User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
CDC calls for masks in schools, hard-hit areas, even if vaccinated
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
The agency has called for masks in K-12 school settings and in areas of the United States experiencing high or substantial SARS-CoV-2 transmission, even for the fully vaccinated.
The move reverses a controversial announcement the agency made in May 2021 that fully vaccinated Americans could skip wearing a mask in most settings.
Unlike the increasing vaccination rates and decreasing case numbers reported in May, however, some regions of the United States are now reporting large jumps in COVID-19 case numbers. And the Delta variant as well as new evidence of transmission from breakthrough cases are largely driving these changes.
“Today we have new science related to the [D]elta variant that requires us to update the guidance on what you can do when you are fully vaccinated,” CDC Director Rochelle Walensky, MD, MPH, said during a media briefing July 27.
New evidence has emerged on breakthrough-case transmission risk, for example. “Information on the [D]elta variant from several states and other countries indicates that in rare cases, some people infected with the [D]elta variant after vaccination may be contagious and spread virus to others,” Dr. Walensky said, adding that the viral loads appear to be about the same in vaccinated and unvaccinated individuals.
“This new science is worrisome,” she said.
Even though unvaccinated people represent the vast majority of cases of transmission, Dr. Walensky said, “we thought it was important for [vaccinated] people to understand they have the potential to transmit the virus to others.”
As a result, in addition to continuing to strongly encourage everyone to get vaccinated, the CDC recommends that fully vaccinated people wear masks in public indoor settings to help prevent the spread of the Delta variant in areas with substantial or high transmission, Dr. Walensky said. “This includes schools.”
Masks in schools
The CDC is now recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status. Their goal is to optimize safety and allow children to return to full-time in-person learning in the fall.
The CDC tracks substantial and high transmission rates through the agency’s COVID Data Tracker site. Substantial transmission means between 50 and 100 cases per 100,000 people reported over 7 days and high means more than 100 cases per 100,000 people.
The B.1.617.2, or Delta, variant is believed to be responsible for COVID-19 cases increasing more than 300% nationally from June 19 to July 23, 2021.
“A prudent move”
“I think it’s a prudent move. Given the dominance of the [D]elta variant and the caseloads that we are seeing rising in many locations across the United States, including in my backyard here in San Francisco,” Joe DeRisi, PhD, copresident of the Chan Zuckerberg Biohub and professor of biochemistry and biophysics at the University of California San Francisco, said in an interview.
Dr. DeRisi said he was not surprised that vaccinated people with breakthrough infections could be capable of transmitting the virus. He added that clinical testing done by the Biohub and UCSF produced a lot of data on viral load levels, “and they cover an enormous range.”
What was unexpected to him was the rapid rise of the dominant variant. “The rise of the [D]elta strain is astonishing. It’s happened so fast,” he said.
“I know it’s difficult”
Reacting to the news, Colleen Kraft, MD, said, “One of the things that we’re learning is that if we’re going to have low vaccine uptake or we have a number of people that can’t be vaccinated yet, such as children, that we really need to go back to stopping transmission, which involves mask wearing.”
“I know that it’s very difficult and people feel like we’re sliding backward,” Dr. Kraft said during a media briefing sponsored by Emory University held shortly after the CDC announcement.
She added that the CDC updated guidance seems appropriate. “I don’t think any of us really want to be in this position or want to go back to masking but…we’re finding ourselves in the same place we were a year ago, in July 2020.
“In general we just don’t want anybody to be infected even if there’s a small chance for you to be infected and there’s a small chance for you to transmit it,” said Dr. Kraft, who’s an assistant professor in the department of pathology and associate professor in the department of medicine, division of infectious diseases at Emory University School of Medicine in Atlanta.
Breakthrough transmissions
“The good news is you’re still unlikely to get critically ill if you’re vaccinated. But what has changed with the [D]elta variant is instead of being 90% plus protected from getting the virus at all, you’re probably more in the 70% to 80% range,” James T. McDeavitt, MD, told this news organization.
“So we’re seeing breakthrough infections,” said Dr. McDeavitt, executive vice president and dean of clinical affairs at Baylor College of Medicine in Houston. “We are starting to see [such people] are potentially infectious.” Even if a vaccinated person is individually much less likely to experience serious COVID-19 outcomes, “they can spread it to someone else who spreads it to someone else who is more vulnerable. It puts the more at-risk populations at further risk.”
It breaks down to individual and public health concerns. “I am fully vaccinated. I am very confident I am not going to end up in a hospital,” he said. “Now if I were unvaccinated, with the prevalence of the virus around the country, I’m probably in more danger than I’ve ever been in the course of the pandemic. The unvaccinated are really at risk right now.”
IDSA and AMA support mask change
The Infectious Diseases Society of America (IDSA) has released a statement supporting the new CDC recommendations. “To stay ahead of the spread of the highly transmissible Delta variant, IDSA also urges that in communities with moderate transmission rates, all individuals, even those who are vaccinated, wear masks in indoor public places,” stated IDSA President Barbara D. Alexander, MD, MHS.
“IDSA also supports CDC’s guidance recommending universal indoor masking for all teachers, staff, students, and visitors to K-12 schools, regardless of vaccination status, until vaccines are authorized and widely available to all children and vaccination rates are sufficient to control transmission.”
