User login
The Overweight Child With Hypertension
When presented with an overweight child who has hypertension, collect a detailed history, including a 24-hour food-intake history.
Also assess the child's nutritional habits, such as number of fast-food items typically eaten per week and number of family dinners.
Ask about the fluids these children generally consume. For instance, do they drink any caloric beverages other than low-fat milk?
Take an exercise history. Inquire how many hours per day the child is exposed to television, video games, and other media.
Social interaction can be particularly important with an overweight child. Ask if the child has been teased or bullied at home, in school, or elsewhere in the community.
Next ask the parent(s) and patient what they know about high blood pressure. Also inquire about a family history of hypertension.
Confirm any elevation in the child's blood pressure during a physical examination. If the patient has severe hypertension, it is usually time to refer the child to a specialist.
If the child has hypertension for three consecutive monthly visits, further evaluation with blood work is appropriate. Order a complete metabolic panel, urinalysis, and fasting lipid panel. Urinalysis, for example, is useful as a screen for type 2 diabetes.
On a full review of systems, identify other morbidities associated with obesity and perform appropriate tests.
For instance, the child with daytime sleepiness and snoring may require a sleep study to identify obstructive sleep apnea.
In addition, if liver function tests are elevated, a pediatric ultrasound exam can identify a fatty liver.
You can also order an electrocardiogram to identify heart pathology and refer the child if the findings are abnormal.
Many families request thyroid testing for an overweight child. Full thyroid function tests are not cost effective and need not be done. A thyroid-stimulating hormone test should suffice.
As for behavioral counseling, at the Cleveland Clinic Children's Hospital, we recommend our “5 to GO!” messaging, in which children are told to eat 5-a-day fruits and veggies; give 4 compliments a day to anyone they encounter, including other kids, and get 4 compliments a day from anyone; consume 3 dairy products a day; engage in no more than 2 hours of media/TV time a day; drink 0 sugar-sweetened beverages, and go!
For teenagers, we aim for 4 dairy/calcium servings and 3 compliments a day (not that they need fewer compliments, but they do need more calcium than the under age 10 crowd).
The key is to follow patients monthly. Slow, steady change—with positive motivation tailored to each family—works better than trying to do everything at once.
Follow up, follow up, and follow up—with a lot of cheerleading!
Patient education is also essential. Help patients and their families figure out how to cook a no-added-salt diet, how to shop the periphery of a grocery store where the fresh produce is located, and how to build physical activity and exercise into the family's daily plan.
Consider a weight management program such as our Fit Youth Program. Patients and families who participate in this 12-week program at the Cleveland Clinic receive group counseling sessions led by a psychologist in combination with a pediatrician, a dietitian, and an exercise physiologist.
Multidisciplinary interventions such as this one can accomplish modest weight loss versus progression toward 30 pounds of weight gain per year, as occurs in many of our children who do not receive effective treatment.
When presented with an overweight child who has hypertension, collect a detailed history, including a 24-hour food-intake history.
Also assess the child's nutritional habits, such as number of fast-food items typically eaten per week and number of family dinners.
Ask about the fluids these children generally consume. For instance, do they drink any caloric beverages other than low-fat milk?
Take an exercise history. Inquire how many hours per day the child is exposed to television, video games, and other media.
Social interaction can be particularly important with an overweight child. Ask if the child has been teased or bullied at home, in school, or elsewhere in the community.
Next ask the parent(s) and patient what they know about high blood pressure. Also inquire about a family history of hypertension.
Confirm any elevation in the child's blood pressure during a physical examination. If the patient has severe hypertension, it is usually time to refer the child to a specialist.
If the child has hypertension for three consecutive monthly visits, further evaluation with blood work is appropriate. Order a complete metabolic panel, urinalysis, and fasting lipid panel. Urinalysis, for example, is useful as a screen for type 2 diabetes.
On a full review of systems, identify other morbidities associated with obesity and perform appropriate tests.
For instance, the child with daytime sleepiness and snoring may require a sleep study to identify obstructive sleep apnea.
In addition, if liver function tests are elevated, a pediatric ultrasound exam can identify a fatty liver.
You can also order an electrocardiogram to identify heart pathology and refer the child if the findings are abnormal.
Many families request thyroid testing for an overweight child. Full thyroid function tests are not cost effective and need not be done. A thyroid-stimulating hormone test should suffice.
As for behavioral counseling, at the Cleveland Clinic Children's Hospital, we recommend our “5 to GO!” messaging, in which children are told to eat 5-a-day fruits and veggies; give 4 compliments a day to anyone they encounter, including other kids, and get 4 compliments a day from anyone; consume 3 dairy products a day; engage in no more than 2 hours of media/TV time a day; drink 0 sugar-sweetened beverages, and go!
For teenagers, we aim for 4 dairy/calcium servings and 3 compliments a day (not that they need fewer compliments, but they do need more calcium than the under age 10 crowd).
The key is to follow patients monthly. Slow, steady change—with positive motivation tailored to each family—works better than trying to do everything at once.
Follow up, follow up, and follow up—with a lot of cheerleading!
Patient education is also essential. Help patients and their families figure out how to cook a no-added-salt diet, how to shop the periphery of a grocery store where the fresh produce is located, and how to build physical activity and exercise into the family's daily plan.
Consider a weight management program such as our Fit Youth Program. Patients and families who participate in this 12-week program at the Cleveland Clinic receive group counseling sessions led by a psychologist in combination with a pediatrician, a dietitian, and an exercise physiologist.
Multidisciplinary interventions such as this one can accomplish modest weight loss versus progression toward 30 pounds of weight gain per year, as occurs in many of our children who do not receive effective treatment.
When presented with an overweight child who has hypertension, collect a detailed history, including a 24-hour food-intake history.
Also assess the child's nutritional habits, such as number of fast-food items typically eaten per week and number of family dinners.
Ask about the fluids these children generally consume. For instance, do they drink any caloric beverages other than low-fat milk?
Take an exercise history. Inquire how many hours per day the child is exposed to television, video games, and other media.
Social interaction can be particularly important with an overweight child. Ask if the child has been teased or bullied at home, in school, or elsewhere in the community.
Next ask the parent(s) and patient what they know about high blood pressure. Also inquire about a family history of hypertension.
Confirm any elevation in the child's blood pressure during a physical examination. If the patient has severe hypertension, it is usually time to refer the child to a specialist.
If the child has hypertension for three consecutive monthly visits, further evaluation with blood work is appropriate. Order a complete metabolic panel, urinalysis, and fasting lipid panel. Urinalysis, for example, is useful as a screen for type 2 diabetes.
On a full review of systems, identify other morbidities associated with obesity and perform appropriate tests.
For instance, the child with daytime sleepiness and snoring may require a sleep study to identify obstructive sleep apnea.
In addition, if liver function tests are elevated, a pediatric ultrasound exam can identify a fatty liver.
You can also order an electrocardiogram to identify heart pathology and refer the child if the findings are abnormal.
Many families request thyroid testing for an overweight child. Full thyroid function tests are not cost effective and need not be done. A thyroid-stimulating hormone test should suffice.
