User login
Proceedings of the 4th Annual Perioperative Medicine Summit
Supplement Editor:
Amir K. Jaffer, MD, FHM
Associate Editors:
David L. Hepner, MD, and Franklin A. Michota, MD, FHM
Contents
Public reporting and pay-for-performance programs in perioperative medicine
Peter Lindenauer, MD MSc
Cardiac risk stratification for noncardiac surgery: Update from the American College of Cardiology/American Heart Association 2007 guidelines
Lee A. Fleisher, MD
Perioperative care of the elderly patient: An update
Robert M. Palmer, MD, MPH
The role of testing in the preoperative evaluation
David L. Hepner, MD
Perioperative fluid management: Progress despite lingering controversies
Mark A. Hamilton, MBBS, MRCP, FRCA
Giving anesthesiologists what they want: How to write a useful preoperative consult
David Lubarsky, MD, MBA, and Keith Candiotti, MD
Perioperative management of warfarin and antiplatelet therapy
Amir K. Jaffer, MD, FHM
Prevention of venous thromboembolism after surgery
Franklin A. Michota, MD, FHM
Perioperative management of diabetes: Translating evidence into practice
Luigi F. Meneghini, MD, MBA
Postoperative pulmonary complications: An update on risk assessment and reduction
Gerald W. Smetana, MD
Postoperative gastrointestinal tract dysfunction: An overview of causes and management strategies
Michael G. (Monty) Mythen, MD
Case studies in perioperative management: Challenges, controversies, and common ground
Steven L. Cohn, MD, and BobbieJean Sweitzer, MD
Statins and noncardiac surgery: Current evidence and practical considerations
Don Poldermans, MD, PhD
The experts debate: perioperative beta-blockade for noncardiac surgery patients—proven safe or not?
Don Poldermans, MD, PhD, and P.J. Devereaux, MD, PhD
Perioperative considerations for patients with liver disease
Paul Martin, MD
Perioperative management of obstructive sleep apnea: Ready for prime time?
Shirin Shafazand, MD, MS
Nuts and bolts of preoperative clinics: The view from three institutions
Angela M. Bader, MD, MPH; BobbieJean Sweitzer, MD; and Ajay Kumar, MD
Perioperative management of anemia: Limits of blood transfusion and alternatives to it
Ajay Kumar, MD
Medicolegal issues in perioperative medicine: Lessons from real cases
Franklin A. Michota, MD, FHM, and Matthew J. Donnelly, Esq
Perioperative medication management: General principles and practical applications
Christopher Whinney, MD
Supplement Editor:
Amir K. Jaffer, MD, FHM
Associate Editors:
David L. Hepner, MD, and Franklin A. Michota, MD, FHM
Contents
Public reporting and pay-for-performance programs in perioperative medicine
Peter Lindenauer, MD MSc
Cardiac risk stratification for noncardiac surgery: Update from the American College of Cardiology/American Heart Association 2007 guidelines
Lee A. Fleisher, MD
Perioperative care of the elderly patient: An update
Robert M. Palmer, MD, MPH
The role of testing in the preoperative evaluation
David L. Hepner, MD
Perioperative fluid management: Progress despite lingering controversies
Mark A. Hamilton, MBBS, MRCP, FRCA
Giving anesthesiologists what they want: How to write a useful preoperative consult
David Lubarsky, MD, MBA, and Keith Candiotti, MD
Perioperative management of warfarin and antiplatelet therapy
Amir K. Jaffer, MD, FHM
Prevention of venous thromboembolism after surgery
Franklin A. Michota, MD, FHM
Perioperative management of diabetes: Translating evidence into practice
Luigi F. Meneghini, MD, MBA
Postoperative pulmonary complications: An update on risk assessment and reduction
Gerald W. Smetana, MD
Postoperative gastrointestinal tract dysfunction: An overview of causes and management strategies
Michael G. (Monty) Mythen, MD
Case studies in perioperative management: Challenges, controversies, and common ground
Steven L. Cohn, MD, and BobbieJean Sweitzer, MD
Statins and noncardiac surgery: Current evidence and practical considerations
Don Poldermans, MD, PhD
The experts debate: perioperative beta-blockade for noncardiac surgery patients—proven safe or not?
Don Poldermans, MD, PhD, and P.J. Devereaux, MD, PhD
Perioperative considerations for patients with liver disease
Paul Martin, MD
Perioperative management of obstructive sleep apnea: Ready for prime time?
Shirin Shafazand, MD, MS
Nuts and bolts of preoperative clinics: The view from three institutions
Angela M. Bader, MD, MPH; BobbieJean Sweitzer, MD; and Ajay Kumar, MD
Perioperative management of anemia: Limits of blood transfusion and alternatives to it
Ajay Kumar, MD
Medicolegal issues in perioperative medicine: Lessons from real cases
Franklin A. Michota, MD, FHM, and Matthew J. Donnelly, Esq
Perioperative medication management: General principles and practical applications
Christopher Whinney, MD
Supplement Editor:
Amir K. Jaffer, MD, FHM
Associate Editors:
David L. Hepner, MD, and Franklin A. Michota, MD, FHM
Contents
Public reporting and pay-for-performance programs in perioperative medicine
Peter Lindenauer, MD MSc
Cardiac risk stratification for noncardiac surgery: Update from the American College of Cardiology/American Heart Association 2007 guidelines
Lee A. Fleisher, MD
Perioperative care of the elderly patient: An update
Robert M. Palmer, MD, MPH
The role of testing in the preoperative evaluation
David L. Hepner, MD
Perioperative fluid management: Progress despite lingering controversies
Mark A. Hamilton, MBBS, MRCP, FRCA
Giving anesthesiologists what they want: How to write a useful preoperative consult
David Lubarsky, MD, MBA, and Keith Candiotti, MD
Perioperative management of warfarin and antiplatelet therapy
Amir K. Jaffer, MD, FHM
Prevention of venous thromboembolism after surgery
Franklin A. Michota, MD, FHM
Perioperative management of diabetes: Translating evidence into practice
Luigi F. Meneghini, MD, MBA
Postoperative pulmonary complications: An update on risk assessment and reduction
Gerald W. Smetana, MD
Postoperative gastrointestinal tract dysfunction: An overview of causes and management strategies
Michael G. (Monty) Mythen, MD
Case studies in perioperative management: Challenges, controversies, and common ground
Steven L. Cohn, MD, and BobbieJean Sweitzer, MD
Statins and noncardiac surgery: Current evidence and practical considerations
Don Poldermans, MD, PhD
The experts debate: perioperative beta-blockade for noncardiac surgery patients—proven safe or not?
Don Poldermans, MD, PhD, and P.J. Devereaux, MD, PhD
Perioperative considerations for patients with liver disease
Paul Martin, MD
Perioperative management of obstructive sleep apnea: Ready for prime time?
Shirin Shafazand, MD, MS
Nuts and bolts of preoperative clinics: The view from three institutions
Angela M. Bader, MD, MPH; BobbieJean Sweitzer, MD; and Ajay Kumar, MD
Perioperative management of anemia: Limits of blood transfusion and alternatives to it
Ajay Kumar, MD
Medicolegal issues in perioperative medicine: Lessons from real cases
Franklin A. Michota, MD, FHM, and Matthew J. Donnelly, Esq
Perioperative medication management: General principles and practical applications
Christopher Whinney, MD
Beyond office sphygmomanometry: Ways to better assess blood pressure
Hypertension is difficult to diagnose, and its treatment is difficult to monitor optimally on the basis of traditional office blood pressure measurements. To better protect our patients from the effects of undiagnosed or poorly controlled hypertension, we need to consider other options, such as ambulatory 24-hour blood pressure monitoring, automated measurement in the office, measurement in the patient’s home, and devices that analyze the peripheral pulse wave to estimate the central blood pressure and other indices of arterial stiffness.
MANUAL OFFICE MEASUREMENT HAS INHERENT LIMITATIONS
Office blood pressure measurements do provide enormous information about cardiovascular risk and the risk of death, as shown in epidemiologic studies. A meta-analysis1 of 61 prospective observational studies that included more than 1 million patients showed that office blood pressure levels clearly correlate with increased risk of death from cardiovascular disease and stroke.
