User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
COVID-19 vaccines: Preparing for patient questions
With U.S. approval of one coronavirus vaccine likely imminent and approval of a second one expected soon after, physicians will likely be deluged with questions. Public attitudes about the vaccines vary by demographics, with a recent poll showing that men and older adults are more likely to choose vaccination, and women and people of color evincing more wariness.
Although the reasons for reluctance may vary, questions from patient will likely be similar. Some are related to the “warp speed” language about the vaccines. Other concerns arise from the fact that the platform – mRNA – has not been used in human vaccines before. And as with any vaccine, there are rumors and false claims making the rounds on social media.
In anticipation of the most common questions physicians may encounter, two experts, Krutika Kuppalli, MD, assistant professor of medicine in the division of infectious diseases at the Medical University of South Carolina, Charleston, and Angela Rasmussen, PhD, virologist and nonresident affiliate at Georgetown University’s Center for Global Health Science and Security, Washington, talked in an interview about what clinicians can expect and what evidence-based – as well as compassionate – answers might look like.
Q: Will this vaccine give me COVID-19?
“There is not an intact virus in there,” Dr. Rasmussen said. The mRNA-based vaccines cannot cause COVID-19 because they don’t use any part of the coronavirus itself. Instead, the Moderna and Pfizer vaccines contain manufactured mRNA molecules that carry the instructions for building the virus’ spike protein. After vaccine administration, the recipient’s own cells take up this mRNA, use it to build this bit of protein, and display it on their surfaces. The foreign protein flag triggers the immune system response.
The mRNA does not enter the cell nucleus or interact with the recipient’s DNA. And because it’s so fragile, it degrades quite quickly. To keep that from happening before cell entry, the mRNAs are cushioned in protective fats.
Q: Was this vaccine made too quickly?
“People have been working on this platform for 30 years, so it’s not that this is brand new,” Dr. Kuppalli said.
Researchers began working on mRNA vaccines in the 1990s. Technological developments in the last decade have meant that their use has become feasible, and they have been tested in animals against many viral diseases. The mRNA vaccines are attractive because they’re expected to be safe and easily manufactured from common materials. That’s what we’ve seen in the COVID-19 pandemic, the Centers for Disease Control and Prevention says on its website. Design of the spike protein mRNA component began as soon as the viral genome became available in January.
Usually, rolling out a vaccine takes years, so less than a year under a program called Operation Warp Speed can seem like moving too fast, Dr. Rasmussen acknowledged. “The name has given people the impression that by going at warp speed, we’re cutting all the corners. [But] the reality is that Operation Warp Speed is mostly for manufacturing and distribution.”
What underlies the speed is a restructuring of the normal vaccine development process, Dr. Kuppalli said. The same phases of development – animal testing, a small initial human phase, a second for safety testing, a third large phase for efficacy – were all conducted as for any vaccine. But in this case, some phases were completed in parallel, rather than sequentially. This approach has proved so successful that there is already talk about making it the model for developing future vaccines.
Two other factors contributed to the speed, said Dr. Kuppalli and Dr. Rasmussen. First, gearing up production can slow a rollout, but with these vaccines, companies ramped up production even before anyone knew if the vaccines would work – the “warp speed” part. The second factor has been the large number of cases, making exposures more likely and thus accelerating the results of the efficacy trials. “There is so much COVID being transmitted everywhere in the United States that it did not take long to hit the threshold of events to read out phase 3,” Dr. Rasmussen said.
Q: This vaccine has never been used in humans. How do we know it’s safe?
The Pfizer phase 3 trial included more than 43,000 people, and Moderna’s had more than 30,000. The first humans received mRNA-based COVID-19 vaccines in March. The most common adverse events emerge right after a vaccination, Dr. Kuppalli said.
As with any vaccine that gains approval, monitoring will continue.
UK health officials have reported that two health care workers vaccinated in the initial rollout of the Pfizer vaccine had what seems to have been a severe allergic response. Both recipients had a history of anaphylactic allergic responses and carried EpiPens, and both recovered. During the trial, allergic reaction rates were 0.63% in the vaccine group and 0.51% in the placebo group.
As a result of the two reactions, UK regulators are now recommending that patients with a history of severe allergies not receive the vaccine at the current time.
Q: What are the likely side effects?
So far, the most common side effects are pain at the injection site and an achy, flu-like feeling, Dr. Kuppalli said. More severe reactions have been reported, but were not common in the trials.
Dr. Rasmussen noted that the common side effects are a good sign, and signal that the recipient is generating “a robust immune response.”
“Everybody I’ve talked to who’s had the response has said they would go through it again,” Dr. Kruppalli said. “I definitely plan on lining up and being one of the first people to get the vaccine.”
Q: I already had COVID-19 or had a positive antibody test. Do I still need to get the vaccine?
Dr. Rasmussen said that there are “too many unknowns” to say if a history of COVID-19 would make a difference. “We don’t know how long neutralizing antibodies last” after infection, she said. “What we know is that the vaccine tends to produce antibody titers towards the higher end of the spectrum,” suggesting better immunity with vaccination than after natural infection.
Q: Can patients of color feel safe getting the vaccine?
“People of color might be understandably reluctant to take a vaccine that was developed in a way that appears to be faster [than past development],” said Dr. Rasmussen. She said physicians should acknowledge and understand the history that has led them to feel that way, “everything from Tuskegee to Henrietta Lacks to today.”
Empathy is key, and “providers should meet patients where they are and not condescend to them.”
Dr. Kuppalli agreed. “Clinicians really need to work on trying to strip away their biases.”
Thus far there are no safety signals that differ by race or ethnicity, according to the companies. The Pfizer phase 3 trial enrolled just over 9% Black participants, 0.5% Native American/Alaska Native, 0.2% Native Hawaiian/Pacific Islander, 2.3% multiracial participants, and 28% Hispanic/Latinx. For its part, Moderna says that approximately 37% of participants in its phase 3 trial come from communities of color.
Q: What about children and pregnant women?
Although the trials included participants from many different age groups and backgrounds, children and pregnant or lactating women were not among them. Pfizer gained approval in October to include participants as young as age 12 years, and a Moderna spokesperson said in an interview that the company planned pediatric inclusion at the end of 2020, pending approval.
“Unfortunately, we don’t have data on pregnant and lactating women,” Dr. Kuppalli said. She said she hopes that public health organizations such as the CDC will address that in the coming weeks. Dr. Rasmussen called the lack of data in pregnant women and children “a big oversight.”
Dr. Rasmussen has disclosed no relevant financial relationships. Dr. Kuppalli is a consultant with GlaxoSmithKline.
A version of this article originally appeared on Medscape.com.
