User login
Information Transfer to Rehabilitation
Effective communication among physicians during the hospital discharge process is critical to patient care. Patients are at high risk of having an adverse drug event,1 readmission, or death2 during the transition from hospital to home.3 Ineffective communication between inpatient and outpatient providers has been implicated as a leading cause of adverse events.35 Conversely, efforts to improve communication have been shown to improve compliance with follow‐up tests and decrease readmission rates.6, 7 Recently, the absence of several specific data elements in discharge documentation have been shown to be common and to have potential for patient harm, including test results that are pending at the time of discharge.8, 9 Unexplained discrepancies between preadmission and discharge medication regimens are also common and potentially dangerous.1
According to the Joint Commission for Accreditation of Healthcare Organizations (TJC), the following elements should be included in discharge summaries: the reason for hospitalization; significant findings; procedures performed and care, treatment, and services provided; the patient's condition at discharge; and information provided to the patient and family, as appropriate.10 TJC also advocates medication reconciliation, a process of identifying the most accurate list of all medications a patient is takingincluding name, dosage, frequency, and routeand using this list to provide correct medications for patients anywhere within the health care system.11
Despite the importance of complete communication among providers at hospital discharge, a recent systematic review showed that discharge summaries often lacked important information such as diagnostic test results (missing from 33%‐63%), treatment or hospital course (7%‐22%), discharge medications (2%‐40%), test results pending at discharge (65%), patient or family counseling (90%‐92%), and follow‐up plans (2%‐43%).1
Most of the studies addressing this issue have evaluated communication pitfalls between acute care hospitals and primary care physicians among patients discharged home.17 In contrast, the quality of discharge documentation among patients discharged to rehabilitation centers and other subacute care facilities has been less well studied, perhaps due to relatively smaller numbers of patients discharged to such facilities. This communication is as or more important because these patients are potentially more vulnerable and their medical conditions more active than for patients discharged home.12 Furthermore, discharge information from acute care hospitals will often form the basis for admission orders at subacute facilities. Last, these patients will have a second transition in care (from subacute facility to home) whose quality is dependent at least in part on the quality of communication during the first transition.
The aim of this study was to evaluate the quality of information transfer among patients discharged from acute hospitals to subacute facilities across an integrated healthcare delivery system. The long‐term goals of this effort were to determine the areas most in need of improvement, to guide interventions to address these problems, and to track improvements in these measures over time as interventions are implemented and refined.
Methods
This observational study was conducted as part of a quality improvement project evaluating the quality of information provided during the discharge process across Partners Health Care System. The institutional review boards of the participating institutions approved the study.
Study Sample
We evaluated a sample of discharge documentation packets (eg, discharge summaries, discharge orders, nursing instructions, care coordination, and physical/occupational therapy notes) of patients discharged from all 5 acute care hospitals of the Partners Healthcare System to 30 subacute facilities (rehabilitation hospitals and skilled nursing facilities) from March 2005 through June 2007.
For reviewers at acute sites, discharge documentation packets were randomly selected each quarter using a random number generator within Microsoft Excel (Microsoft, Redmond, WA). At subacute sites, reviewers selected which packets to review, although they were encouraged to review all of them. Random selection of packets could not be achieved at subacute sites because reviews took place on the day of admission to the subacute facility. All reviewers received 1 hour of training on how to evaluate discharge packets, including review of a standardized teaching packet with 1 of the coauthors (J.L.S. or T.O.).
Two of the 5 acute care hospitals in the study are academic medical centers and the other 3 are community hospitals. Reviewers were a mix of trained medical residents or nurse practitioners at acute sites and admitting physicians or nurse practitioners at receiving subacute sites.
Fifty packets were reviewed per acute site per quarter. This provided roughly 10% precision around our estimates (ie, if compliance with a measure were 80%, the 95% confidence interval around this estimate would be 70%‐90%). This sample size is consistent with those used to obtain other national benchmarks, such as those for National Hospital Quality Measures, which generally require at least 35 cases per quarter.13
Measures
A multidisciplinary team at Partners derived, reviewed, and refined a minimum data set required to appropriately care for patients during the first 72 hours after transfer from an acute care hospital to a subacute facility. Several of these measures are required by TJC. Other measures were either modifications of TJC measures made to facilitate uniform data collection (eg, history and physical examination at admission instead of significant findings) or additional data elements (not required by TJC) felt to be important to patient care based on the medical literature and interviews with receiving providers at subacute facilities. All measures were refined by the multidisciplinary team with input from additional subspecialists as needed (see Table 1 for the final list of measures).
| Reason(s) for Admission | |
|---|---|
| Joint Commission requirements | A focused history |
| A focused physical exam | |
| Pertinent past medical history | |
| Treatment rendered | |
| Discharge diagnosis(es) | |
| Condition on discharge | |
| Discharge summary | |
| Any information missing | |
| Non‐Joint Commission requirements | |
| Medication information | Discharge medications |
| Drug allergies | |
| Preadmission medication information | |
| Explanation for any differences between preadmission and discharge medications | |
| Test results information | Latest pertinent laboratory results |
| Pertinent radiology results | |
| Test results pending at time of transfer | |
| Overall assessment | Were management and follow‐up plans adequately described? |
| Did you uncover a significant condition not mentioned in the discharge packet? | |
Data Collection
After reviewing the entire discharge documentation packet, reviewers completed a survey concerning the inclusion of the required data elements. Surveys were completed online using Perseus Survey Solutions 6.0 (Perseus Development Corp., Braintree, MA) in the month following discharge (for reviewers at acute care sites) or within 24 hours of admission to the subacute facility (for reviewers at subacute sites). To verify the accuracy and completeness of packets, reviewers at acute sites were instructed to compare the discharge documentation to a review of the inpatient medical record. Similarly, reviewers at subacute sites were instructed to complete their evaluations after admitting each patient to their facility.
Outcomes
The primary outcome was the proportion of packets that contained each data element. In addition, we calculated the proportion of packets that contained all applicable elements required by TJC and all applicable data elements measured in the study. Last, we evaluated two global (albeit subjective) measures of satisfaction with the packet: Were management and follow‐up plans adequately described? (both components needed to be adequately described to get credit for this question) and Did you uncover a significant condition not mentioned in the discharge packet? Significant conditions were defined as active medical problems requiring management during or immediately following the hospitalization.
Statistical Analysis
Results were calculated as proportions, odds ratios, and 95% confidence intervals (CI), using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Simple logistic regression was used to compare inclusion of data elements between medical and surgical services and between academic medical centers and community hospitals. To evaluate interrater reliability, 2 reviewers (both at acute sites) independently evaluated 29 randomly chosen charts, each with 12 data elements.
Results
A total of 1501 discharge documentation packets were reviewed, including 980 patients (65%) from a medical unit and 521 patients (35%) from a surgical unit. Based on 2007 data, these packets represent approximately 4% of all eligible discharges to subacute facilities. Patients discharged from 1 of the 2 academic medical centers represented 44% of the sample. A total of 644 discharge packets (43%) were reviewed at acute sites and 814 packets (54%) were reviewed at subacute sites. Information about reviewer site was missing in 43 discharge packets (3%). For the 29 charts independently reviewed by 2 reviewers, there was complete agreement for 331 out of 348 data elements (95.1%).
Only 1055 (70%) discharge summaries had all the information required by TJC (Table 2). Physical examination at admission (a component of significant findings, as noted above) and condition at discharge were the 2 elements most often missing. The defect‐free rate varied by site, with a range of 61% to 76% across the 5 acute care hospitals (data not shown).
| Sample Size | Missing [n (%)] | 95% CI Missing % | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Reason(s) for admission | 1497 | 14 (0.9) | 0.41.4 |
| A focused history | 1493 | 65 (4.4) | 3.35.3 |
| A focused physical exam | 1493 | 170 (11.4) | 9.713 |
| Pertinent past medical history | 1494 | 69 (4.6) | 3.55.6 |
| Treatment rendered | 1494 | 33 (2.2) | 1.42.9 |
| Discharge diagnosis(es) | 1480 | 53 (3.6) | 2.64.5 |
| Condition on discharge | 1462 | 208 (14.2) | 12.416.0 |
| Discharge summary | 1475 | 90 (6.1) | 4.87.3 |
| Any information missing | 1501 | 447 (29.7) | 27.432.0 |
| Non‐Joint Commission requirements | |||
| Medication information | |||
| Discharge medications | 1491 | 19 (1.3) | 0.71.8 |
| Drug allergies | 1470 | 88 (6.0) | 4.77.2 |
| Preadmission medication information | 1460 | 297 (20.3) | 18.322.4 |
| Explanation for any differences between preadmission and discharge medications | 1060 | 374 (35.3) | 32.038.1 |
| Test results information | |||
| Latest pertinent lab results | 1460 | 261 (17.9) | 15.919.8 |
| Pertinent radiology results | 1303 | 139 (10.7) | 912.4 |
| Test results pending at time of transfer | 341 | 160 (47.2) | 41.952.5 |
| Overall assessment | |||
| Were management and follow‐up plans adequately described? | 1461 | No (%): 161 (11.1) | 95% CI No %: 9.512.7 |
| Did you uncover a significant condition not mentioned in the discharge packet? | 1469 | Yes (%): 162 (11.0) | 95% CI Yes %: 9.413.0 |
| All applicable elements present | 1501 | 503 (33.5) | 31.135.9 |
The rates of inclusion of other (non‐TJC required) data elements are shown in Table 2. Most often missing were preadmission medication regimens, any documented reason for any difference between preadmission and discharge medications, pertinent laboratory results, and an adequate follow‐up plan (including who to follow up with, when to follow‐up, and a list of tasks to be accomplished at the follow‐up visit). Notation regarding significant test results that were pending at the time of transfer was missing in 160 of 341 applicable patients (47%), and in 162 patients (11%), physicians uncovered a significant condition that was not mentioned in the discharge documentation. Only 503 (33.5%) discharge documentation packets had all applicable measures present. In addition, the discharge summary was not received at all on the day of discharge according to the receiving site in 90 patients (6%).
Reviewers were asked in a separate question which missing data were necessary for patient care. Data elements most often cited were explanations for any medication discrepancies and test results pending at the time of the hospital discharge.
Community hospitals had a higher rate of inclusion of TJC‐required data elements when compared to academic medical centers (Table 3). Also, among non‐TJC required data elements, inclusion rates were higher among the community hospitals, especially regarding information about medication discrepancies, pending test results, and follow‐up information (Table 3).
| Total (n) | All Elements Present [n (%)] | OR (95% CI) | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Hospital type | |||
| Community hospitals | 949 | 826 (87) | 2.7 (2.13.6) |
| Academic medical centers | 541 | 384 (71) | Ref. |
| Service | |||
| Medical services | 1013 | 745 (73) | 1.3 (1.01.7) |
| Surgical services | 488 | 332 (68) | Ref. |
| Explanation for any medication discrepancies | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 718 | 550 (76) | 5.0 (3.86.5) |
| Academic medical centers | 342 | 136 (39) | Ref. |
| Service | |||
| Medical services | 754 | 529 (70) | 2.2 (1.72.9) |
| Surgical services | 306 | 157 (51) | Ref. |
| Test results pending at time of transfer | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 172 | 109 (63) | 2.4 (1.53.7) |
| Academic medical centers | 169 | 71 (42) | Ref. |
| Service | |||
| Medical services | 227 | 146 (64) | 4.2 (2.66.9) |
| Surgical services | 114 | 34 (30) | Ref. |
| Follow‐up plans adequately described | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 968 | 883 (91) | 1.7 (1.22.4) |
| Academic medical centers | 543 | 466 (85) | Ref. |
| Service | |||
| Medical services | 983 | 862 (87) | 0.67 (0.51.0) |
| Surgical services | 478 | 437 (91) | Ref. |
Although no differences were found between medical and surgical services regarding compliance with TJC requirements, a difference was noted in documentation of explanations of medication discrepancies and pending test results, with medical services performing better in both measures (Table 3).
In general, reviewers at subacute sites more often evaluated packets as deficient than reviewers at acute sites, up to an absolute difference of 33% in the proportion of missing data, depending on the data element (see Appendix, Table 1).
Discussion
Our study evaluated the completeness of documentation in the discharge summaries of patients discharged from acute care to subacute care facilities. Our results for the inclusion of TJC‐required data elements were similar to those quoted in the literature for patients discharged home.6 Our results also demonstrated a high rate of other missing data elements that are arguably of equal or greater importance, including reasons for discrepancies between preadmission and discharge medication regimens and tests that are pending at the time of discharge.1, 8, 9 Our results also demonstrated the relatively poorer performance of academic centers compared to community hospitals regarding inclusion of information about medication reconciliation, follow‐up, pending test results, and complete information required by TJC. Finally, we found that patients discharged from surgical services more often lacked documentation of medication discrepancies and pending test results compared with patients from medical services.
To our knowledge, this is one of the first studies looking at the quality of information transfer in patients discharged to subacute care facilities. The results of this study are not surprising given the known problems with general information transfer at hospital discharge.1 The fact that community hospitals provided more complete information than academic medical centers for certain data elements may be due to the difference between residents and more senior physicians preparing discharge documentation. Such differences could reflect differences in experience, training, and degree of appreciation for the importance of discharge documentation, and/or restrictions in work hours among residents (eg, resulting in time‐pressure to complete discharge summaries and/or summaries being written by residents who know the patients less well). These hypotheses deserve further exploration. The differences between medical and surgical services should also be validated and explored in other healthcare systems, including both academic and community settings.
The results of this study should be viewed in light of the study's limitations. Packets evaluated by reviewers at subacute facilities were chosen by the reviewers and may not have been representative of all patients received by that facility (in contrast to those reviewed at the acute sites, which were chosen at random and more likely to be representative, although we did not formally test for this). It is possible that reviewers at subacute sites selected the worst discharge documentation packets for evaluation. Second, evaluations by reviewers at subacute sites did not distinguish between information missing from discharge documentation and failure to receive the documentation at all from the acute care hospital (again in contrast to reviewers at acute sites, who always had access to the documentation). Lastly, reviewers at acute and subacute sites may have graded packets differently due to their different clinical perspectives. These 3 factors may explain the relatively poorer results of discharge packets reviewed by reviewers at subacute sites. Further study would be needed to distinguish among these possibilities (eg, having acute and subacute reviewers answer the same questions for the same discharge packets to allow us to measure interrater reliability between the different kinds of reviewers; explicitly asking subacute reviewers about receipt of each piece of documentation; comparing the distribution of diagnosis‐related group [DRG] codes and hospital length of stay in evaluated vs. total discharge packets as a measure of representativeness). We also cannot rule out the possibility of reviewer bias, but all reviewers were trained in a standardized fashion and we know that reliability of assessments were high, at least among reviewers at acute sites. Last, we did not measure actual or potential adverse events caused by these information deficits.
As part of a Partners‐wide initiative to improve transitions in care, the results were presented to the administrations of each of the 5 acute care hospitals. The Partners High Performance Medicine Transition team then began work with a steering committee (composed of representatives from each hospital) to address these deficiencies. Since then, the hospitals have taken several steps to improve the quality of information transfer for discharged patients, including the following:
Technological improvements to the hospitals' discharge ordering systems to actively solicit and/or autoimport the required information into discharge documentation.
Creation of discharge templates to record the required information on paper.
Provision of feedback to clinicians and their service chiefs regarding the ongoing quality of their discharge documentation.
Creation of an online Partners‐wide curriculum on discharge summary authorship, with a mandatory quiz to be taken by all incoming clinicians.
In conclusion, we found room for improvement in the inclusion of data elements required for the safe transfer of patients from acute hospitals to subacute facilities, especially in areas such as medication reconciliation, pending test results, and adequate follow‐up plans. We also found variation by site and type of service. For patients discharged to rehabilitation and other subacute facilities, improvement is needed in the communication of clinically relevant information to those providing continuing care.
Appendix
| JCAHO Indicators | Reviews from Sub‐Acute Sites (N = 814)* | Reviews from Acute Sites (N = 644)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Missing N | % | 95% CI | Sample Size | Missing | % | 95% CI | |
| ||||||||
| Reason(s) for admission | 812 | 9 | 1.1 | 0.41.8 | 643 | 4 | 0.6 | 0.011.2 |
| A focused history | 810 | 49 | 6.1 | 4.47.7 | 642 | 16 | 2.5 | 1.33.7 |
| A focused physical exam | 810 | 131 | 16.2 | 13.718.7 | 641 | 34 | 5.3 | 3.67.0 |
| Pertinent past medical history | 810 | 50 | 6.2 | 4.57.8 | 642 | 14 | 22.0 | 1.13.3 |
| Treatment rendered | 811 | 29 | 3.6 | 2.34.9 | 641 | 4 | 0.6 | 0.011.2 |
| Discharge diagnosis(es) | 806 | 59 | 7.3 | 5.59.1 | 630 | 7 | 1.1 | 0.31.9 |
| Condition on discharge | 800 | 92 | 11.5 | 9.313.7 | 622 | 109 | 17.5 | 14.520.5 |
| Discharge summary | 809 | 77 | 9.5 | 7.511.5 | 624 | 11 | 1.8 | 0.72.8 |
| Any information missing | ||||||||
| Medication Information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Discharge medications | 811 | 12 | 1.5 | 0.72.3 | 638 | 6 | 0.9 | 0.21.7 |
| Drug allergies | 811 | 47 | 5.8 | 4.27.4 | 639 | 35 | 5.5 | 3.77.2 |
| Explanation for any differences between preadmission and discharge medications | 542 | 275 | 50.7 | 46.555 | 498 | 88 | 17.7 | 14.321.0 |
| Test results information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Latest pertinent lab results | 790 | 178 | 22.5 | 19.625.4 | 629 | 73 | 11.6 | 9.114.1 |
| Pertinent radiology results | 668 | 110 | 16.5 | 13.719.3 | 601 | 27 | 4.5 | 2.86.2 |
| Test results pending at time of transfer | 183 | 87 | 47.5 | 40.354.8 | 152 | 73 | 48.0 | 40.156.0 |
| Management Information | Sample Size | No | % | 95% CI | Sample Size | No | % | 95% CI |
| Were management and follow‐up plans adequately described? | 794 | 121 | 15.2 | 12.717.7 | 631 | 79 | 12.5 | 9.915.1 |
| Sample Size | Yes | % | 95% CI | Sample Size | Yes | % | 95% CI | |
| Did you uncover a significant condition not mentioned in the discharge packet? | 793 | 117 | 14.8 | 12.317.2 | 635 | 38 | 6.0 | 4.47.8 |
- , , , et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.1989;19:624–631.
- , , , , , .Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- , , , .Effect of discharge summary availability during post‐discharge visits on hospital readmission.JGen Intern Med.2002;17:186–192.
- , , , .Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , et al.Impact of a standardized communication system on continuity of care between family physicians and the emergency department.CJEM.2007;9:79–86.
- , , , et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- , , .Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- Standard IM.6.10: Hospital Accreditation Standards.Oakbrook Terrace, IL:Joint Commission on Accreditation of Healthcare Organizations;2006:338–340.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed July 2009.
- , .Effectiveness of multidisciplinary rehabilitation services in post acute care: state‐of‐the‐science. A review.Arch Phys Med Rehabil.2007;88:1526–1534.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures: Population and Sampling Specifications Version 2.4. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed July 2009.
Effective communication among physicians during the hospital discharge process is critical to patient care. Patients are at high risk of having an adverse drug event,1 readmission, or death2 during the transition from hospital to home.3 Ineffective communication between inpatient and outpatient providers has been implicated as a leading cause of adverse events.35 Conversely, efforts to improve communication have been shown to improve compliance with follow‐up tests and decrease readmission rates.6, 7 Recently, the absence of several specific data elements in discharge documentation have been shown to be common and to have potential for patient harm, including test results that are pending at the time of discharge.8, 9 Unexplained discrepancies between preadmission and discharge medication regimens are also common and potentially dangerous.1
According to the Joint Commission for Accreditation of Healthcare Organizations (TJC), the following elements should be included in discharge summaries: the reason for hospitalization; significant findings; procedures performed and care, treatment, and services provided; the patient's condition at discharge; and information provided to the patient and family, as appropriate.10 TJC also advocates medication reconciliation, a process of identifying the most accurate list of all medications a patient is takingincluding name, dosage, frequency, and routeand using this list to provide correct medications for patients anywhere within the health care system.11
Despite the importance of complete communication among providers at hospital discharge, a recent systematic review showed that discharge summaries often lacked important information such as diagnostic test results (missing from 33%‐63%), treatment or hospital course (7%‐22%), discharge medications (2%‐40%), test results pending at discharge (65%), patient or family counseling (90%‐92%), and follow‐up plans (2%‐43%).1
Most of the studies addressing this issue have evaluated communication pitfalls between acute care hospitals and primary care physicians among patients discharged home.17 In contrast, the quality of discharge documentation among patients discharged to rehabilitation centers and other subacute care facilities has been less well studied, perhaps due to relatively smaller numbers of patients discharged to such facilities. This communication is as or more important because these patients are potentially more vulnerable and their medical conditions more active than for patients discharged home.12 Furthermore, discharge information from acute care hospitals will often form the basis for admission orders at subacute facilities. Last, these patients will have a second transition in care (from subacute facility to home) whose quality is dependent at least in part on the quality of communication during the first transition.
The aim of this study was to evaluate the quality of information transfer among patients discharged from acute hospitals to subacute facilities across an integrated healthcare delivery system. The long‐term goals of this effort were to determine the areas most in need of improvement, to guide interventions to address these problems, and to track improvements in these measures over time as interventions are implemented and refined.
Methods
This observational study was conducted as part of a quality improvement project evaluating the quality of information provided during the discharge process across Partners Health Care System. The institutional review boards of the participating institutions approved the study.
Study Sample
We evaluated a sample of discharge documentation packets (eg, discharge summaries, discharge orders, nursing instructions, care coordination, and physical/occupational therapy notes) of patients discharged from all 5 acute care hospitals of the Partners Healthcare System to 30 subacute facilities (rehabilitation hospitals and skilled nursing facilities) from March 2005 through June 2007.
For reviewers at acute sites, discharge documentation packets were randomly selected each quarter using a random number generator within Microsoft Excel (Microsoft, Redmond, WA). At subacute sites, reviewers selected which packets to review, although they were encouraged to review all of them. Random selection of packets could not be achieved at subacute sites because reviews took place on the day of admission to the subacute facility. All reviewers received 1 hour of training on how to evaluate discharge packets, including review of a standardized teaching packet with 1 of the coauthors (J.L.S. or T.O.).
Two of the 5 acute care hospitals in the study are academic medical centers and the other 3 are community hospitals. Reviewers were a mix of trained medical residents or nurse practitioners at acute sites and admitting physicians or nurse practitioners at receiving subacute sites.
Fifty packets were reviewed per acute site per quarter. This provided roughly 10% precision around our estimates (ie, if compliance with a measure were 80%, the 95% confidence interval around this estimate would be 70%‐90%). This sample size is consistent with those used to obtain other national benchmarks, such as those for National Hospital Quality Measures, which generally require at least 35 cases per quarter.13
Measures
A multidisciplinary team at Partners derived, reviewed, and refined a minimum data set required to appropriately care for patients during the first 72 hours after transfer from an acute care hospital to a subacute facility. Several of these measures are required by TJC. Other measures were either modifications of TJC measures made to facilitate uniform data collection (eg, history and physical examination at admission instead of significant findings) or additional data elements (not required by TJC) felt to be important to patient care based on the medical literature and interviews with receiving providers at subacute facilities. All measures were refined by the multidisciplinary team with input from additional subspecialists as needed (see Table 1 for the final list of measures).
| Reason(s) for Admission | |
|---|---|
| Joint Commission requirements | A focused history |
| A focused physical exam | |
| Pertinent past medical history | |
| Treatment rendered | |
| Discharge diagnosis(es) | |
| Condition on discharge | |
| Discharge summary | |
| Any information missing | |
| Non‐Joint Commission requirements | |
| Medication information | Discharge medications |
| Drug allergies | |
| Preadmission medication information | |
| Explanation for any differences between preadmission and discharge medications | |
| Test results information | Latest pertinent laboratory results |
| Pertinent radiology results | |
| Test results pending at time of transfer | |
| Overall assessment | Were management and follow‐up plans adequately described? |
| Did you uncover a significant condition not mentioned in the discharge packet? | |
Data Collection
After reviewing the entire discharge documentation packet, reviewers completed a survey concerning the inclusion of the required data elements. Surveys were completed online using Perseus Survey Solutions 6.0 (Perseus Development Corp., Braintree, MA) in the month following discharge (for reviewers at acute care sites) or within 24 hours of admission to the subacute facility (for reviewers at subacute sites). To verify the accuracy and completeness of packets, reviewers at acute sites were instructed to compare the discharge documentation to a review of the inpatient medical record. Similarly, reviewers at subacute sites were instructed to complete their evaluations after admitting each patient to their facility.
Outcomes
The primary outcome was the proportion of packets that contained each data element. In addition, we calculated the proportion of packets that contained all applicable elements required by TJC and all applicable data elements measured in the study. Last, we evaluated two global (albeit subjective) measures of satisfaction with the packet: Were management and follow‐up plans adequately described? (both components needed to be adequately described to get credit for this question) and Did you uncover a significant condition not mentioned in the discharge packet? Significant conditions were defined as active medical problems requiring management during or immediately following the hospitalization.
Statistical Analysis
Results were calculated as proportions, odds ratios, and 95% confidence intervals (CI), using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Simple logistic regression was used to compare inclusion of data elements between medical and surgical services and between academic medical centers and community hospitals. To evaluate interrater reliability, 2 reviewers (both at acute sites) independently evaluated 29 randomly chosen charts, each with 12 data elements.
Results
A total of 1501 discharge documentation packets were reviewed, including 980 patients (65%) from a medical unit and 521 patients (35%) from a surgical unit. Based on 2007 data, these packets represent approximately 4% of all eligible discharges to subacute facilities. Patients discharged from 1 of the 2 academic medical centers represented 44% of the sample. A total of 644 discharge packets (43%) were reviewed at acute sites and 814 packets (54%) were reviewed at subacute sites. Information about reviewer site was missing in 43 discharge packets (3%). For the 29 charts independently reviewed by 2 reviewers, there was complete agreement for 331 out of 348 data elements (95.1%).
Only 1055 (70%) discharge summaries had all the information required by TJC (Table 2). Physical examination at admission (a component of significant findings, as noted above) and condition at discharge were the 2 elements most often missing. The defect‐free rate varied by site, with a range of 61% to 76% across the 5 acute care hospitals (data not shown).
| Sample Size | Missing [n (%)] | 95% CI Missing % | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Reason(s) for admission | 1497 | 14 (0.9) | 0.41.4 |
| A focused history | 1493 | 65 (4.4) | 3.35.3 |
| A focused physical exam | 1493 | 170 (11.4) | 9.713 |
| Pertinent past medical history | 1494 | 69 (4.6) | 3.55.6 |
| Treatment rendered | 1494 | 33 (2.2) | 1.42.9 |
| Discharge diagnosis(es) | 1480 | 53 (3.6) | 2.64.5 |
| Condition on discharge | 1462 | 208 (14.2) | 12.416.0 |
| Discharge summary | 1475 | 90 (6.1) | 4.87.3 |
| Any information missing | 1501 | 447 (29.7) | 27.432.0 |
| Non‐Joint Commission requirements | |||
| Medication information | |||
| Discharge medications | 1491 | 19 (1.3) | 0.71.8 |
| Drug allergies | 1470 | 88 (6.0) | 4.77.2 |
| Preadmission medication information | 1460 | 297 (20.3) | 18.322.4 |
| Explanation for any differences between preadmission and discharge medications | 1060 | 374 (35.3) | 32.038.1 |
| Test results information | |||
| Latest pertinent lab results | 1460 | 261 (17.9) | 15.919.8 |
| Pertinent radiology results | 1303 | 139 (10.7) | 912.4 |
| Test results pending at time of transfer | 341 | 160 (47.2) | 41.952.5 |
| Overall assessment | |||
| Were management and follow‐up plans adequately described? | 1461 | No (%): 161 (11.1) | 95% CI No %: 9.512.7 |
| Did you uncover a significant condition not mentioned in the discharge packet? | 1469 | Yes (%): 162 (11.0) | 95% CI Yes %: 9.413.0 |
| All applicable elements present | 1501 | 503 (33.5) | 31.135.9 |
The rates of inclusion of other (non‐TJC required) data elements are shown in Table 2. Most often missing were preadmission medication regimens, any documented reason for any difference between preadmission and discharge medications, pertinent laboratory results, and an adequate follow‐up plan (including who to follow up with, when to follow‐up, and a list of tasks to be accomplished at the follow‐up visit). Notation regarding significant test results that were pending at the time of transfer was missing in 160 of 341 applicable patients (47%), and in 162 patients (11%), physicians uncovered a significant condition that was not mentioned in the discharge documentation. Only 503 (33.5%) discharge documentation packets had all applicable measures present. In addition, the discharge summary was not received at all on the day of discharge according to the receiving site in 90 patients (6%).
Reviewers were asked in a separate question which missing data were necessary for patient care. Data elements most often cited were explanations for any medication discrepancies and test results pending at the time of the hospital discharge.
Community hospitals had a higher rate of inclusion of TJC‐required data elements when compared to academic medical centers (Table 3). Also, among non‐TJC required data elements, inclusion rates were higher among the community hospitals, especially regarding information about medication discrepancies, pending test results, and follow‐up information (Table 3).
| Total (n) | All Elements Present [n (%)] | OR (95% CI) | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Hospital type | |||
| Community hospitals | 949 | 826 (87) | 2.7 (2.13.6) |
| Academic medical centers | 541 | 384 (71) | Ref. |
| Service | |||
| Medical services | 1013 | 745 (73) | 1.3 (1.01.7) |
| Surgical services | 488 | 332 (68) | Ref. |
| Explanation for any medication discrepancies | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 718 | 550 (76) | 5.0 (3.86.5) |
| Academic medical centers | 342 | 136 (39) | Ref. |
| Service | |||
| Medical services | 754 | 529 (70) | 2.2 (1.72.9) |
| Surgical services | 306 | 157 (51) | Ref. |
| Test results pending at time of transfer | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 172 | 109 (63) | 2.4 (1.53.7) |
| Academic medical centers | 169 | 71 (42) | Ref. |
| Service | |||
| Medical services | 227 | 146 (64) | 4.2 (2.66.9) |
| Surgical services | 114 | 34 (30) | Ref. |
| Follow‐up plans adequately described | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 968 | 883 (91) | 1.7 (1.22.4) |
| Academic medical centers | 543 | 466 (85) | Ref. |
| Service | |||
| Medical services | 983 | 862 (87) | 0.67 (0.51.0) |
| Surgical services | 478 | 437 (91) | Ref. |
Although no differences were found between medical and surgical services regarding compliance with TJC requirements, a difference was noted in documentation of explanations of medication discrepancies and pending test results, with medical services performing better in both measures (Table 3).
In general, reviewers at subacute sites more often evaluated packets as deficient than reviewers at acute sites, up to an absolute difference of 33% in the proportion of missing data, depending on the data element (see Appendix, Table 1).
Discussion
Our study evaluated the completeness of documentation in the discharge summaries of patients discharged from acute care to subacute care facilities. Our results for the inclusion of TJC‐required data elements were similar to those quoted in the literature for patients discharged home.6 Our results also demonstrated a high rate of other missing data elements that are arguably of equal or greater importance, including reasons for discrepancies between preadmission and discharge medication regimens and tests that are pending at the time of discharge.1, 8, 9 Our results also demonstrated the relatively poorer performance of academic centers compared to community hospitals regarding inclusion of information about medication reconciliation, follow‐up, pending test results, and complete information required by TJC. Finally, we found that patients discharged from surgical services more often lacked documentation of medication discrepancies and pending test results compared with patients from medical services.
To our knowledge, this is one of the first studies looking at the quality of information transfer in patients discharged to subacute care facilities. The results of this study are not surprising given the known problems with general information transfer at hospital discharge.1 The fact that community hospitals provided more complete information than academic medical centers for certain data elements may be due to the difference between residents and more senior physicians preparing discharge documentation. Such differences could reflect differences in experience, training, and degree of appreciation for the importance of discharge documentation, and/or restrictions in work hours among residents (eg, resulting in time‐pressure to complete discharge summaries and/or summaries being written by residents who know the patients less well). These hypotheses deserve further exploration. The differences between medical and surgical services should also be validated and explored in other healthcare systems, including both academic and community settings.
The results of this study should be viewed in light of the study's limitations. Packets evaluated by reviewers at subacute facilities were chosen by the reviewers and may not have been representative of all patients received by that facility (in contrast to those reviewed at the acute sites, which were chosen at random and more likely to be representative, although we did not formally test for this). It is possible that reviewers at subacute sites selected the worst discharge documentation packets for evaluation. Second, evaluations by reviewers at subacute sites did not distinguish between information missing from discharge documentation and failure to receive the documentation at all from the acute care hospital (again in contrast to reviewers at acute sites, who always had access to the documentation). Lastly, reviewers at acute and subacute sites may have graded packets differently due to their different clinical perspectives. These 3 factors may explain the relatively poorer results of discharge packets reviewed by reviewers at subacute sites. Further study would be needed to distinguish among these possibilities (eg, having acute and subacute reviewers answer the same questions for the same discharge packets to allow us to measure interrater reliability between the different kinds of reviewers; explicitly asking subacute reviewers about receipt of each piece of documentation; comparing the distribution of diagnosis‐related group [DRG] codes and hospital length of stay in evaluated vs. total discharge packets as a measure of representativeness). We also cannot rule out the possibility of reviewer bias, but all reviewers were trained in a standardized fashion and we know that reliability of assessments were high, at least among reviewers at acute sites. Last, we did not measure actual or potential adverse events caused by these information deficits.
As part of a Partners‐wide initiative to improve transitions in care, the results were presented to the administrations of each of the 5 acute care hospitals. The Partners High Performance Medicine Transition team then began work with a steering committee (composed of representatives from each hospital) to address these deficiencies. Since then, the hospitals have taken several steps to improve the quality of information transfer for discharged patients, including the following:
Technological improvements to the hospitals' discharge ordering systems to actively solicit and/or autoimport the required information into discharge documentation.
Creation of discharge templates to record the required information on paper.
Provision of feedback to clinicians and their service chiefs regarding the ongoing quality of their discharge documentation.
Creation of an online Partners‐wide curriculum on discharge summary authorship, with a mandatory quiz to be taken by all incoming clinicians.
In conclusion, we found room for improvement in the inclusion of data elements required for the safe transfer of patients from acute hospitals to subacute facilities, especially in areas such as medication reconciliation, pending test results, and adequate follow‐up plans. We also found variation by site and type of service. For patients discharged to rehabilitation and other subacute facilities, improvement is needed in the communication of clinically relevant information to those providing continuing care.
Appendix
| JCAHO Indicators | Reviews from Sub‐Acute Sites (N = 814)* | Reviews from Acute Sites (N = 644)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Missing N | % | 95% CI | Sample Size | Missing | % | 95% CI | |
| ||||||||
| Reason(s) for admission | 812 | 9 | 1.1 | 0.41.8 | 643 | 4 | 0.6 | 0.011.2 |
| A focused history | 810 | 49 | 6.1 | 4.47.7 | 642 | 16 | 2.5 | 1.33.7 |
| A focused physical exam | 810 | 131 | 16.2 | 13.718.7 | 641 | 34 | 5.3 | 3.67.0 |
| Pertinent past medical history | 810 | 50 | 6.2 | 4.57.8 | 642 | 14 | 22.0 | 1.13.3 |
| Treatment rendered | 811 | 29 | 3.6 | 2.34.9 | 641 | 4 | 0.6 | 0.011.2 |
| Discharge diagnosis(es) | 806 | 59 | 7.3 | 5.59.1 | 630 | 7 | 1.1 | 0.31.9 |
| Condition on discharge | 800 | 92 | 11.5 | 9.313.7 | 622 | 109 | 17.5 | 14.520.5 |
| Discharge summary | 809 | 77 | 9.5 | 7.511.5 | 624 | 11 | 1.8 | 0.72.8 |
| Any information missing | ||||||||
| Medication Information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Discharge medications | 811 | 12 | 1.5 | 0.72.3 | 638 | 6 | 0.9 | 0.21.7 |
| Drug allergies | 811 | 47 | 5.8 | 4.27.4 | 639 | 35 | 5.5 | 3.77.2 |
| Explanation for any differences between preadmission and discharge medications | 542 | 275 | 50.7 | 46.555 | 498 | 88 | 17.7 | 14.321.0 |
| Test results information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Latest pertinent lab results | 790 | 178 | 22.5 | 19.625.4 | 629 | 73 | 11.6 | 9.114.1 |
| Pertinent radiology results | 668 | 110 | 16.5 | 13.719.3 | 601 | 27 | 4.5 | 2.86.2 |
| Test results pending at time of transfer | 183 | 87 | 47.5 | 40.354.8 | 152 | 73 | 48.0 | 40.156.0 |
| Management Information | Sample Size | No | % | 95% CI | Sample Size | No | % | 95% CI |
| Were management and follow‐up plans adequately described? | 794 | 121 | 15.2 | 12.717.7 | 631 | 79 | 12.5 | 9.915.1 |
| Sample Size | Yes | % | 95% CI | Sample Size | Yes | % | 95% CI | |
| Did you uncover a significant condition not mentioned in the discharge packet? | 793 | 117 | 14.8 | 12.317.2 | 635 | 38 | 6.0 | 4.47.8 |
Effective communication among physicians during the hospital discharge process is critical to patient care. Patients are at high risk of having an adverse drug event,1 readmission, or death2 during the transition from hospital to home.3 Ineffective communication between inpatient and outpatient providers has been implicated as a leading cause of adverse events.35 Conversely, efforts to improve communication have been shown to improve compliance with follow‐up tests and decrease readmission rates.6, 7 Recently, the absence of several specific data elements in discharge documentation have been shown to be common and to have potential for patient harm, including test results that are pending at the time of discharge.8, 9 Unexplained discrepancies between preadmission and discharge medication regimens are also common and potentially dangerous.1
According to the Joint Commission for Accreditation of Healthcare Organizations (TJC), the following elements should be included in discharge summaries: the reason for hospitalization; significant findings; procedures performed and care, treatment, and services provided; the patient's condition at discharge; and information provided to the patient and family, as appropriate.10 TJC also advocates medication reconciliation, a process of identifying the most accurate list of all medications a patient is takingincluding name, dosage, frequency, and routeand using this list to provide correct medications for patients anywhere within the health care system.11
Despite the importance of complete communication among providers at hospital discharge, a recent systematic review showed that discharge summaries often lacked important information such as diagnostic test results (missing from 33%‐63%), treatment or hospital course (7%‐22%), discharge medications (2%‐40%), test results pending at discharge (65%), patient or family counseling (90%‐92%), and follow‐up plans (2%‐43%).1
Most of the studies addressing this issue have evaluated communication pitfalls between acute care hospitals and primary care physicians among patients discharged home.17 In contrast, the quality of discharge documentation among patients discharged to rehabilitation centers and other subacute care facilities has been less well studied, perhaps due to relatively smaller numbers of patients discharged to such facilities. This communication is as or more important because these patients are potentially more vulnerable and their medical conditions more active than for patients discharged home.12 Furthermore, discharge information from acute care hospitals will often form the basis for admission orders at subacute facilities. Last, these patients will have a second transition in care (from subacute facility to home) whose quality is dependent at least in part on the quality of communication during the first transition.
The aim of this study was to evaluate the quality of information transfer among patients discharged from acute hospitals to subacute facilities across an integrated healthcare delivery system. The long‐term goals of this effort were to determine the areas most in need of improvement, to guide interventions to address these problems, and to track improvements in these measures over time as interventions are implemented and refined.
Methods
This observational study was conducted as part of a quality improvement project evaluating the quality of information provided during the discharge process across Partners Health Care System. The institutional review boards of the participating institutions approved the study.
Study Sample
We evaluated a sample of discharge documentation packets (eg, discharge summaries, discharge orders, nursing instructions, care coordination, and physical/occupational therapy notes) of patients discharged from all 5 acute care hospitals of the Partners Healthcare System to 30 subacute facilities (rehabilitation hospitals and skilled nursing facilities) from March 2005 through June 2007.
For reviewers at acute sites, discharge documentation packets were randomly selected each quarter using a random number generator within Microsoft Excel (Microsoft, Redmond, WA). At subacute sites, reviewers selected which packets to review, although they were encouraged to review all of them. Random selection of packets could not be achieved at subacute sites because reviews took place on the day of admission to the subacute facility. All reviewers received 1 hour of training on how to evaluate discharge packets, including review of a standardized teaching packet with 1 of the coauthors (J.L.S. or T.O.).
Two of the 5 acute care hospitals in the study are academic medical centers and the other 3 are community hospitals. Reviewers were a mix of trained medical residents or nurse practitioners at acute sites and admitting physicians or nurse practitioners at receiving subacute sites.
Fifty packets were reviewed per acute site per quarter. This provided roughly 10% precision around our estimates (ie, if compliance with a measure were 80%, the 95% confidence interval around this estimate would be 70%‐90%). This sample size is consistent with those used to obtain other national benchmarks, such as those for National Hospital Quality Measures, which generally require at least 35 cases per quarter.13
Measures
A multidisciplinary team at Partners derived, reviewed, and refined a minimum data set required to appropriately care for patients during the first 72 hours after transfer from an acute care hospital to a subacute facility. Several of these measures are required by TJC. Other measures were either modifications of TJC measures made to facilitate uniform data collection (eg, history and physical examination at admission instead of significant findings) or additional data elements (not required by TJC) felt to be important to patient care based on the medical literature and interviews with receiving providers at subacute facilities. All measures were refined by the multidisciplinary team with input from additional subspecialists as needed (see Table 1 for the final list of measures).
| Reason(s) for Admission | |
|---|---|
| Joint Commission requirements | A focused history |
| A focused physical exam | |
| Pertinent past medical history | |
| Treatment rendered | |
| Discharge diagnosis(es) | |
| Condition on discharge | |
| Discharge summary | |
| Any information missing | |
| Non‐Joint Commission requirements | |
| Medication information | Discharge medications |
| Drug allergies | |
| Preadmission medication information | |
| Explanation for any differences between preadmission and discharge medications | |
| Test results information | Latest pertinent laboratory results |
| Pertinent radiology results | |
| Test results pending at time of transfer | |
| Overall assessment | Were management and follow‐up plans adequately described? |
| Did you uncover a significant condition not mentioned in the discharge packet? | |
Data Collection
After reviewing the entire discharge documentation packet, reviewers completed a survey concerning the inclusion of the required data elements. Surveys were completed online using Perseus Survey Solutions 6.0 (Perseus Development Corp., Braintree, MA) in the month following discharge (for reviewers at acute care sites) or within 24 hours of admission to the subacute facility (for reviewers at subacute sites). To verify the accuracy and completeness of packets, reviewers at acute sites were instructed to compare the discharge documentation to a review of the inpatient medical record. Similarly, reviewers at subacute sites were instructed to complete their evaluations after admitting each patient to their facility.
Outcomes
The primary outcome was the proportion of packets that contained each data element. In addition, we calculated the proportion of packets that contained all applicable elements required by TJC and all applicable data elements measured in the study. Last, we evaluated two global (albeit subjective) measures of satisfaction with the packet: Were management and follow‐up plans adequately described? (both components needed to be adequately described to get credit for this question) and Did you uncover a significant condition not mentioned in the discharge packet? Significant conditions were defined as active medical problems requiring management during or immediately following the hospitalization.
Statistical Analysis
Results were calculated as proportions, odds ratios, and 95% confidence intervals (CI), using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Simple logistic regression was used to compare inclusion of data elements between medical and surgical services and between academic medical centers and community hospitals. To evaluate interrater reliability, 2 reviewers (both at acute sites) independently evaluated 29 randomly chosen charts, each with 12 data elements.
Results
A total of 1501 discharge documentation packets were reviewed, including 980 patients (65%) from a medical unit and 521 patients (35%) from a surgical unit. Based on 2007 data, these packets represent approximately 4% of all eligible discharges to subacute facilities. Patients discharged from 1 of the 2 academic medical centers represented 44% of the sample. A total of 644 discharge packets (43%) were reviewed at acute sites and 814 packets (54%) were reviewed at subacute sites. Information about reviewer site was missing in 43 discharge packets (3%). For the 29 charts independently reviewed by 2 reviewers, there was complete agreement for 331 out of 348 data elements (95.1%).
Only 1055 (70%) discharge summaries had all the information required by TJC (Table 2). Physical examination at admission (a component of significant findings, as noted above) and condition at discharge were the 2 elements most often missing. The defect‐free rate varied by site, with a range of 61% to 76% across the 5 acute care hospitals (data not shown).
| Sample Size | Missing [n (%)] | 95% CI Missing % | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Reason(s) for admission | 1497 | 14 (0.9) | 0.41.4 |
| A focused history | 1493 | 65 (4.4) | 3.35.3 |
| A focused physical exam | 1493 | 170 (11.4) | 9.713 |
| Pertinent past medical history | 1494 | 69 (4.6) | 3.55.6 |
| Treatment rendered | 1494 | 33 (2.2) | 1.42.9 |
| Discharge diagnosis(es) | 1480 | 53 (3.6) | 2.64.5 |
| Condition on discharge | 1462 | 208 (14.2) | 12.416.0 |
| Discharge summary | 1475 | 90 (6.1) | 4.87.3 |
| Any information missing | 1501 | 447 (29.7) | 27.432.0 |
| Non‐Joint Commission requirements | |||
| Medication information | |||
| Discharge medications | 1491 | 19 (1.3) | 0.71.8 |
| Drug allergies | 1470 | 88 (6.0) | 4.77.2 |
| Preadmission medication information | 1460 | 297 (20.3) | 18.322.4 |
| Explanation for any differences between preadmission and discharge medications | 1060 | 374 (35.3) | 32.038.1 |
| Test results information | |||
| Latest pertinent lab results | 1460 | 261 (17.9) | 15.919.8 |
| Pertinent radiology results | 1303 | 139 (10.7) | 912.4 |
| Test results pending at time of transfer | 341 | 160 (47.2) | 41.952.5 |
| Overall assessment | |||
| Were management and follow‐up plans adequately described? | 1461 | No (%): 161 (11.1) | 95% CI No %: 9.512.7 |
| Did you uncover a significant condition not mentioned in the discharge packet? | 1469 | Yes (%): 162 (11.0) | 95% CI Yes %: 9.413.0 |
| All applicable elements present | 1501 | 503 (33.5) | 31.135.9 |
The rates of inclusion of other (non‐TJC required) data elements are shown in Table 2. Most often missing were preadmission medication regimens, any documented reason for any difference between preadmission and discharge medications, pertinent laboratory results, and an adequate follow‐up plan (including who to follow up with, when to follow‐up, and a list of tasks to be accomplished at the follow‐up visit). Notation regarding significant test results that were pending at the time of transfer was missing in 160 of 341 applicable patients (47%), and in 162 patients (11%), physicians uncovered a significant condition that was not mentioned in the discharge documentation. Only 503 (33.5%) discharge documentation packets had all applicable measures present. In addition, the discharge summary was not received at all on the day of discharge according to the receiving site in 90 patients (6%).
Reviewers were asked in a separate question which missing data were necessary for patient care. Data elements most often cited were explanations for any medication discrepancies and test results pending at the time of the hospital discharge.
Community hospitals had a higher rate of inclusion of TJC‐required data elements when compared to academic medical centers (Table 3). Also, among non‐TJC required data elements, inclusion rates were higher among the community hospitals, especially regarding information about medication discrepancies, pending test results, and follow‐up information (Table 3).
| Total (n) | All Elements Present [n (%)] | OR (95% CI) | |
|---|---|---|---|
| |||
| Joint Commission requirements | |||
| Hospital type | |||
| Community hospitals | 949 | 826 (87) | 2.7 (2.13.6) |
| Academic medical centers | 541 | 384 (71) | Ref. |
| Service | |||
| Medical services | 1013 | 745 (73) | 1.3 (1.01.7) |
| Surgical services | 488 | 332 (68) | Ref. |
| Explanation for any medication discrepancies | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 718 | 550 (76) | 5.0 (3.86.5) |
| Academic medical centers | 342 | 136 (39) | Ref. |
| Service | |||
| Medical services | 754 | 529 (70) | 2.2 (1.72.9) |
| Surgical services | 306 | 157 (51) | Ref. |
| Test results pending at time of transfer | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 172 | 109 (63) | 2.4 (1.53.7) |
| Academic medical centers | 169 | 71 (42) | Ref. |
| Service | |||
| Medical services | 227 | 146 (64) | 4.2 (2.66.9) |
| Surgical services | 114 | 34 (30) | Ref. |
| Follow‐up plans adequately described | Yes [n (%)] | ||
| Hospital type | |||
| Community hospitals | 968 | 883 (91) | 1.7 (1.22.4) |
| Academic medical centers | 543 | 466 (85) | Ref. |
| Service | |||
| Medical services | 983 | 862 (87) | 0.67 (0.51.0) |
| Surgical services | 478 | 437 (91) | Ref. |
Although no differences were found between medical and surgical services regarding compliance with TJC requirements, a difference was noted in documentation of explanations of medication discrepancies and pending test results, with medical services performing better in both measures (Table 3).
In general, reviewers at subacute sites more often evaluated packets as deficient than reviewers at acute sites, up to an absolute difference of 33% in the proportion of missing data, depending on the data element (see Appendix, Table 1).
Discussion
Our study evaluated the completeness of documentation in the discharge summaries of patients discharged from acute care to subacute care facilities. Our results for the inclusion of TJC‐required data elements were similar to those quoted in the literature for patients discharged home.6 Our results also demonstrated a high rate of other missing data elements that are arguably of equal or greater importance, including reasons for discrepancies between preadmission and discharge medication regimens and tests that are pending at the time of discharge.1, 8, 9 Our results also demonstrated the relatively poorer performance of academic centers compared to community hospitals regarding inclusion of information about medication reconciliation, follow‐up, pending test results, and complete information required by TJC. Finally, we found that patients discharged from surgical services more often lacked documentation of medication discrepancies and pending test results compared with patients from medical services.
To our knowledge, this is one of the first studies looking at the quality of information transfer in patients discharged to subacute care facilities. The results of this study are not surprising given the known problems with general information transfer at hospital discharge.1 The fact that community hospitals provided more complete information than academic medical centers for certain data elements may be due to the difference between residents and more senior physicians preparing discharge documentation. Such differences could reflect differences in experience, training, and degree of appreciation for the importance of discharge documentation, and/or restrictions in work hours among residents (eg, resulting in time‐pressure to complete discharge summaries and/or summaries being written by residents who know the patients less well). These hypotheses deserve further exploration. The differences between medical and surgical services should also be validated and explored in other healthcare systems, including both academic and community settings.
The results of this study should be viewed in light of the study's limitations. Packets evaluated by reviewers at subacute facilities were chosen by the reviewers and may not have been representative of all patients received by that facility (in contrast to those reviewed at the acute sites, which were chosen at random and more likely to be representative, although we did not formally test for this). It is possible that reviewers at subacute sites selected the worst discharge documentation packets for evaluation. Second, evaluations by reviewers at subacute sites did not distinguish between information missing from discharge documentation and failure to receive the documentation at all from the acute care hospital (again in contrast to reviewers at acute sites, who always had access to the documentation). Lastly, reviewers at acute and subacute sites may have graded packets differently due to their different clinical perspectives. These 3 factors may explain the relatively poorer results of discharge packets reviewed by reviewers at subacute sites. Further study would be needed to distinguish among these possibilities (eg, having acute and subacute reviewers answer the same questions for the same discharge packets to allow us to measure interrater reliability between the different kinds of reviewers; explicitly asking subacute reviewers about receipt of each piece of documentation; comparing the distribution of diagnosis‐related group [DRG] codes and hospital length of stay in evaluated vs. total discharge packets as a measure of representativeness). We also cannot rule out the possibility of reviewer bias, but all reviewers were trained in a standardized fashion and we know that reliability of assessments were high, at least among reviewers at acute sites. Last, we did not measure actual or potential adverse events caused by these information deficits.
As part of a Partners‐wide initiative to improve transitions in care, the results were presented to the administrations of each of the 5 acute care hospitals. The Partners High Performance Medicine Transition team then began work with a steering committee (composed of representatives from each hospital) to address these deficiencies. Since then, the hospitals have taken several steps to improve the quality of information transfer for discharged patients, including the following:
Technological improvements to the hospitals' discharge ordering systems to actively solicit and/or autoimport the required information into discharge documentation.
Creation of discharge templates to record the required information on paper.
Provision of feedback to clinicians and their service chiefs regarding the ongoing quality of their discharge documentation.
Creation of an online Partners‐wide curriculum on discharge summary authorship, with a mandatory quiz to be taken by all incoming clinicians.
In conclusion, we found room for improvement in the inclusion of data elements required for the safe transfer of patients from acute hospitals to subacute facilities, especially in areas such as medication reconciliation, pending test results, and adequate follow‐up plans. We also found variation by site and type of service. For patients discharged to rehabilitation and other subacute facilities, improvement is needed in the communication of clinically relevant information to those providing continuing care.
