User login
Hospitalists and ACC in Pandemic Flu
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
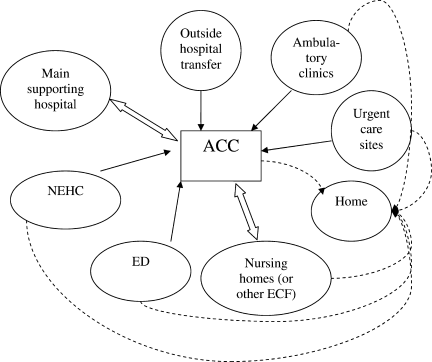
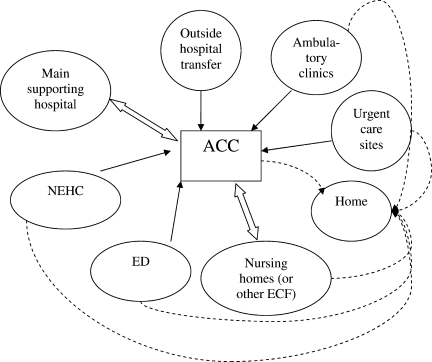
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.
Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.

Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.

Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.
Agent shows promise in acute leukemias

Delivering drugs in combination requires a certain balance, a balance that ensures the drugs act synergistically. And researchers say they have struck the right balance with a new drug that combines two old standbys.
Daunorubicin and cytarabine (or ara-C) have proven activity against acute leukemia. However, neither of the drugs has elicited impressive survival rates when given alone, according to Eric Feldman, MD, of Weill Cornell Medical College.
In a presentation at Chemotherapy Foundation Symposium XXVII, Dr Feldman discussed a new agent comprised of the two drugs that he theorizes will prove more effective than either drug alone.
“When you combine different combinations of cytarabine and daunorubicin, there are some ratios that, in fact, may be antagonistic or just additive,” Dr Feldman said. “But… there are some—particularly this 5-to-1 ara-C-to-daunorubicin—that may be synergistic. And the question is, how do you deliver to the leukemia cell this synergistic combination of drugs?”
For a long time, Dr Feldman said, scientists did not have the appropriate technology to accomplish that. But now they do, and they have made significant strides with the compound CPX-351.
“Basically, this is a liposomal combination of daunorubicin and ara-C,” Dr Feldman said. “But the unique feature is that it fixes a 5-to-1 molar ratio of ara-C with daunorubicin and delivers to the cell this ratio in this concentration.”
To test the tolerability and efficacy of this compound, researchers began a phase 1 trial of CPX-351. The majority of patients on the trial had acute myeloid leukemia, though there were a few with acute lymphocytic leukemia and myelodysplastic syndrome. All were refractory to prior therapy, and most were over the age of 60 years.
The FDA mandated that the initial dose of CPX-351 be very low, so the researchers started with 3 units/m². One unit of CPX-351 is equal to 1 mg of cytarabine and 0.44 mg of daunorubicin. The researchers increased the dose gradually and monitored patients for responses and toxicities.
“We started low… and did not see responses at all until we got to 32 units,” Dr Feldman said. “By 101 [units], we saw multiple responses, and this is the dose that was considered the maximum-tolerated dose.”
This is because, at 134 units, the team observed 3 dose-limiting toxicities. They saw left ventricular systolic dysfunction and 1 patient with hypertensive crisis, although it was not clear whether this event was actually related to the drug.
“The main problem that we found was persistent cytopenias,” Dr Feldman said. “There was 1 patient in this cohort that took over 80 days to achieve a complete remission, meaning recovery of their platelets to 100,000 and neutrophils to 1000. We considered that the true dose-limiting toxicity.”
Apart from this myelosuppression, CPX-351 was well tolerated. Some patients did experience mucositosis, vomiting, and a skin rash, but the rash responded to corticosteroids. Importantly, patients did not experience alopecia.
With these promising results, researchers began a phase 2 study of CPX-351. They enrolled newly diagnosed leukemia patients between 60 and 75 years of age. Patients had high- or intermediate-risk disease.
They were randomized in a 2-to-1 fashion to receive either 100 units of CPX-351 or standard 3 + 7 therapy. The preliminary data from this study were presented at the ASH Annual Meeting in December. ![]()

Delivering drugs in combination requires a certain balance, a balance that ensures the drugs act synergistically. And researchers say they have struck the right balance with a new drug that combines two old standbys.
Daunorubicin and cytarabine (or ara-C) have proven activity against acute leukemia. However, neither of the drugs has elicited impressive survival rates when given alone, according to Eric Feldman, MD, of Weill Cornell Medical College.
In a presentation at Chemotherapy Foundation Symposium XXVII, Dr Feldman discussed a new agent comprised of the two drugs that he theorizes will prove more effective than either drug alone.
“When you combine different combinations of cytarabine and daunorubicin, there are some ratios that, in fact, may be antagonistic or just additive,” Dr Feldman said. “But… there are some—particularly this 5-to-1 ara-C-to-daunorubicin—that may be synergistic. And the question is, how do you deliver to the leukemia cell this synergistic combination of drugs?”
For a long time, Dr Feldman said, scientists did not have the appropriate technology to accomplish that. But now they do, and they have made significant strides with the compound CPX-351.
“Basically, this is a liposomal combination of daunorubicin and ara-C,” Dr Feldman said. “But the unique feature is that it fixes a 5-to-1 molar ratio of ara-C with daunorubicin and delivers to the cell this ratio in this concentration.”
To test the tolerability and efficacy of this compound, researchers began a phase 1 trial of CPX-351. The majority of patients on the trial had acute myeloid leukemia, though there were a few with acute lymphocytic leukemia and myelodysplastic syndrome. All were refractory to prior therapy, and most were over the age of 60 years.
The FDA mandated that the initial dose of CPX-351 be very low, so the researchers started with 3 units/m². One unit of CPX-351 is equal to 1 mg of cytarabine and 0.44 mg of daunorubicin. The researchers increased the dose gradually and monitored patients for responses and toxicities.
“We started low… and did not see responses at all until we got to 32 units,” Dr Feldman said. “By 101 [units], we saw multiple responses, and this is the dose that was considered the maximum-tolerated dose.”
This is because, at 134 units, the team observed 3 dose-limiting toxicities. They saw left ventricular systolic dysfunction and 1 patient with hypertensive crisis, although it was not clear whether this event was actually related to the drug.
“The main problem that we found was persistent cytopenias,” Dr Feldman said. “There was 1 patient in this cohort that took over 80 days to achieve a complete remission, meaning recovery of their platelets to 100,000 and neutrophils to 1000. We considered that the true dose-limiting toxicity.”
Apart from this myelosuppression, CPX-351 was well tolerated. Some patients did experience mucositosis, vomiting, and a skin rash, but the rash responded to corticosteroids. Importantly, patients did not experience alopecia.
With these promising results, researchers began a phase 2 study of CPX-351. They enrolled newly diagnosed leukemia patients between 60 and 75 years of age. Patients had high- or intermediate-risk disease.
They were randomized in a 2-to-1 fashion to receive either 100 units of CPX-351 or standard 3 + 7 therapy. The preliminary data from this study were presented at the ASH Annual Meeting in December. ![]()

Delivering drugs in combination requires a certain balance, a balance that ensures the drugs act synergistically. And researchers say they have struck the right balance with a new drug that combines two old standbys.
Daunorubicin and cytarabine (or ara-C) have proven activity against acute leukemia. However, neither of the drugs has elicited impressive survival rates when given alone, according to Eric Feldman, MD, of Weill Cornell Medical College.
In a presentation at Chemotherapy Foundation Symposium XXVII, Dr Feldman discussed a new agent comprised of the two drugs that he theorizes will prove more effective than either drug alone.
“When you combine different combinations of cytarabine and daunorubicin, there are some ratios that, in fact, may be antagonistic or just additive,” Dr Feldman said. “But… there are some—particularly this 5-to-1 ara-C-to-daunorubicin—that may be synergistic. And the question is, how do you deliver to the leukemia cell this synergistic combination of drugs?”
For a long time, Dr Feldman said, scientists did not have the appropriate technology to accomplish that. But now they do, and they have made significant strides with the compound CPX-351.
“Basically, this is a liposomal combination of daunorubicin and ara-C,” Dr Feldman said. “But the unique feature is that it fixes a 5-to-1 molar ratio of ara-C with daunorubicin and delivers to the cell this ratio in this concentration.”
To test the tolerability and efficacy of this compound, researchers began a phase 1 trial of CPX-351. The majority of patients on the trial had acute myeloid leukemia, though there were a few with acute lymphocytic leukemia and myelodysplastic syndrome. All were refractory to prior therapy, and most were over the age of 60 years.
The FDA mandated that the initial dose of CPX-351 be very low, so the researchers started with 3 units/m². One unit of CPX-351 is equal to 1 mg of cytarabine and 0.44 mg of daunorubicin. The researchers increased the dose gradually and monitored patients for responses and toxicities.
“We started low… and did not see responses at all until we got to 32 units,” Dr Feldman said. “By 101 [units], we saw multiple responses, and this is the dose that was considered the maximum-tolerated dose.”
This is because, at 134 units, the team observed 3 dose-limiting toxicities. They saw left ventricular systolic dysfunction and 1 patient with hypertensive crisis, although it was not clear whether this event was actually related to the drug.
“The main problem that we found was persistent cytopenias,” Dr Feldman said. “There was 1 patient in this cohort that took over 80 days to achieve a complete remission, meaning recovery of their platelets to 100,000 and neutrophils to 1000. We considered that the true dose-limiting toxicity.”
Apart from this myelosuppression, CPX-351 was well tolerated. Some patients did experience mucositosis, vomiting, and a skin rash, but the rash responded to corticosteroids. Importantly, patients did not experience alopecia.
With these promising results, researchers began a phase 2 study of CPX-351. They enrolled newly diagnosed leukemia patients between 60 and 75 years of age. Patients had high- or intermediate-risk disease.
They were randomized in a 2-to-1 fashion to receive either 100 units of CPX-351 or standard 3 + 7 therapy. The preliminary data from this study were presented at the ASH Annual Meeting in December. ![]()
Make The Diagnosis
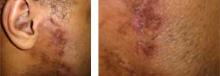
A 51 year-old-male presented with asymptomatic violaceous, indurated plaques on his left and right cheeks. He also had follicular plugging in the right ear. What’s your diagnosis?
Images courtesy Dr. Donna Bilu Martin
Diagnosis: Lupus Erythematosus Panniculitis
Lupus panniculitis, or lupus profundus, represents 2%-3% of all patients with lupus erythematosus. It most commonly occurs in adults aged 20-60. Patients present with tender subcutaneous nodules and plaques that tend to develop on the face, upper outer arms, shoulders, hips, and trunk. The distal extremities are usually spared. The overlying skin can show features of chronic cutaneous lupus including scaling, follicular plugging, atrophy, dyspigmentation, telangiectasias, and ulceration.
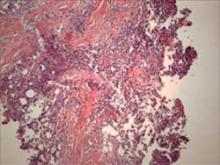
Histopathology reveals a primarily lobular panniculitis with a marked predominance of lymphocytes and scattered plasma cells. One characteristic feature is hyalin necrosis of fat lobules that can extend into the septa.
Treatment options include sunscreen, potent topical and intralesional corticosteroids, antimalarials, systemic steroids (in initial phases of disease), dapsone, cyclophosphamide, and thalidomide. This patient was treated with thalidomide, which resulted in improvement of his lesions.
This case was first presented at Maryland Derm, at the University of Maryland School of Medicine in Baltimore, by Dr. Bilu Martin and Dr. Anthony Gaspari.
