User login
A Look inside Healthcare Transparency
One of the many trends in healthcare today is a move toward making specific quality and pricing information available to the public.
“When you’re buying a car, you can easily compare quality, features, and prices to make an educated guess,” points out Eric Siegal, MD, regional medical director, Cogent Healthcare, Madison, Wis., and chair of SHM’s Public Policy Committee. “In contrast, healthcare is completely opaque. People choose a doctor or a hospital—sometimes for a surgery that’s life threatening—by word of mouth or [based on] proximity. How do you make it possible to choose based on quality of care and on price?”
Known as healthcare transparency, this trend is driven by multiple sources. “The [CMS] Hospital Compare initiative was a first step in this, as were the Leapfrog initiative and the IHI [Institute for Health Improvement] Collaborative,” says Dr. Siegal. “In fact, the government is a little late to the game, but they’re quickly closing the gap.”
Mandate from the President
On August 22, 2006, President George W. Bush signed an executive order requiring key federal agencies to collect information about the quality and cost of the healthcare they provide and to share that data with each another—and with beneficiaries. Agencies included in the order are the Department of Health and Human Services (HHS), the Department of Defense (DoD), the Department of Veterans Affairs (VA), and the Office of Personnel Management (OPM).
The executive order directs these four agencies to work with the private sector and other government agencies to develop programs to measure quality of care. They were required by Jan. 1, 2007, to identify practices that promote high quality care and to compile information on the prices they pay for common services available to their members. Ultimately, the executive order calls for combining that data in a comprehensive source on providers’ quality and prices; this information will then be available to consumers.
President Bush has said that his order sends a message to healthcare providers that “in order to do business with the federal government, you’ve got to show us your prices.” The new requirements for transparency will affect healthcare providers across the country because treating about one-quarter of Americans covered by health insurance entails “doing business with the federal government.” That one-quarter includes Medicare beneficiaries, health insurance beneficiaries at the DoD and the VA, and federal employees. (The order clearly states that the directive does not apply to state-administered or -funded programs.)
House Legislation: Make Prices Public
Comprehensive pricing transparency may also be required on a state level. On Sept. 13, 2006, Representative Michael Burgess (R-Texas) introduced the Health Care Price Transparency Act of 2006 in the House. This American Hospital Association (AHA)-supported legislation would require states to publicly report hospital charges for specific inpatient and outpatient services and would require insurers to give patients, on request, an estimate of their expected out-of-pocket expenses.
—Eric Siegal, MD
The bill would also require the Agency for Healthcare Research and Quality to study what type of healthcare price information consumers would find useful and how that information could be made available in a timely, understandable form.
Thirty-two states already require hospitals to report pricing information, and six more are voluntarily doing so, but this legislation would likely change the information that hospitals and other providers are gathering and providing.
At press time, the legislation had been referred to the House Subcommittee on Health.
How Transparency Will Roll Out
While the House legislation is in limbo, the executive order will have an immediate effect on healthcare, starting this year. The quality measures to be included in reporting will be developed from private and government sources, including local providers, employers, and health plans and insurers.
After the data are gathered and the information technology (IT) infrastructure is set up, consumers will be able to access specific information on pricing and quality of services performed by doctors, hospitals, and other healthcare providers. This information may be available through a variety of sources, including insurance companies, employers, and Medicare-sponsored Web sites.
One of the keys to success will be in the collaboration among the agencies involved. “There’s a keen understanding among the major players that if everyone does their own thing, we’ll have chaos,” says Dr. Siegal. “There has to be a significant degree of harmonization [among] physician measures, hospital measures, inpatient measures, and outpatient measures.”
Where Hospitalists Fit in
Will healthcare transparency affect hospitalists? “It’s already impacting hospitalists,” says Dr. Siegal. “Not on pricing, but on quality reporting. The good news is that hospitalists may be the single best-prepared group of physicians [for transparency] because we’re already doing it. The question will be, as it becomes more pervasive, will it be done in a way that is thoughtful, measured, and practical?”
Hospitals are likely to look to their hospitalists to ensure that their quality measurements are competitive. Dr. Siegal explains, “Hospitals looking to improve quality will be most effective in getting results from the physicians whose financial incentives are aligned with theirs.”
However, additional—or more public—quality indicators will not necessarily create a huge source of income for hospital medicine. “The low-hanging fruit won’t be the patients that hospitalists see; it will be elective surgical cases,” predicts Dr. Siegal. “Those are cleanly defined procedures, with bundled payments and predictable outcomes, where a hospital can understand what happens and what’s included. Then they can say, ‘Why do we charge 20% more for a total elective hip [surgery] than the hospital down the road?’ ”
As transparency is rolled out in U.S. hospitals and healthcare systems, hospitalists will look good. “Hospitalists already live in a quality reporting world, more so than other doctors,” says Dr. Siegal. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
One of the many trends in healthcare today is a move toward making specific quality and pricing information available to the public.
“When you’re buying a car, you can easily compare quality, features, and prices to make an educated guess,” points out Eric Siegal, MD, regional medical director, Cogent Healthcare, Madison, Wis., and chair of SHM’s Public Policy Committee. “In contrast, healthcare is completely opaque. People choose a doctor or a hospital—sometimes for a surgery that’s life threatening—by word of mouth or [based on] proximity. How do you make it possible to choose based on quality of care and on price?”
Known as healthcare transparency, this trend is driven by multiple sources. “The [CMS] Hospital Compare initiative was a first step in this, as were the Leapfrog initiative and the IHI [Institute for Health Improvement] Collaborative,” says Dr. Siegal. “In fact, the government is a little late to the game, but they’re quickly closing the gap.”
Mandate from the President
On August 22, 2006, President George W. Bush signed an executive order requiring key federal agencies to collect information about the quality and cost of the healthcare they provide and to share that data with each another—and with beneficiaries. Agencies included in the order are the Department of Health and Human Services (HHS), the Department of Defense (DoD), the Department of Veterans Affairs (VA), and the Office of Personnel Management (OPM).
The executive order directs these four agencies to work with the private sector and other government agencies to develop programs to measure quality of care. They were required by Jan. 1, 2007, to identify practices that promote high quality care and to compile information on the prices they pay for common services available to their members. Ultimately, the executive order calls for combining that data in a comprehensive source on providers’ quality and prices; this information will then be available to consumers.
President Bush has said that his order sends a message to healthcare providers that “in order to do business with the federal government, you’ve got to show us your prices.” The new requirements for transparency will affect healthcare providers across the country because treating about one-quarter of Americans covered by health insurance entails “doing business with the federal government.” That one-quarter includes Medicare beneficiaries, health insurance beneficiaries at the DoD and the VA, and federal employees. (The order clearly states that the directive does not apply to state-administered or -funded programs.)
House Legislation: Make Prices Public
Comprehensive pricing transparency may also be required on a state level. On Sept. 13, 2006, Representative Michael Burgess (R-Texas) introduced the Health Care Price Transparency Act of 2006 in the House. This American Hospital Association (AHA)-supported legislation would require states to publicly report hospital charges for specific inpatient and outpatient services and would require insurers to give patients, on request, an estimate of their expected out-of-pocket expenses.
—Eric Siegal, MD
The bill would also require the Agency for Healthcare Research and Quality to study what type of healthcare price information consumers would find useful and how that information could be made available in a timely, understandable form.
Thirty-two states already require hospitals to report pricing information, and six more are voluntarily doing so, but this legislation would likely change the information that hospitals and other providers are gathering and providing.
At press time, the legislation had been referred to the House Subcommittee on Health.
How Transparency Will Roll Out
While the House legislation is in limbo, the executive order will have an immediate effect on healthcare, starting this year. The quality measures to be included in reporting will be developed from private and government sources, including local providers, employers, and health plans and insurers.
After the data are gathered and the information technology (IT) infrastructure is set up, consumers will be able to access specific information on pricing and quality of services performed by doctors, hospitals, and other healthcare providers. This information may be available through a variety of sources, including insurance companies, employers, and Medicare-sponsored Web sites.
One of the keys to success will be in the collaboration among the agencies involved. “There’s a keen understanding among the major players that if everyone does their own thing, we’ll have chaos,” says Dr. Siegal. “There has to be a significant degree of harmonization [among] physician measures, hospital measures, inpatient measures, and outpatient measures.”
Where Hospitalists Fit in
Will healthcare transparency affect hospitalists? “It’s already impacting hospitalists,” says Dr. Siegal. “Not on pricing, but on quality reporting. The good news is that hospitalists may be the single best-prepared group of physicians [for transparency] because we’re already doing it. The question will be, as it becomes more pervasive, will it be done in a way that is thoughtful, measured, and practical?”
Hospitals are likely to look to their hospitalists to ensure that their quality measurements are competitive. Dr. Siegal explains, “Hospitals looking to improve quality will be most effective in getting results from the physicians whose financial incentives are aligned with theirs.”
However, additional—or more public—quality indicators will not necessarily create a huge source of income for hospital medicine. “The low-hanging fruit won’t be the patients that hospitalists see; it will be elective surgical cases,” predicts Dr. Siegal. “Those are cleanly defined procedures, with bundled payments and predictable outcomes, where a hospital can understand what happens and what’s included. Then they can say, ‘Why do we charge 20% more for a total elective hip [surgery] than the hospital down the road?’ ”
As transparency is rolled out in U.S. hospitals and healthcare systems, hospitalists will look good. “Hospitalists already live in a quality reporting world, more so than other doctors,” says Dr. Siegal. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
One of the many trends in healthcare today is a move toward making specific quality and pricing information available to the public.
“When you’re buying a car, you can easily compare quality, features, and prices to make an educated guess,” points out Eric Siegal, MD, regional medical director, Cogent Healthcare, Madison, Wis., and chair of SHM’s Public Policy Committee. “In contrast, healthcare is completely opaque. People choose a doctor or a hospital—sometimes for a surgery that’s life threatening—by word of mouth or [based on] proximity. How do you make it possible to choose based on quality of care and on price?”
Known as healthcare transparency, this trend is driven by multiple sources. “The [CMS] Hospital Compare initiative was a first step in this, as were the Leapfrog initiative and the IHI [Institute for Health Improvement] Collaborative,” says Dr. Siegal. “In fact, the government is a little late to the game, but they’re quickly closing the gap.”
Mandate from the President
On August 22, 2006, President George W. Bush signed an executive order requiring key federal agencies to collect information about the quality and cost of the healthcare they provide and to share that data with each another—and with beneficiaries. Agencies included in the order are the Department of Health and Human Services (HHS), the Department of Defense (DoD), the Department of Veterans Affairs (VA), and the Office of Personnel Management (OPM).
The executive order directs these four agencies to work with the private sector and other government agencies to develop programs to measure quality of care. They were required by Jan. 1, 2007, to identify practices that promote high quality care and to compile information on the prices they pay for common services available to their members. Ultimately, the executive order calls for combining that data in a comprehensive source on providers’ quality and prices; this information will then be available to consumers.
