User login
ONLINE EXCLUSIVE: Audio interview with Lee H. Schwamm, MD
Massachusetts General Hospital and Brigham and Women’s Hospital are the Boston-based hubs for the Partners TeleStroke Network. The system connects 27 participating hospitals across three states with an escalating chain of access to stroke resources. Spoke hospitals transmit, through a secure link, such clinical data as noncontrast head CT scans to the hub, where a stroke expert “examines” the patient via live video feed and shares in the responsibility for deciding whether to initiate t-PA.
Massachusetts General Hospital and Brigham and Women’s Hospital are the Boston-based hubs for the Partners TeleStroke Network. The system connects 27 participating hospitals across three states with an escalating chain of access to stroke resources. Spoke hospitals transmit, through a secure link, such clinical data as noncontrast head CT scans to the hub, where a stroke expert “examines” the patient via live video feed and shares in the responsibility for deciding whether to initiate t-PA.
Massachusetts General Hospital and Brigham and Women’s Hospital are the Boston-based hubs for the Partners TeleStroke Network. The system connects 27 participating hospitals across three states with an escalating chain of access to stroke resources. Spoke hospitals transmit, through a secure link, such clinical data as noncontrast head CT scans to the hub, where a stroke expert “examines” the patient via live video feed and shares in the responsibility for deciding whether to initiate t-PA.
The Role of Incretin-Based Therapies in Treating Patients with Type 2 Diabetes Mellitus
Supplement Editor:
Laurence Kennedy, MD
Contents
Incretin-based therapies for type 2 diabetes mellitus: New therapeutic mechanisms
Laurence Kennedy, MD
Current antihyperglycemic treatment strategies for patients with type 2 diabetes mellitus
Lawrence Blonde, MD
Role of the incretin pathway in the pathogenesis of type 2 diabetes mellitus
Jeffrey S. Freeman, DO
Patient and treatment perspectives: Revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk
Anne L. Peters, MD, CDE
Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors
Jaime A. Davidson, MD
Redefining treatment success in type 2 diabetes mellitus: Comprehensive targeting of core defects
William T. Cefalu, MD; Robert J. Richards, MD; and Lydia Y. Melendez-Ramirez, MD
Supplement Editor:
Laurence Kennedy, MD
Contents
Incretin-based therapies for type 2 diabetes mellitus: New therapeutic mechanisms
Laurence Kennedy, MD
Current antihyperglycemic treatment strategies for patients with type 2 diabetes mellitus
Lawrence Blonde, MD
Role of the incretin pathway in the pathogenesis of type 2 diabetes mellitus
Jeffrey S. Freeman, DO
Patient and treatment perspectives: Revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk
Anne L. Peters, MD, CDE
Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors
Jaime A. Davidson, MD
Redefining treatment success in type 2 diabetes mellitus: Comprehensive targeting of core defects
William T. Cefalu, MD; Robert J. Richards, MD; and Lydia Y. Melendez-Ramirez, MD
Supplement Editor:
Laurence Kennedy, MD
Contents
Incretin-based therapies for type 2 diabetes mellitus: New therapeutic mechanisms
Laurence Kennedy, MD
Current antihyperglycemic treatment strategies for patients with type 2 diabetes mellitus
Lawrence Blonde, MD
Role of the incretin pathway in the pathogenesis of type 2 diabetes mellitus
Jeffrey S. Freeman, DO
Patient and treatment perspectives: Revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk
Anne L. Peters, MD, CDE
Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors
Jaime A. Davidson, MD
Redefining treatment success in type 2 diabetes mellitus: Comprehensive targeting of core defects
William T. Cefalu, MD; Robert J. Richards, MD; and Lydia Y. Melendez-Ramirez, MD
Incretin-based therapies for type 2 diabetes mellitus: New therapeutic mechanisms
Almost a decade into the 21st century, the global epidemic of diabetes—which accelerated in the 1970s—shows no sign of slowing. At the same time, our insights into both type 1 and type 2 diabetes mellitus (T2DM) have increased at a similarly rapid rate.
At the beginning of the 1970s, it was far from clear whether improved glycemic control made much difference in the long-term well-being of people with diabetes other than to relieve their symptoms of hyperglycemia and decrease the likelihood of diabetic ketoacidosis or hyperglycemic hyperosmolar nonketotic coma. Concerns were expressed about the risk/benefit ratio of antihyperglycemic drugs—so there is nothing new under the sun! The drugs available in the United States were limited to insulin and sulfonylureas. The rest of the world also had access to metformin, but, in truth, its potential was underestimated until much later.
RECOGNIZING THE VALUE OF GLYCEMIC CONTROL
Out of this milieu of scientific uncertainty grew the two clinical trials that effectively ended the debate about the value of glycemic control: the Diabetes Control and Complications Trial (DCCT)1 for type 1 diabetes, and the United Kingdom Prospective Diabetes Study (UKPDS)2,3 for T2DM. The conduct of these trials was facilitated by the timely demonstration of the utility of glycosylated hemoglobin (HbA1c) as an objective measure of glycemic control, and of microalbuminuria as a marker of early nephropathy.
Both the DCCT and the UKPDS, in their initial “end of study” analyses in the 1990s, established the role of glycemic control in reducing the risk of retinopathy, neuropathy, and nephropathy—the microvascular complications of diabetes. Additionally, the UKPDS demonstrated that in T2DM, hypertension management was at least as important as glycemic control in reducing the risk of microvascular complications.
Neither the DCCT nor the UKPDS was powered to determine initially whether glycemic control was a risk factor for cardiovascular disease; however, careful longer-term surveillance of the patient cohorts in the studies has recently borne fruit in this regard. Reports from both studies have shown that efforts to control glycemia early in the course of diabetes are rewarded many years later by a decreased risk of cardiovascular events and death.4,5 This is true even when excellent glycemic control achieved early on is not sustained indefinitely. It has also become widely recognized that the management of diabetes, with prevention of microvascular and cardiovascular disease as major aims, involves much more than a simple preoccupation with glycemic control—important as that is.
NEW TREATMENT OPTIONS
Concurrent with the DCCT and the UKPDS being conducted with, in effect, the therapeutic tools of the 1970s, considerable strides were being made in the development of new classes of antihyperglycemic agents for use in T2DM. These include the thiazolidinediones (TZDs), alpha-glucosidase inhibitors, nonsulfonylurea insulin secretagogues (also known as glinides), and, more recently, the incretin-based drugs that are the focus of this supplement to the Cleveland Clinic Journal of Medicine.
Understandable enthusiasm for tapping into the hitherto unexploited pathways and mechanisms targeted by a new drug class is inevitably tempered by known, or sometimes unforeseen, adverse effects. Some of the adverse effects typically associated with antihyperglycemic drugs used before the incretin-based therapies became available include hypoglycemia, weight gain, and fluid retention; all of these are perceived as possibly increasing the risk of the very thing we are striving to avoid in diabetes—cardiovascular morbidity and mortality. Such is the concern about this risk—epitomized, rightly or wrongly, in the controversial meta-analysis of clinical trials involving rosiglitazone6—that the US Food and Drug Administration now requires new antihyperglycemic drugs not only to meet efficacy standards for improving glycemia but also to show no sign of increased cardiovascular risk. The requirement must be met in preapproval trials, to be followed by postmarketing studies to prove the lack of cardiovascular risk.
As the contributions in this supplement point out, incretin-based therapies generally are either weight neutral or promote weight loss; by their modes of action, they are unlikely to cause hypoglycemia; and, as shown thus far, they are unassociated with fluid retention or increased likelihood of heart failure. Continued vigilance regarding cardiovascular risk will be important for the new incretin-based therapies, however.
BETA-CELL FUNCTION STILL A CHALLENGE
Another aspect of T2DM highlighted by the UKPDS is the degree of pancreatic beta-cell function loss—typically about 50% or more—at the time of clinical diagnosis, and the steady decline in function thereafter.7 This, as much as the understandable fatigue with lifestyle modification that normal humans experience, accounts for the frequent failure of oral antihyperglycemic monotherapy or dual therapy to maintain satisfactory glycemic control over the years. Relieving hyperglycemia at the time of diagnosis by any means usually leads to a temporary improvement in beta-cell function, but the possibility of slowing or even reversing the long-term decline has been an elusive therapeutic goal.
Although direct quantitative assessment of beta-cell function in humans is difficult in routine practice or outside of strict research protocols, a randomized study comparing different monotherapies for T2DM showed that over several years, the rise in HbA1c was more gradual with rosiglitazone than with glyburide or metformin; this suggests that, at least compared with metformin and sulfonylureas, the TZDs may have some longer-term benefit with respect to beta-cell function.8
That incretin-based treatments may help preserve or improve beta-cell function has been suggested by animal data.9 Proving that that is the case in humans will be much more challenging. A recent randomized study in patients with T2DM already taking metformin showed that addition of exenatide for 1 year resulted in improved beta-cell function, assessed by C-peptide responses to glucose and to arginine during a combined euglycemic-hyperinsulinemic and hyperglycemic clamp procedure. The improvement was evident compared with baseline function and with patients randomized to receive insulin glargine in addition to metformin for a year.10 However, 4 weeks after exenatide and glargine were discontinued, the beta-cell function had reverted to the pretreatment level and was not significantly different in the two groups of patients. Moreover, 3 months after treatment discontinuation, the HbA1c levels, which had decreased during the year to a similar extent in both groups, had returned to pretreatment levels. The investigators acknowledged that it was impossible in their study to “discriminate between acute and long-term effects of exenatide on beta-cell function.”10 So, in my opinion, the challenge remains to show that meaningful long-term effects on beta-cell function can be achieved with incretin-based therapy.
That said, there is no doubt that the incretin-based therapies bring a new dimension to our ability to treat diabetes. The articles in this supplement will provide both the specialist and nonspecialist with a better understanding of these relatively new therapies.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- UK Prospective Diabetes Study Group. UK prospective diabetes study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44:1249–1258.
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355:2427–2443.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
Almost a decade into the 21st century, the global epidemic of diabetes—which accelerated in the 1970s—shows no sign of slowing. At the same time, our insights into both type 1 and type 2 diabetes mellitus (T2DM) have increased at a similarly rapid rate.
At the beginning of the 1970s, it was far from clear whether improved glycemic control made much difference in the long-term well-being of people with diabetes other than to relieve their symptoms of hyperglycemia and decrease the likelihood of diabetic ketoacidosis or hyperglycemic hyperosmolar nonketotic coma. Concerns were expressed about the risk/benefit ratio of antihyperglycemic drugs—so there is nothing new under the sun! The drugs available in the United States were limited to insulin and sulfonylureas. The rest of the world also had access to metformin, but, in truth, its potential was underestimated until much later.
RECOGNIZING THE VALUE OF GLYCEMIC CONTROL
Out of this milieu of scientific uncertainty grew the two clinical trials that effectively ended the debate about the value of glycemic control: the Diabetes Control and Complications Trial (DCCT)1 for type 1 diabetes, and the United Kingdom Prospective Diabetes Study (UKPDS)2,3 for T2DM. The conduct of these trials was facilitated by the timely demonstration of the utility of glycosylated hemoglobin (HbA1c) as an objective measure of glycemic control, and of microalbuminuria as a marker of early nephropathy.
Both the DCCT and the UKPDS, in their initial “end of study” analyses in the 1990s, established the role of glycemic control in reducing the risk of retinopathy, neuropathy, and nephropathy—the microvascular complications of diabetes. Additionally, the UKPDS demonstrated that in T2DM, hypertension management was at least as important as glycemic control in reducing the risk of microvascular complications.
Neither the DCCT nor the UKPDS was powered to determine initially whether glycemic control was a risk factor for cardiovascular disease; however, careful longer-term surveillance of the patient cohorts in the studies has recently borne fruit in this regard. Reports from both studies have shown that efforts to control glycemia early in the course of diabetes are rewarded many years later by a decreased risk of cardiovascular events and death.4,5 This is true even when excellent glycemic control achieved early on is not sustained indefinitely. It has also become widely recognized that the management of diabetes, with prevention of microvascular and cardiovascular disease as major aims, involves much more than a simple preoccupation with glycemic control—important as that is.
NEW TREATMENT OPTIONS
Concurrent with the DCCT and the UKPDS being conducted with, in effect, the therapeutic tools of the 1970s, considerable strides were being made in the development of new classes of antihyperglycemic agents for use in T2DM. These include the thiazolidinediones (TZDs), alpha-glucosidase inhibitors, nonsulfonylurea insulin secretagogues (also known as glinides), and, more recently, the incretin-based drugs that are the focus of this supplement to the Cleveland Clinic Journal of Medicine.
Understandable enthusiasm for tapping into the hitherto unexploited pathways and mechanisms targeted by a new drug class is inevitably tempered by known, or sometimes unforeseen, adverse effects. Some of the adverse effects typically associated with antihyperglycemic drugs used before the incretin-based therapies became available include hypoglycemia, weight gain, and fluid retention; all of these are perceived as possibly increasing the risk of the very thing we are striving to avoid in diabetes—cardiovascular morbidity and mortality. Such is the concern about this risk—epitomized, rightly or wrongly, in the controversial meta-analysis of clinical trials involving rosiglitazone6—that the US Food and Drug Administration now requires new antihyperglycemic drugs not only to meet efficacy standards for improving glycemia but also to show no sign of increased cardiovascular risk. The requirement must be met in preapproval trials, to be followed by postmarketing studies to prove the lack of cardiovascular risk.
As the contributions in this supplement point out, incretin-based therapies generally are either weight neutral or promote weight loss; by their modes of action, they are unlikely to cause hypoglycemia; and, as shown thus far, they are unassociated with fluid retention or increased likelihood of heart failure. Continued vigilance regarding cardiovascular risk will be important for the new incretin-based therapies, however.
BETA-CELL FUNCTION STILL A CHALLENGE
Another aspect of T2DM highlighted by the UKPDS is the degree of pancreatic beta-cell function loss—typically about 50% or more—at the time of clinical diagnosis, and the steady decline in function thereafter.7 This, as much as the understandable fatigue with lifestyle modification that normal humans experience, accounts for the frequent failure of oral antihyperglycemic monotherapy or dual therapy to maintain satisfactory glycemic control over the years. Relieving hyperglycemia at the time of diagnosis by any means usually leads to a temporary improvement in beta-cell function, but the possibility of slowing or even reversing the long-term decline has been an elusive therapeutic goal.
Although direct quantitative assessment of beta-cell function in humans is difficult in routine practice or outside of strict research protocols, a randomized study comparing different monotherapies for T2DM showed that over several years, the rise in HbA1c was more gradual with rosiglitazone than with glyburide or metformin; this suggests that, at least compared with metformin and sulfonylureas, the TZDs may have some longer-term benefit with respect to beta-cell function.8
That incretin-based treatments may help preserve or improve beta-cell function has been suggested by animal data.9 Proving that that is the case in humans will be much more challenging. A recent randomized study in patients with T2DM already taking metformin showed that addition of exenatide for 1 year resulted in improved beta-cell function, assessed by C-peptide responses to glucose and to arginine during a combined euglycemic-hyperinsulinemic and hyperglycemic clamp procedure. The improvement was evident compared with baseline function and with patients randomized to receive insulin glargine in addition to metformin for a year.10 However, 4 weeks after exenatide and glargine were discontinued, the beta-cell function had reverted to the pretreatment level and was not significantly different in the two groups of patients. Moreover, 3 months after treatment discontinuation, the HbA1c levels, which had decreased during the year to a similar extent in both groups, had returned to pretreatment levels. The investigators acknowledged that it was impossible in their study to “discriminate between acute and long-term effects of exenatide on beta-cell function.”10 So, in my opinion, the challenge remains to show that meaningful long-term effects on beta-cell function can be achieved with incretin-based therapy.
That said, there is no doubt that the incretin-based therapies bring a new dimension to our ability to treat diabetes. The articles in this supplement will provide both the specialist and nonspecialist with a better understanding of these relatively new therapies.
Almost a decade into the 21st century, the global epidemic of diabetes—which accelerated in the 1970s—shows no sign of slowing. At the same time, our insights into both type 1 and type 2 diabetes mellitus (T2DM) have increased at a similarly rapid rate.
At the beginning of the 1970s, it was far from clear whether improved glycemic control made much difference in the long-term well-being of people with diabetes other than to relieve their symptoms of hyperglycemia and decrease the likelihood of diabetic ketoacidosis or hyperglycemic hyperosmolar nonketotic coma. Concerns were expressed about the risk/benefit ratio of antihyperglycemic drugs—so there is nothing new under the sun! The drugs available in the United States were limited to insulin and sulfonylureas. The rest of the world also had access to metformin, but, in truth, its potential was underestimated until much later.
RECOGNIZING THE VALUE OF GLYCEMIC CONTROL
Out of this milieu of scientific uncertainty grew the two clinical trials that effectively ended the debate about the value of glycemic control: the Diabetes Control and Complications Trial (DCCT)1 for type 1 diabetes, and the United Kingdom Prospective Diabetes Study (UKPDS)2,3 for T2DM. The conduct of these trials was facilitated by the timely demonstration of the utility of glycosylated hemoglobin (HbA1c) as an objective measure of glycemic control, and of microalbuminuria as a marker of early nephropathy.
Both the DCCT and the UKPDS, in their initial “end of study” analyses in the 1990s, established the role of glycemic control in reducing the risk of retinopathy, neuropathy, and nephropathy—the microvascular complications of diabetes. Additionally, the UKPDS demonstrated that in T2DM, hypertension management was at least as important as glycemic control in reducing the risk of microvascular complications.
Neither the DCCT nor the UKPDS was powered to determine initially whether glycemic control was a risk factor for cardiovascular disease; however, careful longer-term surveillance of the patient cohorts in the studies has recently borne fruit in this regard. Reports from both studies have shown that efforts to control glycemia early in the course of diabetes are rewarded many years later by a decreased risk of cardiovascular events and death.4,5 This is true even when excellent glycemic control achieved early on is not sustained indefinitely. It has also become widely recognized that the management of diabetes, with prevention of microvascular and cardiovascular disease as major aims, involves much more than a simple preoccupation with glycemic control—important as that is.
NEW TREATMENT OPTIONS
Concurrent with the DCCT and the UKPDS being conducted with, in effect, the therapeutic tools of the 1970s, considerable strides were being made in the development of new classes of antihyperglycemic agents for use in T2DM. These include the thiazolidinediones (TZDs), alpha-glucosidase inhibitors, nonsulfonylurea insulin secretagogues (also known as glinides), and, more recently, the incretin-based drugs that are the focus of this supplement to the Cleveland Clinic Journal of Medicine.
Understandable enthusiasm for tapping into the hitherto unexploited pathways and mechanisms targeted by a new drug class is inevitably tempered by known, or sometimes unforeseen, adverse effects. Some of the adverse effects typically associated with antihyperglycemic drugs used before the incretin-based therapies became available include hypoglycemia, weight gain, and fluid retention; all of these are perceived as possibly increasing the risk of the very thing we are striving to avoid in diabetes—cardiovascular morbidity and mortality. Such is the concern about this risk—epitomized, rightly or wrongly, in the controversial meta-analysis of clinical trials involving rosiglitazone6—that the US Food and Drug Administration now requires new antihyperglycemic drugs not only to meet efficacy standards for improving glycemia but also to show no sign of increased cardiovascular risk. The requirement must be met in preapproval trials, to be followed by postmarketing studies to prove the lack of cardiovascular risk.
As the contributions in this supplement point out, incretin-based therapies generally are either weight neutral or promote weight loss; by their modes of action, they are unlikely to cause hypoglycemia; and, as shown thus far, they are unassociated with fluid retention or increased likelihood of heart failure. Continued vigilance regarding cardiovascular risk will be important for the new incretin-based therapies, however.
BETA-CELL FUNCTION STILL A CHALLENGE
Another aspect of T2DM highlighted by the UKPDS is the degree of pancreatic beta-cell function loss—typically about 50% or more—at the time of clinical diagnosis, and the steady decline in function thereafter.7 This, as much as the understandable fatigue with lifestyle modification that normal humans experience, accounts for the frequent failure of oral antihyperglycemic monotherapy or dual therapy to maintain satisfactory glycemic control over the years. Relieving hyperglycemia at the time of diagnosis by any means usually leads to a temporary improvement in beta-cell function, but the possibility of slowing or even reversing the long-term decline has been an elusive therapeutic goal.
Although direct quantitative assessment of beta-cell function in humans is difficult in routine practice or outside of strict research protocols, a randomized study comparing different monotherapies for T2DM showed that over several years, the rise in HbA1c was more gradual with rosiglitazone than with glyburide or metformin; this suggests that, at least compared with metformin and sulfonylureas, the TZDs may have some longer-term benefit with respect to beta-cell function.8
That incretin-based treatments may help preserve or improve beta-cell function has been suggested by animal data.9 Proving that that is the case in humans will be much more challenging. A recent randomized study in patients with T2DM already taking metformin showed that addition of exenatide for 1 year resulted in improved beta-cell function, assessed by C-peptide responses to glucose and to arginine during a combined euglycemic-hyperinsulinemic and hyperglycemic clamp procedure. The improvement was evident compared with baseline function and with patients randomized to receive insulin glargine in addition to metformin for a year.10 However, 4 weeks after exenatide and glargine were discontinued, the beta-cell function had reverted to the pretreatment level and was not significantly different in the two groups of patients. Moreover, 3 months after treatment discontinuation, the HbA1c levels, which had decreased during the year to a similar extent in both groups, had returned to pretreatment levels. The investigators acknowledged that it was impossible in their study to “discriminate between acute and long-term effects of exenatide on beta-cell function.”10 So, in my opinion, the challenge remains to show that meaningful long-term effects on beta-cell function can be achieved with incretin-based therapy.
That said, there is no doubt that the incretin-based therapies bring a new dimension to our ability to treat diabetes. The articles in this supplement will provide both the specialist and nonspecialist with a better understanding of these relatively new therapies.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- UK Prospective Diabetes Study Group. UK prospective diabetes study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44:1249–1258.
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355:2427–2443.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- UK Prospective Diabetes Study Group. UK prospective diabetes study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44:1249–1258.
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355:2427–2443.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
Current antihyperglycemic treatment strategies for patients with type 2 diabetes mellitus
Data from the Centers for Disease Control and Prevention indicate that almost 24 million Americans, or 7.8% of the population, have diabetes; 90% to 95% of these have type 2 diabetes mellitus (T2DM).1 Diabetes and excessive weight often coexist. An analysis of data from the 1999–2002 National Health and Nutrition Examination Survey (NHANES) showed that among individuals with diabetes, 85% were overweight or obese and 55% were obese.2
Gaps remain in the management of T2DM between the goals for clinical parameters of care (eg, control of glucose, blood pressure [BP], and lipids) and actual clinical practice.3 NHANES data reveal that glycemic control improved from a mean glycosylated hemoglobin A1c (HbA1c) of 7.82% in 1999–2000 to 7.18% in 2003–2004.4 Hazard models based on the United Kingdom Prospective Diabetes Study (UKPDS) 10-year outcomes data in 4,320 newly diagnosed T2DM patients suggest that a sustained decrease in HbA1c of 0.511 percentage points could reduce diabetes complications by 10.7%.4,5
Additional analysis of NHANES data showed that in 2003–2004, about 57% of individuals achieved glycemic control, 48% reached BP targets, and 50% achieved target cholesterol goals.Only about 13% of diabetes patients achieved their target goals for all three parameters concurrently.6
This article reviews the association between cardiometabolic risk and the current antihyperglycemic treatments for patients with T2DM, with a focus on the role of incretin-related therapies.
THE IMPORTANCE OF CARDIOMETABOLIC RISK IN T2DM
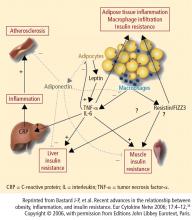
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among people with diabetes and is the reported cause of mortality in up to 65% of deaths in persons with diabetes in the United States.7 The risk of CVD is two- to fourfold greater among adults with diabetes than among adults who do not have diabetes.8 The risk of CVD in patients with T2DM was evident in the UKPDS 17, where macrovascular complications, including CVD, were about twice as common as microvascular complications (20% vs 9%) after 9 years of follow-up.9 A study that involved more than 44,000 patients showed an almost double rate of mortality from all causes among individuals with T2DM compared with those with no diabetes (hazard ratio, 1.93; 95% confidence interval, 1.89 to 1.97).10 Current guidelines recommend aggressive management of CV risk factors, including BP control, correction of atherogenic dyslipidemia, glycemic control, weight reduction for those who are overweight or obese, and smoking cessation for those who smoke.3,11 Lifestyle interventions, including weight reduction and appropriately prescribed physical activity, result in reduced CV risk factors, which can help slow the progression of T2DM.12
GOALS OF T2DM THERAPY
Several studies have demonstrated that glycemic control can delay or prevent the development and progression of microvascular complications.13,14 UKPDS 33 showed that more intensive blood glucose control (median HbA1c 7.0%) in patients with T2DM followed over 10 years significantly (P = .029) reduced the risk for any diabetes-related end point by 12% compared with conventional therapy (median HbA1c 7.9%). Most of the risk reduction was accounted for by a 25% risk reduction in microvascular end points (P = .0099).13 Another report (UKPDS 35) demonstrated that HbA1c was strongly related to microvascular effects, with a 1% reduction in HbA1c associated with a 37% reduction in microvascular complications.14
Does intensive glucose control reduce CV risk?
To resolve the ongoing question of whether intensive glucose control can lead to a reduction in CV risk in patients with T2DM, three large, long-term trials were conducted within the last decade.15–18 Two of these, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trials, each enrolled more than 10,000 previously treated patients with long-standing T2DM. Patients were randomized to standard or intensive glycemic control for 3.5 years in the ACCORD trial and for 5 years in the ADVANCE trial.15,16
The ACCORD and ADVANCE trials, along with the smaller Veterans Administration Diabetes Trial (VADT) (N = 1,791), failed to show that more intensive glycemic control significantly reduced CVD.15–17 Additionally, the glycemic control component of ACCORD was halted because of increased mortality in the intensive arm compared with the standard arm.15 Further analyses of ACCORD data presented at the 69th Scientific Sessions of the American Diabetes Association (ADA) showed that HbA1c values lower than 7.0% did not explain the increased mortality. The 20% higher risk of death for every 1.0% increase in HbA1c greater than 6.0% suggests that glucose concentrations even lower than the general HbA1c goal of less than 7.0% may be appropriate in some patients.18 The most recent finding from VADT was that CV risk was dependent on disease duration and presence of comorbidities. Intensive therapy seemed to work best in patients with diabetes of less than 15 years’ duration, while risk of a CV event was more than doubled with intensive therapy in patients having diabetes for more than 21 years.
Clarification of treatment goals
A position statement of the ADA and a scientific statement of the American College of Cardiology Foundation and the American Heart Association19 concluded that the “evidence obtained from ACCORD, ADVANCE, and VADT does not suggest the need for major changes in glycemic control targets but, rather, additional clarification of the language that has consistently stressed individualization.” They state that while the general HbA1c goal of less than 7.0% seems reasonable, even lower HbA1c goals may be appropriate for some patients if they can be achieved without significant hypoglycemia or other adverse effects. Such patients might include those with diabetes of short duration, long life expectancy, or no significant CVD or hypoglycemia. Conversely, higher HbA1c goals may be appropriate for patients with limited life expectancy, a history of severe hypoglycemia, established microvascular or macrovascular complications, significant other comorbid conditions, or longstanding diabetes in whom an HbA1c of less than 7.0% has been difficult to attain despite optimal treatment and diabetes self-management education.19
Long-term risk reduction
A 10-year, postinterventional follow-up study (UKPDS 80) of the UKPDS survivor cohort was reported recently.20 Results showed that despite an early loss of glycemic differences between patients treated with diet and those treated with intensive regimens (sulfonylurea or insulin; metformin in overweight patients), the pharmacotherapy group demonstrated a prolonged reduction in microvascular risk as well as a significant reduction in the risk for myocardial infarction (15% [P = .01] in the sulfonylurea-insulin group and 33% [P = .005] in the metformin group) and death from any cause.20 This suggests that early improvement in glycemic control is associated with long-term benefits in the micro- and macrovascular health of patients with T2DM.
Additionally, the recent long-term follow-up of the Steno-2 study21 showed that a multifactorial intervention striving for intensive glucose, BP, and lipid control that included the use of renin-angiotensin system blockers, aspirin, and lipid-lowering agents not only reduced the risk of nonfatal CVD among patients with T2DM and microalbuminuria, but also had sustained beneficial effects on vascular complications and on rates of death from any cause and from CV causes. From a health care payer perspective, intensive multifactorial intervention was more likely to be cost-effective than conventional treatment in Denmark, especially if applied in a primary care setting.22
Comprehensive care needed
The lower-than-expected rates of CV outcomes in the ACCORD, ADVANCE, VADT, and Steno-2 studies reinforce the importance of comprehensive diabetes care that treats not only hyperglycemia but also elevated BP and dyslipidemia; these are considered the “ABCs” of diabetes.11,19 The 2009 ADA standards of medical care guidelines recommend that for most T2DM patients, HbA1c should be maintained at less than 7.0%,3 while the American Association of Clinical Endocrinologists (AACE) 2007 guidelines state that HbA1c should be 6.5% or less.11 Both organizations stress the importance of individualized goals, as discussed above, and advocate BP goals of less than 130/80 mm Hg and dyslipidemia goals of low-density lipoprotein cholesterol (LDL-C) less than 100 mg/dL, high-density lipoprotein cholesterol (HDL-C) greater than 40 mg/dL for men and 50 mg/dL for women, and triglycerides less than 150 mg/dL. It is recommended that an optional LDL-C goal of less than 70 mg/dL be considered for individuals with overt CVD.
CURRENT ANTIHYPERGLYCEMIC TREATMENT STRATEGIES
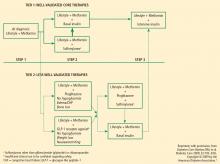
In response to new insights from clinical research and emerging treatment strategies, disease-specific organizations and medical specialty societies regularly revise and update their treatment guidelines and algorithms. These resources recommend that glycemic progress should be regularly monitored and pharmacologic therapy titrated or new drugs added promptly if glycemic goals are not met after 2 to 3 months.
Several algorithms combine scientific evidence with expert clinical opinion to guide physicians in treating their patients with T2DM. The American College of Endocrinology (ACE)/AACE road maps are designed to help develop individualized treatment regimens to achieve an HbA1c of 6.5% or less.23 The algorithm from a writing group assembled by the ADA and the European Association for the Study of Diabetes (EASD) similarly promotes pharmacologic treatment together with lifestyle modifications to maintain a glycemic goal of HbA1c less than 7.0%.24
OVERVIEW OF ANTIHYPERGLYCEMIC TREATMENT APPROACHES
Initial oral therapy
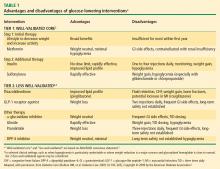
T2DM is usually treated initially with a single oral agent. Consistent with the progressive nature of the disease, patients often eventually require one or more additional oral agents and in many cases insulin.13,27 Choice of specific agents is based on individual patient circumstances, including the need for weight loss and control of fasting versus postprandial glucose, the presence of dyslipidemia and hypertension, and the risk for and potential consequences of hypoglycemia.24 T2DM patients with severely uncontrolled and symptomatic hyperglycemia are best treated, at least initially, with a combination of insulin therapy and lifestyle intervention, often with metformin.
Metformin. The recently revised ADA/EASD writing group algorithm recommends that patients not requiring initial insulin begin treatment with metformin at the time of diagnosis unless there are contraindications.24 Metformin is not associated with hypoglycemia and is considered weight-neutral, although some patients may lose weight.28
Sulfonylureas. Sulfonylureas stimulate insulin secretion from pancreatic beta cells; their use may be associated with hypoglycemia and weight gain. Mechanisms for weight gain with sulfonylureas include reduction of glucosuria and increased caloric intake to prevent or treat hypoglycemia.11,28 Nateglinide and repaglinide are nonsulfonylurea oral insulin secretagogues. They result in rapid and relatively short-lived insulin responses and are usually administered three times a day, before each meal. Their use may be associated with weight gain and hypoglycemia.11
Thiazolidinediones. Thiazolidinediones (TZD) increase insulin sensitivity in muscle, adipose tissue, and the liver. Hypoglycemia is uncommon with TZD monotherapy but weight gain related to increased and redistributed adiposity and fluid retention frequently occurs.
Alpha-glucosidase inhibitors. The alpha-glucosidase inhibitors are administered before meals and primarily reduce postprandial hyperglycemia. They are generally weight-neutral.28
Insulin. Insulin and insulin analogues are the most effective antihyperglycemic agents, but their use can be associated with hypoglycemia and clinically significant weight gain.28
Colesevelam. Colesevelam is a bile acid sequestrant that was recently approved by the US Food and Drug Administration as an antihyperglycemic therapy in people with T2DM. At a dosage of 1.875 g BID or 3.75 g QD in combination with a sulfonylurea, metformin, or insulin therapy, reductions in HbA1c compared with placebo in clinical trials of colesevelam have ranged from –0.5% to –0.7% (P < .02). Frequency of hypoglycemia and weight gain is low with this agent.26
Weight management. Weight reduction is important for overweight or obese patients with T2DM.27,28 Even moderate weight loss (5% of body weight) can be associated with improved insulin action and reduced hyperglycemia.29 Conversely, weight gain has been shown to worsen hyperglycemia and other CV risk factors. Treatment-related weight gain can also lead to decreased regimen adherence, contributing to poor glycemic control.28
THE ROLE OF INCRETIN HORMONES AND INCRETIN-BASED THERAPIES IN T2DM PATIENTS
Over the last few years, the role of incretin hormones and their contribution to diabetes pathophysiology has become more apparent. The incretin effect refers to the observation that orally administered glucose elicits a greater insulin response than does glucose administered intravenously to produce equivalent blood glucose concentrations.30,31 The incretin effect is diminished in patients with T2DM.
Hormone mediation of the incretin effect
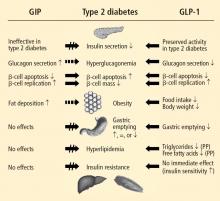
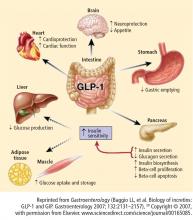
The two hormones that mediate the incretin effect are GIP (also known as gastric inhibitory polypeptide or glucose-dependent insulinotropic polypeptide) and glucagon-like peptide−1 (GLP-1).30,31 GLP-1 has several glucoregulatory actions, including enhancement of endogenous insulin release and suppression of inappropriate glucagon secretion, both in a glucose-dependent manner. Therefore, these effects of GLP-1 occur only when glucose concentrations are elevated, thereby minimizing the risk of hypoglycemia. GLP-1 also regulates gastric emptying; infusions of GLP-1 can slow the accelerated emptying that is often present in T2DM patients. GLP-1 also increases satiety and decreases food intake via a central mechanism.31
Because GLP-1 is rapidly inactivated by the enzyme dipeptidyl peptidase–4 (DPP-4), therapeutic use of GLP-1 would require continuous infusion, which is impractical.30,31 Two strategies have been used to produce incretin-related therapies. One, inhibition of the DPP-4 enzyme, results in a two- to threefold enhancement of endogenous GLP-1. The other, involving agents that resist breakdown by DPP-4 but bind to and activate the GLP-1 receptor, produces glucoregulatory effects similar to those of GLP-1.30
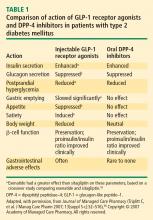
Following subcutaneous (SC) injection, GLP-1 receptor agonists enhance insulin secretion and suppress inappropriately elevated glucagon, both in a glucose-dependent manner, as well as slow gastric emptying and enhance satiety.30 DPP-4 inhibitors provide glucose-dependent enhanced insulin secretion and glucagon suppression, but they do not have the same effects on gastric emptying or satiety.
Clinically, the GLP-1 receptor agonists improve glycemia and are associated with weight loss.32–35 Adverse gastrointestinal symptoms are relatively common during the first few weeks of treatment. DPP-4 inhibitors improve glycemia but are weight-neutral and are not generally associated with significant gastrointestinal symptoms.32,36–38
Incretin-based therapies
Incretin-based therapies are currently part of the antihyperglycemic armamentarium.25,32 The AACE guidelines11 and the ACE/AACE roadmaps23 include the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin among antihyperglycemic therapies for patients with T2DM. The most recent update of the consensus algorithm statement of a joint ADA/EASD writing group included GLP-1 receptor agonists (but not DPP-4 inhibitors) in tier 2 of preferred agents, especially for patients who have concerns related to weight and hypoglycemia.24 They noted that DPP-4 inhibitors may be appropriate choices in selected patients.
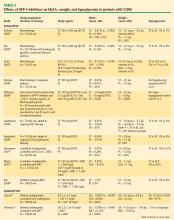
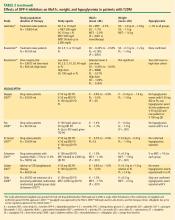
DPP-4 inhibitors: sitagliptin, saxagliptin. Until recently, sitagliptin was the only DPP-4 inhibitor available in the United States. Sitagliptin is approved by the FDA for treatment of T2DM at a recommended oral dosage of 100 mg QD, either as monotherapy or in combination with other oral antihyperglycemic medications. The dosage of sitagliptin should be reduced to 50 mg/day in patients with creatinine clearance (CrCl) levels that are between 30 mL/min and 50 mL/min and to 25 mg/day in those with CrCl less than 30 mL/min.39
In a meta-analysis of incretin-based therapies, DPP-4 inhibitors produced a reduction in HbA1c compared with placebo (weighted mean difference of –0.74%; 95% confidence interval, –0.85% to –0.62%).32 DPP-4 inhibitor antihyperglycemic efficacy has been shown to be similar whether used as a monotherapy or add-on therapy.32,37,38 This same meta-analysis showed DPP-4 inhibitors as having a neutral effect on weight.32 More recently, a single-pill combination of metformin and sitagliptin was approved.40
A study comparing metformin, sitagliptin, and the combination of the two as initial monotherapy in T2DM patients with a baseline HbA1c of 8.8% showed 24-week HbA1c reductions from baseline of –0.66% with sitagliptin 100 mg QD, –0.82% with metformin 500 mg BID, and –1.90% with sitagliptin 50 mg + metformin 1,000 mg BID.41
On July 31, 2009, the FDA approved another DPP-4 inhibitor, saxagliptin, for the treatment of T2DM either as monotherapy or in combination with metformin, a sulfonylurea, or a TZD.42
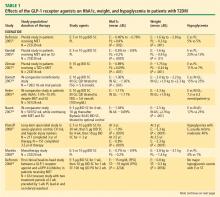
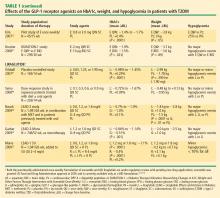
GLP-1 receptor agonist: exenatide. Exenatide, the only FDA-approved GLP-1 receptor agonist, is the synthetic version of exendin-4, which binds to the human GLP-1 receptor and in vitro possesses many of the glucoregulatory effects of endogenous GLP-1.30,32 Exenatide is indicated as monotherapy or adjunctive therapy for patients with T2DM who have not achieved adequate glycemic control with metformin, a sulfonylurea, a TZD, or metformin in combination with a sulfonylurea or a TZD.43 Exenatide is administered by SC injection BID at a starting dosage of 5 mg BID for 4 weeks, followed by an increase to 10 mg BID.
Exenatide has been shown not only to enhance glucose-dependent insulin secretion but also to restore impaired first-phase insulin response in subjects with T2DM. Exenatide also helps control postprandial glycemic excursions by suppressing inappropriate glucagon secretion, slowing accelerated gastric emptying, and enhancing satiety. The increased satiety results in decreased food intake and weight loss.31,44 In a recent head-to-head crossover study, exenatide was shown to be more effective than sitagliptin in lowering postprandial glucose concentrations, increasing insulin secretion, and reducing postprandial glucagon secretion.45 Exenatide also slowed gastric emptying and reduced caloric intake.
Exenatide, in most studies, resulted in a placebo-subtracted HbA1c reduction of approximately –1.0% and in one study lowered HbA1c from baseline by –1.5%. Completer analyses have shown HbA1c reductions of –1.0% up to 3 years and –0.8% up to 3.5 years. Exenatide has also been associated with a mean weight loss of as much as –3.6 kg at 30 weeks and as much as –5.3 kg at 3.5 years.33–35,46,47 A 1-year study showed that exenatide improved beta-cell secretory function compared with insulin glargine in metformin-treated patients with T2DM.48 Long-term data, including findings from completed and intention-to-treat analyses of 82 weeks49 to at least 3 years47 have demonstrated that exenatide improved CV risk factors, including those related to BP, lipids, and hepatic injury biomarkers.
Therapies in development
Incretin-based therapies in development include a novel once-weekly formulation of exenatide; taspoglutide, another once-weekly GLP-1 receptor agonist; and liraglutide, a GLP-1 receptor agonist that is administered once daily.50 Liraglutide is currently being evaluated in clinical trials as a once-daily SC injection.51–53 Liraglutide has been reported to reduce HbA1c by –1.1% at 26 weeks and up to –1.14% at 52 weeks and result in weight loss (up to –2.8 kg at 26 weeks and up to –2.5 kg at 52 weeks) in patients with T2DM who are treatment-naïve or taking other antidiabetes agents, including metformin, sulfonylurea, and TZD.51–53 Evaluation of the once-weekly formulation of exenatide showed reductions in HbA1c of –1.9% at 30 weeks and –2.0% at 52 weeks with a weight loss of –3.7 kg at 30 weeks and –4.1 kg over 52 weeks of treatment.46,54
CONCLUSION
In the United States, the epidemics of excessive weight and T2DM have contributed to an increased medical risk for many individuals. Comprehensive diabetes treatments targeting not only hyperglycemia but also frequently associated overweight/obesity, hypertension, and dyslipidemia will be required to reduce such risk. Current treatment strategies have evolved based on updated clinical guidelines and trials, as well as practice experience, including those related to newer agents. Incretin-based therapies, such as the GLP-1 receptor agonist, exenatide, and the DPP-4 inhibitors, sitagliptin and saxagliptin, are important additions to the treatment armamentarium, offering a reduction in hyperglycemia and beneficial effects on weight (reduction with exenatide and neutral with sitagliptin), and have been shown to improve several CV risk factors.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, 2008. Available at: http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes: United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008; 31:81–86.
- Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 2006; 49:1761–1769.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004; 140:945–950.
- Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004; 292:2495–2499.
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in noninsulin-dependent diabetes mellitus. Ann Intern Med 1996; 124(1 Pt 2):136–145.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Kerr M. ADA 2009: intensive glycemic control not directly linked to excess cardiovascular risk. Medscape Medical News Web site. http://www.medscape.com/viewarticle/704260_print. Published June 11, 2009. Accessed September 16, 2009.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Gaede P, Valentine WJ, Palmer AJ, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008; 31:1510–1515.
- ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus. Endocr Pract 2007; 13:260–268.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- Sonnett TE, Levien TL, Neumiller JJ, Gates BJ, Setter SM. Colesevelam hydrochloride for the treatment of type 2 diabetes mellitus. Clin Ther 2009; 31:245–259.
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131:281–303.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3:153–165.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632−2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Januvia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2048–2054.