“Mask wearing will help reduce infections, prevent serious illnesses and death, limit strain on local hospitals and stave off the development of even more troubling variants,” she added.
The American Medical Association (AMA) also released a statement supporting the CDC’s policy changes.
“According to the CDC, emerging data indicates that vaccinated individuals infected with the Delta variant have similar viral loads as those who are unvaccinated and are capable of transmission,” AMA President Gerald E. Harmon, MD said in the statement.
“However, the science remains clear, the authorized vaccines remain safe and effective in preventing severe complications from COVID-19, including hospitalization and death,” he stated. “We strongly support the updated recommendations, which call for universal masking in areas of high or substantial COVID-19 transmission and in K-12 schools, to help reduce transmission of the virus. Wearing a mask is a small but important protective measure that can help us all stay safer.”
“The highest spread of cases and [most] severe outcomes are happening in places with low vaccination rates and among unvaccinated people,” Dr. Walensky said. “With the [D]elta variant, vaccinating more Americans now is more urgent than ever.”
“This moment, and the associated suffering, illness, and death, could have been avoided with higher vaccination coverage in this country,” she said.
A version of this article first appeared on Medscape.com.
Children and COVID: Vaccinations, new cases both rising
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.
the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
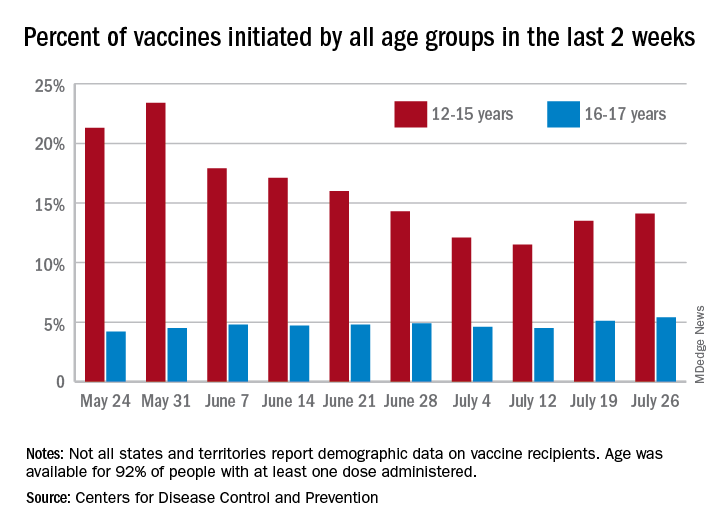
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.

the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.

the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
Vaccine breakthrough cases rising with Delta: Here’s what that means
At a recent town hall meeting in Cincinnati, President Joe Biden was asked about COVID-19 cases, hospitalizations, and deaths rising in response to the Delta variant.
Touting the importance of vaccination, “We have a pandemic for those who haven’t gotten a vaccination. It’s that basic, that simple,” President Biden said at the event, which was broadcast live on CNN.
“If you’re vaccinated, you’re not going to be hospitalized, not going to the ICU unit, and not going to die,” he said, adding “you’re not going to get COVID if you have these vaccinations.”
Unfortunately, it’s not so simple. Fully vaccinated people continue to be well protected against severe disease and death, even with Delta, Because of that, many experts continue to advise caution, even if fully vaccinated.
“I was disappointed,” Leana Wen, MD, MSc, an emergency physician and visiting professor of health policy and management at George Washington University’s Milken School of Public Health in Washington, told CNN in response to the president’s statement.
“I actually thought he was answering questions as if it were a month ago. He’s not really meeting the realities of what’s happening on the ground,” she said. “I think he may have led people astray.”
Vaccines still work
Recent cases support Dr. Wen’s claim. Fully vaccinated Olympic athletes, wedding guests, healthcare workers, and even White House staff have recently tested positive. So what gives?
The vast majority of these illnesses are mild, and public health officials say they are to be expected.
“The vaccines were designed to keep us out of the hospital and to keep us from dying. That was the whole purpose of the vaccine and they’re even more successful than we anticipated,” says William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville.
As good as they are, these shots aren’t perfect. Their protection differs from person to person depending on age and underlying health. People with immune function that’s weakened because of age or a health condition can still become seriously ill, and, in very rare cases, die after vaccination.
When people are infected with Delta, they carry approximately 1,000 times more virus compared with previous versions of the virus, according to a recent study. All that virus can overwhelm even the strong protection from the vaccines.
“Three months ago, breakthroughs didn’t occur nearly at this rate because there was just so much less virus exposure in the community,” said Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota in Minneapolis.
Breakthroughs by the numbers
In Los Angeles County, where 69% of residents over age 12 have been fully vaccinated, COVID-19 cases are rising, and so, too, are cases that break through the protection of the vaccine.
In June, fully vaccinated people accounted for 20%, or 1 in 5, COVID cases in the county, which is the most populous in the United States. The increase mirrors Delta’s rise. The proportion of breakthrough cases is up from 11% in May, 5% in April, and 2% in March, according to the Los Angeles County Department of Public Health.
