User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Placental allograft, cytology processor, cell-free RNA testing, and male infertility
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
VTE prevention: Patient selection and treatment planning throughout pregnancy
Pregnancy and the postpartum period are times of increased risk for venous thromboembolism (VTE). While VTE is a rare event overall, it is responsible for more than 9% of maternal deaths in the United States.1 The increased risk of VTE exists throughout pregnancy, rising in the third trimester.2 The highest-risk period is the first 6 weeks postpartum, likely peaking in the first 2 to 3 weeks and returning to baseline at about 12 weeks postpartum.2,3
To reduce this source of maternal harm, the National Partnership for Maternal Safety and the Council on Patient Safety in Women’s Health Care recommend the use of VTE prevention bundles. Bundles include standard assessment of risk during prenatal care, any admission to the hospital, and postpartum coupled with standard recommendations for treatment.4-6 Multiple published guidelines are available for prevention of VTE in pregnancy, and they provide varying recommendations on patient selection and treatment. Many of these recommendations are based on low quality of evidence, making the choice of standard practice difficult.
In this article, I attempt to simplify patient selection and treatment based on currently published guidelines from the American College of Obstetricians and Gynecologists (ACOG), Royal College of Obstetricians and Gynaecologists (RCOG), American College of Chest Physicians (CHEST), American Society of Hematology (ASH), and expert opinion.
Determining VTE risk and need for prophylaxis
CASE 1 Woman with factor V Leiden
A 25-year-old woman (G1P0) presents for her initial prenatal visit. She says she is a carrier for factor V Leiden but has never had a clot. She was tested after her sister had a VTE. She asks, does she need VTE prophylaxis before her delivery?
What are the considerations and options for this patient?
Options for VTE prophylaxis
Before considering patients at risk for VTE, it is helpful to review the options for prophylaxis. Patients can undergo clinical surveillance or routine care with attention to VTE symptoms and a low threshold for workup.
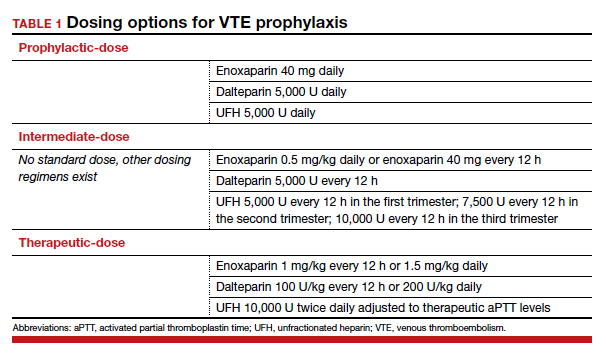
There are 3 categories of chemoprophylaxis for prevention of VTE. (TABLE 1 offers examples of dosing regimens.) No strategy has been proven optimal over another:
- prophylactic-dose: the lowest, fixed dose.
- intermediate-dose: lacks a standard definition and is any dose higher than prophylactic-dose but lower than therapeutic-dose. This includes fixed twice-daily doses, weight-based doses, and incrementally increasing doses.
- therapeutic-dose: typically used for treatment but mentioned here since patients with high-risk conditions may use it for prevention of VTE.
The preferred agent for VTE chemoprophylaxis is low molecular weight heparin (LMWH; dalteparin, enoxaparin). LMWH has a lower risk of complications than unfractionated heparin (UFH) and can be injected once daily. LMWH and UFH do not cross the placenta. LMWH and UFH are safe in breastfeeding. Oral direct thrombin inhibitors and anti-Xa inhibitors are not recommended in pregnancy or lactation at this time. Warfarin is avoided in pregnancy except in situations with mechanical heart valves, which will not be addressed here. Patients taking warfarin for long-term anticoagulation can transition back while breastfeeding with appropriate bridging.
Expert opinion recommends antepartum chemoprophylaxis when there is a 2% to 3% risk of VTE in pregnancy.7-9 This is balanced against an approximately 2% overall risk of bleeding, with less than 1% risk of bleeding antepartum.9
Continue to: Risk factors for VTE...
Risk factors for VTE
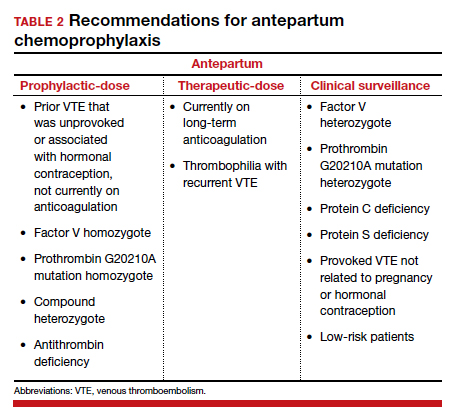
History of VTE. The most important risk factor for VTE is a personal history of prior VTE.6 Recurrence risks have been widely reported and depend on the factors surrounding the initial event. For patients with a prior provoked deep vein thrombosis (DVT; associated with trauma or surgery), the antepartum VTE risk likely is less than 1%, and VTE chemoprophylaxis is not recommended antepartum.7
For patients with a prior VTE that was not associated with surgery or trauma (unprovoked), the risk is approximately 3%; for prior VTE related to pregnancy or hormonal contraception, the risk is approximately 6%.7 For both of these groups, prophylactic-dose antepartum is recommended. Patients with recurrent VTE are often taking long-term anticoagulation. Anyone on long-term anticoagulation should be placed on therapeutic-dose antepartum. For patients not receiving long-term anticoagulation, consider a hematology consultation when available, and begin an intermediate-dose or therapeutic-dose regimen after assessing other risk factors and the risk of bleeding and discussing treatment with the patient.
Thrombophilias. The next most important risk factor is the presence of inherited thrombophilias.6 Factor V homozygote, prothrombin G20210A mutation homozygote, antithrombin deficiency, and combined factor V heterozygote and prothrombin G20210A heterozygote (also called compound heterozygote) have the strongest association with VTE in pregnancy.8 It is recommended that patients with these high-risk thrombophilias receive prophylactic-dose antepartum.8
Factor V heterozygote, prothrombin G20210A mutation heterozygote, and protein C or protein S deficiency are considered low-risk thrombophilias. Patients with low-risk thrombophilias and no personal history of VTE or first-degree relative with VTE can be monitored with clinical surveillance antepartum. However, if a family history of VTE or other risk factors for VTE are present, antepartum prophylactic-dose is recommended. Clinical factors to consider antepartum include obesity, age older than 35 years, parity of 3 or higher, varicose veins, immobility, smoking, assisted reproductive technology use, multiple gestation, and preeclampsia.10
Antiphospholipid syndrome (APS) is another high-risk condition. For patients not taking long-term anticoagulation antepartum, prophylactic-dose is recommended. For patients on long-term anticoagulation, therapeutic-dose is recommended.
Other medical conditions. Patients with medical conditions that place them at high risk for VTE may warrant prophylactic-dose antepartum. These include active cancer, active systemic lupus erythematosus, sickle cell disease, nephropathy, and inflammatory bowel disease.10 This decision can be made in conjunction with other specialists caring for the patient.
Antepartum prophylactic-dose is not recommended for low-risk patients as there is less than 1% risk of VTE.7 (TABLE 2 summarizes antepartum chemoprophylaxis recommendations.)
CASE 1 continued Patient develops another VTE risk factor
The patient is being followed with clinical surveillance. At 19 weeks’ gestation, she presents to the emergency department with shortness of breath and fever. She is diagnosed with COVID-19 and is admitted by a medicine service. They call the OB team to ask for recommendations regarding anticoagulation.
What should the next steps include?
Hospitalization and nonobstetric surgery are risk factors for VTE. Many hospitals use a standardized assessment for all inpatients, such as the Padua or Caprini VTE risk assessment scores. These can be modified for use in pregnant patients, although neither scoring system is currently validated for use in pregnancy.5 For any pregnant patient admitted to the hospital, mechanical prophylaxis is recommended.
COVID-19. Infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated clinical syndrome, COVID-19, is associated with increased rates of VTE. Recommendations for pregnant patients with COVID-19 are the same as for the general population. During hospitalization for COVID-19, pregnant patients should be placed on prophylactic-dose chemoprophylaxis. Patients should not be discharged home on chemoprophylaxis, and patients managed as outpatients for their disease do not need chemoprophylaxis.11
Management approach. Prophylactic-dose administration is recommended during hospital stay for all patients admitted with anticipated length of stay of 3 days or longer and who are not at high risk for bleeding or delivery.10 Both LMWH and UFH are options for inpatients. For any nonobstetric surgery or admission, LMWH may be most appropriate. However, as most obstetrics admissions are at increased risk for delivery, UFH 5,000 U twice daily to 3 times daily is the best option to increase the chances for neuraxial anesthesia. (I review anesthesia considerations for delivery later in this article.) For patients at high risk for bleeding or delivery, mechanical prophylaxis alone, with elastic stockings or pneumatic compression devices, can be used.
Continue to: CASE 1 continued Patient is discharged home...
CASE 1 continued Patient is discharged home
The patient received enoxaparin while she was in the hospital. She is now discharged and doing well. She asks, will she need anticoagulation prophylaxis after delivery?
How would you counsel her?
Chemoprophylaxis in the postpartum period
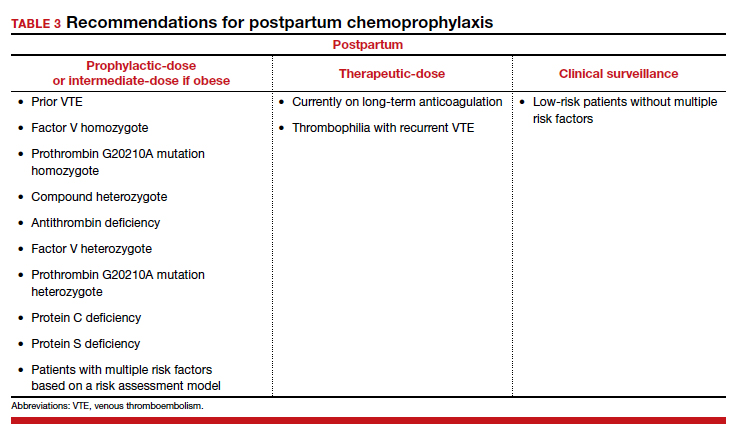
With no risk of fetal harm and a higher risk of VTE per day, the threshold for chemoprophylaxis is lower in the postpartum period. The risk of postpartum bleeding is less than 1%, with the most common complication being wound hematomas (0.61%).9 For this case patient, the COVID-19 diagnosis does not alter the recommendations for postpartum chemoprophylaxis. Additionally, as the need for neuraxial anesthesia has passed, the use of intermediate-dose chemoprophylaxis over prophylactic-dose is advocated in the postpartum period, especially in obese patients.12
As mentioned previously, there is no standard definition of intermediate-dose. Data suggest that a weight-based intermediate-dose is most likely to achieve therapeutic levels of anti-Xa in this high-risk population compared with a fixed dose.13,14 For example, enoxaparin 0.5 mg/kg twice daily is recommended for patients with class 3 obesity or higher by the Society for Maternal-Fetal Medicine.12
As a rule, anyone who was on chemoprophylaxis antepartum should be continued on at least an equivalent dose for 6 weeks postpartum. Postpartum, patients with any prior DVT should take prophylactic-dose or intermediate-dose chemoprophylaxis for 6 weeks. Patients with a known high-risk thrombophilia should receive prophylactic-dose or intermediate-dose chemoprophylaxis postpartum for 6 weeks. For patients with a low-risk thrombophilia, prophylactic-dose or intermediate-dose chemoprophylaxis is recommended for 6 weeks.
For low-risk patients without prior VTE or thrombophilia, standardized risk assessment is recommended.
Cesarean delivery
Cesarean delivery (CD) is a risk factor for postpartum VTE.9 A universal chemoprophylaxis strategy has not been proven in this patient population. Mechanical prophylaxis with sequential compression devices is recommended for all patients undergoing CD pre-procedure and until patients are fully ambulatory.8,9 Early ambulation also should be encouraged.
Many risk assessment models are available for postoperative VTE prevention, and they have widely different chemoprophylaxis rates. Studies have shown chemoprophylaxis rates of 85% by RCOG, 1% by ACOG, 35% by CHEST, 94% by Caprini, and less than 1% by Padua.15,16 In addition to the antepartum patient-specific risk factors mentioned, postpartum risk factors include infection, postpartum hemorrhage, and transfusion. Based on data extrapolated from the nonobstetric literature, chemoprophylaxis is recommended until discharge from the hospital unless risk factors are expected to continue.9
Neuraxial anesthesia
For patients who require postpartum chemoprophylaxis, the Society for Obstetric Anesthesia and Perinatology (SOAP) offers evidence-based guidelines for use after neuraxial anesthesia. UFH can be initiated 1 hour or longer after a neuraxial procedure and 1 hour or longer after catheter removal. Prophylactic-dose LMWH can be restarted at 12 hours or longer after a neuraxial procedure and at 4 to 6 hours or longer after catheter removal. For patients restarting intermediate-dose or therapeutic-dose, the recommendations are to wait 24 hours or longer after a neuraxial procedure and 4 hours or longer after catheter removal.17 Timing can be individualized based on the patient’s risk of hemorrhage and surgical bleeding. Although it may be tempting to delay chemoprophylaxis in the setting of bleeding, postpartum hemorrhage and transfusion increase the risks of VTE. In this setting, it is best to consider the use of UFH, which safely can be started earlier than LMWH.
