User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
COVID-19 vaccines: Safe for immunocompromised patients?
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
To vape or not to vape: Is that really a question?
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at [email protected].
Asthma guidelines update FeNO, intermittent ICS use
The updated guidelines address six priority topics, including refined recommendations for the use of fractional exhaled nitric oxide (FeNO) testing, intermittent inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMA), and bronchial thermoplasty, but notably exclude any recommendations for the use of fast-emerging biological therapy.
“Biological therapy is the major step forward,” said William W. Busse, MD, professor of allergy and immunology at the University of Wisconsin–Madison, and lead author of the previous guidelines (Bethesda, Md.: NHLBI, 2007). “It wasn’t within the scope of work, so it’s not a criticism, but it is an important shortcoming,” he said. The omission identifies the need for the next update. “This is an area that has to be dealt with,” Dr. Busse stated in an interview.
Including biologic agents would have delayed the release of the recommendations for another year or 2, wrote the expert panel working group of the NHLBI, “and this was felt to be unacceptable.” The working group, overseen by the National Asthma Education and Prevention Program Coordinating Committee, also acknowledged the update is “not a complete revision” of the 2007 guidelines.
The update provides an evidenced-based review of six key topics in asthma care, as Mary Cataletto, MD, FCCP, professor of pediatrics at New York University Long Island, Mineola, pointed out: use of FeNO, indoor allergen mitigation, use of intermittent ICS and LAMA for asthma, role of subcutaneous and sublingual immunotherapy in the treatment of allergic asthma, and the use of bronchial thermoplasty.
“It has been 13 years since the last update and substantial progress has been made since then in understanding how to best treat children and adults with asthma,” said working group member Michael Schatz, MD, MS, FCCP, an allergy specialist at Kaiser Permanente Medical Center in San Diego.
According to Dr. Schatz, the most important updated recommendations are:
- Conditional recommendation for the use of ICS in children aged infant to 4 years with recurrent wheezing with respiratory infections.
- Use of combination ICS-formoterol for maintenance and to relieve flares in patients with moderate to severe asthma.
- Addition of the LAMA inhaled bronchodilator as add-on therapy for severe asthma not controlled by long-acting beta-agonist (LABA)/ICS combination medications.
Another important update, Dr. Cataletto said, is “shared decision-making among members of asthma teams in order to improve asthma care across all age groups.”
In all, the update includes 19 recommendations in the six subject areas. Each recommendation is notated with two values: its strength, either strong or conditional, and the certainty of evidence behind it, either low, moderate, or high. For example, the recommendation for ICS in young children that Dr. Schatz referred to has a conditional strength of recommendation with moderate certainty of evidence.
Using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology to determine strength of recommendation is a notable innovation of the latest guidelines, Dr. Busse noted.
Recommendations (strength of recommendation/certainty of evidence) include:
- Use of FeNO in children and adults when the asthma diagnosis is uncertain (conditional/moderate) or in those with allergic asthma and an uncertain course of management (conditional/low).
- Avoid standalone FeNO to evaluate asthma control or the likelihood or severity of future exacerbations, or for in infants to 4-year-olds with recurrent wheezing (strong/low for both).
- Avoid allergen mitigation in routine asthma management for patients who don’t have sensitivity to specific indoor allergens (conditional/low).
- Multicomponent allergen-specific mitigation when specific allergen sensitivity has been identified and pest management alone for symptoms related to specific pest exposure (conditional/low for both).
- Impermeable bedding covers should be part of a multicomponent mitigation strategy, not as a standalone tool, for patients with asthma and dust mite sensitivity (conditional/moderate).
- Daily ICS at onset of a respiratory tract infection along with as-needed short-acting beta-agonists in children aged 4 years and younger with recurrent wheezing but no wheezing between infections rather than as-needed standalone SABA (conditional/high).
- For adults and children aged 12 years and older with mild persistent asthma, either daily low-dose ICS with as-needed SABA or as-needed ICS and SABA concomitantly (conditional/moderate).
- Avoid short-course increased ICS dosing for patients aged 4 years and older with good adherence to daily ICS therapy (conditional/low).
- For patients aged 4 years and older with moderate to severe persistent asthma, a preference for combined ICS-formoterol inhaler over higher dose ICS daily and intermittent SABA or daily ICS-LABA with intermittent SABA (strong/high [aged 12 years and older]; moderate [aged 4-11 years]).
- A preference for combined ICS-formoterol for both daily and relief therapy for patients 12 years and older with severe persistent asthma over higher-dose ICS-LABA daily and intermittent SABA (conditional/high).
- A preference for adding LABA rather than LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma (conditional/moderate).
- If LABA isn’t used, add LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma rather than continuing the same dose of ICS alone (conditional/moderate).
- In those same patients already on combined ICS-LABA therapy, add LAMA rather than continuing the same dose of ICS-LABA (conditional/moderate).
- Use subcutaneous immunotherapy as a potential adjunct to standard drug therapy in patients aged 5 years and older with mild to moderate allergic asthma when their asthma is controlled on immunotherapy (conditional/moderate).
- Avoid sublingual immunotherapy in patients with persistent allergic asthma (conditional/moderate).
- Avoid bronchial thermoplasty in those 18 years and older with persistent asthma, but consider it in patients who can accept the short-term worsening symptoms or unknown long-term side effects in exchange for the potential benefits (conditional/moderate).
One of the key elements of the guidelines is the use of the SMART (single maintenance and reliever therapy) approach to evaluate the comparative effectiveness of intermittent ICS with formoterol, Dr. Busse noted. “I think that’s a very significant advance. The literature is replete with evidence to support this. Secondly, it really makes life convenient for patients; you have one inhaler.”
The recommendation on SABA use is also significant, Dr. Busse said. “Data have emerged to suggest that if you’re having a need for one of these rescue medications, it’s due to an increase in inflammation in the lower airway, and you want to give an ICS which will act on the inflammation along with the bronchodilator. That’s a new concept, and it’s a very significant step forward.”
Dr. Schatz disclosed financial relationships with Merck, Teva, and ALK-Abello, but was recused from the writing, discussion, and voting related to the immunotherapy recommendation. Dr. Cataletto and Dr. Busse have no relevant relationships to disclose.
SOURCE: Schatz M et al. J Allergy Clin Immunol. 2020;146:1217-70.
The updated guidelines address six priority topics, including refined recommendations for the use of fractional exhaled nitric oxide (FeNO) testing, intermittent inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMA), and bronchial thermoplasty, but notably exclude any recommendations for the use of fast-emerging biological therapy.
“Biological therapy is the major step forward,” said William W. Busse, MD, professor of allergy and immunology at the University of Wisconsin–Madison, and lead author of the previous guidelines (Bethesda, Md.: NHLBI, 2007). “It wasn’t within the scope of work, so it’s not a criticism, but it is an important shortcoming,” he said. The omission identifies the need for the next update. “This is an area that has to be dealt with,” Dr. Busse stated in an interview.
Including biologic agents would have delayed the release of the recommendations for another year or 2, wrote the expert panel working group of the NHLBI, “and this was felt to be unacceptable.” The working group, overseen by the National Asthma Education and Prevention Program Coordinating Committee, also acknowledged the update is “not a complete revision” of the 2007 guidelines.
The update provides an evidenced-based review of six key topics in asthma care, as Mary Cataletto, MD, FCCP, professor of pediatrics at New York University Long Island, Mineola, pointed out: use of FeNO, indoor allergen mitigation, use of intermittent ICS and LAMA for asthma, role of subcutaneous and sublingual immunotherapy in the treatment of allergic asthma, and the use of bronchial thermoplasty.
“It has been 13 years since the last update and substantial progress has been made since then in understanding how to best treat children and adults with asthma,” said working group member Michael Schatz, MD, MS, FCCP, an allergy specialist at Kaiser Permanente Medical Center in San Diego.
