User login
Patients on methotrexate show T-cell response to Pfizer vaccine
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study spanning 2 decades offers insights into pediatric psoriasis trends
, while predictors of moderate to severe disease include morphology, non-White race, and culture-confirmed infection.
Those are among the key findings from a retrospective analysis of pediatric psoriasis patients who were seen at the University of California, San Francisco, over a 24-year period.
“Overall, our data support prior findings of age- and sex-based differences in location and morphology and presents new information demonstrating associations with severity,” presenting study author Carmel Aghdasi said during the annual meeting of the Society for Pediatric Dermatology. “We provide evidence of the increased use of systemic and biologic therapies over time, an important step in ensuring pediatric patients are adequately treated.”
To characterize the demographics, clinical features, comorbidities, and treatments, and to determine predictors of severity and changes in treatment patterns over 2 decades in a large cohort of pediatric psoriasis patients, Ms. Aghdasi, a 4th-year medical student at the University of California, San Francisco, and colleagues retrospectively evaluated the records of 754 pediatric patients up to 18 years of age who were seen at UCSF for psoriasis from 1997 to 2021. They collected demographic, clinical, familial, comorbidity, and treatment data and divided the cohort into two groups by date of last visit.
Group 1 consisted of 332 patients whose last visit was between 2001 and 2011, while the second group included 422 patients whose last visit was between 2012 and 2021. The researchers also divided the cohort into three age groups: infants (0-2 years of age), children (3-12 years of age), and adolescents (13-18 years of age).
Slightly more than half of the patients (55%) were female and 67% presented between ages 3 and 12. (Seventy-four patients were in the youngest category, 0-2 years, when they presented.) The average age of disease onset was 7 years, the average age at presentation to pediatric dermatology was 8.8 years, and 37% of the total cohort were overweight or obese. The top four comorbidities were being overweight or obese (37%), followed by atopic dermatitis (19%), psychiatric disease (7%), and arthritis (4%).
Plaque was the most common morphology (56%), while the most common sites of involvement were the head and neck (69%), extremities (61%), and trunk (44%). About half of the cohort (51%) had mild disease, 15% had culture-confirmed infections (9% had Streptococcal infections), and 66% of patients reported itch as a symptom.
The researchers observed that inverse psoriasis was significantly more common in infants and decreased with age. Anogenital involvement was more common in males and in those aged 0-2, while head and neck involvement was more common in females. Nail involvement was more common in childhood.
Topical therapy was the most common treatment overall and by far the most common among those in the 0-2 age category. “Overall, phototherapy was used in childhood and adolescents but almost never in infancy,” Ms. Aghdasi said. “Looking at changes in systemic treatment over time, conventional systemic use increased in infants and children and decreased in adolescents. Biologic use increased in all ages, most notably in children aged 3-12 years old.”
Multivariate regression analyses revealed that the following independent variables predicted moderate to severe psoriasis: adolescent age (adjusted odds ratio, 1.9; P = .03), guttate morphology (aOR, 2.2; P = .006), plaque and guttate morphology (aOR, 7.6; P less than .001), pustular or erythrodermic morphology (aOR, 5; P = .003), culture-confirmed infection (aOR, 2; P = .007), Black race (aOR, 3.3; P = .007), Asian race (aOR, 1.8; P = .04, and Hispanic race (aOR, 1.9; P = .03).
“Further analysis is needed to elucidate the influence of race on severity and of the clinical utility of infection as a marker of severity,” Ms. Aghdasi said. “Interestingly, we did not find that obesity was a marker of severity in our cohort.”
In an interview, senior study author Kelly M. Cordoro, MD, professor of dermatology and pediatrics at UCSF, noted that this finding conflicts with prior studies showing an association between obesity and severe psoriasis in children.
“Though methodologies and patient populations differ among studies, what is striking,” she said, is the percentage of overweight/obese patients (37%; defined as a body mass index ≥ 85th percentile) “in our 2-decade single institution dataset.” This “is nearly identical” to the percentage of patients with excess adiposity – 37.9% (also defined as BMI ≥ 85th percentile) – in an international cross-sectional study, which also identified an association between obesity (BMI ≥ 95th percentile) and psoriasis severity in children, she noted.
“What is clear is the strong association between obesity and childhood psoriasis, as multiple studies, including ours, confirm obesity as a major comorbidity of pediatric psoriasis,” Dr. Cordoro said. “Both conditions must be adequately managed to reduce the risk of adverse health outcomes for obese patients with psoriasis.”
The other study coauthors were Dana Feigenbaum, MD, and Alana Ju, MD. The work was supported by the UCSF Yearlong Inquiry Program. The researchers reported having no relevant financial disclosures.