But blood pressure is a dynamic measure with inherent minute-to-minute variability, and measurement will not be accurate if the correct technique is not followed. Traditional office sphygmomanometry is a snapshot and does not accurately reflect a patient’s blood pressure in the real world and in real time.
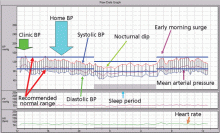
Recently, unique patterns of blood pressure have been identified that may not be detected in the physician’s office. It is clear from several clinical trials that some patients’ blood pressure is transiently elevated in the first few minutes during office measurements (the “white coat effect”). In addition, when office measurements are compared with out-of-office measurements, several patterns of hypertension emerge that have prognostic value. These patterns are white coat hypertension, masked hypertension, nocturnal hypertension, and failure of the blood pressure to dip during sleep.
WHITE COAT EFFECT
The white coat effect is described as a transient elevation in office blood pressure caused by an alerting reaction when the pressure is measured by a physician or a nurse. It may last for several minutes. The magnitude of blood pressure elevation has been noted to be higher when measured by a physician than when measured by a nurse. Multiple blood pressure measurements taken over 5 to 10 minutes help eliminate the white coat effect. In a recent study,2 36% of patients with hypertension demonstrated the white coat effect.
In a study by Mancia et al,3 46 patients underwent intra-arterial blood pressure monitoring for 2 days, during which time a physician or a nurse would check their blood pressure repeatedly over 10 minutes. This study found that most patients demonstrated the white coat effect: the blood pressure was higher in the first few measurements, but came down after 5 minutes. The white coat effect was as much as 22.6 ± 1.8 mm Hg when blood pressure was measured by a physician and was lower when measured by a nurse.
WHITE COAT HYPERTENSION
In contrast to the white coat effect, which is transient, white coat hypertension is defined as persistent elevation of office blood pressure measurements with normal blood pressure levels when measured outside the physician’s office. Depending on the population sampled, the prevalence of white coat hypertension ranges from 12% to 20%, but this is understandably difficult or almost impossible to detect with traditional office blood pressure measurements alone.4–7
MASKED HYPERTENSION
Patients with normal blood pressure in the physician’s office but high blood pressure during daily life were found to have a higher risk of cardiovascular events. This condition is called masked hypertension.8 For clinicians, the danger lies in underestimating the patient’s risk of cardiovascular events and, thus, undertreating the hypertension. Preliminary data on masked hypertension show that the rates of end-organ damage and cardiovascular events are slightly higher in patients with masked hypertension than in patients with sustained hypertension.
NOCTURNAL HYPERTENSION
Elevated nighttime blood pressure (>125/75 mm Hg) is considered nocturnal hypertension and is generally considered a subgroup of masked hypertension.9
In the African American Study of Kidney Disease and Hypertension (AASK),10,11 although most patients achieved their blood pressure goal during the trial, they were noted to have relentless progression of renal disease. On ambulatory 24-hour blood pressure monitoring during the cohort phase of the study,10 a high prevalence of elevated nighttime blood pressure (66%) was found. Further analysis showed that the elevated nighttime blood pressure was associated with worse hypertension-related end-organ damage. It is still unclear if lowering nighttime blood pressure improves clinical outcomes in this high-risk population.
DIPPING VS NONDIPPING
The mean blood pressure during sleep should normally decrease by 10% to 20% compared with daytime readings. “Nondipping,” ie, the lack of this nocturnal dip in blood pressure, carries a higher risk of death from cardiovascular causes, even if the person is otherwise normotensive.12,13 Nondipping is commonly noted in African Americans, patients with diabetes, and those with chronic kidney disease.
A study by Lurbe et al14 of patients with type 1 diabetes mellitus who underwent ambulatory 24-hour blood pressure monitoring found that the onset of the nondipping phenomenon preceded microalbuminuria (a risk factor for kidney disease). Data from our institution15 showed that nondipping was associated with a greater decline in glomerular filtration rate when compared with dipping.
The lack of reproducibility of a person’s dipping status has been a barrier in relying on this as a prognostic measure. White and Larocca16 found that only about half of the patients who appeared to be nondippers on one 24-hour recording still were nondippers on a second recording 4 to 8 weeks later. Compared with nondipping, nocturnal hypertension is a more stable blood pressure pattern that is being increasingly recognized in patients undergoing 24-hour blood pressure monitoring.
AUTOMATIC BLOOD PRESSURE DEVICES
An automated in-office blood pressure measurement device is one way to minimize the white coat effect and obtain a more accurate blood pressure assessment. Devices such as BpTRU (BpTRU Medical Devices Ltd, Coquitlam, BC, Canada) are programmed to take a series of automatic, oscillometric readings at regular intervals while the patient is left alone in a quiet room. BpTRU has been validated in several clinical trials and has been shown to overcome the white coat effect to some extent. Myers et al17 compared 24-hour blood pressure readings with those obtained by a family physician, by a research technician, and by the BpTRU device and found that the BpTRU readings were much closer to the average of awake blood pressure readings on 24-hour blood pressure monitoring.
AMBULATORY 24-HOUR BLOOD PRESSURE MONITORING
- Presence or absence of the nocturnal dip (the normal 10% to 20% drop in blood pressure at night during sleep)
- Morning surge (which in some studies was associated with higher incidence of stroke)
- Supine hypertension and sudden fluctuations in blood pressure seen in patients with autonomic failure.
Studies have shown that basing antihypertensive therapy on ambulatory 24-hour blood pressure monitoring results in better control of hypertension and lowers the rate of cardiovascular events.18,19
Perloff et al18 found that in patients whose hypertension was considered well controlled on the basis of office blood pressure measurements, those with higher blood pressures on ambulatory 24-hour monitoring had higher cardiovascular morbidity and mortality rates.
More recently, Clement et al19 showed that patients being treated for hypertension who have higher average ambulatory 24-hour blood pressures had a higher risk of cardiovascular events and cardiovascular death.
After following 790 patients for 3.7 years, Verdecchia et al20 concluded that controlling hypertension on the basis of ambulatory 24-hour blood pressure readings rather than traditional office measurements lowered the risk of cardiovascular disease.
‘Normal’ blood pressure on ambulatory 24-hour monitoring
It should be noted that the normal average blood pressure on ambulatory 24-hour monitoring tends to be lower than that on traditional office readings. According to the 2007 European guidelines,21 an average 24-hour blood pressure above the range of 125/80 to 130/80 mm Hg is considered diagnostic of hypertension.
The bottom line on ambulatory 24-hour monitoring: Not perfect, but helpful
Ambulatory 24-hour blood pressure monitoring is not perfect. It interferes with the patient’s activities and with sleep, and this can affect the readings. It is also expensive, and Medicare and Medicaid cover it only if the patient is diagnosed with white coat hypertension, based on stringent criteria that include three elevated clinic blood pressure measurements and two normal out-of-clinic blood pressure measurements and no evidence of end-organ damage. Despite these issues, almost all national guidelines for the management of hypertension recommend ambulatory 24-hour blood pressure monitoring to improve cardiovascular risk prediction and to measure the variability in blood pressure levels.
USING THE INTERNET IN MANAGING HYPERTENSION
Green et al22 studied a new model of care using home blood pressure monitoring via the Internet, and provided feedback and intervention to the patient via a pharmacist to achieve blood pressure goals. Patients measured their blood pressure at home on at least 2 days a week (two measurements each time), using an automatic oscillometric monitor (Omron Hem-705-CP, Kyoto, Japan), and entered the results in an electronic medical record on the Internet. In the intervention group, a pharmacist communicated with each patient by either phone or e-mail every 2 weeks, making changes to their antihypertensive regimens as needed.
Patients in the intervention group had an average reduction in blood pressure of 14 mm Hg from baseline, and their blood pressure was much better controlled compared with the control groups, who were being passively monitored or were receiving usual care based on office blood pressure readings.
MEASURING ARTERIAL STIFFNESS TO ASSES RISK OF END-ORGAN DAMAGE
Mean arterial blood pressure, derived from the extremes of systolic and diastolic pressure as measured with a traditional sphygmomanometer, is a product of cardiac output and total peripheral vascular resistance. In contrast, central aortic blood pressure, the central augmentation index, and pulse wave velocity are measures derived from brachial blood pressure as well as arterial pulse wave tracings. They provide additional information on arterial stiffness and help stratify patients at increased cardiovascular risk.
