User login
Curriculum Vitae 101
By now, if you’re a final-year resident, you should be thinking about your plans for when you finish your residency. Before you begin the job search in earnest, it’s a good idea to create or update your curriculum vitae, or CV. You might be thinking, “That’s easy. I haven’t done anything yet!” That might be the case, but in reality, you probably have done more than you realize.
Whether you are just starting out or need to freshen a rough draft, here are some recommendations for creating a CV.
Brainstorm
The first step is to capture all the things you have done. Start by taking a sheet of paper and making columns with the following headings: licensure/documents, honors and awards, presentations/publications, research activities, committees, teaching, community service, and special skills. List each of the things you’ve done in each category.
Don’t be modest. You have to sell yourself. No item is too small for consideration for your CV at this stage. Get together with other people in your residency class and brainstorm together. They might help you think of certain activities that you have not already thought about. Here are some key points to keep in mind as you brainstorm each section:
- Licensure/documents: If you haven’t obtained a license in the state where you want to practice, now is the time to do it. Make sure advanced cardiac life support (ACLS) and BLS are current. If you haven’t taken your board exam, mention that you are board-eligible and include the date you plan to take the exam.
- Honors and awards: You don’t have to receive a trophy at a fancy awards ceremony to fill out this section. Did you ever receive a letter from the department chair, program director, or clerkship director giving you a special commendation? Such recognition might be worth a mention.
- Presentations and publications: If you have been published, include the citation here. Many residents present posters at regional meetings; this information should go in your CV. Have you given a presentation for “Morning Report” or a “Morbidity and Mortality Conference”? If so, these count as presentations, too. Many residents have written Web-based materials. Cite these as well.
- Teaching: Consider all the activities you perform for medical students. Have you given the students any prepared lectures? Have you been a preceptor for their physical exam labs? Have you provided mentorship for a student? Significant time spent mentoring also should be reflected on a resident’s CV.
- Research: QI projects generally count as HM research projects.
- Committees: Think about all the meetings you’ve attended and determine if any of them count as providing services to the residency or hospital.
- Special skills: Proficiency in thoracocentesis or lumbar puncture procedures qualifies for this section. If you speak a second (or third, fourth, etc.) language, include it here.
Rough Draft = First Attempt
Now that you have gathered your information, it’s time to organize it. Web-based resources and templates are plentiful, and many can help you write the CV. If you are applying for an academic position, you will need to keep a detailed CV. If you are not applying for an academic position, it is best to keep your CV at no more than two pages in length; however, you might want to keep a comprehensive (and lengthier) version on file.
Maintain a Career Folder
Once you’ve created your first CV, you will need to develop a system to update and maintain the document. The easiest way to do this is to keep a “career folder” on your desktop or in a filing cabinet. This will help you catalog all the extra things you’ve done throughout your career.
Write notes to yourself, with the date and time spent on certain activities. Then, at regular intervals, document them on your CV. It’s best to update your CV every six months.
The career file also can be used to keep evaluations, letters from patients, or anything else that exemplifies your accomplishments at work. Having a system for organizing your achievements will help you negotiate a raise and assist with future promotions or tenure.
Cover Letter
A cover letter should be no more than three to four paragraphs in length. Keep it simple and to the point. Briefly state how you heard about the job opening and why you are interested in the job.
Take a paragraph to identify the skills and experience you have to offer the HM group. The final paragraph should be used to explain how you intend to follow up and the best way you can be reached (phone, e-mail, etc.) to arrange an interview.
Interview Tips
A well-written CV can lead to several interview offers. Here are some important tips to help you obtain that all-important job offer:
- Have a clear vision. It’s important to know what you are looking for. Having clear goals will help you know exactly the kind of job you want and avoid wasting time and energy.
- Set aside time for a phone interview. You can learn a lot about an HM program during this time; give the interviewer a chance to learn about you, too. Use this step to screen out those places you really want to visit in person.
- Show up on time. Give yourself enough time to reach your destination, park, and find the meeting location. If possible, take a test drive a day or two before.
- Remember, your appearance matters. Dress professionally in conservative business attire. Furthermore, always act professional. Avoid negative talk about past attendings or employers. If you are going out for lunch, avoid ordering alcohol.
- Write down questions to ask. This will give you more clarity and ensure that all of your questions regarding the prospective job are answered.
- Show interest in the program. Ask appropriate questions, even if you have all the information you need. Don’t leave without asking about the next steps in the hiring process.
- Talk about money last. Contrary to popular belief, it’s OK to bring up the topic of money during an interview. Just don’t make it your first—and only—question.
- Check out the town. Bring your spouse or partner to explore a prospective relocation site. Look into housing, schools, your potential commute, and recreational activities. TH
Dr. Garcia is assistant professor in the division of hospital medicine at the University of Texas Health Sciences Center at San Antonio. Dr. Patel is a hospitalist at HPMG Regions Hospital and assistant professor of medicine at the University of Minnesota in St. Paul.
Image Source: CHAGIN/ISTOCKPHOTO.COM
By now, if you’re a final-year resident, you should be thinking about your plans for when you finish your residency. Before you begin the job search in earnest, it’s a good idea to create or update your curriculum vitae, or CV. You might be thinking, “That’s easy. I haven’t done anything yet!” That might be the case, but in reality, you probably have done more than you realize.
Whether you are just starting out or need to freshen a rough draft, here are some recommendations for creating a CV.
Brainstorm
The first step is to capture all the things you have done. Start by taking a sheet of paper and making columns with the following headings: licensure/documents, honors and awards, presentations/publications, research activities, committees, teaching, community service, and special skills. List each of the things you’ve done in each category.
Don’t be modest. You have to sell yourself. No item is too small for consideration for your CV at this stage. Get together with other people in your residency class and brainstorm together. They might help you think of certain activities that you have not already thought about. Here are some key points to keep in mind as you brainstorm each section:
- Licensure/documents: If you haven’t obtained a license in the state where you want to practice, now is the time to do it. Make sure advanced cardiac life support (ACLS) and BLS are current. If you haven’t taken your board exam, mention that you are board-eligible and include the date you plan to take the exam.
- Honors and awards: You don’t have to receive a trophy at a fancy awards ceremony to fill out this section. Did you ever receive a letter from the department chair, program director, or clerkship director giving you a special commendation? Such recognition might be worth a mention.
- Presentations and publications: If you have been published, include the citation here. Many residents present posters at regional meetings; this information should go in your CV. Have you given a presentation for “Morning Report” or a “Morbidity and Mortality Conference”? If so, these count as presentations, too. Many residents have written Web-based materials. Cite these as well.
- Teaching: Consider all the activities you perform for medical students. Have you given the students any prepared lectures? Have you been a preceptor for their physical exam labs? Have you provided mentorship for a student? Significant time spent mentoring also should be reflected on a resident’s CV.
- Research: QI projects generally count as HM research projects.
- Committees: Think about all the meetings you’ve attended and determine if any of them count as providing services to the residency or hospital.
- Special skills: Proficiency in thoracocentesis or lumbar puncture procedures qualifies for this section. If you speak a second (or third, fourth, etc.) language, include it here.
Rough Draft = First Attempt
Now that you have gathered your information, it’s time to organize it. Web-based resources and templates are plentiful, and many can help you write the CV. If you are applying for an academic position, you will need to keep a detailed CV. If you are not applying for an academic position, it is best to keep your CV at no more than two pages in length; however, you might want to keep a comprehensive (and lengthier) version on file.
Maintain a Career Folder
Once you’ve created your first CV, you will need to develop a system to update and maintain the document. The easiest way to do this is to keep a “career folder” on your desktop or in a filing cabinet. This will help you catalog all the extra things you’ve done throughout your career.
Write notes to yourself, with the date and time spent on certain activities. Then, at regular intervals, document them on your CV. It’s best to update your CV every six months.
The career file also can be used to keep evaluations, letters from patients, or anything else that exemplifies your accomplishments at work. Having a system for organizing your achievements will help you negotiate a raise and assist with future promotions or tenure.
Cover Letter
A cover letter should be no more than three to four paragraphs in length. Keep it simple and to the point. Briefly state how you heard about the job opening and why you are interested in the job.
Take a paragraph to identify the skills and experience you have to offer the HM group. The final paragraph should be used to explain how you intend to follow up and the best way you can be reached (phone, e-mail, etc.) to arrange an interview.
Interview Tips
A well-written CV can lead to several interview offers. Here are some important tips to help you obtain that all-important job offer:
- Have a clear vision. It’s important to know what you are looking for. Having clear goals will help you know exactly the kind of job you want and avoid wasting time and energy.
- Set aside time for a phone interview. You can learn a lot about an HM program during this time; give the interviewer a chance to learn about you, too. Use this step to screen out those places you really want to visit in person.
- Show up on time. Give yourself enough time to reach your destination, park, and find the meeting location. If possible, take a test drive a day or two before.
- Remember, your appearance matters. Dress professionally in conservative business attire. Furthermore, always act professional. Avoid negative talk about past attendings or employers. If you are going out for lunch, avoid ordering alcohol.
- Write down questions to ask. This will give you more clarity and ensure that all of your questions regarding the prospective job are answered.
- Show interest in the program. Ask appropriate questions, even if you have all the information you need. Don’t leave without asking about the next steps in the hiring process.
- Talk about money last. Contrary to popular belief, it’s OK to bring up the topic of money during an interview. Just don’t make it your first—and only—question.
- Check out the town. Bring your spouse or partner to explore a prospective relocation site. Look into housing, schools, your potential commute, and recreational activities. TH
Dr. Garcia is assistant professor in the division of hospital medicine at the University of Texas Health Sciences Center at San Antonio. Dr. Patel is a hospitalist at HPMG Regions Hospital and assistant professor of medicine at the University of Minnesota in St. Paul.
Image Source: CHAGIN/ISTOCKPHOTO.COM
By now, if you’re a final-year resident, you should be thinking about your plans for when you finish your residency. Before you begin the job search in earnest, it’s a good idea to create or update your curriculum vitae, or CV. You might be thinking, “That’s easy. I haven’t done anything yet!” That might be the case, but in reality, you probably have done more than you realize.
Whether you are just starting out or need to freshen a rough draft, here are some recommendations for creating a CV.
Brainstorm
The first step is to capture all the things you have done. Start by taking a sheet of paper and making columns with the following headings: licensure/documents, honors and awards, presentations/publications, research activities, committees, teaching, community service, and special skills. List each of the things you’ve done in each category.
Don’t be modest. You have to sell yourself. No item is too small for consideration for your CV at this stage. Get together with other people in your residency class and brainstorm together. They might help you think of certain activities that you have not already thought about. Here are some key points to keep in mind as you brainstorm each section:
- Licensure/documents: If you haven’t obtained a license in the state where you want to practice, now is the time to do it. Make sure advanced cardiac life support (ACLS) and BLS are current. If you haven’t taken your board exam, mention that you are board-eligible and include the date you plan to take the exam.
- Honors and awards: You don’t have to receive a trophy at a fancy awards ceremony to fill out this section. Did you ever receive a letter from the department chair, program director, or clerkship director giving you a special commendation? Such recognition might be worth a mention.
- Presentations and publications: If you have been published, include the citation here. Many residents present posters at regional meetings; this information should go in your CV. Have you given a presentation for “Morning Report” or a “Morbidity and Mortality Conference”? If so, these count as presentations, too. Many residents have written Web-based materials. Cite these as well.
- Teaching: Consider all the activities you perform for medical students. Have you given the students any prepared lectures? Have you been a preceptor for their physical exam labs? Have you provided mentorship for a student? Significant time spent mentoring also should be reflected on a resident’s CV.
- Research: QI projects generally count as HM research projects.
- Committees: Think about all the meetings you’ve attended and determine if any of them count as providing services to the residency or hospital.
- Special skills: Proficiency in thoracocentesis or lumbar puncture procedures qualifies for this section. If you speak a second (or third, fourth, etc.) language, include it here.
Rough Draft = First Attempt
Now that you have gathered your information, it’s time to organize it. Web-based resources and templates are plentiful, and many can help you write the CV. If you are applying for an academic position, you will need to keep a detailed CV. If you are not applying for an academic position, it is best to keep your CV at no more than two pages in length; however, you might want to keep a comprehensive (and lengthier) version on file.
Maintain a Career Folder
Once you’ve created your first CV, you will need to develop a system to update and maintain the document. The easiest way to do this is to keep a “career folder” on your desktop or in a filing cabinet. This will help you catalog all the extra things you’ve done throughout your career.
Write notes to yourself, with the date and time spent on certain activities. Then, at regular intervals, document them on your CV. It’s best to update your CV every six months.
The career file also can be used to keep evaluations, letters from patients, or anything else that exemplifies your accomplishments at work. Having a system for organizing your achievements will help you negotiate a raise and assist with future promotions or tenure.
Cover Letter
A cover letter should be no more than three to four paragraphs in length. Keep it simple and to the point. Briefly state how you heard about the job opening and why you are interested in the job.
Take a paragraph to identify the skills and experience you have to offer the HM group. The final paragraph should be used to explain how you intend to follow up and the best way you can be reached (phone, e-mail, etc.) to arrange an interview.
Interview Tips
A well-written CV can lead to several interview offers. Here are some important tips to help you obtain that all-important job offer:
- Have a clear vision. It’s important to know what you are looking for. Having clear goals will help you know exactly the kind of job you want and avoid wasting time and energy.
- Set aside time for a phone interview. You can learn a lot about an HM program during this time; give the interviewer a chance to learn about you, too. Use this step to screen out those places you really want to visit in person.
- Show up on time. Give yourself enough time to reach your destination, park, and find the meeting location. If possible, take a test drive a day or two before.
- Remember, your appearance matters. Dress professionally in conservative business attire. Furthermore, always act professional. Avoid negative talk about past attendings or employers. If you are going out for lunch, avoid ordering alcohol.
- Write down questions to ask. This will give you more clarity and ensure that all of your questions regarding the prospective job are answered.
- Show interest in the program. Ask appropriate questions, even if you have all the information you need. Don’t leave without asking about the next steps in the hiring process.
- Talk about money last. Contrary to popular belief, it’s OK to bring up the topic of money during an interview. Just don’t make it your first—and only—question.
- Check out the town. Bring your spouse or partner to explore a prospective relocation site. Look into housing, schools, your potential commute, and recreational activities. TH
Dr. Garcia is assistant professor in the division of hospital medicine at the University of Texas Health Sciences Center at San Antonio. Dr. Patel is a hospitalist at HPMG Regions Hospital and assistant professor of medicine at the University of Minnesota in St. Paul.
Image Source: CHAGIN/ISTOCKPHOTO.COM
Market Watch
Discontinued Products
- Amoxicillin powder for oral suspension and pediatric drops for oral suspension1
- Amoxicillin powder for oral suspension (Amoxil brand usually for adults), 250mg/5mL (100mL and 150mL sizes)2
- Insulin isophane suspension (Humulin 50/50), due to limited use.3 Current patient demand and existing inventory note product availability through April 2010. There are about 3,000 patients in the U.S. who will be affected by this action.
- Phenytoin 30 mg (Dilantin Kapseals brand) are being reformulated in a new, extended-release formulation, but Kapseals will be discontinued.4
New Generics
- Tacrolimus (generic Prograf) capsules5
New Drugs, Indications, and Dosage Forms
- Asenapine tablets (Saphris) have been approved by the Food and Drug Administration (FDA) to treat adults with schizophrenia and bipolar I disorder. The most common adverse effects in trials were akathisia, oral hypoesthesia, and somnolence. The most common adverse effects that were reported in the bipolar disorder trials were somnolence, dizziness, movement disorders other than akathisia, and weight gain.6
- Colchicine 0.6 mg tablets (Colcrys) have been approved by the FDA to treat gout flares and familial Mediterranean fever.7 Colchicine has been used for many years but has not received FDA approval until recently. The FDA is re-evaluating some older drugs and drug classes. For example, the pancrelipase products fall under a similar ruling. Now that colchicine is approved, the manufacturer has shown that it meets modern standards for safety, effectiveness, quality, and labeling. Historically, physicians have administered colchicine hourly to treat acute gout flares until symptoms subsided or the patient developed adverse gastrointestinal symptoms. A dosing study determined that one 1.2-mg dose of this formulation followed by 0.6 mg one hour later was as effective as hourly dosing in patients without renal or hepatic dysfunction. This two-dose regimen was less toxic than prior dosing regimens and, therefore, it received the FDA’s approval.8
- Fentanyl buccal soluble film (Onsolis) has been approved by the FDA as an opioid for managing breakthrough cancer pain in patients 18 years and older who already are receiving and are tolerant to opioid therapy.9 It is available in 200-, 400-, 600-, 800- and 1,200-mcg strengths. A Risk Evaluation and Mitigation Strategies (REMS) will be available with dispensing.
- Insulin aspart injection (NovoLog) has undergone a label change. NovoLog can now be used in an insulin pump for up to six days. The infusion set should be changed at least every three days. The updated label includes information about discarding the drug if temperatures exceed 37oC (98.6oF).10
- Interferon beta-1b injection (Extavia): A new brand of interferon has been approved by the FDA for treating relapsing forms of multiple sclerosis (MS), as well as for patients who have experienced a first clinical episode of MS with magnetic resonance imaging (MRI) features consistent of the disease.11
- Morphine/naltrexone capsules (Embeda), a long-acting opioid designed to reduce drug euphoria, have been approved by the FDA to treat moderate to severe chronic pain. It was developed with the abuse-deterrent drug naltrexone, which reduces euphoria when crushed or chewed.12
- Pitavastatin 4 mg (Livalo) has been approved by the FDA to treat hypercholesterolemia and combined dyslipidemia.13 It’s a potent statin with a new base structure. Additionally, it is only minimally metabolized by the cytochrome P450 (CYP) pathway. It will be available in early 2010 in 1-, 2- and 4-mg strengths. Only time will tell whether this is truly a benefit for this new agent.
- Saxagliptin (Onglyza), a new oral dipeptidyl peptidase-4 (DPP-4) inhibitor, has been approved by the FDA to treat Type 2 diabetes mellitus as an adjunct to diet and exercise.14 It is administered once daily at a starting dose of 2.5 mg or 5 mg, without regard to meal.15 The lower dose is recommended in patients with moderate to severe renal impairment or end-stage renal disease (CrCL < 50 mL/min). The lower dose (2.5 mg) also is recommended for patients taking strong CYP3A4/5 inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, or telithromycin). The most common adverse effects in clinical trials were respiratory tract infection, urinary tract infection, and headache.
Pipeline
- Roflumilast (Daxas), a phosphodiesterase 4 enzyme inhibitor, has been submitted to the FDA. It is a once-daily oral treatment for patients with symptomatic chronic obstructive pulmonary disease (COPD).16
- Tocilizumab (Actemra), an interleukin-6 receptor-inhibiting monoclonal antibody to treat rheumatoid arthritis (RA), has been approved for use in Europe. Its manufacturer has announced that the FDA has accepted its reapplication for treating moderate to severe RA. It is available in Japan for treating RA, juvenile idiopathic arthritis, and Castleman’s disease.17
- TZP-102, an investigational oral prokinetic agent for treating diabetic gastroparesis, has received FDA fast-track status. A multinational study is under way for this ghrelin receptor agonist.18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
Discontinued Products
- Amoxicillin powder for oral suspension and pediatric drops for oral suspension1
- Amoxicillin powder for oral suspension (Amoxil brand usually for adults), 250mg/5mL (100mL and 150mL sizes)2
- Insulin isophane suspension (Humulin 50/50), due to limited use.3 Current patient demand and existing inventory note product availability through April 2010. There are about 3,000 patients in the U.S. who will be affected by this action.
- Phenytoin 30 mg (Dilantin Kapseals brand) are being reformulated in a new, extended-release formulation, but Kapseals will be discontinued.4
New Generics
- Tacrolimus (generic Prograf) capsules5
New Drugs, Indications, and Dosage Forms
- Asenapine tablets (Saphris) have been approved by the Food and Drug Administration (FDA) to treat adults with schizophrenia and bipolar I disorder. The most common adverse effects in trials were akathisia, oral hypoesthesia, and somnolence. The most common adverse effects that were reported in the bipolar disorder trials were somnolence, dizziness, movement disorders other than akathisia, and weight gain.6
- Colchicine 0.6 mg tablets (Colcrys) have been approved by the FDA to treat gout flares and familial Mediterranean fever.7 Colchicine has been used for many years but has not received FDA approval until recently. The FDA is re-evaluating some older drugs and drug classes. For example, the pancrelipase products fall under a similar ruling. Now that colchicine is approved, the manufacturer has shown that it meets modern standards for safety, effectiveness, quality, and labeling. Historically, physicians have administered colchicine hourly to treat acute gout flares until symptoms subsided or the patient developed adverse gastrointestinal symptoms. A dosing study determined that one 1.2-mg dose of this formulation followed by 0.6 mg one hour later was as effective as hourly dosing in patients without renal or hepatic dysfunction. This two-dose regimen was less toxic than prior dosing regimens and, therefore, it received the FDA’s approval.8
- Fentanyl buccal soluble film (Onsolis) has been approved by the FDA as an opioid for managing breakthrough cancer pain in patients 18 years and older who already are receiving and are tolerant to opioid therapy.9 It is available in 200-, 400-, 600-, 800- and 1,200-mcg strengths. A Risk Evaluation and Mitigation Strategies (REMS) will be available with dispensing.
- Insulin aspart injection (NovoLog) has undergone a label change. NovoLog can now be used in an insulin pump for up to six days. The infusion set should be changed at least every three days. The updated label includes information about discarding the drug if temperatures exceed 37oC (98.6oF).10
- Interferon beta-1b injection (Extavia): A new brand of interferon has been approved by the FDA for treating relapsing forms of multiple sclerosis (MS), as well as for patients who have experienced a first clinical episode of MS with magnetic resonance imaging (MRI) features consistent of the disease.11
- Morphine/naltrexone capsules (Embeda), a long-acting opioid designed to reduce drug euphoria, have been approved by the FDA to treat moderate to severe chronic pain. It was developed with the abuse-deterrent drug naltrexone, which reduces euphoria when crushed or chewed.12
- Pitavastatin 4 mg (Livalo) has been approved by the FDA to treat hypercholesterolemia and combined dyslipidemia.13 It’s a potent statin with a new base structure. Additionally, it is only minimally metabolized by the cytochrome P450 (CYP) pathway. It will be available in early 2010 in 1-, 2- and 4-mg strengths. Only time will tell whether this is truly a benefit for this new agent.
- Saxagliptin (Onglyza), a new oral dipeptidyl peptidase-4 (DPP-4) inhibitor, has been approved by the FDA to treat Type 2 diabetes mellitus as an adjunct to diet and exercise.14 It is administered once daily at a starting dose of 2.5 mg or 5 mg, without regard to meal.15 The lower dose is recommended in patients with moderate to severe renal impairment or end-stage renal disease (CrCL < 50 mL/min). The lower dose (2.5 mg) also is recommended for patients taking strong CYP3A4/5 inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, or telithromycin). The most common adverse effects in clinical trials were respiratory tract infection, urinary tract infection, and headache.
Pipeline
- Roflumilast (Daxas), a phosphodiesterase 4 enzyme inhibitor, has been submitted to the FDA. It is a once-daily oral treatment for patients with symptomatic chronic obstructive pulmonary disease (COPD).16
- Tocilizumab (Actemra), an interleukin-6 receptor-inhibiting monoclonal antibody to treat rheumatoid arthritis (RA), has been approved for use in Europe. Its manufacturer has announced that the FDA has accepted its reapplication for treating moderate to severe RA. It is available in Japan for treating RA, juvenile idiopathic arthritis, and Castleman’s disease.17
- TZP-102, an investigational oral prokinetic agent for treating diabetic gastroparesis, has received FDA fast-track status. A multinational study is under way for this ghrelin receptor agonist.18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
Discontinued Products
- Amoxicillin powder for oral suspension and pediatric drops for oral suspension1
- Amoxicillin powder for oral suspension (Amoxil brand usually for adults), 250mg/5mL (100mL and 150mL sizes)2
- Insulin isophane suspension (Humulin 50/50), due to limited use.3 Current patient demand and existing inventory note product availability through April 2010. There are about 3,000 patients in the U.S. who will be affected by this action.