Image courtesy Dr. Donna Bilu Martin
Histology shows a lobular panniculitis with a marked predominance of lymphocytes and scattered plasma cells.
A 51 year-old-male presented with asymptomatic violaceous, indurated plaques on his left and right cheeks. He also had follicular plugging in the right ear. What’s your diagnosis?
Images courtesy Dr. Donna Bilu Martin
Diagnosis: Lupus Erythematosus Panniculitis
Lupus panniculitis, or lupus profundus, represents 2%-3% of all patients with lupus erythematosus. It most commonly occurs in adults aged 20-60. Patients present with tender subcutaneous nodules and plaques that tend to develop on the face, upper outer arms, shoulders, hips, and trunk. The distal extremities are usually spared. The overlying skin can show features of chronic cutaneous lupus including scaling, follicular plugging, atrophy, dyspigmentation, telangiectasias, and ulceration.
Histopathology reveals a primarily lobular panniculitis with a marked predominance of lymphocytes and scattered plasma cells. One characteristic feature is hyalin necrosis of fat lobules that can extend into the septa.
Treatment options include sunscreen, potent topical and intralesional corticosteroids, antimalarials, systemic steroids (in initial phases of disease), dapsone, cyclophosphamide, and thalidomide. This patient was treated with thalidomide, which resulted in improvement of his lesions.
This case was first presented at Maryland Derm, at the University of Maryland School of Medicine in Baltimore, by Dr. Bilu Martin and Dr. Anthony Gaspari.
Image courtesy Dr. Donna Bilu Martin
Histology shows a lobular panniculitis with a marked predominance of lymphocytes and scattered plasma cells.
A 51 year-old-male presented with asymptomatic violaceous, indurated plaques on his left and right cheeks. He also had follicular plugging in the right ear. What’s your diagnosis?
Images courtesy Dr. Donna Bilu Martin
Diagnosis: Lupus Erythematosus Panniculitis
Lupus panniculitis, or lupus profundus, represents 2%-3% of all patients with lupus erythematosus. It most commonly occurs in adults aged 20-60. Patients present with tender subcutaneous nodules and plaques that tend to develop on the face, upper outer arms, shoulders, hips, and trunk. The distal extremities are usually spared. The overlying skin can show features of chronic cutaneous lupus including scaling, follicular plugging, atrophy, dyspigmentation, telangiectasias, and ulceration.
Histopathology reveals a primarily lobular panniculitis with a marked predominance of lymphocytes and scattered plasma cells. One characteristic feature is hyalin necrosis of fat lobules that can extend into the septa.
Treatment options include sunscreen, potent topical and intralesional corticosteroids, antimalarials, systemic steroids (in initial phases of disease), dapsone, cyclophosphamide, and thalidomide. This patient was treated with thalidomide, which resulted in improvement of his lesions.
This case was first presented at Maryland Derm, at the University of Maryland School of Medicine in Baltimore, by Dr. Bilu Martin and Dr. Anthony Gaspari.
Image courtesy Dr. Donna Bilu Martin
Histology shows a lobular panniculitis with a marked predominance of lymphocytes and scattered plasma cells.
Incomplete Handoffs Hinder Patient Safety, Workflow
Nearly one in five hospitalists admitted uncertainty about transitional patient-care plans after service change, according to a report to be published in this month’s Journal of Hospital Medicine.
The review, a single-institution study conducted at the University of Chicago, found 18% of respondents acknowledged uncertainty, 13% reported incomplete handoffs, and 16% attributed at least one “near miss” to incomplete communication. The study suggests that “investments in improving service change could not only improve patient safety, but they could improve hospitalists’ daily workflow,” says senior author Vineet Arora, MD, MAPP, an academic hospitalist and associate director of Internal Medicine Residency at the University of Chicago.
Keiki Hinami, MD, MS, instructor of medicine in the Division of Hospital Medicine at the Northwestern University Feinberg School of Medicine, says a unique facet of the report, titled “Understanding Communication During Hospitalist Service Changes: A Mixed Methods Study,” was the understanding by physicians that successful handoffs often involve more than a brief conversation or a pass-through of documentation.
“The outgoing doctor would come back to the incoming doctor and ask for updates, or they would solicit the incoming doctor for more information if they needed it,” says Dr. Hinami, who was one of the study’s authors while employed as a clinical associate by University of Chicago. “The participants of our study naturally adopted a strategy acknowledging that one conversation is not usually sufficient.”
The study measured 60 service changes among 17 hospitalists on a non-teaching service from May to December 2007. Hospitalists who reported incomplete handoffs were more likely to report uncertainty about care plans (71% incomplete vs. 10% complete, P<0.01), discovery of missing information (71% vs. 24%, P=0.01), and near misses/adverse events (57% vs. 10%, P<0.01).
Dr. Arora says work is under way to develop educational programs and evaluation tools to train hospitalists and others to improve service change handoffs.
“How do you teach people to communicate only pertinent information?” Dr. Hinami says. “That’s really a difficult challenge. Even though handoffs are something we do every day, most people have never had any formal training in communicating that.”
Nearly one in five hospitalists admitted uncertainty about transitional patient-care plans after service change, according to a report to be published in this month’s Journal of Hospital Medicine.
The review, a single-institution study conducted at the University of Chicago, found 18% of respondents acknowledged uncertainty, 13% reported incomplete handoffs, and 16% attributed at least one “near miss” to incomplete communication. The study suggests that “investments in improving service change could not only improve patient safety, but they could improve hospitalists’ daily workflow,” says senior author Vineet Arora, MD, MAPP, an academic hospitalist and associate director of Internal Medicine Residency at the University of Chicago.
Keiki Hinami, MD, MS, instructor of medicine in the Division of Hospital Medicine at the Northwestern University Feinberg School of Medicine, says a unique facet of the report, titled “Understanding Communication During Hospitalist Service Changes: A Mixed Methods Study,” was the understanding by physicians that successful handoffs often involve more than a brief conversation or a pass-through of documentation.
“The outgoing doctor would come back to the incoming doctor and ask for updates, or they would solicit the incoming doctor for more information if they needed it,” says Dr. Hinami, who was one of the study’s authors while employed as a clinical associate by University of Chicago. “The participants of our study naturally adopted a strategy acknowledging that one conversation is not usually sufficient.”
The study measured 60 service changes among 17 hospitalists on a non-teaching service from May to December 2007. Hospitalists who reported incomplete handoffs were more likely to report uncertainty about care plans (71% incomplete vs. 10% complete, P<0.01), discovery of missing information (71% vs. 24%, P=0.01), and near misses/adverse events (57% vs. 10%, P<0.01).
Dr. Arora says work is under way to develop educational programs and evaluation tools to train hospitalists and others to improve service change handoffs.
“How do you teach people to communicate only pertinent information?” Dr. Hinami says. “That’s really a difficult challenge. Even though handoffs are something we do every day, most people have never had any formal training in communicating that.”
Nearly one in five hospitalists admitted uncertainty about transitional patient-care plans after service change, according to a report to be published in this month’s Journal of Hospital Medicine.
The review, a single-institution study conducted at the University of Chicago, found 18% of respondents acknowledged uncertainty, 13% reported incomplete handoffs, and 16% attributed at least one “near miss” to incomplete communication. The study suggests that “investments in improving service change could not only improve patient safety, but they could improve hospitalists’ daily workflow,” says senior author Vineet Arora, MD, MAPP, an academic hospitalist and associate director of Internal Medicine Residency at the University of Chicago.
Keiki Hinami, MD, MS, instructor of medicine in the Division of Hospital Medicine at the Northwestern University Feinberg School of Medicine, says a unique facet of the report, titled “Understanding Communication During Hospitalist Service Changes: A Mixed Methods Study,” was the understanding by physicians that successful handoffs often involve more than a brief conversation or a pass-through of documentation.
“The outgoing doctor would come back to the incoming doctor and ask for updates, or they would solicit the incoming doctor for more information if they needed it,” says Dr. Hinami, who was one of the study’s authors while employed as a clinical associate by University of Chicago. “The participants of our study naturally adopted a strategy acknowledging that one conversation is not usually sufficient.”
The study measured 60 service changes among 17 hospitalists on a non-teaching service from May to December 2007. Hospitalists who reported incomplete handoffs were more likely to report uncertainty about care plans (71% incomplete vs. 10% complete, P<0.01), discovery of missing information (71% vs. 24%, P=0.01), and near misses/adverse events (57% vs. 10%, P<0.01).
Dr. Arora says work is under way to develop educational programs and evaluation tools to train hospitalists and others to improve service change handoffs.
“How do you teach people to communicate only pertinent information?” Dr. Hinami says. “That’s really a difficult challenge. Even though handoffs are something we do every day, most people have never had any formal training in communicating that.”
Avoid Social Networking Pitfalls
Although Web sites like Facebook, Linked In, and Ning are touted as valuable tools for social and professional networking, if users aren’t careful, career-related catastrophes can occur. It bears repeating that no online activity is anonymous, especially with more and more healthcare employers and recruiters visiting these sites to learn about job candidates, says Roberta Renaldy, a senior staffing specialist at Northwestern Memorial Hospital in Chicago.
“They’re becoming your resume before your resume,” Renaldy says of social networking sites.
To keep career opportunities open, hospitalists should avoid dishing out “digital dirt”—aka put-downs—about other people, she says. Vulgarity, unsavory photos, incorrect spelling and grammar, angry online disputes, and dispensing medical advice also are taboo. Even strong points of view on controversial issues can run hospitalists the risk of getting passed over for a job or promotion.
“Someone might be willing to take this risk, but I encourage people to really think before they express their opinions,” Renaldy says.
On the flip side, hospitalists should create a personal brand that’s compelling and consistent across their social networking profiles, says E. Chandlee Bryan, a certified career coach at the firm Best Fit Forward in New York City. Be accurate about expertise and keep visitors interested by providing constant career updates, she says. Always thank network contacts for the slightest bit of advice, and don’t hesitate to offer others help, Bryan suggests.
Renaldy emphasizes the old-fashioned approach. “Using the Internet is a way to spark a networking relationship, but many times it doesn’t develop the relationship,” she says. “Nothing replaces face-to-face contact in furthering your professional career.”
Although Web sites like Facebook, Linked In, and Ning are touted as valuable tools for social and professional networking, if users aren’t careful, career-related catastrophes can occur. It bears repeating that no online activity is anonymous, especially with more and more healthcare employers and recruiters visiting these sites to learn about job candidates, says Roberta Renaldy, a senior staffing specialist at Northwestern Memorial Hospital in Chicago.
“They’re becoming your resume before your resume,” Renaldy says of social networking sites.
To keep career opportunities open, hospitalists should avoid dishing out “digital dirt”—aka put-downs—about other people, she says. Vulgarity, unsavory photos, incorrect spelling and grammar, angry online disputes, and dispensing medical advice also are taboo. Even strong points of view on controversial issues can run hospitalists the risk of getting passed over for a job or promotion.
“Someone might be willing to take this risk, but I encourage people to really think before they express their opinions,” Renaldy says.
On the flip side, hospitalists should create a personal brand that’s compelling and consistent across their social networking profiles, says E. Chandlee Bryan, a certified career coach at the firm Best Fit Forward in New York City. Be accurate about expertise and keep visitors interested by providing constant career updates, she says. Always thank network contacts for the slightest bit of advice, and don’t hesitate to offer others help, Bryan suggests.
Renaldy emphasizes the old-fashioned approach. “Using the Internet is a way to spark a networking relationship, but many times it doesn’t develop the relationship,” she says. “Nothing replaces face-to-face contact in furthering your professional career.”
