User login
Flu vaccination rates: How can you do better?
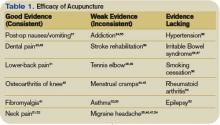
Each year, the flu causes an average of about 36,000 excess deaths and over 200,000 hospitalizations in the US.1,2 Much of this morbidity and mortality is preventable, yet each year, a large proportion of those for whom the vaccine is recommended go unvaccinated (TABLE 1).
TABLE 1
High-risk groups who went unvaccinated with influenza vaccine (2005)
| POPULATION GROUP | PROPORTION UNVACCINATED |
|---|---|
| Household contacts of those at high risk | 83%–91% |
| Pregnant women | 84% |
| Patients, ages 50–64 years | 77% |
| Patients, ages 6–23 months | 67% |
| Those with high-risk medical conditions | 66%–82% |
| Health care workers | 64% |
| Patients, ages ≥65 years | 40% |
Improving rates among health care workers
The recommendations of the Centers for Disease Control and Prevention (CDC) for the 2007–2008 influenza season include a new recommendation that targets health care worker vaccination rates.3 Because of the low rate of vaccination of health care workers, and the potential impact of higher coverage on both worker and patient safety, the CDC now recommends that the level of vaccination coverage be used as one measure of a facility’s patient safety quality program. The CDC also recommends the implementation of policies to encourage acceptance of the vaccine, such as requiring those caregivers who refuse immunization to sign waivers.
Improving rates among patients
To improve vaccination levels among patients, the CDC recommends:
- using reminder/recall systems
- using standing order programs
- administering the vaccine before and during the influenza season to patients during routine health care visits.
For more on improving vaccination coverage, see “Tips to help improve vaccination rates”.
Offer the vaccine to anyone who wants it
While the groups for whom vaccine is recommended are the same as last year (TABLE 2), this year the CDC is emphasizing the importance of:
- offering the vaccine to anyone who wants to reduce their risk of contracting influenza or transmitting the virus to others.
- continuing to offer vaccine to those susceptible throughout the flu season.
A minor change from last year’s recommendations involves children who are 6 months through 8 years of age who receive only 1 dose of vaccine their first year of vaccination. The CDC now recommends that these children receive 2 doses the next year. If they receive only 1 dose 2 years in a row, the CDC recommends only a single dose annually thereafter.
TABLE 2
Who should receive the influenza vaccine?
| Anyone who wants to reduce their risk of contracting the flu or transmitting the virus to others People at high risk for complications from the flu, including:
|
The 2 vaccines: How they differ
The same 2 vaccine types are available this year as last: trivalent influenza vaccine (TIV) and live attenuated influenza vaccine (LAIV). The vaccines include the same viral strain antigens and either can be used annually unless contraindicated (TABLE 3).
The major differences between the 2 vaccine types are:
- LAIV is administered as an intranasal spray while TIV requires an intramuscular injection
- LAIV is approved only for healthy people who are 5 to 49 years of age, whereas TIV is approved for anyone over the age of 6 months
- The interval between 2 doses in children under 9 years of age is 4 weeks for TIV and 6 to 10 weeks for LAIV
- LAIV should not be administered to family members or close contacts of those who are immunosuppressed and require a protective environment, while TIV can be used in this situation
- LAIV, being a live virus vaccine, should be administered simultaneously with, or 4 weeks after, the administration of other live virus vaccines. TIV is not a live virus vaccine, and its timing in relation to other live virus vaccines is not an issue.
TABLE 3
Contraindications and precautions for influenza vaccines
| TIV trivalent influenza vaccine |
| ||
| LAIV live attenuated influenza vaccine |
| ||
Antiviral options remain the same
Once again this year, the CDC does not recommend the use of adamantane antivirals for prophylaxis or treatment of influenza, leaving the 2 neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza), for these purposes. Treating flu patients with these antivirals shortens the duration of symptoms and may reduce viral shedding.
The earlier the treatment is started, the better the results. There appears to be no—or only minimal—benefit for those with uncomplicated influenza if the treatment is started more than 2 days after the onset of illness.
The Task Force on Community Preventive Services (an independent group, whose members are appointed by the director of the CDC) indicates that there is evidence to support the use of the following methods for improving vaccination rates:4
- Provider reminders, including notations, stickers, or other prompts in clients’ charts that notify staff when a client is due for certain vaccinations, including the influenza vaccine
- A recall system to notify patients when vaccines are due, using telephone messages or mailings. (E-mail messages are not mentioned but should also work)
- Standing orders for adults that allow staff to administer vaccines without the patient seeing the physician
- Assessing provider performance in delivering vaccinations and supplying this data to the provider
- Decreasing out-of-pocket costs for vaccinations.
Consider antiviral prophylaxis for these patients
The CDC recommends that antiviral prophylaxis be considered for those who are susceptible, residing in an area with circulating influenza virus, and who:
- have not been vaccinated or were recently vaccinated (since it takes 2 weeks for immunity to develop after vaccination)
- are unvaccinated and providing care for high-risk individuals
- have a contraindication to the vaccine
- have immune deficiencies and may not respond adequately to the vaccine.
The CDC also recommends prophylaxis for all residents and staff in a long-term care facility where influenza is circulating, without regard to vaccine status. More complete information on indications, dose and duration of antivirals for prophylaxis, and treatment can be found in this year’s CDC recommendations.3
Another flu season approaches
The good news for the coming year is that the government expects that the supply of vaccine will exceed 100 million doses. This should be sufficient, unless unforeseen production problems arise.
Each year millions of doses of influenza vaccine go unused and are discarded. By following the CDC’s recommendations, and those of the Task Force on Community Preventive Services4 (top left), each of us can improve vaccination coverage in our area and minimize the number of hospitalizations and deaths from the flu.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 e. van buren, Phoenix, AZ 85004; [email protected].
1. Thompson WW, Shay DK, Wintraub E, et al. Mortality associated with influenza and respiratory synctial virus in the United States. JAMA 2003;289:179-186.
2. Thompson WW, Shay DK, Wintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333-1340.
3. Centers for Disease Control and Prevention. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2007. MMWR Recomm Rep 2007;56(RR-6).-
4. Centers for Disease Control and Prevention. Vaccines. Guide to Community Preventive Services Web site. Available at: www.thecommunityguide.org/vaccine. Accessed on September 10, 2007.
Each year, the flu causes an average of about 36,000 excess deaths and over 200,000 hospitalizations in the US.1,2 Much of this morbidity and mortality is preventable, yet each year, a large proportion of those for whom the vaccine is recommended go unvaccinated (TABLE 1).
TABLE 1
High-risk groups who went unvaccinated with influenza vaccine (2005)
| POPULATION GROUP | PROPORTION UNVACCINATED |
|---|---|
| Household contacts of those at high risk | 83%–91% |
| Pregnant women | 84% |
| Patients, ages 50–64 years | 77% |
| Patients, ages 6–23 months | 67% |
| Those with high-risk medical conditions | 66%–82% |
| Health care workers | 64% |
| Patients, ages ≥65 years | 40% |
Improving rates among health care workers
The recommendations of the Centers for Disease Control and Prevention (CDC) for the 2007–2008 influenza season include a new recommendation that targets health care worker vaccination rates.3 Because of the low rate of vaccination of health care workers, and the potential impact of higher coverage on both worker and patient safety, the CDC now recommends that the level of vaccination coverage be used as one measure of a facility’s patient safety quality program. The CDC also recommends the implementation of policies to encourage acceptance of the vaccine, such as requiring those caregivers who refuse immunization to sign waivers.
Improving rates among patients
To improve vaccination levels among patients, the CDC recommends:
- using reminder/recall systems
- using standing order programs
- administering the vaccine before and during the influenza season to patients during routine health care visits.
For more on improving vaccination coverage, see “Tips to help improve vaccination rates”.
Offer the vaccine to anyone who wants it
While the groups for whom vaccine is recommended are the same as last year (TABLE 2), this year the CDC is emphasizing the importance of:
- offering the vaccine to anyone who wants to reduce their risk of contracting influenza or transmitting the virus to others.
- continuing to offer vaccine to those susceptible throughout the flu season.
A minor change from last year’s recommendations involves children who are 6 months through 8 years of age who receive only 1 dose of vaccine their first year of vaccination. The CDC now recommends that these children receive 2 doses the next year. If they receive only 1 dose 2 years in a row, the CDC recommends only a single dose annually thereafter.
TABLE 2
Who should receive the influenza vaccine?
| Anyone who wants to reduce their risk of contracting the flu or transmitting the virus to others People at high risk for complications from the flu, including:
|
The 2 vaccines: How they differ
The same 2 vaccine types are available this year as last: trivalent influenza vaccine (TIV) and live attenuated influenza vaccine (LAIV). The vaccines include the same viral strain antigens and either can be used annually unless contraindicated (TABLE 3).
The major differences between the 2 vaccine types are:
- LAIV is administered as an intranasal spray while TIV requires an intramuscular injection
- LAIV is approved only for healthy people who are 5 to 49 years of age, whereas TIV is approved for anyone over the age of 6 months
- The interval between 2 doses in children under 9 years of age is 4 weeks for TIV and 6 to 10 weeks for LAIV
- LAIV should not be administered to family members or close contacts of those who are immunosuppressed and require a protective environment, while TIV can be used in this situation
- LAIV, being a live virus vaccine, should be administered simultaneously with, or 4 weeks after, the administration of other live virus vaccines. TIV is not a live virus vaccine, and its timing in relation to other live virus vaccines is not an issue.
TABLE 3
Contraindications and precautions for influenza vaccines
| TIV trivalent influenza vaccine |
| ||
| LAIV live attenuated influenza vaccine |
| ||
Antiviral options remain the same
Once again this year, the CDC does not recommend the use of adamantane antivirals for prophylaxis or treatment of influenza, leaving the 2 neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza), for these purposes. Treating flu patients with these antivirals shortens the duration of symptoms and may reduce viral shedding.
The earlier the treatment is started, the better the results. There appears to be no—or only minimal—benefit for those with uncomplicated influenza if the treatment is started more than 2 days after the onset of illness.
The Task Force on Community Preventive Services (an independent group, whose members are appointed by the director of the CDC) indicates that there is evidence to support the use of the following methods for improving vaccination rates:4
- Provider reminders, including notations, stickers, or other prompts in clients’ charts that notify staff when a client is due for certain vaccinations, including the influenza vaccine
- A recall system to notify patients when vaccines are due, using telephone messages or mailings. (E-mail messages are not mentioned but should also work)
- Standing orders for adults that allow staff to administer vaccines without the patient seeing the physician
- Assessing provider performance in delivering vaccinations and supplying this data to the provider
- Decreasing out-of-pocket costs for vaccinations.
Consider antiviral prophylaxis for these patients
The CDC recommends that antiviral prophylaxis be considered for those who are susceptible, residing in an area with circulating influenza virus, and who:
- have not been vaccinated or were recently vaccinated (since it takes 2 weeks for immunity to develop after vaccination)
- are unvaccinated and providing care for high-risk individuals
- have a contraindication to the vaccine
- have immune deficiencies and may not respond adequately to the vaccine.
The CDC also recommends prophylaxis for all residents and staff in a long-term care facility where influenza is circulating, without regard to vaccine status. More complete information on indications, dose and duration of antivirals for prophylaxis, and treatment can be found in this year’s CDC recommendations.3
Another flu season approaches
The good news for the coming year is that the government expects that the supply of vaccine will exceed 100 million doses. This should be sufficient, unless unforeseen production problems arise.
Each year millions of doses of influenza vaccine go unused and are discarded. By following the CDC’s recommendations, and those of the Task Force on Community Preventive Services4 (top left), each of us can improve vaccination coverage in our area and minimize the number of hospitalizations and deaths from the flu.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 e. van buren, Phoenix, AZ 85004; [email protected].
Each year, the flu causes an average of about 36,000 excess deaths and over 200,000 hospitalizations in the US.1,2 Much of this morbidity and mortality is preventable, yet each year, a large proportion of those for whom the vaccine is recommended go unvaccinated (TABLE 1).
TABLE 1
High-risk groups who went unvaccinated with influenza vaccine (2005)
| POPULATION GROUP | PROPORTION UNVACCINATED |
|---|---|
| Household contacts of those at high risk | 83%–91% |
| Pregnant women | 84% |
| Patients, ages 50–64 years | 77% |
| Patients, ages 6–23 months | 67% |
| Those with high-risk medical conditions | 66%–82% |
| Health care workers | 64% |
| Patients, ages ≥65 years | 40% |
Improving rates among health care workers
The recommendations of the Centers for Disease Control and Prevention (CDC) for the 2007–2008 influenza season include a new recommendation that targets health care worker vaccination rates.3 Because of the low rate of vaccination of health care workers, and the potential impact of higher coverage on both worker and patient safety, the CDC now recommends that the level of vaccination coverage be used as one measure of a facility’s patient safety quality program. The CDC also recommends the implementation of policies to encourage acceptance of the vaccine, such as requiring those caregivers who refuse immunization to sign waivers.
Improving rates among patients
To improve vaccination levels among patients, the CDC recommends:
- using reminder/recall systems
- using standing order programs
- administering the vaccine before and during the influenza season to patients during routine health care visits.
For more on improving vaccination coverage, see “Tips to help improve vaccination rates”.
Offer the vaccine to anyone who wants it
While the groups for whom vaccine is recommended are the same as last year (TABLE 2), this year the CDC is emphasizing the importance of:
- offering the vaccine to anyone who wants to reduce their risk of contracting influenza or transmitting the virus to others.
- continuing to offer vaccine to those susceptible throughout the flu season.
A minor change from last year’s recommendations involves children who are 6 months through 8 years of age who receive only 1 dose of vaccine their first year of vaccination. The CDC now recommends that these children receive 2 doses the next year. If they receive only 1 dose 2 years in a row, the CDC recommends only a single dose annually thereafter.
TABLE 2
Who should receive the influenza vaccine?
| Anyone who wants to reduce their risk of contracting the flu or transmitting the virus to others People at high risk for complications from the flu, including:
|
The 2 vaccines: How they differ
The same 2 vaccine types are available this year as last: trivalent influenza vaccine (TIV) and live attenuated influenza vaccine (LAIV). The vaccines include the same viral strain antigens and either can be used annually unless contraindicated (TABLE 3).
The major differences between the 2 vaccine types are:
- LAIV is administered as an intranasal spray while TIV requires an intramuscular injection
- LAIV is approved only for healthy people who are 5 to 49 years of age, whereas TIV is approved for anyone over the age of 6 months
- The interval between 2 doses in children under 9 years of age is 4 weeks for TIV and 6 to 10 weeks for LAIV
- LAIV should not be administered to family members or close contacts of those who are immunosuppressed and require a protective environment, while TIV can be used in this situation
- LAIV, being a live virus vaccine, should be administered simultaneously with, or 4 weeks after, the administration of other live virus vaccines. TIV is not a live virus vaccine, and its timing in relation to other live virus vaccines is not an issue.
TABLE 3
Contraindications and precautions for influenza vaccines
| TIV trivalent influenza vaccine |
| ||
| LAIV live attenuated influenza vaccine |
| ||
Antiviral options remain the same
Once again this year, the CDC does not recommend the use of adamantane antivirals for prophylaxis or treatment of influenza, leaving the 2 neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza), for these purposes. Treating flu patients with these antivirals shortens the duration of symptoms and may reduce viral shedding.
The earlier the treatment is started, the better the results. There appears to be no—or only minimal—benefit for those with uncomplicated influenza if the treatment is started more than 2 days after the onset of illness.
The Task Force on Community Preventive Services (an independent group, whose members are appointed by the director of the CDC) indicates that there is evidence to support the use of the following methods for improving vaccination rates:4
- Provider reminders, including notations, stickers, or other prompts in clients’ charts that notify staff when a client is due for certain vaccinations, including the influenza vaccine
- A recall system to notify patients when vaccines are due, using telephone messages or mailings. (E-mail messages are not mentioned but should also work)
- Standing orders for adults that allow staff to administer vaccines without the patient seeing the physician
- Assessing provider performance in delivering vaccinations and supplying this data to the provider
- Decreasing out-of-pocket costs for vaccinations.
Consider antiviral prophylaxis for these patients
The CDC recommends that antiviral prophylaxis be considered for those who are susceptible, residing in an area with circulating influenza virus, and who:
- have not been vaccinated or were recently vaccinated (since it takes 2 weeks for immunity to develop after vaccination)
- are unvaccinated and providing care for high-risk individuals
- have a contraindication to the vaccine
- have immune deficiencies and may not respond adequately to the vaccine.
The CDC also recommends prophylaxis for all residents and staff in a long-term care facility where influenza is circulating, without regard to vaccine status. More complete information on indications, dose and duration of antivirals for prophylaxis, and treatment can be found in this year’s CDC recommendations.3
Another flu season approaches
The good news for the coming year is that the government expects that the supply of vaccine will exceed 100 million doses. This should be sufficient, unless unforeseen production problems arise.
Each year millions of doses of influenza vaccine go unused and are discarded. By following the CDC’s recommendations, and those of the Task Force on Community Preventive Services4 (top left), each of us can improve vaccination coverage in our area and minimize the number of hospitalizations and deaths from the flu.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 e. van buren, Phoenix, AZ 85004; [email protected].
1. Thompson WW, Shay DK, Wintraub E, et al. Mortality associated with influenza and respiratory synctial virus in the United States. JAMA 2003;289:179-186.
2. Thompson WW, Shay DK, Wintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333-1340.
3. Centers for Disease Control and Prevention. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2007. MMWR Recomm Rep 2007;56(RR-6).-
4. Centers for Disease Control and Prevention. Vaccines. Guide to Community Preventive Services Web site. Available at: www.thecommunityguide.org/vaccine. Accessed on September 10, 2007.
1. Thompson WW, Shay DK, Wintraub E, et al. Mortality associated with influenza and respiratory synctial virus in the United States. JAMA 2003;289:179-186.
2. Thompson WW, Shay DK, Wintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333-1340.
3. Centers for Disease Control and Prevention. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2007. MMWR Recomm Rep 2007;56(RR-6).-
4. Centers for Disease Control and Prevention. Vaccines. Guide to Community Preventive Services Web site. Available at: www.thecommunityguide.org/vaccine. Accessed on September 10, 2007.
Eltrombopag increases platelet levels in chronic ITP

GENEVA—A recent trial shows that eltrombopag can, with minimal toxicity, improve platelet levels and reduce bleeding in patients with chronic idiopathic thrombocytopenic purpura (ITP).
The phase 3, randomized, controlled trial evaluated the safety and efficacy of eltrombopag treatment in patients with chronic idiopathic thrombocytopenic purpura (ITP).
James B. Bussel, MD, from Weill Medical College of Cornell University in New York, presented the results at the recent meeting of the International Society on Thrombosis and Haemostasis.
A phase 1 study, reported in a 2007 issue of Blood, administered eltrombopag in active doses of 30, 50, and 75 mg. In normal volunteers, it takes a week for platelet counts to increase. Although increases were seen at all 3 dose levels, the optimal dose of 50 mg was chosen for subsequent studies.
In the phase 3 study, patients were randomized to receive placebo (n=38) or 50 mg of eltrombopag (n=76) once daily for 6 weeks (dose increase to 75 mg allowed). Investigators assessed bleeding according to the World Health Organization bleeding scale.
At the end of treatment (day 43), there was a significant increase in platelet levels in the eltrombopag arm vs the placebo arm. The median platelet count at the end of study was 18,000/μL in the placebo arm and 69,000/μL in the eltrombopag arm. The endpoint of >50,000 platelets/μL was reached in up to 42 days of dosing.
Eighteen out of 76 patients achieved platelet increases to >200,000/μL. The platelet counts fell 2 weeks after the end of therapy. There was a reduction in grade 2/4 bleeding of 18% in the placebo arm, and there were no specific findings of adverse events.
Platelet antibodies are still present as platelet levels fall after the cessation of therapy. Dr Bussel said this study confirmed previous findings with eltrombopag. Treatment not only increased platelet levels, but was able to reduce bleeding.
ITP results in reduced production of marrow platelets and platelet destruction by antibodies. Treatment of ITP is necessary to avoid major bleeding by increasing platelets to >50,000/μL. Eltrombopag is a small molecule, can be used orally, binds to a different region of receptor than thrombopoietin, and signals through the JAK/STAT pathway. ![]()

GENEVA—A recent trial shows that eltrombopag can, with minimal toxicity, improve platelet levels and reduce bleeding in patients with chronic idiopathic thrombocytopenic purpura (ITP).
The phase 3, randomized, controlled trial evaluated the safety and efficacy of eltrombopag treatment in patients with chronic idiopathic thrombocytopenic purpura (ITP).
James B. Bussel, MD, from Weill Medical College of Cornell University in New York, presented the results at the recent meeting of the International Society on Thrombosis and Haemostasis.
A phase 1 study, reported in a 2007 issue of Blood, administered eltrombopag in active doses of 30, 50, and 75 mg. In normal volunteers, it takes a week for platelet counts to increase. Although increases were seen at all 3 dose levels, the optimal dose of 50 mg was chosen for subsequent studies.
In the phase 3 study, patients were randomized to receive placebo (n=38) or 50 mg of eltrombopag (n=76) once daily for 6 weeks (dose increase to 75 mg allowed). Investigators assessed bleeding according to the World Health Organization bleeding scale.
At the end of treatment (day 43), there was a significant increase in platelet levels in the eltrombopag arm vs the placebo arm. The median platelet count at the end of study was 18,000/μL in the placebo arm and 69,000/μL in the eltrombopag arm. The endpoint of >50,000 platelets/μL was reached in up to 42 days of dosing.
Eighteen out of 76 patients achieved platelet increases to >200,000/μL. The platelet counts fell 2 weeks after the end of therapy. There was a reduction in grade 2/4 bleeding of 18% in the placebo arm, and there were no specific findings of adverse events.
Platelet antibodies are still present as platelet levels fall after the cessation of therapy. Dr Bussel said this study confirmed previous findings with eltrombopag. Treatment not only increased platelet levels, but was able to reduce bleeding.
ITP results in reduced production of marrow platelets and platelet destruction by antibodies. Treatment of ITP is necessary to avoid major bleeding by increasing platelets to >50,000/μL. Eltrombopag is a small molecule, can be used orally, binds to a different region of receptor than thrombopoietin, and signals through the JAK/STAT pathway. ![]()

GENEVA—A recent trial shows that eltrombopag can, with minimal toxicity, improve platelet levels and reduce bleeding in patients with chronic idiopathic thrombocytopenic purpura (ITP).
The phase 3, randomized, controlled trial evaluated the safety and efficacy of eltrombopag treatment in patients with chronic idiopathic thrombocytopenic purpura (ITP).
James B. Bussel, MD, from Weill Medical College of Cornell University in New York, presented the results at the recent meeting of the International Society on Thrombosis and Haemostasis.
A phase 1 study, reported in a 2007 issue of Blood, administered eltrombopag in active doses of 30, 50, and 75 mg. In normal volunteers, it takes a week for platelet counts to increase. Although increases were seen at all 3 dose levels, the optimal dose of 50 mg was chosen for subsequent studies.
In the phase 3 study, patients were randomized to receive placebo (n=38) or 50 mg of eltrombopag (n=76) once daily for 6 weeks (dose increase to 75 mg allowed). Investigators assessed bleeding according to the World Health Organization bleeding scale.
At the end of treatment (day 43), there was a significant increase in platelet levels in the eltrombopag arm vs the placebo arm. The median platelet count at the end of study was 18,000/μL in the placebo arm and 69,000/μL in the eltrombopag arm. The endpoint of >50,000 platelets/μL was reached in up to 42 days of dosing.
Eighteen out of 76 patients achieved platelet increases to >200,000/μL. The platelet counts fell 2 weeks after the end of therapy. There was a reduction in grade 2/4 bleeding of 18% in the placebo arm, and there were no specific findings of adverse events.
Platelet antibodies are still present as platelet levels fall after the cessation of therapy. Dr Bussel said this study confirmed previous findings with eltrombopag. Treatment not only increased platelet levels, but was able to reduce bleeding.
ITP results in reduced production of marrow platelets and platelet destruction by antibodies. Treatment of ITP is necessary to avoid major bleeding by increasing platelets to >50,000/μL. Eltrombopag is a small molecule, can be used orally, binds to a different region of receptor than thrombopoietin, and signals through the JAK/STAT pathway. ![]()
Curbside Consequences
Question: I’m a hospitalist in a small hospitalist group, so I stay pretty busy and sometimes I have to turn away a lot of requests for help. Recently I did a curbside consult on a surgical patient with diabetes. It was a hectic day, as usual, but I made time to do it because, to be honest, I’m very interested in these kinds of cases (for personal reasons). Problem is, another physician saw me give the consult and then asked me to do one for him about an hour later ... and I said no! I felt bad, but honestly, I had several patients to follow up with and felt I had to press on. I feel like I might have damaged my relationship with this doctor (a cardiologist). How can I help rebuild our relationship?
Boo-Boo in Beloit, Wis.
Dr. Hospitalist responds: Dear Boo Boo: Feel like you worked your way into a pile of doo doo? I generally go by the rule that if I feel something went wrong with an interaction, something probably did and damage control is in order.
Ignoring the encounter may lead the cardiologist to believe that you don’t value your relationship. I suggest you explain your predicament to the cardiologist and let him/her know that you are addressing problems with your schedule. To fix this “problem,” you need to examine why it occurred and understand how you can prevent it.
If you feel like you are “turn(ing) away a lot of requests for help,” I suspect you are missing a significant amount of business. You can view this as a problem or view this as an opportunity. Also recognize this is an opportunity for competitors. Referring physicians will send referrals elsewhere if you are not able to consistently provide the necessary service.
What’s not clear to me is whether your inability to accommodate requests for referrals is due to inadequate staffing, inefficiency, inappropriate billing, or a combination of factors.
To elucidate this situation, you need data. You will never know the facts until you gather the data.
If you are not measuring your program’s performance presently, it is never too late to start. Begin by tracking your work relative value units (wRVUs). This is a commonly used marker of productivity. SHM’s biannual productivity and compensation survey benchmarks this data. Are your wRVU data comparable with hospitalists with similar job descriptions? If so, consider adding staff to your program. If your wRVU data fall short of expectations, does the problem lie in billing? Are you billing for each encounter? Are you billing at appropriate levels for service provided? An audit of your notes and bills can be insightful. Or is inefficiency contributing to your problem? If so, consider an examination of your workflow. A workflow redesign with implementation of tools like templates may markedly improve your efficiency.
Career, Committee Work
Question: My career growth is very important to me. I’m presently the only hospitalist in our program, which I started about a year ago. I have many requests to serve on hospital committees. I’ve tried to serve on as many as possible but feel overstretched. Should I stop serving on committees and concentrate on my clinical work? Would this be bad for my career?
Too Busy in Ohio
Dr. Hospitalist responds: Dear Too Busy: Kudos for taking your job seriously, but don’t feel like you have to serve on all committees. Your concern about burnout is legitimate. There are many reasons hospitalists serve on hospital committees.
Seats at the table of some committees clearly have a higher potential impact than other committees. Participation in some committees can foster relationships with other hospital leaders and better position you and your hospitalist program. Others serve because committee participation may be tied to financial remuneration. I suggest you serve on committees where you believe you can be of most benefit to the hospital and for your program.
Ask yourself, “Does the committee work fit with the goals of the hospital or hospitalist program?” I always remind folks that they should not just think of committees as a way to get paid or better themselves. Some of the most meaningful work comes from serving on committees where members make difficult decisions and often sacrifice their individual goals for the betterment of patients and families. Consider limiting your participation to certain committees. Pick wisely, and you may find that you will cherish your participation.
An Unfortunate Encounter
Question: About a week ago, I was rounding with our CEO and a visiting doctor who is a friend of his. The CEO likes to tag along every so often to stay in touch with ‘‘the trenches.” At one point, they witnessed as I conducted a hand-off. I keep apprised of my peers’ discussions on how to do a proper hand-off, so I was following what I think is a pretty sound checklist of steps.
Unbelievably, my CEO’s doctor pal began ‘‘whispering’’ comments to the CEO about how he thought such and such I was doing wasn’t entirely necessary. (I could hear them clearly, as could the physician to whom I was handing off the patient, and the patient herself!)
I happen to know for a fact that the CEO has mused aloud and behind closed doors about the value of our hospital group. I’ve heard him, and friends have told me they have, too. I feel as though he’s undermining our efforts to adhere to a sound hand-off routine. How can I try to make him understand the value of a sound hand-off plan? Even if I can’t, how can I get my peers to buy in to better hand-off rules?
Angry in Helena, Mont.
Dr. Hospitalist responds: Dear Angry: it is laudable that your CEO makes hospital rounds. More CEOs and hospital administrators should do the same. Whether they are to “keep in touch” or for the appearance of doing so is probably less important. Inevitably they will see things on rounds that will help them understand the challenges doctors and nurses face every day in the hospital.
I share your concern that a colleague—but more importantly, a patient—heard your CEO’s friend make derogatory comments about your work. Although your CEO has made public comments previously about the value of your hospitalist program, he missed the boat on this one. He had a great chance to show you, your colleagues, and your patient how much he values the care you and your colleagues provide.
It may very well be that your CEO did not feel like his relationship with his friend could stand the conflict, but I am concerned that his lack of action may have created irreparable damage to his reputation as a leader. I have seen interactions like this breed gossip, which spreads like wildfire. Soon thereafter, discussions of the situation may bear little resemblance to what actually happened.
Make an appointment to meet with your CEO and describe to him what you saw, heard, and felt. Any smart CEO will understand that aside from an explanation, an apology is in order—if not to appease you, then to save his reputation as a leader. TH
Question: I’m a hospitalist in a small hospitalist group, so I stay pretty busy and sometimes I have to turn away a lot of requests for help. Recently I did a curbside consult on a surgical patient with diabetes. It was a hectic day, as usual, but I made time to do it because, to be honest, I’m very interested in these kinds of cases (for personal reasons). Problem is, another physician saw me give the consult and then asked me to do one for him about an hour later ... and I said no! I felt bad, but honestly, I had several patients to follow up with and felt I had to press on. I feel like I might have damaged my relationship with this doctor (a cardiologist). How can I help rebuild our relationship?
Boo-Boo in Beloit, Wis.
Dr. Hospitalist responds: Dear Boo Boo: Feel like you worked your way into a pile of doo doo? I generally go by the rule that if I feel something went wrong with an interaction, something probably did and damage control is in order.
Ignoring the encounter may lead the cardiologist to believe that you don’t value your relationship. I suggest you explain your predicament to the cardiologist and let him/her know that you are addressing problems with your schedule. To fix this “problem,” you need to examine why it occurred and understand how you can prevent it.
If you feel like you are “turn(ing) away a lot of requests for help,” I suspect you are missing a significant amount of business. You can view this as a problem or view this as an opportunity. Also recognize this is an opportunity for competitors. Referring physicians will send referrals elsewhere if you are not able to consistently provide the necessary service.
What’s not clear to me is whether your inability to accommodate requests for referrals is due to inadequate staffing, inefficiency, inappropriate billing, or a combination of factors.
To elucidate this situation, you need data. You will never know the facts until you gather the data.
If you are not measuring your program’s performance presently, it is never too late to start. Begin by tracking your work relative value units (wRVUs). This is a commonly used marker of productivity. SHM’s biannual productivity and compensation survey benchmarks this data. Are your wRVU data comparable with hospitalists with similar job descriptions? If so, consider adding staff to your program. If your wRVU data fall short of expectations, does the problem lie in billing? Are you billing for each encounter? Are you billing at appropriate levels for service provided? An audit of your notes and bills can be insightful. Or is inefficiency contributing to your problem? If so, consider an examination of your workflow. A workflow redesign with implementation of tools like templates may markedly improve your efficiency.
