User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Hidradenitis Suppurativa: Workup
Shift schedule today could worsen that stroke tomorrow
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Hidradenitis Suppurativa Treatment
Justice Department task force to fight abortion ban overreach
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
FDA grants emergency authorization for Novavax COVID vaccine
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
2022 Update on menopause
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes?
Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295.
Expert Commentary
In the nonpregnant population, medical management of hypertension >140/90 mm Hg is standard practice. By contrast, much higher blood pressures (BPs; up to 160/110 mm Hg) traditionally have been tolerated in pregnant patients with chronic hypertension without initiating treatment, and existing medications are often discontinued during pregnancy. Concern for impaired fetal growth as well as lack of data on improved outcomes have led to different recommendations for the management of mild chronic hypertension in pregnancy. However, chronic hypertension affects a substantial number of pregnant patients and is known to be a risk factor for severe short-term pregnancy and long-term health complications. With preliminary data suggesting that BPs >140/90 mm Hg prior to 20 weeks’ gestation are associated with an increase in adverse outcomes, Tita and colleagues sought to determine the effects of decreasing BP in pregnant patients with mild chronic hypertension.
Details about the study
This is an investigator-initiated, multicenter, pragmatic, open-label, randomized control trial of 2,408 patients with mild chronic hypertension. The active treatment group was treated with antihypertensive medication (including titration of existing medication), targeting a BP of <140/90 mm Hg. The control group only received medication for severe hypertension (≥160 mm Hg systolic or ≥105 mm Hg diastolic). The primary outcome of the study was a composite of preeclampsia with severe features, medically indicated preterm delivery prior to 35 weeks’ gestation (not spontaneous labor or rupture of membranes), placental abruption, and fetal or neonatal death. Birthweight that was less than the 10th percentile was used as a safety outcome. The hypothesis was that treatment would decrease the rate of adverse pregnancy and fetal/neonatal outcomes.
The patient population of singleton pregnancies at a gestational age of less than 23 weeks included 56% with known chronic hypertension on medications, 22% with known chronic hypertension without medications, and 22% with newly diagnosed (during pregnancy) chronic hypertension. The treatment group primarily received labetalol (61.7%) or nifedipine (35.6%); the maximum dose of a single agent was used as tolerated prior to adding a second agent. The control group only received an antihypertensive medication for severe hypertension.
Treatment of chronic hypertension demonstrated a decreased risk of the composite adverse outcome with an adjusted risk ratio of 0.82 (95% confidence interval [CI], 0.74 to 0.92; P<.001) and a number needed to treat (NNT) of 14.7. When analyzed separately, a similar risk reduction was noted for both preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation. There was no statistical difference between the groups for birth weight <10th percentile and <5th percentile (adjusted risk ratio, 1.04 [0.82–1.31] vs 0.89 [0.62–1.26], respectively).
Planned subgroup analysis by type of chronic hypertension, race/ethnic group, diabetes status, gestational age at baseline, and body mass index (BMI) demonstrated a similar treatment effect to the overall composite primary outcome, with the exception of patients with newly diagn
Study strengths and weaknesses
The study strengths cited are a large sample size, multiple study sites, an independent data and safety monitoring board with close oversight, and centralized blinded confirmation of outcomes. Another strength is that the patient population of the study was similar to the overall population of pregnant patients in the United States with chronic hypertension in terms of age, race, and ethnicity.
The weaknesses of the study include the open-label design and the high ratio of screened to enrolled patients. Both of these issues appear related to the study design (ethics and logistics of a blinded treatment and gestational age cutoff) and the physiology of pregnancy (expected decrease in BP in the second trimester rendering patients ineligible due to lower BP). The study was also not powered to assess treatment effect in all of the subgroups, and further evaluation of patients with newly diagnosed chronic hypertension and BMI ≥ 40 kg/m2 is needed. ●
Pregnant patients with chronic hypertension should continue or initiate antihypertensive medication to target a BP goal of <140/90 mm Hg. This substantial practice change is supported by the significant decrease demonstrated in this study in adverse outcomes such as preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation without an increase in small-for-gestational-age newborns.
Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295.
Expert Commentary
In the nonpregnant population, medical management of hypertension >140/90 mm Hg is standard practice. By contrast, much higher blood pressures (BPs; up to 160/110 mm Hg) traditionally have been tolerated in pregnant patients with chronic hypertension without initiating treatment, and existing medications are often discontinued during pregnancy. Concern for impaired fetal growth as well as lack of data on improved outcomes have led to different recommendations for the management of mild chronic hypertension in pregnancy. However, chronic hypertension affects a substantial number of pregnant patients and is known to be a risk factor for severe short-term pregnancy and long-term health complications. With preliminary data suggesting that BPs >140/90 mm Hg prior to 20 weeks’ gestation are associated with an increase in adverse outcomes, Tita and colleagues sought to determine the effects of decreasing BP in pregnant patients with mild chronic hypertension.
Details about the study
This is an investigator-initiated, multicenter, pragmatic, open-label, randomized control trial of 2,408 patients with mild chronic hypertension. The active treatment group was treated with antihypertensive medication (including titration of existing medication), targeting a BP of <140/90 mm Hg. The control group only received medication for severe hypertension (≥160 mm Hg systolic or ≥105 mm Hg diastolic). The primary outcome of the study was a composite of preeclampsia with severe features, medically indicated preterm delivery prior to 35 weeks’ gestation (not spontaneous labor or rupture of membranes), placental abruption, and fetal or neonatal death. Birthweight that was less than the 10th percentile was used as a safety outcome. The hypothesis was that treatment would decrease the rate of adverse pregnancy and fetal/neonatal outcomes.
The patient population of singleton pregnancies at a gestational age of less than 23 weeks included 56% with known chronic hypertension on medications, 22% with known chronic hypertension without medications, and 22% with newly diagnosed (during pregnancy) chronic hypertension. The treatment group primarily received labetalol (61.7%) or nifedipine (35.6%); the maximum dose of a single agent was used as tolerated prior to adding a second agent. The control group only received an antihypertensive medication for severe hypertension.
Treatment of chronic hypertension demonstrated a decreased risk of the composite adverse outcome with an adjusted risk ratio of 0.82 (95% confidence interval [CI], 0.74 to 0.92; P<.001) and a number needed to treat (NNT) of 14.7. When analyzed separately, a similar risk reduction was noted for both preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation. There was no statistical difference between the groups for birth weight <10th percentile and <5th percentile (adjusted risk ratio, 1.04 [0.82–1.31] vs 0.89 [0.62–1.26], respectively).
Planned subgroup analysis by type of chronic hypertension, race/ethnic group, diabetes status, gestational age at baseline, and body mass index (BMI) demonstrated a similar treatment effect to the overall composite primary outcome, with the exception of patients with newly diagn
Study strengths and weaknesses
The study strengths cited are a large sample size, multiple study sites, an independent data and safety monitoring board with close oversight, and centralized blinded confirmation of outcomes. Another strength is that the patient population of the study was similar to the overall population of pregnant patients in the United States with chronic hypertension in terms of age, race, and ethnicity.
The weaknesses of the study include the open-label design and the high ratio of screened to enrolled patients. Both of these issues appear related to the study design (ethics and logistics of a blinded treatment and gestational age cutoff) and the physiology of pregnancy (expected decrease in BP in the second trimester rendering patients ineligible due to lower BP). The study was also not powered to assess treatment effect in all of the subgroups, and further evaluation of patients with newly diagnosed chronic hypertension and BMI ≥ 40 kg/m2 is needed. ●
Pregnant patients with chronic hypertension should continue or initiate antihypertensive medication to target a BP goal of <140/90 mm Hg. This substantial practice change is supported by the significant decrease demonstrated in this study in adverse outcomes such as preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation without an increase in small-for-gestational-age newborns.
Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295.
Expert Commentary
