User login
Standardized Orders Improve Pediatric Care
For many years physicians have created and used various standardized order forms for patient hospital admissions. The increasing popularity of electronic medical records and forms has led to the use of computerized physician order entry (CPOE) as a means of reducing medication errors.13 Crowley et al.,4 Stucky,5 and Garg et al.,6 along with various committees, have recommended standardized order sets and CPOE as a strategy for reducing medication errors. However, implementation of CPOE systems is expensive and not available in most hospitals. According to a recent survey of hospitals in the US, CPOE was only available to physicians at 16% of the participating institutions.7 Until CPOE becomes widespread, standardized preprinted formatted order sets may serve as an inexpensive alternative.
There is anecdotal evidence that standardized admission order forms may improve quality of care and efficiency, and decrease provider variation.8 However, few rigorous studies exist in the pediatric research literature regarding their ability to actually improve patient care.
In 2005, our institution, a large tertiary‐care academic teaching hospital, developed a standardized preprinted pediatric admission order set (PAOS). We did so for 3 reasons. First, there was a desire to improve completeness of orders. Handwritten orders often missed important elements such as weight, allergies, vital sign parameters, activity, etc. Second, there was a need to save time and improve efficiency. Third, it was important to reduce medical errors and the number of clarification requests by decreasing the necessity to decipher physician handwriting. Our PAOS was a convenience order set as opposed to a best practices order set. In other words, our PAOS did not contain evidence‐based management guidelines or protocols for specific admission diagnoses and was created solely to improve the quality and efficiency of workflow.
Documenting improvement in patient outcomes or reduction of medical errors is ultimately needed to establish the effectiveness of a standardized order set. Secondary outcomes, howeverparticularly the perceptions of the staff who are asked to use the order setare equally important, because they may identify real‐life barriers to use that, regardless of effectiveness, could limit dissemination and uptake. With respect to perceptions, 2 groups become paramount: those who write the orders, and those who respond to them. The purpose of the current study was to examine perceived effects of the new PAOS on inpatient care among those who, in our institution, write the ordersresident physicians.
MATERIALS AND METHODS
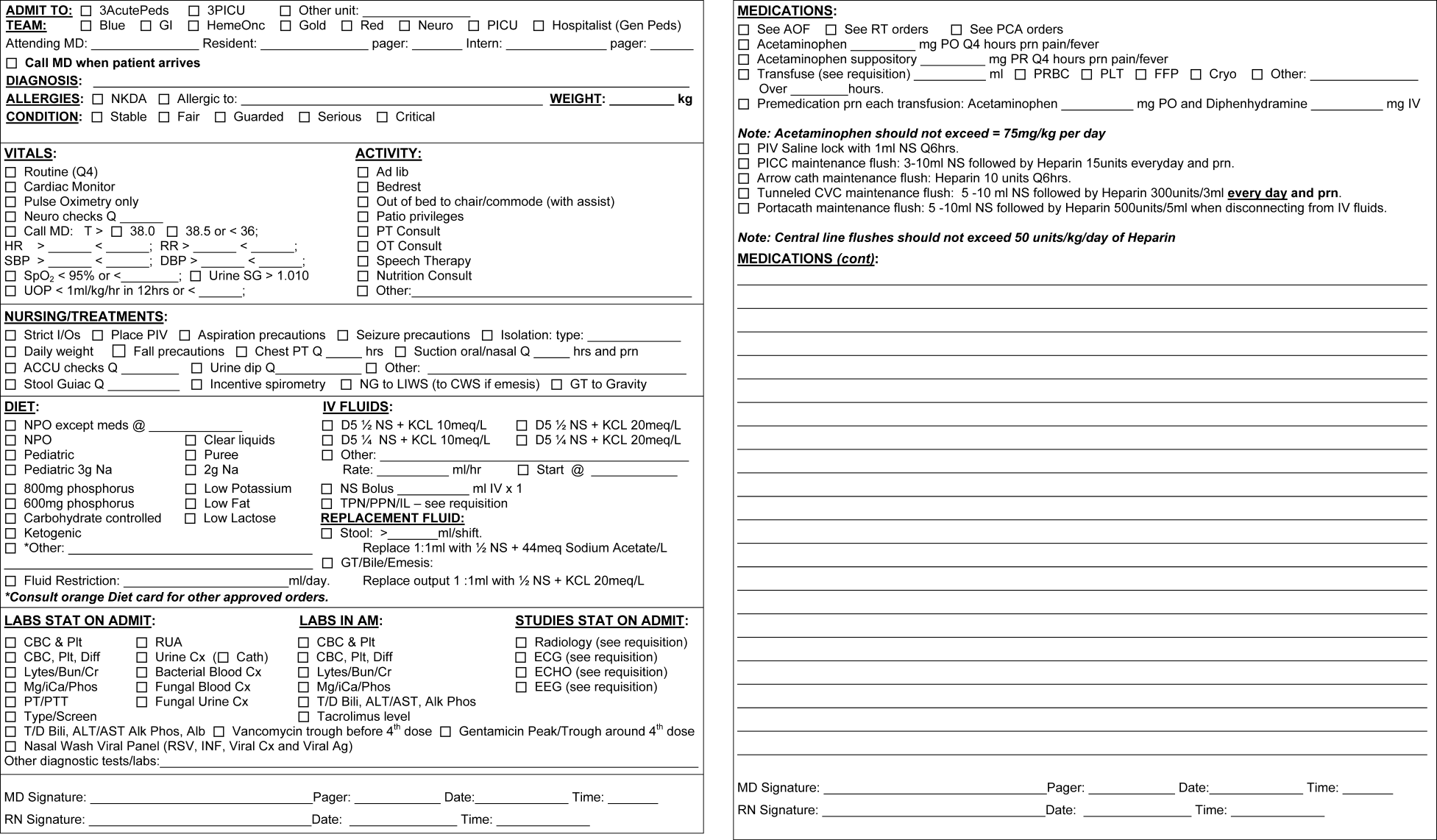
The PAOS was created in August 2005 at the University of California, Los Angeles (UCLA) Medical Center by a committee comprising pediatric hospitalists, nurses, pharmacists, residents, and clerks. The PAOS consisted mainly of check boxes (Figure 1). The PAOS was uploaded to the hospital website and made available for printing from all computers in the hospital, emergency room, and clinics.
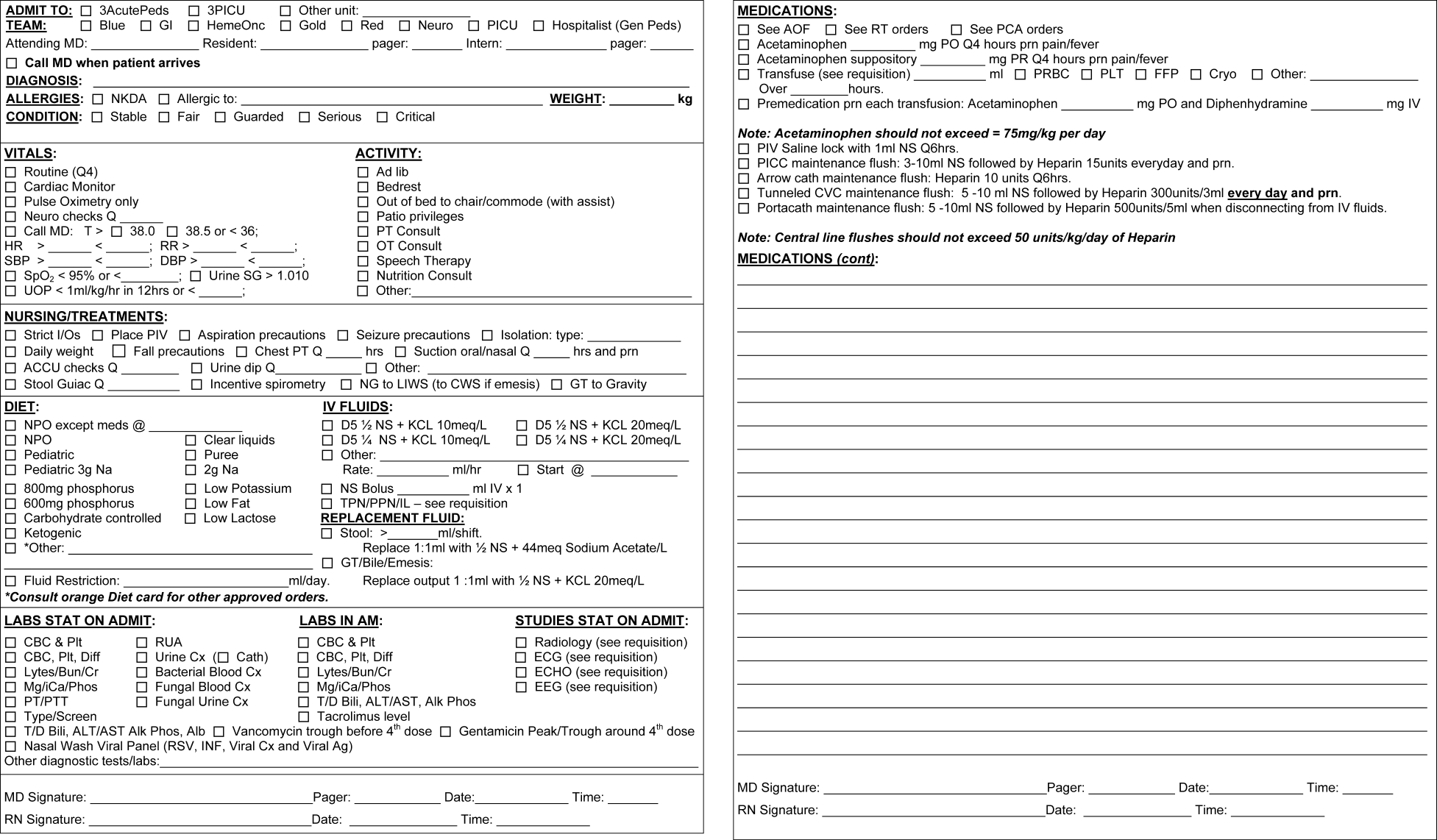
The UCLA Hospital and Medical Center is a nonprofit, 667‐bed tertiary‐care teaching hospital in Los Angeles, California. The pediatric ward has 70 licensed beds with approximately 3,000 admissions per year. The majority of the admissions were done by the pediatric residents. Physicians were free to edit the PAOS to suit a particular patient's needs or to hand‐write orders on a blank order form.
Measures
Fourteen months after the institution of the PAOS, all 97 UCLA pediatric residents (PL‐1, n = 34; PL‐2, n = 33; PL‐3, n = 30) were asked to complete a survey to anonymously evaluate the order set. All residents were US medical school graduates. Resident participation in the research project was voluntary and confidential, and residents were assured that participation would not affect their standing in the pediatric residency program. Each resident completed only 1 survey. Responses were collected October 2006 to June 2007. The residents were asked to rate the PAOS overall and with respect to 9 specific dimensions using a 5‐point Likert scale with 1 indicating strong disagreement and 5 indicating strong agreement (Figure 2).

This study was reviewed and approved by the institutional review board at the UCLA Medical Center.
Statistical Analysis
We used bivariate ordered logistic regression to estimate the association between overall rating and each of the 9 dimensions. Ordered logistic regression, a standard technique for ordered categorical variables, is essentially a weighted average of logistic regressions performed at each potential cut‐point of the outcome variable. For instance, potential cut‐points on our 5‐point Likert scale included strong disagreement versus any other, any disagreement versus nondisagreement, any agreement versus nonagreement, and strong agreement versus any other. We then used multivariate ordered logistic regression to examine which specific dimensions remained independently associated with the overall rating.
RESULTS
From October 2006 to June 2007, 59 residents (from a total of 97 residents; 61%) responded to the survey. Overall, 89% of respondents approved of the PAOS, 58% reported using it 90% of the time, and all said that they would recommend it to their colleagues (Table 1). Eighty‐four percent thought that the PAOS improved inpatient care, and 75% thought that medical errors were reduced. Eighty‐eight percent reported that the POAS saved time; 93% said it was convenient; and most reported less need for clarification with clerks (81%) and nurses (82%).
| Strongly Agree (%) | Agree (%) | Other (%) | |
|---|---|---|---|
| Specific dimensions | |||
| Looks neat | 63 | 32 | 5 |
| User friendly/convenient | 60 | 33 | 7 |
| Readily available | 47 | 26 | 26 |
| Saves time | 56 | 32 | 12 |
| Comprehensive | 40 | 40 | 19 |
| Reduces medical error | 40 | 35 | 25 |
| Fewer clarification phone calls/errors by clerks | 47 | 33 | 19 |
| Fewer clarification phone calls/errors by nurses | 47 | 35 | 18 |
| Improves overall patient care | 46 | 39 | 16 |
| Overall rating | 40 | 49 | 11 |
In bivariate analyses, each of the 9 dimensions was strongly associated with the overall rating (P < 0.001 for each). In multivariate analyses, however, only perceived improvement in patient care was independently associated with overall rating (OR, 3.9; P = 0.04).
We then examined whether perceived improvement in patient care itself was independently predicted by the other 8 dimensions. Residents who said that the form was comprehensive (OR, 5.6; P = 0.01), reduced medical errors (OR, 4.1; P = 0.01), or required less need for clarification with nurses (OR, 9.6; P = 0.01) were more likely to perceive that the form improved patient care than residents who did not.
DISCUSSION
A standardized admission order set is a simple, low‐cost intervention that may benefit patients by reducing medical errors and expediting high‐quality care. In general, residents rated the PAOS favorably. Just as importantly, the PAOS scored well across all specific dimensions, which suggests few perceived barriers to use among residents.
Some dimensions, however, appeared potentially more important than others. Residents who perceived an improvement in patient care tended to rate the PAOS favorably. Perceived improvement in patient care, in turn, was linked to the order set's comprehensiveness, perceived reductions in medical errors, and less need for clarification with nurses.
Even though this study did not directly query those most responsible for responding to the order set (ie, nurses, pharmacists, and clerks), the order set was created through a collaborative partnership of physicians, nurses, pharmacists, and clerks. It is reasonable to infer that resident‐perceived reduction in the need for clarification of orders with nurses and clerks might indicate a broad‐based, multidisciplinary improvement in clarity and workflow. Moreover, the fact that less need for clarification with nurses was strongly associated with resident‐perceived improvement in patient care underscores the importance of including nurses, pharmacists, and clerks in the development of these order sets.
Our experience using the standard admission orders over the past 2 years is congruent with other authors' findings. Most studies, however, have examined standardized order forms only in adult populations, and mainly for specific medical conditions. Micek et al.9 demonstrated that use of a standardized physician order set among adults with septic shock lowered 28‐day mortality and reduced hospital stay. Among stroke patients, rates of optimal treatment significantly improved after the introduction of standardized stroke orders.10 For patients with acute myocardial infarction, standardized admission orders increased early administration of aspirin and beta blockers.11, 12 With respect to cancer, implementation of a preprinted chemotherapy prescription form improved order completeness, prevented medication errors, and reduced time spent by pharmacists clarifying orders.13 Finally, standardized trauma admission orders developed in a surgical‐trauma intensive care unit reduced admission laboratory charges and improved order completeness.14 We found only a single pediatric study examining standardized order forms. Kozer et al.15 found that the use of a preprinted structured medication order form cut medication errors nearly in half in a pediatric emergency department.
Whether our results would have been similar had we implemented a series of best practices order sets rather than a single convenience order set is unclear. Although best practices order sets would have facilitated application of evidence‐based guidelines for common diagnoses, they would also have introduced potentially unwelcome logistical heterogeneity (with a separate form and protocol needed for each diagnosis) that might have reduced acceptability and uptake. In addition, there is a risk that best practices order sets would have been perceived as unduly limiting physician professional autonomy.
Our study has limitations. First, our study was performed within a single institution and may not be easily generalized. However, we believe that the basic format of the PAOS lends it to easy adaptability. Second, we did not survey residents before the order set was introduced to assess baseline perceptions. Instead, many of the questions in the survey ask about perceived improvements compared with the previous system. Conducting a formal pre‐post data collection and analysis might have yielded different results. Third, improvement in patient care was measured indirectly based on resident opinion.
In conclusion, our study suggests that our standardized admission order set prompting physicians to initiate comprehensive care is well‐liked by residents and is thought to benefit patients by reducing medical errors and expediting high‐quality care. The next step is to confirm that the resident‐perceived improvement in patient care correlates with actual improvement in patient care. If improvements can be confirmed, then PAOS adoption could be broadly recommended to pediatric hospitals. In the future, the PAOS may also help guide computerized physician order entry templates that can be further tailored to specific common diagnoses.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280(15):1311–1316.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163(12):1409–1416.
- ,,,,.The effect of computerized physician order entry on medication errors and adverse drug events in pediatric inpatients.Pediatrics.2003;112(3 Pt 1):506–509.
- ,,.Medication errors in children: a descriptive summary of medication error reports submitted to the United States Pharmacopoeia.Curr Ther Res Clin Exp.2001;62:627–640.
- .Prevention of medication errors in the pediatric inpatient setting.Pediatrics.2003;112(2):431–436.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293(10):1223–1238.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11(2):95–99.
- .Using standardized admit orders to improve inpatient care.Fam Pract Manag.1999;6(10):30–32.
- ,,, et al.Before‐after study of a standardized hospital order set for the management of septic shock.Crit Care Med.2006;34(11):2707–2713.
- California Acute Stroke Pilot Registry Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65(3):360–365.
- ,,,,,.Improving quality of care for acute myocardial infarction. The guidelines applied in practice (GAP) initiative.JAMA.2002;287(10):1269–1276.
- ,,,,,.Quality improvement initiative and its impact on the management of patients with acute myocardial infarction.Arch Intern Med.2000;160:3057–3061.
- ,,,,,.Effect of a cancer chemotherapy prescription form on prescription completeness.Am J Hosp Pharm.1989;46(9):1802–1806.
- ,.Standardized trauma admission orders, a pilot project.Int J Trauma Nurs.1996;2(1):13–21.
- ,,,,.Using a preprinted order sheet to reduce prescription errors in a pediatric emergency department: a randomized, controlled trial.Pediatrics.2005;116(6):1299–1302.
For many years physicians have created and used various standardized order forms for patient hospital admissions. The increasing popularity of electronic medical records and forms has led to the use of computerized physician order entry (CPOE) as a means of reducing medication errors.13 Crowley et al.,4 Stucky,5 and Garg et al.,6 along with various committees, have recommended standardized order sets and CPOE as a strategy for reducing medication errors. However, implementation of CPOE systems is expensive and not available in most hospitals. According to a recent survey of hospitals in the US, CPOE was only available to physicians at 16% of the participating institutions.7 Until CPOE becomes widespread, standardized preprinted formatted order sets may serve as an inexpensive alternative.
There is anecdotal evidence that standardized admission order forms may improve quality of care and efficiency, and decrease provider variation.8 However, few rigorous studies exist in the pediatric research literature regarding their ability to actually improve patient care.
In 2005, our institution, a large tertiary‐care academic teaching hospital, developed a standardized preprinted pediatric admission order set (PAOS). We did so for 3 reasons. First, there was a desire to improve completeness of orders. Handwritten orders often missed important elements such as weight, allergies, vital sign parameters, activity, etc. Second, there was a need to save time and improve efficiency. Third, it was important to reduce medical errors and the number of clarification requests by decreasing the necessity to decipher physician handwriting. Our PAOS was a convenience order set as opposed to a best practices order set. In other words, our PAOS did not contain evidence‐based management guidelines or protocols for specific admission diagnoses and was created solely to improve the quality and efficiency of workflow.
Documenting improvement in patient outcomes or reduction of medical errors is ultimately needed to establish the effectiveness of a standardized order set. Secondary outcomes, howeverparticularly the perceptions of the staff who are asked to use the order setare equally important, because they may identify real‐life barriers to use that, regardless of effectiveness, could limit dissemination and uptake. With respect to perceptions, 2 groups become paramount: those who write the orders, and those who respond to them. The purpose of the current study was to examine perceived effects of the new PAOS on inpatient care among those who, in our institution, write the ordersresident physicians.
MATERIALS AND METHODS
The PAOS was created in August 2005 at the University of California, Los Angeles (UCLA) Medical Center by a committee comprising pediatric hospitalists, nurses, pharmacists, residents, and clerks. The PAOS consisted mainly of check boxes (Figure 1). The PAOS was uploaded to the hospital website and made available for printing from all computers in the hospital, emergency room, and clinics.

The UCLA Hospital and Medical Center is a nonprofit, 667‐bed tertiary‐care teaching hospital in Los Angeles, California. The pediatric ward has 70 licensed beds with approximately 3,000 admissions per year. The majority of the admissions were done by the pediatric residents. Physicians were free to edit the PAOS to suit a particular patient's needs or to hand‐write orders on a blank order form.
Measures
Fourteen months after the institution of the PAOS, all 97 UCLA pediatric residents (PL‐1, n = 34; PL‐2, n = 33; PL‐3, n = 30) were asked to complete a survey to anonymously evaluate the order set. All residents were US medical school graduates. Resident participation in the research project was voluntary and confidential, and residents were assured that participation would not affect their standing in the pediatric residency program. Each resident completed only 1 survey. Responses were collected October 2006 to June 2007. The residents were asked to rate the PAOS overall and with respect to 9 specific dimensions using a 5‐point Likert scale with 1 indicating strong disagreement and 5 indicating strong agreement (Figure 2).