Career, Committee Work
Question: My career growth is very important to me. I’m presently the only hospitalist in our program, which I started about a year ago. I have many requests to serve on hospital committees. I’ve tried to serve on as many as possible but feel overstretched. Should I stop serving on committees and concentrate on my clinical work? Would this be bad for my career?
Too Busy in Ohio
Dr. Hospitalist responds: Dear Too Busy: Kudos for taking your job seriously, but don’t feel like you have to serve on all committees. Your concern about burnout is legitimate. There are many reasons hospitalists serve on hospital committees.
Seats at the table of some committees clearly have a higher potential impact than other committees. Participation in some committees can foster relationships with other hospital leaders and better position you and your hospitalist program. Others serve because committee participation may be tied to financial remuneration. I suggest you serve on committees where you believe you can be of most benefit to the hospital and for your program.
Ask yourself, “Does the committee work fit with the goals of the hospital or hospitalist program?” I always remind folks that they should not just think of committees as a way to get paid or better themselves. Some of the most meaningful work comes from serving on committees where members make difficult decisions and often sacrifice their individual goals for the betterment of patients and families. Consider limiting your participation to certain committees. Pick wisely, and you may find that you will cherish your participation.
An Unfortunate Encounter
Question: About a week ago, I was rounding with our CEO and a visiting doctor who is a friend of his. The CEO likes to tag along every so often to stay in touch with ‘‘the trenches.” At one point, they witnessed as I conducted a hand-off. I keep apprised of my peers’ discussions on how to do a proper hand-off, so I was following what I think is a pretty sound checklist of steps.
Unbelievably, my CEO’s doctor pal began ‘‘whispering’’ comments to the CEO about how he thought such and such I was doing wasn’t entirely necessary. (I could hear them clearly, as could the physician to whom I was handing off the patient, and the patient herself!)
I happen to know for a fact that the CEO has mused aloud and behind closed doors about the value of our hospital group. I’ve heard him, and friends have told me they have, too. I feel as though he’s undermining our efforts to adhere to a sound hand-off routine. How can I try to make him understand the value of a sound hand-off plan? Even if I can’t, how can I get my peers to buy in to better hand-off rules?
Angry in Helena, Mont.
Dr. Hospitalist responds: Dear Angry: it is laudable that your CEO makes hospital rounds. More CEOs and hospital administrators should do the same. Whether they are to “keep in touch” or for the appearance of doing so is probably less important. Inevitably they will see things on rounds that will help them understand the challenges doctors and nurses face every day in the hospital.
I share your concern that a colleague—but more importantly, a patient—heard your CEO’s friend make derogatory comments about your work. Although your CEO has made public comments previously about the value of your hospitalist program, he missed the boat on this one. He had a great chance to show you, your colleagues, and your patient how much he values the care you and your colleagues provide.
It may very well be that your CEO did not feel like his relationship with his friend could stand the conflict, but I am concerned that his lack of action may have created irreparable damage to his reputation as a leader. I have seen interactions like this breed gossip, which spreads like wildfire. Soon thereafter, discussions of the situation may bear little resemblance to what actually happened.
Make an appointment to meet with your CEO and describe to him what you saw, heard, and felt. Any smart CEO will understand that aside from an explanation, an apology is in order—if not to appease you, then to save his reputation as a leader. TH
Question: I’m a hospitalist in a small hospitalist group, so I stay pretty busy and sometimes I have to turn away a lot of requests for help. Recently I did a curbside consult on a surgical patient with diabetes. It was a hectic day, as usual, but I made time to do it because, to be honest, I’m very interested in these kinds of cases (for personal reasons). Problem is, another physician saw me give the consult and then asked me to do one for him about an hour later ... and I said no! I felt bad, but honestly, I had several patients to follow up with and felt I had to press on. I feel like I might have damaged my relationship with this doctor (a cardiologist). How can I help rebuild our relationship?
Boo-Boo in Beloit, Wis.
Dr. Hospitalist responds: Dear Boo Boo: Feel like you worked your way into a pile of doo doo? I generally go by the rule that if I feel something went wrong with an interaction, something probably did and damage control is in order.
Ignoring the encounter may lead the cardiologist to believe that you don’t value your relationship. I suggest you explain your predicament to the cardiologist and let him/her know that you are addressing problems with your schedule. To fix this “problem,” you need to examine why it occurred and understand how you can prevent it.
If you feel like you are “turn(ing) away a lot of requests for help,” I suspect you are missing a significant amount of business. You can view this as a problem or view this as an opportunity. Also recognize this is an opportunity for competitors. Referring physicians will send referrals elsewhere if you are not able to consistently provide the necessary service.
What’s not clear to me is whether your inability to accommodate requests for referrals is due to inadequate staffing, inefficiency, inappropriate billing, or a combination of factors.
To elucidate this situation, you need data. You will never know the facts until you gather the data.
If you are not measuring your program’s performance presently, it is never too late to start. Begin by tracking your work relative value units (wRVUs). This is a commonly used marker of productivity. SHM’s biannual productivity and compensation survey benchmarks this data. Are your wRVU data comparable with hospitalists with similar job descriptions? If so, consider adding staff to your program. If your wRVU data fall short of expectations, does the problem lie in billing? Are you billing for each encounter? Are you billing at appropriate levels for service provided? An audit of your notes and bills can be insightful. Or is inefficiency contributing to your problem? If so, consider an examination of your workflow. A workflow redesign with implementation of tools like templates may markedly improve your efficiency.
Career, Committee Work
Question: My career growth is very important to me. I’m presently the only hospitalist in our program, which I started about a year ago. I have many requests to serve on hospital committees. I’ve tried to serve on as many as possible but feel overstretched. Should I stop serving on committees and concentrate on my clinical work? Would this be bad for my career?
Too Busy in Ohio
Dr. Hospitalist responds: Dear Too Busy: Kudos for taking your job seriously, but don’t feel like you have to serve on all committees. Your concern about burnout is legitimate. There are many reasons hospitalists serve on hospital committees.
Seats at the table of some committees clearly have a higher potential impact than other committees. Participation in some committees can foster relationships with other hospital leaders and better position you and your hospitalist program. Others serve because committee participation may be tied to financial remuneration. I suggest you serve on committees where you believe you can be of most benefit to the hospital and for your program.
Ask yourself, “Does the committee work fit with the goals of the hospital or hospitalist program?” I always remind folks that they should not just think of committees as a way to get paid or better themselves. Some of the most meaningful work comes from serving on committees where members make difficult decisions and often sacrifice their individual goals for the betterment of patients and families. Consider limiting your participation to certain committees. Pick wisely, and you may find that you will cherish your participation.
An Unfortunate Encounter
Question: About a week ago, I was rounding with our CEO and a visiting doctor who is a friend of his. The CEO likes to tag along every so often to stay in touch with ‘‘the trenches.” At one point, they witnessed as I conducted a hand-off. I keep apprised of my peers’ discussions on how to do a proper hand-off, so I was following what I think is a pretty sound checklist of steps.
Unbelievably, my CEO’s doctor pal began ‘‘whispering’’ comments to the CEO about how he thought such and such I was doing wasn’t entirely necessary. (I could hear them clearly, as could the physician to whom I was handing off the patient, and the patient herself!)
I happen to know for a fact that the CEO has mused aloud and behind closed doors about the value of our hospital group. I’ve heard him, and friends have told me they have, too. I feel as though he’s undermining our efforts to adhere to a sound hand-off routine. How can I try to make him understand the value of a sound hand-off plan? Even if I can’t, how can I get my peers to buy in to better hand-off rules?
Angry in Helena, Mont.
Dr. Hospitalist responds: Dear Angry: it is laudable that your CEO makes hospital rounds. More CEOs and hospital administrators should do the same. Whether they are to “keep in touch” or for the appearance of doing so is probably less important. Inevitably they will see things on rounds that will help them understand the challenges doctors and nurses face every day in the hospital.
I share your concern that a colleague—but more importantly, a patient—heard your CEO’s friend make derogatory comments about your work. Although your CEO has made public comments previously about the value of your hospitalist program, he missed the boat on this one. He had a great chance to show you, your colleagues, and your patient how much he values the care you and your colleagues provide.
It may very well be that your CEO did not feel like his relationship with his friend could stand the conflict, but I am concerned that his lack of action may have created irreparable damage to his reputation as a leader. I have seen interactions like this breed gossip, which spreads like wildfire. Soon thereafter, discussions of the situation may bear little resemblance to what actually happened.
Make an appointment to meet with your CEO and describe to him what you saw, heard, and felt. Any smart CEO will understand that aside from an explanation, an apology is in order—if not to appease you, then to save his reputation as a leader. TH
A Unit-Based Approach
Would you want all your patients on the same nursing unit? Think about it—no more walking all over the building to see a few patients on each floor.
Because you would be physically present on “your” unit nearly all day, you could develop close working relationships with the nurses and other caregivers, which might improve everyone’s satisfaction with work. Everyone could better anticipate your work flow and communicate this to patients and families. You likely would be paged much less often because the nursing staff could keep track of whether you’re with a patient or off the floor to attend a conference; they could hold non-urgent issues until you get back.
All these things might make you and others more efficient—able to see the same number of patients you see today in less time, while maintaining or improving quality and cost effectiveness.
Sound familiar? The idea that working at only one site leads to efficiency and quality improvement is one of the underpinnings of the hospitalist concept. Instead of covering the outpatient office and hospital every day, doctors can focus on only the hospital or only the office. But what if you extended that idea to focusing your practice on only one unit within the hospital rather than the whole building? Would that be a good idea and lead to the benefits described above, or would that be taking the idea of “focused practice” too far?
Before answering, I should describe the approaches some practices have taken to pursue the benefits of concentrating patients in one part of the hospital. I’ll refer to this idea as “unit-based hospitalists,” the term current SHM President Rusty Holman uses when talking about this idea.
Locate most hospitalist patients in one unit. This is the most common form of unit-based hospitalists. Most institutions find the closest they can get to unit-based hospitalists is to have all hospitalist admissions go to the same floor when that floor has a bed available and the patient doesn’t require placement elsewhere. In such cases, the hospitalist practice might have something like 50% of patients on that floor and 50% dispersed throughout the hospital (telemetry, ICU, surgery floor). So the whole hospitalist practice has a primary “home” within the hospital, while each hospitalist spends part of each day caring for patients elsewhere. This is not very difficult for most hospitals to implement—and many are because most hospitalist patients end up on the “general medical” floor. This lets the hospitalist spend more time on that unit than any other. She can get to know the staff on that floor better, which might lead to many benefits, including improved satisfaction and efficiency.
Locate individual hospitalists on different hospital units. A more pure, but uncommon, form of unit-based hospitalists involves changing the way hospitalists are placed through the institution rather than changing patient placement. Each hospitalist in the group is assigned to a different nursing unit—or perhaps more than one unit—and sees whichever hospitalist patients are placed there. This system has the advantage of the hospitalist working all or most of the day on the same nursing unit, which can foster excellent teamwork. Instead of the nurse having to figure out which hospitalist to page for a particular patient, he simply needs to know, “Who is our hospitalist today?” and can contact that doctor for issues on most patients. Additionally, because the hospitalist will spend nearly the whole day physically on that unit, paging can be reduced significantly.
Despite its advantages, basing an individual hospitalist on a particular unit of the hospital is uncommon because in its purest form it can lead to terrible hospitalist-patient continuity. And, it’s hard to be confident that the disruptions in continuity are worth the benefits of the unit-based system. For example, the practice may have a patient to admit in the ED but can’t know which hospitalist should see the patient until a room is assigned. The fifth-floor hospitalist might go admit the patient in the ED, only to have the patient end up on the third floor, in which case the third-floor hospitalist would take over the next day. And each time the patient transferred to a new unit, either because of medical needs such as telemetry or simply because the hospital is full and needs to move patients, he would get a new hospitalist.
In addition to problems with continuity for patients who occupy more than one unit during their stay, this system would mean individual hospitalist workloads might get far out of balance. One floor might be very busy, while another is slow or limited by nurse staff shortages, and the respective hospitalists would have a correspondingly out-of-balance workload. A group could decide to address these problems by, for example, having the fifth-floor hospitalist see patients in other parts of the hospital in an effort to provide better hospitalist-patient continuity and address out-of-balance patient loads. But if this happens with any regularity it would mean the group has essentially moved back to a non-unit-based system.
In nearly all hospitals it would be unnecessary and unreasonable to assign a hospitalist to each nursing unit because some units tend to have few hospitalist patients. Yet when patients end up in those units because of medical necessity or bed space needs, one of the hospitalists will have to leave his/her unit to see this patient. If this happens often enough, it begins to dilute or negate the benefit of basing a hospitalist in one or two units.
Although one of the potential benefits of the unit-based model is enhanced relationships and integration among hospitalists and other unit-based clinical staff, it would be difficult to ensure that the same one or two hospitalists always work in a particular unit, and would limit scheduling flexibility dramatically. For example, if Dr. Starsky and Dr. Hutch are the unit-based hospitalists for Unit A, what happens if Dr. Starsky and Dr. Hutch are both scheduled to be off for the same block of days? What happens if both are scheduled to work the same block of days? To obtain the benefits of enhanced relationships and better unit integration, the practice would need to ensure that this scheduling overlap rarely happens—and that’s hard to do.
Where is the sweet spot in grouping patients and hospitalists by nursing unit? There is a wide range of opinion about whether unit-based hospital medicine in any form is worth pursuing. Some hospitalists are convinced that grouping all of their patients on the same unit could decrease efficiency because the doctor is nearly always working within view of patients and families and may be regularly interrupted. I am convinced that assigning each hospitalist to a particular unit in the hospital yields the greatest benefits. But I also think most institutions will find that the complexity and costs of this system are simply too high to justify. In that case, the next best approach might be to locate most hospitalist patients on the same unit unless that unit is full or the patient must be placed elsewhere. There is a good chance this is happening in your hospital—even if it isn’t written in the policy manual. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management-consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
Would you want all your patients on the same nursing unit? Think about it—no more walking all over the building to see a few patients on each floor.
Because you would be physically present on “your” unit nearly all day, you could develop close working relationships with the nurses and other caregivers, which might improve everyone’s satisfaction with work. Everyone could better anticipate your work flow and communicate this to patients and families. You likely would be paged much less often because the nursing staff could keep track of whether you’re with a patient or off the floor to attend a conference; they could hold non-urgent issues until you get back.
All these things might make you and others more efficient—able to see the same number of patients you see today in less time, while maintaining or improving quality and cost effectiveness.
Sound familiar? The idea that working at only one site leads to efficiency and quality improvement is one of the underpinnings of the hospitalist concept. Instead of covering the outpatient office and hospital every day, doctors can focus on only the hospital or only the office. But what if you extended that idea to focusing your practice on only one unit within the hospital rather than the whole building? Would that be a good idea and lead to the benefits described above, or would that be taking the idea of “focused practice” too far?
Before answering, I should describe the approaches some practices have taken to pursue the benefits of concentrating patients in one part of the hospital. I’ll refer to this idea as “unit-based hospitalists,” the term current SHM President Rusty Holman uses when talking about this idea.
Locate most hospitalist patients in one unit. This is the most common form of unit-based hospitalists. Most institutions find the closest they can get to unit-based hospitalists is to have all hospitalist admissions go to the same floor when that floor has a bed available and the patient doesn’t require placement elsewhere. In such cases, the hospitalist practice might have something like 50% of patients on that floor and 50% dispersed throughout the hospital (telemetry, ICU, surgery floor). So the whole hospitalist practice has a primary “home” within the hospital, while each hospitalist spends part of each day caring for patients elsewhere. This is not very difficult for most hospitals to implement—and many are because most hospitalist patients end up on the “general medical” floor. This lets the hospitalist spend more time on that unit than any other. She can get to know the staff on that floor better, which might lead to many benefits, including improved satisfaction and efficiency.
Locate individual hospitalists on different hospital units. A more pure, but uncommon, form of unit-based hospitalists involves changing the way hospitalists are placed through the institution rather than changing patient placement. Each hospitalist in the group is assigned to a different nursing unit—or perhaps more than one unit—and sees whichever hospitalist patients are placed there. This system has the advantage of the hospitalist working all or most of the day on the same nursing unit, which can foster excellent teamwork. Instead of the nurse having to figure out which hospitalist to page for a particular patient, he simply needs to know, “Who is our hospitalist today?” and can contact that doctor for issues on most patients. Additionally, because the hospitalist will spend nearly the whole day physically on that unit, paging can be reduced significantly.
Despite its advantages, basing an individual hospitalist on a particular unit of the hospital is uncommon because in its purest form it can lead to terrible hospitalist-patient continuity. And, it’s hard to be confident that the disruptions in continuity are worth the benefits of the unit-based system. For example, the practice may have a patient to admit in the ED but can’t know which hospitalist should see the patient until a room is assigned. The fifth-floor hospitalist might go admit the patient in the ED, only to have the patient end up on the third floor, in which case the third-floor hospitalist would take over the next day. And each time the patient transferred to a new unit, either because of medical needs such as telemetry or simply because the hospital is full and needs to move patients, he would get a new hospitalist.
In addition to problems with continuity for patients who occupy more than one unit during their stay, this system would mean individual hospitalist workloads might get far out of balance. One floor might be very busy, while another is slow or limited by nurse staff shortages, and the respective hospitalists would have a correspondingly out-of-balance workload. A group could decide to address these problems by, for example, having the fifth-floor hospitalist see patients in other parts of the hospital in an effort to provide better hospitalist-patient continuity and address out-of-balance patient loads. But if this happens with any regularity it would mean the group has essentially moved back to a non-unit-based system.
In nearly all hospitals it would be unnecessary and unreasonable to assign a hospitalist to each nursing unit because some units tend to have few hospitalist patients. Yet when patients end up in those units because of medical necessity or bed space needs, one of the hospitalists will have to leave his/her unit to see this patient. If this happens often enough, it begins to dilute or negate the benefit of basing a hospitalist in one or two units.
Although one of the potential benefits of the unit-based model is enhanced relationships and integration among hospitalists and other unit-based clinical staff, it would be difficult to ensure that the same one or two hospitalists always work in a particular unit, and would limit scheduling flexibility dramatically. For example, if Dr. Starsky and Dr. Hutch are the unit-based hospitalists for Unit A, what happens if Dr. Starsky and Dr. Hutch are both scheduled to be off for the same block of days? What happens if both are scheduled to work the same block of days? To obtain the benefits of enhanced relationships and better unit integration, the practice would need to ensure that this scheduling overlap rarely happens—and that’s hard to do.
Where is the sweet spot in grouping patients and hospitalists by nursing unit? There is a wide range of opinion about whether unit-based hospital medicine in any form is worth pursuing. Some hospitalists are convinced that grouping all of their patients on the same unit could decrease efficiency because the doctor is nearly always working within view of patients and families and may be regularly interrupted. I am convinced that assigning each hospitalist to a particular unit in the hospital yields the greatest benefits. But I also think most institutions will find that the complexity and costs of this system are simply too high to justify. In that case, the next best approach might be to locate most hospitalist patients on the same unit unless that unit is full or the patient must be placed elsewhere. There is a good chance this is happening in your hospital—even if it isn’t written in the policy manual. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management-consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
Would you want all your patients on the same nursing unit? Think about it—no more walking all over the building to see a few patients on each floor.
Because you would be physically present on “your” unit nearly all day, you could develop close working relationships with the nurses and other caregivers, which might improve everyone’s satisfaction with work. Everyone could better anticipate your work flow and communicate this to patients and families. You likely would be paged much less often because the nursing staff could keep track of whether you’re with a patient or off the floor to attend a conference; they could hold non-urgent issues until you get back.
All these things might make you and others more efficient—able to see the same number of patients you see today in less time, while maintaining or improving quality and cost effectiveness.
Sound familiar? The idea that working at only one site leads to efficiency and quality improvement is one of the underpinnings of the hospitalist concept. Instead of covering the outpatient office and hospital every day, doctors can focus on only the hospital or only the office. But what if you extended that idea to focusing your practice on only one unit within the hospital rather than the whole building? Would that be a good idea and lead to the benefits described above, or would that be taking the idea of “focused practice” too far?
Before answering, I should describe the approaches some practices have taken to pursue the benefits of concentrating patients in one part of the hospital. I’ll refer to this idea as “unit-based hospitalists,” the term current SHM President Rusty Holman uses when talking about this idea.
Locate most hospitalist patients in one unit. This is the most common form of unit-based hospitalists. Most institutions find the closest they can get to unit-based hospitalists is to have all hospitalist admissions go to the same floor when that floor has a bed available and the patient doesn’t require placement elsewhere. In such cases, the hospitalist practice might have something like 50% of patients on that floor and 50% dispersed throughout the hospital (telemetry, ICU, surgery floor). So the whole hospitalist practice has a primary “home” within the hospital, while each hospitalist spends part of each day caring for patients elsewhere. This is not very difficult for most hospitals to implement—and many are because most hospitalist patients end up on the “general medical” floor. This lets the hospitalist spend more time on that unit than any other. She can get to know the staff on that floor better, which might lead to many benefits, including improved satisfaction and efficiency.
Locate individual hospitalists on different hospital units. A more pure, but uncommon, form of unit-based hospitalists involves changing the way hospitalists are placed through the institution rather than changing patient placement. Each hospitalist in the group is assigned to a different nursing unit—or perhaps more than one unit—and sees whichever hospitalist patients are placed there. This system has the advantage of the hospitalist working all or most of the day on the same nursing unit, which can foster excellent teamwork. Instead of the nurse having to figure out which hospitalist to page for a particular patient, he simply needs to know, “Who is our hospitalist today?” and can contact that doctor for issues on most patients. Additionally, because the hospitalist will spend nearly the whole day physically on that unit, paging can be reduced significantly.
Despite its advantages, basing an individual hospitalist on a particular unit of the hospital is uncommon because in its purest form it can lead to terrible hospitalist-patient continuity. And, it’s hard to be confident that the disruptions in continuity are worth the benefits of the unit-based system. For example, the practice may have a patient to admit in the ED but can’t know which hospitalist should see the patient until a room is assigned. The fifth-floor hospitalist might go admit the patient in the ED, only to have the patient end up on the third floor, in which case the third-floor hospitalist would take over the next day. And each time the patient transferred to a new unit, either because of medical needs such as telemetry or simply because the hospital is full and needs to move patients, he would get a new hospitalist.
In addition to problems with continuity for patients who occupy more than one unit during their stay, this system would mean individual hospitalist workloads might get far out of balance. One floor might be very busy, while another is slow or limited by nurse staff shortages, and the respective hospitalists would have a correspondingly out-of-balance workload. A group could decide to address these problems by, for example, having the fifth-floor hospitalist see patients in other parts of the hospital in an effort to provide better hospitalist-patient continuity and address out-of-balance patient loads. But if this happens with any regularity it would mean the group has essentially moved back to a non-unit-based system.
In nearly all hospitals it would be unnecessary and unreasonable to assign a hospitalist to each nursing unit because some units tend to have few hospitalist patients. Yet when patients end up in those units because of medical necessity or bed space needs, one of the hospitalists will have to leave his/her unit to see this patient. If this happens often enough, it begins to dilute or negate the benefit of basing a hospitalist in one or two units.
Although one of the potential benefits of the unit-based model is enhanced relationships and integration among hospitalists and other unit-based clinical staff, it would be difficult to ensure that the same one or two hospitalists always work in a particular unit, and would limit scheduling flexibility dramatically. For example, if Dr. Starsky and Dr. Hutch are the unit-based hospitalists for Unit A, what happens if Dr. Starsky and Dr. Hutch are both scheduled to be off for the same block of days? What happens if both are scheduled to work the same block of days? To obtain the benefits of enhanced relationships and better unit integration, the practice would need to ensure that this scheduling overlap rarely happens—and that’s hard to do.
Where is the sweet spot in grouping patients and hospitalists by nursing unit? There is a wide range of opinion about whether unit-based hospital medicine in any form is worth pursuing. Some hospitalists are convinced that grouping all of their patients on the same unit could decrease efficiency because the doctor is nearly always working within view of patients and families and may be regularly interrupted. I am convinced that assigning each hospitalist to a particular unit in the hospital yields the greatest benefits. But I also think most institutions will find that the complexity and costs of this system are simply too high to justify. In that case, the next best approach might be to locate most hospitalist patients on the same unit unless that unit is full or the patient must be placed elsewhere. There is a good chance this is happening in your hospital—even if it isn’t written in the policy manual. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management-consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
Transitions of Care
As I embark on my tenure as physician editor of The Hospitalist, I am struck by the similarities between the editor transition and the transitions of care that happen as part of the daily backdrop of our hospitalist lives.
Both scenarios depend on open communication, a multidisciplinary team approach, a well-considered plan, and constituent feedback.
Similar to the communication between providers in patient handoffs, the previous editor, Jamie Newman, MD, and I talked about the history, course, and plan for the publication. Unlike most of my hospital handoffs, this patient is in great condition.
Jamie took over 24 months ago during a major transition for the publication and masterfully shepherded it to the place of prominence it holds today. For this he deserves a ton of credit. The content is top-notch, the reporting timely and noteworthy, and the design compelling.
As a consequence the readership is strong; so strong, it has played an influential role in our transitional communication. Several months ago we asked you to submit feedback about the publication in the form of a reader survey. From that data it was clear The Hospitalist was headed in the right direction but could use a slight “rehab” consult to make it even stronger. You provided several inputs instrumental to enhancing the publication. In short, you clearly desired more clinical content, an easier-to-use “In the Literature” section, and more concise material. These ideas formed the cornerstone of the upcoming changes in the publication.
More Clinical Content
In the near future we will begin to run more articles about the topics that induce the most angina in hospitalists. In general, we will de-emphasize comprehensive topic reviews (e.g., “Congestive heart failure from A to Z: genomics, pathogenesis, presentation, diagnostics, therapeutics and beyond”).
In its place we will introduce shorter articles centered on controversial questions in hospital medicine, the type and scope of questions that by their very nature are common, contentious, and stress-inducing (e.g.,
When should nesiritide therapy be initiated in acute decompensated CHF?).
New “In the Lit”
While this is one of the most well-read sections, many noted it can be difficult to navigate and sometimes seems bloated. To remedy this, we will increase the number of articles reviewed while decreasing the amount of detail per article. What we lose in depth we hope to gain in breadth.
We feel this will provide a general overview of all the pertinent literature so you can be confident you are up to date on take-home points of the most current studies. The department will also be reformatted so it is much easier to find the most crucial information. Look for these changes in the next month or two.
New Departments
The “Legal Eagle” and “Billing and Coding” departments will provide important information on medical malpractice and reimbursement documentation, while the “Hospital Pharmacy” department will offer up-to-the minute highlights of advances in therapeutics.
Finally, an advice column will give you the opportunity to ask experts your questions about the practice of hospital medicine.
Of course, much of The Hospitalist will remain unchanged. We will preserve your favorite features such John Nelson’s “Practice Management” column, Larry Wellikson’s “SHM Point of View” column, and the “Society Pages” and “Public Policy” departments. All this will happen against the backdrop of timely, in-depth reporting that keeps you abreast of the world of hospital medicine.
In all this transitioning it is important to recognize the team effort this publication requires. Indeed, the success of this publication is multidisciplinary and includes the expertise of the many folks at Wiley, notably Lisa Dionne (editorial director), Geoff Giordano (editor), and our colleagues at SHM—Larry Wellikson (CEO of SHM) and Todd Von Deak (director of membership and marketing) as well as the entire editorial and publishing staff at Wiley.
Most importantly, the success of this transition—and indeed the publication itself—depends on feedback from you, the reader. My e-mail box is always open to suggestions on how to improve the publication, including feedback on what we’re doing right and what we need to change. I’m also interested in hearing your ideas about clinical content areas that need to be covered. Just shoot me an e-mail saying, “I’d like to learn more about … .” And, we always welcome offers to contribute content to the publication.
To all of you: Thanks for helping make this transition such a successful one. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver and Health Sciences Center, where he serves as director of the Hospital Medicine Program, Inpatient Clinical Services in the Department of Medicine, and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
As I embark on my tenure as physician editor of The Hospitalist, I am struck by the similarities between the editor transition and the transitions of care that happen as part of the daily backdrop of our hospitalist lives.
Both scenarios depend on open communication, a multidisciplinary team approach, a well-considered plan, and constituent feedback.
Similar to the communication between providers in patient handoffs, the previous editor, Jamie Newman, MD, and I talked about the history, course, and plan for the publication. Unlike most of my hospital handoffs, this patient is in great condition.
Jamie took over 24 months ago during a major transition for the publication and masterfully shepherded it to the place of prominence it holds today. For this he deserves a ton of credit. The content is top-notch, the reporting timely and noteworthy, and the design compelling.
As a consequence the readership is strong; so strong, it has played an influential role in our transitional communication. Several months ago we asked you to submit feedback about the publication in the form of a reader survey. From that data it was clear The Hospitalist was headed in the right direction but could use a slight “rehab” consult to make it even stronger. You provided several inputs instrumental to enhancing the publication. In short, you clearly desired more clinical content, an easier-to-use “In the Literature” section, and more concise material. These ideas formed the cornerstone of the upcoming changes in the publication.
More Clinical Content
In the near future we will begin to run more articles about the topics that induce the most angina in hospitalists. In general, we will de-emphasize comprehensive topic reviews (e.g., “Congestive heart failure from A to Z: genomics, pathogenesis, presentation, diagnostics, therapeutics and beyond”).
In its place we will introduce shorter articles centered on controversial questions in hospital medicine, the type and scope of questions that by their very nature are common, contentious, and stress-inducing (e.g.,
When should nesiritide therapy be initiated in acute decompensated CHF?).
New “In the Lit”
While this is one of the most well-read sections, many noted it can be difficult to navigate and sometimes seems bloated. To remedy this, we will increase the number of articles reviewed while decreasing the amount of detail per article. What we lose in depth we hope to gain in breadth.
We feel this will provide a general overview of all the pertinent literature so you can be confident you are up to date on take-home points of the most current studies. The department will also be reformatted so it is much easier to find the most crucial information. Look for these changes in the next month or two.
New Departments
The “Legal Eagle” and “Billing and Coding” departments will provide important information on medical malpractice and reimbursement documentation, while the “Hospital Pharmacy” department will offer up-to-the minute highlights of advances in therapeutics.
Finally, an advice column will give you the opportunity to ask experts your questions about the practice of hospital medicine.
Of course, much of The Hospitalist will remain unchanged. We will preserve your favorite features such John Nelson’s “Practice Management” column, Larry Wellikson’s “SHM Point of View” column, and the “Society Pages” and “Public Policy” departments. All this will happen against the backdrop of timely, in-depth reporting that keeps you abreast of the world of hospital medicine.
In all this transitioning it is important to recognize the team effort this publication requires. Indeed, the success of this publication is multidisciplinary and includes the expertise of the many folks at Wiley, notably Lisa Dionne (editorial director), Geoff Giordano (editor), and our colleagues at SHM—Larry Wellikson (CEO of SHM) and Todd Von Deak (director of membership and marketing) as well as the entire editorial and publishing staff at Wiley.
Most importantly, the success of this transition—and indeed the publication itself—depends on feedback from you, the reader. My e-mail box is always open to suggestions on how to improve the publication, including feedback on what we’re doing right and what we need to change. I’m also interested in hearing your ideas about clinical content areas that need to be covered. Just shoot me an e-mail saying, “I’d like to learn more about … .” And, we always welcome offers to contribute content to the publication.