, while predictors of moderate to severe disease include morphology, non-White race, and culture-confirmed infection.
Those are among the key findings from a retrospective analysis of pediatric psoriasis patients who were seen at the University of California, San Francisco, over a 24-year period.
“Overall, our data support prior findings of age- and sex-based differences in location and morphology and presents new information demonstrating associations with severity,” presenting study author Carmel Aghdasi said during the annual meeting of the Society for Pediatric Dermatology. “We provide evidence of the increased use of systemic and biologic therapies over time, an important step in ensuring pediatric patients are adequately treated.”
To characterize the demographics, clinical features, comorbidities, and treatments, and to determine predictors of severity and changes in treatment patterns over 2 decades in a large cohort of pediatric psoriasis patients, Ms. Aghdasi, a 4th-year medical student at the University of California, San Francisco, and colleagues retrospectively evaluated the records of 754 pediatric patients up to 18 years of age who were seen at UCSF for psoriasis from 1997 to 2021. They collected demographic, clinical, familial, comorbidity, and treatment data and divided the cohort into two groups by date of last visit.
Group 1 consisted of 332 patients whose last visit was between 2001 and 2011, while the second group included 422 patients whose last visit was between 2012 and 2021. The researchers also divided the cohort into three age groups: infants (0-2 years of age), children (3-12 years of age), and adolescents (13-18 years of age).
Slightly more than half of the patients (55%) were female and 67% presented between ages 3 and 12. (Seventy-four patients were in the youngest category, 0-2 years, when they presented.) The average age of disease onset was 7 years, the average age at presentation to pediatric dermatology was 8.8 years, and 37% of the total cohort were overweight or obese. The top four comorbidities were being overweight or obese (37%), followed by atopic dermatitis (19%), psychiatric disease (7%), and arthritis (4%).
Plaque was the most common morphology (56%), while the most common sites of involvement were the head and neck (69%), extremities (61%), and trunk (44%). About half of the cohort (51%) had mild disease, 15% had culture-confirmed infections (9% had Streptococcal infections), and 66% of patients reported itch as a symptom.
The researchers observed that inverse psoriasis was significantly more common in infants and decreased with age. Anogenital involvement was more common in males and in those aged 0-2, while head and neck involvement was more common in females. Nail involvement was more common in childhood.
Topical therapy was the most common treatment overall and by far the most common among those in the 0-2 age category. “Overall, phototherapy was used in childhood and adolescents but almost never in infancy,” Ms. Aghdasi said. “Looking at changes in systemic treatment over time, conventional systemic use increased in infants and children and decreased in adolescents. Biologic use increased in all ages, most notably in children aged 3-12 years old.”
Multivariate regression analyses revealed that the following independent variables predicted moderate to severe psoriasis: adolescent age (adjusted odds ratio, 1.9; P = .03), guttate morphology (aOR, 2.2; P = .006), plaque and guttate morphology (aOR, 7.6; P less than .001), pustular or erythrodermic morphology (aOR, 5; P = .003), culture-confirmed infection (aOR, 2; P = .007), Black race (aOR, 3.3; P = .007), Asian race (aOR, 1.8; P = .04, and Hispanic race (aOR, 1.9; P = .03).
“Further analysis is needed to elucidate the influence of race on severity and of the clinical utility of infection as a marker of severity,” Ms. Aghdasi said. “Interestingly, we did not find that obesity was a marker of severity in our cohort.”
In an interview, senior study author Kelly M. Cordoro, MD, professor of dermatology and pediatrics at UCSF, noted that this finding conflicts with prior studies showing an association between obesity and severe psoriasis in children.
“Though methodologies and patient populations differ among studies, what is striking,” she said, is the percentage of overweight/obese patients (37%; defined as a body mass index ≥ 85th percentile) “in our 2-decade single institution dataset.” This “is nearly identical” to the percentage of patients with excess adiposity – 37.9% (also defined as BMI ≥ 85th percentile) – in an international cross-sectional study, which also identified an association between obesity (BMI ≥ 95th percentile) and psoriasis severity in children, she noted.
“What is clear is the strong association between obesity and childhood psoriasis, as multiple studies, including ours, confirm obesity as a major comorbidity of pediatric psoriasis,” Dr. Cordoro said. “Both conditions must be adequately managed to reduce the risk of adverse health outcomes for obese patients with psoriasis.”
The other study coauthors were Dana Feigenbaum, MD, and Alana Ju, MD. The work was supported by the UCSF Yearlong Inquiry Program. The researchers reported having no relevant financial disclosures.
