User login
New treatments aim to tame vitiligo
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
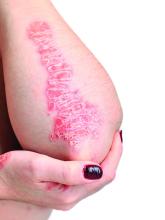
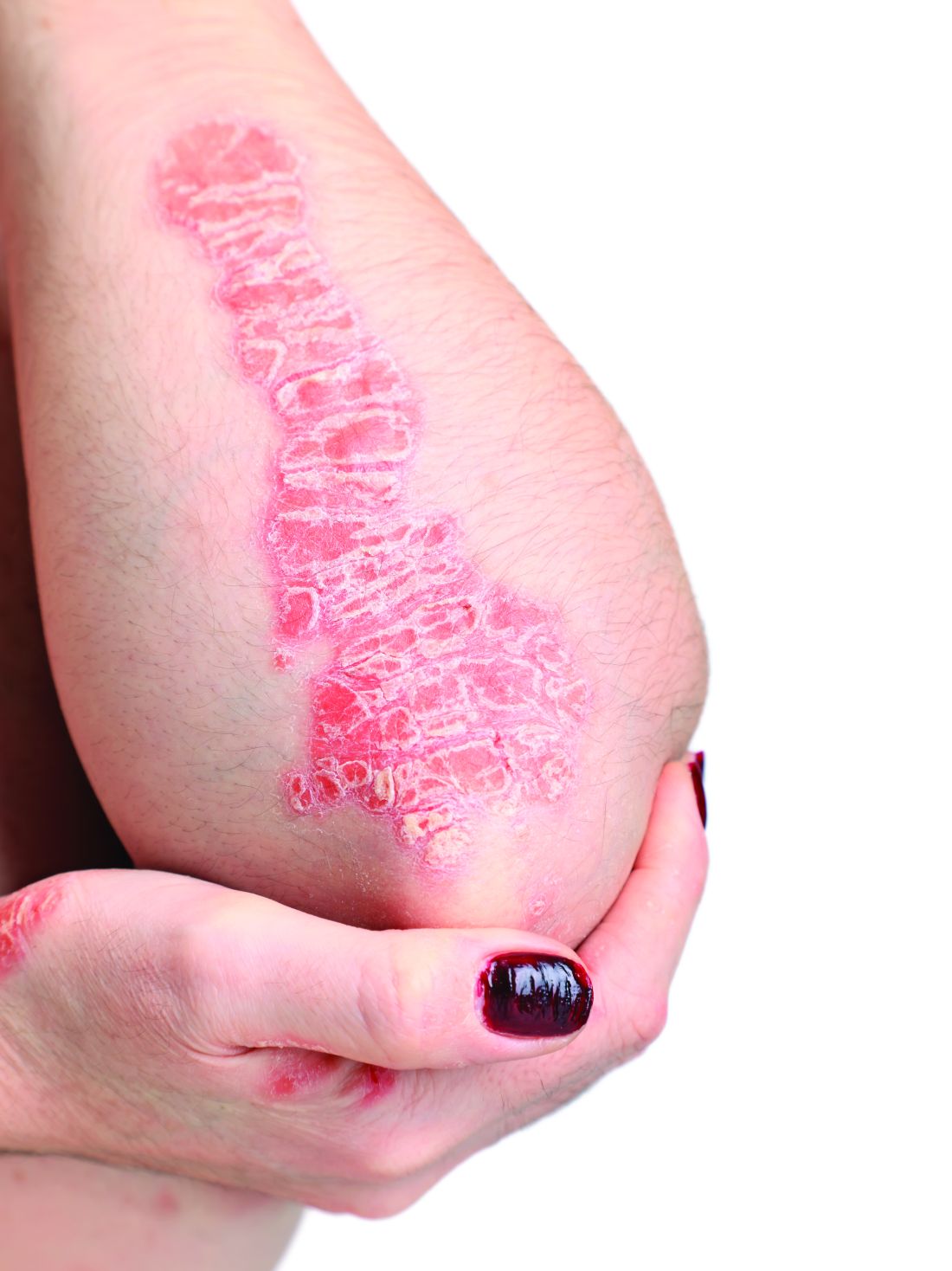
Topical psoriasis treatments
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Updated materials and mentoring can boost diversity in dermatology
LAS VEGAS – in the specialty.
The growing ethnic minority population in the United States “underscores the need for medical education to ensure dermatologists are prepared to provide quality care for patients of diverse racial and ethnic backgrounds,” said Dr. Taylor, the Bernett L. Johnson Jr., MD, Professor, and vice chair for diversity, equity, and inclusion in the department of dermatology at the University of Pennsylvania.