This study was reviewed and approved by the institutional review board at the UCLA Medical Center.
Statistical Analysis
We used bivariate ordered logistic regression to estimate the association between overall rating and each of the 9 dimensions. Ordered logistic regression, a standard technique for ordered categorical variables, is essentially a weighted average of logistic regressions performed at each potential cut‐point of the outcome variable. For instance, potential cut‐points on our 5‐point Likert scale included strong disagreement versus any other, any disagreement versus nondisagreement, any agreement versus nonagreement, and strong agreement versus any other. We then used multivariate ordered logistic regression to examine which specific dimensions remained independently associated with the overall rating.
RESULTS
From October 2006 to June 2007, 59 residents (from a total of 97 residents; 61%) responded to the survey. Overall, 89% of respondents approved of the PAOS, 58% reported using it 90% of the time, and all said that they would recommend it to their colleagues (Table 1). Eighty‐four percent thought that the PAOS improved inpatient care, and 75% thought that medical errors were reduced. Eighty‐eight percent reported that the POAS saved time; 93% said it was convenient; and most reported less need for clarification with clerks (81%) and nurses (82%).
| Strongly Agree (%) | Agree (%) | Other (%) | |
|---|---|---|---|
| Specific dimensions | |||
| Looks neat | 63 | 32 | 5 |
| User friendly/convenient | 60 | 33 | 7 |
| Readily available | 47 | 26 | 26 |
| Saves time | 56 | 32 | 12 |
| Comprehensive | 40 | 40 | 19 |
| Reduces medical error | 40 | 35 | 25 |
| Fewer clarification phone calls/errors by clerks | 47 | 33 | 19 |
| Fewer clarification phone calls/errors by nurses | 47 | 35 | 18 |
| Improves overall patient care | 46 | 39 | 16 |
| Overall rating | 40 | 49 | 11 |
In bivariate analyses, each of the 9 dimensions was strongly associated with the overall rating (P < 0.001 for each). In multivariate analyses, however, only perceived improvement in patient care was independently associated with overall rating (OR, 3.9; P = 0.04).
We then examined whether perceived improvement in patient care itself was independently predicted by the other 8 dimensions. Residents who said that the form was comprehensive (OR, 5.6; P = 0.01), reduced medical errors (OR, 4.1; P = 0.01), or required less need for clarification with nurses (OR, 9.6; P = 0.01) were more likely to perceive that the form improved patient care than residents who did not.
DISCUSSION
A standardized admission order set is a simple, low‐cost intervention that may benefit patients by reducing medical errors and expediting high‐quality care. In general, residents rated the PAOS favorably. Just as importantly, the PAOS scored well across all specific dimensions, which suggests few perceived barriers to use among residents.
Some dimensions, however, appeared potentially more important than others. Residents who perceived an improvement in patient care tended to rate the PAOS favorably. Perceived improvement in patient care, in turn, was linked to the order set's comprehensiveness, perceived reductions in medical errors, and less need for clarification with nurses.
Even though this study did not directly query those most responsible for responding to the order set (ie, nurses, pharmacists, and clerks), the order set was created through a collaborative partnership of physicians, nurses, pharmacists, and clerks. It is reasonable to infer that resident‐perceived reduction in the need for clarification of orders with nurses and clerks might indicate a broad‐based, multidisciplinary improvement in clarity and workflow. Moreover, the fact that less need for clarification with nurses was strongly associated with resident‐perceived improvement in patient care underscores the importance of including nurses, pharmacists, and clerks in the development of these order sets.
Our experience using the standard admission orders over the past 2 years is congruent with other authors' findings. Most studies, however, have examined standardized order forms only in adult populations, and mainly for specific medical conditions. Micek et al.9 demonstrated that use of a standardized physician order set among adults with septic shock lowered 28‐day mortality and reduced hospital stay. Among stroke patients, rates of optimal treatment significantly improved after the introduction of standardized stroke orders.10 For patients with acute myocardial infarction, standardized admission orders increased early administration of aspirin and beta blockers.11, 12 With respect to cancer, implementation of a preprinted chemotherapy prescription form improved order completeness, prevented medication errors, and reduced time spent by pharmacists clarifying orders.13 Finally, standardized trauma admission orders developed in a surgical‐trauma intensive care unit reduced admission laboratory charges and improved order completeness.14 We found only a single pediatric study examining standardized order forms. Kozer et al.15 found that the use of a preprinted structured medication order form cut medication errors nearly in half in a pediatric emergency department.
Whether our results would have been similar had we implemented a series of best practices order sets rather than a single convenience order set is unclear. Although best practices order sets would have facilitated application of evidence‐based guidelines for common diagnoses, they would also have introduced potentially unwelcome logistical heterogeneity (with a separate form and protocol needed for each diagnosis) that might have reduced acceptability and uptake. In addition, there is a risk that best practices order sets would have been perceived as unduly limiting physician professional autonomy.
Our study has limitations. First, our study was performed within a single institution and may not be easily generalized. However, we believe that the basic format of the PAOS lends it to easy adaptability. Second, we did not survey residents before the order set was introduced to assess baseline perceptions. Instead, many of the questions in the survey ask about perceived improvements compared with the previous system. Conducting a formal pre‐post data collection and analysis might have yielded different results. Third, improvement in patient care was measured indirectly based on resident opinion.
In conclusion, our study suggests that our standardized admission order set prompting physicians to initiate comprehensive care is well‐liked by residents and is thought to benefit patients by reducing medical errors and expediting high‐quality care. The next step is to confirm that the resident‐perceived improvement in patient care correlates with actual improvement in patient care. If improvements can be confirmed, then PAOS adoption could be broadly recommended to pediatric hospitals. In the future, the PAOS may also help guide computerized physician order entry templates that can be further tailored to specific common diagnoses.
For many years physicians have created and used various standardized order forms for patient hospital admissions. The increasing popularity of electronic medical records and forms has led to the use of computerized physician order entry (CPOE) as a means of reducing medication errors.13 Crowley et al.,4 Stucky,5 and Garg et al.,6 along with various committees, have recommended standardized order sets and CPOE as a strategy for reducing medication errors. However, implementation of CPOE systems is expensive and not available in most hospitals. According to a recent survey of hospitals in the US, CPOE was only available to physicians at 16% of the participating institutions.7 Until CPOE becomes widespread, standardized preprinted formatted order sets may serve as an inexpensive alternative.
There is anecdotal evidence that standardized admission order forms may improve quality of care and efficiency, and decrease provider variation.8 However, few rigorous studies exist in the pediatric research literature regarding their ability to actually improve patient care.
In 2005, our institution, a large tertiary‐care academic teaching hospital, developed a standardized preprinted pediatric admission order set (PAOS). We did so for 3 reasons. First, there was a desire to improve completeness of orders. Handwritten orders often missed important elements such as weight, allergies, vital sign parameters, activity, etc. Second, there was a need to save time and improve efficiency. Third, it was important to reduce medical errors and the number of clarification requests by decreasing the necessity to decipher physician handwriting. Our PAOS was a convenience order set as opposed to a best practices order set. In other words, our PAOS did not contain evidence‐based management guidelines or protocols for specific admission diagnoses and was created solely to improve the quality and efficiency of workflow.
Documenting improvement in patient outcomes or reduction of medical errors is ultimately needed to establish the effectiveness of a standardized order set. Secondary outcomes, howeverparticularly the perceptions of the staff who are asked to use the order setare equally important, because they may identify real‐life barriers to use that, regardless of effectiveness, could limit dissemination and uptake. With respect to perceptions, 2 groups become paramount: those who write the orders, and those who respond to them. The purpose of the current study was to examine perceived effects of the new PAOS on inpatient care among those who, in our institution, write the ordersresident physicians.
MATERIALS AND METHODS
The PAOS was created in August 2005 at the University of California, Los Angeles (UCLA) Medical Center by a committee comprising pediatric hospitalists, nurses, pharmacists, residents, and clerks. The PAOS consisted mainly of check boxes (Figure 1). The PAOS was uploaded to the hospital website and made available for printing from all computers in the hospital, emergency room, and clinics.

The UCLA Hospital and Medical Center is a nonprofit, 667‐bed tertiary‐care teaching hospital in Los Angeles, California. The pediatric ward has 70 licensed beds with approximately 3,000 admissions per year. The majority of the admissions were done by the pediatric residents. Physicians were free to edit the PAOS to suit a particular patient's needs or to hand‐write orders on a blank order form.
Measures
Fourteen months after the institution of the PAOS, all 97 UCLA pediatric residents (PL‐1, n = 34; PL‐2, n = 33; PL‐3, n = 30) were asked to complete a survey to anonymously evaluate the order set. All residents were US medical school graduates. Resident participation in the research project was voluntary and confidential, and residents were assured that participation would not affect their standing in the pediatric residency program. Each resident completed only 1 survey. Responses were collected October 2006 to June 2007. The residents were asked to rate the PAOS overall and with respect to 9 specific dimensions using a 5‐point Likert scale with 1 indicating strong disagreement and 5 indicating strong agreement (Figure 2).

This study was reviewed and approved by the institutional review board at the UCLA Medical Center.
Statistical Analysis
We used bivariate ordered logistic regression to estimate the association between overall rating and each of the 9 dimensions. Ordered logistic regression, a standard technique for ordered categorical variables, is essentially a weighted average of logistic regressions performed at each potential cut‐point of the outcome variable. For instance, potential cut‐points on our 5‐point Likert scale included strong disagreement versus any other, any disagreement versus nondisagreement, any agreement versus nonagreement, and strong agreement versus any other. We then used multivariate ordered logistic regression to examine which specific dimensions remained independently associated with the overall rating.
RESULTS
From October 2006 to June 2007, 59 residents (from a total of 97 residents; 61%) responded to the survey. Overall, 89% of respondents approved of the PAOS, 58% reported using it 90% of the time, and all said that they would recommend it to their colleagues (Table 1). Eighty‐four percent thought that the PAOS improved inpatient care, and 75% thought that medical errors were reduced. Eighty‐eight percent reported that the POAS saved time; 93% said it was convenient; and most reported less need for clarification with clerks (81%) and nurses (82%).
| Strongly Agree (%) | Agree (%) | Other (%) | |
|---|---|---|---|
| Specific dimensions | |||
| Looks neat | 63 | 32 | 5 |
| User friendly/convenient | 60 | 33 | 7 |
| Readily available | 47 | 26 | 26 |
| Saves time | 56 | 32 | 12 |
| Comprehensive | 40 | 40 | 19 |
| Reduces medical error | 40 | 35 | 25 |
| Fewer clarification phone calls/errors by clerks | 47 | 33 | 19 |
| Fewer clarification phone calls/errors by nurses | 47 | 35 | 18 |
| Improves overall patient care | 46 | 39 | 16 |
| Overall rating | 40 | 49 | 11 |
In bivariate analyses, each of the 9 dimensions was strongly associated with the overall rating (P < 0.001 for each). In multivariate analyses, however, only perceived improvement in patient care was independently associated with overall rating (OR, 3.9; P = 0.04).
We then examined whether perceived improvement in patient care itself was independently predicted by the other 8 dimensions. Residents who said that the form was comprehensive (OR, 5.6; P = 0.01), reduced medical errors (OR, 4.1; P = 0.01), or required less need for clarification with nurses (OR, 9.6; P = 0.01) were more likely to perceive that the form improved patient care than residents who did not.
DISCUSSION
A standardized admission order set is a simple, low‐cost intervention that may benefit patients by reducing medical errors and expediting high‐quality care. In general, residents rated the PAOS favorably. Just as importantly, the PAOS scored well across all specific dimensions, which suggests few perceived barriers to use among residents.
Some dimensions, however, appeared potentially more important than others. Residents who perceived an improvement in patient care tended to rate the PAOS favorably. Perceived improvement in patient care, in turn, was linked to the order set's comprehensiveness, perceived reductions in medical errors, and less need for clarification with nurses.
Even though this study did not directly query those most responsible for responding to the order set (ie, nurses, pharmacists, and clerks), the order set was created through a collaborative partnership of physicians, nurses, pharmacists, and clerks. It is reasonable to infer that resident‐perceived reduction in the need for clarification of orders with nurses and clerks might indicate a broad‐based, multidisciplinary improvement in clarity and workflow. Moreover, the fact that less need for clarification with nurses was strongly associated with resident‐perceived improvement in patient care underscores the importance of including nurses, pharmacists, and clerks in the development of these order sets.
Our experience using the standard admission orders over the past 2 years is congruent with other authors' findings. Most studies, however, have examined standardized order forms only in adult populations, and mainly for specific medical conditions. Micek et al.9 demonstrated that use of a standardized physician order set among adults with septic shock lowered 28‐day mortality and reduced hospital stay. Among stroke patients, rates of optimal treatment significantly improved after the introduction of standardized stroke orders.10 For patients with acute myocardial infarction, standardized admission orders increased early administration of aspirin and beta blockers.11, 12 With respect to cancer, implementation of a preprinted chemotherapy prescription form improved order completeness, prevented medication errors, and reduced time spent by pharmacists clarifying orders.13 Finally, standardized trauma admission orders developed in a surgical‐trauma intensive care unit reduced admission laboratory charges and improved order completeness.14 We found only a single pediatric study examining standardized order forms. Kozer et al.15 found that the use of a preprinted structured medication order form cut medication errors nearly in half in a pediatric emergency department.
Whether our results would have been similar had we implemented a series of best practices order sets rather than a single convenience order set is unclear. Although best practices order sets would have facilitated application of evidence‐based guidelines for common diagnoses, they would also have introduced potentially unwelcome logistical heterogeneity (with a separate form and protocol needed for each diagnosis) that might have reduced acceptability and uptake. In addition, there is a risk that best practices order sets would have been perceived as unduly limiting physician professional autonomy.
Our study has limitations. First, our study was performed within a single institution and may not be easily generalized. However, we believe that the basic format of the PAOS lends it to easy adaptability. Second, we did not survey residents before the order set was introduced to assess baseline perceptions. Instead, many of the questions in the survey ask about perceived improvements compared with the previous system. Conducting a formal pre‐post data collection and analysis might have yielded different results. Third, improvement in patient care was measured indirectly based on resident opinion.
In conclusion, our study suggests that our standardized admission order set prompting physicians to initiate comprehensive care is well‐liked by residents and is thought to benefit patients by reducing medical errors and expediting high‐quality care. The next step is to confirm that the resident‐perceived improvement in patient care correlates with actual improvement in patient care. If improvements can be confirmed, then PAOS adoption could be broadly recommended to pediatric hospitals. In the future, the PAOS may also help guide computerized physician order entry templates that can be further tailored to specific common diagnoses.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280(15):1311–1316.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163(12):1409–1416.
- ,,,,.The effect of computerized physician order entry on medication errors and adverse drug events in pediatric inpatients.Pediatrics.2003;112(3 Pt 1):506–509.
- ,,.Medication errors in children: a descriptive summary of medication error reports submitted to the United States Pharmacopoeia.Curr Ther Res Clin Exp.2001;62:627–640.
- .Prevention of medication errors in the pediatric inpatient setting.Pediatrics.2003;112(2):431–436.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293(10):1223–1238.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11(2):95–99.
- .Using standardized admit orders to improve inpatient care.Fam Pract Manag.1999;6(10):30–32.
- ,,, et al.Before‐after study of a standardized hospital order set for the management of septic shock.Crit Care Med.2006;34(11):2707–2713.
- California Acute Stroke Pilot Registry Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65(3):360–365.
- ,,,,,.Improving quality of care for acute myocardial infarction. The guidelines applied in practice (GAP) initiative.JAMA.2002;287(10):1269–1276.
- ,,,,,.Quality improvement initiative and its impact on the management of patients with acute myocardial infarction.Arch Intern Med.2000;160:3057–3061.
- ,,,,,.Effect of a cancer chemotherapy prescription form on prescription completeness.Am J Hosp Pharm.1989;46(9):1802–1806.
- ,.Standardized trauma admission orders, a pilot project.Int J Trauma Nurs.1996;2(1):13–21.
- ,,,,.Using a preprinted order sheet to reduce prescription errors in a pediatric emergency department: a randomized, controlled trial.Pediatrics.2005;116(6):1299–1302.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280(15):1311–1316.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163(12):1409–1416.
- ,,,,.The effect of computerized physician order entry on medication errors and adverse drug events in pediatric inpatients.Pediatrics.2003;112(3 Pt 1):506–509.
- ,,.Medication errors in children: a descriptive summary of medication error reports submitted to the United States Pharmacopoeia.Curr Ther Res Clin Exp.2001;62:627–640.
- .Prevention of medication errors in the pediatric inpatient setting.Pediatrics.2003;112(2):431–436.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293(10):1223–1238.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11(2):95–99.
- .Using standardized admit orders to improve inpatient care.Fam Pract Manag.1999;6(10):30–32.
- ,,, et al.Before‐after study of a standardized hospital order set for the management of septic shock.Crit Care Med.2006;34(11):2707–2713.
- California Acute Stroke Pilot Registry Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65(3):360–365.
- ,,,,,.Improving quality of care for acute myocardial infarction. The guidelines applied in practice (GAP) initiative.JAMA.2002;287(10):1269–1276.
- ,,,,,.Quality improvement initiative and its impact on the management of patients with acute myocardial infarction.Arch Intern Med.2000;160:3057–3061.
- ,,,,,.Effect of a cancer chemotherapy prescription form on prescription completeness.Am J Hosp Pharm.1989;46(9):1802–1806.
- ,.Standardized trauma admission orders, a pilot project.Int J Trauma Nurs.1996;2(1):13–21.
- ,,,,.Using a preprinted order sheet to reduce prescription errors in a pediatric emergency department: a randomized, controlled trial.Pediatrics.2005;116(6):1299–1302.
Copyright © 2009 Society of Hospital Medicine
A change of heart
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
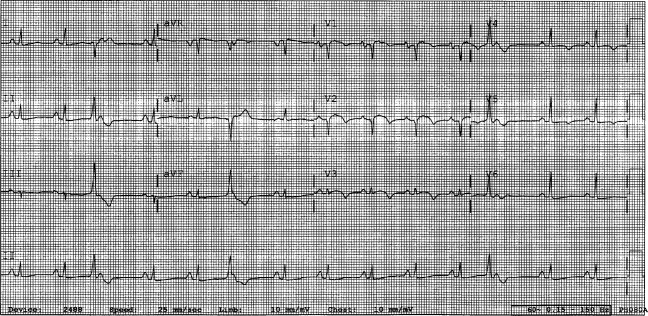
A 29‐year‐old man developed palpitations and dyspnea while loading boxes into a truck. In the emergency department, telemetry demonstrated a wide‐complex tachycardia at a rate of 204 beats per minute. The patient spontaneously cardioverted to sinus rhythm (Figure 1) before direct current cardioversion was performed.
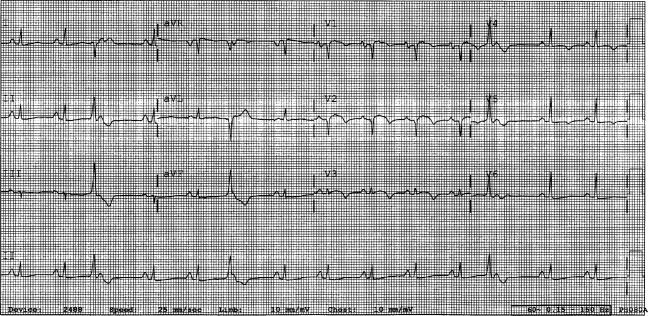
Wide‐complex tachycardia is usually explained by a supraventricular tachycardia with aberrant ventricular conduction or a ventricular tachycardia. Although algorithms exist to guide the clinician in parsing out those etiologies, often the knowledge of underlying structural cardiac disease is most informative. In patients with a history of myocardial infarction, greater than 95% of wide‐complex tachycardia is ventricular tachycardia. The ventricular ectopy, T‐wave inversion or flattening, and poor R‐wave progression are suggestive of a cardiomyopathy, either acute or chronic. A pressing concern, especially with the Q waves and concave ST morphology in V1 and V2, would be coronary ischemia. His age makes this less likely, but an aberrant coronary circulation or drug use could account for it.
Over the past 2 years, the patient had several episodes of sustained palpitations, which terminated after several minutes. Previously, the patient exercised frequently including playing rugby in college. However, over the past year he experienced difficulty climbing stairs due to shortness of breath, which he attributed to deconditioning and smoking. He had no significant medical history, was not taking any medications, nor did he use recreational stimulants. He drank alcohol occasionally. He had no risk factors for the human immunodeficiency virus (HIV). Both of the patient's parents were alive and well. There was no family history of sudden cardiac death.
The duration of symptoms suggests that this is a chronic cardiomyopathy rather than acute myocarditis or acute ischemia, acknowledging that either one could be superimposed. The absence of family history lowers the likelihood of heritable causes of arrhythmia that may accompany a structurally normal (eg, long QT syndrome) or abnormal (eg, hypertrophic cardiomyopathy) heart, although penetrance can be variable. What might account for a cardiomyopathy in a young person? Most cases are probably idiopathic, but etiologies that diverge from the usual suspects of coronary artery disease, hypertension, and valvular disease, which affect an older population, include antecedent viral myocarditis, substance abuse, HIV, or infiltrative disorders such as sarcoidosis.
The patient's pulse was 92 beats per minute and regular and the blood pressure was 96/52 mm Hg. The jugular venous pressure was elevated with prominent v‐waves, the point of maximal impulse was diffuse, there were no extra heart sounds or murmurs, and an enlarged liver was detected. An echocardiogram demonstrated left ventricular dysfunction with an ejection fraction of 30%, severe enlargement of the right atrium and right ventricle, and moderate tricuspid regurgitation. Cardiac catheterization revealed normal coronary arteries without evidence of pulmonary hypertension or intracardiac shunt.
The physical examination and echocardiographic findings of right‐sided failure are unusual given the absence of pulmonary hypertension or intracardiac shunt, and could prompt repeat of the hemodynamic measurements and/or investigations for pulmonary disease that may account for right‐sided pressure overload (in addition to that caused by left ventricular failure). An alternative explanation would be a cardiomyopathic process that preferentially involves the right side of the heart, such as arrhythmogenic right ventricular dysplasia (ARVD), but that would not satisfactorily explain the significant decline in left ventricular function. An acute right ventricular infarction could cause his acute symptoms and his examination and echocardiographic findings, but not the underlying chronic illness. It is common to see patients with long‐standing biventricular failure who present with prominent signs of right‐sided failure (elevated neck veins, hepatomegaly, and edema) but limited or no signs of left‐sided failure (rales) to match their degree of volume overload or dyspnea.
Cardiac magnetic resonance imaging (MRI) revealed a dilated right ventricle with extensive hyperenhancement, a right ventricular ejection fraction of 9%, and moderate left ventricular dysfunction (Figure 2). Electrophysiology testing induced both nonsustained polymorphic and monomorphic ventricular tachycardia. Late potentials were detected on a signal‐averaged electrocardiogram. A single‐chamber cardioverter defibrillator was implanted and the patient was discharged on carvedilol, lisinopril, and spironolactone. An HIV‐1 antibody was negative and a thyroid‐stimulating hormone concentration was within normal limits.
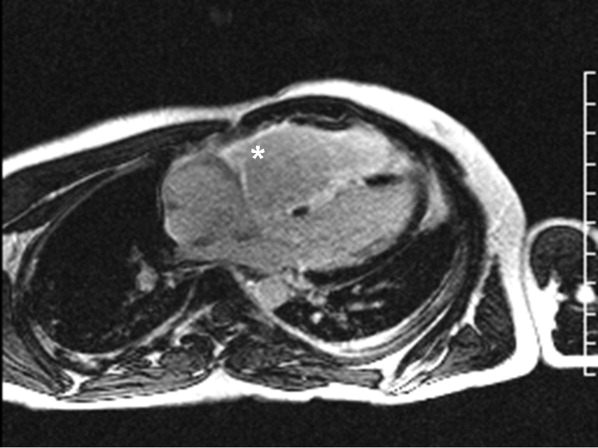
Assuming that accurate evaluation of the pulmonary circulation has been undertaken to exclude pulmonary hypertension, the enlarged and hyperenhanced right ventricle on MRI suggests a process that preferentially infiltrates the right ventricular myocardium, and may secondarily affect the left ventricle either by further infiltration or as a consequence of altered mechanics from the highly dysfunctional right ventricle. ARVD affects the right ventricle, but it is possible that another infiltrative cardiomyopathy, such as sarcoid or an antecedent viral infection, could be restricted in its distribution. Late‐potentials identified on signal average electrocardiograms indicate areas of abnormal conduction that may serve as substrate for reentrant ventricular arrhythmias. They are, however, nonspecific, as they are seen in a variety of myocardial diseases.
The patient continued to have progressive dyspnea and was readmitted after receiving an appropriate implantable cardioverter defibrillator shock for ventricular tachycardia. Recurrent slow ventricular tachycardia (Figure 3) was treated with supplemental beta‐blockade and amiodarone (10 g total). Repeat echocardiography demonstrated severe left ventricular dysfunction with an ejection fraction of less than 15%. There were no recurrences of ventricular arrhythmias and the patient was discharged and referred for cardiac transplant evaluation for ARVD.
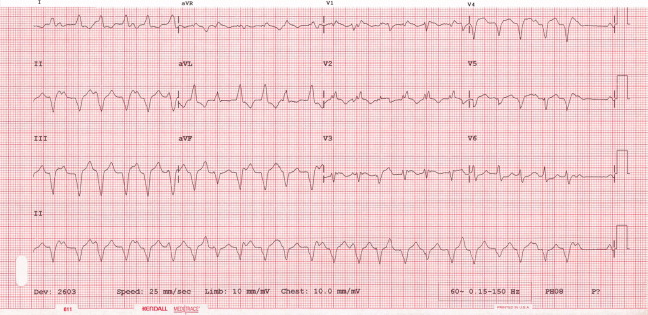
This degree of left ventricular dysfunction is unlikely to be accounted for by altered mechanics and interactions from a failing right ventricle alone and frames this as a biventricular cardiomyopathy, which has an extensive differential diagnosis and requires information from the general medical evaluation.
On routine laboratory testing 6 months later, a serum aspartate aminotransferase of 79 units/L and a serum alanine aminotransferase of 118 units/L were found. Bilirubin, albumin, and alkaline phosphatase were normal. The transaminase levels had been normal on initial evaluation. The patient reported that 2 paternal uncles had end‐stage nonalcoholic cirrhosis. Transjugular liver biopsy was consistent with mild lobular hepatitis with mild portal fibrosis with a few lobular collections of mononuclear cells. There was no evidence of iron overload. The hepatic venogram and transhepatic pressure gradient (2 mm Hg) were normal.
The elevated transaminase levels could be due to amiodarone‐associated hepatotoxicity, hepatic congestion, or a primary liver disease. It is important to consider combined cardiohepatic syndromes such as hemochromatosis, sarcoidosis, or amyloidosis. The relatively normal liver histology and normal hepatic hemodynamics do not suggest a significant primary intrinsic liver disease. The 2 uncles with cirrhosis could suggest a heritable liver disease, although cirrhosis in multiple family members is frequently accounted for by shared habits such as alcohol consumption or excessive caloric intake. Liver disorders with a genetic component, such as hemochromatosis, Wilson's disease, and alpha‐1‐antitrypsin deficiency are mostly autosomal recessive, which would make this pattern of transmission unusual. Furthermore, aside from hemochromatosis, these genetic hepatic disorders have few cardiac manifestations. Right‐sided congestion and amiodarone appear to be the most likely explanations of his liver abnormalities.
Pulmonary function testing revealed normal lung volumes without obstruction, but the diffusing capacity for carbon monoxide was substantially reduced. Computed tomography of the chest identified scattered ground‐glass opacities as well as small nodules with an upper lobe distribution (Figure 4). Although not reported on the initial interpretation, review of a chest x‐ray taken 6 months previously also demonstrated small nodules in the upper lobe distribution. Bronchoscopic examination was normal. Bronchioalveolar lavage fluid stains and cultures for bacteria, mycobacteria, Pneumocystis, and fungus were negative. Transbronchial biopsies of the right middle lobe had no evidence of infection, malignancy, or granulomatous inflammation. The patient continued to have progressive New York Heart Association Class IV heart failure symptoms. Repeat right heart catheterization was notable for a cardiac index of 1.4 L/minute/m2. The mean pulmonary artery pressure was 20 mm Hg. An intraaortic balloon pump was placed for refractory cardiogenic shock.
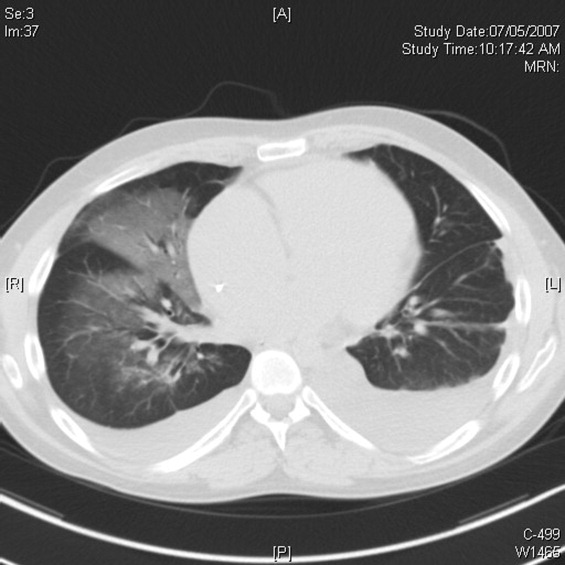
The reduced diffusion capacity and ground‐glass opacities suggest an interstitial process, which may have been missed on transbronchial biopsy because of sampling error. His pulmonary disease is likely another manifestation of his infiltrative cardiac disease. The constellation of cardiac, pulmonary, and hepatic involvement in the context of progressive dyspnea over 2 years is suggestive of sarcoidosis although the absence of hilar lymphadenopathy and 2 biopsy specimens without granulomas argue against the diagnosis, and the effects of amiodarone on the latter 2 organs cannot be ignored. On the limited menu of pharmacologic treatments that may treat this severe and progressive cardiomyopathy are steroids, which makes a diligent search for a steroid‐responsive syndrome important. Therefore, despite the negative studies, sarcoidosis must be investigated to the fullest extent with either an endomyocardial biopsy or surgical lung biopsy.
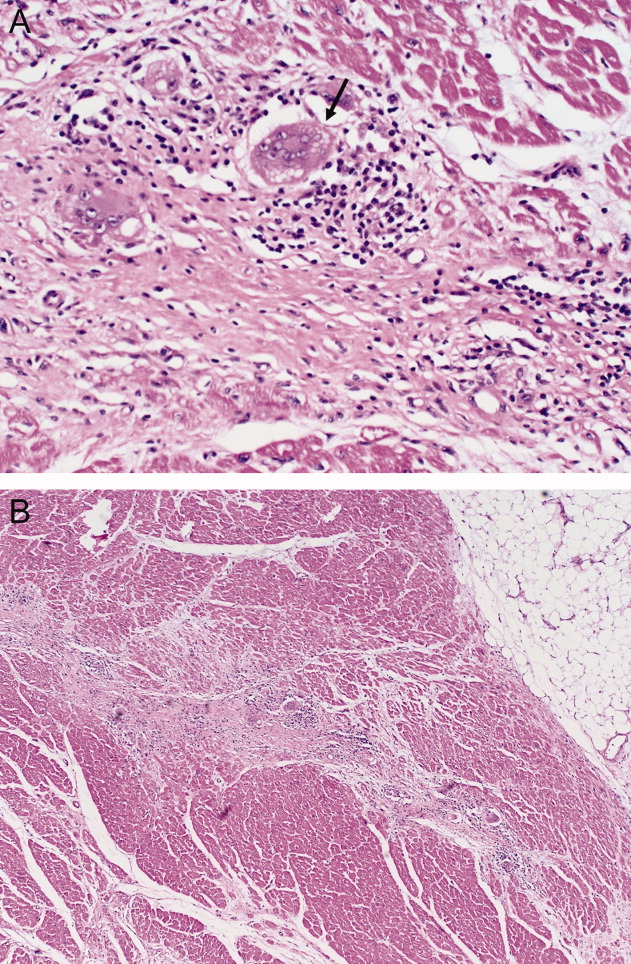
The patient underwent cardiac transplantation. The native heart was found to have right ventricular thinning, which was most notable at the right ventricular outflow tract. Microscopic examination revealed extensive fibrosis and granulomatous inflammation (Figure 5) with scarring typical of cardiac sarcoidosis. Six months after cardiac transplantation, the patient is doing well on prednisone, tacrolimus, and mycophenolate mofetil. Follow‐up chest x‐rays show resolution of the pulmonary nodules.
COMMENTARY
Cardiomyopathy in a young person is a relatively uncommon clinical event that prompts consideration of a broad differential diagnosis that is notably different from the most common etiologies of cardiomyopathy in older adults. This case highlights the challenges of arriving at a diagnosis in the absence of a gold standard, and the greater challenges of modifying initial diagnostic impressions as new clinical data become available.
After encountering ventricular tachycardia and right ventricular dysfunction in a young patient, the clinicians arrived at the diagnosis of ARVD. This rare and progressive disorder is associated with up to 20% of ventricular arrhythmias and sudden death in the young,1, 2 but can be challenging to diagnose. Despite common referrals for cardiac MRI to exclude ARVD, cardiac MRI is not the gold standard for diagnosis and is the most common method of misdiagnosis of ARVD.3 A diagnosis of ARVD requires the presence of 2 major, 1 major and 2 minor, or 4 minor International Task Force criteria (Table 1).4, 5 While the diagnostic criteria provide standardization across populations (eg, in clinical studies), additional considerations are needed in the management of individual patients. Scoring systems serve as a tool, but the final diagnosis requires balancing such criteria with competing hypotheses. This dilemma is familiar to clinicians considering other less common conditions such as amyotrophic lateral sclerosis (World Neurology Foundation), rheumatic fever (Jones criteria), or systemic lupus erythematosus (American College of Rheumatology). This patient's cardiac MRI findings, precordial T‐wave inversions, frequent ventricular ectopy, and late potentials on a signal‐averaged electrocardiogram fulfilled the International Task Force criteria for a diagnosis of ARVD. Discordant information included the right bundle branch pattern of the ventricular tachycardia, which suggested left ventricular origin, as opposed to the more common left bundle branch pattern observed in ARVD, and the absence of a family history. In addition, in U.S. populations only 25% of cases present with heart failure and fewer than 5% develop biventricular failure.6 Nonetheless, this patient's imaging evidence of right ventricular structural abnormalities and dysfunction and electrocardiographic abnormalities coupled with the absence of obvious systemic disease made ARVD the logical working diagnosis.
| Major | Minor | |
|---|---|---|
| ||
| I. Global and/or regional dysfunction and structural alterations | Severe dilation and reduction of right ventricular ejection fraction, localized right ventricular aneurysms | Mild right ventricular dilatation and/or reduced ejection fraction |
| II. Endomyocardial biopsy | Fibrofatty replacement of myocardium | |
| III. Repolarization abnormalities | T‐wave inversion in leads V1‐V3 or beyond | |
| IV. Depolarization/conduction abnormalities | Epsilon waves or localized QRS prolongation (>110 msec) in leads V1‐V3 | Late potentials on signal‐averaged electrocardiogram |
| V. Arrhythmias | Left bundle branch block‐type ventricular tachycardia (sustained and nonsustained) or frequent ventricular extra systoles (>1,000/24 hours) | |
| VI. Family history | Familial disease confirmed at necropsy or surgery | Familial history of premature sudden death (35 years old) or clinical diagnosis based on present criteria |
When more widespread manifestations developed, namely hepatic and pulmonary abnormalities, each was investigated with imaging and biopsy. Once a multisystem illness became apparent, the discussant reframed the patient's illness to include other diagnostic possibilities. In practice it is difficult to reverse a working diagnosis despite contradictory evidence because of the common pitfall of anchoring bias. Tversky and Kahneman7 were the first to describe the cognitive processes behind probability assessment and decision making in time‐sensitive situations. Under these conditions, decision makers tend to focus on the first symptom, striking feature, or diagnosis and anchor subsequent probabilities to that initial presentation. Once a decision or diagnosis has been reached, clinicians tend to interpret subsequent findings in the context of the original diagnosis rather than reevaluating their initial impression. In the setting of a known diagnosis of ARVD, 3 separate diagnoses (ARVD, amiodarone‐associated lung injury, and amiodarone‐induced hepatic dysfunction) were considered by the treating physicians. The initial diagnosis of ARVD followed by the sequential rather than simultaneous manifestations of sarcoidosis made arriving at the revised diagnosis even more challenging.
Cardiac sarcoidosis is a mimic of ARVD and should be considered when evaluating a patient for right ventricular dysplasia.8, 9 The differential diagnosis of ARVD includes idiopathic ventricular tachycardia, myocarditis, idiopathic cardiomyopathy, and sarcoidosis. Cardiac sarcoidosis can present as ventricular ectopy, sustained ventricular arrhythmias, asymptomatic ventricular dysfunction, heart failure, or sudden death.10 Although 25% of patients with sarcoidosis have evidence of cardiac involvement at autopsy, only 5% have clinical manifestations.11 Those patients with clinical evidence of cardiac sarcoidosis have a wide range of clinical findings (Table 2). While the patient's cardiomyopathy was advanced, it is possible that earlier administration of corticosteroid therapy may have arrested his progressive biventricular failure. As clinicians, we should always remember to force ourselves to broaden our differential diagnosis when new findings become available, especially those that point to a systemicrather than an organ‐specificdisorder. In this case, while the original diagnostic findings were accurate and strongly suggested ARVD, a change of heart was needed to arrive at the ultimate diagnosis.
| Clinical Manifestation | Prevalence (%) |
|---|---|
| Atrioventricular block | 40 |
| Bundle branch block | 40 |
| Supraventricular tachycardia | 20 |
| Ventricular arrhythmias | 25 |
| Heart failure | 25 |
| Sudden cardiac death | 35 |
KEY POINTS FOR HOSPITALISTS
-
Cardiomyopathy in a young person requires consideration of a broad differential diagnosis that is notably different from the most common etiologies of cardiomyopathy in the elderly.
-
Anchoring bias is a common pitfall in clinical decision making. When new or contradictory findings are uncovered, clinicians should reevaluate their initial impression to ensure it remains the most likely diagnosis.
-
Cardiac sarcoidosis is a mimic of ARVD and should be considered when evaluating a patient for right ventricular cardiomyopathy. The differential diagnosis of ARVD includes idiopathic ventricular tachycardia, right ventricular outflow tract tachycardia, myocarditis, idiopathic dilated cardiomyopathy, and sarcoidosis.
- ,,, et al.Right ventricular dysplasia: a report of 24 adult cases.Circulation.1982;65:384–398.
- ,,,,.Right ventricular cardiomyopathy and sudden death in young people.N Engl J Med.1988;318:129–133.
- ,,, et al.Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy.J Cardiovasc Electrophysiol.2004;15:300–306.
- ,,, et al.Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology.Br Heart J.1994;71:215–218.
- ,,, et al.Predictors of appropriate implantable defibrillator therapies in patients with arrhythmogenic right ventricular dysplasia.Heart Rhythm.2005;2:1188–1194.
- ,,, et al.Arrhythmogenic right ventricular dysplasia: a United States experience.Circulation.2005;112:3823–3832.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,.Unusual presentation of cardiac sarcoidosis.Congest Heart Fail.2007;13:116–118.
- ,,, et al.Cardiac sarcoidosis mimicking right ventricular dysplasia.Circ J.2003;67:169–171.
- ,,,,.Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation.Heart Rhythm.2006;3:924–929.
- ,.Sarcoid heart disease: clinical course and treatment.Int J Cardiol.2004;97:173–182.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 29‐year‐old man developed palpitations and dyspnea while loading boxes into a truck. In the emergency department, telemetry demonstrated a wide‐complex tachycardia at a rate of 204 beats per minute. The patient spontaneously cardioverted to sinus rhythm (Figure 1) before direct current cardioversion was performed.