For patients without neuraxial anesthesia, ACOG recommends chemoprophylaxis 4 to 6 hours after vaginal delivery and 6 to 12 hours after CD.8 (TABLE 3 summarizes recommendations for postpartum chemoprophylaxis.)
Continue to: Adjusting the anticoagulation regimen...
Adjusting the anticoagulation regimen
CASE 2 Pregnant woman with prior VTE
A 36-year-old woman (G1P0) with prior VTE is taking enoxaparin 40 mg daily. She asks, does she need any blood work for her anticoagulation?
What would you test for?
Increased renal clearance of LMWH and increased volume of distribution during pregnancy has led to the consideration of monitoring anti-Xa levels. There are no published standards or recommendations for dose adjustment. At this time, anti-Xa level monitoring antepartum is not recommended, but it may be considered when a patient is at the extremes of weight. With a weight-based strategy in the postpartum period, monitoring is not recommended as studies show a higher likelihood of therapeutic anti-Xa levels with this approach.13,14 This is an active area of research, and these recommendations may change.
For prophylactic-dose or intermediate-dose anticoagulation, a peak anti-Xa level of 0.2 to 0.6 U/mL is generally accepted as the target. For therapeutic-dose, a peak anti-Xa level of 0.6 to 1.2 U/mL is generally accepted as the therapeutic range. This blood draw must be collected 4 hours after the third dose.
CASE 2 continued Anticoagulation considerations nearing delivery
The patient is now at 36 weeks’ gestation, and she asks, what should be done regarding her anticoagulation prior to delivery?
What would be an appropriate approach?
Traditionally, patients were transitioned to UFH at 36 weeks and allowed to present in spontaneous labor to increase the likelihood of neuraxial anesthesia. The alternative is to continue prophylactic-dose LMWH until a scheduled delivery. While the SOAP guidelines establish the timeframe that is safe to proceed with neuraxial anesthesia, there is variation in practice, so consider discussing this with your anesthesia providers.
SOAP considers prophylactic-dose UFH to be 5,000 U 2 to 3 times per day. In this setting, neuraxial anesthesia can be placed more than 4 to 6 hours from the last dose.17 But due to the pharmacokinetics of pregnancy, ACOG recommends 10,000 U in the third trimester.8 This dose is considered intermediate-dose by SOAP, and 12 hours or longer plus a normal activated partial thromboplastin time (aPTT) or undetectable anti-Xa level are required prior to neuraxial anesthesia. This is the same time allowed for prophylactic-dose LMWH without lab work. Prophylactic-dose LMWH is considered to be enoxaparin 40 mg or less daily or 30 mg twice daily, and dalteparin 5,000 U daily. For therapeutic-dose LMWH or UFH, 24 hours or more from last dose is recommended prior to neuraxial anesthesia. For intermediate-dose LMWH, data are limited to recommend anything between 12 and 24 hours.17
In my practice, we favor a shared decision-making approach with patients. We discuss the likelihood of labor prior to 39 weeks based on a patient’s history, the importance of neuraxial anesthesia to the patient, and the importance of the number of daily injections. Most patients continue enoxaparin until a scheduled induction, and they are instructed to skip their dose if labor symptoms begin. Patients at high risk for preterm delivery can be transitioned to heparin earlier than 36 weeks. ●
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373. doi: 10.1097/AOG.0000000000002114.
- Kourlaba G, Relakis J, Kontodimas S, et al. A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. Int J Gynaecol Obstet. 2016;132:4-10. doi: 10.1016/j.ijgo.2015.06.054.
- Sultan AA, West J, Tata LJ, et al. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156:366-373. doi: 10.1111/j.1365-2141.2011.08956.x.
- American College of Obstetricians and Gynecologists. Council on Patient Safety in Women’s Health Care: maternal venous thromboembolism (+AIM). 2015. https://safehealthcareforeverywoman.org/council/patient-safety-bundles/maternal-safety-bundles/maternal-venous-thromboembolism-aim/. Accessed February 26, 2021.
- Urato AC, Abi-Jaoude E, Abramson J, et al. National Partnership for Maternal Safety: consensus bundle on venous thromboembolism. Obstet Gynecol. 2019;134:1115-1117. doi: 10.1097/AOG.0000000000003540.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17. doi: 10.1097/AOG.0000000000002706.
- Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-3359. doi: 10.1182/bloodadvances.2018024802.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi: 10.1097/AOG.0000000000002703.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2, suppl):e691S-e736S. doi: 10.1378/chest.11-2300.
- Lamont MC, McDermott C, Thomson AJ, et al. United Kingdom recommendations for obstetric venous thromboembolism prophylaxis: evidence and rationale. Semin Perinatol. 2019;43:222-228. doi: 10.1053/j.semperi.2019.03.008.
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed February 26, 2021.
- Society for Maternal-Fetal Medicine (SMFM); Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series #51: thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17. doi: 10.1016/j.ajog.2020.04.032.
- Overcash RT, Somers AT, LaCoursiere DY. Enoxaparin dosing after cesarean delivery in morbidly obese women. Obstet Gynecol. 2015;125:1371-1376. doi: 10.1097/AOG.0000000000000873.
- Hiscock RJ, Casey E, Simmons SW, et al. Peak plasma anti-Xa levels after first and third doses of enoxaparin in women receiving weight-based thromboprophylaxis following caesarean section: a prospective cohort study. Int J Obstet Anesth. 2013;22:280-288. doi: 10.1016/j.ijoa.2013.05.008.
- Palmerola KL, D’Alton ME, Brock CO, et al. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG. 2016;123:2157-2162. doi: 10.1111/1471-0528.13706.
- Tran JP, Stribling SS, Ibezim UC, et al. Performance of risk assessment models for peripartum thromboprophylaxis. Reprod Sci. 2019;26:1243-1248. doi: 10.1177/1933719118813197.
- Leffert L, Butwick A, Carvalho B, et al; members of the SOAP VTE Taskforce. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:928-944. doi: 10.1213/ANE.0000000000002530.

Pregnancy and the postpartum period are times of increased risk for venous thromboembolism (VTE). While VTE is a rare event overall, it is responsible for more than 9% of maternal deaths in the United States.1 The increased risk of VTE exists throughout pregnancy, rising in the third trimester.2 The highest-risk period is the first 6 weeks postpartum, likely peaking in the first 2 to 3 weeks and returning to baseline at about 12 weeks postpartum.2,3
To reduce this source of maternal harm, the National Partnership for Maternal Safety and the Council on Patient Safety in Women’s Health Care recommend the use of VTE prevention bundles. Bundles include standard assessment of risk during prenatal care, any admission to the hospital, and postpartum coupled with standard recommendations for treatment.4-6 Multiple published guidelines are available for prevention of VTE in pregnancy, and they provide varying recommendations on patient selection and treatment. Many of these recommendations are based on low quality of evidence, making the choice of standard practice difficult.
In this article, I attempt to simplify patient selection and treatment based on currently published guidelines from the American College of Obstetricians and Gynecologists (ACOG), Royal College of Obstetricians and Gynaecologists (RCOG), American College of Chest Physicians (CHEST), American Society of Hematology (ASH), and expert opinion.
Determining VTE risk and need for prophylaxis
CASE 1 Woman with factor V Leiden
A 25-year-old woman (G1P0) presents for her initial prenatal visit. She says she is a carrier for factor V Leiden but has never had a clot. She was tested after her sister had a VTE. She asks, does she need VTE prophylaxis before her delivery?
What are the considerations and options for this patient?
Options for VTE prophylaxis
Before considering patients at risk for VTE, it is helpful to review the options for prophylaxis. Patients can undergo clinical surveillance or routine care with attention to VTE symptoms and a low threshold for workup.
There are 3 categories of chemoprophylaxis for prevention of VTE. (TABLE 1 offers examples of dosing regimens.) No strategy has been proven optimal over another:
- prophylactic-dose: the lowest, fixed dose.
- intermediate-dose: lacks a standard definition and is any dose higher than prophylactic-dose but lower than therapeutic-dose. This includes fixed twice-daily doses, weight-based doses, and incrementally increasing doses.
- therapeutic-dose: typically used for treatment but mentioned here since patients with high-risk conditions may use it for prevention of VTE.
The preferred agent for VTE chemoprophylaxis is low molecular weight heparin (LMWH; dalteparin, enoxaparin). LMWH has a lower risk of complications than unfractionated heparin (UFH) and can be injected once daily. LMWH and UFH do not cross the placenta. LMWH and UFH are safe in breastfeeding. Oral direct thrombin inhibitors and anti-Xa inhibitors are not recommended in pregnancy or lactation at this time. Warfarin is avoided in pregnancy except in situations with mechanical heart valves, which will not be addressed here. Patients taking warfarin for long-term anticoagulation can transition back while breastfeeding with appropriate bridging.

Expert opinion recommends antepartum chemoprophylaxis when there is a 2% to 3% risk of VTE in pregnancy.7-9 This is balanced against an approximately 2% overall risk of bleeding, with less than 1% risk of bleeding antepartum.9
Continue to: Risk factors for VTE...
Risk factors for VTE
History of VTE. The most important risk factor for VTE is a personal history of prior VTE.6 Recurrence risks have been widely reported and depend on the factors surrounding the initial event. For patients with a prior provoked deep vein thrombosis (DVT; associated with trauma or surgery), the antepartum VTE risk likely is less than 1%, and VTE chemoprophylaxis is not recommended antepartum.7
For patients with a prior VTE that was not associated with surgery or trauma (unprovoked), the risk is approximately 3%; for prior VTE related to pregnancy or hormonal contraception, the risk is approximately 6%.7 For both of these groups, prophylactic-dose antepartum is recommended. Patients with recurrent VTE are often taking long-term anticoagulation. Anyone on long-term anticoagulation should be placed on therapeutic-dose antepartum. For patients not receiving long-term anticoagulation, consider a hematology consultation when available, and begin an intermediate-dose or therapeutic-dose regimen after assessing other risk factors and the risk of bleeding and discussing treatment with the patient.
Thrombophilias. The next most important risk factor is the presence of inherited thrombophilias.6 Factor V homozygote, prothrombin G20210A mutation homozygote, antithrombin deficiency, and combined factor V heterozygote and prothrombin G20210A heterozygote (also called compound heterozygote) have the strongest association with VTE in pregnancy.8 It is recommended that patients with these high-risk thrombophilias receive prophylactic-dose antepartum.8
Factor V heterozygote, prothrombin G20210A mutation heterozygote, and protein C or protein S deficiency are considered low-risk thrombophilias. Patients with low-risk thrombophilias and no personal history of VTE or first-degree relative with VTE can be monitored with clinical surveillance antepartum. However, if a family history of VTE or other risk factors for VTE are present, antepartum prophylactic-dose is recommended. Clinical factors to consider antepartum include obesity, age older than 35 years, parity of 3 or higher, varicose veins, immobility, smoking, assisted reproductive technology use, multiple gestation, and preeclampsia.10
Antiphospholipid syndrome (APS) is another high-risk condition. For patients not taking long-term anticoagulation antepartum, prophylactic-dose is recommended. For patients on long-term anticoagulation, therapeutic-dose is recommended.
Other medical conditions. Patients with medical conditions that place them at high risk for VTE may warrant prophylactic-dose antepartum. These include active cancer, active systemic lupus erythematosus, sickle cell disease, nephropathy, and inflammatory bowel disease.10 This decision can be made in conjunction with other specialists caring for the patient.
Antepartum prophylactic-dose is not recommended for low-risk patients as there is less than 1% risk of VTE.7 (TABLE 2 summarizes antepartum chemoprophylaxis recommendations.)

CASE 1 continued Patient develops another VTE risk factor
The patient is being followed with clinical surveillance. At 19 weeks’ gestation, she presents to the emergency department with shortness of breath and fever. She is diagnosed with COVID-19 and is admitted by a medicine service. They call the OB team to ask for recommendations regarding anticoagulation.
What should the next steps include?
Hospitalization and nonobstetric surgery are risk factors for VTE. Many hospitals use a standardized assessment for all inpatients, such as the Padua or Caprini VTE risk assessment scores. These can be modified for use in pregnant patients, although neither scoring system is currently validated for use in pregnancy.5 For any pregnant patient admitted to the hospital, mechanical prophylaxis is recommended.
COVID-19. Infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated clinical syndrome, COVID-19, is associated with increased rates of VTE. Recommendations for pregnant patients with COVID-19 are the same as for the general population. During hospitalization for COVID-19, pregnant patients should be placed on prophylactic-dose chemoprophylaxis. Patients should not be discharged home on chemoprophylaxis, and patients managed as outpatients for their disease do not need chemoprophylaxis.11
Management approach. Prophylactic-dose administration is recommended during hospital stay for all patients admitted with anticipated length of stay of 3 days or longer and who are not at high risk for bleeding or delivery.10 Both LMWH and UFH are options for inpatients. For any nonobstetric surgery or admission, LMWH may be most appropriate. However, as most obstetrics admissions are at increased risk for delivery, UFH 5,000 U twice daily to 3 times daily is the best option to increase the chances for neuraxial anesthesia. (I review anesthesia considerations for delivery later in this article.) For patients at high risk for bleeding or delivery, mechanical prophylaxis alone, with elastic stockings or pneumatic compression devices, can be used.