The art of evaluating the arterial pulse wave with the fingertips while examining a patient and diagnosing various ailments was well known and practiced by ancient Greek and Chinese physicians. Although this was less recognized in Western medicine, it was the pulse wave recording on a sphygmograph that was used to measure human blood pressure in the 19th century.23 In the early 20th century, this art was lost with the invention of the mercury sphygmomanometer.
Arterial stiffness indices—ie, central aortic blood pressure, the central augmentation index, and pulse wave velocity—can now be measured noninvasively and have been shown to correlate very well with measurements obtained via a central arterial catheter. In the past, the only way to measure central blood pressure was directly via a central arterial catheter. New devices now measure arterial stiffness indices indirectly by applanation tonometry and pulse wave analysis (reviewed by O’Rourke et al25).
Several trials have shown that these arterial indices have a better prognostic value than the mean arterial pressure or the brachial pulse pressure. For example, the Baltimore Longitudinal Study of Aging26 followed 100 normotensive individuals for 5 years and found that those with a higher pulse wave velocity had a greater chance of developing incident hypertension. Other studies showed that pulse wave velocity and other indices of arterial stiffness are associated with dysfunction of the microvasculature in the brain, with higher cardiovascular risk, and a higher risk of death.
A major limitation in measuring these arterial stiffness indices is that they are derived values and require measurement of brachial blood pressure in addition to the pulse wave tracing.
Recent hypertension guidelines21,27,28 released during the past 2 years in Europe, Latin America, and Japan have recommended measurement of arterial stiffness as part of a comprehensive evaluation of patients with hypertension.
EXCITING TIMES IN HYPERTENSION
These are exciting times in the field of hypertension. With advances in technology, we have new devices and techniques that provide a closer view of the hemodynamic changes and blood pressures experienced by vital organs. In addition, we can now go beyond the physician’s office and evaluate blood pressure changes that occur during the course of a usual day in a patient’s life. This enables us to make better decisions in the management of their hypertension, embodying Dr. Harvey Cushing’s teaching that the physician’s obligation is to “view the man in his world.”29
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Agespecific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Mancia G, Parati G, Pomidossi G, Grassi G, Casadei R, Zanchetti A. Alerting reaction and rise in blood pressure during measurement by physician and nurse. Hypertension 1987; 9:209–215.
- Mancia G, Sega R, Bravi C, et al. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens 1995; 13:1377–1390.
- Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol 2005; 46:508–515.
- Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension.” Am J Hypertens 2008; 21:393–399.
- Obara T, Ohkubo T, Funahashi J, et al. Isolated uncontrolled hypertension at home and in the office among treated hypertensive patients from the J-HOME study. J Hypertens 2005; 23:1653–1660.
- Pickering TG DK, Rafey MA, Schwartz J, Gerin W. Masked hypertension: are those with normal office but elevated ambulatory blood pressure at risk? J Hypertens 2002; 20( suppl 4):176.
- Pickering TG, Hall JE, Appel LJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20:2183–2189.
- Brotman DJ, Davidson MB, Boumitri M, Vidt DG. Impaired diurnal blood pressure variation and all-cause mortality. Am J Hypertens 2008; 21:92–97.
- Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002; 347:797–805.
- Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glo-merular filtration rate. Arch Intern Med 2006; 166:846–852.
- White WB, Larocca GM. Improving the utility of the nocturnal hypertension definition by using absolute sleep blood pressure rather than the “dipping” proportion. Am J Cardiol 2003; 92:1439–1441.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Perloff D, Sokolow M, Cowan R. The prognostic value of ambulatory blood pressures. JAMA 1983; 249:2792–2798.
- Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 2003; 348:2407–2415.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Mansia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 2007; 16:135–232.
- Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008; 299:2857–2867.
- Mohamed F. On chronic Bright’s disease, and its essential symptoms. Lancet 1879; 1:399–401.
- Liew Y, Rafey MA, Allam S, Arrigain S, Butler R, Schreiber M. Blood pressure goals and arterial stiffness in chronic kidney disease. J Clin Hypertens (Greenwich) 2009; 11:201–206.
- O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol 2001; 51:507–522.
- Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 2008; 51:1377–1383.
- Sanchez RA, Ayala M, Baglivo H, et al. Latin American guidelines on hypertension. J Hypertens 2009; 27:905–922.
- Japanese Society of Hypertension. The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension: Measurement and clinical evaluation of blood pressure. Hypertens Res 2009; 32:11–23.
- Dubos RJ. Man Adapting. New Haven, CT: Yale University Press, 1980.
Hypertension is difficult to diagnose, and its treatment is difficult to monitor optimally on the basis of traditional office blood pressure measurements. To better protect our patients from the effects of undiagnosed or poorly controlled hypertension, we need to consider other options, such as ambulatory 24-hour blood pressure monitoring, automated measurement in the office, measurement in the patient’s home, and devices that analyze the peripheral pulse wave to estimate the central blood pressure and other indices of arterial stiffness.
MANUAL OFFICE MEASUREMENT HAS INHERENT LIMITATIONS
Office blood pressure measurements do provide enormous information about cardiovascular risk and the risk of death, as shown in epidemiologic studies. A meta-analysis1 of 61 prospective observational studies that included more than 1 million patients showed that office blood pressure levels clearly correlate with increased risk of death from cardiovascular disease and stroke.
But blood pressure is a dynamic measure with inherent minute-to-minute variability, and measurement will not be accurate if the correct technique is not followed. Traditional office sphygmomanometry is a snapshot and does not accurately reflect a patient’s blood pressure in the real world and in real time.
Recently, unique patterns of blood pressure have been identified that may not be detected in the physician’s office. It is clear from several clinical trials that some patients’ blood pressure is transiently elevated in the first few minutes during office measurements (the “white coat effect”). In addition, when office measurements are compared with out-of-office measurements, several patterns of hypertension emerge that have prognostic value. These patterns are white coat hypertension, masked hypertension, nocturnal hypertension, and failure of the blood pressure to dip during sleep.
WHITE COAT EFFECT
The white coat effect is described as a transient elevation in office blood pressure caused by an alerting reaction when the pressure is measured by a physician or a nurse. It may last for several minutes. The magnitude of blood pressure elevation has been noted to be higher when measured by a physician than when measured by a nurse. Multiple blood pressure measurements taken over 5 to 10 minutes help eliminate the white coat effect. In a recent study,2 36% of patients with hypertension demonstrated the white coat effect.
In a study by Mancia et al,3 46 patients underwent intra-arterial blood pressure monitoring for 2 days, during which time a physician or a nurse would check their blood pressure repeatedly over 10 minutes. This study found that most patients demonstrated the white coat effect: the blood pressure was higher in the first few measurements, but came down after 5 minutes. The white coat effect was as much as 22.6 ± 1.8 mm Hg when blood pressure was measured by a physician and was lower when measured by a nurse.
WHITE COAT HYPERTENSION
In contrast to the white coat effect, which is transient, white coat hypertension is defined as persistent elevation of office blood pressure measurements with normal blood pressure levels when measured outside the physician’s office. Depending on the population sampled, the prevalence of white coat hypertension ranges from 12% to 20%, but this is understandably difficult or almost impossible to detect with traditional office blood pressure measurements alone.4–7
MASKED HYPERTENSION
Patients with normal blood pressure in the physician’s office but high blood pressure during daily life were found to have a higher risk of cardiovascular events. This condition is called masked hypertension.8 For clinicians, the danger lies in underestimating the patient’s risk of cardiovascular events and, thus, undertreating the hypertension. Preliminary data on masked hypertension show that the rates of end-organ damage and cardiovascular events are slightly higher in patients with masked hypertension than in patients with sustained hypertension.
NOCTURNAL HYPERTENSION
Elevated nighttime blood pressure (>125/75 mm Hg) is considered nocturnal hypertension and is generally considered a subgroup of masked hypertension.9
In the African American Study of Kidney Disease and Hypertension (AASK),10,11 although most patients achieved their blood pressure goal during the trial, they were noted to have relentless progression of renal disease. On ambulatory 24-hour blood pressure monitoring during the cohort phase of the study,10 a high prevalence of elevated nighttime blood pressure (66%) was found. Further analysis showed that the elevated nighttime blood pressure was associated with worse hypertension-related end-organ damage. It is still unclear if lowering nighttime blood pressure improves clinical outcomes in this high-risk population.