Improving education includes diversifying resource material, she said. A recent study in the Journal of the American Academy of Dermatology showed the representation of skin tones on Google searches for skin conditions was mostly light skin (91.7%), although non-Hispanic Whites account for less than two-thirds (approximately 60%) of the U.S. population, she said. Many people with darker skin tones “are not finding people who look like themselves” when they search skin conditions online, she noted.
The lack of diversity in images occurs not only on Google, “but in our textbooks, which are the foundational resources for our students,” said Nada M. Elbuluk, MD, founder and director of the Skin of Color and Pigmentary Disorders Program at the University of Southern California, Los Angeles. She also established the Dermatology Diversity and Inclusion Program at USC.
The underrepresentation of teaching images, combined with the lack of data on epidemiology and treatment, can translate to poorer quality of care for skin of color patients and contribute to more misdiagnoses in these populations, Dr. Elbuluk emphasized.
Cultural competency and workforce diversity are ongoing issues in dermatology, added Valerie D. Callender, MD, professor of dermatology at Howard University, Washington, and medical director of the Callender Dermatology & Cosmetic Center in Glenn Dale, Md.
“We know that patients of color seek physicians of color,” she said. “We need to target our residents’ interest in dermatology,” and all physicians need to be comfortable with treating patients of all races, she added.
Although more than 13% of Americans are Black, only 3% of dermatologists in the United States are Black, Dr. Callender noted. Similarly, 4.2% of dermatologists in the United States are Hispanic or Latino, but these groups make up more than 18% of the general U.S. population, according to a recent study, she said.
Cheryl M. Burgess, MD, founder and medical director of the Center for Dermatology and Dermatologic Surgery in Washington, presented a roadmap of strategies for improving diversity in dermatology, starting with increasing STEM education at the high school and college levels among all populations and increasing the pipeline of underrepresented students to medical schools.
Then, faculty should work to increase interest in dermatology among underrepresented medical students and increase the numbers of underrepresented medical students in dermatology residency programs, said Dr. Burgess, assistant clinical professor of dermatology at Georgetown University and George Washington University, Washington.
“The more diversity we have in our specialty, the more we learn from each other,” and increased diversity can promote new research questions, said Andrew F. Alexis, MD, vice chair for diversity and inclusion in the department of dermatology and professor of clinical dermatology at Weill Cornell Medicine, New York.
Increasing the diversity of populations in clinical trials is another important strategy to improve diversity in dermatology, he emphasized.
Mentoring is an excellent way to help underrepresented students develop and pursue a career in dermatology, the panelists agreed. Time is precious for everyone, so don’t hesitate to use Zoom and other technology to help connect with mentees, Dr. Burgess advised.
Dr. Taylor added that mentoring doesn’t have to be a huge time commitment, it can be as simple as volunteering once a year at a school career forum. “It is so gratifying to have these young people looking up to you,” she said.
The panelists disclosed relationships with multiple companies, but none were relevant to this panel discussion. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in the specialty.
The growing ethnic minority population in the United States “underscores the need for medical education to ensure dermatologists are prepared to provide quality care for patients of diverse racial and ethnic backgrounds,” said Dr. Taylor, the Bernett L. Johnson Jr., MD, Professor, and vice chair for diversity, equity, and inclusion in the department of dermatology at the University of Pennsylvania.
Improving education includes diversifying resource material, she said. A recent study in the Journal of the American Academy of Dermatology showed the representation of skin tones on Google searches for skin conditions was mostly light skin (91.7%), although non-Hispanic Whites account for less than two-thirds (approximately 60%) of the U.S. population, she said. Many people with darker skin tones “are not finding people who look like themselves” when they search skin conditions online, she noted.
The lack of diversity in images occurs not only on Google, “but in our textbooks, which are the foundational resources for our students,” said Nada M. Elbuluk, MD, founder and director of the Skin of Color and Pigmentary Disorders Program at the University of Southern California, Los Angeles. She also established the Dermatology Diversity and Inclusion Program at USC.
The underrepresentation of teaching images, combined with the lack of data on epidemiology and treatment, can translate to poorer quality of care for skin of color patients and contribute to more misdiagnoses in these populations, Dr. Elbuluk emphasized.
Cultural competency and workforce diversity are ongoing issues in dermatology, added Valerie D. Callender, MD, professor of dermatology at Howard University, Washington, and medical director of the Callender Dermatology & Cosmetic Center in Glenn Dale, Md.
“We know that patients of color seek physicians of color,” she said. “We need to target our residents’ interest in dermatology,” and all physicians need to be comfortable with treating patients of all races, she added.
Although more than 13% of Americans are Black, only 3% of dermatologists in the United States are Black, Dr. Callender noted. Similarly, 4.2% of dermatologists in the United States are Hispanic or Latino, but these groups make up more than 18% of the general U.S. population, according to a recent study, she said.