With U.S. approval of one coronavirus vaccine likely imminent and approval of a second one expected soon after, physicians will likely be deluged with questions. Public attitudes about the vaccines vary by demographics, with a recent poll showing that men and older adults are more likely to choose vaccination, and women and people of color evincing more wariness.
Although the reasons for reluctance may vary, questions from patient will likely be similar. Some are related to the “warp speed” language about the vaccines. Other concerns arise from the fact that the platform – mRNA – has not been used in human vaccines before. And as with any vaccine, there are rumors and false claims making the rounds on social media.
In anticipation of the most common questions physicians may encounter, two experts, Krutika Kuppalli, MD, assistant professor of medicine in the division of infectious diseases at the Medical University of South Carolina, Charleston, and Angela Rasmussen, PhD, virologist and nonresident affiliate at Georgetown University’s Center for Global Health Science and Security, Washington, talked in an interview about what clinicians can expect and what evidence-based – as well as compassionate – answers might look like.
Q: Will this vaccine give me COVID-19?
“There is not an intact virus in there,” Dr. Rasmussen said. The mRNA-based vaccines cannot cause COVID-19 because they don’t use any part of the coronavirus itself. Instead, the Moderna and Pfizer vaccines contain manufactured mRNA molecules that carry the instructions for building the virus’ spike protein. After vaccine administration, the recipient’s own cells take up this mRNA, use it to build this bit of protein, and display it on their surfaces. The foreign protein flag triggers the immune system response.
The mRNA does not enter the cell nucleus or interact with the recipient’s DNA. And because it’s so fragile, it degrades quite quickly. To keep that from happening before cell entry, the mRNAs are cushioned in protective fats.
Q: Was this vaccine made too quickly?
“People have been working on this platform for 30 years, so it’s not that this is brand new,” Dr. Kuppalli said.
Researchers began working on mRNA vaccines in the 1990s. Technological developments in the last decade have meant that their use has become feasible, and they have been tested in animals against many viral diseases. The mRNA vaccines are attractive because they’re expected to be safe and easily manufactured from common materials. That’s what we’ve seen in the COVID-19 pandemic, the Centers for Disease Control and Prevention says on its website. Design of the spike protein mRNA component began as soon as the viral genome became available in January.
Usually, rolling out a vaccine takes years, so less than a year under a program called Operation Warp Speed can seem like moving too fast, Dr. Rasmussen acknowledged. “The name has given people the impression that by going at warp speed, we’re cutting all the corners. [But] the reality is that Operation Warp Speed is mostly for manufacturing and distribution.”
What underlies the speed is a restructuring of the normal vaccine development process, Dr. Kuppalli said. The same phases of development – animal testing, a small initial human phase, a second for safety testing, a third large phase for efficacy – were all conducted as for any vaccine. But in this case, some phases were completed in parallel, rather than sequentially. This approach has proved so successful that there is already talk about making it the model for developing future vaccines.
Two other factors contributed to the speed, said Dr. Kuppalli and Dr. Rasmussen. First, gearing up production can slow a rollout, but with these vaccines, companies ramped up production even before anyone knew if the vaccines would work – the “warp speed” part. The second factor has been the large number of cases, making exposures more likely and thus accelerating the results of the efficacy trials. “There is so much COVID being transmitted everywhere in the United States that it did not take long to hit the threshold of events to read out phase 3,” Dr. Rasmussen said.
Q: This vaccine has never been used in humans. How do we know it’s safe?
The Pfizer phase 3 trial included more than 43,000 people, and Moderna’s had more than 30,000. The first humans received mRNA-based COVID-19 vaccines in March. The most common adverse events emerge right after a vaccination, Dr. Kuppalli said.
As with any vaccine that gains approval, monitoring will continue.
UK health officials have reported that two health care workers vaccinated in the initial rollout of the Pfizer vaccine had what seems to have been a severe allergic response. Both recipients had a history of anaphylactic allergic responses and carried EpiPens, and both recovered. During the trial, allergic reaction rates were 0.63% in the vaccine group and 0.51% in the placebo group.
As a result of the two reactions, UK regulators are now recommending that patients with a history of severe allergies not receive the vaccine at the current time.
Q: What are the likely side effects?
So far, the most common side effects are pain at the injection site and an achy, flu-like feeling, Dr. Kuppalli said. More severe reactions have been reported, but were not common in the trials.
Dr. Rasmussen noted that the common side effects are a good sign, and signal that the recipient is generating “a robust immune response.”
“Everybody I’ve talked to who’s had the response has said they would go through it again,” Dr. Kruppalli said. “I definitely plan on lining up and being one of the first people to get the vaccine.”
Q: I already had COVID-19 or had a positive antibody test. Do I still need to get the vaccine?
Dr. Rasmussen said that there are “too many unknowns” to say if a history of COVID-19 would make a difference. “We don’t know how long neutralizing antibodies last” after infection, she said. “What we know is that the vaccine tends to produce antibody titers towards the higher end of the spectrum,” suggesting better immunity with vaccination than after natural infection.
Q: Can patients of color feel safe getting the vaccine?
“People of color might be understandably reluctant to take a vaccine that was developed in a way that appears to be faster [than past development],” said Dr. Rasmussen. She said physicians should acknowledge and understand the history that has led them to feel that way, “everything from Tuskegee to Henrietta Lacks to today.”
Empathy is key, and “providers should meet patients where they are and not condescend to them.”
Dr. Kuppalli agreed. “Clinicians really need to work on trying to strip away their biases.”
Thus far there are no safety signals that differ by race or ethnicity, according to the companies. The Pfizer phase 3 trial enrolled just over 9% Black participants, 0.5% Native American/Alaska Native, 0.2% Native Hawaiian/Pacific Islander, 2.3% multiracial participants, and 28% Hispanic/Latinx. For its part, Moderna says that approximately 37% of participants in its phase 3 trial come from communities of color.
Q: What about children and pregnant women?
Although the trials included participants from many different age groups and backgrounds, children and pregnant or lactating women were not among them. Pfizer gained approval in October to include participants as young as age 12 years, and a Moderna spokesperson said in an interview that the company planned pediatric inclusion at the end of 2020, pending approval.
“Unfortunately, we don’t have data on pregnant and lactating women,” Dr. Kuppalli said. She said she hopes that public health organizations such as the CDC will address that in the coming weeks. Dr. Rasmussen called the lack of data in pregnant women and children “a big oversight.”
Dr. Rasmussen has disclosed no relevant financial relationships. Dr. Kuppalli is a consultant with GlaxoSmithKline.
A version of this article originally appeared on Medscape.com.
With U.S. approval of one coronavirus vaccine likely imminent and approval of a second one expected soon after, physicians will likely be deluged with questions. Public attitudes about the vaccines vary by demographics, with a recent poll showing that men and older adults are more likely to choose vaccination, and women and people of color evincing more wariness.
