User login
Improved Prophylaxis Following Education
Venous thromboembolism (VTE), which encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major cause of the morbidity and mortality of hospitalized medical patients.1 Hospitalization for an acute medical illness has been associated with an 8‐fold increase in the relative risk of VTE and is responsible for approximately a quarter of all VTE cases in the general population.2, 3
Current evidence‐based guidelines, including those from the American College of Chest Physicians (ACCP), recommend prophylaxis with low‐dose unfractionated heparin (UFH) or low‐molecular‐weight heparin (LMWH) for medical patients with risk factors for VTE.4, 5 Mechanical prophylaxis methods including graduated compression stockings and intermittent pneumatic compression are recommended for those patients for whom anticoagulant therapy is contraindicated because of a high risk of bleeding.4, 5 However, several studies have shown that adherence to these guidelines is suboptimal, with many at‐risk patients receiving inadequate prophylaxis (range 32%‐87%).610
Physician‐related factors identified as potential barriers to guideline adherence include not being aware or familiar with the guidelines, not agreeing with the guidelines, or believing the guideline recommendations to be ineffective.11 More specific studies have shown that some physicians may lack basic knowledge regarding the current treatment standards for VTE and may underestimate the significance of VTE.1213 As distinct strategies, education aimed at disseminating VTE prophylaxis guidelines, as well as regular audit‐and‐feedback of physician performance, has been shown to improve rates of VTE prophylaxis in clinical practice.6, 1417 Implementation of educational programs significantly increased the level of appropriate VTE prophylaxis from 59% to 70% of patients in an Australian hospital15 and from 73% to 97% of patients in a Scottish hospital.14 Another strategy, the use of point‐of‐care electronically provided reminders with decision support, has been successful not only in increasing the rates of VTE prophylaxis, but also in decreasing the incidence of clinical VTE events.16 Although highly effective, electronic alerts with computerized decision support do not exist in many hospitals, and other methods of intervention are needed.
In this study, we evaluated adherence to the 2001 ACCP guidelines for VTE prophylaxis among medical patients in our teaching hospital. (The guidelines were updated in 2004, after our study was completed.) After determining that our baseline rates of appropriate VTE prophylaxis were suboptimal, we developed, implemented, and evaluated a multifaceted strategy to improve the rates of appropriate thromboprophylaxis among our medical inpatients.
Six categories of quality improvement strategies have been described: provider education, decision support, audit‐and‐feedback, patient education, organization change, and regulation and policy.18 The intervention we developed was a composite of 3 of these: provider education, decision support, and audit‐and‐feedback.
METHODS
Study Design and Patients
This was a before‐and‐after study designed to assess whether implementation of a VTE prophylaxis quality improvement intervention could improve the rate of appropriate thromboprophylaxis in hospitalized medical patients at the State University of New York, Downstate Medical CenterUniversity Hospital of Brooklyn, an urban university teaching hospital of approximately 400 beds. This initiative, conducted as part of a departmental quality assurance and performance improvement program, did not require institutional review board approval. After an informal survey revealed a prophylaxis rate of approximately 50%, a more formal baseline assessment of the rate of medical patients receiving VTE prophylaxis was conducted during October 2002. This assessment was a single sampling of all medical inpatients on 2 of the medical floors on a single day. The results were consistent with those of the informal survey as well as those from an international registry.19 The results from the baseline study indicated that VTE prophylaxis was underused: only 46.9% of our medical inpatients received any form of prophylaxis. The prophylaxis rate was assessed again in 2 sampling periods beginning 12 and 18 months after implementation of the intervention. Data were collected monthly and combined into 3‐month blocks. The first postintervention sample (n = 116 patient charts) was drawn from a period 12‐14 months after implementation and the second (n = 147 patient charts) from a period 18‐20 months after implementation.
On a randomly designated day in the latter half of each month during each sampling period, all charts on 2 primary medical floors were reviewed and included in the retrospective analysis. Patients who were not on the medical service were excluded from analysis. Patients, as well as their medications, were identified using a list generated from our pharmacy database. We chose this method and schedule for several reasons. First, we sought to reduce the likelihood of including a patient more than once in a monthly sample. Second, by waiting for the latter half of the month we sought to allow house staff a chance to acquire knowledge from the educational program introduced on the first day of the month. Third, we wanted to allow house staff the time to actualize new attitudes reinforced by the audit‐and‐feedback element. The house staff included approximately 4 interns and 4 residents each month plus 10‐15 attendings or hospitalists.
Data Collection
For each sampling period we conducted a medical record (paper) review, and the Division Chief of General Internal Medicine also interviewed the medical house staff and attending physicians. Data collected included risk factors for VTE, contraindications to anticoagulant prophylaxis, type of VTE prophylaxis received, and appropriateness of the prophylaxis. Prophylaxis was considered appropriate when it was given in accordance with a risk stratification scheme (Table 1) adapted from the 2001 ACCP guideline recommendations for surgical patients20 and modified for medical inpatients, similar to the risk assessment model by Caprini et al.21 Prophylaxis was also considered appropriate when no prophylaxis was given for low‐risk patients or when full anticoagulation was given for another indication (Table 1). Questionable prophylaxis was defined as UFH given every 12 hours to a high‐risk patient. All other prophylaxis was deemed inappropriate (including no prophylaxis if prophylaxis was indicated, use of enoxaparin at incorrect prophylactic doses such as 60 or 20 mg, IPC alone for a high‐risk patient with no contraindication to pharmacological prophylaxis, and the use of warfarin if no other indication for it). The risk factors for thromboembolism and contraindications to anticoagulant prophylaxis are given in Table 2. Non‐ambulatory was defined as an order for bed rest with or without bathroom privileges or was judged based on information obtained from the medical house staff and nurses about whether the patient was ambulatory or had been observed walking outside his or her room. Data on pharmacological prophylaxis were obtained from the hospital pharmacy. Information on use of mechanical prophylaxis was obtained by house staff interviews or review of the order sheet. The house officer or attending physician of each patient was interviewed retrospectively to determine the reason for admission and the risk factors for VTE present on admission. Patients were classified as having low, moderate, high, or highest risk for VTE based on their age and any major risk factors for VTE (Table 1).19 All collected data were reported to the Department of Medicine Performance Improvement Committee for independent corroboration.
| Risk category | Definition | Dosage of appropriate prophylaxis | ||
|---|---|---|---|---|
| Age (years) | Additional risk factorsb | Low‐dose unfractionated heparin | LMWH | |
| ||||
| Low (0‐1 risk factors) | <40 | 0‐1 factor | None | None |
| Moderate (2 risk factors) | 40‐60 | 1 factor | 5000 units q12h | 40 mg of enoxaparin or 5000 units of dalteparin |
| High (3‐4 risk factors) | >60 | 1‐2 factors or hypercoagulable state | 5000 units q8h or q12h (q8h recommended for surgical patients) | 40 mg of enoxaparin or 5000 units of dalteparin |
| Highest (5 or more factors) | >40 | Malignancy, prior VTE, or CVA | 5000 units q8h plus IPC | enoxaparin or dalteparin plus IPC |
| Risk factors for thromboembolism |
|---|
| Contraindications to anticoagulant prophylaxis |
| Age > 40 years |
| Infection |
| Inflammatory disease |
| Congestive heart failure |
| Chronic obstructive pulmonary disease |
| Prior venous thromboembolism |
| Cancer |
| Cerebrovascular accident |
| End‐stage renal disease |
| Hypercoagulable state |
| Atrial fibrillation |
| Recent surgery |
| Obesity |
| Non‐ambulatory |
| Active gastrointestinal bleed |
| Central nervous system bleed |
| Thrombocytopenia (platelet count <100,000/L) |
Intervention Strategies
The intervention introduced comprised 3 strategies designed to improve VTE prophylaxis: provider education, decision support, and audit‐and‐feedback.
Provider Education
On the first day of every month, an orientation was given to all incoming medicine house staff by the chief resident that included information on the scope, risk factors, and asymptomatic nature of VTE, the importance of risk stratification, the need to provide adequate prophylaxis, and recommended prophylaxis regimens. A nurse educator also provided information to the nursing staff with the expectation that they would remind physicians to prescribe prophylactic treatment if not ordered initially; however, according to the nurses and house staff, this rarely occurred. Large posters showing VTE risk factors and prophylaxis were displayed at 2 nursing stations and physician charting rooms but were not visible to patients.
Decision Support
Pocket cards containing information on VTE risk factors and prophylaxis options were handed out to the house staff at the beginning of each month. These portable decision support tools assisted physicians in the selection of prophylaxis (a more recent, revised version of the material contained in this pocket guide is available at
Audit‐and‐Feedback
Monthly audits were performed by the Division Chief of General Internal Medicine in order to evaluate the type and appropriateness of VTE prophylaxis prescribed (Table 3). During the orientation at the beginning of the month, the chief resident mentioned that an audit would take place sometime during the rotation. This random audit took place during the last 2 weeks of each month on the same day the data were requested from the pharmacy. Over 1‐2 days, physicians were interviewed either one to one or in a group, depending on the availability of house staff. All house staff and hospitalists were queried about the reasons for admission and the presence of VTE risk factors; physicians received feedback from the Division Chief on VTE risk category, prophylaxis, and appropriateness of prophylaxis treatment of their patients.
| Element | Time/effort required |
|---|---|
| |
| Orientation about VTE risk factors and the need to provide adequate prophylaxis given to all incoming house staff by the chief resident on the first day of every month | 10 min/month |
| Introduction of pocket cards containing information on VTE risk factors and prophylaxis options | 5 min/month |
| In‐hospital education of nurses by the nurse educator | 2 sessions of 1 h |
| Large posters presenting VTE risk factors and prophylaxis displayed in nursing stations and physician charting rooms | 5 min one time only |
| Monthly audits by the Division Chief of General Internal Medicine to evaluate the type and suitability of VTE prophylaxis prescribed | 2 h/month for interviews 2 h/month for record review/ data entry |
Statistical Analysis
Differences in pre‐ and post‐intervention VTE prophylaxis and appropriate VTE prophylaxis rates were analyzed using the chi‐square test for categorical variables and the one‐way analysis of variance test for continuous variables. Differences were considered significant at the 5% level (P = .05).
RESULTS
Patients and Demographics
From October 2002 to August 2004 data were collected from 312 hospitalized medical patients: 49 patients in the baseline group during October 2002, and 116 and 147 at the 12‐ to 14‐month and 18‐ to 20‐month time points, respectively. Thus, approximately 40‐50 patients were randomly selected each month, representing 40% of the general medical service census. Patient demographics were similar between groups (Table 4). Overall, most patients were female (65.7%), and mean age was 61.2 years. The most common admission diagnoses were infection/sepsis (29.5%), chest pain/acute coronary syndromes/myocardial infarction (15.7%), heart failure (10.9%), and malignancy (9.6%). Overall, 7.1% (22 patients) had a contraindication to anticoagulant prophylaxis. The most common contraindication was active gastrointestinal bleeding on the current admission, which occurred in 18 of these patients.
| Baseline (n = 49) | 12 months (n = 116) | 18 months (n = 147) | P valuea | |
|---|---|---|---|---|
| ||||
| Patient demographic | ||||
| Mean age, years (SE) | 59.3 (2.6) | 63.3 (1.6) | 60.1 (1.5) | .25b |
| Men, n (%) | 20 (40.8) | 31 (26.7) | 56 (38.1) | .08 |
| Contraindications to pharmacological prophylaxis, n (%) | 7 (14.3) | 5 (4.3) | 10 (6.8) | .07 |
| Gastrointestinal bleeding | 5 (10.2) | 5 (4.3) | 8 (5.4) | |
| CNS bleeding | 1 (2.0) | 0 (0.0) | 0 (0.0) | |
| Low platelet count | 1 (2.0) | 0 (0.0) | 2 (1.4) | |
| Risk factor | ||||
| Mean number of risk factors (SE) | 3.1 (0.2) | 2.7 (0.1) | 3.0 (0.1) | .05b |
| Non‐ambulatoryc | 46 (93.9) | 73 (89.0) | 112 (80.0)d | .03 |
| Age > 40 years | 39 (79.6) | 101 (87.1) | 122 (83.0) | .44 |
| Cancer | 14 (28.6) | 15 (12.9) | 24 (16.3) | .05 |
| End‐stage renal disease | 13 (26.5) | 29 (25.0) | 36 (24.5) | .96 |
| Congestive heart failure | 11 (22.4) | 23 (19.8) | 28 (19.0) | .87 |
| Infection | 8 (16.3) | 24 (20.7) | 46 (31.3) | .04 |
| Cerebrovascular accident | 8 (16.3) | 12 (10.3) | 15 (10.2) | .47 |
| COPD | 5 (10.2) | 9 (7.8) | 14 (9.5) | .84 |
| Sepsis | 3 (6.1) | 6 (5.2) | 21 (14.3) | .03 |
| Atrial fibrillation | 3 (6.1) | 8 (6.9) | 15 (10.2) | .52 |
| Surgery | 1 (2.0) | 1 (0.9) | 2 (1.4) | .82 |
| Previous venous thromboembolism | 0 (0.0) | 6 (5.2) | 8 (5.4) | .25 |
| Obesity (morbid) | 0 (0.0) | 2 (1.7) | 2 (1.4) | .66 |
| Hypercoagulable state | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Risk Factors for VTE
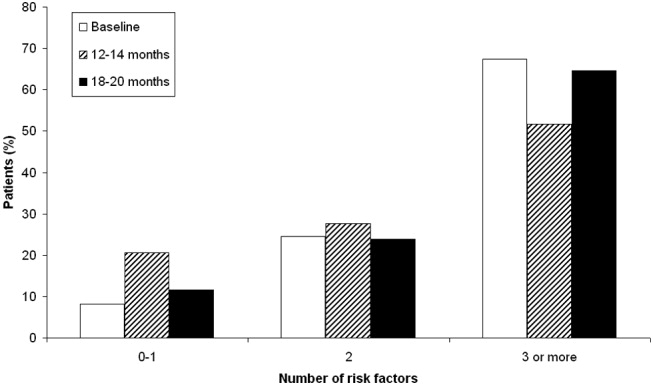
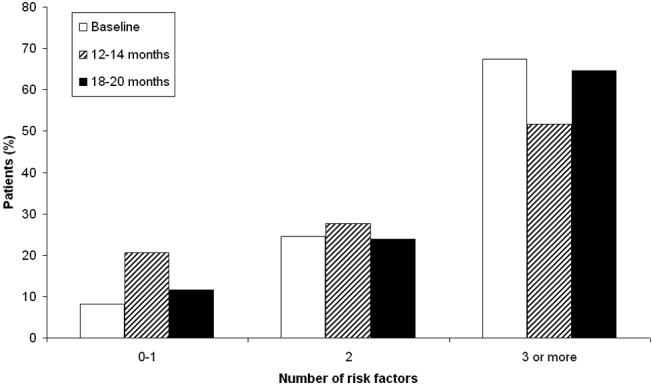
Patient risk factors for VTE in each data collection period are summarized in Table 4. Analysis of this data showed that the most prevalent risk factors for VTE in the 3 patient populations were age older than 40 years (262/312, 84.0% of the total patient population) and nonambulatory state (231/271, 85.2% of the total population). Overall, the average number of risk factors for VTE was approximately 3, with more than 60% of patients having 3 or more VTE risk factors (Fig. 1).
Prophylaxis Use
The types of VTE prophylaxis used and the proportion of patients treated appropriately are summarized for each data collection period in Tables 5 and 6, respectively. In all 3 populations, most patients received pharmacological rather than mechanical prophylaxis, most commonly UFH. At baseline, the prophylaxis decision was appropriate (in accordance with the recommendations of the ACCP guidelines) as often as it was inappropriate (42.9% of patients). The prophylaxis decision was questionable in the remaining 14.3% of patients.
| Prophylaxis type | Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea |
|---|---|---|---|---|---|
| |||||
| Any pharmacological | 22 (44.9) | 94 (81.0) | <.01 | 118 (80.3) | <.01 |
| Any UFH | 17 (34.7) | 61 (52.6) | .04 | 58 (39.5) | .55 |
| IV UFHb | 3 (6.1) | 5 (4.3) | 2 (1.4) | ||
| bid UFHc | 13 (26.5) | 43 (37.1) | 39 (26.5) | ||
| tid UFHc | 1 (2.0) | 10 (8.6) | 16 (10.9) | ||
| qd UFHc | 0 (0.0) | 3 (2.6) | 1 (0.7) | ||
| Any LMWH | 6 (12.2) | 30 (25.9) | .05 | 59 (40.1) | <.01 |
| Mechanical prophylaxis | 1 (2.0) | 7 (6.0) | .28 | 10 (6.8) | .21 |
| Warfarin | 6 (12.2) | 20 (17.2) | .42 | 19 (12.9) | .90 |
| Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea | |
|---|---|---|---|---|---|
| |||||
| Receiving prophylaxis | 23 (46.9) | 100 (86.2) | <.01 | 127 (86.4) | <.01 |
| Appropriate | 21 (42.9) | 79 (68.1) | <.01 | 125 (85.0) | <.01 |
| UFH | 10 (20.4) | 33 (28.4) | .28 | 45 (30.6) | .16 |
| LMWH | 5 (10.2) | 27 (23.3) | .05 | 58 (39.5) | <.01 |
| Questionable | 7 (14.3) | 28 (24.1) | .14 | 14 (9.5) | .35 |
| Inappropriate | 21 (42.9) | 9 (7.8) | <.01 | 8 (5.4) | <.01 |
Change in Prophylaxis Use
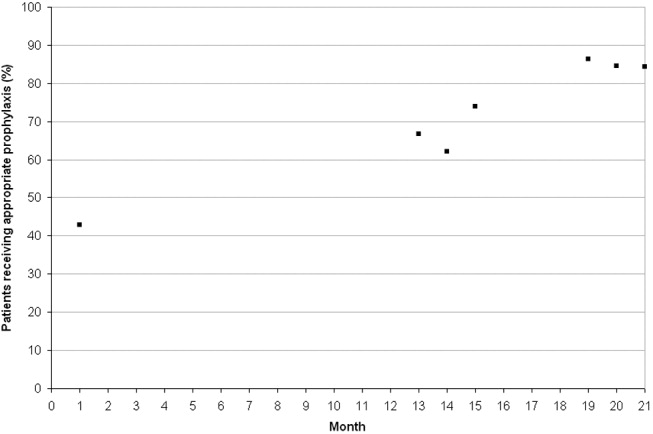
Twelve and 18 months after implementation of the quality improvement program, we observed an increase in the use of any prophylaxis, from 46.9% at baseline to 86.2% and 86.4%, respectively (Table 5; P < .01 in both groups versus baseline). This increase was a result almost entirely of an increase in the proportion of patients receiving pharmacological prophylaxis, which significantly increased, from 44.9% to 81.0% and 80.3%, at the 12‐ and 18‐month time points, respectively (Table 5; P < .01 for both groups versus baseline). Most meaningfully, there was a significant increase in the proportion of patients for whom an appropriate prophylaxis decision was made (from 42.9% to 68.1% and 85.0%, at the 12‐ and 18‐month time points, respectively; Table 6; P < .01 for both groups versus baseline). This represented a trend toward continuing increases in the use of appropriate prophylaxis as the study progressed (Fig. 2). This change was driven mainly by a significant increase in the prescribing of LMWH, almost all of which was prescribed in accordance with the 2001 ACCP guidelines (Table 6).
DISCUSSION
In this study we evaluated the effect of an intervention that combined physician education with a decision support tool and a mechanism for audit‐and‐feedback. We have shown that implementation of such a multifaceted intervention is practical in a teaching hospital and can improve the rates of VTE prophylaxis use in medical patients. In nearly doubling the rate of appropriate prophylaxis, the effect size of our intervention was large, statistically significant, and sustained 18 months after implementation.
More than 60% of our patients had 3 or more risk factors, and more than 80% had at least 2 risk factors. The rate we observed for patients with 3 or more risk factors was 3 times higher than that reported previously.22 Despite the prevalence of high‐risk patients in our study, we observed that the preintervention rate of VTE prophylaxis among medical patients was relatively low at 47%, and only 43% of patients received prophylaxis in accordance with the ACCP guidelines. Our study findings are consistent with those of several other studies that have shown low rates of VTE prophylaxis in medical patients.6, 8, 2324 In a study of 15 hospitals in Massachusetts, only 13%‐19% of medical patients with indications and risk factors for VTE prophylaxis received any prophylaxis prior to an educational intervention.6 Similarly, a study of 368 consecutive medical patients at a Swiss hospital showed that only 22% of those at‐risk received VTE prophylaxis in accordance with the Thromboembolic Risk Factors (THRIFT) I Consensus Group recommendations.8 Results from 2 prospective patient registries also indicated low rates of VTE prophylaxis in medical patients.19, 24 In the IMPROVE registry of acutely ill medical patients, only 39% of patients hospitalized for 3 or more days received VTE prophylaxis19 and in the DVT‐FREE registry only 42% of medical patients with the inpatient diagnosis of DVT had received prophylaxis within 30 days of that diagnosis.24 In a recent retrospective study of 217 medical patients at the University of Utah hospital, just 43% of patients at high risk for VTE received any sort of prophylaxis.23
Physician education was the main intervention in several previous studies aimed at raising rates of VTE prophylaxis. Our study joins those that have also shown significant improvements after implementation of VTE prophylaxis educational initiatives.6, 14, 15, 23 In the study by Anderson et al., a significantly greater increase in the proportion of high‐risk patients receiving effective VTE prophylaxis was seen between 1986 and 1989 in hospitals that participated in a formal continuing medical education program compared with those that did not (increase: 28% versus 11%; P < .001).6 In 3 additional studies, educational interventions were shown to increase the rate of appropriate prophylaxis in at‐risk patients from 59% to 70%, from 55% to 96%, and from 43% to 72%.14, 15, 23
Other studies have cast doubt on the ability of time‐limited educational interventions to achieve a large or sustained effect.27, 28 A recent systematic review of strategies to improve the use of prophylaxis in hospitals concluded that a number of active strategies are likely to achieve optimal outcomes by combining a system for reminding clinicians to assess patients for VTE with assisting the selection of prophylaxis and providing audit‐and‐feedback.29 The large, sustained effect reported in our study might have been a result of the multifaceted and ongoing nature of the intervention, with reintroduction of the material to all incoming house staff each month. An audit from the last quarter of 2005nearly 2 years after the start of our interventionshowed that prophylaxis rates were approaching 100% (data not included in this study).
Another strategy, the use of computerized reminders to physicians, has been shown to increase the rate of VTE prophylaxis in surgical and medical/surgical patients.16, 26 Kucher et al. compared the incidence of DVT or PE in 1255 hospitalized patients whose physicians received an electronic alert of patient risk of DVT with 1251 hospitalized patients whose physicians did not receive such an alert. They found that the computer alert was associated with a significant reduction in the incidence of DVT or PE at 90 days, with a hazard ratio of 0.59 (95% confidence interval: 0.43, 0.81).16 Our study offers one practical alternative for those institutions that, like ours, do not currently have computerized order entry.
We were unable to determine if there was a specific element of the multifaceted VTE prophylaxis intervention program that contributed the most to the improvement in prophylaxis rates. Provider education was ongoing rather than just a single educational campaign. It was further supported by the pocket cards that provided support for decision making on VTE risk factors, risk categories (based on number and type of risk factor), recommended prophylaxis choices, and potential contraindications. In addition, our method of audit‐and‐feedback constructively leveraged the Hawthorne effect: aware that individual behavior was being measured, our physicians likely adjusted their practice accordingly. Taken together, it is likely that the several elements of our intervention were more powerful in combination than they would have been alone.
Although the multifaceted intervention worked well within our urban university teaching hospital, its application and outcome might be different for other types of hospitals. In our audit‐and‐feedback, for instance, review of resident physician performance was conducted by the Division Chief of General Internal Medicine, tapping into a very strong authority gradient. Hierarchical structures are likely to be different in other types of hospitals. It would therefore be valuable to examine whether the audit‐and‐feedback methodology presented in this article can be replicated in other hospital settings.
A potential limitation of this study was the use of retrospective review to determine baseline rates of VTE prophylaxis. This approach relies on medical notes being accurate and complete; such notes may not have been available for each patient. However, random reviews of both patient charts and hospital billing data for comorbidities performed after coding as a quality control step allowed for confirmation of the data or the extraction and addition of missing data. In addition, data collection was limited to a single day in the latter half of the month. It is not clear whether this sampling strategy collects data that are reflective of performance for the entire month. Our study was also limited by the absence of a control group. Without a control group, we cannot exclude the possibility that during the study factors other than the educational intervention might have contributed to the improvement in prophylaxis rates.
In this study we did not address whether an increase in VTE prophylaxis use translates to an improvement in patient outcomes, namely, a reduction in the rate of VTE. Mosen et al. showed that increasing the VTE prophylaxis rate by implementing a computerized reminder system did not decrease the rate of VTE.26 However, the baseline rate of VTE prophylaxis was already very good, and the study was only powered to detect a large difference in VTE rates. Conversely, Kucher et al. recently demonstrated a significant reduction in VTE events 90 days after initiation of a computerized alert program.16 Further studies designed to confirm the inverse relationship between rate of VTE prophylaxis and rate of clinical outcome of VTE would be helpful.
In conclusion, in a setting in which most hospitalized medically ill patients have multiple risk factors for VTE, we have shown that a practical multifaceted intervention can result in a marked increase in the proportion of medical patients receiving VTE prophylaxis, as well as in the proportion of patients receiving prophylaxis commensurate with evidence‐based guidelines.
Acknowledgements
We thank Nicholas Galeota, Director of Pharmacy at SUNY Downstate for his assistance in providing monthly patient medication lists, Helen Wiggett for providing writing support, and Dan Bridges for editorial support for this manuscript.
- .Pulmonary embolism.Lancet.2004;363:1295–1305.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- ,,, et al.,Cardiovascular Disease Educational and Research Trust, International Union of Angiology.Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence.Int Angiol.2001;20:1–37.
- ,,, et al.Changing clinical practice. Prospective study of the impact of continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Venous thromboembolism prophylaxis in a South Australian teaching hospital.Ann Pharmacother.2003;37:1398–1402.
- ,,,.Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,.Audit of surgeon awareness of readmissions with venous thrombo‐embolism.Intern Med J.2003;33:578–580.
- ,,,A survey of physicians' knowledge and management of venous thromboembolism.Vasc Endovascular Surg.2002;36:367–375.
- ,,,.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,,.Educational campaign to improve the prevention of postoperative venous thromboembolism.J Clin Pharm Ther.1999;24:279–287.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,.Implementation of a national guideline on prophylaxisof venous thromboembolism: a survey of acute services in Scotland.Thromboembolism Prevention Evaluation Study Group.Health Bull (Edinb).1999;57:141–147.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,,,,.A multinational observational cohort study in acutely ill medical patients of practices in prevention of venous thromboembolism: findings of the international medical prevention registry on venous thromboembolism (IMPROVE).Blood.2003;102:321a.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 Suppl 5):12–19.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152:1660–1664.
- ,,,,.Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates.Am J Hematol.2005;78:167–172.
- ,.Failure to prophylax for deep vein thrombosis: results from the DVT FREE registry.Blood.2003;102:322a.
- ,,.Improving uptake of prophylaxis for venous thromboembolism in general surgical patients using prospective audit.BMJ.1996;313:917.
- ,,, et al.The effect of a computerized reminder system on the prevention of postoperative venous thromboembolism.Chest.2004;125:1635–1641.
- ,,,,,.Comparative trial of a short workshop designed to enhance appropriate use of screening tests by family physicians.CMAJ.2002;167:1241–1246.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
Venous thromboembolism (VTE), which encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major cause of the morbidity and mortality of hospitalized medical patients.1 Hospitalization for an acute medical illness has been associated with an 8‐fold increase in the relative risk of VTE and is responsible for approximately a quarter of all VTE cases in the general population.2, 3
Current evidence‐based guidelines, including those from the American College of Chest Physicians (ACCP), recommend prophylaxis with low‐dose unfractionated heparin (UFH) or low‐molecular‐weight heparin (LMWH) for medical patients with risk factors for VTE.4, 5 Mechanical prophylaxis methods including graduated compression stockings and intermittent pneumatic compression are recommended for those patients for whom anticoagulant therapy is contraindicated because of a high risk of bleeding.4, 5 However, several studies have shown that adherence to these guidelines is suboptimal, with many at‐risk patients receiving inadequate prophylaxis (range 32%‐87%).610
Physician‐related factors identified as potential barriers to guideline adherence include not being aware or familiar with the guidelines, not agreeing with the guidelines, or believing the guideline recommendations to be ineffective.11 More specific studies have shown that some physicians may lack basic knowledge regarding the current treatment standards for VTE and may underestimate the significance of VTE.1213 As distinct strategies, education aimed at disseminating VTE prophylaxis guidelines, as well as regular audit‐and‐feedback of physician performance, has been shown to improve rates of VTE prophylaxis in clinical practice.6, 1417 Implementation of educational programs significantly increased the level of appropriate VTE prophylaxis from 59% to 70% of patients in an Australian hospital15 and from 73% to 97% of patients in a Scottish hospital.14 Another strategy, the use of point‐of‐care electronically provided reminders with decision support, has been successful not only in increasing the rates of VTE prophylaxis, but also in decreasing the incidence of clinical VTE events.16 Although highly effective, electronic alerts with computerized decision support do not exist in many hospitals, and other methods of intervention are needed.
In this study, we evaluated adherence to the 2001 ACCP guidelines for VTE prophylaxis among medical patients in our teaching hospital. (The guidelines were updated in 2004, after our study was completed.) After determining that our baseline rates of appropriate VTE prophylaxis were suboptimal, we developed, implemented, and evaluated a multifaceted strategy to improve the rates of appropriate thromboprophylaxis among our medical inpatients.
Six categories of quality improvement strategies have been described: provider education, decision support, audit‐and‐feedback, patient education, organization change, and regulation and policy.18 The intervention we developed was a composite of 3 of these: provider education, decision support, and audit‐and‐feedback.
METHODS
Study Design and Patients
This was a before‐and‐after study designed to assess whether implementation of a VTE prophylaxis quality improvement intervention could improve the rate of appropriate thromboprophylaxis in hospitalized medical patients at the State University of New York, Downstate Medical CenterUniversity Hospital of Brooklyn, an urban university teaching hospital of approximately 400 beds. This initiative, conducted as part of a departmental quality assurance and performance improvement program, did not require institutional review board approval. After an informal survey revealed a prophylaxis rate of approximately 50%, a more formal baseline assessment of the rate of medical patients receiving VTE prophylaxis was conducted during October 2002. This assessment was a single sampling of all medical inpatients on 2 of the medical floors on a single day. The results were consistent with those of the informal survey as well as those from an international registry.19 The results from the baseline study indicated that VTE prophylaxis was underused: only 46.9% of our medical inpatients received any form of prophylaxis. The prophylaxis rate was assessed again in 2 sampling periods beginning 12 and 18 months after implementation of the intervention. Data were collected monthly and combined into 3‐month blocks. The first postintervention sample (n = 116 patient charts) was drawn from a period 12‐14 months after implementation and the second (n = 147 patient charts) from a period 18‐20 months after implementation.
On a randomly designated day in the latter half of each month during each sampling period, all charts on 2 primary medical floors were reviewed and included in the retrospective analysis. Patients who were not on the medical service were excluded from analysis. Patients, as well as their medications, were identified using a list generated from our pharmacy database. We chose this method and schedule for several reasons. First, we sought to reduce the likelihood of including a patient more than once in a monthly sample. Second, by waiting for the latter half of the month we sought to allow house staff a chance to acquire knowledge from the educational program introduced on the first day of the month. Third, we wanted to allow house staff the time to actualize new attitudes reinforced by the audit‐and‐feedback element. The house staff included approximately 4 interns and 4 residents each month plus 10‐15 attendings or hospitalists.
Data Collection
For each sampling period we conducted a medical record (paper) review, and the Division Chief of General Internal Medicine also interviewed the medical house staff and attending physicians. Data collected included risk factors for VTE, contraindications to anticoagulant prophylaxis, type of VTE prophylaxis received, and appropriateness of the prophylaxis. Prophylaxis was considered appropriate when it was given in accordance with a risk stratification scheme (Table 1) adapted from the 2001 ACCP guideline recommendations for surgical patients20 and modified for medical inpatients, similar to the risk assessment model by Caprini et al.21 Prophylaxis was also considered appropriate when no prophylaxis was given for low‐risk patients or when full anticoagulation was given for another indication (Table 1). Questionable prophylaxis was defined as UFH given every 12 hours to a high‐risk patient. All other prophylaxis was deemed inappropriate (including no prophylaxis if prophylaxis was indicated, use of enoxaparin at incorrect prophylactic doses such as 60 or 20 mg, IPC alone for a high‐risk patient with no contraindication to pharmacological prophylaxis, and the use of warfarin if no other indication for it). The risk factors for thromboembolism and contraindications to anticoagulant prophylaxis are given in Table 2. Non‐ambulatory was defined as an order for bed rest with or without bathroom privileges or was judged based on information obtained from the medical house staff and nurses about whether the patient was ambulatory or had been observed walking outside his or her room. Data on pharmacological prophylaxis were obtained from the hospital pharmacy. Information on use of mechanical prophylaxis was obtained by house staff interviews or review of the order sheet. The house officer or attending physician of each patient was interviewed retrospectively to determine the reason for admission and the risk factors for VTE present on admission. Patients were classified as having low, moderate, high, or highest risk for VTE based on their age and any major risk factors for VTE (Table 1).19 All collected data were reported to the Department of Medicine Performance Improvement Committee for independent corroboration.
| Risk category | Definition | Dosage of appropriate prophylaxis | ||
|---|---|---|---|---|
| Age (years) | Additional risk factorsb | Low‐dose unfractionated heparin | LMWH | |
| ||||
| Low (0‐1 risk factors) | <40 | 0‐1 factor | None | None |
| Moderate (2 risk factors) | 40‐60 | 1 factor | 5000 units q12h | 40 mg of enoxaparin or 5000 units of dalteparin |
| High (3‐4 risk factors) | >60 | 1‐2 factors or hypercoagulable state | 5000 units q8h or q12h (q8h recommended for surgical patients) | 40 mg of enoxaparin or 5000 units of dalteparin |
| Highest (5 or more factors) | >40 | Malignancy, prior VTE, or CVA | 5000 units q8h plus IPC | enoxaparin or dalteparin plus IPC |
| Risk factors for thromboembolism |
|---|
| Contraindications to anticoagulant prophylaxis |
| Age > 40 years |
| Infection |
| Inflammatory disease |
| Congestive heart failure |
| Chronic obstructive pulmonary disease |
| Prior venous thromboembolism |
| Cancer |
| Cerebrovascular accident |
| End‐stage renal disease |
| Hypercoagulable state |
| Atrial fibrillation |
| Recent surgery |
| Obesity |
| Non‐ambulatory |
| Active gastrointestinal bleed |
| Central nervous system bleed |
| Thrombocytopenia (platelet count <100,000/L) |
Intervention Strategies
The intervention introduced comprised 3 strategies designed to improve VTE prophylaxis: provider education, decision support, and audit‐and‐feedback.
Provider Education
On the first day of every month, an orientation was given to all incoming medicine house staff by the chief resident that included information on the scope, risk factors, and asymptomatic nature of VTE, the importance of risk stratification, the need to provide adequate prophylaxis, and recommended prophylaxis regimens. A nurse educator also provided information to the nursing staff with the expectation that they would remind physicians to prescribe prophylactic treatment if not ordered initially; however, according to the nurses and house staff, this rarely occurred. Large posters showing VTE risk factors and prophylaxis were displayed at 2 nursing stations and physician charting rooms but were not visible to patients.
Decision Support
Pocket cards containing information on VTE risk factors and prophylaxis options were handed out to the house staff at the beginning of each month. These portable decision support tools assisted physicians in the selection of prophylaxis (a more recent, revised version of the material contained in this pocket guide is available at
Audit‐and‐Feedback
Monthly audits were performed by the Division Chief of General Internal Medicine in order to evaluate the type and appropriateness of VTE prophylaxis prescribed (Table 3). During the orientation at the beginning of the month, the chief resident mentioned that an audit would take place sometime during the rotation. This random audit took place during the last 2 weeks of each month on the same day the data were requested from the pharmacy. Over 1‐2 days, physicians were interviewed either one to one or in a group, depending on the availability of house staff. All house staff and hospitalists were queried about the reasons for admission and the presence of VTE risk factors; physicians received feedback from the Division Chief on VTE risk category, prophylaxis, and appropriateness of prophylaxis treatment of their patients.
| Element | Time/effort required |
|---|---|
| |
| Orientation about VTE risk factors and the need to provide adequate prophylaxis given to all incoming house staff by the chief resident on the first day of every month | 10 min/month |
| Introduction of pocket cards containing information on VTE risk factors and prophylaxis options | 5 min/month |
| In‐hospital education of nurses by the nurse educator | 2 sessions of 1 h |
| Large posters presenting VTE risk factors and prophylaxis displayed in nursing stations and physician charting rooms | 5 min one time only |
| Monthly audits by the Division Chief of General Internal Medicine to evaluate the type and suitability of VTE prophylaxis prescribed | 2 h/month for interviews 2 h/month for record review/ data entry |
Statistical Analysis
Differences in pre‐ and post‐intervention VTE prophylaxis and appropriate VTE prophylaxis rates were analyzed using the chi‐square test for categorical variables and the one‐way analysis of variance test for continuous variables. Differences were considered significant at the 5% level (P = .05).