- Janumet. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2041–2048.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, for the Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281:E155–E161.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
Data from the Centers for Disease Control and Prevention indicate that almost 24 million Americans, or 7.8% of the population, have diabetes; 90% to 95% of these have type 2 diabetes mellitus (T2DM).1 Diabetes and excessive weight often coexist. An analysis of data from the 1999–2002 National Health and Nutrition Examination Survey (NHANES) showed that among individuals with diabetes, 85% were overweight or obese and 55% were obese.2
Gaps remain in the management of T2DM between the goals for clinical parameters of care (eg, control of glucose, blood pressure [BP], and lipids) and actual clinical practice.3 NHANES data reveal that glycemic control improved from a mean glycosylated hemoglobin A1c (HbA1c) of 7.82% in 1999–2000 to 7.18% in 2003–2004.4 Hazard models based on the United Kingdom Prospective Diabetes Study (UKPDS) 10-year outcomes data in 4,320 newly diagnosed T2DM patients suggest that a sustained decrease in HbA1c of 0.511 percentage points could reduce diabetes complications by 10.7%.4,5
Additional analysis of NHANES data showed that in 2003–2004, about 57% of individuals achieved glycemic control, 48% reached BP targets, and 50% achieved target cholesterol goals.Only about 13% of diabetes patients achieved their target goals for all three parameters concurrently.6
This article reviews the association between cardiometabolic risk and the current antihyperglycemic treatments for patients with T2DM, with a focus on the role of incretin-related therapies.
THE IMPORTANCE OF CARDIOMETABOLIC RISK IN T2DM
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among people with diabetes and is the reported cause of mortality in up to 65% of deaths in persons with diabetes in the United States.7 The risk of CVD is two- to fourfold greater among adults with diabetes than among adults who do not have diabetes.8 The risk of CVD in patients with T2DM was evident in the UKPDS 17, where macrovascular complications, including CVD, were about twice as common as microvascular complications (20% vs 9%) after 9 years of follow-up.9 A study that involved more than 44,000 patients showed an almost double rate of mortality from all causes among individuals with T2DM compared with those with no diabetes (hazard ratio, 1.93; 95% confidence interval, 1.89 to 1.97).10 Current guidelines recommend aggressive management of CV risk factors, including BP control, correction of atherogenic dyslipidemia, glycemic control, weight reduction for those who are overweight or obese, and smoking cessation for those who smoke.3,11 Lifestyle interventions, including weight reduction and appropriately prescribed physical activity, result in reduced CV risk factors, which can help slow the progression of T2DM.12
GOALS OF T2DM THERAPY
Several studies have demonstrated that glycemic control can delay or prevent the development and progression of microvascular complications.13,14 UKPDS 33 showed that more intensive blood glucose control (median HbA1c 7.0%) in patients with T2DM followed over 10 years significantly (P = .029) reduced the risk for any diabetes-related end point by 12% compared with conventional therapy (median HbA1c 7.9%). Most of the risk reduction was accounted for by a 25% risk reduction in microvascular end points (P = .0099).13 Another report (UKPDS 35) demonstrated that HbA1c was strongly related to microvascular effects, with a 1% reduction in HbA1c associated with a 37% reduction in microvascular complications.14
Does intensive glucose control reduce CV risk?
To resolve the ongoing question of whether intensive glucose control can lead to a reduction in CV risk in patients with T2DM, three large, long-term trials were conducted within the last decade.15–18 Two of these, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trials, each enrolled more than 10,000 previously treated patients with long-standing T2DM. Patients were randomized to standard or intensive glycemic control for 3.5 years in the ACCORD trial and for 5 years in the ADVANCE trial.15,16
The ACCORD and ADVANCE trials, along with the smaller Veterans Administration Diabetes Trial (VADT) (N = 1,791), failed to show that more intensive glycemic control significantly reduced CVD.15–17 Additionally, the glycemic control component of ACCORD was halted because of increased mortality in the intensive arm compared with the standard arm.15 Further analyses of ACCORD data presented at the 69th Scientific Sessions of the American Diabetes Association (ADA) showed that HbA1c values lower than 7.0% did not explain the increased mortality. The 20% higher risk of death for every 1.0% increase in HbA1c greater than 6.0% suggests that glucose concentrations even lower than the general HbA1c goal of less than 7.0% may be appropriate in some patients.18 The most recent finding from VADT was that CV risk was dependent on disease duration and presence of comorbidities. Intensive therapy seemed to work best in patients with diabetes of less than 15 years’ duration, while risk of a CV event was more than doubled with intensive therapy in patients having diabetes for more than 21 years.
Clarification of treatment goals
A position statement of the ADA and a scientific statement of the American College of Cardiology Foundation and the American Heart Association19 concluded that the “evidence obtained from ACCORD, ADVANCE, and VADT does not suggest the need for major changes in glycemic control targets but, rather, additional clarification of the language that has consistently stressed individualization.” They state that while the general HbA1c goal of less than 7.0% seems reasonable, even lower HbA1c goals may be appropriate for some patients if they can be achieved without significant hypoglycemia or other adverse effects. Such patients might include those with diabetes of short duration, long life expectancy, or no significant CVD or hypoglycemia. Conversely, higher HbA1c goals may be appropriate for patients with limited life expectancy, a history of severe hypoglycemia, established microvascular or macrovascular complications, significant other comorbid conditions, or longstanding diabetes in whom an HbA1c of less than 7.0% has been difficult to attain despite optimal treatment and diabetes self-management education.19
Long-term risk reduction
A 10-year, postinterventional follow-up study (UKPDS 80) of the UKPDS survivor cohort was reported recently.20 Results showed that despite an early loss of glycemic differences between patients treated with diet and those treated with intensive regimens (sulfonylurea or insulin; metformin in overweight patients), the pharmacotherapy group demonstrated a prolonged reduction in microvascular risk as well as a significant reduction in the risk for myocardial infarction (15% [P = .01] in the sulfonylurea-insulin group and 33% [P = .005] in the metformin group) and death from any cause.20 This suggests that early improvement in glycemic control is associated with long-term benefits in the micro- and macrovascular health of patients with T2DM.
Additionally, the recent long-term follow-up of the Steno-2 study21 showed that a multifactorial intervention striving for intensive glucose, BP, and lipid control that included the use of renin-angiotensin system blockers, aspirin, and lipid-lowering agents not only reduced the risk of nonfatal CVD among patients with T2DM and microalbuminuria, but also had sustained beneficial effects on vascular complications and on rates of death from any cause and from CV causes. From a health care payer perspective, intensive multifactorial intervention was more likely to be cost-effective than conventional treatment in Denmark, especially if applied in a primary care setting.22
Comprehensive care needed
The lower-than-expected rates of CV outcomes in the ACCORD, ADVANCE, VADT, and Steno-2 studies reinforce the importance of comprehensive diabetes care that treats not only hyperglycemia but also elevated BP and dyslipidemia; these are considered the “ABCs” of diabetes.11,19 The 2009 ADA standards of medical care guidelines recommend that for most T2DM patients, HbA1c should be maintained at less than 7.0%,3 while the American Association of Clinical Endocrinologists (AACE) 2007 guidelines state that HbA1c should be 6.5% or less.11 Both organizations stress the importance of individualized goals, as discussed above, and advocate BP goals of less than 130/80 mm Hg and dyslipidemia goals of low-density lipoprotein cholesterol (LDL-C) less than 100 mg/dL, high-density lipoprotein cholesterol (HDL-C) greater than 40 mg/dL for men and 50 mg/dL for women, and triglycerides less than 150 mg/dL. It is recommended that an optional LDL-C goal of less than 70 mg/dL be considered for individuals with overt CVD.
CURRENT ANTIHYPERGLYCEMIC TREATMENT STRATEGIES
In response to new insights from clinical research and emerging treatment strategies, disease-specific organizations and medical specialty societies regularly revise and update their treatment guidelines and algorithms. These resources recommend that glycemic progress should be regularly monitored and pharmacologic therapy titrated or new drugs added promptly if glycemic goals are not met after 2 to 3 months.
Several algorithms combine scientific evidence with expert clinical opinion to guide physicians in treating their patients with T2DM. The American College of Endocrinology (ACE)/AACE road maps are designed to help develop individualized treatment regimens to achieve an HbA1c of 6.5% or less.23 The algorithm from a writing group assembled by the ADA and the European Association for the Study of Diabetes (EASD) similarly promotes pharmacologic treatment together with lifestyle modifications to maintain a glycemic goal of HbA1c less than 7.0%.24
OVERVIEW OF ANTIHYPERGLYCEMIC TREATMENT APPROACHES
Initial oral therapy
T2DM is usually treated initially with a single oral agent. Consistent with the progressive nature of the disease, patients often eventually require one or more additional oral agents and in many cases insulin.13,27 Choice of specific agents is based on individual patient circumstances, including the need for weight loss and control of fasting versus postprandial glucose, the presence of dyslipidemia and hypertension, and the risk for and potential consequences of hypoglycemia.24 T2DM patients with severely uncontrolled and symptomatic hyperglycemia are best treated, at least initially, with a combination of insulin therapy and lifestyle intervention, often with metformin.
Metformin. The recently revised ADA/EASD writing group algorithm recommends that patients not requiring initial insulin begin treatment with metformin at the time of diagnosis unless there are contraindications.24 Metformin is not associated with hypoglycemia and is considered weight-neutral, although some patients may lose weight.28
Sulfonylureas. Sulfonylureas stimulate insulin secretion from pancreatic beta cells; their use may be associated with hypoglycemia and weight gain. Mechanisms for weight gain with sulfonylureas include reduction of glucosuria and increased caloric intake to prevent or treat hypoglycemia.11,28 Nateglinide and repaglinide are nonsulfonylurea oral insulin secretagogues. They result in rapid and relatively short-lived insulin responses and are usually administered three times a day, before each meal. Their use may be associated with weight gain and hypoglycemia.11
Thiazolidinediones. Thiazolidinediones (TZD) increase insulin sensitivity in muscle, adipose tissue, and the liver. Hypoglycemia is uncommon with TZD monotherapy but weight gain related to increased and redistributed adiposity and fluid retention frequently occurs.
Alpha-glucosidase inhibitors. The alpha-glucosidase inhibitors are administered before meals and primarily reduce postprandial hyperglycemia. They are generally weight-neutral.28
Insulin. Insulin and insulin analogues are the most effective antihyperglycemic agents, but their use can be associated with hypoglycemia and clinically significant weight gain.28
Colesevelam. Colesevelam is a bile acid sequestrant that was recently approved by the US Food and Drug Administration as an antihyperglycemic therapy in people with T2DM. At a dosage of 1.875 g BID or 3.75 g QD in combination with a sulfonylurea, metformin, or insulin therapy, reductions in HbA1c compared with placebo in clinical trials of colesevelam have ranged from –0.5% to –0.7% (P < .02). Frequency of hypoglycemia and weight gain is low with this agent.26
Weight management. Weight reduction is important for overweight or obese patients with T2DM.27,28 Even moderate weight loss (5% of body weight) can be associated with improved insulin action and reduced hyperglycemia.29 Conversely, weight gain has been shown to worsen hyperglycemia and other CV risk factors. Treatment-related weight gain can also lead to decreased regimen adherence, contributing to poor glycemic control.28
THE ROLE OF INCRETIN HORMONES AND INCRETIN-BASED THERAPIES IN T2DM PATIENTS
Over the last few years, the role of incretin hormones and their contribution to diabetes pathophysiology has become more apparent. The incretin effect refers to the observation that orally administered glucose elicits a greater insulin response than does glucose administered intravenously to produce equivalent blood glucose concentrations.30,31 The incretin effect is diminished in patients with T2DM.
Hormone mediation of the incretin effect
The two hormones that mediate the incretin effect are GIP (also known as gastric inhibitory polypeptide or glucose-dependent insulinotropic polypeptide) and glucagon-like peptide−1 (GLP-1).30,31 GLP-1 has several glucoregulatory actions, including enhancement of endogenous insulin release and suppression of inappropriate glucagon secretion, both in a glucose-dependent manner. Therefore, these effects of GLP-1 occur only when glucose concentrations are elevated, thereby minimizing the risk of hypoglycemia. GLP-1 also regulates gastric emptying; infusions of GLP-1 can slow the accelerated emptying that is often present in T2DM patients. GLP-1 also increases satiety and decreases food intake via a central mechanism.31
Because GLP-1 is rapidly inactivated by the enzyme dipeptidyl peptidase–4 (DPP-4), therapeutic use of GLP-1 would require continuous infusion, which is impractical.30,31 Two strategies have been used to produce incretin-related therapies. One, inhibition of the DPP-4 enzyme, results in a two- to threefold enhancement of endogenous GLP-1. The other, involving agents that resist breakdown by DPP-4 but bind to and activate the GLP-1 receptor, produces glucoregulatory effects similar to those of GLP-1.30
Following subcutaneous (SC) injection, GLP-1 receptor agonists enhance insulin secretion and suppress inappropriately elevated glucagon, both in a glucose-dependent manner, as well as slow gastric emptying and enhance satiety.30 DPP-4 inhibitors provide glucose-dependent enhanced insulin secretion and glucagon suppression, but they do not have the same effects on gastric emptying or satiety.
Clinically, the GLP-1 receptor agonists improve glycemia and are associated with weight loss.32–35 Adverse gastrointestinal symptoms are relatively common during the first few weeks of treatment. DPP-4 inhibitors improve glycemia but are weight-neutral and are not generally associated with significant gastrointestinal symptoms.32,36–38
Incretin-based therapies
Incretin-based therapies are currently part of the antihyperglycemic armamentarium.25,32 The AACE guidelines11 and the ACE/AACE roadmaps23 include the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin among antihyperglycemic therapies for patients with T2DM. The most recent update of the consensus algorithm statement of a joint ADA/EASD writing group included GLP-1 receptor agonists (but not DPP-4 inhibitors) in tier 2 of preferred agents, especially for patients who have concerns related to weight and hypoglycemia.24 They noted that DPP-4 inhibitors may be appropriate choices in selected patients.
DPP-4 inhibitors: sitagliptin, saxagliptin. Until recently, sitagliptin was the only DPP-4 inhibitor available in the United States. Sitagliptin is approved by the FDA for treatment of T2DM at a recommended oral dosage of 100 mg QD, either as monotherapy or in combination with other oral antihyperglycemic medications. The dosage of sitagliptin should be reduced to 50 mg/day in patients with creatinine clearance (CrCl) levels that are between 30 mL/min and 50 mL/min and to 25 mg/day in those with CrCl less than 30 mL/min.39
In a meta-analysis of incretin-based therapies, DPP-4 inhibitors produced a reduction in HbA1c compared with placebo (weighted mean difference of –0.74%; 95% confidence interval, –0.85% to –0.62%).32 DPP-4 inhibitor antihyperglycemic efficacy has been shown to be similar whether used as a monotherapy or add-on therapy.32,37,38 This same meta-analysis showed DPP-4 inhibitors as having a neutral effect on weight.32 More recently, a single-pill combination of metformin and sitagliptin was approved.40
A study comparing metformin, sitagliptin, and the combination of the two as initial monotherapy in T2DM patients with a baseline HbA1c of 8.8% showed 24-week HbA1c reductions from baseline of –0.66% with sitagliptin 100 mg QD, –0.82% with metformin 500 mg BID, and –1.90% with sitagliptin 50 mg + metformin 1,000 mg BID.41
On July 31, 2009, the FDA approved another DPP-4 inhibitor, saxagliptin, for the treatment of T2DM either as monotherapy or in combination with metformin, a sulfonylurea, or a TZD.42
GLP-1 receptor agonist: exenatide. Exenatide, the only FDA-approved GLP-1 receptor agonist, is the synthetic version of exendin-4, which binds to the human GLP-1 receptor and in vitro possesses many of the glucoregulatory effects of endogenous GLP-1.30,32 Exenatide is indicated as monotherapy or adjunctive therapy for patients with T2DM who have not achieved adequate glycemic control with metformin, a sulfonylurea, a TZD, or metformin in combination with a sulfonylurea or a TZD.43 Exenatide is administered by SC injection BID at a starting dosage of 5 mg BID for 4 weeks, followed by an increase to 10 mg BID.
Exenatide has been shown not only to enhance glucose-dependent insulin secretion but also to restore impaired first-phase insulin response in subjects with T2DM. Exenatide also helps control postprandial glycemic excursions by suppressing inappropriate glucagon secretion, slowing accelerated gastric emptying, and enhancing satiety. The increased satiety results in decreased food intake and weight loss.31,44 In a recent head-to-head crossover study, exenatide was shown to be more effective than sitagliptin in lowering postprandial glucose concentrations, increasing insulin secretion, and reducing postprandial glucagon secretion.45 Exenatide also slowed gastric emptying and reduced caloric intake.
Exenatide, in most studies, resulted in a placebo-subtracted HbA1c reduction of approximately –1.0% and in one study lowered HbA1c from baseline by –1.5%. Completer analyses have shown HbA1c reductions of –1.0% up to 3 years and –0.8% up to 3.5 years. Exenatide has also been associated with a mean weight loss of as much as –3.6 kg at 30 weeks and as much as –5.3 kg at 3.5 years.33–35,46,47 A 1-year study showed that exenatide improved beta-cell secretory function compared with insulin glargine in metformin-treated patients with T2DM.48 Long-term data, including findings from completed and intention-to-treat analyses of 82 weeks49 to at least 3 years47 have demonstrated that exenatide improved CV risk factors, including those related to BP, lipids, and hepatic injury biomarkers.
Therapies in development
Incretin-based therapies in development include a novel once-weekly formulation of exenatide; taspoglutide, another once-weekly GLP-1 receptor agonist; and liraglutide, a GLP-1 receptor agonist that is administered once daily.50 Liraglutide is currently being evaluated in clinical trials as a once-daily SC injection.51–53 Liraglutide has been reported to reduce HbA1c by –1.1% at 26 weeks and up to –1.14% at 52 weeks and result in weight loss (up to –2.8 kg at 26 weeks and up to –2.5 kg at 52 weeks) in patients with T2DM who are treatment-naïve or taking other antidiabetes agents, including metformin, sulfonylurea, and TZD.51–53 Evaluation of the once-weekly formulation of exenatide showed reductions in HbA1c of –1.9% at 30 weeks and –2.0% at 52 weeks with a weight loss of –3.7 kg at 30 weeks and –4.1 kg over 52 weeks of treatment.46,54
CONCLUSION
In the United States, the epidemics of excessive weight and T2DM have contributed to an increased medical risk for many individuals. Comprehensive diabetes treatments targeting not only hyperglycemia but also frequently associated overweight/obesity, hypertension, and dyslipidemia will be required to reduce such risk. Current treatment strategies have evolved based on updated clinical guidelines and trials, as well as practice experience, including those related to newer agents. Incretin-based therapies, such as the GLP-1 receptor agonist, exenatide, and the DPP-4 inhibitors, sitagliptin and saxagliptin, are important additions to the treatment armamentarium, offering a reduction in hyperglycemia and beneficial effects on weight (reduction with exenatide and neutral with sitagliptin), and have been shown to improve several CV risk factors.
Data from the Centers for Disease Control and Prevention indicate that almost 24 million Americans, or 7.8% of the population, have diabetes; 90% to 95% of these have type 2 diabetes mellitus (T2DM).1 Diabetes and excessive weight often coexist. An analysis of data from the 1999–2002 National Health and Nutrition Examination Survey (NHANES) showed that among individuals with diabetes, 85% were overweight or obese and 55% were obese.2
Gaps remain in the management of T2DM between the goals for clinical parameters of care (eg, control of glucose, blood pressure [BP], and lipids) and actual clinical practice.3 NHANES data reveal that glycemic control improved from a mean glycosylated hemoglobin A1c (HbA1c) of 7.82% in 1999–2000 to 7.18% in 2003–2004.4 Hazard models based on the United Kingdom Prospective Diabetes Study (UKPDS) 10-year outcomes data in 4,320 newly diagnosed T2DM patients suggest that a sustained decrease in HbA1c of 0.511 percentage points could reduce diabetes complications by 10.7%.4,5
Additional analysis of NHANES data showed that in 2003–2004, about 57% of individuals achieved glycemic control, 48% reached BP targets, and 50% achieved target cholesterol goals.Only about 13% of diabetes patients achieved their target goals for all three parameters concurrently.6
This article reviews the association between cardiometabolic risk and the current antihyperglycemic treatments for patients with T2DM, with a focus on the role of incretin-related therapies.
THE IMPORTANCE OF CARDIOMETABOLIC RISK IN T2DM
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among people with diabetes and is the reported cause of mortality in up to 65% of deaths in persons with diabetes in the United States.7 The risk of CVD is two- to fourfold greater among adults with diabetes than among adults who do not have diabetes.8 The risk of CVD in patients with T2DM was evident in the UKPDS 17, where macrovascular complications, including CVD, were about twice as common as microvascular complications (20% vs 9%) after 9 years of follow-up.9 A study that involved more than 44,000 patients showed an almost double rate of mortality from all causes among individuals with T2DM compared with those with no diabetes (hazard ratio, 1.93; 95% confidence interval, 1.89 to 1.97).10 Current guidelines recommend aggressive management of CV risk factors, including BP control, correction of atherogenic dyslipidemia, glycemic control, weight reduction for those who are overweight or obese, and smoking cessation for those who smoke.3,11 Lifestyle interventions, including weight reduction and appropriately prescribed physical activity, result in reduced CV risk factors, which can help slow the progression of T2DM.12
GOALS OF T2DM THERAPY
Several studies have demonstrated that glycemic control can delay or prevent the development and progression of microvascular complications.13,14 UKPDS 33 showed that more intensive blood glucose control (median HbA1c 7.0%) in patients with T2DM followed over 10 years significantly (P = .029) reduced the risk for any diabetes-related end point by 12% compared with conventional therapy (median HbA1c 7.9%). Most of the risk reduction was accounted for by a 25% risk reduction in microvascular end points (P = .0099).13 Another report (UKPDS 35) demonstrated that HbA1c was strongly related to microvascular effects, with a 1% reduction in HbA1c associated with a 37% reduction in microvascular complications.14
Does intensive glucose control reduce CV risk?
To resolve the ongoing question of whether intensive glucose control can lead to a reduction in CV risk in patients with T2DM, three large, long-term trials were conducted within the last decade.15–18 Two of these, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trials, each enrolled more than 10,000 previously treated patients with long-standing T2DM. Patients were randomized to standard or intensive glycemic control for 3.5 years in the ACCORD trial and for 5 years in the ADVANCE trial.15,16
The ACCORD and ADVANCE trials, along with the smaller Veterans Administration Diabetes Trial (VADT) (N = 1,791), failed to show that more intensive glycemic control significantly reduced CVD.15–17 Additionally, the glycemic control component of ACCORD was halted because of increased mortality in the intensive arm compared with the standard arm.15 Further analyses of ACCORD data presented at the 69th Scientific Sessions of the American Diabetes Association (ADA) showed that HbA1c values lower than 7.0% did not explain the increased mortality. The 20% higher risk of death for every 1.0% increase in HbA1c greater than 6.0% suggests that glucose concentrations even lower than the general HbA1c goal of less than 7.0% may be appropriate in some patients.18 The most recent finding from VADT was that CV risk was dependent on disease duration and presence of comorbidities. Intensive therapy seemed to work best in patients with diabetes of less than 15 years’ duration, while risk of a CV event was more than doubled with intensive therapy in patients having diabetes for more than 21 years.
Clarification of treatment goals
A position statement of the ADA and a scientific statement of the American College of Cardiology Foundation and the American Heart Association19 concluded that the “evidence obtained from ACCORD, ADVANCE, and VADT does not suggest the need for major changes in glycemic control targets but, rather, additional clarification of the language that has consistently stressed individualization.” They state that while the general HbA1c goal of less than 7.0% seems reasonable, even lower HbA1c goals may be appropriate for some patients if they can be achieved without significant hypoglycemia or other adverse effects. Such patients might include those with diabetes of short duration, long life expectancy, or no significant CVD or hypoglycemia. Conversely, higher HbA1c goals may be appropriate for patients with limited life expectancy, a history of severe hypoglycemia, established microvascular or macrovascular complications, significant other comorbid conditions, or longstanding diabetes in whom an HbA1c of less than 7.0% has been difficult to attain despite optimal treatment and diabetes self-management education.19
Long-term risk reduction
A 10-year, postinterventional follow-up study (UKPDS 80) of the UKPDS survivor cohort was reported recently.20 Results showed that despite an early loss of glycemic differences between patients treated with diet and those treated with intensive regimens (sulfonylurea or insulin; metformin in overweight patients), the pharmacotherapy group demonstrated a prolonged reduction in microvascular risk as well as a significant reduction in the risk for myocardial infarction (15% [P = .01] in the sulfonylurea-insulin group and 33% [P = .005] in the metformin group) and death from any cause.20 This suggests that early improvement in glycemic control is associated with long-term benefits in the micro- and macrovascular health of patients with T2DM.
Additionally, the recent long-term follow-up of the Steno-2 study21 showed that a multifactorial intervention striving for intensive glucose, BP, and lipid control that included the use of renin-angiotensin system blockers, aspirin, and lipid-lowering agents not only reduced the risk of nonfatal CVD among patients with T2DM and microalbuminuria, but also had sustained beneficial effects on vascular complications and on rates of death from any cause and from CV causes. From a health care payer perspective, intensive multifactorial intervention was more likely to be cost-effective than conventional treatment in Denmark, especially if applied in a primary care setting.22
Comprehensive care needed
The lower-than-expected rates of CV outcomes in the ACCORD, ADVANCE, VADT, and Steno-2 studies reinforce the importance of comprehensive diabetes care that treats not only hyperglycemia but also elevated BP and dyslipidemia; these are considered the “ABCs” of diabetes.11,19 The 2009 ADA standards of medical care guidelines recommend that for most T2DM patients, HbA1c should be maintained at less than 7.0%,3 while the American Association of Clinical Endocrinologists (AACE) 2007 guidelines state that HbA1c should be 6.5% or less.11 Both organizations stress the importance of individualized goals, as discussed above, and advocate BP goals of less than 130/80 mm Hg and dyslipidemia goals of low-density lipoprotein cholesterol (LDL-C) less than 100 mg/dL, high-density lipoprotein cholesterol (HDL-C) greater than 40 mg/dL for men and 50 mg/dL for women, and triglycerides less than 150 mg/dL. It is recommended that an optional LDL-C goal of less than 70 mg/dL be considered for individuals with overt CVD.
CURRENT ANTIHYPERGLYCEMIC TREATMENT STRATEGIES
In response to new insights from clinical research and emerging treatment strategies, disease-specific organizations and medical specialty societies regularly revise and update their treatment guidelines and algorithms. These resources recommend that glycemic progress should be regularly monitored and pharmacologic therapy titrated or new drugs added promptly if glycemic goals are not met after 2 to 3 months.
Several algorithms combine scientific evidence with expert clinical opinion to guide physicians in treating their patients with T2DM. The American College of Endocrinology (ACE)/AACE road maps are designed to help develop individualized treatment regimens to achieve an HbA1c of 6.5% or less.23 The algorithm from a writing group assembled by the ADA and the European Association for the Study of Diabetes (EASD) similarly promotes pharmacologic treatment together with lifestyle modifications to maintain a glycemic goal of HbA1c less than 7.0%.24
OVERVIEW OF ANTIHYPERGLYCEMIC TREATMENT APPROACHES
Initial oral therapy
T2DM is usually treated initially with a single oral agent. Consistent with the progressive nature of the disease, patients often eventually require one or more additional oral agents and in many cases insulin.13,27 Choice of specific agents is based on individual patient circumstances, including the need for weight loss and control of fasting versus postprandial glucose, the presence of dyslipidemia and hypertension, and the risk for and potential consequences of hypoglycemia.24 T2DM patients with severely uncontrolled and symptomatic hyperglycemia are best treated, at least initially, with a combination of insulin therapy and lifestyle intervention, often with metformin.
Metformin. The recently revised ADA/EASD writing group algorithm recommends that patients not requiring initial insulin begin treatment with metformin at the time of diagnosis unless there are contraindications.24 Metformin is not associated with hypoglycemia and is considered weight-neutral, although some patients may lose weight.28
Sulfonylureas. Sulfonylureas stimulate insulin secretion from pancreatic beta cells; their use may be associated with hypoglycemia and weight gain. Mechanisms for weight gain with sulfonylureas include reduction of glucosuria and increased caloric intake to prevent or treat hypoglycemia.11,28 Nateglinide and repaglinide are nonsulfonylurea oral insulin secretagogues. They result in rapid and relatively short-lived insulin responses and are usually administered three times a day, before each meal. Their use may be associated with weight gain and hypoglycemia.11
Thiazolidinediones. Thiazolidinediones (TZD) increase insulin sensitivity in muscle, adipose tissue, and the liver. Hypoglycemia is uncommon with TZD monotherapy but weight gain related to increased and redistributed adiposity and fluid retention frequently occurs.
Alpha-glucosidase inhibitors. The alpha-glucosidase inhibitors are administered before meals and primarily reduce postprandial hyperglycemia. They are generally weight-neutral.28
Insulin. Insulin and insulin analogues are the most effective antihyperglycemic agents, but their use can be associated with hypoglycemia and clinically significant weight gain.28
Colesevelam. Colesevelam is a bile acid sequestrant that was recently approved by the US Food and Drug Administration as an antihyperglycemic therapy in people with T2DM. At a dosage of 1.875 g BID or 3.75 g QD in combination with a sulfonylurea, metformin, or insulin therapy, reductions in HbA1c compared with placebo in clinical trials of colesevelam have ranged from –0.5% to –0.7% (P < .02). Frequency of hypoglycemia and weight gain is low with this agent.26
Weight management. Weight reduction is important for overweight or obese patients with T2DM.27,28 Even moderate weight loss (5% of body weight) can be associated with improved insulin action and reduced hyperglycemia.29 Conversely, weight gain has been shown to worsen hyperglycemia and other CV risk factors. Treatment-related weight gain can also lead to decreased regimen adherence, contributing to poor glycemic control.28
THE ROLE OF INCRETIN HORMONES AND INCRETIN-BASED THERAPIES IN T2DM PATIENTS
Over the last few years, the role of incretin hormones and their contribution to diabetes pathophysiology has become more apparent. The incretin effect refers to the observation that orally administered glucose elicits a greater insulin response than does glucose administered intravenously to produce equivalent blood glucose concentrations.30,31 The incretin effect is diminished in patients with T2DM.
Hormone mediation of the incretin effect
The two hormones that mediate the incretin effect are GIP (also known as gastric inhibitory polypeptide or glucose-dependent insulinotropic polypeptide) and glucagon-like peptide−1 (GLP-1).30,31 GLP-1 has several glucoregulatory actions, including enhancement of endogenous insulin release and suppression of inappropriate glucagon secretion, both in a glucose-dependent manner. Therefore, these effects of GLP-1 occur only when glucose concentrations are elevated, thereby minimizing the risk of hypoglycemia. GLP-1 also regulates gastric emptying; infusions of GLP-1 can slow the accelerated emptying that is often present in T2DM patients. GLP-1 also increases satiety and decreases food intake via a central mechanism.31
Because GLP-1 is rapidly inactivated by the enzyme dipeptidyl peptidase–4 (DPP-4), therapeutic use of GLP-1 would require continuous infusion, which is impractical.30,31 Two strategies have been used to produce incretin-related therapies. One, inhibition of the DPP-4 enzyme, results in a two- to threefold enhancement of endogenous GLP-1. The other, involving agents that resist breakdown by DPP-4 but bind to and activate the GLP-1 receptor, produces glucoregulatory effects similar to those of GLP-1.30
Following subcutaneous (SC) injection, GLP-1 receptor agonists enhance insulin secretion and suppress inappropriately elevated glucagon, both in a glucose-dependent manner, as well as slow gastric emptying and enhance satiety.30 DPP-4 inhibitors provide glucose-dependent enhanced insulin secretion and glucagon suppression, but they do not have the same effects on gastric emptying or satiety.
Clinically, the GLP-1 receptor agonists improve glycemia and are associated with weight loss.32–35 Adverse gastrointestinal symptoms are relatively common during the first few weeks of treatment. DPP-4 inhibitors improve glycemia but are weight-neutral and are not generally associated with significant gastrointestinal symptoms.32,36–38
Incretin-based therapies
Incretin-based therapies are currently part of the antihyperglycemic armamentarium.25,32 The AACE guidelines11 and the ACE/AACE roadmaps23 include the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin among antihyperglycemic therapies for patients with T2DM. The most recent update of the consensus algorithm statement of a joint ADA/EASD writing group included GLP-1 receptor agonists (but not DPP-4 inhibitors) in tier 2 of preferred agents, especially for patients who have concerns related to weight and hypoglycemia.24 They noted that DPP-4 inhibitors may be appropriate choices in selected patients.
DPP-4 inhibitors: sitagliptin, saxagliptin. Until recently, sitagliptin was the only DPP-4 inhibitor available in the United States. Sitagliptin is approved by the FDA for treatment of T2DM at a recommended oral dosage of 100 mg QD, either as monotherapy or in combination with other oral antihyperglycemic medications. The dosage of sitagliptin should be reduced to 50 mg/day in patients with creatinine clearance (CrCl) levels that are between 30 mL/min and 50 mL/min and to 25 mg/day in those with CrCl less than 30 mL/min.39
In a meta-analysis of incretin-based therapies, DPP-4 inhibitors produced a reduction in HbA1c compared with placebo (weighted mean difference of –0.74%; 95% confidence interval, –0.85% to –0.62%).32 DPP-4 inhibitor antihyperglycemic efficacy has been shown to be similar whether used as a monotherapy or add-on therapy.32,37,38 This same meta-analysis showed DPP-4 inhibitors as having a neutral effect on weight.32 More recently, a single-pill combination of metformin and sitagliptin was approved.40
A study comparing metformin, sitagliptin, and the combination of the two as initial monotherapy in T2DM patients with a baseline HbA1c of 8.8% showed 24-week HbA1c reductions from baseline of –0.66% with sitagliptin 100 mg QD, –0.82% with metformin 500 mg BID, and –1.90% with sitagliptin 50 mg + metformin 1,000 mg BID.41
On July 31, 2009, the FDA approved another DPP-4 inhibitor, saxagliptin, for the treatment of T2DM either as monotherapy or in combination with metformin, a sulfonylurea, or a TZD.42
GLP-1 receptor agonist: exenatide. Exenatide, the only FDA-approved GLP-1 receptor agonist, is the synthetic version of exendin-4, which binds to the human GLP-1 receptor and in vitro possesses many of the glucoregulatory effects of endogenous GLP-1.30,32 Exenatide is indicated as monotherapy or adjunctive therapy for patients with T2DM who have not achieved adequate glycemic control with metformin, a sulfonylurea, a TZD, or metformin in combination with a sulfonylurea or a TZD.43 Exenatide is administered by SC injection BID at a starting dosage of 5 mg BID for 4 weeks, followed by an increase to 10 mg BID.
Exenatide has been shown not only to enhance glucose-dependent insulin secretion but also to restore impaired first-phase insulin response in subjects with T2DM. Exenatide also helps control postprandial glycemic excursions by suppressing inappropriate glucagon secretion, slowing accelerated gastric emptying, and enhancing satiety. The increased satiety results in decreased food intake and weight loss.31,44 In a recent head-to-head crossover study, exenatide was shown to be more effective than sitagliptin in lowering postprandial glucose concentrations, increasing insulin secretion, and reducing postprandial glucagon secretion.45 Exenatide also slowed gastric emptying and reduced caloric intake.
Exenatide, in most studies, resulted in a placebo-subtracted HbA1c reduction of approximately –1.0% and in one study lowered HbA1c from baseline by –1.5%. Completer analyses have shown HbA1c reductions of –1.0% up to 3 years and –0.8% up to 3.5 years. Exenatide has also been associated with a mean weight loss of as much as –3.6 kg at 30 weeks and as much as –5.3 kg at 3.5 years.33–35,46,47 A 1-year study showed that exenatide improved beta-cell secretory function compared with insulin glargine in metformin-treated patients with T2DM.48 Long-term data, including findings from completed and intention-to-treat analyses of 82 weeks49 to at least 3 years47 have demonstrated that exenatide improved CV risk factors, including those related to BP, lipids, and hepatic injury biomarkers.
Therapies in development
Incretin-based therapies in development include a novel once-weekly formulation of exenatide; taspoglutide, another once-weekly GLP-1 receptor agonist; and liraglutide, a GLP-1 receptor agonist that is administered once daily.50 Liraglutide is currently being evaluated in clinical trials as a once-daily SC injection.51–53 Liraglutide has been reported to reduce HbA1c by –1.1% at 26 weeks and up to –1.14% at 52 weeks and result in weight loss (up to –2.8 kg at 26 weeks and up to –2.5 kg at 52 weeks) in patients with T2DM who are treatment-naïve or taking other antidiabetes agents, including metformin, sulfonylurea, and TZD.51–53 Evaluation of the once-weekly formulation of exenatide showed reductions in HbA1c of –1.9% at 30 weeks and –2.0% at 52 weeks with a weight loss of –3.7 kg at 30 weeks and –4.1 kg over 52 weeks of treatment.46,54
CONCLUSION
In the United States, the epidemics of excessive weight and T2DM have contributed to an increased medical risk for many individuals. Comprehensive diabetes treatments targeting not only hyperglycemia but also frequently associated overweight/obesity, hypertension, and dyslipidemia will be required to reduce such risk. Current treatment strategies have evolved based on updated clinical guidelines and trials, as well as practice experience, including those related to newer agents. Incretin-based therapies, such as the GLP-1 receptor agonist, exenatide, and the DPP-4 inhibitors, sitagliptin and saxagliptin, are important additions to the treatment armamentarium, offering a reduction in hyperglycemia and beneficial effects on weight (reduction with exenatide and neutral with sitagliptin), and have been shown to improve several CV risk factors.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, 2008. Available at: http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes: United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008; 31:81–86.
- Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 2006; 49:1761–1769.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004; 140:945–950.
- Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004; 292:2495–2499.
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in noninsulin-dependent diabetes mellitus. Ann Intern Med 1996; 124(1 Pt 2):136–145.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Kerr M. ADA 2009: intensive glycemic control not directly linked to excess cardiovascular risk. Medscape Medical News Web site. http://www.medscape.com/viewarticle/704260_print. Published June 11, 2009. Accessed September 16, 2009.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Gaede P, Valentine WJ, Palmer AJ, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008; 31:1510–1515.
- ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus. Endocr Pract 2007; 13:260–268.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- Sonnett TE, Levien TL, Neumiller JJ, Gates BJ, Setter SM. Colesevelam hydrochloride for the treatment of type 2 diabetes mellitus. Clin Ther 2009; 31:245–259.
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131:281–303.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3:153–165.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632−2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Januvia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2048–2054.
- Janumet. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2041–2048.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, for the Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281:E155–E161.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, 2008. Available at: http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes: United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008; 31:81–86.
- Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 2006; 49:1761–1769.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004; 140:945–950.
- Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004; 292:2495–2499.
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in noninsulin-dependent diabetes mellitus. Ann Intern Med 1996; 124(1 Pt 2):136–145.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Kerr M. ADA 2009: intensive glycemic control not directly linked to excess cardiovascular risk. Medscape Medical News Web site. http://www.medscape.com/viewarticle/704260_print. Published June 11, 2009. Accessed September 16, 2009.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Gaede P, Valentine WJ, Palmer AJ, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008; 31:1510–1515.
- ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus. Endocr Pract 2007; 13:260–268.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- Sonnett TE, Levien TL, Neumiller JJ, Gates BJ, Setter SM. Colesevelam hydrochloride for the treatment of type 2 diabetes mellitus. Clin Ther 2009; 31:245–259.
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131:281–303.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3:153–165.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632−2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Januvia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2048–2054.
- Janumet. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008:2041–2048.
- Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, for the Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30:1979–1987.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281:E155–E161.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
KEY POINTS
- Up to 65% of deaths among people with diabetes are caused by cardiovascular disease.
- Glycemic control can delay or slow the progression of microvascular complications.
- In addition to hyperglycemia, comprehensive diabetes therapy must target cardiovascular disease–related risk factors, including excess weight/obesity, elevated blood pressure, and abnormal lipid concentrations.
- Diminished incretin hormonal activity contributes to the pathophysiology of diabetes.
Role of the incretin pathway in the pathogenesis of type 2 diabetes mellitus
It has long been understood that the pathophysiology of type 2 diabetes mellitus (T2DM) is based on the triad of progressive decline in insulin-producing pancreatic beta cells, an increase in insulin resistance, and increased hepatic glucose production.1,2 It is now evident that other factors, including defective actions of the gastrointestinal (GI) incretin hormones glucagon-like peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), also play significant roles.2–5 The uncontrolled hyperglycemia resulting from such defects may lead to microvascular complications, including retinopathy, neuropathy, microangiopathy, and nephropathy, and macrovascular complications, such as coronary artery disease and peripheral vascular disease.
This review explores the growing understanding of the role of the incretins in normal insulin secretion, as well as in the pathogenesis of T2DM, and examines the pathophysiologic basis for the benefits and therapeutic application of incretin-based therapies in T2DM.1,2
THE GI SYSTEM AND GLUCOSE HOMEOSTASIS IN THE HEALTHY STATE
The GI system plays an integral role in glucose homeostasis.6 The observation that orally administered glucose provides a stronger insulinotropic stimulus than an intravenous glucose challenge provided insight into the regulation of plasma glucose by the GI system of healthy individuals.7 The incretin effect, as this is termed, may be responsible for 50% to 70% of the total insulin secreted following oral glucose intake.8
Two GI peptide hormones (the incretins)—GLP-1 and GIP—were found to exert major glucoregulatory actions.3,9,10 Within minutes of nutrient ingestion, GLP-1 is secreted from intestinal L cells in the distal ileum and colon, while GIP is released by intestinal K cells in the duodenum and jejunum.3 GLP-1 and GIP trigger their insulinotropic actions by binding beta-cell receptors.3 GLP-1 receptors are expressed on pancreatic glucagon-containing alpha and delta cells as well as on beta cells, whereas GIP receptors are expressed primarily on beta cells.3,8 GLP-1 receptors are also expressed in the central nervous system (CNS), peripheral nervous system, lung, heart, and GI tract, while GIP receptors are expressed in adipose tissue and the CNS.3 GLP-1 inhibits glucose-dependent glucagon secretion from alpha cells.3 In healthy individuals, fasting glucose is managed by tonic insulin/glucagon secretion, but excursions of postprandial glucose (PPG) are controlled by insulin and the incretin hormones.11
Additionally, in animal studies, GLP-1 has been shown to induce the transcriptional activation of the insulin gene and insulin biosynthesis, thus increasing beta-cell proliferation and decreasing beta-cell apoptosis.12 GLP-1 stimulates a CNS-mediated pathway of insulin secretion, slows gastric emptying, increases CNS-mediated satiety leading to reduced food intake, indirectly increases insulin sensitivity and nutrient uptake in skeletal muscle and adipose tissue, and exerts neuroprotective effects.8
Both GLP-1 and GIP are rapidly degraded by the serine protease dipeptidyl peptidase–4 (DPP-4), which is widely expressed in bound and free forms.14 A recent study in healthy adults showed that GLP-1 concentration declined even during maximal DPP-4 inhibition, suggesting that there may be pathways of GLP-1 elimination other than DPP-4 enzymatic degradation.15
INCRETINS AND THE PATHOGENESIS OF T2DM
Studies have shown that incretin pathways play a role in the progression of T2DM.3,16 The significant reduction in the incretin effect seen in patients with T2DM has been attributed to several factors, including impaired secretion of GLP-1, accelerated metabolism of GLP-1 and GIP, and defective responsiveness to both hormones.16 Many patients with T2DM also have accelerated gastric emptying that may contribute to deterioration of their glycemic control.17
While GIP concentration is normal or modestly increased in patients with T2DM,16,18 the insulinotropic actions of GIP are significantly diminished.19 Thus, patients with T2DM have an impaired responsiveness to GIP with a possible link to GIP-receptor downregulation or desensitization.20
Are secretory defects a cause or result of T2DM?