Although the reasons for reluctance may vary, questions from patient will likely be similar. Some are related to the “warp speed” language about the vaccines. Other concerns arise from the fact that the platform – mRNA – has not been used in human vaccines before. And as with any vaccine, there are rumors and false claims making the rounds on social media.
In anticipation of the most common questions physicians may encounter, two experts, Krutika Kuppalli, MD, assistant professor of medicine in the division of infectious diseases at the Medical University of South Carolina, Charleston, and Angela Rasmussen, PhD, virologist and nonresident affiliate at Georgetown University’s Center for Global Health Science and Security, Washington, talked in an interview about what clinicians can expect and what evidence-based – as well as compassionate – answers might look like.
Q: Will this vaccine give me COVID-19?
“There is not an intact virus in there,” Dr. Rasmussen said. The mRNA-based vaccines cannot cause COVID-19 because they don’t use any part of the coronavirus itself. Instead, the Moderna and Pfizer vaccines contain manufactured mRNA molecules that carry the instructions for building the virus’ spike protein. After vaccine administration, the recipient’s own cells take up this mRNA, use it to build this bit of protein, and display it on their surfaces. The foreign protein flag triggers the immune system response.
The mRNA does not enter the cell nucleus or interact with the recipient’s DNA. And because it’s so fragile, it degrades quite quickly. To keep that from happening before cell entry, the mRNAs are cushioned in protective fats.
Q: Was this vaccine made too quickly?
“People have been working on this platform for 30 years, so it’s not that this is brand new,” Dr. Kuppalli said.
Researchers began working on mRNA vaccines in the 1990s. Technological developments in the last decade have meant that their use has become feasible, and they have been tested in animals against many viral diseases. The mRNA vaccines are attractive because they’re expected to be safe and easily manufactured from common materials. That’s what we’ve seen in the COVID-19 pandemic, the Centers for Disease Control and Prevention says on its website. Design of the spike protein mRNA component began as soon as the viral genome became available in January.
Usually, rolling out a vaccine takes years, so less than a year under a program called Operation Warp Speed can seem like moving too fast, Dr. Rasmussen acknowledged. “The name has given people the impression that by going at warp speed, we’re cutting all the corners. [But] the reality is that Operation Warp Speed is mostly for manufacturing and distribution.”
What underlies the speed is a restructuring of the normal vaccine development process, Dr. Kuppalli said. The same phases of development – animal testing, a small initial human phase, a second for safety testing, a third large phase for efficacy – were all conducted as for any vaccine. But in this case, some phases were completed in parallel, rather than sequentially. This approach has proved so successful that there is already talk about making it the model for developing future vaccines.
Two other factors contributed to the speed, said Dr. Kuppalli and Dr. Rasmussen. First, gearing up production can slow a rollout, but with these vaccines, companies ramped up production even before anyone knew if the vaccines would work – the “warp speed” part. The second factor has been the large number of cases, making exposures more likely and thus accelerating the results of the efficacy trials. “There is so much COVID being transmitted everywhere in the United States that it did not take long to hit the threshold of events to read out phase 3,” Dr. Rasmussen said.
Q: This vaccine has never been used in humans. How do we know it’s safe?
The Pfizer phase 3 trial included more than 43,000 people, and Moderna’s had more than 30,000. The first humans received mRNA-based COVID-19 vaccines in March. The most common adverse events emerge right after a vaccination, Dr. Kuppalli said.
As with any vaccine that gains approval, monitoring will continue.
UK health officials have reported that two health care workers vaccinated in the initial rollout of the Pfizer vaccine had what seems to have been a severe allergic response. Both recipients had a history of anaphylactic allergic responses and carried EpiPens, and both recovered. During the trial, allergic reaction rates were 0.63% in the vaccine group and 0.51% in the placebo group.
As a result of the two reactions, UK regulators are now recommending that patients with a history of severe allergies not receive the vaccine at the current time.
Q: What are the likely side effects?
So far, the most common side effects are pain at the injection site and an achy, flu-like feeling, Dr. Kuppalli said. More severe reactions have been reported, but were not common in the trials.
Dr. Rasmussen noted that the common side effects are a good sign, and signal that the recipient is generating “a robust immune response.”
“Everybody I’ve talked to who’s had the response has said they would go through it again,” Dr. Kruppalli said. “I definitely plan on lining up and being one of the first people to get the vaccine.”
Q: I already had COVID-19 or had a positive antibody test. Do I still need to get the vaccine?
Dr. Rasmussen said that there are “too many unknowns” to say if a history of COVID-19 would make a difference. “We don’t know how long neutralizing antibodies last” after infection, she said. “What we know is that the vaccine tends to produce antibody titers towards the higher end of the spectrum,” suggesting better immunity with vaccination than after natural infection.
Q: Can patients of color feel safe getting the vaccine?
“People of color might be understandably reluctant to take a vaccine that was developed in a way that appears to be faster [than past development],” said Dr. Rasmussen. She said physicians should acknowledge and understand the history that has led them to feel that way, “everything from Tuskegee to Henrietta Lacks to today.”
Empathy is key, and “providers should meet patients where they are and not condescend to them.”
Dr. Kuppalli agreed. “Clinicians really need to work on trying to strip away their biases.”
Thus far there are no safety signals that differ by race or ethnicity, according to the companies. The Pfizer phase 3 trial enrolled just over 9% Black participants, 0.5% Native American/Alaska Native, 0.2% Native Hawaiian/Pacific Islander, 2.3% multiracial participants, and 28% Hispanic/Latinx. For its part, Moderna says that approximately 37% of participants in its phase 3 trial come from communities of color.
Q: What about children and pregnant women?
Although the trials included participants from many different age groups and backgrounds, children and pregnant or lactating women were not among them. Pfizer gained approval in October to include participants as young as age 12 years, and a Moderna spokesperson said in an interview that the company planned pediatric inclusion at the end of 2020, pending approval.
“Unfortunately, we don’t have data on pregnant and lactating women,” Dr. Kuppalli said. She said she hopes that public health organizations such as the CDC will address that in the coming weeks. Dr. Rasmussen called the lack of data in pregnant women and children “a big oversight.”
Dr. Rasmussen has disclosed no relevant financial relationships. Dr. Kuppalli is a consultant with GlaxoSmithKline.
A version of this article originally appeared on Medscape.com.
Just under three million will get COVID-19 vaccine in first week
The federal government says it will distribute only enough doses of Pfizer’s COVID-19 vaccine to immunize 2.9 million Americans in the first week after the US Food and Drug Administration (FDA) authorizes it, far less than the initially discussed 6.4 million doses.
Theoretically, states have already formulated plans for distribution based on the revised lower amount. But in a briefing with reporters on December 9, officials from Operation Warp Speed and the Department of Health and Human Services (HHS) didn’t make clear exactly what the states were expecting.
