User login
Metastatsis to the Bones of the Hand
Etiology, Classification, and Treatment of Urticaria
Urticaria has been recognized since the days of Hippocrates. The name of the condition dates back to the 18th century, when the burning and edema of the skin was likened to that caused by contact with nettles (Urtica dioica). Urticaria affects 10% to 25% of the population worldwide at some point in their lives.1 The condition is characterized by short-lived edema of the skin, mouth, and genitalia related to a transient leakage of plasma from small blood vessels into the surrounding connective tissues. Urticaria may present with superficial edema of the dermis (wheals) or deeper edema of the dermal, subcutaneous, or submucosal tissues (angioedema).2 Wheals typically are itchy with a pale center, maturing into pink superficial plaques. Areas of angioedema tend to be pale and painful; last longer than wheals; and may involve the mouth and rarely the bowel.
Case Report
A 40-year-old woman in otherwise good health presented with a 5-year history of recurrent pruritic light red lesions on her chest and back. She reported that individual lesions would last up to 24 hours in one area before disappearing, while other new crops of lesions would develop in other areas of her body. She had no associated facial edema or lip or throat involvement, and she denied taking any medications. Her history failed to reveal any potential triggers for the eruptions. On physical examination, multiple elevated superficial erythematous papules and plaques were noted, with shapes varying from annular to circinate, areas of central clearing, and targetlike lesions on the trunk and extremities. The lesions blanched with pressure (Figure). The woman had no mucosal involvement, scars, or change in pigmentation. Results from the remainder of the physical examination were unremarkable.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Because of the extent of involvement and the erythematous to violaceous aspect of certain lesions, a 3-mm punch biopsy was performed to rule out urticarial vasculitis. Histology results were consistent with urticaria with red blood extravasation but without vasculitis. Our patient initially was treated with topical clobetasol propionate ointment, 10 mg of cetirizine hydrochloride, and topical calamine lotion. At follow-up one week later, she mentioned that she had improved after 5 days of treatment but began developing new lesions 2 days prior to her second visit. Given the severity of pruritus and after a discussion of the role of corticosteroids for acute urticaria, a taper dose of prednisone was prescribed at 40 mg/d, in addition to 60 mg of fexofenadine hydrochloride twice daily. The patient was lesion- and symptom-free after 7 days of treatment, with no recurrence one month later.
Comment
Urticaria may be acute or chronic. Acute urticaria is idiopathic in more than 50% of patients but can occur as a type 1 hypersensitivity reaction to food or wasp or bee stings; an immunologic response to blood products, infection, or febrile illness; or an adverse effect of drug therapy by various mechanisms, such as penicillin or angiotensin-converting enzyme inhibitors.3 As opposed to acute urticaria, chronic urticaria is defined by recurrent episodes occurring at least twice weekly for 6 weeks.2 Urticaria occurring less frequently than this, over a long period, is more accurately termed episodic because it is more likely to have an identifiable environmental trigger. All chronic urticaria implicitly go through an acute stage (<6 weeks). Although many classification systems of chronic urticaria exist, a concise clinical classification is included in the Table.2 Urticarial vasculitis is a small vessel vasculitis but is included in the classification because it is clinically indistinguishable from other urticarial lesions.
PLEASE REFER TO THE PDF TO VIEW THE TABLE
Urticarial lesions in chronic urticaria typically last 4 to 36 hours and can occur in individuals of any age (though it is most common in women), usually with few systemic symptoms.4 Pruritus is nearly always severe, especially at night, and may prevent sleep. Fifty percent of cases resolve spontaneously by 6 months, but of those that do not, 40% still have symptoms of urticaria 10 years later.4 The severe effect of chronic urticaria on quality of life often is underestimated.5
Ordinary Urticaria
Patients previously classified as having chronic idiopathic or "ordinary" urticaria are now divided into 2 groups: 50% to 60% of these patients have chronic idiopathic urticaria (CIU), and the remainder have chronic autoimmune urticaria (CAU).6 Results from a study in children demonstrated that autoimmune urticaria occurs in children in as many as 30% of chronic cases.7 CAU is caused by an immunoglobulin (Ig) G antibody to the α subunit of the IgE receptor (35%–40% of cases) or to IgE (5%–10% of cases).6 The IgG subclasses that appear to be pathogenic are IgG1; IgG3; and, to a lesser degree, IgG4 (though not IgG2).6 Complement activation augments histamine secretion by release of C5a.8 CAU has been reported to be associated with antithyroid antibodies (27% of cases)6,9; autoimmune conditions such as vitiligo, rheumatoid arthritis, and pernicious anemia; and low vitamin B12 levels.10 Patients with demonstrable histamine-releasing autoantibodies have a very strong association with HLA-DR4 and its associated allele HLA-DQ8.11
Histologically, the 2 groups of urticaria are indistinguishable.6 Advanced techniques show a perivascular nonnecrotizing infiltrate of CD4+ lymphocytes consisting of a mixture of TH1 and TH2 subtypes, plus monocytes, neutrophils, eosinophils, and basophils. These cells are recruited because of interactions with C5a, cell priming cytokines, chemokines, and adhesion molecules.6 A recent study also found inflammatory cells and mediator up-regulation in uninvolved CIU skin as a sign of prolonged and widespread "urticarial status."12
Physical Urticaria
Physical urticaria are classified and induced by a physical stimulus. Most physical urticaria occur within minutes of provocation and resolve within 2 hours, with the exception of delayed pressure urticaria, which may persist for 24 hours or longer.13 Angioedema may occur in all physical urticaria except dermographism. Overlap between groups is common, and physical urticaria often occur as an added feature of chronic urticaria.
The most common type of physical urticaria is simple immediate dermographism, presenting with linear wheals at sites of scratching or friction. It occurs in about 1.4% to 5% of the population worldwide14 and may be viewed as an exaggerated physiologic response. On average, dermographism runs a course of 2 to 3 years before usually resolving spontaneously.15
Delayed-pressure urticaria is a response to sustained pressure to the skin, presenting with deep erythematous edema after a delay of unknown cause lasting 30 minutes to 12 hours.14,16 An increased level of interleukin 6 has been found in suction blister fluid over induced lesions.2,15 The edema tends to be deeper, pruritic, and painful, and it may persist for days. Systemic features such as malaise, flulike symptoms, and arthralgia may occur. The prognosis is variable, but the mean duration is 6 to 9 years.17 The response to antihistamines often is poor, and oral corticosteroids may be needed for disease control.2
Cholinergic urticaria usually presents with multiple, transient, pruritic, small, red macules or papules on the neck, trunk, forearms, wrists, and thighs in response to heat, often surrounded by an obvious flare. It mainly affects young adults, with an overall prevalence of 11% in this group.18 Fifty percent of patients are atopic.15 Angioedema and systemic manifestations such as headache, palpitations, abdominal pain, wheezing, and syncope may occur. The cholinergic sympathetic innervation of sweat glands is involved because the eruption can be blocked by topical anticholinergic drugs,19 but how this leads to urticaria is unclear. The routine treatment is with low-sedation H1-type antihistamines, with or without an anxiolytic such as oral propranolol. In severe cases, the anabolic steroid stanazolol has been used.15
Cold urticaria is a heterogeneous condition in which whealing occurs within minutes in response to cold exposure, most frequently in children and young adults. Wheals usually arise at the site of localized cooling but may be generalized following lowering of the body temperature.16 Diagnosis may be confirmed by applying an ice cube for 5 to 15 minutes to the skin, allowing an interval for skin rewarming, and observing the development of whealing that occurs on skin rewarming. Systemic symptoms such as flushing, headache, abdominal pain, and syncope can occur if large areas are affected. The cause is unknown, but a serum factor, possibly IgM or IgE, has been implicated.20 A heterozygous deficiency of the protease inhibitor α1-antichymotrypsin has been demonstrated and may be etiologically important in some patients.21 The prognosis is good, with spontaneous improvement in an average of 2 to 3 years.15 Ninety-six percent of cases of cold urticaria are primary.22 The diagnosis of secondary acquired cold urticaria depends on being able to demonstrate cryoglobulins, cold agglutinins, or possibly cryofibrinogens.17 These findings should, in turn, lead to investigations for an underlying cause, such as hepatitis B or C infection, lymphoproliferative disease, or infectious mononucleosis.15
Other uncommon forms of physical urticaria include adrenergic urticaria, which develops during phases of stress and has been associated with an increase in the plasma concentrations of norepinephrine, epinephrine, and prolactin.23 Aquagenic urticaria is precipitated by skin contact with water of any temperature.3 Exercise-induced anaphylaxis involves urticaria, respiratory distress, or hypotension after exercise. In localized heat urticaria, wheals occur on skin in direct contact with warm objects. Solar urticaria is a rare condition that occurs within minutes of exposure to UV light waves ranging from 280 to 760 nm14; it usually disappears in less than one hour. Vibratory urticaria occurs after a vibratory stimulus and can be a hereditary autosomaldominant disorder or an acquired sporadic disease.24
Urticarial Vasculitis
Urticarial vasculitis describes a distinct entity in which the gross cutaneous lesions resemble urticaria and histologically show features of a vasculitis. The diagnosis is suggested clinically by wheals lasting more than 24 hours and residual bruising.25 Although the clinical lesions may present as typical urticaria, pathophysiologically, it is a different disease caused by deposition of antigen-antibody complexes in vessel walls, a type 3 reaction causing vascular damage.17 Lesions often occur at pressure points and may resolve with residual purpura. Extracutaneous manifestations include transient and migratory arthralgia (50%); gastrointestinal symptoms (20%); and pulmonary obstructive disease (20%), particularly in smokers and patients with renal disease (5%–10%).17 Normocomplementemic urticarial vasculitis usually is idiopathic, but hypocomplementemic urticarial vasculitis may be associated with underlying systemic lupus erythematosus, Sjögren syndrome, or cryoglobulinemia.2 Primary urticarial vasculitis can occasionally evolve into systemic lupus erythematosus.26 Patients with urticarial vasculitis often improve on nonsteroidal anti-inflammatory drugs (NSAIDs), but some patients may need immunosuppressive therapy.
Contact Urticaria
Contact urticaria develops at the site(s) of contact of an urticant and can be divided into an allergic subgroup caused by an IgE-allergen interaction and a nonallergic subgroup that is IgE independent. The allergic form typically is seen in children with atopic dermatitis sensitized to environmental allergens such as grass, animals, food, or latex, and it may be complicated by anaphylaxis. Natural rubber latex is one of the most important causes today.27 This type appears within minutes, fades within 2 hours, and is partially inhibited by antihistamines. Nonallergic contact urticaria is caused by the direct effect of the urticant on blood vessels and includes irritants such as benzoic acid and cinnamic aldehyde in cosmetics. It may take 45 minutes for lesions to appear and urticaria is partially inhibited by NSAIDs.
Angioedema Without Wheals
It is useful to classify angioedema occurring without wheals as a separate entity because its etiology may be associated with hereditary angioedema, which must be excluded. The condition usually is idiopathic or caused by a drug reaction to angiotensin-converting enzyme inhibitors, aspirin, or NSAIDs. Hereditary angioedema is a rare autosomal-dominant condition with a prevalence between 1:10,000 and 1:150,000 in the general population and is caused by a deficiency (type 1, 85%) or dysfunction (type 2, 15%) of C1 inhibitor.28 A low level of C4 in the serum is a constant and diagnostic feature. A third type affecting primarily women and exacerbated by estrogens recently has been described.28 Patients have lifelong episodic angioedema and may experience colicky abdominal pain. Laryngeal involvement can be life threatening. Treatment is difficult and involves fluid replacement and purified C1 inhibitor concentrate for acute attacks (not approved in the United States) and prophylactic treatment with anabolic androgens and antifibrinolytics.28
Diagnosis
The diagnosis of urticaria is primarily clinical; extensive laboratory tests are very rarely needed—only when indicated by the patient history.3 Some authors argue that laboratory investigations are unnecessary for mild ordinary urticaria responding to antihistamines.29 Taking a thorough patient history has been found to be almost as effective in identifying a cause as a complete diagnostic evaluation.30 In acute urticaria, if the history indicates a type 1 hypersensitivity reaction, confirmation is possible by a prick test or laboratory radioallergosorbent tests.3 Many physical and contact urticaria can be confirmed by a challenge of the offending agent. An initial baseline investigation with a complete blood count and erythrocyte sedimentation rate should be taken in more severe cases to identify any internal disease or raise the possibility of urticarial vasculitis.17 Of note, a biopsy is more sensitive and specific for ruling out urticarial vasculitis than are a complete blood count and erythrocyte sedimentation rate.
A search for thyroid autoantibodies is appropriate for all chronic urticaria not responding to first-line therapies with antihistamines, especially when autoimmune urticaria is suspected.2 Further investigations are guided by clinical suspicion, which may include a skin biopsy, autoimmune screening, urinalysis, serum cryoglobulins, and hepatitis B and C serology.31 The only available test to screen for autoantibodies against the IgE receptor is the autologous serum skin test. This test should be performed with care because infections could be transmitted, particularly if, by mistake, patients were not injected with their own serum.31 Measurement of C4 is indicated only in patients who present with angioedema alone and should be followed by a determination of the levels and function of C1 inhibitor, if C4 is below reference range.29
Management and Treatment
Management of urticaria depends on its cause. Aggravating factors should be identified from the history, and triggering stimuli for physical urticaria should be avoided. Simple cooling lotions such as menthol 1% or 2% in an aqueous cream often are useful.32 Aspirin and NSAIDs should be avoided because they aggravate symptoms in 30% of patients.33 Patients taking low-dose aspirin for its antithrombotic properties usually can continue regular treatment. Avoiding codeine and other opiates also is recommended because an enhanced skin test reaction may be found in chronic urticaria.34 Avoiding dietary pseudoallergens, such as food coloring and natural salicylates, is controversial.14,35 This generally has only a small role unless proven by a double-blinded placebo-controlled challenge.2
The mainstays for treatment of urticaria are oral antihistamines, as they reduce pruritus and wheal duration and numbers. Oral antihistamines have been reported to produce moderate or good response in 44% to 91% of patients with all types of urticaria.36,37 Antihistamines can be grouped into first-generation (sedating), second generation (minimally sedating), third-generation (nonsedating), and H2 antagonists.17 The physiologic and pathologic actions of histamine are mediated through 4 histamine receptor subtypes: H1, H2, H3, and H4.38 The erythema, wheal formation, and itching associated with urticaria are mainly due to activation of H1 receptors and the less contributory role of H2 receptors.38 Histamine H3 receptors are located presynaptically on postganglionic sympathetic norepinephric nerves, including sympathetics innervating the heart and blood vessels. The contribution of H3 receptors to skin responses mediated by histamine has not been fully elucidated. However, in a recent experimental study, the authors reported that the combination of H1 and H3 antagonists might be a novel approach for the treatment of urticaria.38
Initially, a minimally sedating second- or third-generation antihistamine, such as loratadine,39 fexofenadine hydrochloride,40 and cetirizine hydrochloride,41-44 should be given at a once-daily oral dosing. When one antihistamine is not helpful, it is usually worth trying a different one, and some physicians combine 2 or more antihistamines at the same time.3 It is common practice to exceed the licensed dose in severely affected patients.31 High doses of antihistamines have effects beyond the blockade of histamine receptors, and actions that are not due to antagonism of H1 receptors may account for the efficacy of older antihistamines.45 As a general rule, antihistamines are safe and have few substantial adverse effects; drug interactions are rare. If possible, it is best to avoid all antihistamines in pregnancy, though none have been proven teratogenic. If one is used, the consensus is that chlorpheniramine maleate is among the safest.46
Addition of a sedating first-generation antihistamine such as hydroxyzine at night can be helpful, especially if nocturnal pruritus prevents sleep. The use of a sedating antihistamine as monotherapy is less desirable because of impairment of cognitive function, including driving performance and concentration. The addition of a H2 antagonist to conventional H1 antihistamines may be helpful in some patients.3,47 Doxepin hydrochloride at low doses (10–50 mg) is used for its potent H1 and H2 receptor antagonist properties. Doxepin hydrochloride is highly sedative and especially suitable for patients with associated depression.48
Oral corticosteroids given in short reducing courses may be needed for severe exacerbations not responding to full-dose antihistamines. Relatively high doses of up to 40 to 60 mg of prednisone may be needed for disease control. Alternate-day ste-roids may be used for patients with severe disease.6 Long-term administration should be avoided.1
Many patients feel reassured by carrying an epinephrine pen for self-administration if they are prone to severe attacks. Leukotriene antagonists (zafirlukast and montelukast sodium) have been shown to be superior to placebo in the treatment of patients with chronic urticaria.49,50 Nifedipine has a small effect in chronic urticaria and often is used for patients with concomitant hypertension. Thyroxine recently was reported to suppress CIU symptoms associated with antithyroid autoantibodies in some patients.51
Given the role of the immune system in a subset of patients, immunosuppressive therapy is considered for patients with a severe disabling course. Cyclosporine at 2.5 to 5 mg/kg per day is of proven value in autoantibody-positive chronic urticaria52 but also is effective in most cases of severe autoantibody-negative disease.15 Tacrolimus also has shown promise in a recent trial.53 Other options include plasmapheresis54 and intravenous immunoglobulin.55,56 Optimal treatment protocols have yet to be confirmed. Treatments for CIU with only limited or anecdotal supportive evidence include sulfasalazine, methotrexate, rofecoxib, colchicine, dapsone, and cyclophosphamide.3
Future treatment may involve development of selective immunotherapy targeting the IgE receptor or vaccinations to down-regulate and induce tolerance to the IgE receptor. Other potential strategies include blocking formation of C5a and use of therapeutic antibodies such as anti-IgE, anti–tumor necrosis factor α, and anti–interleukin 5.2
Conclusion
There is no single way to manage urticaria and angioedema. Most patients are treated successfully with antihistamines. However, patients with severe antihistamine-resistant urticaria may be very disabled by their disease, and the treatment can pose a major challenge to the physician.
- Henderson RL Jr, Fleischer AB Jr, Feldman SR. Allergists and dermatologists have far more expertise in caring for patients with urticaria than other specialists. J Am Acad Dermatol. 2000;43:1084-1091.
- Grattan CEH, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645-657.
- Kozel MM, Sabroe RA. Chronic urticaria: aetiology, management and current and future treatment options. Drugs. 2004;64:2515-2536.
- Negro-Alvarez JM, Miralles-Lopez JC. Chronic idiopathic urticaria treatment. Allergol Immunopathol (Madr). 2001;29:129-132.
- Grob JJ, Revuz J, Ortonne JP, et al. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol. 2005;152:289-295.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Brunetti L, Francavilla R, Miniello VL, et al. High prevalence of autoimmune urticaria in children with chronic urticaria. J Allergy Clin Immunol. 2004;114:922-927.
- Kikuchi Y, Kaplan AP. A role for C5a in augmenting IgG-dependent histamine release from basophils in chronic urticaria. J Allergy Clin Immunol. 2002;109:114-118.
- Gruber BL, Baeza M, Marchese M, et al. Prevalence and functional role of anti-IgE antibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213-217.
- Mete N, Gulbahar O, Aydin A, et al. Low B12 levels in chronic idiopathic urticaria. J Investig Allergol Clin Immunol. 2004;14:292-299.
- O'Donnell BF, O'Neill CM, Francis DM, et al. Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br J Dermatol. 1999;140:853-858.
- Caproni M, Giomi B, Volpi W, et al. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin Immunol. 2005;114:284-292.
- Black AK, Lawlor F, Greaves MW. Consensus meeting on the definition of physical urticarias and urticarial vasculitis. Clin Exp Dermatol. 1996;21:424-426.
- Henz BM. Physical urticaria. In: Henz BM, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical, Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer Verlag; 1998:55-89.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines: an EAACI position paper. Allergy. 1997;52:503-513.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:287-311.
- Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
- Herxheimer A. The nervous pathway mediating cholinergic urticaria. Clin Sci (Lond). 1956;15:195-204.
- Wanderer AA, Maselli R, Ellis EF, et al. Immunological characterisation of serum factors responsible for cold urticaria.
- Lindmark B, Wallengren J. Heterozygous alpha1-antichymotrypsin deficiency may be associated with cold urticaria. Allergy. 1992;47:456-458.
- Neittaanmaki H. Cold urticaria: clinical findings in 220 patients. J Am Acad Dermatol. 1985;13:636-644.
- Haustein UF. Adrenergic urticaria and adrenergic pruritus. Acta Derm Venereol. 1990;70:82-84.
- Mathelier-Fusade P, Vermeulen C, Leynadier F. Vibratory angioedema. Ann Dermatol Venereol. 2001;128:750-752.
- O’Donnell B, Black AK. Urticarial vasculitis. Int Angiol. 1995;14:166-174.
- Bisaccia E, Adamo V, Rozan SW. Urticarial vasculitis progressing to systemic lupus erythematosus. Arch Dermatol. 1988;124:1088-1090.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Zuraw BL. Current and future therapy for hereditary angioedema. Clin Immunol. 2005;114:10-16.
- Grattan C, Powell S, Humphreys F. Management and diagnostic guidelines for urticaria and angio-oedema. Br J Dermatol. 2001;144:708-714.
- Kozel MM, Mekkes JR, Bossuyt PM, et al. The effectiveness of a history-based diagnostic approach in chronic urticaria and angioedema. Arch Dermatol. 1998;134:1575-1580.
- Zuberbier T, Greaves MW, Juhlin L, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001;6:123-127.
- Bromm B, Scharein E, Darsow U, et al. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett. 1995;187:157-160.
- Doeglas HMG. Reactions to aspirin and food additives in patients with chronic urticaria, including the physical urticarias. Br J Dermatol. 1975;93:135-144.
- Kaufman A, Rosenstreich DL. Mast cell heterogeneity in chronic idiopathic urticaria. Ann Allergy. 1990;65:367-373.
- Ortolani C, Pastorello E, Ispano M, et al. Food allergy diagnosis protocol. Allerg Immunol (Paris). 1988;20:48-50.
- 36. Humphreys F, Hunter JA. The characteristics of urticarial in 390 patients. Br J Dermatol. 1998;138:635-638.
- 37. Nettis E, Pannofino A, D’Aprile C, et al. Clinical and aetiological aspects in urticaria and angio-oedema. Br J Dermatol. 2003;148:501-506.
- 38. McLeod RL, Mingo GG, Kreutner W, et al. Effect of combined histamine H1 and H3 receptor blockade on cutaneous microvascular permeability elicited by compound 48/80. Life Sci. 2005;76:1787-1794.
- 39. Monroe EW. Loratadine in the treatment of urticaria. Clin Ther. 1997;19:232-242.
- 40. Nelson HS, Reynolds R, Mason J. Fexofenadine HCl is safe and effective for treatment of chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2000;84: 517-522.
- 41. Breneman D, Bronsky EA, Bruce S, et al. Cetirizine and astemizole therapy for chronic idiopathic urticaria: a double-blind, placebo-controlled, comparative trial. J Am Acad Dermatol. 1995;33(2 pt 1):192-198.
- 42. Breneman DL. Cetirizine versus hydroxyzine and placebo in chronic idiopathic urticaria. Ann Pharmacother. 1996;30:1075-1079.
- 43. Kalivas J, Breneman D, Tharp M, et al. Urticaria: clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy Clin Immunol. 1990;86(6 pt 2): 1014-1018.
- 44. Andri L, Senna GE, Betteli C, et al. A comparison of the efficacy of cetirizine and terfenadine: a double-blind, controlled study of chronic idiopathic urticaria. Allergy. 1993;48:358-365.
- 45. Kaplan AP. Clinical practice. chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- 46. Andrews AW, Fornwald JA, Lijinsky W. Nitrosation and mutagenicity of some anime drugs. Toxicol Appl Pharmacol. 1980;52:237-244.
- 47. Commens CA, Greaves MW. Cimetidine in chronic idiopathic urticaria: a randomised double-blind study. Br J Dermatol. 1978;99:675-679.
- 48. Furukawa T, McGuire H, Barbui C. Low dosage tricyclic antidepressants for depression. Cochrane Database Syst Rev. 2003;(3):CD003197.
- 49. Ellis MH. Successful treatment of chronic urticaria with leukotriene antagonists. J Allergy Clin Immunol. 1998;102:876-877.
- 50. Spector S, Tan RA. Antileukotrienes in chronic urticaria. J Allergy Clin Immunol. 1998;101(4 pt 1):572.
- 51. Aversano M, Caiazzo P, Iorio G, et al. Improvement of chronic idiopathic urticaria with L-thyroxine: a new TSH role in immune response? Allergy. 2005;60:489-493.
- Grattan CE, O’Donnell BF, Francis DM, et al. Randomized double-blind study of cyclosporin in chronic “idiopathic” urticaria. Br J Dermatol. 2000;143:365-372.
- Kessel A, Bamberger E, Toubi E. Tacrolimus in the treatment of severe chronic idiopathic urticaria: an open-label prospective study. J Am Acad Dermatol. 2005;52:145-148.
- Grattan CEH, Francis DM, Slater NGP, et al. Plasmapheresis for severe unremitting chronic urticaria. Lancet. 1992;339:1078-1080.
- O’Donnell BF, Barr RM, Blac AK, et al. Intravenous immunoglobulin in chronic autoimmune urticaria. Br J Dermatol. 1998;138:101-106.
- Klote MM, Nelson MR, Engler RJ. Autoimmune urticarial response to high-dose intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2005;94:307-308.
Urticaria has been recognized since the days of Hippocrates. The name of the condition dates back to the 18th century, when the burning and edema of the skin was likened to that caused by contact with nettles (Urtica dioica). Urticaria affects 10% to 25% of the population worldwide at some point in their lives.1 The condition is characterized by short-lived edema of the skin, mouth, and genitalia related to a transient leakage of plasma from small blood vessels into the surrounding connective tissues. Urticaria may present with superficial edema of the dermis (wheals) or deeper edema of the dermal, subcutaneous, or submucosal tissues (angioedema).2 Wheals typically are itchy with a pale center, maturing into pink superficial plaques. Areas of angioedema tend to be pale and painful; last longer than wheals; and may involve the mouth and rarely the bowel.
Case Report
A 40-year-old woman in otherwise good health presented with a 5-year history of recurrent pruritic light red lesions on her chest and back. She reported that individual lesions would last up to 24 hours in one area before disappearing, while other new crops of lesions would develop in other areas of her body. She had no associated facial edema or lip or throat involvement, and she denied taking any medications. Her history failed to reveal any potential triggers for the eruptions. On physical examination, multiple elevated superficial erythematous papules and plaques were noted, with shapes varying from annular to circinate, areas of central clearing, and targetlike lesions on the trunk and extremities. The lesions blanched with pressure (Figure). The woman had no mucosal involvement, scars, or change in pigmentation. Results from the remainder of the physical examination were unremarkable.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Because of the extent of involvement and the erythematous to violaceous aspect of certain lesions, a 3-mm punch biopsy was performed to rule out urticarial vasculitis. Histology results were consistent with urticaria with red blood extravasation but without vasculitis. Our patient initially was treated with topical clobetasol propionate ointment, 10 mg of cetirizine hydrochloride, and topical calamine lotion. At follow-up one week later, she mentioned that she had improved after 5 days of treatment but began developing new lesions 2 days prior to her second visit. Given the severity of pruritus and after a discussion of the role of corticosteroids for acute urticaria, a taper dose of prednisone was prescribed at 40 mg/d, in addition to 60 mg of fexofenadine hydrochloride twice daily. The patient was lesion- and symptom-free after 7 days of treatment, with no recurrence one month later.
Comment
Urticaria may be acute or chronic. Acute urticaria is idiopathic in more than 50% of patients but can occur as a type 1 hypersensitivity reaction to food or wasp or bee stings; an immunologic response to blood products, infection, or febrile illness; or an adverse effect of drug therapy by various mechanisms, such as penicillin or angiotensin-converting enzyme inhibitors.3 As opposed to acute urticaria, chronic urticaria is defined by recurrent episodes occurring at least twice weekly for 6 weeks.2 Urticaria occurring less frequently than this, over a long period, is more accurately termed episodic because it is more likely to have an identifiable environmental trigger. All chronic urticaria implicitly go through an acute stage (<6 weeks). Although many classification systems of chronic urticaria exist, a concise clinical classification is included in the Table.2 Urticarial vasculitis is a small vessel vasculitis but is included in the classification because it is clinically indistinguishable from other urticarial lesions.
PLEASE REFER TO THE PDF TO VIEW THE TABLE
Urticarial lesions in chronic urticaria typically last 4 to 36 hours and can occur in individuals of any age (though it is most common in women), usually with few systemic symptoms.4 Pruritus is nearly always severe, especially at night, and may prevent sleep. Fifty percent of cases resolve spontaneously by 6 months, but of those that do not, 40% still have symptoms of urticaria 10 years later.4 The severe effect of chronic urticaria on quality of life often is underestimated.5
Ordinary Urticaria
Patients previously classified as having chronic idiopathic or "ordinary" urticaria are now divided into 2 groups: 50% to 60% of these patients have chronic idiopathic urticaria (CIU), and the remainder have chronic autoimmune urticaria (CAU).6 Results from a study in children demonstrated that autoimmune urticaria occurs in children in as many as 30% of chronic cases.7 CAU is caused by an immunoglobulin (Ig) G antibody to the α subunit of the IgE receptor (35%–40% of cases) or to IgE (5%–10% of cases).6 The IgG subclasses that appear to be pathogenic are IgG1; IgG3; and, to a lesser degree, IgG4 (though not IgG2).6 Complement activation augments histamine secretion by release of C5a.8 CAU has been reported to be associated with antithyroid antibodies (27% of cases)6,9; autoimmune conditions such as vitiligo, rheumatoid arthritis, and pernicious anemia; and low vitamin B12 levels.10 Patients with demonstrable histamine-releasing autoantibodies have a very strong association with HLA-DR4 and its associated allele HLA-DQ8.11
Histologically, the 2 groups of urticaria are indistinguishable.6 Advanced techniques show a perivascular nonnecrotizing infiltrate of CD4+ lymphocytes consisting of a mixture of TH1 and TH2 subtypes, plus monocytes, neutrophils, eosinophils, and basophils. These cells are recruited because of interactions with C5a, cell priming cytokines, chemokines, and adhesion molecules.6 A recent study also found inflammatory cells and mediator up-regulation in uninvolved CIU skin as a sign of prolonged and widespread "urticarial status."12
Physical Urticaria
Physical urticaria are classified and induced by a physical stimulus. Most physical urticaria occur within minutes of provocation and resolve within 2 hours, with the exception of delayed pressure urticaria, which may persist for 24 hours or longer.13 Angioedema may occur in all physical urticaria except dermographism. Overlap between groups is common, and physical urticaria often occur as an added feature of chronic urticaria.
The most common type of physical urticaria is simple immediate dermographism, presenting with linear wheals at sites of scratching or friction. It occurs in about 1.4% to 5% of the population worldwide14 and may be viewed as an exaggerated physiologic response. On average, dermographism runs a course of 2 to 3 years before usually resolving spontaneously.15
Delayed-pressure urticaria is a response to sustained pressure to the skin, presenting with deep erythematous edema after a delay of unknown cause lasting 30 minutes to 12 hours.14,16 An increased level of interleukin 6 has been found in suction blister fluid over induced lesions.2,15 The edema tends to be deeper, pruritic, and painful, and it may persist for days. Systemic features such as malaise, flulike symptoms, and arthralgia may occur. The prognosis is variable, but the mean duration is 6 to 9 years.17 The response to antihistamines often is poor, and oral corticosteroids may be needed for disease control.2
Cholinergic urticaria usually presents with multiple, transient, pruritic, small, red macules or papules on the neck, trunk, forearms, wrists, and thighs in response to heat, often surrounded by an obvious flare. It mainly affects young adults, with an overall prevalence of 11% in this group.18 Fifty percent of patients are atopic.15 Angioedema and systemic manifestations such as headache, palpitations, abdominal pain, wheezing, and syncope may occur. The cholinergic sympathetic innervation of sweat glands is involved because the eruption can be blocked by topical anticholinergic drugs,19 but how this leads to urticaria is unclear. The routine treatment is with low-sedation H1-type antihistamines, with or without an anxiolytic such as oral propranolol. In severe cases, the anabolic steroid stanazolol has been used.15
Cold urticaria is a heterogeneous condition in which whealing occurs within minutes in response to cold exposure, most frequently in children and young adults. Wheals usually arise at the site of localized cooling but may be generalized following lowering of the body temperature.16 Diagnosis may be confirmed by applying an ice cube for 5 to 15 minutes to the skin, allowing an interval for skin rewarming, and observing the development of whealing that occurs on skin rewarming. Systemic symptoms such as flushing, headache, abdominal pain, and syncope can occur if large areas are affected. The cause is unknown, but a serum factor, possibly IgM or IgE, has been implicated.20 A heterozygous deficiency of the protease inhibitor α1-antichymotrypsin has been demonstrated and may be etiologically important in some patients.21 The prognosis is good, with spontaneous improvement in an average of 2 to 3 years.15 Ninety-six percent of cases of cold urticaria are primary.22 The diagnosis of secondary acquired cold urticaria depends on being able to demonstrate cryoglobulins, cold agglutinins, or possibly cryofibrinogens.17 These findings should, in turn, lead to investigations for an underlying cause, such as hepatitis B or C infection, lymphoproliferative disease, or infectious mononucleosis.15
Other uncommon forms of physical urticaria include adrenergic urticaria, which develops during phases of stress and has been associated with an increase in the plasma concentrations of norepinephrine, epinephrine, and prolactin.23 Aquagenic urticaria is precipitated by skin contact with water of any temperature.3 Exercise-induced anaphylaxis involves urticaria, respiratory distress, or hypotension after exercise. In localized heat urticaria, wheals occur on skin in direct contact with warm objects. Solar urticaria is a rare condition that occurs within minutes of exposure to UV light waves ranging from 280 to 760 nm14; it usually disappears in less than one hour. Vibratory urticaria occurs after a vibratory stimulus and can be a hereditary autosomaldominant disorder or an acquired sporadic disease.24
Urticarial Vasculitis
Urticarial vasculitis describes a distinct entity in which the gross cutaneous lesions resemble urticaria and histologically show features of a vasculitis. The diagnosis is suggested clinically by wheals lasting more than 24 hours and residual bruising.25 Although the clinical lesions may present as typical urticaria, pathophysiologically, it is a different disease caused by deposition of antigen-antibody complexes in vessel walls, a type 3 reaction causing vascular damage.17 Lesions often occur at pressure points and may resolve with residual purpura. Extracutaneous manifestations include transient and migratory arthralgia (50%); gastrointestinal symptoms (20%); and pulmonary obstructive disease (20%), particularly in smokers and patients with renal disease (5%–10%).17 Normocomplementemic urticarial vasculitis usually is idiopathic, but hypocomplementemic urticarial vasculitis may be associated with underlying systemic lupus erythematosus, Sjögren syndrome, or cryoglobulinemia.2 Primary urticarial vasculitis can occasionally evolve into systemic lupus erythematosus.26 Patients with urticarial vasculitis often improve on nonsteroidal anti-inflammatory drugs (NSAIDs), but some patients may need immunosuppressive therapy.
Contact Urticaria
Contact urticaria develops at the site(s) of contact of an urticant and can be divided into an allergic subgroup caused by an IgE-allergen interaction and a nonallergic subgroup that is IgE independent. The allergic form typically is seen in children with atopic dermatitis sensitized to environmental allergens such as grass, animals, food, or latex, and it may be complicated by anaphylaxis. Natural rubber latex is one of the most important causes today.27 This type appears within minutes, fades within 2 hours, and is partially inhibited by antihistamines. Nonallergic contact urticaria is caused by the direct effect of the urticant on blood vessels and includes irritants such as benzoic acid and cinnamic aldehyde in cosmetics. It may take 45 minutes for lesions to appear and urticaria is partially inhibited by NSAIDs.
Angioedema Without Wheals
It is useful to classify angioedema occurring without wheals as a separate entity because its etiology may be associated with hereditary angioedema, which must be excluded. The condition usually is idiopathic or caused by a drug reaction to angiotensin-converting enzyme inhibitors, aspirin, or NSAIDs. Hereditary angioedema is a rare autosomal-dominant condition with a prevalence between 1:10,000 and 1:150,000 in the general population and is caused by a deficiency (type 1, 85%) or dysfunction (type 2, 15%) of C1 inhibitor.28 A low level of C4 in the serum is a constant and diagnostic feature. A third type affecting primarily women and exacerbated by estrogens recently has been described.28 Patients have lifelong episodic angioedema and may experience colicky abdominal pain. Laryngeal involvement can be life threatening. Treatment is difficult and involves fluid replacement and purified C1 inhibitor concentrate for acute attacks (not approved in the United States) and prophylactic treatment with anabolic androgens and antifibrinolytics.28
Diagnosis
The diagnosis of urticaria is primarily clinical; extensive laboratory tests are very rarely needed—only when indicated by the patient history.3 Some authors argue that laboratory investigations are unnecessary for mild ordinary urticaria responding to antihistamines.29 Taking a thorough patient history has been found to be almost as effective in identifying a cause as a complete diagnostic evaluation.30 In acute urticaria, if the history indicates a type 1 hypersensitivity reaction, confirmation is possible by a prick test or laboratory radioallergosorbent tests.3 Many physical and contact urticaria can be confirmed by a challenge of the offending agent. An initial baseline investigation with a complete blood count and erythrocyte sedimentation rate should be taken in more severe cases to identify any internal disease or raise the possibility of urticarial vasculitis.17 Of note, a biopsy is more sensitive and specific for ruling out urticarial vasculitis than are a complete blood count and erythrocyte sedimentation rate.
A search for thyroid autoantibodies is appropriate for all chronic urticaria not responding to first-line therapies with antihistamines, especially when autoimmune urticaria is suspected.2 Further investigations are guided by clinical suspicion, which may include a skin biopsy, autoimmune screening, urinalysis, serum cryoglobulins, and hepatitis B and C serology.31 The only available test to screen for autoantibodies against the IgE receptor is the autologous serum skin test. This test should be performed with care because infections could be transmitted, particularly if, by mistake, patients were not injected with their own serum.31 Measurement of C4 is indicated only in patients who present with angioedema alone and should be followed by a determination of the levels and function of C1 inhibitor, if C4 is below reference range.29
Management and Treatment
Management of urticaria depends on its cause. Aggravating factors should be identified from the history, and triggering stimuli for physical urticaria should be avoided. Simple cooling lotions such as menthol 1% or 2% in an aqueous cream often are useful.32 Aspirin and NSAIDs should be avoided because they aggravate symptoms in 30% of patients.33 Patients taking low-dose aspirin for its antithrombotic properties usually can continue regular treatment. Avoiding codeine and other opiates also is recommended because an enhanced skin test reaction may be found in chronic urticaria.34 Avoiding dietary pseudoallergens, such as food coloring and natural salicylates, is controversial.14,35 This generally has only a small role unless proven by a double-blinded placebo-controlled challenge.2
The mainstays for treatment of urticaria are oral antihistamines, as they reduce pruritus and wheal duration and numbers. Oral antihistamines have been reported to produce moderate or good response in 44% to 91% of patients with all types of urticaria.36,37 Antihistamines can be grouped into first-generation (sedating), second generation (minimally sedating), third-generation (nonsedating), and H2 antagonists.17 The physiologic and pathologic actions of histamine are mediated through 4 histamine receptor subtypes: H1, H2, H3, and H4.38 The erythema, wheal formation, and itching associated with urticaria are mainly due to activation of H1 receptors and the less contributory role of H2 receptors.38 Histamine H3 receptors are located presynaptically on postganglionic sympathetic norepinephric nerves, including sympathetics innervating the heart and blood vessels. The contribution of H3 receptors to skin responses mediated by histamine has not been fully elucidated. However, in a recent experimental study, the authors reported that the combination of H1 and H3 antagonists might be a novel approach for the treatment of urticaria.38
Initially, a minimally sedating second- or third-generation antihistamine, such as loratadine,39 fexofenadine hydrochloride,40 and cetirizine hydrochloride,41-44 should be given at a once-daily oral dosing. When one antihistamine is not helpful, it is usually worth trying a different one, and some physicians combine 2 or more antihistamines at the same time.3 It is common practice to exceed the licensed dose in severely affected patients.31 High doses of antihistamines have effects beyond the blockade of histamine receptors, and actions that are not due to antagonism of H1 receptors may account for the efficacy of older antihistamines.45 As a general rule, antihistamines are safe and have few substantial adverse effects; drug interactions are rare. If possible, it is best to avoid all antihistamines in pregnancy, though none have been proven teratogenic. If one is used, the consensus is that chlorpheniramine maleate is among the safest.46
Addition of a sedating first-generation antihistamine such as hydroxyzine at night can be helpful, especially if nocturnal pruritus prevents sleep. The use of a sedating antihistamine as monotherapy is less desirable because of impairment of cognitive function, including driving performance and concentration. The addition of a H2 antagonist to conventional H1 antihistamines may be helpful in some patients.3,47 Doxepin hydrochloride at low doses (10–50 mg) is used for its potent H1 and H2 receptor antagonist properties. Doxepin hydrochloride is highly sedative and especially suitable for patients with associated depression.48
Oral corticosteroids given in short reducing courses may be needed for severe exacerbations not responding to full-dose antihistamines. Relatively high doses of up to 40 to 60 mg of prednisone may be needed for disease control. Alternate-day ste-roids may be used for patients with severe disease.6 Long-term administration should be avoided.1
Many patients feel reassured by carrying an epinephrine pen for self-administration if they are prone to severe attacks. Leukotriene antagonists (zafirlukast and montelukast sodium) have been shown to be superior to placebo in the treatment of patients with chronic urticaria.49,50 Nifedipine has a small effect in chronic urticaria and often is used for patients with concomitant hypertension. Thyroxine recently was reported to suppress CIU symptoms associated with antithyroid autoantibodies in some patients.51
Given the role of the immune system in a subset of patients, immunosuppressive therapy is considered for patients with a severe disabling course. Cyclosporine at 2.5 to 5 mg/kg per day is of proven value in autoantibody-positive chronic urticaria52 but also is effective in most cases of severe autoantibody-negative disease.15 Tacrolimus also has shown promise in a recent trial.53 Other options include plasmapheresis54 and intravenous immunoglobulin.55,56 Optimal treatment protocols have yet to be confirmed. Treatments for CIU with only limited or anecdotal supportive evidence include sulfasalazine, methotrexate, rofecoxib, colchicine, dapsone, and cyclophosphamide.3
Future treatment may involve development of selective immunotherapy targeting the IgE receptor or vaccinations to down-regulate and induce tolerance to the IgE receptor. Other potential strategies include blocking formation of C5a and use of therapeutic antibodies such as anti-IgE, anti–tumor necrosis factor α, and anti–interleukin 5.2
Conclusion
There is no single way to manage urticaria and angioedema. Most patients are treated successfully with antihistamines. However, patients with severe antihistamine-resistant urticaria may be very disabled by their disease, and the treatment can pose a major challenge to the physician.