According to Dr. Schatz, the most important updated recommendations are:
- Conditional recommendation for the use of ICS in children aged infant to 4 years with recurrent wheezing with respiratory infections.
- Use of combination ICS-formoterol for maintenance and to relieve flares in patients with moderate to severe asthma.
- Addition of the LAMA inhaled bronchodilator as add-on therapy for severe asthma not controlled by long-acting beta-agonist (LABA)/ICS combination medications.
Another important update, Dr. Cataletto said, is “shared decision-making among members of asthma teams in order to improve asthma care across all age groups.”
In all, the update includes 19 recommendations in the six subject areas. Each recommendation is notated with two values: its strength, either strong or conditional, and the certainty of evidence behind it, either low, moderate, or high. For example, the recommendation for ICS in young children that Dr. Schatz referred to has a conditional strength of recommendation with moderate certainty of evidence.
Using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology to determine strength of recommendation is a notable innovation of the latest guidelines, Dr. Busse noted.
Recommendations (strength of recommendation/certainty of evidence) include:
- Use of FeNO in children and adults when the asthma diagnosis is uncertain (conditional/moderate) or in those with allergic asthma and an uncertain course of management (conditional/low).
- Avoid standalone FeNO to evaluate asthma control or the likelihood or severity of future exacerbations, or for in infants to 4-year-olds with recurrent wheezing (strong/low for both).
- Avoid allergen mitigation in routine asthma management for patients who don’t have sensitivity to specific indoor allergens (conditional/low).
- Multicomponent allergen-specific mitigation when specific allergen sensitivity has been identified and pest management alone for symptoms related to specific pest exposure (conditional/low for both).
- Impermeable bedding covers should be part of a multicomponent mitigation strategy, not as a standalone tool, for patients with asthma and dust mite sensitivity (conditional/moderate).
- Daily ICS at onset of a respiratory tract infection along with as-needed short-acting beta-agonists in children aged 4 years and younger with recurrent wheezing but no wheezing between infections rather than as-needed standalone SABA (conditional/high).
- For adults and children aged 12 years and older with mild persistent asthma, either daily low-dose ICS with as-needed SABA or as-needed ICS and SABA concomitantly (conditional/moderate).
- Avoid short-course increased ICS dosing for patients aged 4 years and older with good adherence to daily ICS therapy (conditional/low).
- For patients aged 4 years and older with moderate to severe persistent asthma, a preference for combined ICS-formoterol inhaler over higher dose ICS daily and intermittent SABA or daily ICS-LABA with intermittent SABA (strong/high [aged 12 years and older]; moderate [aged 4-11 years]).
- A preference for combined ICS-formoterol for both daily and relief therapy for patients 12 years and older with severe persistent asthma over higher-dose ICS-LABA daily and intermittent SABA (conditional/high).
- A preference for adding LABA rather than LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma (conditional/moderate).
- If LABA isn’t used, add LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma rather than continuing the same dose of ICS alone (conditional/moderate).
- In those same patients already on combined ICS-LABA therapy, add LAMA rather than continuing the same dose of ICS-LABA (conditional/moderate).
- Use subcutaneous immunotherapy as a potential adjunct to standard drug therapy in patients aged 5 years and older with mild to moderate allergic asthma when their asthma is controlled on immunotherapy (conditional/moderate).
- Avoid sublingual immunotherapy in patients with persistent allergic asthma (conditional/moderate).
- Avoid bronchial thermoplasty in those 18 years and older with persistent asthma, but consider it in patients who can accept the short-term worsening symptoms or unknown long-term side effects in exchange for the potential benefits (conditional/moderate).
One of the key elements of the guidelines is the use of the SMART (single maintenance and reliever therapy) approach to evaluate the comparative effectiveness of intermittent ICS with formoterol, Dr. Busse noted. “I think that’s a very significant advance. The literature is replete with evidence to support this. Secondly, it really makes life convenient for patients; you have one inhaler.”
The recommendation on SABA use is also significant, Dr. Busse said. “Data have emerged to suggest that if you’re having a need for one of these rescue medications, it’s due to an increase in inflammation in the lower airway, and you want to give an ICS which will act on the inflammation along with the bronchodilator. That’s a new concept, and it’s a very significant step forward.”
Dr. Schatz disclosed financial relationships with Merck, Teva, and ALK-Abello, but was recused from the writing, discussion, and voting related to the immunotherapy recommendation. Dr. Cataletto and Dr. Busse have no relevant relationships to disclose.
SOURCE: Schatz M et al. J Allergy Clin Immunol. 2020;146:1217-70.
The updated guidelines address six priority topics, including refined recommendations for the use of fractional exhaled nitric oxide (FeNO) testing, intermittent inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMA), and bronchial thermoplasty, but notably exclude any recommendations for the use of fast-emerging biological therapy.
“Biological therapy is the major step forward,” said William W. Busse, MD, professor of allergy and immunology at the University of Wisconsin–Madison, and lead author of the previous guidelines (Bethesda, Md.: NHLBI, 2007). “It wasn’t within the scope of work, so it’s not a criticism, but it is an important shortcoming,” he said. The omission identifies the need for the next update. “This is an area that has to be dealt with,” Dr. Busse stated in an interview.
Including biologic agents would have delayed the release of the recommendations for another year or 2, wrote the expert panel working group of the NHLBI, “and this was felt to be unacceptable.” The working group, overseen by the National Asthma Education and Prevention Program Coordinating Committee, also acknowledged the update is “not a complete revision” of the 2007 guidelines.
The update provides an evidenced-based review of six key topics in asthma care, as Mary Cataletto, MD, FCCP, professor of pediatrics at New York University Long Island, Mineola, pointed out: use of FeNO, indoor allergen mitigation, use of intermittent ICS and LAMA for asthma, role of subcutaneous and sublingual immunotherapy in the treatment of allergic asthma, and the use of bronchial thermoplasty.
“It has been 13 years since the last update and substantial progress has been made since then in understanding how to best treat children and adults with asthma,” said working group member Michael Schatz, MD, MS, FCCP, an allergy specialist at Kaiser Permanente Medical Center in San Diego.
According to Dr. Schatz, the most important updated recommendations are:
- Conditional recommendation for the use of ICS in children aged infant to 4 years with recurrent wheezing with respiratory infections.
- Use of combination ICS-formoterol for maintenance and to relieve flares in patients with moderate to severe asthma.
- Addition of the LAMA inhaled bronchodilator as add-on therapy for severe asthma not controlled by long-acting beta-agonist (LABA)/ICS combination medications.
Another important update, Dr. Cataletto said, is “shared decision-making among members of asthma teams in order to improve asthma care across all age groups.”
In all, the update includes 19 recommendations in the six subject areas. Each recommendation is notated with two values: its strength, either strong or conditional, and the certainty of evidence behind it, either low, moderate, or high. For example, the recommendation for ICS in young children that Dr. Schatz referred to has a conditional strength of recommendation with moderate certainty of evidence.
Using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology to determine strength of recommendation is a notable innovation of the latest guidelines, Dr. Busse noted.
Recommendations (strength of recommendation/certainty of evidence) include:
- Use of FeNO in children and adults when the asthma diagnosis is uncertain (conditional/moderate) or in those with allergic asthma and an uncertain course of management (conditional/low).
- Avoid standalone FeNO to evaluate asthma control or the likelihood or severity of future exacerbations, or for in infants to 4-year-olds with recurrent wheezing (strong/low for both).
- Avoid allergen mitigation in routine asthma management for patients who don’t have sensitivity to specific indoor allergens (conditional/low).
- Multicomponent allergen-specific mitigation when specific allergen sensitivity has been identified and pest management alone for symptoms related to specific pest exposure (conditional/low for both).
- Impermeable bedding covers should be part of a multicomponent mitigation strategy, not as a standalone tool, for patients with asthma and dust mite sensitivity (conditional/moderate).