DIPPING VS NONDIPPING
The mean blood pressure during sleep should normally decrease by 10% to 20% compared with daytime readings. “Nondipping,” ie, the lack of this nocturnal dip in blood pressure, carries a higher risk of death from cardiovascular causes, even if the person is otherwise normotensive.12,13 Nondipping is commonly noted in African Americans, patients with diabetes, and those with chronic kidney disease.
A study by Lurbe et al14 of patients with type 1 diabetes mellitus who underwent ambulatory 24-hour blood pressure monitoring found that the onset of the nondipping phenomenon preceded microalbuminuria (a risk factor for kidney disease). Data from our institution15 showed that nondipping was associated with a greater decline in glomerular filtration rate when compared with dipping.
The lack of reproducibility of a person’s dipping status has been a barrier in relying on this as a prognostic measure. White and Larocca16 found that only about half of the patients who appeared to be nondippers on one 24-hour recording still were nondippers on a second recording 4 to 8 weeks later. Compared with nondipping, nocturnal hypertension is a more stable blood pressure pattern that is being increasingly recognized in patients undergoing 24-hour blood pressure monitoring.
AUTOMATIC BLOOD PRESSURE DEVICES
An automated in-office blood pressure measurement device is one way to minimize the white coat effect and obtain a more accurate blood pressure assessment. Devices such as BpTRU (BpTRU Medical Devices Ltd, Coquitlam, BC, Canada) are programmed to take a series of automatic, oscillometric readings at regular intervals while the patient is left alone in a quiet room. BpTRU has been validated in several clinical trials and has been shown to overcome the white coat effect to some extent. Myers et al17 compared 24-hour blood pressure readings with those obtained by a family physician, by a research technician, and by the BpTRU device and found that the BpTRU readings were much closer to the average of awake blood pressure readings on 24-hour blood pressure monitoring.
AMBULATORY 24-HOUR BLOOD PRESSURE MONITORING
- Presence or absence of the nocturnal dip (the normal 10% to 20% drop in blood pressure at night during sleep)
- Morning surge (which in some studies was associated with higher incidence of stroke)
- Supine hypertension and sudden fluctuations in blood pressure seen in patients with autonomic failure.
Studies have shown that basing antihypertensive therapy on ambulatory 24-hour blood pressure monitoring results in better control of hypertension and lowers the rate of cardiovascular events.18,19
Perloff et al18 found that in patients whose hypertension was considered well controlled on the basis of office blood pressure measurements, those with higher blood pressures on ambulatory 24-hour monitoring had higher cardiovascular morbidity and mortality rates.
More recently, Clement et al19 showed that patients being treated for hypertension who have higher average ambulatory 24-hour blood pressures had a higher risk of cardiovascular events and cardiovascular death.
After following 790 patients for 3.7 years, Verdecchia et al20 concluded that controlling hypertension on the basis of ambulatory 24-hour blood pressure readings rather than traditional office measurements lowered the risk of cardiovascular disease.
‘Normal’ blood pressure on ambulatory 24-hour monitoring
It should be noted that the normal average blood pressure on ambulatory 24-hour monitoring tends to be lower than that on traditional office readings. According to the 2007 European guidelines,21 an average 24-hour blood pressure above the range of 125/80 to 130/80 mm Hg is considered diagnostic of hypertension.
The bottom line on ambulatory 24-hour monitoring: Not perfect, but helpful
Ambulatory 24-hour blood pressure monitoring is not perfect. It interferes with the patient’s activities and with sleep, and this can affect the readings. It is also expensive, and Medicare and Medicaid cover it only if the patient is diagnosed with white coat hypertension, based on stringent criteria that include three elevated clinic blood pressure measurements and two normal out-of-clinic blood pressure measurements and no evidence of end-organ damage. Despite these issues, almost all national guidelines for the management of hypertension recommend ambulatory 24-hour blood pressure monitoring to improve cardiovascular risk prediction and to measure the variability in blood pressure levels.
USING THE INTERNET IN MANAGING HYPERTENSION
Green et al22 studied a new model of care using home blood pressure monitoring via the Internet, and provided feedback and intervention to the patient via a pharmacist to achieve blood pressure goals. Patients measured their blood pressure at home on at least 2 days a week (two measurements each time), using an automatic oscillometric monitor (Omron Hem-705-CP, Kyoto, Japan), and entered the results in an electronic medical record on the Internet. In the intervention group, a pharmacist communicated with each patient by either phone or e-mail every 2 weeks, making changes to their antihypertensive regimens as needed.
Patients in the intervention group had an average reduction in blood pressure of 14 mm Hg from baseline, and their blood pressure was much better controlled compared with the control groups, who were being passively monitored or were receiving usual care based on office blood pressure readings.
MEASURING ARTERIAL STIFFNESS TO ASSES RISK OF END-ORGAN DAMAGE
Mean arterial blood pressure, derived from the extremes of systolic and diastolic pressure as measured with a traditional sphygmomanometer, is a product of cardiac output and total peripheral vascular resistance. In contrast, central aortic blood pressure, the central augmentation index, and pulse wave velocity are measures derived from brachial blood pressure as well as arterial pulse wave tracings. They provide additional information on arterial stiffness and help stratify patients at increased cardiovascular risk.
The art of evaluating the arterial pulse wave with the fingertips while examining a patient and diagnosing various ailments was well known and practiced by ancient Greek and Chinese physicians. Although this was less recognized in Western medicine, it was the pulse wave recording on a sphygmograph that was used to measure human blood pressure in the 19th century.23 In the early 20th century, this art was lost with the invention of the mercury sphygmomanometer.
Arterial stiffness indices—ie, central aortic blood pressure, the central augmentation index, and pulse wave velocity—can now be measured noninvasively and have been shown to correlate very well with measurements obtained via a central arterial catheter. In the past, the only way to measure central blood pressure was directly via a central arterial catheter. New devices now measure arterial stiffness indices indirectly by applanation tonometry and pulse wave analysis (reviewed by O’Rourke et al25).
Several trials have shown that these arterial indices have a better prognostic value than the mean arterial pressure or the brachial pulse pressure. For example, the Baltimore Longitudinal Study of Aging26 followed 100 normotensive individuals for 5 years and found that those with a higher pulse wave velocity had a greater chance of developing incident hypertension. Other studies showed that pulse wave velocity and other indices of arterial stiffness are associated with dysfunction of the microvasculature in the brain, with higher cardiovascular risk, and a higher risk of death.
A major limitation in measuring these arterial stiffness indices is that they are derived values and require measurement of brachial blood pressure in addition to the pulse wave tracing.
Recent hypertension guidelines21,27,28 released during the past 2 years in Europe, Latin America, and Japan have recommended measurement of arterial stiffness as part of a comprehensive evaluation of patients with hypertension.
EXCITING TIMES IN HYPERTENSION
These are exciting times in the field of hypertension. With advances in technology, we have new devices and techniques that provide a closer view of the hemodynamic changes and blood pressures experienced by vital organs. In addition, we can now go beyond the physician’s office and evaluate blood pressure changes that occur during the course of a usual day in a patient’s life. This enables us to make better decisions in the management of their hypertension, embodying Dr. Harvey Cushing’s teaching that the physician’s obligation is to “view the man in his world.”29
Hypertension is difficult to diagnose, and its treatment is difficult to monitor optimally on the basis of traditional office blood pressure measurements. To better protect our patients from the effects of undiagnosed or poorly controlled hypertension, we need to consider other options, such as ambulatory 24-hour blood pressure monitoring, automated measurement in the office, measurement in the patient’s home, and devices that analyze the peripheral pulse wave to estimate the central blood pressure and other indices of arterial stiffness.
MANUAL OFFICE MEASUREMENT HAS INHERENT LIMITATIONS
Office blood pressure measurements do provide enormous information about cardiovascular risk and the risk of death, as shown in epidemiologic studies. A meta-analysis1 of 61 prospective observational studies that included more than 1 million patients showed that office blood pressure levels clearly correlate with increased risk of death from cardiovascular disease and stroke.
But blood pressure is a dynamic measure with inherent minute-to-minute variability, and measurement will not be accurate if the correct technique is not followed. Traditional office sphygmomanometry is a snapshot and does not accurately reflect a patient’s blood pressure in the real world and in real time.
