User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Adult ADHD: Tips for an accurate diagnosis
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
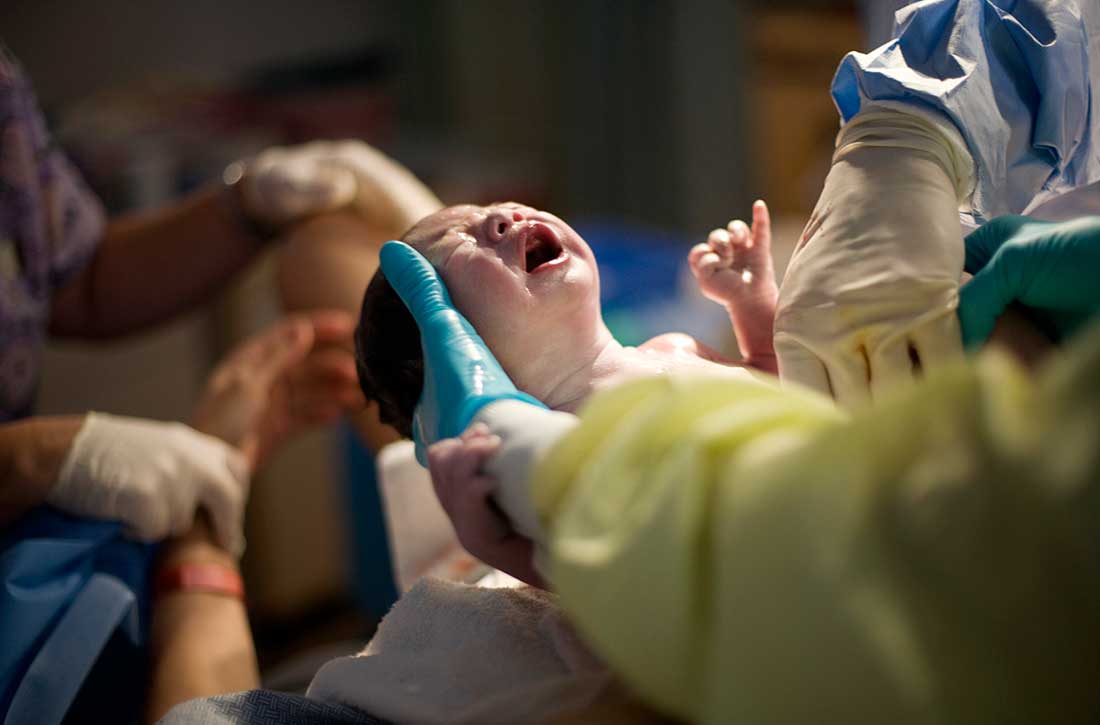
Childbirth-related PTSD: How it differs and who’s at risk
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
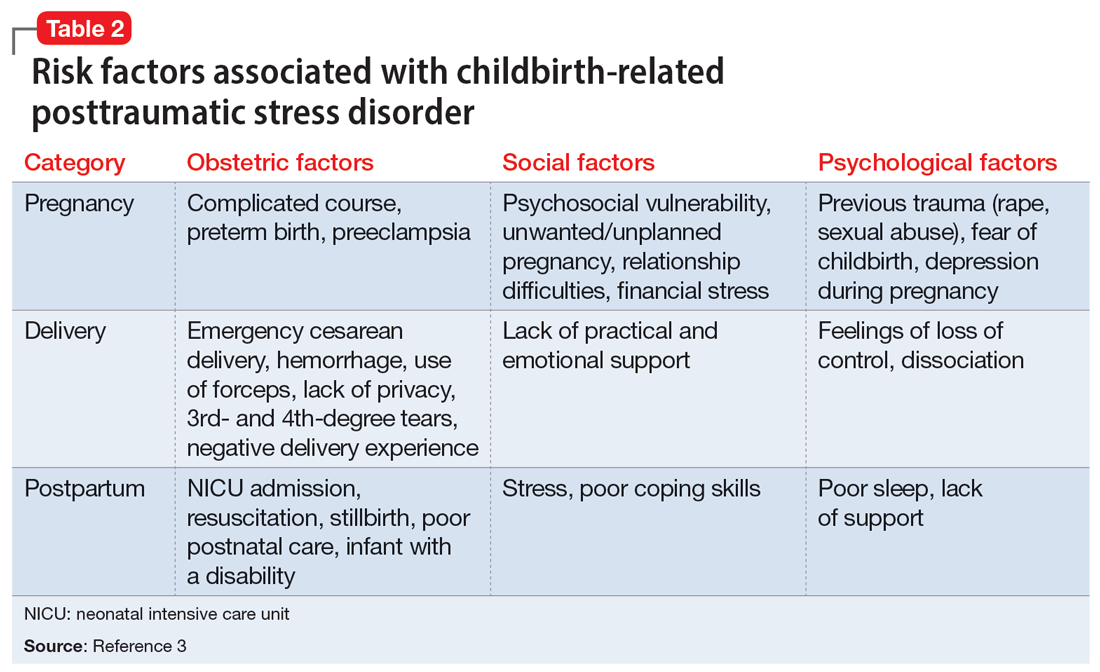
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.
Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.

Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.

Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
Perinatal psychiatric screening: What to ask
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
Brick and mortar: Changes in the therapeutic relationship in a postvirtual world
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.
A new doctor in a COVID mask
As a 2020 graduate, my medical school experience was largely untouched by the coronavirus. However, when I transitioned to residency, the world was 4 months into the COVID-19 pandemic, and I was required to wear an N95 mask. Just as I started calling myself Dr. Petteruti, I stopped seeing my patients’ entire face, and they stopped seeing mine. In this article, I share my reflections on wearing a mask during residency.
Even after 3 years of daily practice, I have found that wearing a mask brings an acute awareness of my face. As a community physician, the spheres of personal and public life intersect as I treat patients. Learning to navigate this is an important and shared experience across many community-based residency programs. However, during the first few years of residency, I have been able to shop at a local grocery store or eat at a nearby restaurant without any concerns of being recognized by a patient. Until recently, my patients had never seen my face. That has now changed.
For a new intern, a mask can be a savior. It can hide most of what is on your face from your patient. It is remarkable how the brain fills in the gaps of the visage and, by extension, aspects of the person. Many times, I was thankful to have my morning yawn or facial expression covered during provoking conversations with patients. Furthermore, masks gave me an opportunity to examine my own reactions, emotions, affect, and countertransference of each interaction on my own time.