Although Web sites like Facebook, Linked In, and Ning are touted as valuable tools for social and professional networking, if users aren’t careful, career-related catastrophes can occur. It bears repeating that no online activity is anonymous, especially with more and more healthcare employers and recruiters visiting these sites to learn about job candidates, says Roberta Renaldy, a senior staffing specialist at Northwestern Memorial Hospital in Chicago.
“They’re becoming your resume before your resume,” Renaldy says of social networking sites.
To keep career opportunities open, hospitalists should avoid dishing out “digital dirt”—aka put-downs—about other people, she says. Vulgarity, unsavory photos, incorrect spelling and grammar, angry online disputes, and dispensing medical advice also are taboo. Even strong points of view on controversial issues can run hospitalists the risk of getting passed over for a job or promotion.
“Someone might be willing to take this risk, but I encourage people to really think before they express their opinions,” Renaldy says.
On the flip side, hospitalists should create a personal brand that’s compelling and consistent across their social networking profiles, says E. Chandlee Bryan, a certified career coach at the firm Best Fit Forward in New York City. Be accurate about expertise and keep visitors interested by providing constant career updates, she says. Always thank network contacts for the slightest bit of advice, and don’t hesitate to offer others help, Bryan suggests.
Renaldy emphasizes the old-fashioned approach. “Using the Internet is a way to spark a networking relationship, but many times it doesn’t develop the relationship,” she says. “Nothing replaces face-to-face contact in furthering your professional career.”
Dr. Hospitalist
“Hospitalism” Isn’t the Same as HM
If hospitalists are doctors who provide care to hospitalized patients, is the correct term for the care they provide “hospitalism”?
P. Doherty, DO
Fort Collins, Colo.
Dr. Hospitalist responds: I am of the belief that the correct term for the general medical care of hospitalized patients is “hospital medicine.” Hospitalism is a term I’ve heard used interchangeably with hospital medicine, but I do not believe it accurately describes the field of medicine practiced by hospitalists.
The dictionary, and online resources like Wikipedia, describes “hospitalism” as a medical condition suffered by children who were “institutionalized for long periods and deprived of substitute maternal care.” This term was first described in the late 1800s and popularized by psychotherapist Rene Spitz in 1945.1
Lee Goldman, MD, and Robert Wachter, MD, FHM, coined the term “hospitalist” in a landmark 1996 New England Journal of Medicine article. Dr. Goldman describes hospitalism as “[a] variety of iatrogenic maladies that were acquired by hospitalized patients and that often were more deadly than the admitting condition itself.” In fact, he described hospitalists as “a cure for hospitalism.”2
I had never heard of the term hospitalism and did not understand its definition before I became a hospitalist. As a hospitalist, I prefer to practice hospital medicine.
Don’t Give Up on Hand-Hygiene Compliance
I am the director of a hospitalist group. How do I convince my colleagues to wash their hands?
B. Hunter, MD
Dr. Hospitalist responds: Dr. Hunter, don’t feel discouraged. You are not alone. Appropriate hand hygiene in the hospital setting is a difficult nut to crack. In some ways, I liken hand-washing noncompliance to smoking or eating junk food: We know that it is bad for us. None of us dispute the facts. There is plenty of research to support the fact that smoking causes chronic obstructive pulmonary disease and lung cancer; junk food causes obesity, which leads to heart disease and other ailments. But the truth of the matter is that many of us have a hard time resisting cigarettes and greasy burgers.
Hand hygiene is no different. It’s habitual. If it is not part of your routine, cleaning your hands before and after you enter a patient’s hospital room is time-consuming. But the truth remains: There is so much at stake.
Setting cost aside, hospital-acquired infections are a significant cause of morbidity and mortality. We know hand hygiene works. We also know that it is the right thing to do. If any of us were hospitalized, would we want our providers to clean their hands before examining us?
If the hospital where you work is like his or mine, hand-cleanser dispensers are conveniently located near the entry to every patient room. Signs urging compliance are plastered all over the place. The rules are clearly outlined and the rationale thoughtfully explained. Despite that fact, some providers, doctors, nurses, and others simply choose to ignore all the facts and reminders.
Some medical leaders believe hand-hygiene noncompliance is a medical error, and rogue providers should be punished for ignoring patient-safety measures. I agree. If your institution does not yet have a hand-hygiene program in place, it is incumbent on you and the hospital to institute one. If you have a program and providers ignore the rules, it is time to monitor compliance and punish the individuals who are putting our patients’ well-being at risk. TH
References
- Crandall FM. Hospitalism. Neonatology on the Web site. Available at: www.neonatology.org/classics/crandall.html. Accessed Sept. 12, 2009.
- Goldman L. Hospitalists as cure for hospitalism. Trans Am Clin Climatol Assoc. 2003;114:37-48.
“Hospitalism” Isn’t the Same as HM
If hospitalists are doctors who provide care to hospitalized patients, is the correct term for the care they provide “hospitalism”?
P. Doherty, DO
Fort Collins, Colo.
Dr. Hospitalist responds: I am of the belief that the correct term for the general medical care of hospitalized patients is “hospital medicine.” Hospitalism is a term I’ve heard used interchangeably with hospital medicine, but I do not believe it accurately describes the field of medicine practiced by hospitalists.
The dictionary, and online resources like Wikipedia, describes “hospitalism” as a medical condition suffered by children who were “institutionalized for long periods and deprived of substitute maternal care.” This term was first described in the late 1800s and popularized by psychotherapist Rene Spitz in 1945.1
Lee Goldman, MD, and Robert Wachter, MD, FHM, coined the term “hospitalist” in a landmark 1996 New England Journal of Medicine article. Dr. Goldman describes hospitalism as “[a] variety of iatrogenic maladies that were acquired by hospitalized patients and that often were more deadly than the admitting condition itself.” In fact, he described hospitalists as “a cure for hospitalism.”2
I had never heard of the term hospitalism and did not understand its definition before I became a hospitalist. As a hospitalist, I prefer to practice hospital medicine.
Don’t Give Up on Hand-Hygiene Compliance
I am the director of a hospitalist group. How do I convince my colleagues to wash their hands?
B. Hunter, MD
Dr. Hospitalist responds: Dr. Hunter, don’t feel discouraged. You are not alone. Appropriate hand hygiene in the hospital setting is a difficult nut to crack. In some ways, I liken hand-washing noncompliance to smoking or eating junk food: We know that it is bad for us. None of us dispute the facts. There is plenty of research to support the fact that smoking causes chronic obstructive pulmonary disease and lung cancer; junk food causes obesity, which leads to heart disease and other ailments. But the truth of the matter is that many of us have a hard time resisting cigarettes and greasy burgers.
Hand hygiene is no different. It’s habitual. If it is not part of your routine, cleaning your hands before and after you enter a patient’s hospital room is time-consuming. But the truth remains: There is so much at stake.
Setting cost aside, hospital-acquired infections are a significant cause of morbidity and mortality. We know hand hygiene works. We also know that it is the right thing to do. If any of us were hospitalized, would we want our providers to clean their hands before examining us?
If the hospital where you work is like his or mine, hand-cleanser dispensers are conveniently located near the entry to every patient room. Signs urging compliance are plastered all over the place. The rules are clearly outlined and the rationale thoughtfully explained. Despite that fact, some providers, doctors, nurses, and others simply choose to ignore all the facts and reminders.
Some medical leaders believe hand-hygiene noncompliance is a medical error, and rogue providers should be punished for ignoring patient-safety measures. I agree. If your institution does not yet have a hand-hygiene program in place, it is incumbent on you and the hospital to institute one. If you have a program and providers ignore the rules, it is time to monitor compliance and punish the individuals who are putting our patients’ well-being at risk. TH
References
- Crandall FM. Hospitalism. Neonatology on the Web site. Available at: www.neonatology.org/classics/crandall.html. Accessed Sept. 12, 2009.
- Goldman L. Hospitalists as cure for hospitalism. Trans Am Clin Climatol Assoc. 2003;114:37-48.
“Hospitalism” Isn’t the Same as HM
If hospitalists are doctors who provide care to hospitalized patients, is the correct term for the care they provide “hospitalism”?
P. Doherty, DO
Fort Collins, Colo.
Dr. Hospitalist responds: I am of the belief that the correct term for the general medical care of hospitalized patients is “hospital medicine.” Hospitalism is a term I’ve heard used interchangeably with hospital medicine, but I do not believe it accurately describes the field of medicine practiced by hospitalists.
The dictionary, and online resources like Wikipedia, describes “hospitalism” as a medical condition suffered by children who were “institutionalized for long periods and deprived of substitute maternal care.” This term was first described in the late 1800s and popularized by psychotherapist Rene Spitz in 1945.1
Lee Goldman, MD, and Robert Wachter, MD, FHM, coined the term “hospitalist” in a landmark 1996 New England Journal of Medicine article. Dr. Goldman describes hospitalism as “[a] variety of iatrogenic maladies that were acquired by hospitalized patients and that often were more deadly than the admitting condition itself.” In fact, he described hospitalists as “a cure for hospitalism.”2
I had never heard of the term hospitalism and did not understand its definition before I became a hospitalist. As a hospitalist, I prefer to practice hospital medicine.
Don’t Give Up on Hand-Hygiene Compliance
I am the director of a hospitalist group. How do I convince my colleagues to wash their hands?
B. Hunter, MD
Dr. Hospitalist responds: Dr. Hunter, don’t feel discouraged. You are not alone. Appropriate hand hygiene in the hospital setting is a difficult nut to crack. In some ways, I liken hand-washing noncompliance to smoking or eating junk food: We know that it is bad for us. None of us dispute the facts. There is plenty of research to support the fact that smoking causes chronic obstructive pulmonary disease and lung cancer; junk food causes obesity, which leads to heart disease and other ailments. But the truth of the matter is that many of us have a hard time resisting cigarettes and greasy burgers.
Hand hygiene is no different. It’s habitual. If it is not part of your routine, cleaning your hands before and after you enter a patient’s hospital room is time-consuming. But the truth remains: There is so much at stake.
Setting cost aside, hospital-acquired infections are a significant cause of morbidity and mortality. We know hand hygiene works. We also know that it is the right thing to do. If any of us were hospitalized, would we want our providers to clean their hands before examining us?
If the hospital where you work is like his or mine, hand-cleanser dispensers are conveniently located near the entry to every patient room. Signs urging compliance are plastered all over the place. The rules are clearly outlined and the rationale thoughtfully explained. Despite that fact, some providers, doctors, nurses, and others simply choose to ignore all the facts and reminders.
Some medical leaders believe hand-hygiene noncompliance is a medical error, and rogue providers should be punished for ignoring patient-safety measures. I agree. If your institution does not yet have a hand-hygiene program in place, it is incumbent on you and the hospital to institute one. If you have a program and providers ignore the rules, it is time to monitor compliance and punish the individuals who are putting our patients’ well-being at risk. TH
References
- Crandall FM. Hospitalism. Neonatology on the Web site. Available at: www.neonatology.org/classics/crandall.html. Accessed Sept. 12, 2009.
- Goldman L. Hospitalists as cure for hospitalism. Trans Am Clin Climatol Assoc. 2003;114:37-48.
Compensation Conundrum
Should hospitalist compensation increase automatically based on how long the physician has been in the practice (i.e., tenure)? Should hospitalist compensation have a cost-of-living provision, in which compensation goes up based on some external measure, such as the consumer price index?
I’m in favor of ever-increasing hospitalist incomes, including mine. And, fortunately, surveys done by SHM and others show hospitalist incomes have been increasing much faster than the cost of living. A portion of this increase can be explained by inflation and the fact that the average productivity for a full-time hospitalist has been increasing. We’re either working harder or more efficiently, but either way, the average full-time hospitalist is seeing more billable encounters than ever before.