Continue to: CASE 1 continued Patient is discharged home...
CASE 1 continued Patient is discharged home
The patient received enoxaparin while she was in the hospital. She is now discharged and doing well. She asks, will she need anticoagulation prophylaxis after delivery?
How would you counsel her?
Chemoprophylaxis in the postpartum period
With no risk of fetal harm and a higher risk of VTE per day, the threshold for chemoprophylaxis is lower in the postpartum period. The risk of postpartum bleeding is less than 1%, with the most common complication being wound hematomas (0.61%).9 For this case patient, the COVID-19 diagnosis does not alter the recommendations for postpartum chemoprophylaxis. Additionally, as the need for neuraxial anesthesia has passed, the use of intermediate-dose chemoprophylaxis over prophylactic-dose is advocated in the postpartum period, especially in obese patients.12
As mentioned previously, there is no standard definition of intermediate-dose. Data suggest that a weight-based intermediate-dose is most likely to achieve therapeutic levels of anti-Xa in this high-risk population compared with a fixed dose.13,14 For example, enoxaparin 0.5 mg/kg twice daily is recommended for patients with class 3 obesity or higher by the Society for Maternal-Fetal Medicine.12
As a rule, anyone who was on chemoprophylaxis antepartum should be continued on at least an equivalent dose for 6 weeks postpartum. Postpartum, patients with any prior DVT should take prophylactic-dose or intermediate-dose chemoprophylaxis for 6 weeks. Patients with a known high-risk thrombophilia should receive prophylactic-dose or intermediate-dose chemoprophylaxis postpartum for 6 weeks. For patients with a low-risk thrombophilia, prophylactic-dose or intermediate-dose chemoprophylaxis is recommended for 6 weeks.
For low-risk patients without prior VTE or thrombophilia, standardized risk assessment is recommended.
Cesarean delivery
Cesarean delivery (CD) is a risk factor for postpartum VTE.9 A universal chemoprophylaxis strategy has not been proven in this patient population. Mechanical prophylaxis with sequential compression devices is recommended for all patients undergoing CD pre-procedure and until patients are fully ambulatory.8,9 Early ambulation also should be encouraged.
Many risk assessment models are available for postoperative VTE prevention, and they have widely different chemoprophylaxis rates. Studies have shown chemoprophylaxis rates of 85% by RCOG, 1% by ACOG, 35% by CHEST, 94% by Caprini, and less than 1% by Padua.15,16 In addition to the antepartum patient-specific risk factors mentioned, postpartum risk factors include infection, postpartum hemorrhage, and transfusion. Based on data extrapolated from the nonobstetric literature, chemoprophylaxis is recommended until discharge from the hospital unless risk factors are expected to continue.9
Neuraxial anesthesia
For patients who require postpartum chemoprophylaxis, the Society for Obstetric Anesthesia and Perinatology (SOAP) offers evidence-based guidelines for use after neuraxial anesthesia. UFH can be initiated 1 hour or longer after a neuraxial procedure and 1 hour or longer after catheter removal. Prophylactic-dose LMWH can be restarted at 12 hours or longer after a neuraxial procedure and at 4 to 6 hours or longer after catheter removal. For patients restarting intermediate-dose or therapeutic-dose, the recommendations are to wait 24 hours or longer after a neuraxial procedure and 4 hours or longer after catheter removal.17 Timing can be individualized based on the patient’s risk of hemorrhage and surgical bleeding. Although it may be tempting to delay chemoprophylaxis in the setting of bleeding, postpartum hemorrhage and transfusion increase the risks of VTE. In this setting, it is best to consider the use of UFH, which safely can be started earlier than LMWH.
For patients without neuraxial anesthesia, ACOG recommends chemoprophylaxis 4 to 6 hours after vaginal delivery and 6 to 12 hours after CD.8 (TABLE 3 summarizes recommendations for postpartum chemoprophylaxis.)

Continue to: Adjusting the anticoagulation regimen...
Adjusting the anticoagulation regimen
CASE 2 Pregnant woman with prior VTE
A 36-year-old woman (G1P0) with prior VTE is taking enoxaparin 40 mg daily. She asks, does she need any blood work for her anticoagulation?
What would you test for?
Increased renal clearance of LMWH and increased volume of distribution during pregnancy has led to the consideration of monitoring anti-Xa levels. There are no published standards or recommendations for dose adjustment. At this time, anti-Xa level monitoring antepartum is not recommended, but it may be considered when a patient is at the extremes of weight. With a weight-based strategy in the postpartum period, monitoring is not recommended as studies show a higher likelihood of therapeutic anti-Xa levels with this approach.13,14 This is an active area of research, and these recommendations may change.
For prophylactic-dose or intermediate-dose anticoagulation, a peak anti-Xa level of 0.2 to 0.6 U/mL is generally accepted as the target. For therapeutic-dose, a peak anti-Xa level of 0.6 to 1.2 U/mL is generally accepted as the therapeutic range. This blood draw must be collected 4 hours after the third dose.
CASE 2 continued Anticoagulation considerations nearing delivery
The patient is now at 36 weeks’ gestation, and she asks, what should be done regarding her anticoagulation prior to delivery?
What would be an appropriate approach?
Traditionally, patients were transitioned to UFH at 36 weeks and allowed to present in spontaneous labor to increase the likelihood of neuraxial anesthesia. The alternative is to continue prophylactic-dose LMWH until a scheduled delivery. While the SOAP guidelines establish the timeframe that is safe to proceed with neuraxial anesthesia, there is variation in practice, so consider discussing this with your anesthesia providers.
SOAP considers prophylactic-dose UFH to be 5,000 U 2 to 3 times per day. In this setting, neuraxial anesthesia can be placed more than 4 to 6 hours from the last dose.17 But due to the pharmacokinetics of pregnancy, ACOG recommends 10,000 U in the third trimester.8 This dose is considered intermediate-dose by SOAP, and 12 hours or longer plus a normal activated partial thromboplastin time (aPTT) or undetectable anti-Xa level are required prior to neuraxial anesthesia. This is the same time allowed for prophylactic-dose LMWH without lab work. Prophylactic-dose LMWH is considered to be enoxaparin 40 mg or less daily or 30 mg twice daily, and dalteparin 5,000 U daily. For therapeutic-dose LMWH or UFH, 24 hours or more from last dose is recommended prior to neuraxial anesthesia. For intermediate-dose LMWH, data are limited to recommend anything between 12 and 24 hours.17
In my practice, we favor a shared decision-making approach with patients. We discuss the likelihood of labor prior to 39 weeks based on a patient’s history, the importance of neuraxial anesthesia to the patient, and the importance of the number of daily injections. Most patients continue enoxaparin until a scheduled induction, and they are instructed to skip their dose if labor symptoms begin. Patients at high risk for preterm delivery can be transitioned to heparin earlier than 36 weeks. ●

Pregnancy and the postpartum period are times of increased risk for venous thromboembolism (VTE). While VTE is a rare event overall, it is responsible for more than 9% of maternal deaths in the United States.1 The increased risk of VTE exists throughout pregnancy, rising in the third trimester.2 The highest-risk period is the first 6 weeks postpartum, likely peaking in the first 2 to 3 weeks and returning to baseline at about 12 weeks postpartum.2,3
To reduce this source of maternal harm, the National Partnership for Maternal Safety and the Council on Patient Safety in Women’s Health Care recommend the use of VTE prevention bundles. Bundles include standard assessment of risk during prenatal care, any admission to the hospital, and postpartum coupled with standard recommendations for treatment.4-6 Multiple published guidelines are available for prevention of VTE in pregnancy, and they provide varying recommendations on patient selection and treatment. Many of these recommendations are based on low quality of evidence, making the choice of standard practice difficult.
In this article, I attempt to simplify patient selection and treatment based on currently published guidelines from the American College of Obstetricians and Gynecologists (ACOG), Royal College of Obstetricians and Gynaecologists (RCOG), American College of Chest Physicians (CHEST), American Society of Hematology (ASH), and expert opinion.
Determining VTE risk and need for prophylaxis
CASE 1 Woman with factor V Leiden
A 25-year-old woman (G1P0) presents for her initial prenatal visit. She says she is a carrier for factor V Leiden but has never had a clot. She was tested after her sister had a VTE. She asks, does she need VTE prophylaxis before her delivery?
What are the considerations and options for this patient?
Options for VTE prophylaxis
Before considering patients at risk for VTE, it is helpful to review the options for prophylaxis. Patients can undergo clinical surveillance or routine care with attention to VTE symptoms and a low threshold for workup.
There are 3 categories of chemoprophylaxis for prevention of VTE. (TABLE 1 offers examples of dosing regimens.) No strategy has been proven optimal over another:
- prophylactic-dose: the lowest, fixed dose.
- intermediate-dose: lacks a standard definition and is any dose higher than prophylactic-dose but lower than therapeutic-dose. This includes fixed twice-daily doses, weight-based doses, and incrementally increasing doses.
- therapeutic-dose: typically used for treatment but mentioned here since patients with high-risk conditions may use it for prevention of VTE.
The preferred agent for VTE chemoprophylaxis is low molecular weight heparin (LMWH; dalteparin, enoxaparin). LMWH has a lower risk of complications than unfractionated heparin (UFH) and can be injected once daily. LMWH and UFH do not cross the placenta. LMWH and UFH are safe in breastfeeding. Oral direct thrombin inhibitors and anti-Xa inhibitors are not recommended in pregnancy or lactation at this time. Warfarin is avoided in pregnancy except in situations with mechanical heart valves, which will not be addressed here. Patients taking warfarin for long-term anticoagulation can transition back while breastfeeding with appropriate bridging.

Expert opinion recommends antepartum chemoprophylaxis when there is a 2% to 3% risk of VTE in pregnancy.7-9 This is balanced against an approximately 2% overall risk of bleeding, with less than 1% risk of bleeding antepartum.9
Continue to: Risk factors for VTE...
Risk factors for VTE
History of VTE. The most important risk factor for VTE is a personal history of prior VTE.6 Recurrence risks have been widely reported and depend on the factors surrounding the initial event. For patients with a prior provoked deep vein thrombosis (DVT; associated with trauma or surgery), the antepartum VTE risk likely is less than 1%, and VTE chemoprophylaxis is not recommended antepartum.7
For patients with a prior VTE that was not associated with surgery or trauma (unprovoked), the risk is approximately 3%; for prior VTE related to pregnancy or hormonal contraception, the risk is approximately 6%.7 For both of these groups, prophylactic-dose antepartum is recommended. Patients with recurrent VTE are often taking long-term anticoagulation. Anyone on long-term anticoagulation should be placed on therapeutic-dose antepartum. For patients not receiving long-term anticoagulation, consider a hematology consultation when available, and begin an intermediate-dose or therapeutic-dose regimen after assessing other risk factors and the risk of bleeding and discussing treatment with the patient.
Thrombophilias. The next most important risk factor is the presence of inherited thrombophilias.6 Factor V homozygote, prothrombin G20210A mutation homozygote, antithrombin deficiency, and combined factor V heterozygote and prothrombin G20210A heterozygote (also called compound heterozygote) have the strongest association with VTE in pregnancy.8 It is recommended that patients with these high-risk thrombophilias receive prophylactic-dose antepartum.8
Factor V heterozygote, prothrombin G20210A mutation heterozygote, and protein C or protein S deficiency are considered low-risk thrombophilias. Patients with low-risk thrombophilias and no personal history of VTE or first-degree relative with VTE can be monitored with clinical surveillance antepartum. However, if a family history of VTE or other risk factors for VTE are present, antepartum prophylactic-dose is recommended. Clinical factors to consider antepartum include obesity, age older than 35 years, parity of 3 or higher, varicose veins, immobility, smoking, assisted reproductive technology use, multiple gestation, and preeclampsia.10
Antiphospholipid syndrome (APS) is another high-risk condition. For patients not taking long-term anticoagulation antepartum, prophylactic-dose is recommended. For patients on long-term anticoagulation, therapeutic-dose is recommended.
Other medical conditions. Patients with medical conditions that place them at high risk for VTE may warrant prophylactic-dose antepartum. These include active cancer, active systemic lupus erythematosus, sickle cell disease, nephropathy, and inflammatory bowel disease.10 This decision can be made in conjunction with other specialists caring for the patient.
Antepartum prophylactic-dose is not recommended for low-risk patients as there is less than 1% risk of VTE.7 (TABLE 2 summarizes antepartum chemoprophylaxis recommendations.)

CASE 1 continued Patient develops another VTE risk factor
The patient is being followed with clinical surveillance. At 19 weeks’ gestation, she presents to the emergency department with shortness of breath and fever. She is diagnosed with COVID-19 and is admitted by a medicine service. They call the OB team to ask for recommendations regarding anticoagulation.
What should the next steps include?
