User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Ivabradine knocks down heart rate, symptoms in POTS
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
CDC chief lays out attack plan for COVID variants
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
New child COVID-19 cases decline as total passes 3 million
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
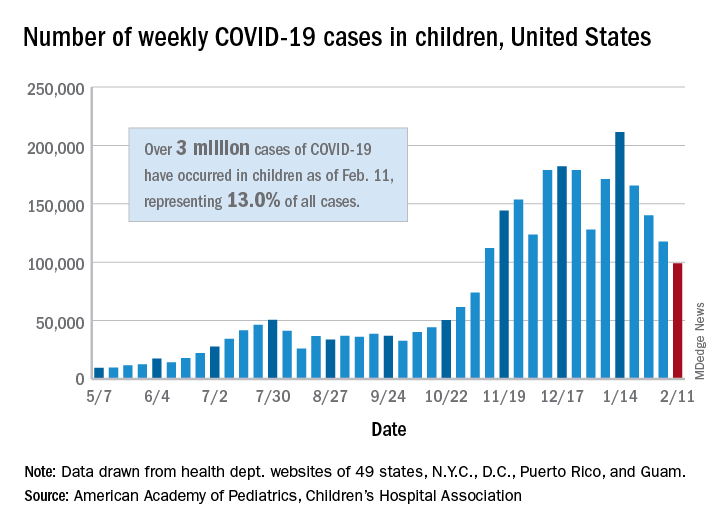
It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
New COVID-19 cases in children continue to drop each week, but the total number of cases has now surpassed 3 million since the start of the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

It was still enough, though, to bring the total to 3.03 million children infected with SARS-CoV-19 in the United States, the AAP and the CHA said in their weekly report.
The nation also hit a couple of other ignominious milestones. The cumulative rate of COVID-19 infection now stands at 4,030 per 100,000, so 4% of all children have been infected. Also, children represented 16.9% of all new cases for the week, which equals the highest proportion seen throughout the pandemic, based on data from health departments in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
There have been 241 COVID-19–related deaths in children so far, with 14 reported during the week of Feb. 5-11. Kansas just recorded its first pediatric death, which leaves 10 states that have had no fatalities. Texas, with 39 deaths, has had more than any other state, among the 43 that are reporting mortality by age, the AAP/CHA report showed.
Outcomes have improved for PAH in connective tissue disease
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
FROM ARTHRITIS & RHEUMATOLOGY
Tocilizumab may improve lung function in early systemic sclerosis
Treatment with tocilizumab (Actemra) could stabilize or improve lung function in people with early interstitial lung disease associated with systemic sclerosis (SSc-ILD), a new study has found.
A paper published online Feb. 3 in Arthritis & Rheumatology presents the results of a post hoc analysis of data from a phase 3, placebo-controlled, double-blind trial of subcutaneous tocilizumab in patients with SSc and progressive skin disease, which included high-resolution chest CT to assess lung involvement and fibrosis.
Tocilizumab is a monoclonal antibody that targets interleukin-6 and is currently approved for the treatment of immune-mediated diseases such as rheumatoid arthritis, giant cell arteritis, cytokine release syndrome, and systemic and polyarticular course juvenile idiopathic arthritis.
Two previous studies of tocilizumab in patients with early, diffuse cutaneous SSc had also found that the treatment was associated with preservation of lung function but did not characterize that effect using radiography.
Of the 210 participants in the trial, called focuSSced, 136 were found to have interstitial lung disease at baseline and were randomized to 162 mg tocilizumab weekly or placebo for 48 weeks.
At baseline, around three-quarters of those with interstitial lung disease had moderate to severe lung involvement, defined as ground glass opacities, honeycombing, and fibrotic reticulation across at least 20% of the whole lung.
Those in the tocilizumab group showed a 0.1% mean decline in forced vital capacity (FVC) over the 48-week study, while those in the placebo group had a mean decline of 6.3%.