The mask mandate also protected some features that illustrated my youth. For the patient, a mask can add a dry, clinical distance to the physician, often emitting a professional interpretation to the encounter. For the physician, the mask serves as a concrete barrier to the otherwise effortless acts of observation. Early in my career, I had to set reminders to have patients who were taking antipsychotic medications remove their masks to assess for tardive dyskinesia. Sometimes this surprised the patient, who was hesitant to expose themselves physically and psychologically. Alternatively, mask wearing has proved to be an additional data point on some patients, such as those with disorganized behavior. If the mask is located on the patient’s head, chin, or eyes, or is otherwise inappropriately placed, this provides the clinician with supplemental information.
After spending most of my third year of residency in an outpatient office diligently learning how to build a sturdy therapeutic patient alliance, the mask mandate was lifted. Patients’ transference began to change right before my newly bared face. People often relate age to wisdom and experience, so my lack of age—and thus, possible perceived lack of knowledge—became glaringly apparent. During our initial encounters without masks, patients I had known for most of the year began discussing their symptoms and treatments with more hesitancy. My established patients suddenly had a noticeable change in the intensity of their eye contact. Some even asked if I had cut my hair or what had changed about my appearance since our previous visit. This change in affect and behavior offers a unique experience for the resident; renovating the patient-doctor relationship based on the physician’s appearance.
As psychiatrists, we would generally assume mask wearing has an undesirable effect on the therapeutic alliance and increases skewed inferences in our evaluations. This held true for my experience in residency. In psychotherapy, we work to help patients remove their own metaphorical “masks” of defense and security in self-exploration. However, as young physicians, rather than creating barriers between us and our patients, the mask mandate seemed to have created a sense of credibility in our practice and trustworthiness in our decisions.
Some questions remain. As clinicians, what are we missing when we can only see our patient’s eyes and forehead? How will the COVID-19 pandemic affect my training and career as a psychiatrist? These may remain unanswered for my generation of trainees for some time, as society will look back and contemplate this period for decades. Though we entered our career in uncertain times, with an increased risk of morbidity and death and high demand for proper personal protective equipment, we were and still are thankful for our masks and for the limited infection exposure afforded by the nature of our specialty.
As a 2020 graduate, my medical school experience was largely untouched by the coronavirus. However, when I transitioned to residency, the world was 4 months into the COVID-19 pandemic, and I was required to wear an N95 mask. Just as I started calling myself Dr. Petteruti, I stopped seeing my patients’ entire face, and they stopped seeing mine. In this article, I share my reflections on wearing a mask during residency.
Even after 3 years of daily practice, I have found that wearing a mask brings an acute awareness of my face. As a community physician, the spheres of personal and public life intersect as I treat patients. Learning to navigate this is an important and shared experience across many community-based residency programs. However, during the first few years of residency, I have been able to shop at a local grocery store or eat at a nearby restaurant without any concerns of being recognized by a patient. Until recently, my patients had never seen my face. That has now changed.
For a new intern, a mask can be a savior. It can hide most of what is on your face from your patient. It is remarkable how the brain fills in the gaps of the visage and, by extension, aspects of the person. Many times, I was thankful to have my morning yawn or facial expression covered during provoking conversations with patients. Furthermore, masks gave me an opportunity to examine my own reactions, emotions, affect, and countertransference of each interaction on my own time.
The mask mandate also protected some features that illustrated my youth. For the patient, a mask can add a dry, clinical distance to the physician, often emitting a professional interpretation to the encounter. For the physician, the mask serves as a concrete barrier to the otherwise effortless acts of observation. Early in my career, I had to set reminders to have patients who were taking antipsychotic medications remove their masks to assess for tardive dyskinesia. Sometimes this surprised the patient, who was hesitant to expose themselves physically and psychologically. Alternatively, mask wearing has proved to be an additional data point on some patients, such as those with disorganized behavior. If the mask is located on the patient’s head, chin, or eyes, or is otherwise inappropriately placed, this provides the clinician with supplemental information.
After spending most of my third year of residency in an outpatient office diligently learning how to build a sturdy therapeutic patient alliance, the mask mandate was lifted. Patients’ transference began to change right before my newly bared face. People often relate age to wisdom and experience, so my lack of age—and thus, possible perceived lack of knowledge—became glaringly apparent. During our initial encounters without masks, patients I had known for most of the year began discussing their symptoms and treatments with more hesitancy. My established patients suddenly had a noticeable change in the intensity of their eye contact. Some even asked if I had cut my hair or what had changed about my appearance since our previous visit. This change in affect and behavior offers a unique experience for the resident; renovating the patient-doctor relationship based on the physician’s appearance.
As psychiatrists, we would generally assume mask wearing has an undesirable effect on the therapeutic alliance and increases skewed inferences in our evaluations. This held true for my experience in residency. In psychotherapy, we work to help patients remove their own metaphorical “masks” of defense and security in self-exploration. However, as young physicians, rather than creating barriers between us and our patients, the mask mandate seemed to have created a sense of credibility in our practice and trustworthiness in our decisions.
Some questions remain. As clinicians, what are we missing when we can only see our patient’s eyes and forehead? How will the COVID-19 pandemic affect my training and career as a psychiatrist? These may remain unanswered for my generation of trainees for some time, as society will look back and contemplate this period for decades. Though we entered our career in uncertain times, with an increased risk of morbidity and death and high demand for proper personal protective equipment, we were and still are thankful for our masks and for the limited infection exposure afforded by the nature of our specialty.
As a 2020 graduate, my medical school experience was largely untouched by the coronavirus. However, when I transitioned to residency, the world was 4 months into the COVID-19 pandemic, and I was required to wear an N95 mask. Just as I started calling myself Dr. Petteruti, I stopped seeing my patients’ entire face, and they stopped seeing mine. In this article, I share my reflections on wearing a mask during residency.
Even after 3 years of daily practice, I have found that wearing a mask brings an acute awareness of my face. As a community physician, the spheres of personal and public life intersect as I treat patients. Learning to navigate this is an important and shared experience across many community-based residency programs. However, during the first few years of residency, I have been able to shop at a local grocery store or eat at a nearby restaurant without any concerns of being recognized by a patient. Until recently, my patients had never seen my face. That has now changed.
For a new intern, a mask can be a savior. It can hide most of what is on your face from your patient. It is remarkable how the brain fills in the gaps of the visage and, by extension, aspects of the person. Many times, I was thankful to have my morning yawn or facial expression covered during provoking conversations with patients. Furthermore, masks gave me an opportunity to examine my own reactions, emotions, affect, and countertransference of each interaction on my own time.
The mask mandate also protected some features that illustrated my youth. For the patient, a mask can add a dry, clinical distance to the physician, often emitting a professional interpretation to the encounter. For the physician, the mask serves as a concrete barrier to the otherwise effortless acts of observation. Early in my career, I had to set reminders to have patients who were taking antipsychotic medications remove their masks to assess for tardive dyskinesia. Sometimes this surprised the patient, who was hesitant to expose themselves physically and psychologically. Alternatively, mask wearing has proved to be an additional data point on some patients, such as those with disorganized behavior. If the mask is located on the patient’s head, chin, or eyes, or is otherwise inappropriately placed, this provides the clinician with supplemental information.