Hospitalization and nonobstetric surgery are risk factors for VTE. Many hospitals use a standardized assessment for all inpatients, such as the Padua or Caprini VTE risk assessment scores. These can be modified for use in pregnant patients, although neither scoring system is currently validated for use in pregnancy.5 For any pregnant patient admitted to the hospital, mechanical prophylaxis is recommended.
COVID-19. Infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated clinical syndrome, COVID-19, is associated with increased rates of VTE. Recommendations for pregnant patients with COVID-19 are the same as for the general population. During hospitalization for COVID-19, pregnant patients should be placed on prophylactic-dose chemoprophylaxis. Patients should not be discharged home on chemoprophylaxis, and patients managed as outpatients for their disease do not need chemoprophylaxis.11
Management approach. Prophylactic-dose administration is recommended during hospital stay for all patients admitted with anticipated length of stay of 3 days or longer and who are not at high risk for bleeding or delivery.10 Both LMWH and UFH are options for inpatients. For any nonobstetric surgery or admission, LMWH may be most appropriate. However, as most obstetrics admissions are at increased risk for delivery, UFH 5,000 U twice daily to 3 times daily is the best option to increase the chances for neuraxial anesthesia. (I review anesthesia considerations for delivery later in this article.) For patients at high risk for bleeding or delivery, mechanical prophylaxis alone, with elastic stockings or pneumatic compression devices, can be used.
Continue to: CASE 1 continued Patient is discharged home...
CASE 1 continued Patient is discharged home
The patient received enoxaparin while she was in the hospital. She is now discharged and doing well. She asks, will she need anticoagulation prophylaxis after delivery?
How would you counsel her?
Chemoprophylaxis in the postpartum period
With no risk of fetal harm and a higher risk of VTE per day, the threshold for chemoprophylaxis is lower in the postpartum period. The risk of postpartum bleeding is less than 1%, with the most common complication being wound hematomas (0.61%).9 For this case patient, the COVID-19 diagnosis does not alter the recommendations for postpartum chemoprophylaxis. Additionally, as the need for neuraxial anesthesia has passed, the use of intermediate-dose chemoprophylaxis over prophylactic-dose is advocated in the postpartum period, especially in obese patients.12
As mentioned previously, there is no standard definition of intermediate-dose. Data suggest that a weight-based intermediate-dose is most likely to achieve therapeutic levels of anti-Xa in this high-risk population compared with a fixed dose.13,14 For example, enoxaparin 0.5 mg/kg twice daily is recommended for patients with class 3 obesity or higher by the Society for Maternal-Fetal Medicine.12
As a rule, anyone who was on chemoprophylaxis antepartum should be continued on at least an equivalent dose for 6 weeks postpartum. Postpartum, patients with any prior DVT should take prophylactic-dose or intermediate-dose chemoprophylaxis for 6 weeks. Patients with a known high-risk thrombophilia should receive prophylactic-dose or intermediate-dose chemoprophylaxis postpartum for 6 weeks. For patients with a low-risk thrombophilia, prophylactic-dose or intermediate-dose chemoprophylaxis is recommended for 6 weeks.
For low-risk patients without prior VTE or thrombophilia, standardized risk assessment is recommended.
Cesarean delivery
Cesarean delivery (CD) is a risk factor for postpartum VTE.9 A universal chemoprophylaxis strategy has not been proven in this patient population. Mechanical prophylaxis with sequential compression devices is recommended for all patients undergoing CD pre-procedure and until patients are fully ambulatory.8,9 Early ambulation also should be encouraged.
Many risk assessment models are available for postoperative VTE prevention, and they have widely different chemoprophylaxis rates. Studies have shown chemoprophylaxis rates of 85% by RCOG, 1% by ACOG, 35% by CHEST, 94% by Caprini, and less than 1% by Padua.15,16 In addition to the antepartum patient-specific risk factors mentioned, postpartum risk factors include infection, postpartum hemorrhage, and transfusion. Based on data extrapolated from the nonobstetric literature, chemoprophylaxis is recommended until discharge from the hospital unless risk factors are expected to continue.9
Neuraxial anesthesia
For patients who require postpartum chemoprophylaxis, the Society for Obstetric Anesthesia and Perinatology (SOAP) offers evidence-based guidelines for use after neuraxial anesthesia. UFH can be initiated 1 hour or longer after a neuraxial procedure and 1 hour or longer after catheter removal. Prophylactic-dose LMWH can be restarted at 12 hours or longer after a neuraxial procedure and at 4 to 6 hours or longer after catheter removal. For patients restarting intermediate-dose or therapeutic-dose, the recommendations are to wait 24 hours or longer after a neuraxial procedure and 4 hours or longer after catheter removal.17 Timing can be individualized based on the patient’s risk of hemorrhage and surgical bleeding. Although it may be tempting to delay chemoprophylaxis in the setting of bleeding, postpartum hemorrhage and transfusion increase the risks of VTE. In this setting, it is best to consider the use of UFH, which safely can be started earlier than LMWH.
For patients without neuraxial anesthesia, ACOG recommends chemoprophylaxis 4 to 6 hours after vaginal delivery and 6 to 12 hours after CD.8 (TABLE 3 summarizes recommendations for postpartum chemoprophylaxis.)

Continue to: Adjusting the anticoagulation regimen...
Adjusting the anticoagulation regimen
CASE 2 Pregnant woman with prior VTE
A 36-year-old woman (G1P0) with prior VTE is taking enoxaparin 40 mg daily. She asks, does she need any blood work for her anticoagulation?
What would you test for?
Increased renal clearance of LMWH and increased volume of distribution during pregnancy has led to the consideration of monitoring anti-Xa levels. There are no published standards or recommendations for dose adjustment. At this time, anti-Xa level monitoring antepartum is not recommended, but it may be considered when a patient is at the extremes of weight. With a weight-based strategy in the postpartum period, monitoring is not recommended as studies show a higher likelihood of therapeutic anti-Xa levels with this approach.13,14 This is an active area of research, and these recommendations may change.
For prophylactic-dose or intermediate-dose anticoagulation, a peak anti-Xa level of 0.2 to 0.6 U/mL is generally accepted as the target. For therapeutic-dose, a peak anti-Xa level of 0.6 to 1.2 U/mL is generally accepted as the therapeutic range. This blood draw must be collected 4 hours after the third dose.
CASE 2 continued Anticoagulation considerations nearing delivery
The patient is now at 36 weeks’ gestation, and she asks, what should be done regarding her anticoagulation prior to delivery?
What would be an appropriate approach?
Traditionally, patients were transitioned to UFH at 36 weeks and allowed to present in spontaneous labor to increase the likelihood of neuraxial anesthesia. The alternative is to continue prophylactic-dose LMWH until a scheduled delivery. While the SOAP guidelines establish the timeframe that is safe to proceed with neuraxial anesthesia, there is variation in practice, so consider discussing this with your anesthesia providers.
SOAP considers prophylactic-dose UFH to be 5,000 U 2 to 3 times per day. In this setting, neuraxial anesthesia can be placed more than 4 to 6 hours from the last dose.17 But due to the pharmacokinetics of pregnancy, ACOG recommends 10,000 U in the third trimester.8 This dose is considered intermediate-dose by SOAP, and 12 hours or longer plus a normal activated partial thromboplastin time (aPTT) or undetectable anti-Xa level are required prior to neuraxial anesthesia. This is the same time allowed for prophylactic-dose LMWH without lab work. Prophylactic-dose LMWH is considered to be enoxaparin 40 mg or less daily or 30 mg twice daily, and dalteparin 5,000 U daily. For therapeutic-dose LMWH or UFH, 24 hours or more from last dose is recommended prior to neuraxial anesthesia. For intermediate-dose LMWH, data are limited to recommend anything between 12 and 24 hours.17
In my practice, we favor a shared decision-making approach with patients. We discuss the likelihood of labor prior to 39 weeks based on a patient’s history, the importance of neuraxial anesthesia to the patient, and the importance of the number of daily injections. Most patients continue enoxaparin until a scheduled induction, and they are instructed to skip their dose if labor symptoms begin. Patients at high risk for preterm delivery can be transitioned to heparin earlier than 36 weeks. ●
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373. doi: 10.1097/AOG.0000000000002114.
- Kourlaba G, Relakis J, Kontodimas S, et al. A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. Int J Gynaecol Obstet. 2016;132:4-10. doi: 10.1016/j.ijgo.2015.06.054.
- Sultan AA, West J, Tata LJ, et al. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156:366-373. doi: 10.1111/j.1365-2141.2011.08956.x.
- American College of Obstetricians and Gynecologists. Council on Patient Safety in Women’s Health Care: maternal venous thromboembolism (+AIM). 2015. https://safehealthcareforeverywoman.org/council/patient-safety-bundles/maternal-safety-bundles/maternal-venous-thromboembolism-aim/. Accessed February 26, 2021.
- Urato AC, Abi-Jaoude E, Abramson J, et al. National Partnership for Maternal Safety: consensus bundle on venous thromboembolism. Obstet Gynecol. 2019;134:1115-1117. doi: 10.1097/AOG.0000000000003540.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17. doi: 10.1097/AOG.0000000000002706.
- Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-3359. doi: 10.1182/bloodadvances.2018024802.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi: 10.1097/AOG.0000000000002703.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2, suppl):e691S-e736S. doi: 10.1378/chest.11-2300.
- Lamont MC, McDermott C, Thomson AJ, et al. United Kingdom recommendations for obstetric venous thromboembolism prophylaxis: evidence and rationale. Semin Perinatol. 2019;43:222-228. doi: 10.1053/j.semperi.2019.03.008.
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed February 26, 2021.
- Society for Maternal-Fetal Medicine (SMFM); Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series #51: thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17. doi: 10.1016/j.ajog.2020.04.032.
- Overcash RT, Somers AT, LaCoursiere DY. Enoxaparin dosing after cesarean delivery in morbidly obese women. Obstet Gynecol. 2015;125:1371-1376. doi: 10.1097/AOG.0000000000000873.
- Hiscock RJ, Casey E, Simmons SW, et al. Peak plasma anti-Xa levels after first and third doses of enoxaparin in women receiving weight-based thromboprophylaxis following caesarean section: a prospective cohort study. Int J Obstet Anesth. 2013;22:280-288. doi: 10.1016/j.ijoa.2013.05.008.
- Palmerola KL, D’Alton ME, Brock CO, et al. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG. 2016;123:2157-2162. doi: 10.1111/1471-0528.13706.
- Tran JP, Stribling SS, Ibezim UC, et al. Performance of risk assessment models for peripartum thromboprophylaxis. Reprod Sci. 2019;26:1243-1248. doi: 10.1177/1933719118813197.
- Leffert L, Butwick A, Carvalho B, et al; members of the SOAP VTE Taskforce. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:928-944. doi: 10.1213/ANE.0000000000002530.
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366-373. doi: 10.1097/AOG.0000000000002114.
- Kourlaba G, Relakis J, Kontodimas S, et al. A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. Int J Gynaecol Obstet. 2016;132:4-10. doi: 10.1016/j.ijgo.2015.06.054.
- Sultan AA, West J, Tata LJ, et al. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156:366-373. doi: 10.1111/j.1365-2141.2011.08956.x.
- American College of Obstetricians and Gynecologists. Council on Patient Safety in Women’s Health Care: maternal venous thromboembolism (+AIM). 2015. https://safehealthcareforeverywoman.org/council/patient-safety-bundles/maternal-safety-bundles/maternal-venous-thromboembolism-aim/. Accessed February 26, 2021.
- Urato AC, Abi-Jaoude E, Abramson J, et al. National Partnership for Maternal Safety: consensus bundle on venous thromboembolism. Obstet Gynecol. 2019;134:1115-1117. doi: 10.1097/AOG.0000000000003540.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17. doi: 10.1097/AOG.0000000000002706.
- Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-3359. doi: 10.1182/bloodadvances.2018024802.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi: 10.1097/AOG.0000000000002703.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2, suppl):e691S-e736S. doi: 10.1378/chest.11-2300.
- Lamont MC, McDermott C, Thomson AJ, et al. United Kingdom recommendations for obstetric venous thromboembolism prophylaxis: evidence and rationale. Semin Perinatol. 2019;43:222-228. doi: 10.1053/j.semperi.2019.03.008.
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed February 26, 2021.
- Society for Maternal-Fetal Medicine (SMFM); Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series #51: thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17. doi: 10.1016/j.ajog.2020.04.032.
- Overcash RT, Somers AT, LaCoursiere DY. Enoxaparin dosing after cesarean delivery in morbidly obese women. Obstet Gynecol. 2015;125:1371-1376. doi: 10.1097/AOG.0000000000000873.
- Hiscock RJ, Casey E, Simmons SW, et al. Peak plasma anti-Xa levels after first and third doses of enoxaparin in women receiving weight-based thromboprophylaxis following caesarean section: a prospective cohort study. Int J Obstet Anesth. 2013;22:280-288. doi: 10.1016/j.ijoa.2013.05.008.
- Palmerola KL, D’Alton ME, Brock CO, et al. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG. 2016;123:2157-2162. doi: 10.1111/1471-0528.13706.