When stratified by severity of lung involvement, those with mild lung disease group treated with tocilizumab had a 4.1% decline in FVC, compared with a 10% decline in the placebo group; those with moderate disease in the treatment group had an 0.7% mean increase in FVC, compared with a 5.7% decrease in the placebo group, and those with severe lung involvement in the treatment arm had a 2.1% increase in FVC, compared with a 6.7% decrease in the placebo arm.
Those treated with tocilizumab also showed a statistically significant 1.8% improvement in the amount of lung involvement, which was largely seen in those with more extensive lung involvement at baseline. Those with more than 20% of the lung affected had a significant 4.9% reduction in lung area affected, while those in the placebo arm showed a significant increase in fibrosis.
First author David Roofeh, MD, of the University of Michigan Scleroderma Program, and colleagues wrote that most patients with SSc will develop interstitial lung disease – particularly those with early, diffuse cutaneous SSc and elevated markers such as C-reactive protein.
“Patients with these high-risk features, especially those with disease in the initial phase of development, represent an important target for early intervention as ILD is largely irreversible in SSc,” the authors wrote.
Findings from a specific patient population may not be generalizable
Commenting on the findings, Lorinda Chung, MD, of Stanford (Calif.) University, said in an interview that the study demonstrated that tocilizumab could prevent radiographic progression of ILD in early diffuse SSc patients with mild to severe lung disease and evidence of active skin disease, as well as elevated inflammatory markers.
“This was a very specific patient population who was studied in the focuSSced clinical trial, and this paper only evaluated a subset of these patients,” Dr. Chung said. “The results may not be generalizable to all SSc-ILD patients and further studies are needed.”
The authors suggested that the patients with progressive skin disease and elevated acute phase reactants may represent a group in the immunoinflammatory phase of the disease rather than the advanced fibrotic stage, and that this might be a “window of therapeutic opportunity to preserve lung function.”
Dr. Chung noted that the radiographic improvement induced by tocilizumab treatment was greatest in those with the most radiographic disease at baseline.
“This may reflect tocilizumab’s impact on decreasing inflammation, but we are not provided the data on the effects of tocilizumab on the individual components of the QILD [quantitative ILD: summation of ground glass opacities, honeycombing, and fibrotic reticulation],” she said.
The study’s authors also made a point about the utility of screening patients with high-resolution chest CT to detect early signs of ILD.
“Our data demonstrate the value of obtaining HRCT at the time of diagnosis: PFTs [pulmonary function tests] are not sensitive enough to accurately assess the presence of ILD and delays in treatment initiation may lead to irreversible disease,” they wrote.
Describing the results as ‘hypothesis-generating’ owing to the post hoc nature of the analysis, the authors said that FVC was an indirect measure of the flow-resistive properties of the lung, and that other aspects of SSc – such as hide-bound chest thickness – could cause thoracic restriction.
Two authors were funded by the National Institutes of Health. Six authors declared grants, funding, and other support from the pharmaceutical sector, including Roche, which sponsored the original focuSSced trial.
Treatment with tocilizumab (Actemra) could stabilize or improve lung function in people with early interstitial lung disease associated with systemic sclerosis (SSc-ILD), a new study has found.
A paper published online Feb. 3 in Arthritis & Rheumatology presents the results of a post hoc analysis of data from a phase 3, placebo-controlled, double-blind trial of subcutaneous tocilizumab in patients with SSc and progressive skin disease, which included high-resolution chest CT to assess lung involvement and fibrosis.
Tocilizumab is a monoclonal antibody that targets interleukin-6 and is currently approved for the treatment of immune-mediated diseases such as rheumatoid arthritis, giant cell arteritis, cytokine release syndrome, and systemic and polyarticular course juvenile idiopathic arthritis.
Two previous studies of tocilizumab in patients with early, diffuse cutaneous SSc had also found that the treatment was associated with preservation of lung function but did not characterize that effect using radiography.
Of the 210 participants in the trial, called focuSSced, 136 were found to have interstitial lung disease at baseline and were randomized to 162 mg tocilizumab weekly or placebo for 48 weeks.
At baseline, around three-quarters of those with interstitial lung disease had moderate to severe lung involvement, defined as ground glass opacities, honeycombing, and fibrotic reticulation across at least 20% of the whole lung.