- Phenytoin 30 mg (Dilantin Kapseals brand) are being reformulated in a new, extended-release formulation, but Kapseals will be discontinued.4
New Generics
- Tacrolimus (generic Prograf) capsules5
New Drugs, Indications, and Dosage Forms
- Asenapine tablets (Saphris) have been approved by the Food and Drug Administration (FDA) to treat adults with schizophrenia and bipolar I disorder. The most common adverse effects in trials were akathisia, oral hypoesthesia, and somnolence. The most common adverse effects that were reported in the bipolar disorder trials were somnolence, dizziness, movement disorders other than akathisia, and weight gain.6
- Colchicine 0.6 mg tablets (Colcrys) have been approved by the FDA to treat gout flares and familial Mediterranean fever.7 Colchicine has been used for many years but has not received FDA approval until recently. The FDA is re-evaluating some older drugs and drug classes. For example, the pancrelipase products fall under a similar ruling. Now that colchicine is approved, the manufacturer has shown that it meets modern standards for safety, effectiveness, quality, and labeling. Historically, physicians have administered colchicine hourly to treat acute gout flares until symptoms subsided or the patient developed adverse gastrointestinal symptoms. A dosing study determined that one 1.2-mg dose of this formulation followed by 0.6 mg one hour later was as effective as hourly dosing in patients without renal or hepatic dysfunction. This two-dose regimen was less toxic than prior dosing regimens and, therefore, it received the FDA’s approval.8
- Fentanyl buccal soluble film (Onsolis) has been approved by the FDA as an opioid for managing breakthrough cancer pain in patients 18 years and older who already are receiving and are tolerant to opioid therapy.9 It is available in 200-, 400-, 600-, 800- and 1,200-mcg strengths. A Risk Evaluation and Mitigation Strategies (REMS) will be available with dispensing.
- Insulin aspart injection (NovoLog) has undergone a label change. NovoLog can now be used in an insulin pump for up to six days. The infusion set should be changed at least every three days. The updated label includes information about discarding the drug if temperatures exceed 37oC (98.6oF).10
- Interferon beta-1b injection (Extavia): A new brand of interferon has been approved by the FDA for treating relapsing forms of multiple sclerosis (MS), as well as for patients who have experienced a first clinical episode of MS with magnetic resonance imaging (MRI) features consistent of the disease.11
- Morphine/naltrexone capsules (Embeda), a long-acting opioid designed to reduce drug euphoria, have been approved by the FDA to treat moderate to severe chronic pain. It was developed with the abuse-deterrent drug naltrexone, which reduces euphoria when crushed or chewed.12
- Pitavastatin 4 mg (Livalo) has been approved by the FDA to treat hypercholesterolemia and combined dyslipidemia.13 It’s a potent statin with a new base structure. Additionally, it is only minimally metabolized by the cytochrome P450 (CYP) pathway. It will be available in early 2010 in 1-, 2- and 4-mg strengths. Only time will tell whether this is truly a benefit for this new agent.
- Saxagliptin (Onglyza), a new oral dipeptidyl peptidase-4 (DPP-4) inhibitor, has been approved by the FDA to treat Type 2 diabetes mellitus as an adjunct to diet and exercise.14 It is administered once daily at a starting dose of 2.5 mg or 5 mg, without regard to meal.15 The lower dose is recommended in patients with moderate to severe renal impairment or end-stage renal disease (CrCL < 50 mL/min). The lower dose (2.5 mg) also is recommended for patients taking strong CYP3A4/5 inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, or telithromycin). The most common adverse effects in clinical trials were respiratory tract infection, urinary tract infection, and headache.
Pipeline
- Roflumilast (Daxas), a phosphodiesterase 4 enzyme inhibitor, has been submitted to the FDA. It is a once-daily oral treatment for patients with symptomatic chronic obstructive pulmonary disease (COPD).16
- Tocilizumab (Actemra), an interleukin-6 receptor-inhibiting monoclonal antibody to treat rheumatoid arthritis (RA), has been approved for use in Europe. Its manufacturer has announced that the FDA has accepted its reapplication for treating moderate to severe RA. It is available in Japan for treating RA, juvenile idiopathic arthritis, and Castleman’s disease.17
- TZP-102, an investigational oral prokinetic agent for treating diabetic gastroparesis, has received FDA fast-track status. A multinational study is under way for this ghrelin receptor agonist.18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
What is the appropriate use of chronic medications in the perioperative setting?
Case
A 72-year-old female with multiple medical problems is admitted with a hip fracture. Surgery is scheduled in 48 hours. The patient’s home medications include aspirin, carbidopa/levodopa, celecoxib, clonidine, estradiol, ginkgo, lisinopril, NPH insulin, sulfasalazine, and prednisone 10 mg a day, which she has been taking for years. How should these and other medications be managed in the perioperative period?
Background
Perioperative management of chronic medications is a complex issue, as physicians are required to balance the beneficial and harmful effects of the individual drugs prescribed to their patients. On one hand, cessation of medications can result in decompensation of disease or withdrawal. On the other hand, continuation of drugs can alter metabolism of anesthetic agents, cause perioperative hemodynamic instability, or result in such post-operative complications as acute renal failure, bleeding, infection, and impaired wound healing.
Certain traits make it reasonable to continue medications during the perioperative period. A long elimination half-life or duration of action makes stopping some medications impractical as it takes four to five half-lives to completely clear the drug from the body; holding the drug for a few days around surgery will not appreciably affect its concentration. Stopping drugs that carry severe withdrawal symptoms can be impractical because of the need for lengthy tapers, which can delay surgery and result in decompensation of underlying disease.
Drugs with no significant interactions with anesthesia or risk of perioperative complications should be continued in order to avoid deterioration of the underlying disease. Conversely, drugs that interact with anesthesia or increase risk for complications should be stopped if this can be accomplished safely. Patient-specific factors should receive consideration, as the risk of complications has to be balanced against the danger of exacerbating the underlying disease.
Overview of the Data
The challenge in providing recommendations on perioperative medication management lies in a dearth of high-quality clinical trials. Thus, much of the information comes from case reports, expert opinion, and sound application of pharmacology.
Antiplatelet therapy: Nuances of perioperative antiplatelet therapy are beyond the scope of this review, but some general principles can be elucidated from the American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines.1 Management of antiplatelet therapy should be done in conjunction with the surgical team, as cardiovascular risk has to be weighed against bleeding risk.
Aspirin therapy should be continued in all patients with a history of coronary artery disease (CAD), balloon angioplasty, or percutaneous coronary intervention (PCI), unless the risk of bleeding complications is felt to exceed the cardioprotective benefits—for example, in some neurosurgical patients.1
Clopidogrel therapy is crucial for prevention of in-stent thrombosis (IST) following PCI because patients who experience IST suffer catastrophic myocardial infarctions with high mortality. Ideally, surgery should be delayed to permit completion of clopidogrel therapy—30 to 45 days after implantation of a bare-metal stent and 365 days after a drug-eluting stent. If surgery has to be performed sooner, guidelines recommend operating on dual antiplatelet therapy with aspirin and clopidogrel.1 Again, this course of treatment has to be balanced against the risk of hemorrhagic complications from surgery.
Both aspirin and clopidogrel irreversibly inhibit platelet aggregation. The recovery of normal coagulation involves formation of new platelets, which necessitates cessation of therapy for seven to 10 days before surgery. Platelet inhibition begins within minutes of restarting aspirin and within hours of taking clopidogrel, although attaining peak clopidogrel effect takes three to seven days, unless a loading dose is used.
Cardiovascular Drugs
Beta-blockers in the perioperative setting are a focus of an ongoing debate beyond the scope of this review (see “What Pre-Operative Cardiac Evaluation of Patients Undergoing Intermediate-Risk Surgery Is Most Effective?,” February 2008, p. 26). Given the current evidence and the latest ACC/AHA guidelines, it is still reasonable to continue therapy in patients who are already taking them to avoid precipitating cardiovascular events by withdrawal. Patients with increased cardiac risk, demonstrated by a Revised Cardiac Risk Index (RCRI) score of ≥2 (see Table 1, p. 12), should be considered for beta-blocker therapy before surgery.1 In either case, the dose should be titrated to a heart rate <65 for optimal cardiac protection.1
Statins should be continued if the patient is taking them, especially because preoperative withdrawal has been associated with a 4.6-fold increase in troponin release and a 7.5-fold increased risk of myocardial infarction (MI) and cardiovascular death following major vascular surgery.2 Patients with increased cardiac risk— RCRI ≥1—can be considered for initiation of statin therapy before surgery, although the benefit of this intervention has not been examined in prospective studies.1
Amiodarone has an exceptionally long half-life of up to 142 days. It should be continued in the perioperative period.
Calcium channel blockers (CCBs) can be continued with no unpleasant perioperative hemodynamic effects.1 CCBs have potential cardioprotective benefits.
Clonidine withdrawal can result in severe rebound hypertension with reports of encephalopathy, stroke, and death. These effects are exacerbated by concomitant beta-blocker therapy. For this reason, if a patient is expected to be NPO for more than 12 hours, they should be converted to a clonidine patch 48-72 hours before surgery with concurrent tapering of the oral dose.3
Digoxin has a long half-life (up to 48 hours) and should be continued with monitoring of levels if there is a change in renal function.
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARBs) have been associated with a 50% increased risk of hypotension requiring vasopressors during induction of anesthesia.4 However, it is worth mentioning that this finding has not been corroborated in other studies. A large retrospective cohort of cardiothoracic surgical patients found a 28% increased risk of post-operative acute renal failure (ARF) with both drug classes, although another cardiothoracic report published the same year demonstrated a 50% reduction in risk with ACEIs.5,6 Although the evidence of harm is not unequivocal, perioperative blood-pressure control can be achieved with other drugs without hemodynamic or renal risk, such as CCBs, and in most cases ACEIs/ARBs should be stopped one day before surgery.
Diuretics carry a risk of volume depletion and electrolyte derangements, and should be stopped once a patient becomes NPO. Excess volume is managed with as-needed intravenous formulations.
Drugs Acting on the Central Nervous System
The majority of central nervous system (CNS)-active drugs, including antiepileptics, antipsychotics, benzodiazepines, bupropion, gabapentin, lithium, mirtazapine, selective serotonin and norepinephrine reuptake inhibitors (SSRIs and SNRIs), tricyclic antidepressants (TCAs), and valproic acid, balance a low risk of perioperative complications against a significant potential for withdrawal and disease decompensation. Therefore, these medications should be continued.
Carbidopa/Levodopa should be continued because abrupt cessation can precipitate systemic withdrawal resembling serotonin syndrome and rapid deterioration of Parkinson’s symptoms.
Monoamine oxidase inhibitor (MAOI) therapy usually indicates refractory psychiatric illness, so these drugs should be continued to avoid decompensation. Importantly, MAOI-safe anesthesia without dextromethorphan, meperidine, epinephrine, or norepinephrine has to be used due to the risk of cardiovascular instability.7
Diabetic Drugs
Insulin therapy should be continued with adjustments. Glargine basal insulin has no peak and can be continued without changing the dose. Likewise, patients with insulin pumps can continue the usual basal rate. Short-acting insulin or such insulin mixes as 70/30 should be stopped four hours before surgery to avoid hypoglycemia. Intermediate-acting insulin (e.g., NPH) can be administered at half the usual dose the day of surgery with a perioperative 5% dextrose infusion. NPH should not be given the day of surgery if the dextrose infusion cannot be used.8
Incretins (exenatide, sitagliptin) rarely cause hypoglycemia in the absence of insulin and may be beneficial in controlling intraoperative hyperglycemia. Therefore, these medications can be continued.8
Thiazolidinediones (TZDs; pioglitazone, rosiglitazone) alter gene transcription with biological duration of action on the order of weeks and low risk of hypoglycemia, and should be continued.
Metformin carries an FDA black-box warning to discontinue therapy before any intravascular radiocontrast studies or surgical procedures due to the risk of severe lactic acidosis if renal failure develops. It should be stopped 24 hours before surgery and restarted at least 48-72 hours after. Normal renal function should be confirmed before restarting therapy.8
Sulfonylureas (glimepiride, glipizide, glyburide) carry a significant risk of hypoglycemia in a patient who is NPO; they should be stopped the night before surgery or before commencement of NPO status.
Hormones
Antithyroid drugs (methimazole, propylthiouracil) and levothyroxine should be continued, as they have no perioperative contraindications.
Oral contraceptives (OCPs), hormone replacement therapy (HRT), and raloxifene can increase the risk of DVT. The largest study on the topic was the HERS trial of postmenopausal women on estrogen/progesterone HRT. The authors reported a 4.9-fold increased risk of DVT for 90 days after surgery.9 Unfortunately, no information was provided on the types of surgery, or whether appropriate and consistent DVT prophylaxis was utilized. HERS authors also reported a 2.5-fold increased risk of DVT for at least 30 days after cessation of HRT.9
Given the data, it is reasonable to stop hormone therapy four weeks before surgery when prolonged immobilization is anticipated and patients are able to tolerate hormone withdrawal, especially if other DVT risk factors are present. If hormone therapy cannot be stopped, strong consideration should be given to higher-intensity DVT prophylaxis (e.g., chemoprophylaxis as opposed to mechanical measures) of longer duration—up to 28 days following general surgery and up to 35 days after orthopedic procedures.10
Perioperative Corticosteroids
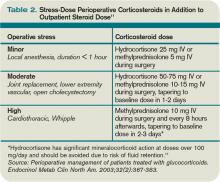
Corticosteroid therapy in excess of prednisone 5 mg/day or equivalent for more than five days in the 30 days preceding surgery might predispose patients to acute adrenal insufficiency in the perioperative period. Surgical procedures typically result in cortisol release of 50-150 mg/day, which returns to baseline within 48 hours.11 Therefore, the recommendation is to continue a patient’s baseline steroid dose and supplement it with stress-dose steroids tailored to the severity of operative stress (see Table 2, above).
Mineralocorticoid supplementation is not necessary, because endogenous production is not suppressed by corticosteroid therapy.11 Although a recent systematic review suggests that routine stress-dose steroids might not be indicated, high-quality prospective data are needed before abandoning this strategy due to complications of acute adrenal insufficiency compared to the risk of a brief corticosteroid burst.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Nonselective cyclooxygenase (COX) inhibitors reversibly decrease platelet aggregation only while the drug is present in the circulation and should be stopped one to three days before surgery due to risk of bleeding.
Selective COX-2 inhibitors do not significantly alter platelet aggregation and can be continued for opioid-sparing perioperative pain control.
Both COX-2-selective and nonselective inhibitors should be held if there are concerns for impaired renal function.
Disease-Modifying Antirheumatic Drugs (DMARDs) and Biological Response Modifiers (BRMs)
Methotrexate increases the risk of wound infections and dehiscence. However, this is offset by a decreased risk of post-operative disease flares with continued use. It can be continued unless the patient has medical comorbidities, advanced age, or chronic therapy with more than 10 mg/day of prednisone, in which case the drug should be stopped two weeks before surgery.12
Azathioprine, leflunomide, and sulfasalazine are renally cleared with a risk of myelosuppression; all of these medications should be stopped. Long half-life of leflunomide necessitates stopping it two weeks before surgery; azathioprine and sulfasalazine can be stopped one day in advance. The drugs can be restarted three days after surgery, assuming stable renal function.13
Anti-TNF-α (adalimumab, etanercept, infliximab), IL1 antagonist (anakinra), and anti-CD20 (rituximab) agents should be stopped one week before surgery and resumed 1-2 weeks afterward, unless risk of complications from disease flareup outweighs the concern for wound infections and dehiscence.14
Herbal Medicines
It is estimated that as much as a third of the U.S. population uses herbal medicines. These substances can result in perioperative hemodynamic instability (ephedra, ginseng, ma huang), hypoglycemia (ginseng), immunosuppression (echinacea, when taken for more than eight weeks), abnormal bleeding (garlic, ginkgo, ginseng), and prolongation of anesthesia (kava, St. John’s wort, valerian). All of these herbal medicine should be stopped one to two weeks before surgery.15,16
Back to the Case
The patient’s Carbidopa/Levodopa should be continued. Celecoxib can be continued if her renal function in stable. If aspirin is taken for a history of coronary artery disease or percutaneous coronary intervention, it should be continued, if possible. Clonidine should be continued or changed to a patch if an extended NPO period is anticipated. Ginkgo, lisinopril, and sulfasalazine should be stopped.
Hospitalization does not provide the luxury of stopping estradiol in advance, so it might be continued with chemical DVT prophylaxis for up to 35 days after surgery. The patient should receive 50-75 mg of IV hydrocortisone during surgery and an additional 25 mg the following day, in addition to her usual prednisone 10 mg/day. She can either receive half her usual NPH dose the morning of surgery with a 5% dextrose infusion in the operating room, or the NPH should be held altogether.
Bottom Line
Perioperative medication use should be tailored to each patient, balancing the risks and benefits of individual drugs. High-quality trials are needed to provide more robust clinical guidelines. TH
Dr. Levin is a hospitalist at the University of Colorado Denver.
References
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):1971-1996.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316-320.
- Spell NO III. Stopping and restarting medications in the perioperative period. Med Clin North Am. 2001;85(5):1117-1128.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325.
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3(5):1266-1273.
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative Angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg. 2008;86(4):1160-1165.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm. 2004;61(9):899-914.
- Kohl BA, Schwartz S. Surgery in the patient with endocrine dysfunction. Med Clin North Am. 2009;93(5):1031-1047.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132(9):689-696.
- Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ; American College of Chest Physicians. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):71S-109S.
- Axelrod L. Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin North Am. 2003;32(2):367-383.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg. 2008;143(12):1222-1226.
- Rosandich PA, Kelley JT III, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Curr Opin Rheumatol. 2004;16(3):192-198.
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286(2):208-216.
- Hodges PJ, Kam PC. The peri-operative implications of herbal medicines. Anaesthesia. 2002;57(9):889-899.
Case
A 72-year-old female with multiple medical problems is admitted with a hip fracture. Surgery is scheduled in 48 hours. The patient’s home medications include aspirin, carbidopa/levodopa, celecoxib, clonidine, estradiol, ginkgo, lisinopril, NPH insulin, sulfasalazine, and prednisone 10 mg a day, which she has been taking for years. How should these and other medications be managed in the perioperative period?
Background
Perioperative management of chronic medications is a complex issue, as physicians are required to balance the beneficial and harmful effects of the individual drugs prescribed to their patients. On one hand, cessation of medications can result in decompensation of disease or withdrawal. On the other hand, continuation of drugs can alter metabolism of anesthetic agents, cause perioperative hemodynamic instability, or result in such post-operative complications as acute renal failure, bleeding, infection, and impaired wound healing.
Certain traits make it reasonable to continue medications during the perioperative period. A long elimination half-life or duration of action makes stopping some medications impractical as it takes four to five half-lives to completely clear the drug from the body; holding the drug for a few days around surgery will not appreciably affect its concentration. Stopping drugs that carry severe withdrawal symptoms can be impractical because of the need for lengthy tapers, which can delay surgery and result in decompensation of underlying disease.
Drugs with no significant interactions with anesthesia or risk of perioperative complications should be continued in order to avoid deterioration of the underlying disease. Conversely, drugs that interact with anesthesia or increase risk for complications should be stopped if this can be accomplished safely. Patient-specific factors should receive consideration, as the risk of complications has to be balanced against the danger of exacerbating the underlying disease.
Overview of the Data
The challenge in providing recommendations on perioperative medication management lies in a dearth of high-quality clinical trials. Thus, much of the information comes from case reports, expert opinion, and sound application of pharmacology.
Antiplatelet therapy: Nuances of perioperative antiplatelet therapy are beyond the scope of this review, but some general principles can be elucidated from the American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines.1 Management of antiplatelet therapy should be done in conjunction with the surgical team, as cardiovascular risk has to be weighed against bleeding risk.
Aspirin therapy should be continued in all patients with a history of coronary artery disease (CAD), balloon angioplasty, or percutaneous coronary intervention (PCI), unless the risk of bleeding complications is felt to exceed the cardioprotective benefits—for example, in some neurosurgical patients.1
Clopidogrel therapy is crucial for prevention of in-stent thrombosis (IST) following PCI because patients who experience IST suffer catastrophic myocardial infarctions with high mortality. Ideally, surgery should be delayed to permit completion of clopidogrel therapy—30 to 45 days after implantation of a bare-metal stent and 365 days after a drug-eluting stent. If surgery has to be performed sooner, guidelines recommend operating on dual antiplatelet therapy with aspirin and clopidogrel.1 Again, this course of treatment has to be balanced against the risk of hemorrhagic complications from surgery.
Both aspirin and clopidogrel irreversibly inhibit platelet aggregation. The recovery of normal coagulation involves formation of new platelets, which necessitates cessation of therapy for seven to 10 days before surgery. Platelet inhibition begins within minutes of restarting aspirin and within hours of taking clopidogrel, although attaining peak clopidogrel effect takes three to seven days, unless a loading dose is used.
Cardiovascular Drugs
Beta-blockers in the perioperative setting are a focus of an ongoing debate beyond the scope of this review (see “What Pre-Operative Cardiac Evaluation of Patients Undergoing Intermediate-Risk Surgery Is Most Effective?,” February 2008, p. 26). Given the current evidence and the latest ACC/AHA guidelines, it is still reasonable to continue therapy in patients who are already taking them to avoid precipitating cardiovascular events by withdrawal. Patients with increased cardiac risk, demonstrated by a Revised Cardiac Risk Index (RCRI) score of ≥2 (see Table 1, p. 12), should be considered for beta-blocker therapy before surgery.1 In either case, the dose should be titrated to a heart rate <65 for optimal cardiac protection.1
Statins should be continued if the patient is taking them, especially because preoperative withdrawal has been associated with a 4.6-fold increase in troponin release and a 7.5-fold increased risk of myocardial infarction (MI) and cardiovascular death following major vascular surgery.2 Patients with increased cardiac risk— RCRI ≥1—can be considered for initiation of statin therapy before surgery, although the benefit of this intervention has not been examined in prospective studies.1
Amiodarone has an exceptionally long half-life of up to 142 days. It should be continued in the perioperative period.
Calcium channel blockers (CCBs) can be continued with no unpleasant perioperative hemodynamic effects.1 CCBs have potential cardioprotective benefits.
Clonidine withdrawal can result in severe rebound hypertension with reports of encephalopathy, stroke, and death. These effects are exacerbated by concomitant beta-blocker therapy. For this reason, if a patient is expected to be NPO for more than 12 hours, they should be converted to a clonidine patch 48-72 hours before surgery with concurrent tapering of the oral dose.3
Digoxin has a long half-life (up to 48 hours) and should be continued with monitoring of levels if there is a change in renal function.
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARBs) have been associated with a 50% increased risk of hypotension requiring vasopressors during induction of anesthesia.4 However, it is worth mentioning that this finding has not been corroborated in other studies. A large retrospective cohort of cardiothoracic surgical patients found a 28% increased risk of post-operative acute renal failure (ARF) with both drug classes, although another cardiothoracic report published the same year demonstrated a 50% reduction in risk with ACEIs.5,6 Although the evidence of harm is not unequivocal, perioperative blood-pressure control can be achieved with other drugs without hemodynamic or renal risk, such as CCBs, and in most cases ACEIs/ARBs should be stopped one day before surgery.
Diuretics carry a risk of volume depletion and electrolyte derangements, and should be stopped once a patient becomes NPO. Excess volume is managed with as-needed intravenous formulations.
Drugs Acting on the Central Nervous System
The majority of central nervous system (CNS)-active drugs, including antiepileptics, antipsychotics, benzodiazepines, bupropion, gabapentin, lithium, mirtazapine, selective serotonin and norepinephrine reuptake inhibitors (SSRIs and SNRIs), tricyclic antidepressants (TCAs), and valproic acid, balance a low risk of perioperative complications against a significant potential for withdrawal and disease decompensation. Therefore, these medications should be continued.
Carbidopa/Levodopa should be continued because abrupt cessation can precipitate systemic withdrawal resembling serotonin syndrome and rapid deterioration of Parkinson’s symptoms.
Monoamine oxidase inhibitor (MAOI) therapy usually indicates refractory psychiatric illness, so these drugs should be continued to avoid decompensation. Importantly, MAOI-safe anesthesia without dextromethorphan, meperidine, epinephrine, or norepinephrine has to be used due to the risk of cardiovascular instability.7
Diabetic Drugs
Insulin therapy should be continued with adjustments. Glargine basal insulin has no peak and can be continued without changing the dose. Likewise, patients with insulin pumps can continue the usual basal rate. Short-acting insulin or such insulin mixes as 70/30 should be stopped four hours before surgery to avoid hypoglycemia. Intermediate-acting insulin (e.g., NPH) can be administered at half the usual dose the day of surgery with a perioperative 5% dextrose infusion. NPH should not be given the day of surgery if the dextrose infusion cannot be used.8
Incretins (exenatide, sitagliptin) rarely cause hypoglycemia in the absence of insulin and may be beneficial in controlling intraoperative hyperglycemia. Therefore, these medications can be continued.8
Thiazolidinediones (TZDs; pioglitazone, rosiglitazone) alter gene transcription with biological duration of action on the order of weeks and low risk of hypoglycemia, and should be continued.
Metformin carries an FDA black-box warning to discontinue therapy before any intravascular radiocontrast studies or surgical procedures due to the risk of severe lactic acidosis if renal failure develops. It should be stopped 24 hours before surgery and restarted at least 48-72 hours after. Normal renal function should be confirmed before restarting therapy.8
Sulfonylureas (glimepiride, glipizide, glyburide) carry a significant risk of hypoglycemia in a patient who is NPO; they should be stopped the night before surgery or before commencement of NPO status.