Appendix
| JCAHO Indicators | Reviews from Sub‐Acute Sites (N = 814)* | Reviews from Acute Sites (N = 644)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Missing N | % | 95% CI | Sample Size | Missing | % | 95% CI | |
| ||||||||
| Reason(s) for admission | 812 | 9 | 1.1 | 0.41.8 | 643 | 4 | 0.6 | 0.011.2 |
| A focused history | 810 | 49 | 6.1 | 4.47.7 | 642 | 16 | 2.5 | 1.33.7 |
| A focused physical exam | 810 | 131 | 16.2 | 13.718.7 | 641 | 34 | 5.3 | 3.67.0 |
| Pertinent past medical history | 810 | 50 | 6.2 | 4.57.8 | 642 | 14 | 22.0 | 1.13.3 |
| Treatment rendered | 811 | 29 | 3.6 | 2.34.9 | 641 | 4 | 0.6 | 0.011.2 |
| Discharge diagnosis(es) | 806 | 59 | 7.3 | 5.59.1 | 630 | 7 | 1.1 | 0.31.9 |
| Condition on discharge | 800 | 92 | 11.5 | 9.313.7 | 622 | 109 | 17.5 | 14.520.5 |
| Discharge summary | 809 | 77 | 9.5 | 7.511.5 | 624 | 11 | 1.8 | 0.72.8 |
| Any information missing | ||||||||
| Medication Information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Discharge medications | 811 | 12 | 1.5 | 0.72.3 | 638 | 6 | 0.9 | 0.21.7 |
| Drug allergies | 811 | 47 | 5.8 | 4.27.4 | 639 | 35 | 5.5 | 3.77.2 |
| Explanation for any differences between preadmission and discharge medications | 542 | 275 | 50.7 | 46.555 | 498 | 88 | 17.7 | 14.321.0 |
| Test results information | Sample Size | Missing | % | 95% CI | Sample Size | Missing | % | 95% CI |
| Latest pertinent lab results | 790 | 178 | 22.5 | 19.625.4 | 629 | 73 | 11.6 | 9.114.1 |
| Pertinent radiology results | 668 | 110 | 16.5 | 13.719.3 | 601 | 27 | 4.5 | 2.86.2 |
| Test results pending at time of transfer | 183 | 87 | 47.5 | 40.354.8 | 152 | 73 | 48.0 | 40.156.0 |
| Management Information | Sample Size | No | % | 95% CI | Sample Size | No | % | 95% CI |
| Were management and follow‐up plans adequately described? | 794 | 121 | 15.2 | 12.717.7 | 631 | 79 | 12.5 | 9.915.1 |
| Sample Size | Yes | % | 95% CI | Sample Size | Yes | % | 95% CI | |
| Did you uncover a significant condition not mentioned in the discharge packet? | 793 | 117 | 14.8 | 12.317.2 | 635 | 38 | 6.0 | 4.47.8 |
- , , , et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.1989;19:624–631.
- , , , , , .Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- , , , .Effect of discharge summary availability during post‐discharge visits on hospital readmission.JGen Intern Med.2002;17:186–192.
- , , , .Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , et al.Impact of a standardized communication system on continuity of care between family physicians and the emergency department.CJEM.2007;9:79–86.
- , , , et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- , , .Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- Standard IM.6.10: Hospital Accreditation Standards.Oakbrook Terrace, IL:Joint Commission on Accreditation of Healthcare Organizations;2006:338–340.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed July 2009.
- , .Effectiveness of multidisciplinary rehabilitation services in post acute care: state‐of‐the‐science. A review.Arch Phys Med Rehabil.2007;88:1526–1534.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures: Population and Sampling Specifications Version 2.4. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed July 2009.
- , , , et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- , , , .Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.1989;19:624–631.
- , , , , , .Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- , , , .Effect of discharge summary availability during post‐discharge visits on hospital readmission.JGen Intern Med.2002;17:186–192.
- , , , .Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18:646–651.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , et al.Impact of a standardized communication system on continuity of care between family physicians and the emergency department.CJEM.2007;9:79–86.
- , , , et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- , , .Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167:1305–1311.
- Standard IM.6.10: Hospital Accreditation Standards.Oakbrook Terrace, IL:Joint Commission on Accreditation of Healthcare Organizations;2006:338–340.
- Joint Commission on Accreditation of Healthcare Organizations. Joint Commission national patient safety goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed July 2009.
- , .Effectiveness of multidisciplinary rehabilitation services in post acute care: state‐of‐the‐science. A review.Arch Phys Med Rehabil.2007;88:1526–1534.
- Joint Commission on Accreditation of Healthcare Organizations. Specification Manual for National Hospital Quality Measures: Population and Sampling Specifications Version 2.4. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Current+NHQM+Manual.htm. Accessed July 2009.
Copyright © 2009 Society of Hospital Medicine
Letters to the Editor
Insulin is designated a high‐alert medication because of its potential to result in harm if it is used incorrectly.1 Despite this, changes in insulin regimens made in the inpatient setting are often poorly communicated to either the patient or his primary care physician at the time of discharge.2 Poor communication of medication instructions at the time of hospital discharge has been linked to medication errors and adverse drug events.3
We conducted a quality improvement project to improve and standardize the communication of insulin instructions to patients (and/or their caregivers) at hospital discharge. Specifically, we developed and implemented a standardized discharge instructions for insulin (DIFI) form and compared the comprehensiveness of insulin instructions and diabetes‐related readmissions before and after the introduction of the form.
Methods
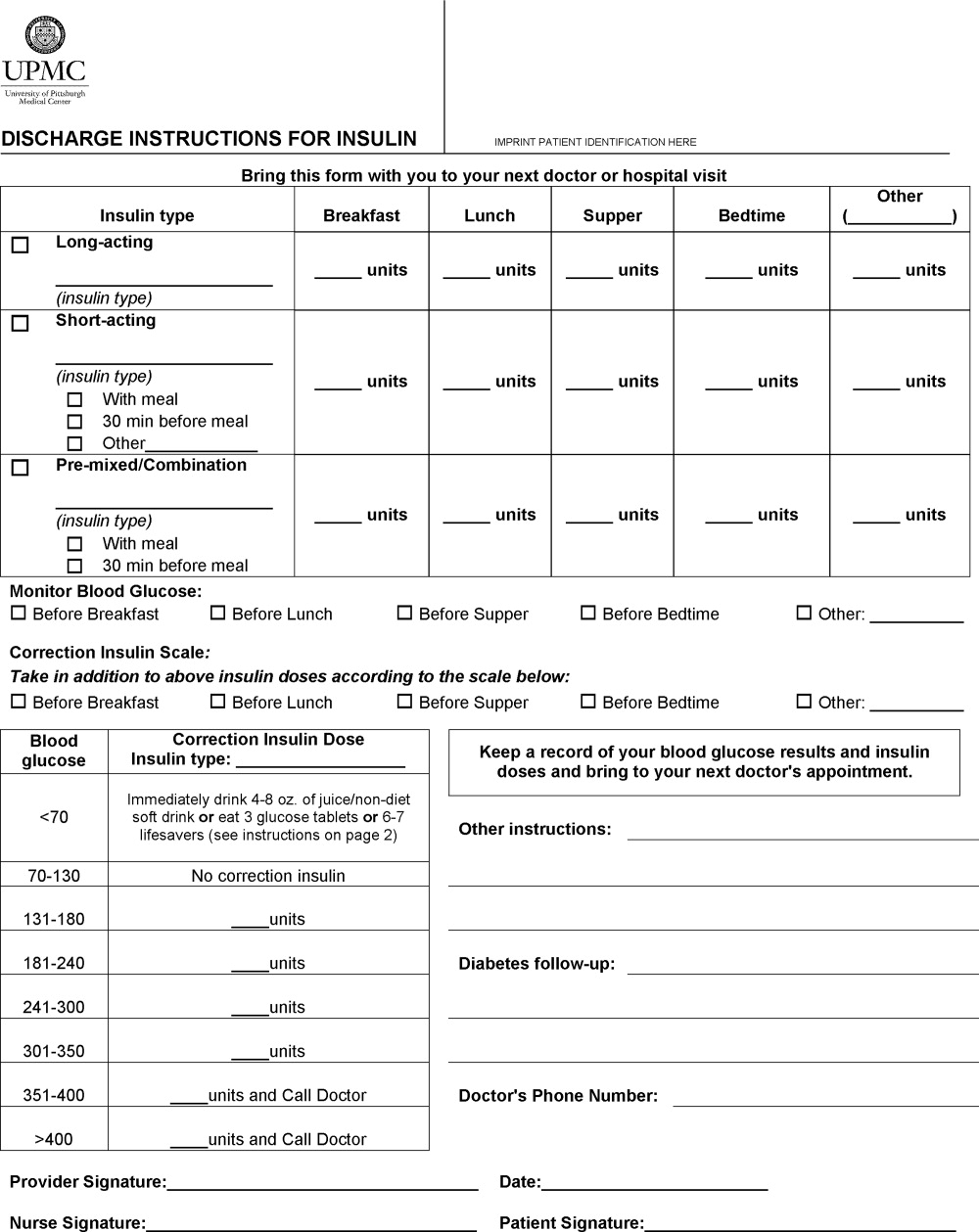
A multidisciplinary team4 created the DIFI form. Page 1 (Figure 1) includes sections for entering all insulin types and doses and the frequency of glucose monitoring and a space for specific diabetes instructions. Page 2 provides general information on symptom recognition and management of hyperglycemia and hypoglycemia. Page 3 is a blank glucose log.
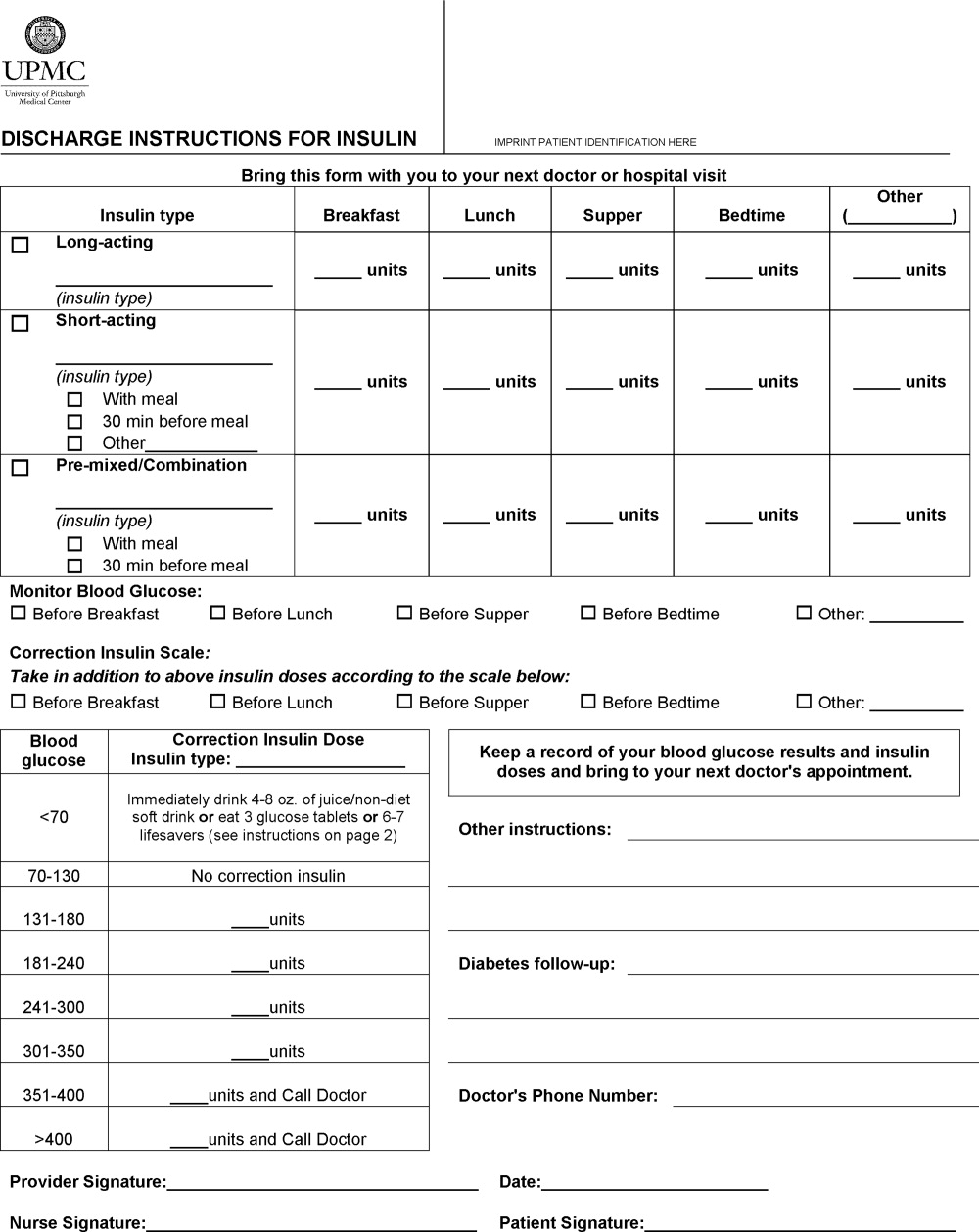
We retrospectively reviewed the records of patients discharged to home on insulin from a general medicine unit during the 3‐month period before availability of the DIFI form and during the 3‐month period afterward. Approval for this project was obtained from the hospital's quality improvement review committee. The percentages of orders with specific instructions for the timing and dosing of basal, prandial, and correction insulin and home glucose monitoring were calculated. The number of patients readmitted within 2 weeks of discharge for a diabetes‐related problem was also determined. Fisher's exact tests were used to compare demographics and indicators in the preimplementation and postimplementation groups.
Results
Chart review was performed for 67 patients with insulin orders at discharge prior to the DIFI form and for 27 patients after implementation. There were no group differences in gender (female gender: 63% pre‐DIFI vs. 63% post‐DIFI, P = 0.49), previous history of diabetes (98.5% vs. 92.6%, P = 0.20), diabetes‐related admitting diagnosis (20.1% vs. 37%, P = 0.12), or insulin use prior to admission (95.5% vs. 85.2%, P = 0.10).
More orders written with the DIFI form contained specific instructions for timing and dosing of basal (67% vs. 100%, P = 0.0003), prandial (51% vs. 100%, P = 0.0008), and correction insulin (14% vs. 95%, P < 0.0001) and for glucose monitoring (17.9% vs. 88.9%, P < 0.0001). There were 4 diabetes‐related readmissions in the preimplementation group and none in the postimplementation group (P = not significant).
Discussion
It is important that patients receive clear directions at the time of hospital discharge to ensure a safe transition of care from the inpatient setting to the outpatient setting. This is particularly true for insulin regimens, which frequently consist of at least 2 different types of insulin and often include instructions for modifying doses on the basis of home glucose readings. The day of hospital discharge is not conducive to recall of verbal communication concerning complicated medication regimens.5 Additionally, our hospital's standard discharge form was not a satisfactory tool for providing detailed directions. We found that the DIFI form prompted a more consistent provision of specific instructions for insulin therapy and glucose monitoring in comparison with previous practice.
- Institute for Safe Medication Practices. ISMP's list of high‐alert medications. Available at: http://www.ismp.org/tools/highalertmedications.pdf. Accessed December 2008.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- , , , , .Evolution of a diabetes inpatient safety committee.Endocr Pract.2006;12(suppl 3):91–99.
- , , , , .Scheduling of pharmacist‐provided medication education for hospitalized patients.Hosp Pharm.2008;43:121–126.
Insulin is designated a high‐alert medication because of its potential to result in harm if it is used incorrectly.1 Despite this, changes in insulin regimens made in the inpatient setting are often poorly communicated to either the patient or his primary care physician at the time of discharge.2 Poor communication of medication instructions at the time of hospital discharge has been linked to medication errors and adverse drug events.3
We conducted a quality improvement project to improve and standardize the communication of insulin instructions to patients (and/or their caregivers) at hospital discharge. Specifically, we developed and implemented a standardized discharge instructions for insulin (DIFI) form and compared the comprehensiveness of insulin instructions and diabetes‐related readmissions before and after the introduction of the form.
Methods
A multidisciplinary team4 created the DIFI form. Page 1 (Figure 1) includes sections for entering all insulin types and doses and the frequency of glucose monitoring and a space for specific diabetes instructions. Page 2 provides general information on symptom recognition and management of hyperglycemia and hypoglycemia. Page 3 is a blank glucose log.

We retrospectively reviewed the records of patients discharged to home on insulin from a general medicine unit during the 3‐month period before availability of the DIFI form and during the 3‐month period afterward. Approval for this project was obtained from the hospital's quality improvement review committee. The percentages of orders with specific instructions for the timing and dosing of basal, prandial, and correction insulin and home glucose monitoring were calculated. The number of patients readmitted within 2 weeks of discharge for a diabetes‐related problem was also determined. Fisher's exact tests were used to compare demographics and indicators in the preimplementation and postimplementation groups.
Results
Chart review was performed for 67 patients with insulin orders at discharge prior to the DIFI form and for 27 patients after implementation. There were no group differences in gender (female gender: 63% pre‐DIFI vs. 63% post‐DIFI, P = 0.49), previous history of diabetes (98.5% vs. 92.6%, P = 0.20), diabetes‐related admitting diagnosis (20.1% vs. 37%, P = 0.12), or insulin use prior to admission (95.5% vs. 85.2%, P = 0.10).
More orders written with the DIFI form contained specific instructions for timing and dosing of basal (67% vs. 100%, P = 0.0003), prandial (51% vs. 100%, P = 0.0008), and correction insulin (14% vs. 95%, P < 0.0001) and for glucose monitoring (17.9% vs. 88.9%, P < 0.0001). There were 4 diabetes‐related readmissions in the preimplementation group and none in the postimplementation group (P = not significant).
Discussion
It is important that patients receive clear directions at the time of hospital discharge to ensure a safe transition of care from the inpatient setting to the outpatient setting. This is particularly true for insulin regimens, which frequently consist of at least 2 different types of insulin and often include instructions for modifying doses on the basis of home glucose readings. The day of hospital discharge is not conducive to recall of verbal communication concerning complicated medication regimens.5 Additionally, our hospital's standard discharge form was not a satisfactory tool for providing detailed directions. We found that the DIFI form prompted a more consistent provision of specific instructions for insulin therapy and glucose monitoring in comparison with previous practice.
Insulin is designated a high‐alert medication because of its potential to result in harm if it is used incorrectly.1 Despite this, changes in insulin regimens made in the inpatient setting are often poorly communicated to either the patient or his primary care physician at the time of discharge.2 Poor communication of medication instructions at the time of hospital discharge has been linked to medication errors and adverse drug events.3
We conducted a quality improvement project to improve and standardize the communication of insulin instructions to patients (and/or their caregivers) at hospital discharge. Specifically, we developed and implemented a standardized discharge instructions for insulin (DIFI) form and compared the comprehensiveness of insulin instructions and diabetes‐related readmissions before and after the introduction of the form.
Methods
A multidisciplinary team4 created the DIFI form. Page 1 (Figure 1) includes sections for entering all insulin types and doses and the frequency of glucose monitoring and a space for specific diabetes instructions. Page 2 provides general information on symptom recognition and management of hyperglycemia and hypoglycemia. Page 3 is a blank glucose log.

We retrospectively reviewed the records of patients discharged to home on insulin from a general medicine unit during the 3‐month period before availability of the DIFI form and during the 3‐month period afterward. Approval for this project was obtained from the hospital's quality improvement review committee. The percentages of orders with specific instructions for the timing and dosing of basal, prandial, and correction insulin and home glucose monitoring were calculated. The number of patients readmitted within 2 weeks of discharge for a diabetes‐related problem was also determined. Fisher's exact tests were used to compare demographics and indicators in the preimplementation and postimplementation groups.
Results
Chart review was performed for 67 patients with insulin orders at discharge prior to the DIFI form and for 27 patients after implementation. There were no group differences in gender (female gender: 63% pre‐DIFI vs. 63% post‐DIFI, P = 0.49), previous history of diabetes (98.5% vs. 92.6%, P = 0.20), diabetes‐related admitting diagnosis (20.1% vs. 37%, P = 0.12), or insulin use prior to admission (95.5% vs. 85.2%, P = 0.10).
More orders written with the DIFI form contained specific instructions for timing and dosing of basal (67% vs. 100%, P = 0.0003), prandial (51% vs. 100%, P = 0.0008), and correction insulin (14% vs. 95%, P < 0.0001) and for glucose monitoring (17.9% vs. 88.9%, P < 0.0001). There were 4 diabetes‐related readmissions in the preimplementation group and none in the postimplementation group (P = not significant).
Discussion
It is important that patients receive clear directions at the time of hospital discharge to ensure a safe transition of care from the inpatient setting to the outpatient setting. This is particularly true for insulin regimens, which frequently consist of at least 2 different types of insulin and often include instructions for modifying doses on the basis of home glucose readings. The day of hospital discharge is not conducive to recall of verbal communication concerning complicated medication regimens.5 Additionally, our hospital's standard discharge form was not a satisfactory tool for providing detailed directions. We found that the DIFI form prompted a more consistent provision of specific instructions for insulin therapy and glucose monitoring in comparison with previous practice.
- Institute for Safe Medication Practices. ISMP's list of high‐alert medications. Available at: http://www.ismp.org/tools/highalertmedications.pdf. Accessed December 2008.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- , , , , .Evolution of a diabetes inpatient safety committee.Endocr Pract.2006;12(suppl 3):91–99.
- , , , , .Scheduling of pharmacist‐provided medication education for hospitalized patients.Hosp Pharm.2008;43:121–126.
- Institute for Safe Medication Practices. ISMP's list of high‐alert medications. Available at: http://www.ismp.org/tools/highalertmedications.pdf. Accessed December 2008.
- , , , .Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- , , , , .The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- , , , , .Evolution of a diabetes inpatient safety committee.Endocr Pract.2006;12(suppl 3):91–99.
- , , , , .Scheduling of pharmacist‐provided medication education for hospitalized patients.Hosp Pharm.2008;43:121–126.
Acid‐Suppressive Therapy
The United States spends a larger share of its gross domestic product (GDP) on healthcare than any other major industrialized country.1 Expenditures for healthcare represent nearly one‐seventh of the nation's GDP, and they continue to be one of the fastest growing components of the federal budget.1 Drug expenditures are one of the most rapidly growing components of total healthcare expenditures.2 Two of the biggest drivers behind this explosive growth of rising drug expenditures are price and use.2, 3
Acid‐suppressive therapy (AST), including histamine‐2 (H2) receptor antagonists and proton pump inhibitors (PPIs), is used extensively in the hospitalized population.4 One of the most common uses of AST in hospitalized patients has been in preventing gastric mucosal damage and bleeding.5 However, published data suggest that the use of AST will be beneficial only in a well‐defined group of critical care patients in preventing stress ulcers and bleeding.68 This perception of benefit has been extrapolated to hospitalized patients in general, with little or no evidence to support its use.7, 9
There have only been limited studies on the overall use or the appropriateness of use of AST in hospitalized patients.7, 9 Also, there have been no studies that have looked at patient or physician factors which can predict the appropriateness of initiation and use of AST in hospitalized patients. The aim of our study was to identify:
The appropriateness of acid suppressive therapy in hospitalized patients admitted to a tertiary teaching institution and the associated cost of inappropriate AST use to the patient.
Patient and physician characteristics which can predict the inappropriate initiation and use of AST in patients.
Methods
This study was conducted at a 308‐bed tertiary academic medical center. On an average, there are approximately 800 to 1000 discharges every month from this hospital. All consecutive discharges over a period of 8 consecutive days were selected for inclusion in the study. All patients were assessed for the use of AST during their hospitalization. Use was defined as any prescription of an acid‐suppressive medication, regardless of dosage regimen, in which the patient received at least 1 dose during their hospitalization.7 The class of agents prescribed for AST was also noted. Ranitidine is the preferred H2 receptor antagonist and pantoprazole is the preferred PPI on the hospital formulary. It was also recorded whether the patient was on the medication at the time of admission. If the patient was on AST prior to admission, the records of the patient were reviewed for the indication for initiation of the AST. The discharge records of all these patients were also reviewed to determine if the patient was continued on AST even after discharge. Patients who were readmitted during the study period were not recounted.
Since the aim of our study was to evaluate the inappropriate initiation of AST in hospitalized patients, the following patients were excluded from the analysis: patients who were on AST prior to admission; patients who had a valid therapeutic indication for AST; and patients who met valid therapeutic indications for AST, such as intensive care unit (ICU) transfers.
Two physicians reviewed the records in order to determine whether there was any indication for AST use. If there was discordance between the 2 physicians, a third physician reviewed the records to assess the appropriateness of AST. Patient and prescribing physician characteristics were collected to assess the predictors of the use of AST.
We used the guidelines published by the American Society of Health‐System Pharmacists (ASHP) to determine appropriateness of gastrointestinal (GI) prophylaxis in patients.10
GI prophylaxis was defined as appropriate if: Patient was in the ICU plus 1 of the following10:
Coagulopathy (ie, platelet count of <50,000 mm3 or international normalized ratio of 1.5, or an activated partial thromboplastin 2 times normal);
Mechanical ventilation for >48 hours;
History of GI ulceration or bleeding within 1 year of admission;
Glasgow coma score of 10;
Thermal injury to >35% of body surface area;
Partial hepatectomy;
Multiple trauma (injury severity score of 16);
Transplantation perioperatively in the ICU;
Spinal cord injury;
Hepatic failure;
Two or more of the following risk factors: sepsis; ICU stay of >1 week; occult bleeding lasting at least 6 days; and high‐dose corticosteroids (>250 mg/day of hydrocortisone or equivalent steroid).
Other indications for the appropriate use of AST were as follows: any documentation of current or past gastroesophageal reflux disease (GERD); active peptic ulcer disease or maintenance therapy in patients with peptic ulcer disease; treatment of esophagitis/gastritis/duodenitis; or patients admitted with upper GI bleeding or melena.
Ranitidine is the preferred H2 receptor antagonist used at this medical center. The cost to the patient of oral ranitidine was $8.54 per day while the cost of intravenous therapy was $135.00 per day. Pantoprazole is the preferred PPI used in this hospital. The cost of oral pantoprazole was $10.57 per day while the cost of intravenous therapy was $57.00 per day (Dr. Joel Reddish, PharmD, Truman Medical Center, Kansas City, MO; Pharmacy Staff; personal communication, September 25, 2007). The cost of intravenous ranitidine was higher than intravenous pantoprazole since ranitidine had to be infused 3 times per day. The cost of AST was calculated by calculating the total number of days during the admission the person was on AST.
Statistical Analysis
All results are expressed as means standard deviations (SDs) or actual frequencies. Univariate logistic regression was used to assess for the predictors of inappropriate use of AST. SAS software version 9.1 (SAS Institute, Inc., Cary, NC) was used for statistical analysis. Multiple logistic regression was used for multivariate analysis. All parameters with a P value of <0.15 were included in the multiple logistic regression model. Backward elimination was done to identify the best‐fitting model for logistic regression.
Previous studies have identified an approximately 50% excessive use of AST.6, 7, 9
A power analysis was performed based upon an alpha level of 0.05, use of a 2‐sided test, and an expected difference between the 2 groups of 25% (75% inappropriate use in one group, 50% inappropriate use in the other). This analysis indicated that 65 patients in each of 2 groups would provide 85% power to detect differences in the prescribing habits of the providers. Therefore it was decided that all discharges over a period of 8 consecutive days would be included in the analysis to meet the required sample size.
Results
There were 207 patients in our study cohort. Of the 207 patients, 103 (49.8%) were males and 71 (34.3%) were Caucasians. Of the 207 patients, AST was used in 164 (79.2%) of the patients. PPI therapy was used in 126 (60.9%) of the patients while 38 (18.4%) of the patients were put on H2 receptor antagonists. In the study cohort, 51 (24.6%) of the patients had a current or a past diagnosis of GERD. Of the 207 patients, 35 patients were on a PPI prior to admission and 16 were on a H2 blocker prior to admission. Table 1 describes the demographic characteristics of the patients.
| Means SD or Actual Frequencies | |
|---|---|
| |
| Patient characteristics | |
| 1) Age (years) | 49.1 16.1 |
| 2) Race (C/AA/H/O) | 71/118/12/6 |
| 3) Gender (male/female) | 103/104 |
| 4) History of diabetes (%) | 52 (25.1) |
| 5) History of hypertension (%) | 116 (56.0) |
| 6) History of CAD (%) | 34 (16.5) |
| 7) ICU stay (%) | 15 (7.3) |
| 8) Current or past GERD (%) | 51 (24.6) |
| 9) Use of PPI/H2 receptor antagonist prior to admission (%) | 51 (24.6) |
| 10) Clopidogrel use (%) | 8 (3.9) |
| 11) Aspirin use (%) | 41 (19.8) |
| 12) Corticosteroid use (%) | 4 (1.9) |
| 13) Coumadin use (%) | 8 (3.9) |
| 14) Hemoglobin (gm/dL) | 12.65 2.55 |
| 15) Platelet count (thousands) | 255 106 |
| 16) Hospital stay (days) | 4.9 6.1 |
| Physician characteristics | |
| 1) PGY1 (%) | 127 (61.4) |
| 2) Medical education (MD) (%) | 161 (77.8) |
| 3) International Medical Graduates (IMGs) (%) | 80 (38.6) |
| 4) Specialty (Medicine) (%) | 158 (76.3) |
The most common primary admitting diagnosis was either cardiovascular or gastrointestinal. Table 2 outlines the most common admitting diagnoses of the patients.
| Diagnoses | Number of Patients (%) |
|---|---|
| |
| 1. Cardiovascular: chest pain/CHF exacerbation/arrhythmias/PVD | 32 (15.5) |
| 2. Gastrointestinal: hematemesis/gastric ulcer/abdominal pain/CLD/pancreatitis | 32 (15.5) |
| 3. Neurologic: syncope/dizziness/stroke/meningitis/altered mental status/seizures | 25 (12.0) |
| 4. Pulmonary: asthma/COPD exacerbation/pneumonia/empyema | 24 (11.6) |
| 5. Trauma/accidents | 15 (7.2) |
| 6. Psychiatric: psychoses/suicidal ideation/substance abuse | 14 (6.8) |
| 7. Infectious: cellulitis/wound infections/ abscesses | 13 (6.3) |
| 8. Oncology | 12 (5.8) |
| 9. Hematologic: sickle cell crises/anemia/thrombocytopenia | 10 (4.8) |
| 10. Renal: renal failure/UTI/hematuria | 8 (3.9) |
| 11. Surgical | 7 (3.4) |
| 12. Others | 15 (7.2) |
To determine the predictors of inappropriate initiation of AST in hospitalized patients, excluding the patients as described in the Methods section, there were 133 patients who met the inclusion criteria for analysis. The reason for inappropriate use of AST in all of the 133 patients included for analysis in our study was for stress ulcer prophylaxis in low‐risk patients. AST was inappropriately used in 92 of the 133 patients (69.2%). On univariate analysis, physician characteristics predictive for inappropriate AST use were being in an early stage of training, physicians in the medicine specialty and physicians who were international medical graduates (Table 3). As far as patient characteristics were concerned, only a higher hemoglobin value was associated with the inappropriate use of AST (see Table 3 for details).
| Parameter | Hazard Ratios | 95% CI (P Value) |
|---|---|---|
| ||
| Patient characteristics | ||
| 1) Age | 1.018 | 0.991.04 (0.15) |
| 2) Race | 1.46 | 0.683.13 (0.32) |
| 3) Gender | 1.03 | 0.492.16 (0.94) |
| 4) History of diabetes | 1.62 | 0.634.14 (0.32) |
| 5) History of hypertension | 1.28 | 0.612.68 (0.52) |
| 6) History of CAD | 1.26 | 0.374.21 (0.71) |
| 7) Nursing home resident | 0.44 | 0.037.20 (0.56) |
| 8) Aspirin use | 1.69 | 0.594.80 (0.33) |
| 9) Clopidogrel use | 0.89 | 0.0810.09 (0.92) |
| 10) Coumadin use | 1.36 | 0.267.04 (0.71) |
| 11) Hemoglobin | 1.24 | 1.061.46 (0.006) |
| 12) Raised WBC count | 0.81 | 0.322.00 (0.64) |
| 13) Platelets | 1.00 | 0.991.001 (0.23) |
| 14) Length of stay | 1.03 | 0.921.15 (0.61) |
| Physician characteristics | ||
| 15) PGY1 (PGY1 vs. others) | 5.18 | 2.3411.50 (<0.0001) |
| 16) Medical education (MD vs. others) | 2.59 | 1.086.17 (0.03) |
| 17) Training (IMG vs.AMG) | 5.34 | 2.0513.93 (0.0006) |
| 18) Specialty (medicine vs. others) | 3.81 | 1.708.55 (0.001) |
On multivariate analysis, as far as patient characteristics were concerned only a higher hemoglobin value was associated with inappropriate AST use. Residents who were in their first year of training as well as physicians with a MD degree were more likely to prescribe AST inappropriately (Table 4).
| Parameter | Hazards Ratio | 95% CI (P value) |
|---|---|---|
| ||
| 1. Hemoglobin (g/dL) | 1.35 | 1.131.62 (0.001) |
| 2. Level of training (PGY‐1 vs. others) | 4.98 | 1.9413.19 (0.0008) |
| 3. Medical education (MD vs. others) | 2.81 | 1.017.83 (0.048) |
The direct calculated patient cost for AST during this time period was $8026. The estimated projected cost for AST over a period of 1 year was $366,000.
Out of the 92 patients in whom AST was used inappropriately, 6 (6.5%) of the patients were discharged on an H2 receptor antagonist while 7 (7.6%) of the patients were discharged on PPI therapy.
Discussion
Prescription drug expenditures are the most rapidly growing component of health care expenditures.2 Two of the biggest drivers behind this explosive growth of rising drug expenditures are price and use.2, 3 PPIs have constantly figured in the national top 20 drug lists for dispensed prescription and drug sales.2
This study found a very high frequency of overuse of acid suppressive therapy in hospitalized patients for stress ulcer prophylaxis. Unfortunately, a large majority (69.2%) of these patients were not at an increased risk of stress‐related mucosal ulceration. One of the reasons for this widespread use of AST is the overestimation of the risk of stress‐related mucosal ulceration in hospitalized patients. However, the fear of stress‐ulcer bleeding seems to largely unjustified, as overall rates of bleeding, as reported previously, have been very low.11 Our results are consistent with the few reports on the overuse of AST reported previously. Nardino et al.,7 in a study of 226 patients, found that 65% of the patients received AST inappropriately. Also in a study from Italy, Parente et al.9 found, in a cohort of 799 hospitalized patients, 68% of the prescriptions for AST were not appropriate.
To date, there has been limited information available on the prescribing characteristics of the physicians, which may help to clarify the inappropriate use of AST. This study was conducted at a tertiary academic medical center and all the admissions are done by residents. This study is the first study that has tried to examine the physician and patient characteristics behind this phenomenon. In multivariate analysis, we found that residents who were in their first year of residency training were more likely to initiate AST inappropriately. This could be secondary to the fact that most of the residents in their first year of training are given blanket orders to put all patients on stress‐ulcer prophylaxis. In a study done by Liberman and Whelan12 at the University of Chicago Hospitals, it was found that house officers learned about stress‐ulcer prophylaxis from their supervising residents. Thus, it is possible that as residents progress through their training, the incidence of inappropriate initiation of stress ulcer prophylaxis decreases. We also found that physicians with an MD degree were more likely to initiate AST inappropriately. The reason behind this not clear, though there may be a difference in the medical education that possibly contributes to this.
One curious finding that was associated with an increased use of AST was a higher hemoglobin level. One possibility is that patients with a low hemoglobin value were more likely to be put on AST appropriately. This could be the reason behind the association of a higher hemoglobin value with inappropriate AST use.
One of the reasons for the widespread use of AST is that most practitioners view AST as harmless.6 However, the use of AST is not without risks. Multiple studies in the past have found an increased risk of Clostridium difficileassociated disease in patients on AST.1316 Also, AST has been associated with an increased risk of community‐acquired pneumonia17 as well as a risk of hip fractures.18 These studies demonstrate that the use of AST is not without its risks and there is a potential for increased morbidity as well as indirect costs for the patient and the community as a whole associated with its use.
The direct cost for this inappropriate use of AST over a period of 8 days was $8026 in our study, with an estimated annual cost close to $366,000. This did not include the cost of patients who were discharged inappropriately with AST. Also, this did not include the indirect costs including the increased risk of community‐acquired pneumonias, hip fractures, and Clostridium infections. Thus, it is possible that the costs of inappropriate use of AST may be much higher than reported.
One of the limitations of our study was that this study was conducted at a single teaching hospital; thus, it is possible that the results could be biased by the prescribing habits of a relatively few physicians. However, since we looked at all specialties, we had a large cohort of physicians in our study. Also, previous multicenter studies as well as single center studies have demonstrated similar results in terms of overprescription.7, 9, 19 Also, the economic impact has been calculated by assessing the cost that is billed to the patients. This may be different from the cost of the medicines to the hospital and insurers.
Conclusions
AST was inappropriately used in 69.2% of the patients studied, leading to an increased direct patient cost of $8026 and projected estimated direct healthcare costs of approximately $366,000 over 1 year. Residents in their first year of training and physicians with an MD degree are more likely to initiate AST inappropriately in patients. Curtailing the inappropriate use of AST therapy may reduce overall costs for the patient and institution.
Acknowledgements
This work was presented in part as an abstract in the Quality Improvement Category at the Missouri State American College of Physicians meeting.
- Agency for Healthcare Research and Quality (AHRQ). Health Care Costs Fact Sheet. Available at: http://www.ahrq.gov/news/costsfact.htm. Accessed March 2009.
- , .Changing prescribing patterns and increasing prescription expenditures in Medicaid.Ann Fam Med.2004;2(5):488–493.
- , , , , .Explaining drug spending trends: does perception match reality?Health Aff (Milwood).2000;19(2):231–239.
- , , .Overuse of acid suppressant drugs in patients with chronic renal failure.Nephrol Dial Transplant.2003;18(3):570–575.
- , , , .Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32(10):2008–2013.
- , .Stress ulcer prophylaxis in hospitalized patients not in intensive care units.Am J Health Syst Pharm.2007;64(13):1396–1400.
- , , .Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95(11):3118–3122.
- , , .Systematic review of the risk of enteric infection in patients taking acid suppression.Am J Gastroenterol.2007;102(9):2047–2056.
- , , , et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17(12):1503–1506.
- ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis.Am J Health Syst Pharm.1999;56(4):347–379.
- , , .Hospital‐acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings.J Hosp Med.2006;1(1):13–20.
- , .Brief report: reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice‐based educational intervention.J Gen Intern Med.2006;21(5):498–500.
- , , , .Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea.J Hosp Infect.2003;54(3):243–245.
- , , , , .Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004;171(1):33–38.
- , , , , , .Proton pump inhibitor therapy is a risk factor for Clostridium difficile‐associated diarrhoea.Aliment Pharmacol Ther.2006;24(4):613–619.
- , , , .Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease.JAMA.2005;294(23):2989–2995.
- , , , , , .Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs.JAMA.2004;292(16):1955–1960.
- , , , .Long‐term proton pump inhibitor therapy and risk of hip fracture.JAMA.2006;296(24):2947–2953.
- , , , et al.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29(4):325–329.
The United States spends a larger share of its gross domestic product (GDP) on healthcare than any other major industrialized country.1 Expenditures for healthcare represent nearly one‐seventh of the nation's GDP, and they continue to be one of the fastest growing components of the federal budget.1 Drug expenditures are one of the most rapidly growing components of total healthcare expenditures.2 Two of the biggest drivers behind this explosive growth of rising drug expenditures are price and use.2, 3
Acid‐suppressive therapy (AST), including histamine‐2 (H2) receptor antagonists and proton pump inhibitors (PPIs), is used extensively in the hospitalized population.4 One of the most common uses of AST in hospitalized patients has been in preventing gastric mucosal damage and bleeding.5 However, published data suggest that the use of AST will be beneficial only in a well‐defined group of critical care patients in preventing stress ulcers and bleeding.68 This perception of benefit has been extrapolated to hospitalized patients in general, with little or no evidence to support its use.7, 9
There have only been limited studies on the overall use or the appropriateness of use of AST in hospitalized patients.7, 9 Also, there have been no studies that have looked at patient or physician factors which can predict the appropriateness of initiation and use of AST in hospitalized patients. The aim of our study was to identify:
The appropriateness of acid suppressive therapy in hospitalized patients admitted to a tertiary teaching institution and the associated cost of inappropriate AST use to the patient.
Patient and physician characteristics which can predict the inappropriate initiation and use of AST in patients.
Methods
This study was conducted at a 308‐bed tertiary academic medical center. On an average, there are approximately 800 to 1000 discharges every month from this hospital. All consecutive discharges over a period of 8 consecutive days were selected for inclusion in the study. All patients were assessed for the use of AST during their hospitalization. Use was defined as any prescription of an acid‐suppressive medication, regardless of dosage regimen, in which the patient received at least 1 dose during their hospitalization.7 The class of agents prescribed for AST was also noted. Ranitidine is the preferred H2 receptor antagonist and pantoprazole is the preferred PPI on the hospital formulary. It was also recorded whether the patient was on the medication at the time of admission. If the patient was on AST prior to admission, the records of the patient were reviewed for the indication for initiation of the AST. The discharge records of all these patients were also reviewed to determine if the patient was continued on AST even after discharge. Patients who were readmitted during the study period were not recounted.
Since the aim of our study was to evaluate the inappropriate initiation of AST in hospitalized patients, the following patients were excluded from the analysis: patients who were on AST prior to admission; patients who had a valid therapeutic indication for AST; and patients who met valid therapeutic indications for AST, such as intensive care unit (ICU) transfers.
Two physicians reviewed the records in order to determine whether there was any indication for AST use. If there was discordance between the 2 physicians, a third physician reviewed the records to assess the appropriateness of AST. Patient and prescribing physician characteristics were collected to assess the predictors of the use of AST.
We used the guidelines published by the American Society of Health‐System Pharmacists (ASHP) to determine appropriateness of gastrointestinal (GI) prophylaxis in patients.10
GI prophylaxis was defined as appropriate if: Patient was in the ICU plus 1 of the following10:
Coagulopathy (ie, platelet count of <50,000 mm3 or international normalized ratio of 1.5, or an activated partial thromboplastin 2 times normal);
Mechanical ventilation for >48 hours;
History of GI ulceration or bleeding within 1 year of admission;
Glasgow coma score of 10;
Thermal injury to >35% of body surface area;
Partial hepatectomy;
Multiple trauma (injury severity score of 16);
Transplantation perioperatively in the ICU;
Spinal cord injury;
Hepatic failure;
Two or more of the following risk factors: sepsis; ICU stay of >1 week; occult bleeding lasting at least 6 days; and high‐dose corticosteroids (>250 mg/day of hydrocortisone or equivalent steroid).
Other indications for the appropriate use of AST were as follows: any documentation of current or past gastroesophageal reflux disease (GERD); active peptic ulcer disease or maintenance therapy in patients with peptic ulcer disease; treatment of esophagitis/gastritis/duodenitis; or patients admitted with upper GI bleeding or melena.
Ranitidine is the preferred H2 receptor antagonist used at this medical center. The cost to the patient of oral ranitidine was $8.54 per day while the cost of intravenous therapy was $135.00 per day. Pantoprazole is the preferred PPI used in this hospital. The cost of oral pantoprazole was $10.57 per day while the cost of intravenous therapy was $57.00 per day (Dr. Joel Reddish, PharmD, Truman Medical Center, Kansas City, MO; Pharmacy Staff; personal communication, September 25, 2007). The cost of intravenous ranitidine was higher than intravenous pantoprazole since ranitidine had to be infused 3 times per day. The cost of AST was calculated by calculating the total number of days during the admission the person was on AST.
Statistical Analysis
All results are expressed as means standard deviations (SDs) or actual frequencies. Univariate logistic regression was used to assess for the predictors of inappropriate use of AST. SAS software version 9.1 (SAS Institute, Inc., Cary, NC) was used for statistical analysis. Multiple logistic regression was used for multivariate analysis. All parameters with a P value of <0.15 were included in the multiple logistic regression model. Backward elimination was done to identify the best‐fitting model for logistic regression.
Previous studies have identified an approximately 50% excessive use of AST.6, 7, 9
A power analysis was performed based upon an alpha level of 0.05, use of a 2‐sided test, and an expected difference between the 2 groups of 25% (75% inappropriate use in one group, 50% inappropriate use in the other). This analysis indicated that 65 patients in each of 2 groups would provide 85% power to detect differences in the prescribing habits of the providers. Therefore it was decided that all discharges over a period of 8 consecutive days would be included in the analysis to meet the required sample size.
Results
There were 207 patients in our study cohort. Of the 207 patients, 103 (49.8%) were males and 71 (34.3%) were Caucasians. Of the 207 patients, AST was used in 164 (79.2%) of the patients. PPI therapy was used in 126 (60.9%) of the patients while 38 (18.4%) of the patients were put on H2 receptor antagonists. In the study cohort, 51 (24.6%) of the patients had a current or a past diagnosis of GERD. Of the 207 patients, 35 patients were on a PPI prior to admission and 16 were on a H2 blocker prior to admission. Table 1 describes the demographic characteristics of the patients.
| Means SD or Actual Frequencies | |
|---|---|
| |
| Patient characteristics | |
| 1) Age (years) | 49.1 16.1 |
| 2) Race (C/AA/H/O) | 71/118/12/6 |
| 3) Gender (male/female) | 103/104 |
| 4) History of diabetes (%) | 52 (25.1) |
| 5) History of hypertension (%) | 116 (56.0) |
| 6) History of CAD (%) | 34 (16.5) |
| 7) ICU stay (%) | 15 (7.3) |
| 8) Current or past GERD (%) | 51 (24.6) |
| 9) Use of PPI/H2 receptor antagonist prior to admission (%) | 51 (24.6) |
| 10) Clopidogrel use (%) | 8 (3.9) |
| 11) Aspirin use (%) | 41 (19.8) |
| 12) Corticosteroid use (%) | 4 (1.9) |
| 13) Coumadin use (%) | 8 (3.9) |
| 14) Hemoglobin (gm/dL) | 12.65 2.55 |
| 15) Platelet count (thousands) | 255 106 |
| 16) Hospital stay (days) | 4.9 6.1 |
| Physician characteristics | |
| 1) PGY1 (%) | 127 (61.4) |
| 2) Medical education (MD) (%) | 161 (77.8) |
| 3) International Medical Graduates (IMGs) (%) | 80 (38.6) |
| 4) Specialty (Medicine) (%) | 158 (76.3) |
The most common primary admitting diagnosis was either cardiovascular or gastrointestinal. Table 2 outlines the most common admitting diagnoses of the patients.
| Diagnoses | Number of Patients (%) |
|---|---|
| |
| 1. Cardiovascular: chest pain/CHF exacerbation/arrhythmias/PVD | 32 (15.5) |
| 2. Gastrointestinal: hematemesis/gastric ulcer/abdominal pain/CLD/pancreatitis | 32 (15.5) |
| 3. Neurologic: syncope/dizziness/stroke/meningitis/altered mental status/seizures | 25 (12.0) |
| 4. Pulmonary: asthma/COPD exacerbation/pneumonia/empyema | 24 (11.6) |
| 5. Trauma/accidents | 15 (7.2) |
| 6. Psychiatric: psychoses/suicidal ideation/substance abuse | 14 (6.8) |
| 7. Infectious: cellulitis/wound infections/ abscesses | 13 (6.3) |
| 8. Oncology | 12 (5.8) |
| 9. Hematologic: sickle cell crises/anemia/thrombocytopenia | 10 (4.8) |
| 10. Renal: renal failure/UTI/hematuria | 8 (3.9) |
| 11. Surgical | 7 (3.4) |
| 12. Others | 15 (7.2) |
To determine the predictors of inappropriate initiation of AST in hospitalized patients, excluding the patients as described in the Methods section, there were 133 patients who met the inclusion criteria for analysis. The reason for inappropriate use of AST in all of the 133 patients included for analysis in our study was for stress ulcer prophylaxis in low‐risk patients. AST was inappropriately used in 92 of the 133 patients (69.2%). On univariate analysis, physician characteristics predictive for inappropriate AST use were being in an early stage of training, physicians in the medicine specialty and physicians who were international medical graduates (Table 3). As far as patient characteristics were concerned, only a higher hemoglobin value was associated with the inappropriate use of AST (see Table 3 for details).
| Parameter | Hazard Ratios | 95% CI (P Value) |
|---|---|---|
| ||
| Patient characteristics | ||
| 1) Age | 1.018 | 0.991.04 (0.15) |
| 2) Race | 1.46 | 0.683.13 (0.32) |
| 3) Gender | 1.03 | 0.492.16 (0.94) |
| 4) History of diabetes | 1.62 | 0.634.14 (0.32) |
| 5) History of hypertension | 1.28 | 0.612.68 (0.52) |
| 6) History of CAD | 1.26 | 0.374.21 (0.71) |
| 7) Nursing home resident | 0.44 | 0.037.20 (0.56) |
| 8) Aspirin use | 1.69 | 0.594.80 (0.33) |
| 9) Clopidogrel use | 0.89 | 0.0810.09 (0.92) |
| 10) Coumadin use | 1.36 | 0.267.04 (0.71) |
| 11) Hemoglobin | 1.24 | 1.061.46 (0.006) |
| 12) Raised WBC count | 0.81 | 0.322.00 (0.64) |
| 13) Platelets | 1.00 | 0.991.001 (0.23) |
| 14) Length of stay | 1.03 | 0.921.15 (0.61) |
| Physician characteristics | ||
| 15) PGY1 (PGY1 vs. others) | 5.18 | 2.3411.50 (<0.0001) |
| 16) Medical education (MD vs. others) | 2.59 | 1.086.17 (0.03) |
| 17) Training (IMG vs.AMG) | 5.34 | 2.0513.93 (0.0006) |
| 18) Specialty (medicine vs. others) | 3.81 | 1.708.55 (0.001) |
On multivariate analysis, as far as patient characteristics were concerned only a higher hemoglobin value was associated with inappropriate AST use. Residents who were in their first year of training as well as physicians with a MD degree were more likely to prescribe AST inappropriately (Table 4).
| Parameter | Hazards Ratio | 95% CI (P value) |
|---|---|---|
| ||
| 1. Hemoglobin (g/dL) | 1.35 | 1.131.62 (0.001) |
| 2. Level of training (PGY‐1 vs. others) | 4.98 | 1.9413.19 (0.0008) |
| 3. Medical education (MD vs. others) | 2.81 | 1.017.83 (0.048) |
The direct calculated patient cost for AST during this time period was $8026. The estimated projected cost for AST over a period of 1 year was $366,000.
Out of the 92 patients in whom AST was used inappropriately, 6 (6.5%) of the patients were discharged on an H2 receptor antagonist while 7 (7.6%) of the patients were discharged on PPI therapy.
Discussion
Prescription drug expenditures are the most rapidly growing component of health care expenditures.2 Two of the biggest drivers behind this explosive growth of rising drug expenditures are price and use.2, 3 PPIs have constantly figured in the national top 20 drug lists for dispensed prescription and drug sales.2
This study found a very high frequency of overuse of acid suppressive therapy in hospitalized patients for stress ulcer prophylaxis. Unfortunately, a large majority (69.2%) of these patients were not at an increased risk of stress‐related mucosal ulceration. One of the reasons for this widespread use of AST is the overestimation of the risk of stress‐related mucosal ulceration in hospitalized patients. However, the fear of stress‐ulcer bleeding seems to largely unjustified, as overall rates of bleeding, as reported previously, have been very low.11 Our results are consistent with the few reports on the overuse of AST reported previously. Nardino et al.,7 in a study of 226 patients, found that 65% of the patients received AST inappropriately. Also in a study from Italy, Parente et al.9 found, in a cohort of 799 hospitalized patients, 68% of the prescriptions for AST were not appropriate.
To date, there has been limited information available on the prescribing characteristics of the physicians, which may help to clarify the inappropriate use of AST. This study was conducted at a tertiary academic medical center and all the admissions are done by residents. This study is the first study that has tried to examine the physician and patient characteristics behind this phenomenon. In multivariate analysis, we found that residents who were in their first year of residency training were more likely to initiate AST inappropriately. This could be secondary to the fact that most of the residents in their first year of training are given blanket orders to put all patients on stress‐ulcer prophylaxis. In a study done by Liberman and Whelan12 at the University of Chicago Hospitals, it was found that house officers learned about stress‐ulcer prophylaxis from their supervising residents. Thus, it is possible that as residents progress through their training, the incidence of inappropriate initiation of stress ulcer prophylaxis decreases. We also found that physicians with an MD degree were more likely to initiate AST inappropriately. The reason behind this not clear, though there may be a difference in the medical education that possibly contributes to this.
One curious finding that was associated with an increased use of AST was a higher hemoglobin level. One possibility is that patients with a low hemoglobin value were more likely to be put on AST appropriately. This could be the reason behind the association of a higher hemoglobin value with inappropriate AST use.
One of the reasons for the widespread use of AST is that most practitioners view AST as harmless.6 However, the use of AST is not without risks. Multiple studies in the past have found an increased risk of Clostridium difficileassociated disease in patients on AST.1316 Also, AST has been associated with an increased risk of community‐acquired pneumonia17 as well as a risk of hip fractures.18 These studies demonstrate that the use of AST is not without its risks and there is a potential for increased morbidity as well as indirect costs for the patient and the community as a whole associated with its use.
The direct cost for this inappropriate use of AST over a period of 8 days was $8026 in our study, with an estimated annual cost close to $366,000. This did not include the cost of patients who were discharged inappropriately with AST. Also, this did not include the indirect costs including the increased risk of community‐acquired pneumonias, hip fractures, and Clostridium infections. Thus, it is possible that the costs of inappropriate use of AST may be much higher than reported.
One of the limitations of our study was that this study was conducted at a single teaching hospital; thus, it is possible that the results could be biased by the prescribing habits of a relatively few physicians. However, since we looked at all specialties, we had a large cohort of physicians in our study. Also, previous multicenter studies as well as single center studies have demonstrated similar results in terms of overprescription.7, 9, 19 Also, the economic impact has been calculated by assessing the cost that is billed to the patients. This may be different from the cost of the medicines to the hospital and insurers.