Urticaria has been recognized since the days of Hippocrates. The name of the condition dates back to the 18th century, when the burning and edema of the skin was likened to that caused by contact with nettles (Urtica dioica). Urticaria affects 10% to 25% of the population worldwide at some point in their lives.1 The condition is characterized by short-lived edema of the skin, mouth, and genitalia related to a transient leakage of plasma from small blood vessels into the surrounding connective tissues. Urticaria may present with superficial edema of the dermis (wheals) or deeper edema of the dermal, subcutaneous, or submucosal tissues (angioedema).2 Wheals typically are itchy with a pale center, maturing into pink superficial plaques. Areas of angioedema tend to be pale and painful; last longer than wheals; and may involve the mouth and rarely the bowel.
Case Report
A 40-year-old woman in otherwise good health presented with a 5-year history of recurrent pruritic light red lesions on her chest and back. She reported that individual lesions would last up to 24 hours in one area before disappearing, while other new crops of lesions would develop in other areas of her body. She had no associated facial edema or lip or throat involvement, and she denied taking any medications. Her history failed to reveal any potential triggers for the eruptions. On physical examination, multiple elevated superficial erythematous papules and plaques were noted, with shapes varying from annular to circinate, areas of central clearing, and targetlike lesions on the trunk and extremities. The lesions blanched with pressure (Figure). The woman had no mucosal involvement, scars, or change in pigmentation. Results from the remainder of the physical examination were unremarkable.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Because of the extent of involvement and the erythematous to violaceous aspect of certain lesions, a 3-mm punch biopsy was performed to rule out urticarial vasculitis. Histology results were consistent with urticaria with red blood extravasation but without vasculitis. Our patient initially was treated with topical clobetasol propionate ointment, 10 mg of cetirizine hydrochloride, and topical calamine lotion. At follow-up one week later, she mentioned that she had improved after 5 days of treatment but began developing new lesions 2 days prior to her second visit. Given the severity of pruritus and after a discussion of the role of corticosteroids for acute urticaria, a taper dose of prednisone was prescribed at 40 mg/d, in addition to 60 mg of fexofenadine hydrochloride twice daily. The patient was lesion- and symptom-free after 7 days of treatment, with no recurrence one month later.
Comment
Urticaria may be acute or chronic. Acute urticaria is idiopathic in more than 50% of patients but can occur as a type 1 hypersensitivity reaction to food or wasp or bee stings; an immunologic response to blood products, infection, or febrile illness; or an adverse effect of drug therapy by various mechanisms, such as penicillin or angiotensin-converting enzyme inhibitors.3 As opposed to acute urticaria, chronic urticaria is defined by recurrent episodes occurring at least twice weekly for 6 weeks.2 Urticaria occurring less frequently than this, over a long period, is more accurately termed episodic because it is more likely to have an identifiable environmental trigger. All chronic urticaria implicitly go through an acute stage (<6 weeks). Although many classification systems of chronic urticaria exist, a concise clinical classification is included in the Table.2 Urticarial vasculitis is a small vessel vasculitis but is included in the classification because it is clinically indistinguishable from other urticarial lesions.
PLEASE REFER TO THE PDF TO VIEW THE TABLE
Urticarial lesions in chronic urticaria typically last 4 to 36 hours and can occur in individuals of any age (though it is most common in women), usually with few systemic symptoms.4 Pruritus is nearly always severe, especially at night, and may prevent sleep. Fifty percent of cases resolve spontaneously by 6 months, but of those that do not, 40% still have symptoms of urticaria 10 years later.4 The severe effect of chronic urticaria on quality of life often is underestimated.5
Ordinary Urticaria
Patients previously classified as having chronic idiopathic or "ordinary" urticaria are now divided into 2 groups: 50% to 60% of these patients have chronic idiopathic urticaria (CIU), and the remainder have chronic autoimmune urticaria (CAU).6 Results from a study in children demonstrated that autoimmune urticaria occurs in children in as many as 30% of chronic cases.7 CAU is caused by an immunoglobulin (Ig) G antibody to the α subunit of the IgE receptor (35%–40% of cases) or to IgE (5%–10% of cases).6 The IgG subclasses that appear to be pathogenic are IgG1; IgG3; and, to a lesser degree, IgG4 (though not IgG2).6 Complement activation augments histamine secretion by release of C5a.8 CAU has been reported to be associated with antithyroid antibodies (27% of cases)6,9; autoimmune conditions such as vitiligo, rheumatoid arthritis, and pernicious anemia; and low vitamin B12 levels.10 Patients with demonstrable histamine-releasing autoantibodies have a very strong association with HLA-DR4 and its associated allele HLA-DQ8.11
Histologically, the 2 groups of urticaria are indistinguishable.6 Advanced techniques show a perivascular nonnecrotizing infiltrate of CD4+ lymphocytes consisting of a mixture of TH1 and TH2 subtypes, plus monocytes, neutrophils, eosinophils, and basophils. These cells are recruited because of interactions with C5a, cell priming cytokines, chemokines, and adhesion molecules.6 A recent study also found inflammatory cells and mediator up-regulation in uninvolved CIU skin as a sign of prolonged and widespread "urticarial status."12
Physical Urticaria
Physical urticaria are classified and induced by a physical stimulus. Most physical urticaria occur within minutes of provocation and resolve within 2 hours, with the exception of delayed pressure urticaria, which may persist for 24 hours or longer.13 Angioedema may occur in all physical urticaria except dermographism. Overlap between groups is common, and physical urticaria often occur as an added feature of chronic urticaria.
The most common type of physical urticaria is simple immediate dermographism, presenting with linear wheals at sites of scratching or friction. It occurs in about 1.4% to 5% of the population worldwide14 and may be viewed as an exaggerated physiologic response. On average, dermographism runs a course of 2 to 3 years before usually resolving spontaneously.15
Delayed-pressure urticaria is a response to sustained pressure to the skin, presenting with deep erythematous edema after a delay of unknown cause lasting 30 minutes to 12 hours.14,16 An increased level of interleukin 6 has been found in suction blister fluid over induced lesions.2,15 The edema tends to be deeper, pruritic, and painful, and it may persist for days. Systemic features such as malaise, flulike symptoms, and arthralgia may occur. The prognosis is variable, but the mean duration is 6 to 9 years.17 The response to antihistamines often is poor, and oral corticosteroids may be needed for disease control.2
Cholinergic urticaria usually presents with multiple, transient, pruritic, small, red macules or papules on the neck, trunk, forearms, wrists, and thighs in response to heat, often surrounded by an obvious flare. It mainly affects young adults, with an overall prevalence of 11% in this group.18 Fifty percent of patients are atopic.15 Angioedema and systemic manifestations such as headache, palpitations, abdominal pain, wheezing, and syncope may occur. The cholinergic sympathetic innervation of sweat glands is involved because the eruption can be blocked by topical anticholinergic drugs,19 but how this leads to urticaria is unclear. The routine treatment is with low-sedation H1-type antihistamines, with or without an anxiolytic such as oral propranolol. In severe cases, the anabolic steroid stanazolol has been used.15
Cold urticaria is a heterogeneous condition in which whealing occurs within minutes in response to cold exposure, most frequently in children and young adults. Wheals usually arise at the site of localized cooling but may be generalized following lowering of the body temperature.16 Diagnosis may be confirmed by applying an ice cube for 5 to 15 minutes to the skin, allowing an interval for skin rewarming, and observing the development of whealing that occurs on skin rewarming. Systemic symptoms such as flushing, headache, abdominal pain, and syncope can occur if large areas are affected. The cause is unknown, but a serum factor, possibly IgM or IgE, has been implicated.20 A heterozygous deficiency of the protease inhibitor α1-antichymotrypsin has been demonstrated and may be etiologically important in some patients.21 The prognosis is good, with spontaneous improvement in an average of 2 to 3 years.15 Ninety-six percent of cases of cold urticaria are primary.22 The diagnosis of secondary acquired cold urticaria depends on being able to demonstrate cryoglobulins, cold agglutinins, or possibly cryofibrinogens.17 These findings should, in turn, lead to investigations for an underlying cause, such as hepatitis B or C infection, lymphoproliferative disease, or infectious mononucleosis.15
Other uncommon forms of physical urticaria include adrenergic urticaria, which develops during phases of stress and has been associated with an increase in the plasma concentrations of norepinephrine, epinephrine, and prolactin.23 Aquagenic urticaria is precipitated by skin contact with water of any temperature.3 Exercise-induced anaphylaxis involves urticaria, respiratory distress, or hypotension after exercise. In localized heat urticaria, wheals occur on skin in direct contact with warm objects. Solar urticaria is a rare condition that occurs within minutes of exposure to UV light waves ranging from 280 to 760 nm14; it usually disappears in less than one hour. Vibratory urticaria occurs after a vibratory stimulus and can be a hereditary autosomaldominant disorder or an acquired sporadic disease.24
Urticarial Vasculitis
Urticarial vasculitis describes a distinct entity in which the gross cutaneous lesions resemble urticaria and histologically show features of a vasculitis. The diagnosis is suggested clinically by wheals lasting more than 24 hours and residual bruising.25 Although the clinical lesions may present as typical urticaria, pathophysiologically, it is a different disease caused by deposition of antigen-antibody complexes in vessel walls, a type 3 reaction causing vascular damage.17 Lesions often occur at pressure points and may resolve with residual purpura. Extracutaneous manifestations include transient and migratory arthralgia (50%); gastrointestinal symptoms (20%); and pulmonary obstructive disease (20%), particularly in smokers and patients with renal disease (5%–10%).17 Normocomplementemic urticarial vasculitis usually is idiopathic, but hypocomplementemic urticarial vasculitis may be associated with underlying systemic lupus erythematosus, Sjögren syndrome, or cryoglobulinemia.2 Primary urticarial vasculitis can occasionally evolve into systemic lupus erythematosus.26 Patients with urticarial vasculitis often improve on nonsteroidal anti-inflammatory drugs (NSAIDs), but some patients may need immunosuppressive therapy.
Contact Urticaria
Contact urticaria develops at the site(s) of contact of an urticant and can be divided into an allergic subgroup caused by an IgE-allergen interaction and a nonallergic subgroup that is IgE independent. The allergic form typically is seen in children with atopic dermatitis sensitized to environmental allergens such as grass, animals, food, or latex, and it may be complicated by anaphylaxis. Natural rubber latex is one of the most important causes today.27 This type appears within minutes, fades within 2 hours, and is partially inhibited by antihistamines. Nonallergic contact urticaria is caused by the direct effect of the urticant on blood vessels and includes irritants such as benzoic acid and cinnamic aldehyde in cosmetics. It may take 45 minutes for lesions to appear and urticaria is partially inhibited by NSAIDs.
Angioedema Without Wheals
It is useful to classify angioedema occurring without wheals as a separate entity because its etiology may be associated with hereditary angioedema, which must be excluded. The condition usually is idiopathic or caused by a drug reaction to angiotensin-converting enzyme inhibitors, aspirin, or NSAIDs. Hereditary angioedema is a rare autosomal-dominant condition with a prevalence between 1:10,000 and 1:150,000 in the general population and is caused by a deficiency (type 1, 85%) or dysfunction (type 2, 15%) of C1 inhibitor.28 A low level of C4 in the serum is a constant and diagnostic feature. A third type affecting primarily women and exacerbated by estrogens recently has been described.28 Patients have lifelong episodic angioedema and may experience colicky abdominal pain. Laryngeal involvement can be life threatening. Treatment is difficult and involves fluid replacement and purified C1 inhibitor concentrate for acute attacks (not approved in the United States) and prophylactic treatment with anabolic androgens and antifibrinolytics.28
Diagnosis
The diagnosis of urticaria is primarily clinical; extensive laboratory tests are very rarely needed—only when indicated by the patient history.3 Some authors argue that laboratory investigations are unnecessary for mild ordinary urticaria responding to antihistamines.29 Taking a thorough patient history has been found to be almost as effective in identifying a cause as a complete diagnostic evaluation.30 In acute urticaria, if the history indicates a type 1 hypersensitivity reaction, confirmation is possible by a prick test or laboratory radioallergosorbent tests.3 Many physical and contact urticaria can be confirmed by a challenge of the offending agent. An initial baseline investigation with a complete blood count and erythrocyte sedimentation rate should be taken in more severe cases to identify any internal disease or raise the possibility of urticarial vasculitis.17 Of note, a biopsy is more sensitive and specific for ruling out urticarial vasculitis than are a complete blood count and erythrocyte sedimentation rate.
A search for thyroid autoantibodies is appropriate for all chronic urticaria not responding to first-line therapies with antihistamines, especially when autoimmune urticaria is suspected.2 Further investigations are guided by clinical suspicion, which may include a skin biopsy, autoimmune screening, urinalysis, serum cryoglobulins, and hepatitis B and C serology.31 The only available test to screen for autoantibodies against the IgE receptor is the autologous serum skin test. This test should be performed with care because infections could be transmitted, particularly if, by mistake, patients were not injected with their own serum.31 Measurement of C4 is indicated only in patients who present with angioedema alone and should be followed by a determination of the levels and function of C1 inhibitor, if C4 is below reference range.29
Management and Treatment
Management of urticaria depends on its cause. Aggravating factors should be identified from the history, and triggering stimuli for physical urticaria should be avoided. Simple cooling lotions such as menthol 1% or 2% in an aqueous cream often are useful.32 Aspirin and NSAIDs should be avoided because they aggravate symptoms in 30% of patients.33 Patients taking low-dose aspirin for its antithrombotic properties usually can continue regular treatment. Avoiding codeine and other opiates also is recommended because an enhanced skin test reaction may be found in chronic urticaria.34 Avoiding dietary pseudoallergens, such as food coloring and natural salicylates, is controversial.14,35 This generally has only a small role unless proven by a double-blinded placebo-controlled challenge.2
The mainstays for treatment of urticaria are oral antihistamines, as they reduce pruritus and wheal duration and numbers. Oral antihistamines have been reported to produce moderate or good response in 44% to 91% of patients with all types of urticaria.36,37 Antihistamines can be grouped into first-generation (sedating), second generation (minimally sedating), third-generation (nonsedating), and H2 antagonists.17 The physiologic and pathologic actions of histamine are mediated through 4 histamine receptor subtypes: H1, H2, H3, and H4.38 The erythema, wheal formation, and itching associated with urticaria are mainly due to activation of H1 receptors and the less contributory role of H2 receptors.38 Histamine H3 receptors are located presynaptically on postganglionic sympathetic norepinephric nerves, including sympathetics innervating the heart and blood vessels. The contribution of H3 receptors to skin responses mediated by histamine has not been fully elucidated. However, in a recent experimental study, the authors reported that the combination of H1 and H3 antagonists might be a novel approach for the treatment of urticaria.38
Initially, a minimally sedating second- or third-generation antihistamine, such as loratadine,39 fexofenadine hydrochloride,40 and cetirizine hydrochloride,41-44 should be given at a once-daily oral dosing. When one antihistamine is not helpful, it is usually worth trying a different one, and some physicians combine 2 or more antihistamines at the same time.3 It is common practice to exceed the licensed dose in severely affected patients.31 High doses of antihistamines have effects beyond the blockade of histamine receptors, and actions that are not due to antagonism of H1 receptors may account for the efficacy of older antihistamines.45 As a general rule, antihistamines are safe and have few substantial adverse effects; drug interactions are rare. If possible, it is best to avoid all antihistamines in pregnancy, though none have been proven teratogenic. If one is used, the consensus is that chlorpheniramine maleate is among the safest.46
Addition of a sedating first-generation antihistamine such as hydroxyzine at night can be helpful, especially if nocturnal pruritus prevents sleep. The use of a sedating antihistamine as monotherapy is less desirable because of impairment of cognitive function, including driving performance and concentration. The addition of a H2 antagonist to conventional H1 antihistamines may be helpful in some patients.3,47 Doxepin hydrochloride at low doses (10–50 mg) is used for its potent H1 and H2 receptor antagonist properties. Doxepin hydrochloride is highly sedative and especially suitable for patients with associated depression.48
Oral corticosteroids given in short reducing courses may be needed for severe exacerbations not responding to full-dose antihistamines. Relatively high doses of up to 40 to 60 mg of prednisone may be needed for disease control. Alternate-day ste-roids may be used for patients with severe disease.6 Long-term administration should be avoided.1
Many patients feel reassured by carrying an epinephrine pen for self-administration if they are prone to severe attacks. Leukotriene antagonists (zafirlukast and montelukast sodium) have been shown to be superior to placebo in the treatment of patients with chronic urticaria.49,50 Nifedipine has a small effect in chronic urticaria and often is used for patients with concomitant hypertension. Thyroxine recently was reported to suppress CIU symptoms associated with antithyroid autoantibodies in some patients.51
Given the role of the immune system in a subset of patients, immunosuppressive therapy is considered for patients with a severe disabling course. Cyclosporine at 2.5 to 5 mg/kg per day is of proven value in autoantibody-positive chronic urticaria52 but also is effective in most cases of severe autoantibody-negative disease.15 Tacrolimus also has shown promise in a recent trial.53 Other options include plasmapheresis54 and intravenous immunoglobulin.55,56 Optimal treatment protocols have yet to be confirmed. Treatments for CIU with only limited or anecdotal supportive evidence include sulfasalazine, methotrexate, rofecoxib, colchicine, dapsone, and cyclophosphamide.3
Future treatment may involve development of selective immunotherapy targeting the IgE receptor or vaccinations to down-regulate and induce tolerance to the IgE receptor. Other potential strategies include blocking formation of C5a and use of therapeutic antibodies such as anti-IgE, anti–tumor necrosis factor α, and anti–interleukin 5.2
Conclusion
There is no single way to manage urticaria and angioedema. Most patients are treated successfully with antihistamines. However, patients with severe antihistamine-resistant urticaria may be very disabled by their disease, and the treatment can pose a major challenge to the physician.
- Henderson RL Jr, Fleischer AB Jr, Feldman SR. Allergists and dermatologists have far more expertise in caring for patients with urticaria than other specialists. J Am Acad Dermatol. 2000;43:1084-1091.
- Grattan CEH, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645-657.
- Kozel MM, Sabroe RA. Chronic urticaria: aetiology, management and current and future treatment options. Drugs. 2004;64:2515-2536.
- Negro-Alvarez JM, Miralles-Lopez JC. Chronic idiopathic urticaria treatment. Allergol Immunopathol (Madr). 2001;29:129-132.
- Grob JJ, Revuz J, Ortonne JP, et al. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol. 2005;152:289-295.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Brunetti L, Francavilla R, Miniello VL, et al. High prevalence of autoimmune urticaria in children with chronic urticaria. J Allergy Clin Immunol. 2004;114:922-927.
- Kikuchi Y, Kaplan AP. A role for C5a in augmenting IgG-dependent histamine release from basophils in chronic urticaria. J Allergy Clin Immunol. 2002;109:114-118.
- Gruber BL, Baeza M, Marchese M, et al. Prevalence and functional role of anti-IgE antibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213-217.
- Mete N, Gulbahar O, Aydin A, et al. Low B12 levels in chronic idiopathic urticaria. J Investig Allergol Clin Immunol. 2004;14:292-299.
- O'Donnell BF, O'Neill CM, Francis DM, et al. Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br J Dermatol. 1999;140:853-858.
- Caproni M, Giomi B, Volpi W, et al. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin Immunol. 2005;114:284-292.
- Black AK, Lawlor F, Greaves MW. Consensus meeting on the definition of physical urticarias and urticarial vasculitis. Clin Exp Dermatol. 1996;21:424-426.
- Henz BM. Physical urticaria. In: Henz BM, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical, Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer Verlag; 1998:55-89.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines: an EAACI position paper. Allergy. 1997;52:503-513.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:287-311.
- Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
- Herxheimer A. The nervous pathway mediating cholinergic urticaria. Clin Sci (Lond). 1956;15:195-204.
- Wanderer AA, Maselli R, Ellis EF, et al. Immunological characterisation of serum factors responsible for cold urticaria.
- Lindmark B, Wallengren J. Heterozygous alpha1-antichymotrypsin deficiency may be associated with cold urticaria. Allergy. 1992;47:456-458.
- Neittaanmaki H. Cold urticaria: clinical findings in 220 patients. J Am Acad Dermatol. 1985;13:636-644.
- Haustein UF. Adrenergic urticaria and adrenergic pruritus. Acta Derm Venereol. 1990;70:82-84.
- Mathelier-Fusade P, Vermeulen C, Leynadier F. Vibratory angioedema. Ann Dermatol Venereol. 2001;128:750-752.
- O’Donnell B, Black AK. Urticarial vasculitis. Int Angiol. 1995;14:166-174.
- Bisaccia E, Adamo V, Rozan SW. Urticarial vasculitis progressing to systemic lupus erythematosus. Arch Dermatol. 1988;124:1088-1090.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Zuraw BL. Current and future therapy for hereditary angioedema. Clin Immunol. 2005;114:10-16.
- Grattan C, Powell S, Humphreys F. Management and diagnostic guidelines for urticaria and angio-oedema. Br J Dermatol. 2001;144:708-714.
- Kozel MM, Mekkes JR, Bossuyt PM, et al. The effectiveness of a history-based diagnostic approach in chronic urticaria and angioedema. Arch Dermatol. 1998;134:1575-1580.
- Zuberbier T, Greaves MW, Juhlin L, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001;6:123-127.
- Bromm B, Scharein E, Darsow U, et al. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett. 1995;187:157-160.
- Doeglas HMG. Reactions to aspirin and food additives in patients with chronic urticaria, including the physical urticarias. Br J Dermatol. 1975;93:135-144.
- Kaufman A, Rosenstreich DL. Mast cell heterogeneity in chronic idiopathic urticaria. Ann Allergy. 1990;65:367-373.
- Ortolani C, Pastorello E, Ispano M, et al. Food allergy diagnosis protocol. Allerg Immunol (Paris). 1988;20:48-50.
- 36. Humphreys F, Hunter JA. The characteristics of urticarial in 390 patients. Br J Dermatol. 1998;138:635-638.
- 37. Nettis E, Pannofino A, D’Aprile C, et al. Clinical and aetiological aspects in urticaria and angio-oedema. Br J Dermatol. 2003;148:501-506.
- 38. McLeod RL, Mingo GG, Kreutner W, et al. Effect of combined histamine H1 and H3 receptor blockade on cutaneous microvascular permeability elicited by compound 48/80. Life Sci. 2005;76:1787-1794.
- 39. Monroe EW. Loratadine in the treatment of urticaria. Clin Ther. 1997;19:232-242.
- 40. Nelson HS, Reynolds R, Mason J. Fexofenadine HCl is safe and effective for treatment of chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2000;84: 517-522.
- 41. Breneman D, Bronsky EA, Bruce S, et al. Cetirizine and astemizole therapy for chronic idiopathic urticaria: a double-blind, placebo-controlled, comparative trial. J Am Acad Dermatol. 1995;33(2 pt 1):192-198.
- 42. Breneman DL. Cetirizine versus hydroxyzine and placebo in chronic idiopathic urticaria. Ann Pharmacother. 1996;30:1075-1079.
- 43. Kalivas J, Breneman D, Tharp M, et al. Urticaria: clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy Clin Immunol. 1990;86(6 pt 2): 1014-1018.
- 44. Andri L, Senna GE, Betteli C, et al. A comparison of the efficacy of cetirizine and terfenadine: a double-blind, controlled study of chronic idiopathic urticaria. Allergy. 1993;48:358-365.
- 45. Kaplan AP. Clinical practice. chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- 46. Andrews AW, Fornwald JA, Lijinsky W. Nitrosation and mutagenicity of some anime drugs. Toxicol Appl Pharmacol. 1980;52:237-244.
- 47. Commens CA, Greaves MW. Cimetidine in chronic idiopathic urticaria: a randomised double-blind study. Br J Dermatol. 1978;99:675-679.
- 48. Furukawa T, McGuire H, Barbui C. Low dosage tricyclic antidepressants for depression. Cochrane Database Syst Rev. 2003;(3):CD003197.
- 49. Ellis MH. Successful treatment of chronic urticaria with leukotriene antagonists. J Allergy Clin Immunol. 1998;102:876-877.
- 50. Spector S, Tan RA. Antileukotrienes in chronic urticaria. J Allergy Clin Immunol. 1998;101(4 pt 1):572.
- 51. Aversano M, Caiazzo P, Iorio G, et al. Improvement of chronic idiopathic urticaria with L-thyroxine: a new TSH role in immune response? Allergy. 2005;60:489-493.
- Grattan CE, O’Donnell BF, Francis DM, et al. Randomized double-blind study of cyclosporin in chronic “idiopathic” urticaria. Br J Dermatol. 2000;143:365-372.
- Kessel A, Bamberger E, Toubi E. Tacrolimus in the treatment of severe chronic idiopathic urticaria: an open-label prospective study. J Am Acad Dermatol. 2005;52:145-148.
- Grattan CEH, Francis DM, Slater NGP, et al. Plasmapheresis for severe unremitting chronic urticaria. Lancet. 1992;339:1078-1080.
- O’Donnell BF, Barr RM, Blac AK, et al. Intravenous immunoglobulin in chronic autoimmune urticaria. Br J Dermatol. 1998;138:101-106.
- Klote MM, Nelson MR, Engler RJ. Autoimmune urticarial response to high-dose intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2005;94:307-308.
- Henderson RL Jr, Fleischer AB Jr, Feldman SR. Allergists and dermatologists have far more expertise in caring for patients with urticaria than other specialists. J Am Acad Dermatol. 2000;43:1084-1091.
- Grattan CEH, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645-657.
- Kozel MM, Sabroe RA. Chronic urticaria: aetiology, management and current and future treatment options. Drugs. 2004;64:2515-2536.
- Negro-Alvarez JM, Miralles-Lopez JC. Chronic idiopathic urticaria treatment. Allergol Immunopathol (Madr). 2001;29:129-132.
- Grob JJ, Revuz J, Ortonne JP, et al. Comparative study of the impact of chronic urticaria, psoriasis and atopic dermatitis on the quality of life. Br J Dermatol. 2005;152:289-295.
- Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465-474.
- Brunetti L, Francavilla R, Miniello VL, et al. High prevalence of autoimmune urticaria in children with chronic urticaria. J Allergy Clin Immunol. 2004;114:922-927.
- Kikuchi Y, Kaplan AP. A role for C5a in augmenting IgG-dependent histamine release from basophils in chronic urticaria. J Allergy Clin Immunol. 2002;109:114-118.
- Gruber BL, Baeza M, Marchese M, et al. Prevalence and functional role of anti-IgE antibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213-217.
- Mete N, Gulbahar O, Aydin A, et al. Low B12 levels in chronic idiopathic urticaria. J Investig Allergol Clin Immunol. 2004;14:292-299.
- O'Donnell BF, O'Neill CM, Francis DM, et al. Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br J Dermatol. 1999;140:853-858.
- Caproni M, Giomi B, Volpi W, et al. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin Immunol. 2005;114:284-292.
- Black AK, Lawlor F, Greaves MW. Consensus meeting on the definition of physical urticarias and urticarial vasculitis. Clin Exp Dermatol. 1996;21:424-426.
- Henz BM. Physical urticaria. In: Henz BM, Zuberbier T, Grabbe J, et al, eds. Urticaria: Clinical, Diagnostic and Therapeutic Aspects. Berlin, Germany: Springer Verlag; 1998:55-89.
- Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-672.
- Kontou-Fili K, Borici-Mazi R, Kapp A, et al. Physical urticaria: classification and diagnostic guidelines: an EAACI position paper. Allergy. 1997;52:503-513.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:287-311.
- Zuberbier T, Althaus C, Chantraine-Hess S, et al. Prevalence of cholinergic urticaria in young adults. J Am Acad Dermatol. 1994;31:978-981.
- Herxheimer A. The nervous pathway mediating cholinergic urticaria. Clin Sci (Lond). 1956;15:195-204.
- Wanderer AA, Maselli R, Ellis EF, et al. Immunological characterisation of serum factors responsible for cold urticaria.
- Lindmark B, Wallengren J. Heterozygous alpha1-antichymotrypsin deficiency may be associated with cold urticaria. Allergy. 1992;47:456-458.
- Neittaanmaki H. Cold urticaria: clinical findings in 220 patients. J Am Acad Dermatol. 1985;13:636-644.
- Haustein UF. Adrenergic urticaria and adrenergic pruritus. Acta Derm Venereol. 1990;70:82-84.
- Mathelier-Fusade P, Vermeulen C, Leynadier F. Vibratory angioedema. Ann Dermatol Venereol. 2001;128:750-752.
- O’Donnell B, Black AK. Urticarial vasculitis. Int Angiol. 1995;14:166-174.
- Bisaccia E, Adamo V, Rozan SW. Urticarial vasculitis progressing to systemic lupus erythematosus. Arch Dermatol. 1988;124:1088-1090.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Zuraw BL. Current and future therapy for hereditary angioedema. Clin Immunol. 2005;114:10-16.
- Grattan C, Powell S, Humphreys F. Management and diagnostic guidelines for urticaria and angio-oedema. Br J Dermatol. 2001;144:708-714.
- Kozel MM, Mekkes JR, Bossuyt PM, et al. The effectiveness of a history-based diagnostic approach in chronic urticaria and angioedema. Arch Dermatol. 1998;134:1575-1580.
- Zuberbier T, Greaves MW, Juhlin L, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001;6:123-127.
- Bromm B, Scharein E, Darsow U, et al. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett. 1995;187:157-160.
- Doeglas HMG. Reactions to aspirin and food additives in patients with chronic urticaria, including the physical urticarias. Br J Dermatol. 1975;93:135-144.
- Kaufman A, Rosenstreich DL. Mast cell heterogeneity in chronic idiopathic urticaria. Ann Allergy. 1990;65:367-373.
- Ortolani C, Pastorello E, Ispano M, et al. Food allergy diagnosis protocol. Allerg Immunol (Paris). 1988;20:48-50.
- 36. Humphreys F, Hunter JA. The characteristics of urticarial in 390 patients. Br J Dermatol. 1998;138:635-638.
- 37. Nettis E, Pannofino A, D’Aprile C, et al. Clinical and aetiological aspects in urticaria and angio-oedema. Br J Dermatol. 2003;148:501-506.
- 38. McLeod RL, Mingo GG, Kreutner W, et al. Effect of combined histamine H1 and H3 receptor blockade on cutaneous microvascular permeability elicited by compound 48/80. Life Sci. 2005;76:1787-1794.
- 39. Monroe EW. Loratadine in the treatment of urticaria. Clin Ther. 1997;19:232-242.
- 40. Nelson HS, Reynolds R, Mason J. Fexofenadine HCl is safe and effective for treatment of chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2000;84: 517-522.
- 41. Breneman D, Bronsky EA, Bruce S, et al. Cetirizine and astemizole therapy for chronic idiopathic urticaria: a double-blind, placebo-controlled, comparative trial. J Am Acad Dermatol. 1995;33(2 pt 1):192-198.
- 42. Breneman DL. Cetirizine versus hydroxyzine and placebo in chronic idiopathic urticaria. Ann Pharmacother. 1996;30:1075-1079.
- 43. Kalivas J, Breneman D, Tharp M, et al. Urticaria: clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy Clin Immunol. 1990;86(6 pt 2): 1014-1018.
- 44. Andri L, Senna GE, Betteli C, et al. A comparison of the efficacy of cetirizine and terfenadine: a double-blind, controlled study of chronic idiopathic urticaria. Allergy. 1993;48:358-365.
- 45. Kaplan AP. Clinical practice. chronic urticaria and angioedema. N Engl J Med. 2002;346:175-179.
- 46. Andrews AW, Fornwald JA, Lijinsky W. Nitrosation and mutagenicity of some anime drugs. Toxicol Appl Pharmacol. 1980;52:237-244.
- 47. Commens CA, Greaves MW. Cimetidine in chronic idiopathic urticaria: a randomised double-blind study. Br J Dermatol. 1978;99:675-679.
- 48. Furukawa T, McGuire H, Barbui C. Low dosage tricyclic antidepressants for depression. Cochrane Database Syst Rev. 2003;(3):CD003197.
- 49. Ellis MH. Successful treatment of chronic urticaria with leukotriene antagonists. J Allergy Clin Immunol. 1998;102:876-877.
- 50. Spector S, Tan RA. Antileukotrienes in chronic urticaria. J Allergy Clin Immunol. 1998;101(4 pt 1):572.
- 51. Aversano M, Caiazzo P, Iorio G, et al. Improvement of chronic idiopathic urticaria with L-thyroxine: a new TSH role in immune response? Allergy. 2005;60:489-493.
- Grattan CE, O’Donnell BF, Francis DM, et al. Randomized double-blind study of cyclosporin in chronic “idiopathic” urticaria. Br J Dermatol. 2000;143:365-372.
- Kessel A, Bamberger E, Toubi E. Tacrolimus in the treatment of severe chronic idiopathic urticaria: an open-label prospective study. J Am Acad Dermatol. 2005;52:145-148.
- Grattan CEH, Francis DM, Slater NGP, et al. Plasmapheresis for severe unremitting chronic urticaria. Lancet. 1992;339:1078-1080.
- O’Donnell BF, Barr RM, Blac AK, et al. Intravenous immunoglobulin in chronic autoimmune urticaria. Br J Dermatol. 1998;138:101-106.
- Klote MM, Nelson MR, Engler RJ. Autoimmune urticarial response to high-dose intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2005;94:307-308.
Traction Folliculitis: An Underreported Entity
CPT 2007: What’s in it for you?
ObGyns stand to benefit from new Current Procedural Terminology (CPT) codes that capture more of the specifics of procedures such as laparoscopic hysterectomy, and provide codes for newer kinds of services such as prenatal nuchal translucency screening and genetic counseling. A downside for 2007—getting accustomed to the renumbered codes for bone density and breast imaging.
Laparoscopic hysterectomy codes get specific
58541 Laparoscopy, surgical, supracervical hysterectomy, for uterus 250 g or less
58542 …with removal of tube(s) and/or ovary(s)
58543 Laparoscopy, surgical, supracervical hysterectomy, for uterus greater than
250 g
58544 …with removal of tube(s) and/or ovary(s)
Nuchal translucency: Document the detail
76813 Ultrasound, pregnant uterus, real time with image documentation, 1st-trimester fetal nuchal translucency measurement, transabdominal or transvaginal approach; single or 1st gestation
76814 …each additional gestation
Reimbursement should become routine for 1st-trimester nuchal translucency ultrasound imaging.
Coding has been a challenge; in fact, ACOG only recommended reporting the unlisted code 76999 (unlisted ultrasound procedure [eg, diagnostic, interventional]), which requires submission of documentation to make the case for payment. The test is normally performed between 11 and 13 weeks’ gestation.
When measured correctly, nuchal translucency thickness is a powerful marker in Down syndrome screening in the late first trimester
Even when the payer does not require it, documentation is important. Nuchal translucency ultrasound documentation should include:
- the fetal crown–rump length
- verification of the sagittal view of the fetal spine
- 3 measurements of the maximum thickness of the subcutaneous translucency between the skin and the soft tissue overlying the cervical spine
- as with all ultrasound procedures, image documentation and a final written report
Special training is required by the sonographer or physician who performs this measurement. So be aware that the payer may have rules to ensure such training.
Different codes for initial and recurrent cancer
58950 Resection (initial) of ovarian, tubal or primary peritoneal malignancy with bilateral salpingo-oophorectomy and omentectomy
Primary malignancy resections will continue to be reported with the existing code numbers 58950 through 58952. To make the point clear, CPT revised the wording of the base code, 58950, to specify the initial operation.
58957 Resection (tumor debulking) of recurrent ovarian, tubal, primary peritoneal, uterine malignancy (intra-abdominal, retroperitoneal tumors), with omentectomy, if performed
58958 …with pelvic lymphadenectomy
Unlike other codes for malignancy in the female genitourinary section of CPT, the above 2 new codes specify a broader range of cancers to include uterine malignancy.
Previously, code 49200 or code 49201 (excision or destruction, open, intra-abdominal or retroperitoneal tumors or cysts or endometriomas) would have been as reported for recurrent uterine malignancy.
Do not report these codes in addition: 38770 and 38780 (removal of pelvic or retroperitoneal lymph nodes), 44005 (enterolysis), 49000 (exploratory laparotomy), 49200–49215 (open excision of tumors), 49255 (omentectomy), or 58900–58960 (removal of tubes and ovaries).
New technologies
Uterine artery embolization
37210 Uterine artery embolization
The new code includes vascular access, vessel selection, injection of the material, intraprocedure mapping, and all radiological supervision and interpretation, including image guidance.
Genetic counseling
96040 Medical genetics and genetic counseling services, each 30 minutes face-to-face with patient/family
This code is good news for practices that use the services of a genetic counselor. Need, content, and total time must be documented in the report. However, Medicare has assigned no physician relative value units to this new code because they consider it bundled into any E/M service. Check with your payers about separate reimbursement for this service.
| OLD | NEW | |
| BONE DENSITY | ||
| CT, bone mineral density study 1 or more sites | ||
| Axial skeleton (eg, hips, pelvis, spine) | 76060 | 77078 |
| Appendicular skeleton (peripheral) (eg, radius, wrist, heel) | 76061 | 77079 |
| Dual-energy X-ray absorptiometry, bone-density study 1 or more sites | ||
| Axial skeleton | 76065 | 77080 |
| Appendicular skeleton | 76067 | 77081 |
| Vertebral fracture assessment | 76077 | 77082 |
| Radiographic absorptiometry (eg, photodensitometry, radiogrammetry) 1 or more sites | 76078 | 77083 |
| MAMMOGRAPHY | ||
| Unilateral | 76090 | 77055 |
| Bilateral | 76091 | 77056 |
| Screening mammography, bilateral (2-view film study of each breast) | 76092 | 77057 |
| INTRAOPERATIVE ULTRASOUND | ||
| Ultrasound guidance, intraoperative | 76986 | 76998 |
Elizabeth W. Woodcock
Atlanta-based Elizabeth W. Woodcock is a speaker, trainer, and author specializing in practice management. Among her recent books is Mastering Patient Flow.
It’s likely you counsel your patients about smoking cessation at least once a day, if not more. Do you know that you can be reimbursed for this important service? Medicare and Medicaid pay for 8 visits annually in a 12-month period, and other payers are rapidly following suit. In 2005, the Centers for Medicare and Medicaid Services (CMS) added procedure codes for intermediate and intensive smoking cessation visits:
G0375 Smoking and tobacco-use cessation counseling visit; intermediate, greater than 3 minutes up to 10 minutes.