Recently, unique patterns of blood pressure have been identified that may not be detected in the physician’s office. It is clear from several clinical trials that some patients’ blood pressure is transiently elevated in the first few minutes during office measurements (the “white coat effect”). In addition, when office measurements are compared with out-of-office measurements, several patterns of hypertension emerge that have prognostic value. These patterns are white coat hypertension, masked hypertension, nocturnal hypertension, and failure of the blood pressure to dip during sleep.
WHITE COAT EFFECT
The white coat effect is described as a transient elevation in office blood pressure caused by an alerting reaction when the pressure is measured by a physician or a nurse. It may last for several minutes. The magnitude of blood pressure elevation has been noted to be higher when measured by a physician than when measured by a nurse. Multiple blood pressure measurements taken over 5 to 10 minutes help eliminate the white coat effect. In a recent study,2 36% of patients with hypertension demonstrated the white coat effect.
In a study by Mancia et al,3 46 patients underwent intra-arterial blood pressure monitoring for 2 days, during which time a physician or a nurse would check their blood pressure repeatedly over 10 minutes. This study found that most patients demonstrated the white coat effect: the blood pressure was higher in the first few measurements, but came down after 5 minutes. The white coat effect was as much as 22.6 ± 1.8 mm Hg when blood pressure was measured by a physician and was lower when measured by a nurse.
WHITE COAT HYPERTENSION
In contrast to the white coat effect, which is transient, white coat hypertension is defined as persistent elevation of office blood pressure measurements with normal blood pressure levels when measured outside the physician’s office. Depending on the population sampled, the prevalence of white coat hypertension ranges from 12% to 20%, but this is understandably difficult or almost impossible to detect with traditional office blood pressure measurements alone.4–7
MASKED HYPERTENSION
Patients with normal blood pressure in the physician’s office but high blood pressure during daily life were found to have a higher risk of cardiovascular events. This condition is called masked hypertension.8 For clinicians, the danger lies in underestimating the patient’s risk of cardiovascular events and, thus, undertreating the hypertension. Preliminary data on masked hypertension show that the rates of end-organ damage and cardiovascular events are slightly higher in patients with masked hypertension than in patients with sustained hypertension.
NOCTURNAL HYPERTENSION
Elevated nighttime blood pressure (>125/75 mm Hg) is considered nocturnal hypertension and is generally considered a subgroup of masked hypertension.9
In the African American Study of Kidney Disease and Hypertension (AASK),10,11 although most patients achieved their blood pressure goal during the trial, they were noted to have relentless progression of renal disease. On ambulatory 24-hour blood pressure monitoring during the cohort phase of the study,10 a high prevalence of elevated nighttime blood pressure (66%) was found. Further analysis showed that the elevated nighttime blood pressure was associated with worse hypertension-related end-organ damage. It is still unclear if lowering nighttime blood pressure improves clinical outcomes in this high-risk population.
DIPPING VS NONDIPPING
The mean blood pressure during sleep should normally decrease by 10% to 20% compared with daytime readings. “Nondipping,” ie, the lack of this nocturnal dip in blood pressure, carries a higher risk of death from cardiovascular causes, even if the person is otherwise normotensive.12,13 Nondipping is commonly noted in African Americans, patients with diabetes, and those with chronic kidney disease.
A study by Lurbe et al14 of patients with type 1 diabetes mellitus who underwent ambulatory 24-hour blood pressure monitoring found that the onset of the nondipping phenomenon preceded microalbuminuria (a risk factor for kidney disease). Data from our institution15 showed that nondipping was associated with a greater decline in glomerular filtration rate when compared with dipping.
The lack of reproducibility of a person’s dipping status has been a barrier in relying on this as a prognostic measure. White and Larocca16 found that only about half of the patients who appeared to be nondippers on one 24-hour recording still were nondippers on a second recording 4 to 8 weeks later. Compared with nondipping, nocturnal hypertension is a more stable blood pressure pattern that is being increasingly recognized in patients undergoing 24-hour blood pressure monitoring.
AUTOMATIC BLOOD PRESSURE DEVICES
An automated in-office blood pressure measurement device is one way to minimize the white coat effect and obtain a more accurate blood pressure assessment. Devices such as BpTRU (BpTRU Medical Devices Ltd, Coquitlam, BC, Canada) are programmed to take a series of automatic, oscillometric readings at regular intervals while the patient is left alone in a quiet room. BpTRU has been validated in several clinical trials and has been shown to overcome the white coat effect to some extent. Myers et al17 compared 24-hour blood pressure readings with those obtained by a family physician, by a research technician, and by the BpTRU device and found that the BpTRU readings were much closer to the average of awake blood pressure readings on 24-hour blood pressure monitoring.
AMBULATORY 24-HOUR BLOOD PRESSURE MONITORING
- Presence or absence of the nocturnal dip (the normal 10% to 20% drop in blood pressure at night during sleep)
- Morning surge (which in some studies was associated with higher incidence of stroke)
- Supine hypertension and sudden fluctuations in blood pressure seen in patients with autonomic failure.
Studies have shown that basing antihypertensive therapy on ambulatory 24-hour blood pressure monitoring results in better control of hypertension and lowers the rate of cardiovascular events.18,19
Perloff et al18 found that in patients whose hypertension was considered well controlled on the basis of office blood pressure measurements, those with higher blood pressures on ambulatory 24-hour monitoring had higher cardiovascular morbidity and mortality rates.
More recently, Clement et al19 showed that patients being treated for hypertension who have higher average ambulatory 24-hour blood pressures had a higher risk of cardiovascular events and cardiovascular death.
After following 790 patients for 3.7 years, Verdecchia et al20 concluded that controlling hypertension on the basis of ambulatory 24-hour blood pressure readings rather than traditional office measurements lowered the risk of cardiovascular disease.
‘Normal’ blood pressure on ambulatory 24-hour monitoring
It should be noted that the normal average blood pressure on ambulatory 24-hour monitoring tends to be lower than that on traditional office readings. According to the 2007 European guidelines,21 an average 24-hour blood pressure above the range of 125/80 to 130/80 mm Hg is considered diagnostic of hypertension.
The bottom line on ambulatory 24-hour monitoring: Not perfect, but helpful
Ambulatory 24-hour blood pressure monitoring is not perfect. It interferes with the patient’s activities and with sleep, and this can affect the readings. It is also expensive, and Medicare and Medicaid cover it only if the patient is diagnosed with white coat hypertension, based on stringent criteria that include three elevated clinic blood pressure measurements and two normal out-of-clinic blood pressure measurements and no evidence of end-organ damage. Despite these issues, almost all national guidelines for the management of hypertension recommend ambulatory 24-hour blood pressure monitoring to improve cardiovascular risk prediction and to measure the variability in blood pressure levels.
USING THE INTERNET IN MANAGING HYPERTENSION
Green et al22 studied a new model of care using home blood pressure monitoring via the Internet, and provided feedback and intervention to the patient via a pharmacist to achieve blood pressure goals. Patients measured their blood pressure at home on at least 2 days a week (two measurements each time), using an automatic oscillometric monitor (Omron Hem-705-CP, Kyoto, Japan), and entered the results in an electronic medical record on the Internet. In the intervention group, a pharmacist communicated with each patient by either phone or e-mail every 2 weeks, making changes to their antihypertensive regimens as needed.
Patients in the intervention group had an average reduction in blood pressure of 14 mm Hg from baseline, and their blood pressure was much better controlled compared with the control groups, who were being passively monitored or were receiving usual care based on office blood pressure readings.
MEASURING ARTERIAL STIFFNESS TO ASSES RISK OF END-ORGAN DAMAGE
Mean arterial blood pressure, derived from the extremes of systolic and diastolic pressure as measured with a traditional sphygmomanometer, is a product of cardiac output and total peripheral vascular resistance. In contrast, central aortic blood pressure, the central augmentation index, and pulse wave velocity are measures derived from brachial blood pressure as well as arterial pulse wave tracings. They provide additional information on arterial stiffness and help stratify patients at increased cardiovascular risk.
The art of evaluating the arterial pulse wave with the fingertips while examining a patient and diagnosing various ailments was well known and practiced by ancient Greek and Chinese physicians. Although this was less recognized in Western medicine, it was the pulse wave recording on a sphygmograph that was used to measure human blood pressure in the 19th century.23 In the early 20th century, this art was lost with the invention of the mercury sphygmomanometer.