Conclusions
AST was inappropriately used in 69.2% of the patients studied, leading to an increased direct patient cost of $8026 and projected estimated direct healthcare costs of approximately $366,000 over 1 year. Residents in their first year of training and physicians with an MD degree are more likely to initiate AST inappropriately in patients. Curtailing the inappropriate use of AST therapy may reduce overall costs for the patient and institution.
Acknowledgements
This work was presented in part as an abstract in the Quality Improvement Category at the Missouri State American College of Physicians meeting.
The United States spends a larger share of its gross domestic product (GDP) on healthcare than any other major industrialized country.1 Expenditures for healthcare represent nearly one‐seventh of the nation's GDP, and they continue to be one of the fastest growing components of the federal budget.1 Drug expenditures are one of the most rapidly growing components of total healthcare expenditures.2 Two of the biggest drivers behind this explosive growth of rising drug expenditures are price and use.2, 3
Acid‐suppressive therapy (AST), including histamine‐2 (H2) receptor antagonists and proton pump inhibitors (PPIs), is used extensively in the hospitalized population.4 One of the most common uses of AST in hospitalized patients has been in preventing gastric mucosal damage and bleeding.5 However, published data suggest that the use of AST will be beneficial only in a well‐defined group of critical care patients in preventing stress ulcers and bleeding.68 This perception of benefit has been extrapolated to hospitalized patients in general, with little or no evidence to support its use.7, 9
There have only been limited studies on the overall use or the appropriateness of use of AST in hospitalized patients.7, 9 Also, there have been no studies that have looked at patient or physician factors which can predict the appropriateness of initiation and use of AST in hospitalized patients. The aim of our study was to identify:
The appropriateness of acid suppressive therapy in hospitalized patients admitted to a tertiary teaching institution and the associated cost of inappropriate AST use to the patient.
Patient and physician characteristics which can predict the inappropriate initiation and use of AST in patients.
Methods
This study was conducted at a 308‐bed tertiary academic medical center. On an average, there are approximately 800 to 1000 discharges every month from this hospital. All consecutive discharges over a period of 8 consecutive days were selected for inclusion in the study. All patients were assessed for the use of AST during their hospitalization. Use was defined as any prescription of an acid‐suppressive medication, regardless of dosage regimen, in which the patient received at least 1 dose during their hospitalization.7 The class of agents prescribed for AST was also noted. Ranitidine is the preferred H2 receptor antagonist and pantoprazole is the preferred PPI on the hospital formulary. It was also recorded whether the patient was on the medication at the time of admission. If the patient was on AST prior to admission, the records of the patient were reviewed for the indication for initiation of the AST. The discharge records of all these patients were also reviewed to determine if the patient was continued on AST even after discharge. Patients who were readmitted during the study period were not recounted.
Since the aim of our study was to evaluate the inappropriate initiation of AST in hospitalized patients, the following patients were excluded from the analysis: patients who were on AST prior to admission; patients who had a valid therapeutic indication for AST; and patients who met valid therapeutic indications for AST, such as intensive care unit (ICU) transfers.
Two physicians reviewed the records in order to determine whether there was any indication for AST use. If there was discordance between the 2 physicians, a third physician reviewed the records to assess the appropriateness of AST. Patient and prescribing physician characteristics were collected to assess the predictors of the use of AST.
We used the guidelines published by the American Society of Health‐System Pharmacists (ASHP) to determine appropriateness of gastrointestinal (GI) prophylaxis in patients.10
GI prophylaxis was defined as appropriate if: Patient was in the ICU plus 1 of the following10:
Coagulopathy (ie, platelet count of <50,000 mm3 or international normalized ratio of 1.5, or an activated partial thromboplastin 2 times normal);
Mechanical ventilation for >48 hours;
History of GI ulceration or bleeding within 1 year of admission;
Glasgow coma score of 10;
Thermal injury to >35% of body surface area;
Partial hepatectomy;
Multiple trauma (injury severity score of 16);
Transplantation perioperatively in the ICU;
Spinal cord injury;
Hepatic failure;
Two or more of the following risk factors: sepsis; ICU stay of >1 week; occult bleeding lasting at least 6 days; and high‐dose corticosteroids (>250 mg/day of hydrocortisone or equivalent steroid).
Other indications for the appropriate use of AST were as follows: any documentation of current or past gastroesophageal reflux disease (GERD); active peptic ulcer disease or maintenance therapy in patients with peptic ulcer disease; treatment of esophagitis/gastritis/duodenitis; or patients admitted with upper GI bleeding or melena.
Ranitidine is the preferred H2 receptor antagonist used at this medical center. The cost to the patient of oral ranitidine was $8.54 per day while the cost of intravenous therapy was $135.00 per day. Pantoprazole is the preferred PPI used in this hospital. The cost of oral pantoprazole was $10.57 per day while the cost of intravenous therapy was $57.00 per day (Dr. Joel Reddish, PharmD, Truman Medical Center, Kansas City, MO; Pharmacy Staff; personal communication, September 25, 2007). The cost of intravenous ranitidine was higher than intravenous pantoprazole since ranitidine had to be infused 3 times per day. The cost of AST was calculated by calculating the total number of days during the admission the person was on AST.
Statistical Analysis
All results are expressed as means standard deviations (SDs) or actual frequencies. Univariate logistic regression was used to assess for the predictors of inappropriate use of AST. SAS software version 9.1 (SAS Institute, Inc., Cary, NC) was used for statistical analysis. Multiple logistic regression was used for multivariate analysis. All parameters with a P value of <0.15 were included in the multiple logistic regression model. Backward elimination was done to identify the best‐fitting model for logistic regression.
Previous studies have identified an approximately 50% excessive use of AST.6, 7, 9
A power analysis was performed based upon an alpha level of 0.05, use of a 2‐sided test, and an expected difference between the 2 groups of 25% (75% inappropriate use in one group, 50% inappropriate use in the other). This analysis indicated that 65 patients in each of 2 groups would provide 85% power to detect differences in the prescribing habits of the providers. Therefore it was decided that all discharges over a period of 8 consecutive days would be included in the analysis to meet the required sample size.
Results
There were 207 patients in our study cohort. Of the 207 patients, 103 (49.8%) were males and 71 (34.3%) were Caucasians. Of the 207 patients, AST was used in 164 (79.2%) of the patients. PPI therapy was used in 126 (60.9%) of the patients while 38 (18.4%) of the patients were put on H2 receptor antagonists. In the study cohort, 51 (24.6%) of the patients had a current or a past diagnosis of GERD. Of the 207 patients, 35 patients were on a PPI prior to admission and 16 were on a H2 blocker prior to admission. Table 1 describes the demographic characteristics of the patients.
| Means SD or Actual Frequencies | |
|---|---|
| |
| Patient characteristics | |
| 1) Age (years) | 49.1 16.1 |
| 2) Race (C/AA/H/O) | 71/118/12/6 |
| 3) Gender (male/female) | 103/104 |
| 4) History of diabetes (%) | 52 (25.1) |
| 5) History of hypertension (%) | 116 (56.0) |
| 6) History of CAD (%) | 34 (16.5) |
| 7) ICU stay (%) | 15 (7.3) |
| 8) Current or past GERD (%) | 51 (24.6) |
| 9) Use of PPI/H2 receptor antagonist prior to admission (%) | 51 (24.6) |
| 10) Clopidogrel use (%) | 8 (3.9) |
| 11) Aspirin use (%) | 41 (19.8) |
| 12) Corticosteroid use (%) | 4 (1.9) |
| 13) Coumadin use (%) | 8 (3.9) |
| 14) Hemoglobin (gm/dL) | 12.65 2.55 |
| 15) Platelet count (thousands) | 255 106 |
| 16) Hospital stay (days) | 4.9 6.1 |
| Physician characteristics | |
| 1) PGY1 (%) | 127 (61.4) |
| 2) Medical education (MD) (%) | 161 (77.8) |
| 3) International Medical Graduates (IMGs) (%) | 80 (38.6) |
| 4) Specialty (Medicine) (%) | 158 (76.3) |
The most common primary admitting diagnosis was either cardiovascular or gastrointestinal. Table 2 outlines the most common admitting diagnoses of the patients.
| Diagnoses | Number of Patients (%) |
|---|---|
| |
| 1. Cardiovascular: chest pain/CHF exacerbation/arrhythmias/PVD | 32 (15.5) |
| 2. Gastrointestinal: hematemesis/gastric ulcer/abdominal pain/CLD/pancreatitis | 32 (15.5) |
| 3. Neurologic: syncope/dizziness/stroke/meningitis/altered mental status/seizures | 25 (12.0) |
| 4. Pulmonary: asthma/COPD exacerbation/pneumonia/empyema | 24 (11.6) |
| 5. Trauma/accidents | 15 (7.2) |
| 6. Psychiatric: psychoses/suicidal ideation/substance abuse | 14 (6.8) |
| 7. Infectious: cellulitis/wound infections/ abscesses | 13 (6.3) |
| 8. Oncology | 12 (5.8) |
| 9. Hematologic: sickle cell crises/anemia/thrombocytopenia | 10 (4.8) |
| 10. Renal: renal failure/UTI/hematuria | 8 (3.9) |
| 11. Surgical | 7 (3.4) |
| 12. Others | 15 (7.2) |
To determine the predictors of inappropriate initiation of AST in hospitalized patients, excluding the patients as described in the Methods section, there were 133 patients who met the inclusion criteria for analysis. The reason for inappropriate use of AST in all of the 133 patients included for analysis in our study was for stress ulcer prophylaxis in low‐risk patients. AST was inappropriately used in 92 of the 133 patients (69.2%). On univariate analysis, physician characteristics predictive for inappropriate AST use were being in an early stage of training, physicians in the medicine specialty and physicians who were international medical graduates (Table 3). As far as patient characteristics were concerned, only a higher hemoglobin value was associated with the inappropriate use of AST (see Table 3 for details).
| Parameter | Hazard Ratios | 95% CI (P Value) |
|---|---|---|
| ||
| Patient characteristics | ||
| 1) Age | 1.018 | 0.991.04 (0.15) |
| 2) Race | 1.46 | 0.683.13 (0.32) |
| 3) Gender | 1.03 | 0.492.16 (0.94) |
| 4) History of diabetes | 1.62 | 0.634.14 (0.32) |
| 5) History of hypertension | 1.28 | 0.612.68 (0.52) |
| 6) History of CAD | 1.26 | 0.374.21 (0.71) |
| 7) Nursing home resident | 0.44 | 0.037.20 (0.56) |
| 8) Aspirin use | 1.69 | 0.594.80 (0.33) |
| 9) Clopidogrel use | 0.89 | 0.0810.09 (0.92) |
| 10) Coumadin use | 1.36 | 0.267.04 (0.71) |
| 11) Hemoglobin | 1.24 | 1.061.46 (0.006) |
| 12) Raised WBC count | 0.81 | 0.322.00 (0.64) |
| 13) Platelets | 1.00 | 0.991.001 (0.23) |
| 14) Length of stay | 1.03 | 0.921.15 (0.61) |
| Physician characteristics | ||
| 15) PGY1 (PGY1 vs. others) | 5.18 | 2.3411.50 (<0.0001) |
| 16) Medical education (MD vs. others) | 2.59 | 1.086.17 (0.03) |
| 17) Training (IMG vs.AMG) | 5.34 | 2.0513.93 (0.0006) |
| 18) Specialty (medicine vs. others) | 3.81 | 1.708.55 (0.001) |
On multivariate analysis, as far as patient characteristics were concerned only a higher hemoglobin value was associated with inappropriate AST use. Residents who were in their first year of training as well as physicians with a MD degree were more likely to prescribe AST inappropriately (Table 4).
| Parameter | Hazards Ratio | 95% CI (P value) |
|---|---|---|
| ||
| 1. Hemoglobin (g/dL) | 1.35 | 1.131.62 (0.001) |
| 2. Level of training (PGY‐1 vs. others) | 4.98 | 1.9413.19 (0.0008) |
| 3. Medical education (MD vs. others) | 2.81 | 1.017.83 (0.048) |
The direct calculated patient cost for AST during this time period was $8026. The estimated projected cost for AST over a period of 1 year was $366,000.
Out of the 92 patients in whom AST was used inappropriately, 6 (6.5%) of the patients were discharged on an H2 receptor antagonist while 7 (7.6%) of the patients were discharged on PPI therapy.
Discussion
Prescription drug expenditures are the most rapidly growing component of health care expenditures.2 Two of the biggest drivers behind this explosive growth of rising drug expenditures are price and use.2, 3 PPIs have constantly figured in the national top 20 drug lists for dispensed prescription and drug sales.2
This study found a very high frequency of overuse of acid suppressive therapy in hospitalized patients for stress ulcer prophylaxis. Unfortunately, a large majority (69.2%) of these patients were not at an increased risk of stress‐related mucosal ulceration. One of the reasons for this widespread use of AST is the overestimation of the risk of stress‐related mucosal ulceration in hospitalized patients. However, the fear of stress‐ulcer bleeding seems to largely unjustified, as overall rates of bleeding, as reported previously, have been very low.11 Our results are consistent with the few reports on the overuse of AST reported previously. Nardino et al.,7 in a study of 226 patients, found that 65% of the patients received AST inappropriately. Also in a study from Italy, Parente et al.9 found, in a cohort of 799 hospitalized patients, 68% of the prescriptions for AST were not appropriate.
To date, there has been limited information available on the prescribing characteristics of the physicians, which may help to clarify the inappropriate use of AST. This study was conducted at a tertiary academic medical center and all the admissions are done by residents. This study is the first study that has tried to examine the physician and patient characteristics behind this phenomenon. In multivariate analysis, we found that residents who were in their first year of residency training were more likely to initiate AST inappropriately. This could be secondary to the fact that most of the residents in their first year of training are given blanket orders to put all patients on stress‐ulcer prophylaxis. In a study done by Liberman and Whelan12 at the University of Chicago Hospitals, it was found that house officers learned about stress‐ulcer prophylaxis from their supervising residents. Thus, it is possible that as residents progress through their training, the incidence of inappropriate initiation of stress ulcer prophylaxis decreases. We also found that physicians with an MD degree were more likely to initiate AST inappropriately. The reason behind this not clear, though there may be a difference in the medical education that possibly contributes to this.
One curious finding that was associated with an increased use of AST was a higher hemoglobin level. One possibility is that patients with a low hemoglobin value were more likely to be put on AST appropriately. This could be the reason behind the association of a higher hemoglobin value with inappropriate AST use.
One of the reasons for the widespread use of AST is that most practitioners view AST as harmless.6 However, the use of AST is not without risks. Multiple studies in the past have found an increased risk of Clostridium difficileassociated disease in patients on AST.1316 Also, AST has been associated with an increased risk of community‐acquired pneumonia17 as well as a risk of hip fractures.18 These studies demonstrate that the use of AST is not without its risks and there is a potential for increased morbidity as well as indirect costs for the patient and the community as a whole associated with its use.
The direct cost for this inappropriate use of AST over a period of 8 days was $8026 in our study, with an estimated annual cost close to $366,000. This did not include the cost of patients who were discharged inappropriately with AST. Also, this did not include the indirect costs including the increased risk of community‐acquired pneumonias, hip fractures, and Clostridium infections. Thus, it is possible that the costs of inappropriate use of AST may be much higher than reported.
One of the limitations of our study was that this study was conducted at a single teaching hospital; thus, it is possible that the results could be biased by the prescribing habits of a relatively few physicians. However, since we looked at all specialties, we had a large cohort of physicians in our study. Also, previous multicenter studies as well as single center studies have demonstrated similar results in terms of overprescription.7, 9, 19 Also, the economic impact has been calculated by assessing the cost that is billed to the patients. This may be different from the cost of the medicines to the hospital and insurers.
Conclusions
AST was inappropriately used in 69.2% of the patients studied, leading to an increased direct patient cost of $8026 and projected estimated direct healthcare costs of approximately $366,000 over 1 year. Residents in their first year of training and physicians with an MD degree are more likely to initiate AST inappropriately in patients. Curtailing the inappropriate use of AST therapy may reduce overall costs for the patient and institution.
Acknowledgements
This work was presented in part as an abstract in the Quality Improvement Category at the Missouri State American College of Physicians meeting.
- Agency for Healthcare Research and Quality (AHRQ). Health Care Costs Fact Sheet. Available at: http://www.ahrq.gov/news/costsfact.htm. Accessed March 2009.
- , .Changing prescribing patterns and increasing prescription expenditures in Medicaid.Ann Fam Med.2004;2(5):488–493.
- , , , , .Explaining drug spending trends: does perception match reality?Health Aff (Milwood).2000;19(2):231–239.
- , , .Overuse of acid suppressant drugs in patients with chronic renal failure.Nephrol Dial Transplant.2003;18(3):570–575.
- , , , .Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32(10):2008–2013.
- , .Stress ulcer prophylaxis in hospitalized patients not in intensive care units.Am J Health Syst Pharm.2007;64(13):1396–1400.
- , , .Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95(11):3118–3122.
- , , .Systematic review of the risk of enteric infection in patients taking acid suppression.Am J Gastroenterol.2007;102(9):2047–2056.
- , , , et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17(12):1503–1506.
- ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis.Am J Health Syst Pharm.1999;56(4):347–379.
- , , .Hospital‐acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings.J Hosp Med.2006;1(1):13–20.
- , .Brief report: reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice‐based educational intervention.J Gen Intern Med.2006;21(5):498–500.
- , , , .Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea.J Hosp Infect.2003;54(3):243–245.
- , , , , .Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004;171(1):33–38.
- , , , , , .Proton pump inhibitor therapy is a risk factor for Clostridium difficile‐associated diarrhoea.Aliment Pharmacol Ther.2006;24(4):613–619.
- , , , .Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease.JAMA.2005;294(23):2989–2995.
- , , , , , .Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs.JAMA.2004;292(16):1955–1960.
- , , , .Long‐term proton pump inhibitor therapy and risk of hip fracture.JAMA.2006;296(24):2947–2953.
- , , , et al.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29(4):325–329.
- Agency for Healthcare Research and Quality (AHRQ). Health Care Costs Fact Sheet. Available at: http://www.ahrq.gov/news/costsfact.htm. Accessed March 2009.
- , .Changing prescribing patterns and increasing prescription expenditures in Medicaid.Ann Fam Med.2004;2(5):488–493.
- , , , , .Explaining drug spending trends: does perception match reality?Health Aff (Milwood).2000;19(2):231–239.
- , , .Overuse of acid suppressant drugs in patients with chronic renal failure.Nephrol Dial Transplant.2003;18(3):570–575.
- , , , .Prevention of stress ulceration: current trends in critical care.Crit Care Med.2004;32(10):2008–2013.
- , .Stress ulcer prophylaxis in hospitalized patients not in intensive care units.Am J Health Syst Pharm.2007;64(13):1396–1400.
- , , .Overuse of acid‐suppressive therapy in hospitalized patients.Am J Gastroenterol.2000;95(11):3118–3122.
- , , .Systematic review of the risk of enteric infection in patients taking acid suppression.Am J Gastroenterol.2007;102(9):2047–2056.
- , , , et al.Hospital use of acid‐suppressive medications and its fall‐out on prescribing in general practice: a 1‐month survey.Aliment Pharmacol Ther.2003;17(12):1503–1506.
- ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis.Am J Health Syst Pharm.1999;56(4):347–379.
- , , .Hospital‐acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings.J Hosp Med.2006;1(1):13–20.
- , .Brief report: reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice‐based educational intervention.J Gen Intern Med.2006;21(5):498–500.
- , , , .Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea.J Hosp Infect.2003;54(3):243–245.
- , , , , .Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case‐control studies.CMAJ.2004;171(1):33–38.
- , , , , , .Proton pump inhibitor therapy is a risk factor for Clostridium difficile‐associated diarrhoea.Aliment Pharmacol Ther.2006;24(4):613–619.
- , , , .Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease.JAMA.2005;294(23):2989–2995.
- , , , , , .Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs.JAMA.2004;292(16):1955–1960.
- , , , .Long‐term proton pump inhibitor therapy and risk of hip fracture.JAMA.2006;296(24):2947–2953.
- , , , et al.Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study.Ital J Gastroenterol Hepatol.1997;29(4):325–329.
Copyright © 2009 Society of Hospital Medicine
Multioccupancy Hospital Rooms
Originally championed in the form of large multibed wards by Florence Nightingale in the nineteenth century, multioccupancy hospital rooms have recently been criticized for concerns about their cost, safety, lack of privacy, and unpopularity among patients.13 Specifically, they have been linked to longer hospital stays and the morbidity that those stays produceinfections, falls, and medical errors.13 In 2006, the Health Guidelines Revision Committee, the body that establishes guidelines for the construction of healthcare facilities in the United States, moved to a position recommending private rooms as the minimum standard for medical/surgical and postpartum hospital beds. The evidence supporting their position, however, has remained limited. Given the substantial cost and effort involved in converting hospital rooms, it is important to understand the impact of such policies on patients.
In a recent literature review by van de Glind et al.,4 the authors were able to identify only 4 randomized controlled trials comparing private and shared rooms. While they found that private rooms had a moderately positive impact on patient satisfaction and privacy, data on infection control was mixed, and data on patient safety was lacking.48 Though suggested, the association between private rooms and shortened hospital stays has not clearly been shown.13, 9 Certain advantages of shared rooms, such as decreased loneliness and increased patient social interaction, have also been identified.10 In addition, specialized multioccupancy wards for dementia and delirium have been demonstrated to be useful in the management of inpatients with those conditions, partly because of increased nursing presence.11 Finally, an additional theoretical benefit of shared rooms is the possibility of assistance between roommates in emergency situations.
For the sake of providing high‐quality patient‐centered care, patient concerns and preferences are also important to consider when establishing health policy regarding rooms. Previously, patient surveys and interviews have been helpful in identifying issues of concern to patients and understanding their preferences. Kirk12 identified privacy, quiet, improved sleep, and ability to have a family member stay in the room as reasons why 18 of 24 hospice patients preferred private hospice rooms.12 Conversely, in a 2002 Welsh survey of palliative patients, 68% wanted to be in an open area (4‐bed bay), identifying companionship as the major reason.13 Most recently, palliative care patients in the United Kingdom expressed a preference for shared rooms while well enough to interact with others but preferred private rooms when very ill or dying.14 These findings, given the lack of solid medical justification for 1 room type, raise multiple concerns about whether universal adherence to 1 room type will lead to the best patient care in the United States, and more specifically the veteran population. First, nearly all of the preference data described has been collected outside of the United States and veteran's populations. Second, studies have targeted highly‐specialized patient populations, and their applicability in a general medical/surgical population is unknown.10, 1217 Finally, the diversity of preferences exhibited in these studies underscores the difficulty in applying preference data to outside populations, such as the veteran population.
With 7.8 million patients enrolled in its healthcare system and operating 153 medical centers, the U.S. Department of Veterans Affairs has a large interest in the ongoing debate about shared vs. private hospital rooms. Because veterans share a common military background, they may place a higher value on companionship relative to privacy, and so they are an important group to study separately from the general U.S. population. To date, we have been unable to identify any studies that have looked at veterans' experiences and preferences in regards to room type. Through an anonymous survey, we sought to learn about these experiences and preferences and assess whether previously found advantages of each room type (eg, companionship in shared rooms, privacy in private rooms) could be confirmed in the veteran population. By doing so, we also aimed to better inform public policy about a general continued role for shared hospital rooms in the United States.
Methods
Our study used a point prevalence survey to examine patient preferences and experiences with shared (2‐person or 3‐person occupancy) vs. private rooms.
Design
From September to November 2007 patients admitted to the Washington DC VA Medical Center medical service (a tertiary care facility, with 76 beds on the medical service, 37 shared and 39 private) were asked to complete anonymous written surveys prior to discharge. Male patients were assigned rooms based on bed availability only, with the exception of those requiring isolation or those receiving chemotherapy, which mandated private rooms. Patients were not able to request specific room types. Planned patient discharges were identified daily by consulting each ward unit's central discharge list. One member of the research team not directly involved in the patient's care would approach the patient to see if he was willing to participate. Participants were asked to fill out the surveys independently, but those who were incapable of doing so due to physical limitations were allowed assistance by family, a member of the research team, or the nurse, if they so requested. Patients were asked to leave completed surveys in provided blank envelopes for pickup by a member of the research team. These were later marked as coming from either private, shared, or isolation rooms. Exclusion criteria included hospitalization for chemotherapy and female sex. Patients who were not able to complete surveys because of cognitive impairment were considered nonparticipants. Although data from patients in private contact isolation rooms was collected, it was left out of analysis, as assignment to these rooms was based upon medical condition rather than solely on bed availability. To account for patients who switched room type during their hospitalization, 1 survey question asked for their predominant room type. Power analysis suggested that a sample size of 70 patients per group would give 80% power to demonstrate differences of 20% (estimated difference expected for key measures) between the groups at a 95% confidence level.
Data Collection
The survey instrument was designed for the purposes of this study as no suitable existing tool could be found. Questions were drawn from issues identified in the literature on loneliness, fear, and anxiety, as well as author hypothesis about potential social benefits of having roommates.1013 Questions included demographics (age, race, education, household income); hospital experiences including adequacy of privacy (Do you feel that the privacy in your room is adequate?), nursing availability (Have you felt that a nurse was available when you needed one?), loneliness (Have you been lonely during this hospital stay?), fear of death, interactions with roommates (Have any of your roommates helped you in any way [like calling for a nurse]?); and private vs. shared room preference. Except for 1 open‐ended question asking the reason for room preference, questions were yes/no.
Data Analysis
Chi‐square testing was performed to determine whether there were any baseline differences between the private vs. shared groups in terms of demographic variables and length of stay; whether room type was associated with differences in perception of privacy, nursing availability, fear of death, and loneliness; and to determine differences in room preference with regard to demographic variables. Fisher 2‐tailed exact testing was performed to evaluate differences in room preference based upon room type, as the sample sizes for this comparison were small. For the open‐ended question, responses were coded into categories by 1 research team member (W.E.) and reviewed by another team member (K.C.). Consensus was reached through discussion.
The protocol received exemption from Institutional Review Board oversight and approval from the Research and Development Committee.
Results
A total of 162 surveys were completed with a participation rate of approximately 73%. Eighty three patients (51%) reported a shared room stay, while 70 patients (43%) reported a private room stay (17 of which were isolation). Nine did not report room type (Figure 1). Excluding isolation patients and those respondents who did not indicate room type, 5% of respondents reported age less than 45 years, 56% between 45 and 65 years, and 39% greater than 65 years. Sixty percent of patients identified themselves as African‐American, 28% as white, and 5% as other (7% did not answer). The majority (56%) reported having some formal higher education after high school. Median total household income was between $11,000 and $20,000.
Private vs. Shared
Comparison of demographic information between the private and shared groups revealed no significant differences in age, race, education, or income distribution (Table 1). There was also no difference in reported length of stay. Because some respondents failed or declined to answer certain questions, the number of responses reported for each category varied. Notably, 79% of patients staying in private rooms reported that they had been in shared rooms before, while 78% of patients in shared rooms reported that they had been in private rooms before, indicating that both groups were familiar with each type of room.
| Private (n = 53) (%) | Shared (n = 83) (%) | P Value* | |
|---|---|---|---|
| |||
| Age (years) | 0.63 | ||
| <45 | 3 (6) | 4 (5) | |
| 45‐65 | 32 (60) | 44 (53) | |
| >65 | 18 (34) | 35 (42) | |
| Race | 0.80 | ||
| Caucasian | 15 (28) | 23 (28) | |
| African‐American | 34 (64) | 48 (58) | |
| Other | 2 (4) | 5 (6) | |
| No response | 2 (4) | 7 (8) | |
| Education | 0.67 | ||
| 4 (8) | 10 (12) | ||
| High school | 16 (30) | 26 (31) | |
| Some college | 20 (38) | 31 (37) | |
| College | 4 (8) | 10 (12) | |
| >College | 6 (11) | 5 (6) | |
| No response | 3 (6) | 1 (1) | |
| Annual household income | 0.17 | ||
| <$10,000 | 10 (19) | 21 (25) | |
| $11,000‐$20,000 | 16 (30) | 19 (23) | |
| $21,000‐$35,000 | 4 (8) | 19 (23) | |
| $36,000‐$60,000 | 9 (17) | 12 (14) | |
| >$60,000 | 6 (11) | 6 (7) | |
| No response | 8 (15) | 6 (7) | |
| Length of stay (days) | 0.90 | ||
| <5 | 35 (66) | 52 (63) | |
| 6‐10 | 12 (23) | 21 (25) | |
| >10 | 5 (9) | 9 (11) | |
| No response | 1 (2) | 1 (1) | |
| Previous experience in opposite room type | 0.73 | ||
| Yes | 42 (79) | 65 (78) | |
| No | 10 (19) | 18 (22) | |
| No response | 1 (2) | 0 | |
In describing their experiences, patients in private rooms were more likely to report adequate privacy (92% vs. 53%; P 0.01) and available nursing (79% vs. 64%; P = 0.025) than patients in shared rooms (Table 2). There was no difference in the amount of reported loneliness or fear of death. For patients with roommate interactions, 66% replied that they enjoyed talking with their roommates (Table 3). A minority (31%) indicated that they had been bothered by sights, noises, or messes made by their roommates. A majority of patients reported giving help to roommates (59%), and a substantial percentage reported receiving help (35%).
| Private (n = 53) (%) | Shared (n = 83) (%) | P Value* | |
|---|---|---|---|
| |||
| Privacy adequate | 49 (92) | 44 (53) | <0.01 |
| Felt nurse was available | 42 (79) | 53 (64) | 0.025 |
| Felt lonely | 11 (21) | 18 (22) | 0.913 |
| Had fear of dying | 5 (9) | 13 (16) | 0.309 |
| Yes (%) | No (%) | N/A or Missing (%) | |
|---|---|---|---|
| |||
| Enjoyed conversation | 69 (66) | 15 (14) | 20 (19) |
| Bothered by roommate | 32 (31) | 70 (67) | 2 (2) |
| Received help from roommate | 36 (35) | 65 (63) | 3 (3) |
| Gave help to roommate | 61 (59) | 39 (38) | 4 (4) |
Patient Preferences
Of the 117 patients who expressed a preference for a private or shared room, 92 (79%) stated that they preferred private rooms (Table 4). Patients in shared rooms were more likely to prefer shared rooms than patients in private rooms. Race did not impact preference. Patients older than 65 years were more likely to prefer shared rooms than patients younger than 65 years (31% vs. 15%; P = 0.042), although the preference for private rooms persisted across all demographic groups.
| Prefer Private (%) | Prefer Shared (%) | P Value* | |
|---|---|---|---|
| |||
| Total (n = 117) | 92 (79) | 25 (21) | |
| Comparison by race | |||
| Caucasian (n = 31) | 25 (81) | 6 (19) | 0.60 |
| African‐American (n = 75) | 57 (76) | 18 (24) | |
| Comparison by age (years) | |||
| Age >65 (n = 45) | 31 (69) | 14 (31) | 0.042 |
| Age <65 (n = 72) | 61 (85) | 11 (15) | |
| Comparison by current room status | |||
| Private (n = 43) | 41 (95) | 2 (5) | <0.01 |
| Shared (n = 71) | 50 (70) | 21 (30) | |
Ninety‐two patients responded to the open‐ended prompt to state reasons for these preferences, most commonly citing privacy and peace and quiet (Table 5). Other reasons included worrying about germs, being unwilling to share the television, wanting a private bathroom, being bothered by smells, feeling a lack of security, and wanting to have space for family. Patients who preferred shared rooms most commonly cited a desire for companionship.
|
| Private (80) |
| Privacy (33): privacy, like to be alone |
| Peace/quiet (28): snoring roommates, sleep better |
| Room amenities (7): television sharing, bathroom is within |
| Germs (6): germs, diseases |
| Security (2): security, belongings |
| Family (2): daughter had to sleep in lounge |
| Smells (2): smells |
| Shared (14) |
| Company (13): someone to talk to |
| Safety (1): safer, someone to look out for you |
| Neutral (6): |
| No preference (5): doesn't matter, what's available |
| Depends on circumstances (1): depends on sickness |
Discussion
In this observational study of U.S. veterans, we found that patients in private rooms were more satisfied with privacy than their shared‐room counterparts, a finding consistent with previous studies.4, 5 We also found that private room patients were more likely to feel that nursing was available, a finding that had not previously been reported. Although increased loneliness and fear of death had been noted in private room patients before, our study did not support such findings.10 We found a strong overall preference for private rooms, adding to previously mixed evidence about patient room preferences.10, 1217 Although we had hypothesized a substantial demand for shared rooms among veterans based upon their common military experience, we found the demand modest at best.
The increased satisfaction with privacy among private room patients was expected.4, 5 That nearly one‐half of the patients in shared rooms found the privacy to be inadequate, however, was a strong finding that points to a fundamental problem with these rooms. That private room patient also felt more that nurses were available was surprising, since the higher number of patients in shared rooms might have been expected to generate a more visible nursing presence. Whether this finding is reproducible and whether it is based upon an objective difference in nursing behavior is something that should be studied further. It may simply have been a marker of increased satisfaction among the private room patients.
Although loneliness and increased fear of death had previously been identified as possible drawbacks of private rooms, our survey found no such suggestion.10 This may have been because our survey instrument simply asked patients to answer yes or no whether they had experienced the particular feelings; the previous study had used interviews. A more sensitive survey instrument would be needed to investigate questions of loneliness and fear of death further.
Except for the problem of privacy, the shared room experience generally appeared to be a positive one for patients, particularly in the exchange of conversation and help. A genuine value of these interactions to the patients was suggested by the finding that patients in shared rooms were more likely to prefer shared rooms than patients in private rooms. It is possible that once in them, patients found shared rooms to be better than expected, or that they warmed to them as a matter of resolving cognitive dissonance between their preconceptions and their placement. Although peace and quiet was a frequently cited reason for preferring private rooms, relatively few patients reported being bothered by their roommates. The high rate of assistance among roommates was perhaps the most surprising positive about the shared room experience, even if patients recalled helping others more than they remembered being helped themselves.
Preference for private rooms was greater than that found in previous studies, and appears to have been based largely upon concerns for privacy.1217 We suspect it may reflect changing societal values that have placed an increased priority on personal privacy; this is suggested by the greater willingness of elderly patients to stay in shared rooms. Whether the preference for private rooms is even higher in the general nonveteran population is something that should be assessed with future studies.
Our study was limited by a number of factors. While room assignments were based on bed availability, it is possible that patients requested particular rooms or were moved due to other factors. By selecting a study population of male veterans of the armed services, we limited the extent to which our results can be generalized. However, this particular population is an important one to study due to the size of the VA healthcare system, the prevalence of shared rooms in VA hospitals, and the unique cultural values of veterans. We were unable to obtain information from approximately 27% of our target population because they were unable or unwilling to participate, thus excluding a population subset from analysis and potentially biasing our results. Although a high prevalence of preference for private rooms was seen, the strength of the preference was not assessed, and patients were unable to convey if they had no preference regarding room type. The survey instrument was designed for the purposes of this study and has not been validated. Some patients were assisted with filling out their surveys and may have responded more positively about their roommates if they thought their roommates could overhear their answers to questions. Because patient enrollment was slower than expected, fewer patients were enrolled than planned and the power achieved was insufficient for detecting differences of 20% or less between groups. This is unlikely to have affected our positive findings, but may have prevented us from detecting additional differences among the study groups.
Our data shows that for a population of veteran patients, private rooms are preferred, and that increased privacy is a primary advantage. It also demonstrates that multioccupancy rooms have several positive aspects. Given the need for sound public policy in regard to this issue, further research is needed to better evaluate objective outcomes of room type, such as fall rates, nosocomial infection rates, and lengths of hospitalization. In the meantime, efforts should be taken to address some of the known problems of shared rooms. For instance, establishing more substantial dividers between beds would be an intervention that could increase privacy, reduce noise, and minimize unwelcome smells. Additionally, given that patients have a wide variety of feelings toward multioccupancy rooms, incorporating patient choice into room assignments when logistically feasible is a step that could lead to increased patient satisfaction with hospitalization.
- , , . The use of single patient rooms vs. multiple occupancy rooms in acute care environments. Avaliable at: http://www.healthdesign.org/research/reports/single_patient_rooms.php. Accessed March,2009.
- , , .Advantages and disadvantages of single‐versus multiple‐occupancy rooms in acute care environments: a review and analysis of the literature.Environ Behav.2005;37:760–786.
- , .Multibed versus single‐bed rooms. In: Kobus R, Skaggs RL, Bobrow M, Thomas J, Payette M, eds.Building Type Basics For Healthcare Facilities.New York:John Wiley;2000:145–157.
- , , .Do patients in hospitals benefit from single rooms? A literature review.Health Policy.2007;84:153–161.
- , , , , .Single room maternity care and client satisfaction.Birth.2000;27(4):235–243.
- , , .Do appealing hospital rooms increase patient evaluation of physicians, nurses, and hospital services?Health Care Manage Rev.2003;28(3):254–264.
- , , .The effect of private isolation rooms on patient care practices, colonization and infection in an intensive care unit.Am J Med.1981;70(3):641–645.
- , , .The effect of increased bed numbers on MRSA transmission in acute medical wards.J Hosp Infect.1998;39(3):213–219.
- , .Streamlining patient care processes through flexible room and equipment design.Crit Care Nurs Q.24(3):59–76.
- , , , .A psychological comparison of patients in “open” and “closed” coronary care units.J Psychosom Res.1972;16:449–457.
- , , , , , .A model for managing delirious older patients.J Am Geriatr Soc.2003;51:1031–1035.
- .Patient preferences for a single or shared room in a hospice.Nurs Times.2002;98(50):39–41.
- , .Do patients and their relatives prefer single cubicles or shared wards?Palliat Med.2002;16(5):445–446.
- , .How does the environment impact on the quality of life of advanced cancer patients? A qualitative study with implications for ward design.Palliat Med.2008;22(6):768–774.
- , .Roommates.Am J Nurs.1973;73(1):104–107.
- .Single rooms and patient choice.Nurs Stand.2005;20(9):41–48.
- .Patients' wishes regarding sickrooms.Nurs Times.1990;86(20):53.
Originally championed in the form of large multibed wards by Florence Nightingale in the nineteenth century, multioccupancy hospital rooms have recently been criticized for concerns about their cost, safety, lack of privacy, and unpopularity among patients.13 Specifically, they have been linked to longer hospital stays and the morbidity that those stays produceinfections, falls, and medical errors.13 In 2006, the Health Guidelines Revision Committee, the body that establishes guidelines for the construction of healthcare facilities in the United States, moved to a position recommending private rooms as the minimum standard for medical/surgical and postpartum hospital beds. The evidence supporting their position, however, has remained limited. Given the substantial cost and effort involved in converting hospital rooms, it is important to understand the impact of such policies on patients.
In a recent literature review by van de Glind et al.,4 the authors were able to identify only 4 randomized controlled trials comparing private and shared rooms. While they found that private rooms had a moderately positive impact on patient satisfaction and privacy, data on infection control was mixed, and data on patient safety was lacking.48 Though suggested, the association between private rooms and shortened hospital stays has not clearly been shown.13, 9 Certain advantages of shared rooms, such as decreased loneliness and increased patient social interaction, have also been identified.10 In addition, specialized multioccupancy wards for dementia and delirium have been demonstrated to be useful in the management of inpatients with those conditions, partly because of increased nursing presence.11 Finally, an additional theoretical benefit of shared rooms is the possibility of assistance between roommates in emergency situations.
For the sake of providing high‐quality patient‐centered care, patient concerns and preferences are also important to consider when establishing health policy regarding rooms. Previously, patient surveys and interviews have been helpful in identifying issues of concern to patients and understanding their preferences. Kirk12 identified privacy, quiet, improved sleep, and ability to have a family member stay in the room as reasons why 18 of 24 hospice patients preferred private hospice rooms.12 Conversely, in a 2002 Welsh survey of palliative patients, 68% wanted to be in an open area (4‐bed bay), identifying companionship as the major reason.13 Most recently, palliative care patients in the United Kingdom expressed a preference for shared rooms while well enough to interact with others but preferred private rooms when very ill or dying.14 These findings, given the lack of solid medical justification for 1 room type, raise multiple concerns about whether universal adherence to 1 room type will lead to the best patient care in the United States, and more specifically the veteran population. First, nearly all of the preference data described has been collected outside of the United States and veteran's populations. Second, studies have targeted highly‐specialized patient populations, and their applicability in a general medical/surgical population is unknown.10, 1217 Finally, the diversity of preferences exhibited in these studies underscores the difficulty in applying preference data to outside populations, such as the veteran population.
With 7.8 million patients enrolled in its healthcare system and operating 153 medical centers, the U.S. Department of Veterans Affairs has a large interest in the ongoing debate about shared vs. private hospital rooms. Because veterans share a common military background, they may place a higher value on companionship relative to privacy, and so they are an important group to study separately from the general U.S. population. To date, we have been unable to identify any studies that have looked at veterans' experiences and preferences in regards to room type. Through an anonymous survey, we sought to learn about these experiences and preferences and assess whether previously found advantages of each room type (eg, companionship in shared rooms, privacy in private rooms) could be confirmed in the veteran population. By doing so, we also aimed to better inform public policy about a general continued role for shared hospital rooms in the United States.
Methods
Our study used a point prevalence survey to examine patient preferences and experiences with shared (2‐person or 3‐person occupancy) vs. private rooms.
Design
From September to November 2007 patients admitted to the Washington DC VA Medical Center medical service (a tertiary care facility, with 76 beds on the medical service, 37 shared and 39 private) were asked to complete anonymous written surveys prior to discharge. Male patients were assigned rooms based on bed availability only, with the exception of those requiring isolation or those receiving chemotherapy, which mandated private rooms. Patients were not able to request specific room types. Planned patient discharges were identified daily by consulting each ward unit's central discharge list. One member of the research team not directly involved in the patient's care would approach the patient to see if he was willing to participate. Participants were asked to fill out the surveys independently, but those who were incapable of doing so due to physical limitations were allowed assistance by family, a member of the research team, or the nurse, if they so requested. Patients were asked to leave completed surveys in provided blank envelopes for pickup by a member of the research team. These were later marked as coming from either private, shared, or isolation rooms. Exclusion criteria included hospitalization for chemotherapy and female sex. Patients who were not able to complete surveys because of cognitive impairment were considered nonparticipants. Although data from patients in private contact isolation rooms was collected, it was left out of analysis, as assignment to these rooms was based upon medical condition rather than solely on bed availability. To account for patients who switched room type during their hospitalization, 1 survey question asked for their predominant room type. Power analysis suggested that a sample size of 70 patients per group would give 80% power to demonstrate differences of 20% (estimated difference expected for key measures) between the groups at a 95% confidence level.
Data Collection
The survey instrument was designed for the purposes of this study as no suitable existing tool could be found. Questions were drawn from issues identified in the literature on loneliness, fear, and anxiety, as well as author hypothesis about potential social benefits of having roommates.1013 Questions included demographics (age, race, education, household income); hospital experiences including adequacy of privacy (Do you feel that the privacy in your room is adequate?), nursing availability (Have you felt that a nurse was available when you needed one?), loneliness (Have you been lonely during this hospital stay?), fear of death, interactions with roommates (Have any of your roommates helped you in any way [like calling for a nurse]?); and private vs. shared room preference. Except for 1 open‐ended question asking the reason for room preference, questions were yes/no.
Data Analysis
Chi‐square testing was performed to determine whether there were any baseline differences between the private vs. shared groups in terms of demographic variables and length of stay; whether room type was associated with differences in perception of privacy, nursing availability, fear of death, and loneliness; and to determine differences in room preference with regard to demographic variables. Fisher 2‐tailed exact testing was performed to evaluate differences in room preference based upon room type, as the sample sizes for this comparison were small. For the open‐ended question, responses were coded into categories by 1 research team member (W.E.) and reviewed by another team member (K.C.). Consensus was reached through discussion.
The protocol received exemption from Institutional Review Board oversight and approval from the Research and Development Committee.
Results
A total of 162 surveys were completed with a participation rate of approximately 73%. Eighty three patients (51%) reported a shared room stay, while 70 patients (43%) reported a private room stay (17 of which were isolation). Nine did not report room type (Figure 1). Excluding isolation patients and those respondents who did not indicate room type, 5% of respondents reported age less than 45 years, 56% between 45 and 65 years, and 39% greater than 65 years. Sixty percent of patients identified themselves as African‐American, 28% as white, and 5% as other (7% did not answer). The majority (56%) reported having some formal higher education after high school. Median total household income was between $11,000 and $20,000.

Private vs. Shared
Comparison of demographic information between the private and shared groups revealed no significant differences in age, race, education, or income distribution (Table 1). There was also no difference in reported length of stay. Because some respondents failed or declined to answer certain questions, the number of responses reported for each category varied. Notably, 79% of patients staying in private rooms reported that they had been in shared rooms before, while 78% of patients in shared rooms reported that they had been in private rooms before, indicating that both groups were familiar with each type of room.
| Private (n = 53) (%) | Shared (n = 83) (%) | P Value* | |
|---|---|---|---|
| |||
| Age (years) | 0.63 | ||
| <45 | 3 (6) | 4 (5) | |
| 45‐65 | 32 (60) | 44 (53) | |
| >65 | 18 (34) | 35 (42) | |
| Race | 0.80 | ||
| Caucasian | 15 (28) | 23 (28) | |
| African‐American | 34 (64) | 48 (58) | |
| Other | 2 (4) | 5 (6) | |
| No response | 2 (4) | 7 (8) | |
| Education | 0.67 | ||
| 4 (8) | 10 (12) | ||
| High school | 16 (30) | 26 (31) | |
| Some college | 20 (38) | 31 (37) | |
| College | 4 (8) | 10 (12) | |
| >College | 6 (11) | 5 (6) | |
| No response | 3 (6) | 1 (1) | |
| Annual household income | 0.17 | ||
| <$10,000 | 10 (19) | 21 (25) | |
| $11,000‐$20,000 | 16 (30) | 19 (23) | |
| $21,000‐$35,000 | 4 (8) | 19 (23) | |
| $36,000‐$60,000 | 9 (17) | 12 (14) | |
| >$60,000 | 6 (11) | 6 (7) | |
| No response | 8 (15) | 6 (7) | |
| Length of stay (days) | 0.90 | ||
| <5 | 35 (66) | 52 (63) | |
| 6‐10 | 12 (23) | 21 (25) | |
| >10 | 5 (9) | 9 (11) | |
| No response | 1 (2) | 1 (1) | |
| Previous experience in opposite room type | 0.73 | ||
| Yes | 42 (79) | 65 (78) | |
| No | 10 (19) | 18 (22) | |
| No response | 1 (2) | 0 | |
In describing their experiences, patients in private rooms were more likely to report adequate privacy (92% vs. 53%; P 0.01) and available nursing (79% vs. 64%; P = 0.025) than patients in shared rooms (Table 2). There was no difference in the amount of reported loneliness or fear of death. For patients with roommate interactions, 66% replied that they enjoyed talking with their roommates (Table 3). A minority (31%) indicated that they had been bothered by sights, noises, or messes made by their roommates. A majority of patients reported giving help to roommates (59%), and a substantial percentage reported receiving help (35%).
| Private (n = 53) (%) | Shared (n = 83) (%) | P Value* | |
|---|---|---|---|
| |||
| Privacy adequate | 49 (92) | 44 (53) | <0.01 |
| Felt nurse was available | 42 (79) | 53 (64) | 0.025 |
| Felt lonely | 11 (21) | 18 (22) | 0.913 |
| Had fear of dying | 5 (9) | 13 (16) | 0.309 |
| Yes (%) | No (%) | N/A or Missing (%) | |
|---|---|---|---|
| |||
| Enjoyed conversation | 69 (66) | 15 (14) | 20 (19) |
| Bothered by roommate | 32 (31) | 70 (67) | 2 (2) |
| Received help from roommate | 36 (35) | 65 (63) | 3 (3) |
| Gave help to roommate | 61 (59) | 39 (38) | 4 (4) |
Patient Preferences
Of the 117 patients who expressed a preference for a private or shared room, 92 (79%) stated that they preferred private rooms (Table 4). Patients in shared rooms were more likely to prefer shared rooms than patients in private rooms. Race did not impact preference. Patients older than 65 years were more likely to prefer shared rooms than patients younger than 65 years (31% vs. 15%; P = 0.042), although the preference for private rooms persisted across all demographic groups.
| Prefer Private (%) | Prefer Shared (%) | P Value* | |
|---|---|---|---|
| |||
| Total (n = 117) | 92 (79) | 25 (21) | |
| Comparison by race | |||
| Caucasian (n = 31) | 25 (81) | 6 (19) | 0.60 |
| African‐American (n = 75) | 57 (76) | 18 (24) | |
| Comparison by age (years) | |||
| Age >65 (n = 45) | 31 (69) | 14 (31) | 0.042 |
| Age <65 (n = 72) | 61 (85) | 11 (15) | |
| Comparison by current room status | |||
| Private (n = 43) | 41 (95) | 2 (5) | <0.01 |
| Shared (n = 71) | 50 (70) | 21 (30) | |
Ninety‐two patients responded to the open‐ended prompt to state reasons for these preferences, most commonly citing privacy and peace and quiet (Table 5). Other reasons included worrying about germs, being unwilling to share the television, wanting a private bathroom, being bothered by smells, feeling a lack of security, and wanting to have space for family. Patients who preferred shared rooms most commonly cited a desire for companionship.
|
| Private (80) |
| Privacy (33): privacy, like to be alone |
| Peace/quiet (28): snoring roommates, sleep better |
| Room amenities (7): television sharing, bathroom is within |
| Germs (6): germs, diseases |
| Security (2): security, belongings |
| Family (2): daughter had to sleep in lounge |
| Smells (2): smells |
| Shared (14) |
| Company (13): someone to talk to |
| Safety (1): safer, someone to look out for you |
| Neutral (6): |
| No preference (5): doesn't matter, what's available |
| Depends on circumstances (1): depends on sickness |
Discussion
In this observational study of U.S. veterans, we found that patients in private rooms were more satisfied with privacy than their shared‐room counterparts, a finding consistent with previous studies.4, 5 We also found that private room patients were more likely to feel that nursing was available, a finding that had not previously been reported. Although increased loneliness and fear of death had been noted in private room patients before, our study did not support such findings.10 We found a strong overall preference for private rooms, adding to previously mixed evidence about patient room preferences.10, 1217 Although we had hypothesized a substantial demand for shared rooms among veterans based upon their common military experience, we found the demand modest at best.
The increased satisfaction with privacy among private room patients was expected.4, 5 That nearly one‐half of the patients in shared rooms found the privacy to be inadequate, however, was a strong finding that points to a fundamental problem with these rooms. That private room patient also felt more that nurses were available was surprising, since the higher number of patients in shared rooms might have been expected to generate a more visible nursing presence. Whether this finding is reproducible and whether it is based upon an objective difference in nursing behavior is something that should be studied further. It may simply have been a marker of increased satisfaction among the private room patients.
Although loneliness and increased fear of death had previously been identified as possible drawbacks of private rooms, our survey found no such suggestion.10 This may have been because our survey instrument simply asked patients to answer yes or no whether they had experienced the particular feelings; the previous study had used interviews. A more sensitive survey instrument would be needed to investigate questions of loneliness and fear of death further.
Except for the problem of privacy, the shared room experience generally appeared to be a positive one for patients, particularly in the exchange of conversation and help. A genuine value of these interactions to the patients was suggested by the finding that patients in shared rooms were more likely to prefer shared rooms than patients in private rooms. It is possible that once in them, patients found shared rooms to be better than expected, or that they warmed to them as a matter of resolving cognitive dissonance between their preconceptions and their placement. Although peace and quiet was a frequently cited reason for preferring private rooms, relatively few patients reported being bothered by their roommates. The high rate of assistance among roommates was perhaps the most surprising positive about the shared room experience, even if patients recalled helping others more than they remembered being helped themselves.
Preference for private rooms was greater than that found in previous studies, and appears to have been based largely upon concerns for privacy.1217 We suspect it may reflect changing societal values that have placed an increased priority on personal privacy; this is suggested by the greater willingness of elderly patients to stay in shared rooms. Whether the preference for private rooms is even higher in the general nonveteran population is something that should be assessed with future studies.
Our study was limited by a number of factors. While room assignments were based on bed availability, it is possible that patients requested particular rooms or were moved due to other factors. By selecting a study population of male veterans of the armed services, we limited the extent to which our results can be generalized. However, this particular population is an important one to study due to the size of the VA healthcare system, the prevalence of shared rooms in VA hospitals, and the unique cultural values of veterans. We were unable to obtain information from approximately 27% of our target population because they were unable or unwilling to participate, thus excluding a population subset from analysis and potentially biasing our results. Although a high prevalence of preference for private rooms was seen, the strength of the preference was not assessed, and patients were unable to convey if they had no preference regarding room type. The survey instrument was designed for the purposes of this study and has not been validated. Some patients were assisted with filling out their surveys and may have responded more positively about their roommates if they thought their roommates could overhear their answers to questions. Because patient enrollment was slower than expected, fewer patients were enrolled than planned and the power achieved was insufficient for detecting differences of 20% or less between groups. This is unlikely to have affected our positive findings, but may have prevented us from detecting additional differences among the study groups.
Our data shows that for a population of veteran patients, private rooms are preferred, and that increased privacy is a primary advantage. It also demonstrates that multioccupancy rooms have several positive aspects. Given the need for sound public policy in regard to this issue, further research is needed to better evaluate objective outcomes of room type, such as fall rates, nosocomial infection rates, and lengths of hospitalization. In the meantime, efforts should be taken to address some of the known problems of shared rooms. For instance, establishing more substantial dividers between beds would be an intervention that could increase privacy, reduce noise, and minimize unwelcome smells. Additionally, given that patients have a wide variety of feelings toward multioccupancy rooms, incorporating patient choice into room assignments when logistically feasible is a step that could lead to increased patient satisfaction with hospitalization.