Those in the tocilizumab group showed a 0.1% mean decline in forced vital capacity (FVC) over the 48-week study, while those in the placebo group had a mean decline of 6.3%.
When stratified by severity of lung involvement, those with mild lung disease group treated with tocilizumab had a 4.1% decline in FVC, compared with a 10% decline in the placebo group; those with moderate disease in the treatment group had an 0.7% mean increase in FVC, compared with a 5.7% decrease in the placebo group, and those with severe lung involvement in the treatment arm had a 2.1% increase in FVC, compared with a 6.7% decrease in the placebo arm.
Those treated with tocilizumab also showed a statistically significant 1.8% improvement in the amount of lung involvement, which was largely seen in those with more extensive lung involvement at baseline. Those with more than 20% of the lung affected had a significant 4.9% reduction in lung area affected, while those in the placebo arm showed a significant increase in fibrosis.
First author David Roofeh, MD, of the University of Michigan Scleroderma Program, and colleagues wrote that most patients with SSc will develop interstitial lung disease – particularly those with early, diffuse cutaneous SSc and elevated markers such as C-reactive protein.
“Patients with these high-risk features, especially those with disease in the initial phase of development, represent an important target for early intervention as ILD is largely irreversible in SSc,” the authors wrote.
Findings from a specific patient population may not be generalizable
Commenting on the findings, Lorinda Chung, MD, of Stanford (Calif.) University, said in an interview that the study demonstrated that tocilizumab could prevent radiographic progression of ILD in early diffuse SSc patients with mild to severe lung disease and evidence of active skin disease, as well as elevated inflammatory markers.
“This was a very specific patient population who was studied in the focuSSced clinical trial, and this paper only evaluated a subset of these patients,” Dr. Chung said. “The results may not be generalizable to all SSc-ILD patients and further studies are needed.”
The authors suggested that the patients with progressive skin disease and elevated acute phase reactants may represent a group in the immunoinflammatory phase of the disease rather than the advanced fibrotic stage, and that this might be a “window of therapeutic opportunity to preserve lung function.”
Dr. Chung noted that the radiographic improvement induced by tocilizumab treatment was greatest in those with the most radiographic disease at baseline.
“This may reflect tocilizumab’s impact on decreasing inflammation, but we are not provided the data on the effects of tocilizumab on the individual components of the QILD [quantitative ILD: summation of ground glass opacities, honeycombing, and fibrotic reticulation],” she said.
The study’s authors also made a point about the utility of screening patients with high-resolution chest CT to detect early signs of ILD.
“Our data demonstrate the value of obtaining HRCT at the time of diagnosis: PFTs [pulmonary function tests] are not sensitive enough to accurately assess the presence of ILD and delays in treatment initiation may lead to irreversible disease,” they wrote.
Describing the results as ‘hypothesis-generating’ owing to the post hoc nature of the analysis, the authors said that FVC was an indirect measure of the flow-resistive properties of the lung, and that other aspects of SSc – such as hide-bound chest thickness – could cause thoracic restriction.
Two authors were funded by the National Institutes of Health. Six authors declared grants, funding, and other support from the pharmaceutical sector, including Roche, which sponsored the original focuSSced trial.
Treatment with tocilizumab (Actemra) could stabilize or improve lung function in people with early interstitial lung disease associated with systemic sclerosis (SSc-ILD), a new study has found.
A paper published online Feb. 3 in Arthritis & Rheumatology presents the results of a post hoc analysis of data from a phase 3, placebo-controlled, double-blind trial of subcutaneous tocilizumab in patients with SSc and progressive skin disease, which included high-resolution chest CT to assess lung involvement and fibrosis.
Tocilizumab is a monoclonal antibody that targets interleukin-6 and is currently approved for the treatment of immune-mediated diseases such as rheumatoid arthritis, giant cell arteritis, cytokine release syndrome, and systemic and polyarticular course juvenile idiopathic arthritis.