- Tran JP, Stribling SS, Ibezim UC, et al. Performance of risk assessment models for peripartum thromboprophylaxis. Reprod Sci. 2019;26:1243-1248. doi: 10.1177/1933719118813197.
- Leffert L, Butwick A, Carvalho B, et al; members of the SOAP VTE Taskforce. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:928-944. doi: 10.1213/ANE.0000000000002530.
Hepatitis in pregnancy: Sorting through the alphabet
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).
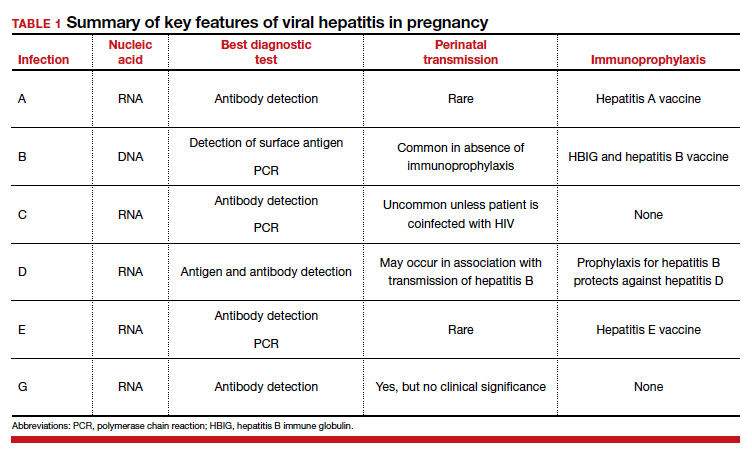
Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).
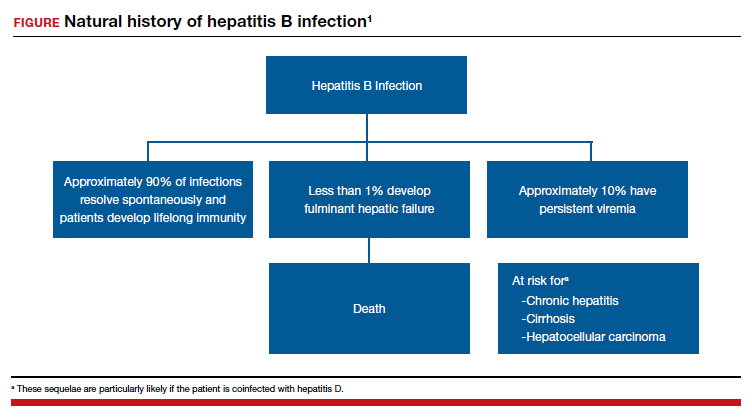

All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
Does prophylactic use of tranexamic acid reduce PPH from cesarean delivery when coupled with uterotonics?
Sentilhes L, Senat MV, Le Lous M, et al; Groupe de Recherche en Obstetrique et Gynecologie. Tranexamic acid for the prevention of blood loss after cesarean delivery. N Engl J Med. 2021;384:1623-1634. doi: 10.1056/NEJMoa2028788.
EXPERT COMMENTARY
Postpartum hemorrhage is the leading cause of maternal mortality worldwide.1 Many preventive strategies, including tranexamic acid administration, have been studied in an attempt to reduce the risk of PPH. Tranexamic acid prevents the conversion of plasminogen to plasmin, preventing the breakdown of fibrin, and ultimately stabilizing the fibrin matrix of clot.2 It has been shown to be an effective approach to treating hemorrhage in patients after trauma as well as cardiac surgery.3,4 The use of tranexamic acid in obstetric hemorrhage has reduced mortality in previous trials,5 but its prophylactic use has had mixed results in preventing obstetric hemorrhage.6-8
Recently, Sentilhes and colleagues published the largest prospective study to date addressing the efficacy of tranexamic acid for the primary prevention of PPH.
Details of the study
Multiple hospitals throughout France participated in the investigators’ double-blind randomized, placebo-controlled trial. Women undergoing CD at 34 or more weeks’ gestation (N = 4,551) were randomly assigned to receive 1 g of intravenous (IV) tranexamic acid or placebo after cord clamping. Both groups received IV prophylactic uterotonics. The primary outcome was PPH, defined by estimated blood loss (EBL) greater than 1 L or receipt of red blood cell transfusion within the first 2 days after surgery.
Results. The rate of PPH was significantly lower in women who received tranexamic acid compared with those who received placebo. Yet, the mean EBL between the 2 groups differed by only 100 mL. The rates of blood transfusions, additional uterotonic administration, arterial embolization, and hysterectomy did not differ between groups.
The clinicians responsible for the care of these patients did not observe a difference in the rate of “clinically significant” PPH between those who received tranexamic acid and those who received placebo. Women who received tranexamic acid were more likely to experience nausea and vomiting, but they did not have any increased risk of venous thromboembolic disease.
Study strengths and limitations
Sentilhes and colleagues’ study findings contradict those of an earlier meta-analysis on the topic.9 This may be due to the effect of publication bias on meta-analyses, which makes them prone to supporting the findings of published positive trials while missing data from negative trials that did not reach publication. The gold standard for addressing a research question such as this is a randomized controlled trial (RCT). The study reviewed here is an excellent example of a well-designed and executed RCT.
There may be a benefit to prophylactic tranexamic acid in certain populations not well captured among these study participants. The inclusion criteria were broad, including both prelabor and intrapartum CDs, making the results generalizable. However, the population studied, with a mean body mass index of 26 kg/m2 and age of 33, may not resemble some readers’ patient population. Prespecified subgroup analyses did not find a benefit to tranexamic acid in patients considered at high risk for PPH or in those undergoing intrapartum CD. ●
Prevention of PPH would reduce the burden of maternal morbidity and mortality dramatically. Unfortunately, the addition of tranexamic acid as a prophylactic agent at CD does not appear to have a clinically significant impact on the outcomes that matter to patients or providers. While tranexamic acid certainly has a role in the treatment of PPH, its benefit as a preventive agent has yet to be demonstrated.
JONATHAN S. HIRSHBERG, MD,
AND ALISON G. CAHILL, MD, MSCI
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Chauncey JM, Wieters JS. Tranexamic Acid. StatPearls Publishing LLC [internet]; 2021.
- Karski JM, Teasdale SJ, Norman P, et al. Prevention of bleeding after cardiopulmonary bypass with high-dose tranexamic acid. Double-blind, randomized clinical trial. J Thorac Cardiovasc Surg. 1995;110:835-842.
- Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1-79.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116.
- Sentilhes L, Winer N, Azria E, et al; Groupe de Recherche en Obstetrique et Gynecologie. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379:731-742.
- Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak. 2013;23;459-462.
- Simonazzi G, Bisulli M, Saccone G, et al. Tranexamic acid for preventing postpartum blood loss after cesarean delivery: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2016;95:28-37.
- Wang Y, Liu S, He L. Prophylactic use of tranexamic acid reduces blood loss and transfusion requirements in patients undergoing cesarean section: a meta-analysis. J Obstet Gynaecol Res. 2019;45:1562-1575.
Sentilhes L, Senat MV, Le Lous M, et al; Groupe de Recherche en Obstetrique et Gynecologie. Tranexamic acid for the prevention of blood loss after cesarean delivery. N Engl J Med. 2021;384:1623-1634. doi: 10.1056/NEJMoa2028788.
EXPERT COMMENTARY
Postpartum hemorrhage is the leading cause of maternal mortality worldwide.1 Many preventive strategies, including tranexamic acid administration, have been studied in an attempt to reduce the risk of PPH. Tranexamic acid prevents the conversion of plasminogen to plasmin, preventing the breakdown of fibrin, and ultimately stabilizing the fibrin matrix of clot.2 It has been shown to be an effective approach to treating hemorrhage in patients after trauma as well as cardiac surgery.3,4 The use of tranexamic acid in obstetric hemorrhage has reduced mortality in previous trials,5 but its prophylactic use has had mixed results in preventing obstetric hemorrhage.6-8
Recently, Sentilhes and colleagues published the largest prospective study to date addressing the efficacy of tranexamic acid for the primary prevention of PPH.
Details of the study
Multiple hospitals throughout France participated in the investigators’ double-blind randomized, placebo-controlled trial. Women undergoing CD at 34 or more weeks’ gestation (N = 4,551) were randomly assigned to receive 1 g of intravenous (IV) tranexamic acid or placebo after cord clamping. Both groups received IV prophylactic uterotonics. The primary outcome was PPH, defined by estimated blood loss (EBL) greater than 1 L or receipt of red blood cell transfusion within the first 2 days after surgery.
Results. The rate of PPH was significantly lower in women who received tranexamic acid compared with those who received placebo. Yet, the mean EBL between the 2 groups differed by only 100 mL. The rates of blood transfusions, additional uterotonic administration, arterial embolization, and hysterectomy did not differ between groups.
The clinicians responsible for the care of these patients did not observe a difference in the rate of “clinically significant” PPH between those who received tranexamic acid and those who received placebo. Women who received tranexamic acid were more likely to experience nausea and vomiting, but they did not have any increased risk of venous thromboembolic disease.
Study strengths and limitations
Sentilhes and colleagues’ study findings contradict those of an earlier meta-analysis on the topic.9 This may be due to the effect of publication bias on meta-analyses, which makes them prone to supporting the findings of published positive trials while missing data from negative trials that did not reach publication. The gold standard for addressing a research question such as this is a randomized controlled trial (RCT). The study reviewed here is an excellent example of a well-designed and executed RCT.
There may be a benefit to prophylactic tranexamic acid in certain populations not well captured among these study participants. The inclusion criteria were broad, including both prelabor and intrapartum CDs, making the results generalizable. However, the population studied, with a mean body mass index of 26 kg/m2 and age of 33, may not resemble some readers’ patient population. Prespecified subgroup analyses did not find a benefit to tranexamic acid in patients considered at high risk for PPH or in those undergoing intrapartum CD. ●
Prevention of PPH would reduce the burden of maternal morbidity and mortality dramatically. Unfortunately, the addition of tranexamic acid as a prophylactic agent at CD does not appear to have a clinically significant impact on the outcomes that matter to patients or providers. While tranexamic acid certainly has a role in the treatment of PPH, its benefit as a preventive agent has yet to be demonstrated.
JONATHAN S. HIRSHBERG, MD,
AND ALISON G. CAHILL, MD, MSCI
Sentilhes L, Senat MV, Le Lous M, et al; Groupe de Recherche en Obstetrique et Gynecologie. Tranexamic acid for the prevention of blood loss after cesarean delivery. N Engl J Med. 2021;384:1623-1634. doi: 10.1056/NEJMoa2028788.
EXPERT COMMENTARY
Postpartum hemorrhage is the leading cause of maternal mortality worldwide.1 Many preventive strategies, including tranexamic acid administration, have been studied in an attempt to reduce the risk of PPH. Tranexamic acid prevents the conversion of plasminogen to plasmin, preventing the breakdown of fibrin, and ultimately stabilizing the fibrin matrix of clot.2 It has been shown to be an effective approach to treating hemorrhage in patients after trauma as well as cardiac surgery.3,4 The use of tranexamic acid in obstetric hemorrhage has reduced mortality in previous trials,5 but its prophylactic use has had mixed results in preventing obstetric hemorrhage.6-8
Recently, Sentilhes and colleagues published the largest prospective study to date addressing the efficacy of tranexamic acid for the primary prevention of PPH.
Details of the study
Multiple hospitals throughout France participated in the investigators’ double-blind randomized, placebo-controlled trial. Women undergoing CD at 34 or more weeks’ gestation (N = 4,551) were randomly assigned to receive 1 g of intravenous (IV) tranexamic acid or placebo after cord clamping. Both groups received IV prophylactic uterotonics. The primary outcome was PPH, defined by estimated blood loss (EBL) greater than 1 L or receipt of red blood cell transfusion within the first 2 days after surgery.
Results. The rate of PPH was significantly lower in women who received tranexamic acid compared with those who received placebo. Yet, the mean EBL between the 2 groups differed by only 100 mL. The rates of blood transfusions, additional uterotonic administration, arterial embolization, and hysterectomy did not differ between groups.
The clinicians responsible for the care of these patients did not observe a difference in the rate of “clinically significant” PPH between those who received tranexamic acid and those who received placebo. Women who received tranexamic acid were more likely to experience nausea and vomiting, but they did not have any increased risk of venous thromboembolic disease.
Study strengths and limitations
Sentilhes and colleagues’ study findings contradict those of an earlier meta-analysis on the topic.9 This may be due to the effect of publication bias on meta-analyses, which makes them prone to supporting the findings of published positive trials while missing data from negative trials that did not reach publication. The gold standard for addressing a research question such as this is a randomized controlled trial (RCT). The study reviewed here is an excellent example of a well-designed and executed RCT.
There may be a benefit to prophylactic tranexamic acid in certain populations not well captured among these study participants. The inclusion criteria were broad, including both prelabor and intrapartum CDs, making the results generalizable. However, the population studied, with a mean body mass index of 26 kg/m2 and age of 33, may not resemble some readers’ patient population. Prespecified subgroup analyses did not find a benefit to tranexamic acid in patients considered at high risk for PPH or in those undergoing intrapartum CD. ●
Prevention of PPH would reduce the burden of maternal morbidity and mortality dramatically. Unfortunately, the addition of tranexamic acid as a prophylactic agent at CD does not appear to have a clinically significant impact on the outcomes that matter to patients or providers. While tranexamic acid certainly has a role in the treatment of PPH, its benefit as a preventive agent has yet to be demonstrated.