Hormones
Antithyroid drugs (methimazole, propylthiouracil) and levothyroxine should be continued, as they have no perioperative contraindications.
Oral contraceptives (OCPs), hormone replacement therapy (HRT), and raloxifene can increase the risk of DVT. The largest study on the topic was the HERS trial of postmenopausal women on estrogen/progesterone HRT. The authors reported a 4.9-fold increased risk of DVT for 90 days after surgery.9 Unfortunately, no information was provided on the types of surgery, or whether appropriate and consistent DVT prophylaxis was utilized. HERS authors also reported a 2.5-fold increased risk of DVT for at least 30 days after cessation of HRT.9
Given the data, it is reasonable to stop hormone therapy four weeks before surgery when prolonged immobilization is anticipated and patients are able to tolerate hormone withdrawal, especially if other DVT risk factors are present. If hormone therapy cannot be stopped, strong consideration should be given to higher-intensity DVT prophylaxis (e.g., chemoprophylaxis as opposed to mechanical measures) of longer duration—up to 28 days following general surgery and up to 35 days after orthopedic procedures.10
Perioperative Corticosteroids
Corticosteroid therapy in excess of prednisone 5 mg/day or equivalent for more than five days in the 30 days preceding surgery might predispose patients to acute adrenal insufficiency in the perioperative period. Surgical procedures typically result in cortisol release of 50-150 mg/day, which returns to baseline within 48 hours.11 Therefore, the recommendation is to continue a patient’s baseline steroid dose and supplement it with stress-dose steroids tailored to the severity of operative stress (see Table 2, above).
Mineralocorticoid supplementation is not necessary, because endogenous production is not suppressed by corticosteroid therapy.11 Although a recent systematic review suggests that routine stress-dose steroids might not be indicated, high-quality prospective data are needed before abandoning this strategy due to complications of acute adrenal insufficiency compared to the risk of a brief corticosteroid burst.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Nonselective cyclooxygenase (COX) inhibitors reversibly decrease platelet aggregation only while the drug is present in the circulation and should be stopped one to three days before surgery due to risk of bleeding.
Selective COX-2 inhibitors do not significantly alter platelet aggregation and can be continued for opioid-sparing perioperative pain control.
Both COX-2-selective and nonselective inhibitors should be held if there are concerns for impaired renal function.
Disease-Modifying Antirheumatic Drugs (DMARDs) and Biological Response Modifiers (BRMs)
Methotrexate increases the risk of wound infections and dehiscence. However, this is offset by a decreased risk of post-operative disease flares with continued use. It can be continued unless the patient has medical comorbidities, advanced age, or chronic therapy with more than 10 mg/day of prednisone, in which case the drug should be stopped two weeks before surgery.12
Azathioprine, leflunomide, and sulfasalazine are renally cleared with a risk of myelosuppression; all of these medications should be stopped. Long half-life of leflunomide necessitates stopping it two weeks before surgery; azathioprine and sulfasalazine can be stopped one day in advance. The drugs can be restarted three days after surgery, assuming stable renal function.13
Anti-TNF-α (adalimumab, etanercept, infliximab), IL1 antagonist (anakinra), and anti-CD20 (rituximab) agents should be stopped one week before surgery and resumed 1-2 weeks afterward, unless risk of complications from disease flareup outweighs the concern for wound infections and dehiscence.14
Herbal Medicines
It is estimated that as much as a third of the U.S. population uses herbal medicines. These substances can result in perioperative hemodynamic instability (ephedra, ginseng, ma huang), hypoglycemia (ginseng), immunosuppression (echinacea, when taken for more than eight weeks), abnormal bleeding (garlic, ginkgo, ginseng), and prolongation of anesthesia (kava, St. John’s wort, valerian). All of these herbal medicine should be stopped one to two weeks before surgery.15,16
Back to the Case
The patient’s Carbidopa/Levodopa should be continued. Celecoxib can be continued if her renal function in stable. If aspirin is taken for a history of coronary artery disease or percutaneous coronary intervention, it should be continued, if possible. Clonidine should be continued or changed to a patch if an extended NPO period is anticipated. Ginkgo, lisinopril, and sulfasalazine should be stopped.
Hospitalization does not provide the luxury of stopping estradiol in advance, so it might be continued with chemical DVT prophylaxis for up to 35 days after surgery. The patient should receive 50-75 mg of IV hydrocortisone during surgery and an additional 25 mg the following day, in addition to her usual prednisone 10 mg/day. She can either receive half her usual NPH dose the morning of surgery with a 5% dextrose infusion in the operating room, or the NPH should be held altogether.
Bottom Line
Perioperative medication use should be tailored to each patient, balancing the risks and benefits of individual drugs. High-quality trials are needed to provide more robust clinical guidelines. TH
Dr. Levin is a hospitalist at the University of Colorado Denver.
References
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):1971-1996.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316-320.
- Spell NO III. Stopping and restarting medications in the perioperative period. Med Clin North Am. 2001;85(5):1117-1128.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325.
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3(5):1266-1273.
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative Angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg. 2008;86(4):1160-1165.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm. 2004;61(9):899-914.
- Kohl BA, Schwartz S. Surgery in the patient with endocrine dysfunction. Med Clin North Am. 2009;93(5):1031-1047.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132(9):689-696.
- Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ; American College of Chest Physicians. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):71S-109S.
- Axelrod L. Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin North Am. 2003;32(2):367-383.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg. 2008;143(12):1222-1226.
- Rosandich PA, Kelley JT III, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Curr Opin Rheumatol. 2004;16(3):192-198.
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286(2):208-216.
- Hodges PJ, Kam PC. The peri-operative implications of herbal medicines. Anaesthesia. 2002;57(9):889-899.
Case
A 72-year-old female with multiple medical problems is admitted with a hip fracture. Surgery is scheduled in 48 hours. The patient’s home medications include aspirin, carbidopa/levodopa, celecoxib, clonidine, estradiol, ginkgo, lisinopril, NPH insulin, sulfasalazine, and prednisone 10 mg a day, which she has been taking for years. How should these and other medications be managed in the perioperative period?
Background
Perioperative management of chronic medications is a complex issue, as physicians are required to balance the beneficial and harmful effects of the individual drugs prescribed to their patients. On one hand, cessation of medications can result in decompensation of disease or withdrawal. On the other hand, continuation of drugs can alter metabolism of anesthetic agents, cause perioperative hemodynamic instability, or result in such post-operative complications as acute renal failure, bleeding, infection, and impaired wound healing.
Certain traits make it reasonable to continue medications during the perioperative period. A long elimination half-life or duration of action makes stopping some medications impractical as it takes four to five half-lives to completely clear the drug from the body; holding the drug for a few days around surgery will not appreciably affect its concentration. Stopping drugs that carry severe withdrawal symptoms can be impractical because of the need for lengthy tapers, which can delay surgery and result in decompensation of underlying disease.
Drugs with no significant interactions with anesthesia or risk of perioperative complications should be continued in order to avoid deterioration of the underlying disease. Conversely, drugs that interact with anesthesia or increase risk for complications should be stopped if this can be accomplished safely. Patient-specific factors should receive consideration, as the risk of complications has to be balanced against the danger of exacerbating the underlying disease.
Overview of the Data
The challenge in providing recommendations on perioperative medication management lies in a dearth of high-quality clinical trials. Thus, much of the information comes from case reports, expert opinion, and sound application of pharmacology.
Antiplatelet therapy: Nuances of perioperative antiplatelet therapy are beyond the scope of this review, but some general principles can be elucidated from the American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines.1 Management of antiplatelet therapy should be done in conjunction with the surgical team, as cardiovascular risk has to be weighed against bleeding risk.
Aspirin therapy should be continued in all patients with a history of coronary artery disease (CAD), balloon angioplasty, or percutaneous coronary intervention (PCI), unless the risk of bleeding complications is felt to exceed the cardioprotective benefits—for example, in some neurosurgical patients.1
Clopidogrel therapy is crucial for prevention of in-stent thrombosis (IST) following PCI because patients who experience IST suffer catastrophic myocardial infarctions with high mortality. Ideally, surgery should be delayed to permit completion of clopidogrel therapy—30 to 45 days after implantation of a bare-metal stent and 365 days after a drug-eluting stent. If surgery has to be performed sooner, guidelines recommend operating on dual antiplatelet therapy with aspirin and clopidogrel.1 Again, this course of treatment has to be balanced against the risk of hemorrhagic complications from surgery.
Both aspirin and clopidogrel irreversibly inhibit platelet aggregation. The recovery of normal coagulation involves formation of new platelets, which necessitates cessation of therapy for seven to 10 days before surgery. Platelet inhibition begins within minutes of restarting aspirin and within hours of taking clopidogrel, although attaining peak clopidogrel effect takes three to seven days, unless a loading dose is used.
Cardiovascular Drugs
Beta-blockers in the perioperative setting are a focus of an ongoing debate beyond the scope of this review (see “What Pre-Operative Cardiac Evaluation of Patients Undergoing Intermediate-Risk Surgery Is Most Effective?,” February 2008, p. 26). Given the current evidence and the latest ACC/AHA guidelines, it is still reasonable to continue therapy in patients who are already taking them to avoid precipitating cardiovascular events by withdrawal. Patients with increased cardiac risk, demonstrated by a Revised Cardiac Risk Index (RCRI) score of ≥2 (see Table 1, p. 12), should be considered for beta-blocker therapy before surgery.1 In either case, the dose should be titrated to a heart rate <65 for optimal cardiac protection.1
Statins should be continued if the patient is taking them, especially because preoperative withdrawal has been associated with a 4.6-fold increase in troponin release and a 7.5-fold increased risk of myocardial infarction (MI) and cardiovascular death following major vascular surgery.2 Patients with increased cardiac risk— RCRI ≥1—can be considered for initiation of statin therapy before surgery, although the benefit of this intervention has not been examined in prospective studies.1
Amiodarone has an exceptionally long half-life of up to 142 days. It should be continued in the perioperative period.
Calcium channel blockers (CCBs) can be continued with no unpleasant perioperative hemodynamic effects.1 CCBs have potential cardioprotective benefits.
Clonidine withdrawal can result in severe rebound hypertension with reports of encephalopathy, stroke, and death. These effects are exacerbated by concomitant beta-blocker therapy. For this reason, if a patient is expected to be NPO for more than 12 hours, they should be converted to a clonidine patch 48-72 hours before surgery with concurrent tapering of the oral dose.3
Digoxin has a long half-life (up to 48 hours) and should be continued with monitoring of levels if there is a change in renal function.
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARBs) have been associated with a 50% increased risk of hypotension requiring vasopressors during induction of anesthesia.4 However, it is worth mentioning that this finding has not been corroborated in other studies. A large retrospective cohort of cardiothoracic surgical patients found a 28% increased risk of post-operative acute renal failure (ARF) with both drug classes, although another cardiothoracic report published the same year demonstrated a 50% reduction in risk with ACEIs.5,6 Although the evidence of harm is not unequivocal, perioperative blood-pressure control can be achieved with other drugs without hemodynamic or renal risk, such as CCBs, and in most cases ACEIs/ARBs should be stopped one day before surgery.
Diuretics carry a risk of volume depletion and electrolyte derangements, and should be stopped once a patient becomes NPO. Excess volume is managed with as-needed intravenous formulations.
Drugs Acting on the Central Nervous System
The majority of central nervous system (CNS)-active drugs, including antiepileptics, antipsychotics, benzodiazepines, bupropion, gabapentin, lithium, mirtazapine, selective serotonin and norepinephrine reuptake inhibitors (SSRIs and SNRIs), tricyclic antidepressants (TCAs), and valproic acid, balance a low risk of perioperative complications against a significant potential for withdrawal and disease decompensation. Therefore, these medications should be continued.
Carbidopa/Levodopa should be continued because abrupt cessation can precipitate systemic withdrawal resembling serotonin syndrome and rapid deterioration of Parkinson’s symptoms.
Monoamine oxidase inhibitor (MAOI) therapy usually indicates refractory psychiatric illness, so these drugs should be continued to avoid decompensation. Importantly, MAOI-safe anesthesia without dextromethorphan, meperidine, epinephrine, or norepinephrine has to be used due to the risk of cardiovascular instability.7
Diabetic Drugs
Insulin therapy should be continued with adjustments. Glargine basal insulin has no peak and can be continued without changing the dose. Likewise, patients with insulin pumps can continue the usual basal rate. Short-acting insulin or such insulin mixes as 70/30 should be stopped four hours before surgery to avoid hypoglycemia. Intermediate-acting insulin (e.g., NPH) can be administered at half the usual dose the day of surgery with a perioperative 5% dextrose infusion. NPH should not be given the day of surgery if the dextrose infusion cannot be used.8
Incretins (exenatide, sitagliptin) rarely cause hypoglycemia in the absence of insulin and may be beneficial in controlling intraoperative hyperglycemia. Therefore, these medications can be continued.8
Thiazolidinediones (TZDs; pioglitazone, rosiglitazone) alter gene transcription with biological duration of action on the order of weeks and low risk of hypoglycemia, and should be continued.
Metformin carries an FDA black-box warning to discontinue therapy before any intravascular radiocontrast studies or surgical procedures due to the risk of severe lactic acidosis if renal failure develops. It should be stopped 24 hours before surgery and restarted at least 48-72 hours after. Normal renal function should be confirmed before restarting therapy.8
Sulfonylureas (glimepiride, glipizide, glyburide) carry a significant risk of hypoglycemia in a patient who is NPO; they should be stopped the night before surgery or before commencement of NPO status.
Hormones
Antithyroid drugs (methimazole, propylthiouracil) and levothyroxine should be continued, as they have no perioperative contraindications.
Oral contraceptives (OCPs), hormone replacement therapy (HRT), and raloxifene can increase the risk of DVT. The largest study on the topic was the HERS trial of postmenopausal women on estrogen/progesterone HRT. The authors reported a 4.9-fold increased risk of DVT for 90 days after surgery.9 Unfortunately, no information was provided on the types of surgery, or whether appropriate and consistent DVT prophylaxis was utilized. HERS authors also reported a 2.5-fold increased risk of DVT for at least 30 days after cessation of HRT.9
Given the data, it is reasonable to stop hormone therapy four weeks before surgery when prolonged immobilization is anticipated and patients are able to tolerate hormone withdrawal, especially if other DVT risk factors are present. If hormone therapy cannot be stopped, strong consideration should be given to higher-intensity DVT prophylaxis (e.g., chemoprophylaxis as opposed to mechanical measures) of longer duration—up to 28 days following general surgery and up to 35 days after orthopedic procedures.10
Perioperative Corticosteroids
Corticosteroid therapy in excess of prednisone 5 mg/day or equivalent for more than five days in the 30 days preceding surgery might predispose patients to acute adrenal insufficiency in the perioperative period. Surgical procedures typically result in cortisol release of 50-150 mg/day, which returns to baseline within 48 hours.11 Therefore, the recommendation is to continue a patient’s baseline steroid dose and supplement it with stress-dose steroids tailored to the severity of operative stress (see Table 2, above).
Mineralocorticoid supplementation is not necessary, because endogenous production is not suppressed by corticosteroid therapy.11 Although a recent systematic review suggests that routine stress-dose steroids might not be indicated, high-quality prospective data are needed before abandoning this strategy due to complications of acute adrenal insufficiency compared to the risk of a brief corticosteroid burst.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Nonselective cyclooxygenase (COX) inhibitors reversibly decrease platelet aggregation only while the drug is present in the circulation and should be stopped one to three days before surgery due to risk of bleeding.
Selective COX-2 inhibitors do not significantly alter platelet aggregation and can be continued for opioid-sparing perioperative pain control.
Both COX-2-selective and nonselective inhibitors should be held if there are concerns for impaired renal function.
Disease-Modifying Antirheumatic Drugs (DMARDs) and Biological Response Modifiers (BRMs)
Methotrexate increases the risk of wound infections and dehiscence. However, this is offset by a decreased risk of post-operative disease flares with continued use. It can be continued unless the patient has medical comorbidities, advanced age, or chronic therapy with more than 10 mg/day of prednisone, in which case the drug should be stopped two weeks before surgery.12
Azathioprine, leflunomide, and sulfasalazine are renally cleared with a risk of myelosuppression; all of these medications should be stopped. Long half-life of leflunomide necessitates stopping it two weeks before surgery; azathioprine and sulfasalazine can be stopped one day in advance. The drugs can be restarted three days after surgery, assuming stable renal function.13
Anti-TNF-α (adalimumab, etanercept, infliximab), IL1 antagonist (anakinra), and anti-CD20 (rituximab) agents should be stopped one week before surgery and resumed 1-2 weeks afterward, unless risk of complications from disease flareup outweighs the concern for wound infections and dehiscence.14
Herbal Medicines
It is estimated that as much as a third of the U.S. population uses herbal medicines. These substances can result in perioperative hemodynamic instability (ephedra, ginseng, ma huang), hypoglycemia (ginseng), immunosuppression (echinacea, when taken for more than eight weeks), abnormal bleeding (garlic, ginkgo, ginseng), and prolongation of anesthesia (kava, St. John’s wort, valerian). All of these herbal medicine should be stopped one to two weeks before surgery.15,16
Back to the Case
The patient’s Carbidopa/Levodopa should be continued. Celecoxib can be continued if her renal function in stable. If aspirin is taken for a history of coronary artery disease or percutaneous coronary intervention, it should be continued, if possible. Clonidine should be continued or changed to a patch if an extended NPO period is anticipated. Ginkgo, lisinopril, and sulfasalazine should be stopped.
Hospitalization does not provide the luxury of stopping estradiol in advance, so it might be continued with chemical DVT prophylaxis for up to 35 days after surgery. The patient should receive 50-75 mg of IV hydrocortisone during surgery and an additional 25 mg the following day, in addition to her usual prednisone 10 mg/day. She can either receive half her usual NPH dose the morning of surgery with a 5% dextrose infusion in the operating room, or the NPH should be held altogether.
Bottom Line
Perioperative medication use should be tailored to each patient, balancing the risks and benefits of individual drugs. High-quality trials are needed to provide more robust clinical guidelines. TH
Dr. Levin is a hospitalist at the University of Colorado Denver.
References
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):1971-1996.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316-320.
- Spell NO III. Stopping and restarting medications in the perioperative period. Med Clin North Am. 2001;85(5):1117-1128.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325.
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3(5):1266-1273.
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative Angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg. 2008;86(4):1160-1165.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm. 2004;61(9):899-914.
- Kohl BA, Schwartz S. Surgery in the patient with endocrine dysfunction. Med Clin North Am. 2009;93(5):1031-1047.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132(9):689-696.
- Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ; American College of Chest Physicians. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):71S-109S.
- Axelrod L. Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin North Am. 2003;32(2):367-383.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg. 2008;143(12):1222-1226.
- Rosandich PA, Kelley JT III, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Curr Opin Rheumatol. 2004;16(3):192-198.
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286(2):208-216.
- Hodges PJ, Kam PC. The peri-operative implications of herbal medicines. Anaesthesia. 2002;57(9):889-899.
In the Literature
In This Edition
Literature at a Glance
A guide to this month’s studies
- CPOE and quality outcomes
- Outcomes of standardized management of endocarditis
- Effect of tPA three to 4.5 hours after stroke onset
- Failure to notify patients of significant test results
- PFO repair and stroke rate
- Predictors of delay in defibrillation for in-hospital arrest
- H. pylori eradication and risk of future gastric cancer
- Bleeding risk with fondaparinux vs. enoxaparin in ACS
- Perceptions of physician ability to predict medical futility
CPOE Is Associated with Improvement in Quality Measures
Clinical question: Is computerized physician order entry (CPOE) associated with improved outcomes across a large, nationally representative sample of hospitals?
Background: Several single-institution studies suggest CPOE leads to better outcomes in quality measures for heart failure, acute myocardial infarction, and pneumonia as defined by the Hospital Quality Alliance (HQA) initiative, led by the Centers for Medicare and Medicaid Services (CMS). Little systematic information is known about the effects of CPOE on quality of care.
Study design: Cross-sectional study.
Setting: The Health Information Management System Society (HIMSS) analytics database of 3,364 hospitals throughout the U.S.
Synopsis: Of the hospitals that reported CPOE utilization to HIMSS, 264 (7.8%) fully implement CPOE throughout their institutions. These CPOE hospitals outperformed their peers on five of 11 quality measures related to ordering medications, and in one of nine non-medication-related measures. No difference was noted in the other measures, except CPOE hospitals were less effective at providing antibiotics within four hours of pneumonia diagnosis. Hospitals that utilized CPOE were generally academic, larger, and nonprofit. After adjusting for these differences, benefits were still preserved.
The authors indicate that the lack of systematic outperformance by CPOE hospitals in all 20 of the quality categories inherently suggests that other factors (e.g., concomitant QI efforts) are not affecting these results. Given the observational nature of this study, no causal relationship can be established between CPOE and the observed benefits. CPOE might represent the commitment of certain hospitals to quality measures, but further study is needed.
Bottom line: Enhanced compliance in several CMS-established quality measures is seen in hospitals that utilize CPOE throughout their institutions.
Citation: Yu FB, Menachemi N, Berner ES, Allison JJ, Weissman NW, Houston TK. Full implementation of computerized physician order entry and medication-related quality outcomes: a study of 3,364 hospitals. Am J Med Qual. 2009;24(4):278-286.
Standardized Management of Endocarditis Leads to Significant Mortality Benefit
Clinical question: Does a standardized approach to the treatment of infective endocarditis reduce mortality and morbidity?
Background: Despite epidemiological changes to the inciting bacteria and improvements in available antibiotics, mortality and morbidity associated with endocarditis remain high. The contribution of inconsistent or inaccurate treatment of endocarditis is unclear.
Study design: Case series with historical controls from 1994 to 2001, compared with protocolized patients from 2002 to 2006.
Setting: Single teaching tertiary-care hospital in France.
Synopsis: The authors established a diagnostic protocol for infectious endocarditis from 1994 to 2001 (period 1) and established a treatment protocol from 2002 to 2006 (period 2). Despite a statistically significant sicker population (older, higher comorbidities, higher coagulase-negative staphylococcal infections, and fewer healthy valves), the period-2 patients had a dramatically lower mortality rate of 8.2% (P<0.001), compared with 18.5% in period-1 patients. Fewer episodes of renal failure, organ failure, and deaths associated with embolism were noted in period 2.
Whether these results are due to more frequent care, more aggressive care (patients were “summoned” if they did not show for appointments), standardized medication and surgical options, or the effects of long-term collaboration, these results appear durable, remarkable, and reproducible.
This study is limited by its lack of randomization and extensive time frame, with concomitant changes in medical treatment and observed infectious organisms.
Bottom line: Implementation of a standardized approach to endocarditis has significant benefit on mortality and morbidity.
Citation: Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298.
Treatment with tPA in the Three- to 4.5-Hour Time Window after Stroke Is Beneficial
Clinical question: What is the effect of tissue plasminogen activator (tPA) on outcomes in patients treated in the three- to 4.5-hour window after stroke?
Background: The third European Cooperative Acute Stroke Study 3 (ECASS-3) demonstrated benefit of treatment of acute stroke with tPA in the three- to 4.5-hour time window. Prior studies, however, did not show superiority of tPA over placebo, and there is a lack of a confirmatory randomized, controlled trial of tPA in this time frame.
Study design: Meta-analysis of randomized, controlled trials.
Setting: Four studies involving 1,622 patients who were treated with intravenous tPA for acute ischemic stroke from three to 4.5 hours after stroke compared with placebo.
Synopsis: Of the randomized, controlled trials of intravenous tPA for treatment of acute ischemic stroke from three to 4.5 hours after stroke, four trials (ECASS-1, ECASS-2, ECASS-3, and ATLANTIS) were included in the analysis. Treatment with tPA in the three- to 4.5-hour time window is associated with increased favorable outcomes based on the global outcome measure (OR 1.31; 95% CI: 1.10-1.56, P=0.002) and the modified Rankin Scale (OR 1.31; 95% CI: 1.07-1.59, P=0.01), compared with placebo. The 90-day mortality rate was not significantly different between the treatment and placebo groups (OR 1.04; 95% CI 0.75-1.43, P=0.83).
Due to the relatively high dose of tPA (1.1 mg/kg) administered in the ECASS-1 trial, a separate meta-analysis looking at the other three trials (tPA dose of 0.9 mg/kg) was conducted, and the favorable outcome with tPA remained.
Bottom line: Treatment of acute ischemic stroke with tPA in the three- to 4.5-hour time window results in an increased rate of favorable functional outcomes without a significant difference in mortality.
Citation: Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
Outpatients Often Are Not Notified of Clinically Significant Test Results
Clinical question: How frequently do primary-care physicians (PCPs) fail to inform patients of clinically significant outpatient test results?
Background: Diagnostic errors are the most common cause of malpractice claims in the U.S. It is unclear how often providers fail to either inform patients of abnormal test results or document that patients have been notified.
Study design: Retrospective chart review.