Cheryl M. Burgess, MD, founder and medical director of the Center for Dermatology and Dermatologic Surgery in Washington, presented a roadmap of strategies for improving diversity in dermatology, starting with increasing STEM education at the high school and college levels among all populations and increasing the pipeline of underrepresented students to medical schools.
Then, faculty should work to increase interest in dermatology among underrepresented medical students and increase the numbers of underrepresented medical students in dermatology residency programs, said Dr. Burgess, assistant clinical professor of dermatology at Georgetown University and George Washington University, Washington.
“The more diversity we have in our specialty, the more we learn from each other,” and increased diversity can promote new research questions, said Andrew F. Alexis, MD, vice chair for diversity and inclusion in the department of dermatology and professor of clinical dermatology at Weill Cornell Medicine, New York.
Increasing the diversity of populations in clinical trials is another important strategy to improve diversity in dermatology, he emphasized.
Mentoring is an excellent way to help underrepresented students develop and pursue a career in dermatology, the panelists agreed. Time is precious for everyone, so don’t hesitate to use Zoom and other technology to help connect with mentees, Dr. Burgess advised.
Dr. Taylor added that mentoring doesn’t have to be a huge time commitment, it can be as simple as volunteering once a year at a school career forum. “It is so gratifying to have these young people looking up to you,” she said.
The panelists disclosed relationships with multiple companies, but none were relevant to this panel discussion. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in the specialty.
The growing ethnic minority population in the United States “underscores the need for medical education to ensure dermatologists are prepared to provide quality care for patients of diverse racial and ethnic backgrounds,” said Dr. Taylor, the Bernett L. Johnson Jr., MD, Professor, and vice chair for diversity, equity, and inclusion in the department of dermatology at the University of Pennsylvania.
Improving education includes diversifying resource material, she said. A recent study in the Journal of the American Academy of Dermatology showed the representation of skin tones on Google searches for skin conditions was mostly light skin (91.7%), although non-Hispanic Whites account for less than two-thirds (approximately 60%) of the U.S. population, she said. Many people with darker skin tones “are not finding people who look like themselves” when they search skin conditions online, she noted.
The lack of diversity in images occurs not only on Google, “but in our textbooks, which are the foundational resources for our students,” said Nada M. Elbuluk, MD, founder and director of the Skin of Color and Pigmentary Disorders Program at the University of Southern California, Los Angeles. She also established the Dermatology Diversity and Inclusion Program at USC.
The underrepresentation of teaching images, combined with the lack of data on epidemiology and treatment, can translate to poorer quality of care for skin of color patients and contribute to more misdiagnoses in these populations, Dr. Elbuluk emphasized.
Cultural competency and workforce diversity are ongoing issues in dermatology, added Valerie D. Callender, MD, professor of dermatology at Howard University, Washington, and medical director of the Callender Dermatology & Cosmetic Center in Glenn Dale, Md.
“We know that patients of color seek physicians of color,” she said. “We need to target our residents’ interest in dermatology,” and all physicians need to be comfortable with treating patients of all races, she added.
Although more than 13% of Americans are Black, only 3% of dermatologists in the United States are Black, Dr. Callender noted. Similarly, 4.2% of dermatologists in the United States are Hispanic or Latino, but these groups make up more than 18% of the general U.S. population, according to a recent study, she said.
Cheryl M. Burgess, MD, founder and medical director of the Center for Dermatology and Dermatologic Surgery in Washington, presented a roadmap of strategies for improving diversity in dermatology, starting with increasing STEM education at the high school and college levels among all populations and increasing the pipeline of underrepresented students to medical schools.
Then, faculty should work to increase interest in dermatology among underrepresented medical students and increase the numbers of underrepresented medical students in dermatology residency programs, said Dr. Burgess, assistant clinical professor of dermatology at Georgetown University and George Washington University, Washington.
“The more diversity we have in our specialty, the more we learn from each other,” and increased diversity can promote new research questions, said Andrew F. Alexis, MD, vice chair for diversity and inclusion in the department of dermatology and professor of clinical dermatology at Weill Cornell Medicine, New York.
Increasing the diversity of populations in clinical trials is another important strategy to improve diversity in dermatology, he emphasized.
Mentoring is an excellent way to help underrepresented students develop and pursue a career in dermatology, the panelists agreed. Time is precious for everyone, so don’t hesitate to use Zoom and other technology to help connect with mentees, Dr. Burgess advised.
Dr. Taylor added that mentoring doesn’t have to be a huge time commitment, it can be as simple as volunteering once a year at a school career forum. “It is so gratifying to have these young people looking up to you,” she said.