In the United Kingdom, which is collecting the best information on infections caused by variants, the estimated effectiveness of the vaccines to prevent an illness that causes symptoms dropped by about 10 points against Delta compared with Alpha (or B.1.1.7).
After two doses, vaccines prevent symptomatic infection about 79% of the time against Delta, according to data compiled by Public Health England. They are still highly effective at preventing hospitalization, 96% after two doses.
Out of 229,218 COVID infections in the United Kingdom between February and July 19, 28,773 — or 12.5% — were in fully vaccinated people. Of those breakthrough infections, 1,101, or 3.8%, required a visit to an emergency room, according to Public Health England. Just 474, or 2.9%, of fully vaccinated people required hospital admission, and 229, or less than 1%, died.
Unanswered questions
One of the biggest questions about breakthrough cases is how often people who have it may pass the virus to others.
“We know the vaccine reduces the likelihood of carrying the virus and the amount of virus you would carry,” Dr. Wen told CNN. But we don’t yet know whether a vaccinated person with a breakthrough infection may still be contagious to others.
For that reason, the Centers for Disease Control and Prevention says that fully vaccinated people still need to be tested if they have symptoms and shouldn’t be out in public for at least 10 days after a positive test.
How should fully vaccinated people behave? That depends a lot on their underlying health and whether or not they have vulnerable people around them.
If you’re older or immunocompromised, Dr. Schaffner recommends what he calls the “belt-and-suspenders approach,” in other words, do everything you can to stay safe.
“Get vaccinated for sure, but since we can’t be absolutely certain that the vaccines are going to be optimally protective and you are particularly susceptible to serious disease, you would be well advised to adopt at least one and perhaps more of the other mitigation measures,” he said.
These include wearing a mask, social distancing, making sure your spaces are well ventilated, and not spending prolonged periods of time indoors in crowded places.
Taking young children to visit vaccinated, elderly grandparents demands extra caution, again, with Delta circulating, particularly as they go back to school and start mixing with other kids.
Dr. Schaffner recommends explaining the ground rules before the visit: Hugs around the waist. No kissing. Wearing a mask while indoors with them.
Other important unanswered questions are whether breakthrough infections can lead to prolonged symptoms, or “long covid.” Most experts think that’s less likely in vaccinated people.
And Dr. Osterholm said it will be important to see whether there’s anything unusual about the breakthrough cases happening in the community.
“I think some of us have been challenged by the number of clusters that we’ve seen,” he said. “I think that really needs to be examined more.”
A version of this article first appeared on Medscape.com.
At a recent town hall meeting in Cincinnati, President Joe Biden was asked about COVID-19 cases, hospitalizations, and deaths rising in response to the Delta variant.
Touting the importance of vaccination, “We have a pandemic for those who haven’t gotten a vaccination. It’s that basic, that simple,” President Biden said at the event, which was broadcast live on CNN.
“If you’re vaccinated, you’re not going to be hospitalized, not going to the ICU unit, and not going to die,” he said, adding “you’re not going to get COVID if you have these vaccinations.”
Unfortunately, it’s not so simple. Fully vaccinated people continue to be well protected against severe disease and death, even with Delta, Because of that, many experts continue to advise caution, even if fully vaccinated.
“I was disappointed,” Leana Wen, MD, MSc, an emergency physician and visiting professor of health policy and management at George Washington University’s Milken School of Public Health in Washington, told CNN in response to the president’s statement.
“I actually thought he was answering questions as if it were a month ago. He’s not really meeting the realities of what’s happening on the ground,” she said. “I think he may have led people astray.”
Vaccines still work
Recent cases support Dr. Wen’s claim. Fully vaccinated Olympic athletes, wedding guests, healthcare workers, and even White House staff have recently tested positive. So what gives?
The vast majority of these illnesses are mild, and public health officials say they are to be expected.
“The vaccines were designed to keep us out of the hospital and to keep us from dying. That was the whole purpose of the vaccine and they’re even more successful than we anticipated,” says William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville.
As good as they are, these shots aren’t perfect. Their protection differs from person to person depending on age and underlying health. People with immune function that’s weakened because of age or a health condition can still become seriously ill, and, in very rare cases, die after vaccination.
When people are infected with Delta, they carry approximately 1,000 times more virus compared with previous versions of the virus, according to a recent study. All that virus can overwhelm even the strong protection from the vaccines.
“Three months ago, breakthroughs didn’t occur nearly at this rate because there was just so much less virus exposure in the community,” said Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota in Minneapolis.
Breakthroughs by the numbers
In Los Angeles County, where 69% of residents over age 12 have been fully vaccinated, COVID-19 cases are rising, and so, too, are cases that break through the protection of the vaccine.
In June, fully vaccinated people accounted for 20%, or 1 in 5, COVID cases in the county, which is the most populous in the United States. The increase mirrors Delta’s rise. The proportion of breakthrough cases is up from 11% in May, 5% in April, and 2% in March, according to the Los Angeles County Department of Public Health.
In the United Kingdom, which is collecting the best information on infections caused by variants, the estimated effectiveness of the vaccines to prevent an illness that causes symptoms dropped by about 10 points against Delta compared with Alpha (or B.1.1.7).