As for behavioral counseling, at the Cleveland Clinic Children's Hospital, we recommend our “5 to GO!” messaging, in which children are told to eat 5-a-day fruits and veggies; give 4 compliments a day to anyone they encounter, including other kids, and get 4 compliments a day from anyone; consume 3 dairy products a day; engage in no more than 2 hours of media/TV time a day; drink 0 sugar-sweetened beverages, and go!
For teenagers, we aim for 4 dairy/calcium servings and 3 compliments a day (not that they need fewer compliments, but they do need more calcium than the under age 10 crowd).
The key is to follow patients monthly. Slow, steady change—with positive motivation tailored to each family—works better than trying to do everything at once.
Follow up, follow up, and follow up—with a lot of cheerleading!
Patient education is also essential. Help patients and their families figure out how to cook a no-added-salt diet, how to shop the periphery of a grocery store where the fresh produce is located, and how to build physical activity and exercise into the family's daily plan.
Consider a weight management program such as our Fit Youth Program. Patients and families who participate in this 12-week program at the Cleveland Clinic receive group counseling sessions led by a psychologist in combination with a pediatrician, a dietitian, and an exercise physiologist.
Multidisciplinary interventions such as this one can accomplish modest weight loss versus progression toward 30 pounds of weight gain per year, as occurs in many of our children who do not receive effective treatment.
Mentorship Matters
A hospitalist-led initiative to boost the implementation of glycemic controls has exceeded initial goals at an Alabama hospital, an early sign of success for the SHM-sponsored pilot program.
Steven C. Smith, MD, FHM, medical director of hospitalist services at Healthcare Authority for Medical West in Bessemer, Ala., says that after the Glycemic Control Mentored Implementation (GCMI) program was put in place earlier this year, his group set a two-week goal of 5% utilization of the program's evidence-based order set. He also set a three-month goal of 25% compliance with the order set.
"Much to my surprise, we achieved 16% utilization at two weeks," Dr. Smith says, adding that three-month data are still being tabulated. "The involvement by SHM is what made the difference. Being able to tell people on the medical staff that this is part of a national-level QI project, we're participating in something bigger—that made a big difference in getting people interested in the order set."
Representatives from several of the 30 pilot sites have reported similar success in the early stages of the yearlong project. Among other issues, the GCMI program tackles subcutaneous insulin protocols, transition from subcutaneous to infusion, care coordination, improving follow-up care, and hypoglycemia management.
Although institutions entered the program in April, Dr. Smith and his colleagues didn't begin their formal mentoring relationship until July. Since then, his HM group has stayed in touch with program mentors through teleconferences and direct e-mail exchanges. The service also has access to a data-aggregation system through the Yale Center for Medical Informatics, which encourages more attention and utilization of newly created order sets.
"We are in the process of collecting data from a period of about one year prior to our project and comparing that to data since the implementation of our order set," Dr. Smith says. The analysis "will allow us, and our mentor, to tailor our efforts to our particular institution in an ongoing fashion. The ongoing measures of our success with this project will include outcomes measures like length of stay, cost of stay, mortality and morbidity, ICU length of stay, ventilator days, and others."
A hospitalist-led initiative to boost the implementation of glycemic controls has exceeded initial goals at an Alabama hospital, an early sign of success for the SHM-sponsored pilot program.
Steven C. Smith, MD, FHM, medical director of hospitalist services at Healthcare Authority for Medical West in Bessemer, Ala., says that after the Glycemic Control Mentored Implementation (GCMI) program was put in place earlier this year, his group set a two-week goal of 5% utilization of the program's evidence-based order set. He also set a three-month goal of 25% compliance with the order set.
"Much to my surprise, we achieved 16% utilization at two weeks," Dr. Smith says, adding that three-month data are still being tabulated. "The involvement by SHM is what made the difference. Being able to tell people on the medical staff that this is part of a national-level QI project, we're participating in something bigger—that made a big difference in getting people interested in the order set."
Representatives from several of the 30 pilot sites have reported similar success in the early stages of the yearlong project. Among other issues, the GCMI program tackles subcutaneous insulin protocols, transition from subcutaneous to infusion, care coordination, improving follow-up care, and hypoglycemia management.
Although institutions entered the program in April, Dr. Smith and his colleagues didn't begin their formal mentoring relationship until July. Since then, his HM group has stayed in touch with program mentors through teleconferences and direct e-mail exchanges. The service also has access to a data-aggregation system through the Yale Center for Medical Informatics, which encourages more attention and utilization of newly created order sets.
"We are in the process of collecting data from a period of about one year prior to our project and comparing that to data since the implementation of our order set," Dr. Smith says. The analysis "will allow us, and our mentor, to tailor our efforts to our particular institution in an ongoing fashion. The ongoing measures of our success with this project will include outcomes measures like length of stay, cost of stay, mortality and morbidity, ICU length of stay, ventilator days, and others."
A hospitalist-led initiative to boost the implementation of glycemic controls has exceeded initial goals at an Alabama hospital, an early sign of success for the SHM-sponsored pilot program.
Steven C. Smith, MD, FHM, medical director of hospitalist services at Healthcare Authority for Medical West in Bessemer, Ala., says that after the Glycemic Control Mentored Implementation (GCMI) program was put in place earlier this year, his group set a two-week goal of 5% utilization of the program's evidence-based order set. He also set a three-month goal of 25% compliance with the order set.
"Much to my surprise, we achieved 16% utilization at two weeks," Dr. Smith says, adding that three-month data are still being tabulated. "The involvement by SHM is what made the difference. Being able to tell people on the medical staff that this is part of a national-level QI project, we're participating in something bigger—that made a big difference in getting people interested in the order set."
Representatives from several of the 30 pilot sites have reported similar success in the early stages of the yearlong project. Among other issues, the GCMI program tackles subcutaneous insulin protocols, transition from subcutaneous to infusion, care coordination, improving follow-up care, and hypoglycemia management.
Although institutions entered the program in April, Dr. Smith and his colleagues didn't begin their formal mentoring relationship until July. Since then, his HM group has stayed in touch with program mentors through teleconferences and direct e-mail exchanges. The service also has access to a data-aggregation system through the Yale Center for Medical Informatics, which encourages more attention and utilization of newly created order sets.
"We are in the process of collecting data from a period of about one year prior to our project and comparing that to data since the implementation of our order set," Dr. Smith says. The analysis "will allow us, and our mentor, to tailor our efforts to our particular institution in an ongoing fashion. The ongoing measures of our success with this project will include outcomes measures like length of stay, cost of stay, mortality and morbidity, ICU length of stay, ventilator days, and others."
In the Literature: Research You Need to Know
Clinical question: What factors are associated with methicillin-resistant Staphylococcus aureus (MRSA) carriage at hospital discharge to home health care, prolonged MRSA colonization, and MRSA transmission to household contacts?
Background: Previous studies have reported prolonged colonization with hospital-acquired MRSA after discharge, as well as transmission of MRSA to household contacts. However, the rates and risk factors for MRSA carriage and transmission are unknown.