, while predictors of moderate to severe disease include morphology, non-White race, and culture-confirmed infection.
Those are among the key findings from a retrospective analysis of pediatric psoriasis patients who were seen at the University of California, San Francisco, over a 24-year period.
“Overall, our data support prior findings of age- and sex-based differences in location and morphology and presents new information demonstrating associations with severity,” presenting study author Carmel Aghdasi said during the annual meeting of the Society for Pediatric Dermatology. “We provide evidence of the increased use of systemic and biologic therapies over time, an important step in ensuring pediatric patients are adequately treated.”
To characterize the demographics, clinical features, comorbidities, and treatments, and to determine predictors of severity and changes in treatment patterns over 2 decades in a large cohort of pediatric psoriasis patients, Ms. Aghdasi, a 4th-year medical student at the University of California, San Francisco, and colleagues retrospectively evaluated the records of 754 pediatric patients up to 18 years of age who were seen at UCSF for psoriasis from 1997 to 2021. They collected demographic, clinical, familial, comorbidity, and treatment data and divided the cohort into two groups by date of last visit.
Group 1 consisted of 332 patients whose last visit was between 2001 and 2011, while the second group included 422 patients whose last visit was between 2012 and 2021. The researchers also divided the cohort into three age groups: infants (0-2 years of age), children (3-12 years of age), and adolescents (13-18 years of age).
Slightly more than half of the patients (55%) were female and 67% presented between ages 3 and 12. (Seventy-four patients were in the youngest category, 0-2 years, when they presented.) The average age of disease onset was 7 years, the average age at presentation to pediatric dermatology was 8.8 years, and 37% of the total cohort were overweight or obese. The top four comorbidities were being overweight or obese (37%), followed by atopic dermatitis (19%), psychiatric disease (7%), and arthritis (4%).
Plaque was the most common morphology (56%), while the most common sites of involvement were the head and neck (69%), extremities (61%), and trunk (44%). About half of the cohort (51%) had mild disease, 15% had culture-confirmed infections (9% had Streptococcal infections), and 66% of patients reported itch as a symptom.
The researchers observed that inverse psoriasis was significantly more common in infants and decreased with age. Anogenital involvement was more common in males and in those aged 0-2, while head and neck involvement was more common in females. Nail involvement was more common in childhood.
Topical therapy was the most common treatment overall and by far the most common among those in the 0-2 age category. “Overall, phototherapy was used in childhood and adolescents but almost never in infancy,” Ms. Aghdasi said. “Looking at changes in systemic treatment over time, conventional systemic use increased in infants and children and decreased in adolescents. Biologic use increased in all ages, most notably in children aged 3-12 years old.”
Multivariate regression analyses revealed that the following independent variables predicted moderate to severe psoriasis: adolescent age (adjusted odds ratio, 1.9; P = .03), guttate morphology (aOR, 2.2; P = .006), plaque and guttate morphology (aOR, 7.6; P less than .001), pustular or erythrodermic morphology (aOR, 5; P = .003), culture-confirmed infection (aOR, 2; P = .007), Black race (aOR, 3.3; P = .007), Asian race (aOR, 1.8; P = .04, and Hispanic race (aOR, 1.9; P = .03).
“Further analysis is needed to elucidate the influence of race on severity and of the clinical utility of infection as a marker of severity,” Ms. Aghdasi said. “Interestingly, we did not find that obesity was a marker of severity in our cohort.”
In an interview, senior study author Kelly M. Cordoro, MD, professor of dermatology and pediatrics at UCSF, noted that this finding conflicts with prior studies showing an association between obesity and severe psoriasis in children.
“Though methodologies and patient populations differ among studies, what is striking,” she said, is the percentage of overweight/obese patients (37%; defined as a body mass index ≥ 85th percentile) “in our 2-decade single institution dataset.” This “is nearly identical” to the percentage of patients with excess adiposity – 37.9% (also defined as BMI ≥ 85th percentile) – in an international cross-sectional study, which also identified an association between obesity (BMI ≥ 95th percentile) and psoriasis severity in children, she noted.
“What is clear is the strong association between obesity and childhood psoriasis, as multiple studies, including ours, confirm obesity as a major comorbidity of pediatric psoriasis,” Dr. Cordoro said. “Both conditions must be adequately managed to reduce the risk of adverse health outcomes for obese patients with psoriasis.”
The other study coauthors were Dana Feigenbaum, MD, and Alana Ju, MD. The work was supported by the UCSF Yearlong Inquiry Program. The researchers reported having no relevant financial disclosures.