- Daily ICS at onset of a respiratory tract infection along with as-needed short-acting beta-agonists in children aged 4 years and younger with recurrent wheezing but no wheezing between infections rather than as-needed standalone SABA (conditional/high).
- For adults and children aged 12 years and older with mild persistent asthma, either daily low-dose ICS with as-needed SABA or as-needed ICS and SABA concomitantly (conditional/moderate).
- Avoid short-course increased ICS dosing for patients aged 4 years and older with good adherence to daily ICS therapy (conditional/low).
- For patients aged 4 years and older with moderate to severe persistent asthma, a preference for combined ICS-formoterol inhaler over higher dose ICS daily and intermittent SABA or daily ICS-LABA with intermittent SABA (strong/high [aged 12 years and older]; moderate [aged 4-11 years]).
- A preference for combined ICS-formoterol for both daily and relief therapy for patients 12 years and older with severe persistent asthma over higher-dose ICS-LABA daily and intermittent SABA (conditional/high).
- A preference for adding LABA rather than LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma (conditional/moderate).
- If LABA isn’t used, add LAMA to ICS in patients aged 12 years and older with uncontrolled persistent asthma rather than continuing the same dose of ICS alone (conditional/moderate).
- In those same patients already on combined ICS-LABA therapy, add LAMA rather than continuing the same dose of ICS-LABA (conditional/moderate).
- Use subcutaneous immunotherapy as a potential adjunct to standard drug therapy in patients aged 5 years and older with mild to moderate allergic asthma when their asthma is controlled on immunotherapy (conditional/moderate).
- Avoid sublingual immunotherapy in patients with persistent allergic asthma (conditional/moderate).
- Avoid bronchial thermoplasty in those 18 years and older with persistent asthma, but consider it in patients who can accept the short-term worsening symptoms or unknown long-term side effects in exchange for the potential benefits (conditional/moderate).
One of the key elements of the guidelines is the use of the SMART (single maintenance and reliever therapy) approach to evaluate the comparative effectiveness of intermittent ICS with formoterol, Dr. Busse noted. “I think that’s a very significant advance. The literature is replete with evidence to support this. Secondly, it really makes life convenient for patients; you have one inhaler.”
The recommendation on SABA use is also significant, Dr. Busse said. “Data have emerged to suggest that if you’re having a need for one of these rescue medications, it’s due to an increase in inflammation in the lower airway, and you want to give an ICS which will act on the inflammation along with the bronchodilator. That’s a new concept, and it’s a very significant step forward.”
Dr. Schatz disclosed financial relationships with Merck, Teva, and ALK-Abello, but was recused from the writing, discussion, and voting related to the immunotherapy recommendation. Dr. Cataletto and Dr. Busse have no relevant relationships to disclose.
SOURCE: Schatz M et al. J Allergy Clin Immunol. 2020;146:1217-70.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Coronavirus has infected over 2% of U.S. children
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.
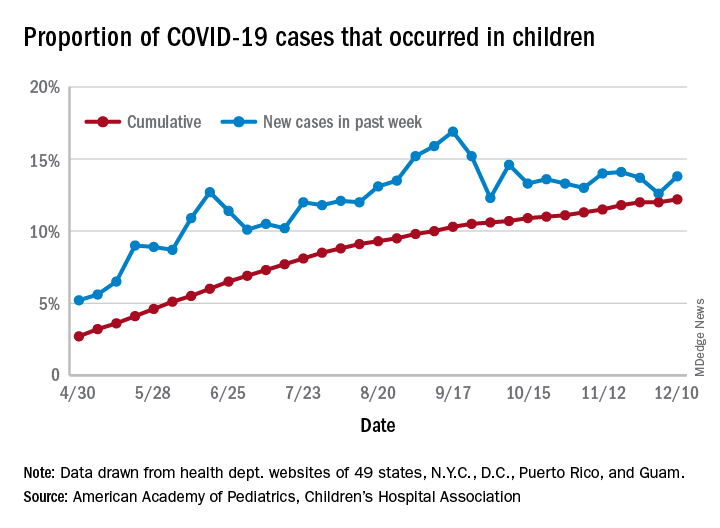
For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
FDA gives guidance on allergy, pregnancy concerns for Pfizer COVID vaccine
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
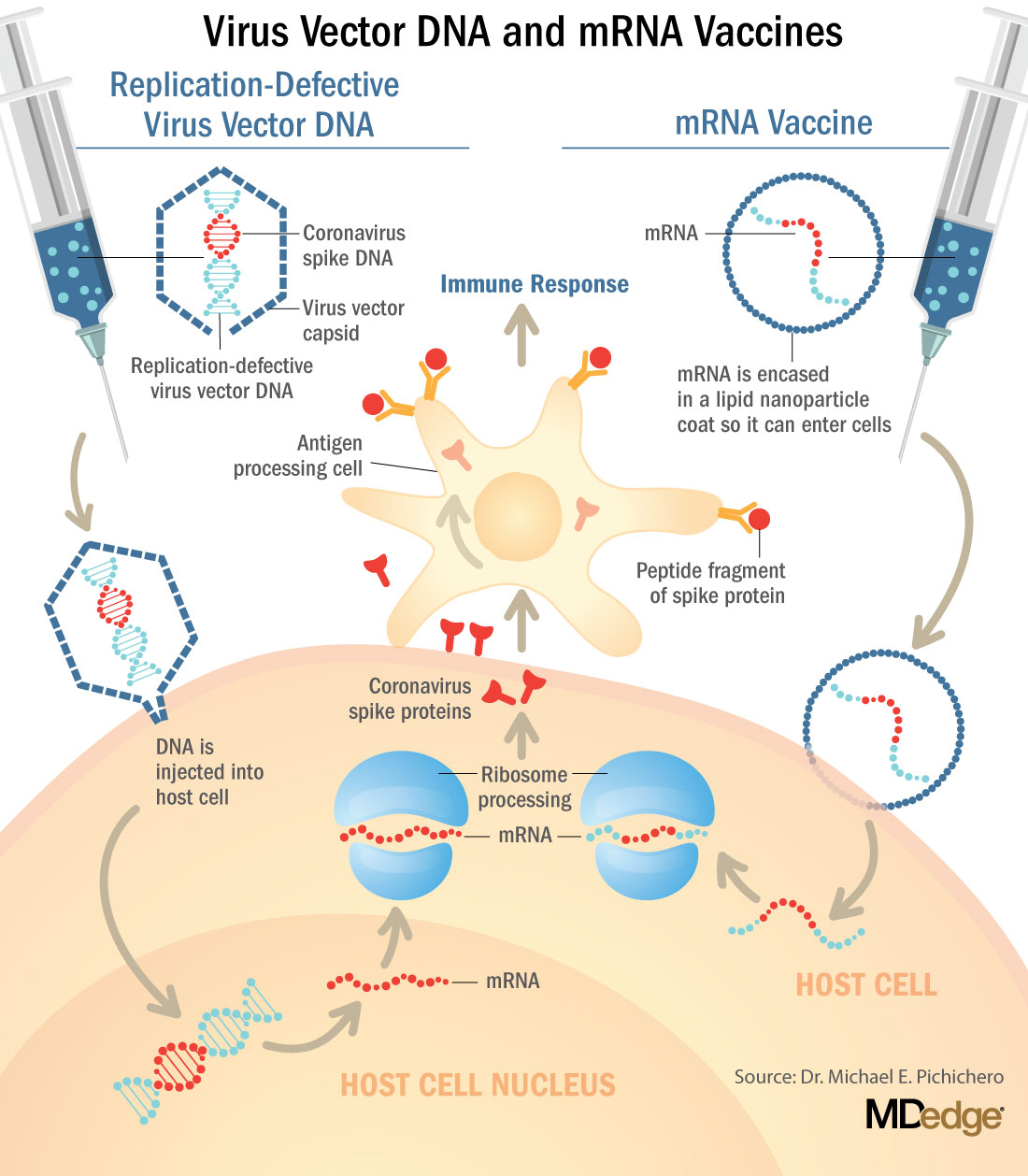
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
COVID-19 neurologic fallout not limited to the severely ill
Serious neurologic complications in patients with COVID-19 are not limited to the severely ill, new research confirms.