In contrast to GIP, the secretion of GLP-1 has been shown to be deficient in patients with T2DM.18 As with GIP, it is unknown to what degree this defect is a cause or consequence of T2DM. In a study of identical twins, defective GLP-1 secretion was observed only in the one sibling with T2DM, suggesting that GLP-1 secretory deficits may be secondary to the development of T2DM.21 Despite the diminished secretion of GLP-1 in patients with T2DM, the insulinotropic actions of GLP-1 are preserved.19 It has also been shown that the effects of GLP-1 on gastric emptying and glucagon secretion are maintained in patients with T2DM.19,22,23
Whether this incretin dysregulation is responsible for or is the end result of hyperglycemia remains a subject of continued investigation. A recent study confirmed that the incretin effect is reduced in patients with T2DM, but advanced the concept that it may be a consequence of the diabetic state.16,24 Notably, impaired actions of GLP-1 and GIP and diminished concentrations of GLP-1 may be partially restored by improved glycemic control.24
Recent preclinical and clinical studies continue to clarify the roles of incretin hormones in T2DM. The findings from a study of obese diabetic mice suggest that the effect of GLP-1 therapy on the long-term remission of diabetes may be caused by improvements in beta-cell function and insulin sensitivity, as well as by a reduction in gluconeogenesis in the liver.25
Incretin effect and glucose tolerance, body mass index
Another study was conducted to evaluate quantitatively the separate impacts of obesity and hyperglycemia on the incretin effect in patients with T2DM, patients with impaired glucose tolerance, and patients with normal glucose tolerance.26 There was a significant (P ≤ .05) reduction in the incretin effect in terms of total insulin secretion, beta-cell glucose sensitivity, and the GLP-1 response to oral glucose in patients with T2DM compared with individuals whose glucose tolerance was normal or impaired. Each manifestation of the incretin effect was inversely related to both glucose tolerance and body mass index in an independent, additive manner (P ≤ .05); thus, glucose tolerance and obesity attenuate the incretin effect on beta-cell function and GLP-1 response independently of each other.
Exogenous GLP-1 has been shown to restore the regulation of blood glucose to near-normal concentrations in patients with T2DM.27 Several studies of patients with T2DM have shown that synthetic GLP-1 administration induces insulin secretion,19,27 slows gastric emptying (which is accelerated in patients with T2DM), and decreases inappropriately elevated glucagon secretion.19,23,28 Acute GLP-1 infusion studies showed that GLP-1 improved fasting plasma glucose (FPG) and PPG concentrations23,27; long-term studies showed that this hormone exerts euglycemic effects, leading to improvements in glycosylated hemoglobin (HbA1c), and induces weight loss.29
TARGETING FUNDAMENTAL DEFECTS OF T2DM WITH INCRETIN-BASED THERAPIES
Recognition and a better understanding of the role of the incretins and the enzyme involved in their degradation have led to the development of two incretin-based treatments: the GLP-1 receptor agonists, which possess many of the glucoregulatory actions of incretin peptides, and the DPP-4 inhibitors.5 Both the GLP-1 receptor agonists and the DPP-4 inhibitors have demonstrated safety and efficacy in the management of hyperglycemia in patients with T2DM.
GLP-1 receptor agonists
The GLP-1 receptor agonist exenatide is a synthetic form of exendin-4 and has a unique amino acid sequence that renders it resistant to degradation by DPP-4, making its actions longer lasting than endogenous GLP-1.5,30 Exenatide has a half-life of 2.4 hours and is detectable for up to 10 hours after subcutaneous (SC) injection.5,30 It is administered BID and has been approved as monotherapy or an adjunct therapy in patients with T2DM who have inadequate glycemic control following treatment with metformin, a sulfonylurea, a thiazolidinedione (TZD), or metformin in combination with a sulfonylurea or a TZD.31–35
In both human and animal studies, exenatide has been shown to enhance glucose-dependent insulin secretion and suppress inappropriate glucagon secretion in a glucose-dependent manner, reduce food intake and body weight, and acutely improve beta-cell function by enhancing first- and second-phase insulin secretion.5,36,37
In a small study involving 17 patients with T2DM, exenatide was shown to slow gastric emptying, which could be an important mechanism contributing to its beneficial effects on PPG concentration.38 Exenatide also has been shown to attenuate postprandial hyperglycemia, a risk factor for cardiovascular disease (CVD), by reducing endogenous glucose production by about 50% in patients with T2DM.39 Another mechanism for glycemic control may exist, as a recent animal study has shown that exenatide, similar to endogenous GLP-1, lowers blood glucose concentration independent of changes in pancreatic islet hormone secretion or delayed gastric emptying.40
A formulation of exenatide that is administered once weekly—exenatide long-acting release (LAR)—is in clinical evaluation and under review by the US Food and Drug Administration (FDA). In a short-term study, exenatide-LAR (0.8 mg or 2.0 mg) was administered once weekly for 15 weeks to patients with T2DM whose glycemia was suboptimally controlled with metformin alone or in combination with diet and exercise. Compared with placebo, treatment with exenatide once weekly was associated with markedly reduced HbA1c, FPG, PPG and body weight.41 In a larger, 30-week, phase 3 trial, Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide ONce Weekly (DURATION-1), exenatide-LAR 2 mg once weekly was compared with exenatide 10 mg BID in patients with T2DM. Exenatide-LAR once weekly was associated with a significantly greater reduction in HbA1c (–1.9% vs –1.5%, P = .0023), and with a similar low risk of hypoglycemia and reduction in body weight (–3.7 kg vs –3.6 kg, P = .89) compared with the BID formulation.42
Liraglutide, recently approved in the European Union for T2DM and also under regulatory review in the United States, is a DPP-4–resistant human analogue GLP-1 receptor agonist in clinical development that has a 97% homology to native GLP-1.43–45 In contrast to exenatide, the acetylated liraglutide molecule allows binding to serum albumin and provides resistance to DPP-4 degradation, thus prolonging the half-life of liraglutide to approximately 12 hours. Liraglutide is administered SC QD as monotherapy or in combination with other antidiabetes agents such as metformin or sulfonylurea to patients with T2DM.44–47 Liraglutide has been shown to reduce HbA1c, decrease body weight, and lead to a lower incidence of hypoglycemia compared with the sulfonylurea glimepiride.
DPP-4 inhibitors
Sitagliptin is a DPP-4 inhibitor indicated as monotherapy or in combination with metformin or a TZD in patients with T2DM with inadequate glycemic control.48–51 Given orally, sitagliptin does not bind to the GLP-1 receptor agonist and has been shown to inhibit circulating DPP-4 activity by about 80%.52,53 Sitagliptin has been associated with an approximate twofold increase in postprandial GLP-1 plasma concentrations compared with placebo in healthy human subjects and in patients with T2DM.53 Saxagliptin, another potent DPP-4 inhibitor, significantly reduced HbA1c and FPG concentrations in patients with T2DM54 with a neutral effect on weight; it was recently approved by the FDA for treatment of T2DM.55
The DPP-4 inhibitor vildagliptin is currently being used in the European Union and Latin America but has yet to receive regulatory approval in the United States.54 Alogliptin, a novel, high-affinity, high-specificity DPP-4 inhibitor currently in development, provides rapid and sustained DPP-4 inhibition and significantly reduces HbA1c, FPG, and PPG concentrations with no change in body weight in patients with T2DM.56,57
Incretin-based therapies compared
Effects of incretin-based therapies
The number of people with T2DM, overweight/obesity, or CVD, alone or in combination, is approaching epidemic proportions, with the mechanisms of these conditions interrelated. Approximately 24 million Americans have diabetes, and T2DM accounts for more than 90% of these cases.61 Most patients with T2DM are not achieving HbA1c targets.62–64 About 60% of deaths among patients with T2DM are caused by CVD.65 Compounding the problem, overweight/obesity enhances the risk for CV-related morbidities in patients with diabetes.66 A cluster of metabolic disorders referred to as the metabolic syndrome (which includes hyperglycemia, measures of central obesity, and a series of significant CV risk factors) is common in patients with T2DM and CVD.67 Unfortunately, many antidiabetes drugs that successfully manage glycemic control also cause weight gain, which in theory may increase CV risk in patients with T2DM.68
Data from studies of patients with T2DM show that exenatide improves glycemic control and reduces body weight. Exenatide administered BID significantly reduced HbA1c (–0.40% to –0.86%) and weight (–1.6 kg to –2.8 kg) relative to baseline in three 30-week, placebo-controlled clinical trials.31,33,34 In subsequent 2-year, open-label extension studies, exenatide produced significant reductions from baseline in HbA1c (–20.9% at 30 weeks) and weight (–2.1 kg at 30 weeks). Both decreases were sustained through 2 years (HbA1c –1.1%, weight –4.7 kg) with a low incidence of hypoglycemia.31 Further post hoc analysis of the open-label extension of the 30-week trials followed patients treated with exenatide BID for 3 years or longer.69 In addition to markedly decreasing HbA1c from baseline levels (–1.1% at 3 years and –0.8% at up to 3.5 years; P < .0001), adjunctive exenatide produced significant reductions in body weight—up to –5.3 kg after 3.5 years of therapy.31,69 At 3.5 years, continued exenatide therapy resulted in a –6% reduction in low-density lipoprotein cholesterol, a 24% mean increase in high-density lipoprotein cholesterol, and a mean reduction in blood pressure of –2% to –4% from baseline levels. Improvements in hepatic biomarkers and homeostasis model assessment-B, a measure of beta-cell function, were seen after 2 and 3 years of exenatide treatment.31 Hypoglycemia was generally mild and transient.
In comparative head-to-head studies, exenatide BID and insulin analogues reduced HbA1c by similar magnitudes; yet exenatide treatment resulted in better control in terms of PPG and weight loss, while insulin glargine and insulin aspart produced weight gain.70–73
Mechanisms of cardioprotective effects
Although the mechanisms for the potential cardioprotective effects of GLP-1 and its receptor agonists remain to be fully elucidated, a recent study suggested that two novel pathways could be involved—one that is dependent on the known GLP-1 receptor pathway, and one that is independent of the GLP-1 receptor pathway.74 Correlating with observations of a potential cardioprotective effect, an infusion of recombinant GLP-1 in patients with acute myocardial infarction, when added to standard therapy, resulted in improved left ventricular function and was associated with reduced mortality.75 Evidence continues to accumulate for potential cardioprotective effects of the GLP-1 receptor agonists, indicating that they may have a positive impact on macrovascular complications in patients with T2DM.
CONCLUSION
T2DM, which is often associated with overweight and obesity, remains a significant challenge worldwide. The broad spectrum of glucoregulatory actions of the incretin hormones GLP-1 and GIP, and their importance in maintaining glucose homeostasis, have been recognized and correlated with the pathogenesis of T2DM. An improved understanding of the roles played by GLP-1 and GIP in the pathogenesis of T2DM may provide clinicians with important details regarding the therapeutic application of incretin-based therapies, including the GLP-1 receptor agonist exenatide and the DPP-4 inhibitors sitagliptin and saxagliptin. Antidiabetes agents whose development is based on the multiple pharmacologic effects of incretin hormones can address the multifaceted nature of T2DM and overcome some current limitations of traditional therapies, especially those related to weight. This becomes more compelling given the close link among T2DM, obesity, and increased CV risk.
- Boyle PJ, Freeman JS. Application of incretin mimetics and dipeptidyl peptidase IV inhibitors in managing type 2 diabetes mellitus. J Am Osteopath Assoc 2007; 107(suppl 3):S10–S16.
- Freeman JS. The pathophysiologic role of incretins. J Am Osteopath Assoc 2007; 107(suppl 3):S6–S9.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev 2006; 7:163–182.
- Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 1964; 24:1076–1082.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Brown JC, Dryburgh JR, Ross SA, Dupré J. Identification and actions of gastric inhibitory polypeptide. Recent Prog Horm Res 1975; 31:487–532.
- Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987; 2:1300–1304.
- Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986; 63:492–498.
- Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003; 144:5149–5158.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995; 44:1126–1131.
- Dai H, Gustavson SM, Preston GM, Eskra JD, Calle R, Hirshberg B. Non-linear increase in GLP-1 levels in response to DPP-IV inhibition in healthy adult subjects. Diabetes Obes Metab 2008; 10:506–513.
- Nauck MA, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Phillips WT, Schwartz JG, McMahan CA. Rapid gastric emptying of an oral glucose solution in type 2 diabetic patients. J Nucl Med 1992; 33:1496–1500.
- Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001; 86:3717–3723.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Lynn FC, Thompson SA, Pospisilik JA, et al. A novel pathway for regulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta cells. FASEB J 2003; 17:91–93.
- Vaag AA, Holst JJ, Vølund A, Beck-Nielsen HB. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)—evidence for decreased glucagon-like peptide-1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol 1996; 135:425–432.
- Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:2719–2725.
- Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36:741–744.
- Knop FK, Vilsbøll T, Højberg PV, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 2007; 56:1951–1959.
- Lee YS, Shin S, Shigihara T, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes 2007; 56:1671–1679.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care 1992; 15:270–276.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002; 359:824–830.
- Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2005; 62:173–181.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005; 90:5991–5997.
- Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism 2001; 50:583–589.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2008; 294:E846–E852.
- Ionut V, Zheng D, Stefanovski D, Bergman RN. Exenatide can reduce glucose independent of islet hormones or gastric emptying. Am J Physiol Endocrinol Metab 2008; 295:E269–E277.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43:1664–1669.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Committee for Medicinal Products for Human Use: Summary of Positive Opinion for Victoza. European Medicines Agency Web site. http://www.emea.europa.eu/pdfs/human/opinion/Victoza_14168909en.pdf. Published April 23, 2009. Accessed September 21, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Aschner P, Kipnes MS, Lunceford JK, et al; for the Sitagliptin 021 Study Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Covington P, Christopher R, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther 2008; 30:499–512.
- DeFronzo RA, Fleck PR, Wilson CA, Mekki Q; on behalf of the Alogliptin Study 010 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care 2008; 31:2315–2317.
- Triplitt CL, McGill JB, Porte D Jr, Conner CS. The changing landscape of type 2 diabetes: the role of incretin-based therapies in managed care outcomes. J Manag Care Pharm 2007; 13(9 suppl C):S2–S16.
- Garber AJ, Spann SJ. An overview of incretin clinical trials. J Fam Pract 2008; 57(9 suppl):S10–S18.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Published 2008. Accessed September 21, 2009.
- Ong KL, Cheung BM, Wong LY, Wt NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Sanders CL, Yesupriya AJ, Curtin LR. Analysis of population structure and stratification in NHANES III self-reported race/ethnicities. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/genomics/events/file/print/10year/08_pop_struct_ab.pdf. Accessed September 21, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:480–486.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008; 117:2340–2350.
- Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004; 109:962–965.
It has long been understood that the pathophysiology of type 2 diabetes mellitus (T2DM) is based on the triad of progressive decline in insulin-producing pancreatic beta cells, an increase in insulin resistance, and increased hepatic glucose production.1,2 It is now evident that other factors, including defective actions of the gastrointestinal (GI) incretin hormones glucagon-like peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), also play significant roles.2–5 The uncontrolled hyperglycemia resulting from such defects may lead to microvascular complications, including retinopathy, neuropathy, microangiopathy, and nephropathy, and macrovascular complications, such as coronary artery disease and peripheral vascular disease.
This review explores the growing understanding of the role of the incretins in normal insulin secretion, as well as in the pathogenesis of T2DM, and examines the pathophysiologic basis for the benefits and therapeutic application of incretin-based therapies in T2DM.1,2
THE GI SYSTEM AND GLUCOSE HOMEOSTASIS IN THE HEALTHY STATE
The GI system plays an integral role in glucose homeostasis.6 The observation that orally administered glucose provides a stronger insulinotropic stimulus than an intravenous glucose challenge provided insight into the regulation of plasma glucose by the GI system of healthy individuals.7 The incretin effect, as this is termed, may be responsible for 50% to 70% of the total insulin secreted following oral glucose intake.8
Two GI peptide hormones (the incretins)—GLP-1 and GIP—were found to exert major glucoregulatory actions.3,9,10 Within minutes of nutrient ingestion, GLP-1 is secreted from intestinal L cells in the distal ileum and colon, while GIP is released by intestinal K cells in the duodenum and jejunum.3 GLP-1 and GIP trigger their insulinotropic actions by binding beta-cell receptors.3 GLP-1 receptors are expressed on pancreatic glucagon-containing alpha and delta cells as well as on beta cells, whereas GIP receptors are expressed primarily on beta cells.3,8 GLP-1 receptors are also expressed in the central nervous system (CNS), peripheral nervous system, lung, heart, and GI tract, while GIP receptors are expressed in adipose tissue and the CNS.3 GLP-1 inhibits glucose-dependent glucagon secretion from alpha cells.3 In healthy individuals, fasting glucose is managed by tonic insulin/glucagon secretion, but excursions of postprandial glucose (PPG) are controlled by insulin and the incretin hormones.11
Additionally, in animal studies, GLP-1 has been shown to induce the transcriptional activation of the insulin gene and insulin biosynthesis, thus increasing beta-cell proliferation and decreasing beta-cell apoptosis.12 GLP-1 stimulates a CNS-mediated pathway of insulin secretion, slows gastric emptying, increases CNS-mediated satiety leading to reduced food intake, indirectly increases insulin sensitivity and nutrient uptake in skeletal muscle and adipose tissue, and exerts neuroprotective effects.8
Both GLP-1 and GIP are rapidly degraded by the serine protease dipeptidyl peptidase–4 (DPP-4), which is widely expressed in bound and free forms.14 A recent study in healthy adults showed that GLP-1 concentration declined even during maximal DPP-4 inhibition, suggesting that there may be pathways of GLP-1 elimination other than DPP-4 enzymatic degradation.15
INCRETINS AND THE PATHOGENESIS OF T2DM
Studies have shown that incretin pathways play a role in the progression of T2DM.3,16 The significant reduction in the incretin effect seen in patients with T2DM has been attributed to several factors, including impaired secretion of GLP-1, accelerated metabolism of GLP-1 and GIP, and defective responsiveness to both hormones.16 Many patients with T2DM also have accelerated gastric emptying that may contribute to deterioration of their glycemic control.17
While GIP concentration is normal or modestly increased in patients with T2DM,16,18 the insulinotropic actions of GIP are significantly diminished.19 Thus, patients with T2DM have an impaired responsiveness to GIP with a possible link to GIP-receptor downregulation or desensitization.20
Are secretory defects a cause or result of T2DM?
In contrast to GIP, the secretion of GLP-1 has been shown to be deficient in patients with T2DM.18 As with GIP, it is unknown to what degree this defect is a cause or consequence of T2DM. In a study of identical twins, defective GLP-1 secretion was observed only in the one sibling with T2DM, suggesting that GLP-1 secretory deficits may be secondary to the development of T2DM.21 Despite the diminished secretion of GLP-1 in patients with T2DM, the insulinotropic actions of GLP-1 are preserved.19 It has also been shown that the effects of GLP-1 on gastric emptying and glucagon secretion are maintained in patients with T2DM.19,22,23
Whether this incretin dysregulation is responsible for or is the end result of hyperglycemia remains a subject of continued investigation. A recent study confirmed that the incretin effect is reduced in patients with T2DM, but advanced the concept that it may be a consequence of the diabetic state.16,24 Notably, impaired actions of GLP-1 and GIP and diminished concentrations of GLP-1 may be partially restored by improved glycemic control.24
Recent preclinical and clinical studies continue to clarify the roles of incretin hormones in T2DM. The findings from a study of obese diabetic mice suggest that the effect of GLP-1 therapy on the long-term remission of diabetes may be caused by improvements in beta-cell function and insulin sensitivity, as well as by a reduction in gluconeogenesis in the liver.25
Incretin effect and glucose tolerance, body mass index
Another study was conducted to evaluate quantitatively the separate impacts of obesity and hyperglycemia on the incretin effect in patients with T2DM, patients with impaired glucose tolerance, and patients with normal glucose tolerance.26 There was a significant (P ≤ .05) reduction in the incretin effect in terms of total insulin secretion, beta-cell glucose sensitivity, and the GLP-1 response to oral glucose in patients with T2DM compared with individuals whose glucose tolerance was normal or impaired. Each manifestation of the incretin effect was inversely related to both glucose tolerance and body mass index in an independent, additive manner (P ≤ .05); thus, glucose tolerance and obesity attenuate the incretin effect on beta-cell function and GLP-1 response independently of each other.
Exogenous GLP-1 has been shown to restore the regulation of blood glucose to near-normal concentrations in patients with T2DM.27 Several studies of patients with T2DM have shown that synthetic GLP-1 administration induces insulin secretion,19,27 slows gastric emptying (which is accelerated in patients with T2DM), and decreases inappropriately elevated glucagon secretion.19,23,28 Acute GLP-1 infusion studies showed that GLP-1 improved fasting plasma glucose (FPG) and PPG concentrations23,27; long-term studies showed that this hormone exerts euglycemic effects, leading to improvements in glycosylated hemoglobin (HbA1c), and induces weight loss.29
TARGETING FUNDAMENTAL DEFECTS OF T2DM WITH INCRETIN-BASED THERAPIES
Recognition and a better understanding of the role of the incretins and the enzyme involved in their degradation have led to the development of two incretin-based treatments: the GLP-1 receptor agonists, which possess many of the glucoregulatory actions of incretin peptides, and the DPP-4 inhibitors.5 Both the GLP-1 receptor agonists and the DPP-4 inhibitors have demonstrated safety and efficacy in the management of hyperglycemia in patients with T2DM.
GLP-1 receptor agonists
The GLP-1 receptor agonist exenatide is a synthetic form of exendin-4 and has a unique amino acid sequence that renders it resistant to degradation by DPP-4, making its actions longer lasting than endogenous GLP-1.5,30 Exenatide has a half-life of 2.4 hours and is detectable for up to 10 hours after subcutaneous (SC) injection.5,30 It is administered BID and has been approved as monotherapy or an adjunct therapy in patients with T2DM who have inadequate glycemic control following treatment with metformin, a sulfonylurea, a thiazolidinedione (TZD), or metformin in combination with a sulfonylurea or a TZD.31–35
In both human and animal studies, exenatide has been shown to enhance glucose-dependent insulin secretion and suppress inappropriate glucagon secretion in a glucose-dependent manner, reduce food intake and body weight, and acutely improve beta-cell function by enhancing first- and second-phase insulin secretion.5,36,37
In a small study involving 17 patients with T2DM, exenatide was shown to slow gastric emptying, which could be an important mechanism contributing to its beneficial effects on PPG concentration.38 Exenatide also has been shown to attenuate postprandial hyperglycemia, a risk factor for cardiovascular disease (CVD), by reducing endogenous glucose production by about 50% in patients with T2DM.39 Another mechanism for glycemic control may exist, as a recent animal study has shown that exenatide, similar to endogenous GLP-1, lowers blood glucose concentration independent of changes in pancreatic islet hormone secretion or delayed gastric emptying.40
A formulation of exenatide that is administered once weekly—exenatide long-acting release (LAR)—is in clinical evaluation and under review by the US Food and Drug Administration (FDA). In a short-term study, exenatide-LAR (0.8 mg or 2.0 mg) was administered once weekly for 15 weeks to patients with T2DM whose glycemia was suboptimally controlled with metformin alone or in combination with diet and exercise. Compared with placebo, treatment with exenatide once weekly was associated with markedly reduced HbA1c, FPG, PPG and body weight.41 In a larger, 30-week, phase 3 trial, Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide ONce Weekly (DURATION-1), exenatide-LAR 2 mg once weekly was compared with exenatide 10 mg BID in patients with T2DM. Exenatide-LAR once weekly was associated with a significantly greater reduction in HbA1c (–1.9% vs –1.5%, P = .0023), and with a similar low risk of hypoglycemia and reduction in body weight (–3.7 kg vs –3.6 kg, P = .89) compared with the BID formulation.42
Liraglutide, recently approved in the European Union for T2DM and also under regulatory review in the United States, is a DPP-4–resistant human analogue GLP-1 receptor agonist in clinical development that has a 97% homology to native GLP-1.43–45 In contrast to exenatide, the acetylated liraglutide molecule allows binding to serum albumin and provides resistance to DPP-4 degradation, thus prolonging the half-life of liraglutide to approximately 12 hours. Liraglutide is administered SC QD as monotherapy or in combination with other antidiabetes agents such as metformin or sulfonylurea to patients with T2DM.44–47 Liraglutide has been shown to reduce HbA1c, decrease body weight, and lead to a lower incidence of hypoglycemia compared with the sulfonylurea glimepiride.
DPP-4 inhibitors
Sitagliptin is a DPP-4 inhibitor indicated as monotherapy or in combination with metformin or a TZD in patients with T2DM with inadequate glycemic control.48–51 Given orally, sitagliptin does not bind to the GLP-1 receptor agonist and has been shown to inhibit circulating DPP-4 activity by about 80%.52,53 Sitagliptin has been associated with an approximate twofold increase in postprandial GLP-1 plasma concentrations compared with placebo in healthy human subjects and in patients with T2DM.53 Saxagliptin, another potent DPP-4 inhibitor, significantly reduced HbA1c and FPG concentrations in patients with T2DM54 with a neutral effect on weight; it was recently approved by the FDA for treatment of T2DM.55
The DPP-4 inhibitor vildagliptin is currently being used in the European Union and Latin America but has yet to receive regulatory approval in the United States.54 Alogliptin, a novel, high-affinity, high-specificity DPP-4 inhibitor currently in development, provides rapid and sustained DPP-4 inhibition and significantly reduces HbA1c, FPG, and PPG concentrations with no change in body weight in patients with T2DM.56,57
Incretin-based therapies compared
Effects of incretin-based therapies
The number of people with T2DM, overweight/obesity, or CVD, alone or in combination, is approaching epidemic proportions, with the mechanisms of these conditions interrelated. Approximately 24 million Americans have diabetes, and T2DM accounts for more than 90% of these cases.61 Most patients with T2DM are not achieving HbA1c targets.62–64 About 60% of deaths among patients with T2DM are caused by CVD.65 Compounding the problem, overweight/obesity enhances the risk for CV-related morbidities in patients with diabetes.66 A cluster of metabolic disorders referred to as the metabolic syndrome (which includes hyperglycemia, measures of central obesity, and a series of significant CV risk factors) is common in patients with T2DM and CVD.67 Unfortunately, many antidiabetes drugs that successfully manage glycemic control also cause weight gain, which in theory may increase CV risk in patients with T2DM.68
Data from studies of patients with T2DM show that exenatide improves glycemic control and reduces body weight. Exenatide administered BID significantly reduced HbA1c (–0.40% to –0.86%) and weight (–1.6 kg to –2.8 kg) relative to baseline in three 30-week, placebo-controlled clinical trials.31,33,34 In subsequent 2-year, open-label extension studies, exenatide produced significant reductions from baseline in HbA1c (–20.9% at 30 weeks) and weight (–2.1 kg at 30 weeks). Both decreases were sustained through 2 years (HbA1c –1.1%, weight –4.7 kg) with a low incidence of hypoglycemia.31 Further post hoc analysis of the open-label extension of the 30-week trials followed patients treated with exenatide BID for 3 years or longer.69 In addition to markedly decreasing HbA1c from baseline levels (–1.1% at 3 years and –0.8% at up to 3.5 years; P < .0001), adjunctive exenatide produced significant reductions in body weight—up to –5.3 kg after 3.5 years of therapy.31,69 At 3.5 years, continued exenatide therapy resulted in a –6% reduction in low-density lipoprotein cholesterol, a 24% mean increase in high-density lipoprotein cholesterol, and a mean reduction in blood pressure of –2% to –4% from baseline levels. Improvements in hepatic biomarkers and homeostasis model assessment-B, a measure of beta-cell function, were seen after 2 and 3 years of exenatide treatment.31 Hypoglycemia was generally mild and transient.
In comparative head-to-head studies, exenatide BID and insulin analogues reduced HbA1c by similar magnitudes; yet exenatide treatment resulted in better control in terms of PPG and weight loss, while insulin glargine and insulin aspart produced weight gain.70–73
Mechanisms of cardioprotective effects
Although the mechanisms for the potential cardioprotective effects of GLP-1 and its receptor agonists remain to be fully elucidated, a recent study suggested that two novel pathways could be involved—one that is dependent on the known GLP-1 receptor pathway, and one that is independent of the GLP-1 receptor pathway.74 Correlating with observations of a potential cardioprotective effect, an infusion of recombinant GLP-1 in patients with acute myocardial infarction, when added to standard therapy, resulted in improved left ventricular function and was associated with reduced mortality.75 Evidence continues to accumulate for potential cardioprotective effects of the GLP-1 receptor agonists, indicating that they may have a positive impact on macrovascular complications in patients with T2DM.
CONCLUSION
T2DM, which is often associated with overweight and obesity, remains a significant challenge worldwide. The broad spectrum of glucoregulatory actions of the incretin hormones GLP-1 and GIP, and their importance in maintaining glucose homeostasis, have been recognized and correlated with the pathogenesis of T2DM. An improved understanding of the roles played by GLP-1 and GIP in the pathogenesis of T2DM may provide clinicians with important details regarding the therapeutic application of incretin-based therapies, including the GLP-1 receptor agonist exenatide and the DPP-4 inhibitors sitagliptin and saxagliptin. Antidiabetes agents whose development is based on the multiple pharmacologic effects of incretin hormones can address the multifaceted nature of T2DM and overcome some current limitations of traditional therapies, especially those related to weight. This becomes more compelling given the close link among T2DM, obesity, and increased CV risk.
It has long been understood that the pathophysiology of type 2 diabetes mellitus (T2DM) is based on the triad of progressive decline in insulin-producing pancreatic beta cells, an increase in insulin resistance, and increased hepatic glucose production.1,2 It is now evident that other factors, including defective actions of the gastrointestinal (GI) incretin hormones glucagon-like peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), also play significant roles.2–5 The uncontrolled hyperglycemia resulting from such defects may lead to microvascular complications, including retinopathy, neuropathy, microangiopathy, and nephropathy, and macrovascular complications, such as coronary artery disease and peripheral vascular disease.
This review explores the growing understanding of the role of the incretins in normal insulin secretion, as well as in the pathogenesis of T2DM, and examines the pathophysiologic basis for the benefits and therapeutic application of incretin-based therapies in T2DM.1,2
THE GI SYSTEM AND GLUCOSE HOMEOSTASIS IN THE HEALTHY STATE
The GI system plays an integral role in glucose homeostasis.6 The observation that orally administered glucose provides a stronger insulinotropic stimulus than an intravenous glucose challenge provided insight into the regulation of plasma glucose by the GI system of healthy individuals.7 The incretin effect, as this is termed, may be responsible for 50% to 70% of the total insulin secreted following oral glucose intake.8
Two GI peptide hormones (the incretins)—GLP-1 and GIP—were found to exert major glucoregulatory actions.3,9,10 Within minutes of nutrient ingestion, GLP-1 is secreted from intestinal L cells in the distal ileum and colon, while GIP is released by intestinal K cells in the duodenum and jejunum.3 GLP-1 and GIP trigger their insulinotropic actions by binding beta-cell receptors.3 GLP-1 receptors are expressed on pancreatic glucagon-containing alpha and delta cells as well as on beta cells, whereas GIP receptors are expressed primarily on beta cells.3,8 GLP-1 receptors are also expressed in the central nervous system (CNS), peripheral nervous system, lung, heart, and GI tract, while GIP receptors are expressed in adipose tissue and the CNS.3 GLP-1 inhibits glucose-dependent glucagon secretion from alpha cells.3 In healthy individuals, fasting glucose is managed by tonic insulin/glucagon secretion, but excursions of postprandial glucose (PPG) are controlled by insulin and the incretin hormones.11
Additionally, in animal studies, GLP-1 has been shown to induce the transcriptional activation of the insulin gene and insulin biosynthesis, thus increasing beta-cell proliferation and decreasing beta-cell apoptosis.12 GLP-1 stimulates a CNS-mediated pathway of insulin secretion, slows gastric emptying, increases CNS-mediated satiety leading to reduced food intake, indirectly increases insulin sensitivity and nutrient uptake in skeletal muscle and adipose tissue, and exerts neuroprotective effects.8
Both GLP-1 and GIP are rapidly degraded by the serine protease dipeptidyl peptidase–4 (DPP-4), which is widely expressed in bound and free forms.14 A recent study in healthy adults showed that GLP-1 concentration declined even during maximal DPP-4 inhibition, suggesting that there may be pathways of GLP-1 elimination other than DPP-4 enzymatic degradation.15
INCRETINS AND THE PATHOGENESIS OF T2DM
Studies have shown that incretin pathways play a role in the progression of T2DM.3,16 The significant reduction in the incretin effect seen in patients with T2DM has been attributed to several factors, including impaired secretion of GLP-1, accelerated metabolism of GLP-1 and GIP, and defective responsiveness to both hormones.16 Many patients with T2DM also have accelerated gastric emptying that may contribute to deterioration of their glycemic control.17
While GIP concentration is normal or modestly increased in patients with T2DM,16,18 the insulinotropic actions of GIP are significantly diminished.19 Thus, patients with T2DM have an impaired responsiveness to GIP with a possible link to GIP-receptor downregulation or desensitization.20
Are secretory defects a cause or result of T2DM?
In contrast to GIP, the secretion of GLP-1 has been shown to be deficient in patients with T2DM.18 As with GIP, it is unknown to what degree this defect is a cause or consequence of T2DM. In a study of identical twins, defective GLP-1 secretion was observed only in the one sibling with T2DM, suggesting that GLP-1 secretory deficits may be secondary to the development of T2DM.21 Despite the diminished secretion of GLP-1 in patients with T2DM, the insulinotropic actions of GLP-1 are preserved.19 It has also been shown that the effects of GLP-1 on gastric emptying and glucagon secretion are maintained in patients with T2DM.19,22,23
Whether this incretin dysregulation is responsible for or is the end result of hyperglycemia remains a subject of continued investigation. A recent study confirmed that the incretin effect is reduced in patients with T2DM, but advanced the concept that it may be a consequence of the diabetic state.16,24 Notably, impaired actions of GLP-1 and GIP and diminished concentrations of GLP-1 may be partially restored by improved glycemic control.24
Recent preclinical and clinical studies continue to clarify the roles of incretin hormones in T2DM. The findings from a study of obese diabetic mice suggest that the effect of GLP-1 therapy on the long-term remission of diabetes may be caused by improvements in beta-cell function and insulin sensitivity, as well as by a reduction in gluconeogenesis in the liver.25
Incretin effect and glucose tolerance, body mass index
Another study was conducted to evaluate quantitatively the separate impacts of obesity and hyperglycemia on the incretin effect in patients with T2DM, patients with impaired glucose tolerance, and patients with normal glucose tolerance.26 There was a significant (P ≤ .05) reduction in the incretin effect in terms of total insulin secretion, beta-cell glucose sensitivity, and the GLP-1 response to oral glucose in patients with T2DM compared with individuals whose glucose tolerance was normal or impaired. Each manifestation of the incretin effect was inversely related to both glucose tolerance and body mass index in an independent, additive manner (P ≤ .05); thus, glucose tolerance and obesity attenuate the incretin effect on beta-cell function and GLP-1 response independently of each other.
Exogenous GLP-1 has been shown to restore the regulation of blood glucose to near-normal concentrations in patients with T2DM.27 Several studies of patients with T2DM have shown that synthetic GLP-1 administration induces insulin secretion,19,27 slows gastric emptying (which is accelerated in patients with T2DM), and decreases inappropriately elevated glucagon secretion.19,23,28 Acute GLP-1 infusion studies showed that GLP-1 improved fasting plasma glucose (FPG) and PPG concentrations23,27; long-term studies showed that this hormone exerts euglycemic effects, leading to improvements in glycosylated hemoglobin (HbA1c), and induces weight loss.29
TARGETING FUNDAMENTAL DEFECTS OF T2DM WITH INCRETIN-BASED THERAPIES
Recognition and a better understanding of the role of the incretins and the enzyme involved in their degradation have led to the development of two incretin-based treatments: the GLP-1 receptor agonists, which possess many of the glucoregulatory actions of incretin peptides, and the DPP-4 inhibitors.5 Both the GLP-1 receptor agonists and the DPP-4 inhibitors have demonstrated safety and efficacy in the management of hyperglycemia in patients with T2DM.
GLP-1 receptor agonists
The GLP-1 receptor agonist exenatide is a synthetic form of exendin-4 and has a unique amino acid sequence that renders it resistant to degradation by DPP-4, making its actions longer lasting than endogenous GLP-1.5,30 Exenatide has a half-life of 2.4 hours and is detectable for up to 10 hours after subcutaneous (SC) injection.5,30 It is administered BID and has been approved as monotherapy or an adjunct therapy in patients with T2DM who have inadequate glycemic control following treatment with metformin, a sulfonylurea, a thiazolidinedione (TZD), or metformin in combination with a sulfonylurea or a TZD.31–35
In both human and animal studies, exenatide has been shown to enhance glucose-dependent insulin secretion and suppress inappropriate glucagon secretion in a glucose-dependent manner, reduce food intake and body weight, and acutely improve beta-cell function by enhancing first- and second-phase insulin secretion.5,36,37
In a small study involving 17 patients with T2DM, exenatide was shown to slow gastric emptying, which could be an important mechanism contributing to its beneficial effects on PPG concentration.38 Exenatide also has been shown to attenuate postprandial hyperglycemia, a risk factor for cardiovascular disease (CVD), by reducing endogenous glucose production by about 50% in patients with T2DM.39 Another mechanism for glycemic control may exist, as a recent animal study has shown that exenatide, similar to endogenous GLP-1, lowers blood glucose concentration independent of changes in pancreatic islet hormone secretion or delayed gastric emptying.40
A formulation of exenatide that is administered once weekly—exenatide long-acting release (LAR)—is in clinical evaluation and under review by the US Food and Drug Administration (FDA). In a short-term study, exenatide-LAR (0.8 mg or 2.0 mg) was administered once weekly for 15 weeks to patients with T2DM whose glycemia was suboptimally controlled with metformin alone or in combination with diet and exercise. Compared with placebo, treatment with exenatide once weekly was associated with markedly reduced HbA1c, FPG, PPG and body weight.41 In a larger, 30-week, phase 3 trial, Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide ONce Weekly (DURATION-1), exenatide-LAR 2 mg once weekly was compared with exenatide 10 mg BID in patients with T2DM. Exenatide-LAR once weekly was associated with a significantly greater reduction in HbA1c (–1.9% vs –1.5%, P = .0023), and with a similar low risk of hypoglycemia and reduction in body weight (–3.7 kg vs –3.6 kg, P = .89) compared with the BID formulation.42
Liraglutide, recently approved in the European Union for T2DM and also under regulatory review in the United States, is a DPP-4–resistant human analogue GLP-1 receptor agonist in clinical development that has a 97% homology to native GLP-1.43–45 In contrast to exenatide, the acetylated liraglutide molecule allows binding to serum albumin and provides resistance to DPP-4 degradation, thus prolonging the half-life of liraglutide to approximately 12 hours. Liraglutide is administered SC QD as monotherapy or in combination with other antidiabetes agents such as metformin or sulfonylurea to patients with T2DM.44–47 Liraglutide has been shown to reduce HbA1c, decrease body weight, and lead to a lower incidence of hypoglycemia compared with the sulfonylurea glimepiride.
DPP-4 inhibitors
Sitagliptin is a DPP-4 inhibitor indicated as monotherapy or in combination with metformin or a TZD in patients with T2DM with inadequate glycemic control.48–51 Given orally, sitagliptin does not bind to the GLP-1 receptor agonist and has been shown to inhibit circulating DPP-4 activity by about 80%.52,53 Sitagliptin has been associated with an approximate twofold increase in postprandial GLP-1 plasma concentrations compared with placebo in healthy human subjects and in patients with T2DM.53 Saxagliptin, another potent DPP-4 inhibitor, significantly reduced HbA1c and FPG concentrations in patients with T2DM54 with a neutral effect on weight; it was recently approved by the FDA for treatment of T2DM.55
The DPP-4 inhibitor vildagliptin is currently being used in the European Union and Latin America but has yet to receive regulatory approval in the United States.54 Alogliptin, a novel, high-affinity, high-specificity DPP-4 inhibitor currently in development, provides rapid and sustained DPP-4 inhibition and significantly reduces HbA1c, FPG, and PPG concentrations with no change in body weight in patients with T2DM.56,57
Incretin-based therapies compared
Effects of incretin-based therapies
The number of people with T2DM, overweight/obesity, or CVD, alone or in combination, is approaching epidemic proportions, with the mechanisms of these conditions interrelated. Approximately 24 million Americans have diabetes, and T2DM accounts for more than 90% of these cases.61 Most patients with T2DM are not achieving HbA1c targets.62–64 About 60% of deaths among patients with T2DM are caused by CVD.65 Compounding the problem, overweight/obesity enhances the risk for CV-related morbidities in patients with diabetes.66 A cluster of metabolic disorders referred to as the metabolic syndrome (which includes hyperglycemia, measures of central obesity, and a series of significant CV risk factors) is common in patients with T2DM and CVD.67 Unfortunately, many antidiabetes drugs that successfully manage glycemic control also cause weight gain, which in theory may increase CV risk in patients with T2DM.68
Data from studies of patients with T2DM show that exenatide improves glycemic control and reduces body weight. Exenatide administered BID significantly reduced HbA1c (–0.40% to –0.86%) and weight (–1.6 kg to –2.8 kg) relative to baseline in three 30-week, placebo-controlled clinical trials.31,33,34 In subsequent 2-year, open-label extension studies, exenatide produced significant reductions from baseline in HbA1c (–20.9% at 30 weeks) and weight (–2.1 kg at 30 weeks). Both decreases were sustained through 2 years (HbA1c –1.1%, weight –4.7 kg) with a low incidence of hypoglycemia.31 Further post hoc analysis of the open-label extension of the 30-week trials followed patients treated with exenatide BID for 3 years or longer.69 In addition to markedly decreasing HbA1c from baseline levels (–1.1% at 3 years and –0.8% at up to 3.5 years; P < .0001), adjunctive exenatide produced significant reductions in body weight—up to –5.3 kg after 3.5 years of therapy.31,69 At 3.5 years, continued exenatide therapy resulted in a –6% reduction in low-density lipoprotein cholesterol, a 24% mean increase in high-density lipoprotein cholesterol, and a mean reduction in blood pressure of –2% to –4% from baseline levels. Improvements in hepatic biomarkers and homeostasis model assessment-B, a measure of beta-cell function, were seen after 2 and 3 years of exenatide treatment.31 Hypoglycemia was generally mild and transient.
In comparative head-to-head studies, exenatide BID and insulin analogues reduced HbA1c by similar magnitudes; yet exenatide treatment resulted in better control in terms of PPG and weight loss, while insulin glargine and insulin aspart produced weight gain.70–73
Mechanisms of cardioprotective effects
Although the mechanisms for the potential cardioprotective effects of GLP-1 and its receptor agonists remain to be fully elucidated, a recent study suggested that two novel pathways could be involved—one that is dependent on the known GLP-1 receptor pathway, and one that is independent of the GLP-1 receptor pathway.74 Correlating with observations of a potential cardioprotective effect, an infusion of recombinant GLP-1 in patients with acute myocardial infarction, when added to standard therapy, resulted in improved left ventricular function and was associated with reduced mortality.75 Evidence continues to accumulate for potential cardioprotective effects of the GLP-1 receptor agonists, indicating that they may have a positive impact on macrovascular complications in patients with T2DM.