Study design: Multicenter prospective cohort.
Setting: Home-health-care system associated with 47 public teaching hospitals in France.
Synopsis: More than 1,500 hospitalized adult patients with planned discharge to home health care were screened for MRSA colonization, and 12.7% were MRSA-positive by nasal or skin lesion swab.
Factors independently associated with MRSA carriage at hospital discharge were chronic skin lesions, older age, longer duration of hospitalization, and neurologic and cardiovascular primary diagnoses.
Surveillance sampling for up to one year showed that approximately half of these patients had clearance of MRSA, with a median time to clearance of 282 days. Lack of self-sufficiency in daily activities was associated with persistent MRSA carriage.
Nineteen percent of household contacts acquired MRSA during the study period. Risks included older age and providing healthcare to the index patient.
Bottom line: Hospital-acquired MRSA colonization is prevalent at discharge to home health settings, is frequently prolonged, and commonly results in transmission to household contacts, particularly those providing healthcare to the index patient.
Citation: Lucet JC, Paoletti X, Demontpion C, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med. 2009;169(15):1372-1378.
—Reviewed for TH eWire by Kelly Cunningham, MD, Elizabeth Rice, MD, Eduard Vasilevskis, MD, Joshua LaBrin, MD, Kelly Sopko, MD, Shelley Ellis, MD, MPH, and Sunil Kripalani, MD, MSc, Section of Hospital Medicine, Vanderbilt University, Nashville, Tenn.
Clinical question: What factors are associated with methicillin-resistant Staphylococcus aureus (MRSA) carriage at hospital discharge to home health care, prolonged MRSA colonization, and MRSA transmission to household contacts?
Background: Previous studies have reported prolonged colonization with hospital-acquired MRSA after discharge, as well as transmission of MRSA to household contacts. However, the rates and risk factors for MRSA carriage and transmission are unknown.
Study design: Multicenter prospective cohort.
Setting: Home-health-care system associated with 47 public teaching hospitals in France.
Synopsis: More than 1,500 hospitalized adult patients with planned discharge to home health care were screened for MRSA colonization, and 12.7% were MRSA-positive by nasal or skin lesion swab.
Factors independently associated with MRSA carriage at hospital discharge were chronic skin lesions, older age, longer duration of hospitalization, and neurologic and cardiovascular primary diagnoses.
Surveillance sampling for up to one year showed that approximately half of these patients had clearance of MRSA, with a median time to clearance of 282 days. Lack of self-sufficiency in daily activities was associated with persistent MRSA carriage.
Nineteen percent of household contacts acquired MRSA during the study period. Risks included older age and providing healthcare to the index patient.
Bottom line: Hospital-acquired MRSA colonization is prevalent at discharge to home health settings, is frequently prolonged, and commonly results in transmission to household contacts, particularly those providing healthcare to the index patient.
Citation: Lucet JC, Paoletti X, Demontpion C, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med. 2009;169(15):1372-1378.
—Reviewed for TH eWire by Kelly Cunningham, MD, Elizabeth Rice, MD, Eduard Vasilevskis, MD, Joshua LaBrin, MD, Kelly Sopko, MD, Shelley Ellis, MD, MPH, and Sunil Kripalani, MD, MSc, Section of Hospital Medicine, Vanderbilt University, Nashville, Tenn.
Clinical question: What factors are associated with methicillin-resistant Staphylococcus aureus (MRSA) carriage at hospital discharge to home health care, prolonged MRSA colonization, and MRSA transmission to household contacts?
Background: Previous studies have reported prolonged colonization with hospital-acquired MRSA after discharge, as well as transmission of MRSA to household contacts. However, the rates and risk factors for MRSA carriage and transmission are unknown.
Study design: Multicenter prospective cohort.
Setting: Home-health-care system associated with 47 public teaching hospitals in France.
Synopsis: More than 1,500 hospitalized adult patients with planned discharge to home health care were screened for MRSA colonization, and 12.7% were MRSA-positive by nasal or skin lesion swab.
Factors independently associated with MRSA carriage at hospital discharge were chronic skin lesions, older age, longer duration of hospitalization, and neurologic and cardiovascular primary diagnoses.
Surveillance sampling for up to one year showed that approximately half of these patients had clearance of MRSA, with a median time to clearance of 282 days. Lack of self-sufficiency in daily activities was associated with persistent MRSA carriage.
Nineteen percent of household contacts acquired MRSA during the study period. Risks included older age and providing healthcare to the index patient.
Bottom line: Hospital-acquired MRSA colonization is prevalent at discharge to home health settings, is frequently prolonged, and commonly results in transmission to household contacts, particularly those providing healthcare to the index patient.
Citation: Lucet JC, Paoletti X, Demontpion C, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med. 2009;169(15):1372-1378.
—Reviewed for TH eWire by Kelly Cunningham, MD, Elizabeth Rice, MD, Eduard Vasilevskis, MD, Joshua LaBrin, MD, Kelly Sopko, MD, Shelley Ellis, MD, MPH, and Sunil Kripalani, MD, MSc, Section of Hospital Medicine, Vanderbilt University, Nashville, Tenn.
Communication, interdisciplinary approaches key to effective transitions
Improving patient discharges and reducing preventable "bounce-back" readmissions will have increasing implications for hospitals’ bottom lines—especially if proposed Medicare payment bundling reforms are adopted by Congress. Discharge improvement also enhances a hospital’s reputation with patients and providers, says Neil Gupta, MD, a hospitalist at the University of California at San Francisco.
"I think we all recognize in our daily practice that there are things we can do better for patients at discharge," Dr. Gupta says. "It's sometimes hard to get the resources and motivation to do this. But we know it could really impact patient care and make it better."
Dr. Gupta's hospital is one of 24 participating in SHM's Project BOOST (Better Outcomes for Older Adults through Safe Transitions). The mentorship program is developing a consensus and resources for best practices in patient discharges. National data show that about one in five hospitalized Medicare patients are readmitted within 30 days. While some of these readmissions are appropriate, the sheer quantity of readmissions shows room for improvement, says Dr. Gupta's colleague, Arpana Vidyarthi, MD, a hospitalist and director of quality at UCSF. "In reality, no one in the United States is doing it very well," she says.
Drs. Gupta and Vidyarthi suggest focusing your HM group's communication with primary-care physicians (PCPs): Study whether the hospitalist's messages are getting through to the PCPs and ask for their feedback. Other targets should include identifying high-risk patients, reconciling medications, scheduling the patient's first outpatient visit prior to discharge, and confirming the patient's understanding of the discharge plan.
The first step is to form an interdisciplinary team that approaches discharges as a QI project, Dr. Gupta explains. "Building that team is huge," he says. "It adds a whole new perspective." At UCSF, the team meets monthly and includes hospitalists, PCPs, staff nurses and nursing supervisors, pharmacists, care managers, and patients.
For more information about Project BOOST, visit SHM's resource room.