JONATHAN S. HIRSHBERG, MD,
AND ALISON G. CAHILL, MD, MSCI
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Chauncey JM, Wieters JS. Tranexamic Acid. StatPearls Publishing LLC [internet]; 2021.
- Karski JM, Teasdale SJ, Norman P, et al. Prevention of bleeding after cardiopulmonary bypass with high-dose tranexamic acid. Double-blind, randomized clinical trial. J Thorac Cardiovasc Surg. 1995;110:835-842.
- Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1-79.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116.
- Sentilhes L, Winer N, Azria E, et al; Groupe de Recherche en Obstetrique et Gynecologie. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379:731-742.
- Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak. 2013;23;459-462.
- Simonazzi G, Bisulli M, Saccone G, et al. Tranexamic acid for preventing postpartum blood loss after cesarean delivery: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2016;95:28-37.
- Wang Y, Liu S, He L. Prophylactic use of tranexamic acid reduces blood loss and transfusion requirements in patients undergoing cesarean section: a meta-analysis. J Obstet Gynaecol Res. 2019;45:1562-1575.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Chauncey JM, Wieters JS. Tranexamic Acid. StatPearls Publishing LLC [internet]; 2021.
- Karski JM, Teasdale SJ, Norman P, et al. Prevention of bleeding after cardiopulmonary bypass with high-dose tranexamic acid. Double-blind, randomized clinical trial. J Thorac Cardiovasc Surg. 1995;110:835-842.
- Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1-79.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116.
- Sentilhes L, Winer N, Azria E, et al; Groupe de Recherche en Obstetrique et Gynecologie. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379:731-742.
- Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak. 2013;23;459-462.
- Simonazzi G, Bisulli M, Saccone G, et al. Tranexamic acid for preventing postpartum blood loss after cesarean delivery: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2016;95:28-37.
- Wang Y, Liu S, He L. Prophylactic use of tranexamic acid reduces blood loss and transfusion requirements in patients undergoing cesarean section: a meta-analysis. J Obstet Gynaecol Res. 2019;45:1562-1575.
3 cases of hormone therapy optimized to match the patient problem
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
Focus on cancer risk
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
Hereditary cancer risk assessment is the key to identifying patients and families who are at increased risk for developing cancer. The knowledge generated by cancer risk assessment impacts clinical decisions that obstetricians and gynecologists and their patients make every day. Previvors—patients predisposed to developing cancer, because of their family history or a pathogenic gene variant, who have not had cancer—benefit from counseling, heightened surveillance, and medical and surgical options.
For the last 25 years, this field has been growing dramatically, and although the scientific advances are present, only 15.3% of patients with a personal history of breast or ovarian cancer who meet hereditary cancer testing criteria have been tested.1 As many as 1 in 4 women who present for a gynecologic examination may have a personal history or a family history that qualifies them for genetic testing.2
Cancer risk app considerations
The ability to leverage mobile device applications can provide clinicians and patients with a useful screening tool to identify women who are at increased cancer risk. Only a handful of apps are available today and most are geared to patients. Such apps explore the different testing modalities, including genetic testing, as well as treatment options. When evaluating the best app for patients, using the ACOG-recommended rubric shown on page 35, the qualities to keep in mind and that should score 4 out of 4 include design, authority, usefulness, and accuracy.
A few apps provide reminders for appointments, such as mammograms, magnetic resonance imaging, or breast self-exams, and allow patients to track treatment plans. To date, no app addresses prevention and treatment opportunities that are specific to patients who have a hereditary predisposition. At least one app lists hereditary cancer testing guidelines. Many more apps are geared toward individuals with cancer rather than toward previvors.
As ObGyns, we have an opportunity to educate and identify women and, subsequently, better counsel women identified as at increased risk for developing cancer. We can utilize medical apps to efficiently incorporate this screening into clinical practice. ●
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.
- Childers P, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- DeFrancesco M, Waldman RN, Pearlstone MM, et al. Hereditary cancer risk assessment and genetic testing in a community practice setting. Obstet Gynecol. 2018;132:1121-1129.
Mobile apps in ObGyn practice: Tools for enhancing women’s preventive health care
Evolutionary changes in ObGyn
Preventive medicine guidelines have evolved to reflect enhanced cervical cancer screening tests, longer-acting contraceptive options, and better data on the lack of utility of the annual pelvic exam that has changed the focus of the annual visit for both physicians and patients.1 These changes allow us to pivot and leverage the trust we build with our patients to make meaningful impacts in preventing chronic disease, improving prepregnancy health, reducing maternal mortality and morbidity, and improving the quality and longevity of our patients’ lives. New guidelines, coupled with the knowledge of the leading causes of morbidity for women, provide the chance to incorporate areas of screening and intervention that, while we are capable of addressing, we traditionally have not done so for various reasons.
The ACOG Presidential Task Force identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. ObGyns are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas. To make this daunting task more manageable, the Task Force not only published webinars to address the clinical knowledge pertaining to these areas of health but also specifically looked at how to use technology to aid obstetrician-gynecologists in addressing them with patients.
Making use of technology in clinical practice
Technology is emerging as an influential player in health care. Major corporations, such as Amazon, Google, Apple, and Facebook, are making headlines in health care as they consider strategies (moves) to revolutionize technology and, in turn, patient visits like we have never seen before. Examples include incorporating artificial intelligence in a patient’s care and allowing better access for primary care.
The changes that we will see over the next 10 years, influenced by industry, will be more than those seen in our lifetime. To prepare for these changes, we need to incorporate technology into our daily practice. This encompasses much more than just the electronic medical record. Consequently, the Task Force intentionally looked at mobile medical apps to aid physicians in addressing the 5 specific areas of preventive health identified.
While a small step compared with what is to come, apps are a great resource to leverage in making this transition. However, with hundreds of thousands of medical apps available in app stores and the constant updates and iterations of each, it would be impossible to recommend any single app. There is much value in having a framework to use to efficiently measure the benefit of an app that you or your patient comes across in clinical practice. The objective of this series was to provide clinicians with an effective tool to evaluate a medical app that could be used, for example, when addressing obesity or optimizing prepregnancy health.
Continue to: The recommended rubric for evaluating apps...
The recommended rubric for evaluating apps
To evaluate mobile drug information apps, the Task Force members recommend a user-friendly, convenient rubric developed by the American Society of Health-System Pharmacists (ASHP) (see page 35). The rubric can help obstetrician-gynecologists evaluate and compare the value of various medical apps that specifically address obesity, diabetes mellitus, cardiovascular disease, improving maternal morbidity with enhanced preconception counseling, and cancer risk assessment.
The authors of this Task Force series have attempted to highlight the key features of an app as it pertains to a particular area of focus. It is important to keep in mind the primary user and the goal when choosing or recommending an app for practice or for patient use. The ASHP’s rubric is a tool meant to aid clinicians in evaluating medical apps, but it is ultimately the user’s decision to determine if the deficiencies of an app should deter its use. Although all the criteria are relevant and important, as medical experts it is incumbent on us to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice.
While integrating the use of medical apps into clinical practice will be novel for some, for others, junior Fellows in particular, it has become part of their practice and education. Dr. Eva Hoffmann, Chief Resident in the NYU Langone Health System, offers this perspective: “As medical trainees we use mobile apps to enhance our patient interaction and guide high-quality, continuous care. In today’s modern technological world, apps help keep us up to date with the ever-changing guidelines in pregnancy and routine gynecologic care as well as communicate directly and discreetly with a patient whenever the need arises. The most significant apps provide guidance on abnormal Pap results, indicated deliveries prior to 39 weeks, and the ability to respond to obstetrical emergencies. They also allow for quick society-endorsed references in seconds. Apps have changed the way that we practice by providing evidence-based medicine literally at our fingertips—in a shareable and communicable way—making the practice of medicine even more efficient and effective.”
Opportunity to reaffirm expertise
Dr. Chalas’ initiative was meant to shed light on the opportunity obstetrician-gynecologists have to reassert themselves as women’s health experts, to consider redefining their practice by incorporating new preventive guidelines, and to leverage medical apps for achieving better health outcomes for women across their lifetime. We hope that by opening a dialogue about how ubiquitous medical apps are (for both physicians and patients) in today’s health arena, how many apps are inaccurate and/or misused, and how a simple rubric can be used to assess an app’s value, you are inspired and feel more comfortable to incorporate medical apps into your practice.
Health care will continually undergo advancements, and as a specialty we must evolve to address women’s needs. Obstetrician-gynecologists are well suited to contribute significantly to the well-being of women and mothers. We can leverage technology-based apps to help us redefine our roles and priorities at the patient’s annual visit. We can reaffirm ourselves as the leading women’s health care physicians.
An additional resource
To enhance your understanding of apps and how to evaluate them, Dr. Katherine Chen’s App Review series in
In appreciation
The members of this Task Force want to thank the Editorial Board and staff of
- Women’s Preventive Services Initiative website. Recommendations for well-woman care: a well-woman chart. https:// www.womenspreventivehealth.org/wellwomanchart/. Accessed June 11, 2021.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Evolutionary changes in ObGyn
Preventive medicine guidelines have evolved to reflect enhanced cervical cancer screening tests, longer-acting contraceptive options, and better data on the lack of utility of the annual pelvic exam that has changed the focus of the annual visit for both physicians and patients.1 These changes allow us to pivot and leverage the trust we build with our patients to make meaningful impacts in preventing chronic disease, improving prepregnancy health, reducing maternal mortality and morbidity, and improving the quality and longevity of our patients’ lives. New guidelines, coupled with the knowledge of the leading causes of morbidity for women, provide the chance to incorporate areas of screening and intervention that, while we are capable of addressing, we traditionally have not done so for various reasons.
The ACOG Presidential Task Force identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. ObGyns are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas. To make this daunting task more manageable, the Task Force not only published webinars to address the clinical knowledge pertaining to these areas of health but also specifically looked at how to use technology to aid obstetrician-gynecologists in addressing them with patients.
Making use of technology in clinical practice
Technology is emerging as an influential player in health care. Major corporations, such as Amazon, Google, Apple, and Facebook, are making headlines in health care as they consider strategies (moves) to revolutionize technology and, in turn, patient visits like we have never seen before. Examples include incorporating artificial intelligence in a patient’s care and allowing better access for primary care.
The changes that we will see over the next 10 years, influenced by industry, will be more than those seen in our lifetime. To prepare for these changes, we need to incorporate technology into our daily practice. This encompasses much more than just the electronic medical record. Consequently, the Task Force intentionally looked at mobile medical apps to aid physicians in addressing the 5 specific areas of preventive health identified.
While a small step compared with what is to come, apps are a great resource to leverage in making this transition. However, with hundreds of thousands of medical apps available in app stores and the constant updates and iterations of each, it would be impossible to recommend any single app. There is much value in having a framework to use to efficiently measure the benefit of an app that you or your patient comes across in clinical practice. The objective of this series was to provide clinicians with an effective tool to evaluate a medical app that could be used, for example, when addressing obesity or optimizing prepregnancy health.
Continue to: The recommended rubric for evaluating apps...
The recommended rubric for evaluating apps
To evaluate mobile drug information apps, the Task Force members recommend a user-friendly, convenient rubric developed by the American Society of Health-System Pharmacists (ASHP) (see page 35). The rubric can help obstetrician-gynecologists evaluate and compare the value of various medical apps that specifically address obesity, diabetes mellitus, cardiovascular disease, improving maternal morbidity with enhanced preconception counseling, and cancer risk assessment.
The authors of this Task Force series have attempted to highlight the key features of an app as it pertains to a particular area of focus. It is important to keep in mind the primary user and the goal when choosing or recommending an app for practice or for patient use. The ASHP’s rubric is a tool meant to aid clinicians in evaluating medical apps, but it is ultimately the user’s decision to determine if the deficiencies of an app should deter its use. Although all the criteria are relevant and important, as medical experts it is incumbent on us to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice.
While integrating the use of medical apps into clinical practice will be novel for some, for others, junior Fellows in particular, it has become part of their practice and education. Dr. Eva Hoffmann, Chief Resident in the NYU Langone Health System, offers this perspective: “As medical trainees we use mobile apps to enhance our patient interaction and guide high-quality, continuous care. In today’s modern technological world, apps help keep us up to date with the ever-changing guidelines in pregnancy and routine gynecologic care as well as communicate directly and discreetly with a patient whenever the need arises. The most significant apps provide guidance on abnormal Pap results, indicated deliveries prior to 39 weeks, and the ability to respond to obstetrical emergencies. They also allow for quick society-endorsed references in seconds. Apps have changed the way that we practice by providing evidence-based medicine literally at our fingertips—in a shareable and communicable way—making the practice of medicine even more efficient and effective.”