Originally championed in the form of large multibed wards by Florence Nightingale in the nineteenth century, multioccupancy hospital rooms have recently been criticized for concerns about their cost, safety, lack of privacy, and unpopularity among patients.13 Specifically, they have been linked to longer hospital stays and the morbidity that those stays produceinfections, falls, and medical errors.13 In 2006, the Health Guidelines Revision Committee, the body that establishes guidelines for the construction of healthcare facilities in the United States, moved to a position recommending private rooms as the minimum standard for medical/surgical and postpartum hospital beds. The evidence supporting their position, however, has remained limited. Given the substantial cost and effort involved in converting hospital rooms, it is important to understand the impact of such policies on patients.
In a recent literature review by van de Glind et al.,4 the authors were able to identify only 4 randomized controlled trials comparing private and shared rooms. While they found that private rooms had a moderately positive impact on patient satisfaction and privacy, data on infection control was mixed, and data on patient safety was lacking.48 Though suggested, the association between private rooms and shortened hospital stays has not clearly been shown.13, 9 Certain advantages of shared rooms, such as decreased loneliness and increased patient social interaction, have also been identified.10 In addition, specialized multioccupancy wards for dementia and delirium have been demonstrated to be useful in the management of inpatients with those conditions, partly because of increased nursing presence.11 Finally, an additional theoretical benefit of shared rooms is the possibility of assistance between roommates in emergency situations.
For the sake of providing high‐quality patient‐centered care, patient concerns and preferences are also important to consider when establishing health policy regarding rooms. Previously, patient surveys and interviews have been helpful in identifying issues of concern to patients and understanding their preferences. Kirk12 identified privacy, quiet, improved sleep, and ability to have a family member stay in the room as reasons why 18 of 24 hospice patients preferred private hospice rooms.12 Conversely, in a 2002 Welsh survey of palliative patients, 68% wanted to be in an open area (4‐bed bay), identifying companionship as the major reason.13 Most recently, palliative care patients in the United Kingdom expressed a preference for shared rooms while well enough to interact with others but preferred private rooms when very ill or dying.14 These findings, given the lack of solid medical justification for 1 room type, raise multiple concerns about whether universal adherence to 1 room type will lead to the best patient care in the United States, and more specifically the veteran population. First, nearly all of the preference data described has been collected outside of the United States and veteran's populations. Second, studies have targeted highly‐specialized patient populations, and their applicability in a general medical/surgical population is unknown.10, 1217 Finally, the diversity of preferences exhibited in these studies underscores the difficulty in applying preference data to outside populations, such as the veteran population.
With 7.8 million patients enrolled in its healthcare system and operating 153 medical centers, the U.S. Department of Veterans Affairs has a large interest in the ongoing debate about shared vs. private hospital rooms. Because veterans share a common military background, they may place a higher value on companionship relative to privacy, and so they are an important group to study separately from the general U.S. population. To date, we have been unable to identify any studies that have looked at veterans' experiences and preferences in regards to room type. Through an anonymous survey, we sought to learn about these experiences and preferences and assess whether previously found advantages of each room type (eg, companionship in shared rooms, privacy in private rooms) could be confirmed in the veteran population. By doing so, we also aimed to better inform public policy about a general continued role for shared hospital rooms in the United States.
Methods
Our study used a point prevalence survey to examine patient preferences and experiences with shared (2‐person or 3‐person occupancy) vs. private rooms.
Design
From September to November 2007 patients admitted to the Washington DC VA Medical Center medical service (a tertiary care facility, with 76 beds on the medical service, 37 shared and 39 private) were asked to complete anonymous written surveys prior to discharge. Male patients were assigned rooms based on bed availability only, with the exception of those requiring isolation or those receiving chemotherapy, which mandated private rooms. Patients were not able to request specific room types. Planned patient discharges were identified daily by consulting each ward unit's central discharge list. One member of the research team not directly involved in the patient's care would approach the patient to see if he was willing to participate. Participants were asked to fill out the surveys independently, but those who were incapable of doing so due to physical limitations were allowed assistance by family, a member of the research team, or the nurse, if they so requested. Patients were asked to leave completed surveys in provided blank envelopes for pickup by a member of the research team. These were later marked as coming from either private, shared, or isolation rooms. Exclusion criteria included hospitalization for chemotherapy and female sex. Patients who were not able to complete surveys because of cognitive impairment were considered nonparticipants. Although data from patients in private contact isolation rooms was collected, it was left out of analysis, as assignment to these rooms was based upon medical condition rather than solely on bed availability. To account for patients who switched room type during their hospitalization, 1 survey question asked for their predominant room type. Power analysis suggested that a sample size of 70 patients per group would give 80% power to demonstrate differences of 20% (estimated difference expected for key measures) between the groups at a 95% confidence level.
Data Collection
The survey instrument was designed for the purposes of this study as no suitable existing tool could be found. Questions were drawn from issues identified in the literature on loneliness, fear, and anxiety, as well as author hypothesis about potential social benefits of having roommates.1013 Questions included demographics (age, race, education, household income); hospital experiences including adequacy of privacy (Do you feel that the privacy in your room is adequate?), nursing availability (Have you felt that a nurse was available when you needed one?), loneliness (Have you been lonely during this hospital stay?), fear of death, interactions with roommates (Have any of your roommates helped you in any way [like calling for a nurse]?); and private vs. shared room preference. Except for 1 open‐ended question asking the reason for room preference, questions were yes/no.
Data Analysis
Chi‐square testing was performed to determine whether there were any baseline differences between the private vs. shared groups in terms of demographic variables and length of stay; whether room type was associated with differences in perception of privacy, nursing availability, fear of death, and loneliness; and to determine differences in room preference with regard to demographic variables. Fisher 2‐tailed exact testing was performed to evaluate differences in room preference based upon room type, as the sample sizes for this comparison were small. For the open‐ended question, responses were coded into categories by 1 research team member (W.E.) and reviewed by another team member (K.C.). Consensus was reached through discussion.
The protocol received exemption from Institutional Review Board oversight and approval from the Research and Development Committee.
Results
A total of 162 surveys were completed with a participation rate of approximately 73%. Eighty three patients (51%) reported a shared room stay, while 70 patients (43%) reported a private room stay (17 of which were isolation). Nine did not report room type (Figure 1). Excluding isolation patients and those respondents who did not indicate room type, 5% of respondents reported age less than 45 years, 56% between 45 and 65 years, and 39% greater than 65 years. Sixty percent of patients identified themselves as African‐American, 28% as white, and 5% as other (7% did not answer). The majority (56%) reported having some formal higher education after high school. Median total household income was between $11,000 and $20,000.

Private vs. Shared
Comparison of demographic information between the private and shared groups revealed no significant differences in age, race, education, or income distribution (Table 1). There was also no difference in reported length of stay. Because some respondents failed or declined to answer certain questions, the number of responses reported for each category varied. Notably, 79% of patients staying in private rooms reported that they had been in shared rooms before, while 78% of patients in shared rooms reported that they had been in private rooms before, indicating that both groups were familiar with each type of room.
| Private (n = 53) (%) | Shared (n = 83) (%) | P Value* | |
|---|---|---|---|
| |||
| Age (years) | 0.63 | ||
| <45 | 3 (6) | 4 (5) | |
| 45‐65 | 32 (60) | 44 (53) | |
| >65 | 18 (34) | 35 (42) | |
| Race | 0.80 | ||
| Caucasian | 15 (28) | 23 (28) | |
| African‐American | 34 (64) | 48 (58) | |
| Other | 2 (4) | 5 (6) | |
| No response | 2 (4) | 7 (8) | |
| Education | 0.67 | ||
| 4 (8) | 10 (12) | ||
| High school | 16 (30) | 26 (31) | |
| Some college | 20 (38) | 31 (37) | |
| College | 4 (8) | 10 (12) | |
| >College | 6 (11) | 5 (6) | |
| No response | 3 (6) | 1 (1) | |
| Annual household income | 0.17 | ||
| <$10,000 | 10 (19) | 21 (25) | |
| $11,000‐$20,000 | 16 (30) | 19 (23) | |
| $21,000‐$35,000 | 4 (8) | 19 (23) | |
| $36,000‐$60,000 | 9 (17) | 12 (14) | |
| >$60,000 | 6 (11) | 6 (7) | |
| No response | 8 (15) | 6 (7) | |
| Length of stay (days) | 0.90 | ||
| <5 | 35 (66) | 52 (63) | |
| 6‐10 | 12 (23) | 21 (25) | |
| >10 | 5 (9) | 9 (11) | |
| No response | 1 (2) | 1 (1) | |
| Previous experience in opposite room type | 0.73 | ||
| Yes | 42 (79) | 65 (78) | |
| No | 10 (19) | 18 (22) | |
| No response | 1 (2) | 0 | |
In describing their experiences, patients in private rooms were more likely to report adequate privacy (92% vs. 53%; P 0.01) and available nursing (79% vs. 64%; P = 0.025) than patients in shared rooms (Table 2). There was no difference in the amount of reported loneliness or fear of death. For patients with roommate interactions, 66% replied that they enjoyed talking with their roommates (Table 3). A minority (31%) indicated that they had been bothered by sights, noises, or messes made by their roommates. A majority of patients reported giving help to roommates (59%), and a substantial percentage reported receiving help (35%).
| Private (n = 53) (%) | Shared (n = 83) (%) | P Value* | |
|---|---|---|---|
| |||
| Privacy adequate | 49 (92) | 44 (53) | <0.01 |
| Felt nurse was available | 42 (79) | 53 (64) | 0.025 |
| Felt lonely | 11 (21) | 18 (22) | 0.913 |
| Had fear of dying | 5 (9) | 13 (16) | 0.309 |
| Yes (%) | No (%) | N/A or Missing (%) | |
|---|---|---|---|
| |||
| Enjoyed conversation | 69 (66) | 15 (14) | 20 (19) |
| Bothered by roommate | 32 (31) | 70 (67) | 2 (2) |
| Received help from roommate | 36 (35) | 65 (63) | 3 (3) |
| Gave help to roommate | 61 (59) | 39 (38) | 4 (4) |
Patient Preferences
Of the 117 patients who expressed a preference for a private or shared room, 92 (79%) stated that they preferred private rooms (Table 4). Patients in shared rooms were more likely to prefer shared rooms than patients in private rooms. Race did not impact preference. Patients older than 65 years were more likely to prefer shared rooms than patients younger than 65 years (31% vs. 15%; P = 0.042), although the preference for private rooms persisted across all demographic groups.
| Prefer Private (%) | Prefer Shared (%) | P Value* | |
|---|---|---|---|
| |||
| Total (n = 117) | 92 (79) | 25 (21) | |
| Comparison by race | |||
| Caucasian (n = 31) | 25 (81) | 6 (19) | 0.60 |
| African‐American (n = 75) | 57 (76) | 18 (24) | |
| Comparison by age (years) | |||
| Age >65 (n = 45) | 31 (69) | 14 (31) | 0.042 |
| Age <65 (n = 72) | 61 (85) | 11 (15) | |
| Comparison by current room status | |||
| Private (n = 43) | 41 (95) | 2 (5) | <0.01 |
| Shared (n = 71) | 50 (70) | 21 (30) | |
Ninety‐two patients responded to the open‐ended prompt to state reasons for these preferences, most commonly citing privacy and peace and quiet (Table 5). Other reasons included worrying about germs, being unwilling to share the television, wanting a private bathroom, being bothered by smells, feeling a lack of security, and wanting to have space for family. Patients who preferred shared rooms most commonly cited a desire for companionship.
|
| Private (80) |
| Privacy (33): privacy, like to be alone |
| Peace/quiet (28): snoring roommates, sleep better |
| Room amenities (7): television sharing, bathroom is within |
| Germs (6): germs, diseases |
| Security (2): security, belongings |
| Family (2): daughter had to sleep in lounge |
| Smells (2): smells |
| Shared (14) |
| Company (13): someone to talk to |
| Safety (1): safer, someone to look out for you |
| Neutral (6): |
| No preference (5): doesn't matter, what's available |
| Depends on circumstances (1): depends on sickness |
Discussion
In this observational study of U.S. veterans, we found that patients in private rooms were more satisfied with privacy than their shared‐room counterparts, a finding consistent with previous studies.4, 5 We also found that private room patients were more likely to feel that nursing was available, a finding that had not previously been reported. Although increased loneliness and fear of death had been noted in private room patients before, our study did not support such findings.10 We found a strong overall preference for private rooms, adding to previously mixed evidence about patient room preferences.10, 1217 Although we had hypothesized a substantial demand for shared rooms among veterans based upon their common military experience, we found the demand modest at best.
The increased satisfaction with privacy among private room patients was expected.4, 5 That nearly one‐half of the patients in shared rooms found the privacy to be inadequate, however, was a strong finding that points to a fundamental problem with these rooms. That private room patient also felt more that nurses were available was surprising, since the higher number of patients in shared rooms might have been expected to generate a more visible nursing presence. Whether this finding is reproducible and whether it is based upon an objective difference in nursing behavior is something that should be studied further. It may simply have been a marker of increased satisfaction among the private room patients.
Although loneliness and increased fear of death had previously been identified as possible drawbacks of private rooms, our survey found no such suggestion.10 This may have been because our survey instrument simply asked patients to answer yes or no whether they had experienced the particular feelings; the previous study had used interviews. A more sensitive survey instrument would be needed to investigate questions of loneliness and fear of death further.
Except for the problem of privacy, the shared room experience generally appeared to be a positive one for patients, particularly in the exchange of conversation and help. A genuine value of these interactions to the patients was suggested by the finding that patients in shared rooms were more likely to prefer shared rooms than patients in private rooms. It is possible that once in them, patients found shared rooms to be better than expected, or that they warmed to them as a matter of resolving cognitive dissonance between their preconceptions and their placement. Although peace and quiet was a frequently cited reason for preferring private rooms, relatively few patients reported being bothered by their roommates. The high rate of assistance among roommates was perhaps the most surprising positive about the shared room experience, even if patients recalled helping others more than they remembered being helped themselves.
Preference for private rooms was greater than that found in previous studies, and appears to have been based largely upon concerns for privacy.1217 We suspect it may reflect changing societal values that have placed an increased priority on personal privacy; this is suggested by the greater willingness of elderly patients to stay in shared rooms. Whether the preference for private rooms is even higher in the general nonveteran population is something that should be assessed with future studies.
Our study was limited by a number of factors. While room assignments were based on bed availability, it is possible that patients requested particular rooms or were moved due to other factors. By selecting a study population of male veterans of the armed services, we limited the extent to which our results can be generalized. However, this particular population is an important one to study due to the size of the VA healthcare system, the prevalence of shared rooms in VA hospitals, and the unique cultural values of veterans. We were unable to obtain information from approximately 27% of our target population because they were unable or unwilling to participate, thus excluding a population subset from analysis and potentially biasing our results. Although a high prevalence of preference for private rooms was seen, the strength of the preference was not assessed, and patients were unable to convey if they had no preference regarding room type. The survey instrument was designed for the purposes of this study and has not been validated. Some patients were assisted with filling out their surveys and may have responded more positively about their roommates if they thought their roommates could overhear their answers to questions. Because patient enrollment was slower than expected, fewer patients were enrolled than planned and the power achieved was insufficient for detecting differences of 20% or less between groups. This is unlikely to have affected our positive findings, but may have prevented us from detecting additional differences among the study groups.
Our data shows that for a population of veteran patients, private rooms are preferred, and that increased privacy is a primary advantage. It also demonstrates that multioccupancy rooms have several positive aspects. Given the need for sound public policy in regard to this issue, further research is needed to better evaluate objective outcomes of room type, such as fall rates, nosocomial infection rates, and lengths of hospitalization. In the meantime, efforts should be taken to address some of the known problems of shared rooms. For instance, establishing more substantial dividers between beds would be an intervention that could increase privacy, reduce noise, and minimize unwelcome smells. Additionally, given that patients have a wide variety of feelings toward multioccupancy rooms, incorporating patient choice into room assignments when logistically feasible is a step that could lead to increased patient satisfaction with hospitalization.
- , , . The use of single patient rooms vs. multiple occupancy rooms in acute care environments. Avaliable at: http://www.healthdesign.org/research/reports/single_patient_rooms.php. Accessed March,2009.
- , , .Advantages and disadvantages of single‐versus multiple‐occupancy rooms in acute care environments: a review and analysis of the literature.Environ Behav.2005;37:760–786.
- , .Multibed versus single‐bed rooms. In: Kobus R, Skaggs RL, Bobrow M, Thomas J, Payette M, eds.Building Type Basics For Healthcare Facilities.New York:John Wiley;2000:145–157.
- , , .Do patients in hospitals benefit from single rooms? A literature review.Health Policy.2007;84:153–161.
- , , , , .Single room maternity care and client satisfaction.Birth.2000;27(4):235–243.
- , , .Do appealing hospital rooms increase patient evaluation of physicians, nurses, and hospital services?Health Care Manage Rev.2003;28(3):254–264.
- , , .The effect of private isolation rooms on patient care practices, colonization and infection in an intensive care unit.Am J Med.1981;70(3):641–645.
- , , .The effect of increased bed numbers on MRSA transmission in acute medical wards.J Hosp Infect.1998;39(3):213–219.
- , .Streamlining patient care processes through flexible room and equipment design.Crit Care Nurs Q.24(3):59–76.
- , , , .A psychological comparison of patients in “open” and “closed” coronary care units.J Psychosom Res.1972;16:449–457.
- , , , , , .A model for managing delirious older patients.J Am Geriatr Soc.2003;51:1031–1035.
- .Patient preferences for a single or shared room in a hospice.Nurs Times.2002;98(50):39–41.
- , .Do patients and their relatives prefer single cubicles or shared wards?Palliat Med.2002;16(5):445–446.
- , .How does the environment impact on the quality of life of advanced cancer patients? A qualitative study with implications for ward design.Palliat Med.2008;22(6):768–774.
- , .Roommates.Am J Nurs.1973;73(1):104–107.
- .Single rooms and patient choice.Nurs Stand.2005;20(9):41–48.
- .Patients' wishes regarding sickrooms.Nurs Times.1990;86(20):53.
- , , . The use of single patient rooms vs. multiple occupancy rooms in acute care environments. Avaliable at: http://www.healthdesign.org/research/reports/single_patient_rooms.php. Accessed March,2009.
- , , .Advantages and disadvantages of single‐versus multiple‐occupancy rooms in acute care environments: a review and analysis of the literature.Environ Behav.2005;37:760–786.
- , .Multibed versus single‐bed rooms. In: Kobus R, Skaggs RL, Bobrow M, Thomas J, Payette M, eds.Building Type Basics For Healthcare Facilities.New York:John Wiley;2000:145–157.
- , , .Do patients in hospitals benefit from single rooms? A literature review.Health Policy.2007;84:153–161.
- , , , , .Single room maternity care and client satisfaction.Birth.2000;27(4):235–243.
- , , .Do appealing hospital rooms increase patient evaluation of physicians, nurses, and hospital services?Health Care Manage Rev.2003;28(3):254–264.
- , , .The effect of private isolation rooms on patient care practices, colonization and infection in an intensive care unit.Am J Med.1981;70(3):641–645.
- , , .The effect of increased bed numbers on MRSA transmission in acute medical wards.J Hosp Infect.1998;39(3):213–219.
- , .Streamlining patient care processes through flexible room and equipment design.Crit Care Nurs Q.24(3):59–76.
- , , , .A psychological comparison of patients in “open” and “closed” coronary care units.J Psychosom Res.1972;16:449–457.
- , , , , , .A model for managing delirious older patients.J Am Geriatr Soc.2003;51:1031–1035.
- .Patient preferences for a single or shared room in a hospice.Nurs Times.2002;98(50):39–41.
- , .Do patients and their relatives prefer single cubicles or shared wards?Palliat Med.2002;16(5):445–446.
- , .How does the environment impact on the quality of life of advanced cancer patients? A qualitative study with implications for ward design.Palliat Med.2008;22(6):768–774.
- , .Roommates.Am J Nurs.1973;73(1):104–107.
- .Single rooms and patient choice.Nurs Stand.2005;20(9):41–48.
- .Patients' wishes regarding sickrooms.Nurs Times.1990;86(20):53.
Copyright © 2009 Society of Hospital Medicine
Implementing an Alphanumeric Paging System
Effective communication between healthcare providers is essential to patient safety and quality of care.1, 2 Numeric pagers are commonly used communication devices in healthcare, but cannot convey important information such as the reason for the page, urgency of the page, or sender name. Physicians must respond to numeric pages, often disrupting patient encounters or educational activities.36 In a study of medical interns, disruptions to patient care occurred with up to 65% of pages received, two‐thirds of which were not felt to be urgent.5 In addition to causing frustration, frequent disruptions can contribute to medical errors.7, 8
Alphanumeric pagers can display both numbers and text, and may address some of the communication problems associated with numeric pagers. They also lay the groundwork for other patient safety initiatives such as automated paging of critical laboratory values9 and real‐time reporting of user‐requested laboratory data.10 Implementation of alphanumeric paging on a general surgery teaching service reduced disruptions to patient care and the number of pages requiring a return call.11
Our primary aim was to implement an alphanumeric paging system. We will describe our implementation strategies and barriers identified. We evaluated the implementation of alphanumeric paging by measuring (1) the proportion of pages sent as text pages, (2) the source of the pages (other physicians or from the general medicine [GM] ward), (3) the content of the text pages, (4) the number of pages that disrupted scheduled education activities, and (5) satisfaction with the alphanumeric paging system.
Materials and Methods
Setting
Sunnybrook Health Sciences Centre is a tertiary care academic teaching hospital affiliated with the University of Toronto (Toronto, Ontario, Canada). There are 4 physician teams that provide hospitalist care to admitted patients on the General Internal Medicine service. Each physician team consists of 1 attending physician, 1 second‐year or third‐year resident, 2 to 3 first‐year residents, and 3 to 4 third‐year and fourth‐year medical students. In total, 12 to 13 residents rotate through the General Internal Medicine service per month. Each physician team is assigned to 1 of 4 GM wards, which are staffed with nurses and allied health staff. Five to eight Internet‐enabled computer stations are located on each GM ward. All physicians, nurses, and allied health staff who worked on the 4 GM wards participated in the study.
Existing Paging System
Prior to July 2006, all physicians at our hospital carried numeric pagers. A physician could be paged by 3 methods: (1) through the hospital operator; (2) using the telephone; or (3) using an Internet‐based paging system (Smart Web 3.6.2, AmCom Software Inc.). Most pages were sent through the hospital operator or by telephone.
Intervention
The intervention included: (1) equipping resident physicians with alphanumeric pagers and (2) increasing the use of the existing Internet‐based paging system to send text pages. We equipped each resident with an alphanumeric pager (Motorola Flex Alphanumeric Pager). Users could send a text page using the existing Internet‐based application (Smart Web 3.6.2). This application allows users to search for a specific physician either by name or by on‐call assignment, and send a page up to 125 characters long from any Internet‐enabled computer in the hospital. Numeric pages could be sent by telephone, through the hospital operator, or by using the web‐based paging system throughout the study period.
Implementation Process
We provided alphanumeric pagers to the residents on the General Internal Medicine service in July 2006. Alphanumeric pagers were limited, so each resident traded their numeric pager for an alphanumeric pager at the start of each rotation. Once their rotation ended, they returned their alphanumeric pager for their original numeric pager. The communications department coordinated this process. The chief medical resident spent 10 minutes to teach the residents how to use the system at the beginning of the rotation. In August and September 2006, a member of the communications department trained the nurses on the 4 GM wards how to send a text page using the Internet‐based paging application. We scheduled these 15‐minute sessions throughout the day and evening in order to capture as many nurses as possible. We encouraged the nurses to include standardized information in the text message (eg, patient ID, issue, level of urgency, sender name, call‐back number).
We used rapid‐cycle change methods12 to implement the alphanumeric system (Figure 1). The first change cycle in August 2006 consisted of providing pagers to residents and training the nurses and physicians to send text pages. Users reported that the paging interface was difficult to use. For the second change cycle in September 2006, the communications department modified the paging interface to improve usability and created shortcut icons on the GM ward computers. While the system was easier to access, the nurses reported that 1‐time training was insufficient. For the next change cycle in September 2006, we developed Internet‐based tutorials that could be accessed at any time, and made expert users (charge nurses) available for just‐in‐time training. We asked these charge nurses what they believed would encourage adoption of the system. They suggested that contests worked well with other initiatives. For the final change cycle in October and November 2006, we held a contest and rewarded the GM ward that sent the highest percentage of text pages with a team lunch.
Our results from November 2006 were presented to our hospital medical leaders, who approved widespread implementation of alphanumeric pagers for all residents and medical students in all programs. The cost of this upgrade was approximately $35,000 per year to lease 500 alphanumeric pagers. By July 2007, all residents and students in our hospital had alphanumeric pagers.
Measures
Our primary outcome measure was the percentage of pages sent as text pages. We chose November 2005 as our before implementation period to account for temporal variations in patient load, and secular trends in resident knowledge and experience during an academic year. We collected data during our rapid‐cycle improvement periods of implementation and testing in September to November 2006. We assessed sustainability after implementation by collecting data in January 2008, 6 months after hospital‐wide implementation of alphanumeric pagers, and 14 months after the initial implementation on the GIM service.
We reviewed weekday paging records from our communications department for each study period. For text pages, we reviewed the text message to determine whether the page was sent by a physician or by another health care professional. We established 5 mutually exclusive categories of messages prior to the study: (1) A GM ward‐to‐physician (GM ward‐to‐Doc) numeric page was any page that contained only a phone number for 1 of the 4 GM ward main telephone numbers; (2) A Doc‐to‐Doc numeric page was any page that was preceded by 000‐ (a convention used at our hospital to indicate a physician sender), or that contained a phone number used only by physicians, such as the doctor's lounge; (3) A GM ward‐to‐Doc text page was a text page sent by any GM ward health care professional; (4) A Doc‐to‐Doc text page was a text page sent by any physician or medical student; and (5) All other numeric pages, such as those with phone numbers from other hospital wards, were classified as numeric non‐GM ward and non‐Doc in‐hospital page.
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds that occur every weekday from 12:00 to 1:00 PM. We classified a page as disruptive if it required an immediate call‐back (ie, all numeric pages and urgent text pages).
We surveyed residents and daytime GM ward nursing staff during the implementation period (October and November 2006) to assess satisfaction with the alphanumeric paging system using a 5‐point Likert scale (1 = strongly disagree, 5 = strongly agree). We distributed paper surveys to nurses, and used an electronic web survey for residents.13
Statistical Analysis
We compared the paging data after implementation (January 2008) to the period before implementation (November 2005) using a Student t‐test for comparison of means, and chi‐square and Fisher's exact tests for categorical value comparisons. We assigned a significance level of P < 0.05 for the t tests and chi‐square tests, and P < 0.01 for the Fisher's exact tests. We used SPSS 11.0 to perform statistical analyses (Chicago, IL).
Results
Paging Patterns Before, During, and After Implementation
The number of pages per resident was similar before and during implementation, but higher afterwards. (46 16 pages/resident/week in November 2005, 47 20 pages/resident/week in November 2006, and 59 27 pages/resident/week in January 2008; P = 0.17; Table 1). The mean number of admissions per night was 8.0 2.7 before implementation, compared to 10.2 3.5 after implementation (P = 0.009).
| Before Implementation (all residents had numeric pagers) | During Implementation (all residents had alphanumeric pagers) | After Implementation (all residents had alphanumeric pagers) | |||
|---|---|---|---|---|---|
| Paging Characteristics | November 2005 | October 2006 | November 2006 | January 2008 | P Value |
| |||||
| Total number of pages | 1431 | 1879 | 1813 | 1269 | |
| Total number of resident weeks worked | 29 | 33 | 33 | 21 | |
| Pages per resident week, mean (SD) | 46.2 (16.3) | 57.9 (19.2) | 46.5 (20.2) | 59.0 (26.5) | 0.17* |
| Number of patients admitted per night, mean (SD) | 8.0 (2.7) | 10.2 (3.5) | 11.0 (2.9) | 10.2 (3.5) | 0.009* |
| Type of page, n (%) | |||||
| Numeric pages | 751 (53) | 462 (25) | 580 (32) | 374 (30) | <0.001 |
| GM wards‐to‐Doc | 584 (41) | 393 (21) | 538 (30) | 352 (28) | <0.001 |
| Doc‐to‐Doc | 167 (12) | 69 (4) | 42 (2) | 22 (2) | <0.001 |
| Non‐GM ward/Doc pages | 680 (47) | 1107 (59) | 809 (45) | 487 (38) | <0.001 |
| Text pages | 0 (0) | 310 (16) | 424 (23) | 408 (32) | <0.001 |
| GM wards‐to‐Doc | 0 (0) | 175 (9) | 221 (12) | 129 (10) | <0.001 |
| Doc‐to‐Doc | 0 (0) | 135 (7) | 203 (11) | 279 (22) | <0.001 |
We observed a significant and sustained increase in the use of text paging during the study (Table 1). After implementation, 32% of all pages sent to our residents were text messages (P < 0.001). Physicians almost exclusively sent text pages by the end of implementation (increase from 0% to 83% text paging rate during implementation, and 93% after implementation; P < 0.001; Figure 2). GM ward text paging rates also increased from 0% to 29% during implementation, and 27% after implementation (P < 0.001; Figure 2). The alphanumeric paging system was used to a greater degree by physicians compared to other workers on the GM ward after full implementation (93% vs. 27%; P < 0.001). We explored the proportion of GM ward‐to‐Doc pages sent as text from different GM wards during implementation, and found significant variation, ranging from 14% to 57% (P < 0.001).
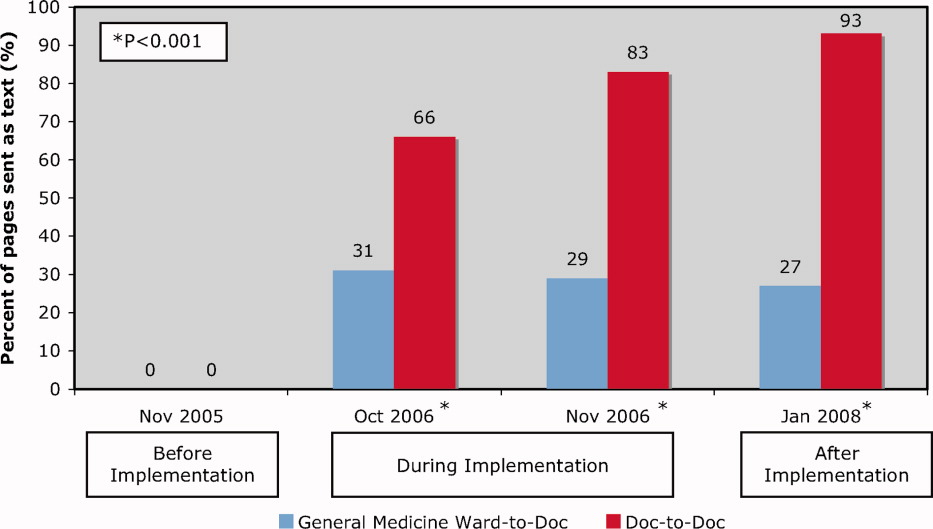
The most common reasons for text paging from GM wards were to request a patient assessment or for notification of a patient's clinical status (25%), to clarify written orders (20%), and to request a medication prescription (13%) (Table 2). Among physicians, the most common reasons for text paging were to set up meetings for work or teaching rounds (33%), to relay patient‐care related messages (27%), and to sign‐over patients at the end of the day (23%) (Table 3). The remainder of the other Doc‐to‐Doc pages (18%) were mostly personal messages or team communication that was not related to clinical work.
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Requests for patient assessment or notification of a patient's clinical status | 55 (25) | Patient X. Temp 38.5. No other symptoms. [Nurse's name]. |
| The repeat CXR on Patient X has been completed. Please call [ward] if NG can be used. Thank you. | ||
| Clarification of a written order | 45 (20) | Would you like Patient X to get a second dose of Lasix? He has already had 100 mg and his output thus far is 500 cc. [Nurse's name] |
| Patient X. BP = 100/62, pulse 54. Patient is supposed to have metoprolol 50 mg tonight. Do you want me to hold it? [Nurse's name] | ||
| Request for a medication prescription | 28 (13) | Patient X needs an analgesic for pain in his arms and legs. Please call [ward]. |
| Patient X has a daily coumadin order. INR 2.14. Please call with dosage for 1800. Ask for [Nurse]. | ||
| Cosigning written order | 25 (11) | Please co‐sign neurology suggested orders for Patient X. [Nurse's name] |
| Not urgent at your leisure, please co‐sign Patient X orders on [ward] for medical student. Thanks. | ||
| Notification of a recent laboratory result | 23 (11) | Patient X's potassium is 3.0 today. Please call [ward]. |
| Patient X. Sodium 163. Troponin unchanged at 1.54. [ward]. | ||
| Arranging meetings with patients and/or family | 18 (8) | Meeting with Patient X's family and social worker at 2 pm tomorrow on [ward]. |
| [Social worker] is here. [Physiotherapist] expected any minute. We are going to meet in family room for Patient X. | ||
| Request to complete paperwork | 15 (7) | Patient X is ready to go home, and just needs discharge orders and prescriptions |
| Referral form for community palliative doctor on the front of the chart fill in where marked by arrows. Can you please put on form prognosis as well? [Social worker] | ||
| Other | 12 (6) | |
| Total | 221 (100) | |
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Setting up meetings for work or teaching rounds | 67 (33) | Confirmed diabetes teaching at 1400 hr in [room]. Please let medical students know. Thanks. |
| Please come to [lecture theatre] if you can in the next 10 minutes for the teleconferenced noon rounds. Thanks. | ||
| We are in the Emergency Department with [attending staff]. Come to meet us here if you can. | ||
| Relaying patient‐care related messages | 54 (27) | Patient X US query cholangitis, can we ask for a surgical consult. He may benefit from surgery or percutaneous drain. Please repeat his blood work. |
| Patient X in [room X]. Presented with DKA. pH 7.2. pCO2 23, bicarbonate 9. Lytes pending. Got IV insulin and NS. Need to check to clinical stability. [Resident] | ||
| Signing‐over patients at the end of the day | 46 (23) | I'm ready to sign out to you. Where are you? [Resident]. |
| Patient X is back from cardiac cath, and is stable. Please check Cr over WE, and watch for CHF. | ||
| Other | 36 (18) | |
| Total | 203 (100) | |
Impact of Alphanumeric Paging System on Disruptions
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds (Table 4). Prior to implementation, residents were paged 2.9 2.4 times per week during educational rounds, compared to 3.4 3.6 times per week after implementation (P = 0.66). Prior to implementation, all pages were numeric necessitating an immediate call‐back, causing an educational disruption. During the implementation period, 13% of pages received during educational rounds were nonurgent text pages that did not require an immediate call‐back, increasing to 29% after implementation (P < 0.001).
| November 2005 (3 weeks)* | November 2006 (3 weeks) | January 2008 (2 weeks) | P Value* | |
|---|---|---|---|---|
| ||||
| Total number of pages received during scheduled educational rounds | 104 | 129 | 103 | |
| Total pages from GM ward or Doc | 61 (59%) | 76 (59%) | 62 (60%) | 0.888 |
| Numeric | 61 (59%) | 43 (33%) | 25 (24%) | <0.001 |
| Text | 0 (0%) | 33 (26%) | 37 (36%) | <0.001 |
| Urgent | 0 (0%) | 16 (13%) | 7 (7%) | 0.007 |
| Nonurgent | 0 (0%) | 17 (13%) | 30 (29%) | <0.001 |
| Numeric pages non‐GM ward and non‐Doc | 43 (41%) | 53 (41%) | 41 (40%) | 0.888 |
| Pages requiring an immediate call back | 104 (100%) | 112 (87%) | 73 (71%) | <0.001 |
User Satisfaction
Physicians (18/25; response rate = 72%) were very satisfied with the alphanumeric paging system (mean, 4.6/5), felt that the alphanumeric paging system minimized disruptions to patient care duties (4.1/5) as well as educational rounds (4.2/5), and allowed them to prioritize their tasks effectively (4.6/5). Nursing staff (32/80, response rate = 40%) were also satisfied with the alphanumeric paging system (4.1/5), and found the technology very easy to use (4.5/5).
Potential Barriers to and Unintended Downsides of Implementation
We identified a number of barriers that limited the broader adoption of alphanumeric paging at our hospital. Nursing staff expressed concerns about limited computer and typographical skills. We addressed this by involving nursing champions to promote the alphanumeric paging system and to assist with nurse training. There were insufficient computers available for the nurses to send text pages, so many opted to page the physician using the conventional telephone system. The limited number of alphanumeric pagers during the implementation period meant that cross‐covering and off‐service residents were not carrying alphanumeric pagers. This undermined our ability to encourage use of a single paging system. We addressed this by convincing the hospital to provide alphanumeric pagers to all residents and medical students at our institution, a practice that was adopted in July 2007.
We also identified several potential unintended downsides to the implementation of alphanumeric paging. Nurses received no confirmation that nonurgent pages had reached the residents. We asked the residents to close the communication loop by making a phone call or confirming in person at the next convenient opportunity. Pagers store confidential transmitted patient information unless the resident deletes it. Communication using the pagers may replace discussions that should occur in person. For example, residents might send a text page with brief updates about patients as the only form of sign‐over. Even though the majority of sign‐over pages in our study were simply a text message to arrange a place to meet for face‐to‐face sign‐over, we did encounter a small number of pages where it is unclear whether provision of actual sign‐over information via text message was in lieu of a formal handoff, or whether it was accompanied by an in‐person handoff as well. Finally, nurses had to leave the patient's bedside to send a text page from a computer workstation. We highlighted that sending a nonurgent text page allows nurses to return to the bedside rather than wait at the nursing station for a call‐back.
Our opinions regarding key elements of an alphanumeric paging system implementation are summarized in Table 5.
| Equip all members of the healthcare team with alphanumeric pagers | |
| Use a web‐based paging program that allows easy and accurate identification of the responsible physician 24 hours a day, 7 days a week | |
| Install sufficient computer terminals for accessing the paging program | |
| Provide 2‐way communication so the page recipient can acknowledge the receipt of the message | |
| Maintain patient confidentiality by encrypting or encoding messages and sending them via a secure server | |
| Choose pager technology that ensures reliable delivery of messages without dropped pages | |
| Ensure ongoing technical support and training services for health care team members |
Discussion
We successfully implemented an alphanumeric paging system on a resident inpatient internal medicine teaching service, with 42% of pages from our GM wards or physicians sent as text pages during implementation period. Six months after widespread use of alphanumeric pagers at our hospital (and 14 months after the initial implementation on the General Internal Medicine service) the text paging rate was 52%. Physicians have nearly universally adopted the use of alphanumeric paging as a means of communicating with one another, while the adoption by nursing staff was modest. The implementation of the alphanumeric paging system was associated with a significant reduction in disruptive pages.
We could identify only one prior study of alphanumeric paging implementation in a hospital setting. A general surgery teaching service in the United States demonstrated that 35% of all pages received by residents were text pages 3 months after implementation,11 similar to our result of 32% text paging rate after full implementation. We found a greater use of text paging among physicians in our study (93% of Doc‐to‐Doc pages were text), compared to 55% in this prior study. This difference may be partially explained by varied methods for identifying Doc‐to‐Doc pages between the studies.
A number of factors influence the adoption of new technology, such as the technology's features, end‐user characteristics, and dissemination strategies.14 During the implementation, even though the web‐based paging system was deemed easy to use, there was a lack of computers available to send text messages at each of our nursing stations. Residents were generally more familiar with technology and the use of computers for communication than nurses, and therefore more likely to use the technology. The presence of innovators influenced the success of adoption, evidenced by the fact that the GM ward that sent the highest percentage of pages as text was also the GM ward where the project leader (B.W.) worked as the attending staff during the implementation period.
Our study has several limitations. First, our method for classifying the source of numeric pages was imperfect. Our method systematically underestimates Doc‐to‐Doc numeric pages, because we assumed that all pages from GM ward phone numbers were not from physicians. We also may have misclassified the origin of some text messages. These limitations would not affect our conclusion that text messaging increased, but may overestimate the increase in Doc‐to‐Doc text messaging, and underestimate the increase in GM ward‐to‐Doc text messaging. The reasons for paging are not known for the numeric pages. The number of pages received per resident increased after implementation, so it is possible that alphanumeric pages increased calls for certain nonurgent issues, such as co‐signing orders. Finally, we assumed that residents were attending scheduled educational rounds, but were unable to confirm attendance, so we cannot be sure that disruptions actually were reduced.
In summary, we implemented an alphanumeric paging system, and observed a sustained use of text messaging after 1 year. The implementation of alphanumeric paging was associated with a reduction in disruptive pages sent during scheduled educational rounds.
Acknowledgements
Twenty alphanumeric pagers were provided in kind for the duration of the implementation period by PageNet Canada. The authors thank the Advance Practice Nurses from the 4 GM wards (Sonia Dyal, Jackie Griffin‐White, Tracey Kitchen‐Clark, and Trish Trieu) and members of the communications department (Jonathon Tunstead, Howard Golding, Joan Moodie, and Myles Leicester) for the instrumental role they played in the implementation of the alphanumeric paging system.
- , , , , .Teams: communication in multidisciplinary care.Oncologist.2006;11:520–526.
- .14,000 preventable deaths in Australian hospitals.BMJ.1995;310:1487.
- , .Interrupted care. The effects of paging on pediatric resident activities.Am J Dis Child.1992;146:806–808.
- , , .Patterns of paging medical interns during night calls at two teaching hospitals.CMAJ.1994;151:307–311.
- , .The sounds of the hospital. Paging patterns in three teaching hospitals.N Engl J Med.1988;319:1585–1589.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- , .Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- , , et al.Improving response to critical laboratory results with automation: results of a randomized controlled trial.J Am Med Inform Assoc.1999;6:512–522.
- , , , .Real‐time notification of laboratory data requested by users through alphanumeric pagers.J Am Med Inform Assoc.2002;9:217–222.
- , , , , .Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191:561–565.
- .A primer on leading the improvement of systems.BMJ.1996;312:619–622.
- SurveyMonkey.com. The simple way to create surveys. Available at: http://www.surveymonkey.com. Accessed September 2009.
- .Diffusion of innovations.New York:Free Press;1995.
Effective communication between healthcare providers is essential to patient safety and quality of care.1, 2 Numeric pagers are commonly used communication devices in healthcare, but cannot convey important information such as the reason for the page, urgency of the page, or sender name. Physicians must respond to numeric pages, often disrupting patient encounters or educational activities.36 In a study of medical interns, disruptions to patient care occurred with up to 65% of pages received, two‐thirds of which were not felt to be urgent.5 In addition to causing frustration, frequent disruptions can contribute to medical errors.7, 8
Alphanumeric pagers can display both numbers and text, and may address some of the communication problems associated with numeric pagers. They also lay the groundwork for other patient safety initiatives such as automated paging of critical laboratory values9 and real‐time reporting of user‐requested laboratory data.10 Implementation of alphanumeric paging on a general surgery teaching service reduced disruptions to patient care and the number of pages requiring a return call.11
Our primary aim was to implement an alphanumeric paging system. We will describe our implementation strategies and barriers identified. We evaluated the implementation of alphanumeric paging by measuring (1) the proportion of pages sent as text pages, (2) the source of the pages (other physicians or from the general medicine [GM] ward), (3) the content of the text pages, (4) the number of pages that disrupted scheduled education activities, and (5) satisfaction with the alphanumeric paging system.
Materials and Methods
Setting
Sunnybrook Health Sciences Centre is a tertiary care academic teaching hospital affiliated with the University of Toronto (Toronto, Ontario, Canada). There are 4 physician teams that provide hospitalist care to admitted patients on the General Internal Medicine service. Each physician team consists of 1 attending physician, 1 second‐year or third‐year resident, 2 to 3 first‐year residents, and 3 to 4 third‐year and fourth‐year medical students. In total, 12 to 13 residents rotate through the General Internal Medicine service per month. Each physician team is assigned to 1 of 4 GM wards, which are staffed with nurses and allied health staff. Five to eight Internet‐enabled computer stations are located on each GM ward. All physicians, nurses, and allied health staff who worked on the 4 GM wards participated in the study.
Existing Paging System
Prior to July 2006, all physicians at our hospital carried numeric pagers. A physician could be paged by 3 methods: (1) through the hospital operator; (2) using the telephone; or (3) using an Internet‐based paging system (Smart Web 3.6.2, AmCom Software Inc.). Most pages were sent through the hospital operator or by telephone.
Intervention
The intervention included: (1) equipping resident physicians with alphanumeric pagers and (2) increasing the use of the existing Internet‐based paging system to send text pages. We equipped each resident with an alphanumeric pager (Motorola Flex Alphanumeric Pager). Users could send a text page using the existing Internet‐based application (Smart Web 3.6.2). This application allows users to search for a specific physician either by name or by on‐call assignment, and send a page up to 125 characters long from any Internet‐enabled computer in the hospital. Numeric pages could be sent by telephone, through the hospital operator, or by using the web‐based paging system throughout the study period.
Implementation Process
We provided alphanumeric pagers to the residents on the General Internal Medicine service in July 2006. Alphanumeric pagers were limited, so each resident traded their numeric pager for an alphanumeric pager at the start of each rotation. Once their rotation ended, they returned their alphanumeric pager for their original numeric pager. The communications department coordinated this process. The chief medical resident spent 10 minutes to teach the residents how to use the system at the beginning of the rotation. In August and September 2006, a member of the communications department trained the nurses on the 4 GM wards how to send a text page using the Internet‐based paging application. We scheduled these 15‐minute sessions throughout the day and evening in order to capture as many nurses as possible. We encouraged the nurses to include standardized information in the text message (eg, patient ID, issue, level of urgency, sender name, call‐back number).
We used rapid‐cycle change methods12 to implement the alphanumeric system (Figure 1). The first change cycle in August 2006 consisted of providing pagers to residents and training the nurses and physicians to send text pages. Users reported that the paging interface was difficult to use. For the second change cycle in September 2006, the communications department modified the paging interface to improve usability and created shortcut icons on the GM ward computers. While the system was easier to access, the nurses reported that 1‐time training was insufficient. For the next change cycle in September 2006, we developed Internet‐based tutorials that could be accessed at any time, and made expert users (charge nurses) available for just‐in‐time training. We asked these charge nurses what they believed would encourage adoption of the system. They suggested that contests worked well with other initiatives. For the final change cycle in October and November 2006, we held a contest and rewarded the GM ward that sent the highest percentage of text pages with a team lunch.

Our results from November 2006 were presented to our hospital medical leaders, who approved widespread implementation of alphanumeric pagers for all residents and medical students in all programs. The cost of this upgrade was approximately $35,000 per year to lease 500 alphanumeric pagers. By July 2007, all residents and students in our hospital had alphanumeric pagers.
Measures
Our primary outcome measure was the percentage of pages sent as text pages. We chose November 2005 as our before implementation period to account for temporal variations in patient load, and secular trends in resident knowledge and experience during an academic year. We collected data during our rapid‐cycle improvement periods of implementation and testing in September to November 2006. We assessed sustainability after implementation by collecting data in January 2008, 6 months after hospital‐wide implementation of alphanumeric pagers, and 14 months after the initial implementation on the GIM service.
We reviewed weekday paging records from our communications department for each study period. For text pages, we reviewed the text message to determine whether the page was sent by a physician or by another health care professional. We established 5 mutually exclusive categories of messages prior to the study: (1) A GM ward‐to‐physician (GM ward‐to‐Doc) numeric page was any page that contained only a phone number for 1 of the 4 GM ward main telephone numbers; (2) A Doc‐to‐Doc numeric page was any page that was preceded by 000‐ (a convention used at our hospital to indicate a physician sender), or that contained a phone number used only by physicians, such as the doctor's lounge; (3) A GM ward‐to‐Doc text page was a text page sent by any GM ward health care professional; (4) A Doc‐to‐Doc text page was a text page sent by any physician or medical student; and (5) All other numeric pages, such as those with phone numbers from other hospital wards, were classified as numeric non‐GM ward and non‐Doc in‐hospital page.
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds that occur every weekday from 12:00 to 1:00 PM. We classified a page as disruptive if it required an immediate call‐back (ie, all numeric pages and urgent text pages).
We surveyed residents and daytime GM ward nursing staff during the implementation period (October and November 2006) to assess satisfaction with the alphanumeric paging system using a 5‐point Likert scale (1 = strongly disagree, 5 = strongly agree). We distributed paper surveys to nurses, and used an electronic web survey for residents.13
Statistical Analysis
We compared the paging data after implementation (January 2008) to the period before implementation (November 2005) using a Student t‐test for comparison of means, and chi‐square and Fisher's exact tests for categorical value comparisons. We assigned a significance level of P < 0.05 for the t tests and chi‐square tests, and P < 0.01 for the Fisher's exact tests. We used SPSS 11.0 to perform statistical analyses (Chicago, IL).
Results
Paging Patterns Before, During, and After Implementation
The number of pages per resident was similar before and during implementation, but higher afterwards. (46 16 pages/resident/week in November 2005, 47 20 pages/resident/week in November 2006, and 59 27 pages/resident/week in January 2008; P = 0.17; Table 1). The mean number of admissions per night was 8.0 2.7 before implementation, compared to 10.2 3.5 after implementation (P = 0.009).
| Before Implementation (all residents had numeric pagers) | During Implementation (all residents had alphanumeric pagers) | After Implementation (all residents had alphanumeric pagers) | |||
|---|---|---|---|---|---|
| Paging Characteristics | November 2005 | October 2006 | November 2006 | January 2008 | P Value |
| |||||
| Total number of pages | 1431 | 1879 | 1813 | 1269 | |
| Total number of resident weeks worked | 29 | 33 | 33 | 21 | |
| Pages per resident week, mean (SD) | 46.2 (16.3) | 57.9 (19.2) | 46.5 (20.2) | 59.0 (26.5) | 0.17* |
| Number of patients admitted per night, mean (SD) | 8.0 (2.7) | 10.2 (3.5) | 11.0 (2.9) | 10.2 (3.5) | 0.009* |
| Type of page, n (%) | |||||
| Numeric pages | 751 (53) | 462 (25) | 580 (32) | 374 (30) | <0.001 |
| GM wards‐to‐Doc | 584 (41) | 393 (21) | 538 (30) | 352 (28) | <0.001 |
| Doc‐to‐Doc | 167 (12) | 69 (4) | 42 (2) | 22 (2) | <0.001 |
| Non‐GM ward/Doc pages | 680 (47) | 1107 (59) | 809 (45) | 487 (38) | <0.001 |
| Text pages | 0 (0) | 310 (16) | 424 (23) | 408 (32) | <0.001 |
| GM wards‐to‐Doc | 0 (0) | 175 (9) | 221 (12) | 129 (10) | <0.001 |
| Doc‐to‐Doc | 0 (0) | 135 (7) | 203 (11) | 279 (22) | <0.001 |
We observed a significant and sustained increase in the use of text paging during the study (Table 1). After implementation, 32% of all pages sent to our residents were text messages (P < 0.001). Physicians almost exclusively sent text pages by the end of implementation (increase from 0% to 83% text paging rate during implementation, and 93% after implementation; P < 0.001; Figure 2). GM ward text paging rates also increased from 0% to 29% during implementation, and 27% after implementation (P < 0.001; Figure 2). The alphanumeric paging system was used to a greater degree by physicians compared to other workers on the GM ward after full implementation (93% vs. 27%; P < 0.001). We explored the proportion of GM ward‐to‐Doc pages sent as text from different GM wards during implementation, and found significant variation, ranging from 14% to 57% (P < 0.001).

The most common reasons for text paging from GM wards were to request a patient assessment or for notification of a patient's clinical status (25%), to clarify written orders (20%), and to request a medication prescription (13%) (Table 2). Among physicians, the most common reasons for text paging were to set up meetings for work or teaching rounds (33%), to relay patient‐care related messages (27%), and to sign‐over patients at the end of the day (23%) (Table 3). The remainder of the other Doc‐to‐Doc pages (18%) were mostly personal messages or team communication that was not related to clinical work.