Arterial stiffness indices—ie, central aortic blood pressure, the central augmentation index, and pulse wave velocity—can now be measured noninvasively and have been shown to correlate very well with measurements obtained via a central arterial catheter. In the past, the only way to measure central blood pressure was directly via a central arterial catheter. New devices now measure arterial stiffness indices indirectly by applanation tonometry and pulse wave analysis (reviewed by O’Rourke et al25).
Several trials have shown that these arterial indices have a better prognostic value than the mean arterial pressure or the brachial pulse pressure. For example, the Baltimore Longitudinal Study of Aging26 followed 100 normotensive individuals for 5 years and found that those with a higher pulse wave velocity had a greater chance of developing incident hypertension. Other studies showed that pulse wave velocity and other indices of arterial stiffness are associated with dysfunction of the microvasculature in the brain, with higher cardiovascular risk, and a higher risk of death.
A major limitation in measuring these arterial stiffness indices is that they are derived values and require measurement of brachial blood pressure in addition to the pulse wave tracing.
Recent hypertension guidelines21,27,28 released during the past 2 years in Europe, Latin America, and Japan have recommended measurement of arterial stiffness as part of a comprehensive evaluation of patients with hypertension.
EXCITING TIMES IN HYPERTENSION
These are exciting times in the field of hypertension. With advances in technology, we have new devices and techniques that provide a closer view of the hemodynamic changes and blood pressures experienced by vital organs. In addition, we can now go beyond the physician’s office and evaluate blood pressure changes that occur during the course of a usual day in a patient’s life. This enables us to make better decisions in the management of their hypertension, embodying Dr. Harvey Cushing’s teaching that the physician’s obligation is to “view the man in his world.”29
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Agespecific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Mancia G, Parati G, Pomidossi G, Grassi G, Casadei R, Zanchetti A. Alerting reaction and rise in blood pressure during measurement by physician and nurse. Hypertension 1987; 9:209–215.
- Mancia G, Sega R, Bravi C, et al. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens 1995; 13:1377–1390.
- Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol 2005; 46:508–515.
- Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension.” Am J Hypertens 2008; 21:393–399.
- Obara T, Ohkubo T, Funahashi J, et al. Isolated uncontrolled hypertension at home and in the office among treated hypertensive patients from the J-HOME study. J Hypertens 2005; 23:1653–1660.
- Pickering TG DK, Rafey MA, Schwartz J, Gerin W. Masked hypertension: are those with normal office but elevated ambulatory blood pressure at risk? J Hypertens 2002; 20( suppl 4):176.
- Pickering TG, Hall JE, Appel LJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20:2183–2189.
- Brotman DJ, Davidson MB, Boumitri M, Vidt DG. Impaired diurnal blood pressure variation and all-cause mortality. Am J Hypertens 2008; 21:92–97.
- Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002; 347:797–805.
- Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glo-merular filtration rate. Arch Intern Med 2006; 166:846–852.
- White WB, Larocca GM. Improving the utility of the nocturnal hypertension definition by using absolute sleep blood pressure rather than the “dipping” proportion. Am J Cardiol 2003; 92:1439–1441.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Perloff D, Sokolow M, Cowan R. The prognostic value of ambulatory blood pressures. JAMA 1983; 249:2792–2798.
- Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 2003; 348:2407–2415.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Mansia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 2007; 16:135–232.
- Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008; 299:2857–2867.
- Mohamed F. On chronic Bright’s disease, and its essential symptoms. Lancet 1879; 1:399–401.
- Liew Y, Rafey MA, Allam S, Arrigain S, Butler R, Schreiber M. Blood pressure goals and arterial stiffness in chronic kidney disease. J Clin Hypertens (Greenwich) 2009; 11:201–206.
- O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol 2001; 51:507–522.
- Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 2008; 51:1377–1383.
- Sanchez RA, Ayala M, Baglivo H, et al. Latin American guidelines on hypertension. J Hypertens 2009; 27:905–922.
- Japanese Society of Hypertension. The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension: Measurement and clinical evaluation of blood pressure. Hypertens Res 2009; 32:11–23.
- Dubos RJ. Man Adapting. New Haven, CT: Yale University Press, 1980.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Agespecific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Culleton BF, McKay DW, Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood Press Monit 2006; 11:37–42.
- Mancia G, Parati G, Pomidossi G, Grassi G, Casadei R, Zanchetti A. Alerting reaction and rise in blood pressure during measurement by physician and nurse. Hypertension 1987; 9:209–215.
- Mancia G, Sega R, Bravi C, et al. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens 1995; 13:1377–1390.
- Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol 2005; 46:508–515.
- Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension.” Am J Hypertens 2008; 21:393–399.
- Obara T, Ohkubo T, Funahashi J, et al. Isolated uncontrolled hypertension at home and in the office among treated hypertensive patients from the J-HOME study. J Hypertens 2005; 23:1653–1660.
- Pickering TG DK, Rafey MA, Schwartz J, Gerin W. Masked hypertension: are those with normal office but elevated ambulatory blood pressure at risk? J Hypertens 2002; 20( suppl 4):176.
- Pickering TG, Hall JE, Appel LJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27.
- Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20:2183–2189.
- Brotman DJ, Davidson MB, Boumitri M, Vidt DG. Impaired diurnal blood pressure variation and all-cause mortality. Am J Hypertens 2008; 21:92–97.
- Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002; 347:797–805.
- Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glo-merular filtration rate. Arch Intern Med 2006; 166:846–852.
- White WB, Larocca GM. Improving the utility of the nocturnal hypertension definition by using absolute sleep blood pressure rather than the “dipping” proportion. Am J Cardiol 2003; 92:1439–1441.
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens 2009; 27:280–286.
- Perloff D, Sokolow M, Cowan R. The prognostic value of ambulatory blood pressures. JAMA 1983; 249:2792–2798.
- Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 2003; 348:2407–2415.
- Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002; 39:878–885.
- Mansia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 2007; 16:135–232.
- Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008; 299:2857–2867.
- Mohamed F. On chronic Bright’s disease, and its essential symptoms. Lancet 1879; 1:399–401.
- Liew Y, Rafey MA, Allam S, Arrigain S, Butler R, Schreiber M. Blood pressure goals and arterial stiffness in chronic kidney disease. J Clin Hypertens (Greenwich) 2009; 11:201–206.
- O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol 2001; 51:507–522.
- Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 2008; 51:1377–1383.
- Sanchez RA, Ayala M, Baglivo H, et al. Latin American guidelines on hypertension. J Hypertens 2009; 27:905–922.
- Japanese Society of Hypertension. The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension: Measurement and clinical evaluation of blood pressure. Hypertens Res 2009; 32:11–23.
- Dubos RJ. Man Adapting. New Haven, CT: Yale University Press, 1980.
KEY POINTS
- Traditional office blood pressure measurements have diagnostic limitations, since they are only snapshots of a very dynamic variable.
- Ambulatory 24-hour blood pressure monitoring is a useful and proven tool and can reveal nocturnal hypertension, a possible new marker of risk.
- Automatic devices can be used in the clinician’s office to minimize the “white coat effect” and measure blood pressure accurately.
- Pulse-wave analysis provides physiologic data on central blood pressure and arterial stiffness. This information may help in the early identification and management of patients at risk for end-organ damage.
Intraosseous and Extraosseous Attachments of Flexor Tendon to Bone: A Biomechanical In Vivo Study in Rabbits
Effects of Recombinant Human Bone Morphogenetic Protein 2 on Surgical Infections in a Rabbit Posterolateral Lumbar Fusion Model
Porcine Small Intestine Submucosa Xenograft Augmentation in Repair of Massive Rotator Cuff Tears
Atrophic Nonunion of Humeral Diaphysis Treated With Locking Plate and Recombinant Bone Morphogenetic Protein: Nine Cases
Self-induced Skin Lesions: A Review of Dermatitis Artefacta
Teens With an Autism Spectrum Disorder
The general pediatrician's role in managing an adolescent with an autism spectrum disorder depends largely on his or her comfort with doing counseling, testing, and medication management.
Most adolescents with an autism spectrum disorder already have a pretty thick chart from a history of pediatrician and pediatric subspecialist consultations, but the etiology question may remain. If a child's intelligence is within the normal range, a lot of elaborate medical testing generally is not necessary. If the child has cognitive dysfunction, such as an IQ below 70, there is a greater probability of finding an underlying cause for the disorder.