RESULTS
Patients and Demographics
From October 2002 to August 2004 data were collected from 312 hospitalized medical patients: 49 patients in the baseline group during October 2002, and 116 and 147 at the 12‐ to 14‐month and 18‐ to 20‐month time points, respectively. Thus, approximately 40‐50 patients were randomly selected each month, representing 40% of the general medical service census. Patient demographics were similar between groups (Table 4). Overall, most patients were female (65.7%), and mean age was 61.2 years. The most common admission diagnoses were infection/sepsis (29.5%), chest pain/acute coronary syndromes/myocardial infarction (15.7%), heart failure (10.9%), and malignancy (9.6%). Overall, 7.1% (22 patients) had a contraindication to anticoagulant prophylaxis. The most common contraindication was active gastrointestinal bleeding on the current admission, which occurred in 18 of these patients.
| Baseline (n = 49) | 12 months (n = 116) | 18 months (n = 147) | P valuea | |
|---|---|---|---|---|
| ||||
| Patient demographic | ||||
| Mean age, years (SE) | 59.3 (2.6) | 63.3 (1.6) | 60.1 (1.5) | .25b |
| Men, n (%) | 20 (40.8) | 31 (26.7) | 56 (38.1) | .08 |
| Contraindications to pharmacological prophylaxis, n (%) | 7 (14.3) | 5 (4.3) | 10 (6.8) | .07 |
| Gastrointestinal bleeding | 5 (10.2) | 5 (4.3) | 8 (5.4) | |
| CNS bleeding | 1 (2.0) | 0 (0.0) | 0 (0.0) | |
| Low platelet count | 1 (2.0) | 0 (0.0) | 2 (1.4) | |
| Risk factor | ||||
| Mean number of risk factors (SE) | 3.1 (0.2) | 2.7 (0.1) | 3.0 (0.1) | .05b |
| Non‐ambulatoryc | 46 (93.9) | 73 (89.0) | 112 (80.0)d | .03 |
| Age > 40 years | 39 (79.6) | 101 (87.1) | 122 (83.0) | .44 |
| Cancer | 14 (28.6) | 15 (12.9) | 24 (16.3) | .05 |
| End‐stage renal disease | 13 (26.5) | 29 (25.0) | 36 (24.5) | .96 |
| Congestive heart failure | 11 (22.4) | 23 (19.8) | 28 (19.0) | .87 |
| Infection | 8 (16.3) | 24 (20.7) | 46 (31.3) | .04 |
| Cerebrovascular accident | 8 (16.3) | 12 (10.3) | 15 (10.2) | .47 |
| COPD | 5 (10.2) | 9 (7.8) | 14 (9.5) | .84 |
| Sepsis | 3 (6.1) | 6 (5.2) | 21 (14.3) | .03 |
| Atrial fibrillation | 3 (6.1) | 8 (6.9) | 15 (10.2) | .52 |
| Surgery | 1 (2.0) | 1 (0.9) | 2 (1.4) | .82 |
| Previous venous thromboembolism | 0 (0.0) | 6 (5.2) | 8 (5.4) | .25 |
| Obesity (morbid) | 0 (0.0) | 2 (1.7) | 2 (1.4) | .66 |
| Hypercoagulable state | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Risk Factors for VTE
Patient risk factors for VTE in each data collection period are summarized in Table 4. Analysis of this data showed that the most prevalent risk factors for VTE in the 3 patient populations were age older than 40 years (262/312, 84.0% of the total patient population) and nonambulatory state (231/271, 85.2% of the total population). Overall, the average number of risk factors for VTE was approximately 3, with more than 60% of patients having 3 or more VTE risk factors (Fig. 1).

Prophylaxis Use
The types of VTE prophylaxis used and the proportion of patients treated appropriately are summarized for each data collection period in Tables 5 and 6, respectively. In all 3 populations, most patients received pharmacological rather than mechanical prophylaxis, most commonly UFH. At baseline, the prophylaxis decision was appropriate (in accordance with the recommendations of the ACCP guidelines) as often as it was inappropriate (42.9% of patients). The prophylaxis decision was questionable in the remaining 14.3% of patients.
| Prophylaxis type | Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea |
|---|---|---|---|---|---|
| |||||
| Any pharmacological | 22 (44.9) | 94 (81.0) | <.01 | 118 (80.3) | <.01 |
| Any UFH | 17 (34.7) | 61 (52.6) | .04 | 58 (39.5) | .55 |
| IV UFHb | 3 (6.1) | 5 (4.3) | 2 (1.4) | ||
| bid UFHc | 13 (26.5) | 43 (37.1) | 39 (26.5) | ||
| tid UFHc | 1 (2.0) | 10 (8.6) | 16 (10.9) | ||
| qd UFHc | 0 (0.0) | 3 (2.6) | 1 (0.7) | ||
| Any LMWH | 6 (12.2) | 30 (25.9) | .05 | 59 (40.1) | <.01 |
| Mechanical prophylaxis | 1 (2.0) | 7 (6.0) | .28 | 10 (6.8) | .21 |
| Warfarin | 6 (12.2) | 20 (17.2) | .42 | 19 (12.9) | .90 |
| Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea | |
|---|---|---|---|---|---|
| |||||
| Receiving prophylaxis | 23 (46.9) | 100 (86.2) | <.01 | 127 (86.4) | <.01 |
| Appropriate | 21 (42.9) | 79 (68.1) | <.01 | 125 (85.0) | <.01 |
| UFH | 10 (20.4) | 33 (28.4) | .28 | 45 (30.6) | .16 |
| LMWH | 5 (10.2) | 27 (23.3) | .05 | 58 (39.5) | <.01 |
| Questionable | 7 (14.3) | 28 (24.1) | .14 | 14 (9.5) | .35 |
| Inappropriate | 21 (42.9) | 9 (7.8) | <.01 | 8 (5.4) | <.01 |
Change in Prophylaxis Use
Twelve and 18 months after implementation of the quality improvement program, we observed an increase in the use of any prophylaxis, from 46.9% at baseline to 86.2% and 86.4%, respectively (Table 5; P < .01 in both groups versus baseline). This increase was a result almost entirely of an increase in the proportion of patients receiving pharmacological prophylaxis, which significantly increased, from 44.9% to 81.0% and 80.3%, at the 12‐ and 18‐month time points, respectively (Table 5; P < .01 for both groups versus baseline). Most meaningfully, there was a significant increase in the proportion of patients for whom an appropriate prophylaxis decision was made (from 42.9% to 68.1% and 85.0%, at the 12‐ and 18‐month time points, respectively; Table 6; P < .01 for both groups versus baseline). This represented a trend toward continuing increases in the use of appropriate prophylaxis as the study progressed (Fig. 2). This change was driven mainly by a significant increase in the prescribing of LMWH, almost all of which was prescribed in accordance with the 2001 ACCP guidelines (Table 6).

DISCUSSION
In this study we evaluated the effect of an intervention that combined physician education with a decision support tool and a mechanism for audit‐and‐feedback. We have shown that implementation of such a multifaceted intervention is practical in a teaching hospital and can improve the rates of VTE prophylaxis use in medical patients. In nearly doubling the rate of appropriate prophylaxis, the effect size of our intervention was large, statistically significant, and sustained 18 months after implementation.
More than 60% of our patients had 3 or more risk factors, and more than 80% had at least 2 risk factors. The rate we observed for patients with 3 or more risk factors was 3 times higher than that reported previously.22 Despite the prevalence of high‐risk patients in our study, we observed that the preintervention rate of VTE prophylaxis among medical patients was relatively low at 47%, and only 43% of patients received prophylaxis in accordance with the ACCP guidelines. Our study findings are consistent with those of several other studies that have shown low rates of VTE prophylaxis in medical patients.6, 8, 2324 In a study of 15 hospitals in Massachusetts, only 13%‐19% of medical patients with indications and risk factors for VTE prophylaxis received any prophylaxis prior to an educational intervention.6 Similarly, a study of 368 consecutive medical patients at a Swiss hospital showed that only 22% of those at‐risk received VTE prophylaxis in accordance with the Thromboembolic Risk Factors (THRIFT) I Consensus Group recommendations.8 Results from 2 prospective patient registries also indicated low rates of VTE prophylaxis in medical patients.19, 24 In the IMPROVE registry of acutely ill medical patients, only 39% of patients hospitalized for 3 or more days received VTE prophylaxis19 and in the DVT‐FREE registry only 42% of medical patients with the inpatient diagnosis of DVT had received prophylaxis within 30 days of that diagnosis.24 In a recent retrospective study of 217 medical patients at the University of Utah hospital, just 43% of patients at high risk for VTE received any sort of prophylaxis.23
Physician education was the main intervention in several previous studies aimed at raising rates of VTE prophylaxis. Our study joins those that have also shown significant improvements after implementation of VTE prophylaxis educational initiatives.6, 14, 15, 23 In the study by Anderson et al., a significantly greater increase in the proportion of high‐risk patients receiving effective VTE prophylaxis was seen between 1986 and 1989 in hospitals that participated in a formal continuing medical education program compared with those that did not (increase: 28% versus 11%; P < .001).6 In 3 additional studies, educational interventions were shown to increase the rate of appropriate prophylaxis in at‐risk patients from 59% to 70%, from 55% to 96%, and from 43% to 72%.14, 15, 23
Other studies have cast doubt on the ability of time‐limited educational interventions to achieve a large or sustained effect.27, 28 A recent systematic review of strategies to improve the use of prophylaxis in hospitals concluded that a number of active strategies are likely to achieve optimal outcomes by combining a system for reminding clinicians to assess patients for VTE with assisting the selection of prophylaxis and providing audit‐and‐feedback.29 The large, sustained effect reported in our study might have been a result of the multifaceted and ongoing nature of the intervention, with reintroduction of the material to all incoming house staff each month. An audit from the last quarter of 2005nearly 2 years after the start of our interventionshowed that prophylaxis rates were approaching 100% (data not included in this study).
Another strategy, the use of computerized reminders to physicians, has been shown to increase the rate of VTE prophylaxis in surgical and medical/surgical patients.16, 26 Kucher et al. compared the incidence of DVT or PE in 1255 hospitalized patients whose physicians received an electronic alert of patient risk of DVT with 1251 hospitalized patients whose physicians did not receive such an alert. They found that the computer alert was associated with a significant reduction in the incidence of DVT or PE at 90 days, with a hazard ratio of 0.59 (95% confidence interval: 0.43, 0.81).16 Our study offers one practical alternative for those institutions that, like ours, do not currently have computerized order entry.
We were unable to determine if there was a specific element of the multifaceted VTE prophylaxis intervention program that contributed the most to the improvement in prophylaxis rates. Provider education was ongoing rather than just a single educational campaign. It was further supported by the pocket cards that provided support for decision making on VTE risk factors, risk categories (based on number and type of risk factor), recommended prophylaxis choices, and potential contraindications. In addition, our method of audit‐and‐feedback constructively leveraged the Hawthorne effect: aware that individual behavior was being measured, our physicians likely adjusted their practice accordingly. Taken together, it is likely that the several elements of our intervention were more powerful in combination than they would have been alone.
Although the multifaceted intervention worked well within our urban university teaching hospital, its application and outcome might be different for other types of hospitals. In our audit‐and‐feedback, for instance, review of resident physician performance was conducted by the Division Chief of General Internal Medicine, tapping into a very strong authority gradient. Hierarchical structures are likely to be different in other types of hospitals. It would therefore be valuable to examine whether the audit‐and‐feedback methodology presented in this article can be replicated in other hospital settings.
A potential limitation of this study was the use of retrospective review to determine baseline rates of VTE prophylaxis. This approach relies on medical notes being accurate and complete; such notes may not have been available for each patient. However, random reviews of both patient charts and hospital billing data for comorbidities performed after coding as a quality control step allowed for confirmation of the data or the extraction and addition of missing data. In addition, data collection was limited to a single day in the latter half of the month. It is not clear whether this sampling strategy collects data that are reflective of performance for the entire month. Our study was also limited by the absence of a control group. Without a control group, we cannot exclude the possibility that during the study factors other than the educational intervention might have contributed to the improvement in prophylaxis rates.
In this study we did not address whether an increase in VTE prophylaxis use translates to an improvement in patient outcomes, namely, a reduction in the rate of VTE. Mosen et al. showed that increasing the VTE prophylaxis rate by implementing a computerized reminder system did not decrease the rate of VTE.26 However, the baseline rate of VTE prophylaxis was already very good, and the study was only powered to detect a large difference in VTE rates. Conversely, Kucher et al. recently demonstrated a significant reduction in VTE events 90 days after initiation of a computerized alert program.16 Further studies designed to confirm the inverse relationship between rate of VTE prophylaxis and rate of clinical outcome of VTE would be helpful.
In conclusion, in a setting in which most hospitalized medically ill patients have multiple risk factors for VTE, we have shown that a practical multifaceted intervention can result in a marked increase in the proportion of medical patients receiving VTE prophylaxis, as well as in the proportion of patients receiving prophylaxis commensurate with evidence‐based guidelines.
Acknowledgements
We thank Nicholas Galeota, Director of Pharmacy at SUNY Downstate for his assistance in providing monthly patient medication lists, Helen Wiggett for providing writing support, and Dan Bridges for editorial support for this manuscript.
Venous thromboembolism (VTE), which encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major cause of the morbidity and mortality of hospitalized medical patients.1 Hospitalization for an acute medical illness has been associated with an 8‐fold increase in the relative risk of VTE and is responsible for approximately a quarter of all VTE cases in the general population.2, 3
Current evidence‐based guidelines, including those from the American College of Chest Physicians (ACCP), recommend prophylaxis with low‐dose unfractionated heparin (UFH) or low‐molecular‐weight heparin (LMWH) for medical patients with risk factors for VTE.4, 5 Mechanical prophylaxis methods including graduated compression stockings and intermittent pneumatic compression are recommended for those patients for whom anticoagulant therapy is contraindicated because of a high risk of bleeding.4, 5 However, several studies have shown that adherence to these guidelines is suboptimal, with many at‐risk patients receiving inadequate prophylaxis (range 32%‐87%).610
Physician‐related factors identified as potential barriers to guideline adherence include not being aware or familiar with the guidelines, not agreeing with the guidelines, or believing the guideline recommendations to be ineffective.11 More specific studies have shown that some physicians may lack basic knowledge regarding the current treatment standards for VTE and may underestimate the significance of VTE.1213 As distinct strategies, education aimed at disseminating VTE prophylaxis guidelines, as well as regular audit‐and‐feedback of physician performance, has been shown to improve rates of VTE prophylaxis in clinical practice.6, 1417 Implementation of educational programs significantly increased the level of appropriate VTE prophylaxis from 59% to 70% of patients in an Australian hospital15 and from 73% to 97% of patients in a Scottish hospital.14 Another strategy, the use of point‐of‐care electronically provided reminders with decision support, has been successful not only in increasing the rates of VTE prophylaxis, but also in decreasing the incidence of clinical VTE events.16 Although highly effective, electronic alerts with computerized decision support do not exist in many hospitals, and other methods of intervention are needed.
In this study, we evaluated adherence to the 2001 ACCP guidelines for VTE prophylaxis among medical patients in our teaching hospital. (The guidelines were updated in 2004, after our study was completed.) After determining that our baseline rates of appropriate VTE prophylaxis were suboptimal, we developed, implemented, and evaluated a multifaceted strategy to improve the rates of appropriate thromboprophylaxis among our medical inpatients.
Six categories of quality improvement strategies have been described: provider education, decision support, audit‐and‐feedback, patient education, organization change, and regulation and policy.18 The intervention we developed was a composite of 3 of these: provider education, decision support, and audit‐and‐feedback.
METHODS
Study Design and Patients
This was a before‐and‐after study designed to assess whether implementation of a VTE prophylaxis quality improvement intervention could improve the rate of appropriate thromboprophylaxis in hospitalized medical patients at the State University of New York, Downstate Medical CenterUniversity Hospital of Brooklyn, an urban university teaching hospital of approximately 400 beds. This initiative, conducted as part of a departmental quality assurance and performance improvement program, did not require institutional review board approval. After an informal survey revealed a prophylaxis rate of approximately 50%, a more formal baseline assessment of the rate of medical patients receiving VTE prophylaxis was conducted during October 2002. This assessment was a single sampling of all medical inpatients on 2 of the medical floors on a single day. The results were consistent with those of the informal survey as well as those from an international registry.19 The results from the baseline study indicated that VTE prophylaxis was underused: only 46.9% of our medical inpatients received any form of prophylaxis. The prophylaxis rate was assessed again in 2 sampling periods beginning 12 and 18 months after implementation of the intervention. Data were collected monthly and combined into 3‐month blocks. The first postintervention sample (n = 116 patient charts) was drawn from a period 12‐14 months after implementation and the second (n = 147 patient charts) from a period 18‐20 months after implementation.
On a randomly designated day in the latter half of each month during each sampling period, all charts on 2 primary medical floors were reviewed and included in the retrospective analysis. Patients who were not on the medical service were excluded from analysis. Patients, as well as their medications, were identified using a list generated from our pharmacy database. We chose this method and schedule for several reasons. First, we sought to reduce the likelihood of including a patient more than once in a monthly sample. Second, by waiting for the latter half of the month we sought to allow house staff a chance to acquire knowledge from the educational program introduced on the first day of the month. Third, we wanted to allow house staff the time to actualize new attitudes reinforced by the audit‐and‐feedback element. The house staff included approximately 4 interns and 4 residents each month plus 10‐15 attendings or hospitalists.
Data Collection
For each sampling period we conducted a medical record (paper) review, and the Division Chief of General Internal Medicine also interviewed the medical house staff and attending physicians. Data collected included risk factors for VTE, contraindications to anticoagulant prophylaxis, type of VTE prophylaxis received, and appropriateness of the prophylaxis. Prophylaxis was considered appropriate when it was given in accordance with a risk stratification scheme (Table 1) adapted from the 2001 ACCP guideline recommendations for surgical patients20 and modified for medical inpatients, similar to the risk assessment model by Caprini et al.21 Prophylaxis was also considered appropriate when no prophylaxis was given for low‐risk patients or when full anticoagulation was given for another indication (Table 1). Questionable prophylaxis was defined as UFH given every 12 hours to a high‐risk patient. All other prophylaxis was deemed inappropriate (including no prophylaxis if prophylaxis was indicated, use of enoxaparin at incorrect prophylactic doses such as 60 or 20 mg, IPC alone for a high‐risk patient with no contraindication to pharmacological prophylaxis, and the use of warfarin if no other indication for it). The risk factors for thromboembolism and contraindications to anticoagulant prophylaxis are given in Table 2. Non‐ambulatory was defined as an order for bed rest with or without bathroom privileges or was judged based on information obtained from the medical house staff and nurses about whether the patient was ambulatory or had been observed walking outside his or her room. Data on pharmacological prophylaxis were obtained from the hospital pharmacy. Information on use of mechanical prophylaxis was obtained by house staff interviews or review of the order sheet. The house officer or attending physician of each patient was interviewed retrospectively to determine the reason for admission and the risk factors for VTE present on admission. Patients were classified as having low, moderate, high, or highest risk for VTE based on their age and any major risk factors for VTE (Table 1).19 All collected data were reported to the Department of Medicine Performance Improvement Committee for independent corroboration.
| Risk category | Definition | Dosage of appropriate prophylaxis | ||
|---|---|---|---|---|
| Age (years) | Additional risk factorsb | Low‐dose unfractionated heparin | LMWH | |
| ||||
| Low (0‐1 risk factors) | <40 | 0‐1 factor | None | None |
| Moderate (2 risk factors) | 40‐60 | 1 factor | 5000 units q12h | 40 mg of enoxaparin or 5000 units of dalteparin |
| High (3‐4 risk factors) | >60 | 1‐2 factors or hypercoagulable state | 5000 units q8h or q12h (q8h recommended for surgical patients) | 40 mg of enoxaparin or 5000 units of dalteparin |
| Highest (5 or more factors) | >40 | Malignancy, prior VTE, or CVA | 5000 units q8h plus IPC | enoxaparin or dalteparin plus IPC |
| Risk factors for thromboembolism |
|---|
| Contraindications to anticoagulant prophylaxis |
| Age > 40 years |
| Infection |
| Inflammatory disease |
| Congestive heart failure |
| Chronic obstructive pulmonary disease |
| Prior venous thromboembolism |
| Cancer |
| Cerebrovascular accident |
| End‐stage renal disease |
| Hypercoagulable state |
| Atrial fibrillation |
| Recent surgery |
| Obesity |
| Non‐ambulatory |
| Active gastrointestinal bleed |
| Central nervous system bleed |
| Thrombocytopenia (platelet count <100,000/L) |
Intervention Strategies
The intervention introduced comprised 3 strategies designed to improve VTE prophylaxis: provider education, decision support, and audit‐and‐feedback.
Provider Education
On the first day of every month, an orientation was given to all incoming medicine house staff by the chief resident that included information on the scope, risk factors, and asymptomatic nature of VTE, the importance of risk stratification, the need to provide adequate prophylaxis, and recommended prophylaxis regimens. A nurse educator also provided information to the nursing staff with the expectation that they would remind physicians to prescribe prophylactic treatment if not ordered initially; however, according to the nurses and house staff, this rarely occurred. Large posters showing VTE risk factors and prophylaxis were displayed at 2 nursing stations and physician charting rooms but were not visible to patients.
Decision Support
Pocket cards containing information on VTE risk factors and prophylaxis options were handed out to the house staff at the beginning of each month. These portable decision support tools assisted physicians in the selection of prophylaxis (a more recent, revised version of the material contained in this pocket guide is available at
Audit‐and‐Feedback
Monthly audits were performed by the Division Chief of General Internal Medicine in order to evaluate the type and appropriateness of VTE prophylaxis prescribed (Table 3). During the orientation at the beginning of the month, the chief resident mentioned that an audit would take place sometime during the rotation. This random audit took place during the last 2 weeks of each month on the same day the data were requested from the pharmacy. Over 1‐2 days, physicians were interviewed either one to one or in a group, depending on the availability of house staff. All house staff and hospitalists were queried about the reasons for admission and the presence of VTE risk factors; physicians received feedback from the Division Chief on VTE risk category, prophylaxis, and appropriateness of prophylaxis treatment of their patients.
| Element | Time/effort required |
|---|---|
| |
| Orientation about VTE risk factors and the need to provide adequate prophylaxis given to all incoming house staff by the chief resident on the first day of every month | 10 min/month |
| Introduction of pocket cards containing information on VTE risk factors and prophylaxis options | 5 min/month |
| In‐hospital education of nurses by the nurse educator | 2 sessions of 1 h |
| Large posters presenting VTE risk factors and prophylaxis displayed in nursing stations and physician charting rooms | 5 min one time only |
| Monthly audits by the Division Chief of General Internal Medicine to evaluate the type and suitability of VTE prophylaxis prescribed | 2 h/month for interviews 2 h/month for record review/ data entry |
Statistical Analysis
Differences in pre‐ and post‐intervention VTE prophylaxis and appropriate VTE prophylaxis rates were analyzed using the chi‐square test for categorical variables and the one‐way analysis of variance test for continuous variables. Differences were considered significant at the 5% level (P = .05).
RESULTS
Patients and Demographics
From October 2002 to August 2004 data were collected from 312 hospitalized medical patients: 49 patients in the baseline group during October 2002, and 116 and 147 at the 12‐ to 14‐month and 18‐ to 20‐month time points, respectively. Thus, approximately 40‐50 patients were randomly selected each month, representing 40% of the general medical service census. Patient demographics were similar between groups (Table 4). Overall, most patients were female (65.7%), and mean age was 61.2 years. The most common admission diagnoses were infection/sepsis (29.5%), chest pain/acute coronary syndromes/myocardial infarction (15.7%), heart failure (10.9%), and malignancy (9.6%). Overall, 7.1% (22 patients) had a contraindication to anticoagulant prophylaxis. The most common contraindication was active gastrointestinal bleeding on the current admission, which occurred in 18 of these patients.
| Baseline (n = 49) | 12 months (n = 116) | 18 months (n = 147) | P valuea | |
|---|---|---|---|---|
| ||||
| Patient demographic | ||||
| Mean age, years (SE) | 59.3 (2.6) | 63.3 (1.6) | 60.1 (1.5) | .25b |
| Men, n (%) | 20 (40.8) | 31 (26.7) | 56 (38.1) | .08 |
| Contraindications to pharmacological prophylaxis, n (%) | 7 (14.3) | 5 (4.3) | 10 (6.8) | .07 |
| Gastrointestinal bleeding | 5 (10.2) | 5 (4.3) | 8 (5.4) | |
| CNS bleeding | 1 (2.0) | 0 (0.0) | 0 (0.0) | |
| Low platelet count | 1 (2.0) | 0 (0.0) | 2 (1.4) | |
| Risk factor | ||||
| Mean number of risk factors (SE) | 3.1 (0.2) | 2.7 (0.1) | 3.0 (0.1) | .05b |
| Non‐ambulatoryc | 46 (93.9) | 73 (89.0) | 112 (80.0)d | .03 |
| Age > 40 years | 39 (79.6) | 101 (87.1) | 122 (83.0) | .44 |
| Cancer | 14 (28.6) | 15 (12.9) | 24 (16.3) | .05 |
| End‐stage renal disease | 13 (26.5) | 29 (25.0) | 36 (24.5) | .96 |
| Congestive heart failure | 11 (22.4) | 23 (19.8) | 28 (19.0) | .87 |
| Infection | 8 (16.3) | 24 (20.7) | 46 (31.3) | .04 |
| Cerebrovascular accident | 8 (16.3) | 12 (10.3) | 15 (10.2) | .47 |
| COPD | 5 (10.2) | 9 (7.8) | 14 (9.5) | .84 |
| Sepsis | 3 (6.1) | 6 (5.2) | 21 (14.3) | .03 |
| Atrial fibrillation | 3 (6.1) | 8 (6.9) | 15 (10.2) | .52 |
| Surgery | 1 (2.0) | 1 (0.9) | 2 (1.4) | .82 |
| Previous venous thromboembolism | 0 (0.0) | 6 (5.2) | 8 (5.4) | .25 |
| Obesity (morbid) | 0 (0.0) | 2 (1.7) | 2 (1.4) | .66 |
| Hypercoagulable state | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Risk Factors for VTE
Patient risk factors for VTE in each data collection period are summarized in Table 4. Analysis of this data showed that the most prevalent risk factors for VTE in the 3 patient populations were age older than 40 years (262/312, 84.0% of the total patient population) and nonambulatory state (231/271, 85.2% of the total population). Overall, the average number of risk factors for VTE was approximately 3, with more than 60% of patients having 3 or more VTE risk factors (Fig. 1).

Prophylaxis Use
The types of VTE prophylaxis used and the proportion of patients treated appropriately are summarized for each data collection period in Tables 5 and 6, respectively. In all 3 populations, most patients received pharmacological rather than mechanical prophylaxis, most commonly UFH. At baseline, the prophylaxis decision was appropriate (in accordance with the recommendations of the ACCP guidelines) as often as it was inappropriate (42.9% of patients). The prophylaxis decision was questionable in the remaining 14.3% of patients.
| Prophylaxis type | Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea |
|---|---|---|---|---|---|
| |||||
| Any pharmacological | 22 (44.9) | 94 (81.0) | <.01 | 118 (80.3) | <.01 |
| Any UFH | 17 (34.7) | 61 (52.6) | .04 | 58 (39.5) | .55 |
| IV UFHb | 3 (6.1) | 5 (4.3) | 2 (1.4) | ||
| bid UFHc | 13 (26.5) | 43 (37.1) | 39 (26.5) | ||
| tid UFHc | 1 (2.0) | 10 (8.6) | 16 (10.9) | ||
| qd UFHc | 0 (0.0) | 3 (2.6) | 1 (0.7) | ||
| Any LMWH | 6 (12.2) | 30 (25.9) | .05 | 59 (40.1) | <.01 |
| Mechanical prophylaxis | 1 (2.0) | 7 (6.0) | .28 | 10 (6.8) | .21 |
| Warfarin | 6 (12.2) | 20 (17.2) | .42 | 19 (12.9) | .90 |
| Baseline (n = 49), n (%) | 12 months (n = 116), n (%) | P valuea | 18 months (n = 147), n (%) | P valuea | |
|---|---|---|---|---|---|
| |||||
| Receiving prophylaxis | 23 (46.9) | 100 (86.2) | <.01 | 127 (86.4) | <.01 |
| Appropriate | 21 (42.9) | 79 (68.1) | <.01 | 125 (85.0) | <.01 |
| UFH | 10 (20.4) | 33 (28.4) | .28 | 45 (30.6) | .16 |
| LMWH | 5 (10.2) | 27 (23.3) | .05 | 58 (39.5) | <.01 |
| Questionable | 7 (14.3) | 28 (24.1) | .14 | 14 (9.5) | .35 |
| Inappropriate | 21 (42.9) | 9 (7.8) | <.01 | 8 (5.4) | <.01 |
Change in Prophylaxis Use
Twelve and 18 months after implementation of the quality improvement program, we observed an increase in the use of any prophylaxis, from 46.9% at baseline to 86.2% and 86.4%, respectively (Table 5; P < .01 in both groups versus baseline). This increase was a result almost entirely of an increase in the proportion of patients receiving pharmacological prophylaxis, which significantly increased, from 44.9% to 81.0% and 80.3%, at the 12‐ and 18‐month time points, respectively (Table 5; P < .01 for both groups versus baseline). Most meaningfully, there was a significant increase in the proportion of patients for whom an appropriate prophylaxis decision was made (from 42.9% to 68.1% and 85.0%, at the 12‐ and 18‐month time points, respectively; Table 6; P < .01 for both groups versus baseline). This represented a trend toward continuing increases in the use of appropriate prophylaxis as the study progressed (Fig. 2). This change was driven mainly by a significant increase in the prescribing of LMWH, almost all of which was prescribed in accordance with the 2001 ACCP guidelines (Table 6).

DISCUSSION
In this study we evaluated the effect of an intervention that combined physician education with a decision support tool and a mechanism for audit‐and‐feedback. We have shown that implementation of such a multifaceted intervention is practical in a teaching hospital and can improve the rates of VTE prophylaxis use in medical patients. In nearly doubling the rate of appropriate prophylaxis, the effect size of our intervention was large, statistically significant, and sustained 18 months after implementation.
More than 60% of our patients had 3 or more risk factors, and more than 80% had at least 2 risk factors. The rate we observed for patients with 3 or more risk factors was 3 times higher than that reported previously.22 Despite the prevalence of high‐risk patients in our study, we observed that the preintervention rate of VTE prophylaxis among medical patients was relatively low at 47%, and only 43% of patients received prophylaxis in accordance with the ACCP guidelines. Our study findings are consistent with those of several other studies that have shown low rates of VTE prophylaxis in medical patients.6, 8, 2324 In a study of 15 hospitals in Massachusetts, only 13%‐19% of medical patients with indications and risk factors for VTE prophylaxis received any prophylaxis prior to an educational intervention.6 Similarly, a study of 368 consecutive medical patients at a Swiss hospital showed that only 22% of those at‐risk received VTE prophylaxis in accordance with the Thromboembolic Risk Factors (THRIFT) I Consensus Group recommendations.8 Results from 2 prospective patient registries also indicated low rates of VTE prophylaxis in medical patients.19, 24 In the IMPROVE registry of acutely ill medical patients, only 39% of patients hospitalized for 3 or more days received VTE prophylaxis19 and in the DVT‐FREE registry only 42% of medical patients with the inpatient diagnosis of DVT had received prophylaxis within 30 days of that diagnosis.24 In a recent retrospective study of 217 medical patients at the University of Utah hospital, just 43% of patients at high risk for VTE received any sort of prophylaxis.23
Physician education was the main intervention in several previous studies aimed at raising rates of VTE prophylaxis. Our study joins those that have also shown significant improvements after implementation of VTE prophylaxis educational initiatives.6, 14, 15, 23 In the study by Anderson et al., a significantly greater increase in the proportion of high‐risk patients receiving effective VTE prophylaxis was seen between 1986 and 1989 in hospitals that participated in a formal continuing medical education program compared with those that did not (increase: 28% versus 11%; P < .001).6 In 3 additional studies, educational interventions were shown to increase the rate of appropriate prophylaxis in at‐risk patients from 59% to 70%, from 55% to 96%, and from 43% to 72%.14, 15, 23
Other studies have cast doubt on the ability of time‐limited educational interventions to achieve a large or sustained effect.27, 28 A recent systematic review of strategies to improve the use of prophylaxis in hospitals concluded that a number of active strategies are likely to achieve optimal outcomes by combining a system for reminding clinicians to assess patients for VTE with assisting the selection of prophylaxis and providing audit‐and‐feedback.29 The large, sustained effect reported in our study might have been a result of the multifaceted and ongoing nature of the intervention, with reintroduction of the material to all incoming house staff each month. An audit from the last quarter of 2005nearly 2 years after the start of our interventionshowed that prophylaxis rates were approaching 100% (data not included in this study).
Another strategy, the use of computerized reminders to physicians, has been shown to increase the rate of VTE prophylaxis in surgical and medical/surgical patients.16, 26 Kucher et al. compared the incidence of DVT or PE in 1255 hospitalized patients whose physicians received an electronic alert of patient risk of DVT with 1251 hospitalized patients whose physicians did not receive such an alert. They found that the computer alert was associated with a significant reduction in the incidence of DVT or PE at 90 days, with a hazard ratio of 0.59 (95% confidence interval: 0.43, 0.81).16 Our study offers one practical alternative for those institutions that, like ours, do not currently have computerized order entry.
We were unable to determine if there was a specific element of the multifaceted VTE prophylaxis intervention program that contributed the most to the improvement in prophylaxis rates. Provider education was ongoing rather than just a single educational campaign. It was further supported by the pocket cards that provided support for decision making on VTE risk factors, risk categories (based on number and type of risk factor), recommended prophylaxis choices, and potential contraindications. In addition, our method of audit‐and‐feedback constructively leveraged the Hawthorne effect: aware that individual behavior was being measured, our physicians likely adjusted their practice accordingly. Taken together, it is likely that the several elements of our intervention were more powerful in combination than they would have been alone.
Although the multifaceted intervention worked well within our urban university teaching hospital, its application and outcome might be different for other types of hospitals. In our audit‐and‐feedback, for instance, review of resident physician performance was conducted by the Division Chief of General Internal Medicine, tapping into a very strong authority gradient. Hierarchical structures are likely to be different in other types of hospitals. It would therefore be valuable to examine whether the audit‐and‐feedback methodology presented in this article can be replicated in other hospital settings.
A potential limitation of this study was the use of retrospective review to determine baseline rates of VTE prophylaxis. This approach relies on medical notes being accurate and complete; such notes may not have been available for each patient. However, random reviews of both patient charts and hospital billing data for comorbidities performed after coding as a quality control step allowed for confirmation of the data or the extraction and addition of missing data. In addition, data collection was limited to a single day in the latter half of the month. It is not clear whether this sampling strategy collects data that are reflective of performance for the entire month. Our study was also limited by the absence of a control group. Without a control group, we cannot exclude the possibility that during the study factors other than the educational intervention might have contributed to the improvement in prophylaxis rates.
In this study we did not address whether an increase in VTE prophylaxis use translates to an improvement in patient outcomes, namely, a reduction in the rate of VTE. Mosen et al. showed that increasing the VTE prophylaxis rate by implementing a computerized reminder system did not decrease the rate of VTE.26 However, the baseline rate of VTE prophylaxis was already very good, and the study was only powered to detect a large difference in VTE rates. Conversely, Kucher et al. recently demonstrated a significant reduction in VTE events 90 days after initiation of a computerized alert program.16 Further studies designed to confirm the inverse relationship between rate of VTE prophylaxis and rate of clinical outcome of VTE would be helpful.
In conclusion, in a setting in which most hospitalized medically ill patients have multiple risk factors for VTE, we have shown that a practical multifaceted intervention can result in a marked increase in the proportion of medical patients receiving VTE prophylaxis, as well as in the proportion of patients receiving prophylaxis commensurate with evidence‐based guidelines.
Acknowledgements
We thank Nicholas Galeota, Director of Pharmacy at SUNY Downstate for his assistance in providing monthly patient medication lists, Helen Wiggett for providing writing support, and Dan Bridges for editorial support for this manuscript.
- .Pulmonary embolism.Lancet.2004;363:1295–1305.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- ,,, et al.,Cardiovascular Disease Educational and Research Trust, International Union of Angiology.Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence.Int Angiol.2001;20:1–37.
- ,,, et al.Changing clinical practice. Prospective study of the impact of continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Venous thromboembolism prophylaxis in a South Australian teaching hospital.Ann Pharmacother.2003;37:1398–1402.
- ,,,.Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,.Audit of surgeon awareness of readmissions with venous thrombo‐embolism.Intern Med J.2003;33:578–580.
- ,,,A survey of physicians' knowledge and management of venous thromboembolism.Vasc Endovascular Surg.2002;36:367–375.
- ,,,.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,,.Educational campaign to improve the prevention of postoperative venous thromboembolism.J Clin Pharm Ther.1999;24:279–287.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,.Implementation of a national guideline on prophylaxisof venous thromboembolism: a survey of acute services in Scotland.Thromboembolism Prevention Evaluation Study Group.Health Bull (Edinb).1999;57:141–147.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,,,,.A multinational observational cohort study in acutely ill medical patients of practices in prevention of venous thromboembolism: findings of the international medical prevention registry on venous thromboembolism (IMPROVE).Blood.2003;102:321a.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 Suppl 5):12–19.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152:1660–1664.