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Requests for patient assessment or notification of a patient's clinical status | 55 (25) | Patient X. Temp 38.5. No other symptoms. [Nurse's name]. |
| The repeat CXR on Patient X has been completed. Please call [ward] if NG can be used. Thank you. | ||
| Clarification of a written order | 45 (20) | Would you like Patient X to get a second dose of Lasix? He has already had 100 mg and his output thus far is 500 cc. [Nurse's name] |
| Patient X. BP = 100/62, pulse 54. Patient is supposed to have metoprolol 50 mg tonight. Do you want me to hold it? [Nurse's name] | ||
| Request for a medication prescription | 28 (13) | Patient X needs an analgesic for pain in his arms and legs. Please call [ward]. |
| Patient X has a daily coumadin order. INR 2.14. Please call with dosage for 1800. Ask for [Nurse]. | ||
| Cosigning written order | 25 (11) | Please co‐sign neurology suggested orders for Patient X. [Nurse's name] |
| Not urgent at your leisure, please co‐sign Patient X orders on [ward] for medical student. Thanks. | ||
| Notification of a recent laboratory result | 23 (11) | Patient X's potassium is 3.0 today. Please call [ward]. |
| Patient X. Sodium 163. Troponin unchanged at 1.54. [ward]. | ||
| Arranging meetings with patients and/or family | 18 (8) | Meeting with Patient X's family and social worker at 2 pm tomorrow on [ward]. |
| [Social worker] is here. [Physiotherapist] expected any minute. We are going to meet in family room for Patient X. | ||
| Request to complete paperwork | 15 (7) | Patient X is ready to go home, and just needs discharge orders and prescriptions |
| Referral form for community palliative doctor on the front of the chart fill in where marked by arrows. Can you please put on form prognosis as well? [Social worker] | ||
| Other | 12 (6) | |
| Total | 221 (100) | |
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Setting up meetings for work or teaching rounds | 67 (33) | Confirmed diabetes teaching at 1400 hr in [room]. Please let medical students know. Thanks. |
| Please come to [lecture theatre] if you can in the next 10 minutes for the teleconferenced noon rounds. Thanks. | ||
| We are in the Emergency Department with [attending staff]. Come to meet us here if you can. | ||
| Relaying patient‐care related messages | 54 (27) | Patient X US query cholangitis, can we ask for a surgical consult. He may benefit from surgery or percutaneous drain. Please repeat his blood work. |
| Patient X in [room X]. Presented with DKA. pH 7.2. pCO2 23, bicarbonate 9. Lytes pending. Got IV insulin and NS. Need to check to clinical stability. [Resident] | ||
| Signing‐over patients at the end of the day | 46 (23) | I'm ready to sign out to you. Where are you? [Resident]. |
| Patient X is back from cardiac cath, and is stable. Please check Cr over WE, and watch for CHF. | ||
| Other | 36 (18) | |
| Total | 203 (100) | |
Impact of Alphanumeric Paging System on Disruptions
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds (Table 4). Prior to implementation, residents were paged 2.9 2.4 times per week during educational rounds, compared to 3.4 3.6 times per week after implementation (P = 0.66). Prior to implementation, all pages were numeric necessitating an immediate call‐back, causing an educational disruption. During the implementation period, 13% of pages received during educational rounds were nonurgent text pages that did not require an immediate call‐back, increasing to 29% after implementation (P < 0.001).
| November 2005 (3 weeks)* | November 2006 (3 weeks) | January 2008 (2 weeks) | P Value* | |
|---|---|---|---|---|
| ||||
| Total number of pages received during scheduled educational rounds | 104 | 129 | 103 | |
| Total pages from GM ward or Doc | 61 (59%) | 76 (59%) | 62 (60%) | 0.888 |
| Numeric | 61 (59%) | 43 (33%) | 25 (24%) | <0.001 |
| Text | 0 (0%) | 33 (26%) | 37 (36%) | <0.001 |
| Urgent | 0 (0%) | 16 (13%) | 7 (7%) | 0.007 |
| Nonurgent | 0 (0%) | 17 (13%) | 30 (29%) | <0.001 |
| Numeric pages non‐GM ward and non‐Doc | 43 (41%) | 53 (41%) | 41 (40%) | 0.888 |
| Pages requiring an immediate call back | 104 (100%) | 112 (87%) | 73 (71%) | <0.001 |
User Satisfaction
Physicians (18/25; response rate = 72%) were very satisfied with the alphanumeric paging system (mean, 4.6/5), felt that the alphanumeric paging system minimized disruptions to patient care duties (4.1/5) as well as educational rounds (4.2/5), and allowed them to prioritize their tasks effectively (4.6/5). Nursing staff (32/80, response rate = 40%) were also satisfied with the alphanumeric paging system (4.1/5), and found the technology very easy to use (4.5/5).
Potential Barriers to and Unintended Downsides of Implementation
We identified a number of barriers that limited the broader adoption of alphanumeric paging at our hospital. Nursing staff expressed concerns about limited computer and typographical skills. We addressed this by involving nursing champions to promote the alphanumeric paging system and to assist with nurse training. There were insufficient computers available for the nurses to send text pages, so many opted to page the physician using the conventional telephone system. The limited number of alphanumeric pagers during the implementation period meant that cross‐covering and off‐service residents were not carrying alphanumeric pagers. This undermined our ability to encourage use of a single paging system. We addressed this by convincing the hospital to provide alphanumeric pagers to all residents and medical students at our institution, a practice that was adopted in July 2007.
We also identified several potential unintended downsides to the implementation of alphanumeric paging. Nurses received no confirmation that nonurgent pages had reached the residents. We asked the residents to close the communication loop by making a phone call or confirming in person at the next convenient opportunity. Pagers store confidential transmitted patient information unless the resident deletes it. Communication using the pagers may replace discussions that should occur in person. For example, residents might send a text page with brief updates about patients as the only form of sign‐over. Even though the majority of sign‐over pages in our study were simply a text message to arrange a place to meet for face‐to‐face sign‐over, we did encounter a small number of pages where it is unclear whether provision of actual sign‐over information via text message was in lieu of a formal handoff, or whether it was accompanied by an in‐person handoff as well. Finally, nurses had to leave the patient's bedside to send a text page from a computer workstation. We highlighted that sending a nonurgent text page allows nurses to return to the bedside rather than wait at the nursing station for a call‐back.
Our opinions regarding key elements of an alphanumeric paging system implementation are summarized in Table 5.
| Equip all members of the healthcare team with alphanumeric pagers | |
| Use a web‐based paging program that allows easy and accurate identification of the responsible physician 24 hours a day, 7 days a week | |
| Install sufficient computer terminals for accessing the paging program | |
| Provide 2‐way communication so the page recipient can acknowledge the receipt of the message | |
| Maintain patient confidentiality by encrypting or encoding messages and sending them via a secure server | |
| Choose pager technology that ensures reliable delivery of messages without dropped pages | |
| Ensure ongoing technical support and training services for health care team members |
Discussion
We successfully implemented an alphanumeric paging system on a resident inpatient internal medicine teaching service, with 42% of pages from our GM wards or physicians sent as text pages during implementation period. Six months after widespread use of alphanumeric pagers at our hospital (and 14 months after the initial implementation on the General Internal Medicine service) the text paging rate was 52%. Physicians have nearly universally adopted the use of alphanumeric paging as a means of communicating with one another, while the adoption by nursing staff was modest. The implementation of the alphanumeric paging system was associated with a significant reduction in disruptive pages.
We could identify only one prior study of alphanumeric paging implementation in a hospital setting. A general surgery teaching service in the United States demonstrated that 35% of all pages received by residents were text pages 3 months after implementation,11 similar to our result of 32% text paging rate after full implementation. We found a greater use of text paging among physicians in our study (93% of Doc‐to‐Doc pages were text), compared to 55% in this prior study. This difference may be partially explained by varied methods for identifying Doc‐to‐Doc pages between the studies.
A number of factors influence the adoption of new technology, such as the technology's features, end‐user characteristics, and dissemination strategies.14 During the implementation, even though the web‐based paging system was deemed easy to use, there was a lack of computers available to send text messages at each of our nursing stations. Residents were generally more familiar with technology and the use of computers for communication than nurses, and therefore more likely to use the technology. The presence of innovators influenced the success of adoption, evidenced by the fact that the GM ward that sent the highest percentage of pages as text was also the GM ward where the project leader (B.W.) worked as the attending staff during the implementation period.
Our study has several limitations. First, our method for classifying the source of numeric pages was imperfect. Our method systematically underestimates Doc‐to‐Doc numeric pages, because we assumed that all pages from GM ward phone numbers were not from physicians. We also may have misclassified the origin of some text messages. These limitations would not affect our conclusion that text messaging increased, but may overestimate the increase in Doc‐to‐Doc text messaging, and underestimate the increase in GM ward‐to‐Doc text messaging. The reasons for paging are not known for the numeric pages. The number of pages received per resident increased after implementation, so it is possible that alphanumeric pages increased calls for certain nonurgent issues, such as co‐signing orders. Finally, we assumed that residents were attending scheduled educational rounds, but were unable to confirm attendance, so we cannot be sure that disruptions actually were reduced.
In summary, we implemented an alphanumeric paging system, and observed a sustained use of text messaging after 1 year. The implementation of alphanumeric paging was associated with a reduction in disruptive pages sent during scheduled educational rounds.
Acknowledgements
Twenty alphanumeric pagers were provided in kind for the duration of the implementation period by PageNet Canada. The authors thank the Advance Practice Nurses from the 4 GM wards (Sonia Dyal, Jackie Griffin‐White, Tracey Kitchen‐Clark, and Trish Trieu) and members of the communications department (Jonathon Tunstead, Howard Golding, Joan Moodie, and Myles Leicester) for the instrumental role they played in the implementation of the alphanumeric paging system.
Effective communication between healthcare providers is essential to patient safety and quality of care.1, 2 Numeric pagers are commonly used communication devices in healthcare, but cannot convey important information such as the reason for the page, urgency of the page, or sender name. Physicians must respond to numeric pages, often disrupting patient encounters or educational activities.36 In a study of medical interns, disruptions to patient care occurred with up to 65% of pages received, two‐thirds of which were not felt to be urgent.5 In addition to causing frustration, frequent disruptions can contribute to medical errors.7, 8
Alphanumeric pagers can display both numbers and text, and may address some of the communication problems associated with numeric pagers. They also lay the groundwork for other patient safety initiatives such as automated paging of critical laboratory values9 and real‐time reporting of user‐requested laboratory data.10 Implementation of alphanumeric paging on a general surgery teaching service reduced disruptions to patient care and the number of pages requiring a return call.11
Our primary aim was to implement an alphanumeric paging system. We will describe our implementation strategies and barriers identified. We evaluated the implementation of alphanumeric paging by measuring (1) the proportion of pages sent as text pages, (2) the source of the pages (other physicians or from the general medicine [GM] ward), (3) the content of the text pages, (4) the number of pages that disrupted scheduled education activities, and (5) satisfaction with the alphanumeric paging system.
Materials and Methods
Setting
Sunnybrook Health Sciences Centre is a tertiary care academic teaching hospital affiliated with the University of Toronto (Toronto, Ontario, Canada). There are 4 physician teams that provide hospitalist care to admitted patients on the General Internal Medicine service. Each physician team consists of 1 attending physician, 1 second‐year or third‐year resident, 2 to 3 first‐year residents, and 3 to 4 third‐year and fourth‐year medical students. In total, 12 to 13 residents rotate through the General Internal Medicine service per month. Each physician team is assigned to 1 of 4 GM wards, which are staffed with nurses and allied health staff. Five to eight Internet‐enabled computer stations are located on each GM ward. All physicians, nurses, and allied health staff who worked on the 4 GM wards participated in the study.
Existing Paging System
Prior to July 2006, all physicians at our hospital carried numeric pagers. A physician could be paged by 3 methods: (1) through the hospital operator; (2) using the telephone; or (3) using an Internet‐based paging system (Smart Web 3.6.2, AmCom Software Inc.). Most pages were sent through the hospital operator or by telephone.
Intervention
The intervention included: (1) equipping resident physicians with alphanumeric pagers and (2) increasing the use of the existing Internet‐based paging system to send text pages. We equipped each resident with an alphanumeric pager (Motorola Flex Alphanumeric Pager). Users could send a text page using the existing Internet‐based application (Smart Web 3.6.2). This application allows users to search for a specific physician either by name or by on‐call assignment, and send a page up to 125 characters long from any Internet‐enabled computer in the hospital. Numeric pages could be sent by telephone, through the hospital operator, or by using the web‐based paging system throughout the study period.
Implementation Process
We provided alphanumeric pagers to the residents on the General Internal Medicine service in July 2006. Alphanumeric pagers were limited, so each resident traded their numeric pager for an alphanumeric pager at the start of each rotation. Once their rotation ended, they returned their alphanumeric pager for their original numeric pager. The communications department coordinated this process. The chief medical resident spent 10 minutes to teach the residents how to use the system at the beginning of the rotation. In August and September 2006, a member of the communications department trained the nurses on the 4 GM wards how to send a text page using the Internet‐based paging application. We scheduled these 15‐minute sessions throughout the day and evening in order to capture as many nurses as possible. We encouraged the nurses to include standardized information in the text message (eg, patient ID, issue, level of urgency, sender name, call‐back number).
We used rapid‐cycle change methods12 to implement the alphanumeric system (Figure 1). The first change cycle in August 2006 consisted of providing pagers to residents and training the nurses and physicians to send text pages. Users reported that the paging interface was difficult to use. For the second change cycle in September 2006, the communications department modified the paging interface to improve usability and created shortcut icons on the GM ward computers. While the system was easier to access, the nurses reported that 1‐time training was insufficient. For the next change cycle in September 2006, we developed Internet‐based tutorials that could be accessed at any time, and made expert users (charge nurses) available for just‐in‐time training. We asked these charge nurses what they believed would encourage adoption of the system. They suggested that contests worked well with other initiatives. For the final change cycle in October and November 2006, we held a contest and rewarded the GM ward that sent the highest percentage of text pages with a team lunch.

Our results from November 2006 were presented to our hospital medical leaders, who approved widespread implementation of alphanumeric pagers for all residents and medical students in all programs. The cost of this upgrade was approximately $35,000 per year to lease 500 alphanumeric pagers. By July 2007, all residents and students in our hospital had alphanumeric pagers.
Measures
Our primary outcome measure was the percentage of pages sent as text pages. We chose November 2005 as our before implementation period to account for temporal variations in patient load, and secular trends in resident knowledge and experience during an academic year. We collected data during our rapid‐cycle improvement periods of implementation and testing in September to November 2006. We assessed sustainability after implementation by collecting data in January 2008, 6 months after hospital‐wide implementation of alphanumeric pagers, and 14 months after the initial implementation on the GIM service.
We reviewed weekday paging records from our communications department for each study period. For text pages, we reviewed the text message to determine whether the page was sent by a physician or by another health care professional. We established 5 mutually exclusive categories of messages prior to the study: (1) A GM ward‐to‐physician (GM ward‐to‐Doc) numeric page was any page that contained only a phone number for 1 of the 4 GM ward main telephone numbers; (2) A Doc‐to‐Doc numeric page was any page that was preceded by 000‐ (a convention used at our hospital to indicate a physician sender), or that contained a phone number used only by physicians, such as the doctor's lounge; (3) A GM ward‐to‐Doc text page was a text page sent by any GM ward health care professional; (4) A Doc‐to‐Doc text page was a text page sent by any physician or medical student; and (5) All other numeric pages, such as those with phone numbers from other hospital wards, were classified as numeric non‐GM ward and non‐Doc in‐hospital page.
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds that occur every weekday from 12:00 to 1:00 PM. We classified a page as disruptive if it required an immediate call‐back (ie, all numeric pages and urgent text pages).
We surveyed residents and daytime GM ward nursing staff during the implementation period (October and November 2006) to assess satisfaction with the alphanumeric paging system using a 5‐point Likert scale (1 = strongly disagree, 5 = strongly agree). We distributed paper surveys to nurses, and used an electronic web survey for residents.13
Statistical Analysis
We compared the paging data after implementation (January 2008) to the period before implementation (November 2005) using a Student t‐test for comparison of means, and chi‐square and Fisher's exact tests for categorical value comparisons. We assigned a significance level of P < 0.05 for the t tests and chi‐square tests, and P < 0.01 for the Fisher's exact tests. We used SPSS 11.0 to perform statistical analyses (Chicago, IL).
Results
Paging Patterns Before, During, and After Implementation
The number of pages per resident was similar before and during implementation, but higher afterwards. (46 16 pages/resident/week in November 2005, 47 20 pages/resident/week in November 2006, and 59 27 pages/resident/week in January 2008; P = 0.17; Table 1). The mean number of admissions per night was 8.0 2.7 before implementation, compared to 10.2 3.5 after implementation (P = 0.009).
| Before Implementation (all residents had numeric pagers) | During Implementation (all residents had alphanumeric pagers) | After Implementation (all residents had alphanumeric pagers) | |||
|---|---|---|---|---|---|
| Paging Characteristics | November 2005 | October 2006 | November 2006 | January 2008 | P Value |
| |||||
| Total number of pages | 1431 | 1879 | 1813 | 1269 | |
| Total number of resident weeks worked | 29 | 33 | 33 | 21 | |
| Pages per resident week, mean (SD) | 46.2 (16.3) | 57.9 (19.2) | 46.5 (20.2) | 59.0 (26.5) | 0.17* |
| Number of patients admitted per night, mean (SD) | 8.0 (2.7) | 10.2 (3.5) | 11.0 (2.9) | 10.2 (3.5) | 0.009* |
| Type of page, n (%) | |||||
| Numeric pages | 751 (53) | 462 (25) | 580 (32) | 374 (30) | <0.001 |
| GM wards‐to‐Doc | 584 (41) | 393 (21) | 538 (30) | 352 (28) | <0.001 |
| Doc‐to‐Doc | 167 (12) | 69 (4) | 42 (2) | 22 (2) | <0.001 |
| Non‐GM ward/Doc pages | 680 (47) | 1107 (59) | 809 (45) | 487 (38) | <0.001 |
| Text pages | 0 (0) | 310 (16) | 424 (23) | 408 (32) | <0.001 |
| GM wards‐to‐Doc | 0 (0) | 175 (9) | 221 (12) | 129 (10) | <0.001 |
| Doc‐to‐Doc | 0 (0) | 135 (7) | 203 (11) | 279 (22) | <0.001 |
We observed a significant and sustained increase in the use of text paging during the study (Table 1). After implementation, 32% of all pages sent to our residents were text messages (P < 0.001). Physicians almost exclusively sent text pages by the end of implementation (increase from 0% to 83% text paging rate during implementation, and 93% after implementation; P < 0.001; Figure 2). GM ward text paging rates also increased from 0% to 29% during implementation, and 27% after implementation (P < 0.001; Figure 2). The alphanumeric paging system was used to a greater degree by physicians compared to other workers on the GM ward after full implementation (93% vs. 27%; P < 0.001). We explored the proportion of GM ward‐to‐Doc pages sent as text from different GM wards during implementation, and found significant variation, ranging from 14% to 57% (P < 0.001).

The most common reasons for text paging from GM wards were to request a patient assessment or for notification of a patient's clinical status (25%), to clarify written orders (20%), and to request a medication prescription (13%) (Table 2). Among physicians, the most common reasons for text paging were to set up meetings for work or teaching rounds (33%), to relay patient‐care related messages (27%), and to sign‐over patients at the end of the day (23%) (Table 3). The remainder of the other Doc‐to‐Doc pages (18%) were mostly personal messages or team communication that was not related to clinical work.
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Requests for patient assessment or notification of a patient's clinical status | 55 (25) | Patient X. Temp 38.5. No other symptoms. [Nurse's name]. |
| The repeat CXR on Patient X has been completed. Please call [ward] if NG can be used. Thank you. | ||
| Clarification of a written order | 45 (20) | Would you like Patient X to get a second dose of Lasix? He has already had 100 mg and his output thus far is 500 cc. [Nurse's name] |
| Patient X. BP = 100/62, pulse 54. Patient is supposed to have metoprolol 50 mg tonight. Do you want me to hold it? [Nurse's name] | ||
| Request for a medication prescription | 28 (13) | Patient X needs an analgesic for pain in his arms and legs. Please call [ward]. |
| Patient X has a daily coumadin order. INR 2.14. Please call with dosage for 1800. Ask for [Nurse]. | ||
| Cosigning written order | 25 (11) | Please co‐sign neurology suggested orders for Patient X. [Nurse's name] |
| Not urgent at your leisure, please co‐sign Patient X orders on [ward] for medical student. Thanks. | ||
| Notification of a recent laboratory result | 23 (11) | Patient X's potassium is 3.0 today. Please call [ward]. |
| Patient X. Sodium 163. Troponin unchanged at 1.54. [ward]. | ||
| Arranging meetings with patients and/or family | 18 (8) | Meeting with Patient X's family and social worker at 2 pm tomorrow on [ward]. |
| [Social worker] is here. [Physiotherapist] expected any minute. We are going to meet in family room for Patient X. | ||
| Request to complete paperwork | 15 (7) | Patient X is ready to go home, and just needs discharge orders and prescriptions |
| Referral form for community palliative doctor on the front of the chart fill in where marked by arrows. Can you please put on form prognosis as well? [Social worker] | ||
| Other | 12 (6) | |
| Total | 221 (100) | |
| Reason for Paging | Number (%) | Examples |
|---|---|---|
| ||
| Setting up meetings for work or teaching rounds | 67 (33) | Confirmed diabetes teaching at 1400 hr in [room]. Please let medical students know. Thanks. |
| Please come to [lecture theatre] if you can in the next 10 minutes for the teleconferenced noon rounds. Thanks. | ||
| We are in the Emergency Department with [attending staff]. Come to meet us here if you can. | ||
| Relaying patient‐care related messages | 54 (27) | Patient X US query cholangitis, can we ask for a surgical consult. He may benefit from surgery or percutaneous drain. Please repeat his blood work. |
| Patient X in [room X]. Presented with DKA. pH 7.2. pCO2 23, bicarbonate 9. Lytes pending. Got IV insulin and NS. Need to check to clinical stability. [Resident] | ||
| Signing‐over patients at the end of the day | 46 (23) | I'm ready to sign out to you. Where are you? [Resident]. |
| Patient X is back from cardiac cath, and is stable. Please check Cr over WE, and watch for CHF. | ||
| Other | 36 (18) | |
| Total | 203 (100) | |
Impact of Alphanumeric Paging System on Disruptions
We evaluated the impact of the alphanumeric system on disruptions by studying pages received during scheduled educational rounds (Table 4). Prior to implementation, residents were paged 2.9 2.4 times per week during educational rounds, compared to 3.4 3.6 times per week after implementation (P = 0.66). Prior to implementation, all pages were numeric necessitating an immediate call‐back, causing an educational disruption. During the implementation period, 13% of pages received during educational rounds were nonurgent text pages that did not require an immediate call‐back, increasing to 29% after implementation (P < 0.001).
| November 2005 (3 weeks)* | November 2006 (3 weeks) | January 2008 (2 weeks) | P Value* | |
|---|---|---|---|---|
| ||||
| Total number of pages received during scheduled educational rounds | 104 | 129 | 103 | |
| Total pages from GM ward or Doc | 61 (59%) | 76 (59%) | 62 (60%) | 0.888 |
| Numeric | 61 (59%) | 43 (33%) | 25 (24%) | <0.001 |
| Text | 0 (0%) | 33 (26%) | 37 (36%) | <0.001 |
| Urgent | 0 (0%) | 16 (13%) | 7 (7%) | 0.007 |
| Nonurgent | 0 (0%) | 17 (13%) | 30 (29%) | <0.001 |
| Numeric pages non‐GM ward and non‐Doc | 43 (41%) | 53 (41%) | 41 (40%) | 0.888 |
| Pages requiring an immediate call back | 104 (100%) | 112 (87%) | 73 (71%) | <0.001 |
User Satisfaction
Physicians (18/25; response rate = 72%) were very satisfied with the alphanumeric paging system (mean, 4.6/5), felt that the alphanumeric paging system minimized disruptions to patient care duties (4.1/5) as well as educational rounds (4.2/5), and allowed them to prioritize their tasks effectively (4.6/5). Nursing staff (32/80, response rate = 40%) were also satisfied with the alphanumeric paging system (4.1/5), and found the technology very easy to use (4.5/5).
Potential Barriers to and Unintended Downsides of Implementation
We identified a number of barriers that limited the broader adoption of alphanumeric paging at our hospital. Nursing staff expressed concerns about limited computer and typographical skills. We addressed this by involving nursing champions to promote the alphanumeric paging system and to assist with nurse training. There were insufficient computers available for the nurses to send text pages, so many opted to page the physician using the conventional telephone system. The limited number of alphanumeric pagers during the implementation period meant that cross‐covering and off‐service residents were not carrying alphanumeric pagers. This undermined our ability to encourage use of a single paging system. We addressed this by convincing the hospital to provide alphanumeric pagers to all residents and medical students at our institution, a practice that was adopted in July 2007.
We also identified several potential unintended downsides to the implementation of alphanumeric paging. Nurses received no confirmation that nonurgent pages had reached the residents. We asked the residents to close the communication loop by making a phone call or confirming in person at the next convenient opportunity. Pagers store confidential transmitted patient information unless the resident deletes it. Communication using the pagers may replace discussions that should occur in person. For example, residents might send a text page with brief updates about patients as the only form of sign‐over. Even though the majority of sign‐over pages in our study were simply a text message to arrange a place to meet for face‐to‐face sign‐over, we did encounter a small number of pages where it is unclear whether provision of actual sign‐over information via text message was in lieu of a formal handoff, or whether it was accompanied by an in‐person handoff as well. Finally, nurses had to leave the patient's bedside to send a text page from a computer workstation. We highlighted that sending a nonurgent text page allows nurses to return to the bedside rather than wait at the nursing station for a call‐back.
Our opinions regarding key elements of an alphanumeric paging system implementation are summarized in Table 5.
| Equip all members of the healthcare team with alphanumeric pagers | |
| Use a web‐based paging program that allows easy and accurate identification of the responsible physician 24 hours a day, 7 days a week | |
| Install sufficient computer terminals for accessing the paging program | |
| Provide 2‐way communication so the page recipient can acknowledge the receipt of the message | |
| Maintain patient confidentiality by encrypting or encoding messages and sending them via a secure server | |
| Choose pager technology that ensures reliable delivery of messages without dropped pages | |
| Ensure ongoing technical support and training services for health care team members |
Discussion
We successfully implemented an alphanumeric paging system on a resident inpatient internal medicine teaching service, with 42% of pages from our GM wards or physicians sent as text pages during implementation period. Six months after widespread use of alphanumeric pagers at our hospital (and 14 months after the initial implementation on the General Internal Medicine service) the text paging rate was 52%. Physicians have nearly universally adopted the use of alphanumeric paging as a means of communicating with one another, while the adoption by nursing staff was modest. The implementation of the alphanumeric paging system was associated with a significant reduction in disruptive pages.
We could identify only one prior study of alphanumeric paging implementation in a hospital setting. A general surgery teaching service in the United States demonstrated that 35% of all pages received by residents were text pages 3 months after implementation,11 similar to our result of 32% text paging rate after full implementation. We found a greater use of text paging among physicians in our study (93% of Doc‐to‐Doc pages were text), compared to 55% in this prior study. This difference may be partially explained by varied methods for identifying Doc‐to‐Doc pages between the studies.
A number of factors influence the adoption of new technology, such as the technology's features, end‐user characteristics, and dissemination strategies.14 During the implementation, even though the web‐based paging system was deemed easy to use, there was a lack of computers available to send text messages at each of our nursing stations. Residents were generally more familiar with technology and the use of computers for communication than nurses, and therefore more likely to use the technology. The presence of innovators influenced the success of adoption, evidenced by the fact that the GM ward that sent the highest percentage of pages as text was also the GM ward where the project leader (B.W.) worked as the attending staff during the implementation period.
Our study has several limitations. First, our method for classifying the source of numeric pages was imperfect. Our method systematically underestimates Doc‐to‐Doc numeric pages, because we assumed that all pages from GM ward phone numbers were not from physicians. We also may have misclassified the origin of some text messages. These limitations would not affect our conclusion that text messaging increased, but may overestimate the increase in Doc‐to‐Doc text messaging, and underestimate the increase in GM ward‐to‐Doc text messaging. The reasons for paging are not known for the numeric pages. The number of pages received per resident increased after implementation, so it is possible that alphanumeric pages increased calls for certain nonurgent issues, such as co‐signing orders. Finally, we assumed that residents were attending scheduled educational rounds, but were unable to confirm attendance, so we cannot be sure that disruptions actually were reduced.
In summary, we implemented an alphanumeric paging system, and observed a sustained use of text messaging after 1 year. The implementation of alphanumeric paging was associated with a reduction in disruptive pages sent during scheduled educational rounds.
Acknowledgements
Twenty alphanumeric pagers were provided in kind for the duration of the implementation period by PageNet Canada. The authors thank the Advance Practice Nurses from the 4 GM wards (Sonia Dyal, Jackie Griffin‐White, Tracey Kitchen‐Clark, and Trish Trieu) and members of the communications department (Jonathon Tunstead, Howard Golding, Joan Moodie, and Myles Leicester) for the instrumental role they played in the implementation of the alphanumeric paging system.
- , , , , .Teams: communication in multidisciplinary care.Oncologist.2006;11:520–526.
- .14,000 preventable deaths in Australian hospitals.BMJ.1995;310:1487.
- , .Interrupted care. The effects of paging on pediatric resident activities.Am J Dis Child.1992;146:806–808.
- , , .Patterns of paging medical interns during night calls at two teaching hospitals.CMAJ.1994;151:307–311.
- , .The sounds of the hospital. Paging patterns in three teaching hospitals.N Engl J Med.1988;319:1585–1589.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- , .Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- , , et al.Improving response to critical laboratory results with automation: results of a randomized controlled trial.J Am Med Inform Assoc.1999;6:512–522.
- , , , .Real‐time notification of laboratory data requested by users through alphanumeric pagers.J Am Med Inform Assoc.2002;9:217–222.
- , , , , .Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191:561–565.
- .A primer on leading the improvement of systems.BMJ.1996;312:619–622.
- SurveyMonkey.com. The simple way to create surveys. Available at: http://www.surveymonkey.com. Accessed September 2009.
- .Diffusion of innovations.New York:Free Press;1995.
- , , , , .Teams: communication in multidisciplinary care.Oncologist.2006;11:520–526.
- .14,000 preventable deaths in Australian hospitals.BMJ.1995;310:1487.
- , .Interrupted care. The effects of paging on pediatric resident activities.Am J Dis Child.1992;146:806–808.
- , , .Patterns of paging medical interns during night calls at two teaching hospitals.CMAJ.1994;151:307–311.
- , .The sounds of the hospital. Paging patterns in three teaching hospitals.N Engl J Med.1988;319:1585–1589.
- , , .How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- , .Communication behaviours in a hospital setting: an observational study.BMJ.1998;316:673–676.
- , .Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- , , et al.Improving response to critical laboratory results with automation: results of a randomized controlled trial.J Am Med Inform Assoc.1999;6:512–522.
- , , , .Real‐time notification of laboratory data requested by users through alphanumeric pagers.J Am Med Inform Assoc.2002;9:217–222.
- , , , , .Alphanumeric paging in an academic hospital setting.Am J Surg.2006;191:561–565.
- .A primer on leading the improvement of systems.BMJ.1996;312:619–622.
- SurveyMonkey.com. The simple way to create surveys. Available at: http://www.surveymonkey.com. Accessed September 2009.
- .Diffusion of innovations.New York:Free Press;1995.
Statin therapy: New data suggest effects on plaque volume and stability
| ||||||||||||||||
| ||||||||||||||||
Value‐Driven Health Care
It is estimated that the Medicare Part A trust fund will be exhausted by 2016 to 2019; also, the quality of care delivered in the United States is highly variable.13 Value is typically defined as the quality achieved for a given cost (ie, value = quality/cost). The focus on the 3 related concepts of value, quality, and cost of health care is likely to continue to increase. Previously, the U.S. Department of Health and Human Services (HHS) made value‐driven health care one of the Department's top priorities.4 Policymakers are in a period of transition but the publicly available plans of the President and Senate leadership indicate that the focus on value‐based initiatives will likely continue to increase as our nation strives to achieve better outcomes for our health care dollar.5, 6 Specifically, the federal government and other payers increasingly align payment incentives with value and quality, encourage public reporting on quality and Medicare payment costs, such as on the Hospital Compare website (
Since hospital care represented $648 billion in 2006, which is 37% of the total patient‐related U.S. health care expenditure, the trend to pay for value will likely have significant impact on hospitals and hospitalists.7 The Society of Hospital Medicine has a public policy committee that provides feedback to government on programs and policies related to value‐driven health care. The policies and programs need consideration and input from the broader community of hospitalists. This work outlines some of the major national initiatives and policies focused on value‐driven health care and their implications for hospitalists. Hospitalists will need to understand the policy landscape and trends, lead improvement in their individual hospitals to receive value‐based incentives, and assess the opportunities and challenges of current and potential payer programs and policies.
Policies and Initiatives: Implications for Hospitals and Hospitalists
Within the portfolio of value‐driven health care, there are at least 6 major government programs, initiatives, and policies with implications for hospitals and hospitalists: value‐based purchasing (VBP), quality and cost public reporting, Medicare demonstrations, hospital‐acquired conditions, incentives for use of effective HIT, and the physician quality reporting initiative (PQRI) (Table 1).
| Initiative or Policy | Description | Specific Examples | Potential Implications |
|---|---|---|---|
| Value‐based purchasing | At least a portion of hospital payment related to value instead of the traditional fee‐for‐service model | Performance score is based on domains such as process measures (eg, beta‐blocker at discharge for acute myocardial), outcome measures (eg, 30‐day AMI mortality), and satisfaction measures (eg, Consumer Assessment of Healthcare Providers and Hospital Survey, aka HCAHPS) | Hospitalists will influence hospital performance on Total Performance Score and could lead quality improvement efforts to improve score |
| Payment based on hospital Total Performance Score | |||
| Public reporting of quality and cost | Websites such as CMS HospitalCompare increasingly report on select quality and cost metrics | HospitalCompare reports process measures (eg, percent of heart failure patients given discharge instructions), outcome measures (eg, 30‐day risk‐adjusted mortality for AMI), survey of patients' hospital experience, and Medicare payment and volume for certain conditions | Many of these measures are directly related to care provided by hospitalists |
| Hospitalists could lead quality improvement initiatives focused on these measures | |||
| Medicare demonstrations | CMS has demonstration projects to test concepts, such as value‐based purchasing, incentive payments, and care management, aimed at improving the value of care delivered | Premier Hospital Quality Incentive Demonstration: Differentiates payment to hospitals based on performance on 30 metrics | Hospitals with their associated hospitalists can apply to participate in these and other demonstrations |
| Acute Care Episode (ACE) Demonstration: Provides bundled payments for select orthopedic and cardiovascular inpatient procedures | |||
| Medicare Hospital Gainsharing Demonstration: Hospitals can provide gainsharing payments to physicians based on savings from improving quality and efficiency | Successful demonstrations can be expanded or components incorporated into payment policy | ||
| Hospital‐acquired conditions | CMS rule that the presence of a select group of reasonably preventable conditions as the only complicating condition will not cause a higher‐paying diagnosis‐related group (DRG) to be assigned to the case | First 10 implemented: | Hospitalists can lead or participate in safety initiatives to decrease or eliminate these complications |
| Foreign object retained after surgery; | |||
| Air embolism; | |||
| Blood incompatibility; | |||
| Stage III and IV pressure ulcers; | |||
| Falls and trauma; | |||
| Manifestations of poor glycemic control; | |||
| Catheter‐associated urinary tract infections; | |||
| Vascular catheter‐associated infection; | |||
| Surgical site infection following specific surgeries; | |||
| Deep vein thrombosis and pulmonary embolus | |||
| Incentives for use of effective health information technology | Incentives for HIT use, often focused on certified interoperable systems and/or quality reporting, are provided by federal and private payers | The American Recovery and Reinvestment Act included over $17 billion of HIT incentives | Front line providers, including hospitalists, need to help guide implementation of HIT to be successful |
| Incentive money was directed at hospitals (hospital‐based physicians were excluded) and ambulatory physicians are eligible for direct incentives | Systems that include physician order entry, clinical decision support, and quality measurement capture and reporting can help hospitalists deliver higher quality care | ||
| Physician Quality Reporting Initiative (PQRI) | Quality measures linked to 2% incentive payment to physicians and other practitioners for reporting quality measures via claims or registry | Examples of measures hospitalists can report on include: | Hospitalists can drive performance on these measures, lead reporting efforts, and share in the financial rewards |
| Deep vein thrombosis prophylaxis for ischemic stroke or intracranial hemorrhage; | |||
| Medication reconciliation at discharge; | |||
| Advance care plan documented; | |||
| Oral antiplatelet therapy for patients discharged with stroke diagnosis |
Value‐Based Purchasing
The Deficit Reduction Act Section 5001(b) authorized the Centers for Medicare and Medicaid Services (CMS) to develop a Medicare hospital VBP plan. The VBP plan is currently in the Presidents FY10 budget and the senate Committee's specification for health reform. VBP involves at least a portion of the payment being related to value instead of the traditional fee‐for‐service (FFS) model. CMS has consulted with external stakeholders on the measures, data infrastructure and validation, and incentive structure for the proposed program. Hospitals would submit data for all VBP measures that apply and performance scores would be given based on both attainment and improvement. The higher of the 2 scores (ie, attainment or improvement) would represent the hospital's performance in a given domain and the weighted domain scores would determine the hospital's total performance score. This total performance score would then be translated into an incentive payment for the hospital. Measure domains would include process measures (eg, beta‐blocker at discharge for acute myocardial infarction [AMI]), outcome measures (eg, 30‐day AMI mortality), and satisfaction measures (eg, Consumer Assessment of Healthcare Providers and Systems Hospital survey [HCAHPS]). Hospitalists are in a unique position to impact and improve performance in all of the above domains for hospitals. This has the potential to increase the value of high‐performing hospitalist groups to their respective hospitals. Most importantly, this program aligns incentives with rewarding the provision of high‐quality care as defined by these measures. Over time, CMS may consider expanding the measures to other domains such as efficiency, more patient‐centered outcomes, and care coordination. In terms of efficiency measurement, the Medicare Improvements for Patients and Providers Act (MIPPA) passed in 2008 called for CMS to provide confidential reports to physicians or groups of physicians on their relative resource use. This legislation also called for HHS to develop a plan for transitioning to VBP for Medicare professional services and a report to Congress on this plan.9 This lays the foundation for transforming Medicare to focus more on quality, resource use, and value and less on FFS.
Public Reporting of Quality and Cost
CMS is beginning to empower consumers with information on quality and Medicare payment costs so they can make educated decisions on where and how they seek care. The CMS website focused on hospitals is HospitalCompare (
Medicare Demonstrations
Several Medicare demonstration projects already implemented or in the planning stages are particularly relevant to hospitals and hospitalist practice. These demonstrations test concepts, such as VBP, incentive payments, and care management, aimed at improving the value of care delivered. If demonstrations are successful, they have potential for incorporation into federal policy. The Premier Hospital Quality Incentive Demonstration is an incentive program that differentiates payment to hospitals based on performance on 30 quality measures. Participation is voluntary. The top 20% of hospitals receive incentive payments.11 The success of this demonstration led to its current second phase. The Acute Care Episode (ACE) Demonstration will provide bundled payments (including Part A and B services) for ACEs within Medicare FFS. The demonstration includes gainsharing by allowing sites to reward individual clinicians, teams of clinicians, or other hospital staff who show measurable clinical quality improvement. The focus is on select orthopedic and cardiovascular inpatient procedures (eg, hip/knee replacement surgery and coronary artery bypass graft surgery).12 The demonstration also has potential to increase volume to participant physician‐hospital organizations through financial incentives to beneficiaries via payments to offset their Medicare cost‐sharing obligations. If this demonstration is successful and the concept of bundled payment is expanded to other conditions and additional geographies, this could have significant impact on hospitalists. Specifically, programs for care coordination and reducing readmissions and complications after discharge would be directly incentivized. The demonstration focuses on Texas, Oklahoma, Colorado, and New Mexico. The Medicare Hospital Gainsharing Demonstration program to test and evaluate arrangements between hospitals and physicians is designed improve the quality and efficiency of care provided to beneficiaries. The demonstration allows hospitals to provide gainsharing payments to physicians that represent solely a share of the savings incurred as a result of collaborative efforts to improve overall quality and efficiency. The demonstration was launched in 2007.13 Finally, the upcoming Medicare Medical Home Demonstration has the potential for direct or indirect hospitalist participation as facilitators in Tier 2 medical homes that take into account care coordination across inpatient and outpatient settings.14 These demonstrations represent attempts by the federal government to align incentives with high‐quality, high‐value delivery of care.
Hospital‐Acquired Conditions
Hospital‐acquired conditions (HACs) have significant cost and quality implications for U.S. healthcare, such as the estimated 99,000 deaths associated with hospital‐acquired infections annually.15 Therefore, CMS received statutory authority to not pay additional charges for reasonably preventable HACs. Beginning October 1, 2008, CMS implemented a rule that the presence of selected reasonably preventable conditions as the only complicating condition would not cause a higher‐paying DRG to be assigned to the case; therefore, the case would be paid as though the secondary diagnosis were not present. CMS also required the reporting of a new data element to delineate HACs from conditions present on admission. Hospitals can indicate to CMS if a condition was present on admission as a secondary diagnosis, allowing reimbursement for care provided to treat any condition present on admission. The first 10 HACs to be implemented were: foreign object retained after surgery, air embolism, blood incompatibility, stage III and IV pressure ulcers, falls and trauma, manifestations of poor glycemic control, catheter‐associated urinary tract infections, vascular catheterassociated infection, surgical site infection following specific surgeries, and deep vein thrombosis and pulmonary embolus.16 Since some complications are not absolutely preventable and evidence‐based guidelines for the prevention of some complications are lacking, this has generated some resistance from the provider community.17 The HAC payment policy is a step toward aligning incentives with quality performance, but any further HACs will need to be evaluated for their level of preventability and potential for unintended consequences.
Incentives for Use of Effective HIT
The use of HIT can be incentivized in 3 main ways, all of which are likely to increase over time. First, incentives can be implemented to reward reporting quality metrics via electronic health records or registries. Second, incentives based on quality performance may indirectly encourage the adoption of HIT because an electronic medical record, especially with computerized provider order entry and decision support, may enable higher performance on quality metrics. Finally, CMS has provided direct incentives for information technology adoption, such as certified electronic health records.18 The American Recovery and Reinvestment Act of 2009 created over $17 billion of potential incentive payments for HIT use by physicians and hospitals.19 Hospitals are eligible for significant incentives reaching estimates over $6 million per year, phasing out by 2015, so Congress excluded hospital‐based physicians from direct payments. Ambulatory physicians are eligible for up to $18,000 per year, phasing out by 2015, with subsequent payment reductions for nonuse. Since hospitalists often function at the nexus of clinical care, quality improvement, and technology use, they have the opportunity to lead or facilitate effective implementation of information technology in their hospitals. These efforts may be rewarded by hospitals.
PQRI
PQRI was authorized in 2006 and included a 1.5% incentive payment for satisfactorily reporting quality data. The incentive payment will increase to 2% in 2009. There are 153 PQRI measures in 2009 and a significant number of the measures focus on hospital‐based care. Examples of measures hospitalists can help report include: deep vein thrombosis prophylaxis for ischemic stroke or intracranial hemorrhage, medication reconciliation at discharge, advance care plan documentation, oral antiplatelet therapy for patients discharged with stroke diagnosis, and anticoagulant therapy prescribed for atrial fibrillation in stroke patients at discharge. PQRI measures can be reported through claims‐based or registry‐based reporting. Reporting can be done on individual measures or for measure groups associated with specific conditions.20 Hospitalists have the potential to drive performance on these measures, lead reporting efforts, and share in the financial rewards.
Future Considerations
The political leadership at the federal and state level is beginning a new transition; however, the focus on quality and value for our health care dollar will likely continue to increase.5, 6 The U.S. health care system has untenable cost estimates, significant quality gaps, and a fractured payment system that fails to reward effective care coordination.2, 21, 22 This increased focus on quality and value should be viewed as an opportunity for hospitalists and hospitals. Hospitalist groups that can achieve high‐quality performance will be increasingly valued, and hospitals should further recognize the critical role hospitalists play in achieving high performance and the associated financial rewards. Hospitalists often lead quality improvement and safety programs in hospitals, and these programs are likely to be seen as progressively more important as payment is linked to performance. The Society of Hospital Medicine engages with policymakers and this role is increasingly significant as more policy and payment decisions impact hospitalists. The Society has focused on collaborative work with payers, policymakers, and other providers to find joint shared solutions. Hospitalists can serve as a link between providers and a focal point of care coordination, especially for the hospitalized patient. Finally, as our system and its incentives continue to progress toward alignment with value‐based high quality care, hospitalists should be leading the change and be an essential part of the solution to transform our health care system to provide high‐quality, efficient care to all Americans.
Acknowledgements
Dr. Tom Valuck is recognized for his thoughtful comments and edits in preparation, submission, and revision of this manuscript.
- Medicare Board of Trustees. A Summary of the 2008 Report. Available at: http://www.ssa.gov/OACT/TRSUM/trsummary.html. Accessed April 2009.
- , , , et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- Economy Likely to Move up Medicare's Insolvency. Available at: http://abcnews.go.com/Politics/Health/wireStory?id=6369658. Accessed April 2009.
- Value‐Driven Health Care. Available at: http://www.hhs.gov/valuedriven. Accessed April 2009.
- Obama‐Biden Full Health Care Plan. Available at: http://www.barackobama.com/issues/healthcare. Accessed April 2009.
- Senate Chairman Max Baucus Call to Action Health Reform 2009. Available at: http://finance.senate.gov/healthreform2009/finalwhitepaper.pdf. Accessed April 2009.
- , , , .National health spending in 2006: a year of change for prescription drugs.Health Aff (Millwood).2008;27(1):14–29.
- Medicare Hospital Value‐Based Purchasing Plan. October 31,2008. Available at: http://www.cms.hhs.gov/AcuteInpatientPPS/downloads/hospital_VBP_plan_issues_paper.pdf. Accessed April 2009.
- Medicare Improvements for Patients and Providers Act of 2008. Available at: http://www.asm.org/ASM/files/ccLibraryFiles/Filename/000000004120/HR6331.pdf. Accessed April 2009.
- HospitalCompare. Available at: www.hospitalcompare.hhs.gov. Accessed April 2009.
- Premier Hospital Quality Incentive Demonstration. Available at: http://www.cms.hhs.gov/HospitalQualityInits/35_hospitalpremier.asp. Accessed April 2009.
- Acute Care Episode Demonstration. Available at: http://www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none122(2):160–166.
- Hospital Acquired Conditions. Available at: http://www.cms.hhs.gov/HospitalAcqCond/06_Hospital‐Acquired_Conditions.asp. Accessed April 2009.
- , , .The wisdom and justice of not paying for “preventable complications”.JAMA.2008;299(18):2197–2199.
- E‐prescribing incentive program. Available at: http://www.cms.hhs.gov/ERXincentive. Accessed April 2009.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills299(19):2319–2321.
It is estimated that the Medicare Part A trust fund will be exhausted by 2016 to 2019; also, the quality of care delivered in the United States is highly variable.13 Value is typically defined as the quality achieved for a given cost (ie, value = quality/cost). The focus on the 3 related concepts of value, quality, and cost of health care is likely to continue to increase. Previously, the U.S. Department of Health and Human Services (HHS) made value‐driven health care one of the Department's top priorities.4 Policymakers are in a period of transition but the publicly available plans of the President and Senate leadership indicate that the focus on value‐based initiatives will likely continue to increase as our nation strives to achieve better outcomes for our health care dollar.5, 6 Specifically, the federal government and other payers increasingly align payment incentives with value and quality, encourage public reporting on quality and Medicare payment costs, such as on the Hospital Compare website (
Since hospital care represented $648 billion in 2006, which is 37% of the total patient‐related U.S. health care expenditure, the trend to pay for value will likely have significant impact on hospitals and hospitalists.7 The Society of Hospital Medicine has a public policy committee that provides feedback to government on programs and policies related to value‐driven health care. The policies and programs need consideration and input from the broader community of hospitalists. This work outlines some of the major national initiatives and policies focused on value‐driven health care and their implications for hospitalists. Hospitalists will need to understand the policy landscape and trends, lead improvement in their individual hospitals to receive value‐based incentives, and assess the opportunities and challenges of current and potential payer programs and policies.
Policies and Initiatives: Implications for Hospitals and Hospitalists
Within the portfolio of value‐driven health care, there are at least 6 major government programs, initiatives, and policies with implications for hospitals and hospitalists: value‐based purchasing (VBP), quality and cost public reporting, Medicare demonstrations, hospital‐acquired conditions, incentives for use of effective HIT, and the physician quality reporting initiative (PQRI) (Table 1).
| Initiative or Policy | Description | Specific Examples | Potential Implications |
|---|---|---|---|
| Value‐based purchasing | At least a portion of hospital payment related to value instead of the traditional fee‐for‐service model | Performance score is based on domains such as process measures (eg, beta‐blocker at discharge for acute myocardial), outcome measures (eg, 30‐day AMI mortality), and satisfaction measures (eg, Consumer Assessment of Healthcare Providers and Hospital Survey, aka HCAHPS) | Hospitalists will influence hospital performance on Total Performance Score and could lead quality improvement efforts to improve score |
| Payment based on hospital Total Performance Score | |||
| Public reporting of quality and cost | Websites such as CMS HospitalCompare increasingly report on select quality and cost metrics | HospitalCompare reports process measures (eg, percent of heart failure patients given discharge instructions), outcome measures (eg, 30‐day risk‐adjusted mortality for AMI), survey of patients' hospital experience, and Medicare payment and volume for certain conditions | Many of these measures are directly related to care provided by hospitalists |
| Hospitalists could lead quality improvement initiatives focused on these measures | |||
| Medicare demonstrations | CMS has demonstration projects to test concepts, such as value‐based purchasing, incentive payments, and care management, aimed at improving the value of care delivered | Premier Hospital Quality Incentive Demonstration: Differentiates payment to hospitals based on performance on 30 metrics | Hospitals with their associated hospitalists can apply to participate in these and other demonstrations |
| Acute Care Episode (ACE) Demonstration: Provides bundled payments for select orthopedic and cardiovascular inpatient procedures | |||
| Medicare Hospital Gainsharing Demonstration: Hospitals can provide gainsharing payments to physicians based on savings from improving quality and efficiency | Successful demonstrations can be expanded or components incorporated into payment policy | ||
| Hospital‐acquired conditions | CMS rule that the presence of a select group of reasonably preventable conditions as the only complicating condition will not cause a higher‐paying diagnosis‐related group (DRG) to be assigned to the case | First 10 implemented: | Hospitalists can lead or participate in safety initiatives to decrease or eliminate these complications |
| Foreign object retained after surgery; | |||
| Air embolism; | |||
| Blood incompatibility; | |||
| Stage III and IV pressure ulcers; | |||
| Falls and trauma; | |||
| Manifestations of poor glycemic control; | |||
| Catheter‐associated urinary tract infections; | |||
| Vascular catheter‐associated infection; | |||
| Surgical site infection following specific surgeries; | |||
| Deep vein thrombosis and pulmonary embolus | |||
| Incentives for use of effective health information technology | Incentives for HIT use, often focused on certified interoperable systems and/or quality reporting, are provided by federal and private payers | The American Recovery and Reinvestment Act included over $17 billion of HIT incentives | Front line providers, including hospitalists, need to help guide implementation of HIT to be successful |
| Incentive money was directed at hospitals (hospital‐based physicians were excluded) and ambulatory physicians are eligible for direct incentives | Systems that include physician order entry, clinical decision support, and quality measurement capture and reporting can help hospitalists deliver higher quality care | ||
| Physician Quality Reporting Initiative (PQRI) | Quality measures linked to 2% incentive payment to physicians and other practitioners for reporting quality measures via claims or registry | Examples of measures hospitalists can report on include: | Hospitalists can drive performance on these measures, lead reporting efforts, and share in the financial rewards |
| Deep vein thrombosis prophylaxis for ischemic stroke or intracranial hemorrhage; | |||
| Medication reconciliation at discharge; | |||
| Advance care plan documented; | |||
| Oral antiplatelet therapy for patients discharged with stroke diagnosis |
Value‐Based Purchasing
The Deficit Reduction Act Section 5001(b) authorized the Centers for Medicare and Medicaid Services (CMS) to develop a Medicare hospital VBP plan. The VBP plan is currently in the Presidents FY10 budget and the senate Committee's specification for health reform. VBP involves at least a portion of the payment being related to value instead of the traditional fee‐for‐service (FFS) model. CMS has consulted with external stakeholders on the measures, data infrastructure and validation, and incentive structure for the proposed program. Hospitals would submit data for all VBP measures that apply and performance scores would be given based on both attainment and improvement. The higher of the 2 scores (ie, attainment or improvement) would represent the hospital's performance in a given domain and the weighted domain scores would determine the hospital's total performance score. This total performance score would then be translated into an incentive payment for the hospital. Measure domains would include process measures (eg, beta‐blocker at discharge for acute myocardial infarction [AMI]), outcome measures (eg, 30‐day AMI mortality), and satisfaction measures (eg, Consumer Assessment of Healthcare Providers and Systems Hospital survey [HCAHPS]). Hospitalists are in a unique position to impact and improve performance in all of the above domains for hospitals. This has the potential to increase the value of high‐performing hospitalist groups to their respective hospitals. Most importantly, this program aligns incentives with rewarding the provision of high‐quality care as defined by these measures. Over time, CMS may consider expanding the measures to other domains such as efficiency, more patient‐centered outcomes, and care coordination. In terms of efficiency measurement, the Medicare Improvements for Patients and Providers Act (MIPPA) passed in 2008 called for CMS to provide confidential reports to physicians or groups of physicians on their relative resource use. This legislation also called for HHS to develop a plan for transitioning to VBP for Medicare professional services and a report to Congress on this plan.9 This lays the foundation for transforming Medicare to focus more on quality, resource use, and value and less on FFS.