Wide‐complex tachycardia is usually explained by a supraventricular tachycardia with aberrant ventricular conduction or a ventricular tachycardia. Although algorithms exist to guide the clinician in parsing out those etiologies, often the knowledge of underlying structural cardiac disease is most informative. In patients with a history of myocardial infarction, greater than 95% of wide‐complex tachycardia is ventricular tachycardia. The ventricular ectopy, T‐wave inversion or flattening, and poor R‐wave progression are suggestive of a cardiomyopathy, either acute or chronic. A pressing concern, especially with the Q waves and concave ST morphology in V1 and V2, would be coronary ischemia. His age makes this less likely, but an aberrant coronary circulation or drug use could account for it.
Over the past 2 years, the patient had several episodes of sustained palpitations, which terminated after several minutes. Previously, the patient exercised frequently including playing rugby in college. However, over the past year he experienced difficulty climbing stairs due to shortness of breath, which he attributed to deconditioning and smoking. He had no significant medical history, was not taking any medications, nor did he use recreational stimulants. He drank alcohol occasionally. He had no risk factors for the human immunodeficiency virus (HIV). Both of the patient's parents were alive and well. There was no family history of sudden cardiac death.
The duration of symptoms suggests that this is a chronic cardiomyopathy rather than acute myocarditis or acute ischemia, acknowledging that either one could be superimposed. The absence of family history lowers the likelihood of heritable causes of arrhythmia that may accompany a structurally normal (eg, long QT syndrome) or abnormal (eg, hypertrophic cardiomyopathy) heart, although penetrance can be variable. What might account for a cardiomyopathy in a young person? Most cases are probably idiopathic, but etiologies that diverge from the usual suspects of coronary artery disease, hypertension, and valvular disease, which affect an older population, include antecedent viral myocarditis, substance abuse, HIV, or infiltrative disorders such as sarcoidosis.
The patient's pulse was 92 beats per minute and regular and the blood pressure was 96/52 mm Hg. The jugular venous pressure was elevated with prominent v‐waves, the point of maximal impulse was diffuse, there were no extra heart sounds or murmurs, and an enlarged liver was detected. An echocardiogram demonstrated left ventricular dysfunction with an ejection fraction of 30%, severe enlargement of the right atrium and right ventricle, and moderate tricuspid regurgitation. Cardiac catheterization revealed normal coronary arteries without evidence of pulmonary hypertension or intracardiac shunt.
The physical examination and echocardiographic findings of right‐sided failure are unusual given the absence of pulmonary hypertension or intracardiac shunt, and could prompt repeat of the hemodynamic measurements and/or investigations for pulmonary disease that may account for right‐sided pressure overload (in addition to that caused by left ventricular failure). An alternative explanation would be a cardiomyopathic process that preferentially involves the right side of the heart, such as arrhythmogenic right ventricular dysplasia (ARVD), but that would not satisfactorily explain the significant decline in left ventricular function. An acute right ventricular infarction could cause his acute symptoms and his examination and echocardiographic findings, but not the underlying chronic illness. It is common to see patients with long‐standing biventricular failure who present with prominent signs of right‐sided failure (elevated neck veins, hepatomegaly, and edema) but limited or no signs of left‐sided failure (rales) to match their degree of volume overload or dyspnea.
Cardiac magnetic resonance imaging (MRI) revealed a dilated right ventricle with extensive hyperenhancement, a right ventricular ejection fraction of 9%, and moderate left ventricular dysfunction (Figure 2). Electrophysiology testing induced both nonsustained polymorphic and monomorphic ventricular tachycardia. Late potentials were detected on a signal‐averaged electrocardiogram. A single‐chamber cardioverter defibrillator was implanted and the patient was discharged on carvedilol, lisinopril, and spironolactone. An HIV‐1 antibody was negative and a thyroid‐stimulating hormone concentration was within normal limits.

Assuming that accurate evaluation of the pulmonary circulation has been undertaken to exclude pulmonary hypertension, the enlarged and hyperenhanced right ventricle on MRI suggests a process that preferentially infiltrates the right ventricular myocardium, and may secondarily affect the left ventricle either by further infiltration or as a consequence of altered mechanics from the highly dysfunctional right ventricle. ARVD affects the right ventricle, but it is possible that another infiltrative cardiomyopathy, such as sarcoid or an antecedent viral infection, could be restricted in its distribution. Late‐potentials identified on signal average electrocardiograms indicate areas of abnormal conduction that may serve as substrate for reentrant ventricular arrhythmias. They are, however, nonspecific, as they are seen in a variety of myocardial diseases.
The patient continued to have progressive dyspnea and was readmitted after receiving an appropriate implantable cardioverter defibrillator shock for ventricular tachycardia. Recurrent slow ventricular tachycardia (Figure 3) was treated with supplemental beta‐blockade and amiodarone (10 g total). Repeat echocardiography demonstrated severe left ventricular dysfunction with an ejection fraction of less than 15%. There were no recurrences of ventricular arrhythmias and the patient was discharged and referred for cardiac transplant evaluation for ARVD.

This degree of left ventricular dysfunction is unlikely to be accounted for by altered mechanics and interactions from a failing right ventricle alone and frames this as a biventricular cardiomyopathy, which has an extensive differential diagnosis and requires information from the general medical evaluation.
On routine laboratory testing 6 months later, a serum aspartate aminotransferase of 79 units/L and a serum alanine aminotransferase of 118 units/L were found. Bilirubin, albumin, and alkaline phosphatase were normal. The transaminase levels had been normal on initial evaluation. The patient reported that 2 paternal uncles had end‐stage nonalcoholic cirrhosis. Transjugular liver biopsy was consistent with mild lobular hepatitis with mild portal fibrosis with a few lobular collections of mononuclear cells. There was no evidence of iron overload. The hepatic venogram and transhepatic pressure gradient (2 mm Hg) were normal.
The elevated transaminase levels could be due to amiodarone‐associated hepatotoxicity, hepatic congestion, or a primary liver disease. It is important to consider combined cardiohepatic syndromes such as hemochromatosis, sarcoidosis, or amyloidosis. The relatively normal liver histology and normal hepatic hemodynamics do not suggest a significant primary intrinsic liver disease. The 2 uncles with cirrhosis could suggest a heritable liver disease, although cirrhosis in multiple family members is frequently accounted for by shared habits such as alcohol consumption or excessive caloric intake. Liver disorders with a genetic component, such as hemochromatosis, Wilson's disease, and alpha‐1‐antitrypsin deficiency are mostly autosomal recessive, which would make this pattern of transmission unusual. Furthermore, aside from hemochromatosis, these genetic hepatic disorders have few cardiac manifestations. Right‐sided congestion and amiodarone appear to be the most likely explanations of his liver abnormalities.
Pulmonary function testing revealed normal lung volumes without obstruction, but the diffusing capacity for carbon monoxide was substantially reduced. Computed tomography of the chest identified scattered ground‐glass opacities as well as small nodules with an upper lobe distribution (Figure 4). Although not reported on the initial interpretation, review of a chest x‐ray taken 6 months previously also demonstrated small nodules in the upper lobe distribution. Bronchoscopic examination was normal. Bronchioalveolar lavage fluid stains and cultures for bacteria, mycobacteria, Pneumocystis, and fungus were negative. Transbronchial biopsies of the right middle lobe had no evidence of infection, malignancy, or granulomatous inflammation. The patient continued to have progressive New York Heart Association Class IV heart failure symptoms. Repeat right heart catheterization was notable for a cardiac index of 1.4 L/minute/m2. The mean pulmonary artery pressure was 20 mm Hg. An intraaortic balloon pump was placed for refractory cardiogenic shock.

The reduced diffusion capacity and ground‐glass opacities suggest an interstitial process, which may have been missed on transbronchial biopsy because of sampling error. His pulmonary disease is likely another manifestation of his infiltrative cardiac disease. The constellation of cardiac, pulmonary, and hepatic involvement in the context of progressive dyspnea over 2 years is suggestive of sarcoidosis although the absence of hilar lymphadenopathy and 2 biopsy specimens without granulomas argue against the diagnosis, and the effects of amiodarone on the latter 2 organs cannot be ignored. On the limited menu of pharmacologic treatments that may treat this severe and progressive cardiomyopathy are steroids, which makes a diligent search for a steroid‐responsive syndrome important. Therefore, despite the negative studies, sarcoidosis must be investigated to the fullest extent with either an endomyocardial biopsy or surgical lung biopsy.
The patient underwent cardiac transplantation. The native heart was found to have right ventricular thinning, which was most notable at the right ventricular outflow tract. Microscopic examination revealed extensive fibrosis and granulomatous inflammation (Figure 5) with scarring typical of cardiac sarcoidosis. Six months after cardiac transplantation, the patient is doing well on prednisone, tacrolimus, and mycophenolate mofetil. Follow‐up chest x‐rays show resolution of the pulmonary nodules.

COMMENTARY
Cardiomyopathy in a young person is a relatively uncommon clinical event that prompts consideration of a broad differential diagnosis that is notably different from the most common etiologies of cardiomyopathy in older adults. This case highlights the challenges of arriving at a diagnosis in the absence of a gold standard, and the greater challenges of modifying initial diagnostic impressions as new clinical data become available.
After encountering ventricular tachycardia and right ventricular dysfunction in a young patient, the clinicians arrived at the diagnosis of ARVD. This rare and progressive disorder is associated with up to 20% of ventricular arrhythmias and sudden death in the young,1, 2 but can be challenging to diagnose. Despite common referrals for cardiac MRI to exclude ARVD, cardiac MRI is not the gold standard for diagnosis and is the most common method of misdiagnosis of ARVD.3 A diagnosis of ARVD requires the presence of 2 major, 1 major and 2 minor, or 4 minor International Task Force criteria (Table 1).4, 5 While the diagnostic criteria provide standardization across populations (eg, in clinical studies), additional considerations are needed in the management of individual patients. Scoring systems serve as a tool, but the final diagnosis requires balancing such criteria with competing hypotheses. This dilemma is familiar to clinicians considering other less common conditions such as amyotrophic lateral sclerosis (World Neurology Foundation), rheumatic fever (Jones criteria), or systemic lupus erythematosus (American College of Rheumatology). This patient's cardiac MRI findings, precordial T‐wave inversions, frequent ventricular ectopy, and late potentials on a signal‐averaged electrocardiogram fulfilled the International Task Force criteria for a diagnosis of ARVD. Discordant information included the right bundle branch pattern of the ventricular tachycardia, which suggested left ventricular origin, as opposed to the more common left bundle branch pattern observed in ARVD, and the absence of a family history. In addition, in U.S. populations only 25% of cases present with heart failure and fewer than 5% develop biventricular failure.6 Nonetheless, this patient's imaging evidence of right ventricular structural abnormalities and dysfunction and electrocardiographic abnormalities coupled with the absence of obvious systemic disease made ARVD the logical working diagnosis.
| Major | Minor | |
|---|---|---|
| ||
| I. Global and/or regional dysfunction and structural alterations | Severe dilation and reduction of right ventricular ejection fraction, localized right ventricular aneurysms | Mild right ventricular dilatation and/or reduced ejection fraction |
| II. Endomyocardial biopsy | Fibrofatty replacement of myocardium | |
| III. Repolarization abnormalities | T‐wave inversion in leads V1‐V3 or beyond | |
| IV. Depolarization/conduction abnormalities | Epsilon waves or localized QRS prolongation (>110 msec) in leads V1‐V3 | Late potentials on signal‐averaged electrocardiogram |
| V. Arrhythmias | Left bundle branch block‐type ventricular tachycardia (sustained and nonsustained) or frequent ventricular extra systoles (>1,000/24 hours) | |
| VI. Family history | Familial disease confirmed at necropsy or surgery | Familial history of premature sudden death (35 years old) or clinical diagnosis based on present criteria |
When more widespread manifestations developed, namely hepatic and pulmonary abnormalities, each was investigated with imaging and biopsy. Once a multisystem illness became apparent, the discussant reframed the patient's illness to include other diagnostic possibilities. In practice it is difficult to reverse a working diagnosis despite contradictory evidence because of the common pitfall of anchoring bias. Tversky and Kahneman7 were the first to describe the cognitive processes behind probability assessment and decision making in time‐sensitive situations. Under these conditions, decision makers tend to focus on the first symptom, striking feature, or diagnosis and anchor subsequent probabilities to that initial presentation. Once a decision or diagnosis has been reached, clinicians tend to interpret subsequent findings in the context of the original diagnosis rather than reevaluating their initial impression. In the setting of a known diagnosis of ARVD, 3 separate diagnoses (ARVD, amiodarone‐associated lung injury, and amiodarone‐induced hepatic dysfunction) were considered by the treating physicians. The initial diagnosis of ARVD followed by the sequential rather than simultaneous manifestations of sarcoidosis made arriving at the revised diagnosis even more challenging.
Cardiac sarcoidosis is a mimic of ARVD and should be considered when evaluating a patient for right ventricular dysplasia.8, 9 The differential diagnosis of ARVD includes idiopathic ventricular tachycardia, myocarditis, idiopathic cardiomyopathy, and sarcoidosis. Cardiac sarcoidosis can present as ventricular ectopy, sustained ventricular arrhythmias, asymptomatic ventricular dysfunction, heart failure, or sudden death.10 Although 25% of patients with sarcoidosis have evidence of cardiac involvement at autopsy, only 5% have clinical manifestations.11 Those patients with clinical evidence of cardiac sarcoidosis have a wide range of clinical findings (Table 2). While the patient's cardiomyopathy was advanced, it is possible that earlier administration of corticosteroid therapy may have arrested his progressive biventricular failure. As clinicians, we should always remember to force ourselves to broaden our differential diagnosis when new findings become available, especially those that point to a systemicrather than an organ‐specificdisorder. In this case, while the original diagnostic findings were accurate and strongly suggested ARVD, a change of heart was needed to arrive at the ultimate diagnosis.
| Clinical Manifestation | Prevalence (%) |
|---|---|
| Atrioventricular block | 40 |
| Bundle branch block | 40 |
| Supraventricular tachycardia | 20 |
| Ventricular arrhythmias | 25 |
| Heart failure | 25 |
| Sudden cardiac death | 35 |
KEY POINTS FOR HOSPITALISTS
-
Cardiomyopathy in a young person requires consideration of a broad differential diagnosis that is notably different from the most common etiologies of cardiomyopathy in the elderly.
-
Anchoring bias is a common pitfall in clinical decision making. When new or contradictory findings are uncovered, clinicians should reevaluate their initial impression to ensure it remains the most likely diagnosis.
-
Cardiac sarcoidosis is a mimic of ARVD and should be considered when evaluating a patient for right ventricular cardiomyopathy. The differential diagnosis of ARVD includes idiopathic ventricular tachycardia, right ventricular outflow tract tachycardia, myocarditis, idiopathic dilated cardiomyopathy, and sarcoidosis.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 29‐year‐old man developed palpitations and dyspnea while loading boxes into a truck. In the emergency department, telemetry demonstrated a wide‐complex tachycardia at a rate of 204 beats per minute. The patient spontaneously cardioverted to sinus rhythm (Figure 1) before direct current cardioversion was performed.

