a resume or CV, references, and a statement of interest is included, Dr. Percelay says. “This is not supposed to be some exclusive club that no one can get into,” he says. “We are committed to a transparent process.”
While the application deadline has passed, organizers expect additional calls for volunteers in the future as strategic projects move forward, projects are added, and current volunteers depart (see “How to Get Involved,” above).
“They will be the next volunteer go-tos. We will essentially build them into new projects that come up or if gaps emerge,” Dr. Daru says. “We want to have as many people as possible who are really motivated.”
Group Effort
You don’t necessarily have to volunteer for workgroups to be a part of the broader effort. You can read and comment on draft reports released by some of the project teams, or review the roundtable’s executive summary and find ways to apply the vision and goals to your own PHM program, says Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas, and pediatric editor of The Hospitalist.
“If each pediatric hospitalist set strategic initiatives for their own group or hospital, chances are they would find remarkable similarity between what they came up with and what the strategic planning roundtable came up with,” says Dr. Shen, who is directing one of the quality and safety workgroup’s initiatives. “There are plenty of ways to think globally and act locally.” TH
Lisa Ryan is a freelance writer based in New Jersey.
Top Image Source: HOMER SYKES/ALAMY