To all of you: Thanks for helping make this transition such a successful one. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver and Health Sciences Center, where he serves as director of the Hospital Medicine Program, Inpatient Clinical Services in the Department of Medicine, and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
As I embark on my tenure as physician editor of The Hospitalist, I am struck by the similarities between the editor transition and the transitions of care that happen as part of the daily backdrop of our hospitalist lives.
Both scenarios depend on open communication, a multidisciplinary team approach, a well-considered plan, and constituent feedback.
Similar to the communication between providers in patient handoffs, the previous editor, Jamie Newman, MD, and I talked about the history, course, and plan for the publication. Unlike most of my hospital handoffs, this patient is in great condition.
Jamie took over 24 months ago during a major transition for the publication and masterfully shepherded it to the place of prominence it holds today. For this he deserves a ton of credit. The content is top-notch, the reporting timely and noteworthy, and the design compelling.
As a consequence the readership is strong; so strong, it has played an influential role in our transitional communication. Several months ago we asked you to submit feedback about the publication in the form of a reader survey. From that data it was clear The Hospitalist was headed in the right direction but could use a slight “rehab” consult to make it even stronger. You provided several inputs instrumental to enhancing the publication. In short, you clearly desired more clinical content, an easier-to-use “In the Literature” section, and more concise material. These ideas formed the cornerstone of the upcoming changes in the publication.
More Clinical Content
In the near future we will begin to run more articles about the topics that induce the most angina in hospitalists. In general, we will de-emphasize comprehensive topic reviews (e.g., “Congestive heart failure from A to Z: genomics, pathogenesis, presentation, diagnostics, therapeutics and beyond”).
In its place we will introduce shorter articles centered on controversial questions in hospital medicine, the type and scope of questions that by their very nature are common, contentious, and stress-inducing (e.g.,
When should nesiritide therapy be initiated in acute decompensated CHF?).
New “In the Lit”
While this is one of the most well-read sections, many noted it can be difficult to navigate and sometimes seems bloated. To remedy this, we will increase the number of articles reviewed while decreasing the amount of detail per article. What we lose in depth we hope to gain in breadth.
We feel this will provide a general overview of all the pertinent literature so you can be confident you are up to date on take-home points of the most current studies. The department will also be reformatted so it is much easier to find the most crucial information. Look for these changes in the next month or two.
New Departments
The “Legal Eagle” and “Billing and Coding” departments will provide important information on medical malpractice and reimbursement documentation, while the “Hospital Pharmacy” department will offer up-to-the minute highlights of advances in therapeutics.
Finally, an advice column will give you the opportunity to ask experts your questions about the practice of hospital medicine.
Of course, much of The Hospitalist will remain unchanged. We will preserve your favorite features such John Nelson’s “Practice Management” column, Larry Wellikson’s “SHM Point of View” column, and the “Society Pages” and “Public Policy” departments. All this will happen against the backdrop of timely, in-depth reporting that keeps you abreast of the world of hospital medicine.
In all this transitioning it is important to recognize the team effort this publication requires. Indeed, the success of this publication is multidisciplinary and includes the expertise of the many folks at Wiley, notably Lisa Dionne (editorial director), Geoff Giordano (editor), and our colleagues at SHM—Larry Wellikson (CEO of SHM) and Todd Von Deak (director of membership and marketing) as well as the entire editorial and publishing staff at Wiley.
Most importantly, the success of this transition—and indeed the publication itself—depends on feedback from you, the reader. My e-mail box is always open to suggestions on how to improve the publication, including feedback on what we’re doing right and what we need to change. I’m also interested in hearing your ideas about clinical content areas that need to be covered. Just shoot me an e-mail saying, “I’d like to learn more about … .” And, we always welcome offers to contribute content to the publication.
To all of you: Thanks for helping make this transition such a successful one. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver and Health Sciences Center, where he serves as director of the Hospital Medicine Program, Inpatient Clinical Services in the Department of Medicine, and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Readmission or the Egg?
If you heat water sufficiently, you get steam. When you cool the steam, you get water again. Using the same process for chocolate—more “busy” than water and composed of multiple ingredients—will take you from solid to liquid and back again. So why won’t this technique also apply to the egg?
Eggs, or egg whites to be specific, are made up almost exclusively of the protein albumin. The chains of amino acids in proteins are normally configured in elaborate and precise folds, spirals, and sheets. Upon heating, the albumin becomes denatured and alters its molecular structure in such a way that it unfolds and adheres to itself in a dysfunctional manner known as aggregates. In the end, albumin permanently changes from clear to white and retains a rigid form.
No intervention has been successful in returning albumin to its original viscosity and color—not cooling, not anything. The caveat is that there is exciting research being done with naturally existing heat shock proteins called chaperones that have the potential to return proteins to their native state. This research has enormous implications for treating diseases such as cystic fibrosis and Alzheimer’s.
It may seem idiosyncratic that while we can manipulate water and chocolate, we can’t unfry an egg. The answer is, simply, that proteins are too complex for simple logic or techniques.
In its June report, the Medicare Payment Advisory Commission (MedPAC), the group that advises Congress on issues affecting the Medicare program, formulated recommendations for amending the construct for payments to hospitals based on their readmission rates. The rationale for targeting readmission rates, according to MedPAC, is to create favorable financial incentives for hospitals that achieve lower readmissions. Sounds simple enough. The MedPAC report identified potential savings of $12 billion given a 13.3% rate of “potentially preventable readmissions” within 30 days. Not only does it sound simple, it sounds compelling for quality and financial imperatives.
SHM has long identified transitions of care as one of the most vulnerable events for patients. Some of the earliest presentations at SHM meetings vividly described the “voltage drop” of information that can occur when a patient enters or leaves the hospital—not to mention during intra-hospital transitions. SHM has embraced transitions of care as a core competency in hospital medicine, has built a quality improvement resource room online for care transitions of older adults, and in July year co-sponsored a summit on transitions of care.
Readmission rates are commonly considered a proxy outcome measure to reflect the broader issue of quality of care transitions at the time of hospital discharge; however, we must be clear that these two entities are not nearly synonymous. A hospital readmission does not necessarily reflect a poorly executed hospital discharge, and high-quality discharges do not absolutely prevent hospital readmissions. The challenge with improving transitions of care and reducing preventable readmissions lies in the systems, processes, facilities, and people involved.
To drive lower readmission rates, MedPAC is suggesting a bifurcated strategy: public reporting and altering payment schedules to hospitals. I believe the former, combined with appropriate public education on the multifactorial nature of readmissions and how to interpret the data, can be a positive step toward improving care transitions. The more transparent the healthcare system becomes, the more frank conversations we can have in the pursuit of higher quality care. Those conversations open the door to understanding the complexity of care processes and the dependency of various resources and stakeholders on one another. They also help to confront the brutal truth of care transitions: that there must be shared accountability for ensuring our patients receive the support they need, where and when they need it.
By recommending changes in payment methodology to hospitals, however, MedPAC is isolating the facility from the intricate composite of systems and processes involved in transitions of care. While I realize the analogy is a bit of a stretch, this approach strikes me as similar to applying the logic of cooling to unfry an egg. A generally simple tactic to address a highly complex issue. Just as albumin has precise folds, spirals, and sheets to allow it to perform its proper function as a protein, so too must the healthcare system provide proper coordination, communication and support services to ensure proper health and well-being of patients.
Restructuring hospital payments in no way addresses the role of physicians in the hospital, physicians in the ambulatory or sub-acute setting, home-care agencies, other vendors, caregiver compliance, patient self-care, or chronic disease management. MedPAC’s proposal holds one party accountable in a scenario where only joint accountability will render the results we desire. In a recent article in the Harvard Business Review, Roger Martin eloquently describes a common coping mechanism people use to address complexity and ambiguity—simplification whenever possible. Within organizations, “When a colleague admonishes us to ‘quit complicating the issue,’ it’s not just an impatient reminder to get on with the damn job—it’s also a plea to keep the complexity at a comfortable level.”
I do not mean to imply incentives are not important; they are vital to stimulate change and manage behavior. That being said, I also believe incentive programs often beget unintended consequences and may drive undesirable behavior.
Would MedPAC’s proposal cause hospitals to become apprehensive about accepting more complex cases? Even the best severity-adjustment methods account for only a fraction of the variations among patients, so hospitals may feel compelled to screen or select out certain complex populations as opposed to relying on severity-adjustment measures to account for true differences in patient outcomes.
Don Berwick, MD, the CEO of the Institute of Healthcare Improvement (IHI), is often quoted as saying, “Every system is perfectly designed to achieve the results it gets.” If this is so, a singular focus on incentives and penalties directed toward hospitals will bring either unilateral facility actions and/or a lack of leverage to effect needed improvements in the rest of the care system.
Alternatively, the Centers for Medicare and Medicaid Systems (CMS) could focus on several areas that constructively address the interdependent systems and multiple stakeholders involved in transitions of care. CMS could:
- Adopt a public reporting system for readmission rates for hospitals according to select discharge diagnoses. Transparency likely will drive some improvements via the “Hawthorne effect,” and it will serve as a common basis for key parties discussing the issues to drive improvement;
- Advocate that public reporting should be accompanied by rigorous public education on transitions of care. Such education should include a clear outline of the complexities, interdependencies, and pitfalls common to care transitions, and should also include clear steps patients and caregivers can take to play an effective role in the process;
- Participate in the development of improvement tools to address readmission rates. IHI is a terrific example of an organization that has created such a device to improve hospital mortality rates. Their Mortality Diagnostic Tool identifies potentially avoidable hospital deaths;
- Sponsor collaborative meetings with key industry organizations to discuss the issues, gain consensus on standards and expectations, and promote necessary change; and
- Take the framework of reporting, education, improvement tools and practice standards to create aligned incentives across facilities, providers, vendors, and beneficiaries.
While it’s tempting to seek simple answers to complex issues, they often fall short of the best solution. As leaders in healthcare, we must embrace complexity and find answers that reflect an integrated and aligned approach. We must acknowledge that accountability for high-quality transitions of care and reductions in readmissions has to be shared. With the support of CMS, SHM, and other agencies and professional organizations, we have every resource available to improve this vulnerable time in the lives of our patients. Only then will we have an environment suitable to unfry the egg. Or perhaps we’ll engineer an environment where the egg is never fried in the first place. TH
Dr. Holman is the president of SHM.
If you heat water sufficiently, you get steam. When you cool the steam, you get water again. Using the same process for chocolate—more “busy” than water and composed of multiple ingredients—will take you from solid to liquid and back again. So why won’t this technique also apply to the egg?
Eggs, or egg whites to be specific, are made up almost exclusively of the protein albumin. The chains of amino acids in proteins are normally configured in elaborate and precise folds, spirals, and sheets. Upon heating, the albumin becomes denatured and alters its molecular structure in such a way that it unfolds and adheres to itself in a dysfunctional manner known as aggregates. In the end, albumin permanently changes from clear to white and retains a rigid form.
No intervention has been successful in returning albumin to its original viscosity and color—not cooling, not anything. The caveat is that there is exciting research being done with naturally existing heat shock proteins called chaperones that have the potential to return proteins to their native state. This research has enormous implications for treating diseases such as cystic fibrosis and Alzheimer’s.
It may seem idiosyncratic that while we can manipulate water and chocolate, we can’t unfry an egg. The answer is, simply, that proteins are too complex for simple logic or techniques.
In its June report, the Medicare Payment Advisory Commission (MedPAC), the group that advises Congress on issues affecting the Medicare program, formulated recommendations for amending the construct for payments to hospitals based on their readmission rates. The rationale for targeting readmission rates, according to MedPAC, is to create favorable financial incentives for hospitals that achieve lower readmissions. Sounds simple enough. The MedPAC report identified potential savings of $12 billion given a 13.3% rate of “potentially preventable readmissions” within 30 days. Not only does it sound simple, it sounds compelling for quality and financial imperatives.
SHM has long identified transitions of care as one of the most vulnerable events for patients. Some of the earliest presentations at SHM meetings vividly described the “voltage drop” of information that can occur when a patient enters or leaves the hospital—not to mention during intra-hospital transitions. SHM has embraced transitions of care as a core competency in hospital medicine, has built a quality improvement resource room online for care transitions of older adults, and in July year co-sponsored a summit on transitions of care.
Readmission rates are commonly considered a proxy outcome measure to reflect the broader issue of quality of care transitions at the time of hospital discharge; however, we must be clear that these two entities are not nearly synonymous. A hospital readmission does not necessarily reflect a poorly executed hospital discharge, and high-quality discharges do not absolutely prevent hospital readmissions. The challenge with improving transitions of care and reducing preventable readmissions lies in the systems, processes, facilities, and people involved.
To drive lower readmission rates, MedPAC is suggesting a bifurcated strategy: public reporting and altering payment schedules to hospitals. I believe the former, combined with appropriate public education on the multifactorial nature of readmissions and how to interpret the data, can be a positive step toward improving care transitions. The more transparent the healthcare system becomes, the more frank conversations we can have in the pursuit of higher quality care. Those conversations open the door to understanding the complexity of care processes and the dependency of various resources and stakeholders on one another. They also help to confront the brutal truth of care transitions: that there must be shared accountability for ensuring our patients receive the support they need, where and when they need it.
By recommending changes in payment methodology to hospitals, however, MedPAC is isolating the facility from the intricate composite of systems and processes involved in transitions of care. While I realize the analogy is a bit of a stretch, this approach strikes me as similar to applying the logic of cooling to unfry an egg. A generally simple tactic to address a highly complex issue. Just as albumin has precise folds, spirals, and sheets to allow it to perform its proper function as a protein, so too must the healthcare system provide proper coordination, communication and support services to ensure proper health and well-being of patients.
Restructuring hospital payments in no way addresses the role of physicians in the hospital, physicians in the ambulatory or sub-acute setting, home-care agencies, other vendors, caregiver compliance, patient self-care, or chronic disease management. MedPAC’s proposal holds one party accountable in a scenario where only joint accountability will render the results we desire. In a recent article in the Harvard Business Review, Roger Martin eloquently describes a common coping mechanism people use to address complexity and ambiguity—simplification whenever possible. Within organizations, “When a colleague admonishes us to ‘quit complicating the issue,’ it’s not just an impatient reminder to get on with the damn job—it’s also a plea to keep the complexity at a comfortable level.”
I do not mean to imply incentives are not important; they are vital to stimulate change and manage behavior. That being said, I also believe incentive programs often beget unintended consequences and may drive undesirable behavior.
Would MedPAC’s proposal cause hospitals to become apprehensive about accepting more complex cases? Even the best severity-adjustment methods account for only a fraction of the variations among patients, so hospitals may feel compelled to screen or select out certain complex populations as opposed to relying on severity-adjustment measures to account for true differences in patient outcomes.
Don Berwick, MD, the CEO of the Institute of Healthcare Improvement (IHI), is often quoted as saying, “Every system is perfectly designed to achieve the results it gets.” If this is so, a singular focus on incentives and penalties directed toward hospitals will bring either unilateral facility actions and/or a lack of leverage to effect needed improvements in the rest of the care system.
Alternatively, the Centers for Medicare and Medicaid Systems (CMS) could focus on several areas that constructively address the interdependent systems and multiple stakeholders involved in transitions of care. CMS could:
- Adopt a public reporting system for readmission rates for hospitals according to select discharge diagnoses. Transparency likely will drive some improvements via the “Hawthorne effect,” and it will serve as a common basis for key parties discussing the issues to drive improvement;
- Advocate that public reporting should be accompanied by rigorous public education on transitions of care. Such education should include a clear outline of the complexities, interdependencies, and pitfalls common to care transitions, and should also include clear steps patients and caregivers can take to play an effective role in the process;
- Participate in the development of improvement tools to address readmission rates. IHI is a terrific example of an organization that has created such a device to improve hospital mortality rates. Their Mortality Diagnostic Tool identifies potentially avoidable hospital deaths;
- Sponsor collaborative meetings with key industry organizations to discuss the issues, gain consensus on standards and expectations, and promote necessary change; and
- Take the framework of reporting, education, improvement tools and practice standards to create aligned incentives across facilities, providers, vendors, and beneficiaries.
While it’s tempting to seek simple answers to complex issues, they often fall short of the best solution. As leaders in healthcare, we must embrace complexity and find answers that reflect an integrated and aligned approach. We must acknowledge that accountability for high-quality transitions of care and reductions in readmissions has to be shared. With the support of CMS, SHM, and other agencies and professional organizations, we have every resource available to improve this vulnerable time in the lives of our patients. Only then will we have an environment suitable to unfry the egg. Or perhaps we’ll engineer an environment where the egg is never fried in the first place. TH
Dr. Holman is the president of SHM.
If you heat water sufficiently, you get steam. When you cool the steam, you get water again. Using the same process for chocolate—more “busy” than water and composed of multiple ingredients—will take you from solid to liquid and back again. So why won’t this technique also apply to the egg?
Eggs, or egg whites to be specific, are made up almost exclusively of the protein albumin. The chains of amino acids in proteins are normally configured in elaborate and precise folds, spirals, and sheets. Upon heating, the albumin becomes denatured and alters its molecular structure in such a way that it unfolds and adheres to itself in a dysfunctional manner known as aggregates. In the end, albumin permanently changes from clear to white and retains a rigid form.
No intervention has been successful in returning albumin to its original viscosity and color—not cooling, not anything. The caveat is that there is exciting research being done with naturally existing heat shock proteins called chaperones that have the potential to return proteins to their native state. This research has enormous implications for treating diseases such as cystic fibrosis and Alzheimer’s.
It may seem idiosyncratic that while we can manipulate water and chocolate, we can’t unfry an egg. The answer is, simply, that proteins are too complex for simple logic or techniques.
In its June report, the Medicare Payment Advisory Commission (MedPAC), the group that advises Congress on issues affecting the Medicare program, formulated recommendations for amending the construct for payments to hospitals based on their readmission rates. The rationale for targeting readmission rates, according to MedPAC, is to create favorable financial incentives for hospitals that achieve lower readmissions. Sounds simple enough. The MedPAC report identified potential savings of $12 billion given a 13.3% rate of “potentially preventable readmissions” within 30 days. Not only does it sound simple, it sounds compelling for quality and financial imperatives.
SHM has long identified transitions of care as one of the most vulnerable events for patients. Some of the earliest presentations at SHM meetings vividly described the “voltage drop” of information that can occur when a patient enters or leaves the hospital—not to mention during intra-hospital transitions. SHM has embraced transitions of care as a core competency in hospital medicine, has built a quality improvement resource room online for care transitions of older adults, and in July year co-sponsored a summit on transitions of care.
Readmission rates are commonly considered a proxy outcome measure to reflect the broader issue of quality of care transitions at the time of hospital discharge; however, we must be clear that these two entities are not nearly synonymous. A hospital readmission does not necessarily reflect a poorly executed hospital discharge, and high-quality discharges do not absolutely prevent hospital readmissions. The challenge with improving transitions of care and reducing preventable readmissions lies in the systems, processes, facilities, and people involved.
To drive lower readmission rates, MedPAC is suggesting a bifurcated strategy: public reporting and altering payment schedules to hospitals. I believe the former, combined with appropriate public education on the multifactorial nature of readmissions and how to interpret the data, can be a positive step toward improving care transitions. The more transparent the healthcare system becomes, the more frank conversations we can have in the pursuit of higher quality care. Those conversations open the door to understanding the complexity of care processes and the dependency of various resources and stakeholders on one another. They also help to confront the brutal truth of care transitions: that there must be shared accountability for ensuring our patients receive the support they need, where and when they need it.
By recommending changes in payment methodology to hospitals, however, MedPAC is isolating the facility from the intricate composite of systems and processes involved in transitions of care. While I realize the analogy is a bit of a stretch, this approach strikes me as similar to applying the logic of cooling to unfry an egg. A generally simple tactic to address a highly complex issue. Just as albumin has precise folds, spirals, and sheets to allow it to perform its proper function as a protein, so too must the healthcare system provide proper coordination, communication and support services to ensure proper health and well-being of patients.
Restructuring hospital payments in no way addresses the role of physicians in the hospital, physicians in the ambulatory or sub-acute setting, home-care agencies, other vendors, caregiver compliance, patient self-care, or chronic disease management. MedPAC’s proposal holds one party accountable in a scenario where only joint accountability will render the results we desire. In a recent article in the Harvard Business Review, Roger Martin eloquently describes a common coping mechanism people use to address complexity and ambiguity—simplification whenever possible. Within organizations, “When a colleague admonishes us to ‘quit complicating the issue,’ it’s not just an impatient reminder to get on with the damn job—it’s also a plea to keep the complexity at a comfortable level.”
I do not mean to imply incentives are not important; they are vital to stimulate change and manage behavior. That being said, I also believe incentive programs often beget unintended consequences and may drive undesirable behavior.
Would MedPAC’s proposal cause hospitals to become apprehensive about accepting more complex cases? Even the best severity-adjustment methods account for only a fraction of the variations among patients, so hospitals may feel compelled to screen or select out certain complex populations as opposed to relying on severity-adjustment measures to account for true differences in patient outcomes.
Don Berwick, MD, the CEO of the Institute of Healthcare Improvement (IHI), is often quoted as saying, “Every system is perfectly designed to achieve the results it gets.” If this is so, a singular focus on incentives and penalties directed toward hospitals will bring either unilateral facility actions and/or a lack of leverage to effect needed improvements in the rest of the care system.
Alternatively, the Centers for Medicare and Medicaid Systems (CMS) could focus on several areas that constructively address the interdependent systems and multiple stakeholders involved in transitions of care. CMS could:
- Adopt a public reporting system for readmission rates for hospitals according to select discharge diagnoses. Transparency likely will drive some improvements via the “Hawthorne effect,” and it will serve as a common basis for key parties discussing the issues to drive improvement;
- Advocate that public reporting should be accompanied by rigorous public education on transitions of care. Such education should include a clear outline of the complexities, interdependencies, and pitfalls common to care transitions, and should also include clear steps patients and caregivers can take to play an effective role in the process;
- Participate in the development of improvement tools to address readmission rates. IHI is a terrific example of an organization that has created such a device to improve hospital mortality rates. Their Mortality Diagnostic Tool identifies potentially avoidable hospital deaths;
- Sponsor collaborative meetings with key industry organizations to discuss the issues, gain consensus on standards and expectations, and promote necessary change; and
- Take the framework of reporting, education, improvement tools and practice standards to create aligned incentives across facilities, providers, vendors, and beneficiaries.
While it’s tempting to seek simple answers to complex issues, they often fall short of the best solution. As leaders in healthcare, we must embrace complexity and find answers that reflect an integrated and aligned approach. We must acknowledge that accountability for high-quality transitions of care and reductions in readmissions has to be shared. With the support of CMS, SHM, and other agencies and professional organizations, we have every resource available to improve this vulnerable time in the lives of our patients. Only then will we have an environment suitable to unfry the egg. Or perhaps we’ll engineer an environment where the egg is never fried in the first place. TH
Dr. Holman is the president of SHM.
More Patients Pick Acupuncture
In 2004, 370 of 1,394 reporting hospitals offered some complementary alternative medicine (CAM) services in the U.S. Of the 370 hospitals reporting CAM services, 11.5% (42 hospitals) reported inpatient acupuncture services.1
This threefold increase since 1998 demonstrates the growing use of and demand for acupuncture services in hospitals. This trend is driven by patient demand and clinical effectiveness. Acupuncture is a safe treatment modality hospital physicians should be familiar because it can benefit patients in the inpatient setting.
Origins of Acupuncture
The first use of acupuncture is not known. The earliest medical textbook on acupuncture was The Medical Classic of the Yellow Emperor, written about 100 B.C. The first translation of this text into English was in 1949.2
The book outlined the theory of a system of six sets of symmetrical channels on the body’s surface, which it called meridians; along these, it posited an intricate network of points.3 Needling the points was supposed to manipulate or release the flow of energy or life force—Qi—to the internal organs, thereby alleviating symptoms. Heating acupuncture points with burning herbs—moxibustion—was also purported to relieve pain.
Acupuncture in the U.S.
The first documented use of acupuncture in the United States occurred in the 19th century. In 1826, Bache used it to treat lumbago.4 During that same era, William Mosley used acupuncture to treat patients with lumbago and sciatica.5
In 1971, a first-person account of the use of acupuncture by New York Times reporter James Reston excited great interest in the technique. Reston was introduced to the procedure to relieve pain after an emergency appendectomy during a trip to China with Henry A. Kissinger.6
Since then, there has been a steady increase in the use of acupuncture by physicians. The American Academy of Medical Acupuncture, the only physician-based acupuncture society in North America, was formed in 1987; in 1992, the Office of Alternative Medicine was created within the NIH. In November 1997, the Food and Drug Administration (FDA) removed the experimental designation for acupuncture needles and approved their use by licensed practitioners. By 1993, the FDA had a record of more than 9,000 licensed acupuncturists, estimated to be providing more than 10 million treatments annually at a cost in excess of $500 million.7
Acupuncture is part of the quasi-medical area of complementary and alternative medicine, whose practitioners field more visits annually than all primary-care physicians in this country combined.8 Most acupuncturists practice the Chinese technique.9
Licensing requirements vary by state.10 As acupuncture has gained popularity and respect, and as its benefit for various medical conditions has been proved in high-quality studies, many well-established medical institutions and universities have begun to integrate it with more traditional Western medical treatments.
Acupuncture Theories
The early Chinese theories about how best to perform acupuncture were varied and sometimes conflicting.11 Early treatments using heat, bloodletting, and crude stone implementation evolved over centuries into the intricate practice known today.
Western scientists first became seriously interested in researching the effects of acupuncture in the 1970s. Many of the early studies were poorly designed, and the results were often not reproducible. They were not sufficiently randomized or blinded, and placebo controls were unreliable or nonexistent. To date, no single theory has been put forth that can explain all the phenomena associated with acupuncture treatment.
In 1991, the World Health Organization proposed a standard nomenclature for the 400 acupuncture points and the 20 meridians connecting those points.12 The precise anatomical locations of these areas have not yet been identified definitively. They have a low electrical resistance compared with surrounding tissue. Theories attempting to correlate the acupuncture points with neurovascular bundles have been postulated but remain unproved. The existence of acupuncture points has been verified with galvanometer scanning. These devices measure electrical conductance and emit an audio signal when an area of low resistance is encountered. New points have been added and the location of some of the original ones redefined by this technique.
In some of the earliest research conducted, French acupuncturists Niboyet and Grall mapped many of the points.13,14 Darras attempted to prove the existence of the meridians by tracing the flow of the radionuclide technetium TC 99m sulfur colloid after it was injected into them.15 No published reports in the English-language medical literature have reliably confirmed scientific studies documenting either the existence or location of the meridians.16
The neurohumoral theory postulates that the analgesic effects of acupuncture are related to the release of neurotransmitters such as endogenous opioids. In addition, acupuncture appears to inhibit the transmission of C-fiber pain at the level of the spinal cord.17,18 Other physiological phenomena have also been observed with acupuncture by needling. They include vasodilation, increased serum cortisol, variations in serum glucose and cholesterol levels, increased white blood cell counts, and acid suppression.5 Their significance continues to be questioned.
Evidence-Based Approach
Many studies of acupuncture have methodological flaws. The biggest problem as yet unresolved is an appropriate placebo control.19 Sham acupuncture, which involves needling non-acupuncture points, is frequently the control of choice but has serious limitations.
In 1997, the landmark NIH consensus statement was probably the most important presentation of evidence supporting the efficacy of acupuncture.20 Conclusions made about the effectiveness of acupuncture were based on evidence from reliable studies. Many promising results emerged. Specific indications for use of acupuncture were identified on the basis of published reports of its effectiveness. Efficacy in treating dental pain and post-operative and chemotherapy-induced nausea were demonstrated. Research suggested its usefulness as an adjunct or alternative treatment for lower-back pain, osteoarthritis, addiction, and stroke rehabilitation. The panel also concluded that further research would likely uncover additional uses for acupuncture.
From the standpoint of acupuncture’s effectiveness, it can clearly benefit specific patient groups. It is most commonly used as a treatment for back pain.21 Since the NIH conference, further research has confirmed its effectiveness in treating a variety of medical conditions. (See Table 1, above)
Much of the ongoing research on acupuncture has focused on the use of functional magnetic resonance imaging of the brain, specifically on the areas that light up, or show brain activity, during activities or a state of pain.22-24 Acupuncture has been found to reduce the intensity of signals in such areas. The mechanism for the analgesic effects of acupuncture may be the result of reduced blood flow to the brain.24 Several studies have identified specific areas of the brain affected by pressure on various acupuncture points.25
Practical Aspects
Acupuncture treatments are extremely time efficient and require minimal equipment. They can be administered with the patient in the recumbent position or sitting upright. For initial sessions, I prefer the former, especially for younger males, who are more prone to vasovagal reactions. Any of several different methods of acupuncture can be used to stimulate points. In addition to needling, acupuncture can be conducted by electro-acupuncture, moxibustion, cupping, scraping, tapping, acupressure, or laser.
Most inpatient referrals are for pain management. Other common indications include post-operative or chemotherapy-induced nausea (emesis), anxiety, and prevention of withdrawal symptoms from narcotics.
Acupuncture Safety
Overall, acupuncture is a safe treatment method. Many large studies have confirmed that most types of acupuncture have a low rate of complications and that most of these complications are transient and minor in nature.28,29 They are incident-reporting studies, however, and have the limitations inherent in these studies. Nausea, dizziness, bruising, and needle pain are some of the most commonly reported. The rare but serious adverse events, such as pneumothorax, usually occur as a result of the practitioner’s poor training or technique.30
Future of Acupuncture
Public acceptance of, and demand for, acupuncture for pain relief is increasing. Additional clinical studies are needed, however, to expand the types of conditions for which acupuncture may be useful. It is essential to maintain a constant focus on safe practice, which would be aided by the establishment of a standardized accreditation and training system. Hospitals need to establish uniform credentialing guidelines similar to those for other procedures that require evidence of medical competence and safety.31
In February 2005, the Federal Acupuncture Coverage Act was introduced to Congress. If enacted, the measure would allow acupuncture to be covered under Part B for Medicare recipients.
The trend toward an integrated approach to patient therapy in large academic medical institutions is encouraging. The incorporation of the teaching of acupuncture within the current medical school curricula would no doubt complement this approach. TH
Joseph C. Charles, MD, FACP, is an assistant professor of medicine and division education coordinator for the Department of Hospital Internal Medicine at the Mayo Clinic Hospital Arizona.
References
- Ananth, S. Health Forum 2005 Complementary and Alternative Medicine Survey of Hospitals, July 19, 2006. News release, American Hospital Association.
- Veith I (trans). The Yellow Emperor’s Classic of Internal Medicine. Baltimore; Lippincott, Williams & Wilkins: 1949.
- Ming Z (trans). The Medical Classic of the Yellow Emperor. Beijing; Foreign Languages Press: 2001.
- Cassedy JH. Early uses of acupuncture in the United States, with an addendum (1826) by Franklin Bache, M.D. Bull N Y Acad Med. 1974 Sep;50(8):892-906.
- Osler W. The Principles and Practice of Medicine. New York: D. Appleton and Company; 1892.
- Reston J. Now, let me tell you about my appendectomy in Peking. New York Times. July 26, 1971.
- Mitchell BB. Educational and licensing requirements for acupuncturists. J Altern Complement Med. 1996 spring;2(1):33-35.
- Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993 Jan 28;328(4):246-252.