- ,,,,.Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates.Am J Hematol.2005;78:167–172.
- ,.Failure to prophylax for deep vein thrombosis: results from the DVT FREE registry.Blood.2003;102:322a.
- ,,.Improving uptake of prophylaxis for venous thromboembolism in general surgical patients using prospective audit.BMJ.1996;313:917.
- ,,, et al.The effect of a computerized reminder system on the prevention of postoperative venous thromboembolism.Chest.2004;125:1635–1641.
- ,,,,,.Comparative trial of a short workshop designed to enhance appropriate use of screening tests by family physicians.CMAJ.2002;167:1241–1246.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
- .Pulmonary embolism.Lancet.2004;363:1295–1305.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:338S–400S.
- ,,, et al.,Cardiovascular Disease Educational and Research Trust, International Union of Angiology.Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence.Int Angiol.2001;20:1–37.
- ,,, et al.Changing clinical practice. Prospective study of the impact of continuing medical education and quality assurance programs on use of prophylaxis for venous thromboembolism.Arch Intern Med.1994;154:669–677.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120:1964–1971.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17:788–791.
- ,.Venous thromboembolism prophylaxis in a South Australian teaching hospital.Ann Pharmacother.2003;37:1398–1402.
- ,,,.Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross‐sectional study.Thromb J.2004;2:3–9.
- ,,, et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282:1458–1465.
- ,.Audit of surgeon awareness of readmissions with venous thrombo‐embolism.Intern Med J.2003;33:578–580.
- ,,,A survey of physicians' knowledge and management of venous thromboembolism.Vasc Endovascular Surg.2002;36:367–375.
- ,,,.Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43:23–25.
- ,,,,.Educational campaign to improve the prevention of postoperative venous thromboembolism.J Clin Pharm Ther.1999;24:279–287.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977.
- ,,.Implementation of a national guideline on prophylaxisof venous thromboembolism: a survey of acute services in Scotland.Thromboembolism Prevention Evaluation Study Group.Health Bull (Edinb).1999;57:141–147.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,,,,.A multinational observational cohort study in acutely ill medical patients of practices in prevention of venous thromboembolism: findings of the international medical prevention registry on venous thromboembolism (IMPROVE).Blood.2003;102:321a.
- ,,, et al.Prevention of venous thromboembolism.Chest.2001;119:132S–175S.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 Suppl 5):12–19.
- ,,,,.The prevalence of risk factors for venous thromboembolism among hospital patients.Arch Intern Med.1992;152:1660–1664.
- ,,,,.Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates.Am J Hematol.2005;78:167–172.
- ,.Failure to prophylax for deep vein thrombosis: results from the DVT FREE registry.Blood.2003;102:322a.
- ,,.Improving uptake of prophylaxis for venous thromboembolism in general surgical patients using prospective audit.BMJ.1996;313:917.
- ,,, et al.The effect of a computerized reminder system on the prevention of postoperative venous thromboembolism.Chest.2004;125:1635–1641.
- ,,,,,.Comparative trial of a short workshop designed to enhance appropriate use of screening tests by family physicians.CMAJ.2002;167:1241–1246.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.CMAJ.1995;153:1423–1431.
- ,,, et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241:397–415.
Copyright © 2006 Society of Hospital Medicine
Moxibustion burns
A 53‐year‐old Korean woman was admitted to the hospital with a diagnosis of cellulitis (thin arrow) and rule out vasculitis. Further history obtained with the assistance of a Korean translator revealed that the patient, though untrained in Chinese medicine, had attempted scarring direct moxibustion for intermittent headaches. She was treated with intravenous antibiotics for 24 hours for her cellulitis and discharged in good condition on oral antibiotics.
Moxibustion is a traditional Chinese medical technique that involves burning the herb mugwort (Artemesia vulgaris) to relieve cold or stagnant conditions by stimulating circulation. Moxibustion can be performed indirectly or directly. Indirect moxibustion involves application of the burning moxa to the end of an acupuncture needle or by holding the moxa close to the skin. In direct moxibustion, a cone‐shaped moxa is held over an acupuncture point. Direct moxibustion can be divided into scarring and nonscarring types. With nonscarring direct moxibustion, moxa is placed on top of an acupuncture point, lit, and then removed before it burns the skin. With scarring moxibustion, the burning moxa is left on the skin until it burns out, leading to burns and scarring.
This case demonstrates the importance of obtaining an accurate history when making a clinical diagnosis and, in patients who are not fluent in English, the critical role that translators serve in the management of patients. The differential diagnosis of skin ulcers encompasses many other conditions in addition to infection, including iatrogenic causes of traditional as well as alternative medical therapies. 0

A 53‐year‐old Korean woman was admitted to the hospital with a diagnosis of cellulitis (thin arrow) and rule out vasculitis. Further history obtained with the assistance of a Korean translator revealed that the patient, though untrained in Chinese medicine, had attempted scarring direct moxibustion for intermittent headaches. She was treated with intravenous antibiotics for 24 hours for her cellulitis and discharged in good condition on oral antibiotics.
Moxibustion is a traditional Chinese medical technique that involves burning the herb mugwort (Artemesia vulgaris) to relieve cold or stagnant conditions by stimulating circulation. Moxibustion can be performed indirectly or directly. Indirect moxibustion involves application of the burning moxa to the end of an acupuncture needle or by holding the moxa close to the skin. In direct moxibustion, a cone‐shaped moxa is held over an acupuncture point. Direct moxibustion can be divided into scarring and nonscarring types. With nonscarring direct moxibustion, moxa is placed on top of an acupuncture point, lit, and then removed before it burns the skin. With scarring moxibustion, the burning moxa is left on the skin until it burns out, leading to burns and scarring.
This case demonstrates the importance of obtaining an accurate history when making a clinical diagnosis and, in patients who are not fluent in English, the critical role that translators serve in the management of patients. The differential diagnosis of skin ulcers encompasses many other conditions in addition to infection, including iatrogenic causes of traditional as well as alternative medical therapies. 0


A 53‐year‐old Korean woman was admitted to the hospital with a diagnosis of cellulitis (thin arrow) and rule out vasculitis. Further history obtained with the assistance of a Korean translator revealed that the patient, though untrained in Chinese medicine, had attempted scarring direct moxibustion for intermittent headaches. She was treated with intravenous antibiotics for 24 hours for her cellulitis and discharged in good condition on oral antibiotics.
Moxibustion is a traditional Chinese medical technique that involves burning the herb mugwort (Artemesia vulgaris) to relieve cold or stagnant conditions by stimulating circulation. Moxibustion can be performed indirectly or directly. Indirect moxibustion involves application of the burning moxa to the end of an acupuncture needle or by holding the moxa close to the skin. In direct moxibustion, a cone‐shaped moxa is held over an acupuncture point. Direct moxibustion can be divided into scarring and nonscarring types. With nonscarring direct moxibustion, moxa is placed on top of an acupuncture point, lit, and then removed before it burns the skin. With scarring moxibustion, the burning moxa is left on the skin until it burns out, leading to burns and scarring.
This case demonstrates the importance of obtaining an accurate history when making a clinical diagnosis and, in patients who are not fluent in English, the critical role that translators serve in the management of patients. The differential diagnosis of skin ulcers encompasses many other conditions in addition to infection, including iatrogenic causes of traditional as well as alternative medical therapies. 0


Clinical Conundrum
A 20‐year‐old woman presented to the emergency department after 2 days of epistaxis and vaginal bleeding.
A young woman is more likely to present with infection, toxic exposure, or rheumatologic disease than with a degenerative disease or malignancy. Her bleeding may relate to a platelet abnormality, either quantitative or qualitative. I would pursue her bleeding and menstrual history further.
The patient was healthy until 2 months previously, when she noted arthralgia of her shoulders, wrists, elbows, knees, and ankles. She was examined by a rheumatologist who detected mild arthritis in her left wrist and proximal interphalangeal joints. The rest of her joints were normal. Rheumatoid factor and ANA were positive, and the erythrocyte sedimentation rate was 122 mm/hour. She was diagnosed with possible systemic lupus erythematosus and was placed on a nonsteroidal anti‐inflammatory agent. At a follow‐up visit 1 month prior to admission, her arthralgia had markedly improved. Two weeks prior to admission, the patient began to feel fatigued. Two days prior to admission, she developed epistaxis and what she thought was her menses, though bleeding was heavier than usual and associated with the passage of red clots. On the day of admission the vaginal bleeding worsened, and emergency personnel transported the patient to the hospital.
The diagnosis of systemic lupus erythematosus (SLE) is not engraved in stone. One must be vigilant for other diseases masquerading as SLE while continuing to build a case for it. As more criteria are fulfilled, the probability of lupus increases, yet no findings, alone or in combination, are pathognomonic of this protean disease. This patient's age, sex, and serology are compatible with SLE; otherwise, her presentation is nonspecific. I would request a complete blood count, coagulation tests, and additional serological tests.
The quantity of the bleeding is described, but this does not help decipher its etiology. Excess bleeding may be a result of one or more of 3 broad etiologies: problems with platelets (quantitative or qualitative), with clotting factors (quantitative or qualitative), or with blood vessels (trauma, vasculitis, or diseases affecting collagen). Because quantitative and qualitative factor disorders generally do not present with mucosal bleeding, I am thinking more about platelet problems and about processes that damage the microvasculature. If this woman has lupus, immunologic thrombocytopenia may be the cause of mucosal bleeding.
The patient had no previous medical problems and had never been pregnant. Her only medication was sulindac twice daily for the past month. She was born in Hong Kong, graduated from high school in San Francisco, and attended junior college. She lived with her parents and brother and denied alcohol, tobacco, or recreational drug use but had recently obtained a tattoo on her lower back. There was no family history of autoimmune or bleeding disorders, and a review of systems was notable for dyspnea with minimal exertion and fatigue which worsened in the past 2 days. She had no prior episodes of abnormal bleeding or clotting.
Tattoos may be surrogates for other high‐risk behaviors and suggest an increased risk of hepatitis and sexually transmitted diseases. I want to know her sexual history and other risk factors for human immunodeficiency virus infection. The dyspnea and fatigue are likely the result of anemia, but I am also considering cardiac disease. Though SLE remains a possibility, I cannot assume the presence of a lupus anticoagulant with antiphospholipid syndrome without a history of infertility or recurrent miscarriages.
On arrival at the emergency department, the patient had a blood pressure of 78/46 mm Hg, a pulse of 120 beats/min, a temperature of 34C, 14 respirations per minute, and oxygen saturation of 99% while breathing supplemental oxygen through a nonrebreather mask. Systolic blood pressure improved to 90 mmHg after 4 L of normal saline was administered. The patient was pale but alert. There was crusted blood in her mouth and nostrils without active bleeding or petechiae. Her tongue was pierced with a ring, and sclerae were anicteric. Bleeding was noted from both nipples. There was no heart murmur or gallop, and jugular venous pressure was not elevated. Pulmonary exam revealed bibasilar crackles. Abdomen was soft, not tender, and without hepatosplenomegaly, and her umbilicus was pierced by a ring. Genitourinary exam revealed scant vaginal discharge and clotted blood in the vagina. Skin demonstrated no petechiae, ecchymoses, or stigmata of liver disease. Neurological and joint exams were normal.
It is hard to conceive of vaginal bleeding producing this profound a degree of hypotension. The patient may have additional occult sites of bleeding, or she may have a distributive cause of hypotension such as sepsis or adrenal hemorrhage with resultant adrenal insufficiency. Breast bleeding is unusual, even with profound thrombocytopenia, and I wonder about a concomitant factor deficiency. Furthermore, if thrombocytopenia was the sole reason for the bleeding, I would have expected petechiae. Diffuse vascular injury, such as from lupus or vasculitis, would be an unusual cause of profound bleeding unless there was also disseminated intravascular coagulation.
Laboratory studies revealed a white count of 2000/mm3, of which 42% were neutrophils, 40% bands, 8% lymphocytes, and 10% monocytes. Hematocrit was 17.6%, platelets 35,000/mm3. Sodium was 124 mmol/L, potassium 6 mmol/L, chloride 92 mmol/L, bicarbonate 10 mmol/L, blood urea nitrogen 122 mg/dL (43.5 mmol/L), and creatinine 3.4 mg/dL (300 mol/L). Blood glucose was 44 mg/dL (2.44 mmol/L). Total bilirubin was 3.0 mg/dL (51.3 mol/L; normal range, 0.1‐1.5), alkaline phosphatase 105 U/L (normal range, 39‐117), aspartate aminotransferase 849 U/L (normal range, 8‐31), alanine aminotransferase 261 U/L (normal range, 7‐31), international normalized unit (INR) 2.9, and partial thromboplastin time (PTT) 34.2 seconds.
The combination of profound hypotension, electrolyte abnormalities, hypoglycemia, and hypothermia makes adrenal insufficiency a consideration. I would perform a cortrosyn stimulation test and start glucocorticoid and perhaps mineralocorticoid replacement. In addition, there is renal failure and metabolic acidosis, with a calculated anion gap of 22. The anion gap may be from lactic acidosis secondary to hypotension and hypoperfusion. The abnormal transaminases and bilirubin could relate to infectious hepatitis or systemic infection. Although ischemia could explain these findings, it is rare for a 20‐year‐old to develop ischemic hepatopathy. Thrombocytopenia this moderate may augment the volume of blood loss, but spontaneous bleeding because of thrombocytopenia is unusual until the platelet count falls below 20,000/mm3. Furthermore, the elevated INR points to a mixed coagulopathy. Interpretation of the INR is complicated by the fact she has liver disease, and I am most concerned about acute disseminated intravascular coagulation (DIC) or impending fulminant hepatic failure. This is not the pattern seen with antiphospholipid antibody syndrome, in which the INR tends to be preserved and the PTT prolonged.
Urine dipstick testing demonstrated a specific gravity of 1.015, trace leukocyte esterase, 2+ protein, and 3+ blood, and microscopy revealed 2 white blood cells and 38 red blood cells per high‐power field, many bacteria, and no casts. Creatine kinase was 20,599 U/L, with a myocardial fraction of 1.4%. Lipase was normal, lactate was 7.3 mmol/L, and serum pregnancy test was negative.
Although there is proteinuria and hematuria, we do not have solid evidence of glomerulonephritis. Although the red cells could be a contaminant from her vaginal bleeding, I would examine her sediment carefully for dysmorphic red cells, recognizing that only a quarter of people with glomerulonephritis have red‐cell casts. A urine protein‐to‐creatinine ratio would be useful for estimating the degree of proteinuria. The elevated creatine kinase indicates rhabdomyolysis. In a previously healthy young woman without evidence of cardiogenic shock, it would be unusual for hypotension to result in rhabdomyolysis. Infection and metabolic derangements are possible etiologies of rhabdomyolysis. Alternatively, coagulopathy might have produced intramuscular bleeding. The constellation of thrombocytopenia, anemia, and renal failure raises my suspicion that there is a thrombotic microangiopathy, such as thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS). I would inspect a peripheral‐blood smear for schistocytes and evidence of microangiopathy.
The chest radiograph demonstrated low lung volumes, patchy areas of consolidation, and pulmonary edema. Heart size was normal, and there were no pleural effusions. On the first hospital day the patient required mechanical ventilation because of respiratory failure. She received 5 units of packed red blood cells, 2 units of fresh frozen plasma, and 1 unit of platelets. Vasopressor infusion was started, and a vascular catheter was placed for hemodialysis. Blood, respiratory, and urine cultures were sent, and methylprednisolone, piperacillin/tazobactam, and vancomycin were administered. D‐dimer was greater than 10,000 ng/mL, fibrinogen was 178 mg/dL, and lactate dehydrogenase was 1671 U/L (27 kat/L). The peripheral‐blood smear demonstrated 1+ schistocytes and no spherocytes. There were fewer white blood cells with bands and myelocytes, but no blasts.
The presence of schistocytes and the elevated lactate dehydrogenase point to a microangiopathic hemolytic process. Causes of microangiopathic hemolytic anemia include TTP, HUS, DIC, paraneoplastic conditions, and endothelial damage from malignant hypertension or scleroderma renal crisis. The INR and PTT will usually be normal in TTP and HUS. The depressed fibrinogen and elevated D‐dimer suggest that in response to severe bleeding, she is also clotting. DIC, possibly from a severe infection, would explain these findings. Alternatively, the multisystem organ failure may represent progression of SLE.
Additional serology studies detected antinuclear antibodies at 1:320 with a speckled pattern. Rheumatoid factor was not present, but antidouble‐stranded DNA and antiSmith antibodies were elevated. C3 was 30 mg/dL (normal range, 90‐180), C4 was 24 mg/dL (normal range, 16‐47), and the erythrocyte sedimentation rate was 53 mm/h.
The results of the additional lab tests support a diagnosis of lupus and thus a lupus flare, but I agree that antibiotics should be empirically administered while searching for an underlying infection that might mimic lupus. Apart from infection, severe lupus may be complicated by widespread vasculitis or catastrophic antiphospholipid antibody syndrome, which would necessitate high‐dose immunosuppressive therapy and anticoagulation, respectively.
Tests for antiphospholipid antibodies including lupus anticoagulant and for anticardiolipin antibodies were negative. The patient continued to require vasopressors, hemodialysis, and mechanical ventilation. On the fourth hospital day she developed a morbilliform rash over her trunk, face, and extremities. Skin over her right buttock became indurated and tender. On the sixth day of hospitalization the skin on her face, extremities, and palms began to desquamate (Fig. 1).
Regarding the rash, it is hard to differentiate the chicken from the egg. The rash may be a reaction to medication, or it may be a clue to a multiorgan disease. I am considering severe skin reactions like Stevens‐Johnson as well as bacterial toxin‐mediated diseases such as toxic shock syndrome. The criteria for toxic shock syndrome with multisystem involvement are very similar to those for lupus. In this case, a desquamating rash occurring on the heel of a multiorgan illness definitely points to toxic shock syndrome. In staphylococcal toxic shock cases, blood cultures are frequently negative, and the origin may elude detection, but of the sources identified, most have been wounds and soft‐tissue infections.
On hospital day 4, blood cultures from admission grew oxacillin‐sensitive Staphylococcus aureus in 4 of the 4 bottles. Magnetic resonance imaging of the thigh demonstrated extensive necrosis of multiple muscles (Fig. 2). The patient underwent muscle debridement in the operating room, and Gram's stain of the debrided muscle revealed Gram‐positive cocci. Following surgery, she rapidly improved. She no longer required dialysis and was eventually discharged home after completing a prolonged course of intravenous anti‐Staphylococcal antibiotics at a rehabilitation facility. Follow‐up urine testing on 2 occasions revealed 1.6 and 1.4 g of protein in 24‐hour collections, but serum creatinine remained normal, and microscopy demonstrated no dysmorphic red cells or red‐cell casts. Performance of a kidney biopsy was deferred. Other than transient arthralgia and malar rash, her lupus has been quiescent, and her prednisone dose was tapered to 5 mg daily. Six months after discharge she returned to school.

COMMENTARY
Using the American College of Rheumatology (ACR) definition, systemic lupus erythematosus (SLE) is diagnosed when at least 4 criteria are met with a sensitivity and specificity above 95%. These criteria were developed for study purposes to differentiate SLE from other rheumatic diseases. At disease onset a patient may not meet the ACR threshold, but delaying treatment may be harmful. Data conflict on the probability of such patients eventually being classified as having SLE, with estimates ranging from less than 10% to more than 60%.1, 2 With SLE prominent in the differential diagnosis of a critically ill patient, hospitalists must consider the 3 most common causes of death in lupus patients: lupus crisis, severe infection, and thrombosis.3
Most exacerbations of SLE occur in one system, most commonly the musculoskeletal system, and are mild. However, 10% of patients a year will require high‐dose corticosteroids or cytotoxic agents for severe flares that can occur in any system affected by lupus and in 15% of cases may involve multiple sites simultaneously.4, 5 Diagnosing lupus flares remains challenging. Although pulmonary hemorrhage and red blood cell casts may strongly implicate active lupus in the lungs or kidneys, specific clinical and laboratory markers of lupus crisis are lacking. Several global indices reliably measure current disease status but are cumbersome, cannot be relied on solely for treatment decisions and have not been well studied in hospitalized patients.68 Fever, once a dependable harbinger of active lupus,9 cannot reliably discriminate lupus flares from infection. In 2 studies, Rovin et al. found that infection accounted for fever in all but one SLE outpatient taking prednisone and that in hospitalized SLE patients, failure of fevers to resolve within 48 hours of administering 20‐40 mg of prednisone daily strongly suggested infection.10 The laboratory findings provided general support for there being an SLE flare or an infection, but, as the discussant pointed out, these cannot be relied on exclusively to discriminate between the two. Results that suggest infection in an SLE patient include leukocytosis, increased band forms or metamyelocytes, and possibly elevated C‐reactive protein. Findings favoring SLE flare include leukopenia, low C3 or C4 (particularly for nephritis or hematologic flares) and elevated anti‐double‐stranded DNA antibodies for nephritis.1113 Without a clear gold standard for definitively determining a lupus crisis, it is diagnosed when clinical manifestations fit a pattern seen in SLE (nephritis, cerebritis, serositis, vasculitis, pneumonitis), the results of serology studies support this conclusion, and other plausible diagnoses are excluded.
Infection and active disease account for most ICU admissions of lupus patients. SLE and infection intertwine in 3 ways. First, SLE patients are predisposed to infection, possibly because of a variety of identified genetic abnormalities of immune function.14 Although community‐acquired bacteria and viruses account for most infections, lupus patients are vulnerable to a wide array of atypical and opportunistic pathogens. Clinical factors that augment this intrinsic risk include severity of the underlying SLE, flares of the central nervous system or kidneys, and use of immunosuppressive agents.14 The latter deserves particular attention, as a recent study found more than 90% of SLE patients admitted to an ICU with severe infection were taking corticosteroids prior to hospitalization.15 Second, infection may trigger a lupus flare. Third, features of severe lupus flares and infection may overlap. Differentiating between the 2 may be difficult, and the stakes are high, as SLE patients admitted to ICUs have a risk of death that is substantially higher (47%) than that of those without SLE (29%) and much greater than the overall risk of death for those with SLE, for whom 10‐year survival exceeds 90%.15
In addition to lupus crisis and infection, the differential diagnosis of acute multisystem disease in a patient with SLE includes catastrophic antiphospholipid syndrome (APS) and thrombotic thrombocytopenic purpura, 2 thrombotic microangiopathies to which SLE patients are predisposed. Thrombocytopenia and hemolytic anemia with schistocytes should raise suspicion of these diagnoses. Additional findings for TTP include fevers, altered mental status, acute renal failure, and elevated serum lactate dehydrogenase; however, prothrombin time should not be prolonged. Lupus anticoagulant or anticardiolipin antibodies are found in up to 30% of lupus patients, of whom 50%‐70% develop APS within 20 years, characterized by thrombosis or spontaneous abortions in the presence of antiphospholipid antibodies.16 Catastrophic APS is a rare subset of APS involving thromboses of multiple organs simultaneously and has a mortality rate of 50%.
In the present patient, an elevated INR, bleeding, hypotension, and the absence of antiphospholipid antibodies argued against TTP and APS, leading the discussant to focus on SLE and sepsis. Arthralgia, cytopenia, and the results of serology studies suggested a lupus crisis, but hypothermia, hypotension, and DIC pointed to severe infection. Empiric treatment of both conditions with corticosteroids and broad‐spectrum antibiotics was indicated, and ultimately the patient's condition was found to meet criteria for toxic shock syndrome (TSS) and SLE. TSS has rarely been reported in SLE1718 and poses a particularly difficult diagnostic challenge because a severe lupus flare can meet the diagnostic criteria for TSS (Table 1), especially early on, before the characteristic desquamating rash appears. Acuity of the illness increased the ante in this challenging case. Afraid not to treat a potentially life‐threatening condition, empiric treatment of severe lupus and sepsis was initiated. Attention then shifted to fraying, or unraveling, the knot linking infection and lupus. Ultimately, diagnoses of both TSS and SLE were established.
|
| 1. Fever > 38.9C |
| 2. Hypotension (SBP 90 mm Hg) |
| 3. Diffuse erythroderma |
| 4. Desquamation, particularly of palms and soles (occurring 1‐2 weeks after onset of illness) |
| 5. Involvement of 3 or more systems: |
| GI (vomiting or diarrhea at onset) |
| Muscular (CK > twice the upper limit of normal or severe myalgia) |
| Mucus membranes (vaginal, oropharyngeal, or conjunctival hyperemia) |
| Renal (pyuria; BUN or creatinine > twice the upper limit of normal) |
| Hepatic (bilirubin or transaminases > twice the upper limit of normal) |
| Hematologic (platelets 100,000/mm3) |
| Central nervous system (altered mental status without localizing deficits unexplained by hypotension or fever) |
| In addition, negative cultures of blood, throat, and cerebrospinal fluid are expected (except for blood cultures in S. aureus TSS, which may be positive). |
Acknowledgements
The authors thank Michael Chan, MD, and Shelley Gordon, MD, for their input on this manuscript.
- ,.Incomplete lupus erythematosus.Arch Intern Med.1989;149:2473–2476.
- ,,.Systemic lupus erythematosus. Differences between patients who do, and who do not, fulfill classification criteria at the time of diagnosis.J Rheumatol.1980;7:831–837.
- ,,, et al.Morbidity and mortality in systemic lupus erythematosus during a 10‐year period: a comparison of early and late manifestations in a cohort of 1,000 patients.Medicine (Baltimore).2003;82:299–308.
- ,,,,.Definition and treatment of lupus flares measured by the BILAG index.Rheumatology.2003;42:1372–1379.
- ,,,,.The occurrence, nature and distributions of flares in a cohort of patients with systemic lupus erythematosus: a rheumatologic view.Br J Rheumatol.1995;34:257–260.
- ,,.Comparison of the validity and sensitivity to change of 5 activity indices in systemic lupus erythematosus.J Rheumatol.2000;27:664–670.
- ,,.Serologically active clinically quiescent systemic lupus erythematosus—predictors of clinical flares.J Rheumatol.1994;21:2239–2241.
- ,,,,,.Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail.Arthritis Rheum.1996;39:370–378.
- ,,.Fever in systemic lupus erythematosus.Am J Med.1979;67:935–940.
- ,,, et al.Clinical significance of fever in the systemic lupus erythematosus patient receiving steroid therapy.Kidney Int.2005;68:747–759.
- ,,.Lupus nephritis flares.Lupus.2005;14:49–52.
- ,,,.A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus.Arthritis Rheum.2001;44:2350–2357.
- ,,,.Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study.Arthritis Rheum.1991;34:937–944.
- ,.Infections and SLE.Autoimmunity.2005;38:473–485.
- ,,, et al.Outcome and prognostic factors in critically ill patients with systemic lupus erythematosus: a retrospective study.Critical Care.2005;9:R177–R183.
- ,,.The Antiphospholipid Syndrome.N Engl J Med.2002;346:752–763.
- ,,.Toxic shock syndrome in a patient with systemic lupus erythematosus.Can Med Assoc J.1983;129:1201–1202.
- ,,.Toxic shock syndrome in a patient with breast cancer and systemic lupus erythematosus.Eur J Surg Oncol.2001;27:330–331.
- Case definitions for infectious conditions under public health surveillance.MMWR Recomm Rep.1997;46(RR‐10):39.
A 20‐year‐old woman presented to the emergency department after 2 days of epistaxis and vaginal bleeding.
A young woman is more likely to present with infection, toxic exposure, or rheumatologic disease than with a degenerative disease or malignancy. Her bleeding may relate to a platelet abnormality, either quantitative or qualitative. I would pursue her bleeding and menstrual history further.
The patient was healthy until 2 months previously, when she noted arthralgia of her shoulders, wrists, elbows, knees, and ankles. She was examined by a rheumatologist who detected mild arthritis in her left wrist and proximal interphalangeal joints. The rest of her joints were normal. Rheumatoid factor and ANA were positive, and the erythrocyte sedimentation rate was 122 mm/hour. She was diagnosed with possible systemic lupus erythematosus and was placed on a nonsteroidal anti‐inflammatory agent. At a follow‐up visit 1 month prior to admission, her arthralgia had markedly improved. Two weeks prior to admission, the patient began to feel fatigued. Two days prior to admission, she developed epistaxis and what she thought was her menses, though bleeding was heavier than usual and associated with the passage of red clots. On the day of admission the vaginal bleeding worsened, and emergency personnel transported the patient to the hospital.
The diagnosis of systemic lupus erythematosus (SLE) is not engraved in stone. One must be vigilant for other diseases masquerading as SLE while continuing to build a case for it. As more criteria are fulfilled, the probability of lupus increases, yet no findings, alone or in combination, are pathognomonic of this protean disease. This patient's age, sex, and serology are compatible with SLE; otherwise, her presentation is nonspecific. I would request a complete blood count, coagulation tests, and additional serological tests.
The quantity of the bleeding is described, but this does not help decipher its etiology. Excess bleeding may be a result of one or more of 3 broad etiologies: problems with platelets (quantitative or qualitative), with clotting factors (quantitative or qualitative), or with blood vessels (trauma, vasculitis, or diseases affecting collagen). Because quantitative and qualitative factor disorders generally do not present with mucosal bleeding, I am thinking more about platelet problems and about processes that damage the microvasculature. If this woman has lupus, immunologic thrombocytopenia may be the cause of mucosal bleeding.
The patient had no previous medical problems and had never been pregnant. Her only medication was sulindac twice daily for the past month. She was born in Hong Kong, graduated from high school in San Francisco, and attended junior college. She lived with her parents and brother and denied alcohol, tobacco, or recreational drug use but had recently obtained a tattoo on her lower back. There was no family history of autoimmune or bleeding disorders, and a review of systems was notable for dyspnea with minimal exertion and fatigue which worsened in the past 2 days. She had no prior episodes of abnormal bleeding or clotting.
Tattoos may be surrogates for other high‐risk behaviors and suggest an increased risk of hepatitis and sexually transmitted diseases. I want to know her sexual history and other risk factors for human immunodeficiency virus infection. The dyspnea and fatigue are likely the result of anemia, but I am also considering cardiac disease. Though SLE remains a possibility, I cannot assume the presence of a lupus anticoagulant with antiphospholipid syndrome without a history of infertility or recurrent miscarriages.
On arrival at the emergency department, the patient had a blood pressure of 78/46 mm Hg, a pulse of 120 beats/min, a temperature of 34C, 14 respirations per minute, and oxygen saturation of 99% while breathing supplemental oxygen through a nonrebreather mask. Systolic blood pressure improved to 90 mmHg after 4 L of normal saline was administered. The patient was pale but alert. There was crusted blood in her mouth and nostrils without active bleeding or petechiae. Her tongue was pierced with a ring, and sclerae were anicteric. Bleeding was noted from both nipples. There was no heart murmur or gallop, and jugular venous pressure was not elevated. Pulmonary exam revealed bibasilar crackles. Abdomen was soft, not tender, and without hepatosplenomegaly, and her umbilicus was pierced by a ring. Genitourinary exam revealed scant vaginal discharge and clotted blood in the vagina. Skin demonstrated no petechiae, ecchymoses, or stigmata of liver disease. Neurological and joint exams were normal.
It is hard to conceive of vaginal bleeding producing this profound a degree of hypotension. The patient may have additional occult sites of bleeding, or she may have a distributive cause of hypotension such as sepsis or adrenal hemorrhage with resultant adrenal insufficiency. Breast bleeding is unusual, even with profound thrombocytopenia, and I wonder about a concomitant factor deficiency. Furthermore, if thrombocytopenia was the sole reason for the bleeding, I would have expected petechiae. Diffuse vascular injury, such as from lupus or vasculitis, would be an unusual cause of profound bleeding unless there was also disseminated intravascular coagulation.
Laboratory studies revealed a white count of 2000/mm3, of which 42% were neutrophils, 40% bands, 8% lymphocytes, and 10% monocytes. Hematocrit was 17.6%, platelets 35,000/mm3. Sodium was 124 mmol/L, potassium 6 mmol/L, chloride 92 mmol/L, bicarbonate 10 mmol/L, blood urea nitrogen 122 mg/dL (43.5 mmol/L), and creatinine 3.4 mg/dL (300 mol/L). Blood glucose was 44 mg/dL (2.44 mmol/L). Total bilirubin was 3.0 mg/dL (51.3 mol/L; normal range, 0.1‐1.5), alkaline phosphatase 105 U/L (normal range, 39‐117), aspartate aminotransferase 849 U/L (normal range, 8‐31), alanine aminotransferase 261 U/L (normal range, 7‐31), international normalized unit (INR) 2.9, and partial thromboplastin time (PTT) 34.2 seconds.
The combination of profound hypotension, electrolyte abnormalities, hypoglycemia, and hypothermia makes adrenal insufficiency a consideration. I would perform a cortrosyn stimulation test and start glucocorticoid and perhaps mineralocorticoid replacement. In addition, there is renal failure and metabolic acidosis, with a calculated anion gap of 22. The anion gap may be from lactic acidosis secondary to hypotension and hypoperfusion. The abnormal transaminases and bilirubin could relate to infectious hepatitis or systemic infection. Although ischemia could explain these findings, it is rare for a 20‐year‐old to develop ischemic hepatopathy. Thrombocytopenia this moderate may augment the volume of blood loss, but spontaneous bleeding because of thrombocytopenia is unusual until the platelet count falls below 20,000/mm3. Furthermore, the elevated INR points to a mixed coagulopathy. Interpretation of the INR is complicated by the fact she has liver disease, and I am most concerned about acute disseminated intravascular coagulation (DIC) or impending fulminant hepatic failure. This is not the pattern seen with antiphospholipid antibody syndrome, in which the INR tends to be preserved and the PTT prolonged.
Urine dipstick testing demonstrated a specific gravity of 1.015, trace leukocyte esterase, 2+ protein, and 3+ blood, and microscopy revealed 2 white blood cells and 38 red blood cells per high‐power field, many bacteria, and no casts. Creatine kinase was 20,599 U/L, with a myocardial fraction of 1.4%. Lipase was normal, lactate was 7.3 mmol/L, and serum pregnancy test was negative.
Although there is proteinuria and hematuria, we do not have solid evidence of glomerulonephritis. Although the red cells could be a contaminant from her vaginal bleeding, I would examine her sediment carefully for dysmorphic red cells, recognizing that only a quarter of people with glomerulonephritis have red‐cell casts. A urine protein‐to‐creatinine ratio would be useful for estimating the degree of proteinuria. The elevated creatine kinase indicates rhabdomyolysis. In a previously healthy young woman without evidence of cardiogenic shock, it would be unusual for hypotension to result in rhabdomyolysis. Infection and metabolic derangements are possible etiologies of rhabdomyolysis. Alternatively, coagulopathy might have produced intramuscular bleeding. The constellation of thrombocytopenia, anemia, and renal failure raises my suspicion that there is a thrombotic microangiopathy, such as thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS). I would inspect a peripheral‐blood smear for schistocytes and evidence of microangiopathy.
The chest radiograph demonstrated low lung volumes, patchy areas of consolidation, and pulmonary edema. Heart size was normal, and there were no pleural effusions. On the first hospital day the patient required mechanical ventilation because of respiratory failure. She received 5 units of packed red blood cells, 2 units of fresh frozen plasma, and 1 unit of platelets. Vasopressor infusion was started, and a vascular catheter was placed for hemodialysis. Blood, respiratory, and urine cultures were sent, and methylprednisolone, piperacillin/tazobactam, and vancomycin were administered. D‐dimer was greater than 10,000 ng/mL, fibrinogen was 178 mg/dL, and lactate dehydrogenase was 1671 U/L (27 kat/L). The peripheral‐blood smear demonstrated 1+ schistocytes and no spherocytes. There were fewer white blood cells with bands and myelocytes, but no blasts.
The presence of schistocytes and the elevated lactate dehydrogenase point to a microangiopathic hemolytic process. Causes of microangiopathic hemolytic anemia include TTP, HUS, DIC, paraneoplastic conditions, and endothelial damage from malignant hypertension or scleroderma renal crisis. The INR and PTT will usually be normal in TTP and HUS. The depressed fibrinogen and elevated D‐dimer suggest that in response to severe bleeding, she is also clotting. DIC, possibly from a severe infection, would explain these findings. Alternatively, the multisystem organ failure may represent progression of SLE.
Additional serology studies detected antinuclear antibodies at 1:320 with a speckled pattern. Rheumatoid factor was not present, but antidouble‐stranded DNA and antiSmith antibodies were elevated. C3 was 30 mg/dL (normal range, 90‐180), C4 was 24 mg/dL (normal range, 16‐47), and the erythrocyte sedimentation rate was 53 mm/h.
The results of the additional lab tests support a diagnosis of lupus and thus a lupus flare, but I agree that antibiotics should be empirically administered while searching for an underlying infection that might mimic lupus. Apart from infection, severe lupus may be complicated by widespread vasculitis or catastrophic antiphospholipid antibody syndrome, which would necessitate high‐dose immunosuppressive therapy and anticoagulation, respectively.
Tests for antiphospholipid antibodies including lupus anticoagulant and for anticardiolipin antibodies were negative. The patient continued to require vasopressors, hemodialysis, and mechanical ventilation. On the fourth hospital day she developed a morbilliform rash over her trunk, face, and extremities. Skin over her right buttock became indurated and tender. On the sixth day of hospitalization the skin on her face, extremities, and palms began to desquamate (Fig. 1).