Short descriptor Smoke/tobacco counseling 3-10
G0376 Smoking and tobacco-use cessation visit; intensive, greater than 10 minutes.
Short descriptor Smoke/tobacco counseling greater than 10
G0375 pays approximately $13; G0376 pays approximately $25. The exact payment depends on your geographic practice cost index (GPCI) as determined by CMS.
These codes do not modify coverage for minimal smoking cessation counseling (3 minutes or less in duration), which is considered covered as part of each evaluation and management (E/M) visit, and therefore is not separately billable.
ObGyns stand to benefit from new Current Procedural Terminology (CPT) codes that capture more of the specifics of procedures such as laparoscopic hysterectomy, and provide codes for newer kinds of services such as prenatal nuchal translucency screening and genetic counseling. A downside for 2007—getting accustomed to the renumbered codes for bone density and breast imaging.
Laparoscopic hysterectomy codes get specific
58541 Laparoscopy, surgical, supracervical hysterectomy, for uterus 250 g or less
58542 …with removal of tube(s) and/or ovary(s)
58543 Laparoscopy, surgical, supracervical hysterectomy, for uterus greater than
250 g
58544 …with removal of tube(s) and/or ovary(s)
Nuchal translucency: Document the detail
76813 Ultrasound, pregnant uterus, real time with image documentation, 1st-trimester fetal nuchal translucency measurement, transabdominal or transvaginal approach; single or 1st gestation
76814 …each additional gestation
Reimbursement should become routine for 1st-trimester nuchal translucency ultrasound imaging.
Coding has been a challenge; in fact, ACOG only recommended reporting the unlisted code 76999 (unlisted ultrasound procedure [eg, diagnostic, interventional]), which requires submission of documentation to make the case for payment. The test is normally performed between 11 and 13 weeks’ gestation.
When measured correctly, nuchal translucency thickness is a powerful marker in Down syndrome screening in the late first trimester
Even when the payer does not require it, documentation is important. Nuchal translucency ultrasound documentation should include:
- the fetal crown–rump length
- verification of the sagittal view of the fetal spine
- 3 measurements of the maximum thickness of the subcutaneous translucency between the skin and the soft tissue overlying the cervical spine
- as with all ultrasound procedures, image documentation and a final written report
Special training is required by the sonographer or physician who performs this measurement. So be aware that the payer may have rules to ensure such training.
Different codes for initial and recurrent cancer
58950 Resection (initial) of ovarian, tubal or primary peritoneal malignancy with bilateral salpingo-oophorectomy and omentectomy
Primary malignancy resections will continue to be reported with the existing code numbers 58950 through 58952. To make the point clear, CPT revised the wording of the base code, 58950, to specify the initial operation.
58957 Resection (tumor debulking) of recurrent ovarian, tubal, primary peritoneal, uterine malignancy (intra-abdominal, retroperitoneal tumors), with omentectomy, if performed
58958 …with pelvic lymphadenectomy
Unlike other codes for malignancy in the female genitourinary section of CPT, the above 2 new codes specify a broader range of cancers to include uterine malignancy.
Previously, code 49200 or code 49201 (excision or destruction, open, intra-abdominal or retroperitoneal tumors or cysts or endometriomas) would have been as reported for recurrent uterine malignancy.
Do not report these codes in addition: 38770 and 38780 (removal of pelvic or retroperitoneal lymph nodes), 44005 (enterolysis), 49000 (exploratory laparotomy), 49200–49215 (open excision of tumors), 49255 (omentectomy), or 58900–58960 (removal of tubes and ovaries).
New technologies
Uterine artery embolization
37210 Uterine artery embolization
The new code includes vascular access, vessel selection, injection of the material, intraprocedure mapping, and all radiological supervision and interpretation, including image guidance.
Genetic counseling
96040 Medical genetics and genetic counseling services, each 30 minutes face-to-face with patient/family
This code is good news for practices that use the services of a genetic counselor. Need, content, and total time must be documented in the report. However, Medicare has assigned no physician relative value units to this new code because they consider it bundled into any E/M service. Check with your payers about separate reimbursement for this service.
| OLD | NEW | |
| BONE DENSITY | ||
| CT, bone mineral density study 1 or more sites | ||
| Axial skeleton (eg, hips, pelvis, spine) | 76060 | 77078 |
| Appendicular skeleton (peripheral) (eg, radius, wrist, heel) | 76061 | 77079 |
| Dual-energy X-ray absorptiometry, bone-density study 1 or more sites | ||
| Axial skeleton | 76065 | 77080 |
| Appendicular skeleton | 76067 | 77081 |
| Vertebral fracture assessment | 76077 | 77082 |
| Radiographic absorptiometry (eg, photodensitometry, radiogrammetry) 1 or more sites | 76078 | 77083 |
| MAMMOGRAPHY | ||
| Unilateral | 76090 | 77055 |
| Bilateral | 76091 | 77056 |
| Screening mammography, bilateral (2-view film study of each breast) | 76092 | 77057 |
| INTRAOPERATIVE ULTRASOUND | ||
| Ultrasound guidance, intraoperative | 76986 | 76998 |
Elizabeth W. Woodcock
Atlanta-based Elizabeth W. Woodcock is a speaker, trainer, and author specializing in practice management. Among her recent books is Mastering Patient Flow.
It’s likely you counsel your patients about smoking cessation at least once a day, if not more. Do you know that you can be reimbursed for this important service? Medicare and Medicaid pay for 8 visits annually in a 12-month period, and other payers are rapidly following suit. In 2005, the Centers for Medicare and Medicaid Services (CMS) added procedure codes for intermediate and intensive smoking cessation visits:
G0375 Smoking and tobacco-use cessation counseling visit; intermediate, greater than 3 minutes up to 10 minutes.
Short descriptor Smoke/tobacco counseling 3-10
G0376 Smoking and tobacco-use cessation visit; intensive, greater than 10 minutes.
Short descriptor Smoke/tobacco counseling greater than 10
G0375 pays approximately $13; G0376 pays approximately $25. The exact payment depends on your geographic practice cost index (GPCI) as determined by CMS.
These codes do not modify coverage for minimal smoking cessation counseling (3 minutes or less in duration), which is considered covered as part of each evaluation and management (E/M) visit, and therefore is not separately billable.
ObGyns stand to benefit from new Current Procedural Terminology (CPT) codes that capture more of the specifics of procedures such as laparoscopic hysterectomy, and provide codes for newer kinds of services such as prenatal nuchal translucency screening and genetic counseling. A downside for 2007—getting accustomed to the renumbered codes for bone density and breast imaging.
Laparoscopic hysterectomy codes get specific
58541 Laparoscopy, surgical, supracervical hysterectomy, for uterus 250 g or less
58542 …with removal of tube(s) and/or ovary(s)
58543 Laparoscopy, surgical, supracervical hysterectomy, for uterus greater than
250 g
58544 …with removal of tube(s) and/or ovary(s)
Nuchal translucency: Document the detail
76813 Ultrasound, pregnant uterus, real time with image documentation, 1st-trimester fetal nuchal translucency measurement, transabdominal or transvaginal approach; single or 1st gestation
76814 …each additional gestation
Reimbursement should become routine for 1st-trimester nuchal translucency ultrasound imaging.
Coding has been a challenge; in fact, ACOG only recommended reporting the unlisted code 76999 (unlisted ultrasound procedure [eg, diagnostic, interventional]), which requires submission of documentation to make the case for payment. The test is normally performed between 11 and 13 weeks’ gestation.
When measured correctly, nuchal translucency thickness is a powerful marker in Down syndrome screening in the late first trimester
Even when the payer does not require it, documentation is important. Nuchal translucency ultrasound documentation should include:
- the fetal crown–rump length
- verification of the sagittal view of the fetal spine
- 3 measurements of the maximum thickness of the subcutaneous translucency between the skin and the soft tissue overlying the cervical spine
- as with all ultrasound procedures, image documentation and a final written report
Special training is required by the sonographer or physician who performs this measurement. So be aware that the payer may have rules to ensure such training.
Different codes for initial and recurrent cancer
58950 Resection (initial) of ovarian, tubal or primary peritoneal malignancy with bilateral salpingo-oophorectomy and omentectomy
Primary malignancy resections will continue to be reported with the existing code numbers 58950 through 58952. To make the point clear, CPT revised the wording of the base code, 58950, to specify the initial operation.
58957 Resection (tumor debulking) of recurrent ovarian, tubal, primary peritoneal, uterine malignancy (intra-abdominal, retroperitoneal tumors), with omentectomy, if performed
58958 …with pelvic lymphadenectomy
Unlike other codes for malignancy in the female genitourinary section of CPT, the above 2 new codes specify a broader range of cancers to include uterine malignancy.
Previously, code 49200 or code 49201 (excision or destruction, open, intra-abdominal or retroperitoneal tumors or cysts or endometriomas) would have been as reported for recurrent uterine malignancy.
Do not report these codes in addition: 38770 and 38780 (removal of pelvic or retroperitoneal lymph nodes), 44005 (enterolysis), 49000 (exploratory laparotomy), 49200–49215 (open excision of tumors), 49255 (omentectomy), or 58900–58960 (removal of tubes and ovaries).
New technologies
Uterine artery embolization
37210 Uterine artery embolization
The new code includes vascular access, vessel selection, injection of the material, intraprocedure mapping, and all radiological supervision and interpretation, including image guidance.
Genetic counseling
96040 Medical genetics and genetic counseling services, each 30 minutes face-to-face with patient/family
This code is good news for practices that use the services of a genetic counselor. Need, content, and total time must be documented in the report. However, Medicare has assigned no physician relative value units to this new code because they consider it bundled into any E/M service. Check with your payers about separate reimbursement for this service.
| OLD | NEW | |
| BONE DENSITY | ||
| CT, bone mineral density study 1 or more sites | ||
| Axial skeleton (eg, hips, pelvis, spine) | 76060 | 77078 |
| Appendicular skeleton (peripheral) (eg, radius, wrist, heel) | 76061 | 77079 |
| Dual-energy X-ray absorptiometry, bone-density study 1 or more sites | ||
| Axial skeleton | 76065 | 77080 |
| Appendicular skeleton | 76067 | 77081 |
| Vertebral fracture assessment | 76077 | 77082 |
| Radiographic absorptiometry (eg, photodensitometry, radiogrammetry) 1 or more sites | 76078 | 77083 |
| MAMMOGRAPHY | ||
| Unilateral | 76090 | 77055 |
| Bilateral | 76091 | 77056 |
| Screening mammography, bilateral (2-view film study of each breast) | 76092 | 77057 |
| INTRAOPERATIVE ULTRASOUND | ||
| Ultrasound guidance, intraoperative | 76986 | 76998 |
Elizabeth W. Woodcock
Atlanta-based Elizabeth W. Woodcock is a speaker, trainer, and author specializing in practice management. Among her recent books is Mastering Patient Flow.
It’s likely you counsel your patients about smoking cessation at least once a day, if not more. Do you know that you can be reimbursed for this important service? Medicare and Medicaid pay for 8 visits annually in a 12-month period, and other payers are rapidly following suit. In 2005, the Centers for Medicare and Medicaid Services (CMS) added procedure codes for intermediate and intensive smoking cessation visits:
G0375 Smoking and tobacco-use cessation counseling visit; intermediate, greater than 3 minutes up to 10 minutes.
Short descriptor Smoke/tobacco counseling 3-10
G0376 Smoking and tobacco-use cessation visit; intensive, greater than 10 minutes.
Short descriptor Smoke/tobacco counseling greater than 10
G0375 pays approximately $13; G0376 pays approximately $25. The exact payment depends on your geographic practice cost index (GPCI) as determined by CMS.
These codes do not modify coverage for minimal smoking cessation counseling (3 minutes or less in duration), which is considered covered as part of each evaluation and management (E/M) visit, and therefore is not separately billable.
Multidetector CT accurate for PE, but requires clinical context
-
CLINICAL QUESTION: How accurate is multidetector computed tomography for pulmonary embolism?
-
BOTTOM LINE: Patients with high or intermediate probability of pulmonary embolism (PE) and an abnormal result on computed tomographic angiography (CTA) or CTA combined with venous‐phase imaging (CTA‐CTV) are very likely to have PE. Those with low or intermediate probability and a negative CTA or CTA‐CTV result are unlikely to have PE. All other patients that is, those with discordant findings between the clinical examination and CTA or CTA‐CTV need either further testing or close clinical follow‐up to confirm or exclude the diagnosis. Clinical evaluation using a validated decision rule remains an important part of the evaluation. (LOE = 2b)
-
REFERENCE: Stein PD, Fowler SE, Goodman LR, et al, for the PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:23172327.
-
STUDY DESIGN: Diagnostic test evaluation
-
FUNDING: Government
-
SETTING: Emergency department
-
SYNOPSIS: CT technology continues to evolve, now moving from single slice CT to 4‐slice or 16‐slice multidetector scans. In this study, 824 patients with suspected PE underwent a standard clinical evaluation using the Wells clinical decision rule, CTA, CTA‐CTV, ventilation perfusion (VQ) scanning, venous compression ultrasound of the legs, and pulmonary digital subtraction angiography (DSA), if necessary. Patients were drawn from a group of 7284 patients with suspected PE, but large numbers were excluded because they couldn't complete testing within 36 hours, had abnormal renal function, declined to participate, were using anticoagulants, or were otherwise unable to complete the protocol. The mean age of participants was 51 years, 65% were white, and 62% were women. Defined by the composite reference standard (high probability VQ scan, abnormal DSA result, or abnormal venous ultrasound and nondiagnostic VQ scan), 192 (23%) had a PE. Among those who'd had PE ruled out using this reference standard, only 2 had a likely PE during the 6‐month follow‐up. Also, 51 had CTA that was of insufficient quality and 87 had a CTA‐CTV of poor quality, and they were excluded from the analysis. The CTA was 83% sensitive and 96% specific (positive likelihood ratio [LR+] = 19.6; negative likelihood ratio [LR‐] = 0.18) and the CTA‐CTV was 90% sensitive and 95% specific (LR+ = 16.5; LR‐ = 0.11). It's important to note that the predictive value of the tests depended on the clinical assessment. The Wells rule was used to stratify patients as high, intermediate, or low risk. The positive predictive value of CTA and CTA‐CTV was 96%, but the negative predictive value was only 60% to 82% for those tests. However, for patients with a low clinical probability, the positive predictive value was only 57% to 58%, while the negative predictive value was a robust 96% to 97%. Values for positive and negative predictive value in intermedate probability patients were between 89% and 92%.
-
CLINICAL QUESTION: How accurate is multidetector computed tomography for pulmonary embolism?
-
BOTTOM LINE: Patients with high or intermediate probability of pulmonary embolism (PE) and an abnormal result on computed tomographic angiography (CTA) or CTA combined with venous‐phase imaging (CTA‐CTV) are very likely to have PE. Those with low or intermediate probability and a negative CTA or CTA‐CTV result are unlikely to have PE. All other patients that is, those with discordant findings between the clinical examination and CTA or CTA‐CTV need either further testing or close clinical follow‐up to confirm or exclude the diagnosis. Clinical evaluation using a validated decision rule remains an important part of the evaluation. (LOE = 2b)
-
REFERENCE: Stein PD, Fowler SE, Goodman LR, et al, for the PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:23172327.
-
STUDY DESIGN: Diagnostic test evaluation
-
FUNDING: Government
-
SETTING: Emergency department
-
SYNOPSIS: CT technology continues to evolve, now moving from single slice CT to 4‐slice or 16‐slice multidetector scans. In this study, 824 patients with suspected PE underwent a standard clinical evaluation using the Wells clinical decision rule, CTA, CTA‐CTV, ventilation perfusion (VQ) scanning, venous compression ultrasound of the legs, and pulmonary digital subtraction angiography (DSA), if necessary. Patients were drawn from a group of 7284 patients with suspected PE, but large numbers were excluded because they couldn't complete testing within 36 hours, had abnormal renal function, declined to participate, were using anticoagulants, or were otherwise unable to complete the protocol. The mean age of participants was 51 years, 65% were white, and 62% were women. Defined by the composite reference standard (high probability VQ scan, abnormal DSA result, or abnormal venous ultrasound and nondiagnostic VQ scan), 192 (23%) had a PE. Among those who'd had PE ruled out using this reference standard, only 2 had a likely PE during the 6‐month follow‐up. Also, 51 had CTA that was of insufficient quality and 87 had a CTA‐CTV of poor quality, and they were excluded from the analysis. The CTA was 83% sensitive and 96% specific (positive likelihood ratio [LR+] = 19.6; negative likelihood ratio [LR‐] = 0.18) and the CTA‐CTV was 90% sensitive and 95% specific (LR+ = 16.5; LR‐ = 0.11). It's important to note that the predictive value of the tests depended on the clinical assessment. The Wells rule was used to stratify patients as high, intermediate, or low risk. The positive predictive value of CTA and CTA‐CTV was 96%, but the negative predictive value was only 60% to 82% for those tests. However, for patients with a low clinical probability, the positive predictive value was only 57% to 58%, while the negative predictive value was a robust 96% to 97%. Values for positive and negative predictive value in intermedate probability patients were between 89% and 92%.
-
CLINICAL QUESTION: How accurate is multidetector computed tomography for pulmonary embolism?
-
BOTTOM LINE: Patients with high or intermediate probability of pulmonary embolism (PE) and an abnormal result on computed tomographic angiography (CTA) or CTA combined with venous‐phase imaging (CTA‐CTV) are very likely to have PE. Those with low or intermediate probability and a negative CTA or CTA‐CTV result are unlikely to have PE. All other patients that is, those with discordant findings between the clinical examination and CTA or CTA‐CTV need either further testing or close clinical follow‐up to confirm or exclude the diagnosis. Clinical evaluation using a validated decision rule remains an important part of the evaluation. (LOE = 2b)
-
REFERENCE: Stein PD, Fowler SE, Goodman LR, et al, for the PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:23172327.
-
STUDY DESIGN: Diagnostic test evaluation
-
FUNDING: Government
-
SETTING: Emergency department
-
SYNOPSIS: CT technology continues to evolve, now moving from single slice CT to 4‐slice or 16‐slice multidetector scans. In this study, 824 patients with suspected PE underwent a standard clinical evaluation using the Wells clinical decision rule, CTA, CTA‐CTV, ventilation perfusion (VQ) scanning, venous compression ultrasound of the legs, and pulmonary digital subtraction angiography (DSA), if necessary. Patients were drawn from a group of 7284 patients with suspected PE, but large numbers were excluded because they couldn't complete testing within 36 hours, had abnormal renal function, declined to participate, were using anticoagulants, or were otherwise unable to complete the protocol. The mean age of participants was 51 years, 65% were white, and 62% were women. Defined by the composite reference standard (high probability VQ scan, abnormal DSA result, or abnormal venous ultrasound and nondiagnostic VQ scan), 192 (23%) had a PE. Among those who'd had PE ruled out using this reference standard, only 2 had a likely PE during the 6‐month follow‐up. Also, 51 had CTA that was of insufficient quality and 87 had a CTA‐CTV of poor quality, and they were excluded from the analysis. The CTA was 83% sensitive and 96% specific (positive likelihood ratio [LR+] = 19.6; negative likelihood ratio [LR‐] = 0.18) and the CTA‐CTV was 90% sensitive and 95% specific (LR+ = 16.5; LR‐ = 0.11). It's important to note that the predictive value of the tests depended on the clinical assessment. The Wells rule was used to stratify patients as high, intermediate, or low risk. The positive predictive value of CTA and CTA‐CTV was 96%, but the negative predictive value was only 60% to 82% for those tests. However, for patients with a low clinical probability, the positive predictive value was only 57% to 58%, while the negative predictive value was a robust 96% to 97%. Values for positive and negative predictive value in intermedate probability patients were between 89% and 92%.
Training Opportunities for Academic Hospitalists
There is a growing demand for hospitalists in the United States. In academic settings, hospitalists are called on to perform a variety of duties, from leading quality improvement initiatives to serving on hospital committees to helping to offset restrictions on work hours of the house staff.1 Although hospitalists may be well positioned to take on these roles, obtaining adequate protected time and recognition for such contributions remains a challenge. The existing promotion and tenure processes at academic institutions may not give adequate consideration to such responsibilities. Hospitalists who do not meet the traditional benchmarks of teaching and research may suffer in their career advancement and, ultimately, in their desire to remain in academics. Developing a sustainable and long‐term career in hospital medicine is important not only from a professional developmental standpoint, but also because it may lead to better patient care; evidence from a large multicenter hospitalist study suggests that physician experience is linked to improved patient care and outcomes.2 Thus, it behooves academic medical centers that employ hospitalists to create rewarding hospitalist career paths.
Goldman described academic hospital medicine as comprising periods of systole, during which hospitalists provide clinical care, and periods of diastole, the portion of a hospitalist's time spent in nonclinical activities.3 Far from being a period of relaxation, diastole is an active component of a hospitalist's work, the time devoted to the pursuit of complementary interests, career advancement, and job diversity. A well‐thought‐out plan for the diastolic phase of a hospitalist job description can lead to significant improvement in quality, education, research, and outcomes for an academic medical center.4 A good balance of systole and diastole allows for focus on career development and advancement and has the potential to be very helpful in preventing burnout. This is of particular concern to academic hospitalists, who report working longer hours, feeling more stress, and worrying more about burnout than their nonhospitalist colleagues.5 This suggests the diastolic phase is an important part of creating a sustainable hospitalist job and should be funded as part of an academic hospitalist position.
Although the optimal balance of systole and diastole to prevent burnout is not known, outlining clear expectations is an important strategy for preparing physicians for a sustainable academic hospitalist career. This is an important issue, given the increasing number of residency graduates who are choosing careers in hospital medicine.6 Based on the reported career plans of residents taking internal medicine in‐training exams from 2002 through 2006, the number of residents going into hospital medicine has more than doubled, from 3% (in 2002) to 6.5% (in 2006). The goal of this article is to compare and contrast several career paths that balance systole and diastole in academic hospital medicine. Specifically, we review training opportunities for becoming a successful hospitalist‐educator, hospitalistquality expert, hospitalist‐investigator, and hospitalist‐administrator.
EDUCATION (THE HOSPITALIST‐EDUCATOR)
Hospitalists in academic centers often play central roles as teachers and leaders in medical education. This is not surprising given that most teaching of medical trainees occurs in the inpatient setting.7 Furthermore, several studies have consistently demonstrated that trainee satisfaction with teaching by hospitalists is high, and hospitalists are rated as more effective teachers than traditional subspecialist ward attendings.810
A typical hospitalist‐educator position is 80%‐90% clinical time, with 10%‐20% set aside for teaching. However, academic hospitalists are often expected to teach medical trainees concurrently with their clinical care activities, rather than during a separate, protected time.11 Thus, most hospitalist‐educator responsibilities do not occur during diastole, as may be conceived, but instead are add to the systole. Small amounts of protected diastolic time for a hospitalist‐educator can be used for related administrative activities, such as writing letters of recommendation, mentoring students and residents, doing creative thinking and curriculum development, and conducting educational research, such as evaluating a new educational program or curriculum. Some hospitalist‐educator positions, such as director of the residency program or internal medicine clerkship, are exceptions in that they generally include a greater amount of protected time, which may be earmarked for administrative activities and hands‐on teaching.
Education and Training
One possibility for advanced training in education is the addition of a chief resident year, either at a physician's own institution or at another academic center. Such a year provides an opportunity to consolidate knowledge, build a teaching portfolio, and accumulate expertise in an area such as evidence‐based medicine or perioperative care. Serving as a chief resident can enhance subsequent applications by being able to demonstrate the ability to teach and, more importantly, to assume a leadership role within an organization. These skills can be applied to a number of activities in an academic hospitalist program, such as heading a committee, teaching during inpatient service time, or developing a new course for students, residents, or faculty.
An advanced training program in medical education is also an option (Table 1). Offerings include medical education fellowship training, formal degree‐granting programs (such as a master's in health professions education), or short‐term intensive coursework. Fellowships and degree‐granting programs are generally 2‐year programs designed for health professionals who want to better prepare for educational leadership roles. Core topics include curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. An alternative option for busy clinician‐educators is online or distance learning courses in medical education, which cover similar topics and skill sets. In early 2006 the Society of Hospital Medicine released the Core Competencies in Hospital Medicine, which can serve as a useful framework for developing novel inpatient curricula for faculty, residents, and students.12, 13
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in health professional education (MHPE): Preparation for educational leadership roles. Typical coursework in curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. | Varies according to program | Tuition ranges from approximately $1500‐$4300 | Example: University of Illinois |
| Fellowship in medical education: Prepares faculty to pursue scholarship in medical education or educational leadership or to become effective teachers through workshops, coursework, and/or a mentored project. Often affiliated with a department of medical education. | Varies according to program. Generally 1 year. | Varies. May be subsidized in certain institutions as part of internal faculty development. | Example: University of Michigan |
| Short‐term coursework | |||
| Harvard Macy Institute: Programs designed to promote leadership and scholarship in medical education | 1‐ or 2‐week programs | Fees for the year 2006 are $4500 USD. | |
| Stanford Faculty Development Center (SFDC): Train‐the‐trainer approach for clinical teaching and professionalism in contemporary practice | 4‐week training sessions | The institutions of faculty selected for the month‐long training programs are asked to pay a fee of $5000. Transportation, housing and food are not included. | |
Short‐term extramural courses offered by institutions such as the Harvard Macy Institute for Medical Educators and the Stanford Faculty Development Program in Teaching can also provide advanced instruction to hospitalist‐educators.14, 15 In addition to these training programs, the Society of General Internal Medicine, along with other professional societies, offers career development workshops for clinician educators on topics such as curriculum development and teaching skills.
Regardless of the type of training, adequate mentorship and resources are critical to the successful application of new skills to the design or evaluation of hospital‐based curricula. Mentorship may be available from institutional leaders in medical education, even those not formally affiliated with the hospitalist program. For instance, medical school leaders, such as deans, division chiefs, chairpersons, program directors, and clerkship directors, can often be helpful in guiding junior faculty in obtaining skills and time for teaching.
We encourage those interested in a career in medical education to begin volunteering at their institution early on. Volunteering to directly teach residents and students (eg, assisting in introduction to clinical medicine, giving lectures to third‐year clerks) can be a valuable way of becoming distinguished as a qualified teacher. Likewise, joining a professional medical society of individuals with similar interests can facilitate mentorship and skill acquisition. Certain professional medical societies, such as the American College of Physicians, promote national recognition through awarding fellowships, an honor for those physicians who have demonstrated superior competence in internal medicine, professional accomplishment, and scholarship.16 Developing concrete examples of expertise in the field, such as through the publication of abstracts and articles on medical education and development of curricula, help lead to advancement in the educational track. Clear focus on a career path, development of an intellectual product, positive learner evaluation of educational activities, and national recognition can all be used by an academic institution to evaluate suitability for promotion.
Rewards and Challenges
One of the rewards of a hospitalist‐educator career is being able to meaningfully interact with a variety of trainees, including medical students and residents. As teaching attendings, hospitalist‐educators are likely to engage students and residents for short‐term but intensive periods, resulting in the ability to influence career choice and professional growth as a physician.17 Hospitalists may be called on by trainees to serve as mentors or advisers and to write letters of recommendation. In addition, with experience, hospitalist‐educators are well positioned to serve in administrative roles in medical education, such as clerkship director or program director.
Burnout is a particular concern for hospitalist‐educators, given the heavy clinical demands of inpatient academic service combined with the additional pressure to be academically productive.5 Because of this, it is important to design academic hospitalist‐educator positions with a diastole that contains time to recover from the heavy clinical demands of inpatient service, in addition to providing time for career development activities.
Successful career development as an educator can be difficult. There are relatively few venues at which educational work can be peer‐evaluated and published, which are keys to successful academic promotion.18 Because some educational journals are highly competitive, one possibility way to get educational work disseminated is through the MedEd Portal, sponsored by the Association of American Medical Colleges, which allows peer review of medical educational materials, including innovative curricula.19 In addition to original research contributions, many scientific meetings and medical education journals also accept descriptions of interesting clinical vignettes and innovations in medical education. New online education journals, such as BMC Medical Education and Seminars in Medical Practice, have expanded publication opportunities.20
Limited opportunities are available to help fund research in medical education. Although funding may be more readily available to educators who focus on a particular clinical entity or patient population, most medical education research is conducted with inadequate funding and requires extensive donated time by committed faculty.21 For this reason, securing advanced training in medical education and having protected time will allow hospitalists on the educator track to compete more successfully for limited educational research dollars and to have sufficient time to produce and publish scholarly work, thus improving their chances of academic success and career satisfaction.
CLINICAL QUALITY AND OPERATIONS IMPROVEMENT (THE HOSPITALISTQUALITY EXPERT)
Hospitalists are increasingly being called on to lead clinical quality and operations improvement at academic teaching hospitals. Benefits to the institution include the consistent presence of a committed physician who is able to plan and execute change in the context of clinical care. This is in contrast to the transient nature of residents and nonhospitalist attending physicians, whose ability to participate in such initiatives is impaired by the scheduling of their rotations. Hospitalists, however, are often able to cultivate long‐standing relationships with nurses, case managers, and hospital administrators, thereby building the institutional clout to lead such initiatives while considering views from all the necessary stakeholders.22 Thus, they are in a good position to serve as physician champions and expedite the adoption of new innovations within hospitalist groups and among other physician groups and clinical staff.23, 24
Education and Training
Being a successful agent of change requires knowledge of the science of quality improvement coupled with the skills necessary to make such changes, such as the ability to perform a needs assessment, to develop measures of performance, to negotiate and motivate others to change behaviors, to adopt new tools and practices, and to implement and test interventions designed to improve care. It is possible for residents or junior faculty members to gain this experience through designing and implementing a quality improvement project during residency training under the direction of a mentor.25, 26 However, given the likely variability in such experience, there is no substitute for formal training in these core areas of hospital medicine.
A broad range of opportunities for advanced training in quality and operations improvement are available (Table 2). Choosing the correct program may depend on baseline expertise, availability, and the desired level of involvement. For example, introductions to these skills can be obtained through precourses or workshops at medical conferences such as the Institute of Healthcare Improvement or the Society of Hospital Medicine. For more in‐depth training, the Advanced Training Program (ATP) in Health Care Delivery Improvement, sponsored by Intermountain Healthcare, offers 12‐ to 21‐day in‐depth minicourses designed to train individuals for leadership positions in quality and safety.27 Lastly, more structured fellowships, such as the Veterans Affairs Quality Scholars Program or the George W. Merck Fellowships in Health Care Improvement, offer junior and midcareer faculty the opportunity to obtain formal training in the science of quality improvement.28, 29 Because early‐career hospitalists may face geographic and financial restrictions, exploration of local or institutional opportunities for advanced education in quality improvement can be particularly important.
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Veterans Association National Quality Scholars: Fellowship to learn and apply knowledge for improvement of health care | 2 years | No cost, application to fellowship program required | |
| George W. Merck Fellowship: Mentored research or improvement project at Institute of Healthcare Improvement with a plan to return to home institution to execute change | 1 year | No cost, application to fellowship program required | |
| Short‐term coursework | |||
| Intermountain Health Care: Designed to give executives and quality improvement leaders the necessary tools to conduct clinical practice improvement projects. | 20‐ and 12‐day training programs in Salt Lake City, UT | Tuition for the 20‐day program:
| |
Rewards and Challenges
Engaging in successful clinical or process improvement can be very rewarding, both professionally and personally. Professional gains include building new interdisciplinary relationships and infrastructure to continually monitor and improve key performance measures. In addition, a rigorous evaluation of this type of work can result in being able to make presentations at national meetings or to be published in a variety of peer‐reviewed medical journals, including specialty journals for quality improvement work, such as Quality and Safety in Healthcare and the Joint Commission Journal on Quality Improvement. Many national medical meetings, such as the Institute for Healthcare Improvement, the Society of Hospital Medicine and other subspecialty society meetings, also provide an opportunity to showcase innovations in practice.
Despite the potential rewards, it can also be challenging for academic hospitalists to participate in or lead quality improvement projects. One major challenge is ensuring that hospitalists are engaged in improvement work that is aligned with the interests of the hospital. Because most hospital administrators and frontline staff are employed by the hospital, whereas those comprising the academic faculty are employed by the university, this alignment is not always guaranteed. For example, an area of interest to a hospitalist that also could lead to academic productivity and career advancement might not be considered a priority area of improvement for the hospital because of competing clinical or operations improvements. In this scenario, it can be extremely difficult to engage other stakeholders such as nurses or administrative support staff in order to make a meaningful, sustainable change or improvement. To avoid this situation, it can be helpful from the outset to partner with hospital quality leaders in discussing priority areas, with attention to any potential interface in which hospitalist expertise is needed. In the event a potential project or area is identified, a hospitalist is particularly well positioned to serve as a physician champion, which is often key to the success of any hospitalwide initiative. In some cases, hospital funding may be available for these types of initiatives, increasing the likelihood of resource development for sustainable change.
RESEARCH (THE HOSPITALIST‐INVESTIGATOR)
Few hospitalists devote most of their time to clinical research. Having a strong research base is essential for the field of hospital medicine to gain credibility as a distinct specialty.4 Although the initial research in hospital medicine sought to prove the value of the field itself, hospitalists have now begun to focus on quality improvement and outcomes research.3032 Because of their unique position in clinical care, hospitalists are well situated to oversee inpatient data collection and perform research on a variety of conditions ranging from acute coronary syndromes to venous thromboembolism. Another potential area of research for hospitalists is participation in clinical trials focused on the inpatient setting. Although the proportion of time spent in research can vary widely, to become an independently successful clinical researcher typically requires a substantial amount of time be devoted to research. In general, at least 50% protected time, greater if possible, is recommended.
Education and Training
To develop a career around research generally requires advanced training in research methods. The most frequently used option for obtaining such training is through completing a clinical research fellowship in general internal medicine or an equivalent program, such as the fellowships administered by the Robert Wood Johnson Clinical Scholars Program (Table 3).33 Several academic centers also have developed such hospital medicine fellowships, which often can be tailored to provide the desired experience in research ethics, methodology, and statistical analysis.34, 35 In selecting a training program, prospective hospitalist‐researchers should consider the availability of suitable research mentors. Because hospital medicine as a field is relatively new, research mentors within the group of hospitalists may be scarce; if so, researchers should seek appropriate mentorship from established investigators in other programs or departments. Effective mentorship is a strong predictor of future research success.36
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Hospital or General Medicine Fellowships: Designed to provide clinical research training through mentored projects and coursework with possible master's degree | Generally 2‐year programs | No cost, application to program is required. Stipends vary. No cost, application to program is required | Hospital Medicine: |
| Robert Wood Johnson Clinical‐Scholars Program: Training in health services research with an emphasis on community‐based research and leadership training. | 2 years | Stipends currently range from $48,000 to $50,000 per year, depending on the training site. | Robert Wood Johnson: |
| Short‐term coursework | |||
| University‐based summer programs in clinical research (eg, Harvard University Summer Session for Public Health Studies which features graduate courses in epidemiology, biostatistics, economics, health care management, etc.) | Intensive 3‐week courses in Harvard University Summer Session | 2004 tuition for each 2.5‐credit course was $1830. There is a nonrefundable deposit/registration fee of $125. These fees do not include certain course materials (ie, texts estimated at $60 per course). | Example: Harvard School of Public Health |
Negotiating protected time can be challenging for new investigators, particularly when hospitalist salaries are generated by clinical activity. Some academic programs are willing to provide a few years of departmental support to promising young investigators in order to allow them to develop their research program and obtain additional funding. Several career development awards are available through the National Institutes of Health and through nonfederally funded sources.37, 38 These awards generally protect 3‐5 years of a researcher's time for research and require that a substantial proportion of time be devoted to that purpose, often at least 75%.
To gain visibility as a researcher, it is advantageous to present original findings at national meetings, such as those of the Society of Hospital Medicine, the Society of General Internal Medicine, and other subspecialty meetings.39, 40 These meetings not only increase awareness of a hospitalist's research but also provide opportunities for networking and developing collaboration on research. Many societies, including the Society of Hospital Medicine, have research abstract competitions and offer research grants for investigators that can help to fund projects and support protected time.
Rewards and Challenges
There are many rewards and opportunities for a hospitalist investigator, particularly because the field is young and there are many unanswered research questions related to inpatient medicine. There are also the intrinsic rewards of being devoted to scientific inquiry and having greater autonomy over how time is spent. A hospitalist's schedule can be well suited to research. Although attending on the wards can be very time‐consuming, time off the wards is often free of outpatient duties and can be entirely devoted to research.
There are also several challenges to becoming a successful researcher. The pressure to obtain grant funding and publish high‐quality scientific manuscripts is high. Obtaining sufficient protected time may be difficult in busy clinical departments, and applying for grant funding is both time‐consuming and highly competitive. It is very important to be familiar with the specific criteria for academic promotion at one's institution. Understanding these expectations can help to effectively prioritize activities. Standard requirements generally include number and quality of articles published in peer‐reviewed journals, successful application for research funding, national recognition in the field, service to the institution and research community, and evidence of research independence. One significant challenge is the lack of a single large funding source for hospital‐related research. Although the Agency for Healthcare Research and Quality funds studies related to hospital care, such as on the quality of care or cost effectiveness of various system‐based hospital care interventions, their budget for investigator‐initiated proposals is limited.41 One promising funding source for research in hospital care is from agencies and foundations dedicated to the aging population, such as the National Institute for Aging (NIA), the Hartford Foundation, and the Aetna Foundation, to name a few.42, 43 Yet research on hospital care alone, without detailed attention to issues unique to geriatric‐specific conditions or populations, is unlikely to be funded by these avenues. With few federal grant programs directly suited to the emerging research agenda in hospital medicine, hospitalist‐investigators may be at a disadvantage for obtaining tenure‐track positions, compared with their subspecialist colleagues, who may receive funding from NIH agencies or foundations dedicated to their own field.
ADMINISTRATION (THE HOSPITALIST‐ADMINISTRATOR)
Physician leaders in hospital administration are not new. Many hospitals already include physicians in senior management positions, such as chief medical officer.44 Naturally, a career in hospital administration is another potential path for diastole in academic medical centers.
Education and Training
Although a master of business, health administration, or medical management is not a prerequisite for the physician who wants to move into management, it is an increasingly important credential for senior administrative positions (Table 4). Primarily, it serves as a signal that a physician is committed to management and has a working knowledge of strategic planning, business models, human resources, leadership, and clinical operations. For physicians without formal business training who are interested in management, exploring internal opportunities is a necessary first step. Likewise, getting a business degree is not as important as management experience. The successful application of business skills requires practice, mentoring, and on‐the‐job experience. For hospitalists, this experience could be obtained by volunteering to serve on committees such as utilization review, quality assurance, credentialing, or medical staff executive committees. In lieu of a graduate degree, physicians may wish to participate in one of the many fellowships in health services administration. These programs generally aim to provide practical mentored learning experience in a health care organization and may last up to 2 years.45
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in business administration (MBA): General management core with option for courses specializing in health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MBA): |
| Master's in health administration (MHA): Studies in analytic and management needs of health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MHA): |
| Fellowship in health services administration: Preceptor‐directed program that provides practical learning experience in a health care organization beyond graduate‐level academic instruction. | Usually lasts 1‐2 years. | Compensation varies. Median reported as $39,055. | Directory (American College of Healthcare Executives): |
| Short‐term coursework | |||
| Society of Hospital Medicine Leadership Academy: Instruction for hospitalists in leading change, communicating effectively, handling conflict and negotiation, doing strategic planning, and interpreting hospital business drivers. Held biannually. | 3‐ to 4‐day program | $1400‐$1600. Discounted rate for members of Society of Hospital Medicine | |
For hospitalists and trainees considering a career as an executive, the American College of Physician Executives can serve as a valuable resource.46 This organization, founded in 1975, offers educational resources, including publications, comprehensive CD‐ROM products, and 1‐day courses and master's degree programs in conjunction with several leading business schools in medical management. In addition, the Society of Hospital Medicine offers a Leadership Academy designed to assist practicing hospitalists in evaluating their leadership strengths and applying them to everyday management challenges.47 Such a program also can facilitate the development of a peer network and the mentoring relationships needed to achieve these goals.
Rewards and Challenges
The life of the physician executive can be rewarding, but making the transition may prove challenging. However, if physicians can navigate this transition successfully, they will likely find a wide array of opportunities, as demand for physician‐executives remains high.