Setting: Twenty-three primary-care practices: 19 private, four academic.
Synopsis: More than 5,400 charts were reviewed, and 1,889 abnormal test results were identified in this study. Failure to inform or document notification was identified in 135 cases (7.1%). The failure rates in the practices ranged from 0.0% to 26.2%. Practices with the best processes for managing test results had the lowest failure rates; these processes included: all results being routed to the responsible physician; the physician signing off on all results; the practice informing patients of all results, both normal and abnormal; documenting when the patient is informed; and instructing patients to call if not notified of test results within a certain time interval.
Limitations of this study include the potential of over- or underreporting of failures to inform as a chart review was used, and only practices that agreed to participate were included.
Bottom line: Failure to notify outpatients of test results is common but can be minimized by creating a systematic management of test results that include best practices.
Citation: Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med. 2009;169(12):1123-1129.
Repair of Incidental PFO Discovered During Cardiothoracic Surgery Repair Increases Postoperative Stroke Risk
Clinical question: What is the impact of closing incidentally discovered patent foramen ovale (PFO) defects during cardiothoracic surgery?
Background: PFO’s role in cryptogenic stroke remains controversial. Incidental PFO is commonly detected by transesophageal echocardiography (TEE) during cardiothoracic surgery. Routine PFO closure has been recommended when almost no alteration of the surgical plan is required.
Study design: Retrospective chart review.
Setting: The Cleveland Clinic.
Synopsis: Between 1995 and 2006, 13,092 patients undergoing cardiothoracic surgery had TEE data with no previous diagnosis of PFO, but the review found that 2,277 (17%) had PFO discovered intraoperatively. Of these, 639 (28%) had the PFO repaired.
Patients with an intraoperative diagnosis of PFO had similar rates of in-hospital stroke and hospital death compared with those without PFO. Patients who had their PFO repaired had a greater in-hospital stroke risk (2.8% vs. 1.2%; P=0.04) compared with those with a non-repaired PFO, representing nearly 2.5 times greater odds of having an in-hospital stroke. No other difference was noted in perioperative outcomes for patients who underwent intraoperative repair compared with those who did not, including risk of in-hospital death, hospital length of stay, ICU length of stay, and time on cardiopulmonary bypass. Long-term analysis demonstrated that PFO repair was associated with no survival difference.
The study is limited by its retrospective nature.
Bottom line: Routine surgical closure of incidental PFO detected during intraoperative imaging should be discouraged.
Citation: Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of interoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA. 2009;302(3):290-297.
Hospital-Level Differences Are Strong Predictors of Time to Defibrillation Delay In Cardiac Arrest
Clinical question: What are the predictors of delay in the time to defibrillation after in-hospital cardiac arrest?
Background: Thirty percent of in-hospital cardiac arrests from ventricular arrhythmias are not treated within the American Heart Association’s recommendation of two minutes. This delay is associated with a 50% lower rate of in-hospital survival. Exploring the hospital-level variation in delays to defibrillation is a critical step toward sharing the best practices.
Study design: Retrospective review of registry data.
Setting: The National Registry of Cardiopulmonary Resuscitation (NRCPR) survey of 200 acute-care, nonpediatric hospitals.
Synopsis: The registry identified 7,479 patients who experienced cardiac arrest from ventricular tachycardia or pulseless ventricular fibrillation. The primary outcome was the hospital rate of delayed defibrillation (time to defibrillation > two minutes), which ranged from 2% to 51%.
Time to defibrillation was found to be a major predictor of survival after a cardiac arrest. Only bed volume and arrest location were associated with differences in rates of delayed defibrillation (lower rates in larger hospitals and in ICUs). The variability was not due to differences in patient characteristics, but was due to hospital-level effects. Academic status, geographical location, arrest volume, and daily admission volume did not affect the time to defibrillation.
The study was able to identify only a few facility characteristics that account for the variability between hospitals in the rate of delayed defibrillation. The study emphasizes the need for new approaches to identifying hospital innovations in process-of-care measures that are associated with improved performance in defibrillation times.
Bottom Line: Future research is needed to better understand the reason for the wide variation between hospitals in the rate of delayed defibrillation after in-hospital cardiac arrest.
Citation: Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265-1273.
Treating for H. Pylori Reduces the Risk for Developing Gastric Cancer in High-Risk Patients
Clinical question: In patients with high-baseline incidence of gastric cancer, does H. pylori eradication reduce the risk for developing gastric cancer?
Background: Gastric cancer remains a major health problem in Asia. The link of H. pylori and gastric cancer has been established, but it remains unclear whether treatment for H. pylori is effective primary prevention for the development of gastric cancer.
Study design: Meta-analysis of six studies.
Setting: All but one trial was performed in Asia.
Synopsis: Seven studies met inclusion criteria, one of which was excluded due to heterogeneity. The six remaining studies were pooled, with 37 of 3,388 (1.1%) treated patients developing a new gastric cancer, compared with 56 of 3,307 (1.7%) patients who received placebo or were in the control group (RR 0.65; 0.43-0.98). Most patients received gastric biopsy prior to enrollment, and most of those demonstrated gastric atrophy or intestinal metaplasia.
These patients, despite more advanced precancerous pathology findings, still benefited from eradication. The seventh study, which was excluded, enrolled patients with early gastric cancer; these patients still benefited from H. pylori eradication and, when included in the meta-analysis, the RR was even lower, 0.57 (0.49-0.81).
Only two trials were double-blinded, but all of the studies employed the same definition of gastric cancer and held to excellent data reporting standards. This study encourages screening and treatment in high-risk patients given the widespread incidence of H. pylori.
Bottom Line: Treatment of H. pylori reduces the risk of gastric cancer in high-risk patients.
Citation: Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121-128.
Patients on Anti-Platelet Agents with Acute Coronary Syndrome Have a Lower Bleeding Risk When Treated with Fondaparinux
Clinical question: Is there a difference in bleeding risk with fondaparinux and enoxaparin when used with GPIIb/IIIa inhibitors or thienopyridines in NSTEMI-ACS?
Background: The OASIS 5 study reported a 50% reduction in severe bleeding when comparing fondaparinux to enoxaparin in ACS while maintaining a similar efficacy. This subgroup analysis was performed to evaluate whether reduced bleeding risk with fondaparinux remains in patients treated with additional anti-platelet agents.
Study design: Subgroup analysis of a large, multicenter, randomized, double-blind trial.
Setting: Acute-care hospitals in North America, Eastern and Western Europe, Latin America, Australia, and Asia.
Synopsis: Patients with NSTE-ACS received either fondaparinux or enoxaparin and were treated with GPIIb/IIIa inhibitors or thienopyridines at the discretion of their physician. At 30 days, the fondaparinux group had similar efficacy and decreased bleeding risk in both the GPIIb/IIIa and the thienopyridine groups. Of the 3,630 patients in the GPIIb/IIIa group, the risk for major bleeding with fondaparinux was 5.2%, whereas the risk with enoxaparin was 8.3% (HR 0.61; P<0.001) compared with enoxaparin. Of the 1,352 patients treated with thienopyridines, the risk for major bleeding with fondaparinux was 3.4%, whereas the risk with enoxaparin was 5.4% (HR 0.62; P<0.001).
Bottom Line: This subgroup analysis suggests there are less-severe bleeding complications in patients treated with fondaparinux when compared with enoxaparin in the setting of cotreatment with GPIIb/IIIa inhibitors, thienopyridines, or both.
Citation: Jolly SS, Faxon DP, Fox KA, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors of thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009;54(5):468-476.
Surrogate Decision-Makers Frequently Doubt Clinicians’ Ability to Predict Medical Futility
Clinical question: What attitudes do surrogate decision-makers hold toward clinicians’ predictions of medical futility in critically-ill patients?
Background: The clinical judgment of medical futility leading to the withdrawal of life-sustaining treatment—despite the objections of surrogate decision-makers—is controversial. Very little is known about how surrogate decision-makers view the futility rationale when physicians suggest limiting the use of life-sustaining treatment.
Study design: Multicenter, mixed, qualitative and quantitative study.
Setting: Three ICUs in three different California hospitals from 2006 to 2007.
Synopsis: Semi-structured interviews of surrogate decision-makers for 50 incapacitated, critically-ill patients were performed to ascertain their beliefs about medical futility in response to hypothetical situations. Of the surrogates surveyed, 64% expressed doubt about physicians’ futility predictions.
The interviewees gave four main reasons for their doubts. Two reasons not previously described were doubts about the accuracy of physicians’ predictions and the need for surrogates to see futility themselves. Previously described sources of conflict included a misunderstanding about prognosis and religious-based objections. Surrogates with religious objections were more likely to request continuation of life-sustaining treatments than those with secular or experiential objections (OR 4; 95% CI 1.2-14.0; P=0.03). Nearly a third (32%) of surrogates elected to continue life support with a <1% survival estimate; 18% elected to continue life support when physicians thought there was no chance of survival.
This study has several limitations: a small sample size, the use of hypothetical situations, and the inability to assess attitudes as they change over time.
Bottom line: The nature of surrogate decision-makers’ doubts about medical futility can help predict whether they accept predictions of medical futility from physicians.
Citation: Zier LS, Burack JH, Micco G, Chipman AK, Frank JA, White DB. Surrogate decision makers’ responses to physicians’ predictions of medical futility. Chest. 2009;136:110-117. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- CPOE and quality outcomes
- Outcomes of standardized management of endocarditis
- Effect of tPA three to 4.5 hours after stroke onset
- Failure to notify patients of significant test results
- PFO repair and stroke rate
- Predictors of delay in defibrillation for in-hospital arrest
- H. pylori eradication and risk of future gastric cancer
- Bleeding risk with fondaparinux vs. enoxaparin in ACS
- Perceptions of physician ability to predict medical futility
CPOE Is Associated with Improvement in Quality Measures
Clinical question: Is computerized physician order entry (CPOE) associated with improved outcomes across a large, nationally representative sample of hospitals?
Background: Several single-institution studies suggest CPOE leads to better outcomes in quality measures for heart failure, acute myocardial infarction, and pneumonia as defined by the Hospital Quality Alliance (HQA) initiative, led by the Centers for Medicare and Medicaid Services (CMS). Little systematic information is known about the effects of CPOE on quality of care.
Study design: Cross-sectional study.
Setting: The Health Information Management System Society (HIMSS) analytics database of 3,364 hospitals throughout the U.S.
Synopsis: Of the hospitals that reported CPOE utilization to HIMSS, 264 (7.8%) fully implement CPOE throughout their institutions. These CPOE hospitals outperformed their peers on five of 11 quality measures related to ordering medications, and in one of nine non-medication-related measures. No difference was noted in the other measures, except CPOE hospitals were less effective at providing antibiotics within four hours of pneumonia diagnosis. Hospitals that utilized CPOE were generally academic, larger, and nonprofit. After adjusting for these differences, benefits were still preserved.
The authors indicate that the lack of systematic outperformance by CPOE hospitals in all 20 of the quality categories inherently suggests that other factors (e.g., concomitant QI efforts) are not affecting these results. Given the observational nature of this study, no causal relationship can be established between CPOE and the observed benefits. CPOE might represent the commitment of certain hospitals to quality measures, but further study is needed.
Bottom line: Enhanced compliance in several CMS-established quality measures is seen in hospitals that utilize CPOE throughout their institutions.
Citation: Yu FB, Menachemi N, Berner ES, Allison JJ, Weissman NW, Houston TK. Full implementation of computerized physician order entry and medication-related quality outcomes: a study of 3,364 hospitals. Am J Med Qual. 2009;24(4):278-286.
Standardized Management of Endocarditis Leads to Significant Mortality Benefit
Clinical question: Does a standardized approach to the treatment of infective endocarditis reduce mortality and morbidity?
Background: Despite epidemiological changes to the inciting bacteria and improvements in available antibiotics, mortality and morbidity associated with endocarditis remain high. The contribution of inconsistent or inaccurate treatment of endocarditis is unclear.
Study design: Case series with historical controls from 1994 to 2001, compared with protocolized patients from 2002 to 2006.
Setting: Single teaching tertiary-care hospital in France.
Synopsis: The authors established a diagnostic protocol for infectious endocarditis from 1994 to 2001 (period 1) and established a treatment protocol from 2002 to 2006 (period 2). Despite a statistically significant sicker population (older, higher comorbidities, higher coagulase-negative staphylococcal infections, and fewer healthy valves), the period-2 patients had a dramatically lower mortality rate of 8.2% (P<0.001), compared with 18.5% in period-1 patients. Fewer episodes of renal failure, organ failure, and deaths associated with embolism were noted in period 2.
Whether these results are due to more frequent care, more aggressive care (patients were “summoned” if they did not show for appointments), standardized medication and surgical options, or the effects of long-term collaboration, these results appear durable, remarkable, and reproducible.
This study is limited by its lack of randomization and extensive time frame, with concomitant changes in medical treatment and observed infectious organisms.
Bottom line: Implementation of a standardized approach to endocarditis has significant benefit on mortality and morbidity.
Citation: Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298.
Treatment with tPA in the Three- to 4.5-Hour Time Window after Stroke Is Beneficial
Clinical question: What is the effect of tissue plasminogen activator (tPA) on outcomes in patients treated in the three- to 4.5-hour window after stroke?
Background: The third European Cooperative Acute Stroke Study 3 (ECASS-3) demonstrated benefit of treatment of acute stroke with tPA in the three- to 4.5-hour time window. Prior studies, however, did not show superiority of tPA over placebo, and there is a lack of a confirmatory randomized, controlled trial of tPA in this time frame.
Study design: Meta-analysis of randomized, controlled trials.
Setting: Four studies involving 1,622 patients who were treated with intravenous tPA for acute ischemic stroke from three to 4.5 hours after stroke compared with placebo.
Synopsis: Of the randomized, controlled trials of intravenous tPA for treatment of acute ischemic stroke from three to 4.5 hours after stroke, four trials (ECASS-1, ECASS-2, ECASS-3, and ATLANTIS) were included in the analysis. Treatment with tPA in the three- to 4.5-hour time window is associated with increased favorable outcomes based on the global outcome measure (OR 1.31; 95% CI: 1.10-1.56, P=0.002) and the modified Rankin Scale (OR 1.31; 95% CI: 1.07-1.59, P=0.01), compared with placebo. The 90-day mortality rate was not significantly different between the treatment and placebo groups (OR 1.04; 95% CI 0.75-1.43, P=0.83).
Due to the relatively high dose of tPA (1.1 mg/kg) administered in the ECASS-1 trial, a separate meta-analysis looking at the other three trials (tPA dose of 0.9 mg/kg) was conducted, and the favorable outcome with tPA remained.
Bottom line: Treatment of acute ischemic stroke with tPA in the three- to 4.5-hour time window results in an increased rate of favorable functional outcomes without a significant difference in mortality.
Citation: Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
Outpatients Often Are Not Notified of Clinically Significant Test Results
Clinical question: How frequently do primary-care physicians (PCPs) fail to inform patients of clinically significant outpatient test results?
Background: Diagnostic errors are the most common cause of malpractice claims in the U.S. It is unclear how often providers fail to either inform patients of abnormal test results or document that patients have been notified.
Study design: Retrospective chart review.
Setting: Twenty-three primary-care practices: 19 private, four academic.
Synopsis: More than 5,400 charts were reviewed, and 1,889 abnormal test results were identified in this study. Failure to inform or document notification was identified in 135 cases (7.1%). The failure rates in the practices ranged from 0.0% to 26.2%. Practices with the best processes for managing test results had the lowest failure rates; these processes included: all results being routed to the responsible physician; the physician signing off on all results; the practice informing patients of all results, both normal and abnormal; documenting when the patient is informed; and instructing patients to call if not notified of test results within a certain time interval.
Limitations of this study include the potential of over- or underreporting of failures to inform as a chart review was used, and only practices that agreed to participate were included.
Bottom line: Failure to notify outpatients of test results is common but can be minimized by creating a systematic management of test results that include best practices.
Citation: Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med. 2009;169(12):1123-1129.
Repair of Incidental PFO Discovered During Cardiothoracic Surgery Repair Increases Postoperative Stroke Risk
Clinical question: What is the impact of closing incidentally discovered patent foramen ovale (PFO) defects during cardiothoracic surgery?
Background: PFO’s role in cryptogenic stroke remains controversial. Incidental PFO is commonly detected by transesophageal echocardiography (TEE) during cardiothoracic surgery. Routine PFO closure has been recommended when almost no alteration of the surgical plan is required.
Study design: Retrospective chart review.
Setting: The Cleveland Clinic.
Synopsis: Between 1995 and 2006, 13,092 patients undergoing cardiothoracic surgery had TEE data with no previous diagnosis of PFO, but the review found that 2,277 (17%) had PFO discovered intraoperatively. Of these, 639 (28%) had the PFO repaired.
Patients with an intraoperative diagnosis of PFO had similar rates of in-hospital stroke and hospital death compared with those without PFO. Patients who had their PFO repaired had a greater in-hospital stroke risk (2.8% vs. 1.2%; P=0.04) compared with those with a non-repaired PFO, representing nearly 2.5 times greater odds of having an in-hospital stroke. No other difference was noted in perioperative outcomes for patients who underwent intraoperative repair compared with those who did not, including risk of in-hospital death, hospital length of stay, ICU length of stay, and time on cardiopulmonary bypass. Long-term analysis demonstrated that PFO repair was associated with no survival difference.
The study is limited by its retrospective nature.
Bottom line: Routine surgical closure of incidental PFO detected during intraoperative imaging should be discouraged.
Citation: Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of interoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA. 2009;302(3):290-297.
Hospital-Level Differences Are Strong Predictors of Time to Defibrillation Delay In Cardiac Arrest
Clinical question: What are the predictors of delay in the time to defibrillation after in-hospital cardiac arrest?
Background: Thirty percent of in-hospital cardiac arrests from ventricular arrhythmias are not treated within the American Heart Association’s recommendation of two minutes. This delay is associated with a 50% lower rate of in-hospital survival. Exploring the hospital-level variation in delays to defibrillation is a critical step toward sharing the best practices.
Study design: Retrospective review of registry data.
Setting: The National Registry of Cardiopulmonary Resuscitation (NRCPR) survey of 200 acute-care, nonpediatric hospitals.
Synopsis: The registry identified 7,479 patients who experienced cardiac arrest from ventricular tachycardia or pulseless ventricular fibrillation. The primary outcome was the hospital rate of delayed defibrillation (time to defibrillation > two minutes), which ranged from 2% to 51%.
Time to defibrillation was found to be a major predictor of survival after a cardiac arrest. Only bed volume and arrest location were associated with differences in rates of delayed defibrillation (lower rates in larger hospitals and in ICUs). The variability was not due to differences in patient characteristics, but was due to hospital-level effects. Academic status, geographical location, arrest volume, and daily admission volume did not affect the time to defibrillation.
The study was able to identify only a few facility characteristics that account for the variability between hospitals in the rate of delayed defibrillation. The study emphasizes the need for new approaches to identifying hospital innovations in process-of-care measures that are associated with improved performance in defibrillation times.
Bottom Line: Future research is needed to better understand the reason for the wide variation between hospitals in the rate of delayed defibrillation after in-hospital cardiac arrest.
Citation: Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265-1273.
Treating for H. Pylori Reduces the Risk for Developing Gastric Cancer in High-Risk Patients
Clinical question: In patients with high-baseline incidence of gastric cancer, does H. pylori eradication reduce the risk for developing gastric cancer?
Background: Gastric cancer remains a major health problem in Asia. The link of H. pylori and gastric cancer has been established, but it remains unclear whether treatment for H. pylori is effective primary prevention for the development of gastric cancer.
Study design: Meta-analysis of six studies.
Setting: All but one trial was performed in Asia.
Synopsis: Seven studies met inclusion criteria, one of which was excluded due to heterogeneity. The six remaining studies were pooled, with 37 of 3,388 (1.1%) treated patients developing a new gastric cancer, compared with 56 of 3,307 (1.7%) patients who received placebo or were in the control group (RR 0.65; 0.43-0.98). Most patients received gastric biopsy prior to enrollment, and most of those demonstrated gastric atrophy or intestinal metaplasia.
These patients, despite more advanced precancerous pathology findings, still benefited from eradication. The seventh study, which was excluded, enrolled patients with early gastric cancer; these patients still benefited from H. pylori eradication and, when included in the meta-analysis, the RR was even lower, 0.57 (0.49-0.81).
Only two trials were double-blinded, but all of the studies employed the same definition of gastric cancer and held to excellent data reporting standards. This study encourages screening and treatment in high-risk patients given the widespread incidence of H. pylori.
Bottom Line: Treatment of H. pylori reduces the risk of gastric cancer in high-risk patients.
Citation: Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121-128.
Patients on Anti-Platelet Agents with Acute Coronary Syndrome Have a Lower Bleeding Risk When Treated with Fondaparinux
Clinical question: Is there a difference in bleeding risk with fondaparinux and enoxaparin when used with GPIIb/IIIa inhibitors or thienopyridines in NSTEMI-ACS?
Background: The OASIS 5 study reported a 50% reduction in severe bleeding when comparing fondaparinux to enoxaparin in ACS while maintaining a similar efficacy. This subgroup analysis was performed to evaluate whether reduced bleeding risk with fondaparinux remains in patients treated with additional anti-platelet agents.
Study design: Subgroup analysis of a large, multicenter, randomized, double-blind trial.
Setting: Acute-care hospitals in North America, Eastern and Western Europe, Latin America, Australia, and Asia.
Synopsis: Patients with NSTE-ACS received either fondaparinux or enoxaparin and were treated with GPIIb/IIIa inhibitors or thienopyridines at the discretion of their physician. At 30 days, the fondaparinux group had similar efficacy and decreased bleeding risk in both the GPIIb/IIIa and the thienopyridine groups. Of the 3,630 patients in the GPIIb/IIIa group, the risk for major bleeding with fondaparinux was 5.2%, whereas the risk with enoxaparin was 8.3% (HR 0.61; P<0.001) compared with enoxaparin. Of the 1,352 patients treated with thienopyridines, the risk for major bleeding with fondaparinux was 3.4%, whereas the risk with enoxaparin was 5.4% (HR 0.62; P<0.001).
Bottom Line: This subgroup analysis suggests there are less-severe bleeding complications in patients treated with fondaparinux when compared with enoxaparin in the setting of cotreatment with GPIIb/IIIa inhibitors, thienopyridines, or both.
Citation: Jolly SS, Faxon DP, Fox KA, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors of thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009;54(5):468-476.
Surrogate Decision-Makers Frequently Doubt Clinicians’ Ability to Predict Medical Futility
Clinical question: What attitudes do surrogate decision-makers hold toward clinicians’ predictions of medical futility in critically-ill patients?
Background: The clinical judgment of medical futility leading to the withdrawal of life-sustaining treatment—despite the objections of surrogate decision-makers—is controversial. Very little is known about how surrogate decision-makers view the futility rationale when physicians suggest limiting the use of life-sustaining treatment.
Study design: Multicenter, mixed, qualitative and quantitative study.
Setting: Three ICUs in three different California hospitals from 2006 to 2007.
Synopsis: Semi-structured interviews of surrogate decision-makers for 50 incapacitated, critically-ill patients were performed to ascertain their beliefs about medical futility in response to hypothetical situations. Of the surrogates surveyed, 64% expressed doubt about physicians’ futility predictions.
The interviewees gave four main reasons for their doubts. Two reasons not previously described were doubts about the accuracy of physicians’ predictions and the need for surrogates to see futility themselves. Previously described sources of conflict included a misunderstanding about prognosis and religious-based objections. Surrogates with religious objections were more likely to request continuation of life-sustaining treatments than those with secular or experiential objections (OR 4; 95% CI 1.2-14.0; P=0.03). Nearly a third (32%) of surrogates elected to continue life support with a <1% survival estimate; 18% elected to continue life support when physicians thought there was no chance of survival.
This study has several limitations: a small sample size, the use of hypothetical situations, and the inability to assess attitudes as they change over time.
Bottom line: The nature of surrogate decision-makers’ doubts about medical futility can help predict whether they accept predictions of medical futility from physicians.
Citation: Zier LS, Burack JH, Micco G, Chipman AK, Frank JA, White DB. Surrogate decision makers’ responses to physicians’ predictions of medical futility. Chest. 2009;136:110-117. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- CPOE and quality outcomes
- Outcomes of standardized management of endocarditis
- Effect of tPA three to 4.5 hours after stroke onset
- Failure to notify patients of significant test results
- PFO repair and stroke rate
- Predictors of delay in defibrillation for in-hospital arrest
- H. pylori eradication and risk of future gastric cancer
- Bleeding risk with fondaparinux vs. enoxaparin in ACS
- Perceptions of physician ability to predict medical futility
CPOE Is Associated with Improvement in Quality Measures
Clinical question: Is computerized physician order entry (CPOE) associated with improved outcomes across a large, nationally representative sample of hospitals?