Regarding the rash, it is hard to differentiate the chicken from the egg. The rash may be a reaction to medication, or it may be a clue to a multiorgan disease. I am considering severe skin reactions like Stevens‐Johnson as well as bacterial toxin‐mediated diseases such as toxic shock syndrome. The criteria for toxic shock syndrome with multisystem involvement are very similar to those for lupus. In this case, a desquamating rash occurring on the heel of a multiorgan illness definitely points to toxic shock syndrome. In staphylococcal toxic shock cases, blood cultures are frequently negative, and the origin may elude detection, but of the sources identified, most have been wounds and soft‐tissue infections.
On hospital day 4, blood cultures from admission grew oxacillin‐sensitive Staphylococcus aureus in 4 of the 4 bottles. Magnetic resonance imaging of the thigh demonstrated extensive necrosis of multiple muscles (Fig. 2). The patient underwent muscle debridement in the operating room, and Gram's stain of the debrided muscle revealed Gram‐positive cocci. Following surgery, she rapidly improved. She no longer required dialysis and was eventually discharged home after completing a prolonged course of intravenous anti‐Staphylococcal antibiotics at a rehabilitation facility. Follow‐up urine testing on 2 occasions revealed 1.6 and 1.4 g of protein in 24‐hour collections, but serum creatinine remained normal, and microscopy demonstrated no dysmorphic red cells or red‐cell casts. Performance of a kidney biopsy was deferred. Other than transient arthralgia and malar rash, her lupus has been quiescent, and her prednisone dose was tapered to 5 mg daily. Six months after discharge she returned to school.

COMMENTARY
Using the American College of Rheumatology (ACR) definition, systemic lupus erythematosus (SLE) is diagnosed when at least 4 criteria are met with a sensitivity and specificity above 95%. These criteria were developed for study purposes to differentiate SLE from other rheumatic diseases. At disease onset a patient may not meet the ACR threshold, but delaying treatment may be harmful. Data conflict on the probability of such patients eventually being classified as having SLE, with estimates ranging from less than 10% to more than 60%.1, 2 With SLE prominent in the differential diagnosis of a critically ill patient, hospitalists must consider the 3 most common causes of death in lupus patients: lupus crisis, severe infection, and thrombosis.3
Most exacerbations of SLE occur in one system, most commonly the musculoskeletal system, and are mild. However, 10% of patients a year will require high‐dose corticosteroids or cytotoxic agents for severe flares that can occur in any system affected by lupus and in 15% of cases may involve multiple sites simultaneously.4, 5 Diagnosing lupus flares remains challenging. Although pulmonary hemorrhage and red blood cell casts may strongly implicate active lupus in the lungs or kidneys, specific clinical and laboratory markers of lupus crisis are lacking. Several global indices reliably measure current disease status but are cumbersome, cannot be relied on solely for treatment decisions and have not been well studied in hospitalized patients.68 Fever, once a dependable harbinger of active lupus,9 cannot reliably discriminate lupus flares from infection. In 2 studies, Rovin et al. found that infection accounted for fever in all but one SLE outpatient taking prednisone and that in hospitalized SLE patients, failure of fevers to resolve within 48 hours of administering 20‐40 mg of prednisone daily strongly suggested infection.10 The laboratory findings provided general support for there being an SLE flare or an infection, but, as the discussant pointed out, these cannot be relied on exclusively to discriminate between the two. Results that suggest infection in an SLE patient include leukocytosis, increased band forms or metamyelocytes, and possibly elevated C‐reactive protein. Findings favoring SLE flare include leukopenia, low C3 or C4 (particularly for nephritis or hematologic flares) and elevated anti‐double‐stranded DNA antibodies for nephritis.1113 Without a clear gold standard for definitively determining a lupus crisis, it is diagnosed when clinical manifestations fit a pattern seen in SLE (nephritis, cerebritis, serositis, vasculitis, pneumonitis), the results of serology studies support this conclusion, and other plausible diagnoses are excluded.
Infection and active disease account for most ICU admissions of lupus patients. SLE and infection intertwine in 3 ways. First, SLE patients are predisposed to infection, possibly because of a variety of identified genetic abnormalities of immune function.14 Although community‐acquired bacteria and viruses account for most infections, lupus patients are vulnerable to a wide array of atypical and opportunistic pathogens. Clinical factors that augment this intrinsic risk include severity of the underlying SLE, flares of the central nervous system or kidneys, and use of immunosuppressive agents.14 The latter deserves particular attention, as a recent study found more than 90% of SLE patients admitted to an ICU with severe infection were taking corticosteroids prior to hospitalization.15 Second, infection may trigger a lupus flare. Third, features of severe lupus flares and infection may overlap. Differentiating between the 2 may be difficult, and the stakes are high, as SLE patients admitted to ICUs have a risk of death that is substantially higher (47%) than that of those without SLE (29%) and much greater than the overall risk of death for those with SLE, for whom 10‐year survival exceeds 90%.15
In addition to lupus crisis and infection, the differential diagnosis of acute multisystem disease in a patient with SLE includes catastrophic antiphospholipid syndrome (APS) and thrombotic thrombocytopenic purpura, 2 thrombotic microangiopathies to which SLE patients are predisposed. Thrombocytopenia and hemolytic anemia with schistocytes should raise suspicion of these diagnoses. Additional findings for TTP include fevers, altered mental status, acute renal failure, and elevated serum lactate dehydrogenase; however, prothrombin time should not be prolonged. Lupus anticoagulant or anticardiolipin antibodies are found in up to 30% of lupus patients, of whom 50%‐70% develop APS within 20 years, characterized by thrombosis or spontaneous abortions in the presence of antiphospholipid antibodies.16 Catastrophic APS is a rare subset of APS involving thromboses of multiple organs simultaneously and has a mortality rate of 50%.
In the present patient, an elevated INR, bleeding, hypotension, and the absence of antiphospholipid antibodies argued against TTP and APS, leading the discussant to focus on SLE and sepsis. Arthralgia, cytopenia, and the results of serology studies suggested a lupus crisis, but hypothermia, hypotension, and DIC pointed to severe infection. Empiric treatment of both conditions with corticosteroids and broad‐spectrum antibiotics was indicated, and ultimately the patient's condition was found to meet criteria for toxic shock syndrome (TSS) and SLE. TSS has rarely been reported in SLE1718 and poses a particularly difficult diagnostic challenge because a severe lupus flare can meet the diagnostic criteria for TSS (Table 1), especially early on, before the characteristic desquamating rash appears. Acuity of the illness increased the ante in this challenging case. Afraid not to treat a potentially life‐threatening condition, empiric treatment of severe lupus and sepsis was initiated. Attention then shifted to fraying, or unraveling, the knot linking infection and lupus. Ultimately, diagnoses of both TSS and SLE were established.
|
| 1. Fever > 38.9C |
| 2. Hypotension (SBP 90 mm Hg) |
| 3. Diffuse erythroderma |
| 4. Desquamation, particularly of palms and soles (occurring 1‐2 weeks after onset of illness) |
| 5. Involvement of 3 or more systems: |
| GI (vomiting or diarrhea at onset) |
| Muscular (CK > twice the upper limit of normal or severe myalgia) |
| Mucus membranes (vaginal, oropharyngeal, or conjunctival hyperemia) |
| Renal (pyuria; BUN or creatinine > twice the upper limit of normal) |
| Hepatic (bilirubin or transaminases > twice the upper limit of normal) |
| Hematologic (platelets 100,000/mm3) |
| Central nervous system (altered mental status without localizing deficits unexplained by hypotension or fever) |
| In addition, negative cultures of blood, throat, and cerebrospinal fluid are expected (except for blood cultures in S. aureus TSS, which may be positive). |
Acknowledgements
The authors thank Michael Chan, MD, and Shelley Gordon, MD, for their input on this manuscript.
A 20‐year‐old woman presented to the emergency department after 2 days of epistaxis and vaginal bleeding.
A young woman is more likely to present with infection, toxic exposure, or rheumatologic disease than with a degenerative disease or malignancy. Her bleeding may relate to a platelet abnormality, either quantitative or qualitative. I would pursue her bleeding and menstrual history further.
The patient was healthy until 2 months previously, when she noted arthralgia of her shoulders, wrists, elbows, knees, and ankles. She was examined by a rheumatologist who detected mild arthritis in her left wrist and proximal interphalangeal joints. The rest of her joints were normal. Rheumatoid factor and ANA were positive, and the erythrocyte sedimentation rate was 122 mm/hour. She was diagnosed with possible systemic lupus erythematosus and was placed on a nonsteroidal anti‐inflammatory agent. At a follow‐up visit 1 month prior to admission, her arthralgia had markedly improved. Two weeks prior to admission, the patient began to feel fatigued. Two days prior to admission, she developed epistaxis and what she thought was her menses, though bleeding was heavier than usual and associated with the passage of red clots. On the day of admission the vaginal bleeding worsened, and emergency personnel transported the patient to the hospital.
The diagnosis of systemic lupus erythematosus (SLE) is not engraved in stone. One must be vigilant for other diseases masquerading as SLE while continuing to build a case for it. As more criteria are fulfilled, the probability of lupus increases, yet no findings, alone or in combination, are pathognomonic of this protean disease. This patient's age, sex, and serology are compatible with SLE; otherwise, her presentation is nonspecific. I would request a complete blood count, coagulation tests, and additional serological tests.
The quantity of the bleeding is described, but this does not help decipher its etiology. Excess bleeding may be a result of one or more of 3 broad etiologies: problems with platelets (quantitative or qualitative), with clotting factors (quantitative or qualitative), or with blood vessels (trauma, vasculitis, or diseases affecting collagen). Because quantitative and qualitative factor disorders generally do not present with mucosal bleeding, I am thinking more about platelet problems and about processes that damage the microvasculature. If this woman has lupus, immunologic thrombocytopenia may be the cause of mucosal bleeding.
The patient had no previous medical problems and had never been pregnant. Her only medication was sulindac twice daily for the past month. She was born in Hong Kong, graduated from high school in San Francisco, and attended junior college. She lived with her parents and brother and denied alcohol, tobacco, or recreational drug use but had recently obtained a tattoo on her lower back. There was no family history of autoimmune or bleeding disorders, and a review of systems was notable for dyspnea with minimal exertion and fatigue which worsened in the past 2 days. She had no prior episodes of abnormal bleeding or clotting.
Tattoos may be surrogates for other high‐risk behaviors and suggest an increased risk of hepatitis and sexually transmitted diseases. I want to know her sexual history and other risk factors for human immunodeficiency virus infection. The dyspnea and fatigue are likely the result of anemia, but I am also considering cardiac disease. Though SLE remains a possibility, I cannot assume the presence of a lupus anticoagulant with antiphospholipid syndrome without a history of infertility or recurrent miscarriages.
On arrival at the emergency department, the patient had a blood pressure of 78/46 mm Hg, a pulse of 120 beats/min, a temperature of 34C, 14 respirations per minute, and oxygen saturation of 99% while breathing supplemental oxygen through a nonrebreather mask. Systolic blood pressure improved to 90 mmHg after 4 L of normal saline was administered. The patient was pale but alert. There was crusted blood in her mouth and nostrils without active bleeding or petechiae. Her tongue was pierced with a ring, and sclerae were anicteric. Bleeding was noted from both nipples. There was no heart murmur or gallop, and jugular venous pressure was not elevated. Pulmonary exam revealed bibasilar crackles. Abdomen was soft, not tender, and without hepatosplenomegaly, and her umbilicus was pierced by a ring. Genitourinary exam revealed scant vaginal discharge and clotted blood in the vagina. Skin demonstrated no petechiae, ecchymoses, or stigmata of liver disease. Neurological and joint exams were normal.
It is hard to conceive of vaginal bleeding producing this profound a degree of hypotension. The patient may have additional occult sites of bleeding, or she may have a distributive cause of hypotension such as sepsis or adrenal hemorrhage with resultant adrenal insufficiency. Breast bleeding is unusual, even with profound thrombocytopenia, and I wonder about a concomitant factor deficiency. Furthermore, if thrombocytopenia was the sole reason for the bleeding, I would have expected petechiae. Diffuse vascular injury, such as from lupus or vasculitis, would be an unusual cause of profound bleeding unless there was also disseminated intravascular coagulation.
Laboratory studies revealed a white count of 2000/mm3, of which 42% were neutrophils, 40% bands, 8% lymphocytes, and 10% monocytes. Hematocrit was 17.6%, platelets 35,000/mm3. Sodium was 124 mmol/L, potassium 6 mmol/L, chloride 92 mmol/L, bicarbonate 10 mmol/L, blood urea nitrogen 122 mg/dL (43.5 mmol/L), and creatinine 3.4 mg/dL (300 mol/L). Blood glucose was 44 mg/dL (2.44 mmol/L). Total bilirubin was 3.0 mg/dL (51.3 mol/L; normal range, 0.1‐1.5), alkaline phosphatase 105 U/L (normal range, 39‐117), aspartate aminotransferase 849 U/L (normal range, 8‐31), alanine aminotransferase 261 U/L (normal range, 7‐31), international normalized unit (INR) 2.9, and partial thromboplastin time (PTT) 34.2 seconds.
The combination of profound hypotension, electrolyte abnormalities, hypoglycemia, and hypothermia makes adrenal insufficiency a consideration. I would perform a cortrosyn stimulation test and start glucocorticoid and perhaps mineralocorticoid replacement. In addition, there is renal failure and metabolic acidosis, with a calculated anion gap of 22. The anion gap may be from lactic acidosis secondary to hypotension and hypoperfusion. The abnormal transaminases and bilirubin could relate to infectious hepatitis or systemic infection. Although ischemia could explain these findings, it is rare for a 20‐year‐old to develop ischemic hepatopathy. Thrombocytopenia this moderate may augment the volume of blood loss, but spontaneous bleeding because of thrombocytopenia is unusual until the platelet count falls below 20,000/mm3. Furthermore, the elevated INR points to a mixed coagulopathy. Interpretation of the INR is complicated by the fact she has liver disease, and I am most concerned about acute disseminated intravascular coagulation (DIC) or impending fulminant hepatic failure. This is not the pattern seen with antiphospholipid antibody syndrome, in which the INR tends to be preserved and the PTT prolonged.
Urine dipstick testing demonstrated a specific gravity of 1.015, trace leukocyte esterase, 2+ protein, and 3+ blood, and microscopy revealed 2 white blood cells and 38 red blood cells per high‐power field, many bacteria, and no casts. Creatine kinase was 20,599 U/L, with a myocardial fraction of 1.4%. Lipase was normal, lactate was 7.3 mmol/L, and serum pregnancy test was negative.
Although there is proteinuria and hematuria, we do not have solid evidence of glomerulonephritis. Although the red cells could be a contaminant from her vaginal bleeding, I would examine her sediment carefully for dysmorphic red cells, recognizing that only a quarter of people with glomerulonephritis have red‐cell casts. A urine protein‐to‐creatinine ratio would be useful for estimating the degree of proteinuria. The elevated creatine kinase indicates rhabdomyolysis. In a previously healthy young woman without evidence of cardiogenic shock, it would be unusual for hypotension to result in rhabdomyolysis. Infection and metabolic derangements are possible etiologies of rhabdomyolysis. Alternatively, coagulopathy might have produced intramuscular bleeding. The constellation of thrombocytopenia, anemia, and renal failure raises my suspicion that there is a thrombotic microangiopathy, such as thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS). I would inspect a peripheral‐blood smear for schistocytes and evidence of microangiopathy.
The chest radiograph demonstrated low lung volumes, patchy areas of consolidation, and pulmonary edema. Heart size was normal, and there were no pleural effusions. On the first hospital day the patient required mechanical ventilation because of respiratory failure. She received 5 units of packed red blood cells, 2 units of fresh frozen plasma, and 1 unit of platelets. Vasopressor infusion was started, and a vascular catheter was placed for hemodialysis. Blood, respiratory, and urine cultures were sent, and methylprednisolone, piperacillin/tazobactam, and vancomycin were administered. D‐dimer was greater than 10,000 ng/mL, fibrinogen was 178 mg/dL, and lactate dehydrogenase was 1671 U/L (27 kat/L). The peripheral‐blood smear demonstrated 1+ schistocytes and no spherocytes. There were fewer white blood cells with bands and myelocytes, but no blasts.
The presence of schistocytes and the elevated lactate dehydrogenase point to a microangiopathic hemolytic process. Causes of microangiopathic hemolytic anemia include TTP, HUS, DIC, paraneoplastic conditions, and endothelial damage from malignant hypertension or scleroderma renal crisis. The INR and PTT will usually be normal in TTP and HUS. The depressed fibrinogen and elevated D‐dimer suggest that in response to severe bleeding, she is also clotting. DIC, possibly from a severe infection, would explain these findings. Alternatively, the multisystem organ failure may represent progression of SLE.
Additional serology studies detected antinuclear antibodies at 1:320 with a speckled pattern. Rheumatoid factor was not present, but antidouble‐stranded DNA and antiSmith antibodies were elevated. C3 was 30 mg/dL (normal range, 90‐180), C4 was 24 mg/dL (normal range, 16‐47), and the erythrocyte sedimentation rate was 53 mm/h.
The results of the additional lab tests support a diagnosis of lupus and thus a lupus flare, but I agree that antibiotics should be empirically administered while searching for an underlying infection that might mimic lupus. Apart from infection, severe lupus may be complicated by widespread vasculitis or catastrophic antiphospholipid antibody syndrome, which would necessitate high‐dose immunosuppressive therapy and anticoagulation, respectively.
Tests for antiphospholipid antibodies including lupus anticoagulant and for anticardiolipin antibodies were negative. The patient continued to require vasopressors, hemodialysis, and mechanical ventilation. On the fourth hospital day she developed a morbilliform rash over her trunk, face, and extremities. Skin over her right buttock became indurated and tender. On the sixth day of hospitalization the skin on her face, extremities, and palms began to desquamate (Fig. 1).

Regarding the rash, it is hard to differentiate the chicken from the egg. The rash may be a reaction to medication, or it may be a clue to a multiorgan disease. I am considering severe skin reactions like Stevens‐Johnson as well as bacterial toxin‐mediated diseases such as toxic shock syndrome. The criteria for toxic shock syndrome with multisystem involvement are very similar to those for lupus. In this case, a desquamating rash occurring on the heel of a multiorgan illness definitely points to toxic shock syndrome. In staphylococcal toxic shock cases, blood cultures are frequently negative, and the origin may elude detection, but of the sources identified, most have been wounds and soft‐tissue infections.
On hospital day 4, blood cultures from admission grew oxacillin‐sensitive Staphylococcus aureus in 4 of the 4 bottles. Magnetic resonance imaging of the thigh demonstrated extensive necrosis of multiple muscles (Fig. 2). The patient underwent muscle debridement in the operating room, and Gram's stain of the debrided muscle revealed Gram‐positive cocci. Following surgery, she rapidly improved. She no longer required dialysis and was eventually discharged home after completing a prolonged course of intravenous anti‐Staphylococcal antibiotics at a rehabilitation facility. Follow‐up urine testing on 2 occasions revealed 1.6 and 1.4 g of protein in 24‐hour collections, but serum creatinine remained normal, and microscopy demonstrated no dysmorphic red cells or red‐cell casts. Performance of a kidney biopsy was deferred. Other than transient arthralgia and malar rash, her lupus has been quiescent, and her prednisone dose was tapered to 5 mg daily. Six months after discharge she returned to school.

COMMENTARY
Using the American College of Rheumatology (ACR) definition, systemic lupus erythematosus (SLE) is diagnosed when at least 4 criteria are met with a sensitivity and specificity above 95%. These criteria were developed for study purposes to differentiate SLE from other rheumatic diseases. At disease onset a patient may not meet the ACR threshold, but delaying treatment may be harmful. Data conflict on the probability of such patients eventually being classified as having SLE, with estimates ranging from less than 10% to more than 60%.1, 2 With SLE prominent in the differential diagnosis of a critically ill patient, hospitalists must consider the 3 most common causes of death in lupus patients: lupus crisis, severe infection, and thrombosis.3
Most exacerbations of SLE occur in one system, most commonly the musculoskeletal system, and are mild. However, 10% of patients a year will require high‐dose corticosteroids or cytotoxic agents for severe flares that can occur in any system affected by lupus and in 15% of cases may involve multiple sites simultaneously.4, 5 Diagnosing lupus flares remains challenging. Although pulmonary hemorrhage and red blood cell casts may strongly implicate active lupus in the lungs or kidneys, specific clinical and laboratory markers of lupus crisis are lacking. Several global indices reliably measure current disease status but are cumbersome, cannot be relied on solely for treatment decisions and have not been well studied in hospitalized patients.68 Fever, once a dependable harbinger of active lupus,9 cannot reliably discriminate lupus flares from infection. In 2 studies, Rovin et al. found that infection accounted for fever in all but one SLE outpatient taking prednisone and that in hospitalized SLE patients, failure of fevers to resolve within 48 hours of administering 20‐40 mg of prednisone daily strongly suggested infection.10 The laboratory findings provided general support for there being an SLE flare or an infection, but, as the discussant pointed out, these cannot be relied on exclusively to discriminate between the two. Results that suggest infection in an SLE patient include leukocytosis, increased band forms or metamyelocytes, and possibly elevated C‐reactive protein. Findings favoring SLE flare include leukopenia, low C3 or C4 (particularly for nephritis or hematologic flares) and elevated anti‐double‐stranded DNA antibodies for nephritis.1113 Without a clear gold standard for definitively determining a lupus crisis, it is diagnosed when clinical manifestations fit a pattern seen in SLE (nephritis, cerebritis, serositis, vasculitis, pneumonitis), the results of serology studies support this conclusion, and other plausible diagnoses are excluded.
Infection and active disease account for most ICU admissions of lupus patients. SLE and infection intertwine in 3 ways. First, SLE patients are predisposed to infection, possibly because of a variety of identified genetic abnormalities of immune function.14 Although community‐acquired bacteria and viruses account for most infections, lupus patients are vulnerable to a wide array of atypical and opportunistic pathogens. Clinical factors that augment this intrinsic risk include severity of the underlying SLE, flares of the central nervous system or kidneys, and use of immunosuppressive agents.14 The latter deserves particular attention, as a recent study found more than 90% of SLE patients admitted to an ICU with severe infection were taking corticosteroids prior to hospitalization.15 Second, infection may trigger a lupus flare. Third, features of severe lupus flares and infection may overlap. Differentiating between the 2 may be difficult, and the stakes are high, as SLE patients admitted to ICUs have a risk of death that is substantially higher (47%) than that of those without SLE (29%) and much greater than the overall risk of death for those with SLE, for whom 10‐year survival exceeds 90%.15
In addition to lupus crisis and infection, the differential diagnosis of acute multisystem disease in a patient with SLE includes catastrophic antiphospholipid syndrome (APS) and thrombotic thrombocytopenic purpura, 2 thrombotic microangiopathies to which SLE patients are predisposed. Thrombocytopenia and hemolytic anemia with schistocytes should raise suspicion of these diagnoses. Additional findings for TTP include fevers, altered mental status, acute renal failure, and elevated serum lactate dehydrogenase; however, prothrombin time should not be prolonged. Lupus anticoagulant or anticardiolipin antibodies are found in up to 30% of lupus patients, of whom 50%‐70% develop APS within 20 years, characterized by thrombosis or spontaneous abortions in the presence of antiphospholipid antibodies.16 Catastrophic APS is a rare subset of APS involving thromboses of multiple organs simultaneously and has a mortality rate of 50%.
In the present patient, an elevated INR, bleeding, hypotension, and the absence of antiphospholipid antibodies argued against TTP and APS, leading the discussant to focus on SLE and sepsis. Arthralgia, cytopenia, and the results of serology studies suggested a lupus crisis, but hypothermia, hypotension, and DIC pointed to severe infection. Empiric treatment of both conditions with corticosteroids and broad‐spectrum antibiotics was indicated, and ultimately the patient's condition was found to meet criteria for toxic shock syndrome (TSS) and SLE. TSS has rarely been reported in SLE1718 and poses a particularly difficult diagnostic challenge because a severe lupus flare can meet the diagnostic criteria for TSS (Table 1), especially early on, before the characteristic desquamating rash appears. Acuity of the illness increased the ante in this challenging case. Afraid not to treat a potentially life‐threatening condition, empiric treatment of severe lupus and sepsis was initiated. Attention then shifted to fraying, or unraveling, the knot linking infection and lupus. Ultimately, diagnoses of both TSS and SLE were established.
|
| 1. Fever > 38.9C |
| 2. Hypotension (SBP 90 mm Hg) |
| 3. Diffuse erythroderma |
| 4. Desquamation, particularly of palms and soles (occurring 1‐2 weeks after onset of illness) |
| 5. Involvement of 3 or more systems: |
| GI (vomiting or diarrhea at onset) |
| Muscular (CK > twice the upper limit of normal or severe myalgia) |
| Mucus membranes (vaginal, oropharyngeal, or conjunctival hyperemia) |
| Renal (pyuria; BUN or creatinine > twice the upper limit of normal) |
| Hepatic (bilirubin or transaminases > twice the upper limit of normal) |
| Hematologic (platelets 100,000/mm3) |
| Central nervous system (altered mental status without localizing deficits unexplained by hypotension or fever) |
| In addition, negative cultures of blood, throat, and cerebrospinal fluid are expected (except for blood cultures in S. aureus TSS, which may be positive). |
Acknowledgements
The authors thank Michael Chan, MD, and Shelley Gordon, MD, for their input on this manuscript.
- ,.Incomplete lupus erythematosus.Arch Intern Med.1989;149:2473–2476.
- ,,.Systemic lupus erythematosus. Differences between patients who do, and who do not, fulfill classification criteria at the time of diagnosis.J Rheumatol.1980;7:831–837.
- ,,, et al.Morbidity and mortality in systemic lupus erythematosus during a 10‐year period: a comparison of early and late manifestations in a cohort of 1,000 patients.Medicine (Baltimore).2003;82:299–308.
- ,,,,.Definition and treatment of lupus flares measured by the BILAG index.Rheumatology.2003;42:1372–1379.
- ,,,,.The occurrence, nature and distributions of flares in a cohort of patients with systemic lupus erythematosus: a rheumatologic view.Br J Rheumatol.1995;34:257–260.
- ,,.Comparison of the validity and sensitivity to change of 5 activity indices in systemic lupus erythematosus.J Rheumatol.2000;27:664–670.
- ,,.Serologically active clinically quiescent systemic lupus erythematosus—predictors of clinical flares.J Rheumatol.1994;21:2239–2241.
- ,,,,,.Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail.Arthritis Rheum.1996;39:370–378.
- ,,.Fever in systemic lupus erythematosus.Am J Med.1979;67:935–940.
- ,,, et al.Clinical significance of fever in the systemic lupus erythematosus patient receiving steroid therapy.Kidney Int.2005;68:747–759.
- ,,.Lupus nephritis flares.Lupus.2005;14:49–52.
- ,,,.A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus.Arthritis Rheum.2001;44:2350–2357.
- ,,,.Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study.Arthritis Rheum.1991;34:937–944.
- ,.Infections and SLE.Autoimmunity.2005;38:473–485.
- ,,, et al.Outcome and prognostic factors in critically ill patients with systemic lupus erythematosus: a retrospective study.Critical Care.2005;9:R177–R183.
- ,,.The Antiphospholipid Syndrome.N Engl J Med.2002;346:752–763.
- ,,.Toxic shock syndrome in a patient with systemic lupus erythematosus.Can Med Assoc J.1983;129:1201–1202.
- ,,.Toxic shock syndrome in a patient with breast cancer and systemic lupus erythematosus.Eur J Surg Oncol.2001;27:330–331.
- Case definitions for infectious conditions under public health surveillance.MMWR Recomm Rep.1997;46(RR‐10):39.
- ,.Incomplete lupus erythematosus.Arch Intern Med.1989;149:2473–2476.
- ,,.Systemic lupus erythematosus. Differences between patients who do, and who do not, fulfill classification criteria at the time of diagnosis.J Rheumatol.1980;7:831–837.
- ,,, et al.Morbidity and mortality in systemic lupus erythematosus during a 10‐year period: a comparison of early and late manifestations in a cohort of 1,000 patients.Medicine (Baltimore).2003;82:299–308.
- ,,,,.Definition and treatment of lupus flares measured by the BILAG index.Rheumatology.2003;42:1372–1379.
- ,,,,.The occurrence, nature and distributions of flares in a cohort of patients with systemic lupus erythematosus: a rheumatologic view.Br J Rheumatol.1995;34:257–260.
- ,,.Comparison of the validity and sensitivity to change of 5 activity indices in systemic lupus erythematosus.J Rheumatol.2000;27:664–670.
- ,,.Serologically active clinically quiescent systemic lupus erythematosus—predictors of clinical flares.J Rheumatol.1994;21:2239–2241.
- ,,,,,.Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail.Arthritis Rheum.1996;39:370–378.
- ,,.Fever in systemic lupus erythematosus.Am J Med.1979;67:935–940.
- ,,, et al.Clinical significance of fever in the systemic lupus erythematosus patient receiving steroid therapy.Kidney Int.2005;68:747–759.
- ,,.Lupus nephritis flares.Lupus.2005;14:49–52.
- ,,,.A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus.Arthritis Rheum.2001;44:2350–2357.
- ,,,.Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study.Arthritis Rheum.1991;34:937–944.
- ,.Infections and SLE.Autoimmunity.2005;38:473–485.
- ,,, et al.Outcome and prognostic factors in critically ill patients with systemic lupus erythematosus: a retrospective study.Critical Care.2005;9:R177–R183.
- ,,.The Antiphospholipid Syndrome.N Engl J Med.2002;346:752–763.
- ,,.Toxic shock syndrome in a patient with systemic lupus erythematosus.Can Med Assoc J.1983;129:1201–1202.
- ,,.Toxic shock syndrome in a patient with breast cancer and systemic lupus erythematosus.Eur J Surg Oncol.2001;27:330–331.
- Case definitions for infectious conditions under public health surveillance.MMWR Recomm Rep.1997;46(RR‐10):39.
Handoffs
It was the second week after finishing my internal medicine residency. What a daunting experience it was to be a newly appointed attending physician. My hospital rounds were painfully slow because I would consult the Tarsacon pharmacopoeia and Uptodate prior to writing any orders or making clinical decisions. During this keystone phase of my career I had the privilege of taking care of Ms. S. It has been almost 2 years since, and I still think of her and what I learned taking care of her.
Ms. S was a woman in her eighties with end‐stage chronic obstructive pulmonary disease (COPD). She was a frequent flyer, as evidenced by the multiple discharge summaries appended to her chart. Her hospital course was predictably punctuated by frequent inpatient exacerbations of COPD, and every time I told her that she'd be discharged the next day I had to eat my own words. After much effort and pharmaceutical gymnastics she finally seemed to be improving. She went nearly 3 days without a significant exacerbation of her condition. I believed that with my medical prowess, I would be mankind's next savior. As I told her yet again that she'd be discharged the next day, she thanked me and said she hoped not to be readmitted any time soon. Making small talk I learned that she had been a nurse at the very same hospital, where she had spent close to 40 years discharging her responsibilities. We didn't know smoking was bad back theneverybody did it, she said. I commiserated and assured her that she was well on her way to recovery.
The next day as I zealously sauntered into the hospital, I thought of the fantastic job I had done managing Ms. S's COPD. I mentally patted myself on the back; after all I was a smart guy. As I went into her room, I saw to my utter horror that she was in the midst of a severe COPD exacerbation. I swung into action, barked orders to start nebulizers, gave her a huge dose of steroids, and put her on a monitor. Through the clutter of nurses starting their IVs and the beeping of monitors, she said her time had come and she was ready to die. I gently chided her, assuring her this was just another episode, no different from her previous ones. She looked frail and tired, and her eyes appeared sad and forlorn. I departed from her room to call the resident in the ICU, where I thought surely she would be better served.
The unit unsurprisingly had no beds immediately available. She would be moved as soon as the unit had a bed; meanwhile, the pulmonary physician was on his way to see her. I dashed back to her room to check on her. To my dismay she was in respiratory distress, unable to talk and using all she had just to breathe. On the table next to the bed she had scribbled on a piece of scrap paper: Let me go please, it is OK. Knowing how exhausted she was, it must have taken superhuman effort to write this. She already had advance directives and was a DNR/DNI, but now she was precipitously declining in front of my eyes. Visibly trembling, I went to the nursing station and called her sister to apprise her of the waning of Ms. S's condition. I had never been faced with a scenario like this before. Taking time to compose myself, I wrote an order to put Ms. S on a morphine drip. All the while I couldn't shake off a sense of being ineffectual. Was modern medicine powerless to help people like her? I hoped I was doing the right thing. The pulmonary physician concurred with what I was doing, giving me the validation I was seeking.
Her sister arrived expeditiously, and I filled her in on what had happened. She nodded in understanding and stated her sister had always said when her time was up, she wanted to be let go. Her next question was the one I dreaded: How long do you think she has? I was evasive, reflecting my discomfiture at being totally unprepared in such situations. It is hard to tell, maybe 24 to 48 hours, but these things are hard to predict, I answered her. Writing orders for comfort measures, I couldn't help feeling unqualified to be a doctor. This wasn't something I thought I'd have to grapple with.
Soon all of Ms. S's relatives near and far came in to see her. They spent time at her bedside, but the morphine had taken effect. She was sleeping and looked comfortable but was unable to participate in conversation.
The next day as I arrived at the hospital, there was a cloud of dread in my mind. Ms. S probably had passed away some time during the night. At the nursing station, I was informed that the predictable hadn't happened. I went into her room to find it full of her loved ones. Her sister looked haggard but calm, and Ms. S was sleeping. I asked how things were, and Ms. S's sister's eyes lit up. She woke up at 1 a.m. and spoke to us for 2 hours. We were all able to tell her how much we loved her. She asked about all the children in the family and told us how much she loved them. Thereafter she went to sleep. She woke up at 6 a.m., looked around, and said, Why the hell am I still alive? She went to sleep soon and has been sleeping ever since. Laughter broke out in the room. I laughed with them. This was all a new experience. So Ms. S had closure before she died. Her family seemed content in this sad hour.
Ms. S passed away shortly after that. Her family thanked me for making her comfortable. But initially I felt I had done nothing much. Then the realization of my role as a doctor finally hit home. Sometimes it is apposite to hold a patient's hand and let the inevitable happen rather than fret on the shortcomings of medicine. It is all right not to overintellectualize every clinical event. After all it is all about treating people, not a constellation of bodily organs. Sometimes less is more and all that is needed. I always look back on this experience every time I feel smug. Since then I have incorporated discussion about goals of care into my repertoire. Patients with multiple admissions for incurable diseases need special attention. I hope my personal growth involves approaching such patients with the empathy and compassion they need. Having such discussions also helps to prevent the frenzied panicking that usually accompanies the inevitable decline of patients with terminal illness. The increasing emphasis of medical school curricula on end‐of‐life care issues reflects these sentiments. A growing geriatric cohort with multiple and often chronic medical diagnoses makes these skills indispensable.
My personal credo has been profoundly influenced by Sir Robert Hutchison's Physician's Prayer. I first read it in medical school but revisited it shortly after my experience with Ms S. To my mind, it is still relevant and serves to keep me on an even keel when making difficult decisions.
From inability to let well alone, from too much zeal for the new and contempt for what is old, from putting knowledge before wisdom, science before art and cleverness before common sense, from treating patients as cases and from making the cure of the disease more grievous than the endurance of the same, good Lord deliver us.
Sir Robert Hutchison (1871‐1960)
Print and Online Resources
-
Oxford Textbook of Palliative Medicine.
-
Center to Advance Palliative Care:
www.capc.org . -
American Academy of Hospice and Palliative Medicine:
www.abhpm.org . -
International Association for Hospice and Palliative Care:
www.hospicecare.com .
It was the second week after finishing my internal medicine residency. What a daunting experience it was to be a newly appointed attending physician. My hospital rounds were painfully slow because I would consult the Tarsacon pharmacopoeia and Uptodate prior to writing any orders or making clinical decisions. During this keystone phase of my career I had the privilege of taking care of Ms. S. It has been almost 2 years since, and I still think of her and what I learned taking care of her.
Ms. S was a woman in her eighties with end‐stage chronic obstructive pulmonary disease (COPD). She was a frequent flyer, as evidenced by the multiple discharge summaries appended to her chart. Her hospital course was predictably punctuated by frequent inpatient exacerbations of COPD, and every time I told her that she'd be discharged the next day I had to eat my own words. After much effort and pharmaceutical gymnastics she finally seemed to be improving. She went nearly 3 days without a significant exacerbation of her condition. I believed that with my medical prowess, I would be mankind's next savior. As I told her yet again that she'd be discharged the next day, she thanked me and said she hoped not to be readmitted any time soon. Making small talk I learned that she had been a nurse at the very same hospital, where she had spent close to 40 years discharging her responsibilities. We didn't know smoking was bad back theneverybody did it, she said. I commiserated and assured her that she was well on her way to recovery.
The next day as I zealously sauntered into the hospital, I thought of the fantastic job I had done managing Ms. S's COPD. I mentally patted myself on the back; after all I was a smart guy. As I went into her room, I saw to my utter horror that she was in the midst of a severe COPD exacerbation. I swung into action, barked orders to start nebulizers, gave her a huge dose of steroids, and put her on a monitor. Through the clutter of nurses starting their IVs and the beeping of monitors, she said her time had come and she was ready to die. I gently chided her, assuring her this was just another episode, no different from her previous ones. She looked frail and tired, and her eyes appeared sad and forlorn. I departed from her room to call the resident in the ICU, where I thought surely she would be better served.