One major challenge to becoming a physician‐executive is reconciling the administrative role with the initial desire to enter a career in clinical medicine.48 Physician‐executives who continue to see patients are more likely to be satisfied with their jobs than physician‐executives who do not.49 Physician‐executives also may feel they are being criticized by their purely clinical colleagues for working in the business or management of medicine.50 Actual or perceived lack of support may promote isolation and burnout.51 In addition, the constantly shifting landscape of health care administration results in a much more unstable environment than that found in clinical medicine. For example, the risk of termination for a physician‐executive is 20‐40 times higher than that for a practicing physician.50 The reasons for this higher risk include personal conflict with a boss, reorganization (ie, downsizing, merging, etc.), and immediate departure of a supervisor. Access to mentors, support groups, and the option to practice part time are all potential mechanisms to ensure long‐term success as a physician‐administrator.
CONCLUSIONS
As hospital medicine continues to grow and evolve, designing sustainable and rewarding academic careers will be crucial to the success of the field. Being able to balance clinical systole time with obtaining the skills to support nonclinical diastole time is important to ensuring a successful career as an academic hospitalist. We have described several possible career paths in teaching, research, quality improvement, and administration. By preparing future hospitalists with the knowledge and skills required to assume a variety of roles during their diastolic time, we hope to encourage the growth of hospitalist leaders with well‐developed skill sets. If hospitalists adequately prepare themselves, academic hospital medicine will likely remain sustainable and rewarding, and future generations of trainees will be inspired and prepared to enter the field.
Acknowledgements
We are grateful to Jennifer Higa and Kimberly Alvarez for their assistance in preparing this manuscript.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80:39–43.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(s1):141–142.
- .The hospitalist movement.Ann Intern Med.1999;131:545.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,, et al.Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations.J Gen Intern Med.2006;21(s4):128.
- ,,.Career plans for trainees in internal medicine residency programs.Acad Med.2005;80:507–512.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Amer J Med.2005;118:680–687.
- ,,, et al..Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,,,,.Resident satisfaction on an academic hospitalist service: time to teach.Am J Med.2002;112:597–601.
- ,.The present and future of appointment, tenure, and compensation policies for medical school clinical faculty.Acad Med.2001;76:993–1004.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(1).
- Harvard Macy Institute. Harvard College. Available at: http://www.harvardmacy.org/programs.asp?DocumentID=1. Accessed October 3,2005.
- Stanford Faculty Development Center. Stanford University. Available at http://sfdc.stanford.edu/. Accessed January 23,2006.
- American College of Physicians. Available at: http://www.acponline.org/college/membership/classes.htm#fellow. Accessed June 10,2006
- ,,, et al.Effect of the inpatient general medicine rotation on student pursuit of a generalist career.J Gen Intern Med.2006;21:471–475.
- ,.Graduate medical education research in the 21st century and JAMA on call.JAMA.2004;292:2913–2915.
- Association of American Medical Colleges. MedEd (PORTAL); Providing Online Resources to Advance Learning in Medical Education. Available at: http://www.aamc.org/meded/mededportal/start.htm. Accessed January 23,2006.
- BioMed Central. BMC Medical Education. Available at: http://www.biomedcentral.com/bmcmededuc/.Accessed January 23,2006.
- ,,,.Costs and funding for published medical education research.JAMA.2005;294(9):1052–7.
- .Teamwork and hospital medicine. A vision for the future.Crit Care Nurse.2003;23(3):8,10–11.
- . (1995)Diffusion of Innovations.4th ed.The Free Press:,Toronto.
- ,,.Clarifying the concepts in knowledge transfer: a literature review.J Adv Nurs.2006;53:691–701.
- ,,,,.Creating a quality improvement elective for medical house officers.J Gen Intern Med.2004;19:861–867.
- ,,.A continuous quality improvement curriculum for residents: addressing core competency, improving systems.Acad Med.2004;79(10 Suppl):S65–7.
- Institute for Healthcare Delivery Research. Advanced Training Program in Health Care Delivery Improvement (ATP). Available at: http://www.ihc.com/xp/ihc/institute/education/atp/. Accessed October 3,2005.
- Veterans Health Administration. VA Quality Scholars Program. Available at: http://www.dartmouth.edu/∼cecs/fellowships/vaqs.html
- Institute for Healthcare Improvement. George W. Merck Fellowships. Available at: http://www.ihi.org/ihi. Accessed October 3,2005.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Robert Wood Johnson Clinical Scholars Program. Stanford University (Palo Alto, CA). Available at: http://rwjcsp.stanford.edu/. Accessed October 3,2005.
- ,.Hospital Medicine Fellowship Update.Society of Hospital Medicine.The Hospitalist.2004;8(5):38.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119(1):72.e1–e7.
- ,,, et al.Mentorship in Academic General Internal Medicine.J Gen Intern Med.2005;2(34):1–5.
- ,,,,.Getting funded. Career development awards for aspiring clinical investigators.J Gen Intern Med.2004;19(5 Pt 1):472–478.
- K Kiosk—Information about NIH Career Development Awards. Available at: http://grants.nih.gov/training/careerdevelopmentawards.htm. Accessed March 20,2006.
- Research Career Development Awards for Junior Faculty and Fellows in General Internal Medicine. Available at: http://www.sgim.org/careerdevelopment.cfm. Accessed March 24,2006.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org//AM/Template.cfm?Section=Home. Accessed October 4,2005.
- ,.What is an academic general internist? Career options and training pathways.JAMA.2002;288:2045–2048.
- U.S. National Institutes of Health.National Institute on Aging. Available at: http://www.nia.nih.gov/. Accessed January 25,2006.
- .Hartford Foundation. Available from: http://www.jhartfound.org/. Accessed January 25,2006.
- .Why will physicians in this new environment replace MHAs?Physician Exec.1996;22(2):5–10.
- Directory of Fellowships in Health Services Administration. Available at: http://www.ache.org/pgfd/purpose.cfm. Accessed March 24,2006.
- American College of Physician Executives. Available at: http://www.acpe.org/. Accessed October 3,2005.
- Society of Hospital Medicine. Leadership Academy statement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Search_Advanced_Search6(7):37–40.
- ,,,.Satisfaction higher for physician executives who treat patients, survey finds.Physician Exec.2002;28(3):17–21.
- .Physician executives don't have to go it alone.Managed Care Magazine.2003. Available at: http://www.managedcaremag.com/archives/0307/0307.viewpoint_lazarus.html.Accessed January 25,year="2006"2006.
- .Controlled burn! Physician executives must be ready to handle job burnout, career stress.Physician Exec.2001;27(4):42–45.
There is a growing demand for hospitalists in the United States. In academic settings, hospitalists are called on to perform a variety of duties, from leading quality improvement initiatives to serving on hospital committees to helping to offset restrictions on work hours of the house staff.1 Although hospitalists may be well positioned to take on these roles, obtaining adequate protected time and recognition for such contributions remains a challenge. The existing promotion and tenure processes at academic institutions may not give adequate consideration to such responsibilities. Hospitalists who do not meet the traditional benchmarks of teaching and research may suffer in their career advancement and, ultimately, in their desire to remain in academics. Developing a sustainable and long‐term career in hospital medicine is important not only from a professional developmental standpoint, but also because it may lead to better patient care; evidence from a large multicenter hospitalist study suggests that physician experience is linked to improved patient care and outcomes.2 Thus, it behooves academic medical centers that employ hospitalists to create rewarding hospitalist career paths.
Goldman described academic hospital medicine as comprising periods of systole, during which hospitalists provide clinical care, and periods of diastole, the portion of a hospitalist's time spent in nonclinical activities.3 Far from being a period of relaxation, diastole is an active component of a hospitalist's work, the time devoted to the pursuit of complementary interests, career advancement, and job diversity. A well‐thought‐out plan for the diastolic phase of a hospitalist job description can lead to significant improvement in quality, education, research, and outcomes for an academic medical center.4 A good balance of systole and diastole allows for focus on career development and advancement and has the potential to be very helpful in preventing burnout. This is of particular concern to academic hospitalists, who report working longer hours, feeling more stress, and worrying more about burnout than their nonhospitalist colleagues.5 This suggests the diastolic phase is an important part of creating a sustainable hospitalist job and should be funded as part of an academic hospitalist position.
Although the optimal balance of systole and diastole to prevent burnout is not known, outlining clear expectations is an important strategy for preparing physicians for a sustainable academic hospitalist career. This is an important issue, given the increasing number of residency graduates who are choosing careers in hospital medicine.6 Based on the reported career plans of residents taking internal medicine in‐training exams from 2002 through 2006, the number of residents going into hospital medicine has more than doubled, from 3% (in 2002) to 6.5% (in 2006). The goal of this article is to compare and contrast several career paths that balance systole and diastole in academic hospital medicine. Specifically, we review training opportunities for becoming a successful hospitalist‐educator, hospitalistquality expert, hospitalist‐investigator, and hospitalist‐administrator.
EDUCATION (THE HOSPITALIST‐EDUCATOR)
Hospitalists in academic centers often play central roles as teachers and leaders in medical education. This is not surprising given that most teaching of medical trainees occurs in the inpatient setting.7 Furthermore, several studies have consistently demonstrated that trainee satisfaction with teaching by hospitalists is high, and hospitalists are rated as more effective teachers than traditional subspecialist ward attendings.810
A typical hospitalist‐educator position is 80%‐90% clinical time, with 10%‐20% set aside for teaching. However, academic hospitalists are often expected to teach medical trainees concurrently with their clinical care activities, rather than during a separate, protected time.11 Thus, most hospitalist‐educator responsibilities do not occur during diastole, as may be conceived, but instead are add to the systole. Small amounts of protected diastolic time for a hospitalist‐educator can be used for related administrative activities, such as writing letters of recommendation, mentoring students and residents, doing creative thinking and curriculum development, and conducting educational research, such as evaluating a new educational program or curriculum. Some hospitalist‐educator positions, such as director of the residency program or internal medicine clerkship, are exceptions in that they generally include a greater amount of protected time, which may be earmarked for administrative activities and hands‐on teaching.
Education and Training
One possibility for advanced training in education is the addition of a chief resident year, either at a physician's own institution or at another academic center. Such a year provides an opportunity to consolidate knowledge, build a teaching portfolio, and accumulate expertise in an area such as evidence‐based medicine or perioperative care. Serving as a chief resident can enhance subsequent applications by being able to demonstrate the ability to teach and, more importantly, to assume a leadership role within an organization. These skills can be applied to a number of activities in an academic hospitalist program, such as heading a committee, teaching during inpatient service time, or developing a new course for students, residents, or faculty.
An advanced training program in medical education is also an option (Table 1). Offerings include medical education fellowship training, formal degree‐granting programs (such as a master's in health professions education), or short‐term intensive coursework. Fellowships and degree‐granting programs are generally 2‐year programs designed for health professionals who want to better prepare for educational leadership roles. Core topics include curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. An alternative option for busy clinician‐educators is online or distance learning courses in medical education, which cover similar topics and skill sets. In early 2006 the Society of Hospital Medicine released the Core Competencies in Hospital Medicine, which can serve as a useful framework for developing novel inpatient curricula for faculty, residents, and students.12, 13
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in health professional education (MHPE): Preparation for educational leadership roles. Typical coursework in curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. | Varies according to program | Tuition ranges from approximately $1500‐$4300 | Example: University of Illinois |
| Fellowship in medical education: Prepares faculty to pursue scholarship in medical education or educational leadership or to become effective teachers through workshops, coursework, and/or a mentored project. Often affiliated with a department of medical education. | Varies according to program. Generally 1 year. | Varies. May be subsidized in certain institutions as part of internal faculty development. | Example: University of Michigan |
| Short‐term coursework | |||
| Harvard Macy Institute: Programs designed to promote leadership and scholarship in medical education | 1‐ or 2‐week programs | Fees for the year 2006 are $4500 USD. | |
| Stanford Faculty Development Center (SFDC): Train‐the‐trainer approach for clinical teaching and professionalism in contemporary practice | 4‐week training sessions | The institutions of faculty selected for the month‐long training programs are asked to pay a fee of $5000. Transportation, housing and food are not included. | |
Short‐term extramural courses offered by institutions such as the Harvard Macy Institute for Medical Educators and the Stanford Faculty Development Program in Teaching can also provide advanced instruction to hospitalist‐educators.14, 15 In addition to these training programs, the Society of General Internal Medicine, along with other professional societies, offers career development workshops for clinician educators on topics such as curriculum development and teaching skills.
Regardless of the type of training, adequate mentorship and resources are critical to the successful application of new skills to the design or evaluation of hospital‐based curricula. Mentorship may be available from institutional leaders in medical education, even those not formally affiliated with the hospitalist program. For instance, medical school leaders, such as deans, division chiefs, chairpersons, program directors, and clerkship directors, can often be helpful in guiding junior faculty in obtaining skills and time for teaching.
We encourage those interested in a career in medical education to begin volunteering at their institution early on. Volunteering to directly teach residents and students (eg, assisting in introduction to clinical medicine, giving lectures to third‐year clerks) can be a valuable way of becoming distinguished as a qualified teacher. Likewise, joining a professional medical society of individuals with similar interests can facilitate mentorship and skill acquisition. Certain professional medical societies, such as the American College of Physicians, promote national recognition through awarding fellowships, an honor for those physicians who have demonstrated superior competence in internal medicine, professional accomplishment, and scholarship.16 Developing concrete examples of expertise in the field, such as through the publication of abstracts and articles on medical education and development of curricula, help lead to advancement in the educational track. Clear focus on a career path, development of an intellectual product, positive learner evaluation of educational activities, and national recognition can all be used by an academic institution to evaluate suitability for promotion.
Rewards and Challenges
One of the rewards of a hospitalist‐educator career is being able to meaningfully interact with a variety of trainees, including medical students and residents. As teaching attendings, hospitalist‐educators are likely to engage students and residents for short‐term but intensive periods, resulting in the ability to influence career choice and professional growth as a physician.17 Hospitalists may be called on by trainees to serve as mentors or advisers and to write letters of recommendation. In addition, with experience, hospitalist‐educators are well positioned to serve in administrative roles in medical education, such as clerkship director or program director.
Burnout is a particular concern for hospitalist‐educators, given the heavy clinical demands of inpatient academic service combined with the additional pressure to be academically productive.5 Because of this, it is important to design academic hospitalist‐educator positions with a diastole that contains time to recover from the heavy clinical demands of inpatient service, in addition to providing time for career development activities.
Successful career development as an educator can be difficult. There are relatively few venues at which educational work can be peer‐evaluated and published, which are keys to successful academic promotion.18 Because some educational journals are highly competitive, one possibility way to get educational work disseminated is through the MedEd Portal, sponsored by the Association of American Medical Colleges, which allows peer review of medical educational materials, including innovative curricula.19 In addition to original research contributions, many scientific meetings and medical education journals also accept descriptions of interesting clinical vignettes and innovations in medical education. New online education journals, such as BMC Medical Education and Seminars in Medical Practice, have expanded publication opportunities.20
Limited opportunities are available to help fund research in medical education. Although funding may be more readily available to educators who focus on a particular clinical entity or patient population, most medical education research is conducted with inadequate funding and requires extensive donated time by committed faculty.21 For this reason, securing advanced training in medical education and having protected time will allow hospitalists on the educator track to compete more successfully for limited educational research dollars and to have sufficient time to produce and publish scholarly work, thus improving their chances of academic success and career satisfaction.
CLINICAL QUALITY AND OPERATIONS IMPROVEMENT (THE HOSPITALISTQUALITY EXPERT)
Hospitalists are increasingly being called on to lead clinical quality and operations improvement at academic teaching hospitals. Benefits to the institution include the consistent presence of a committed physician who is able to plan and execute change in the context of clinical care. This is in contrast to the transient nature of residents and nonhospitalist attending physicians, whose ability to participate in such initiatives is impaired by the scheduling of their rotations. Hospitalists, however, are often able to cultivate long‐standing relationships with nurses, case managers, and hospital administrators, thereby building the institutional clout to lead such initiatives while considering views from all the necessary stakeholders.22 Thus, they are in a good position to serve as physician champions and expedite the adoption of new innovations within hospitalist groups and among other physician groups and clinical staff.23, 24
Education and Training
Being a successful agent of change requires knowledge of the science of quality improvement coupled with the skills necessary to make such changes, such as the ability to perform a needs assessment, to develop measures of performance, to negotiate and motivate others to change behaviors, to adopt new tools and practices, and to implement and test interventions designed to improve care. It is possible for residents or junior faculty members to gain this experience through designing and implementing a quality improvement project during residency training under the direction of a mentor.25, 26 However, given the likely variability in such experience, there is no substitute for formal training in these core areas of hospital medicine.
A broad range of opportunities for advanced training in quality and operations improvement are available (Table 2). Choosing the correct program may depend on baseline expertise, availability, and the desired level of involvement. For example, introductions to these skills can be obtained through precourses or workshops at medical conferences such as the Institute of Healthcare Improvement or the Society of Hospital Medicine. For more in‐depth training, the Advanced Training Program (ATP) in Health Care Delivery Improvement, sponsored by Intermountain Healthcare, offers 12‐ to 21‐day in‐depth minicourses designed to train individuals for leadership positions in quality and safety.27 Lastly, more structured fellowships, such as the Veterans Affairs Quality Scholars Program or the George W. Merck Fellowships in Health Care Improvement, offer junior and midcareer faculty the opportunity to obtain formal training in the science of quality improvement.28, 29 Because early‐career hospitalists may face geographic and financial restrictions, exploration of local or institutional opportunities for advanced education in quality improvement can be particularly important.
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Veterans Association National Quality Scholars: Fellowship to learn and apply knowledge for improvement of health care | 2 years | No cost, application to fellowship program required | |
| George W. Merck Fellowship: Mentored research or improvement project at Institute of Healthcare Improvement with a plan to return to home institution to execute change | 1 year | No cost, application to fellowship program required | |
| Short‐term coursework | |||
| Intermountain Health Care: Designed to give executives and quality improvement leaders the necessary tools to conduct clinical practice improvement projects. | 20‐ and 12‐day training programs in Salt Lake City, UT | Tuition for the 20‐day program:
| |
Rewards and Challenges
Engaging in successful clinical or process improvement can be very rewarding, both professionally and personally. Professional gains include building new interdisciplinary relationships and infrastructure to continually monitor and improve key performance measures. In addition, a rigorous evaluation of this type of work can result in being able to make presentations at national meetings or to be published in a variety of peer‐reviewed medical journals, including specialty journals for quality improvement work, such as Quality and Safety in Healthcare and the Joint Commission Journal on Quality Improvement. Many national medical meetings, such as the Institute for Healthcare Improvement, the Society of Hospital Medicine and other subspecialty society meetings, also provide an opportunity to showcase innovations in practice.
Despite the potential rewards, it can also be challenging for academic hospitalists to participate in or lead quality improvement projects. One major challenge is ensuring that hospitalists are engaged in improvement work that is aligned with the interests of the hospital. Because most hospital administrators and frontline staff are employed by the hospital, whereas those comprising the academic faculty are employed by the university, this alignment is not always guaranteed. For example, an area of interest to a hospitalist that also could lead to academic productivity and career advancement might not be considered a priority area of improvement for the hospital because of competing clinical or operations improvements. In this scenario, it can be extremely difficult to engage other stakeholders such as nurses or administrative support staff in order to make a meaningful, sustainable change or improvement. To avoid this situation, it can be helpful from the outset to partner with hospital quality leaders in discussing priority areas, with attention to any potential interface in which hospitalist expertise is needed. In the event a potential project or area is identified, a hospitalist is particularly well positioned to serve as a physician champion, which is often key to the success of any hospitalwide initiative. In some cases, hospital funding may be available for these types of initiatives, increasing the likelihood of resource development for sustainable change.
RESEARCH (THE HOSPITALIST‐INVESTIGATOR)
Few hospitalists devote most of their time to clinical research. Having a strong research base is essential for the field of hospital medicine to gain credibility as a distinct specialty.4 Although the initial research in hospital medicine sought to prove the value of the field itself, hospitalists have now begun to focus on quality improvement and outcomes research.3032 Because of their unique position in clinical care, hospitalists are well situated to oversee inpatient data collection and perform research on a variety of conditions ranging from acute coronary syndromes to venous thromboembolism. Another potential area of research for hospitalists is participation in clinical trials focused on the inpatient setting. Although the proportion of time spent in research can vary widely, to become an independently successful clinical researcher typically requires a substantial amount of time be devoted to research. In general, at least 50% protected time, greater if possible, is recommended.
Education and Training
To develop a career around research generally requires advanced training in research methods. The most frequently used option for obtaining such training is through completing a clinical research fellowship in general internal medicine or an equivalent program, such as the fellowships administered by the Robert Wood Johnson Clinical Scholars Program (Table 3).33 Several academic centers also have developed such hospital medicine fellowships, which often can be tailored to provide the desired experience in research ethics, methodology, and statistical analysis.34, 35 In selecting a training program, prospective hospitalist‐researchers should consider the availability of suitable research mentors. Because hospital medicine as a field is relatively new, research mentors within the group of hospitalists may be scarce; if so, researchers should seek appropriate mentorship from established investigators in other programs or departments. Effective mentorship is a strong predictor of future research success.36
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Hospital or General Medicine Fellowships: Designed to provide clinical research training through mentored projects and coursework with possible master's degree | Generally 2‐year programs | No cost, application to program is required. Stipends vary. No cost, application to program is required | Hospital Medicine: |
| Robert Wood Johnson Clinical‐Scholars Program: Training in health services research with an emphasis on community‐based research and leadership training. | 2 years | Stipends currently range from $48,000 to $50,000 per year, depending on the training site. | Robert Wood Johnson: |
| Short‐term coursework | |||
| University‐based summer programs in clinical research (eg, Harvard University Summer Session for Public Health Studies which features graduate courses in epidemiology, biostatistics, economics, health care management, etc.) | Intensive 3‐week courses in Harvard University Summer Session | 2004 tuition for each 2.5‐credit course was $1830. There is a nonrefundable deposit/registration fee of $125. These fees do not include certain course materials (ie, texts estimated at $60 per course). | Example: Harvard School of Public Health |
Negotiating protected time can be challenging for new investigators, particularly when hospitalist salaries are generated by clinical activity. Some academic programs are willing to provide a few years of departmental support to promising young investigators in order to allow them to develop their research program and obtain additional funding. Several career development awards are available through the National Institutes of Health and through nonfederally funded sources.37, 38 These awards generally protect 3‐5 years of a researcher's time for research and require that a substantial proportion of time be devoted to that purpose, often at least 75%.
To gain visibility as a researcher, it is advantageous to present original findings at national meetings, such as those of the Society of Hospital Medicine, the Society of General Internal Medicine, and other subspecialty meetings.39, 40 These meetings not only increase awareness of a hospitalist's research but also provide opportunities for networking and developing collaboration on research. Many societies, including the Society of Hospital Medicine, have research abstract competitions and offer research grants for investigators that can help to fund projects and support protected time.
Rewards and Challenges
There are many rewards and opportunities for a hospitalist investigator, particularly because the field is young and there are many unanswered research questions related to inpatient medicine. There are also the intrinsic rewards of being devoted to scientific inquiry and having greater autonomy over how time is spent. A hospitalist's schedule can be well suited to research. Although attending on the wards can be very time‐consuming, time off the wards is often free of outpatient duties and can be entirely devoted to research.
There are also several challenges to becoming a successful researcher. The pressure to obtain grant funding and publish high‐quality scientific manuscripts is high. Obtaining sufficient protected time may be difficult in busy clinical departments, and applying for grant funding is both time‐consuming and highly competitive. It is very important to be familiar with the specific criteria for academic promotion at one's institution. Understanding these expectations can help to effectively prioritize activities. Standard requirements generally include number and quality of articles published in peer‐reviewed journals, successful application for research funding, national recognition in the field, service to the institution and research community, and evidence of research independence. One significant challenge is the lack of a single large funding source for hospital‐related research. Although the Agency for Healthcare Research and Quality funds studies related to hospital care, such as on the quality of care or cost effectiveness of various system‐based hospital care interventions, their budget for investigator‐initiated proposals is limited.41 One promising funding source for research in hospital care is from agencies and foundations dedicated to the aging population, such as the National Institute for Aging (NIA), the Hartford Foundation, and the Aetna Foundation, to name a few.42, 43 Yet research on hospital care alone, without detailed attention to issues unique to geriatric‐specific conditions or populations, is unlikely to be funded by these avenues. With few federal grant programs directly suited to the emerging research agenda in hospital medicine, hospitalist‐investigators may be at a disadvantage for obtaining tenure‐track positions, compared with their subspecialist colleagues, who may receive funding from NIH agencies or foundations dedicated to their own field.
ADMINISTRATION (THE HOSPITALIST‐ADMINISTRATOR)
Physician leaders in hospital administration are not new. Many hospitals already include physicians in senior management positions, such as chief medical officer.44 Naturally, a career in hospital administration is another potential path for diastole in academic medical centers.
Education and Training
Although a master of business, health administration, or medical management is not a prerequisite for the physician who wants to move into management, it is an increasingly important credential for senior administrative positions (Table 4). Primarily, it serves as a signal that a physician is committed to management and has a working knowledge of strategic planning, business models, human resources, leadership, and clinical operations. For physicians without formal business training who are interested in management, exploring internal opportunities is a necessary first step. Likewise, getting a business degree is not as important as management experience. The successful application of business skills requires practice, mentoring, and on‐the‐job experience. For hospitalists, this experience could be obtained by volunteering to serve on committees such as utilization review, quality assurance, credentialing, or medical staff executive committees. In lieu of a graduate degree, physicians may wish to participate in one of the many fellowships in health services administration. These programs generally aim to provide practical mentored learning experience in a health care organization and may last up to 2 years.45
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in business administration (MBA): General management core with option for courses specializing in health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MBA): |
| Master's in health administration (MHA): Studies in analytic and management needs of health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MHA): |
| Fellowship in health services administration: Preceptor‐directed program that provides practical learning experience in a health care organization beyond graduate‐level academic instruction. | Usually lasts 1‐2 years. | Compensation varies. Median reported as $39,055. | Directory (American College of Healthcare Executives): |
| Short‐term coursework | |||
| Society of Hospital Medicine Leadership Academy: Instruction for hospitalists in leading change, communicating effectively, handling conflict and negotiation, doing strategic planning, and interpreting hospital business drivers. Held biannually. | 3‐ to 4‐day program | $1400‐$1600. Discounted rate for members of Society of Hospital Medicine | |
For hospitalists and trainees considering a career as an executive, the American College of Physician Executives can serve as a valuable resource.46 This organization, founded in 1975, offers educational resources, including publications, comprehensive CD‐ROM products, and 1‐day courses and master's degree programs in conjunction with several leading business schools in medical management. In addition, the Society of Hospital Medicine offers a Leadership Academy designed to assist practicing hospitalists in evaluating their leadership strengths and applying them to everyday management challenges.47 Such a program also can facilitate the development of a peer network and the mentoring relationships needed to achieve these goals.
Rewards and Challenges
The life of the physician executive can be rewarding, but making the transition may prove challenging. However, if physicians can navigate this transition successfully, they will likely find a wide array of opportunities, as demand for physician‐executives remains high.
One major challenge to becoming a physician‐executive is reconciling the administrative role with the initial desire to enter a career in clinical medicine.48 Physician‐executives who continue to see patients are more likely to be satisfied with their jobs than physician‐executives who do not.49 Physician‐executives also may feel they are being criticized by their purely clinical colleagues for working in the business or management of medicine.50 Actual or perceived lack of support may promote isolation and burnout.51 In addition, the constantly shifting landscape of health care administration results in a much more unstable environment than that found in clinical medicine. For example, the risk of termination for a physician‐executive is 20‐40 times higher than that for a practicing physician.50 The reasons for this higher risk include personal conflict with a boss, reorganization (ie, downsizing, merging, etc.), and immediate departure of a supervisor. Access to mentors, support groups, and the option to practice part time are all potential mechanisms to ensure long‐term success as a physician‐administrator.
CONCLUSIONS
As hospital medicine continues to grow and evolve, designing sustainable and rewarding academic careers will be crucial to the success of the field. Being able to balance clinical systole time with obtaining the skills to support nonclinical diastole time is important to ensuring a successful career as an academic hospitalist. We have described several possible career paths in teaching, research, quality improvement, and administration. By preparing future hospitalists with the knowledge and skills required to assume a variety of roles during their diastolic time, we hope to encourage the growth of hospitalist leaders with well‐developed skill sets. If hospitalists adequately prepare themselves, academic hospital medicine will likely remain sustainable and rewarding, and future generations of trainees will be inspired and prepared to enter the field.
Acknowledgements
We are grateful to Jennifer Higa and Kimberly Alvarez for their assistance in preparing this manuscript.
There is a growing demand for hospitalists in the United States. In academic settings, hospitalists are called on to perform a variety of duties, from leading quality improvement initiatives to serving on hospital committees to helping to offset restrictions on work hours of the house staff.1 Although hospitalists may be well positioned to take on these roles, obtaining adequate protected time and recognition for such contributions remains a challenge. The existing promotion and tenure processes at academic institutions may not give adequate consideration to such responsibilities. Hospitalists who do not meet the traditional benchmarks of teaching and research may suffer in their career advancement and, ultimately, in their desire to remain in academics. Developing a sustainable and long‐term career in hospital medicine is important not only from a professional developmental standpoint, but also because it may lead to better patient care; evidence from a large multicenter hospitalist study suggests that physician experience is linked to improved patient care and outcomes.2 Thus, it behooves academic medical centers that employ hospitalists to create rewarding hospitalist career paths.
Goldman described academic hospital medicine as comprising periods of systole, during which hospitalists provide clinical care, and periods of diastole, the portion of a hospitalist's time spent in nonclinical activities.3 Far from being a period of relaxation, diastole is an active component of a hospitalist's work, the time devoted to the pursuit of complementary interests, career advancement, and job diversity. A well‐thought‐out plan for the diastolic phase of a hospitalist job description can lead to significant improvement in quality, education, research, and outcomes for an academic medical center.4 A good balance of systole and diastole allows for focus on career development and advancement and has the potential to be very helpful in preventing burnout. This is of particular concern to academic hospitalists, who report working longer hours, feeling more stress, and worrying more about burnout than their nonhospitalist colleagues.5 This suggests the diastolic phase is an important part of creating a sustainable hospitalist job and should be funded as part of an academic hospitalist position.
Although the optimal balance of systole and diastole to prevent burnout is not known, outlining clear expectations is an important strategy for preparing physicians for a sustainable academic hospitalist career. This is an important issue, given the increasing number of residency graduates who are choosing careers in hospital medicine.6 Based on the reported career plans of residents taking internal medicine in‐training exams from 2002 through 2006, the number of residents going into hospital medicine has more than doubled, from 3% (in 2002) to 6.5% (in 2006). The goal of this article is to compare and contrast several career paths that balance systole and diastole in academic hospital medicine. Specifically, we review training opportunities for becoming a successful hospitalist‐educator, hospitalistquality expert, hospitalist‐investigator, and hospitalist‐administrator.
EDUCATION (THE HOSPITALIST‐EDUCATOR)
Hospitalists in academic centers often play central roles as teachers and leaders in medical education. This is not surprising given that most teaching of medical trainees occurs in the inpatient setting.7 Furthermore, several studies have consistently demonstrated that trainee satisfaction with teaching by hospitalists is high, and hospitalists are rated as more effective teachers than traditional subspecialist ward attendings.810
A typical hospitalist‐educator position is 80%‐90% clinical time, with 10%‐20% set aside for teaching. However, academic hospitalists are often expected to teach medical trainees concurrently with their clinical care activities, rather than during a separate, protected time.11 Thus, most hospitalist‐educator responsibilities do not occur during diastole, as may be conceived, but instead are add to the systole. Small amounts of protected diastolic time for a hospitalist‐educator can be used for related administrative activities, such as writing letters of recommendation, mentoring students and residents, doing creative thinking and curriculum development, and conducting educational research, such as evaluating a new educational program or curriculum. Some hospitalist‐educator positions, such as director of the residency program or internal medicine clerkship, are exceptions in that they generally include a greater amount of protected time, which may be earmarked for administrative activities and hands‐on teaching.
Education and Training
One possibility for advanced training in education is the addition of a chief resident year, either at a physician's own institution or at another academic center. Such a year provides an opportunity to consolidate knowledge, build a teaching portfolio, and accumulate expertise in an area such as evidence‐based medicine or perioperative care. Serving as a chief resident can enhance subsequent applications by being able to demonstrate the ability to teach and, more importantly, to assume a leadership role within an organization. These skills can be applied to a number of activities in an academic hospitalist program, such as heading a committee, teaching during inpatient service time, or developing a new course for students, residents, or faculty.
An advanced training program in medical education is also an option (Table 1). Offerings include medical education fellowship training, formal degree‐granting programs (such as a master's in health professions education), or short‐term intensive coursework. Fellowships and degree‐granting programs are generally 2‐year programs designed for health professionals who want to better prepare for educational leadership roles. Core topics include curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. An alternative option for busy clinician‐educators is online or distance learning courses in medical education, which cover similar topics and skill sets. In early 2006 the Society of Hospital Medicine released the Core Competencies in Hospital Medicine, which can serve as a useful framework for developing novel inpatient curricula for faculty, residents, and students.12, 13
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in health professional education (MHPE): Preparation for educational leadership roles. Typical coursework in curriculum development, program evaluation, instruction, student assessment, current educational issues, research methods, and leadership. | Varies according to program | Tuition ranges from approximately $1500‐$4300 | Example: University of Illinois |
| Fellowship in medical education: Prepares faculty to pursue scholarship in medical education or educational leadership or to become effective teachers through workshops, coursework, and/or a mentored project. Often affiliated with a department of medical education. | Varies according to program. Generally 1 year. | Varies. May be subsidized in certain institutions as part of internal faculty development. | Example: University of Michigan |
| Short‐term coursework | |||
| Harvard Macy Institute: Programs designed to promote leadership and scholarship in medical education | 1‐ or 2‐week programs | Fees for the year 2006 are $4500 USD. | |
| Stanford Faculty Development Center (SFDC): Train‐the‐trainer approach for clinical teaching and professionalism in contemporary practice | 4‐week training sessions | The institutions of faculty selected for the month‐long training programs are asked to pay a fee of $5000. Transportation, housing and food are not included. | |
Short‐term extramural courses offered by institutions such as the Harvard Macy Institute for Medical Educators and the Stanford Faculty Development Program in Teaching can also provide advanced instruction to hospitalist‐educators.14, 15 In addition to these training programs, the Society of General Internal Medicine, along with other professional societies, offers career development workshops for clinician educators on topics such as curriculum development and teaching skills.
Regardless of the type of training, adequate mentorship and resources are critical to the successful application of new skills to the design or evaluation of hospital‐based curricula. Mentorship may be available from institutional leaders in medical education, even those not formally affiliated with the hospitalist program. For instance, medical school leaders, such as deans, division chiefs, chairpersons, program directors, and clerkship directors, can often be helpful in guiding junior faculty in obtaining skills and time for teaching.
We encourage those interested in a career in medical education to begin volunteering at their institution early on. Volunteering to directly teach residents and students (eg, assisting in introduction to clinical medicine, giving lectures to third‐year clerks) can be a valuable way of becoming distinguished as a qualified teacher. Likewise, joining a professional medical society of individuals with similar interests can facilitate mentorship and skill acquisition. Certain professional medical societies, such as the American College of Physicians, promote national recognition through awarding fellowships, an honor for those physicians who have demonstrated superior competence in internal medicine, professional accomplishment, and scholarship.16 Developing concrete examples of expertise in the field, such as through the publication of abstracts and articles on medical education and development of curricula, help lead to advancement in the educational track. Clear focus on a career path, development of an intellectual product, positive learner evaluation of educational activities, and national recognition can all be used by an academic institution to evaluate suitability for promotion.
Rewards and Challenges
One of the rewards of a hospitalist‐educator career is being able to meaningfully interact with a variety of trainees, including medical students and residents. As teaching attendings, hospitalist‐educators are likely to engage students and residents for short‐term but intensive periods, resulting in the ability to influence career choice and professional growth as a physician.17 Hospitalists may be called on by trainees to serve as mentors or advisers and to write letters of recommendation. In addition, with experience, hospitalist‐educators are well positioned to serve in administrative roles in medical education, such as clerkship director or program director.
Burnout is a particular concern for hospitalist‐educators, given the heavy clinical demands of inpatient academic service combined with the additional pressure to be academically productive.5 Because of this, it is important to design academic hospitalist‐educator positions with a diastole that contains time to recover from the heavy clinical demands of inpatient service, in addition to providing time for career development activities.
Successful career development as an educator can be difficult. There are relatively few venues at which educational work can be peer‐evaluated and published, which are keys to successful academic promotion.18 Because some educational journals are highly competitive, one possibility way to get educational work disseminated is through the MedEd Portal, sponsored by the Association of American Medical Colleges, which allows peer review of medical educational materials, including innovative curricula.19 In addition to original research contributions, many scientific meetings and medical education journals also accept descriptions of interesting clinical vignettes and innovations in medical education. New online education journals, such as BMC Medical Education and Seminars in Medical Practice, have expanded publication opportunities.20
Limited opportunities are available to help fund research in medical education. Although funding may be more readily available to educators who focus on a particular clinical entity or patient population, most medical education research is conducted with inadequate funding and requires extensive donated time by committed faculty.21 For this reason, securing advanced training in medical education and having protected time will allow hospitalists on the educator track to compete more successfully for limited educational research dollars and to have sufficient time to produce and publish scholarly work, thus improving their chances of academic success and career satisfaction.
CLINICAL QUALITY AND OPERATIONS IMPROVEMENT (THE HOSPITALISTQUALITY EXPERT)
Hospitalists are increasingly being called on to lead clinical quality and operations improvement at academic teaching hospitals. Benefits to the institution include the consistent presence of a committed physician who is able to plan and execute change in the context of clinical care. This is in contrast to the transient nature of residents and nonhospitalist attending physicians, whose ability to participate in such initiatives is impaired by the scheduling of their rotations. Hospitalists, however, are often able to cultivate long‐standing relationships with nurses, case managers, and hospital administrators, thereby building the institutional clout to lead such initiatives while considering views from all the necessary stakeholders.22 Thus, they are in a good position to serve as physician champions and expedite the adoption of new innovations within hospitalist groups and among other physician groups and clinical staff.23, 24
Education and Training
Being a successful agent of change requires knowledge of the science of quality improvement coupled with the skills necessary to make such changes, such as the ability to perform a needs assessment, to develop measures of performance, to negotiate and motivate others to change behaviors, to adopt new tools and practices, and to implement and test interventions designed to improve care. It is possible for residents or junior faculty members to gain this experience through designing and implementing a quality improvement project during residency training under the direction of a mentor.25, 26 However, given the likely variability in such experience, there is no substitute for formal training in these core areas of hospital medicine.
A broad range of opportunities for advanced training in quality and operations improvement are available (Table 2). Choosing the correct program may depend on baseline expertise, availability, and the desired level of involvement. For example, introductions to these skills can be obtained through precourses or workshops at medical conferences such as the Institute of Healthcare Improvement or the Society of Hospital Medicine. For more in‐depth training, the Advanced Training Program (ATP) in Health Care Delivery Improvement, sponsored by Intermountain Healthcare, offers 12‐ to 21‐day in‐depth minicourses designed to train individuals for leadership positions in quality and safety.27 Lastly, more structured fellowships, such as the Veterans Affairs Quality Scholars Program or the George W. Merck Fellowships in Health Care Improvement, offer junior and midcareer faculty the opportunity to obtain formal training in the science of quality improvement.28, 29 Because early‐career hospitalists may face geographic and financial restrictions, exploration of local or institutional opportunities for advanced education in quality improvement can be particularly important.
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Veterans Association National Quality Scholars: Fellowship to learn and apply knowledge for improvement of health care | 2 years | No cost, application to fellowship program required | |
| George W. Merck Fellowship: Mentored research or improvement project at Institute of Healthcare Improvement with a plan to return to home institution to execute change | 1 year | No cost, application to fellowship program required | |
| Short‐term coursework | |||
| Intermountain Health Care: Designed to give executives and quality improvement leaders the necessary tools to conduct clinical practice improvement projects. | 20‐ and 12‐day training programs in Salt Lake City, UT | Tuition for the 20‐day program:
| |
Rewards and Challenges
Engaging in successful clinical or process improvement can be very rewarding, both professionally and personally. Professional gains include building new interdisciplinary relationships and infrastructure to continually monitor and improve key performance measures. In addition, a rigorous evaluation of this type of work can result in being able to make presentations at national meetings or to be published in a variety of peer‐reviewed medical journals, including specialty journals for quality improvement work, such as Quality and Safety in Healthcare and the Joint Commission Journal on Quality Improvement. Many national medical meetings, such as the Institute for Healthcare Improvement, the Society of Hospital Medicine and other subspecialty society meetings, also provide an opportunity to showcase innovations in practice.