But inflation and workload account for only a portion of the historical increase in hospitalist salaries. Market forces—principally, the demand for hospitalists exceeding the supply—probably are the biggest factors leading to rising salaries.
When Is a Hospitalist Most Valuable?
In this column, I’m going to discuss compensation philosophy for standard work as a staff hospitalist. That is mostly direct patient care, with the typical amount of such additional work as committee participation, protocol development, etc. A hospitalist who takes on a new role, such as group leader, medical director of the quality department, chief medical officer, etc., should expect his or her salary to change as a result of the promotion, and I’m excluding that situation from this article.
So back to my original question. If surveys demonstrate hospitalist salaries are increasing, then it is reasonable for your practice to ensure your hospitalist salaries keep up with established market rates. But independent of changes in the market, should hospitalist salaries in your group go up based on years of service in the group or years of experience as a hospitalist, even if some of that time was in another practice?
There are a number of ways to approach this question, but for me the key question is, at what point in an HM career is the hospitalist most valuable to the practice? Is it the first year out of residency? What about five years into your practice as a hospitalist? Maybe 10 years? Longer? I think it makes the most sense for salary to increase as long as the hospitalist’s value to the practice is increasing, but routine increases beyond that probably don’t make sense.
A hospitalist straight out of residency is almost always less valuable than someone with experience in your practice. But one point of view is that the new residency grad usually catches up to experienced hospitalists within six to 18 months. This is often followed by a long plateau phase, and, eventually, some of the more “senior” physicians become a little less valuable than those with just a few years of experience. One orthopedist told me, “New hospitalists are so eager to help and are flexible and are committed to the success of the team. But after a while, a lot of them tend to ossify and become more difficult to work with.”
For very understandable reasons, physicians who are more established in their careers could have less scheduling flexibility and might be less willing to adjust their scope of practice to take on new roles. Some senior hospitalists—like doctors in any specialty—lose value if they don’t adequately keep up with advances in medicine. Offsetting this potential decline in value with tenure are the increase in institutional knowledge as well as relationships that a hospitalist develops by staying in the same practice for years (see Figure 1).
This point of view matches a compensation structure that might have a new residency grad start at a lower salary that increases to the “mature” level after 12 to 18 months, and remains there without future tenure-based increases—that is, a single step up in salary based on tenure. I think this makes sense for most practices.
Exceptions to the Rule
I can think of two reasonably common situations in which a group might deviate from a single tenure-based salary increase. The first is private groups in which the physicians are the contractual owners of their practice (i.e., they’re employed by a corporation they own, and not employed by the hospital or another large entity). In this structure, new physicians typically have a lower salary until they become a partner, usually after a year or two with the group. Becoming partner could require buying into the practice with tens of thousands of dollars, and might include the opportunity to share in future profit distributions. (Although buying into physician practices has been a common and appropriate model for decades, I think a new hospitalist should think carefully about whether what they will own after buying in is really worth the cost of the buy-in.)
Another exception is when hospitalists are part of a large multi-specialty physician group that offers multiple tenure-based salary increases for all physicians in all specialties. It probably makes sense to structure the hospitalist compensation plan the same way. But remember, it might take several years for primary-care physicians and surgeons to build robust patient populations and referral streams, and annual increases in salary for the first five years might mirror the pace by which a typical doctor builds his or her practice.
A new hospitalist, even one just out of residency, has an essentially mature referral stream within days of starting. So a five-year, tenure-based increase in salary could look more like the practice is simply underpaying the hospitalist until their fifth anniversary with the practice.
Automatic Increases
Some hospitalists have automatic cost-of-living salary increases. In some cases, the increases are tied to an external benchmark (e.g., the consumer price index), but it is probably more common that the future increase is simply estimated and compensation is contractually assured of increasing by a few percentage points annually.
Most hospitalists don’t have such a provision in their contracts. Indeed, most working Americans with salaries in the hospitalist range and higher don’t have cost-of-living increases. Instead, future salary adjustments are made based on market data, such as the results of salary surveys. TH
Dr. Nelson has been a hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Should hospitalist compensation increase automatically based on how long the physician has been in the practice (i.e., tenure)? Should hospitalist compensation have a cost-of-living provision, in which compensation goes up based on some external measure, such as the consumer price index?
I’m in favor of ever-increasing hospitalist incomes, including mine. And, fortunately, surveys done by SHM and others show hospitalist incomes have been increasing much faster than the cost of living. A portion of this increase can be explained by inflation and the fact that the average productivity for a full-time hospitalist has been increasing. We’re either working harder or more efficiently, but either way, the average full-time hospitalist is seeing more billable encounters than ever before.
But inflation and workload account for only a portion of the historical increase in hospitalist salaries. Market forces—principally, the demand for hospitalists exceeding the supply—probably are the biggest factors leading to rising salaries.
When Is a Hospitalist Most Valuable?
In this column, I’m going to discuss compensation philosophy for standard work as a staff hospitalist. That is mostly direct patient care, with the typical amount of such additional work as committee participation, protocol development, etc. A hospitalist who takes on a new role, such as group leader, medical director of the quality department, chief medical officer, etc., should expect his or her salary to change as a result of the promotion, and I’m excluding that situation from this article.
So back to my original question. If surveys demonstrate hospitalist salaries are increasing, then it is reasonable for your practice to ensure your hospitalist salaries keep up with established market rates. But independent of changes in the market, should hospitalist salaries in your group go up based on years of service in the group or years of experience as a hospitalist, even if some of that time was in another practice?
There are a number of ways to approach this question, but for me the key question is, at what point in an HM career is the hospitalist most valuable to the practice? Is it the first year out of residency? What about five years into your practice as a hospitalist? Maybe 10 years? Longer? I think it makes the most sense for salary to increase as long as the hospitalist’s value to the practice is increasing, but routine increases beyond that probably don’t make sense.
A hospitalist straight out of residency is almost always less valuable than someone with experience in your practice. But one point of view is that the new residency grad usually catches up to experienced hospitalists within six to 18 months. This is often followed by a long plateau phase, and, eventually, some of the more “senior” physicians become a little less valuable than those with just a few years of experience. One orthopedist told me, “New hospitalists are so eager to help and are flexible and are committed to the success of the team. But after a while, a lot of them tend to ossify and become more difficult to work with.”
For very understandable reasons, physicians who are more established in their careers could have less scheduling flexibility and might be less willing to adjust their scope of practice to take on new roles. Some senior hospitalists—like doctors in any specialty—lose value if they don’t adequately keep up with advances in medicine. Offsetting this potential decline in value with tenure are the increase in institutional knowledge as well as relationships that a hospitalist develops by staying in the same practice for years (see Figure 1).
This point of view matches a compensation structure that might have a new residency grad start at a lower salary that increases to the “mature” level after 12 to 18 months, and remains there without future tenure-based increases—that is, a single step up in salary based on tenure. I think this makes sense for most practices.
Exceptions to the Rule
I can think of two reasonably common situations in which a group might deviate from a single tenure-based salary increase. The first is private groups in which the physicians are the contractual owners of their practice (i.e., they’re employed by a corporation they own, and not employed by the hospital or another large entity). In this structure, new physicians typically have a lower salary until they become a partner, usually after a year or two with the group. Becoming partner could require buying into the practice with tens of thousands of dollars, and might include the opportunity to share in future profit distributions. (Although buying into physician practices has been a common and appropriate model for decades, I think a new hospitalist should think carefully about whether what they will own after buying in is really worth the cost of the buy-in.)
Another exception is when hospitalists are part of a large multi-specialty physician group that offers multiple tenure-based salary increases for all physicians in all specialties. It probably makes sense to structure the hospitalist compensation plan the same way. But remember, it might take several years for primary-care physicians and surgeons to build robust patient populations and referral streams, and annual increases in salary for the first five years might mirror the pace by which a typical doctor builds his or her practice.
A new hospitalist, even one just out of residency, has an essentially mature referral stream within days of starting. So a five-year, tenure-based increase in salary could look more like the practice is simply underpaying the hospitalist until their fifth anniversary with the practice.
Automatic Increases
Some hospitalists have automatic cost-of-living salary increases. In some cases, the increases are tied to an external benchmark (e.g., the consumer price index), but it is probably more common that the future increase is simply estimated and compensation is contractually assured of increasing by a few percentage points annually.
Most hospitalists don’t have such a provision in their contracts. Indeed, most working Americans with salaries in the hospitalist range and higher don’t have cost-of-living increases. Instead, future salary adjustments are made based on market data, such as the results of salary surveys. TH
Dr. Nelson has been a hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Should hospitalist compensation increase automatically based on how long the physician has been in the practice (i.e., tenure)? Should hospitalist compensation have a cost-of-living provision, in which compensation goes up based on some external measure, such as the consumer price index?
I’m in favor of ever-increasing hospitalist incomes, including mine. And, fortunately, surveys done by SHM and others show hospitalist incomes have been increasing much faster than the cost of living. A portion of this increase can be explained by inflation and the fact that the average productivity for a full-time hospitalist has been increasing. We’re either working harder or more efficiently, but either way, the average full-time hospitalist is seeing more billable encounters than ever before.
But inflation and workload account for only a portion of the historical increase in hospitalist salaries. Market forces—principally, the demand for hospitalists exceeding the supply—probably are the biggest factors leading to rising salaries.
When Is a Hospitalist Most Valuable?
In this column, I’m going to discuss compensation philosophy for standard work as a staff hospitalist. That is mostly direct patient care, with the typical amount of such additional work as committee participation, protocol development, etc. A hospitalist who takes on a new role, such as group leader, medical director of the quality department, chief medical officer, etc., should expect his or her salary to change as a result of the promotion, and I’m excluding that situation from this article.
So back to my original question. If surveys demonstrate hospitalist salaries are increasing, then it is reasonable for your practice to ensure your hospitalist salaries keep up with established market rates. But independent of changes in the market, should hospitalist salaries in your group go up based on years of service in the group or years of experience as a hospitalist, even if some of that time was in another practice?
There are a number of ways to approach this question, but for me the key question is, at what point in an HM career is the hospitalist most valuable to the practice? Is it the first year out of residency? What about five years into your practice as a hospitalist? Maybe 10 years? Longer? I think it makes the most sense for salary to increase as long as the hospitalist’s value to the practice is increasing, but routine increases beyond that probably don’t make sense.
A hospitalist straight out of residency is almost always less valuable than someone with experience in your practice. But one point of view is that the new residency grad usually catches up to experienced hospitalists within six to 18 months. This is often followed by a long plateau phase, and, eventually, some of the more “senior” physicians become a little less valuable than those with just a few years of experience. One orthopedist told me, “New hospitalists are so eager to help and are flexible and are committed to the success of the team. But after a while, a lot of them tend to ossify and become more difficult to work with.”
For very understandable reasons, physicians who are more established in their careers could have less scheduling flexibility and might be less willing to adjust their scope of practice to take on new roles. Some senior hospitalists—like doctors in any specialty—lose value if they don’t adequately keep up with advances in medicine. Offsetting this potential decline in value with tenure are the increase in institutional knowledge as well as relationships that a hospitalist develops by staying in the same practice for years (see Figure 1).
This point of view matches a compensation structure that might have a new residency grad start at a lower salary that increases to the “mature” level after 12 to 18 months, and remains there without future tenure-based increases—that is, a single step up in salary based on tenure. I think this makes sense for most practices.
Exceptions to the Rule
I can think of two reasonably common situations in which a group might deviate from a single tenure-based salary increase. The first is private groups in which the physicians are the contractual owners of their practice (i.e., they’re employed by a corporation they own, and not employed by the hospital or another large entity). In this structure, new physicians typically have a lower salary until they become a partner, usually after a year or two with the group. Becoming partner could require buying into the practice with tens of thousands of dollars, and might include the opportunity to share in future profit distributions. (Although buying into physician practices has been a common and appropriate model for decades, I think a new hospitalist should think carefully about whether what they will own after buying in is really worth the cost of the buy-in.)