Vaccine will be shipped to and allocated by 64 jurisdictions and five federal agencies — the Bureau of Prisons, the Department of Defense, the Department of State, the Indian Health Service, and the Veterans Health Administration — according to the Centers for Disease Control and Prevention’s COVID-19 Vaccination Program Interim Playbook.
It will be up to states — which will receive a supply prorated to population — and these agencies to determine how to prioritize distribution of the 2.9 million doses. Each state and agency has its own plan. Gen. Gustave Perna, the chief operating officer for Operation Warp Speed, said in the briefing that 30 states have told the federal government they will prioritize initial doses for residents and staff of long-term care facilities.
The distribution is contingent on FDA authorization, which could happen soon. The FDA’s Vaccines and Related Biologics Advisory Committee weighed the effectiveness data for the Pfizer vaccine on December 10 and recommended that the agency grant emergency authorization. The FDA could issue a decision at any time.
Fewer doses out of the gate
Perna said the federal government will begin shipping the Pfizer vaccine within 24 hours of an FDA authorization.
He said those shipments will include a total of 2.9 million doses — not the 6.4 million that will be available. The government is holding 500,000 doses in reserve and another 2.9 million to guarantee that the first few million people who are vaccinated will be able to receive a second dose 21 days later, said Perna.
In part, that is because the FDA labeling will require that a first dose be followed by a second exactly 21 days later, said HHS Secretary Alex Azar in the briefing.
Federal officials have calculated how much to hold back on the basis of Pfizer’s production, said Azar. At least initially, “we will not distribute a vaccine knowing that the booster will not be available either from reserve supply by us or ongoing expected predicted production,” he said.
Even with Pfizer having reduced its estimates of how much vaccine it can deliver in December, Azar said, “There will be enough vaccine available for 20 million first vaccinations in the month of December.”
That estimate is predicated, however, on the idea that a vaccine under development by Moderna will receive clearance shortly after the FDA assesses that vaccine’s safety and effectiveness on December 17.
This article first appeared on Medscape.com.
The federal government says it will distribute only enough doses of Pfizer’s COVID-19 vaccine to immunize 2.9 million Americans in the first week after the US Food and Drug Administration (FDA) authorizes it, far less than the initially discussed 6.4 million doses.
Theoretically, states have already formulated plans for distribution based on the revised lower amount. But in a briefing with reporters on December 9, officials from Operation Warp Speed and the Department of Health and Human Services (HHS) didn’t make clear exactly what the states were expecting.
Vaccine will be shipped to and allocated by 64 jurisdictions and five federal agencies — the Bureau of Prisons, the Department of Defense, the Department of State, the Indian Health Service, and the Veterans Health Administration — according to the Centers for Disease Control and Prevention’s COVID-19 Vaccination Program Interim Playbook.
It will be up to states — which will receive a supply prorated to population — and these agencies to determine how to prioritize distribution of the 2.9 million doses. Each state and agency has its own plan. Gen. Gustave Perna, the chief operating officer for Operation Warp Speed, said in the briefing that 30 states have told the federal government they will prioritize initial doses for residents and staff of long-term care facilities.
The distribution is contingent on FDA authorization, which could happen soon. The FDA’s Vaccines and Related Biologics Advisory Committee weighed the effectiveness data for the Pfizer vaccine on December 10 and recommended that the agency grant emergency authorization. The FDA could issue a decision at any time.
Fewer doses out of the gate
Perna said the federal government will begin shipping the Pfizer vaccine within 24 hours of an FDA authorization.
He said those shipments will include a total of 2.9 million doses — not the 6.4 million that will be available. The government is holding 500,000 doses in reserve and another 2.9 million to guarantee that the first few million people who are vaccinated will be able to receive a second dose 21 days later, said Perna.
In part, that is because the FDA labeling will require that a first dose be followed by a second exactly 21 days later, said HHS Secretary Alex Azar in the briefing.
Federal officials have calculated how much to hold back on the basis of Pfizer’s production, said Azar. At least initially, “we will not distribute a vaccine knowing that the booster will not be available either from reserve supply by us or ongoing expected predicted production,” he said.
Even with Pfizer having reduced its estimates of how much vaccine it can deliver in December, Azar said, “There will be enough vaccine available for 20 million first vaccinations in the month of December.”
That estimate is predicated, however, on the idea that a vaccine under development by Moderna will receive clearance shortly after the FDA assesses that vaccine’s safety and effectiveness on December 17.
This article first appeared on Medscape.com.
The federal government says it will distribute only enough doses of Pfizer’s COVID-19 vaccine to immunize 2.9 million Americans in the first week after the US Food and Drug Administration (FDA) authorizes it, far less than the initially discussed 6.4 million doses.
Theoretically, states have already formulated plans for distribution based on the revised lower amount. But in a briefing with reporters on December 9, officials from Operation Warp Speed and the Department of Health and Human Services (HHS) didn’t make clear exactly what the states were expecting.
Vaccine will be shipped to and allocated by 64 jurisdictions and five federal agencies — the Bureau of Prisons, the Department of Defense, the Department of State, the Indian Health Service, and the Veterans Health Administration — according to the Centers for Disease Control and Prevention’s COVID-19 Vaccination Program Interim Playbook.
It will be up to states — which will receive a supply prorated to population — and these agencies to determine how to prioritize distribution of the 2.9 million doses. Each state and agency has its own plan. Gen. Gustave Perna, the chief operating officer for Operation Warp Speed, said in the briefing that 30 states have told the federal government they will prioritize initial doses for residents and staff of long-term care facilities.
The distribution is contingent on FDA authorization, which could happen soon. The FDA’s Vaccines and Related Biologics Advisory Committee weighed the effectiveness data for the Pfizer vaccine on December 10 and recommended that the agency grant emergency authorization. The FDA could issue a decision at any time.
Fewer doses out of the gate
Perna said the federal government will begin shipping the Pfizer vaccine within 24 hours of an FDA authorization.
He said those shipments will include a total of 2.9 million doses — not the 6.4 million that will be available. The government is holding 500,000 doses in reserve and another 2.9 million to guarantee that the first few million people who are vaccinated will be able to receive a second dose 21 days later, said Perna.
In part, that is because the FDA labeling will require that a first dose be followed by a second exactly 21 days later, said HHS Secretary Alex Azar in the briefing.
Federal officials have calculated how much to hold back on the basis of Pfizer’s production, said Azar. At least initially, “we will not distribute a vaccine knowing that the booster will not be available either from reserve supply by us or ongoing expected predicted production,” he said.
Even with Pfizer having reduced its estimates of how much vaccine it can deliver in December, Azar said, “There will be enough vaccine available for 20 million first vaccinations in the month of December.”