Opportunity to reaffirm expertise
Dr. Chalas’ initiative was meant to shed light on the opportunity obstetrician-gynecologists have to reassert themselves as women’s health experts, to consider redefining their practice by incorporating new preventive guidelines, and to leverage medical apps for achieving better health outcomes for women across their lifetime. We hope that by opening a dialogue about how ubiquitous medical apps are (for both physicians and patients) in today’s health arena, how many apps are inaccurate and/or misused, and how a simple rubric can be used to assess an app’s value, you are inspired and feel more comfortable to incorporate medical apps into your practice.
Health care will continually undergo advancements, and as a specialty we must evolve to address women’s needs. Obstetrician-gynecologists are well suited to contribute significantly to the well-being of women and mothers. We can leverage technology-based apps to help us redefine our roles and priorities at the patient’s annual visit. We can reaffirm ourselves as the leading women’s health care physicians.
An additional resource
To enhance your understanding of apps and how to evaluate them, Dr. Katherine Chen’s App Review series in
In appreciation
The members of this Task Force want to thank the Editorial Board and staff of
Evolutionary changes in ObGyn
Preventive medicine guidelines have evolved to reflect enhanced cervical cancer screening tests, longer-acting contraceptive options, and better data on the lack of utility of the annual pelvic exam that has changed the focus of the annual visit for both physicians and patients.1 These changes allow us to pivot and leverage the trust we build with our patients to make meaningful impacts in preventing chronic disease, improving prepregnancy health, reducing maternal mortality and morbidity, and improving the quality and longevity of our patients’ lives. New guidelines, coupled with the knowledge of the leading causes of morbidity for women, provide the chance to incorporate areas of screening and intervention that, while we are capable of addressing, we traditionally have not done so for various reasons.
The ACOG Presidential Task Force identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. ObGyns are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas. To make this daunting task more manageable, the Task Force not only published webinars to address the clinical knowledge pertaining to these areas of health but also specifically looked at how to use technology to aid obstetrician-gynecologists in addressing them with patients.
Making use of technology in clinical practice
Technology is emerging as an influential player in health care. Major corporations, such as Amazon, Google, Apple, and Facebook, are making headlines in health care as they consider strategies (moves) to revolutionize technology and, in turn, patient visits like we have never seen before. Examples include incorporating artificial intelligence in a patient’s care and allowing better access for primary care.
The changes that we will see over the next 10 years, influenced by industry, will be more than those seen in our lifetime. To prepare for these changes, we need to incorporate technology into our daily practice. This encompasses much more than just the electronic medical record. Consequently, the Task Force intentionally looked at mobile medical apps to aid physicians in addressing the 5 specific areas of preventive health identified.
While a small step compared with what is to come, apps are a great resource to leverage in making this transition. However, with hundreds of thousands of medical apps available in app stores and the constant updates and iterations of each, it would be impossible to recommend any single app. There is much value in having a framework to use to efficiently measure the benefit of an app that you or your patient comes across in clinical practice. The objective of this series was to provide clinicians with an effective tool to evaluate a medical app that could be used, for example, when addressing obesity or optimizing prepregnancy health.
Continue to: The recommended rubric for evaluating apps...
The recommended rubric for evaluating apps
To evaluate mobile drug information apps, the Task Force members recommend a user-friendly, convenient rubric developed by the American Society of Health-System Pharmacists (ASHP) (see page 35). The rubric can help obstetrician-gynecologists evaluate and compare the value of various medical apps that specifically address obesity, diabetes mellitus, cardiovascular disease, improving maternal morbidity with enhanced preconception counseling, and cancer risk assessment.
The authors of this Task Force series have attempted to highlight the key features of an app as it pertains to a particular area of focus. It is important to keep in mind the primary user and the goal when choosing or recommending an app for practice or for patient use. The ASHP’s rubric is a tool meant to aid clinicians in evaluating medical apps, but it is ultimately the user’s decision to determine if the deficiencies of an app should deter its use. Although all the criteria are relevant and important, as medical experts it is incumbent on us to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice.
While integrating the use of medical apps into clinical practice will be novel for some, for others, junior Fellows in particular, it has become part of their practice and education. Dr. Eva Hoffmann, Chief Resident in the NYU Langone Health System, offers this perspective: “As medical trainees we use mobile apps to enhance our patient interaction and guide high-quality, continuous care. In today’s modern technological world, apps help keep us up to date with the ever-changing guidelines in pregnancy and routine gynecologic care as well as communicate directly and discreetly with a patient whenever the need arises. The most significant apps provide guidance on abnormal Pap results, indicated deliveries prior to 39 weeks, and the ability to respond to obstetrical emergencies. They also allow for quick society-endorsed references in seconds. Apps have changed the way that we practice by providing evidence-based medicine literally at our fingertips—in a shareable and communicable way—making the practice of medicine even more efficient and effective.”
Opportunity to reaffirm expertise
Dr. Chalas’ initiative was meant to shed light on the opportunity obstetrician-gynecologists have to reassert themselves as women’s health experts, to consider redefining their practice by incorporating new preventive guidelines, and to leverage medical apps for achieving better health outcomes for women across their lifetime. We hope that by opening a dialogue about how ubiquitous medical apps are (for both physicians and patients) in today’s health arena, how many apps are inaccurate and/or misused, and how a simple rubric can be used to assess an app’s value, you are inspired and feel more comfortable to incorporate medical apps into your practice.
Health care will continually undergo advancements, and as a specialty we must evolve to address women’s needs. Obstetrician-gynecologists are well suited to contribute significantly to the well-being of women and mothers. We can leverage technology-based apps to help us redefine our roles and priorities at the patient’s annual visit. We can reaffirm ourselves as the leading women’s health care physicians.
An additional resource
To enhance your understanding of apps and how to evaluate them, Dr. Katherine Chen’s App Review series in
In appreciation
The members of this Task Force want to thank the Editorial Board and staff of
- Women’s Preventive Services Initiative website. Recommendations for well-woman care: a well-woman chart. https:// www.womenspreventivehealth.org/wellwomanchart/. Accessed June 11, 2021.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
- Women’s Preventive Services Initiative website. Recommendations for well-woman care: a well-woman chart. https:// www.womenspreventivehealth.org/wellwomanchart/. Accessed June 11, 2021.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
What’s my number? Do I really need $10 million to retire from my medical practice?
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
Greater travel distance reduces rates of abortion
Travel distance is an important determinant of access to abortion care in the United States, new findings show.
Increases in median travel distance to the nearest abortion care facility were associated with significant reductions in median abortion rate.
The abortion rate was 21.1 per 1,000 female residents of reproductive age among those who lived less than 5 miles from a facility, but that number dropped to 3.9/1,000 for those living 120 miles or further away.
Overall, in a model of 3,107 U.S. counties that included 62.5 million women of reproductive age, there were an estimated 696,760 abortions (at a mean rate of 11.1/1,000). The authors estimate that if abortion services were integrated into primary care, an additional 18,190 abortions would be performed (mean rate, 11.4/1,000).
Similarly, if telemedicine became widely available in this setting, this would allow approximately 70,920 abortions (mean rate 12.3/1,000). The study was published online in JAMA Open Network.
Reducing travel distances to abortion facilities would increase access, but additional clinics and providers would be needed to meet the demand. But as the population density of many counties with poor access is low, innovative strategies are also needed.
Integrating abortion into primary care or making medication abortion care available by telemedicine may decrease this unmet need, and lead author Kirsten Thompson, MPH, noted that there is growing evidence that both solutions are quite feasible to implement.
“A study published in 2018 has led primary care providers to adopt the same regimen for miscarriage care, showing that they are interested and capable, despite the barriers posed by the mifepristone [Risk Evaluation and Mitigation Strategy] program for these patients,” said Ms. Thompson, who is program and communications director, Bixby Center for Global Reproductive Health, University of California, San Francisco. “Medical education programs designed specifically for primary care providers have trained family medicine and other clinicians in abortion care for over a decade.”
As for telemedicine, Ms. Thompson explained that, during the pandemic, a preliminary injunction in a federal court case and then the Food and Drug Administration suspended enforcement of the in-person requirements of the mifepristone REMS. “In states that allow medical abortion care by telemedicine, providers have been able to offer remote care when medically appropriate, including mailing medical abortion pills to patients at home,” she said. “Researchers have already published evidence on the safety of and patient satisfaction with this approach.”
However, there are two main barriers to the widespread adoption of medical abortion by telemedicine in the United States. “One is the potentially temporary nature of the FDA’s enforcement discretion and second, are the 19 states with laws that ban it, singling out medical abortion as somehow different from other forms of care by telemedicine,” she said.
Study details
About one in four women in the United States will terminate a pregnancy during their lifetime, but the issue is highly contentious and many states have implemented policies that restrict access to abortion care. The authors pointed out that studies have documented clinic closures and women being unable to obtain abortion care, with low-income women and non-White women being disproportionately affected. Increased travel to a provider has also been associated with delays in care as well as increased costs and stress.
Prior research has shown that the further a woman lives from a facility, the less likely she is to obtain abortion care. In this study, Ms. Thompson and colleagues examined the association between travel distance to the nearest abortion care facility and the abortion rate, and then modeled the effect of reduced travel distance on rates.
They first conducted a cross-sectional geographic analysis using the American Community Survey and the U.S. Census to calculate county-level abortion rates per 1,000 women aged between 15 and 44 years. The 2015 data covered 1,948 counties in 27 states.
Abortion rates were then estimated for 3,107 counties in 48 states and the effect of different travel distance scenarios on the abortion rate was also estimated by multivariable model. Data were collected from April 2018 to October 2019.
There were 37.3 million women of reproductive age residing in the 27 states, and a total of 428,720 reported abortions (mean rate, 11.5/1,000; median rate, 9.9/1,000 women).
When looking at all 48 states, the population-weighted mean travel distance to the nearest facility was 25.6 miles, with a median travel distance of 8.2 miles.
A multivariable model showed that a greater travel distance was associated with lower abortion rates. When compared with traveling less than 5 miles, the abortion rate declined by 0.05/1,000 for women traveling between 5 to less than 15 miles for care, 0.22 for those traveling 15 to less than 30 miles, 0.34 for 30 to less than 60 miles, 0.43 for 60 to less than 120 miles, and 0.73 for those traveling 120 miles or more.
They estimated that, if all travel was under 30 miles, there would be a 2.6% increase or 18,190 additional abortions. A simulation also showed that there would be a 10.2% increase (70,920 additional abortions) using medication via telemedicine.
Solutions are feasible
Approached for an independent comment, Sarah W. Prager, MD, MAS, professor of obstetrics and gynecology and division chief, complex family planning, at the University of Washington, Seattle, agreed that the solutions proposed by the authors were feasible.
“More than a third of abortions that are eligible are now done with medication,” she said, “And 89% of abortions are done in the first trimester.”
What this means is that early first-trimester abortions can conceivably be performed in the primary care setting. “Any primary care clinician – whether it’s a family practice or internal medicine physician, or nurse practitioner or nurse midwife – can all be trained to do aspiration or prescribe medication in the first trimester,” said Dr. Prager. “So it could easily be integrated into primary care settings if there was motivation for that to happen.”
However, she emphasized that more is involved than just training the provider. “The whole clinic has to buy into it,” Dr. Prager explained. “The nurses have to be willing to assist, you need the medical assistants, the scheduler or person who works the front desk – the whole clinic system has to buy into it and that’s where it becomes more challenging.”
The individual provider may be willing, but the system may still not be allowing that to happen. “This is also where telemedicine can come in, where the medication can be mailed so it can circumvent the problem to a certain extent,” Dr. Prager added. “You don’t have to have the infrastructure in the same way.”
But many states already have laws in place to make that illegal, especially for abortion care even if they allow it for similar types of care.
Another expert also weighed in and agreed that these two solutions can potentially be implemented.
“The concept of decreased rates of abortion associated with greater distances traveled is not new, but what is unique to this manuscript is the estimations that the authors conducted in understanding the impact of expanding access to abortion among primary care and telehealth providers,” said Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology, University of California, Davis.
“The study provides convincing evidence regarding the need to strengthen infrastructures that support expansion of these services in primary care settings, among physicians and advanced care practitioners,” she said. “Training to provide medical abortion and first-trimester surgical abortion is simple. Many primary care providers are already doing gynecologic procedures – IUD insertions, colposcopies, endometrial biopsies.”
Thus, she noted, adding abortion care “to their toolkit isn’t too far of a stretch.”
As for telemedicine, Dr. Cansino pointed out how the COVID-19 pandemic has also expanded what both patients and providers think are safe options for providing and receiving good care. “Consultations through telemedicine coupled with access to medications for medical abortion through local pharmacies or express mail is definitely safe and feasible.”
The study was supported by the William and Flora Hewlett Foundation and by an anonymous foundation for general operating support (Ms Thompson). Ms. Thompson reported receiving personal fees from GenBioPro outside the submitted work. Dr. Cansino and Dr. Prager have no disclosures.
Travel distance is an important determinant of access to abortion care in the United States, new findings show.
Increases in median travel distance to the nearest abortion care facility were associated with significant reductions in median abortion rate.
The abortion rate was 21.1 per 1,000 female residents of reproductive age among those who lived less than 5 miles from a facility, but that number dropped to 3.9/1,000 for those living 120 miles or further away.