Another exception is when hospitalists are part of a large multi-specialty physician group that offers multiple tenure-based salary increases for all physicians in all specialties. It probably makes sense to structure the hospitalist compensation plan the same way. But remember, it might take several years for primary-care physicians and surgeons to build robust patient populations and referral streams, and annual increases in salary for the first five years might mirror the pace by which a typical doctor builds his or her practice.
A new hospitalist, even one just out of residency, has an essentially mature referral stream within days of starting. So a five-year, tenure-based increase in salary could look more like the practice is simply underpaying the hospitalist until their fifth anniversary with the practice.
Automatic Increases
Some hospitalists have automatic cost-of-living salary increases. In some cases, the increases are tied to an external benchmark (e.g., the consumer price index), but it is probably more common that the future increase is simply estimated and compensation is contractually assured of increasing by a few percentage points annually.
Most hospitalists don’t have such a provision in their contracts. Indeed, most working Americans with salaries in the hospitalist range and higher don’t have cost-of-living increases. Instead, future salary adjustments are made based on market data, such as the results of salary surveys. TH
Dr. Nelson has been a hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Undercover, MD
I had a baby. OK, that may not be entirely accurate: My wife, ever the stickler for details, likes to irritatingly point out that she had the baby and I just stood around, unearthing innovative means to get in the way while nervously asking inappropriate questions seemingly aimed only at annoying the hospital staff.
A baby was had, nonetheless.
And while that is remarkable, the really notable story is what happened during our hospital stay.
An Inauspicious Start
It’s hard to read a newspaper, view a television news program, or have a conversation in public without knowing the situation: The U.S. healthcare system is in shambles—nearly 50 million uninsured, 16% of GDP spent on healthcare, and a World Health Organization (WHO) overall ranking of 37th in the world based on multiple health indicators. And, of course, there’s the thorny data suggesting that as many as 98,000 patients die annually from hospital-induced medical errors.
As such, I was prepared and ever vigilant for this admission. This was my chance to see, from the patient’s perspective, this massive, impersonal, error-riddled, sputtering system. I would expose it for what it was, and take names.
We started our odyssey a bit early, as these things are wont to happen—two weeks early, in fact. A somewhat inauspicious start, some would say. Admittedly, that’s what my buddy grumbled as I called him 90 minutes before the first pitch of Game 4 of the National League Divisional Series to tell him he’d have to watch the Phillies play the Rockies without me.
The Death Trap Beckons
My wife went to check in while I parked the car. Well, sort of park the car. It turns out this unwelcoming beast known as “the hospital” actually employed valets to park my car. “Parking is the last thing you need to worry about right now,” the attendant said. I, however, saw through this ruse. Aaron—according to his nametag—is up to no good, or so I thought, counting the pennies in my ashtray.
After checking in, the first person my wife and I encountered was Jane, our nurse. She came in all bubbly, effusing that “things will go well and you’ll be with your new addition very soon.” All heart-warming encouragement and smiles aside, she actually appeared unaware of this death trap cavorting as a hospital—a jumbo jet’s worth of patients dying from medical errors every day is no laughing matter, missy. “I’ve got my eye on you,” I whispered conspiratorially to no one; a knowing smirk appeared as I took down her name.
Later, this Jane would interrupt my attempts to make my wife laugh through expertly executed 1980s dance moves. Jane implored her to just “let it out and cry for a few minutes—then you’ll feel better.” Somewhat embarrassingly, this automaton was so caught up in her own medical world that she misinterpreted my wife’s tears of laughter for tears of apprehension. Oh, how misguided these medical personnel can be. “Stick to the nursing and I’ll comfort my wife,” I thought to myself. My wife, meanwhile, was in the process of “letting it out,” after which she did indeed report feeling better. Oh, she’s good. If I didn’t know her so well, she would have led me to believe Nurse Jane’s advice was sage, caring, and spot-on. I wasn’t fooled; my wife’s a sucker for my dance moves.
Procedural Missteps: I’ll Be Outside
Next up: Steve, a second-year anesthesia resident intent on plunging a catheter into my wife’s spine for pain relief. Little did young Steve know that I was on to him and his attempts to give my wife Brown-Séquard syndrome. After all, procedures are a common source of hospital error. Under the guise of “informed consent,” he spouted the pros and cons of the procedure, all the while failing to mention the near certainty with which my wife would develop paralysis from a spinal hematoma.
Of course, I am no boob. I relentlessly exposed this neophyte with my knowing questions, finishing the undressing with a curt “there is no way you’re coming near my wife with that needle!” To which my wife, in her ever-tender way, ordered me to get the hell out of the room so she could get some (unprintable expletive) pain relief. See, this young doctor was getting to her as well.
Undeterred, I continued to prowl for medical errors. Knowing communication to be fertile ground for hospital slip-ups, I watched intently the handoff of care the day nurse, Jane, gave to the night nurse, Sarah. Surprisingly, Jane appeared to get it all right—at least that’s how it would appear to the untrained eye. She succinctly overviewed our history and course, documented the medications my wife received, gave the plan, and told her what to do if things didn’t follow that plan. Sarah repeated back the salient points as she reviewed the written chart and asked a few questions.
All good on the surface, but she spent so much time doting over my wife’s emotional needs that she failed to notice my discontent over the Rockies’ series-ending loss to the Phillies. I helpfully pointed out this blunder to her, at which time my wife rolled her eyes and asked, “Why can’t guys have babies?” Assuming my wife missed that class in medical school, I immediately began to overview basic reproductive physiology, at which point I was again asked to leave the room—this time by Nurse Sarah.
Waiting-Room Reflections
Not one to be satisfied with my victories, I stalked the halls looking for a less-than-15-second hand wash or an HIPAA violation. Seeing none, I pondered my wife’s fate at the hands of this massive, impersonal, error-riddled healthcare machine. I just couldn’t surmise a scenario in which she’d get out of there alive.
Just then, Susan, our obstetrician, came out to let me know that my daughter would soon be making her entrance. She informed me that it would be OK to film the procedure, if I liked. “But what about malpractice? Aren’t you worried about having this on film?” I asked. “Not a bit,” she replied. “What I’m worried about is your horsing around causing us both to miss this delivery.”
With that, we were back in the room, reunited in our common purpose. Moments later, I was the proud owner of a freshly minted baby girl.
Putting down the video camera, the state of the healthcare system finally came into focus. To be certain, we have our problems. But this wasn’t the massive, impersonal, sputtering system I was led to expect. Rather, I found it filled with caring, compassionate, highly skilled professionals with names like Aaron, Jane, Sarah, Steve, and Susan. Together, they had engineered a true miracle.
A miracle named Kaiya. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
I had a baby. OK, that may not be entirely accurate: My wife, ever the stickler for details, likes to irritatingly point out that she had the baby and I just stood around, unearthing innovative means to get in the way while nervously asking inappropriate questions seemingly aimed only at annoying the hospital staff.
A baby was had, nonetheless.
And while that is remarkable, the really notable story is what happened during our hospital stay.
An Inauspicious Start
It’s hard to read a newspaper, view a television news program, or have a conversation in public without knowing the situation: The U.S. healthcare system is in shambles—nearly 50 million uninsured, 16% of GDP spent on healthcare, and a World Health Organization (WHO) overall ranking of 37th in the world based on multiple health indicators. And, of course, there’s the thorny data suggesting that as many as 98,000 patients die annually from hospital-induced medical errors.
As such, I was prepared and ever vigilant for this admission. This was my chance to see, from the patient’s perspective, this massive, impersonal, error-riddled, sputtering system. I would expose it for what it was, and take names.
We started our odyssey a bit early, as these things are wont to happen—two weeks early, in fact. A somewhat inauspicious start, some would say. Admittedly, that’s what my buddy grumbled as I called him 90 minutes before the first pitch of Game 4 of the National League Divisional Series to tell him he’d have to watch the Phillies play the Rockies without me.
The Death Trap Beckons
My wife went to check in while I parked the car. Well, sort of park the car. It turns out this unwelcoming beast known as “the hospital” actually employed valets to park my car. “Parking is the last thing you need to worry about right now,” the attendant said. I, however, saw through this ruse. Aaron—according to his nametag—is up to no good, or so I thought, counting the pennies in my ashtray.
After checking in, the first person my wife and I encountered was Jane, our nurse. She came in all bubbly, effusing that “things will go well and you’ll be with your new addition very soon.” All heart-warming encouragement and smiles aside, she actually appeared unaware of this death trap cavorting as a hospital—a jumbo jet’s worth of patients dying from medical errors every day is no laughing matter, missy. “I’ve got my eye on you,” I whispered conspiratorially to no one; a knowing smirk appeared as I took down her name.
Later, this Jane would interrupt my attempts to make my wife laugh through expertly executed 1980s dance moves. Jane implored her to just “let it out and cry for a few minutes—then you’ll feel better.” Somewhat embarrassingly, this automaton was so caught up in her own medical world that she misinterpreted my wife’s tears of laughter for tears of apprehension. Oh, how misguided these medical personnel can be. “Stick to the nursing and I’ll comfort my wife,” I thought to myself. My wife, meanwhile, was in the process of “letting it out,” after which she did indeed report feeling better. Oh, she’s good. If I didn’t know her so well, she would have led me to believe Nurse Jane’s advice was sage, caring, and spot-on. I wasn’t fooled; my wife’s a sucker for my dance moves.
Procedural Missteps: I’ll Be Outside
Next up: Steve, a second-year anesthesia resident intent on plunging a catheter into my wife’s spine for pain relief. Little did young Steve know that I was on to him and his attempts to give my wife Brown-Séquard syndrome. After all, procedures are a common source of hospital error. Under the guise of “informed consent,” he spouted the pros and cons of the procedure, all the while failing to mention the near certainty with which my wife would develop paralysis from a spinal hematoma.
Of course, I am no boob. I relentlessly exposed this neophyte with my knowing questions, finishing the undressing with a curt “there is no way you’re coming near my wife with that needle!” To which my wife, in her ever-tender way, ordered me to get the hell out of the room so she could get some (unprintable expletive) pain relief. See, this young doctor was getting to her as well.
Undeterred, I continued to prowl for medical errors. Knowing communication to be fertile ground for hospital slip-ups, I watched intently the handoff of care the day nurse, Jane, gave to the night nurse, Sarah. Surprisingly, Jane appeared to get it all right—at least that’s how it would appear to the untrained eye. She succinctly overviewed our history and course, documented the medications my wife received, gave the plan, and told her what to do if things didn’t follow that plan. Sarah repeated back the salient points as she reviewed the written chart and asked a few questions.
All good on the surface, but she spent so much time doting over my wife’s emotional needs that she failed to notice my discontent over the Rockies’ series-ending loss to the Phillies. I helpfully pointed out this blunder to her, at which time my wife rolled her eyes and asked, “Why can’t guys have babies?” Assuming my wife missed that class in medical school, I immediately began to overview basic reproductive physiology, at which point I was again asked to leave the room—this time by Nurse Sarah.
Waiting-Room Reflections
Not one to be satisfied with my victories, I stalked the halls looking for a less-than-15-second hand wash or an HIPAA violation. Seeing none, I pondered my wife’s fate at the hands of this massive, impersonal, error-riddled healthcare machine. I just couldn’t surmise a scenario in which she’d get out of there alive.