Wide‐complex tachycardia is usually explained by a supraventricular tachycardia with aberrant ventricular conduction or a ventricular tachycardia. Although algorithms exist to guide the clinician in parsing out those etiologies, often the knowledge of underlying structural cardiac disease is most informative. In patients with a history of myocardial infarction, greater than 95% of wide‐complex tachycardia is ventricular tachycardia. The ventricular ectopy, T‐wave inversion or flattening, and poor R‐wave progression are suggestive of a cardiomyopathy, either acute or chronic. A pressing concern, especially with the Q waves and concave ST morphology in V1 and V2, would be coronary ischemia. His age makes this less likely, but an aberrant coronary circulation or drug use could account for it.
Over the past 2 years, the patient had several episodes of sustained palpitations, which terminated after several minutes. Previously, the patient exercised frequently including playing rugby in college. However, over the past year he experienced difficulty climbing stairs due to shortness of breath, which he attributed to deconditioning and smoking. He had no significant medical history, was not taking any medications, nor did he use recreational stimulants. He drank alcohol occasionally. He had no risk factors for the human immunodeficiency virus (HIV). Both of the patient's parents were alive and well. There was no family history of sudden cardiac death.
The duration of symptoms suggests that this is a chronic cardiomyopathy rather than acute myocarditis or acute ischemia, acknowledging that either one could be superimposed. The absence of family history lowers the likelihood of heritable causes of arrhythmia that may accompany a structurally normal (eg, long QT syndrome) or abnormal (eg, hypertrophic cardiomyopathy) heart, although penetrance can be variable. What might account for a cardiomyopathy in a young person? Most cases are probably idiopathic, but etiologies that diverge from the usual suspects of coronary artery disease, hypertension, and valvular disease, which affect an older population, include antecedent viral myocarditis, substance abuse, HIV, or infiltrative disorders such as sarcoidosis.
The patient's pulse was 92 beats per minute and regular and the blood pressure was 96/52 mm Hg. The jugular venous pressure was elevated with prominent v‐waves, the point of maximal impulse was diffuse, there were no extra heart sounds or murmurs, and an enlarged liver was detected. An echocardiogram demonstrated left ventricular dysfunction with an ejection fraction of 30%, severe enlargement of the right atrium and right ventricle, and moderate tricuspid regurgitation. Cardiac catheterization revealed normal coronary arteries without evidence of pulmonary hypertension or intracardiac shunt.
The physical examination and echocardiographic findings of right‐sided failure are unusual given the absence of pulmonary hypertension or intracardiac shunt, and could prompt repeat of the hemodynamic measurements and/or investigations for pulmonary disease that may account for right‐sided pressure overload (in addition to that caused by left ventricular failure). An alternative explanation would be a cardiomyopathic process that preferentially involves the right side of the heart, such as arrhythmogenic right ventricular dysplasia (ARVD), but that would not satisfactorily explain the significant decline in left ventricular function. An acute right ventricular infarction could cause his acute symptoms and his examination and echocardiographic findings, but not the underlying chronic illness. It is common to see patients with long‐standing biventricular failure who present with prominent signs of right‐sided failure (elevated neck veins, hepatomegaly, and edema) but limited or no signs of left‐sided failure (rales) to match their degree of volume overload or dyspnea.
Cardiac magnetic resonance imaging (MRI) revealed a dilated right ventricle with extensive hyperenhancement, a right ventricular ejection fraction of 9%, and moderate left ventricular dysfunction (Figure 2). Electrophysiology testing induced both nonsustained polymorphic and monomorphic ventricular tachycardia. Late potentials were detected on a signal‐averaged electrocardiogram. A single‐chamber cardioverter defibrillator was implanted and the patient was discharged on carvedilol, lisinopril, and spironolactone. An HIV‐1 antibody was negative and a thyroid‐stimulating hormone concentration was within normal limits.

Assuming that accurate evaluation of the pulmonary circulation has been undertaken to exclude pulmonary hypertension, the enlarged and hyperenhanced right ventricle on MRI suggests a process that preferentially infiltrates the right ventricular myocardium, and may secondarily affect the left ventricle either by further infiltration or as a consequence of altered mechanics from the highly dysfunctional right ventricle. ARVD affects the right ventricle, but it is possible that another infiltrative cardiomyopathy, such as sarcoid or an antecedent viral infection, could be restricted in its distribution. Late‐potentials identified on signal average electrocardiograms indicate areas of abnormal conduction that may serve as substrate for reentrant ventricular arrhythmias. They are, however, nonspecific, as they are seen in a variety of myocardial diseases.
The patient continued to have progressive dyspnea and was readmitted after receiving an appropriate implantable cardioverter defibrillator shock for ventricular tachycardia. Recurrent slow ventricular tachycardia (Figure 3) was treated with supplemental beta‐blockade and amiodarone (10 g total). Repeat echocardiography demonstrated severe left ventricular dysfunction with an ejection fraction of less than 15%. There were no recurrences of ventricular arrhythmias and the patient was discharged and referred for cardiac transplant evaluation for ARVD.

This degree of left ventricular dysfunction is unlikely to be accounted for by altered mechanics and interactions from a failing right ventricle alone and frames this as a biventricular cardiomyopathy, which has an extensive differential diagnosis and requires information from the general medical evaluation.
On routine laboratory testing 6 months later, a serum aspartate aminotransferase of 79 units/L and a serum alanine aminotransferase of 118 units/L were found. Bilirubin, albumin, and alkaline phosphatase were normal. The transaminase levels had been normal on initial evaluation. The patient reported that 2 paternal uncles had end‐stage nonalcoholic cirrhosis. Transjugular liver biopsy was consistent with mild lobular hepatitis with mild portal fibrosis with a few lobular collections of mononuclear cells. There was no evidence of iron overload. The hepatic venogram and transhepatic pressure gradient (2 mm Hg) were normal.
The elevated transaminase levels could be due to amiodarone‐associated hepatotoxicity, hepatic congestion, or a primary liver disease. It is important to consider combined cardiohepatic syndromes such as hemochromatosis, sarcoidosis, or amyloidosis. The relatively normal liver histology and normal hepatic hemodynamics do not suggest a significant primary intrinsic liver disease. The 2 uncles with cirrhosis could suggest a heritable liver disease, although cirrhosis in multiple family members is frequently accounted for by shared habits such as alcohol consumption or excessive caloric intake. Liver disorders with a genetic component, such as hemochromatosis, Wilson's disease, and alpha‐1‐antitrypsin deficiency are mostly autosomal recessive, which would make this pattern of transmission unusual. Furthermore, aside from hemochromatosis, these genetic hepatic disorders have few cardiac manifestations. Right‐sided congestion and amiodarone appear to be the most likely explanations of his liver abnormalities.
Pulmonary function testing revealed normal lung volumes without obstruction, but the diffusing capacity for carbon monoxide was substantially reduced. Computed tomography of the chest identified scattered ground‐glass opacities as well as small nodules with an upper lobe distribution (Figure 4). Although not reported on the initial interpretation, review of a chest x‐ray taken 6 months previously also demonstrated small nodules in the upper lobe distribution. Bronchoscopic examination was normal. Bronchioalveolar lavage fluid stains and cultures for bacteria, mycobacteria, Pneumocystis, and fungus were negative. Transbronchial biopsies of the right middle lobe had no evidence of infection, malignancy, or granulomatous inflammation. The patient continued to have progressive New York Heart Association Class IV heart failure symptoms. Repeat right heart catheterization was notable for a cardiac index of 1.4 L/minute/m2. The mean pulmonary artery pressure was 20 mm Hg. An intraaortic balloon pump was placed for refractory cardiogenic shock.

The reduced diffusion capacity and ground‐glass opacities suggest an interstitial process, which may have been missed on transbronchial biopsy because of sampling error. His pulmonary disease is likely another manifestation of his infiltrative cardiac disease. The constellation of cardiac, pulmonary, and hepatic involvement in the context of progressive dyspnea over 2 years is suggestive of sarcoidosis although the absence of hilar lymphadenopathy and 2 biopsy specimens without granulomas argue against the diagnosis, and the effects of amiodarone on the latter 2 organs cannot be ignored. On the limited menu of pharmacologic treatments that may treat this severe and progressive cardiomyopathy are steroids, which makes a diligent search for a steroid‐responsive syndrome important. Therefore, despite the negative studies, sarcoidosis must be investigated to the fullest extent with either an endomyocardial biopsy or surgical lung biopsy.
The patient underwent cardiac transplantation. The native heart was found to have right ventricular thinning, which was most notable at the right ventricular outflow tract. Microscopic examination revealed extensive fibrosis and granulomatous inflammation (Figure 5) with scarring typical of cardiac sarcoidosis. Six months after cardiac transplantation, the patient is doing well on prednisone, tacrolimus, and mycophenolate mofetil. Follow‐up chest x‐rays show resolution of the pulmonary nodules.