“We found a range of neurologic diagnoses, including stroke and seizures, among hospitalized patients with COVID-19 and the majority were not critically ill, suggesting that these complications are not limited just to those patients who require ICU care or a ventilator,” study investigator Pria Anand, MD, division of neuro-infectious diseases, Boston University, said in an interview.
The study was published online Dec. 9 in Neurology Clinical Practice.
‘Moderately severe’ disability
For the study, the investigators reviewed the medical records of 74 adults (mean age, 64 years) who were hospitalized with COVID-19 and evaluated for neurologic conditions at Boston Medical Center, a safety-net hospital caring primarily for underserved, low-income, racial and ethnic minority populations.
The most common COVID-19 symptoms on arrival to the hospital were cough (39%), dyspnea (36%), and fever (34%). Eleven patients required intubation (15%) and 28 required some form of supplemental oxygen (38%). Thirty-four patients required intensive care (46%).
The most common neurologic COVID-19 symptoms at presentation were altered mental status (53%), myalgia (24%), fatigue (24%), and headache (18%).
After neurologic assessment, the most common final neurologic diagnosis was multifactorial or toxic-metabolic encephalopathy (35%), followed by seizure (20%), ischemic stroke (20%), primary movement disorder (9%), peripheral neuropathy (8%), and hemorrhagic stroke (4%).
Three patients (4%) suffered traumatic brain injuries after falling in their homes after developing COVID-19.
Ten (14%) patients died in the hospital. Survivors had “moderately severe” disability at discharge (median modified Rankin Scale score of 4 from a preadmission mRS score of 2) and many were discharged to nursing facilities or rehabilitation hospitals.
“Although we do not have data on their posthospital course, this suggests that patients with neurologic complications of COVID-19 are likely to require ongoing rehabilitation, even after they leave the hospital,” Dr. Anand, a member of the American Academy of Neurology, said in an interview.
“There are a diverse range of mechanisms by which COVID-19 can cause neurologic complications,” Dr. Anand said.
“These complications can result from the body’s immunological response to the virus (e.g., Guillain-Barré syndrome, an autoimmune disorder affecting the nerves), from having a systemic severe illness (e.g., brain injury as a result of insufficient oxygenation), from the increased tendency to form blood clots (e.g., stroke), from worsening of preexisting neurologic disorders, and possibly from involvement of the nervous system by the virus itself,” she explained.
The researchers said more study is needed to characterize the infectious and postinfectious neurologic complications of COVID-19 in diverse patient populations.
Lingering issues
In an interview, Kenneth L. Tyler, MD, chair of neurology, University of Colorado, Denver, noted that this is one of the larger series published to date of the neurologic complications associated with COVID-19, and the first to come from a U.S. safety-net hospital in a large metropolitan area.
“Overall, the types and categories of neurological complications reported including encephalopathy (35%) and acute cerebrovascular events (~20%) are similar to those reported elsewhere,” said Dr. Tyler.
However, the frequency of stroke (~20%) is higher than in some other reports, “likely reflecting the comorbidities such as diabetes, hypertension, limited access to care [that are] present in this population,” he said.
Dr. Tyler also noted that the “relatively high frequency” of primary movement disorders, notably myoclonus, “hasn’t been particularly well recognized or described, although one of the authors has written on this in COVID-19, so perhaps there is a bit of an ‘ascertainment bias’ – as they were looking harder for it?”
Finally, he noted, it’s important to understand that all the published studies “vary tremendously in the populations they examine, so direct comparisons can be difficult.”
Also weighing in on the report in an interview, Richard Temes, MD, director, Northwell Health’s Center for Neurocritical Care in Manhasset, N.Y., said neurologic problems have been noted since the start of COVID-19 and have been well described.
“It’s common for patients to present with very nonspecific neurological complaints like confusion, disorientation, altered mental status, lethargy, but also neurological disease such as strokes, brain hemorrhages, and seizures are quite common as well,” said Dr. Temes.
He also noted that a number of patients with COVID-19 will have “lingering effects, especially patients who are hospitalized, that can range from memory deficit, cognitive slowing, and trouble with activities of daily living and depression.
“These effects can occur with any patient who is hospitalized for a [significant] period of time, especially in the intensive care unit, so it’s hard to tease out whether or not this is truly from COVID itself or if it’s just being a survivor from a very severe, critical illness. We don’t know yet. We need more data on that,” he cautioned.
A version of this article originally appeared on Medscape.com.
Serious neurologic complications in patients with COVID-19 are not limited to the severely ill, new research confirms.
“We found a range of neurologic diagnoses, including stroke and seizures, among hospitalized patients with COVID-19 and the majority were not critically ill, suggesting that these complications are not limited just to those patients who require ICU care or a ventilator,” study investigator Pria Anand, MD, division of neuro-infectious diseases, Boston University, said in an interview.
The study was published online Dec. 9 in Neurology Clinical Practice.
‘Moderately severe’ disability
For the study, the investigators reviewed the medical records of 74 adults (mean age, 64 years) who were hospitalized with COVID-19 and evaluated for neurologic conditions at Boston Medical Center, a safety-net hospital caring primarily for underserved, low-income, racial and ethnic minority populations.
The most common COVID-19 symptoms on arrival to the hospital were cough (39%), dyspnea (36%), and fever (34%). Eleven patients required intubation (15%) and 28 required some form of supplemental oxygen (38%). Thirty-four patients required intensive care (46%).
The most common neurologic COVID-19 symptoms at presentation were altered mental status (53%), myalgia (24%), fatigue (24%), and headache (18%).
After neurologic assessment, the most common final neurologic diagnosis was multifactorial or toxic-metabolic encephalopathy (35%), followed by seizure (20%), ischemic stroke (20%), primary movement disorder (9%), peripheral neuropathy (8%), and hemorrhagic stroke (4%).
Three patients (4%) suffered traumatic brain injuries after falling in their homes after developing COVID-19.
Ten (14%) patients died in the hospital. Survivors had “moderately severe” disability at discharge (median modified Rankin Scale score of 4 from a preadmission mRS score of 2) and many were discharged to nursing facilities or rehabilitation hospitals.
“Although we do not have data on their posthospital course, this suggests that patients with neurologic complications of COVID-19 are likely to require ongoing rehabilitation, even after they leave the hospital,” Dr. Anand, a member of the American Academy of Neurology, said in an interview.
“There are a diverse range of mechanisms by which COVID-19 can cause neurologic complications,” Dr. Anand said.
“These complications can result from the body’s immunological response to the virus (e.g., Guillain-Barré syndrome, an autoimmune disorder affecting the nerves), from having a systemic severe illness (e.g., brain injury as a result of insufficient oxygenation), from the increased tendency to form blood clots (e.g., stroke), from worsening of preexisting neurologic disorders, and possibly from involvement of the nervous system by the virus itself,” she explained.
The researchers said more study is needed to characterize the infectious and postinfectious neurologic complications of COVID-19 in diverse patient populations.
Lingering issues
In an interview, Kenneth L. Tyler, MD, chair of neurology, University of Colorado, Denver, noted that this is one of the larger series published to date of the neurologic complications associated with COVID-19, and the first to come from a U.S. safety-net hospital in a large metropolitan area.
“Overall, the types and categories of neurological complications reported including encephalopathy (35%) and acute cerebrovascular events (~20%) are similar to those reported elsewhere,” said Dr. Tyler.
However, the frequency of stroke (~20%) is higher than in some other reports, “likely reflecting the comorbidities such as diabetes, hypertension, limited access to care [that are] present in this population,” he said.