FROM SPD 2021
New analysis puts U.S. psoriasis prevalence at 3%
, according to an analysis of national survey data from 2011 to 2014.
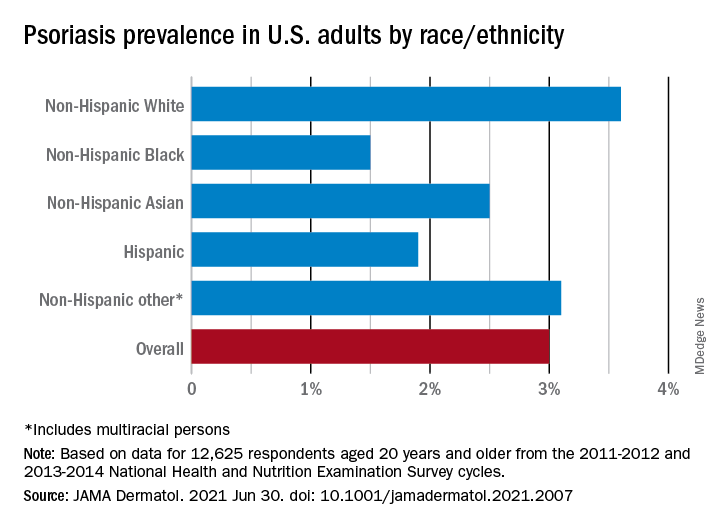
“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
, according to an analysis of national survey data from 2011 to 2014.

“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
, according to an analysis of national survey data from 2011 to 2014.

“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
FROM JAMA DERMATOLOGY
Malignancy risk: Secukinumab shows long-term safety for psoriasis, PsA, ankylosing spondylitis
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Predictive factors for development of axial involvement in PsA
Key clinical point: Axial involvement in psoriatic arthritis (axPsA) is a unique phenotype with characteristics lying between axial spondyloarthritis and pure peripheral PsA. Male gender, elevated C-reactive protein (CRP), and absence of psoriasis were associated with axPsA.
Major finding: Axial involvement was observed in 35.5% of patients with PsA, and they were thus classified as axPsA. Being male (odds ratio [OR], 1.68; 95% confidence interval [CI], 1.09-2.61), having elevated CRP (OR, 2.87; 95% CI, 1.80-4.60), and absence of psoriasis (OR, 0.33; 95% CI, 0.15-0.72) were independently associated with axPsA.
Study details: The data come from an observational, cross-sectional ASAS-perSpA study of 3,684 patients with axial spondyloarthritis or PsA.
Disclosures: The ASAS-PerSpA study was funded by Pfizer, Lilly, AbbVie, Novartis, UCB, Janssen, and Merck. The authors including the lead author reported receiving consulting fees, speaking fees, and/or honoraria from various sources including AbbVie. Three authors reported no conflicts of interest.
Source: Benavent D et al. Semin Arthritis Rheum. 2021 May 5. doi: 10.1016/j.semarthrit.2021.04.018.
Key clinical point: Axial involvement in psoriatic arthritis (axPsA) is a unique phenotype with characteristics lying between axial spondyloarthritis and pure peripheral PsA. Male gender, elevated C-reactive protein (CRP), and absence of psoriasis were associated with axPsA.
Major finding: Axial involvement was observed in 35.5% of patients with PsA, and they were thus classified as axPsA. Being male (odds ratio [OR], 1.68; 95% confidence interval [CI], 1.09-2.61), having elevated CRP (OR, 2.87; 95% CI, 1.80-4.60), and absence of psoriasis (OR, 0.33; 95% CI, 0.15-0.72) were independently associated with axPsA.
Study details: The data come from an observational, cross-sectional ASAS-perSpA study of 3,684 patients with axial spondyloarthritis or PsA.
Disclosures: The ASAS-PerSpA study was funded by Pfizer, Lilly, AbbVie, Novartis, UCB, Janssen, and Merck. The authors including the lead author reported receiving consulting fees, speaking fees, and/or honoraria from various sources including AbbVie. Three authors reported no conflicts of interest.
Source: Benavent D et al. Semin Arthritis Rheum. 2021 May 5. doi: 10.1016/j.semarthrit.2021.04.018.
Key clinical point: Axial involvement in psoriatic arthritis (axPsA) is a unique phenotype with characteristics lying between axial spondyloarthritis and pure peripheral PsA. Male gender, elevated C-reactive protein (CRP), and absence of psoriasis were associated with axPsA.