Background: Several single-institution studies suggest CPOE leads to better outcomes in quality measures for heart failure, acute myocardial infarction, and pneumonia as defined by the Hospital Quality Alliance (HQA) initiative, led by the Centers for Medicare and Medicaid Services (CMS). Little systematic information is known about the effects of CPOE on quality of care.
Study design: Cross-sectional study.
Setting: The Health Information Management System Society (HIMSS) analytics database of 3,364 hospitals throughout the U.S.
Synopsis: Of the hospitals that reported CPOE utilization to HIMSS, 264 (7.8%) fully implement CPOE throughout their institutions. These CPOE hospitals outperformed their peers on five of 11 quality measures related to ordering medications, and in one of nine non-medication-related measures. No difference was noted in the other measures, except CPOE hospitals were less effective at providing antibiotics within four hours of pneumonia diagnosis. Hospitals that utilized CPOE were generally academic, larger, and nonprofit. After adjusting for these differences, benefits were still preserved.
The authors indicate that the lack of systematic outperformance by CPOE hospitals in all 20 of the quality categories inherently suggests that other factors (e.g., concomitant QI efforts) are not affecting these results. Given the observational nature of this study, no causal relationship can be established between CPOE and the observed benefits. CPOE might represent the commitment of certain hospitals to quality measures, but further study is needed.
Bottom line: Enhanced compliance in several CMS-established quality measures is seen in hospitals that utilize CPOE throughout their institutions.
Citation: Yu FB, Menachemi N, Berner ES, Allison JJ, Weissman NW, Houston TK. Full implementation of computerized physician order entry and medication-related quality outcomes: a study of 3,364 hospitals. Am J Med Qual. 2009;24(4):278-286.
Standardized Management of Endocarditis Leads to Significant Mortality Benefit
Clinical question: Does a standardized approach to the treatment of infective endocarditis reduce mortality and morbidity?
Background: Despite epidemiological changes to the inciting bacteria and improvements in available antibiotics, mortality and morbidity associated with endocarditis remain high. The contribution of inconsistent or inaccurate treatment of endocarditis is unclear.
Study design: Case series with historical controls from 1994 to 2001, compared with protocolized patients from 2002 to 2006.
Setting: Single teaching tertiary-care hospital in France.
Synopsis: The authors established a diagnostic protocol for infectious endocarditis from 1994 to 2001 (period 1) and established a treatment protocol from 2002 to 2006 (period 2). Despite a statistically significant sicker population (older, higher comorbidities, higher coagulase-negative staphylococcal infections, and fewer healthy valves), the period-2 patients had a dramatically lower mortality rate of 8.2% (P<0.001), compared with 18.5% in period-1 patients. Fewer episodes of renal failure, organ failure, and deaths associated with embolism were noted in period 2.
Whether these results are due to more frequent care, more aggressive care (patients were “summoned” if they did not show for appointments), standardized medication and surgical options, or the effects of long-term collaboration, these results appear durable, remarkable, and reproducible.
This study is limited by its lack of randomization and extensive time frame, with concomitant changes in medical treatment and observed infectious organisms.
Bottom line: Implementation of a standardized approach to endocarditis has significant benefit on mortality and morbidity.
Citation: Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298.
Treatment with tPA in the Three- to 4.5-Hour Time Window after Stroke Is Beneficial
Clinical question: What is the effect of tissue plasminogen activator (tPA) on outcomes in patients treated in the three- to 4.5-hour window after stroke?
Background: The third European Cooperative Acute Stroke Study 3 (ECASS-3) demonstrated benefit of treatment of acute stroke with tPA in the three- to 4.5-hour time window. Prior studies, however, did not show superiority of tPA over placebo, and there is a lack of a confirmatory randomized, controlled trial of tPA in this time frame.
Study design: Meta-analysis of randomized, controlled trials.
Setting: Four studies involving 1,622 patients who were treated with intravenous tPA for acute ischemic stroke from three to 4.5 hours after stroke compared with placebo.
Synopsis: Of the randomized, controlled trials of intravenous tPA for treatment of acute ischemic stroke from three to 4.5 hours after stroke, four trials (ECASS-1, ECASS-2, ECASS-3, and ATLANTIS) were included in the analysis. Treatment with tPA in the three- to 4.5-hour time window is associated with increased favorable outcomes based on the global outcome measure (OR 1.31; 95% CI: 1.10-1.56, P=0.002) and the modified Rankin Scale (OR 1.31; 95% CI: 1.07-1.59, P=0.01), compared with placebo. The 90-day mortality rate was not significantly different between the treatment and placebo groups (OR 1.04; 95% CI 0.75-1.43, P=0.83).
Due to the relatively high dose of tPA (1.1 mg/kg) administered in the ECASS-1 trial, a separate meta-analysis looking at the other three trials (tPA dose of 0.9 mg/kg) was conducted, and the favorable outcome with tPA remained.
Bottom line: Treatment of acute ischemic stroke with tPA in the three- to 4.5-hour time window results in an increased rate of favorable functional outcomes without a significant difference in mortality.
Citation: Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
Outpatients Often Are Not Notified of Clinically Significant Test Results
Clinical question: How frequently do primary-care physicians (PCPs) fail to inform patients of clinically significant outpatient test results?
Background: Diagnostic errors are the most common cause of malpractice claims in the U.S. It is unclear how often providers fail to either inform patients of abnormal test results or document that patients have been notified.
Study design: Retrospective chart review.
Setting: Twenty-three primary-care practices: 19 private, four academic.
Synopsis: More than 5,400 charts were reviewed, and 1,889 abnormal test results were identified in this study. Failure to inform or document notification was identified in 135 cases (7.1%). The failure rates in the practices ranged from 0.0% to 26.2%. Practices with the best processes for managing test results had the lowest failure rates; these processes included: all results being routed to the responsible physician; the physician signing off on all results; the practice informing patients of all results, both normal and abnormal; documenting when the patient is informed; and instructing patients to call if not notified of test results within a certain time interval.
Limitations of this study include the potential of over- or underreporting of failures to inform as a chart review was used, and only practices that agreed to participate were included.
Bottom line: Failure to notify outpatients of test results is common but can be minimized by creating a systematic management of test results that include best practices.
Citation: Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med. 2009;169(12):1123-1129.
Repair of Incidental PFO Discovered During Cardiothoracic Surgery Repair Increases Postoperative Stroke Risk
Clinical question: What is the impact of closing incidentally discovered patent foramen ovale (PFO) defects during cardiothoracic surgery?
Background: PFO’s role in cryptogenic stroke remains controversial. Incidental PFO is commonly detected by transesophageal echocardiography (TEE) during cardiothoracic surgery. Routine PFO closure has been recommended when almost no alteration of the surgical plan is required.
Study design: Retrospective chart review.
Setting: The Cleveland Clinic.
Synopsis: Between 1995 and 2006, 13,092 patients undergoing cardiothoracic surgery had TEE data with no previous diagnosis of PFO, but the review found that 2,277 (17%) had PFO discovered intraoperatively. Of these, 639 (28%) had the PFO repaired.
Patients with an intraoperative diagnosis of PFO had similar rates of in-hospital stroke and hospital death compared with those without PFO. Patients who had their PFO repaired had a greater in-hospital stroke risk (2.8% vs. 1.2%; P=0.04) compared with those with a non-repaired PFO, representing nearly 2.5 times greater odds of having an in-hospital stroke. No other difference was noted in perioperative outcomes for patients who underwent intraoperative repair compared with those who did not, including risk of in-hospital death, hospital length of stay, ICU length of stay, and time on cardiopulmonary bypass. Long-term analysis demonstrated that PFO repair was associated with no survival difference.
The study is limited by its retrospective nature.
Bottom line: Routine surgical closure of incidental PFO detected during intraoperative imaging should be discouraged.
Citation: Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of interoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA. 2009;302(3):290-297.
Hospital-Level Differences Are Strong Predictors of Time to Defibrillation Delay In Cardiac Arrest
Clinical question: What are the predictors of delay in the time to defibrillation after in-hospital cardiac arrest?
Background: Thirty percent of in-hospital cardiac arrests from ventricular arrhythmias are not treated within the American Heart Association’s recommendation of two minutes. This delay is associated with a 50% lower rate of in-hospital survival. Exploring the hospital-level variation in delays to defibrillation is a critical step toward sharing the best practices.
Study design: Retrospective review of registry data.
Setting: The National Registry of Cardiopulmonary Resuscitation (NRCPR) survey of 200 acute-care, nonpediatric hospitals.
Synopsis: The registry identified 7,479 patients who experienced cardiac arrest from ventricular tachycardia or pulseless ventricular fibrillation. The primary outcome was the hospital rate of delayed defibrillation (time to defibrillation > two minutes), which ranged from 2% to 51%.
Time to defibrillation was found to be a major predictor of survival after a cardiac arrest. Only bed volume and arrest location were associated with differences in rates of delayed defibrillation (lower rates in larger hospitals and in ICUs). The variability was not due to differences in patient characteristics, but was due to hospital-level effects. Academic status, geographical location, arrest volume, and daily admission volume did not affect the time to defibrillation.
The study was able to identify only a few facility characteristics that account for the variability between hospitals in the rate of delayed defibrillation. The study emphasizes the need for new approaches to identifying hospital innovations in process-of-care measures that are associated with improved performance in defibrillation times.
Bottom Line: Future research is needed to better understand the reason for the wide variation between hospitals in the rate of delayed defibrillation after in-hospital cardiac arrest.
Citation: Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265-1273.
Treating for H. Pylori Reduces the Risk for Developing Gastric Cancer in High-Risk Patients
Clinical question: In patients with high-baseline incidence of gastric cancer, does H. pylori eradication reduce the risk for developing gastric cancer?
Background: Gastric cancer remains a major health problem in Asia. The link of H. pylori and gastric cancer has been established, but it remains unclear whether treatment for H. pylori is effective primary prevention for the development of gastric cancer.
Study design: Meta-analysis of six studies.
Setting: All but one trial was performed in Asia.
Synopsis: Seven studies met inclusion criteria, one of which was excluded due to heterogeneity. The six remaining studies were pooled, with 37 of 3,388 (1.1%) treated patients developing a new gastric cancer, compared with 56 of 3,307 (1.7%) patients who received placebo or were in the control group (RR 0.65; 0.43-0.98). Most patients received gastric biopsy prior to enrollment, and most of those demonstrated gastric atrophy or intestinal metaplasia.
These patients, despite more advanced precancerous pathology findings, still benefited from eradication. The seventh study, which was excluded, enrolled patients with early gastric cancer; these patients still benefited from H. pylori eradication and, when included in the meta-analysis, the RR was even lower, 0.57 (0.49-0.81).
Only two trials were double-blinded, but all of the studies employed the same definition of gastric cancer and held to excellent data reporting standards. This study encourages screening and treatment in high-risk patients given the widespread incidence of H. pylori.
Bottom Line: Treatment of H. pylori reduces the risk of gastric cancer in high-risk patients.
Citation: Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121-128.
Patients on Anti-Platelet Agents with Acute Coronary Syndrome Have a Lower Bleeding Risk When Treated with Fondaparinux
Clinical question: Is there a difference in bleeding risk with fondaparinux and enoxaparin when used with GPIIb/IIIa inhibitors or thienopyridines in NSTEMI-ACS?
Background: The OASIS 5 study reported a 50% reduction in severe bleeding when comparing fondaparinux to enoxaparin in ACS while maintaining a similar efficacy. This subgroup analysis was performed to evaluate whether reduced bleeding risk with fondaparinux remains in patients treated with additional anti-platelet agents.
Study design: Subgroup analysis of a large, multicenter, randomized, double-blind trial.
Setting: Acute-care hospitals in North America, Eastern and Western Europe, Latin America, Australia, and Asia.
Synopsis: Patients with NSTE-ACS received either fondaparinux or enoxaparin and were treated with GPIIb/IIIa inhibitors or thienopyridines at the discretion of their physician. At 30 days, the fondaparinux group had similar efficacy and decreased bleeding risk in both the GPIIb/IIIa and the thienopyridine groups. Of the 3,630 patients in the GPIIb/IIIa group, the risk for major bleeding with fondaparinux was 5.2%, whereas the risk with enoxaparin was 8.3% (HR 0.61; P<0.001) compared with enoxaparin. Of the 1,352 patients treated with thienopyridines, the risk for major bleeding with fondaparinux was 3.4%, whereas the risk with enoxaparin was 5.4% (HR 0.62; P<0.001).
Bottom Line: This subgroup analysis suggests there are less-severe bleeding complications in patients treated with fondaparinux when compared with enoxaparin in the setting of cotreatment with GPIIb/IIIa inhibitors, thienopyridines, or both.
Citation: Jolly SS, Faxon DP, Fox KA, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors of thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009;54(5):468-476.
Surrogate Decision-Makers Frequently Doubt Clinicians’ Ability to Predict Medical Futility
Clinical question: What attitudes do surrogate decision-makers hold toward clinicians’ predictions of medical futility in critically-ill patients?
Background: The clinical judgment of medical futility leading to the withdrawal of life-sustaining treatment—despite the objections of surrogate decision-makers—is controversial. Very little is known about how surrogate decision-makers view the futility rationale when physicians suggest limiting the use of life-sustaining treatment.
Study design: Multicenter, mixed, qualitative and quantitative study.
Setting: Three ICUs in three different California hospitals from 2006 to 2007.
Synopsis: Semi-structured interviews of surrogate decision-makers for 50 incapacitated, critically-ill patients were performed to ascertain their beliefs about medical futility in response to hypothetical situations. Of the surrogates surveyed, 64% expressed doubt about physicians’ futility predictions.
The interviewees gave four main reasons for their doubts. Two reasons not previously described were doubts about the accuracy of physicians’ predictions and the need for surrogates to see futility themselves. Previously described sources of conflict included a misunderstanding about prognosis and religious-based objections. Surrogates with religious objections were more likely to request continuation of life-sustaining treatments than those with secular or experiential objections (OR 4; 95% CI 1.2-14.0; P=0.03). Nearly a third (32%) of surrogates elected to continue life support with a <1% survival estimate; 18% elected to continue life support when physicians thought there was no chance of survival.
This study has several limitations: a small sample size, the use of hypothetical situations, and the inability to assess attitudes as they change over time.
Bottom line: The nature of surrogate decision-makers’ doubts about medical futility can help predict whether they accept predictions of medical futility from physicians.
Citation: Zier LS, Burack JH, Micco G, Chipman AK, Frank JA, White DB. Surrogate decision makers’ responses to physicians’ predictions of medical futility. Chest. 2009;136:110-117. TH
Dr. Flanders discusses the challenges of hospitalized patient care
Scott Flanders, MD, FHM, director of the hospitalist division, University of Michigan Health System, Ann Arbor; thinks HM has tackled many of the patient-safety issues noted in the 1999 IOM report. He also says HM should continue to redesign strategies to continue to improve outcomes; "This is a long road—a marathon, not a sprint."
Click here to listen to the audio file.
Scott Flanders, MD, FHM, director of the hospitalist division, University of Michigan Health System, Ann Arbor; thinks HM has tackled many of the patient-safety issues noted in the 1999 IOM report. He also says HM should continue to redesign strategies to continue to improve outcomes; "This is a long road—a marathon, not a sprint."
Click here to listen to the audio file.
Scott Flanders, MD, FHM, director of the hospitalist division, University of Michigan Health System, Ann Arbor; thinks HM has tackled many of the patient-safety issues noted in the 1999 IOM report. He also says HM should continue to redesign strategies to continue to improve outcomes; "This is a long road—a marathon, not a sprint."
Click here to listen to the audio file.
Dr. Arora talks about HM's role in patient safety
Vineet Arora, MD, MA, FHM, assistant professor in the Department of Medicine at the University of Chicago, believes HM has played a part in patient-safety reform, but she also believes HM can do more to preserve continuity of care for patients as they transition in and out of the hospital.
Click here to listen to the audio file.
Vineet Arora, MD, MA, FHM, assistant professor in the Department of Medicine at the University of Chicago, believes HM has played a part in patient-safety reform, but she also believes HM can do more to preserve continuity of care for patients as they transition in and out of the hospital.
Click here to listen to the audio file.
Vineet Arora, MD, MA, FHM, assistant professor in the Department of Medicine at the University of Chicago, believes HM has played a part in patient-safety reform, but she also believes HM can do more to preserve continuity of care for patients as they transition in and out of the hospital.
Click here to listen to the audio file.
Dr. Wachter talks about patient safety
Robert Wachter, MD, FHM, chief of the hospitalist division, professor and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president, and author of the blog "Wachter's World," is outspoken in his belief that hospital medicine should be one the solutions to medical mistakes in U.S. hospitals.
Click here to listen to the audio file.
Robert Wachter, MD, FHM, chief of the hospitalist division, professor and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president, and author of the blog "Wachter's World," is outspoken in his belief that hospital medicine should be one the solutions to medical mistakes in U.S. hospitals.
Click here to listen to the audio file.
Robert Wachter, MD, FHM, chief of the hospitalist division, professor and associate chair of the Department of Medicine at the University of California at San Francisco, former SHM president, and author of the blog "Wachter's World," is outspoken in his belief that hospital medicine should be one the solutions to medical mistakes in U.S. hospitals.
Click here to listen to the audio file.
Mentored Implementation
When Kendall Rogers, MD, signed up for his first mentored implementation project, he remembers being skeptical. After all, it seemed too good to be true. “I wanted to ask, ‘What’s the catch? Are you trying to get us to adopt a certain practice?’ ” says Dr. Rogers, a hospitalist at the University of New Mexico Health Science Center School of Medicine in Albuquerque.
Now, after participating in SHM’s Venous Thromboembolism (VTE) Prevention Collaborative and later mentoring other hospitalists in SHM’s Glycemic Control Mentored Implemen-tation (GCMI) program, he understands the motivation.
“Mentored implementation is unique in that it accomplishes two goals,” he says. “It improves the nuts and bolts of a project, and it also creates new hospitalist leaders and quality-improvement [QI] experts.”
Prior to his work in the VTE Prevention Collaborative, Dr. Rogers had little exposure to QI programs. He has since implemented a VTE prevention program at his hospital, and his mentorship of hospitalists in the GCMI program is helping to create custom programs to optimize glycemic control protocols. He also is a faculty member for SHM’s QI and patient-safety pre-course and is leading SHM training sessions on VTE prevention.
The mentored implementation model, he says, is an effective way to get over many of the daunting roadblocks that can stand in the way of a hospitalist-led QI program. “Many people need that spark,” Dr. Rogers says. “This is a highly effective way to be that spark. I’ve seen too many people get disillusioned and frustrated with quality-improvement programs and give up. In these programs, the mentor can help identify and address roadblocks.”
What is Mentored Implementation?
In theory, mentored implementation is a unique and simple approach to both education and QI in healthcare. At its core, mentored implementation is the pairing of a program participant with a subject-matter expert who already has been involved in similar programs and will help the participant implement a QI program of their own.
The concept is new to QI initiatives. Although SHM has embraced the idea, mentored implementation programs first started at the Center to Advance Palliative Care in New York City, says Kathleen Kerr, SHM’s program manager for mentored implementation programs and senior research analyst in the Department of Medicine at the University of California at San Francisco. The model is an alternative to more traditional educational approaches that rely exclusively on lectures or educational sessions.
“You could sit in a session and it’s very valuable, but also very different from actually doing it,” Kerr says. “It’s hard to process so much information in a session. You don’t understand the complexity of something like gathering data until you’re actually doing it. The mentor can tailor what they’re teaching to the exact stage of the project.”
In practice, the most effective mentored implementation projects create multiple layers of support for both the mentor and the participant. SHM’s mentored implementation programs include online resource rooms on the topic (e.g., glycemic control or hospital discharge) and collaboration between participants. Rather than being just repositories of information on the subject, SHM’s resource rooms are roadmaps for new programs.
“SHM’s resource rooms define an intervention that can be implemented,” says Geri Barnes, SHM’s senior director of education and meetings.
Those resources, plus ongoing guidance from mentors, help hospitalists implement QI programs at their hospitals. Many hospitalists are early in their careers and benefit from all of the resources available. The energy that early-career hospitalists bring to QI is one of the key components the program harnesses, Kerr says.
“Junior staff are really motivated to do things in their scope, but there aren’t really a lot of mid-career local mentors” who can provide the guidance they need, Kerr says.
Training Days
Given SHM’s focus on QI and the relative youth of both HM as a specialty and hospitalists in relation to their peers, the mentored implementation model seems particularly suited to hospitalists. Launched in 2007, the VTE Prevention Collaborative was SHM’s first mentored implementation program. It was designed to help hospitalists create custom programs to prevent VTE. The collaborative included mentors, an online resource room, and on-site consultations with experts.

—Kendall Rogers, MD, University of New Mexico Health Science Center School of Medicine, Albuquerque
SHM created Project BOOST (Better Outcomes for Older adults through Safe Transitions) in 2008. Project BOOST began with six pilot sites and has now expanded to 30 sites. Each hospital site can participate in daylong training sessions and yearlong mentorships. Sites also receive the Project BOOST implementation guide from SHM’s resource room. Since it was posted in July 2008, more than 250 hospitals have downloaded the guide.
In 2009, SHM and hospitalists are teaming up in 30 different sites across the country to improve early detection and treatment of hyperglycemia in hospitalized patients through the Glycemic Control Mentored Implementation program. Each participant in the two-year program receives a toolkit, access to Web-based resources, and is assigned a mentor to guide implementation.
MI 2.0
Despite early successes with SHM’s mentored implementation programs, those closest to them acknowledge there is room for improvement. Among a host of factors is the success of the next generation of programs, which will hinge on the idea’s scalability.
“We’re looking at testing models where we have a one-to-one mentoring program, compared to a one-to-five mentoring program,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives.
Kerr also sees opportunities to expand the scope without sacrificing the customized approach. “We are looking for ways to expand the reach of each individual effort. Right now, customization means that mentored implementation is more like building a Ferrari than a Ford,” she says. “We need to do some ‘train the trainer’ models and explore ways to reach more hospitals simultaneously.”
For Dr. Rogers, his experience with mentored implementation and QI has strengthened his resolve to help hospitalists get it right.
“We have a lot to learn to do this effectively. We have 5,000 hospitals out there and hospitalists are naturally looked at as leaders within the institution,” he says. “The failure of one hospitalist quality-improvement program affects all of us, so success is key. This is one of the most effective tools for doing it.” TH
Brandon Shank is a freelance writer based in Philadelphia.
Letters
The Unique Potential of Hospitalists as Leaders in Healthcare Reform
The usual first response when a physician is asked, “Why do you practice medicine?” is “to help people.” This is especially true for younger practitioners. A frequent second response is “I like the independence.” As physicians, we enjoy being our own boss and calling the shots.
Therein rests the cultural healthcare quandary. Physicians need to accept the fact that standardization of medicine is going to happen, as it allows for improved efficiencies with a resultant decrease in healthcare expenditures. Yet the independent and entrepreneurial nature of physicians has caused them to resist the standardization of medicine for years. After all, while one fellow physician might treat a disease or perform a procedure differently than another, as long as it is efficacious, we all believe our peers should be able to practice the way they want.
Hospitalists are no different, as they are independent, too. They are simply working under the hospital umbrella. This relationship of working in hospitals positions HM practitioners, as a group, to be central players in the healthcare reform debate. This truly is a unique opportunity.
Looking demographically at the generational makeup of all physicians, we have four familiar groups represented: baby boomers, Gen X’ers, Gen Y’ers, and millennials. There are certain broad yet defining characteristics of these four generational groups. The baby boomers, being the offspring of the World War II generation, the generation that rebuilt the world and kept their “nose to the grindstone,” are defined by their work ethic. Simply put, boomers live to work. As children and students of the 1960s, they also value individuality.
Gen X’ers focus more on themselves, and often are referred to as the “me generation.” They expect to have a range of choices within their expression of individuality.
Gen Y’ers have a different work ethic, one their managers often find alarming. They are defined by the adage “work to live.” This dilemma, while difficult for their managers, allows Gen Y’ers to adapt to workplace practices, as their individuality is no longer of primary concern. After all, “it is only work.”
Millennials, having been brought up in the digital age, are bombarded with information and entertainment 24 hours a day. From birth on, they have heard that the future is uncertain. Demographically, they are more aligned with the work ethic of their great-grandparents, the World War II generation, and they are more willing to serve the common good. Thus, millennials, like Generation Y, are less individualistic and more willing to adapt to the work environment.
In considering hospitalists and their roles in the current healthcare debate and medical standards, this young specialty is uniquely poised to implement the upcoming standardizations required for three reasons. First, HM has an unusually large representation of Gen Y’ers and millennials—more than other medical specialties. These younger physicians, with their adaptability for the common good, are less resistant to the standardization of medicine.
Second, unlike most practitioners, hospitalists tend to practice in larger medical groups. Thus, they are familiar with standardization and the uniformity necessary for the group to practice effectively.
Third, with the Centers for Medicare and Medicaid Services (CMS) adopting the experimental payment mechanism known as value-based purchasing, hospitals will insist on standardization to maximize reimbursement.