Just then, Susan, our obstetrician, came out to let me know that my daughter would soon be making her entrance. She informed me that it would be OK to film the procedure, if I liked. “But what about malpractice? Aren’t you worried about having this on film?” I asked. “Not a bit,” she replied. “What I’m worried about is your horsing around causing us both to miss this delivery.”
With that, we were back in the room, reunited in our common purpose. Moments later, I was the proud owner of a freshly minted baby girl.
Putting down the video camera, the state of the healthcare system finally came into focus. To be certain, we have our problems. But this wasn’t the massive, impersonal, sputtering system I was led to expect. Rather, I found it filled with caring, compassionate, highly skilled professionals with names like Aaron, Jane, Sarah, Steve, and Susan. Together, they had engineered a true miracle.
A miracle named Kaiya. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
I had a baby. OK, that may not be entirely accurate: My wife, ever the stickler for details, likes to irritatingly point out that she had the baby and I just stood around, unearthing innovative means to get in the way while nervously asking inappropriate questions seemingly aimed only at annoying the hospital staff.
A baby was had, nonetheless.
And while that is remarkable, the really notable story is what happened during our hospital stay.
An Inauspicious Start
It’s hard to read a newspaper, view a television news program, or have a conversation in public without knowing the situation: The U.S. healthcare system is in shambles—nearly 50 million uninsured, 16% of GDP spent on healthcare, and a World Health Organization (WHO) overall ranking of 37th in the world based on multiple health indicators. And, of course, there’s the thorny data suggesting that as many as 98,000 patients die annually from hospital-induced medical errors.
As such, I was prepared and ever vigilant for this admission. This was my chance to see, from the patient’s perspective, this massive, impersonal, error-riddled, sputtering system. I would expose it for what it was, and take names.
We started our odyssey a bit early, as these things are wont to happen—two weeks early, in fact. A somewhat inauspicious start, some would say. Admittedly, that’s what my buddy grumbled as I called him 90 minutes before the first pitch of Game 4 of the National League Divisional Series to tell him he’d have to watch the Phillies play the Rockies without me.
The Death Trap Beckons
My wife went to check in while I parked the car. Well, sort of park the car. It turns out this unwelcoming beast known as “the hospital” actually employed valets to park my car. “Parking is the last thing you need to worry about right now,” the attendant said. I, however, saw through this ruse. Aaron—according to his nametag—is up to no good, or so I thought, counting the pennies in my ashtray.
After checking in, the first person my wife and I encountered was Jane, our nurse. She came in all bubbly, effusing that “things will go well and you’ll be with your new addition very soon.” All heart-warming encouragement and smiles aside, she actually appeared unaware of this death trap cavorting as a hospital—a jumbo jet’s worth of patients dying from medical errors every day is no laughing matter, missy. “I’ve got my eye on you,” I whispered conspiratorially to no one; a knowing smirk appeared as I took down her name.
Later, this Jane would interrupt my attempts to make my wife laugh through expertly executed 1980s dance moves. Jane implored her to just “let it out and cry for a few minutes—then you’ll feel better.” Somewhat embarrassingly, this automaton was so caught up in her own medical world that she misinterpreted my wife’s tears of laughter for tears of apprehension. Oh, how misguided these medical personnel can be. “Stick to the nursing and I’ll comfort my wife,” I thought to myself. My wife, meanwhile, was in the process of “letting it out,” after which she did indeed report feeling better. Oh, she’s good. If I didn’t know her so well, she would have led me to believe Nurse Jane’s advice was sage, caring, and spot-on. I wasn’t fooled; my wife’s a sucker for my dance moves.
Procedural Missteps: I’ll Be Outside
Next up: Steve, a second-year anesthesia resident intent on plunging a catheter into my wife’s spine for pain relief. Little did young Steve know that I was on to him and his attempts to give my wife Brown-Séquard syndrome. After all, procedures are a common source of hospital error. Under the guise of “informed consent,” he spouted the pros and cons of the procedure, all the while failing to mention the near certainty with which my wife would develop paralysis from a spinal hematoma.
Of course, I am no boob. I relentlessly exposed this neophyte with my knowing questions, finishing the undressing with a curt “there is no way you’re coming near my wife with that needle!” To which my wife, in her ever-tender way, ordered me to get the hell out of the room so she could get some (unprintable expletive) pain relief. See, this young doctor was getting to her as well.
Undeterred, I continued to prowl for medical errors. Knowing communication to be fertile ground for hospital slip-ups, I watched intently the handoff of care the day nurse, Jane, gave to the night nurse, Sarah. Surprisingly, Jane appeared to get it all right—at least that’s how it would appear to the untrained eye. She succinctly overviewed our history and course, documented the medications my wife received, gave the plan, and told her what to do if things didn’t follow that plan. Sarah repeated back the salient points as she reviewed the written chart and asked a few questions.
All good on the surface, but she spent so much time doting over my wife’s emotional needs that she failed to notice my discontent over the Rockies’ series-ending loss to the Phillies. I helpfully pointed out this blunder to her, at which time my wife rolled her eyes and asked, “Why can’t guys have babies?” Assuming my wife missed that class in medical school, I immediately began to overview basic reproductive physiology, at which point I was again asked to leave the room—this time by Nurse Sarah.
Waiting-Room Reflections
Not one to be satisfied with my victories, I stalked the halls looking for a less-than-15-second hand wash or an HIPAA violation. Seeing none, I pondered my wife’s fate at the hands of this massive, impersonal, error-riddled healthcare machine. I just couldn’t surmise a scenario in which she’d get out of there alive.
Just then, Susan, our obstetrician, came out to let me know that my daughter would soon be making her entrance. She informed me that it would be OK to film the procedure, if I liked. “But what about malpractice? Aren’t you worried about having this on film?” I asked. “Not a bit,” she replied. “What I’m worried about is your horsing around causing us both to miss this delivery.”
With that, we were back in the room, reunited in our common purpose. Moments later, I was the proud owner of a freshly minted baby girl.
Putting down the video camera, the state of the healthcare system finally came into focus. To be certain, we have our problems. But this wasn’t the massive, impersonal, sputtering system I was led to expect. Rather, I found it filled with caring, compassionate, highly skilled professionals with names like Aaron, Jane, Sarah, Steve, and Susan. Together, they had engineered a true miracle.
A miracle named Kaiya. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
A Watershed Moment
The announcement was made Sept. 23, 2009. The American Board of Medical Specialties (ABMS) had approved a “pilot program” for Recognition of Focused Practice (RFP) in Hospital Medicine. ABMS-sanctioned board certification for hospitalists had finally arrived.
When the announcement came, I was at the University of California at San Francisco’s CME conference with 600 other hospitalists. Robert Wachter, MD, FHM, chief of the hospital medicine division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World, announced the RFP in HM to the audience—it received resounding applause. Dr. Wachter, the UCSF conference chair, then went on to recap the history behind RFP and how we got here. As he spoke, I realized that I had forgotten how long this process had taken and how much work had been done by so many people.
To practicing hospitalists, the creation of some sort of certification in HM seems like the proverbial no-brainer. But think about it from the American Board of Internal Medicine (ABIM) perspective: All “specialties” with board certification have training programs or fellowships that help define them. We do not have hospitalist residency programs or fellowships that are necessary for practicing as a hospitalist. Our expertise is acquired during practice.
So if the training path for hospitalists is not different—at least for now—from that of other general internists, how do we create board certification? When a group, such as hospitalists, “asks” to be recognized as a specialty with their own certification, the ABIM needs to consider the implications of creating such recognition. For hospitalists, the question was: If we do this for hospitalists, do we have to create a certificate for officists or outpatient physicians? For the broader questions around recognition of focused practice, the board needed to ask, “What if a group comes forward and asks for recognition of their focus and expertise in caring for diabetes or sepsis?”
ABIM thought very carefully about these questions. For the latter, ABIM says the field asking for recognition of focused practice must have a lot of physicians who only practice in this field while also having large numbers of physicians who never practice in the field. And they said, “Yes, if we do this for hospitalists, we will ultimately need to create a similar pathway for outpatient physicians.” Fortunately for us, they did not wait for RFP in outpatient medicine to be developed before proceeding with RFP for HM. (Figuring out how to do this on the outpatient side is even harder, and even though ABIM has been very supportive of RFP for hospitalists, the ABMS, which oversees all the specialty boards, still had to sign off on this approach. In September, they did.)
RFP Formula
So what will RFP in HM look like? The process is described on ABIM’s Web site (www.abim.org/news/news/focused-practice-hospital-medicine-qa.aspx), but to qualify, a hospitalist must:
- Be certified in internal medicine;
- Have practiced for a sufficient period of time to have achieved certain volume thresholds for patients with inpatient diagnoses;
- Participate in hospitalist-based practice improvement and self-assessment modules; and
- Pass a secure exam, which SHM president-elect Jeff Wiese, MD, FHM, and his committee have been hard at work in creating.
All told, the bar has been set high. And from what I hear, the exam has a strong QI focus—a discipline most residency programs skimp on when designing their curriculums.
SHM recognizes that we will have to ramp up our educational efforts to prepare hospitalists for RFP. We will need to develop QI assessment modules, continue developing and enhancing QI education at our annual meeting, and we’ll need to consider more regional efforts at providing the education hospitalists need to do this QI work in their hospitals.
A Bright Future
So what does this really mean for our field? Quite a bit, actually.
We have long tracked all the elements traditionally required to call HM a specialty, and one by one we have ticked them off: large numbers of physicians in the field, a separate body of knowledge (i.e., core competencies), textbooks, a journal, national meetings, training programs. But the one element that remained unchecked was a separate certification. Cross that one off the list. Granted, training programs remain few and underdeveloped, but I expect that, too, will change and grow over time.
There are additional questions raised by this new certification. One might ask: “Will hospitals or payors require RFP in HM in order to practice or be paid for what we do?” Maybe. I expect this will not happen rapidly, as many hospitalists are likely to wait until they need to recertify before choosing to pursue RFP in HM. But if all of our colleagues start getting certified, or programs hiring hospitalists begin to require this certification (e.g., advertise the fact that all of their physicians are “certified hospitalists”), the floodgates will open.
Questions also arise about what happens now for family practice or pediatrics. Well, I am pleased to say that the American Board of Family Medicine (ABFM) has announced its intent to collaborate with ABIM to establish a similar pilot program for RFP in HM for family-practice physicians. And the pediatric societies and board-certifying agencies are watching closely to see if they might consider the same approach for pediatric hospitalists. Stay tuned.
ABMS’ recent approval of the RFP pathway is what many have been working tirelessly toward for years. It is a testament to the maturity, breadth, and importance of our field. And it validates what many of us have known for years: Hospitalists have unique skill sets that are vital to the U.S. healthcare system, and, as a result, we have been integrated into the fabric of hospital care delivery in every state.
With the coming redesign of the healthcare system, hospitalists will be counted on to lead and facilitate the work required to deliver high-quality, efficient hospital care across the country. RFP provides both a means to get hospitalists up to speed to do this important work and recognizes those who are ready to go out and lead.
This truly is a watershed moment for HM. TH
Dr. Flanders is president of SHM.
The announcement was made Sept. 23, 2009. The American Board of Medical Specialties (ABMS) had approved a “pilot program” for Recognition of Focused Practice (RFP) in Hospital Medicine. ABMS-sanctioned board certification for hospitalists had finally arrived.
When the announcement came, I was at the University of California at San Francisco’s CME conference with 600 other hospitalists. Robert Wachter, MD, FHM, chief of the hospital medicine division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World, announced the RFP in HM to the audience—it received resounding applause. Dr. Wachter, the UCSF conference chair, then went on to recap the history behind RFP and how we got here. As he spoke, I realized that I had forgotten how long this process had taken and how much work had been done by so many people.