The unit unsurprisingly had no beds immediately available. She would be moved as soon as the unit had a bed; meanwhile, the pulmonary physician was on his way to see her. I dashed back to her room to check on her. To my dismay she was in respiratory distress, unable to talk and using all she had just to breathe. On the table next to the bed she had scribbled on a piece of scrap paper: Let me go please, it is OK. Knowing how exhausted she was, it must have taken superhuman effort to write this. She already had advance directives and was a DNR/DNI, but now she was precipitously declining in front of my eyes. Visibly trembling, I went to the nursing station and called her sister to apprise her of the waning of Ms. S's condition. I had never been faced with a scenario like this before. Taking time to compose myself, I wrote an order to put Ms. S on a morphine drip. All the while I couldn't shake off a sense of being ineffectual. Was modern medicine powerless to help people like her? I hoped I was doing the right thing. The pulmonary physician concurred with what I was doing, giving me the validation I was seeking.
Her sister arrived expeditiously, and I filled her in on what had happened. She nodded in understanding and stated her sister had always said when her time was up, she wanted to be let go. Her next question was the one I dreaded: How long do you think she has? I was evasive, reflecting my discomfiture at being totally unprepared in such situations. It is hard to tell, maybe 24 to 48 hours, but these things are hard to predict, I answered her. Writing orders for comfort measures, I couldn't help feeling unqualified to be a doctor. This wasn't something I thought I'd have to grapple with.
Soon all of Ms. S's relatives near and far came in to see her. They spent time at her bedside, but the morphine had taken effect. She was sleeping and looked comfortable but was unable to participate in conversation.
The next day as I arrived at the hospital, there was a cloud of dread in my mind. Ms. S probably had passed away some time during the night. At the nursing station, I was informed that the predictable hadn't happened. I went into her room to find it full of her loved ones. Her sister looked haggard but calm, and Ms. S was sleeping. I asked how things were, and Ms. S's sister's eyes lit up. She woke up at 1 a.m. and spoke to us for 2 hours. We were all able to tell her how much we loved her. She asked about all the children in the family and told us how much she loved them. Thereafter she went to sleep. She woke up at 6 a.m., looked around, and said, Why the hell am I still alive? She went to sleep soon and has been sleeping ever since. Laughter broke out in the room. I laughed with them. This was all a new experience. So Ms. S had closure before she died. Her family seemed content in this sad hour.
Ms. S passed away shortly after that. Her family thanked me for making her comfortable. But initially I felt I had done nothing much. Then the realization of my role as a doctor finally hit home. Sometimes it is apposite to hold a patient's hand and let the inevitable happen rather than fret on the shortcomings of medicine. It is all right not to overintellectualize every clinical event. After all it is all about treating people, not a constellation of bodily organs. Sometimes less is more and all that is needed. I always look back on this experience every time I feel smug. Since then I have incorporated discussion about goals of care into my repertoire. Patients with multiple admissions for incurable diseases need special attention. I hope my personal growth involves approaching such patients with the empathy and compassion they need. Having such discussions also helps to prevent the frenzied panicking that usually accompanies the inevitable decline of patients with terminal illness. The increasing emphasis of medical school curricula on end‐of‐life care issues reflects these sentiments. A growing geriatric cohort with multiple and often chronic medical diagnoses makes these skills indispensable.
My personal credo has been profoundly influenced by Sir Robert Hutchison's Physician's Prayer. I first read it in medical school but revisited it shortly after my experience with Ms S. To my mind, it is still relevant and serves to keep me on an even keel when making difficult decisions.
From inability to let well alone, from too much zeal for the new and contempt for what is old, from putting knowledge before wisdom, science before art and cleverness before common sense, from treating patients as cases and from making the cure of the disease more grievous than the endurance of the same, good Lord deliver us.
Sir Robert Hutchison (1871‐1960)
Print and Online Resources
-
Oxford Textbook of Palliative Medicine.
-
Center to Advance Palliative Care:
www.capc.org . -
American Academy of Hospice and Palliative Medicine:
www.abhpm.org . -
International Association for Hospice and Palliative Care:
www.hospicecare.com .
It was the second week after finishing my internal medicine residency. What a daunting experience it was to be a newly appointed attending physician. My hospital rounds were painfully slow because I would consult the Tarsacon pharmacopoeia and Uptodate prior to writing any orders or making clinical decisions. During this keystone phase of my career I had the privilege of taking care of Ms. S. It has been almost 2 years since, and I still think of her and what I learned taking care of her.
Ms. S was a woman in her eighties with end‐stage chronic obstructive pulmonary disease (COPD). She was a frequent flyer, as evidenced by the multiple discharge summaries appended to her chart. Her hospital course was predictably punctuated by frequent inpatient exacerbations of COPD, and every time I told her that she'd be discharged the next day I had to eat my own words. After much effort and pharmaceutical gymnastics she finally seemed to be improving. She went nearly 3 days without a significant exacerbation of her condition. I believed that with my medical prowess, I would be mankind's next savior. As I told her yet again that she'd be discharged the next day, she thanked me and said she hoped not to be readmitted any time soon. Making small talk I learned that she had been a nurse at the very same hospital, where she had spent close to 40 years discharging her responsibilities. We didn't know smoking was bad back theneverybody did it, she said. I commiserated and assured her that she was well on her way to recovery.
The next day as I zealously sauntered into the hospital, I thought of the fantastic job I had done managing Ms. S's COPD. I mentally patted myself on the back; after all I was a smart guy. As I went into her room, I saw to my utter horror that she was in the midst of a severe COPD exacerbation. I swung into action, barked orders to start nebulizers, gave her a huge dose of steroids, and put her on a monitor. Through the clutter of nurses starting their IVs and the beeping of monitors, she said her time had come and she was ready to die. I gently chided her, assuring her this was just another episode, no different from her previous ones. She looked frail and tired, and her eyes appeared sad and forlorn. I departed from her room to call the resident in the ICU, where I thought surely she would be better served.
The unit unsurprisingly had no beds immediately available. She would be moved as soon as the unit had a bed; meanwhile, the pulmonary physician was on his way to see her. I dashed back to her room to check on her. To my dismay she was in respiratory distress, unable to talk and using all she had just to breathe. On the table next to the bed she had scribbled on a piece of scrap paper: Let me go please, it is OK. Knowing how exhausted she was, it must have taken superhuman effort to write this. She already had advance directives and was a DNR/DNI, but now she was precipitously declining in front of my eyes. Visibly trembling, I went to the nursing station and called her sister to apprise her of the waning of Ms. S's condition. I had never been faced with a scenario like this before. Taking time to compose myself, I wrote an order to put Ms. S on a morphine drip. All the while I couldn't shake off a sense of being ineffectual. Was modern medicine powerless to help people like her? I hoped I was doing the right thing. The pulmonary physician concurred with what I was doing, giving me the validation I was seeking.
Her sister arrived expeditiously, and I filled her in on what had happened. She nodded in understanding and stated her sister had always said when her time was up, she wanted to be let go. Her next question was the one I dreaded: How long do you think she has? I was evasive, reflecting my discomfiture at being totally unprepared in such situations. It is hard to tell, maybe 24 to 48 hours, but these things are hard to predict, I answered her. Writing orders for comfort measures, I couldn't help feeling unqualified to be a doctor. This wasn't something I thought I'd have to grapple with.
Soon all of Ms. S's relatives near and far came in to see her. They spent time at her bedside, but the morphine had taken effect. She was sleeping and looked comfortable but was unable to participate in conversation.
The next day as I arrived at the hospital, there was a cloud of dread in my mind. Ms. S probably had passed away some time during the night. At the nursing station, I was informed that the predictable hadn't happened. I went into her room to find it full of her loved ones. Her sister looked haggard but calm, and Ms. S was sleeping. I asked how things were, and Ms. S's sister's eyes lit up. She woke up at 1 a.m. and spoke to us for 2 hours. We were all able to tell her how much we loved her. She asked about all the children in the family and told us how much she loved them. Thereafter she went to sleep. She woke up at 6 a.m., looked around, and said, Why the hell am I still alive? She went to sleep soon and has been sleeping ever since. Laughter broke out in the room. I laughed with them. This was all a new experience. So Ms. S had closure before she died. Her family seemed content in this sad hour.
Ms. S passed away shortly after that. Her family thanked me for making her comfortable. But initially I felt I had done nothing much. Then the realization of my role as a doctor finally hit home. Sometimes it is apposite to hold a patient's hand and let the inevitable happen rather than fret on the shortcomings of medicine. It is all right not to overintellectualize every clinical event. After all it is all about treating people, not a constellation of bodily organs. Sometimes less is more and all that is needed. I always look back on this experience every time I feel smug. Since then I have incorporated discussion about goals of care into my repertoire. Patients with multiple admissions for incurable diseases need special attention. I hope my personal growth involves approaching such patients with the empathy and compassion they need. Having such discussions also helps to prevent the frenzied panicking that usually accompanies the inevitable decline of patients with terminal illness. The increasing emphasis of medical school curricula on end‐of‐life care issues reflects these sentiments. A growing geriatric cohort with multiple and often chronic medical diagnoses makes these skills indispensable.
My personal credo has been profoundly influenced by Sir Robert Hutchison's Physician's Prayer. I first read it in medical school but revisited it shortly after my experience with Ms S. To my mind, it is still relevant and serves to keep me on an even keel when making difficult decisions.
From inability to let well alone, from too much zeal for the new and contempt for what is old, from putting knowledge before wisdom, science before art and cleverness before common sense, from treating patients as cases and from making the cure of the disease more grievous than the endurance of the same, good Lord deliver us.
Sir Robert Hutchison (1871‐1960)
Print and Online Resources
-
Oxford Textbook of Palliative Medicine.
-
Center to Advance Palliative Care:
www.capc.org . -
American Academy of Hospice and Palliative Medicine:
www.abhpm.org . -
International Association for Hospice and Palliative Care:
www.hospicecare.com .
Editorial
As we approach the 6th year since the Institute of Medicine's Crossing the Quality Chasm offered a new vision for the American health care system, we still have a marked mismatch between the demand for health care quality and the supply of know‐how to deliver it. What the field of quality improvement (QI) still needs is merely this: QI practitioners in every care setting, a working vocabulary, a predictive framework for the mechanisms of reliable care, and rational therapies rigorously studied.13 Fortunately, the field of QI has attracted enough empiricistsworking in the lab of the hospital and other care settingsto lurch forward. But few would argue that we still have far less insight into the delivery of quality care than into the delivery of myocardial blood flow.
For ischemic heart disease we have classes of therapies, each of which is grounded in basic and clinical science: antiplatelets, beta‐blockers, vasodilators, lipid‐lowering agents. For care delivery we have the makings of analogous therapy classes, derived and introduced rather recently in a large review, Closing the Quality Gap: A Critical Analysis of the Quality Improvement Literature.4, 5 To facilitate their review of the evidence, the authors, including 2 prominent hospitalists, developed a new taxonomy of QI strategies (see Table 1). Though their effect size, relative efficacy, and interactions are not yet clear, many of these strategies can be applied to the inpatient setting, perhaps no less rationally than a well‐constructed antianginal regimen.
| QI Strategies | Examples |
|---|---|
| |
| Provider education | Conferences and workshops |
| Educational outreach visits (eg, academic detailing) | |
| Distributed educational materials | |
| Provider reminder systems | Reminders in charts for providers |
| Computer‐based reminders for providers | |
| Computer‐based decision support | |
| Facilitated relay of clinical data to providers | Transmission of clinical data from data source to hospital physician by means other than medical record, eg, page, e‐mail, phone call to hospitalist about clinically significant findings in postdischarge period |
| Audit and feedback of performance to providers | Feedback of performance to individual providers |
| Quality indicators and reports | |
| National/state quality report cards | |
| Publicly released performance data | |
| Benchmarkingprovision of outcomes data from top performers for comparison with provider's own data | |
| Patient education | Classes |
| Parent and family education | |
| Patient pamphlets | |
| Intensive education strategies promoting self‐management of chronic conditions | |
| Promotion of self‐management | Materials and devices promoting self‐management, eg, diabetes educator, pharmacist‐facilitated teaching of discharge medications |
| Patient reminder systems | Postcards or calls to patients |
| Organizational or team change | Case management, disease management |
| Multidisciplinary teams | |
| Change from paper to computer‐based records | |
| Increased staffing | |
| Skill mix changes | |
| Continuous quality improvement | Interventions using an iterative process for assessing quality problems, developing solutions, testing their impacts, and then reassessing the need for further action, eg, PlanDoStudyAct |
| Financial incentives, regulation, and policy | Provider directed: |
| Financial incentives based on achievement of performance goals | |
| Alternative reimbursement systems (eg, fee‐for‐service, capitated payments) | |
| Licensure requirements | |
| Health system directed: | |
| Initiatives by accreditation bodies (eg, residency work hour limits) | |
| Changes in reimbursement schemes (eg, capitation, prospective payment, salaried providers) | |
Where in the pathophysiology of a hospital do these QI therapies act? A plurality target the level of the provider: provider education, provider reminders, audit‐and‐feedback of provider performance, and facilitated relay of clinical data to providers. Remaining strategies target the patient (patient education, promotion of self‐management, and patient reminders), the immediate system within which care is delivered (organizational change), and the methodology of problem solving (continuous quality improvement). Only one strategy (financial incentives, regulation, and policy) fails to act directly at the level of the patient or provider, arguably the only level at which care actually can be improved.6
The value of the Quality Gap taxonomy is still largely untapped. If QI researchers and practitioners were to adopt its language as a standard, we could ramp up the power with which we communicate, interpret, and ultimately conduct improvement initiatives. In this issue of the Journal of Hospital Medicine, Cohn and colleagues profile a quality improvement initiative that achieved an impressive new level of performance. For an inpatient metric with a baseline institutional performance of 47%and an international benchmark of 39%the investigators executed a QI initiative that appears to have raised the rate of VTE prophylaxis to 85%.7 Despite a study design that weakens validity (beforeafter without controls) and a setting that diminishes applicability (medical patients in a single academic center), the authors have made a solid contribution to the QI literature simply by using the Quality Gap taxonomy. The authors specifically name and profile at least 3 distinct classes of QI strategies: provider education, a provider reminder element (ie, decision support), and an audit‐and‐feedback layer.
Even though provider education is unlikely to be sufficient as a lone QI strategya large review showed consistent but only modest benefitsit is often necessary.8 The provider education executed by Cohn and colleagues was frequent and regular. In the beginning of each month the chief resident oriented incoming house staff about venous thromboembolism (VTE) risk factors and the need for prophylaxis. They were given decision support pocket cards. Posters on display in nurse and physician work areas highlighted VTE risk factors. The provider education element also included discussions with the division chief about the topic. As robust as it was, however, the provider education was just a single component of the larger QI effort.
The second element, decision support, included VTE risk factor pocket cards with prophylaxis options listed. Introduced initially with the provider education, the pocket cards were handed out monthly by the chief resident. It is critical to recognize this decision support layer as a distinct core QI strategyand that it may even be fundamental to the success of the other strategies. Placed into the clinical workflow as a durable item, the decision support pocket card has the power to overcome provider uncertainty at moments of medical decision making. Generally speaking, a decision support layer, whether a pocket card, computer alert, or algorithm on a preprinted order form, can function as a shared baseline. Shared baselines or protocols reduce unnecessary variation in practice, a common source of poor quality care. Any mechanism that encourages groups of providers to deliver the same recommended care to groups of similarly at‐risk patients, while allowing customization of the protocol to meet the special needs of any individual patient, will have the net effect of raising overall quality of care.
This QI initiative may have achieved its greatest performance gainsas well as its greatest loss in terms of applicability to other settingsfrom its third facet, the audit‐and‐feedback layer. As a QI strategy audit‐and‐feedback has been defined as a summary of clinical performance for health care providers or institutions, performed for a specific period of time and reported either publicly or confidentially.1 It has demonstrated small to moderate benefits, with variations in effect most likely related to the format.9 As profiled in this study, it is hard to imagine a more powerful audit‐and‐feedback arrangement. The division chief of General Internal Medicine not only performed the audits, but also directly delivered the feedback to the house staff. In a deliberate, systematic, and successful way the investigators constructively used an existing authority gradient to leverage the Hawthorne effect, a change in worker behavior triggered by knowledge of being observed. Although it contributed to the impressive new VTE prophylaxis rates, this component did diminish generalizability and sustainability. Nonacademic centers may struggle to replicate these results, a point the authors dutifully point out. But even other academic centers might struggle in the absence of an authority figure with comparable influence and dedication to VTE prophylaxis. At the study hospital itself, similar rates of improvement would not be expected in patient populations outside the purview of the division chief.
Several alternatives to the before‐after study design could have produced richer information. Simultaneous data on VTE prophylaxis rates in a nonintervention population in the same or a similar hospital could have controlled for background or secular effects. An interrupted time series design may even have been feasible and could have provided more confidence in causality and more information on effect size. For example, what would be the effect on performance, if any, with removal of the decision support pocket card at 10 or 15 months? How much would performance rebound after its reintroduction? What could we have learned had the authors chosen instead to measure performance after sequentially introducing each component?
Using the language of the Quality Gap taxonomy, what conclusions can we draw from this improvement initiative? The introduction of a portable provider reminder (the decision support pocket card), when preceded by a program of provider education and followed by high‐intensity audit‐and‐feedback within an existing provider hierarchy, may have the power to raise VTE prophylaxis rates to 85% over an 18‐month period. With the large effect size somewhat mitigating the design flaws that weaken causality, we might risk an inference that these 3 classes of QI strategies can be reasonably successful in combination. But would we introduce them in our own medical centers? Using the clarity afforded by the taxonomy, we can identify several potential limitations, all attributable to the specifics of the audit‐and‐feedback arrangement: stringent preconditions of the practice setting, guaranteed inability to spread the initiative to other patient populations within the same medical center, limited scalability to include other QI projects, and reliance on the role of a single individual. Although clopidogrel 300 mg daily in the last week of every month is one way to pursue antiplatelet activity, other schedules or alternative agents may be preferable for the vast majority of patients.
The taxonomy can be used to compare, contrast, and more fully understand other QI studies. For example, among acutely ill medical inpatients not receiving VTE prophylaxis, Kucher and colleagues found that an electronic alert nearly doubled prophylaxis rates compared to those in a control group.10 Before trying to emulate their experience, a similarly equipped hospital would do well to recognize that the electronic alert was deployed as a composite of provider education, provider reminder, and facilitated relay of clinical information strategies, increasing prophylaxis rates for high risk patients from a baseline of 85% to 88%.
On the 21st‐century side of the quality chasm there is still something to be learned from QI research that falls short of recently proposed standards.3 This may be true as long as the key question remains: what are the mechanisms of reliable and sustainable performance improvement? We have not yet reached the day where a predictive framework, the clarity of our inquiry, the rigor of our study design, and the strength of our evidence churn out coherent answers. But we do have insights from a wealth of ongoing QI activity triggered by such forces as the Institute for Healthcare Improvement's 100,000 Lives Campaign and the advent of mandatory public reporting of hospital performance measures. By adding to this primordial mix the taxonomy offered by Closing the Quality Gap and its uptake into our vernacular by reports such as the one by Cohn in this issue of the Journal of Hospital Medicine, we are acquiring the language and experience to conduct intelligent and intelligible QI research.
- ,,.Designing a quality improvement intervention: a systematic approach.Qual Saf Health Care.2003;12;215–220.
- ,,.Implementing clinical guidelines: current evidence and future implications.J Contin Educ Health Prof.2004;24(Suppl 1):S31–S37.
- ,.Toward stronger evidence on quality improvement. Draft publication guidelines: the beginning of a consensus project.Qual Saf Health Care.2005;14:319–325.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol.1, Series Overview and Methodology. Technical Review 9 (Contract No. 290‐02‐0017 to the Stanford University–UCSF Evidence‐based Practices Center). AHRQ Publication No. 04‐0051‐1.Rockville, MD:Agency for Healthcare Research and Quality,2004.
- ,,, et al.Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta‐regression analysis.JAMA.2006;296:427–440.
- ,,, et al.Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units.Jt Comm J Qual Improv.2002;28:472–493.
- ,,, et al.A multinational observational cohort study in acutely ill medical patients of Pharmacological thromboembolic prophylaxis practices in prevention of venous thromboembolism: findings of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE).Blood.2003;102(Suppl):321a.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;6:1–84.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977
As we approach the 6th year since the Institute of Medicine's Crossing the Quality Chasm offered a new vision for the American health care system, we still have a marked mismatch between the demand for health care quality and the supply of know‐how to deliver it. What the field of quality improvement (QI) still needs is merely this: QI practitioners in every care setting, a working vocabulary, a predictive framework for the mechanisms of reliable care, and rational therapies rigorously studied.13 Fortunately, the field of QI has attracted enough empiricistsworking in the lab of the hospital and other care settingsto lurch forward. But few would argue that we still have far less insight into the delivery of quality care than into the delivery of myocardial blood flow.
For ischemic heart disease we have classes of therapies, each of which is grounded in basic and clinical science: antiplatelets, beta‐blockers, vasodilators, lipid‐lowering agents. For care delivery we have the makings of analogous therapy classes, derived and introduced rather recently in a large review, Closing the Quality Gap: A Critical Analysis of the Quality Improvement Literature.4, 5 To facilitate their review of the evidence, the authors, including 2 prominent hospitalists, developed a new taxonomy of QI strategies (see Table 1). Though their effect size, relative efficacy, and interactions are not yet clear, many of these strategies can be applied to the inpatient setting, perhaps no less rationally than a well‐constructed antianginal regimen.
| QI Strategies | Examples |
|---|---|
| |
| Provider education | Conferences and workshops |
| Educational outreach visits (eg, academic detailing) | |
| Distributed educational materials | |
| Provider reminder systems | Reminders in charts for providers |
| Computer‐based reminders for providers | |
| Computer‐based decision support | |
| Facilitated relay of clinical data to providers | Transmission of clinical data from data source to hospital physician by means other than medical record, eg, page, e‐mail, phone call to hospitalist about clinically significant findings in postdischarge period |
| Audit and feedback of performance to providers | Feedback of performance to individual providers |
| Quality indicators and reports | |
| National/state quality report cards | |
| Publicly released performance data | |
| Benchmarkingprovision of outcomes data from top performers for comparison with provider's own data | |
| Patient education | Classes |
| Parent and family education | |
| Patient pamphlets | |
| Intensive education strategies promoting self‐management of chronic conditions | |
| Promotion of self‐management | Materials and devices promoting self‐management, eg, diabetes educator, pharmacist‐facilitated teaching of discharge medications |
| Patient reminder systems | Postcards or calls to patients |
| Organizational or team change | Case management, disease management |
| Multidisciplinary teams | |
| Change from paper to computer‐based records | |
| Increased staffing | |
| Skill mix changes | |
| Continuous quality improvement | Interventions using an iterative process for assessing quality problems, developing solutions, testing their impacts, and then reassessing the need for further action, eg, PlanDoStudyAct |
| Financial incentives, regulation, and policy | Provider directed: |
| Financial incentives based on achievement of performance goals | |
| Alternative reimbursement systems (eg, fee‐for‐service, capitated payments) | |
| Licensure requirements | |
| Health system directed: | |
| Initiatives by accreditation bodies (eg, residency work hour limits) | |
| Changes in reimbursement schemes (eg, capitation, prospective payment, salaried providers) | |
Where in the pathophysiology of a hospital do these QI therapies act? A plurality target the level of the provider: provider education, provider reminders, audit‐and‐feedback of provider performance, and facilitated relay of clinical data to providers. Remaining strategies target the patient (patient education, promotion of self‐management, and patient reminders), the immediate system within which care is delivered (organizational change), and the methodology of problem solving (continuous quality improvement). Only one strategy (financial incentives, regulation, and policy) fails to act directly at the level of the patient or provider, arguably the only level at which care actually can be improved.6
The value of the Quality Gap taxonomy is still largely untapped. If QI researchers and practitioners were to adopt its language as a standard, we could ramp up the power with which we communicate, interpret, and ultimately conduct improvement initiatives. In this issue of the Journal of Hospital Medicine, Cohn and colleagues profile a quality improvement initiative that achieved an impressive new level of performance. For an inpatient metric with a baseline institutional performance of 47%and an international benchmark of 39%the investigators executed a QI initiative that appears to have raised the rate of VTE prophylaxis to 85%.7 Despite a study design that weakens validity (beforeafter without controls) and a setting that diminishes applicability (medical patients in a single academic center), the authors have made a solid contribution to the QI literature simply by using the Quality Gap taxonomy. The authors specifically name and profile at least 3 distinct classes of QI strategies: provider education, a provider reminder element (ie, decision support), and an audit‐and‐feedback layer.
Even though provider education is unlikely to be sufficient as a lone QI strategya large review showed consistent but only modest benefitsit is often necessary.8 The provider education executed by Cohn and colleagues was frequent and regular. In the beginning of each month the chief resident oriented incoming house staff about venous thromboembolism (VTE) risk factors and the need for prophylaxis. They were given decision support pocket cards. Posters on display in nurse and physician work areas highlighted VTE risk factors. The provider education element also included discussions with the division chief about the topic. As robust as it was, however, the provider education was just a single component of the larger QI effort.
The second element, decision support, included VTE risk factor pocket cards with prophylaxis options listed. Introduced initially with the provider education, the pocket cards were handed out monthly by the chief resident. It is critical to recognize this decision support layer as a distinct core QI strategyand that it may even be fundamental to the success of the other strategies. Placed into the clinical workflow as a durable item, the decision support pocket card has the power to overcome provider uncertainty at moments of medical decision making. Generally speaking, a decision support layer, whether a pocket card, computer alert, or algorithm on a preprinted order form, can function as a shared baseline. Shared baselines or protocols reduce unnecessary variation in practice, a common source of poor quality care. Any mechanism that encourages groups of providers to deliver the same recommended care to groups of similarly at‐risk patients, while allowing customization of the protocol to meet the special needs of any individual patient, will have the net effect of raising overall quality of care.
This QI initiative may have achieved its greatest performance gainsas well as its greatest loss in terms of applicability to other settingsfrom its third facet, the audit‐and‐feedback layer. As a QI strategy audit‐and‐feedback has been defined as a summary of clinical performance for health care providers or institutions, performed for a specific period of time and reported either publicly or confidentially.1 It has demonstrated small to moderate benefits, with variations in effect most likely related to the format.9 As profiled in this study, it is hard to imagine a more powerful audit‐and‐feedback arrangement. The division chief of General Internal Medicine not only performed the audits, but also directly delivered the feedback to the house staff. In a deliberate, systematic, and successful way the investigators constructively used an existing authority gradient to leverage the Hawthorne effect, a change in worker behavior triggered by knowledge of being observed. Although it contributed to the impressive new VTE prophylaxis rates, this component did diminish generalizability and sustainability. Nonacademic centers may struggle to replicate these results, a point the authors dutifully point out. But even other academic centers might struggle in the absence of an authority figure with comparable influence and dedication to VTE prophylaxis. At the study hospital itself, similar rates of improvement would not be expected in patient populations outside the purview of the division chief.
Several alternatives to the before‐after study design could have produced richer information. Simultaneous data on VTE prophylaxis rates in a nonintervention population in the same or a similar hospital could have controlled for background or secular effects. An interrupted time series design may even have been feasible and could have provided more confidence in causality and more information on effect size. For example, what would be the effect on performance, if any, with removal of the decision support pocket card at 10 or 15 months? How much would performance rebound after its reintroduction? What could we have learned had the authors chosen instead to measure performance after sequentially introducing each component?
Using the language of the Quality Gap taxonomy, what conclusions can we draw from this improvement initiative? The introduction of a portable provider reminder (the decision support pocket card), when preceded by a program of provider education and followed by high‐intensity audit‐and‐feedback within an existing provider hierarchy, may have the power to raise VTE prophylaxis rates to 85% over an 18‐month period. With the large effect size somewhat mitigating the design flaws that weaken causality, we might risk an inference that these 3 classes of QI strategies can be reasonably successful in combination. But would we introduce them in our own medical centers? Using the clarity afforded by the taxonomy, we can identify several potential limitations, all attributable to the specifics of the audit‐and‐feedback arrangement: stringent preconditions of the practice setting, guaranteed inability to spread the initiative to other patient populations within the same medical center, limited scalability to include other QI projects, and reliance on the role of a single individual. Although clopidogrel 300 mg daily in the last week of every month is one way to pursue antiplatelet activity, other schedules or alternative agents may be preferable for the vast majority of patients.
The taxonomy can be used to compare, contrast, and more fully understand other QI studies. For example, among acutely ill medical inpatients not receiving VTE prophylaxis, Kucher and colleagues found that an electronic alert nearly doubled prophylaxis rates compared to those in a control group.10 Before trying to emulate their experience, a similarly equipped hospital would do well to recognize that the electronic alert was deployed as a composite of provider education, provider reminder, and facilitated relay of clinical information strategies, increasing prophylaxis rates for high risk patients from a baseline of 85% to 88%.
On the 21st‐century side of the quality chasm there is still something to be learned from QI research that falls short of recently proposed standards.3 This may be true as long as the key question remains: what are the mechanisms of reliable and sustainable performance improvement? We have not yet reached the day where a predictive framework, the clarity of our inquiry, the rigor of our study design, and the strength of our evidence churn out coherent answers. But we do have insights from a wealth of ongoing QI activity triggered by such forces as the Institute for Healthcare Improvement's 100,000 Lives Campaign and the advent of mandatory public reporting of hospital performance measures. By adding to this primordial mix the taxonomy offered by Closing the Quality Gap and its uptake into our vernacular by reports such as the one by Cohn in this issue of the Journal of Hospital Medicine, we are acquiring the language and experience to conduct intelligent and intelligible QI research.
As we approach the 6th year since the Institute of Medicine's Crossing the Quality Chasm offered a new vision for the American health care system, we still have a marked mismatch between the demand for health care quality and the supply of know‐how to deliver it. What the field of quality improvement (QI) still needs is merely this: QI practitioners in every care setting, a working vocabulary, a predictive framework for the mechanisms of reliable care, and rational therapies rigorously studied.13 Fortunately, the field of QI has attracted enough empiricistsworking in the lab of the hospital and other care settingsto lurch forward. But few would argue that we still have far less insight into the delivery of quality care than into the delivery of myocardial blood flow.
For ischemic heart disease we have classes of therapies, each of which is grounded in basic and clinical science: antiplatelets, beta‐blockers, vasodilators, lipid‐lowering agents. For care delivery we have the makings of analogous therapy classes, derived and introduced rather recently in a large review, Closing the Quality Gap: A Critical Analysis of the Quality Improvement Literature.4, 5 To facilitate their review of the evidence, the authors, including 2 prominent hospitalists, developed a new taxonomy of QI strategies (see Table 1). Though their effect size, relative efficacy, and interactions are not yet clear, many of these strategies can be applied to the inpatient setting, perhaps no less rationally than a well‐constructed antianginal regimen.
| QI Strategies | Examples |
|---|---|
| |
| Provider education | Conferences and workshops |
| Educational outreach visits (eg, academic detailing) | |
| Distributed educational materials | |
| Provider reminder systems | Reminders in charts for providers |
| Computer‐based reminders for providers | |
| Computer‐based decision support | |
| Facilitated relay of clinical data to providers | Transmission of clinical data from data source to hospital physician by means other than medical record, eg, page, e‐mail, phone call to hospitalist about clinically significant findings in postdischarge period |
| Audit and feedback of performance to providers | Feedback of performance to individual providers |
| Quality indicators and reports | |
| National/state quality report cards | |
| Publicly released performance data | |
| Benchmarkingprovision of outcomes data from top performers for comparison with provider's own data | |
| Patient education | Classes |
| Parent and family education | |
| Patient pamphlets | |
| Intensive education strategies promoting self‐management of chronic conditions | |
| Promotion of self‐management | Materials and devices promoting self‐management, eg, diabetes educator, pharmacist‐facilitated teaching of discharge medications |
| Patient reminder systems | Postcards or calls to patients |
| Organizational or team change | Case management, disease management |
| Multidisciplinary teams | |
| Change from paper to computer‐based records | |
| Increased staffing | |
| Skill mix changes | |
| Continuous quality improvement | Interventions using an iterative process for assessing quality problems, developing solutions, testing their impacts, and then reassessing the need for further action, eg, PlanDoStudyAct |
| Financial incentives, regulation, and policy | Provider directed: |
| Financial incentives based on achievement of performance goals | |
| Alternative reimbursement systems (eg, fee‐for‐service, capitated payments) | |
| Licensure requirements | |
| Health system directed: | |
| Initiatives by accreditation bodies (eg, residency work hour limits) | |
| Changes in reimbursement schemes (eg, capitation, prospective payment, salaried providers) | |
Where in the pathophysiology of a hospital do these QI therapies act? A plurality target the level of the provider: provider education, provider reminders, audit‐and‐feedback of provider performance, and facilitated relay of clinical data to providers. Remaining strategies target the patient (patient education, promotion of self‐management, and patient reminders), the immediate system within which care is delivered (organizational change), and the methodology of problem solving (continuous quality improvement). Only one strategy (financial incentives, regulation, and policy) fails to act directly at the level of the patient or provider, arguably the only level at which care actually can be improved.6
The value of the Quality Gap taxonomy is still largely untapped. If QI researchers and practitioners were to adopt its language as a standard, we could ramp up the power with which we communicate, interpret, and ultimately conduct improvement initiatives. In this issue of the Journal of Hospital Medicine, Cohn and colleagues profile a quality improvement initiative that achieved an impressive new level of performance. For an inpatient metric with a baseline institutional performance of 47%and an international benchmark of 39%the investigators executed a QI initiative that appears to have raised the rate of VTE prophylaxis to 85%.7 Despite a study design that weakens validity (beforeafter without controls) and a setting that diminishes applicability (medical patients in a single academic center), the authors have made a solid contribution to the QI literature simply by using the Quality Gap taxonomy. The authors specifically name and profile at least 3 distinct classes of QI strategies: provider education, a provider reminder element (ie, decision support), and an audit‐and‐feedback layer.
Even though provider education is unlikely to be sufficient as a lone QI strategya large review showed consistent but only modest benefitsit is often necessary.8 The provider education executed by Cohn and colleagues was frequent and regular. In the beginning of each month the chief resident oriented incoming house staff about venous thromboembolism (VTE) risk factors and the need for prophylaxis. They were given decision support pocket cards. Posters on display in nurse and physician work areas highlighted VTE risk factors. The provider education element also included discussions with the division chief about the topic. As robust as it was, however, the provider education was just a single component of the larger QI effort.
The second element, decision support, included VTE risk factor pocket cards with prophylaxis options listed. Introduced initially with the provider education, the pocket cards were handed out monthly by the chief resident. It is critical to recognize this decision support layer as a distinct core QI strategyand that it may even be fundamental to the success of the other strategies. Placed into the clinical workflow as a durable item, the decision support pocket card has the power to overcome provider uncertainty at moments of medical decision making. Generally speaking, a decision support layer, whether a pocket card, computer alert, or algorithm on a preprinted order form, can function as a shared baseline. Shared baselines or protocols reduce unnecessary variation in practice, a common source of poor quality care. Any mechanism that encourages groups of providers to deliver the same recommended care to groups of similarly at‐risk patients, while allowing customization of the protocol to meet the special needs of any individual patient, will have the net effect of raising overall quality of care.
This QI initiative may have achieved its greatest performance gainsas well as its greatest loss in terms of applicability to other settingsfrom its third facet, the audit‐and‐feedback layer. As a QI strategy audit‐and‐feedback has been defined as a summary of clinical performance for health care providers or institutions, performed for a specific period of time and reported either publicly or confidentially.1 It has demonstrated small to moderate benefits, with variations in effect most likely related to the format.9 As profiled in this study, it is hard to imagine a more powerful audit‐and‐feedback arrangement. The division chief of General Internal Medicine not only performed the audits, but also directly delivered the feedback to the house staff. In a deliberate, systematic, and successful way the investigators constructively used an existing authority gradient to leverage the Hawthorne effect, a change in worker behavior triggered by knowledge of being observed. Although it contributed to the impressive new VTE prophylaxis rates, this component did diminish generalizability and sustainability. Nonacademic centers may struggle to replicate these results, a point the authors dutifully point out. But even other academic centers might struggle in the absence of an authority figure with comparable influence and dedication to VTE prophylaxis. At the study hospital itself, similar rates of improvement would not be expected in patient populations outside the purview of the division chief.
Several alternatives to the before‐after study design could have produced richer information. Simultaneous data on VTE prophylaxis rates in a nonintervention population in the same or a similar hospital could have controlled for background or secular effects. An interrupted time series design may even have been feasible and could have provided more confidence in causality and more information on effect size. For example, what would be the effect on performance, if any, with removal of the decision support pocket card at 10 or 15 months? How much would performance rebound after its reintroduction? What could we have learned had the authors chosen instead to measure performance after sequentially introducing each component?
Using the language of the Quality Gap taxonomy, what conclusions can we draw from this improvement initiative? The introduction of a portable provider reminder (the decision support pocket card), when preceded by a program of provider education and followed by high‐intensity audit‐and‐feedback within an existing provider hierarchy, may have the power to raise VTE prophylaxis rates to 85% over an 18‐month period. With the large effect size somewhat mitigating the design flaws that weaken causality, we might risk an inference that these 3 classes of QI strategies can be reasonably successful in combination. But would we introduce them in our own medical centers? Using the clarity afforded by the taxonomy, we can identify several potential limitations, all attributable to the specifics of the audit‐and‐feedback arrangement: stringent preconditions of the practice setting, guaranteed inability to spread the initiative to other patient populations within the same medical center, limited scalability to include other QI projects, and reliance on the role of a single individual. Although clopidogrel 300 mg daily in the last week of every month is one way to pursue antiplatelet activity, other schedules or alternative agents may be preferable for the vast majority of patients.