CONCLUSION
T2DM, which is often associated with overweight and obesity, remains a significant challenge worldwide. The broad spectrum of glucoregulatory actions of the incretin hormones GLP-1 and GIP, and their importance in maintaining glucose homeostasis, have been recognized and correlated with the pathogenesis of T2DM. An improved understanding of the roles played by GLP-1 and GIP in the pathogenesis of T2DM may provide clinicians with important details regarding the therapeutic application of incretin-based therapies, including the GLP-1 receptor agonist exenatide and the DPP-4 inhibitors sitagliptin and saxagliptin. Antidiabetes agents whose development is based on the multiple pharmacologic effects of incretin hormones can address the multifaceted nature of T2DM and overcome some current limitations of traditional therapies, especially those related to weight. This becomes more compelling given the close link among T2DM, obesity, and increased CV risk.
- Boyle PJ, Freeman JS. Application of incretin mimetics and dipeptidyl peptidase IV inhibitors in managing type 2 diabetes mellitus. J Am Osteopath Assoc 2007; 107(suppl 3):S10–S16.
- Freeman JS. The pathophysiologic role of incretins. J Am Osteopath Assoc 2007; 107(suppl 3):S6–S9.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev 2006; 7:163–182.
- Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 1964; 24:1076–1082.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Brown JC, Dryburgh JR, Ross SA, Dupré J. Identification and actions of gastric inhibitory polypeptide. Recent Prog Horm Res 1975; 31:487–532.
- Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987; 2:1300–1304.
- Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986; 63:492–498.
- Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003; 144:5149–5158.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995; 44:1126–1131.
- Dai H, Gustavson SM, Preston GM, Eskra JD, Calle R, Hirshberg B. Non-linear increase in GLP-1 levels in response to DPP-IV inhibition in healthy adult subjects. Diabetes Obes Metab 2008; 10:506–513.
- Nauck MA, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Phillips WT, Schwartz JG, McMahan CA. Rapid gastric emptying of an oral glucose solution in type 2 diabetic patients. J Nucl Med 1992; 33:1496–1500.
- Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001; 86:3717–3723.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Lynn FC, Thompson SA, Pospisilik JA, et al. A novel pathway for regulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta cells. FASEB J 2003; 17:91–93.
- Vaag AA, Holst JJ, Vølund A, Beck-Nielsen HB. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)—evidence for decreased glucagon-like peptide-1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol 1996; 135:425–432.
- Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:2719–2725.
- Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36:741–744.
- Knop FK, Vilsbøll T, Højberg PV, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 2007; 56:1951–1959.
- Lee YS, Shin S, Shigihara T, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes 2007; 56:1671–1679.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care 1992; 15:270–276.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002; 359:824–830.
- Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2005; 62:173–181.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005; 90:5991–5997.
- Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism 2001; 50:583–589.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2008; 294:E846–E852.
- Ionut V, Zheng D, Stefanovski D, Bergman RN. Exenatide can reduce glucose independent of islet hormones or gastric emptying. Am J Physiol Endocrinol Metab 2008; 295:E269–E277.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43:1664–1669.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Committee for Medicinal Products for Human Use: Summary of Positive Opinion for Victoza. European Medicines Agency Web site. http://www.emea.europa.eu/pdfs/human/opinion/Victoza_14168909en.pdf. Published April 23, 2009. Accessed September 21, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Aschner P, Kipnes MS, Lunceford JK, et al; for the Sitagliptin 021 Study Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Covington P, Christopher R, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther 2008; 30:499–512.
- DeFronzo RA, Fleck PR, Wilson CA, Mekki Q; on behalf of the Alogliptin Study 010 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care 2008; 31:2315–2317.
- Triplitt CL, McGill JB, Porte D Jr, Conner CS. The changing landscape of type 2 diabetes: the role of incretin-based therapies in managed care outcomes. J Manag Care Pharm 2007; 13(9 suppl C):S2–S16.
- Garber AJ, Spann SJ. An overview of incretin clinical trials. J Fam Pract 2008; 57(9 suppl):S10–S18.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Published 2008. Accessed September 21, 2009.
- Ong KL, Cheung BM, Wong LY, Wt NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Sanders CL, Yesupriya AJ, Curtin LR. Analysis of population structure and stratification in NHANES III self-reported race/ethnicities. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/genomics/events/file/print/10year/08_pop_struct_ab.pdf. Accessed September 21, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:480–486.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008; 117:2340–2350.
- Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004; 109:962–965.
- Boyle PJ, Freeman JS. Application of incretin mimetics and dipeptidyl peptidase IV inhibitors in managing type 2 diabetes mellitus. J Am Osteopath Assoc 2007; 107(suppl 3):S10–S16.
- Freeman JS. The pathophysiologic role of incretins. J Am Osteopath Assoc 2007; 107(suppl 3):S6–S9.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev 2006; 7:163–182.
- Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 1964; 24:1076–1082.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Brown JC, Dryburgh JR, Ross SA, Dupré J. Identification and actions of gastric inhibitory polypeptide. Recent Prog Horm Res 1975; 31:487–532.
- Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987; 2:1300–1304.
- Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986; 63:492–498.
- Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003; 144:5149–5158.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995; 44:1126–1131.
- Dai H, Gustavson SM, Preston GM, Eskra JD, Calle R, Hirshberg B. Non-linear increase in GLP-1 levels in response to DPP-IV inhibition in healthy adult subjects. Diabetes Obes Metab 2008; 10:506–513.
- Nauck MA, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Phillips WT, Schwartz JG, McMahan CA. Rapid gastric emptying of an oral glucose solution in type 2 diabetic patients. J Nucl Med 1992; 33:1496–1500.
- Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001; 86:3717–3723.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Lynn FC, Thompson SA, Pospisilik JA, et al. A novel pathway for regulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta cells. FASEB J 2003; 17:91–93.
- Vaag AA, Holst JJ, Vølund A, Beck-Nielsen HB. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)—evidence for decreased glucagon-like peptide-1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol 1996; 135:425–432.
- Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:2719–2725.
- Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36:741–744.
- Knop FK, Vilsbøll T, Højberg PV, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 2007; 56:1951–1959.
- Lee YS, Shin S, Shigihara T, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes 2007; 56:1671–1679.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care 1992; 15:270–276.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002; 359:824–830.
- Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2005; 62:173–181.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005; 90:5991–5997.
- Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism 2001; 50:583–589.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2008; 294:E846–E852.
- Ionut V, Zheng D, Stefanovski D, Bergman RN. Exenatide can reduce glucose independent of islet hormones or gastric emptying. Am J Physiol Endocrinol Metab 2008; 295:E269–E277.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43:1664–1669.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Committee for Medicinal Products for Human Use: Summary of Positive Opinion for Victoza. European Medicines Agency Web site. http://www.emea.europa.eu/pdfs/human/opinion/Victoza_14168909en.pdf. Published April 23, 2009. Accessed September 21, 2009.
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278.
- Aschner P, Kipnes MS, Lunceford JK, et al; for the Sitagliptin 021 Study Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Covington P, Christopher R, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther 2008; 30:499–512.
- DeFronzo RA, Fleck PR, Wilson CA, Mekki Q; on behalf of the Alogliptin Study 010 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care 2008; 31:2315–2317.
- Triplitt CL, McGill JB, Porte D Jr, Conner CS. The changing landscape of type 2 diabetes: the role of incretin-based therapies in managed care outcomes. J Manag Care Pharm 2007; 13(9 suppl C):S2–S16.
- Garber AJ, Spann SJ. An overview of incretin clinical trials. J Fam Pract 2008; 57(9 suppl):S10–S18.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Published 2008. Accessed September 21, 2009.
- Ong KL, Cheung BM, Wong LY, Wt NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Sanders CL, Yesupriya AJ, Curtin LR. Analysis of population structure and stratification in NHANES III self-reported race/ethnicities. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/genomics/events/file/print/10year/08_pop_struct_ab.pdf. Accessed September 21, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:480–486.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol 2003; 2:33–47.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008; 117:2340–2350.
- Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004; 109:962–965.
KEY POINTS
- The incretin effect may be responsible for up to 70% of insulin secretion following oral glucose ingestion; reduction of the incretin effect contributes to T2DM pathophysiology.
- It is unknown whether incretin defects are a cause or consequence of T2DM.
- Incretin therapies effectively lower glucose with concomitant favorable effects on body weight. GLP-1 receptor agonists reduce weight, while DPP-4 inhibitors are weight neutral.
Patient and treatment perspectives: Revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk
Type 2 diabetes mellitus (T2DM), excess weight, and obesity are increasing in prevalence at alarming rates.1–3 Concurrent with the increased prevalence is increased risk of morbidity and mortality. A healthy diet and exercise in conjunction with antidiabetes medications can help lower glucose concentration in patients with T2DM. Because these patients are at increased risk of cardiovascular (CV) morbidity and mortality, however, treatment strategies should address the CV risk factors, including blood pressure (BP), lipids, and body weight, as well as glycemic aspects of the disease.
To help clinicians manage the complex issues in treating patients with T2DM, this article presents an overview of patient and treatment perspectives relevant to overweight/obesity and CV disease (CVD). It includes an examination of the latest guidelines and algorithms for the management of T2DM, which continue to be updated and modified.
T2DM, WEIGHT GAIN OR OBESITY, AND CV RISK: A CHALLENGING TRIAD
Despite therapeutic advances in the diagnosis and treatment of diabetes and CVD over the last decade, the estimated number of persons in the United States older than 35 years with self-reported diabetes (with T2DM accounting for 90% to 95% of diagnosed cases) and CVD has increased from 4.2 million in 1997 to 5.7 million in 2005.3,4 The CV risk for patients with T2DM who have not had a CV event such as a myocardial infarction (MI) is similar to that of individuals without diabetes who have had a prior MI.5 Patients with T2DM have nearly double the mortality of those without the disease.6 Adding to their risk, about 80% of patients with T2DM are overweight or obese, conditions associated with worsened insulin resistance and increased CV risk and disease burden.7,8 Even a modest weight gain (5 kg) may increase the risk of coronary heart disease (CHD) by 30%, while associated changes in lipids and BP can increase the risk by another 20%.9
It is as important to control CV risk factors as it is to control glycemia in patients with T2DM, and both are difficult to achieve. Data from a recent nationwide Norwegian survey showed that only 13% of patients with T2DM achieved study-defined target levels; ie, glycosylated hemoglobin (HbA1c) less than 7.5%, BP less than 140/85 mm Hg, and total cholesterol/high-density lipoprotein (HDL-C) ratio less than 4.0.10
BENEFITS OF MANAGING GLYCEMIA, WEIGHT REDUCTION, AND CV RISK FACTORS
Several large studies, many ongoing, are generating data on the relationships among glycemia, weight reduction, and CV risk. It is well established that individuals with T2DM need aggressive risk factor reduction (glucose control, blood pressure management, and treatment of dyslipidemia) to optimize outcomes. However, characterization of the benefits of various components of risk factor reduction, particularly over many years, is only now occurring.
Results from the United Kingdom Prospective Diabetes Studies (UKPDS) showed the benefits and risks of pharmacologic glycemic control—essentially monotherapy with insulin or a sulfonylurea—compared with conventional dietary therapy in reducing diabetic complications in patients with newly diagnosed T2DM. In UKPDS 33, both insulin and sulfonylureas (intensive treatment) reduced the risk of microvascular end points (retinopathy, nephropathy) in patients whose median HbA1c was lowered to 7.0% at 10 years of follow-up, compared with patients who reached an HbA1c of 7.9%. However, intensive glycemic control did not translate into a statistically significant reduction in macrovascular complications, including MI, stroke, CVD, and death. Additionally, patients assigned to insulin had greater weight gain (+4.0 kg) than did patients assigned to receive the sulfonylurea chlorpropamide (+2.6 kg) or glyburide (+1.7 kg) (P < .01).11
The UKPDS showed that intensive treatment with metformin reduced the risk of T2DM-related end points compared with conventional treatment (primarily diet alone) in overweight patients.12 Although there were fewer patients in the metformin-treated subset (n = 342) than in the conventional treatment cohort, a secondary analysis showed that metformin was associated with less weight gain and fewer hypoglycemic episodes than either insulin or sulfonylurea therapy.12 Since HbA1c levels in the treatment groups were equal, the additional benefits seen with metformin in overweight patients with T2DM were not based solely on glycemic control.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial involved 10,000 individuals with T2DM. The primary outcome measure was a composite of CV events. The intensively treated group was controlled to a target HbA1c of less than 6.0%, with most patients receiving insulin. The trial was terminated early because an increased risk of sudden death was observed.13 A similar study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), evaluated more than 11,000 patients with T2DM, starting with a sulfonylurea-based regimen. In this study, there was no reduction in macrovascular events, but there was a reduction in nephropathy in the intensively treated group.14 In both studies, hypoglycemia and weight gain were more frequent in intensively treated patients; and in ACCORD, there were more episodes of severe hypoglycemia in the intensive-treatment group.13,14
The Veterans Affairs Diabetes Trial (VADT) evaluated the effect of intensive glucose control on CVD in 1,791 patients (mean age, 60 years) with poorly controlled T2DM (average duration, 11.5 years). The primary end points included MI, stroke, new or worsening congestive heart failure (CHF), limb amputation, and invasive intervention for coronary or peripheral arterial disease. The hazard ratio for these end points in the intensive-treatment group was 0.88 (95% confidence interval [CI], 0.74 to 1.05).15,16 Specifically, the following beneficial effects were achieved:
- HbA1c reduced by –1.0% to –2.5% in absolute units,
- systolic BP (SBP) reduced by –4 to –7 mm Hg,
- diastolic BP (DBP) reduced by –7 to –8 mm Hg,
- low-density lipoprotein cholesterol (LDL-C) reduced by –27 to –28 mg/dL,
- triglycerides reduced by –44 to –50 mg/dL, and
- HDL-C increased by 4 to 5 mg/dL.
Despite these benefits, body weight increased approximately 9 to 18 lb (4 to 8 kg) during therapy.15
Since overweight and obesity are independent risk factors for CHD and CVD in patients with T2DM,17 weight management is an integral component in treatment. In the Action for Health in Diabetes (Look AHEAD) trial, an intensive exercise and weight-loss program resulted in clinically significant (P < .001) weight loss at 1 year in patients who had T2DM and a body mass index (BMI) greater than 25 kg2/m (> 27 kg2/m if receiving insulin).18 When compared with patients who received less structured, infrequent support and minimal education about diabetes, participants in the intensive program showed more weight loss, improved glucose control, decreased CV events, and reduced medicine use. The Look AHEAD trial is currently evaluating whether these improvements will continue to result in lower CV risk.
PATIENT ADHERENCE AND SATISFACTION
It is often challenging for patients with T2DM to adhere to their treatment regimens. The Diabetes Attitude, Wishes, and Needs (DAWN) study examined psychosocial barriers to self-care in patients with diabetes and found that while 78% of patients with T2DM adhered to their medications, only 39% achieved complete success in at least two-thirds of their self-care domains.19 A multicenter, randomized, clinical trial examined the correlates of treatment satisfaction, including body weight, on patients’ appraisal of treatment satisfaction with injectable insulin. The 14.5% of patients who experienced a reduction in BMI reported systematic improvement in treatment satisfaction.20 Similarly, a cross-sectionally designed study (n = 99) that analyzed the interrelation of adherence, BMI, and depression in adults with T2DM found that patients with higher BMI and poor adherence also had depression, which was mediated by lower self-efficacy perceptions and increased diabetes symptoms.21 The results from these studies show a clear relationship between adherence with treatment regimens and achievement of HbA1c goals.22
RECENT DEVELOPMENTS IN T2DM MANAGEMENT: STRATEGIES TO REDUCE CV RISK
Because excess weight and obesity are prominent features of T2DM, it is important to use an antidiabetes agent that does not induce unnecessary weight gain (particularly central weight gain, which is thought to be most atherogenic).23 Metformin, considered the first-line agent for treatment of T2DM, is generally weight neutral with a low level of hypoglycemia.24,25 Sulfonylureas, insulin, and thiazolidinediones (TZDs) are all associated with weight gain, although newer-analogue insulins may cause less weight gain than older agents. TZDs, especially pioglitazone, are associated with improvements in long-term beta-cell function and CV risk factors despite weight gain.26,27
The newer antidiabetes agents belong to the dipeptidyl peptidase–4 (DPP-4) inhibitor and the glucagon-like peptide–1 (GLP-1) receptor agonist therapeutic classes and have been shown to be either weight neutral (DPP-4 inhibitors) or to cause weight loss (GLP-1 receptor agonists).28
Obesity and the incretin effect
Two recent studies showed that surgically induced weight loss enhances the physiologic “incretin effect.” In one study, obese individuals with T2DM whose weight loss was secondary to bariatric surgery combined with caloric restriction showed improved insulin sensitivity, improved carbohydrate metabolism, and elevated levels of adiponectin and GLP-1, all of which may reduce the incidence of T2DM.36 In the other study, bariatric surgery in morbidly obese individuals with T2DM improved insulin secretion and ameliorated insulin resistance.37
DPP-4 inhibitors
DPP-4 inhibitors such as sitagliptin and saxagliptin inhibit the enzymatic activity of DPP-4 and increase endogenous concentrations of GLP-1.28 Sitagliptin has been compared with placebo as monotherapy and has been studied in combination with other therapies.
In an 18-week study, sitagliptin monotherapy, 100 and 200 mg QD, significantly reduced HbA1c compared with placebo (placebo-subtracted HbA1c reduction, –0.60% and –0.48%, respectively) in patients with T2DM. Sitagliptin also significantly decreased fasting plasma glucose (FPG) concentration relative to placebo.38 Twelve weeks of sitagliptin monotherapy at dosages of 5, 12.5, 25, and 50 mg BID led to significant (P < .001) reductions in HbA1c compared with placebo. Sitagliptin also produced significant reductions in FPG and mean daily glucose concentrations across the doses studied.39 Similar results were reported in other 12-week studies: 50 mg BID and 100 mg QD sitagliptin monotherapy significantly (P < .05) reduced HbA1c –0.39% to –0.56% and FPG concentration –11.0 to –17.2 mg/dL compared with placebo40; sitagliptin 100 mg QD compared with placebo produced a least-squares mean change from baseline HbA1c of –0.65% versus 0.41% (P < .001) and FPG of –22.5 versus 9.4 mg/dL (P < .001).41
Sitagliptin also has been studied in combination with other therapies. After 24 weeks, sitagliptin combined with pioglitazone significantly reduced HbA1c by –0.70% and FPG by –17.7 mg/dL (P < .001 for both) compared with placebo.42 In another 24-week study, 100 mg sitagliptin QD significantly improved glycemic control and beta-cell function (P < .05 for both) in patients with T2DM who had inadequate glycemic control with glimepiride or glimepiride plus metformin.43
In addition to significantly reducing HbA1c, sitagliptin 100 and 200 mg QD produced only small differences in body weight relative to placebo: least-squares mean change from baseline for sitagliptin 100 mg was –0.7 kg (95% CI, –1.3 to –0.1) and for 200 mg was –0.6 kg (95% CI, –1.0 to –0.2); for placebo it was –0.2 kg (95% CI, –0.7 to 0.2).38 These findings were consistent with those from another 24-week monotherapy study where sitagliptin produced weight loss of up to –0.2 kg44 and a 30-week study of sitagliptin added to ongoing metformin therapy. In the latter study, both sitagliptin and placebo resulted in weight reductions of –0.5 kg.45
The effects of sitagliptin on lipids and BP have been reported in clinical studies in patients with and without T2DM. In one study of patients with T2DM, the addition of sitagliptin to metformin increased total cholesterol (+8.1 mg/dL), LDL-C (+9.2 mg/dL), and HDL-C (+1.8 mg/dL) but lowered triglyceride (–14.5 mg/dL) after 18 weeks of treatment (24-week data).46 Data from a small (n = 19) study in nondiabetic patients with mild to moderate hypertension showed that sitagliptin produced small reductions (–2 to –3 mm Hg) in 24-hour ambulatory BP measurements.47
Another DPP-4 inhibitor, saxagliptin, with efficacy similar to that described for sitagliptin, was recently approved by the US Food and Drug Administration (FDA) for treatment of T2DM.48
GLP-1 receptor agonists
Many of the GLP-1 receptor agonists developed or under development have glucoregulatory effects similar to GLP-1 but are resistant to degradation by DPP-4.28 Exenatide, an exendin-4 receptor agonist, has compared favorably with sitagliptin and with insulin analogues. Long-acting (once-weekly and once-daily) GLP-1 receptor agonists are under development.
In a 2-week, head-to-head study in metformin-treated patients with T2DM, exenatide had a greater effect than sitagliptin in lowering PPG and was more potent in increasing insulin secretion and reducing postprandial glucagon secretion. In contrast to sitagliptin, exenatide slowed gastric emptying and reduced caloric intake.49
In two studies of patients treated with exenatide, on a background of either metformin alone or metformin plus a sulfonylurea, patients who received metformin lost more weight (–1.6 to –2.8 kg; P ≤ .01) and experienced more significant decreases from baseline HbA1c (–0.4% to –0.8%; P < .002) at 30 weeks than did patients who received placebo.50,51 In a 16-week trial of exenatide in patients previously treated with a TZD with or without metformin, exenatide reduced HbA1c –0.98%, fasting blood glucose –1.69 mmol/L, and body weight –1.51 kg.52
When compared with insulin analogues, exenatide has been associated with weight loss (~ –3 kg) while the insulin analogues were associated with weight gain (~ +3 kg).53 After 26 weeks, body weight decreased –2.3 kg with exenatide and increased +1.8 kg with insulin glargine.54 Similar results were found in a crossover noninferiority trial, where the least-squares mean difference in weight change was significantly (P < .001) different (2.2 kg) between the treatments.55 When exenatide was compared with insulin aspart in an open-label, noninferiority trial, there was a between-group difference in weight of –5.4 kg after 52 weeks.32
Exenatide has also demonstrated these benefits in open-label extension studies. After 2 years, mean HbA1c reductions of –1.1% from baseline were sustained (P < .05), and weight loss of –4.7 kg was maintained (P < .001).56 After 82 weeks, similar HbA1c decreases (–1.1%) and weight loss (–4.4 kg) were exhibited.57 Even after 3 years, these benefits were maintained in patients who remained on the drug (HbA1c reduction from baseline, –1.0%; weight loss, –5.3 kg [P < .0001 for both]).58
Long-acting formulations of GLP-1 receptor agonists are in clinical development; two of these are once-weekly exenatide and once-daily liraglutide. Exenatide once weekly has the advantage of less frequent dosing and has elicited greater reductions in HbA1c than exenatide BID. After 15 weeks of once-weekly administration, the 0.8-mg formulation reduced HbA1c –1.4% and the 2-mg formulation reduced it –1.7% (P < .0001 for both compared with placebo). Body weight was lowered –3.8 kg (P < .05 compared with placebo) with the 2-mg formulation.59 Compared with exenatide BID, exenatide 2 mg once weekly showed greater reductions in HbA1c (–1.9% vs –1.5%; P = .0023) after 30 weeks of therapy.60 In a 1-year noncomparative trial, treatment with exenatide once weekly improved HbA1c (–2.0%) and weight (–4.1 kg), as well as BP and lipid profiles compared with baseline.61
Liraglutide, a once-daily human analogue GLP-1 receptor agonist, is under review by the FDA.28 In a 26-week study of patients with T2DM, liraglutide was associated with reductions in HbA1c (mean, –1.04%; P = 0.067 compared with insulin) and body weight (mean, –2.5 kg; P < .001 compared with insulin) at dosages of 0.6 to 1.8 mg/day SC. Liraglutide produced a decline in SBP from 0.6 to 3 mm Hg but was not associated with a decrease in DBP.62 In a 52-week study comparing liraglutide with glimepiride monotherapy, liraglutide 1.2 mg was associated with an HbA1c reduction of –0.84% (P = .0014) and the 1.8-mg dose with a reduction of –1.14% (P < .0001) compared with –0.51% for glimepiride. SBP decreased –0.7 mm Hg with glimepiride compared with –2.1 mm Hg for liraglutide 1.2 mg (P = .2912) and –3.6 mm Hg for liraglutide 1.8 mg (P < .0118). Mean DBP fell slightly but not significantly in all treatment groups.63 No effects on lipid parameters were reported in these two liraglutide studies.
The Liraglutide Effect and Action in Diabetes (LEAD-6) trial was undertaken to compare exenatide (10 mg BID SC) and liraglutide (1.8 mg/day SC) as add-on therapy to metformin, a sulfonylurea, or a combination of both in 464 patients with T2DM. After 26 weeks of treatment, liraglutide was associated with a significant reduction in HbA1c of –1.12%, compared with –0.79% with exenatide (P < .0001). Patients treated with liraglutide lost –3.2 kg while those on exenatide lost –2.9 kg. Among patients previously treated with metformin alone, there was a 1-kg difference in favor of liraglutide (P = NS).64
Safety profile
All of the drugs discussed have potential adverse effects. Metformin continues to have a black box warning for lactic acidosis.65 Sulfonylureas and insulin can cause hypoglycemia. TZDs can cause fluid retention and, in rare cases, CHF (for which these drugs also carry a black box warning).66,67 TZDs also increase the risk of distal fracture.66,67 The most common side effects of exenatide are gastrointestinal, but there have been reported cases of pancreatitis, some of which have been fatal.68,69 It has been difficult to prove whether exenatide increases the risk of pancreatitis, as patients with T2DM are already at an increased (three- to fourfold) risk for this condition compared with persons who do not have T2DM.69 Exenatide should not be used in patients with severe renal impairment or end-stage renal disease; it should be used with caution in patients who have undergone renal transplantation and in patients with moderate renal impairment.
The prescribing information for sitagliptin includes pancreatitis among the adverse reactions identified during the drug’s postapproval use.70 As with exenatide, it is not fully known whether a true association exists between the agent and pancreatitis. However, since pancreatitis can occur in this patient population, it is recommended that abdominal pain be fully evaluated to rule out pancreatitis. Continued postmarketing surveillance is important for all of these agents.
THE ROLE OF GUIDELINES
The American Association of Clinical Endocrinologists (AACE),26 the American Diabetes Association (ADA),71 and the ADA in conjunction with the European Association for the Study of Diabetes (EASD)24 have recently revised their recommendations for the management of patients with diabetes. The guidelines are unanimous in setting a glycemic goal (HbA1c < 7.0% for the ADA, HbA1c ≤ 6.5% for the AACE) and advocating individualized care for a treatment goal of HbA1c lower than 6.0% in patients who stand to benefit from near euglycemia without inducing severe hypoglycemia.24,26,71
CVD is the major cause of morbidity and mortality associated with T2DM and is a source of increasing concern.5 Accordingly, special consideration should be given to patients with coexisting CV risk factors, including hypertension and dyslipidemia. The ADA and the EASD advocate lifestyle modification to decrease body weight and the concurrent initiation of metformin as first-line therapy.24 If that strategy is insufficient, then two tiers of treatment guide the choice of next steps24:
- Tier 1, in addition to metformin, includes the sulfonylureas and insulin. Although these are excellent glucose-lowering drugs, they are associated with weight gain, hypoglycemia, and no improvement in BP or lipid levels. They are relatively low in cost and have been used for many years. Their main drawback is evidence that despite their use, beta-cell failure continues unabated over time.
- Tier 2 treatments include pioglitazone and the GLP-1 receptor agonist exenatide. Consideration may be given to the use of pioglitazone or exenatide when hypoglycemia is of concern, with exenatide being preferred when weight loss is a major objective and HbA1c is close to target (< 8.0%).24 Additionally, both the TZDs and exenatide probably help slow the rate of beta-cell failure, particularly if they are used early in the course of the disease.72,73 The AACE recommends different pharmacologic approaches based on HbA1c at diagnosis.26
The American Heart Association and the ADA have issued a joint scientific statement on the primary prevention of CVD in patients with diabetes.74 They advocate lifestyle management of body weight, nutrition, and physical activity.74 In addition, they stress the need for attention to BP, lipid levels, and smoking status, and the use of antiplatelet agents in patients at increased CV risk (> 40 years of age and a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria).
CONCLUSION
T2DM, weight gain/obesity, and CV risk present a continuing challenge to patients and clinicians. Antidiabetes agents have varying degrees of evidence to support their effects on HbA1c, body weight, BP, and lipid levels. A better understanding of the pathophysiology of T2DM has led to the development of newer antidiabetes agents that target the fundamental defects of the disease. Evidence continues to accumulate for the improved benefits of glycemic control and weight loss in T2DM with GLP-1 receptor agonists such as exenatide currently having robust data in terms of beneficial effects on weight and CV risk factors. As clinicians continue to incorporate this knowledge into their practice patterns, patient adherence and clinical outcomes are expected to improve. Newer agents, such as incretin-based therapies, address T2DM as well as other factors that increase cardiometabolic risk through their effects not only on glycemic control but on body weight, BP, and lipids.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of self-reported cardiovascular disease among persons aged >35 years with diabetes—United States, 1997–2005. MMWR Morb Mortal Wkly Rep 2007; 56:1129–1132.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Bonora E, Targher G, Formentini G, et al. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabet Med 2004; 21:52–58.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Jenssen TG, Tonstad S, Claudi T, Midthejell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005; 22:1379–1385.
- Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes 2007; 5:8.
- Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: the mediational role of diabetes symptoms and self-efficacy. Health Psychol 2007; 26:693–700.
- Ruelas V, Roybal GM, Lu Y, Goldman D, Peters A. Clinical and behavioral correlates of achieving and maintaining glycemic targets in an underserved population with type 2 diabetes. Diabetes Care 2009; 32:54–56.
- Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 2003; 52:172–179.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007; 30:1127–1142.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 2001; 86:3853–3860.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17:4–12.
- de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 2009; 19:313–320.
- Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis 2008; 18:574–579.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Hanefeld M, Herman G, Wu M, Mickel C, Sanchez M, Stein PP; for the Sitagliptin Study 014 Investigators. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007; 23:1329–1339.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol 2008; 48:592–598.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29:139–153.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Garber A, Henry R. Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Fortamet [package insert]. Ft. Lauderdale, FL: Watson Laboratories; 2008.
- Actos. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008; 3077–3082.
- Avandia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2009; 1351–1359.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Idris I. News and views: FDA reviews incidences of acute pancreatitis in patients taking Byetta. Diabetes Obes Metab 2008; 10:96–98.
- Januvia [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2006, 2007.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292:E871–E883.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007; 30:162–172.
Type 2 diabetes mellitus (T2DM), excess weight, and obesity are increasing in prevalence at alarming rates.1–3 Concurrent with the increased prevalence is increased risk of morbidity and mortality. A healthy diet and exercise in conjunction with antidiabetes medications can help lower glucose concentration in patients with T2DM. Because these patients are at increased risk of cardiovascular (CV) morbidity and mortality, however, treatment strategies should address the CV risk factors, including blood pressure (BP), lipids, and body weight, as well as glycemic aspects of the disease.
To help clinicians manage the complex issues in treating patients with T2DM, this article presents an overview of patient and treatment perspectives relevant to overweight/obesity and CV disease (CVD). It includes an examination of the latest guidelines and algorithms for the management of T2DM, which continue to be updated and modified.
T2DM, WEIGHT GAIN OR OBESITY, AND CV RISK: A CHALLENGING TRIAD
Despite therapeutic advances in the diagnosis and treatment of diabetes and CVD over the last decade, the estimated number of persons in the United States older than 35 years with self-reported diabetes (with T2DM accounting for 90% to 95% of diagnosed cases) and CVD has increased from 4.2 million in 1997 to 5.7 million in 2005.3,4 The CV risk for patients with T2DM who have not had a CV event such as a myocardial infarction (MI) is similar to that of individuals without diabetes who have had a prior MI.5 Patients with T2DM have nearly double the mortality of those without the disease.6 Adding to their risk, about 80% of patients with T2DM are overweight or obese, conditions associated with worsened insulin resistance and increased CV risk and disease burden.7,8 Even a modest weight gain (5 kg) may increase the risk of coronary heart disease (CHD) by 30%, while associated changes in lipids and BP can increase the risk by another 20%.9
It is as important to control CV risk factors as it is to control glycemia in patients with T2DM, and both are difficult to achieve. Data from a recent nationwide Norwegian survey showed that only 13% of patients with T2DM achieved study-defined target levels; ie, glycosylated hemoglobin (HbA1c) less than 7.5%, BP less than 140/85 mm Hg, and total cholesterol/high-density lipoprotein (HDL-C) ratio less than 4.0.10
BENEFITS OF MANAGING GLYCEMIA, WEIGHT REDUCTION, AND CV RISK FACTORS
Several large studies, many ongoing, are generating data on the relationships among glycemia, weight reduction, and CV risk. It is well established that individuals with T2DM need aggressive risk factor reduction (glucose control, blood pressure management, and treatment of dyslipidemia) to optimize outcomes. However, characterization of the benefits of various components of risk factor reduction, particularly over many years, is only now occurring.
Results from the United Kingdom Prospective Diabetes Studies (UKPDS) showed the benefits and risks of pharmacologic glycemic control—essentially monotherapy with insulin or a sulfonylurea—compared with conventional dietary therapy in reducing diabetic complications in patients with newly diagnosed T2DM. In UKPDS 33, both insulin and sulfonylureas (intensive treatment) reduced the risk of microvascular end points (retinopathy, nephropathy) in patients whose median HbA1c was lowered to 7.0% at 10 years of follow-up, compared with patients who reached an HbA1c of 7.9%. However, intensive glycemic control did not translate into a statistically significant reduction in macrovascular complications, including MI, stroke, CVD, and death. Additionally, patients assigned to insulin had greater weight gain (+4.0 kg) than did patients assigned to receive the sulfonylurea chlorpropamide (+2.6 kg) or glyburide (+1.7 kg) (P < .01).11
The UKPDS showed that intensive treatment with metformin reduced the risk of T2DM-related end points compared with conventional treatment (primarily diet alone) in overweight patients.12 Although there were fewer patients in the metformin-treated subset (n = 342) than in the conventional treatment cohort, a secondary analysis showed that metformin was associated with less weight gain and fewer hypoglycemic episodes than either insulin or sulfonylurea therapy.12 Since HbA1c levels in the treatment groups were equal, the additional benefits seen with metformin in overweight patients with T2DM were not based solely on glycemic control.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial involved 10,000 individuals with T2DM. The primary outcome measure was a composite of CV events. The intensively treated group was controlled to a target HbA1c of less than 6.0%, with most patients receiving insulin. The trial was terminated early because an increased risk of sudden death was observed.13 A similar study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), evaluated more than 11,000 patients with T2DM, starting with a sulfonylurea-based regimen. In this study, there was no reduction in macrovascular events, but there was a reduction in nephropathy in the intensively treated group.14 In both studies, hypoglycemia and weight gain were more frequent in intensively treated patients; and in ACCORD, there were more episodes of severe hypoglycemia in the intensive-treatment group.13,14
The Veterans Affairs Diabetes Trial (VADT) evaluated the effect of intensive glucose control on CVD in 1,791 patients (mean age, 60 years) with poorly controlled T2DM (average duration, 11.5 years). The primary end points included MI, stroke, new or worsening congestive heart failure (CHF), limb amputation, and invasive intervention for coronary or peripheral arterial disease. The hazard ratio for these end points in the intensive-treatment group was 0.88 (95% confidence interval [CI], 0.74 to 1.05).15,16 Specifically, the following beneficial effects were achieved:
- HbA1c reduced by –1.0% to –2.5% in absolute units,
- systolic BP (SBP) reduced by –4 to –7 mm Hg,
- diastolic BP (DBP) reduced by –7 to –8 mm Hg,
- low-density lipoprotein cholesterol (LDL-C) reduced by –27 to –28 mg/dL,
- triglycerides reduced by –44 to –50 mg/dL, and
- HDL-C increased by 4 to 5 mg/dL.
Despite these benefits, body weight increased approximately 9 to 18 lb (4 to 8 kg) during therapy.15
Since overweight and obesity are independent risk factors for CHD and CVD in patients with T2DM,17 weight management is an integral component in treatment. In the Action for Health in Diabetes (Look AHEAD) trial, an intensive exercise and weight-loss program resulted in clinically significant (P < .001) weight loss at 1 year in patients who had T2DM and a body mass index (BMI) greater than 25 kg2/m (> 27 kg2/m if receiving insulin).18 When compared with patients who received less structured, infrequent support and minimal education about diabetes, participants in the intensive program showed more weight loss, improved glucose control, decreased CV events, and reduced medicine use. The Look AHEAD trial is currently evaluating whether these improvements will continue to result in lower CV risk.
PATIENT ADHERENCE AND SATISFACTION
It is often challenging for patients with T2DM to adhere to their treatment regimens. The Diabetes Attitude, Wishes, and Needs (DAWN) study examined psychosocial barriers to self-care in patients with diabetes and found that while 78% of patients with T2DM adhered to their medications, only 39% achieved complete success in at least two-thirds of their self-care domains.19 A multicenter, randomized, clinical trial examined the correlates of treatment satisfaction, including body weight, on patients’ appraisal of treatment satisfaction with injectable insulin. The 14.5% of patients who experienced a reduction in BMI reported systematic improvement in treatment satisfaction.20 Similarly, a cross-sectionally designed study (n = 99) that analyzed the interrelation of adherence, BMI, and depression in adults with T2DM found that patients with higher BMI and poor adherence also had depression, which was mediated by lower self-efficacy perceptions and increased diabetes symptoms.21 The results from these studies show a clear relationship between adherence with treatment regimens and achievement of HbA1c goals.22
RECENT DEVELOPMENTS IN T2DM MANAGEMENT: STRATEGIES TO REDUCE CV RISK
Because excess weight and obesity are prominent features of T2DM, it is important to use an antidiabetes agent that does not induce unnecessary weight gain (particularly central weight gain, which is thought to be most atherogenic).23 Metformin, considered the first-line agent for treatment of T2DM, is generally weight neutral with a low level of hypoglycemia.24,25 Sulfonylureas, insulin, and thiazolidinediones (TZDs) are all associated with weight gain, although newer-analogue insulins may cause less weight gain than older agents. TZDs, especially pioglitazone, are associated with improvements in long-term beta-cell function and CV risk factors despite weight gain.26,27
The newer antidiabetes agents belong to the dipeptidyl peptidase–4 (DPP-4) inhibitor and the glucagon-like peptide–1 (GLP-1) receptor agonist therapeutic classes and have been shown to be either weight neutral (DPP-4 inhibitors) or to cause weight loss (GLP-1 receptor agonists).28
Obesity and the incretin effect
Two recent studies showed that surgically induced weight loss enhances the physiologic “incretin effect.” In one study, obese individuals with T2DM whose weight loss was secondary to bariatric surgery combined with caloric restriction showed improved insulin sensitivity, improved carbohydrate metabolism, and elevated levels of adiponectin and GLP-1, all of which may reduce the incidence of T2DM.36 In the other study, bariatric surgery in morbidly obese individuals with T2DM improved insulin secretion and ameliorated insulin resistance.37
DPP-4 inhibitors
DPP-4 inhibitors such as sitagliptin and saxagliptin inhibit the enzymatic activity of DPP-4 and increase endogenous concentrations of GLP-1.28 Sitagliptin has been compared with placebo as monotherapy and has been studied in combination with other therapies.
In an 18-week study, sitagliptin monotherapy, 100 and 200 mg QD, significantly reduced HbA1c compared with placebo (placebo-subtracted HbA1c reduction, –0.60% and –0.48%, respectively) in patients with T2DM. Sitagliptin also significantly decreased fasting plasma glucose (FPG) concentration relative to placebo.38 Twelve weeks of sitagliptin monotherapy at dosages of 5, 12.5, 25, and 50 mg BID led to significant (P < .001) reductions in HbA1c compared with placebo. Sitagliptin also produced significant reductions in FPG and mean daily glucose concentrations across the doses studied.39 Similar results were reported in other 12-week studies: 50 mg BID and 100 mg QD sitagliptin monotherapy significantly (P < .05) reduced HbA1c –0.39% to –0.56% and FPG concentration –11.0 to –17.2 mg/dL compared with placebo40; sitagliptin 100 mg QD compared with placebo produced a least-squares mean change from baseline HbA1c of –0.65% versus 0.41% (P < .001) and FPG of –22.5 versus 9.4 mg/dL (P < .001).41
Sitagliptin also has been studied in combination with other therapies. After 24 weeks, sitagliptin combined with pioglitazone significantly reduced HbA1c by –0.70% and FPG by –17.7 mg/dL (P < .001 for both) compared with placebo.42 In another 24-week study, 100 mg sitagliptin QD significantly improved glycemic control and beta-cell function (P < .05 for both) in patients with T2DM who had inadequate glycemic control with glimepiride or glimepiride plus metformin.43
In addition to significantly reducing HbA1c, sitagliptin 100 and 200 mg QD produced only small differences in body weight relative to placebo: least-squares mean change from baseline for sitagliptin 100 mg was –0.7 kg (95% CI, –1.3 to –0.1) and for 200 mg was –0.6 kg (95% CI, –1.0 to –0.2); for placebo it was –0.2 kg (95% CI, –0.7 to 0.2).38 These findings were consistent with those from another 24-week monotherapy study where sitagliptin produced weight loss of up to –0.2 kg44 and a 30-week study of sitagliptin added to ongoing metformin therapy. In the latter study, both sitagliptin and placebo resulted in weight reductions of –0.5 kg.45
The effects of sitagliptin on lipids and BP have been reported in clinical studies in patients with and without T2DM. In one study of patients with T2DM, the addition of sitagliptin to metformin increased total cholesterol (+8.1 mg/dL), LDL-C (+9.2 mg/dL), and HDL-C (+1.8 mg/dL) but lowered triglyceride (–14.5 mg/dL) after 18 weeks of treatment (24-week data).46 Data from a small (n = 19) study in nondiabetic patients with mild to moderate hypertension showed that sitagliptin produced small reductions (–2 to –3 mm Hg) in 24-hour ambulatory BP measurements.47
Another DPP-4 inhibitor, saxagliptin, with efficacy similar to that described for sitagliptin, was recently approved by the US Food and Drug Administration (FDA) for treatment of T2DM.48
GLP-1 receptor agonists
Many of the GLP-1 receptor agonists developed or under development have glucoregulatory effects similar to GLP-1 but are resistant to degradation by DPP-4.28 Exenatide, an exendin-4 receptor agonist, has compared favorably with sitagliptin and with insulin analogues. Long-acting (once-weekly and once-daily) GLP-1 receptor agonists are under development.