To practicing hospitalists, the creation of some sort of certification in HM seems like the proverbial no-brainer. But think about it from the American Board of Internal Medicine (ABIM) perspective: All “specialties” with board certification have training programs or fellowships that help define them. We do not have hospitalist residency programs or fellowships that are necessary for practicing as a hospitalist. Our expertise is acquired during practice.
So if the training path for hospitalists is not different—at least for now—from that of other general internists, how do we create board certification? When a group, such as hospitalists, “asks” to be recognized as a specialty with their own certification, the ABIM needs to consider the implications of creating such recognition. For hospitalists, the question was: If we do this for hospitalists, do we have to create a certificate for officists or outpatient physicians? For the broader questions around recognition of focused practice, the board needed to ask, “What if a group comes forward and asks for recognition of their focus and expertise in caring for diabetes or sepsis?”
ABIM thought very carefully about these questions. For the latter, ABIM says the field asking for recognition of focused practice must have a lot of physicians who only practice in this field while also having large numbers of physicians who never practice in the field. And they said, “Yes, if we do this for hospitalists, we will ultimately need to create a similar pathway for outpatient physicians.” Fortunately for us, they did not wait for RFP in outpatient medicine to be developed before proceeding with RFP for HM. (Figuring out how to do this on the outpatient side is even harder, and even though ABIM has been very supportive of RFP for hospitalists, the ABMS, which oversees all the specialty boards, still had to sign off on this approach. In September, they did.)
RFP Formula
So what will RFP in HM look like? The process is described on ABIM’s Web site (www.abim.org/news/news/focused-practice-hospital-medicine-qa.aspx), but to qualify, a hospitalist must:
- Be certified in internal medicine;
- Have practiced for a sufficient period of time to have achieved certain volume thresholds for patients with inpatient diagnoses;
- Participate in hospitalist-based practice improvement and self-assessment modules; and
- Pass a secure exam, which SHM president-elect Jeff Wiese, MD, FHM, and his committee have been hard at work in creating.
All told, the bar has been set high. And from what I hear, the exam has a strong QI focus—a discipline most residency programs skimp on when designing their curriculums.
SHM recognizes that we will have to ramp up our educational efforts to prepare hospitalists for RFP. We will need to develop QI assessment modules, continue developing and enhancing QI education at our annual meeting, and we’ll need to consider more regional efforts at providing the education hospitalists need to do this QI work in their hospitals.
A Bright Future
So what does this really mean for our field? Quite a bit, actually.
We have long tracked all the elements traditionally required to call HM a specialty, and one by one we have ticked them off: large numbers of physicians in the field, a separate body of knowledge (i.e., core competencies), textbooks, a journal, national meetings, training programs. But the one element that remained unchecked was a separate certification. Cross that one off the list. Granted, training programs remain few and underdeveloped, but I expect that, too, will change and grow over time.
There are additional questions raised by this new certification. One might ask: “Will hospitals or payors require RFP in HM in order to practice or be paid for what we do?” Maybe. I expect this will not happen rapidly, as many hospitalists are likely to wait until they need to recertify before choosing to pursue RFP in HM. But if all of our colleagues start getting certified, or programs hiring hospitalists begin to require this certification (e.g., advertise the fact that all of their physicians are “certified hospitalists”), the floodgates will open.
Questions also arise about what happens now for family practice or pediatrics. Well, I am pleased to say that the American Board of Family Medicine (ABFM) has announced its intent to collaborate with ABIM to establish a similar pilot program for RFP in HM for family-practice physicians. And the pediatric societies and board-certifying agencies are watching closely to see if they might consider the same approach for pediatric hospitalists. Stay tuned.
ABMS’ recent approval of the RFP pathway is what many have been working tirelessly toward for years. It is a testament to the maturity, breadth, and importance of our field. And it validates what many of us have known for years: Hospitalists have unique skill sets that are vital to the U.S. healthcare system, and, as a result, we have been integrated into the fabric of hospital care delivery in every state.
With the coming redesign of the healthcare system, hospitalists will be counted on to lead and facilitate the work required to deliver high-quality, efficient hospital care across the country. RFP provides both a means to get hospitalists up to speed to do this important work and recognizes those who are ready to go out and lead.
This truly is a watershed moment for HM. TH
Dr. Flanders is president of SHM.
The announcement was made Sept. 23, 2009. The American Board of Medical Specialties (ABMS) had approved a “pilot program” for Recognition of Focused Practice (RFP) in Hospital Medicine. ABMS-sanctioned board certification for hospitalists had finally arrived.
When the announcement came, I was at the University of California at San Francisco’s CME conference with 600 other hospitalists. Robert Wachter, MD, FHM, chief of the hospital medicine division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World, announced the RFP in HM to the audience—it received resounding applause. Dr. Wachter, the UCSF conference chair, then went on to recap the history behind RFP and how we got here. As he spoke, I realized that I had forgotten how long this process had taken and how much work had been done by so many people.
To practicing hospitalists, the creation of some sort of certification in HM seems like the proverbial no-brainer. But think about it from the American Board of Internal Medicine (ABIM) perspective: All “specialties” with board certification have training programs or fellowships that help define them. We do not have hospitalist residency programs or fellowships that are necessary for practicing as a hospitalist. Our expertise is acquired during practice.
So if the training path for hospitalists is not different—at least for now—from that of other general internists, how do we create board certification? When a group, such as hospitalists, “asks” to be recognized as a specialty with their own certification, the ABIM needs to consider the implications of creating such recognition. For hospitalists, the question was: If we do this for hospitalists, do we have to create a certificate for officists or outpatient physicians? For the broader questions around recognition of focused practice, the board needed to ask, “What if a group comes forward and asks for recognition of their focus and expertise in caring for diabetes or sepsis?”
ABIM thought very carefully about these questions. For the latter, ABIM says the field asking for recognition of focused practice must have a lot of physicians who only practice in this field while also having large numbers of physicians who never practice in the field. And they said, “Yes, if we do this for hospitalists, we will ultimately need to create a similar pathway for outpatient physicians.” Fortunately for us, they did not wait for RFP in outpatient medicine to be developed before proceeding with RFP for HM. (Figuring out how to do this on the outpatient side is even harder, and even though ABIM has been very supportive of RFP for hospitalists, the ABMS, which oversees all the specialty boards, still had to sign off on this approach. In September, they did.)
RFP Formula
So what will RFP in HM look like? The process is described on ABIM’s Web site (www.abim.org/news/news/focused-practice-hospital-medicine-qa.aspx), but to qualify, a hospitalist must:
- Be certified in internal medicine;
- Have practiced for a sufficient period of time to have achieved certain volume thresholds for patients with inpatient diagnoses;
- Participate in hospitalist-based practice improvement and self-assessment modules; and
- Pass a secure exam, which SHM president-elect Jeff Wiese, MD, FHM, and his committee have been hard at work in creating.
All told, the bar has been set high. And from what I hear, the exam has a strong QI focus—a discipline most residency programs skimp on when designing their curriculums.
SHM recognizes that we will have to ramp up our educational efforts to prepare hospitalists for RFP. We will need to develop QI assessment modules, continue developing and enhancing QI education at our annual meeting, and we’ll need to consider more regional efforts at providing the education hospitalists need to do this QI work in their hospitals.
A Bright Future
So what does this really mean for our field? Quite a bit, actually.
We have long tracked all the elements traditionally required to call HM a specialty, and one by one we have ticked them off: large numbers of physicians in the field, a separate body of knowledge (i.e., core competencies), textbooks, a journal, national meetings, training programs. But the one element that remained unchecked was a separate certification. Cross that one off the list. Granted, training programs remain few and underdeveloped, but I expect that, too, will change and grow over time.
There are additional questions raised by this new certification. One might ask: “Will hospitals or payors require RFP in HM in order to practice or be paid for what we do?” Maybe. I expect this will not happen rapidly, as many hospitalists are likely to wait until they need to recertify before choosing to pursue RFP in HM. But if all of our colleagues start getting certified, or programs hiring hospitalists begin to require this certification (e.g., advertise the fact that all of their physicians are “certified hospitalists”), the floodgates will open.
Questions also arise about what happens now for family practice or pediatrics. Well, I am pleased to say that the American Board of Family Medicine (ABFM) has announced its intent to collaborate with ABIM to establish a similar pilot program for RFP in HM for family-practice physicians. And the pediatric societies and board-certifying agencies are watching closely to see if they might consider the same approach for pediatric hospitalists. Stay tuned.
ABMS’ recent approval of the RFP pathway is what many have been working tirelessly toward for years. It is a testament to the maturity, breadth, and importance of our field. And it validates what many of us have known for years: Hospitalists have unique skill sets that are vital to the U.S. healthcare system, and, as a result, we have been integrated into the fabric of hospital care delivery in every state.
With the coming redesign of the healthcare system, hospitalists will be counted on to lead and facilitate the work required to deliver high-quality, efficient hospital care across the country. RFP provides both a means to get hospitalists up to speed to do this important work and recognizes those who are ready to go out and lead.
This truly is a watershed moment for HM. TH
Dr. Flanders is president of SHM.
(Fish) Food for Thought
The putrid smell of vomit wafted behind me, flowing in and out of my nostrils with each up and down of our boat. Two in our deep-sea-fishing party already had lost their breakfast; I was focused on keeping mine down. The ocean seemed fairly calm, but I didn’t feel very steady. In fact, I felt like I was on a bamboo raft that had been tied together with palm fronds.
In between thoughts of how I would have been ostracized as a seafaring Polynesian, I had one thought on my mind. “Keep your eyes on the horizon,” our captain had said as we boarded the boat. My eyes were not going anywhere else that day. The horizon, whether the coastline of Oahu or just the thin line between ocean blue and sky blue, provided an unwavering constant as the waves changed our position minute by minute.
Our daily work as hospitalists is filled with ups and downs—waves, if you will. At times they threaten to capsize us; at others, they provide a short boost of momentum. These waves come in many forms, whether a busy teaching service, an interaction with a consultant, or your personal schedule. And all too often, that constant cyclical motion becomes hypnotizing. All of us have encountered colleagues that get lost at sea; they seem to always focus on that constant sense of unsteadiness. We recognize this form of despair as whining, and it’s not far removed from motion sickness. The only difference is the specific sense that is assaulted when the victim can no longer handle the ride.
Chart a Course to Success
If the captain of our fishing charter had been a business instructor, the lesson for the day would have been strategic planning. If he had been a medical school professor—well, there probably is no suitable analogy, as the path to organizational success isn’t yet a part of our core curriculum. Strategic planning is the deceptively simple process by which you ensure that you are headed toward your ultimate vision; it’s how you, your group, or your field charts its course toward the horizon.
Medicine has been in the habit of learning from business lately. Toyota’s strategy is a prime example. Their core strategic plan is termed “Lean” production or practices. Continuous quality improvement, though an oversimplification, is a substitute phrase that all hospitalists should recognize. Amazingly, Toyota’s strategic plan extends 50 to 100 years into the future and is intertwined into each and every phase of the company. Although the Lean system is being carefully studied and applied by many in the healthcare industry, the true hidden curriculum lies not in the details of their practices, but rather in their choice and execution of strategy. Toyota’s impressive history of achievement contains a few valuable lessons applicable to your own future success.
At one time, Toyota was a newcomer to the established field of automobile manufacturing, not dissimilar to the current state of most pediatric hospitalists. Like us, they undoubtedly faced uphill battles surrounding established cultural barriers and rigid practice patterns. And despite giving up more than half a century to Ford and the concept of mass production, Toyota has become the leading manufacturer of automobiles in the world.