The benefits to HM practitioners are twofold. The hospitalist will share in reimbursement of pay-for-performance, thereby gaining a financial incentive for the greater efficiencies that standardization yields. This is evidenced by the trend that hospitalist contracts are increasingly based on pay-for-performance, rather than payment based on relative value units.
The second benefit, and perhaps the most important, is that the influence and power of hospitalists will greatly increase, particularly in formulating the standards of medical treatment, procedures, and, more importantly, QI and patient safety.
As the practice of HM matures from infancy into adolescence, recognizing the opportunity at hand and deciding how to proceed is paramount to its future position and existence.
Michael G. Cassatly, DMD
Certified business coach,
American Board of Oral and Maxillofacial Surgery diplomate
When Kendall Rogers, MD, signed up for his first mentored implementation project, he remembers being skeptical. After all, it seemed too good to be true. “I wanted to ask, ‘What’s the catch? Are you trying to get us to adopt a certain practice?’ ” says Dr. Rogers, a hospitalist at the University of New Mexico Health Science Center School of Medicine in Albuquerque.
Now, after participating in SHM’s Venous Thromboembolism (VTE) Prevention Collaborative and later mentoring other hospitalists in SHM’s Glycemic Control Mentored Implemen-tation (GCMI) program, he understands the motivation.
“Mentored implementation is unique in that it accomplishes two goals,” he says. “It improves the nuts and bolts of a project, and it also creates new hospitalist leaders and quality-improvement [QI] experts.”
Prior to his work in the VTE Prevention Collaborative, Dr. Rogers had little exposure to QI programs. He has since implemented a VTE prevention program at his hospital, and his mentorship of hospitalists in the GCMI program is helping to create custom programs to optimize glycemic control protocols. He also is a faculty member for SHM’s QI and patient-safety pre-course and is leading SHM training sessions on VTE prevention.
The mentored implementation model, he says, is an effective way to get over many of the daunting roadblocks that can stand in the way of a hospitalist-led QI program. “Many people need that spark,” Dr. Rogers says. “This is a highly effective way to be that spark. I’ve seen too many people get disillusioned and frustrated with quality-improvement programs and give up. In these programs, the mentor can help identify and address roadblocks.”
What is Mentored Implementation?
In theory, mentored implementation is a unique and simple approach to both education and QI in healthcare. At its core, mentored implementation is the pairing of a program participant with a subject-matter expert who already has been involved in similar programs and will help the participant implement a QI program of their own.
The concept is new to QI initiatives. Although SHM has embraced the idea, mentored implementation programs first started at the Center to Advance Palliative Care in New York City, says Kathleen Kerr, SHM’s program manager for mentored implementation programs and senior research analyst in the Department of Medicine at the University of California at San Francisco. The model is an alternative to more traditional educational approaches that rely exclusively on lectures or educational sessions.
“You could sit in a session and it’s very valuable, but also very different from actually doing it,” Kerr says. “It’s hard to process so much information in a session. You don’t understand the complexity of something like gathering data until you’re actually doing it. The mentor can tailor what they’re teaching to the exact stage of the project.”
In practice, the most effective mentored implementation projects create multiple layers of support for both the mentor and the participant. SHM’s mentored implementation programs include online resource rooms on the topic (e.g., glycemic control or hospital discharge) and collaboration between participants. Rather than being just repositories of information on the subject, SHM’s resource rooms are roadmaps for new programs.
“SHM’s resource rooms define an intervention that can be implemented,” says Geri Barnes, SHM’s senior director of education and meetings.
Those resources, plus ongoing guidance from mentors, help hospitalists implement QI programs at their hospitals. Many hospitalists are early in their careers and benefit from all of the resources available. The energy that early-career hospitalists bring to QI is one of the key components the program harnesses, Kerr says.
“Junior staff are really motivated to do things in their scope, but there aren’t really a lot of mid-career local mentors” who can provide the guidance they need, Kerr says.
Training Days
Given SHM’s focus on QI and the relative youth of both HM as a specialty and hospitalists in relation to their peers, the mentored implementation model seems particularly suited to hospitalists. Launched in 2007, the VTE Prevention Collaborative was SHM’s first mentored implementation program. It was designed to help hospitalists create custom programs to prevent VTE. The collaborative included mentors, an online resource room, and on-site consultations with experts.

—Kendall Rogers, MD, University of New Mexico Health Science Center School of Medicine, Albuquerque
SHM created Project BOOST (Better Outcomes for Older adults through Safe Transitions) in 2008. Project BOOST began with six pilot sites and has now expanded to 30 sites. Each hospital site can participate in daylong training sessions and yearlong mentorships. Sites also receive the Project BOOST implementation guide from SHM’s resource room. Since it was posted in July 2008, more than 250 hospitals have downloaded the guide.
In 2009, SHM and hospitalists are teaming up in 30 different sites across the country to improve early detection and treatment of hyperglycemia in hospitalized patients through the Glycemic Control Mentored Implementation program. Each participant in the two-year program receives a toolkit, access to Web-based resources, and is assigned a mentor to guide implementation.
MI 2.0
Despite early successes with SHM’s mentored implementation programs, those closest to them acknowledge there is room for improvement. Among a host of factors is the success of the next generation of programs, which will hinge on the idea’s scalability.
“We’re looking at testing models where we have a one-to-one mentoring program, compared to a one-to-five mentoring program,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives.
Kerr also sees opportunities to expand the scope without sacrificing the customized approach. “We are looking for ways to expand the reach of each individual effort. Right now, customization means that mentored implementation is more like building a Ferrari than a Ford,” she says. “We need to do some ‘train the trainer’ models and explore ways to reach more hospitals simultaneously.”
For Dr. Rogers, his experience with mentored implementation and QI has strengthened his resolve to help hospitalists get it right.
“We have a lot to learn to do this effectively. We have 5,000 hospitals out there and hospitalists are naturally looked at as leaders within the institution,” he says. “The failure of one hospitalist quality-improvement program affects all of us, so success is key. This is one of the most effective tools for doing it.” TH
Brandon Shank is a freelance writer based in Philadelphia.
Letters
The Unique Potential of Hospitalists as Leaders in Healthcare Reform
The usual first response when a physician is asked, “Why do you practice medicine?” is “to help people.” This is especially true for younger practitioners. A frequent second response is “I like the independence.” As physicians, we enjoy being our own boss and calling the shots.
Therein rests the cultural healthcare quandary. Physicians need to accept the fact that standardization of medicine is going to happen, as it allows for improved efficiencies with a resultant decrease in healthcare expenditures. Yet the independent and entrepreneurial nature of physicians has caused them to resist the standardization of medicine for years. After all, while one fellow physician might treat a disease or perform a procedure differently than another, as long as it is efficacious, we all believe our peers should be able to practice the way they want.
Hospitalists are no different, as they are independent, too. They are simply working under the hospital umbrella. This relationship of working in hospitals positions HM practitioners, as a group, to be central players in the healthcare reform debate. This truly is a unique opportunity.
Looking demographically at the generational makeup of all physicians, we have four familiar groups represented: baby boomers, Gen X’ers, Gen Y’ers, and millennials. There are certain broad yet defining characteristics of these four generational groups. The baby boomers, being the offspring of the World War II generation, the generation that rebuilt the world and kept their “nose to the grindstone,” are defined by their work ethic. Simply put, boomers live to work. As children and students of the 1960s, they also value individuality.
Gen X’ers focus more on themselves, and often are referred to as the “me generation.” They expect to have a range of choices within their expression of individuality.
Gen Y’ers have a different work ethic, one their managers often find alarming. They are defined by the adage “work to live.” This dilemma, while difficult for their managers, allows Gen Y’ers to adapt to workplace practices, as their individuality is no longer of primary concern. After all, “it is only work.”
Millennials, having been brought up in the digital age, are bombarded with information and entertainment 24 hours a day. From birth on, they have heard that the future is uncertain. Demographically, they are more aligned with the work ethic of their great-grandparents, the World War II generation, and they are more willing to serve the common good. Thus, millennials, like Generation Y, are less individualistic and more willing to adapt to the work environment.
In considering hospitalists and their roles in the current healthcare debate and medical standards, this young specialty is uniquely poised to implement the upcoming standardizations required for three reasons. First, HM has an unusually large representation of Gen Y’ers and millennials—more than other medical specialties. These younger physicians, with their adaptability for the common good, are less resistant to the standardization of medicine.
Second, unlike most practitioners, hospitalists tend to practice in larger medical groups. Thus, they are familiar with standardization and the uniformity necessary for the group to practice effectively.
Third, with the Centers for Medicare and Medicaid Services (CMS) adopting the experimental payment mechanism known as value-based purchasing, hospitals will insist on standardization to maximize reimbursement.
The benefits to HM practitioners are twofold. The hospitalist will share in reimbursement of pay-for-performance, thereby gaining a financial incentive for the greater efficiencies that standardization yields. This is evidenced by the trend that hospitalist contracts are increasingly based on pay-for-performance, rather than payment based on relative value units.
The second benefit, and perhaps the most important, is that the influence and power of hospitalists will greatly increase, particularly in formulating the standards of medical treatment, procedures, and, more importantly, QI and patient safety.
As the practice of HM matures from infancy into adolescence, recognizing the opportunity at hand and deciding how to proceed is paramount to its future position and existence.
Michael G. Cassatly, DMD
Certified business coach,
American Board of Oral and Maxillofacial Surgery diplomate
When Kendall Rogers, MD, signed up for his first mentored implementation project, he remembers being skeptical. After all, it seemed too good to be true. “I wanted to ask, ‘What’s the catch? Are you trying to get us to adopt a certain practice?’ ” says Dr. Rogers, a hospitalist at the University of New Mexico Health Science Center School of Medicine in Albuquerque.
Now, after participating in SHM’s Venous Thromboembolism (VTE) Prevention Collaborative and later mentoring other hospitalists in SHM’s Glycemic Control Mentored Implemen-tation (GCMI) program, he understands the motivation.
“Mentored implementation is unique in that it accomplishes two goals,” he says. “It improves the nuts and bolts of a project, and it also creates new hospitalist leaders and quality-improvement [QI] experts.”
Prior to his work in the VTE Prevention Collaborative, Dr. Rogers had little exposure to QI programs. He has since implemented a VTE prevention program at his hospital, and his mentorship of hospitalists in the GCMI program is helping to create custom programs to optimize glycemic control protocols. He also is a faculty member for SHM’s QI and patient-safety pre-course and is leading SHM training sessions on VTE prevention.
The mentored implementation model, he says, is an effective way to get over many of the daunting roadblocks that can stand in the way of a hospitalist-led QI program. “Many people need that spark,” Dr. Rogers says. “This is a highly effective way to be that spark. I’ve seen too many people get disillusioned and frustrated with quality-improvement programs and give up. In these programs, the mentor can help identify and address roadblocks.”
What is Mentored Implementation?
In theory, mentored implementation is a unique and simple approach to both education and QI in healthcare. At its core, mentored implementation is the pairing of a program participant with a subject-matter expert who already has been involved in similar programs and will help the participant implement a QI program of their own.
The concept is new to QI initiatives. Although SHM has embraced the idea, mentored implementation programs first started at the Center to Advance Palliative Care in New York City, says Kathleen Kerr, SHM’s program manager for mentored implementation programs and senior research analyst in the Department of Medicine at the University of California at San Francisco. The model is an alternative to more traditional educational approaches that rely exclusively on lectures or educational sessions.
“You could sit in a session and it’s very valuable, but also very different from actually doing it,” Kerr says. “It’s hard to process so much information in a session. You don’t understand the complexity of something like gathering data until you’re actually doing it. The mentor can tailor what they’re teaching to the exact stage of the project.”
In practice, the most effective mentored implementation projects create multiple layers of support for both the mentor and the participant. SHM’s mentored implementation programs include online resource rooms on the topic (e.g., glycemic control or hospital discharge) and collaboration between participants. Rather than being just repositories of information on the subject, SHM’s resource rooms are roadmaps for new programs.
“SHM’s resource rooms define an intervention that can be implemented,” says Geri Barnes, SHM’s senior director of education and meetings.
Those resources, plus ongoing guidance from mentors, help hospitalists implement QI programs at their hospitals. Many hospitalists are early in their careers and benefit from all of the resources available. The energy that early-career hospitalists bring to QI is one of the key components the program harnesses, Kerr says.
“Junior staff are really motivated to do things in their scope, but there aren’t really a lot of mid-career local mentors” who can provide the guidance they need, Kerr says.
Training Days
Given SHM’s focus on QI and the relative youth of both HM as a specialty and hospitalists in relation to their peers, the mentored implementation model seems particularly suited to hospitalists. Launched in 2007, the VTE Prevention Collaborative was SHM’s first mentored implementation program. It was designed to help hospitalists create custom programs to prevent VTE. The collaborative included mentors, an online resource room, and on-site consultations with experts.

—Kendall Rogers, MD, University of New Mexico Health Science Center School of Medicine, Albuquerque
SHM created Project BOOST (Better Outcomes for Older adults through Safe Transitions) in 2008. Project BOOST began with six pilot sites and has now expanded to 30 sites. Each hospital site can participate in daylong training sessions and yearlong mentorships. Sites also receive the Project BOOST implementation guide from SHM’s resource room. Since it was posted in July 2008, more than 250 hospitals have downloaded the guide.
In 2009, SHM and hospitalists are teaming up in 30 different sites across the country to improve early detection and treatment of hyperglycemia in hospitalized patients through the Glycemic Control Mentored Implementation program. Each participant in the two-year program receives a toolkit, access to Web-based resources, and is assigned a mentor to guide implementation.
MI 2.0
Despite early successes with SHM’s mentored implementation programs, those closest to them acknowledge there is room for improvement. Among a host of factors is the success of the next generation of programs, which will hinge on the idea’s scalability.
“We’re looking at testing models where we have a one-to-one mentoring program, compared to a one-to-five mentoring program,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives.
Kerr also sees opportunities to expand the scope without sacrificing the customized approach. “We are looking for ways to expand the reach of each individual effort. Right now, customization means that mentored implementation is more like building a Ferrari than a Ford,” she says. “We need to do some ‘train the trainer’ models and explore ways to reach more hospitals simultaneously.”
For Dr. Rogers, his experience with mentored implementation and QI has strengthened his resolve to help hospitalists get it right.
“We have a lot to learn to do this effectively. We have 5,000 hospitals out there and hospitalists are naturally looked at as leaders within the institution,” he says. “The failure of one hospitalist quality-improvement program affects all of us, so success is key. This is one of the most effective tools for doing it.” TH
Brandon Shank is a freelance writer based in Philadelphia.
Letters
The Unique Potential of Hospitalists as Leaders in Healthcare Reform
The usual first response when a physician is asked, “Why do you practice medicine?” is “to help people.” This is especially true for younger practitioners. A frequent second response is “I like the independence.” As physicians, we enjoy being our own boss and calling the shots.
Therein rests the cultural healthcare quandary. Physicians need to accept the fact that standardization of medicine is going to happen, as it allows for improved efficiencies with a resultant decrease in healthcare expenditures. Yet the independent and entrepreneurial nature of physicians has caused them to resist the standardization of medicine for years. After all, while one fellow physician might treat a disease or perform a procedure differently than another, as long as it is efficacious, we all believe our peers should be able to practice the way they want.
Hospitalists are no different, as they are independent, too. They are simply working under the hospital umbrella. This relationship of working in hospitals positions HM practitioners, as a group, to be central players in the healthcare reform debate. This truly is a unique opportunity.
Looking demographically at the generational makeup of all physicians, we have four familiar groups represented: baby boomers, Gen X’ers, Gen Y’ers, and millennials. There are certain broad yet defining characteristics of these four generational groups. The baby boomers, being the offspring of the World War II generation, the generation that rebuilt the world and kept their “nose to the grindstone,” are defined by their work ethic. Simply put, boomers live to work. As children and students of the 1960s, they also value individuality.
Gen X’ers focus more on themselves, and often are referred to as the “me generation.” They expect to have a range of choices within their expression of individuality.
Gen Y’ers have a different work ethic, one their managers often find alarming. They are defined by the adage “work to live.” This dilemma, while difficult for their managers, allows Gen Y’ers to adapt to workplace practices, as their individuality is no longer of primary concern. After all, “it is only work.”
Millennials, having been brought up in the digital age, are bombarded with information and entertainment 24 hours a day. From birth on, they have heard that the future is uncertain. Demographically, they are more aligned with the work ethic of their great-grandparents, the World War II generation, and they are more willing to serve the common good. Thus, millennials, like Generation Y, are less individualistic and more willing to adapt to the work environment.
In considering hospitalists and their roles in the current healthcare debate and medical standards, this young specialty is uniquely poised to implement the upcoming standardizations required for three reasons. First, HM has an unusually large representation of Gen Y’ers and millennials—more than other medical specialties. These younger physicians, with their adaptability for the common good, are less resistant to the standardization of medicine.
Second, unlike most practitioners, hospitalists tend to practice in larger medical groups. Thus, they are familiar with standardization and the uniformity necessary for the group to practice effectively.
Third, with the Centers for Medicare and Medicaid Services (CMS) adopting the experimental payment mechanism known as value-based purchasing, hospitals will insist on standardization to maximize reimbursement.
The benefits to HM practitioners are twofold. The hospitalist will share in reimbursement of pay-for-performance, thereby gaining a financial incentive for the greater efficiencies that standardization yields. This is evidenced by the trend that hospitalist contracts are increasingly based on pay-for-performance, rather than payment based on relative value units.
The second benefit, and perhaps the most important, is that the influence and power of hospitalists will greatly increase, particularly in formulating the standards of medical treatment, procedures, and, more importantly, QI and patient safety.
As the practice of HM matures from infancy into adolescence, recognizing the opportunity at hand and deciding how to proceed is paramount to its future position and existence.
Michael G. Cassatly, DMD
Certified business coach,
American Board of Oral and Maxillofacial Surgery diplomate
A-Plus Achievement
Timing is everything. Christopher Columbus, Abraham Lincoln, Harry Truman … each benefited from perfect timing and, in turn, helped change the course of history.
HM has had great timing, too. With numbers now estimated at more than 30,000 hospitalists nationwide, HM is systematically changing the way patients are cared for in the hospital. The maturation process is equally evident. In less than two decades, HM has organized annual meetings, developed educational programs, established a peer-reviewed journal, and published core competencies.
The next step in the evolutionary process: the Recognition of Focused Practice (RFP) in Hospital Medicine through the American Board of Internal Medicine’s (ABIM) maintenance of certification (MOC) program. Registration for the RFP in HM should be available by May 2010, with the first MOC in fall 2010.
“The timing is perfect. It’s appropriate,” says Robert Wachter, MD, FHM, chief of the hospital medicine
division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World. “We knew we needed to ripen and mature. It’s like watching your child growing up: their first steps, first day of school, graduating high school. This has a lot of the same feeling. This is one more statement that the field is real, here to stay, and vitally important to medicine.”
The new pathway to board recertification is as meaningful to HM’s founding fathers as it will be to the next generation of hospitalists. It represents validation to physicians who have chosen a career in HM, and it offers early-career physicians a specialized path to recertification. Moreover, hospitalists agree the RFP in HM provides accountability to the profession and patients.
“Hospitalists must be able to exhibit clinical competence in the areas of patient safety, quality, and clinical care transitions,” says Jeff Wiese, MD, FACP, FHM, associate dean of Graduate Medical Education and professor of medicine at Tulane University in New Orleans, SHM president-elect, and chair of the ABIM Hospital Medicine Maintenance of Certification Question Writing Committee. “Everything is driving at those issues. Individuals who go through the four parts of this certification will be able to say to their patients, with confidence, that one, they are a competent internist knowledgeable in hospital-based care, and two, they are an expert in patient safety, quality, and transitions of care.”
ABIM, for its part, expects to learn from this “new paradigm,” says Eric Holmbloe, MD, ABIM’s senior vice president and chief medical officer.
“The major change is the ability to implement the concept of a focused practice. It’s the first time, and it does recognize that the world has changed,” Dr. Holmbloe says, noting his group is “working feverishly” to complete the examination and build the online infrastructure needed for testing. “For those individuals whose [certificates] expire in 2010 or 2011, this is a viable pathway for recertification.”
Satisfaction Guaranteed
Ask any long-in-the-tooth hospitalist what the RFP in HM means to them, and more often than not the answer centers on professional self-regulation and career validation. Few will argue the specialty has, at times, suffered from an identity crisis. Sans fellowships, specialized training, or a particular organ to hang their hat on, HM physicians have labored to carve their niche and gain a seat at the specialist’s table.
The RFP in HM, many hospitalists envision, solves a number of those acceptance-related issues (see “Certified Special,” p. 53). Most importantly, it identifies physicians who have chosen to make HM their career.
“Hospitalists have always struggled, especially in academic settings,” says Scott Flanders, MD, FHM, SHM president, associate professor and director of the hospitalist program at the University of Michigan Health System in Ann Arbor. “I think this is a major, major moment for the field. It validates the field, and the belief that HM is a positive [for medicine].”
The new designation likely has greater meaning to older HM physicians, those who remember the early days of society formation (i.e., the National Association of Inpatient Physicians) and annual meeting attendance in the hundreds, not thousands.
“With the older docs, validation is particularly meaningful,” says Dr. Wachter, who, along with Lee Goldman, MD, first coined the term “hospitalist” in a 1996 article in the New England Journal of Medicine.1 “Paradoxically, most of the older physicians don’t have to take this test.”
That might be true, but only a small percentage of the 30,000-plus hospitalists in the U.S. are grandfathered into time-unlimited IM certificates. ABIM began time-limited certificates in 1990. The average hospitalist is 40, according to SHM’s “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement.”
John Nelson, MD, doesn’t have to recertify, but the co-founder of SHM is planning to be among the first to take the test. “It’s the first way hospitalists will be able to show their competence,” says Dr. Nelson, FACP, FHM, past president of SHM, and a principal in the national practice management firm Nelson Flores Hospital Medicine Consultants. A regular contributor to The Hospitalist, Dr. Nelson says the RFP in HM “will help people take our field more seriously.”
Inclusive of IM
The RFP in HM pathway is not a way to distance hospitalists from their internal medicine (IM) training and certification. Nearly 85% of hospitalists are IM-trained, according to SHM’s 2007-2008 bi-annual survey, and clinical competence as an internist will be a requisite for recertification through the new ABIM test.
“HM is borne of internal medicine … and has evolved to something unique. Therefore, your credentials should reflect that uniqueness,” says Larry Wellikson, MD, FHM, CEO of SHM. “This [process] is going to make the whole maintenance of certification process more relevant to hospitalists. This is recognition that hospitalists should be accountable for learning new things after they complete their formal training—things not emphasized in training like patient safety, quality, and care transitions.”
The language used on ABIM’s Web site to announce the new testing program furthers the message: “ABIM is developing a program to assess, set standards for, and recognize the proficiency of general internists who focus their practice in the care of hospitalized patients. The board’s decision to advance this program is consistent with its primary role of certifying internal-medicine physicians who meet the profession’s standards for focused practice in internal medicine.”
In addition to quality, patient safety, and transitions, “accountability to the patient” is a popular catchphrase used by members of the committee dedicated to writing the new certification test. Licensure and certification provide public accountability to patients, colleagues, and stakeholders, says Dr. Wiese. What differentiates career hospitalists from those making a pit stop during an alternate career path is increased accessibility to patients, expertise in patient safety and quality initiatives, and knowledge of clinical care transitions, he explains.
“Everything is driving at those issues,” he says. “From the beginning, there has been no agenda to change residency training. Any information contrary is a complete myth. Principle No. 1 is that you have to be competent internist. This new [MOC process] is much closer to an MBA.”
How hospitalists who are recertifying through the RFP in HM will refer to themselves remains up for debate. Although some physicians might say they are “board-certified in hospital medicine,” Dr. Wachter hopes hospitalists will use the phrase “board-certified in internal medicine with a recognized focused practice in hospital medicine.” “I hope to think our physicians will still say ‘IM,’ ” he says. “The goal here is not to sever ties with IM. That is a concern.”
Dr. Wiese, who, along with Dr. Wachter and other members of the test-writing committee, was required to complete the IM maintenance of certification (MOC), envisions a four-part process (see “Board Certification, With a Healthy Dose of HM,” p. 31) that challenges hospitalists in the core competencies of hospital-based practice.
“The [IM] recertification was the one test in my career that made me a better physician. I think this HM examination is going to be the same way,” Dr. Wiese says. “I want hospitalists to say, ‘When I go back to take care of my patients, they’ll be better off for it.’ ”
Target Audience
Dr. Flanders has about 40 hospitalists in his HM group at the University of Michigan. Although he recertified in IM in 2006, he knows many of his hospitalists are itching to take ABIM’s new HM-focused test. “They will be thrilled. They have all said to me that we can’t get this done fast enough,” he says, noting three or four of his hospitalists probably will recertify through the new test in fall 2010. “Our field is young; I suspect there are a lot of hospitalists out there who are within the 10-year window for recertification. I plan to do it at my next MOC … and that date could be moved up the way things are going.”