Dr. Tyler also noted that the “relatively high frequency” of primary movement disorders, notably myoclonus, “hasn’t been particularly well recognized or described, although one of the authors has written on this in COVID-19, so perhaps there is a bit of an ‘ascertainment bias’ – as they were looking harder for it?”
Finally, he noted, it’s important to understand that all the published studies “vary tremendously in the populations they examine, so direct comparisons can be difficult.”
Also weighing in on the report in an interview, Richard Temes, MD, director, Northwell Health’s Center for Neurocritical Care in Manhasset, N.Y., said neurologic problems have been noted since the start of COVID-19 and have been well described.
“It’s common for patients to present with very nonspecific neurological complaints like confusion, disorientation, altered mental status, lethargy, but also neurological disease such as strokes, brain hemorrhages, and seizures are quite common as well,” said Dr. Temes.
He also noted that a number of patients with COVID-19 will have “lingering effects, especially patients who are hospitalized, that can range from memory deficit, cognitive slowing, and trouble with activities of daily living and depression.
“These effects can occur with any patient who is hospitalized for a [significant] period of time, especially in the intensive care unit, so it’s hard to tease out whether or not this is truly from COVID itself or if it’s just being a survivor from a very severe, critical illness. We don’t know yet. We need more data on that,” he cautioned.
A version of this article originally appeared on Medscape.com.
Serious neurologic complications in patients with COVID-19 are not limited to the severely ill, new research confirms.
“We found a range of neurologic diagnoses, including stroke and seizures, among hospitalized patients with COVID-19 and the majority were not critically ill, suggesting that these complications are not limited just to those patients who require ICU care or a ventilator,” study investigator Pria Anand, MD, division of neuro-infectious diseases, Boston University, said in an interview.
The study was published online Dec. 9 in Neurology Clinical Practice.
‘Moderately severe’ disability
For the study, the investigators reviewed the medical records of 74 adults (mean age, 64 years) who were hospitalized with COVID-19 and evaluated for neurologic conditions at Boston Medical Center, a safety-net hospital caring primarily for underserved, low-income, racial and ethnic minority populations.
The most common COVID-19 symptoms on arrival to the hospital were cough (39%), dyspnea (36%), and fever (34%). Eleven patients required intubation (15%) and 28 required some form of supplemental oxygen (38%). Thirty-four patients required intensive care (46%).
The most common neurologic COVID-19 symptoms at presentation were altered mental status (53%), myalgia (24%), fatigue (24%), and headache (18%).
After neurologic assessment, the most common final neurologic diagnosis was multifactorial or toxic-metabolic encephalopathy (35%), followed by seizure (20%), ischemic stroke (20%), primary movement disorder (9%), peripheral neuropathy (8%), and hemorrhagic stroke (4%).
Three patients (4%) suffered traumatic brain injuries after falling in their homes after developing COVID-19.
Ten (14%) patients died in the hospital. Survivors had “moderately severe” disability at discharge (median modified Rankin Scale score of 4 from a preadmission mRS score of 2) and many were discharged to nursing facilities or rehabilitation hospitals.
“Although we do not have data on their posthospital course, this suggests that patients with neurologic complications of COVID-19 are likely to require ongoing rehabilitation, even after they leave the hospital,” Dr. Anand, a member of the American Academy of Neurology, said in an interview.
“There are a diverse range of mechanisms by which COVID-19 can cause neurologic complications,” Dr. Anand said.
“These complications can result from the body’s immunological response to the virus (e.g., Guillain-Barré syndrome, an autoimmune disorder affecting the nerves), from having a systemic severe illness (e.g., brain injury as a result of insufficient oxygenation), from the increased tendency to form blood clots (e.g., stroke), from worsening of preexisting neurologic disorders, and possibly from involvement of the nervous system by the virus itself,” she explained.
The researchers said more study is needed to characterize the infectious and postinfectious neurologic complications of COVID-19 in diverse patient populations.
Lingering issues
In an interview, Kenneth L. Tyler, MD, chair of neurology, University of Colorado, Denver, noted that this is one of the larger series published to date of the neurologic complications associated with COVID-19, and the first to come from a U.S. safety-net hospital in a large metropolitan area.
“Overall, the types and categories of neurological complications reported including encephalopathy (35%) and acute cerebrovascular events (~20%) are similar to those reported elsewhere,” said Dr. Tyler.
However, the frequency of stroke (~20%) is higher than in some other reports, “likely reflecting the comorbidities such as diabetes, hypertension, limited access to care [that are] present in this population,” he said.
Dr. Tyler also noted that the “relatively high frequency” of primary movement disorders, notably myoclonus, “hasn’t been particularly well recognized or described, although one of the authors has written on this in COVID-19, so perhaps there is a bit of an ‘ascertainment bias’ – as they were looking harder for it?”
Finally, he noted, it’s important to understand that all the published studies “vary tremendously in the populations they examine, so direct comparisons can be difficult.”
Also weighing in on the report in an interview, Richard Temes, MD, director, Northwell Health’s Center for Neurocritical Care in Manhasset, N.Y., said neurologic problems have been noted since the start of COVID-19 and have been well described.
“It’s common for patients to present with very nonspecific neurological complaints like confusion, disorientation, altered mental status, lethargy, but also neurological disease such as strokes, brain hemorrhages, and seizures are quite common as well,” said Dr. Temes.
He also noted that a number of patients with COVID-19 will have “lingering effects, especially patients who are hospitalized, that can range from memory deficit, cognitive slowing, and trouble with activities of daily living and depression.
“These effects can occur with any patient who is hospitalized for a [significant] period of time, especially in the intensive care unit, so it’s hard to tease out whether or not this is truly from COVID itself or if it’s just being a survivor from a very severe, critical illness. We don’t know yet. We need more data on that,” he cautioned.
A version of this article originally appeared on Medscape.com.
Baricitinib combo for COVID-19 accelerates recovery, study shows
according to trial results published Dec. 11 in the New England Journal of Medicine.
Median time to recovery was 7 days for patients who received baricitinib versus 8 days for patients who received placebo.
The difference was greater in patients who required high-flow oxygen or noninvasive ventilation during their hospitalization. In this group, baricitinib shortened median time to recovery from 18 days to 10 days.
“Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status, notably among patients receiving high-flow oxygen or noninvasive mechanical ventilation,” reported Andre C. Kalil, MD, MPH, from the University of Nebraska Medical Center, Omaha, and colleagues. In addition, the combination was associated with fewer adverse events.
The study details data from the ACTT-2 trial that the Food and Drug Administration used to issue an emergency-use authorization for baricitinib in combination with remdesivir on Nov. 19.
Under the emergency-use authorization, baricitinib (Olumiant, Eli Lilly), a Janus kinase inhibitor approved for the treatment of rheumatoid arthritis, may be used in combination with remdesivir (Veklury, Gilead), an antiviral, for treating hospitalized adults and children aged at least 2 years with suspected or confirmed COVID-19.
The combination is intended for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation.
Combo treatment favored
It is unclear how baricitinib compares with dexamethasone, which improved survival and led to a 1-day shorter hospital stay in another trial. There are differences between the drugs and trial designs, and only a “head-to-head comparison ... will allow the efficacy and safety differences between these two approaches to be fully understood,” Dr. Kalil and coauthors wrote.
“Dexamethasone has a long half-life, acts on glucocorticoid receptors, and reduces inflammation through a broad-pathway approach that has been associated with immunosuppression, hospital-acquired infections, gastrointestinal bleeding, hyperglycemia, and neuromuscular weakness, even with short courses,” they wrote. “Baricitinib has a short half-life, acts on targeted critical pathways to reduce inflammation while minimizing biologic redundancy with less immunosuppression, and may have antiviral activity.”
The ACTT-2 trial started in May and enrolled 1,033 patients in eight countries. Participants were randomly assigned to receive oral baricitinib tablets plus intravenous remdesivir or oral placebo tablets plus remdesivir.