That estimate is predicated, however, on the idea that a vaccine under development by Moderna will receive clearance shortly after the FDA assesses that vaccine’s safety and effectiveness on December 17.
This article first appeared on Medscape.com.
FDA panel overwhelmingly backs emergency authorization for Pfizer COVID vaccine
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
Patients with lung and blood cancers most vulnerable to COVID-19
Patients with cancer are at significantly increased risk for COVID-19 and worse outcomes, a new review confirms. It also found that patients with leukemia, non-Hodgkin lymphoma, and lung cancer are at greatest risk.
Blacks with cancer are at even higher risk, and for patients with colorectal cancer and non-Hodgkin lymphoma, the risk is higher for women than for men. (This contrasts with findings in noncancer populations, where men are more at risk from COVID-19 and severe outcomes than women.)
These findings come from a huge review of electronic health records of 73.4 million patients in the United States. They “highlight the need to protect and monitor patients with cancer as part of the strategy to control the pandemic,” the authors wrote.
The review was published online Dec. 10 in JAMA Oncology.
The greater risk for COVID-19 among patients with cancer is well known, but breaking the risk down by cancer type is novel, wrote the investigators, led by Quanqiu Wang, MS, Center for Artificial Intelligence in Drug Discovery, Case Western Reserve University, Cleveland.
Cancer patients are immunocompromised and have more contact with the health care system, which increases their risk for COVID-19. But which bodily systems are affected by cancer seems to matter. In patients with blood cancer, for example, COVID-19 is probably more dangerous, because blood cancer weakens the immune system directly, the authors suggested.
The increased risk for infection and hospitalization with SARS-CoV-2 among Black patients with cancer might be because of biology, but it is more likely because of factors that weren’t captured in the database review. Such factors include social adversity, economic status, access to health care, and lifestyle, the researchers noted.
For this study, the investigators analyzed electronic health records held in the IBM Watson Health Explorys system, which captures about 15% of new cancer diagnoses in the United States.
The analysis found that, as of Aug. 14, 2020, 16,570 patients (0.02%) had been diagnosed with COVID-19; about 1,200 also had been diagnosed with cancer. Of those, 690 were diagnosed with cancer in the previous year, which counted as a recent cancer diagnosis in the analysis. The study included 13 common cancers, including endometrial, kidney, liver, lung, gastrointestinal, prostate, skin, and thyroid cancers, among others.
Patients with any cancer diagnosis (adjusted odds ratio, 1.46) as well as those with a recent cancer diagnosis (aOR, 7.14) had a significantly higher risk for COVID-19 than those without cancer, after adjusting for asthma, cardiovascular diseases, nursing home stays, and other risk factors.
The risk for COVID-19 was highest among patients recently diagnosed with leukemia (aOR, 12.16), non-Hodgkin lymphoma (aOR, 8.54), and lung cancer (aOR 7.66). The risk for COVID-19 was lower for patients with cancers associated with worse prognoses, including pancreatic (aOR, 6.26) and liver (aOR, 6.49) cancer. It was weakest for patients with thyroid cancer (aOR, 3.10; P for all < .001).
Hospitalization was more common in recent cancer patients with COVID-19 than in COVID-19 patients without cancer (47.46% vs. 24.6%), as was COVID-19–related death (14.93% vs. 5.26%). Among cancer patients who did not have COVID-19, 12.39% were hospitalized, and 4.03% died. The findings suggest a synergistic effect between the COVID-19 and cancer, the team noted.
Among patients recently diagnosed with cancer, Black patients – 10.3% of the overall study population – had a significantly higher risk for COVID-19 than White patients. The racial disparity was largest for patients with breast cancer (aOR, 5.44), followed by patients with prostate cancer (aOR, 5.10), colorectal cancer (aOR, 3.30), and lung cancer (aOR, 2.53; P for all < .001).
Hospitalizations were more common among Black patients with cancer and COVID-19 than White patients. There was also a trend toward higher mortality among Black patients (18.52% vs. 13.51%; P = .11)
However, these differences may not be related to race, oncologist Aakash Desai, MBBS, of the Mayo Clinic, Rochester, Minn., and colleagues noted in an accompanying commentary. “Interestingly, a previous study of hospitalized patients with COVID-19 without cancer demonstrated that mortality rates for Black patients were comparable to those for White patients after adjustment for both comorbidities and deprivation index, suggesting that observed differences are mainly owing to societal disparities rather than biology.”
The editorialists also noted that the finding that Black patients with cancer are at greater risk for COVID-19 (aOR, 1.58-5.44, depending on cancer) echoes the findings in the general population. The Centers for Disease Control and Prevention estimates a severalfold increased risk among Black patients. These higher rates may largely be explained by social determinants, they suggested. Such factors include increased burden of comorbidities, crowded living conditions (inner cities, multigenerational homes, etc.), dependence on public transportation or child care, and higher work-related exposures. “Until such societal disparities are accounted for, we cannot presume these findings are caused by any inherent differences among racial groups,” the editorialists wrote.
“Clearly, the haunting spotlight of COVID-19 has dramatically illuminated known U.S. health care and societal disparities,” Dr. Desai and colleagues wrote. “This situation should be a wake-up call that brings much-needed improvements in U.S. equity policies, including but not limited to better health care access. Nothing appears more critical for alleviating these disparate clinical outcomes in this time of crisis and beyond,” they declared.
The study was funded by the National Institutes of Health, the American Cancer Society, and other organizations. The investigators disclosed having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with cancer are at significantly increased risk for COVID-19 and worse outcomes, a new review confirms. It also found that patients with leukemia, non-Hodgkin lymphoma, and lung cancer are at greatest risk.
Blacks with cancer are at even higher risk, and for patients with colorectal cancer and non-Hodgkin lymphoma, the risk is higher for women than for men. (This contrasts with findings in noncancer populations, where men are more at risk from COVID-19 and severe outcomes than women.)
These findings come from a huge review of electronic health records of 73.4 million patients in the United States. They “highlight the need to protect and monitor patients with cancer as part of the strategy to control the pandemic,” the authors wrote.
The review was published online Dec. 10 in JAMA Oncology.
The greater risk for COVID-19 among patients with cancer is well known, but breaking the risk down by cancer type is novel, wrote the investigators, led by Quanqiu Wang, MS, Center for Artificial Intelligence in Drug Discovery, Case Western Reserve University, Cleveland.
Cancer patients are immunocompromised and have more contact with the health care system, which increases their risk for COVID-19. But which bodily systems are affected by cancer seems to matter. In patients with blood cancer, for example, COVID-19 is probably more dangerous, because blood cancer weakens the immune system directly, the authors suggested.
The increased risk for infection and hospitalization with SARS-CoV-2 among Black patients with cancer might be because of biology, but it is more likely because of factors that weren’t captured in the database review. Such factors include social adversity, economic status, access to health care, and lifestyle, the researchers noted.