Improving patient discharges and reducing preventable "bounce-back" readmissions will have increasing implications for hospitals’ bottom lines—especially if proposed Medicare payment bundling reforms are adopted by Congress. Discharge improvement also enhances a hospital’s reputation with patients and providers, says Neil Gupta, MD, a hospitalist at the University of California at San Francisco.
"I think we all recognize in our daily practice that there are things we can do better for patients at discharge," Dr. Gupta says. "It's sometimes hard to get the resources and motivation to do this. But we know it could really impact patient care and make it better."
Dr. Gupta's hospital is one of 24 participating in SHM's Project BOOST (Better Outcomes for Older Adults through Safe Transitions). The mentorship program is developing a consensus and resources for best practices in patient discharges. National data show that about one in five hospitalized Medicare patients are readmitted within 30 days. While some of these readmissions are appropriate, the sheer quantity of readmissions shows room for improvement, says Dr. Gupta's colleague, Arpana Vidyarthi, MD, a hospitalist and director of quality at UCSF. "In reality, no one in the United States is doing it very well," she says.
Drs. Gupta and Vidyarthi suggest focusing your HM group's communication with primary-care physicians (PCPs): Study whether the hospitalist's messages are getting through to the PCPs and ask for their feedback. Other targets should include identifying high-risk patients, reconciling medications, scheduling the patient's first outpatient visit prior to discharge, and confirming the patient's understanding of the discharge plan.
The first step is to form an interdisciplinary team that approaches discharges as a QI project, Dr. Gupta explains. "Building that team is huge," he says. "It adds a whole new perspective." At UCSF, the team meets monthly and includes hospitalists, PCPs, staff nurses and nursing supervisors, pharmacists, care managers, and patients.
For more information about Project BOOST, visit SHM's resource room.
Improving patient discharges and reducing preventable "bounce-back" readmissions will have increasing implications for hospitals’ bottom lines—especially if proposed Medicare payment bundling reforms are adopted by Congress. Discharge improvement also enhances a hospital’s reputation with patients and providers, says Neil Gupta, MD, a hospitalist at the University of California at San Francisco.
"I think we all recognize in our daily practice that there are things we can do better for patients at discharge," Dr. Gupta says. "It's sometimes hard to get the resources and motivation to do this. But we know it could really impact patient care and make it better."
Dr. Gupta's hospital is one of 24 participating in SHM's Project BOOST (Better Outcomes for Older Adults through Safe Transitions). The mentorship program is developing a consensus and resources for best practices in patient discharges. National data show that about one in five hospitalized Medicare patients are readmitted within 30 days. While some of these readmissions are appropriate, the sheer quantity of readmissions shows room for improvement, says Dr. Gupta's colleague, Arpana Vidyarthi, MD, a hospitalist and director of quality at UCSF. "In reality, no one in the United States is doing it very well," she says.
Drs. Gupta and Vidyarthi suggest focusing your HM group's communication with primary-care physicians (PCPs): Study whether the hospitalist's messages are getting through to the PCPs and ask for their feedback. Other targets should include identifying high-risk patients, reconciling medications, scheduling the patient's first outpatient visit prior to discharge, and confirming the patient's understanding of the discharge plan.
The first step is to form an interdisciplinary team that approaches discharges as a QI project, Dr. Gupta explains. "Building that team is huge," he says. "It adds a whole new perspective." At UCSF, the team meets monthly and includes hospitalists, PCPs, staff nurses and nursing supervisors, pharmacists, care managers, and patients.
For more information about Project BOOST, visit SHM's resource room.
Mission: Medicine
Every six months, physicians and nurses from Geisinger Medical Center in Danville, Pa., travel to a rural area outside of Chiquimula, Guatemala, to care for people who live without access to modern medicine. Lice, tooth decay, poor eyesight, and asthma are common. Some of the locals suffer from more severe health issues, including parasitic infections, pneumonia, malnutrition, and malaria.
Joel L. Strohecker, DO, a family-medicine-trained hospitalist practicing at Lutheran Medical Center and St. Anthony Hospital in Denver, joined the Geisinger group in June. He recently spoke with TH eWire about his weeklong trip.
Question: About 70% of your patients were children. When did you really feel like you were able to help?
Answer: A couple of little kids who had asthma were brought back a couple days later and were so much better. And the joint injections you could do for the men who worked in the fields. Those were gratifying experiences because they immediately feel better. Then they bring their families the next day because we’ve done something good for them.
Q: You mentioned one memorable patient, a woman with a spinal cord injury. Tell me more about her.
A: She's someone that brought tears to my eyes. It had taken her a couple of hours to walk the trails to get there. She had fallen 20 years earlier and had never been able to really walk well since then. It was very obvious, just by her exam, that she had an upper cervical spinal cord injury. It was just so sad. I mean, what can you do? We gave her steroids, but she had diminished function in her legs.
Q: What was the most rewarding part of the trip?
A: The work was tiring and not necessarily uplifting at times, but I think the best part for me was when we would go back to Chiquimula every night. … We would all go back and play soccer. For me, that was probably the biggest treat and most enjoyable time of the trip. You weren't just a tourist; you were really embedded with the people.
For more information about volunteer opportunities in Guatemala, contact Dr. Strohecker at [email protected].
Every six months, physicians and nurses from Geisinger Medical Center in Danville, Pa., travel to a rural area outside of Chiquimula, Guatemala, to care for people who live without access to modern medicine. Lice, tooth decay, poor eyesight, and asthma are common. Some of the locals suffer from more severe health issues, including parasitic infections, pneumonia, malnutrition, and malaria.
Joel L. Strohecker, DO, a family-medicine-trained hospitalist practicing at Lutheran Medical Center and St. Anthony Hospital in Denver, joined the Geisinger group in June. He recently spoke with TH eWire about his weeklong trip.
Question: About 70% of your patients were children. When did you really feel like you were able to help?
Answer: A couple of little kids who had asthma were brought back a couple days later and were so much better. And the joint injections you could do for the men who worked in the fields. Those were gratifying experiences because they immediately feel better. Then they bring their families the next day because we’ve done something good for them.
Q: You mentioned one memorable patient, a woman with a spinal cord injury. Tell me more about her.
A: She's someone that brought tears to my eyes. It had taken her a couple of hours to walk the trails to get there. She had fallen 20 years earlier and had never been able to really walk well since then. It was very obvious, just by her exam, that she had an upper cervical spinal cord injury. It was just so sad. I mean, what can you do? We gave her steroids, but she had diminished function in her legs.
Q: What was the most rewarding part of the trip?
A: The work was tiring and not necessarily uplifting at times, but I think the best part for me was when we would go back to Chiquimula every night. … We would all go back and play soccer. For me, that was probably the biggest treat and most enjoyable time of the trip. You weren't just a tourist; you were really embedded with the people.
For more information about volunteer opportunities in Guatemala, contact Dr. Strohecker at [email protected].
Every six months, physicians and nurses from Geisinger Medical Center in Danville, Pa., travel to a rural area outside of Chiquimula, Guatemala, to care for people who live without access to modern medicine. Lice, tooth decay, poor eyesight, and asthma are common. Some of the locals suffer from more severe health issues, including parasitic infections, pneumonia, malnutrition, and malaria.