In the nonpregnant population, medical management of hypertension >140/90 mm Hg is standard practice. By contrast, much higher blood pressures (BPs; up to 160/110 mm Hg) traditionally have been tolerated in pregnant patients with chronic hypertension without initiating treatment, and existing medications are often discontinued during pregnancy. Concern for impaired fetal growth as well as lack of data on improved outcomes have led to different recommendations for the management of mild chronic hypertension in pregnancy. However, chronic hypertension affects a substantial number of pregnant patients and is known to be a risk factor for severe short-term pregnancy and long-term health complications. With preliminary data suggesting that BPs >140/90 mm Hg prior to 20 weeks’ gestation are associated with an increase in adverse outcomes, Tita and colleagues sought to determine the effects of decreasing BP in pregnant patients with mild chronic hypertension.
Details about the study
This is an investigator-initiated, multicenter, pragmatic, open-label, randomized control trial of 2,408 patients with mild chronic hypertension. The active treatment group was treated with antihypertensive medication (including titration of existing medication), targeting a BP of <140/90 mm Hg. The control group only received medication for severe hypertension (≥160 mm Hg systolic or ≥105 mm Hg diastolic). The primary outcome of the study was a composite of preeclampsia with severe features, medically indicated preterm delivery prior to 35 weeks’ gestation (not spontaneous labor or rupture of membranes), placental abruption, and fetal or neonatal death. Birthweight that was less than the 10th percentile was used as a safety outcome. The hypothesis was that treatment would decrease the rate of adverse pregnancy and fetal/neonatal outcomes.
The patient population of singleton pregnancies at a gestational age of less than 23 weeks included 56% with known chronic hypertension on medications, 22% with known chronic hypertension without medications, and 22% with newly diagnosed (during pregnancy) chronic hypertension. The treatment group primarily received labetalol (61.7%) or nifedipine (35.6%); the maximum dose of a single agent was used as tolerated prior to adding a second agent. The control group only received an antihypertensive medication for severe hypertension.
Treatment of chronic hypertension demonstrated a decreased risk of the composite adverse outcome with an adjusted risk ratio of 0.82 (95% confidence interval [CI], 0.74 to 0.92; P<.001) and a number needed to treat (NNT) of 14.7. When analyzed separately, a similar risk reduction was noted for both preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation. There was no statistical difference between the groups for birth weight <10th percentile and <5th percentile (adjusted risk ratio, 1.04 [0.82–1.31] vs 0.89 [0.62–1.26], respectively).
Planned subgroup analysis by type of chronic hypertension, race/ethnic group, diabetes status, gestational age at baseline, and body mass index (BMI) demonstrated a similar treatment effect to the overall composite primary outcome, with the exception of patients with newly diagn
Study strengths and weaknesses
The study strengths cited are a large sample size, multiple study sites, an independent data and safety monitoring board with close oversight, and centralized blinded confirmation of outcomes. Another strength is that the patient population of the study was similar to the overall population of pregnant patients in the United States with chronic hypertension in terms of age, race, and ethnicity.
The weaknesses of the study include the open-label design and the high ratio of screened to enrolled patients. Both of these issues appear related to the study design (ethics and logistics of a blinded treatment and gestational age cutoff) and the physiology of pregnancy (expected decrease in BP in the second trimester rendering patients ineligible due to lower BP). The study was also not powered to assess treatment effect in all of the subgroups, and further evaluation of patients with newly diagnosed chronic hypertension and BMI ≥ 40 kg/m2 is needed. ●
Pregnant patients with chronic hypertension should continue or initiate antihypertensive medication to target a BP goal of <140/90 mm Hg. This substantial practice change is supported by the significant decrease demonstrated in this study in adverse outcomes such as preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation without an increase in small-for-gestational-age newborns.
Best practices for evaluating pelvic pain in patients with Essure tubal occlusion devices
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.
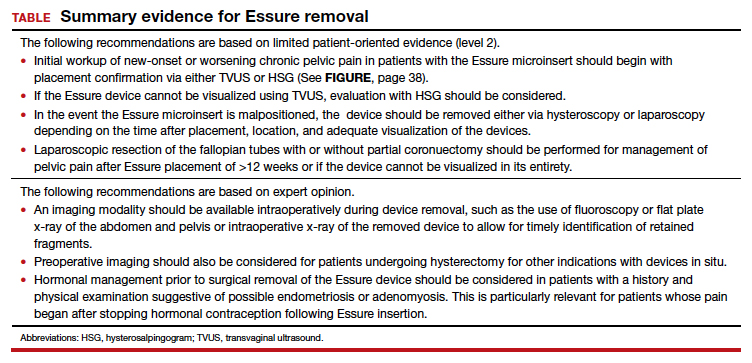
Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.
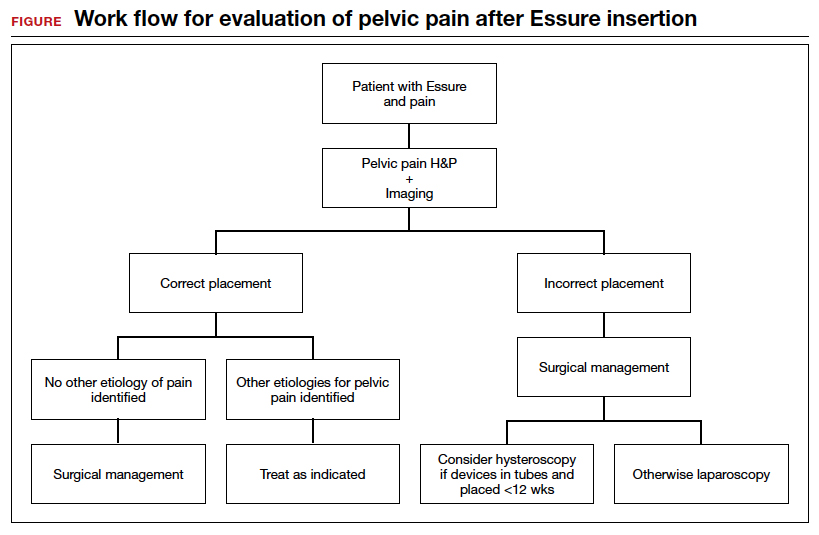
Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
Misoprostol: Clinical pharmacology in obstetrics and gynecology
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.
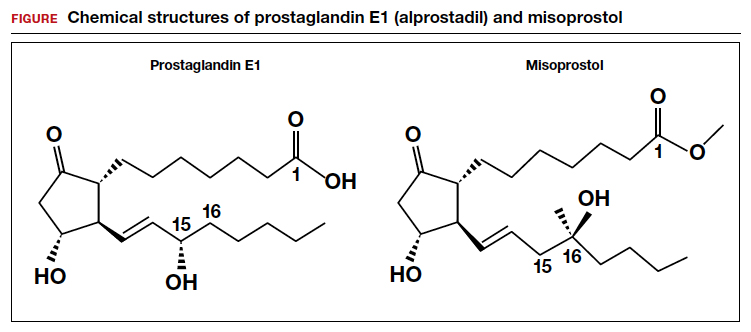
Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.

Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.

Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
Appropriate antibiotic selection for 12 common infections in obstetric patients
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13
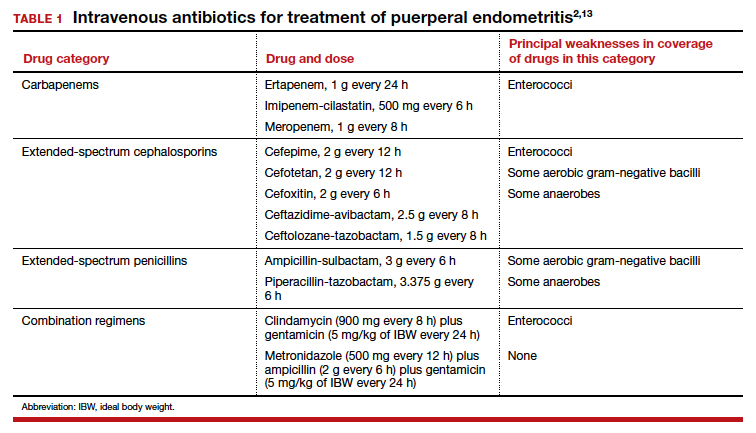
7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17
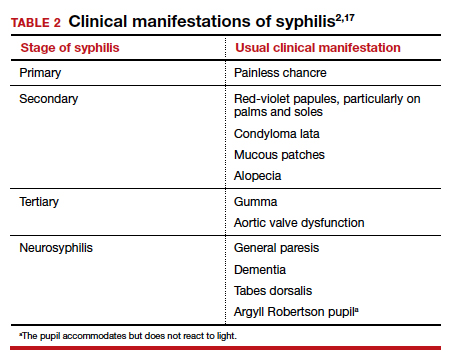
Antibiotic selection
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17
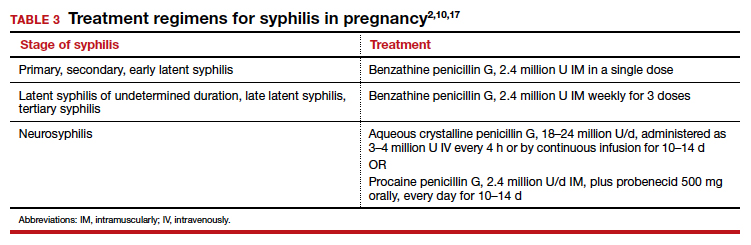
11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13

7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17

Antibiotic selection
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17

11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13

7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17

Antibiotic selection
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17

11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.