Despite the potential rewards, it can also be challenging for academic hospitalists to participate in or lead quality improvement projects. One major challenge is ensuring that hospitalists are engaged in improvement work that is aligned with the interests of the hospital. Because most hospital administrators and frontline staff are employed by the hospital, whereas those comprising the academic faculty are employed by the university, this alignment is not always guaranteed. For example, an area of interest to a hospitalist that also could lead to academic productivity and career advancement might not be considered a priority area of improvement for the hospital because of competing clinical or operations improvements. In this scenario, it can be extremely difficult to engage other stakeholders such as nurses or administrative support staff in order to make a meaningful, sustainable change or improvement. To avoid this situation, it can be helpful from the outset to partner with hospital quality leaders in discussing priority areas, with attention to any potential interface in which hospitalist expertise is needed. In the event a potential project or area is identified, a hospitalist is particularly well positioned to serve as a physician champion, which is often key to the success of any hospitalwide initiative. In some cases, hospital funding may be available for these types of initiatives, increasing the likelihood of resource development for sustainable change.
RESEARCH (THE HOSPITALIST‐INVESTIGATOR)
Few hospitalists devote most of their time to clinical research. Having a strong research base is essential for the field of hospital medicine to gain credibility as a distinct specialty.4 Although the initial research in hospital medicine sought to prove the value of the field itself, hospitalists have now begun to focus on quality improvement and outcomes research.3032 Because of their unique position in clinical care, hospitalists are well situated to oversee inpatient data collection and perform research on a variety of conditions ranging from acute coronary syndromes to venous thromboembolism. Another potential area of research for hospitalists is participation in clinical trials focused on the inpatient setting. Although the proportion of time spent in research can vary widely, to become an independently successful clinical researcher typically requires a substantial amount of time be devoted to research. In general, at least 50% protected time, greater if possible, is recommended.
Education and Training
To develop a career around research generally requires advanced training in research methods. The most frequently used option for obtaining such training is through completing a clinical research fellowship in general internal medicine or an equivalent program, such as the fellowships administered by the Robert Wood Johnson Clinical Scholars Program (Table 3).33 Several academic centers also have developed such hospital medicine fellowships, which often can be tailored to provide the desired experience in research ethics, methodology, and statistical analysis.34, 35 In selecting a training program, prospective hospitalist‐researchers should consider the availability of suitable research mentors. Because hospital medicine as a field is relatively new, research mentors within the group of hospitalists may be scarce; if so, researchers should seek appropriate mentorship from established investigators in other programs or departments. Effective mentorship is a strong predictor of future research success.36
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Hospital or General Medicine Fellowships: Designed to provide clinical research training through mentored projects and coursework with possible master's degree | Generally 2‐year programs | No cost, application to program is required. Stipends vary. No cost, application to program is required | Hospital Medicine: |
| Robert Wood Johnson Clinical‐Scholars Program: Training in health services research with an emphasis on community‐based research and leadership training. | 2 years | Stipends currently range from $48,000 to $50,000 per year, depending on the training site. | Robert Wood Johnson: |
| Short‐term coursework | |||
| University‐based summer programs in clinical research (eg, Harvard University Summer Session for Public Health Studies which features graduate courses in epidemiology, biostatistics, economics, health care management, etc.) | Intensive 3‐week courses in Harvard University Summer Session | 2004 tuition for each 2.5‐credit course was $1830. There is a nonrefundable deposit/registration fee of $125. These fees do not include certain course materials (ie, texts estimated at $60 per course). | Example: Harvard School of Public Health |
Negotiating protected time can be challenging for new investigators, particularly when hospitalist salaries are generated by clinical activity. Some academic programs are willing to provide a few years of departmental support to promising young investigators in order to allow them to develop their research program and obtain additional funding. Several career development awards are available through the National Institutes of Health and through nonfederally funded sources.37, 38 These awards generally protect 3‐5 years of a researcher's time for research and require that a substantial proportion of time be devoted to that purpose, often at least 75%.
To gain visibility as a researcher, it is advantageous to present original findings at national meetings, such as those of the Society of Hospital Medicine, the Society of General Internal Medicine, and other subspecialty meetings.39, 40 These meetings not only increase awareness of a hospitalist's research but also provide opportunities for networking and developing collaboration on research. Many societies, including the Society of Hospital Medicine, have research abstract competitions and offer research grants for investigators that can help to fund projects and support protected time.
Rewards and Challenges
There are many rewards and opportunities for a hospitalist investigator, particularly because the field is young and there are many unanswered research questions related to inpatient medicine. There are also the intrinsic rewards of being devoted to scientific inquiry and having greater autonomy over how time is spent. A hospitalist's schedule can be well suited to research. Although attending on the wards can be very time‐consuming, time off the wards is often free of outpatient duties and can be entirely devoted to research.
There are also several challenges to becoming a successful researcher. The pressure to obtain grant funding and publish high‐quality scientific manuscripts is high. Obtaining sufficient protected time may be difficult in busy clinical departments, and applying for grant funding is both time‐consuming and highly competitive. It is very important to be familiar with the specific criteria for academic promotion at one's institution. Understanding these expectations can help to effectively prioritize activities. Standard requirements generally include number and quality of articles published in peer‐reviewed journals, successful application for research funding, national recognition in the field, service to the institution and research community, and evidence of research independence. One significant challenge is the lack of a single large funding source for hospital‐related research. Although the Agency for Healthcare Research and Quality funds studies related to hospital care, such as on the quality of care or cost effectiveness of various system‐based hospital care interventions, their budget for investigator‐initiated proposals is limited.41 One promising funding source for research in hospital care is from agencies and foundations dedicated to the aging population, such as the National Institute for Aging (NIA), the Hartford Foundation, and the Aetna Foundation, to name a few.42, 43 Yet research on hospital care alone, without detailed attention to issues unique to geriatric‐specific conditions or populations, is unlikely to be funded by these avenues. With few federal grant programs directly suited to the emerging research agenda in hospital medicine, hospitalist‐investigators may be at a disadvantage for obtaining tenure‐track positions, compared with their subspecialist colleagues, who may receive funding from NIH agencies or foundations dedicated to their own field.
ADMINISTRATION (THE HOSPITALIST‐ADMINISTRATOR)
Physician leaders in hospital administration are not new. Many hospitals already include physicians in senior management positions, such as chief medical officer.44 Naturally, a career in hospital administration is another potential path for diastole in academic medical centers.
Education and Training
Although a master of business, health administration, or medical management is not a prerequisite for the physician who wants to move into management, it is an increasingly important credential for senior administrative positions (Table 4). Primarily, it serves as a signal that a physician is committed to management and has a working knowledge of strategic planning, business models, human resources, leadership, and clinical operations. For physicians without formal business training who are interested in management, exploring internal opportunities is a necessary first step. Likewise, getting a business degree is not as important as management experience. The successful application of business skills requires practice, mentoring, and on‐the‐job experience. For hospitalists, this experience could be obtained by volunteering to serve on committees such as utilization review, quality assurance, credentialing, or medical staff executive committees. In lieu of a graduate degree, physicians may wish to participate in one of the many fellowships in health services administration. These programs generally aim to provide practical mentored learning experience in a health care organization and may last up to 2 years.45
| Description | Length of time | Cost | Source/website |
|---|---|---|---|
| Degrees/fellowships | |||
| Master's in business administration (MBA): General management core with option for courses specializing in health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MBA): |
| Master's in health administration (MHA): Studies in analytic and management needs of health care. | Generally 2‐year program | Varies in accordance with each institution. | Directory websites (MHA): |
| Fellowship in health services administration: Preceptor‐directed program that provides practical learning experience in a health care organization beyond graduate‐level academic instruction. | Usually lasts 1‐2 years. | Compensation varies. Median reported as $39,055. | Directory (American College of Healthcare Executives): |
| Short‐term coursework | |||
| Society of Hospital Medicine Leadership Academy: Instruction for hospitalists in leading change, communicating effectively, handling conflict and negotiation, doing strategic planning, and interpreting hospital business drivers. Held biannually. | 3‐ to 4‐day program | $1400‐$1600. Discounted rate for members of Society of Hospital Medicine | |
For hospitalists and trainees considering a career as an executive, the American College of Physician Executives can serve as a valuable resource.46 This organization, founded in 1975, offers educational resources, including publications, comprehensive CD‐ROM products, and 1‐day courses and master's degree programs in conjunction with several leading business schools in medical management. In addition, the Society of Hospital Medicine offers a Leadership Academy designed to assist practicing hospitalists in evaluating their leadership strengths and applying them to everyday management challenges.47 Such a program also can facilitate the development of a peer network and the mentoring relationships needed to achieve these goals.
Rewards and Challenges
The life of the physician executive can be rewarding, but making the transition may prove challenging. However, if physicians can navigate this transition successfully, they will likely find a wide array of opportunities, as demand for physician‐executives remains high.
One major challenge to becoming a physician‐executive is reconciling the administrative role with the initial desire to enter a career in clinical medicine.48 Physician‐executives who continue to see patients are more likely to be satisfied with their jobs than physician‐executives who do not.49 Physician‐executives also may feel they are being criticized by their purely clinical colleagues for working in the business or management of medicine.50 Actual or perceived lack of support may promote isolation and burnout.51 In addition, the constantly shifting landscape of health care administration results in a much more unstable environment than that found in clinical medicine. For example, the risk of termination for a physician‐executive is 20‐40 times higher than that for a practicing physician.50 The reasons for this higher risk include personal conflict with a boss, reorganization (ie, downsizing, merging, etc.), and immediate departure of a supervisor. Access to mentors, support groups, and the option to practice part time are all potential mechanisms to ensure long‐term success as a physician‐administrator.
CONCLUSIONS
As hospital medicine continues to grow and evolve, designing sustainable and rewarding academic careers will be crucial to the success of the field. Being able to balance clinical systole time with obtaining the skills to support nonclinical diastole time is important to ensuring a successful career as an academic hospitalist. We have described several possible career paths in teaching, research, quality improvement, and administration. By preparing future hospitalists with the knowledge and skills required to assume a variety of roles during their diastolic time, we hope to encourage the growth of hospitalist leaders with well‐developed skill sets. If hospitalists adequately prepare themselves, academic hospital medicine will likely remain sustainable and rewarding, and future generations of trainees will be inspired and prepared to enter the field.
Acknowledgements
We are grateful to Jennifer Higa and Kimberly Alvarez for their assistance in preparing this manuscript.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80:39–43.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(s1):141–142.
- .The hospitalist movement.Ann Intern Med.1999;131:545.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,, et al.Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations.J Gen Intern Med.2006;21(s4):128.
- ,,.Career plans for trainees in internal medicine residency programs.Acad Med.2005;80:507–512.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Amer J Med.2005;118:680–687.
- ,,, et al..Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,,,,.Resident satisfaction on an academic hospitalist service: time to teach.Am J Med.2002;112:597–601.
- ,.The present and future of appointment, tenure, and compensation policies for medical school clinical faculty.Acad Med.2001;76:993–1004.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(1).
- Harvard Macy Institute. Harvard College. Available at: http://www.harvardmacy.org/programs.asp?DocumentID=1. Accessed October 3,2005.
- Stanford Faculty Development Center. Stanford University. Available at http://sfdc.stanford.edu/. Accessed January 23,2006.
- American College of Physicians. Available at: http://www.acponline.org/college/membership/classes.htm#fellow. Accessed June 10,2006
- ,,, et al.Effect of the inpatient general medicine rotation on student pursuit of a generalist career.J Gen Intern Med.2006;21:471–475.
- ,.Graduate medical education research in the 21st century and JAMA on call.JAMA.2004;292:2913–2915.
- Association of American Medical Colleges. MedEd (PORTAL); Providing Online Resources to Advance Learning in Medical Education. Available at: http://www.aamc.org/meded/mededportal/start.htm. Accessed January 23,2006.
- BioMed Central. BMC Medical Education. Available at: http://www.biomedcentral.com/bmcmededuc/.Accessed January 23,2006.
- ,,,.Costs and funding for published medical education research.JAMA.2005;294(9):1052–7.
- .Teamwork and hospital medicine. A vision for the future.Crit Care Nurse.2003;23(3):8,10–11.
- . (1995)Diffusion of Innovations.4th ed.The Free Press:,Toronto.
- ,,.Clarifying the concepts in knowledge transfer: a literature review.J Adv Nurs.2006;53:691–701.
- ,,,,.Creating a quality improvement elective for medical house officers.J Gen Intern Med.2004;19:861–867.
- ,,.A continuous quality improvement curriculum for residents: addressing core competency, improving systems.Acad Med.2004;79(10 Suppl):S65–7.
- Institute for Healthcare Delivery Research. Advanced Training Program in Health Care Delivery Improvement (ATP). Available at: http://www.ihc.com/xp/ihc/institute/education/atp/. Accessed October 3,2005.
- Veterans Health Administration. VA Quality Scholars Program. Available at: http://www.dartmouth.edu/∼cecs/fellowships/vaqs.html
- Institute for Healthcare Improvement. George W. Merck Fellowships. Available at: http://www.ihi.org/ihi. Accessed October 3,2005.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Robert Wood Johnson Clinical Scholars Program. Stanford University (Palo Alto, CA). Available at: http://rwjcsp.stanford.edu/. Accessed October 3,2005.
- ,.Hospital Medicine Fellowship Update.Society of Hospital Medicine.The Hospitalist.2004;8(5):38.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119(1):72.e1–e7.
- ,,, et al.Mentorship in Academic General Internal Medicine.J Gen Intern Med.2005;2(34):1–5.
- ,,,,.Getting funded. Career development awards for aspiring clinical investigators.J Gen Intern Med.2004;19(5 Pt 1):472–478.
- K Kiosk—Information about NIH Career Development Awards. Available at: http://grants.nih.gov/training/careerdevelopmentawards.htm. Accessed March 20,2006.
- Research Career Development Awards for Junior Faculty and Fellows in General Internal Medicine. Available at: http://www.sgim.org/careerdevelopment.cfm. Accessed March 24,2006.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org//AM/Template.cfm?Section=Home. Accessed October 4,2005.
- ,.What is an academic general internist? Career options and training pathways.JAMA.2002;288:2045–2048.
- U.S. National Institutes of Health.National Institute on Aging. Available at: http://www.nia.nih.gov/. Accessed January 25,2006.
- .Hartford Foundation. Available from: http://www.jhartfound.org/. Accessed January 25,2006.
- .Why will physicians in this new environment replace MHAs?Physician Exec.1996;22(2):5–10.
- Directory of Fellowships in Health Services Administration. Available at: http://www.ache.org/pgfd/purpose.cfm. Accessed March 24,2006.
- American College of Physician Executives. Available at: http://www.acpe.org/. Accessed October 3,2005.
- Society of Hospital Medicine. Leadership Academy statement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Search_Advanced_Search6(7):37–40.
- ,,,.Satisfaction higher for physician executives who treat patients, survey finds.Physician Exec.2002;28(3):17–21.
- .Physician executives don't have to go it alone.Managed Care Magazine.2003. Available at: http://www.managedcaremag.com/archives/0307/0307.viewpoint_lazarus.html.Accessed January 25,year="2006"2006.
- .Controlled burn! Physician executives must be ready to handle job burnout, career stress.Physician Exec.2001;27(4):42–45.
- ,,.Balancing continuity of care with residents' limited work hours: defining the implications.Acad Med.2005;80:39–43.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.J Gen Intern Med.2005;20(s1):141–142.
- .The hospitalist movement.Ann Intern Med.1999;131:545.
- .The future of hospital medicine: evolution or revolution?Am J Med.2004;117:446–450.
- ,,, et al.Satisfaction and worklife of academic hospitalist and non‐hospitalist attendings on general medical inpatient rotations.J Gen Intern Med.2006;21(s4):128.
- ,,.Career plans for trainees in internal medicine residency programs.Acad Med.2005;80:507–512.
- ,,,,.Closing the gap between internal medicine training and practice: recommendations from recent graduates.Amer J Med.2005;118:680–687.
- ,,, et al..Hospitalists as teachers.J Gen Intern Med.2004;19(1):8–15.
- ,.Implications of the hospitalist model for medical students' education.Acad Med.2001;76:324–330.
- ,,,,,.Resident satisfaction on an academic hospitalist service: time to teach.Am J Med.2002;112:597–601.
- ,.The present and future of appointment, tenure, and compensation policies for medical school clinical faculty.Acad Med.2001;76:993–1004.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1(1):48–56.
- ,,,,.The core competencies in hospital medicine.J Hosp Med.2006;1(1).
- Harvard Macy Institute. Harvard College. Available at: http://www.harvardmacy.org/programs.asp?DocumentID=1. Accessed October 3,2005.
- Stanford Faculty Development Center. Stanford University. Available at http://sfdc.stanford.edu/. Accessed January 23,2006.
- American College of Physicians. Available at: http://www.acponline.org/college/membership/classes.htm#fellow. Accessed June 10,2006
- ,,, et al.Effect of the inpatient general medicine rotation on student pursuit of a generalist career.J Gen Intern Med.2006;21:471–475.
- ,.Graduate medical education research in the 21st century and JAMA on call.JAMA.2004;292:2913–2915.
- Association of American Medical Colleges. MedEd (PORTAL); Providing Online Resources to Advance Learning in Medical Education. Available at: http://www.aamc.org/meded/mededportal/start.htm. Accessed January 23,2006.
- BioMed Central. BMC Medical Education. Available at: http://www.biomedcentral.com/bmcmededuc/.Accessed January 23,2006.
- ,,,.Costs and funding for published medical education research.JAMA.2005;294(9):1052–7.
- .Teamwork and hospital medicine. A vision for the future.Crit Care Nurse.2003;23(3):8,10–11.
- . (1995)Diffusion of Innovations.4th ed.The Free Press:,Toronto.
- ,,.Clarifying the concepts in knowledge transfer: a literature review.J Adv Nurs.2006;53:691–701.
- ,,,,.Creating a quality improvement elective for medical house officers.J Gen Intern Med.2004;19:861–867.
- ,,.A continuous quality improvement curriculum for residents: addressing core competency, improving systems.Acad Med.2004;79(10 Suppl):S65–7.
- Institute for Healthcare Delivery Research. Advanced Training Program in Health Care Delivery Improvement (ATP). Available at: http://www.ihc.com/xp/ihc/institute/education/atp/. Accessed October 3,2005.
- Veterans Health Administration. VA Quality Scholars Program. Available at: http://www.dartmouth.edu/∼cecs/fellowships/vaqs.html
- Institute for Healthcare Improvement. George W. Merck Fellowships. Available at: http://www.ihi.org/ihi. Accessed October 3,2005.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Robert Wood Johnson Clinical Scholars Program. Stanford University (Palo Alto, CA). Available at: http://rwjcsp.stanford.edu/. Accessed October 3,2005.
- ,.Hospital Medicine Fellowship Update.Society of Hospital Medicine.The Hospitalist.2004;8(5):38.
- ,,,.Hospital medicine fellowships: works in progress.Am J Med.2006;119(1):72.e1–e7.
- ,,, et al.Mentorship in Academic General Internal Medicine.J Gen Intern Med.2005;2(34):1–5.
- ,,,,.Getting funded. Career development awards for aspiring clinical investigators.J Gen Intern Med.2004;19(5 Pt 1):472–478.
- K Kiosk—Information about NIH Career Development Awards. Available at: http://grants.nih.gov/training/careerdevelopmentawards.htm. Accessed March 20,2006.
- Research Career Development Awards for Junior Faculty and Fellows in General Internal Medicine. Available at: http://www.sgim.org/careerdevelopment.cfm. Accessed March 24,2006.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org//AM/Template.cfm?Section=Home. Accessed October 4,2005.
- ,.What is an academic general internist? Career options and training pathways.JAMA.2002;288:2045–2048.
- U.S. National Institutes of Health.National Institute on Aging. Available at: http://www.nia.nih.gov/. Accessed January 25,2006.
- .Hartford Foundation. Available from: http://www.jhartfound.org/. Accessed January 25,2006.
- .Why will physicians in this new environment replace MHAs?Physician Exec.1996;22(2):5–10.
- Directory of Fellowships in Health Services Administration. Available at: http://www.ache.org/pgfd/purpose.cfm. Accessed March 24,2006.
- American College of Physician Executives. Available at: http://www.acpe.org/. Accessed October 3,2005.
- Society of Hospital Medicine. Leadership Academy statement. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Search_Advanced_Search6(7):37–40.
- ,,,.Satisfaction higher for physician executives who treat patients, survey finds.Physician Exec.2002;28(3):17–21.
- .Physician executives don't have to go it alone.Managed Care Magazine.2003. Available at: http://www.managedcaremag.com/archives/0307/0307.viewpoint_lazarus.html.Accessed January 25,year="2006"2006.
- .Controlled burn! Physician executives must be ready to handle job burnout, career stress.Physician Exec.2001;27(4):42–45.
Copyright © 2006 Society of Hospital Medicine
CAP Quality Care / Seymann
The quality movement has spawned efforts to define and measure best practices for clinical conditions commonly cared for by hospitalists. Pneumonia is the most frequent infectious cause of death in the United States, and it accounts for more than 1 million hospitalizations annually at an estimated annual cost of $12.2 billion, most of it incurred by inpatients.1 The morbidity and mortality of the elderly are particularly burdensome.2, 3 For these reasons, attention has been focused on improving the quality of care of inpatients with community‐acquired pneumonia (CAP).
Credentialing agencies such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) require hospitals to report performance on predefined core measures of pneumonia care that they have identified as best practices (see Table 1).4, 5 The performance of individual organizations on these measures is now publicly reported at a website (
|
| Collection of blood cultures before antibiotic therapy. |
| Collection of blood cultures within 24 hours of admission. |
| Mean time of less than 4 hours from arrival to initial administration of antibiotics. |
| Choice of initial antibiotics according to established guidelines.* |
| Pneumococcal screening and vaccination of eligible patients by discharge. |
| Influenza screening and vaccination of eligible patients during flu season. |
| Oxygenation assessment within 24 hours of admission. |
| Smoking cessation counseling to all smokers. |
Performance on core measures for pneumonia is less consistent across hospitals than the other conditions currently being monitored.7 It is instructive, then, to review the evidence base for the existing pneumonia quality measures, which can inform decisions about prioritizing interventions to provide the most effective care for inpatients with CAP.
BLOOD CULTURES
In a large multicenter retrospective study of Medicare patients hospitalized with CAP, Meehan et al.10 found the performance of blood cultures within 24 hours of arrival to be associated with reduced 30‐day mortality. Despite the large sample size of more than 14,000 patients, the risk‐adjusted mortality reduction was of only borderline significance (RR 0.9 [0.81‐1.00]). The unadjusted data did not show a significant mortality reduction. Notably, collection of blood cultures prior to antibiotic administration did not affect mortality, even excluding patients receiving prehospital antibiotics.
A smaller review of 38 U.S. academic medical centers showed relatively high compliance with blood culture performance, but no mortality reduction, even after adjustment for severity of illness. Similarly, performing blood cultures before administration of antibiotics yielded no significant effect.11
Several studies call into question the clinical utility of performing blood cultures drawn from patients with CAP. Combined, these studies evaluated almost 3000 pneumonia patients who had blood cultures drawn; the likelihood of a change in therapy based on results was at most 5%. Among the patients with positive cultures, only 20%‐40% had a treatment change based on the result.1215
The more severely ill patients with CAP may benefit from blood cultures, though the findings reported in the literature vary.12, 16 Using the Pneumonia Severity Index (PSI) score17 to classify severity of illness, an observational study of 209 inpatients with CAP found the yield of blood cultures increased from 10% in the lowest‐risk groups to 27% in the most severely ill.16 In contrast, two larger studies with a combined enrollment of almost 14,000 patients were unable to demonstrate a difference in the incidence of bacteremia despite adjustment for the PSI score.12, 18 It is clear from these and other studies that patients in PSI classes I‐III derive very little benefit from the performance of blood cultures.12, 16, 19
Metersky et al.18 described a prospectively validated risk assessment tool that reliably predicted bacteremia in Medicare patients with CAP and explored its utility in reducing unnecessary blood cultures. Independent risk factors for bacteremia included prior antibiotic use, liver disease, hypotension, tachycardia, fever or hypothermia, BUN > 30 mg/dL, sodium 130 mmol/L, and WBC 5000 or > 20,000/mm2. Use of this tool predicted bacteremia in 89% of patients and avoided 39% of unnecessary blood cultures. The authors also tested a modified version of the tool that excluded the laboratory abnormalities, so rapid assessment could be made at the initial patient presentation. This version advocated a single blood culture for most patients, and 2 blood cultures for patients with 2 or more risk factors. The modified tool accurately identified 88% of the patients with bacteremia and enabled a 44% reduction in unnecessary cultures.
In summary, blood cultures occasionally provide useful clinical information about etiology and resistance patterns, but they do not seem to reliably influence therapeutic decisions. It seems inappropriate to recommend against their use in practice, but they are not a solid benchmark for evidence‐based quality care. Measures that mandate risk assessment of all inpatients with CAP and require blood cultures only for older patients or those judged at high risk by PSI may better reflect quality. Alternatively, performing blood cultures on patients deemed to be high risk by the model of Metersky et al.18 may suffice.
ANTIBIOTIC TIMING
In a study of Medicare patients by Meehan et al.,10 the 30‐day mortality rate was reduced by 15% in the subset of patients who received antibiotics within 8 hours of arrival at the hospital. However, a trend toward mortality reduction was noted for those receiving antibiotics as early as 6 hours after arrival. Rapid administration of antibiotics was thus deemed an important measure of the quality of care of patients with CAP.
Additional studies attempted to confirm this observation. Battleman et al.20 evaluated 700 patients admitted for CAP through the emergency department. They found that a delay of more than 8 hours in the administration of antibiotics was correlated with a prolonged inpatient stay. Mortality rates were not reported. Achieving rapid delivery of antibiotics was closely linked to administration of the first dose of antibiotics in the emergency department.
Conversely, a large retrospective review by Dedier et al.11 found no reduction in inpatient mortality or in length of stay based on rapid antibiotic delivery, despite adjustment for severity of illness. They did not evaluate 30‐day mortality.
The effect of antibiotic timing on the time to clinical stability has also been investigated. Clinical stability was defined as 24 hours of a systolic blood pressure 90 mm Hg, heart rate 100 beats/min, respiratory rate 24 breaths/min, temperature 38.3C (101F), room air oxygen saturation 90%, and the ability to eat. Silber et al.,21 in a review of the records of 409 inpatients with moderate to severe CAP by PSI score, compared patients receiving antibiotics less than 4 hours, between 4 and 8 hours, and more than 8 hours after hospital admission. There was no difference between groups in time to clinical stability, even with adjustment for PSI.
Marrie and Wu22 attempted to define the factors that influenced inpatient mortality of patients with CAP not admitted to the intensive care unit (ICU). In a prospective study of 3043 patients evaluating a clinical pathway, a multivariate analysis showed antibiotic administration within 4 hours was not correlated with reduced mortality.
Although most studies supporting rapid antibiotic delivery used a target of 8 hours, administration in less than 4 hours is the consensus standard for pneumonia care set by CMS and JCAHO.23, 24
A benefit of timing antibiotic administration less than 4 hours after admission has been confirmed by a single, very large retrospective study of Medicare patients at least 65 years old.25 Analysis of a random sample of more than 18,000 patients with CAP who had not received prehospital antibiotics showed that the relative risk reduction for inpatient mortality was 15% in the group receiving antibiotics within 4 hours. Thirty‐day mortality was similarly reduced, and benefits continued for every hour of early antibiotic administration up to 9 hours.
The absolute risk reduction was small, however (0.6%), yielding a number needed to treat of 167 patients to prevent 1 death.
Randomized controlled trials, which would more definitively address the issue of antibiotic timing, are unlikely, as intentionally delaying administration of antibiotics to patients with known CAP is unethical. Hence, reliance on observational data must suffice. Intuitively, it makes sense to begin treatment of a bacterial infection at the earliest time possible. However, it is also known that not all patients present in a typical fashion, and diagnosis is uncertain at least 20% of the time.26 Anecdotal reports suggest that incentivizing physicians on performance measures encourages premature administration of empiric antibiotics to all patients presenting with cough, prior to confirmation of pneumonia.27, 28 Such practices promote further antibiotic resistance, arguably a larger health issue than delay in antibiotic delivery.29, 30
Houck31 offers potential solutions to this problem, such as eliminating the pressure on hospitals to perform at 100% on this measure by reporting performance within acceptable ranges (eg, 70%‐84% and 85%‐100%) Targeting a benchmark of 80% or a duration of 6 hours may also be appropriate. Finally, a 4‐hour benchmark has not been shown to benefit younger patients, so it is important to apply this target only to patients more than 65 years of age.
CHOICE OF ANTIBIOTIC
A retrospective review of 12,945 cases of inpatients with CAP found that, in comparison to ceftriaxone alone, initial antibiotic regimens consisting either of a second‐ or third‐generation cephalosporin plus a macrolide or of a fluoroquinolone alone were associated with an approximately 30% reduction in 30‐day mortality.32 Hence, current guidelines recommend the combination of a B‐lactam and macrolide, a B‐lactam and doxycycline, or a respiratory fluoroquinolone for inpatients with CAP not admitted to the ICU.3335
The results of subsequent studies supported the contention that guideline‐compliant antibiotics improve outcomes. A prospective multicenter study of a clinical pathway that encouraged use of either levofloxacin or cefuroxime plus azithromycin for the initial treatment of inpatient CAP showed significantly reduced mortality. Compared with any other antibiotic regimen, the odds ratio for death was 0.22 with the cephalosporin/macrolide combination and 0.43 with the fluoroquinolone. Of note, early mortality (within 5 days of admission) was not reduced by antibiotic choice.22 Similar results were found in a retrospective analysis, which found the odds of 30‐day mortality increased by 5.7 in patients not receiving guideline‐compliant therapy.36 A third study found guideline‐compliant antibiotics reduced the likelihood of a prolonged length of stay by 45%.20
Of note, data on the effectiveness of the cephalosporin/doxycycline combination are limited, and the major guidelines differ about whether this regimen is appropriate for inpatients with CAP.33, 34 Important findings from a recent retrospective cohort study showed that initial therapy with ceftriaxone plus doxycycline was associated with reduced inpatient mortality (OR = 0.26) as well as reduced 30‐day mortality (OR = 0.37) compared with other guideline‐compliant therapies for CAP.37 When patients who would not have been considered appropriate for initial doxycycline therapy (those resident in nursing homes, with aspiration pneumonia, or in the ICU) were excluded, a large reduction in inpatient mortality remained (OR = 0.17), without any increase in length of stay or readmission rate. Interestingly, this study suggests the potential superiority of this regimen, though a randomized controlled trial is needed to confirm this. The current core measures do include doxycycline as an acceptable option for CAP therapy (see Table 1).
Currently, controversy remains about whether the benefit of these selected regimens results from their activity against atypical pathogens (Mycoplasma, Legionella, Chlamydia) and whether there is additional benefit from using combination antibiotic therapy.38, 39 Waterer40 described 225 inpatients with bacteremic pneumococcal pneumonia, noting the antibiotic regimen received during the first 24 hours of hospitalization. Patients were classified retrospectively into 3 groupssingle effective therapy (SET), dual effective therapy (DET), or more than dual effective therapy (MET)on the basis of the concordance of pneumococcal sensitivity with the initial antibiotics. Patients on 2 antibiotics were classified in the DET group if the organism was sensitive to both and in the SET group if the organism was resistant to 1 of the 2. Those in the MET group were analyzed separately, as they were found to have a higher baseline severity of illness based on the PSI score; the SET and DET groups were equivalent.
Surprisingly, the SET group was found to have a 3‐fold increase in inpatient mortality; adjustment for severity of illness increased the odds ratio for death to 6.4. Of note, all deaths were in the most severely ill patients (PSI IV‐V). The protective effects of receiving DET were not specifically limited to those receiving a macrolide as the second agent, and multivariate analysis did not find coverage of atypical organisms to be an independent predictor of mortality.
A recent prospective multicenter trial of 844 patients with bacteremic pneumococcal pneumonia at 21 hospitals confirmed these findings.41 A significant 14‐day survival advantage (23% versus 55%) was found in the subgroup of critically ill patients who received at least 2 effective antibiotics. Though survival benefit was restricted to the sickest patients, severity of illness was similar among the groups.
The specific importance of macrolides in combination therapy remains under investigation. A review of a database of inpatients with bacteremic pneumococcal pneumonia over a 10‐year period found that 58% received initial empiric therapy with a B‐lactam/macrolide combination and 42% received B‐lactam without a macrolide (though other antibiotic combinations were not excluded).42 After logistic regression analysis, the investigators found a relative reduction in inpatient mortality of 60% in the patients receiving combination therapy with macrolides. Unfortunately, neither comparison to fluoroquinolone monotherapy nor risk stratification by PSI was reported. A similar study from Canada that did stratify for risk confirmed a mortality benefit of combination therapy.43
A subsequent, extremely large study of more than 44,000 patients from a hospital claims‐made database lent support to these findings.44 This study included all CAP patients regardless of microbiology and was not restricted to those with bacteremia. Outcomes among groups receiving monotherapy with any of the standard agents for CAP were compared with those in groups receiving combination therapy with a macrolide as the second agent. Statistically significant reductions in 30‐day mortality were observed in all groups receiving dual therapy with macrolides. Consistent with other studies, the benefit applied only to patients with more severe CAP. The percentage of patients with bacteremia was not specified.
Of note, this study did not allow direct comparison of fluoroquinolone monotherapy to combination therapy with a B‐lactam and a macrolide. However, the fluoroquinolone/macrolide combination conferred no additional benefit beyond fluoroquinolone monotherapy when adjusted for severity of illness or age. This implies that fluoroquinolone monotherapy is adequate, at least in some subpopulations. This is consistent with initial studies that established the superiority of the antibiotic combinations recommended by the guidelines.20, 22, 32
The potential benefit of combination therapy appears limited to patients with higher severity of illness and pneumococcal bacteremia. However, outcomes are affected by the antibiotic regimen selected in the initial 24‐48 hours of hospitalization, before results of blood cultures are routinely available. At present, clinical prediction of patients who will benefit from combination therapy is difficult.
Coverage of undiagnosed mixed infections with atypical organisms is probably not a major factor benefiting patients receiving combination therapy. Several recent meta‐analyses found no reduction in mortality or the rate of clinical failure among patients receiving antibiotics covering atypical organisms compared with those for patients whose regimens did not have such coverage.4547 Subgroups of patients with Legionella pneumonia do benefit from antibiotics with targeted activity against atypical organisms, but fewer than 1% of all patients were so identified. Evidence for antibiotic synergy is similarly lacking.48, 49 The immunomodulatory effects of macrolides, which decrease cytokine production and inflammation and subsequently reduce the severity of lung injury and other complications of sepsis, are considered potential factors in the reduction of mortality.50
The definition of severe CAP and the indications for ICU admission remain controversial, evidence for which is reviewed elsewhere.34, 51, 52 Antibiotic recommendations for ICU patients are included in Table 1 for completeness, but a detailed review of the evidence is lacking because current guidelines are based on consensus opinion.34 The use of fluoroquinolone monotherapy in severe CAP is not currently recommended because of limitations of the existing evidence. The majority of quinolone trials have excluded severely ill patients, and approval trials of newer respiratory fluoroquinolones have used levofloxacin as a comparator. Studies comparing fluoroquinolones typically allowed investigators in the B‐lactam arm the option of adding macrolides or tetracycline at their discretion. In addition, such trials have been designed as noniferiority trials.38 Clearly, randomized controlled trials are needed to resolve this issue.
Currently, selecting appropriate antibiotics should follow established guidelines, with consideration of using combination therapy for patients with a higher severity of illness. Emphasis on this measure should be stronger than that on antibiotic timing, as the bulk of the evidence favors significant mortality reduction from following guidelines for antibiotic therapy.
VACCINATION
Guidelines recommend all eligible adults hospitalized with CAP receive pneumococcal vaccination on discharge,3335, 53 though there is no evidence this reduces the incidence of pneumonia or death.54, 55 Retrospective studies have shown reduced incidence of invasive disease (bacteremia and meningitis), but not of other end points.5457 The estimated mortality from pneumococcal bacteremia remain as high as 20%‐30%, with no evidence that this rate has decreased over the last 30 years.5861 Despite this, a recent meta‐analysis from the Cochrane database that included only randomized, controlled trials (75,197 patients in 15 trials) was unable to show significant reductions in all‐cause pneumonia or mortality for vaccinated subjects.62 Cohort studies, evaluated separately in this analysis, showed an efficacy of 53% in reducing the incidence of invasive pneumococcal disease. Given the relatively low incidence of invasive disease in the general population, the number needed to treat was estimated at 20,000, or 4000 if only older patients were considered. A subsequent retrospective cohort study showed no reduction in pneumonia hospitalizations, cases of outpatient pneumonia, or mortality among 45,365 elderly vaccinees.56 Some specific subgroups may benefit, however. Vaccinated patients with chronic lung disease did show a reduction in hospitalization for pneumonia (RR 0.57 [0.38‐0.84]) and in mortality (RR 0.7 [0.56‐0.9]) in a retrospective study of HMO patients older than age 65.63
It is of interest that since the licensure of the pediatric 7‐valent protein‐polysaccharide conjugate vaccine in 2000, the incidence of invasive pneumococcal disease among adults has dropped significantly. Overall reduction in invasive disease in adults more than 50 years old was 11% from 1998 to 2003 (relative risk reduction [RRR] = 28%). This is likely the result of decreased transmission from colonized or infected children and not a coincidental increase in adult pneumococcal vaccination, as the rates of disease caused by the 16 strains unique to the 23‐valent vaccine did not change.64, 65 The overall reduction in the incidence of invasive disease is still superior with the adult vaccine, up to 30% in vaccinated subjects (RRR = 44%).56 Invasive disease caused specifically by penicillin‐nonsusceptible serotypes has dropped by 49% in the elderly since introduction of the vaccine.66 Thus, the combined impact of the 2 vaccines may be significant. It is not yet clear what effect, if any, the 7‐valent vaccine will have on the hospitalization rate or mortality.
In contrast to the results for pneumococcal vaccination, studies of the benefits of influenza vaccination have shown clear and consistent reductions in mortality, respiratory illness, hospitalization, and pneumonia, especially among patients with comorbidities.6771 Cost effectiveness has been demonstrated for all populations,72, 73 and the reduction in mortality among high‐risk patients younger than age 65 has been estimated to be as high as 78%.68 Among the elderly, reduction in mortality of about 50% has been reported, along with 20%‐30% reductions in hospitalizations for pneumonia, influenza, cardiac disease, and stroke.70 Reduced incidence of pneumonia in vaccinated patients has even been documented among elderly patients without specific comorbidities.67 Annual revaccination has the most significant impact on mortality.74
The pneumococcal vaccine remains important in the effort to reduce the severity of and complications from invasive pneumococcal disease in the elderly, but the lack of significant benefits on hard end points such as mortality or hospitalizations makes it a less robust measure of quality pneumonia care. In contrast, influenza vaccination has a much larger impact on outcomes in the population at risk. Emphasis should be shifted from pneumococcal to influenza vaccine in pneumonia performance measures.
OXYGENATION ASSESSMENT
It seems intuitive that oxygenation assessment is important in the initial evaluation of patients with CAP, though there is not direct evidence to support this. The recommendation for oxygenation assessment in the published guidelines for CAP is by consensus.3335 Documented hypoxemia is associated with increased pneumonia‐related mortality,17, 75 and clinical judgment does not adequately predict hypoxemia.76 Though the assessment of oxygenation has been found to vary widely among practitioners,77 performance has remained consistent since the advent of monitoring and reporting of quality measures, with compliance rates of 99%.4, 7 Monitoring performance of this measure should continue, though high compliance rates limit its ability to discriminate among institutions.
SMOKING CESSATION COUNSELING
Counseling patients to stop smoking was found to be modestly (2%) but statistically significantly effective in promoting abstinence at 1 year.78 In its report on treating tobacco use, the U.S. Public Health Services recommended that all smokers receive hospital‐ and system‐based interventions at every visit.79 As part of the Pneumonia Patient Outcomes Research Team (PORT) study, smokers with pneumonia underwent a tobacco cessation interview. Though only 15% of these patients quit smoking, 93% of those who quit did so at the time they developed CAP.80 A retrospective study of patients with bacteremic pneumococcal pneumonia found tobacco exposure, including passive smoking, to be a strong independent risk factor for invasive disease.81 The most recent CAP guidelines from the Infectious Disease Society of America (IDSA) recommend smoking cessation counseling for all hospitalized patients who smoke.33 However, hospitals are not likely to have the impact that a more comprehensive, outpatient‐based smoking cessation program would. Without ongoing support, counseling, and pharmacotherapy, the effects of an intervention would be expected to be small.79 Though evidence of benefit is limited, smoking cessation interventions should be encouraged at all sites of care. Quality care merits this regardless of admitting diagnosis, but benefits specific to CAP outcomes have not yet been demonstrated.
