User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Drug-resistant TB trial stopped early after successful results
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Vitamin D may protect against COVID-19, especially in Black patients
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 variants now detected in more animals, may find hosts in mice
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
Here we go again? Rate of COVID-19 in children takes a turn for the worse
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
Black nonsmokers still at high risk for secondhand smoke exposure
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.
While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
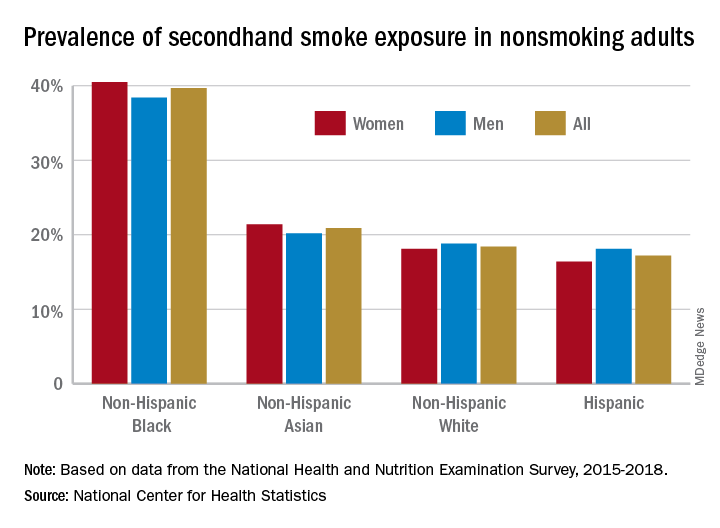
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Update: U.S. regulators question AstraZeneca vaccine trial data
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
High obesity rates in Southern states magnify COVID threats
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
COVID-19 virus reinfections rare; riskiest after age 65
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Could pollen be driving COVID-19 infections?
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Decline in child COVID-19 cases picks up after 2-week slowdown
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.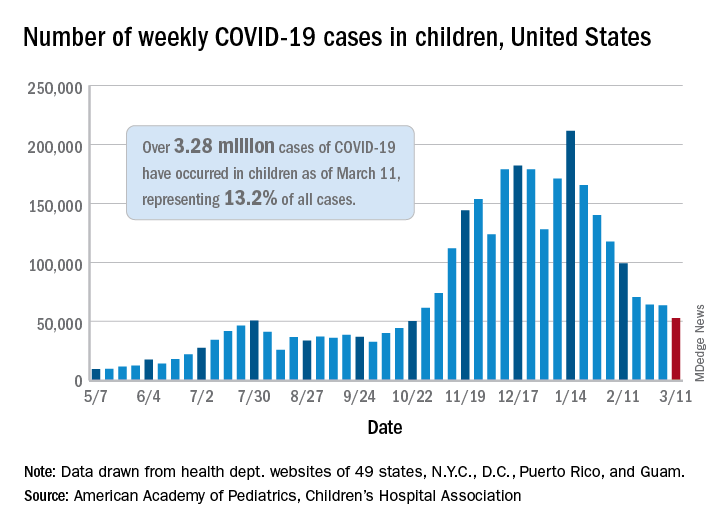
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.