Overall, in a model of 3,107 U.S. counties that included 62.5 million women of reproductive age, there were an estimated 696,760 abortions (at a mean rate of 11.1/1,000). The authors estimate that if abortion services were integrated into primary care, an additional 18,190 abortions would be performed (mean rate, 11.4/1,000).
Similarly, if telemedicine became widely available in this setting, this would allow approximately 70,920 abortions (mean rate 12.3/1,000). The study was published online in JAMA Open Network.
Reducing travel distances to abortion facilities would increase access, but additional clinics and providers would be needed to meet the demand. But as the population density of many counties with poor access is low, innovative strategies are also needed.
Integrating abortion into primary care or making medication abortion care available by telemedicine may decrease this unmet need, and lead author Kirsten Thompson, MPH, noted that there is growing evidence that both solutions are quite feasible to implement.
“A study published in 2018 has led primary care providers to adopt the same regimen for miscarriage care, showing that they are interested and capable, despite the barriers posed by the mifepristone [Risk Evaluation and Mitigation Strategy] program for these patients,” said Ms. Thompson, who is program and communications director, Bixby Center for Global Reproductive Health, University of California, San Francisco. “Medical education programs designed specifically for primary care providers have trained family medicine and other clinicians in abortion care for over a decade.”
As for telemedicine, Ms. Thompson explained that, during the pandemic, a preliminary injunction in a federal court case and then the Food and Drug Administration suspended enforcement of the in-person requirements of the mifepristone REMS. “In states that allow medical abortion care by telemedicine, providers have been able to offer remote care when medically appropriate, including mailing medical abortion pills to patients at home,” she said. “Researchers have already published evidence on the safety of and patient satisfaction with this approach.”
However, there are two main barriers to the widespread adoption of medical abortion by telemedicine in the United States. “One is the potentially temporary nature of the FDA’s enforcement discretion and second, are the 19 states with laws that ban it, singling out medical abortion as somehow different from other forms of care by telemedicine,” she said.
Study details
About one in four women in the United States will terminate a pregnancy during their lifetime, but the issue is highly contentious and many states have implemented policies that restrict access to abortion care. The authors pointed out that studies have documented clinic closures and women being unable to obtain abortion care, with low-income women and non-White women being disproportionately affected. Increased travel to a provider has also been associated with delays in care as well as increased costs and stress.
Prior research has shown that the further a woman lives from a facility, the less likely she is to obtain abortion care. In this study, Ms. Thompson and colleagues examined the association between travel distance to the nearest abortion care facility and the abortion rate, and then modeled the effect of reduced travel distance on rates.
They first conducted a cross-sectional geographic analysis using the American Community Survey and the U.S. Census to calculate county-level abortion rates per 1,000 women aged between 15 and 44 years. The 2015 data covered 1,948 counties in 27 states.
Abortion rates were then estimated for 3,107 counties in 48 states and the effect of different travel distance scenarios on the abortion rate was also estimated by multivariable model. Data were collected from April 2018 to October 2019.
There were 37.3 million women of reproductive age residing in the 27 states, and a total of 428,720 reported abortions (mean rate, 11.5/1,000; median rate, 9.9/1,000 women).
When looking at all 48 states, the population-weighted mean travel distance to the nearest facility was 25.6 miles, with a median travel distance of 8.2 miles.
A multivariable model showed that a greater travel distance was associated with lower abortion rates. When compared with traveling less than 5 miles, the abortion rate declined by 0.05/1,000 for women traveling between 5 to less than 15 miles for care, 0.22 for those traveling 15 to less than 30 miles, 0.34 for 30 to less than 60 miles, 0.43 for 60 to less than 120 miles, and 0.73 for those traveling 120 miles or more.
They estimated that, if all travel was under 30 miles, there would be a 2.6% increase or 18,190 additional abortions. A simulation also showed that there would be a 10.2% increase (70,920 additional abortions) using medication via telemedicine.
Solutions are feasible
Approached for an independent comment, Sarah W. Prager, MD, MAS, professor of obstetrics and gynecology and division chief, complex family planning, at the University of Washington, Seattle, agreed that the solutions proposed by the authors were feasible.
“More than a third of abortions that are eligible are now done with medication,” she said, “And 89% of abortions are done in the first trimester.”
What this means is that early first-trimester abortions can conceivably be performed in the primary care setting. “Any primary care clinician – whether it’s a family practice or internal medicine physician, or nurse practitioner or nurse midwife – can all be trained to do aspiration or prescribe medication in the first trimester,” said Dr. Prager. “So it could easily be integrated into primary care settings if there was motivation for that to happen.”
However, she emphasized that more is involved than just training the provider. “The whole clinic has to buy into it,” Dr. Prager explained. “The nurses have to be willing to assist, you need the medical assistants, the scheduler or person who works the front desk – the whole clinic system has to buy into it and that’s where it becomes more challenging.”
The individual provider may be willing, but the system may still not be allowing that to happen. “This is also where telemedicine can come in, where the medication can be mailed so it can circumvent the problem to a certain extent,” Dr. Prager added. “You don’t have to have the infrastructure in the same way.”
But many states already have laws in place to make that illegal, especially for abortion care even if they allow it for similar types of care.
Another expert also weighed in and agreed that these two solutions can potentially be implemented.
“The concept of decreased rates of abortion associated with greater distances traveled is not new, but what is unique to this manuscript is the estimations that the authors conducted in understanding the impact of expanding access to abortion among primary care and telehealth providers,” said Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology, University of California, Davis.
“The study provides convincing evidence regarding the need to strengthen infrastructures that support expansion of these services in primary care settings, among physicians and advanced care practitioners,” she said. “Training to provide medical abortion and first-trimester surgical abortion is simple. Many primary care providers are already doing gynecologic procedures – IUD insertions, colposcopies, endometrial biopsies.”
Thus, she noted, adding abortion care “to their toolkit isn’t too far of a stretch.”
As for telemedicine, Dr. Cansino pointed out how the COVID-19 pandemic has also expanded what both patients and providers think are safe options for providing and receiving good care. “Consultations through telemedicine coupled with access to medications for medical abortion through local pharmacies or express mail is definitely safe and feasible.”
The study was supported by the William and Flora Hewlett Foundation and by an anonymous foundation for general operating support (Ms Thompson). Ms. Thompson reported receiving personal fees from GenBioPro outside the submitted work. Dr. Cansino and Dr. Prager have no disclosures.
Travel distance is an important determinant of access to abortion care in the United States, new findings show.
Increases in median travel distance to the nearest abortion care facility were associated with significant reductions in median abortion rate.
The abortion rate was 21.1 per 1,000 female residents of reproductive age among those who lived less than 5 miles from a facility, but that number dropped to 3.9/1,000 for those living 120 miles or further away.
Overall, in a model of 3,107 U.S. counties that included 62.5 million women of reproductive age, there were an estimated 696,760 abortions (at a mean rate of 11.1/1,000). The authors estimate that if abortion services were integrated into primary care, an additional 18,190 abortions would be performed (mean rate, 11.4/1,000).
Similarly, if telemedicine became widely available in this setting, this would allow approximately 70,920 abortions (mean rate 12.3/1,000). The study was published online in JAMA Open Network.
Reducing travel distances to abortion facilities would increase access, but additional clinics and providers would be needed to meet the demand. But as the population density of many counties with poor access is low, innovative strategies are also needed.
Integrating abortion into primary care or making medication abortion care available by telemedicine may decrease this unmet need, and lead author Kirsten Thompson, MPH, noted that there is growing evidence that both solutions are quite feasible to implement.
“A study published in 2018 has led primary care providers to adopt the same regimen for miscarriage care, showing that they are interested and capable, despite the barriers posed by the mifepristone [Risk Evaluation and Mitigation Strategy] program for these patients,” said Ms. Thompson, who is program and communications director, Bixby Center for Global Reproductive Health, University of California, San Francisco. “Medical education programs designed specifically for primary care providers have trained family medicine and other clinicians in abortion care for over a decade.”
As for telemedicine, Ms. Thompson explained that, during the pandemic, a preliminary injunction in a federal court case and then the Food and Drug Administration suspended enforcement of the in-person requirements of the mifepristone REMS. “In states that allow medical abortion care by telemedicine, providers have been able to offer remote care when medically appropriate, including mailing medical abortion pills to patients at home,” she said. “Researchers have already published evidence on the safety of and patient satisfaction with this approach.”
However, there are two main barriers to the widespread adoption of medical abortion by telemedicine in the United States. “One is the potentially temporary nature of the FDA’s enforcement discretion and second, are the 19 states with laws that ban it, singling out medical abortion as somehow different from other forms of care by telemedicine,” she said.
Study details
About one in four women in the United States will terminate a pregnancy during their lifetime, but the issue is highly contentious and many states have implemented policies that restrict access to abortion care. The authors pointed out that studies have documented clinic closures and women being unable to obtain abortion care, with low-income women and non-White women being disproportionately affected. Increased travel to a provider has also been associated with delays in care as well as increased costs and stress.
Prior research has shown that the further a woman lives from a facility, the less likely she is to obtain abortion care. In this study, Ms. Thompson and colleagues examined the association between travel distance to the nearest abortion care facility and the abortion rate, and then modeled the effect of reduced travel distance on rates.
They first conducted a cross-sectional geographic analysis using the American Community Survey and the U.S. Census to calculate county-level abortion rates per 1,000 women aged between 15 and 44 years. The 2015 data covered 1,948 counties in 27 states.
Abortion rates were then estimated for 3,107 counties in 48 states and the effect of different travel distance scenarios on the abortion rate was also estimated by multivariable model. Data were collected from April 2018 to October 2019.
There were 37.3 million women of reproductive age residing in the 27 states, and a total of 428,720 reported abortions (mean rate, 11.5/1,000; median rate, 9.9/1,000 women).
When looking at all 48 states, the population-weighted mean travel distance to the nearest facility was 25.6 miles, with a median travel distance of 8.2 miles.
A multivariable model showed that a greater travel distance was associated with lower abortion rates. When compared with traveling less than 5 miles, the abortion rate declined by 0.05/1,000 for women traveling between 5 to less than 15 miles for care, 0.22 for those traveling 15 to less than 30 miles, 0.34 for 30 to less than 60 miles, 0.43 for 60 to less than 120 miles, and 0.73 for those traveling 120 miles or more.
They estimated that, if all travel was under 30 miles, there would be a 2.6% increase or 18,190 additional abortions. A simulation also showed that there would be a 10.2% increase (70,920 additional abortions) using medication via telemedicine.
Solutions are feasible
Approached for an independent comment, Sarah W. Prager, MD, MAS, professor of obstetrics and gynecology and division chief, complex family planning, at the University of Washington, Seattle, agreed that the solutions proposed by the authors were feasible.
“More than a third of abortions that are eligible are now done with medication,” she said, “And 89% of abortions are done in the first trimester.”
What this means is that early first-trimester abortions can conceivably be performed in the primary care setting. “Any primary care clinician – whether it’s a family practice or internal medicine physician, or nurse practitioner or nurse midwife – can all be trained to do aspiration or prescribe medication in the first trimester,” said Dr. Prager. “So it could easily be integrated into primary care settings if there was motivation for that to happen.”
However, she emphasized that more is involved than just training the provider. “The whole clinic has to buy into it,” Dr. Prager explained. “The nurses have to be willing to assist, you need the medical assistants, the scheduler or person who works the front desk – the whole clinic system has to buy into it and that’s where it becomes more challenging.”
The individual provider may be willing, but the system may still not be allowing that to happen. “This is also where telemedicine can come in, where the medication can be mailed so it can circumvent the problem to a certain extent,” Dr. Prager added. “You don’t have to have the infrastructure in the same way.”
But many states already have laws in place to make that illegal, especially for abortion care even if they allow it for similar types of care.
Another expert also weighed in and agreed that these two solutions can potentially be implemented.
“The concept of decreased rates of abortion associated with greater distances traveled is not new, but what is unique to this manuscript is the estimations that the authors conducted in understanding the impact of expanding access to abortion among primary care and telehealth providers,” said Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology, University of California, Davis.
“The study provides convincing evidence regarding the need to strengthen infrastructures that support expansion of these services in primary care settings, among physicians and advanced care practitioners,” she said. “Training to provide medical abortion and first-trimester surgical abortion is simple. Many primary care providers are already doing gynecologic procedures – IUD insertions, colposcopies, endometrial biopsies.”
Thus, she noted, adding abortion care “to their toolkit isn’t too far of a stretch.”
As for telemedicine, Dr. Cansino pointed out how the COVID-19 pandemic has also expanded what both patients and providers think are safe options for providing and receiving good care. “Consultations through telemedicine coupled with access to medications for medical abortion through local pharmacies or express mail is definitely safe and feasible.”
The study was supported by the William and Flora Hewlett Foundation and by an anonymous foundation for general operating support (Ms Thompson). Ms. Thompson reported receiving personal fees from GenBioPro outside the submitted work. Dr. Cansino and Dr. Prager have no disclosures.
FROM JAMA NETWORK OPEN
Do you plan to incorporate dynamic ultrasonography (use of the vaginal probe to help examine a patient, for pelvic pain for instance) into your practice?
[polldaddy:10873738]
[polldaddy:10873738]
[polldaddy:10873738]