The taxonomy can be used to compare, contrast, and more fully understand other QI studies. For example, among acutely ill medical inpatients not receiving VTE prophylaxis, Kucher and colleagues found that an electronic alert nearly doubled prophylaxis rates compared to those in a control group.10 Before trying to emulate their experience, a similarly equipped hospital would do well to recognize that the electronic alert was deployed as a composite of provider education, provider reminder, and facilitated relay of clinical information strategies, increasing prophylaxis rates for high risk patients from a baseline of 85% to 88%.
On the 21st‐century side of the quality chasm there is still something to be learned from QI research that falls short of recently proposed standards.3 This may be true as long as the key question remains: what are the mechanisms of reliable and sustainable performance improvement? We have not yet reached the day where a predictive framework, the clarity of our inquiry, the rigor of our study design, and the strength of our evidence churn out coherent answers. But we do have insights from a wealth of ongoing QI activity triggered by such forces as the Institute for Healthcare Improvement's 100,000 Lives Campaign and the advent of mandatory public reporting of hospital performance measures. By adding to this primordial mix the taxonomy offered by Closing the Quality Gap and its uptake into our vernacular by reports such as the one by Cohn in this issue of the Journal of Hospital Medicine, we are acquiring the language and experience to conduct intelligent and intelligible QI research.
- ,,.Designing a quality improvement intervention: a systematic approach.Qual Saf Health Care.2003;12;215–220.
- ,,.Implementing clinical guidelines: current evidence and future implications.J Contin Educ Health Prof.2004;24(Suppl 1):S31–S37.
- ,.Toward stronger evidence on quality improvement. Draft publication guidelines: the beginning of a consensus project.Qual Saf Health Care.2005;14:319–325.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol.1, Series Overview and Methodology. Technical Review 9 (Contract No. 290‐02‐0017 to the Stanford University–UCSF Evidence‐based Practices Center). AHRQ Publication No. 04‐0051‐1.Rockville, MD:Agency for Healthcare Research and Quality,2004.
- ,,, et al.Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta‐regression analysis.JAMA.2006;296:427–440.
- ,,, et al.Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units.Jt Comm J Qual Improv.2002;28:472–493.
- ,,, et al.A multinational observational cohort study in acutely ill medical patients of Pharmacological thromboembolic prophylaxis practices in prevention of venous thromboembolism: findings of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE).Blood.2003;102(Suppl):321a.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;6:1–84.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977
- ,,.Designing a quality improvement intervention: a systematic approach.Qual Saf Health Care.2003;12;215–220.
- ,,.Implementing clinical guidelines: current evidence and future implications.J Contin Educ Health Prof.2004;24(Suppl 1):S31–S37.
- ,.Toward stronger evidence on quality improvement. Draft publication guidelines: the beginning of a consensus project.Qual Saf Health Care.2005;14:319–325.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol.1, Series Overview and Methodology. Technical Review 9 (Contract No. 290‐02‐0017 to the Stanford University–UCSF Evidence‐based Practices Center). AHRQ Publication No. 04‐0051‐1.Rockville, MD:Agency for Healthcare Research and Quality,2004.
- ,,, et al.Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta‐regression analysis.JAMA.2006;296:427–440.
- ,,, et al.Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units.Jt Comm J Qual Improv.2002;28:472–493.
- ,,, et al.A multinational observational cohort study in acutely ill medical patients of Pharmacological thromboembolic prophylaxis practices in prevention of venous thromboembolism: findings of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE).Blood.2003;102(Suppl):321a.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;6:1–84.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977
Newman's Handy Dandy Admit Note
(Circle the appropriate answers.)
HISTORY OF PRESENT ILLNESS
This (0-109)-year-old (Asian, Black, Caucasian, Aleutian, Venusian) (male, female, other) presents with a (1, 7, 100)-(second, minute, day, year, century) history of (pain, swelling, itching, enlargement) of the (arm, chest, scrotum, uvula, pineal gland). (She/He/It) rates it as (11, 12, 20) out of 10. It is aggravated by (breathing, thinking, hang gliding, vigorous dancing, ennui) and alleviated by (acetaminophen, chocolate, high-dose morphine).
PAST MEDICAL HISTORY
Diabetes, hypertension, pineal insufficiency, Kluver-Bucy syndrome, rectal prolapse, Chagas disease, visceral larval migrans, ichthyosis, all of the above
PAST SURGICAL HISTORY
- Transplant of the (heart, kidney, pancreas, amygdala, pineal gland)
- ORIF of (humerus, femur, rear axle)
- Hemorrhoidectomy, cholecystectomy, pancreatectomy, cerumen removal, all of the above
ALLERGIES
(Penicillin, sulfa, every known drug in existence except Demerol)
CURRENT MEDICATION
- Insulin, metformin, Gila monster venom
- Alpha blocker, calcium blocker, beta blocker, blocker blocker
- Amoxicillin, “gorillacillin,” maggot extract, “mickeymycin”
- SSRI, MAOI, TRIAD, ECT, DOA
- (Thyroid, adrenal, pineal) gland extract
FAMILY HISTORY
Adopted, old age, some kind of cancer
SOCIAL HISTORY
- Alcohol: (teetotaler, tippler, boozer, lush, no alcohol—only beer)—multiply by 10
- Smoking: (never; three packs a day; not tobacco; old stogies I have found, short but not too big around)
- Drugs: (Is this confidential?, Freon, whatever I can get my hands on)
- Employment: (meter maid, sewer maintenance worker, JCAHO auditor, forensic proctologist, hospitologist)
REVIEW OF SYMPTOMS
- (Chest pain, more chest pain, even more chest pain), (rectal, urinary, salivary) incontinence, (ears itch with urination, nose runs with defecation, small bugs crawling out of my skin), (short of breath, short of patience)
- Stool is (sticky, floating, malodorous, frequent, shaped like the Statue of Liberty)
- Double vision, tunnel vision, television, hyperacusis, hearing loss, could you repeat that?, halitosis, dysgeusia, dysphonia, “datphonia”
- Abdominal (pain, cramping, crunches)
PHYSICAL EXAM
- BP (0, 90, 140, 230, 290)/(0, 3, 90, 160)
- Pulse (absent, irregularly irregularly irregular, tachycardic, tacky dresser) rate (0, 3, 84, 112, 190, 280)
- Temperature (32.2, 36.8, 25 minutes at 450—baste often)
- Normocephalic/atraumatic, bullet-headed, pointy-headed, hatchet in skull
- Eyes (PERRLA, anisocoria, bloodshot, pinpoint dude)
- Fundus exam (never can see them, cotton wool spots, cotton candy)
- Ears (present, “hyperceruminic,” absent)
- Mouth (macroglossia, foot in mouth, black hairy tongue, halitotic, skin of the teeth, wooden teeth)
- Neck (supple without adenopathy, thick, multiple hickies, red)
- Lungs (clear to auscultation and percussion—OK, I never really percussed; Velcro rales; egophonic; wheezy)
- Heart (systolic, 5/6, even a medical student can hear) murmur (no, pericardial, aye there’s the) rub
- Abdomen (scaphoid, pendulous, six-pack); bowel sounds (absent—was that you?)
- Umbilicus (surgically absent, Sister Mary Joseph nodule, high lint content)
- Extremities (extreme, all six present, night-clubbing)
NEURO EXAM
- Reflexes (cremaster positive, anal wink intact)
- Mentation (alert and oriented x3, catatonic, dogatonic)
- Psychiatric (appropriate, psychotic, truly weird, bipolar, tripolar)
- Skin (present, hideous thing growing on the patient’s face)
LABORATORY FINDINGS
Results of (CBC, ionized calcium, “citrulated ceruloplasmin,” complement levels, insult levels, saliva electrophoresis, urinary zinc level, melatonin level, and protein Q) all markedly abnormal.
IMAGING
(CAT scan, PET scan, DOG scan, plain film, complex film, KUB, IVP, XYZ, molybdenum scan, Afghaniscan)—all suggest need for further imaging.
ASSESSMENT AND PLAN
- “Hyperlabemia:” Likely iatrogenic etiology, or not. Correct with supplemental lab results.
- Pain: Source unclear but probably malingering. Treat with excessive narcotics, thereby causing problems 3 and 4 (see below).
- Bowel obstruction: Discontinue narcotics. Place (NG tube, rectal tube, boob tube, multiple consults).
- Altered mental status: Baseline worsened by narcotics. Give benzodiazepines, antipsychotics, antihistamine, more narcotics; then intubate.
- Discharge planning: The patient is a rock and will be on service forever.
- Abnormal imaging: Perform further scans.
- Code status: (Full code, no code, Morse code)
- Pineal: Gland abnormal—consult the pineal gland service TH
Jamie Newman, MD, FACP, is the physician editor of The Hospitalist, consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine at the Mayo Clinic College of Medicine, Rochester, Minn.
(Circle the appropriate answers.)
HISTORY OF PRESENT ILLNESS
This (0-109)-year-old (Asian, Black, Caucasian, Aleutian, Venusian) (male, female, other) presents with a (1, 7, 100)-(second, minute, day, year, century) history of (pain, swelling, itching, enlargement) of the (arm, chest, scrotum, uvula, pineal gland). (She/He/It) rates it as (11, 12, 20) out of 10. It is aggravated by (breathing, thinking, hang gliding, vigorous dancing, ennui) and alleviated by (acetaminophen, chocolate, high-dose morphine).
PAST MEDICAL HISTORY
Diabetes, hypertension, pineal insufficiency, Kluver-Bucy syndrome, rectal prolapse, Chagas disease, visceral larval migrans, ichthyosis, all of the above
PAST SURGICAL HISTORY
- Transplant of the (heart, kidney, pancreas, amygdala, pineal gland)
- ORIF of (humerus, femur, rear axle)
- Hemorrhoidectomy, cholecystectomy, pancreatectomy, cerumen removal, all of the above
ALLERGIES
(Penicillin, sulfa, every known drug in existence except Demerol)
CURRENT MEDICATION
- Insulin, metformin, Gila monster venom
- Alpha blocker, calcium blocker, beta blocker, blocker blocker
- Amoxicillin, “gorillacillin,” maggot extract, “mickeymycin”
- SSRI, MAOI, TRIAD, ECT, DOA
- (Thyroid, adrenal, pineal) gland extract
FAMILY HISTORY
Adopted, old age, some kind of cancer
SOCIAL HISTORY
- Alcohol: (teetotaler, tippler, boozer, lush, no alcohol—only beer)—multiply by 10
- Smoking: (never; three packs a day; not tobacco; old stogies I have found, short but not too big around)
- Drugs: (Is this confidential?, Freon, whatever I can get my hands on)
- Employment: (meter maid, sewer maintenance worker, JCAHO auditor, forensic proctologist, hospitologist)
REVIEW OF SYMPTOMS
- (Chest pain, more chest pain, even more chest pain), (rectal, urinary, salivary) incontinence, (ears itch with urination, nose runs with defecation, small bugs crawling out of my skin), (short of breath, short of patience)
- Stool is (sticky, floating, malodorous, frequent, shaped like the Statue of Liberty)
- Double vision, tunnel vision, television, hyperacusis, hearing loss, could you repeat that?, halitosis, dysgeusia, dysphonia, “datphonia”
- Abdominal (pain, cramping, crunches)
PHYSICAL EXAM
- BP (0, 90, 140, 230, 290)/(0, 3, 90, 160)
- Pulse (absent, irregularly irregularly irregular, tachycardic, tacky dresser) rate (0, 3, 84, 112, 190, 280)
- Temperature (32.2, 36.8, 25 minutes at 450—baste often)
- Normocephalic/atraumatic, bullet-headed, pointy-headed, hatchet in skull
- Eyes (PERRLA, anisocoria, bloodshot, pinpoint dude)
- Fundus exam (never can see them, cotton wool spots, cotton candy)
- Ears (present, “hyperceruminic,” absent)
- Mouth (macroglossia, foot in mouth, black hairy tongue, halitotic, skin of the teeth, wooden teeth)
- Neck (supple without adenopathy, thick, multiple hickies, red)
- Lungs (clear to auscultation and percussion—OK, I never really percussed; Velcro rales; egophonic; wheezy)
- Heart (systolic, 5/6, even a medical student can hear) murmur (no, pericardial, aye there’s the) rub
- Abdomen (scaphoid, pendulous, six-pack); bowel sounds (absent—was that you?)
- Umbilicus (surgically absent, Sister Mary Joseph nodule, high lint content)
- Extremities (extreme, all six present, night-clubbing)
NEURO EXAM
- Reflexes (cremaster positive, anal wink intact)
- Mentation (alert and oriented x3, catatonic, dogatonic)
- Psychiatric (appropriate, psychotic, truly weird, bipolar, tripolar)
- Skin (present, hideous thing growing on the patient’s face)
LABORATORY FINDINGS
Results of (CBC, ionized calcium, “citrulated ceruloplasmin,” complement levels, insult levels, saliva electrophoresis, urinary zinc level, melatonin level, and protein Q) all markedly abnormal.
IMAGING
(CAT scan, PET scan, DOG scan, plain film, complex film, KUB, IVP, XYZ, molybdenum scan, Afghaniscan)—all suggest need for further imaging.
ASSESSMENT AND PLAN
- “Hyperlabemia:” Likely iatrogenic etiology, or not. Correct with supplemental lab results.
- Pain: Source unclear but probably malingering. Treat with excessive narcotics, thereby causing problems 3 and 4 (see below).
- Bowel obstruction: Discontinue narcotics. Place (NG tube, rectal tube, boob tube, multiple consults).
- Altered mental status: Baseline worsened by narcotics. Give benzodiazepines, antipsychotics, antihistamine, more narcotics; then intubate.
- Discharge planning: The patient is a rock and will be on service forever.
- Abnormal imaging: Perform further scans.
- Code status: (Full code, no code, Morse code)
- Pineal: Gland abnormal—consult the pineal gland service TH
Jamie Newman, MD, FACP, is the physician editor of The Hospitalist, consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine at the Mayo Clinic College of Medicine, Rochester, Minn.
(Circle the appropriate answers.)
HISTORY OF PRESENT ILLNESS
This (0-109)-year-old (Asian, Black, Caucasian, Aleutian, Venusian) (male, female, other) presents with a (1, 7, 100)-(second, minute, day, year, century) history of (pain, swelling, itching, enlargement) of the (arm, chest, scrotum, uvula, pineal gland). (She/He/It) rates it as (11, 12, 20) out of 10. It is aggravated by (breathing, thinking, hang gliding, vigorous dancing, ennui) and alleviated by (acetaminophen, chocolate, high-dose morphine).
PAST MEDICAL HISTORY
Diabetes, hypertension, pineal insufficiency, Kluver-Bucy syndrome, rectal prolapse, Chagas disease, visceral larval migrans, ichthyosis, all of the above
PAST SURGICAL HISTORY
- Transplant of the (heart, kidney, pancreas, amygdala, pineal gland)
- ORIF of (humerus, femur, rear axle)
- Hemorrhoidectomy, cholecystectomy, pancreatectomy, cerumen removal, all of the above
ALLERGIES
(Penicillin, sulfa, every known drug in existence except Demerol)
CURRENT MEDICATION
- Insulin, metformin, Gila monster venom
- Alpha blocker, calcium blocker, beta blocker, blocker blocker
- Amoxicillin, “gorillacillin,” maggot extract, “mickeymycin”
- SSRI, MAOI, TRIAD, ECT, DOA
- (Thyroid, adrenal, pineal) gland extract
FAMILY HISTORY
Adopted, old age, some kind of cancer
SOCIAL HISTORY
- Alcohol: (teetotaler, tippler, boozer, lush, no alcohol—only beer)—multiply by 10
- Smoking: (never; three packs a day; not tobacco; old stogies I have found, short but not too big around)
- Drugs: (Is this confidential?, Freon, whatever I can get my hands on)
- Employment: (meter maid, sewer maintenance worker, JCAHO auditor, forensic proctologist, hospitologist)
REVIEW OF SYMPTOMS
- (Chest pain, more chest pain, even more chest pain), (rectal, urinary, salivary) incontinence, (ears itch with urination, nose runs with defecation, small bugs crawling out of my skin), (short of breath, short of patience)
- Stool is (sticky, floating, malodorous, frequent, shaped like the Statue of Liberty)
- Double vision, tunnel vision, television, hyperacusis, hearing loss, could you repeat that?, halitosis, dysgeusia, dysphonia, “datphonia”
- Abdominal (pain, cramping, crunches)
PHYSICAL EXAM
- BP (0, 90, 140, 230, 290)/(0, 3, 90, 160)
- Pulse (absent, irregularly irregularly irregular, tachycardic, tacky dresser) rate (0, 3, 84, 112, 190, 280)
- Temperature (32.2, 36.8, 25 minutes at 450—baste often)
- Normocephalic/atraumatic, bullet-headed, pointy-headed, hatchet in skull
- Eyes (PERRLA, anisocoria, bloodshot, pinpoint dude)
- Fundus exam (never can see them, cotton wool spots, cotton candy)
- Ears (present, “hyperceruminic,” absent)
- Mouth (macroglossia, foot in mouth, black hairy tongue, halitotic, skin of the teeth, wooden teeth)
- Neck (supple without adenopathy, thick, multiple hickies, red)
- Lungs (clear to auscultation and percussion—OK, I never really percussed; Velcro rales; egophonic; wheezy)
- Heart (systolic, 5/6, even a medical student can hear) murmur (no, pericardial, aye there’s the) rub
- Abdomen (scaphoid, pendulous, six-pack); bowel sounds (absent—was that you?)
- Umbilicus (surgically absent, Sister Mary Joseph nodule, high lint content)
- Extremities (extreme, all six present, night-clubbing)
NEURO EXAM
- Reflexes (cremaster positive, anal wink intact)
- Mentation (alert and oriented x3, catatonic, dogatonic)
- Psychiatric (appropriate, psychotic, truly weird, bipolar, tripolar)
- Skin (present, hideous thing growing on the patient’s face)
LABORATORY FINDINGS
Results of (CBC, ionized calcium, “citrulated ceruloplasmin,” complement levels, insult levels, saliva electrophoresis, urinary zinc level, melatonin level, and protein Q) all markedly abnormal.
IMAGING
(CAT scan, PET scan, DOG scan, plain film, complex film, KUB, IVP, XYZ, molybdenum scan, Afghaniscan)—all suggest need for further imaging.
ASSESSMENT AND PLAN
- “Hyperlabemia:” Likely iatrogenic etiology, or not. Correct with supplemental lab results.
- Pain: Source unclear but probably malingering. Treat with excessive narcotics, thereby causing problems 3 and 4 (see below).
- Bowel obstruction: Discontinue narcotics. Place (NG tube, rectal tube, boob tube, multiple consults).
- Altered mental status: Baseline worsened by narcotics. Give benzodiazepines, antipsychotics, antihistamine, more narcotics; then intubate.
- Discharge planning: The patient is a rock and will be on service forever.
- Abnormal imaging: Perform further scans.
- Code status: (Full code, no code, Morse code)
- Pineal: Gland abnormal—consult the pineal gland service TH
Jamie Newman, MD, FACP, is the physician editor of The Hospitalist, consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine at the Mayo Clinic College of Medicine, Rochester, Minn.
The First Catheterization
It was very painful. I felt that I had planted an apple orchard and other men who had gathered the harvest stood at the wall, laughing at me.” Dr. Werner Forssmann said these words toward the end of his life on his unexpected scientific exile after having laid the foundations of modern cardiology.
Pacemaker Insertion, angioplasty, and valve repair might now be impossible without the daring of Dr. Forssmann, whose humble roots set into sharp relief the depth of his accomplishments.
Leading physicians in turn-of-the-century Europe said that investigation and treatment of conditions affecting the heart were anathema to mainstream medical society; the heart was off-limits. In 1896 Sir Stephen Paget went so far as to say that “no new method, and no new discovery, can overcome the natural difficulties that attend a wound of the heart.”
Enter Dr. Forssmann. Raised by in Berlin by his mother in a middle-class household after his father was killed in WWI, this young surgeon in training broached a bold idea with his surgeon-mentor Dr. Richard Schneider, a friend of the Forssmann family, in summer 1929.
Far from fantasy, Dr. Forssmann’s inspiration to perform what is now called cardiac catheterization came from a sketch in his physiology textbook depicting a long, thin tube being placed into a horse’s jugular vein and guided into the animal’s heart with balloon-assisted measurements of intracardiac pressures. Dr. Forssmann proposed to reach the heart of man—not through the jugular, but through the veins in the crease of the arm, which was more accessible
But how would this experiment happen? Dr. Forssmann elicited the help of Gerda Ditzen, a surgical nurse at Auguste Viktoria Home (Hospital), Eberswalde, near Berlin. In a month, Dr. Forssmann had convinced her to be his first human guinea pig. Dr. Forssmann, unbeknownst to Ditzen, planned on experimenting on himself. She held the keys to the closet, which was needed to obtain a long enough catheter.
As nurse Ditzen was strapped to the surgical table in the small operating room, sweating from both excitement and the sweltering
heat, Dr. Forssmann walked the distance of the OR and began his self-experimentation. With an incision in his left elbow crease, Dr. Forssmann identified the predominant vein and inserted the 65-cm-long ureteral tube into his arm, feeling progressive painless warmth as the tube coursed along. He had determined this was the only tube thin and long enough to safely and adequately reach the endocardium. However he still needed her help to conceal the tube hanging out of his arm. They went—tube in place—to the fluoroscopic X-ray facility, where images were obtained in the hospital basement.
The initial X-ray clearly indicated that the tube had not yet reached its destination. Dr. Forssmann forced the tube farther, resisting at one point the overwhelming urge to cough when the tube collided against his vein. When the tube was shown to be in the right auricle Dr. Forssmann had the technician snap the picture, finally obtaining the proof that he needed. Dr. Forssmann uneventfully removed the tube.
The real incident involved in this daring experiment was to come: Dr. Forssmann had to face the reactions—not only of his mentor Dr. Schneider, but also the medical community. The majority was ostensibly displeased with his methods, rationale, and approach, believing them too dangerous. After repeated self-experiments, Dr. Forssmann learned that his self-cath procedure could be safely performed; he submitted his findings with fluoroscopic proof to the German medical community at large. At least one prior researcher, the surgeon Ernst Unger, repudiated Dr. Forssmann’s claim, saying he had done the same thing many years earlier, but without hard evidence to back his claim.
Despite the rising tide of opposition to his findings, Dr. Forssmann pushed on. His subsequent experiments with rabbits and dogs (and ultimately himself) proved that catheterization angiography could not be achieved with simply sodium iodide. He developed the use of groin catheterization to reach the inferior vena cava through the femoral veins. Dr. Forssmann’s further experiments in aortography proved unfruitful. By this time, he had decided to stop his self-experimentation, having reached his limits with exploration. Instead he decided to seek work as a local urologist in a small German farming community.
In 1956, Forssmann was awarded the Nobel Prize in Medicine, which he shared with André Cournand, MD, and Dickinson W. Richards, MD, who were affiliated with Columbia University, New York City. When offered a job to head a German cardiovascular institute, Dr. Forssmann declined, citing his lack of knowledge about advancements in the field since his last self-experimentation in 1935. TH
Reference
- Altman, Lawrence K. Who Goes First: The Story of Self-Experimentation in Medicine. New York: Random House; 1987.
It was very painful. I felt that I had planted an apple orchard and other men who had gathered the harvest stood at the wall, laughing at me.” Dr. Werner Forssmann said these words toward the end of his life on his unexpected scientific exile after having laid the foundations of modern cardiology.
Pacemaker Insertion, angioplasty, and valve repair might now be impossible without the daring of Dr. Forssmann, whose humble roots set into sharp relief the depth of his accomplishments.
Leading physicians in turn-of-the-century Europe said that investigation and treatment of conditions affecting the heart were anathema to mainstream medical society; the heart was off-limits. In 1896 Sir Stephen Paget went so far as to say that “no new method, and no new discovery, can overcome the natural difficulties that attend a wound of the heart.”
Enter Dr. Forssmann. Raised by in Berlin by his mother in a middle-class household after his father was killed in WWI, this young surgeon in training broached a bold idea with his surgeon-mentor Dr. Richard Schneider, a friend of the Forssmann family, in summer 1929.
Far from fantasy, Dr. Forssmann’s inspiration to perform what is now called cardiac catheterization came from a sketch in his physiology textbook depicting a long, thin tube being placed into a horse’s jugular vein and guided into the animal’s heart with balloon-assisted measurements of intracardiac pressures. Dr. Forssmann proposed to reach the heart of man—not through the jugular, but through the veins in the crease of the arm, which was more accessible
But how would this experiment happen? Dr. Forssmann elicited the help of Gerda Ditzen, a surgical nurse at Auguste Viktoria Home (Hospital), Eberswalde, near Berlin. In a month, Dr. Forssmann had convinced her to be his first human guinea pig. Dr. Forssmann, unbeknownst to Ditzen, planned on experimenting on himself. She held the keys to the closet, which was needed to obtain a long enough catheter.
As nurse Ditzen was strapped to the surgical table in the small operating room, sweating from both excitement and the sweltering
heat, Dr. Forssmann walked the distance of the OR and began his self-experimentation. With an incision in his left elbow crease, Dr. Forssmann identified the predominant vein and inserted the 65-cm-long ureteral tube into his arm, feeling progressive painless warmth as the tube coursed along. He had determined this was the only tube thin and long enough to safely and adequately reach the endocardium. However he still needed her help to conceal the tube hanging out of his arm. They went—tube in place—to the fluoroscopic X-ray facility, where images were obtained in the hospital basement.
The initial X-ray clearly indicated that the tube had not yet reached its destination. Dr. Forssmann forced the tube farther, resisting at one point the overwhelming urge to cough when the tube collided against his vein. When the tube was shown to be in the right auricle Dr. Forssmann had the technician snap the picture, finally obtaining the proof that he needed. Dr. Forssmann uneventfully removed the tube.
The real incident involved in this daring experiment was to come: Dr. Forssmann had to face the reactions—not only of his mentor Dr. Schneider, but also the medical community. The majority was ostensibly displeased with his methods, rationale, and approach, believing them too dangerous. After repeated self-experiments, Dr. Forssmann learned that his self-cath procedure could be safely performed; he submitted his findings with fluoroscopic proof to the German medical community at large. At least one prior researcher, the surgeon Ernst Unger, repudiated Dr. Forssmann’s claim, saying he had done the same thing many years earlier, but without hard evidence to back his claim.
Despite the rising tide of opposition to his findings, Dr. Forssmann pushed on. His subsequent experiments with rabbits and dogs (and ultimately himself) proved that catheterization angiography could not be achieved with simply sodium iodide. He developed the use of groin catheterization to reach the inferior vena cava through the femoral veins. Dr. Forssmann’s further experiments in aortography proved unfruitful. By this time, he had decided to stop his self-experimentation, having reached his limits with exploration. Instead he decided to seek work as a local urologist in a small German farming community.
In 1956, Forssmann was awarded the Nobel Prize in Medicine, which he shared with André Cournand, MD, and Dickinson W. Richards, MD, who were affiliated with Columbia University, New York City. When offered a job to head a German cardiovascular institute, Dr. Forssmann declined, citing his lack of knowledge about advancements in the field since his last self-experimentation in 1935. TH
Reference
- Altman, Lawrence K. Who Goes First: The Story of Self-Experimentation in Medicine. New York: Random House; 1987.
It was very painful. I felt that I had planted an apple orchard and other men who had gathered the harvest stood at the wall, laughing at me.” Dr. Werner Forssmann said these words toward the end of his life on his unexpected scientific exile after having laid the foundations of modern cardiology.
Pacemaker Insertion, angioplasty, and valve repair might now be impossible without the daring of Dr. Forssmann, whose humble roots set into sharp relief the depth of his accomplishments.
Leading physicians in turn-of-the-century Europe said that investigation and treatment of conditions affecting the heart were anathema to mainstream medical society; the heart was off-limits. In 1896 Sir Stephen Paget went so far as to say that “no new method, and no new discovery, can overcome the natural difficulties that attend a wound of the heart.”
Enter Dr. Forssmann. Raised by in Berlin by his mother in a middle-class household after his father was killed in WWI, this young surgeon in training broached a bold idea with his surgeon-mentor Dr. Richard Schneider, a friend of the Forssmann family, in summer 1929.
Far from fantasy, Dr. Forssmann’s inspiration to perform what is now called cardiac catheterization came from a sketch in his physiology textbook depicting a long, thin tube being placed into a horse’s jugular vein and guided into the animal’s heart with balloon-assisted measurements of intracardiac pressures. Dr. Forssmann proposed to reach the heart of man—not through the jugular, but through the veins in the crease of the arm, which was more accessible
But how would this experiment happen? Dr. Forssmann elicited the help of Gerda Ditzen, a surgical nurse at Auguste Viktoria Home (Hospital), Eberswalde, near Berlin. In a month, Dr. Forssmann had convinced her to be his first human guinea pig. Dr. Forssmann, unbeknownst to Ditzen, planned on experimenting on himself. She held the keys to the closet, which was needed to obtain a long enough catheter.
As nurse Ditzen was strapped to the surgical table in the small operating room, sweating from both excitement and the sweltering
heat, Dr. Forssmann walked the distance of the OR and began his self-experimentation. With an incision in his left elbow crease, Dr. Forssmann identified the predominant vein and inserted the 65-cm-long ureteral tube into his arm, feeling progressive painless warmth as the tube coursed along. He had determined this was the only tube thin and long enough to safely and adequately reach the endocardium. However he still needed her help to conceal the tube hanging out of his arm. They went—tube in place—to the fluoroscopic X-ray facility, where images were obtained in the hospital basement.
The initial X-ray clearly indicated that the tube had not yet reached its destination. Dr. Forssmann forced the tube farther, resisting at one point the overwhelming urge to cough when the tube collided against his vein. When the tube was shown to be in the right auricle Dr. Forssmann had the technician snap the picture, finally obtaining the proof that he needed. Dr. Forssmann uneventfully removed the tube.
The real incident involved in this daring experiment was to come: Dr. Forssmann had to face the reactions—not only of his mentor Dr. Schneider, but also the medical community. The majority was ostensibly displeased with his methods, rationale, and approach, believing them too dangerous. After repeated self-experiments, Dr. Forssmann learned that his self-cath procedure could be safely performed; he submitted his findings with fluoroscopic proof to the German medical community at large. At least one prior researcher, the surgeon Ernst Unger, repudiated Dr. Forssmann’s claim, saying he had done the same thing many years earlier, but without hard evidence to back his claim.
Despite the rising tide of opposition to his findings, Dr. Forssmann pushed on. His subsequent experiments with rabbits and dogs (and ultimately himself) proved that catheterization angiography could not be achieved with simply sodium iodide. He developed the use of groin catheterization to reach the inferior vena cava through the femoral veins. Dr. Forssmann’s further experiments in aortography proved unfruitful. By this time, he had decided to stop his self-experimentation, having reached his limits with exploration. Instead he decided to seek work as a local urologist in a small German farming community.
In 1956, Forssmann was awarded the Nobel Prize in Medicine, which he shared with André Cournand, MD, and Dickinson W. Richards, MD, who were affiliated with Columbia University, New York City. When offered a job to head a German cardiovascular institute, Dr. Forssmann declined, citing his lack of knowledge about advancements in the field since his last self-experimentation in 1935. TH
Reference
- Altman, Lawrence K. Who Goes First: The Story of Self-Experimentation in Medicine. New York: Random House; 1987.
In the Literature
Semi-Recumbent Position to Prevent Ventilator-Associated Pneumonia: Is It Possible?
By Joseph Ming Wah Li, MD
Van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006 Feb;34(2):396-402.
Ventilator-associated pneumonia (VAP) is a cause of significant morbidity and mortality among mechanically ventilated patients. Studies with radioactive-labeled enteral feeds have demonstrated an increased frequency of endotracheal aspiration of gastric contents in supine patients. The CDC guidelines for prevention of nosocomial pneumonia advise placement of mechanically ventilated patients in a semi-recumbent position as a VAP prevention measure.
Only one previous study, by Drakulovic and colleagues, has assessed this strategy to prevent VAP.1 That study demonstrated a 75% decrease in the incidence of VAP. But van Nieuwenhoven and colleagues raised two important questions about the findings from the previous study: First, the Drakulovic study placed control patients in a horizontal (zero degrees) position, which is not the standard of care in most ICUs. Most patients are placed at 10 degrees, and this position is elevated as patients are weaned. Second, the Drakulovic study measured patients only once daily but did not monitor their body positions in between the daily measurements.
Dr. van Nieuwenhoven and colleagues set out to determine whether it is feasible to keep mechanically ventilated patients in a semi-recumbent position on a continual basis and whether this measure would prevent VAP. This was a prospective multi-centered trial in which mechanically ventilated patients were randomly assigned to the semi-recumbent position with a target backrest elevation of 45 degrees or standard of care (supine position) with a backrest elevation of 10 degrees. They used a transducer with a pendulum, which was placed on the bed frame to measure the backrest elevation every 60 seconds for up to seven days. They calculated a mean degree of elevation for each patient daily. Nurses always respected the patient’s request for positioning, but a dedicated research nurse restored backrest position to the randomized position whenever possible.
Baseline characteristics for both groups were similar. For the supine (control) group, average elevations were 9.8 degrees on day one and 16.1 degrees on day seven. For the semi-recumbent group, average elevations were 28.1 degrees on day one and 22.6 degrees on day seven. There were no significant differences in numbers of patients who developed VAP in either group.
This study suggests that, despite the use of dedicated research nurses to maintain positioning, it may not be possible to keep patients’ backrests elevated to 45 degrees. Keeping patients’ backrests at an elevation of nearly 30 degrees does not appear to prevent VAP more than keeping patients’ backrests at 10 degrees, the present standard of care.
Reference
- Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851-1858.
Bar Codes in Medicine: An Opportunity for Quality Improvement
By Alex Carbo, MD
Poon EG, Cina JL, Churchill W, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145:426-434.
Medication errors and adverse drug events (ADEs) have received much attention in the literature; the use of health information technology to mitigate these errors and ADEs has now been proposed in many areas of healthcare. In an effort to decrease medication-dispensing errors, the U.S. Food and Drug Administration (FDA) mandated bar code use for all medications in hospitals, beginning in April 2006. While this technology has been extensively studied in other industries, there is little data describing its effects in the healthcare system.
Poon and colleagues set out to evaluate whether implementation of bar code technology reduced dispensing errors and the ADEs that might be caused by these miscalculations. In a before-and-after evaluation, they studied more than 350,000 dispensed medication doses in an academic medical center between February 2003 and September 2004.
During the bar code conversion process, the hospital pharmacy built a dedicated repackaging center, which was responsible for affixing a bar code to every dose of medication. These medications were then dispensed in three different configurations: two configurations required staff to verify all doses at least once using bar code scanning, and the third configuration—for commonly dispensed medications that could not be accommodated in a standard carousel machine because of their size or need for refrigeration—required scanning only one dose from each batch.
The authors found a 93% to 96% relative reduction in the incidence of target dispensing errors (P<0.001) and an 86% to 97% relative reduction in the incidence of potential ADEs (P<0.001) in the two configurations that required staff to verify all doses by scanning. The greatest reductions were seen in wrong medication errors (56%), wrong strength/dose errors (71%), wrong formulation errors (90%), and expired medication errors (100%).
In the configuration that did not require scanning of every dose, however, there was a 60% relative reduction in the incidence of target dispensing errors (P<0.001), but a 2.4-fold increase in the incidence of target potential ADEs. This included new errors attributable to wrong strength and wrong medication dispensing.
In light of the FDA’s mandate regarding bar codes, it seems that every hospital has the opportunity to improve patient safety and decrease medication error rates with the use of bar code technology. This study suggests that in order to achieve this benefit these systems should be designed to ensure that every medication dose is verified by scanning during the dispensing process.
Evaluation of a Guideline to Guide Resuscitation
By Cindy Lien, MD
Morrison LJ, Visentin LM, Kiss A, et al. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2006 Aug 3;355(5):478-487.
The survival rate of patients with out-of-hospital cardiac arrest is very low. Thus, guidelines have been developed for termination of resuscitation for those patients who have had no response to advanced cardiac life support provided by emergency medical service (EMS) personnel. Similar guidelines have not yet been developed, however, for situations in which patients receive basic life support from emergency workers trained in the use of an automated external cardiac defibrillator. Patients with little potential for survival are routinely transported to emergency departments, at significant cost to the healthcare system.
Morrison and colleagues present results from the Termination of Resuscitation (TOR) study, a prospective evaluation of a clinical prediction rule for the termination of basic life support by emergency medical personnel trained in the use of automated external defibrillators. The clinical prediction rule, previously developed in a retrospective review of case records from a large urban EMS system, recommends termination of resuscitation if there is no return of spontaneous circulation, no shock administered, and no witness of the arrest by EMS personnel.