Joel L. Strohecker, DO, a family-medicine-trained hospitalist practicing at Lutheran Medical Center and St. Anthony Hospital in Denver, joined the Geisinger group in June. He recently spoke with TH eWire about his weeklong trip.
Question: About 70% of your patients were children. When did you really feel like you were able to help?
Answer: A couple of little kids who had asthma were brought back a couple days later and were so much better. And the joint injections you could do for the men who worked in the fields. Those were gratifying experiences because they immediately feel better. Then they bring their families the next day because we’ve done something good for them.
Q: You mentioned one memorable patient, a woman with a spinal cord injury. Tell me more about her.
A: She's someone that brought tears to my eyes. It had taken her a couple of hours to walk the trails to get there. She had fallen 20 years earlier and had never been able to really walk well since then. It was very obvious, just by her exam, that she had an upper cervical spinal cord injury. It was just so sad. I mean, what can you do? We gave her steroids, but she had diminished function in her legs.
Q: What was the most rewarding part of the trip?
A: The work was tiring and not necessarily uplifting at times, but I think the best part for me was when we would go back to Chiquimula every night. … We would all go back and play soccer. For me, that was probably the biggest treat and most enjoyable time of the trip. You weren't just a tourist; you were really embedded with the people.
For more information about volunteer opportunities in Guatemala, contact Dr. Strohecker at [email protected].
Medicare Cuts Could Hit HM Hard
The final 2010 Medicare physician fee schedule presents a mixed bag for hospitalists. As officials from the Centers for Medicare and Medicaid Services (CMS) warned, the update carries a hefty 21.2% fee schedule cut. Congressional action to avert that cut is expected, though wrangling over healthcare reform may force a stopgap measure to prevent the cuts from taking effect Jan. 1.
In a statement, Jonathan Blum, director of the CMS Center for Medicare Management, said the Obama administration is committed to repealing the sustainable growth rate formula that resulted in the substantial cut. In the meantime, he said, CMS is finalizing its proposal to drop physician-administered drugs from the definition of "physician services," which is used to formulate future fee updates. SHM has strongly supported both efforts and is calling on members to contact their legislators before a Nov. 16 vote.
Another huge change for hospitalists: The use of consultation codes has been discontinued, with the exception of codes related to telemedicine. In their place, healthcare providers must bill under initial hospital care, initial nursing facility care, or initial office visits. All transfers of care, for example, will now require billing under an initial visit code rather than a subsequent visit code. Consultation documentation requirements will no longer apply, though initial codes could be valued somewhat lower than similar consultation codes despite proposed adjustments to the relative value units (RVUs). Although bad for traditional consultations, some analysts see the net change as good for the comanagement of patients.
To help smooth the transition to this new coding system, SHM will be hosting a webinar, "Hot Topics in Evaluation and Management Coding," on Dec. 2.
The final 2010 Medicare physician fee schedule presents a mixed bag for hospitalists. As officials from the Centers for Medicare and Medicaid Services (CMS) warned, the update carries a hefty 21.2% fee schedule cut. Congressional action to avert that cut is expected, though wrangling over healthcare reform may force a stopgap measure to prevent the cuts from taking effect Jan. 1.
In a statement, Jonathan Blum, director of the CMS Center for Medicare Management, said the Obama administration is committed to repealing the sustainable growth rate formula that resulted in the substantial cut. In the meantime, he said, CMS is finalizing its proposal to drop physician-administered drugs from the definition of "physician services," which is used to formulate future fee updates. SHM has strongly supported both efforts and is calling on members to contact their legislators before a Nov. 16 vote.
Another huge change for hospitalists: The use of consultation codes has been discontinued, with the exception of codes related to telemedicine. In their place, healthcare providers must bill under initial hospital care, initial nursing facility care, or initial office visits. All transfers of care, for example, will now require billing under an initial visit code rather than a subsequent visit code. Consultation documentation requirements will no longer apply, though initial codes could be valued somewhat lower than similar consultation codes despite proposed adjustments to the relative value units (RVUs). Although bad for traditional consultations, some analysts see the net change as good for the comanagement of patients.
To help smooth the transition to this new coding system, SHM will be hosting a webinar, "Hot Topics in Evaluation and Management Coding," on Dec. 2.
The final 2010 Medicare physician fee schedule presents a mixed bag for hospitalists. As officials from the Centers for Medicare and Medicaid Services (CMS) warned, the update carries a hefty 21.2% fee schedule cut. Congressional action to avert that cut is expected, though wrangling over healthcare reform may force a stopgap measure to prevent the cuts from taking effect Jan. 1.
In a statement, Jonathan Blum, director of the CMS Center for Medicare Management, said the Obama administration is committed to repealing the sustainable growth rate formula that resulted in the substantial cut. In the meantime, he said, CMS is finalizing its proposal to drop physician-administered drugs from the definition of "physician services," which is used to formulate future fee updates. SHM has strongly supported both efforts and is calling on members to contact their legislators before a Nov. 16 vote.
Another huge change for hospitalists: The use of consultation codes has been discontinued, with the exception of codes related to telemedicine. In their place, healthcare providers must bill under initial hospital care, initial nursing facility care, or initial office visits. All transfers of care, for example, will now require billing under an initial visit code rather than a subsequent visit code. Consultation documentation requirements will no longer apply, though initial codes could be valued somewhat lower than similar consultation codes despite proposed adjustments to the relative value units (RVUs). Although bad for traditional consultations, some analysts see the net change as good for the comanagement of patients.
To help smooth the transition to this new coding system, SHM will be hosting a webinar, "Hot Topics in Evaluation and Management Coding," on Dec. 2.
Academics Energized
Frank Marquez, MD, wasn't told he was going to the Academic Hospitalist Academy (AHA) until a couple of days before the start of the four-day training program in Atlanta. Short notice aside, Dr. Marquez was elated he was able to attend.
"There are a lot of practical tips. For me, the biggest thing is the academy has shown me that I have to stop being passive and start being proactive. I need to take an active role, serve on committees," says Dr. Marquez, a third-year academic hospitalist at St. Joseph's Hospital and Medical Center in Phoenix. "I think that's going to help my career."
Dr. Marquez, who leads a five-person team of residents, interns, and medical students, was one of nearly 80 early-career physicians—the average attendee had two years of HM experience—who attended AHA. The inaugural event was co-sponsored by SHM, the Society of General Internal Medicine and the Associate Chiefs of General Internal Medicine. The program featured top-flight HM faculty, but Dr. Marquez especially enjoyed the emphasis on small-group workshops and interactive teaching.
"When we first took our jobs as hospitalists, no one took the time to explain to us how to be an effective leader, mentor people, implement change," he says. "Here you have an opportunity to learn that and to participate. It’s not a lecture; it’s not intimidating. You can speak up."
Frank Marquez, MD, wasn't told he was going to the Academic Hospitalist Academy (AHA) until a couple of days before the start of the four-day training program in Atlanta. Short notice aside, Dr. Marquez was elated he was able to attend.