Major finding: Axial involvement was observed in 35.5% of patients with PsA, and they were thus classified as axPsA. Being male (odds ratio [OR], 1.68; 95% confidence interval [CI], 1.09-2.61), having elevated CRP (OR, 2.87; 95% CI, 1.80-4.60), and absence of psoriasis (OR, 0.33; 95% CI, 0.15-0.72) were independently associated with axPsA.
Study details: The data come from an observational, cross-sectional ASAS-perSpA study of 3,684 patients with axial spondyloarthritis or PsA.
Disclosures: The ASAS-PerSpA study was funded by Pfizer, Lilly, AbbVie, Novartis, UCB, Janssen, and Merck. The authors including the lead author reported receiving consulting fees, speaking fees, and/or honoraria from various sources including AbbVie. Three authors reported no conflicts of interest.
Source: Benavent D et al. Semin Arthritis Rheum. 2021 May 5. doi: 10.1016/j.semarthrit.2021.04.018.
Metabolic syndrome is more prevalent in PsA than in psoriasis and RA
Key clinical point: The prevalence of metabolic syndrome (MetS) was significantly higher in patients with psoriatic arthritis (PsA) than those with psoriasis or rheumatoid arthritis (RA).
Major finding: Patients with PsA were 1.61 (95% confidence interval [CI], 1.49-1.74) and 1.66 (95% CI, 1.54-1.79) times more likely to have MetS than patients with psoriasis and RA, respectively.
Study details: Findings are from a systematic review and meta-analysis of 24, 89, and 53 studies on PsA, psoriasis, and RA, respectively.
Disclosures: The study reported no source of funding and conflicts of interest.
Source: Loganathan A et al. Int J Rheum Dis. 2021 Jun 2. doi: 10.1111/1 756-185X.14147.
Key clinical point: The prevalence of metabolic syndrome (MetS) was significantly higher in patients with psoriatic arthritis (PsA) than those with psoriasis or rheumatoid arthritis (RA).
Major finding: Patients with PsA were 1.61 (95% confidence interval [CI], 1.49-1.74) and 1.66 (95% CI, 1.54-1.79) times more likely to have MetS than patients with psoriasis and RA, respectively.
Study details: Findings are from a systematic review and meta-analysis of 24, 89, and 53 studies on PsA, psoriasis, and RA, respectively.
Disclosures: The study reported no source of funding and conflicts of interest.
Source: Loganathan A et al. Int J Rheum Dis. 2021 Jun 2. doi: 10.1111/1 756-185X.14147.
Key clinical point: The prevalence of metabolic syndrome (MetS) was significantly higher in patients with psoriatic arthritis (PsA) than those with psoriasis or rheumatoid arthritis (RA).
Major finding: Patients with PsA were 1.61 (95% confidence interval [CI], 1.49-1.74) and 1.66 (95% CI, 1.54-1.79) times more likely to have MetS than patients with psoriasis and RA, respectively.
Study details: Findings are from a systematic review and meta-analysis of 24, 89, and 53 studies on PsA, psoriasis, and RA, respectively.
Disclosures: The study reported no source of funding and conflicts of interest.
Source: Loganathan A et al. Int J Rheum Dis. 2021 Jun 2. doi: 10.1111/1 756-185X.14147.
Women with PsA more likely to discontinue b/ts DMARDs
Key clinical point: Women with psoriatic arthritis (PsA) were at greater risk of discontinuing biologic or targeted synthetic disease‐modifying antirheumatic drugs (b/ts DMARDs) because of both lack of efficacy and adverse events. Moreover, the first-line treatment was associated with a lower risk for treatment discontinuation.
Major finding: Women (hazard ratio [HR], 1.36; 95% confidence interval [CI], 1.15-1.62) and patients receiving the second and further lines of treatment (HR, 1.69; 95% CI, 1.41-2.03) were at greater risk of discontinuing treatment because of lack of efficacy. The risk for discontinuation because of adverse events was higher in women (HR, 1.92; 95% CI, 1.44-2.56) and older patients (HR, 1.01; 95% CI, 1.00-1.03).
Study details: Findings are from a real-world multicenter prospective study of 4,752 patients with rheumatic disease from the BIODASER registry who were initiated on b/ts DMARDs, of which 1,250 patients had PsA.
Disclosures: BIOBADASER is supported by the Spanish Agency of Medicines and Medical Devices, Biogen, Bristol Myers Squibb, Celltrion Healthcare, Lilly, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, and Samsung Bioepis. The authors declared no conflicts of interest.
Source: Prior-Español A et al. Sci Rep. 2021 May 27. doi: 10.1038/s41598-021-90442-w.