Public Reporting of Quality and Cost
CMS is beginning to empower consumers with information on quality and Medicare payment costs so they can make educated decisions on where and how they seek care. The CMS website focused on hospitals is HospitalCompare (
Medicare Demonstrations
Several Medicare demonstration projects already implemented or in the planning stages are particularly relevant to hospitals and hospitalist practice. These demonstrations test concepts, such as VBP, incentive payments, and care management, aimed at improving the value of care delivered. If demonstrations are successful, they have potential for incorporation into federal policy. The Premier Hospital Quality Incentive Demonstration is an incentive program that differentiates payment to hospitals based on performance on 30 quality measures. Participation is voluntary. The top 20% of hospitals receive incentive payments.11 The success of this demonstration led to its current second phase. The Acute Care Episode (ACE) Demonstration will provide bundled payments (including Part A and B services) for ACEs within Medicare FFS. The demonstration includes gainsharing by allowing sites to reward individual clinicians, teams of clinicians, or other hospital staff who show measurable clinical quality improvement. The focus is on select orthopedic and cardiovascular inpatient procedures (eg, hip/knee replacement surgery and coronary artery bypass graft surgery).12 The demonstration also has potential to increase volume to participant physician‐hospital organizations through financial incentives to beneficiaries via payments to offset their Medicare cost‐sharing obligations. If this demonstration is successful and the concept of bundled payment is expanded to other conditions and additional geographies, this could have significant impact on hospitalists. Specifically, programs for care coordination and reducing readmissions and complications after discharge would be directly incentivized. The demonstration focuses on Texas, Oklahoma, Colorado, and New Mexico. The Medicare Hospital Gainsharing Demonstration program to test and evaluate arrangements between hospitals and physicians is designed improve the quality and efficiency of care provided to beneficiaries. The demonstration allows hospitals to provide gainsharing payments to physicians that represent solely a share of the savings incurred as a result of collaborative efforts to improve overall quality and efficiency. The demonstration was launched in 2007.13 Finally, the upcoming Medicare Medical Home Demonstration has the potential for direct or indirect hospitalist participation as facilitators in Tier 2 medical homes that take into account care coordination across inpatient and outpatient settings.14 These demonstrations represent attempts by the federal government to align incentives with high‐quality, high‐value delivery of care.
Hospital‐Acquired Conditions
Hospital‐acquired conditions (HACs) have significant cost and quality implications for U.S. healthcare, such as the estimated 99,000 deaths associated with hospital‐acquired infections annually.15 Therefore, CMS received statutory authority to not pay additional charges for reasonably preventable HACs. Beginning October 1, 2008, CMS implemented a rule that the presence of selected reasonably preventable conditions as the only complicating condition would not cause a higher‐paying DRG to be assigned to the case; therefore, the case would be paid as though the secondary diagnosis were not present. CMS also required the reporting of a new data element to delineate HACs from conditions present on admission. Hospitals can indicate to CMS if a condition was present on admission as a secondary diagnosis, allowing reimbursement for care provided to treat any condition present on admission. The first 10 HACs to be implemented were: foreign object retained after surgery, air embolism, blood incompatibility, stage III and IV pressure ulcers, falls and trauma, manifestations of poor glycemic control, catheter‐associated urinary tract infections, vascular catheterassociated infection, surgical site infection following specific surgeries, and deep vein thrombosis and pulmonary embolus.16 Since some complications are not absolutely preventable and evidence‐based guidelines for the prevention of some complications are lacking, this has generated some resistance from the provider community.17 The HAC payment policy is a step toward aligning incentives with quality performance, but any further HACs will need to be evaluated for their level of preventability and potential for unintended consequences.
Incentives for Use of Effective HIT
The use of HIT can be incentivized in 3 main ways, all of which are likely to increase over time. First, incentives can be implemented to reward reporting quality metrics via electronic health records or registries. Second, incentives based on quality performance may indirectly encourage the adoption of HIT because an electronic medical record, especially with computerized provider order entry and decision support, may enable higher performance on quality metrics. Finally, CMS has provided direct incentives for information technology adoption, such as certified electronic health records.18 The American Recovery and Reinvestment Act of 2009 created over $17 billion of potential incentive payments for HIT use by physicians and hospitals.19 Hospitals are eligible for significant incentives reaching estimates over $6 million per year, phasing out by 2015, so Congress excluded hospital‐based physicians from direct payments. Ambulatory physicians are eligible for up to $18,000 per year, phasing out by 2015, with subsequent payment reductions for nonuse. Since hospitalists often function at the nexus of clinical care, quality improvement, and technology use, they have the opportunity to lead or facilitate effective implementation of information technology in their hospitals. These efforts may be rewarded by hospitals.
PQRI
PQRI was authorized in 2006 and included a 1.5% incentive payment for satisfactorily reporting quality data. The incentive payment will increase to 2% in 2009. There are 153 PQRI measures in 2009 and a significant number of the measures focus on hospital‐based care. Examples of measures hospitalists can help report include: deep vein thrombosis prophylaxis for ischemic stroke or intracranial hemorrhage, medication reconciliation at discharge, advance care plan documentation, oral antiplatelet therapy for patients discharged with stroke diagnosis, and anticoagulant therapy prescribed for atrial fibrillation in stroke patients at discharge. PQRI measures can be reported through claims‐based or registry‐based reporting. Reporting can be done on individual measures or for measure groups associated with specific conditions.20 Hospitalists have the potential to drive performance on these measures, lead reporting efforts, and share in the financial rewards.
Future Considerations
The political leadership at the federal and state level is beginning a new transition; however, the focus on quality and value for our health care dollar will likely continue to increase.5, 6 The U.S. health care system has untenable cost estimates, significant quality gaps, and a fractured payment system that fails to reward effective care coordination.2, 21, 22 This increased focus on quality and value should be viewed as an opportunity for hospitalists and hospitals. Hospitalist groups that can achieve high‐quality performance will be increasingly valued, and hospitals should further recognize the critical role hospitalists play in achieving high performance and the associated financial rewards. Hospitalists often lead quality improvement and safety programs in hospitals, and these programs are likely to be seen as progressively more important as payment is linked to performance. The Society of Hospital Medicine engages with policymakers and this role is increasingly significant as more policy and payment decisions impact hospitalists. The Society has focused on collaborative work with payers, policymakers, and other providers to find joint shared solutions. Hospitalists can serve as a link between providers and a focal point of care coordination, especially for the hospitalized patient. Finally, as our system and its incentives continue to progress toward alignment with value‐based high quality care, hospitalists should be leading the change and be an essential part of the solution to transform our health care system to provide high‐quality, efficient care to all Americans.
Acknowledgements
Dr. Tom Valuck is recognized for his thoughtful comments and edits in preparation, submission, and revision of this manuscript.
It is estimated that the Medicare Part A trust fund will be exhausted by 2016 to 2019; also, the quality of care delivered in the United States is highly variable.13 Value is typically defined as the quality achieved for a given cost (ie, value = quality/cost). The focus on the 3 related concepts of value, quality, and cost of health care is likely to continue to increase. Previously, the U.S. Department of Health and Human Services (HHS) made value‐driven health care one of the Department's top priorities.4 Policymakers are in a period of transition but the publicly available plans of the President and Senate leadership indicate that the focus on value‐based initiatives will likely continue to increase as our nation strives to achieve better outcomes for our health care dollar.5, 6 Specifically, the federal government and other payers increasingly align payment incentives with value and quality, encourage public reporting on quality and Medicare payment costs, such as on the Hospital Compare website (
Since hospital care represented $648 billion in 2006, which is 37% of the total patient‐related U.S. health care expenditure, the trend to pay for value will likely have significant impact on hospitals and hospitalists.7 The Society of Hospital Medicine has a public policy committee that provides feedback to government on programs and policies related to value‐driven health care. The policies and programs need consideration and input from the broader community of hospitalists. This work outlines some of the major national initiatives and policies focused on value‐driven health care and their implications for hospitalists. Hospitalists will need to understand the policy landscape and trends, lead improvement in their individual hospitals to receive value‐based incentives, and assess the opportunities and challenges of current and potential payer programs and policies.
Policies and Initiatives: Implications for Hospitals and Hospitalists
Within the portfolio of value‐driven health care, there are at least 6 major government programs, initiatives, and policies with implications for hospitals and hospitalists: value‐based purchasing (VBP), quality and cost public reporting, Medicare demonstrations, hospital‐acquired conditions, incentives for use of effective HIT, and the physician quality reporting initiative (PQRI) (Table 1).
| Initiative or Policy | Description | Specific Examples | Potential Implications |
|---|---|---|---|
| Value‐based purchasing | At least a portion of hospital payment related to value instead of the traditional fee‐for‐service model | Performance score is based on domains such as process measures (eg, beta‐blocker at discharge for acute myocardial), outcome measures (eg, 30‐day AMI mortality), and satisfaction measures (eg, Consumer Assessment of Healthcare Providers and Hospital Survey, aka HCAHPS) | Hospitalists will influence hospital performance on Total Performance Score and could lead quality improvement efforts to improve score |
| Payment based on hospital Total Performance Score | |||
| Public reporting of quality and cost | Websites such as CMS HospitalCompare increasingly report on select quality and cost metrics | HospitalCompare reports process measures (eg, percent of heart failure patients given discharge instructions), outcome measures (eg, 30‐day risk‐adjusted mortality for AMI), survey of patients' hospital experience, and Medicare payment and volume for certain conditions | Many of these measures are directly related to care provided by hospitalists |
| Hospitalists could lead quality improvement initiatives focused on these measures | |||
| Medicare demonstrations | CMS has demonstration projects to test concepts, such as value‐based purchasing, incentive payments, and care management, aimed at improving the value of care delivered | Premier Hospital Quality Incentive Demonstration: Differentiates payment to hospitals based on performance on 30 metrics | Hospitals with their associated hospitalists can apply to participate in these and other demonstrations |
| Acute Care Episode (ACE) Demonstration: Provides bundled payments for select orthopedic and cardiovascular inpatient procedures | |||
| Medicare Hospital Gainsharing Demonstration: Hospitals can provide gainsharing payments to physicians based on savings from improving quality and efficiency | Successful demonstrations can be expanded or components incorporated into payment policy | ||
| Hospital‐acquired conditions | CMS rule that the presence of a select group of reasonably preventable conditions as the only complicating condition will not cause a higher‐paying diagnosis‐related group (DRG) to be assigned to the case | First 10 implemented: | Hospitalists can lead or participate in safety initiatives to decrease or eliminate these complications |
| Foreign object retained after surgery; | |||
| Air embolism; | |||
| Blood incompatibility; | |||
| Stage III and IV pressure ulcers; | |||
| Falls and trauma; | |||
| Manifestations of poor glycemic control; | |||
| Catheter‐associated urinary tract infections; | |||
| Vascular catheter‐associated infection; | |||
| Surgical site infection following specific surgeries; | |||
| Deep vein thrombosis and pulmonary embolus | |||
| Incentives for use of effective health information technology | Incentives for HIT use, often focused on certified interoperable systems and/or quality reporting, are provided by federal and private payers | The American Recovery and Reinvestment Act included over $17 billion of HIT incentives | Front line providers, including hospitalists, need to help guide implementation of HIT to be successful |
| Incentive money was directed at hospitals (hospital‐based physicians were excluded) and ambulatory physicians are eligible for direct incentives | Systems that include physician order entry, clinical decision support, and quality measurement capture and reporting can help hospitalists deliver higher quality care | ||
| Physician Quality Reporting Initiative (PQRI) | Quality measures linked to 2% incentive payment to physicians and other practitioners for reporting quality measures via claims or registry | Examples of measures hospitalists can report on include: | Hospitalists can drive performance on these measures, lead reporting efforts, and share in the financial rewards |
| Deep vein thrombosis prophylaxis for ischemic stroke or intracranial hemorrhage; | |||
| Medication reconciliation at discharge; | |||
| Advance care plan documented; | |||
| Oral antiplatelet therapy for patients discharged with stroke diagnosis |
Value‐Based Purchasing
The Deficit Reduction Act Section 5001(b) authorized the Centers for Medicare and Medicaid Services (CMS) to develop a Medicare hospital VBP plan. The VBP plan is currently in the Presidents FY10 budget and the senate Committee's specification for health reform. VBP involves at least a portion of the payment being related to value instead of the traditional fee‐for‐service (FFS) model. CMS has consulted with external stakeholders on the measures, data infrastructure and validation, and incentive structure for the proposed program. Hospitals would submit data for all VBP measures that apply and performance scores would be given based on both attainment and improvement. The higher of the 2 scores (ie, attainment or improvement) would represent the hospital's performance in a given domain and the weighted domain scores would determine the hospital's total performance score. This total performance score would then be translated into an incentive payment for the hospital. Measure domains would include process measures (eg, beta‐blocker at discharge for acute myocardial infarction [AMI]), outcome measures (eg, 30‐day AMI mortality), and satisfaction measures (eg, Consumer Assessment of Healthcare Providers and Systems Hospital survey [HCAHPS]). Hospitalists are in a unique position to impact and improve performance in all of the above domains for hospitals. This has the potential to increase the value of high‐performing hospitalist groups to their respective hospitals. Most importantly, this program aligns incentives with rewarding the provision of high‐quality care as defined by these measures. Over time, CMS may consider expanding the measures to other domains such as efficiency, more patient‐centered outcomes, and care coordination. In terms of efficiency measurement, the Medicare Improvements for Patients and Providers Act (MIPPA) passed in 2008 called for CMS to provide confidential reports to physicians or groups of physicians on their relative resource use. This legislation also called for HHS to develop a plan for transitioning to VBP for Medicare professional services and a report to Congress on this plan.9 This lays the foundation for transforming Medicare to focus more on quality, resource use, and value and less on FFS.
Public Reporting of Quality and Cost
CMS is beginning to empower consumers with information on quality and Medicare payment costs so they can make educated decisions on where and how they seek care. The CMS website focused on hospitals is HospitalCompare (
Medicare Demonstrations
Several Medicare demonstration projects already implemented or in the planning stages are particularly relevant to hospitals and hospitalist practice. These demonstrations test concepts, such as VBP, incentive payments, and care management, aimed at improving the value of care delivered. If demonstrations are successful, they have potential for incorporation into federal policy. The Premier Hospital Quality Incentive Demonstration is an incentive program that differentiates payment to hospitals based on performance on 30 quality measures. Participation is voluntary. The top 20% of hospitals receive incentive payments.11 The success of this demonstration led to its current second phase. The Acute Care Episode (ACE) Demonstration will provide bundled payments (including Part A and B services) for ACEs within Medicare FFS. The demonstration includes gainsharing by allowing sites to reward individual clinicians, teams of clinicians, or other hospital staff who show measurable clinical quality improvement. The focus is on select orthopedic and cardiovascular inpatient procedures (eg, hip/knee replacement surgery and coronary artery bypass graft surgery).12 The demonstration also has potential to increase volume to participant physician‐hospital organizations through financial incentives to beneficiaries via payments to offset their Medicare cost‐sharing obligations. If this demonstration is successful and the concept of bundled payment is expanded to other conditions and additional geographies, this could have significant impact on hospitalists. Specifically, programs for care coordination and reducing readmissions and complications after discharge would be directly incentivized. The demonstration focuses on Texas, Oklahoma, Colorado, and New Mexico. The Medicare Hospital Gainsharing Demonstration program to test and evaluate arrangements between hospitals and physicians is designed improve the quality and efficiency of care provided to beneficiaries. The demonstration allows hospitals to provide gainsharing payments to physicians that represent solely a share of the savings incurred as a result of collaborative efforts to improve overall quality and efficiency. The demonstration was launched in 2007.13 Finally, the upcoming Medicare Medical Home Demonstration has the potential for direct or indirect hospitalist participation as facilitators in Tier 2 medical homes that take into account care coordination across inpatient and outpatient settings.14 These demonstrations represent attempts by the federal government to align incentives with high‐quality, high‐value delivery of care.
Hospital‐Acquired Conditions
Hospital‐acquired conditions (HACs) have significant cost and quality implications for U.S. healthcare, such as the estimated 99,000 deaths associated with hospital‐acquired infections annually.15 Therefore, CMS received statutory authority to not pay additional charges for reasonably preventable HACs. Beginning October 1, 2008, CMS implemented a rule that the presence of selected reasonably preventable conditions as the only complicating condition would not cause a higher‐paying DRG to be assigned to the case; therefore, the case would be paid as though the secondary diagnosis were not present. CMS also required the reporting of a new data element to delineate HACs from conditions present on admission. Hospitals can indicate to CMS if a condition was present on admission as a secondary diagnosis, allowing reimbursement for care provided to treat any condition present on admission. The first 10 HACs to be implemented were: foreign object retained after surgery, air embolism, blood incompatibility, stage III and IV pressure ulcers, falls and trauma, manifestations of poor glycemic control, catheter‐associated urinary tract infections, vascular catheterassociated infection, surgical site infection following specific surgeries, and deep vein thrombosis and pulmonary embolus.16 Since some complications are not absolutely preventable and evidence‐based guidelines for the prevention of some complications are lacking, this has generated some resistance from the provider community.17 The HAC payment policy is a step toward aligning incentives with quality performance, but any further HACs will need to be evaluated for their level of preventability and potential for unintended consequences.
Incentives for Use of Effective HIT
The use of HIT can be incentivized in 3 main ways, all of which are likely to increase over time. First, incentives can be implemented to reward reporting quality metrics via electronic health records or registries. Second, incentives based on quality performance may indirectly encourage the adoption of HIT because an electronic medical record, especially with computerized provider order entry and decision support, may enable higher performance on quality metrics. Finally, CMS has provided direct incentives for information technology adoption, such as certified electronic health records.18 The American Recovery and Reinvestment Act of 2009 created over $17 billion of potential incentive payments for HIT use by physicians and hospitals.19 Hospitals are eligible for significant incentives reaching estimates over $6 million per year, phasing out by 2015, so Congress excluded hospital‐based physicians from direct payments. Ambulatory physicians are eligible for up to $18,000 per year, phasing out by 2015, with subsequent payment reductions for nonuse. Since hospitalists often function at the nexus of clinical care, quality improvement, and technology use, they have the opportunity to lead or facilitate effective implementation of information technology in their hospitals. These efforts may be rewarded by hospitals.
PQRI
PQRI was authorized in 2006 and included a 1.5% incentive payment for satisfactorily reporting quality data. The incentive payment will increase to 2% in 2009. There are 153 PQRI measures in 2009 and a significant number of the measures focus on hospital‐based care. Examples of measures hospitalists can help report include: deep vein thrombosis prophylaxis for ischemic stroke or intracranial hemorrhage, medication reconciliation at discharge, advance care plan documentation, oral antiplatelet therapy for patients discharged with stroke diagnosis, and anticoagulant therapy prescribed for atrial fibrillation in stroke patients at discharge. PQRI measures can be reported through claims‐based or registry‐based reporting. Reporting can be done on individual measures or for measure groups associated with specific conditions.20 Hospitalists have the potential to drive performance on these measures, lead reporting efforts, and share in the financial rewards.
Future Considerations
The political leadership at the federal and state level is beginning a new transition; however, the focus on quality and value for our health care dollar will likely continue to increase.5, 6 The U.S. health care system has untenable cost estimates, significant quality gaps, and a fractured payment system that fails to reward effective care coordination.2, 21, 22 This increased focus on quality and value should be viewed as an opportunity for hospitalists and hospitals. Hospitalist groups that can achieve high‐quality performance will be increasingly valued, and hospitals should further recognize the critical role hospitalists play in achieving high performance and the associated financial rewards. Hospitalists often lead quality improvement and safety programs in hospitals, and these programs are likely to be seen as progressively more important as payment is linked to performance. The Society of Hospital Medicine engages with policymakers and this role is increasingly significant as more policy and payment decisions impact hospitalists. The Society has focused on collaborative work with payers, policymakers, and other providers to find joint shared solutions. Hospitalists can serve as a link between providers and a focal point of care coordination, especially for the hospitalized patient. Finally, as our system and its incentives continue to progress toward alignment with value‐based high quality care, hospitalists should be leading the change and be an essential part of the solution to transform our health care system to provide high‐quality, efficient care to all Americans.
Acknowledgements
Dr. Tom Valuck is recognized for his thoughtful comments and edits in preparation, submission, and revision of this manuscript.
- Medicare Board of Trustees. A Summary of the 2008 Report. Available at: http://www.ssa.gov/OACT/TRSUM/trsummary.html. Accessed April 2009.
- , , , et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- Economy Likely to Move up Medicare's Insolvency. Available at: http://abcnews.go.com/Politics/Health/wireStory?id=6369658. Accessed April 2009.
- Value‐Driven Health Care. Available at: http://www.hhs.gov/valuedriven. Accessed April 2009.
- Obama‐Biden Full Health Care Plan. Available at: http://www.barackobama.com/issues/healthcare. Accessed April 2009.
- Senate Chairman Max Baucus Call to Action Health Reform 2009. Available at: http://finance.senate.gov/healthreform2009/finalwhitepaper.pdf. Accessed April 2009.
- , , , .National health spending in 2006: a year of change for prescription drugs.Health Aff (Millwood).2008;27(1):14–29.
- Medicare Hospital Value‐Based Purchasing Plan. October 31,2008. Available at: http://www.cms.hhs.gov/AcuteInpatientPPS/downloads/hospital_VBP_plan_issues_paper.pdf. Accessed April 2009.
- Medicare Improvements for Patients and Providers Act of 2008. Available at: http://www.asm.org/ASM/files/ccLibraryFiles/Filename/000000004120/HR6331.pdf. Accessed April 2009.
- HospitalCompare. Available at: www.hospitalcompare.hhs.gov. Accessed April 2009.
- Premier Hospital Quality Incentive Demonstration. Available at: http://www.cms.hhs.gov/HospitalQualityInits/35_hospitalpremier.asp. Accessed April 2009.
- Acute Care Episode Demonstration. Available at: http://www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none122(2):160–166.
- Hospital Acquired Conditions. Available at: http://www.cms.hhs.gov/HospitalAcqCond/06_Hospital‐Acquired_Conditions.asp. Accessed April 2009.
- , , .The wisdom and justice of not paying for “preventable complications”.JAMA.2008;299(18):2197–2199.
- E‐prescribing incentive program. Available at: http://www.cms.hhs.gov/ERXincentive. Accessed April 2009.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills299(19):2319–2321.
- Medicare Board of Trustees. A Summary of the 2008 Report. Available at: http://www.ssa.gov/OACT/TRSUM/trsummary.html. Accessed April 2009.
- , , , et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- Economy Likely to Move up Medicare's Insolvency. Available at: http://abcnews.go.com/Politics/Health/wireStory?id=6369658. Accessed April 2009.
- Value‐Driven Health Care. Available at: http://www.hhs.gov/valuedriven. Accessed April 2009.
- Obama‐Biden Full Health Care Plan. Available at: http://www.barackobama.com/issues/healthcare. Accessed April 2009.
- Senate Chairman Max Baucus Call to Action Health Reform 2009. Available at: http://finance.senate.gov/healthreform2009/finalwhitepaper.pdf. Accessed April 2009.
- , , , .National health spending in 2006: a year of change for prescription drugs.Health Aff (Millwood).2008;27(1):14–29.
- Medicare Hospital Value‐Based Purchasing Plan. October 31,2008. Available at: http://www.cms.hhs.gov/AcuteInpatientPPS/downloads/hospital_VBP_plan_issues_paper.pdf. Accessed April 2009.
- Medicare Improvements for Patients and Providers Act of 2008. Available at: http://www.asm.org/ASM/files/ccLibraryFiles/Filename/000000004120/HR6331.pdf. Accessed April 2009.
- HospitalCompare. Available at: www.hospitalcompare.hhs.gov. Accessed April 2009.
- Premier Hospital Quality Incentive Demonstration. Available at: http://www.cms.hhs.gov/HospitalQualityInits/35_hospitalpremier.asp. Accessed April 2009.
- Acute Care Episode Demonstration. Available at: http://www.cms.hhs.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?filterType=none122(2):160–166.
- Hospital Acquired Conditions. Available at: http://www.cms.hhs.gov/HospitalAcqCond/06_Hospital‐Acquired_Conditions.asp. Accessed April 2009.
- , , .The wisdom and justice of not paying for “preventable complications”.JAMA.2008;299(18):2197–2199.
- E‐prescribing incentive program. Available at: http://www.cms.hhs.gov/ERXincentive. Accessed April 2009.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills299(19):2319–2321.
Hospitalists in Internal Medicine Residency
By the year 2010, more than 20,000 hospitalists will be in practice, compared to 5000 rheumatologists and 8000 pulmonologists.13 The growth of this career option has been driven by an industry need to reduce healthcare costs, increase the emphasis on quality improvement of healthcare services, and improve the efficiency and delivery of care between that provided in the hospital and that provided by the primary care physician.49
While hospitalists' roots can be traced back to community hospitals in the late 1980s and early 1990s, this career option is now flourishing in academic centers, with the rise of hospitalist faculty and hospitalist faculty tracks.10, 11 One potential advantage of having faculty who are hospitalists is the availability and expertise of physicians who specialize in the care of hospitalized patients.8, 12 Additionally, hospitalist faculty have been reported to achieve high resident satisfaction scores, increase understanding of cost‐effective measures, and improve the supervision of hospital procedures.12 To our knowledge, the medical literature does not provide an estimate of the percentage of internal medicine residency programs utilizing hospitalist faculty.
Critics of hospitalist faculty point to the potential loss of teaching opportunities from shorter hospital stays, bemoan the decreased physician‐patient continuity between inpatient and outpatient arenas, and fear that the hospitalists' presence may decrease resident autonomy and decrease subspecialty consultations by fellows.5, 13, 14 Hospitalist faculty need development, education, and training to match the teaching activities they are expected to fulfill. The challenge is for hospitalist societies and national residency organizations to define, plan for, and meet their faculty development needs.
Goals
The goals of this study were to describe the current involvement of hospitalists in internal medicine residencies. More specifically, we wanted to determine: (1) the percentage of programs with hospitalists as faculty, (2) the teaching activities of hospitalists, (3) regional differences in academic hospitalist activity, and (4) the number of programs with hospitalist training tracks.
Materials and Methods
Questionnaire Development
The Survey Committee of the Association of Program Directors in Internal Medicine (APDIM) is charged with developing questionnaires to track the baseline characteristics of the 391 internal medicine residencies in the United States as well as to address current issues facing residencies and residency directors. The Survey Committee's goal is to create a longitudinal data warehouse to: (1) track changes over time, (2) create valid outcome measures, and (3) facilitate educational studies and interventions. Two of the authors (B.B. and F.M.) were members of this committee. This work contains results from 2 successive questionnaires.
The first questionnaire, completed in 2005, and its administration have been described in our previous reports.1517 The second, completed in 2007, repeated many of the baseline characteristic questions, and introduced new questions regarding current residency issues, in particular hospitalism in residencies. Whereas the 2005 questionnaire was sent as an e‐mail attachment to the residency programs, the 2007 questionnaire used a web‐based format for completion and data collection. We e‐mailed a notification of the questionnaire with a link to the website in November 2006 to each member program of APDIM (total = 381 programs in 2006), representing 97% of the training programs in internal medicine. The directions and glossary for the questionnaire provided definitions and explained that the first section about the baseline characteristics could be completed by a program administrator or an associate program director. In both surveys, we defined the term faculty as any physician who serves as an attending or preceptor, provides lectures, noon conferences, physical diagnosis rounds, etc., or attends educational conferences (eg, morning report) on a regular basis. The survey assumed that program directors identified hospitalists as physicians whose primary professional focus is the general medical care of hospitalized patients. We asked that the program director review and approve the first section, and complete the remaining questions on his or her own. We sent subsequent request e‐mails in December 2006 and January 2007. The survey was confidential with respondents tracked by numerical codes.
Data Analysis
We used SPSS for Windows 15.0.0 (SPSS, Inc., Chicago, IL) statistics program for all analyses. Each program was categorized by setting (university‐based, community‐based, military, Veterans Administration, multispecialty group), number of residents, and the state in which the program was located. Respondents were assigned a region based upon the categorization used by the U.S. Census Bureau.18 We combined response categories for variables when we found sparsely selected responses. We examined continuous variables for evidence of skewness, outliers, and nonnormality.
In order to avoid misinterpretation of the results of multiple comparisons, we are reporting only bivariate associations that are significant at the P < 0.01 level. We used Spearman's rho to find correlations with the number of hospitalists and continuous variables. Chi‐square analyses were used to compare nominal variables. Fisher's exact test was used to compare the increase in the prevalence of hospitalists at primary teaching hospitals over time. For all the analyses we used 2‐sided tests.
Results
A total of 272 (response rate 70%) programs completed the 2005 survey, and 236 (response rate 62%) completed the 2007 survey. A total of 171 programs completed both surveys. In 2007, 15 (6%) program directors reported that they were hospitalists while 118 (50%) claimed to be traditional general internists.
For the program directors who answered both surveys, 57% indicated that their primary teaching hospital employed hospitalists before the residency work‐hour limits were implemented (before June 2002). At the time of the survey in 2005, 77% said that hospitalists were employed, a 20% increase in 3 years. When we surveyed these same programs again in 2007, the proportion had risen to 81% (Fisher's exact P = 0.02 compared to before work‐hour limits; Figure 1).
Hospitalist Data from 2007
Eighty‐three percent of program directors identified using hospitalists as a part of their residency faculty. There was no significant difference between community‐based and university‐based residency programs (Table 1). There was an expected positive correlation (Spearman rho = 0.39, P < 0.001) between the total number of hospital beds and the number of hospitalists. The number and proportion of hospitalists had no correlation with residency program size, Residency Review Committee (RRC) cycle length (P = 0.99), or the American Board of Internal Medicine (ABIM) board exam pass rate (P = 0.60).
| Community‐Based (n = 130) | University‐Based (n = 63) | Northeast Region (n = 76) | Midwest Region (n = 53) | Southern Region (n = 59) | Western Region (n = 30) | |
|---|---|---|---|---|---|---|
| ||||||
| Does your primary teaching hospital employ hospitalists now?* | 98 (75) | 54 (86) | 61 (80) | 41 (77) | 48 (81) | 22 (73) |
| Are the hospitalists involved in teaching residents?* | 90 (91) | 47 (87) | 60 (98) | 35 (85) | 38 (79) | 22 (100) |
| If Yes, what teaching activities?* | ||||||
| Hospitalists serve as attending on resident service | 81 (92) | 43 (91) | 57 (95) | 32 (91) | 33 (87) | 20 (91) |
| Hospitalists conduct teaching rounds | 71 (79) | 39 (83) | 55 (92) | 30 (86) | 24 (63) | 18 (82) |
| Hospitalists perform direct observation of inpatient clinical skills | 60 (67) | 32 (68) | 45 (75) | 26 (74) | 20 (53) | 17 (77) |
| Hospitalists provide lectures | 56 (67) | 35 (74) | 43 (72) | 26 (74) | 19 (50) | 18 (82) |
| Hospitalists attend morning report | 43 (49) | 27 (59) | 35 (58) | 19 (54) | 19 (50) | 13 (57) |
| Hospitalists teach physical diagnosis | 41 (46) | 23 (49) | 28 (47) | 21 (60) | 14 (37) | 13 (59) |
| Hospitalists conduct interdisciplinary education rounds | 25 (28) | 18 (38) | 24 (40) | 10 (29) | 11 (29) | 7 (32) |
| Do you have a hospitalist track/focus? | 13 (11) | 6 (10) | 6 (8) | 5 (9) | 7 (12) | 5 (17) |
Teaching hospitals of the university‐based residencies employed hospitalists more often than those of community‐based programs (87% vs. 76%, P = 0.07; Table 1). And, while programs across the United States employed hospitalists at near the same proportions, programs in the Northeast (92%) and the West coast (92%) trended toward involving them in teaching residents more often (vs. Midwest 78% and South 76%, P = 0.04). Compared to programs in the Northeast, those in the Southern region generally utilize hospitalists less for common teaching activities, and in particular, significantly less (63%, P = 0.001) for conducting teaching rounds. Eleven percent of residencies had a hospitalist track or a hospitalist training focus, and this did not vary between community‐based and university‐based programs, but the Western region trended toward a higher percentage (17%, P = 0.18, compared to the Northeast 8%).
Of those programs that utilize hospitalists, program directors indicated that hospitalists contributed the following teaching activities to their residency programs (Table 1): serve as attending on resident service (92%), conduct teaching rounds (81%), perform direct observation of inpatient clinical skills (67%), provide lectures (68%), attend morning report (52%), teach physical diagnosis (48%), and conduct interdisciplinary education rounds (31%). Notable comments provided by program directors about other teaching activities of hospitalists included: accept and review night float patients, residents do inpatient consultations with hospitalists, serve as one of the associate program directors, and write curriculum updates and develop evaluation methods (ie, oral exams, multiple choice questions, etc.).
Discussion
This is the first study to document the national rise of hospitalist faculty in internal medicine residency programs. Program directors noted a 20% increase in teaching hospitals that employed hospitalists after the work‐hour regulations went into effecta trend that continued to rise. The tendency was seen first on the coasts, where managed care has higher penetration, and particularly in the Northeast, where New York's resident work‐hour reforms occurred by state mandate prior to the residency accreditation action that affected the rest of the country. Not only have hospitalists picked up the burden of service at these hospitals,19, 20 but the vast majority of programs (>80%) have utilized hospitalists as teachers in important areas of their residency. The magnitude of hospitalist involvement in residency training may have important implications.
Beyond the financial significance of hospitalists at academic teaching hospitals,21 only a few studies have addressed their impact on resident education. On the monthly evaluations at the University of California, San Francisco (San Francisco, CA), residents' satisfaction with their attendings was significantly higher when the physician was a hospitalist rather than a traditional faculty member.22 Residents believed hospitalists were more effective teachers, and provided more effective feedback. At Emory University (Atlanta, GA), a methodologically more rigorous study of postrotation assessment of faculty demonstrated that ratings of hospitalists were not different from traditional general internists; both scored higher than subspecialists.23 The hospitalists as a group had completed training more recently, which also was associated with higher scores.
Even community hospitals that sponsor residency programs have benefited from hospitalist faculty. At Norwalk Hospital (Norwalk, CT), the program had used resident teams led by a group of community physicians and a small group of employed internists. But time pressures and reimbursement concerns created tension between the workload and education balance. After hiring 2 hospitalist clinician‐educators, the length of stay and cost per case were substantially reduced, while resident evaluations indicated improved teaching rounds, conferences, and bedside teaching.24
The results of our study fit with the role of hospitalists as well as what individual programs have reported about hospitalist faculty in the past. Hospitalist faculty serve by and large (92%) as attendings on the hospital ward services. Theoretically, who better to have round with residents in the hospital than the specialists of hospital medicine. For a pulmonary curricular experience, residents work with pulmonologists. But beyond serving as attendings in the hospital, they perform the traditional functions of hospital attendings: providing teaching rounds (>80%), evaluating clinical skills (67%), and even lecturing to residents (>65%). The Southern region trends toward slower adoption of hospitalists as faculty, particularly compared with the Northeast. Overall, what is striking is how much hospitalist faculty already are filling the roles expected of all academic faculty.
We found that only 11% of programs have a hospitalist track through which internal medicine residents may develop the specialized skills and knowledge needed to function optimally in a hospitalist career.25 But given the rapid growth of this specialty, we might expect to see a similar rise in programs providing such specialized training.
Are there risks to having hospitalists teaching residents? One concern is the potential to model fragmented medical care to trainees when hospitals and ambulatory health systems neglect to ensure quality handoffs.26 In an era that heralds the demise of the primary care general internist,27 the impact of hospitalist faculty on general internal medicine nationally, the gravitation of residents toward or away from hospitalist and ambulatory careers, and the role of the traditional general internist in residency training programs in the future remain to be seen. These were not addressed by our data, but are ripe areas for study.
This study has several limitations. It relies on self‐reported data from program directors that, while knowing the intimate details of their educational program, may not have exact knowledge of the number of hospitalists employed by their hospitals. There is also the potential for recall bias by asking the group to remember the number of hospitalists before duty‐hour implementation. Both points in time of our 2 surveys (2005 and 2007) were after the incident growth of hospital medicine as evidenced by the high prevalence of hospitalists in both surveys. Yet, most program directors know that the service needs of the hospitals were acutely increased when the duty‐hours policies went into affect, and probably were fairly involved in hospital decisions to utilize hospitalist physicians to meet these needs. Finally, our study does not address hospitalism within the family medicine and pediatric specialties, both of which have a significant stake in hospital medicine.
In conclusion, our study documents the recent growth and current prevalence of hospitalists' activities in the teaching hospitals of internal medicine residencies in the United States, the duties they perform in resident education, and the magnitude of their penetration in the geographic regions of the country, both in community‐based and university‐based programs. The high degree of involvement of hospitalists in resident education may have important implications for the future of internal medicine as a discipline both with regard to the need for academic faculty development of this important sector of the education community as well as for the education and career development of the residents whom they train.
Acknowledgements
The authors thank the Mayo Clinic Survey Research Center for their assistance with survey design and data collection.
- , , , et al.Implementation of a voluntary hospitalist service at a community teaching hospital; improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- , , , et al.The United States rheumatology workforce: supply and demand, 2005–2025.Arthritis Rheum.2007;56(3):722–729.
- , , , , ;Committee on Manpower for Pulmonary and Critical Care Societies.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284(21):2762–2770.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- , , , , .Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- , , .The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- , , , , .What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medical service?J Gen Intern Med.2004;19:395–401.
- , , , et al.The presence of hospitalists in medical education.Acad Med.2000;75(suppl):S34–S36.
- , , , et al.Assessing the value of hospitalists to academic health centers: Brigham and Women's Hospital and Harvard Medical School.Am J Med.1999;106:134–137.
- , .Role of hospitalists in medical education.Am J Med.1999;107(4):305–309.
- , .The hospitalists: new boon for internal medicine or retreat from primary care?Ann Intern Med.1999;130:382–387.
- .The hospitalist movement: caution lights flashing at the crossroads.Am J Med.1999;107:409–413.
- , , .The state of competency evaluation in internal medicine residency.J Gen Intern Med.2008;23(7):1010–1015.
- , , .Sources of satisfaction for residency program directors: a second administration of the PD‐Sat.Am J Med.2009;122(2):196–201.
- , , .What predicts residency accreditation cycle length?Acad Med.2009;84(3):356–361.
- U.S. Census Bureau. Census Regions and Divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed May 2009.
- , , , et al.Complying with ACGME resident duty hours restrictions: restructuring the 80‐hour workweek to enhance education and patient safety at Texas A81(12):1026–1031.
- Association of Program Directors in Internal Medicine;, , , , .Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- , , , et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- , , , , .Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- , , , et al.Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- , , , et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–301.
- , , , , .How to use The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:48–56.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- American College of Physicians. The impending collapse of primary care medicine and its implications for the state of the nation's health care: a report from the American College of Physicians. January 30,2006. Available at: http://www.acponline.org/advocacy/events/state_of_healthcare/statehc06_1.pdf. Accessed May 2009.
By the year 2010, more than 20,000 hospitalists will be in practice, compared to 5000 rheumatologists and 8000 pulmonologists.13 The growth of this career option has been driven by an industry need to reduce healthcare costs, increase the emphasis on quality improvement of healthcare services, and improve the efficiency and delivery of care between that provided in the hospital and that provided by the primary care physician.49
While hospitalists' roots can be traced back to community hospitals in the late 1980s and early 1990s, this career option is now flourishing in academic centers, with the rise of hospitalist faculty and hospitalist faculty tracks.10, 11 One potential advantage of having faculty who are hospitalists is the availability and expertise of physicians who specialize in the care of hospitalized patients.8, 12 Additionally, hospitalist faculty have been reported to achieve high resident satisfaction scores, increase understanding of cost‐effective measures, and improve the supervision of hospital procedures.12 To our knowledge, the medical literature does not provide an estimate of the percentage of internal medicine residency programs utilizing hospitalist faculty.
Critics of hospitalist faculty point to the potential loss of teaching opportunities from shorter hospital stays, bemoan the decreased physician‐patient continuity between inpatient and outpatient arenas, and fear that the hospitalists' presence may decrease resident autonomy and decrease subspecialty consultations by fellows.5, 13, 14 Hospitalist faculty need development, education, and training to match the teaching activities they are expected to fulfill. The challenge is for hospitalist societies and national residency organizations to define, plan for, and meet their faculty development needs.
Goals
The goals of this study were to describe the current involvement of hospitalists in internal medicine residencies. More specifically, we wanted to determine: (1) the percentage of programs with hospitalists as faculty, (2) the teaching activities of hospitalists, (3) regional differences in academic hospitalist activity, and (4) the number of programs with hospitalist training tracks.
Materials and Methods
Questionnaire Development
The Survey Committee of the Association of Program Directors in Internal Medicine (APDIM) is charged with developing questionnaires to track the baseline characteristics of the 391 internal medicine residencies in the United States as well as to address current issues facing residencies and residency directors. The Survey Committee's goal is to create a longitudinal data warehouse to: (1) track changes over time, (2) create valid outcome measures, and (3) facilitate educational studies and interventions. Two of the authors (B.B. and F.M.) were members of this committee. This work contains results from 2 successive questionnaires.
The first questionnaire, completed in 2005, and its administration have been described in our previous reports.1517 The second, completed in 2007, repeated many of the baseline characteristic questions, and introduced new questions regarding current residency issues, in particular hospitalism in residencies. Whereas the 2005 questionnaire was sent as an e‐mail attachment to the residency programs, the 2007 questionnaire used a web‐based format for completion and data collection. We e‐mailed a notification of the questionnaire with a link to the website in November 2006 to each member program of APDIM (total = 381 programs in 2006), representing 97% of the training programs in internal medicine. The directions and glossary for the questionnaire provided definitions and explained that the first section about the baseline characteristics could be completed by a program administrator or an associate program director. In both surveys, we defined the term faculty as any physician who serves as an attending or preceptor, provides lectures, noon conferences, physical diagnosis rounds, etc., or attends educational conferences (eg, morning report) on a regular basis. The survey assumed that program directors identified hospitalists as physicians whose primary professional focus is the general medical care of hospitalized patients. We asked that the program director review and approve the first section, and complete the remaining questions on his or her own. We sent subsequent request e‐mails in December 2006 and January 2007. The survey was confidential with respondents tracked by numerical codes.
Data Analysis
We used SPSS for Windows 15.0.0 (SPSS, Inc., Chicago, IL) statistics program for all analyses. Each program was categorized by setting (university‐based, community‐based, military, Veterans Administration, multispecialty group), number of residents, and the state in which the program was located. Respondents were assigned a region based upon the categorization used by the U.S. Census Bureau.18 We combined response categories for variables when we found sparsely selected responses. We examined continuous variables for evidence of skewness, outliers, and nonnormality.
In order to avoid misinterpretation of the results of multiple comparisons, we are reporting only bivariate associations that are significant at the P < 0.01 level. We used Spearman's rho to find correlations with the number of hospitalists and continuous variables. Chi‐square analyses were used to compare nominal variables. Fisher's exact test was used to compare the increase in the prevalence of hospitalists at primary teaching hospitals over time. For all the analyses we used 2‐sided tests.
Results
A total of 272 (response rate 70%) programs completed the 2005 survey, and 236 (response rate 62%) completed the 2007 survey. A total of 171 programs completed both surveys. In 2007, 15 (6%) program directors reported that they were hospitalists while 118 (50%) claimed to be traditional general internists.
For the program directors who answered both surveys, 57% indicated that their primary teaching hospital employed hospitalists before the residency work‐hour limits were implemented (before June 2002). At the time of the survey in 2005, 77% said that hospitalists were employed, a 20% increase in 3 years. When we surveyed these same programs again in 2007, the proportion had risen to 81% (Fisher's exact P = 0.02 compared to before work‐hour limits; Figure 1).

Hospitalist Data from 2007
Eighty‐three percent of program directors identified using hospitalists as a part of their residency faculty. There was no significant difference between community‐based and university‐based residency programs (Table 1). There was an expected positive correlation (Spearman rho = 0.39, P < 0.001) between the total number of hospital beds and the number of hospitalists. The number and proportion of hospitalists had no correlation with residency program size, Residency Review Committee (RRC) cycle length (P = 0.99), or the American Board of Internal Medicine (ABIM) board exam pass rate (P = 0.60).
| Community‐Based (n = 130) | University‐Based (n = 63) | Northeast Region (n = 76) | Midwest Region (n = 53) | Southern Region (n = 59) | Western Region (n = 30) | |
|---|---|---|---|---|---|---|
| ||||||
| Does your primary teaching hospital employ hospitalists now?* | 98 (75) | 54 (86) | 61 (80) | 41 (77) | 48 (81) | 22 (73) |
| Are the hospitalists involved in teaching residents?* | 90 (91) | 47 (87) | 60 (98) | 35 (85) | 38 (79) | 22 (100) |
| If Yes, what teaching activities?* | ||||||
| Hospitalists serve as attending on resident service | 81 (92) | 43 (91) | 57 (95) | 32 (91) | 33 (87) | 20 (91) |
| Hospitalists conduct teaching rounds | 71 (79) | 39 (83) | 55 (92) | 30 (86) | 24 (63) | 18 (82) |
| Hospitalists perform direct observation of inpatient clinical skills | 60 (67) | 32 (68) | 45 (75) | 26 (74) | 20 (53) | 17 (77) |
| Hospitalists provide lectures | 56 (67) | 35 (74) | 43 (72) | 26 (74) | 19 (50) | 18 (82) |
| Hospitalists attend morning report | 43 (49) | 27 (59) | 35 (58) | 19 (54) | 19 (50) | 13 (57) |
| Hospitalists teach physical diagnosis | 41 (46) | 23 (49) | 28 (47) | 21 (60) | 14 (37) | 13 (59) |
| Hospitalists conduct interdisciplinary education rounds | 25 (28) | 18 (38) | 24 (40) | 10 (29) | 11 (29) | 7 (32) |
| Do you have a hospitalist track/focus? | 13 (11) | 6 (10) | 6 (8) | 5 (9) | 7 (12) | 5 (17) |
Teaching hospitals of the university‐based residencies employed hospitalists more often than those of community‐based programs (87% vs. 76%, P = 0.07; Table 1). And, while programs across the United States employed hospitalists at near the same proportions, programs in the Northeast (92%) and the West coast (92%) trended toward involving them in teaching residents more often (vs. Midwest 78% and South 76%, P = 0.04). Compared to programs in the Northeast, those in the Southern region generally utilize hospitalists less for common teaching activities, and in particular, significantly less (63%, P = 0.001) for conducting teaching rounds. Eleven percent of residencies had a hospitalist track or a hospitalist training focus, and this did not vary between community‐based and university‐based programs, but the Western region trended toward a higher percentage (17%, P = 0.18, compared to the Northeast 8%).
Of those programs that utilize hospitalists, program directors indicated that hospitalists contributed the following teaching activities to their residency programs (Table 1): serve as attending on resident service (92%), conduct teaching rounds (81%), perform direct observation of inpatient clinical skills (67%), provide lectures (68%), attend morning report (52%), teach physical diagnosis (48%), and conduct interdisciplinary education rounds (31%). Notable comments provided by program directors about other teaching activities of hospitalists included: accept and review night float patients, residents do inpatient consultations with hospitalists, serve as one of the associate program directors, and write curriculum updates and develop evaluation methods (ie, oral exams, multiple choice questions, etc.).
Discussion
This is the first study to document the national rise of hospitalist faculty in internal medicine residency programs. Program directors noted a 20% increase in teaching hospitals that employed hospitalists after the work‐hour regulations went into effecta trend that continued to rise. The tendency was seen first on the coasts, where managed care has higher penetration, and particularly in the Northeast, where New York's resident work‐hour reforms occurred by state mandate prior to the residency accreditation action that affected the rest of the country. Not only have hospitalists picked up the burden of service at these hospitals,19, 20 but the vast majority of programs (>80%) have utilized hospitalists as teachers in important areas of their residency. The magnitude of hospitalist involvement in residency training may have important implications.
Beyond the financial significance of hospitalists at academic teaching hospitals,21 only a few studies have addressed their impact on resident education. On the monthly evaluations at the University of California, San Francisco (San Francisco, CA), residents' satisfaction with their attendings was significantly higher when the physician was a hospitalist rather than a traditional faculty member.22 Residents believed hospitalists were more effective teachers, and provided more effective feedback. At Emory University (Atlanta, GA), a methodologically more rigorous study of postrotation assessment of faculty demonstrated that ratings of hospitalists were not different from traditional general internists; both scored higher than subspecialists.23 The hospitalists as a group had completed training more recently, which also was associated with higher scores.
Even community hospitals that sponsor residency programs have benefited from hospitalist faculty. At Norwalk Hospital (Norwalk, CT), the program had used resident teams led by a group of community physicians and a small group of employed internists. But time pressures and reimbursement concerns created tension between the workload and education balance. After hiring 2 hospitalist clinician‐educators, the length of stay and cost per case were substantially reduced, while resident evaluations indicated improved teaching rounds, conferences, and bedside teaching.24
The results of our study fit with the role of hospitalists as well as what individual programs have reported about hospitalist faculty in the past. Hospitalist faculty serve by and large (92%) as attendings on the hospital ward services. Theoretically, who better to have round with residents in the hospital than the specialists of hospital medicine. For a pulmonary curricular experience, residents work with pulmonologists. But beyond serving as attendings in the hospital, they perform the traditional functions of hospital attendings: providing teaching rounds (>80%), evaluating clinical skills (67%), and even lecturing to residents (>65%). The Southern region trends toward slower adoption of hospitalists as faculty, particularly compared with the Northeast. Overall, what is striking is how much hospitalist faculty already are filling the roles expected of all academic faculty.
We found that only 11% of programs have a hospitalist track through which internal medicine residents may develop the specialized skills and knowledge needed to function optimally in a hospitalist career.25 But given the rapid growth of this specialty, we might expect to see a similar rise in programs providing such specialized training.
Are there risks to having hospitalists teaching residents? One concern is the potential to model fragmented medical care to trainees when hospitals and ambulatory health systems neglect to ensure quality handoffs.26 In an era that heralds the demise of the primary care general internist,27 the impact of hospitalist faculty on general internal medicine nationally, the gravitation of residents toward or away from hospitalist and ambulatory careers, and the role of the traditional general internist in residency training programs in the future remain to be seen. These were not addressed by our data, but are ripe areas for study.
This study has several limitations. It relies on self‐reported data from program directors that, while knowing the intimate details of their educational program, may not have exact knowledge of the number of hospitalists employed by their hospitals. There is also the potential for recall bias by asking the group to remember the number of hospitalists before duty‐hour implementation. Both points in time of our 2 surveys (2005 and 2007) were after the incident growth of hospital medicine as evidenced by the high prevalence of hospitalists in both surveys. Yet, most program directors know that the service needs of the hospitals were acutely increased when the duty‐hours policies went into affect, and probably were fairly involved in hospital decisions to utilize hospitalist physicians to meet these needs. Finally, our study does not address hospitalism within the family medicine and pediatric specialties, both of which have a significant stake in hospital medicine.
In conclusion, our study documents the recent growth and current prevalence of hospitalists' activities in the teaching hospitals of internal medicine residencies in the United States, the duties they perform in resident education, and the magnitude of their penetration in the geographic regions of the country, both in community‐based and university‐based programs. The high degree of involvement of hospitalists in resident education may have important implications for the future of internal medicine as a discipline both with regard to the need for academic faculty development of this important sector of the education community as well as for the education and career development of the residents whom they train.
Acknowledgements
The authors thank the Mayo Clinic Survey Research Center for their assistance with survey design and data collection.
By the year 2010, more than 20,000 hospitalists will be in practice, compared to 5000 rheumatologists and 8000 pulmonologists.13 The growth of this career option has been driven by an industry need to reduce healthcare costs, increase the emphasis on quality improvement of healthcare services, and improve the efficiency and delivery of care between that provided in the hospital and that provided by the primary care physician.49
While hospitalists' roots can be traced back to community hospitals in the late 1980s and early 1990s, this career option is now flourishing in academic centers, with the rise of hospitalist faculty and hospitalist faculty tracks.10, 11 One potential advantage of having faculty who are hospitalists is the availability and expertise of physicians who specialize in the care of hospitalized patients.8, 12 Additionally, hospitalist faculty have been reported to achieve high resident satisfaction scores, increase understanding of cost‐effective measures, and improve the supervision of hospital procedures.12 To our knowledge, the medical literature does not provide an estimate of the percentage of internal medicine residency programs utilizing hospitalist faculty.
Critics of hospitalist faculty point to the potential loss of teaching opportunities from shorter hospital stays, bemoan the decreased physician‐patient continuity between inpatient and outpatient arenas, and fear that the hospitalists' presence may decrease resident autonomy and decrease subspecialty consultations by fellows.5, 13, 14 Hospitalist faculty need development, education, and training to match the teaching activities they are expected to fulfill. The challenge is for hospitalist societies and national residency organizations to define, plan for, and meet their faculty development needs.
Goals
The goals of this study were to describe the current involvement of hospitalists in internal medicine residencies. More specifically, we wanted to determine: (1) the percentage of programs with hospitalists as faculty, (2) the teaching activities of hospitalists, (3) regional differences in academic hospitalist activity, and (4) the number of programs with hospitalist training tracks.
Materials and Methods
Questionnaire Development
The Survey Committee of the Association of Program Directors in Internal Medicine (APDIM) is charged with developing questionnaires to track the baseline characteristics of the 391 internal medicine residencies in the United States as well as to address current issues facing residencies and residency directors. The Survey Committee's goal is to create a longitudinal data warehouse to: (1) track changes over time, (2) create valid outcome measures, and (3) facilitate educational studies and interventions. Two of the authors (B.B. and F.M.) were members of this committee. This work contains results from 2 successive questionnaires.
The first questionnaire, completed in 2005, and its administration have been described in our previous reports.1517 The second, completed in 2007, repeated many of the baseline characteristic questions, and introduced new questions regarding current residency issues, in particular hospitalism in residencies. Whereas the 2005 questionnaire was sent as an e‐mail attachment to the residency programs, the 2007 questionnaire used a web‐based format for completion and data collection. We e‐mailed a notification of the questionnaire with a link to the website in November 2006 to each member program of APDIM (total = 381 programs in 2006), representing 97% of the training programs in internal medicine. The directions and glossary for the questionnaire provided definitions and explained that the first section about the baseline characteristics could be completed by a program administrator or an associate program director. In both surveys, we defined the term faculty as any physician who serves as an attending or preceptor, provides lectures, noon conferences, physical diagnosis rounds, etc., or attends educational conferences (eg, morning report) on a regular basis. The survey assumed that program directors identified hospitalists as physicians whose primary professional focus is the general medical care of hospitalized patients. We asked that the program director review and approve the first section, and complete the remaining questions on his or her own. We sent subsequent request e‐mails in December 2006 and January 2007. The survey was confidential with respondents tracked by numerical codes.