How did Toyota choose and execute a strategy that allowed it to thrive in the face of such obstacles? In the beginning, there probably were many strategic options. They could have decided to focus on creating a specific product, such as the “ultimate driving machine,” or cars that are boxy but safe. They could have opted to cater to a specific consumer class, perhaps building a strong fleet of affordable autos. Or they could have looked to improve their purchasing power and distribution methods (think Dell and Walmart).
Instead, they made a conscious decision to pursue excellence in reliability, quality, and value (sound familiar?), then followed through beautifully.
Strategy at Home
Despite differences in industry and scale, all of these same sorts of decisions are critical to the success of your career, your HM group, or even the field of pediatric HM. Are you aware of the specific strategies in place for your group’s success? Have you been involved in the process? Before this year, I was probably like most of you. I had some vague notion of success. It involved increasing relative value units, making everyone happy, and completing a big QI or research project.
In the past 12 months, however, I have taken part in three strategic planning sessions: one for a regional pediatric society, one for my hospital, and one for my hospitalist group. The importance of these processes crystallized for me. Apparently, the leaders in our field have had the same thoughts. They convened the Pediatric Hospital Medicine Roundtable, a strategic planning session for our field (see “All Grown Up,” p. 1). Clearly, 2009 is the year of the strategic plan.
Despite the unifying theme, the processes and products of all of these plans have been unique. Strategic plans must be developed organically, out of local context and environment, and can only be created by those who live and breathe the work. What works for group safety at the university hospital of quality focus might not work for group communication experts at suburban community hospitals. Differences in institutional, organizational, and cultural beliefs should affect the decision-making process. When a strategy has been devised, it should be carefully chosen and explicitly implemented.
Does your group’s strategy come to mind? Or are you just treading water, unable to see beyond the next looming wave? If you have a vision of what you want, whether it’s money, fame, or protected time, then this same line of reasoning should apply to the strategic plan for your individual career, as well as the future of pediatric HM.
The lesson here is simple: Success requires a plan. Strategic planning is how you set a vision for the future and chart that course. Unexpected political waves are sure to come, and not every victory will come with a prize catch. But if you can create that beautiful Impressionist painting on the horizon and maintain that course, you are less likely to lose your breakfast and go without lunch. TH
Dr. Shen is The Hospitalist’s pediatric editor.
The putrid smell of vomit wafted behind me, flowing in and out of my nostrils with each up and down of our boat. Two in our deep-sea-fishing party already had lost their breakfast; I was focused on keeping mine down. The ocean seemed fairly calm, but I didn’t feel very steady. In fact, I felt like I was on a bamboo raft that had been tied together with palm fronds.
In between thoughts of how I would have been ostracized as a seafaring Polynesian, I had one thought on my mind. “Keep your eyes on the horizon,” our captain had said as we boarded the boat. My eyes were not going anywhere else that day. The horizon, whether the coastline of Oahu or just the thin line between ocean blue and sky blue, provided an unwavering constant as the waves changed our position minute by minute.
Our daily work as hospitalists is filled with ups and downs—waves, if you will. At times they threaten to capsize us; at others, they provide a short boost of momentum. These waves come in many forms, whether a busy teaching service, an interaction with a consultant, or your personal schedule. And all too often, that constant cyclical motion becomes hypnotizing. All of us have encountered colleagues that get lost at sea; they seem to always focus on that constant sense of unsteadiness. We recognize this form of despair as whining, and it’s not far removed from motion sickness. The only difference is the specific sense that is assaulted when the victim can no longer handle the ride.
Chart a Course to Success
If the captain of our fishing charter had been a business instructor, the lesson for the day would have been strategic planning. If he had been a medical school professor—well, there probably is no suitable analogy, as the path to organizational success isn’t yet a part of our core curriculum. Strategic planning is the deceptively simple process by which you ensure that you are headed toward your ultimate vision; it’s how you, your group, or your field charts its course toward the horizon.
Medicine has been in the habit of learning from business lately. Toyota’s strategy is a prime example. Their core strategic plan is termed “Lean” production or practices. Continuous quality improvement, though an oversimplification, is a substitute phrase that all hospitalists should recognize. Amazingly, Toyota’s strategic plan extends 50 to 100 years into the future and is intertwined into each and every phase of the company. Although the Lean system is being carefully studied and applied by many in the healthcare industry, the true hidden curriculum lies not in the details of their practices, but rather in their choice and execution of strategy. Toyota’s impressive history of achievement contains a few valuable lessons applicable to your own future success.
At one time, Toyota was a newcomer to the established field of automobile manufacturing, not dissimilar to the current state of most pediatric hospitalists. Like us, they undoubtedly faced uphill battles surrounding established cultural barriers and rigid practice patterns. And despite giving up more than half a century to Ford and the concept of mass production, Toyota has become the leading manufacturer of automobiles in the world.
How did Toyota choose and execute a strategy that allowed it to thrive in the face of such obstacles? In the beginning, there probably were many strategic options. They could have decided to focus on creating a specific product, such as the “ultimate driving machine,” or cars that are boxy but safe. They could have opted to cater to a specific consumer class, perhaps building a strong fleet of affordable autos. Or they could have looked to improve their purchasing power and distribution methods (think Dell and Walmart).
Instead, they made a conscious decision to pursue excellence in reliability, quality, and value (sound familiar?), then followed through beautifully.
Strategy at Home
Despite differences in industry and scale, all of these same sorts of decisions are critical to the success of your career, your HM group, or even the field of pediatric HM. Are you aware of the specific strategies in place for your group’s success? Have you been involved in the process? Before this year, I was probably like most of you. I had some vague notion of success. It involved increasing relative value units, making everyone happy, and completing a big QI or research project.
In the past 12 months, however, I have taken part in three strategic planning sessions: one for a regional pediatric society, one for my hospital, and one for my hospitalist group. The importance of these processes crystallized for me. Apparently, the leaders in our field have had the same thoughts. They convened the Pediatric Hospital Medicine Roundtable, a strategic planning session for our field (see “All Grown Up,” p. 1). Clearly, 2009 is the year of the strategic plan.
Despite the unifying theme, the processes and products of all of these plans have been unique. Strategic plans must be developed organically, out of local context and environment, and can only be created by those who live and breathe the work. What works for group safety at the university hospital of quality focus might not work for group communication experts at suburban community hospitals. Differences in institutional, organizational, and cultural beliefs should affect the decision-making process. When a strategy has been devised, it should be carefully chosen and explicitly implemented.
Does your group’s strategy come to mind? Or are you just treading water, unable to see beyond the next looming wave? If you have a vision of what you want, whether it’s money, fame, or protected time, then this same line of reasoning should apply to the strategic plan for your individual career, as well as the future of pediatric HM.
The lesson here is simple: Success requires a plan. Strategic planning is how you set a vision for the future and chart that course. Unexpected political waves are sure to come, and not every victory will come with a prize catch. But if you can create that beautiful Impressionist painting on the horizon and maintain that course, you are less likely to lose your breakfast and go without lunch. TH
Dr. Shen is The Hospitalist’s pediatric editor.
The putrid smell of vomit wafted behind me, flowing in and out of my nostrils with each up and down of our boat. Two in our deep-sea-fishing party already had lost their breakfast; I was focused on keeping mine down. The ocean seemed fairly calm, but I didn’t feel very steady. In fact, I felt like I was on a bamboo raft that had been tied together with palm fronds.
In between thoughts of how I would have been ostracized as a seafaring Polynesian, I had one thought on my mind. “Keep your eyes on the horizon,” our captain had said as we boarded the boat. My eyes were not going anywhere else that day. The horizon, whether the coastline of Oahu or just the thin line between ocean blue and sky blue, provided an unwavering constant as the waves changed our position minute by minute.
Our daily work as hospitalists is filled with ups and downs—waves, if you will. At times they threaten to capsize us; at others, they provide a short boost of momentum. These waves come in many forms, whether a busy teaching service, an interaction with a consultant, or your personal schedule. And all too often, that constant cyclical motion becomes hypnotizing. All of us have encountered colleagues that get lost at sea; they seem to always focus on that constant sense of unsteadiness. We recognize this form of despair as whining, and it’s not far removed from motion sickness. The only difference is the specific sense that is assaulted when the victim can no longer handle the ride.
Chart a Course to Success
If the captain of our fishing charter had been a business instructor, the lesson for the day would have been strategic planning. If he had been a medical school professor—well, there probably is no suitable analogy, as the path to organizational success isn’t yet a part of our core curriculum. Strategic planning is the deceptively simple process by which you ensure that you are headed toward your ultimate vision; it’s how you, your group, or your field charts its course toward the horizon.
Medicine has been in the habit of learning from business lately. Toyota’s strategy is a prime example. Their core strategic plan is termed “Lean” production or practices. Continuous quality improvement, though an oversimplification, is a substitute phrase that all hospitalists should recognize. Amazingly, Toyota’s strategic plan extends 50 to 100 years into the future and is intertwined into each and every phase of the company. Although the Lean system is being carefully studied and applied by many in the healthcare industry, the true hidden curriculum lies not in the details of their practices, but rather in their choice and execution of strategy. Toyota’s impressive history of achievement contains a few valuable lessons applicable to your own future success.
At one time, Toyota was a newcomer to the established field of automobile manufacturing, not dissimilar to the current state of most pediatric hospitalists. Like us, they undoubtedly faced uphill battles surrounding established cultural barriers and rigid practice patterns. And despite giving up more than half a century to Ford and the concept of mass production, Toyota has become the leading manufacturer of automobiles in the world.
How did Toyota choose and execute a strategy that allowed it to thrive in the face of such obstacles? In the beginning, there probably were many strategic options. They could have decided to focus on creating a specific product, such as the “ultimate driving machine,” or cars that are boxy but safe. They could have opted to cater to a specific consumer class, perhaps building a strong fleet of affordable autos. Or they could have looked to improve their purchasing power and distribution methods (think Dell and Walmart).
Instead, they made a conscious decision to pursue excellence in reliability, quality, and value (sound familiar?), then followed through beautifully.
Strategy at Home
Despite differences in industry and scale, all of these same sorts of decisions are critical to the success of your career, your HM group, or even the field of pediatric HM. Are you aware of the specific strategies in place for your group’s success? Have you been involved in the process? Before this year, I was probably like most of you. I had some vague notion of success. It involved increasing relative value units, making everyone happy, and completing a big QI or research project.
In the past 12 months, however, I have taken part in three strategic planning sessions: one for a regional pediatric society, one for my hospital, and one for my hospitalist group. The importance of these processes crystallized for me. Apparently, the leaders in our field have had the same thoughts. They convened the Pediatric Hospital Medicine Roundtable, a strategic planning session for our field (see “All Grown Up,” p. 1). Clearly, 2009 is the year of the strategic plan.
Despite the unifying theme, the processes and products of all of these plans have been unique. Strategic plans must be developed organically, out of local context and environment, and can only be created by those who live and breathe the work. What works for group safety at the university hospital of quality focus might not work for group communication experts at suburban community hospitals. Differences in institutional, organizational, and cultural beliefs should affect the decision-making process. When a strategy has been devised, it should be carefully chosen and explicitly implemented.
Does your group’s strategy come to mind? Or are you just treading water, unable to see beyond the next looming wave? If you have a vision of what you want, whether it’s money, fame, or protected time, then this same line of reasoning should apply to the strategic plan for your individual career, as well as the future of pediatric HM.
The lesson here is simple: Success requires a plan. Strategic planning is how you set a vision for the future and chart that course. Unexpected political waves are sure to come, and not every victory will come with a prize catch. But if you can create that beautiful Impressionist painting on the horizon and maintain that course, you are less likely to lose your breakfast and go without lunch. TH
Dr. Shen is The Hospitalist’s pediatric editor.