COMMENTARY
Cardiomyopathy in a young person is a relatively uncommon clinical event that prompts consideration of a broad differential diagnosis that is notably different from the most common etiologies of cardiomyopathy in older adults. This case highlights the challenges of arriving at a diagnosis in the absence of a gold standard, and the greater challenges of modifying initial diagnostic impressions as new clinical data become available.
After encountering ventricular tachycardia and right ventricular dysfunction in a young patient, the clinicians arrived at the diagnosis of ARVD. This rare and progressive disorder is associated with up to 20% of ventricular arrhythmias and sudden death in the young,1, 2 but can be challenging to diagnose. Despite common referrals for cardiac MRI to exclude ARVD, cardiac MRI is not the gold standard for diagnosis and is the most common method of misdiagnosis of ARVD.3 A diagnosis of ARVD requires the presence of 2 major, 1 major and 2 minor, or 4 minor International Task Force criteria (Table 1).4, 5 While the diagnostic criteria provide standardization across populations (eg, in clinical studies), additional considerations are needed in the management of individual patients. Scoring systems serve as a tool, but the final diagnosis requires balancing such criteria with competing hypotheses. This dilemma is familiar to clinicians considering other less common conditions such as amyotrophic lateral sclerosis (World Neurology Foundation), rheumatic fever (Jones criteria), or systemic lupus erythematosus (American College of Rheumatology). This patient's cardiac MRI findings, precordial T‐wave inversions, frequent ventricular ectopy, and late potentials on a signal‐averaged electrocardiogram fulfilled the International Task Force criteria for a diagnosis of ARVD. Discordant information included the right bundle branch pattern of the ventricular tachycardia, which suggested left ventricular origin, as opposed to the more common left bundle branch pattern observed in ARVD, and the absence of a family history. In addition, in U.S. populations only 25% of cases present with heart failure and fewer than 5% develop biventricular failure.6 Nonetheless, this patient's imaging evidence of right ventricular structural abnormalities and dysfunction and electrocardiographic abnormalities coupled with the absence of obvious systemic disease made ARVD the logical working diagnosis.
| Major | Minor | |
|---|---|---|
| ||
| I. Global and/or regional dysfunction and structural alterations | Severe dilation and reduction of right ventricular ejection fraction, localized right ventricular aneurysms | Mild right ventricular dilatation and/or reduced ejection fraction |
| II. Endomyocardial biopsy | Fibrofatty replacement of myocardium | |
| III. Repolarization abnormalities | T‐wave inversion in leads V1‐V3 or beyond | |
| IV. Depolarization/conduction abnormalities | Epsilon waves or localized QRS prolongation (>110 msec) in leads V1‐V3 | Late potentials on signal‐averaged electrocardiogram |
| V. Arrhythmias | Left bundle branch block‐type ventricular tachycardia (sustained and nonsustained) or frequent ventricular extra systoles (>1,000/24 hours) | |
| VI. Family history | Familial disease confirmed at necropsy or surgery | Familial history of premature sudden death (35 years old) or clinical diagnosis based on present criteria |
When more widespread manifestations developed, namely hepatic and pulmonary abnormalities, each was investigated with imaging and biopsy. Once a multisystem illness became apparent, the discussant reframed the patient's illness to include other diagnostic possibilities. In practice it is difficult to reverse a working diagnosis despite contradictory evidence because of the common pitfall of anchoring bias. Tversky and Kahneman7 were the first to describe the cognitive processes behind probability assessment and decision making in time‐sensitive situations. Under these conditions, decision makers tend to focus on the first symptom, striking feature, or diagnosis and anchor subsequent probabilities to that initial presentation. Once a decision or diagnosis has been reached, clinicians tend to interpret subsequent findings in the context of the original diagnosis rather than reevaluating their initial impression. In the setting of a known diagnosis of ARVD, 3 separate diagnoses (ARVD, amiodarone‐associated lung injury, and amiodarone‐induced hepatic dysfunction) were considered by the treating physicians. The initial diagnosis of ARVD followed by the sequential rather than simultaneous manifestations of sarcoidosis made arriving at the revised diagnosis even more challenging.
Cardiac sarcoidosis is a mimic of ARVD and should be considered when evaluating a patient for right ventricular dysplasia.8, 9 The differential diagnosis of ARVD includes idiopathic ventricular tachycardia, myocarditis, idiopathic cardiomyopathy, and sarcoidosis. Cardiac sarcoidosis can present as ventricular ectopy, sustained ventricular arrhythmias, asymptomatic ventricular dysfunction, heart failure, or sudden death.10 Although 25% of patients with sarcoidosis have evidence of cardiac involvement at autopsy, only 5% have clinical manifestations.11 Those patients with clinical evidence of cardiac sarcoidosis have a wide range of clinical findings (Table 2). While the patient's cardiomyopathy was advanced, it is possible that earlier administration of corticosteroid therapy may have arrested his progressive biventricular failure. As clinicians, we should always remember to force ourselves to broaden our differential diagnosis when new findings become available, especially those that point to a systemicrather than an organ‐specificdisorder. In this case, while the original diagnostic findings were accurate and strongly suggested ARVD, a change of heart was needed to arrive at the ultimate diagnosis.
| Clinical Manifestation | Prevalence (%) |
|---|---|
| Atrioventricular block | 40 |
| Bundle branch block | 40 |
| Supraventricular tachycardia | 20 |
| Ventricular arrhythmias | 25 |
| Heart failure | 25 |
| Sudden cardiac death | 35 |
KEY POINTS FOR HOSPITALISTS
-
Cardiomyopathy in a young person requires consideration of a broad differential diagnosis that is notably different from the most common etiologies of cardiomyopathy in the elderly.
-
Anchoring bias is a common pitfall in clinical decision making. When new or contradictory findings are uncovered, clinicians should reevaluate their initial impression to ensure it remains the most likely diagnosis.
-
Cardiac sarcoidosis is a mimic of ARVD and should be considered when evaluating a patient for right ventricular cardiomyopathy. The differential diagnosis of ARVD includes idiopathic ventricular tachycardia, right ventricular outflow tract tachycardia, myocarditis, idiopathic dilated cardiomyopathy, and sarcoidosis.
- ,,, et al.Right ventricular dysplasia: a report of 24 adult cases.Circulation.1982;65:384–398.
- ,,,,.Right ventricular cardiomyopathy and sudden death in young people.N Engl J Med.1988;318:129–133.
- ,,, et al.Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy.J Cardiovasc Electrophysiol.2004;15:300–306.
- ,,, et al.Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology.Br Heart J.1994;71:215–218.
- ,,, et al.Predictors of appropriate implantable defibrillator therapies in patients with arrhythmogenic right ventricular dysplasia.Heart Rhythm.2005;2:1188–1194.
- ,,, et al.Arrhythmogenic right ventricular dysplasia: a United States experience.Circulation.2005;112:3823–3832.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,.Unusual presentation of cardiac sarcoidosis.Congest Heart Fail.2007;13:116–118.
- ,,, et al.Cardiac sarcoidosis mimicking right ventricular dysplasia.Circ J.2003;67:169–171.
- ,,,,.Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation.Heart Rhythm.2006;3:924–929.
- ,.Sarcoid heart disease: clinical course and treatment.Int J Cardiol.2004;97:173–182.
- ,,, et al.Right ventricular dysplasia: a report of 24 adult cases.Circulation.1982;65:384–398.
- ,,,,.Right ventricular cardiomyopathy and sudden death in young people.N Engl J Med.1988;318:129–133.
- ,,, et al.Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy.J Cardiovasc Electrophysiol.2004;15:300–306.
- ,,, et al.Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology.Br Heart J.1994;71:215–218.
- ,,, et al.Predictors of appropriate implantable defibrillator therapies in patients with arrhythmogenic right ventricular dysplasia.Heart Rhythm.2005;2:1188–1194.
- ,,, et al.Arrhythmogenic right ventricular dysplasia: a United States experience.Circulation.2005;112:3823–3832.
- ,.Judgment under uncertainty: heuristics and biases.Science.1974;185:1124–1131.
- ,,.Unusual presentation of cardiac sarcoidosis.Congest Heart Fail.2007;13:116–118.
- ,,, et al.Cardiac sarcoidosis mimicking right ventricular dysplasia.Circ J.2003;67:169–171.
- ,,,,.Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation.Heart Rhythm.2006;3:924–929.
- ,.Sarcoid heart disease: clinical course and treatment.Int J Cardiol.2004;97:173–182.
Admission Order Sets for DVT Prophylaxis
The use of clinical decision support, despite great promise to improve health care, remains preliminary.1 The broad scope of quality and safety challenges facing clinicians2, 3 requires this situation to change. There is an urgent need to develop decision support tools and strategies that are effective, address many quality issues simultaneously, and are easy to implement in both academic and community settings.
One decision support tool that could help to meet this challenge is the order set. An order set is a group of orders with a common functional purpose that is used directly by a physician to create orders for a specific patient. Order sets can be used with either paper‐based or computerized provider order entry (CPOE) systems. Several studies have investigated the delivery of focused evidence‐based treatments to patients admitted using disease‐specific order sets compared with either historical or concurrent controls and have demonstrated increased use of therapies such as aspirin for acute myocardial infarction admissions,4 systemic corticosteroids, metered‐dose inhalers and pulse oximetry for pediatric asthma admissions,5 and venous thromboembolism prophylaxis for adult emergency department admissions.6 However, the ability of order sets to improve multiple quality measures in a diverse patient population has not been evaluated previously.
This study examined the effect of paper‐based order sets on the quality of admission orders for general medical patients in a community hospital. The primary hypothesis was that order set use would increase the proportion of general medical patients ordered deep venous thrombosis (DVT) prophylaxis. We chose this primary endpoint because DVT prophylaxis continues to be significantly underused in hospitalized patients.7, 8 Secondary hypotheses were that order sets would improve other admission order quality of care measures. We studied paper‐based order sets because the study hospital, along with the vast majority of North American hospitals, uses paper for order entry.9
PATIENTS AND METHODS
Study Setting
The study took place in a 750‐bed community hospital in Mississauga, Ontario, Canada. The study included only general medical patients and excluded cardiology, neurology, and intensive care unit patients. Approximately 30 different internists admitted patients during the study period from April 1, 2003 to March 31, 2005. The internists were not aware that this study was being conducted. Order sets were implemented as an option for writing admission orders in December 2003. Prior to the implementation of order sets, physicians wrote all admission orders using traditional free‐text handwritten orders on blank paper order sheets. Essentially all general medical patients are admitted through the emergency room. The hospital's Research Ethics Board approved this study.
Order Set Development
Local specialists developed order set content (evidence‐based where possible) using informal consensus methods, without explicitly grading evidence. This process created a general admission order set and six diagnosis‐specific order sets (community acquired pneumonia, chronic obstructive pulmonary disease [COPD], febrile neutropenia, soft tissue infection, upper gastrointestinal [GI] bleeding, and urinary tract infection [UTI]). All order sets contained the same orders pertaining to the primary and secondary outcomes, except for the GI bleed admission order set, which did not contain a DVT prophylaxis section.
Order sets were paper‐based and consisted of a menu of orders typically required for a medical admission. These included admitting service, admitting physician, allergies, resuscitation status, diet, activity level, frequency of vital sign measurement, laboratory investigations, diagnostic imaging, intravenous fluid therapy, and medications. The orders were either optional (requiring the physician to check a box to be performed) or default (enacted unless specifically crossed out by the physician). Both order types could consist of a single order (for example, heparin for DVT prophylaxis) or several orders simultaneously (for example, measurement of serum sodium, potassium, and creatinine). All order sets included space for additional free‐text handwritten orders to meet individual patient needs.
The DVT prophylaxis section contained optional orders for 5,000 units of heparin subcutaneously (sc) twice daily (BID) and compression stockings. The ordering physician could select 1, both, or neither of these options. Initiating other forms of DVT prophylaxis or therapeutic anticoagulation required additional free‐text handwritten orders.
Informal clinician feedback led to improved order set content and formatting in August 2004. Orders pertaining to study outcomes were unchanged in this upgrade.
Implementation
In December 2003, we placed the order sets near the stacks of blank paper order sheets used by internists admitting patients in the Emergency Department. We notified physicians by e‐mail when order sets became available but provided no formal education about order sets, DVT prophylaxis, or other study outcomes. The use of order sets was voluntary. We developed a website to facilitate timely reordering of depleted order sets from the hospital's print shop and trained all emergency room clerks regarding website access and storage of the order sets in convenient locations for physicians. Although order set availability was not formally assessed, there were no reports by physicians or observations by study investigators of order sets being unavailable at any time.
Data Collection
To assess the effect of order sets on the ordering of DVT prophylaxis, we retrospectively and randomly selected patient admissions and reviewed these patients' charts from 3 time periods during the study period: OctoberNovember 2003 (period 1, immediately prior to availability of order sets; 113 charts available of 120 discharged patients randomly selected from a total of 1,169 discharges); AprilDecember 2004 (period 2, 412 months after order set availability; 291 charts available of 300 discharged patients randomly selected from a total of 4,620 discharges); and FebruaryMarch 2005 (period 3, 1415 months after order set availability; 283 charts available of 290 randomly selected discharges out of a total of 1,057 discharges). We conducted an additional chart audit just prior to final submission of the manuscript (108 charts available of 120 discharged patients randomly selected from a total of 1,060 discharges in OctoberNovember 2007) to determine the sustainability of the improvements. The same patient could be selected in different time periods. One author (C.O. or K.D.) reviewed each chart using a jointly developed data collection form.
We assessed the admission orders of each chart for the use of an order set and the ordering of DVT prophylaxis, defined as 5,000 units of heparin sc BID or compression stockings (no patient received sc heparin 3 times daily, heparin sc BID in doses greater than 5,000 units, prophylactic doses of low molecular‐weight heparin, or low dose warfarin). We recorded the ordering of therapeutic anticoagulation, defined as intravenous heparin, full‐dose low molecular‐weight heparin, or warfarin with a target international normalized ratio 2.
Independent from the chart review, we examined the overall administration of heparin doses for DVT prophylaxis to all medical inpatients using the hospital pharmacy database. We estimated the overall administration of heparin for DVT prophylaxis in medical inpatients (136 medical beds, 4 wards) on a monthly basis from April 1, 2003 (8 months prior to order set availability) to March 30, 2005 (15 months after order set availability). We calculated monthly utilization as the proportion of patient‐days for which DVT prophylaxis was administered, as follows: (number of doses of subcutaneous heparin dispensed by the hospital pharmacy to the 4 wards)/(2 [since there are 2 doses per patient‐day] number of patient‐days).
We collected additional data from the charts selected during period 2 (AprilDecember 2004) to evaluate the effect of order sets on the following secondary outcomes: (1) the documentation of admission diagnosis, allergies, and code status; (2) general care orders (electrocardiogram [ECG] and notification of physician for chest pain, allied health consultation, standard hospital potassium replacement protocol [already available in the hospital], standard hospital diabetic diet and standard hospital insulin sliding scale [for patients with diabetes], night time sedation diet or nil per os, activity level and vital sign frequency); (3) blood urea nitrogen (BUN), a laboratory test often inappropriately ordered according to local guidelines10; (4) order formatting (numbering, dating, timing, and signing of all order pages); and (5) organization of orders in the standardized arrangement used in the order sets. This standardized arrangement of content was as follows: attending physician, admitting diagnosis, requests for consultation, diet, activity, vital signs, oxygen, nasogastric tube, urinary catheter, investigations, intravenous fluids, and medications. Free‐text admission orders and order set orders that maintained this arrangement were recorded as standardized. We did not assess order appropriateness.
We recorded the characteristics of all medical patients admitted to the hospital in two 1‐year periods during the study (April 1, 2003 to March 30, 2004 and April 1, 2004 to March 30, 2005), including age, gender, length of stay, diagnosis (defined by case management group [CMG]), and resource intensity weight (RIW). CMG defines groups of patients who are similar in diagnosis or procedure and RIW is a measure of resources used during a patient's hospital stay.11 The definitions of CMG and RIW did not change during the study.
Statistical Analysis
Baseline characteristics were compared using Student t‐test for normally distributed continuous variables (patient age) and the Mann‐Whitney U test for skewed continuous variables (length of stay and RIW). Chi square or Fisher's exact tests were used to compare categorical variables. Relative risks (RR) and 95% confidence intervals (CI) were calculated and compared using a z‐test. A 2‐sided P value <0.05 was taken to be statistically significant. All calculations were carried out using SAS Version 8.2 (SAS Institute, Cary, NC).
RESULTS
As shown in Table 1, there were no clinically important differences in demographic or clinical characteristics of medical patients between the 2 years of the study. There were small but statistically significant increases in patient illness complexity (as reflected in median RIW) (P = 0.003) and length of stay (P = 0.0002).
| Patient Characteristic | April 1, 2003 to March 30, 2004 (n = 4,415) | April 1, 2004 to March 30, 2005 (n = 4,287) |
|---|---|---|
| ||
| Age, mean SD | 67.2 17.7 | 67.6 17.5 |
| Length of stay, median days (IQR) | 6 (3‐12) | 6 (3‐13) |
| RIW,11 median (IQR) | 0.96 (0.68‐1.73) | 1.03 (0.72‐1.88) |
| Females, number (% of total) | 2,276 (52) | 2,223 (52) |
| Case mix group,11 number of patients (% of total) | ||
| Chronic obstructive pulmonary disease | 357 (8.1) | 385 (9.0) |
| Simple pneumonia and pleurisy | 322 (7.3) | 217 (5.1) |
| Esophageal, gastrointestinal, and miscellaneous digestive disease | 223 (5.1) | 239 (5.6) |
| Gastrointestinal hemorrhage | 185 (4.2) | 198 (4.6) |
| Respiratory neoplasm | 127 (2.9) | 144 (3.4) |
| Total | 1,214 (27.5) | 1,183 (27.7) |
Clinicians used order sets in 32.3% of admissions during period 2 (AprilDecember 2004, 412 months after order set availability), increasing to 51.6% in period 3 (FebruaryMarch 2005, 1415 months after order set availability). The results of the chart audit assessing the impact of order set use on DVT prophylaxis are shown in Figure 1. Prior to order set introduction, 10.9% of patients received orders for DVT prophylaxis. Subsequently, ordering of DVT prophylaxis in patients admitted with order sets increased (period 2: 35.6%; P < 0.001; RR, 3.27; 95% CI, 1.806.12 and period 3: 44.0%; P < 0.001; RR, 4.04; 95% CI, 2.327.31). In contrast, DVT prophylaxis ordering in the nonorder set group was initially unchanged (period 2: 10.6%; P = 0.93; RR, 0.97; 95% CI, 0.491.95), although later it increased to a smaller extent (period 3: 20.6%; P = 0.049; RR, 1.90; 95% CI, 1.013.65). As a result of this differential increase, patients admitted with order sets were more likely to be ordered DVT prophylaxis in both study periods (period 2: 35.6% versus 10.6%; P < 0.0001; RR, 3.38; 95% CI, 2.035.62 and period 3: 44.0% versus 20.6%; P < 0.0001; RR, 2.13; 95% CI, 1.443.16). The use of therapeutic anticoagulation was similar in patients admitted with and without order sets and did not change between time periods.
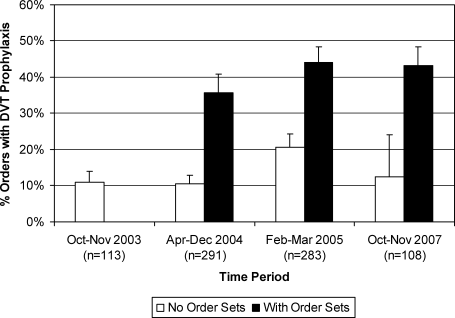
The hospital‐wide monthly utilization of heparin for DVT prophylaxis in medical inpatients increased from an average of 12.8% (range, 9.7%16.1%) of patient‐days before order set implementation (AprilNovember 2003) to 18.5% (range, 16.4%20.0%) of patient‐days in the 8 months after order sets were first implemented (DecemberJuly 2004, P < 0.0001 compared to the preorder set time period). After August 2004, when upgraded order sets were introduced, DVT prophylaxis utilization increased further in the last 7 months of the study to 25.8% (range, 22.4%32.2%; P < 0.0001 compared to preorder set time period; Figure 2).
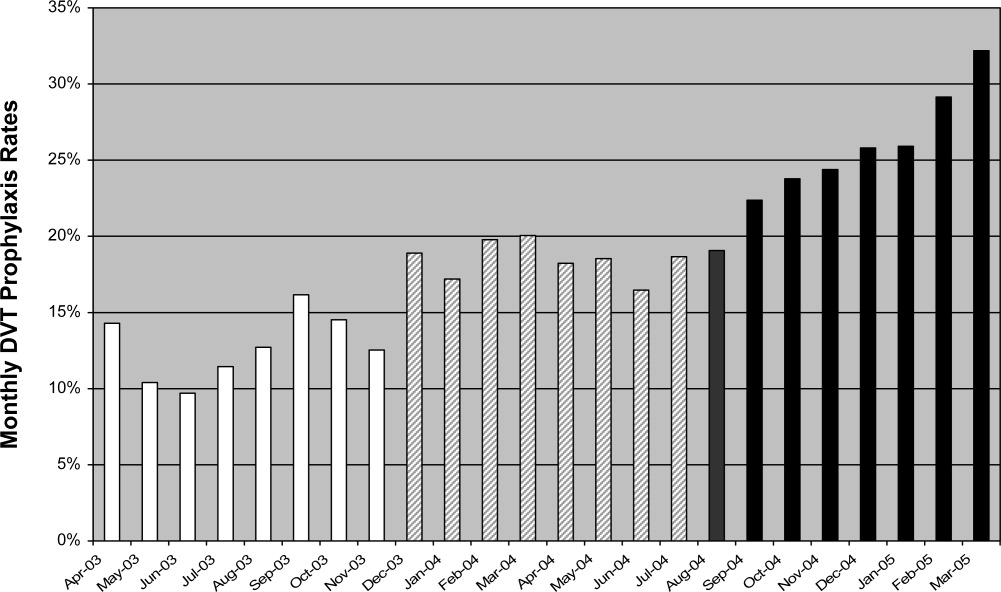
Table 2 shows the impact of order sets on secondary outcomes. Admissions completed with order sets had statistically significant increases in general care orders (ECG and notification of physician for chest pain, allied health consultations and standard hospital diabetic diet, insulin scale, and potassium replacement protocol orders), documentation of allergies and code status, numbering of pages, and use of a standardized arrangement for orders. Ordering of BUN decreased significantly.
| Outcome | Optional or Default | Order Set [n = 94 (%)] | No Order Set [n = 197 (%)] | P Value |
|---|---|---|---|---|
| ||||
| Documentation | ||||
| Admitting diagnosis | Optional | 91 (96.8) | 187 (94.9) | 0.47 |
| Allergies | Optional | 51 (54.3) | 19 (9.6) | <0.0001 |
| Resuscitation status | Optional | 54 (57.4) | 20 (10.2) | <0.0001 |
| General care orders | ||||
| ECG and call MD for chest pain | Default* | 80 (85.1) | 0 (0.0) | <0.0001 |
| Allied health consult | 59 (62.8) | 25 (12.7) | <0.0001 | |
| Diet | Optional | 90 (95.7) | 188 (95.4) | 0.90 |
| Activity | Optional | 80 (85.1) | 150 (76.1) | 0.08 |
| Vitals signs and frequency | Optional | 91 (96.8) | 178 (90.4) | 0.052 |
| Standard hospital diabetic diet | Optional | 16 (17.0) | 10 (5.1) | 0.0008 |
| Standard hospital insulin sliding scale | Optional | 18 (19.1) | 15 (7.6) | 0.004 |
| Standard hospital potassium protocol | Optional | 60 (63.8) | 1 (0.51) | <0.0001 |
| Nighttime sedation | ||||
| Zopiclone as needed | Optional | 43 (45.7) | 2 (1.0) | < 0.0001 |
| Lorazepam as needed | Optional | 12 (12.8) | 15 (7.6) | 0.16 |
| Laboratory test order | ||||
| Blood urea nitrogen | Optional | 37 (39.4) | 117 (59.0) | 0.0014 |
| Order formatting | ||||
| Numbering of pages | Default | 94 (100) | 4 (2.0) | <0.0001 |
| Dating of orders | Optional | 79 (84.0) | 185 (93.9) | 0.0067 |
| Timing of orders | Optional | 14 (14.9) | 29 (14.7) | 0.97 |
| Signing of orders | Optional | 93 (98.9) | 196 (99.5) | 0.54 |
| Standard arrangement of orders | 81 (86.2) | 66 (33.5) | <0.0001 | |
Order sets were not associated with changes in diet, activity, or vital sign orders, documentation of admission diagnosis, or the signing and timing of orders. Apart from order timing, these orders were present in >75% of admissions completed without order sets. The only negative effect of order sets was a reduction in the dating of orders (84.0% of order set admissions versus 93.9% of nonorder set admissions, P = 0.007). Finally, order sets had both an intended and unintended effect on nighttime sedation orders. Relative frequency of ordering of zopiclone compared to lorazepam increased (43/55 in the order set group vs. 2/17 in the no order set group [P < 0.0001], the intended effect), and increased overall frequency of ordering of nighttime sedation (55/94 vs. 17/197 [P < 0.0001], an unintended effect).
The additional chart audit in OctoberNovember 2007, just prior to final submission of the manuscript, determined that clinician use of order sets had increased to 92.6% of admitted medical patients, and that ordering of DVT prophylaxis in patients admitted with order sets had been sustained at 43.2% (P = 0.90 compared to period 3) (Figure 1).
DISCUSSION
We found that paper‐based order sets were associated with markedly increased use of DVT prophylaxis and made physician ordering more consistent with hospital consensus guidelines in multiple other areas, including laboratory test utilization and general care, while also increasing completeness of documentation. Given the difficulties and limited resources frequently associated with guideline development, dissemination, and implementation,12 it is worth noting that our improvements were achieved in a community hospital with voluntary physician adoption and no dedicated project funding, care process redesign, or healthcare worker education. The broad impact of order sets combined with minimal organizational resources required for implementation in this study suggests that this clinical decision support tool may have wide applicability.
The study hospital used paper‐based orders rather than CPOE, similar to 90% of U.S. hospitals at the time of the study.9 Order sets can be deployed in either paper‐based or computerized ordering systems. By providing a mechanism for entering large blocks of orders in an efficient manner, paper‐based order sets may be a necessary first step to facilitate the paper to CPOE transition, making them well suited to the current care delivery environment. Successful use of paper‐based order sets may help accelerate adoption of CPOE, which appears to be many years away from full implementation in the majority of U.S. hospitals.13
The most clinically important outcome in our study was a more than 4‐fold increase in ordering of DVT prophylaxis (last study period compared with baseline) in medical patients admitted with order sets, compared to a smaller increase in patients admitted without order sets. Our result is particularly significant as this study was performed in a community hospital, a setting with a lower adherence to DVT prophylaxis guidelines compared to academic centers.8, 14 The increase in DVT prophylaxis in patients admitted without order sets could be the result of a secular trend or a passive educational effect of order sets on physicians who only used order sets intermittently. The study was not publicized and thus was unlikely in itself to contribute to the increased performance.
We did not assess clinical outcomes of DVT or pulmonary embolism, but the clinical efficacy of improving adherence to DVT prophylaxis has been previously established.15 We also did not assess the appropriateness of DVT prophylaxis (or any other order). However, a recent multicenter Canadian observational study, using the American College of Chest Physician's Consensus Guidelines on Antithrombotic Therapy16 as a reference standard, found that 90% of medical patients admitted to hospital meeting study criteria had indications for thromboprophylaxis, but only 16% of eligible patients actually received it.8 In addition, multivariable regression analysis demonstrated even lower utilization in community hospitals compared to academic hospitals. These data suggest that the study hospital is typical of Canadian hospitals, and that the low overall utilization of DVT prophylaxis (13% of hospital patient‐days) prior to the availability of order sets in the study hospital is a significant gap between optimal and actual practice.
In addition, order sets had an impact on many secondary outcomes, such as standardization and completeness of orders (for example documentation of allergies and resuscitation status). While these effects appear to be beneficial in terms of quality of care and patient safety, the relationship of our secondary outcomes to patient‐important outcomes has not been established.
Furthermore, our before‐after design does not exclude the possibility of unknown confounding effects as explanations of improved performance in the order set group. For example, the change could have been driven by a small number of admitting physicians, since it is likely that order sets were adopted more readily by some physicians than others, and this group could have been responsible for a greater proportion of the admissions at different times. Unfortunately, we did not record the identity of the admitting physician. However, data from OctoberNovember 2007 show that >90% of medical patients were admitted using order sets, suggesting that voluntary clinician adoption of order sets has become nearly universal. Nevertheless, there still appear to be a few physicians who rarely or never used orders sets. Motivating these physicians to prescribe appropriate DVT prophylaxis remains a challenge.
Although this study was conducted in 1 center, other hospitals have similarly low rates of thromboprophylaxis,8 and our order set implementation strategy consumed few resources, improving the generalizability of our results. While most changes were beneficial, order set use was associated with decreased dating of orders and with an unintended effect or overall increase ordering of nightime sedation. Although the reasons for this are unclear, it highlights the importance of systematically evaluating the impact of order sets to identify unintended consequences and areas in which the order set may need to be redesigned to address these issues.
The study of order sets is preliminary despite their role as a key enabler for CPOE17 and their suggested usefulness to reduce medical error.18 For example, order sets were not considered in recent analyses of factors predicting success of computerized decision support systems19, 20 and have not been reviewed by the Cochrane Effective Practice and Organisation of Care Group.21 As discussed in the introduction, several studies have demonstrated that disease‐specific order sets can increase the use of evidence‐based treatments.46 Our study extends this work by demonstrating that admission order sets can improve performance hospital‐wide for a broad range of outcomes simultaneously, including DVT prophylaxis. Although most studies have demonstrated increased utilization of evidence‐based therapies, at least 1 study found no increased use of aspirin, heparin, or beta‐blockers in acute coronary syndrome admissions with the introduction of order sets.22 This suggests that the way order sets are structured or introduced is important to ensure that they achieve the desired changes in practice. Finally, our study suggests that improved outcomes using order sets can still be achieved with minimal organizational resources.
Order sets may potentially complement other decision support tools such as alerts and reminders. Alerts are an effective decision support tool12, 23, 24 but risk disrupting clinician workflow. Moreover, excessive alerts can lead to alert fatigue, resulting in many alerts being ignored.25 This phenomenon reduces alert effectiveness and limits the number of issues that alerts can address simultaneously. In contrast, order sets are broad in scope due to integration with clinical workflow, but lack the ability of alerts to apply rules to a specific patient's data. A potentially effective 2‐staged decision support strategy would use order sets as the primary admission decision support tool and selective alerts for remaining issues. This approach may increase the overall scope, physician adoption, and effectiveness of clinical decision support, and should be evaluated.
Our postintervention rate of DVT prophylaxis, while substantially improved from baseline, is still below ideal practice. Order sets were simply made available to clinicians admitting medical patients, who had the option to select DVT prophylaxis. Given limited resources, we did not develop and implement education programs regarding the appropriate use of DVT prophylaxis or make available any DVT risk assessment evaluations (available in Ref.26). Our study methodology thus provides a realistic assessment of improvements attainable in other hospitals with similarly limited resources. Additional increases in DVT prophylaxis rates would likely require a more comprehensive and resource‐intensive multifaceted quality improvement initiative. Detailed guidelines and supporting references for implementing such an initiative are available from the Society of Hospital Medicine.26 As described in their Venous Thromboembolism (VTE) Resource Room,26 such an initiative should include a standardized DVT risk assessment to guide the need for DVT prophylaxis integrated into admission order sets; prompts to order DVT prophylaxis when completing admission orders; and a system to audit adverse events and variations from best practice and return this information to clinicians.26
CONCLUSIONS
This is the largest and most comprehensive evaluation of the effectiveness of order sets as a clinical decision support tool. We found that order sets improved the quality of multiple patient orders and improved hospital‐wide DVT prophylaxis rates. These improvements were achieved in a community hospital with voluntary physician adoption and no dedicated project funding, care process redesign, or healthcare worker education. Although used in a paper‐based format in this study, order sets can also be employed in a computerized ordering environment. By providing a mechanism for entering large blocks of orders in an efficient manner, paper‐based order sets may be a necessary first step to facilitate the paper‐to‐CPOE transition. These attributes make order sets an attractive quality improvement tool in community and academic settings. More research is needed on the optimal design and use of this promising decision support tool.
- .Health Information Management: Integrating Information Technology in Health Care Work.London:Routledge;2004.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;2006.
- National Healthcare Quality Report.2004.Rockville, MD:Agency for Healthcare Research and Quality;2006.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13:188–196.
- ,,,,,.The role of computerized order sets in pediatric inpatient asthma treatment.Ped Allergy Immunol.2006;17:199–206.
- ,,,.Venous thromboembolism prophylaxis in emergency department admissions.J Hosp Med.2007;2:79–85.
- .DVT prevention: what is happening in the “real world”?Semin Thromb Hemost.2003;29(Suppl 1):23–31.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,,,.Computerized physician order entry in US hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- Ontario Association of Medical Laboratories. Guidelines for the Use of Serum Tests to Detect Renal Dysfunction. Available at:http://www.oaml.com/PDF/CLP007.pdf. Accessed 12 May2008.
- ,.Physicians in health care management: 3. Case mix groups and resource intensity weights: an overview for physicians.CMAJ.1994;150:889–894.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;8(6):iii–iv,1–72.
- ,,,,.Predicting computerized physician order entry system adoption in US hospitals: can the federal mandate be met?Int J Med Inform.2008;77(8):539–545.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119(Suppl 1):132S–175S.
- ,,.A consensus statement on considerations for a successful CPOE implementation.J Am Med Inform Assoc.2003;10:229–234.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,,,.Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success.BMJ.2005;330:765.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- Cochrane Reviews by the Effective Practice and Organisation of Care Group. Available at: http://www.cochrane.org/reviews/en/topics/61_reviews.html. Accessed 12 May2008.
- ,,.Embedded guideline information without patient specificity in a commercial emergency department computerized order‐entry system.Acad Emer Med.2006;13:452–458.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,.A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior.JAMA.2000;283:2816–2821.
- ,,, et al.Computerized physician order entry and online decision support.Acad Emerg Med.2004;11:1135–1141.
- Society of Hospital Medicine VTE Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm. Accessed 12 May2008.
The use of clinical decision support, despite great promise to improve health care, remains preliminary.1 The broad scope of quality and safety challenges facing clinicians2, 3 requires this situation to change. There is an urgent need to develop decision support tools and strategies that are effective, address many quality issues simultaneously, and are easy to implement in both academic and community settings.
One decision support tool that could help to meet this challenge is the order set. An order set is a group of orders with a common functional purpose that is used directly by a physician to create orders for a specific patient. Order sets can be used with either paper‐based or computerized provider order entry (CPOE) systems. Several studies have investigated the delivery of focused evidence‐based treatments to patients admitted using disease‐specific order sets compared with either historical or concurrent controls and have demonstrated increased use of therapies such as aspirin for acute myocardial infarction admissions,4 systemic corticosteroids, metered‐dose inhalers and pulse oximetry for pediatric asthma admissions,5 and venous thromboembolism prophylaxis for adult emergency department admissions.6 However, the ability of order sets to improve multiple quality measures in a diverse patient population has not been evaluated previously.
This study examined the effect of paper‐based order sets on the quality of admission orders for general medical patients in a community hospital. The primary hypothesis was that order set use would increase the proportion of general medical patients ordered deep venous thrombosis (DVT) prophylaxis. We chose this primary endpoint because DVT prophylaxis continues to be significantly underused in hospitalized patients.7, 8 Secondary hypotheses were that order sets would improve other admission order quality of care measures. We studied paper‐based order sets because the study hospital, along with the vast majority of North American hospitals, uses paper for order entry.9
PATIENTS AND METHODS
Study Setting
The study took place in a 750‐bed community hospital in Mississauga, Ontario, Canada. The study included only general medical patients and excluded cardiology, neurology, and intensive care unit patients. Approximately 30 different internists admitted patients during the study period from April 1, 2003 to March 31, 2005. The internists were not aware that this study was being conducted. Order sets were implemented as an option for writing admission orders in December 2003. Prior to the implementation of order sets, physicians wrote all admission orders using traditional free‐text handwritten orders on blank paper order sheets. Essentially all general medical patients are admitted through the emergency room. The hospital's Research Ethics Board approved this study.
Order Set Development
Local specialists developed order set content (evidence‐based where possible) using informal consensus methods, without explicitly grading evidence. This process created a general admission order set and six diagnosis‐specific order sets (community acquired pneumonia, chronic obstructive pulmonary disease [COPD], febrile neutropenia, soft tissue infection, upper gastrointestinal [GI] bleeding, and urinary tract infection [UTI]). All order sets contained the same orders pertaining to the primary and secondary outcomes, except for the GI bleed admission order set, which did not contain a DVT prophylaxis section.
Order sets were paper‐based and consisted of a menu of orders typically required for a medical admission. These included admitting service, admitting physician, allergies, resuscitation status, diet, activity level, frequency of vital sign measurement, laboratory investigations, diagnostic imaging, intravenous fluid therapy, and medications. The orders were either optional (requiring the physician to check a box to be performed) or default (enacted unless specifically crossed out by the physician). Both order types could consist of a single order (for example, heparin for DVT prophylaxis) or several orders simultaneously (for example, measurement of serum sodium, potassium, and creatinine). All order sets included space for additional free‐text handwritten orders to meet individual patient needs.
The DVT prophylaxis section contained optional orders for 5,000 units of heparin subcutaneously (sc) twice daily (BID) and compression stockings. The ordering physician could select 1, both, or neither of these options. Initiating other forms of DVT prophylaxis or therapeutic anticoagulation required additional free‐text handwritten orders.
Informal clinician feedback led to improved order set content and formatting in August 2004. Orders pertaining to study outcomes were unchanged in this upgrade.
Implementation
In December 2003, we placed the order sets near the stacks of blank paper order sheets used by internists admitting patients in the Emergency Department. We notified physicians by e‐mail when order sets became available but provided no formal education about order sets, DVT prophylaxis, or other study outcomes. The use of order sets was voluntary. We developed a website to facilitate timely reordering of depleted order sets from the hospital's print shop and trained all emergency room clerks regarding website access and storage of the order sets in convenient locations for physicians. Although order set availability was not formally assessed, there were no reports by physicians or observations by study investigators of order sets being unavailable at any time.
Data Collection
To assess the effect of order sets on the ordering of DVT prophylaxis, we retrospectively and randomly selected patient admissions and reviewed these patients' charts from 3 time periods during the study period: OctoberNovember 2003 (period 1, immediately prior to availability of order sets; 113 charts available of 120 discharged patients randomly selected from a total of 1,169 discharges); AprilDecember 2004 (period 2, 412 months after order set availability; 291 charts available of 300 discharged patients randomly selected from a total of 4,620 discharges); and FebruaryMarch 2005 (period 3, 1415 months after order set availability; 283 charts available of 290 randomly selected discharges out of a total of 1,057 discharges). We conducted an additional chart audit just prior to final submission of the manuscript (108 charts available of 120 discharged patients randomly selected from a total of 1,060 discharges in OctoberNovember 2007) to determine the sustainability of the improvements. The same patient could be selected in different time periods. One author (C.O. or K.D.) reviewed each chart using a jointly developed data collection form.
We assessed the admission orders of each chart for the use of an order set and the ordering of DVT prophylaxis, defined as 5,000 units of heparin sc BID or compression stockings (no patient received sc heparin 3 times daily, heparin sc BID in doses greater than 5,000 units, prophylactic doses of low molecular‐weight heparin, or low dose warfarin). We recorded the ordering of therapeutic anticoagulation, defined as intravenous heparin, full‐dose low molecular‐weight heparin, or warfarin with a target international normalized ratio 2.
Independent from the chart review, we examined the overall administration of heparin doses for DVT prophylaxis to all medical inpatients using the hospital pharmacy database. We estimated the overall administration of heparin for DVT prophylaxis in medical inpatients (136 medical beds, 4 wards) on a monthly basis from April 1, 2003 (8 months prior to order set availability) to March 30, 2005 (15 months after order set availability). We calculated monthly utilization as the proportion of patient‐days for which DVT prophylaxis was administered, as follows: (number of doses of subcutaneous heparin dispensed by the hospital pharmacy to the 4 wards)/(2 [since there are 2 doses per patient‐day] number of patient‐days).
We collected additional data from the charts selected during period 2 (AprilDecember 2004) to evaluate the effect of order sets on the following secondary outcomes: (1) the documentation of admission diagnosis, allergies, and code status; (2) general care orders (electrocardiogram [ECG] and notification of physician for chest pain, allied health consultation, standard hospital potassium replacement protocol [already available in the hospital], standard hospital diabetic diet and standard hospital insulin sliding scale [for patients with diabetes], night time sedation diet or nil per os, activity level and vital sign frequency); (3) blood urea nitrogen (BUN), a laboratory test often inappropriately ordered according to local guidelines10; (4) order formatting (numbering, dating, timing, and signing of all order pages); and (5) organization of orders in the standardized arrangement used in the order sets. This standardized arrangement of content was as follows: attending physician, admitting diagnosis, requests for consultation, diet, activity, vital signs, oxygen, nasogastric tube, urinary catheter, investigations, intravenous fluids, and medications. Free‐text admission orders and order set orders that maintained this arrangement were recorded as standardized. We did not assess order appropriateness.
We recorded the characteristics of all medical patients admitted to the hospital in two 1‐year periods during the study (April 1, 2003 to March 30, 2004 and April 1, 2004 to March 30, 2005), including age, gender, length of stay, diagnosis (defined by case management group [CMG]), and resource intensity weight (RIW). CMG defines groups of patients who are similar in diagnosis or procedure and RIW is a measure of resources used during a patient's hospital stay.11 The definitions of CMG and RIW did not change during the study.
Statistical Analysis
Baseline characteristics were compared using Student t‐test for normally distributed continuous variables (patient age) and the Mann‐Whitney U test for skewed continuous variables (length of stay and RIW). Chi square or Fisher's exact tests were used to compare categorical variables. Relative risks (RR) and 95% confidence intervals (CI) were calculated and compared using a z‐test. A 2‐sided P value <0.05 was taken to be statistically significant. All calculations were carried out using SAS Version 8.2 (SAS Institute, Cary, NC).
RESULTS
As shown in Table 1, there were no clinically important differences in demographic or clinical characteristics of medical patients between the 2 years of the study. There were small but statistically significant increases in patient illness complexity (as reflected in median RIW) (P = 0.003) and length of stay (P = 0.0002).
| Patient Characteristic | April 1, 2003 to March 30, 2004 (n = 4,415) | April 1, 2004 to March 30, 2005 (n = 4,287) |
|---|---|---|
| ||
| Age, mean SD | 67.2 17.7 | 67.6 17.5 |
| Length of stay, median days (IQR) | 6 (3‐12) | 6 (3‐13) |
| RIW,11 median (IQR) | 0.96 (0.68‐1.73) | 1.03 (0.72‐1.88) |
| Females, number (% of total) | 2,276 (52) | 2,223 (52) |
| Case mix group,11 number of patients (% of total) | ||
| Chronic obstructive pulmonary disease | 357 (8.1) | 385 (9.0) |
| Simple pneumonia and pleurisy | 322 (7.3) | 217 (5.1) |
| Esophageal, gastrointestinal, and miscellaneous digestive disease | 223 (5.1) | 239 (5.6) |
| Gastrointestinal hemorrhage | 185 (4.2) | 198 (4.6) |
| Respiratory neoplasm | 127 (2.9) | 144 (3.4) |
| Total | 1,214 (27.5) | 1,183 (27.7) |
Clinicians used order sets in 32.3% of admissions during period 2 (AprilDecember 2004, 412 months after order set availability), increasing to 51.6% in period 3 (FebruaryMarch 2005, 1415 months after order set availability). The results of the chart audit assessing the impact of order set use on DVT prophylaxis are shown in Figure 1. Prior to order set introduction, 10.9% of patients received orders for DVT prophylaxis. Subsequently, ordering of DVT prophylaxis in patients admitted with order sets increased (period 2: 35.6%; P < 0.001; RR, 3.27; 95% CI, 1.806.12 and period 3: 44.0%; P < 0.001; RR, 4.04; 95% CI, 2.327.31). In contrast, DVT prophylaxis ordering in the nonorder set group was initially unchanged (period 2: 10.6%; P = 0.93; RR, 0.97; 95% CI, 0.491.95), although later it increased to a smaller extent (period 3: 20.6%; P = 0.049; RR, 1.90; 95% CI, 1.013.65). As a result of this differential increase, patients admitted with order sets were more likely to be ordered DVT prophylaxis in both study periods (period 2: 35.6% versus 10.6%; P < 0.0001; RR, 3.38; 95% CI, 2.035.62 and period 3: 44.0% versus 20.6%; P < 0.0001; RR, 2.13; 95% CI, 1.443.16). The use of therapeutic anticoagulation was similar in patients admitted with and without order sets and did not change between time periods.