Two previous studies of tocilizumab in patients with early, diffuse cutaneous SSc had also found that the treatment was associated with preservation of lung function but did not characterize that effect using radiography.
Of the 210 participants in the trial, called focuSSced, 136 were found to have interstitial lung disease at baseline and were randomized to 162 mg tocilizumab weekly or placebo for 48 weeks.
At baseline, around three-quarters of those with interstitial lung disease had moderate to severe lung involvement, defined as ground glass opacities, honeycombing, and fibrotic reticulation across at least 20% of the whole lung.
Those in the tocilizumab group showed a 0.1% mean decline in forced vital capacity (FVC) over the 48-week study, while those in the placebo group had a mean decline of 6.3%.
When stratified by severity of lung involvement, those with mild lung disease group treated with tocilizumab had a 4.1% decline in FVC, compared with a 10% decline in the placebo group; those with moderate disease in the treatment group had an 0.7% mean increase in FVC, compared with a 5.7% decrease in the placebo group, and those with severe lung involvement in the treatment arm had a 2.1% increase in FVC, compared with a 6.7% decrease in the placebo arm.
Those treated with tocilizumab also showed a statistically significant 1.8% improvement in the amount of lung involvement, which was largely seen in those with more extensive lung involvement at baseline. Those with more than 20% of the lung affected had a significant 4.9% reduction in lung area affected, while those in the placebo arm showed a significant increase in fibrosis.
First author David Roofeh, MD, of the University of Michigan Scleroderma Program, and colleagues wrote that most patients with SSc will develop interstitial lung disease – particularly those with early, diffuse cutaneous SSc and elevated markers such as C-reactive protein.
“Patients with these high-risk features, especially those with disease in the initial phase of development, represent an important target for early intervention as ILD is largely irreversible in SSc,” the authors wrote.
Findings from a specific patient population may not be generalizable
Commenting on the findings, Lorinda Chung, MD, of Stanford (Calif.) University, said in an interview that the study demonstrated that tocilizumab could prevent radiographic progression of ILD in early diffuse SSc patients with mild to severe lung disease and evidence of active skin disease, as well as elevated inflammatory markers.
“This was a very specific patient population who was studied in the focuSSced clinical trial, and this paper only evaluated a subset of these patients,” Dr. Chung said. “The results may not be generalizable to all SSc-ILD patients and further studies are needed.”
The authors suggested that the patients with progressive skin disease and elevated acute phase reactants may represent a group in the immunoinflammatory phase of the disease rather than the advanced fibrotic stage, and that this might be a “window of therapeutic opportunity to preserve lung function.”
Dr. Chung noted that the radiographic improvement induced by tocilizumab treatment was greatest in those with the most radiographic disease at baseline.
“This may reflect tocilizumab’s impact on decreasing inflammation, but we are not provided the data on the effects of tocilizumab on the individual components of the QILD [quantitative ILD: summation of ground glass opacities, honeycombing, and fibrotic reticulation],” she said.
The study’s authors also made a point about the utility of screening patients with high-resolution chest CT to detect early signs of ILD.
“Our data demonstrate the value of obtaining HRCT at the time of diagnosis: PFTs [pulmonary function tests] are not sensitive enough to accurately assess the presence of ILD and delays in treatment initiation may lead to irreversible disease,” they wrote.
Describing the results as ‘hypothesis-generating’ owing to the post hoc nature of the analysis, the authors said that FVC was an indirect measure of the flow-resistive properties of the lung, and that other aspects of SSc – such as hide-bound chest thickness – could cause thoracic restriction.
Two authors were funded by the National Institutes of Health. Six authors declared grants, funding, and other support from the pharmaceutical sector, including Roche, which sponsored the original focuSSced trial.
FROM ARTHRITIS & RHEUMATOLOGY
What to do if an employee tests positive for COVID-19
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
Prospective data support delaying antibiotics for pediatric respiratory infections
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
FROM PEDIATRICS
PPE protected critical care staff from COVID-19 transmission
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
FROM CCC50
ColCORONA: More questions than answers for colchicine in COVID-19
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Study: COVID cases have been ‘severely undercounted’
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.