Participants who received both drugs had significantly improved clinical status at day 15. Patients who received both treatments also had fewer serious adverse events.
“Although ACTT-2 was not powered to detect a difference in mortality between the two groups, both the survival rate and the time-to-death analyses favored combination treatment,” the researchers wrote.
The trial was sponsored by the National Institute of Allergy and Infectious Diseases. Some of the authors disclosed funding from government grants and financial ties to Eli Lilly, Gilead, and other companies.
A version of this article originally appeared on Medscape.com.
according to trial results published Dec. 11 in the New England Journal of Medicine.
Median time to recovery was 7 days for patients who received baricitinib versus 8 days for patients who received placebo.
The difference was greater in patients who required high-flow oxygen or noninvasive ventilation during their hospitalization. In this group, baricitinib shortened median time to recovery from 18 days to 10 days.
“Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status, notably among patients receiving high-flow oxygen or noninvasive mechanical ventilation,” reported Andre C. Kalil, MD, MPH, from the University of Nebraska Medical Center, Omaha, and colleagues. In addition, the combination was associated with fewer adverse events.
The study details data from the ACTT-2 trial that the Food and Drug Administration used to issue an emergency-use authorization for baricitinib in combination with remdesivir on Nov. 19.
Under the emergency-use authorization, baricitinib (Olumiant, Eli Lilly), a Janus kinase inhibitor approved for the treatment of rheumatoid arthritis, may be used in combination with remdesivir (Veklury, Gilead), an antiviral, for treating hospitalized adults and children aged at least 2 years with suspected or confirmed COVID-19.
The combination is intended for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation.
Combo treatment favored
It is unclear how baricitinib compares with dexamethasone, which improved survival and led to a 1-day shorter hospital stay in another trial. There are differences between the drugs and trial designs, and only a “head-to-head comparison ... will allow the efficacy and safety differences between these two approaches to be fully understood,” Dr. Kalil and coauthors wrote.
“Dexamethasone has a long half-life, acts on glucocorticoid receptors, and reduces inflammation through a broad-pathway approach that has been associated with immunosuppression, hospital-acquired infections, gastrointestinal bleeding, hyperglycemia, and neuromuscular weakness, even with short courses,” they wrote. “Baricitinib has a short half-life, acts on targeted critical pathways to reduce inflammation while minimizing biologic redundancy with less immunosuppression, and may have antiviral activity.”
The ACTT-2 trial started in May and enrolled 1,033 patients in eight countries. Participants were randomly assigned to receive oral baricitinib tablets plus intravenous remdesivir or oral placebo tablets plus remdesivir.
Participants who received both drugs had significantly improved clinical status at day 15. Patients who received both treatments also had fewer serious adverse events.
“Although ACTT-2 was not powered to detect a difference in mortality between the two groups, both the survival rate and the time-to-death analyses favored combination treatment,” the researchers wrote.
The trial was sponsored by the National Institute of Allergy and Infectious Diseases. Some of the authors disclosed funding from government grants and financial ties to Eli Lilly, Gilead, and other companies.
A version of this article originally appeared on Medscape.com.
according to trial results published Dec. 11 in the New England Journal of Medicine.
Median time to recovery was 7 days for patients who received baricitinib versus 8 days for patients who received placebo.
The difference was greater in patients who required high-flow oxygen or noninvasive ventilation during their hospitalization. In this group, baricitinib shortened median time to recovery from 18 days to 10 days.
“Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status, notably among patients receiving high-flow oxygen or noninvasive mechanical ventilation,” reported Andre C. Kalil, MD, MPH, from the University of Nebraska Medical Center, Omaha, and colleagues. In addition, the combination was associated with fewer adverse events.
The study details data from the ACTT-2 trial that the Food and Drug Administration used to issue an emergency-use authorization for baricitinib in combination with remdesivir on Nov. 19.
Under the emergency-use authorization, baricitinib (Olumiant, Eli Lilly), a Janus kinase inhibitor approved for the treatment of rheumatoid arthritis, may be used in combination with remdesivir (Veklury, Gilead), an antiviral, for treating hospitalized adults and children aged at least 2 years with suspected or confirmed COVID-19.
The combination is intended for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation.
Combo treatment favored
It is unclear how baricitinib compares with dexamethasone, which improved survival and led to a 1-day shorter hospital stay in another trial. There are differences between the drugs and trial designs, and only a “head-to-head comparison ... will allow the efficacy and safety differences between these two approaches to be fully understood,” Dr. Kalil and coauthors wrote.
“Dexamethasone has a long half-life, acts on glucocorticoid receptors, and reduces inflammation through a broad-pathway approach that has been associated with immunosuppression, hospital-acquired infections, gastrointestinal bleeding, hyperglycemia, and neuromuscular weakness, even with short courses,” they wrote. “Baricitinib has a short half-life, acts on targeted critical pathways to reduce inflammation while minimizing biologic redundancy with less immunosuppression, and may have antiviral activity.”
The ACTT-2 trial started in May and enrolled 1,033 patients in eight countries. Participants were randomly assigned to receive oral baricitinib tablets plus intravenous remdesivir or oral placebo tablets plus remdesivir.
Participants who received both drugs had significantly improved clinical status at day 15. Patients who received both treatments also had fewer serious adverse events.
“Although ACTT-2 was not powered to detect a difference in mortality between the two groups, both the survival rate and the time-to-death analyses favored combination treatment,” the researchers wrote.
The trial was sponsored by the National Institute of Allergy and Infectious Diseases. Some of the authors disclosed funding from government grants and financial ties to Eli Lilly, Gilead, and other companies.
A version of this article originally appeared on Medscape.com.
CDC panel recommends Pfizer’s COVID-19 vaccine for people 16 and over
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
FDA OKs emergency use of Pfizer COVID-19 vaccine
The much-anticipated emergency use authorization (EUA) of this vaccine — the first such approval in the United States — was greeted with optimism by infectious disease and pulmonary experts, although unanswered questions remain regarding use in people with allergic hypersensitivity, safety in pregnant women, and how smooth distribution will be.
“I am delighted. This is a first, firm step on a long path to getting this COVID pandemic under control,” William Schaffner, MD, professor of infectious diseases at the Vanderbilt University School of Medicine in Nashville, Tennessee, said in an interview.
The FDA gave the green light after the December 10 recommendation from the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. The committee voted 17-4 in favor of the emergency authorization.
The COVID-19 vaccine is “going to have a major impact here in the US. I’m very optimistic about it,” Dial Hewlett, MD, a spokesperson for the Infectious Diseases Society of American (IDSA), told this news organization.
Daniel Culver, DO, chair of medicine at the Cleveland Clinic in Ohio, is likewise hopeful. “My understanding is that supplies of the vaccine are already in place in hubs and will be shipped relatively quickly. The hope would be we can start vaccinating people as early as next week.”
Allergic reactions reported in the UK
After vaccinations with the Pfizer vaccine began in the UK on December 8, reports surfaced of two healthcare workers who experienced allergic reactions. They have since recovered, but officials warned that people with a history of severe allergic reactions should not receive the Pfizer vaccine at this time.
“For the moment, they are asking people who have had notable allergic reactions to step aside while this is investigated. It shows you that the system is working,” Schaffner said.
Both vaccine recipients who experienced anaphylaxis carried EpiPens, as they were at high risk for allergic reactions, Hewlett said. Also, if other COVID-19 vaccines are approved for use in the future, people allergic to the Pfizer vaccine might have another option, he added.
Reassuring role models
Schaffner supports the CDC Advisory Committee on Immunization Practices (ACIP) decision to start vaccinations with healthcare workers and residents of long-term care facilities.
“Vaccinating healthcare workers, in particular, will be a model for the general public,” said Schaffner, who is also a former member of the IDSA board of directors. “If they see those of us in white coats and blue scrubs lining up for the vaccine, that will provide confidence.”
To further increase acceptance of the COVID-19 vaccine, public health officials need to provide information and reassure the general public, Schaffner said.