President Bush has said that his order sends a message to healthcare providers that “in order to do business with the federal government, you’ve got to show us your prices.” The new requirements for transparency will affect healthcare providers across the country because treating about one-quarter of Americans covered by health insurance entails “doing business with the federal government.” That one-quarter includes Medicare beneficiaries, health insurance beneficiaries at the DoD and the VA, and federal employees. (The order clearly states that the directive does not apply to state-administered or -funded programs.)
House Legislation: Make Prices Public
Comprehensive pricing transparency may also be required on a state level. On Sept. 13, 2006, Representative Michael Burgess (R-Texas) introduced the Health Care Price Transparency Act of 2006 in the House. This American Hospital Association (AHA)-supported legislation would require states to publicly report hospital charges for specific inpatient and outpatient services and would require insurers to give patients, on request, an estimate of their expected out-of-pocket expenses.
—Eric Siegal, MD
The bill would also require the Agency for Healthcare Research and Quality to study what type of healthcare price information consumers would find useful and how that information could be made available in a timely, understandable form.
Thirty-two states already require hospitals to report pricing information, and six more are voluntarily doing so, but this legislation would likely change the information that hospitals and other providers are gathering and providing.
At press time, the legislation had been referred to the House Subcommittee on Health.
How Transparency Will Roll Out
While the House legislation is in limbo, the executive order will have an immediate effect on healthcare, starting this year. The quality measures to be included in reporting will be developed from private and government sources, including local providers, employers, and health plans and insurers.
After the data are gathered and the information technology (IT) infrastructure is set up, consumers will be able to access specific information on pricing and quality of services performed by doctors, hospitals, and other healthcare providers. This information may be available through a variety of sources, including insurance companies, employers, and Medicare-sponsored Web sites.
One of the keys to success will be in the collaboration among the agencies involved. “There’s a keen understanding among the major players that if everyone does their own thing, we’ll have chaos,” says Dr. Siegal. “There has to be a significant degree of harmonization [among] physician measures, hospital measures, inpatient measures, and outpatient measures.”
Where Hospitalists Fit in
Will healthcare transparency affect hospitalists? “It’s already impacting hospitalists,” says Dr. Siegal. “Not on pricing, but on quality reporting. The good news is that hospitalists may be the single best-prepared group of physicians [for transparency] because we’re already doing it. The question will be, as it becomes more pervasive, will it be done in a way that is thoughtful, measured, and practical?”
Hospitals are likely to look to their hospitalists to ensure that their quality measurements are competitive. Dr. Siegal explains, “Hospitals looking to improve quality will be most effective in getting results from the physicians whose financial incentives are aligned with theirs.”
However, additional—or more public—quality indicators will not necessarily create a huge source of income for hospital medicine. “The low-hanging fruit won’t be the patients that hospitalists see; it will be elective surgical cases,” predicts Dr. Siegal. “Those are cleanly defined procedures, with bundled payments and predictable outcomes, where a hospital can understand what happens and what’s included. Then they can say, ‘Why do we charge 20% more for a total elective hip [surgery] than the hospital down the road?’ ”
As transparency is rolled out in U.S. hospitals and healthcare systems, hospitalists will look good. “Hospitalists already live in a quality reporting world, more so than other doctors,” says Dr. Siegal. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
Register Now for February’s SHM Leadership Academy
SHM’s Level I Leadership Academy is back. If you were unable to register for the sold-out September Academy, now is your chance. This semi-annual course will be held during the week of February 26–March 1, 2007, at the Gaylord Palms Resort and Convention Center in Orlando, Fla. This course gives attendees hands-on experience and a unique opportunity to learn from the best in the field. All previous Level I academies have sold out weeks in advance, so reserve your spot today by visiting www.hospitalmedicine.org for more information.
ASHP Foundation Launches New Hospital Pharmacist-Hospitalist Team Research Grant
Foundation seeks to encourage collaborative studies of VTE prevention
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in hospitals. It mostly affects patients with primary medical conditions; those who have had surgery for gynecologic, orthopedic, urologic, and vascular conditions; and those receiving care in critical care settings.
The American Society of Health-System Pharmacists (ASHP) Research and Education Foundation has created a new grant program sponsored by the Sanofi Aventis Group to support multidisciplinary research studies conducted by hospital pharmacists and hospitalists to prevent and treat VTE in hospitalized patients.
“Because the focus of a hospitalist is providing quality medical care for hospitalized patients and the unique medical problems that they may face as a result of being hospitalized, the ASHP Foundation felt it was important to offer hospital pharmacists an opportunity to partner with this group of physicians,” says Daniel J. Cobaugh, PharmD, FAACT, DABAT, ASHP Foundation director of research. “We wanted to focus the research grant on a major patient care issue that can be effectively addressed through hospital pharmacist-hospitalist collaborations. We believe this collaborative approach will have far-reaching implications for improving patient care and patient safety.”
“The hospital of the future will be based on patient-centered care, with measurable quality outcomes and delivered by teams of health professionals,” explains Larry Wellikson, MD, FACP, SHM CEO. “This collaborative process with hospital-based pharmacists and hospitalists working together to improve VTE care is just the kind of interdisciplinary teamwork that can serve as a beacon to lead us to a better future.”
Applications should emphasize the following:
- Project objectives that address health services research related to the prevention and treatment of VTE;
- Sound research methods that support the study objectives;
- Interdisciplinary collaboration between hospital pharmacists and hospitalist physicians;
- The potential for findings to be replicated in other healthcare facilities; and
- Prudent use of grant funds.
Potential areas of research focus include the use of appropriate interventions to prevent VTE, optimization and monitoring of therapies used for VTE, ensuring continuity of care, provision of literacy-sensitive education to patients and caregivers, and health professional education.
Applications and detailed instructions for the Hospital Pharmacist-Hospitalist Collaboration: VTE Prevention and Treatment Team Grant is available on the ASHP Foundation Web site at www.ashpfoundation.org. The deadline for completed applications is March 1.
SHM Launches New Grassroots Advocacy Tool
Grassroots involvement by SHM members is critical to our ability to influence health policy in Washington. That is why SHM has launched a powerful new advocacy tool designed to help you communicate quickly and effectively with your congressional representatives. Capwiz·XC, located in the “Advocacy” section of our Web site, enables you to take action on any issue important to hospital medicine by sending personalized communications to your elected officials.
Advocacy doesn’t have to require a big time commitment. Communicating with your representatives in Congress now takes just a few minutes at SHM’s Legislative Action Center. You can send an e-mail whenever it is convenient for you, and our action alerts contain sample text for you to use and personalize as desired. Physician payment reform, quality improvement, palliative care, and funding levels for the National Institutes of Health and the Agency for Healthcare Research and Quality are just a few of the many issues before the 110th Congress. You can help influence the debate, improve patient care, and increase the visibility of hospitalists by making your voice heard through Capwiz.
Capwiz has many other features that will help keep you informed and educated about the legislative process. These include an interactive map to help you find your elected officials. Simply enter your ZIP code or click on your state to find out who your elected officials are. From there, you can easily select one of the listed state or congressional officials to see the full legislative biography page. Each bio page includes direct links you can use to contact the legislator, look up his or her key votes, and find staff contact information. You’ll also find a plethora of information about each elected representative, including:
- Office term;
- Co-sponsorship status;
- Contact information;
- Party affiliation;
- Political background;
- Committee(s); and
- PAC contributions.
Are you interested in looking up a piece of legislation recently mentioned in the news? Or do you want to monitor the various bills that will affect issues important to your practice? The “Legislative Action Center” in the “Advocacy” section contains “Issues and Legislation” to help you to stay on top of current legislation affecting healthcare. Keep track of any bill’s name, summary, co-sponsor(s), and key votes, while also monitoring SHM’s position on the legislation.
SHM is pleased to provide you with the opportunity to become more familiar with the political process and actively participate in influencing the policies that affect hospitalists and their patients. Capwiz contains up-to-the-minute legislative data and online tools to enable you to make a difference in the political process. Please visit our Legislative Action Center today at www.hospitalmedicine.org. TH
A response to the needs:
Products and Initiatives
- Annual Awards
- Antimicrobial Resistance Resource Room
- Career Center
- Chapters
- Coding and Documentation Precourse
- Collaboration with IHI and Other Organizations
- Core Competencies
- Credentialing by ABIM
- Dashboard White Paper
- Discharge Planning Checklist
- DVT Awareness Campaign
- DVT Mentored Implementation
- e-Newsletter
- Geriatric Resource Room
- Glycemic Control Resource Room
- Heart Failure Resource Room
- Journal of Hospital Medicine
- Leadership Academies
- Legislative Action Day
- Letters to Congress
- List Serves
- Mentoring
- Practice Management Precourse
- Productivity and Compensation Survey
- Public Policy White Paper
- Quality Precourse
- Research Grants
- Resource Rooms
- RIV Competition
- SEPs Workshop
- Stroke Resource Room
- Survey Collaboration with AHA
- The Hospitalist
- Value Added White Paper
- VTE Resource Room
- Web site
A response to the needs:
Committees and Task Forces
- Annual Meeting Committee
- Awards Committee
- Benchmarks Committee
- Board of Directors
- Career Satisfaction Task Force
- Certification Task Force
- Curriculum Task Force
- Education Committee
- Ethics Committee
- Executive Committee of the Board of Directors
- Executive Review Committee
- Family Practice Task Force
- Finance Committee of the Board of Directors
- Hospital Quality and Patient Safety Committee
- Heart Failure Award Task Force
- Leadership Committee
- Membership Committee
- Nominations Committee
- Non Physician Provider Committee
- Palliative Care Task Force
- Pediatric Committee
- Pediatric Core Curriculum Task Force
- Performance and Standards Task Force
- Public Policy Committee
- Research Committee
- Resource Room Oversight Committee
- Research, Innovation, and Clinical Vignettes Committee
- Women in Hospital Medicine
- Young Physicians Committee TH
SHM’s Level I Leadership Academy is back. If you were unable to register for the sold-out September Academy, now is your chance. This semi-annual course will be held during the week of February 26–March 1, 2007, at the Gaylord Palms Resort and Convention Center in Orlando, Fla. This course gives attendees hands-on experience and a unique opportunity to learn from the best in the field. All previous Level I academies have sold out weeks in advance, so reserve your spot today by visiting www.hospitalmedicine.org for more information.
ASHP Foundation Launches New Hospital Pharmacist-Hospitalist Team Research Grant
Foundation seeks to encourage collaborative studies of VTE prevention
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in hospitals. It mostly affects patients with primary medical conditions; those who have had surgery for gynecologic, orthopedic, urologic, and vascular conditions; and those receiving care in critical care settings.
The American Society of Health-System Pharmacists (ASHP) Research and Education Foundation has created a new grant program sponsored by the Sanofi Aventis Group to support multidisciplinary research studies conducted by hospital pharmacists and hospitalists to prevent and treat VTE in hospitalized patients.