After spending most of my third year of residency in an outpatient office diligently learning how to build a sturdy therapeutic patient alliance, the mask mandate was lifted. Patients’ transference began to change right before my newly bared face. People often relate age to wisdom and experience, so my lack of age—and thus, possible perceived lack of knowledge—became glaringly apparent. During our initial encounters without masks, patients I had known for most of the year began discussing their symptoms and treatments with more hesitancy. My established patients suddenly had a noticeable change in the intensity of their eye contact. Some even asked if I had cut my hair or what had changed about my appearance since our previous visit. This change in affect and behavior offers a unique experience for the resident; renovating the patient-doctor relationship based on the physician’s appearance.
As psychiatrists, we would generally assume mask wearing has an undesirable effect on the therapeutic alliance and increases skewed inferences in our evaluations. This held true for my experience in residency. In psychotherapy, we work to help patients remove their own metaphorical “masks” of defense and security in self-exploration. However, as young physicians, rather than creating barriers between us and our patients, the mask mandate seemed to have created a sense of credibility in our practice and trustworthiness in our decisions.
Some questions remain. As clinicians, what are we missing when we can only see our patient’s eyes and forehead? How will the COVID-19 pandemic affect my training and career as a psychiatrist? These may remain unanswered for my generation of trainees for some time, as society will look back and contemplate this period for decades. Though we entered our career in uncertain times, with an increased risk of morbidity and death and high demand for proper personal protective equipment, we were and still are thankful for our masks and for the limited infection exposure afforded by the nature of our specialty.
Worsening mania while receiving low-dose quetiapine: A case report
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1A antagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1A antagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1A antagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
Navigating the challenges of patients with substance use disorders who leave AMA
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Working closely with individuals with substance use disorders (SUDs), we’ve observed a worrisome trend of patients leaving the hospital against medical advice (AMA). This issue is not only prevalent in psychiatric settings, but also in emergency departments, medical and surgical floors, and even intensive care units.1
Compared to individuals without such disorders, individuals with SUDs—particularly those with opioid use disorders—are up to 3 times more likely to leave the hospital AMA.1,2 Leaving AMA can lead to multiple complications, including an increased risk of readmission, suboptimal treatment outcomes, and an increased use of health care resources.1-3
It is critical to understand why patients elect to leave a hospital AMA. In a qualitative study, Simon et al1 found that individuals with SUDs often leave AMA due to uncontrolled withdrawal symptoms and pain, perceived stigma and discrimination, and dissatisfaction with care. Predictors of patients leaving the hospital AMA include the severity of their drug dependence and previous negative treatment experiences.4 A systematic review found housing instability and a lack of social support influence an individual’s decision to leave AMA.5
Recommendations for managing patients who leave AMA
Enhancing your understanding of withdrawal symptoms may allow you to offer patients more effective symptom control, possibly with methadone or buprenorphine.2 Injectable opioid agonist treatment may also help to retain a patient in care. In a case report, a 47-year-old man with a severe opioid use disorder who had left the hospital AMA due to uncontrolled opioid withdrawal was readmitted, treated with IV hydromorphone, and enrolled in ongoing community injectable opioid agonist treatment.6
Clinicians must address the stigma and discrimination patients with SUDs often face in health care institutions. Additional training for clinicians to improve their understanding of these disorders and foster a more compassionate and nonjudgmental approach to care may be beneficial.
Like most medicolegal conflicts, leaving AMA is often a clinical and interpersonal problem disguised as a legal one. When assessing these patients’ decision-making capacity, we often find they are angry and dissatisfied with the care they have (or have not) received. The most useful intervention may be to restore communication between the patient and their treatment team.
Even after a patient leaves AMA, the treatment team may experience countertransference issues, such as heightened emotional reactions or biases, that could compromise their clinical judgment. Addressing these dynamics may require team debriefings, supervision, or further training in managing transference and countertransference, particularly since patients who leave AMA may return for subsequent care.7
Integrated care models, which feature close collaboration between clinicians from different specialties, can help ensure that a patient’s diverse health needs are met and reduce the likelihood of them leaving AMA. Integrated care models may be particularly effective for patients with co-occurring conditions such as HIV and SUDs.8
Implementing these recommendations can be challenging. Barriers to addressing AMA departures span several domains, including patient-specific barriers (eg, stigma and discrimination), clinical barriers (eg, lack of resources and training for clinicians), institutional hurdles (eg, systemic inefficiencies), and broader social barriers (eg, housing instability and inadequate social support). Overcoming these barriers requires a multifaceted approach involving clinicians, policymakers, and the community that considers medical, psychological, and social factors.
1. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525.
2. Kenne DR, Boros AP, Fischbein RL. Characteristics of opiate users leaving detoxification treatment against medical advice. J Addict Dis. 2010;29(3):383-394.
3. Mahajan RK, Gautam PL, Paul G, et al. Retrospective evaluation of patients leaving against medical advice in a tertiary care teaching hospital. Indian J Crit Care Med. 2019;23(3):139-142.
4. Armenian SH, Chutuape MA, Stitzer ML. Predictors of discharges against medical advice from a short-term hospital detoxification unit. Drug Alcohol Depend. 1999;56(1):1-8.
5. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59.
6. McAdam M, Brar R, Young S. Initiation of injectable opioid agonist treatment in hospital: a case report. Drug Alcohol Rev. 2020;39(2):138-141.
7. Schouten R, Weintraub BR. Legal aspects of consultation. In: Stern TA, Freudenreich O, Smith FA, et al, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 7th ed. Elsevier; 2018:578-579.
8. Vallecillo G, Robles MJ, Fonseca F, et al. Integrated care on leaving hospital against medical advice among HIV-infected people with substance use disorders. AIDS Res Hum Retroviruses. 2018;34(12):1044-1049.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Working closely with individuals with substance use disorders (SUDs), we’ve observed a worrisome trend of patients leaving the hospital against medical advice (AMA). This issue is not only prevalent in psychiatric settings, but also in emergency departments, medical and surgical floors, and even intensive care units.1
Compared to individuals without such disorders, individuals with SUDs—particularly those with opioid use disorders—are up to 3 times more likely to leave the hospital AMA.1,2 Leaving AMA can lead to multiple complications, including an increased risk of readmission, suboptimal treatment outcomes, and an increased use of health care resources.1-3
It is critical to understand why patients elect to leave a hospital AMA. In a qualitative study, Simon et al1 found that individuals with SUDs often leave AMA due to uncontrolled withdrawal symptoms and pain, perceived stigma and discrimination, and dissatisfaction with care. Predictors of patients leaving the hospital AMA include the severity of their drug dependence and previous negative treatment experiences.4 A systematic review found housing instability and a lack of social support influence an individual’s decision to leave AMA.5
Recommendations for managing patients who leave AMA
Enhancing your understanding of withdrawal symptoms may allow you to offer patients more effective symptom control, possibly with methadone or buprenorphine.2 Injectable opioid agonist treatment may also help to retain a patient in care. In a case report, a 47-year-old man with a severe opioid use disorder who had left the hospital AMA due to uncontrolled opioid withdrawal was readmitted, treated with IV hydromorphone, and enrolled in ongoing community injectable opioid agonist treatment.6
Clinicians must address the stigma and discrimination patients with SUDs often face in health care institutions. Additional training for clinicians to improve their understanding of these disorders and foster a more compassionate and nonjudgmental approach to care may be beneficial.