Hewlett agreed. “I know there are a lot of people in the population who are very hesitant about vaccines. As infection disease specialists and people in public health, we are trying to allay a lot of concerns people have.”
Reassurance will be especially important in minority communities. “They have been disproportionately affected by the virus, and they have a traditional history of not being optimally vaccinated,” Schaffner said. “We need to reach them in particular with good information and reassurance…so they can make good decisions for themselves and their families.”
No vaccine is 100% effective or completely free of side effects. “There is always a chance there can be adverse reactions, but we think for the most part this is going to be a safe and effective vaccine,” said Hewlett, medical director at the Division of Disease Control and deputy to commissioner of health at the Westchester County Department of Health in White Plains, New York.
Distribution: Smooth or full of strife?
In addition to the concern that some people will not take advantage of vaccination against COVID-19, there could be vaccine supply issues down the road, Schaffner said.
Culver agreed. “In the early phases, I expect that there will be some kinks to work out, but because the numbers are relatively small, this should be okay,” he said.
“I think when we start to get into larger-scale vaccination programs — the supply chain, transport, and storage will be a Herculean undertaking,” Culver added. “It will take careful coordination between healthcare providers, distributors, suppliers, and public health officials to pull this off.”
Planning and distribution also should focus beyond US borders. Any issues in vaccine distribution or administration in the United States “will only be multiplied in several other parts of the world,” Culver said. Because COVID-19 is a pandemic, “we need to think about vaccinating globally.”
Investigating adverse events
Adverse events common to vaccinations in general — injection site pain, headaches, and fever — would not be unexpected with the COVID-19 vaccines. However, experts remain concerned that other, unrelated adverse events might be erroneously attributed to vaccination. For example, if a fall, heart attack, or death occurs within days of immunization, some might immediately blame the vaccine product.
“It’s important to remember that any new, highly touted medical therapy like this will receive a lot of scrutiny, so it would be unusual not to hear about something happening to somebody,” Culver said. Vaccine companies and health agencies will be carefully evaluating any reported adverse events to ensure no safety signal was missed in the trials.
“Fortunately, there are systems in place to investigate these events immediately,” Schaffner said.
Pregnancy recommendations pending
One question still looms: Is the COVID-19 vaccination safe for pregnant women? This isn’t just a question for the general public, either, Schaffner said. He estimated that about 70 percent of healthcare workers are women, and data suggests about 300,000 of these healthcare workers are pregnant.
“The CDC’s Advisory Committee on Immunization Practices will speak to that just as soon as the EUA is issued,” he added.
Patients are asking Culver about the priority order for vaccination. He said it’s difficult to provide firm guidance at this point.
People also have “lingering skepticism” about whether vaccine development was done in a prudent way, Culver said. Some people question whether the Pfizer vaccine and others were rushed to market. “So we try to spend time with the patients, reassuring them that all the usual safety evaluations were carefully done,” he said.
Another concern is whether mRNA vaccines can interact with human DNA. “The quick, short, and definitive answer is no,” Schaffner said. The m stands for messenger — the vaccines transmit information. "Once it gets into a cell, the mRNA does not go anywhere near the DNA, and once it transmits its information to the cell appropriately, it gets metabolized, and we excrete all the remnants."
Hewlett pointed out that investigations and surveillance will continue. Because this is an EUA and not full approval, “that essentially means they will still be obligated to collect a lot more data than they would ordinarily,” he said.
How long immunoprotection will last also remains an unknown. “The big question left on the table now is the durability,” Culver said. “Of course, we won’t know the answer to that for quite some time.”
Schaffner and Culver have disclosed no relevant financial relationships. Hewlett was an employee of Pfizer until mid-2019. His previous work as Pfizer’s senior medical director of global medical product evaluation was not associated with development of the COVID-19 vaccine.
This article first appeared on Medscape.com.
The much-anticipated emergency use authorization (EUA) of this vaccine — the first such approval in the United States — was greeted with optimism by infectious disease and pulmonary experts, although unanswered questions remain regarding use in people with allergic hypersensitivity, safety in pregnant women, and how smooth distribution will be.
“I am delighted. This is a first, firm step on a long path to getting this COVID pandemic under control,” William Schaffner, MD, professor of infectious diseases at the Vanderbilt University School of Medicine in Nashville, Tennessee, said in an interview.
The FDA gave the green light after the December 10 recommendation from the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. The committee voted 17-4 in favor of the emergency authorization.
The COVID-19 vaccine is “going to have a major impact here in the US. I’m very optimistic about it,” Dial Hewlett, MD, a spokesperson for the Infectious Diseases Society of American (IDSA), told this news organization.
Daniel Culver, DO, chair of medicine at the Cleveland Clinic in Ohio, is likewise hopeful. “My understanding is that supplies of the vaccine are already in place in hubs and will be shipped relatively quickly. The hope would be we can start vaccinating people as early as next week.”
Allergic reactions reported in the UK
After vaccinations with the Pfizer vaccine began in the UK on December 8, reports surfaced of two healthcare workers who experienced allergic reactions. They have since recovered, but officials warned that people with a history of severe allergic reactions should not receive the Pfizer vaccine at this time.
“For the moment, they are asking people who have had notable allergic reactions to step aside while this is investigated. It shows you that the system is working,” Schaffner said.
Both vaccine recipients who experienced anaphylaxis carried EpiPens, as they were at high risk for allergic reactions, Hewlett said. Also, if other COVID-19 vaccines are approved for use in the future, people allergic to the Pfizer vaccine might have another option, he added.
Reassuring role models
Schaffner supports the CDC Advisory Committee on Immunization Practices (ACIP) decision to start vaccinations with healthcare workers and residents of long-term care facilities.
“Vaccinating healthcare workers, in particular, will be a model for the general public,” said Schaffner, who is also a former member of the IDSA board of directors. “If they see those of us in white coats and blue scrubs lining up for the vaccine, that will provide confidence.”
To further increase acceptance of the COVID-19 vaccine, public health officials need to provide information and reassure the general public, Schaffner said.
Hewlett agreed. “I know there are a lot of people in the population who are very hesitant about vaccines. As infection disease specialists and people in public health, we are trying to allay a lot of concerns people have.”
Reassurance will be especially important in minority communities. “They have been disproportionately affected by the virus, and they have a traditional history of not being optimally vaccinated,” Schaffner said. “We need to reach them in particular with good information and reassurance…so they can make good decisions for themselves and their families.”
No vaccine is 100% effective or completely free of side effects. “There is always a chance there can be adverse reactions, but we think for the most part this is going to be a safe and effective vaccine,” said Hewlett, medical director at the Division of Disease Control and deputy to commissioner of health at the Westchester County Department of Health in White Plains, New York.
Distribution: Smooth or full of strife?
In addition to the concern that some people will not take advantage of vaccination against COVID-19, there could be vaccine supply issues down the road, Schaffner said.
Culver agreed. “In the early phases, I expect that there will be some kinks to work out, but because the numbers are relatively small, this should be okay,” he said.
“I think when we start to get into larger-scale vaccination programs — the supply chain, transport, and storage will be a Herculean undertaking,” Culver added. “It will take careful coordination between healthcare providers, distributors, suppliers, and public health officials to pull this off.”
Planning and distribution also should focus beyond US borders. Any issues in vaccine distribution or administration in the United States “will only be multiplied in several other parts of the world,” Culver said. Because COVID-19 is a pandemic, “we need to think about vaccinating globally.”
Investigating adverse events
Adverse events common to vaccinations in general — injection site pain, headaches, and fever — would not be unexpected with the COVID-19 vaccines. However, experts remain concerned that other, unrelated adverse events might be erroneously attributed to vaccination. For example, if a fall, heart attack, or death occurs within days of immunization, some might immediately blame the vaccine product.