“Because the focus of a hospitalist is providing quality medical care for hospitalized patients and the unique medical problems that they may face as a result of being hospitalized, the ASHP Foundation felt it was important to offer hospital pharmacists an opportunity to partner with this group of physicians,” says Daniel J. Cobaugh, PharmD, FAACT, DABAT, ASHP Foundation director of research. “We wanted to focus the research grant on a major patient care issue that can be effectively addressed through hospital pharmacist-hospitalist collaborations. We believe this collaborative approach will have far-reaching implications for improving patient care and patient safety.”
“The hospital of the future will be based on patient-centered care, with measurable quality outcomes and delivered by teams of health professionals,” explains Larry Wellikson, MD, FACP, SHM CEO. “This collaborative process with hospital-based pharmacists and hospitalists working together to improve VTE care is just the kind of interdisciplinary teamwork that can serve as a beacon to lead us to a better future.”
Applications should emphasize the following:
- Project objectives that address health services research related to the prevention and treatment of VTE;
- Sound research methods that support the study objectives;
- Interdisciplinary collaboration between hospital pharmacists and hospitalist physicians;
- The potential for findings to be replicated in other healthcare facilities; and
- Prudent use of grant funds.
Potential areas of research focus include the use of appropriate interventions to prevent VTE, optimization and monitoring of therapies used for VTE, ensuring continuity of care, provision of literacy-sensitive education to patients and caregivers, and health professional education.
Applications and detailed instructions for the Hospital Pharmacist-Hospitalist Collaboration: VTE Prevention and Treatment Team Grant is available on the ASHP Foundation Web site at www.ashpfoundation.org. The deadline for completed applications is March 1.
SHM Launches New Grassroots Advocacy Tool
Grassroots involvement by SHM members is critical to our ability to influence health policy in Washington. That is why SHM has launched a powerful new advocacy tool designed to help you communicate quickly and effectively with your congressional representatives. Capwiz·XC, located in the “Advocacy” section of our Web site, enables you to take action on any issue important to hospital medicine by sending personalized communications to your elected officials.
Advocacy doesn’t have to require a big time commitment. Communicating with your representatives in Congress now takes just a few minutes at SHM’s Legislative Action Center. You can send an e-mail whenever it is convenient for you, and our action alerts contain sample text for you to use and personalize as desired. Physician payment reform, quality improvement, palliative care, and funding levels for the National Institutes of Health and the Agency for Healthcare Research and Quality are just a few of the many issues before the 110th Congress. You can help influence the debate, improve patient care, and increase the visibility of hospitalists by making your voice heard through Capwiz.
Capwiz has many other features that will help keep you informed and educated about the legislative process. These include an interactive map to help you find your elected officials. Simply enter your ZIP code or click on your state to find out who your elected officials are. From there, you can easily select one of the listed state or congressional officials to see the full legislative biography page. Each bio page includes direct links you can use to contact the legislator, look up his or her key votes, and find staff contact information. You’ll also find a plethora of information about each elected representative, including:
- Office term;
- Co-sponsorship status;
- Contact information;
- Party affiliation;
- Political background;
- Committee(s); and
- PAC contributions.
Are you interested in looking up a piece of legislation recently mentioned in the news? Or do you want to monitor the various bills that will affect issues important to your practice? The “Legislative Action Center” in the “Advocacy” section contains “Issues and Legislation” to help you to stay on top of current legislation affecting healthcare. Keep track of any bill’s name, summary, co-sponsor(s), and key votes, while also monitoring SHM’s position on the legislation.
SHM is pleased to provide you with the opportunity to become more familiar with the political process and actively participate in influencing the policies that affect hospitalists and their patients. Capwiz contains up-to-the-minute legislative data and online tools to enable you to make a difference in the political process. Please visit our Legislative Action Center today at www.hospitalmedicine.org. TH
A response to the needs:
Products and Initiatives
- Annual Awards
- Antimicrobial Resistance Resource Room
- Career Center
- Chapters
- Coding and Documentation Precourse
- Collaboration with IHI and Other Organizations
- Core Competencies
- Credentialing by ABIM
- Dashboard White Paper
- Discharge Planning Checklist
- DVT Awareness Campaign
- DVT Mentored Implementation
- e-Newsletter
- Geriatric Resource Room
- Glycemic Control Resource Room
- Heart Failure Resource Room
- Journal of Hospital Medicine
- Leadership Academies
- Legislative Action Day
- Letters to Congress
- List Serves
- Mentoring
- Practice Management Precourse
- Productivity and Compensation Survey
- Public Policy White Paper
- Quality Precourse
- Research Grants
- Resource Rooms
- RIV Competition
- SEPs Workshop
- Stroke Resource Room
- Survey Collaboration with AHA
- The Hospitalist
- Value Added White Paper
- VTE Resource Room
- Web site
A response to the needs:
Committees and Task Forces
- Annual Meeting Committee
- Awards Committee
- Benchmarks Committee
- Board of Directors
- Career Satisfaction Task Force
- Certification Task Force
- Curriculum Task Force
- Education Committee
- Ethics Committee
- Executive Committee of the Board of Directors
- Executive Review Committee
- Family Practice Task Force
- Finance Committee of the Board of Directors
- Hospital Quality and Patient Safety Committee
- Heart Failure Award Task Force
- Leadership Committee
- Membership Committee
- Nominations Committee
- Non Physician Provider Committee
- Palliative Care Task Force
- Pediatric Committee
- Pediatric Core Curriculum Task Force
- Performance and Standards Task Force
- Public Policy Committee
- Research Committee
- Resource Room Oversight Committee
- Research, Innovation, and Clinical Vignettes Committee
- Women in Hospital Medicine
- Young Physicians Committee TH
SHM’s Level I Leadership Academy is back. If you were unable to register for the sold-out September Academy, now is your chance. This semi-annual course will be held during the week of February 26–March 1, 2007, at the Gaylord Palms Resort and Convention Center in Orlando, Fla. This course gives attendees hands-on experience and a unique opportunity to learn from the best in the field. All previous Level I academies have sold out weeks in advance, so reserve your spot today by visiting www.hospitalmedicine.org for more information.
ASHP Foundation Launches New Hospital Pharmacist-Hospitalist Team Research Grant
Foundation seeks to encourage collaborative studies of VTE prevention
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in hospitals. It mostly affects patients with primary medical conditions; those who have had surgery for gynecologic, orthopedic, urologic, and vascular conditions; and those receiving care in critical care settings.
The American Society of Health-System Pharmacists (ASHP) Research and Education Foundation has created a new grant program sponsored by the Sanofi Aventis Group to support multidisciplinary research studies conducted by hospital pharmacists and hospitalists to prevent and treat VTE in hospitalized patients.
“Because the focus of a hospitalist is providing quality medical care for hospitalized patients and the unique medical problems that they may face as a result of being hospitalized, the ASHP Foundation felt it was important to offer hospital pharmacists an opportunity to partner with this group of physicians,” says Daniel J. Cobaugh, PharmD, FAACT, DABAT, ASHP Foundation director of research. “We wanted to focus the research grant on a major patient care issue that can be effectively addressed through hospital pharmacist-hospitalist collaborations. We believe this collaborative approach will have far-reaching implications for improving patient care and patient safety.”
“The hospital of the future will be based on patient-centered care, with measurable quality outcomes and delivered by teams of health professionals,” explains Larry Wellikson, MD, FACP, SHM CEO. “This collaborative process with hospital-based pharmacists and hospitalists working together to improve VTE care is just the kind of interdisciplinary teamwork that can serve as a beacon to lead us to a better future.”
Applications should emphasize the following:
- Project objectives that address health services research related to the prevention and treatment of VTE;
- Sound research methods that support the study objectives;
- Interdisciplinary collaboration between hospital pharmacists and hospitalist physicians;
- The potential for findings to be replicated in other healthcare facilities; and
- Prudent use of grant funds.
Potential areas of research focus include the use of appropriate interventions to prevent VTE, optimization and monitoring of therapies used for VTE, ensuring continuity of care, provision of literacy-sensitive education to patients and caregivers, and health professional education.
Applications and detailed instructions for the Hospital Pharmacist-Hospitalist Collaboration: VTE Prevention and Treatment Team Grant is available on the ASHP Foundation Web site at www.ashpfoundation.org. The deadline for completed applications is March 1.
SHM Launches New Grassroots Advocacy Tool
Grassroots involvement by SHM members is critical to our ability to influence health policy in Washington. That is why SHM has launched a powerful new advocacy tool designed to help you communicate quickly and effectively with your congressional representatives. Capwiz·XC, located in the “Advocacy” section of our Web site, enables you to take action on any issue important to hospital medicine by sending personalized communications to your elected officials.
Advocacy doesn’t have to require a big time commitment. Communicating with your representatives in Congress now takes just a few minutes at SHM’s Legislative Action Center. You can send an e-mail whenever it is convenient for you, and our action alerts contain sample text for you to use and personalize as desired. Physician payment reform, quality improvement, palliative care, and funding levels for the National Institutes of Health and the Agency for Healthcare Research and Quality are just a few of the many issues before the 110th Congress. You can help influence the debate, improve patient care, and increase the visibility of hospitalists by making your voice heard through Capwiz.
Capwiz has many other features that will help keep you informed and educated about the legislative process. These include an interactive map to help you find your elected officials. Simply enter your ZIP code or click on your state to find out who your elected officials are. From there, you can easily select one of the listed state or congressional officials to see the full legislative biography page. Each bio page includes direct links you can use to contact the legislator, look up his or her key votes, and find staff contact information. You’ll also find a plethora of information about each elected representative, including:
- Office term;
- Co-sponsorship status;
- Contact information;
- Party affiliation;
- Political background;
- Committee(s); and
- PAC contributions.
Are you interested in looking up a piece of legislation recently mentioned in the news? Or do you want to monitor the various bills that will affect issues important to your practice? The “Legislative Action Center” in the “Advocacy” section contains “Issues and Legislation” to help you to stay on top of current legislation affecting healthcare. Keep track of any bill’s name, summary, co-sponsor(s), and key votes, while also monitoring SHM’s position on the legislation.