Like most medicolegal conflicts, leaving AMA is often a clinical and interpersonal problem disguised as a legal one. When assessing these patients’ decision-making capacity, we often find they are angry and dissatisfied with the care they have (or have not) received. The most useful intervention may be to restore communication between the patient and their treatment team.
Even after a patient leaves AMA, the treatment team may experience countertransference issues, such as heightened emotional reactions or biases, that could compromise their clinical judgment. Addressing these dynamics may require team debriefings, supervision, or further training in managing transference and countertransference, particularly since patients who leave AMA may return for subsequent care.7
Integrated care models, which feature close collaboration between clinicians from different specialties, can help ensure that a patient’s diverse health needs are met and reduce the likelihood of them leaving AMA. Integrated care models may be particularly effective for patients with co-occurring conditions such as HIV and SUDs.8
Implementing these recommendations can be challenging. Barriers to addressing AMA departures span several domains, including patient-specific barriers (eg, stigma and discrimination), clinical barriers (eg, lack of resources and training for clinicians), institutional hurdles (eg, systemic inefficiencies), and broader social barriers (eg, housing instability and inadequate social support). Overcoming these barriers requires a multifaceted approach involving clinicians, policymakers, and the community that considers medical, psychological, and social factors.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Working closely with individuals with substance use disorders (SUDs), we’ve observed a worrisome trend of patients leaving the hospital against medical advice (AMA). This issue is not only prevalent in psychiatric settings, but also in emergency departments, medical and surgical floors, and even intensive care units.1
Compared to individuals without such disorders, individuals with SUDs—particularly those with opioid use disorders—are up to 3 times more likely to leave the hospital AMA.1,2 Leaving AMA can lead to multiple complications, including an increased risk of readmission, suboptimal treatment outcomes, and an increased use of health care resources.1-3
It is critical to understand why patients elect to leave a hospital AMA. In a qualitative study, Simon et al1 found that individuals with SUDs often leave AMA due to uncontrolled withdrawal symptoms and pain, perceived stigma and discrimination, and dissatisfaction with care. Predictors of patients leaving the hospital AMA include the severity of their drug dependence and previous negative treatment experiences.4 A systematic review found housing instability and a lack of social support influence an individual’s decision to leave AMA.5
Recommendations for managing patients who leave AMA
Enhancing your understanding of withdrawal symptoms may allow you to offer patients more effective symptom control, possibly with methadone or buprenorphine.2 Injectable opioid agonist treatment may also help to retain a patient in care. In a case report, a 47-year-old man with a severe opioid use disorder who had left the hospital AMA due to uncontrolled opioid withdrawal was readmitted, treated with IV hydromorphone, and enrolled in ongoing community injectable opioid agonist treatment.6
Clinicians must address the stigma and discrimination patients with SUDs often face in health care institutions. Additional training for clinicians to improve their understanding of these disorders and foster a more compassionate and nonjudgmental approach to care may be beneficial.
Like most medicolegal conflicts, leaving AMA is often a clinical and interpersonal problem disguised as a legal one. When assessing these patients’ decision-making capacity, we often find they are angry and dissatisfied with the care they have (or have not) received. The most useful intervention may be to restore communication between the patient and their treatment team.
Even after a patient leaves AMA, the treatment team may experience countertransference issues, such as heightened emotional reactions or biases, that could compromise their clinical judgment. Addressing these dynamics may require team debriefings, supervision, or further training in managing transference and countertransference, particularly since patients who leave AMA may return for subsequent care.7
Integrated care models, which feature close collaboration between clinicians from different specialties, can help ensure that a patient’s diverse health needs are met and reduce the likelihood of them leaving AMA. Integrated care models may be particularly effective for patients with co-occurring conditions such as HIV and SUDs.8
Implementing these recommendations can be challenging. Barriers to addressing AMA departures span several domains, including patient-specific barriers (eg, stigma and discrimination), clinical barriers (eg, lack of resources and training for clinicians), institutional hurdles (eg, systemic inefficiencies), and broader social barriers (eg, housing instability and inadequate social support). Overcoming these barriers requires a multifaceted approach involving clinicians, policymakers, and the community that considers medical, psychological, and social factors.
1. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525.
2. Kenne DR, Boros AP, Fischbein RL. Characteristics of opiate users leaving detoxification treatment against medical advice. J Addict Dis. 2010;29(3):383-394.
3. Mahajan RK, Gautam PL, Paul G, et al. Retrospective evaluation of patients leaving against medical advice in a tertiary care teaching hospital. Indian J Crit Care Med. 2019;23(3):139-142.
4. Armenian SH, Chutuape MA, Stitzer ML. Predictors of discharges against medical advice from a short-term hospital detoxification unit. Drug Alcohol Depend. 1999;56(1):1-8.
5. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59.
6. McAdam M, Brar R, Young S. Initiation of injectable opioid agonist treatment in hospital: a case report. Drug Alcohol Rev. 2020;39(2):138-141.
7. Schouten R, Weintraub BR. Legal aspects of consultation. In: Stern TA, Freudenreich O, Smith FA, et al, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 7th ed. Elsevier; 2018:578-579.
8. Vallecillo G, Robles MJ, Fonseca F, et al. Integrated care on leaving hospital against medical advice among HIV-infected people with substance use disorders. AIDS Res Hum Retroviruses. 2018;34(12):1044-1049.
1. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525.
2. Kenne DR, Boros AP, Fischbein RL. Characteristics of opiate users leaving detoxification treatment against medical advice. J Addict Dis. 2010;29(3):383-394.
3. Mahajan RK, Gautam PL, Paul G, et al. Retrospective evaluation of patients leaving against medical advice in a tertiary care teaching hospital. Indian J Crit Care Med. 2019;23(3):139-142.
4. Armenian SH, Chutuape MA, Stitzer ML. Predictors of discharges against medical advice from a short-term hospital detoxification unit. Drug Alcohol Depend. 1999;56(1):1-8.
5. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59.
6. McAdam M, Brar R, Young S. Initiation of injectable opioid agonist treatment in hospital: a case report. Drug Alcohol Rev. 2020;39(2):138-141.
7. Schouten R, Weintraub BR. Legal aspects of consultation. In: Stern TA, Freudenreich O, Smith FA, et al, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 7th ed. Elsevier; 2018:578-579.
8. Vallecillo G, Robles MJ, Fonseca F, et al. Integrated care on leaving hospital against medical advice among HIV-infected people with substance use disorders. AIDS Res Hum Retroviruses. 2018;34(12):1044-1049.
New tests may finally diagnose long COVID
One of the biggest challenges facing clinicians who treat long COVID is a lack of consensus when it comes to recognizing and diagnosing the condition. But
Effective diagnostic testing would be a game-changer in the long COVID fight, for it’s not just the fatigue, brain fog, heart palpitations, and other persistent symptoms that affect patients. Two out of three people with long COVID also suffer mental health challenges like depression and anxiety. Some patients say their symptoms are not taken seriously by their doctors. And as many as 12% of long COVID patients are unemployed because of the severity of their illness and their employers may be skeptical of their condition.
Quick, accurate diagnosis would eliminate all that. Now a new preprint study suggests that the elevation of certain immune system proteins are a commonality in long COVID patients and identifying them may be an accurate way to diagnose the condition.
Researchers at Cardiff (Wales) University, tracked 166 patients, 79 of whom had been diagnosed with long COVID and 87 who had not. All participants had recovered from a severe bout of acute COVID-19.
In an analysis of the blood plasma of the study participants, researchers found elevated levels of certain components. Four proteins in particular – Ba, iC3b, C5a, and TCC – predicted the presence of long COVID with 78.5% accuracy.
“I was gobsmacked by the results. We’re seeing a massive dysregulation in those four biomarkers,” says study author Wioleta Zelek, PhD, a research fellow at Cardiff University. “It’s a combination that we showed was predictive of long COVID.”
The study revealed that long COVID was associated with inflammation of the immune system causing these complement proteins to remain dysregulated. Proteins like C3, C4, and C5 are important parts of the immune system because they recruit phagocytes, cells that attack and engulf bacteria and viruses at the site of infection to destroy pathogens like SARS-coV-2.
In the case of long COVID, these proteins remain chronically elevated. While the symptoms of long COVID have seemed largely unrelated to one another, researchers point to elevated inflammation as a connecting factor that causes various systems in the body to go haywire.
“Anything that could help to better diagnose patients with long COVID is research we’re greatly appreciative of within the clinical community,” said Nisha Viswanathan, MD, director of the University of California, Los Angeles, Long COVID program at UCLA Health.
Testing for biomarkers highlighted in the study, as well as others like serotonin and cortisol, may help doctors separate patients who have long COVID from patients who have similar symptoms caused by other conditions, said Dr. Viswanathan. For example, a recent study published in the journal Cell found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but recovered from the condition.
Dr. Viswanathan cautions that the biomarker test does not answer all the questions about diagnosing long COVID. For example, Dr. Viswanathan said scientists don’t know whether complement dysregulation is caused by long COVID and not another underlying medical issue that patients had prior to infection, because “we don’t know where patients’ levels were prior to developing long COVID.” For example, those with autoimmune issues are more likely to develop long COVID, which means their levels could have been elevated prior to a COVID infection.
It is increasingly likely, said Dr. Viswanathan, that long COVID is an umbrella term for a host of conditions that could be caused by different impacts of the virus. Other research has pointed to the different phenotypes of long COVID. For example, some are focused on cardiopulmonary issues and others on fatigue and gastrointestinal problems.
“It looks like these different phenotypes have a different mechanism for disease,” she said. This means that it’s less likely to be a one-size-fits-all condition and the next step in the research should be identifying which biomarker is aligned with which phenotype of the disease.
Better diagnostics will open the door to better treatments, Dr. Zelek said. The more doctors understand about the mechanism causing immune dysregulation in long COVID patients, the more they can treat it with existing medications. Dr. Zelek’s lab has been studying certain medications like pegcetacoplan (C3 blocker), danicopan (anti-factor D), and iptacopan (anti-factor B) that can be used to break the body’s cycle of inflammation and reduce symptoms experienced in those with long COVID.
These drugs are approved by the U.S. Food and Drug Administration for the treatment of a rare blood disease called paroxysmal nocturnal hemoglobinuria. The C5 inhibitor zilucoplan has also been used in patients hospitalized with COVID-19 and researchers have found that the drug lowered serum C5 and interleukin-8 concentration in the blood, seeming to reduce certain aspects of the immune system’s inflammatory response to the virus.
The Cardiff University research is one of the most detailed studies to highlight long COVID biomarkers to date, said infectious disease specialist Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. The research needs to be duplicated in a larger study population that might include the other biomarkers like serotonin and cortisol to see if they’re related, she said.
Researchers are learning more everyday about the various biomarkers that may be linked to long COVID, she added. This Cardiff study showed that a huge percentage of those patients had elevated levels of certain complements. The next step, said Dr. McComsey, “is to put all these puzzle pieces together” so that clinicians have a common diagnostic tool or tools that provide patients with some peace of mind in starting their road to recovery.
A version of this article first appeared on Medscape.com.
One of the biggest challenges facing clinicians who treat long COVID is a lack of consensus when it comes to recognizing and diagnosing the condition. But
Effective diagnostic testing would be a game-changer in the long COVID fight, for it’s not just the fatigue, brain fog, heart palpitations, and other persistent symptoms that affect patients. Two out of three people with long COVID also suffer mental health challenges like depression and anxiety. Some patients say their symptoms are not taken seriously by their doctors. And as many as 12% of long COVID patients are unemployed because of the severity of their illness and their employers may be skeptical of their condition.
Quick, accurate diagnosis would eliminate all that. Now a new preprint study suggests that the elevation of certain immune system proteins are a commonality in long COVID patients and identifying them may be an accurate way to diagnose the condition.
Researchers at Cardiff (Wales) University, tracked 166 patients, 79 of whom had been diagnosed with long COVID and 87 who had not. All participants had recovered from a severe bout of acute COVID-19.
In an analysis of the blood plasma of the study participants, researchers found elevated levels of certain components. Four proteins in particular – Ba, iC3b, C5a, and TCC – predicted the presence of long COVID with 78.5% accuracy.
“I was gobsmacked by the results. We’re seeing a massive dysregulation in those four biomarkers,” says study author Wioleta Zelek, PhD, a research fellow at Cardiff University. “It’s a combination that we showed was predictive of long COVID.”