Consider separate counseling and procedural visits. Like many pediatric patients, one with an autism spectrum disorder can be anxious about vaccinations, needles, or any other unpleasant procedure. So if they know a particular visit is limited to a discussion of concerns, they are more likely to relax and be more communicative.
Counseling will depend on the cognitive and language abilities of the patient. Discussions will have to be tailored to the level of understanding of the teen with autism spectrum disorder.
Counseling can include addressing sexuality issues. Discuss physical and emotional changes associated with adolescence and the effects they can have on the patient and the family. Teenagers in general are often confused about these changes, and those with autism spectrum disorder are no exception.
Discussions of the sort of sexual interest the patient has or does not have will depend on the patient's expressive capacity. Ask open-ended questions about any situations that may have arisen or about any concerns the patient may have about the future.
Also, educate the teen about the prevention of unwanted pregnancy and sexually transmitted infections.
Medication management is important in this population. Many adolescents on the autism spectrum already take psychopharmacologic agents. There is a wide range of comfort levels among pediatricians regarding prescription of psychopharmacologic agents and management of behavioral challenges. Refer the patient to a specialist if you are not at ease in these situations.
Similarly, some pediatricians will be more comfortable than others in ordering and evaluating genetic testing.
Technology has advanced from general karyotype testing a decade ago to more accurate molecular fragile X assays and chromosome microarray analyses that are available today.
If you feel up to date based on your training and experience, go ahead and order initial testing or updated testing as indicated.
General pediatricians are well equipped to manage any underlying medical issues. For example, if a patient has spells that might suggest seizures, an EEG might be in order, especially in this higher-risk population.
Start a transition plan once the adolescent is in high school. Pediatricians are integral in creating this plan, along with family physicians, internists, and/or other adult care providers.
Also work with school personnel to ensure an optimal outcome. Specific goals can include preparing the patient for postsecondary education or having the patient get necessary vocational skills as he or she becomes more independent and joins the workforce.
Work with parents to clarify goals for future living arrangements. Also suggest that parents establish a special needs trust to protect assets designated for the adolescent while still maintaining eligibility for government benefit programs.
The general pediatrician's role in managing an adolescent with an autism spectrum disorder depends largely on his or her comfort with doing counseling, testing, and medication management.
Most adolescents with an autism spectrum disorder already have a pretty thick chart from a history of pediatrician and pediatric subspecialist consultations, but the etiology question may remain. If a child's intelligence is within the normal range, a lot of elaborate medical testing generally is not necessary. If the child has cognitive dysfunction, such as an IQ below 70, there is a greater probability of finding an underlying cause for the disorder.
Consider separate counseling and procedural visits. Like many pediatric patients, one with an autism spectrum disorder can be anxious about vaccinations, needles, or any other unpleasant procedure. So if they know a particular visit is limited to a discussion of concerns, they are more likely to relax and be more communicative.
Counseling will depend on the cognitive and language abilities of the patient. Discussions will have to be tailored to the level of understanding of the teen with autism spectrum disorder.
Counseling can include addressing sexuality issues. Discuss physical and emotional changes associated with adolescence and the effects they can have on the patient and the family. Teenagers in general are often confused about these changes, and those with autism spectrum disorder are no exception.
Discussions of the sort of sexual interest the patient has or does not have will depend on the patient's expressive capacity. Ask open-ended questions about any situations that may have arisen or about any concerns the patient may have about the future.
Also, educate the teen about the prevention of unwanted pregnancy and sexually transmitted infections.
Medication management is important in this population. Many adolescents on the autism spectrum already take psychopharmacologic agents. There is a wide range of comfort levels among pediatricians regarding prescription of psychopharmacologic agents and management of behavioral challenges. Refer the patient to a specialist if you are not at ease in these situations.
Similarly, some pediatricians will be more comfortable than others in ordering and evaluating genetic testing.
Technology has advanced from general karyotype testing a decade ago to more accurate molecular fragile X assays and chromosome microarray analyses that are available today.
If you feel up to date based on your training and experience, go ahead and order initial testing or updated testing as indicated.
General pediatricians are well equipped to manage any underlying medical issues. For example, if a patient has spells that might suggest seizures, an EEG might be in order, especially in this higher-risk population.
Start a transition plan once the adolescent is in high school. Pediatricians are integral in creating this plan, along with family physicians, internists, and/or other adult care providers.
Also work with school personnel to ensure an optimal outcome. Specific goals can include preparing the patient for postsecondary education or having the patient get necessary vocational skills as he or she becomes more independent and joins the workforce.
Work with parents to clarify goals for future living arrangements. Also suggest that parents establish a special needs trust to protect assets designated for the adolescent while still maintaining eligibility for government benefit programs.
The general pediatrician's role in managing an adolescent with an autism spectrum disorder depends largely on his or her comfort with doing counseling, testing, and medication management.
Most adolescents with an autism spectrum disorder already have a pretty thick chart from a history of pediatrician and pediatric subspecialist consultations, but the etiology question may remain. If a child's intelligence is within the normal range, a lot of elaborate medical testing generally is not necessary. If the child has cognitive dysfunction, such as an IQ below 70, there is a greater probability of finding an underlying cause for the disorder.
Consider separate counseling and procedural visits. Like many pediatric patients, one with an autism spectrum disorder can be anxious about vaccinations, needles, or any other unpleasant procedure. So if they know a particular visit is limited to a discussion of concerns, they are more likely to relax and be more communicative.
Counseling will depend on the cognitive and language abilities of the patient. Discussions will have to be tailored to the level of understanding of the teen with autism spectrum disorder.
Counseling can include addressing sexuality issues. Discuss physical and emotional changes associated with adolescence and the effects they can have on the patient and the family. Teenagers in general are often confused about these changes, and those with autism spectrum disorder are no exception.
Discussions of the sort of sexual interest the patient has or does not have will depend on the patient's expressive capacity. Ask open-ended questions about any situations that may have arisen or about any concerns the patient may have about the future.
Also, educate the teen about the prevention of unwanted pregnancy and sexually transmitted infections.
Medication management is important in this population. Many adolescents on the autism spectrum already take psychopharmacologic agents. There is a wide range of comfort levels among pediatricians regarding prescription of psychopharmacologic agents and management of behavioral challenges. Refer the patient to a specialist if you are not at ease in these situations.
Similarly, some pediatricians will be more comfortable than others in ordering and evaluating genetic testing.
Technology has advanced from general karyotype testing a decade ago to more accurate molecular fragile X assays and chromosome microarray analyses that are available today.
If you feel up to date based on your training and experience, go ahead and order initial testing or updated testing as indicated.
General pediatricians are well equipped to manage any underlying medical issues. For example, if a patient has spells that might suggest seizures, an EEG might be in order, especially in this higher-risk population.
Start a transition plan once the adolescent is in high school. Pediatricians are integral in creating this plan, along with family physicians, internists, and/or other adult care providers.
Also work with school personnel to ensure an optimal outcome. Specific goals can include preparing the patient for postsecondary education or having the patient get necessary vocational skills as he or she becomes more independent and joins the workforce.
Work with parents to clarify goals for future living arrangements. Also suggest that parents establish a special needs trust to protect assets designated for the adolescent while still maintaining eligibility for government benefit programs.
N95 Mask Doesn’t Prevent Flu's Spread
A new study on the efficacy of surgical masks compared with respirator masks in combating the spread of influenza shouldn’t lead directly to increased prophylactic mask usage, one hospitalist group leader says. In fact, with hospitals and patients fully aware of another potential H1N1 flu pandemic this winter, HM groups should focus more on traditional hygiene issues and staff management to stem the impact of flu season, says William Ford, MD, FHM, medical director at Cogent Healthcare and director of the hospitalist program at Temple University in Philadelphia.
The randomized controlled trial published online (JAMA. October 2009. doi:10.1001/jama.2009.1466) ) tracked 446 nurses in EDs, medical units, and pediatric units in eight tertiary-care hospitals in Ontario. Researchers found that influenza infection occurred in 23.6% of nurses in the surgical-mask group and in 22.9% of nurses in the N95 respirator group (absolute risk difference –0.73%; 95% CI, –8.8% to 7.3%; P=0.86).