“It’s important to remember that any new, highly touted medical therapy like this will receive a lot of scrutiny, so it would be unusual not to hear about something happening to somebody,” Culver said. Vaccine companies and health agencies will be carefully evaluating any reported adverse events to ensure no safety signal was missed in the trials.
“Fortunately, there are systems in place to investigate these events immediately,” Schaffner said.
Pregnancy recommendations pending
One question still looms: Is the COVID-19 vaccination safe for pregnant women? This isn’t just a question for the general public, either, Schaffner said. He estimated that about 70 percent of healthcare workers are women, and data suggests about 300,000 of these healthcare workers are pregnant.
“The CDC’s Advisory Committee on Immunization Practices will speak to that just as soon as the EUA is issued,” he added.
Patients are asking Culver about the priority order for vaccination. He said it’s difficult to provide firm guidance at this point.
People also have “lingering skepticism” about whether vaccine development was done in a prudent way, Culver said. Some people question whether the Pfizer vaccine and others were rushed to market. “So we try to spend time with the patients, reassuring them that all the usual safety evaluations were carefully done,” he said.
Another concern is whether mRNA vaccines can interact with human DNA. “The quick, short, and definitive answer is no,” Schaffner said. The m stands for messenger — the vaccines transmit information. "Once it gets into a cell, the mRNA does not go anywhere near the DNA, and once it transmits its information to the cell appropriately, it gets metabolized, and we excrete all the remnants."
Hewlett pointed out that investigations and surveillance will continue. Because this is an EUA and not full approval, “that essentially means they will still be obligated to collect a lot more data than they would ordinarily,” he said.
How long immunoprotection will last also remains an unknown. “The big question left on the table now is the durability,” Culver said. “Of course, we won’t know the answer to that for quite some time.”
Schaffner and Culver have disclosed no relevant financial relationships. Hewlett was an employee of Pfizer until mid-2019. His previous work as Pfizer’s senior medical director of global medical product evaluation was not associated with development of the COVID-19 vaccine.
This article first appeared on Medscape.com.
The much-anticipated emergency use authorization (EUA) of this vaccine — the first such approval in the United States — was greeted with optimism by infectious disease and pulmonary experts, although unanswered questions remain regarding use in people with allergic hypersensitivity, safety in pregnant women, and how smooth distribution will be.
“I am delighted. This is a first, firm step on a long path to getting this COVID pandemic under control,” William Schaffner, MD, professor of infectious diseases at the Vanderbilt University School of Medicine in Nashville, Tennessee, said in an interview.
The FDA gave the green light after the December 10 recommendation from the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. The committee voted 17-4 in favor of the emergency authorization.
The COVID-19 vaccine is “going to have a major impact here in the US. I’m very optimistic about it,” Dial Hewlett, MD, a spokesperson for the Infectious Diseases Society of American (IDSA), told this news organization.
Daniel Culver, DO, chair of medicine at the Cleveland Clinic in Ohio, is likewise hopeful. “My understanding is that supplies of the vaccine are already in place in hubs and will be shipped relatively quickly. The hope would be we can start vaccinating people as early as next week.”
Allergic reactions reported in the UK
After vaccinations with the Pfizer vaccine began in the UK on December 8, reports surfaced of two healthcare workers who experienced allergic reactions. They have since recovered, but officials warned that people with a history of severe allergic reactions should not receive the Pfizer vaccine at this time.
“For the moment, they are asking people who have had notable allergic reactions to step aside while this is investigated. It shows you that the system is working,” Schaffner said.
Both vaccine recipients who experienced anaphylaxis carried EpiPens, as they were at high risk for allergic reactions, Hewlett said. Also, if other COVID-19 vaccines are approved for use in the future, people allergic to the Pfizer vaccine might have another option, he added.
Reassuring role models
Schaffner supports the CDC Advisory Committee on Immunization Practices (ACIP) decision to start vaccinations with healthcare workers and residents of long-term care facilities.
“Vaccinating healthcare workers, in particular, will be a model for the general public,” said Schaffner, who is also a former member of the IDSA board of directors. “If they see those of us in white coats and blue scrubs lining up for the vaccine, that will provide confidence.”
To further increase acceptance of the COVID-19 vaccine, public health officials need to provide information and reassure the general public, Schaffner said.
Hewlett agreed. “I know there are a lot of people in the population who are very hesitant about vaccines. As infection disease specialists and people in public health, we are trying to allay a lot of concerns people have.”
Reassurance will be especially important in minority communities. “They have been disproportionately affected by the virus, and they have a traditional history of not being optimally vaccinated,” Schaffner said. “We need to reach them in particular with good information and reassurance…so they can make good decisions for themselves and their families.”
No vaccine is 100% effective or completely free of side effects. “There is always a chance there can be adverse reactions, but we think for the most part this is going to be a safe and effective vaccine,” said Hewlett, medical director at the Division of Disease Control and deputy to commissioner of health at the Westchester County Department of Health in White Plains, New York.
Distribution: Smooth or full of strife?
In addition to the concern that some people will not take advantage of vaccination against COVID-19, there could be vaccine supply issues down the road, Schaffner said.
Culver agreed. “In the early phases, I expect that there will be some kinks to work out, but because the numbers are relatively small, this should be okay,” he said.
“I think when we start to get into larger-scale vaccination programs — the supply chain, transport, and storage will be a Herculean undertaking,” Culver added. “It will take careful coordination between healthcare providers, distributors, suppliers, and public health officials to pull this off.”
Planning and distribution also should focus beyond US borders. Any issues in vaccine distribution or administration in the United States “will only be multiplied in several other parts of the world,” Culver said. Because COVID-19 is a pandemic, “we need to think about vaccinating globally.”
Investigating adverse events
Adverse events common to vaccinations in general — injection site pain, headaches, and fever — would not be unexpected with the COVID-19 vaccines. However, experts remain concerned that other, unrelated adverse events might be erroneously attributed to vaccination. For example, if a fall, heart attack, or death occurs within days of immunization, some might immediately blame the vaccine product.
“It’s important to remember that any new, highly touted medical therapy like this will receive a lot of scrutiny, so it would be unusual not to hear about something happening to somebody,” Culver said. Vaccine companies and health agencies will be carefully evaluating any reported adverse events to ensure no safety signal was missed in the trials.
“Fortunately, there are systems in place to investigate these events immediately,” Schaffner said.
Pregnancy recommendations pending
One question still looms: Is the COVID-19 vaccination safe for pregnant women? This isn’t just a question for the general public, either, Schaffner said. He estimated that about 70 percent of healthcare workers are women, and data suggests about 300,000 of these healthcare workers are pregnant.
“The CDC’s Advisory Committee on Immunization Practices will speak to that just as soon as the EUA is issued,” he added.
Patients are asking Culver about the priority order for vaccination. He said it’s difficult to provide firm guidance at this point.
People also have “lingering skepticism” about whether vaccine development was done in a prudent way, Culver said. Some people question whether the Pfizer vaccine and others were rushed to market. “So we try to spend time with the patients, reassuring them that all the usual safety evaluations were carefully done,” he said.
Another concern is whether mRNA vaccines can interact with human DNA. “The quick, short, and definitive answer is no,” Schaffner said. The m stands for messenger — the vaccines transmit information. "Once it gets into a cell, the mRNA does not go anywhere near the DNA, and once it transmits its information to the cell appropriately, it gets metabolized, and we excrete all the remnants."
Hewlett pointed out that investigations and surveillance will continue. Because this is an EUA and not full approval, “that essentially means they will still be obligated to collect a lot more data than they would ordinarily,” he said.
How long immunoprotection will last also remains an unknown. “The big question left on the table now is the durability,” Culver said. “Of course, we won’t know the answer to that for quite some time.”
Schaffner and Culver have disclosed no relevant financial relationships. Hewlett was an employee of Pfizer until mid-2019. His previous work as Pfizer’s senior medical director of global medical product evaluation was not associated with development of the COVID-19 vaccine.
This article first appeared on Medscape.com.