In a 2-week, head-to-head study in metformin-treated patients with T2DM, exenatide had a greater effect than sitagliptin in lowering PPG and was more potent in increasing insulin secretion and reducing postprandial glucagon secretion. In contrast to sitagliptin, exenatide slowed gastric emptying and reduced caloric intake.49
In two studies of patients treated with exenatide, on a background of either metformin alone or metformin plus a sulfonylurea, patients who received metformin lost more weight (–1.6 to –2.8 kg; P ≤ .01) and experienced more significant decreases from baseline HbA1c (–0.4% to –0.8%; P < .002) at 30 weeks than did patients who received placebo.50,51 In a 16-week trial of exenatide in patients previously treated with a TZD with or without metformin, exenatide reduced HbA1c –0.98%, fasting blood glucose –1.69 mmol/L, and body weight –1.51 kg.52
When compared with insulin analogues, exenatide has been associated with weight loss (~ –3 kg) while the insulin analogues were associated with weight gain (~ +3 kg).53 After 26 weeks, body weight decreased –2.3 kg with exenatide and increased +1.8 kg with insulin glargine.54 Similar results were found in a crossover noninferiority trial, where the least-squares mean difference in weight change was significantly (P < .001) different (2.2 kg) between the treatments.55 When exenatide was compared with insulin aspart in an open-label, noninferiority trial, there was a between-group difference in weight of –5.4 kg after 52 weeks.32
Exenatide has also demonstrated these benefits in open-label extension studies. After 2 years, mean HbA1c reductions of –1.1% from baseline were sustained (P < .05), and weight loss of –4.7 kg was maintained (P < .001).56 After 82 weeks, similar HbA1c decreases (–1.1%) and weight loss (–4.4 kg) were exhibited.57 Even after 3 years, these benefits were maintained in patients who remained on the drug (HbA1c reduction from baseline, –1.0%; weight loss, –5.3 kg [P < .0001 for both]).58
Long-acting formulations of GLP-1 receptor agonists are in clinical development; two of these are once-weekly exenatide and once-daily liraglutide. Exenatide once weekly has the advantage of less frequent dosing and has elicited greater reductions in HbA1c than exenatide BID. After 15 weeks of once-weekly administration, the 0.8-mg formulation reduced HbA1c –1.4% and the 2-mg formulation reduced it –1.7% (P < .0001 for both compared with placebo). Body weight was lowered –3.8 kg (P < .05 compared with placebo) with the 2-mg formulation.59 Compared with exenatide BID, exenatide 2 mg once weekly showed greater reductions in HbA1c (–1.9% vs –1.5%; P = .0023) after 30 weeks of therapy.60 In a 1-year noncomparative trial, treatment with exenatide once weekly improved HbA1c (–2.0%) and weight (–4.1 kg), as well as BP and lipid profiles compared with baseline.61
Liraglutide, a once-daily human analogue GLP-1 receptor agonist, is under review by the FDA.28 In a 26-week study of patients with T2DM, liraglutide was associated with reductions in HbA1c (mean, –1.04%; P = 0.067 compared with insulin) and body weight (mean, –2.5 kg; P < .001 compared with insulin) at dosages of 0.6 to 1.8 mg/day SC. Liraglutide produced a decline in SBP from 0.6 to 3 mm Hg but was not associated with a decrease in DBP.62 In a 52-week study comparing liraglutide with glimepiride monotherapy, liraglutide 1.2 mg was associated with an HbA1c reduction of –0.84% (P = .0014) and the 1.8-mg dose with a reduction of –1.14% (P < .0001) compared with –0.51% for glimepiride. SBP decreased –0.7 mm Hg with glimepiride compared with –2.1 mm Hg for liraglutide 1.2 mg (P = .2912) and –3.6 mm Hg for liraglutide 1.8 mg (P < .0118). Mean DBP fell slightly but not significantly in all treatment groups.63 No effects on lipid parameters were reported in these two liraglutide studies.
The Liraglutide Effect and Action in Diabetes (LEAD-6) trial was undertaken to compare exenatide (10 mg BID SC) and liraglutide (1.8 mg/day SC) as add-on therapy to metformin, a sulfonylurea, or a combination of both in 464 patients with T2DM. After 26 weeks of treatment, liraglutide was associated with a significant reduction in HbA1c of –1.12%, compared with –0.79% with exenatide (P < .0001). Patients treated with liraglutide lost –3.2 kg while those on exenatide lost –2.9 kg. Among patients previously treated with metformin alone, there was a 1-kg difference in favor of liraglutide (P = NS).64
Safety profile
All of the drugs discussed have potential adverse effects. Metformin continues to have a black box warning for lactic acidosis.65 Sulfonylureas and insulin can cause hypoglycemia. TZDs can cause fluid retention and, in rare cases, CHF (for which these drugs also carry a black box warning).66,67 TZDs also increase the risk of distal fracture.66,67 The most common side effects of exenatide are gastrointestinal, but there have been reported cases of pancreatitis, some of which have been fatal.68,69 It has been difficult to prove whether exenatide increases the risk of pancreatitis, as patients with T2DM are already at an increased (three- to fourfold) risk for this condition compared with persons who do not have T2DM.69 Exenatide should not be used in patients with severe renal impairment or end-stage renal disease; it should be used with caution in patients who have undergone renal transplantation and in patients with moderate renal impairment.
The prescribing information for sitagliptin includes pancreatitis among the adverse reactions identified during the drug’s postapproval use.70 As with exenatide, it is not fully known whether a true association exists between the agent and pancreatitis. However, since pancreatitis can occur in this patient population, it is recommended that abdominal pain be fully evaluated to rule out pancreatitis. Continued postmarketing surveillance is important for all of these agents.
THE ROLE OF GUIDELINES
The American Association of Clinical Endocrinologists (AACE),26 the American Diabetes Association (ADA),71 and the ADA in conjunction with the European Association for the Study of Diabetes (EASD)24 have recently revised their recommendations for the management of patients with diabetes. The guidelines are unanimous in setting a glycemic goal (HbA1c < 7.0% for the ADA, HbA1c ≤ 6.5% for the AACE) and advocating individualized care for a treatment goal of HbA1c lower than 6.0% in patients who stand to benefit from near euglycemia without inducing severe hypoglycemia.24,26,71
CVD is the major cause of morbidity and mortality associated with T2DM and is a source of increasing concern.5 Accordingly, special consideration should be given to patients with coexisting CV risk factors, including hypertension and dyslipidemia. The ADA and the EASD advocate lifestyle modification to decrease body weight and the concurrent initiation of metformin as first-line therapy.24 If that strategy is insufficient, then two tiers of treatment guide the choice of next steps24:
- Tier 1, in addition to metformin, includes the sulfonylureas and insulin. Although these are excellent glucose-lowering drugs, they are associated with weight gain, hypoglycemia, and no improvement in BP or lipid levels. They are relatively low in cost and have been used for many years. Their main drawback is evidence that despite their use, beta-cell failure continues unabated over time.
- Tier 2 treatments include pioglitazone and the GLP-1 receptor agonist exenatide. Consideration may be given to the use of pioglitazone or exenatide when hypoglycemia is of concern, with exenatide being preferred when weight loss is a major objective and HbA1c is close to target (< 8.0%).24 Additionally, both the TZDs and exenatide probably help slow the rate of beta-cell failure, particularly if they are used early in the course of the disease.72,73 The AACE recommends different pharmacologic approaches based on HbA1c at diagnosis.26
The American Heart Association and the ADA have issued a joint scientific statement on the primary prevention of CVD in patients with diabetes.74 They advocate lifestyle management of body weight, nutrition, and physical activity.74 In addition, they stress the need for attention to BP, lipid levels, and smoking status, and the use of antiplatelet agents in patients at increased CV risk (> 40 years of age and a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria).
CONCLUSION
T2DM, weight gain/obesity, and CV risk present a continuing challenge to patients and clinicians. Antidiabetes agents have varying degrees of evidence to support their effects on HbA1c, body weight, BP, and lipid levels. A better understanding of the pathophysiology of T2DM has led to the development of newer antidiabetes agents that target the fundamental defects of the disease. Evidence continues to accumulate for the improved benefits of glycemic control and weight loss in T2DM with GLP-1 receptor agonists such as exenatide currently having robust data in terms of beneficial effects on weight and CV risk factors. As clinicians continue to incorporate this knowledge into their practice patterns, patient adherence and clinical outcomes are expected to improve. Newer agents, such as incretin-based therapies, address T2DM as well as other factors that increase cardiometabolic risk through their effects not only on glycemic control but on body weight, BP, and lipids.
Type 2 diabetes mellitus (T2DM), excess weight, and obesity are increasing in prevalence at alarming rates.1–3 Concurrent with the increased prevalence is increased risk of morbidity and mortality. A healthy diet and exercise in conjunction with antidiabetes medications can help lower glucose concentration in patients with T2DM. Because these patients are at increased risk of cardiovascular (CV) morbidity and mortality, however, treatment strategies should address the CV risk factors, including blood pressure (BP), lipids, and body weight, as well as glycemic aspects of the disease.
To help clinicians manage the complex issues in treating patients with T2DM, this article presents an overview of patient and treatment perspectives relevant to overweight/obesity and CV disease (CVD). It includes an examination of the latest guidelines and algorithms for the management of T2DM, which continue to be updated and modified.
T2DM, WEIGHT GAIN OR OBESITY, AND CV RISK: A CHALLENGING TRIAD
Despite therapeutic advances in the diagnosis and treatment of diabetes and CVD over the last decade, the estimated number of persons in the United States older than 35 years with self-reported diabetes (with T2DM accounting for 90% to 95% of diagnosed cases) and CVD has increased from 4.2 million in 1997 to 5.7 million in 2005.3,4 The CV risk for patients with T2DM who have not had a CV event such as a myocardial infarction (MI) is similar to that of individuals without diabetes who have had a prior MI.5 Patients with T2DM have nearly double the mortality of those without the disease.6 Adding to their risk, about 80% of patients with T2DM are overweight or obese, conditions associated with worsened insulin resistance and increased CV risk and disease burden.7,8 Even a modest weight gain (5 kg) may increase the risk of coronary heart disease (CHD) by 30%, while associated changes in lipids and BP can increase the risk by another 20%.9
It is as important to control CV risk factors as it is to control glycemia in patients with T2DM, and both are difficult to achieve. Data from a recent nationwide Norwegian survey showed that only 13% of patients with T2DM achieved study-defined target levels; ie, glycosylated hemoglobin (HbA1c) less than 7.5%, BP less than 140/85 mm Hg, and total cholesterol/high-density lipoprotein (HDL-C) ratio less than 4.0.10
BENEFITS OF MANAGING GLYCEMIA, WEIGHT REDUCTION, AND CV RISK FACTORS
Several large studies, many ongoing, are generating data on the relationships among glycemia, weight reduction, and CV risk. It is well established that individuals with T2DM need aggressive risk factor reduction (glucose control, blood pressure management, and treatment of dyslipidemia) to optimize outcomes. However, characterization of the benefits of various components of risk factor reduction, particularly over many years, is only now occurring.
Results from the United Kingdom Prospective Diabetes Studies (UKPDS) showed the benefits and risks of pharmacologic glycemic control—essentially monotherapy with insulin or a sulfonylurea—compared with conventional dietary therapy in reducing diabetic complications in patients with newly diagnosed T2DM. In UKPDS 33, both insulin and sulfonylureas (intensive treatment) reduced the risk of microvascular end points (retinopathy, nephropathy) in patients whose median HbA1c was lowered to 7.0% at 10 years of follow-up, compared with patients who reached an HbA1c of 7.9%. However, intensive glycemic control did not translate into a statistically significant reduction in macrovascular complications, including MI, stroke, CVD, and death. Additionally, patients assigned to insulin had greater weight gain (+4.0 kg) than did patients assigned to receive the sulfonylurea chlorpropamide (+2.6 kg) or glyburide (+1.7 kg) (P < .01).11
The UKPDS showed that intensive treatment with metformin reduced the risk of T2DM-related end points compared with conventional treatment (primarily diet alone) in overweight patients.12 Although there were fewer patients in the metformin-treated subset (n = 342) than in the conventional treatment cohort, a secondary analysis showed that metformin was associated with less weight gain and fewer hypoglycemic episodes than either insulin or sulfonylurea therapy.12 Since HbA1c levels in the treatment groups were equal, the additional benefits seen with metformin in overweight patients with T2DM were not based solely on glycemic control.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial involved 10,000 individuals with T2DM. The primary outcome measure was a composite of CV events. The intensively treated group was controlled to a target HbA1c of less than 6.0%, with most patients receiving insulin. The trial was terminated early because an increased risk of sudden death was observed.13 A similar study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), evaluated more than 11,000 patients with T2DM, starting with a sulfonylurea-based regimen. In this study, there was no reduction in macrovascular events, but there was a reduction in nephropathy in the intensively treated group.14 In both studies, hypoglycemia and weight gain were more frequent in intensively treated patients; and in ACCORD, there were more episodes of severe hypoglycemia in the intensive-treatment group.13,14
The Veterans Affairs Diabetes Trial (VADT) evaluated the effect of intensive glucose control on CVD in 1,791 patients (mean age, 60 years) with poorly controlled T2DM (average duration, 11.5 years). The primary end points included MI, stroke, new or worsening congestive heart failure (CHF), limb amputation, and invasive intervention for coronary or peripheral arterial disease. The hazard ratio for these end points in the intensive-treatment group was 0.88 (95% confidence interval [CI], 0.74 to 1.05).15,16 Specifically, the following beneficial effects were achieved:
- HbA1c reduced by –1.0% to –2.5% in absolute units,
- systolic BP (SBP) reduced by –4 to –7 mm Hg,
- diastolic BP (DBP) reduced by –7 to –8 mm Hg,
- low-density lipoprotein cholesterol (LDL-C) reduced by –27 to –28 mg/dL,
- triglycerides reduced by –44 to –50 mg/dL, and
- HDL-C increased by 4 to 5 mg/dL.
Despite these benefits, body weight increased approximately 9 to 18 lb (4 to 8 kg) during therapy.15
Since overweight and obesity are independent risk factors for CHD and CVD in patients with T2DM,17 weight management is an integral component in treatment. In the Action for Health in Diabetes (Look AHEAD) trial, an intensive exercise and weight-loss program resulted in clinically significant (P < .001) weight loss at 1 year in patients who had T2DM and a body mass index (BMI) greater than 25 kg2/m (> 27 kg2/m if receiving insulin).18 When compared with patients who received less structured, infrequent support and minimal education about diabetes, participants in the intensive program showed more weight loss, improved glucose control, decreased CV events, and reduced medicine use. The Look AHEAD trial is currently evaluating whether these improvements will continue to result in lower CV risk.
PATIENT ADHERENCE AND SATISFACTION
It is often challenging for patients with T2DM to adhere to their treatment regimens. The Diabetes Attitude, Wishes, and Needs (DAWN) study examined psychosocial barriers to self-care in patients with diabetes and found that while 78% of patients with T2DM adhered to their medications, only 39% achieved complete success in at least two-thirds of their self-care domains.19 A multicenter, randomized, clinical trial examined the correlates of treatment satisfaction, including body weight, on patients’ appraisal of treatment satisfaction with injectable insulin. The 14.5% of patients who experienced a reduction in BMI reported systematic improvement in treatment satisfaction.20 Similarly, a cross-sectionally designed study (n = 99) that analyzed the interrelation of adherence, BMI, and depression in adults with T2DM found that patients with higher BMI and poor adherence also had depression, which was mediated by lower self-efficacy perceptions and increased diabetes symptoms.21 The results from these studies show a clear relationship between adherence with treatment regimens and achievement of HbA1c goals.22
RECENT DEVELOPMENTS IN T2DM MANAGEMENT: STRATEGIES TO REDUCE CV RISK
Because excess weight and obesity are prominent features of T2DM, it is important to use an antidiabetes agent that does not induce unnecessary weight gain (particularly central weight gain, which is thought to be most atherogenic).23 Metformin, considered the first-line agent for treatment of T2DM, is generally weight neutral with a low level of hypoglycemia.24,25 Sulfonylureas, insulin, and thiazolidinediones (TZDs) are all associated with weight gain, although newer-analogue insulins may cause less weight gain than older agents. TZDs, especially pioglitazone, are associated with improvements in long-term beta-cell function and CV risk factors despite weight gain.26,27
The newer antidiabetes agents belong to the dipeptidyl peptidase–4 (DPP-4) inhibitor and the glucagon-like peptide–1 (GLP-1) receptor agonist therapeutic classes and have been shown to be either weight neutral (DPP-4 inhibitors) or to cause weight loss (GLP-1 receptor agonists).28
Obesity and the incretin effect
Two recent studies showed that surgically induced weight loss enhances the physiologic “incretin effect.” In one study, obese individuals with T2DM whose weight loss was secondary to bariatric surgery combined with caloric restriction showed improved insulin sensitivity, improved carbohydrate metabolism, and elevated levels of adiponectin and GLP-1, all of which may reduce the incidence of T2DM.36 In the other study, bariatric surgery in morbidly obese individuals with T2DM improved insulin secretion and ameliorated insulin resistance.37
DPP-4 inhibitors
DPP-4 inhibitors such as sitagliptin and saxagliptin inhibit the enzymatic activity of DPP-4 and increase endogenous concentrations of GLP-1.28 Sitagliptin has been compared with placebo as monotherapy and has been studied in combination with other therapies.
In an 18-week study, sitagliptin monotherapy, 100 and 200 mg QD, significantly reduced HbA1c compared with placebo (placebo-subtracted HbA1c reduction, –0.60% and –0.48%, respectively) in patients with T2DM. Sitagliptin also significantly decreased fasting plasma glucose (FPG) concentration relative to placebo.38 Twelve weeks of sitagliptin monotherapy at dosages of 5, 12.5, 25, and 50 mg BID led to significant (P < .001) reductions in HbA1c compared with placebo. Sitagliptin also produced significant reductions in FPG and mean daily glucose concentrations across the doses studied.39 Similar results were reported in other 12-week studies: 50 mg BID and 100 mg QD sitagliptin monotherapy significantly (P < .05) reduced HbA1c –0.39% to –0.56% and FPG concentration –11.0 to –17.2 mg/dL compared with placebo40; sitagliptin 100 mg QD compared with placebo produced a least-squares mean change from baseline HbA1c of –0.65% versus 0.41% (P < .001) and FPG of –22.5 versus 9.4 mg/dL (P < .001).41
Sitagliptin also has been studied in combination with other therapies. After 24 weeks, sitagliptin combined with pioglitazone significantly reduced HbA1c by –0.70% and FPG by –17.7 mg/dL (P < .001 for both) compared with placebo.42 In another 24-week study, 100 mg sitagliptin QD significantly improved glycemic control and beta-cell function (P < .05 for both) in patients with T2DM who had inadequate glycemic control with glimepiride or glimepiride plus metformin.43
In addition to significantly reducing HbA1c, sitagliptin 100 and 200 mg QD produced only small differences in body weight relative to placebo: least-squares mean change from baseline for sitagliptin 100 mg was –0.7 kg (95% CI, –1.3 to –0.1) and for 200 mg was –0.6 kg (95% CI, –1.0 to –0.2); for placebo it was –0.2 kg (95% CI, –0.7 to 0.2).38 These findings were consistent with those from another 24-week monotherapy study where sitagliptin produced weight loss of up to –0.2 kg44 and a 30-week study of sitagliptin added to ongoing metformin therapy. In the latter study, both sitagliptin and placebo resulted in weight reductions of –0.5 kg.45
The effects of sitagliptin on lipids and BP have been reported in clinical studies in patients with and without T2DM. In one study of patients with T2DM, the addition of sitagliptin to metformin increased total cholesterol (+8.1 mg/dL), LDL-C (+9.2 mg/dL), and HDL-C (+1.8 mg/dL) but lowered triglyceride (–14.5 mg/dL) after 18 weeks of treatment (24-week data).46 Data from a small (n = 19) study in nondiabetic patients with mild to moderate hypertension showed that sitagliptin produced small reductions (–2 to –3 mm Hg) in 24-hour ambulatory BP measurements.47
Another DPP-4 inhibitor, saxagliptin, with efficacy similar to that described for sitagliptin, was recently approved by the US Food and Drug Administration (FDA) for treatment of T2DM.48
GLP-1 receptor agonists
Many of the GLP-1 receptor agonists developed or under development have glucoregulatory effects similar to GLP-1 but are resistant to degradation by DPP-4.28 Exenatide, an exendin-4 receptor agonist, has compared favorably with sitagliptin and with insulin analogues. Long-acting (once-weekly and once-daily) GLP-1 receptor agonists are under development.
In a 2-week, head-to-head study in metformin-treated patients with T2DM, exenatide had a greater effect than sitagliptin in lowering PPG and was more potent in increasing insulin secretion and reducing postprandial glucagon secretion. In contrast to sitagliptin, exenatide slowed gastric emptying and reduced caloric intake.49
In two studies of patients treated with exenatide, on a background of either metformin alone or metformin plus a sulfonylurea, patients who received metformin lost more weight (–1.6 to –2.8 kg; P ≤ .01) and experienced more significant decreases from baseline HbA1c (–0.4% to –0.8%; P < .002) at 30 weeks than did patients who received placebo.50,51 In a 16-week trial of exenatide in patients previously treated with a TZD with or without metformin, exenatide reduced HbA1c –0.98%, fasting blood glucose –1.69 mmol/L, and body weight –1.51 kg.52
When compared with insulin analogues, exenatide has been associated with weight loss (~ –3 kg) while the insulin analogues were associated with weight gain (~ +3 kg).53 After 26 weeks, body weight decreased –2.3 kg with exenatide and increased +1.8 kg with insulin glargine.54 Similar results were found in a crossover noninferiority trial, where the least-squares mean difference in weight change was significantly (P < .001) different (2.2 kg) between the treatments.55 When exenatide was compared with insulin aspart in an open-label, noninferiority trial, there was a between-group difference in weight of –5.4 kg after 52 weeks.32
Exenatide has also demonstrated these benefits in open-label extension studies. After 2 years, mean HbA1c reductions of –1.1% from baseline were sustained (P < .05), and weight loss of –4.7 kg was maintained (P < .001).56 After 82 weeks, similar HbA1c decreases (–1.1%) and weight loss (–4.4 kg) were exhibited.57 Even after 3 years, these benefits were maintained in patients who remained on the drug (HbA1c reduction from baseline, –1.0%; weight loss, –5.3 kg [P < .0001 for both]).58
Long-acting formulations of GLP-1 receptor agonists are in clinical development; two of these are once-weekly exenatide and once-daily liraglutide. Exenatide once weekly has the advantage of less frequent dosing and has elicited greater reductions in HbA1c than exenatide BID. After 15 weeks of once-weekly administration, the 0.8-mg formulation reduced HbA1c –1.4% and the 2-mg formulation reduced it –1.7% (P < .0001 for both compared with placebo). Body weight was lowered –3.8 kg (P < .05 compared with placebo) with the 2-mg formulation.59 Compared with exenatide BID, exenatide 2 mg once weekly showed greater reductions in HbA1c (–1.9% vs –1.5%; P = .0023) after 30 weeks of therapy.60 In a 1-year noncomparative trial, treatment with exenatide once weekly improved HbA1c (–2.0%) and weight (–4.1 kg), as well as BP and lipid profiles compared with baseline.61
Liraglutide, a once-daily human analogue GLP-1 receptor agonist, is under review by the FDA.28 In a 26-week study of patients with T2DM, liraglutide was associated with reductions in HbA1c (mean, –1.04%; P = 0.067 compared with insulin) and body weight (mean, –2.5 kg; P < .001 compared with insulin) at dosages of 0.6 to 1.8 mg/day SC. Liraglutide produced a decline in SBP from 0.6 to 3 mm Hg but was not associated with a decrease in DBP.62 In a 52-week study comparing liraglutide with glimepiride monotherapy, liraglutide 1.2 mg was associated with an HbA1c reduction of –0.84% (P = .0014) and the 1.8-mg dose with a reduction of –1.14% (P < .0001) compared with –0.51% for glimepiride. SBP decreased –0.7 mm Hg with glimepiride compared with –2.1 mm Hg for liraglutide 1.2 mg (P = .2912) and –3.6 mm Hg for liraglutide 1.8 mg (P < .0118). Mean DBP fell slightly but not significantly in all treatment groups.63 No effects on lipid parameters were reported in these two liraglutide studies.
The Liraglutide Effect and Action in Diabetes (LEAD-6) trial was undertaken to compare exenatide (10 mg BID SC) and liraglutide (1.8 mg/day SC) as add-on therapy to metformin, a sulfonylurea, or a combination of both in 464 patients with T2DM. After 26 weeks of treatment, liraglutide was associated with a significant reduction in HbA1c of –1.12%, compared with –0.79% with exenatide (P < .0001). Patients treated with liraglutide lost –3.2 kg while those on exenatide lost –2.9 kg. Among patients previously treated with metformin alone, there was a 1-kg difference in favor of liraglutide (P = NS).64
Safety profile
All of the drugs discussed have potential adverse effects. Metformin continues to have a black box warning for lactic acidosis.65 Sulfonylureas and insulin can cause hypoglycemia. TZDs can cause fluid retention and, in rare cases, CHF (for which these drugs also carry a black box warning).66,67 TZDs also increase the risk of distal fracture.66,67 The most common side effects of exenatide are gastrointestinal, but there have been reported cases of pancreatitis, some of which have been fatal.68,69 It has been difficult to prove whether exenatide increases the risk of pancreatitis, as patients with T2DM are already at an increased (three- to fourfold) risk for this condition compared with persons who do not have T2DM.69 Exenatide should not be used in patients with severe renal impairment or end-stage renal disease; it should be used with caution in patients who have undergone renal transplantation and in patients with moderate renal impairment.
The prescribing information for sitagliptin includes pancreatitis among the adverse reactions identified during the drug’s postapproval use.70 As with exenatide, it is not fully known whether a true association exists between the agent and pancreatitis. However, since pancreatitis can occur in this patient population, it is recommended that abdominal pain be fully evaluated to rule out pancreatitis. Continued postmarketing surveillance is important for all of these agents.
THE ROLE OF GUIDELINES
The American Association of Clinical Endocrinologists (AACE),26 the American Diabetes Association (ADA),71 and the ADA in conjunction with the European Association for the Study of Diabetes (EASD)24 have recently revised their recommendations for the management of patients with diabetes. The guidelines are unanimous in setting a glycemic goal (HbA1c < 7.0% for the ADA, HbA1c ≤ 6.5% for the AACE) and advocating individualized care for a treatment goal of HbA1c lower than 6.0% in patients who stand to benefit from near euglycemia without inducing severe hypoglycemia.24,26,71
CVD is the major cause of morbidity and mortality associated with T2DM and is a source of increasing concern.5 Accordingly, special consideration should be given to patients with coexisting CV risk factors, including hypertension and dyslipidemia. The ADA and the EASD advocate lifestyle modification to decrease body weight and the concurrent initiation of metformin as first-line therapy.24 If that strategy is insufficient, then two tiers of treatment guide the choice of next steps24:
- Tier 1, in addition to metformin, includes the sulfonylureas and insulin. Although these are excellent glucose-lowering drugs, they are associated with weight gain, hypoglycemia, and no improvement in BP or lipid levels. They are relatively low in cost and have been used for many years. Their main drawback is evidence that despite their use, beta-cell failure continues unabated over time.
- Tier 2 treatments include pioglitazone and the GLP-1 receptor agonist exenatide. Consideration may be given to the use of pioglitazone or exenatide when hypoglycemia is of concern, with exenatide being preferred when weight loss is a major objective and HbA1c is close to target (< 8.0%).24 Additionally, both the TZDs and exenatide probably help slow the rate of beta-cell failure, particularly if they are used early in the course of the disease.72,73 The AACE recommends different pharmacologic approaches based on HbA1c at diagnosis.26
The American Heart Association and the ADA have issued a joint scientific statement on the primary prevention of CVD in patients with diabetes.74 They advocate lifestyle management of body weight, nutrition, and physical activity.74 In addition, they stress the need for attention to BP, lipid levels, and smoking status, and the use of antiplatelet agents in patients at increased CV risk (> 40 years of age and a family history of CVD, hypertension, smoking, dyslipidemia, or albuminuria).
CONCLUSION
T2DM, weight gain/obesity, and CV risk present a continuing challenge to patients and clinicians. Antidiabetes agents have varying degrees of evidence to support their effects on HbA1c, body weight, BP, and lipid levels. A better understanding of the pathophysiology of T2DM has led to the development of newer antidiabetes agents that target the fundamental defects of the disease. Evidence continues to accumulate for the improved benefits of glycemic control and weight loss in T2DM with GLP-1 receptor agonists such as exenatide currently having robust data in terms of beneficial effects on weight and CV risk factors. As clinicians continue to incorporate this knowledge into their practice patterns, patient adherence and clinical outcomes are expected to improve. Newer agents, such as incretin-based therapies, address T2DM as well as other factors that increase cardiometabolic risk through their effects not only on glycemic control but on body weight, BP, and lipids.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of self-reported cardiovascular disease among persons aged >35 years with diabetes—United States, 1997–2005. MMWR Morb Mortal Wkly Rep 2007; 56:1129–1132.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Bonora E, Targher G, Formentini G, et al. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabet Med 2004; 21:52–58.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Jenssen TG, Tonstad S, Claudi T, Midthejell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005; 22:1379–1385.
- Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes 2007; 5:8.
- Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: the mediational role of diabetes symptoms and self-efficacy. Health Psychol 2007; 26:693–700.
- Ruelas V, Roybal GM, Lu Y, Goldman D, Peters A. Clinical and behavioral correlates of achieving and maintaining glycemic targets in an underserved population with type 2 diabetes. Diabetes Care 2009; 32:54–56.
- Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 2003; 52:172–179.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007; 30:1127–1142.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 2001; 86:3853–3860.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17:4–12.
- de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 2009; 19:313–320.
- Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis 2008; 18:574–579.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Hanefeld M, Herman G, Wu M, Mickel C, Sanchez M, Stein PP; for the Sitagliptin Study 014 Investigators. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007; 23:1329–1339.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol 2008; 48:592–598.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29:139–153.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Garber A, Henry R. Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Fortamet [package insert]. Ft. Lauderdale, FL: Watson Laboratories; 2008.
- Actos. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008; 3077–3082.
- Avandia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2009; 1351–1359.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Idris I. News and views: FDA reviews incidences of acute pancreatitis in patients taking Byetta. Diabetes Obes Metab 2008; 10:96–98.
- Januvia [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2006, 2007.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292:E871–E883.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007; 30:162–172.
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep 2004; 53:1066–1068.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Centers for Disease Control and Prevention (CDC). Prevalence of self-reported cardiovascular disease among persons aged >35 years with diabetes—United States, 1997–2005. MMWR Morb Mortal Wkly Rep 2007; 56:1129–1132.
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006; 23:516–521.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur J Endocrinol 2008; 158:773–784.
- Bonora E, Targher G, Formentini G, et al. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabet Med 2004; 21:52–58.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Jenssen TG, Tonstad S, Claudi T, Midthejell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854–865.
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; for the VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009; 32:187–192.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005; 22:1379–1385.
- Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes 2007; 5:8.
- Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: the mediational role of diabetes symptoms and self-efficacy. Health Psychol 2007; 26:693–700.
- Ruelas V, Roybal GM, Lu Y, Goldman D, Peters A. Clinical and behavioral correlates of achieving and maintaining glycemic targets in an underserved population with type 2 diabetes. Diabetes Care 2009; 32:54–56.
- Nieves DJ, Cnop M, Retzlaff B, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 2003; 52:172–179.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007; 30:1127–1142.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132:2131–2157.
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004; 53(suppl 3):S190–S196.
- Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 2001; 86:3853–3860.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008; 57:1340–1348.
- Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17:4–12.
- de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 2009; 19:313–320.
- Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis 2008; 18:574–579.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Hanefeld M, Herman G, Wu M, Mickel C, Sanchez M, Stein PP; for the Sitagliptin Study 014 Investigators. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007; 23:1329–1339.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol 2008; 48:592–598.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29:139–153.
- Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8:436–447.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Bergenstal RM, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation 2008; 118:S1086. Abstract 1239.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Garber A, Henry R. Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Buse JB, Rosenstock J, Sesti G, et al; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374:39–47.
- Fortamet [package insert]. Ft. Lauderdale, FL: Watson Laboratories; 2008.
- Actos. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2008; 3077–3082.
- Avandia. Physicians’ Desk Reference. 63rd edition. Montvale, NJ: Physicians’ Desk Reference Inc; 2009; 1351–1359.
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
- Idris I. News and views: FDA reviews incidences of acute pancreatitis in patients taking Byetta. Diabetes Obes Metab 2008; 10:96–98.
- Januvia [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2006, 2007.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292:E871–E883.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007; 30:162–172.
KEY POINTS
- Control of cardiovascular risk factors is as important as glycemic control in patients with T2DM.
- Intensive glucose control has shown mixed results in terms of correlation with improved cardiovascular risk factors.
- Newer agents target the fundamental pathophysiologic defects of T2DM, with beneficial effects on weight and other cardiovascular risk factors.
Advances in therapy for type 2 diabetes: GLP–1 receptor agonists and DPP–4 inhibitors
The prevalence of type 2 diabetes mellitus (T2DM) is increasing exponentially worldwide. According to the Centers for Disease Control and Prevention, more than 23 million Americans had diabetes in 2007.1 Globally, the prevalence of diabetes, of which T2DM accounts for 90% to 95% of cases,1 is expected to increase from 171 million in 2000 to 366 million in 2030.2 The National Health and Nutrition Examination Survey (NHANES) showed that about 66% of Americans were overweight or obese between 2003–2004.3 Data from a Swedish National Diabetes Register study showed both overweight and obesity as independent risk factors for cardiovascular disease (CVD) in patients with T2DM.4
This article presents an overview of the evolving concepts of the pathophysiology of T2DM, with a focus on two new therapeutic classes: the glucagon-like peptide–1 (GLP-1) receptor agonists and the dipeptidyl peptidase–4 (DPP-4) inhibitors.
THE PATHOPHYSIOLOGY OF T2DM
The American Association of Clinical Endocrinologists (AACE) describes T2DM as “a progressive, complex metabolic disorder characterized by coexisting defects of multiple organ sites including insulin resistance in muscle and adipose tissue, a progressive decline in pancreatic insulin secretion, unrestrained hepatic glucose production, and other hormonal deficiencies.”5 Other defects include accelerated gastric emptying in patients with T2DM, especially those who are obese or who have the disease for a long duration.6,7
Hormonal deficiencies in T2DM are related to abnormalities in the secretion of the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP).8,9 In addition to the triumvirate of core defects associated with T2DM (involvement of the pancreatic beta cell, muscle, and liver), other mechanisms of disease onset have been advanced, including accelerated lipolysis, hyperglucagonemia, and incretin deficiency/resistance.9 Also, the rate of basal hepatic glucose production is markedly increased in patients with T2DM, which is closely correlated with elevations in fasting plasma glucagon concentration.9
The incretin effect—the intestinal augmentation of secretion of insulin—attributed to GLP-1 and GIP is reduced in patients with T2DM.10 The secretion of GIP may be normal or elevated in patients with T2DM while the secretion of GLP-1 is deficient; however, cellular responsiveness to GLP-1 is preserved while responsiveness to GIP is diminished.11
Both endogenous and exogenous GLP-1 and GIP are degraded in vivo and in vitro by the enzyme DPP-4,12
a ubiquitous, membrane-spanning, cell-surface aminopeptidase that preferentially cleaves peptides with a proline or alanine residue in the second amino-terminal position. DPP-4 is widely expressed (eg, in the liver, lungs, kidney, lymphocytes, epithelial cells, endothelial cells). The role of DPP-4 in the immune system stems from its exopeptidase activity and its interactions with various molecules, including cytokines and chemokines.13
INCRETIN-BASED THERAPIES: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Exenatide is a GLP-1 receptor agonist that is resistant to DPP-4 degradation. Based on preclinical studies, exenatide, which shares a 53% amino acid sequence identity with human GLP-1, is approximately 5,500 times more potent than endogenous GLP-1 in glucose lowering.14,15 Among the acute actions of exenatide is glucose-dependent insulinotropism, the end result of which may be a reduced risk of hypoglycemia.16 This contrasts with insulin secretagogues (eg, sulfonylureas), which increase insulin secretion regardless of glucose concentrations.
Exenatide received US Food and Drug Administration (FDA) approval in 2005 and is indicated for the treatment of patients with T2DM.13,17 Exenatide is administered BID as a subcutaneous (SC) injection in doses of 5 or 10 μg within 1 hour before the two major meals of the day, which should be eaten about 6 hours apart.18
Approved in 2006, sitagliptin was the first DPP-4 inhibitor indicated for adjunctive therapy to lifestyle modifications for the treatment of patients with T2DM.17 The recommended dosage of oral sitagliptin is 100 mg QD. A single-tablet formulation of the combination of sitagliptin and metformin was approved by the FDA in 2007.19 Another DPP-4 inhibitor, saxagliptin, was approved in July 2009 for treatment of patients with T2DM either as monotherapy or in combination with metformin, sulfonylurea, or a thiazolidinedione (TZD).20 The DPP-4 inhibitor vildagliptin is approved in the European Union and Latin America but not in the United States. Vildagliptin is available as a 50- or 100-mg daily dosage; it has been recommended for use at 50 mg QD in combination with a sulfonylurea or at 50 mg BID with either metformin or a TZD.18
GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS IN DEVELOPMENT
Exenatide is currently being evaluated as a once-weekly formulation.21,22 Compared with the BID formulation, exenatide once weekly has been shown to produce significantly greater improvements in glycemic control, with similar reductions in body weight and no increased risk of hypoglycemia.21
Also undergoing regulatory review is the partly DPP-4–resistant acylated GLP-1 receptor agonist liraglutide.13 Liraglutide, a human analogue GLP-1 receptor agonist, has 97% linear amino acid sequence homology to human GLP-1.23,24 Based on its prolonged degradation time and resulting 10- to 14-hour half-life, liraglutide is anticipated to be dosed once daily.13,25,26
Other GLP-1 receptor agonists and DPP-4 inhibitors are in varying stages of development.27 Albiglutide is a long-acting GLP-1 receptor agonist that is generated by the genetic fusion of a DPP-4–resistant GLP-1 to human albumin. Based on pharmacokinetic studies, albiglutide has a half-life of 6 to 8 days. AVE0010, an exendin-4-based GLP-1 receptor agonist, was shown in a 28-day T2DM clinical trial to have an affinity four times greater than native GLP-1 for the human GLP-1 receptor.27 Taspoglutide (R1583), a human analogue GLP-1 receptor agonist, was evaluated in three randomized, placebo-controlled studies as a GLP-1 receptor agonist. Alogliptin, a DPP-4 inhibitor currently in development, has been shown to be safe and effective in studies as monotherapy and in combination with other antidiabetes agents.28–30
CLINICAL TRIALS: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Effects on HbA1c and weight
Weight reduction with GLP-1 receptor agonists. In addition to effective glucose lowering, the GLP-1 receptor agonists, particularly exendin-4 agonists, produced beneficial effects on weight (Table 1). Exenatide BID elicited mean weight reductions up to –3.6 kg at 30 weeks21,31,32 and –5.3 kg at 3.5 years.37 Exenatide once weekly resulted in mean weight reductions of up to –3.8 kg at 15 weeks22 and –3.7 kg at 30 weeks.21 Effects on weight with liraglutide varied from a mean reduction of up to –2.99 kg to a slight gain of up to +0.13 kg at 14 weeks40,41 and with weight loss of up to –2.8 kg at 26 weeks23,26 and up to –2.5 kg at 52 weeks.25 In this review, only exenatide has been assessed in insulin-comparator studies, where it was shown to reduce weight compared with the insulin analogues, which led to weight gain.34–36
Hypoglycemia. Patients receiving exenatide experienced lower rates of hypoglycemia (up to 17%) than patients treated with either insulin glargine or insulin aspart (~25%).34,36 The rate of hypoglycemia with exenatide is comparable to that seen with metformin (up to 21%) in a systematic review of oral antidiabetes agents conducted by the Agency for Healthcare Research and Quality.62 No major hypoglycemic events were reported in the liraglutide studies reviewed. The incidence of hypoglycemia reported with DPP-4 inhibitors (Table 2) is also low (2% or less in most studies). The glucose-dependent mechanisms of the incretin-based therapies minimizes the risk of hypoglycemia.
DPP-4 inhibitors and sustained HbA1c reduction. The effects of the DPP-4 inhibitors on HbA1c and weight, either as monotherapy or in combination with other agents, were evaluated in studies ranging in duration from 12 to 52 weeks (Table 2). No studies were identified that compared the glycemic control effects of DPP-4 inhibitors and insulin analogues. Sitagliptin led to a mean reduction in HbA1c from baseline of up to –0.65% at 12 weeks,43,45 up to –0.48% at 18 weeks,44 up to –0.85% at 24 weeks,42,46,47,50 up to –1.0% at 30 weeks,49 and up to –0.67% at 52 weeks.48 Saxagliptin mean reductions in HbA1c ranged from –0.43% to –1.17%.51–54 Data from four 24-week T2DM studies56–60 showed vildagliptin reducing HbA1c up to –1.4% at 24 weeks, with the greatest reduction in a study that involved drug-naïve patients with a relatively short duration of disease (mean, 1.2 years).59 Reductions in HbA1c of –1.0% were sustained in a 52-week study61 and its 52-week extension.58
DPP-4 inhibitors: weight neutral. The DPP-4 inhibitors appear to have a weight-neutral effect (Table 2). The effects of sitagliptin on weight ranged from a loss of –1.5 kg48 at 52 weeks to a gain of +1.8 kg at 24 weeks.50 Weight changes with saxagliptin ranged from a mean reduction of –1.8 kg53 to a gain of +0.7 kg.51 Two vildagliptin studies showed varying effects on weight ranging from a loss of up to –1.8 kg from baseline56 to a gain of up to +1.3 kg57 relative to placebo, both at 24 weeks.
Potential for CV risk reduction
Potentially beneficial effects on CV risk factors, including blood pressure (ie, reduction) and lipid concentrations (ie, differential effects on low-density lipoprotein and high-density lipoprotein cholesterol), were identified in seven GLP-1 receptor studies—three with exenatide (two with exenatide BID,37,38 and one with the investigational exenatide once weekly21) and four with liraglutide.23,25,26,41 For the DPP-4 inhibitors, three studies were identified—two with sitagliptin45,50 and one with vildagliptin61—in which potentially beneficial effects on CV risk factors were demonstrated.The data have been encouraging, although the clinical implications have yet to be fully understood.
Head-to-head comparison
A recent study compared the effects of the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin on postprandial glucose (PPG) concentrations, insulin and glucagon secretion, gastric intake, and caloric intake.39 Although limited by a short treatment duration (2 weeks), the study showed that the GLP-1 receptor agonist had a greater effect than the DPP-4 inhibitor in reducing PPG concentrations, a more potent effect in increasing insulin secretion and decreasing postprandial glucagon secretion, and a relatively greater effect in reducing caloric intake; and that it decreased the rate of gastric emptying (sitagliptin had no effect). These differences suggest that exenatide may provide a greater degree of GLP-1 receptor activation than the more physiologic concentrations of GLP-1 reached with DPP-4 inhibition.39 Results of a scintigraphic study showed that exenatide substantially slows the gastric emptying that is accelerated in patients with T2DM. This could be another beneficial mechanism in treating postprandial glycemia.63
Adverse effects
Exenatide has shown effects on hepatic injury markers (ie, improvement in alanine and aspartate aminotransferases) for up to 3.5 years of treatment.37 For the GLP-1 receptor agonist and DPP-4 inhibitor studies reviewed, the adverse events were generally mild and included nausea and vomiting, nasopharyngitis, and mild hypoglycemia.