Key clinical point: Women with psoriatic arthritis (PsA) were at greater risk of discontinuing biologic or targeted synthetic disease‐modifying antirheumatic drugs (b/ts DMARDs) because of both lack of efficacy and adverse events. Moreover, the first-line treatment was associated with a lower risk for treatment discontinuation.
Major finding: Women (hazard ratio [HR], 1.36; 95% confidence interval [CI], 1.15-1.62) and patients receiving the second and further lines of treatment (HR, 1.69; 95% CI, 1.41-2.03) were at greater risk of discontinuing treatment because of lack of efficacy. The risk for discontinuation because of adverse events was higher in women (HR, 1.92; 95% CI, 1.44-2.56) and older patients (HR, 1.01; 95% CI, 1.00-1.03).
Study details: Findings are from a real-world multicenter prospective study of 4,752 patients with rheumatic disease from the BIODASER registry who were initiated on b/ts DMARDs, of which 1,250 patients had PsA.
Disclosures: BIOBADASER is supported by the Spanish Agency of Medicines and Medical Devices, Biogen, Bristol Myers Squibb, Celltrion Healthcare, Lilly, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, and Samsung Bioepis. The authors declared no conflicts of interest.
Source: Prior-Español A et al. Sci Rep. 2021 May 27. doi: 10.1038/s41598-021-90442-w.
Key clinical point: Women with psoriatic arthritis (PsA) were at greater risk of discontinuing biologic or targeted synthetic disease‐modifying antirheumatic drugs (b/ts DMARDs) because of both lack of efficacy and adverse events. Moreover, the first-line treatment was associated with a lower risk for treatment discontinuation.
Major finding: Women (hazard ratio [HR], 1.36; 95% confidence interval [CI], 1.15-1.62) and patients receiving the second and further lines of treatment (HR, 1.69; 95% CI, 1.41-2.03) were at greater risk of discontinuing treatment because of lack of efficacy. The risk for discontinuation because of adverse events was higher in women (HR, 1.92; 95% CI, 1.44-2.56) and older patients (HR, 1.01; 95% CI, 1.00-1.03).
Study details: Findings are from a real-world multicenter prospective study of 4,752 patients with rheumatic disease from the BIODASER registry who were initiated on b/ts DMARDs, of which 1,250 patients had PsA.
Disclosures: BIOBADASER is supported by the Spanish Agency of Medicines and Medical Devices, Biogen, Bristol Myers Squibb, Celltrion Healthcare, Lilly, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, and Samsung Bioepis. The authors declared no conflicts of interest.
Source: Prior-Español A et al. Sci Rep. 2021 May 27. doi: 10.1038/s41598-021-90442-w.
PsA: Tildrakizumab shows promise in phase 2b trial
Key clinical point: Treatment with tildrakizumab was more effective than placebo and was well tolerated through 52 weeks of treatment in patients with active psoriatic arthritis (PsA).
Major finding: At week 24, the proportion of patients achieving at least 20% improvement in the American College of Rheumatology response was significantly higher for any dose of tildrakizumab vs. placebo (71.4%-79.5% vs. 50.6%; all P less than or equal to .0125). Treatment-emergent adverse events (TEAEs) and serious TEAEs occurred in 64.5% and 3.3%, respectively, and were comparable among treatment arms.
Study details: Findings are from a 52-week phase 2b study of 391 patients with PsA who were randomly assigned to tildrakizumab 200 mg every 4 weeks (Q4W), tildrakizumab 200 mg, 100 mg, or 20 mg every 12 weeks or placebo Q4W.
Disclosures: This study was funded by Sun Pharma Global FZE, and the analyses were funded by Sun Pharmaceutical Industries, Princeton, NJ, USA. Some of the authors reported receiving research grants, honoraria, consulting fees, and/or speaker fees from various sources. AM Mendelsohn and SJ Rozzo reported being an employee of Sun Pharmaceutical Industries, Inc. and/or holding shares in Johnson and Johnson.
Source: Mease PJ et al. Ann Rheum Dis. 2021 May 13. doi: 10.1136/annrheumdis-2020-219014.
Key clinical point: Treatment with tildrakizumab was more effective than placebo and was well tolerated through 52 weeks of treatment in patients with active psoriatic arthritis (PsA).
Major finding: At week 24, the proportion of patients achieving at least 20% improvement in the American College of Rheumatology response was significantly higher for any dose of tildrakizumab vs. placebo (71.4%-79.5% vs. 50.6%; all P less than or equal to .0125). Treatment-emergent adverse events (TEAEs) and serious TEAEs occurred in 64.5% and 3.3%, respectively, and were comparable among treatment arms.