SHM is pleased to provide you with the opportunity to become more familiar with the political process and actively participate in influencing the policies that affect hospitalists and their patients. Capwiz contains up-to-the-minute legislative data and online tools to enable you to make a difference in the political process. Please visit our Legislative Action Center today at www.hospitalmedicine.org. TH
A response to the needs:
Products and Initiatives
- Annual Awards
- Antimicrobial Resistance Resource Room
- Career Center
- Chapters
- Coding and Documentation Precourse
- Collaboration with IHI and Other Organizations
- Core Competencies
- Credentialing by ABIM
- Dashboard White Paper
- Discharge Planning Checklist
- DVT Awareness Campaign
- DVT Mentored Implementation
- e-Newsletter
- Geriatric Resource Room
- Glycemic Control Resource Room
- Heart Failure Resource Room
- Journal of Hospital Medicine
- Leadership Academies
- Legislative Action Day
- Letters to Congress
- List Serves
- Mentoring
- Practice Management Precourse
- Productivity and Compensation Survey
- Public Policy White Paper
- Quality Precourse
- Research Grants
- Resource Rooms
- RIV Competition
- SEPs Workshop
- Stroke Resource Room
- Survey Collaboration with AHA
- The Hospitalist
- Value Added White Paper
- VTE Resource Room
- Web site
A response to the needs:
Committees and Task Forces
- Annual Meeting Committee
- Awards Committee
- Benchmarks Committee
- Board of Directors
- Career Satisfaction Task Force
- Certification Task Force
- Curriculum Task Force
- Education Committee
- Ethics Committee
- Executive Committee of the Board of Directors
- Executive Review Committee
- Family Practice Task Force
- Finance Committee of the Board of Directors
- Hospital Quality and Patient Safety Committee
- Heart Failure Award Task Force
- Leadership Committee
- Membership Committee
- Nominations Committee
- Non Physician Provider Committee
- Palliative Care Task Force
- Pediatric Committee
- Pediatric Core Curriculum Task Force
- Performance and Standards Task Force
- Public Policy Committee
- Research Committee
- Resource Room Oversight Committee
- Research, Innovation, and Clinical Vignettes Committee
- Women in Hospital Medicine
- Young Physicians Committee TH
A Case of Pruritis Rash
History of Present Illness
A55-year-old male presented with a one and one-half week history of a sore throat, shortness of breath on exertion, ankle edema, and arthralgias that began in the ankles and subsequently spread to involve the elbows and wrists.
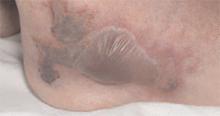
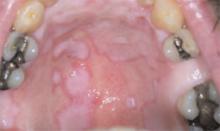
He had also developed a pruritic eruption involving the lower extremities, which consisted of erythematous palpable purpuric lesions and patches with superficial and central necrosis and ulceration, as well as a large 4-cm bulla of the right lateral ankle. (See Figures 1 and 2, below.) Other skin findings included petechiae of the palms and multiple ulcerations of the hard palate. Laboratory evaluation demonstrated c-ANCA antibody positivity (1:512), a proteinase 3 antibody level of greater than 100 U/ml, and a creatinine of 1.0mg/dl. TH
What is the most appropriate treatment for this condition?
- Prednisone;
- Azathioprine;
- Cyclophosphamide;
- Prednisone combined with cyclophosphamide; or
- Vancomycin combined with rifampin
Discussion
The answer is D: Wegener’s granulomatosis (WG) is a chronic granulomatous inflammatory response of unknown etiology that usually presents with the classic triad of systemic vasculitis, necrotizing granulomatous inflammation of the upper and lower respiratory tracts, and glomerulonephritis. The generalized or classic form of WG can progress rapidly to cause irreversible organ dysfunction and death. Although the pathogenesis remains unknown, it is felt that WG may result from an exaggerated cell-mediated response to an unknown antigen.1
The average age of onset for WG is 45.2 years, with 63.5% of patients male and 91% Caucasian.2 A WG diagnosis can be very difficult, and elements of the classic triad may not all be present initially. Pulmonary infiltrates or nodules are seen via chest X-ray or CT scan in just less than half of patients as an early manifestation of WG.
Occasionally WG presents with skin lesions (13%) or oral ulcers (6%), however, 40% of patients eventually develop skin involvement consisting of painful subcutaneous nodules, papules, vesicles or bullae, petechiae, palpable purpura, and pyoderma gangrenosum-like lesions.3 Histologic evaluation of these skin lesions reveals non-specific perivascular lymphocytic inflammation, leukocytoclastic vasculitis-like changes, palisading granulomas, and granulomatous vasculitis; however, it is rare to see granulomatous vasculitis or palisading necrotizing granulomas in skin specimens.4,5
WG can also affect the eyes, heart, respiratory system, nervous system, kidneys, and joints.6 The upper respiratory tract is involved in the majority of patients, and symptoms reflecting otitis, epistaxis, rhinorrhea, or sinusitis are common and may be the first manifestation of disease. When mucosal necrotizing granulomas occur, they can result in the typical saddle nose deformity seen in patients with WG. Lower respiratory tract involvement is also common and can present with cough, dyspnea, chest pain, and hemoptysis.1
Patients with WG usually have a positive c-ANCA, however this is not specific for WG and may also indicate Churg-Strauss Syndrome and microscopic polyarteritis. The median survival of patients with untreated WG is five months, and corticosteroids used alone do not change this median survival. When corticosteroids are combined with cytotoxic agents, such as cyclophosphamide, the prognosis significantly improves in greater than 90% of patients, with a 75% remission rate, and an 87% survival of patients followed from six months to 24 years.3 TH
References
- Hannon CW, Swerlick RA. Vasculitis. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Vol 1. New York: Elsevier Limited; 2003: 393-395.
- Cotch MF, Hoffman GS, Yerg DE, et al. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum. 1996 Jan;39(1):87-92.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. [see comments]. Ann Int Med. 1992;116:488-498.
- Hu CH, O’Loughlin S, Winkelmann RK. Cutaneous manifestations of Wegener granulomatosis. Arch Dermatol. 1997;113(2):175-182.
- Lie JT. Wegener’s granulomatosis: histological documentation of common and uncommon manifestations in 216 patients. Vasa. 1997;26:261-270.
- Yi ES, Colby TV. Wegener’s granulomatosis. Semin Diagn Pathol. 2001 Feb;18(1):34-46.
History of Present Illness
A55-year-old male presented with a one and one-half week history of a sore throat, shortness of breath on exertion, ankle edema, and arthralgias that began in the ankles and subsequently spread to involve the elbows and wrists.
He had also developed a pruritic eruption involving the lower extremities, which consisted of erythematous palpable purpuric lesions and patches with superficial and central necrosis and ulceration, as well as a large 4-cm bulla of the right lateral ankle. (See Figures 1 and 2, below.) Other skin findings included petechiae of the palms and multiple ulcerations of the hard palate. Laboratory evaluation demonstrated c-ANCA antibody positivity (1:512), a proteinase 3 antibody level of greater than 100 U/ml, and a creatinine of 1.0mg/dl. TH
What is the most appropriate treatment for this condition?
- Prednisone;
- Azathioprine;
- Cyclophosphamide;
- Prednisone combined with cyclophosphamide; or
- Vancomycin combined with rifampin
Discussion
The answer is D: Wegener’s granulomatosis (WG) is a chronic granulomatous inflammatory response of unknown etiology that usually presents with the classic triad of systemic vasculitis, necrotizing granulomatous inflammation of the upper and lower respiratory tracts, and glomerulonephritis. The generalized or classic form of WG can progress rapidly to cause irreversible organ dysfunction and death. Although the pathogenesis remains unknown, it is felt that WG may result from an exaggerated cell-mediated response to an unknown antigen.1
The average age of onset for WG is 45.2 years, with 63.5% of patients male and 91% Caucasian.2 A WG diagnosis can be very difficult, and elements of the classic triad may not all be present initially. Pulmonary infiltrates or nodules are seen via chest X-ray or CT scan in just less than half of patients as an early manifestation of WG.
Occasionally WG presents with skin lesions (13%) or oral ulcers (6%), however, 40% of patients eventually develop skin involvement consisting of painful subcutaneous nodules, papules, vesicles or bullae, petechiae, palpable purpura, and pyoderma gangrenosum-like lesions.3 Histologic evaluation of these skin lesions reveals non-specific perivascular lymphocytic inflammation, leukocytoclastic vasculitis-like changes, palisading granulomas, and granulomatous vasculitis; however, it is rare to see granulomatous vasculitis or palisading necrotizing granulomas in skin specimens.4,5
WG can also affect the eyes, heart, respiratory system, nervous system, kidneys, and joints.6 The upper respiratory tract is involved in the majority of patients, and symptoms reflecting otitis, epistaxis, rhinorrhea, or sinusitis are common and may be the first manifestation of disease. When mucosal necrotizing granulomas occur, they can result in the typical saddle nose deformity seen in patients with WG. Lower respiratory tract involvement is also common and can present with cough, dyspnea, chest pain, and hemoptysis.1
Patients with WG usually have a positive c-ANCA, however this is not specific for WG and may also indicate Churg-Strauss Syndrome and microscopic polyarteritis. The median survival of patients with untreated WG is five months, and corticosteroids used alone do not change this median survival. When corticosteroids are combined with cytotoxic agents, such as cyclophosphamide, the prognosis significantly improves in greater than 90% of patients, with a 75% remission rate, and an 87% survival of patients followed from six months to 24 years.3 TH
References
- Hannon CW, Swerlick RA. Vasculitis. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Vol 1. New York: Elsevier Limited; 2003: 393-395.
- Cotch MF, Hoffman GS, Yerg DE, et al. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum. 1996 Jan;39(1):87-92.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. [see comments]. Ann Int Med. 1992;116:488-498.
- Hu CH, O’Loughlin S, Winkelmann RK. Cutaneous manifestations of Wegener granulomatosis. Arch Dermatol. 1997;113(2):175-182.
- Lie JT. Wegener’s granulomatosis: histological documentation of common and uncommon manifestations in 216 patients. Vasa. 1997;26:261-270.
- Yi ES, Colby TV. Wegener’s granulomatosis. Semin Diagn Pathol. 2001 Feb;18(1):34-46.
History of Present Illness
A55-year-old male presented with a one and one-half week history of a sore throat, shortness of breath on exertion, ankle edema, and arthralgias that began in the ankles and subsequently spread to involve the elbows and wrists.
He had also developed a pruritic eruption involving the lower extremities, which consisted of erythematous palpable purpuric lesions and patches with superficial and central necrosis and ulceration, as well as a large 4-cm bulla of the right lateral ankle. (See Figures 1 and 2, below.) Other skin findings included petechiae of the palms and multiple ulcerations of the hard palate. Laboratory evaluation demonstrated c-ANCA antibody positivity (1:512), a proteinase 3 antibody level of greater than 100 U/ml, and a creatinine of 1.0mg/dl. TH
What is the most appropriate treatment for this condition?
- Prednisone;
- Azathioprine;
- Cyclophosphamide;
- Prednisone combined with cyclophosphamide; or
- Vancomycin combined with rifampin
Discussion
The answer is D: Wegener’s granulomatosis (WG) is a chronic granulomatous inflammatory response of unknown etiology that usually presents with the classic triad of systemic vasculitis, necrotizing granulomatous inflammation of the upper and lower respiratory tracts, and glomerulonephritis. The generalized or classic form of WG can progress rapidly to cause irreversible organ dysfunction and death. Although the pathogenesis remains unknown, it is felt that WG may result from an exaggerated cell-mediated response to an unknown antigen.1
The average age of onset for WG is 45.2 years, with 63.5% of patients male and 91% Caucasian.2 A WG diagnosis can be very difficult, and elements of the classic triad may not all be present initially. Pulmonary infiltrates or nodules are seen via chest X-ray or CT scan in just less than half of patients as an early manifestation of WG.