CONCLUSIONS
The burden of illness caused by CAP mandates that clinicians strive to deliver the highest quality of care to afflicted patients. Critical evaluation of the strength of the evidence will continue to guide such endeavors, and changes in practice will follow as new information surfaces. Standards of care, as adopted by consensus groups such as the IDSA and American Thoracic Society, will continue to inform the practice of hospitalists.
How quality is defined for public reporting requires particularly careful assessment. The definition of quality should be based on evidence more rigorous than that ascribed to consensus guidelines. Within the profession, guidelines offer reasonable standards of care and delineate areas for further research and are invaluable tools for practicing clinicians. In the public arena, however, proclaiming practices as good or bad sets expectations of health care consumers not educated in the nuances of evaluating clinical evidence and can unfairly bias them against conscientious and effective providers whose standards reflect different interpretations of controversial issues. Regulatory agencies should publicly target interventions using only the most solid evidentiary foundation while internally striving to monitor the effects of different practice patterns and report measurable differences in outcomes revealed by careful investigation. Areas where controversy remains should be the primary targets of further research but should not be offered as benchmarks for public scrutiny until the medical community has settled on a position.
Furthermore, when evidence remains questionable, financial incentives should be linked to performance indicators with extreme caution. It would be counterproductive if health care organizations, driven to achieve optimal antibiotic timing to obtain payment updates from CMS, began to administer antibiotics prior to completing workups on all patients with respiratory complaints, as this would likely lead to antibiotic overuse. Similarly, institutions pushed to collect blood cultures before antibiotics are given may inappropriately delay administration in order to perform well on quality measures, resulting in potential harm to patients.
The measures of quality care for CAP for which the evidence on outcomes is the most convincing are antibiotic selection (mortality benefit, reduction in LOS) and influenza vaccination (mortality benefit, reduction in hospitalizations, reduction in respiratory illness). Antibiotic timing also shows a smaller but convincing reduction in mortality, though the advantages of receiving antibiotics within 4 hours instead of 8 hours are not clearly established for younger patients. These measures should be emphasized most heavily in the arena of public reporting and incentives for quality care, with additions and modifications guided by emerging evidence. Revision of the other measures to conform with current evidence would allow public reporting to more accurately reflect quality.
- ,,,.Treatment costs of community‐acquired pneumonia in an employed population.Chest.2004;125:2140–2145.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,,.Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988‐2002.JAMA.2005;294:2712–2719.
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002‐2004.N Engl J Med.2005;353:255–264.
- Performance Measurement: Core Measures. Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/index.htm. Accessed November 7, 2005.
- Medicare Quality Improvement Program Priorities: DRAFT, August2005.Centers for Medicare 353:265–274.
- Medicare demonstration shows hospital quality of care improves with payments tied to quality.Centers for Medicare 278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95(1):78–82.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community‐acquired pneumonia: link between quality of care and resource utilization.Arch Intern Med.2002;162:682–688.
- ,,, et al.Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia.Chest.2003;124:1798–1804.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127:1260–1270.
- Hospital Quality Measures:2004‐2007. Available at: http://www.cms.hhs.gov/quality/hospital/. Accessed November 17, 2005.
- National Hospital Quality Measures Specification Manual version 1.02.Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/aligned_manual.htm.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,,,,.Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours?Chest.2006;130:16–21.
- ,.Administration of first hospital antibiotics for community‐acquired pneumonia: does timeliness affect outcomes?Curr Opin Infect Dis.2005;18(2):151–156.
- .The pneumonia controversy: hospitals grapple with 4 hour benchmark.Ann Emerg Med.2006;47:259–261.
- ,,, et al.Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods.Ann Intern Med.2001;134:479–486.
- .The hidden impact of antibacterial resistance in respiratory tract infection. Clinical failures: the tip of the iceberg?Respir Med2001;95Suppl A:S5–11; discussion S26–S27.
- .Antibiotics and pneumonia: is timing everything or just a cause of more problems?Chest.2006;130:1–3.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults.Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,,.Effects of guideline‐concordant antimicrobial therapy on mortality among patients with community‐acquired pneumonia.Am J Med.2004;117:726–731.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- .Monotherapy versus combination antimicrobial therapy for pneumococcal pneumonia.Curr Opin Infect Dis.2005;18:157–163.
- ,.The controversy of combination vs monotherapy in the treatment of hospitalized community‐acquired pneumonia.Chest.2005;128:940–946.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults.Cochrane Database Syst. Rev.2005:CD004418.
- ,,.Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,.Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro.Antimicrob Agents Chemother.2003;47:1151–1153.
- ,,,,.Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo.J Antimicrob Chemother.2000;46:973–980.
- .The effects of macrolides on inflammatory cells.Chest.2004;125(2 Suppl):41S–50S; quiz 1S.
- ,,, et al.Severe community‐acquired pneumonia. Risk factors and follow‐up epidemiology.Am J Respir Crit Care Med.1999;160:923–929.
- ,,, et al.Severe community‐acquired pneumonia. Assessment of severity criteria.Am J Respir Crit Care Med.1998;158:1102–1108.
- ,,,.Improving influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among adults aged 65 years at high risk: a report on recommendations of the Task Force on Community Preventive Services.MMWR Recomm Rep.2005;54(RR‐5):1–11.
- ,,,.The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials.Vaccine.2004;22:3214–3224.
- ,,.Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults.Vaccine.2002;20:2166–2173.
- ,,, et al.Effectiveness of pneumococcal polysaccharide vaccine in older adults.N Engl J Med.2003;348:1747–1755.
- ,,,,,.Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations.JAMA.1993;270:1826–1831.
- ,,,.Early predictors of mortality in pneumococcal bacteraemia.J Infect.2000;40:256–261.
- ,,.Pneumococcal bacteremia in adults: a 14‐year experience in an inner‐city university hospital.Clin Infect Dis.1995;21:345–351.
- ,,,,,.Pneumococcal bacteraemia and meningitis in England and Wales, 1993 to 1995.Commun Dis Public Health.1998;1(1):22–27.
- ,,,,.Pneumococcal bacteremia—no change in mortality in 30 years: analysis of 104 cases and review of the literature.Isr J Med Sci.1987;23:174–180.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003:CD000422.
- ,,,.The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease.Arch Intern Med.1999;159:2437–2442.
- ,,, et al.Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine.JAMA.2005;294:2043–2051.
- ,,, et al.Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine.N Engl J Med.2003;348:1737–1746.
- ,,, et al.Effect of introduction of the pneumococcal conjugate vaccine on drug‐resistant Streptococcus pneumoniae.N Engl J Med.2006;354:1455–1463.
- ,,,,,.Influenza vaccination in community‐dwelling elderly: impact on mortality and influenza‐associated morbidity.Arch Intern Med.2003;163:1089–1094.
- ,,, et al.Clinical effectiveness of influenza vaccination in persons younger than 65 years with high‐risk medical conditions: the PRISMA study.Arch Intern Med.2005;165:274–280.
- ,,, et al.Influence of high‐risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed‐care organizations.Clin Infect Dis.2002;35:370–377.
- ,,,,,.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,,,.Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study.Chest.2004;125:2011–2020.
- ,,,,,.Economic analysis of influenza vaccination and antiviral treatment for healthy working adults.Ann Intern Med.2002;137:225–331.
- ,,,,.The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Annual revaccination against influenza and mortality risk in community‐dwelling elderly persons.JAMA.2004;292:2089–2095.
- ,,, et al.Causes of death for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study.Arch Intern Med.2002;162:1059–1064.
- ,,.Contribution of routine pulse oximetry to evaluation and management of patients with respiratory illness in a pediatric emergency department.Ann Emerg Med.1995;25(1):36–40.
- ,,, et al.Arterial blood gas and pulse oximetry in initial management of patients with community‐acquired pneumonia.J Gen Intern Med.2001;16:590–598.
- ,.An analysis of the effectiveness of interventions intended to help people stop smoking.Arch Intern Med.1995;155:1933–1941.
- A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report.The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives.JAMA.2000;283:3244–3254.
- .Quality indicators for the management of pneumonia in vulnerable elders.Ann Intern Med.2001;135:736–743.
- ,,, et al.Cigarette smoking and invasive pneumococcal disease.Active Bacterial Core Surveillance Team.N Engl J Med.2000;342:681–689.
The quality movement has spawned efforts to define and measure best practices for clinical conditions commonly cared for by hospitalists. Pneumonia is the most frequent infectious cause of death in the United States, and it accounts for more than 1 million hospitalizations annually at an estimated annual cost of $12.2 billion, most of it incurred by inpatients.1 The morbidity and mortality of the elderly are particularly burdensome.2, 3 For these reasons, attention has been focused on improving the quality of care of inpatients with community‐acquired pneumonia (CAP).
Credentialing agencies such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) require hospitals to report performance on predefined core measures of pneumonia care that they have identified as best practices (see Table 1).4, 5 The performance of individual organizations on these measures is now publicly reported at a website (
|
| Collection of blood cultures before antibiotic therapy. |
| Collection of blood cultures within 24 hours of admission. |
| Mean time of less than 4 hours from arrival to initial administration of antibiotics. |
| Choice of initial antibiotics according to established guidelines.* |
| Pneumococcal screening and vaccination of eligible patients by discharge. |
| Influenza screening and vaccination of eligible patients during flu season. |
| Oxygenation assessment within 24 hours of admission. |
| Smoking cessation counseling to all smokers. |
Performance on core measures for pneumonia is less consistent across hospitals than the other conditions currently being monitored.7 It is instructive, then, to review the evidence base for the existing pneumonia quality measures, which can inform decisions about prioritizing interventions to provide the most effective care for inpatients with CAP.
BLOOD CULTURES
In a large multicenter retrospective study of Medicare patients hospitalized with CAP, Meehan et al.10 found the performance of blood cultures within 24 hours of arrival to be associated with reduced 30‐day mortality. Despite the large sample size of more than 14,000 patients, the risk‐adjusted mortality reduction was of only borderline significance (RR 0.9 [0.81‐1.00]). The unadjusted data did not show a significant mortality reduction. Notably, collection of blood cultures prior to antibiotic administration did not affect mortality, even excluding patients receiving prehospital antibiotics.
A smaller review of 38 U.S. academic medical centers showed relatively high compliance with blood culture performance, but no mortality reduction, even after adjustment for severity of illness. Similarly, performing blood cultures before administration of antibiotics yielded no significant effect.11
Several studies call into question the clinical utility of performing blood cultures drawn from patients with CAP. Combined, these studies evaluated almost 3000 pneumonia patients who had blood cultures drawn; the likelihood of a change in therapy based on results was at most 5%. Among the patients with positive cultures, only 20%‐40% had a treatment change based on the result.1215
The more severely ill patients with CAP may benefit from blood cultures, though the findings reported in the literature vary.12, 16 Using the Pneumonia Severity Index (PSI) score17 to classify severity of illness, an observational study of 209 inpatients with CAP found the yield of blood cultures increased from 10% in the lowest‐risk groups to 27% in the most severely ill.16 In contrast, two larger studies with a combined enrollment of almost 14,000 patients were unable to demonstrate a difference in the incidence of bacteremia despite adjustment for the PSI score.12, 18 It is clear from these and other studies that patients in PSI classes I‐III derive very little benefit from the performance of blood cultures.12, 16, 19
Metersky et al.18 described a prospectively validated risk assessment tool that reliably predicted bacteremia in Medicare patients with CAP and explored its utility in reducing unnecessary blood cultures. Independent risk factors for bacteremia included prior antibiotic use, liver disease, hypotension, tachycardia, fever or hypothermia, BUN > 30 mg/dL, sodium 130 mmol/L, and WBC 5000 or > 20,000/mm2. Use of this tool predicted bacteremia in 89% of patients and avoided 39% of unnecessary blood cultures. The authors also tested a modified version of the tool that excluded the laboratory abnormalities, so rapid assessment could be made at the initial patient presentation. This version advocated a single blood culture for most patients, and 2 blood cultures for patients with 2 or more risk factors. The modified tool accurately identified 88% of the patients with bacteremia and enabled a 44% reduction in unnecessary cultures.
In summary, blood cultures occasionally provide useful clinical information about etiology and resistance patterns, but they do not seem to reliably influence therapeutic decisions. It seems inappropriate to recommend against their use in practice, but they are not a solid benchmark for evidence‐based quality care. Measures that mandate risk assessment of all inpatients with CAP and require blood cultures only for older patients or those judged at high risk by PSI may better reflect quality. Alternatively, performing blood cultures on patients deemed to be high risk by the model of Metersky et al.18 may suffice.
ANTIBIOTIC TIMING
In a study of Medicare patients by Meehan et al.,10 the 30‐day mortality rate was reduced by 15% in the subset of patients who received antibiotics within 8 hours of arrival at the hospital. However, a trend toward mortality reduction was noted for those receiving antibiotics as early as 6 hours after arrival. Rapid administration of antibiotics was thus deemed an important measure of the quality of care of patients with CAP.
Additional studies attempted to confirm this observation. Battleman et al.20 evaluated 700 patients admitted for CAP through the emergency department. They found that a delay of more than 8 hours in the administration of antibiotics was correlated with a prolonged inpatient stay. Mortality rates were not reported. Achieving rapid delivery of antibiotics was closely linked to administration of the first dose of antibiotics in the emergency department.
Conversely, a large retrospective review by Dedier et al.11 found no reduction in inpatient mortality or in length of stay based on rapid antibiotic delivery, despite adjustment for severity of illness. They did not evaluate 30‐day mortality.
The effect of antibiotic timing on the time to clinical stability has also been investigated. Clinical stability was defined as 24 hours of a systolic blood pressure 90 mm Hg, heart rate 100 beats/min, respiratory rate 24 breaths/min, temperature 38.3C (101F), room air oxygen saturation 90%, and the ability to eat. Silber et al.,21 in a review of the records of 409 inpatients with moderate to severe CAP by PSI score, compared patients receiving antibiotics less than 4 hours, between 4 and 8 hours, and more than 8 hours after hospital admission. There was no difference between groups in time to clinical stability, even with adjustment for PSI.
Marrie and Wu22 attempted to define the factors that influenced inpatient mortality of patients with CAP not admitted to the intensive care unit (ICU). In a prospective study of 3043 patients evaluating a clinical pathway, a multivariate analysis showed antibiotic administration within 4 hours was not correlated with reduced mortality.
Although most studies supporting rapid antibiotic delivery used a target of 8 hours, administration in less than 4 hours is the consensus standard for pneumonia care set by CMS and JCAHO.23, 24
A benefit of timing antibiotic administration less than 4 hours after admission has been confirmed by a single, very large retrospective study of Medicare patients at least 65 years old.25 Analysis of a random sample of more than 18,000 patients with CAP who had not received prehospital antibiotics showed that the relative risk reduction for inpatient mortality was 15% in the group receiving antibiotics within 4 hours. Thirty‐day mortality was similarly reduced, and benefits continued for every hour of early antibiotic administration up to 9 hours.
The absolute risk reduction was small, however (0.6%), yielding a number needed to treat of 167 patients to prevent 1 death.
Randomized controlled trials, which would more definitively address the issue of antibiotic timing, are unlikely, as intentionally delaying administration of antibiotics to patients with known CAP is unethical. Hence, reliance on observational data must suffice. Intuitively, it makes sense to begin treatment of a bacterial infection at the earliest time possible. However, it is also known that not all patients present in a typical fashion, and diagnosis is uncertain at least 20% of the time.26 Anecdotal reports suggest that incentivizing physicians on performance measures encourages premature administration of empiric antibiotics to all patients presenting with cough, prior to confirmation of pneumonia.27, 28 Such practices promote further antibiotic resistance, arguably a larger health issue than delay in antibiotic delivery.29, 30
Houck31 offers potential solutions to this problem, such as eliminating the pressure on hospitals to perform at 100% on this measure by reporting performance within acceptable ranges (eg, 70%‐84% and 85%‐100%) Targeting a benchmark of 80% or a duration of 6 hours may also be appropriate. Finally, a 4‐hour benchmark has not been shown to benefit younger patients, so it is important to apply this target only to patients more than 65 years of age.
CHOICE OF ANTIBIOTIC
A retrospective review of 12,945 cases of inpatients with CAP found that, in comparison to ceftriaxone alone, initial antibiotic regimens consisting either of a second‐ or third‐generation cephalosporin plus a macrolide or of a fluoroquinolone alone were associated with an approximately 30% reduction in 30‐day mortality.32 Hence, current guidelines recommend the combination of a B‐lactam and macrolide, a B‐lactam and doxycycline, or a respiratory fluoroquinolone for inpatients with CAP not admitted to the ICU.3335
The results of subsequent studies supported the contention that guideline‐compliant antibiotics improve outcomes. A prospective multicenter study of a clinical pathway that encouraged use of either levofloxacin or cefuroxime plus azithromycin for the initial treatment of inpatient CAP showed significantly reduced mortality. Compared with any other antibiotic regimen, the odds ratio for death was 0.22 with the cephalosporin/macrolide combination and 0.43 with the fluoroquinolone. Of note, early mortality (within 5 days of admission) was not reduced by antibiotic choice.22 Similar results were found in a retrospective analysis, which found the odds of 30‐day mortality increased by 5.7 in patients not receiving guideline‐compliant therapy.36 A third study found guideline‐compliant antibiotics reduced the likelihood of a prolonged length of stay by 45%.20
Of note, data on the effectiveness of the cephalosporin/doxycycline combination are limited, and the major guidelines differ about whether this regimen is appropriate for inpatients with CAP.33, 34 Important findings from a recent retrospective cohort study showed that initial therapy with ceftriaxone plus doxycycline was associated with reduced inpatient mortality (OR = 0.26) as well as reduced 30‐day mortality (OR = 0.37) compared with other guideline‐compliant therapies for CAP.37 When patients who would not have been considered appropriate for initial doxycycline therapy (those resident in nursing homes, with aspiration pneumonia, or in the ICU) were excluded, a large reduction in inpatient mortality remained (OR = 0.17), without any increase in length of stay or readmission rate. Interestingly, this study suggests the potential superiority of this regimen, though a randomized controlled trial is needed to confirm this. The current core measures do include doxycycline as an acceptable option for CAP therapy (see Table 1).
Currently, controversy remains about whether the benefit of these selected regimens results from their activity against atypical pathogens (Mycoplasma, Legionella, Chlamydia) and whether there is additional benefit from using combination antibiotic therapy.38, 39 Waterer40 described 225 inpatients with bacteremic pneumococcal pneumonia, noting the antibiotic regimen received during the first 24 hours of hospitalization. Patients were classified retrospectively into 3 groupssingle effective therapy (SET), dual effective therapy (DET), or more than dual effective therapy (MET)on the basis of the concordance of pneumococcal sensitivity with the initial antibiotics. Patients on 2 antibiotics were classified in the DET group if the organism was sensitive to both and in the SET group if the organism was resistant to 1 of the 2. Those in the MET group were analyzed separately, as they were found to have a higher baseline severity of illness based on the PSI score; the SET and DET groups were equivalent.
Surprisingly, the SET group was found to have a 3‐fold increase in inpatient mortality; adjustment for severity of illness increased the odds ratio for death to 6.4. Of note, all deaths were in the most severely ill patients (PSI IV‐V). The protective effects of receiving DET were not specifically limited to those receiving a macrolide as the second agent, and multivariate analysis did not find coverage of atypical organisms to be an independent predictor of mortality.
A recent prospective multicenter trial of 844 patients with bacteremic pneumococcal pneumonia at 21 hospitals confirmed these findings.41 A significant 14‐day survival advantage (23% versus 55%) was found in the subgroup of critically ill patients who received at least 2 effective antibiotics. Though survival benefit was restricted to the sickest patients, severity of illness was similar among the groups.
The specific importance of macrolides in combination therapy remains under investigation. A review of a database of inpatients with bacteremic pneumococcal pneumonia over a 10‐year period found that 58% received initial empiric therapy with a B‐lactam/macrolide combination and 42% received B‐lactam without a macrolide (though other antibiotic combinations were not excluded).42 After logistic regression analysis, the investigators found a relative reduction in inpatient mortality of 60% in the patients receiving combination therapy with macrolides. Unfortunately, neither comparison to fluoroquinolone monotherapy nor risk stratification by PSI was reported. A similar study from Canada that did stratify for risk confirmed a mortality benefit of combination therapy.43
A subsequent, extremely large study of more than 44,000 patients from a hospital claims‐made database lent support to these findings.44 This study included all CAP patients regardless of microbiology and was not restricted to those with bacteremia. Outcomes among groups receiving monotherapy with any of the standard agents for CAP were compared with those in groups receiving combination therapy with a macrolide as the second agent. Statistically significant reductions in 30‐day mortality were observed in all groups receiving dual therapy with macrolides. Consistent with other studies, the benefit applied only to patients with more severe CAP. The percentage of patients with bacteremia was not specified.
Of note, this study did not allow direct comparison of fluoroquinolone monotherapy to combination therapy with a B‐lactam and a macrolide. However, the fluoroquinolone/macrolide combination conferred no additional benefit beyond fluoroquinolone monotherapy when adjusted for severity of illness or age. This implies that fluoroquinolone monotherapy is adequate, at least in some subpopulations. This is consistent with initial studies that established the superiority of the antibiotic combinations recommended by the guidelines.20, 22, 32
The potential benefit of combination therapy appears limited to patients with higher severity of illness and pneumococcal bacteremia. However, outcomes are affected by the antibiotic regimen selected in the initial 24‐48 hours of hospitalization, before results of blood cultures are routinely available. At present, clinical prediction of patients who will benefit from combination therapy is difficult.
Coverage of undiagnosed mixed infections with atypical organisms is probably not a major factor benefiting patients receiving combination therapy. Several recent meta‐analyses found no reduction in mortality or the rate of clinical failure among patients receiving antibiotics covering atypical organisms compared with those for patients whose regimens did not have such coverage.4547 Subgroups of patients with Legionella pneumonia do benefit from antibiotics with targeted activity against atypical organisms, but fewer than 1% of all patients were so identified. Evidence for antibiotic synergy is similarly lacking.48, 49 The immunomodulatory effects of macrolides, which decrease cytokine production and inflammation and subsequently reduce the severity of lung injury and other complications of sepsis, are considered potential factors in the reduction of mortality.50
The definition of severe CAP and the indications for ICU admission remain controversial, evidence for which is reviewed elsewhere.34, 51, 52 Antibiotic recommendations for ICU patients are included in Table 1 for completeness, but a detailed review of the evidence is lacking because current guidelines are based on consensus opinion.34 The use of fluoroquinolone monotherapy in severe CAP is not currently recommended because of limitations of the existing evidence. The majority of quinolone trials have excluded severely ill patients, and approval trials of newer respiratory fluoroquinolones have used levofloxacin as a comparator. Studies comparing fluoroquinolones typically allowed investigators in the B‐lactam arm the option of adding macrolides or tetracycline at their discretion. In addition, such trials have been designed as noniferiority trials.38 Clearly, randomized controlled trials are needed to resolve this issue.
Currently, selecting appropriate antibiotics should follow established guidelines, with consideration of using combination therapy for patients with a higher severity of illness. Emphasis on this measure should be stronger than that on antibiotic timing, as the bulk of the evidence favors significant mortality reduction from following guidelines for antibiotic therapy.
VACCINATION
Guidelines recommend all eligible adults hospitalized with CAP receive pneumococcal vaccination on discharge,3335, 53 though there is no evidence this reduces the incidence of pneumonia or death.54, 55 Retrospective studies have shown reduced incidence of invasive disease (bacteremia and meningitis), but not of other end points.5457 The estimated mortality from pneumococcal bacteremia remain as high as 20%‐30%, with no evidence that this rate has decreased over the last 30 years.5861 Despite this, a recent meta‐analysis from the Cochrane database that included only randomized, controlled trials (75,197 patients in 15 trials) was unable to show significant reductions in all‐cause pneumonia or mortality for vaccinated subjects.62 Cohort studies, evaluated separately in this analysis, showed an efficacy of 53% in reducing the incidence of invasive pneumococcal disease. Given the relatively low incidence of invasive disease in the general population, the number needed to treat was estimated at 20,000, or 4000 if only older patients were considered. A subsequent retrospective cohort study showed no reduction in pneumonia hospitalizations, cases of outpatient pneumonia, or mortality among 45,365 elderly vaccinees.56 Some specific subgroups may benefit, however. Vaccinated patients with chronic lung disease did show a reduction in hospitalization for pneumonia (RR 0.57 [0.38‐0.84]) and in mortality (RR 0.7 [0.56‐0.9]) in a retrospective study of HMO patients older than age 65.63
It is of interest that since the licensure of the pediatric 7‐valent protein‐polysaccharide conjugate vaccine in 2000, the incidence of invasive pneumococcal disease among adults has dropped significantly. Overall reduction in invasive disease in adults more than 50 years old was 11% from 1998 to 2003 (relative risk reduction [RRR] = 28%). This is likely the result of decreased transmission from colonized or infected children and not a coincidental increase in adult pneumococcal vaccination, as the rates of disease caused by the 16 strains unique to the 23‐valent vaccine did not change.64, 65 The overall reduction in the incidence of invasive disease is still superior with the adult vaccine, up to 30% in vaccinated subjects (RRR = 44%).56 Invasive disease caused specifically by penicillin‐nonsusceptible serotypes has dropped by 49% in the elderly since introduction of the vaccine.66 Thus, the combined impact of the 2 vaccines may be significant. It is not yet clear what effect, if any, the 7‐valent vaccine will have on the hospitalization rate or mortality.
In contrast to the results for pneumococcal vaccination, studies of the benefits of influenza vaccination have shown clear and consistent reductions in mortality, respiratory illness, hospitalization, and pneumonia, especially among patients with comorbidities.6771 Cost effectiveness has been demonstrated for all populations,72, 73 and the reduction in mortality among high‐risk patients younger than age 65 has been estimated to be as high as 78%.68 Among the elderly, reduction in mortality of about 50% has been reported, along with 20%‐30% reductions in hospitalizations for pneumonia, influenza, cardiac disease, and stroke.70 Reduced incidence of pneumonia in vaccinated patients has even been documented among elderly patients without specific comorbidities.67 Annual revaccination has the most significant impact on mortality.74
The pneumococcal vaccine remains important in the effort to reduce the severity of and complications from invasive pneumococcal disease in the elderly, but the lack of significant benefits on hard end points such as mortality or hospitalizations makes it a less robust measure of quality pneumonia care. In contrast, influenza vaccination has a much larger impact on outcomes in the population at risk. Emphasis should be shifted from pneumococcal to influenza vaccine in pneumonia performance measures.
OXYGENATION ASSESSMENT
It seems intuitive that oxygenation assessment is important in the initial evaluation of patients with CAP, though there is not direct evidence to support this. The recommendation for oxygenation assessment in the published guidelines for CAP is by consensus.3335 Documented hypoxemia is associated with increased pneumonia‐related mortality,17, 75 and clinical judgment does not adequately predict hypoxemia.76 Though the assessment of oxygenation has been found to vary widely among practitioners,77 performance has remained consistent since the advent of monitoring and reporting of quality measures, with compliance rates of 99%.4, 7 Monitoring performance of this measure should continue, though high compliance rates limit its ability to discriminate among institutions.
SMOKING CESSATION COUNSELING
Counseling patients to stop smoking was found to be modestly (2%) but statistically significantly effective in promoting abstinence at 1 year.78 In its report on treating tobacco use, the U.S. Public Health Services recommended that all smokers receive hospital‐ and system‐based interventions at every visit.79 As part of the Pneumonia Patient Outcomes Research Team (PORT) study, smokers with pneumonia underwent a tobacco cessation interview. Though only 15% of these patients quit smoking, 93% of those who quit did so at the time they developed CAP.80 A retrospective study of patients with bacteremic pneumococcal pneumonia found tobacco exposure, including passive smoking, to be a strong independent risk factor for invasive disease.81 The most recent CAP guidelines from the Infectious Disease Society of America (IDSA) recommend smoking cessation counseling for all hospitalized patients who smoke.33 However, hospitals are not likely to have the impact that a more comprehensive, outpatient‐based smoking cessation program would. Without ongoing support, counseling, and pharmacotherapy, the effects of an intervention would be expected to be small.79 Though evidence of benefit is limited, smoking cessation interventions should be encouraged at all sites of care. Quality care merits this regardless of admitting diagnosis, but benefits specific to CAP outcomes have not yet been demonstrated.
CONCLUSIONS
The burden of illness caused by CAP mandates that clinicians strive to deliver the highest quality of care to afflicted patients. Critical evaluation of the strength of the evidence will continue to guide such endeavors, and changes in practice will follow as new information surfaces. Standards of care, as adopted by consensus groups such as the IDSA and American Thoracic Society, will continue to inform the practice of hospitalists.
How quality is defined for public reporting requires particularly careful assessment. The definition of quality should be based on evidence more rigorous than that ascribed to consensus guidelines. Within the profession, guidelines offer reasonable standards of care and delineate areas for further research and are invaluable tools for practicing clinicians. In the public arena, however, proclaiming practices as good or bad sets expectations of health care consumers not educated in the nuances of evaluating clinical evidence and can unfairly bias them against conscientious and effective providers whose standards reflect different interpretations of controversial issues. Regulatory agencies should publicly target interventions using only the most solid evidentiary foundation while internally striving to monitor the effects of different practice patterns and report measurable differences in outcomes revealed by careful investigation. Areas where controversy remains should be the primary targets of further research but should not be offered as benchmarks for public scrutiny until the medical community has settled on a position.
Furthermore, when evidence remains questionable, financial incentives should be linked to performance indicators with extreme caution. It would be counterproductive if health care organizations, driven to achieve optimal antibiotic timing to obtain payment updates from CMS, began to administer antibiotics prior to completing workups on all patients with respiratory complaints, as this would likely lead to antibiotic overuse. Similarly, institutions pushed to collect blood cultures before antibiotics are given may inappropriately delay administration in order to perform well on quality measures, resulting in potential harm to patients.
The measures of quality care for CAP for which the evidence on outcomes is the most convincing are antibiotic selection (mortality benefit, reduction in LOS) and influenza vaccination (mortality benefit, reduction in hospitalizations, reduction in respiratory illness). Antibiotic timing also shows a smaller but convincing reduction in mortality, though the advantages of receiving antibiotics within 4 hours instead of 8 hours are not clearly established for younger patients. These measures should be emphasized most heavily in the arena of public reporting and incentives for quality care, with additions and modifications guided by emerging evidence. Revision of the other measures to conform with current evidence would allow public reporting to more accurately reflect quality.
The quality movement has spawned efforts to define and measure best practices for clinical conditions commonly cared for by hospitalists. Pneumonia is the most frequent infectious cause of death in the United States, and it accounts for more than 1 million hospitalizations annually at an estimated annual cost of $12.2 billion, most of it incurred by inpatients.1 The morbidity and mortality of the elderly are particularly burdensome.2, 3 For these reasons, attention has been focused on improving the quality of care of inpatients with community‐acquired pneumonia (CAP).
Credentialing agencies such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) require hospitals to report performance on predefined core measures of pneumonia care that they have identified as best practices (see Table 1).4, 5 The performance of individual organizations on these measures is now publicly reported at a website (
|
| Collection of blood cultures before antibiotic therapy. |
| Collection of blood cultures within 24 hours of admission. |
| Mean time of less than 4 hours from arrival to initial administration of antibiotics. |
| Choice of initial antibiotics according to established guidelines.* |
| Pneumococcal screening and vaccination of eligible patients by discharge. |
| Influenza screening and vaccination of eligible patients during flu season. |
| Oxygenation assessment within 24 hours of admission. |
| Smoking cessation counseling to all smokers. |
Performance on core measures for pneumonia is less consistent across hospitals than the other conditions currently being monitored.7 It is instructive, then, to review the evidence base for the existing pneumonia quality measures, which can inform decisions about prioritizing interventions to provide the most effective care for inpatients with CAP.
BLOOD CULTURES
In a large multicenter retrospective study of Medicare patients hospitalized with CAP, Meehan et al.10 found the performance of blood cultures within 24 hours of arrival to be associated with reduced 30‐day mortality. Despite the large sample size of more than 14,000 patients, the risk‐adjusted mortality reduction was of only borderline significance (RR 0.9 [0.81‐1.00]). The unadjusted data did not show a significant mortality reduction. Notably, collection of blood cultures prior to antibiotic administration did not affect mortality, even excluding patients receiving prehospital antibiotics.
A smaller review of 38 U.S. academic medical centers showed relatively high compliance with blood culture performance, but no mortality reduction, even after adjustment for severity of illness. Similarly, performing blood cultures before administration of antibiotics yielded no significant effect.11
Several studies call into question the clinical utility of performing blood cultures drawn from patients with CAP. Combined, these studies evaluated almost 3000 pneumonia patients who had blood cultures drawn; the likelihood of a change in therapy based on results was at most 5%. Among the patients with positive cultures, only 20%‐40% had a treatment change based on the result.1215
The more severely ill patients with CAP may benefit from blood cultures, though the findings reported in the literature vary.12, 16 Using the Pneumonia Severity Index (PSI) score17 to classify severity of illness, an observational study of 209 inpatients with CAP found the yield of blood cultures increased from 10% in the lowest‐risk groups to 27% in the most severely ill.16 In contrast, two larger studies with a combined enrollment of almost 14,000 patients were unable to demonstrate a difference in the incidence of bacteremia despite adjustment for the PSI score.12, 18 It is clear from these and other studies that patients in PSI classes I‐III derive very little benefit from the performance of blood cultures.12, 16, 19
Metersky et al.18 described a prospectively validated risk assessment tool that reliably predicted bacteremia in Medicare patients with CAP and explored its utility in reducing unnecessary blood cultures. Independent risk factors for bacteremia included prior antibiotic use, liver disease, hypotension, tachycardia, fever or hypothermia, BUN > 30 mg/dL, sodium 130 mmol/L, and WBC 5000 or > 20,000/mm2. Use of this tool predicted bacteremia in 89% of patients and avoided 39% of unnecessary blood cultures. The authors also tested a modified version of the tool that excluded the laboratory abnormalities, so rapid assessment could be made at the initial patient presentation. This version advocated a single blood culture for most patients, and 2 blood cultures for patients with 2 or more risk factors. The modified tool accurately identified 88% of the patients with bacteremia and enabled a 44% reduction in unnecessary cultures.
In summary, blood cultures occasionally provide useful clinical information about etiology and resistance patterns, but they do not seem to reliably influence therapeutic decisions. It seems inappropriate to recommend against their use in practice, but they are not a solid benchmark for evidence‐based quality care. Measures that mandate risk assessment of all inpatients with CAP and require blood cultures only for older patients or those judged at high risk by PSI may better reflect quality. Alternatively, performing blood cultures on patients deemed to be high risk by the model of Metersky et al.18 may suffice.
ANTIBIOTIC TIMING
In a study of Medicare patients by Meehan et al.,10 the 30‐day mortality rate was reduced by 15% in the subset of patients who received antibiotics within 8 hours of arrival at the hospital. However, a trend toward mortality reduction was noted for those receiving antibiotics as early as 6 hours after arrival. Rapid administration of antibiotics was thus deemed an important measure of the quality of care of patients with CAP.
Additional studies attempted to confirm this observation. Battleman et al.20 evaluated 700 patients admitted for CAP through the emergency department. They found that a delay of more than 8 hours in the administration of antibiotics was correlated with a prolonged inpatient stay. Mortality rates were not reported. Achieving rapid delivery of antibiotics was closely linked to administration of the first dose of antibiotics in the emergency department.
Conversely, a large retrospective review by Dedier et al.11 found no reduction in inpatient mortality or in length of stay based on rapid antibiotic delivery, despite adjustment for severity of illness. They did not evaluate 30‐day mortality.
The effect of antibiotic timing on the time to clinical stability has also been investigated. Clinical stability was defined as 24 hours of a systolic blood pressure 90 mm Hg, heart rate 100 beats/min, respiratory rate 24 breaths/min, temperature 38.3C (101F), room air oxygen saturation 90%, and the ability to eat. Silber et al.,21 in a review of the records of 409 inpatients with moderate to severe CAP by PSI score, compared patients receiving antibiotics less than 4 hours, between 4 and 8 hours, and more than 8 hours after hospital admission. There was no difference between groups in time to clinical stability, even with adjustment for PSI.
Marrie and Wu22 attempted to define the factors that influenced inpatient mortality of patients with CAP not admitted to the intensive care unit (ICU). In a prospective study of 3043 patients evaluating a clinical pathway, a multivariate analysis showed antibiotic administration within 4 hours was not correlated with reduced mortality.
Although most studies supporting rapid antibiotic delivery used a target of 8 hours, administration in less than 4 hours is the consensus standard for pneumonia care set by CMS and JCAHO.23, 24
A benefit of timing antibiotic administration less than 4 hours after admission has been confirmed by a single, very large retrospective study of Medicare patients at least 65 years old.25 Analysis of a random sample of more than 18,000 patients with CAP who had not received prehospital antibiotics showed that the relative risk reduction for inpatient mortality was 15% in the group receiving antibiotics within 4 hours. Thirty‐day mortality was similarly reduced, and benefits continued for every hour of early antibiotic administration up to 9 hours.
The absolute risk reduction was small, however (0.6%), yielding a number needed to treat of 167 patients to prevent 1 death.
Randomized controlled trials, which would more definitively address the issue of antibiotic timing, are unlikely, as intentionally delaying administration of antibiotics to patients with known CAP is unethical. Hence, reliance on observational data must suffice. Intuitively, it makes sense to begin treatment of a bacterial infection at the earliest time possible. However, it is also known that not all patients present in a typical fashion, and diagnosis is uncertain at least 20% of the time.26 Anecdotal reports suggest that incentivizing physicians on performance measures encourages premature administration of empiric antibiotics to all patients presenting with cough, prior to confirmation of pneumonia.27, 28 Such practices promote further antibiotic resistance, arguably a larger health issue than delay in antibiotic delivery.29, 30
Houck31 offers potential solutions to this problem, such as eliminating the pressure on hospitals to perform at 100% on this measure by reporting performance within acceptable ranges (eg, 70%‐84% and 85%‐100%) Targeting a benchmark of 80% or a duration of 6 hours may also be appropriate. Finally, a 4‐hour benchmark has not been shown to benefit younger patients, so it is important to apply this target only to patients more than 65 years of age.
CHOICE OF ANTIBIOTIC
A retrospective review of 12,945 cases of inpatients with CAP found that, in comparison to ceftriaxone alone, initial antibiotic regimens consisting either of a second‐ or third‐generation cephalosporin plus a macrolide or of a fluoroquinolone alone were associated with an approximately 30% reduction in 30‐day mortality.32 Hence, current guidelines recommend the combination of a B‐lactam and macrolide, a B‐lactam and doxycycline, or a respiratory fluoroquinolone for inpatients with CAP not admitted to the ICU.3335
The results of subsequent studies supported the contention that guideline‐compliant antibiotics improve outcomes. A prospective multicenter study of a clinical pathway that encouraged use of either levofloxacin or cefuroxime plus azithromycin for the initial treatment of inpatient CAP showed significantly reduced mortality. Compared with any other antibiotic regimen, the odds ratio for death was 0.22 with the cephalosporin/macrolide combination and 0.43 with the fluoroquinolone. Of note, early mortality (within 5 days of admission) was not reduced by antibiotic choice.22 Similar results were found in a retrospective analysis, which found the odds of 30‐day mortality increased by 5.7 in patients not receiving guideline‐compliant therapy.36 A third study found guideline‐compliant antibiotics reduced the likelihood of a prolonged length of stay by 45%.20
Of note, data on the effectiveness of the cephalosporin/doxycycline combination are limited, and the major guidelines differ about whether this regimen is appropriate for inpatients with CAP.33, 34 Important findings from a recent retrospective cohort study showed that initial therapy with ceftriaxone plus doxycycline was associated with reduced inpatient mortality (OR = 0.26) as well as reduced 30‐day mortality (OR = 0.37) compared with other guideline‐compliant therapies for CAP.37 When patients who would not have been considered appropriate for initial doxycycline therapy (those resident in nursing homes, with aspiration pneumonia, or in the ICU) were excluded, a large reduction in inpatient mortality remained (OR = 0.17), without any increase in length of stay or readmission rate. Interestingly, this study suggests the potential superiority of this regimen, though a randomized controlled trial is needed to confirm this. The current core measures do include doxycycline as an acceptable option for CAP therapy (see Table 1).