Meta-analysis conclusions
The published clinical trial data presented in this review expand the body of evidence on the safety and efficacy of incretin-based therapy in patients with T2DM. These data include the results of a meta-analysis by Amori et al,17 which examined randomized controlled trials of 12 weeks’ or longer duration that compared incretin-based therapy with placebo or other diabetes medications and reported HbA1c changes in adults with T2DM. The meta-analysis showed that incretin-based therapies reduced HbA1c more than placebo (weighted mean difference, –0.97% [95% confidence interval (CI), –1.13% to –0.81%] for GLP-1 receptor agonists and –0.74% [95% CI, –0.85% to –0.62%] for DPP-4 inhibitors) and were noninferior to other antidiabetes agents. Treatment with a GLP-1 receptor agonist (ie, exenatide) caused weight loss (–1.4 kg and –4.8 kg vs placebo and insulin, respectively) while DPP-4 inhibitors (ie, sitagliptin, vildagliptin) were weight neutral.17
Beta-cell function
Evidence regarding the effects of incretin-based therapies, particularly the exendin-4 GLP-1 receptor agonists, on beta-cell function in patients with T2DM continues to accumulate. When assessing long-term (1 year) exenatide treatment in patients with T2DM, a trial (n = 69) comparing exenatide with the basal insulin analogue insulin glargine showed that exenatide and insulin glargine resulted in similar reductions in HbA1c (–0.8% vs –0.7%; P = .55).64 However, exenatide significantly reduced body weight while insulin glargine resulted in weight gain (–3.6 kg vs +1.0 kg; P < .0001). In terms of beta-cell function, arginine-stimulated C-peptide secretion during hyperglycemia increased 2.46-fold from baseline after 52 weeks of exenatide treatment compared with 1.31-fold with insulin glargine treatment (P < .0001).64
With respect to the direct beta-cell effects of liraglutide, a preclinical study reported that liraglutide improved glucose homeostasis in marginal mass islet transplantation in diabetic mice.65 In this study, liraglutide was shown, in a mouse model, to reduce the time to normoglycemia after islet cell transplantation (median time, 1 vs 72.5 days; P < .0001). The effects of liraglutide on beta-cell function also were assessed in 13 patients with T2DM. After 7 days of treatment, liraglutide improved beta-cell function, which was associated with improvement in glucose concentration.66 Liraglutide improved potentiation of insulin secretion during the first meal, owing in part to restoration of the potentiation peak (which is markedly blunted in T2DM), in a phenomenon similar to that observed with exenatide.67
Beneficial effects on beta-cell function have also been reported with DPP-4 inhibitors. In a model-based analysis of patients with T2DM, it was shown that sitagliptin improved basal, static, and dynamic responsiveness of pancreatic beta cells to glucose. The results were observed when sitagliptin was administered both as an add-on to metformin therapy and as monotherapy.68 A 52-week, double-blind, randomized, parallel-group study compared vildagliptin 50 mg/day and placebo in 306 patients with T2DM and mild hyperglycemia (HbA1c, 6.2% to 7.5%). Vildagliptin was shown to significantly increase fasting insulin secretory tone, glucose sensitivity, and rate sensitivity, all of which are aspects of beta-cell function.69
Summary
Based on the ability of incretin-based therapies to address various disease mechanisms, including beta-cell defects (ie, hyperglycemia), hormone-related abnormalities (ie, hyperglucagonemia, incretin deficiency/resistance), and accelerated gastric emptying (especially with GLP-1 receptor agonists); their favorable effects on weight (reduction with GLP-1 receptor agonists and neutral with DPP-4 inhibitors); their beneficial effects on CV risk factors; and their good safety profile (ie, hypoglycemia risk comparable with metformin), these agents could be considered therapeutic advances for the treatment of patients with T2DM.
INCRETIN-BASED THERAPIES IN GUIDELINES AND ALGORITHMS
The 2007 AACE medical guidelines for clinical practice for the management of diabetes recognized the place of the incretin-based therapies and included them among the pharmacologic options.5 Exenatide was specifically recommended for combination therapy with metformin, a sulfonylurea (secretagogue), a sulfonylurea plus metformin, or a TZD. Sitagliptin was recommended for use as monotherapy or in combination with metformin or a TZD.5
In 2009, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes convened a consensus panel to produce an algorithm for the initiation and adjustment of therapy for patients with T2DM. In this algorithm, GLP-1 receptor agonists were considered appropriate in certain clinical scenarios (eg, when hypoglycemia was an issue or weight loss was a major consideration during treatment). However, the groups also noted a need for more data on long-term safety and the cost of treatment with incretin-based therapies.70
The AACE and the American College of Endocrinology recently developed “road maps” for managing patients with T2DM. In patients with T2DM who are naïve to therapy, DPP-4 inhibitors are among the recommended first options when the initial HbA1c is 6.0% to 7.0% and as a combination therapy component when HbA1c reaches 7.0% to 9.0%. In patients who have already received monotherapy for 2 to 3 months and whose HbA1c is 6.5% to 8.5%, treatment options include combination therapy with a DPP-4 inhibitor and metformin or a TZD. Another option includes the initiation of treatment with a GLP-1 receptor agonist in combination with a TZD, with metformin or a sulfonylurea, or with metformin and a sulfonylurea.71
The role of GLP-1 receptor agonist therapies and their incorporation into T2DM treatment algorithms was noted at the 2008 annual meeting of the ADA. In the Banting lecture, Ralph A. DeFronzo, MD, advocated the early use of triple-drug therapy with metformin, exenatide, and a TZD in the management of patients with T2DM.9
CONCLUSION
T2DM, which is linked to weight gain and obesity, is a complex disease that predisposes patients to and is associated with CVD. A better understanding and appreciation of the role of the incretin system in the pathogenesis of T2DM has led to the development of incretin-based therapies, such as the GLP-1 receptor agonists and DPP-4 inhibitors. As more experimental and clinical evidence becomes available, subtle nuances are emerging that distinguish the roles of these two therapeutic classes.
- 2007 National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/estimates07.htm. Updated: July 23, 2008. Accessed September 25, 2009.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Bertin E, Schneider N, Abdelli N, et al. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes Metab 2001; 27:357–364.
- Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term type 2 diabetes mellitus. Diabet Med 1998; 15:1022–1027.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995; 80:952–957.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Eng J, Kleinman WA, Singh L, Singh G, Raufman J-P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom: further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992; 267:7402–7405.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Davidson JA, Parente EB, Gross JL. Incretin mimetics and dipeptidyl peptidase-4 inhibitors: innovative treatment therapies for type 2 diabetes. Arq Bras Endocrinol Metabol 2008; 52:1039–1049.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Marre M, Shaw J, Brändle M, et al; for the LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetes Med 2009; 26:268–278.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide 1 with pharmacokinetic properties suitable for once-daily administration. J Med Chem 2000; 43:1664–1669.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Fleck P, Christopher R, Covington P, Wilson C, Mekki Q. Efficacy and safety of alogliptin monotherapy over 12 weeks in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 479-P.
- DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Effect of alogliptin combined with pioglitazone on glycemic control in metformin-treated patients with type 2 diabetes. Paper presented at: 69th Annual Meeting of the American Diabetes Association; June 5–9, 2009; New Orleans, LA. Abstract 2024-PO.
- Nauck M, Ellis G, Fleck P, Wilson C, Mekki Q. Efficacy and safety of alogliptin added to metformin therapy in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 477-P.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30:1448–1460.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81:161–168.
- Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007; 30:1608–1610.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R; for the CV181-040 Investigators. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 2009; 63:1395–1406.
- DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009; 32:1649–1655.
- Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R; for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009; 11:611–622.
- Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R; for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin 2009; 25:2401–2411.
- Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naïve patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:376–386.
- Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 2007; 39:218–223.
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metformin. Horm Metab Res 2008; 40:892–895.
- Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med 2008; 25:435–441.
- Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract 2007; 76:132–138.
- Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with type 2 diabetes. Diabetes Med 2007; 24:955–961.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Merani S, Truong W, Emamaullee JA, Toso C, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology 2008; 149:4322–4328.
- Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care 2007; 30:2032–2033.
- Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res 2006; 38:838–844.
- Xu L, Man CD, Charbonnel B, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab 2008; 10:1212–1220.
- Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed b-cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab 2008; 93:103–109.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Jellinger PS, Davidson JA, Blonde L, et al; for the ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract 2007; 13:260–268.
The prevalence of type 2 diabetes mellitus (T2DM) is increasing exponentially worldwide. According to the Centers for Disease Control and Prevention, more than 23 million Americans had diabetes in 2007.1 Globally, the prevalence of diabetes, of which T2DM accounts for 90% to 95% of cases,1 is expected to increase from 171 million in 2000 to 366 million in 2030.2 The National Health and Nutrition Examination Survey (NHANES) showed that about 66% of Americans were overweight or obese between 2003–2004.3 Data from a Swedish National Diabetes Register study showed both overweight and obesity as independent risk factors for cardiovascular disease (CVD) in patients with T2DM.4
This article presents an overview of the evolving concepts of the pathophysiology of T2DM, with a focus on two new therapeutic classes: the glucagon-like peptide–1 (GLP-1) receptor agonists and the dipeptidyl peptidase–4 (DPP-4) inhibitors.
THE PATHOPHYSIOLOGY OF T2DM
The American Association of Clinical Endocrinologists (AACE) describes T2DM as “a progressive, complex metabolic disorder characterized by coexisting defects of multiple organ sites including insulin resistance in muscle and adipose tissue, a progressive decline in pancreatic insulin secretion, unrestrained hepatic glucose production, and other hormonal deficiencies.”5 Other defects include accelerated gastric emptying in patients with T2DM, especially those who are obese or who have the disease for a long duration.6,7
Hormonal deficiencies in T2DM are related to abnormalities in the secretion of the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP).8,9 In addition to the triumvirate of core defects associated with T2DM (involvement of the pancreatic beta cell, muscle, and liver), other mechanisms of disease onset have been advanced, including accelerated lipolysis, hyperglucagonemia, and incretin deficiency/resistance.9 Also, the rate of basal hepatic glucose production is markedly increased in patients with T2DM, which is closely correlated with elevations in fasting plasma glucagon concentration.9
The incretin effect—the intestinal augmentation of secretion of insulin—attributed to GLP-1 and GIP is reduced in patients with T2DM.10 The secretion of GIP may be normal or elevated in patients with T2DM while the secretion of GLP-1 is deficient; however, cellular responsiveness to GLP-1 is preserved while responsiveness to GIP is diminished.11
Both endogenous and exogenous GLP-1 and GIP are degraded in vivo and in vitro by the enzyme DPP-4,12
a ubiquitous, membrane-spanning, cell-surface aminopeptidase that preferentially cleaves peptides with a proline or alanine residue in the second amino-terminal position. DPP-4 is widely expressed (eg, in the liver, lungs, kidney, lymphocytes, epithelial cells, endothelial cells). The role of DPP-4 in the immune system stems from its exopeptidase activity and its interactions with various molecules, including cytokines and chemokines.13
INCRETIN-BASED THERAPIES: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Exenatide is a GLP-1 receptor agonist that is resistant to DPP-4 degradation. Based on preclinical studies, exenatide, which shares a 53% amino acid sequence identity with human GLP-1, is approximately 5,500 times more potent than endogenous GLP-1 in glucose lowering.14,15 Among the acute actions of exenatide is glucose-dependent insulinotropism, the end result of which may be a reduced risk of hypoglycemia.16 This contrasts with insulin secretagogues (eg, sulfonylureas), which increase insulin secretion regardless of glucose concentrations.
Exenatide received US Food and Drug Administration (FDA) approval in 2005 and is indicated for the treatment of patients with T2DM.13,17 Exenatide is administered BID as a subcutaneous (SC) injection in doses of 5 or 10 μg within 1 hour before the two major meals of the day, which should be eaten about 6 hours apart.18
Approved in 2006, sitagliptin was the first DPP-4 inhibitor indicated for adjunctive therapy to lifestyle modifications for the treatment of patients with T2DM.17 The recommended dosage of oral sitagliptin is 100 mg QD. A single-tablet formulation of the combination of sitagliptin and metformin was approved by the FDA in 2007.19 Another DPP-4 inhibitor, saxagliptin, was approved in July 2009 for treatment of patients with T2DM either as monotherapy or in combination with metformin, sulfonylurea, or a thiazolidinedione (TZD).20 The DPP-4 inhibitor vildagliptin is approved in the European Union and Latin America but not in the United States. Vildagliptin is available as a 50- or 100-mg daily dosage; it has been recommended for use at 50 mg QD in combination with a sulfonylurea or at 50 mg BID with either metformin or a TZD.18
GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS IN DEVELOPMENT
Exenatide is currently being evaluated as a once-weekly formulation.21,22 Compared with the BID formulation, exenatide once weekly has been shown to produce significantly greater improvements in glycemic control, with similar reductions in body weight and no increased risk of hypoglycemia.21
Also undergoing regulatory review is the partly DPP-4–resistant acylated GLP-1 receptor agonist liraglutide.13 Liraglutide, a human analogue GLP-1 receptor agonist, has 97% linear amino acid sequence homology to human GLP-1.23,24 Based on its prolonged degradation time and resulting 10- to 14-hour half-life, liraglutide is anticipated to be dosed once daily.13,25,26
Other GLP-1 receptor agonists and DPP-4 inhibitors are in varying stages of development.27 Albiglutide is a long-acting GLP-1 receptor agonist that is generated by the genetic fusion of a DPP-4–resistant GLP-1 to human albumin. Based on pharmacokinetic studies, albiglutide has a half-life of 6 to 8 days. AVE0010, an exendin-4-based GLP-1 receptor agonist, was shown in a 28-day T2DM clinical trial to have an affinity four times greater than native GLP-1 for the human GLP-1 receptor.27 Taspoglutide (R1583), a human analogue GLP-1 receptor agonist, was evaluated in three randomized, placebo-controlled studies as a GLP-1 receptor agonist. Alogliptin, a DPP-4 inhibitor currently in development, has been shown to be safe and effective in studies as monotherapy and in combination with other antidiabetes agents.28–30
CLINICAL TRIALS: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Effects on HbA1c and weight
Weight reduction with GLP-1 receptor agonists. In addition to effective glucose lowering, the GLP-1 receptor agonists, particularly exendin-4 agonists, produced beneficial effects on weight (Table 1). Exenatide BID elicited mean weight reductions up to –3.6 kg at 30 weeks21,31,32 and –5.3 kg at 3.5 years.37 Exenatide once weekly resulted in mean weight reductions of up to –3.8 kg at 15 weeks22 and –3.7 kg at 30 weeks.21 Effects on weight with liraglutide varied from a mean reduction of up to –2.99 kg to a slight gain of up to +0.13 kg at 14 weeks40,41 and with weight loss of up to –2.8 kg at 26 weeks23,26 and up to –2.5 kg at 52 weeks.25 In this review, only exenatide has been assessed in insulin-comparator studies, where it was shown to reduce weight compared with the insulin analogues, which led to weight gain.34–36
Hypoglycemia. Patients receiving exenatide experienced lower rates of hypoglycemia (up to 17%) than patients treated with either insulin glargine or insulin aspart (~25%).34,36 The rate of hypoglycemia with exenatide is comparable to that seen with metformin (up to 21%) in a systematic review of oral antidiabetes agents conducted by the Agency for Healthcare Research and Quality.62 No major hypoglycemic events were reported in the liraglutide studies reviewed. The incidence of hypoglycemia reported with DPP-4 inhibitors (Table 2) is also low (2% or less in most studies). The glucose-dependent mechanisms of the incretin-based therapies minimizes the risk of hypoglycemia.
DPP-4 inhibitors and sustained HbA1c reduction. The effects of the DPP-4 inhibitors on HbA1c and weight, either as monotherapy or in combination with other agents, were evaluated in studies ranging in duration from 12 to 52 weeks (Table 2). No studies were identified that compared the glycemic control effects of DPP-4 inhibitors and insulin analogues. Sitagliptin led to a mean reduction in HbA1c from baseline of up to –0.65% at 12 weeks,43,45 up to –0.48% at 18 weeks,44 up to –0.85% at 24 weeks,42,46,47,50 up to –1.0% at 30 weeks,49 and up to –0.67% at 52 weeks.48 Saxagliptin mean reductions in HbA1c ranged from –0.43% to –1.17%.51–54 Data from four 24-week T2DM studies56–60 showed vildagliptin reducing HbA1c up to –1.4% at 24 weeks, with the greatest reduction in a study that involved drug-naïve patients with a relatively short duration of disease (mean, 1.2 years).59 Reductions in HbA1c of –1.0% were sustained in a 52-week study61 and its 52-week extension.58
DPP-4 inhibitors: weight neutral. The DPP-4 inhibitors appear to have a weight-neutral effect (Table 2). The effects of sitagliptin on weight ranged from a loss of –1.5 kg48 at 52 weeks to a gain of +1.8 kg at 24 weeks.50 Weight changes with saxagliptin ranged from a mean reduction of –1.8 kg53 to a gain of +0.7 kg.51 Two vildagliptin studies showed varying effects on weight ranging from a loss of up to –1.8 kg from baseline56 to a gain of up to +1.3 kg57 relative to placebo, both at 24 weeks.
Potential for CV risk reduction
Potentially beneficial effects on CV risk factors, including blood pressure (ie, reduction) and lipid concentrations (ie, differential effects on low-density lipoprotein and high-density lipoprotein cholesterol), were identified in seven GLP-1 receptor studies—three with exenatide (two with exenatide BID,37,38 and one with the investigational exenatide once weekly21) and four with liraglutide.23,25,26,41 For the DPP-4 inhibitors, three studies were identified—two with sitagliptin45,50 and one with vildagliptin61—in which potentially beneficial effects on CV risk factors were demonstrated.The data have been encouraging, although the clinical implications have yet to be fully understood.
Head-to-head comparison
A recent study compared the effects of the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin on postprandial glucose (PPG) concentrations, insulin and glucagon secretion, gastric intake, and caloric intake.39 Although limited by a short treatment duration (2 weeks), the study showed that the GLP-1 receptor agonist had a greater effect than the DPP-4 inhibitor in reducing PPG concentrations, a more potent effect in increasing insulin secretion and decreasing postprandial glucagon secretion, and a relatively greater effect in reducing caloric intake; and that it decreased the rate of gastric emptying (sitagliptin had no effect). These differences suggest that exenatide may provide a greater degree of GLP-1 receptor activation than the more physiologic concentrations of GLP-1 reached with DPP-4 inhibition.39 Results of a scintigraphic study showed that exenatide substantially slows the gastric emptying that is accelerated in patients with T2DM. This could be another beneficial mechanism in treating postprandial glycemia.63
Adverse effects
Exenatide has shown effects on hepatic injury markers (ie, improvement in alanine and aspartate aminotransferases) for up to 3.5 years of treatment.37 For the GLP-1 receptor agonist and DPP-4 inhibitor studies reviewed, the adverse events were generally mild and included nausea and vomiting, nasopharyngitis, and mild hypoglycemia.
Meta-analysis conclusions
The published clinical trial data presented in this review expand the body of evidence on the safety and efficacy of incretin-based therapy in patients with T2DM. These data include the results of a meta-analysis by Amori et al,17 which examined randomized controlled trials of 12 weeks’ or longer duration that compared incretin-based therapy with placebo or other diabetes medications and reported HbA1c changes in adults with T2DM. The meta-analysis showed that incretin-based therapies reduced HbA1c more than placebo (weighted mean difference, –0.97% [95% confidence interval (CI), –1.13% to –0.81%] for GLP-1 receptor agonists and –0.74% [95% CI, –0.85% to –0.62%] for DPP-4 inhibitors) and were noninferior to other antidiabetes agents. Treatment with a GLP-1 receptor agonist (ie, exenatide) caused weight loss (–1.4 kg and –4.8 kg vs placebo and insulin, respectively) while DPP-4 inhibitors (ie, sitagliptin, vildagliptin) were weight neutral.17
Beta-cell function
Evidence regarding the effects of incretin-based therapies, particularly the exendin-4 GLP-1 receptor agonists, on beta-cell function in patients with T2DM continues to accumulate. When assessing long-term (1 year) exenatide treatment in patients with T2DM, a trial (n = 69) comparing exenatide with the basal insulin analogue insulin glargine showed that exenatide and insulin glargine resulted in similar reductions in HbA1c (–0.8% vs –0.7%; P = .55).64 However, exenatide significantly reduced body weight while insulin glargine resulted in weight gain (–3.6 kg vs +1.0 kg; P < .0001). In terms of beta-cell function, arginine-stimulated C-peptide secretion during hyperglycemia increased 2.46-fold from baseline after 52 weeks of exenatide treatment compared with 1.31-fold with insulin glargine treatment (P < .0001).64
With respect to the direct beta-cell effects of liraglutide, a preclinical study reported that liraglutide improved glucose homeostasis in marginal mass islet transplantation in diabetic mice.65 In this study, liraglutide was shown, in a mouse model, to reduce the time to normoglycemia after islet cell transplantation (median time, 1 vs 72.5 days; P < .0001). The effects of liraglutide on beta-cell function also were assessed in 13 patients with T2DM. After 7 days of treatment, liraglutide improved beta-cell function, which was associated with improvement in glucose concentration.66 Liraglutide improved potentiation of insulin secretion during the first meal, owing in part to restoration of the potentiation peak (which is markedly blunted in T2DM), in a phenomenon similar to that observed with exenatide.67
Beneficial effects on beta-cell function have also been reported with DPP-4 inhibitors. In a model-based analysis of patients with T2DM, it was shown that sitagliptin improved basal, static, and dynamic responsiveness of pancreatic beta cells to glucose. The results were observed when sitagliptin was administered both as an add-on to metformin therapy and as monotherapy.68 A 52-week, double-blind, randomized, parallel-group study compared vildagliptin 50 mg/day and placebo in 306 patients with T2DM and mild hyperglycemia (HbA1c, 6.2% to 7.5%). Vildagliptin was shown to significantly increase fasting insulin secretory tone, glucose sensitivity, and rate sensitivity, all of which are aspects of beta-cell function.69
Summary
Based on the ability of incretin-based therapies to address various disease mechanisms, including beta-cell defects (ie, hyperglycemia), hormone-related abnormalities (ie, hyperglucagonemia, incretin deficiency/resistance), and accelerated gastric emptying (especially with GLP-1 receptor agonists); their favorable effects on weight (reduction with GLP-1 receptor agonists and neutral with DPP-4 inhibitors); their beneficial effects on CV risk factors; and their good safety profile (ie, hypoglycemia risk comparable with metformin), these agents could be considered therapeutic advances for the treatment of patients with T2DM.
INCRETIN-BASED THERAPIES IN GUIDELINES AND ALGORITHMS
The 2007 AACE medical guidelines for clinical practice for the management of diabetes recognized the place of the incretin-based therapies and included them among the pharmacologic options.5 Exenatide was specifically recommended for combination therapy with metformin, a sulfonylurea (secretagogue), a sulfonylurea plus metformin, or a TZD. Sitagliptin was recommended for use as monotherapy or in combination with metformin or a TZD.5
In 2009, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes convened a consensus panel to produce an algorithm for the initiation and adjustment of therapy for patients with T2DM. In this algorithm, GLP-1 receptor agonists were considered appropriate in certain clinical scenarios (eg, when hypoglycemia was an issue or weight loss was a major consideration during treatment). However, the groups also noted a need for more data on long-term safety and the cost of treatment with incretin-based therapies.70
The AACE and the American College of Endocrinology recently developed “road maps” for managing patients with T2DM. In patients with T2DM who are naïve to therapy, DPP-4 inhibitors are among the recommended first options when the initial HbA1c is 6.0% to 7.0% and as a combination therapy component when HbA1c reaches 7.0% to 9.0%. In patients who have already received monotherapy for 2 to 3 months and whose HbA1c is 6.5% to 8.5%, treatment options include combination therapy with a DPP-4 inhibitor and metformin or a TZD. Another option includes the initiation of treatment with a GLP-1 receptor agonist in combination with a TZD, with metformin or a sulfonylurea, or with metformin and a sulfonylurea.71
The role of GLP-1 receptor agonist therapies and their incorporation into T2DM treatment algorithms was noted at the 2008 annual meeting of the ADA. In the Banting lecture, Ralph A. DeFronzo, MD, advocated the early use of triple-drug therapy with metformin, exenatide, and a TZD in the management of patients with T2DM.9
CONCLUSION
T2DM, which is linked to weight gain and obesity, is a complex disease that predisposes patients to and is associated with CVD. A better understanding and appreciation of the role of the incretin system in the pathogenesis of T2DM has led to the development of incretin-based therapies, such as the GLP-1 receptor agonists and DPP-4 inhibitors. As more experimental and clinical evidence becomes available, subtle nuances are emerging that distinguish the roles of these two therapeutic classes.
The prevalence of type 2 diabetes mellitus (T2DM) is increasing exponentially worldwide. According to the Centers for Disease Control and Prevention, more than 23 million Americans had diabetes in 2007.1 Globally, the prevalence of diabetes, of which T2DM accounts for 90% to 95% of cases,1 is expected to increase from 171 million in 2000 to 366 million in 2030.2 The National Health and Nutrition Examination Survey (NHANES) showed that about 66% of Americans were overweight or obese between 2003–2004.3 Data from a Swedish National Diabetes Register study showed both overweight and obesity as independent risk factors for cardiovascular disease (CVD) in patients with T2DM.4
This article presents an overview of the evolving concepts of the pathophysiology of T2DM, with a focus on two new therapeutic classes: the glucagon-like peptide–1 (GLP-1) receptor agonists and the dipeptidyl peptidase–4 (DPP-4) inhibitors.
THE PATHOPHYSIOLOGY OF T2DM
The American Association of Clinical Endocrinologists (AACE) describes T2DM as “a progressive, complex metabolic disorder characterized by coexisting defects of multiple organ sites including insulin resistance in muscle and adipose tissue, a progressive decline in pancreatic insulin secretion, unrestrained hepatic glucose production, and other hormonal deficiencies.”5 Other defects include accelerated gastric emptying in patients with T2DM, especially those who are obese or who have the disease for a long duration.6,7
Hormonal deficiencies in T2DM are related to abnormalities in the secretion of the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP).8,9 In addition to the triumvirate of core defects associated with T2DM (involvement of the pancreatic beta cell, muscle, and liver), other mechanisms of disease onset have been advanced, including accelerated lipolysis, hyperglucagonemia, and incretin deficiency/resistance.9 Also, the rate of basal hepatic glucose production is markedly increased in patients with T2DM, which is closely correlated with elevations in fasting plasma glucagon concentration.9
The incretin effect—the intestinal augmentation of secretion of insulin—attributed to GLP-1 and GIP is reduced in patients with T2DM.10 The secretion of GIP may be normal or elevated in patients with T2DM while the secretion of GLP-1 is deficient; however, cellular responsiveness to GLP-1 is preserved while responsiveness to GIP is diminished.11
Both endogenous and exogenous GLP-1 and GIP are degraded in vivo and in vitro by the enzyme DPP-4,12
a ubiquitous, membrane-spanning, cell-surface aminopeptidase that preferentially cleaves peptides with a proline or alanine residue in the second amino-terminal position. DPP-4 is widely expressed (eg, in the liver, lungs, kidney, lymphocytes, epithelial cells, endothelial cells). The role of DPP-4 in the immune system stems from its exopeptidase activity and its interactions with various molecules, including cytokines and chemokines.13
INCRETIN-BASED THERAPIES: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Exenatide is a GLP-1 receptor agonist that is resistant to DPP-4 degradation. Based on preclinical studies, exenatide, which shares a 53% amino acid sequence identity with human GLP-1, is approximately 5,500 times more potent than endogenous GLP-1 in glucose lowering.14,15 Among the acute actions of exenatide is glucose-dependent insulinotropism, the end result of which may be a reduced risk of hypoglycemia.16 This contrasts with insulin secretagogues (eg, sulfonylureas), which increase insulin secretion regardless of glucose concentrations.
Exenatide received US Food and Drug Administration (FDA) approval in 2005 and is indicated for the treatment of patients with T2DM.13,17 Exenatide is administered BID as a subcutaneous (SC) injection in doses of 5 or 10 μg within 1 hour before the two major meals of the day, which should be eaten about 6 hours apart.18
Approved in 2006, sitagliptin was the first DPP-4 inhibitor indicated for adjunctive therapy to lifestyle modifications for the treatment of patients with T2DM.17 The recommended dosage of oral sitagliptin is 100 mg QD. A single-tablet formulation of the combination of sitagliptin and metformin was approved by the FDA in 2007.19 Another DPP-4 inhibitor, saxagliptin, was approved in July 2009 for treatment of patients with T2DM either as monotherapy or in combination with metformin, sulfonylurea, or a thiazolidinedione (TZD).20 The DPP-4 inhibitor vildagliptin is approved in the European Union and Latin America but not in the United States. Vildagliptin is available as a 50- or 100-mg daily dosage; it has been recommended for use at 50 mg QD in combination with a sulfonylurea or at 50 mg BID with either metformin or a TZD.18
GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS IN DEVELOPMENT
Exenatide is currently being evaluated as a once-weekly formulation.21,22 Compared with the BID formulation, exenatide once weekly has been shown to produce significantly greater improvements in glycemic control, with similar reductions in body weight and no increased risk of hypoglycemia.21
Also undergoing regulatory review is the partly DPP-4–resistant acylated GLP-1 receptor agonist liraglutide.13 Liraglutide, a human analogue GLP-1 receptor agonist, has 97% linear amino acid sequence homology to human GLP-1.23,24 Based on its prolonged degradation time and resulting 10- to 14-hour half-life, liraglutide is anticipated to be dosed once daily.13,25,26
Other GLP-1 receptor agonists and DPP-4 inhibitors are in varying stages of development.27 Albiglutide is a long-acting GLP-1 receptor agonist that is generated by the genetic fusion of a DPP-4–resistant GLP-1 to human albumin. Based on pharmacokinetic studies, albiglutide has a half-life of 6 to 8 days. AVE0010, an exendin-4-based GLP-1 receptor agonist, was shown in a 28-day T2DM clinical trial to have an affinity four times greater than native GLP-1 for the human GLP-1 receptor.27 Taspoglutide (R1583), a human analogue GLP-1 receptor agonist, was evaluated in three randomized, placebo-controlled studies as a GLP-1 receptor agonist. Alogliptin, a DPP-4 inhibitor currently in development, has been shown to be safe and effective in studies as monotherapy and in combination with other antidiabetes agents.28–30
CLINICAL TRIALS: GLP-1 RECEPTOR AGONISTS AND DPP-4 INHIBITORS
Effects on HbA1c and weight
Weight reduction with GLP-1 receptor agonists. In addition to effective glucose lowering, the GLP-1 receptor agonists, particularly exendin-4 agonists, produced beneficial effects on weight (Table 1). Exenatide BID elicited mean weight reductions up to –3.6 kg at 30 weeks21,31,32 and –5.3 kg at 3.5 years.37 Exenatide once weekly resulted in mean weight reductions of up to –3.8 kg at 15 weeks22 and –3.7 kg at 30 weeks.21 Effects on weight with liraglutide varied from a mean reduction of up to –2.99 kg to a slight gain of up to +0.13 kg at 14 weeks40,41 and with weight loss of up to –2.8 kg at 26 weeks23,26 and up to –2.5 kg at 52 weeks.25 In this review, only exenatide has been assessed in insulin-comparator studies, where it was shown to reduce weight compared with the insulin analogues, which led to weight gain.34–36
Hypoglycemia. Patients receiving exenatide experienced lower rates of hypoglycemia (up to 17%) than patients treated with either insulin glargine or insulin aspart (~25%).34,36 The rate of hypoglycemia with exenatide is comparable to that seen with metformin (up to 21%) in a systematic review of oral antidiabetes agents conducted by the Agency for Healthcare Research and Quality.62 No major hypoglycemic events were reported in the liraglutide studies reviewed. The incidence of hypoglycemia reported with DPP-4 inhibitors (Table 2) is also low (2% or less in most studies). The glucose-dependent mechanisms of the incretin-based therapies minimizes the risk of hypoglycemia.
DPP-4 inhibitors and sustained HbA1c reduction. The effects of the DPP-4 inhibitors on HbA1c and weight, either as monotherapy or in combination with other agents, were evaluated in studies ranging in duration from 12 to 52 weeks (Table 2). No studies were identified that compared the glycemic control effects of DPP-4 inhibitors and insulin analogues. Sitagliptin led to a mean reduction in HbA1c from baseline of up to –0.65% at 12 weeks,43,45 up to –0.48% at 18 weeks,44 up to –0.85% at 24 weeks,42,46,47,50 up to –1.0% at 30 weeks,49 and up to –0.67% at 52 weeks.48 Saxagliptin mean reductions in HbA1c ranged from –0.43% to –1.17%.51–54 Data from four 24-week T2DM studies56–60 showed vildagliptin reducing HbA1c up to –1.4% at 24 weeks, with the greatest reduction in a study that involved drug-naïve patients with a relatively short duration of disease (mean, 1.2 years).59 Reductions in HbA1c of –1.0% were sustained in a 52-week study61 and its 52-week extension.58
DPP-4 inhibitors: weight neutral. The DPP-4 inhibitors appear to have a weight-neutral effect (Table 2). The effects of sitagliptin on weight ranged from a loss of –1.5 kg48 at 52 weeks to a gain of +1.8 kg at 24 weeks.50 Weight changes with saxagliptin ranged from a mean reduction of –1.8 kg53 to a gain of +0.7 kg.51 Two vildagliptin studies showed varying effects on weight ranging from a loss of up to –1.8 kg from baseline56 to a gain of up to +1.3 kg57 relative to placebo, both at 24 weeks.
Potential for CV risk reduction
Potentially beneficial effects on CV risk factors, including blood pressure (ie, reduction) and lipid concentrations (ie, differential effects on low-density lipoprotein and high-density lipoprotein cholesterol), were identified in seven GLP-1 receptor studies—three with exenatide (two with exenatide BID,37,38 and one with the investigational exenatide once weekly21) and four with liraglutide.23,25,26,41 For the DPP-4 inhibitors, three studies were identified—two with sitagliptin45,50 and one with vildagliptin61—in which potentially beneficial effects on CV risk factors were demonstrated.The data have been encouraging, although the clinical implications have yet to be fully understood.
Head-to-head comparison
A recent study compared the effects of the GLP-1 receptor agonist exenatide and the DPP-4 inhibitor sitagliptin on postprandial glucose (PPG) concentrations, insulin and glucagon secretion, gastric intake, and caloric intake.39 Although limited by a short treatment duration (2 weeks), the study showed that the GLP-1 receptor agonist had a greater effect than the DPP-4 inhibitor in reducing PPG concentrations, a more potent effect in increasing insulin secretion and decreasing postprandial glucagon secretion, and a relatively greater effect in reducing caloric intake; and that it decreased the rate of gastric emptying (sitagliptin had no effect). These differences suggest that exenatide may provide a greater degree of GLP-1 receptor activation than the more physiologic concentrations of GLP-1 reached with DPP-4 inhibition.39 Results of a scintigraphic study showed that exenatide substantially slows the gastric emptying that is accelerated in patients with T2DM. This could be another beneficial mechanism in treating postprandial glycemia.63
Adverse effects
Exenatide has shown effects on hepatic injury markers (ie, improvement in alanine and aspartate aminotransferases) for up to 3.5 years of treatment.37 For the GLP-1 receptor agonist and DPP-4 inhibitor studies reviewed, the adverse events were generally mild and included nausea and vomiting, nasopharyngitis, and mild hypoglycemia.
Meta-analysis conclusions
The published clinical trial data presented in this review expand the body of evidence on the safety and efficacy of incretin-based therapy in patients with T2DM. These data include the results of a meta-analysis by Amori et al,17 which examined randomized controlled trials of 12 weeks’ or longer duration that compared incretin-based therapy with placebo or other diabetes medications and reported HbA1c changes in adults with T2DM. The meta-analysis showed that incretin-based therapies reduced HbA1c more than placebo (weighted mean difference, –0.97% [95% confidence interval (CI), –1.13% to –0.81%] for GLP-1 receptor agonists and –0.74% [95% CI, –0.85% to –0.62%] for DPP-4 inhibitors) and were noninferior to other antidiabetes agents. Treatment with a GLP-1 receptor agonist (ie, exenatide) caused weight loss (–1.4 kg and –4.8 kg vs placebo and insulin, respectively) while DPP-4 inhibitors (ie, sitagliptin, vildagliptin) were weight neutral.17
Beta-cell function
Evidence regarding the effects of incretin-based therapies, particularly the exendin-4 GLP-1 receptor agonists, on beta-cell function in patients with T2DM continues to accumulate. When assessing long-term (1 year) exenatide treatment in patients with T2DM, a trial (n = 69) comparing exenatide with the basal insulin analogue insulin glargine showed that exenatide and insulin glargine resulted in similar reductions in HbA1c (–0.8% vs –0.7%; P = .55).64 However, exenatide significantly reduced body weight while insulin glargine resulted in weight gain (–3.6 kg vs +1.0 kg; P < .0001). In terms of beta-cell function, arginine-stimulated C-peptide secretion during hyperglycemia increased 2.46-fold from baseline after 52 weeks of exenatide treatment compared with 1.31-fold with insulin glargine treatment (P < .0001).64
With respect to the direct beta-cell effects of liraglutide, a preclinical study reported that liraglutide improved glucose homeostasis in marginal mass islet transplantation in diabetic mice.65 In this study, liraglutide was shown, in a mouse model, to reduce the time to normoglycemia after islet cell transplantation (median time, 1 vs 72.5 days; P < .0001). The effects of liraglutide on beta-cell function also were assessed in 13 patients with T2DM. After 7 days of treatment, liraglutide improved beta-cell function, which was associated with improvement in glucose concentration.66 Liraglutide improved potentiation of insulin secretion during the first meal, owing in part to restoration of the potentiation peak (which is markedly blunted in T2DM), in a phenomenon similar to that observed with exenatide.67
Beneficial effects on beta-cell function have also been reported with DPP-4 inhibitors. In a model-based analysis of patients with T2DM, it was shown that sitagliptin improved basal, static, and dynamic responsiveness of pancreatic beta cells to glucose. The results were observed when sitagliptin was administered both as an add-on to metformin therapy and as monotherapy.68 A 52-week, double-blind, randomized, parallel-group study compared vildagliptin 50 mg/day and placebo in 306 patients with T2DM and mild hyperglycemia (HbA1c, 6.2% to 7.5%). Vildagliptin was shown to significantly increase fasting insulin secretory tone, glucose sensitivity, and rate sensitivity, all of which are aspects of beta-cell function.69
Summary
Based on the ability of incretin-based therapies to address various disease mechanisms, including beta-cell defects (ie, hyperglycemia), hormone-related abnormalities (ie, hyperglucagonemia, incretin deficiency/resistance), and accelerated gastric emptying (especially with GLP-1 receptor agonists); their favorable effects on weight (reduction with GLP-1 receptor agonists and neutral with DPP-4 inhibitors); their beneficial effects on CV risk factors; and their good safety profile (ie, hypoglycemia risk comparable with metformin), these agents could be considered therapeutic advances for the treatment of patients with T2DM.
INCRETIN-BASED THERAPIES IN GUIDELINES AND ALGORITHMS
The 2007 AACE medical guidelines for clinical practice for the management of diabetes recognized the place of the incretin-based therapies and included them among the pharmacologic options.5 Exenatide was specifically recommended for combination therapy with metformin, a sulfonylurea (secretagogue), a sulfonylurea plus metformin, or a TZD. Sitagliptin was recommended for use as monotherapy or in combination with metformin or a TZD.5
In 2009, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes convened a consensus panel to produce an algorithm for the initiation and adjustment of therapy for patients with T2DM. In this algorithm, GLP-1 receptor agonists were considered appropriate in certain clinical scenarios (eg, when hypoglycemia was an issue or weight loss was a major consideration during treatment). However, the groups also noted a need for more data on long-term safety and the cost of treatment with incretin-based therapies.70
The AACE and the American College of Endocrinology recently developed “road maps” for managing patients with T2DM. In patients with T2DM who are naïve to therapy, DPP-4 inhibitors are among the recommended first options when the initial HbA1c is 6.0% to 7.0% and as a combination therapy component when HbA1c reaches 7.0% to 9.0%. In patients who have already received monotherapy for 2 to 3 months and whose HbA1c is 6.5% to 8.5%, treatment options include combination therapy with a DPP-4 inhibitor and metformin or a TZD. Another option includes the initiation of treatment with a GLP-1 receptor agonist in combination with a TZD, with metformin or a sulfonylurea, or with metformin and a sulfonylurea.71
The role of GLP-1 receptor agonist therapies and their incorporation into T2DM treatment algorithms was noted at the 2008 annual meeting of the ADA. In the Banting lecture, Ralph A. DeFronzo, MD, advocated the early use of triple-drug therapy with metformin, exenatide, and a TZD in the management of patients with T2DM.9
CONCLUSION
T2DM, which is linked to weight gain and obesity, is a complex disease that predisposes patients to and is associated with CVD. A better understanding and appreciation of the role of the incretin system in the pathogenesis of T2DM has led to the development of incretin-based therapies, such as the GLP-1 receptor agonists and DPP-4 inhibitors. As more experimental and clinical evidence becomes available, subtle nuances are emerging that distinguish the roles of these two therapeutic classes.
- 2007 National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/estimates07.htm. Updated: July 23, 2008. Accessed September 25, 2009.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Bertin E, Schneider N, Abdelli N, et al. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes Metab 2001; 27:357–364.
- Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term type 2 diabetes mellitus. Diabet Med 1998; 15:1022–1027.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995; 80:952–957.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Eng J, Kleinman WA, Singh L, Singh G, Raufman J-P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom: further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992; 267:7402–7405.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Davidson JA, Parente EB, Gross JL. Incretin mimetics and dipeptidyl peptidase-4 inhibitors: innovative treatment therapies for type 2 diabetes. Arq Bras Endocrinol Metabol 2008; 52:1039–1049.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Marre M, Shaw J, Brändle M, et al; for the LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetes Med 2009; 26:268–278.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide 1 with pharmacokinetic properties suitable for once-daily administration. J Med Chem 2000; 43:1664–1669.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Fleck P, Christopher R, Covington P, Wilson C, Mekki Q. Efficacy and safety of alogliptin monotherapy over 12 weeks in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 479-P.
- DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Effect of alogliptin combined with pioglitazone on glycemic control in metformin-treated patients with type 2 diabetes. Paper presented at: 69th Annual Meeting of the American Diabetes Association; June 5–9, 2009; New Orleans, LA. Abstract 2024-PO.
- Nauck M, Ellis G, Fleck P, Wilson C, Mekki Q. Efficacy and safety of alogliptin added to metformin therapy in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 477-P.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30:1448–1460.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81:161–168.
- Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007; 30:1608–1610.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R; for the CV181-040 Investigators. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 2009; 63:1395–1406.
- DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009; 32:1649–1655.
- Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R; for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009; 11:611–622.
- Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R; for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin 2009; 25:2401–2411.
- Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naïve patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:376–386.
- Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 2007; 39:218–223.
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metformin. Horm Metab Res 2008; 40:892–895.
- Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med 2008; 25:435–441.
- Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract 2007; 76:132–138.
- Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with type 2 diabetes. Diabetes Med 2007; 24:955–961.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Merani S, Truong W, Emamaullee JA, Toso C, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology 2008; 149:4322–4328.
- Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care 2007; 30:2032–2033.
- Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res 2006; 38:838–844.
- Xu L, Man CD, Charbonnel B, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab 2008; 10:1212–1220.
- Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed b-cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab 2008; 93:103–109.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Jellinger PS, Davidson JA, Blonde L, et al; for the ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract 2007; 13:260–268.
- 2007 National Diabetes Fact Sheet. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/diabetes/pubs/estimates07.htm. Updated: July 23, 2008. Accessed September 25, 2009.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009; 52:65–73.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- Bertin E, Schneider N, Abdelli N, et al. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes Metab 2001; 27:357–364.
- Weytjens C, Keymeulen B, Van Haleweyn C, Somers G, Bossuyt A. Rapid gastric emptying of a liquid meal in long-term type 2 diabetes mellitus. Diabet Med 1998; 15:1022–1027.
- Stonehouse AH, Holcombe JH, Kendall DM. GLP-1 analogues, DPP-IV inhibitors and the metabolic syndrome. In: Fonseca V, ed. Therapeutic Strategies in Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008: 137–157.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91:301–307.
- Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995; 80:952–957.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696–1705.
- Eng J, Kleinman WA, Singh L, Singh G, Raufman J-P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom: further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992; 267:7402–7405.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88:3082–3089.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Davidson JA, Parente EB, Gross JL. Incretin mimetics and dipeptidyl peptidase-4 inhibitors: innovative treatment therapies for type 2 diabetes. Arq Bras Endocrinol Metabol 2008; 52:1039–1049.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Marre M, Shaw J, Brändle M, et al; for the LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabetes Med 2009; 26:268–278.
- Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide 1 with pharmacokinetic properties suitable for once-daily administration. J Med Chem 2000; 43:1664–1669.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Baggio LL, Drucker DJ, Maida A, Lamont BJ. ADA 2008: incretin-based therapeutics. MedscapeCME Web site. http://www.medscape.com/viewprogram/15786. Accessed September 18, 2009.
- Fleck P, Christopher R, Covington P, Wilson C, Mekki Q. Efficacy and safety of alogliptin monotherapy over 12 weeks in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 479-P.
- DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Effect of alogliptin combined with pioglitazone on glycemic control in metformin-treated patients with type 2 diabetes. Paper presented at: 69th Annual Meeting of the American Diabetes Association; June 5–9, 2009; New Orleans, LA. Abstract 2024-PO.
- Nauck M, Ellis G, Fleck P, Wilson C, Mekki Q. Efficacy and safety of alogliptin added to metformin therapy in patients with type 2 diabetes. Paper presented at: 68th Annual Meeting of the American Diabetes Association; June 6–10, 2008; San Francisco, CA. Abstract 477-P.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30:1448–1460.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81:161–168.
- Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007; 30:1608–1610.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79:291–298.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; for the Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; for the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9:733–745.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; for the Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24:537–550.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; for the Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R; for the CV181-040 Investigators. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 2009; 63:1395–1406.
- DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009; 32:1649–1655.
- Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R; for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009; 11:611–622.
- Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R; for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin 2009; 25:2401–2411.
- Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naïve patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:376–386.
- Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 2007; 39:218–223.
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naïve patients with type 2 diabetes: comparison with metformin. Horm Metab Res 2008; 40:892–895.
- Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med 2008; 25:435–441.
- Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract 2007; 76:132–138.
- Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with type 2 diabetes. Diabetes Med 2007; 24:955–961.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept 2008; 151:123–129.
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32:762–768.
- Merani S, Truong W, Emamaullee JA, Toso C, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology 2008; 149:4322–4328.
- Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care 2007; 30:2032–2033.
- Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res 2006; 38:838–844.
- Xu L, Man CD, Charbonnel B, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab 2008; 10:1212–1220.
- Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed b-cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab 2008; 93:103–109.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Jellinger PS, Davidson JA, Blonde L, et al; for the ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus: ACE/AACE Diabetes Road Map Task Force. Endocr Pract 2007; 13:260–268.
KEY POINTS
- Hormonal deficiencies in T2DM are related to abnormalities in the secretion of amylin, glucagon, and incretin hormones.
- In clinical trials, GLP-1 receptor agonists reduced HbA1c levels, had beneficial effects on weight, and caused less hypoglycemia than insulin analogues.
- Both GLP-1 receptor agonists and DPP-4 inhibitors improve pancreatic beta-cell function.
- Incretin-based therapies have been incorporated into recently updated clinical guidelines for treatment of T2DM.
Redefining treatment success in type 2 diabetes mellitus: Comprehensive targeting of core defects
According to the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), glycosylated hemoglobin (HbA1c) in patients with diabetes should be maintained at 6.5% or less (AACE) or at less than 7.0% (ADA). Both organizations support an aggressive stepwise approach that includes medication and lifestyle modification, with strategies and clinical attention devoted to avoiding significant hypoglycemia.1,2 Yet, despite the introduction of new antidiabetes agents, most current management strategies are offset by limitations in achieving and maintaining glycemic targets needed to provide optimal care for patients with diabetes, more than 90% of whom have type 2 diabetes mellitus (T2DM).3,4
Nationally, glycemic control among patients with T2DM has improved but is still far from optimal. According to data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), glycemic control (HbA1c < 7.0%) rates were 35.8% for patients with T2DM.5 In a more recent report (NHANES 1999–2004), fewer than half (48.4%) of adult patients with diagnosed diabetes achieved HbA1c levels below 7.0%.5,6 Factors contributing to these data include earlier onset and earlier detection of T2DM.7
CHANGING TREATMENT TRENDS
Available treatments for patients with T2DM include secretagogues, such as sulfonylureas and “glinides” (repaglinide and nateglinide), metformin, thiazolidinediones (TZDs), and dipeptidyl peptidase–4 (DPP-4) inhibitors among oral medications, and insulin and glucagon-like peptide–1 (GLP-1) receptor agonists among parenterally administered agents. According to the latest published data on prescribing patterns for patients with T2DM, analyses of the National Disease and Therapeutic Index (1994–2007) and the National Prescription Audit (2001–2007), sulfonylurea use decreased from 67% of treatment visits in 1994 to 34% of visits in 2007.8 By 2007, metformin, used in 54% of treatment visits, and TZDs, used in 28%, were the most frequently administered antidiabetes agents. Insulin use declined from 38% of visits during which a treatment was administered in 1994 to 25% of visits in 2000, but had increased subsequently to 28% of visits in 2007.
SIGNIFICANCE OF CARDIOVASCULAR RISK
Clinical research has suggested that focusing solely on improving glycemic control may be insufficient to reduce overall morbidity and mortality associated with diabetes. Specifically, data from recent studies, including the Action to Control Cardiovascular Risk in Diabetes (ACCORD), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT), emphasized that lowering HbA1c below 7% in a high-risk population of individuals with T2DM did not improve cardiovascular (CV) outcomes.9–11 The observations confirm that risk factors, including weight, blood pressure (BP), and lipid levels, are vitally important in reducing morbidity and mortality in this population. This perception is further underscored by the NHANES 1999–2004 data, which showed poor concurrent control of HbA1c, BP, and lipids; only 13.2% of patients with diagnosed diabetes achieved all three target goals simultaneously.6 Similarly, a nationwide survey in Norway showed that only 13% of patients with T2DM concurrently achieved goals for HbA1c, BP, and lipids.12
In the Danish Steno-2 Study, patients with T2DM and persistent microalbuminuria were treated with either intensive target-driven therapy using multiple drugs or conventional multifactorial treatment. Over a mean period of 13.3 years (7.8 years of treatment plus 5.5 years of follow-up), intensive multifactorial intervention to control multiple CV risk factors, including HbA1c, BP, and lipids, was associated with a lower risk of death from CV causes (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.19 to 0.94; P = .04) and a lower risk of CV events (HR, 0.41; 95% CI, 0.25 to 0.67; P < .001) than was conventional therapy.13
This article clarifies the redefinition of treatment success in patients with T2DM based on targeting the underlying physiologic defects of the disease.
T2DM, OVERWEIGHT/OBESITY, AND CV DISEASE: CLOSELY LINKED
The incidence and prevalence of T2DM, overweight/obesity, and CV disease (CVD) are increasing worldwide. It is estimated that the worldwide prevalence of diabetes will increase from 171 million in 2000 to 366 million by 203014; T2DM increases the risk of morbidity and mortality from microvascular (eg, neuropathic, retinopathic, nephropathic) and macrovascular (eg, coronary, peripheral vascular disease) complications.15 According to a Michigan health maintenance organization study (N = 1,364), the median annual direct cost of medical care for Caucasian patients with T2DM who were diet controlled, had a body mass index (BMI) of 30 kg/m2 or higher, and had no vascular complications was estimated to be $1,700 for men and $2,100 for women.16 The actual cost of care for patients with T2DM may be much higher, since most patients present with multiple CV risk factors in addition to being overweight.
NHANES data show that approximately two-thirds of Americans are either overweight or obese17; overweight/obesity affects about 80% of adults diagnosed with T2DM.18 Overweight or obesity can increase the risk for developing T2DM by more than 90-fold and, in women, it can increase the risk for developing coronary heart disease (CHD) by sixfold.19 The close link between T2DM and CVD is underscored further with recent data from the Framingham Heart Study, which showed a high lifetime risk of CVD in patients with diabetes, heightened further by obesity. During the 30-year study period, the lifetime risk of CVD in normal-weight people with diabetes was 78.6% in men and 54.8% in women; the risk increased to 86.9% in obese men with diabetes and to 78.8% in obese women with diabetes.20 The NHANES data also showed that the prevalence of T2DM increased in the past decade and that patients are being diagnosed at a younger age, from a mean age of 52 years in 1988–1994 to 46 years in 1999–2000.7
BRIDGING THE GAP FROM PATHOPHYSIOLOGY TO UNMET NEEDS
The paradigm behind the pathophysiology of T2DM has shifted from its perception as a simple “dual-defect” disease (ie, deficiency in insulin secretion and peripheral tissue insulin resistance) to a multidimensional disorder.1,21 This new model includes overweight/obesity, insulin resistance, qualitative and quantitative defects in insulin secretion, and dysregulation in the secretion of other hormones, including the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the gastrointestinal incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide.21–23
CLINICAL GUIDELINES AND CV RISK FACTOR MANAGEMENT
The best strategy for managing T2DM is a comprehensive approach that addresses the fundamental core defects plus associated factors that contribute to increased CV risk. Several specialty groups have suggested guidelines and algorithms for the management of T2DM and its comorbidities. These guidelines, including the ADA standards of medical care, the AACE standards in tandem with the American College of Endocrinology guidelines, and the recent joint statement from the ADA and the European Association for the Study of Diabetes (EASD), acknowledge that the core defects of T2DM and the associated CV risk factors (eg, weight gain, obesity, hypertension, dyslipidemia) are important in developing optimal treatment strategies.1–3 Medical nutrition guidelines advocate weight loss as a key initial step in managing T2DM and the comorbidities that lead to elevated CV risk.25,26 The National Institutes of Health and the US Department of Health and Human Services/US Department of Agriculture advocate regular physical activity, dietary assessment, and periodic comorbidity and weight assessment for all people, not just those with T2DM or CVD.26,27
Weight reduction
Evidence in support of effective lifestyle intervention was demonstrated in the Action for Health in Diabetes (Look AHEAD) study. After 1 year, patients with T2DM treated with intensive lifestyle intervention lost an average of 8.6% of their initial weight compared with 0.7% in patients treated only with diabetes support and education (P < 0.001). The intensive-intervention patients also had a significant drop in HbA1c (from 7.3% to 6.6%; P < 0.001) and were able to reduce their antidiabetes, antihypertensive, and lipid-lowering medications.28 More recent data from the Look AHEAD study reported that overweight patients with T2DM enrolled in a weight management program experienced significant weight loss, improved physical fitness, reduced physical symptoms, and overall improvement in health-related quality of life.29 Thus, weight reduction appears to be a key component in reducing CV risk and improving quality of life in most patients with T2DM.28–30
Hypertension
Hypertension is a major risk factor for microvascular complications and CVD, and may be associated with, or be the underlying result of, nephropathy.2 BP control is clearly important in reducing the morbidity and mortality associated with T2DM. The recommended BP goal in patients with T2DM is less than 130/80 mm Hg.1,2
Hyperlipidemia
According to the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III [ATP III]), diabetes is considered a CHD risk equivalent because it confers a high risk of new CHD developing within 10 years.31 In addition to the NCEP–ATP III guidelines, the ADA and the AACE have set target levels for lipids in patients with diabetes, including T2DM.1,2,31 All three organizations have defined 100 mg/dL as the target level for low-density lipoprotein.
HbA1c and lifestyle intervention
EVOLUTION OF ANTIDIABETES THERAPIES
Traditional antidiabetes agents used in the treatment of patients with T2DM have focused mainly on insulin secretion and insulin resistance, with treatment success defined as achieving HbA1c goals with a reduced incidence of hypoglycemia.23 Secretagogues, such as sulfonylureas and glinides, stimulate the pancreas to release insulin. Insulin sensitizers, such as TZDs and metformin, enhance the action of insulin in muscle and fat1,3,23 and lower hepatic glucose production. The alpha-glucosidase inhibitors alter carbohydrate absorption from the gastrointestinal tract.1 The extent to which each agent achieves treatment success in terms of glucose lowering depends on several factors, including intrinsic attributes, duration of disease, and baseline glycemic control.3
GLP-1 receptor agonists
Exenatide effects. Although many agents are in development, to date exenatide is the only GLP-1 receptor agonist approved by the US Food and Drug Administration (FDA).8,33 Exenatide is an exendin-4 GLP-1 receptor agonist with multiple glucoregulatory effects, including enhanced glucose-dependent insulin secretion, reduced glucagon secretion and food intake, and slowed gastric emptying.22,34 Exenatide is detectable in the circulation for up to 10 hours following subcutaneous (SC) administration22 and has a greater potency in reducing plasma glucose than GLP-1 in preclinical studies.35,36
By virtue of its beneficial effects on glycemic control, weight, BP, and lipids, exenatide addresses some of the components of the metabolic syndrome.37–41 In pivotal 30-week studies, exenatide was associated with HbA1c reductions that ranged from –0.40% to –0.86% from baseline and decreases in body weight of approximately –1 kg to –3 kg from baseline, without severe hypoglycemia.37–39 The percentage of patients who reached the ADA goal of HbA1c less than 7.0% at 30 weeks ranged from 24% to 34%. The addition of exenatide to TZD therapy in a 16-week study was associated with mean reductions in HbA1c of –0.98%, fasting plasma glucose (FPG) concentration of –1.69 mmol/L (–30.42 mg/dL), and body weight of –1.51 kg.40
A posthoc analysis of an open-label extension study involving patients who completed the original 30-week placebo-controlled studies showed that 46% of patients who remained on exenatide achieved the ADA goal of HbA1c less than 7.0% at 3 years.41 Exenatide administered for up to 3.5 years was associated with sustained reductions in HbA1c of –1.0% (P < .0001) and body weight of –5.3 kg (P < .001). Pancreatic beta-cell function, assessed by homeostasis model assessment, improved, as did BP, triglyceride, high-density lipoprotein, low-density lipoprotein, and aspartate aminotransferase levels.41
Comparison with insulin analogues. Comparative studies have highlighted the contrasting effects of exenatide and insulin analogues (eg, insulin glargine and fixed-ratio insulin).42–45 In a 26-week trial comparing exenatide with insulin glargine in subjects with T2DM, both agents resulted in similar decreases in HbA1c. Exenatide was also associated with a –2.3-kg weight reduction, whereas insulin glargine was associated with a +1.8-kg weight gain.42 Although rates of symptomatic hypoglycemia were similar, there were fewer cases of nocturnal hypoglycemia with exenatide (0.9 event/patient-year vs 2.4 events/patient-year with insulin).
In a 32-week study comparing exenatide BID with titrated insulin glargine QD, the HbA1c reductions for exenatide and insulin glargine were comparable. However, body weight decreased –4.2 kg over two 16-week treatment periods with exenatide, but increased +3.3 kg over the same periods with the basal insulin analogue.43 The incidence of hypoglycemia was lower with exenatide than with insulin glargine (14.7% vs 25.2%), although the difference was not statistically significant.
In another study that compared exenatide with biphasic insulin aspart, patients who were treated with exenatide also lost weight while those who received the fast-acting insulin analogue gained weight (between-group difference, –5.4 kg). Patients treated with exenatide also demonstrated greater reductions in postprandial plasma glucose (PPG) excursions following their morning (P < .001), midday (P = .002), and evening meals (P < .001).44 Overall, hypoglycemia rates were similar at study end between exenatide and insulin aspart (4.7 events/patient-year vs 5.6 events/patient-year). In all of these studies, significant gastrointestinal adverse events (nausea and vomiting) occurred more frequently with exenatide, and more patients withdrew from exenatide than from insulin.
Formulations in development. Other advances in GLP-1 receptor agonist therapy include novel formulations under clinical development, such as exenatide once weekly36,46 and liraglutide, a human analogue GLP-1 receptor agonist formulated for once-daily administration.47,48 In a 52-week study in patients with T2DM, liraglutide significantly reduced HbA1c; the 1.2-mg SC QD dosage reduced HBA1c by –0.84% (P = .0014) and the 1.8-mg SC QD dosage by –1.14% (P < .0001). In comparison, glimepiride 8 mg orally QD achieved a –0.51% reduction. Liraglutide was also associated with greater reductions in weight, hypoglycemia, and systolic BP than glimepiride.47
A 26-week study compared liraglutide (0.6, 1.2, and 1.8 mg SC QD), placebo, and glimepiride 4 mg QD in combination with metformin 1 g BID. HbA1c was reduced significantly in all liraglutide groups compared with placebo (P < .0001). Mean HbA1c decreased –1.0% with liraglutide 1.2 mg and 1.8 mg and with glimepiride; it decreased –0.7% with liraglutide 0.6 mg; and it increased +0.1% with placebo. Body weight decreased –1.8 kg to –2.8 kg in all liraglutide groups but increased +1.0 kg in the glimepiride group (P < .0001). The incidence of minor hypoglycemia with liraglutide (~3%) was comparable to that observed with placebo but less than that with glimepiride (17%; P < .001).48
A once-weekly long-acting release (LAR) formulation of exenatide submitted to the FDA for approval may provide enhanced glycemic and weight control, potentially improving patient acceptance and adherence.36,46 In a 15-week study, exenatide once weekly produced significant reductions in HbA1c, FPG, PPG, and body weight. There were no withdrawals due to adverse events, and the formation of anti-exenatide antibodies was not predictive of therapeutic end point response or adverse safety outcome. Instances of hypoglycemia were mild and not dose related.36 In a 30-week study comparing exenatide LAR once weekly with exenatide BID, patients given exenatide LAR once weekly had significantly greater HbA1c reductions than did patients given exenatide BID (–1.9% vs –1.5%; P = .0023). Treatment adherence was 98% with both exenatide regimens, and no episodes of major hypoglycemia occurred with either formulation regardless of background sulfonylurea use. Favorable effects on BP and lipid profile were observed with both exenatide regimens.46
DPP-4 inhibitors
The DPP-4 inhibitors (commonly called gliptins) inhibit the proteolytic cleavage of circulating GLP-1 by binding to the DPP-4 enzyme, increasing the concentration of endogenous GLP-1 approximately two- to threefold.49–51 These concentrations result in more prompt and appropriate secretion of insulin and suppression of glucagon in response to a carbohydrate-containing snack or meal, with the change in glucagon correlating linearly with improved glucose tolerance.51
DPP-4 inhibitors, which are given orally, include sitagliptin and saxagliptin (approved in the United States) and vildagliptin (not approved in the United States but used in the European Union and Latin America).8,22,33,52 Sitagliptin can be used either as monotherapy or in combination with metformin or a TZD.8,49–55 Recently, a single-tablet formulation of sitagliptin plus metformin was granted regulatory approval.8
When used alone or in combination with metformin or pioglitazone, sitagliptin has been associated with significant reductions in HbA1c (of ~0.5% to 0.6% when used alone, ~0.7% with metformin, and ~0.9% with pioglitazone [P < .001 vs placebo]), with hypoglycemia occurring in 1.3% or less of the population.54 In an 18-week study in which patients with T2DM who were inadequately controlled with metformin monotherapy were randomized to receive add-on sitagliptin (100 mg QD), rosiglitazone (8 mg QD), or placebo, sitagliptin reduced HbA1c –0.73% (P < .001 vs placebo) and reduced body weight –0.4 kg, while rosiglitazone reduced HbA1c –0.79% and increased body weight +1.5 kg.55
To evaluate the effectiveness of sitagliptin and metformin as initial therapy, a 54-week study was completed in 885 patients with T2DM and inadequate glycemic control (HbA1c 7.5–11%) on diet and exercise.56 Patients were evaluated on monotherapy with either sitagliptin (100 mg QD) or metformin (1 g or 2 g QD), or on initial therapy with the two in combination (sitagliptin 100 mg + metformin 1 mg or 2 mg QD). At week 54, in the all-patients-treated analysis, mean changes in HbA1c from baseline were –1.8% with sitagliptin plus metformin 2 g QD, –1.4% with sitagliptin plus metformin 1 g QD, –1.3% with metformin 2 g QD monotherapy, –1.0% with metformin 1 g QD monotherapy, and –0.8% with sitagliptin 100 mg QD monotherapy.
All treatments improved measures of beta-cell function (eg, homeostasis model assessment [HOMA]-beta, proinsulin/insulin ratio). Mean body weight decreased from baseline in the combination and metformin monotherapy groups and was unchanged from baseline in the sitagliptin monotherapy group. The incidence of hypoglycemia was low (1%–3%) across treatment groups. The incidence of gastrointestinal adverse experiences was evaluated with the coadministration of sitagliptin and metformin and appeared similar to that observed with use of metformin as monotherapy.56 Thus, this study suggested that an initial combination of a DPP-IV inhibitor with metformin can improve glycemic control and markers of beta-cell function in patients with T2DM.
Incretin-based therapies compared
Studies in both healthy individuals and in patients with T2DM have shown that oral DPP-4 inhibitors such as sitagliptin increase endogenous GLP-1 concentrations by about twofold compared with placebo.22,50 The pharmacologic concentration of subcutaneously administered exenatide available for activating the GLP-1 receptor is significantly greater than the increased endogenous GLP-1 concentrations achieved with sitagliptin. In a recent clinical study comparing exenatide and sitagliptin in patients with T2DM, the mean 2-hour plasma concentration for exenatide was 64 pM compared with the mean 2-hour postprandial GLP-1 concentration of 15 pM for sitagliptin (baseline GLP-1 concentration was 7.2 pM).57 While both agents were shown to be effective, exenatide appeared to have had a greater effect than sitagliptin in increasing insulin secretion and reducing postprandial glucagon secretion, leading to significantly (P < 0.0001) greater reductions in PPG.57
Sitagliptin has been minimally associated with nausea, whereas patients who take exenatide need to be informed of the risk of usually mild to moderate, but sometimes severe, nausea and vomiting that tends to decrease over time.
For a detailed comparison of the effects of GLP-1 receptor agonists and DPP-4 inhibitors on HbA1c, weight, and hypoglycemia, see “Advances in therapy for type 2 diabetes: GLP–1 receptor agonists and DPP–4 inhibitors.”
CONCLUSION
Despite advances in diagnosis and treatment, T2DM, overweight/obesity, CVD, and their complications remain major public health burdens worldwide. The concepts that explain the pathophysiology of T2DM include the contribution of various factors beyond insulin secretion and insulin resistance, such as the role of incretin hormones in disease progression. A comprehensive approach to managing patients with T2DM requires targeting the fundamental defects of the disease and its comorbidities. Newer agents, including incretin-based therapies such as GLP-1 receptor agonists and DPP-4 inhibitors, address the fundamental defects of T2DM. The definition of treatment success in the management of T2DM will be redefined as more data become available on agents that exert beneficial effects not only on glycemia but on parameters that may influence overall CV health, such as weight, BP, and lipid profiles.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the US National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Koopman RJ, Mainous AG III, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005; 3:60–63.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Jenssen TG, Tonstad S, Claudi T, Midthjell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003; 26:2300–2304.
- National Center for Health Statistics. Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control. Eur J Endocrinol 2008; 158:773–784.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care 2008; 31:1582–1584.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Cefalu WT. Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: rationale and specific agents. Clin Pharmacol Ther 2007; 81:636–649.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- US Department of Health and Human Services (HHS) and US Department of Agriculture. Dietary guidelines for Americans, 2005. US Department of HHS Web site. http://www.health.gov/DietaryGuidelines/dga2005/document/default.htm. Published January 2005. Accessed September 25, 2009.
- National Heart, Lung, and Blood Institute. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health Web site. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Updated: October 2000. Accessed September 28, 2009.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K; for the Look AHEAD Research Group. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009; 169:163–171.
- Klein S, Sheard NF, Pi-Sunyer X, et al; for the American Diabetes Association; North American Association for the Study of Obesity; American Society for Clinical Nutrition. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004; 117:77–88.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004; 89:2078–2084.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Bohannon N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad Med 2009; 121:40–45.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Zerilli T, Pyon EY. Sitagliptin phosphate: a DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Ther 2007; 29:2614–2634.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin 2009; 25:569–583.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
According to the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), glycosylated hemoglobin (HbA1c) in patients with diabetes should be maintained at 6.5% or less (AACE) or at less than 7.0% (ADA). Both organizations support an aggressive stepwise approach that includes medication and lifestyle modification, with strategies and clinical attention devoted to avoiding significant hypoglycemia.1,2 Yet, despite the introduction of new antidiabetes agents, most current management strategies are offset by limitations in achieving and maintaining glycemic targets needed to provide optimal care for patients with diabetes, more than 90% of whom have type 2 diabetes mellitus (T2DM).3,4
Nationally, glycemic control among patients with T2DM has improved but is still far from optimal. According to data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), glycemic control (HbA1c < 7.0%) rates were 35.8% for patients with T2DM.5 In a more recent report (NHANES 1999–2004), fewer than half (48.4%) of adult patients with diagnosed diabetes achieved HbA1c levels below 7.0%.5,6 Factors contributing to these data include earlier onset and earlier detection of T2DM.7
CHANGING TREATMENT TRENDS
Available treatments for patients with T2DM include secretagogues, such as sulfonylureas and “glinides” (repaglinide and nateglinide), metformin, thiazolidinediones (TZDs), and dipeptidyl peptidase–4 (DPP-4) inhibitors among oral medications, and insulin and glucagon-like peptide–1 (GLP-1) receptor agonists among parenterally administered agents. According to the latest published data on prescribing patterns for patients with T2DM, analyses of the National Disease and Therapeutic Index (1994–2007) and the National Prescription Audit (2001–2007), sulfonylurea use decreased from 67% of treatment visits in 1994 to 34% of visits in 2007.8 By 2007, metformin, used in 54% of treatment visits, and TZDs, used in 28%, were the most frequently administered antidiabetes agents. Insulin use declined from 38% of visits during which a treatment was administered in 1994 to 25% of visits in 2000, but had increased subsequently to 28% of visits in 2007.
SIGNIFICANCE OF CARDIOVASCULAR RISK
Clinical research has suggested that focusing solely on improving glycemic control may be insufficient to reduce overall morbidity and mortality associated with diabetes. Specifically, data from recent studies, including the Action to Control Cardiovascular Risk in Diabetes (ACCORD), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT), emphasized that lowering HbA1c below 7% in a high-risk population of individuals with T2DM did not improve cardiovascular (CV) outcomes.9–11 The observations confirm that risk factors, including weight, blood pressure (BP), and lipid levels, are vitally important in reducing morbidity and mortality in this population. This perception is further underscored by the NHANES 1999–2004 data, which showed poor concurrent control of HbA1c, BP, and lipids; only 13.2% of patients with diagnosed diabetes achieved all three target goals simultaneously.6 Similarly, a nationwide survey in Norway showed that only 13% of patients with T2DM concurrently achieved goals for HbA1c, BP, and lipids.12
In the Danish Steno-2 Study, patients with T2DM and persistent microalbuminuria were treated with either intensive target-driven therapy using multiple drugs or conventional multifactorial treatment. Over a mean period of 13.3 years (7.8 years of treatment plus 5.5 years of follow-up), intensive multifactorial intervention to control multiple CV risk factors, including HbA1c, BP, and lipids, was associated with a lower risk of death from CV causes (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.19 to 0.94; P = .04) and a lower risk of CV events (HR, 0.41; 95% CI, 0.25 to 0.67; P < .001) than was conventional therapy.13
This article clarifies the redefinition of treatment success in patients with T2DM based on targeting the underlying physiologic defects of the disease.
T2DM, OVERWEIGHT/OBESITY, AND CV DISEASE: CLOSELY LINKED
The incidence and prevalence of T2DM, overweight/obesity, and CV disease (CVD) are increasing worldwide. It is estimated that the worldwide prevalence of diabetes will increase from 171 million in 2000 to 366 million by 203014; T2DM increases the risk of morbidity and mortality from microvascular (eg, neuropathic, retinopathic, nephropathic) and macrovascular (eg, coronary, peripheral vascular disease) complications.15 According to a Michigan health maintenance organization study (N = 1,364), the median annual direct cost of medical care for Caucasian patients with T2DM who were diet controlled, had a body mass index (BMI) of 30 kg/m2 or higher, and had no vascular complications was estimated to be $1,700 for men and $2,100 for women.16 The actual cost of care for patients with T2DM may be much higher, since most patients present with multiple CV risk factors in addition to being overweight.
NHANES data show that approximately two-thirds of Americans are either overweight or obese17; overweight/obesity affects about 80% of adults diagnosed with T2DM.18 Overweight or obesity can increase the risk for developing T2DM by more than 90-fold and, in women, it can increase the risk for developing coronary heart disease (CHD) by sixfold.19 The close link between T2DM and CVD is underscored further with recent data from the Framingham Heart Study, which showed a high lifetime risk of CVD in patients with diabetes, heightened further by obesity. During the 30-year study period, the lifetime risk of CVD in normal-weight people with diabetes was 78.6% in men and 54.8% in women; the risk increased to 86.9% in obese men with diabetes and to 78.8% in obese women with diabetes.20 The NHANES data also showed that the prevalence of T2DM increased in the past decade and that patients are being diagnosed at a younger age, from a mean age of 52 years in 1988–1994 to 46 years in 1999–2000.7
BRIDGING THE GAP FROM PATHOPHYSIOLOGY TO UNMET NEEDS
The paradigm behind the pathophysiology of T2DM has shifted from its perception as a simple “dual-defect” disease (ie, deficiency in insulin secretion and peripheral tissue insulin resistance) to a multidimensional disorder.1,21 This new model includes overweight/obesity, insulin resistance, qualitative and quantitative defects in insulin secretion, and dysregulation in the secretion of other hormones, including the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the gastrointestinal incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide.21–23
CLINICAL GUIDELINES AND CV RISK FACTOR MANAGEMENT
The best strategy for managing T2DM is a comprehensive approach that addresses the fundamental core defects plus associated factors that contribute to increased CV risk. Several specialty groups have suggested guidelines and algorithms for the management of T2DM and its comorbidities. These guidelines, including the ADA standards of medical care, the AACE standards in tandem with the American College of Endocrinology guidelines, and the recent joint statement from the ADA and the European Association for the Study of Diabetes (EASD), acknowledge that the core defects of T2DM and the associated CV risk factors (eg, weight gain, obesity, hypertension, dyslipidemia) are important in developing optimal treatment strategies.1–3 Medical nutrition guidelines advocate weight loss as a key initial step in managing T2DM and the comorbidities that lead to elevated CV risk.25,26 The National Institutes of Health and the US Department of Health and Human Services/US Department of Agriculture advocate regular physical activity, dietary assessment, and periodic comorbidity and weight assessment for all people, not just those with T2DM or CVD.26,27
Weight reduction
Evidence in support of effective lifestyle intervention was demonstrated in the Action for Health in Diabetes (Look AHEAD) study. After 1 year, patients with T2DM treated with intensive lifestyle intervention lost an average of 8.6% of their initial weight compared with 0.7% in patients treated only with diabetes support and education (P < 0.001). The intensive-intervention patients also had a significant drop in HbA1c (from 7.3% to 6.6%; P < 0.001) and were able to reduce their antidiabetes, antihypertensive, and lipid-lowering medications.28 More recent data from the Look AHEAD study reported that overweight patients with T2DM enrolled in a weight management program experienced significant weight loss, improved physical fitness, reduced physical symptoms, and overall improvement in health-related quality of life.29 Thus, weight reduction appears to be a key component in reducing CV risk and improving quality of life in most patients with T2DM.28–30
Hypertension
Hypertension is a major risk factor for microvascular complications and CVD, and may be associated with, or be the underlying result of, nephropathy.2 BP control is clearly important in reducing the morbidity and mortality associated with T2DM. The recommended BP goal in patients with T2DM is less than 130/80 mm Hg.1,2
Hyperlipidemia
According to the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III [ATP III]), diabetes is considered a CHD risk equivalent because it confers a high risk of new CHD developing within 10 years.31 In addition to the NCEP–ATP III guidelines, the ADA and the AACE have set target levels for lipids in patients with diabetes, including T2DM.1,2,31 All three organizations have defined 100 mg/dL as the target level for low-density lipoprotein.
HbA1c and lifestyle intervention
EVOLUTION OF ANTIDIABETES THERAPIES
Traditional antidiabetes agents used in the treatment of patients with T2DM have focused mainly on insulin secretion and insulin resistance, with treatment success defined as achieving HbA1c goals with a reduced incidence of hypoglycemia.23 Secretagogues, such as sulfonylureas and glinides, stimulate the pancreas to release insulin. Insulin sensitizers, such as TZDs and metformin, enhance the action of insulin in muscle and fat1,3,23 and lower hepatic glucose production. The alpha-glucosidase inhibitors alter carbohydrate absorption from the gastrointestinal tract.1 The extent to which each agent achieves treatment success in terms of glucose lowering depends on several factors, including intrinsic attributes, duration of disease, and baseline glycemic control.3
GLP-1 receptor agonists
Exenatide effects. Although many agents are in development, to date exenatide is the only GLP-1 receptor agonist approved by the US Food and Drug Administration (FDA).8,33 Exenatide is an exendin-4 GLP-1 receptor agonist with multiple glucoregulatory effects, including enhanced glucose-dependent insulin secretion, reduced glucagon secretion and food intake, and slowed gastric emptying.22,34 Exenatide is detectable in the circulation for up to 10 hours following subcutaneous (SC) administration22 and has a greater potency in reducing plasma glucose than GLP-1 in preclinical studies.35,36
By virtue of its beneficial effects on glycemic control, weight, BP, and lipids, exenatide addresses some of the components of the metabolic syndrome.37–41 In pivotal 30-week studies, exenatide was associated with HbA1c reductions that ranged from –0.40% to –0.86% from baseline and decreases in body weight of approximately –1 kg to –3 kg from baseline, without severe hypoglycemia.37–39 The percentage of patients who reached the ADA goal of HbA1c less than 7.0% at 30 weeks ranged from 24% to 34%. The addition of exenatide to TZD therapy in a 16-week study was associated with mean reductions in HbA1c of –0.98%, fasting plasma glucose (FPG) concentration of –1.69 mmol/L (–30.42 mg/dL), and body weight of –1.51 kg.40
A posthoc analysis of an open-label extension study involving patients who completed the original 30-week placebo-controlled studies showed that 46% of patients who remained on exenatide achieved the ADA goal of HbA1c less than 7.0% at 3 years.41 Exenatide administered for up to 3.5 years was associated with sustained reductions in HbA1c of –1.0% (P < .0001) and body weight of –5.3 kg (P < .001). Pancreatic beta-cell function, assessed by homeostasis model assessment, improved, as did BP, triglyceride, high-density lipoprotein, low-density lipoprotein, and aspartate aminotransferase levels.41
Comparison with insulin analogues. Comparative studies have highlighted the contrasting effects of exenatide and insulin analogues (eg, insulin glargine and fixed-ratio insulin).42–45 In a 26-week trial comparing exenatide with insulin glargine in subjects with T2DM, both agents resulted in similar decreases in HbA1c. Exenatide was also associated with a –2.3-kg weight reduction, whereas insulin glargine was associated with a +1.8-kg weight gain.42 Although rates of symptomatic hypoglycemia were similar, there were fewer cases of nocturnal hypoglycemia with exenatide (0.9 event/patient-year vs 2.4 events/patient-year with insulin).
In a 32-week study comparing exenatide BID with titrated insulin glargine QD, the HbA1c reductions for exenatide and insulin glargine were comparable. However, body weight decreased –4.2 kg over two 16-week treatment periods with exenatide, but increased +3.3 kg over the same periods with the basal insulin analogue.43 The incidence of hypoglycemia was lower with exenatide than with insulin glargine (14.7% vs 25.2%), although the difference was not statistically significant.
In another study that compared exenatide with biphasic insulin aspart, patients who were treated with exenatide also lost weight while those who received the fast-acting insulin analogue gained weight (between-group difference, –5.4 kg). Patients treated with exenatide also demonstrated greater reductions in postprandial plasma glucose (PPG) excursions following their morning (P < .001), midday (P = .002), and evening meals (P < .001).44 Overall, hypoglycemia rates were similar at study end between exenatide and insulin aspart (4.7 events/patient-year vs 5.6 events/patient-year). In all of these studies, significant gastrointestinal adverse events (nausea and vomiting) occurred more frequently with exenatide, and more patients withdrew from exenatide than from insulin.
Formulations in development. Other advances in GLP-1 receptor agonist therapy include novel formulations under clinical development, such as exenatide once weekly36,46 and liraglutide, a human analogue GLP-1 receptor agonist formulated for once-daily administration.47,48 In a 52-week study in patients with T2DM, liraglutide significantly reduced HbA1c; the 1.2-mg SC QD dosage reduced HBA1c by –0.84% (P = .0014) and the 1.8-mg SC QD dosage by –1.14% (P < .0001). In comparison, glimepiride 8 mg orally QD achieved a –0.51% reduction. Liraglutide was also associated with greater reductions in weight, hypoglycemia, and systolic BP than glimepiride.47
A 26-week study compared liraglutide (0.6, 1.2, and 1.8 mg SC QD), placebo, and glimepiride 4 mg QD in combination with metformin 1 g BID. HbA1c was reduced significantly in all liraglutide groups compared with placebo (P < .0001). Mean HbA1c decreased –1.0% with liraglutide 1.2 mg and 1.8 mg and with glimepiride; it decreased –0.7% with liraglutide 0.6 mg; and it increased +0.1% with placebo. Body weight decreased –1.8 kg to –2.8 kg in all liraglutide groups but increased +1.0 kg in the glimepiride group (P < .0001). The incidence of minor hypoglycemia with liraglutide (~3%) was comparable to that observed with placebo but less than that with glimepiride (17%; P < .001).48
A once-weekly long-acting release (LAR) formulation of exenatide submitted to the FDA for approval may provide enhanced glycemic and weight control, potentially improving patient acceptance and adherence.36,46 In a 15-week study, exenatide once weekly produced significant reductions in HbA1c, FPG, PPG, and body weight. There were no withdrawals due to adverse events, and the formation of anti-exenatide antibodies was not predictive of therapeutic end point response or adverse safety outcome. Instances of hypoglycemia were mild and not dose related.36 In a 30-week study comparing exenatide LAR once weekly with exenatide BID, patients given exenatide LAR once weekly had significantly greater HbA1c reductions than did patients given exenatide BID (–1.9% vs –1.5%; P = .0023). Treatment adherence was 98% with both exenatide regimens, and no episodes of major hypoglycemia occurred with either formulation regardless of background sulfonylurea use. Favorable effects on BP and lipid profile were observed with both exenatide regimens.46
DPP-4 inhibitors
The DPP-4 inhibitors (commonly called gliptins) inhibit the proteolytic cleavage of circulating GLP-1 by binding to the DPP-4 enzyme, increasing the concentration of endogenous GLP-1 approximately two- to threefold.49–51 These concentrations result in more prompt and appropriate secretion of insulin and suppression of glucagon in response to a carbohydrate-containing snack or meal, with the change in glucagon correlating linearly with improved glucose tolerance.51
DPP-4 inhibitors, which are given orally, include sitagliptin and saxagliptin (approved in the United States) and vildagliptin (not approved in the United States but used in the European Union and Latin America).8,22,33,52 Sitagliptin can be used either as monotherapy or in combination with metformin or a TZD.8,49–55 Recently, a single-tablet formulation of sitagliptin plus metformin was granted regulatory approval.8
When used alone or in combination with metformin or pioglitazone, sitagliptin has been associated with significant reductions in HbA1c (of ~0.5% to 0.6% when used alone, ~0.7% with metformin, and ~0.9% with pioglitazone [P < .001 vs placebo]), with hypoglycemia occurring in 1.3% or less of the population.54 In an 18-week study in which patients with T2DM who were inadequately controlled with metformin monotherapy were randomized to receive add-on sitagliptin (100 mg QD), rosiglitazone (8 mg QD), or placebo, sitagliptin reduced HbA1c –0.73% (P < .001 vs placebo) and reduced body weight –0.4 kg, while rosiglitazone reduced HbA1c –0.79% and increased body weight +1.5 kg.55
To evaluate the effectiveness of sitagliptin and metformin as initial therapy, a 54-week study was completed in 885 patients with T2DM and inadequate glycemic control (HbA1c 7.5–11%) on diet and exercise.56 Patients were evaluated on monotherapy with either sitagliptin (100 mg QD) or metformin (1 g or 2 g QD), or on initial therapy with the two in combination (sitagliptin 100 mg + metformin 1 mg or 2 mg QD). At week 54, in the all-patients-treated analysis, mean changes in HbA1c from baseline were –1.8% with sitagliptin plus metformin 2 g QD, –1.4% with sitagliptin plus metformin 1 g QD, –1.3% with metformin 2 g QD monotherapy, –1.0% with metformin 1 g QD monotherapy, and –0.8% with sitagliptin 100 mg QD monotherapy.
All treatments improved measures of beta-cell function (eg, homeostasis model assessment [HOMA]-beta, proinsulin/insulin ratio). Mean body weight decreased from baseline in the combination and metformin monotherapy groups and was unchanged from baseline in the sitagliptin monotherapy group. The incidence of hypoglycemia was low (1%–3%) across treatment groups. The incidence of gastrointestinal adverse experiences was evaluated with the coadministration of sitagliptin and metformin and appeared similar to that observed with use of metformin as monotherapy.56 Thus, this study suggested that an initial combination of a DPP-IV inhibitor with metformin can improve glycemic control and markers of beta-cell function in patients with T2DM.
Incretin-based therapies compared
Studies in both healthy individuals and in patients with T2DM have shown that oral DPP-4 inhibitors such as sitagliptin increase endogenous GLP-1 concentrations by about twofold compared with placebo.22,50 The pharmacologic concentration of subcutaneously administered exenatide available for activating the GLP-1 receptor is significantly greater than the increased endogenous GLP-1 concentrations achieved with sitagliptin. In a recent clinical study comparing exenatide and sitagliptin in patients with T2DM, the mean 2-hour plasma concentration for exenatide was 64 pM compared with the mean 2-hour postprandial GLP-1 concentration of 15 pM for sitagliptin (baseline GLP-1 concentration was 7.2 pM).57 While both agents were shown to be effective, exenatide appeared to have had a greater effect than sitagliptin in increasing insulin secretion and reducing postprandial glucagon secretion, leading to significantly (P < 0.0001) greater reductions in PPG.57
Sitagliptin has been minimally associated with nausea, whereas patients who take exenatide need to be informed of the risk of usually mild to moderate, but sometimes severe, nausea and vomiting that tends to decrease over time.
For a detailed comparison of the effects of GLP-1 receptor agonists and DPP-4 inhibitors on HbA1c, weight, and hypoglycemia, see “Advances in therapy for type 2 diabetes: GLP–1 receptor agonists and DPP–4 inhibitors.”
CONCLUSION
Despite advances in diagnosis and treatment, T2DM, overweight/obesity, CVD, and their complications remain major public health burdens worldwide. The concepts that explain the pathophysiology of T2DM include the contribution of various factors beyond insulin secretion and insulin resistance, such as the role of incretin hormones in disease progression. A comprehensive approach to managing patients with T2DM requires targeting the fundamental defects of the disease and its comorbidities. Newer agents, including incretin-based therapies such as GLP-1 receptor agonists and DPP-4 inhibitors, address the fundamental defects of T2DM. The definition of treatment success in the management of T2DM will be redefined as more data become available on agents that exert beneficial effects not only on glycemia but on parameters that may influence overall CV health, such as weight, BP, and lipid profiles.