The hospital‐wide monthly utilization of heparin for DVT prophylaxis in medical inpatients increased from an average of 12.8% (range, 9.7%16.1%) of patient‐days before order set implementation (AprilNovember 2003) to 18.5% (range, 16.4%20.0%) of patient‐days in the 8 months after order sets were first implemented (DecemberJuly 2004, P < 0.0001 compared to the preorder set time period). After August 2004, when upgraded order sets were introduced, DVT prophylaxis utilization increased further in the last 7 months of the study to 25.8% (range, 22.4%32.2%; P < 0.0001 compared to preorder set time period; Figure 2).

Table 2 shows the impact of order sets on secondary outcomes. Admissions completed with order sets had statistically significant increases in general care orders (ECG and notification of physician for chest pain, allied health consultations and standard hospital diabetic diet, insulin scale, and potassium replacement protocol orders), documentation of allergies and code status, numbering of pages, and use of a standardized arrangement for orders. Ordering of BUN decreased significantly.
| Outcome | Optional or Default | Order Set [n = 94 (%)] | No Order Set [n = 197 (%)] | P Value |
|---|---|---|---|---|
| ||||
| Documentation | ||||
| Admitting diagnosis | Optional | 91 (96.8) | 187 (94.9) | 0.47 |
| Allergies | Optional | 51 (54.3) | 19 (9.6) | <0.0001 |
| Resuscitation status | Optional | 54 (57.4) | 20 (10.2) | <0.0001 |
| General care orders | ||||
| ECG and call MD for chest pain | Default* | 80 (85.1) | 0 (0.0) | <0.0001 |
| Allied health consult | 59 (62.8) | 25 (12.7) | <0.0001 | |
| Diet | Optional | 90 (95.7) | 188 (95.4) | 0.90 |
| Activity | Optional | 80 (85.1) | 150 (76.1) | 0.08 |
| Vitals signs and frequency | Optional | 91 (96.8) | 178 (90.4) | 0.052 |
| Standard hospital diabetic diet | Optional | 16 (17.0) | 10 (5.1) | 0.0008 |
| Standard hospital insulin sliding scale | Optional | 18 (19.1) | 15 (7.6) | 0.004 |
| Standard hospital potassium protocol | Optional | 60 (63.8) | 1 (0.51) | <0.0001 |
| Nighttime sedation | ||||
| Zopiclone as needed | Optional | 43 (45.7) | 2 (1.0) | < 0.0001 |
| Lorazepam as needed | Optional | 12 (12.8) | 15 (7.6) | 0.16 |
| Laboratory test order | ||||
| Blood urea nitrogen | Optional | 37 (39.4) | 117 (59.0) | 0.0014 |
| Order formatting | ||||
| Numbering of pages | Default | 94 (100) | 4 (2.0) | <0.0001 |
| Dating of orders | Optional | 79 (84.0) | 185 (93.9) | 0.0067 |
| Timing of orders | Optional | 14 (14.9) | 29 (14.7) | 0.97 |
| Signing of orders | Optional | 93 (98.9) | 196 (99.5) | 0.54 |
| Standard arrangement of orders | 81 (86.2) | 66 (33.5) | <0.0001 | |
Order sets were not associated with changes in diet, activity, or vital sign orders, documentation of admission diagnosis, or the signing and timing of orders. Apart from order timing, these orders were present in >75% of admissions completed without order sets. The only negative effect of order sets was a reduction in the dating of orders (84.0% of order set admissions versus 93.9% of nonorder set admissions, P = 0.007). Finally, order sets had both an intended and unintended effect on nighttime sedation orders. Relative frequency of ordering of zopiclone compared to lorazepam increased (43/55 in the order set group vs. 2/17 in the no order set group [P < 0.0001], the intended effect), and increased overall frequency of ordering of nighttime sedation (55/94 vs. 17/197 [P < 0.0001], an unintended effect).
The additional chart audit in OctoberNovember 2007, just prior to final submission of the manuscript, determined that clinician use of order sets had increased to 92.6% of admitted medical patients, and that ordering of DVT prophylaxis in patients admitted with order sets had been sustained at 43.2% (P = 0.90 compared to period 3) (Figure 1).
DISCUSSION
We found that paper‐based order sets were associated with markedly increased use of DVT prophylaxis and made physician ordering more consistent with hospital consensus guidelines in multiple other areas, including laboratory test utilization and general care, while also increasing completeness of documentation. Given the difficulties and limited resources frequently associated with guideline development, dissemination, and implementation,12 it is worth noting that our improvements were achieved in a community hospital with voluntary physician adoption and no dedicated project funding, care process redesign, or healthcare worker education. The broad impact of order sets combined with minimal organizational resources required for implementation in this study suggests that this clinical decision support tool may have wide applicability.
The study hospital used paper‐based orders rather than CPOE, similar to 90% of U.S. hospitals at the time of the study.9 Order sets can be deployed in either paper‐based or computerized ordering systems. By providing a mechanism for entering large blocks of orders in an efficient manner, paper‐based order sets may be a necessary first step to facilitate the paper to CPOE transition, making them well suited to the current care delivery environment. Successful use of paper‐based order sets may help accelerate adoption of CPOE, which appears to be many years away from full implementation in the majority of U.S. hospitals.13
The most clinically important outcome in our study was a more than 4‐fold increase in ordering of DVT prophylaxis (last study period compared with baseline) in medical patients admitted with order sets, compared to a smaller increase in patients admitted without order sets. Our result is particularly significant as this study was performed in a community hospital, a setting with a lower adherence to DVT prophylaxis guidelines compared to academic centers.8, 14 The increase in DVT prophylaxis in patients admitted without order sets could be the result of a secular trend or a passive educational effect of order sets on physicians who only used order sets intermittently. The study was not publicized and thus was unlikely in itself to contribute to the increased performance.
We did not assess clinical outcomes of DVT or pulmonary embolism, but the clinical efficacy of improving adherence to DVT prophylaxis has been previously established.15 We also did not assess the appropriateness of DVT prophylaxis (or any other order). However, a recent multicenter Canadian observational study, using the American College of Chest Physician's Consensus Guidelines on Antithrombotic Therapy16 as a reference standard, found that 90% of medical patients admitted to hospital meeting study criteria had indications for thromboprophylaxis, but only 16% of eligible patients actually received it.8 In addition, multivariable regression analysis demonstrated even lower utilization in community hospitals compared to academic hospitals. These data suggest that the study hospital is typical of Canadian hospitals, and that the low overall utilization of DVT prophylaxis (13% of hospital patient‐days) prior to the availability of order sets in the study hospital is a significant gap between optimal and actual practice.
In addition, order sets had an impact on many secondary outcomes, such as standardization and completeness of orders (for example documentation of allergies and resuscitation status). While these effects appear to be beneficial in terms of quality of care and patient safety, the relationship of our secondary outcomes to patient‐important outcomes has not been established.
Furthermore, our before‐after design does not exclude the possibility of unknown confounding effects as explanations of improved performance in the order set group. For example, the change could have been driven by a small number of admitting physicians, since it is likely that order sets were adopted more readily by some physicians than others, and this group could have been responsible for a greater proportion of the admissions at different times. Unfortunately, we did not record the identity of the admitting physician. However, data from OctoberNovember 2007 show that >90% of medical patients were admitted using order sets, suggesting that voluntary clinician adoption of order sets has become nearly universal. Nevertheless, there still appear to be a few physicians who rarely or never used orders sets. Motivating these physicians to prescribe appropriate DVT prophylaxis remains a challenge.
Although this study was conducted in 1 center, other hospitals have similarly low rates of thromboprophylaxis,8 and our order set implementation strategy consumed few resources, improving the generalizability of our results. While most changes were beneficial, order set use was associated with decreased dating of orders and with an unintended effect or overall increase ordering of nightime sedation. Although the reasons for this are unclear, it highlights the importance of systematically evaluating the impact of order sets to identify unintended consequences and areas in which the order set may need to be redesigned to address these issues.
The study of order sets is preliminary despite their role as a key enabler for CPOE17 and their suggested usefulness to reduce medical error.18 For example, order sets were not considered in recent analyses of factors predicting success of computerized decision support systems19, 20 and have not been reviewed by the Cochrane Effective Practice and Organisation of Care Group.21 As discussed in the introduction, several studies have demonstrated that disease‐specific order sets can increase the use of evidence‐based treatments.46 Our study extends this work by demonstrating that admission order sets can improve performance hospital‐wide for a broad range of outcomes simultaneously, including DVT prophylaxis. Although most studies have demonstrated increased utilization of evidence‐based therapies, at least 1 study found no increased use of aspirin, heparin, or beta‐blockers in acute coronary syndrome admissions with the introduction of order sets.22 This suggests that the way order sets are structured or introduced is important to ensure that they achieve the desired changes in practice. Finally, our study suggests that improved outcomes using order sets can still be achieved with minimal organizational resources.
Order sets may potentially complement other decision support tools such as alerts and reminders. Alerts are an effective decision support tool12, 23, 24 but risk disrupting clinician workflow. Moreover, excessive alerts can lead to alert fatigue, resulting in many alerts being ignored.25 This phenomenon reduces alert effectiveness and limits the number of issues that alerts can address simultaneously. In contrast, order sets are broad in scope due to integration with clinical workflow, but lack the ability of alerts to apply rules to a specific patient's data. A potentially effective 2‐staged decision support strategy would use order sets as the primary admission decision support tool and selective alerts for remaining issues. This approach may increase the overall scope, physician adoption, and effectiveness of clinical decision support, and should be evaluated.
Our postintervention rate of DVT prophylaxis, while substantially improved from baseline, is still below ideal practice. Order sets were simply made available to clinicians admitting medical patients, who had the option to select DVT prophylaxis. Given limited resources, we did not develop and implement education programs regarding the appropriate use of DVT prophylaxis or make available any DVT risk assessment evaluations (available in Ref.26). Our study methodology thus provides a realistic assessment of improvements attainable in other hospitals with similarly limited resources. Additional increases in DVT prophylaxis rates would likely require a more comprehensive and resource‐intensive multifaceted quality improvement initiative. Detailed guidelines and supporting references for implementing such an initiative are available from the Society of Hospital Medicine.26 As described in their Venous Thromboembolism (VTE) Resource Room,26 such an initiative should include a standardized DVT risk assessment to guide the need for DVT prophylaxis integrated into admission order sets; prompts to order DVT prophylaxis when completing admission orders; and a system to audit adverse events and variations from best practice and return this information to clinicians.26
CONCLUSIONS
This is the largest and most comprehensive evaluation of the effectiveness of order sets as a clinical decision support tool. We found that order sets improved the quality of multiple patient orders and improved hospital‐wide DVT prophylaxis rates. These improvements were achieved in a community hospital with voluntary physician adoption and no dedicated project funding, care process redesign, or healthcare worker education. Although used in a paper‐based format in this study, order sets can also be employed in a computerized ordering environment. By providing a mechanism for entering large blocks of orders in an efficient manner, paper‐based order sets may be a necessary first step to facilitate the paper‐to‐CPOE transition. These attributes make order sets an attractive quality improvement tool in community and academic settings. More research is needed on the optimal design and use of this promising decision support tool.
The use of clinical decision support, despite great promise to improve health care, remains preliminary.1 The broad scope of quality and safety challenges facing clinicians2, 3 requires this situation to change. There is an urgent need to develop decision support tools and strategies that are effective, address many quality issues simultaneously, and are easy to implement in both academic and community settings.
One decision support tool that could help to meet this challenge is the order set. An order set is a group of orders with a common functional purpose that is used directly by a physician to create orders for a specific patient. Order sets can be used with either paper‐based or computerized provider order entry (CPOE) systems. Several studies have investigated the delivery of focused evidence‐based treatments to patients admitted using disease‐specific order sets compared with either historical or concurrent controls and have demonstrated increased use of therapies such as aspirin for acute myocardial infarction admissions,4 systemic corticosteroids, metered‐dose inhalers and pulse oximetry for pediatric asthma admissions,5 and venous thromboembolism prophylaxis for adult emergency department admissions.6 However, the ability of order sets to improve multiple quality measures in a diverse patient population has not been evaluated previously.
This study examined the effect of paper‐based order sets on the quality of admission orders for general medical patients in a community hospital. The primary hypothesis was that order set use would increase the proportion of general medical patients ordered deep venous thrombosis (DVT) prophylaxis. We chose this primary endpoint because DVT prophylaxis continues to be significantly underused in hospitalized patients.7, 8 Secondary hypotheses were that order sets would improve other admission order quality of care measures. We studied paper‐based order sets because the study hospital, along with the vast majority of North American hospitals, uses paper for order entry.9
PATIENTS AND METHODS
Study Setting
The study took place in a 750‐bed community hospital in Mississauga, Ontario, Canada. The study included only general medical patients and excluded cardiology, neurology, and intensive care unit patients. Approximately 30 different internists admitted patients during the study period from April 1, 2003 to March 31, 2005. The internists were not aware that this study was being conducted. Order sets were implemented as an option for writing admission orders in December 2003. Prior to the implementation of order sets, physicians wrote all admission orders using traditional free‐text handwritten orders on blank paper order sheets. Essentially all general medical patients are admitted through the emergency room. The hospital's Research Ethics Board approved this study.
Order Set Development
Local specialists developed order set content (evidence‐based where possible) using informal consensus methods, without explicitly grading evidence. This process created a general admission order set and six diagnosis‐specific order sets (community acquired pneumonia, chronic obstructive pulmonary disease [COPD], febrile neutropenia, soft tissue infection, upper gastrointestinal [GI] bleeding, and urinary tract infection [UTI]). All order sets contained the same orders pertaining to the primary and secondary outcomes, except for the GI bleed admission order set, which did not contain a DVT prophylaxis section.
Order sets were paper‐based and consisted of a menu of orders typically required for a medical admission. These included admitting service, admitting physician, allergies, resuscitation status, diet, activity level, frequency of vital sign measurement, laboratory investigations, diagnostic imaging, intravenous fluid therapy, and medications. The orders were either optional (requiring the physician to check a box to be performed) or default (enacted unless specifically crossed out by the physician). Both order types could consist of a single order (for example, heparin for DVT prophylaxis) or several orders simultaneously (for example, measurement of serum sodium, potassium, and creatinine). All order sets included space for additional free‐text handwritten orders to meet individual patient needs.
The DVT prophylaxis section contained optional orders for 5,000 units of heparin subcutaneously (sc) twice daily (BID) and compression stockings. The ordering physician could select 1, both, or neither of these options. Initiating other forms of DVT prophylaxis or therapeutic anticoagulation required additional free‐text handwritten orders.
Informal clinician feedback led to improved order set content and formatting in August 2004. Orders pertaining to study outcomes were unchanged in this upgrade.
Implementation
In December 2003, we placed the order sets near the stacks of blank paper order sheets used by internists admitting patients in the Emergency Department. We notified physicians by e‐mail when order sets became available but provided no formal education about order sets, DVT prophylaxis, or other study outcomes. The use of order sets was voluntary. We developed a website to facilitate timely reordering of depleted order sets from the hospital's print shop and trained all emergency room clerks regarding website access and storage of the order sets in convenient locations for physicians. Although order set availability was not formally assessed, there were no reports by physicians or observations by study investigators of order sets being unavailable at any time.
Data Collection
To assess the effect of order sets on the ordering of DVT prophylaxis, we retrospectively and randomly selected patient admissions and reviewed these patients' charts from 3 time periods during the study period: OctoberNovember 2003 (period 1, immediately prior to availability of order sets; 113 charts available of 120 discharged patients randomly selected from a total of 1,169 discharges); AprilDecember 2004 (period 2, 412 months after order set availability; 291 charts available of 300 discharged patients randomly selected from a total of 4,620 discharges); and FebruaryMarch 2005 (period 3, 1415 months after order set availability; 283 charts available of 290 randomly selected discharges out of a total of 1,057 discharges). We conducted an additional chart audit just prior to final submission of the manuscript (108 charts available of 120 discharged patients randomly selected from a total of 1,060 discharges in OctoberNovember 2007) to determine the sustainability of the improvements. The same patient could be selected in different time periods. One author (C.O. or K.D.) reviewed each chart using a jointly developed data collection form.
We assessed the admission orders of each chart for the use of an order set and the ordering of DVT prophylaxis, defined as 5,000 units of heparin sc BID or compression stockings (no patient received sc heparin 3 times daily, heparin sc BID in doses greater than 5,000 units, prophylactic doses of low molecular‐weight heparin, or low dose warfarin). We recorded the ordering of therapeutic anticoagulation, defined as intravenous heparin, full‐dose low molecular‐weight heparin, or warfarin with a target international normalized ratio 2.
Independent from the chart review, we examined the overall administration of heparin doses for DVT prophylaxis to all medical inpatients using the hospital pharmacy database. We estimated the overall administration of heparin for DVT prophylaxis in medical inpatients (136 medical beds, 4 wards) on a monthly basis from April 1, 2003 (8 months prior to order set availability) to March 30, 2005 (15 months after order set availability). We calculated monthly utilization as the proportion of patient‐days for which DVT prophylaxis was administered, as follows: (number of doses of subcutaneous heparin dispensed by the hospital pharmacy to the 4 wards)/(2 [since there are 2 doses per patient‐day] number of patient‐days).
We collected additional data from the charts selected during period 2 (AprilDecember 2004) to evaluate the effect of order sets on the following secondary outcomes: (1) the documentation of admission diagnosis, allergies, and code status; (2) general care orders (electrocardiogram [ECG] and notification of physician for chest pain, allied health consultation, standard hospital potassium replacement protocol [already available in the hospital], standard hospital diabetic diet and standard hospital insulin sliding scale [for patients with diabetes], night time sedation diet or nil per os, activity level and vital sign frequency); (3) blood urea nitrogen (BUN), a laboratory test often inappropriately ordered according to local guidelines10; (4) order formatting (numbering, dating, timing, and signing of all order pages); and (5) organization of orders in the standardized arrangement used in the order sets. This standardized arrangement of content was as follows: attending physician, admitting diagnosis, requests for consultation, diet, activity, vital signs, oxygen, nasogastric tube, urinary catheter, investigations, intravenous fluids, and medications. Free‐text admission orders and order set orders that maintained this arrangement were recorded as standardized. We did not assess order appropriateness.
We recorded the characteristics of all medical patients admitted to the hospital in two 1‐year periods during the study (April 1, 2003 to March 30, 2004 and April 1, 2004 to March 30, 2005), including age, gender, length of stay, diagnosis (defined by case management group [CMG]), and resource intensity weight (RIW). CMG defines groups of patients who are similar in diagnosis or procedure and RIW is a measure of resources used during a patient's hospital stay.11 The definitions of CMG and RIW did not change during the study.
Statistical Analysis
Baseline characteristics were compared using Student t‐test for normally distributed continuous variables (patient age) and the Mann‐Whitney U test for skewed continuous variables (length of stay and RIW). Chi square or Fisher's exact tests were used to compare categorical variables. Relative risks (RR) and 95% confidence intervals (CI) were calculated and compared using a z‐test. A 2‐sided P value <0.05 was taken to be statistically significant. All calculations were carried out using SAS Version 8.2 (SAS Institute, Cary, NC).
RESULTS
As shown in Table 1, there were no clinically important differences in demographic or clinical characteristics of medical patients between the 2 years of the study. There were small but statistically significant increases in patient illness complexity (as reflected in median RIW) (P = 0.003) and length of stay (P = 0.0002).
| Patient Characteristic | April 1, 2003 to March 30, 2004 (n = 4,415) | April 1, 2004 to March 30, 2005 (n = 4,287) |
|---|---|---|
| ||
| Age, mean SD | 67.2 17.7 | 67.6 17.5 |
| Length of stay, median days (IQR) | 6 (3‐12) | 6 (3‐13) |
| RIW,11 median (IQR) | 0.96 (0.68‐1.73) | 1.03 (0.72‐1.88) |
| Females, number (% of total) | 2,276 (52) | 2,223 (52) |
| Case mix group,11 number of patients (% of total) | ||
| Chronic obstructive pulmonary disease | 357 (8.1) | 385 (9.0) |
| Simple pneumonia and pleurisy | 322 (7.3) | 217 (5.1) |
| Esophageal, gastrointestinal, and miscellaneous digestive disease | 223 (5.1) | 239 (5.6) |
| Gastrointestinal hemorrhage | 185 (4.2) | 198 (4.6) |
| Respiratory neoplasm | 127 (2.9) | 144 (3.4) |
| Total | 1,214 (27.5) | 1,183 (27.7) |
Clinicians used order sets in 32.3% of admissions during period 2 (AprilDecember 2004, 412 months after order set availability), increasing to 51.6% in period 3 (FebruaryMarch 2005, 1415 months after order set availability). The results of the chart audit assessing the impact of order set use on DVT prophylaxis are shown in Figure 1. Prior to order set introduction, 10.9% of patients received orders for DVT prophylaxis. Subsequently, ordering of DVT prophylaxis in patients admitted with order sets increased (period 2: 35.6%; P < 0.001; RR, 3.27; 95% CI, 1.806.12 and period 3: 44.0%; P < 0.001; RR, 4.04; 95% CI, 2.327.31). In contrast, DVT prophylaxis ordering in the nonorder set group was initially unchanged (period 2: 10.6%; P = 0.93; RR, 0.97; 95% CI, 0.491.95), although later it increased to a smaller extent (period 3: 20.6%; P = 0.049; RR, 1.90; 95% CI, 1.013.65). As a result of this differential increase, patients admitted with order sets were more likely to be ordered DVT prophylaxis in both study periods (period 2: 35.6% versus 10.6%; P < 0.0001; RR, 3.38; 95% CI, 2.035.62 and period 3: 44.0% versus 20.6%; P < 0.0001; RR, 2.13; 95% CI, 1.443.16). The use of therapeutic anticoagulation was similar in patients admitted with and without order sets and did not change between time periods.