Occasionally WG presents with skin lesions (13%) or oral ulcers (6%), however, 40% of patients eventually develop skin involvement consisting of painful subcutaneous nodules, papules, vesicles or bullae, petechiae, palpable purpura, and pyoderma gangrenosum-like lesions.3 Histologic evaluation of these skin lesions reveals non-specific perivascular lymphocytic inflammation, leukocytoclastic vasculitis-like changes, palisading granulomas, and granulomatous vasculitis; however, it is rare to see granulomatous vasculitis or palisading necrotizing granulomas in skin specimens.4,5
WG can also affect the eyes, heart, respiratory system, nervous system, kidneys, and joints.6 The upper respiratory tract is involved in the majority of patients, and symptoms reflecting otitis, epistaxis, rhinorrhea, or sinusitis are common and may be the first manifestation of disease. When mucosal necrotizing granulomas occur, they can result in the typical saddle nose deformity seen in patients with WG. Lower respiratory tract involvement is also common and can present with cough, dyspnea, chest pain, and hemoptysis.1
Patients with WG usually have a positive c-ANCA, however this is not specific for WG and may also indicate Churg-Strauss Syndrome and microscopic polyarteritis. The median survival of patients with untreated WG is five months, and corticosteroids used alone do not change this median survival. When corticosteroids are combined with cytotoxic agents, such as cyclophosphamide, the prognosis significantly improves in greater than 90% of patients, with a 75% remission rate, and an 87% survival of patients followed from six months to 24 years.3 TH
References
- Hannon CW, Swerlick RA. Vasculitis. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Vol 1. New York: Elsevier Limited; 2003: 393-395.
- Cotch MF, Hoffman GS, Yerg DE, et al. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum. 1996 Jan;39(1):87-92.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. [see comments]. Ann Int Med. 1992;116:488-498.
- Hu CH, O’Loughlin S, Winkelmann RK. Cutaneous manifestations of Wegener granulomatosis. Arch Dermatol. 1997;113(2):175-182.
- Lie JT. Wegener’s granulomatosis: histological documentation of common and uncommon manifestations in 216 patients. Vasa. 1997;26:261-270.
- Yi ES, Colby TV. Wegener’s granulomatosis. Semin Diagn Pathol. 2001 Feb;18(1):34-46.
JHM Accepted for Indexing in MEDLINE
In December the Journal of Hospital Medicine (JHM, the peer-reviewed sister publication of The Hospitalist) was selected for indexing and inclusion in the National Library of Medicine’s MEDLINE (Medical Literature Analysis and Retrieval System Online). MEDLINE is a bibliographic database that contains 13 million references to journal articles in medicine, nursing, dentistry, veterinary medicine, healthcare systems, and preclinical sciences. It’s the primary component of PubMed, part of the Entrez series of databases provided by the Library’s National Center for Biotechnology Information (NCBI).
“The Journal’s acceptance is a profound recognition that hospital medicine has developed its own sphere of medical knowledge and that hospitalists are making a significant impact on our modern healthcare delivery system,” says Larry Wellikson, MD, FACP, SHM CEO.
JHM, an official SHM publication, debuted in February 2006 and is the premier forum for peer-reviewed research articles and evidence-based reviews in the specialty of hospital medicine. For more information about JHM, visit www.interscience.wiley.com/journal/jhm.
In December the Journal of Hospital Medicine (JHM, the peer-reviewed sister publication of The Hospitalist) was selected for indexing and inclusion in the National Library of Medicine’s MEDLINE (Medical Literature Analysis and Retrieval System Online). MEDLINE is a bibliographic database that contains 13 million references to journal articles in medicine, nursing, dentistry, veterinary medicine, healthcare systems, and preclinical sciences. It’s the primary component of PubMed, part of the Entrez series of databases provided by the Library’s National Center for Biotechnology Information (NCBI).
“The Journal’s acceptance is a profound recognition that hospital medicine has developed its own sphere of medical knowledge and that hospitalists are making a significant impact on our modern healthcare delivery system,” says Larry Wellikson, MD, FACP, SHM CEO.
JHM, an official SHM publication, debuted in February 2006 and is the premier forum for peer-reviewed research articles and evidence-based reviews in the specialty of hospital medicine. For more information about JHM, visit www.interscience.wiley.com/journal/jhm.
In December the Journal of Hospital Medicine (JHM, the peer-reviewed sister publication of The Hospitalist) was selected for indexing and inclusion in the National Library of Medicine’s MEDLINE (Medical Literature Analysis and Retrieval System Online). MEDLINE is a bibliographic database that contains 13 million references to journal articles in medicine, nursing, dentistry, veterinary medicine, healthcare systems, and preclinical sciences. It’s the primary component of PubMed, part of the Entrez series of databases provided by the Library’s National Center for Biotechnology Information (NCBI).
“The Journal’s acceptance is a profound recognition that hospital medicine has developed its own sphere of medical knowledge and that hospitalists are making a significant impact on our modern healthcare delivery system,” says Larry Wellikson, MD, FACP, SHM CEO.
JHM, an official SHM publication, debuted in February 2006 and is the premier forum for peer-reviewed research articles and evidence-based reviews in the specialty of hospital medicine. For more information about JHM, visit www.interscience.wiley.com/journal/jhm.
A Stake in the Sand
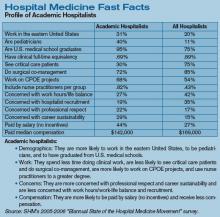
Productivity and compensation benchmarks can be useful when negotiating with hospital administrators for increased reimbursements and support resources, when recruiting hospitalists, and when conducting self-evaluations. For many of these processes, hospitalists—and, indeed, hospital administrators—turn to the information contained in the voluminous SHM 2005-2006 Survey, “The Authoritative Source on the State of the Hospital Medicine Movement.” (See “For More Information,” p. 32.)
With a response rate of 26%, the survey represents some 2,550 hospitalists across the nation, and its variables present a more comprehensive aerial view of hospital medicine than did previous surveys. But on the ground and in the trenches, hospital medicine groups must be careful to look at the survey’s metrics with a discerning eye.
When applying the survey metrics to one’s own practice, there can be benefits as well as pitfalls, cautions Joe Miller, SHM senior vice president and principal analyst of the survey data. He emphasizes the great variation among hospital medicine groups and warns against looking at survey medians as representing a “typical” hospital medicine practice.
“When you’ve seen one hospital medicine group, you’ve seen one hospital medicine group,” he quips. In several recent conversations, hospital medicine group leaders and SHM leaders involved in compiling the survey discussed the survey’s strengths and limitations as a benchmarking tool.
Healthy to Negotiate
According to the survey 97% of hospitalist programs receive some type of financial support. “Virtually every program in the country is challenged to defend the amount of money [they receive] or to negotiate for support dollars,” says Miller, who believes that negotiation can be a healthy dynamic. “There is a sense of equality of both sides of the table, a mutual respect between hospitalists and the hospital.” In the process of such negotiations, it will be important not to pin one’s position entirely to the survey metrics.
John Nelson, MD, medical director of the hospitalist practice at Overlake Hospital in Bellevue, Wash., a consultant for hospitalist practices with Nelson/Flores Associates, a columnist for The Hospitalist (“Practice Management”), and a co-founder and past president of SHM, believes that some hospitalists mistakenly view the survey as SHM’s position on what a hospitalist should make. “The survey is the best information we have about what hospitalists do make—there is no better source—but it’s still a survey.”
Using compensation medians as yardsticks for actual salaries and compensation packages is analogous to “learning the average weight of an American and deciding that’s what we all should weigh—and that’s a big mistake,” he says. “If you hold up the survey as the governing document, then each party will use it to their advantage.”
Because the survey is regarded as the most authoritative existing source on hospitalists’ compensation and productivity, it nevertheless ends up being used as a benchmark, says Robin L. Dauterive, assistant director of the clinical hospitalist service at Massachusetts General Hospital in Boston.
“Whenever I’m preparing billings reports or dashboard measures—anything that shows my group’s workload—sooner or later, I always have to include something in there that states, ‘This is what other people are doing,’ ” says Dauterive. “It’s something that you can’t get away from, unfortunately, in medicine.”
She realizes that the survey does not purport to set any national standards, and yet, “all administrations want comparisons.” Dr. Nelson has also noted this phenomenon with the survey. In the absence of additional guidance, hospital executives and hospitalists often find that they’re just arguing about the survey. “And that’s unfortunate,” he says. “It means they’ve lost sight of the unique attributes of a given practice that might support higher or lower incomes and higher or lower workloads.”
View in Context
Hospitalists reading the survey for the first time might first seek to analyze the metrics regarding billings and collections. Here it is especially important not to view the reported numbers in isolation, says Dr. Nelson. For instance, to learn how a hospitalist’s annual gross charges (billings) compare with others across the country (question 12 of the Individual Hospitalist questionnaire—p. 87, Appendix 2), details on pages 251 and 252 supply pertinent variables. For instance, in comparing the four regions of the country, Table 056-A shows that the median annual gross charges for physicians in the south are highest, at $354,000. Hospitalists compensated by a 100% incentive method report higher charges per year ($392,000) than those who are on a 100% salary or a mix of the two methods of payment. Turning to Table 056-B, on page 252 of the published survey, hospitalists can find annual gross charges according to practitioner type, specialty, and employment model. Hospitalists should not stop their reading there, however, as a comparison of others’ annual gross collections might give a more complete picture.
Still, the SHM Survey does not reference all possible explanatory variables. Collections can be influenced by location and payer mix. Hospitalists practicing in a large urban hospital are likely to see more indigent patients for whom the hospital is not reimbursed. A careful reading of the survey should include the questionnaire and the tables supporting chapter conclusions, and the reader must recognize the survey’s limitations.
Apples to Oranges
IPC–The Hospitalist Company participates in the SHM survey and also uses it as a recruitment tool, reports IPC Vice President of Physician Staffing Timothy Lary. “We look at the income averages, and we’re able to demonstrate how our averages are, for the most part, higher than the averages,” he explains. “We also look at the survey from an internal viewpoint, but oftentimes you are comparing apples to oranges.”
Like individual hospitalists, hospital medicine group leaders seek comparisons when they read the survey. For her part, Dr. Dauterive has found the data on starting salaries for new hospitalists useful. For example, page 259, detailed table 060-A on hospitalists’ compensation by category and total, breaks out median yearly income by years as a hospitalist, from less than a year to six or more years. (Many of the detailed “A” tables in Chapter 8 on compensation include the “years as a hospitalist” category.) Dr. Dauterive praises the wealth of data in the survey, pointing to examples of the many variables she was surprised to learn. One of those factors was that 48% of surveyed hospitalist programs were at non-teaching hospitals. (See page 7 of the survey, Executive Summary, “Teaching status of affiliated hospital.”)
Those interviewed for this article agree that productivity data are probably more telling about the day-to-day clinical realities for hospitalists. Productivity metrics figure prominently in Dr. Dauterive’s uses for the survey. Accordingly, the annual number of billable patient encounters seen by the hospitalist (Table 58-B, page 256) and the annual number of work relative value units (RVUs) worked by the hospitalist (Table 59-B, page 258) caught her interest.