- Sherman KJ, Cherkin DC, Eisenberg DM, et al. The practice of acupuncture: who are the providers and what do they do? Ann Fam Med. 2005 Mar-Apr;3(2):151-158.
- Leake R, Broderick JE. Current licensure for acupuncture in the United States. Altern Ther Health Med. 1999 Jul;5(4):94-96.
- Shang C. The past, present, and future of meridian system research. In: Stux G, Hammerschlag R, eds. Clinical Acupuncture: Scientific Basis. Berlin: Springer; 2001:69-82.
- WHO Scientific Group on International Acupuncture Nomenclature. A proposed standard international acupuncture nomenclature: report of a WHO Scientific Group. Geneva: World Health Organization; 1991.
- Niboyet JEH. Nouvelles constatations sur les proprietes electriques des points chinois. Bull Soc Acupunct. 1938;4:30-79.
- Helms JM. Acupuncture Energetics: A Clinical Approach for Physicians. Berkeley, Calif.: Medical Acupuncture Publishers; 1995:23-24.
- De Vernejoul P, Albarede P, Darras JC. Study of acupuncture meridians using radioactive tracers [in French]. Bull Acad Natl Med. 1985 Oct;169(7):1071-1075.
- Simon J, Guiraud G, Esquerre JP, et al. Acupuncture meridians demythified. Contribution of radiotracer methodology [in French]. Presse Med. 1988 Jul 2;17(26):1341-1344.
- Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: endorphin implicated. Life Sci. 1976 Dec 1;19(11):1757-1762.
- Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374-383.
- Vincent C, Lewith G. Placebo controls for acupuncture studies. J R Soc Med. 1995 Apr;88(4):199-202.
- Acupuncture. NIH Consensus Statement 1997; 15:1-34
- Manheimer E, White A, Berman B, et al. Meta-analysis: acupuncture for low back pain. Ann Intern Med. 2005;142:651-663.
- Tank DW, Oqawa S, Uqurbil K. Mapping the brain with MRI. Curr Biol. 1992;525-528.
- Salvatore S. Brain imaging suggests acupuncture works, study says. [monograph on the Internet]. CNN.com with WebMD.com. Dec. 1, 1999. Available at http://archives.cnn.com/1999/HEALTH/alternative/12/01/brain.acupuncture/index.html. Last accessed April 14, 2007.
- Fang JL, Krings T, Weidemann J, et al. Functional MRI in healthy subjects during acupuncture: different effects of needle rotation in real and false acupoints. Neuroradiology. 2004;46:359-362.
- Cho ZH, Chung SC, Jones JP, et al. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci USA. 1998 Mar;95(5):2670-2673. Retraction in Cho ZH, Chung SC, Lee HJ, Wong EK, Min BI. Proc Natl Acad Sci USA. 2006 Jul 5;103(27):10527.
- Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998;44:129-138.
- Gam AN, Thorsen H, Lonnberg F. The effect of low-level laser therapy on musculoskeletal pain: a meta-analysis. Pain. 1993;52:63-66.
- White A, Hayhoe S, Hart A, et al. Adverse events following acupuncture: prospective survey of 32, 000 consultations with doctors and physiotherapists. BMJ. 2001 Sep 1;323(7311):485-486.
- MacPherson H, Thomas K, Walters S, et al. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ. 2001;323:486-487. Comment in BMJ. 2001 Sep 1;323(7311):467-8. BMJ. 2002 Jan 19;324(7330):170-1.
- Chauffe RJ, Duskin AL. Pneumothorax secondary to acupuncture therapy. South Med J. 2006;99:1297-1299.
- Cohen MH, Hrbek A, Davis RB, et al. Emerging credentialing practices, malpractice liability policies, and guidelines governing complementary and alternative medical practices and dietary supplement recommendations: a descriptive study of 19 integrative health care centers in the United States. Arch Intern Med. 2005;165:289-295.
- Linde K, Jobst K, Panton J. Acupuncture for chronic asthma. Cochrane Database Syst Rev. 2000;(2):CD000008.
- Kleijnen J, Ter Riet G, Knipschild P. Acupuncture and asthma: a review of controlled trials. Thorax. 1991;46:799-802.
- Ter Reit G, Kleijnen J, Knipschild P. A meta-analysis of studies into the effect of acupuncture on addiction. Br J Gen Pract. 1990;40:379-382.
- Vincent CA. A controlled trial of the treatment of migraine by acupuncture. Clin J Pain. 1989;5:305-312.
- White AR, Rampes H, Ernst E. Acupuncture for smoking cessation. Cochrane Database Syst Rev. 2002;(2):CD000009.
- Mann E. Using acupuncture and acupressure to treat postoperative emesis. Prof Nurse. 1999; 14:691-694.
- Macklin EA, Wayne PM, Kalish LA, et al. Stop hypertension with the acupuncture research program (SHARP): results of a randomized, controlled clinical trial. Hypertension. 2006;48:838-845.
- Lee JD, Chon JS, Jeong HK, et al. The cerebrovascular response to traditional acupuncture after stroke. Neuroradiology. 2003;45:780-784.
- Scharf HP, Mansmann U, Streitberger K, et al. Acupuncture and knee osteoarthritis: a three-armed randomized trial. Ann Intern Med. 2006;145:12-20.
- Martin DP, Sletten CD, Williams BA, et al. Improvement in fibromyalgia symptoms with acupuncture: results of a randomized controlled trial. Mayo Clin Proc. 2006;81:749-757.
- Lu DP, Lu GP. Anatomical relevance of some acupuncture points in the head and neck region that dictate medical or dental application depending on depth of needle insertion. Acupunct Electrother Res. 2003;28(3-4):145-156.
- Ernst E, Pittler MH. The effectiveness of acupuncture in treating acute dental pain: a systemic review. Br Dent J. 1998;184:443-447.
- Chen HM, Chen CH. Effects of acupressure at the Sanyinjiao point on primary dysmenorrhoea. J Adv Nurs. 2004;48(4):380-387.
- Pouresmail Z, Ibrahimzadeh R. Effects of acupressure and ibuprofen on the severity of primary dysmenorrhea. J Tradit Chin Med. 2002 Sep; 22(3):205-210.
- Linde K, Streng A, Jurgens S, et al. Acupuncture for patients with migraine: a randomized controlled trial. JAMA. 2005 May 4;293(17):2118-2125.
- Allais G, De Lorenzo C, Quirico PE, et al. Acupuncture in the prophylactic treatment of migraine without aura: a comparison with flunarizine. Headache. 2002 Oct;42(9):855-861.
- Green S, Buchbinder R, Barnsley L, et al. Acupuncture for lateral elbow pain. Cochrane Database Syst Rev. 2002;(1):CD003527. Review.
- Trinh KV, Phillips SD. Acupuncture for the alleviation of lateral epicondyle pain: a systematic review. Rheumatology (Oxford). 2004:43:1085-1090.
- David J, Townsend S, Sathanathan R, et al. The effect of acupuncture on patients with rheumatoid arthritis: a randomized, placebo-controlled cross-over study. Rheumatology (Oxford). 1999 Sep;38(9):864-869. Comment in Rheumatology (Oxford). 2000 Oct;39(10):1153-1154.
- Irnich D, Behrens N, Molzen H, et al. Randomised trial of acupuncture compared with conventional massage and “sham” laser acupuncture for treatment of chronic neck pain. BMJ. 2001 Jun 30;322(7302):1574-1578. Comment in BMJ. 2001 Dec 1;323(7324):1306-7.
- White P, Lewith G, Prescott P, et al. Acupuncture versus placebo for the treatment of chronic mechanical neck pain: a randomized, controlled trial. Ann Intern Med. 2004 Dec 21;141(12):911-919. Comment in Ann Intern Med. 2004 Dec 21;141(12):957-958. Ann Intern Med. 2005 May 17;142(10):873; author reply 873-874.
- Cheuk DK, Wong V. Acupuncture for epilepsy. Cochrane Database Syst Rev. 2006;(2):CD005062.
- Griggs C, Jensen J. Effectiveness of acupuncture for migraine: critical literature review. J Adv Nurs. 2006 May;54(4):491-501.
- Kim YH, Schiff E, Waalen J, et al. Efficacy of acupuncture for treating cocaine addiction: a review paper. J Addict Dis. 2005;24(4):115-132.
- Forbes A, Jackson S, Walter C, et al. Acupuncture for irritable bowel syndrome: a blinded placebo-controlled trial. World J Gastroenterol. 2005 Jul 14;11(26):4040-4044.
- Schneider A, Enck P, Streitberger K, et al. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649-654.
In 2004, 370 of 1,394 reporting hospitals offered some complementary alternative medicine (CAM) services in the U.S. Of the 370 hospitals reporting CAM services, 11.5% (42 hospitals) reported inpatient acupuncture services.1
This threefold increase since 1998 demonstrates the growing use of and demand for acupuncture services in hospitals. This trend is driven by patient demand and clinical effectiveness. Acupuncture is a safe treatment modality hospital physicians should be familiar because it can benefit patients in the inpatient setting.
Origins of Acupuncture
The first use of acupuncture is not known. The earliest medical textbook on acupuncture was The Medical Classic of the Yellow Emperor, written about 100 B.C. The first translation of this text into English was in 1949.2
The book outlined the theory of a system of six sets of symmetrical channels on the body’s surface, which it called meridians; along these, it posited an intricate network of points.3 Needling the points was supposed to manipulate or release the flow of energy or life force—Qi—to the internal organs, thereby alleviating symptoms. Heating acupuncture points with burning herbs—moxibustion—was also purported to relieve pain.
Acupuncture in the U.S.
The first documented use of acupuncture in the United States occurred in the 19th century. In 1826, Bache used it to treat lumbago.4 During that same era, William Mosley used acupuncture to treat patients with lumbago and sciatica.5
In 1971, a first-person account of the use of acupuncture by New York Times reporter James Reston excited great interest in the technique. Reston was introduced to the procedure to relieve pain after an emergency appendectomy during a trip to China with Henry A. Kissinger.6
Since then, there has been a steady increase in the use of acupuncture by physicians. The American Academy of Medical Acupuncture, the only physician-based acupuncture society in North America, was formed in 1987; in 1992, the Office of Alternative Medicine was created within the NIH. In November 1997, the Food and Drug Administration (FDA) removed the experimental designation for acupuncture needles and approved their use by licensed practitioners. By 1993, the FDA had a record of more than 9,000 licensed acupuncturists, estimated to be providing more than 10 million treatments annually at a cost in excess of $500 million.7
Acupuncture is part of the quasi-medical area of complementary and alternative medicine, whose practitioners field more visits annually than all primary-care physicians in this country combined.8 Most acupuncturists practice the Chinese technique.9
Licensing requirements vary by state.10 As acupuncture has gained popularity and respect, and as its benefit for various medical conditions has been proved in high-quality studies, many well-established medical institutions and universities have begun to integrate it with more traditional Western medical treatments.
Acupuncture Theories
The early Chinese theories about how best to perform acupuncture were varied and sometimes conflicting.11 Early treatments using heat, bloodletting, and crude stone implementation evolved over centuries into the intricate practice known today.
Western scientists first became seriously interested in researching the effects of acupuncture in the 1970s. Many of the early studies were poorly designed, and the results were often not reproducible. They were not sufficiently randomized or blinded, and placebo controls were unreliable or nonexistent. To date, no single theory has been put forth that can explain all the phenomena associated with acupuncture treatment.
In 1991, the World Health Organization proposed a standard nomenclature for the 400 acupuncture points and the 20 meridians connecting those points.12 The precise anatomical locations of these areas have not yet been identified definitively. They have a low electrical resistance compared with surrounding tissue. Theories attempting to correlate the acupuncture points with neurovascular bundles have been postulated but remain unproved. The existence of acupuncture points has been verified with galvanometer scanning. These devices measure electrical conductance and emit an audio signal when an area of low resistance is encountered. New points have been added and the location of some of the original ones redefined by this technique.
In some of the earliest research conducted, French acupuncturists Niboyet and Grall mapped many of the points.13,14 Darras attempted to prove the existence of the meridians by tracing the flow of the radionuclide technetium TC 99m sulfur colloid after it was injected into them.15 No published reports in the English-language medical literature have reliably confirmed scientific studies documenting either the existence or location of the meridians.16
The neurohumoral theory postulates that the analgesic effects of acupuncture are related to the release of neurotransmitters such as endogenous opioids. In addition, acupuncture appears to inhibit the transmission of C-fiber pain at the level of the spinal cord.17,18 Other physiological phenomena have also been observed with acupuncture by needling. They include vasodilation, increased serum cortisol, variations in serum glucose and cholesterol levels, increased white blood cell counts, and acid suppression.5 Their significance continues to be questioned.
Evidence-Based Approach
Many studies of acupuncture have methodological flaws. The biggest problem as yet unresolved is an appropriate placebo control.19 Sham acupuncture, which involves needling non-acupuncture points, is frequently the control of choice but has serious limitations.
In 1997, the landmark NIH consensus statement was probably the most important presentation of evidence supporting the efficacy of acupuncture.20 Conclusions made about the effectiveness of acupuncture were based on evidence from reliable studies. Many promising results emerged. Specific indications for use of acupuncture were identified on the basis of published reports of its effectiveness. Efficacy in treating dental pain and post-operative and chemotherapy-induced nausea were demonstrated. Research suggested its usefulness as an adjunct or alternative treatment for lower-back pain, osteoarthritis, addiction, and stroke rehabilitation. The panel also concluded that further research would likely uncover additional uses for acupuncture.
From the standpoint of acupuncture’s effectiveness, it can clearly benefit specific patient groups. It is most commonly used as a treatment for back pain.21 Since the NIH conference, further research has confirmed its effectiveness in treating a variety of medical conditions. (See Table 1, above)
Much of the ongoing research on acupuncture has focused on the use of functional magnetic resonance imaging of the brain, specifically on the areas that light up, or show brain activity, during activities or a state of pain.22-24 Acupuncture has been found to reduce the intensity of signals in such areas. The mechanism for the analgesic effects of acupuncture may be the result of reduced blood flow to the brain.24 Several studies have identified specific areas of the brain affected by pressure on various acupuncture points.25
Practical Aspects
Acupuncture treatments are extremely time efficient and require minimal equipment. They can be administered with the patient in the recumbent position or sitting upright. For initial sessions, I prefer the former, especially for younger males, who are more prone to vasovagal reactions. Any of several different methods of acupuncture can be used to stimulate points. In addition to needling, acupuncture can be conducted by electro-acupuncture, moxibustion, cupping, scraping, tapping, acupressure, or laser.
Most inpatient referrals are for pain management. Other common indications include post-operative or chemotherapy-induced nausea (emesis), anxiety, and prevention of withdrawal symptoms from narcotics.
Acupuncture Safety
Overall, acupuncture is a safe treatment method. Many large studies have confirmed that most types of acupuncture have a low rate of complications and that most of these complications are transient and minor in nature.28,29 They are incident-reporting studies, however, and have the limitations inherent in these studies. Nausea, dizziness, bruising, and needle pain are some of the most commonly reported. The rare but serious adverse events, such as pneumothorax, usually occur as a result of the practitioner’s poor training or technique.30
Future of Acupuncture
Public acceptance of, and demand for, acupuncture for pain relief is increasing. Additional clinical studies are needed, however, to expand the types of conditions for which acupuncture may be useful. It is essential to maintain a constant focus on safe practice, which would be aided by the establishment of a standardized accreditation and training system. Hospitals need to establish uniform credentialing guidelines similar to those for other procedures that require evidence of medical competence and safety.31
In February 2005, the Federal Acupuncture Coverage Act was introduced to Congress. If enacted, the measure would allow acupuncture to be covered under Part B for Medicare recipients.
The trend toward an integrated approach to patient therapy in large academic medical institutions is encouraging. The incorporation of the teaching of acupuncture within the current medical school curricula would no doubt complement this approach. TH
Joseph C. Charles, MD, FACP, is an assistant professor of medicine and division education coordinator for the Department of Hospital Internal Medicine at the Mayo Clinic Hospital Arizona.
References
- Ananth, S. Health Forum 2005 Complementary and Alternative Medicine Survey of Hospitals, July 19, 2006. News release, American Hospital Association.
- Veith I (trans). The Yellow Emperor’s Classic of Internal Medicine. Baltimore; Lippincott, Williams & Wilkins: 1949.
- Ming Z (trans). The Medical Classic of the Yellow Emperor. Beijing; Foreign Languages Press: 2001.
- Cassedy JH. Early uses of acupuncture in the United States, with an addendum (1826) by Franklin Bache, M.D. Bull N Y Acad Med. 1974 Sep;50(8):892-906.
- Osler W. The Principles and Practice of Medicine. New York: D. Appleton and Company; 1892.
- Reston J. Now, let me tell you about my appendectomy in Peking. New York Times. July 26, 1971.
- Mitchell BB. Educational and licensing requirements for acupuncturists. J Altern Complement Med. 1996 spring;2(1):33-35.
- Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993 Jan 28;328(4):246-252.
- Sherman KJ, Cherkin DC, Eisenberg DM, et al. The practice of acupuncture: who are the providers and what do they do? Ann Fam Med. 2005 Mar-Apr;3(2):151-158.
- Leake R, Broderick JE. Current licensure for acupuncture in the United States. Altern Ther Health Med. 1999 Jul;5(4):94-96.
- Shang C. The past, present, and future of meridian system research. In: Stux G, Hammerschlag R, eds. Clinical Acupuncture: Scientific Basis. Berlin: Springer; 2001:69-82.
- WHO Scientific Group on International Acupuncture Nomenclature. A proposed standard international acupuncture nomenclature: report of a WHO Scientific Group. Geneva: World Health Organization; 1991.
- Niboyet JEH. Nouvelles constatations sur les proprietes electriques des points chinois. Bull Soc Acupunct. 1938;4:30-79.
- Helms JM. Acupuncture Energetics: A Clinical Approach for Physicians. Berkeley, Calif.: Medical Acupuncture Publishers; 1995:23-24.
- De Vernejoul P, Albarede P, Darras JC. Study of acupuncture meridians using radioactive tracers [in French]. Bull Acad Natl Med. 1985 Oct;169(7):1071-1075.
- Simon J, Guiraud G, Esquerre JP, et al. Acupuncture meridians demythified. Contribution of radiotracer methodology [in French]. Presse Med. 1988 Jul 2;17(26):1341-1344.
- Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: endorphin implicated. Life Sci. 1976 Dec 1;19(11):1757-1762.
- Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374-383.
- Vincent C, Lewith G. Placebo controls for acupuncture studies. J R Soc Med. 1995 Apr;88(4):199-202.
- Acupuncture. NIH Consensus Statement 1997; 15:1-34
- Manheimer E, White A, Berman B, et al. Meta-analysis: acupuncture for low back pain. Ann Intern Med. 2005;142:651-663.
- Tank DW, Oqawa S, Uqurbil K. Mapping the brain with MRI. Curr Biol. 1992;525-528.
- Salvatore S. Brain imaging suggests acupuncture works, study says. [monograph on the Internet]. CNN.com with WebMD.com. Dec. 1, 1999. Available at http://archives.cnn.com/1999/HEALTH/alternative/12/01/brain.acupuncture/index.html. Last accessed April 14, 2007.
- Fang JL, Krings T, Weidemann J, et al. Functional MRI in healthy subjects during acupuncture: different effects of needle rotation in real and false acupoints. Neuroradiology. 2004;46:359-362.
- Cho ZH, Chung SC, Jones JP, et al. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci USA. 1998 Mar;95(5):2670-2673. Retraction in Cho ZH, Chung SC, Lee HJ, Wong EK, Min BI. Proc Natl Acad Sci USA. 2006 Jul 5;103(27):10527.
- Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998;44:129-138.
- Gam AN, Thorsen H, Lonnberg F. The effect of low-level laser therapy on musculoskeletal pain: a meta-analysis. Pain. 1993;52:63-66.
- White A, Hayhoe S, Hart A, et al. Adverse events following acupuncture: prospective survey of 32, 000 consultations with doctors and physiotherapists. BMJ. 2001 Sep 1;323(7311):485-486.
- MacPherson H, Thomas K, Walters S, et al. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ. 2001;323:486-487. Comment in BMJ. 2001 Sep 1;323(7311):467-8. BMJ. 2002 Jan 19;324(7330):170-1.
- Chauffe RJ, Duskin AL. Pneumothorax secondary to acupuncture therapy. South Med J. 2006;99:1297-1299.
- Cohen MH, Hrbek A, Davis RB, et al. Emerging credentialing practices, malpractice liability policies, and guidelines governing complementary and alternative medical practices and dietary supplement recommendations: a descriptive study of 19 integrative health care centers in the United States. Arch Intern Med. 2005;165:289-295.
- Linde K, Jobst K, Panton J. Acupuncture for chronic asthma. Cochrane Database Syst Rev. 2000;(2):CD000008.
- Kleijnen J, Ter Riet G, Knipschild P. Acupuncture and asthma: a review of controlled trials. Thorax. 1991;46:799-802.
- Ter Reit G, Kleijnen J, Knipschild P. A meta-analysis of studies into the effect of acupuncture on addiction. Br J Gen Pract. 1990;40:379-382.
- Vincent CA. A controlled trial of the treatment of migraine by acupuncture. Clin J Pain. 1989;5:305-312.
- White AR, Rampes H, Ernst E. Acupuncture for smoking cessation. Cochrane Database Syst Rev. 2002;(2):CD000009.
- Mann E. Using acupuncture and acupressure to treat postoperative emesis. Prof Nurse. 1999; 14:691-694.
- Macklin EA, Wayne PM, Kalish LA, et al. Stop hypertension with the acupuncture research program (SHARP): results of a randomized, controlled clinical trial. Hypertension. 2006;48:838-845.
- Lee JD, Chon JS, Jeong HK, et al. The cerebrovascular response to traditional acupuncture after stroke. Neuroradiology. 2003;45:780-784.
- Scharf HP, Mansmann U, Streitberger K, et al. Acupuncture and knee osteoarthritis: a three-armed randomized trial. Ann Intern Med. 2006;145:12-20.
- Martin DP, Sletten CD, Williams BA, et al. Improvement in fibromyalgia symptoms with acupuncture: results of a randomized controlled trial. Mayo Clin Proc. 2006;81:749-757.
- Lu DP, Lu GP. Anatomical relevance of some acupuncture points in the head and neck region that dictate medical or dental application depending on depth of needle insertion. Acupunct Electrother Res. 2003;28(3-4):145-156.
- Ernst E, Pittler MH. The effectiveness of acupuncture in treating acute dental pain: a systemic review. Br Dent J. 1998;184:443-447.
- Chen HM, Chen CH. Effects of acupressure at the Sanyinjiao point on primary dysmenorrhoea. J Adv Nurs. 2004;48(4):380-387.
- Pouresmail Z, Ibrahimzadeh R. Effects of acupressure and ibuprofen on the severity of primary dysmenorrhea. J Tradit Chin Med. 2002 Sep; 22(3):205-210.
- Linde K, Streng A, Jurgens S, et al. Acupuncture for patients with migraine: a randomized controlled trial. JAMA. 2005 May 4;293(17):2118-2125.
- Allais G, De Lorenzo C, Quirico PE, et al. Acupuncture in the prophylactic treatment of migraine without aura: a comparison with flunarizine. Headache. 2002 Oct;42(9):855-861.
- Green S, Buchbinder R, Barnsley L, et al. Acupuncture for lateral elbow pain. Cochrane Database Syst Rev. 2002;(1):CD003527. Review.
- Trinh KV, Phillips SD. Acupuncture for the alleviation of lateral epicondyle pain: a systematic review. Rheumatology (Oxford). 2004:43:1085-1090.
- David J, Townsend S, Sathanathan R, et al. The effect of acupuncture on patients with rheumatoid arthritis: a randomized, placebo-controlled cross-over study. Rheumatology (Oxford). 1999 Sep;38(9):864-869. Comment in Rheumatology (Oxford). 2000 Oct;39(10):1153-1154.
- Irnich D, Behrens N, Molzen H, et al. Randomised trial of acupuncture compared with conventional massage and “sham” laser acupuncture for treatment of chronic neck pain. BMJ. 2001 Jun 30;322(7302):1574-1578. Comment in BMJ. 2001 Dec 1;323(7324):1306-7.
- White P, Lewith G, Prescott P, et al. Acupuncture versus placebo for the treatment of chronic mechanical neck pain: a randomized, controlled trial. Ann Intern Med. 2004 Dec 21;141(12):911-919. Comment in Ann Intern Med. 2004 Dec 21;141(12):957-958. Ann Intern Med. 2005 May 17;142(10):873; author reply 873-874.
- Cheuk DK, Wong V. Acupuncture for epilepsy. Cochrane Database Syst Rev. 2006;(2):CD005062.
- Griggs C, Jensen J. Effectiveness of acupuncture for migraine: critical literature review. J Adv Nurs. 2006 May;54(4):491-501.
- Kim YH, Schiff E, Waalen J, et al. Efficacy of acupuncture for treating cocaine addiction: a review paper. J Addict Dis. 2005;24(4):115-132.
- Forbes A, Jackson S, Walter C, et al. Acupuncture for irritable bowel syndrome: a blinded placebo-controlled trial. World J Gastroenterol. 2005 Jul 14;11(26):4040-4044.
- Schneider A, Enck P, Streitberger K, et al. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649-654.
In 2004, 370 of 1,394 reporting hospitals offered some complementary alternative medicine (CAM) services in the U.S. Of the 370 hospitals reporting CAM services, 11.5% (42 hospitals) reported inpatient acupuncture services.1
This threefold increase since 1998 demonstrates the growing use of and demand for acupuncture services in hospitals. This trend is driven by patient demand and clinical effectiveness. Acupuncture is a safe treatment modality hospital physicians should be familiar because it can benefit patients in the inpatient setting.
Origins of Acupuncture
The first use of acupuncture is not known. The earliest medical textbook on acupuncture was The Medical Classic of the Yellow Emperor, written about 100 B.C. The first translation of this text into English was in 1949.2
The book outlined the theory of a system of six sets of symmetrical channels on the body’s surface, which it called meridians; along these, it posited an intricate network of points.3 Needling the points was supposed to manipulate or release the flow of energy or life force—Qi—to the internal organs, thereby alleviating symptoms. Heating acupuncture points with burning herbs—moxibustion—was also purported to relieve pain.
Acupuncture in the U.S.
The first documented use of acupuncture in the United States occurred in the 19th century. In 1826, Bache used it to treat lumbago.4 During that same era, William Mosley used acupuncture to treat patients with lumbago and sciatica.5
In 1971, a first-person account of the use of acupuncture by New York Times reporter James Reston excited great interest in the technique. Reston was introduced to the procedure to relieve pain after an emergency appendectomy during a trip to China with Henry A. Kissinger.6
Since then, there has been a steady increase in the use of acupuncture by physicians. The American Academy of Medical Acupuncture, the only physician-based acupuncture society in North America, was formed in 1987; in 1992, the Office of Alternative Medicine was created within the NIH. In November 1997, the Food and Drug Administration (FDA) removed the experimental designation for acupuncture needles and approved their use by licensed practitioners. By 1993, the FDA had a record of more than 9,000 licensed acupuncturists, estimated to be providing more than 10 million treatments annually at a cost in excess of $500 million.7
Acupuncture is part of the quasi-medical area of complementary and alternative medicine, whose practitioners field more visits annually than all primary-care physicians in this country combined.8 Most acupuncturists practice the Chinese technique.9
Licensing requirements vary by state.10 As acupuncture has gained popularity and respect, and as its benefit for various medical conditions has been proved in high-quality studies, many well-established medical institutions and universities have begun to integrate it with more traditional Western medical treatments.
Acupuncture Theories
The early Chinese theories about how best to perform acupuncture were varied and sometimes conflicting.11 Early treatments using heat, bloodletting, and crude stone implementation evolved over centuries into the intricate practice known today.
Western scientists first became seriously interested in researching the effects of acupuncture in the 1970s. Many of the early studies were poorly designed, and the results were often not reproducible. They were not sufficiently randomized or blinded, and placebo controls were unreliable or nonexistent. To date, no single theory has been put forth that can explain all the phenomena associated with acupuncture treatment.
In 1991, the World Health Organization proposed a standard nomenclature for the 400 acupuncture points and the 20 meridians connecting those points.12 The precise anatomical locations of these areas have not yet been identified definitively. They have a low electrical resistance compared with surrounding tissue. Theories attempting to correlate the acupuncture points with neurovascular bundles have been postulated but remain unproved. The existence of acupuncture points has been verified with galvanometer scanning. These devices measure electrical conductance and emit an audio signal when an area of low resistance is encountered. New points have been added and the location of some of the original ones redefined by this technique.
In some of the earliest research conducted, French acupuncturists Niboyet and Grall mapped many of the points.13,14 Darras attempted to prove the existence of the meridians by tracing the flow of the radionuclide technetium TC 99m sulfur colloid after it was injected into them.15 No published reports in the English-language medical literature have reliably confirmed scientific studies documenting either the existence or location of the meridians.16
The neurohumoral theory postulates that the analgesic effects of acupuncture are related to the release of neurotransmitters such as endogenous opioids. In addition, acupuncture appears to inhibit the transmission of C-fiber pain at the level of the spinal cord.17,18 Other physiological phenomena have also been observed with acupuncture by needling. They include vasodilation, increased serum cortisol, variations in serum glucose and cholesterol levels, increased white blood cell counts, and acid suppression.5 Their significance continues to be questioned.
Evidence-Based Approach
Many studies of acupuncture have methodological flaws. The biggest problem as yet unresolved is an appropriate placebo control.19 Sham acupuncture, which involves needling non-acupuncture points, is frequently the control of choice but has serious limitations.
In 1997, the landmark NIH consensus statement was probably the most important presentation of evidence supporting the efficacy of acupuncture.20 Conclusions made about the effectiveness of acupuncture were based on evidence from reliable studies. Many promising results emerged. Specific indications for use of acupuncture were identified on the basis of published reports of its effectiveness. Efficacy in treating dental pain and post-operative and chemotherapy-induced nausea were demonstrated. Research suggested its usefulness as an adjunct or alternative treatment for lower-back pain, osteoarthritis, addiction, and stroke rehabilitation. The panel also concluded that further research would likely uncover additional uses for acupuncture.
From the standpoint of acupuncture’s effectiveness, it can clearly benefit specific patient groups. It is most commonly used as a treatment for back pain.21 Since the NIH conference, further research has confirmed its effectiveness in treating a variety of medical conditions. (See Table 1, above)
Much of the ongoing research on acupuncture has focused on the use of functional magnetic resonance imaging of the brain, specifically on the areas that light up, or show brain activity, during activities or a state of pain.22-24 Acupuncture has been found to reduce the intensity of signals in such areas. The mechanism for the analgesic effects of acupuncture may be the result of reduced blood flow to the brain.24 Several studies have identified specific areas of the brain affected by pressure on various acupuncture points.25
Practical Aspects
Acupuncture treatments are extremely time efficient and require minimal equipment. They can be administered with the patient in the recumbent position or sitting upright. For initial sessions, I prefer the former, especially for younger males, who are more prone to vasovagal reactions. Any of several different methods of acupuncture can be used to stimulate points. In addition to needling, acupuncture can be conducted by electro-acupuncture, moxibustion, cupping, scraping, tapping, acupressure, or laser.
Most inpatient referrals are for pain management. Other common indications include post-operative or chemotherapy-induced nausea (emesis), anxiety, and prevention of withdrawal symptoms from narcotics.