The hospital‐wide monthly utilization of heparin for DVT prophylaxis in medical inpatients increased from an average of 12.8% (range, 9.7%16.1%) of patient‐days before order set implementation (AprilNovember 2003) to 18.5% (range, 16.4%20.0%) of patient‐days in the 8 months after order sets were first implemented (DecemberJuly 2004, P < 0.0001 compared to the preorder set time period). After August 2004, when upgraded order sets were introduced, DVT prophylaxis utilization increased further in the last 7 months of the study to 25.8% (range, 22.4%32.2%; P < 0.0001 compared to preorder set time period; Figure 2).

Table 2 shows the impact of order sets on secondary outcomes. Admissions completed with order sets had statistically significant increases in general care orders (ECG and notification of physician for chest pain, allied health consultations and standard hospital diabetic diet, insulin scale, and potassium replacement protocol orders), documentation of allergies and code status, numbering of pages, and use of a standardized arrangement for orders. Ordering of BUN decreased significantly.
| Outcome | Optional or Default | Order Set [n = 94 (%)] | No Order Set [n = 197 (%)] | P Value |
|---|---|---|---|---|
| ||||
| Documentation | ||||
| Admitting diagnosis | Optional | 91 (96.8) | 187 (94.9) | 0.47 |
| Allergies | Optional | 51 (54.3) | 19 (9.6) | <0.0001 |
| Resuscitation status | Optional | 54 (57.4) | 20 (10.2) | <0.0001 |
| General care orders | ||||
| ECG and call MD for chest pain | Default* | 80 (85.1) | 0 (0.0) | <0.0001 |
| Allied health consult | 59 (62.8) | 25 (12.7) | <0.0001 | |
| Diet | Optional | 90 (95.7) | 188 (95.4) | 0.90 |
| Activity | Optional | 80 (85.1) | 150 (76.1) | 0.08 |
| Vitals signs and frequency | Optional | 91 (96.8) | 178 (90.4) | 0.052 |
| Standard hospital diabetic diet | Optional | 16 (17.0) | 10 (5.1) | 0.0008 |
| Standard hospital insulin sliding scale | Optional | 18 (19.1) | 15 (7.6) | 0.004 |
| Standard hospital potassium protocol | Optional | 60 (63.8) | 1 (0.51) | <0.0001 |
| Nighttime sedation | ||||
| Zopiclone as needed | Optional | 43 (45.7) | 2 (1.0) | < 0.0001 |
| Lorazepam as needed | Optional | 12 (12.8) | 15 (7.6) | 0.16 |
| Laboratory test order | ||||
| Blood urea nitrogen | Optional | 37 (39.4) | 117 (59.0) | 0.0014 |
| Order formatting | ||||
| Numbering of pages | Default | 94 (100) | 4 (2.0) | <0.0001 |
| Dating of orders | Optional | 79 (84.0) | 185 (93.9) | 0.0067 |
| Timing of orders | Optional | 14 (14.9) | 29 (14.7) | 0.97 |
| Signing of orders | Optional | 93 (98.9) | 196 (99.5) | 0.54 |
| Standard arrangement of orders | 81 (86.2) | 66 (33.5) | <0.0001 | |
Order sets were not associated with changes in diet, activity, or vital sign orders, documentation of admission diagnosis, or the signing and timing of orders. Apart from order timing, these orders were present in >75% of admissions completed without order sets. The only negative effect of order sets was a reduction in the dating of orders (84.0% of order set admissions versus 93.9% of nonorder set admissions, P = 0.007). Finally, order sets had both an intended and unintended effect on nighttime sedation orders. Relative frequency of ordering of zopiclone compared to lorazepam increased (43/55 in the order set group vs. 2/17 in the no order set group [P < 0.0001], the intended effect), and increased overall frequency of ordering of nighttime sedation (55/94 vs. 17/197 [P < 0.0001], an unintended effect).
The additional chart audit in OctoberNovember 2007, just prior to final submission of the manuscript, determined that clinician use of order sets had increased to 92.6% of admitted medical patients, and that ordering of DVT prophylaxis in patients admitted with order sets had been sustained at 43.2% (P = 0.90 compared to period 3) (Figure 1).
DISCUSSION
We found that paper‐based order sets were associated with markedly increased use of DVT prophylaxis and made physician ordering more consistent with hospital consensus guidelines in multiple other areas, including laboratory test utilization and general care, while also increasing completeness of documentation. Given the difficulties and limited resources frequently associated with guideline development, dissemination, and implementation,12 it is worth noting that our improvements were achieved in a community hospital with voluntary physician adoption and no dedicated project funding, care process redesign, or healthcare worker education. The broad impact of order sets combined with minimal organizational resources required for implementation in this study suggests that this clinical decision support tool may have wide applicability.
The study hospital used paper‐based orders rather than CPOE, similar to 90% of U.S. hospitals at the time of the study.9 Order sets can be deployed in either paper‐based or computerized ordering systems. By providing a mechanism for entering large blocks of orders in an efficient manner, paper‐based order sets may be a necessary first step to facilitate the paper to CPOE transition, making them well suited to the current care delivery environment. Successful use of paper‐based order sets may help accelerate adoption of CPOE, which appears to be many years away from full implementation in the majority of U.S. hospitals.13
The most clinically important outcome in our study was a more than 4‐fold increase in ordering of DVT prophylaxis (last study period compared with baseline) in medical patients admitted with order sets, compared to a smaller increase in patients admitted without order sets. Our result is particularly significant as this study was performed in a community hospital, a setting with a lower adherence to DVT prophylaxis guidelines compared to academic centers.8, 14 The increase in DVT prophylaxis in patients admitted without order sets could be the result of a secular trend or a passive educational effect of order sets on physicians who only used order sets intermittently. The study was not publicized and thus was unlikely in itself to contribute to the increased performance.
We did not assess clinical outcomes of DVT or pulmonary embolism, but the clinical efficacy of improving adherence to DVT prophylaxis has been previously established.15 We also did not assess the appropriateness of DVT prophylaxis (or any other order). However, a recent multicenter Canadian observational study, using the American College of Chest Physician's Consensus Guidelines on Antithrombotic Therapy16 as a reference standard, found that 90% of medical patients admitted to hospital meeting study criteria had indications for thromboprophylaxis, but only 16% of eligible patients actually received it.8 In addition, multivariable regression analysis demonstrated even lower utilization in community hospitals compared to academic hospitals. These data suggest that the study hospital is typical of Canadian hospitals, and that the low overall utilization of DVT prophylaxis (13% of hospital patient‐days) prior to the availability of order sets in the study hospital is a significant gap between optimal and actual practice.
In addition, order sets had an impact on many secondary outcomes, such as standardization and completeness of orders (for example documentation of allergies and resuscitation status). While these effects appear to be beneficial in terms of quality of care and patient safety, the relationship of our secondary outcomes to patient‐important outcomes has not been established.
Furthermore, our before‐after design does not exclude the possibility of unknown confounding effects as explanations of improved performance in the order set group. For example, the change could have been driven by a small number of admitting physicians, since it is likely that order sets were adopted more readily by some physicians than others, and this group could have been responsible for a greater proportion of the admissions at different times. Unfortunately, we did not record the identity of the admitting physician. However, data from OctoberNovember 2007 show that >90% of medical patients were admitted using order sets, suggesting that voluntary clinician adoption of order sets has become nearly universal. Nevertheless, there still appear to be a few physicians who rarely or never used orders sets. Motivating these physicians to prescribe appropriate DVT prophylaxis remains a challenge.
Although this study was conducted in 1 center, other hospitals have similarly low rates of thromboprophylaxis,8 and our order set implementation strategy consumed few resources, improving the generalizability of our results. While most changes were beneficial, order set use was associated with decreased dating of orders and with an unintended effect or overall increase ordering of nightime sedation. Although the reasons for this are unclear, it highlights the importance of systematically evaluating the impact of order sets to identify unintended consequences and areas in which the order set may need to be redesigned to address these issues.
The study of order sets is preliminary despite their role as a key enabler for CPOE17 and their suggested usefulness to reduce medical error.18 For example, order sets were not considered in recent analyses of factors predicting success of computerized decision support systems19, 20 and have not been reviewed by the Cochrane Effective Practice and Organisation of Care Group.21 As discussed in the introduction, several studies have demonstrated that disease‐specific order sets can increase the use of evidence‐based treatments.46 Our study extends this work by demonstrating that admission order sets can improve performance hospital‐wide for a broad range of outcomes simultaneously, including DVT prophylaxis. Although most studies have demonstrated increased utilization of evidence‐based therapies, at least 1 study found no increased use of aspirin, heparin, or beta‐blockers in acute coronary syndrome admissions with the introduction of order sets.22 This suggests that the way order sets are structured or introduced is important to ensure that they achieve the desired changes in practice. Finally, our study suggests that improved outcomes using order sets can still be achieved with minimal organizational resources.
Order sets may potentially complement other decision support tools such as alerts and reminders. Alerts are an effective decision support tool12, 23, 24 but risk disrupting clinician workflow. Moreover, excessive alerts can lead to alert fatigue, resulting in many alerts being ignored.25 This phenomenon reduces alert effectiveness and limits the number of issues that alerts can address simultaneously. In contrast, order sets are broad in scope due to integration with clinical workflow, but lack the ability of alerts to apply rules to a specific patient's data. A potentially effective 2‐staged decision support strategy would use order sets as the primary admission decision support tool and selective alerts for remaining issues. This approach may increase the overall scope, physician adoption, and effectiveness of clinical decision support, and should be evaluated.
Our postintervention rate of DVT prophylaxis, while substantially improved from baseline, is still below ideal practice. Order sets were simply made available to clinicians admitting medical patients, who had the option to select DVT prophylaxis. Given limited resources, we did not develop and implement education programs regarding the appropriate use of DVT prophylaxis or make available any DVT risk assessment evaluations (available in Ref.26). Our study methodology thus provides a realistic assessment of improvements attainable in other hospitals with similarly limited resources. Additional increases in DVT prophylaxis rates would likely require a more comprehensive and resource‐intensive multifaceted quality improvement initiative. Detailed guidelines and supporting references for implementing such an initiative are available from the Society of Hospital Medicine.26 As described in their Venous Thromboembolism (VTE) Resource Room,26 such an initiative should include a standardized DVT risk assessment to guide the need for DVT prophylaxis integrated into admission order sets; prompts to order DVT prophylaxis when completing admission orders; and a system to audit adverse events and variations from best practice and return this information to clinicians.26
CONCLUSIONS
This is the largest and most comprehensive evaluation of the effectiveness of order sets as a clinical decision support tool. We found that order sets improved the quality of multiple patient orders and improved hospital‐wide DVT prophylaxis rates. These improvements were achieved in a community hospital with voluntary physician adoption and no dedicated project funding, care process redesign, or healthcare worker education. Although used in a paper‐based format in this study, order sets can also be employed in a computerized ordering environment. By providing a mechanism for entering large blocks of orders in an efficient manner, paper‐based order sets may be a necessary first step to facilitate the paper‐to‐CPOE transition. These attributes make order sets an attractive quality improvement tool in community and academic settings. More research is needed on the optimal design and use of this promising decision support tool.
- .Health Information Management: Integrating Information Technology in Health Care Work.London:Routledge;2004.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;2006.
- National Healthcare Quality Report.2004.Rockville, MD:Agency for Healthcare Research and Quality;2006.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13:188–196.
- ,,,,,.The role of computerized order sets in pediatric inpatient asthma treatment.Ped Allergy Immunol.2006;17:199–206.
- ,,,.Venous thromboembolism prophylaxis in emergency department admissions.J Hosp Med.2007;2:79–85.
- .DVT prevention: what is happening in the “real world”?Semin Thromb Hemost.2003;29(Suppl 1):23–31.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,,,.Computerized physician order entry in US hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- Ontario Association of Medical Laboratories. Guidelines for the Use of Serum Tests to Detect Renal Dysfunction. Available at:http://www.oaml.com/PDF/CLP007.pdf. Accessed 12 May2008.
- ,.Physicians in health care management: 3. Case mix groups and resource intensity weights: an overview for physicians.CMAJ.1994;150:889–894.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;8(6):iii–iv,1–72.
- ,,,,.Predicting computerized physician order entry system adoption in US hospitals: can the federal mandate be met?Int J Med Inform.2008;77(8):539–545.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119(Suppl 1):132S–175S.
- ,,.A consensus statement on considerations for a successful CPOE implementation.J Am Med Inform Assoc.2003;10:229–234.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,,,.Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success.BMJ.2005;330:765.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- Cochrane Reviews by the Effective Practice and Organisation of Care Group. Available at: http://www.cochrane.org/reviews/en/topics/61_reviews.html. Accessed 12 May2008.
- ,,.Embedded guideline information without patient specificity in a commercial emergency department computerized order‐entry system.Acad Emer Med.2006;13:452–458.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,.A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior.JAMA.2000;283:2816–2821.
- ,,, et al.Computerized physician order entry and online decision support.Acad Emerg Med.2004;11:1135–1141.
- Society of Hospital Medicine VTE Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm. Accessed 12 May2008.
- .Health Information Management: Integrating Information Technology in Health Care Work.London:Routledge;2004.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;2006.
- National Healthcare Quality Report.2004.Rockville, MD:Agency for Healthcare Research and Quality;2006.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13:188–196.
- ,,,,,.The role of computerized order sets in pediatric inpatient asthma treatment.Ped Allergy Immunol.2006;17:199–206.
- ,,,.Venous thromboembolism prophylaxis in emergency department admissions.J Hosp Med.2007;2:79–85.
- .DVT prevention: what is happening in the “real world”?Semin Thromb Hemost.2003;29(Suppl 1):23–31.
- ,,, et al.Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada.Thromb Res.2007;119:145–155.
- ,,,.Computerized physician order entry in US hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- Ontario Association of Medical Laboratories. Guidelines for the Use of Serum Tests to Detect Renal Dysfunction. Available at:http://www.oaml.com/PDF/CLP007.pdf. Accessed 12 May2008.
- ,.Physicians in health care management: 3. Case mix groups and resource intensity weights: an overview for physicians.CMAJ.1994;150:889–894.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;8(6):iii–iv,1–72.
- ,,,,.Predicting computerized physician order entry system adoption in US hospitals: can the federal mandate be met?Int J Med Inform.2008;77(8):539–545.
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Ann Intern Med.1991;115:591–595.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119(Suppl 1):132S–175S.
- ,,.A consensus statement on considerations for a successful CPOE implementation.J Am Med Inform Assoc.2003;10:229–234.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,,,.Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success.BMJ.2005;330:765.
- ,,, et al.Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review.JAMA.2005;293:1223–1238.
- Cochrane Reviews by the Effective Practice and Organisation of Care Group. Available at: http://www.cochrane.org/reviews/en/topics/61_reviews.html. Accessed 12 May2008.
- ,,.Embedded guideline information without patient specificity in a commercial emergency department computerized order‐entry system.Acad Emer Med.2006;13:452–458.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,,.A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior.JAMA.2000;283:2816–2821.
- ,,, et al.Computerized physician order entry and online decision support.Acad Emerg Med.2004;11:1135–1141.
- Society of Hospital Medicine VTE Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm. Accessed 12 May2008.
Copyright © 2009 Society of Hospital Medicine
Allogeneic Stem Cell Transplant
Dr. Sergio Giralt discusses his findings that suggest age should no longer be a contraindication to allogeneic stem cell transplantation. Dr. Armand Keating, press conference moderator, comments on the implications of this study during a briefing at the annual meeting of the American Society of Hematology.
Dr. Sergio Giralt discusses his findings that suggest age should no longer be a contraindication to allogeneic stem cell transplantation. Dr. Armand Keating, press conference moderator, comments on the implications of this study during a briefing at the annual meeting of the American Society of Hematology.
Dr. Sergio Giralt discusses his findings that suggest age should no longer be a contraindication to allogeneic stem cell transplantation. Dr. Armand Keating, press conference moderator, comments on the implications of this study during a briefing at the annual meeting of the American Society of Hematology.
Hospitalists Applaud SCHIP Expansion
Pediatric hospitalists are praising a new bill that expands the funding and scope of the State Children's Health Insurance Program (SCHIP), a program jointly funded by federal and state governments for children in families with incomes too high to qualify for Medicaid.
"Ideally, this will lead to better primary care, more immunizations, and disease prevention," says Jack Percelay, MD, MPH, a hospitalist at E.L.M.O. Pediatrics in New York City, Treasurer of SHM's board of directors, and a member of SHM's Public Policy Committee. Percelay foresees a twofold benefit of the new legislation: a likely decrease in uninsured pediatric patients using the ED and more fee recovery from patients who otherwise couldn't pay.
David Rappaport, MD, FAAP, a hospitalist at Alfred I. Dupont Hospital for Children in Wilmington, Del., agrees. "Children's health is more than the cuddly factor—it's a smart investment in healthcare," he says, explaining that paying for preventive measures in children, such as inoculations, can save on their healthcare costs in the future.
Signed by President Obama on Feb. 4, the bill reauthorizes SCHIP for four years and expands eligibility to children in families with incomes of up to three times the federal poverty level. It also covers legal immigrant pregnant women and children who have been in the country less than five years. The expansion will cover an additional 4 million children, raising the total to 11 million uninsured children enrolled in the program. Most of the $32.8 billion increase in federal funding for the program is to be covered by a 62-cent-per-pack increase in the federal cigarette tax.
Pediatric hospitalists are praising a new bill that expands the funding and scope of the State Children's Health Insurance Program (SCHIP), a program jointly funded by federal and state governments for children in families with incomes too high to qualify for Medicaid.
"Ideally, this will lead to better primary care, more immunizations, and disease prevention," says Jack Percelay, MD, MPH, a hospitalist at E.L.M.O. Pediatrics in New York City, Treasurer of SHM's board of directors, and a member of SHM's Public Policy Committee. Percelay foresees a twofold benefit of the new legislation: a likely decrease in uninsured pediatric patients using the ED and more fee recovery from patients who otherwise couldn't pay.
David Rappaport, MD, FAAP, a hospitalist at Alfred I. Dupont Hospital for Children in Wilmington, Del., agrees. "Children's health is more than the cuddly factor—it's a smart investment in healthcare," he says, explaining that paying for preventive measures in children, such as inoculations, can save on their healthcare costs in the future.
Signed by President Obama on Feb. 4, the bill reauthorizes SCHIP for four years and expands eligibility to children in families with incomes of up to three times the federal poverty level. It also covers legal immigrant pregnant women and children who have been in the country less than five years. The expansion will cover an additional 4 million children, raising the total to 11 million uninsured children enrolled in the program. Most of the $32.8 billion increase in federal funding for the program is to be covered by a 62-cent-per-pack increase in the federal cigarette tax.
Pediatric hospitalists are praising a new bill that expands the funding and scope of the State Children's Health Insurance Program (SCHIP), a program jointly funded by federal and state governments for children in families with incomes too high to qualify for Medicaid.
"Ideally, this will lead to better primary care, more immunizations, and disease prevention," says Jack Percelay, MD, MPH, a hospitalist at E.L.M.O. Pediatrics in New York City, Treasurer of SHM's board of directors, and a member of SHM's Public Policy Committee. Percelay foresees a twofold benefit of the new legislation: a likely decrease in uninsured pediatric patients using the ED and more fee recovery from patients who otherwise couldn't pay.
David Rappaport, MD, FAAP, a hospitalist at Alfred I. Dupont Hospital for Children in Wilmington, Del., agrees. "Children's health is more than the cuddly factor—it's a smart investment in healthcare," he says, explaining that paying for preventive measures in children, such as inoculations, can save on their healthcare costs in the future.
Signed by President Obama on Feb. 4, the bill reauthorizes SCHIP for four years and expands eligibility to children in families with incomes of up to three times the federal poverty level. It also covers legal immigrant pregnant women and children who have been in the country less than five years. The expansion will cover an additional 4 million children, raising the total to 11 million uninsured children enrolled in the program. Most of the $32.8 billion increase in federal funding for the program is to be covered by a 62-cent-per-pack increase in the federal cigarette tax.
Back to School
Patience Agborbesong, MD, didn't go to SHM's "Essential Procedures for the Hospitalist" seminar on a whim. In fact, the medical director of Wake Forest Inpatient Physicians of Winston-Salem, N.C., skipped her own hospital's career day to attend the society's One-Day Hospitalist University (ODHU) session where she received four hours of hands-on training in the use of ultrasound equipment for vascular access, paracentesis, and thoracentesis.
"When I was training, we didn't use ultrasounds to routinely do central lines," says Dr. Agborbesong, an ODHU rookie. "Now that is something that is recommended as a patient safety measure. When we do the procedure without ultrasounds, you're blind-sticking and going by the anatomic landmarks."
The course was one of four one-day seminars that drew nearly 200 hospitalists to Atlanta. CME credit is awarded for all of the ODHU courses. The program also included "Best Practices in Managing a Hospital Medicine Program," "Critical Care Medicine for the Hospitalist," and "Fundamentals of Inpatient Coding and Documentation."
Like many hospitalists, Dr. Agborbesong used ODHU as a chance to expand the skill set of her 15-hospitalist group. In addition to relaying what she learned during the ultrasound course to her colleagues, she's also planning to hone her skills with help from radiologists at Wake Forest University Baptist Medical Center.
"I didn't come here thinking I would be an expert," she says. "It was a very good place to start."
To stay updated on SHM-sponsored training programs, visit the SHM Web site.
Patience Agborbesong, MD, didn't go to SHM's "Essential Procedures for the Hospitalist" seminar on a whim. In fact, the medical director of Wake Forest Inpatient Physicians of Winston-Salem, N.C., skipped her own hospital's career day to attend the society's One-Day Hospitalist University (ODHU) session where she received four hours of hands-on training in the use of ultrasound equipment for vascular access, paracentesis, and thoracentesis.
"When I was training, we didn't use ultrasounds to routinely do central lines," says Dr. Agborbesong, an ODHU rookie. "Now that is something that is recommended as a patient safety measure. When we do the procedure without ultrasounds, you're blind-sticking and going by the anatomic landmarks."
The course was one of four one-day seminars that drew nearly 200 hospitalists to Atlanta. CME credit is awarded for all of the ODHU courses. The program also included "Best Practices in Managing a Hospital Medicine Program," "Critical Care Medicine for the Hospitalist," and "Fundamentals of Inpatient Coding and Documentation."
Like many hospitalists, Dr. Agborbesong used ODHU as a chance to expand the skill set of her 15-hospitalist group. In addition to relaying what she learned during the ultrasound course to her colleagues, she's also planning to hone her skills with help from radiologists at Wake Forest University Baptist Medical Center.
"I didn't come here thinking I would be an expert," she says. "It was a very good place to start."
To stay updated on SHM-sponsored training programs, visit the SHM Web site.
Patience Agborbesong, MD, didn't go to SHM's "Essential Procedures for the Hospitalist" seminar on a whim. In fact, the medical director of Wake Forest Inpatient Physicians of Winston-Salem, N.C., skipped her own hospital's career day to attend the society's One-Day Hospitalist University (ODHU) session where she received four hours of hands-on training in the use of ultrasound equipment for vascular access, paracentesis, and thoracentesis.
"When I was training, we didn't use ultrasounds to routinely do central lines," says Dr. Agborbesong, an ODHU rookie. "Now that is something that is recommended as a patient safety measure. When we do the procedure without ultrasounds, you're blind-sticking and going by the anatomic landmarks."
The course was one of four one-day seminars that drew nearly 200 hospitalists to Atlanta. CME credit is awarded for all of the ODHU courses. The program also included "Best Practices in Managing a Hospital Medicine Program," "Critical Care Medicine for the Hospitalist," and "Fundamentals of Inpatient Coding and Documentation."