Still, Dr. Dauterive found herself wanting more data to shed light on those numbers. In negotiations for resources with hospital administrators, Dr. Dauterive would like to be able to pinpoint the reasons behind reported numbers of clinical encounters seen by the hospitalist. If the median number of billable patient encounters seen by the hospitalist in a teaching service was 1,668 (based on 107 responses; page 256, Detailed Table 058-B), what were some of the influences on this number? What was the acuity level of patients? Did the hospitalist have group resources, such as physician extenders, to help with patient admissions and rounds?
“For groups that have low lengths of stay, it would be important for me to know why,” she says. “Did they have extra supports? Do their [doctors] use Palm Pilots? You don’t always know from looking at the numbers how to apply them, make the connection, and justify the resources you’re trying to achieve,” she says.
No Perfect Measure
The ideal survey for Dr. Dauterive would include specific structured models, providing links between categories so that she could compare characteristics that more closely align with her group’s situation.
“Our program is very mixed, so it would be helpful for me to know how work RVUs were being reported,” she says. Pointing to results showing higher productivity (work RVUs) in practices compensated by 100% incentive (Table 060-A, page 259 of the survey), Dr. Dauterive wonders what factors drive these results. While the 100% incentive might appear to be the most important factor, perhaps these groups also have physician extenders or are located in a geographic location that boosts their productivity.
“I’m in a nonprofit hospital, in a clinical hospitalist service, and I want to be able to approach the administration and say, ‘If you want us to see the most patients, these are the kinds of services that see the most patients,’ ”says Dr. Dauterive. “But, if you are more interested in physician retention and work/life, then these are the characteristics of those successful programs.”
This level of detail can be difficult to interpolate from the survey, agrees Dr. Nelson. Patient acuity, for instance, is not specifically queried in the survey questionnaire. “I agree, in the ideal world, this is all information that you would want to know,” he says. Answers to the following questions could help refine product metrics:
- Does your group have teaching responsibilities for residents?
- Do you take a lot of calls from home, or do you have a separate night shift?
- Do you cover more than one physical hospital on the same day?
- Does your group do more than the typical amount of committee and administrative work?
“All these factors,” notes Dr. Nelson, “would influence productivity. There is no perfect way to know the answer to any of those things.” And, he adds, the survey already comprises 292 pages, including numerous detailed tables of data. To include all pertinent variables would entail a longer questionnaire, which might affect the response rate.
Healthcare Delivery Is Local
In his consultations with hospitalist groups, Dr. Nelson always emphasizes that the survey is “a starting point” and not the goal of what hospitalists should make. He favors adjunctive methods for benchmarking practices: “I think that when you’re benchmarking your practice, it’s as important to gather as much local and regional data as you can—in addition to the SHM survey.” He tries to network with other Seattle hospitalist programs to learn about their patterns of work hours, patient loads, and the like.
Thomas Baudendistel, MD, FACP, associate program director of the Internal Medicine Residency Program at Sutter Health’s California Pacific Medical Center in San Francisco, notes that regional markets differ widely. The healthcare market in the Northern California Bay area is very different from the one in Los Angeles in terms of financial remuneration and incentivization.
“The survey,” he says, “gives a global gestalt of the regional flavor of hospital medicine” and reveals general ballpark medians that can be a good starting point for practice benchmarking. “I think what our administration [at California Pacific Medical Center] wants to see is our data compared to the people across the street and down the road, because that’s a closer comparison in terms of payer mix and insurance reimbursements.”
IPC’s Lary agrees. “When I compete, I don’t compete against people across the country; I compete with people across the street,” he says. “As large as IPC is, we realize that healthcare is delivered locally. What we try to do [with the survey] is take the information and, to the best or our ability, figure out how it applies to our individual settings and [to the] different markets that we are in.”
A Stake in the Sand
“I think the benchmarks we have in the survey are just a piece of information—[the survey] is a context, it’s a stake in the sand,” concludes Miller. “We do have variations by type of program, by size of hospital, by geographic location, by size of program. There are numbers for each one of those, and you can clue in as to what some of the more important variations are. We could list probably 25 to 50 variables that would affect hospitalists’ productivity in one way or the other—and that’s not taking into account the individual styles of hospitalists.”
For instance, some hospitalists want to work and earn as much money as possible, while others are searching for a work/life balance that will allow them time with their families.
The survey, says Lary, supplies a piece of information in a complex puzzle about a highly variable profession. “There are so many different ways this business is being conducted right now,” he says. “One medical community may be willing to subsidize a hospital medicine program, and another may not be willing.”
Hospitalists’ professional goals vary widely as well. As far as Dr. Nelson is concerned, the bottom line for hospitalists is to structure independent practices tailored to fit their goals. This means that hospitalists are connected to the economic consequences of their staffing and workload decisions. In that way, he says, rather than approaching administrators about hiring more physicians, the practice itself can decide whether it is worth the decrease in individual hospitalists’ incomes to hire another doctor.
Because their specialty is still evolving, hospitalists will find themselves educating their clients about the profession’s services and advantages. And for that process, the survey can be a helpful adjunct. Miller agrees that the use of the survey requires a certain amount of interpolation on the part of hospitalist leaders. They should be careful, he emphasizes, not to lose sight of the individuality of their own practices.
“If you hold up the survey as the governing document when you negotiate with your hospital, then each party will use it to their advantage,” says Dr. Nelson. “This can push you towards being ‘average’ when that might not be appropriate for your practice.” TH
Gretchen Henkel is a frequent contributor to The Hospitalist.
Productivity and compensation benchmarks can be useful when negotiating with hospital administrators for increased reimbursements and support resources, when recruiting hospitalists, and when conducting self-evaluations. For many of these processes, hospitalists—and, indeed, hospital administrators—turn to the information contained in the voluminous SHM 2005-2006 Survey, “The Authoritative Source on the State of the Hospital Medicine Movement.” (See “For More Information,” p. 32.)
With a response rate of 26%, the survey represents some 2,550 hospitalists across the nation, and its variables present a more comprehensive aerial view of hospital medicine than did previous surveys. But on the ground and in the trenches, hospital medicine groups must be careful to look at the survey’s metrics with a discerning eye.
When applying the survey metrics to one’s own practice, there can be benefits as well as pitfalls, cautions Joe Miller, SHM senior vice president and principal analyst of the survey data. He emphasizes the great variation among hospital medicine groups and warns against looking at survey medians as representing a “typical” hospital medicine practice.
“When you’ve seen one hospital medicine group, you’ve seen one hospital medicine group,” he quips. In several recent conversations, hospital medicine group leaders and SHM leaders involved in compiling the survey discussed the survey’s strengths and limitations as a benchmarking tool.
Healthy to Negotiate
According to the survey 97% of hospitalist programs receive some type of financial support. “Virtually every program in the country is challenged to defend the amount of money [they receive] or to negotiate for support dollars,” says Miller, who believes that negotiation can be a healthy dynamic. “There is a sense of equality of both sides of the table, a mutual respect between hospitalists and the hospital.” In the process of such negotiations, it will be important not to pin one’s position entirely to the survey metrics.
John Nelson, MD, medical director of the hospitalist practice at Overlake Hospital in Bellevue, Wash., a consultant for hospitalist practices with Nelson/Flores Associates, a columnist for The Hospitalist (“Practice Management”), and a co-founder and past president of SHM, believes that some hospitalists mistakenly view the survey as SHM’s position on what a hospitalist should make. “The survey is the best information we have about what hospitalists do make—there is no better source—but it’s still a survey.”
Using compensation medians as yardsticks for actual salaries and compensation packages is analogous to “learning the average weight of an American and deciding that’s what we all should weigh—and that’s a big mistake,” he says. “If you hold up the survey as the governing document, then each party will use it to their advantage.”
Because the survey is regarded as the most authoritative existing source on hospitalists’ compensation and productivity, it nevertheless ends up being used as a benchmark, says Robin L. Dauterive, assistant director of the clinical hospitalist service at Massachusetts General Hospital in Boston.
“Whenever I’m preparing billings reports or dashboard measures—anything that shows my group’s workload—sooner or later, I always have to include something in there that states, ‘This is what other people are doing,’ ” says Dauterive. “It’s something that you can’t get away from, unfortunately, in medicine.”
She realizes that the survey does not purport to set any national standards, and yet, “all administrations want comparisons.” Dr. Nelson has also noted this phenomenon with the survey. In the absence of additional guidance, hospital executives and hospitalists often find that they’re just arguing about the survey. “And that’s unfortunate,” he says. “It means they’ve lost sight of the unique attributes of a given practice that might support higher or lower incomes and higher or lower workloads.”
View in Context
Hospitalists reading the survey for the first time might first seek to analyze the metrics regarding billings and collections. Here it is especially important not to view the reported numbers in isolation, says Dr. Nelson. For instance, to learn how a hospitalist’s annual gross charges (billings) compare with others across the country (question 12 of the Individual Hospitalist questionnaire—p. 87, Appendix 2), details on pages 251 and 252 supply pertinent variables. For instance, in comparing the four regions of the country, Table 056-A shows that the median annual gross charges for physicians in the south are highest, at $354,000. Hospitalists compensated by a 100% incentive method report higher charges per year ($392,000) than those who are on a 100% salary or a mix of the two methods of payment. Turning to Table 056-B, on page 252 of the published survey, hospitalists can find annual gross charges according to practitioner type, specialty, and employment model. Hospitalists should not stop their reading there, however, as a comparison of others’ annual gross collections might give a more complete picture.
Still, the SHM Survey does not reference all possible explanatory variables. Collections can be influenced by location and payer mix. Hospitalists practicing in a large urban hospital are likely to see more indigent patients for whom the hospital is not reimbursed. A careful reading of the survey should include the questionnaire and the tables supporting chapter conclusions, and the reader must recognize the survey’s limitations.
Apples to Oranges
IPC–The Hospitalist Company participates in the SHM survey and also uses it as a recruitment tool, reports IPC Vice President of Physician Staffing Timothy Lary. “We look at the income averages, and we’re able to demonstrate how our averages are, for the most part, higher than the averages,” he explains. “We also look at the survey from an internal viewpoint, but oftentimes you are comparing apples to oranges.”
Like individual hospitalists, hospital medicine group leaders seek comparisons when they read the survey. For her part, Dr. Dauterive has found the data on starting salaries for new hospitalists useful. For example, page 259, detailed table 060-A on hospitalists’ compensation by category and total, breaks out median yearly income by years as a hospitalist, from less than a year to six or more years. (Many of the detailed “A” tables in Chapter 8 on compensation include the “years as a hospitalist” category.) Dr. Dauterive praises the wealth of data in the survey, pointing to examples of the many variables she was surprised to learn. One of those factors was that 48% of surveyed hospitalist programs were at non-teaching hospitals. (See page 7 of the survey, Executive Summary, “Teaching status of affiliated hospital.”)