In the current study, the authors obtained follow-up data for 1,240 adult patients in Ontario, Canada, who had suffered an arrest of presumed cardiac cause and were subsequently transported to the emergency department after resuscitative efforts. Twenty-four EMS systems participated in the study. The study found that only 0.5% of the patients for whom the clinical prediction rule recommended termination survived (four out of 776 patients). Of the 1,240 total study patients, 41 (3%) survived. The clinical prediction rule recommended continuation of resuscitative efforts for 37 of these 41 patients, resulting in a specificity of 90.2%. The positive predictive value for death was calculated to be 99.5% when termination was recommended.
The TOR trial also determined whether the addition of other criteria to the original prediction rule could further refine the specificity and positive predictive value. They found that the addition to the criteria of a response time greater than eight minutes increased the positive predictive value and specificity to 99.7% and 97.6%, respectively. When the variable “not witnessed by bystander” was added to the clinical prediction rule, both the positive predictive value and specificity increased to 100%. In other words, no patients survived if they had had a completely unwitnessed arrest, no return of spontaneous circulation, and no shocks delivered.
This study identifies a subpopulation of patients with presumed cardiac arrest for whom termination of resuscitative efforts in the field appears reasonable. The authors note that a survival rate of 1% or less has been suggested in past literature as reflective of medical futility. The TOR investigators acknowledge that their study took place before the 2005 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care were released and that their study protocols were consistent with the 2000 resuscitation guidelines. In light of this information, continued validity testing of the clinical prediction rule under the 2005 AHA protocols is needed.
Nonetheless, it is quite impressive that use of their clinical prediction rule would have resulted in transportation of only 37% of patients (464 of 1,240), rather than 100% of patients, as is currently the practice. If the guidelines described in this article are to be implemented, further studies are necessary to address the training of EMS personnel, who would carry responsibility for terminating resuscitation and notifying families of patients’ deaths.
Prevention of Ventilator-Associated Pneumonia
By Diane Sliwka, MD
Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006 Jun;173(12):1348-1355. Epub 2006 Apr 7.
Ventilator-associated pneumonia (VAP) is an important nosocomial source of morbidity and mortality. The use of prophylactic antimicrobials to decrease VAP raises concern for antimicrobial resistance. This study evaluates the topical antiseptic chlorhexidine (CHX) as an alternative prophylactic intervention for VAP. CHX has previously been shown to decrease VAP in cardiac surgical patients, but has not been studied in higher risk, long-term-ventilated patients. Because CHX works better for gram-positive organisms, the combination of colistin and CHX (COL + CHX) was also studied for improved gram-negative coverage.
This multi-center, randomized, double-blind, placebo-controlled trial enrolled 385 adult patients. Patients who were expected to be intubated for longer than 48 hours were randomized to 3 arms: CHX alone, CHX + COL, and placebo. Exclusion criteria included known preadmission immunocompromised state, pregnancy, and physical limitation to oral application. Pneumonia was defined by clinical decision-making, which was later confirmed by three blinded intensivists’ reviews of the case records and supported by daily clinical pulmonary infection scores.
The primary endpoint of VAP was diagnosed in 52 of 385 patients: 18% placebo, 13% CHX, and 10% CHX + COL. Rate of VAP in the two treatment groups was lower than placebo and reached statistical significance when compared to placebo. The daily hazard ratio for CHX versus placebo was .352 (95% CI .160, .791); for CHX + COL versus placebo, it was .454 (95% CI .224, .925), showing a 65% and 55% reduction in the rate of pneumonia development. Multivariate analysis of variables such as gender, pulmonary admission diagnosis, colonization at time of admission, and antimicrobial use on admission did not affect the data.
The secondary endpoint of endotracheal colonization was evaluated by a twice-weekly endotracheal culture. There was no statistically significant difference in colonization among the three groups in the first (days 1-4) or third (days 9-12) time frames. During the second time frame (days 5-8), there was a statistically significant decrease in colonization for the CHX + COL treatment group when compared to both placebo (16% versus 40% p<.007) and to CHX (16% versus 38%, p<.011); this decrease is thought to be due to gram-negative coverage by COL.
The secondary endpoint of oropharyngeal colonization was evaluated for 87% of all patient days. CHX and CHX + COL were similarly effective for gram-positive bacteria when compared to placebo, with 30% and 27% reduction in rates of colonization, respectively: HR 0.695 for CHX (95% CI, 0.606, 0.796; p < 0.001) and 0.732 (95% CI, 0.640, 0.838; p < 0.001) for CHX + COL. The CHX + COL combination was more effective for gram-negative bacteria: daily HR .534 (95% CI, 0.455, 0.626; p <0.001) alone with a 47% reduction in gram-negative colonization compared to CHX.
No difference was seen in ICU mortality, duration of mechanical ventilation, or duration of ICU stay. One adverse event (tongue swelling) occurred in the CHX + COL group.
Limitations of the study include the following:
- Daily assessments on all patients were not performed;
- The placebo group had more males and more infections on admission than the other two groups, raising the question of randomization error;
- Clinical versus quantitative diagnosis of pneumonia may overestimate VAP in this study;
- It is not known how many patients were not enrolled in the study due to short anticipated ventilator times, but who later had prolonged ventilations; and
- The lack of effect on ventilator time, ICU length of stay, and mortality raises the question of the significance of these findings.
Despite these limitations, the low cost of these treatments, minimal adverse events, low risk of promoting significant antimicrobial resistance, and the finding of decreased VAP and bacterial colonization risk shown in this study support the potential benefit of topical decontamination with CHX and COL in conjunction with other measures of VAP prevention. TH
Reference
- De Riso AJ II, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556-1561.
Semi-Recumbent Position to Prevent Ventilator-Associated Pneumonia: Is It Possible?
By Joseph Ming Wah Li, MD
Van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006 Feb;34(2):396-402.
Ventilator-associated pneumonia (VAP) is a cause of significant morbidity and mortality among mechanically ventilated patients. Studies with radioactive-labeled enteral feeds have demonstrated an increased frequency of endotracheal aspiration of gastric contents in supine patients. The CDC guidelines for prevention of nosocomial pneumonia advise placement of mechanically ventilated patients in a semi-recumbent position as a VAP prevention measure.
Only one previous study, by Drakulovic and colleagues, has assessed this strategy to prevent VAP.1 That study demonstrated a 75% decrease in the incidence of VAP. But van Nieuwenhoven and colleagues raised two important questions about the findings from the previous study: First, the Drakulovic study placed control patients in a horizontal (zero degrees) position, which is not the standard of care in most ICUs. Most patients are placed at 10 degrees, and this position is elevated as patients are weaned. Second, the Drakulovic study measured patients only once daily but did not monitor their body positions in between the daily measurements.
Dr. van Nieuwenhoven and colleagues set out to determine whether it is feasible to keep mechanically ventilated patients in a semi-recumbent position on a continual basis and whether this measure would prevent VAP. This was a prospective multi-centered trial in which mechanically ventilated patients were randomly assigned to the semi-recumbent position with a target backrest elevation of 45 degrees or standard of care (supine position) with a backrest elevation of 10 degrees. They used a transducer with a pendulum, which was placed on the bed frame to measure the backrest elevation every 60 seconds for up to seven days. They calculated a mean degree of elevation for each patient daily. Nurses always respected the patient’s request for positioning, but a dedicated research nurse restored backrest position to the randomized position whenever possible.
Baseline characteristics for both groups were similar. For the supine (control) group, average elevations were 9.8 degrees on day one and 16.1 degrees on day seven. For the semi-recumbent group, average elevations were 28.1 degrees on day one and 22.6 degrees on day seven. There were no significant differences in numbers of patients who developed VAP in either group.
This study suggests that, despite the use of dedicated research nurses to maintain positioning, it may not be possible to keep patients’ backrests elevated to 45 degrees. Keeping patients’ backrests at an elevation of nearly 30 degrees does not appear to prevent VAP more than keeping patients’ backrests at 10 degrees, the present standard of care.
Reference
- Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851-1858.
Bar Codes in Medicine: An Opportunity for Quality Improvement
By Alex Carbo, MD
Poon EG, Cina JL, Churchill W, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145:426-434.
Medication errors and adverse drug events (ADEs) have received much attention in the literature; the use of health information technology to mitigate these errors and ADEs has now been proposed in many areas of healthcare. In an effort to decrease medication-dispensing errors, the U.S. Food and Drug Administration (FDA) mandated bar code use for all medications in hospitals, beginning in April 2006. While this technology has been extensively studied in other industries, there is little data describing its effects in the healthcare system.
Poon and colleagues set out to evaluate whether implementation of bar code technology reduced dispensing errors and the ADEs that might be caused by these miscalculations. In a before-and-after evaluation, they studied more than 350,000 dispensed medication doses in an academic medical center between February 2003 and September 2004.
During the bar code conversion process, the hospital pharmacy built a dedicated repackaging center, which was responsible for affixing a bar code to every dose of medication. These medications were then dispensed in three different configurations: two configurations required staff to verify all doses at least once using bar code scanning, and the third configuration—for commonly dispensed medications that could not be accommodated in a standard carousel machine because of their size or need for refrigeration—required scanning only one dose from each batch.
The authors found a 93% to 96% relative reduction in the incidence of target dispensing errors (P<0.001) and an 86% to 97% relative reduction in the incidence of potential ADEs (P<0.001) in the two configurations that required staff to verify all doses by scanning. The greatest reductions were seen in wrong medication errors (56%), wrong strength/dose errors (71%), wrong formulation errors (90%), and expired medication errors (100%).
In the configuration that did not require scanning of every dose, however, there was a 60% relative reduction in the incidence of target dispensing errors (P<0.001), but a 2.4-fold increase in the incidence of target potential ADEs. This included new errors attributable to wrong strength and wrong medication dispensing.
In light of the FDA’s mandate regarding bar codes, it seems that every hospital has the opportunity to improve patient safety and decrease medication error rates with the use of bar code technology. This study suggests that in order to achieve this benefit these systems should be designed to ensure that every medication dose is verified by scanning during the dispensing process.
Evaluation of a Guideline to Guide Resuscitation
By Cindy Lien, MD
Morrison LJ, Visentin LM, Kiss A, et al. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2006 Aug 3;355(5):478-487.
The survival rate of patients with out-of-hospital cardiac arrest is very low. Thus, guidelines have been developed for termination of resuscitation for those patients who have had no response to advanced cardiac life support provided by emergency medical service (EMS) personnel. Similar guidelines have not yet been developed, however, for situations in which patients receive basic life support from emergency workers trained in the use of an automated external cardiac defibrillator. Patients with little potential for survival are routinely transported to emergency departments, at significant cost to the healthcare system.
Morrison and colleagues present results from the Termination of Resuscitation (TOR) study, a prospective evaluation of a clinical prediction rule for the termination of basic life support by emergency medical personnel trained in the use of automated external defibrillators. The clinical prediction rule, previously developed in a retrospective review of case records from a large urban EMS system, recommends termination of resuscitation if there is no return of spontaneous circulation, no shock administered, and no witness of the arrest by EMS personnel.
In the current study, the authors obtained follow-up data for 1,240 adult patients in Ontario, Canada, who had suffered an arrest of presumed cardiac cause and were subsequently transported to the emergency department after resuscitative efforts. Twenty-four EMS systems participated in the study. The study found that only 0.5% of the patients for whom the clinical prediction rule recommended termination survived (four out of 776 patients). Of the 1,240 total study patients, 41 (3%) survived. The clinical prediction rule recommended continuation of resuscitative efforts for 37 of these 41 patients, resulting in a specificity of 90.2%. The positive predictive value for death was calculated to be 99.5% when termination was recommended.
The TOR trial also determined whether the addition of other criteria to the original prediction rule could further refine the specificity and positive predictive value. They found that the addition to the criteria of a response time greater than eight minutes increased the positive predictive value and specificity to 99.7% and 97.6%, respectively. When the variable “not witnessed by bystander” was added to the clinical prediction rule, both the positive predictive value and specificity increased to 100%. In other words, no patients survived if they had had a completely unwitnessed arrest, no return of spontaneous circulation, and no shocks delivered.
This study identifies a subpopulation of patients with presumed cardiac arrest for whom termination of resuscitative efforts in the field appears reasonable. The authors note that a survival rate of 1% or less has been suggested in past literature as reflective of medical futility. The TOR investigators acknowledge that their study took place before the 2005 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care were released and that their study protocols were consistent with the 2000 resuscitation guidelines. In light of this information, continued validity testing of the clinical prediction rule under the 2005 AHA protocols is needed.
Nonetheless, it is quite impressive that use of their clinical prediction rule would have resulted in transportation of only 37% of patients (464 of 1,240), rather than 100% of patients, as is currently the practice. If the guidelines described in this article are to be implemented, further studies are necessary to address the training of EMS personnel, who would carry responsibility for terminating resuscitation and notifying families of patients’ deaths.
Prevention of Ventilator-Associated Pneumonia
By Diane Sliwka, MD
Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006 Jun;173(12):1348-1355. Epub 2006 Apr 7.
Ventilator-associated pneumonia (VAP) is an important nosocomial source of morbidity and mortality. The use of prophylactic antimicrobials to decrease VAP raises concern for antimicrobial resistance. This study evaluates the topical antiseptic chlorhexidine (CHX) as an alternative prophylactic intervention for VAP. CHX has previously been shown to decrease VAP in cardiac surgical patients, but has not been studied in higher risk, long-term-ventilated patients. Because CHX works better for gram-positive organisms, the combination of colistin and CHX (COL + CHX) was also studied for improved gram-negative coverage.
This multi-center, randomized, double-blind, placebo-controlled trial enrolled 385 adult patients. Patients who were expected to be intubated for longer than 48 hours were randomized to 3 arms: CHX alone, CHX + COL, and placebo. Exclusion criteria included known preadmission immunocompromised state, pregnancy, and physical limitation to oral application. Pneumonia was defined by clinical decision-making, which was later confirmed by three blinded intensivists’ reviews of the case records and supported by daily clinical pulmonary infection scores.
The primary endpoint of VAP was diagnosed in 52 of 385 patients: 18% placebo, 13% CHX, and 10% CHX + COL. Rate of VAP in the two treatment groups was lower than placebo and reached statistical significance when compared to placebo. The daily hazard ratio for CHX versus placebo was .352 (95% CI .160, .791); for CHX + COL versus placebo, it was .454 (95% CI .224, .925), showing a 65% and 55% reduction in the rate of pneumonia development. Multivariate analysis of variables such as gender, pulmonary admission diagnosis, colonization at time of admission, and antimicrobial use on admission did not affect the data.
The secondary endpoint of endotracheal colonization was evaluated by a twice-weekly endotracheal culture. There was no statistically significant difference in colonization among the three groups in the first (days 1-4) or third (days 9-12) time frames. During the second time frame (days 5-8), there was a statistically significant decrease in colonization for the CHX + COL treatment group when compared to both placebo (16% versus 40% p<.007) and to CHX (16% versus 38%, p<.011); this decrease is thought to be due to gram-negative coverage by COL.
The secondary endpoint of oropharyngeal colonization was evaluated for 87% of all patient days. CHX and CHX + COL were similarly effective for gram-positive bacteria when compared to placebo, with 30% and 27% reduction in rates of colonization, respectively: HR 0.695 for CHX (95% CI, 0.606, 0.796; p < 0.001) and 0.732 (95% CI, 0.640, 0.838; p < 0.001) for CHX + COL. The CHX + COL combination was more effective for gram-negative bacteria: daily HR .534 (95% CI, 0.455, 0.626; p <0.001) alone with a 47% reduction in gram-negative colonization compared to CHX.
No difference was seen in ICU mortality, duration of mechanical ventilation, or duration of ICU stay. One adverse event (tongue swelling) occurred in the CHX + COL group.
Limitations of the study include the following:
- Daily assessments on all patients were not performed;
- The placebo group had more males and more infections on admission than the other two groups, raising the question of randomization error;
- Clinical versus quantitative diagnosis of pneumonia may overestimate VAP in this study;
- It is not known how many patients were not enrolled in the study due to short anticipated ventilator times, but who later had prolonged ventilations; and
- The lack of effect on ventilator time, ICU length of stay, and mortality raises the question of the significance of these findings.
Despite these limitations, the low cost of these treatments, minimal adverse events, low risk of promoting significant antimicrobial resistance, and the finding of decreased VAP and bacterial colonization risk shown in this study support the potential benefit of topical decontamination with CHX and COL in conjunction with other measures of VAP prevention. TH
Reference
- De Riso AJ II, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556-1561.
Semi-Recumbent Position to Prevent Ventilator-Associated Pneumonia: Is It Possible?
By Joseph Ming Wah Li, MD
Van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006 Feb;34(2):396-402.
Ventilator-associated pneumonia (VAP) is a cause of significant morbidity and mortality among mechanically ventilated patients. Studies with radioactive-labeled enteral feeds have demonstrated an increased frequency of endotracheal aspiration of gastric contents in supine patients. The CDC guidelines for prevention of nosocomial pneumonia advise placement of mechanically ventilated patients in a semi-recumbent position as a VAP prevention measure.
Only one previous study, by Drakulovic and colleagues, has assessed this strategy to prevent VAP.1 That study demonstrated a 75% decrease in the incidence of VAP. But van Nieuwenhoven and colleagues raised two important questions about the findings from the previous study: First, the Drakulovic study placed control patients in a horizontal (zero degrees) position, which is not the standard of care in most ICUs. Most patients are placed at 10 degrees, and this position is elevated as patients are weaned. Second, the Drakulovic study measured patients only once daily but did not monitor their body positions in between the daily measurements.
Dr. van Nieuwenhoven and colleagues set out to determine whether it is feasible to keep mechanically ventilated patients in a semi-recumbent position on a continual basis and whether this measure would prevent VAP. This was a prospective multi-centered trial in which mechanically ventilated patients were randomly assigned to the semi-recumbent position with a target backrest elevation of 45 degrees or standard of care (supine position) with a backrest elevation of 10 degrees. They used a transducer with a pendulum, which was placed on the bed frame to measure the backrest elevation every 60 seconds for up to seven days. They calculated a mean degree of elevation for each patient daily. Nurses always respected the patient’s request for positioning, but a dedicated research nurse restored backrest position to the randomized position whenever possible.
Baseline characteristics for both groups were similar. For the supine (control) group, average elevations were 9.8 degrees on day one and 16.1 degrees on day seven. For the semi-recumbent group, average elevations were 28.1 degrees on day one and 22.6 degrees on day seven. There were no significant differences in numbers of patients who developed VAP in either group.
This study suggests that, despite the use of dedicated research nurses to maintain positioning, it may not be possible to keep patients’ backrests elevated to 45 degrees. Keeping patients’ backrests at an elevation of nearly 30 degrees does not appear to prevent VAP more than keeping patients’ backrests at 10 degrees, the present standard of care.
Reference
- Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851-1858.
Bar Codes in Medicine: An Opportunity for Quality Improvement
By Alex Carbo, MD
Poon EG, Cina JL, Churchill W, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145:426-434.
Medication errors and adverse drug events (ADEs) have received much attention in the literature; the use of health information technology to mitigate these errors and ADEs has now been proposed in many areas of healthcare. In an effort to decrease medication-dispensing errors, the U.S. Food and Drug Administration (FDA) mandated bar code use for all medications in hospitals, beginning in April 2006. While this technology has been extensively studied in other industries, there is little data describing its effects in the healthcare system.
Poon and colleagues set out to evaluate whether implementation of bar code technology reduced dispensing errors and the ADEs that might be caused by these miscalculations. In a before-and-after evaluation, they studied more than 350,000 dispensed medication doses in an academic medical center between February 2003 and September 2004.
During the bar code conversion process, the hospital pharmacy built a dedicated repackaging center, which was responsible for affixing a bar code to every dose of medication. These medications were then dispensed in three different configurations: two configurations required staff to verify all doses at least once using bar code scanning, and the third configuration—for commonly dispensed medications that could not be accommodated in a standard carousel machine because of their size or need for refrigeration—required scanning only one dose from each batch.
The authors found a 93% to 96% relative reduction in the incidence of target dispensing errors (P<0.001) and an 86% to 97% relative reduction in the incidence of potential ADEs (P<0.001) in the two configurations that required staff to verify all doses by scanning. The greatest reductions were seen in wrong medication errors (56%), wrong strength/dose errors (71%), wrong formulation errors (90%), and expired medication errors (100%).
In the configuration that did not require scanning of every dose, however, there was a 60% relative reduction in the incidence of target dispensing errors (P<0.001), but a 2.4-fold increase in the incidence of target potential ADEs. This included new errors attributable to wrong strength and wrong medication dispensing.
In light of the FDA’s mandate regarding bar codes, it seems that every hospital has the opportunity to improve patient safety and decrease medication error rates with the use of bar code technology. This study suggests that in order to achieve this benefit these systems should be designed to ensure that every medication dose is verified by scanning during the dispensing process.
Evaluation of a Guideline to Guide Resuscitation
By Cindy Lien, MD
Morrison LJ, Visentin LM, Kiss A, et al. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2006 Aug 3;355(5):478-487.
The survival rate of patients with out-of-hospital cardiac arrest is very low. Thus, guidelines have been developed for termination of resuscitation for those patients who have had no response to advanced cardiac life support provided by emergency medical service (EMS) personnel. Similar guidelines have not yet been developed, however, for situations in which patients receive basic life support from emergency workers trained in the use of an automated external cardiac defibrillator. Patients with little potential for survival are routinely transported to emergency departments, at significant cost to the healthcare system.
Morrison and colleagues present results from the Termination of Resuscitation (TOR) study, a prospective evaluation of a clinical prediction rule for the termination of basic life support by emergency medical personnel trained in the use of automated external defibrillators. The clinical prediction rule, previously developed in a retrospective review of case records from a large urban EMS system, recommends termination of resuscitation if there is no return of spontaneous circulation, no shock administered, and no witness of the arrest by EMS personnel.
In the current study, the authors obtained follow-up data for 1,240 adult patients in Ontario, Canada, who had suffered an arrest of presumed cardiac cause and were subsequently transported to the emergency department after resuscitative efforts. Twenty-four EMS systems participated in the study. The study found that only 0.5% of the patients for whom the clinical prediction rule recommended termination survived (four out of 776 patients). Of the 1,240 total study patients, 41 (3%) survived. The clinical prediction rule recommended continuation of resuscitative efforts for 37 of these 41 patients, resulting in a specificity of 90.2%. The positive predictive value for death was calculated to be 99.5% when termination was recommended.
The TOR trial also determined whether the addition of other criteria to the original prediction rule could further refine the specificity and positive predictive value. They found that the addition to the criteria of a response time greater than eight minutes increased the positive predictive value and specificity to 99.7% and 97.6%, respectively. When the variable “not witnessed by bystander” was added to the clinical prediction rule, both the positive predictive value and specificity increased to 100%. In other words, no patients survived if they had had a completely unwitnessed arrest, no return of spontaneous circulation, and no shocks delivered.
This study identifies a subpopulation of patients with presumed cardiac arrest for whom termination of resuscitative efforts in the field appears reasonable. The authors note that a survival rate of 1% or less has been suggested in past literature as reflective of medical futility. The TOR investigators acknowledge that their study took place before the 2005 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care were released and that their study protocols were consistent with the 2000 resuscitation guidelines. In light of this information, continued validity testing of the clinical prediction rule under the 2005 AHA protocols is needed.
Nonetheless, it is quite impressive that use of their clinical prediction rule would have resulted in transportation of only 37% of patients (464 of 1,240), rather than 100% of patients, as is currently the practice. If the guidelines described in this article are to be implemented, further studies are necessary to address the training of EMS personnel, who would carry responsibility for terminating resuscitation and notifying families of patients’ deaths.
Prevention of Ventilator-Associated Pneumonia
By Diane Sliwka, MD
Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006 Jun;173(12):1348-1355. Epub 2006 Apr 7.
Ventilator-associated pneumonia (VAP) is an important nosocomial source of morbidity and mortality. The use of prophylactic antimicrobials to decrease VAP raises concern for antimicrobial resistance. This study evaluates the topical antiseptic chlorhexidine (CHX) as an alternative prophylactic intervention for VAP. CHX has previously been shown to decrease VAP in cardiac surgical patients, but has not been studied in higher risk, long-term-ventilated patients. Because CHX works better for gram-positive organisms, the combination of colistin and CHX (COL + CHX) was also studied for improved gram-negative coverage.
This multi-center, randomized, double-blind, placebo-controlled trial enrolled 385 adult patients. Patients who were expected to be intubated for longer than 48 hours were randomized to 3 arms: CHX alone, CHX + COL, and placebo. Exclusion criteria included known preadmission immunocompromised state, pregnancy, and physical limitation to oral application. Pneumonia was defined by clinical decision-making, which was later confirmed by three blinded intensivists’ reviews of the case records and supported by daily clinical pulmonary infection scores.
The primary endpoint of VAP was diagnosed in 52 of 385 patients: 18% placebo, 13% CHX, and 10% CHX + COL. Rate of VAP in the two treatment groups was lower than placebo and reached statistical significance when compared to placebo. The daily hazard ratio for CHX versus placebo was .352 (95% CI .160, .791); for CHX + COL versus placebo, it was .454 (95% CI .224, .925), showing a 65% and 55% reduction in the rate of pneumonia development. Multivariate analysis of variables such as gender, pulmonary admission diagnosis, colonization at time of admission, and antimicrobial use on admission did not affect the data.
The secondary endpoint of endotracheal colonization was evaluated by a twice-weekly endotracheal culture. There was no statistically significant difference in colonization among the three groups in the first (days 1-4) or third (days 9-12) time frames. During the second time frame (days 5-8), there was a statistically significant decrease in colonization for the CHX + COL treatment group when compared to both placebo (16% versus 40% p<.007) and to CHX (16% versus 38%, p<.011); this decrease is thought to be due to gram-negative coverage by COL.
The secondary endpoint of oropharyngeal colonization was evaluated for 87% of all patient days. CHX and CHX + COL were similarly effective for gram-positive bacteria when compared to placebo, with 30% and 27% reduction in rates of colonization, respectively: HR 0.695 for CHX (95% CI, 0.606, 0.796; p < 0.001) and 0.732 (95% CI, 0.640, 0.838; p < 0.001) for CHX + COL. The CHX + COL combination was more effective for gram-negative bacteria: daily HR .534 (95% CI, 0.455, 0.626; p <0.001) alone with a 47% reduction in gram-negative colonization compared to CHX.
No difference was seen in ICU mortality, duration of mechanical ventilation, or duration of ICU stay. One adverse event (tongue swelling) occurred in the CHX + COL group.
Limitations of the study include the following:
- Daily assessments on all patients were not performed;
- The placebo group had more males and more infections on admission than the other two groups, raising the question of randomization error;
- Clinical versus quantitative diagnosis of pneumonia may overestimate VAP in this study;
- It is not known how many patients were not enrolled in the study due to short anticipated ventilator times, but who later had prolonged ventilations; and
- The lack of effect on ventilator time, ICU length of stay, and mortality raises the question of the significance of these findings.
Despite these limitations, the low cost of these treatments, minimal adverse events, low risk of promoting significant antimicrobial resistance, and the finding of decreased VAP and bacterial colonization risk shown in this study support the potential benefit of topical decontamination with CHX and COL in conjunction with other measures of VAP prevention. TH
Reference
- De Riso AJ II, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556-1561.
Protocol Pilot
Evidence from randomized controlled trials and historical series convincingly demonstrates that tight glycemic control in the critical care setting reduces mortality, length of hospital stay, and other morbidities.1-6 Several protocols targeting a range of glucoses have been published.7-10 We modified a protocol used at the University of Washington (Seattle) that targeted glucoses of 80-180 mg/dl to aim for a tighter glycemic goal range of 80-110 mg/dl and effectively lowered blood glucoses in our intensive care unit (ICU) with a low rate of hypoglycemia.9 We share here our protocol and results over the past year.
Methods
Column protocols have the advantage of accommodating rates of insulin infusion adjustment to the individual patient’s degree of insulin resistance. If glucoses are not falling appropriately using one column, a protocol is followed to move to a more aggressive column. If glucoses fall precipitously, a similar process guides the nurse to a less aggressive column. The column format avoids the need for nurses to do calculations, which reduces the possibility of error.
In August 2005, in our ICU, we piloted a four-column protocol used at the University of Washington. This protocol was designed with an initial target glucose range of 80-180 mg/dl, and during our pilot it did not work well because we were targeting a glucose range of 80-110 mg/dl. Our ICU nursing staff at Southwest Washington Medical Center (SWMC), Vancouver, preferred to revise the column format rather than switch to a different type of protocol such as the Portland Protocol. We therefore created a new six-column protocol targeting the 80-110 mg/dl glucose range. (See Figure 1, above.)
In creating this protocol, we reviewed infusion rates in an unpublished Oregon Health and Sciences University (OHSU), Portland, six-column protocol and the Georgia Hospital Association, Marietta, 10-column protocol.11,12 Our current version includes new glucose ranges of 70-89 mg/dl, 90-99 mg/dl, and 100-109 mg/dl to allow for more rate adjustments within the overall target range, while our previous version had only one glucose category of 70-109 mg/dl within the target range.
Nursing feedback also led us to modify criteria for moving to the right (more aggressive) column as follows: If blood glucose is lower than 200, algorithm failure is defined as glucose outside of goal range and not decreased since previous reading; if blood glucose is greater than or equal to 200, algorithm failure is defined as glucose outside of goal range and has not decreased by at least 60 mg/dl within one hour. The longest interval we allow between glucometer checks for stable patients is two hours. Automatic triggers for implementing the protocol include two consecutive glucoses over 140 mg/dl or a single glucose over 180 mg/dl.
Our revised protocol succeeded when we intensified nursing education, solicited frequent nursing feedback, and organized the procedure so that ICU nurse managers drove the process with a responsive physician/pharmacist team.
Results
In March 2006 we instituted the revised six-column insulin infusion protocol in our ICU. The percent of glucometer readings in the ICU between 70-150 mg/dl increased from 58% in February 2006 to 78% in August of 2006. (See Figure 2, above.) In our cardiac care unit (CCU), where an older, non-column insulin infusion protocol continued to be used, glucoses went from 50% in the 70-150 mg/dl range in February 2006 to 61% in that range in July 2006. When we began the six-column ICU insulin infusion protocol in the CCU in August 2006, 66% of glucoses were in the 70-150 range. (See Figure 3, p. 43.)
The percentage of glucoses below 70 mg/dl increased from 2%-3% using the four-column protocol to 4%-5% using the six-column protocol, as the percentage of glucoses between 70-150 mg/dl rose above 70%.
The main complaint from nurses relates to the need for frequent (hourly to twice hourly) glucose monitoring, which was also a complaint with earlier protocols in our institution.
Discussion
These results compare favorably with those achieved by acknowledged leaders in critical care glycemic control in the United States.13 We have achieved them with the use of a safe column protocol that our critical care nurses are now comfortable using. The hypoglycemia rates with our protocol have been lower than those seen in some other published protocols. The Berghe protocol, for example, reported 5% of patients experiencing glucoses lower than or equal to 40 mg/dl, while our protocol resulted in 4%-5% of glucose values lower than 70 mg/dl.
Mortality rates in our ICU have fallen from 14% in September 2005 to 11% in February 2006 to 8% in August 2006. Although this decline in mortality has occurred over the range of time in which we have improved glycemic control in our ICU, multiple other mortality reduction initiatives, any or all of which could account for the decline, occurred simultaneously in our hospital; in addition, mortality rates were falling before the introduction of the new insulin infusion protocol.
Head-to-head comparisons of existing protocols, using agreed upon glucometrics such as time-to-goal range and degree of glycemic variability, are needed.14 Testing new protocols, including proactive insulin infusion adjustments at the time of administration of known caloric loads—such as antibiotics delivered as a bolus in D5W solution—or at the time of initiation of high dose steroids or epinephrine drips, may be a means to keep a higher percentage of blood sugars in range. Ultimately, continuous blood sensing devices with closed loop insulin infusion responses are desired.
For now, our protocol offers a user-friendly means of getting a high percentage of critical care patients into a reasonable glycemic range with a low risk of hypoglycemia. Others are free to use and/or modify it. TH
The authors work at Southwest Washington Medical Center, Vancouver, Wash.
References
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359-1367.
- Malmberg K, Norhammar A, Wedel H, et al. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626-2632.
- Furnary AP, Wu Y, Bookin SO. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project. Endocr Pract. 2006 Mar-Apr;12 Suppl 3:22-26.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006 Feb 2;354(5):449-461.
- Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004 Aug;79(8):992-1000.
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004 Oct 11;164(18):2005-2011.
- Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12 Suppl 3:79-85.
- Markovitz LJ, Weichmann RJ, Harris N, et al. Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery. Endocr Pract. 2002 Jan-Feb;8(1):10-18.
- Ku SY, Sayre CA, Hirsch IB, et al. New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia. Jt Comm J Qual Patient Saf. 2005 Mar;31(3):141-147.
- Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004 Mar-Apr;10 Suppl 2:21-33.
- In a written communication from Andy Ahmann, MD, OHSU, in 2006.
- Georgia Hospital Association Diabetes Protocols. Chart 6b. Available at: www.adaendo.com/GHA/index.html. Accessed October 29, 2006.
- In a written communication from Terry Clemmer, MD, director, Critical Care Medicine, LDS Hospital, Salt Lake City, Utah, in October 2006.
- Braithwaite SS, Godara H, Song HJ, et al. No patient left behind: evaluation and design of intravenous insulin infusion algorithms. Endocr Pract. 2006 Jul-Aug;12 Suppl 3:72-78.
Evidence from randomized controlled trials and historical series convincingly demonstrates that tight glycemic control in the critical care setting reduces mortality, length of hospital stay, and other morbidities.1-6 Several protocols targeting a range of glucoses have been published.7-10 We modified a protocol used at the University of Washington (Seattle) that targeted glucoses of 80-180 mg/dl to aim for a tighter glycemic goal range of 80-110 mg/dl and effectively lowered blood glucoses in our intensive care unit (ICU) with a low rate of hypoglycemia.9 We share here our protocol and results over the past year.
Methods
Column protocols have the advantage of accommodating rates of insulin infusion adjustment to the individual patient’s degree of insulin resistance. If glucoses are not falling appropriately using one column, a protocol is followed to move to a more aggressive column. If glucoses fall precipitously, a similar process guides the nurse to a less aggressive column. The column format avoids the need for nurses to do calculations, which reduces the possibility of error.
In August 2005, in our ICU, we piloted a four-column protocol used at the University of Washington. This protocol was designed with an initial target glucose range of 80-180 mg/dl, and during our pilot it did not work well because we were targeting a glucose range of 80-110 mg/dl. Our ICU nursing staff at Southwest Washington Medical Center (SWMC), Vancouver, preferred to revise the column format rather than switch to a different type of protocol such as the Portland Protocol. We therefore created a new six-column protocol targeting the 80-110 mg/dl glucose range. (See Figure 1, above.)
In creating this protocol, we reviewed infusion rates in an unpublished Oregon Health and Sciences University (OHSU), Portland, six-column protocol and the Georgia Hospital Association, Marietta, 10-column protocol.11,12 Our current version includes new glucose ranges of 70-89 mg/dl, 90-99 mg/dl, and 100-109 mg/dl to allow for more rate adjustments within the overall target range, while our previous version had only one glucose category of 70-109 mg/dl within the target range.
Nursing feedback also led us to modify criteria for moving to the right (more aggressive) column as follows: If blood glucose is lower than 200, algorithm failure is defined as glucose outside of goal range and not decreased since previous reading; if blood glucose is greater than or equal to 200, algorithm failure is defined as glucose outside of goal range and has not decreased by at least 60 mg/dl within one hour. The longest interval we allow between glucometer checks for stable patients is two hours. Automatic triggers for implementing the protocol include two consecutive glucoses over 140 mg/dl or a single glucose over 180 mg/dl.
Our revised protocol succeeded when we intensified nursing education, solicited frequent nursing feedback, and organized the procedure so that ICU nurse managers drove the process with a responsive physician/pharmacist team.
Results
In March 2006 we instituted the revised six-column insulin infusion protocol in our ICU. The percent of glucometer readings in the ICU between 70-150 mg/dl increased from 58% in February 2006 to 78% in August of 2006. (See Figure 2, above.) In our cardiac care unit (CCU), where an older, non-column insulin infusion protocol continued to be used, glucoses went from 50% in the 70-150 mg/dl range in February 2006 to 61% in that range in July 2006. When we began the six-column ICU insulin infusion protocol in the CCU in August 2006, 66% of glucoses were in the 70-150 range. (See Figure 3, p. 43.)
The percentage of glucoses below 70 mg/dl increased from 2%-3% using the four-column protocol to 4%-5% using the six-column protocol, as the percentage of glucoses between 70-150 mg/dl rose above 70%.
The main complaint from nurses relates to the need for frequent (hourly to twice hourly) glucose monitoring, which was also a complaint with earlier protocols in our institution.
Discussion
These results compare favorably with those achieved by acknowledged leaders in critical care glycemic control in the United States.13 We have achieved them with the use of a safe column protocol that our critical care nurses are now comfortable using. The hypoglycemia rates with our protocol have been lower than those seen in some other published protocols. The Berghe protocol, for example, reported 5% of patients experiencing glucoses lower than or equal to 40 mg/dl, while our protocol resulted in 4%-5% of glucose values lower than 70 mg/dl.
Mortality rates in our ICU have fallen from 14% in September 2005 to 11% in February 2006 to 8% in August 2006. Although this decline in mortality has occurred over the range of time in which we have improved glycemic control in our ICU, multiple other mortality reduction initiatives, any or all of which could account for the decline, occurred simultaneously in our hospital; in addition, mortality rates were falling before the introduction of the new insulin infusion protocol.