For this study, the investigators analyzed electronic health records held in the IBM Watson Health Explorys system, which captures about 15% of new cancer diagnoses in the United States.
The analysis found that, as of Aug. 14, 2020, 16,570 patients (0.02%) had been diagnosed with COVID-19; about 1,200 also had been diagnosed with cancer. Of those, 690 were diagnosed with cancer in the previous year, which counted as a recent cancer diagnosis in the analysis. The study included 13 common cancers, including endometrial, kidney, liver, lung, gastrointestinal, prostate, skin, and thyroid cancers, among others.
Patients with any cancer diagnosis (adjusted odds ratio, 1.46) as well as those with a recent cancer diagnosis (aOR, 7.14) had a significantly higher risk for COVID-19 than those without cancer, after adjusting for asthma, cardiovascular diseases, nursing home stays, and other risk factors.
The risk for COVID-19 was highest among patients recently diagnosed with leukemia (aOR, 12.16), non-Hodgkin lymphoma (aOR, 8.54), and lung cancer (aOR 7.66). The risk for COVID-19 was lower for patients with cancers associated with worse prognoses, including pancreatic (aOR, 6.26) and liver (aOR, 6.49) cancer. It was weakest for patients with thyroid cancer (aOR, 3.10; P for all < .001).
Hospitalization was more common in recent cancer patients with COVID-19 than in COVID-19 patients without cancer (47.46% vs. 24.6%), as was COVID-19–related death (14.93% vs. 5.26%). Among cancer patients who did not have COVID-19, 12.39% were hospitalized, and 4.03% died. The findings suggest a synergistic effect between the COVID-19 and cancer, the team noted.
Among patients recently diagnosed with cancer, Black patients – 10.3% of the overall study population – had a significantly higher risk for COVID-19 than White patients. The racial disparity was largest for patients with breast cancer (aOR, 5.44), followed by patients with prostate cancer (aOR, 5.10), colorectal cancer (aOR, 3.30), and lung cancer (aOR, 2.53; P for all < .001).
Hospitalizations were more common among Black patients with cancer and COVID-19 than White patients. There was also a trend toward higher mortality among Black patients (18.52% vs. 13.51%; P = .11)
However, these differences may not be related to race, oncologist Aakash Desai, MBBS, of the Mayo Clinic, Rochester, Minn., and colleagues noted in an accompanying commentary. “Interestingly, a previous study of hospitalized patients with COVID-19 without cancer demonstrated that mortality rates for Black patients were comparable to those for White patients after adjustment for both comorbidities and deprivation index, suggesting that observed differences are mainly owing to societal disparities rather than biology.”
The editorialists also noted that the finding that Black patients with cancer are at greater risk for COVID-19 (aOR, 1.58-5.44, depending on cancer) echoes the findings in the general population. The Centers for Disease Control and Prevention estimates a severalfold increased risk among Black patients. These higher rates may largely be explained by social determinants, they suggested. Such factors include increased burden of comorbidities, crowded living conditions (inner cities, multigenerational homes, etc.), dependence on public transportation or child care, and higher work-related exposures. “Until such societal disparities are accounted for, we cannot presume these findings are caused by any inherent differences among racial groups,” the editorialists wrote.
“Clearly, the haunting spotlight of COVID-19 has dramatically illuminated known U.S. health care and societal disparities,” Dr. Desai and colleagues wrote. “This situation should be a wake-up call that brings much-needed improvements in U.S. equity policies, including but not limited to better health care access. Nothing appears more critical for alleviating these disparate clinical outcomes in this time of crisis and beyond,” they declared.
The study was funded by the National Institutes of Health, the American Cancer Society, and other organizations. The investigators disclosed having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with cancer are at significantly increased risk for COVID-19 and worse outcomes, a new review confirms. It also found that patients with leukemia, non-Hodgkin lymphoma, and lung cancer are at greatest risk.
Blacks with cancer are at even higher risk, and for patients with colorectal cancer and non-Hodgkin lymphoma, the risk is higher for women than for men. (This contrasts with findings in noncancer populations, where men are more at risk from COVID-19 and severe outcomes than women.)
These findings come from a huge review of electronic health records of 73.4 million patients in the United States. They “highlight the need to protect and monitor patients with cancer as part of the strategy to control the pandemic,” the authors wrote.
The review was published online Dec. 10 in JAMA Oncology.
The greater risk for COVID-19 among patients with cancer is well known, but breaking the risk down by cancer type is novel, wrote the investigators, led by Quanqiu Wang, MS, Center for Artificial Intelligence in Drug Discovery, Case Western Reserve University, Cleveland.
Cancer patients are immunocompromised and have more contact with the health care system, which increases their risk for COVID-19. But which bodily systems are affected by cancer seems to matter. In patients with blood cancer, for example, COVID-19 is probably more dangerous, because blood cancer weakens the immune system directly, the authors suggested.
The increased risk for infection and hospitalization with SARS-CoV-2 among Black patients with cancer might be because of biology, but it is more likely because of factors that weren’t captured in the database review. Such factors include social adversity, economic status, access to health care, and lifestyle, the researchers noted.
For this study, the investigators analyzed electronic health records held in the IBM Watson Health Explorys system, which captures about 15% of new cancer diagnoses in the United States.
The analysis found that, as of Aug. 14, 2020, 16,570 patients (0.02%) had been diagnosed with COVID-19; about 1,200 also had been diagnosed with cancer. Of those, 690 were diagnosed with cancer in the previous year, which counted as a recent cancer diagnosis in the analysis. The study included 13 common cancers, including endometrial, kidney, liver, lung, gastrointestinal, prostate, skin, and thyroid cancers, among others.
Patients with any cancer diagnosis (adjusted odds ratio, 1.46) as well as those with a recent cancer diagnosis (aOR, 7.14) had a significantly higher risk for COVID-19 than those without cancer, after adjusting for asthma, cardiovascular diseases, nursing home stays, and other risk factors.
The risk for COVID-19 was highest among patients recently diagnosed with leukemia (aOR, 12.16), non-Hodgkin lymphoma (aOR, 8.54), and lung cancer (aOR 7.66). The risk for COVID-19 was lower for patients with cancers associated with worse prognoses, including pancreatic (aOR, 6.26) and liver (aOR, 6.49) cancer. It was weakest for patients with thyroid cancer (aOR, 3.10; P for all < .001).
Hospitalization was more common in recent cancer patients with COVID-19 than in COVID-19 patients without cancer (47.46% vs. 24.6%), as was COVID-19–related death (14.93% vs. 5.26%). Among cancer patients who did not have COVID-19, 12.39% were hospitalized, and 4.03% died. The findings suggest a synergistic effect between the COVID-19 and cancer, the team noted.