Acupuncture Safety
Overall, acupuncture is a safe treatment method. Many large studies have confirmed that most types of acupuncture have a low rate of complications and that most of these complications are transient and minor in nature.28,29 They are incident-reporting studies, however, and have the limitations inherent in these studies. Nausea, dizziness, bruising, and needle pain are some of the most commonly reported. The rare but serious adverse events, such as pneumothorax, usually occur as a result of the practitioner’s poor training or technique.30
Future of Acupuncture
Public acceptance of, and demand for, acupuncture for pain relief is increasing. Additional clinical studies are needed, however, to expand the types of conditions for which acupuncture may be useful. It is essential to maintain a constant focus on safe practice, which would be aided by the establishment of a standardized accreditation and training system. Hospitals need to establish uniform credentialing guidelines similar to those for other procedures that require evidence of medical competence and safety.31
In February 2005, the Federal Acupuncture Coverage Act was introduced to Congress. If enacted, the measure would allow acupuncture to be covered under Part B for Medicare recipients.
The trend toward an integrated approach to patient therapy in large academic medical institutions is encouraging. The incorporation of the teaching of acupuncture within the current medical school curricula would no doubt complement this approach. TH
Joseph C. Charles, MD, FACP, is an assistant professor of medicine and division education coordinator for the Department of Hospital Internal Medicine at the Mayo Clinic Hospital Arizona.
References
- Ananth, S. Health Forum 2005 Complementary and Alternative Medicine Survey of Hospitals, July 19, 2006. News release, American Hospital Association.
- Veith I (trans). The Yellow Emperor’s Classic of Internal Medicine. Baltimore; Lippincott, Williams & Wilkins: 1949.
- Ming Z (trans). The Medical Classic of the Yellow Emperor. Beijing; Foreign Languages Press: 2001.
- Cassedy JH. Early uses of acupuncture in the United States, with an addendum (1826) by Franklin Bache, M.D. Bull N Y Acad Med. 1974 Sep;50(8):892-906.
- Osler W. The Principles and Practice of Medicine. New York: D. Appleton and Company; 1892.
- Reston J. Now, let me tell you about my appendectomy in Peking. New York Times. July 26, 1971.
- Mitchell BB. Educational and licensing requirements for acupuncturists. J Altern Complement Med. 1996 spring;2(1):33-35.
- Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993 Jan 28;328(4):246-252.
- Sherman KJ, Cherkin DC, Eisenberg DM, et al. The practice of acupuncture: who are the providers and what do they do? Ann Fam Med. 2005 Mar-Apr;3(2):151-158.
- Leake R, Broderick JE. Current licensure for acupuncture in the United States. Altern Ther Health Med. 1999 Jul;5(4):94-96.
- Shang C. The past, present, and future of meridian system research. In: Stux G, Hammerschlag R, eds. Clinical Acupuncture: Scientific Basis. Berlin: Springer; 2001:69-82.
- WHO Scientific Group on International Acupuncture Nomenclature. A proposed standard international acupuncture nomenclature: report of a WHO Scientific Group. Geneva: World Health Organization; 1991.
- Niboyet JEH. Nouvelles constatations sur les proprietes electriques des points chinois. Bull Soc Acupunct. 1938;4:30-79.
- Helms JM. Acupuncture Energetics: A Clinical Approach for Physicians. Berkeley, Calif.: Medical Acupuncture Publishers; 1995:23-24.
- De Vernejoul P, Albarede P, Darras JC. Study of acupuncture meridians using radioactive tracers [in French]. Bull Acad Natl Med. 1985 Oct;169(7):1071-1075.
- Simon J, Guiraud G, Esquerre JP, et al. Acupuncture meridians demythified. Contribution of radiotracer methodology [in French]. Presse Med. 1988 Jul 2;17(26):1341-1344.
- Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: endorphin implicated. Life Sci. 1976 Dec 1;19(11):1757-1762.
- Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374-383.
- Vincent C, Lewith G. Placebo controls for acupuncture studies. J R Soc Med. 1995 Apr;88(4):199-202.
- Acupuncture. NIH Consensus Statement 1997; 15:1-34
- Manheimer E, White A, Berman B, et al. Meta-analysis: acupuncture for low back pain. Ann Intern Med. 2005;142:651-663.
- Tank DW, Oqawa S, Uqurbil K. Mapping the brain with MRI. Curr Biol. 1992;525-528.
- Salvatore S. Brain imaging suggests acupuncture works, study says. [monograph on the Internet]. CNN.com with WebMD.com. Dec. 1, 1999. Available at http://archives.cnn.com/1999/HEALTH/alternative/12/01/brain.acupuncture/index.html. Last accessed April 14, 2007.
- Fang JL, Krings T, Weidemann J, et al. Functional MRI in healthy subjects during acupuncture: different effects of needle rotation in real and false acupoints. Neuroradiology. 2004;46:359-362.
- Cho ZH, Chung SC, Jones JP, et al. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci USA. 1998 Mar;95(5):2670-2673. Retraction in Cho ZH, Chung SC, Lee HJ, Wong EK, Min BI. Proc Natl Acad Sci USA. 2006 Jul 5;103(27):10527.
- Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998;44:129-138.
- Gam AN, Thorsen H, Lonnberg F. The effect of low-level laser therapy on musculoskeletal pain: a meta-analysis. Pain. 1993;52:63-66.
- White A, Hayhoe S, Hart A, et al. Adverse events following acupuncture: prospective survey of 32, 000 consultations with doctors and physiotherapists. BMJ. 2001 Sep 1;323(7311):485-486.
- MacPherson H, Thomas K, Walters S, et al. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ. 2001;323:486-487. Comment in BMJ. 2001 Sep 1;323(7311):467-8. BMJ. 2002 Jan 19;324(7330):170-1.
- Chauffe RJ, Duskin AL. Pneumothorax secondary to acupuncture therapy. South Med J. 2006;99:1297-1299.
- Cohen MH, Hrbek A, Davis RB, et al. Emerging credentialing practices, malpractice liability policies, and guidelines governing complementary and alternative medical practices and dietary supplement recommendations: a descriptive study of 19 integrative health care centers in the United States. Arch Intern Med. 2005;165:289-295.
- Linde K, Jobst K, Panton J. Acupuncture for chronic asthma. Cochrane Database Syst Rev. 2000;(2):CD000008.
- Kleijnen J, Ter Riet G, Knipschild P. Acupuncture and asthma: a review of controlled trials. Thorax. 1991;46:799-802.
- Ter Reit G, Kleijnen J, Knipschild P. A meta-analysis of studies into the effect of acupuncture on addiction. Br J Gen Pract. 1990;40:379-382.
- Vincent CA. A controlled trial of the treatment of migraine by acupuncture. Clin J Pain. 1989;5:305-312.
- White AR, Rampes H, Ernst E. Acupuncture for smoking cessation. Cochrane Database Syst Rev. 2002;(2):CD000009.
- Mann E. Using acupuncture and acupressure to treat postoperative emesis. Prof Nurse. 1999; 14:691-694.
- Macklin EA, Wayne PM, Kalish LA, et al. Stop hypertension with the acupuncture research program (SHARP): results of a randomized, controlled clinical trial. Hypertension. 2006;48:838-845.
- Lee JD, Chon JS, Jeong HK, et al. The cerebrovascular response to traditional acupuncture after stroke. Neuroradiology. 2003;45:780-784.
- Scharf HP, Mansmann U, Streitberger K, et al. Acupuncture and knee osteoarthritis: a three-armed randomized trial. Ann Intern Med. 2006;145:12-20.
- Martin DP, Sletten CD, Williams BA, et al. Improvement in fibromyalgia symptoms with acupuncture: results of a randomized controlled trial. Mayo Clin Proc. 2006;81:749-757.
- Lu DP, Lu GP. Anatomical relevance of some acupuncture points in the head and neck region that dictate medical or dental application depending on depth of needle insertion. Acupunct Electrother Res. 2003;28(3-4):145-156.
- Ernst E, Pittler MH. The effectiveness of acupuncture in treating acute dental pain: a systemic review. Br Dent J. 1998;184:443-447.
- Chen HM, Chen CH. Effects of acupressure at the Sanyinjiao point on primary dysmenorrhoea. J Adv Nurs. 2004;48(4):380-387.
- Pouresmail Z, Ibrahimzadeh R. Effects of acupressure and ibuprofen on the severity of primary dysmenorrhea. J Tradit Chin Med. 2002 Sep; 22(3):205-210.
- Linde K, Streng A, Jurgens S, et al. Acupuncture for patients with migraine: a randomized controlled trial. JAMA. 2005 May 4;293(17):2118-2125.
- Allais G, De Lorenzo C, Quirico PE, et al. Acupuncture in the prophylactic treatment of migraine without aura: a comparison with flunarizine. Headache. 2002 Oct;42(9):855-861.
- Green S, Buchbinder R, Barnsley L, et al. Acupuncture for lateral elbow pain. Cochrane Database Syst Rev. 2002;(1):CD003527. Review.
- Trinh KV, Phillips SD. Acupuncture for the alleviation of lateral epicondyle pain: a systematic review. Rheumatology (Oxford). 2004:43:1085-1090.
- David J, Townsend S, Sathanathan R, et al. The effect of acupuncture on patients with rheumatoid arthritis: a randomized, placebo-controlled cross-over study. Rheumatology (Oxford). 1999 Sep;38(9):864-869. Comment in Rheumatology (Oxford). 2000 Oct;39(10):1153-1154.
- Irnich D, Behrens N, Molzen H, et al. Randomised trial of acupuncture compared with conventional massage and “sham” laser acupuncture for treatment of chronic neck pain. BMJ. 2001 Jun 30;322(7302):1574-1578. Comment in BMJ. 2001 Dec 1;323(7324):1306-7.
- White P, Lewith G, Prescott P, et al. Acupuncture versus placebo for the treatment of chronic mechanical neck pain: a randomized, controlled trial. Ann Intern Med. 2004 Dec 21;141(12):911-919. Comment in Ann Intern Med. 2004 Dec 21;141(12):957-958. Ann Intern Med. 2005 May 17;142(10):873; author reply 873-874.
- Cheuk DK, Wong V. Acupuncture for epilepsy. Cochrane Database Syst Rev. 2006;(2):CD005062.
- Griggs C, Jensen J. Effectiveness of acupuncture for migraine: critical literature review. J Adv Nurs. 2006 May;54(4):491-501.
- Kim YH, Schiff E, Waalen J, et al. Efficacy of acupuncture for treating cocaine addiction: a review paper. J Addict Dis. 2005;24(4):115-132.
- Forbes A, Jackson S, Walter C, et al. Acupuncture for irritable bowel syndrome: a blinded placebo-controlled trial. World J Gastroenterol. 2005 Jul 14;11(26):4040-4044.
- Schneider A, Enck P, Streitberger K, et al. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649-654.
Drugs and the Elderly
Never before have doctors had such an abundance of therapeutic options. And—not surprisingly—elderly patients are taking more medications than ever.
A national survey from 1998 revealed that more than 40% of elderly American adults take five or more medications a day—and that’s at home. Meantime, drug-related complications have risen steadily.
In 2005, the United States spent $177 billion in the management of drug-related problems—$34 billion more than was spent on the drugs themselves.1 Because up to a third of adverse medication effects warrant a hospital admission, hospitalists are the front line in the diagnosis and treatment of these syndromes.
Additionally, medication-related consequences can complicate hospitalizations required for other reasons. They can be observed as frequently as weekly according to hospitalist Balazs Zsenits, MD, FACP, of Rochester (N.Y.) General Hospital—and they’re often serious. In fact, medication reactions are so frequently fatal they represent the fifth-leading cause of death in the United States.
As one might expect, the elderly are disproportionately affected by the potentially toxic consequences of medication. In fact, a 2005 study published in Pharmacotherapy revealed that more than two-thirds of hospitalized elderly adults had an adverse drug effect over a four-year period.2 Among the more common outcomes were constipation, falls, immobility, confusion, hip fractures, and a decline in functional status requiring nursing home placement. Moreover, the authors noted that drug side effects frequently mimicked other geriatric syndromes, prompting physicians to prescribe additional medication.
While multiple medications may be necessary to prevent the progression of disease in older people, the overuse and misuse of drugs has been linked to serious health problems, including hospitalizations and death.
Polypharmacy
Patients at greatest risk for a polypharmacy-associated medical complication are those taking five or more concurrent drugs, those with multiple physicians, patients with significant medical comorbidities or impairments in vision or dexterity, and individuals who have recently been hospitalized.4-5 At least 25% of elderly Americans fall into at least one of these categories
But polypharmacy is not the only reason elderly patients experience a disproportionately high rate of adverse medication effects. Age-related altered drug metabolism is also responsible for unexpected drug consequences in this age group.
Aging influences every aspect of physiologic drug processing. While the absorption of oral medications from the GI tract remains relatively constant in the absence of disease states and gastric pH altering medications, bioavailability and clearance dramatically change with aging. These changes become the most pronounced after age 75, when kidney and liver function become limited.
As people age, their total body water decreases, their lean body mass is reduced, and their percentage of body fat increases. This increase in body fat expands the volume of distribution for lipophilic drugs and also decreases the volume of distribution for hydrophilic drugs.6 The result is that water-soluble medications have an elevated active serum concentration, and lipid-soluble agents, while they may have a decreased serum concentration, have a prolonged half-life.
These effects are best exemplified by examining what happens after a geriatric patient takes diazepam. A lipid-soluble drug, diazepam and its metabolites will be stored in an increasingly large body compartment. This will temporarily decrease the serum level of the drug, but will prolong the half-life from an average of 20 hours to greater than 50 hours. Repeated dosing will quickly result in toxic serum levels, at which point the patient is at risk for CNS side effects as well as falls and fractures.
The aging process also affects the role of drug-binding serum proteins. The total serum protein level is usually maintained (while albumin levels may diminish slightly, increasing levels of alpha 1 antitrypsin keeps the total protein level normal). More significantly, the affinity of the serum proteins for protein-bound drugs lessens as patients age. The degree of plasma protein binding has a significant impact on the pharmacologic activity of the drug, because it is the free drug that is physiologically active and exerts the pharmacologic effect.
In treating patients with highly protein-bound drugs, like phenytoin, one should expect toxic reactions at a normal serum level because more of the drug is unbound, and, hence, active. Elderly patients with low albumin levels secondary to malnutrition or liver disease will have an even more pronounced effect.
Effects of Metabolism
Many drugs undergo hepatic metabolism to produce more soluble forms for subsequent elimination through renal excretion. Though hepatic metabolism is affected by multiple variables including genotype, lifestyle, hepatic blood flow, hepatic diseases, and interactions with other medications, aging also plays a significant role.7
Of the two biotransformation systems through which hepatic metabolism occurs, it is the cytochrome P450 system (Phase I) most affected by increasing years. For most drugs, this leads to increased serum levels of the unmetabolized entity, leading to a greater potential for toxicity. Disease states that reduce blood flow to the liver, like congestive heart failure and cirrhosis, further inhibit this process. For drugs whose pharmacological activity requires biotransformation from a pro-drug form, inhibition can lead to decreased efficacy.
In contrast, Phase II metabolism, including acetylation, sulfonation, conjugation, and glucuronidation, is little influenced by advanced age.
Drug Elimination
The renal elimination of drugs is altered by aging, although there is significant variation between individuals for any given decade.8 Drug excretion does correlate with creatinine clearance, which declines by 50% by age 75. However, because lean body mass decreases with aging, the serum creatinine level tends to overestimate the creatinine clearance of older adults.
Utilization of the Cockroft-Gault formula (Figure 1, above) allows for an accurate estimation of the creatinine clearance in these patients.9 For example, a 25-year-old man and an 85-year-old man, each weighing 158 pounds and having a serum creatinine value of 1 mg per dL, would have different estimated creatinine clearance even though their serum creatinine value is the same. The younger man would have an estimated creatinine clearance of 115 mL per minute, while the older man’s would be 55 mL per minute.
Approximating creatinine clearance is particularly important when prescribing medications that have a narrow therapeutic index (aminoglycosides, lithium, digoxin, procainamide, vancomycin). Even minimally excessive doses of these drugs will result in a prolonged the half-life, and an increased potential for toxic effects.
Expect and account for these alterations in drug metabolism in elderly patients. Typical changes result in increased active serum concentrations of the drug and extended half-life. Elevated drug concentrations result in more adverse drug events, and these include not only known complications, but also uncommon problems such as blood dyscrasias. If a rare adverse drug reaction does occur, it is most likely to happen in an elderly person.
The Acute Care Setting
In light of the physiologic changes associated with aging, as well as the problems posed by taking multiple medications, it is clear that active intervention is required to prevent adverse drug reactions in geriatric patients.
A large cohort study of Medicare enrollees with more than 30,000 patient-years of observation found that 28% of adverse drug reactions were potentially avoidable. Most errors occurred during prescribing and monitoring. A number of strategies have been proposed for reducing these unwanted medication consequences in the hospital setting, including:
- Avoid inappropriate drug prescribing;
- Avoid overprescribing;
- Implement age-appropriate dosing; and
- Encourage a multidisciplinary ap-proach.
Drugs to Avoid
Though precise clinical data regarding which medications are harmful to elderly patients in the acute care setting is lacking, multiple expert panels have attempted to delineate which drugs should be generally avoided in this population (Table 1, above).
The most notable of these evaluations is the Beers criteria, a frequently updated set of medications deemed inappropriate for use in geriatric patients. Most recently amended in 2003, this list is formulated by experts in pharmacology and geriatrics, and has been validated in large studies as a useful tool for decreasing medication-related problems in the nursing home setting.10
Though a 2006 study of hospital morbidity found that adverse drug reactions in the acute care setting often occur from drugs not listed in the Beers criteria, avoiding medications like those listed above is still a useful tool in preventing side effects.11-12
Avoid Overprescribing
To prevent a polypharmacy-induced iatrogenic illness, it is important to consider any new signs and symptoms to be a possible consequence of current drug therapy. Steps for reducing polypharmacy include:
- Get into the habit of identifying all drugs by generic name and drug class;
- Make certain the drug being prescribed has a clinical indication;
- Know the side-effect profile of the drugs being prescribed;
- Understand how changes in drug distribution, metabolism, and elimination associated with aging increase the risk of adverse drug events;
- Stop any drug without known benefit;
- Stop any drug without a clinical indication;
- Attempt to substitute a less-toxic drug; and
- Be aware of the prescribing-cascade treating an adverse drug reaction as an illness with another drug.
Age-Appropriate Dosing
When starting a new drug, start with a low dose and titrate slowly to the desired clinical effect. While the manufacturers of many commonly used medications do not delineate the lower-dosage recommendations necessary for elderly patients, you can bypass this problem by starting with one-third to half the recommended dosage.
After observing that the patient tolerates the new drug, slowly increase the dose until the desired result is obtained. This approach is particularly important in minimizing potential harmful drug effects in patients with severely reduced renal function.14
Multidisciplinary Approach
In its 2001 report “Crossing the Quality Chasm: A New Health System of the 21st Century,” the U.S. Institute of Medicine declared: “The current care systems cannot do the job. Trying harder will not work. If we want safer, higher-quality care, we will need to have redesigned systems of care, including the use of information technology to support clinical and administrative processes.”
While hospitalists are on the front line for preventing adverse drug reactions, they can’t do it by themselves. Here are a few tips for making your job easier:
- Request that medications inappropriate for geriatric patients (based on the Beers criteria) be notated as such by the pharmacist;
- Ask for a geriatric dosing option in the computer-based medication ordering system;
- Flag charts of patients with previous adverse drug effects with the name of the offending drug;
- Warn nurses and other caregivers to monitor for specific side effects; and
- Advocate that midlevel providers receive hospital-based training in the prevention of medication-related adverse events.
The elderly portion of the population is expanding more rapidly than the population as a whole, and the recognition and prevention of medication side effects in this group is one of the most critical safety and economic issues facing the healthcare system today. While the magnitude of this problem demands multidisciplinary involvement, hospitalists can be key players in making a difference. TH
Dr. Landis is a rheumatologist and a freelance writer
References
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22-29;277(4):307-311. Comment in: JAMA. 1997 Jan 22-29;277(4):341-3422: JAMA. 1997 May 7;277(17):1351-1352; author reply 1353-1354.
- Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25(11):1636-1645. Comment in: Pharmacotherapy. 2006 Jun;26(6):886-887; discussion 887.
- Byron C, Hochberg MC. Changing the patterns of Coxibs/NSAIDs prescribing: balancing CV and GI risks. Medscape. Available at www.medscape.com/viewprogram/5060. Last accessed May 2, 2007.
- Shapiro K. The Complexities of Geriatric Pain Management. 20th Annual Meeting of the American Pain Society. Medscape CME. Available at www.medscape.com/viewarticle/416593. Last accessed May 2, 2007.
- Lau DT, Kasper JD, Potter DE, et al. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res. 2004 Oct; 39(5):1257-1276.
- Longa GJ, Cross RE. Laboratory Monitoring of Drug Therapy. Part II: Variable Protein Binding and Free (Unbound) Drug Concentration. Bull Lab Me. 1984;80:1-6. 7. Chutka DS, Evans JM, Fleming KC, et al. Symposium on geriatrics—Part I: Drug prescribing for elderly patients. Mayo Clin Proc. 1995 Jul;70(7):685-693.
- Feely J, Coakley D. Altered pharmacodynamics in the elderly. Clin Geriatr Med. 1990 May; 6(2): 269-283.
- Williams CM. Using medications appropriately in older adults. Am Fam Phys. 2002 Nov 15;66(10):1917-1924.
- Fick DN, Cooper JW, Wade WE. Updating the Beers criteria for potentially inappropriate medication use in older adults. Arch Intern Med. 2003 Dec 8-22;163(22):2716-2724. Erratum in: Arch Intern Med. 2004 Feb 9;164(3):298. Comment in: Arch Intern Med. 2004 Aug 9-23;164(15):1701.
- Johnston PE, France DJ, Byrne DW, et al. Assessment of adverse drug events among patients in a tertiary care medical center. Am J Health Syst Pharm., 2006;63(22):2218-2227.
- Page RL, Ruscin JM. The risk of adverse drug events and hospital related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006 Dec;4(4):297-305.
- Avidan AY. Sleep changes and disorders in the elderly patient. Curr Neurol Neurosci Rep. 2002 Mar;2(2):178-185.
- Pugh MJV, Fincke G, Bierman AS, et al. Potentially inappropriate prescribing in elderly veterans: Are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc. 2005 Aug;53(8):1282-1289.
Never before have doctors had such an abundance of therapeutic options. And—not surprisingly—elderly patients are taking more medications than ever.
A national survey from 1998 revealed that more than 40% of elderly American adults take five or more medications a day—and that’s at home. Meantime, drug-related complications have risen steadily.
In 2005, the United States spent $177 billion in the management of drug-related problems—$34 billion more than was spent on the drugs themselves.1 Because up to a third of adverse medication effects warrant a hospital admission, hospitalists are the front line in the diagnosis and treatment of these syndromes.
Additionally, medication-related consequences can complicate hospitalizations required for other reasons. They can be observed as frequently as weekly according to hospitalist Balazs Zsenits, MD, FACP, of Rochester (N.Y.) General Hospital—and they’re often serious. In fact, medication reactions are so frequently fatal they represent the fifth-leading cause of death in the United States.
As one might expect, the elderly are disproportionately affected by the potentially toxic consequences of medication. In fact, a 2005 study published in Pharmacotherapy revealed that more than two-thirds of hospitalized elderly adults had an adverse drug effect over a four-year period.2 Among the more common outcomes were constipation, falls, immobility, confusion, hip fractures, and a decline in functional status requiring nursing home placement. Moreover, the authors noted that drug side effects frequently mimicked other geriatric syndromes, prompting physicians to prescribe additional medication.
While multiple medications may be necessary to prevent the progression of disease in older people, the overuse and misuse of drugs has been linked to serious health problems, including hospitalizations and death.
Polypharmacy
Patients at greatest risk for a polypharmacy-associated medical complication are those taking five or more concurrent drugs, those with multiple physicians, patients with significant medical comorbidities or impairments in vision or dexterity, and individuals who have recently been hospitalized.4-5 At least 25% of elderly Americans fall into at least one of these categories
But polypharmacy is not the only reason elderly patients experience a disproportionately high rate of adverse medication effects. Age-related altered drug metabolism is also responsible for unexpected drug consequences in this age group.
Aging influences every aspect of physiologic drug processing. While the absorption of oral medications from the GI tract remains relatively constant in the absence of disease states and gastric pH altering medications, bioavailability and clearance dramatically change with aging. These changes become the most pronounced after age 75, when kidney and liver function become limited.
As people age, their total body water decreases, their lean body mass is reduced, and their percentage of body fat increases. This increase in body fat expands the volume of distribution for lipophilic drugs and also decreases the volume of distribution for hydrophilic drugs.6 The result is that water-soluble medications have an elevated active serum concentration, and lipid-soluble agents, while they may have a decreased serum concentration, have a prolonged half-life.
These effects are best exemplified by examining what happens after a geriatric patient takes diazepam. A lipid-soluble drug, diazepam and its metabolites will be stored in an increasingly large body compartment. This will temporarily decrease the serum level of the drug, but will prolong the half-life from an average of 20 hours to greater than 50 hours. Repeated dosing will quickly result in toxic serum levels, at which point the patient is at risk for CNS side effects as well as falls and fractures.
The aging process also affects the role of drug-binding serum proteins. The total serum protein level is usually maintained (while albumin levels may diminish slightly, increasing levels of alpha 1 antitrypsin keeps the total protein level normal). More significantly, the affinity of the serum proteins for protein-bound drugs lessens as patients age. The degree of plasma protein binding has a significant impact on the pharmacologic activity of the drug, because it is the free drug that is physiologically active and exerts the pharmacologic effect.
In treating patients with highly protein-bound drugs, like phenytoin, one should expect toxic reactions at a normal serum level because more of the drug is unbound, and, hence, active. Elderly patients with low albumin levels secondary to malnutrition or liver disease will have an even more pronounced effect.
Effects of Metabolism
Many drugs undergo hepatic metabolism to produce more soluble forms for subsequent elimination through renal excretion. Though hepatic metabolism is affected by multiple variables including genotype, lifestyle, hepatic blood flow, hepatic diseases, and interactions with other medications, aging also plays a significant role.7
Of the two biotransformation systems through which hepatic metabolism occurs, it is the cytochrome P450 system (Phase I) most affected by increasing years. For most drugs, this leads to increased serum levels of the unmetabolized entity, leading to a greater potential for toxicity. Disease states that reduce blood flow to the liver, like congestive heart failure and cirrhosis, further inhibit this process. For drugs whose pharmacological activity requires biotransformation from a pro-drug form, inhibition can lead to decreased efficacy.
In contrast, Phase II metabolism, including acetylation, sulfonation, conjugation, and glucuronidation, is little influenced by advanced age.
Drug Elimination
The renal elimination of drugs is altered by aging, although there is significant variation between individuals for any given decade.8 Drug excretion does correlate with creatinine clearance, which declines by 50% by age 75. However, because lean body mass decreases with aging, the serum creatinine level tends to overestimate the creatinine clearance of older adults.
Utilization of the Cockroft-Gault formula (Figure 1, above) allows for an accurate estimation of the creatinine clearance in these patients.9 For example, a 25-year-old man and an 85-year-old man, each weighing 158 pounds and having a serum creatinine value of 1 mg per dL, would have different estimated creatinine clearance even though their serum creatinine value is the same. The younger man would have an estimated creatinine clearance of 115 mL per minute, while the older man’s would be 55 mL per minute.
Approximating creatinine clearance is particularly important when prescribing medications that have a narrow therapeutic index (aminoglycosides, lithium, digoxin, procainamide, vancomycin). Even minimally excessive doses of these drugs will result in a prolonged the half-life, and an increased potential for toxic effects.
Expect and account for these alterations in drug metabolism in elderly patients. Typical changes result in increased active serum concentrations of the drug and extended half-life. Elevated drug concentrations result in more adverse drug events, and these include not only known complications, but also uncommon problems such as blood dyscrasias. If a rare adverse drug reaction does occur, it is most likely to happen in an elderly person.
The Acute Care Setting
In light of the physiologic changes associated with aging, as well as the problems posed by taking multiple medications, it is clear that active intervention is required to prevent adverse drug reactions in geriatric patients.
A large cohort study of Medicare enrollees with more than 30,000 patient-years of observation found that 28% of adverse drug reactions were potentially avoidable. Most errors occurred during prescribing and monitoring. A number of strategies have been proposed for reducing these unwanted medication consequences in the hospital setting, including:
- Avoid inappropriate drug prescribing;
- Avoid overprescribing;
- Implement age-appropriate dosing; and
- Encourage a multidisciplinary ap-proach.
Drugs to Avoid
Though precise clinical data regarding which medications are harmful to elderly patients in the acute care setting is lacking, multiple expert panels have attempted to delineate which drugs should be generally avoided in this population (Table 1, above).
The most notable of these evaluations is the Beers criteria, a frequently updated set of medications deemed inappropriate for use in geriatric patients. Most recently amended in 2003, this list is formulated by experts in pharmacology and geriatrics, and has been validated in large studies as a useful tool for decreasing medication-related problems in the nursing home setting.10
Though a 2006 study of hospital morbidity found that adverse drug reactions in the acute care setting often occur from drugs not listed in the Beers criteria, avoiding medications like those listed above is still a useful tool in preventing side effects.11-12
Avoid Overprescribing
To prevent a polypharmacy-induced iatrogenic illness, it is important to consider any new signs and symptoms to be a possible consequence of current drug therapy. Steps for reducing polypharmacy include:
- Get into the habit of identifying all drugs by generic name and drug class;
- Make certain the drug being prescribed has a clinical indication;
- Know the side-effect profile of the drugs being prescribed;
- Understand how changes in drug distribution, metabolism, and elimination associated with aging increase the risk of adverse drug events;
- Stop any drug without known benefit;
- Stop any drug without a clinical indication;
- Attempt to substitute a less-toxic drug; and
- Be aware of the prescribing-cascade treating an adverse drug reaction as an illness with another drug.
Age-Appropriate Dosing
When starting a new drug, start with a low dose and titrate slowly to the desired clinical effect. While the manufacturers of many commonly used medications do not delineate the lower-dosage recommendations necessary for elderly patients, you can bypass this problem by starting with one-third to half the recommended dosage.
After observing that the patient tolerates the new drug, slowly increase the dose until the desired result is obtained. This approach is particularly important in minimizing potential harmful drug effects in patients with severely reduced renal function.14
Multidisciplinary Approach
In its 2001 report “Crossing the Quality Chasm: A New Health System of the 21st Century,” the U.S. Institute of Medicine declared: “The current care systems cannot do the job. Trying harder will not work. If we want safer, higher-quality care, we will need to have redesigned systems of care, including the use of information technology to support clinical and administrative processes.”
While hospitalists are on the front line for preventing adverse drug reactions, they can’t do it by themselves. Here are a few tips for making your job easier:
- Request that medications inappropriate for geriatric patients (based on the Beers criteria) be notated as such by the pharmacist;
- Ask for a geriatric dosing option in the computer-based medication ordering system;
- Flag charts of patients with previous adverse drug effects with the name of the offending drug;
- Warn nurses and other caregivers to monitor for specific side effects; and
- Advocate that midlevel providers receive hospital-based training in the prevention of medication-related adverse events.
The elderly portion of the population is expanding more rapidly than the population as a whole, and the recognition and prevention of medication side effects in this group is one of the most critical safety and economic issues facing the healthcare system today. While the magnitude of this problem demands multidisciplinary involvement, hospitalists can be key players in making a difference. TH
Dr. Landis is a rheumatologist and a freelance writer
References
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22-29;277(4):307-311. Comment in: JAMA. 1997 Jan 22-29;277(4):341-3422: JAMA. 1997 May 7;277(17):1351-1352; author reply 1353-1354.
- Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25(11):1636-1645. Comment in: Pharmacotherapy. 2006 Jun;26(6):886-887; discussion 887.
- Byron C, Hochberg MC. Changing the patterns of Coxibs/NSAIDs prescribing: balancing CV and GI risks. Medscape. Available at www.medscape.com/viewprogram/5060. Last accessed May 2, 2007.
- Shapiro K. The Complexities of Geriatric Pain Management. 20th Annual Meeting of the American Pain Society. Medscape CME. Available at www.medscape.com/viewarticle/416593. Last accessed May 2, 2007.
- Lau DT, Kasper JD, Potter DE, et al. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res. 2004 Oct; 39(5):1257-1276.
- Longa GJ, Cross RE. Laboratory Monitoring of Drug Therapy. Part II: Variable Protein Binding and Free (Unbound) Drug Concentration. Bull Lab Me. 1984;80:1-6. 7. Chutka DS, Evans JM, Fleming KC, et al. Symposium on geriatrics—Part I: Drug prescribing for elderly patients. Mayo Clin Proc. 1995 Jul;70(7):685-693.
- Feely J, Coakley D. Altered pharmacodynamics in the elderly. Clin Geriatr Med. 1990 May; 6(2): 269-283.
- Williams CM. Using medications appropriately in older adults. Am Fam Phys. 2002 Nov 15;66(10):1917-1924.
- Fick DN, Cooper JW, Wade WE. Updating the Beers criteria for potentially inappropriate medication use in older adults. Arch Intern Med. 2003 Dec 8-22;163(22):2716-2724. Erratum in: Arch Intern Med. 2004 Feb 9;164(3):298. Comment in: Arch Intern Med. 2004 Aug 9-23;164(15):1701.
- Johnston PE, France DJ, Byrne DW, et al. Assessment of adverse drug events among patients in a tertiary care medical center. Am J Health Syst Pharm., 2006;63(22):2218-2227.
- Page RL, Ruscin JM. The risk of adverse drug events and hospital related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006 Dec;4(4):297-305.
- Avidan AY. Sleep changes and disorders in the elderly patient. Curr Neurol Neurosci Rep. 2002 Mar;2(2):178-185.
- Pugh MJV, Fincke G, Bierman AS, et al. Potentially inappropriate prescribing in elderly veterans: Are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc. 2005 Aug;53(8):1282-1289.
Never before have doctors had such an abundance of therapeutic options. And—not surprisingly—elderly patients are taking more medications than ever.
A national survey from 1998 revealed that more than 40% of elderly American adults take five or more medications a day—and that’s at home. Meantime, drug-related complications have risen steadily.
In 2005, the United States spent $177 billion in the management of drug-related problems—$34 billion more than was spent on the drugs themselves.1 Because up to a third of adverse medication effects warrant a hospital admission, hospitalists are the front line in the diagnosis and treatment of these syndromes.
Additionally, medication-related consequences can complicate hospitalizations required for other reasons. They can be observed as frequently as weekly according to hospitalist Balazs Zsenits, MD, FACP, of Rochester (N.Y.) General Hospital—and they’re often serious. In fact, medication reactions are so frequently fatal they represent the fifth-leading cause of death in the United States.
As one might expect, the elderly are disproportionately affected by the potentially toxic consequences of medication. In fact, a 2005 study published in Pharmacotherapy revealed that more than two-thirds of hospitalized elderly adults had an adverse drug effect over a four-year period.2 Among the more common outcomes were constipation, falls, immobility, confusion, hip fractures, and a decline in functional status requiring nursing home placement. Moreover, the authors noted that drug side effects frequently mimicked other geriatric syndromes, prompting physicians to prescribe additional medication.
While multiple medications may be necessary to prevent the progression of disease in older people, the overuse and misuse of drugs has been linked to serious health problems, including hospitalizations and death.
Polypharmacy
Patients at greatest risk for a polypharmacy-associated medical complication are those taking five or more concurrent drugs, those with multiple physicians, patients with significant medical comorbidities or impairments in vision or dexterity, and individuals who have recently been hospitalized.4-5 At least 25% of elderly Americans fall into at least one of these categories
But polypharmacy is not the only reason elderly patients experience a disproportionately high rate of adverse medication effects. Age-related altered drug metabolism is also responsible for unexpected drug consequences in this age group.
Aging influences every aspect of physiologic drug processing. While the absorption of oral medications from the GI tract remains relatively constant in the absence of disease states and gastric pH altering medications, bioavailability and clearance dramatically change with aging. These changes become the most pronounced after age 75, when kidney and liver function become limited.
As people age, their total body water decreases, their lean body mass is reduced, and their percentage of body fat increases. This increase in body fat expands the volume of distribution for lipophilic drugs and also decreases the volume of distribution for hydrophilic drugs.6 The result is that water-soluble medications have an elevated active serum concentration, and lipid-soluble agents, while they may have a decreased serum concentration, have a prolonged half-life.
These effects are best exemplified by examining what happens after a geriatric patient takes diazepam. A lipid-soluble drug, diazepam and its metabolites will be stored in an increasingly large body compartment. This will temporarily decrease the serum level of the drug, but will prolong the half-life from an average of 20 hours to greater than 50 hours. Repeated dosing will quickly result in toxic serum levels, at which point the patient is at risk for CNS side effects as well as falls and fractures.
The aging process also affects the role of drug-binding serum proteins. The total serum protein level is usually maintained (while albumin levels may diminish slightly, increasing levels of alpha 1 antitrypsin keeps the total protein level normal). More significantly, the affinity of the serum proteins for protein-bound drugs lessens as patients age. The degree of plasma protein binding has a significant impact on the pharmacologic activity of the drug, because it is the free drug that is physiologically active and exerts the pharmacologic effect.
In treating patients with highly protein-bound drugs, like phenytoin, one should expect toxic reactions at a normal serum level because more of the drug is unbound, and, hence, active. Elderly patients with low albumin levels secondary to malnutrition or liver disease will have an even more pronounced effect.
Effects of Metabolism
Many drugs undergo hepatic metabolism to produce more soluble forms for subsequent elimination through renal excretion. Though hepatic metabolism is affected by multiple variables including genotype, lifestyle, hepatic blood flow, hepatic diseases, and interactions with other medications, aging also plays a significant role.7
Of the two biotransformation systems through which hepatic metabolism occurs, it is the cytochrome P450 system (Phase I) most affected by increasing years. For most drugs, this leads to increased serum levels of the unmetabolized entity, leading to a greater potential for toxicity. Disease states that reduce blood flow to the liver, like congestive heart failure and cirrhosis, further inhibit this process. For drugs whose pharmacological activity requires biotransformation from a pro-drug form, inhibition can lead to decreased efficacy.
In contrast, Phase II metabolism, including acetylation, sulfonation, conjugation, and glucuronidation, is little influenced by advanced age.
Drug Elimination
The renal elimination of drugs is altered by aging, although there is significant variation between individuals for any given decade.8 Drug excretion does correlate with creatinine clearance, which declines by 50% by age 75. However, because lean body mass decreases with aging, the serum creatinine level tends to overestimate the creatinine clearance of older adults.
Utilization of the Cockroft-Gault formula (Figure 1, above) allows for an accurate estimation of the creatinine clearance in these patients.9 For example, a 25-year-old man and an 85-year-old man, each weighing 158 pounds and having a serum creatinine value of 1 mg per dL, would have different estimated creatinine clearance even though their serum creatinine value is the same. The younger man would have an estimated creatinine clearance of 115 mL per minute, while the older man’s would be 55 mL per minute.
Approximating creatinine clearance is particularly important when prescribing medications that have a narrow therapeutic index (aminoglycosides, lithium, digoxin, procainamide, vancomycin). Even minimally excessive doses of these drugs will result in a prolonged the half-life, and an increased potential for toxic effects.
Expect and account for these alterations in drug metabolism in elderly patients. Typical changes result in increased active serum concentrations of the drug and extended half-life. Elevated drug concentrations result in more adverse drug events, and these include not only known complications, but also uncommon problems such as blood dyscrasias. If a rare adverse drug reaction does occur, it is most likely to happen in an elderly person.
The Acute Care Setting
In light of the physiologic changes associated with aging, as well as the problems posed by taking multiple medications, it is clear that active intervention is required to prevent adverse drug reactions in geriatric patients.
A large cohort study of Medicare enrollees with more than 30,000 patient-years of observation found that 28% of adverse drug reactions were potentially avoidable. Most errors occurred during prescribing and monitoring. A number of strategies have been proposed for reducing these unwanted medication consequences in the hospital setting, including:
- Avoid inappropriate drug prescribing;
- Avoid overprescribing;
- Implement age-appropriate dosing; and
- Encourage a multidisciplinary ap-proach.
Drugs to Avoid
Though precise clinical data regarding which medications are harmful to elderly patients in the acute care setting is lacking, multiple expert panels have attempted to delineate which drugs should be generally avoided in this population (Table 1, above).
The most notable of these evaluations is the Beers criteria, a frequently updated set of medications deemed inappropriate for use in geriatric patients. Most recently amended in 2003, this list is formulated by experts in pharmacology and geriatrics, and has been validated in large studies as a useful tool for decreasing medication-related problems in the nursing home setting.10
Though a 2006 study of hospital morbidity found that adverse drug reactions in the acute care setting often occur from drugs not listed in the Beers criteria, avoiding medications like those listed above is still a useful tool in preventing side effects.11-12
Avoid Overprescribing
To prevent a polypharmacy-induced iatrogenic illness, it is important to consider any new signs and symptoms to be a possible consequence of current drug therapy. Steps for reducing polypharmacy include:
- Get into the habit of identifying all drugs by generic name and drug class;
- Make certain the drug being prescribed has a clinical indication;
- Know the side-effect profile of the drugs being prescribed;
- Understand how changes in drug distribution, metabolism, and elimination associated with aging increase the risk of adverse drug events;
- Stop any drug without known benefit;
- Stop any drug without a clinical indication;
- Attempt to substitute a less-toxic drug; and
- Be aware of the prescribing-cascade treating an adverse drug reaction as an illness with another drug.
Age-Appropriate Dosing
When starting a new drug, start with a low dose and titrate slowly to the desired clinical effect. While the manufacturers of many commonly used medications do not delineate the lower-dosage recommendations necessary for elderly patients, you can bypass this problem by starting with one-third to half the recommended dosage.
After observing that the patient tolerates the new drug, slowly increase the dose until the desired result is obtained. This approach is particularly important in minimizing potential harmful drug effects in patients with severely reduced renal function.14
Multidisciplinary Approach
In its 2001 report “Crossing the Quality Chasm: A New Health System of the 21st Century,” the U.S. Institute of Medicine declared: “The current care systems cannot do the job. Trying harder will not work. If we want safer, higher-quality care, we will need to have redesigned systems of care, including the use of information technology to support clinical and administrative processes.”
While hospitalists are on the front line for preventing adverse drug reactions, they can’t do it by themselves. Here are a few tips for making your job easier:
- Request that medications inappropriate for geriatric patients (based on the Beers criteria) be notated as such by the pharmacist;
- Ask for a geriatric dosing option in the computer-based medication ordering system;
- Flag charts of patients with previous adverse drug effects with the name of the offending drug;
- Warn nurses and other caregivers to monitor for specific side effects; and
- Advocate that midlevel providers receive hospital-based training in the prevention of medication-related adverse events.
The elderly portion of the population is expanding more rapidly than the population as a whole, and the recognition and prevention of medication side effects in this group is one of the most critical safety and economic issues facing the healthcare system today. While the magnitude of this problem demands multidisciplinary involvement, hospitalists can be key players in making a difference. TH
Dr. Landis is a rheumatologist and a freelance writer
References
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22-29;277(4):307-311. Comment in: JAMA. 1997 Jan 22-29;277(4):341-3422: JAMA. 1997 May 7;277(17):1351-1352; author reply 1353-1354.
- Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25(11):1636-1645. Comment in: Pharmacotherapy. 2006 Jun;26(6):886-887; discussion 887.
- Byron C, Hochberg MC. Changing the patterns of Coxibs/NSAIDs prescribing: balancing CV and GI risks. Medscape. Available at www.medscape.com/viewprogram/5060. Last accessed May 2, 2007.
- Shapiro K. The Complexities of Geriatric Pain Management. 20th Annual Meeting of the American Pain Society. Medscape CME. Available at www.medscape.com/viewarticle/416593. Last accessed May 2, 2007.
- Lau DT, Kasper JD, Potter DE, et al. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res. 2004 Oct; 39(5):1257-1276.
- Longa GJ, Cross RE. Laboratory Monitoring of Drug Therapy. Part II: Variable Protein Binding and Free (Unbound) Drug Concentration. Bull Lab Me. 1984;80:1-6. 7. Chutka DS, Evans JM, Fleming KC, et al. Symposium on geriatrics—Part I: Drug prescribing for elderly patients. Mayo Clin Proc. 1995 Jul;70(7):685-693.
- Feely J, Coakley D. Altered pharmacodynamics in the elderly. Clin Geriatr Med. 1990 May; 6(2): 269-283.
- Williams CM. Using medications appropriately in older adults. Am Fam Phys. 2002 Nov 15;66(10):1917-1924.
- Fick DN, Cooper JW, Wade WE. Updating the Beers criteria for potentially inappropriate medication use in older adults. Arch Intern Med. 2003 Dec 8-22;163(22):2716-2724. Erratum in: Arch Intern Med. 2004 Feb 9;164(3):298. Comment in: Arch Intern Med. 2004 Aug 9-23;164(15):1701.
- Johnston PE, France DJ, Byrne DW, et al. Assessment of adverse drug events among patients in a tertiary care medical center. Am J Health Syst Pharm., 2006;63(22):2218-2227.
- Page RL, Ruscin JM. The risk of adverse drug events and hospital related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006 Dec;4(4):297-305.
- Avidan AY. Sleep changes and disorders in the elderly patient. Curr Neurol Neurosci Rep. 2002 Mar;2(2):178-185.
- Pugh MJV, Fincke G, Bierman AS, et al. Potentially inappropriate prescribing in elderly veterans: Are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc. 2005 Aug;53(8):1282-1289.
Mining for Data
The expansion of information technology (IT) in U.S. hospitals is an evolutionary process. Billing, collections, and admission and discharge records have long been computerized, but now electronic medical administration records, patient electronic health records, and computerized physician order entry (CPOE) systems are joining the ranks.
Hospitalists are more likely to encounter sophisticated IT systems in larger, urban, or teaching hospitals, according to a 2005 survey by the American Hospital Association.1
The Hospitalist’s first installment about hospital informatics (“Charts to Screens,” January 2007, p. 25) focused on the challenges of health IT and the barriers to effective adoption of computer-based documentation systems. This installment explores the potentially rich vein of data available to hospitalists from those information systems and the opportunities for research and QI applications.
The mechanics of conducting clinical research and QI projects will depend to a large extent on the progress each hospital medical group’s institution has made in the IT adoption process. Some say hospitalists have powerful contributions to make in influencing how the IT process evolves so their research opportunities will also improve.
QI Topics
Data in information systems differ from hospital to hospital, says Tejal K. Gandhi, MD, MPH, director of patient safety at Boston’s Brigham and Women’s Hospital (BWH) and assistant professor of medicine in the Department of Medicine at Harvard Medical School in Boston.
Dr. Gandhi’s research focuses on redesigning hospital and outpatient processes to improve patient safety. She notes that hospitalists can take advantage of data the hospital is collecting to satisfy its reporting requirements to spearhead more quality-improvement efforts.
“For example,” says Dr. Gandhi, “the hospital has to document how it’s doing on pneumonia measures, acute myocardial infarction measures (was the patient having a heart attack given aspirin and a beta-blocker?), and others. These are fruitful topics for quality-improvement projects.”
Hospitalist Andrew Karson, MD, MPH, associate director of the Decision Support and Quality Management Unit and associate program director for the Internal Medicine Residency Program at Massachusetts General Hospital, Boston, also focuses on patient safety issues in his research. Given hospitalists’ knowledge of decision-making systems in the hospital, they are in a unique situation to initiate such projects, he believes.
For example, Dr. Karson participated in a study initiated by colleague Christopher L. Roy, MD, associate director of the hospitalist program at BWH.2 Dr. Roy posited that pending test results could be an important patient safety issue and, at the very least, might affect continuity of care. The researchers identified 2,644 consecutive patients discharged from BWH and Massachusetts General between February and June 2004. During that time, a mixture of hospitalists and non-hospitalists were responsible for discharging patients on house staff and non-house-staff services. Using a Results Manager application integrated into each patient’s electronic medical record (EMR) at the hospitals, the team identified and tracked pending laboratory and/or radiologic test results that had been returned after the patients were discharged.
The team used physician reviewers to determine whether the pending test results were potentially actionable. They found that 41% (1,095) of the discharged patients had a total of 2,033 test results return after their discharge. Of those tests, the physician reviewer determined that 9.4% (191) were potentially actionable. Examples of actionable results of which discharging physicians had been unaware included a levofloxacin-resistant Klebsiella infection in a patient being treated with levofloxacin, and a thyroid-stimulating hormone level that was dangerously low in a patient with rapid atrial fibrillation. A coauthor of the study, Eric G. Poon, MD, MPH, Division of General Medicine and Primary Care at BWH, is working on a results-management system that will automatically alert hospitalists and other physicians in the process of discharging patients when those patients are awaiting test results.
Research Potential
CPOE systems afford opportunities to delve further into clinical research and QI projects.
The flow of care in hospitals is inextricably linked with writing orders—for medications, tests, consultations, or interventional care processes. “Interfacing with CPOEs, therefore, can help influence the way care is practiced more broadly for our patients,” says Dr. Karson. “By embedding rules and decision support elements within our CPOE systems, we can improve the quality and safety of the care that we provide.”
The effect of CPOE on ICU patient care was highlighted in a 2005 study conducted by intensivist Stephen P. Hoffmann, MD, medical director, ICU, and associate professor of medicine at Ohio State University Medical Center, Columbus, and his colleagues. The team compared orders for ICU care before and after modification of a CPOE system and found that use of higher-efficiency CPOE order paths led to significant reductions in orders for vasoactive infusions, sedative infusions, and ventilator management.3
Paul D. Hain, MD, interim chief of staff and director of the Pediatric Hospitalist Program at Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn., and his colleagues at Vanderbilt University’s School of Medicine have been able to use their institution’s advanced CPOE to increase adherence to evidence-based treatments.
With the help of IT support staff, Dr. Hain inserted a pop-up window into the CPOE to remind providers that bronchodilators (albuterol) and steroids are ineffective for the treatment of bronchiolitis. Working with a third-year medical student, Ryan Bailey, Dr. Hain compared orders for these treatments in the years preceding installation of his pop-up reminder with those afterward. There was a significant drop in the non-evidence-based treatments, he notes, based on the installation of the pop-up window. “The reminder actually worked!” he exclaims. “It got people to stop using inappropriate therapies.”
This type of quality improvement, says Dr. Hain, is good for the hospital, for the hospitalists, and for their non-hospitalist colleagues. “This type of reminder allows us to share evidence-based guidelines with other admitting physicians in real time, and it appears to be a much more effective way to communicate information, as evidenced by our success in decreasing non-evidence-based treatments for bronchiolitis,” he asserts. The pop-up window includes a link to the treatment guidelines, so it also offers users an educational opportunity.
Close the Loop
Dr. Hoffmann and others caution about the limitations of using CPOE data. Most CPOE systems, notes Dr. Hoffmann, do not have a way of capturing whether an order or intervention was actually carried out.
“With CPOE, you can get a very good handle on how many order sets for processes of care have been ordered, but it doesn’t complete the loop—it doesn’t tell you whether that process of care happened once it has been ordered,” Dr. Hoffman says. “If you use the CPOE data set alone and stop there, the process is going to be fraught with unreliable information.”
CPOE can be a good tool for organizing clinical improvement projects but may not be the perfect tool for verifying outcomes of the order set. This was underscored by a project Dr. Hoffmann and his team conducted in collaboration with the University HealthSystem Consortium (UHC) on ventilator-associated pneumonia (VAP). The team wrote policy and processes based on current evidence for preventing VAP—such as raising the heads of patients’ beds to 30 degrees when they are mechanically ventilated—and created a flowchart of those processes. The aim of the project was to tie these care processes to the order for a ventilator, so that each time one was ordered, the other care items were bundled with it to trigger changes at the bedside. Now, it won’t be possible for a provider to order a ventilator without at least reviewing and ordering the additional care processes.
For the UHC project, Dr. Hoffmann and his team had to manually review charts and documentation to verify that the VAP bundle had been ordered and then utilized. “We looked at what documentation needed to be done, and we have modified nursing and respiratory therapy documentation to ensure that all these bundled-care process steps are adequately documented,” he says. The next step will be to tie CPOE with bedside charting and decision support, so that verification of order sets will also be stored in the hospital system’s information warehouse and will be accessible to physician researchers. The project is ongoing, states Dr. Hoffmann, and another evaluation will be conducted manually after a six-month interval to validate the data collection points that will be most useful in the automation process.
There are other ways to verify CPOE implementation. At BWH, says Dr. Gandhi, the electronic medication administration record (eMAR) provides a powerful adjunct to the CPOE.
“The fact that we have eMAR data is really advantageous,” notes Dr. Gandhi, “because now we can actually tell what patients have received.” For example, she says, Narcan (naloxone hydrochloride) is usually ordered and kept at the bedside for a patient receiving opioids in case the patient develops respiratory depression. Before institution of the hospital’s eMAR, “we could never tell how much Narcan was actually being given without doing laborious chart reviews. Now, with our eMAR, we can easily track how many times it was given, and this supplies a much better indicator of potential problems with the use of narcotics.”
At Vanderbilt, says Dr. Hain, a dosage checker application installed behind the CPOE has allowed his colleague Neal Patel, MD, MPH, to verify that medication errors in the Pediatric ICU dropped dramatically after its implementation. But, on other projects, researchers must know in advance that they intend to follow up on order entries so that they can convert order entries into binary procedures. The CPOE and EMR systems have the capability of inserting text boxes, drop-down menus, and click buttons for verifying medications, procedures, or even safety check-offs. If the CPOE is not set up in advance for this feature, however, it’s back to manual extraction to confirm the data—“a painful process, just as it is from paper charts,” Dr. Hain notes.
Privacy and Other Issues
Are there privacy issues of which hospitalists should be aware when using their hospital information system databases for their research?
“In general, if you’re doing quality improvement projects solely for the sake of improving the quality of patient care at your institution,” says Dr. Karson, “you do not need IRB [institutional review board] approval.” Whenever hospitalists plan to publish or present the data to external audiences, however, prior IRB approval must be obtained to show that patients’ identities will be protected and that use of the data will cause no harm.
There could be wrinkles in following these guidelines if the results of a QI project reveal surprisingly good results or important lessons about quality patient care that researchers think are worth sharing. Although it is possible to apply post-hoc for IRB approval, Dr. Gandhi and others suggest obtaining approval prior to the start of the project if researchers think there is any chance they may want to share results externally. Researchers must also adhere to the quality rules during QI projects, asserts Dr. Hain, to make sure patients’ identities are protected.
The IT/MD Interface
Whether hospitals use off-the-shelf or custom-built, institution-specific CPOEs, hospitalists are well positioned to play important roles in enhancing their designs, believes Dr. Karson. “If you’re going to support [clinicians’] decisions with computerized decision support, then CPOE systems are a great way to broadly affect the care of patients,” he says.
As those CPOE systems are designed, they require decisions along the way so they will achieve the quality, safety, and efficiency goals for the hospital and for the patients that the hospital cares for. Who better to interface with information systems designers than process-oriented hospitalists? As a hospitalist, Dr. Karson is taking a lead role in updating the pneumonia order set in his hospital’s provider order entry system.
It is sometimes possible for hospitalists to extract data manually to effect a proof of concept as justification for an IT system upgrade, says Dr. Hain. For example, in Vanderbilt’s outpatient clinic, one physician wanted to know whether all diabetic patients received foot exams at their regular visits. They inserted a paper form with check boxes into patients’ charts and then aggregated these forms to show it was possible to track quality measures for diabetics. This has led to a diabetics dashboard on the outpatient clinic computers that tracks foot exams by the day, week, or month.
Hospitalists report varying degrees of expertise with IT. Dr. Hoffmann’s introduction to IT came when he assumed the medical directorship of Ohio State University’s ICU. Since that time, he has been charged with collaborating with the medical center’s information systems (IS) personnel to improve the CPOE. “We have a group here that embraces the system—so much so that the IS people sometimes are inundated with our enthusiasm to make changes,” Dr. Hoffman says.
Dr. Hain, who has a background in engineering, relies on IT support when designing changes to the CPOE. “Our IT department here has done a really good job of reaching out to its users,” he says. Several physicians in the medical informatics department specialize in the CPOE, as is the case in many academic institutions. “It’s important that the gap be bridged between computer programmers and MDs,” he says. “The best way to do that is to have MDs with master’s degrees in informatics working with the programmers, making it all the more seamless.” TH
Gretchen Henkel is a frequent contributor to The Hospitalist.
References
- American Hospital Association. Forward momentum: hospital use of information technology. October 2005. Available at www.aha.org/aha/content/2005/pdf/FINALNonEmbITSurvey105.pdf. Last accessed April 10, 2007.
- Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005 Jul 19; 143(2):121-128.
- Ali NA, Mekhjian HS, Kuehn PL, et al. Specificity of computerized physician order entry has a significant effect on the efficiency of workflow for critically ill patients. Crit Care Med. 2005 Jan;33(1):110-114.
The expansion of information technology (IT) in U.S. hospitals is an evolutionary process. Billing, collections, and admission and discharge records have long been computerized, but now electronic medical administration records, patient electronic health records, and computerized physician order entry (CPOE) systems are joining the ranks.
Hospitalists are more likely to encounter sophisticated IT systems in larger, urban, or teaching hospitals, according to a 2005 survey by the American Hospital Association.1
The Hospitalist’s first installment about hospital informatics (“Charts to Screens,” January 2007, p. 25) focused on the challenges of health IT and the barriers to effective adoption of computer-based documentation systems. This installment explores the potentially rich vein of data available to hospitalists from those information systems and the opportunities for research and QI applications.
The mechanics of conducting clinical research and QI projects will depend to a large extent on the progress each hospital medical group’s institution has made in the IT adoption process. Some say hospitalists have powerful contributions to make in influencing how the IT process evolves so their research opportunities will also improve.
QI Topics
Data in information systems differ from hospital to hospital, says Tejal K. Gandhi, MD, MPH, director of patient safety at Boston’s Brigham and Women’s Hospital (BWH) and assistant professor of medicine in the Department of Medicine at Harvard Medical School in Boston.
Dr. Gandhi’s research focuses on redesigning hospital and outpatient processes to improve patient safety. She notes that hospitalists can take advantage of data the hospital is collecting to satisfy its reporting requirements to spearhead more quality-improvement efforts.
“For example,” says Dr. Gandhi, “the hospital has to document how it’s doing on pneumonia measures, acute myocardial infarction measures (was the patient having a heart attack given aspirin and a beta-blocker?), and others. These are fruitful topics for quality-improvement projects.”
Hospitalist Andrew Karson, MD, MPH, associate director of the Decision Support and Quality Management Unit and associate program director for the Internal Medicine Residency Program at Massachusetts General Hospital, Boston, also focuses on patient safety issues in his research. Given hospitalists’ knowledge of decision-making systems in the hospital, they are in a unique situation to initiate such projects, he believes.
For example, Dr. Karson participated in a study initiated by colleague Christopher L. Roy, MD, associate director of the hospitalist program at BWH.2 Dr. Roy posited that pending test results could be an important patient safety issue and, at the very least, might affect continuity of care. The researchers identified 2,644 consecutive patients discharged from BWH and Massachusetts General between February and June 2004. During that time, a mixture of hospitalists and non-hospitalists were responsible for discharging patients on house staff and non-house-staff services. Using a Results Manager application integrated into each patient’s electronic medical record (EMR) at the hospitals, the team identified and tracked pending laboratory and/or radiologic test results that had been returned after the patients were discharged.
The team used physician reviewers to determine whether the pending test results were potentially actionable. They found that 41% (1,095) of the discharged patients had a total of 2,033 test results return after their discharge. Of those tests, the physician reviewer determined that 9.4% (191) were potentially actionable. Examples of actionable results of which discharging physicians had been unaware included a levofloxacin-resistant Klebsiella infection in a patient being treated with levofloxacin, and a thyroid-stimulating hormone level that was dangerously low in a patient with rapid atrial fibrillation. A coauthor of the study, Eric G. Poon, MD, MPH, Division of General Medicine and Primary Care at BWH, is working on a results-management system that will automatically alert hospitalists and other physicians in the process of discharging patients when those patients are awaiting test results.
Research Potential
CPOE systems afford opportunities to delve further into clinical research and QI projects.
The flow of care in hospitals is inextricably linked with writing orders—for medications, tests, consultations, or interventional care processes. “Interfacing with CPOEs, therefore, can help influence the way care is practiced more broadly for our patients,” says Dr. Karson. “By embedding rules and decision support elements within our CPOE systems, we can improve the quality and safety of the care that we provide.”
The effect of CPOE on ICU patient care was highlighted in a 2005 study conducted by intensivist Stephen P. Hoffmann, MD, medical director, ICU, and associate professor of medicine at Ohio State University Medical Center, Columbus, and his colleagues. The team compared orders for ICU care before and after modification of a CPOE system and found that use of higher-efficiency CPOE order paths led to significant reductions in orders for vasoactive infusions, sedative infusions, and ventilator management.3
Paul D. Hain, MD, interim chief of staff and director of the Pediatric Hospitalist Program at Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn., and his colleagues at Vanderbilt University’s School of Medicine have been able to use their institution’s advanced CPOE to increase adherence to evidence-based treatments.
With the help of IT support staff, Dr. Hain inserted a pop-up window into the CPOE to remind providers that bronchodilators (albuterol) and steroids are ineffective for the treatment of bronchiolitis. Working with a third-year medical student, Ryan Bailey, Dr. Hain compared orders for these treatments in the years preceding installation of his pop-up reminder with those afterward. There was a significant drop in the non-evidence-based treatments, he notes, based on the installation of the pop-up window. “The reminder actually worked!” he exclaims. “It got people to stop using inappropriate therapies.”
This type of quality improvement, says Dr. Hain, is good for the hospital, for the hospitalists, and for their non-hospitalist colleagues. “This type of reminder allows us to share evidence-based guidelines with other admitting physicians in real time, and it appears to be a much more effective way to communicate information, as evidenced by our success in decreasing non-evidence-based treatments for bronchiolitis,” he asserts. The pop-up window includes a link to the treatment guidelines, so it also offers users an educational opportunity.
Close the Loop
Dr. Hoffmann and others caution about the limitations of using CPOE data. Most CPOE systems, notes Dr. Hoffmann, do not have a way of capturing whether an order or intervention was actually carried out.
“With CPOE, you can get a very good handle on how many order sets for processes of care have been ordered, but it doesn’t complete the loop—it doesn’t tell you whether that process of care happened once it has been ordered,” Dr. Hoffman says. “If you use the CPOE data set alone and stop there, the process is going to be fraught with unreliable information.”