The study revealed that long COVID was associated with inflammation of the immune system causing these complement proteins to remain dysregulated. Proteins like C3, C4, and C5 are important parts of the immune system because they recruit phagocytes, cells that attack and engulf bacteria and viruses at the site of infection to destroy pathogens like SARS-coV-2.
In the case of long COVID, these proteins remain chronically elevated. While the symptoms of long COVID have seemed largely unrelated to one another, researchers point to elevated inflammation as a connecting factor that causes various systems in the body to go haywire.
“Anything that could help to better diagnose patients with long COVID is research we’re greatly appreciative of within the clinical community,” said Nisha Viswanathan, MD, director of the University of California, Los Angeles, Long COVID program at UCLA Health.
Testing for biomarkers highlighted in the study, as well as others like serotonin and cortisol, may help doctors separate patients who have long COVID from patients who have similar symptoms caused by other conditions, said Dr. Viswanathan. For example, a recent study published in the journal Cell found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but recovered from the condition.
Dr. Viswanathan cautions that the biomarker test does not answer all the questions about diagnosing long COVID. For example, Dr. Viswanathan said scientists don’t know whether complement dysregulation is caused by long COVID and not another underlying medical issue that patients had prior to infection, because “we don’t know where patients’ levels were prior to developing long COVID.” For example, those with autoimmune issues are more likely to develop long COVID, which means their levels could have been elevated prior to a COVID infection.
It is increasingly likely, said Dr. Viswanathan, that long COVID is an umbrella term for a host of conditions that could be caused by different impacts of the virus. Other research has pointed to the different phenotypes of long COVID. For example, some are focused on cardiopulmonary issues and others on fatigue and gastrointestinal problems.
“It looks like these different phenotypes have a different mechanism for disease,” she said. This means that it’s less likely to be a one-size-fits-all condition and the next step in the research should be identifying which biomarker is aligned with which phenotype of the disease.
Better diagnostics will open the door to better treatments, Dr. Zelek said. The more doctors understand about the mechanism causing immune dysregulation in long COVID patients, the more they can treat it with existing medications. Dr. Zelek’s lab has been studying certain medications like pegcetacoplan (C3 blocker), danicopan (anti-factor D), and iptacopan (anti-factor B) that can be used to break the body’s cycle of inflammation and reduce symptoms experienced in those with long COVID.
These drugs are approved by the U.S. Food and Drug Administration for the treatment of a rare blood disease called paroxysmal nocturnal hemoglobinuria. The C5 inhibitor zilucoplan has also been used in patients hospitalized with COVID-19 and researchers have found that the drug lowered serum C5 and interleukin-8 concentration in the blood, seeming to reduce certain aspects of the immune system’s inflammatory response to the virus.
The Cardiff University research is one of the most detailed studies to highlight long COVID biomarkers to date, said infectious disease specialist Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. The research needs to be duplicated in a larger study population that might include the other biomarkers like serotonin and cortisol to see if they’re related, she said.
Researchers are learning more everyday about the various biomarkers that may be linked to long COVID, she added. This Cardiff study showed that a huge percentage of those patients had elevated levels of certain complements. The next step, said Dr. McComsey, “is to put all these puzzle pieces together” so that clinicians have a common diagnostic tool or tools that provide patients with some peace of mind in starting their road to recovery.
A version of this article first appeared on Medscape.com.
One of the biggest challenges facing clinicians who treat long COVID is a lack of consensus when it comes to recognizing and diagnosing the condition. But
Effective diagnostic testing would be a game-changer in the long COVID fight, for it’s not just the fatigue, brain fog, heart palpitations, and other persistent symptoms that affect patients. Two out of three people with long COVID also suffer mental health challenges like depression and anxiety. Some patients say their symptoms are not taken seriously by their doctors. And as many as 12% of long COVID patients are unemployed because of the severity of their illness and their employers may be skeptical of their condition.
Quick, accurate diagnosis would eliminate all that. Now a new preprint study suggests that the elevation of certain immune system proteins are a commonality in long COVID patients and identifying them may be an accurate way to diagnose the condition.
Researchers at Cardiff (Wales) University, tracked 166 patients, 79 of whom had been diagnosed with long COVID and 87 who had not. All participants had recovered from a severe bout of acute COVID-19.
In an analysis of the blood plasma of the study participants, researchers found elevated levels of certain components. Four proteins in particular – Ba, iC3b, C5a, and TCC – predicted the presence of long COVID with 78.5% accuracy.
“I was gobsmacked by the results. We’re seeing a massive dysregulation in those four biomarkers,” says study author Wioleta Zelek, PhD, a research fellow at Cardiff University. “It’s a combination that we showed was predictive of long COVID.”
The study revealed that long COVID was associated with inflammation of the immune system causing these complement proteins to remain dysregulated. Proteins like C3, C4, and C5 are important parts of the immune system because they recruit phagocytes, cells that attack and engulf bacteria and viruses at the site of infection to destroy pathogens like SARS-coV-2.
In the case of long COVID, these proteins remain chronically elevated. While the symptoms of long COVID have seemed largely unrelated to one another, researchers point to elevated inflammation as a connecting factor that causes various systems in the body to go haywire.
“Anything that could help to better diagnose patients with long COVID is research we’re greatly appreciative of within the clinical community,” said Nisha Viswanathan, MD, director of the University of California, Los Angeles, Long COVID program at UCLA Health.
Testing for biomarkers highlighted in the study, as well as others like serotonin and cortisol, may help doctors separate patients who have long COVID from patients who have similar symptoms caused by other conditions, said Dr. Viswanathan. For example, a recent study published in the journal Cell found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but recovered from the condition.
Dr. Viswanathan cautions that the biomarker test does not answer all the questions about diagnosing long COVID. For example, Dr. Viswanathan said scientists don’t know whether complement dysregulation is caused by long COVID and not another underlying medical issue that patients had prior to infection, because “we don’t know where patients’ levels were prior to developing long COVID.” For example, those with autoimmune issues are more likely to develop long COVID, which means their levels could have been elevated prior to a COVID infection.
It is increasingly likely, said Dr. Viswanathan, that long COVID is an umbrella term for a host of conditions that could be caused by different impacts of the virus. Other research has pointed to the different phenotypes of long COVID. For example, some are focused on cardiopulmonary issues and others on fatigue and gastrointestinal problems.
“It looks like these different phenotypes have a different mechanism for disease,” she said. This means that it’s less likely to be a one-size-fits-all condition and the next step in the research should be identifying which biomarker is aligned with which phenotype of the disease.
Better diagnostics will open the door to better treatments, Dr. Zelek said. The more doctors understand about the mechanism causing immune dysregulation in long COVID patients, the more they can treat it with existing medications. Dr. Zelek’s lab has been studying certain medications like pegcetacoplan (C3 blocker), danicopan (anti-factor D), and iptacopan (anti-factor B) that can be used to break the body’s cycle of inflammation and reduce symptoms experienced in those with long COVID.