Those interviewed for this article agree that productivity data are probably more telling about the day-to-day clinical realities for hospitalists. Productivity metrics figure prominently in Dr. Dauterive’s uses for the survey. Accordingly, the annual number of billable patient encounters seen by the hospitalist (Table 58-B, page 256) and the annual number of work relative value units (RVUs) worked by the hospitalist (Table 59-B, page 258) caught her interest.
Still, Dr. Dauterive found herself wanting more data to shed light on those numbers. In negotiations for resources with hospital administrators, Dr. Dauterive would like to be able to pinpoint the reasons behind reported numbers of clinical encounters seen by the hospitalist. If the median number of billable patient encounters seen by the hospitalist in a teaching service was 1,668 (based on 107 responses; page 256, Detailed Table 058-B), what were some of the influences on this number? What was the acuity level of patients? Did the hospitalist have group resources, such as physician extenders, to help with patient admissions and rounds?
“For groups that have low lengths of stay, it would be important for me to know why,” she says. “Did they have extra supports? Do their [doctors] use Palm Pilots? You don’t always know from looking at the numbers how to apply them, make the connection, and justify the resources you’re trying to achieve,” she says.
No Perfect Measure
The ideal survey for Dr. Dauterive would include specific structured models, providing links between categories so that she could compare characteristics that more closely align with her group’s situation.
“Our program is very mixed, so it would be helpful for me to know how work RVUs were being reported,” she says. Pointing to results showing higher productivity (work RVUs) in practices compensated by 100% incentive (Table 060-A, page 259 of the survey), Dr. Dauterive wonders what factors drive these results. While the 100% incentive might appear to be the most important factor, perhaps these groups also have physician extenders or are located in a geographic location that boosts their productivity.
“I’m in a nonprofit hospital, in a clinical hospitalist service, and I want to be able to approach the administration and say, ‘If you want us to see the most patients, these are the kinds of services that see the most patients,’ ”says Dr. Dauterive. “But, if you are more interested in physician retention and work/life, then these are the characteristics of those successful programs.”
This level of detail can be difficult to interpolate from the survey, agrees Dr. Nelson. Patient acuity, for instance, is not specifically queried in the survey questionnaire. “I agree, in the ideal world, this is all information that you would want to know,” he says. Answers to the following questions could help refine product metrics:
- Does your group have teaching responsibilities for residents?
- Do you take a lot of calls from home, or do you have a separate night shift?
- Do you cover more than one physical hospital on the same day?
- Does your group do more than the typical amount of committee and administrative work?
“All these factors,” notes Dr. Nelson, “would influence productivity. There is no perfect way to know the answer to any of those things.” And, he adds, the survey already comprises 292 pages, including numerous detailed tables of data. To include all pertinent variables would entail a longer questionnaire, which might affect the response rate.
Healthcare Delivery Is Local
In his consultations with hospitalist groups, Dr. Nelson always emphasizes that the survey is “a starting point” and not the goal of what hospitalists should make. He favors adjunctive methods for benchmarking practices: “I think that when you’re benchmarking your practice, it’s as important to gather as much local and regional data as you can—in addition to the SHM survey.” He tries to network with other Seattle hospitalist programs to learn about their patterns of work hours, patient loads, and the like.
Thomas Baudendistel, MD, FACP, associate program director of the Internal Medicine Residency Program at Sutter Health’s California Pacific Medical Center in San Francisco, notes that regional markets differ widely. The healthcare market in the Northern California Bay area is very different from the one in Los Angeles in terms of financial remuneration and incentivization.
“The survey,” he says, “gives a global gestalt of the regional flavor of hospital medicine” and reveals general ballpark medians that can be a good starting point for practice benchmarking. “I think what our administration [at California Pacific Medical Center] wants to see is our data compared to the people across the street and down the road, because that’s a closer comparison in terms of payer mix and insurance reimbursements.”
IPC’s Lary agrees. “When I compete, I don’t compete against people across the country; I compete with people across the street,” he says. “As large as IPC is, we realize that healthcare is delivered locally. What we try to do [with the survey] is take the information and, to the best or our ability, figure out how it applies to our individual settings and [to the] different markets that we are in.”
A Stake in the Sand
“I think the benchmarks we have in the survey are just a piece of information—[the survey] is a context, it’s a stake in the sand,” concludes Miller. “We do have variations by type of program, by size of hospital, by geographic location, by size of program. There are numbers for each one of those, and you can clue in as to what some of the more important variations are. We could list probably 25 to 50 variables that would affect hospitalists’ productivity in one way or the other—and that’s not taking into account the individual styles of hospitalists.”
For instance, some hospitalists want to work and earn as much money as possible, while others are searching for a work/life balance that will allow them time with their families.
The survey, says Lary, supplies a piece of information in a complex puzzle about a highly variable profession. “There are so many different ways this business is being conducted right now,” he says. “One medical community may be willing to subsidize a hospital medicine program, and another may not be willing.”
Hospitalists’ professional goals vary widely as well. As far as Dr. Nelson is concerned, the bottom line for hospitalists is to structure independent practices tailored to fit their goals. This means that hospitalists are connected to the economic consequences of their staffing and workload decisions. In that way, he says, rather than approaching administrators about hiring more physicians, the practice itself can decide whether it is worth the decrease in individual hospitalists’ incomes to hire another doctor.
Because their specialty is still evolving, hospitalists will find themselves educating their clients about the profession’s services and advantages. And for that process, the survey can be a helpful adjunct. Miller agrees that the use of the survey requires a certain amount of interpolation on the part of hospitalist leaders. They should be careful, he emphasizes, not to lose sight of the individuality of their own practices.
“If you hold up the survey as the governing document when you negotiate with your hospital, then each party will use it to their advantage,” says Dr. Nelson. “This can push you towards being ‘average’ when that might not be appropriate for your practice.” TH
Gretchen Henkel is a frequent contributor to The Hospitalist.
Productivity and compensation benchmarks can be useful when negotiating with hospital administrators for increased reimbursements and support resources, when recruiting hospitalists, and when conducting self-evaluations. For many of these processes, hospitalists—and, indeed, hospital administrators—turn to the information contained in the voluminous SHM 2005-2006 Survey, “The Authoritative Source on the State of the Hospital Medicine Movement.” (See “For More Information,” p. 32.)
With a response rate of 26%, the survey represents some 2,550 hospitalists across the nation, and its variables present a more comprehensive aerial view of hospital medicine than did previous surveys. But on the ground and in the trenches, hospital medicine groups must be careful to look at the survey’s metrics with a discerning eye.
When applying the survey metrics to one’s own practice, there can be benefits as well as pitfalls, cautions Joe Miller, SHM senior vice president and principal analyst of the survey data. He emphasizes the great variation among hospital medicine groups and warns against looking at survey medians as representing a “typical” hospital medicine practice.
“When you’ve seen one hospital medicine group, you’ve seen one hospital medicine group,” he quips. In several recent conversations, hospital medicine group leaders and SHM leaders involved in compiling the survey discussed the survey’s strengths and limitations as a benchmarking tool.
Healthy to Negotiate
According to the survey 97% of hospitalist programs receive some type of financial support. “Virtually every program in the country is challenged to defend the amount of money [they receive] or to negotiate for support dollars,” says Miller, who believes that negotiation can be a healthy dynamic. “There is a sense of equality of both sides of the table, a mutual respect between hospitalists and the hospital.” In the process of such negotiations, it will be important not to pin one’s position entirely to the survey metrics.
John Nelson, MD, medical director of the hospitalist practice at Overlake Hospital in Bellevue, Wash., a consultant for hospitalist practices with Nelson/Flores Associates, a columnist for The Hospitalist (“Practice Management”), and a co-founder and past president of SHM, believes that some hospitalists mistakenly view the survey as SHM’s position on what a hospitalist should make. “The survey is the best information we have about what hospitalists do make—there is no better source—but it’s still a survey.”
Using compensation medians as yardsticks for actual salaries and compensation packages is analogous to “learning the average weight of an American and deciding that’s what we all should weigh—and that’s a big mistake,” he says. “If you hold up the survey as the governing document, then each party will use it to their advantage.”
Because the survey is regarded as the most authoritative existing source on hospitalists’ compensation and productivity, it nevertheless ends up being used as a benchmark, says Robin L. Dauterive, assistant director of the clinical hospitalist service at Massachusetts General Hospital in Boston.
“Whenever I’m preparing billings reports or dashboard measures—anything that shows my group’s workload—sooner or later, I always have to include something in there that states, ‘This is what other people are doing,’ ” says Dauterive. “It’s something that you can’t get away from, unfortunately, in medicine.”
She realizes that the survey does not purport to set any national standards, and yet, “all administrations want comparisons.” Dr. Nelson has also noted this phenomenon with the survey. In the absence of additional guidance, hospital executives and hospitalists often find that they’re just arguing about the survey. “And that’s unfortunate,” he says. “It means they’ve lost sight of the unique attributes of a given practice that might support higher or lower incomes and higher or lower workloads.”
View in Context
Hospitalists reading the survey for the first time might first seek to analyze the metrics regarding billings and collections. Here it is especially important not to view the reported numbers in isolation, says Dr. Nelson. For instance, to learn how a hospitalist’s annual gross charges (billings) compare with others across the country (question 12 of the Individual Hospitalist questionnaire—p. 87, Appendix 2), details on pages 251 and 252 supply pertinent variables. For instance, in comparing the four regions of the country, Table 056-A shows that the median annual gross charges for physicians in the south are highest, at $354,000. Hospitalists compensated by a 100% incentive method report higher charges per year ($392,000) than those who are on a 100% salary or a mix of the two methods of payment. Turning to Table 056-B, on page 252 of the published survey, hospitalists can find annual gross charges according to practitioner type, specialty, and employment model. Hospitalists should not stop their reading there, however, as a comparison of others’ annual gross collections might give a more complete picture.
Still, the SHM Survey does not reference all possible explanatory variables. Collections can be influenced by location and payer mix. Hospitalists practicing in a large urban hospital are likely to see more indigent patients for whom the hospital is not reimbursed. A careful reading of the survey should include the questionnaire and the tables supporting chapter conclusions, and the reader must recognize the survey’s limitations.
Apples to Oranges
IPC–The Hospitalist Company participates in the SHM survey and also uses it as a recruitment tool, reports IPC Vice President of Physician Staffing Timothy Lary. “We look at the income averages, and we’re able to demonstrate how our averages are, for the most part, higher than the averages,” he explains. “We also look at the survey from an internal viewpoint, but oftentimes you are comparing apples to oranges.”
Like individual hospitalists, hospital medicine group leaders seek comparisons when they read the survey. For her part, Dr. Dauterive has found the data on starting salaries for new hospitalists useful. For example, page 259, detailed table 060-A on hospitalists’ compensation by category and total, breaks out median yearly income by years as a hospitalist, from less than a year to six or more years. (Many of the detailed “A” tables in Chapter 8 on compensation include the “years as a hospitalist” category.) Dr. Dauterive praises the wealth of data in the survey, pointing to examples of the many variables she was surprised to learn. One of those factors was that 48% of surveyed hospitalist programs were at non-teaching hospitals. (See page 7 of the survey, Executive Summary, “Teaching status of affiliated hospital.”)