"There are a lot of practical tips. For me, the biggest thing is the academy has shown me that I have to stop being passive and start being proactive. I need to take an active role, serve on committees," says Dr. Marquez, a third-year academic hospitalist at St. Joseph's Hospital and Medical Center in Phoenix. "I think that's going to help my career."
Dr. Marquez, who leads a five-person team of residents, interns, and medical students, was one of nearly 80 early-career physicians—the average attendee had two years of HM experience—who attended AHA. The inaugural event was co-sponsored by SHM, the Society of General Internal Medicine and the Associate Chiefs of General Internal Medicine. The program featured top-flight HM faculty, but Dr. Marquez especially enjoyed the emphasis on small-group workshops and interactive teaching.
"When we first took our jobs as hospitalists, no one took the time to explain to us how to be an effective leader, mentor people, implement change," he says. "Here you have an opportunity to learn that and to participate. It’s not a lecture; it’s not intimidating. You can speak up."
Frank Marquez, MD, wasn't told he was going to the Academic Hospitalist Academy (AHA) until a couple of days before the start of the four-day training program in Atlanta. Short notice aside, Dr. Marquez was elated he was able to attend.
"There are a lot of practical tips. For me, the biggest thing is the academy has shown me that I have to stop being passive and start being proactive. I need to take an active role, serve on committees," says Dr. Marquez, a third-year academic hospitalist at St. Joseph's Hospital and Medical Center in Phoenix. "I think that's going to help my career."
Dr. Marquez, who leads a five-person team of residents, interns, and medical students, was one of nearly 80 early-career physicians—the average attendee had two years of HM experience—who attended AHA. The inaugural event was co-sponsored by SHM, the Society of General Internal Medicine and the Associate Chiefs of General Internal Medicine. The program featured top-flight HM faculty, but Dr. Marquez especially enjoyed the emphasis on small-group workshops and interactive teaching.
"When we first took our jobs as hospitalists, no one took the time to explain to us how to be an effective leader, mentor people, implement change," he says. "Here you have an opportunity to learn that and to participate. It’s not a lecture; it’s not intimidating. You can speak up."
Make the Diagnosis
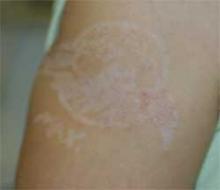
Diagnosis: Contact Dermatitis to Paraphenylenediamine
The patient’s mother reported blisters, erythema, and scabbing in the area of the tattoo. Six months later, the patient underwent paraphenylenediamine patch testing and exhibited a reaction.
The patient was treated with mild topical steroids and a 4-day prednisone course prior to presentation. A week of clobetasol ointment improved the pruritus and erythema.
Henna is a green powdered extract derived from the leaves of the Lawsonia alba plant. The active ingredient is lawsone. Middle Eastern and Indian cultures use the extract to dye the hair, skin, and nails. Contact with the skin for an extended period of time yields a brownish orange pigment. In Western countries, Henna tattoos have gained popularity as a temporary alternative to ink tattoos.
Henna may be used in its pure form, however, paraphenylenediamine (PPD) is often added to darken the pigment, expedite drying time, and improve design accuracy. PPD is an allergen found in hair dyes and photographic film processing. It is a potent T-cell stimulator, and its efficacy is directly related to concentration and duration of exposure. Patch tests among individuals with henna contact dermatitis are negative to pure henna powder but react strongly to PPD, which has lead to the assumption that PPD is the main allergen in henna paste.
Henna tattoo inks have been found to have PPD concentrations as high as 15%-30%, and, often, the inks are in contact with the skin for several days after application. The hypersensitivity can sensitize individuals to PPD-containing substances such as dark hair dyes and dark clothing. Cross reaction may cause hypersensitivity to natural rubber latex, azo dyes, thiurams, PABA sunscreen, para-aminosalicylic acid, and benzocaine.
The initial inflammatory response may present as erythematous, eczematous, pruritic, or papulovesicular eruption in the area or boundary of the original design. Edema, anaphylaxis, and collapse are less common manifestations. The inflammation can result in scarring, keloid formation, and permanent, post-inflammatory pigment changes.
As demonstrated in my patient, hypopigmentation occurs more frequently in children than adults. Therapy includes protection of the blistered area, antihistamines, treatment of infection, and aggressive topical corticosteroid therapy.
This case was first presented at Maryland Derm, at the University of Maryland School of Medicine in Baltimore, by Dr. Martin, Dr. Vera David, and Dr. Anthony Gaspari.
Diagnosis: Contact Dermatitis to Paraphenylenediamine
The patient’s mother reported blisters, erythema, and scabbing in the area of the tattoo. Six months later, the patient underwent paraphenylenediamine patch testing and exhibited a reaction.
The patient was treated with mild topical steroids and a 4-day prednisone course prior to presentation. A week of clobetasol ointment improved the pruritus and erythema.
Henna is a green powdered extract derived from the leaves of the Lawsonia alba plant. The active ingredient is lawsone. Middle Eastern and Indian cultures use the extract to dye the hair, skin, and nails. Contact with the skin for an extended period of time yields a brownish orange pigment. In Western countries, Henna tattoos have gained popularity as a temporary alternative to ink tattoos.
Henna may be used in its pure form, however, paraphenylenediamine (PPD) is often added to darken the pigment, expedite drying time, and improve design accuracy. PPD is an allergen found in hair dyes and photographic film processing. It is a potent T-cell stimulator, and its efficacy is directly related to concentration and duration of exposure. Patch tests among individuals with henna contact dermatitis are negative to pure henna powder but react strongly to PPD, which has lead to the assumption that PPD is the main allergen in henna paste.
Henna tattoo inks have been found to have PPD concentrations as high as 15%-30%, and, often, the inks are in contact with the skin for several days after application. The hypersensitivity can sensitize individuals to PPD-containing substances such as dark hair dyes and dark clothing. Cross reaction may cause hypersensitivity to natural rubber latex, azo dyes, thiurams, PABA sunscreen, para-aminosalicylic acid, and benzocaine.
The initial inflammatory response may present as erythematous, eczematous, pruritic, or papulovesicular eruption in the area or boundary of the original design. Edema, anaphylaxis, and collapse are less common manifestations. The inflammation can result in scarring, keloid formation, and permanent, post-inflammatory pigment changes.
As demonstrated in my patient, hypopigmentation occurs more frequently in children than adults. Therapy includes protection of the blistered area, antihistamines, treatment of infection, and aggressive topical corticosteroid therapy.
This case was first presented at Maryland Derm, at the University of Maryland School of Medicine in Baltimore, by Dr. Martin, Dr. Vera David, and Dr. Anthony Gaspari.
Diagnosis: Contact Dermatitis to Paraphenylenediamine
The patient’s mother reported blisters, erythema, and scabbing in the area of the tattoo. Six months later, the patient underwent paraphenylenediamine patch testing and exhibited a reaction.
The patient was treated with mild topical steroids and a 4-day prednisone course prior to presentation. A week of clobetasol ointment improved the pruritus and erythema.