Dr. Wachter agrees the RFP in HM is an “attractive” option to hospitalists, especially those whose recertification is looming in the next two or three years. This MOC, he explains, “offers a pathway that is more in sync with the medicine [they are practicing] day in and day out.”
ABIM plans to have comprehensive information about the process available on its Web site (www.abim.org) this month and online registration available in May 2010 (see “FAQs,” left). While the test-writing committee finishes its tasks, Dr. Holmbloe says, ABIM’s systems department is working to build the online infrastructure. The first RFP in HM tests should go live in fall 2010.
“For those individuals [whose certificates] expire in 2010 or 2011, this is a viable pathway for recertification. If HM is their passion, this is for them,” Dr. Holmbloe says. “The major change, from ABIM’s perspective, is the ability to implement the concept of a focused practice. It’s the first time, a new paradigm. This does recognize that the world has changed.”
Crash Course
Every physician, sometime in his or her career, has crammed for a test. ABIM, however, recommends physicians start this process two or three years before their certificate expires. That timetable might work for some hospitalists, not so much for others. In any event, Dr. Flanders says hospitalists can count on SHM to help them prepare for the HM-specific examination.
“SHM has to help develop the tools and resources hospitalists will need to successfully prepare for and pass this test,” he says. He expects educational resources and self-assessment modules will be available on SHM’s Web site (www.hospitalmedicine.org) and at HM10, April 8-11 in Washington, D.C.
Dr. Wellikson says MOC preparedness “should match [SHM’s] educational projects,” and his staff “will continue to develop” tools and resources to assist hospitalists. He also recognizes the moment: the notch on HM’s evolutionary timeline where a once-fledgling group of inpatient physicians helped chart a new course for American medicine.
“Obviously, 10 years ago was too early. Now there are 30,000 hospitalists. Many of them are making HM a career. It’s evolving as a discipline,” Dr. Wellikson says. “We’ve moved beyond the idea of HM to the reality of HM.” TH
Jason Carris is editor of The Hospitalist.
Reference
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517.
Image Source: PORCOREX/ISTOCKPHOTO.COM
Timing is everything. Christopher Columbus, Abraham Lincoln, Harry Truman … each benefited from perfect timing and, in turn, helped change the course of history.
HM has had great timing, too. With numbers now estimated at more than 30,000 hospitalists nationwide, HM is systematically changing the way patients are cared for in the hospital. The maturation process is equally evident. In less than two decades, HM has organized annual meetings, developed educational programs, established a peer-reviewed journal, and published core competencies.
The next step in the evolutionary process: the Recognition of Focused Practice (RFP) in Hospital Medicine through the American Board of Internal Medicine’s (ABIM) maintenance of certification (MOC) program. Registration for the RFP in HM should be available by May 2010, with the first MOC in fall 2010.
“The timing is perfect. It’s appropriate,” says Robert Wachter, MD, FHM, chief of the hospital medicine
division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World. “We knew we needed to ripen and mature. It’s like watching your child growing up: their first steps, first day of school, graduating high school. This has a lot of the same feeling. This is one more statement that the field is real, here to stay, and vitally important to medicine.”
The new pathway to board recertification is as meaningful to HM’s founding fathers as it will be to the next generation of hospitalists. It represents validation to physicians who have chosen a career in HM, and it offers early-career physicians a specialized path to recertification. Moreover, hospitalists agree the RFP in HM provides accountability to the profession and patients.
“Hospitalists must be able to exhibit clinical competence in the areas of patient safety, quality, and clinical care transitions,” says Jeff Wiese, MD, FACP, FHM, associate dean of Graduate Medical Education and professor of medicine at Tulane University in New Orleans, SHM president-elect, and chair of the ABIM Hospital Medicine Maintenance of Certification Question Writing Committee. “Everything is driving at those issues. Individuals who go through the four parts of this certification will be able to say to their patients, with confidence, that one, they are a competent internist knowledgeable in hospital-based care, and two, they are an expert in patient safety, quality, and transitions of care.”
ABIM, for its part, expects to learn from this “new paradigm,” says Eric Holmbloe, MD, ABIM’s senior vice president and chief medical officer.
“The major change is the ability to implement the concept of a focused practice. It’s the first time, and it does recognize that the world has changed,” Dr. Holmbloe says, noting his group is “working feverishly” to complete the examination and build the online infrastructure needed for testing. “For those individuals whose [certificates] expire in 2010 or 2011, this is a viable pathway for recertification.”
Satisfaction Guaranteed
Ask any long-in-the-tooth hospitalist what the RFP in HM means to them, and more often than not the answer centers on professional self-regulation and career validation. Few will argue the specialty has, at times, suffered from an identity crisis. Sans fellowships, specialized training, or a particular organ to hang their hat on, HM physicians have labored to carve their niche and gain a seat at the specialist’s table.
The RFP in HM, many hospitalists envision, solves a number of those acceptance-related issues (see “Certified Special,” p. 53). Most importantly, it identifies physicians who have chosen to make HM their career.
“Hospitalists have always struggled, especially in academic settings,” says Scott Flanders, MD, FHM, SHM president, associate professor and director of the hospitalist program at the University of Michigan Health System in Ann Arbor. “I think this is a major, major moment for the field. It validates the field, and the belief that HM is a positive [for medicine].”
The new designation likely has greater meaning to older HM physicians, those who remember the early days of society formation (i.e., the National Association of Inpatient Physicians) and annual meeting attendance in the hundreds, not thousands.
“With the older docs, validation is particularly meaningful,” says Dr. Wachter, who, along with Lee Goldman, MD, first coined the term “hospitalist” in a 1996 article in the New England Journal of Medicine.1 “Paradoxically, most of the older physicians don’t have to take this test.”
That might be true, but only a small percentage of the 30,000-plus hospitalists in the U.S. are grandfathered into time-unlimited IM certificates. ABIM began time-limited certificates in 1990. The average hospitalist is 40, according to SHM’s “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement.”
John Nelson, MD, doesn’t have to recertify, but the co-founder of SHM is planning to be among the first to take the test. “It’s the first way hospitalists will be able to show their competence,” says Dr. Nelson, FACP, FHM, past president of SHM, and a principal in the national practice management firm Nelson Flores Hospital Medicine Consultants. A regular contributor to The Hospitalist, Dr. Nelson says the RFP in HM “will help people take our field more seriously.”
Inclusive of IM
The RFP in HM pathway is not a way to distance hospitalists from their internal medicine (IM) training and certification. Nearly 85% of hospitalists are IM-trained, according to SHM’s 2007-2008 bi-annual survey, and clinical competence as an internist will be a requisite for recertification through the new ABIM test.
“HM is borne of internal medicine … and has evolved to something unique. Therefore, your credentials should reflect that uniqueness,” says Larry Wellikson, MD, FHM, CEO of SHM. “This [process] is going to make the whole maintenance of certification process more relevant to hospitalists. This is recognition that hospitalists should be accountable for learning new things after they complete their formal training—things not emphasized in training like patient safety, quality, and care transitions.”
The language used on ABIM’s Web site to announce the new testing program furthers the message: “ABIM is developing a program to assess, set standards for, and recognize the proficiency of general internists who focus their practice in the care of hospitalized patients. The board’s decision to advance this program is consistent with its primary role of certifying internal-medicine physicians who meet the profession’s standards for focused practice in internal medicine.”
In addition to quality, patient safety, and transitions, “accountability to the patient” is a popular catchphrase used by members of the committee dedicated to writing the new certification test. Licensure and certification provide public accountability to patients, colleagues, and stakeholders, says Dr. Wiese. What differentiates career hospitalists from those making a pit stop during an alternate career path is increased accessibility to patients, expertise in patient safety and quality initiatives, and knowledge of clinical care transitions, he explains.
“Everything is driving at those issues,” he says. “From the beginning, there has been no agenda to change residency training. Any information contrary is a complete myth. Principle No. 1 is that you have to be competent internist. This new [MOC process] is much closer to an MBA.”
How hospitalists who are recertifying through the RFP in HM will refer to themselves remains up for debate. Although some physicians might say they are “board-certified in hospital medicine,” Dr. Wachter hopes hospitalists will use the phrase “board-certified in internal medicine with a recognized focused practice in hospital medicine.” “I hope to think our physicians will still say ‘IM,’ ” he says. “The goal here is not to sever ties with IM. That is a concern.”
Dr. Wiese, who, along with Dr. Wachter and other members of the test-writing committee, was required to complete the IM maintenance of certification (MOC), envisions a four-part process (see “Board Certification, With a Healthy Dose of HM,” p. 31) that challenges hospitalists in the core competencies of hospital-based practice.
“The [IM] recertification was the one test in my career that made me a better physician. I think this HM examination is going to be the same way,” Dr. Wiese says. “I want hospitalists to say, ‘When I go back to take care of my patients, they’ll be better off for it.’ ”
Target Audience
Dr. Flanders has about 40 hospitalists in his HM group at the University of Michigan. Although he recertified in IM in 2006, he knows many of his hospitalists are itching to take ABIM’s new HM-focused test. “They will be thrilled. They have all said to me that we can’t get this done fast enough,” he says, noting three or four of his hospitalists probably will recertify through the new test in fall 2010. “Our field is young; I suspect there are a lot of hospitalists out there who are within the 10-year window for recertification. I plan to do it at my next MOC … and that date could be moved up the way things are going.”
Dr. Wachter agrees the RFP in HM is an “attractive” option to hospitalists, especially those whose recertification is looming in the next two or three years. This MOC, he explains, “offers a pathway that is more in sync with the medicine [they are practicing] day in and day out.”
ABIM plans to have comprehensive information about the process available on its Web site (www.abim.org) this month and online registration available in May 2010 (see “FAQs,” left). While the test-writing committee finishes its tasks, Dr. Holmbloe says, ABIM’s systems department is working to build the online infrastructure. The first RFP in HM tests should go live in fall 2010.
“For those individuals [whose certificates] expire in 2010 or 2011, this is a viable pathway for recertification. If HM is their passion, this is for them,” Dr. Holmbloe says. “The major change, from ABIM’s perspective, is the ability to implement the concept of a focused practice. It’s the first time, a new paradigm. This does recognize that the world has changed.”
Crash Course
Every physician, sometime in his or her career, has crammed for a test. ABIM, however, recommends physicians start this process two or three years before their certificate expires. That timetable might work for some hospitalists, not so much for others. In any event, Dr. Flanders says hospitalists can count on SHM to help them prepare for the HM-specific examination.
“SHM has to help develop the tools and resources hospitalists will need to successfully prepare for and pass this test,” he says. He expects educational resources and self-assessment modules will be available on SHM’s Web site (www.hospitalmedicine.org) and at HM10, April 8-11 in Washington, D.C.
Dr. Wellikson says MOC preparedness “should match [SHM’s] educational projects,” and his staff “will continue to develop” tools and resources to assist hospitalists. He also recognizes the moment: the notch on HM’s evolutionary timeline where a once-fledgling group of inpatient physicians helped chart a new course for American medicine.
“Obviously, 10 years ago was too early. Now there are 30,000 hospitalists. Many of them are making HM a career. It’s evolving as a discipline,” Dr. Wellikson says. “We’ve moved beyond the idea of HM to the reality of HM.” TH
Jason Carris is editor of The Hospitalist.
Reference
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517.
Image Source: PORCOREX/ISTOCKPHOTO.COM
Timing is everything. Christopher Columbus, Abraham Lincoln, Harry Truman … each benefited from perfect timing and, in turn, helped change the course of history.
HM has had great timing, too. With numbers now estimated at more than 30,000 hospitalists nationwide, HM is systematically changing the way patients are cared for in the hospital. The maturation process is equally evident. In less than two decades, HM has organized annual meetings, developed educational programs, established a peer-reviewed journal, and published core competencies.
The next step in the evolutionary process: the Recognition of Focused Practice (RFP) in Hospital Medicine through the American Board of Internal Medicine’s (ABIM) maintenance of certification (MOC) program. Registration for the RFP in HM should be available by May 2010, with the first MOC in fall 2010.
“The timing is perfect. It’s appropriate,” says Robert Wachter, MD, FHM, chief of the hospital medicine
division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World. “We knew we needed to ripen and mature. It’s like watching your child growing up: their first steps, first day of school, graduating high school. This has a lot of the same feeling. This is one more statement that the field is real, here to stay, and vitally important to medicine.”
The new pathway to board recertification is as meaningful to HM’s founding fathers as it will be to the next generation of hospitalists. It represents validation to physicians who have chosen a career in HM, and it offers early-career physicians a specialized path to recertification. Moreover, hospitalists agree the RFP in HM provides accountability to the profession and patients.
“Hospitalists must be able to exhibit clinical competence in the areas of patient safety, quality, and clinical care transitions,” says Jeff Wiese, MD, FACP, FHM, associate dean of Graduate Medical Education and professor of medicine at Tulane University in New Orleans, SHM president-elect, and chair of the ABIM Hospital Medicine Maintenance of Certification Question Writing Committee. “Everything is driving at those issues. Individuals who go through the four parts of this certification will be able to say to their patients, with confidence, that one, they are a competent internist knowledgeable in hospital-based care, and two, they are an expert in patient safety, quality, and transitions of care.”
ABIM, for its part, expects to learn from this “new paradigm,” says Eric Holmbloe, MD, ABIM’s senior vice president and chief medical officer.
“The major change is the ability to implement the concept of a focused practice. It’s the first time, and it does recognize that the world has changed,” Dr. Holmbloe says, noting his group is “working feverishly” to complete the examination and build the online infrastructure needed for testing. “For those individuals whose [certificates] expire in 2010 or 2011, this is a viable pathway for recertification.”
Satisfaction Guaranteed
Ask any long-in-the-tooth hospitalist what the RFP in HM means to them, and more often than not the answer centers on professional self-regulation and career validation. Few will argue the specialty has, at times, suffered from an identity crisis. Sans fellowships, specialized training, or a particular organ to hang their hat on, HM physicians have labored to carve their niche and gain a seat at the specialist’s table.
The RFP in HM, many hospitalists envision, solves a number of those acceptance-related issues (see “Certified Special,” p. 53). Most importantly, it identifies physicians who have chosen to make HM their career.
“Hospitalists have always struggled, especially in academic settings,” says Scott Flanders, MD, FHM, SHM president, associate professor and director of the hospitalist program at the University of Michigan Health System in Ann Arbor. “I think this is a major, major moment for the field. It validates the field, and the belief that HM is a positive [for medicine].”
The new designation likely has greater meaning to older HM physicians, those who remember the early days of society formation (i.e., the National Association of Inpatient Physicians) and annual meeting attendance in the hundreds, not thousands.
“With the older docs, validation is particularly meaningful,” says Dr. Wachter, who, along with Lee Goldman, MD, first coined the term “hospitalist” in a 1996 article in the New England Journal of Medicine.1 “Paradoxically, most of the older physicians don’t have to take this test.”
That might be true, but only a small percentage of the 30,000-plus hospitalists in the U.S. are grandfathered into time-unlimited IM certificates. ABIM began time-limited certificates in 1990. The average hospitalist is 40, according to SHM’s “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement.”
John Nelson, MD, doesn’t have to recertify, but the co-founder of SHM is planning to be among the first to take the test. “It’s the first way hospitalists will be able to show their competence,” says Dr. Nelson, FACP, FHM, past president of SHM, and a principal in the national practice management firm Nelson Flores Hospital Medicine Consultants. A regular contributor to The Hospitalist, Dr. Nelson says the RFP in HM “will help people take our field more seriously.”
Inclusive of IM
The RFP in HM pathway is not a way to distance hospitalists from their internal medicine (IM) training and certification. Nearly 85% of hospitalists are IM-trained, according to SHM’s 2007-2008 bi-annual survey, and clinical competence as an internist will be a requisite for recertification through the new ABIM test.
“HM is borne of internal medicine … and has evolved to something unique. Therefore, your credentials should reflect that uniqueness,” says Larry Wellikson, MD, FHM, CEO of SHM. “This [process] is going to make the whole maintenance of certification process more relevant to hospitalists. This is recognition that hospitalists should be accountable for learning new things after they complete their formal training—things not emphasized in training like patient safety, quality, and care transitions.”
The language used on ABIM’s Web site to announce the new testing program furthers the message: “ABIM is developing a program to assess, set standards for, and recognize the proficiency of general internists who focus their practice in the care of hospitalized patients. The board’s decision to advance this program is consistent with its primary role of certifying internal-medicine physicians who meet the profession’s standards for focused practice in internal medicine.”
In addition to quality, patient safety, and transitions, “accountability to the patient” is a popular catchphrase used by members of the committee dedicated to writing the new certification test. Licensure and certification provide public accountability to patients, colleagues, and stakeholders, says Dr. Wiese. What differentiates career hospitalists from those making a pit stop during an alternate career path is increased accessibility to patients, expertise in patient safety and quality initiatives, and knowledge of clinical care transitions, he explains.
“Everything is driving at those issues,” he says. “From the beginning, there has been no agenda to change residency training. Any information contrary is a complete myth. Principle No. 1 is that you have to be competent internist. This new [MOC process] is much closer to an MBA.”
How hospitalists who are recertifying through the RFP in HM will refer to themselves remains up for debate. Although some physicians might say they are “board-certified in hospital medicine,” Dr. Wachter hopes hospitalists will use the phrase “board-certified in internal medicine with a recognized focused practice in hospital medicine.” “I hope to think our physicians will still say ‘IM,’ ” he says. “The goal here is not to sever ties with IM. That is a concern.”
Dr. Wiese, who, along with Dr. Wachter and other members of the test-writing committee, was required to complete the IM maintenance of certification (MOC), envisions a four-part process (see “Board Certification, With a Healthy Dose of HM,” p. 31) that challenges hospitalists in the core competencies of hospital-based practice.
“The [IM] recertification was the one test in my career that made me a better physician. I think this HM examination is going to be the same way,” Dr. Wiese says. “I want hospitalists to say, ‘When I go back to take care of my patients, they’ll be better off for it.’ ”
Target Audience
Dr. Flanders has about 40 hospitalists in his HM group at the University of Michigan. Although he recertified in IM in 2006, he knows many of his hospitalists are itching to take ABIM’s new HM-focused test. “They will be thrilled. They have all said to me that we can’t get this done fast enough,” he says, noting three or four of his hospitalists probably will recertify through the new test in fall 2010. “Our field is young; I suspect there are a lot of hospitalists out there who are within the 10-year window for recertification. I plan to do it at my next MOC … and that date could be moved up the way things are going.”
Dr. Wachter agrees the RFP in HM is an “attractive” option to hospitalists, especially those whose recertification is looming in the next two or three years. This MOC, he explains, “offers a pathway that is more in sync with the medicine [they are practicing] day in and day out.”
ABIM plans to have comprehensive information about the process available on its Web site (www.abim.org) this month and online registration available in May 2010 (see “FAQs,” left). While the test-writing committee finishes its tasks, Dr. Holmbloe says, ABIM’s systems department is working to build the online infrastructure. The first RFP in HM tests should go live in fall 2010.
“For those individuals [whose certificates] expire in 2010 or 2011, this is a viable pathway for recertification. If HM is their passion, this is for them,” Dr. Holmbloe says. “The major change, from ABIM’s perspective, is the ability to implement the concept of a focused practice. It’s the first time, a new paradigm. This does recognize that the world has changed.”
Crash Course
Every physician, sometime in his or her career, has crammed for a test. ABIM, however, recommends physicians start this process two or three years before their certificate expires. That timetable might work for some hospitalists, not so much for others. In any event, Dr. Flanders says hospitalists can count on SHM to help them prepare for the HM-specific examination.
“SHM has to help develop the tools and resources hospitalists will need to successfully prepare for and pass this test,” he says. He expects educational resources and self-assessment modules will be available on SHM’s Web site (www.hospitalmedicine.org) and at HM10, April 8-11 in Washington, D.C.
Dr. Wellikson says MOC preparedness “should match [SHM’s] educational projects,” and his staff “will continue to develop” tools and resources to assist hospitalists. He also recognizes the moment: the notch on HM’s evolutionary timeline where a once-fledgling group of inpatient physicians helped chart a new course for American medicine.
“Obviously, 10 years ago was too early. Now there are 30,000 hospitalists. Many of them are making HM a career. It’s evolving as a discipline,” Dr. Wellikson says. “We’ve moved beyond the idea of HM to the reality of HM.” TH
Jason Carris is editor of The Hospitalist.
Reference
- Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517.
Image Source: PORCOREX/ISTOCKPHOTO.COM
Medical Mistakes, 10 Years Post-Op
It’s November 1999, and the release of an advance copy of a breakthrough Institute of Medicine (IOM) report on patient safety provokes headlines around the world with its estimate that as many as 98,000 people per year die from medical errors in U.S. hospitals. The report and subsequent book, To Err is Human: Building a Safer Health System, already is labeled a landmark event for modern medicine.1 It launches a nationwide effort to systematically improve patient safety and reduce errors.
Believe it or not, the IOM report celebrates its 10th anniversary this month. Many healthcare leaders point out that the QI and patient-safety revolution birthed by the IOM report has paralleled the simultaneous—and seismic—growth of HM.
The IOM report drew upon data from Harvard Medical Practice Studies and other existing research for its shocking estimates of error-induced deaths. The report, to a large degree, focused on prescribing errors, with less emphasis on hospital-acquired infections and other safety and quality issues that have emerged since its publication. The report also proposed a comprehensive safety strategy for government, industry, consumers, and healthcare providers—a proposal that has been adopted only in pieces.
In commemorating the 10th anniversary of the IOM report, industry leaders agree that HM more than any other medical specialty will continue to play a leading role in pushing the quality and patient-safety agenda in hospitals throughout America.
IOM’s Committee on Quality of Healthcare in America, which was made up of physicians, researchers, and healthcare leaders, authored the breakthrough report on medical errors, and followed up two years later with Crossing the Quality Chasm: A New Health System for the 21st Century (www.iom.edu/?id=12736).2
The Hospitalist caught up with two of the original committee members, Donald Berwick, MD, MPP, FRCP, president and CEO of the Institute for Healthcare Improvement (IHI), and Christine Cassel, MD, president and CEO of the American Board of Internal Medicine (ABIM), to discuss how far medicine has come—and how far it has to go—in the areas of hospital quality and patient safety.

—Christine Cassel, MD, president, CEO, American Board of Internal Medicine, Philadelphia
Question: What is the legacy of the IOM report?
Dr. Berwick: It didn’t launch the patient-safety movement, but it was the most important single contributor to that movement. In one step, it took the focus on safety as a goal in medicine from a relatively fringe concern to a central issue, and a central task for health providers.
Its most important element was the focus on systems improvement, rather than exhortations to individual health professionals to do a better job with patient safety. It is a cultural norm to blame someone when something goes wrong. That hasn’t changed fundamentally. But the IOM report made the point that it’s not people who are to blame for problems in patient safety, and blame won’t get us where we need to go.
Q: How do you rate the impact of To Err is Human on the medical industry as a whole?
Dr. Cassel: The Agency for Healthcare Research and Quality, five years after the IOM report, said we hadn’t made enough progress. We have, most importantly, been able to talk about it and understand some of the approaches to safety and quality. But that’s not nearly enough, in my opinion.
Dr. Berwick: I’d give it a C-minus. There has been a change in awareness of medical safety. Before the IOM report, you just didn’t hear about it. A scientific basis for the statement of the problem was created, and we can never go back. Prototypes of what could be achieved have started to emerge, not just in this country but worldwide. The problem is that the success is just in pockets—not fundamental change in the nature of the American healthcare industry. That level of execution just is not there yet. Now it’s game time—time to take safety and quality mainstream.
Q: In retrospect, what was missed in the report?
Dr. Berwick: If we missed any boat in our analysis, the idea of “no blame” is not meant to relieve everyone of responsibility for medical errors, but to relocate responsibility for safety in the offices and work of leaders of healthcare institutions. The finger points to the executive suite. There’s more and more evidence that safety does not improve without the clear commitment of leaders.
Dr. Cassel: When we think about how we train doctors, which I spend a lot of time doing, they just aren’t trained to think of root-cause analysis or how to work in teams to reduce errors. That needs to change. ABIM’s new pathway for hospitalists, which will be rolled out in another year or so (see “A-Plus Achievement,” p. 1), treats questions of how … to identify patient-safety issues as core knowledge.
Q: What is the relationship of the patient-safety movement to the hospitalist movement?
Dr. Cassel: The development and growth of patient safety has paralleled the growth of hospital medicine, and I think that’s a good thing. Most of the literature on available errors focuses on the hospital because that’s the easiest place to find numbers of patients and shine a light on safety. Specialists in hospital medicine have a unique opportunity and responsibility to be leaders in continuing to advance the cause of patient safety.
Q: What should HM’s patient-safety agenda look like going forward?
Dr. Berwick: No. 1, aim for zero. There are types of injuries and infections that can be nearly eliminated in the hospital. When you look at safety-oriented efforts in other industries, they strive to get to the point where they’re no longer talking about ratios, only numerators (how many actual incidents).