Study details: Findings are from a 52-week phase 2b study of 391 patients with PsA who were randomly assigned to tildrakizumab 200 mg every 4 weeks (Q4W), tildrakizumab 200 mg, 100 mg, or 20 mg every 12 weeks or placebo Q4W.
Disclosures: This study was funded by Sun Pharma Global FZE, and the analyses were funded by Sun Pharmaceutical Industries, Princeton, NJ, USA. Some of the authors reported receiving research grants, honoraria, consulting fees, and/or speaker fees from various sources. AM Mendelsohn and SJ Rozzo reported being an employee of Sun Pharmaceutical Industries, Inc. and/or holding shares in Johnson and Johnson.
Source: Mease PJ et al. Ann Rheum Dis. 2021 May 13. doi: 10.1136/annrheumdis-2020-219014.
Key clinical point: Treatment with tildrakizumab was more effective than placebo and was well tolerated through 52 weeks of treatment in patients with active psoriatic arthritis (PsA).
Major finding: At week 24, the proportion of patients achieving at least 20% improvement in the American College of Rheumatology response was significantly higher for any dose of tildrakizumab vs. placebo (71.4%-79.5% vs. 50.6%; all P less than or equal to .0125). Treatment-emergent adverse events (TEAEs) and serious TEAEs occurred in 64.5% and 3.3%, respectively, and were comparable among treatment arms.
Study details: Findings are from a 52-week phase 2b study of 391 patients with PsA who were randomly assigned to tildrakizumab 200 mg every 4 weeks (Q4W), tildrakizumab 200 mg, 100 mg, or 20 mg every 12 weeks or placebo Q4W.
Disclosures: This study was funded by Sun Pharma Global FZE, and the analyses were funded by Sun Pharmaceutical Industries, Princeton, NJ, USA. Some of the authors reported receiving research grants, honoraria, consulting fees, and/or speaker fees from various sources. AM Mendelsohn and SJ Rozzo reported being an employee of Sun Pharmaceutical Industries, Inc. and/or holding shares in Johnson and Johnson.
Source: Mease PJ et al. Ann Rheum Dis. 2021 May 13. doi: 10.1136/annrheumdis-2020-219014.
Targeted metabolomic profiling predicts CV risk in psoriasis and PsA
Key clinical point: A range of novel metabolite markers associated with the risk for cardiovascular (CV) events when combined in a model matched with age and sex showed improved performance in predicting CV diseases in patients with psoriasis and psoriatic disease (PsA).
Major finding: Alanine, tyrosine, degree of unsaturation of fatty acids, and high-density lipoprotein particles were associated with decreased CV risk, whereas glycoprotein acetyls, apolipoprotein B, and cholesterol remnants were associated with increased CV risk (all P less than .05). The addition of 13 metabolites in the expanded model improved CV risk prediction beyond the base model with only age and sex (area under the receiver operator characteristic curve, 79.9 vs. 72.6; P = .02).
Study details: This was a prospective study of 977 patients with psoriasis and PsA.
Disclosures: The study was supported by a grant from the National Psoriasis Foundation and Arthritis Society. Some of the authors including the lead author declared receiving grants, personal fees, and/or advisory roles for various sources.
Source: Colaco K et al. Ann Rheum Dis. 2021 May 28. doi: 10.1136/annrheumdis-2021-220168.
Key clinical point: A range of novel metabolite markers associated with the risk for cardiovascular (CV) events when combined in a model matched with age and sex showed improved performance in predicting CV diseases in patients with psoriasis and psoriatic disease (PsA).
Major finding: Alanine, tyrosine, degree of unsaturation of fatty acids, and high-density lipoprotein particles were associated with decreased CV risk, whereas glycoprotein acetyls, apolipoprotein B, and cholesterol remnants were associated with increased CV risk (all P less than .05). The addition of 13 metabolites in the expanded model improved CV risk prediction beyond the base model with only age and sex (area under the receiver operator characteristic curve, 79.9 vs. 72.6; P = .02).
Study details: This was a prospective study of 977 patients with psoriasis and PsA.
Disclosures: The study was supported by a grant from the National Psoriasis Foundation and Arthritis Society. Some of the authors including the lead author declared receiving grants, personal fees, and/or advisory roles for various sources.
Source: Colaco K et al. Ann Rheum Dis. 2021 May 28. doi: 10.1136/annrheumdis-2021-220168.
Key clinical point: A range of novel metabolite markers associated with the risk for cardiovascular (CV) events when combined in a model matched with age and sex showed improved performance in predicting CV diseases in patients with psoriasis and psoriatic disease (PsA).