Currently, controversy remains about whether the benefit of these selected regimens results from their activity against atypical pathogens (Mycoplasma, Legionella, Chlamydia) and whether there is additional benefit from using combination antibiotic therapy.38, 39 Waterer40 described 225 inpatients with bacteremic pneumococcal pneumonia, noting the antibiotic regimen received during the first 24 hours of hospitalization. Patients were classified retrospectively into 3 groupssingle effective therapy (SET), dual effective therapy (DET), or more than dual effective therapy (MET)on the basis of the concordance of pneumococcal sensitivity with the initial antibiotics. Patients on 2 antibiotics were classified in the DET group if the organism was sensitive to both and in the SET group if the organism was resistant to 1 of the 2. Those in the MET group were analyzed separately, as they were found to have a higher baseline severity of illness based on the PSI score; the SET and DET groups were equivalent.
Surprisingly, the SET group was found to have a 3‐fold increase in inpatient mortality; adjustment for severity of illness increased the odds ratio for death to 6.4. Of note, all deaths were in the most severely ill patients (PSI IV‐V). The protective effects of receiving DET were not specifically limited to those receiving a macrolide as the second agent, and multivariate analysis did not find coverage of atypical organisms to be an independent predictor of mortality.
A recent prospective multicenter trial of 844 patients with bacteremic pneumococcal pneumonia at 21 hospitals confirmed these findings.41 A significant 14‐day survival advantage (23% versus 55%) was found in the subgroup of critically ill patients who received at least 2 effective antibiotics. Though survival benefit was restricted to the sickest patients, severity of illness was similar among the groups.
The specific importance of macrolides in combination therapy remains under investigation. A review of a database of inpatients with bacteremic pneumococcal pneumonia over a 10‐year period found that 58% received initial empiric therapy with a B‐lactam/macrolide combination and 42% received B‐lactam without a macrolide (though other antibiotic combinations were not excluded).42 After logistic regression analysis, the investigators found a relative reduction in inpatient mortality of 60% in the patients receiving combination therapy with macrolides. Unfortunately, neither comparison to fluoroquinolone monotherapy nor risk stratification by PSI was reported. A similar study from Canada that did stratify for risk confirmed a mortality benefit of combination therapy.43
A subsequent, extremely large study of more than 44,000 patients from a hospital claims‐made database lent support to these findings.44 This study included all CAP patients regardless of microbiology and was not restricted to those with bacteremia. Outcomes among groups receiving monotherapy with any of the standard agents for CAP were compared with those in groups receiving combination therapy with a macrolide as the second agent. Statistically significant reductions in 30‐day mortality were observed in all groups receiving dual therapy with macrolides. Consistent with other studies, the benefit applied only to patients with more severe CAP. The percentage of patients with bacteremia was not specified.
Of note, this study did not allow direct comparison of fluoroquinolone monotherapy to combination therapy with a B‐lactam and a macrolide. However, the fluoroquinolone/macrolide combination conferred no additional benefit beyond fluoroquinolone monotherapy when adjusted for severity of illness or age. This implies that fluoroquinolone monotherapy is adequate, at least in some subpopulations. This is consistent with initial studies that established the superiority of the antibiotic combinations recommended by the guidelines.20, 22, 32
The potential benefit of combination therapy appears limited to patients with higher severity of illness and pneumococcal bacteremia. However, outcomes are affected by the antibiotic regimen selected in the initial 24‐48 hours of hospitalization, before results of blood cultures are routinely available. At present, clinical prediction of patients who will benefit from combination therapy is difficult.
Coverage of undiagnosed mixed infections with atypical organisms is probably not a major factor benefiting patients receiving combination therapy. Several recent meta‐analyses found no reduction in mortality or the rate of clinical failure among patients receiving antibiotics covering atypical organisms compared with those for patients whose regimens did not have such coverage.4547 Subgroups of patients with Legionella pneumonia do benefit from antibiotics with targeted activity against atypical organisms, but fewer than 1% of all patients were so identified. Evidence for antibiotic synergy is similarly lacking.48, 49 The immunomodulatory effects of macrolides, which decrease cytokine production and inflammation and subsequently reduce the severity of lung injury and other complications of sepsis, are considered potential factors in the reduction of mortality.50
The definition of severe CAP and the indications for ICU admission remain controversial, evidence for which is reviewed elsewhere.34, 51, 52 Antibiotic recommendations for ICU patients are included in Table 1 for completeness, but a detailed review of the evidence is lacking because current guidelines are based on consensus opinion.34 The use of fluoroquinolone monotherapy in severe CAP is not currently recommended because of limitations of the existing evidence. The majority of quinolone trials have excluded severely ill patients, and approval trials of newer respiratory fluoroquinolones have used levofloxacin as a comparator. Studies comparing fluoroquinolones typically allowed investigators in the B‐lactam arm the option of adding macrolides or tetracycline at their discretion. In addition, such trials have been designed as noniferiority trials.38 Clearly, randomized controlled trials are needed to resolve this issue.
Currently, selecting appropriate antibiotics should follow established guidelines, with consideration of using combination therapy for patients with a higher severity of illness. Emphasis on this measure should be stronger than that on antibiotic timing, as the bulk of the evidence favors significant mortality reduction from following guidelines for antibiotic therapy.
VACCINATION
Guidelines recommend all eligible adults hospitalized with CAP receive pneumococcal vaccination on discharge,3335, 53 though there is no evidence this reduces the incidence of pneumonia or death.54, 55 Retrospective studies have shown reduced incidence of invasive disease (bacteremia and meningitis), but not of other end points.5457 The estimated mortality from pneumococcal bacteremia remain as high as 20%‐30%, with no evidence that this rate has decreased over the last 30 years.5861 Despite this, a recent meta‐analysis from the Cochrane database that included only randomized, controlled trials (75,197 patients in 15 trials) was unable to show significant reductions in all‐cause pneumonia or mortality for vaccinated subjects.62 Cohort studies, evaluated separately in this analysis, showed an efficacy of 53% in reducing the incidence of invasive pneumococcal disease. Given the relatively low incidence of invasive disease in the general population, the number needed to treat was estimated at 20,000, or 4000 if only older patients were considered. A subsequent retrospective cohort study showed no reduction in pneumonia hospitalizations, cases of outpatient pneumonia, or mortality among 45,365 elderly vaccinees.56 Some specific subgroups may benefit, however. Vaccinated patients with chronic lung disease did show a reduction in hospitalization for pneumonia (RR 0.57 [0.38‐0.84]) and in mortality (RR 0.7 [0.56‐0.9]) in a retrospective study of HMO patients older than age 65.63
It is of interest that since the licensure of the pediatric 7‐valent protein‐polysaccharide conjugate vaccine in 2000, the incidence of invasive pneumococcal disease among adults has dropped significantly. Overall reduction in invasive disease in adults more than 50 years old was 11% from 1998 to 2003 (relative risk reduction [RRR] = 28%). This is likely the result of decreased transmission from colonized or infected children and not a coincidental increase in adult pneumococcal vaccination, as the rates of disease caused by the 16 strains unique to the 23‐valent vaccine did not change.64, 65 The overall reduction in the incidence of invasive disease is still superior with the adult vaccine, up to 30% in vaccinated subjects (RRR = 44%).56 Invasive disease caused specifically by penicillin‐nonsusceptible serotypes has dropped by 49% in the elderly since introduction of the vaccine.66 Thus, the combined impact of the 2 vaccines may be significant. It is not yet clear what effect, if any, the 7‐valent vaccine will have on the hospitalization rate or mortality.
In contrast to the results for pneumococcal vaccination, studies of the benefits of influenza vaccination have shown clear and consistent reductions in mortality, respiratory illness, hospitalization, and pneumonia, especially among patients with comorbidities.6771 Cost effectiveness has been demonstrated for all populations,72, 73 and the reduction in mortality among high‐risk patients younger than age 65 has been estimated to be as high as 78%.68 Among the elderly, reduction in mortality of about 50% has been reported, along with 20%‐30% reductions in hospitalizations for pneumonia, influenza, cardiac disease, and stroke.70 Reduced incidence of pneumonia in vaccinated patients has even been documented among elderly patients without specific comorbidities.67 Annual revaccination has the most significant impact on mortality.74
The pneumococcal vaccine remains important in the effort to reduce the severity of and complications from invasive pneumococcal disease in the elderly, but the lack of significant benefits on hard end points such as mortality or hospitalizations makes it a less robust measure of quality pneumonia care. In contrast, influenza vaccination has a much larger impact on outcomes in the population at risk. Emphasis should be shifted from pneumococcal to influenza vaccine in pneumonia performance measures.
OXYGENATION ASSESSMENT
It seems intuitive that oxygenation assessment is important in the initial evaluation of patients with CAP, though there is not direct evidence to support this. The recommendation for oxygenation assessment in the published guidelines for CAP is by consensus.3335 Documented hypoxemia is associated with increased pneumonia‐related mortality,17, 75 and clinical judgment does not adequately predict hypoxemia.76 Though the assessment of oxygenation has been found to vary widely among practitioners,77 performance has remained consistent since the advent of monitoring and reporting of quality measures, with compliance rates of 99%.4, 7 Monitoring performance of this measure should continue, though high compliance rates limit its ability to discriminate among institutions.
SMOKING CESSATION COUNSELING
Counseling patients to stop smoking was found to be modestly (2%) but statistically significantly effective in promoting abstinence at 1 year.78 In its report on treating tobacco use, the U.S. Public Health Services recommended that all smokers receive hospital‐ and system‐based interventions at every visit.79 As part of the Pneumonia Patient Outcomes Research Team (PORT) study, smokers with pneumonia underwent a tobacco cessation interview. Though only 15% of these patients quit smoking, 93% of those who quit did so at the time they developed CAP.80 A retrospective study of patients with bacteremic pneumococcal pneumonia found tobacco exposure, including passive smoking, to be a strong independent risk factor for invasive disease.81 The most recent CAP guidelines from the Infectious Disease Society of America (IDSA) recommend smoking cessation counseling for all hospitalized patients who smoke.33 However, hospitals are not likely to have the impact that a more comprehensive, outpatient‐based smoking cessation program would. Without ongoing support, counseling, and pharmacotherapy, the effects of an intervention would be expected to be small.79 Though evidence of benefit is limited, smoking cessation interventions should be encouraged at all sites of care. Quality care merits this regardless of admitting diagnosis, but benefits specific to CAP outcomes have not yet been demonstrated.
CONCLUSIONS
The burden of illness caused by CAP mandates that clinicians strive to deliver the highest quality of care to afflicted patients. Critical evaluation of the strength of the evidence will continue to guide such endeavors, and changes in practice will follow as new information surfaces. Standards of care, as adopted by consensus groups such as the IDSA and American Thoracic Society, will continue to inform the practice of hospitalists.
How quality is defined for public reporting requires particularly careful assessment. The definition of quality should be based on evidence more rigorous than that ascribed to consensus guidelines. Within the profession, guidelines offer reasonable standards of care and delineate areas for further research and are invaluable tools for practicing clinicians. In the public arena, however, proclaiming practices as good or bad sets expectations of health care consumers not educated in the nuances of evaluating clinical evidence and can unfairly bias them against conscientious and effective providers whose standards reflect different interpretations of controversial issues. Regulatory agencies should publicly target interventions using only the most solid evidentiary foundation while internally striving to monitor the effects of different practice patterns and report measurable differences in outcomes revealed by careful investigation. Areas where controversy remains should be the primary targets of further research but should not be offered as benchmarks for public scrutiny until the medical community has settled on a position.
Furthermore, when evidence remains questionable, financial incentives should be linked to performance indicators with extreme caution. It would be counterproductive if health care organizations, driven to achieve optimal antibiotic timing to obtain payment updates from CMS, began to administer antibiotics prior to completing workups on all patients with respiratory complaints, as this would likely lead to antibiotic overuse. Similarly, institutions pushed to collect blood cultures before antibiotics are given may inappropriately delay administration in order to perform well on quality measures, resulting in potential harm to patients.
The measures of quality care for CAP for which the evidence on outcomes is the most convincing are antibiotic selection (mortality benefit, reduction in LOS) and influenza vaccination (mortality benefit, reduction in hospitalizations, reduction in respiratory illness). Antibiotic timing also shows a smaller but convincing reduction in mortality, though the advantages of receiving antibiotics within 4 hours instead of 8 hours are not clearly established for younger patients. These measures should be emphasized most heavily in the arena of public reporting and incentives for quality care, with additions and modifications guided by emerging evidence. Revision of the other measures to conform with current evidence would allow public reporting to more accurately reflect quality.
- ,,,.Treatment costs of community‐acquired pneumonia in an employed population.Chest.2004;125:2140–2145.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,,.Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988‐2002.JAMA.2005;294:2712–2719.
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002‐2004.N Engl J Med.2005;353:255–264.
- Performance Measurement: Core Measures. Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/index.htm. Accessed November 7, 2005.
- Medicare Quality Improvement Program Priorities: DRAFT, August2005.Centers for Medicare 353:265–274.
- Medicare demonstration shows hospital quality of care improves with payments tied to quality.Centers for Medicare 278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95(1):78–82.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community‐acquired pneumonia: link between quality of care and resource utilization.Arch Intern Med.2002;162:682–688.
- ,,, et al.Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia.Chest.2003;124:1798–1804.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127:1260–1270.
- Hospital Quality Measures:2004‐2007. Available at: http://www.cms.hhs.gov/quality/hospital/. Accessed November 17, 2005.
- National Hospital Quality Measures Specification Manual version 1.02.Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/aligned_manual.htm.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,,,,.Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours?Chest.2006;130:16–21.
- ,.Administration of first hospital antibiotics for community‐acquired pneumonia: does timeliness affect outcomes?Curr Opin Infect Dis.2005;18(2):151–156.
- .The pneumonia controversy: hospitals grapple with 4 hour benchmark.Ann Emerg Med.2006;47:259–261.
- ,,, et al.Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods.Ann Intern Med.2001;134:479–486.
- .The hidden impact of antibacterial resistance in respiratory tract infection. Clinical failures: the tip of the iceberg?Respir Med2001;95Suppl A:S5–11; discussion S26–S27.
- .Antibiotics and pneumonia: is timing everything or just a cause of more problems?Chest.2006;130:1–3.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults.Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,,.Effects of guideline‐concordant antimicrobial therapy on mortality among patients with community‐acquired pneumonia.Am J Med.2004;117:726–731.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- .Monotherapy versus combination antimicrobial therapy for pneumococcal pneumonia.Curr Opin Infect Dis.2005;18:157–163.
- ,.The controversy of combination vs monotherapy in the treatment of hospitalized community‐acquired pneumonia.Chest.2005;128:940–946.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults.Cochrane Database Syst. Rev.2005:CD004418.
- ,,.Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,.Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro.Antimicrob Agents Chemother.2003;47:1151–1153.
- ,,,,.Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo.J Antimicrob Chemother.2000;46:973–980.
- .The effects of macrolides on inflammatory cells.Chest.2004;125(2 Suppl):41S–50S; quiz 1S.
- ,,, et al.Severe community‐acquired pneumonia. Risk factors and follow‐up epidemiology.Am J Respir Crit Care Med.1999;160:923–929.
- ,,, et al.Severe community‐acquired pneumonia. Assessment of severity criteria.Am J Respir Crit Care Med.1998;158:1102–1108.
- ,,,.Improving influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among adults aged 65 years at high risk: a report on recommendations of the Task Force on Community Preventive Services.MMWR Recomm Rep.2005;54(RR‐5):1–11.
- ,,,.The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials.Vaccine.2004;22:3214–3224.
- ,,.Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults.Vaccine.2002;20:2166–2173.
- ,,, et al.Effectiveness of pneumococcal polysaccharide vaccine in older adults.N Engl J Med.2003;348:1747–1755.
- ,,,,,.Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations.JAMA.1993;270:1826–1831.
- ,,,.Early predictors of mortality in pneumococcal bacteraemia.J Infect.2000;40:256–261.
- ,,.Pneumococcal bacteremia in adults: a 14‐year experience in an inner‐city university hospital.Clin Infect Dis.1995;21:345–351.
- ,,,,,.Pneumococcal bacteraemia and meningitis in England and Wales, 1993 to 1995.Commun Dis Public Health.1998;1(1):22–27.
- ,,,,.Pneumococcal bacteremia—no change in mortality in 30 years: analysis of 104 cases and review of the literature.Isr J Med Sci.1987;23:174–180.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003:CD000422.
- ,,,.The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease.Arch Intern Med.1999;159:2437–2442.
- ,,, et al.Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine.JAMA.2005;294:2043–2051.
- ,,, et al.Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine.N Engl J Med.2003;348:1737–1746.
- ,,, et al.Effect of introduction of the pneumococcal conjugate vaccine on drug‐resistant Streptococcus pneumoniae.N Engl J Med.2006;354:1455–1463.
- ,,,,,.Influenza vaccination in community‐dwelling elderly: impact on mortality and influenza‐associated morbidity.Arch Intern Med.2003;163:1089–1094.
- ,,, et al.Clinical effectiveness of influenza vaccination in persons younger than 65 years with high‐risk medical conditions: the PRISMA study.Arch Intern Med.2005;165:274–280.
- ,,, et al.Influence of high‐risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed‐care organizations.Clin Infect Dis.2002;35:370–377.
- ,,,,,.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,,,.Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study.Chest.2004;125:2011–2020.
- ,,,,,.Economic analysis of influenza vaccination and antiviral treatment for healthy working adults.Ann Intern Med.2002;137:225–331.
- ,,,,.The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Annual revaccination against influenza and mortality risk in community‐dwelling elderly persons.JAMA.2004;292:2089–2095.
- ,,, et al.Causes of death for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study.Arch Intern Med.2002;162:1059–1064.
- ,,.Contribution of routine pulse oximetry to evaluation and management of patients with respiratory illness in a pediatric emergency department.Ann Emerg Med.1995;25(1):36–40.
- ,,, et al.Arterial blood gas and pulse oximetry in initial management of patients with community‐acquired pneumonia.J Gen Intern Med.2001;16:590–598.
- ,.An analysis of the effectiveness of interventions intended to help people stop smoking.Arch Intern Med.1995;155:1933–1941.
- A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report.The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives.JAMA.2000;283:3244–3254.
- .Quality indicators for the management of pneumonia in vulnerable elders.Ann Intern Med.2001;135:736–743.
- ,,, et al.Cigarette smoking and invasive pneumococcal disease.Active Bacterial Core Surveillance Team.N Engl J Med.2000;342:681–689.
- ,,,.Treatment costs of community‐acquired pneumonia in an employed population.Chest.2004;125:2140–2145.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,,.Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988‐2002.JAMA.2005;294:2712–2719.
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002‐2004.N Engl J Med.2005;353:255–264.
- Performance Measurement: Core Measures. Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/index.htm. Accessed November 7, 2005.
- Medicare Quality Improvement Program Priorities: DRAFT, August2005.Centers for Medicare 353:265–274.
- Medicare demonstration shows hospital quality of care improves with payments tied to quality.Centers for Medicare 278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95(1):78–82.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community‐acquired pneumonia: link between quality of care and resource utilization.Arch Intern Med.2002;162:682–688.
- ,,, et al.Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia.Chest.2003;124:1798–1804.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127:1260–1270.
- Hospital Quality Measures:2004‐2007. Available at: http://www.cms.hhs.gov/quality/hospital/. Accessed November 17, 2005.
- National Hospital Quality Measures Specification Manual version 1.02.Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/aligned_manual.htm.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,,,,.Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours?Chest.2006;130:16–21.
- ,.Administration of first hospital antibiotics for community‐acquired pneumonia: does timeliness affect outcomes?Curr Opin Infect Dis.2005;18(2):151–156.
- .The pneumonia controversy: hospitals grapple with 4 hour benchmark.Ann Emerg Med.2006;47:259–261.
- ,,, et al.Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods.Ann Intern Med.2001;134:479–486.
- .The hidden impact of antibacterial resistance in respiratory tract infection. Clinical failures: the tip of the iceberg?Respir Med2001;95Suppl A:S5–11; discussion S26–S27.
- .Antibiotics and pneumonia: is timing everything or just a cause of more problems?Chest.2006;130:1–3.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults.Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,,.Effects of guideline‐concordant antimicrobial therapy on mortality among patients with community‐acquired pneumonia.Am J Med.2004;117:726–731.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- .Monotherapy versus combination antimicrobial therapy for pneumococcal pneumonia.Curr Opin Infect Dis.2005;18:157–163.
- ,.The controversy of combination vs monotherapy in the treatment of hospitalized community‐acquired pneumonia.Chest.2005;128:940–946.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults.Cochrane Database Syst. Rev.2005:CD004418.
- ,,.Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,.Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro.Antimicrob Agents Chemother.2003;47:1151–1153.
- ,,,,.Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo.J Antimicrob Chemother.2000;46:973–980.
- .The effects of macrolides on inflammatory cells.Chest.2004;125(2 Suppl):41S–50S; quiz 1S.
- ,,, et al.Severe community‐acquired pneumonia. Risk factors and follow‐up epidemiology.Am J Respir Crit Care Med.1999;160:923–929.
- ,,, et al.Severe community‐acquired pneumonia. Assessment of severity criteria.Am J Respir Crit Care Med.1998;158:1102–1108.
- ,,,.Improving influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among adults aged 65 years at high risk: a report on recommendations of the Task Force on Community Preventive Services.MMWR Recomm Rep.2005;54(RR‐5):1–11.
- ,,,.The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials.Vaccine.2004;22:3214–3224.
- ,,.Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults.Vaccine.2002;20:2166–2173.
- ,,, et al.Effectiveness of pneumococcal polysaccharide vaccine in older adults.N Engl J Med.2003;348:1747–1755.
- ,,,,,.Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations.JAMA.1993;270:1826–1831.
- ,,,.Early predictors of mortality in pneumococcal bacteraemia.J Infect.2000;40:256–261.
- ,,.Pneumococcal bacteremia in adults: a 14‐year experience in an inner‐city university hospital.Clin Infect Dis.1995;21:345–351.
- ,,,,,.Pneumococcal bacteraemia and meningitis in England and Wales, 1993 to 1995.Commun Dis Public Health.1998;1(1):22–27.
- ,,,,.Pneumococcal bacteremia—no change in mortality in 30 years: analysis of 104 cases and review of the literature.Isr J Med Sci.1987;23:174–180.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003:CD000422.
- ,,,.The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease.Arch Intern Med.1999;159:2437–2442.
- ,,, et al.Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine.JAMA.2005;294:2043–2051.
- ,,, et al.Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine.N Engl J Med.2003;348:1737–1746.
- ,,, et al.Effect of introduction of the pneumococcal conjugate vaccine on drug‐resistant Streptococcus pneumoniae.N Engl J Med.2006;354:1455–1463.
- ,,,,,.Influenza vaccination in community‐dwelling elderly: impact on mortality and influenza‐associated morbidity.Arch Intern Med.2003;163:1089–1094.
- ,,, et al.Clinical effectiveness of influenza vaccination in persons younger than 65 years with high‐risk medical conditions: the PRISMA study.Arch Intern Med.2005;165:274–280.
- ,,, et al.Influence of high‐risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed‐care organizations.Clin Infect Dis.2002;35:370–377.
- ,,,,,.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,,,.Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study.Chest.2004;125:2011–2020.
- ,,,,,.Economic analysis of influenza vaccination and antiviral treatment for healthy working adults.Ann Intern Med.2002;137:225–331.
- ,,,,.The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Annual revaccination against influenza and mortality risk in community‐dwelling elderly persons.JAMA.2004;292:2089–2095.
- ,,, et al.Causes of death for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study.Arch Intern Med.2002;162:1059–1064.
- ,,.Contribution of routine pulse oximetry to evaluation and management of patients with respiratory illness in a pediatric emergency department.Ann Emerg Med.1995;25(1):36–40.
- ,,, et al.Arterial blood gas and pulse oximetry in initial management of patients with community‐acquired pneumonia.J Gen Intern Med.2001;16:590–598.
- ,.An analysis of the effectiveness of interventions intended to help people stop smoking.Arch Intern Med.1995;155:1933–1941.
- A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report.The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives.JAMA.2000;283:3244–3254.
- .Quality indicators for the management of pneumonia in vulnerable elders.Ann Intern Med.2001;135:736–743.
- ,,, et al.Cigarette smoking and invasive pneumococcal disease.Active Bacterial Core Surveillance Team.N Engl J Med.2000;342:681–689.
Effect of Educational Intervention on Intern Confidence
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).
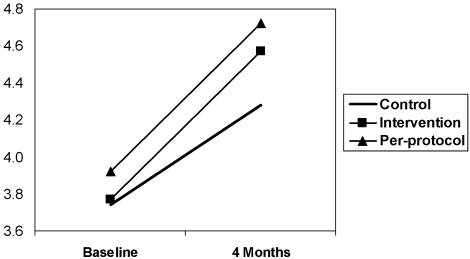
Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).
DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).

Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).

DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
Cross‐cover is defined as an on‐call physician managing acute problems such as chest pain, dyspnea, and hypoxemia for patients primarily cared for by another physician. Cross‐cover problems are commonly encountered with hospitalized patients, and inappropriate evaluation and management can result in misdiagnosis. Residents in many internal medicine residency programs receive only informal instruction about how to manage cross‐cover problems, usually from senior medical residents. Unfortunately, instruction is often provided while a patient is experiencing a problem, a frequent occurrence in the chaotic circumstances of a stressful learning environment. Furthermore, the knowledge base, experience, and teaching skills of senior residents vary substantially, and typically senior residents receive no formal instruction to guide them in how or what to teach more junior residents. If formal instruction is provided to residents, it is typically through often poorly attended didactic lectures that have been shown to be an ineffective forum for acquiring skills or changing physician behavior.15
Although previous studies did find that educational interventions can improve confidence and increase knowledge about various aspects of residency training, many of these studies were not randomized,68 or they involved complex interventions requiring a significant amount of resident and teaching staff time.911 The few randomized studies that used simple educational interventions focused on outpatient education, but most of a resident's time is spent in an inpatient setting.1213
Therefore, we designed a simple, randomized educational intervention consisting of 2 formal small‐group, case‐based discussion sessions addressing 1 cross‐cover situation: a hospitalized patient with acute dyspnea. We hypothesized that the addition of small‐group sessions would improve intern knowledge about and confidence in managing acute dyspnea above that gained from a combination of informal education and formal but lecture‐based education.
METHODS
Thirty‐eight internal medicine residents in their first year of postgraduate training (interns) at the University of Michigan were approached to participate in the study. Twenty‐six interns signed informed consent forms and were randomized using a random number generator to receive either the standard education (the control group) or the standard education plus the educational intervention (the intervention group). The standard education was informal teaching by senior medical residents on the wards and a 1‐hour lecture on Approach to the Patient with Acute Dyspnea, taught by an attending physician from the Department of Pulmonary and Critical Care Medicine. The educational intervention included the standard education as well as 2 small‐group, case‐based interactive sessions on acute dyspnea management. Both sessions were developed and taught by the first author (T.M.R.), a third‐year resident in internal medicine. A senior resident taught the sessions to try to make the information more relevant and practical and to make asking questions less intimidating. The first session, which lasted 50 minutes, discussed cases of bronchospasm, pulmonary edema, and pulmonary embolism as causes of acute dyspnea. It addressed several concepts: knowing when and how quickly to evaluate a dyspneic patient, formulating a differential diagnosis, appropriately evaluating acute dyspnea, providing empiric therapy, and recognizing indications for intubation. The second small‐group session occurred approximately 1 month after the first session and lasted 30 minutes. In this session key concepts learned during the first session were reviewed, and a case of ventricular tachycardia presenting as acute dyspnea was discussed. In an effort to increase attendance, free food and drink were provided at each session, and participants were sent reminders via e‐mail and the paging system prior to each session.
All study participants completed pre‐ and postintervention surveys that assessed their knowledge of acute dyspnea management and their confidence in managing patients with this condition. The pretests were conducted just before the first small‐group session was held. The post‐tests were conducted 4 months later. Knowledge was assessed by the score on the 45‐point test, which contained both open‐ and closed‐ended questions derived from 10 case‐based items. The number of points that a question was worth varied depending on how many elements made up a correct answer. For example, one question asked, What tests (if any) do you plan to order immediately after you examine the patient? As 3 tests should have been obtained (EKG, CXR, and ABG), this item had a maximum score of 3 points. Confidence was assessed by averaging 17 items scored on a 5‐point Likert scale (from strongly agree to strongly disagree). The items measured the physician's confidence in managing various aspects of the dyspneic patient (eg, confidence in knowing when to intubate a patient, when to obtain an ABG/CXR/EKG, and when to transfer a patient to the ICU). Data were analyzed using repeated‐measures analysis of variance. Primary analysis was based on the intention‐to‐treat principle, with alpha set to .05 (2‐sided). A secondary, per‐protocol analysis was also performed. In this analysis, study participants who attended both small‐group sessions (ie, completed the entire intervention) were compared with the control group. The protocol was approved by the institutional review board at the University of Michigan Health System.
RESULTS
All participants completed the study. Overall, only 3 of the 26 interns attended the lecture on Approach to the Patient with Acute Dyspnea. Fourteen of the 16 interns assigned to the intervention group attended 1 of the 2 small‐group sessions (11 attended the first session, and 10 attended the second session). Seven interns attended both sessions. The study period was 4 months. Both the intervention and control groups reported managing a similar number of patients with acute dyspnea, both prior to the study (mean of 5.9 in the intervention group and 7.4 in the control group, P = .51) and at the end of study (mean 10.6 in the intervention group and 10.2 in the control group, P = .91). There was no significant difference in the total number of completed inpatient months (mean of 4.9 in the intervention group and 4.7 in the control group, P =. 32) or in the number of inpatient months completed prior to the start of the study (mean of 2 in the intervention group and 2.4 in the control group, P = .15).
Confidence
Subjects in both the intervention and control groups showed increased confidence over time. The mean score of the intervention group increased from 3.77 to 4.57 (a 21.2% increase) and that of the control group increased from 3.74 to 4.28 (a 14.4% increase). Although the trend over time was highly significant for both groups (P < .001), the effect of the intervention was not significant (P = .19). However, the power to detect a difference between the groups was low (0.25). In the per‐protocol analysis, there was no significant difference between the groups (P = .26; see Fig. 1).

Knowledge
In the primary analysis, results for knowledge were similar to those obtained for the confidence outcome. In the intervention group, the mean score increased from 35.6 to 38.3 (a 7.6% increase); in the control group, the mean increased from 36.2 to 38.2 (a 5.5% increase). Scores ranged from 31 to 42. Again, the trend for both groups was significant (P < .01), but the effect of the intervention was not significant (P = .65). The power to detect a difference between groups was again low (0.07). In the per‐protocol analysis a trend toward significance was seen, with mean scores increasing from 34.6 to 40.0, a 15.6% increase (P = .067; see Fig. 2).

DISCUSSION
Our randomized controlled trial found that intern confidence and knowledge about acute dyspnea management both increased significantly over time; however, no significant differences between the intervention and control groups were observed. The complete intervention was not administered to the vast majority of those in the intervention group, however, likely skewing results toward the null. As suggested by the per‐protocol analysis, there was a trend toward a significant increase in the knowledge of the interns who had received the entire intervention. This is similar to results found in a randomized study by Schroy et al., which demonstrated a significant increase in resident knowledge of colorectal cancer screening after an educational intervention that used an interactive, case‐based seminar.13
Our study had several strengths. First, we employed the most robust design to detect efficacy, a randomized controlled study design. Second, we had complete follow‐up because all participants finished the study. Finally, our intervention is easily reproducible.
Our findings should also be considered within the context of several limitations. Despite the use of a random number generator, the control and intervention groups were unequal in number, which may have affected the results, particularly with such a small sample size.
Second, the intervention did not occur until 3 months after the start of each participant's internship. The intention was to implement the intervention at the start of internship, but institutional review board approval did not occur for an additional 3 months. This late timing might have been unfortunate because interns may already have had an established management plan for acute dyspnea, making their behavior more difficult to alter, even with additional education.
Third, because we were unaware of available test instruments to assess resident knowledge of acute dyspnea in the hospitalized patient, we needed to create our own. Unfortunately, the instrument yielded only a small variance in test scores, which may have made it difficult to detect an effect on scores if present.
Fourth, attendance at each session was suboptimal, and thus the complete intervention was not administered to the vast majority of those in the intervention group. Because the first small‐group session was the main teaching session, interns who only attended the second session were exposed to just one case discussion and only a review, rather than a full formal discussion, of the material presented during the first session. Therefore, it is not known if the intervention really had no effect or if no differences were detected simply because the complete intervention was not received. The trend toward significance observed in the per‐protocol analysis suggests that compliance with the intervention may be the key to improving knowledge.
Given the small differences observed in this study, future interventions ideally should use a more sensitive testing instrument, a larger sample, and a more powerful intervention that occurs early in training. Future efforts should also be designed to improve attendance at educational interventions. In the setting of reduced resident work hours and increased demands on resident time, this will prove to be a true challenge for all educators and residency programs.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
- ,,,.A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills.Acad Med.1995;70(1):52–58.
- ,,.Teaching smoking cessation skills to senior medical students: a block‐randomized controlled trial of four different approaches.Prev Med.1996;25:251–258.
- ,,,,,.Impact of formal continuing medical education: Do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282:867–874.
- ,,,,,.Problem‐based learning versus lecture‐based learning in postgraduate medical education.Scand J Work Environ Health.2003;29:280–287.
- ,,,,,,,.Better Prescribing Project: A randomized controlled trial of the impact of case‐based educational modules and personal prescribing feedback on prescribing for hypertension in primary care.Family Pract.2004;21:575–581.
- ,.Effect of an educational intervention about breastfeeding on the knowledge, confidence, and behaviors of pediatric resident physicians.Pediatrics.2002;110(5):e59.
- ,,,,,.Effects of a depression education program on residents' knowledge, attitudes, and clinical skills.Obstet Gynecol.2003;101(1):167–174.
- ,,,.Implementation of a web‐ and simulation‐based curriculum to ease the transition from medical school to surgical internship.Am J Surg.2005;190(1):137–140.
- ,,, et al.The effectiveness of intensive training for residents in interviewing.Ann Intern Med.1998;128(2):118–126.
- ,.Structured teaching methods enhance skill acquisition but not problem‐solving abilities: an evaluation of the “silent run through.”Med Educ.1999;33:019–023.
- ,,,,,.An educational intervention to improve physician violence screening skills.Pediatrics.2001;107(5):e68.
- ,,,,,,.Improving emergency medicine residents' approach to patients with alcohol problems: a controlled educational trial.Ann Emerg Med.2002;40(1):50–62.
- ,,,,,.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100:677–684.
Copyright © 2006 Society of Hospital Medicine
Transition of Care for Hospitalized Elderly
Hospital discharge is a critical transition point for many inpatients. Patient recovery from diseases requiring hospitalization is frequently incomplete and requires ongoing management and evaluation after discharge. For hospitalists who focus their practice primarily on inpatient care, the handoff to the outpatient setting frequently involves a change in health care provider and care team. Changes in care environment and care goals can lead to adverse patient‐ and system‐level events.1 High‐risk patients with multiple medical issues and elderly patients are especially vulnerable to the consequences of ineffective discharge handoffs.2, 3
Several studies have identified the errors that commonly occur around the time of hospital discharge. Forster et al.4 found that 1 in 5 patients experiences an adverse event (defined as an injury resulting from medical management rather than from the underlying disease) in the transition from hospital to home. They also found that approximately 62% of adverse events could be either prevented or ameliorated.4 Roy et al. examined test results pending at the time of discharge and determined that posthospital providers were frequently unaware of pending test results, with a potentially serious clinical impact.5 In an analysis of adverse events at 2 large hospitals in the United Kingdom, Neale et al. found that almost 11% of those hospitalized had an adverse event, 18% of which were attributable to the discharge process.6
Communication of important transitional care issues to the posthospital care team and to the patient is essential to a safe transition. Studies by van Walraven et al. found that patient follow‐up with a physician who had access to the hospital discharge summary was associated with a decreased risk of rehospitalization.7 Patients not understanding discharge medications,8 dietary restrictions, or other lifestyle changes such as smoking cessation and exercise can lead to ineffective care transitions. Furthermore, the health system's barriers to effective patient self‐management may exacerbate the risk in the transition from the hospital setting.2, 913
In addition to the growing research literature that has identified gaps in the discharge process, the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) has included discharge instructions as a core performance measure in the care of heart failure patients. Hospital performance on this measure is reported publicly on the Centers for Medicare and Medicaid Services website (
Older adults are considered more vulnerable to adverse events after discharge.8 They account for approximately 12% of the total U.S. population, but they make up 70% of hospitalized patients.17 With these factors in mind, the Society of Hospital Medicine (SHM) identified the elderly as a group especially vulnerable to the clinical care handoffs that occur in the hospital discharge process and therefore the patient population targeted in constructing the required elements of an ideal hospital discharge.
In addition to identifying a target patient population, inclusion of stakeholders primarily responsible for implementation is critical to developing a new process standard. In this instance, the hospitalist was identified as a critical architect of the development of the ideal discharge process, although clearly the hospitalist is just one of many people ultimately responsible for coordinating an effective hospital discharge. Finally, the organizations within which the elderly receive care and at which hospitalists practice are important partners in the implementation of systems of care that facilitate seamless care transitions.
In examining the myriad stakeholders involved in the discharge transitionthe patient, the hospitalist, other caregivers involved in hospital care, and the organizationSHM's Hospital Quality & Patient Safety (HQPS) Committee undertook an initiative to develop a practical list of important elements for hospitalists to include in the discharge of elderly inpatients, referred to in this article as the discharge checklist.
METHODS
In a process similar to that used by professional societies in the development of clinical guidelines, the SHM HQPS Committee used a combination of evidence‐based review and expert panels to develop a discharge checklist for elderly patients. Given that the focus of this project was a process improvement rather than a specific clinical condition, the SHM also believed it was critical to share the draft checklist with academic and community‐based practitioners knowledgeable in both the myriad logistical issues of and potential barriers to improving the discharge transition. The detailed process for checklist development is outlined below and summarized in Figure 1.
Literature Review
A Medline search was performed using the keywords patient discharge and either quality indicators, health care, or quality of health care. Articles included were those written in English and published between January 1975 and January 2005. We also reviewed the abstracts submitted to the SHM's 2003‐2005 annual and regional meetings in and reviewed those that included the designated keywords in their content focus. The number of articles selected was narrowed from 274 to 32 by including only studies of specific discharge elements, articles describing adverse events associated with but not including specific discharge elements, descriptions of tools to gather and report important data at the time of hospital discharge, or recommendations of experts or medical associations about methods of improving the discharge process.
DRAFT Checklist and Expert Review
Two members of the discharge checklist team (S.K. and D.M.) reviewed all 32 relevant reports and assembled a list of possible items for inclusion in an ideal hospital discharge. Inclusion of items was based on clinical relevance to elderly patients and impact on postdischarge care. This list initially contained 24 items in 3 domainsdischarge planning, medications, and the discharge summary document.
The list was sent to 3 experts, selected on the basis of their academic expertise in the fields of geriatrics and care transitions. Each independently reviewed the list, and then all 3 experts met several times by conference call. Items approved by at least 2 of the 3 experts were retained. The revised checklist transformed the 3 domains originally identified into 9 main elements, each with 2‐5 subelements.
Peer Review at SHM Annual Meeting
In a workshop at the 2005 SHM Annual Meeting, a facilitator presented the checklist and moderated a discussion among several experts from the task force and expert panel. The expert panel shared relevant background literature and key findings from their own research. Audience members included community and academic hospitalists, case managers, and pharmacists. Many attendees responded to the checklist and raised relevant issues. In all, 120 clinicians participated in this 90‐minute workshop.
After reviewing the checklist, workshop attendees gave both formal and informal input into the checklist content. Through group discussion and individual suggestions, items were added to the checklist. This process resulted in the addition of 1 main element and 3 subelements. At its completion, each workshop participant was allowed up to 3 votes for items that they believed should be removed from the modified checklist. The results were tallied, and the checklist was further reviewed and critiqued by the workshop faculty. Elements of the discharge checklist were designated as required for optimal handoffs if there was consensus among the committee members and workshop attendees. Elements that did not have unanimous support were discussed further and designated as optional. The final checklist was then developed with both required and optional elements and endorsed by the HQPS Committee and the SHM board.
RESULTS
The final discharge checklist is shown in Figure 2. It contains required and optional data elements and processes for 3 types of discharge documents: the discharge summary, patient instructions, and communication (by phone, e‐mail, or fax) on the day of discharge to the receiving provider. Other documents, such as transfer orders for a rehabilitation facility or nursing home, were considered outside the scope of this project.
