User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Cannabinoids may pose death risk for older patients with COPD
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
FROM THORAX
First pill for COVID-19 could be ready by year’s end
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
Pregnant patients with severe COVID-19 disease at increased risk of complications
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
FROM THE PREGNANCY MEETING
Don’t discontinue osteoporosis meds for COVID-19 vaccines, expert guidance says
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
Inpatient sodium imbalances linked to adverse COVID-19 outcomes
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Delay surgery by 7 weeks after COVID-19 diagnosis, study shows
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
CDC data strengthen link between obesity and severe COVID
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
FDA authorizes first molecular at-home, OTC COVID-19 test
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
Decline in weekly child COVID-19 cases has almost stopped
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.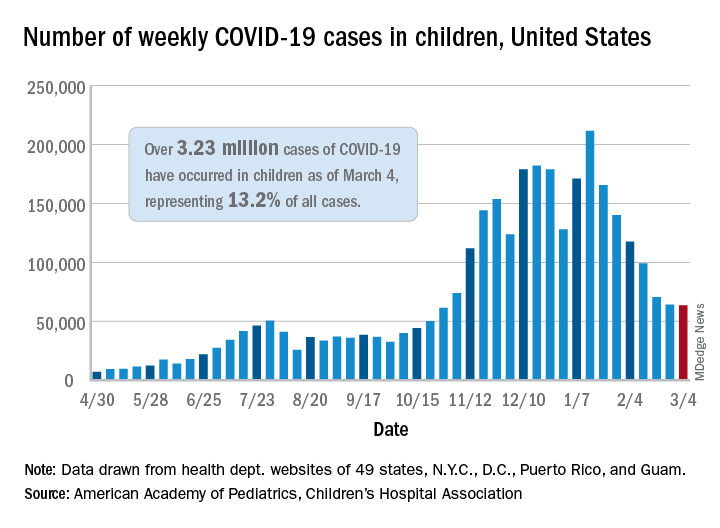
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
Call to action on obesity amid COVID-19 pandemic
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.