Those interviewed for this article agree that productivity data are probably more telling about the day-to-day clinical realities for hospitalists. Productivity metrics figure prominently in Dr. Dauterive’s uses for the survey. Accordingly, the annual number of billable patient encounters seen by the hospitalist (Table 58-B, page 256) and the annual number of work relative value units (RVUs) worked by the hospitalist (Table 59-B, page 258) caught her interest.
Still, Dr. Dauterive found herself wanting more data to shed light on those numbers. In negotiations for resources with hospital administrators, Dr. Dauterive would like to be able to pinpoint the reasons behind reported numbers of clinical encounters seen by the hospitalist. If the median number of billable patient encounters seen by the hospitalist in a teaching service was 1,668 (based on 107 responses; page 256, Detailed Table 058-B), what were some of the influences on this number? What was the acuity level of patients? Did the hospitalist have group resources, such as physician extenders, to help with patient admissions and rounds?
“For groups that have low lengths of stay, it would be important for me to know why,” she says. “Did they have extra supports? Do their [doctors] use Palm Pilots? You don’t always know from looking at the numbers how to apply them, make the connection, and justify the resources you’re trying to achieve,” she says.
No Perfect Measure
The ideal survey for Dr. Dauterive would include specific structured models, providing links between categories so that she could compare characteristics that more closely align with her group’s situation.
“Our program is very mixed, so it would be helpful for me to know how work RVUs were being reported,” she says. Pointing to results showing higher productivity (work RVUs) in practices compensated by 100% incentive (Table 060-A, page 259 of the survey), Dr. Dauterive wonders what factors drive these results. While the 100% incentive might appear to be the most important factor, perhaps these groups also have physician extenders or are located in a geographic location that boosts their productivity.
“I’m in a nonprofit hospital, in a clinical hospitalist service, and I want to be able to approach the administration and say, ‘If you want us to see the most patients, these are the kinds of services that see the most patients,’ ”says Dr. Dauterive. “But, if you are more interested in physician retention and work/life, then these are the characteristics of those successful programs.”
This level of detail can be difficult to interpolate from the survey, agrees Dr. Nelson. Patient acuity, for instance, is not specifically queried in the survey questionnaire. “I agree, in the ideal world, this is all information that you would want to know,” he says. Answers to the following questions could help refine product metrics:
- Does your group have teaching responsibilities for residents?
- Do you take a lot of calls from home, or do you have a separate night shift?
- Do you cover more than one physical hospital on the same day?
- Does your group do more than the typical amount of committee and administrative work?
“All these factors,” notes Dr. Nelson, “would influence productivity. There is no perfect way to know the answer to any of those things.” And, he adds, the survey already comprises 292 pages, including numerous detailed tables of data. To include all pertinent variables would entail a longer questionnaire, which might affect the response rate.
Healthcare Delivery Is Local
In his consultations with hospitalist groups, Dr. Nelson always emphasizes that the survey is “a starting point” and not the goal of what hospitalists should make. He favors adjunctive methods for benchmarking practices: “I think that when you’re benchmarking your practice, it’s as important to gather as much local and regional data as you can—in addition to the SHM survey.” He tries to network with other Seattle hospitalist programs to learn about their patterns of work hours, patient loads, and the like.
Thomas Baudendistel, MD, FACP, associate program director of the Internal Medicine Residency Program at Sutter Health’s California Pacific Medical Center in San Francisco, notes that regional markets differ widely. The healthcare market in the Northern California Bay area is very different from the one in Los Angeles in terms of financial remuneration and incentivization.
“The survey,” he says, “gives a global gestalt of the regional flavor of hospital medicine” and reveals general ballpark medians that can be a good starting point for practice benchmarking. “I think what our administration [at California Pacific Medical Center] wants to see is our data compared to the people across the street and down the road, because that’s a closer comparison in terms of payer mix and insurance reimbursements.”
IPC’s Lary agrees. “When I compete, I don’t compete against people across the country; I compete with people across the street,” he says. “As large as IPC is, we realize that healthcare is delivered locally. What we try to do [with the survey] is take the information and, to the best or our ability, figure out how it applies to our individual settings and [to the] different markets that we are in.”
A Stake in the Sand
“I think the benchmarks we have in the survey are just a piece of information—[the survey] is a context, it’s a stake in the sand,” concludes Miller. “We do have variations by type of program, by size of hospital, by geographic location, by size of program. There are numbers for each one of those, and you can clue in as to what some of the more important variations are. We could list probably 25 to 50 variables that would affect hospitalists’ productivity in one way or the other—and that’s not taking into account the individual styles of hospitalists.”
For instance, some hospitalists want to work and earn as much money as possible, while others are searching for a work/life balance that will allow them time with their families.
The survey, says Lary, supplies a piece of information in a complex puzzle about a highly variable profession. “There are so many different ways this business is being conducted right now,” he says. “One medical community may be willing to subsidize a hospital medicine program, and another may not be willing.”
Hospitalists’ professional goals vary widely as well. As far as Dr. Nelson is concerned, the bottom line for hospitalists is to structure independent practices tailored to fit their goals. This means that hospitalists are connected to the economic consequences of their staffing and workload decisions. In that way, he says, rather than approaching administrators about hiring more physicians, the practice itself can decide whether it is worth the decrease in individual hospitalists’ incomes to hire another doctor.
Because their specialty is still evolving, hospitalists will find themselves educating their clients about the profession’s services and advantages. And for that process, the survey can be a helpful adjunct. Miller agrees that the use of the survey requires a certain amount of interpolation on the part of hospitalist leaders. They should be careful, he emphasizes, not to lose sight of the individuality of their own practices.
“If you hold up the survey as the governing document when you negotiate with your hospital, then each party will use it to their advantage,” says Dr. Nelson. “This can push you towards being ‘average’ when that might not be appropriate for your practice.” TH
Gretchen Henkel is a frequent contributor to The Hospitalist.
Dabigatran can prevent thromboembolism
ORLANDO—A new oral anticoagulant in development, dabigatran etexilate, was shown to be as effective and safe as the low-molecular-weight enoxaparin in prophylaxis of thromboembolism in patients undergoing total knee replacement surgery.
Bengt I. Eriksson, MD, reported these results on behalf of the RE-MODEL study group during the 2006 annual meeting of the American Society of Hematology in December.
The RE-MODEL study was a large multicenter study conducted in 2010 patients undergoing elective total knee surgery in Europe, South Africa, and Australia.
Patients were randomized to receive subcutaneous injections of enoxaparin (40 mg, once daily) or to one of 2 doses of oral dabigatran etexilate (150 mg or 220 mg once daily) for 8 ± 2 days. Enoxaparin was administered 12 hours prior to surgery while dabigatran was administered 1-4 hours after the surgery.
Dabigatran etexilate met the prespecified primary endpoint of noninferiority versus enoxaparin. There was no difference in the incidence of total venous thromboembolism and all-cause mortality between the dabigatran 220 mg and 150 mg treatment arms and enoxaparin (36%, 41%, 38%, respectively).
The secondary efficacy endpoint, the incidence of proximal deep vein thrombosis and/or pulmonary embolism was similar in all treatment arms: 4%, 3%, and 4% of patients receiving dabigatran 220 mg, dabigatran 150 mg, and enoxaparin, respectively.
There was no difference among all treatment arms in bleeding rates (either major or any bleedings), and there were no signs of liver toxicity in the dabigatran arm.
Patients undergoing knee replacement surgery are at an increased risk of developing thromboembolism. Dabigatran etexilate is an oral direct thrombin inhibitor, and offers a convenient fixed oral dosing with no need for coagulation monitoring. ![]()
ORLANDO—A new oral anticoagulant in development, dabigatran etexilate, was shown to be as effective and safe as the low-molecular-weight enoxaparin in prophylaxis of thromboembolism in patients undergoing total knee replacement surgery.
Bengt I. Eriksson, MD, reported these results on behalf of the RE-MODEL study group during the 2006 annual meeting of the American Society of Hematology in December.
The RE-MODEL study was a large multicenter study conducted in 2010 patients undergoing elective total knee surgery in Europe, South Africa, and Australia.
Patients were randomized to receive subcutaneous injections of enoxaparin (40 mg, once daily) or to one of 2 doses of oral dabigatran etexilate (150 mg or 220 mg once daily) for 8 ± 2 days. Enoxaparin was administered 12 hours prior to surgery while dabigatran was administered 1-4 hours after the surgery.
Dabigatran etexilate met the prespecified primary endpoint of noninferiority versus enoxaparin. There was no difference in the incidence of total venous thromboembolism and all-cause mortality between the dabigatran 220 mg and 150 mg treatment arms and enoxaparin (36%, 41%, 38%, respectively).
The secondary efficacy endpoint, the incidence of proximal deep vein thrombosis and/or pulmonary embolism was similar in all treatment arms: 4%, 3%, and 4% of patients receiving dabigatran 220 mg, dabigatran 150 mg, and enoxaparin, respectively.
There was no difference among all treatment arms in bleeding rates (either major or any bleedings), and there were no signs of liver toxicity in the dabigatran arm.
Patients undergoing knee replacement surgery are at an increased risk of developing thromboembolism. Dabigatran etexilate is an oral direct thrombin inhibitor, and offers a convenient fixed oral dosing with no need for coagulation monitoring. ![]()
ORLANDO—A new oral anticoagulant in development, dabigatran etexilate, was shown to be as effective and safe as the low-molecular-weight enoxaparin in prophylaxis of thromboembolism in patients undergoing total knee replacement surgery.
Bengt I. Eriksson, MD, reported these results on behalf of the RE-MODEL study group during the 2006 annual meeting of the American Society of Hematology in December.
The RE-MODEL study was a large multicenter study conducted in 2010 patients undergoing elective total knee surgery in Europe, South Africa, and Australia.
Patients were randomized to receive subcutaneous injections of enoxaparin (40 mg, once daily) or to one of 2 doses of oral dabigatran etexilate (150 mg or 220 mg once daily) for 8 ± 2 days. Enoxaparin was administered 12 hours prior to surgery while dabigatran was administered 1-4 hours after the surgery.
Dabigatran etexilate met the prespecified primary endpoint of noninferiority versus enoxaparin. There was no difference in the incidence of total venous thromboembolism and all-cause mortality between the dabigatran 220 mg and 150 mg treatment arms and enoxaparin (36%, 41%, 38%, respectively).
The secondary efficacy endpoint, the incidence of proximal deep vein thrombosis and/or pulmonary embolism was similar in all treatment arms: 4%, 3%, and 4% of patients receiving dabigatran 220 mg, dabigatran 150 mg, and enoxaparin, respectively.
There was no difference among all treatment arms in bleeding rates (either major or any bleedings), and there were no signs of liver toxicity in the dabigatran arm.
Patients undergoing knee replacement surgery are at an increased risk of developing thromboembolism. Dabigatran etexilate is an oral direct thrombin inhibitor, and offers a convenient fixed oral dosing with no need for coagulation monitoring. ![]()