After two doses, vaccines prevent symptomatic infection about 79% of the time against Delta, according to data compiled by Public Health England. They are still highly effective at preventing hospitalization, 96% after two doses.
Out of 229,218 COVID infections in the United Kingdom between February and July 19, 28,773 — or 12.5% — were in fully vaccinated people. Of those breakthrough infections, 1,101, or 3.8%, required a visit to an emergency room, according to Public Health England. Just 474, or 2.9%, of fully vaccinated people required hospital admission, and 229, or less than 1%, died.
Unanswered questions
One of the biggest questions about breakthrough cases is how often people who have it may pass the virus to others.
“We know the vaccine reduces the likelihood of carrying the virus and the amount of virus you would carry,” Dr. Wen told CNN. But we don’t yet know whether a vaccinated person with a breakthrough infection may still be contagious to others.
For that reason, the Centers for Disease Control and Prevention says that fully vaccinated people still need to be tested if they have symptoms and shouldn’t be out in public for at least 10 days after a positive test.
How should fully vaccinated people behave? That depends a lot on their underlying health and whether or not they have vulnerable people around them.
If you’re older or immunocompromised, Dr. Schaffner recommends what he calls the “belt-and-suspenders approach,” in other words, do everything you can to stay safe.
“Get vaccinated for sure, but since we can’t be absolutely certain that the vaccines are going to be optimally protective and you are particularly susceptible to serious disease, you would be well advised to adopt at least one and perhaps more of the other mitigation measures,” he said.
These include wearing a mask, social distancing, making sure your spaces are well ventilated, and not spending prolonged periods of time indoors in crowded places.
Taking young children to visit vaccinated, elderly grandparents demands extra caution, again, with Delta circulating, particularly as they go back to school and start mixing with other kids.
Dr. Schaffner recommends explaining the ground rules before the visit: Hugs around the waist. No kissing. Wearing a mask while indoors with them.
Other important unanswered questions are whether breakthrough infections can lead to prolonged symptoms, or “long covid.” Most experts think that’s less likely in vaccinated people.
And Dr. Osterholm said it will be important to see whether there’s anything unusual about the breakthrough cases happening in the community.
“I think some of us have been challenged by the number of clusters that we’ve seen,” he said. “I think that really needs to be examined more.”
A version of this article first appeared on Medscape.com.
At a recent town hall meeting in Cincinnati, President Joe Biden was asked about COVID-19 cases, hospitalizations, and deaths rising in response to the Delta variant.
Touting the importance of vaccination, “We have a pandemic for those who haven’t gotten a vaccination. It’s that basic, that simple,” President Biden said at the event, which was broadcast live on CNN.
“If you’re vaccinated, you’re not going to be hospitalized, not going to the ICU unit, and not going to die,” he said, adding “you’re not going to get COVID if you have these vaccinations.”
Unfortunately, it’s not so simple. Fully vaccinated people continue to be well protected against severe disease and death, even with Delta, Because of that, many experts continue to advise caution, even if fully vaccinated.
“I was disappointed,” Leana Wen, MD, MSc, an emergency physician and visiting professor of health policy and management at George Washington University’s Milken School of Public Health in Washington, told CNN in response to the president’s statement.
“I actually thought he was answering questions as if it were a month ago. He’s not really meeting the realities of what’s happening on the ground,” she said. “I think he may have led people astray.”
Vaccines still work
Recent cases support Dr. Wen’s claim. Fully vaccinated Olympic athletes, wedding guests, healthcare workers, and even White House staff have recently tested positive. So what gives?
The vast majority of these illnesses are mild, and public health officials say they are to be expected.
“The vaccines were designed to keep us out of the hospital and to keep us from dying. That was the whole purpose of the vaccine and they’re even more successful than we anticipated,” says William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville.
As good as they are, these shots aren’t perfect. Their protection differs from person to person depending on age and underlying health. People with immune function that’s weakened because of age or a health condition can still become seriously ill, and, in very rare cases, die after vaccination.
When people are infected with Delta, they carry approximately 1,000 times more virus compared with previous versions of the virus, according to a recent study. All that virus can overwhelm even the strong protection from the vaccines.
“Three months ago, breakthroughs didn’t occur nearly at this rate because there was just so much less virus exposure in the community,” said Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota in Minneapolis.
Breakthroughs by the numbers
In Los Angeles County, where 69% of residents over age 12 have been fully vaccinated, COVID-19 cases are rising, and so, too, are cases that break through the protection of the vaccine.
In June, fully vaccinated people accounted for 20%, or 1 in 5, COVID cases in the county, which is the most populous in the United States. The increase mirrors Delta’s rise. The proportion of breakthrough cases is up from 11% in May, 5% in April, and 2% in March, according to the Los Angeles County Department of Public Health.
In the United Kingdom, which is collecting the best information on infections caused by variants, the estimated effectiveness of the vaccines to prevent an illness that causes symptoms dropped by about 10 points against Delta compared with Alpha (or B.1.1.7).
After two doses, vaccines prevent symptomatic infection about 79% of the time against Delta, according to data compiled by Public Health England. They are still highly effective at preventing hospitalization, 96% after two doses.