Dr. Ford says masks "can't hurt" as helpful barriers against the spread of influenza among hospital workers, but HM directors would be better served planning for staffing issues and emphasizing prevention. That includes harping on "hand-washing, hand-washing, and hand-washing," as well as being prepared to implement emergency schedules to rotate physicians into floor shifts should rank-and-file hospitalists call out sick.
"As hospitalist directors, I'd be very cognizant of my backup contingency plan," Dr. Ford says. "We have to take certain steps this year in a worst-case scenario."
A new study on the efficacy of surgical masks compared with respirator masks in combating the spread of influenza shouldn’t lead directly to increased prophylactic mask usage, one hospitalist group leader says. In fact, with hospitals and patients fully aware of another potential H1N1 flu pandemic this winter, HM groups should focus more on traditional hygiene issues and staff management to stem the impact of flu season, says William Ford, MD, FHM, medical director at Cogent Healthcare and director of the hospitalist program at Temple University in Philadelphia.
The randomized controlled trial published online (JAMA. October 2009. doi:10.1001/jama.2009.1466) ) tracked 446 nurses in EDs, medical units, and pediatric units in eight tertiary-care hospitals in Ontario. Researchers found that influenza infection occurred in 23.6% of nurses in the surgical-mask group and in 22.9% of nurses in the N95 respirator group (absolute risk difference –0.73%; 95% CI, –8.8% to 7.3%; P=0.86).
Dr. Ford says masks "can't hurt" as helpful barriers against the spread of influenza among hospital workers, but HM directors would be better served planning for staffing issues and emphasizing prevention. That includes harping on "hand-washing, hand-washing, and hand-washing," as well as being prepared to implement emergency schedules to rotate physicians into floor shifts should rank-and-file hospitalists call out sick.
"As hospitalist directors, I'd be very cognizant of my backup contingency plan," Dr. Ford says. "We have to take certain steps this year in a worst-case scenario."
A new study on the efficacy of surgical masks compared with respirator masks in combating the spread of influenza shouldn’t lead directly to increased prophylactic mask usage, one hospitalist group leader says. In fact, with hospitals and patients fully aware of another potential H1N1 flu pandemic this winter, HM groups should focus more on traditional hygiene issues and staff management to stem the impact of flu season, says William Ford, MD, FHM, medical director at Cogent Healthcare and director of the hospitalist program at Temple University in Philadelphia.
The randomized controlled trial published online (JAMA. October 2009. doi:10.1001/jama.2009.1466) ) tracked 446 nurses in EDs, medical units, and pediatric units in eight tertiary-care hospitals in Ontario. Researchers found that influenza infection occurred in 23.6% of nurses in the surgical-mask group and in 22.9% of nurses in the N95 respirator group (absolute risk difference –0.73%; 95% CI, –8.8% to 7.3%; P=0.86).
Dr. Ford says masks "can't hurt" as helpful barriers against the spread of influenza among hospital workers, but HM directors would be better served planning for staffing issues and emphasizing prevention. That includes harping on "hand-washing, hand-washing, and hand-washing," as well as being prepared to implement emergency schedules to rotate physicians into floor shifts should rank-and-file hospitalists call out sick.
"As hospitalist directors, I'd be very cognizant of my backup contingency plan," Dr. Ford says. "We have to take certain steps this year in a worst-case scenario."
Subcutaneous Rehydration Useful Alternative in Kids
Obtaining intravenous access to treat dehydrated infants and youths can be a challenge for the everyday hospitalist. One alternative is subcutaneous rehydration. Interim results from the Increased Flow Utilizing Subcutaneously-Enabled Pediatric Rehydration I (INFUSE) study presented at the recent American College of Emergency Physicians meeting in Boston suggests subcutaneous rehydration as a viable alternative in children with mild to moderate dehydration.
Complete study results were published in the October issue of Pediatrics (doi:10.1542/peds.2008-3588).
"The study included 51 children admitted to emergency rooms throughout the country who were given Hylenex (hyaluronidase human injection-Baxter)," says Sharon Mace, MD, director of pediatric education and quality improvement at the Cleveland Clinic. Hylenex is a purified preparation of the hyaluronidase enzyme; after being administered subcutaneously, it facilitates the infusion of subcutaneous
fluids. "The majority of the patients were able to be given subcutaneous fluids and then sent home," Dr. Mace adds. "In 86% of patients, the catheter was successfully placed on the first attempt. This contrasts to other studies suggesting that the success rate for IV placement in young children is 50% at best."
Shawn L. Ralston, MD, a pediatric hospitalist at the University of Texas Health Sciences Center in San Antonio, notes that most of the time the question of access already has been addressed in the ED. However, there is a subset of medically complex patients in which subcutaneous rehydration is a useful technique to consider.
"The great thing for the hospitalist is that subcutaneous rehydration is almost always one-stick," Dr. Ralston says. "We also find that after rehydration the kids are able to soon begin taking nutrition and water by mouth again."
According to Dr. Mace, only such minor adverse events as swelling and redness at the injection site were observed. No allergic responses were noticed.
Obtaining intravenous access to treat dehydrated infants and youths can be a challenge for the everyday hospitalist. One alternative is subcutaneous rehydration. Interim results from the Increased Flow Utilizing Subcutaneously-Enabled Pediatric Rehydration I (INFUSE) study presented at the recent American College of Emergency Physicians meeting in Boston suggests subcutaneous rehydration as a viable alternative in children with mild to moderate dehydration.
Complete study results were published in the October issue of Pediatrics (doi:10.1542/peds.2008-3588).
"The study included 51 children admitted to emergency rooms throughout the country who were given Hylenex (hyaluronidase human injection-Baxter)," says Sharon Mace, MD, director of pediatric education and quality improvement at the Cleveland Clinic. Hylenex is a purified preparation of the hyaluronidase enzyme; after being administered subcutaneously, it facilitates the infusion of subcutaneous
fluids. "The majority of the patients were able to be given subcutaneous fluids and then sent home," Dr. Mace adds. "In 86% of patients, the catheter was successfully placed on the first attempt. This contrasts to other studies suggesting that the success rate for IV placement in young children is 50% at best."
Shawn L. Ralston, MD, a pediatric hospitalist at the University of Texas Health Sciences Center in San Antonio, notes that most of the time the question of access already has been addressed in the ED. However, there is a subset of medically complex patients in which subcutaneous rehydration is a useful technique to consider.
"The great thing for the hospitalist is that subcutaneous rehydration is almost always one-stick," Dr. Ralston says. "We also find that after rehydration the kids are able to soon begin taking nutrition and water by mouth again."
According to Dr. Mace, only such minor adverse events as swelling and redness at the injection site were observed. No allergic responses were noticed.
Obtaining intravenous access to treat dehydrated infants and youths can be a challenge for the everyday hospitalist. One alternative is subcutaneous rehydration. Interim results from the Increased Flow Utilizing Subcutaneously-Enabled Pediatric Rehydration I (INFUSE) study presented at the recent American College of Emergency Physicians meeting in Boston suggests subcutaneous rehydration as a viable alternative in children with mild to moderate dehydration.
Complete study results were published in the October issue of Pediatrics (doi:10.1542/peds.2008-3588).
"The study included 51 children admitted to emergency rooms throughout the country who were given Hylenex (hyaluronidase human injection-Baxter)," says Sharon Mace, MD, director of pediatric education and quality improvement at the Cleveland Clinic. Hylenex is a purified preparation of the hyaluronidase enzyme; after being administered subcutaneously, it facilitates the infusion of subcutaneous
fluids. "The majority of the patients were able to be given subcutaneous fluids and then sent home," Dr. Mace adds. "In 86% of patients, the catheter was successfully placed on the first attempt. This contrasts to other studies suggesting that the success rate for IV placement in young children is 50% at best."
Shawn L. Ralston, MD, a pediatric hospitalist at the University of Texas Health Sciences Center in San Antonio, notes that most of the time the question of access already has been addressed in the ED. However, there is a subset of medically complex patients in which subcutaneous rehydration is a useful technique to consider.
"The great thing for the hospitalist is that subcutaneous rehydration is almost always one-stick," Dr. Ralston says. "We also find that after rehydration the kids are able to soon begin taking nutrition and water by mouth again."
According to Dr. Mace, only such minor adverse events as swelling and redness at the injection site were observed. No allergic responses were noticed.