Among patients recently diagnosed with cancer, Black patients – 10.3% of the overall study population – had a significantly higher risk for COVID-19 than White patients. The racial disparity was largest for patients with breast cancer (aOR, 5.44), followed by patients with prostate cancer (aOR, 5.10), colorectal cancer (aOR, 3.30), and lung cancer (aOR, 2.53; P for all < .001).
Hospitalizations were more common among Black patients with cancer and COVID-19 than White patients. There was also a trend toward higher mortality among Black patients (18.52% vs. 13.51%; P = .11)
However, these differences may not be related to race, oncologist Aakash Desai, MBBS, of the Mayo Clinic, Rochester, Minn., and colleagues noted in an accompanying commentary. “Interestingly, a previous study of hospitalized patients with COVID-19 without cancer demonstrated that mortality rates for Black patients were comparable to those for White patients after adjustment for both comorbidities and deprivation index, suggesting that observed differences are mainly owing to societal disparities rather than biology.”
The editorialists also noted that the finding that Black patients with cancer are at greater risk for COVID-19 (aOR, 1.58-5.44, depending on cancer) echoes the findings in the general population. The Centers for Disease Control and Prevention estimates a severalfold increased risk among Black patients. These higher rates may largely be explained by social determinants, they suggested. Such factors include increased burden of comorbidities, crowded living conditions (inner cities, multigenerational homes, etc.), dependence on public transportation or child care, and higher work-related exposures. “Until such societal disparities are accounted for, we cannot presume these findings are caused by any inherent differences among racial groups,” the editorialists wrote.
“Clearly, the haunting spotlight of COVID-19 has dramatically illuminated known U.S. health care and societal disparities,” Dr. Desai and colleagues wrote. “This situation should be a wake-up call that brings much-needed improvements in U.S. equity policies, including but not limited to better health care access. Nothing appears more critical for alleviating these disparate clinical outcomes in this time of crisis and beyond,” they declared.
The study was funded by the National Institutes of Health, the American Cancer Society, and other organizations. The investigators disclosed having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pfizer can’t supply additional vaccines to U.S. until June
Pfizer won’t be able to provide more COVID-19 vaccine doses to the United States until late June or July because other countries have bought up the available supply, according to The Washington Post.
The U.S. government signed a deal with the giant pharmaceutical company earlier this year to provide 100 million doses for $1.95 billion – enough for 50 million Americans to receive the two-dose vaccine. At that time, Pfizer officials encouraged Operation Warp Speed officials to purchase an additional 100 million doses, The New York Times first reported Dec. 7, but the federal officials declined.
Since then, other countries have signed vaccine deals with Pfizer, so the U.S. may not be able to receive a second major allotment until the summer of 2021, The Washington Post reported. Without a substantial number of additional doses, the U.S. may not be able to follow its schedule of vaccinating the majority of Americans against COVID-19 by April or May.
However, Trump administration officials told the newspaper that there won’t be issues, citing other vaccine companies such as Moderna.
“I’m not concerned about our ability to buy vaccines to offer to all of the American public,” Gen. Paul Ostrowski, who oversees logistics for Operation Warp Speed, told The Washington Post.
“It’s clear that Pfizer made plans with other countries. Many have been announced. We understand those pieces,” he said.
With Pfizer’s COVID-19 vaccine on the verge of FDA approval, federal officials contacted the company last weekend to buy another 100 million doses, but the company said its current supply is already committed, the newspaper reported.
The vaccine from Pfizer and BioNTech is expected to win emergency approval within days and has been shown to be effective against COVID-19.
Pfizer added that it may be able to provide 50 million doses at the end of the second quarter and another 50 million doses during the third quarter. However, the company can’t offer anything “substantial” until next summer.
Beyond the initial 100 million doses that the U.S. has already secured, Pfizer and federal officials would need to negotiate a new, “separate and mutually acceptable agreement,” Amy Rose, a spokeswoman for Pfizer, told the newspaper.
On Dec. 8, President Donald Trump was expected to sign an executive order prioritizing vaccination for Americans first before providing doses to other countries, according to Fox News.
The order will provide guidelines to the Department of Health and Human Services, the U.S. Agency for International Development and the U.S. International Development Finance Corporation for foreign assistance with vaccines, the news outlet reported.
It’s unclear whether the executive order is related to the Pfizer issue, whether the president can prevent a private company from fulfilling contracts with other countries, and whether President-elect Joe Biden will create his own policy, according to CNBC. The order may prove to be mostly symbolic.
The FDA could issue an emergency use authorization for Pfizer’s coronavirus vaccine this week and will likely approve Moderna’s vaccine next week. The U.S. has signed a contract with Moderna for 100 million doses.
During a call with reporters on Dec. 7, a spokeswoman for the Department of Health and Human Services said, “We are confident that we will have 100 million doses of Pfizer’s vaccine as agreed to in our contract, and beyond that, we have five other vaccine candidates, including 100 million doses on the way from Moderna.”
Federal officials are counting on vaccine candidates from AstraZeneca and Johnson & Johnson to seek FDA approval in January and be ready for shipment in February.
“We could have all of them,” Moncef Slaoui, the chief science adviser for Operation Warp Speed, told The Washington Post on Dec. 7.
“And for this reason, we feel confident we could cover the needs without a specific cliff,” he said. “We have planned things in such a way as we would indeed avoid a cliff.”
This article first appeared on WebMD.com.
Pfizer won’t be able to provide more COVID-19 vaccine doses to the United States until late June or July because other countries have bought up the available supply, according to The Washington Post.
The U.S. government signed a deal with the giant pharmaceutical company earlier this year to provide 100 million doses for $1.95 billion – enough for 50 million Americans to receive the two-dose vaccine. At that time, Pfizer officials encouraged Operation Warp Speed officials to purchase an additional 100 million doses, The New York Times first reported Dec. 7, but the federal officials declined.
Since then, other countries have signed vaccine deals with Pfizer, so the U.S. may not be able to receive a second major allotment until the summer of 2021, The Washington Post reported. Without a substantial number of additional doses, the U.S. may not be able to follow its schedule of vaccinating the majority of Americans against COVID-19 by April or May.
However, Trump administration officials told the newspaper that there won’t be issues, citing other vaccine companies such as Moderna.
“I’m not concerned about our ability to buy vaccines to offer to all of the American public,” Gen. Paul Ostrowski, who oversees logistics for Operation Warp Speed, told The Washington Post.
“It’s clear that Pfizer made plans with other countries. Many have been announced. We understand those pieces,” he said.
With Pfizer’s COVID-19 vaccine on the verge of FDA approval, federal officials contacted the company last weekend to buy another 100 million doses, but the company said its current supply is already committed, the newspaper reported.