Like many hospitalists, Dr. Agborbesong used ODHU as a chance to expand the skill set of her 15-hospitalist group. In addition to relaying what she learned during the ultrasound course to her colleagues, she's also planning to hone her skills with help from radiologists at Wake Forest University Baptist Medical Center.
"I didn't come here thinking I would be an expert," she says. "It was a very good place to start."
To stay updated on SHM-sponsored training programs, visit the SHM Web site.
Transparent Hospitalists
Six weeks ago hospitalist Frank Michota Jr., MD, posted on the Cleveland Clinic's Web site that he receives at least $5,000 per year in fees from pharmaceutical firms Sanofi-Aventis U.S. Inc. and Scios Inc. Since then, not one of the 100 or so patients he's encountered has asked about it.
Still, hospitalists and specialists at the teaching hospital now are required to publicly disclose their financial ties to the pharmaceutical and medical device industries. The effort is aimed at increasing physician transparency and avoiding conflicts of interest—real or perceived.
As director of academic affairs for the hospital's Department of Hospital Medicine, Dr. Michota appreciates the credibility that the disclosure provides for research. But he thinks it does little to forward patient care. Because most hospitalists are required to use drugs or devices based on formularies, whether they have financial ties to the companies making the drugs or devices is irrelevant, he says.
"It’s not the patient that's looking at this stuff," Dr. Michota says, adding, "the disclosures are more for the ethereal discussions."
He emphasizes most physicians don’t seek out relationships with drug- and device-makers.
"I use Drug A because it's on my formulary," he says. "I have a lot of experience with [Drug A] because it's on my formulary. I'm then asked by the company to research it, because I have experience with Drug A. That's how it works, not the other way around."
Check out the Cleveland Clinic’s physician directory at http://my.clevelandclinic.org/staff_directory/default.aspx.
Six weeks ago hospitalist Frank Michota Jr., MD, posted on the Cleveland Clinic's Web site that he receives at least $5,000 per year in fees from pharmaceutical firms Sanofi-Aventis U.S. Inc. and Scios Inc. Since then, not one of the 100 or so patients he's encountered has asked about it.
Still, hospitalists and specialists at the teaching hospital now are required to publicly disclose their financial ties to the pharmaceutical and medical device industries. The effort is aimed at increasing physician transparency and avoiding conflicts of interest—real or perceived.
As director of academic affairs for the hospital's Department of Hospital Medicine, Dr. Michota appreciates the credibility that the disclosure provides for research. But he thinks it does little to forward patient care. Because most hospitalists are required to use drugs or devices based on formularies, whether they have financial ties to the companies making the drugs or devices is irrelevant, he says.
"It’s not the patient that's looking at this stuff," Dr. Michota says, adding, "the disclosures are more for the ethereal discussions."
He emphasizes most physicians don’t seek out relationships with drug- and device-makers.
"I use Drug A because it's on my formulary," he says. "I have a lot of experience with [Drug A] because it's on my formulary. I'm then asked by the company to research it, because I have experience with Drug A. That's how it works, not the other way around."
Check out the Cleveland Clinic’s physician directory at http://my.clevelandclinic.org/staff_directory/default.aspx.
Six weeks ago hospitalist Frank Michota Jr., MD, posted on the Cleveland Clinic's Web site that he receives at least $5,000 per year in fees from pharmaceutical firms Sanofi-Aventis U.S. Inc. and Scios Inc. Since then, not one of the 100 or so patients he's encountered has asked about it.
Still, hospitalists and specialists at the teaching hospital now are required to publicly disclose their financial ties to the pharmaceutical and medical device industries. The effort is aimed at increasing physician transparency and avoiding conflicts of interest—real or perceived.
As director of academic affairs for the hospital's Department of Hospital Medicine, Dr. Michota appreciates the credibility that the disclosure provides for research. But he thinks it does little to forward patient care. Because most hospitalists are required to use drugs or devices based on formularies, whether they have financial ties to the companies making the drugs or devices is irrelevant, he says.
"It’s not the patient that's looking at this stuff," Dr. Michota says, adding, "the disclosures are more for the ethereal discussions."
He emphasizes most physicians don’t seek out relationships with drug- and device-makers.
"I use Drug A because it's on my formulary," he says. "I have a lot of experience with [Drug A] because it's on my formulary. I'm then asked by the company to research it, because I have experience with Drug A. That's how it works, not the other way around."
Check out the Cleveland Clinic’s physician directory at http://my.clevelandclinic.org/staff_directory/default.aspx.
Research Roundup
Clinical question: Is low molecular weight heparin (LMWH) safe for deep vein thrombosis (DVT) prophylaxis in patients with severe renal insufficiency?
Background: LMWH is avoided in patients with severe renal insufficiency due to concerns about increased bleeding risk caused by delayed renal excretion.
Study design: Multi center, prospective, single-arm trial.
Setting: Several Canadian intensive-care units (ICUs).
Synopsis: 138 critically ill patients with a creatinine clearance lower than 30 mL/min received open-labeled dalteparin, 5,000 IU once daily for DVT prophylaxis for a median duration of seven days. Peak and trough anti-Xa levels were measured to assess for efficacy and evidence of bioaccumulation, respectively. Patients were monitored for DVT development, heparin-induced thrombocytopenia (HIT), and bleeding. None of the patients (0%, CI, 0%-3%) had evidence of bioaccumulation. Seven patients (5.1%) developed DVT and only two patients (1.4%) developed HIT. Of the 10 patients who bled (7.2%), two died.
Of note, 62% of patients in this study had acute renal failure and their improvement in renal function during their ICU stay might have contributed to lack of bioaccumulation. Another limitation is the lack of comparison with other DVT prophylaxis strategies, especially unfractionated heparin.
Bottom line: Using dalteparin for DVT prophylaxis is unlikely to increase bleeding risk in patients with severe renal insufficiency. However, this finding cannot be generalized to dialysis-dependent patients or those with chronic kidney disease.
Citation: Arch Intern Med. 2008;168(16):1805-1812
—Reviewed by Rebecca Allyn, MD, Smitha Chadaga, MD, Mary Dedecker, MD, Vignesh Narayanan, MD, Eugene S. Chu, MD, Division of Hospital Medicine, Denver Health and Hospital Authority
Clinical question: Is low molecular weight heparin (LMWH) safe for deep vein thrombosis (DVT) prophylaxis in patients with severe renal insufficiency?
Background: LMWH is avoided in patients with severe renal insufficiency due to concerns about increased bleeding risk caused by delayed renal excretion.
Study design: Multi center, prospective, single-arm trial.
Setting: Several Canadian intensive-care units (ICUs).
Synopsis: 138 critically ill patients with a creatinine clearance lower than 30 mL/min received open-labeled dalteparin, 5,000 IU once daily for DVT prophylaxis for a median duration of seven days. Peak and trough anti-Xa levels were measured to assess for efficacy and evidence of bioaccumulation, respectively. Patients were monitored for DVT development, heparin-induced thrombocytopenia (HIT), and bleeding. None of the patients (0%, CI, 0%-3%) had evidence of bioaccumulation. Seven patients (5.1%) developed DVT and only two patients (1.4%) developed HIT. Of the 10 patients who bled (7.2%), two died.
Of note, 62% of patients in this study had acute renal failure and their improvement in renal function during their ICU stay might have contributed to lack of bioaccumulation. Another limitation is the lack of comparison with other DVT prophylaxis strategies, especially unfractionated heparin.
Bottom line: Using dalteparin for DVT prophylaxis is unlikely to increase bleeding risk in patients with severe renal insufficiency. However, this finding cannot be generalized to dialysis-dependent patients or those with chronic kidney disease.
Citation: Arch Intern Med. 2008;168(16):1805-1812
—Reviewed by Rebecca Allyn, MD, Smitha Chadaga, MD, Mary Dedecker, MD, Vignesh Narayanan, MD, Eugene S. Chu, MD, Division of Hospital Medicine, Denver Health and Hospital Authority
Clinical question: Is low molecular weight heparin (LMWH) safe for deep vein thrombosis (DVT) prophylaxis in patients with severe renal insufficiency?
Background: LMWH is avoided in patients with severe renal insufficiency due to concerns about increased bleeding risk caused by delayed renal excretion.
Study design: Multi center, prospective, single-arm trial.
Setting: Several Canadian intensive-care units (ICUs).
Synopsis: 138 critically ill patients with a creatinine clearance lower than 30 mL/min received open-labeled dalteparin, 5,000 IU once daily for DVT prophylaxis for a median duration of seven days. Peak and trough anti-Xa levels were measured to assess for efficacy and evidence of bioaccumulation, respectively. Patients were monitored for DVT development, heparin-induced thrombocytopenia (HIT), and bleeding. None of the patients (0%, CI, 0%-3%) had evidence of bioaccumulation. Seven patients (5.1%) developed DVT and only two patients (1.4%) developed HIT. Of the 10 patients who bled (7.2%), two died.
Of note, 62% of patients in this study had acute renal failure and their improvement in renal function during their ICU stay might have contributed to lack of bioaccumulation. Another limitation is the lack of comparison with other DVT prophylaxis strategies, especially unfractionated heparin.
Bottom line: Using dalteparin for DVT prophylaxis is unlikely to increase bleeding risk in patients with severe renal insufficiency. However, this finding cannot be generalized to dialysis-dependent patients or those with chronic kidney disease.
Citation: Arch Intern Med. 2008;168(16):1805-1812
—Reviewed by Rebecca Allyn, MD, Smitha Chadaga, MD, Mary Dedecker, MD, Vignesh Narayanan, MD, Eugene S. Chu, MD, Division of Hospital Medicine, Denver Health and Hospital Authority
Mismatched Cord Blood
Dr. Mary Eapen reports findings that mismatched umbilical cord blood is as effective as mismatched bone marrow or peripheral blood in allogeneic stem cell transplantation in adults with acute leukemia.
Dr. Mary Eapen reports findings that mismatched umbilical cord blood is as effective as mismatched bone marrow or peripheral blood in allogeneic stem cell transplantation in adults with acute leukemia.
Dr. Mary Eapen reports findings that mismatched umbilical cord blood is as effective as mismatched bone marrow or peripheral blood in allogeneic stem cell transplantation in adults with acute leukemia.
Prepare to react to a medical malpractice lawsuit
Prepare to react to a medical malpractice lawsuit
One of my colleagues is being sued for medical malpractice. After he was notified of the lawsuit, his lawyer immediately advised him of some things he should and should not do. Can you help me understand what those might be?
J. Boggs, Fort Smith, Ark.
Dr. Hospitalist responds: Lawsuits alleging medical malpractice are common—probably more common than most of us realize. Most physicians either know someone who has been sued or is currently being sued for medical malpractice. Unfortunately, malpractice is not something most medical schools or postgraduate training programs address sufficiently as part of a curriculum.
To be clear, I am not an attorney. I advise you to speak with an attorney familiar with medical malpractice statutes in your state regarding any legal action. But in my discussions with medical malpractice lawyers and with physicians who have been sued, I have come to understand the process can be an emotional and lengthy experience for everyone involved.
Attorneys often advise medical malpractice defendants of several things:
- Immediately contact your risk manager. If you do not know who your risk manager is or do not have one, contact your medical malpractice carrier. Your risk manager will open a file and notify your medical malpractice carrier. They will ask an attorney to contact you. This attorney will advise you of the most appropriate steps to take.
- It is normal to feel a wide range of emotions when you learn you are being sued. You might be very angry with the plaintiff (the party filing the suit), especially if you believe you provided appropriate care. At times like this, many physicians want to vent and speak with friends, family, colleagues, or even the plaintiff. That leads to a second piece of advice: Don’t speak with ANYONE except your attorney about the case. This might be difficult and seem counterintuitive, but you can harm your case if you talk to others about it. As the lawsuit evolves, both parties will undergo a discovery process to learn everyone’s perspectives. Defendants likely will be asked to provide answers at a deposition. Plaintiff’s counsel will ask defendants whether they spoke with anyone about the case. If they learn defendants spoke with others about the case, they may depose those individuals to learn what was discussed in those private conversations with the defendants. As you can imagine, it can get messy when private conversations are revealed in depositions.
- The last piece of advice attorneys often given new clients: Never alter the record. This seems crazy, right? What intelligent doctor would actually go back and alter the record? Well, silly as it might sound, many providers do go back and change something in the record. Most of the time, the change was something the physician thought was innocuous, such as adding a date to a note. By the time a physician has been sued, the medical record in question probably has been copied, likely more than once. It’s fairly simple to recognize when the original record has been altered. But regardless of how innocent any change may seem, the perception is the alteration was meant to deceive. Jurors normally do not view such instances favorably. Defendants can only hurt themselves when they alter the record.
So, if you are ever a defendant in a case, contact your risk manager immediately, don’t talk to anyone about the case, and stay away from the medical records office.
Take extra precautions to prevent C. difficile infections
I work at a hospital where the infection control officer advocates universal use of alcohol-based hand gels to prevent transmission of infectious pathogens. I previously had been told alcohol-based gels might be insufficient to kill C. difficile. Is this true?
C. Nelson, Atlanta
Dr. Hospitalist responds: You bring up an important question. The role of hand hygiene as a measure to control hospital-acquired infections has become increasingly visible. This is long overdue. The thought of healthcare providers transmitting diseases because they didn’t clean their hands is abhorrent.
Many institutions around the country have adopted policies similar to your hospital’s, encouraging the use of alcohol-based hand gels over the use of soap and water. Hospitals have done this for several reasons:
- Healthcare providers are more likely to use alcohol-based gels than cleanse with soap and water;
- Rubbing your hands with gel takes less time than washing with soap and water;
- Hospitals can place gel dispensers in convenient locations outside each doorway, whereas there are only so many faucets and sinks on any given floor; and
- Even those who do wash their hands with soap and water often do not spend enough time adequately cleaning them.
Alcohol-based gels are effective against a wide range of bacteria that cause hospital-acquired infections, particularly against Staph, including MRSA. But C. difficile may be different. The control of C. difficile in hospitals is difficult because the organism can produce highly resistant spores, which can survive for long periods of time in a hospital environment, such as in mattresses, equipment, furniture, etc. Alcohol-based gels might be less effective against C. difficile spores than other organisms that cause healthcare-associated infections. Providers caring for patients with C. difficile should wear protective clothing, such as gloves and gowns, as well as clean their hands with soap and water.
For additional information on this subject, I suggest you check out Morbidity and Mortality Weekly Report’s “Guideline for Hand Hygiene in Health-Care Settings” (Oct. 25, 2002). You can access it online at the Infectious Disease Society of America’s Web site at www.idsociety.org/ content.aspx?id=4434#hh. TH
Prepare to react to a medical malpractice lawsuit
One of my colleagues is being sued for medical malpractice. After he was notified of the lawsuit, his lawyer immediately advised him of some things he should and should not do. Can you help me understand what those might be?
J. Boggs, Fort Smith, Ark.
Dr. Hospitalist responds: Lawsuits alleging medical malpractice are common—probably more common than most of us realize. Most physicians either know someone who has been sued or is currently being sued for medical malpractice. Unfortunately, malpractice is not something most medical schools or postgraduate training programs address sufficiently as part of a curriculum.
To be clear, I am not an attorney. I advise you to speak with an attorney familiar with medical malpractice statutes in your state regarding any legal action. But in my discussions with medical malpractice lawyers and with physicians who have been sued, I have come to understand the process can be an emotional and lengthy experience for everyone involved.
Attorneys often advise medical malpractice defendants of several things:
- Immediately contact your risk manager. If you do not know who your risk manager is or do not have one, contact your medical malpractice carrier. Your risk manager will open a file and notify your medical malpractice carrier. They will ask an attorney to contact you. This attorney will advise you of the most appropriate steps to take.
- It is normal to feel a wide range of emotions when you learn you are being sued. You might be very angry with the plaintiff (the party filing the suit), especially if you believe you provided appropriate care. At times like this, many physicians want to vent and speak with friends, family, colleagues, or even the plaintiff. That leads to a second piece of advice: Don’t speak with ANYONE except your attorney about the case. This might be difficult and seem counterintuitive, but you can harm your case if you talk to others about it. As the lawsuit evolves, both parties will undergo a discovery process to learn everyone’s perspectives. Defendants likely will be asked to provide answers at a deposition. Plaintiff’s counsel will ask defendants whether they spoke with anyone about the case. If they learn defendants spoke with others about the case, they may depose those individuals to learn what was discussed in those private conversations with the defendants. As you can imagine, it can get messy when private conversations are revealed in depositions.
- The last piece of advice attorneys often given new clients: Never alter the record. This seems crazy, right? What intelligent doctor would actually go back and alter the record? Well, silly as it might sound, many providers do go back and change something in the record. Most of the time, the change was something the physician thought was innocuous, such as adding a date to a note. By the time a physician has been sued, the medical record in question probably has been copied, likely more than once. It’s fairly simple to recognize when the original record has been altered. But regardless of how innocent any change may seem, the perception is the alteration was meant to deceive. Jurors normally do not view such instances favorably. Defendants can only hurt themselves when they alter the record.
So, if you are ever a defendant in a case, contact your risk manager immediately, don’t talk to anyone about the case, and stay away from the medical records office.
Take extra precautions to prevent C. difficile infections
I work at a hospital where the infection control officer advocates universal use of alcohol-based hand gels to prevent transmission of infectious pathogens. I previously had been told alcohol-based gels might be insufficient to kill C. difficile. Is this true?
C. Nelson, Atlanta
Dr. Hospitalist responds: You bring up an important question. The role of hand hygiene as a measure to control hospital-acquired infections has become increasingly visible. This is long overdue. The thought of healthcare providers transmitting diseases because they didn’t clean their hands is abhorrent.
Many institutions around the country have adopted policies similar to your hospital’s, encouraging the use of alcohol-based hand gels over the use of soap and water. Hospitals have done this for several reasons:
- Healthcare providers are more likely to use alcohol-based gels than cleanse with soap and water;
- Rubbing your hands with gel takes less time than washing with soap and water;
- Hospitals can place gel dispensers in convenient locations outside each doorway, whereas there are only so many faucets and sinks on any given floor; and
- Even those who do wash their hands with soap and water often do not spend enough time adequately cleaning them.
Alcohol-based gels are effective against a wide range of bacteria that cause hospital-acquired infections, particularly against Staph, including MRSA. But C. difficile may be different. The control of C. difficile in hospitals is difficult because the organism can produce highly resistant spores, which can survive for long periods of time in a hospital environment, such as in mattresses, equipment, furniture, etc. Alcohol-based gels might be less effective against C. difficile spores than other organisms that cause healthcare-associated infections. Providers caring for patients with C. difficile should wear protective clothing, such as gloves and gowns, as well as clean their hands with soap and water.
For additional information on this subject, I suggest you check out Morbidity and Mortality Weekly Report’s “Guideline for Hand Hygiene in Health-Care Settings” (Oct. 25, 2002). You can access it online at the Infectious Disease Society of America’s Web site at www.idsociety.org/ content.aspx?id=4434#hh. TH
Prepare to react to a medical malpractice lawsuit
One of my colleagues is being sued for medical malpractice. After he was notified of the lawsuit, his lawyer immediately advised him of some things he should and should not do. Can you help me understand what those might be?
J. Boggs, Fort Smith, Ark.
Dr. Hospitalist responds: Lawsuits alleging medical malpractice are common—probably more common than most of us realize. Most physicians either know someone who has been sued or is currently being sued for medical malpractice. Unfortunately, malpractice is not something most medical schools or postgraduate training programs address sufficiently as part of a curriculum.
To be clear, I am not an attorney. I advise you to speak with an attorney familiar with medical malpractice statutes in your state regarding any legal action. But in my discussions with medical malpractice lawyers and with physicians who have been sued, I have come to understand the process can be an emotional and lengthy experience for everyone involved.
Attorneys often advise medical malpractice defendants of several things:
- Immediately contact your risk manager. If you do not know who your risk manager is or do not have one, contact your medical malpractice carrier. Your risk manager will open a file and notify your medical malpractice carrier. They will ask an attorney to contact you. This attorney will advise you of the most appropriate steps to take.
- It is normal to feel a wide range of emotions when you learn you are being sued. You might be very angry with the plaintiff (the party filing the suit), especially if you believe you provided appropriate care. At times like this, many physicians want to vent and speak with friends, family, colleagues, or even the plaintiff. That leads to a second piece of advice: Don’t speak with ANYONE except your attorney about the case. This might be difficult and seem counterintuitive, but you can harm your case if you talk to others about it. As the lawsuit evolves, both parties will undergo a discovery process to learn everyone’s perspectives. Defendants likely will be asked to provide answers at a deposition. Plaintiff’s counsel will ask defendants whether they spoke with anyone about the case. If they learn defendants spoke with others about the case, they may depose those individuals to learn what was discussed in those private conversations with the defendants. As you can imagine, it can get messy when private conversations are revealed in depositions.
- The last piece of advice attorneys often given new clients: Never alter the record. This seems crazy, right? What intelligent doctor would actually go back and alter the record? Well, silly as it might sound, many providers do go back and change something in the record. Most of the time, the change was something the physician thought was innocuous, such as adding a date to a note. By the time a physician has been sued, the medical record in question probably has been copied, likely more than once. It’s fairly simple to recognize when the original record has been altered. But regardless of how innocent any change may seem, the perception is the alteration was meant to deceive. Jurors normally do not view such instances favorably. Defendants can only hurt themselves when they alter the record.
So, if you are ever a defendant in a case, contact your risk manager immediately, don’t talk to anyone about the case, and stay away from the medical records office.
Take extra precautions to prevent C. difficile infections
I work at a hospital where the infection control officer advocates universal use of alcohol-based hand gels to prevent transmission of infectious pathogens. I previously had been told alcohol-based gels might be insufficient to kill C. difficile. Is this true?
C. Nelson, Atlanta
Dr. Hospitalist responds: You bring up an important question. The role of hand hygiene as a measure to control hospital-acquired infections has become increasingly visible. This is long overdue. The thought of healthcare providers transmitting diseases because they didn’t clean their hands is abhorrent.
Many institutions around the country have adopted policies similar to your hospital’s, encouraging the use of alcohol-based hand gels over the use of soap and water. Hospitals have done this for several reasons:
- Healthcare providers are more likely to use alcohol-based gels than cleanse with soap and water;
- Rubbing your hands with gel takes less time than washing with soap and water;
- Hospitals can place gel dispensers in convenient locations outside each doorway, whereas there are only so many faucets and sinks on any given floor; and
- Even those who do wash their hands with soap and water often do not spend enough time adequately cleaning them.
Alcohol-based gels are effective against a wide range of bacteria that cause hospital-acquired infections, particularly against Staph, including MRSA. But C. difficile may be different. The control of C. difficile in hospitals is difficult because the organism can produce highly resistant spores, which can survive for long periods of time in a hospital environment, such as in mattresses, equipment, furniture, etc. Alcohol-based gels might be less effective against C. difficile spores than other organisms that cause healthcare-associated infections. Providers caring for patients with C. difficile should wear protective clothing, such as gloves and gowns, as well as clean their hands with soap and water.
For additional information on this subject, I suggest you check out Morbidity and Mortality Weekly Report’s “Guideline for Hand Hygiene in Health-Care Settings” (Oct. 25, 2002). You can access it online at the Infectious Disease Society of America’s Web site at www.idsociety.org/ content.aspx?id=4434#hh. TH