According to the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), glycosylated hemoglobin (HbA1c) in patients with diabetes should be maintained at 6.5% or less (AACE) or at less than 7.0% (ADA). Both organizations support an aggressive stepwise approach that includes medication and lifestyle modification, with strategies and clinical attention devoted to avoiding significant hypoglycemia.1,2 Yet, despite the introduction of new antidiabetes agents, most current management strategies are offset by limitations in achieving and maintaining glycemic targets needed to provide optimal care for patients with diabetes, more than 90% of whom have type 2 diabetes mellitus (T2DM).3,4
Nationally, glycemic control among patients with T2DM has improved but is still far from optimal. According to data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), glycemic control (HbA1c < 7.0%) rates were 35.8% for patients with T2DM.5 In a more recent report (NHANES 1999–2004), fewer than half (48.4%) of adult patients with diagnosed diabetes achieved HbA1c levels below 7.0%.5,6 Factors contributing to these data include earlier onset and earlier detection of T2DM.7
CHANGING TREATMENT TRENDS
Available treatments for patients with T2DM include secretagogues, such as sulfonylureas and “glinides” (repaglinide and nateglinide), metformin, thiazolidinediones (TZDs), and dipeptidyl peptidase–4 (DPP-4) inhibitors among oral medications, and insulin and glucagon-like peptide–1 (GLP-1) receptor agonists among parenterally administered agents. According to the latest published data on prescribing patterns for patients with T2DM, analyses of the National Disease and Therapeutic Index (1994–2007) and the National Prescription Audit (2001–2007), sulfonylurea use decreased from 67% of treatment visits in 1994 to 34% of visits in 2007.8 By 2007, metformin, used in 54% of treatment visits, and TZDs, used in 28%, were the most frequently administered antidiabetes agents. Insulin use declined from 38% of visits during which a treatment was administered in 1994 to 25% of visits in 2000, but had increased subsequently to 28% of visits in 2007.
SIGNIFICANCE OF CARDIOVASCULAR RISK
Clinical research has suggested that focusing solely on improving glycemic control may be insufficient to reduce overall morbidity and mortality associated with diabetes. Specifically, data from recent studies, including the Action to Control Cardiovascular Risk in Diabetes (ACCORD), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT), emphasized that lowering HbA1c below 7% in a high-risk population of individuals with T2DM did not improve cardiovascular (CV) outcomes.9–11 The observations confirm that risk factors, including weight, blood pressure (BP), and lipid levels, are vitally important in reducing morbidity and mortality in this population. This perception is further underscored by the NHANES 1999–2004 data, which showed poor concurrent control of HbA1c, BP, and lipids; only 13.2% of patients with diagnosed diabetes achieved all three target goals simultaneously.6 Similarly, a nationwide survey in Norway showed that only 13% of patients with T2DM concurrently achieved goals for HbA1c, BP, and lipids.12
In the Danish Steno-2 Study, patients with T2DM and persistent microalbuminuria were treated with either intensive target-driven therapy using multiple drugs or conventional multifactorial treatment. Over a mean period of 13.3 years (7.8 years of treatment plus 5.5 years of follow-up), intensive multifactorial intervention to control multiple CV risk factors, including HbA1c, BP, and lipids, was associated with a lower risk of death from CV causes (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.19 to 0.94; P = .04) and a lower risk of CV events (HR, 0.41; 95% CI, 0.25 to 0.67; P < .001) than was conventional therapy.13
This article clarifies the redefinition of treatment success in patients with T2DM based on targeting the underlying physiologic defects of the disease.
T2DM, OVERWEIGHT/OBESITY, AND CV DISEASE: CLOSELY LINKED
The incidence and prevalence of T2DM, overweight/obesity, and CV disease (CVD) are increasing worldwide. It is estimated that the worldwide prevalence of diabetes will increase from 171 million in 2000 to 366 million by 203014; T2DM increases the risk of morbidity and mortality from microvascular (eg, neuropathic, retinopathic, nephropathic) and macrovascular (eg, coronary, peripheral vascular disease) complications.15 According to a Michigan health maintenance organization study (N = 1,364), the median annual direct cost of medical care for Caucasian patients with T2DM who were diet controlled, had a body mass index (BMI) of 30 kg/m2 or higher, and had no vascular complications was estimated to be $1,700 for men and $2,100 for women.16 The actual cost of care for patients with T2DM may be much higher, since most patients present with multiple CV risk factors in addition to being overweight.
NHANES data show that approximately two-thirds of Americans are either overweight or obese17; overweight/obesity affects about 80% of adults diagnosed with T2DM.18 Overweight or obesity can increase the risk for developing T2DM by more than 90-fold and, in women, it can increase the risk for developing coronary heart disease (CHD) by sixfold.19 The close link between T2DM and CVD is underscored further with recent data from the Framingham Heart Study, which showed a high lifetime risk of CVD in patients with diabetes, heightened further by obesity. During the 30-year study period, the lifetime risk of CVD in normal-weight people with diabetes was 78.6% in men and 54.8% in women; the risk increased to 86.9% in obese men with diabetes and to 78.8% in obese women with diabetes.20 The NHANES data also showed that the prevalence of T2DM increased in the past decade and that patients are being diagnosed at a younger age, from a mean age of 52 years in 1988–1994 to 46 years in 1999–2000.7
BRIDGING THE GAP FROM PATHOPHYSIOLOGY TO UNMET NEEDS
The paradigm behind the pathophysiology of T2DM has shifted from its perception as a simple “dual-defect” disease (ie, deficiency in insulin secretion and peripheral tissue insulin resistance) to a multidimensional disorder.1,21 This new model includes overweight/obesity, insulin resistance, qualitative and quantitative defects in insulin secretion, and dysregulation in the secretion of other hormones, including the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the gastrointestinal incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide.21–23
CLINICAL GUIDELINES AND CV RISK FACTOR MANAGEMENT
The best strategy for managing T2DM is a comprehensive approach that addresses the fundamental core defects plus associated factors that contribute to increased CV risk. Several specialty groups have suggested guidelines and algorithms for the management of T2DM and its comorbidities. These guidelines, including the ADA standards of medical care, the AACE standards in tandem with the American College of Endocrinology guidelines, and the recent joint statement from the ADA and the European Association for the Study of Diabetes (EASD), acknowledge that the core defects of T2DM and the associated CV risk factors (eg, weight gain, obesity, hypertension, dyslipidemia) are important in developing optimal treatment strategies.1–3 Medical nutrition guidelines advocate weight loss as a key initial step in managing T2DM and the comorbidities that lead to elevated CV risk.25,26 The National Institutes of Health and the US Department of Health and Human Services/US Department of Agriculture advocate regular physical activity, dietary assessment, and periodic comorbidity and weight assessment for all people, not just those with T2DM or CVD.26,27
Weight reduction
Evidence in support of effective lifestyle intervention was demonstrated in the Action for Health in Diabetes (Look AHEAD) study. After 1 year, patients with T2DM treated with intensive lifestyle intervention lost an average of 8.6% of their initial weight compared with 0.7% in patients treated only with diabetes support and education (P < 0.001). The intensive-intervention patients also had a significant drop in HbA1c (from 7.3% to 6.6%; P < 0.001) and were able to reduce their antidiabetes, antihypertensive, and lipid-lowering medications.28 More recent data from the Look AHEAD study reported that overweight patients with T2DM enrolled in a weight management program experienced significant weight loss, improved physical fitness, reduced physical symptoms, and overall improvement in health-related quality of life.29 Thus, weight reduction appears to be a key component in reducing CV risk and improving quality of life in most patients with T2DM.28–30
Hypertension
Hypertension is a major risk factor for microvascular complications and CVD, and may be associated with, or be the underlying result of, nephropathy.2 BP control is clearly important in reducing the morbidity and mortality associated with T2DM. The recommended BP goal in patients with T2DM is less than 130/80 mm Hg.1,2
Hyperlipidemia
According to the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III [ATP III]), diabetes is considered a CHD risk equivalent because it confers a high risk of new CHD developing within 10 years.31 In addition to the NCEP–ATP III guidelines, the ADA and the AACE have set target levels for lipids in patients with diabetes, including T2DM.1,2,31 All three organizations have defined 100 mg/dL as the target level for low-density lipoprotein.
HbA1c and lifestyle intervention
EVOLUTION OF ANTIDIABETES THERAPIES
Traditional antidiabetes agents used in the treatment of patients with T2DM have focused mainly on insulin secretion and insulin resistance, with treatment success defined as achieving HbA1c goals with a reduced incidence of hypoglycemia.23 Secretagogues, such as sulfonylureas and glinides, stimulate the pancreas to release insulin. Insulin sensitizers, such as TZDs and metformin, enhance the action of insulin in muscle and fat1,3,23 and lower hepatic glucose production. The alpha-glucosidase inhibitors alter carbohydrate absorption from the gastrointestinal tract.1 The extent to which each agent achieves treatment success in terms of glucose lowering depends on several factors, including intrinsic attributes, duration of disease, and baseline glycemic control.3
GLP-1 receptor agonists
Exenatide effects. Although many agents are in development, to date exenatide is the only GLP-1 receptor agonist approved by the US Food and Drug Administration (FDA).8,33 Exenatide is an exendin-4 GLP-1 receptor agonist with multiple glucoregulatory effects, including enhanced glucose-dependent insulin secretion, reduced glucagon secretion and food intake, and slowed gastric emptying.22,34 Exenatide is detectable in the circulation for up to 10 hours following subcutaneous (SC) administration22 and has a greater potency in reducing plasma glucose than GLP-1 in preclinical studies.35,36
By virtue of its beneficial effects on glycemic control, weight, BP, and lipids, exenatide addresses some of the components of the metabolic syndrome.37–41 In pivotal 30-week studies, exenatide was associated with HbA1c reductions that ranged from –0.40% to –0.86% from baseline and decreases in body weight of approximately –1 kg to –3 kg from baseline, without severe hypoglycemia.37–39 The percentage of patients who reached the ADA goal of HbA1c less than 7.0% at 30 weeks ranged from 24% to 34%. The addition of exenatide to TZD therapy in a 16-week study was associated with mean reductions in HbA1c of –0.98%, fasting plasma glucose (FPG) concentration of –1.69 mmol/L (–30.42 mg/dL), and body weight of –1.51 kg.40
A posthoc analysis of an open-label extension study involving patients who completed the original 30-week placebo-controlled studies showed that 46% of patients who remained on exenatide achieved the ADA goal of HbA1c less than 7.0% at 3 years.41 Exenatide administered for up to 3.5 years was associated with sustained reductions in HbA1c of –1.0% (P < .0001) and body weight of –5.3 kg (P < .001). Pancreatic beta-cell function, assessed by homeostasis model assessment, improved, as did BP, triglyceride, high-density lipoprotein, low-density lipoprotein, and aspartate aminotransferase levels.41
Comparison with insulin analogues. Comparative studies have highlighted the contrasting effects of exenatide and insulin analogues (eg, insulin glargine and fixed-ratio insulin).42–45 In a 26-week trial comparing exenatide with insulin glargine in subjects with T2DM, both agents resulted in similar decreases in HbA1c. Exenatide was also associated with a –2.3-kg weight reduction, whereas insulin glargine was associated with a +1.8-kg weight gain.42 Although rates of symptomatic hypoglycemia were similar, there were fewer cases of nocturnal hypoglycemia with exenatide (0.9 event/patient-year vs 2.4 events/patient-year with insulin).
In a 32-week study comparing exenatide BID with titrated insulin glargine QD, the HbA1c reductions for exenatide and insulin glargine were comparable. However, body weight decreased –4.2 kg over two 16-week treatment periods with exenatide, but increased +3.3 kg over the same periods with the basal insulin analogue.43 The incidence of hypoglycemia was lower with exenatide than with insulin glargine (14.7% vs 25.2%), although the difference was not statistically significant.
In another study that compared exenatide with biphasic insulin aspart, patients who were treated with exenatide also lost weight while those who received the fast-acting insulin analogue gained weight (between-group difference, –5.4 kg). Patients treated with exenatide also demonstrated greater reductions in postprandial plasma glucose (PPG) excursions following their morning (P < .001), midday (P = .002), and evening meals (P < .001).44 Overall, hypoglycemia rates were similar at study end between exenatide and insulin aspart (4.7 events/patient-year vs 5.6 events/patient-year). In all of these studies, significant gastrointestinal adverse events (nausea and vomiting) occurred more frequently with exenatide, and more patients withdrew from exenatide than from insulin.
Formulations in development. Other advances in GLP-1 receptor agonist therapy include novel formulations under clinical development, such as exenatide once weekly36,46 and liraglutide, a human analogue GLP-1 receptor agonist formulated for once-daily administration.47,48 In a 52-week study in patients with T2DM, liraglutide significantly reduced HbA1c; the 1.2-mg SC QD dosage reduced HBA1c by –0.84% (P = .0014) and the 1.8-mg SC QD dosage by –1.14% (P < .0001). In comparison, glimepiride 8 mg orally QD achieved a –0.51% reduction. Liraglutide was also associated with greater reductions in weight, hypoglycemia, and systolic BP than glimepiride.47
A 26-week study compared liraglutide (0.6, 1.2, and 1.8 mg SC QD), placebo, and glimepiride 4 mg QD in combination with metformin 1 g BID. HbA1c was reduced significantly in all liraglutide groups compared with placebo (P < .0001). Mean HbA1c decreased –1.0% with liraglutide 1.2 mg and 1.8 mg and with glimepiride; it decreased –0.7% with liraglutide 0.6 mg; and it increased +0.1% with placebo. Body weight decreased –1.8 kg to –2.8 kg in all liraglutide groups but increased +1.0 kg in the glimepiride group (P < .0001). The incidence of minor hypoglycemia with liraglutide (~3%) was comparable to that observed with placebo but less than that with glimepiride (17%; P < .001).48
A once-weekly long-acting release (LAR) formulation of exenatide submitted to the FDA for approval may provide enhanced glycemic and weight control, potentially improving patient acceptance and adherence.36,46 In a 15-week study, exenatide once weekly produced significant reductions in HbA1c, FPG, PPG, and body weight. There were no withdrawals due to adverse events, and the formation of anti-exenatide antibodies was not predictive of therapeutic end point response or adverse safety outcome. Instances of hypoglycemia were mild and not dose related.36 In a 30-week study comparing exenatide LAR once weekly with exenatide BID, patients given exenatide LAR once weekly had significantly greater HbA1c reductions than did patients given exenatide BID (–1.9% vs –1.5%; P = .0023). Treatment adherence was 98% with both exenatide regimens, and no episodes of major hypoglycemia occurred with either formulation regardless of background sulfonylurea use. Favorable effects on BP and lipid profile were observed with both exenatide regimens.46
DPP-4 inhibitors
The DPP-4 inhibitors (commonly called gliptins) inhibit the proteolytic cleavage of circulating GLP-1 by binding to the DPP-4 enzyme, increasing the concentration of endogenous GLP-1 approximately two- to threefold.49–51 These concentrations result in more prompt and appropriate secretion of insulin and suppression of glucagon in response to a carbohydrate-containing snack or meal, with the change in glucagon correlating linearly with improved glucose tolerance.51
DPP-4 inhibitors, which are given orally, include sitagliptin and saxagliptin (approved in the United States) and vildagliptin (not approved in the United States but used in the European Union and Latin America).8,22,33,52 Sitagliptin can be used either as monotherapy or in combination with metformin or a TZD.8,49–55 Recently, a single-tablet formulation of sitagliptin plus metformin was granted regulatory approval.8
When used alone or in combination with metformin or pioglitazone, sitagliptin has been associated with significant reductions in HbA1c (of ~0.5% to 0.6% when used alone, ~0.7% with metformin, and ~0.9% with pioglitazone [P < .001 vs placebo]), with hypoglycemia occurring in 1.3% or less of the population.54 In an 18-week study in which patients with T2DM who were inadequately controlled with metformin monotherapy were randomized to receive add-on sitagliptin (100 mg QD), rosiglitazone (8 mg QD), or placebo, sitagliptin reduced HbA1c –0.73% (P < .001 vs placebo) and reduced body weight –0.4 kg, while rosiglitazone reduced HbA1c –0.79% and increased body weight +1.5 kg.55
To evaluate the effectiveness of sitagliptin and metformin as initial therapy, a 54-week study was completed in 885 patients with T2DM and inadequate glycemic control (HbA1c 7.5–11%) on diet and exercise.56 Patients were evaluated on monotherapy with either sitagliptin (100 mg QD) or metformin (1 g or 2 g QD), or on initial therapy with the two in combination (sitagliptin 100 mg + metformin 1 mg or 2 mg QD). At week 54, in the all-patients-treated analysis, mean changes in HbA1c from baseline were –1.8% with sitagliptin plus metformin 2 g QD, –1.4% with sitagliptin plus metformin 1 g QD, –1.3% with metformin 2 g QD monotherapy, –1.0% with metformin 1 g QD monotherapy, and –0.8% with sitagliptin 100 mg QD monotherapy.
All treatments improved measures of beta-cell function (eg, homeostasis model assessment [HOMA]-beta, proinsulin/insulin ratio). Mean body weight decreased from baseline in the combination and metformin monotherapy groups and was unchanged from baseline in the sitagliptin monotherapy group. The incidence of hypoglycemia was low (1%–3%) across treatment groups. The incidence of gastrointestinal adverse experiences was evaluated with the coadministration of sitagliptin and metformin and appeared similar to that observed with use of metformin as monotherapy.56 Thus, this study suggested that an initial combination of a DPP-IV inhibitor with metformin can improve glycemic control and markers of beta-cell function in patients with T2DM.
Incretin-based therapies compared
Studies in both healthy individuals and in patients with T2DM have shown that oral DPP-4 inhibitors such as sitagliptin increase endogenous GLP-1 concentrations by about twofold compared with placebo.22,50 The pharmacologic concentration of subcutaneously administered exenatide available for activating the GLP-1 receptor is significantly greater than the increased endogenous GLP-1 concentrations achieved with sitagliptin. In a recent clinical study comparing exenatide and sitagliptin in patients with T2DM, the mean 2-hour plasma concentration for exenatide was 64 pM compared with the mean 2-hour postprandial GLP-1 concentration of 15 pM for sitagliptin (baseline GLP-1 concentration was 7.2 pM).57 While both agents were shown to be effective, exenatide appeared to have had a greater effect than sitagliptin in increasing insulin secretion and reducing postprandial glucagon secretion, leading to significantly (P < 0.0001) greater reductions in PPG.57
Sitagliptin has been minimally associated with nausea, whereas patients who take exenatide need to be informed of the risk of usually mild to moderate, but sometimes severe, nausea and vomiting that tends to decrease over time.
For a detailed comparison of the effects of GLP-1 receptor agonists and DPP-4 inhibitors on HbA1c, weight, and hypoglycemia, see “Advances in therapy for type 2 diabetes: GLP–1 receptor agonists and DPP–4 inhibitors.”
CONCLUSION
Despite advances in diagnosis and treatment, T2DM, overweight/obesity, CVD, and their complications remain major public health burdens worldwide. The concepts that explain the pathophysiology of T2DM include the contribution of various factors beyond insulin secretion and insulin resistance, such as the role of incretin hormones in disease progression. A comprehensive approach to managing patients with T2DM requires targeting the fundamental defects of the disease and its comorbidities. Newer agents, including incretin-based therapies such as GLP-1 receptor agonists and DPP-4 inhibitors, address the fundamental defects of T2DM. The definition of treatment success in the management of T2DM will be redefined as more data become available on agents that exert beneficial effects not only on glycemia but on parameters that may influence overall CV health, such as weight, BP, and lipid profiles.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the US National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Koopman RJ, Mainous AG III, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005; 3:60–63.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Jenssen TG, Tonstad S, Claudi T, Midthjell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003; 26:2300–2304.
- National Center for Health Statistics. Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control. Eur J Endocrinol 2008; 158:773–784.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care 2008; 31:1582–1584.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Cefalu WT. Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: rationale and specific agents. Clin Pharmacol Ther 2007; 81:636–649.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- US Department of Health and Human Services (HHS) and US Department of Agriculture. Dietary guidelines for Americans, 2005. US Department of HHS Web site. http://www.health.gov/DietaryGuidelines/dga2005/document/default.htm. Published January 2005. Accessed September 25, 2009.
- National Heart, Lung, and Blood Institute. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health Web site. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Updated: October 2000. Accessed September 28, 2009.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K; for the Look AHEAD Research Group. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009; 169:163–171.
- Klein S, Sheard NF, Pi-Sunyer X, et al; for the American Diabetes Association; North American Association for the Study of Obesity; American Society for Clinical Nutrition. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004; 117:77–88.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004; 89:2078–2084.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Bohannon N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad Med 2009; 121:40–45.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Zerilli T, Pyon EY. Sitagliptin phosphate: a DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Ther 2007; 29:2614–2634.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin 2009; 25:569–583.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the US National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Koopman RJ, Mainous AG III, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005; 3:60–63.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Jenssen TG, Tonstad S, Claudi T, Midthjell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003; 26:2300–2304.
- National Center for Health Statistics. Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control. Eur J Endocrinol 2008; 158:773–784.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care 2008; 31:1582–1584.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Cefalu WT. Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: rationale and specific agents. Clin Pharmacol Ther 2007; 81:636–649.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- US Department of Health and Human Services (HHS) and US Department of Agriculture. Dietary guidelines for Americans, 2005. US Department of HHS Web site. http://www.health.gov/DietaryGuidelines/dga2005/document/default.htm. Published January 2005. Accessed September 25, 2009.
- National Heart, Lung, and Blood Institute. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health Web site. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Updated: October 2000. Accessed September 28, 2009.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K; for the Look AHEAD Research Group. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009; 169:163–171.
- Klein S, Sheard NF, Pi-Sunyer X, et al; for the American Diabetes Association; North American Association for the Study of Obesity; American Society for Clinical Nutrition. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004; 117:77–88.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004; 89:2078–2084.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Bohannon N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad Med 2009; 121:40–45.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Zerilli T, Pyon EY. Sitagliptin phosphate: a DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Ther 2007; 29:2614–2634.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin 2009; 25:569–583.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
KEY POINTS
- The NHANES 1999–2004 data showed that only 13.2% of patients with diagnosed diabetes achieved concurrent weight, blood pressure, and lipid level goals.
- Among patients with T2DM, lifestyle intervention (control of weight, blood pressure, lipid levels) should be reinforced at every physician visit; glycosylated hemoglobin (HbA1c) should be monitored every 3 months until it is less than 7.0%, and then rechecked every 6 months.
- The effects of GLP-1 agonists on HbA1c are comparable to insulin analogues, but GLP-1 agonists are associated with weight reduction, while insulin is associated with weight gain.
- DPP-4 inhibitors have been associated with significant reductions in HbA1c when used alone or with metformin or pioglitazone.
Clin-Admin Balance
As hospitalists take on more demanding leadership roles, the climb up the career ladder evolves into a juggling act: Hospitalists typically try to handle a full patient load as well as new administrative duties.
If a hospitalist continues to ascend, those administrative duties can begin to consume the schedule. The individual—and the group—could face important decisions about priorities, schedules, and money.
“Hospital medicine is only ten years old; we’re still trying to figure this out,” says Mary Jo Gorman, MD, MBA, chief executive officer of Advanced ICU Care in St. Louis and a past president of SHM. “It’s always a challenge. You identify that you have a need for someone to take charge of an administrative task, but it can take as long as a year to free up [the hospitalist’s] time so that it can get done.”
If you have found yourself in this position, you know that something has to give. “I’ve seen high-energy physicians who think that they can do it all—and they had to,” says Joan C. Faro, MD, FACP, MBA, chief medical officer at John T. Mather Memorial Hospital in Port Jefferson, N.Y. “That is not sustainable. It can’t last forever.”
The question is, how can a hospitalist effectively balance their clinical and administrative duties? Furthermore, what happens when the scales tip in favor—and to the detriment—of one or the other?
When the Juggling Begins
Hospitalists usually add “extra” duties to their normal workloads to advance their careers. Few relinquish their clinical duties as they join committees, further their training, lead a research project, or take on administrative duties.
Dr. Faro says a hospitalist should be able to “head up a focused project or serve on committees” and still be able to meet all their clinical duties. “Once you get beyond that, you need a certain amount of protected time” for administrative or project work, she says. “And when you start to have people reporting to you, you absolutely need that protected time.”
—Joan C. Faro, MD, FACP, MBA, chief medical officer, John T. Mather Memorial Hospital, Port Jefferson, N.Y.
Assigning administrative tasks to physicians who regularly see patients depends on the group structure and requires a clearly defined job description. “If a group is really going to make this work, then you have to pay people for that extra time,” Dr. Gorman says.
Ideally, HM groups have job descriptions for physicians who are called upon to see patients and handle administrative duties. Contracts should include specifications for “protected time,” as well as compensation for new responsibilities.
Clinical-Hour Cutbacks
As administrative duties grow, something has to give. Hospitalists who want to pursue positions of leadership know that that something is hours spent delivering patient care. “If you’re a hospitalist-administrator who wants to make the leap to vice president or department chair or chief medical officer, you need to devote a lot of time to your administrative work,” Dr. Faro says. “You can’t make that leap without putting in those hours.”
So what is a reasonable division of time for, say, the director of an HM program or department? “It’s impossible to pinpoint, but I’d say roughly that [a director] should spend not less than 25% or 30% of their time, and certainly not more than 50% of their time, on clinical work,” Dr. Faro estimates.
Even upper-level physician-administrators should maintain a clinical practice simply to monitor the work their department is doing. “It’s not about [clinical] skills as much as it is about whether you can relate to physicians’ day-to-day work, to their frustrations,” Dr. Gorman says. “That’s a management challenge no matter who you are. For example, if hospitalists are complaining about a new EMR [electronic medical record] system, are you going to say, ‘Oh, just put up with it; it’s not that bad. It will be fine’? Or are you out there trying it and saying, ‘Holy cow, this is really inefficient. We have to change this’?”
On the flip side, how much time should be devoted to administrative tasks? The answer depends on the size of your program and the amount of work you have to do, Dr. Faro says. Group directors and department heads normally make themselves available during regular weekday hours. That usually means you’ll have to fit in your clinical work around meetings, budgets, and presentations.
Can You Give Up Clinical Duties?
It’s natural for physicians to be reluctant to relinquish patient care; some reach a point where they have to make the tough decision to stop clinical work altogether.
“You may figure out that you want to pursue an administrative role, but you don’t want to give up clinical work,” says Dr. Gorman, who spent 15 years juggling a full clinical schedule with administrative duties before she became a full-time administrator. “You get plenty of opportunities to make that decision as you’re crossing back and forth.”
You might want to evaluate your options and make the choice sooner rather than later. Once you’re in administration, the decision might be forced upon you. “Eventually, you’ll find that critical things are happening all hours of the day, any given day of the week, in administration as well as clinical practice,” Dr. Faro says. “There’s a point at which you realize that part-time [administrative work] just doesn’t work. You realize that your expertise and guidance are needed.”
Dr. Gorman warns that there are risks and changes involved with becoming a full-time administrator. Once you decide to give up your clinical practice and go the leadership route, your career is “in the hands of someone else,” she points out. “Your position could be eliminated. You could be fired or replaced. … That is a concern. A lot of people keep their hand in on clinical skills for that reason.” You also might find that advancing a management career requires moving to a new organization or a different part of the country.
On the other hand, the rewards of a career in administration can’t be overlooked. “It’s very satisfying personally,” Dr. Faro says. “It’s inventive; you’re constantly solving problems that didn’t exist yesterday.
“It’s a different kind of job satisfaction. It’s a very personal decision. There are people who realize that this just isn’t for them.” TH
Jane Jerrard is a freelance writer based in Chicago.
As hospitalists take on more demanding leadership roles, the climb up the career ladder evolves into a juggling act: Hospitalists typically try to handle a full patient load as well as new administrative duties.
If a hospitalist continues to ascend, those administrative duties can begin to consume the schedule. The individual—and the group—could face important decisions about priorities, schedules, and money.
“Hospital medicine is only ten years old; we’re still trying to figure this out,” says Mary Jo Gorman, MD, MBA, chief executive officer of Advanced ICU Care in St. Louis and a past president of SHM. “It’s always a challenge. You identify that you have a need for someone to take charge of an administrative task, but it can take as long as a year to free up [the hospitalist’s] time so that it can get done.”
If you have found yourself in this position, you know that something has to give. “I’ve seen high-energy physicians who think that they can do it all—and they had to,” says Joan C. Faro, MD, FACP, MBA, chief medical officer at John T. Mather Memorial Hospital in Port Jefferson, N.Y. “That is not sustainable. It can’t last forever.”
The question is, how can a hospitalist effectively balance their clinical and administrative duties? Furthermore, what happens when the scales tip in favor—and to the detriment—of one or the other?
When the Juggling Begins
Hospitalists usually add “extra” duties to their normal workloads to advance their careers. Few relinquish their clinical duties as they join committees, further their training, lead a research project, or take on administrative duties.
Dr. Faro says a hospitalist should be able to “head up a focused project or serve on committees” and still be able to meet all their clinical duties. “Once you get beyond that, you need a certain amount of protected time” for administrative or project work, she says. “And when you start to have people reporting to you, you absolutely need that protected time.”
—Joan C. Faro, MD, FACP, MBA, chief medical officer, John T. Mather Memorial Hospital, Port Jefferson, N.Y.
Assigning administrative tasks to physicians who regularly see patients depends on the group structure and requires a clearly defined job description. “If a group is really going to make this work, then you have to pay people for that extra time,” Dr. Gorman says.
Ideally, HM groups have job descriptions for physicians who are called upon to see patients and handle administrative duties. Contracts should include specifications for “protected time,” as well as compensation for new responsibilities.
Clinical-Hour Cutbacks
As administrative duties grow, something has to give. Hospitalists who want to pursue positions of leadership know that that something is hours spent delivering patient care. “If you’re a hospitalist-administrator who wants to make the leap to vice president or department chair or chief medical officer, you need to devote a lot of time to your administrative work,” Dr. Faro says. “You can’t make that leap without putting in those hours.”
So what is a reasonable division of time for, say, the director of an HM program or department? “It’s impossible to pinpoint, but I’d say roughly that [a director] should spend not less than 25% or 30% of their time, and certainly not more than 50% of their time, on clinical work,” Dr. Faro estimates.
Even upper-level physician-administrators should maintain a clinical practice simply to monitor the work their department is doing. “It’s not about [clinical] skills as much as it is about whether you can relate to physicians’ day-to-day work, to their frustrations,” Dr. Gorman says. “That’s a management challenge no matter who you are. For example, if hospitalists are complaining about a new EMR [electronic medical record] system, are you going to say, ‘Oh, just put up with it; it’s not that bad. It will be fine’? Or are you out there trying it and saying, ‘Holy cow, this is really inefficient. We have to change this’?”
On the flip side, how much time should be devoted to administrative tasks? The answer depends on the size of your program and the amount of work you have to do, Dr. Faro says. Group directors and department heads normally make themselves available during regular weekday hours. That usually means you’ll have to fit in your clinical work around meetings, budgets, and presentations.
Can You Give Up Clinical Duties?
It’s natural for physicians to be reluctant to relinquish patient care; some reach a point where they have to make the tough decision to stop clinical work altogether.
“You may figure out that you want to pursue an administrative role, but you don’t want to give up clinical work,” says Dr. Gorman, who spent 15 years juggling a full clinical schedule with administrative duties before she became a full-time administrator. “You get plenty of opportunities to make that decision as you’re crossing back and forth.”
You might want to evaluate your options and make the choice sooner rather than later. Once you’re in administration, the decision might be forced upon you. “Eventually, you’ll find that critical things are happening all hours of the day, any given day of the week, in administration as well as clinical practice,” Dr. Faro says. “There’s a point at which you realize that part-time [administrative work] just doesn’t work. You realize that your expertise and guidance are needed.”
Dr. Gorman warns that there are risks and changes involved with becoming a full-time administrator. Once you decide to give up your clinical practice and go the leadership route, your career is “in the hands of someone else,” she points out. “Your position could be eliminated. You could be fired or replaced. … That is a concern. A lot of people keep their hand in on clinical skills for that reason.” You also might find that advancing a management career requires moving to a new organization or a different part of the country.
On the other hand, the rewards of a career in administration can’t be overlooked. “It’s very satisfying personally,” Dr. Faro says. “It’s inventive; you’re constantly solving problems that didn’t exist yesterday.
“It’s a different kind of job satisfaction. It’s a very personal decision. There are people who realize that this just isn’t for them.” TH
Jane Jerrard is a freelance writer based in Chicago.
As hospitalists take on more demanding leadership roles, the climb up the career ladder evolves into a juggling act: Hospitalists typically try to handle a full patient load as well as new administrative duties.
If a hospitalist continues to ascend, those administrative duties can begin to consume the schedule. The individual—and the group—could face important decisions about priorities, schedules, and money.
“Hospital medicine is only ten years old; we’re still trying to figure this out,” says Mary Jo Gorman, MD, MBA, chief executive officer of Advanced ICU Care in St. Louis and a past president of SHM. “It’s always a challenge. You identify that you have a need for someone to take charge of an administrative task, but it can take as long as a year to free up [the hospitalist’s] time so that it can get done.”
If you have found yourself in this position, you know that something has to give. “I’ve seen high-energy physicians who think that they can do it all—and they had to,” says Joan C. Faro, MD, FACP, MBA, chief medical officer at John T. Mather Memorial Hospital in Port Jefferson, N.Y. “That is not sustainable. It can’t last forever.”
The question is, how can a hospitalist effectively balance their clinical and administrative duties? Furthermore, what happens when the scales tip in favor—and to the detriment—of one or the other?
When the Juggling Begins
Hospitalists usually add “extra” duties to their normal workloads to advance their careers. Few relinquish their clinical duties as they join committees, further their training, lead a research project, or take on administrative duties.
Dr. Faro says a hospitalist should be able to “head up a focused project or serve on committees” and still be able to meet all their clinical duties. “Once you get beyond that, you need a certain amount of protected time” for administrative or project work, she says. “And when you start to have people reporting to you, you absolutely need that protected time.”
—Joan C. Faro, MD, FACP, MBA, chief medical officer, John T. Mather Memorial Hospital, Port Jefferson, N.Y.
Assigning administrative tasks to physicians who regularly see patients depends on the group structure and requires a clearly defined job description. “If a group is really going to make this work, then you have to pay people for that extra time,” Dr. Gorman says.
Ideally, HM groups have job descriptions for physicians who are called upon to see patients and handle administrative duties. Contracts should include specifications for “protected time,” as well as compensation for new responsibilities.
Clinical-Hour Cutbacks
As administrative duties grow, something has to give. Hospitalists who want to pursue positions of leadership know that that something is hours spent delivering patient care. “If you’re a hospitalist-administrator who wants to make the leap to vice president or department chair or chief medical officer, you need to devote a lot of time to your administrative work,” Dr. Faro says. “You can’t make that leap without putting in those hours.”
So what is a reasonable division of time for, say, the director of an HM program or department? “It’s impossible to pinpoint, but I’d say roughly that [a director] should spend not less than 25% or 30% of their time, and certainly not more than 50% of their time, on clinical work,” Dr. Faro estimates.
Even upper-level physician-administrators should maintain a clinical practice simply to monitor the work their department is doing. “It’s not about [clinical] skills as much as it is about whether you can relate to physicians’ day-to-day work, to their frustrations,” Dr. Gorman says. “That’s a management challenge no matter who you are. For example, if hospitalists are complaining about a new EMR [electronic medical record] system, are you going to say, ‘Oh, just put up with it; it’s not that bad. It will be fine’? Or are you out there trying it and saying, ‘Holy cow, this is really inefficient. We have to change this’?”
On the flip side, how much time should be devoted to administrative tasks? The answer depends on the size of your program and the amount of work you have to do, Dr. Faro says. Group directors and department heads normally make themselves available during regular weekday hours. That usually means you’ll have to fit in your clinical work around meetings, budgets, and presentations.
Can You Give Up Clinical Duties?
It’s natural for physicians to be reluctant to relinquish patient care; some reach a point where they have to make the tough decision to stop clinical work altogether.
“You may figure out that you want to pursue an administrative role, but you don’t want to give up clinical work,” says Dr. Gorman, who spent 15 years juggling a full clinical schedule with administrative duties before she became a full-time administrator. “You get plenty of opportunities to make that decision as you’re crossing back and forth.”
You might want to evaluate your options and make the choice sooner rather than later. Once you’re in administration, the decision might be forced upon you. “Eventually, you’ll find that critical things are happening all hours of the day, any given day of the week, in administration as well as clinical practice,” Dr. Faro says. “There’s a point at which you realize that part-time [administrative work] just doesn’t work. You realize that your expertise and guidance are needed.”
Dr. Gorman warns that there are risks and changes involved with becoming a full-time administrator. Once you decide to give up your clinical practice and go the leadership route, your career is “in the hands of someone else,” she points out. “Your position could be eliminated. You could be fired or replaced. … That is a concern. A lot of people keep their hand in on clinical skills for that reason.” You also might find that advancing a management career requires moving to a new organization or a different part of the country.
On the other hand, the rewards of a career in administration can’t be overlooked. “It’s very satisfying personally,” Dr. Faro says. “It’s inventive; you’re constantly solving problems that didn’t exist yesterday.
“It’s a different kind of job satisfaction. It’s a very personal decision. There are people who realize that this just isn’t for them.” TH
Jane Jerrard is a freelance writer based in Chicago.
ONLINE EXCLUSIVE: Hub and Spoke For Stroke
Given the varying access to acute-stroke expertise and the roles hospitalists play in treatment (see “Spotlight on Stroke,” p. 1), stroke protocol differs from hospital to hospital throughout the U.S. One response is known as “drip and ship.” Physicians at remote hospitals consult experts at a tertiary-care medical center by phone or video before initiating clot-busting intravenous recombinant tissue plasminogen activator (t-PA) within its three- to 4.5-hour therapeutic window. Once t-PA is administered, the patient is transferred to the medical center for ongoing care.
—Lee Schwamm, MD, director of acute-stroke services, Massachusetts General Hospital, Boston
“But what is the best way to provide that expertise at the bedside to support the first-responding physician who is not a stroke expert?” asks Lee Schwamm, MD, director of acute-stroke services at Massachusetts General Hospital (MGH) in Boston. While the goal is to disseminate stroke treatment expertise as widely as possible, there are other benefits to the arrangement, from the quality of the infrastructure, ongoing education, and a growing relationship that is more than just “transactional” telemedicine.
MGH and Brigham and Women’s Hospital are the hubs for the relationship-building Partners TeleStroke Network. It connects 27 participating hospitals across three states with an escalating chain of access to stroke resources. Spoke hospitals transmit, through a secure link, such clinical data as noncontrast head CT scans to the hub, where a stroke expert “examines” the patient via live video feed and shares in the responsibility for deciding whether to initiate t-PA. The network’s resources include clinical and information technology advocates at the hub and spokes; managers of business processes, contracts, licensure, and credentialing; consultation recording for quality purposes; regular telemedicine grand rounds; and the network’s leadership in an alliance of hub-and-spokes stroke networks at other academic medical centers. “This is not a game to play casually. It’s about developing new healthcare delivery models, with lots of complicating factors,” Dr. Schwamm says.
Hospitalists should not only note that stroke care is coming under greater regulatory scrutiny, but also that stroke information increasingly is available on the Web, Dr. Schwamm says. He also urges hospitals to participate in one of the national quality programs for stroke care, including the American Stroke Association’s Get with the Guidelines: Stroke, the Joint Commission’s primary stroke center accreditation, or the CDC’s Paul Coverdell National Acute Stroke Registry. “Each of these provides a structure for improving the quality of stroke care,” Dr. Schwamm explains, “and is money well spent by the hospital.”
Given the varying access to acute-stroke expertise and the roles hospitalists play in treatment (see “Spotlight on Stroke,” p. 1), stroke protocol differs from hospital to hospital throughout the U.S. One response is known as “drip and ship.” Physicians at remote hospitals consult experts at a tertiary-care medical center by phone or video before initiating clot-busting intravenous recombinant tissue plasminogen activator (t-PA) within its three- to 4.5-hour therapeutic window. Once t-PA is administered, the patient is transferred to the medical center for ongoing care.
—Lee Schwamm, MD, director of acute-stroke services, Massachusetts General Hospital, Boston
“But what is the best way to provide that expertise at the bedside to support the first-responding physician who is not a stroke expert?” asks Lee Schwamm, MD, director of acute-stroke services at Massachusetts General Hospital (MGH) in Boston. While the goal is to disseminate stroke treatment expertise as widely as possible, there are other benefits to the arrangement, from the quality of the infrastructure, ongoing education, and a growing relationship that is more than just “transactional” telemedicine.
MGH and Brigham and Women’s Hospital are the hubs for the relationship-building Partners TeleStroke Network. It connects 27 participating hospitals across three states with an escalating chain of access to stroke resources. Spoke hospitals transmit, through a secure link, such clinical data as noncontrast head CT scans to the hub, where a stroke expert “examines” the patient via live video feed and shares in the responsibility for deciding whether to initiate t-PA. The network’s resources include clinical and information technology advocates at the hub and spokes; managers of business processes, contracts, licensure, and credentialing; consultation recording for quality purposes; regular telemedicine grand rounds; and the network’s leadership in an alliance of hub-and-spokes stroke networks at other academic medical centers. “This is not a game to play casually. It’s about developing new healthcare delivery models, with lots of complicating factors,” Dr. Schwamm says.
Hospitalists should not only note that stroke care is coming under greater regulatory scrutiny, but also that stroke information increasingly is available on the Web, Dr. Schwamm says. He also urges hospitals to participate in one of the national quality programs for stroke care, including the American Stroke Association’s Get with the Guidelines: Stroke, the Joint Commission’s primary stroke center accreditation, or the CDC’s Paul Coverdell National Acute Stroke Registry. “Each of these provides a structure for improving the quality of stroke care,” Dr. Schwamm explains, “and is money well spent by the hospital.”
Given the varying access to acute-stroke expertise and the roles hospitalists play in treatment (see “Spotlight on Stroke,” p. 1), stroke protocol differs from hospital to hospital throughout the U.S. One response is known as “drip and ship.” Physicians at remote hospitals consult experts at a tertiary-care medical center by phone or video before initiating clot-busting intravenous recombinant tissue plasminogen activator (t-PA) within its three- to 4.5-hour therapeutic window. Once t-PA is administered, the patient is transferred to the medical center for ongoing care.
—Lee Schwamm, MD, director of acute-stroke services, Massachusetts General Hospital, Boston
“But what is the best way to provide that expertise at the bedside to support the first-responding physician who is not a stroke expert?” asks Lee Schwamm, MD, director of acute-stroke services at Massachusetts General Hospital (MGH) in Boston. While the goal is to disseminate stroke treatment expertise as widely as possible, there are other benefits to the arrangement, from the quality of the infrastructure, ongoing education, and a growing relationship that is more than just “transactional” telemedicine.
MGH and Brigham and Women’s Hospital are the hubs for the relationship-building Partners TeleStroke Network. It connects 27 participating hospitals across three states with an escalating chain of access to stroke resources. Spoke hospitals transmit, through a secure link, such clinical data as noncontrast head CT scans to the hub, where a stroke expert “examines” the patient via live video feed and shares in the responsibility for deciding whether to initiate t-PA. The network’s resources include clinical and information technology advocates at the hub and spokes; managers of business processes, contracts, licensure, and credentialing; consultation recording for quality purposes; regular telemedicine grand rounds; and the network’s leadership in an alliance of hub-and-spokes stroke networks at other academic medical centers. “This is not a game to play casually. It’s about developing new healthcare delivery models, with lots of complicating factors,” Dr. Schwamm says.
Hospitalists should not only note that stroke care is coming under greater regulatory scrutiny, but also that stroke information increasingly is available on the Web, Dr. Schwamm says. He also urges hospitals to participate in one of the national quality programs for stroke care, including the American Stroke Association’s Get with the Guidelines: Stroke, the Joint Commission’s primary stroke center accreditation, or the CDC’s Paul Coverdell National Acute Stroke Registry. “Each of these provides a structure for improving the quality of stroke care,” Dr. Schwamm explains, “and is money well spent by the hospital.”