The panelists disclosed relationships with multiple companies, but none were relevant to this panel discussion. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
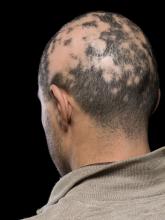
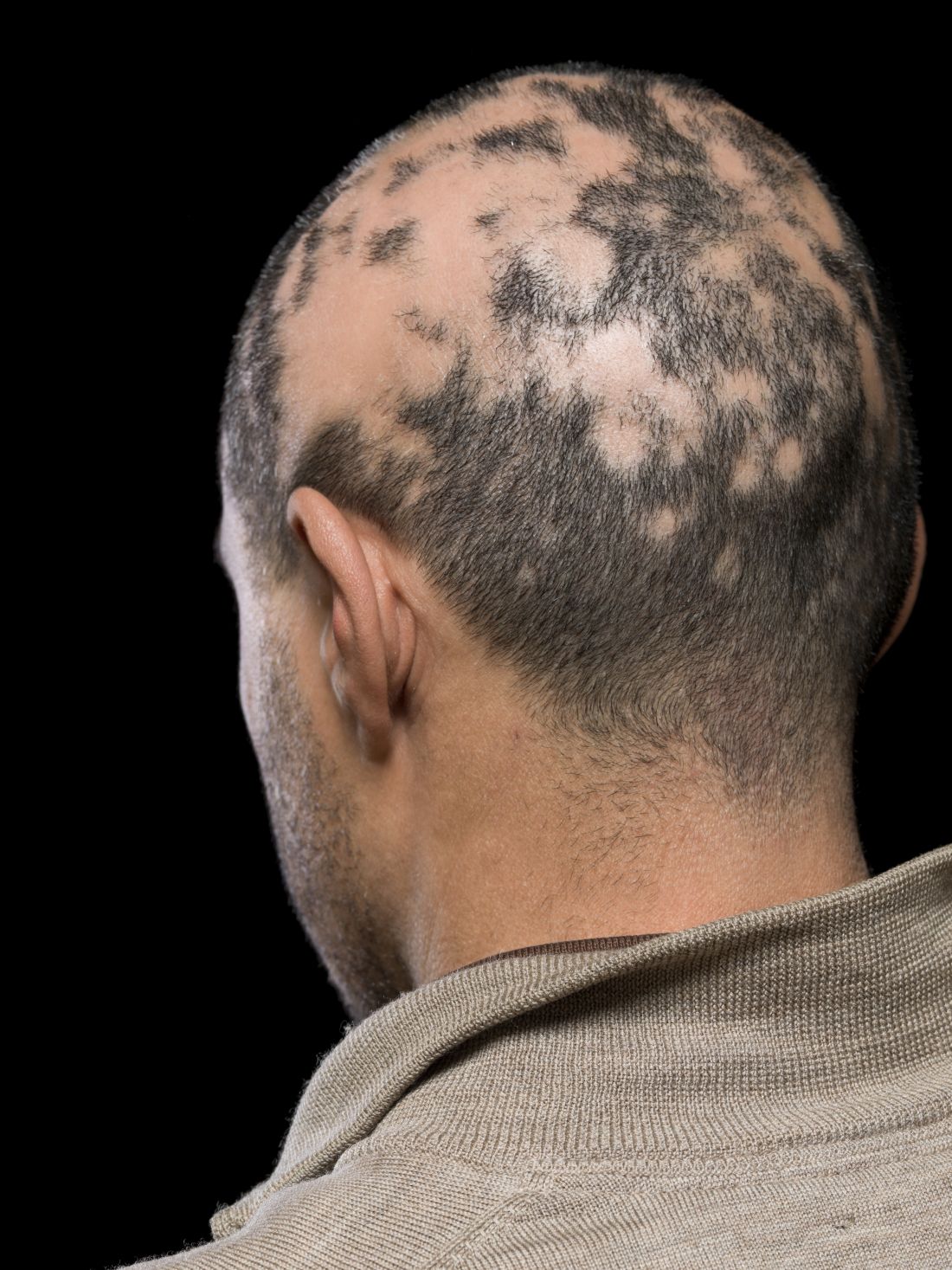
Current alopecia areata options include old and new therapies
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
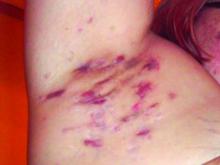
Consider quality of life, comorbidities in hidradenitis suppurativa
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Consider gaps in access and knowledge in diagnosis and treatment in skin of color
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Practical pearls guide treatment of psoriasis in tricky areas
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Education about OTC tools key for patients with acne and rosacea
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY