User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Routine vaccinations missed by older adults during pandemic
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
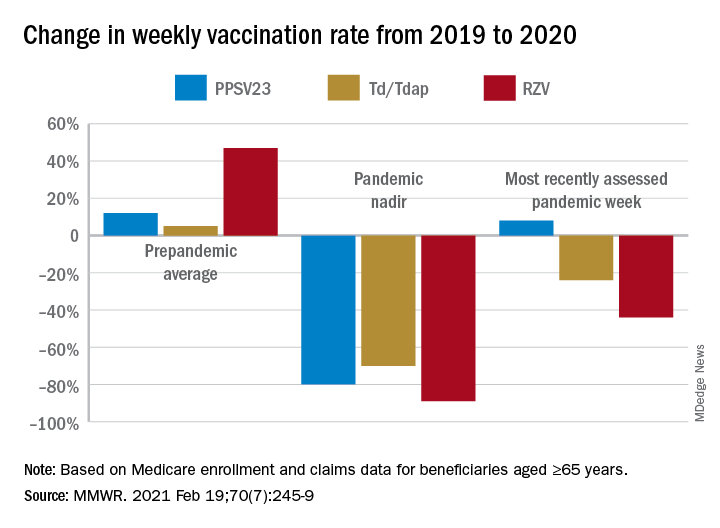
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.
In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
FROM MMWR
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
Study clarifies who gets post–COVID-19 interstitial lung disease
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Novel agent shows promise against cat allergy
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAAAI
Mepolizumab reduced exacerbations in patients with asthma and atopy, depression comorbidities
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
FROM AAAAI 2021
Decline in children’s COVID-19 cases slows
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
Fired for good judgment a sign of physicians’ lost respect
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
What happened to Hasan Gokal, MD, should stick painfully in the craws of all physicians. It should serve as a call to action, because Dr. Gokal is sitting at home today without a job and under threat of further legal action while we continue about our day.
Dr. Gokal’s “crime” is that he vaccinated 10 strangers and acquaintances with soon-to-expire doses of the Moderna COVID-19 vaccine. He drove to the homes of some in the dark of night and injected others on his Sugar Land, Texas, lawn. He spent hours in a frantic search for willing recipients to beat the expiration clock. With minutes to spare, he gave the last dose to his at-risk wife, who has symptomatic pulmonary sarcoidosis, but whose age meant she did not fall into a vaccine priority tier.
According to the New York Times, Dr. Gokal’s wife was hesitant, afraid he might get into trouble. But why would she be hesitant? He wasn’t doing anything immoral. Perhaps she knew how far physicians have fallen and how bitterly they both could suffer.
In Barren County, Ky., where I live, a state of emergency was declared by our judge executive because of inclement weather. This directive allows our emergency management to “waive procedures and formalities otherwise required by the law.” It’s too bad that the same courtesy was not afforded to Dr. Gokal in Texas. It’s a shame that ice and snow didn’t drive his actions. Perhaps that would have protected him against the harsh criticism. Rather, it was his oath to patients and dedication to his fellow humans that motivated him, and for that, he was made to suffer.
Dr. Gokal was right to think that pouring the last 10 vaccine doses down the toilet would be an egregious act. But he was wrong in thinking his decision to find takers for the vaccine would be viewed as expedient. Instead, he was accused of graft and even nepotism. And there is the rub. That he was fired and charged with the theft of $137 worth of vaccines says everything about how physicians are treated in the year 2021. Dr. Gokal’s lawyer says the charge carried a maximum penalty of 1 year in prison and a fine of nearly $4,000.
Thank God a sage judge threw out the case and “rebuked” the office of District Attorney Kim Ogg. That hasn’t stopped her from threatening to bring the case to a grand jury. That threat invites anyone faced with the same scenario to flush the extra vaccine doses into the septic system. It encourages us to choose the toilet handle to avoid a mug shot.
And we can’t ignore the racial slant to this story. The Times reported that Dr. Gokal asked the officials, “Are you suggesting that there were too many Indian names in this group?”
“Exactly” was the answer. Let that sink in.
None of this would have happened 20 years ago. Back then, no one would have questioned the wisdom a physician gains from all our years of training and residency. In an age when anyone who conducts an office visit is now called “doctor,” respect for the letters “MD” has been leveled. We physicians have lost our autonomy and been cowed into submission.
But whatever his profession, Hasan Gokal was fired for being a good human. Today, the sun rose on 10 individuals who now enjoy better protection against a deadly pandemic. They include a bed-bound nonagenarian. A woman in her 80s with dementia. A mother with a child who uses a ventilator. All now have antibodies against SARS-CoV2 because of the tireless actions of Dr. Gokal.
Yet Dr. Gokal’s future is uncertain. Will we help him, or will we leave him to the wolves? In an email exchange with his lawyer’s office, I learned that Dr. Gokal has received offers of employment but is unable to entertain them because the actions by the Harris County District Attorney triggered an automatic review by the Texas Medical Board. A GoFundMe page was launched, but an appreciative Dr. Gokal stated publicly that he’d rather the money go to a needy charity.
In the last paragraph of the Times article, Dr. Gokal asks, “How can I take it back?” referencing stories about “the Pakistani doctor in Houston who stole all those vaccines.”
Let’s help him take back his story. In helping him, perhaps we can take back a little control. We could start with letters of support that could be mailed to his lawyer, Paul Doyle, Esq., of Houston, or tweet, respectfully of course, to the district attorney @Kimoggforda.
We can also let the Harris County Public Health Department in Houston know what we think of their actions.
On Martin Luther King Day, Kim Ogg, the district attorney who charged Dr. Gokal, tweeted MLK’s famous quote: “Injustice anywhere is a threat to justice everywhere.”
Let that motivate us to action.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. In addition to opinion writing, she enjoys spending time with her husband, daughters and parents, and sidelines as a backing vocalist for local rock bands. A version of this article first appeared on Medscape.com.
Patients with asthma say most doctors don’t ask about cannabis use
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
FROM AAAAI 2021
Asthma not an independent risk factor for severe COVID-19, hospitalization
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
Asthma is not an independent risk factor for more severe disease or hospitalization due to COVID-19, according to recent research presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“In our cohort of patients tested for SARS-CoV-2 at Stanford between March and September, asthma was not an independent risk factor in and of itself for hospitalization or more severe disease from COVID,” Lauren E. Eggert, MD, of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, said in a poster presentation at the meeting. “What’s more, allergic asthma actually decreased the risk of hospitalization by nearly half.”
Dr. Eggert noted that there have been conflicting data on whether comorbid asthma is or is not a risk factor for more severe COVID-19. “The general thought at the beginning of the pandemic was that because COVID-19 is predominantly a viral respiratory illness, and viral illnesses are known to cause asthma exacerbations, that patients with asthma may be at higher risk if they got COVID infection,” she explained. “But some of the data also showed that Th2 inflammation downregulates ACE2 receptor [expression], which has been shown to be the port of entry for the SARS-CoV-2 virus, so maybe allergy might have a protective effect.”
The researchers at Stanford University identified 168,190 patients at Stanford Health Care who had a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between March and September 2020 and collected data from their electronic medical records on their history of asthma, if they were hospitalized, comorbid conditions, and laboratory values. Patients who had no other data available except for a positive SARS-CoV-2 result, or were younger than 28 days, were excluded from the study. Dr. Eggert and colleagues used COVID-19 treatment guidelines from the National Institutes of Health to assess disease severity, which grades COVID-19 severity as asymptomatic or presymptomatic infection, mild illness, moderate illness, severe illness, and critical illness.
In total, the researchers analyzed 5,596 patients who were SARS-CoV-2 positive, with 605 patients (10.8%) hospitalized within 14 days of receiving a positive test. Of these, 100 patients (16.5%) were patients with asthma. There were no significant differences between groups hospitalized and not hospitalized due to COVID-19 in patients with asthma and with no asthma.
Among patients with asthma and COVID-19, 28.0% had asymptomatic illness, 19.0% had moderate disease, 33.0% had severe disease, and 20.0% had critical COVID-19, compared with 36.0% of patients without asthma who had asymptomatic illness, 12.0% with moderate disease, 30.0% with severe disease, and 21.0% with critical COVID-19. Dr. Eggert and colleagues performed a univariate analysis, which showed a significant association between asthma and COVID-19 related hospitalization (odds ratio, 1.53; 95% confidence interval, 1.2-1.93; P < .001), but when adjusting for factors such as diabetes, obesity coronary heart disease, and hypertension, they found there was not a significant association between asthma and hospitalization due to COVID-19 (OR, 1.12; 95% CI, 0.86-1.45; P < .40).
In a univariate analysis, asthma was associated with more severe disease in patients hospitalized for COVID-19, but the results were not significant (OR, 1.21; 95% CI, 0.8-1.85; P = .37). When analyzing allergic asthma alone in a univariate analysis, the researchers found a significant association between allergic asthma and lower hospitalization risk, compared with patients who had nonallergic asthma (OR, 0.55; 95% CI, 0.31-0.92; P = .029), and this association remained after they performed a multivariate analysis as well.
“When we stratified by allergic asthma versus nonallergic asthma, we found that having a diagnosis of allergic asthma actually conferred a protective effect, and there was almost half the risk of hospitalization in asthmatics with allergic asthma as compared to others, which we thought was very interesting,” Dr. Eggert said.
“Eosinophil levels during hospitalization, even when adjusted for systemic steroid use – and we followed patients out through September, when dexamethasone was standard of care – also correlated with better outcomes,” she explained. “This is independent of asthmatic status.”