Out of 229,218 COVID infections in the United Kingdom between February and July 19, 28,773 — or 12.5% — were in fully vaccinated people. Of those breakthrough infections, 1,101, or 3.8%, required a visit to an emergency room, according to Public Health England. Just 474, or 2.9%, of fully vaccinated people required hospital admission, and 229, or less than 1%, died.
Unanswered questions
One of the biggest questions about breakthrough cases is how often people who have it may pass the virus to others.
“We know the vaccine reduces the likelihood of carrying the virus and the amount of virus you would carry,” Dr. Wen told CNN. But we don’t yet know whether a vaccinated person with a breakthrough infection may still be contagious to others.
For that reason, the Centers for Disease Control and Prevention says that fully vaccinated people still need to be tested if they have symptoms and shouldn’t be out in public for at least 10 days after a positive test.
How should fully vaccinated people behave? That depends a lot on their underlying health and whether or not they have vulnerable people around them.
If you’re older or immunocompromised, Dr. Schaffner recommends what he calls the “belt-and-suspenders approach,” in other words, do everything you can to stay safe.
“Get vaccinated for sure, but since we can’t be absolutely certain that the vaccines are going to be optimally protective and you are particularly susceptible to serious disease, you would be well advised to adopt at least one and perhaps more of the other mitigation measures,” he said.
These include wearing a mask, social distancing, making sure your spaces are well ventilated, and not spending prolonged periods of time indoors in crowded places.
Taking young children to visit vaccinated, elderly grandparents demands extra caution, again, with Delta circulating, particularly as they go back to school and start mixing with other kids.
Dr. Schaffner recommends explaining the ground rules before the visit: Hugs around the waist. No kissing. Wearing a mask while indoors with them.
Other important unanswered questions are whether breakthrough infections can lead to prolonged symptoms, or “long covid.” Most experts think that’s less likely in vaccinated people.
And Dr. Osterholm said it will be important to see whether there’s anything unusual about the breakthrough cases happening in the community.
“I think some of us have been challenged by the number of clusters that we’ve seen,” he said. “I think that really needs to be examined more.”
A version of this article first appeared on Medscape.com.
CDC panel updates info on rare side effect after J&J vaccine
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Despite recent reports of Guillain-Barré Syndrome (GBS) after the Johnson & Johnson vaccine,
The company also presented new data suggesting that the shots generate strong immune responses against circulating variants and that antibodies generated by the vaccine stay elevated for at least 8 months.
Members of the Advisory Committee on Immunization Practices (ACIP) did not vote, but discussed and affirmed their support for recent decisions by the Food and Drug Administration and CDC to update patient information about the very low risk of GBS that appears to be associated with the vaccine, but to continue offering the vaccine to people in the United States.
The Johnson & Johnson shot has been a minor player in the U.S. vaccination campaign, accounting for less than 4% of all vaccine doses given in this country. Still, the single-dose inoculation, which doesn’t require ultra-cold storage, has been important for reaching people in rural areas, through mobile clinics, at colleges and primary care offices, and in vulnerable populations – those who are incarcerated or homeless.
The FDA says it has received reports of 100 cases of GBS after the Johnson & Johnson vaccine in its Vaccine Adverse Event Reporting System database through the end of June. The cases are still under investigation.
To date, more than 12 million doses of the vaccine have been administered, making the rate of GBS 8.1 cases for every million doses administered.
Although it is still extremely rare, that’s above the expected background rate of GBS of 1.6 cases for every million people, said Grace Lee, MD, a Stanford, Calif., pediatrician who chairs the ACIP’s Vaccine Safety Technical Work Group.
So far, most GBS cases (61%) have been among men. The midpoint age of the cases was 57 years. The average time to onset was 14 days, and 98% of cases occurred within 42 days of the shot. Facial paralysis has been associated with an estimated 30%-50% of cases. One person, who had heart failure, high blood pressure, and diabetes, has died.
Still, the benefits of the vaccine far outweigh its risks. For every million doses given to people over age 50, the vaccine prevents nearly 7,500 COVID-19 hospitalizations and nearly 100 deaths in women, and more than 13,000 COVID-19 hospitalizations and more than 2,400 deaths in men.
Rates of GBS after the mRNA vaccines made by Pfizer and Moderna were around 1 case for every 1 million doses given, which is not above the rate that would be expected without vaccination.
The link to the Johnson & Johnson vaccine prompted the FDA to add a warning to the vaccine’s patient safety information on July 12.
Also in July, the European Medicines Agency recommended a similar warning for the product information of the AstraZeneca vaccine Vaxzevria, which relies on similar technology.
Good against variants
Johnson & Johnson also presented new information showing its vaccine maintained high levels of neutralizing antibodies against four of the so-called “variants of concern” – Alpha, Gamma, Beta, and Delta. The protection generated by the vaccine lasted for at least 8 months after the shot, the company said.
“We’re still learning about the duration of protection and the breadth of coverage against this evolving variant landscape for each of the authorized vaccines,” said Mathai Mammen, MD, PhD, global head of research and development at Janssen, the company that makes the vaccine for J&J.
The company also said that its vaccine generated very strong T-cell responses. T cells destroy infected cells and, along with antibodies, are an important part of the body’s immune response.
Antibody levels and T-cell responses are markers for immunity. Measuring these levels isn’t the same as proving that shots can fend off an infection.
It’s still unclear exactly which component of the immune response is most important for fighting off COVID-19.
Dr. Mammen said the companies are still gathering that clinical data, and would present it soon.
“We will have a better view of the clinical efficacy in the coming weeks,” he said.
A version of this article first appeared on Medscape.com.
Necessary or not, COVID booster shots are probably on the horizon
The drug maker Pfizer recently announced that vaccinated people are likely to need a booster shot to be effectively protected against new variants of COVID-19 and that the company would apply for Food and Drug Administration emergency use authorization for the shot. Top government health officials immediately and emphatically announced that the booster isn’t needed right now – and held firm to that position even after Pfizer’s top scientist made his case and shared preliminary data with them on July 12.
This has led to confusion. Is the protection that has allowed them to see loved ones and go out to dinner fading?
Ultimately, the question of whether a booster is needed is unlikely to determine the FDA’s decision. If recent history is predictive, booster shots will be here before long. That’s because of the outdated, 60-year-old basic standard the FDA uses to authorize medicines for sale: Is a new drug “safe and effective?”
The FDA, using that standard, will very likely have to authorize Pfizer’s booster for emergency use, as it did the company’s prior COVID shot. The booster is likely to be safe – hundreds of millions have taken the earlier shots – and Pfizer reported that it dramatically increases a vaccinated person’s antibodies against SARS-CoV-2. From that perspective, it may also be considered very effective.
But does that kind of efficacy matter? Is a higher level of antibodies needed to protect vaccinated Americans? Though antibody levels may wane some over time, the current vaccines deliver perfectly good immunity so far.
What if a booster is safe and effective in one sense but simply not needed – at least for now?
Reliance on the simple “safe and effective” standard – which certainly sounds reasonable – is a relic of a time when there were far fewer and simpler medicines available to treat diseases and before pharmaceutical manufacturing became one of the world’s biggest businesses.
The FDA’s 1938 landmark legislation focused primarily on safety after more than 100 Americans died from a raspberry-flavored liquid form of an early antibiotic because one of its ingredients was used as antifreeze. The 1962 Kefauver-Harris Amendments to the Federal Food, Drug, and Cosmetic Act set out more specific requirements for drug approval: Companies must scientifically prove a drug’s effectiveness through “adequate and well-controlled studies.”
In today’s pharmaceutical universe, a simple “safe and effective” determination is not always an adequate bar, and it can be manipulated to sell drugs of questionable value. There’s also big money involved: Pfizer is already projecting $26 billion in COVID revenue in 2021.
The United States’ continued use of this standard to let drugs into the market has led to the approval of expensive, not necessarily very effective drugs. In 2014, for example, the FDA approved a toenail fungus drug that can cost up to $1,500 a month and that studies showed cured fewer than 10% of patients after a year of treatment. That’s more effective than doing nothing but less effective and more costly than a number of other treatments for this bothersome malady.
It has also led to a plethora of high-priced drugs to treat diseases like cancers, multiple sclerosis and type 2 diabetes that are all more effective than a placebo but have often not been tested very much against one another to determine which are most effective.
In today’s complex world, clarification is needed to determine just what kind of effectiveness the FDA should demand. And should that be the job of the FDA alone?
For example, should drugmakers prove a drug is significantly more effective than products already on the market? Or demonstrate cost-effectiveness – the health value of a product relative to its price – a metric used by Britain’s health system? And in which cases is effectiveness against a surrogate marker – like an antibody level – a good enough stand-in for whether a drug will have a significant impact on a patient’s health?
In most industrialized countries, broad access to the national market is a two-step process, said Aaron Kesselheim, a professor of medicine at Harvard Medical School, Boston, who studies drug development, marketing and law and recently served on an FDA advisory committee. The first part certifies that a drug is sufficiently safe and effective. That is immediately followed by an independent health technology assessment to see where it fits in the treatment armamentarium, including, in some countries, whether it is useful enough to be sold at all at the stated price. But there’s no such automatic process in the United States.
When Pfizer applies for authorization, the FDA may well clear a booster for the U.S. market. The Centers for Disease Control and Prevention, likely with advice from National Institutes of Health experts, will then have to decide whether to recommend it and for whom. This judgment call usually determines whether insurers will cover it. Pfizer is likely to profit handsomely from a government authorization, and the company will gain some revenue even if only the worried well, who can pay out of pocket, decide to get the shot.
To make any recommendation on a booster, government experts say they need more data. They could, for example, as Anthony S. Fauci, MD, has suggested, eventually green-light the additional vaccine shot only for a small group of patients at high risk for a deadly infection, such as the very old or transplant recipients who take immunosuppressant drugs, as some other countries have done.
But until the United States refines the FDA’s “safe and effective” standard or adds a second layer of vetting, when new products hit the market and manufacturers promote them, Americans will be left to decipher whose version of effective and necessary matters to them.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The drug maker Pfizer recently announced that vaccinated people are likely to need a booster shot to be effectively protected against new variants of COVID-19 and that the company would apply for Food and Drug Administration emergency use authorization for the shot. Top government health officials immediately and emphatically announced that the booster isn’t needed right now – and held firm to that position even after Pfizer’s top scientist made his case and shared preliminary data with them on July 12.
This has led to confusion. Is the protection that has allowed them to see loved ones and go out to dinner fading?
Ultimately, the question of whether a booster is needed is unlikely to determine the FDA’s decision. If recent history is predictive, booster shots will be here before long. That’s because of the outdated, 60-year-old basic standard the FDA uses to authorize medicines for sale: Is a new drug “safe and effective?”
The FDA, using that standard, will very likely have to authorize Pfizer’s booster for emergency use, as it did the company’s prior COVID shot. The booster is likely to be safe – hundreds of millions have taken the earlier shots – and Pfizer reported that it dramatically increases a vaccinated person’s antibodies against SARS-CoV-2. From that perspective, it may also be considered very effective.
But does that kind of efficacy matter? Is a higher level of antibodies needed to protect vaccinated Americans? Though antibody levels may wane some over time, the current vaccines deliver perfectly good immunity so far.
What if a booster is safe and effective in one sense but simply not needed – at least for now?
Reliance on the simple “safe and effective” standard – which certainly sounds reasonable – is a relic of a time when there were far fewer and simpler medicines available to treat diseases and before pharmaceutical manufacturing became one of the world’s biggest businesses.
The FDA’s 1938 landmark legislation focused primarily on safety after more than 100 Americans died from a raspberry-flavored liquid form of an early antibiotic because one of its ingredients was used as antifreeze. The 1962 Kefauver-Harris Amendments to the Federal Food, Drug, and Cosmetic Act set out more specific requirements for drug approval: Companies must scientifically prove a drug’s effectiveness through “adequate and well-controlled studies.”
In today’s pharmaceutical universe, a simple “safe and effective” determination is not always an adequate bar, and it can be manipulated to sell drugs of questionable value. There’s also big money involved: Pfizer is already projecting $26 billion in COVID revenue in 2021.
The United States’ continued use of this standard to let drugs into the market has led to the approval of expensive, not necessarily very effective drugs. In 2014, for example, the FDA approved a toenail fungus drug that can cost up to $1,500 a month and that studies showed cured fewer than 10% of patients after a year of treatment. That’s more effective than doing nothing but less effective and more costly than a number of other treatments for this bothersome malady.
It has also led to a plethora of high-priced drugs to treat diseases like cancers, multiple sclerosis and type 2 diabetes that are all more effective than a placebo but have often not been tested very much against one another to determine which are most effective.
In today’s complex world, clarification is needed to determine just what kind of effectiveness the FDA should demand. And should that be the job of the FDA alone?
For example, should drugmakers prove a drug is significantly more effective than products already on the market? Or demonstrate cost-effectiveness – the health value of a product relative to its price – a metric used by Britain’s health system? And in which cases is effectiveness against a surrogate marker – like an antibody level – a good enough stand-in for whether a drug will have a significant impact on a patient’s health?
In most industrialized countries, broad access to the national market is a two-step process, said Aaron Kesselheim, a professor of medicine at Harvard Medical School, Boston, who studies drug development, marketing and law and recently served on an FDA advisory committee. The first part certifies that a drug is sufficiently safe and effective. That is immediately followed by an independent health technology assessment to see where it fits in the treatment armamentarium, including, in some countries, whether it is useful enough to be sold at all at the stated price. But there’s no such automatic process in the United States.
When Pfizer applies for authorization, the FDA may well clear a booster for the U.S. market. The Centers for Disease Control and Prevention, likely with advice from National Institutes of Health experts, will then have to decide whether to recommend it and for whom. This judgment call usually determines whether insurers will cover it. Pfizer is likely to profit handsomely from a government authorization, and the company will gain some revenue even if only the worried well, who can pay out of pocket, decide to get the shot.
To make any recommendation on a booster, government experts say they need more data. They could, for example, as Anthony S. Fauci, MD, has suggested, eventually green-light the additional vaccine shot only for a small group of patients at high risk for a deadly infection, such as the very old or transplant recipients who take immunosuppressant drugs, as some other countries have done.
But until the United States refines the FDA’s “safe and effective” standard or adds a second layer of vetting, when new products hit the market and manufacturers promote them, Americans will be left to decipher whose version of effective and necessary matters to them.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The drug maker Pfizer recently announced that vaccinated people are likely to need a booster shot to be effectively protected against new variants of COVID-19 and that the company would apply for Food and Drug Administration emergency use authorization for the shot. Top government health officials immediately and emphatically announced that the booster isn’t needed right now – and held firm to that position even after Pfizer’s top scientist made his case and shared preliminary data with them on July 12.
This has led to confusion. Is the protection that has allowed them to see loved ones and go out to dinner fading?
Ultimately, the question of whether a booster is needed is unlikely to determine the FDA’s decision. If recent history is predictive, booster shots will be here before long. That’s because of the outdated, 60-year-old basic standard the FDA uses to authorize medicines for sale: Is a new drug “safe and effective?”
The FDA, using that standard, will very likely have to authorize Pfizer’s booster for emergency use, as it did the company’s prior COVID shot. The booster is likely to be safe – hundreds of millions have taken the earlier shots – and Pfizer reported that it dramatically increases a vaccinated person’s antibodies against SARS-CoV-2. From that perspective, it may also be considered very effective.
But does that kind of efficacy matter? Is a higher level of antibodies needed to protect vaccinated Americans? Though antibody levels may wane some over time, the current vaccines deliver perfectly good immunity so far.
What if a booster is safe and effective in one sense but simply not needed – at least for now?
Reliance on the simple “safe and effective” standard – which certainly sounds reasonable – is a relic of a time when there were far fewer and simpler medicines available to treat diseases and before pharmaceutical manufacturing became one of the world’s biggest businesses.
The FDA’s 1938 landmark legislation focused primarily on safety after more than 100 Americans died from a raspberry-flavored liquid form of an early antibiotic because one of its ingredients was used as antifreeze. The 1962 Kefauver-Harris Amendments to the Federal Food, Drug, and Cosmetic Act set out more specific requirements for drug approval: Companies must scientifically prove a drug’s effectiveness through “adequate and well-controlled studies.”
In today’s pharmaceutical universe, a simple “safe and effective” determination is not always an adequate bar, and it can be manipulated to sell drugs of questionable value. There’s also big money involved: Pfizer is already projecting $26 billion in COVID revenue in 2021.
The United States’ continued use of this standard to let drugs into the market has led to the approval of expensive, not necessarily very effective drugs. In 2014, for example, the FDA approved a toenail fungus drug that can cost up to $1,500 a month and that studies showed cured fewer than 10% of patients after a year of treatment. That’s more effective than doing nothing but less effective and more costly than a number of other treatments for this bothersome malady.
It has also led to a plethora of high-priced drugs to treat diseases like cancers, multiple sclerosis and type 2 diabetes that are all more effective than a placebo but have often not been tested very much against one another to determine which are most effective.
In today’s complex world, clarification is needed to determine just what kind of effectiveness the FDA should demand. And should that be the job of the FDA alone?
For example, should drugmakers prove a drug is significantly more effective than products already on the market? Or demonstrate cost-effectiveness – the health value of a product relative to its price – a metric used by Britain’s health system? And in which cases is effectiveness against a surrogate marker – like an antibody level – a good enough stand-in for whether a drug will have a significant impact on a patient’s health?
In most industrialized countries, broad access to the national market is a two-step process, said Aaron Kesselheim, a professor of medicine at Harvard Medical School, Boston, who studies drug development, marketing and law and recently served on an FDA advisory committee. The first part certifies that a drug is sufficiently safe and effective. That is immediately followed by an independent health technology assessment to see where it fits in the treatment armamentarium, including, in some countries, whether it is useful enough to be sold at all at the stated price. But there’s no such automatic process in the United States.
When Pfizer applies for authorization, the FDA may well clear a booster for the U.S. market. The Centers for Disease Control and Prevention, likely with advice from National Institutes of Health experts, will then have to decide whether to recommend it and for whom. This judgment call usually determines whether insurers will cover it. Pfizer is likely to profit handsomely from a government authorization, and the company will gain some revenue even if only the worried well, who can pay out of pocket, decide to get the shot.
To make any recommendation on a booster, government experts say they need more data. They could, for example, as Anthony S. Fauci, MD, has suggested, eventually green-light the additional vaccine shot only for a small group of patients at high risk for a deadly infection, such as the very old or transplant recipients who take immunosuppressant drugs, as some other countries have done.
But until the United States refines the FDA’s “safe and effective” standard or adds a second layer of vetting, when new products hit the market and manufacturers promote them, Americans will be left to decipher whose version of effective and necessary matters to them.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Delta variant among the most infectious respiratory viruses, CDC says
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
Sickle cell disease, trait may up risk for poor COVID outcomes
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Statins again linked to lower COVID-19 mortality
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
FROM PLOS ONE
‘Dealing with a different beast’: Why Delta has doctors worried
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
Children and COVID: New vaccinations increase as cases continue to climb
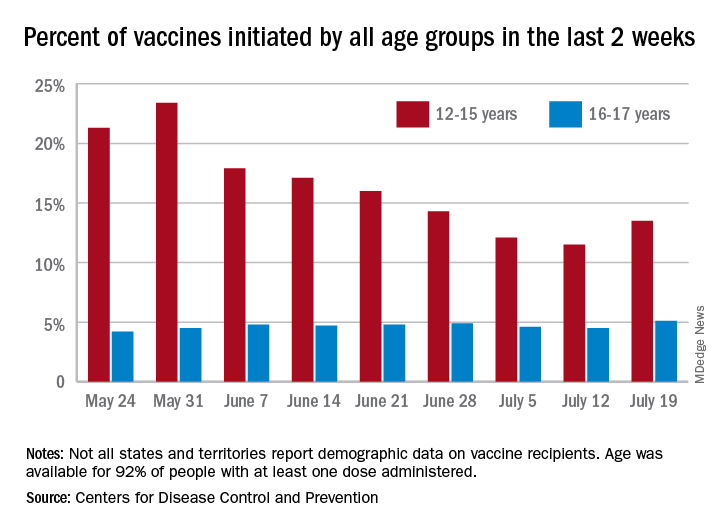
Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”

Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”

Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”