These drugs are approved by the U.S. Food and Drug Administration for the treatment of a rare blood disease called paroxysmal nocturnal hemoglobinuria. The C5 inhibitor zilucoplan has also been used in patients hospitalized with COVID-19 and researchers have found that the drug lowered serum C5 and interleukin-8 concentration in the blood, seeming to reduce certain aspects of the immune system’s inflammatory response to the virus.
The Cardiff University research is one of the most detailed studies to highlight long COVID biomarkers to date, said infectious disease specialist Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. The research needs to be duplicated in a larger study population that might include the other biomarkers like serotonin and cortisol to see if they’re related, she said.
Researchers are learning more everyday about the various biomarkers that may be linked to long COVID, she added. This Cardiff study showed that a huge percentage of those patients had elevated levels of certain complements. The next step, said Dr. McComsey, “is to put all these puzzle pieces together” so that clinicians have a common diagnostic tool or tools that provide patients with some peace of mind in starting their road to recovery.
A version of this article first appeared on Medscape.com.
FROM MEDRXIV
Psychosocial environmental factors may drive persistent childhood asthma
TOPLINE:
Children with asthma exposed to worsening psychosocial environmental factors during childhood were more likely to have more severe asthma symptoms than those without such exposures.
METHODOLOGY:
- The researchers reviewed data from the Longitudinal Study of Australian Children, a nationally representative cohort that also collects data on the health, psychosocial, and environmental status of parents, and used three multivariate models to assess the impact of psychosocial environmental factors on asthma symptoms at ages 1 year, 4-5 years, and 14-15 years.
- The study population included 3,917 children aged 0-15 years who were sorted into three asthma symptom trajectory groups (low/no asthma, transient high asthma, and persistent high asthma); asthma symptoms were defined as a history of chest wheezing lasting at least a week within the past 12 months.
- The researchers identified several psychosocial environmental factors as exposure variables on the basis of literature reviews; these factors were maternal depression, parents’ financial hardship, parental availability, and parental stressful life events.
TAKEAWAY:
- The mean scores of psychosocial factors for the overall study population remained stable over time, but groups of children exposed to bad trajectories of psychosocial factors were significantly more likely to have transient high and persistent high asthma symptoms.
- In the first year of life, only parents’ stressful life events were significantly associated with the persistent high asthma symptom trajectory group in an adjusted analysis.
- At age 4-5 years, maternal depression, low parental availability, and parents’ stressful life events were significantly associated with persistent high asthma; parents’ financial hardship was significantly associated with transient high asthma symptoms.
- At age 14-15 years, children exposed to “moderate and increasing” maternal depression, “moderate and declining” parents’ financial hardship, and “moderate and increasing” parents’ stressful life events were significantly associated with persistent high asthma versus no or low asthma, with relative risk ratios of 1.55, 1.40, and 1.77, respectively.
IN PRACTICE:
The study findings highlight the need for policy makers to take action to improve asthma control in children by reducing exposure to harmful psychosocial environmental factors, the researchers concluded.
SOURCE:
The lead author of the study was K.M. Shahunja, MBBS, PhD candidate at the University of Queensland, Brisbane, Australia. The study was published online in Pediatric Pulmonology.
LIMITATIONS:
The study is the first known to examine asthma symptom trajectories at different developmental stages, but participant attrition and missing values were limiting factors, as was the inability to account for all potential psychosocial environmental factors that might influence asthma symptoms in childhood.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
Children with asthma exposed to worsening psychosocial environmental factors during childhood were more likely to have more severe asthma symptoms than those without such exposures.
METHODOLOGY:
- The researchers reviewed data from the Longitudinal Study of Australian Children, a nationally representative cohort that also collects data on the health, psychosocial, and environmental status of parents, and used three multivariate models to assess the impact of psychosocial environmental factors on asthma symptoms at ages 1 year, 4-5 years, and 14-15 years.
- The study population included 3,917 children aged 0-15 years who were sorted into three asthma symptom trajectory groups (low/no asthma, transient high asthma, and persistent high asthma); asthma symptoms were defined as a history of chest wheezing lasting at least a week within the past 12 months.
- The researchers identified several psychosocial environmental factors as exposure variables on the basis of literature reviews; these factors were maternal depression, parents’ financial hardship, parental availability, and parental stressful life events.
TAKEAWAY:
- The mean scores of psychosocial factors for the overall study population remained stable over time, but groups of children exposed to bad trajectories of psychosocial factors were significantly more likely to have transient high and persistent high asthma symptoms.
- In the first year of life, only parents’ stressful life events were significantly associated with the persistent high asthma symptom trajectory group in an adjusted analysis.
- At age 4-5 years, maternal depression, low parental availability, and parents’ stressful life events were significantly associated with persistent high asthma; parents’ financial hardship was significantly associated with transient high asthma symptoms.
- At age 14-15 years, children exposed to “moderate and increasing” maternal depression, “moderate and declining” parents’ financial hardship, and “moderate and increasing” parents’ stressful life events were significantly associated with persistent high asthma versus no or low asthma, with relative risk ratios of 1.55, 1.40, and 1.77, respectively.
IN PRACTICE:
The study findings highlight the need for policy makers to take action to improve asthma control in children by reducing exposure to harmful psychosocial environmental factors, the researchers concluded.
SOURCE:
The lead author of the study was K.M. Shahunja, MBBS, PhD candidate at the University of Queensland, Brisbane, Australia. The study was published online in Pediatric Pulmonology.
LIMITATIONS:
The study is the first known to examine asthma symptom trajectories at different developmental stages, but participant attrition and missing values were limiting factors, as was the inability to account for all potential psychosocial environmental factors that might influence asthma symptoms in childhood.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
TOPLINE:
Children with asthma exposed to worsening psychosocial environmental factors during childhood were more likely to have more severe asthma symptoms than those without such exposures.
METHODOLOGY:
- The researchers reviewed data from the Longitudinal Study of Australian Children, a nationally representative cohort that also collects data on the health, psychosocial, and environmental status of parents, and used three multivariate models to assess the impact of psychosocial environmental factors on asthma symptoms at ages 1 year, 4-5 years, and 14-15 years.
- The study population included 3,917 children aged 0-15 years who were sorted into three asthma symptom trajectory groups (low/no asthma, transient high asthma, and persistent high asthma); asthma symptoms were defined as a history of chest wheezing lasting at least a week within the past 12 months.
- The researchers identified several psychosocial environmental factors as exposure variables on the basis of literature reviews; these factors were maternal depression, parents’ financial hardship, parental availability, and parental stressful life events.
TAKEAWAY:
- The mean scores of psychosocial factors for the overall study population remained stable over time, but groups of children exposed to bad trajectories of psychosocial factors were significantly more likely to have transient high and persistent high asthma symptoms.
- In the first year of life, only parents’ stressful life events were significantly associated with the persistent high asthma symptom trajectory group in an adjusted analysis.
- At age 4-5 years, maternal depression, low parental availability, and parents’ stressful life events were significantly associated with persistent high asthma; parents’ financial hardship was significantly associated with transient high asthma symptoms.
- At age 14-15 years, children exposed to “moderate and increasing” maternal depression, “moderate and declining” parents’ financial hardship, and “moderate and increasing” parents’ stressful life events were significantly associated with persistent high asthma versus no or low asthma, with relative risk ratios of 1.55, 1.40, and 1.77, respectively.
IN PRACTICE:
The study findings highlight the need for policy makers to take action to improve asthma control in children by reducing exposure to harmful psychosocial environmental factors, the researchers concluded.
SOURCE:
The lead author of the study was K.M. Shahunja, MBBS, PhD candidate at the University of Queensland, Brisbane, Australia. The study was published online in Pediatric Pulmonology.
LIMITATIONS:
The study is the first known to examine asthma symptom trajectories at different developmental stages, but participant attrition and missing values were limiting factors, as was the inability to account for all potential psychosocial environmental factors that might influence asthma symptoms in childhood.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Food insecurity a dementia risk factor?
TOPLINE:
Food insecurity among older adults is associated with increased dementia risk, poorer memory function, and faster memory decline, new research indicates.
METHODOLOGY:
- Researchers analyzed data on 7,012 adults (mean age, 67 years; 59% women) from the U.S. Health and Retirement Study.
- Food security status was assessed in 2013 using a validated survey, with cognitive outcomes evaluated between 2014 and 2018.
- Analyses were adjusted for demographics, socioeconomics, and health factors.
TAKEAWAY:
- About 18% of adults were food insecure, with 10% reporting low food security and 8% very low food security. About 11% of those aged 65+ in 2013 were food insecure.
- The odds of dementia were 38% higher (odds ratio, 1.38; 95% confidence interval [CI], 1.15-1.67) in adults with low food security and 37% higher (OR, 1.37; 95% CI, 1.11-1.59) in those with very low food security, compared with food-secure adults.
- Translated to years of excess cognitive aging, food insecurity was associated with increased dementia risk equivalent to roughly 1.3 excess years of aging.
- Low and very low food security were also associated with lower memory levels and faster age-related memory decline.
IN PRACTICE:
“Our study contributes to a limited literature by capitalizing on a large and diverse sample, validated exposure and outcome measures, and longitudinal data to robustly evaluate these associations, providing evidence in support of the connection between food insecurity in older adulthood and subsequent brain health,” the authors wrote. “Our findings highlight the need to improve food security in older adults and that doing so may protect individuals from cognitive decline and dementia.”
SOURCE:
The study, with first author Haobing Qian, PhD, with the University of California, San Francisco, was published online in JAMA Network Open.
LIMITATIONS:
Residual confounding cannot be ruled out. Food insecurity was not assessed prior to 2013. The researchers lacked information on clinical dementia diagnoses.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Food insecurity among older adults is associated with increased dementia risk, poorer memory function, and faster memory decline, new research indicates.
METHODOLOGY:
- Researchers analyzed data on 7,012 adults (mean age, 67 years; 59% women) from the U.S. Health and Retirement Study.
- Food security status was assessed in 2013 using a validated survey, with cognitive outcomes evaluated between 2014 and 2018.
- Analyses were adjusted for demographics, socioeconomics, and health factors.
TAKEAWAY:
- About 18% of adults were food insecure, with 10% reporting low food security and 8% very low food security. About 11% of those aged 65+ in 2013 were food insecure.
- The odds of dementia were 38% higher (odds ratio, 1.38; 95% confidence interval [CI], 1.15-1.67) in adults with low food security and 37% higher (OR, 1.37; 95% CI, 1.11-1.59) in those with very low food security, compared with food-secure adults.
- Translated to years of excess cognitive aging, food insecurity was associated with increased dementia risk equivalent to roughly 1.3 excess years of aging.
- Low and very low food security were also associated with lower memory levels and faster age-related memory decline.
IN PRACTICE:
“Our study contributes to a limited literature by capitalizing on a large and diverse sample, validated exposure and outcome measures, and longitudinal data to robustly evaluate these associations, providing evidence in support of the connection between food insecurity in older adulthood and subsequent brain health,” the authors wrote. “Our findings highlight the need to improve food security in older adults and that doing so may protect individuals from cognitive decline and dementia.”
SOURCE:
The study, with first author Haobing Qian, PhD, with the University of California, San Francisco, was published online in JAMA Network Open.
LIMITATIONS:
Residual confounding cannot be ruled out. Food insecurity was not assessed prior to 2013. The researchers lacked information on clinical dementia diagnoses.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Food insecurity among older adults is associated with increased dementia risk, poorer memory function, and faster memory decline, new research indicates.
METHODOLOGY:
- Researchers analyzed data on 7,012 adults (mean age, 67 years; 59% women) from the U.S. Health and Retirement Study.
- Food security status was assessed in 2013 using a validated survey, with cognitive outcomes evaluated between 2014 and 2018.
- Analyses were adjusted for demographics, socioeconomics, and health factors.
TAKEAWAY:
- About 18% of adults were food insecure, with 10% reporting low food security and 8% very low food security. About 11% of those aged 65+ in 2013 were food insecure.
- The odds of dementia were 38% higher (odds ratio, 1.38; 95% confidence interval [CI], 1.15-1.67) in adults with low food security and 37% higher (OR, 1.37; 95% CI, 1.11-1.59) in those with very low food security, compared with food-secure adults.
- Translated to years of excess cognitive aging, food insecurity was associated with increased dementia risk equivalent to roughly 1.3 excess years of aging.
- Low and very low food security were also associated with lower memory levels and faster age-related memory decline.
IN PRACTICE:
“Our study contributes to a limited literature by capitalizing on a large and diverse sample, validated exposure and outcome measures, and longitudinal data to robustly evaluate these associations, providing evidence in support of the connection between food insecurity in older adulthood and subsequent brain health,” the authors wrote. “Our findings highlight the need to improve food security in older adults and that doing so may protect individuals from cognitive decline and dementia.”
SOURCE:
The study, with first author Haobing Qian, PhD, with the University of California, San Francisco, was published online in JAMA Network Open.
LIMITATIONS:
Residual confounding cannot be ruled out. Food insecurity was not assessed prior to 2013. The researchers lacked information on clinical dementia diagnoses.
DISCLOSURES:
The study was supported by grants from the National Institutes of Health. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.