Henna is a green powdered extract derived from the leaves of the Lawsonia alba plant. The active ingredient is lawsone. Middle Eastern and Indian cultures use the extract to dye the hair, skin, and nails. Contact with the skin for an extended period of time yields a brownish orange pigment. In Western countries, Henna tattoos have gained popularity as a temporary alternative to ink tattoos.
Henna may be used in its pure form, however, paraphenylenediamine (PPD) is often added to darken the pigment, expedite drying time, and improve design accuracy. PPD is an allergen found in hair dyes and photographic film processing. It is a potent T-cell stimulator, and its efficacy is directly related to concentration and duration of exposure. Patch tests among individuals with henna contact dermatitis are negative to pure henna powder but react strongly to PPD, which has lead to the assumption that PPD is the main allergen in henna paste.
Henna tattoo inks have been found to have PPD concentrations as high as 15%-30%, and, often, the inks are in contact with the skin for several days after application. The hypersensitivity can sensitize individuals to PPD-containing substances such as dark hair dyes and dark clothing. Cross reaction may cause hypersensitivity to natural rubber latex, azo dyes, thiurams, PABA sunscreen, para-aminosalicylic acid, and benzocaine.
The initial inflammatory response may present as erythematous, eczematous, pruritic, or papulovesicular eruption in the area or boundary of the original design. Edema, anaphylaxis, and collapse are less common manifestations. The inflammation can result in scarring, keloid formation, and permanent, post-inflammatory pigment changes.
As demonstrated in my patient, hypopigmentation occurs more frequently in children than adults. Therapy includes protection of the blistered area, antihistamines, treatment of infection, and aggressive topical corticosteroid therapy.
This case was first presented at Maryland Derm, at the University of Maryland School of Medicine in Baltimore, by Dr. Martin, Dr. Vera David, and Dr. Anthony Gaspari.
VTE Prophylaxis Compliance Lacking
Only one in six medical discharges receives venous thromboembolism (VTE) prophylaxis that conforms to the seventh American College of Chest Physicians (ACCP) guidelines, according to a report in the Journal of Hospital Medicine.
The study reported that, overall, 65.9% of medical discharges and 77.7% of surgical discharges received at least one order for VTE prophylaxis during hospitalization. However, when ACCP guidelines for type, dose, and duration are overlaid on the same data set, the percentage of "appropriate prophylaxis" dropped to 16.4% for medical discharges and 12.7% for surgical discharges (JHM 2009;doi 10.1002/jhm.526).
"If we're going to be in the business of healthcare safety and quality … that's not good enough," says lead investigator Alpesh Amin, MD, MBA, FHM, FACP, professor and chairman of the Department of Medicine and executive director of the hospitalist program at the University of California at Irvine. "We're only doing it appropriately [part] of the time."
Dr. Amin has turned VTE research into an area of focus, and is in San Diego today presenting two additional VTE studies at CHEST 2009. One study, "Analysis of Inpatient and Outpatient Venous Thromboembolism Prophylaxis Patterns in U.S. Critical Care Patients," found that of 1,279 discharges analyzed, only 4% continued prophylaxis. The other study, "VTE Prophylaxis Across the Continuum of Care in U.S. Medical and Surgical Patients at Risk of Venous Thromboembolism," reported nearly 90% of patients received no outpatient prophylaxis.
All three studies were supported by Sanofi-Aventis U.S. Inc. The CHEST 2009 presentations have not been published yet. Dr. Amin says the studies show hospitalists can take charge of VTE orders to assure treatment is delivered in line with approved protocols.
"The idea of these studies was to say, 'We've got these national recommendations; how well are we actually doing?' " Dr. Amin says. "You can do something, but you ought to do it according to national guidelines."
For more information on the essential elements of VTE prevention and performance improvement, visit SHM's VTE Resource Room.
Only one in six medical discharges receives venous thromboembolism (VTE) prophylaxis that conforms to the seventh American College of Chest Physicians (ACCP) guidelines, according to a report in the Journal of Hospital Medicine.
The study reported that, overall, 65.9% of medical discharges and 77.7% of surgical discharges received at least one order for VTE prophylaxis during hospitalization. However, when ACCP guidelines for type, dose, and duration are overlaid on the same data set, the percentage of "appropriate prophylaxis" dropped to 16.4% for medical discharges and 12.7% for surgical discharges (JHM 2009;doi 10.1002/jhm.526).
"If we're going to be in the business of healthcare safety and quality … that's not good enough," says lead investigator Alpesh Amin, MD, MBA, FHM, FACP, professor and chairman of the Department of Medicine and executive director of the hospitalist program at the University of California at Irvine. "We're only doing it appropriately [part] of the time."
Dr. Amin has turned VTE research into an area of focus, and is in San Diego today presenting two additional VTE studies at CHEST 2009. One study, "Analysis of Inpatient and Outpatient Venous Thromboembolism Prophylaxis Patterns in U.S. Critical Care Patients," found that of 1,279 discharges analyzed, only 4% continued prophylaxis. The other study, "VTE Prophylaxis Across the Continuum of Care in U.S. Medical and Surgical Patients at Risk of Venous Thromboembolism," reported nearly 90% of patients received no outpatient prophylaxis.
All three studies were supported by Sanofi-Aventis U.S. Inc. The CHEST 2009 presentations have not been published yet. Dr. Amin says the studies show hospitalists can take charge of VTE orders to assure treatment is delivered in line with approved protocols.
"The idea of these studies was to say, 'We've got these national recommendations; how well are we actually doing?' " Dr. Amin says. "You can do something, but you ought to do it according to national guidelines."
For more information on the essential elements of VTE prevention and performance improvement, visit SHM's VTE Resource Room.
Only one in six medical discharges receives venous thromboembolism (VTE) prophylaxis that conforms to the seventh American College of Chest Physicians (ACCP) guidelines, according to a report in the Journal of Hospital Medicine.
The study reported that, overall, 65.9% of medical discharges and 77.7% of surgical discharges received at least one order for VTE prophylaxis during hospitalization. However, when ACCP guidelines for type, dose, and duration are overlaid on the same data set, the percentage of "appropriate prophylaxis" dropped to 16.4% for medical discharges and 12.7% for surgical discharges (JHM 2009;doi 10.1002/jhm.526).
"If we're going to be in the business of healthcare safety and quality … that's not good enough," says lead investigator Alpesh Amin, MD, MBA, FHM, FACP, professor and chairman of the Department of Medicine and executive director of the hospitalist program at the University of California at Irvine. "We're only doing it appropriately [part] of the time."
Dr. Amin has turned VTE research into an area of focus, and is in San Diego today presenting two additional VTE studies at CHEST 2009. One study, "Analysis of Inpatient and Outpatient Venous Thromboembolism Prophylaxis Patterns in U.S. Critical Care Patients," found that of 1,279 discharges analyzed, only 4% continued prophylaxis. The other study, "VTE Prophylaxis Across the Continuum of Care in U.S. Medical and Surgical Patients at Risk of Venous Thromboembolism," reported nearly 90% of patients received no outpatient prophylaxis.