Second is to broaden the focus from safety to all the other dimensions of quality. Think about reliability, processes and performance across the board.
Third is to be authentic about teamwork across professions. In the medical culture at large, there still is too much focus on turf issues between doctors and nurses. I believe in the long run new safety initiatives will be fostered by teams working at unprecedented levels of collaboration, reaching across traditional boundaries.
Dr. Cassel: The issue of diagnostic error is also emerging as another kind of medical error.
In order for patients to get the right treatment, they need to get the right diagnosis. That’s where all of your medical training, knowledge, and judgment come into play. For ABIM, that’s how we evaluate physicians’ judgment.
The next frontier in patient safety is the handoff, from ambulatory to hospital and back, but also with long-term care, which is a black box. An enlightened and energetic hospitalist movement could decide to take that issue on.
Where it would happen is at the community level, although some of the healthcare reform legislation includes ideas about innovation zones and how to create payment mechanisms to support continuity of care. TH
Larry Beresford is a freelance writer based in Oakland, Calif.
References
- Kohn LT, Corrigan JM, Donaldson MS, et al. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press, 2000.
- Institute of Medicine Committee on Quality Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press, 2001.
- Moser R. Diseases of Medical Progress: A Contemporary Analysis of Illnesses Produced by Drugs and Other Therapeutic Procedures. Springfield, Ill.: Charles C. Thomas, 1959.
- Reason, J. Human Error. Cambridge, England: Cambridge University Press, 1990.
- Leape LL. Error in medicine. JAMA. 1994;272(23):1851-1857.
- United Kingdom Department of Health. An Organisation with a Memory. 2000.
- Jerrard J. No fee for errors. The Hospitalist. 2008;(5):18.
- Pronovost PJ, Berenholtz SM, Goeschel C, et al. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008;23(2):207-221.
HM Leaders Weigh In
Question 1: What was hospital medicine’s contribution and role in the patient-safety movement that ensued following the IOM report?
“I believe that hospitalists have been integral to improving patient safety and reducing medical errors in those hospitals. Patients are safer and better off if there is a physician in the house ready to respond should patients have a change in health status. Hospitalists see the hospital as their office, if you will, and they focus not only on treating the patient in the bed, but treating the hospital itself by becoming engaged with quality improvement and patient-safety initiatives that improve the system of care.”—Mark Williams, MD, FHM, chief, division of hospital medicine, Northwestern University Feinberg School of Medicine, Chicago; SHM past president; editor of the Journal of Hospital Medicine
“The role hospital medicine has filled has been as a major supplier of physicians to quality-improvement teams and other hospital teams at the front lines, prior to which physicians were conspicuously absent. If you look, for example, at nurses and other healthcare professionals, they came to the party much earlier than we did. Physicians have only recently on a broad scale become involved on these teams, and I think the major contributors have been hospitalists.”—Winthrop Whitcomb, MD, FHM, director of performance improvement, Mercy Medical Center, Springfield, Mass.; SHM co-founder
“There was a tremendous kind of synergy where hospital medicine was defining itself by its focus on systems of care, safety culture, error reporting, collaboration, interdisciplinary teams and so forth. The IOM report did a beautiful job of taking the knowledge and literature, not just from within medicine but more importantly from outside, and showing how a lot of those concepts that had been implemented successfully elsewhere were lacking in medicine in general. That really just teed it up for hospital medicine to take the impetus and framework IOM supplied and use it as a rubric for what hospital medicine could do for its part of the health system.”—Russ Cucina, MD, assistant professor of medicine and associate medical director for information technology, University of California at San Francisco
Question 2: What is the most important unfinished business for hospitalists regarding the patient-safety movement?
“I think we have made tremendous strides but there is much more to do. Although we have pockets of success, what we need to do is make those successes more uniform, so they happen in every hospital, not just some hospitals that have the right hospitalist leader or the right skill set or the right culture. We want to create the right culture and skill set and team in every hospital, and one of our challenges at SHM is to work on a mentoring program for hospitalists. That means using those who have been successful to mentor other sites and bring them on board to reproduce and replicate the good work.”—Janet Nagamine, MD, FHM, hospitalist, Kaiser Permanente Medical Center, Santa Clara, Calif.; SHM Hospital Quality/Patient Safety Committee chairwoman
“The patients who enter hospitals today are incredibly sick, with multiple organ failures and other complications. Taking care of these patients is incredibly challenging, and there are always going to be things that do not go well. Hospitalists have begun to uncover and tackle a lot of these problems, but even as they eliminate one problem, new treatments, devices, procedures and strategies for caring for patients—all designed to improve care—may have unintended consequences. It is hospitalists’ job to try to mitigate those consequences and redesign the strategies to continue to improve outcomes. But this is a long road—a marathon, not a sprint.”—Scott Flanders, MD, FHM, director of the hospitalist division, University of Michigan Health System, Ann Arbor; president of SHM
“The greatest area of unfinished business I see is preserving continuity of care for our patients as they transition in and out of the hospital. So much is happening, and there is a great need to get information quickly and communicate between the inpatient and community-based practitioner. I should say we’ve come a long way, but there’s a lot more to do in this area, and that’s why six medical societies, including SHM, came together to produce the recent Transitions of Care Consensus Statement, acknowledging that this is a crucial part of patient safety and describing what are effective transitions of care in and out of the hospital.”—Vineet Arora, MD, MA, FHM, assistant professor, Department of Medicine, University of Chicago
—LB
It’s November 1999, and the release of an advance copy of a breakthrough Institute of Medicine (IOM) report on patient safety provokes headlines around the world with its estimate that as many as 98,000 people per year die from medical errors in U.S. hospitals. The report and subsequent book, To Err is Human: Building a Safer Health System, already is labeled a landmark event for modern medicine.1 It launches a nationwide effort to systematically improve patient safety and reduce errors.
Believe it or not, the IOM report celebrates its 10th anniversary this month. Many healthcare leaders point out that the QI and patient-safety revolution birthed by the IOM report has paralleled the simultaneous—and seismic—growth of HM.
The IOM report drew upon data from Harvard Medical Practice Studies and other existing research for its shocking estimates of error-induced deaths. The report, to a large degree, focused on prescribing errors, with less emphasis on hospital-acquired infections and other safety and quality issues that have emerged since its publication. The report also proposed a comprehensive safety strategy for government, industry, consumers, and healthcare providers—a proposal that has been adopted only in pieces.
In commemorating the 10th anniversary of the IOM report, industry leaders agree that HM more than any other medical specialty will continue to play a leading role in pushing the quality and patient-safety agenda in hospitals throughout America.
IOM’s Committee on Quality of Healthcare in America, which was made up of physicians, researchers, and healthcare leaders, authored the breakthrough report on medical errors, and followed up two years later with Crossing the Quality Chasm: A New Health System for the 21st Century (www.iom.edu/?id=12736).2
The Hospitalist caught up with two of the original committee members, Donald Berwick, MD, MPP, FRCP, president and CEO of the Institute for Healthcare Improvement (IHI), and Christine Cassel, MD, president and CEO of the American Board of Internal Medicine (ABIM), to discuss how far medicine has come—and how far it has to go—in the areas of hospital quality and patient safety.

—Christine Cassel, MD, president, CEO, American Board of Internal Medicine, Philadelphia
Question: What is the legacy of the IOM report?
Dr. Berwick: It didn’t launch the patient-safety movement, but it was the most important single contributor to that movement. In one step, it took the focus on safety as a goal in medicine from a relatively fringe concern to a central issue, and a central task for health providers.
Its most important element was the focus on systems improvement, rather than exhortations to individual health professionals to do a better job with patient safety. It is a cultural norm to blame someone when something goes wrong. That hasn’t changed fundamentally. But the IOM report made the point that it’s not people who are to blame for problems in patient safety, and blame won’t get us where we need to go.
Q: How do you rate the impact of To Err is Human on the medical industry as a whole?
Dr. Cassel: The Agency for Healthcare Research and Quality, five years after the IOM report, said we hadn’t made enough progress. We have, most importantly, been able to talk about it and understand some of the approaches to safety and quality. But that’s not nearly enough, in my opinion.
Dr. Berwick: I’d give it a C-minus. There has been a change in awareness of medical safety. Before the IOM report, you just didn’t hear about it. A scientific basis for the statement of the problem was created, and we can never go back. Prototypes of what could be achieved have started to emerge, not just in this country but worldwide. The problem is that the success is just in pockets—not fundamental change in the nature of the American healthcare industry. That level of execution just is not there yet. Now it’s game time—time to take safety and quality mainstream.
Q: In retrospect, what was missed in the report?
Dr. Berwick: If we missed any boat in our analysis, the idea of “no blame” is not meant to relieve everyone of responsibility for medical errors, but to relocate responsibility for safety in the offices and work of leaders of healthcare institutions. The finger points to the executive suite. There’s more and more evidence that safety does not improve without the clear commitment of leaders.
Dr. Cassel: When we think about how we train doctors, which I spend a lot of time doing, they just aren’t trained to think of root-cause analysis or how to work in teams to reduce errors. That needs to change. ABIM’s new pathway for hospitalists, which will be rolled out in another year or so (see “A-Plus Achievement,” p. 1), treats questions of how … to identify patient-safety issues as core knowledge.
Q: What is the relationship of the patient-safety movement to the hospitalist movement?
Dr. Cassel: The development and growth of patient safety has paralleled the growth of hospital medicine, and I think that’s a good thing. Most of the literature on available errors focuses on the hospital because that’s the easiest place to find numbers of patients and shine a light on safety. Specialists in hospital medicine have a unique opportunity and responsibility to be leaders in continuing to advance the cause of patient safety.
Q: What should HM’s patient-safety agenda look like going forward?
Dr. Berwick: No. 1, aim for zero. There are types of injuries and infections that can be nearly eliminated in the hospital. When you look at safety-oriented efforts in other industries, they strive to get to the point where they’re no longer talking about ratios, only numerators (how many actual incidents).
Second is to broaden the focus from safety to all the other dimensions of quality. Think about reliability, processes and performance across the board.
Third is to be authentic about teamwork across professions. In the medical culture at large, there still is too much focus on turf issues between doctors and nurses. I believe in the long run new safety initiatives will be fostered by teams working at unprecedented levels of collaboration, reaching across traditional boundaries.
Dr. Cassel: The issue of diagnostic error is also emerging as another kind of medical error.
In order for patients to get the right treatment, they need to get the right diagnosis. That’s where all of your medical training, knowledge, and judgment come into play. For ABIM, that’s how we evaluate physicians’ judgment.
The next frontier in patient safety is the handoff, from ambulatory to hospital and back, but also with long-term care, which is a black box. An enlightened and energetic hospitalist movement could decide to take that issue on.
Where it would happen is at the community level, although some of the healthcare reform legislation includes ideas about innovation zones and how to create payment mechanisms to support continuity of care. TH
Larry Beresford is a freelance writer based in Oakland, Calif.
References
- Kohn LT, Corrigan JM, Donaldson MS, et al. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press, 2000.
- Institute of Medicine Committee on Quality Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press, 2001.
- Moser R. Diseases of Medical Progress: A Contemporary Analysis of Illnesses Produced by Drugs and Other Therapeutic Procedures. Springfield, Ill.: Charles C. Thomas, 1959.
- Reason, J. Human Error. Cambridge, England: Cambridge University Press, 1990.
- Leape LL. Error in medicine. JAMA. 1994;272(23):1851-1857.
- United Kingdom Department of Health. An Organisation with a Memory. 2000.
- Jerrard J. No fee for errors. The Hospitalist. 2008;(5):18.
- Pronovost PJ, Berenholtz SM, Goeschel C, et al. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008;23(2):207-221.
HM Leaders Weigh In
Question 1: What was hospital medicine’s contribution and role in the patient-safety movement that ensued following the IOM report?
“I believe that hospitalists have been integral to improving patient safety and reducing medical errors in those hospitals. Patients are safer and better off if there is a physician in the house ready to respond should patients have a change in health status. Hospitalists see the hospital as their office, if you will, and they focus not only on treating the patient in the bed, but treating the hospital itself by becoming engaged with quality improvement and patient-safety initiatives that improve the system of care.”—Mark Williams, MD, FHM, chief, division of hospital medicine, Northwestern University Feinberg School of Medicine, Chicago; SHM past president; editor of the Journal of Hospital Medicine
“The role hospital medicine has filled has been as a major supplier of physicians to quality-improvement teams and other hospital teams at the front lines, prior to which physicians were conspicuously absent. If you look, for example, at nurses and other healthcare professionals, they came to the party much earlier than we did. Physicians have only recently on a broad scale become involved on these teams, and I think the major contributors have been hospitalists.”—Winthrop Whitcomb, MD, FHM, director of performance improvement, Mercy Medical Center, Springfield, Mass.; SHM co-founder
“There was a tremendous kind of synergy where hospital medicine was defining itself by its focus on systems of care, safety culture, error reporting, collaboration, interdisciplinary teams and so forth. The IOM report did a beautiful job of taking the knowledge and literature, not just from within medicine but more importantly from outside, and showing how a lot of those concepts that had been implemented successfully elsewhere were lacking in medicine in general. That really just teed it up for hospital medicine to take the impetus and framework IOM supplied and use it as a rubric for what hospital medicine could do for its part of the health system.”—Russ Cucina, MD, assistant professor of medicine and associate medical director for information technology, University of California at San Francisco
Question 2: What is the most important unfinished business for hospitalists regarding the patient-safety movement?
“I think we have made tremendous strides but there is much more to do. Although we have pockets of success, what we need to do is make those successes more uniform, so they happen in every hospital, not just some hospitals that have the right hospitalist leader or the right skill set or the right culture. We want to create the right culture and skill set and team in every hospital, and one of our challenges at SHM is to work on a mentoring program for hospitalists. That means using those who have been successful to mentor other sites and bring them on board to reproduce and replicate the good work.”—Janet Nagamine, MD, FHM, hospitalist, Kaiser Permanente Medical Center, Santa Clara, Calif.; SHM Hospital Quality/Patient Safety Committee chairwoman
“The patients who enter hospitals today are incredibly sick, with multiple organ failures and other complications. Taking care of these patients is incredibly challenging, and there are always going to be things that do not go well. Hospitalists have begun to uncover and tackle a lot of these problems, but even as they eliminate one problem, new treatments, devices, procedures and strategies for caring for patients—all designed to improve care—may have unintended consequences. It is hospitalists’ job to try to mitigate those consequences and redesign the strategies to continue to improve outcomes. But this is a long road—a marathon, not a sprint.”—Scott Flanders, MD, FHM, director of the hospitalist division, University of Michigan Health System, Ann Arbor; president of SHM
“The greatest area of unfinished business I see is preserving continuity of care for our patients as they transition in and out of the hospital. So much is happening, and there is a great need to get information quickly and communicate between the inpatient and community-based practitioner. I should say we’ve come a long way, but there’s a lot more to do in this area, and that’s why six medical societies, including SHM, came together to produce the recent Transitions of Care Consensus Statement, acknowledging that this is a crucial part of patient safety and describing what are effective transitions of care in and out of the hospital.”—Vineet Arora, MD, MA, FHM, assistant professor, Department of Medicine, University of Chicago
—LB
It’s November 1999, and the release of an advance copy of a breakthrough Institute of Medicine (IOM) report on patient safety provokes headlines around the world with its estimate that as many as 98,000 people per year die from medical errors in U.S. hospitals. The report and subsequent book, To Err is Human: Building a Safer Health System, already is labeled a landmark event for modern medicine.1 It launches a nationwide effort to systematically improve patient safety and reduce errors.
Believe it or not, the IOM report celebrates its 10th anniversary this month. Many healthcare leaders point out that the QI and patient-safety revolution birthed by the IOM report has paralleled the simultaneous—and seismic—growth of HM.
The IOM report drew upon data from Harvard Medical Practice Studies and other existing research for its shocking estimates of error-induced deaths. The report, to a large degree, focused on prescribing errors, with less emphasis on hospital-acquired infections and other safety and quality issues that have emerged since its publication. The report also proposed a comprehensive safety strategy for government, industry, consumers, and healthcare providers—a proposal that has been adopted only in pieces.
In commemorating the 10th anniversary of the IOM report, industry leaders agree that HM more than any other medical specialty will continue to play a leading role in pushing the quality and patient-safety agenda in hospitals throughout America.
IOM’s Committee on Quality of Healthcare in America, which was made up of physicians, researchers, and healthcare leaders, authored the breakthrough report on medical errors, and followed up two years later with Crossing the Quality Chasm: A New Health System for the 21st Century (www.iom.edu/?id=12736).2
The Hospitalist caught up with two of the original committee members, Donald Berwick, MD, MPP, FRCP, president and CEO of the Institute for Healthcare Improvement (IHI), and Christine Cassel, MD, president and CEO of the American Board of Internal Medicine (ABIM), to discuss how far medicine has come—and how far it has to go—in the areas of hospital quality and patient safety.

—Christine Cassel, MD, president, CEO, American Board of Internal Medicine, Philadelphia
Question: What is the legacy of the IOM report?
Dr. Berwick: It didn’t launch the patient-safety movement, but it was the most important single contributor to that movement. In one step, it took the focus on safety as a goal in medicine from a relatively fringe concern to a central issue, and a central task for health providers.
Its most important element was the focus on systems improvement, rather than exhortations to individual health professionals to do a better job with patient safety. It is a cultural norm to blame someone when something goes wrong. That hasn’t changed fundamentally. But the IOM report made the point that it’s not people who are to blame for problems in patient safety, and blame won’t get us where we need to go.
Q: How do you rate the impact of To Err is Human on the medical industry as a whole?
Dr. Cassel: The Agency for Healthcare Research and Quality, five years after the IOM report, said we hadn’t made enough progress. We have, most importantly, been able to talk about it and understand some of the approaches to safety and quality. But that’s not nearly enough, in my opinion.
Dr. Berwick: I’d give it a C-minus. There has been a change in awareness of medical safety. Before the IOM report, you just didn’t hear about it. A scientific basis for the statement of the problem was created, and we can never go back. Prototypes of what could be achieved have started to emerge, not just in this country but worldwide. The problem is that the success is just in pockets—not fundamental change in the nature of the American healthcare industry. That level of execution just is not there yet. Now it’s game time—time to take safety and quality mainstream.
Q: In retrospect, what was missed in the report?
Dr. Berwick: If we missed any boat in our analysis, the idea of “no blame” is not meant to relieve everyone of responsibility for medical errors, but to relocate responsibility for safety in the offices and work of leaders of healthcare institutions. The finger points to the executive suite. There’s more and more evidence that safety does not improve without the clear commitment of leaders.
Dr. Cassel: When we think about how we train doctors, which I spend a lot of time doing, they just aren’t trained to think of root-cause analysis or how to work in teams to reduce errors. That needs to change. ABIM’s new pathway for hospitalists, which will be rolled out in another year or so (see “A-Plus Achievement,” p. 1), treats questions of how … to identify patient-safety issues as core knowledge.
Q: What is the relationship of the patient-safety movement to the hospitalist movement?
Dr. Cassel: The development and growth of patient safety has paralleled the growth of hospital medicine, and I think that’s a good thing. Most of the literature on available errors focuses on the hospital because that’s the easiest place to find numbers of patients and shine a light on safety. Specialists in hospital medicine have a unique opportunity and responsibility to be leaders in continuing to advance the cause of patient safety.
Q: What should HM’s patient-safety agenda look like going forward?
Dr. Berwick: No. 1, aim for zero. There are types of injuries and infections that can be nearly eliminated in the hospital. When you look at safety-oriented efforts in other industries, they strive to get to the point where they’re no longer talking about ratios, only numerators (how many actual incidents).
Second is to broaden the focus from safety to all the other dimensions of quality. Think about reliability, processes and performance across the board.
Third is to be authentic about teamwork across professions. In the medical culture at large, there still is too much focus on turf issues between doctors and nurses. I believe in the long run new safety initiatives will be fostered by teams working at unprecedented levels of collaboration, reaching across traditional boundaries.
Dr. Cassel: The issue of diagnostic error is also emerging as another kind of medical error.
In order for patients to get the right treatment, they need to get the right diagnosis. That’s where all of your medical training, knowledge, and judgment come into play. For ABIM, that’s how we evaluate physicians’ judgment.
The next frontier in patient safety is the handoff, from ambulatory to hospital and back, but also with long-term care, which is a black box. An enlightened and energetic hospitalist movement could decide to take that issue on.
Where it would happen is at the community level, although some of the healthcare reform legislation includes ideas about innovation zones and how to create payment mechanisms to support continuity of care. TH
Larry Beresford is a freelance writer based in Oakland, Calif.
References
- Kohn LT, Corrigan JM, Donaldson MS, et al. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press, 2000.
- Institute of Medicine Committee on Quality Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press, 2001.
- Moser R. Diseases of Medical Progress: A Contemporary Analysis of Illnesses Produced by Drugs and Other Therapeutic Procedures. Springfield, Ill.: Charles C. Thomas, 1959.
- Reason, J. Human Error. Cambridge, England: Cambridge University Press, 1990.
- Leape LL. Error in medicine. JAMA. 1994;272(23):1851-1857.
- United Kingdom Department of Health. An Organisation with a Memory. 2000.
- Jerrard J. No fee for errors. The Hospitalist. 2008;(5):18.
- Pronovost PJ, Berenholtz SM, Goeschel C, et al. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008;23(2):207-221.
HM Leaders Weigh In
Question 1: What was hospital medicine’s contribution and role in the patient-safety movement that ensued following the IOM report?
“I believe that hospitalists have been integral to improving patient safety and reducing medical errors in those hospitals. Patients are safer and better off if there is a physician in the house ready to respond should patients have a change in health status. Hospitalists see the hospital as their office, if you will, and they focus not only on treating the patient in the bed, but treating the hospital itself by becoming engaged with quality improvement and patient-safety initiatives that improve the system of care.”—Mark Williams, MD, FHM, chief, division of hospital medicine, Northwestern University Feinberg School of Medicine, Chicago; SHM past president; editor of the Journal of Hospital Medicine
“The role hospital medicine has filled has been as a major supplier of physicians to quality-improvement teams and other hospital teams at the front lines, prior to which physicians were conspicuously absent. If you look, for example, at nurses and other healthcare professionals, they came to the party much earlier than we did. Physicians have only recently on a broad scale become involved on these teams, and I think the major contributors have been hospitalists.”—Winthrop Whitcomb, MD, FHM, director of performance improvement, Mercy Medical Center, Springfield, Mass.; SHM co-founder
“There was a tremendous kind of synergy where hospital medicine was defining itself by its focus on systems of care, safety culture, error reporting, collaboration, interdisciplinary teams and so forth. The IOM report did a beautiful job of taking the knowledge and literature, not just from within medicine but more importantly from outside, and showing how a lot of those concepts that had been implemented successfully elsewhere were lacking in medicine in general. That really just teed it up for hospital medicine to take the impetus and framework IOM supplied and use it as a rubric for what hospital medicine could do for its part of the health system.”—Russ Cucina, MD, assistant professor of medicine and associate medical director for information technology, University of California at San Francisco
Question 2: What is the most important unfinished business for hospitalists regarding the patient-safety movement?
“I think we have made tremendous strides but there is much more to do. Although we have pockets of success, what we need to do is make those successes more uniform, so they happen in every hospital, not just some hospitals that have the right hospitalist leader or the right skill set or the right culture. We want to create the right culture and skill set and team in every hospital, and one of our challenges at SHM is to work on a mentoring program for hospitalists. That means using those who have been successful to mentor other sites and bring them on board to reproduce and replicate the good work.”—Janet Nagamine, MD, FHM, hospitalist, Kaiser Permanente Medical Center, Santa Clara, Calif.; SHM Hospital Quality/Patient Safety Committee chairwoman
“The patients who enter hospitals today are incredibly sick, with multiple organ failures and other complications. Taking care of these patients is incredibly challenging, and there are always going to be things that do not go well. Hospitalists have begun to uncover and tackle a lot of these problems, but even as they eliminate one problem, new treatments, devices, procedures and strategies for caring for patients—all designed to improve care—may have unintended consequences. It is hospitalists’ job to try to mitigate those consequences and redesign the strategies to continue to improve outcomes. But this is a long road—a marathon, not a sprint.”—Scott Flanders, MD, FHM, director of the hospitalist division, University of Michigan Health System, Ann Arbor; president of SHM
“The greatest area of unfinished business I see is preserving continuity of care for our patients as they transition in and out of the hospital. So much is happening, and there is a great need to get information quickly and communicate between the inpatient and community-based practitioner. I should say we’ve come a long way, but there’s a lot more to do in this area, and that’s why six medical societies, including SHM, came together to produce the recent Transitions of Care Consensus Statement, acknowledging that this is a crucial part of patient safety and describing what are effective transitions of care in and out of the hospital.”—Vineet Arora, MD, MA, FHM, assistant professor, Department of Medicine, University of Chicago
—LB