Data Analysis
We used SPSS for Windows 15.0.0 (SPSS, Inc., Chicago, IL) statistics program for all analyses. Each program was categorized by setting (university‐based, community‐based, military, Veterans Administration, multispecialty group), number of residents, and the state in which the program was located. Respondents were assigned a region based upon the categorization used by the U.S. Census Bureau.18 We combined response categories for variables when we found sparsely selected responses. We examined continuous variables for evidence of skewness, outliers, and nonnormality.
In order to avoid misinterpretation of the results of multiple comparisons, we are reporting only bivariate associations that are significant at the P < 0.01 level. We used Spearman's rho to find correlations with the number of hospitalists and continuous variables. Chi‐square analyses were used to compare nominal variables. Fisher's exact test was used to compare the increase in the prevalence of hospitalists at primary teaching hospitals over time. For all the analyses we used 2‐sided tests.
Results
A total of 272 (response rate 70%) programs completed the 2005 survey, and 236 (response rate 62%) completed the 2007 survey. A total of 171 programs completed both surveys. In 2007, 15 (6%) program directors reported that they were hospitalists while 118 (50%) claimed to be traditional general internists.
For the program directors who answered both surveys, 57% indicated that their primary teaching hospital employed hospitalists before the residency work‐hour limits were implemented (before June 2002). At the time of the survey in 2005, 77% said that hospitalists were employed, a 20% increase in 3 years. When we surveyed these same programs again in 2007, the proportion had risen to 81% (Fisher's exact P = 0.02 compared to before work‐hour limits; Figure 1).

Hospitalist Data from 2007
Eighty‐three percent of program directors identified using hospitalists as a part of their residency faculty. There was no significant difference between community‐based and university‐based residency programs (Table 1). There was an expected positive correlation (Spearman rho = 0.39, P < 0.001) between the total number of hospital beds and the number of hospitalists. The number and proportion of hospitalists had no correlation with residency program size, Residency Review Committee (RRC) cycle length (P = 0.99), or the American Board of Internal Medicine (ABIM) board exam pass rate (P = 0.60).
| Community‐Based (n = 130) | University‐Based (n = 63) | Northeast Region (n = 76) | Midwest Region (n = 53) | Southern Region (n = 59) | Western Region (n = 30) | |
|---|---|---|---|---|---|---|
| ||||||
| Does your primary teaching hospital employ hospitalists now?* | 98 (75) | 54 (86) | 61 (80) | 41 (77) | 48 (81) | 22 (73) |
| Are the hospitalists involved in teaching residents?* | 90 (91) | 47 (87) | 60 (98) | 35 (85) | 38 (79) | 22 (100) |
| If Yes, what teaching activities?* | ||||||
| Hospitalists serve as attending on resident service | 81 (92) | 43 (91) | 57 (95) | 32 (91) | 33 (87) | 20 (91) |
| Hospitalists conduct teaching rounds | 71 (79) | 39 (83) | 55 (92) | 30 (86) | 24 (63) | 18 (82) |
| Hospitalists perform direct observation of inpatient clinical skills | 60 (67) | 32 (68) | 45 (75) | 26 (74) | 20 (53) | 17 (77) |
| Hospitalists provide lectures | 56 (67) | 35 (74) | 43 (72) | 26 (74) | 19 (50) | 18 (82) |
| Hospitalists attend morning report | 43 (49) | 27 (59) | 35 (58) | 19 (54) | 19 (50) | 13 (57) |
| Hospitalists teach physical diagnosis | 41 (46) | 23 (49) | 28 (47) | 21 (60) | 14 (37) | 13 (59) |
| Hospitalists conduct interdisciplinary education rounds | 25 (28) | 18 (38) | 24 (40) | 10 (29) | 11 (29) | 7 (32) |
| Do you have a hospitalist track/focus? | 13 (11) | 6 (10) | 6 (8) | 5 (9) | 7 (12) | 5 (17) |
Teaching hospitals of the university‐based residencies employed hospitalists more often than those of community‐based programs (87% vs. 76%, P = 0.07; Table 1). And, while programs across the United States employed hospitalists at near the same proportions, programs in the Northeast (92%) and the West coast (92%) trended toward involving them in teaching residents more often (vs. Midwest 78% and South 76%, P = 0.04). Compared to programs in the Northeast, those in the Southern region generally utilize hospitalists less for common teaching activities, and in particular, significantly less (63%, P = 0.001) for conducting teaching rounds. Eleven percent of residencies had a hospitalist track or a hospitalist training focus, and this did not vary between community‐based and university‐based programs, but the Western region trended toward a higher percentage (17%, P = 0.18, compared to the Northeast 8%).
Of those programs that utilize hospitalists, program directors indicated that hospitalists contributed the following teaching activities to their residency programs (Table 1): serve as attending on resident service (92%), conduct teaching rounds (81%), perform direct observation of inpatient clinical skills (67%), provide lectures (68%), attend morning report (52%), teach physical diagnosis (48%), and conduct interdisciplinary education rounds (31%). Notable comments provided by program directors about other teaching activities of hospitalists included: accept and review night float patients, residents do inpatient consultations with hospitalists, serve as one of the associate program directors, and write curriculum updates and develop evaluation methods (ie, oral exams, multiple choice questions, etc.).
Discussion
This is the first study to document the national rise of hospitalist faculty in internal medicine residency programs. Program directors noted a 20% increase in teaching hospitals that employed hospitalists after the work‐hour regulations went into effecta trend that continued to rise. The tendency was seen first on the coasts, where managed care has higher penetration, and particularly in the Northeast, where New York's resident work‐hour reforms occurred by state mandate prior to the residency accreditation action that affected the rest of the country. Not only have hospitalists picked up the burden of service at these hospitals,19, 20 but the vast majority of programs (>80%) have utilized hospitalists as teachers in important areas of their residency. The magnitude of hospitalist involvement in residency training may have important implications.
Beyond the financial significance of hospitalists at academic teaching hospitals,21 only a few studies have addressed their impact on resident education. On the monthly evaluations at the University of California, San Francisco (San Francisco, CA), residents' satisfaction with their attendings was significantly higher when the physician was a hospitalist rather than a traditional faculty member.22 Residents believed hospitalists were more effective teachers, and provided more effective feedback. At Emory University (Atlanta, GA), a methodologically more rigorous study of postrotation assessment of faculty demonstrated that ratings of hospitalists were not different from traditional general internists; both scored higher than subspecialists.23 The hospitalists as a group had completed training more recently, which also was associated with higher scores.
Even community hospitals that sponsor residency programs have benefited from hospitalist faculty. At Norwalk Hospital (Norwalk, CT), the program had used resident teams led by a group of community physicians and a small group of employed internists. But time pressures and reimbursement concerns created tension between the workload and education balance. After hiring 2 hospitalist clinician‐educators, the length of stay and cost per case were substantially reduced, while resident evaluations indicated improved teaching rounds, conferences, and bedside teaching.24
The results of our study fit with the role of hospitalists as well as what individual programs have reported about hospitalist faculty in the past. Hospitalist faculty serve by and large (92%) as attendings on the hospital ward services. Theoretically, who better to have round with residents in the hospital than the specialists of hospital medicine. For a pulmonary curricular experience, residents work with pulmonologists. But beyond serving as attendings in the hospital, they perform the traditional functions of hospital attendings: providing teaching rounds (>80%), evaluating clinical skills (67%), and even lecturing to residents (>65%). The Southern region trends toward slower adoption of hospitalists as faculty, particularly compared with the Northeast. Overall, what is striking is how much hospitalist faculty already are filling the roles expected of all academic faculty.
We found that only 11% of programs have a hospitalist track through which internal medicine residents may develop the specialized skills and knowledge needed to function optimally in a hospitalist career.25 But given the rapid growth of this specialty, we might expect to see a similar rise in programs providing such specialized training.
Are there risks to having hospitalists teaching residents? One concern is the potential to model fragmented medical care to trainees when hospitals and ambulatory health systems neglect to ensure quality handoffs.26 In an era that heralds the demise of the primary care general internist,27 the impact of hospitalist faculty on general internal medicine nationally, the gravitation of residents toward or away from hospitalist and ambulatory careers, and the role of the traditional general internist in residency training programs in the future remain to be seen. These were not addressed by our data, but are ripe areas for study.
This study has several limitations. It relies on self‐reported data from program directors that, while knowing the intimate details of their educational program, may not have exact knowledge of the number of hospitalists employed by their hospitals. There is also the potential for recall bias by asking the group to remember the number of hospitalists before duty‐hour implementation. Both points in time of our 2 surveys (2005 and 2007) were after the incident growth of hospital medicine as evidenced by the high prevalence of hospitalists in both surveys. Yet, most program directors know that the service needs of the hospitals were acutely increased when the duty‐hours policies went into affect, and probably were fairly involved in hospital decisions to utilize hospitalist physicians to meet these needs. Finally, our study does not address hospitalism within the family medicine and pediatric specialties, both of which have a significant stake in hospital medicine.
In conclusion, our study documents the recent growth and current prevalence of hospitalists' activities in the teaching hospitals of internal medicine residencies in the United States, the duties they perform in resident education, and the magnitude of their penetration in the geographic regions of the country, both in community‐based and university‐based programs. The high degree of involvement of hospitalists in resident education may have important implications for the future of internal medicine as a discipline both with regard to the need for academic faculty development of this important sector of the education community as well as for the education and career development of the residents whom they train.
Acknowledgements
The authors thank the Mayo Clinic Survey Research Center for their assistance with survey design and data collection.
- , , , et al.Implementation of a voluntary hospitalist service at a community teaching hospital; improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- , , , et al.The United States rheumatology workforce: supply and demand, 2005–2025.Arthritis Rheum.2007;56(3):722–729.
- , , , , ;Committee on Manpower for Pulmonary and Critical Care Societies.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284(21):2762–2770.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- , , , , .Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- , , .The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- , , , , .What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medical service?J Gen Intern Med.2004;19:395–401.
- , , , et al.The presence of hospitalists in medical education.Acad Med.2000;75(suppl):S34–S36.
- , , , et al.Assessing the value of hospitalists to academic health centers: Brigham and Women's Hospital and Harvard Medical School.Am J Med.1999;106:134–137.
- , .Role of hospitalists in medical education.Am J Med.1999;107(4):305–309.
- , .The hospitalists: new boon for internal medicine or retreat from primary care?Ann Intern Med.1999;130:382–387.
- .The hospitalist movement: caution lights flashing at the crossroads.Am J Med.1999;107:409–413.
- , , .The state of competency evaluation in internal medicine residency.J Gen Intern Med.2008;23(7):1010–1015.
- , , .Sources of satisfaction for residency program directors: a second administration of the PD‐Sat.Am J Med.2009;122(2):196–201.
- , , .What predicts residency accreditation cycle length?Acad Med.2009;84(3):356–361.
- U.S. Census Bureau. Census Regions and Divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed May 2009.
- , , , et al.Complying with ACGME resident duty hours restrictions: restructuring the 80‐hour workweek to enhance education and patient safety at Texas A81(12):1026–1031.
- Association of Program Directors in Internal Medicine;, , , , .Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- , , , et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- , , , , .Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- , , , et al.Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- , , , et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–301.
- , , , , .How to use The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:48–56.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- American College of Physicians. The impending collapse of primary care medicine and its implications for the state of the nation's health care: a report from the American College of Physicians. January 30,2006. Available at: http://www.acponline.org/advocacy/events/state_of_healthcare/statehc06_1.pdf. Accessed May 2009.
- , , , et al.Implementation of a voluntary hospitalist service at a community teaching hospital; improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- , , , et al.The United States rheumatology workforce: supply and demand, 2005–2025.Arthritis Rheum.2007;56(3):722–729.
- , , , , ;Committee on Manpower for Pulmonary and Critical Care Societies.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284(21):2762–2770.
- , .The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- , , , , .Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- , , .The effect of full‐time faculty hospitalists on the efficiency of care at a community teaching hospital.Ann Intern Med.1998;129:197–203.
- .An introduction to the hospitalist model.Ann Intern Med.1999;130:338–342.
- , .The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- , , , , .What effect does inpatient physician specialty and experience have on clinical outcomes and resource utilization on a general medical service?J Gen Intern Med.2004;19:395–401.
- , , , et al.The presence of hospitalists in medical education.Acad Med.2000;75(suppl):S34–S36.
- , , , et al.Assessing the value of hospitalists to academic health centers: Brigham and Women's Hospital and Harvard Medical School.Am J Med.1999;106:134–137.
- , .Role of hospitalists in medical education.Am J Med.1999;107(4):305–309.
- , .The hospitalists: new boon for internal medicine or retreat from primary care?Ann Intern Med.1999;130:382–387.
- .The hospitalist movement: caution lights flashing at the crossroads.Am J Med.1999;107:409–413.
- , , .The state of competency evaluation in internal medicine residency.J Gen Intern Med.2008;23(7):1010–1015.
- , , .Sources of satisfaction for residency program directors: a second administration of the PD‐Sat.Am J Med.2009;122(2):196–201.
- , , .What predicts residency accreditation cycle length?Acad Med.2009;84(3):356–361.
- U.S. Census Bureau. Census Regions and Divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed May 2009.
- , , , et al.Complying with ACGME resident duty hours restrictions: restructuring the 80‐hour workweek to enhance education and patient safety at Texas A81(12):1026–1031.
- Association of Program Directors in Internal Medicine;, , , , .Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144(12):920–926.
- , , , et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- , , , , .Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- , , , et al.Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- , , , et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19(4):293–301.
- , , , , .How to use The core competencies in hospital medicine: a framework for curriculum development.J Hosp Med.2006;1:48–56.
- .The hospitalist model: perspectives of the patient, the internist, and internal medicine.Ann Intern Med.1999;130:368–372.
- American College of Physicians. The impending collapse of primary care medicine and its implications for the state of the nation's health care: a report from the American College of Physicians. January 30,2006. Available at: http://www.acponline.org/advocacy/events/state_of_healthcare/statehc06_1.pdf. Accessed May 2009.
Copyright © 2009 Society of Hospital Medicine
Hospitalists Best Educators in the Hospital
Hospitalists are increasingly assuming a primary role in medical education in the hospital setting, as they also steadily care for a larger portion of hospitalized patients.1 This issue of the Journal of Hospital Medicine highlights the role of hospitalists as teachers in academic medical centers, confirming their expanding and positive role in resident and medical student education. A survey of academic medical centers, a systematic review, an evaluation of the implementation of an educational curriculum, and a survey of residents hint at the challenges hospitalists face in teaching, but also expose us to a more advanced yet facile approach to evaluating the effectiveness of a teaching intervention.25 These publications provoke interesting questions about clinical teaching that hospital medicine educators and researchers should pursue answering. I believe they will also encourage us to innovate in medical education and assessment of that teaching.
Traditionally, teaching attendings for resident teams on medicine or pediatric services rotated through these duties for 1 to 3 months each year, while spending the majority of their time in clinic or research activities. The increasing complexity of hospitalized patients and the pressure to reduce length of stay prompted closer oversight of trainees. With the advent of resident work‐hour restrictions, the need for greater clinical involvement by attending physicians made it increasingly difficult to maintain the traditional model of limited engagement by faculty attendings. Simply put, the dwindling pool of willing and able teaching attendings encouraged teaching hospitals to employ hospitalists to fill the gap in teaching and supervision, as well as clinical coverage.6
Beasley et al.2 report that resident work‐hour restrictions were associated with an increase in the number of teaching hospitals employing hospitalists to 79% of 193 surveyed hospitals in 2007. Of those hospitals with hospitalists, 92% reported that hospitalists serve as attendings on the teaching service. Hospitalists also teach in a number of other venues within these programs, including formal teaching rounds without direct care responsibility, along with delivering didactic lectures and clinical skills education.
How well are teaching hospitalists performing compared to traditional teaching attendings? Natarajan et al.4 provide an important summary of the evidence in a systematic review of studies comparing teaching efforts of hospitalist attendings to those of nonhospitalist attendings. Eight studies from a variety of institutions measured trainee (resident or medical student) attitudes. It is gratifying to learn that hospitalists were generally rated higher at overall teaching effectiveness, provision of feedback, knowledge base, and involvement of the learner in patient care. It seems likely that publication bias would overestimate the positive effect of hospitalists on learner attitudes. However, there are plausible reasons that the positive effect is accurate. Because their professional responsibilities are focused in the hospital, hospitalists should naturally be more available to learners for teaching and feedback. Hospitalists tend to be younger in their academic careers, placing them closer to the cutting edge of knowledge gained during residency and possibly fellowship. They may be more in tune with the needs and pressures faced by their learners, having dealt with these same challenges either during recent training or during nonteaching rotations.
As a relatively young specialty with young and developing academic hospitalists, will the advantage suggested by the Natarajan et al.4 systematic review be sustained over the long term as careers in hospital medicine mature? A 2005 systematic review studying this question among practicing clinicians found, somewhat paradoxically, that older, more experienced clinicians appeared to be at risk for providing lower‐quality care.7 To avoid this decline in clinical effectiveness, hospitalists should proactively seek innovative ways to refresh and update their knowledge and skills throughout their careers. This is particularly critical for teaching physicians. We should seize the opportunity to study the relationship between advancing clinical/teaching experience and educational quality within our teaching programs.
The review by Natarajan et al.4 should also challenge the hospitalist community to achieve even higher levels of proficiency as teachers of medicine. The review alludes to bedside teaching and attention to psychosocial aspects of care as opportunities for improvement by hospitalist teachers. A recent study suggested that physical examination instruction receives declining attention from inpatient teachers and that there are opportunities to increase the amount of bedside teaching.8 A provocative study of inpatients admitted to a teaching service found that physical examination could substantially impact patient care, but that trainees often failed to appreciate significant findings on initial examination.9 How do teaching hospitalists become proficient at physical examination and bedside teaching? Are there models around the country that are successfully developing outstanding clinician educators, incorporating teach‐the‐teacher models to improve physical examination and bedside teaching?
A practical limitation of attitude surveys and learner evaluation is the well‐known phenomenon of grade inflation that resulted in high ratings for all attending groups in the studies summarized by Natarajan et al.4 This limits the ability of surveys or evaluations to distinguish truly outstanding teachers and consequently makes it difficult to analyze the attributes of these teachers. We need better tools to detect and learn teaching techniques from great teachers in the clinical environment. We need studies evaluating the effect of teaching hospitalists on learner knowledge or, even more importantly, learner outcomes. Ultimately, we need studies of educational interventions that evaluate the impact of these interventions on patient outcome.
Wright et al.5 provide guidance as they describe the evaluation of a teaching intervention that moves beyond measurement of knowledge or attitudes. The Johns Hopkins Bayview hospitalist group sought to improve the quality of medical consultations performed by hospitalists and by residents rotating on the consultation service using a case‐based teaching module with audits of recent notes. The participants then audited their most recent consultation notes with feedback from the module teacher. The study employed pretests and posttests of knowledgea standard evaluation for educational interventions. This tells us little about the true impact of the teaching module. However, the study then assessed the quality of written consultations done by hospitalists before and after the educational interventions. Scores of consult notes improved significantly after the intervention, although the number of assessments for each physician was limited. Importantly, we need to know if interventions such as this are sustained over time. Wright's well‐established medical education research group's study design assessed the impact of an intervention on physician performance and moves us closer to assessment of the impact on actual patient outcomes. As clinical teachers, we would like to believe that our teaching and our educational innovations are having a positive impact on patient care. Can we demonstrate this?
As academic medical centers contend with further resident work‐hour restrictions proposed by the Institute of Medicine (IOM),10 how will this affect hospitalist teachers? The study by Mazotti et al.3 from the University of California at San Francisco residency program found that about one‐quarter of residents reported spending less time teaching after implementation of the Accreditation Council for Graduate Medical Education (ACGME) duty‐hour restrictions in 2003. Interestingly, those residents reporting less time spent teaching also reported less emotional exhaustion and perceived that they were delivering higher‐quality patient care. This raises a fascinating question for academic hospitalists. Would these findings be similar among teaching hospitalists and nonteaching hospitalists? What about hospitalists who rotate through months of teaching and nonteaching services? Is teaching emotionally exhausting for experienced teachers? A Mayo Clinic study suggested that the extent that faculty physicians are able to engage in work that is most meaningful to them as individuals is a strong determinant of faculty burnout.11 Is the hospitalist who finds teaching most rewarding at risk of burnout if they are assigned only 2 weeks a year as a teaching attending? The answers to these questions will be critical to hospitalist program leaders trying to assure sustainable careers for hospitalists in their programs.
Although the study by Mazotti et al.3 did not assess the impact of the reduction in resident teaching time on the teaching responsibilities for academic hospitalists, previous studies suggest that faculty are also teaching less since the introduction of work‐hour restrictions.12, 13 If the new IOM recommendations are enacted, who will teach? Although the reported experience following the 2003 work‐hour restrictions begs pessimism, the anticipated changes represent an opportunity for creative hospitalist teachers to demonstrate effective adaptations to the changing and compressed inpatient teaching environment.
In summary, this issue of the Journal presents studies that praise the role hospitalists play in teaching the next generation of physicians, but also gives a glimpse of future challenges and opportunities. We should take advantage of hospitalists' central position in clinical education in the hospital to innovate, study the effect on both learner outcomes and patient outcomes, and share our experiences with the hospitalist and medical education communities.
- , , , .Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- , , .Hospitalists involvement in internal medicine residencies.J Hosp Med.2009;4(8):471–475.
- , , , , .Impact of duty hour restriction on resident inpatient teaching.J Hosp Med.2009;4(8):476–480.
- , , , .Effect of hospitalist attending physicians on trainee educational experiences: a systematic review.J Hosp Med.2009;4(8):490–498.
- , , , , , .A case‐based teaching module combined with audit and feedback to improve the quality of consultations.J Hosp Med.2009;4(8):486–489.
- , , , , .Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- , , .Systematic review: the relationship between clinical experience and quality of health care.Ann Intern Med.2005;142(4):260–273.
- , , .Quantification of bedside teaching by an academic hospitalist group.J Hosp Med.2009;4(5):304–307.
- .Physical examination in the care of medical inpatients: an observational study.Lancet.2003;362(9390):1100–1105.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. 2008. Available at: http://www.iom.edu/CMS/3809/48553/60449.aspx. Accessed September2009.
- , , , et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
- , .Teaching internal medicine residents in the new era. Inpatient attending with duty‐hour regulations.J Gen Intern Med.2006;21(5):447–452.
- , , , .Effects of resident work hour limitations on faculty professional lives.J Gen Intern Med.2008;23(7):1077–1083.
Hospitalists are increasingly assuming a primary role in medical education in the hospital setting, as they also steadily care for a larger portion of hospitalized patients.1 This issue of the Journal of Hospital Medicine highlights the role of hospitalists as teachers in academic medical centers, confirming their expanding and positive role in resident and medical student education. A survey of academic medical centers, a systematic review, an evaluation of the implementation of an educational curriculum, and a survey of residents hint at the challenges hospitalists face in teaching, but also expose us to a more advanced yet facile approach to evaluating the effectiveness of a teaching intervention.25 These publications provoke interesting questions about clinical teaching that hospital medicine educators and researchers should pursue answering. I believe they will also encourage us to innovate in medical education and assessment of that teaching.
Traditionally, teaching attendings for resident teams on medicine or pediatric services rotated through these duties for 1 to 3 months each year, while spending the majority of their time in clinic or research activities. The increasing complexity of hospitalized patients and the pressure to reduce length of stay prompted closer oversight of trainees. With the advent of resident work‐hour restrictions, the need for greater clinical involvement by attending physicians made it increasingly difficult to maintain the traditional model of limited engagement by faculty attendings. Simply put, the dwindling pool of willing and able teaching attendings encouraged teaching hospitals to employ hospitalists to fill the gap in teaching and supervision, as well as clinical coverage.6
Beasley et al.2 report that resident work‐hour restrictions were associated with an increase in the number of teaching hospitals employing hospitalists to 79% of 193 surveyed hospitals in 2007. Of those hospitals with hospitalists, 92% reported that hospitalists serve as attendings on the teaching service. Hospitalists also teach in a number of other venues within these programs, including formal teaching rounds without direct care responsibility, along with delivering didactic lectures and clinical skills education.
How well are teaching hospitalists performing compared to traditional teaching attendings? Natarajan et al.4 provide an important summary of the evidence in a systematic review of studies comparing teaching efforts of hospitalist attendings to those of nonhospitalist attendings. Eight studies from a variety of institutions measured trainee (resident or medical student) attitudes. It is gratifying to learn that hospitalists were generally rated higher at overall teaching effectiveness, provision of feedback, knowledge base, and involvement of the learner in patient care. It seems likely that publication bias would overestimate the positive effect of hospitalists on learner attitudes. However, there are plausible reasons that the positive effect is accurate. Because their professional responsibilities are focused in the hospital, hospitalists should naturally be more available to learners for teaching and feedback. Hospitalists tend to be younger in their academic careers, placing them closer to the cutting edge of knowledge gained during residency and possibly fellowship. They may be more in tune with the needs and pressures faced by their learners, having dealt with these same challenges either during recent training or during nonteaching rotations.
As a relatively young specialty with young and developing academic hospitalists, will the advantage suggested by the Natarajan et al.4 systematic review be sustained over the long term as careers in hospital medicine mature? A 2005 systematic review studying this question among practicing clinicians found, somewhat paradoxically, that older, more experienced clinicians appeared to be at risk for providing lower‐quality care.7 To avoid this decline in clinical effectiveness, hospitalists should proactively seek innovative ways to refresh and update their knowledge and skills throughout their careers. This is particularly critical for teaching physicians. We should seize the opportunity to study the relationship between advancing clinical/teaching experience and educational quality within our teaching programs.
The review by Natarajan et al.4 should also challenge the hospitalist community to achieve even higher levels of proficiency as teachers of medicine. The review alludes to bedside teaching and attention to psychosocial aspects of care as opportunities for improvement by hospitalist teachers. A recent study suggested that physical examination instruction receives declining attention from inpatient teachers and that there are opportunities to increase the amount of bedside teaching.8 A provocative study of inpatients admitted to a teaching service found that physical examination could substantially impact patient care, but that trainees often failed to appreciate significant findings on initial examination.9 How do teaching hospitalists become proficient at physical examination and bedside teaching? Are there models around the country that are successfully developing outstanding clinician educators, incorporating teach‐the‐teacher models to improve physical examination and bedside teaching?
A practical limitation of attitude surveys and learner evaluation is the well‐known phenomenon of grade inflation that resulted in high ratings for all attending groups in the studies summarized by Natarajan et al.4 This limits the ability of surveys or evaluations to distinguish truly outstanding teachers and consequently makes it difficult to analyze the attributes of these teachers. We need better tools to detect and learn teaching techniques from great teachers in the clinical environment. We need studies evaluating the effect of teaching hospitalists on learner knowledge or, even more importantly, learner outcomes. Ultimately, we need studies of educational interventions that evaluate the impact of these interventions on patient outcome.
Wright et al.5 provide guidance as they describe the evaluation of a teaching intervention that moves beyond measurement of knowledge or attitudes. The Johns Hopkins Bayview hospitalist group sought to improve the quality of medical consultations performed by hospitalists and by residents rotating on the consultation service using a case‐based teaching module with audits of recent notes. The participants then audited their most recent consultation notes with feedback from the module teacher. The study employed pretests and posttests of knowledgea standard evaluation for educational interventions. This tells us little about the true impact of the teaching module. However, the study then assessed the quality of written consultations done by hospitalists before and after the educational interventions. Scores of consult notes improved significantly after the intervention, although the number of assessments for each physician was limited. Importantly, we need to know if interventions such as this are sustained over time. Wright's well‐established medical education research group's study design assessed the impact of an intervention on physician performance and moves us closer to assessment of the impact on actual patient outcomes. As clinical teachers, we would like to believe that our teaching and our educational innovations are having a positive impact on patient care. Can we demonstrate this?
As academic medical centers contend with further resident work‐hour restrictions proposed by the Institute of Medicine (IOM),10 how will this affect hospitalist teachers? The study by Mazotti et al.3 from the University of California at San Francisco residency program found that about one‐quarter of residents reported spending less time teaching after implementation of the Accreditation Council for Graduate Medical Education (ACGME) duty‐hour restrictions in 2003. Interestingly, those residents reporting less time spent teaching also reported less emotional exhaustion and perceived that they were delivering higher‐quality patient care. This raises a fascinating question for academic hospitalists. Would these findings be similar among teaching hospitalists and nonteaching hospitalists? What about hospitalists who rotate through months of teaching and nonteaching services? Is teaching emotionally exhausting for experienced teachers? A Mayo Clinic study suggested that the extent that faculty physicians are able to engage in work that is most meaningful to them as individuals is a strong determinant of faculty burnout.11 Is the hospitalist who finds teaching most rewarding at risk of burnout if they are assigned only 2 weeks a year as a teaching attending? The answers to these questions will be critical to hospitalist program leaders trying to assure sustainable careers for hospitalists in their programs.
Although the study by Mazotti et al.3 did not assess the impact of the reduction in resident teaching time on the teaching responsibilities for academic hospitalists, previous studies suggest that faculty are also teaching less since the introduction of work‐hour restrictions.12, 13 If the new IOM recommendations are enacted, who will teach? Although the reported experience following the 2003 work‐hour restrictions begs pessimism, the anticipated changes represent an opportunity for creative hospitalist teachers to demonstrate effective adaptations to the changing and compressed inpatient teaching environment.
In summary, this issue of the Journal presents studies that praise the role hospitalists play in teaching the next generation of physicians, but also gives a glimpse of future challenges and opportunities. We should take advantage of hospitalists' central position in clinical education in the hospital to innovate, study the effect on both learner outcomes and patient outcomes, and share our experiences with the hospitalist and medical education communities.
Hospitalists are increasingly assuming a primary role in medical education in the hospital setting, as they also steadily care for a larger portion of hospitalized patients.1 This issue of the Journal of Hospital Medicine highlights the role of hospitalists as teachers in academic medical centers, confirming their expanding and positive role in resident and medical student education. A survey of academic medical centers, a systematic review, an evaluation of the implementation of an educational curriculum, and a survey of residents hint at the challenges hospitalists face in teaching, but also expose us to a more advanced yet facile approach to evaluating the effectiveness of a teaching intervention.25 These publications provoke interesting questions about clinical teaching that hospital medicine educators and researchers should pursue answering. I believe they will also encourage us to innovate in medical education and assessment of that teaching.
Traditionally, teaching attendings for resident teams on medicine or pediatric services rotated through these duties for 1 to 3 months each year, while spending the majority of their time in clinic or research activities. The increasing complexity of hospitalized patients and the pressure to reduce length of stay prompted closer oversight of trainees. With the advent of resident work‐hour restrictions, the need for greater clinical involvement by attending physicians made it increasingly difficult to maintain the traditional model of limited engagement by faculty attendings. Simply put, the dwindling pool of willing and able teaching attendings encouraged teaching hospitals to employ hospitalists to fill the gap in teaching and supervision, as well as clinical coverage.6
Beasley et al.2 report that resident work‐hour restrictions were associated with an increase in the number of teaching hospitals employing hospitalists to 79% of 193 surveyed hospitals in 2007. Of those hospitals with hospitalists, 92% reported that hospitalists serve as attendings on the teaching service. Hospitalists also teach in a number of other venues within these programs, including formal teaching rounds without direct care responsibility, along with delivering didactic lectures and clinical skills education.
How well are teaching hospitalists performing compared to traditional teaching attendings? Natarajan et al.4 provide an important summary of the evidence in a systematic review of studies comparing teaching efforts of hospitalist attendings to those of nonhospitalist attendings. Eight studies from a variety of institutions measured trainee (resident or medical student) attitudes. It is gratifying to learn that hospitalists were generally rated higher at overall teaching effectiveness, provision of feedback, knowledge base, and involvement of the learner in patient care. It seems likely that publication bias would overestimate the positive effect of hospitalists on learner attitudes. However, there are plausible reasons that the positive effect is accurate. Because their professional responsibilities are focused in the hospital, hospitalists should naturally be more available to learners for teaching and feedback. Hospitalists tend to be younger in their academic careers, placing them closer to the cutting edge of knowledge gained during residency and possibly fellowship. They may be more in tune with the needs and pressures faced by their learners, having dealt with these same challenges either during recent training or during nonteaching rotations.
As a relatively young specialty with young and developing academic hospitalists, will the advantage suggested by the Natarajan et al.4 systematic review be sustained over the long term as careers in hospital medicine mature? A 2005 systematic review studying this question among practicing clinicians found, somewhat paradoxically, that older, more experienced clinicians appeared to be at risk for providing lower‐quality care.7 To avoid this decline in clinical effectiveness, hospitalists should proactively seek innovative ways to refresh and update their knowledge and skills throughout their careers. This is particularly critical for teaching physicians. We should seize the opportunity to study the relationship between advancing clinical/teaching experience and educational quality within our teaching programs.
The review by Natarajan et al.4 should also challenge the hospitalist community to achieve even higher levels of proficiency as teachers of medicine. The review alludes to bedside teaching and attention to psychosocial aspects of care as opportunities for improvement by hospitalist teachers. A recent study suggested that physical examination instruction receives declining attention from inpatient teachers and that there are opportunities to increase the amount of bedside teaching.8 A provocative study of inpatients admitted to a teaching service found that physical examination could substantially impact patient care, but that trainees often failed to appreciate significant findings on initial examination.9 How do teaching hospitalists become proficient at physical examination and bedside teaching? Are there models around the country that are successfully developing outstanding clinician educators, incorporating teach‐the‐teacher models to improve physical examination and bedside teaching?
A practical limitation of attitude surveys and learner evaluation is the well‐known phenomenon of grade inflation that resulted in high ratings for all attending groups in the studies summarized by Natarajan et al.4 This limits the ability of surveys or evaluations to distinguish truly outstanding teachers and consequently makes it difficult to analyze the attributes of these teachers. We need better tools to detect and learn teaching techniques from great teachers in the clinical environment. We need studies evaluating the effect of teaching hospitalists on learner knowledge or, even more importantly, learner outcomes. Ultimately, we need studies of educational interventions that evaluate the impact of these interventions on patient outcome.
Wright et al.5 provide guidance as they describe the evaluation of a teaching intervention that moves beyond measurement of knowledge or attitudes. The Johns Hopkins Bayview hospitalist group sought to improve the quality of medical consultations performed by hospitalists and by residents rotating on the consultation service using a case‐based teaching module with audits of recent notes. The participants then audited their most recent consultation notes with feedback from the module teacher. The study employed pretests and posttests of knowledgea standard evaluation for educational interventions. This tells us little about the true impact of the teaching module. However, the study then assessed the quality of written consultations done by hospitalists before and after the educational interventions. Scores of consult notes improved significantly after the intervention, although the number of assessments for each physician was limited. Importantly, we need to know if interventions such as this are sustained over time. Wright's well‐established medical education research group's study design assessed the impact of an intervention on physician performance and moves us closer to assessment of the impact on actual patient outcomes. As clinical teachers, we would like to believe that our teaching and our educational innovations are having a positive impact on patient care. Can we demonstrate this?
As academic medical centers contend with further resident work‐hour restrictions proposed by the Institute of Medicine (IOM),10 how will this affect hospitalist teachers? The study by Mazotti et al.3 from the University of California at San Francisco residency program found that about one‐quarter of residents reported spending less time teaching after implementation of the Accreditation Council for Graduate Medical Education (ACGME) duty‐hour restrictions in 2003. Interestingly, those residents reporting less time spent teaching also reported less emotional exhaustion and perceived that they were delivering higher‐quality patient care. This raises a fascinating question for academic hospitalists. Would these findings be similar among teaching hospitalists and nonteaching hospitalists? What about hospitalists who rotate through months of teaching and nonteaching services? Is teaching emotionally exhausting for experienced teachers? A Mayo Clinic study suggested that the extent that faculty physicians are able to engage in work that is most meaningful to them as individuals is a strong determinant of faculty burnout.11 Is the hospitalist who finds teaching most rewarding at risk of burnout if they are assigned only 2 weeks a year as a teaching attending? The answers to these questions will be critical to hospitalist program leaders trying to assure sustainable careers for hospitalists in their programs.
Although the study by Mazotti et al.3 did not assess the impact of the reduction in resident teaching time on the teaching responsibilities for academic hospitalists, previous studies suggest that faculty are also teaching less since the introduction of work‐hour restrictions.12, 13 If the new IOM recommendations are enacted, who will teach? Although the reported experience following the 2003 work‐hour restrictions begs pessimism, the anticipated changes represent an opportunity for creative hospitalist teachers to demonstrate effective adaptations to the changing and compressed inpatient teaching environment.
In summary, this issue of the Journal presents studies that praise the role hospitalists play in teaching the next generation of physicians, but also gives a glimpse of future challenges and opportunities. We should take advantage of hospitalists' central position in clinical education in the hospital to innovate, study the effect on both learner outcomes and patient outcomes, and share our experiences with the hospitalist and medical education communities.
- , , , .Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- , , .Hospitalists involvement in internal medicine residencies.J Hosp Med.2009;4(8):471–475.
- , , , , .Impact of duty hour restriction on resident inpatient teaching.J Hosp Med.2009;4(8):476–480.
- , , , .Effect of hospitalist attending physicians on trainee educational experiences: a systematic review.J Hosp Med.2009;4(8):490–498.
- , , , , , .A case‐based teaching module combined with audit and feedback to improve the quality of consultations.J Hosp Med.2009;4(8):486–489.
- , , , , .Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- , , .Systematic review: the relationship between clinical experience and quality of health care.Ann Intern Med.2005;142(4):260–273.
- , , .Quantification of bedside teaching by an academic hospitalist group.J Hosp Med.2009;4(5):304–307.
- .Physical examination in the care of medical inpatients: an observational study.Lancet.2003;362(9390):1100–1105.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. 2008. Available at: http://www.iom.edu/CMS/3809/48553/60449.aspx. Accessed September2009.
- , , , et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
- , .Teaching internal medicine residents in the new era. Inpatient attending with duty‐hour regulations.J Gen Intern Med.2006;21(5):447–452.
- , , , .Effects of resident work hour limitations on faculty professional lives.J Gen Intern Med.2008;23(7):1077–1083.
- , , , .Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- , , .Hospitalists involvement in internal medicine residencies.J Hosp Med.2009;4(8):471–475.
- , , , , .Impact of duty hour restriction on resident inpatient teaching.J Hosp Med.2009;4(8):476–480.
- , , , .Effect of hospitalist attending physicians on trainee educational experiences: a systematic review.J Hosp Med.2009;4(8):490–498.
- , , , , , .A case‐based teaching module combined with audit and feedback to improve the quality of consultations.J Hosp Med.2009;4(8):486–489.
- , , , , .Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- , , .Systematic review: the relationship between clinical experience and quality of health care.Ann Intern Med.2005;142(4):260–273.
- , , .Quantification of bedside teaching by an academic hospitalist group.J Hosp Med.2009;4(5):304–307.
- .Physical examination in the care of medical inpatients: an observational study.Lancet.2003;362(9390):1100–1105.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. 2008. Available at: http://www.iom.edu/CMS/3809/48553/60449.aspx. Accessed September2009.
- , , , et al.Career fit and burnout among academic faculty.Arch Intern Med.2009;169(10):990–995.
- , .Teaching internal medicine residents in the new era. Inpatient attending with duty‐hour regulations.J Gen Intern Med.2006;21(5):447–452.
- , , , .Effects of resident work hour limitations on faculty professional lives.J Gen Intern Med.2008;23(7):1077–1083.
The Third Time's the Charm
A 58‐year old woman was brought to the emergency department with confusion. Her husband stated that for several hours she had been drifting in and out at home, and that he had to shout to get her attention. He described no seizure activity, weakness, incontinence, or difficulty speaking, and had noted no complaints of headache, fevers, chest pain, shortness of breath, or gastrointestinal complaints.
Altered mental status in a middle‐aged woman can result from a diverse set of etiologies. A key distinction in the neurological examination will be to assure that the complaint of confusion is accurate as opposed to aphasia; the former is usually indicative of diffuse cerebral dysfunction while the latter suggests a focal lesion in the dominant hemisphere.
The acuity of the change in mental status is important, as are the fluctuations described by the husband. Unwitnessed or nonconvulsive seizure activity can present this way. Toxic/metabolic etiologies, infectious and inflammatory disorders of the central nervous system (CNS), and vascular diseases are also important considerations. Although stroke does not typically present with global encephalopathy, intermittent large vessel occlusion, especially in the posterior circulation, can disrupt cognition in this manner. Following a physical examination, initial workup should focus on toxic/metabolic etiologies, followed rapidly by head imaging if no cause is identified.
Her past medical history was notable for type 2 diabetes mellitus, coronary artery disease, hyperlipidemia, and an unspecified seizure disorder, which according to her husband was diagnosed during a recent hospitalization for a similar presentation. She also had a remote history of venous thromboembolism and antithrombin‐III deficiency. She was unemployed, lived with her husband, and spent most of her time at home. She never smoked, and rarely drank alcohol. Her family history was unobtainable, and her husband denied that she used any illicit drugs. Her medications included pioglitazone, aspirin, simvastatin, pregabalin, ferrous sulfate, levetiracetam, warfarin, and magnesium oxide, and she was allergic to sulfa.
While the differential diagnosis remains broad, 3 elements of the history are potentially relevant. The history of epilepsy based on a similar prior presentation increases the likelihood that the current spell is ictal in nature; examination of previous records would be important in order to document whether these spells have indeed been proven to be epileptic, as many conditions can mimic seizures. Given the history of venous thromboembolism and hypercoagulability, one must consider cerebral venous sinus thrombosis, which can present with global neurologic dysfunction and seizures. Prompt identification, usually via computed tomography (CT) or magnetic resonance angiography, is vital, because anticoagulation can mitigate this potentially life‐threatening illness. Finally, although many medications can cause encephalopathy in overdose, levetiracetam has well‐described cognitive side effects even at usual doses, including encephalopathy, irritability, and depression.
The records from that recent hospitalization remarked that she had presented confused and stuporous. Her potassium had been 2.7 mmol/L, international normalized ration (INR) 3.4, and hemoglobin 8 g/dL; other routine laboratory studies were normal. CT and magnetic resonance imaging (MRI) of the brain had been negative, and electroencephalogram (EEG) reportedly was performed but specific results were unknown. She was discharged alert and oriented 1 week prior to the current presentation on the above medications, including levetiracetam for this newly‐diagnosed seizure disorder.
Previous records confirm that the current presentation is that of a relapsing acute alteration in mental status. Regardless of the EEG findings or response to antiepileptic medications, a seizure disorder should remain a primary consideration, although relapsing inflammatory, toxic/metabolic conditions, and, rarely, vascular disorders can also present in this manner.
The neurologic manifestations of hypokalemia are usually peripheral in nature, including periodic paralysis; confusion accompanying hypokalemia is usually not a result of the low potassium itself but rather due to an underlying toxic or endocrinologic cause. Various causes of anemia can lead to mental status changes; the mean corpuscular volume (MCV) will be particularly helpful given known associations between megaloblastic anemia and confusional states.
On examination, she appeared to be in good health and in no distress. She was afebrile. Her blood pressure was 93/57, pulse 90 beats per minute, respiratory rate 16 per minute, and room air oxygen saturation 100%. She was oriented to her surroundings, but slow in her responses to questioning. There were no cranial nerve, motor, or sensory deficits, or abnormal reflexes or movements. Examination of the head, skin, chest, cardiovascular system, abdomen, and extremities was normal. Serum sodium was 136 mmol/L, creatinine 1.2 mg/dL, calcium 9.3 mg/dL, and glucose 81 mg/dL; other routine blood chemistries were normal. Her white blood cell (WBC) count was 7100/L, hemoglobin 9.2 g/dL with normal MCV, and platelet count 275,000/L. INR was 3.4, and liver function tests were normal. CT of the brain demonstrated no evidence of acute pathology.
Given that her laboratory results (aside from the hemoglobin) and CT were essentially normal, the most common etiology of a recurrent encephalopathy would be a toxic exposure including drugs, alcohol, and environmental toxins or poisons. A comprehensive serum drug screen, including heavy metals, could follow a basic urinary screen for drugs of abuse; specific etiologies may be suggested by patterns of injury seen on MRI such as those seen with carbon monoxide or methanol exposure. Other recurrent metabolic processes include the porphyrias and relapsing inflammatory disorders, which could be entertained if further diagnostics are unrevealing.
An EEG is warranted at this point and is a test that is underutilized in the workup of altered mental status. Patients who have a spell and do not quickly awaken should be considered to be in nonconvulsive status epilepticus until proven otherwise. This can be easily identified on the EEG and is an important entity to recognize quickly. Additional findings on EEG may suggest focal cerebral dysfunction (such as that following a seizure or acute unilateral injury), diffuse encephalopathy (eg, triphasic waves), or fairly specific diagnoses (eg, periodic lateralized epileptiform discharges from the temporal lobes in suspected herpes simplex meningoencephalitis). While the CT of the brain is a reasonable initial screen, MRI is more sensitive for structural disease and should be obtained if no etiology is rapidly identified.
Finally, acute infectious etiologies such as abscess, encephalitis, or meningoencephalitis need to be excluded via lumbar puncture. Spinal fluid examination also can be helpful in the consideration of inflammatory and autoimmune disorders.
Over the next several hours, while still in the emergency department, she became increasingly obtunded, to the point that she was unresponsive to all stimuli. No seizure activity was witnessed, her vital signs were unchanged, and no medications had been administered. She was urgently transferred to a tertiary care center, where, at the time of arrival, she was obtunded and nonverbal, and opened her eyes only to noxious stimuli. She would withdraw all 4 extremities in response to pain. Pupils were 2 mm and symmetrically reactive. Corneal reflexes were normal, and her gag reflex was diminished. Motor tone was decreased in all 4 extremities. No fasciculations were noted. Deep tendon reflexes were present but symmetrically diminished throughout, and Babinski testing demonstrated a withdrawal response bilaterally.
Coma is a state of profound unconsciousness where the patient is unarousable and unaware of her surroundings. Coma can result either from bihemispheric dysfunction or diffuse injury to the reticular activating system in the brainstem, and the physical examination should focus on distinguishing between these 2 sites. Because the nuclei of cranial nerves III through XII (excepting XI) reside in the brainstem, the coma examination emphasizes testing the cranial nerves; although all cranial nerves are not tested in this patient, the ones that are appear to be normal, making bihemispheric dysfunction most likely. Bihemispheric coma most commonly results from diffuse toxic or metabolic etiologies such as intoxication or hepatic encephalopathy, but it can also be caused by bilateral structural lesions (including the bilateral thalami) or ongoing seizure activity.
Although an EEG remains the key test in this patient given her past history and an MRI would prove extremely useful, her deterioration warrants a workup for CNS infection. Since the head CT was negative, it would be prudent to proceed with urgent lumbar puncture (although it should never be performed in a patient with significant coagulopathy due to risks of hemorrhage leading to spinal cord injury). She should be covered empirically with broad spectrum meningeal‐dose antibiotics, including acyclovir, until the results of the spinal fluid examination are known, given that bacterial meningitis and herpes meningoencephalitis carry a high morbidity and mortality if not treated promptly.
Routine blood tests were similar to her labs at the referring emergency room. Ammonia level was 10 mol/L. Urine toxicology screen was negative, and blood tests for ethanol, salicylates, lithium, and acetaminophen were negative. Chest X‐ray and urinalysis were normal, and electrocardiogram was notable only for a sinus tachycardia. Cultures of the blood were obtained and the patient was admitted to the intensive care unit.
Levetiracetam, vancomycin, piperacillin‐tazobactam, and acyclovir were initiated. A lumbar puncture was performed without reversing the anticoagulation, and the procedure was traumatic. The cerebrospinal fluid was bloody, with a clear supernatant. Cell count demonstrated a red blood cell (RBC) count of 1250/L and a WBC count of 9/L, with a WBC differential of 42% neutrophils, 48% lymphocytes, and 8% monocytes. The cerebrospinal fluid (CSF) glucose was 62 mg/dL (with a serum glucose of 74 mg/dL) and protein 41 mg/dL. The CSF Gram stain demonstrated no organisms, and fluid was sent for routine culture and polymerase chain reaction (PCR) to detect herpes simplex virus (HSV). A neurology consultation was urgently requested.
As mentioned, it would have been more appropriate to reverse the patient's anticoagulation prior to lumbar puncture. The absence of xanthochromia suggests that the RBCs seen in the sample were introduced at the time of the lumbar puncture, arguing against a hemorrhagic disorder of the CNS (occasionally seen with herpes simplex encephalitis) or spinal fluid (eg, subarachnoid hemorrhage).
A reasonable rule of thumb to correct for the number of RBCs in a traumatic lumbar puncture is to allow 1 WBC for every 700 RBCs/L. Given this conversion, there are still too many WBCs in this sample, indicating a mild pleocytosis that is approximately one‐half neutrophilic and one‐half lymphocytic. This profile is nonspecific and can occur with a variety of conditions including stroke, seizure, inflammatory disorders, and infections, including viruses such as West Nile virus.
While coverage with acyclovir and broad‐spectrum antibacterials is appropriate, it should be noted that piperacillin‐tazobactam has poor CSF penetration and therefore is not a good choice for empiric coverage of CNS infections.
The neurologist's examination additionally noted multifocal myoclonus with noxious stimuli, most prominent in the face and toes. An urgent EEG demonstrated continuous, slow, generalized triphasic wave activity (Figures 1 and 2); no epileptiform discharges were seen. The erythrocyte sedimentation rate (ESR) was 66 mm/hour (normal, 0‐30), and tests for antinuclear antibodies, serum levetiracetam level, and thyroid function studies were ordered.
Stimulus‐evoked multifocal myoclonus is a general marker of encephalopathy found in many conditions, including hepatic and renal failure, drug intoxication (eg, opiates), neurodegenerative disorders (eg, Creutzfeldt‐Jakob disease [CJD]), and postanoxic injury, the latter of which is termed the Lance‐Adams syndrome.
Triphasic waves on EEG, while commonly associated with hepatic encephalopathy, have a similarly broad differential diagnosis, although in a comatose patient, they must first and foremost be distinguished from the repetitive discharges characteristic of nonconvulsive status epilepticus. In addition to hepatic and renal failure, triphasic waves have also been described in medication toxicity (especially with anticonvulsants, lithium, and cephalosporins), CNS infections (including Lyme disease and West Nile virus), strokes involving the bilateral thalami (usually from deep venous thrombosis), inflammatory disorders (such as Hashimoto's encephalopathy [HE]), and neurodegenerative diseases. It is important to remember that a single EEG does not exclude the possibility of an episodic ictal disorder and longer‐term monitoring would be required to definitively exclude seizures.
At this point, although the myoclonus and triphasic waves most commonly would indicate a toxic/metabolic process, the elevated ESR and CSF pleocytosis argue for an inflammatory or infectious condition. An MRI remains the next most useful test to guide further workup because many such conditions have distinct signatures on MRI.
The following day, she was noted to have periods of alertnessopening her eyes and following some commandsbut at other times she was difficult to arouse or obtunded. Tremulous movements and sporadic myoclonic jerks continued but no focal neurologic signs were found. Although there was increased muscle tone throughout, she was intermittently seen moving her limbs spontaneously, but not to command. No new findings were appreciated on routine laboratory tests. Antinuclear antibody testing was negative. Serum levetiracetam level was 23.5 g/mL (reference range, 545). Serum thyroid‐stimulating hormone was less than 0.005 U/mL, but free T3 was 3.5 pg/mL (normal, 1.8‐4.6) and free T4 was 2.0 ng/dL (normal, 0.71.8). An MRI of the brain was compromised by motion artifact but no significant abnormalities were appreciated.
At this point, a family member in another state disclosed that the patient had also been hospitalized 2 months previously while visiting him. Her chief complaint had been shortness of breath. The records were obtained; a cardiac catheterization had revealed nonobstructive coronary disease, and medical management was recommended. The notes mentioned that during the hospitalization she developed altered mental status with disorientation and shaking. CT and MRI of the brain had been unremarkable. The confusion was not explained, but she was discharged in good condition, alert and fully‐oriented.
The additional history confirms a relapsing encephalopathy, now with at least 3 occurrences. The most common etiologies in the face of a normal MRI and basic labs would be recurrent intoxication or exposures, but the inflammatory CSF profile and elevated ESR are not consistent with this. A variety of inflammatory disorders can present with recurrent encephalopathy, including demyelinating diseases and neurosarcoidosis. Some systemic rheumatologic conditions, such as systemic lupus erythematosus, can present with relapsing neurologic symptoms due to seizures, vasculitis, or cerebritis. Vasculitis would fit this picture as well, except for the normal findings on 2 MRIs. In a patient with such dramatic symptoms of neurologic dysfunction, one would expect to see changes on the MRI of cerebral inflammation with probable ischemia.
Therefore, given the CSF, ESR, clinical course, and unrevealing MRI and EEG, the most likely group of disorders responsible would be the nonvasculitic autoimmune meningoencephalitides, which present with recurrent encephalopathy and feature spontaneous remissions and/or often‐dramatic responses to corticosteroids. Key disorders in this category include Sjogren's disease, lupus, and steroid responsive encephalopathy associated with autoimmune thyroiditis (Hashimoto's encephalopathy). The latter condition is the most common of the group and is suggested by the abnormal thyroid‐stimulating hormone testing, although it may occur in the setting of normal thyroid function. The diagnosis can be confirmed with thyroperoxidase and thyroglobulin antibody testing.