The literature review identified medications as a significant source of adverse events for patients upon hospital discharge.4, 8, 14, 1820 The expert panel and workshop participants all endorsed the need for additional detailed attention to reviewing and reconciling medications during the discharge process. The use of standardized tools was suggested by the group to improve the medication review process.21 The required elements include not only a list of discharge medications but also attention to high‐risk medications that require closer postdischarge follow‐up and monitoring (such as warfarin,22 diuretics, nephrotoxic medications, corticosteroids, hypoglycemic medications, and narcotic analgesics), reconciliation of the discharge medication regimen with preadmission medications and designation of medications as new, modified, or discontinued,23 and emphasis on assessing patient understanding of medication self‐management plans.24 Several published studies found that pharmacist oversight of discharge medications or postdischarge telephone calls improved patient outcomes.1820 However, not every health system has the resources and infrastructure necessary to implement these types of programs. Moreover, methods of implementation of each of these discharge elements were believed to be beyond the scope of this project, so pharmacist involvement was not specifically included in this checklist.
The expert panel and workshop participants found items related to cognition and functional status to be important for patients whose usual cognitive or functional status was changed or whose status at discharge was not within normal limits.25, 26 Clinicians seeing patients in follow‐up would then have an important reference point for evaluating progress and the need for additional home support or therapy. Patients with limited literacy or language barriers may need these issues assessed with the help of family members and/or translators to identify changes from their baseline level of functioning.
In addition, resuscitation status was viewed by the group as an important data element for some patients,27 particularly those who had been critically ill. Development of disease or population‐specific content, for example, for patients with heart failure or pneumonia, was also identified as critical to the safe discharge of elderly patient, with the understanding that there may be a need to modify and individualize the content for patients with complex conditions and multiple comorbidities.
The content of the hospital discharge summary deemphasized the need for a complete history of the present illness at the time of hospital admission or an exhaustive hospital course. Instead, it highlighted the need to identify a patient's condition at discharge, pending issues and interventions requiring ongoing and focused monitoring, contingency planning, and contact information of knowledgeable providers in case questions arise after discharge.2830
Postdischarge care was emphasized with the need for a follow‐up appointment within at most 2 weeks of discharge or sooner for patients with fragile clinical conditions.31 Although this was not recommended because of a published study, it was the consensus of the expert panel and peer review process that close follow‐up after hospital discharge was critical in ensuring medication safety. Transportation limitations and other logistical problems with access to a follow‐up clinician were identified as important issues to be resolved in the discharge planning process in order for timely follow‐up to occur. In addition, it was deemed critical that the follow‐up provider receive the key information about the hospitalization with any necessary follow‐up instructions as soon after discharge as possible13 and certainly before the first postdischarge visit. Instructions to patients about medication schedules and follow‐up care need to be in writing at a 6th‐grade reading level; furthermore, processes to identify a patient's level of understanding of the follow‐up plan and areas for targeted education need to be established.24
DISCUSSION
We believe the development of a checklist of required elements to be communicated at discharge is a key step toward standardizing the hospital discharge process. The checklist highlights what is believed to be the key information about the transition of care and its process. The checklist is intended to standardize what is required for a successful hospital discharge. However, each institution will need to further refine this list according to local factors such as patient population, resources, and culture and to determine how best to implement the necessary changes to their current discharge process. Local modification of the checklist also allows for the addition of other elements that are patient‐ or population specific. Elderly patients discharged home from the hospital are the primary patient population targeted by this checklist ; there may be unique and additional elements necessary for an ideal discharge for a patient who is discharged to a subacute or acute rehabilitation facility. These elements are not described in this checklist but will be the focus of future work.
Establishing the critical elements of a hospital discharge transition sets the stage for improving patient outcomes in the immediate postdischarge period. Most important, the checklist conveys the message that the discharge process requires critical thinking, collaboration, and goal setting and that this coordination of care takes time. However, the discharge checklist must reside within a hospital culture that in general does not value the discharge process in the same way it values the admission process. The latter is more standardized and incorporates expectations about content and communication. In the same way, the current discharge is an admission to the next health care setting and deserves at least as much time and effort as a hospital admission. Furthermore, if institutions examine their current discharge processes, they may find that the time necessary to complete the discharge may be similar to the time necessary to admit a patient to the hospital. Finally, organizations need to develop internal policies and procedures that monitor and provide feedback about important dimensions of the discharge process, including content, patient understanding, information transfer, and clinical and service outcomes including satisfaction of the patient and the postdischarge provider. Hospital discharge is truly a team process involving nurses, pharmacists, case managers, and other hospital personnel, so performance measurement should be at the team or unit level, unlike other areas for which individual physicians may receive feedback on performance.
The limitations of the checklist development process include the paucity of randomized, controlled trials focused on the study of health care delivery processes and the lack of an industry gold standard. Furthermore, the heterogeneity of health care delivery systems makes it difficult to recommend specific interventions without understanding the myriad local issues. Those who provided input into this checklist included members of the inpatient team, a scope that can be broadened in the future to include outpatient physicians, patients, and caregivers in the home and long‐term care environments. However, the elements defined through the checklist serve as a starting point for developing discharge transition standards for older adults.
As leaders in hospital care, hospitalists are positioned to raise awareness of the importance of hospital discharge and to lead multidisciplinary efforts to improve the discharge process within their organizations. The first step in that process should be understanding the required elements and local facilitating factors and barriers in achieving a predictable, seamless transition of care for hospitalized patients.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J. R. Soc Med.2001;94:553.
- ,,,.Effect of discharge summary availability during the post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- .An ethnographic study of the process of medication discharge education (MDE).J Adv Nurs.1998;27:341–348.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,.Improving discharge data: lessons from the National Hospital Discharge Survey.Med Care1981;19:1030–1040.
- ,,.An investigation of patient satisfaction following discharge after total hip replacement surgery.Orthop Nurs.2003;22:429–436.
- ,,, et al.Payer‐hospital collaboration to improve patient satisfaction with hospital discharge.Jt Comm J QuaI Improv.1996;22:336–344.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- .Will, ideas, and execution: their role in reducing adverse medication events.J Pediatr.2005;147:727–728.
- .Why we need to learn standardisation.Aust Fam Physician.2005;34(1‐2):67–68.
- Joint Commission on Accreditation of Healthcare Organizations.2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg/06_npsg_cah_hap.htm.
- Census Bureau of Statistics,2000.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Ann Intern Med.2001;111(9B):26S–30S.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .Using the NO TEARS tool for medication review.BMJ.2004;329:434.
- .Toward safer warfarin therapy: does precise daily dosing improve INR control?Mayo Clinic Proc.2002;77:873–875.
- .Institute for Healthcare Improvement, personal communication.
- Institute of Medicine.Kindig DA, editor.Health Literacy: A Prescription to End Confusion.Washington, DC:National Academies Press,2004.
- ,,.Independent Mobility Validation Exam (I‐MOVE): a tool for periodic reassessment of fall‐risk and discharge planning. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
- ,,.Balance in elderly patients: the “get‐up‐and‐go” test.Arch Phys Med Rehabil.1986;67:387–389.
- AMA, Council on Ethical and Judicial Affairs.Guidelines for appropriate use of “do‐not‐resuscitate” orders.JAMA.1991;265:1868–1871.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999.14:160–169.
- JCAHO Manual: Information Management (IM) 6.10 and Patient Care (PC) 15.30
- ,.Specific appointments after pneumonia hospitalization reduce readmissions. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
Hospital discharge is a critical transition point for many inpatients. Patient recovery from diseases requiring hospitalization is frequently incomplete and requires ongoing management and evaluation after discharge. For hospitalists who focus their practice primarily on inpatient care, the handoff to the outpatient setting frequently involves a change in health care provider and care team. Changes in care environment and care goals can lead to adverse patient‐ and system‐level events.1 High‐risk patients with multiple medical issues and elderly patients are especially vulnerable to the consequences of ineffective discharge handoffs.2, 3
Several studies have identified the errors that commonly occur around the time of hospital discharge. Forster et al.4 found that 1 in 5 patients experiences an adverse event (defined as an injury resulting from medical management rather than from the underlying disease) in the transition from hospital to home. They also found that approximately 62% of adverse events could be either prevented or ameliorated.4 Roy et al. examined test results pending at the time of discharge and determined that posthospital providers were frequently unaware of pending test results, with a potentially serious clinical impact.5 In an analysis of adverse events at 2 large hospitals in the United Kingdom, Neale et al. found that almost 11% of those hospitalized had an adverse event, 18% of which were attributable to the discharge process.6
Communication of important transitional care issues to the posthospital care team and to the patient is essential to a safe transition. Studies by van Walraven et al. found that patient follow‐up with a physician who had access to the hospital discharge summary was associated with a decreased risk of rehospitalization.7 Patients not understanding discharge medications,8 dietary restrictions, or other lifestyle changes such as smoking cessation and exercise can lead to ineffective care transitions. Furthermore, the health system's barriers to effective patient self‐management may exacerbate the risk in the transition from the hospital setting.2, 913
In addition to the growing research literature that has identified gaps in the discharge process, the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) has included discharge instructions as a core performance measure in the care of heart failure patients. Hospital performance on this measure is reported publicly on the Centers for Medicare and Medicaid Services website (
Older adults are considered more vulnerable to adverse events after discharge.8 They account for approximately 12% of the total U.S. population, but they make up 70% of hospitalized patients.17 With these factors in mind, the Society of Hospital Medicine (SHM) identified the elderly as a group especially vulnerable to the clinical care handoffs that occur in the hospital discharge process and therefore the patient population targeted in constructing the required elements of an ideal hospital discharge.
In addition to identifying a target patient population, inclusion of stakeholders primarily responsible for implementation is critical to developing a new process standard. In this instance, the hospitalist was identified as a critical architect of the development of the ideal discharge process, although clearly the hospitalist is just one of many people ultimately responsible for coordinating an effective hospital discharge. Finally, the organizations within which the elderly receive care and at which hospitalists practice are important partners in the implementation of systems of care that facilitate seamless care transitions.
In examining the myriad stakeholders involved in the discharge transitionthe patient, the hospitalist, other caregivers involved in hospital care, and the organizationSHM's Hospital Quality & Patient Safety (HQPS) Committee undertook an initiative to develop a practical list of important elements for hospitalists to include in the discharge of elderly inpatients, referred to in this article as the discharge checklist.
METHODS
In a process similar to that used by professional societies in the development of clinical guidelines, the SHM HQPS Committee used a combination of evidence‐based review and expert panels to develop a discharge checklist for elderly patients. Given that the focus of this project was a process improvement rather than a specific clinical condition, the SHM also believed it was critical to share the draft checklist with academic and community‐based practitioners knowledgeable in both the myriad logistical issues of and potential barriers to improving the discharge transition. The detailed process for checklist development is outlined below and summarized in Figure 1.

Literature Review
A Medline search was performed using the keywords patient discharge and either quality indicators, health care, or quality of health care. Articles included were those written in English and published between January 1975 and January 2005. We also reviewed the abstracts submitted to the SHM's 2003‐2005 annual and regional meetings in and reviewed those that included the designated keywords in their content focus. The number of articles selected was narrowed from 274 to 32 by including only studies of specific discharge elements, articles describing adverse events associated with but not including specific discharge elements, descriptions of tools to gather and report important data at the time of hospital discharge, or recommendations of experts or medical associations about methods of improving the discharge process.
DRAFT Checklist and Expert Review
Two members of the discharge checklist team (S.K. and D.M.) reviewed all 32 relevant reports and assembled a list of possible items for inclusion in an ideal hospital discharge. Inclusion of items was based on clinical relevance to elderly patients and impact on postdischarge care. This list initially contained 24 items in 3 domainsdischarge planning, medications, and the discharge summary document.
The list was sent to 3 experts, selected on the basis of their academic expertise in the fields of geriatrics and care transitions. Each independently reviewed the list, and then all 3 experts met several times by conference call. Items approved by at least 2 of the 3 experts were retained. The revised checklist transformed the 3 domains originally identified into 9 main elements, each with 2‐5 subelements.
Peer Review at SHM Annual Meeting
In a workshop at the 2005 SHM Annual Meeting, a facilitator presented the checklist and moderated a discussion among several experts from the task force and expert panel. The expert panel shared relevant background literature and key findings from their own research. Audience members included community and academic hospitalists, case managers, and pharmacists. Many attendees responded to the checklist and raised relevant issues. In all, 120 clinicians participated in this 90‐minute workshop.
After reviewing the checklist, workshop attendees gave both formal and informal input into the checklist content. Through group discussion and individual suggestions, items were added to the checklist. This process resulted in the addition of 1 main element and 3 subelements. At its completion, each workshop participant was allowed up to 3 votes for items that they believed should be removed from the modified checklist. The results were tallied, and the checklist was further reviewed and critiqued by the workshop faculty. Elements of the discharge checklist were designated as required for optimal handoffs if there was consensus among the committee members and workshop attendees. Elements that did not have unanimous support were discussed further and designated as optional. The final checklist was then developed with both required and optional elements and endorsed by the HQPS Committee and the SHM board.
RESULTS
The final discharge checklist is shown in Figure 2. It contains required and optional data elements and processes for 3 types of discharge documents: the discharge summary, patient instructions, and communication (by phone, e‐mail, or fax) on the day of discharge to the receiving provider. Other documents, such as transfer orders for a rehabilitation facility or nursing home, were considered outside the scope of this project.

The literature review identified medications as a significant source of adverse events for patients upon hospital discharge.4, 8, 14, 1820 The expert panel and workshop participants all endorsed the need for additional detailed attention to reviewing and reconciling medications during the discharge process. The use of standardized tools was suggested by the group to improve the medication review process.21 The required elements include not only a list of discharge medications but also attention to high‐risk medications that require closer postdischarge follow‐up and monitoring (such as warfarin,22 diuretics, nephrotoxic medications, corticosteroids, hypoglycemic medications, and narcotic analgesics), reconciliation of the discharge medication regimen with preadmission medications and designation of medications as new, modified, or discontinued,23 and emphasis on assessing patient understanding of medication self‐management plans.24 Several published studies found that pharmacist oversight of discharge medications or postdischarge telephone calls improved patient outcomes.1820 However, not every health system has the resources and infrastructure necessary to implement these types of programs. Moreover, methods of implementation of each of these discharge elements were believed to be beyond the scope of this project, so pharmacist involvement was not specifically included in this checklist.
The expert panel and workshop participants found items related to cognition and functional status to be important for patients whose usual cognitive or functional status was changed or whose status at discharge was not within normal limits.25, 26 Clinicians seeing patients in follow‐up would then have an important reference point for evaluating progress and the need for additional home support or therapy. Patients with limited literacy or language barriers may need these issues assessed with the help of family members and/or translators to identify changes from their baseline level of functioning.
In addition, resuscitation status was viewed by the group as an important data element for some patients,27 particularly those who had been critically ill. Development of disease or population‐specific content, for example, for patients with heart failure or pneumonia, was also identified as critical to the safe discharge of elderly patient, with the understanding that there may be a need to modify and individualize the content for patients with complex conditions and multiple comorbidities.
The content of the hospital discharge summary deemphasized the need for a complete history of the present illness at the time of hospital admission or an exhaustive hospital course. Instead, it highlighted the need to identify a patient's condition at discharge, pending issues and interventions requiring ongoing and focused monitoring, contingency planning, and contact information of knowledgeable providers in case questions arise after discharge.2830
Postdischarge care was emphasized with the need for a follow‐up appointment within at most 2 weeks of discharge or sooner for patients with fragile clinical conditions.31 Although this was not recommended because of a published study, it was the consensus of the expert panel and peer review process that close follow‐up after hospital discharge was critical in ensuring medication safety. Transportation limitations and other logistical problems with access to a follow‐up clinician were identified as important issues to be resolved in the discharge planning process in order for timely follow‐up to occur. In addition, it was deemed critical that the follow‐up provider receive the key information about the hospitalization with any necessary follow‐up instructions as soon after discharge as possible13 and certainly before the first postdischarge visit. Instructions to patients about medication schedules and follow‐up care need to be in writing at a 6th‐grade reading level; furthermore, processes to identify a patient's level of understanding of the follow‐up plan and areas for targeted education need to be established.24
DISCUSSION
We believe the development of a checklist of required elements to be communicated at discharge is a key step toward standardizing the hospital discharge process. The checklist highlights what is believed to be the key information about the transition of care and its process. The checklist is intended to standardize what is required for a successful hospital discharge. However, each institution will need to further refine this list according to local factors such as patient population, resources, and culture and to determine how best to implement the necessary changes to their current discharge process. Local modification of the checklist also allows for the addition of other elements that are patient‐ or population specific. Elderly patients discharged home from the hospital are the primary patient population targeted by this checklist ; there may be unique and additional elements necessary for an ideal discharge for a patient who is discharged to a subacute or acute rehabilitation facility. These elements are not described in this checklist but will be the focus of future work.
Establishing the critical elements of a hospital discharge transition sets the stage for improving patient outcomes in the immediate postdischarge period. Most important, the checklist conveys the message that the discharge process requires critical thinking, collaboration, and goal setting and that this coordination of care takes time. However, the discharge checklist must reside within a hospital culture that in general does not value the discharge process in the same way it values the admission process. The latter is more standardized and incorporates expectations about content and communication. In the same way, the current discharge is an admission to the next health care setting and deserves at least as much time and effort as a hospital admission. Furthermore, if institutions examine their current discharge processes, they may find that the time necessary to complete the discharge may be similar to the time necessary to admit a patient to the hospital. Finally, organizations need to develop internal policies and procedures that monitor and provide feedback about important dimensions of the discharge process, including content, patient understanding, information transfer, and clinical and service outcomes including satisfaction of the patient and the postdischarge provider. Hospital discharge is truly a team process involving nurses, pharmacists, case managers, and other hospital personnel, so performance measurement should be at the team or unit level, unlike other areas for which individual physicians may receive feedback on performance.
The limitations of the checklist development process include the paucity of randomized, controlled trials focused on the study of health care delivery processes and the lack of an industry gold standard. Furthermore, the heterogeneity of health care delivery systems makes it difficult to recommend specific interventions without understanding the myriad local issues. Those who provided input into this checklist included members of the inpatient team, a scope that can be broadened in the future to include outpatient physicians, patients, and caregivers in the home and long‐term care environments. However, the elements defined through the checklist serve as a starting point for developing discharge transition standards for older adults.
As leaders in hospital care, hospitalists are positioned to raise awareness of the importance of hospital discharge and to lead multidisciplinary efforts to improve the discharge process within their organizations. The first step in that process should be understanding the required elements and local facilitating factors and barriers in achieving a predictable, seamless transition of care for hospitalized patients.
Hospital discharge is a critical transition point for many inpatients. Patient recovery from diseases requiring hospitalization is frequently incomplete and requires ongoing management and evaluation after discharge. For hospitalists who focus their practice primarily on inpatient care, the handoff to the outpatient setting frequently involves a change in health care provider and care team. Changes in care environment and care goals can lead to adverse patient‐ and system‐level events.1 High‐risk patients with multiple medical issues and elderly patients are especially vulnerable to the consequences of ineffective discharge handoffs.2, 3
Several studies have identified the errors that commonly occur around the time of hospital discharge. Forster et al.4 found that 1 in 5 patients experiences an adverse event (defined as an injury resulting from medical management rather than from the underlying disease) in the transition from hospital to home. They also found that approximately 62% of adverse events could be either prevented or ameliorated.4 Roy et al. examined test results pending at the time of discharge and determined that posthospital providers were frequently unaware of pending test results, with a potentially serious clinical impact.5 In an analysis of adverse events at 2 large hospitals in the United Kingdom, Neale et al. found that almost 11% of those hospitalized had an adverse event, 18% of which were attributable to the discharge process.6
Communication of important transitional care issues to the posthospital care team and to the patient is essential to a safe transition. Studies by van Walraven et al. found that patient follow‐up with a physician who had access to the hospital discharge summary was associated with a decreased risk of rehospitalization.7 Patients not understanding discharge medications,8 dietary restrictions, or other lifestyle changes such as smoking cessation and exercise can lead to ineffective care transitions. Furthermore, the health system's barriers to effective patient self‐management may exacerbate the risk in the transition from the hospital setting.2, 913
In addition to the growing research literature that has identified gaps in the discharge process, the Joint Commission for the Accreditation of Healthcare Organizations (JCAHO) has included discharge instructions as a core performance measure in the care of heart failure patients. Hospital performance on this measure is reported publicly on the Centers for Medicare and Medicaid Services website (
Older adults are considered more vulnerable to adverse events after discharge.8 They account for approximately 12% of the total U.S. population, but they make up 70% of hospitalized patients.17 With these factors in mind, the Society of Hospital Medicine (SHM) identified the elderly as a group especially vulnerable to the clinical care handoffs that occur in the hospital discharge process and therefore the patient population targeted in constructing the required elements of an ideal hospital discharge.
In addition to identifying a target patient population, inclusion of stakeholders primarily responsible for implementation is critical to developing a new process standard. In this instance, the hospitalist was identified as a critical architect of the development of the ideal discharge process, although clearly the hospitalist is just one of many people ultimately responsible for coordinating an effective hospital discharge. Finally, the organizations within which the elderly receive care and at which hospitalists practice are important partners in the implementation of systems of care that facilitate seamless care transitions.
In examining the myriad stakeholders involved in the discharge transitionthe patient, the hospitalist, other caregivers involved in hospital care, and the organizationSHM's Hospital Quality & Patient Safety (HQPS) Committee undertook an initiative to develop a practical list of important elements for hospitalists to include in the discharge of elderly inpatients, referred to in this article as the discharge checklist.
METHODS
In a process similar to that used by professional societies in the development of clinical guidelines, the SHM HQPS Committee used a combination of evidence‐based review and expert panels to develop a discharge checklist for elderly patients. Given that the focus of this project was a process improvement rather than a specific clinical condition, the SHM also believed it was critical to share the draft checklist with academic and community‐based practitioners knowledgeable in both the myriad logistical issues of and potential barriers to improving the discharge transition. The detailed process for checklist development is outlined below and summarized in Figure 1.

Literature Review
A Medline search was performed using the keywords patient discharge and either quality indicators, health care, or quality of health care. Articles included were those written in English and published between January 1975 and January 2005. We also reviewed the abstracts submitted to the SHM's 2003‐2005 annual and regional meetings in and reviewed those that included the designated keywords in their content focus. The number of articles selected was narrowed from 274 to 32 by including only studies of specific discharge elements, articles describing adverse events associated with but not including specific discharge elements, descriptions of tools to gather and report important data at the time of hospital discharge, or recommendations of experts or medical associations about methods of improving the discharge process.
DRAFT Checklist and Expert Review
Two members of the discharge checklist team (S.K. and D.M.) reviewed all 32 relevant reports and assembled a list of possible items for inclusion in an ideal hospital discharge. Inclusion of items was based on clinical relevance to elderly patients and impact on postdischarge care. This list initially contained 24 items in 3 domainsdischarge planning, medications, and the discharge summary document.
The list was sent to 3 experts, selected on the basis of their academic expertise in the fields of geriatrics and care transitions. Each independently reviewed the list, and then all 3 experts met several times by conference call. Items approved by at least 2 of the 3 experts were retained. The revised checklist transformed the 3 domains originally identified into 9 main elements, each with 2‐5 subelements.
Peer Review at SHM Annual Meeting
In a workshop at the 2005 SHM Annual Meeting, a facilitator presented the checklist and moderated a discussion among several experts from the task force and expert panel. The expert panel shared relevant background literature and key findings from their own research. Audience members included community and academic hospitalists, case managers, and pharmacists. Many attendees responded to the checklist and raised relevant issues. In all, 120 clinicians participated in this 90‐minute workshop.
After reviewing the checklist, workshop attendees gave both formal and informal input into the checklist content. Through group discussion and individual suggestions, items were added to the checklist. This process resulted in the addition of 1 main element and 3 subelements. At its completion, each workshop participant was allowed up to 3 votes for items that they believed should be removed from the modified checklist. The results were tallied, and the checklist was further reviewed and critiqued by the workshop faculty. Elements of the discharge checklist were designated as required for optimal handoffs if there was consensus among the committee members and workshop attendees. Elements that did not have unanimous support were discussed further and designated as optional. The final checklist was then developed with both required and optional elements and endorsed by the HQPS Committee and the SHM board.
RESULTS
The final discharge checklist is shown in Figure 2. It contains required and optional data elements and processes for 3 types of discharge documents: the discharge summary, patient instructions, and communication (by phone, e‐mail, or fax) on the day of discharge to the receiving provider. Other documents, such as transfer orders for a rehabilitation facility or nursing home, were considered outside the scope of this project.

The literature review identified medications as a significant source of adverse events for patients upon hospital discharge.4, 8, 14, 1820 The expert panel and workshop participants all endorsed the need for additional detailed attention to reviewing and reconciling medications during the discharge process. The use of standardized tools was suggested by the group to improve the medication review process.21 The required elements include not only a list of discharge medications but also attention to high‐risk medications that require closer postdischarge follow‐up and monitoring (such as warfarin,22 diuretics, nephrotoxic medications, corticosteroids, hypoglycemic medications, and narcotic analgesics), reconciliation of the discharge medication regimen with preadmission medications and designation of medications as new, modified, or discontinued,23 and emphasis on assessing patient understanding of medication self‐management plans.24 Several published studies found that pharmacist oversight of discharge medications or postdischarge telephone calls improved patient outcomes.1820 However, not every health system has the resources and infrastructure necessary to implement these types of programs. Moreover, methods of implementation of each of these discharge elements were believed to be beyond the scope of this project, so pharmacist involvement was not specifically included in this checklist.
The expert panel and workshop participants found items related to cognition and functional status to be important for patients whose usual cognitive or functional status was changed or whose status at discharge was not within normal limits.25, 26 Clinicians seeing patients in follow‐up would then have an important reference point for evaluating progress and the need for additional home support or therapy. Patients with limited literacy or language barriers may need these issues assessed with the help of family members and/or translators to identify changes from their baseline level of functioning.
In addition, resuscitation status was viewed by the group as an important data element for some patients,27 particularly those who had been critically ill. Development of disease or population‐specific content, for example, for patients with heart failure or pneumonia, was also identified as critical to the safe discharge of elderly patient, with the understanding that there may be a need to modify and individualize the content for patients with complex conditions and multiple comorbidities.
The content of the hospital discharge summary deemphasized the need for a complete history of the present illness at the time of hospital admission or an exhaustive hospital course. Instead, it highlighted the need to identify a patient's condition at discharge, pending issues and interventions requiring ongoing and focused monitoring, contingency planning, and contact information of knowledgeable providers in case questions arise after discharge.2830
Postdischarge care was emphasized with the need for a follow‐up appointment within at most 2 weeks of discharge or sooner for patients with fragile clinical conditions.31 Although this was not recommended because of a published study, it was the consensus of the expert panel and peer review process that close follow‐up after hospital discharge was critical in ensuring medication safety. Transportation limitations and other logistical problems with access to a follow‐up clinician were identified as important issues to be resolved in the discharge planning process in order for timely follow‐up to occur. In addition, it was deemed critical that the follow‐up provider receive the key information about the hospitalization with any necessary follow‐up instructions as soon after discharge as possible13 and certainly before the first postdischarge visit. Instructions to patients about medication schedules and follow‐up care need to be in writing at a 6th‐grade reading level; furthermore, processes to identify a patient's level of understanding of the follow‐up plan and areas for targeted education need to be established.24
DISCUSSION
We believe the development of a checklist of required elements to be communicated at discharge is a key step toward standardizing the hospital discharge process. The checklist highlights what is believed to be the key information about the transition of care and its process. The checklist is intended to standardize what is required for a successful hospital discharge. However, each institution will need to further refine this list according to local factors such as patient population, resources, and culture and to determine how best to implement the necessary changes to their current discharge process. Local modification of the checklist also allows for the addition of other elements that are patient‐ or population specific. Elderly patients discharged home from the hospital are the primary patient population targeted by this checklist ; there may be unique and additional elements necessary for an ideal discharge for a patient who is discharged to a subacute or acute rehabilitation facility. These elements are not described in this checklist but will be the focus of future work.
Establishing the critical elements of a hospital discharge transition sets the stage for improving patient outcomes in the immediate postdischarge period. Most important, the checklist conveys the message that the discharge process requires critical thinking, collaboration, and goal setting and that this coordination of care takes time. However, the discharge checklist must reside within a hospital culture that in general does not value the discharge process in the same way it values the admission process. The latter is more standardized and incorporates expectations about content and communication. In the same way, the current discharge is an admission to the next health care setting and deserves at least as much time and effort as a hospital admission. Furthermore, if institutions examine their current discharge processes, they may find that the time necessary to complete the discharge may be similar to the time necessary to admit a patient to the hospital. Finally, organizations need to develop internal policies and procedures that monitor and provide feedback about important dimensions of the discharge process, including content, patient understanding, information transfer, and clinical and service outcomes including satisfaction of the patient and the postdischarge provider. Hospital discharge is truly a team process involving nurses, pharmacists, case managers, and other hospital personnel, so performance measurement should be at the team or unit level, unlike other areas for which individual physicians may receive feedback on performance.
The limitations of the checklist development process include the paucity of randomized, controlled trials focused on the study of health care delivery processes and the lack of an industry gold standard. Furthermore, the heterogeneity of health care delivery systems makes it difficult to recommend specific interventions without understanding the myriad local issues. Those who provided input into this checklist included members of the inpatient team, a scope that can be broadened in the future to include outpatient physicians, patients, and caregivers in the home and long‐term care environments. However, the elements defined through the checklist serve as a starting point for developing discharge transition standards for older adults.
As leaders in hospital care, hospitalists are positioned to raise awareness of the importance of hospital discharge and to lead multidisciplinary efforts to improve the discharge process within their organizations. The first step in that process should be understanding the required elements and local facilitating factors and barriers in achieving a predictable, seamless transition of care for hospitalized patients.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J. R. Soc Med.2001;94:553.
- ,,,.Effect of discharge summary availability during the post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- .An ethnographic study of the process of medication discharge education (MDE).J Adv Nurs.1998;27:341–348.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,.Improving discharge data: lessons from the National Hospital Discharge Survey.Med Care1981;19:1030–1040.
- ,,.An investigation of patient satisfaction following discharge after total hip replacement surgery.Orthop Nurs.2003;22:429–436.
- ,,, et al.Payer‐hospital collaboration to improve patient satisfaction with hospital discharge.Jt Comm J QuaI Improv.1996;22:336–344.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- .Will, ideas, and execution: their role in reducing adverse medication events.J Pediatr.2005;147:727–728.
- .Why we need to learn standardisation.Aust Fam Physician.2005;34(1‐2):67–68.
- Joint Commission on Accreditation of Healthcare Organizations.2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg/06_npsg_cah_hap.htm.
- Census Bureau of Statistics,2000.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Ann Intern Med.2001;111(9B):26S–30S.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .Using the NO TEARS tool for medication review.BMJ.2004;329:434.
- .Toward safer warfarin therapy: does precise daily dosing improve INR control?Mayo Clinic Proc.2002;77:873–875.
- .Institute for Healthcare Improvement, personal communication.
- Institute of Medicine.Kindig DA, editor.Health Literacy: A Prescription to End Confusion.Washington, DC:National Academies Press,2004.
- ,,.Independent Mobility Validation Exam (I‐MOVE): a tool for periodic reassessment of fall‐risk and discharge planning. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
- ,,.Balance in elderly patients: the “get‐up‐and‐go” test.Arch Phys Med Rehabil.1986;67:387–389.
- AMA, Council on Ethical and Judicial Affairs.Guidelines for appropriate use of “do‐not‐resuscitate” orders.JAMA.1991;265:1868–1871.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999.14:160–169.
- JCAHO Manual: Information Management (IM) 6.10 and Patient Care (PC) 15.30
- ,.Specific appointments after pneumonia hospitalization reduce readmissions. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,.Predictors of elder and family caregiver satisfaction with discharge planning.J Cardiovasc Nurs.2000;14:76–87.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,.Exploring the causes of adverse events in NHS hospital practice.J. R. Soc Med.2001;94:553.
- ,,,.Effect of discharge summary availability during the post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- .An ethnographic study of the process of medication discharge education (MDE).J Adv Nurs.1998;27:341–348.
- .A hospitalization from hell: a patient's perspective on quality.Ann Intern Med.2003;138:33–39.
- ,.Improving discharge data: lessons from the National Hospital Discharge Survey.Med Care1981;19:1030–1040.
- ,,.An investigation of patient satisfaction following discharge after total hip replacement surgery.Orthop Nurs.2003;22:429–436.
- ,,, et al.Payer‐hospital collaboration to improve patient satisfaction with hospital discharge.Jt Comm J QuaI Improv.1996;22:336–344.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen Intern Med.2004;19:624–631.
- .Will, ideas, and execution: their role in reducing adverse medication events.J Pediatr.2005;147:727–728.
- .Why we need to learn standardisation.Aust Fam Physician.2005;34(1‐2):67–68.
- Joint Commission on Accreditation of Healthcare Organizations.2006 Critical Access Hospital and Hospital National Patient Safety Goals. Available at: http://www.jcaho.org/accredited+organizations/patient+safety/06_npsg/06_npsg_cah_hap.htm.
- Census Bureau of Statistics,2000.
- ,,, et al.Pharmacists on rounding teams reduce preventable adverse events in hospital general medicine units.Arch Intern Med.2003;163:2014–2018.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Ann Intern Med.2001;111(9B):26S–30S.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .Using the NO TEARS tool for medication review.BMJ.2004;329:434.
- .Toward safer warfarin therapy: does precise daily dosing improve INR control?Mayo Clinic Proc.2002;77:873–875.
- .Institute for Healthcare Improvement, personal communication.
- Institute of Medicine.Kindig DA, editor.Health Literacy: A Prescription to End Confusion.Washington, DC:National Academies Press,2004.
- ,,.Independent Mobility Validation Exam (I‐MOVE): a tool for periodic reassessment of fall‐risk and discharge planning. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
- ,,.Balance in elderly patients: the “get‐up‐and‐go” test.Arch Phys Med Rehabil.1986;67:387–389.
- AMA, Council on Ethical and Judicial Affairs.Guidelines for appropriate use of “do‐not‐resuscitate” orders.JAMA.1991;265:1868–1871.
- ,.Quality assessment of a discharge summary system.CMAJ.1995;152:1437–1442.
- ,.What is necessary for high‐quality discharge summaries?Am J Med Qual.1999.14:160–169.
- JCAHO Manual: Information Management (IM) 6.10 and Patient Care (PC) 15.30
- ,.Specific appointments after pneumonia hospitalization reduce readmissions. Abstract and Poster presentation at SHM (formerly NAIP) 5th Annual Meeting, Philadelphia, PA, April 9,2002.
Pneumonia: 3 days of antibiotics for uncomplicated course
-
CLINICAL QUESTION: In patients hospitalized for treatment of community‐acquired pneumonia, can treatment be stopped after 3 days if the patient has substantially improved?
-
BOTTOM LINE: Dogma successfully challenged: In patients who respond well to initial treatment, stopping antibiotic therapy after 3 days is just as effective as continuing treatment for the standard 8 days. (LOE = 1b)
-
REFERENCE: el Moussaoui R, de Borgie CA, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderatesevere community acquired pneumonia: randomised, double blind study. BMJ 2006;332:13551358.
-
STUDY DESIGN: Randomized controlled trial (doubleblinded)
-
FUNDING: Industry
-
ALLOCATION: Uncertain
-
SETTING: Inpatient (any location)
-
SYNOPSIS: The treatment of pneumonia for 7 days to 10 days is based on tradition, not scientific evidence. The researchers conducting this study challenged the status quo by enrolling 119 adults with mild to moderatesevere communityacquired pneumonia with a severity index score of 110 or less. On admission, all patients were started on intravenous amoxicillin, the preferred empirical treatment in the Netherlands. After 72 hours of treatment, patients who showed improvement in symptoms, had a temperature of less than 38 C, and could take oral drugs were randomized to treatment with placebo or amoxicillin 750 mg 3 times daily for 5 days. Using modified intentiontotreat analysis, after 10 days 89% of patients in both groups were clinically cured. In followup at 28 days, clinical cure rates were also similar between the 2 approaches, as was bacteriologic success and radiologic success. This study was designed to find a difference in success rates of at least 10%. There are a couple of notable limitations to this study. First, the patients in the shorttreatment group had a median age of 54 years compared with 60 years in the 8day group, and these younger patients may be more likely to respond to the short course, thus skewing the results. Second, the study was conducted in the Netherlands, where resistance patterns may be different than in other countries. Finally, the study was conducted in 9 hospitals over 3 years, which works out to less than 5 patients per hospital per year recruited into the study. Given the imbalance in age and this sparse representation, these patients could be highly selected and not representative of the typical patient admitted to a community hospital.
-
CLINICAL QUESTION: In patients hospitalized for treatment of community‐acquired pneumonia, can treatment be stopped after 3 days if the patient has substantially improved?
-
BOTTOM LINE: Dogma successfully challenged: In patients who respond well to initial treatment, stopping antibiotic therapy after 3 days is just as effective as continuing treatment for the standard 8 days. (LOE = 1b)
-
REFERENCE: el Moussaoui R, de Borgie CA, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderatesevere community acquired pneumonia: randomised, double blind study. BMJ 2006;332:13551358.
-
STUDY DESIGN: Randomized controlled trial (doubleblinded)
-
FUNDING: Industry
-
ALLOCATION: Uncertain
-
SETTING: Inpatient (any location)
-
SYNOPSIS: The treatment of pneumonia for 7 days to 10 days is based on tradition, not scientific evidence. The researchers conducting this study challenged the status quo by enrolling 119 adults with mild to moderatesevere communityacquired pneumonia with a severity index score of 110 or less. On admission, all patients were started on intravenous amoxicillin, the preferred empirical treatment in the Netherlands. After 72 hours of treatment, patients who showed improvement in symptoms, had a temperature of less than 38 C, and could take oral drugs were randomized to treatment with placebo or amoxicillin 750 mg 3 times daily for 5 days. Using modified intentiontotreat analysis, after 10 days 89% of patients in both groups were clinically cured. In followup at 28 days, clinical cure rates were also similar between the 2 approaches, as was bacteriologic success and radiologic success. This study was designed to find a difference in success rates of at least 10%. There are a couple of notable limitations to this study. First, the patients in the shorttreatment group had a median age of 54 years compared with 60 years in the 8day group, and these younger patients may be more likely to respond to the short course, thus skewing the results. Second, the study was conducted in the Netherlands, where resistance patterns may be different than in other countries. Finally, the study was conducted in 9 hospitals over 3 years, which works out to less than 5 patients per hospital per year recruited into the study. Given the imbalance in age and this sparse representation, these patients could be highly selected and not representative of the typical patient admitted to a community hospital.
-
CLINICAL QUESTION: In patients hospitalized for treatment of community‐acquired pneumonia, can treatment be stopped after 3 days if the patient has substantially improved?
-
BOTTOM LINE: Dogma successfully challenged: In patients who respond well to initial treatment, stopping antibiotic therapy after 3 days is just as effective as continuing treatment for the standard 8 days. (LOE = 1b)
-
REFERENCE: el Moussaoui R, de Borgie CA, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderatesevere community acquired pneumonia: randomised, double blind study. BMJ 2006;332:13551358.
-
STUDY DESIGN: Randomized controlled trial (doubleblinded)
-
FUNDING: Industry
-
ALLOCATION: Uncertain
-
SETTING: Inpatient (any location)
-
SYNOPSIS: The treatment of pneumonia for 7 days to 10 days is based on tradition, not scientific evidence. The researchers conducting this study challenged the status quo by enrolling 119 adults with mild to moderatesevere communityacquired pneumonia with a severity index score of 110 or less. On admission, all patients were started on intravenous amoxicillin, the preferred empirical treatment in the Netherlands. After 72 hours of treatment, patients who showed improvement in symptoms, had a temperature of less than 38 C, and could take oral drugs were randomized to treatment with placebo or amoxicillin 750 mg 3 times daily for 5 days. Using modified intentiontotreat analysis, after 10 days 89% of patients in both groups were clinically cured. In followup at 28 days, clinical cure rates were also similar between the 2 approaches, as was bacteriologic success and radiologic success. This study was designed to find a difference in success rates of at least 10%. There are a couple of notable limitations to this study. First, the patients in the shorttreatment group had a median age of 54 years compared with 60 years in the 8day group, and these younger patients may be more likely to respond to the short course, thus skewing the results. Second, the study was conducted in the Netherlands, where resistance patterns may be different than in other countries. Finally, the study was conducted in 9 hospitals over 3 years, which works out to less than 5 patients per hospital per year recruited into the study. Given the imbalance in age and this sparse representation, these patients could be highly selected and not representative of the typical patient admitted to a community hospital.