The vaccine from Pfizer and BioNTech is expected to win emergency approval within days and has been shown to be effective against COVID-19.
Pfizer added that it may be able to provide 50 million doses at the end of the second quarter and another 50 million doses during the third quarter. However, the company can’t offer anything “substantial” until next summer.
Beyond the initial 100 million doses that the U.S. has already secured, Pfizer and federal officials would need to negotiate a new, “separate and mutually acceptable agreement,” Amy Rose, a spokeswoman for Pfizer, told the newspaper.
On Dec. 8, President Donald Trump was expected to sign an executive order prioritizing vaccination for Americans first before providing doses to other countries, according to Fox News.
The order will provide guidelines to the Department of Health and Human Services, the U.S. Agency for International Development and the U.S. International Development Finance Corporation for foreign assistance with vaccines, the news outlet reported.
It’s unclear whether the executive order is related to the Pfizer issue, whether the president can prevent a private company from fulfilling contracts with other countries, and whether President-elect Joe Biden will create his own policy, according to CNBC. The order may prove to be mostly symbolic.
The FDA could issue an emergency use authorization for Pfizer’s coronavirus vaccine this week and will likely approve Moderna’s vaccine next week. The U.S. has signed a contract with Moderna for 100 million doses.
During a call with reporters on Dec. 7, a spokeswoman for the Department of Health and Human Services said, “We are confident that we will have 100 million doses of Pfizer’s vaccine as agreed to in our contract, and beyond that, we have five other vaccine candidates, including 100 million doses on the way from Moderna.”
Federal officials are counting on vaccine candidates from AstraZeneca and Johnson & Johnson to seek FDA approval in January and be ready for shipment in February.
“We could have all of them,” Moncef Slaoui, the chief science adviser for Operation Warp Speed, told The Washington Post on Dec. 7.
“And for this reason, we feel confident we could cover the needs without a specific cliff,” he said. “We have planned things in such a way as we would indeed avoid a cliff.”
This article first appeared on WebMD.com.
Pfizer won’t be able to provide more COVID-19 vaccine doses to the United States until late June or July because other countries have bought up the available supply, according to The Washington Post.
The U.S. government signed a deal with the giant pharmaceutical company earlier this year to provide 100 million doses for $1.95 billion – enough for 50 million Americans to receive the two-dose vaccine. At that time, Pfizer officials encouraged Operation Warp Speed officials to purchase an additional 100 million doses, The New York Times first reported Dec. 7, but the federal officials declined.
Since then, other countries have signed vaccine deals with Pfizer, so the U.S. may not be able to receive a second major allotment until the summer of 2021, The Washington Post reported. Without a substantial number of additional doses, the U.S. may not be able to follow its schedule of vaccinating the majority of Americans against COVID-19 by April or May.
However, Trump administration officials told the newspaper that there won’t be issues, citing other vaccine companies such as Moderna.
“I’m not concerned about our ability to buy vaccines to offer to all of the American public,” Gen. Paul Ostrowski, who oversees logistics for Operation Warp Speed, told The Washington Post.
“It’s clear that Pfizer made plans with other countries. Many have been announced. We understand those pieces,” he said.
With Pfizer’s COVID-19 vaccine on the verge of FDA approval, federal officials contacted the company last weekend to buy another 100 million doses, but the company said its current supply is already committed, the newspaper reported.
The vaccine from Pfizer and BioNTech is expected to win emergency approval within days and has been shown to be effective against COVID-19.
Pfizer added that it may be able to provide 50 million doses at the end of the second quarter and another 50 million doses during the third quarter. However, the company can’t offer anything “substantial” until next summer.
Beyond the initial 100 million doses that the U.S. has already secured, Pfizer and federal officials would need to negotiate a new, “separate and mutually acceptable agreement,” Amy Rose, a spokeswoman for Pfizer, told the newspaper.
On Dec. 8, President Donald Trump was expected to sign an executive order prioritizing vaccination for Americans first before providing doses to other countries, according to Fox News.
The order will provide guidelines to the Department of Health and Human Services, the U.S. Agency for International Development and the U.S. International Development Finance Corporation for foreign assistance with vaccines, the news outlet reported.
It’s unclear whether the executive order is related to the Pfizer issue, whether the president can prevent a private company from fulfilling contracts with other countries, and whether President-elect Joe Biden will create his own policy, according to CNBC. The order may prove to be mostly symbolic.
The FDA could issue an emergency use authorization for Pfizer’s coronavirus vaccine this week and will likely approve Moderna’s vaccine next week. The U.S. has signed a contract with Moderna for 100 million doses.
During a call with reporters on Dec. 7, a spokeswoman for the Department of Health and Human Services said, “We are confident that we will have 100 million doses of Pfizer’s vaccine as agreed to in our contract, and beyond that, we have five other vaccine candidates, including 100 million doses on the way from Moderna.”
Federal officials are counting on vaccine candidates from AstraZeneca and Johnson & Johnson to seek FDA approval in January and be ready for shipment in February.
“We could have all of them,” Moncef Slaoui, the chief science adviser for Operation Warp Speed, told The Washington Post on Dec. 7.
“And for this reason, we feel confident we could cover the needs without a specific cliff,” he said. “We have planned things in such a way as we would indeed avoid a cliff.”
This article first appeared on WebMD.com.
Prioritize COVID-19 vaccination in both types of diabetes, say docs
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Demand for COVID vaccines expected to get heated – and fast
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
New child COVID-19 cases down in last weekly count
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 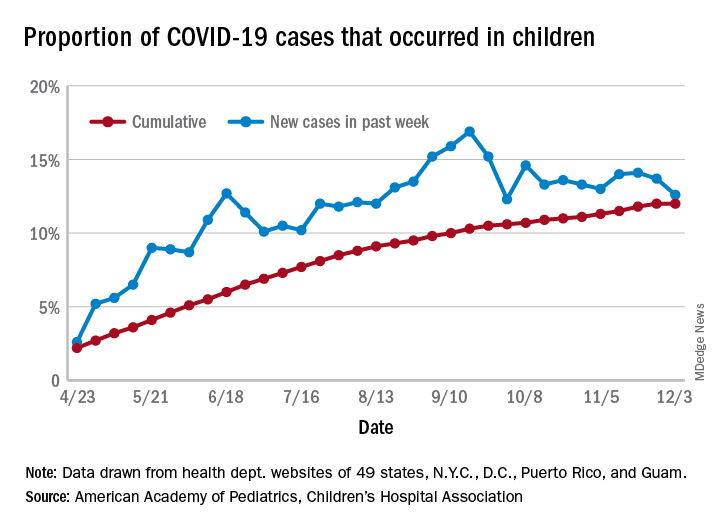
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
PPE shortage crisis continues at most hospitals, survey shows
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.