Three days into the hospitalization, her mental status had gradually improved such that she was more consistently awake and oriented to person and place, and she was transferred to a regular nursing unit. Final results from the CSF and blood cultures were negative, as was PCR for HSV. The antimicrobials were discontinued. Routine serum chemistries continued to be unremarkable. Additional studies recommended by the neurologist demonstrated an antithyroperoxidase antibody concentration of 587.1 IU/mL (normal, <5), and antithyroglobulin antibody level of 52.2 IU/mL (normal, <10).
These results confirm the diagnosis of HE which, in addition to its presentation as a recurrent illness, is an important treatable cause of dementia and should be considered in young patients, those with autoimmune and thyroid disorders, and those whose dementia is rapidly progressive. Most cases are thought to be steroid‐responsive, but some studies have defined the disorder based on this responsiveness, resulting in some nonresponders likely being overlooked.
A trial of corticosteroids should be considered if the patient does not quickly return to baseline given the potential morbidities associated with prolonged altered mental status to this degree. Whether initiation of chronic immunosuppression could prevent these attacks in the future is unclear from the literature but should be considered given the recurrent, dramatic presentation in this patient.
A diagnosis of HE was made, and she was prescribed corticosteroids. Twenty‐four hours later, she was alert and fully‐oriented. She was discharged to home on prednisone and seen in follow‐up in neurology clinic 1 month later. She had had no further episodes of confusion or stupor, but because of steroid‐induced hyperglycemia, her corticosteroids were decreased and mycophenolate mofetil added for chronic immunosuppression. Four months after discharge she was neurologically stable but continued to struggle with the adverse effects of chronic corticosteroid treatment.
COMMENTARY
HE is an uncommon condition that can present with a rapidly progressive decline and should be considered in patients who present with recurrent mental status change in the setting of normal imaging studies and routine laboratory results. The entity was initially described by Lord William Russell Brain in 1966, and in the most recent terminology is known as steroid‐responsive encephalopathy associated with autoimmune thyroiditis (SREAT).1 It is characterized by an acute or subacute encephalopathy associated with thyroid autoimmunity. Patients typically present with fluctuating symptoms, episodes of confusion, alterations of consciousness, and rapid cognitive decline.2 Common features include myoclonus, tremor, ataxia, speech disturbance, stroke‐like episodes, increased muscle tone, neuropsychiatric manifestations, and seizures, that in some cases may progress to status epilepticus.3, 4
Although serum antithyroglobulin and antithyroperoxidase antibodies are elevated in HE, their presence is thought to be an epiphenomenon of the condition rather than the direct cause. Supporting this are the facts that the incidence of encephalopathy is not increased in patients with established autoimmune thyroiditis, and the presence of antithyroid antibodies ranges from 5% to 20% in the general population.2, 5 There is also no evidence that thyroid antibodies directly react with brain tissue, and the levels of these antibodies do not correlate with either neurologic manifestations or clinical improvement.2, 4, 5 As HE has been reported in patients with euthyroidism, hypothyroidism, and hyperthyroidism (with hypothyroidismeither subclinical or activemost common), it is also unlikely that the level of thyroid hormones play a role in the etiology of this disease.2, 4, 6
The etiology and pathogenesis of HE are unclear, although an immune‐mediated process is generally implicated, either from an inflammatory vasculitis or as a form of acute disseminated encephalomyelitis.7‐9 Global hypoperfusion on single‐photon emission computed tomography (SPECT) studies has also been reported.10, 11 Patients with HE may have nonspecific evidence of inflammation, including an elevated ESR, CRP, and CSF protein.12 Other laboratory abnormalities may include a mild elevation of liver aminotransferase levels; renal impairment has also been reported in a few cases of HE in the form of glomerulonephritis, and may be related to deposition of immune complexes containing thyroglobulin antigen.6, 12‐14 MRI of the brain is normal or nonspecific in most cases, and the EEG most commonly shows diffuse slowing.
The differential for a rapidly progressive cognitive decline includes CJD, CNS vasculitis, paraneoplastic syndromes, and autoimmune and subacute infectious encephalopathies. In patients with CJD, T2‐weighted imaging may show hyperintense signals in the basal ganglia, while diffusion‐weighted sequences may reveal changes in the cortical ribbon and bilateral thalami.15 In CNS vasculitis, the imaging findings are variable and range from discrete areas of vascular infarcts to hemorrhagic lesions.16 In paraneoplastic and autoimmune encephalopathies (excluding HE), MRI often shows nonenhancing signal intensity changes in the mesial temporal lobes.12 This patient had repeatedly normal MRI studies of the brain, which in combination with the history of tremor, myoclonus, seizures, and interval return to baseline status, helped point to the diagnosis of HE.
Different approaches to treatment of HE have been recommended. As the acronym SREAT suggests, patients typically respond dramatically to high‐dose steroid therapy. Although a number of patients also improve spontaneously, up to 60% of patients experience a relapsing course and require chronic immunosuppressive agents for maintenance therapy, including long‐term steroids and azathioprine.2, 17 Treatment with plasma exchange and intravenous immune globulin have also been reported, but with mixed results.18, 19 Due to her history of multiple relapses, the patient was placed on mycophenolate mofetil for additional maintenance immunosuppression, as her corticosteroid dose was reduced due to adverse effects.
Acute mental status change is a potentially emergent situation that must be evaluated with careful history and studies to exclude life‐threatening metabolic, infectious, and vascular conditions. This patient presented similarly on 2 prior occasions, and each time her physician team evaluated what appeared to be a new onset of altered consciousness, reaching a plausible but ultimately incorrect diagnosis. The patient's third presentation was finally the charm, as her physicians learned of the repeated history of a confusional state, and in particular the return to baseline status, allowing them to create a differential that focused on etiologies of relapsing encephalopathy and make the correct diagnosis.
Key Points
-
Recurrent acute or subacute cognitive deterioration invokes a differential diagnosis of toxic/metabolic disorders and unusual inflammatory conditions.
-
The nonvasculitic autoimmune encephalopathies are a group of uncommon conditions characterized by nonspecific findings of inflammation and generally unremarkable CNS imaging studies.
-
HE, or SREAT, is the most common of these conditions, and is notable for mental status changes, various findings of increased muscular tone, thyroid autoimmunity, and generally a dramatic response to corticosteroids.
- , , .Hashimoto's disease and encephalopathy.Lancet.1966;2:512–514.
- , , .Hashimoto encephalopathy: syndrome or myth?Arch Neurol.2003;60:164–171.
- , , .Pisani F. Recurrent status epilepticus as the main feature of Hashimoto's encephalopathy.Epilepsy Behav.2006;8:328–330.
- , , , et al.Steroid‐responsive encephalopathy associated with autoimmune thyroiditis.Arch Neurol.2006;63:197–202.
- , , , , .Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment.J Neurol.1996;243:585–593.
- , , , , .Hashimoto's encephalopathy: a steroid‐responsive disorder associated with high anti‐thyroid antibody titers‐report of 5 cases.Neurology.1991;41:228–233.
- , , , , .Hashimoto encephalopathy: a brainstem vasculitis?Neurology.2000;54:769–770.
- , , , , .Nonvasculitic autoimmune inflammatory meningoencephalitis (NAIM): A reversible form of encephalopathy.Neurology.1999;53:1579–1581.
- , , , .Hashimoto's encephalopathy: postmortem findings after fatal status epilepticus.Neurology.2003;61:1124–1126.
- , , .Autoimmune thyroiditis and a rapidly progressive dementia: global hypoperfusion on SPECT scanning suggests a possible mechanism.Neurology.1997;49:623–626.
- , , , , .Hashimoto's encephalopathy: clinical, SPECT and neurophysiologic data.QJM.2003;96:455–457.
- , , .Autoimmune Encephalopathies.The Neurologist.2007;13:140–147.
- , , , , , .Thyroid antigen‐antibody nephritis.Clin Immunol Immunopathol1976;6:341–346.
- , , .Immune complex glomerulonephritis mediated by thyroid antigens.Arch Pathol Lab Med1978;102:530–533.
- , , , et al.Diffusion‐weighted MR imaging of early‐stage Creutzfeldt‐Jakob disease: typical and atypical manifestations.Radiographics.2006;26:S191–S204.
- , , , , .CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography.AJNR Am J Neuroradiol.1999;20:75–85.
- , .Long‐Term Treatment of Hashimoto's Encephalopathy.J Neuropsychiatry Clin Neurosci.2006;18:14–20.
- , .Hashimoto's encephalopathy: steroid resistance and response to intravenouc immunoglobulins.J Neurol Neurosurg Psychiatry.2005;76:455–456.
- , .Hashimoto's encephalopathy responding to plasmapheresis.J Neurol Neurosurg Psychiatry.2001;70:132.
A 58‐year old woman was brought to the emergency department with confusion. Her husband stated that for several hours she had been drifting in and out at home, and that he had to shout to get her attention. He described no seizure activity, weakness, incontinence, or difficulty speaking, and had noted no complaints of headache, fevers, chest pain, shortness of breath, or gastrointestinal complaints.
Altered mental status in a middle‐aged woman can result from a diverse set of etiologies. A key distinction in the neurological examination will be to assure that the complaint of confusion is accurate as opposed to aphasia; the former is usually indicative of diffuse cerebral dysfunction while the latter suggests a focal lesion in the dominant hemisphere.
The acuity of the change in mental status is important, as are the fluctuations described by the husband. Unwitnessed or nonconvulsive seizure activity can present this way. Toxic/metabolic etiologies, infectious and inflammatory disorders of the central nervous system (CNS), and vascular diseases are also important considerations. Although stroke does not typically present with global encephalopathy, intermittent large vessel occlusion, especially in the posterior circulation, can disrupt cognition in this manner. Following a physical examination, initial workup should focus on toxic/metabolic etiologies, followed rapidly by head imaging if no cause is identified.
Her past medical history was notable for type 2 diabetes mellitus, coronary artery disease, hyperlipidemia, and an unspecified seizure disorder, which according to her husband was diagnosed during a recent hospitalization for a similar presentation. She also had a remote history of venous thromboembolism and antithrombin‐III deficiency. She was unemployed, lived with her husband, and spent most of her time at home. She never smoked, and rarely drank alcohol. Her family history was unobtainable, and her husband denied that she used any illicit drugs. Her medications included pioglitazone, aspirin, simvastatin, pregabalin, ferrous sulfate, levetiracetam, warfarin, and magnesium oxide, and she was allergic to sulfa.
While the differential diagnosis remains broad, 3 elements of the history are potentially relevant. The history of epilepsy based on a similar prior presentation increases the likelihood that the current spell is ictal in nature; examination of previous records would be important in order to document whether these spells have indeed been proven to be epileptic, as many conditions can mimic seizures. Given the history of venous thromboembolism and hypercoagulability, one must consider cerebral venous sinus thrombosis, which can present with global neurologic dysfunction and seizures. Prompt identification, usually via computed tomography (CT) or magnetic resonance angiography, is vital, because anticoagulation can mitigate this potentially life‐threatening illness. Finally, although many medications can cause encephalopathy in overdose, levetiracetam has well‐described cognitive side effects even at usual doses, including encephalopathy, irritability, and depression.
The records from that recent hospitalization remarked that she had presented confused and stuporous. Her potassium had been 2.7 mmol/L, international normalized ration (INR) 3.4, and hemoglobin 8 g/dL; other routine laboratory studies were normal. CT and magnetic resonance imaging (MRI) of the brain had been negative, and electroencephalogram (EEG) reportedly was performed but specific results were unknown. She was discharged alert and oriented 1 week prior to the current presentation on the above medications, including levetiracetam for this newly‐diagnosed seizure disorder.
Previous records confirm that the current presentation is that of a relapsing acute alteration in mental status. Regardless of the EEG findings or response to antiepileptic medications, a seizure disorder should remain a primary consideration, although relapsing inflammatory, toxic/metabolic conditions, and, rarely, vascular disorders can also present in this manner.
The neurologic manifestations of hypokalemia are usually peripheral in nature, including periodic paralysis; confusion accompanying hypokalemia is usually not a result of the low potassium itself but rather due to an underlying toxic or endocrinologic cause. Various causes of anemia can lead to mental status changes; the mean corpuscular volume (MCV) will be particularly helpful given known associations between megaloblastic anemia and confusional states.
On examination, she appeared to be in good health and in no distress. She was afebrile. Her blood pressure was 93/57, pulse 90 beats per minute, respiratory rate 16 per minute, and room air oxygen saturation 100%. She was oriented to her surroundings, but slow in her responses to questioning. There were no cranial nerve, motor, or sensory deficits, or abnormal reflexes or movements. Examination of the head, skin, chest, cardiovascular system, abdomen, and extremities was normal. Serum sodium was 136 mmol/L, creatinine 1.2 mg/dL, calcium 9.3 mg/dL, and glucose 81 mg/dL; other routine blood chemistries were normal. Her white blood cell (WBC) count was 7100/L, hemoglobin 9.2 g/dL with normal MCV, and platelet count 275,000/L. INR was 3.4, and liver function tests were normal. CT of the brain demonstrated no evidence of acute pathology.
Given that her laboratory results (aside from the hemoglobin) and CT were essentially normal, the most common etiology of a recurrent encephalopathy would be a toxic exposure including drugs, alcohol, and environmental toxins or poisons. A comprehensive serum drug screen, including heavy metals, could follow a basic urinary screen for drugs of abuse; specific etiologies may be suggested by patterns of injury seen on MRI such as those seen with carbon monoxide or methanol exposure. Other recurrent metabolic processes include the porphyrias and relapsing inflammatory disorders, which could be entertained if further diagnostics are unrevealing.
An EEG is warranted at this point and is a test that is underutilized in the workup of altered mental status. Patients who have a spell and do not quickly awaken should be considered to be in nonconvulsive status epilepticus until proven otherwise. This can be easily identified on the EEG and is an important entity to recognize quickly. Additional findings on EEG may suggest focal cerebral dysfunction (such as that following a seizure or acute unilateral injury), diffuse encephalopathy (eg, triphasic waves), or fairly specific diagnoses (eg, periodic lateralized epileptiform discharges from the temporal lobes in suspected herpes simplex meningoencephalitis). While the CT of the brain is a reasonable initial screen, MRI is more sensitive for structural disease and should be obtained if no etiology is rapidly identified.
Finally, acute infectious etiologies such as abscess, encephalitis, or meningoencephalitis need to be excluded via lumbar puncture. Spinal fluid examination also can be helpful in the consideration of inflammatory and autoimmune disorders.
Over the next several hours, while still in the emergency department, she became increasingly obtunded, to the point that she was unresponsive to all stimuli. No seizure activity was witnessed, her vital signs were unchanged, and no medications had been administered. She was urgently transferred to a tertiary care center, where, at the time of arrival, she was obtunded and nonverbal, and opened her eyes only to noxious stimuli. She would withdraw all 4 extremities in response to pain. Pupils were 2 mm and symmetrically reactive. Corneal reflexes were normal, and her gag reflex was diminished. Motor tone was decreased in all 4 extremities. No fasciculations were noted. Deep tendon reflexes were present but symmetrically diminished throughout, and Babinski testing demonstrated a withdrawal response bilaterally.
Coma is a state of profound unconsciousness where the patient is unarousable and unaware of her surroundings. Coma can result either from bihemispheric dysfunction or diffuse injury to the reticular activating system in the brainstem, and the physical examination should focus on distinguishing between these 2 sites. Because the nuclei of cranial nerves III through XII (excepting XI) reside in the brainstem, the coma examination emphasizes testing the cranial nerves; although all cranial nerves are not tested in this patient, the ones that are appear to be normal, making bihemispheric dysfunction most likely. Bihemispheric coma most commonly results from diffuse toxic or metabolic etiologies such as intoxication or hepatic encephalopathy, but it can also be caused by bilateral structural lesions (including the bilateral thalami) or ongoing seizure activity.
Although an EEG remains the key test in this patient given her past history and an MRI would prove extremely useful, her deterioration warrants a workup for CNS infection. Since the head CT was negative, it would be prudent to proceed with urgent lumbar puncture (although it should never be performed in a patient with significant coagulopathy due to risks of hemorrhage leading to spinal cord injury). She should be covered empirically with broad spectrum meningeal‐dose antibiotics, including acyclovir, until the results of the spinal fluid examination are known, given that bacterial meningitis and herpes meningoencephalitis carry a high morbidity and mortality if not treated promptly.
Routine blood tests were similar to her labs at the referring emergency room. Ammonia level was 10 mol/L. Urine toxicology screen was negative, and blood tests for ethanol, salicylates, lithium, and acetaminophen were negative. Chest X‐ray and urinalysis were normal, and electrocardiogram was notable only for a sinus tachycardia. Cultures of the blood were obtained and the patient was admitted to the intensive care unit.
Levetiracetam, vancomycin, piperacillin‐tazobactam, and acyclovir were initiated. A lumbar puncture was performed without reversing the anticoagulation, and the procedure was traumatic. The cerebrospinal fluid was bloody, with a clear supernatant. Cell count demonstrated a red blood cell (RBC) count of 1250/L and a WBC count of 9/L, with a WBC differential of 42% neutrophils, 48% lymphocytes, and 8% monocytes. The cerebrospinal fluid (CSF) glucose was 62 mg/dL (with a serum glucose of 74 mg/dL) and protein 41 mg/dL. The CSF Gram stain demonstrated no organisms, and fluid was sent for routine culture and polymerase chain reaction (PCR) to detect herpes simplex virus (HSV). A neurology consultation was urgently requested.
As mentioned, it would have been more appropriate to reverse the patient's anticoagulation prior to lumbar puncture. The absence of xanthochromia suggests that the RBCs seen in the sample were introduced at the time of the lumbar puncture, arguing against a hemorrhagic disorder of the CNS (occasionally seen with herpes simplex encephalitis) or spinal fluid (eg, subarachnoid hemorrhage).
A reasonable rule of thumb to correct for the number of RBCs in a traumatic lumbar puncture is to allow 1 WBC for every 700 RBCs/L. Given this conversion, there are still too many WBCs in this sample, indicating a mild pleocytosis that is approximately one‐half neutrophilic and one‐half lymphocytic. This profile is nonspecific and can occur with a variety of conditions including stroke, seizure, inflammatory disorders, and infections, including viruses such as West Nile virus.
While coverage with acyclovir and broad‐spectrum antibacterials is appropriate, it should be noted that piperacillin‐tazobactam has poor CSF penetration and therefore is not a good choice for empiric coverage of CNS infections.
The neurologist's examination additionally noted multifocal myoclonus with noxious stimuli, most prominent in the face and toes. An urgent EEG demonstrated continuous, slow, generalized triphasic wave activity (Figures 1 and 2); no epileptiform discharges were seen. The erythrocyte sedimentation rate (ESR) was 66 mm/hour (normal, 0‐30), and tests for antinuclear antibodies, serum levetiracetam level, and thyroid function studies were ordered.


Stimulus‐evoked multifocal myoclonus is a general marker of encephalopathy found in many conditions, including hepatic and renal failure, drug intoxication (eg, opiates), neurodegenerative disorders (eg, Creutzfeldt‐Jakob disease [CJD]), and postanoxic injury, the latter of which is termed the Lance‐Adams syndrome.
Triphasic waves on EEG, while commonly associated with hepatic encephalopathy, have a similarly broad differential diagnosis, although in a comatose patient, they must first and foremost be distinguished from the repetitive discharges characteristic of nonconvulsive status epilepticus. In addition to hepatic and renal failure, triphasic waves have also been described in medication toxicity (especially with anticonvulsants, lithium, and cephalosporins), CNS infections (including Lyme disease and West Nile virus), strokes involving the bilateral thalami (usually from deep venous thrombosis), inflammatory disorders (such as Hashimoto's encephalopathy [HE]), and neurodegenerative diseases. It is important to remember that a single EEG does not exclude the possibility of an episodic ictal disorder and longer‐term monitoring would be required to definitively exclude seizures.
At this point, although the myoclonus and triphasic waves most commonly would indicate a toxic/metabolic process, the elevated ESR and CSF pleocytosis argue for an inflammatory or infectious condition. An MRI remains the next most useful test to guide further workup because many such conditions have distinct signatures on MRI.
The following day, she was noted to have periods of alertnessopening her eyes and following some commandsbut at other times she was difficult to arouse or obtunded. Tremulous movements and sporadic myoclonic jerks continued but no focal neurologic signs were found. Although there was increased muscle tone throughout, she was intermittently seen moving her limbs spontaneously, but not to command. No new findings were appreciated on routine laboratory tests. Antinuclear antibody testing was negative. Serum levetiracetam level was 23.5 g/mL (reference range, 545). Serum thyroid‐stimulating hormone was less than 0.005 U/mL, but free T3 was 3.5 pg/mL (normal, 1.8‐4.6) and free T4 was 2.0 ng/dL (normal, 0.71.8). An MRI of the brain was compromised by motion artifact but no significant abnormalities were appreciated.
At this point, a family member in another state disclosed that the patient had also been hospitalized 2 months previously while visiting him. Her chief complaint had been shortness of breath. The records were obtained; a cardiac catheterization had revealed nonobstructive coronary disease, and medical management was recommended. The notes mentioned that during the hospitalization she developed altered mental status with disorientation and shaking. CT and MRI of the brain had been unremarkable. The confusion was not explained, but she was discharged in good condition, alert and fully‐oriented.
The additional history confirms a relapsing encephalopathy, now with at least 3 occurrences. The most common etiologies in the face of a normal MRI and basic labs would be recurrent intoxication or exposures, but the inflammatory CSF profile and elevated ESR are not consistent with this. A variety of inflammatory disorders can present with recurrent encephalopathy, including demyelinating diseases and neurosarcoidosis. Some systemic rheumatologic conditions, such as systemic lupus erythematosus, can present with relapsing neurologic symptoms due to seizures, vasculitis, or cerebritis. Vasculitis would fit this picture as well, except for the normal findings on 2 MRIs. In a patient with such dramatic symptoms of neurologic dysfunction, one would expect to see changes on the MRI of cerebral inflammation with probable ischemia.
Therefore, given the CSF, ESR, clinical course, and unrevealing MRI and EEG, the most likely group of disorders responsible would be the nonvasculitic autoimmune meningoencephalitides, which present with recurrent encephalopathy and feature spontaneous remissions and/or often‐dramatic responses to corticosteroids. Key disorders in this category include Sjogren's disease, lupus, and steroid responsive encephalopathy associated with autoimmune thyroiditis (Hashimoto's encephalopathy). The latter condition is the most common of the group and is suggested by the abnormal thyroid‐stimulating hormone testing, although it may occur in the setting of normal thyroid function. The diagnosis can be confirmed with thyroperoxidase and thyroglobulin antibody testing.
Three days into the hospitalization, her mental status had gradually improved such that she was more consistently awake and oriented to person and place, and she was transferred to a regular nursing unit. Final results from the CSF and blood cultures were negative, as was PCR for HSV. The antimicrobials were discontinued. Routine serum chemistries continued to be unremarkable. Additional studies recommended by the neurologist demonstrated an antithyroperoxidase antibody concentration of 587.1 IU/mL (normal, <5), and antithyroglobulin antibody level of 52.2 IU/mL (normal, <10).
These results confirm the diagnosis of HE which, in addition to its presentation as a recurrent illness, is an important treatable cause of dementia and should be considered in young patients, those with autoimmune and thyroid disorders, and those whose dementia is rapidly progressive. Most cases are thought to be steroid‐responsive, but some studies have defined the disorder based on this responsiveness, resulting in some nonresponders likely being overlooked.
A trial of corticosteroids should be considered if the patient does not quickly return to baseline given the potential morbidities associated with prolonged altered mental status to this degree. Whether initiation of chronic immunosuppression could prevent these attacks in the future is unclear from the literature but should be considered given the recurrent, dramatic presentation in this patient.
A diagnosis of HE was made, and she was prescribed corticosteroids. Twenty‐four hours later, she was alert and fully‐oriented. She was discharged to home on prednisone and seen in follow‐up in neurology clinic 1 month later. She had had no further episodes of confusion or stupor, but because of steroid‐induced hyperglycemia, her corticosteroids were decreased and mycophenolate mofetil added for chronic immunosuppression. Four months after discharge she was neurologically stable but continued to struggle with the adverse effects of chronic corticosteroid treatment.
COMMENTARY
HE is an uncommon condition that can present with a rapidly progressive decline and should be considered in patients who present with recurrent mental status change in the setting of normal imaging studies and routine laboratory results. The entity was initially described by Lord William Russell Brain in 1966, and in the most recent terminology is known as steroid‐responsive encephalopathy associated with autoimmune thyroiditis (SREAT).1 It is characterized by an acute or subacute encephalopathy associated with thyroid autoimmunity. Patients typically present with fluctuating symptoms, episodes of confusion, alterations of consciousness, and rapid cognitive decline.2 Common features include myoclonus, tremor, ataxia, speech disturbance, stroke‐like episodes, increased muscle tone, neuropsychiatric manifestations, and seizures, that in some cases may progress to status epilepticus.3, 4
Although serum antithyroglobulin and antithyroperoxidase antibodies are elevated in HE, their presence is thought to be an epiphenomenon of the condition rather than the direct cause. Supporting this are the facts that the incidence of encephalopathy is not increased in patients with established autoimmune thyroiditis, and the presence of antithyroid antibodies ranges from 5% to 20% in the general population.2, 5 There is also no evidence that thyroid antibodies directly react with brain tissue, and the levels of these antibodies do not correlate with either neurologic manifestations or clinical improvement.2, 4, 5 As HE has been reported in patients with euthyroidism, hypothyroidism, and hyperthyroidism (with hypothyroidismeither subclinical or activemost common), it is also unlikely that the level of thyroid hormones play a role in the etiology of this disease.2, 4, 6
The etiology and pathogenesis of HE are unclear, although an immune‐mediated process is generally implicated, either from an inflammatory vasculitis or as a form of acute disseminated encephalomyelitis.7‐9 Global hypoperfusion on single‐photon emission computed tomography (SPECT) studies has also been reported.10, 11 Patients with HE may have nonspecific evidence of inflammation, including an elevated ESR, CRP, and CSF protein.12 Other laboratory abnormalities may include a mild elevation of liver aminotransferase levels; renal impairment has also been reported in a few cases of HE in the form of glomerulonephritis, and may be related to deposition of immune complexes containing thyroglobulin antigen.6, 12‐14 MRI of the brain is normal or nonspecific in most cases, and the EEG most commonly shows diffuse slowing.
The differential for a rapidly progressive cognitive decline includes CJD, CNS vasculitis, paraneoplastic syndromes, and autoimmune and subacute infectious encephalopathies. In patients with CJD, T2‐weighted imaging may show hyperintense signals in the basal ganglia, while diffusion‐weighted sequences may reveal changes in the cortical ribbon and bilateral thalami.15 In CNS vasculitis, the imaging findings are variable and range from discrete areas of vascular infarcts to hemorrhagic lesions.16 In paraneoplastic and autoimmune encephalopathies (excluding HE), MRI often shows nonenhancing signal intensity changes in the mesial temporal lobes.12 This patient had repeatedly normal MRI studies of the brain, which in combination with the history of tremor, myoclonus, seizures, and interval return to baseline status, helped point to the diagnosis of HE.
Different approaches to treatment of HE have been recommended. As the acronym SREAT suggests, patients typically respond dramatically to high‐dose steroid therapy. Although a number of patients also improve spontaneously, up to 60% of patients experience a relapsing course and require chronic immunosuppressive agents for maintenance therapy, including long‐term steroids and azathioprine.2, 17 Treatment with plasma exchange and intravenous immune globulin have also been reported, but with mixed results.18, 19 Due to her history of multiple relapses, the patient was placed on mycophenolate mofetil for additional maintenance immunosuppression, as her corticosteroid dose was reduced due to adverse effects.
Acute mental status change is a potentially emergent situation that must be evaluated with careful history and studies to exclude life‐threatening metabolic, infectious, and vascular conditions. This patient presented similarly on 2 prior occasions, and each time her physician team evaluated what appeared to be a new onset of altered consciousness, reaching a plausible but ultimately incorrect diagnosis. The patient's third presentation was finally the charm, as her physicians learned of the repeated history of a confusional state, and in particular the return to baseline status, allowing them to create a differential that focused on etiologies of relapsing encephalopathy and make the correct diagnosis.
Key Points
-
Recurrent acute or subacute cognitive deterioration invokes a differential diagnosis of toxic/metabolic disorders and unusual inflammatory conditions.
-
The nonvasculitic autoimmune encephalopathies are a group of uncommon conditions characterized by nonspecific findings of inflammation and generally unremarkable CNS imaging studies.
-
HE, or SREAT, is the most common of these conditions, and is notable for mental status changes, various findings of increased muscular tone, thyroid autoimmunity, and generally a dramatic response to corticosteroids.
A 58‐year old woman was brought to the emergency department with confusion. Her husband stated that for several hours she had been drifting in and out at home, and that he had to shout to get her attention. He described no seizure activity, weakness, incontinence, or difficulty speaking, and had noted no complaints of headache, fevers, chest pain, shortness of breath, or gastrointestinal complaints.
Altered mental status in a middle‐aged woman can result from a diverse set of etiologies. A key distinction in the neurological examination will be to assure that the complaint of confusion is accurate as opposed to aphasia; the former is usually indicative of diffuse cerebral dysfunction while the latter suggests a focal lesion in the dominant hemisphere.
The acuity of the change in mental status is important, as are the fluctuations described by the husband. Unwitnessed or nonconvulsive seizure activity can present this way. Toxic/metabolic etiologies, infectious and inflammatory disorders of the central nervous system (CNS), and vascular diseases are also important considerations. Although stroke does not typically present with global encephalopathy, intermittent large vessel occlusion, especially in the posterior circulation, can disrupt cognition in this manner. Following a physical examination, initial workup should focus on toxic/metabolic etiologies, followed rapidly by head imaging if no cause is identified.
Her past medical history was notable for type 2 diabetes mellitus, coronary artery disease, hyperlipidemia, and an unspecified seizure disorder, which according to her husband was diagnosed during a recent hospitalization for a similar presentation. She also had a remote history of venous thromboembolism and antithrombin‐III deficiency. She was unemployed, lived with her husband, and spent most of her time at home. She never smoked, and rarely drank alcohol. Her family history was unobtainable, and her husband denied that she used any illicit drugs. Her medications included pioglitazone, aspirin, simvastatin, pregabalin, ferrous sulfate, levetiracetam, warfarin, and magnesium oxide, and she was allergic to sulfa.
While the differential diagnosis remains broad, 3 elements of the history are potentially relevant. The history of epilepsy based on a similar prior presentation increases the likelihood that the current spell is ictal in nature; examination of previous records would be important in order to document whether these spells have indeed been proven to be epileptic, as many conditions can mimic seizures. Given the history of venous thromboembolism and hypercoagulability, one must consider cerebral venous sinus thrombosis, which can present with global neurologic dysfunction and seizures. Prompt identification, usually via computed tomography (CT) or magnetic resonance angiography, is vital, because anticoagulation can mitigate this potentially life‐threatening illness. Finally, although many medications can cause encephalopathy in overdose, levetiracetam has well‐described cognitive side effects even at usual doses, including encephalopathy, irritability, and depression.
The records from that recent hospitalization remarked that she had presented confused and stuporous. Her potassium had been 2.7 mmol/L, international normalized ration (INR) 3.4, and hemoglobin 8 g/dL; other routine laboratory studies were normal. CT and magnetic resonance imaging (MRI) of the brain had been negative, and electroencephalogram (EEG) reportedly was performed but specific results were unknown. She was discharged alert and oriented 1 week prior to the current presentation on the above medications, including levetiracetam for this newly‐diagnosed seizure disorder.
Previous records confirm that the current presentation is that of a relapsing acute alteration in mental status. Regardless of the EEG findings or response to antiepileptic medications, a seizure disorder should remain a primary consideration, although relapsing inflammatory, toxic/metabolic conditions, and, rarely, vascular disorders can also present in this manner.
The neurologic manifestations of hypokalemia are usually peripheral in nature, including periodic paralysis; confusion accompanying hypokalemia is usually not a result of the low potassium itself but rather due to an underlying toxic or endocrinologic cause. Various causes of anemia can lead to mental status changes; the mean corpuscular volume (MCV) will be particularly helpful given known associations between megaloblastic anemia and confusional states.
On examination, she appeared to be in good health and in no distress. She was afebrile. Her blood pressure was 93/57, pulse 90 beats per minute, respiratory rate 16 per minute, and room air oxygen saturation 100%. She was oriented to her surroundings, but slow in her responses to questioning. There were no cranial nerve, motor, or sensory deficits, or abnormal reflexes or movements. Examination of the head, skin, chest, cardiovascular system, abdomen, and extremities was normal. Serum sodium was 136 mmol/L, creatinine 1.2 mg/dL, calcium 9.3 mg/dL, and glucose 81 mg/dL; other routine blood chemistries were normal. Her white blood cell (WBC) count was 7100/L, hemoglobin 9.2 g/dL with normal MCV, and platelet count 275,000/L. INR was 3.4, and liver function tests were normal. CT of the brain demonstrated no evidence of acute pathology.
Given that her laboratory results (aside from the hemoglobin) and CT were essentially normal, the most common etiology of a recurrent encephalopathy would be a toxic exposure including drugs, alcohol, and environmental toxins or poisons. A comprehensive serum drug screen, including heavy metals, could follow a basic urinary screen for drugs of abuse; specific etiologies may be suggested by patterns of injury seen on MRI such as those seen with carbon monoxide or methanol exposure. Other recurrent metabolic processes include the porphyrias and relapsing inflammatory disorders, which could be entertained if further diagnostics are unrevealing.
An EEG is warranted at this point and is a test that is underutilized in the workup of altered mental status. Patients who have a spell and do not quickly awaken should be considered to be in nonconvulsive status epilepticus until proven otherwise. This can be easily identified on the EEG and is an important entity to recognize quickly. Additional findings on EEG may suggest focal cerebral dysfunction (such as that following a seizure or acute unilateral injury), diffuse encephalopathy (eg, triphasic waves), or fairly specific diagnoses (eg, periodic lateralized epileptiform discharges from the temporal lobes in suspected herpes simplex meningoencephalitis). While the CT of the brain is a reasonable initial screen, MRI is more sensitive for structural disease and should be obtained if no etiology is rapidly identified.
Finally, acute infectious etiologies such as abscess, encephalitis, or meningoencephalitis need to be excluded via lumbar puncture. Spinal fluid examination also can be helpful in the consideration of inflammatory and autoimmune disorders.
Over the next several hours, while still in the emergency department, she became increasingly obtunded, to the point that she was unresponsive to all stimuli. No seizure activity was witnessed, her vital signs were unchanged, and no medications had been administered. She was urgently transferred to a tertiary care center, where, at the time of arrival, she was obtunded and nonverbal, and opened her eyes only to noxious stimuli. She would withdraw all 4 extremities in response to pain. Pupils were 2 mm and symmetrically reactive. Corneal reflexes were normal, and her gag reflex was diminished. Motor tone was decreased in all 4 extremities. No fasciculations were noted. Deep tendon reflexes were present but symmetrically diminished throughout, and Babinski testing demonstrated a withdrawal response bilaterally.
Coma is a state of profound unconsciousness where the patient is unarousable and unaware of her surroundings. Coma can result either from bihemispheric dysfunction or diffuse injury to the reticular activating system in the brainstem, and the physical examination should focus on distinguishing between these 2 sites. Because the nuclei of cranial nerves III through XII (excepting XI) reside in the brainstem, the coma examination emphasizes testing the cranial nerves; although all cranial nerves are not tested in this patient, the ones that are appear to be normal, making bihemispheric dysfunction most likely. Bihemispheric coma most commonly results from diffuse toxic or metabolic etiologies such as intoxication or hepatic encephalopathy, but it can also be caused by bilateral structural lesions (including the bilateral thalami) or ongoing seizure activity.
Although an EEG remains the key test in this patient given her past history and an MRI would prove extremely useful, her deterioration warrants a workup for CNS infection. Since the head CT was negative, it would be prudent to proceed with urgent lumbar puncture (although it should never be performed in a patient with significant coagulopathy due to risks of hemorrhage leading to spinal cord injury). She should be covered empirically with broad spectrum meningeal‐dose antibiotics, including acyclovir, until the results of the spinal fluid examination are known, given that bacterial meningitis and herpes meningoencephalitis carry a high morbidity and mortality if not treated promptly.
Routine blood tests were similar to her labs at the referring emergency room. Ammonia level was 10 mol/L. Urine toxicology screen was negative, and blood tests for ethanol, salicylates, lithium, and acetaminophen were negative. Chest X‐ray and urinalysis were normal, and electrocardiogram was notable only for a sinus tachycardia. Cultures of the blood were obtained and the patient was admitted to the intensive care unit.
Levetiracetam, vancomycin, piperacillin‐tazobactam, and acyclovir were initiated. A lumbar puncture was performed without reversing the anticoagulation, and the procedure was traumatic. The cerebrospinal fluid was bloody, with a clear supernatant. Cell count demonstrated a red blood cell (RBC) count of 1250/L and a WBC count of 9/L, with a WBC differential of 42% neutrophils, 48% lymphocytes, and 8% monocytes. The cerebrospinal fluid (CSF) glucose was 62 mg/dL (with a serum glucose of 74 mg/dL) and protein 41 mg/dL. The CSF Gram stain demonstrated no organisms, and fluid was sent for routine culture and polymerase chain reaction (PCR) to detect herpes simplex virus (HSV). A neurology consultation was urgently requested.
As mentioned, it would have been more appropriate to reverse the patient's anticoagulation prior to lumbar puncture. The absence of xanthochromia suggests that the RBCs seen in the sample were introduced at the time of the lumbar puncture, arguing against a hemorrhagic disorder of the CNS (occasionally seen with herpes simplex encephalitis) or spinal fluid (eg, subarachnoid hemorrhage).
A reasonable rule of thumb to correct for the number of RBCs in a traumatic lumbar puncture is to allow 1 WBC for every 700 RBCs/L. Given this conversion, there are still too many WBCs in this sample, indicating a mild pleocytosis that is approximately one‐half neutrophilic and one‐half lymphocytic. This profile is nonspecific and can occur with a variety of conditions including stroke, seizure, inflammatory disorders, and infections, including viruses such as West Nile virus.
While coverage with acyclovir and broad‐spectrum antibacterials is appropriate, it should be noted that piperacillin‐tazobactam has poor CSF penetration and therefore is not a good choice for empiric coverage of CNS infections.
The neurologist's examination additionally noted multifocal myoclonus with noxious stimuli, most prominent in the face and toes. An urgent EEG demonstrated continuous, slow, generalized triphasic wave activity (Figures 1 and 2); no epileptiform discharges were seen. The erythrocyte sedimentation rate (ESR) was 66 mm/hour (normal, 0‐30), and tests for antinuclear antibodies, serum levetiracetam level, and thyroid function studies were ordered.


Stimulus‐evoked multifocal myoclonus is a general marker of encephalopathy found in many conditions, including hepatic and renal failure, drug intoxication (eg, opiates), neurodegenerative disorders (eg, Creutzfeldt‐Jakob disease [CJD]), and postanoxic injury, the latter of which is termed the Lance‐Adams syndrome.
Triphasic waves on EEG, while commonly associated with hepatic encephalopathy, have a similarly broad differential diagnosis, although in a comatose patient, they must first and foremost be distinguished from the repetitive discharges characteristic of nonconvulsive status epilepticus. In addition to hepatic and renal failure, triphasic waves have also been described in medication toxicity (especially with anticonvulsants, lithium, and cephalosporins), CNS infections (including Lyme disease and West Nile virus), strokes involving the bilateral thalami (usually from deep venous thrombosis), inflammatory disorders (such as Hashimoto's encephalopathy [HE]), and neurodegenerative diseases. It is important to remember that a single EEG does not exclude the possibility of an episodic ictal disorder and longer‐term monitoring would be required to definitively exclude seizures.
At this point, although the myoclonus and triphasic waves most commonly would indicate a toxic/metabolic process, the elevated ESR and CSF pleocytosis argue for an inflammatory or infectious condition. An MRI remains the next most useful test to guide further workup because many such conditions have distinct signatures on MRI.
The following day, she was noted to have periods of alertnessopening her eyes and following some commandsbut at other times she was difficult to arouse or obtunded. Tremulous movements and sporadic myoclonic jerks continued but no focal neurologic signs were found. Although there was increased muscle tone throughout, she was intermittently seen moving her limbs spontaneously, but not to command. No new findings were appreciated on routine laboratory tests. Antinuclear antibody testing was negative. Serum levetiracetam level was 23.5 g/mL (reference range, 545). Serum thyroid‐stimulating hormone was less than 0.005 U/mL, but free T3 was 3.5 pg/mL (normal, 1.8‐4.6) and free T4 was 2.0 ng/dL (normal, 0.71.8). An MRI of the brain was compromised by motion artifact but no significant abnormalities were appreciated.
At this point, a family member in another state disclosed that the patient had also been hospitalized 2 months previously while visiting him. Her chief complaint had been shortness of breath. The records were obtained; a cardiac catheterization had revealed nonobstructive coronary disease, and medical management was recommended. The notes mentioned that during the hospitalization she developed altered mental status with disorientation and shaking. CT and MRI of the brain had been unremarkable. The confusion was not explained, but she was discharged in good condition, alert and fully‐oriented.
The additional history confirms a relapsing encephalopathy, now with at least 3 occurrences. The most common etiologies in the face of a normal MRI and basic labs would be recurrent intoxication or exposures, but the inflammatory CSF profile and elevated ESR are not consistent with this. A variety of inflammatory disorders can present with recurrent encephalopathy, including demyelinating diseases and neurosarcoidosis. Some systemic rheumatologic conditions, such as systemic lupus erythematosus, can present with relapsing neurologic symptoms due to seizures, vasculitis, or cerebritis. Vasculitis would fit this picture as well, except for the normal findings on 2 MRIs. In a patient with such dramatic symptoms of neurologic dysfunction, one would expect to see changes on the MRI of cerebral inflammation with probable ischemia.
Therefore, given the CSF, ESR, clinical course, and unrevealing MRI and EEG, the most likely group of disorders responsible would be the nonvasculitic autoimmune meningoencephalitides, which present with recurrent encephalopathy and feature spontaneous remissions and/or often‐dramatic responses to corticosteroids. Key disorders in this category include Sjogren's disease, lupus, and steroid responsive encephalopathy associated with autoimmune thyroiditis (Hashimoto's encephalopathy). The latter condition is the most common of the group and is suggested by the abnormal thyroid‐stimulating hormone testing, although it may occur in the setting of normal thyroid function. The diagnosis can be confirmed with thyroperoxidase and thyroglobulin antibody testing.
Three days into the hospitalization, her mental status had gradually improved such that she was more consistently awake and oriented to person and place, and she was transferred to a regular nursing unit. Final results from the CSF and blood cultures were negative, as was PCR for HSV. The antimicrobials were discontinued. Routine serum chemistries continued to be unremarkable. Additional studies recommended by the neurologist demonstrated an antithyroperoxidase antibody concentration of 587.1 IU/mL (normal, <5), and antithyroglobulin antibody level of 52.2 IU/mL (normal, <10).
These results confirm the diagnosis of HE which, in addition to its presentation as a recurrent illness, is an important treatable cause of dementia and should be considered in young patients, those with autoimmune and thyroid disorders, and those whose dementia is rapidly progressive. Most cases are thought to be steroid‐responsive, but some studies have defined the disorder based on this responsiveness, resulting in some nonresponders likely being overlooked.
A trial of corticosteroids should be considered if the patient does not quickly return to baseline given the potential morbidities associated with prolonged altered mental status to this degree. Whether initiation of chronic immunosuppression could prevent these attacks in the future is unclear from the literature but should be considered given the recurrent, dramatic presentation in this patient.
A diagnosis of HE was made, and she was prescribed corticosteroids. Twenty‐four hours later, she was alert and fully‐oriented. She was discharged to home on prednisone and seen in follow‐up in neurology clinic 1 month later. She had had no further episodes of confusion or stupor, but because of steroid‐induced hyperglycemia, her corticosteroids were decreased and mycophenolate mofetil added for chronic immunosuppression. Four months after discharge she was neurologically stable but continued to struggle with the adverse effects of chronic corticosteroid treatment.
COMMENTARY
HE is an uncommon condition that can present with a rapidly progressive decline and should be considered in patients who present with recurrent mental status change in the setting of normal imaging studies and routine laboratory results. The entity was initially described by Lord William Russell Brain in 1966, and in the most recent terminology is known as steroid‐responsive encephalopathy associated with autoimmune thyroiditis (SREAT).1 It is characterized by an acute or subacute encephalopathy associated with thyroid autoimmunity. Patients typically present with fluctuating symptoms, episodes of confusion, alterations of consciousness, and rapid cognitive decline.2 Common features include myoclonus, tremor, ataxia, speech disturbance, stroke‐like episodes, increased muscle tone, neuropsychiatric manifestations, and seizures, that in some cases may progress to status epilepticus.3, 4
Although serum antithyroglobulin and antithyroperoxidase antibodies are elevated in HE, their presence is thought to be an epiphenomenon of the condition rather than the direct cause. Supporting this are the facts that the incidence of encephalopathy is not increased in patients with established autoimmune thyroiditis, and the presence of antithyroid antibodies ranges from 5% to 20% in the general population.2, 5 There is also no evidence that thyroid antibodies directly react with brain tissue, and the levels of these antibodies do not correlate with either neurologic manifestations or clinical improvement.2, 4, 5 As HE has been reported in patients with euthyroidism, hypothyroidism, and hyperthyroidism (with hypothyroidismeither subclinical or activemost common), it is also unlikely that the level of thyroid hormones play a role in the etiology of this disease.2, 4, 6
The etiology and pathogenesis of HE are unclear, although an immune‐mediated process is generally implicated, either from an inflammatory vasculitis or as a form of acute disseminated encephalomyelitis.7‐9 Global hypoperfusion on single‐photon emission computed tomography (SPECT) studies has also been reported.10, 11 Patients with HE may have nonspecific evidence of inflammation, including an elevated ESR, CRP, and CSF protein.12 Other laboratory abnormalities may include a mild elevation of liver aminotransferase levels; renal impairment has also been reported in a few cases of HE in the form of glomerulonephritis, and may be related to deposition of immune complexes containing thyroglobulin antigen.6, 12‐14 MRI of the brain is normal or nonspecific in most cases, and the EEG most commonly shows diffuse slowing.
The differential for a rapidly progressive cognitive decline includes CJD, CNS vasculitis, paraneoplastic syndromes, and autoimmune and subacute infectious encephalopathies. In patients with CJD, T2‐weighted imaging may show hyperintense signals in the basal ganglia, while diffusion‐weighted sequences may reveal changes in the cortical ribbon and bilateral thalami.15 In CNS vasculitis, the imaging findings are variable and range from discrete areas of vascular infarcts to hemorrhagic lesions.16 In paraneoplastic and autoimmune encephalopathies (excluding HE), MRI often shows nonenhancing signal intensity changes in the mesial temporal lobes.12 This patient had repeatedly normal MRI studies of the brain, which in combination with the history of tremor, myoclonus, seizures, and interval return to baseline status, helped point to the diagnosis of HE.
Different approaches to treatment of HE have been recommended. As the acronym SREAT suggests, patients typically respond dramatically to high‐dose steroid therapy. Although a number of patients also improve spontaneously, up to 60% of patients experience a relapsing course and require chronic immunosuppressive agents for maintenance therapy, including long‐term steroids and azathioprine.2, 17 Treatment with plasma exchange and intravenous immune globulin have also been reported, but with mixed results.18, 19 Due to her history of multiple relapses, the patient was placed on mycophenolate mofetil for additional maintenance immunosuppression, as her corticosteroid dose was reduced due to adverse effects.
Acute mental status change is a potentially emergent situation that must be evaluated with careful history and studies to exclude life‐threatening metabolic, infectious, and vascular conditions. This patient presented similarly on 2 prior occasions, and each time her physician team evaluated what appeared to be a new onset of altered consciousness, reaching a plausible but ultimately incorrect diagnosis. The patient's third presentation was finally the charm, as her physicians learned of the repeated history of a confusional state, and in particular the return to baseline status, allowing them to create a differential that focused on etiologies of relapsing encephalopathy and make the correct diagnosis.
Key Points
-
Recurrent acute or subacute cognitive deterioration invokes a differential diagnosis of toxic/metabolic disorders and unusual inflammatory conditions.
-
The nonvasculitic autoimmune encephalopathies are a group of uncommon conditions characterized by nonspecific findings of inflammation and generally unremarkable CNS imaging studies.
-
HE, or SREAT, is the most common of these conditions, and is notable for mental status changes, various findings of increased muscular tone, thyroid autoimmunity, and generally a dramatic response to corticosteroids.
- , , .Hashimoto's disease and encephalopathy.Lancet.1966;2:512–514.
- , , .Hashimoto encephalopathy: syndrome or myth?Arch Neurol.2003;60:164–171.
- , , .Pisani F. Recurrent status epilepticus as the main feature of Hashimoto's encephalopathy.Epilepsy Behav.2006;8:328–330.
- , , , et al.Steroid‐responsive encephalopathy associated with autoimmune thyroiditis.Arch Neurol.2006;63:197–202.
- , , , , .Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment.J Neurol.1996;243:585–593.
- , , , , .Hashimoto's encephalopathy: a steroid‐responsive disorder associated with high anti‐thyroid antibody titers‐report of 5 cases.Neurology.1991;41:228–233.
- , , , , .Hashimoto encephalopathy: a brainstem vasculitis?Neurology.2000;54:769–770.
- , , , , .Nonvasculitic autoimmune inflammatory meningoencephalitis (NAIM): A reversible form of encephalopathy.Neurology.1999;53:1579–1581.
- , , , .Hashimoto's encephalopathy: postmortem findings after fatal status epilepticus.Neurology.2003;61:1124–1126.
- , , .Autoimmune thyroiditis and a rapidly progressive dementia: global hypoperfusion on SPECT scanning suggests a possible mechanism.Neurology.1997;49:623–626.
- , , , , .Hashimoto's encephalopathy: clinical, SPECT and neurophysiologic data.QJM.2003;96:455–457.
- , , .Autoimmune Encephalopathies.The Neurologist.2007;13:140–147.
- , , , , , .Thyroid antigen‐antibody nephritis.Clin Immunol Immunopathol1976;6:341–346.
- , , .Immune complex glomerulonephritis mediated by thyroid antigens.Arch Pathol Lab Med1978;102:530–533.
- , , , et al.Diffusion‐weighted MR imaging of early‐stage Creutzfeldt‐Jakob disease: typical and atypical manifestations.Radiographics.2006;26:S191–S204.
- , , , , .CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography.AJNR Am J Neuroradiol.1999;20:75–85.
- , .Long‐Term Treatment of Hashimoto's Encephalopathy.J Neuropsychiatry Clin Neurosci.2006;18:14–20.
- , .Hashimoto's encephalopathy: steroid resistance and response to intravenouc immunoglobulins.J Neurol Neurosurg Psychiatry.2005;76:455–456.
- , .Hashimoto's encephalopathy responding to plasmapheresis.J Neurol Neurosurg Psychiatry.2001;70:132.
- , , .Hashimoto's disease and encephalopathy.Lancet.1966;2:512–514.
- , , .Hashimoto encephalopathy: syndrome or myth?Arch Neurol.2003;60:164–171.
- , , .Pisani F. Recurrent status epilepticus as the main feature of Hashimoto's encephalopathy.Epilepsy Behav.2006;8:328–330.
- , , , et al.Steroid‐responsive encephalopathy associated with autoimmune thyroiditis.Arch Neurol.2006;63:197–202.
- , , , , .Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment.J Neurol.1996;243:585–593.
- , , , , .Hashimoto's encephalopathy: a steroid‐responsive disorder associated with high anti‐thyroid antibody titers‐report of 5 cases.Neurology.1991;41:228–233.
- , , , , .Hashimoto encephalopathy: a brainstem vasculitis?Neurology.2000;54:769–770.
- , , , , .Nonvasculitic autoimmune inflammatory meningoencephalitis (NAIM): A reversible form of encephalopathy.Neurology.1999;53:1579–1581.
- , , , .Hashimoto's encephalopathy: postmortem findings after fatal status epilepticus.Neurology.2003;61:1124–1126.
- , , .Autoimmune thyroiditis and a rapidly progressive dementia: global hypoperfusion on SPECT scanning suggests a possible mechanism.Neurology.1997;49:623–626.
- , , , , .Hashimoto's encephalopathy: clinical, SPECT and neurophysiologic data.QJM.2003;96:455–457.
- , , .Autoimmune Encephalopathies.The Neurologist.2007;13:140–147.
- , , , , , .Thyroid antigen‐antibody nephritis.Clin Immunol Immunopathol1976;6:341–346.
- , , .Immune complex glomerulonephritis mediated by thyroid antigens.Arch Pathol Lab Med1978;102:530–533.
- , , , et al.Diffusion‐weighted MR imaging of early‐stage Creutzfeldt‐Jakob disease: typical and atypical manifestations.Radiographics.2006;26:S191–S204.
- , , , , .CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography.AJNR Am J Neuroradiol.1999;20:75–85.
- , .Long‐Term Treatment of Hashimoto's Encephalopathy.J Neuropsychiatry Clin Neurosci.2006;18:14–20.
- , .Hashimoto's encephalopathy: steroid resistance and response to intravenouc immunoglobulins.J Neurol Neurosurg Psychiatry.2005;76:455–456.
- , .Hashimoto's encephalopathy responding to plasmapheresis.J Neurol Neurosurg Psychiatry.2001;70:132.