Head-to-head comparisons of existing protocols, using agreed upon glucometrics such as time-to-goal range and degree of glycemic variability, are needed.14 Testing new protocols, including proactive insulin infusion adjustments at the time of administration of known caloric loads—such as antibiotics delivered as a bolus in D5W solution—or at the time of initiation of high dose steroids or epinephrine drips, may be a means to keep a higher percentage of blood sugars in range. Ultimately, continuous blood sensing devices with closed loop insulin infusion responses are desired.
For now, our protocol offers a user-friendly means of getting a high percentage of critical care patients into a reasonable glycemic range with a low risk of hypoglycemia. Others are free to use and/or modify it. TH
The authors work at Southwest Washington Medical Center, Vancouver, Wash.
References
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359-1367.
- Malmberg K, Norhammar A, Wedel H, et al. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626-2632.
- Furnary AP, Wu Y, Bookin SO. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project. Endocr Pract. 2006 Mar-Apr;12 Suppl 3:22-26.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006 Feb 2;354(5):449-461.
- Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004 Aug;79(8):992-1000.
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004 Oct 11;164(18):2005-2011.
- Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12 Suppl 3:79-85.
- Markovitz LJ, Weichmann RJ, Harris N, et al. Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery. Endocr Pract. 2002 Jan-Feb;8(1):10-18.
- Ku SY, Sayre CA, Hirsch IB, et al. New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia. Jt Comm J Qual Patient Saf. 2005 Mar;31(3):141-147.
- Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004 Mar-Apr;10 Suppl 2:21-33.
- In a written communication from Andy Ahmann, MD, OHSU, in 2006.
- Georgia Hospital Association Diabetes Protocols. Chart 6b. Available at: www.adaendo.com/GHA/index.html. Accessed October 29, 2006.
- In a written communication from Terry Clemmer, MD, director, Critical Care Medicine, LDS Hospital, Salt Lake City, Utah, in October 2006.
- Braithwaite SS, Godara H, Song HJ, et al. No patient left behind: evaluation and design of intravenous insulin infusion algorithms. Endocr Pract. 2006 Jul-Aug;12 Suppl 3:72-78.
Evidence from randomized controlled trials and historical series convincingly demonstrates that tight glycemic control in the critical care setting reduces mortality, length of hospital stay, and other morbidities.1-6 Several protocols targeting a range of glucoses have been published.7-10 We modified a protocol used at the University of Washington (Seattle) that targeted glucoses of 80-180 mg/dl to aim for a tighter glycemic goal range of 80-110 mg/dl and effectively lowered blood glucoses in our intensive care unit (ICU) with a low rate of hypoglycemia.9 We share here our protocol and results over the past year.
Methods
Column protocols have the advantage of accommodating rates of insulin infusion adjustment to the individual patient’s degree of insulin resistance. If glucoses are not falling appropriately using one column, a protocol is followed to move to a more aggressive column. If glucoses fall precipitously, a similar process guides the nurse to a less aggressive column. The column format avoids the need for nurses to do calculations, which reduces the possibility of error.
In August 2005, in our ICU, we piloted a four-column protocol used at the University of Washington. This protocol was designed with an initial target glucose range of 80-180 mg/dl, and during our pilot it did not work well because we were targeting a glucose range of 80-110 mg/dl. Our ICU nursing staff at Southwest Washington Medical Center (SWMC), Vancouver, preferred to revise the column format rather than switch to a different type of protocol such as the Portland Protocol. We therefore created a new six-column protocol targeting the 80-110 mg/dl glucose range. (See Figure 1, above.)
In creating this protocol, we reviewed infusion rates in an unpublished Oregon Health and Sciences University (OHSU), Portland, six-column protocol and the Georgia Hospital Association, Marietta, 10-column protocol.11,12 Our current version includes new glucose ranges of 70-89 mg/dl, 90-99 mg/dl, and 100-109 mg/dl to allow for more rate adjustments within the overall target range, while our previous version had only one glucose category of 70-109 mg/dl within the target range.
Nursing feedback also led us to modify criteria for moving to the right (more aggressive) column as follows: If blood glucose is lower than 200, algorithm failure is defined as glucose outside of goal range and not decreased since previous reading; if blood glucose is greater than or equal to 200, algorithm failure is defined as glucose outside of goal range and has not decreased by at least 60 mg/dl within one hour. The longest interval we allow between glucometer checks for stable patients is two hours. Automatic triggers for implementing the protocol include two consecutive glucoses over 140 mg/dl or a single glucose over 180 mg/dl.
Our revised protocol succeeded when we intensified nursing education, solicited frequent nursing feedback, and organized the procedure so that ICU nurse managers drove the process with a responsive physician/pharmacist team.
Results
In March 2006 we instituted the revised six-column insulin infusion protocol in our ICU. The percent of glucometer readings in the ICU between 70-150 mg/dl increased from 58% in February 2006 to 78% in August of 2006. (See Figure 2, above.) In our cardiac care unit (CCU), where an older, non-column insulin infusion protocol continued to be used, glucoses went from 50% in the 70-150 mg/dl range in February 2006 to 61% in that range in July 2006. When we began the six-column ICU insulin infusion protocol in the CCU in August 2006, 66% of glucoses were in the 70-150 range. (See Figure 3, p. 43.)
The percentage of glucoses below 70 mg/dl increased from 2%-3% using the four-column protocol to 4%-5% using the six-column protocol, as the percentage of glucoses between 70-150 mg/dl rose above 70%.
The main complaint from nurses relates to the need for frequent (hourly to twice hourly) glucose monitoring, which was also a complaint with earlier protocols in our institution.
Discussion
These results compare favorably with those achieved by acknowledged leaders in critical care glycemic control in the United States.13 We have achieved them with the use of a safe column protocol that our critical care nurses are now comfortable using. The hypoglycemia rates with our protocol have been lower than those seen in some other published protocols. The Berghe protocol, for example, reported 5% of patients experiencing glucoses lower than or equal to 40 mg/dl, while our protocol resulted in 4%-5% of glucose values lower than 70 mg/dl.
Mortality rates in our ICU have fallen from 14% in September 2005 to 11% in February 2006 to 8% in August 2006. Although this decline in mortality has occurred over the range of time in which we have improved glycemic control in our ICU, multiple other mortality reduction initiatives, any or all of which could account for the decline, occurred simultaneously in our hospital; in addition, mortality rates were falling before the introduction of the new insulin infusion protocol.
Head-to-head comparisons of existing protocols, using agreed upon glucometrics such as time-to-goal range and degree of glycemic variability, are needed.14 Testing new protocols, including proactive insulin infusion adjustments at the time of administration of known caloric loads—such as antibiotics delivered as a bolus in D5W solution—or at the time of initiation of high dose steroids or epinephrine drips, may be a means to keep a higher percentage of blood sugars in range. Ultimately, continuous blood sensing devices with closed loop insulin infusion responses are desired.
For now, our protocol offers a user-friendly means of getting a high percentage of critical care patients into a reasonable glycemic range with a low risk of hypoglycemia. Others are free to use and/or modify it. TH
The authors work at Southwest Washington Medical Center, Vancouver, Wash.
References
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359-1367.
- Malmberg K, Norhammar A, Wedel H, et al. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626-2632.
- Furnary AP, Wu Y, Bookin SO. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project. Endocr Pract. 2006 Mar-Apr;12 Suppl 3:22-26.
- Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006 Feb 2;354(5):449-461.
- Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004 Aug;79(8):992-1000.
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004 Oct 11;164(18):2005-2011.
- Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12 Suppl 3:79-85.
- Markovitz LJ, Weichmann RJ, Harris N, et al. Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery. Endocr Pract. 2002 Jan-Feb;8(1):10-18.
- Ku SY, Sayre CA, Hirsch IB, et al. New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia. Jt Comm J Qual Patient Saf. 2005 Mar;31(3):141-147.
- Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004 Mar-Apr;10 Suppl 2:21-33.
- In a written communication from Andy Ahmann, MD, OHSU, in 2006.
- Georgia Hospital Association Diabetes Protocols. Chart 6b. Available at: www.adaendo.com/GHA/index.html. Accessed October 29, 2006.
- In a written communication from Terry Clemmer, MD, director, Critical Care Medicine, LDS Hospital, Salt Lake City, Utah, in October 2006.
- Braithwaite SS, Godara H, Song HJ, et al. No patient left behind: evaluation and design of intravenous insulin infusion algorithms. Endocr Pract. 2006 Jul-Aug;12 Suppl 3:72-78.
Focus on Family
Many physicians see being a hospitalist as an opportunity to focus on direct patient care. For Mary Ottolini, MD, it is a way to have it all. Every day, she cares for patients at Children’s National Medical Center in Washington, D.C., teaches at Children’s and at George Washington University School of Medicine in Washington, D.C., serves on a variety of committees and in numerous organizations, and works as a department head.
Early Start, Full Day
“It’s just another typical, crazy day,” says Dr. Ottolini, division chief of the hospitalist division and director of pediatric medical student education at Children’s National Medical Center and professor of pediatrics at George Washington, as she walks briskly down the hall at 9 a.m. on her way to radiology rounds. Her day actually starts at about 7 a.m., when she sees overnight admissions and addresses any urgent problems that require her attention. By 9 a.m., her day is in full swing.
During radiology rounds she and her team, which consists of residents, interns, and third-year medical students, review films from the previous day with the radiologist. The group addresses issues such as what additional tests might be useful. Sometimes they try to determine whether a condition is the result of an illness or injury.
Leaving radiology, Dr. Ottolini and her team head to the ward for patient rounds. As they walk to the first room, they pass cheerful murals featuring cartoon characters and several paintings and drawings created by children. A third-year resident leads the rounds, filling the group in on each patient’s condition and progress. Dr. Ottolini conducts the physical exams and talks to family members when they are present. When she offers her thoughts and comments to the group, the students listen attentively and take notes as she talks.
At one point, the team has to send for a translator for a non-English speaking family. Dr. Ottolini explains that this is common. In fact, Children’s has translators readily available who speak several common languages, including Spanish, French, and Japanese. Additionally, they have access to individuals who speak just about any language that arises.
Despite the ready availability of translators, these family discussions can be challenging. “When we have the translator, we are trying to balance efficiency with effective family communication,” explains Dr. Ottolini. “The translator adds a time factor because everything has to be repeated, and then there’s a lag time when we are looking at each other and waiting for the translation. It works, but it adds a layer of complexity to the situation, especially when you are trying to teach trainees while addressing parental concerns.”
—Mary Ottolini, MD, division chief, Hospitalist Division, and director of pediatric medical student education, Children’s National Medical Center, Washington, D.C.
Family Matters
Family communication is an important part of Dr. Ottolini’s daily activities. Because she doesn’t have a previous relationship with the patient or family, Dr. Ottolini faces the task of establishing rapport quickly—often in the midst of a crisis. “Especially when the child is seriously ill, it can be challenging to establish a level of trust,” she says. “For me, it’s a matter of trying to put myself in the parents’ shoes.”
Dr. Ottolini has also gained insights from being on the other side of the doctor-patient relationship. “I had an amazing relationship with a doctor who helped me tremendously, and I think of that,” she says. “Part of it is listening and trying to understand what is concerning the family the most. Sometimes, this is not what we think is the greatest concern. If we can get past what’s troubling them, it helps to move the care plan forward and establish a trusting relationship.
“There is no substitute for spending time with the family and getting to know them a little,” she continues. “It is important to understand how the illness is affecting the family’s routines, and helping to resolve these issues is useful as well.”
Dr. Ottolini’s concern for the family is evident in her interactions with them. She speaks with them gently, asks questions, and listens compassionately. Occasionally, Dr. Ottolini will schedule a family conference to address family concerns or other issues. “Family meetings are based on patient complexity—when there is multi-organ system involvement,” she says. “Sometimes, if the parents’ long-term expectations for their child’s prognosis are unrealistic, we want to have a meeting so that they can hear—from different sub-specialists involved—our rationale for what we are recommending— and [so that we can] clarify issues they don’t understand.”
Another level of concern involves families with limited resources. For example, “We have many recent immigrants for whom navigating the system is challenging,” says Dr. Ottolini. “We help them ensure that their child gets the best possible care, and we work to address work schedules and other issues.”
First and foremost, she and her team are patient and family advocates. “If we think it is important or necessary, we will keep a child here even if the insurance company says no,” she says.
Busy Afternoons, Late Days
By 1 p.m., Dr. Ottolini’s day is far from over. Her afternoon is filled with a variety of activities. In addition to seeing new patients, she spends time on billing and administrative activities, holds teaching sessions with medical students and residents at bedside and in the classroom, and writes notes.
“I still write my notes by hand,” she says. “However, this will be computerized in a year or so. When I finish with all of my clinical work and teaching responsibilities, I can catch up on administrative responsibilities or work on one of my research projects.”
Currently she is studying “ways to best conduct rounds and ensure that residents and students can take the information they get and put it all together to clinically problem solve and to see the big picture.”
Committee work is a big part of Dr. Ottolini’s work life. In addition to serving on several hospital committees, she also serves on the George Washington University faculty senate. Elsewhere, she is involved in several national organizations, including SHM.
Talking with families isn’t the only communication activity that takes Dr. Ottolini’s time. She works hard to keep referring physicians informed and to ensure they are involved in the patient’s care as necessary.
“We keep the patient’s pediatrician in the loop as much as possible. We make sure he or she understands how the disease process was managed, what new diagnoses arose, what prescription changes there were, and what follow-up is recommended,” Dr. Ottolini says. She especially wants to involve the pediatrician when a patient is critically ill or when the family is upset or in crisis. “Having a family voice to talk to helps the family feel as if they are getting support from someone they trust,” she explains. “This can be very reassuring for them.”
Dr. Ottolini encourages her students to appreciate the role and involvement of the pediatrician in a hospitalized patient’s care. “I try to make sure that residents and students have some sense of what it is like to be on the other side of things,” she offers. “I encourage them to think about how they would like to be treated if they were the pediatrician.”
Some physicians choose to become hospitalists because they want to spend the majority of their time on direct patient care. While Dr. Ottolini takes great satisfaction from this part of her work, it currently comprises only 30% of her professional time. Forty percent of her time involves medical education and research, which Dr. Ottolini greatly enjoys; administrative activities take up the remaining 30% of her time.
Many hospitalists appreciate the opportunities they have to teach, and Dr. Ottolini is no exception. She proudly observes that several physicians she has taught or mentored have become hospitalists: “For me, this is one of the most satisfying things.”
Admit and Discharge Issues
Dr. Ottolini has some involvement with admission and discharge issues. These decisions are simplified by the involvement of an expert team, however. For example, “We have case managers on rounds with us, and this helps them understand nuances of what we are doing that may not be exclusive in the notes and why it may be important for a patient to stay or appropriate for him or her to be discharged,” she says. “We look at patients’ criteria for discharge and anticipate, [on] the day before, any potential delays that could affect their release—such as getting tests performed and results back.”
Discharge planning is key. “We plan ahead for discharge and communicate goals to the family—such as getting the child off oxygen, getting cultures finalized, and so on,” says Dr. Ottolini. “We assign a discharge time the day before and make sure that the discharge summary, all necessary paperwork, and prescriptions are ready to go.”
For Dr. Ottolini, involvement in admission is limited. “The majority of our patients come through the emergency department,” she says. “However, we do admit patients coming in from the community, and we have input with community physicians if it’s not a clear-cut decision.”
The length of stay (LOS) for the nearly 300 beds in the hospital varies based on the patient’s condition. The average LOS for patients in the short-stay unit is three days. Facility-wide, the average LOS ranges from three to five days.
Challenges, Frustrations, Rewards, and Successes
“Challenges—such as dealing with very ill children who are not going to survive and addressing social situations where children are abused—also are rewarding, [and] we know we have worked in the best interest of the children,” says Dr. Ottolini. She and her team have the satisfaction of knowing they did everything they could to protect their patients, provide them with excellent care, and maximize their quality of life.
Dr. Ottolini says that she faces many of the same frustrations as others who work in a large organization. “With medically fragile children, a lot of coordination and communication needs to take place,” she says. “Sometimes, when lines of communication break down, you think something is happening when it isn’t. For example, after you have prepared a patient for an MRI, you find out that he or she has been bumped because of a more urgent situation. This frustrates the family and affects all of us.”
Pride of a Seasoned Hospitalist
A hospitalist since 1992, Dr. Ottolini is proud to have the title. She enjoys the teamwork she experiences on a daily basis, and even the challenges she experiences bring her tremendous professional and personal satisfaction.
While she sees herself as a generalist, Dr. Ottolini says her work “has enabled me to become especially good at those diagnoses we see a lot of—such as infectious disease problems and dehydration and fluid imbalance.”
An area in which Dr. Ottolini has become something of an expert is one that she would rather not have to see. “Sometimes we are lucky and see no abuse and neglect cases. The majority of the time, there is at least one admitted in a two-week period,” she says. “Out of necessity, I have learned quite a bit about abuse and neglect and caring for children who are abused and neglected. And, in presenting testimony on various cases, I have learned a bit about the court system.”
Helping students deal with this difficult reality is an important part of her teaching and mentoring activities. “From a clinical viewpoint, I help my students understand how to evaluate patients and look for red flags suggesting abuse or neglect,” she says. “However, it also is important for them to consider abuse in terms of different problems.”
Dr. Ottolini teaches her students “not to be closed-minded and not to be prejudiced concerning patients’ socieoeconomic status. They need to understand that abuse and neglect don’t just happen to poor children.”
From a personal standpoint, “we really need to focus on caring for children and not focus on who’s to blame. We want to work in a therapeutic relationship with parents as well as the child,” explains Dr. Ottolini. “It is not for us to figure out who is responsible for the abuse or neglect but to care for the child and work with the parent who is there. It is our job to make it clear to the police when abuse has occurred. Then we make sure that the situation to which the child is being sent when he or she leaves the hospital is reasonable and safe.”
A Happy Hospitalist
Dr. Ottolini rushes down the hall to see a patient as two residents hurry to keep up with her. They pepper her with questions as they walk, and she answers between glances at the chart in front of her. The smile on her face makes it clear that she is enjoying every minute of her “busy, crazy day.” TH
Joanne Kaldy writes regularly for The Hospitalist.
Many physicians see being a hospitalist as an opportunity to focus on direct patient care. For Mary Ottolini, MD, it is a way to have it all. Every day, she cares for patients at Children’s National Medical Center in Washington, D.C., teaches at Children’s and at George Washington University School of Medicine in Washington, D.C., serves on a variety of committees and in numerous organizations, and works as a department head.
Early Start, Full Day
“It’s just another typical, crazy day,” says Dr. Ottolini, division chief of the hospitalist division and director of pediatric medical student education at Children’s National Medical Center and professor of pediatrics at George Washington, as she walks briskly down the hall at 9 a.m. on her way to radiology rounds. Her day actually starts at about 7 a.m., when she sees overnight admissions and addresses any urgent problems that require her attention. By 9 a.m., her day is in full swing.
During radiology rounds she and her team, which consists of residents, interns, and third-year medical students, review films from the previous day with the radiologist. The group addresses issues such as what additional tests might be useful. Sometimes they try to determine whether a condition is the result of an illness or injury.
Leaving radiology, Dr. Ottolini and her team head to the ward for patient rounds. As they walk to the first room, they pass cheerful murals featuring cartoon characters and several paintings and drawings created by children. A third-year resident leads the rounds, filling the group in on each patient’s condition and progress. Dr. Ottolini conducts the physical exams and talks to family members when they are present. When she offers her thoughts and comments to the group, the students listen attentively and take notes as she talks.
At one point, the team has to send for a translator for a non-English speaking family. Dr. Ottolini explains that this is common. In fact, Children’s has translators readily available who speak several common languages, including Spanish, French, and Japanese. Additionally, they have access to individuals who speak just about any language that arises.
Despite the ready availability of translators, these family discussions can be challenging. “When we have the translator, we are trying to balance efficiency with effective family communication,” explains Dr. Ottolini. “The translator adds a time factor because everything has to be repeated, and then there’s a lag time when we are looking at each other and waiting for the translation. It works, but it adds a layer of complexity to the situation, especially when you are trying to teach trainees while addressing parental concerns.”
—Mary Ottolini, MD, division chief, Hospitalist Division, and director of pediatric medical student education, Children’s National Medical Center, Washington, D.C.
Family Matters
Family communication is an important part of Dr. Ottolini’s daily activities. Because she doesn’t have a previous relationship with the patient or family, Dr. Ottolini faces the task of establishing rapport quickly—often in the midst of a crisis. “Especially when the child is seriously ill, it can be challenging to establish a level of trust,” she says. “For me, it’s a matter of trying to put myself in the parents’ shoes.”
Dr. Ottolini has also gained insights from being on the other side of the doctor-patient relationship. “I had an amazing relationship with a doctor who helped me tremendously, and I think of that,” she says. “Part of it is listening and trying to understand what is concerning the family the most. Sometimes, this is not what we think is the greatest concern. If we can get past what’s troubling them, it helps to move the care plan forward and establish a trusting relationship.
“There is no substitute for spending time with the family and getting to know them a little,” she continues. “It is important to understand how the illness is affecting the family’s routines, and helping to resolve these issues is useful as well.”
Dr. Ottolini’s concern for the family is evident in her interactions with them. She speaks with them gently, asks questions, and listens compassionately. Occasionally, Dr. Ottolini will schedule a family conference to address family concerns or other issues. “Family meetings are based on patient complexity—when there is multi-organ system involvement,” she says. “Sometimes, if the parents’ long-term expectations for their child’s prognosis are unrealistic, we want to have a meeting so that they can hear—from different sub-specialists involved—our rationale for what we are recommending— and [so that we can] clarify issues they don’t understand.”
Another level of concern involves families with limited resources. For example, “We have many recent immigrants for whom navigating the system is challenging,” says Dr. Ottolini. “We help them ensure that their child gets the best possible care, and we work to address work schedules and other issues.”
First and foremost, she and her team are patient and family advocates. “If we think it is important or necessary, we will keep a child here even if the insurance company says no,” she says.
Busy Afternoons, Late Days
By 1 p.m., Dr. Ottolini’s day is far from over. Her afternoon is filled with a variety of activities. In addition to seeing new patients, she spends time on billing and administrative activities, holds teaching sessions with medical students and residents at bedside and in the classroom, and writes notes.
“I still write my notes by hand,” she says. “However, this will be computerized in a year or so. When I finish with all of my clinical work and teaching responsibilities, I can catch up on administrative responsibilities or work on one of my research projects.”
Currently she is studying “ways to best conduct rounds and ensure that residents and students can take the information they get and put it all together to clinically problem solve and to see the big picture.”
Committee work is a big part of Dr. Ottolini’s work life. In addition to serving on several hospital committees, she also serves on the George Washington University faculty senate. Elsewhere, she is involved in several national organizations, including SHM.
Talking with families isn’t the only communication activity that takes Dr. Ottolini’s time. She works hard to keep referring physicians informed and to ensure they are involved in the patient’s care as necessary.
“We keep the patient’s pediatrician in the loop as much as possible. We make sure he or she understands how the disease process was managed, what new diagnoses arose, what prescription changes there were, and what follow-up is recommended,” Dr. Ottolini says. She especially wants to involve the pediatrician when a patient is critically ill or when the family is upset or in crisis. “Having a family voice to talk to helps the family feel as if they are getting support from someone they trust,” she explains. “This can be very reassuring for them.”
Dr. Ottolini encourages her students to appreciate the role and involvement of the pediatrician in a hospitalized patient’s care. “I try to make sure that residents and students have some sense of what it is like to be on the other side of things,” she offers. “I encourage them to think about how they would like to be treated if they were the pediatrician.”
Some physicians choose to become hospitalists because they want to spend the majority of their time on direct patient care. While Dr. Ottolini takes great satisfaction from this part of her work, it currently comprises only 30% of her professional time. Forty percent of her time involves medical education and research, which Dr. Ottolini greatly enjoys; administrative activities take up the remaining 30% of her time.
Many hospitalists appreciate the opportunities they have to teach, and Dr. Ottolini is no exception. She proudly observes that several physicians she has taught or mentored have become hospitalists: “For me, this is one of the most satisfying things.”
Admit and Discharge Issues
Dr. Ottolini has some involvement with admission and discharge issues. These decisions are simplified by the involvement of an expert team, however. For example, “We have case managers on rounds with us, and this helps them understand nuances of what we are doing that may not be exclusive in the notes and why it may be important for a patient to stay or appropriate for him or her to be discharged,” she says. “We look at patients’ criteria for discharge and anticipate, [on] the day before, any potential delays that could affect their release—such as getting tests performed and results back.”
Discharge planning is key. “We plan ahead for discharge and communicate goals to the family—such as getting the child off oxygen, getting cultures finalized, and so on,” says Dr. Ottolini. “We assign a discharge time the day before and make sure that the discharge summary, all necessary paperwork, and prescriptions are ready to go.”
For Dr. Ottolini, involvement in admission is limited. “The majority of our patients come through the emergency department,” she says. “However, we do admit patients coming in from the community, and we have input with community physicians if it’s not a clear-cut decision.”
The length of stay (LOS) for the nearly 300 beds in the hospital varies based on the patient’s condition. The average LOS for patients in the short-stay unit is three days. Facility-wide, the average LOS ranges from three to five days.
Challenges, Frustrations, Rewards, and Successes
“Challenges—such as dealing with very ill children who are not going to survive and addressing social situations where children are abused—also are rewarding, [and] we know we have worked in the best interest of the children,” says Dr. Ottolini. She and her team have the satisfaction of knowing they did everything they could to protect their patients, provide them with excellent care, and maximize their quality of life.
Dr. Ottolini says that she faces many of the same frustrations as others who work in a large organization. “With medically fragile children, a lot of coordination and communication needs to take place,” she says. “Sometimes, when lines of communication break down, you think something is happening when it isn’t. For example, after you have prepared a patient for an MRI, you find out that he or she has been bumped because of a more urgent situation. This frustrates the family and affects all of us.”
Pride of a Seasoned Hospitalist
A hospitalist since 1992, Dr. Ottolini is proud to have the title. She enjoys the teamwork she experiences on a daily basis, and even the challenges she experiences bring her tremendous professional and personal satisfaction.
While she sees herself as a generalist, Dr. Ottolini says her work “has enabled me to become especially good at those diagnoses we see a lot of—such as infectious disease problems and dehydration and fluid imbalance.”
An area in which Dr. Ottolini has become something of an expert is one that she would rather not have to see. “Sometimes we are lucky and see no abuse and neglect cases. The majority of the time, there is at least one admitted in a two-week period,” she says. “Out of necessity, I have learned quite a bit about abuse and neglect and caring for children who are abused and neglected. And, in presenting testimony on various cases, I have learned a bit about the court system.”
Helping students deal with this difficult reality is an important part of her teaching and mentoring activities. “From a clinical viewpoint, I help my students understand how to evaluate patients and look for red flags suggesting abuse or neglect,” she says. “However, it also is important for them to consider abuse in terms of different problems.”
Dr. Ottolini teaches her students “not to be closed-minded and not to be prejudiced concerning patients’ socieoeconomic status. They need to understand that abuse and neglect don’t just happen to poor children.”
From a personal standpoint, “we really need to focus on caring for children and not focus on who’s to blame. We want to work in a therapeutic relationship with parents as well as the child,” explains Dr. Ottolini. “It is not for us to figure out who is responsible for the abuse or neglect but to care for the child and work with the parent who is there. It is our job to make it clear to the police when abuse has occurred. Then we make sure that the situation to which the child is being sent when he or she leaves the hospital is reasonable and safe.”
A Happy Hospitalist
Dr. Ottolini rushes down the hall to see a patient as two residents hurry to keep up with her. They pepper her with questions as they walk, and she answers between glances at the chart in front of her. The smile on her face makes it clear that she is enjoying every minute of her “busy, crazy day.” TH
Joanne Kaldy writes regularly for The Hospitalist.
Many physicians see being a hospitalist as an opportunity to focus on direct patient care. For Mary Ottolini, MD, it is a way to have it all. Every day, she cares for patients at Children’s National Medical Center in Washington, D.C., teaches at Children’s and at George Washington University School of Medicine in Washington, D.C., serves on a variety of committees and in numerous organizations, and works as a department head.
Early Start, Full Day
“It’s just another typical, crazy day,” says Dr. Ottolini, division chief of the hospitalist division and director of pediatric medical student education at Children’s National Medical Center and professor of pediatrics at George Washington, as she walks briskly down the hall at 9 a.m. on her way to radiology rounds. Her day actually starts at about 7 a.m., when she sees overnight admissions and addresses any urgent problems that require her attention. By 9 a.m., her day is in full swing.
During radiology rounds she and her team, which consists of residents, interns, and third-year medical students, review films from the previous day with the radiologist. The group addresses issues such as what additional tests might be useful. Sometimes they try to determine whether a condition is the result of an illness or injury.
Leaving radiology, Dr. Ottolini and her team head to the ward for patient rounds. As they walk to the first room, they pass cheerful murals featuring cartoon characters and several paintings and drawings created by children. A third-year resident leads the rounds, filling the group in on each patient’s condition and progress. Dr. Ottolini conducts the physical exams and talks to family members when they are present. When she offers her thoughts and comments to the group, the students listen attentively and take notes as she talks.
At one point, the team has to send for a translator for a non-English speaking family. Dr. Ottolini explains that this is common. In fact, Children’s has translators readily available who speak several common languages, including Spanish, French, and Japanese. Additionally, they have access to individuals who speak just about any language that arises.
Despite the ready availability of translators, these family discussions can be challenging. “When we have the translator, we are trying to balance efficiency with effective family communication,” explains Dr. Ottolini. “The translator adds a time factor because everything has to be repeated, and then there’s a lag time when we are looking at each other and waiting for the translation. It works, but it adds a layer of complexity to the situation, especially when you are trying to teach trainees while addressing parental concerns.”
—Mary Ottolini, MD, division chief, Hospitalist Division, and director of pediatric medical student education, Children’s National Medical Center, Washington, D.C.
Family Matters
Family communication is an important part of Dr. Ottolini’s daily activities. Because she doesn’t have a previous relationship with the patient or family, Dr. Ottolini faces the task of establishing rapport quickly—often in the midst of a crisis. “Especially when the child is seriously ill, it can be challenging to establish a level of trust,” she says. “For me, it’s a matter of trying to put myself in the parents’ shoes.”
Dr. Ottolini has also gained insights from being on the other side of the doctor-patient relationship. “I had an amazing relationship with a doctor who helped me tremendously, and I think of that,” she says. “Part of it is listening and trying to understand what is concerning the family the most. Sometimes, this is not what we think is the greatest concern. If we can get past what’s troubling them, it helps to move the care plan forward and establish a trusting relationship.
“There is no substitute for spending time with the family and getting to know them a little,” she continues. “It is important to understand how the illness is affecting the family’s routines, and helping to resolve these issues is useful as well.”
Dr. Ottolini’s concern for the family is evident in her interactions with them. She speaks with them gently, asks questions, and listens compassionately. Occasionally, Dr. Ottolini will schedule a family conference to address family concerns or other issues. “Family meetings are based on patient complexity—when there is multi-organ system involvement,” she says. “Sometimes, if the parents’ long-term expectations for their child’s prognosis are unrealistic, we want to have a meeting so that they can hear—from different sub-specialists involved—our rationale for what we are recommending— and [so that we can] clarify issues they don’t understand.”
Another level of concern involves families with limited resources. For example, “We have many recent immigrants for whom navigating the system is challenging,” says Dr. Ottolini. “We help them ensure that their child gets the best possible care, and we work to address work schedules and other issues.”
First and foremost, she and her team are patient and family advocates. “If we think it is important or necessary, we will keep a child here even if the insurance company says no,” she says.
Busy Afternoons, Late Days
By 1 p.m., Dr. Ottolini’s day is far from over. Her afternoon is filled with a variety of activities. In addition to seeing new patients, she spends time on billing and administrative activities, holds teaching sessions with medical students and residents at bedside and in the classroom, and writes notes.
“I still write my notes by hand,” she says. “However, this will be computerized in a year or so. When I finish with all of my clinical work and teaching responsibilities, I can catch up on administrative responsibilities or work on one of my research projects.”
Currently she is studying “ways to best conduct rounds and ensure that residents and students can take the information they get and put it all together to clinically problem solve and to see the big picture.”
Committee work is a big part of Dr. Ottolini’s work life. In addition to serving on several hospital committees, she also serves on the George Washington University faculty senate. Elsewhere, she is involved in several national organizations, including SHM.
Talking with families isn’t the only communication activity that takes Dr. Ottolini’s time. She works hard to keep referring physicians informed and to ensure they are involved in the patient’s care as necessary.
“We keep the patient’s pediatrician in the loop as much as possible. We make sure he or she understands how the disease process was managed, what new diagnoses arose, what prescription changes there were, and what follow-up is recommended,” Dr. Ottolini says. She especially wants to involve the pediatrician when a patient is critically ill or when the family is upset or in crisis. “Having a family voice to talk to helps the family feel as if they are getting support from someone they trust,” she explains. “This can be very reassuring for them.”
Dr. Ottolini encourages her students to appreciate the role and involvement of the pediatrician in a hospitalized patient’s care. “I try to make sure that residents and students have some sense of what it is like to be on the other side of things,” she offers. “I encourage them to think about how they would like to be treated if they were the pediatrician.”
Some physicians choose to become hospitalists because they want to spend the majority of their time on direct patient care. While Dr. Ottolini takes great satisfaction from this part of her work, it currently comprises only 30% of her professional time. Forty percent of her time involves medical education and research, which Dr. Ottolini greatly enjoys; administrative activities take up the remaining 30% of her time.
Many hospitalists appreciate the opportunities they have to teach, and Dr. Ottolini is no exception. She proudly observes that several physicians she has taught or mentored have become hospitalists: “For me, this is one of the most satisfying things.”
Admit and Discharge Issues
Dr. Ottolini has some involvement with admission and discharge issues. These decisions are simplified by the involvement of an expert team, however. For example, “We have case managers on rounds with us, and this helps them understand nuances of what we are doing that may not be exclusive in the notes and why it may be important for a patient to stay or appropriate for him or her to be discharged,” she says. “We look at patients’ criteria for discharge and anticipate, [on] the day before, any potential delays that could affect their release—such as getting tests performed and results back.”
Discharge planning is key. “We plan ahead for discharge and communicate goals to the family—such as getting the child off oxygen, getting cultures finalized, and so on,” says Dr. Ottolini. “We assign a discharge time the day before and make sure that the discharge summary, all necessary paperwork, and prescriptions are ready to go.”
For Dr. Ottolini, involvement in admission is limited. “The majority of our patients come through the emergency department,” she says. “However, we do admit patients coming in from the community, and we have input with community physicians if it’s not a clear-cut decision.”
The length of stay (LOS) for the nearly 300 beds in the hospital varies based on the patient’s condition. The average LOS for patients in the short-stay unit is three days. Facility-wide, the average LOS ranges from three to five days.
Challenges, Frustrations, Rewards, and Successes
“Challenges—such as dealing with very ill children who are not going to survive and addressing social situations where children are abused—also are rewarding, [and] we know we have worked in the best interest of the children,” says Dr. Ottolini. She and her team have the satisfaction of knowing they did everything they could to protect their patients, provide them with excellent care, and maximize their quality of life.
Dr. Ottolini says that she faces many of the same frustrations as others who work in a large organization. “With medically fragile children, a lot of coordination and communication needs to take place,” she says. “Sometimes, when lines of communication break down, you think something is happening when it isn’t. For example, after you have prepared a patient for an MRI, you find out that he or she has been bumped because of a more urgent situation. This frustrates the family and affects all of us.”
Pride of a Seasoned Hospitalist
A hospitalist since 1992, Dr. Ottolini is proud to have the title. She enjoys the teamwork she experiences on a daily basis, and even the challenges she experiences bring her tremendous professional and personal satisfaction.
While she sees herself as a generalist, Dr. Ottolini says her work “has enabled me to become especially good at those diagnoses we see a lot of—such as infectious disease problems and dehydration and fluid imbalance.”
An area in which Dr. Ottolini has become something of an expert is one that she would rather not have to see. “Sometimes we are lucky and see no abuse and neglect cases. The majority of the time, there is at least one admitted in a two-week period,” she says. “Out of necessity, I have learned quite a bit about abuse and neglect and caring for children who are abused and neglected. And, in presenting testimony on various cases, I have learned a bit about the court system.”
Helping students deal with this difficult reality is an important part of her teaching and mentoring activities. “From a clinical viewpoint, I help my students understand how to evaluate patients and look for red flags suggesting abuse or neglect,” she says. “However, it also is important for them to consider abuse in terms of different problems.”
Dr. Ottolini teaches her students “not to be closed-minded and not to be prejudiced concerning patients’ socieoeconomic status. They need to understand that abuse and neglect don’t just happen to poor children.”
From a personal standpoint, “we really need to focus on caring for children and not focus on who’s to blame. We want to work in a therapeutic relationship with parents as well as the child,” explains Dr. Ottolini. “It is not for us to figure out who is responsible for the abuse or neglect but to care for the child and work with the parent who is there. It is our job to make it clear to the police when abuse has occurred. Then we make sure that the situation to which the child is being sent when he or she leaves the hospital is reasonable and safe.”
A Happy Hospitalist
Dr. Ottolini rushes down the hall to see a patient as two residents hurry to keep up with her. They pepper her with questions as they walk, and she answers between glances at the chart in front of her. The smile on her face makes it clear that she is enjoying every minute of her “busy, crazy day.” TH
Joanne Kaldy writes regularly for The Hospitalist.