The researchers noted that confirmation of these results are needed through large, multicenter cohort studies, particularly with regard to how allergic asthma might have a protective effect against SARS-CoV-2 infection. “I think going forward, these findings are very interesting and need to be looked at further to explain the mechanism behind them better,” Dr. Eggert said.
“I think there is also a lot of interest in how this might affect our patients on biologics, which deplete the eosinophils and get rid of that allergic phenotype,” she added. “Does that have any effect on disease severity? Unfortunately, the number of patents on biologics was very small in our cohort, but I do think this is an interesting area for exploration.”
This study was funded in part by the Sean N. Parker Center for Allergy & Asthma Research, Stanford University, Sunshine Foundation, Crown Foundation, and the Parker Foundation.
FROM AAAAI
Frequent medication refills show some patients not achieving asthma control
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
While most commercially insured patients with asthma have good disease control, some patients may not, according to a recent review of U.S. administrative claims data.
The results of the retrospective analysis, presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year, showed some patients with asthma had two or more refills for prescribed systemic corticosteroids (SCS) or short-acting beta agonists (SABA) within a period of 12 months.
“,” Randall Brown, MD, MPH, pulmonologist and senior director of Global Respiratory Medical Affairs at Teva Pharmaceuticals in West Chester, Penn., said in a presentation at the meeting. “Understanding the extent of systemic steroid and SABA prescriptions among patients with asthma and the distribution of those prescriptions across disease severity can be useful in determining the degree of disease control.”
Global Initiative for Asthma (GINA) guidelines consider factors such as symptom control and risk of exacerbation when determining asthma severity, but uncontrolled asthma can still be difficult to assess. Dr. Brown and colleagues set out to determine the prevalence of uncontrolled asthma for patients in the IBM/Watson MarketScan U.S. claims database as well as the rate of uncontrolled asthma by GINA classification. In total, 597,955 patients who had an asthma diagnosis between 12 months before or up to 3 months after the index data of filling a SABA prescription were included for analysis. Patients were at least 12 years old with commercial insurance for at least 12 months, and had no other respiratory diseases other than asthma during the 12 months prior to the index date and during the study period.
The researchers then measured each patient’s 2018 GINA classification of asthma severity based on the number of SCS and SABA prescription claims made between January and December 2017. Overall, 54.3% patients were GINA Step 1, 14.6% were Step 2, 10.2% were Step 3, 19.8% were Step 4, and 1.1% were Step 5.
Dr. Brown and colleagues found that, regardless of GINA disease severity, 18.8% of patients filled two or more SCS prescriptions in 1 year, 27.4% filled three or more SABA prescriptions in 1 year, and 38.7% filled two or more SCS and/or three or more SABA prescriptions in 1 year. “[A] large proportion of these patients did not meet the GINA goal of disease control,” Dr. Brown said.
The researchers found 13% of patients with uncontrolled asthma categorized as GINA Step 1, 20% of patients categorized as GINA Step 2, 19% of patients who were GINA Step 3, 31% of patients who were GINA Step 4, and 54% of patients categorized as GINA Step 5 filled two or more two or more SCS prescriptions per year.
The proportion of patients with uncontrolled asthma who filled three or more SABA prescriptions per year included 19% in GINA Step 1, 29% in GINA Step 2, 35% in GINA Step 3, 44% in GINA Step 4, and 57% in GINA Step 5 groups. For patients who filled both two or more SCS and/or three or more SABA prescriptions per year, the proportion of patients with uncontrolled asthma by GINA category was 29% in GINA Step 1, 42% in GINA Step 2, 46% in GINA Step 3, 58% in GINA Step 4, and 76% of patients in GINA Step 5.
While “poor control was seen across all of the GINA disease severity classifications, the greatest proportion of uncontrolled disease was seen at the highest disease severity, which was also true when we used a stricter definition of uncontrolled disease,” Dr. Brown said. When the researchers applied stricter criteria for patients categorized as GINA Step 5, 39% of patients filled three or more SCS, 41% filled four or more SABA, and 60% filled three or more SCS and/or four or more SABA prescriptions over 12 months.
Dr. Brown said that the analysis “highlights the need for improved asthma management strategies within each of the asthma GINA classification steps.”
“While this population that was studied may be reflective of the wider insured U.S. population, the proportions of uncontrolled asthma may be even greater in non–commercially insured patients within the United States,” he said. “Updates to GINA guidelines incorporate recent consensus [and] recent scientific information and therapies, but many patients in the U.S. are not meeting the GINA goal of disease control. Newer paradigms for systemic corticosteroid-free asthma control as a target of disease ‘remission’ are becoming more commonplace. Such changes and goals may lead to improved asthma management strategies and advancement in treatment.”
This study was funded in part by Teva Branded Pharmaceutical Products R&D, which also provided funding for medical writing assistance from Ashfield MedComms. The authors report being employees of Teva Pharmaceuticals.
FROM AAAAI