CPOE can be a good tool for organizing clinical improvement projects but may not be the perfect tool for verifying outcomes of the order set. This was underscored by a project Dr. Hoffmann and his team conducted in collaboration with the University HealthSystem Consortium (UHC) on ventilator-associated pneumonia (VAP). The team wrote policy and processes based on current evidence for preventing VAP—such as raising the heads of patients’ beds to 30 degrees when they are mechanically ventilated—and created a flowchart of those processes. The aim of the project was to tie these care processes to the order for a ventilator, so that each time one was ordered, the other care items were bundled with it to trigger changes at the bedside. Now, it won’t be possible for a provider to order a ventilator without at least reviewing and ordering the additional care processes.
For the UHC project, Dr. Hoffmann and his team had to manually review charts and documentation to verify that the VAP bundle had been ordered and then utilized. “We looked at what documentation needed to be done, and we have modified nursing and respiratory therapy documentation to ensure that all these bundled-care process steps are adequately documented,” he says. The next step will be to tie CPOE with bedside charting and decision support, so that verification of order sets will also be stored in the hospital system’s information warehouse and will be accessible to physician researchers. The project is ongoing, states Dr. Hoffmann, and another evaluation will be conducted manually after a six-month interval to validate the data collection points that will be most useful in the automation process.
There are other ways to verify CPOE implementation. At BWH, says Dr. Gandhi, the electronic medication administration record (eMAR) provides a powerful adjunct to the CPOE.
“The fact that we have eMAR data is really advantageous,” notes Dr. Gandhi, “because now we can actually tell what patients have received.” For example, she says, Narcan (naloxone hydrochloride) is usually ordered and kept at the bedside for a patient receiving opioids in case the patient develops respiratory depression. Before institution of the hospital’s eMAR, “we could never tell how much Narcan was actually being given without doing laborious chart reviews. Now, with our eMAR, we can easily track how many times it was given, and this supplies a much better indicator of potential problems with the use of narcotics.”
At Vanderbilt, says Dr. Hain, a dosage checker application installed behind the CPOE has allowed his colleague Neal Patel, MD, MPH, to verify that medication errors in the Pediatric ICU dropped dramatically after its implementation. But, on other projects, researchers must know in advance that they intend to follow up on order entries so that they can convert order entries into binary procedures. The CPOE and EMR systems have the capability of inserting text boxes, drop-down menus, and click buttons for verifying medications, procedures, or even safety check-offs. If the CPOE is not set up in advance for this feature, however, it’s back to manual extraction to confirm the data—“a painful process, just as it is from paper charts,” Dr. Hain notes.
Privacy and Other Issues
Are there privacy issues of which hospitalists should be aware when using their hospital information system databases for their research?
“In general, if you’re doing quality improvement projects solely for the sake of improving the quality of patient care at your institution,” says Dr. Karson, “you do not need IRB [institutional review board] approval.” Whenever hospitalists plan to publish or present the data to external audiences, however, prior IRB approval must be obtained to show that patients’ identities will be protected and that use of the data will cause no harm.
There could be wrinkles in following these guidelines if the results of a QI project reveal surprisingly good results or important lessons about quality patient care that researchers think are worth sharing. Although it is possible to apply post-hoc for IRB approval, Dr. Gandhi and others suggest obtaining approval prior to the start of the project if researchers think there is any chance they may want to share results externally. Researchers must also adhere to the quality rules during QI projects, asserts Dr. Hain, to make sure patients’ identities are protected.
The IT/MD Interface
Whether hospitals use off-the-shelf or custom-built, institution-specific CPOEs, hospitalists are well positioned to play important roles in enhancing their designs, believes Dr. Karson. “If you’re going to support [clinicians’] decisions with computerized decision support, then CPOE systems are a great way to broadly affect the care of patients,” he says.
As those CPOE systems are designed, they require decisions along the way so they will achieve the quality, safety, and efficiency goals for the hospital and for the patients that the hospital cares for. Who better to interface with information systems designers than process-oriented hospitalists? As a hospitalist, Dr. Karson is taking a lead role in updating the pneumonia order set in his hospital’s provider order entry system.
It is sometimes possible for hospitalists to extract data manually to effect a proof of concept as justification for an IT system upgrade, says Dr. Hain. For example, in Vanderbilt’s outpatient clinic, one physician wanted to know whether all diabetic patients received foot exams at their regular visits. They inserted a paper form with check boxes into patients’ charts and then aggregated these forms to show it was possible to track quality measures for diabetics. This has led to a diabetics dashboard on the outpatient clinic computers that tracks foot exams by the day, week, or month.
Hospitalists report varying degrees of expertise with IT. Dr. Hoffmann’s introduction to IT came when he assumed the medical directorship of Ohio State University’s ICU. Since that time, he has been charged with collaborating with the medical center’s information systems (IS) personnel to improve the CPOE. “We have a group here that embraces the system—so much so that the IS people sometimes are inundated with our enthusiasm to make changes,” Dr. Hoffman says.
Dr. Hain, who has a background in engineering, relies on IT support when designing changes to the CPOE. “Our IT department here has done a really good job of reaching out to its users,” he says. Several physicians in the medical informatics department specialize in the CPOE, as is the case in many academic institutions. “It’s important that the gap be bridged between computer programmers and MDs,” he says. “The best way to do that is to have MDs with master’s degrees in informatics working with the programmers, making it all the more seamless.” TH
Gretchen Henkel is a frequent contributor to The Hospitalist.
References
- American Hospital Association. Forward momentum: hospital use of information technology. October 2005. Available at www.aha.org/aha/content/2005/pdf/FINALNonEmbITSurvey105.pdf. Last accessed April 10, 2007.
- Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005 Jul 19; 143(2):121-128.
- Ali NA, Mekhjian HS, Kuehn PL, et al. Specificity of computerized physician order entry has a significant effect on the efficiency of workflow for critically ill patients. Crit Care Med. 2005 Jan;33(1):110-114.
The expansion of information technology (IT) in U.S. hospitals is an evolutionary process. Billing, collections, and admission and discharge records have long been computerized, but now electronic medical administration records, patient electronic health records, and computerized physician order entry (CPOE) systems are joining the ranks.
Hospitalists are more likely to encounter sophisticated IT systems in larger, urban, or teaching hospitals, according to a 2005 survey by the American Hospital Association.1
The Hospitalist’s first installment about hospital informatics (“Charts to Screens,” January 2007, p. 25) focused on the challenges of health IT and the barriers to effective adoption of computer-based documentation systems. This installment explores the potentially rich vein of data available to hospitalists from those information systems and the opportunities for research and QI applications.
The mechanics of conducting clinical research and QI projects will depend to a large extent on the progress each hospital medical group’s institution has made in the IT adoption process. Some say hospitalists have powerful contributions to make in influencing how the IT process evolves so their research opportunities will also improve.
QI Topics
Data in information systems differ from hospital to hospital, says Tejal K. Gandhi, MD, MPH, director of patient safety at Boston’s Brigham and Women’s Hospital (BWH) and assistant professor of medicine in the Department of Medicine at Harvard Medical School in Boston.
Dr. Gandhi’s research focuses on redesigning hospital and outpatient processes to improve patient safety. She notes that hospitalists can take advantage of data the hospital is collecting to satisfy its reporting requirements to spearhead more quality-improvement efforts.
“For example,” says Dr. Gandhi, “the hospital has to document how it’s doing on pneumonia measures, acute myocardial infarction measures (was the patient having a heart attack given aspirin and a beta-blocker?), and others. These are fruitful topics for quality-improvement projects.”
Hospitalist Andrew Karson, MD, MPH, associate director of the Decision Support and Quality Management Unit and associate program director for the Internal Medicine Residency Program at Massachusetts General Hospital, Boston, also focuses on patient safety issues in his research. Given hospitalists’ knowledge of decision-making systems in the hospital, they are in a unique situation to initiate such projects, he believes.
For example, Dr. Karson participated in a study initiated by colleague Christopher L. Roy, MD, associate director of the hospitalist program at BWH.2 Dr. Roy posited that pending test results could be an important patient safety issue and, at the very least, might affect continuity of care. The researchers identified 2,644 consecutive patients discharged from BWH and Massachusetts General between February and June 2004. During that time, a mixture of hospitalists and non-hospitalists were responsible for discharging patients on house staff and non-house-staff services. Using a Results Manager application integrated into each patient’s electronic medical record (EMR) at the hospitals, the team identified and tracked pending laboratory and/or radiologic test results that had been returned after the patients were discharged.
The team used physician reviewers to determine whether the pending test results were potentially actionable. They found that 41% (1,095) of the discharged patients had a total of 2,033 test results return after their discharge. Of those tests, the physician reviewer determined that 9.4% (191) were potentially actionable. Examples of actionable results of which discharging physicians had been unaware included a levofloxacin-resistant Klebsiella infection in a patient being treated with levofloxacin, and a thyroid-stimulating hormone level that was dangerously low in a patient with rapid atrial fibrillation. A coauthor of the study, Eric G. Poon, MD, MPH, Division of General Medicine and Primary Care at BWH, is working on a results-management system that will automatically alert hospitalists and other physicians in the process of discharging patients when those patients are awaiting test results.
Research Potential
CPOE systems afford opportunities to delve further into clinical research and QI projects.
The flow of care in hospitals is inextricably linked with writing orders—for medications, tests, consultations, or interventional care processes. “Interfacing with CPOEs, therefore, can help influence the way care is practiced more broadly for our patients,” says Dr. Karson. “By embedding rules and decision support elements within our CPOE systems, we can improve the quality and safety of the care that we provide.”
The effect of CPOE on ICU patient care was highlighted in a 2005 study conducted by intensivist Stephen P. Hoffmann, MD, medical director, ICU, and associate professor of medicine at Ohio State University Medical Center, Columbus, and his colleagues. The team compared orders for ICU care before and after modification of a CPOE system and found that use of higher-efficiency CPOE order paths led to significant reductions in orders for vasoactive infusions, sedative infusions, and ventilator management.3
Paul D. Hain, MD, interim chief of staff and director of the Pediatric Hospitalist Program at Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn., and his colleagues at Vanderbilt University’s School of Medicine have been able to use their institution’s advanced CPOE to increase adherence to evidence-based treatments.
With the help of IT support staff, Dr. Hain inserted a pop-up window into the CPOE to remind providers that bronchodilators (albuterol) and steroids are ineffective for the treatment of bronchiolitis. Working with a third-year medical student, Ryan Bailey, Dr. Hain compared orders for these treatments in the years preceding installation of his pop-up reminder with those afterward. There was a significant drop in the non-evidence-based treatments, he notes, based on the installation of the pop-up window. “The reminder actually worked!” he exclaims. “It got people to stop using inappropriate therapies.”
This type of quality improvement, says Dr. Hain, is good for the hospital, for the hospitalists, and for their non-hospitalist colleagues. “This type of reminder allows us to share evidence-based guidelines with other admitting physicians in real time, and it appears to be a much more effective way to communicate information, as evidenced by our success in decreasing non-evidence-based treatments for bronchiolitis,” he asserts. The pop-up window includes a link to the treatment guidelines, so it also offers users an educational opportunity.
Close the Loop
Dr. Hoffmann and others caution about the limitations of using CPOE data. Most CPOE systems, notes Dr. Hoffmann, do not have a way of capturing whether an order or intervention was actually carried out.
“With CPOE, you can get a very good handle on how many order sets for processes of care have been ordered, but it doesn’t complete the loop—it doesn’t tell you whether that process of care happened once it has been ordered,” Dr. Hoffman says. “If you use the CPOE data set alone and stop there, the process is going to be fraught with unreliable information.”
CPOE can be a good tool for organizing clinical improvement projects but may not be the perfect tool for verifying outcomes of the order set. This was underscored by a project Dr. Hoffmann and his team conducted in collaboration with the University HealthSystem Consortium (UHC) on ventilator-associated pneumonia (VAP). The team wrote policy and processes based on current evidence for preventing VAP—such as raising the heads of patients’ beds to 30 degrees when they are mechanically ventilated—and created a flowchart of those processes. The aim of the project was to tie these care processes to the order for a ventilator, so that each time one was ordered, the other care items were bundled with it to trigger changes at the bedside. Now, it won’t be possible for a provider to order a ventilator without at least reviewing and ordering the additional care processes.
For the UHC project, Dr. Hoffmann and his team had to manually review charts and documentation to verify that the VAP bundle had been ordered and then utilized. “We looked at what documentation needed to be done, and we have modified nursing and respiratory therapy documentation to ensure that all these bundled-care process steps are adequately documented,” he says. The next step will be to tie CPOE with bedside charting and decision support, so that verification of order sets will also be stored in the hospital system’s information warehouse and will be accessible to physician researchers. The project is ongoing, states Dr. Hoffmann, and another evaluation will be conducted manually after a six-month interval to validate the data collection points that will be most useful in the automation process.
There are other ways to verify CPOE implementation. At BWH, says Dr. Gandhi, the electronic medication administration record (eMAR) provides a powerful adjunct to the CPOE.
“The fact that we have eMAR data is really advantageous,” notes Dr. Gandhi, “because now we can actually tell what patients have received.” For example, she says, Narcan (naloxone hydrochloride) is usually ordered and kept at the bedside for a patient receiving opioids in case the patient develops respiratory depression. Before institution of the hospital’s eMAR, “we could never tell how much Narcan was actually being given without doing laborious chart reviews. Now, with our eMAR, we can easily track how many times it was given, and this supplies a much better indicator of potential problems with the use of narcotics.”
At Vanderbilt, says Dr. Hain, a dosage checker application installed behind the CPOE has allowed his colleague Neal Patel, MD, MPH, to verify that medication errors in the Pediatric ICU dropped dramatically after its implementation. But, on other projects, researchers must know in advance that they intend to follow up on order entries so that they can convert order entries into binary procedures. The CPOE and EMR systems have the capability of inserting text boxes, drop-down menus, and click buttons for verifying medications, procedures, or even safety check-offs. If the CPOE is not set up in advance for this feature, however, it’s back to manual extraction to confirm the data—“a painful process, just as it is from paper charts,” Dr. Hain notes.
Privacy and Other Issues
Are there privacy issues of which hospitalists should be aware when using their hospital information system databases for their research?
“In general, if you’re doing quality improvement projects solely for the sake of improving the quality of patient care at your institution,” says Dr. Karson, “you do not need IRB [institutional review board] approval.” Whenever hospitalists plan to publish or present the data to external audiences, however, prior IRB approval must be obtained to show that patients’ identities will be protected and that use of the data will cause no harm.
There could be wrinkles in following these guidelines if the results of a QI project reveal surprisingly good results or important lessons about quality patient care that researchers think are worth sharing. Although it is possible to apply post-hoc for IRB approval, Dr. Gandhi and others suggest obtaining approval prior to the start of the project if researchers think there is any chance they may want to share results externally. Researchers must also adhere to the quality rules during QI projects, asserts Dr. Hain, to make sure patients’ identities are protected.
The IT/MD Interface
Whether hospitals use off-the-shelf or custom-built, institution-specific CPOEs, hospitalists are well positioned to play important roles in enhancing their designs, believes Dr. Karson. “If you’re going to support [clinicians’] decisions with computerized decision support, then CPOE systems are a great way to broadly affect the care of patients,” he says.
As those CPOE systems are designed, they require decisions along the way so they will achieve the quality, safety, and efficiency goals for the hospital and for the patients that the hospital cares for. Who better to interface with information systems designers than process-oriented hospitalists? As a hospitalist, Dr. Karson is taking a lead role in updating the pneumonia order set in his hospital’s provider order entry system.
It is sometimes possible for hospitalists to extract data manually to effect a proof of concept as justification for an IT system upgrade, says Dr. Hain. For example, in Vanderbilt’s outpatient clinic, one physician wanted to know whether all diabetic patients received foot exams at their regular visits. They inserted a paper form with check boxes into patients’ charts and then aggregated these forms to show it was possible to track quality measures for diabetics. This has led to a diabetics dashboard on the outpatient clinic computers that tracks foot exams by the day, week, or month.
Hospitalists report varying degrees of expertise with IT. Dr. Hoffmann’s introduction to IT came when he assumed the medical directorship of Ohio State University’s ICU. Since that time, he has been charged with collaborating with the medical center’s information systems (IS) personnel to improve the CPOE. “We have a group here that embraces the system—so much so that the IS people sometimes are inundated with our enthusiasm to make changes,” Dr. Hoffman says.
Dr. Hain, who has a background in engineering, relies on IT support when designing changes to the CPOE. “Our IT department here has done a really good job of reaching out to its users,” he says. Several physicians in the medical informatics department specialize in the CPOE, as is the case in many academic institutions. “It’s important that the gap be bridged between computer programmers and MDs,” he says. “The best way to do that is to have MDs with master’s degrees in informatics working with the programmers, making it all the more seamless.” TH
Gretchen Henkel is a frequent contributor to The Hospitalist.
References
- American Hospital Association. Forward momentum: hospital use of information technology. October 2005. Available at www.aha.org/aha/content/2005/pdf/FINALNonEmbITSurvey105.pdf. Last accessed April 10, 2007.
- Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005 Jul 19; 143(2):121-128.
- Ali NA, Mekhjian HS, Kuehn PL, et al. Specificity of computerized physician order entry has a significant effect on the efficiency of workflow for critically ill patients. Crit Care Med. 2005 Jan;33(1):110-114.
Methadone: Handle with Care
Few topics in hospital-based pain management generate such diverse viewpoints as the use of methadone as an analgesic. Increasingly ordered by hospice physicians and some hospitalists as a tool for managing difficult pain cases, it is also coming under scrutiny for risks related to cardiac complications, respiratory depression, and the challenges of determining appropriate doses.
For some, the risks are grave enough to contraindicate methadone prescription for use in routine hospital practice, unless the hospitalist is well-versed in its use and has access to a pharmacist or other pain expert to review medication orders. Hospitalists should also be aware of the Food and Drug Administration’s strongly worded November 2006 Public Health Advisory, “Methadone Use for Pain Control May Result in Death and Life-Threatening Changes in Breathing and Heart Beat.”1
The FDA stopped short of recommending against the use of methadone as an analgesic, but it admonished physicians to use caution when prescribing it for patients unused to the drug—particularly during drug initiation, conversion from another opioid, or titration upward. Patients should be cautioned to take the drug exactly as prescribed. The advisory also recommended a new maximum initial dose of 30 mg per day (typically prescribed 10 mg tid), when initiating methadone for pain management.
Jean Youngwerth, MD, associate program director of the University of Colorado at Denver and Health Sciences Center, is well aware of the benefits and risks of prescribing methadone in her dual roles as hospitalist and palliative care consultant. “It is a great analgesic, becoming a lot more popular in recent years,” Dr. Youngwerth says. “It’s also ridiculously cheap [often under a dollar a day], which is an important consideration for some of our patients returning home with chronic pain. For other patients, it’s an extra analgesic tool, giving good pain relief, especially for refractory somatic or neuropathic pain.”
But methadone also has downsides. “Its pharmaco-kinetics are so complex and poorly understood that people unfamiliar with prescribing it can get in trouble in a hurry,” Dr. Youngwerth says. “You can kill people with oversedation. I do not prescribe it in my hospitalist practice and I discourage other hospitalists from prescribing it for their patients without consulting a pain or palliative care specialist. You don’t see its maximal effects until at least three to four days out, and that’s usually too long for the hospitalist. When I start palliative care patients on methadone, I normally keep them in the hospital for three or four days so that I can monitor the effects.”
Dr. Youngwerth believes her experience in pain management justifies prescribing methadone for palliative care patients. Even so, she always calls one of the local physician pain experts she works with or a hospital pharmacist to make sure she is ordering it safely and correctly. “I don’t think doctors should be scared off by all the bad publicity about methadone, but they need to realize these are valid concerns,” she says. “People run into problems when they assume that it is just another opioid.”
Hospitalists must recognize the stark realities of using the drug.
“Methadone is the easiest opioid to kill someone with,” says Gail Gazelle, MD, palliative care physician with MD Can Help and Harvard Medical School in Boston. “At the same time, its unique properties can give analgesic effects you can get from no other drug.”
For hospitalists, these issues are complicated by their short involvement with hospitalized patients, who are quickly prepped for discharge back to various community settings and living situations.
Dr. Gazelle wonders, “Is a short hospital stay the right place to initiate methadone treatment, given all of the complications?” If the answer is yes, communicating with the attending and agreeing on a plan for its continued use after discharge from the hospital are essential.
Although narcotic abuse is notoriously difficult to manage, with high relapse rates in every setting, methadone maintenance therapy (MMT) has been shown to reduce overall rates of abuse of other drugs, overdose and death, criminal activity, needle sharing, and commercial sex work. Methadone maintenance is a long-term strategy. The drug is provided as a substitute, not a cure, for narcotic abuse. Patients may continue to receive their daily maintenance dose for years. One-year retention rates in several large studies of MMT have ranged from 25% to 60%, while rates of relapse after leaving MMT are high. Stopping methadone use poses the same challenges as quitting any narcotic and should only be done under a doctor’s care.
Methadone is also used to treat heroin withdrawal, an issue for some hospitalized patients. Prescribing methadone for maintenance therapy is limited to federally licensed methadone treatment programs.
Complications
There are several critical facets of this drug hospitalists must be aware of:
Unpredictable half-life: Methadone, relative to other opioids, has high lipid solubility, slow metabolism, and a typical half-life ranging from 15 to 60 hours—although it can be longer. Methadone’s analgesic effect is shorter-lived, so analgesic doses should be given two, three, or four times daily. But the longer half-life means it can take three days or more after the initial dose before the drug’s full effect—on respiration for example—is known. That is why therapeutic doses can build to toxic levels. There is also wide variation in its effects among patients. Guidelines suggest titrating methadone upward for increased analgesic effect should not be attempted until at least three days after the first dose.
Respiratory depression: This is an issue when the drug is initiated in an opioid-naïve patient or is too rapidly titrated. Deaths from methadone have been seen at doses once considered safe. Physicians are cautioned to start patients on low doses while using other, short-acting opioids for breakthrough pain and frequently assessing for signs of overdose or respiratory depression, such as difficulty in breathing, shallow breathing, extreme sleepiness, or inability to think, talk, or walk normally.
Effect on heart arrhythmias: Methadone can prolong the QTc interval in heart function, leading to a potentially serious cardiac abnormality known as torsade de pointes. The potential for cardiac deaths is another complicating factor that may contraindicate methadone for patients at risk for developing a prolonged QTc interval, including patients with cardiac hypertrophy, hypokalemia, or hypomagnesemia, or a history of cardiac conduction abnormalities or taking medications affecting cardiac conduction. A current EKG may be an appropriate precaution when initiating methadone in elderly patients who have a cardiac history or are receiving methadone in high doses or by intravenous administration.
Drug interactions: The list of drugs that interact with methadone is long, with potential for unwanted side effects and increased or decreased potency. This list includes most anti-retroviral treatments for HIV; sedatives, tranquilizers, barbiturates, seizure medications, muscle relaxants, or any central nervous system depressants; certain steroids and anti-fungals; even over-the-counter cough and cold medications. Also watch for medications that treat irregular heartbeat or prolong the QTc interval. Pharmaceutical company labels for methadone, as well as the FDA advisory, contain a more complete list of drugs that interact with methadone. Always review with patients the other medications they take, including over-the-counter medications and alternative treatments.
Other complications: Methadone should not be prescribed when opioids in general are contraindicated. It can be a management challenge to convert patients from methadone back to other opioids. Another complication of prescribing methadone is the negative publicity it has received in recent years, combined with the stigma of its associations with drug treatment. Some patients, families, or attending physicians may be leery of using it as a pain reliever. It may not be worth titrating methadone to the correct dose in the hospital if it is going to be discontinued post-discharge.
A series of articles in The Charleston (W.Va.) Gazette starting in June 2006 alerted many to the fact methadone is listed by medical examiners nationwide as a cause of death more often than any other prescription pain reliever.10 Methadone was implicated in nearly 4,000 deaths in 2006, four times as many as in 1999. Most of the deaths were considered accidental, and many involved combinations with other drugs—although some were in patients taking methadone as prescribed.
Hospitalists can expect that the diverse caseloads they see likely will include some patients taking methadone. Some may be getting it as an analgesic prescribed by a community physician, pain clinic, or hospice. Others in MMT and receiving a daily dose to manage their addiction disorder may present at the hospital with a different medical problem and perhaps new pain issues.
The hospitalist should not take it for granted that patients on MMT are not also intoxicated or abusing methadone or other drugs, says Michael Weaver, MD, pain and addiction specialist at Virginia Commonwealth University Medical Center in Richmond. Nor should they assume MMT doses are providing adequate analgesia.
“The bottom line in all of these situations is communication,” Dr. Weaver says. Talk to the medical director of the methadone clinic or the community physician who prescribed methadone as an analgesic. Verify the patient’s status, confirm dosage, and discuss the pain issues that need to be addressed—while recognizing pain relief is an appropriate expectation of any hospitalized patient, regardless of drug history or treatment.
Generally, Dr. Weaver says, the maintenance dose of methadone would continue during the hospital stay, and a different analgesic would be ordered for the pain—although the clinic physician may have other ideas. Changing methadone dose or schedule—or attempting to wean a patient off methadone—is not a decision a hospitalist should make unilaterally.11
Methadone’s Merits
Why would a hospitalist want to prescribe a drug that comes with so many caveats?
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., uses methadone—often in combination with the anti-depressant desipramine or the anticonvulsant gabapentin—to treat complex regional pain syndromes and neuropathic pain.
Dr. Jessop carefully assesses patients for neuropathic pain, listening for descriptors such as burning, stinging, or numbing. These are the patients for whom she most often receives palliative care consultations, often following years of out-of-control pain or lack of response to high doses of other opioids. “My job is much easier now that I understand the difference between nocioceptive and neuropathic pain,” she notes.
“I think methadone is magic, perhaps due to its effect on the NMDA (n-methyl, d-asparte) receptors,” Dr. Jessop says. “I’m also convinced from my clinical experience that there can be nerve healing going on when these pain syndromes are effectively treated. I had a patient with horrible phantom pain following multiple hip surgeries and amputation of his leg. He was referred by a family practitioner, who said, ‘I cannot get this man’s pain under control.’ ” The patient’s pain is now controlled with methadone, 30 mg three times a day. He rarely needs to take his hydromorphone (Dilaudid) for breakthrough pain, and he is able to use his prosthetic leg—which would have been unthinkable before.
Dr. Jessop believes low doses of methadone—even lower than the conversion charts recommend—can have a big effect. “I don’t have problems with methadone because I’m so careful in prescribing it,” she says. “I also work closely with the attending physician and give patients my cell phone number when they return home. It is important to get the family involved and to be clear about the risks and benefits.”
Brad Stuart, MD, senior medical director of Sutter VNA and Hospice in Northern California, also believes methadone can be a wonderful pain management tool. “There is no substitute, in my estimation, to adding a little methadone to the opioid regimen—even just 5 mg of liquid three times a day—for difficult neuropathic pain cases,” Dr. Stuart says. “It’s true that you don’t want to raise the dose too quickly. But I find that it’s unusual not to see benefit in these kinds of patients. I disagree with those who would advise hospitalists to stay away from methadone for treating refractory neuropathic pain. If you start slow and go slow, the risks are small relative to the gain.”
Eduardo Bruera, MD, a palliative care physician at M.D. Anderson Cancer Center in Houston, is another believer in methadone for difficult pain cases, although he emphasizes that his experience is limited to the pain associated with cancer. Dr. Bruera does not use methadone as a first-line analgesic, but he finds it effective when other opioids have not been. “Patients who continue to have a lot of pain after multiple escalating opioid doses or signs of opioid toxicity may be signaling that the opioid you’re using is not working,” he says. “If we make three or four dose changes without response, it’s time to change the opioid.”
Dr. Bruera acknowledges that persuasive research studies to establish methadone’s purported efficacy in treating neuropathic pain have not been conducted. “Unfortunately, methadone is an orphan drug, so we don’t know who would pay for those studies. Should we consider it as a first-line opioid for cancer pain? Again, that is an unanswered question.”
Dr. Bruera has been involved in a number of the few published studies and reviews of methadone’s analgesic efficacy, and he is engaged in ongoing orphan drug status research.12, 13
Methadone as Analgesic
Stephen Bekanich, MD, hospitalist and palliative care consultant at the University of Utah Medical Center in Salt Lake City, falls in the middle range of opinions on methadone.
“From the hospitalist’s standpoint, there are downsides,” Dr. Bekanich says. “People who don’t understand how to titrate it may change doses on a daily basis or more often, which is dangerous. They may not understand the dosing equivalents or pay enough attention to drug interactions.
“Of all the opioids, careful assessment and follow-up may be the most important with methadone. Always make sure you have concrete post-discharge plans. If I didn’t have a pharmacist to collaborate with, as a hospitalist I’d probably stay away from it. But it’s different when I put on my palliative care hat.”
Rachelle Bernacki, MD, a hospitalist, palliative care physician and geriatrician at the University of California-San Francisco Medical Center, agrees methadone can be a useful analgesic—particularly when other opioids have failed to relieve the pain. “But I don’t start with it; I may add a small dose of methadone to the existing regimen for complex pain,” she says.
“I caution my residents not to try methadone without consulting with someone familiar with the drug,” explains Dr. Bernacki, who adds that she is fortunate to work with a pharmacist at UCSF who is an expert in pain management and palliative medicine. “Having taught residents, I can confirm that there is a lot of confusion about its use. But I have also used methadone in my outpatient geriatric practice—with fantastic results.”
Paresh Patel, MD, a hospitalist at VCU Medical Center, says he and his colleagues use methadone as a second-line analgesic when pain is not well managed with morphine. He always keeps an eye out for the risks, including potential interactions with psychiatric medications and the need to look at EKGs.
Dr. Patel says conversion from other opioids is one of the biggest challenges in using methadone. He is not satisfied with the various published opioid conversion charts and relies on experience and trial and error. “I always wait 48 hours before titrating up,” he says.
More research is needed in this area, Dr. Patel says, and he is thinking of getting involved in a methadone research project. TH
Larry Beresford is a frequent contributor to The Hospitalist.
References
- Food and Drug Administration. FDA Public Health Advisory: Methadone use for pain control may result in death and life-threatening changes in breathing and heart beat. Available at: www.fda.gov/cder/drug/advisory/methadone.htm. Last accessed June 4, 2007.
- Moulin DE, Palma D, Watling C, et al. Methadone in the management of intractable neuropathic noncancer pain. Can J Neurol Sci. 2005 Aug: 32(3); 340-343.
- Altier N, Dion D, Boulanger A, et al. Management of chronic neuropathic pain with methadone: A review of 13 cases. Clin J Pain. 2005 Jul-Aug;21(4):364-369.
- Morley JS, Bridson J, Nash TP, et al. Low-dose methadone has an analgesic effect in neuropathic pain: A double-blind randomized controlled crossover trial. Palliat Med. 2003 Oct;17(7):576-587.
- Lawlor PG, Turner KS, Hanson J, et al. Dose ratio between morphine and methadone in patients with cancer pain: A retrospective study. Cancer. 1998 Mar;82(6):1167-1173.
- Ripamonti C, De Conno F, Groff L, et al. Equianal-gesic dose/ratio between methadone and other opioid agonists in cancer pain: Comparison of two clinical experiences. Ann Oncol. 1998 Jan;9(1):79-83.
- Gazelle G, Fine PG. Fast Fact and Concept #75: Methadone for the treatment of pain. End-of-Life/Palliative Education Resource Center, Medical College of Wisconsin, Milwaukee, www.eperc.mcw.edu/ff_index.htm; and Roxane Laboratories, Inc., label for dolophine hydrochloride CH (methadone hydrochloride tablets).
- American Methadone Treatment Association. 1998 Methadone Maintenance Program and Patient Census in the U.S., New York, NY, April 1999.
- Office of National Drug Control Policy. Fact sheet. Available at: www.whitehousedrugpolicy.gov. Last accessed June 4, 2007.
- Finn S, Tuckwiller T. The Killer Cure. The Charlotte (W. Va.) Gazette. Available at: www.wvgazette.com. Last accessed June 29, 2007.
- Weaver MF, Schnoll SH. Opioid treatment of chronic pain in patients with addiction. J Pain Palliat Care Pharmacother. 2002:16(3);5-26.
- Bruera E, Sweeney C. Methadone use in cancer patients with pain: A review. J Palliat Med. 2002 Feb;5(1):127-137.
- Bruera E, Palmer JL, Bosnjak S, et al. Methadone versus morphine as a first-line strong opioid for cancer pain: A randomized, double-blind study. J Clin Oncol. 2004 Jan 1;22(1):185-192.
Few topics in hospital-based pain management generate such diverse viewpoints as the use of methadone as an analgesic. Increasingly ordered by hospice physicians and some hospitalists as a tool for managing difficult pain cases, it is also coming under scrutiny for risks related to cardiac complications, respiratory depression, and the challenges of determining appropriate doses.
For some, the risks are grave enough to contraindicate methadone prescription for use in routine hospital practice, unless the hospitalist is well-versed in its use and has access to a pharmacist or other pain expert to review medication orders. Hospitalists should also be aware of the Food and Drug Administration’s strongly worded November 2006 Public Health Advisory, “Methadone Use for Pain Control May Result in Death and Life-Threatening Changes in Breathing and Heart Beat.”1
The FDA stopped short of recommending against the use of methadone as an analgesic, but it admonished physicians to use caution when prescribing it for patients unused to the drug—particularly during drug initiation, conversion from another opioid, or titration upward. Patients should be cautioned to take the drug exactly as prescribed. The advisory also recommended a new maximum initial dose of 30 mg per day (typically prescribed 10 mg tid), when initiating methadone for pain management.
Jean Youngwerth, MD, associate program director of the University of Colorado at Denver and Health Sciences Center, is well aware of the benefits and risks of prescribing methadone in her dual roles as hospitalist and palliative care consultant. “It is a great analgesic, becoming a lot more popular in recent years,” Dr. Youngwerth says. “It’s also ridiculously cheap [often under a dollar a day], which is an important consideration for some of our patients returning home with chronic pain. For other patients, it’s an extra analgesic tool, giving good pain relief, especially for refractory somatic or neuropathic pain.”
But methadone also has downsides. “Its pharmaco-kinetics are so complex and poorly understood that people unfamiliar with prescribing it can get in trouble in a hurry,” Dr. Youngwerth says. “You can kill people with oversedation. I do not prescribe it in my hospitalist practice and I discourage other hospitalists from prescribing it for their patients without consulting a pain or palliative care specialist. You don’t see its maximal effects until at least three to four days out, and that’s usually too long for the hospitalist. When I start palliative care patients on methadone, I normally keep them in the hospital for three or four days so that I can monitor the effects.”
Dr. Youngwerth believes her experience in pain management justifies prescribing methadone for palliative care patients. Even so, she always calls one of the local physician pain experts she works with or a hospital pharmacist to make sure she is ordering it safely and correctly. “I don’t think doctors should be scared off by all the bad publicity about methadone, but they need to realize these are valid concerns,” she says. “People run into problems when they assume that it is just another opioid.”
Hospitalists must recognize the stark realities of using the drug.
“Methadone is the easiest opioid to kill someone with,” says Gail Gazelle, MD, palliative care physician with MD Can Help and Harvard Medical School in Boston. “At the same time, its unique properties can give analgesic effects you can get from no other drug.”
For hospitalists, these issues are complicated by their short involvement with hospitalized patients, who are quickly prepped for discharge back to various community settings and living situations.
Dr. Gazelle wonders, “Is a short hospital stay the right place to initiate methadone treatment, given all of the complications?” If the answer is yes, communicating with the attending and agreeing on a plan for its continued use after discharge from the hospital are essential.
Although narcotic abuse is notoriously difficult to manage, with high relapse rates in every setting, methadone maintenance therapy (MMT) has been shown to reduce overall rates of abuse of other drugs, overdose and death, criminal activity, needle sharing, and commercial sex work. Methadone maintenance is a long-term strategy. The drug is provided as a substitute, not a cure, for narcotic abuse. Patients may continue to receive their daily maintenance dose for years. One-year retention rates in several large studies of MMT have ranged from 25% to 60%, while rates of relapse after leaving MMT are high. Stopping methadone use poses the same challenges as quitting any narcotic and should only be done under a doctor’s care.
Methadone is also used to treat heroin withdrawal, an issue for some hospitalized patients. Prescribing methadone for maintenance therapy is limited to federally licensed methadone treatment programs.
Complications
There are several critical facets of this drug hospitalists must be aware of:
Unpredictable half-life: Methadone, relative to other opioids, has high lipid solubility, slow metabolism, and a typical half-life ranging from 15 to 60 hours—although it can be longer. Methadone’s analgesic effect is shorter-lived, so analgesic doses should be given two, three, or four times daily. But the longer half-life means it can take three days or more after the initial dose before the drug’s full effect—on respiration for example—is known. That is why therapeutic doses can build to toxic levels. There is also wide variation in its effects among patients. Guidelines suggest titrating methadone upward for increased analgesic effect should not be attempted until at least three days after the first dose.
Respiratory depression: This is an issue when the drug is initiated in an opioid-naïve patient or is too rapidly titrated. Deaths from methadone have been seen at doses once considered safe. Physicians are cautioned to start patients on low doses while using other, short-acting opioids for breakthrough pain and frequently assessing for signs of overdose or respiratory depression, such as difficulty in breathing, shallow breathing, extreme sleepiness, or inability to think, talk, or walk normally.
Effect on heart arrhythmias: Methadone can prolong the QTc interval in heart function, leading to a potentially serious cardiac abnormality known as torsade de pointes. The potential for cardiac deaths is another complicating factor that may contraindicate methadone for patients at risk for developing a prolonged QTc interval, including patients with cardiac hypertrophy, hypokalemia, or hypomagnesemia, or a history of cardiac conduction abnormalities or taking medications affecting cardiac conduction. A current EKG may be an appropriate precaution when initiating methadone in elderly patients who have a cardiac history or are receiving methadone in high doses or by intravenous administration.
Drug interactions: The list of drugs that interact with methadone is long, with potential for unwanted side effects and increased or decreased potency. This list includes most anti-retroviral treatments for HIV; sedatives, tranquilizers, barbiturates, seizure medications, muscle relaxants, or any central nervous system depressants; certain steroids and anti-fungals; even over-the-counter cough and cold medications. Also watch for medications that treat irregular heartbeat or prolong the QTc interval. Pharmaceutical company labels for methadone, as well as the FDA advisory, contain a more complete list of drugs that interact with methadone. Always review with patients the other medications they take, including over-the-counter medications and alternative treatments.
Other complications: Methadone should not be prescribed when opioids in general are contraindicated. It can be a management challenge to convert patients from methadone back to other opioids. Another complication of prescribing methadone is the negative publicity it has received in recent years, combined with the stigma of its associations with drug treatment. Some patients, families, or attending physicians may be leery of using it as a pain reliever. It may not be worth titrating methadone to the correct dose in the hospital if it is going to be discontinued post-discharge.
A series of articles in The Charleston (W.Va.) Gazette starting in June 2006 alerted many to the fact methadone is listed by medical examiners nationwide as a cause of death more often than any other prescription pain reliever.10 Methadone was implicated in nearly 4,000 deaths in 2006, four times as many as in 1999. Most of the deaths were considered accidental, and many involved combinations with other drugs—although some were in patients taking methadone as prescribed.
Hospitalists can expect that the diverse caseloads they see likely will include some patients taking methadone. Some may be getting it as an analgesic prescribed by a community physician, pain clinic, or hospice. Others in MMT and receiving a daily dose to manage their addiction disorder may present at the hospital with a different medical problem and perhaps new pain issues.
The hospitalist should not take it for granted that patients on MMT are not also intoxicated or abusing methadone or other drugs, says Michael Weaver, MD, pain and addiction specialist at Virginia Commonwealth University Medical Center in Richmond. Nor should they assume MMT doses are providing adequate analgesia.
“The bottom line in all of these situations is communication,” Dr. Weaver says. Talk to the medical director of the methadone clinic or the community physician who prescribed methadone as an analgesic. Verify the patient’s status, confirm dosage, and discuss the pain issues that need to be addressed—while recognizing pain relief is an appropriate expectation of any hospitalized patient, regardless of drug history or treatment.
Generally, Dr. Weaver says, the maintenance dose of methadone would continue during the hospital stay, and a different analgesic would be ordered for the pain—although the clinic physician may have other ideas. Changing methadone dose or schedule—or attempting to wean a patient off methadone—is not a decision a hospitalist should make unilaterally.11
Methadone’s Merits
Why would a hospitalist want to prescribe a drug that comes with so many caveats?
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., uses methadone—often in combination with the anti-depressant desipramine or the anticonvulsant gabapentin—to treat complex regional pain syndromes and neuropathic pain.
Dr. Jessop carefully assesses patients for neuropathic pain, listening for descriptors such as burning, stinging, or numbing. These are the patients for whom she most often receives palliative care consultations, often following years of out-of-control pain or lack of response to high doses of other opioids. “My job is much easier now that I understand the difference between nocioceptive and neuropathic pain,” she notes.
“I think methadone is magic, perhaps due to its effect on the NMDA (n-methyl, d-asparte) receptors,” Dr. Jessop says. “I’m also convinced from my clinical experience that there can be nerve healing going on when these pain syndromes are effectively treated. I had a patient with horrible phantom pain following multiple hip surgeries and amputation of his leg. He was referred by a family practitioner, who said, ‘I cannot get this man’s pain under control.’ ” The patient’s pain is now controlled with methadone, 30 mg three times a day. He rarely needs to take his hydromorphone (Dilaudid) for breakthrough pain, and he is able to use his prosthetic leg—which would have been unthinkable before.
Dr. Jessop believes low doses of methadone—even lower than the conversion charts recommend—can have a big effect. “I don’t have problems with methadone because I’m so careful in prescribing it,” she says. “I also work closely with the attending physician and give patients my cell phone number when they return home. It is important to get the family involved and to be clear about the risks and benefits.”
Brad Stuart, MD, senior medical director of Sutter VNA and Hospice in Northern California, also believes methadone can be a wonderful pain management tool. “There is no substitute, in my estimation, to adding a little methadone to the opioid regimen—even just 5 mg of liquid three times a day—for difficult neuropathic pain cases,” Dr. Stuart says. “It’s true that you don’t want to raise the dose too quickly. But I find that it’s unusual not to see benefit in these kinds of patients. I disagree with those who would advise hospitalists to stay away from methadone for treating refractory neuropathic pain. If you start slow and go slow, the risks are small relative to the gain.”
Eduardo Bruera, MD, a palliative care physician at M.D. Anderson Cancer Center in Houston, is another believer in methadone for difficult pain cases, although he emphasizes that his experience is limited to the pain associated with cancer. Dr. Bruera does not use methadone as a first-line analgesic, but he finds it effective when other opioids have not been. “Patients who continue to have a lot of pain after multiple escalating opioid doses or signs of opioid toxicity may be signaling that the opioid you’re using is not working,” he says. “If we make three or four dose changes without response, it’s time to change the opioid.”
Dr. Bruera acknowledges that persuasive research studies to establish methadone’s purported efficacy in treating neuropathic pain have not been conducted. “Unfortunately, methadone is an orphan drug, so we don’t know who would pay for those studies. Should we consider it as a first-line opioid for cancer pain? Again, that is an unanswered question.”
Dr. Bruera has been involved in a number of the few published studies and reviews of methadone’s analgesic efficacy, and he is engaged in ongoing orphan drug status research.12, 13
Methadone as Analgesic
Stephen Bekanich, MD, hospitalist and palliative care consultant at the University of Utah Medical Center in Salt Lake City, falls in the middle range of opinions on methadone.
“From the hospitalist’s standpoint, there are downsides,” Dr. Bekanich says. “People who don’t understand how to titrate it may change doses on a daily basis or more often, which is dangerous. They may not understand the dosing equivalents or pay enough attention to drug interactions.
“Of all the opioids, careful assessment and follow-up may be the most important with methadone. Always make sure you have concrete post-discharge plans. If I didn’t have a pharmacist to collaborate with, as a hospitalist I’d probably stay away from it. But it’s different when I put on my palliative care hat.”
Rachelle Bernacki, MD, a hospitalist, palliative care physician and geriatrician at the University of California-San Francisco Medical Center, agrees methadone can be a useful analgesic—particularly when other opioids have failed to relieve the pain. “But I don’t start with it; I may add a small dose of methadone to the existing regimen for complex pain,” she says.
“I caution my residents not to try methadone without consulting with someone familiar with the drug,” explains Dr. Bernacki, who adds that she is fortunate to work with a pharmacist at UCSF who is an expert in pain management and palliative medicine. “Having taught residents, I can confirm that there is a lot of confusion about its use. But I have also used methadone in my outpatient geriatric practice—with fantastic results.”
Paresh Patel, MD, a hospitalist at VCU Medical Center, says he and his colleagues use methadone as a second-line analgesic when pain is not well managed with morphine. He always keeps an eye out for the risks, including potential interactions with psychiatric medications and the need to look at EKGs.
Dr. Patel says conversion from other opioids is one of the biggest challenges in using methadone. He is not satisfied with the various published opioid conversion charts and relies on experience and trial and error. “I always wait 48 hours before titrating up,” he says.
More research is needed in this area, Dr. Patel says, and he is thinking of getting involved in a methadone research project. TH
Larry Beresford is a frequent contributor to The Hospitalist.
References
- Food and Drug Administration. FDA Public Health Advisory: Methadone use for pain control may result in death and life-threatening changes in breathing and heart beat. Available at: www.fda.gov/cder/drug/advisory/methadone.htm. Last accessed June 4, 2007.
- Moulin DE, Palma D, Watling C, et al. Methadone in the management of intractable neuropathic noncancer pain. Can J Neurol Sci. 2005 Aug: 32(3); 340-343.
- Altier N, Dion D, Boulanger A, et al. Management of chronic neuropathic pain with methadone: A review of 13 cases. Clin J Pain. 2005 Jul-Aug;21(4):364-369.
- Morley JS, Bridson J, Nash TP, et al. Low-dose methadone has an analgesic effect in neuropathic pain: A double-blind randomized controlled crossover trial. Palliat Med. 2003 Oct;17(7):576-587.
- Lawlor PG, Turner KS, Hanson J, et al. Dose ratio between morphine and methadone in patients with cancer pain: A retrospective study. Cancer. 1998 Mar;82(6):1167-1173.
- Ripamonti C, De Conno F, Groff L, et al. Equianal-gesic dose/ratio between methadone and other opioid agonists in cancer pain: Comparison of two clinical experiences. Ann Oncol. 1998 Jan;9(1):79-83.
- Gazelle G, Fine PG. Fast Fact and Concept #75: Methadone for the treatment of pain. End-of-Life/Palliative Education Resource Center, Medical College of Wisconsin, Milwaukee, www.eperc.mcw.edu/ff_index.htm; and Roxane Laboratories, Inc., label for dolophine hydrochloride CH (methadone hydrochloride tablets).
- American Methadone Treatment Association. 1998 Methadone Maintenance Program and Patient Census in the U.S., New York, NY, April 1999.
- Office of National Drug Control Policy. Fact sheet. Available at: www.whitehousedrugpolicy.gov. Last accessed June 4, 2007.
- Finn S, Tuckwiller T. The Killer Cure. The Charlotte (W. Va.) Gazette. Available at: www.wvgazette.com. Last accessed June 29, 2007.
- Weaver MF, Schnoll SH. Opioid treatment of chronic pain in patients with addiction. J Pain Palliat Care Pharmacother. 2002:16(3);5-26.
- Bruera E, Sweeney C. Methadone use in cancer patients with pain: A review. J Palliat Med. 2002 Feb;5(1):127-137.
- Bruera E, Palmer JL, Bosnjak S, et al. Methadone versus morphine as a first-line strong opioid for cancer pain: A randomized, double-blind study. J Clin Oncol. 2004 Jan 1;22(1):185-192.
Few topics in hospital-based pain management generate such diverse viewpoints as the use of methadone as an analgesic. Increasingly ordered by hospice physicians and some hospitalists as a tool for managing difficult pain cases, it is also coming under scrutiny for risks related to cardiac complications, respiratory depression, and the challenges of determining appropriate doses.
For some, the risks are grave enough to contraindicate methadone prescription for use in routine hospital practice, unless the hospitalist is well-versed in its use and has access to a pharmacist or other pain expert to review medication orders. Hospitalists should also be aware of the Food and Drug Administration’s strongly worded November 2006 Public Health Advisory, “Methadone Use for Pain Control May Result in Death and Life-Threatening Changes in Breathing and Heart Beat.”1
The FDA stopped short of recommending against the use of methadone as an analgesic, but it admonished physicians to use caution when prescribing it for patients unused to the drug—particularly during drug initiation, conversion from another opioid, or titration upward. Patients should be cautioned to take the drug exactly as prescribed. The advisory also recommended a new maximum initial dose of 30 mg per day (typically prescribed 10 mg tid), when initiating methadone for pain management.
Jean Youngwerth, MD, associate program director of the University of Colorado at Denver and Health Sciences Center, is well aware of the benefits and risks of prescribing methadone in her dual roles as hospitalist and palliative care consultant. “It is a great analgesic, becoming a lot more popular in recent years,” Dr. Youngwerth says. “It’s also ridiculously cheap [often under a dollar a day], which is an important consideration for some of our patients returning home with chronic pain. For other patients, it’s an extra analgesic tool, giving good pain relief, especially for refractory somatic or neuropathic pain.”
But methadone also has downsides. “Its pharmaco-kinetics are so complex and poorly understood that people unfamiliar with prescribing it can get in trouble in a hurry,” Dr. Youngwerth says. “You can kill people with oversedation. I do not prescribe it in my hospitalist practice and I discourage other hospitalists from prescribing it for their patients without consulting a pain or palliative care specialist. You don’t see its maximal effects until at least three to four days out, and that’s usually too long for the hospitalist. When I start palliative care patients on methadone, I normally keep them in the hospital for three or four days so that I can monitor the effects.”
Dr. Youngwerth believes her experience in pain management justifies prescribing methadone for palliative care patients. Even so, she always calls one of the local physician pain experts she works with or a hospital pharmacist to make sure she is ordering it safely and correctly. “I don’t think doctors should be scared off by all the bad publicity about methadone, but they need to realize these are valid concerns,” she says. “People run into problems when they assume that it is just another opioid.”
Hospitalists must recognize the stark realities of using the drug.
“Methadone is the easiest opioid to kill someone with,” says Gail Gazelle, MD, palliative care physician with MD Can Help and Harvard Medical School in Boston. “At the same time, its unique properties can give analgesic effects you can get from no other drug.”
For hospitalists, these issues are complicated by their short involvement with hospitalized patients, who are quickly prepped for discharge back to various community settings and living situations.
Dr. Gazelle wonders, “Is a short hospital stay the right place to initiate methadone treatment, given all of the complications?” If the answer is yes, communicating with the attending and agreeing on a plan for its continued use after discharge from the hospital are essential.
Although narcotic abuse is notoriously difficult to manage, with high relapse rates in every setting, methadone maintenance therapy (MMT) has been shown to reduce overall rates of abuse of other drugs, overdose and death, criminal activity, needle sharing, and commercial sex work. Methadone maintenance is a long-term strategy. The drug is provided as a substitute, not a cure, for narcotic abuse. Patients may continue to receive their daily maintenance dose for years. One-year retention rates in several large studies of MMT have ranged from 25% to 60%, while rates of relapse after leaving MMT are high. Stopping methadone use poses the same challenges as quitting any narcotic and should only be done under a doctor’s care.
Methadone is also used to treat heroin withdrawal, an issue for some hospitalized patients. Prescribing methadone for maintenance therapy is limited to federally licensed methadone treatment programs.
Complications
There are several critical facets of this drug hospitalists must be aware of:
Unpredictable half-life: Methadone, relative to other opioids, has high lipid solubility, slow metabolism, and a typical half-life ranging from 15 to 60 hours—although it can be longer. Methadone’s analgesic effect is shorter-lived, so analgesic doses should be given two, three, or four times daily. But the longer half-life means it can take three days or more after the initial dose before the drug’s full effect—on respiration for example—is known. That is why therapeutic doses can build to toxic levels. There is also wide variation in its effects among patients. Guidelines suggest titrating methadone upward for increased analgesic effect should not be attempted until at least three days after the first dose.
Respiratory depression: This is an issue when the drug is initiated in an opioid-naïve patient or is too rapidly titrated. Deaths from methadone have been seen at doses once considered safe. Physicians are cautioned to start patients on low doses while using other, short-acting opioids for breakthrough pain and frequently assessing for signs of overdose or respiratory depression, such as difficulty in breathing, shallow breathing, extreme sleepiness, or inability to think, talk, or walk normally.
Effect on heart arrhythmias: Methadone can prolong the QTc interval in heart function, leading to a potentially serious cardiac abnormality known as torsade de pointes. The potential for cardiac deaths is another complicating factor that may contraindicate methadone for patients at risk for developing a prolonged QTc interval, including patients with cardiac hypertrophy, hypokalemia, or hypomagnesemia, or a history of cardiac conduction abnormalities or taking medications affecting cardiac conduction. A current EKG may be an appropriate precaution when initiating methadone in elderly patients who have a cardiac history or are receiving methadone in high doses or by intravenous administration.
Drug interactions: The list of drugs that interact with methadone is long, with potential for unwanted side effects and increased or decreased potency. This list includes most anti-retroviral treatments for HIV; sedatives, tranquilizers, barbiturates, seizure medications, muscle relaxants, or any central nervous system depressants; certain steroids and anti-fungals; even over-the-counter cough and cold medications. Also watch for medications that treat irregular heartbeat or prolong the QTc interval. Pharmaceutical company labels for methadone, as well as the FDA advisory, contain a more complete list of drugs that interact with methadone. Always review with patients the other medications they take, including over-the-counter medications and alternative treatments.
Other complications: Methadone should not be prescribed when opioids in general are contraindicated. It can be a management challenge to convert patients from methadone back to other opioids. Another complication of prescribing methadone is the negative publicity it has received in recent years, combined with the stigma of its associations with drug treatment. Some patients, families, or attending physicians may be leery of using it as a pain reliever. It may not be worth titrating methadone to the correct dose in the hospital if it is going to be discontinued post-discharge.
A series of articles in The Charleston (W.Va.) Gazette starting in June 2006 alerted many to the fact methadone is listed by medical examiners nationwide as a cause of death more often than any other prescription pain reliever.10 Methadone was implicated in nearly 4,000 deaths in 2006, four times as many as in 1999. Most of the deaths were considered accidental, and many involved combinations with other drugs—although some were in patients taking methadone as prescribed.
Hospitalists can expect that the diverse caseloads they see likely will include some patients taking methadone. Some may be getting it as an analgesic prescribed by a community physician, pain clinic, or hospice. Others in MMT and receiving a daily dose to manage their addiction disorder may present at the hospital with a different medical problem and perhaps new pain issues.
The hospitalist should not take it for granted that patients on MMT are not also intoxicated or abusing methadone or other drugs, says Michael Weaver, MD, pain and addiction specialist at Virginia Commonwealth University Medical Center in Richmond. Nor should they assume MMT doses are providing adequate analgesia.
“The bottom line in all of these situations is communication,” Dr. Weaver says. Talk to the medical director of the methadone clinic or the community physician who prescribed methadone as an analgesic. Verify the patient’s status, confirm dosage, and discuss the pain issues that need to be addressed—while recognizing pain relief is an appropriate expectation of any hospitalized patient, regardless of drug history or treatment.
Generally, Dr. Weaver says, the maintenance dose of methadone would continue during the hospital stay, and a different analgesic would be ordered for the pain—although the clinic physician may have other ideas. Changing methadone dose or schedule—or attempting to wean a patient off methadone—is not a decision a hospitalist should make unilaterally.11
Methadone’s Merits
Why would a hospitalist want to prescribe a drug that comes with so many caveats?
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., uses methadone—often in combination with the anti-depressant desipramine or the anticonvulsant gabapentin—to treat complex regional pain syndromes and neuropathic pain.
Dr. Jessop carefully assesses patients for neuropathic pain, listening for descriptors such as burning, stinging, or numbing. These are the patients for whom she most often receives palliative care consultations, often following years of out-of-control pain or lack of response to high doses of other opioids. “My job is much easier now that I understand the difference between nocioceptive and neuropathic pain,” she notes.
“I think methadone is magic, perhaps due to its effect on the NMDA (n-methyl, d-asparte) receptors,” Dr. Jessop says. “I’m also convinced from my clinical experience that there can be nerve healing going on when these pain syndromes are effectively treated. I had a patient with horrible phantom pain following multiple hip surgeries and amputation of his leg. He was referred by a family practitioner, who said, ‘I cannot get this man’s pain under control.’ ” The patient’s pain is now controlled with methadone, 30 mg three times a day. He rarely needs to take his hydromorphone (Dilaudid) for breakthrough pain, and he is able to use his prosthetic leg—which would have been unthinkable before.
Dr. Jessop believes low doses of methadone—even lower than the conversion charts recommend—can have a big effect. “I don’t have problems with methadone because I’m so careful in prescribing it,” she says. “I also work closely with the attending physician and give patients my cell phone number when they return home. It is important to get the family involved and to be clear about the risks and benefits.”
Brad Stuart, MD, senior medical director of Sutter VNA and Hospice in Northern California, also believes methadone can be a wonderful pain management tool. “There is no substitute, in my estimation, to adding a little methadone to the opioid regimen—even just 5 mg of liquid three times a day—for difficult neuropathic pain cases,” Dr. Stuart says. “It’s true that you don’t want to raise the dose too quickly. But I find that it’s unusual not to see benefit in these kinds of patients. I disagree with those who would advise hospitalists to stay away from methadone for treating refractory neuropathic pain. If you start slow and go slow, the risks are small relative to the gain.”
Eduardo Bruera, MD, a palliative care physician at M.D. Anderson Cancer Center in Houston, is another believer in methadone for difficult pain cases, although he emphasizes that his experience is limited to the pain associated with cancer. Dr. Bruera does not use methadone as a first-line analgesic, but he finds it effective when other opioids have not been. “Patients who continue to have a lot of pain after multiple escalating opioid doses or signs of opioid toxicity may be signaling that the opioid you’re using is not working,” he says. “If we make three or four dose changes without response, it’s time to change the opioid.”
Dr. Bruera acknowledges that persuasive research studies to establish methadone’s purported efficacy in treating neuropathic pain have not been conducted. “Unfortunately, methadone is an orphan drug, so we don’t know who would pay for those studies. Should we consider it as a first-line opioid for cancer pain? Again, that is an unanswered question.”
Dr. Bruera has been involved in a number of the few published studies and reviews of methadone’s analgesic efficacy, and he is engaged in ongoing orphan drug status research.12, 13
Methadone as Analgesic
Stephen Bekanich, MD, hospitalist and palliative care consultant at the University of Utah Medical Center in Salt Lake City, falls in the middle range of opinions on methadone.
“From the hospitalist’s standpoint, there are downsides,” Dr. Bekanich says. “People who don’t understand how to titrate it may change doses on a daily basis or more often, which is dangerous. They may not understand the dosing equivalents or pay enough attention to drug interactions.
“Of all the opioids, careful assessment and follow-up may be the most important with methadone. Always make sure you have concrete post-discharge plans. If I didn’t have a pharmacist to collaborate with, as a hospitalist I’d probably stay away from it. But it’s different when I put on my palliative care hat.”
Rachelle Bernacki, MD, a hospitalist, palliative care physician and geriatrician at the University of California-San Francisco Medical Center, agrees methadone can be a useful analgesic—particularly when other opioids have failed to relieve the pain. “But I don’t start with it; I may add a small dose of methadone to the existing regimen for complex pain,” she says.
“I caution my residents not to try methadone without consulting with someone familiar with the drug,” explains Dr. Bernacki, who adds that she is fortunate to work with a pharmacist at UCSF who is an expert in pain management and palliative medicine. “Having taught residents, I can confirm that there is a lot of confusion about its use. But I have also used methadone in my outpatient geriatric practice—with fantastic results.”
Paresh Patel, MD, a hospitalist at VCU Medical Center, says he and his colleagues use methadone as a second-line analgesic when pain is not well managed with morphine. He always keeps an eye out for the risks, including potential interactions with psychiatric medications and the need to look at EKGs.
Dr. Patel says conversion from other opioids is one of the biggest challenges in using methadone. He is not satisfied with the various published opioid conversion charts and relies on experience and trial and error. “I always wait 48 hours before titrating up,” he says.
More research is needed in this area, Dr. Patel says, and he is thinking of getting involved in a methadone research project. TH
Larry Beresford is a frequent contributor to The Hospitalist.
References
- Food and Drug Administration. FDA Public Health Advisory: Methadone use for pain control may result in death and life-threatening changes in breathing and heart beat. Available at: www.fda.gov/cder/drug/advisory/methadone.htm. Last accessed June 4, 2007.
- Moulin DE, Palma D, Watling C, et al. Methadone in the management of intractable neuropathic noncancer pain. Can J Neurol Sci. 2005 Aug: 32(3); 340-343.
- Altier N, Dion D, Boulanger A, et al. Management of chronic neuropathic pain with methadone: A review of 13 cases. Clin J Pain. 2005 Jul-Aug;21(4):364-369.
- Morley JS, Bridson J, Nash TP, et al. Low-dose methadone has an analgesic effect in neuropathic pain: A double-blind randomized controlled crossover trial. Palliat Med. 2003 Oct;17(7):576-587.
- Lawlor PG, Turner KS, Hanson J, et al. Dose ratio between morphine and methadone in patients with cancer pain: A retrospective study. Cancer. 1998 Mar;82(6):1167-1173.
- Ripamonti C, De Conno F, Groff L, et al. Equianal-gesic dose/ratio between methadone and other opioid agonists in cancer pain: Comparison of two clinical experiences. Ann Oncol. 1998 Jan;9(1):79-83.
- Gazelle G, Fine PG. Fast Fact and Concept #75: Methadone for the treatment of pain. End-of-Life/Palliative Education Resource Center, Medical College of Wisconsin, Milwaukee, www.eperc.mcw.edu/ff_index.htm; and Roxane Laboratories, Inc., label for dolophine hydrochloride CH (methadone hydrochloride tablets).
- American Methadone Treatment Association. 1998 Methadone Maintenance Program and Patient Census in the U.S., New York, NY, April 1999.
- Office of National Drug Control Policy. Fact sheet. Available at: www.whitehousedrugpolicy.gov. Last accessed June 4, 2007.
- Finn S, Tuckwiller T. The Killer Cure. The Charlotte (W. Va.) Gazette. Available at: www.wvgazette.com. Last accessed June 29, 2007.
- Weaver MF, Schnoll SH. Opioid treatment of chronic pain in patients with addiction. J Pain Palliat Care Pharmacother. 2002:16(3);5-26.
- Bruera E, Sweeney C. Methadone use in cancer patients with pain: A review. J Palliat Med. 2002 Feb;5(1):127-137.
- Bruera E, Palmer JL, Bosnjak S, et al. Methadone versus morphine as a first-line strong opioid for cancer pain: A randomized, double-blind study. J Clin Oncol. 2004 Jan 1;22(1):185-192.