Major finding: Alanine, tyrosine, degree of unsaturation of fatty acids, and high-density lipoprotein particles were associated with decreased CV risk, whereas glycoprotein acetyls, apolipoprotein B, and cholesterol remnants were associated with increased CV risk (all P less than .05). The addition of 13 metabolites in the expanded model improved CV risk prediction beyond the base model with only age and sex (area under the receiver operator characteristic curve, 79.9 vs. 72.6; P = .02).
Study details: This was a prospective study of 977 patients with psoriasis and PsA.
Disclosures: The study was supported by a grant from the National Psoriasis Foundation and Arthritis Society. Some of the authors including the lead author declared receiving grants, personal fees, and/or advisory roles for various sources.
Source: Colaco K et al. Ann Rheum Dis. 2021 May 28. doi: 10.1136/annrheumdis-2021-220168.
ABP 501 safe and effective for PsA and plaque-type psoriasis
Key clinical point: ABP 501, an adalimumab biosimilar, was safe and effective for the treatment of plaque-type psoriasis and psoriatic arthritis (PsA) irrespective of whether patients were originator-naive or switched from the reference adalimumab.
Major finding: In originator-naive patients, mean Psoriasis Area and Severity Index (PASI) significantly improved from baseline to week 24 (P less than .0001). Among patients who underwent nonmedical switch from adalimumab to ABP 501, PASI (0.45 vs. 0.45) and 28-joints Disease Activity Score erythrocyte sedimentation rate (2.35 vs. 2.31) were not significantly different at 16 weeks before to 24 weeks after the switch. The incidence of adverse events was similar in ABP 501 vs. adalimumab groups (67.2% vs. 63.6%).
Study details: Data come from a retrospective, real-life study of 94 patients with psoriasis and PsA who received ABP 501, of which 46 patients underwent a nonmedical switch from the adalimumab reference product to ABP 501.
Disclosures: The study did not receive any funding. A Giunta reported serving as a consultant, board member, and/or speaker for various sources. No other disclosures were reported.
Source: Giunta A et al. Curr Med Res Opin. 2021 May 20. doi: 10.1080/03007995.2021.1923467.
Key clinical point: ABP 501, an adalimumab biosimilar, was safe and effective for the treatment of plaque-type psoriasis and psoriatic arthritis (PsA) irrespective of whether patients were originator-naive or switched from the reference adalimumab.
Major finding: In originator-naive patients, mean Psoriasis Area and Severity Index (PASI) significantly improved from baseline to week 24 (P less than .0001). Among patients who underwent nonmedical switch from adalimumab to ABP 501, PASI (0.45 vs. 0.45) and 28-joints Disease Activity Score erythrocyte sedimentation rate (2.35 vs. 2.31) were not significantly different at 16 weeks before to 24 weeks after the switch. The incidence of adverse events was similar in ABP 501 vs. adalimumab groups (67.2% vs. 63.6%).
Study details: Data come from a retrospective, real-life study of 94 patients with psoriasis and PsA who received ABP 501, of which 46 patients underwent a nonmedical switch from the adalimumab reference product to ABP 501.
Disclosures: The study did not receive any funding. A Giunta reported serving as a consultant, board member, and/or speaker for various sources. No other disclosures were reported.
Source: Giunta A et al. Curr Med Res Opin. 2021 May 20. doi: 10.1080/03007995.2021.1923467.
Key clinical point: ABP 501, an adalimumab biosimilar, was safe and effective for the treatment of plaque-type psoriasis and psoriatic arthritis (PsA) irrespective of whether patients were originator-naive or switched from the reference adalimumab.
Major finding: In originator-naive patients, mean Psoriasis Area and Severity Index (PASI) significantly improved from baseline to week 24 (P less than .0001). Among patients who underwent nonmedical switch from adalimumab to ABP 501, PASI (0.45 vs. 0.45) and 28-joints Disease Activity Score erythrocyte sedimentation rate (2.35 vs. 2.31) were not significantly different at 16 weeks before to 24 weeks after the switch. The incidence of adverse events was similar in ABP 501 vs. adalimumab groups (67.2% vs. 63.6%).
Study details: Data come from a retrospective, real-life study of 94 patients with psoriasis and PsA who received ABP 501, of which 46 patients underwent a nonmedical switch from the adalimumab reference product to ABP 501.
Disclosures: The study did not receive any funding. A Giunta reported serving as a consultant, board member, and/or speaker for various sources. No other disclosures were reported.
Source: Giunta A et al. Curr Med Res Opin. 2021 May 20. doi: 10.1080/03007995.2021.1923467.