All three studies were supported by Sanofi-Aventis U.S. Inc. The CHEST 2009 presentations have not been published yet. Dr. Amin says the studies show hospitalists can take charge of VTE orders to assure treatment is delivered in line with approved protocols.
"The idea of these studies was to say, 'We've got these national recommendations; how well are we actually doing?' " Dr. Amin says. "You can do something, but you ought to do it according to national guidelines."
For more information on the essential elements of VTE prevention and performance improvement, visit SHM's VTE Resource Room.
In the Literature: Research You Need to Know
Clinical question: Does PR prolongation have any clinical significance in ambulatory adults?
Background: Several studies have suggested that first-degree atrio-ventricular block (AVB) is associated with a benign prognosis. However, these studies were based on young, active men in the military. Another study, which was based on middle-aged men, has suggested that AVB may be associated with coronary artery disease. Little is known about AVB prognosis in ambulatory individuals older than 20 years of age.
Study design: Prospective cohort study.
Setting: Community-hospital-based patients.
Synopsis: A subset population of 7,575 individuals older than 20 from the Framingham Heart Study showed that a prolonged PR interval of more than 200 msec is associated with an increased risk of atrial fibrillation/flutter, pacemaker implantation, and all-cause mortality.
When adjusted for age, sex, cardiovascular disease status, body mass index, hypertension, smoking, diabetes, and ratio of total to high-density lipoprotein cholesterol, individuals with first-degree AVB had a twofold adjusted risk of atrial fibrillation (HR, 2.06; 95% CI, 1.36-3.12; P<0.001), a threefold adjusted risk of pacemaker implantation (HR, 2.89; 95% CI, 1.83-4.57; P<0.001), and 1.4-fold adjusted risk of all-cause mortality (HR, 1.44, 95% CI, 1.09-1.91; P=0.01).
This study was confounded by the usual limitations of the Framingham Study Database. Most notably, this study focused specifically on ambulatory patients with prolonged PR interval demonstrated on routine electrocardiogram and, therefore, does not account for factors commonly related to the inpatient setting, such as electrolyte abnormalities. Hospitalists should neither prognosticate nor plan more frequent follow-up for patients based on a prolonged PR interval based on an EKG obtained during acute illness.
Bottom line: PR prolongation is associated with increased risks of atrial fibrillation/flutter, pacemaker implantation, and all-cause mortality in ambulatory adults.
Citation: Cheng S, Keyes M, Larson M, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301(24):2571-2577.
—Reviewed for The Hospitalist by Robert Chang, MD; Nabil Alkhoury-Fallouh, MD; Anita Hart, MD; Hae-won Kim, MD; Francis McBee-Orzulak, MD; Helena Pasieka, MD; Division of General Medicine, University of Michigan, Ann Arbor
Clinical question: Does PR prolongation have any clinical significance in ambulatory adults?
Background: Several studies have suggested that first-degree atrio-ventricular block (AVB) is associated with a benign prognosis. However, these studies were based on young, active men in the military. Another study, which was based on middle-aged men, has suggested that AVB may be associated with coronary artery disease. Little is known about AVB prognosis in ambulatory individuals older than 20 years of age.
Study design: Prospective cohort study.
Setting: Community-hospital-based patients.
Synopsis: A subset population of 7,575 individuals older than 20 from the Framingham Heart Study showed that a prolonged PR interval of more than 200 msec is associated with an increased risk of atrial fibrillation/flutter, pacemaker implantation, and all-cause mortality.
When adjusted for age, sex, cardiovascular disease status, body mass index, hypertension, smoking, diabetes, and ratio of total to high-density lipoprotein cholesterol, individuals with first-degree AVB had a twofold adjusted risk of atrial fibrillation (HR, 2.06; 95% CI, 1.36-3.12; P<0.001), a threefold adjusted risk of pacemaker implantation (HR, 2.89; 95% CI, 1.83-4.57; P<0.001), and 1.4-fold adjusted risk of all-cause mortality (HR, 1.44, 95% CI, 1.09-1.91; P=0.01).
This study was confounded by the usual limitations of the Framingham Study Database. Most notably, this study focused specifically on ambulatory patients with prolonged PR interval demonstrated on routine electrocardiogram and, therefore, does not account for factors commonly related to the inpatient setting, such as electrolyte abnormalities. Hospitalists should neither prognosticate nor plan more frequent follow-up for patients based on a prolonged PR interval based on an EKG obtained during acute illness.
Bottom line: PR prolongation is associated with increased risks of atrial fibrillation/flutter, pacemaker implantation, and all-cause mortality in ambulatory adults.
Citation: Cheng S, Keyes M, Larson M, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301(24):2571-2577.
—Reviewed for The Hospitalist by Robert Chang, MD; Nabil Alkhoury-Fallouh, MD; Anita Hart, MD; Hae-won Kim, MD; Francis McBee-Orzulak, MD; Helena Pasieka, MD; Division of General Medicine, University of Michigan, Ann Arbor
Clinical question: Does PR prolongation have any clinical significance in ambulatory adults?
Background: Several studies have suggested that first-degree atrio-ventricular block (AVB) is associated with a benign prognosis. However, these studies were based on young, active men in the military. Another study, which was based on middle-aged men, has suggested that AVB may be associated with coronary artery disease. Little is known about AVB prognosis in ambulatory individuals older than 20 years of age.
Study design: Prospective cohort study.
Setting: Community-hospital-based patients.
Synopsis: A subset population of 7,575 individuals older than 20 from the Framingham Heart Study showed that a prolonged PR interval of more than 200 msec is associated with an increased risk of atrial fibrillation/flutter, pacemaker implantation, and all-cause mortality.
When adjusted for age, sex, cardiovascular disease status, body mass index, hypertension, smoking, diabetes, and ratio of total to high-density lipoprotein cholesterol, individuals with first-degree AVB had a twofold adjusted risk of atrial fibrillation (HR, 2.06; 95% CI, 1.36-3.12; P<0.001), a threefold adjusted risk of pacemaker implantation (HR, 2.89; 95% CI, 1.83-4.57; P<0.001), and 1.4-fold adjusted risk of all-cause mortality (HR, 1.44, 95% CI, 1.09-1.91; P=0.01).
This study was confounded by the usual limitations of the Framingham Study Database. Most notably, this study focused specifically on ambulatory patients with prolonged PR interval demonstrated on routine electrocardiogram and, therefore, does not account for factors commonly related to the inpatient setting, such as electrolyte abnormalities. Hospitalists should neither prognosticate nor plan more frequent follow-up for patients based on a prolonged PR interval based on an EKG obtained during acute illness.
Bottom line: PR prolongation is associated with increased risks of atrial fibrillation/flutter, pacemaker implantation, and all-cause mortality in ambulatory adults.
Citation: Cheng S, Keyes M, Larson M, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301(24):2571-2577.
—Reviewed for The Hospitalist by Robert Chang, MD; Nabil Alkhoury-Fallouh, MD; Anita Hart, MD; Hae-won Kim, MD; Francis McBee-Orzulak, MD; Helena Pasieka, MD; Division of General Medicine, University of Michigan, Ann Arbor